6
Changing the Regulatory Landscape
The first prong of the committee’s blueprint for reducing tobacco use, set forth in Chapter 5, envisions strengthening traditional tobacco control measures. If the plan set forth in Chapter 5 is successfully implemented and sustained, it could have a significant impact on tobacco use; but even an optimistic projection leaves prevalence at 10 percent in 2025, and a more realistic projection might be 15 percent. The main argument presented in this chapter is that a more substantial long-term impact requires a change in the current legal framework of tobacco control to a new, innovative regulatory approach that takes into account the unique history and characteristics of tobacco.
Over the past two decades, tobacco control policy has developed incrementally as the nation has moved step-by-step from a laissez-faire legal system embedded in a smoking culture to a system with coherent antismoking policy grounded in public health. Incremental reforms, however, will not end the nation’s tobacco problem. A more fundamental shift must occur. It is time for Congress and other policy makers to change the legal structure of tobacco policy, thereby laying the foundation for a strategic initiative to end the nation’s tobacco problem, that is, reducing tobacco use to a level that is insignificant from a public health standpoint. In this chapter, the committee sets forth the blueprint for a new regulatory approach.
Currently (and under the interventions of the blueprint outlined in Chapter 5), the implicit model of tobacco control is demand reduction combined with reactive efforts to prevent the tobacco industry from impeding demand-reducing policies. This leaves in place the supply side (the product and its existing channels of distribution) as well as all the existing
incentives for attracting more consumers and selling more cigarettes. A more ambitious strategy would ask these questions: Can anything be done to substantially curtail the availability of tobacco products? Can anything be done to change tobacco products to make them less hazardous? Is it possible to bring the industry’s incentives into closer alignment with the public health goals of tobacco control? No existing regulatory statute provides a model for tobacco products because there is no other lawful product for which the declared public goal is to suppress its use altogether. A new legal regime, new models, and new policy paradigms are needed.
The challenge, then, is to craft a policy framework that is aligned with the unique aim of tobacco control policy: to substantially reduce, if not eliminate, the use of this unusually damaging product without replicating the problems associated with the prohibition of alcohol in the 1920s and with the contemporary prohibitions of illegal drugs (e.g., widespread noncompliance, violent black markets, corruption, and high rates of arrest). In this chapter and Chapter 7, the committee offers several ideas for more fundamental change.
FEDERAL REGULATORY AUTHORITY IS NEEDED
It is clear that the U.S. Congress has the constitutional authority to enact national legislation bearing on all domains of tobacco control, including banning the distribution and use of tobacco products, analogous to the authority that it exercises over controlled substances such as peyote and marijuana. The pertinent policy questions are (1) whether Congress should exercise its constitutional authority (by prescribing national rules or by delegating the authority to do so to a federal agency, such as the Food and Drug Administration [FDA]), or, conversely, whether it should allow the states to exercise primary authority to regulate the production, distribution, marketing, and use of tobacco; and (2) if Congress does exercise federal regulatory authority in any of these domains, whether it should leave any room for supplemental state regulation and, if so, within what constraints. The committee believes that the time has come for Congress to exercise its acknowledged authority to regulate the production, marketing, and distribution of tobacco products while freeing the states to supplement federal action with their own measures that aim to suppress tobacco use and that are compatible with federal law.
Relationship Between Federal and State Tobacco Regulation
Setting aside the complexities of a complicated body of federal law, Congress has essentially three options: (1) state control, in which the federal government leaves regulation in a particular domain to the states (and,
depending on state law, to localities); (2) federal preemption, in which the federal government has complete regulatory control and precludes any state regulation in the area; and (3) complementary regulation, in which the federal government establishes regulations on a particular subject but does not exclude the states from also establishing regulations. Under the third approach, the federal action typically establishes minimum requirements for the whole country (the basic requirements) while allowing the states to adopt more stringent regulations. Familiar examples of these three approaches include the state regulation of the retail distribution of alcohol, federal preemption of the state regulation of employee benefit plans under the Employee Retirement and Income Security Act, and complementary regulation of controlled substances.
At present, tobacco regulation in the United States is characterized by plenary state control except in the federally preempted domain of information or warnings “based on smoking and health,” as defined in the 1969 Cigarette Labeling Act. The preemption language adopted by the Congress has been construed broadly by the U.S. Supreme Court to preempt “failure to warn” product liability suits under state tort law and direct state or local regulation of tobacco advertising, as well as the obviously preempted area of mandated warnings on packages or in advertisements (Cipollone v. Liggett Group I1992;505 U.S. 504; Lorillard Tobacco Company v. Reilly 2001;533 U.S. 525).
Whether federal regulation is needed and, if so, whether and to what extent the federal regulatory statute should preempt compatible state regulation are complex inquiries involving highly contextual judgments. However, the general contours of the analysis can be summarized succinctly. On the one hand, a decision to preempt state regulation in favor of exclusive federal authority may impose an overly rigid and inefficient uniform nationwide approach that is contrary to public preferences in many jurisdictions, especially when the benefits and costs from regulation vary considerably across jurisdictions. For example, a state with no tobacco farmers to protect may want to impose stricter regulations than a tobacco-growing state, or a state with a large Hispanic population might want to insist on labels in both English and Spanish. As another example, because the prevalence of smoking varies by as much as threefold among the states, the voters in a state with more smokers may find public smoking regulations more oppressive than the voters in another state with fewer smokers.
Nationwide rules can be especially inefficient when it comes to regulatory matters that fall almost exclusively within a state’s geographic boundaries. Thus the regulation of smoking in public places is primarily a local matter, with individual business establishments and local governments bearing the brunt of the compliance costs. The same might also be said for restrictions on point-of-sale advertising, including the in-store placement of
placards and the proximity of external store window signs to schoolyards. Consider in this respect a hypothetical national ban on certain activity (such as the sale of alcohol) within a certain radius of a school. What seems like a perfectly reasonable distance, say, 1,000 yards, would have a very different impact on lawful access to alcohol in a dense urban area, where the ban might cover an entire city, than it would in a less densely populated suburban or rural area. Finally, the imposition of a nationwide approach can increase the costs of a regulatory error, especially when there is uncertainty over the most appropriate form of regulation and when the scientific evidence and business environment are changing.
As these observations suggest, there are many reasons why regulation of retail distribution, marketing, and use of tobacco should presumptively be left to state regulation and, to the extent that federal regulation is adopted in these domains, that supplementary state regulation should be permitted as long as it is compatible with the federal regulatory objectives.
However, in some contexts, there are strong arguments for a national regulatory approach, and perhaps for national uniformity. One such context is the regulation of commercial products for which there is a national market and where the regulated products are easily transported across state lines. For example, if there are substantial differences in regulatory standards from one state to another, strong incentives would exist for people to purchase less heavily regulated products in one state and sell them in a state with more demanding regulatory requirements, thereby potentially undermining the latter state’s regulatory objectives, creating a black market, and possibly attracting the participation of criminal syndicates. Another concern that sometimes arises in situations involving diverse state regulation of product characteristics is inefficiency attributable to the need for manufacturers to comply with 50 different requirements. A third consideration is the need for continuing oversight and research by experts, as is often the case in environmental protection.
Regulation of pharmaceutical products, where national standards have prevailed since adoption of the Pure Food and Drugs Act of 1906, is pertinent illustration of a strong national regulatory interest, potentially high costs to state-by-state variations, and need for ongoing regulatory oversight. Over time, the Congress has tended to both increase the scope of federal regulatory authority and precluded additional state regulation in areas in which the FDA has acted. Explicit statutory preemption has been adopted in connection with FDA regulation of medical devices, over-the-counter drugs, and cosmetics.
As will be explained below, the committee favors strong regulation of tobacco product characteristics, packaging, labeling, and promotion and distribution. The colossal failure of the tobacco market to produce accurate information regarding the health effects of tobacco products and
to produce adequate incentives for companies to develop less hazardous products, the national scope of the problem, and the need for creating and sustaining regulatory expertise argue strongly for national regulation. The residual question is whether supplemental, compatible state regulation should be permitted. In the committee’s view, a uniform national approach preempting state regulation altogether makes the most sense in relation to regulations governing tobacco product design and labeling, as well as in relation to marketing through national media. A uniform national approach makes the least sense in relation to the retail distribution, local marketing, and consumption of tobacco. Federal regulation may not be needed in some of these areas at all, but even if federal regulations are adopted, the federal rules should set the floor, and supplemental state regulation should be permitted. In this latter respect, the current federal preemption provision unduly constrains the prerogatives of the states in regulating the local marketing of tobacco products.
Recommendation 23: Congress should repeal the existing statute preempting state tobacco regulation of advertising and promotion “based on smoking and health” and should enact a new provision that precludes all direct state regulation only in relation to tobacco product characteristics and packaging while allowing complementary state regulation in all other domains of tobacco regulation, including marketing and distribution. Under this approach, federal regulation sets a floor while allowing states to be more restrictive.
This approach was embodied in the proposed “Family Smoking Prevention and Tobacco Control Act” (S. 625 and HR 1008), introduced on February 15, 2007, by Senators Kennedy and Cornyn with 29 other Senators and Congressmen Waxman, Davis, and Palone. This will be referred to as the proposed Tobacco Control legislation in this report.
Any federal statute preempting direct state regulation of the product and its packaging should not preempt any private or public causes of action, in state or federal courts, based on a failure to warn consumers about any risks (of which the company was aware) not covered by the federally prescribed warnings or based on claims of fraud or conspiracy. Congress should make its intentions regarding the narrow scope of preemption clear in the legislative record.
EMPOWERING FDA TO REGULATE TOBACCO
In 1994, the Institute of Medicine (IOM) report Growing Up Tobacco Free urged Congress to adopt a regulatory framework for tobacco products and to empower a federal regulatory agency to implement it:
Congress should confer upon an administrative agency the authority to regulate the design and constituents of tobacco products whenever it determines that such regulation would reduce the prevalence of dependence or disease associated with use of the product or would otherwise promote the public health. The agency should be specifically authorized to prescribe ceilings on the yields of tar, nicotine, or any other harmful constituent of a tobacco product (IOM 1994, p. 246-247).
Soon after the IOM report was released, the FDA surprised many observers by claiming that Congress had already given the agency the authority to regulate cigarettes as nicotine delivery devices under the Food, Drug, and Cosmetic Act (FDCA) and by issuing a rule regulating the marketing and distribution of cigarettes to youth. Eventually, the U.S. Supreme Court rejected the FDA’s position, ruling that the FDCA did not authorize the FDA to regulate tobacco products and striking down the 1996 FDA Tobacco Rule (FDA v. Brown & Williamson Tobacco Corp., 529 U.S. 120, 2000).
Since the U.S. Supreme Court’s decision, proposals have been pending in the Congress to authorize the FDA to regulate tobacco products. The version pending at the time of the final version of the committee’s report is the proposed Tobacco Control legislation, which would give the FDA wide-ranging authority over the manufacture, distribution, and promotion of tobacco products with a few exceptions, which will be described below. The power granted to FDA in the proposed Tobacco Control legislation is even more extensive than that envisioned in Chapter 8 of Growing Up Tobacco Free (IOM 1994).
Under the proposed Tobacco Control legislation:
-
The 1996 FDA Tobacco Rule relating to youth access and marketing to youth would be revived.
-
FDA would have broad authority to promulgate tobacco product standards whenever such a standard is found to be “appropriate for protection of the public health,” taking into consideration “the risks and benefits to the population as a whole, including users and non-users of tobacco products.” (The bill embodies the principles relating to products purporting to reduce exposure to toxins and to reduce disease risk developed by the IOM in Clearing the Smoke [2001].)
-
Legally required package warnings would be strengthened immediately and FDA would have the authority to revise these requirements upon finding “that such a change would promote greater public understanding of the risks associated with tobacco.”
-
FDA would have authority to “restrict … the sale and distribution of a tobacco product if the Secretary [of the U.S. Department of Health and Human Services] determines that such regulation would be appropriate for the protection of the public health.”
-
FDA would be empowered to restrict the advertising and promotion of tobacco products “to the full extent permitted by the First Amendment” upon finding “that such regulation would be appropriate for the protection of the public health.”
Each of these topics is discussed below. Overall, however, the committee reiterates the view taken by two previous IOM committees (IOM 1994, 2001): broad federal regulatory authority over the manufacture, distribution, marketing, and use of tobacco products is an essential element of a comprehensive public health approach to tobacco control.1 Congress does not have the institutional capacity to monitor and respond to ongoing innovations in product design (especially those purporting to reduce exposure to tobacco-related toxins), or to changes in marketing or patterns of consumption, whereas FDA personnel are well-positioned to gather the necessary information and to make evidence-based scientific judgments about the likely public health consequences of alternative regulatory approaches. Overall, the potential benefits of agency regulation in this context seem to far outweigh its potential costs. Specifically, the committee does not agree with the view expressed by some tobacco control advocates that empowering FDA to regulate the manufacture, distribution, promotion, and use of tobacco products necessarily “legitimizes” these products or that it will unavoidably lead to the “regulatory capture” of the agency by the tobacco industry. Other objections to the federal regulation and to particular features of the proposed Tobacco Control legislation are discussed below in the specific contexts in which they arise.
Recommendation 24: Congress should confer upon the FDA broad regulatory authority over the manufacture, distribution, marketing, and use of tobacco products.
A REGULATORY PHILOSOPHY
The ultimate regulatory goal is to reduce smoking-related mortality and morbidity to a level that is acceptable to a well-informed American public.
Under the present circumstances, given what is known about the health consequences of tobacco use, reducing the prevalence of smoking and the level of per-capita cigarette consumption are reasonable proxies for reducing harm. In the context of other health and safety regulations, the goal of reducing cigarette consumption to a socially acceptable level might mean reducing it to the lowest feasible level or the level at which the social costs of trying to reduce it further exceed the public health benefits of doing so. Either way, feasibility is a significant constraint on the regulatory measures that can sensibly be adopted. Most importantly, a total prohibition against tobacco manufacture and distribution is not a realistic option at present or for the foreseeable future.
The United States now has 44.5 million adult smokers (CDC 2005), the vast majority of whom are addicted to the nicotine in cigarette smoke. If it were possible to prevent smoking initiation altogether, it might be feasible to embrace a nicotine maintenance approach, under which tobacco products would be lawfully available only by prescription to people who are addicted to nicotine. However, the experience with marijuana and other illegal drugs demonstrates that prohibition does not eliminate initiation even when there is no lawful market for a psychoactive drug, and it is implausible to expect that tobacco products lawfully available to more than 40 million addicts would not spill over into a large “gray” market that would sustain ongoing initiation, especially by young people. Nor is it plausible that the billions of cigarettes produced for the international market would not find their way into the United States.2
The challenge, then, is to frame a policy for tobacco production and distribution within the context of a regulated market. What should be the aims of the regulatory agency over the short term? In the committee’s view, the following aims should guide tobacco policy for the foreseeable future:
-
Undertake significant and sustained efforts to reduce the rate of initiation of smoking
-
Maximize the options available to addicted smokers to help them quit or reduce their risk
-
Prevent people from becoming addicted to tobacco products if they use them
-
Reduce the risks of using tobacco products to the users and to others
|
2 |
Although prohibition accompanied by prescription-only cigarettes for already addicted smokers might reduce smoking initiation, the committee anticipates that the costs of enforcing such an approach would be substantial and would exceed the potential public health gains. However, such an approach may be worthy of consideration at some future time, especially if it is combined with the nicotine reduction concept sketched in Chapter 7. Innovative strategies of this kind should be explored by the tobacco policy research office recommended in that chapter. |
Policies designed to achieve these aims must be formulated and implemented on the basis of a careful consideration of their potential effectiveness and potential costs. A key factor is the need to avoid creating a substantial black market and its associated costs.
The remainder of this chapter sets forth several elements of the comprehensive regulatory strategy that should supplement the traditional tobacco control approaches described in Chapter 5. Specifically:
-
Tobacco product characteristics should be regulated to protect the public health.
-
Messages on tobacco packages should promote health.
-
The retail environment for tobacco sales should be transformed to promote the public health.
-
New models for regulating the retail market should be explored.
-
The federal government should mandate industry payments for tobacco control and should support and coordinate state funding.
-
Tobacco advertising should be further restricted.
-
Targeting of youth by tobacco manufacturers for any purpose should be banned.
-
Youth exposure to smoking in movies and other media should be reduced.
-
Surveillance and evaluation should be enhanced.
TOBACCO PRODUCT CHARACTERISTICS SHOULD BE REGULATED
As noted above, the proposed Tobacco Control legislation would grant FDA the authority to regulate tobacco products. FDA was selected because it is the only existing regulatory agency with expertise both in scientific and health issues and in product regulation. The authority that would be conferred on FDA for tobacco regulation in the proposed Tobacco Control Legislation parallels in many ways current FDA authority for the regulation of drugs, although different regulatory criteria are needed. Requiring tobacco products to be “safe” is not an available option, of course, and prohibition of the existing products is not a feasible regulatory strategy. Overall, the regulatory standard should be to “protect the public health” by reducing initiation, promoting cessation, preventing relapse, reducing consumption, and reducing product hazards. This standard incorporates its own limitation because it will require the agency to evaluate the likely consumer responses to any proposed regulation, including the likelihood of product substitution and the creation of black markets that could nullify the anticipated public health benefits of the regulation.
FDA regulates many consumer products, including drugs and foods, and the agency has many tools at its disposal that can be deployed in tobacco regulation. FDA regulation serves to inform consumers about the constituents of the products that they consume and to enhance the safety of these products. FDA can require existing and new products to meet toxicant exposure standards and can promote new standards and make products less hazardous. FDA can also ensure that the claims made about products are truthful and are not misleading. If FDA is authorized to regulate tobacco products, the task should be undertaken in the context of a comprehensive framework that includes the regulation of novel tobacco products and other nicotine delivery products, the delivery of tobacco smoke constituents, and the regulation of medications used to treat tobacco addiction.
