2
Geological and Geophysical Setting
Despite the cold surface, large areas of the bed of the Antarctic ice sheet are at the ice pressure melting temperature and actively melting through the combined influence of the insulating cover of glacial ice and the flow of geothermal heat into the base of the ice sheet. In regions where the subglacial rate of meltwater production exceeds the rate of removal by freezing to the base, some kind of drainage system must form to transfer water from regions of net production to regions where freeze-on dominates or to the ice sheet periphery. The presence of water at the base of the ice sheet has been confirmed by deep drilling to the bed and by radar imaging of subglacial lakes (Siegert et al. 2005a). New evidence indicates that some of these lakes are interconnected (Wingham et al. 2006; Fricker et al. 2007). The geometry, storage, and transport processes of this subglacial drainage system are incompletely understood, but it seems reasonable to expect that, in most cases, the lakes are not isolated entities but should be viewed as components of an extensive subglacial aquatic environment.
BASIN SETTINGS: RIFT AND NON-RIFT
Lake Classifications
At least three classification schemes have emerged from recent studies of the Antarctic subglacial lakes, based largely on interpretations of geophysical images. Two of these classifications focus on the description of morphology and physiography, whereas the other scheme distinguishes different types of environments by focusing on the characteristics of the radio-echo sounding data. One of the first schemes was developed by Dowdeswell and Siegert (2003) based on the glaciological and/or topographic setting and the physiography of the subglacial lakes. This classification resulted in three categories of subglacial aquatic environments: (1) lakes within subglacial basins located in the ice sheet interior; (2) lakes perched on the sides of subglacial mountains; and (3) lakes located in areas of enhanced ice flow.
The majority of the lakes are located in subglacial basins within roughly 100 km of ice divides in the ice sheet interior. These basins are typically separated by mountain ranges and their topography can either be relatively subdued, often near the center of subglacial basins, or relatively steep, occupying significant subglacial depressions, often near subglacial basin margins (Dowdeswell and Siegert 2003). Deep subglacial lakes are likely to develop in areas where the topography is subdued. Lake Vostok is the only known lake that occupies a large section of a subglacial trough. Perched lakes are located primarily in the interior of the ice sheet on the flanks of subglacial mountain ranges. These perched lakes are frequently small, measuring less than 10 km in length. Many other lakes occur in areas where the ice flow is enhanced, hundreds of kilometers from the ice sheet crest, such as the fast-flowing Byrd Glacier, which drains a very large interior drainage basin into the Ross Ice Shelf. These lakes are expected to be similar in size and depth to the small and most likely shallow subglacial aquatic environments found in the major subglacial basins in the ice sheet interior (Siegert 2002).
Another of these schemes, based on description of surface morphology and shape, has identified three categories of lakes: basin, relief, and trench (Tabacco et al. 2006). Basin lakes occur at the bottom of large depressions, usually in association with areas of considerable relief in the bedrock. They have irregular shapes, but no preferred elongation directions. This group of lakes is therefore thought of as resulting from glacial scouring. Relief lakes occur in subglacial mountainous regions, at fairly high bedrock elevation. They, therefore, have thinner ice cover than the basin lakes. Trench lakes, as their name implies, have an elongated shape controlled by the nature of the depressions in which they are found. These depressions are long and narrow and typically have steep cross-sectional profiles that may be fault bounded.
Lake Vostok and others in the Dome C region may belong to the trench lakes category. Studinger et al. (2003) have modeled Lake Vostok as the product of overthrust faulting followed by a small amount of extensional faulting during reactivation with the opposite-to-original sense of motion (Figure 2.1). However, this interpretation remains controversial because the majority of Antarctic subglacial lakes are developed on basement rocks that became part of the stable craton in the Precambrian, leaving few opportunities for more recent faulting to create basins in which subglacial lakes can form.
The third classification scheme of subglacial lakes is based on the characteristics of the return signals from each one of these features using ice-penetrating radar as displayed on radargrams. With this approach, three categories of lakes have been identified: great lakes, dim lakes, and fuzzy lakes (e.g., Blankenship et al. 2006).
Great lakes are ones that yield well-defined radar images with flat tops and flat bottoms. Their radar echo is bright, and they all seem to be at least 500 m across. Blankenship et al. (2006) estimate that there are about 30 great lakes among the subset of the 130 lakes that were surveyed systematically using ~51,000 line km of radar data.
Dim lakes are characterized by less clear tops and bottoms on radargrams compared to great lakes. Dim lakes may have started as “great lakes” but have since frozen over. Alternatively, their images may be artifacts of the ice temperature correction applied in the calculation being wrong. The evolution from great lake to dim lake may be caused by flow regime change (e.g., changes in the temperature of the ice).
Fuzzy lakes are distinguished by having hydrologically “flat” radar images that are, however, full of reflections that do not appear to be water. Terms such as mushy swamps or wetlands have been used in descriptions of these features.
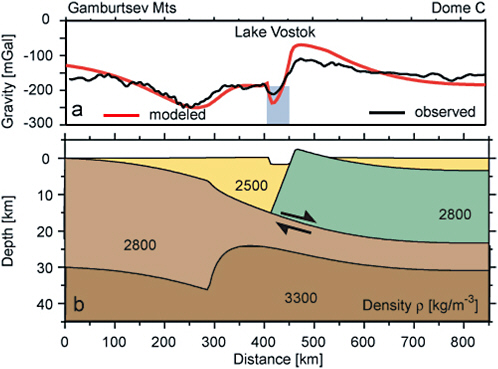
FIGURE 2.1 Diagram from Studinger et al. (2003b) illustrating that Lake Vostok has been modeled as a 50-km-wide tectonic basin developed in a continental collision zone between the Gamburtsev Subglacial Mountains and the Dome C region. SOURCE: Reprinted from Earth and Planetary Science Letters, 216 (4): Studinger et al. (2003b) with permission from Elsevier.
Other Subglacial Environments
Little is known about the ice-bedrock interface in large regions of Antarctica because of the poor airborne radar survey coverage and the lack of drilling to bedrock. In areas that have been surveyed, there is also the potential complication of surface roughness scattering the radar signal and thus preventing accurate bedrock surface mapping and characterization of the nature of the ice-bedrock interface. Enough is known, however, for the glaciology community to have accepted that in addition to well-defined subglacial lakes, there is a substantial capacity for liquid-water storage in water-saturated subglacial sediments (e.g., Siegert and Bamber 2000).
BASEMENT ROCK CHARACTERISTICS
An understanding of the bedrock geology beneath the ice sheets is important to help determine how and when subglacial aquatic environments formed and whether these
environments are connected. Like much information about the Antarctic continent, the basement rocks of the East Antarctic Shield are still poorly understood compared to other continents. This lack of knowledge is a result of the fact that 98 percent of the area is ice covered, and the 2 percent of it that is exposed, generally in coastal regions, is challenging to access. For this reason, current understanding of the basement geology of East Antarctica is constrained largely by seismic data, modeling of gravity and aeromagnetic data, and extrapolations of coastal rock exposures and deformational structures. However, recent examination of the assembly of the East Antarctic Craton suggests that Neoproterozoic to Cambrian rocks (900 million to 543 million years ago), similar to those in the Pinjarra Orogen of Western Australia (Figure 2.2), may exist in the vicinity of some subglacial lakes, including Lake Vostok (Fitzsimons 2003).
A similar age for the basement rocks beneath the ice sheet was indicated from geochemical (87Sr/86Sr versus 143Nd/144Nd) and mineralogical characterization as well as dating (neodymium model age) of a millimeter-size bedrock inclusion recovered from the accreted ice section of the Lake Vostok core (Delmonte et al. 2004). This study made the assumption that the inclusion samarium to neodymium ratio is representative of the basement in spite of its small size. In agreement with the neodymium model ages, new uranium-thorium-lead ion microprobe determinations on detrital zircon and monazite, also from the Lake Vostok core, have yielded ages of between 1800 million and 600 million years (Rodionov et al. 2006). This range is similar to that observed at the surface in the Prince Charles Mountains, Prydz Bay, and Wilkes Land.
