2
Moving Beyond Compliance: Can Industry Get Ahead of the Curve?1
Although environmental management systems (EMSs) are initially implemented to maintain compliance with regulations, they often have implications for lowering environmental costs, training employees, and developing indicators to measure and reduce environmental impact. An effective EMS enables an organization’s officials and stakeholders to examine its values, priorities, policies, strategies, objectives, methods for allocating resources for delivering performance, and also learning. Some research suggests that EMSs can manage risks, gain competitive advantages, and achieve environmental improvements at lower costs. During the workshop the speakers, Roundtable members, and participants considered how companies could use EMSs and other tools and policies to achieve greater impact beyond regulatory compliance.
USING MANAGEMENT SYSTEMS TO IMPROVE PERFORMANCE IN THE CHEMICAL INDUSTRY
Today the chemical industry is increasingly sensitive and responsive to the growing number of public concerns regarding the use of chemical products, said Terry F. Yosie, formerly of the American Chemistry Council (ACC). The chemical industry attempts to design programs that focus on product safety and health. One of the programs that was adopted in the United States in 1988 was Responsible Care®. At the time when the program was created, it focused largely on environmental health and safety issues of manufacturing facilities. During more recent years, this program has undergone some significant changes and today Responsible Care® also addresses the need to integrate the initiative with
the actual business operations, not only within chemical companies but also their business partners.
Yosie noted that all chemical companies who are members of the ACC—ACC members represent about 90 percent of the manufacturing capacity of chemicals in the United States—are required, as an obligation of membership, to participate in Responsible Care®. The scope of Responsible Care® is tailored specifically to the chemical industry and includes such aspects as continuous environmental, health, and safety performance improvement, enhanced security, increased product stewardship, improved performance across the value chain, communication with stakeholders, integration with sustainable development, and improved chemical industry reputation. ACC members are required to obtain a management system certification from independent third party auditors, and they provided the leadership to getting the industry as a whole to adopt this approach. Certification is one of the tools that can help promote cross-integration among government, industry, and non-government organizations (NGOs). The chemical industry is an industry that exists in a global market. Customer expectations have to be met and the industry is expecting its service providers and suppliers to adopt internationally recognized management systems with certification.
Responsible Care® is tailored specifically to the chemical industry and includes such aspects as: continuous performance improvement, enhanced security, increased product stewardship, improved performance across the value chain, communication with stakeholders, integration with sustainable development, and improved chemical industry reputation.
—Terry F. Yosie
Also, ACC is exploring the opportunities to integrate Responsible Care® with other business partners such as the suppliers and logistics providers. There are many opportunities to achieve efficiency across the entire business supply chain, said Yosie. The benefits of this approach are that customers are going to prefer to work with companies that can demonstrate better performance along with competitive costs. ACC collaborates with outside parties such as the U.S. Coast Guard, the Environmental Protection Agency (EPA), the Occupational Safety and Health Administration, and the insurance industry to incorporate elements of Responsible Care® in their decision making. In May 2004, the ACC began publishing a set of performance measures and the performance of all its members against these measures. This transparency creates a dynamic where the academic community, the media, NGOs, and the government can look directly in the Responsible Care® website to determine the performance of ACC members. Also, this initiative provides a good incentive to drive performance because the whole world is watching, said Yosie.
Relationship Between ISO 14001 and Responsible Care®
Responsible Care®’s management system includes environmental health, safety, and security elements. Unlike Responsible Care®, ISO 14001 focuses on environmental issues and does not have fixed metrics nor does it require public reporting. The ISO 14001 standard does not explicitly encourage stakeholder dialog and interaction, whereas Responsible Care® includes it as an auditable function within its management system. Further, ISO-accredited auditors who conduct the certification must obtain additional training to ensure their competency in safety, health, and security issues. Finally, ISO 14001 is facility-based, and Responsible Care® is both facility- and headquarters-based.
Responsible Care®on the Global Stage
Responsible Care® is currently implemented in 52 countries. Its implementation area includes most of Latin America, Southeast Asia, Western Europe, and major North American countries. The number of participating countries is expected to grow in the future to include China, the Middle East, and Russia. In February 2006, the leaders of the major chemical industry trade associations and global chemical companies endorsed a document called the Responsible Care® Global Charter (ICCA, 2006). The charter adds new performance commitments for chemical operations around the world. It also harmonizes and makes the 52 national programs more consistent as a singular set of commitments and obligations to perform the following:
-
Adopt global Responsible Care® core principles.