The need for a tobacco regulatory authority is illustrated by the history of the low yield cigarette, which has been described in detail in a recent IOM report and in National Cancer Institute (NCI) Monograph 13 (IOM 2001; NCI 2001). In brief, low-yield cigarettes were developed after scientific evidence indicated that cigarette tar contributed to cancer. Low-tar cigarettes were implicitly promoted by the tobacco industry as a way to reduce the health risks of smoking. A large majority of smokers believe that low-yield cigarettes were less harmful, and many have switched to low-yield cigarettes rather than quit smoking (Kozlowski et al. 1998). However, because of the engineering characteristics of the cigarettes and the tendency of the smokers to maintain their desired levels of nicotine in their bodies, smokers easily compensate for low-yield cigarettes by smoking more intensively and by smoking more cigarettes (NCI 2001). One strategy used to decrease cigarette tar delivery was to change tobacco blends. However the tobacco contained higher levels of nitrogenous chemicals that resulted in the generation of larger amounts of NNK, a tobacco-derived substance that is known to be a pulmonary carcinogen. The ultimate result of the shift to low-yield cigarettes was no reduction of toxic exposures and no impact on smoking-related disease risks. Unfortunately, however, low yield cigarettes were promoted for more than 30 years before it was publicly understood that they have no beneficial effect on smoking-related risk (NCI 2001).
Evidence introduced in the U.S. Department of Justice Racketeering Influenced and Corrupt Organization (RICO) suit against the tobacco manufacturers indicated that for many years company scientists and company officials had internal information indicating that “light” cigarettes might not deliver lower doses of toxicants and that such cigarettes delivered lower dosages to machines than they did to human smokers (NCI 2001). Moreover, tobacco company researchers had information that the tar from a “light” cigarette might be qualitatively more toxic on a milligram-per-milligram basis than the tar from a regular filtered cigarette. However, public health professionals and officials in other government agencies failed to appreciate
the seriousness of the problem for several reasons. First, lacking the information possessed by tobacco manufacturers, they mistakenly assumed that smoking machines could accurately gauge the relative toxicity of cigarettes. They underestimated the complexity of the cigarette product and the ability of manufacturers to change the product in ways not reflected in the machine-based measurements. Second, lacking complete information about smoker compensation possessed by tobacco manufacturers, they underestimated the behavioral inclination of smokers to maintain their desired levels of nicotine intake. As a result of industry deception, there was a massive regulatory failure, as nothing was done to control how these products were marketed.
The best way to prevent such a sequence of events from occurring again is to have a regulatory body that can systematically assess toxic exposures, make judgments about potential risks from tobacco products, regulate industry claims about the products to ensure that they are accurate and not misleading, set minimum standards, and provide relevant surveillance to determine actual human exposures and risks. This is particularly important because a number of tobacco companies are developing and marketing tobacco products that are intended to reduce the harm from smoking and presumably will be promoted as such.
Goals of Tobacco Product Regulation
The regulation of tobacco product characteristics can be seen as having two primary goals (IOM 2001). One is to reduce the harm from the continued use of tobacco products. This might be achieved by reducing the toxic emissions from cigarettes or the toxic constituents of smokeless tobacco. Reducing toxic exposures would potentially lower the risk and severity of disease in people who continue to smoke. It is essential, however, that the federal government assure that consumers are informed about what is and what is not known about the risks of using products that result in reduced toxic exposures (reduced exposure products). Moreover, regulators must take steps to reduce the likelihood that the availability of reduced-exposure products will increase initiation or reduce the number of users who quit. The danger that the marketing of reduced-exposure products could lead to an increase in smoking prevalence by altering risk perceptions about smoking is one of the greatest challenges that the FDA will need to address.
The second goal of regulating tobacco product characteristics is to reduce consumption. The most promising way of reducing consumption through product regulation would be to make cigarettes less addictive, thereby making quitting easier and preventing initiating smokers from becoming addicted. Another promising strategy is the development of new medications for the treatment of nicotine addiction. To the extent that harm
reduction policies are pursued, it would be desirable to bring modified tobacco products and medications for smoking cessation within a common regulatory framework.
Reducing Harm from Continued Use
The regulation of tobacco products could potentially reduce the harm of tobacco use in smokers who continue to use these products. The approaches to and the pitfalls associated with harm reduction have been reviewed extensively in a recent IOM report (IOM 2001). Some of the ways in which the harm of tobacco products might be reduced include (1) setting performance standards to reduce toxic emissions from cigarettes, (2) evaluating novel and potential reduced exposure products (PREPs), (3) educating users about the risks and benefits of novel products, and (4) encouraging the development of medication that can substantially reduce cigarette consumption (for example, maintaining abstinence through the use of medications). In addition, a national regulatory program would conduct ongoing surveillance of the use of novel and traditional tobacco products.
Making Tobacco Products Less Addictive
The manufacture of cigarettes allows for the control of the nicotine content of the tobacco. Nicotine can be extracted from tobacco and then added back to tobacco to achieve any desired level of nicotine. Although nicotine-free cigarettes were marketed in the past, they were not commercially successful.
For people addicted to controlled substances or alcohol, a common approach to reducing their drug use and minimizing withdrawal symptoms is to gradually taper the use of an addictive drug over time. Such gradual tapering allows a gradual reduction of the dose, a decrease in the level of tolerance, and minimization of the severity of withdrawal symptoms. This type of treatment has been used to treat heroin addicts by gradually increasing the doses of methadone that they receive and to treat alcohol and sedative drug addicts by using drugs such as phenobarbital and benzodiazepines.
An analogous approach could be the basis for a regulatory strategy to reduce the addictiveness of cigarettes (Benowitz and Henningfield 1994; Henningfield et al. 1998). The idea would be to reduce the nicotine content of cigarette tobacco gradually over time. This would result in a lowering of the level of nicotine intake and, presumably, a lowering of the level of nicotine dependence. As nicotine levels become very low, cigarettes would become much less addicting. As a consequence, fewer novice smokers would become regular lifelong smokers. For previously addicted smokers,
a reduction of nicotine dependence would be expected to facilitate quitting. This approach is discussed in more detail in Chapter 7.
Another proposed approach to making tobacco products less addictive includes removing flavorants and additives that enhance the sensory characteristics of cigarettes, as sensory characteristics are thought to contribute to the reinforcing qualities and the addictiveness of cigarettes.
Increasing Medicinal Nicotine Alternatives
Another way to deal with potential compensation would be to make medicinal nicotine more readily available. Currently, nicotine-containing medications are available both over the counter and by prescription, but they tend to be more expensive than cigarettes and are more difficult to obtain. With the ready availability of nicotine-containing medications, smokers could obtain supplemental nicotine to compensate for reduced nicotine intake from low-nicotine–content cigarettes (Henningfield et al. 1998). After complete smoking cessation, the nicotine dose in the medication could be tapered down over time to finally eliminate all dependence on the drug.
The Proposed Tobacco Control Legislation Provisions for FDA Product Regulation
The proposed Tobacco Control legislation would give the FDA authority to “restrict the sale and distribution of a tobacco product if the Secretary determines that such regulation would be appropriate for the protection of the public health.” This broad authority is limited only in the following ways: the FDA (1) may not prohibit the sale of tobacco products altogether, (2) may not require a prescription for tobacco products, (3) may not adopt a minimum purchase age higher than 18 years, and (4) may not ban any particular category of retail outlet from selling tobacco products. As general principles guiding FDA authority, decisions would be based on sound science; the goals would be both to reduce consumption and to reduce the mortality and morbidity caused by tobacco use, and the FDA efforts are expected to complement (and not replace) proven prevention and cessation efforts. The proposed Tobacco Control legislation provisions for FDA product regulation has the key elements described in the following sections.
Disclosure
The bill would require tobacco companies to disclose to the FDA all chemical compounds found in both their tobacco products and the products’ smoke, whether these compounds are added or occur naturally, by quantity. Tobacco companies would be required to disclose the content
and form a delivery based on standards established by the FDA, to disclose research on their product as well as behavioral aspects of its use, and to notify the FDA whenever there is a change in a product.
Testing
The bill would grant the FDA the authority to promulgate regulations on cigarette testing methods, including how the cigarettes are tested and which smoke constituents must be measured. The FDA would also determine what product test data are disclosed to the public to inform consumers, without misleading them, about the risks of tobacco-related disease.
Product Standards
The FDA would be given broad authority to promulgate tobacco product standards whenever such a standard is found to be “appropriate for protection of the public health,” taking into consideration the risks and benefits to the population as a whole, including users and nonusers of tobacco products. The FDA is specifically directed to take into account the increased or decreased likelihood that existing users of tobacco products would stop using such products and the increased or decreased likelihood that those that do not use tobacco products would start using such products. As indicated earlier, the proposed Tobacco Control legislation reflects a statutory formulation of the principles developed by the IOM in Clearing the Smoke. The FDA is specifically authorized to promulgate standards requiring “reduction of nicotine yields of the product” as long as it does not require that nicotine be reduced “to zero.” (The bill stipulates that only an act of Congress can require that nicotine yields be reduced to zero.) The agency is also empowered to promulgate standards “for reduction or elimination of other constituents, including smoke constituents or harmful components of the product.” In short, the FDA is empowered to embrace a harm reduction approach by reducing the toxicity of tobacco products.
Potential Reduced-Exposure Products
The bill would authorize the FDA to develop specific standards for evaluating novel products that companies intend to promote as reducedexposure or reduced-risk products. Such products would be those, as indicated by the manufacturer explicitly or implicitly, that present a lower risk of tobacco-related diseases or that are less harmful than other commercially-marketed tobacco products; tobacco products or their smoke that contain a reduced level of a substance or whose use results in a reduced exposure to a substance; or tobacco products that, when smoked, do not contain or are
free of a particular substance. The FDA would be granted the authority to regulate reduced-exposure and reduced-risk health claims and to ensure that there is a scientific basis for the claims that are permitted.
Public Health Concerns Regarding Federal Tobacco Product Regulation
Proposed FDA regulation has received mixed support from the public health community and from the tobacco industry. Some public health groups, such as the Campaign for Tobacco-Free Kids, have worked with members of Congress in developing the proposed legislation and have been highly supportive of its content. Other public health groups have also endorsed the proposal (Tobacco Free Kids 2007a, 2007b). However, some tobacco control advocates have opposed the bill (AAPHP 2007). They have done so on two quite different grounds. Some opposed the product regulation features of the bill because adoption of a regulatory approach will, in some sense, legitimize a product that should be banned outright. For these opponents, the goal is prohibition, and the most likely political path to prohibition is state-by-state action. Others have argued that the proposed regulatory framework for PREPs, based on the recommendations in Clearing the Smoke, is too demanding and may impede the development of reduced-exposure products by stifling innovation and retarding competition with safer products, especially by small companies and new entrants into the market. For these opponents, the goal is harm reduction, and the federal government should be giving companies incentives to develop PREPs rather than putting regulatory obstacles in their paths.
A third line of objection to federal tobacco product regulation arises out of a deep-seated skepticism about the ultimate utility of federal tobacco regulation. Tobacco control advocates are mindful of the tobacco industry’s past successes in using federal legislation to obstruct tobacco control efforts, and their concerns are reinforced by general skepticism about the natural history of regulation. Under this view, regulatory agencies rarely have as much information as the regulated companies and therefore are prone to regulatory mistakes. Eventually, regulatory agencies tend to be captured by the industry that they are regulating, performance standards tend to be set by the industry itself, and consumers are often worse off than they would have been without regulation.
For some of the critics who hold this perspective, the main problem with the tobacco industry is not under-regulation, but, rather, oligopolistic concentration, which tends to encourage collusion and suppress competition. These critics believe that the industry has engaged in collusive activity throughout the century—even since the political and legal tide turned against it—and there is no reason to think that this will change. As long as this is true, the argument continues, there is a need to be realistic about
the poor prognosis for getting positive results through federal regulation. Rather, ways should be found to break up the industry. From this perspective, competition, not regulation, is the answer.
Committee Response to Public Health Concerns
The committee has given careful consideration to the concerns raised by some public health advocates about the proposed Tobacco Control legislation’s product regulation features. In the end, we think the fears are overstated and that federal tobacco product regulation is an essential element of a long-term strategy for achieving substantial reductions in tobacco use and in tobacco-related morbidity and mortality. One of the major reasons for this conclusion is that active federal monitoring and regulation of the tobacco market is needed to prevent, curtail, and counteract industry efforts to undermine tobacco control policies. A second reason is that the market for reduced-risk products is likely to replicate the “light” cigarette experience in the absence of aggressive regulation of claims about these products.
Reducing use and reducing harm Although harm reduction might be a useful adjunct to a comprehensive tobacco control strategy, it is not at the center of the committee’s charge, which is to propose a blueprint for reducing tobacco use in the United States. Reduced-exposure cigarette products may not reduce the prevalence of tobacco use and may have little public health payoff in the short term; moreover, given the numerous uncertainties identified in Clearing the Smoke, even an optimist would have to be reserved about the long-term payoff. A recent simulation study by Tengs and colleagues reinforces the cautious perspective enunciated in Clearing the Smoke (Tengs et al. 2004). They conclude that even if the new products do reduce harm to smokers by as much as 20 percent, the long-term consequences are highly likely to be negative if the rate of cessation drops by 20 to 40 percent.
Whatever the long-term prospects for harm reduction as a tobacco control strategy, however, industry efforts to develop and market reduced-risk or reduced-exposure products must not be left unattended, because their promotion and use could interfere with efforts to reduce tobacco use. This was the sad lesson of so-called “light” cigarettes. From this standpoint, it is imperative that Congress empower the FDA to regulate the claims that may be made regarding PREPs. In this respect, the FDA’s regulatory jurisdiction over PREPs is an essential component of the blueprint.
Risks and Benefits of Regulation
The general arguments against product regulation enunciated by opponents of the proposed Tobacco Control legislation strike the committee as
overstated in the specific context of tobacco regulation. First, the committee believes that the FDA, a public health agency, is unlikely to become allied with, much less captured by, the tobacco industry. Admittedly, the tobacco industry’s successful efforts to fend off regulatory action in the 1970s and 1980s give one pause. For example, the NCI’s harm reduction program (the Tobacco Working Group [TWG]), which operated from 1968 to 1977, failed because many of its key members were scientists with direct ties to tobacco manufacturers. The NCI did not assemble a working group of independent scientists in great part because there was insufficient expertise in cigarette design and evaluation outside the industry. As documents produced in litigation have revealed, industry scientists, notwithstanding their pronouncements that they were participating solely as individuals rather than as company representatives, repeatedly informed their companies’ legal counsel of any contemplated action by the TWG that might threaten the industry’s interests. Company scientists, acting on instructions from counsel, successfully blocked promising research projects that had been proposed to the TWG. Once the TWG was disbanded, the tobacco companies retained two of its government representatives as consultants. In particular, the director of NCI’s program, Gio Gori, went on to represent the Brown and Williamson Corporation in various regulatory proceedings.
Likewise, the Federal Trade Commission (FTC) has been criticized for its failure to stand up to the tobacco manufacturers. As documents produced in litigation reveal, the FTC passively allowed the industry to obscure the fact that the machine-tested tar and nicotine testing bore little relationship to human exposure. The FTC had no independent knowledge of cigarette design, and the FTC did not regulate the amounts of tar and nicotine delivered. However, its perpetuation of the testing regime merely served as an instrument for disciplining firms that threatened the industry-wide agreement to stick to machine-measured tar and nicotine numbers and avoid any suggestion that the machines did not accurately gauge human exposure. Moreover, the fact that the Master Settlement Agreement (MSA) had to include a variety of restrictions on misleading and youth-oriented advertising provides evidence of regulatory default by the FTC. Finally, the fact that Judge Gladys Kessler, in her final judgment and order in the government’s RICO suit, enjoined the major tobacco manufacturers from using such words as “low tar,” “light,” “ultra-light,” “mild,” and “natural” provides even further evidence that the FTC has failed to carry out its legislative mandate (Tobacco Free Kids 2006).
The committee acknowledges the regrettable history of federal regulatory default. However, the committee believes that the widespread condemnation of the tobacco industry’s deceit during the 1970s and 1980s, most recently documented in Judge Kessler’s opinion in the federal government’s RICO suit, makes it less likely that any federal regulatory agency, especially
an agency charged with protecting the public health such as the FDA, will be captured by the tobacco industry. There is no doubt that the agency could still be misled, but the proposed Tobacco Control legislation’s disclosure requirements are designed to reduce the informational disparity between the industry and the regulators, and the FDA scientists can be expected to be highly skeptical of the data and the interpretations of those data provided by the tobacco industry.
Aside from regulatory capture, the other major criticism of the proposed Tobacco Control legislation is that the agency might retard innovation in the development of PREPs by insisting on high standards of proof regarding the effects of reduced exposure to tobacco toxins. In the committee’s view, however, even if the regulatory criteria for permitting reduced-exposure and reduced-risk claims might be applied too cautiously, the dangers of unregulated competition over safety are greater than the dangers of retarding safety innovation. The underlying question regarding harm reduction is whether it is better to err in one direction or the other. In the committee’s view, the highest priority is to prevent another “light” cigarette disaster.
The committee concludes that product regulation by the FDA will advance tobacco control efforts in the United States and around the world. The proposed Tobacco Control legislation embodies the principles that should govern the regulation of tobacco products in the coming years. The disclosure and testing requirements are needed to correct massive information failures in the tobacco market. The IOM’s approach to reduced-exposure products, which is embodied in the bill, strikes the right balance between encouraging innovation and protecting the public from misleading claims. Empowering FDA to reduce the nicotine content of tobacco products has great potential (see Chapter 7).
Recommendation 25: Congress should empower FDA to regulate the design and characteristics of tobacco products to promote the public health. Specific authority should be conferred
-
to require tobacco manufacturers to disclose to the agency all chemical compounds found in both product and the product’s smoke, whether added or occurring naturally, by quantity; to disclose to the public the amount of nicotine in the product and the amount delivered to the consumer based on standards established by the agency; to disclose to the pubic research on their product, as well as behavioral aspects of its use; and to notify the agency whenever there is a change in a product;
-
to prescribe cigarette testing methods, including how the cigarettes are tested and which smoke constituents must be measured;
-
to promulgate tobacco product standards, including reduction of
-
nicotine yields and reduction or elimination of other constituents, wherever such a standard is found to be appropriate for protection of the public health, taking into consideration the risks and benefits to the population as a whole, including users and non-users of tobacco products; and
-
to develop specific standards for evaluating novel products that companies intend to promote as reduced-exposure or reduced-risk products, and to regulate reduced-exposure and reduced-risk health claims, assuring that there is a scientific basis for claims that are permitted.
These recommendations are generally compatible with Articles 9-11 of the WHO Framework Convention on Tobacco Control (WHO 2003).