The fact that “young” ages in the range of 1800 million to 600 million years are being recognized from materials recovered in the Vostok core, suggests that faults and sutures exist that are juxtaposing basement rocks of a variety of ages. This is relevant to the study of subglacial aquatic environments because if the interior of Antarctica has fractured rocks as suggested by Figure 2.2, then it is not only likely but inevitable that these subglacial aquatic environments constitute a connected hydrologic system.
Further evidence for a potentially connected hydrologic system comes from the hydraulic conductivities1 of bedrock beneath the ice sheet. The hydraulic conductivities of unfractured, igneous, and metamorphic rocks comparable to those observed in East Antarctic coastal exposures are low (3 × 10−14 to 2 × 10−10 m s−1), which means that water does not flow through these rocks readily. However, if the interior of the East Antarctic Craton in the vicinity of Ridge B are dominated by fractured crystalline rocks, as suggested by models 2 and 3 of Fitzsimmons (2003) in Figure 2.2, then the local hydraulic conductivity is likely to be several orders of magnitude greater (8 × 10−9 to 3 × 10−4 m s−1) than that of solid rocks (Domenico and Schwartz 1998).
From a number of studies, it is evident that the East Antarctic basement rocks are Precambrian in age (3800 million to 543 million years ago), but definitive ages of large sections of the basement rocks remain scarce. Palinspastic reconstructions1 show that the East Antarctic basement rocks were formerly the nucleus around which the southern continents were amalgamated to form the supercontinent Gondwanaland. Therefore, this part of the continent is generally regarded as a tectonically stable cratonic region stabilized by ~800 million years when the supercontinent Rodinia fragmented. The Gamburtzev Mountains, which lie beneath the ice just west of Lake Vostok, have an estimated relief of 3500-4000 m. This relief, which is anomalously high for a cratonic
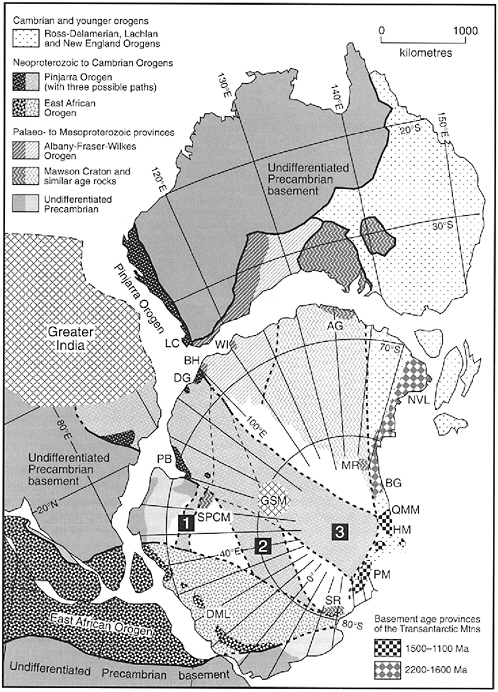
FIGURE 2.2 The bedrock geology beneath most of interior East Antarctica is not well known, and three possible interpretations for the architectural makeup of the crust beneath the East Antarctic lakes have been proposed and are labeled 1, 2 and 3. Interpretations 1 and 2 place Paleoproterozoic (2500 million to 1600 million years ago) to Mesoproterozoic (1600 million to 1900 million years ago) rocks of the Mawson Craton beneath the Lake Vostok region. Interpretation 3 on the other hand places Neoproterozoic to Cambrian (900 million to 543 million years ago) rocks similar to those in the Pinjarra Orogen in Western Australia beneath the area. This figure illustrates that the interior of the East Antarctic Craton may not be homogeneous, but rather is faulted and may comprise terranes of different ages and origins. The structural relationships may be a boundary condition for how and where lakes form and the extent to which they might be connected. SOURCE: Fitzsimons 2003.
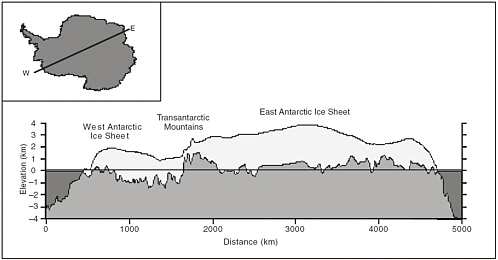
FIGURE 2.3 Cross-sectional profile of the Antarctic ice sheet based on BEDMAP bed topography (Lythe et al. 2001) and surface topography from Liu et al. (1999). Inset: Location of profile end points. SOURCE: Lythe, M. B., D. G. Vaughan, and C. BEDMAP: A new ice thickness and subglacial topographic model of Antarctica, Journal of Geophysical Research B: Solid Earth, 106 (B6): pp. 11335-11351 (2001). Reproduced with permission of American Geophysical Union. SOURCE: G. Clarke, committee member.
interior, has led to speculation that volcanism may have erupted through the craton as recently as Cenozoic times (Dalziel 2006). This hypothesized volcanism would likely be in a zone of high heat flow with potential to accelerate melting of the ice sheet in certain areas. It should be noted that most of these ideas are based on very few concrete data from the underlying rocks, and until samples of these rocks are obtained, speculation about the timing and processes by which these environments could have formed will remain.
ICE SHEET DESCRIPTION
The Antarctic ice sheet accounts for roughly 90 percent of Earth’s present ice volume (Church et al. 2001); it is bisected by the Transantarctic Mountains, which divide it into the high-standing and voluminous East Antarctic ice sheet (EAIS) and the low-standing but more dynamic West Antarctic ice sheet (WAIS). The platform upon which these two ice sheets lie is predominantly above sea level for the EAIS and below sea level for the WAIS, leading to the generalization that the EAIS is a terrestrial ice sheet and the WAIS is a marine ice sheet (Figure 2.3). Maximum ice thicknesses, exceeding 4 km, are encountered in the interior regions of the EAIS where the surface elevation is high and the resulting surface temperature is low.
The ice sheet flows by a combination of processes, some occurring within the ice mass and others at the boundary between the ice and its base. Plastic creep contributes significantly to the flow of the interior regions and tends to be a slow process, yielding flow rates in the range 1 cm to 10 m per year. Closer to the margins, a dendritic network of fast-flow arteries, termed “ice streams,” can develop. These have typical flow rates between 500 and 2000 m per year, and their fast flow is attributed to a combination of sliding over the bed and deformation of subglacial sediment. Where the Antarctic ice sheet flows into the surrounding ocean, ice can come afloat but remain intact to form an ice shelf that flows by thinning and spreading under the influence of gravity. Prominent examples are the Ross and Filchner-Ronne ice shelves that fringe the WAIS. The modes of flow outlined above are referred to as sheet flow, stream flow, and shelf flow, where different flow mechanisms dominate for each case. For the most part, shelf flow is relevant only to the floating margins of the Antarctic ice sheet, but it also operates over large subglacial lakes such as Lake Vostok where, as for ice shelves, the ice column is afloat and the lower boundary is free of frictional resistance.
The temperature distribution within an ice sheet is determined by surface temperature, ice thickness, ice flow, and geothermal heat flux. The mean annual surface air temperature for Antarctica is −36°C (Giovinetto et al. 1990), with mean temperatures of the high interior region being as low as −55°C. For the most part, the sediment and rock that form the continental platform of Antarctica are much warmer because they are shielded from cold surface temperatures by a kilometers-thick insulating blanket of glacier ice. The general tendency is for ice temperature to increase with depth until the bed is reached or the ice melting temperature is attained (Figure 2.4). This melting temperature depends on the ambient pressure (which is determined by the height of the ice column) and on the impurity content of the ice, both of which lower the melting temperature. For ice sheets, the pressure influence dominates and accounts for a 0.0742°C decrease in melting temperature per megapascal of pressure increase, roughly equivalent to a decrease of 0.654°C per kilometer of ice thickness.