-
Implement fundamental features.
-
Advance sustainable development.
-
Continuously improve and report performance.
-
Enhance product stewardship.
-
Extend Responsible Care® through the value chain.
-
Support national and global Responsible Care® governance processes.
-
Address stakeholder expectations.
-
Provide appropriate resources.
Extending Responsible Care® through the business process globally is important because, when the industry operates in a developing country, industry standards and practices are frequently well beyond existing national or local standards. With the aid of Responsible Care®, developing countries will be able to build capacity and institutions that will increase the level of skills within their population and help develop regulatory processes. For example, countries such as Latvia, Poland, and the Czech Republic were using Responsible Care® to meet the European Union entry requirements. Responsible Care® enables countries to track and influence not only how major companies perform but also how they
interact and meet societal expectations. This process facilitates the ability to track and influence how companies set their own objectives and how they participate as constructive members of civil society, concluded Yosie.
THE IMPLICATION OF TECHNOLOGY FOR ENVIRONMENTAL HEALTH
Technological advances have the potential to significantly impact how businesses operate. Although not specifically intended to reduce waste, these advances and practices can affect health and the environment. One of the advantages of technology is that it enables companies to allow their employees to telework. There are many social, economic, health, and cultural benefits of telework. Telework significantly increases employees’ productivity. It increases efficiency and use of real estate. It also increases retention of personnel, reduces stress, and the employee families are happier because they can spend more time together, noted Braden Allenby of Arizona State University. However, the concept of teleworking brings out many issues that need to be addressed, said Allenby.
Environmental Impact of Telework
Due to virtual office activities, a company may no longer need excess real estate; therefore, it can consolidate its holdings, which is good for the environment and is also cost effective. When Allenby was head of health and safety at AT&T, the company did some calculations of the impact of telework. They found that because the employees did not have to commute, they cut back 100 million miles of unnecessary commuting, which came out to 5 million gallons of gasoline, which equals 50,000 tons of CO2 that was not emitted into the atmosphere. Also, most of transportation infrastructure is designed for rush hour period; thus when people telework, rush hour period is shaved, and the demand for infrastructure is reduced.
Challenges of Telework
Despite the advantages that telework offers to employees, there are many challenges. Today, we do not know what the long-term impact of telework is going to be, said Allenby. Telework is a fundamental change in the way people behave, and it may take many years to understand what the impacts of those behaviors are. It is possible that urban sprawl may increase because, due to technological opportunities, people will not be compelled to live close to where they work. From the social perspective, a significant number of parameters are changing in the way people think about work. When the distance between family and work is collapsed, the impacts on the family and the worker are not known. When the worker’s computer is on all the time, it may be difficult to divide between
work, play, education, and family—or the divide may become obsolete. Even today, vacations are becoming obsolete, said Allenby, because when people go on vacation they take their laptops, palmtops, and cell phones to be able to keep up with their work. Otherwise, if they don’t do that, they will be overwhelmed with the amount of information they will have to process when they get back from their vacation.
Telework changes the pattern of life profoundly. It enables older persons and disabled people to come and work as equals in an organization. It changes the concept of retirement and the pension system. Taxation of teleworkers is also challenging. The tax system in the United States is place-based, meaning one gets taxed where they live or work. The challenge is to determine where one works if one is virtual. Another challenge is legal issues. Most of the workplace law today is based upon a manufacturing assumption; therefore, lawmakers need to face the challenge of working out new workplace law that is applicable to telework.
The challenges of telework are more profound than just environmental impact, said Allenby. It is also important to understand the operation of the institutional organization well enough to identify the opportunities to make strategic advances that also benefit the environment and the society, concluded Allenby.
CRADLE TO GRAVE: UPSTREAM SOURCES
Xerox Corporation has a waste-free strategy that has been in development for approximately 13 years, said Jack Azar of Xerox Corporation. The strategy started out with waste-free facilities, expanded into waste-free products, and, ideally, it plans to allow its customers to be waste free as well. In the past 10 years, Xerox avoided approximately 1.5 billion pounds of landfill waste. The calculations on the amount of hazardous materials (solid waste such as lead, chromium, mercury, and cadmium after it is separated from recyclable waste) are about 0.3 percent. Avoiding landfill and the use of raw material is energy efficient and helps reduce CO2 emissions.