MESSAGES ON TOBACCO PACKAGES SHOULD PROMOTE HEALTH
The tobacco industry has long used cigarette packaging to identify and market its products, and governments have long used cigarette packaging to convey messages about tobacco risk and exposure. As legal restrictions have increasingly reduced or eliminated media advertising, the importance of the package as a vehicle for promotion has increased (Slade 1997). The packages carried by smokers serve as mobile advertisements for particular products. Promotional displays of packages in retail outlets are also key marketing tools. In response to the increasing importance of the package in promotion, governments have begun to exert more control over packaging characteristics for the dual purposes of reducing this form of marketing and communicating directly with consumers.
Among the reasons for regulatory interest in tobacco packaging are
-
communicating product information to consumers and potential consumers,
-
warning consumers about hazards and thereby discouraging consumption,
-
communicating other health information (e.g., cessation hotline numbers),
-
preventing smuggling (by requiring documentation of excise tax payment),
-
preventing misleading messages by tobacco companies and providing corrective information to counteract previous deceptions,
-
preventing promotional messages by tobacco companies as other avenues of advertising are curtailed, and
-
“denormalizing” tobacco products.
The use of packages to convey tobacco-related health risks has a number of potential advantages over other forms of communication. The frequency of exposure is high. The messages are delivered at the moment a smoker desires another cigarette. The messages on packages also communicate information to the public at large, and not merely the consumer.
Package Warnings Regarding Tobacco-Related Health Risks
Congress first required health warnings on cigarette packages in 1966 and in advertisements in 1972. By 1985, four rotating warnings were required on both packages and in advertisements. However, U.S. package warnings are still not prominent and are located on the side of the package in small print (see Figure 6-1). In 1994, a previous IOM committee made the following observation about this country’s tobacco health warnings:
The adequacy of the current cigarette warnings has been repeatedly questioned by public health specialists. Moreover, in the committee’s view federal cigarette labeling legislation has reflected an unsatisfactory compromise between the public’s health and the tobacco industry’s desire to avoid concurrent state regulation and to reduce its exposure to tort liability. Negotiations in the legislative process have tended to favor the industry…. The inadequacy of current labeling policy is clearly revealed in the declaration of congressional purpose in the Comprehensive Smoking Education Act of 1984: It is the purpose of this Act to provide a

FIGURE 6-1 An example of U.S. government’s warning on cigarette packages.
new strategy of making Americans more aware of any adverse health effects of smoking, to assure the timely and widespread dissemination of research findings, and to enable individuals to make informed decisions about smoking. It is time to state unequivocally that the primary objective of tobacco regulation is not to promote informed choice but rather to discourage consumption of tobacco products, especially by children and youths, as a means of reducing tobacco-related death and disease. Even though tobacco products are legally available to adults, the paramount public health aim is to reduce the number of people who use and become addicted to these products, through a focus on children and youths. The warnings must be designed to promote this objective. In the committee’s view, the current warnings are inadequate even when measured against an informed choice standard, but they are woefully deficient when evaluated in terms of proper public health criteria” (IOM 1994, p. 236-237).
This committee agrees. Although federal law has remained unchanged for more than 20 years, evidence regarding the ineffectiveness of the prescribed warnings has continued to accumulate. As Krugman and colleagues note, the U.S. package warnings have served the tobacco industry well by reducing their liability exposure while communicating ineffectively with smokers and potential smokers (Krugman et al. 1999). The basic problems with the U.S. warnings are that they are unnoticed and stale, and they fail to convey relevant information in an effective way. In contrast to the messages used in other countries, the United States requires one of four text messages in black and white that occupy only 50 percent of the side of a pack. These messages have not changed in 20 years. They therefore have little effect on decision making or behavior (see Ferrence, Appendix C).
In contrast to the experience with such warnings in the United States, the experiences with these warnings in Canada and other countries have been more promising.
The Canadian Experience
Voluntary health package warnings were introduced in Canada in 1972 but were first imposed by federal law in 1989. Initially, they included four text messages. Five years later, eight stronger messages were introduced, and these messages occupied the top 35 percent of the front and back panels of the pack. These messages clearly specified the diseases and conditions caused by smoking and confirmed that “cigarettes are addictive.” These messages were soon adopted in Australia, Thailand, and Poland.
The most important innovation in package regulation is requiring companies to print graphic messages with pictorial content. Graphic warnings were first introduced in Canada in 2001. The manufacturers of cigarettes for sale in Canada are now required to print 1 of 16 health warnings on each pack of

FIGURE 6-2 Example of one of Health Canada’s 16 graphic warnings.
SOURCE: (Health Canada 2005) http://www.hc-sc.gc.ca/ahc-asc/media/photogal/label-etiquette/img0010_e.html. Licensed under Health Canada copyright.
cigarettes (see Figure 6-2 for an example of such a warning). The new warning system extends to carton wrappers, which now include a warning on each of their six surfaces. The top 50 percent of each main panel on the package (as opposed to the side panel) must be used for the outside warning. These warnings include a photograph or other illustration, a marker word “Warning,” a short summary statement of the warning, and a brief explanation. Inside each pack, there must be 1 of 16 other detailed messages that provide information about quitting or health damage. Warning labels also include information on damage to nonsmokers exposed to smoke from cigarettes. Other tobacco products have similar requirements for warning labels.
Other Countries
Since 2001, several other countries have adopted graphic package warnings including Brazil, Singapore, Thailand, Australia, and Venezuela. Members of the European Union are now permitted, but not required, to prescribe graphic warnings, and the European Union has also developed a standard set of pictorial warnings for consideration by its members. Several other countries (Bangladesh, Hong Kong, India, Malaysia, New Zealand, South Africa, and Taiwan) are currently considering graphic warnings. The World Health Organization Framework Convention for Tobacco Control (FCTC) requires that warnings cover 30 percent of the front and the back of the package and recommends package coverage of 50 percent or more. A series of messages must be rotated. Graphic warnings are permitted but are not required.
Package warning size and placement vary considerably by country. The front of the package is considered the most prominent location
(Cunningham 2005), and it is probably important to have some health message on all sides, since retailers may position packages to hide the warnings if all sides are not covered.
There are considerable variations in the types of graphics used and in potential emotional impacts of particular graphics. In Brazil, for example, the warnings are more colorful and more dramatic than the Canadian warnings, most showing smokers with obvious health conditions (see Figure 6-3).
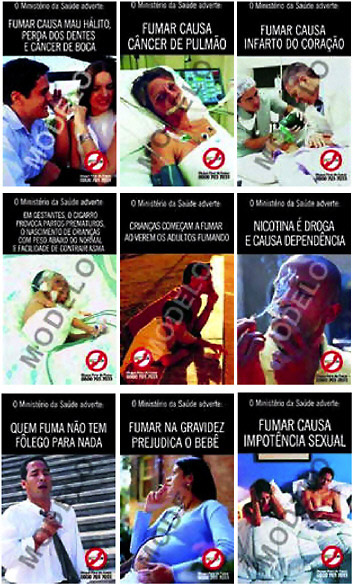
FIGURE 6-3 Examples of Brazil’s graphic warnings.
SOURCE: See www.anvisa.gov.br/eng/informs/news/281003.htm.
Evidence Regarding Effectiveness
Ferrence and colleagues (Appendix C) have reviewed the scientific evidence regarding the effectiveness of tobacco package warnings in getting the attention of consumers and potential consumers (salience), influencing their awareness of tobacco-related health risks (risk perception), and affecting their self-reported smoking intentions and behaviors. In general, the evidence shows that the salience of warnings is affected by their placement, sizes, and other design features, and that salient warnings affect the consumer’s awareness of risks. Although few studies have been able to parse out the effects of warnings on smoking behavior, the available data suggest a beneficial effect on consumption and cessation.
For the committee’s present purposes, the question of greatest importance is what is known about the effects of pictorial warnings. Given that Canada was the first country to introduce pictorial warnings, all of the available evidence derives from Canadian smokers. A study conducted with Canadian smokers in 2001 found that more than half reported that the pictorial warnings have made them more likely to think about the health risks of smoking (Hammond et al. 2004). National surveys conducted on behalf of Health Canada also indicate that approximately 95 percent of youth smokers and 75 percent of adult smokers report that the pictorial warnings have been effective in providing them with important health information (Health Canada 2005a; Health Canada 2005b).
The International Tobacco Control Policy Evaluation Survey—a cohort survey of a representative sample of more than 8,000 adult smokers from Canada, Australia, the United States, and the United Kingdom—also provides suggestive findings. When smokers were asked to cite the sources of smoking-related health information, approximately two-thirds of all smokers cited cigarette packages; this proportion was more than radio, print, and electronic sources, and cigarette packages were the second most common source after television (Hammond et al. 2005) However, the results varied substantially by country: respondents living in countries with more comprehensive warnings were more likely to cite packages as a source of health information. For example, 85 percent of Canadian respondents cited packages as a source of health information; in contrast, 47 percent of U.S. smokers cited packages as a source of health information. In addition, specific health warnings were associated with knowledge about specific diseases. For example, in Canada, where package warnings include information about the risks of impotence, smokers were more than twice as likely as smokers from the other three countries to agree that smoking causes impotence. Overall, the study found that warnings that are graphic, larger, and more comprehensive in content were associated with greater health knowledge.
Finally, there is evidence that smokers with less education are less likely to recall health information in text-based messages than people with more education (Millar 1996). Given the inverse association between smoking and educational status, pictorial warnings may be particularly important for communicating with those most at risk. Indeed, preliminary evidence suggests that countries with pictorial warnings demonstrate fewer disparities in health knowledge across educational levels (Siahpush et al. 2006). Pictorial warnings may also be particularly effective in educating people who are illiterate, and could have a significant population impact in developing countries with low literacy rates, as well as regions where numerous languages and dialects are used.
In a series of papers, Hammond and colleagues (2004) have examined the impact of Canadian graphic warning labels on smoking behavior. Smokers who had read, thought about, and discussed the new labels were more likely to have quit, tried to quit, or reduced their smoking at the 3-month follow-up, after adjustment for intention to quit and smoking status at baseline (Hammond et al. 2004). One-fifth of Canadian smokers said that they smoked less because of the labels, whereas only 1 percent said that they smoked more and one-third said that they were more likely to quit because of the warnings. In addition, former smokers identified the pictorial warnings as important factors in their quitting and in subsequently maintaining abstinence (Hammond et al. 2004). Results from the International Tobacco Control Policy Evaluation Survey are consistent with these findings: at least one quarter of respondents from Canada, Australia, the United Kingdom, and the United States reported that package warnings have made them more likely to quit, although Canadian smokers were significantly more likely to report cessation benefits from the warnings than smokers in the other three countries that have text-only warnings (Fong et al. 2004).
As recommended in Growing Up Tobacco Free (IOM 1994) the proposed Tobacco Control legislation would strengthen the required package warnings immediately and would confer authority on the FDA to revise these requirements upon finding “that such a change would promote greater public understanding of the risks associated with tobacco.” (The 1994 committee stated that the agency should also be authorized to modify the warnings upon finding that so doing would reduce consumption, such as by making the risks more salient or strengthening the resolve of smokers to quit, and this committee agrees.) The bill would specifically authorize the agency to increase the required label area up to 50 percent of the package and to require color graphics. On the basis of the evidence accumulated thus far, graphic warnings of the kind required in Canada, Brazil, and Thailand “would promote greater public understanding of the risks” of using tobacco and would help reduce consumption.
Recommendation 26: Congress should strengthen the federally mandated warning labels for tobacco products immediately and should delegate authority to the FDA to update and revise these warnings on a regular basis upon finding that doing so would promote greater public understanding of the risks of using tobacco products or reduce tobacco consumption. Congress should require or authorize the FDA to require rotating color graphic warnings covering 50 percent of the package equivalent to those required in Canada.
Using Packages to Convey Other Health Information
Aside from printed health warnings, regulatory authorities can use the tobacco package to convey health-related information in other ways. For example, so-called package onserts (printed matter that is affixed to the package, and that is equivalent to inserts in drug product packaging) provide an appropriate vehicle for supplementing the health warnings printed on the package with information on ingredients and details regarding specific health hazards. In addition, the package can be used creatively to promote smoking cessation by displaying a quitline number and by including coupons for nicotine replacement products (e.g., patches and gum).
Recommendation 27: Congress should empower the FDA to require manufacturers to include in or on tobacco packages information about the health effects of tobacco use and about products that can be used to help people quit.
Restricting Misleading Messages on Tobacco Packages
Tobacco manufacturers have traditionally used the words and trademarks on the package as a channel for conveying messages about product characteristics. Some of these messages are misleading and are not protected by the First Amendment, because they falsely imply that smoking a particular brand of cigarette is less harmful than smoking other brands.
As Wakefield and colleagues (Wakefield et al. 2004) have noted, package design can help to shape perceptions of a tobacco product’s performance and its sensory attributes, even among experienced smokers. This phenomenon is best illustrated by the use of brand descriptors and colors to promote perceptions that the tobacco product is safer than other tobacco products. Tobacco manufacturers commonly pair brand descriptors such as “light” and “mild” with cigarettes that generate low tar yields under the machine testing protocols. Although the industry has argued that these terms refer only to the “taste” of a product, these descriptors help to promote these brands as “healthier” products (Pollay 2001; Pollay
and Dewhirst 2002). Indeed, surveys of smokers in the United States and Canada indicate that a substantial proportion of “light” cigarette smokers believe that their cigarettes are less hazardous (Kozlowski et al. 1998; Shiffman et al. 2001). Adolescents have also been found to have similar misconceptions that “light” cigarettes are less hazardous (Borland et al. 2004; Kropp and Halpern-Felsher 2004; see also Chapter 2).
Ashley and colleagues (2001) reported that in Ontario, Canada, in 1996, one in five smokers of “light” cigarettes incorrectly believed that smoking “light” and “mild” cigarettes lowered the risk of cancer and heart disease. In 2000, 27 percent of Ontario smokers said that they smoked “light” cigarettes to reduce health risks, 40 percent as a step toward quitting, and 41 percent said that they would be more likely to quit if they knew that “light” cigarettes provided the same amount of tar and nicotine as regular cigarettes (Ashley et al. 2001). In a study of smokers’ responses to advertisements for potentially reduced-exposure tobacco products, “light” cigarettes, and regular cigarettes, Hamilton and colleagues found that the respondents incorrectly perceived “light” cigarettes as having significantly lower health risks and carcinogen levels than regular cigarettes (Hamilton et al. 2004).
Article 11 of the FCTC calls for the removal of brand descriptors that “directly or indirectly create the false impression that a particular tobacco product is less harmful than other tobacco products,” including terms such as “low tar,” “light,” or “mild.” Several jurisdictions have already banned deceptive descriptors. For example, in September 2003, the European Union banned the use of a number of brand descriptors, such as “low-tar,” “light,” “ultra-light,” and “mild,” in accordance with Directive 2001/37/EC. Findings from the International Tobacco Control Policy Evaluation Survey suggest that this ban has been effective in reducing misconceptions about the health benefits of brands labeled “light” and “mild” (Fong 2005). However, as the experience in the United Kingdom has demonstrated, tobacco manufacturers have proven adept at substituting numbers and colors for the banned descriptors. For example, pale blue and the number “one” are now being used to indicate a “light” or “mild” cigarette. In Brazil and the United Kingdom, manufacturers openly provided translation guides for this substitution. Because the evidence clearly shows that terms such as “mild,” “light,” “ultra-light,” and similar words are interpreted by consumers to imply reduced risk, the use of these terms should be barred.
In her recent remedial order in the federal government’s RICO suit against the big U.S. tobacco manufacturers, Judge Kessler permanently enjoined the companies from “conveying any express or implied health message or health descriptor for any cigarette brand either in the brand name or on any packaging, advertising or other promotional, informational or other
material.” She specifically enjoined use of the words “low tar,” “light,” “ultra-light,” “mild,” “natural,” and “any other words which reasonably could be expected to result in a consumer believing that smoking the cigarette brand using that descriptor may result in a lower risk of disease or be less hazardous to health than smoking other brands of cigarettes.” Judge Kessler’s order is very important, but it has two limitations: it does not apply to all manufacturers and it will require continuing interpretation regarding its application to words and images other than the ones specifically banned in the order.
The committee believes that Congress should supplement Judge Kessler’s order with a statutory restriction banning the use of these specific terms and should empower the regulatory agency to ban any other descriptors, signals, or practices that the companies may subsequently use that have the purpose or effect of leading consumers to believe believing that smoking the cigarette brand with that descriptor may result in a lower risk of disease or may be less hazardous to their health than smoking other brands of cigarettes.
Recommendation 28: Congress should ban, or empower the FDA to ban, terms such as “mild,” “lights,” “ultra-lights,” and other misleading terms mistakenly interpreted by consumers to imply reduced risk, as well as other techniques, such as color codes, that have the purpose or effect of conveying false or misleading impressions about the relative harmfulness of the product.
Using Packages to Convey Corrective Communications
Judge Kessler’s remedial order in the RICO suit also requires the defendant manufacturers to make various “corrective communications” on their websites, at the point of sale and on package inserts (Tobacco Free Kids 2006). These messages would address the adverse health effects of smoking, the addictiveness of smoking and nicotine, the effects of so-called low-tar cigarettes, the adverse effects of exposure to secondhand smoke, and the impact of marketing on youth smoking. Some of these proposed messages would be substantially equivalent to the health warnings contained in the proposed Tobacco Control legislation, although they would sometimes be more lengthy than package warnings. Some of the messages embody admissions of past deception by the manufacturers.
Recommendation 29: Whenever a court or administrative agency has found that a tobacco company has made false or misleading communications regarding the effects of tobacco products, or has engaged in conduct promoting tobacco use among youth or discouraging cessation
by tobacco users of any age, the court or agency should consider using its remedial authority to require manufacturers to include corrective communications on or with the tobacco package as well as at the point of sale.
THE RETAIL ENVIRONMENT FOR TOBACCO SALES SHOULD BE TRANSFORMED TO PROMOTE THE PUBLIC HEALTH
At present, tobacco use is actively promoted in retail outlets, with little regard to the public interest in discouraging smoking initiation (aside from the occasional warning sign that sale to a minor is prohibited) or in helping people quit. In the committee’s view, the retail environment for tobacco should be radically transformed. Effective measures of restricting the commercial distribution of tobacco products to youth are only a starting point. Tobacco is not an ordinary consumer product and should not be treated as such. Although the sale of tobacco products to adults is permitted, it is disfavored as a matter of public policy. The retail environment should be designed to effectuate the public health goals of discouraging tobacco use and reducing tobacco-related disease.