Much of the base of the ice sheet is thought to be at or near the ice melting temperature. This conjecture is consistent with the bottom temperatures at the Byrd Station, Vostok, and Dome Concordia deep ice core sites (Gow et al. 1968; Petit et al. 1999; EPICA community members 2004) and is likely to be the case at the Dome Fuji site as well (Saito and Abe-Ouchi 2004). Melting temperatures also prevail at the bottom of all holes thus far drilled to the bed of active ice streams. As described earlier, extensive airborne radar sounding surveys, which allow the base of the ice sheet to be mapped in detail (e.g., Lythe et al. 2001), also indicate the extent of melting as revealed by the existence of subglacial lakes (Siegert et al. 2005a).
To extend these observations, other sources of information must be used. Computational models of the dynamics of the Antarctic ice sheet (Figures 2.5 and 2.6) indicate that large areas of the ice sheet base are at the ice melting temperature and that most areas are within 5°C of the melting point (which depends on ice pressure). The distribution of Antarctic subglacial lakes (Figure 2.6, black triangles) is broadly consistent with the predicted bed temperatures in Figure 2.6 whereas Figure 2.5 seems to be cold biased potentially resulting from modeling parameters. It is reasonable to conclude that over large areas the underlying sediment and rock are unfrozen and water saturated, and that storage and transport of subglacial water are to be expected.
The rate of basal melting corresponds to the rate at which a subglacial meltwater layer would thicken (e.g., in millimeters per year) if water flow was prevented. This
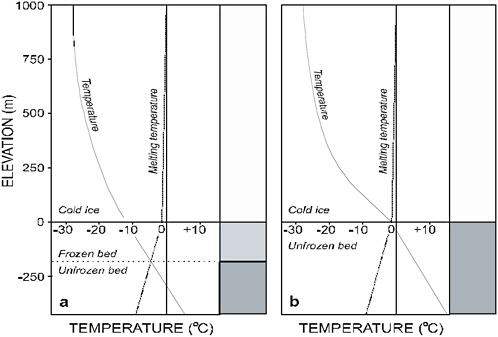
FIGURE 2.4 Diagrammatic profiles of ice temperature and ice melting temperature through an idealized ice sheet and its bed. (a) Frozen glacier bed with subglacial permafrost layer. (b) Melting glacier bed. SOURCE: G. Clarke, Committee Member.
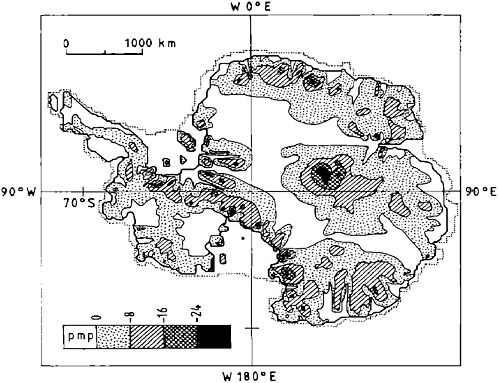
FIGURE 2.5 Map of bed temperature of Antarctic ice sheet (in degrees Celsius relative to the pressure melting temperature) as predicted by the numerical ice dynamics model. SOURCE: Huybrechts (1990).
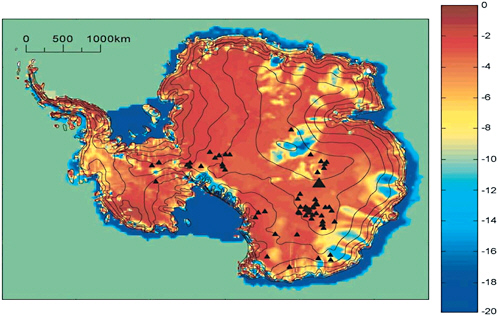
FIGURE 2.6 Map of basal temperature of Antarctic ice sheet (in degrees Celsius) as predicted by the numerical ice dynamics model of Siegert et al. (2005). Black triangles indicate the locations of known subglacial lakes, with the largest triangle corresponding to Lake Vostok. Because of the dependence of ice melting temperature on pressure, the melting temperature over large areas of the ice sheet interior is at or below −2°C. SOURCE: Global and Planetary Change, 45, Martin J. Siegert, Justin Taylor, Antony J. Payne, Spectral roughness of subglacial topography and implications for former ice-sheet dynamics in East Antarctica Bristol Glaciology Centre, School of Geographical Sciences, 249-263, 2005, with permission from Elsevier.
rate is not directly observable but can be predicted using a thermomechanical ice dynamics model. Figure 2.7 presents results from one such model (Siegert et al. 2005). The figure shows the simulated average depth of the subglacial water layer but should be interpreted qualitatively rather than quantitatively because the calculation assumes a highly simplified subglacial hydrologic system. Nevertheless, to a first approximation, regions where the simulated water depth is large correspond to regions where the estimated melt rate is high.
GEOGRAPHICAL LOCATION OF ANTARCTIC SUBGLACIAL LAKES
Main Traits of Locations and Settings
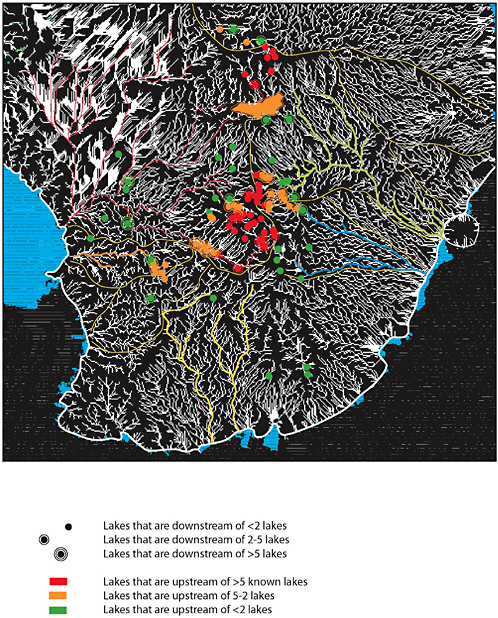
About 80 percent of subglacial lakes reported to date in Antarctica lie at elevations less than a few hundred meters above sea level, while the majority of the remaining lakes are “perched” at higher elevations (Blankenship et al. 2006). Most of these lakes are in East Antarctica (Figure 2.8) with a few located along the continental margin between Victoria Land and Wilkes Land, and in the Transantarctic Mountains region where Precambrian basement was uplifted during the Cenozoic. Only four lakes have
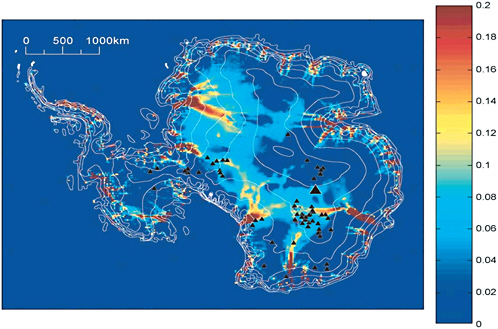
FIGURE 2.7 Average thickness of basal meltwater (in meters) as predicted by the numerical ice dynamics model of Siegert et al. (2005). The model assumes a highly simplified subglacial hydrological system and is best viewed as a qualitative indicator of regions where the basal melt rate is high. SOURCE: Global and Planetary Change, 45, Martin J. Siegert, Justin Taylor, Antony J. Payne, Spectral roughness of subglacial topography and implications for former ice-sheet dynamics in East Antarctica Bristol Glaciology Centre, School of Geographical Sciences, 249-263, 2005, with permission from Elsevier.
been reported so far in West Antarctica. One of these lies in the Byrd Subglacial Basin, between two crustal blocks, the Ellsworth-Whitmore Mountains and Marie Byrd Land. The other three lakes are located on the Ellsworth-Whitmore Mountains block and near its margin (Lake Ellsworth). This block is a former fragment of the Gondwanaland craton that rotated to its present position from the Weddell Sea margin of the continent. Hence all but one of the lakes reported to date are located on Precambrian crust (Dalziel 2006).
