3
Global Implication of Environmental Standards1
Central to any country’s environmental program is their management of the tens of thousands of chemicals used daily in commerce. Although a sound chemical management program is the keystone for ensuring both public health and healthy environments, determining which chemicals to monitor and how to implement the program provides a challenge for all countries. This is true whether they are developing or developed. During the workshop, Roundtable members, speakers, and participants discussed the management approaches in Europe, the United States, and Canada and the implications for improving management of chemicals around the world.
BALANCING RISK ASSESSMENT WITH THE REALITIES OF UNCERTAINTY
The challenges of risk and risk assessment in protecting public health through regulation of chemicals requires looking at the changes in Europe, according to Bernard Goldstein, Graduate School of Public Health, University of Pittsburgh. Central to the current debate about environmental control in the European Union is the precautionary principle. The Rio Declaration defined the precautionary principle as: “Nations shall use the precautionary approach to protect the environment where there are threats of serious or irreversible damage. Scientific uncertainty shall not be used to postpone cost-effective measures to prevent environmental degradation” (Rio Declaration, 1992). According to Goldstein, the precautionary principle is one of those positive statements with which, in principle, everyone can agree. It is similar to the idea of sustainable development—something that is
loosely defined. However, he noted that the use of the precautionary principle in a legal framework suggests the need for further scrutiny.
The basics of risk assessment comprise hazard identification, dose-response evaluation, human exposure evaluation, and risk characterization. Currently, there is some debate as to whether the precautionary principle is already incorporated into risk assessment, should be incorporated into risk assessment, or if the precautionary principle and risk assessment are completely antithetical. Individuals such as Joel Tickner and Lee Ketelsen (2001) and Mary O’Brian (O’Brian, 2000) question current approaches to risk assessment and lay out the argument for the use of the precautionary principle. O’Brian noted that risk assessment obscures and removes the fundamental right to say “no” to unnecessary poisoning of one’s body and environment. Goldstein noted that these statements suggest that risk assessment has failed and we need a new approach.
However, Goldstein questioned the use of the precautionary principle used by Europeans. Although the EU has stated its support of the principle, it has carefully avoided defining it. Some individuals, he noted, question whether the precautionary principle is intentionally nebulous so the EU can use it to form trade barriers. For example, the European Union used the precautionary principle to develop the most stringent aflatoxin standard in the world (European Commission, 1997). It excluded $700 million of sub-Saharan produce to the advantage of European growers. The difference in risk is less than one cancer incident per year in Europe (Majone, 2002). In another example, based on the precautionary principle, the European Union banned imports of all beef from hormone-treated cattle, even though the European Union’s own scientific committees did not find risk. The European Union lost this World Trade Organization case primarily due to the lack of risk assessment, as well as an inconsistent application of the precautionary principle seemingly to form a trade barrier. Goldstein further noted that these types of cases are going to continue with genetically modified foods and with other health and safety issues for which the EU uses the precautionary principle to form trade barriers. These cases are generalizations, but illustrate the different approaches to risk taken by the United States and by Europe. One obvious difference is that Europe does a better job of coupling environmental and trade policies. Further, one of the driving forces in the development of the precautionary principle in Europe has been a growing distrust of government and science as a result of mad cow disease, the French hemophilia scandal, and others.
The definition of the precautionary principle used by Europeans is not fully defined for legal purposes. Some individuals question if the precautionary principle is something nebulous to erect trade barriers.
—Bernard Goldstein
In a paper with Russellyn Carruth, Goldstein looked at whether the precautionary principle and whether it achieves its stated goals (Goldstein and Carruth, 2003). They asserted that the hazardous air pollutant components listed in the 1990 Clean Air Act is a form of the precautionary principle in practice (Table 3-1). Prior to 1990, the Environmental Protection Agency (EPA) had to determine which chemicals were “bad actors.” Only after the EPA listed a chemical and went through a regulatory action could they begin to regulate it. In 1990, the Congress shifted the burden of proof by listing 185 chemicals to regulate and allowing the lengthy EPA rulemaking process only as a way to remove one of those chemicals from the list. The 1990 hazardous air pollutant amendments also specified that the best emissions standards need to be achieved regardless of the location of the source. This meant that a plant in the Mohave Desert had to achieve the same emission standards as those in the middle of Washington, DC. This regulation shifted the approach from a risk-based approach to a precautionary approach. Unfortunately, the result of the use of the precautionary approach is that EPA’s expenditures for research and development in the area of hazardous air pollutants has gone down. This may be due to the notion that it isn’t of regulatory interest to obtain the information.
Invoking the precautionary principle requires some degree of scientific uncertainty about the worst case. If there was scientific certainty, there would be no need to invoke the precautionary principle. Further, the precautionary action needs to have significant economic or social costs—if the costs were trivial the action would be taken without the need to invoke the precautionary principle. In essence, the precautionary principle is used for situations in which resources are to be invested despite there being no surety that adverse consequences will occur. Thus, the more precautionary a country is, the more often that it is going to spend money, resources, and social capital for the wrong reason. Goldstein argued that one needs to build in an evaluation to determine if the precautionary approach is warranted.