Current Retail Promotional Activities
With the adoption of the MSA in 1998, there was a dramatic shift in the tobacco industry’s advertising and promotion budgets, and retail marketing became the dominant strategy (Pierce and Gilpin 2004). The categories of promotional expenditures by tobacco manufacturers since 1980, as reported to the FTC, are presented in Box 6-1 (see also Figure 6-4 and Table 6-1).
Point-of-Sale Advertising
In 2003, tobacco manufacturers spent $165.6 million in payments for the purchase of point-of-sale advertising (FTC 2005). The main venues of such advertising are convenience stores, small grocery stores (often in tandem with the sale of gas), liquor stores, chain supermarkets, and chain pharmacies, with youth exposure especially concentrated at the first two of these locations. The amount spent on point-of-sale advertising in 2003 represents a 41.7 percent decline from that in 2001, when companies spent $284.3 million on point-of-sale advertising, and a decline of 58.6 percent since their peak in 1993 at $400.9 million (FTC 2005). The prime advertising space within most retail stores is the radius around the checkout counter. A study conducted in California found nearly 90 percent of tobacco marketing materials within 4 feet of store checkout counters (Feighery et
|
BOX 6-1 Domestic Cigarette Advertising and Promotional Expenditures for 2003 (Dollars in Thousands)
NOTE: The twenty-four spending categories are listed as they appear in the Federal Trade Commission’s Cigarette Report for 2003. The Committee designated the four main expenditure groups and which spending categories were included in each. |
al. 2001). A similar study found that nearly 50 percent of the California retailers surveyed posted tobacco product advertisements 3 feet or lower in height, which is easy eye-level for young children.
Under current law, state restriction of tobacco advertising (“based on smoking and health”) at the point of sale is preempted by the 1969 Ciga-
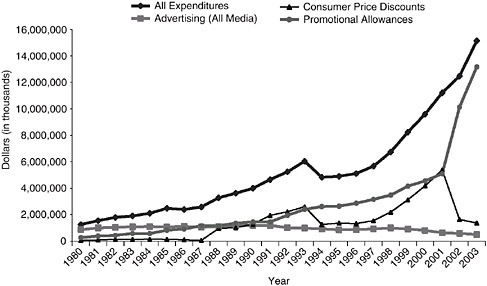
FIGURE 6-4 Domestic cigarette advertising and promotional expenditures for 1980–2003.
rette Labeling Act. However, the committee believes that the states should be free to regulate advertising at the point of sale as long as the regulation is no less restrictive than whatever federal regulation may have been adopted. In fact, this is the approach taken in the proposed Tobacco Control legislation, which would allow the states to ban point-of-sale advertising and other restrictions that would have been preempted under the Cigarette Labeling Act.
Retail Promotional Allowances
Promotional allowances paid to retailers now constitute the lion’s share of manufacturers’ marketing expenditures (see Figure 6-4 and Table 6-1). They are broadly defined by the FTC to include all payments or allowances to retailers “in order to facilitate the sale of any cigarette.” So defined, they include so-called slotting fees, which are industry fees paid to retailers—in the form of discounts—linked to advantageous placement and promotion vis-à-vis competing brands. In addition to product placement itself, these merchandising strategies address an array of product accessories: signage (e.g., regarding discount deals), logos, banners, display racks, and window posters. Another type of retail allowance involves pricing policies. So-called buy-downs feature inventory clearance deals, which are time-constrained discounts. To participate in the buy-down, a retailer must agree to erect special product displays and other promotional signs. In addition to buy-
TABLE 6-1 Promotional Expenditures by Tobacco Manufacturers
downs, there is the most basic of pricing strategies: straight volume discounts for retailers.
Although these practices are common marketing practices for other retail goods, such as food and soft drinks, they are problematic from a tobacco control standpoint for two reasons. First, when the manufacturers purchase display space or other promotional services from the retailer, they are promoting smoking as well as the use of the particular brands displayed or advertised. As discussed below, the committee believes that, aside from properly restrained black-and-white–text only price advertising, all promotional displays should be prohibited, including so-called power walls (large displays of packages of a single brand).
Second, the purchase of space through the payment of slotting fees could reduce or even eliminate the space available for smaller manufactur-
ers who are producing PREPs and who cannot afford to pay the same fees or give similar discounts as the major manufacturers. Even if this practice does not amount to an antitrust violation, it certainly tends to reduce the available shelf space for brands with the smallest market share, such as PREPs introduced by new entrant firms. In the committee’s view, the overall public health objective of reducing tobacco use justifies the more aggressive regulation of retail marketing and sales practices. Instead of allowing market forces to give prioritized access to ordinary tobacco products, society should direct retailers who choose to sell tobacco products to give prioritized display space to products that tend to reduce tobacco-related disease, including smoking cessation products and, as they are introduced, to tobacco products that have been found to have genuine potential for reducing tobacco-related disease.
Recommendation 30: Congress and state legislatures should enact legislation regulating the retail point of sale of tobacco products for the purpose of discouraging consumption of these products and encouraging cessation. Specifically:
-
All retail outlets choosing to carry tobacco products should be licensed and monitored (see also youth access section in Chapter 5).
-
Commercial displays or other activity promoting tobacco use by or in retail outlets should be banned, although text-only informational displays (e.g., price or health-related product characteristics) may be permitted within prescribed regulatory constraints.
-
Retail outlets choosing to carry tobacco products should be required to display and distribute prescribed warnings about the health consequences of tobacco use, information regarding products and services for cessation, and corrective messages designed to offset misstatements or implied claims regarding the health effects of tobacco use (e.g., that “light” cigarettes are less harmful than other cigarettes).
-
Retail outlets choosing to carry tobacco products should be required to allocate a proportionate amount of space to cessation aids and nicotine replacement products and, after regulatory clearance by the FDA or a designated state agency, to “qualifying” exposure-reduction products. (The FDA or a suitable state health agency should promulgate a list of “qualifying” exposure-reducing products.)
States are now preempted from implementing some aspects of these recommendations. However, as explained above, the committee believes that the federal preemption should be repealed. In addition, as explained below, the committee also believes that the proposed recommendation is compatible with the First Amendment.
Retail Sales on Indian Reservations
From a tobacco control standpoint, the main concern about retail cigarette outlets on Indian reservations is that the marketing and the distribution of tobacco products by tribes could impinge upon or undermine state efforts to increase the price (and collect its revenues) through excise taxes and to reduce the promotion and availability of tobacco.
Recommendation 31: Congress should explicitly and unmistakably include production, marketing, and distribution of tobacco products on Indian reservations by Indian tribes within the regulatory jurisdiction of FDA. Authority to investigate and enforce the Jenkins Act should be transferred to the Bureau of Alcohol, Tobacco, Firearms and Explosives. State restrictions on retail outlets should apply to all outlets on Indian reservations.
New Models for Regulating the Retail Market Should Be Explored
The recommendations just presented aim to inject public health into the existing retail market structure, which comprises a diverse array of privately owned businesses selling tobacco as one of many products in numerous locations. Two additional tools for transforming the retail environment for tobacco sales should also be explored. One is restricting the number and location of the retail outlets, and the other is shifting retail ownership to public control through direct public operation or through a chartered nonprofit monopoly. Because state-level regulation of retail distribution of alcohol after repeal of the 19th Amendment provides the most analogous policy experience, the effects of the alternative models developed by states after Prohibition are briefly reviewed before tobacco outlets are addressed.
The Alcohol Experience
Following the passage of the 21st Amendment, which repealed Prohibition, 17 states adopted a public monopoly system for alcohol distribution. Monopoly methods applied primarily to the retail sales of distilled spirits, considered to be the “primary root of social abuse,” whereas wine and beer, thought to be “nonintoxicating,” were often excluded from monopoly control (Munshi 1997; Shipman 1940). In addition to retail sales, most state systems included wholesale businesses in distilled liquors, and Wyoming operated only a wholesale monopoly (Munshi 1997; Shipman 1940). The alternative to a monopoly system was a licensing system in which the state granted licenses for a fee to individuals or companies to dispense liquor under government supervision. Both systems were intended primarily to reduce organized crime and prevent the reemergence of the saloon; however,
another objective was to contain consumption and reduce its associated harms (Shankar 1999; Woeste 2004).
Proponents of the monopoly system advocated it in preference to the licensing system for its ability to remove the private profit motive, which, it was feared, “might defeat effective public control” (Munshi 1997; Shipman 1940). In short, a driving insight was to erase at the retail level the vested private interests that tend to increase consumption. Under the monopoly arrangements, “sales promotion is neither necessary nor socially desirable, the products sold are standardized and do not require diversified handling, and the strategy of customer appeal in store premises and equipment is unnecessary” (Shipman 1940). In addition, price controls would be easier to implement under a monopoly system, as most products would be under direct state control (Munshi 1997; Rutledge 1989). Finally, advocates of the monopoly approach believed that because the state would be more concerned with social welfare than with profit—in contrast to the interests of privately licensed bodies—the state would be better positioned to manage the appearance and location of its stores (Munshi 1997).
Although both the public monopoly and the licensing systems were initially intended to contain the number of liquor outlets and promote temperance, these objectives shifted over the course of the 20th century as states saw opportunities to leverage liquor distribution systems to boost state revenues (Spaeth 1991). Furthermore, as public opposition to heavily regulated liquor sales grew, state officials responded by advocating liberalization of stringent control systems. For example, in a debate regarding the future of Pennsylvania’s state liquor stores, a state senator cited “poor selection, inconvenience, and high prices” as justification for privatization of the state monopoly system (Munshi 1997). Expressing similar distaste with his state’s restrictive licensing scheme, Governor John Carlin of Kansas lobbied for reforming the state’s liquor laws out of concern that the state’s reputation for radical temperance was thwarting efforts to bring new business to the state (Swain 1996).
Although the recent trend of liberalizing retail access to alcohol, even in the states with monopolies on retail sales, has tended to obscure the differences between the two legal regimes, there is a body of research on the relationship between alcohol consumption and the type of regulation and the number of outlets. Several studies have found that privatization of wine sales and the elimination of a state monopoly on retail sales of distilled spirits led to an increase in overall consumption (Holder and Wagenaar 1990; Wagenaar and Holder 1995).
Reducing the convenience of retail alcohol accessibility typically increases the opportunity cost to the drinker, (i.e., the cost in time and money to actually obtain alcohol from retail sources). Specifically, the number and concentration of alcohol retail outlets affect the convenience of obtaining
alcohol, and the distance between outlets increases the cost of doing so. Gruenewald and colleagues (1993) conducted a time series cross-sectional analysis of alcohol consumption and the density of alcohol outlets over the 50 U.S. states. The results indicated that a 10 percent reduction in the density of alcohol outlets would reduce the consumption of spirits from 1 percent to 3 percent and the consumption of wine by 4 percent.
Licensing of alcohol outlets can be used to restrict the number or density of outlets in a given area, as well as the hours of sale, the types of beverages, and the size of beverage containers. Reducing the days and times of alcohol sales restricts the opportunities for alcohol purchasing and can reduce heavy consumption. Licensing is thus a common strategy for reducing drinking-related problems, although the trend in recent years has been to liberalize such restrictions in many countries (Drummond 2000). In general, it appears that changes in licensing provisions that substantially reduce hours of service can have a significant impact on drinking and drinking-related problems overall (Holder 2004).
Options for Restructuring Retail Tobacco Sales
A state retail monopoly for tobacco sales would have advantages and disadvantages. Although it would have the advantage of exerting direct and complete control over the retail environment, it could have the undesirable effect of giving the state a vested economic interest in increased tobacco sales, a concern that would be accentuated if tobacco sales were combined with liquor sales in states that retain retail monopolies over some aspect of alcohol sales. Because states have gradually liberalized (and even encouraged) alcohol sales in recent years, the inclusion of tobacco sales (which should be discouraged) in a consumption-promoting retail alcohol monopoly would send the wrong message altogether. A better option would be to establish a retail monopoly of tobacco-only outlets operated either by the state or by a state-chartered nonprofit corporation. The chartered-nonprofit approach would have the additional advantage of distancing the state from direct participation in tobacco sales. Under either approach, the legislated retail system would have to be carefully structured so that it would have no incentive to engage in marketing and promotional activities. It would be absolutely necessary for the legislature to declare that the sole statutory purpose of the retail outlets would be to facilitate smoking cessation.
Many public health experts will be skeptical about the likelihood that a publicly chartered retail monopoly for tobacco sales could be successfully operated to promote the public health. An alternative to a retail monopoly would be to license private outlets according to a population-based formula. Under this approach, it would be important to structure the system to enable the regulatory authority to reduce the number of outlets when it
determines that doing so would promote the public health. The key would be to restrict the license to a reasonably short term, say, 5 years, without any legal presumption of renewal. If it were commercially feasible, licensure could be restricted to outlets that sell only tobacco. Outlets that sell only tobacco would have the additional advantage of being able to facilitate the enforcement of youth-access restrictions.
Under any of the approaches (public monopoly, chartered non-profit monopoly, or a private licensing system), the decisions regarding number and location of outlets should be made by a public health agency, taking into account the potential benefits (in reducing tobacco use) and the possible costs, including the risk of stimulating a black market. Concerns about black-market supply can be reduced by narrowing the wide variation in state tobacco excise taxes. This problem is addressed below.
Recommendation 32: State governments should develop and, if feasible, implement and evaluate legal mechanisms for restructuring retail tobacco sales and restricting the number of tobacco outlets.
The Federal Role
The committee believes the states should take the lead in exploring innovation in tobacco retail regulation. However, the federal government should play a facilitative role in the near term and should be empowered to take a more directive role over the long term.
The proposed Tobacco Control legislation would permit state innovations in retail sale regulation while restricting FDA authority. Although the bill would give the FDA the authority to restrict “the sale and distribution of a tobacco product … if the Secretary determines that such regulation would be appropriate for the protection of the public health,” it would specifically deny FDA the authority to require a prescription for tobacco products or to ban any particular category of retail outlet from selling tobacco products. The latter limitation would effectively prevent the FDA from adopting a strategy of curtailing the sales of tobacco at retail outlets. However, under the preemption provision, the bill would explicitly leave the states free to adopt restrictions “in addition to or more stringent than” the federal requirements in connection with the sale and distribution of tobacco products. Accordingly, a state could prohibit tobacco products altogether or, more to the point for the present purposes, could require prescriptions or limit the number of retail outlets that sell tobacco.
Recommendation 33: Congress should empower FDA to restrict outlets in order to limit access and facilitate regulation of the retail environment, and thereby protect the public health.
FDA should have the authority to ban categories of retail outlets if it determines that doing so is necessary to implement the regulatory policy, and the proposed Tobacco Control legislation should be modified accordingly. However, the immediate implementation of a federal regulatory scheme would be premature. The first step is to enable and encourage state and local innovation.
THE FEDERAL GOVERNMENT SHOULD MANDATE INDUSTRY PAYMENTS FOR TOBACCO CONTROL AND SHOULD SUPPORT AND COORDINATE STATE FUNDING
This section addresses two distinct problems with current state tobacco control policies as described in Chapter 5: inadequate funding of tobacco control programs and substantial variations in the per-pack rate of state tobacco excise taxes. These problems could be corrected by the states themselves if they were to implement the policies proposed in Recommendations 1 and 2. This section presents a plan for solving both of these problems through federal coordination of state tobacco control policies if the states fail to do so on their own.
As discussed in Chapter 5, substantial state excise taxes on tobacco are an essential component of a comprehensive state tobacco control program, not only as a tool for raising the price and reducing consumption but also as a way of raising revenues that can be used to fund tobacco control programs. Most states allocate insufficient funding for tobacco control programs. Table 6-2 shows each state’s proposed tobacco prevention spending for FY 2006, as well as the Centers for Disease Control and Prevention’s (CDC) minimum spending targets for prevention programs. With the exception of Delaware, Maine, and Mississippi, the states do not spend up to the CDC minimum target for tobacco prevention programs. The District of Columbia, Michigan, Missouri, New Hampshire, South Carolina, and Tennessee did not allocate any state funds for tobacco control in FY 2006. When each state’s actual spending on tobacco use prevention is expressed as a percentage of the CDC’s minimum target, the median is 31.2 percent. Accordingly, Recommendation 1 emphasizes the need for states to fund tobacco control programs at the level recommended by the CDC, earmarking funds generated by state tobacco excises as a way of assuring continued funding if doing so is permissible under the state constitution. If this recommendation were fully implemented by the states, the federal role in tobacco control funding could be limited to those programs that are national in scope (such as the youth-oriented national media campaign proposed in Recommendation 15). However, the plan outlined in this section is designed to give states an additional incentive to fund tobacco control programs.