Lake Districts
Figure 2.8 shows that Antarctic subglacial aquatic environments are clustered near the South Pole, and the Dome C, Ridge B, and Ellsworth-Whitmore Mountains areas. Blankenship et al. (2006) have found that 66 percent of all known Antarctic subglacial aquatic environments lie within 50 km of an ice divide, and some 88 percent of them are within 100 km of a local ice divide. Clusters of these environments in the Dome C and Ridge B areas are close enough to each other for interconnectedness to be possible, if not an absolute certainty. These groups of lakes are now sometimes referred to as “lake districts.”
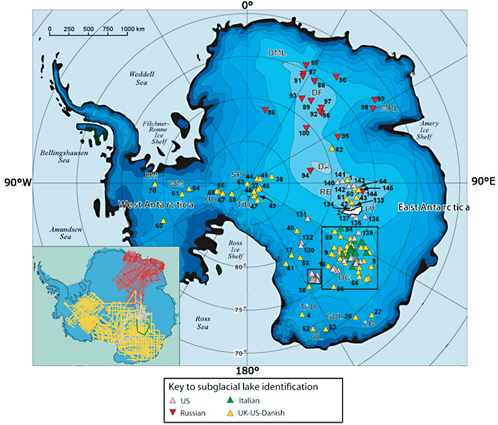
FIGURE 2.8 Locations of 145 Antarctic subglacial lakes. The distribution of subglacial aquatic environments is determined by the distribution of water and the availability of basins to collect water. The vast majority of subglacial aquatic environments identified to date are within 100 km of an ice divide. Major clusters of these environments are located in Dome C (DC) and Ridge B (RB) areas. The majority of these environments are small (less 20 km in length) with an average depth of 100 m. A few larger lakes may be up to 1000 m deep. Map inset shows distribution of radio-echo sounding flight lines. SOURCE: Modified from Siegert et al. (2005a). Reprinted with the permission of Cambridge University Press.
Lake Size and Volume
Very few lakes, among them Lake Vostok, have been purposefully mapped, so in many cases a lake is traversed by a single radar flight line that provides no constraint on lake area, let alone lake volume. To estimate lake volume, radio-echo sounding (RES) measurements, which image the lake surface, must be complemented by some other geophysical technique such as seismic reflection sounding or gravimetric measurement. The former method is capable of mapping the topography of the lake floor, which returns a seismic reflection to the ice surface. The latter method is less precise and relies on the density difference between lake water and subglacial bedrock. It is not capable of resolving the detailed topography of the lake floor and works best for large lakes that
produce a large mass deficit. The subglacial lake with the best-determined geometry, Lake Vostok, is also the largest. The data used for this determination include several airborne radar surveys, one seismic reflection survey, and an aerogravity survey.
PRE-ICE SHEET LAKES AND SEDIMENTS
There are no published studies on lakes that may have formed and sediments deposited prior to the development of the Antarctic ice sheet. At this stage, therefore, only questions exist about this possibility. The spatial extent and thickness of the sediments are unknown as are their physical and chemical characteristics. Are they primarily hemipelagic, turbiditic, or deltaic,—or a combination of all three? Are the sediments disrupted by faults, which would indicate relatively recent tectonic activity? Are the sediments conformable or do discontinuities exist? Does the geometry of these discontinuities reveal any information about the environment in which the sediments were deposited? Distinct groupings of sediment types indicate changing sediment input directions and/or tectonic-induced rotations. Erosional unconformities are likely to be associated with freezing of an entire lake and compaction and reworking of the sediments.
It has been suggested that Lake Vostok had a preglacial antecedent and, more controversially, that the lake has existed continuously from a time before Antarctica was extensively glaciated to the present day (Duxbury et al. 2004). Obviously, this would increase scientific interest in the lake and its biota, but there is uncertainty as to how such a lake might survive inundation by an ice sheet. Glaciological arguments for lake survival (Pattyn 2004) and against it (Siegert et al. 2004) have been advanced, but the issue remains unresolved. Quite independently, Erlingsson (1994a,b) developed the idea of a “captured ice shelf” overlying a “captured lake” to support his hypothesis that the Weichselian glaciation (90–15 kyr2) of the Baltic Sea resulted from expansion of a floating ice shelf rather than the advance of a grounded ice sheet. More recently, Alley et al. (2006) proposed a similar idea to explain how, during the same time period, repeated subglacial outburst floods might have originated from Hudson Bay.
AGE OF LAKES AND WATER RESIDENCE TIME
Several different time scales are relevant to discussion of subglacial lake environments. These constrain the answers to the following questions: (1) What is the transit time for water deposited as snow on the surface of the ice sheet to be melted off the base of the Antarctic ice sheet and delivered to a subglacial lake? This establishes the degree of temporal separation between the surface and the subglacial environment and thus the age of microorganisms that are deposited in the lake by bottom melting. (2) What is the turnover time of water in a subglacial lake reservoir? This has relevance to how rapidly a subglacial lake can recover from accidental contamination. (3) What is the lag time between water exiting the lake and returning to a subaerial or submarine environment? This corresponds to the delivery time of contaminants to the global ocean and to the age of microorganisms flowing from the lake to the global ocean.
The age of the oldest ice at the base of the Antarctic ice sheet is comparable to that of the oldest ice in Antarctic ice cores, roughly 1 million years (EPICA 2004; Peplow
2006), plus the transit time from the interior to the periphery, which adds perhaps another 1 million years. Estimates of the residence time for water in a subglacial lake depend on the reservoir volume and the rate of water discharge into and out of the reservoir.
Water input to the lake comes from the influx of subglacial meltwater from the surroundings and from melting of the ice roof. Water output is the sum of water spilling out from the lake to the surroundings and water freezing onto the roof. Figure 2.9 presents the general case, in which all sources of input and output are active, and several limiting cases. The general case is likely to be the most prevalent because it is the least restrictive.
Estimates of the bulk turnover time τ are independent of the specific nature of the influxes and are based on the ratio of volume V and input discharge Qin as follows: τ = V / Qin. Conventionally, V is taken as the reservoir volume, but this would not be an appropriate choice if the reservoir is poorly mixed—for example, if input meltwater remains in close proximity to the lake roof. Thus, both V and Qin may be poorly determined, but the main objective is to obtain rough rather than exact estimates of τ, so for the present purposes this will suffice. Siegert (2005) estimates the residence time for Lake Vostok to be approximately 100,000 years.
The transit time for water that exits a subglacial lake to flow out to the ice sheet perimeter (escaping either as subaerial outflow or as direct submarine discharge to the Southern Ocean) depends on whether lake water is transported in solid form with the flow of the ice sheet (if the water is frozen onto the base of the ice sheet) or in liquid form via a subglacial water flow system. Solid-phase transport is the slow process, and for this process in Antarctica, the maximum transport time is roughly 1 million years. For the fast process, assuming a water flow velocity of 1 mm s−1 and a travel distance of 2000 km (Figure 2.3), the transport time is roughly 50 years. Here, the major point is that when water from subglacial lakes flows to the global ocean it is unlikely to be more than several million years older than the snow falling on the ice sheet surface. This time scale is relevant to the maximum degree of genetic divergence between organisms deposited on the ice sheet surface and organisms transferred from the ice sheet to the global ocean.