The most important factors to make the product life cycle work, with respect to hardware, equipment, and information technology, is to design up front for the product life cycle, observed Azar. Developing environmental benefits works better if it is done in segments, noted Azar. If a company just takes a product, introduces it as the identical product back to commerce, the approach can only go so far. Xerox found that product conversion developed for remanufacturing, reuse, and parts commonality right up front is a better approach. This process leads to significant waste reduction, extends the life cycle of products, and brings out new features and qualities in a product set, said Azar (Figure 2-1).
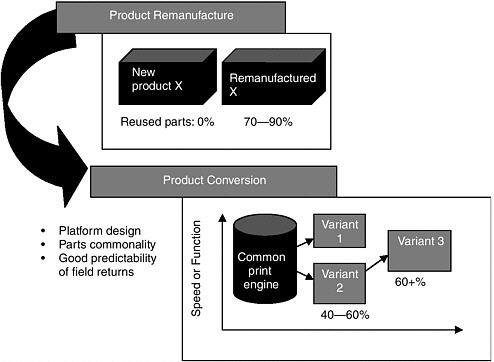
FIGURE 2-1 Xerox Corporation’s approach to product conversion developed for remanufacturing, reuse, and parts commonality. This process leads to significant waste reduction, extends the life cycle of products, and brings out new features and qualities in a product set. SOURCE: Azar, unpublished.
Outsourcing of Manufacturing
Xerox is trying to discover a way to use paper responsibly. Xerox does not manufacture paper; it buys finished and already packaged paper and distributes it. Manufacture of paper is a highly energy-intensive industry; it takes 10 watts to produce every sheet of paper; creating that energy contributes to pollution. Even though the manufacturing of the paper is technically not in the company’s control, Xerox requires that various paper suppliers’ operating policies and manufacturing procedures are consistent with the company’s reputation of its brand and environmental values and encourages them to be more environmentally responsible. Therefore, Xerox’s suppliers have to meet the following requirements: (1) compliance, wherever the supplier is operating; (2) effective paper mill EMS; (3) manufacturers that control their own forests have to have those forests third-party certified; and (4) manufacturers that buy fiber and convert it through their
mills into finished paper have to receive a third-party chain of custody certification. For example, if the wood came from Brazil, Canada, Indonesia, or Finland, Xerox needs to know its origin and that the supplier is certified by a third party.
Today, 82 percent of the 60 suppliers worldwide that Xerox uses for paper are in compliance with the requirements. Cultural and trade barriers around the world may make this approach challenging; however, the company is trying to do what it can to minimize the risks and conduct its business appropriately, concluded Azar.
EMBRACING SUSTAINABLE DEVELOPMENT: GREEN CHEMISTRY
The pharmaceutical industry is devoted to discovering and developing new medicines that enable patients to live longer, healthier, and more productive lives. Sustainability and environmental health are important to the industry for its environmental, economic, and social performance, said Berkeley Cue of the Green Chemistry Institute’s Governing Board. Sustainability indexes measure the performance of chemical industry cohorts that manage large sums of investment capital on the order of hundreds of billions of dollars a year.
Green Chemistry
Green chemistry was created by Paul Anastas from the EPA and John Warner from the University of Massachusetts as a set of principles that reduces or eliminates the generation of hazardous substances in the design, manufacture, and application of chemical products (Anastas and Warner, 1998). By looking at the companies who are performing well and identifying what attributes they exhibit in the design of their chemical products or their chemical manufacturing processes, Anastas and Warner described 12 principles that encompass the framework of green chemistry:
-
Prevention
-
Atom economy
-
Less hazardous chemical synthesis
-
Design safer chemicals
-
Safety solvents and auxiliaries
-
Design for energy efficiency
-
Use renewable feed stocks
-
Reduce derivatives
-
Catalysis
-
Design for degradation
-
Real-time analysis for pollution prevention
-
Inherently safer chemistry for accident prevention
Design for degradation is very challenging for the pharmaceutical industry, noted Cue. The industry would like to have the molecules that they produce go out into the environment completely degraded to innocuous entities, and must have them stable in the dosage form and in the patient’s body until they get to the site of biological activity without degrading.
GlaxoSmithKline found that about 80 percent of the waste that is generated in a production process is solvent, and 20 percent is solid waste. Sometimes the solvent can be recycled and recovered, but it is a very inefficient process with only 40 to 50 percent yield recoveries, and it is very energy intensive. Sometimes solvents can be burned as a coadditive to fuel oil to generate energy; however, burning creates greenhouse gases. Further, the pharmaceutical industry pays twice for the solvent: when they buy it and when they dispose of it, observed Cue. In many cases, the disposal costs from the hazardous solvents are substantially greater than the purchase costs of the solvent. Thus adopting green chemistry at all phases of research and development could have a tremendous impact on the economic bottom line and the environmental bottom line for the industry, said Cue.