TABLE 3-1 Control of Hazardous Air Pollutants in the United States
|
|
Before 1990 |
After 1990 |
|
Burden of proof |
To list chemical, EPA must demonstrate that ambient levels of pollutant produce risk |
To remove chemical from list, industry must demonstrate that chemical does not produce risk |
|
Regulatory control for listed pollutant |
Risk-based application of control technology |
Maximum available control technology |
|
Role of risk assessment |
Primary |
Secondary |
|
SOURCE: Goldstein and Carruth, 2003. |
||
Goldstein concluded that the precautionary principle, if done well, is primary prevention, and risk assessment is secondary prevention. (Secondary prevention would state the risk of breathing a chemical in a room, while primary prevention would never have the chemical in the room for people to breathe). However, he noted that one should not use the precautionary principle as a place to hide behind, a way to avoid understanding science, or a shortcut to avoid a trade-off decision.
GLOBAL CORPORATE POLICIES ON HEALTH, SAFETY, AND THE ENVIRONMENT
Voluntary corporate policies can provide improved protection of human health and the environment, particularly in poor countries, noted Barry Castleman, Environmental Consultant. The vacuum of regulation and liability in many countries has allowed global corporations to operate without applying safeguards required of them in Europe and the United States.
The tragedy in Bhopal, India, in 1984 brought the issue of corporate “double standards” to the world’s attention. Numerous safeguards in effect in the United States such as plant design, safety systems, and maintenance had been neglected at the company’s plant in India, noted Castleman. A corporate audit by Union Carbide Corporation 2.5 years prior to the Bhopal disaster had identified many of these problems (Ives, 1985).
The more precautionary a country is, the more often that it is going to spend money, resources, and social capital for the wrong reason. The precautionary principle, if done well, is primary prevention, and risk assessment is secondary prevention.
—Bernard Goldstein
After the tragedy in Bhopal, multinational corporations began to issue global corporate policy statements based on the premise that there was no justification for operating a chemical process under less strict conditions of pollution control and worker protection in one country than another. In order to be to be successful, these company standards have to be applied to all aspects of production and marketing, stated Castleman. Some corporations assert responsibility for not only their subsidiaries but also their suppliers by auditing the occupational and environmental conditions of these suppliers and requiring conformity with corporate standards. On the other hand, companies that transfer environmentally dangerous production to other ones, where they appear as the customer but not the manufacturer, can make no claim to corporate social responsibility.
Further, the same care needs to be applied in marketing of the products. “Double standards” issues arise in labeling, worker training, and product stewardship. For example, pesticides withdrawn from use in the United States should be removed from use worldwide, asserted Castleman. Another example is hazardous
waste disposal in countries that do not have the proper regulation or facilities set up by the government. In such countries, a responsible company should practice same policies of hazardous waste disposal as required in the United States.
In addition, there needs to be public disclosure of toxic releases worldwide, stressed Castleman. Corporations in the United States often have policies to not sell chemicals to companies that do not use them in a reasonably sound manner. This practice needs to be corporate policy in other areas of the world, regardless of liability considerations, asserted Castleman.
How to Prevent Double Standards Around the World?
Castleman proposed a comparative survey of global policies and practices of leading firms. The practices of the leasing corporations can be used as guidance to ensure that industries and products taking root in industrializing countries are not hazardous and polluting cast-offs from other countries. Leading corporations can demonstrate their sincerity about “no double standards” by participating in a comparative survey by questionnaire and interview. The survey could provide a composite-best model, with enormous benefits to health and the environment worldwide.
Another approach for sharing knowledge and experience would be to hold a conference at which corporate health, safety, and environment officials present their proudest achievements in transferring “green” technology and assuring conformity with high standards of performance worldwide, he said.
Corporate social responsibility needs to be more than vague platitudes, flexible work hours, and well-publicized charitable gifts. We need for corporations to apply their highest standards to protect human health and the environment worldwide, concluded Castleman. By understanding how leading firms try to avoid double standards, we can best encourage other companies to improve their performance and provide ideas to people in developing countries dealing with foreign investors.
THE REACH INITIATIVE
The European Union has the same issues as the United States but in a much more crowded situation, noted Robert Donkers of the delegation of the European Commission to the United States. The European Union has more than 450 million people in an area half the size of the United States. REACH (Registration, Evaluation, and Authorization of Chemicals) is a response to the opinion in the EU that the burden of proof of what chemicals are not safe is no longer on the authorities. Rather, it is on industry to prove that its chemicals can be used safely. Currently, the burden lies with the government, which needs to spend enormous resources to ensure that the chemicals can be used safely, noted Donkers. The European Union
is looking at REACH as an opportunity to ensure that industry is doing what they promised for years—responsible care and product stewardship.
Donkers noted that there is vast lack of information on the properties of chemicals. The current system is not working as it is impossible for authorities to get all the information on properties, uses and risks, which is needed to support risk management measures. Presently, there are more than 70,000 chemicals out there about which there is scant information. It takes time to collect information required for current regulations, and then it takes time to react to the information. At the same time, the European Union does not want to hamper companies from doing business, noted Donkers. The dilemma is that people need to be able to use the chemicals while the harmful impacts of such use are minimized. However, when we don’t know all the impacts we can’t make all the linkages necessary.