TABLE 6-2 Recommended and Proposed Tobacco Control Program Spending
|
State |
CDC Minimum Prevention Spending Target ($ in millions) |
FY 2006 Tobacco Prevention Proposed Spending ($ in millions) |
|
Alabama |
26.7 |
|
|
Alaska |
8.1 |
5.7 |
|
Arizona |
27.8 |
23.1 |
|
Arkansas |
17.9 |
17.7 |
|
California |
165.1 |
79.7 |
|
Colorado |
24.5 |
27 |
|
Connecticut |
21.2 |
0.04 |
|
Delaware |
8.6 |
9.2 |
|
District of Columbia |
7.5 |
0 |
|
Florida |
78.4 |
1 |
|
Georgia |
42.6 |
3.1 |
|
Hawaii |
10.8 |
5.8 |
|
Idaho |
11 |
|
|
Illinois |
64.9 |
11 |
|
Indiana |
34.8 |
10.8 |
|
Iowa |
19.3 |
5.6 |
|
Kansas |
18.1 |
1 |
|
Kentucky |
25.1 |
2.7 |
|
Louisiana |
27.1 |
8 |
|
Maine |
11.2 |
14.2 |
|
Maryland |
30.3 |
9.2 |
|
Massachusetts |
35.2 |
4.3 |
|
Michigan |
54.8 |
0 |
|
Minnesota |
28.6 |
22.1 |
|
Mississippi |
18.8 |
20 |
|
Missouri |
32.8 |
0 |
|
Montana |
9.4 |
6.8 |
|
Nebraska |
13.3 |
3 |
|
Nevada |
13.5 |
4.2 |
|
New Hampshire |
10.9 |
0 |
|
New Jersey |
45.1 |
11.5 |
|
New Mexico |
13.7 |
6 |
|
New York |
95.8 |
43.4 |
|
North Carolina |
42.6 |
15 |
|
North Dakota |
8.2 |
3.1 |
|
Ohio |
61.7 |
47.2 |
|
Oklahoma |
21.8 |
8.9 |
|
Oregon |
21.1 |
3.5 |
|
Pennsylvania |
65.6 |
32.9 |
|
Rhode Island |
9.9 |
2.1 |
|
South Carolina |
23.9 |
0 |
|
South Dakota |
8.7 |
|
|
State |
CDC Minimum Prevention Spending Target ($ in millions) |
FY 2006 Tobacco Prevention Proposed Spending ($ in millions) |
|
Tennessee |
32.2 |
0 |
|
Texas |
103.2 |
7 |
|
Utah |
15.2 |
7.2 |
|
Vermont |
7.9 |
4.9 |
|
Virginia |
38.9 |
12.8 |
|
Washington |
33.3 |
27.2 |
|
West Virginia |
14.2 |
5.9 |
|
Wisconsin |
31.2 |
10 |
|
Wyoming |
7.4 |
5.9 |
|
NOTE: Federal Tax rate is $0.39 per pack. SOURCE: Adapted from Campaign for Tobacco Free Kids. http://www.tobaccofreekids.org/reports/settlements/2006/fullreport.pdf. |
||
A second problem identified in Chapter 5 is that the wide variation in tobacco excise tax rates among the states tends to encourage tax evasion and smuggling through interstate shipments. Table 6-3 shows state cigarette excise tax rates as of January 1, 2006, and reveals a wide disparity in tax rates, ranging from 7 cents to $2.46 per pack, with a median tax of 80 cents per pack. The absolute wide range of state excise tax rates has been increasing over time. Figure 6-5 shows vertical box plots of state excise tax rates in 1998 and 2006.3 The plots show the extreme values as well as the quartile ranges. The median excise tax rate rose from 34 to 80 cents per pack over this period, while the spread between the 25th and the 75th percentiles rose from 40 cents in 1998 to 82.5 cents in 2006. In Recommendation 2, the committee urges the low-tax states to raise their excise taxes to what is now the upper quintile of state tax rates. If that recommendation were implemented by all the states, it would substantially decrease, if not eliminate, the incentive for cross-state smuggling. However, if the states do not deal successfully with this problem on their own, the increasing variation in state tobacco excise taxes should be addressed by the federal government.
In this section, the committee offers a new federal funding scheme (the National Tobacco Control Funding Plan, described below) as a back-up plan to support and coordinate state tobacco control programs, while giving the states with low tobacco excise taxes the incentive to raise them. The basic outline of such a scheme is as follows: the Congress would levy a supplementary remedial assessment on tobacco manufacturers through a per-pack fee, much like the current federal excise tax. The federal gov-
TABLE 6-3 State Cigarette Excise Tax Rates
|
State |
State Cigarette Excise Tax Rates (in cents per pack)—Updated January 1, 2006 |
State |
State Cigarette Excise Tax Rates (in cents per pack)—Updated January 1, 2006 |
|
Rhode Island |
246 |
Kansas |
79 |
|
New Jersey |
240 |
Wisconsin |
77 |
|
Washington |
202.5 |
Utah |
69.5 |
|
Maine |
200 |
Nebraska |
64 |
|
Michigan |
200 |
Wyoming |
60 |
|
Montana |
170 |
Arkansas (2) |
59 |
|
Alaska |
160 |
Idaho |
57 |
|
Connecticut |
151 |
Indiana |
55.5 |
|
Massachusetts |
151 |
Delaware |
55 |
|
New York (1) |
150 |
West Virginia |
55 |
|
Hawaii |
140 |
South Dakota |
53 |
|
Pennsylvania |
135 |
North Dakota |
44 |
|
Ohio |
125 |
Alabama (1) |
42.5 |
|
Minnesota (3) |
123 |
Texas |
41 |
|
Vermont |
119 |
Georgia |
37 |
|
Arizona |
118 |
Iowa |
36 |
|
Oregon |
118 |
Louisiana |
36 |
|
Oklahoma |
103 |
North Carolina |
35 |
|
District of Columbia |
100 |
Florida |
33.9 |
|
Maryland |
100 |
Kentucky (2) |
30 |
|
Illinois (1) |
98 |
Virginia (1) |
30 |
|
New Mexico |
91 |
Tennessee (1,2) |
20 |
|
California |
87 |
Mississippi |
18 |
|
Colorado |
84 |
Missouri (1) |
17 |
|
Nevada |
80 |
South Carolina |
7 |
|
New Hampshire |
80 |
|
|
|
NOTES: *Counties and cities may impose an additional tax on a pack of cigarettes in AL, 1¢ to 6¢; IL, 10¢ to 15¢; MO, 4¢ to 7¢; NYC $1.50; TN, 1¢; and VA, 2¢ to 15¢. *Dealers pay an additional enforcement and administrative fee of 0.1¢ per pack in KY and 0.05¢ in TN. In AR, a $1.25/1,000 cigarette fee is imposed. *Plus an additional 25.5 cent sales tax is added to the wholesale price of a tax stamp (total $1.485). SOURCE: Adapted from Federation of Tax Administration (http://www.taxadmin.org/fta/rate/cigarett.html) |
|||
ernment would use a portion of these funds to support national tobacco control programs (for example, a national media-based educational and prevention program and a national quitline and cessation services network, as recommended in Chapter 5). The remainder of the funds would be distributed to the states based on a formula that rewards states with high excise taxes or high levels of tobacco control spending. This would give states
FIGURE 6-5 Box-whisker plots of state cigarette excise tax rates, 1998–2006.
an incentive to increase their spending on tobacco prevention and would give states with low excise taxes an incentive to raise them, thereby narrowing the current wide variation in excise tax rates among the states. The precise details of the formula would have to be based on further research, but the committee has developed an illustrative formula to demonstrate the principles that it has in mind.
Federal Remedial Assessment
Under the committee’s proposed National Tobacco Control Funding Plan, the federal government would raise funds for tobacco control on a nationwide basis through a per-pack remedial assessment on cigarettes sold in the United States. These assessments would be based on congressional findings that cigarettes are unreasonably hazardous products, that most smokers are addicted to nicotine, that most addicted smokers began smoking as adolescents, that most adult smokers desire to quit, and that the promotional activities of the tobacco companies have created and sustained addiction to cigarettes.
The precise amount of the assessment would be selected by the Congress to yield a target amount of overall funding for tobacco control, whereas it would produce the optimal incentives for the states to raise tobacco excise taxes and spend state funds on tobacco control.
It will be recalled that the committee recommended a substantial increase in the federal tobacco excise tax rate in Chapter 5 (Recommendation 3). It would be compatible with the committee’s recommendation to regard a portion of a new federal excise tax increase as the source of funds for the new tobacco control funding plan. However, the committee has conceptualized the levy as a “remedial assessment” rather than simply as an increase in the excise tax in order to provide a strong rationale for directing the proceeds to tobacco control funding. In addition, it is worth noting that, despite the impact of current federal and state taxes and the regular payments mandated by the MSA, the major U.S. cigarette manufacturers remain highly profitable and still have a substantial ability to help finance the prevention and cessation programs recommended in this report. Table 6-4 shows the pretax domestic operating profits of the three largest U.S.-based tobacco manufacturers for calendar year 2005, as derived from Forms 10-K submitted to the U.S. Securities and Exchange Commission. Their combined pretax operating profits from the sale of cigarettes within the United States exceeded $7 billion in 2005.
An Illustrative Allocation Formula
An example of a formula that would determine the federal funds that would be distributed to the states and that would achieve the committee’s basic objectives is ![]() where G represents the annual grant to a particular state (in millions of dollars); a is a constant that depends on the total funds available for distribution nationwide; S is the state’s annual spending on tobacco prevention (in millions of dollars); and T is the state’s excise tax rate on cigarettes (in cents per pack), exclusive of sales taxes. The symbol
where G represents the annual grant to a particular state (in millions of dollars); a is a constant that depends on the total funds available for distribution nationwide; S is the state’s annual spending on tobacco prevention (in millions of dollars); and T is the state’s excise tax rate on cigarettes (in cents per pack), exclusive of sales taxes. The symbol ![]() refers to the square root of the excise tax rate.
refers to the square root of the excise tax rate.
The formula implies that the state will receive ![]() dollars in federal payments for each dollar spent on tobacco control activities. This means
dollars in federal payments for each dollar spent on tobacco control activities. This means
TABLE 6-4 Pre-Tax Domestic Tobacco Operating Profits of the Three Largest U.S.-Based Cigarette Manufacturers, 2004–2005 (in $millions)
|
Parent Company (Division) |
2004 |
2005 |
|
Altria (Philip Morris USA) |
4,405 |
4,581 |
|
Reynolds American (RJ Reynolds) |
882 |
1,459 |
|
Loews (Lorillard) |
1,040 |
1,151 |
|
Total |
6,327 |
7,191 |
that states with high tobacco excise tax rates receive a higher federal matching rate for tobacco control spending. Moreover, the formula implies that each state would have an incentive to raise its cigarette excise tax, but states with low taxes have the greatest incentive. If a state with a baseline excise tax of 25 cents per pack raised its cigarette tax by 25 cents to 50 cents per pack, it would receive a 41.4 percent increase in federal payments. By contrast, if a state with a baseline excise tax of $1 per pack raised its cigarette tax by 25 cents to $1.25 per pack, it would receive an 11.8 percent increase in federal payments. There is precedent for the use of nonlinear formulas in the distribution of federal funds to the states. For example, the federal medical assistance percentage rates in the Medicaid program are based on the square of each state’s per capita income (see Section 1905(b) of the Social Security Act).4
Table 6-5 shows how the formula would be used to distribute $500 million (an arbitrarily designated amount), based on the values of S (in Table 6-3) and T (in Table 6-4). At current cigarette prices, an additional federal levy of about 2.75 cents per pack would be required to generate such revenue.5 The last column of Table 6-5 shows the effective federal matching rate per dollar of state tobacco prevention spending. Thus the state of Georgia has a relatively low tax rate of 37 cents per pack (Table 6-3) and will spend $3.1 million on tobacco prevention in FY 2006. Under a federal incentive scheme that awards a total of $500 million to the states, Georgia would receive a payment of $0.56 per dollar of tobacco control spending, which comes to a grant of $1.72 million for the year (Table 6-5). By contrast, the state of Minnesota has a relatively high tax rate of $1.23 per pack (Table 6-3) and plans on spending $22.1 million on tobacco prevention in FY 2006 (Table 6-5). Under the same federal scheme, Minnesota would receive a payment of $1.01 per dollar of tobacco control spending, which comes to a grant of $22.37 million for the year (Table 6-5). In effect, the federal grant would subsidize all of Minnesota’s tobacco control spending. Finally, consider the state of Michigan, which has a much higher tax rate of $2.00 per pack (Table 6-3), but will have made no expenditures on tobacco prevention in FY 2006 (Table 6-5). Under the same federal scheme, Michigan would be eligible to receive a payment of $1.29 per dollar spent
|
4 |
For the most recent rates, see Federal Register Vol. 70, No. 229, p. 71856, November 30, 2005. |
|
5 |
According to the USDA Tobacco Situation, total federal taxable removals for 2005 were an estimated 366.7 billion pieces or, equivalently, 18.335 billion packs. The estimated average retail price of a pack of cigarettes in 2005 was $4.32. (See Campaign for Tobacco-Free Kids: http://www.tobaccofreekids.org/research/factsheets/pdf/0207.pdf.) If the short-run price elasticity of demand is −0.4, then a federal levy of about 2.75 cents per pack would generate an additional $500 million in revenue. |
TABLE 6-5 State Grants and Matching Payment Rates Under Proposed Federal Schemea
|
State |
FY 2006 Tobacco Prevention Proposed Spending (in $ millions) |
State Grant Under Proposed Federal Plan (in $ millions) |
Matching Payment per Dollar of State Tobacco Control Spending (in $) |
|
Alabama |
0.5 |
0.19 |
0.6 |
|
Alaska |
5.7 |
6.58 |
1.15 |
|
Arizona |
23.1 |
22.91 |
0.99 |
|
Arkansas |
17.7 |
12.41 |
0.7 |
|
California |
79.732 |
67.86 |
0.85 |
|
Colorado |
27 |
22.59 |
0.84 |
|
Connecticut |
0.04 |
0.04 |
1.12 |
|
Delaware |
9.2 |
6.23 |
0.68 |
|
District of Columbia |
0 |
0 |
0.91 |
|
Florida |
1 |
0.53 |
0.53 |
|
Georgia |
3.1 |
1.72 |
0.56 |
|
Hawaii |
5.8 |
6.26 |
1.08 |
|
Idaho |
0.544 |
0.37 |
0.69 |
|
Illinois |
11 |
9.94 |
0.9 |
|
Indiana |
10.8 |
7.34 |
0.68 |
|
Iowa |
5.6 |
3.07 |
0.55 |
|
Kansas |
1 |
0.81 |
0.81 |
|
Kentucky |
2.7 |
1.35 |
0.5 |
|
Louisiana |
8 |
4.38 |
0.55 |
|
Maine |
14.2 |
18.33 |
1.29 |
|
Maryland |
9.2 |
8.4 |
0.91 |
|
Massachusetts |
4.3 |
4.82 |
1.12 |
|
Michigan |
0 |
0 |
1.29 |
|
Minnesota |
22.1 |
22.37 |
1.01 |
|
Mississippi |
20 |
7.75 |
0.39 |
|
Missouri |
0 |
0 |
0.38 |
|
Montana |
6.8 |
8.09 |
1.19 |
|
Nebraska |
3 |
2.19 |
0.73 |
|
Nevada |
4.2 |
3.43 |
0.82 |
|
New Hampshire |
0 |
0 |
0.82 |
|
New Jersey |
11.5 |
16.26 |
1.41 |
|
New Mexico |
6 |
5.22 |
0.87 |
|
New York |
43.4 |
48.52 |
1.12 |
|
North Carolina |
15 |
7.5 |
0.5 |
|
North Dakota |
3.1 |
1.88 |
0.61 |
|
Ohio |
47.2 |
48.17 |
1.02 |
|
Oklahoma |
8.9 |
8.25 |
0.93 |
|
Oregon |
3.5 |
3.47 |
0.99 |
|
Pennsylvania |
32.9 |
34.9 |
1.06 |
|
Rhode Island |
2.1 |
3.01 |
1.43 |
|
South Carolina |
0 |
0 |
0.24 |
on tobacco control activities, but because it has no current tobacco control spending, it would receive no federal grant.
To illustrate how this federal allocation program would work, we consider three separate cases: (1) Georgia raises its cigarette excise tax by 25 cents per pack, (2) Minnesota raises its cigarette excise tax by 25 cents per pack, and (3) Michigan raises its cigarette excise tax by 25 cents per pack and spends $10 million on tobacco control activities.
If Georgia raised its cigarette tax by 25 cents to 62 cents per pack but did not increase its spending on tobacco control activities, its federal matching rate would still increase from $.56 to $.72 cents per dollar of tobacco prevention spending. As a result, Georgia’s enactment of an increase in its cigarette excise tax would result in an increase in its federal grant from $1.72 million to $2.23 million. By contrast, if Minnesota raised its cigarette tax by 25 cents to $1.48 per pack but did not increase its spending on tobacco control activities, its federal matching rate would still increase from $1.01 to $1.11 per dollar of tobacco prevention spending. As a result, Minnesota’s enactment of an increase in its cigarette excise tax would result in an increase in its federal grant from $22.37 million to $24.54 million. In these two cases, Georgia receives an incentive bonus of approximately $0.5 million for raising its excise tax by 25 cents, whereas Minnesota receives an incentive bonus of more than $2 million for raising its excise tax by the same amount. The explanation is that states with high rates of spending on tobacco prevention get a greater reward for raising their cigarette excise taxes.
Finally, consider the consequences of Michigan’s raising its excise tax by 25 cents and, at the same time, increasing its spending on tobacco control activities by $10 million. As a consequence of the excise tax increase, its federal matching rate would increase from $1.29 to $1.37 per dollar of spending on tobacco prevention. Accordingly, the state’s newly increased spending of $10 million on tobacco prevention would result in a federal grant of $13.7 million, a grant that entirely subsidizes its tobacco control spending and adds additional funds to the state’s coffers. This example illustrates an important feature of the federal allocation formula, namely, that states with higher excise tax rates receive a greater reward for increasing their tobacco prevention spending.
Possible Criticisms of Proposed Allocation Formula
It is arguable that the proposed incentive scheme may not provide sufficient incentives for states with low excise taxes and low spending on tobacco prevention to alter their policies. Thus, consider a state such as South Carolina, which has a very low cigarette excise tax of 7 cents per pack (Table 6-3) and will spend no state funds on tobacco prevention in FY 2006 (Table 6-2). South Carolina would essentially receive a federal subsidy of 24 percent for each dollar that it devoted to tobacco prevention spending, which may not be enough to induce the state to allocate funds for tobacco prevention. However, the size of the effective federal matching rate depends on the amount of funds to be allocated. In a program that allocated $2 billion in federal incentive funds, South Carolina would receive a 97 cent match for every dollar that it decided to invest in tobacco control. Its effective subsidy would be 97 percent for each new dollar of spending on tobacco control. That is, although South Carolina has no current tobacco use prevention program, the federal government would essentially be paying the state to establish a tobacco use prevention program.
Alternatively, one might argue that the incentive system outlined above provides too much reward for past good behavior and too little incentive for future increases in cigarette taxes or tobacco use prevention spending. This is a matter of equity. The grant formula could be modified so that only prospective changes in spending S or cigarette taxes T are rewarded. On the other hand, the formula could be modified to take into account a combination of past and prospective changes in taxes or spending on prevention.
Finally, it could be argued that the nonlinear square root formula does not offer sufficiently strong incentives to equalize tax rates. In that case, the formula could be modified to provide greater incentives to states with lower cigarette excise taxes. For example, if the formula were changed to ![]() a scheme to allocate $500 million would give Georgia a higher subsidy rate of 66 cents per dollar of tobacco control spending, whereas
a scheme to allocate $500 million would give Georgia a higher subsidy rate of 66 cents per dollar of tobacco control spending, whereas
Minnesota would receive a lower subsidy rate of 98 cents per dollar of tobacco control spending.
