E
Additional Remark on Clinical Use of Rare Isotopes
The medical community continues to investigate new isotopes for use in radiation therapy. Recently, studies of the rare isotope 149Tb (terbium) were reported in the European Journal of Nuclear Medicine and Molecular Imaging.1 The research, headed by G.-J. Beyer, involved a collaboration between the On-Line Isotope Mass Separator (ISOLDE) group at the European Organization for Nuclear Research (CERN) and medical researchers from a variety of institutions, including the Memorial Sloan-Kettering Cancer Center. The primary aim of the research was to examine the efficiency of 149Tb-labeled rituximab in specifically killing circulating single cancer cells or small cell clusters in vivo. 149Tb decays to alpha particles 17 percent of the time and has a half-life of 4.1 hours, which is conveniently longer than some other alpha-emitting radionuclides (e.g., 213Bi). Lower-energy alpha particles, such as in 149Tb decays, have been shown to be very efficient in killing cells, and their short range means that minimal damage is caused in the neighborhood of the target cells.
The 149Tb for this study was produced by the on-line isotope separator facility ISOLDE at CERN. The study injected 26 female mice with 5 × 106 Daudi cells, which would normally cause the mice to quickly develop lethal lymphoma disease.
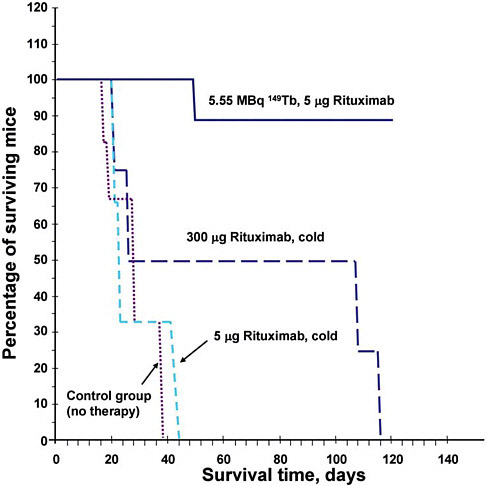
FIGURE E.1 Survival plot of mice grafted with 5 × 106 Daudi cells followed by different intravenous treatments 2 days subsequent. From European Journal of Nuclear Medicine and Molecular Imaging 31(4), April 2004, G.-J. Beyer, M. Miederer, S. Vranješ-Đurić, J.J. Čomor, G. Künzi, O. Hartley, R. Senekowitsch-Schmidtke, D. Soloviev, F. Bechegger, and the ISOLDE Collaboration, “Targeted Alpha Therapy in Vivo: Direct Evidence for Single Cancer Cell Kill Using 149Tb-rituximab,” Figure 3, Copyright Springer-Verlag 2004 with kind permission of Springer Science and Business Media.
The mice were separated into 4 groups: 6 received no further injection (control group), 6 received 5 µg of rituximab, 4 received 300 µg of rituximab, and 10 received 5 µg of rituximab labeled with radioactive 149Tb with a decay rate of 5.5 × 106 decays per second. These second injections were administered 2 days after the Daudi cell inoculation. Rituximab is a monoclonal antibody that targets CD20 antigens, which are expressed in large numbers by the Daudi cells.
The dramatic results of the study are shown in Figure E.1, which indicates the mice’s survival in days in terms of the percentage surviving. All of the mice except those receiving the 149Tb-labeled rituximab had perished by 120 days, and approximately half had developed macroscopic tumors. In the group treated with the 149Tb-labeled rituximab, only one of the nine had died; the remainder showed no pathological changes upon further examination.
The low-energy alpha particles and longer lifetime properties of 149Tb made it the best isotope available for performing this research. Rare-isotope facilities can examine many more isotopes and can be expected to discover more particular isotopes with the ideal chemical and radiological characteristics for the treatment of disease.