The European Union is looking at REACH as an opportunity to ensure that industry is doing what they promised—responsible care in public use.
—Robert Donkers
To begin to address the issue, the European Union is attempting to centralize the system so that the EU can avoid 25 different legal systems for a chemical, which would result from a country-by-country approach, if there will not be soon a functioning and harmonized instrument for all 25 EU member states. Donkers asserted that the European Union sees a need for REACH because the current system for chemical management is inefficient:
-
It is difficult to identify risks.
-
There is a lack of information about most substances on the market.
-
The burden of proof lies with public authorities.
-
There is no efficient instrumentation to address problematic substances.
-
There is a lack of incentives for innovation.
The solution, the European Union believes, is a new chemical policy. This policy, according to Donkers, has substitution and precaution as the underpin of the system. It is based on principles of sustainable development and protection of human health and the environment, also taking into account the economic and social importance of the EU chemical sector and downstream user industry. It is to maintain and enhance competitiveness, but also increases transparency and will be in conformity with the EU obligations under the World Trade Organization’s rules. In addition, it provides opportunities to integrate with international efforts. The REACH initiative, according to Donkers, will be based on information and science to be provided by industry and checked by authorities to determine if we need to take management action. The precautionary principle will be
invoked when industry will not play its role and does not deliver the information necessary; and, on the basis of information available, it would be irresponsible to wait to take action. Measures enacted on the basis of the Precautionary Principle are not permanent, are on a case-to-case basis, and are very regularly reviewed as more scientific information comes available.
As in the United States, current legislation created an artificial divide between old and new chemicals (those chemicals introduced into the market place after September 1981). REACH will do away with this divide. Registration will be required for all new or existing substances above 1 metric tonne (1,000 kg) per year per manufacturer/importer, which will be around 30,000 substances. The registration process will start with the High Production Volume (HPV) chemicals. The higher the volume produced and marketed, the more information will be required (Figure 3-1). The phase in period of existing chemicals is an 11-year plan and registration of the chemicals below the 1,000-tonne limit will be required at the end of the period around 2018. An important element of REACH will be data sharing, observed Donkers—there is a need to avoid duplication of tests where animals are involved. Not all chemicals will go through the evaluation process, which would only be for chemicals where industry proposes animal testing to complete the information or when a specific chemical is singled out for in-depth examination. For the large majority of chemicals, the information on the registration is all that will be required.
A specific group of about 1,500 chemicals will be subject to the authorization process. To get an authorization, the applicant has to show that for each use the risks can be adequately controlled. The chemicals in case are either carcinogenic, mutagenic, reproductive toxicants or persistent, bioaccumulative and toxic. If users could not demonstrate that they can manage 100 percent of the risk, a temporary authorization can be given if there is a clear higher societal benefit, which is greater than the cost, asserted Donkers. The selection of the chemicals for authorization is hazard based, but the decision (authorization) will be risk based.
Downstream use is a part of the registration process. The manufacturer or importer needs to cover all uses identified by downstream users.
—Robert Donkers
Identification of downstream use is a part of the process. The manufacturer/ importer needs to cover all uses identified by downstream users, noted Donkers. The downstream users and their providers must implement the supplier’s risk reduction measures for identified uses. If they choose not to share their use information with suppliers, downstream users have to perform chemical safety assessments for “unidentified uses,” and inform the agency accordingly. Finally, Donkers noted that downstream users need to enter into dialogue with their supplies and consider taking part in consortia of providers and cost-sharing practices.
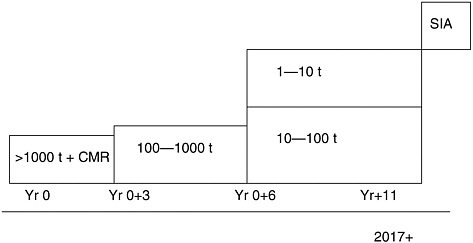
FIGURE 3-1 Schematic of the European Union’s registration of substances. Not all chemicals will require registration. SOURCE: European Commission, 2003.
The cost of the program is currently estimated to be close to 6 billion, according to Donkers. The benefit of the program is still being studied, but initial figures from the World Health Organization estimate the avoided costs in terms of fatal cancer cases among the workers population alone are around 50 billion over a 30-year period. The environmental and health benefits will be evident, but will be hard to monetize as there isn’t a good science base. Donkers concluded that the goal is to have an agreed legislation between the European Parliament and the Council of Ministers by 2006. Implementation will be monitored to ensure that it is consistent across the industrial sectors and provides transparency to make informed decisions about the use of chemicals by all stakeholders.
WORKING WITH REACH: PRACTICAL OBSERVATIONS
James Bus of Dow Chemical Company suggested that industry should not be viewed as pushing back on the REACH initiative in the context that it should not move forward. Rather, he noted that the initiative is a complex new piece of regulation that affects the marketing of chemicals in the European environment. It is reasonable to have a robust dialogue between the government agencies, affected parties, industry groups, and other stakeholders to ensure that, ultimately, there is a legislative outcome that achieves the purpose that was originally intended.