Recommendation 34: If most states fail to increase tobacco control funding and reduce variations in tobacco excise tax rates as proposed in Recommendations 1 and 2, Congress should enact a National Tobacco Control Funding Plan raising funds through a per-pack remedial assessment on cigarettes sold in the United States. Part of the proceeds should be used to support national tobacco control programs and the remainder of the funds should be distributed to the states to subsidize state tobacco control programs according to a formula based on the level of state tobacco control expenditures and state tobacco excise rates. The plan should be designed to give states an incentive not only to increase state spending on tobacco control, but also to raise cigarette taxes, especially in low-tax states. Congress should assure that any federal coordination mechanism affecting the coverage and collection of state tobacco excise taxes applies to Indian tribes.
Prevalence-Based Penalties
The committee’s proposed National Tobacco Control Funding Plan is not the only approach that could be used to tap tobacco industry revenues for the purpose of funding tobacco control activities. For example, various financial formulas have been proposed as devices for penalizing tobacco manufacturers for failing to take steps to reduce the prevalence of smoking among youth. In the proposed final judgment in its RICO suit, the U.S. Department of Justice recommended that the court set specified targets for each defendant on the basis of the 2003 baseline rate of smoking their brands and to reduce the prevalence of smoking among youth by 6 percent each year for 7 years. Under the government’s proposed judgment, a manufacturer failing to reach its target in any given year would be assessed $3,000 (adjusted for inflation) for each young person by which the target was missed. According to the U.S. Department of Justice, these penalties would be justified by the specific finding that the companies intentionally marketed cigarettes to youth while denying that they were doing so. Eighty percent of the recommended assessments would have been used to support the National Cessation Quitline Network, which would have been established under the proposed order, and 20 percent would have been used to support prevention activities. Although Judge Kessler found the tobacco company defendants liable under the RICO Act, she declined to order these proposed remedies on the ground that they are precluded by an earlier ruling by the Court of Appeals for the District of Columbia.
In any case, the committee’s proposed National Tobacco Control Funding Plan is not meant to be an alternative to other plans that are based on prevalence-based penalties; it is meant to stand on its own and can be used to complement a penalty-based approach if such an approach were to be ordered by a court or embraced by Congress. Accordingly, if the Court of Appeals for the District of Columbia were to take a broader view of the district court’s remedial authority under the RICO Act, and the district court were to enter an order similar to that recommended by the federal government, Congress could use the funds raised under the committee’s proposed plan to supplement these funds as needed to carry out the national cessation and prevention programs established by the court’s order.
TOBACCO ADVERTISING SHOULD BE FURTHER RESTRICTED
The Cigarette Smoking Act of 1969 banned the advertising of cigarettes on television and radio. The FDA’s 1996 Tobacco Rule would have limited magazine advertising to a black-and-white–text only format, restricted the use of the trade or brand name of certain tobacco products; prohibited the sale or distribution of promotional brand-identified non-tobacco items, such as hats and tee shirts; and prohibited the use of the brand name of a tobacco product when a tobacco company sponsors entries, teams, and sporting and other events. These restrictions never went into effect. Under the MSA, the participating manufacturers agreed to eliminate billboard advertising, significantly limit brand item advertising, and sharply restrict public advertising in entertainment forms. However, other types of advertising and promotion were not affected. Efforts by the states to restrict point-of-sale advertising have been found to be preempted by the 1969 Cigarette Labeling Act (Lorillard Tobacco Company v. Reilly 2001;533 U.S. 525).
As noted earlier, the proposed Tobacco Control legislation would revive the 1996 FDA Tobacco Rule and would empower the FDA to restrict the advertising and promotion of tobacco products “to the full extent permitted by the First Amendment” upon finding “that such regulation would be appropriate for the protection of the public health.” The questions raised are whether restrictions on tobacco advertising and other forms of marketing would reduce the level of smoking in the population, thereby promoting the public health, and whether these restrictions would be constitutionally permissible.
Current Advertising
As noted above, the tobacco industry’s advertising and promotion budgets shifted dramatically after the MSA was executed in 1998. Retail
marketing became the dominant strategy (Pierce and Gilpin 2004), and traditional forms of advertising in mass media have declined. Magazine advertising has not been abandoned, however, and the industry still spends $107 million per year to produce advertisements containing colorful, attractive, and prominently placed imagery that appeals to both youth and adult consumers (King and Siegel 2001). Expenditures for magazine advertisements have declined dramatically since their peak in 1984, however, when tobacco companies reported spending $425.9 million on advertising in this medium, which then represented more than 20 percent of their marketing and promotion budgets. Magazine advertisements now represent a much smaller percentage of total spending: less than 1 percent, if retail consumer price discounting is included (FTC 2005).
The amount of cigarette advertising in magazines with high youth readership has declined since tobacco companies agreed to avoid targeting youth as a condition of the MSA (Hamilton et al. 2002). Researchers credit this trend to public pressure from advocacy groups and the popular press, as the proportion of advertising expenditures in magazines with at least 15 percent youth readership declined more after public pressure was applied than immediately following the execution of the MSA (Hamilton et al. 2002). Nevertheless, a recent court decision may make tobacco companies take greater care in adhering to the MSA advertising requirements; in 2004, a California court of appeals affirmed a trial court ruling that the R.J. Reynolds Company violated the agreement by placing cigarette advertisements in magazines with high youth readerships (People of the State of California ex reI. Lockyer v. R.J. Reynolds Tobacco Company, 116 CalAppAth 1253, 1291, 2004).
Notwithstanding the decline in industry expenditures on advertising in youth-oriented publications, however, youth exposure to cigarette advertisements remains high. One study found that magazine advertisements for brands of cigarettes preferred by youth (those smoked by more than 5 percent of the smokers in the 8th, 10th, and 12th grades) reached more than 80 percent of young people in the United States an average of 17 times each in 2000 (King and Siegel 2001). Moreover, studies have shown that the MSA itself had little, if any, effect on the exposure of young people to magazine advertisements in the years following the agreement (Hamilton et al. 2002; King and Siegel 2001; Krugman et al. 2005). Cigarette companies continue to promote their products in magazines that reach high percentages and numbers of youth readers (Krugman et al. 2005).
Like magazine advertising, point-of-sale advertising (advertisements at the retail location, excluding outdoor signs on retailer property) also represents a smaller percentage of the promotional spending for tobacco companies than it did in years past. In 2003, tobacco companies reported spending $165.8 million on point-of-sale promotions. This amount rep-
resents a 41.7 percent decline from that in 2001, when companies spent $284.3 million on point-of-sale advertising. Current expenditures on point-of-sale advertising have declined 58.6 percent since their peak in 1993 at $400.9 million (FTC 2005).
In this section, the committee addresses advertising (in magazines and at the point of sale). The committee does not address price discounting to either consumers or retailers. The pricing of cigarettes is addressed in connection with cigarette excise taxes in Chapter 5.
Effects of Advertising and Other Promotional Exposures
No one doubts the government’s powerful interest in preventing the initiation of tobacco use by youth. A core element of the tobacco control policy agenda for decades has been the elimination of youth exposure to tobacco advertising. However, the long-standing industry position has been that advertising does not create new demand but rather affects the market share among existing smokers. This general position has also long been accompanied by an industry-wide insistence that advertising did not “target” youth and that exposure to advertising by youth was the incidental consequence of a spillover effect of targeting young adult smokers.
In 1994, the IOM reached the following conclusions after reviewing the available scientific literature:
The images typically associated with advertising and promotion convey the message that tobacco use is a desirable, socially approved, safe and healthful, and widely practiced behavior among young adults, whom children and youths want to emulate. As a result, tobacco advertising and promotion undoubtedly contribute to the multiple and convergent psychosocial influences that lead children and youths to begin using these products and to become addicted to them (IOM 1994, p. 131).
Since 1994, the available literature has been augmented in two important ways. First, internal industry documents disclosed in the course of litigation have yielded substantial evidence that tobacco companies did, in fact, target youth, including teenagers, to create new demand. Second, scientific evidence documenting the relationship between advertising exposure and consumption has accumulated.
Econometric studies examining the link between advertising and demand for tobacco products have provided mixed results, with a majority finding that cigarette advertising is an insignificant determinant of demand and others concluding that cigarette advertising had a positive and significant impact on consumption (Chaloupka and Warner 1999; Tauras et al. 2005). However, a recent review of these studies by Saffer and Chaloupka
demonstrated that the study results depended on which of three alternative empirical measures of advertising was used (Saffer and Chaloupka 2000). Most of the studies finding that advertising was not an important predictor of cigarette demand used annual or quarterly national aggregate expenditure data. The investigators argue that these studies lacked statistical power and were thus likely to find insignificant results because national expenditures lose variance because of aggregation effects and measure advertising where the marginal effect of advertising is near zero. In contrast, studies using cross-sectional data (typically measured at the local level for periods of less than a year) have greater variation in the advertising data and greater statistical power and thus are more likely to identify a positive relationship between advertising and consumption. Finally, studies that measure advertising on the basis of advertising bans produced various results that depended on the scope of advertising restrictions, leading the investigators to conclude that comprehensive advertising bans can reduce tobacco consumption but that a limited set of advertising bans will have little or no effect.
Saffer and Chaloupka caution that attempts to restrict advertising must be sufficiently comprehensive to eliminate the possibility that tobacco companies will simply substitute the remaining legal forms of advertising and promotion (Saffer and Chaloupka 2000). Advertising bans achieve the greatest success when they eliminate a wide range of media outlets, which diminishes opportunities for substitution, and which defeats industry efforts to replace advertising in the banned media with advertising in alternative channels. For example, the ban on outdoor advertising required by the MSA may have little effect on consumption because other forms of promotion, including print advertising, point-of-sale advertising, sponsorships, and other forms of retail promotion, will not be prohibited.
From the standpoint of the initiation of smoking by youth, the most important feature of tobacco advertising is its noninformational characteristics. The most compelling data are those that link positive feelings toward smoking with exposure to tobacco advertising and to ownership of commodities with tobacco company logos and paraphernalia.
The very purpose of noninformational tobacco advertising is to associate smoking with positive attributes and consequences and to create a positive affect toward smoking and people who smoke. In addition, advertising in magazines and retail displays creates the impression that smoking is a widespread and normal social practice and that tobacco is a normal consumer product. The images used in tobacco marketing associate smoking with lifestyles and experiences that appeal to young people, and these positive associations tend to displace or override risk information in adolescent decision making. The evidence clearly shows that youth exposure to images that create a positive association with smoking is associated with a
higher likelihood of smoking. Although it is difficult to isolate the effect of any particular strand in the web of influences that encourage adolescents to smoke, prevailing scientific opinion regards the relationship between promotional exposures and smoking to be a causal one.
Cigarette advertising also affects demand by current and former smokers. Tobacco advertisements and promotional campaigns may reduce current smokers’ willingness to quit smoking and may induce former smokers to resume their habit by reinforcing the attractions of smoking (Chaloupka and Warner 1999; Warner 1986). A review of tobacco industry documents confirmed that the companies have actively researched the determinants of cessation, and based upon their findings, they engaged in marketing efforts expressly designed to discourage current smokers from quitting and to encourage former smokers to relapse (Ling and Glantz 2004; Pollay and Dewhirst 2002). For example, upon discovering that health was the most frequently reported reason for quitting, the companies sought to address potential quitters’ concerns by developing and promoting more socially acceptable products (Ling and Glantz 2004) and advertising filtered and low-tar cigarettes as alternatives to quitting (Pollay and Dewhirst 2002). The companies have also attempted to encourage former smokers to resume smoking by increasing the number of advertisements appearing in popular magazines during periods when former smokers may be particularly vulnerable. A review of studies on cigarette advertising revealed that since 1984, advertising for cigarettes is more prevalent in January and February than it is in other months (Basil et al. 2000). Researchers believe that this trend likely reflects an attempt to counter New Year’s resolutions by targeting recent quitters when their withdrawal symptoms are peaking.
Tobacco Advertising Should Be Limited to Black-and-White–Text Only
A text-only regulatory approach to tobacco advertising, recommended by the IOM in 1994, is suitably tailored to promote the government’s interests in reducing the initiation of smoking by youth, and in reducing the level of smoking in general while respecting the industry’s interests in communicating product and price information. The government’s compelling interest in preventing the initiation of smoking by youth justifies constraints on the use of promotional messages and images that have a unique appeal to youth (such as cartoon characters) and the placement of commercial messages depicting smoking in a positive light in venues attracting substantial numbers of youth. Under the FDA’s 1996 Tobacco Rule, the ban applied to magazines with a youth readerships of greater than 15 percent. However, in light of the overt purpose of all non-informational tobacco advertising to make smoking appear to be attractive to smokers and nonsmokers alike, including youngsters and former smokers, the committee believes that all
commercial messages promoting smoking should be limited to a black-and-white–text only format, even if the level of youth exposure is less than 15 percent.
Recommendation 35: Congress and state legislatures should enact legislation limiting visually displayed tobacco advertising in all venues, including mass media and at the point-of-sale, to a text-only, black-and-white format.
The proposed restriction on advertising in mass media would apply to magazines and broadcast media (if the current ban were invalidated) and to advertising over the Internet through third parties. However, the committee recognizes that direct communication with customers through the Internet cannot feasibly be restricted.
Is a Black-and-White–Text Only Restriction Constitutional?
It is by no means clear that restrictions on tobacco advertising of the kind recommended above would survive a constitutional challenge.6 However, the committee believes that the proposed restriction on non-informational advertising is justified not only by the government’s powerful interest in suppressing the use of tobacco, an unreasonably dangerous product, but also by the unique history of deception and manipulation by the tobacco industry. Furthermore, allowing informational advertising in a black-and-white–text only format fully respects the genuine constitutional interests of tobacco companies and consumers. Accordingly, the committee believes that there is a reasonable prospect that the U.S. Supreme Court can be persuaded to uphold restrictions for tobacco advertising that would not be constitutionally permissible in other contexts.
The committee acknowledges that smokers have a legitimate interest in receiving accurate information from the manufacturers regarding the characteristics of their product and from the retailers regarding the prices of those products. In addition, the tobacco companies have a correlative interest in supplying such information, subject to appropriate regulation to prevent deception and unfair competition. Indeed, truthful, non-misleading information about tobacco products, including products that reduce exposure to harmful toxicants and purport to reduce the risks of smoking, can promote the public health. However, in the committee’s view, the tobacco industry does not have a constitutionally protected interest in encouraging or promoting smoking, recruiting new smokers, or sustaining the demand
of existing smokers. As the committee has previously noted, tobacco appears to be the only lawful consumer product for which the acknowledged governmental objective is to suppress all consumption. In this light, it would be constitutionally confusing if the tobacco companies’ desire to promote smoking were held to have any constitutional value under the First Amendment in the context of a pubic policy aiming to suppress consumption.
Admittedly, individuals and companies have a First Amendment right to promote public policies that the government opposes, and to promote viewpoints that are strongly objectionable to their fellow citizens. Moreover, tobacco companies have the First Amendment right to express their opposition to laws and policies aiming to suppress tobacco use—in colorful images if they choose to do so. Spending money to promote political viewpoints on issues and candidates is constitutionally protected speech. However, in the committee’s view, spending billions of dollars to promote the use of tobacco products should not be regarded as an exercise of political freedom or as its constitutional equivalent.
The federal and state governments have the constitutional authority to ban tobacco products altogether to protect the public health (see Gonzales v. Raich, 545 U.S. 1, 2005). However, no one believes that prohibition is a viable option in a country with 45 million addicted smokers. Under these circumstances, the federal and state governments have a compelling interest in reducing the prevalence of smoking by preventing smoking initiation and encouraging smoking cessation. The underlying issue, in a nutshell, is whether the U.S. constitutional system creates a fundamental contradiction—empowering the government to take aggressive measures to discourage smoking while simultaneously denying it the authority to restrict industry efforts to promote smoking. To put it another way, is the government barred by the First Amendment from restraining the marketing of an inherently harmful, although legal, product?
The U.S. Supreme Court has rejected the idea that the power to prohibit the sale of a product or service necessarily entails the lesser power to prohibit all commercial speech. If the product is lawful, the First Amendment provides some protection to commercial speech. The committee does not dispute that proposition. However, the question is what protection the First Amendment actually provides. On this point, the committee believes that the First Amendment protects the interests of sellers and buyers in conveying information about the product but does not protect the interest of sellers in promoting the use of a product that the government has a compelling interest in suppressing.
The explicit goal of tobacco policy is to reduce the use of this highly hazardous product in order to reduce tobacco-related mortality and morbidity. The powerful governmental interest in suppressing tobacco use should be sufficient to override whatever economic interest the tobacco
manufacturers and retailers have in encouraging people to smoke, an interest devoid of constitutional value. At the very least, the government’s compelling interest in preventing youth from smoking justifies a black-and-white–text only restriction of tobacco advertising in any venue where substantial numbers of youth would be exposed to the advertising, defined quantitatively (as an absolute number such as 2 million) or as a percentage of the exposed audience (King and Siegel 2001).
The alternative understanding of the First Amendment would allow no distinctions to be drawn among lawful products with respect to commercial advertising by those who sell them. In effect, such a view would leave legislatures with only two choices: banning the product altogether, or allowing it to be aggressively marketed under the shield of the First Amendment. In the committee’s opinion, this view is misguided, and tobacco is the test case.
In sum, the committee is drawing a crucial distinction between promoting tobacco use and informing consumers about tobacco use. Manufacturers and retailers do not have a constitutionally protected interest in promoting the use of their products, but they do have a protected interest in communicating truthful, non-misleading information about their products to consumers. Manufacturers and retailers also have a virtually absolute right to criticize the government’s policies toward tobacco use. Neither of these interests is infringed by a black-and-white–text only restriction.
Admittedly, a picture can be worth a thousand words, and it is conceivable that a text-only restriction could suppress commercial expression with informational value and therefore be unconstitutional as it is applied to a specific advertisement. However, the committee regards this prospect as a marginal one. In the committee’s opinion, few images in contemporary tobacco advertising convey truthful, non-misleading information about tobacco products. A good indication of the challenge that a tobacco company would have to overcome to support a claim that a visual advertisement is constitutionally protected would be to ask the company to describe the nonverbal message in words.