LAKE CONNECTIVITY
The extent to which Antarctic subglacial lakes are interconnected has important implications for their scientific interest as well as for their environmental stewardship. If the lakes are hydraulically isolated, then in principle a variety of distinct subglacial environments could develop and the consequences of chemical or biological contamination would be localized. If the lakes are highly interconnected, as they would be if they were part of a pervasive subglacial hydrologic system, then environmental diversity would be decreased, the effects of dilution would be increased, and in terms of impacted area, the consequences of contamination would be increased. These extremes of connectivity might be termed the “repository model” (as in Figure 2.9) and the “watershed model” (as in Figure 2.9).
Much of the speculation concerning the scientific interest of Lake Vostok has been guided by the repository model. Yet there is little compelling evidence to support this assumption. The behavior of water beneath warm-based valley glaciers and ice caps is well described by the watershed model, and examples of long-term subglacial water
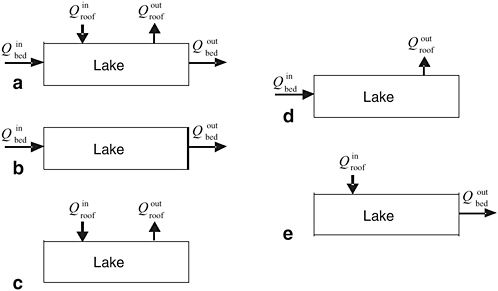
FIGURE 2.9 Water balance in subglacial reservoirs. (a) The general case in which water is received and delivered from the subglacial environment and from melting and freezing processes operating at the roof. (b) Input discharge from upstream subglacial melt sources is balanced by output discharge to the downstream subglacial environment. (c) Melting and freezing processes in the lake exactly balance with no external sources or sinks. (d) Input discharge from the subglacial environment is balanced by freeze-on at the lake roof. (e) Roof melting is balanced by discharge from the lake to the downstream subglacial environment. SOURCE: G. Clarke, Committee Member.
repositories are rare or nonexistent. Evidence suggests that Lake Vostok is long-lived and, on the millennial scale, stable, but this cannot be generally true of subglacial lakes. Wingham et al. (2006) present strong evidence for the episodic discharge from a deeply buried Antarctic subglacial lake into two receiver lakes situated at least 290 km downstream from the source lake. This observation is consistent with previous (Gray et al. 2005) and very recent (Fricker et al. 2007) observations of subglacial water discharge events occurring beneath West Antarctic ice streams. As a default position, one must accept that lake-to-lake connectivity could be the norm rather than the exception. The implication of this is that a basin-scale analysis of hydrologic catchments is necessary before lake-to-lake interactions can be critically examined.
Water System at Ice-Bed Interface
The fate of flowing subglacial water is strongly constrained by the well-established physics of fluid mechanics. It can be shown that the flow of water at the ice-bed interface is driven by the bed slope gradient γbed and the ice surface slope gradient γsurf and that the surface slope ρice /(ρwater − ρice) is approximately 8 times more effective in directing subglacial water flow than the bed slope. This result has important ramifications for subglacial water routing because it implies that, to a first approximation, subglacial
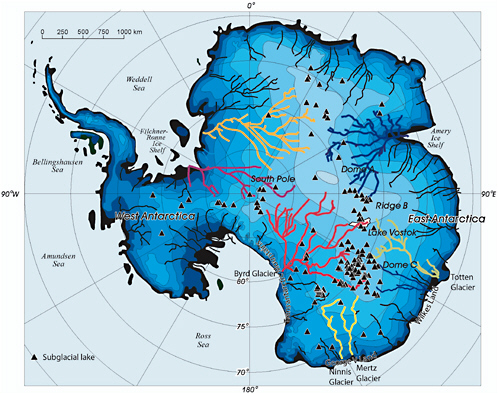
FIGURE 2.10 Locations of known Antarctic subglacial lakes and predicted major drainage routings. SOURCE: Siegert et al. 2007.
water flow follows the ice surface slope. Ice flow is known to follow the surface slope so the implication of the above is that subglacial water routing is closely similar to the routing of the ice flow, both being determined by the ice surface topography.
With knowledge of both the ice surface topography (e.g., Liu et al. 1999) and the bed topography (e.g., Lythe et al. 2000) and the use of a standard water flow routing algorithm, it is possible to delineate large-scale drainage basins for subglacial water flow (Figure 2.10). The same approach can be applied at a finer spatial resolution in regions where high-resolution surface and bed elevation data are available (Figure 2.11).
It has yet to be demonstrated that flow routing algorithms that have been developed for subaerial flows can reliably predict subglacial routings because the situations are not completely analogous. Subglacial flows are not exclusively guided by geometry but are subject to permeability barriers (e.g., frozen beds) that have no subaerial counterpart. Nevertheless such analyses can provide useful information on the expected water pathways and the possibility of interconnection between lakes. For example, according to Figure 2.10, water flowing from Lake Vostok would be routed toward the outlet
of Byrd Glacier in West Antarctica, and according to Figure 2.11, lakes in the Ridge B-Dome C region of East Antarctica would be expected to manifest a large degree of interconnectivity.
Finally, using satellite radar altimetry, very accurate digital elevation models (DEMs) of the ice sheet surface can be generated, and from these, the faint traces of subglacial hydrological networks can be extracted using a surface curvature detection algorithm (Rémy and Legrésy 2004). Whether interconnectivity is continuous or sporadic is a separate question. The observation of Wingham et al. (2006), is best interpreted as a subglacial outburst flood that, over the course of 16 months, transferred 1.8 km3 of water from one basin to others downslope from it. The situation is somewhat analogous to jökulhlaups that catastrophically release meltwater stored beneath Icelandic ice caps (Björnsson 2002) and for which there is a sizable observational and theoretical literature. Inspired in part by the Wingham et al. result, efforts are under way to adapt existing theory to deal with such floods (Evatt et al. 2006).
Groundwater Routing
Groundwater flow is driven by gradients in the hydraulic potential that, in turn, are governed by the surface and bottom geometry of the ice sheet. Water transport following a groundwater routing is driven by the same hydraulic potential gradient that drives water flow along the ice-bed interface, so the relative importance of groundwater flow depends on which mechanism is more effective and under what circumstances.
In regions where the bed is at the melting temperature, the sheet and groundwater flow systems can coexist and both systems experience roughly the same hydraulic potential gradient. Which system dominates will depend on the hydraulic conductivity of the subglacial materials. As is apparent from Table 2.1, the natural range for hydraulic conductivity is extremely large. For back-of-the-envelope estimates, a value of 10−6 ms−1 is taken. There are doubtless regions in which significantly higher values apply, but regions where the hydraulic conductivity is low are likely to control the overall flow rate. By a simple calculation it can be shown that a 1-mm-thick water sheet is as effective as a 460-m-thick aquifer, having a hydraulic conductivity of 10−6 ms−1. Thus, when sheet flow is possible it is far more effective at transporting water than groundwater flow unless the aquifer is highly conductive or very thick.