Bus suggested that the need for reform is real, both in Europe and the United States—where there is a distinction between new and existing chemicals. Dow does have an extensive database that has information on where their chemicals
are being utilized. However, it is not a perfect system. There is an opportunity for improvement. Any system that gives a greater degree of confidence of the full breadth of uses of a chemical can help to elucidate the potential risks, acknowledged Bus.
The decentralized process of looking at chemical evaluations across the scattered governments has resulted in some inefficiency. Bus concurred that certainly high on everyone’s agenda is an environment that offers a credible, scientifically based policy, where we have a good understanding of whether we are adequately protecting human health and the environment with the current use of chemicals in commerce. Further, he noted that a clear objective of any new system should be a system that creates greater transparency to customers, workers, and the stakeholder community.
It is reasonable to have a robust dialogue between the government agencies, affected parties, industry groups, and other stakeholders to ensure that, ultimately, there is a legislative outcome that achieves the purpose that was originally intended.
—James Bus
We live and interact in a global world. Thus any environment that is created in the European Union needs to achieve appropriate international integration and harmonization and, at the same time, also needs to be consistent with the expectation of the World Trade Organization. Bus questioned whether the government can identify mechanisms to set priorities for those chemical selections. For the 30,000 chemicals, how do we drive a process that focuses as rapidly as possible on those chemicals that truly represent the highest risk to either health or the environment? Those chemicals that clearly have lower risks because of their properties, exposures, hazards, and so on, can be set aside for consideration at a later time during the evaluation process, noted Bus. Risk also should not focus just on production volume or drive the testing requirement for decision making, asserted Bus. The chemical industry recognizes that the risk equation does enter the REACH process; however, fundamentally the testing requirements are driven by production volume.
Certainly high on everyone’s agenda is an environment that offers a credible, scientifically based policy, where we have a good understanding of whether we are adequately protecting human health and the environment with the current use of chemicals in commerce.
—James Bus
Bus suggested that the consideration of hazard alone should be more effectively and openly targeted with exposure and use information. This needs to occur not only in the registration and the licensing phases, but also during the authori-
zation phase. When it comes to maximizing use of all existing data, models are needed to help set priorities for the 30,000 chemicals through data sharing with the industry and with regulators.
Coupling information with a better understanding of exposure can allow for better decisions to be made. Bus suggested that one could use a tier-based risk screening process to set priorities for chemicals that undergo registration and authorization. In the tier-based process, one could fundamentally make conservative assumptions based on an exposure, hazard properties, or use scenarios of a chemical. From these assumptions, a number of questions about the level of risk could be asked. If the chemical doesn’t pass the initial screen, the additional testing would be warranted, noted Bus. This tiered evaluation allows for reframing the assumptions and considering risk management decisions. The European Centre for Ecotoxicology and Toxicology of Chemicals is an organization in Europe, which is partly funded by the chemical industry, that has been exploring how this type of tiered-approached system might work. It is a stepwise process that will help everyone understand how we might approach risk characterization when considerations of exposure and use are factored in.
Coupling information with a better understanding of exposure can allow for better decisions to be made.
—James Bus
In practice, the tier-based process will assume a set of conservative assumptions with respect to exposure, hazard properties, and use scenarios. From these assumptions, questions are formulated to ask if the chemical represents a high level of risk even under those conservative levels of assumptions, noted Bus. If the chemical doesn’t pass the initial screen, the value of a screening approach is that it allows further evaluation in the tiered process. This means that the assumptions could be refined with additional data, such as hazard date or other activities that could be undertaken. This additional screening might not require further testing, because one could decide that one could comply with those conservative risk evaluations simply by taking risk management decisions, concluded Bus.
Another issue of importance is substances that might be applicable for exemptions. For example, polymers are substances with large molecular weights that could be potentially excluded from meeting the health and environment requirements. The understanding is that, as the molecular weight increases, the biological activity of the polymers decreases, noted Bus. There was a debate earlier on in the process about what should be the molecular weight of the polymer in order to be considered for exemption. The current REACH proposal has decided to exempt most polymers. This, according to Bus, is valuable to help set the priorities again on the chemicals of most interest. Bus further suggested that research and development substances should be exempted because they are
contained in the environments in which they are used. Finally, he suggested that substances found in waste streams and other types of materials present a very complex scenario. These materials may be adequately covered by other regulatory initiatives that are in place in the European framework.
Isolated intermediates are another area that needs priorities set. The chemical industry uses a number of intermediate chemicals to make their final products. These intermediates are separated out of the process and are either stored or transported. The REACH proposal does have a series of considerations of when these intermediates should be tested. The question that the chemical industry has raised is not necessarily related to the process, but rather what are the implications for international trade, reamarked Bus. Potentially, it can create a different environment for those intermediates that come into a country via the importer.