The pharmaceutical industry is exploring the possibilities to make existing commercial manufacturing processes more environment-friendly. According to Cue, the real battle yet to be fought is going to be in the laboratories, especially for the chemists who design the molecules; the chemists will be the ones to set the strategy for how the molecules will be synthesized throughout the entire life of the product.
In 1996, the EPA Presidential Green Chemistry Board created an initiative to promulgate the practices of green chemistry by giving industries an award in five categories: (1) alternate synthetic pathways; (2) alternate reaction conditions; (3) design of safer chemicals; (4) small business focus, which can be any of the focus areas above; and (5) an academic investigator award. Since 1996, four pharmaceutical companies won the award: Lilly Research Laboratories in 1999, Roche Colorado Corporation for alternative synthetic pathways in 2000, Pfizer Inc. in 2002, and Bristol-Myers Squibb Company in 2004. Lilly’s award was given to the company for chemistry work they did developing a candidate for a non-competitive alpha-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid (AMPA) antagonist for treating epilepsy and neurodegenerative diseases. The new process consisted of seven manufacturing steps with only three isolations. It eliminated 34,000 liters of solvent, 300 kilos of chromium waste per 100 kilos of the active pharmaceutical ingredient, more than tripled the overall yield, and used an innovative bioreduction. Interestingly, this drug candidate did not make it to the marketplace. Cue noted that less than 5 percent of the drugs that start out in development make it all the way to the market. Therefore, there is a disincentive to work on the commercial manufacturing process too soon. However, companies need to establish their manufacturing process early on to meet their business and regulatory requirement, and green chemistry allows them to do this.
According to the Wall Street Journal and the Consortium for the Advancement of Manufacturing in Pharmaceuticals (CAMP) the pharmaceutical industry members spend $90 billion to manufacture drugs (Scherzer, 2003 and CAMP, 2002). Cue noted that Roche Colorado targets and achieves a 25 percent reduction in the manufacturing parts of their processes using green chemistry. Not all companies meet the same target as Roche, but Cue noted that if other companies achieve a 10 percent reduction of the process, it would save $9 billion a year that would be available to the pharmaceutical industry for reinvestment in research and development to bring new products along. Thus according to Cue, green chemistry makes good business sense.
Cue noted the multiple opportunities for the pharmaceutical industry:
-
The industry needs to adopt a “benign first time” mind-set, with no exemptions for lab chemists.
-
The industry needs to move from fossil fuel–based raw materials and start looking at biomass-based raw materials (organic material such as crops, crop wastes, trees, wood waste, and animal waste).
-
The industry needs to measure and publish the pertinent environmental factors as a way of establishing metrics and setting improvement goals.
-
The industry needs to apply a more extensive use of benign solvents such as water, supercritical CO2, ionic liquids, and solvent-free chemistry.
-
The industry needs to collaborate more closely with academia, government, and NGOs through more industrial seminars, communication, participation in workshops, and by recruiting and hiring chemists who are trained in green chemistry.
-
The industry needs to integrate chemical synthesis and chemical engineering concepts.
Cue pointed out that many companies in the pharmaceutical industry are using green chemistry principles at commercial scale, but possibly hundreds of millions of kilograms of waste could still be prevented by broadly adopting green chemistry. Some examples are making their way into the research and development process, especially in development. Discovery labs are the next great opportunity for us. Emerging technologies are largely untapped in this industry, which could revolutionize how active pharmaceutical ingredients are manufactured at every scale. He noted that Einstein once said, “Significant problems we face today cannot be solved at the same level of thinking we were at when we created them.” This is true for industry as sustainability continues to evolve.
ANALYZING RISK PRIOR TO PRODUCTION
Being a $450 billion-a-year enterprise, the chemical industry in the United States is a key element of the country’s economy and nation’s largest exporter,
accounting for 10 cents out of every dollar in the U.S. exports, said Gregory Bond of Dow Chemical Company. The chemical industry is critical to a wide variety of markets essential to human needs, such as food, transportation, electronics, health and medicine, personal and home care, and building and construction. In addition, chemistry companies invest more in research and development than any other business sector.