TABLE 2.1 Representative Values of Hydraulic Conductivity of Various Rock Types
|
Rock Type |
Hydraulic conductivity (m s−1) |
|
Limestone, dolomite |
1×10−9 − 6×10−6 |
|
Sandstone |
3×10−10 − 6×10−6 |
|
Siltstone |
1×10−11 − 1.4×10−8 |
|
Shale |
1×10−13 − 2×10−9 |
|
Permeable basalt |
4×10−7 − 2×10−2 |
|
Fractured igneous and metamorphic rock |
8×10−9 − 3×10−4 |
|
Weathered granite |
3.3×10−6 − 5.2×10−5 |
|
Weathered gabbro |
5.5×10−7 − 3.8×10−6 |
|
Basalt |
2×10−11 − 4.2×10−7 |
|
Unfractured igneous and metamorphic rocks |
3×10−14 − 2×10−10 |
|
SOURCE: Excerpted from Domenico and Schwartz (1990). |
|
Where the ice-bed interface is below the freezing point, a sheet flow system cannot develop, but groundwater flow can proceed through unfrozen material beneath the ice sheet (Figure 2.4). A representative value of the geothermal gradient in frozen subglacial rock or sediment is 30°C km−1.
Thus, if the base of the ice sheet is 3°C below the freezing point, the subglacial material is expected to be frozen to a depth of approximately 100 m below the base of the ice sheet. For this case, the upper boundary of the groundwater flow system would therefore lie 100 m below the bed of the glacier.
CIRCULATION AND STRATIFICATION
Although subglacial lakes are isolated from direct wind-induced mixing, a variety of other hydrodynamic mechanisms can operate within the lakes. Subglacial lake circulation results from endogenous processes that drive water from one part of the lake to another and exogenous processes such as “riverine” fluxes to and from a subglacial hydrologic system that extends beyond the lake margins. Subglacial water flow is well known from Arctic glaciological studies and has been inferred in many parts of Antarctica (e.g. Gray et al. 2005) including into subglacial Lake Concordia (Tikku et al. 2005). There is also evidence of water flow between adjacent subglacial lakes in Antarctica (Wingham et al. 2006; Fricker et al. 2007). Such transfers provide an additional source of momentum to generate within-lake advection and turbulent diffusion, as well as a mechanism that may disperse contaminants among lakes. If the inflow is denser than the lake water at the point of entry because of its temperature, salinity, or suspended sediments content, then it will sink to the bottom of the lake or to a depth of equal density. River-derived overflows, underflows, and interflows are features of lakes at all latitudes, and the resultant density plumes can be much larger than the inflow discharge because of turbulent entrainment of the surrounding water (e.g., Vincent et al. 1991).
The endogenous circulation of subglacial lakes is driven by small gradients in the potential energy of lake water that can result from vertical or lateral gradients in water density. This density depends on the pressure, temperature, and salinity of water, which can be approximated by experimentally based equations of state (e.g., Chen and Millero 1986). The pressure of subglacial meltwater is well constrained and should closely approximate the hydrostatic pressure of the overlying ice and water. Temperature is also reasonably well constrained, but not without complication. For example Souchez et al. (2000) extrapolated the ice temperature profile for the Vostok core hole and estimated the lake temperature to be −3.1°C, some 0.6°C lower than the freezing temperature of freshwater at pressures comparable to that in the lake. Salinity is the least constrained of the three density dependents.
The combination of a sloping ice ceiling with large-scale lake circulation can establish conditions for vigorous melting and freezing at the ice-water contact. These “glaciohydraulic” processes are associated with the pressure dependence of the ice melting temperature and are known to be active beneath floating ice shelves (e.g., Jenkins and Doake 1991) and in subglacial drainage channels that have adverse slopes (Alley et al. 1998; Lawson et al. 1998). Their importance to subglacial lakes is that they can lead to unexpectedly high rates of melting and accretion—far exceeding the rates that might be explained by plausible variations in geothermal flux. The consequence for subglacial Lake Vostok is that parts of the ice ceiling are subjected to melt rates as high
as 38 cm per year while other parts are subjected to freeze-on at as much as 6 cm per year (Siegert et al. 2000). By coincidence, the Vostok 5G core hole is situated above a region where the upstream accretion rate is high and the total thickness of accreted lake ice exceeds 200 m (Jouzel et al. 1999).
Salt inputs to subglacial lakes must come from one of two sources: (1) the melt of glacier ice entering the lake, or (2) chemical weathering and dissolution of previously deposited subglacial salts by groundwaters (including sediment pore water). Several indirect lines of evidence suggest that the solute content of Lake Vostok is very low. Chemical analysis of lake ice recovered from the Vostok ice core at depths below 3539 m indicates that the accreted ice has a salinity of roughly 0.001 parts per thousand. The freezing process tends to concentrate solute in the liquid phase so that water salinity will exceed ice salinity, with the chemical partitioning between the two phases governed by a distribution coefficient. On this basis, Souchez et al. (2000) predicted that the average salinity of Lake Vostok was 0.4-1.2 parts per thousand (in contrast to 35 parts per thousand for ocean water). Using different reasoning, Gorman and Siegert (1999) found that Lake Vostok was comparatively transparent to 60-MHz electromagnetic waves and argued that the electrical conductivity, and thus the salinity, of the lake water must be extremely low. Using RES data of ice thickness, and satellite altimetry, Kapitsa et al. (1996) evaluated the flotation of the ice over Lake Vostok and concluded that the lake water is “relatively fresh.”
Although measurements of the chemistry of accreted ice near the base of the Vostok ice core provide strong evidence for low-salinity lake water, the hypothesis is not free of difficulties. If the temperature extrapolation of Souchez et al. (2000) is correct, higher-salinity water is inferred because increased salinity depresses the temperature of freezing. The low-salinity hypothesis also raises questions about the residence time of lake water and how low salinity can be maintained. If the accretion of lake water ice to the base of the ice sheet concentrates solute in the lake, then salinity should rise over time in the same way that evapoconcentration causes the accumulation of salt in endorheic lakes (terminal lakes that have no outlet). For a lake to maintain itself as a low-salinity water body there must be processes by which salts can be removed, for example, by outflow of lake water to the subglacial surroundings. Furthermore, the accretion ice is formed from the uppermost waters of Lake Vostok where the lowest-density water will occur. If any salt stratification is present in the lake, the accretion ice will capture the signature of the lowest-salinity water in the lake.
As recognized by Wüest and Carmack (2000), the nature of the circulation of subglacial lakes is dependent on the depth of burial of the lake; the chemistry of its water; the magnitude of the geothermal flux through the lake; and the size, geometry, and geographic latitude of the reservoir (Figure 2.12). The omnipresent geothermal flux establishes a temperature gradient between the floor and ceiling of the lake. An important issue is whether heat and solutes are transported mainly by thermal and chemical diffusion or by convection because this determines whether vertical mixing is weak or vigorous. If diffusion processes dominate, the lake can maintain large thermal and chemical gradients and a stratified structure can develop. If the lake is vigorously convecting, temperature and salinity contrasts will be suppressed. It should be noted that Wüest and Carmack (2000) created their model for a single basin, and Lake Vostok is now known to have two basins divided by a shallow sill (Studinger et al. 2004).
Whether Antarctic subglacial aquatic environments are “lake-like” or “ocean-like” in their structure and convective circulation depends strongly on their salinity and depth
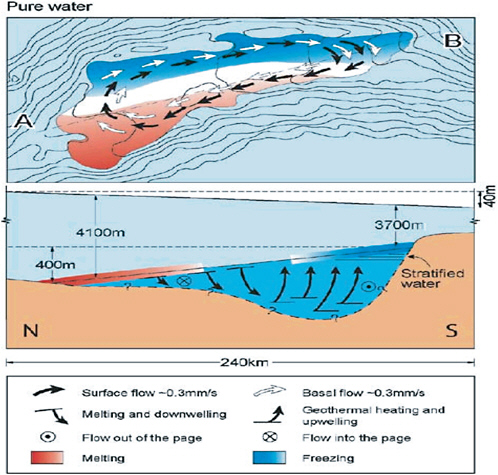
FIGURE 2.12 Conjectured circulation pattern for Lake Vostok assuming that it has low salinity and the flow is driven by thermally induced density gradients in the lake (after Siegert 2005). SOURCE: Reprinted, with permission, from the Annual Review of Earth and Planetary Sciences, Volume 33 ©2005 by Annual Reviews www.annualreviews.org.
of burial (Wuest and Carmack 2000). At pressures exceeding 28.4 MPa (3170 m of ice cover) the maximum density of freshwater occurs at the freezing temperature and the water body is ocean-like, irrespective of its salinity. This should be the case for Lake Vostok, which lies beneath 3700-4300 m of ice. Furthermore, the mean water depth for Lake Vostok is around 360 m (Studinger et al. 2004), so even a conservative estimate of the flow Rayleigh number (an indicator of the degree of convective instability) would suggest that vigorous thermal convection is occurring over much of the lake. If this is the case, then vertical mixing should be effective and reduce any vertical gradients in chemical and biological properties.