Creating a smooth, efficient process is a fundamental concern of industry, noted Bus. The REACH process today has many lengthy, complex processes. In particular, observed Bus, the registration process is proposed to be done by a central agency, while the evaluation process is to be done by the member states. That division of activities can result in a cumbersome process, and there will need to be consistency across the member states on their decisions. Having a centralized process is one consideration that the industry would like to see as REACH moves forward, asserted Bus.
In the European Union, approximately 75 percent of chemicals are not produced in high volumes, observed Bus. These low-volume chemicals are produced by a limited number of companies; thus forming consortia to share experiences in terms of challenges and opportunities is not necessarily feasible. This will result in a particular burden of cost on the small to medium-sized enterprises that constitute approximately 90 percent of the European Union industrial sector. These companies will have to bear the research costs alone. In addition to the cost factor, there are some implications of sharing proprietary data, and there needs to be additional dialogue around these issues.
In the European Union, approximately 75 percent of chemicals are not high volume productions, noted Bus. The low volume chemicals are produced by limited number of companies; thus forming consortia to share experiences in terms of challenges and opportunities is not necessarily feasible.
—James Bus
In conclusion, Bus suggested that, as the REACH program moves forward, there is a need to have a productive dialogue between industry and the European Union authorities. This continued dialogue can help to achieve REACH’s objectives by putting in place a chemical management program that achieves improvements and refinements in understanding human health and risk.
THE CANADIAN ENVIRONMENTAL PROTECTION ACT: TIERED APPROACH TOWARD REGULATION
The Canadian Environmental Protection Act (CEPA) is the primary federal legislation in Canada focusing on environmental protection and on the protection of human health from environmental risks. Broadly defined, environmental substances of concern under CEPA include both organic and inorganic matter, effectively spanning almost anything in the environment that could be harmful to human health. CEPA is not intended to compete with other statutes, such as the Food and Drug Act, the Hazardous Products Act, and the Pest Control Products Act, but rather comes into force where gaps exist in these or other statutes, noted Daniel Krewski, McLaughlin Center for Population Risk Assessment, University of Ottawa. In this regard, CEPA effectively provides a safety net for protecting both human health and the environment. For example, Krewski suggested that pharmaceuticals in Canada are regulated under their Food and Drug Act; however, should the disposal of pharmaceuticals in the environment pose a potential threat to human health or the environment, then CEPA would apply.
CEPA is not intended to compete with other statutes, such as the Food and Drug Act, the Hazardous Products Act, and the Pest Control Products Act, but rather is an umbrella safety net piece of legislation where gaps exist in the statutes.
—Daniel Krewski
CEPA was introduced in 1988 and is required to be reviewed periodically by law. CEPA is focused on national environmental issues, which are addressed in cooperation with the provinces. Provincial governments are responsible for the provision of health services and have considerable authority in environmental and health protection, but transboundary issues, including air quality, fall within the scope of CEPA. The provinces and the federal government work jointly to implement the intent of CEPA through federal/provincial/territorial committees (e.g., the Committee on Environmental Health, the Committee on Drinking Water). The act contains a number of key features, including jurisdiction and management. There is a shared jurisdiction of implementing CEPA between the federal departments of health and environment with Health Canada overseeing the health assessments, and Environmental Canada handling environmental assessments. However, decisions on control measures are determined jointly by the two ministers of these departments following consultation with a broad range of stakeholders, according to Krewski.
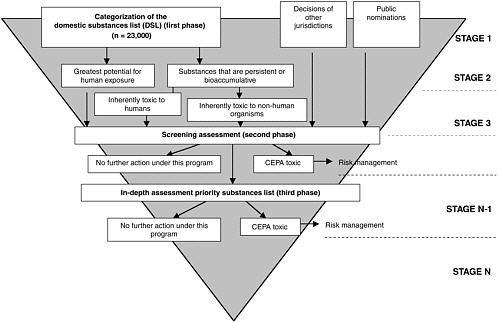
Environmental substances of concern under CEPA include existing substances—those in use in Canada in the period 1984 to 1986, and are listed on the domestic substances list (DSL), noted Krewski. The approximately 23,000 chemicals that fall under this classification require some form of efficient retrospective assessment of risk and subsequent risk management as appropriate
(Figure 3-2). He further noted that newer chemical substances are also of concern. Currently between 800 and 1,000 chemicals are introduced each year. Since 1994, the Canadian government has addressed these newly marketed chemicals through new substances regulations. Currently, no new substances can be introduced into the Canadian marketplace until prescribed information has been provided to the government and assessed within specified periods for potential and health impacts under CEPA.
When CEPA was first promulgated in 1988, first priority substances list (PSL I) containing 44 different substances was put forward, representing environmental agents of particular concern, as identified by an external multidisciplinary advisory committee. A complete, detailed assessment of the chemicals on this list was required to be finished within five years, which included a full review of the scientific literature on toxicity of the agent, leading to an assessment of the health risks associated with the substance. For substances determined to be “toxic,” as defined in the Act (definition provided below), an appropriate risk management strategy must be developed within two years of completion of the risk assessment.