The industry has had a long history of conducting testing, exposure, and risk assessment in practicing product stewardship. It is aware of and has recognized for a long time that some of their products are inherently hazardous; therefore, is continuing its commitment to evaluate risk responsibly. The industry is participating in developing sound public policy and trying to improve its communications; however, there is room for improvement, said Bond. Sixteen years ago, the industry recognized the importance of improving environmental health and safety performance and their dialogue with the public, and launched Responsible Care® which evolved into an environmental health and safety management systems approach.
Milestones of Environmental Health and Safety Management in the Chemical Industry
The industry has recognized that its product intermediates and wastes are inherently hazardous and must be responsibly managed. In 1934, Dow Chemical Company’s leadership recognized the importance of safety evaluation of products’ intermediates and wastes. Dow’s contribution to the field of toxicology has come a long way since that time when the first toxicology studies were conducted in modified 55-gallon drums, said Bond.
Being a $450 billion a year enterprise, the chemical business in the United States is a key element of the country’s economy and nation’s largest exporter, accounting for 10 cents out of every dollar in the U.S. exports.
—Greg Bond
In 2004, the company celebrated the 70th anniversary of its toxicology and environmental fate laboratories. In the late 1970s, the leading companies in the chemical industry founded the Chemical Industry Institute of Technology (CIIT) to conduct fundamental toxicology research to improve testing methods and their basic understanding of mechanisms of toxicity. In 1999, this effort evolved into a global initiative called the Long-Range Research Initiative (LRI), which funds CIIT and research at university to conduct independent research into the interaction between chemicals, human health, and the environment.
Other significant environmental health and safety milestones in the chemical industry include formalization of industrial hygiene in 1930s, epidemiology studies that began in the 1960s, emissions reduction starting in 1980s, the Product
Stewardship Code adopted in early 1990s, and finally, industry metrics adopted in 2002.
From the very beginning there has been an emphasis by the companies on publishing the results of their studies in peer-reviewed literature. However, in spite of the efforts, environmental health and safety programs in companies have been far from perfect, said Bond. They were not of uniform quality across the industry, and there have been notable incidents that have occurred in the industry that have led to some important and useful regulations.
Federal Regulations That Help Ensure Chemical Safety
In the United States, there is a strict comprehensive set of rules that is found in more than a dozen federal laws, said Bond. Similar laws exist in other developed countries and are being rapidly promulgated in the developing countries around the world. The Toxic Substances and Control Act (TSCA) is the primary piece of legislation that governs industrial chemistry. It gives the EPA broad authority to screen and regulate new and existing chemicals. The EPA has the authority to prohibit manufacture and distribution of a substance if it is found to pose an unreasonable risk, noted Bond. A company must notify the EPA prior to bringing any new chemical to market and must provide the EPA with the new chemical’s identity, properties, available hazard data, anticipated production volume, by-products, use, environmental release, disposal practices, and human exposure limits. EPA scientists then determine if the chemical poses an unacceptable risk.
According to the EPA’s data, the agency screened more than 30,000 new chemicals between 1979 and 2001 (EPA, 2004a). More than 1,200 of those were subject to legal restrictions imposed by EPA (EPA, 2004a). The EPA has prohibited certain uses of more than 900 additional substances, using tools such as significant new use rules, consent orders, and other TSCA authorities. In more than 300 cases, companies have voluntarily agreed to conduct additional testing in response to the EPA’s informal requests, and for more than 1,500 chemicals, companies have voluntarily withdrawn their request to manufacture in the face of EPA concerns (EPA, 2004a).
TSCA mandates that every four years companies need to report to the EPA their updated product volume and other detailed information on existing chemicals. Newly enacted rules require companies to submit to the EPA a significantly larger amount of use and exposure information on these chemicals. The EPA has the authority to request even more detailed information and has exercised this request for more than 1,500 chemicals.
The TSCA requires manufacturers and importers to submit within 30 days any information that reasonably supports the conclusion of risk to human health and the environment. Failure to comply is subject to criminal or civil penalties of $25,000 to $50,000 per day and imprisonment.
The Federal Insecticide, Fungicide, and Rodenticide Act (FIFRA) requires more than 120 scientific tests on any new product, and only after the information has been rigorously reviewed by the EPA can the product be approved for use to protect crops or public health. The process can take as long as 8 to 10 years and cost in excess of $30 million per product, noted Bond. Only about one in 20,000 chemicals that are screened and developed for potential use actually makes it from discovery to commercial use as a pesticide. Biocidal products are also regulated under FIFRA and must undergo a rigorous evaluation before they are approved for use.