The effect of bottom heating through the usual geothermal flux of about 0.05 W m−2 will differ among subglacial lakes depending on the thickness of the overlying ice cover (Wuest and Carmack 2000). Because the temperature of freezing (TF; water at the ice-water interface should be close to this temperature) and the temperature of the maximum density of water (TMD) are both functions of pressure. For freshwater at pressures below 2840 dbar (equivalent to 3170 m thickness of ice, assuming an ice density of 913 kg m−3), TMD is greater than TF. The geothermal heat flux will warm the bottom waters to temperatures above TF thereby increasing its density toward that of TMD, and this will result in a stable, density-stratified water column. This stratification pattern is commonly observed in ice-covered freshwater lakes (e.g., Anguissaq Lake, Greenland, beneath 3 m of perennial ice; from Hobbie 1973) where the inverse temperature gradient is maintained by heat losses from the top of the water column to the overlying ice and atmosphere. A large number of the subglacial lakes detected so far have ice thicknesses less than 3170 m and could be stratified; however many have much thicker ice, including Lake Vostok. In this class of freshwater subglacial lakes, TMD is less than TF. Geothermal heating of the bottom water will decrease its density and result in buoyant turbulent plumes. For a freshwater column in Lake Vostok, Wüest and Carmack (2000) calculated that the vertical velocities within these convective plumes would be around 0.3 mm s−1, which would completely mix the water column over a period of days. Geothermal vents and localized hot spots in the lake would increase these rates of mixing substantially, and the input of gas bubbles and warm hydrothermal fluids would further amplify the degree of vertical circulation.
Density currents induced by differential heating and cooling are well known in temperate latitude lakes (e.g., Monismith et al. 1990) and have also been observed in ice-capped lakes caused by spatial variations in solar heating (Matthews and Heaney 1987). In subglacial lakes, horizontal differences in temperature and thus density could be induced by inflows, local variations in the geothermal heat flux, or any tilting of the ice ceiling over the lake. The latter effect has been modeled for Lake Vostok where there is a 460-m difference in ice thickness between the north and south ends of the lake, leading to an estimated 0.31°C difference in upper water temperatures along its 230-km length. Wüest and Carmack (2000) calculated that this would lead to pressure-driven horizontal flows of 0.3 to 0.6 mm s−1 (up to about 20 km per year), with the strength of this horizontal circulation dictated by heat fluxes at the ice-water interface, the extent of tilting of the ice, and Coriolis force. These calculations were subsequently extended using a numerical, three-dimensional ocean circulation model, which confirmed that weak basin-wide circulation would take place, with local variations in the velocity field and a strong influence of bathymetry (Williams 2001).
The same approach using the most accurate bathymetric data available for Lake Vostok predicted anticyclonic flow of about 4500 m3 s−1 in the northern and southern parts of the lake and a cyclonic gyre in its center with velocities of around 0.1 cm s−1 (Mayer et al. 2003). Modification of the model from freshwater to saline conditions showed that even low salinities (1.2°/oo, about 3.4 percent seawater) resulted in a stratified water column in which large vertical gradients in temperature could develop. The gain or loss of freshwater through melting and freeze-up at the ice-water interface under these saline conditions drove a more intense horizontal circulation pattern and reduced the horizontal turnover time scale from 20 years (freshwater conditions) to 11 years (Mayer et al. 2003).
Density currents can also be generated by localized high solute and particle concentrations. Salts and other materials are partially excluded from the ice during freeze-up (e.g., Belzile et al. 2002), and this can give rise to dense water at the ice-water interface that then sinks deeper into the water column.
This mechanism is well known in polar oceans, for example in the Siberian flaw polynyas where this ice exclusion process results in saline, organic-rich plumes that transport water from the surface to more than 2000-m depth in the Arctic Ocean (Dittmar 2004). This mechanism also contributes to thermohaline convection and the formation of hypersaline bottom waters in meromictic high-latitude lakes that experience large quantities of ice production each year (e.g., Ace Lake in the Vestfold Hills Antarctica [Gibson et al. 2000] and Romulus Lake in the Canadian High Arctic [Van Hove et al. 2006]).
Small-scale double diffusion cells caused by the two-order-of-magnitude difference in molecular diffusion rates for salt versus heat (conduction) can expand and extend over considerable depth. This process has attracted considerable interest in perennially ice-covered McMurdo Dry Valley lakes, particularly Lake Vanda where a staircase series of isohaline, isothermal convection cells up to 20 m thick occur in the upper water column, each cell sandwiched between layers of temperature and salinity gradients (Spigel and Priscu 1998). Radiotracer experiments on the largest of these cells showed that there were strong horizontal currents with velocities up to 1 cm s−1 accompanied by vertical turbulent mixing (Ragotzkie and Likens 1964).
Although this variety of hydrodynamic mechanisms is likely to disperse contaminants both vertically and horizontally, full homogeneous mixing throughout the water body seems unlikely for all but the smallest of subglacial lakes, and dilution of any contaminant may be much lower than the mixing ratio calculated for the total lake volume. Many of these mechanisms generate local variations in temperature, salinity, and mixing regimes, and the rate of dispersion will vary within as well as among subglacial water bodies.
Stratification of the water column by salinity gradients will greatly dampen vertical mixing processes. For example, some Arctic (Van Hove et al. 2006) and Antarctic (Spigel and Priscu 1998) ice-capped lakes have unusual temperature profiles, with deep thermal maxima that are well above their mean or even maximum summer air temperatures. These profiles are stabilized by strong salinity gradients, from freshwater beneath the ice to salinities at or above seawater at the bottom of the lake. Both the shape of the profiles and the magnitude of the thermal maxima can be simulated accurately by models that describe long-term (decades to millennia) solar heating in the absence of turbulent mixing (Shirtcliffe and Benseman 1964).
Despite the apparent stability of such density-stratified lakes, however, there is evidence of some vertical exchange. Studies on Lake Fryxell, an ice-covered, meromictic lake in the McMurdo Dry Valleys, have shown the presence of the atmospheric contaminant chloroflurocarbon-113 (CFC-113) even in the saline bottom waters of the lake, implying some transport to depth by convective mixing processes (Tyler et al. 1998).
Refrozen Ice
In 1999, deep ice core drilling at Vostok was halted at a depth of 3623 m, some 127 m above the upper surface of Lake Vostok. Below 3539 m, lake ice was encoun-
tered in the ice core and the scientific motivation for continued drilling and ice core analysis switched from climatology to limnology and microbiology. Airborne radar sounding surveys over Lake Vostok reveal that the lake surface is characterized by distinctive zones where ice melting or freeze-on dominate (Siegert et al. 2000; Bell et al. 2002; Tikku et al. 2004). These regions are thought to be related to the sloping ceiling of the lake and the associated pattern of water circulation (Wüest and Carmack 2000). It is entirely fortuitous that the Vostok core site is situated over a region where ice freeze-on dominates; at other sites, basal melting might have prevailed.