CEPA Toxic
A substance is toxic under CEPA if it is entering or may enter the environment in a quantity or concentration that is hazardous to either human health or the environment. Thus, according to Krewski, exposure is part of the definition of toxicity. If the exposure is sufficiently low that one would not expect an adverse health or environmental impact, then a substance would not be defined as legally toxic under CEPA—even though it may well be a toxic substance in traditional toxicological terms.
Once a substance is defined as toxic, it is placed on Schedule 1—the list of toxic substances. If it is determined to be highly toxic, it is scheduled for virtual elimination by reducing levels in the environment down to essentially zero. To do so, the government utilizes a cost-effective strategy over a period of years to virtually eliminate the substance from the environment.
The risk management strategies that are developed for DSL substances are done using a multistakeholder issues table.
—Daniel Krewski
The risk management strategies that are developed for existing toxic substances are done using a multistakeholder issues table. After a risk assessment is completed and the results have been summarized in a CEPA assessment report, a group of individuals representing the public, nongovernment organizations, government, industry, and other relevant stakeholders is convened to discuss possible strategies for reducing risk. This may include financial incentives or disincentives, regulatory sanctions, communications strategies, community-based approaches,
or any other innovative strategy that may be useful in addressing the specific risk management issue. The recommendations, formulated by the multistakeholder issues table are submitted jointly to the ministers of health and the environment for their consideration. These recommendations may be accepted, declined or accepted with modifications to become the risk management strategy for addressing the substances that are identified as toxic under the statute, noted Krewski.
Challenges of the Canadian Environmental Protection Act
One of the challenges in implementing the Canadian Environmental Protection Act is to coordinate the activities of Health Canada and Environment Canada. There is a need to ensure that numerous and sometimes complex human health risk assessments and environmental assessments of CEPA-toxic are coordinated and integrated in tandem. There was also the challenge of categorizing approximately 23,000 substances on the DSL, a task which has recently been completed according to innovative processes developed by Health Canada and Environment Canada. Finally, there are the operational challenges involved in administering such a broad-based statute, which covers emissions and effluents, new commercial chemicals, and existing substances on the DSL. CEPA also provides the legislative basis for the establishment of national objectives, guidelines and codes of practice, for example, for air and water quality in Canada.
Media-specific approaches are not very conducive to looking at a chemical from cradle to grave, which can consider the entire life cycle of the chemical or a process. Media approaches can push a chemical from one medium to another, but never quite address the life cycle and what the alternatives might be.
—Lynn Goldman
CEPA differs from the U.S. Toxic Substance Control Act (TSCA). Krewski suggested that one of the main differences is that, under CEPA, there is a broader scope for looking at nonregulatory options for risk management. For example, the use of multistakeholder issue tables, which provide for participation of industry, the public, and other stakeholders, can generate a wide range of proposed risk management actions.
U.S. APPROACH TO REGULATION: THE TOXIC SUBSTANCE CONTROL ACT AND PUBLIC HEALTH
It is important to note that, when discussing chemicals management, some of the management is outside of chemical statutes, and their management occurs in media-specific statutes, noted Lynn Goldman, Bloomberg School of Public Health, Johns Hopkins University. One example would be certain air pollutants
covered by the Clean Air Act. These chemicals are interpreted through specific approaches that are often based on an engineering approach and are not usually a risk-based approach. Media-specific approaches are not very conducive to looking at a chemical from cradle to grave, which considers the entire life cycle of the chemical or a process. Media approaches can push a chemical from one medium to another, but never quite address the life cycle and what the alternatives might be, noted Goldman.
As discussed earlier, the life cycle of a chemical starts with research and development, through production, and then use by workers. The use of the chemical can often be just as important as the production. For example, there may be minimal exposure in the production of certain paints, but the workers in the auto body shop who use that paint have a greater exposure. Often regulators do not have full information about a chemical because they lack information about its use. Goldman further noted that it is hard to assess a chemical’s risk if you don’t know about its use and exposure.
The central tenet to any law is the standard that the government is trying to achieve through its efforts, whether the tools are regulatory or voluntary. For example, the 1996 Food Quality Protection Act for food sets the standard to be a “reasonable certainty of no harm”; however, for nonfood uses, the standard is to avoid “unreasonable risk to health or the environment,” observed Goldman. For the TSCA for all chemicals, the standard is the unreasonable risk standard. In addition to the factor of risk, it also includes whether the risk is reasonable in proportion to the costs that are required to control it. Thus the standard in TSCA is more than a common denominator—it doesn’t differentiate between the types of exposure, the quantities of exposures, or the scenarios for exposures. The Food Quality Protection Act and several other statutes have specific provisions requiring that sensitive populations be protected. However, there isn’t similar protection under TSCA. According to Goldman, clear direction has not been given to the EPA that sensitive population must be protected, whether those are children, the elderly, or those with genetic susceptibilities.
Thus, the standard in TSCA is more than a common denominator—it doesn’t differentiate between the types of exposure, the quantities of exposures, or the scenarios for exposures.