The Federal Food, Drug, and Cosmetic Act establishes safety parameters for chemical content of various products and premarket approval of new chemicals, food additives, materials intended for contact with food, and coloring agents. The federal government has also developed stringent regulations for how chemical tests are conducted to ensure that the methods are consistent, reliable, and credible no matter who conducts the testing.
EPA data indicate that there are approximately 9,000 to 15,000 chemicals in U.S. commerce, about 2,800 of which are produced or imported in quantities of a million pounds per year and which account for more than 90 percent of the volume of chemicals in commerce (EPA, 2004a). Although the regulations described above are an important backstop to assure the public of the safety of chemical products, many companies in the industry go well above and beyond what is required by law to assure the safety of their products, said Bond.
Chemical Industry’s Product Stewardship and Trends of Public Expectations
Dow and many leading companies have publicly committed to operate according to local standards or company standards, whichever are stricter, around the globe (Figure 2-2). It prevents costly employee illness and injuries, increases customer loyalty, and helps to avoid costly litigation, noted Bond.
According to Bond, in the past decade, the chemical industry has witnessed a successful shift in attention away from its operations toward its products. The focus on the toxic release inventory (TRI) and emissions reduction has lowered many point source pollution sites and has shifted focus to nonpoint sources. However, the industry still faces challenges.
The lack of transparency in the past has caused people to assume the worst about the chemical industry.
—Greg Bond
First, the lack of transparency in the past has caused people to assume the worst about the chemical industry. Demands for more product testing continue, and legislation now being promulgated in the European Union will make this a reality, said Bond.
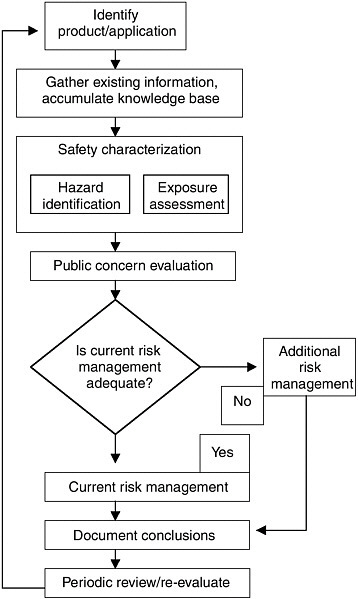
FIGURE 2-2 Among many leading chemical companies, Dow is committed to operate according to the environmental standards. The figure illustrates the general approach to product safety taken at Dow. SOURCE: The Dow Chemical Company, 2004.
In addition, the industry has to add new end points such as endocrine screening and testing to its repertoire. At the same time, there is a highly visible and vocal animal rights community demanding that the industry reduce the use of or ban all animal testing, making for a difficult balancing of competing demands.
Second, there are concerns that the existing safety margins already built into the safety assessment and risk management processes are not adequate to protect children, elderly people, or other vulnerable populations who may have special susceptibility. The public continues to raise questions about chemicals in the environment that are responsible for many human diseases, particularly cancer, asthma, and developmental disabilities such as attention deficit disorder and autism. The causes of such diseases are typically not known, and epidemiologists doubt whether existing tools can answer one way or the other if chemicals are the cause.
Finally, the industry’s risk assessment paradigm is also under discussion, with some opponents arguing for a purely hazard-based approach. Some question if industry-sponsored scientists are inherently conflicted and not trustworthy, while others want to exclude industry-sponsored scientists from scientific panels and federal advisory panels. All the stakeholders need to be less divisive, urged Bond.
Industry’s Response to Challenges
The industry continues to base its efforts on improving its performance and research. Transparency, developing and promoting credible science-based policy, and more outreach to stakeholders continues to be important, too, noted Bond.
In 1998 the chemical industry, working with the EPA, Environmental Defense, and others, launched the High Production Volume (HPV) chemical challenge program, which was an unprecedented voluntary initiative through which industry will make uniform hazard screening information on about 2,200 HPV chemicals available to the EPA and the public by 2005. The latest report, current as of January 31, 2005, is available on the EPA’s website. (EPA, 2004b).