Diagrams depicting specific aspects of cigarette design to promote reduced-exposure products might convey important information to consumers. For example, Philip Morris and the R.J. Reynolds Company have test marketed cigarette-like products that purport to heat rather than burn tobacco. One might expect some advertisements for such products to show a diagram of the heating element, tobacco column, specialized lighter, and other aspects of design. There is a plausible claim for First Amendment protection here, but the constitutionality of the text-only restriction as applied to such an advertisement can be adjudicated on a case-by-case basis. Relevant considerations would include whether the necessary information can be conveyed effectively in words. Moreover, a regulatory agency charged with adopting rules to implement a text-only restriction might well decide
to make an exception for depictions of the product design as they relate to the health effects of smoking. Such an exception would be consistent with the committee’s intent and could be written into the authorizing legislation (e.g., all advertising would have to be in black-and-white text except depictions of the product itself).
Under the committee’s proposal, a company would not be entitled to show a color picture of a cigarette pack on the advertisement, even for the asserted purpose of informing consumers that its particular brand can be distinguished by a specific logo or color. The reason for holding the line on logos and colors is that these logos and colors are selected not only to convey information but also to affect the attitudes and behaviors of consumers toward the product. To allow such displays would threaten to unravel the constitutionally critical distinction between informational advertising and promotional advertising.
Targeting of Youth by Tobacco Manufacturers for Any Purpose Should Be Banned
For more than two decades, tobacco companies have promoted youth smoking education and prevention programs (Davidson 1998; Landman et al. 2002). Early efforts, introduced in the mid-1980s, were aimed at both children and parents; sample themes included “Talk to Your Kids,” “Kids Don’t Smoke,” “Smoking Isn’t Cool,” and “Wait Until You’re Older.” The youth programs portrayed smoking as an adult activity that was inappropriate for teenagers, whereas the parent-oriented messages urged adults to be involved in their children’s decision making regarding smoking.
Despite touting these programs as being designed to discourage teenagers from smoking, internal industry documents now reveal that, from their inception, these campaigns were developed largely to fend off increased regulation and to deflect public scrutiny of industry marketing practices. Industry representatives hoped that their youth prevention programs (which ignored the health effects of smoking) would displace the educational initiatives developed by public health groups, which frequently presented smoking as distasteful and unhealthy.
In the early 1990s, tobacco companies shifted their youth smoking prevention efforts to retailers, launching promotional efforts that included messages such as “It’s the Law,” “We Card,” and “Support the Law.” These campaigns implied that, in addition to age, upholding the law was an important reason not to smoke; moreover, the programs served to shift attention away from the industry’s contributions to youth smoking. Through these youth smoking prevention programs, the industry was able to recruit a network of retailers to assist it in detecting and defeating local tobacco control legislation, such as youth-access measures, advertising restrictions,
and clear-indoor-air laws. The industry also used the presence of this retailer network to fight national legislation, arguing that FDA regulation of tobacco advertising was unnecessary because the We Card program was making a “measurable difference” (Landman et al. 2002).
By the late 1990s, the tobacco companies sought the assistance of third parties to disseminate youth smoking prevention messages. By building alliances with youth organizations such as 4-H and Boys and Girls Clubs, tobacco companies sought to gain credibility with the public and create an aura of legitimacy for their prevention efforts. Industry documents reveal that the companies expended very little (if any) effort to study the effect of their campaigns on reducing the rate of smoking among youth; yet, the industry carefully assessed the public relations outcomes associated with the third-party programs (Landman et al. 2002).
Industry documents also reveal that the youth smoking prevention campaigns also enabled the companies to obtain useful data about the teen market that was otherwise practically inaccessible to them through standard marketing surveys (Landman et al. 2002). For example, the Philip Morris company learned that a smoking prevention advertisement directed at young teens would likely receive little attention from older youth if the message were delivered by members of the younger age group. Thus the company chose not to target teens in the 15- to 18-year-old age group— those at the highest risk for smoking—with their prevention campaigns. The Lorillard company developed a similarly innovative approach, inviting teenagers to visit the company’s website to learn more about its youth smoking prevention campaign. When these individuals enter personal information to qualify for sweepstakes, the company also obtains potentially useful data about the youth market.
A company’s efforts to disseminate informational materials about its programs may constitute no more than veiled attempts to promote its corporate identity among children. The California Departments of Education and Justice confronted such an attempt by Philip Morris in 2000, when the company distributed book covers promoting its youth prevention campaign to schools in California (Landman et al. 2002). This fairly blatant commercial ploy aroused opposition among educators statewide who argued that Philip Morris could have supported existing programs proven to be effective if it had been sincerely interested in helping to reduce the rate of smoking among youth (Landman et al. 2002).
Within the last decade, tobacco companies have expanded youth smoking prevention programs worldwide and have increased their financial commitments to these programs. Philip Morris announced a $100 million “Think. Don’t Smoke.” campaign in 1998 (Tobacco Free Kids 2005), and provided more than $125 million in grants to schools and youth organizations to support youth smoking prevention, youth development, and youth
smoking cessation programs between 1999 and 2004 (Philip Morris USA 2006). Similarly, Lorillard has contributed more than $80 million to youth smoking prevention programs since 1999 (Lorillard Tobacco Company 2007). As in the United States, international campaigns have frequently sent messages that have focused on decision making rather than on the negative health effects of smoking and that have presented smoking as an adult activity.
When these expenditures are viewed in terms most favorable to the companies, these expenditures are designed to demonstrate good corporate citizenship on the youth smoking issue and, perhaps, to weaken political support for stronger regulation. However, tobacco control advocates have a more skeptical view of the industry’s motivation. According to tobacco control advocates, these “youth prevention” programs are not really designed to prevent youth smoking at all. Instead, they are designed to promote smoking by facilitating industry access to young people through marketing surveys, by counteracting the anti-industry message of tobacco control media efforts by portraying the industry as trustworthy, and finally, by beginning to establish brand identification for future smokers.
In the committee’s view, it is not necessary to resolve this dispute regarding the industry’s motivation. In light of the history of past industry practices, industry messages targeted at children and adolescents should be regarded as presumptively suspect. The only acceptable justification for an industry-sponsored youth-oriented program is to prevent youth smoking. However, there is no evidence that the industry’s prevention programs actually do reduce youth smoking, and there is some evidence that they do not (Wakefield et al. 2006). If the tobacco manufacturers are genuinely interested in preventing youth from smoking, they should support programs known to be effective and should contract with an independent nonprofit organization with the necessary expertise to carry out the program (Warner 2002). To the extent that the companies have a legitimate interest in demonstrating good corporate citizenship, this interest can be served by requiring the recipients of company funding to acknowledge company support for its activities.
Recommendation 36: Congress and state legislatures should prohibit tobacco companies from targeting youth under 18 for any purpose, including dissemination of messages about smoking (whether ostensibly to promote or discourage it) or to survey youth opinions, attitudes and behaviors of any kind. If a tobacco company wishes to support youth prevention programs, the company should contribute funds to an independent non-profit organization with expertise in the prevention field. The independent organization should have exclusive responsibility for designing, executing and evaluating the program.
The constitutionality of the proposed restriction is not free from doubt, since it curtails the freedom of tobacco companies to communicate with young people for any purpose.7 However, the proposal does not ban all communication with minors, and the mere exposure of minors to advertising would not be a violation of the proposed ban. Instead, the restriction bans “targeting” of young people (conduct that is also banned by the MSA when it is explicitly promotional). The committee’s proposal extends the MSA ban to all targeting of youth, based on the presumption that any communications that target young people are highly likely to reflect a promotional motivation. Any legislation seeking to implement this restriction could certainly allow room for a company to prove that a specific communication had a legitimate purpose and did not have the purpose or effect of promoting tobacco use. On the basis of this analysis and on the unique history of tobacco company efforts to promote youth smoking, the committee believes that the proposed restriction would survive a constitutional challenge.
YOUTH EXPOSURE TO SMOKING IN MOVIES AND OTHER MEDIA SHOULD BE REDUCED
One of the biggest challenges of modern life for parents is to minimize the exposure of their children and impressionable teens to images and messages in the media that encourage or even glorify unhealthy and risky behaviors. Although the values of a free and open society preclude strong measures to cleanse the cultural environment of images and messages that are unfit for children, properly tailored legal restrictions on the time, place, and manner of display of such images and messages are permissible. The fact remains, however, that the authority of the state in this area is limited. These observations highlight the heavy responsibility borne by the entertainment media for formulating and enforcing industry regulations to facilitate parental efforts to protect their children from potentially harmful exposures to images and messages that tend to promote unhealthy (indeed, unlawful) behavior. A recent IOM/National Research Council report on underage drinking reviewed the evidence bearing on depictions and messages encouraging or glorifying drinking and urged stronger industry self-regulation backed up by monitoring of media content by the federal government (IOM/NRC 2004). This committee believes that a similar approach is needed regarding youth exposure to smoking in the entertainment media, especially in the movies.
The scientific literature on smoking in the movies is reviewed by Halpern-Felsher and Cornell in Appendix H, and the following material is drawn
from that review. Depictions of smoking in the movies doubled in the 1990s, bringing exposure rates closer to those observed in the 1950s (Glantz et al. 2004). Although recent data suggest that depictions of smoking in the movies declined from 2000 to 2004 (Worth et al. 2006), youth exposure to smoking in the movies remains high. In addition to its inclusion in R-rated movies, smoking can readily be observed in many movies rated appropriate for youth, including movies rated G, PG, and PG-13 (Charlesworth and Glantz 2005). Studies that have used content analysis have documented that smoking was portrayed in approximately 87 percent of movies produced from 1988 to 1997 (Dalton et al. 2002), in 77 percent of movies in 2004 (Worth et al. 2006), and in more than 66 percent of children’s animated movies released between 1937 and 1997 (Goldstein et al. 1999).
Healthcare professionals and tobacco control advocates are concerned that youth exposure to smoking in the movies will have an impact on adolescents’ attitudes toward smoking as well as smoking behavior itself (Charlesworth and Glantz 2005; Sargent 2005; Worth et al. 2006). These concerns are consistent with social cognitive theory, which indicates that adolescents are especially vulnerable to social modeling influences on behavior, including risky behaviors such as the use of tobacco and other drugs (Akers and Lee 1996; Bandura 1986).
Research investigating the impact of youth exposure to smoking in movies has yielded three important findings:
-
Exposure to depictions of smoking in movies is associated with more favorable attitudes toward smoking and characters who smoke, and these positive views are particularly prevalent among youth who themselves smoke. As the information in the previous section demonstrates, there is little doubt that youth are being exposed to smoking in the media, including movies, television, magazines, and newspapers, and that such exposures influence youth smoking-related perceptions.
-
Exposure to smoking in movies increases the risk for smoking initiation. Cross-sectional and longitudinal studies provide clear support that youth report greater susceptibility and intentions to smoke and are more likely to actually try smoking following exposure to smoking in the movies and on television. Furthermore, even after controlling for other factors known to be associated with adolescent smoking intention and tobacco use, studies show a clear dose effect, whereby greater exposure to smoking in the movies is associated with a greater chance of smoking. Studies have not yet been conducted to determine whether such a relationship between viewing smoking in the movies and tobacco use continues after initial tobacco use (Sargent 2005).
-
The increased risk for smoking initiation as a result of exposure to smoking in the movies can be reduced by antismoking advertisements and parental restriction of which movies their children watch.
On the basis of these findings, the committee encourages the entertainment industries to formulate and implement a set of strategies to limit and monitor youth exposure to smoking in the movies, television programming, and videos and to combat the effect of tobacco exposure on youth’s smoking attitudes and behaviors. These strategies should both guide and educate the movie industry about the evidence linking smoking in the movies and adolescent tobacco use (Dalton et al. 2003; Sargent et al. 2005), as well as spark a cogent discussion within the movie industry and between the movie industry and policymakers.
A ratings board, which is appointed by the president of the Motion Picture Association of America (MPAA), decides on the ratings assigned to each movie. Currently, such ratings are based on the extent to which there is violence, language, nudity, sensuality, and drug abuse in the film. Tobacco use is not considered. Assigning films with tobacco use a mature (R) rating increases the likelihood that parents will restrict their children’s access to such films, a strategy that has been shown to reduce the rate of smoking initiation (Dalton et al. 2002; Sargent et al. 2004, 2003).
The effects of youth viewing of smoking in the movies were found to be reduced among youth who first viewed an antismoking advertisement (Edwards et al. 2004; Pechmann and Shih 1999). Investigations of the effectiveness of antismoking advertisements with adolescents indicate strategies that are effective in reducing the influence of the viewing of smoking depictions in the media in general and that can be applied to smoking depictions in the movies as well. Goldman and Glantz (1998) showed that messages that are aggressive, delegitimize the tobacco industry, deglamorize smoking, and portray the negative effects of secondhand smoke were the most effective at changing perceptions about the normality of smoking and reducing cigarette consumption (Goldman and Glantz 1998). The results of a recent study of a specific antismoking advertising campaign (the truth® campaign) echoes those findings. That study found that this counter-industry media campaign was effective in increasing negative beliefs and attitudes about the tobacco industry and were associated with lower receptivity to protobacco advertising and less progression of smoking intention and behavior (Hershey et al. 2005).
Recommendation 37: The Motion Picture Association of America (MPAA) should encourage and facilitate the showing of anti-smoking advertisements before any film in which smoking is depicted in more than an incidental manner. The film rating board of the MPAA
should consider the use of tobacco in the movies as a factor in assigning mature film ratings (e.g., an R-rating indicating Restricted: no one under age 17 admitted without parent or guardian) to films that depict tobacco use.
This recommendation urges the MPAA to take smoking into account “as a factor” in its rating system; it does not suggest, categorically, that all movies with smoking receive an R-rating. The objective is to encourage directors and producers to take into account the possible impact of displays of smoking on a teenage audience and give serious consideration whether depicting characters smoking contributes to the artistic aims of the film or is needed for historical or cultural accuracy.
Independent oversight of the industry’s standards and strategies is warranted. Such oversight of industry accountability should be facilitated through public monitoring and awareness of industry practices. Accordingly, the committee recommends that the U.S. Department of Health and Human Services be authorized and funded to monitor these media practices and report to Congress and the public. This approach echoes a similar recommendation made by the IOM Committee on Preventing and Reducing Underage Drinking in 2004 (IOM/NRC 2004).
Recommendation 38: Congress should appropriate the necessary funds to enable the U.S. Department of Health and Human Services to conduct a periodic review of a representative sample of movies, television programs, and videos that are offered at times or in venues in which there is likely to be a significant youth audience (e.g., 15 percent) in order to ascertain the nature and frequency of images portraying tobacco use. The results of these reviews should be reported to Congress and to the public.
SURVEILLANCE AND EVALUATION SHOULD BE ENHANCED
Central to successful tobacco control is surveillance for antismoking program design and outcomes. An in-depth discussion of the elements of surveillance for tobacco control is included in Clearing the Smoke (IOM 2001). CDC offers the following definition of surveillance: “Public health surveillance is the ongoing, systematic collection, analysis, and interpretation of health data essential to the planning, implementation, and evaluation of public health practice, closely integrated with the timely dissemination of these data to those who need to know” (Thacker and Berkelman 1988). The extent of tobacco control activity surveillance depends on the goals of the program, the breadth of control activities and methods, the size of the geographic area being evaluated, the availability and accuracy of data
elements being sought, and the amount of funding available to conduct surveillance procedures.
Surveillance for tobacco use takes several forms. Most frequently, surveillance is underpinned by assessing tobacco use through household surveys. Such surveys, usually conducted by telephone, often include cigarette smoking behaviors; the intensity, amount and patterns of smoking; the brands of cigarettes and other tobacco products consumed; the sources of tobacco purchase or other acquisition; smoking history; personal smoking cessation attempts and the source and methods of such attempts; indicators of tobacco dependence, tobacco-related consultations as part of interactions within the health care system; family and peer use of tobacco; exposure to formal tobacco education programs during schooling; and environmental exposures to tobacco smoke, including venues where smoking occurs and where it is banned. Survey information may be obtained from dedicated tobacco use surveys or at least in part through multipurpose surveys that may contain additional relevant health and behavioral information.
Household surveys have certain limitations, including less than full and possibly biased participation rates, a high respondent time burden, limited access to minors, the recurring need to demonstrate valid responses, and substantial costs. Validation studies are conducted periodically to determine the accuracy of personal reports and may be supplemented by the use of tests for biological markers of tobacco use. More critically, however, many elements of tobacco control programs cannot be comprehensively determined from household information, and information must be derived from other sources. School-based surveys may be required to monitor adolescent tobacco use. Internet-based surveys may also be valuable, as they reduce the time burden for respondents. Tobacco use among special populations, such as prisoners, patients with major mental illnesses, and homeless people, usually require special institutional surveys and sampling procedures and often require the collection of more detailed informed consent. Surveillance of healthcare professional practices pertaining to tobacco-related education and smoking cessation may best be ascertained through reviews of medical records or the presence of institutional clinical guidelines that are themselves periodically evaluated. Data on the commercial distribution and sales of tobacco and tobacco control products often provide considerable insight into control efforts and can also be used to validate population survey information. Monitoring of jurisdictional environmental regulations and compliance with those regulations, tobacco use in the media and countermarketing activities, seizures of illegal tobacco products smuggled into the United States, the delivery of school-based antismoking educational programs, the resources being spent on community-based tobacco control efforts (public and private), the enforcement of youth-access restrictions, and the activities and management of community-based tobacco control
programs themselves are all examples of important surveillance activities occurring outside of household surveys.
Yet another important element of a national control program is toxicological assessment of the contents of tobacco products, both in the distributed product and after the product is burned or other tobacco-containing products are used. Finally, although it may be a long-term endeavor, surveillance for tobacco-related disease outcomes is a critical component of evaluations of tobacco control programs. Such surveillance may take many forms, but most jurisdictions are able to conduct surveillance on the occurrence of smoking-related cancers. Nevertheless, a comprehensive discussion of surveillance elements and methods is beyond the scope of this document.
General guidance for constructing a local and state surveillance program can be found in materials prepared by CDC. The basic elements of existing tobacco control efforts, based on best-practice elements (CDC 1999), provide important guidance to the elements of tobacco control surveillance. However, many state and local control programs have surveillance programs in place and have tailored these programs to their special activities, such as surveillance in workplaces, eating, and drinking establishments, college campuses, urban public places and motor vehicles. Most importantly, surveillance programs allow assessment of the progress that tobacco control programs have made. In turn, surveillance findings are used to drive changes in program activities, direction, and intensity.