—Lynn Goldman
The statutes also differ on cost and benefits. Costs and benefits for pesticides are a consideration for nonfood use, but not for food uses. For food uses, it is a public health decision. With TSCA, it is the same standard regardless of the exposure scenario. Further, noted Goldman, there is a significant burden on the government to prove that a standard has been met, which has rendered much of TSCA ineffective. One of the challenges under TSCA is for new chemical approvals. There is not a level playing field between new chemical and existing
chemicals; they are treated differently by the regulators. It is easier to continue to use existing ones, because they will hardly ever be evaluated. Thus there is a bias in the law against bringing new chemicals into the market. For pesticides (under the Pesticides Act), a company cannot bring a new chemical on the market without testing, without approval; but the EPA can establish categories of exemptions. For TSCA, there isn’t a testing requirement prior to submitting them to EPA.
Enforceable consent agreements are agreements that are made between the EPA and the companies that have become the tool under which the agency can require further testing of new chemicals. These agreements are predicated on the fact that a hazard closely resembles something that is already on the market, which has been well studied, thus triggering a concern based on quantitative structure-activity relationship (QSAR). The problem with this approach is that some compounds do not have an extensive database, according to Goldman. Thus the process isn’t going to inform regulators about completely novel risks as the chemicals come to market, such as risks of nanomaterials. It is easy to conclude, said Goldman, that a more robust data set such as the screening inventory data set (SIDS) used by the EU would be preferable. The government could also learn from the pesticide experience in the United States by having categories of exemptions to certain categories.
A second challenge under TSCA is existing chemicals. At the time that TSCA went into effect, approximately 70,000 chemicals were grandfathered into use and placed on the inventory. This is not a true list as some of the chemicals are mixtures, some chemicals have overlapping structures, and many are not in commerce. However, there is a large number of chemicals in commerce; for practical purposes EPA has primarily put the focus on high-production chemicals, those produced in the amount of at least one million pounds per year. This again is a limitation of TSCA as it has not been very productive in providing new data. Every year, only a few chemicals undergo testing through the use of test rules and agreements. Under TSCA, the government needs to make a finding of unreasonable risk or exposure in order to have the chemical tested. In the absence of direct information about hazard and exposure data, the government has used production volume as a surrogate for potential exposure. Although this allows for data to be collected, it is not exposure information.
All the risk management for chemicals occurs under statutes like the Clean Air Act and not under the regulations directly covering these chemicals. The result is that the government focuses on end-of-the-pipe solutions rather than pollution prevention-related solutions.
—Lynn Goldman
While tiering of testing and priority setting is one way to begin to address concerns, TSCA provides no clear direction about which priority should be addressed first. The EPA has begun to address the persistent bioaccumulative
chemicals, endocrine activity, mutagenicity, as well as the presences in some locations (e.g., home or school environments).
There is very little rulemaking and risk management coming out of the EPA as a result of TSCA. This means that virtually all the risk management for chemicals occurs under statutes like the Clean Air Act and not under the regulations directly covering these chemicals. The result is that the government too often focuses on end-of-the-pipe solutions rather than pollution prevention-related solutions. It further creates problems with shifting pollutants between media as discussed earlier. TSCA does not reward efforts to develop safer processes of resource reduction, nor does it replace media-specific regulations for all media.
INTERNATIONAL COOPERATION ON REGULATORY ISSUES: STRATEGIC APPROACH TO INTERNATIONAL CHEMICAL MANAGEMENT
The public needs to recognize that almost all man-made products involve the use of intentionally produced chemicals. Every year tens of thousands of chemicals are produced and used in commercial activities, noted John Buccini of the United Nations Environmental Program. Each year, the numbers will vary, but some estimate up to approximately 1,000 new chemicals enter into production each year, while older ones are rotated out. Thus there are a constantly changing number of products, articles, and chemicals. This is one of the primary problems with the management of chemicals: how does one hit a moving target, where the nature of the target is evolving as one is trying to take sight of it?
Many of the discussions in the workshop have focused on specific sectors or countries; however, there is a need for a more generalized approach to the global pursuit of sound management of chemicals. These are the man-made laws, but once a chemical is released, the laws of nature govern the chemical.
—John Buccini
Many of the discussions in the workshop have focused on specific sectors or countries; however, there is a need for a more generalized approach to the global pursuit of sound management of chemicals, observed Buccini. There are the manmade laws, but once a chemical is released, the laws of nature govern the chemical. This is an important point, noted Buccini, because once the chemical is released, it can’t be returned. Following release, it will be subject to either short- or long-range transport and undergo degradation or transformation processes that will result in either a safer or more problematic chemical. Depending on the chemical and the nature of release, the chemical may result in local, regional, or global contamination. This can result in exposure of humans and wildlife with potential acute or chronic health or other adverse effects, noted Buccini.
In addition, it is important to note that all industry sectors release chemicals into the environment—whether intentional or not. While considering the management of chemicals, it is important to note that this is a cross-sectoral issue, including food and feed production, transportation, telecommunications, high tech, and so on. However, Buccini noted that many of the discussions have used a narrow interpretation of corporate. He suggested that it needs to be broadened to include the defense and military establishments within a country. He further noted that the idea of government as a polluter is something that hasn’t really been addressed. Chemicals are released by all sectors, including the private and public sectors. He asserted that all sectors need to be held accountable.