The industry has also has been participating in a voluntary EPA program called the Voluntary Children’s Chemical Evaluation Program (VCCEP) (EPA, 2004c), and is sponsoring 20 chemicals in this assessment. In 2001, the American Chemistry Council (ACC) and the International Council of Chemical Associations both adopted a global chemicals management policy. The intent is to take industry product stewardship to the next level of performance, noted Bond. The policy lays out basic obligations of members to conduct product risk characterizations, to engage partners in the value chain, to understand uses and exposure, and to inform about risk characterizations. Many companies, including Dow, are building these obligations into their websites. Eventually, one will be able to go to www.dow.com and learn about their process for conducting product safety evaluations and get a summary and a layered look at the results of those product safety evaluations.
To summarize, Bond noted that the challenges that we are likely to face in the future continue to grow, even as we witness improved environmental health and safety protection. To meet these challenges requires all stakeholders to work
together in new ways. He called for the need for more collaboration and cooperation and less confrontation. He challenged that we need to get beyond the labels and the rhetoric to find practical solutions that are based on balancing sustainable economic, social, and environmental development. Just as the public is demanding more transparency and accountability from industry, the industry also needs to demand more transparency and accountability from all stakeholders in the process. The industry needs to set its priorities carefully and ground its decisions in risk assessment and science, concluded Bond.
VOLUNTARY PROGRAMS: CHALLENGES AND NEEDS
Industry plays an essential but limited role in the generation of hazard data, and there are very important roles that government needs to play in conjunction, said John Balbus of Environmental Defense. Industry volunteerism does not preclude the need for government oversight, monitoring, tracking, validation, and dissemination of results, all of which require significant government resources. Moreover, voluntary programs only work well when there is a good regulatory backstop, noted Balbus. The chemical industry has volunteered to develop hazard and exposure data in a number of programs. Such programs include the High Production Volume (HPV) Challenge, the Organization for Economic Cooperation and Development Screening Information Data Set (OECD-SIDS) program, and the Voluntary Children’s Chemical Evaluation Program (VCCEP).
Overview of High Production Volume (HPV) Program
The realities of chemical safety and regulation are daunting, said Balbus. The first reality is that under the Toxic Substances Control Act, EPA must be able to demonstrate that an existing chemical poses a potential risk in order to require that its producer perform any toxicity testing—a true Catch 22, since making the risk finding is exceedingly difficult in the absence of toxicity data. As a result, over the 30-year history of TSCA, EPA has required testing for fewer than 200 of the tens of thousands of chemicals in commerce. It has turned to voluntary programs to try to bridge the gap.
According to analyses by the EPA and by the American Chemistry Council in the late 1990s, there was a striking lack of publicly available basic toxicity data for the approximately 3,000 chemicals (excluding polymers and inorganic chemicals) that were produced or imported in the highest quantities (over 1 million pounds per year) in the United States. The EPA documented that 43 percent of these high production volume (HPV) chemicals had no publicly available basic toxicity data, and that 93 percent of them were missing data for one or more of these basic tests (EPA, HPV). This situation led EPA to challenge the industry
to fill the gaps which led to the launch of the High Production Volume (HPV) Chemical Challenge in 1998. EPA indicated its intent to develop test rules for any HPV chemicals that were not voluntarily sponsored by their producers. This regulatory backstop helped motivate companies to volunteer their chemicals initially, but to date, EPA has issued only one test rule covering 17 of the nearly 300 unsponsored HPV chemicals.
Another challenge is that there are a lot of unassessed chemicals on the market, but due to limited resources, government is progressing very slowly in assessing them, noted Balbus. Therefore, it is essential for the manufacturers of those products to participate in generating the hazard information about them. The HPV program included options to reduce the cost burden on industry in generating basic screening toxicity information on their products. These options included sponsorship through consortia of producers to pool data and resources, and the encouragement of the use of quantitative structure activity relationship (QSAR) models and interpolation between members of categories of chemicals grouped together based on structural similarities, as a means of estimating rather than directly measuring toxicity data.
There are a lot of unassessed chemicals on the market, but due to limited resources, government is progressing very slowly in assessing them.
—John Balbus
Additionally, the HPV program has focused solely on generating hazard data. At that time, it was purposely set up not to include exposure data, in part because there were no adequate guidelines for how extensive that exposure data should be. Moreover, in contrast to the OECD SIDS process, there was no requirement to do any kind of prioritization or hazard assessment based on the information generated. It was strictly a hazard data generation and public dissemination exercise.