Recommendation 39: State tobacco control agencies should conduct surveillance of tobacco sales and use and the effects of tobacco control interventions, in order to assess local trends in usage patterns; identify special groups at high risk for tobacco use; determine compliance with state and local tobacco-related laws, policies, and ordinances; and evaluate overall programmatic success.
Recommendation 40: The Secretary of HHS, through FDA or other agencies, should establish a national comprehensive tobacco surveillance system to collect information on a broad range of elements needed to understand and track the population impact of all tobacco products and the effects of national interventions (such as attitudes, beliefs, product characteristics, product distribution and usage patterns, and marketing messages and exposures to them).
SUMMARY
This chapter recommends a fundamental change in the current legal framework of tobacco control: a new, innovative regulatory approach that
takes into account the unique history and characteristics of tobacco and its uses. Under the plan envisioned by the committee, the federal government would assume broad regulatory responsibility for tobacco control to augment the traditional state-centered tobacco control approaches described in Chapter 5. Because a total prohibition against the manufacture and distribution of tobacco products is not a realistic option for the foreseeable future, the new legal structure for tobacco control must be framed within the context of a regulated market. Accordingly, Congress should confer upon the FDA broad regulatory authority over the manufacture, distribution, marketing, and use of tobacco products. Such a broad federal regulatory authority would free the states to supplement federal action with their own measures that aim to suppress tobacco use and that are compatible with federal law.
Within this general framework, the committee recommends federal regulation of tobacco product characteristics and product packaging, aggressive state regulation of the retail environment to promote public health objectives, implementation of a federal tobacco control funding plan to coordinate state funding and reduce the wide range of state excise tax rates, and strong state and federal measures to reduce tobacco advertising and promotion and otherwise reduce initiation of tobacco use by youth.
REFERENCES
AAPHP (American Association of Public Health Physicians). 2007. American Association of Public Health Physicians. Web Page. Available at: http://www.aaphp.org/WebLinks/stakeholders.html; accessed May 11, 2007.
Akers RL, Lee G. 1996. A longitudinal test of social learning theory: adolescent smoking. Journal of Drug Issues 26(2):317-343.
Ashley MJ, Cohen J, Ferrence R. 2001. “Light” and “mild” cigarettes: who smokes them? Are they being misled? Canadian Journal of Public Health 92(6):407-411.
Bandura A. 1986. Social Foundations for Thought and Action: A Social-Cognitive Model. Englewood Cliffs, NJ: Prentice Hall.
Basil MD, Basil DZ, Schooler C. 2000. Cigarette advertising to counter New Year’s resolutions. Journal of Health Communication 5(2):161-174.
Benowitz NL, Henningfield JE. 1994. Establishing a nicotine threshold for addiction. The implications for tobacco regulation. New Engand Journal of Medicine 331(2):123-125.
Borland R, Yong HH, King B, Cummings KM, Fong GT, Elton-Marshall T, Hammond D, McNeill A. 2004. Use of and beliefs about light cigarettes in four countries: findings from the International Tobacco Control Policy Evaluation Survey. Nicotine and Tobacco Research 6(Suppl 3):S311-S321.
CDC (Centers for Disease Control and Prevention). 1999. Best Practices for Comprehensive Tobacco Control Programs—August 1999. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health.
CDC. 2005. Cigarette smoking among adults—United States, 2004. MMWR (Morbidity and Mortality Weekly Report) 54(44):1121-1124.
Chaloupka FJ and Warner KE. 1999. The Economics of Smoking. National Bureau of Economic Research, Working Paper No. W7047. Web Page. Available at: http://www.nber.org/papers/w7047; accessed September 13, 2006.
Charlesworth A, Glantz SA. 2005. Smoking in the movies increases adolescent smoking: a review. Pediatrics 116(6):1516-1528.
Cunningham R. 2005. Package Warnings: Overview of International Developments. Ottowa, Ontario: Canadian Cancer Society.
Dalton MA, Ahrens MB, Sargent JD, Mott LA, Beach ML, Tickle JJ, Heatherton TF. 2002. Relation between parental restrictions on movies and adolescent use of tobacco and alcohol. Effective Clinical Practice 5(1):1-10.
Dalton MA, Sargent JD, Beach ML, Titus-Ernstoff L, Gibson JJ, Ahrens MB, Tickle JJ, Heatherton TF. 2003. Effect of viewing smoking in movies on adolescent smoking initiation: a cohort study. Lancet 362(9380):281-285.
Dalton MA, Tickle JJ, Sargent JD, Beach ML, Ahrens MB, Heatherton TF. 2002. The incidence and context of tobacco use in popular movies from 1988 to 1997. Preventive Medicine 34(5):516-523.
Davidson PA. 1998. Tales from the tobacco wars: industry advertising targets teenage girls. Wisconsin Women’s Law Journal 13(1).
Drummond DC. 2000. UK government announces first major relaxation in the alcohol licensing laws for nearly a century: drinking in the UK goes 24-7. Addiction 95(7):997-998.
Edwards CA, Harris WC, Cook DR, Bedford KF, Zuo Y. 2004. Out of the smokescreen: does an anti-smoking advertisement affect young women’s perception of smoking in movies and their intention to smoke? Tobacco Control 13(3):277-282.
Feighery EC, Ribisl KM, Schleicher N, Lee RE, Halvorson S. 2001. Cigarette advertising and promotional strategies in retail outlets: results of a statewide survey in California. Tobacco Control 10(2):184-188.
Fong GT. 2005. Evaluating the Effects of the September 2003 European Union Policy Banning “Light/Mild” Cigarette Brand Descriptors: Findings from the International Tobacco Control Policy Evaluation Survey. Health Canada.
Fong GT, Hammond D, Laux FL, Zanna MP, Cummings KM, Borland R, Ross H. 2004. The near-universal experience of regret among smokers in four countries: findings from the International Tobacco Control Policy Evaluation Survey. Nicotine and Tobacco Research 6(Suppl 3):S341-S351.
FTC (Federal Trade Commision). 2005. Cigarette Report for 2003. Washington, DC: Federal Trade Commission.
Glantz SA, Kacirk KW, McCulloch C. 2004. Back to the future: smoking in movies in 2002 compared with 1950 levels. American Journal of Public Health 94(2):261-263.
Goldman LK, Glantz SA. 1998. Evaluation of antismoking advertising campaigns. Journal of the American Medical Association 279(10):772-777.
Goldstein AO, Sobel RA, Newman GR. 1999. Tobacco and alcohol use in G-rated children’s animated films. Journal of the American Medical Association 281(12):1131-1136.
Gruenewald PJ, Ponicki WB, Holder HD. 1993. The relationship of outlet densities to alcohol consumption: a time series cross-sectional analysis. Alcoholism: Clinical and Experimental Research 17(1):38-47.
Hamilton WL, Norton G, Ouellette TK, Rhodes WM, Kling R, Connolly GN. 2004. Smokers’ responses to advertisements for regular and light cigarettes and potential reduced-exposure tobacco products. Nicotine and Tobacco Research 6(Suppl 3):S353-S362.
Hamilton WL, Turner-Bowker DM, Celebucki CC, Connolly GN. 2002. Cigarette advertising in magazines: the tobacco industry response to the Master Settlement Agreement and to public pressure. Tobacco Control 11(Suppl 2):ii54-ii58.
Hammond D, Fong GT, McDonald PW, Brown KS, Cameron R. 2004. Graphic Canadian ciga
rette warning labels and adverse outcomes: evidence from Canadian smokers. American Journal of Public Health 94(8):1442-1445.
Hammond D, Fong GT, McNeill A, Borland R, Cummings KM. 2005. Effectiveness of Cigarettes Warning Labels in Informing Smokers About the Risks of Smoking: Findings From the International Tobacco Control (ITC) Four Country Survey. Web Page. Available at: http://tc.bmjjournals.com/cgi/content/full/15/suppl_3/iii19; accessed June 21, 2006.
Hammond D, McDonald PW, Fong GT, Brown KS, Cameron R. 2004. The impact of cigarette warning labels and smoke-free bylaws on smoking cessation: evidence from former smokers. Canadian Journal of Public Health 95(3):201-204.
Health Canada. 2005a. The Health Effects of Tobacco and Health Warning Messages on Cigarette Packages—Survey of Adults and Adult Smokers. Web Page. Available at: http://www.hc-sc.gc.ca/hl-vs/tobac-tabac/research-recherche/por-rop/impact/2003_e.html; accessed June 23, 2007.
Health Canada. 2005b. The Health Effects of Tobacco and Health Warning Messages on Cigarette Packages—Survey of Youth. Ottawa, Ontario: Health Canada.
Henningfield JE, Benowitz NL, Slade J, Houston TP, Davis RM, Deitchman SD. 1998. Reducing the addictiveness of cigarettes. Tobacco Control 7(3):281-293.
Hershey JC, Niederdeppe J, Evans WD, Nonnemaker J, Blahut S, Holden D, Messeri P, Haviland ML. 2005. The theory of “truth”: how counterindustry campaigns affect smoking behavior among teens. Health Psychology 24(1):22-31.
Holder HD. 2004. Supply side approaches to reducing underage drinking: an assessment of the scientific evidence. Reducing Underage Drinking: A Collective Responsibility. Washington, DC: National Academy Press. Pp. 458-489.
Holder HD, Wagenaar AC. 1990. Effects of the elimination of a state monopoly on distilled spirits’ retail sales: a time-series analysis of Iowa. British Journal of Addiction 85(12):1615-1625.
IOM (Institue of Medicine). 1994. Growing Up Tobacco Free: Preventing Nicotine Addiction in Children and Youth. Washington, DC: National Academy Press.
IOM. 2001. Clearing the Smoke: Assessing the Science Base for Tobacco Harm Reduction. Washington, DC: National Academy Press.
IOM/NRC (National Research Council). 2004. Reducing Underage Drinking: A Collective Responsibility. Washington, DC: The National Academies Press.
King C, Siegel M. 2001. The Master Settlement Agreement with the tobacco industry and cigarette advertising in magazines. New England Journal of Medicine 345(7):504-511.
Kozlowski LT, Goldberg ME, Yost BA, White EL, Sweeney CT, Pillitteri JL. 1998. Smokers’ misperceptions of light and ultra-light cigarettes may keep them smoking. American Journal of Prevtive Medicine 15(1):9-16.
Kropp RY, Halpern-Felsher BL. 2004. Adolescents’ beliefs about the risks involved in smoking “light” cigarettes. Pediatrics 114(4):e445-e451.
Krugman DM, Fox RJ, Fischer PM. 1999. Do cigarette warnings warn? Understanding what it will take to develop more effective warnings. Journal of Health Communication 4(2):95-104.
Krugman DM, Quinn WH, Sung Y, Morrison M. 2005. Understanding the role of cigarette promotion and youth smoking in a changing marketing environment. Journal of Health Communication 10(3):261-278.
Landman A, Ling PM, Glantz SA. 2002. Tobacco industry youth smoking prevention programs: protecting the industry and hurting tobacco control. American Journal of Public Health 92(6):917-930.
Ling PM, Glantz SA. 2004. Tobacco industry research on smoking cessation. Recapturing young adults and other recent quitters. Journal of General Internal Medicine 19(5 Pt 1):419-426.
Lorillard Tobacco Company. 2007. Lorillard Tobacco Company: Youth Smoking Prevention.
Web Page. Available at: http://www.lorillard.com/index.php?id=5; accessed June 15, 2007.
Millar WJ. 1996. Reaching smokers with lower educational attainment. Health Reports 8(2):11-19(Eng); 13-22(Fre).
Munshi MA. 1997. Share the wine—liquor control in Pennsylvania: a time for reform. University of Pittsburgh Law Review 58(2):507-547.
NCI (National Cancer Institute). 2001. Risks Associated with Smoking Cigarettes with Low Machine-Measured Yields of Tar and Nicotine (Monograph 13). Bethesda, MD: National Institutes of Health.
Pechmann C, Shih CF. 1999. Smoking scenes in movies and antismoking advertisements before movies: effects on youth. Journal of Marketing 63(1-13).
Philip Morris USA. 2006. Youth Smoking Prevention: Grant Programs. Web Page. Available at: http://www.philipmorrisusa.com/en/our_initiatives/ysp/grant_programs.asp; accessed September 13, 2006.
Pierce JP, Gilpin EA. 2004. How did the Master Settlement Agreement change tobacco industry expenditures for cigarette advertising and promotions? Health Promotion Practice 5(3 Suppl):84S-90S.
Pollay RW. 2001. JTI-McDonald, Imperial Tobacco Canada Ltd and Rothmans, Bencon & Hedges InC v. Attorney General of Canada and Canadian Cancer Society. The Role of Packaging Seen Through Industry Documents. Supreme Court, Province of Quebec, District of Montreal.
Pollay RW, Dewhirst T. 2002. The dark side of marketing seemingly “Light” cigarettes: successful images and failed fact. Tob Control 11 (Suppl 1):118-131.
Rutledge TE. 1989. The questionable viability of the Des Moines warranty in light of Brown-Forman Corp v. New York. Kentucky Law Journal 78(1):209-235.
Saffer H, Chaloupka F. 2000. The effect of tobacco advertising bans on tobacco consumption. Journal of Health Economics 19(6):1117-1137.
Sargent JD. 2005. Smoking in movies: impact on adolescent smoking. Adolescent Medicine Clinics 16(2):345-370, ix.
Sargent JD, Beach ML, Adachi-Mejia AM, Gibson JJ, Titus-Ernstoff LT, Carusi CP, Swain SD, Heatherton TF, Dalton MA. 2005. Exposure to movie smoking: its relation to smoking initiation among U.S. adolescents. Pediatrics 116(5):1183-1191.
Sargent JD, Beach ML, Dalton MA, Ernstoff LT, Gibson JJ, Tickle JJ, Heatherton TF. 2004. Effect of parental R-rated movie restriction on adolescent smoking initiation: a prospective study. Pediatrics 114(1):149-156.
Sargent JD, Dalton MA, Heatherton T, Beach M. 2003. Modifying exposure to smoking depicted in movies: a novel approach to preventing adolescent smoking. Archives of Pediatric and Adolescent Medicine 157(7):643-648.
Shankar V. 1999. Alcohol direct shipment laws, the commerce clause, and the twenty-first ammendment. The Virginia Law Review 85(2):353-383.
Shiffman S, Pillitteri JL, Burton SL, Rohay JM, Gitchell JG. 2001. Smokers’ beliefs about “Light” and “Ultra Light” cigarettes. Tobacco Control 10(Suppl 1):i17-i23.
Shipman GA. 1940. State administrative machinery for liquor control. Law and Contemporary Problems 7:600-620.
Siahpush M, McNeill A, Hammond D, Fong GT. 2006. Socioeconomic and country variations in knowledge of health risks of tobacco smoking and toxic constituents of smoke: results from the 2002 International Tobacco Control (ITC) Four Country Survey. Tobacco Control 15:65-70.
Slade J. 1997. The pack as advertisement. Tobacco Control 6(3):169-170.
Spaeth SJ. 1991. The twenty-first ammendment and state control over intoxicating liquor: accommodating the federal interest. California Law Review 79(1):161-204.
Swain KW. 1996. Liquor by the book in Kansas: the ghost of temperance past. Washburn Law Journal 35(2):322-345.
Tauras JA, Chaloupka FJ, Emery S. 2005. The impact of advertising on nicotine replacement therapy demand. Social Science and Medicine 60(10):2351-2358.
Tengs TO, Ahmad S, Moore R, Gage E. 2004. Federal policy mandating safer cigarettes: a hypothetical simulation of the anticipated population health gains or losses. Journal of Policy Analysis and Management 23(4):857-872.
Thacker SB, Berkelman RL. 1988. Public health surveillance in the United States. Epidemiology Review 10:164-190.
Tobacco Free Kids. 2005. A Long History of Empty Promises: The Cigarette Companies’ Ineffective Youth Anti-Smoking Programs. Web Page. Available at: http://www.tobaccofreekids.org/research/factsheets/pdf/0010.pdf; accessed September 13, 2006.
Tobacco Free Kids. 2006. Tobacco Free Kids Action Fund, American Cancer Society, American Heart Association, American Lung Association, Americans for Nonsmokers’ Rights, and National African American Tobacco Prevention Network V. Philip Morris, Inc. Web Page. Available at: http://tobaccofreekids.org/reports/doj/JudgmentOrder.pdf; accessed May 30, 2007.
Tobacco Free Kids. 2007a. Congress Has Historic Opportunity to Save Children and Save Lives by Granting FDA Authority Over Tobacco Products. Web Page. Available at: http://tobaccofreekids.org/Script/DisplayPressRelease.php3?Display=966; accessed May 11, 2007.
Tobacco Free Kids. 2007b. Letter to Representative. Web Page. Available at: http://tobaccofreekids.org/organization/letters/092804.pdf; accessed May 11, 2007b.
Wagenaar AC, Holder HD. 1995. Changes in alcohol consumption resulting from the elimination of retail wine monopolies: Results from five U.S. states. Journal of Studies on Alcohol 56(5):566-572.
Wakefield M, Kloska DD, O’Malley PM, Johnston LD, Chaloupka F, Pierce J, Giovino G, Ruel E, Flay BR. 2004. The role of smoking intentions in predicting future smoking among youth: findings from Monitoring the Future data. Addiction 99(7):914-922.
Wakefield M, Terry-McElrath Y, Emery S, Saffer H, Chaloupka FJ, Szczypka G, Flay B, O’Malley PM, Johnston LD. 2006. Effect of televised, tobacco company-funded smoking prevention advertising on youth smoking-related beliefs, intentions, and behavior. American Journal of Public Health 96(12):2154-2160.
Warner KE. 1986. Selling Smoke: Cigarette Advertising and Public Health. Washington, DC: American Public Health Association.
Warner KE. 2002. What’s a cigarette company to do? American Journal of Public Health 92(6):897-900.
WHO (World Health Organization). 2003. WHO Framework Convention on Tobacco Control. Geneva, Switzerland: WHO.
Woeste K. 2004. Reds, whites, and roses: the dormant commerce clause, the twenty-first ammendment, and the direct shipment of wine. University of Cincinnati Law Review 72(4):1821-1848.
Worth K, Tanski S, Sargent J. 2006. Trends in Top Box Office Movie Tobacco Use, 1996– 2004. First Look Report. Washington, DC: American Legacy Foundation.






































