Chemicals have emerged as a separate issue in recent years; however, it is also being recognized that chemicals constitute an element in issues such as biodiversity, climate change, international waters, land degradation, and ozone depletion, observed Buccini. Although some chemical issues are straightforward (e.g., persistent organic pollutants), it becomes complex when one sees the such linkages as those that exist between poverty and toxins. The dilemma, according to Buccini, is that chemicals can serve a wide variety of roles that establish or preserve an elevated standard of living in countries at all stages of development. They can contribute to resolving many modern issues. Both directly and indirectly, the public has come to view them as essential components of our modern life.
Despite the progress that has been made over many years, concerns exist that population-level effects may be occurring in present and future generations of wildlife and/or humans as a result of the widespread presence in the environment of complex mixtures of pesticides, industrial chemicals, and unintentionally produced substances. In the future, attention will be focused on those substances that are persistent, bioaccumulative, and toxic. Policies for the sound management of chemicals are recognized as essential components of overall public policy in countries at all stages of development, and they should be reflected in national sustainable development plans. There is a trend in industry to shift the production of high-volume chemicals from developed to developing countries. Thus one of the key global chemical issues is whether society can balance the benefits associated with the uses of chemicals with the immediate and long-term hazards posed by chemicals in the environment.
In the future, additional needs will focus on those substances that are persistent, bioaccumulative, and toxic. Policies for the sound management of chemicals are recognized as essential components of overall public policy in countries at all stages of development, and they should be reflected in national sustainable development plans.
—John Buccini
In 1992, as preparations were underway for the UN Conference on Environment and Development (UNCED), there was a heightened interest and activity in addressing toxic chemical issues. There were six program areas articulated as the items of urgent interest:
-
Strengthen international risk assessment.
-
Harmonize classification and labelling systems.
-
Increase the exchange of information.
-
Eliminate unacceptable/unreasonable risks posed by toxic chemicals.
-
Strengthen national capabilities and capacities for managing chemicals.
-
Prevent illegal international traffic in toxic and dangerous products.
There have been some international mechanisms established to coordinate the efforts of intergovernmental organizations and other international stakeholders in addressing the UNCED’s goals and many international agreements have been developed. Currently, there are at least 52 global and regional agreements that address the use of chemicals. There were seven agreements developed in the 1970s, 13 in the 1980s, and 30 since 1990. These agreements cover air pollution, water pollution, biodiversity, specific toxic chemicals, chemical weapons, industrial accidents, storage and transportation, trade in chemicals, and transboundary waste. One problem facing the international arena is how to work with the plethora of agreements. At the World Summit on Sustainable Development in Johannesburg in 2002, the world leaders agreed on a goal of “aiming to achieve, by 2020, that chemicals are used and produced in ways that lead to the minimization of significant adverse effects on human health and the environment” (United Nations World Summit on Sustainable Development, 2002). It was noted in the specific recommendations that both technical and financial assistance will be needed for developing countries and economies in transition to build up their capacity. Whether it is to implement sound toxics policies or conventions, these countries need to have access to clean technologies, trained human resources, policies and legislation, and enforcement capacity. To help address the growing needs, the strategic approach to international chemicals management (SAICM) is beginning to consider strategic approaches to help with the sound management of chemicals. SAICM will include a high-level political declaration and an overarching policy
Specific recommendations noted that both technical and financial assistance will be needed for developing countries and economies in transition to build up their capacity. Whether it is to implement sound toxics policies and conventions, these countries need to have access to clean technologies, trained human resources, policies and legislation, and enforcement capacity.
—John Buccini
strategy that is going to lay out the needs, the objectives, and the strategies for meeting the objectives, and a global plan of action to identify what would be done, when, and by whom. The expectation is that the development of SAICM will raise the visibility of the issue and will clarify the needs of countries. It will likely lead to increased control and/or regulation of chemicals in all countries, but it is hoped in a more coordinated and consistent fashion across the globe.
Legacy Chemicals and Encouraging the Drive to Sustainability
During the discussion, one participant noted the real need to encourage sustainable chemicals by embedding economic advantages to the next generation of chemical innovation—more than simply chemical management. Goldman noted that any new chemical is going to receive more scrutiny than something currently on the market. Although this creates a disincentive to bring forward something new, the expectation is that more stringent regulation does drive people toward improvement. This reasoning will drive industry toward green chemistry and alternatives. Goldman noted that the opposite could also occur. When REACH is enacted in the European Union, the United States, with a weaker chemical law, may become a dumping ground for chemicals that are no longer acceptable for use in Europe. Buccini noted that this has happened in the past. When the OECD decided to exclude the production of brominated flame retardants, the production was shifted to the developing world where the manufacturing processes were poor and finished goods were imported into OECD countries. Buccini concluded that if one wants to race toward sustainability in a country, one has to look at the global implications. The approach needs to reflect developments at a global level; otherwise, one may be merely redistributing risk to other countries.