Today, about 1,900 of the original 2,800 chemicals have been sponsored, said Balbus; however, there are still nearly 300 chemicals that remain unsponsored, and some 700 have been shifted out of the US HPV program and into the OECD SIDS program, which is slower-paced (Environmental Defense, 2004). Nonetheless, the program is generating an unprecedented amount of publicly available toxicity information. A key lesson learned from the HPV Challenge is that while industry can deliver data relatively quickly, the quality of test plans and toxicity data is uneven and requires careful review by government. Public access to such data should also be provided promptly, to ensure accountability of both industry and government in such a voluntary initiative. Unfortunately, declining resources at EPA have meant long delays in making data publicly accessible. Ironically, one reason for the lack of resources for a voluntary program like the HPV is that it has to compete with regulatory programs.
Organization for Economic Cooperation and Development (OECD) Screening Information Data Set (SIDS) Program
According to the EPA, the Screening Information Data Set (SIDS) program, operated under the auspices of the Organization for Economic Cooperation and Development (OECD), is a voluntary cooperative international testing program that began in 1989. Like the HPV Challenge, the SIDS program is focused on developing screening-level toxicity information on HPV chemicals. The SIDS data are used to screen the chemicals and set priorities for further testing or risk assessment/management activities (EPA, HPV). However, unlike the HPV Challenge, the OECD SIDS program allows use of exposure information in formulating its recommendations as to whether a chemical is a priority for further work or not.
Unfortunately, there have been some serious deficiencies in many of the exposure analyses that have been done in this program, according to Balbus. While the OECD prescribes that exposure analyses must include all manufacturing facilities, at least within the sponsoring country, sweeping generalizations about low exposure potential are made based on incomplete data from just one or two facilities. Often the exposure analyses are based on unpublished data generated by the company, and in some cases, even the sponsoring government has not reviewed the data. Because the prioritization of the chemical is based on both exposure and hazard data, there have been several situations where very questionable exposure analyses have been allowed to trump clear evidence of hazard, Balbus said.
Voluntary Children’s Chemical Evaluation Program (VCCEP)
VCCEP was designed to provide data to enable the public to understand the potential health risks to children from chemical exposures. For the pilot phase of the VCCEP, the EPA asked companies that manufactured or imported 23 chemicals, including acetone, benzene, ethylene dibromide, and others that have been found in children’s bodies or their environments, to volunteer to sponsor a Tier 1 evaluation. The hazard evaluation part of VCCEP consists of three tiers shown in Table 2-1. One complication of the VCCEP pilot program has been that all of the chemicals evaluated thus far have had data from all three hazard tiers available and considered in the program. While the tiers for hazard data are clearly defined, the different tiers for exposure data are less clear; the result is some confusion as to what truly constitutes a Tier 1 evaluation under the VCCEP. In fact, it is unclear whether an evaluation of children’s risk should even go forward based on limited first tier hazard data.
Under the VCCEP, the sponsoring company produces a document that reviews all of the exposure and all of the hazard data, models children’s potential exposures, combines them in a risk assessment, and then makes a judgment as to whether or not there is sufficient information available to adequately judge risks
TABLE 2-1 Three Tiers of Voluntary Children’s Chemical Evaluation Program
|
Tier 1 |
Tier 2 |
Tier 3 |
|
Acute toxicity |
Subchronic toxicity |
Neurotoxicity screening battery |
|
Repeated dose toxicity with reproductive and developmental toxicity screens |
Prenatal developmental toxicity |
Carcinogenicity |
|
Reproductive and fertility effects |
Developmental neurotoxicity |
|
|
Bacterial reverse mutation assay |
Immunotoxicity |
|
|
In vitro or in vivo chromosomal aberrations or in vivo micronucleus test |
In vivo chromosomal aberrations or in vivo micronucleus test |
|
|
Metabolism and pharmacokinetics |
|
|
|
SOURCE: EPA, 2004c. |
||
to children, said Balbus. He noted that this is a situation that creates a potential conflict of interest, since the sponsoring company would have strong disincentives to write in a public document that their product was posing risks to children. Some of the expert peer consultation panelists reviewing the VCCEP studies have questioned several of the assumptions within the risk assessment documents, as well as some of the interpretations of the toxicity studies, observed Balbus. Furthermore, the pace of the VCCEP pilot has been very slow. Of 23 chemicals identified for this pilot back in 1999, the first chemical was evaluated in January of 2003, and the EPA did not finalize their review of that first chemical until late in 2005.
In conclusion, Balbus said that Environmental Defense feels strongly that well-designed voluntary programs can have an important role in speeding the pace of data generation, but they are not able to completely replace regulatory frameworks, and they demand dedication of government resources to ensure proper oversight and quality of the output.


