Freeze-on of lake water to the ice ceiling of subglacial lakes results in partitioning of water isotopes and chemical impurities between the solid and liquid phases. The heavy isotopes of hydrogen and oxygen are concentrated in the solid phase, whereas chemical impurities are concentrated in the lake. In contrast, the ice melting process is completely unselective: the isotopic and chemical composition of the meltwater is identical to that of melted ice.
The concentrations of particulates and solutes in the surface waters of Lake Vostok have been estimated from analyses of lake-derived accretion ice and from partition coefficients that express the ratio of concentration of each substance in the ice relative to water. During freeze-up, the crystal lattice of the ice expels and concentrates materials into the remaining water, and partition coefficients are therefore always less than 1. For Lake Vostok calculations, Christner et al. (2006) used the following values derived from measurements in ice and water in Lake Bonney, in the McMurdo Dry Valleys: 0.40 (particulate organic carbon), 0.56 (bacterial cell concentrations), 0.0021 (Na+), 0.022 (K+), 0.0026 (Ca2+), 0.0022 (Mg2+), 0.0023 (Cl−), and 0.00083 (SO42−). Larger solutes including complex organic molecules are excluded to a greater extent than smaller solutes (Belzile et al. 2002), and it is therefore puzzling that the coefficients for large particulates such as bacterial cells are so much higher than for solutes, suggesting that other processes are operating in the Bonney environment (e.g., bacterial growth in the ice) or that particulate incorporation is governed by more complex interactions during freeze-up than simple size exclusion (e.g., ice nucleation by POC and bacteria). The degree of freeze concentration increases with decreasing rates of freeze-up (Killawee et al. 1998): slow ice formation results in more efficient exclusion of materials. Christner et al. (2006) note that freeze-up in Lake Vostok is likely to be an order of magnitude slower than in Lake Bonney; therefore, application of the Bonney partition coefficients will result in potentially large underestimates of the dissolved and particulate concentrations that actually occur in the Vostok surface waters.
Inclusions
Somewhat surprisingly, mineral inclusions have been identified in the lake ice section of the Vostok core. Inclusions are likely to come mostly from the edges of the lake or from shoals (areas close to the pressure melting point). These inclusions are most highly concentrated in the upper portion of the accreted ice and appear to be absent below 3609 m (Jouzel et al. 1999). The ice flow trajectory in this region of Lake Vostok is south-southeast (Tikku et al. 2004), and the flow line that passes through the Vostok core site is presumed to traverse a shoal along the western shore of the lake where ice accretion is occurring. Toward the Vostok site, and farther from the shoreline, the freeze-on process is still active, but ice is afloat and no longer in mechanical contact with portions of the glacier bed.
SEDIMENT ENVIRONMENT
Seismic data have been used to estimate a 300-m thickness of sediments near Vostok Station (Popkov et al. 1998). The lake sediments could contain an unparalleled record of Antarctic paleoenvironmental information, extending well beyond the limit of ice core records.
Lake sediments enter subglacial systems by one of two primary pathways: (1) melt-out of ice-entrained sediment as glaciers melt at their base, or (2) “bulldozing” of basal sediment into the lake basin by subglacial sliding. Authigenic sedimentation could also be occurring in the lakes. Sediment delivered by the first mechanism may contain refrozen basal sediment and atmospheric fallout from the time the glacial ice formed, and it may also contain micrometeorites and cosmic dust (e.g., interplanetary dust particles and cometary debris).
From a stratigraphic and geochronological perspective, the sediment of subglacial lakes is very different from sediments previously studied in lakes and oceans. A complication of inferring paleoclimate from Lake Vostok sediments is that any atmospheric input to the lake is delayed by hundreds of thousands of years, possibly up to 1 million years, which is the expected maximum age of basal ice (Siegert et al. 2001). The length of this delay will vary with internal ice sheet dynamics, and the contributions from the different sediment sources will be on very different time scales. Certainly particle-size-specific analysis will play a key role, since we would expect the atmospheric contribution to be very fine-grained in comparison to the sediments generated through bulldozing of basal sediments.
Because of the unique depositional pathways of subglacial lake sediments, most standard geochronological techniques cannot be used in these environments. If the goal is to establish when given layers in a sediment profile were laid down, paleo-magnetism seems particularly well suited to this problem because as grains settle to the lake bottom, they will be aligned with the contemporary magnetic field. A more thorough consideration of the Lake Vostok sediment environment is given by Doran et al. (2004).
Sediment Flux and Rate of Reservoir Sedimentation
The rate of geologic transport of subglacial sediments into subglacial lakes determines the time required to fill the lake with sediment (hence a limit on its lifetime). Additionally, the comparative importance of the influx of chemicals and microbes to subglacial lakes by subglacial sediment transport and the influx, by melting, require assessment. The situation will vary from lake to lake, but Lake Vostok can be taken as the type example. For rough calculations, take the long axis of Lake Vostok to be 250 km, the volume 5000 km3, and the surface ice flow rate transverse to the long axis 4 m per year. If this surface ice flow rate is attributed entirely to the shear deformation of a 1-m-thick layer of subglacial sediment (as opposed to shear deformation of the overlying ice column, which is the more likely case), the sediment flux into the lake is 0.012 m3 s−1 and the sediment filling time for the reservoir is 13 million years. Most glaciologists would view the assumed deformation rate and layer thickness to be wildly excessive, even as upper limits, and a maximum sediment filling time of the order to 109 years is probably more realistic.
An alternative mode of sediment transport is as suspended sediment in water flowing into the reservoir. For Lake Vostok the magnitude of this water discharge has been estimated as roughly 1 m3 s−1, and most or all of this is attributed to roof melting rather than in-flow from the surroundings. If the flow was from the surroundings and the concentration of suspended sediment was 0.001 (by volume), the rate of sediment influx to the reservoir would be 0.001 m3 s−1, giving a sediment filling time of roughly 150 million years. Higher concentrations of suspended sediment would be associated with episodic flood filling of the reservoir. This is an unlikely scenario for Lake Vostok, but it could apply to some Antarctic subglacial lakes.
Sediments and Paleoclimatic Records
At present, the paleoclimate and ice sheet history of Antarctica is being assessed by the analysis of cores recovered at the edges of the continent because only a few boreholes have accessed the beds beneath the ice sheets (Figure 1.4). The sediment records contained in subglacial aquatic environments, however, may provide a muchneeded detailed paleoenvironmental record from the interior of the ice sheet. It may be a challenge to decipher these records if the sediments contained in these subglacial aquatic environments are a mixture of materials that are deposited on different time scales or if the stratigraphic chronologies are disturbed or destroyed.
Geological proxies will have to be identified to decipher subglacial sedimentary processes within sediment cores to ensure that changes are recognized; methods must be developed to determine the maximum age of subglacial aquatic environments; and chronological methods are needed to date the sediments within these environments. Biological and chemical proxies will also have to be developed to help interpret the timing of paleoenvironmental and climate changes contained in the sedimentary records (SCAR SALE 2006).
If a mechanism exists for protecting the sediments from the overriding glacier—for example, the formation of perennially ice-sealed lakes such as those found in the McMurdo Dry Valleys (Doran et al. 2003, 2004),—the sediment records from subglacial lakes may provide an unparalleled record of Antarctic paleoenvironmental information, extending well beyond the limit of ice core records. Most subglacial lakes are relatively small and fairly shallow, but these shorter sediment records will provide valuable information about subglacial lakes. Longer records, however, may provide the key to understanding the origins of subglacial aquatic environments because this information may be at the bottom of sedimentary sequences (SCAR SALE 2006). Seismic data from Vostok Station (Popkov et al. 1998) indicate that approximately 300 m of sediments may be contained in the southern end of Lake Vostok. The lake sediments could contain a high-resolution record of conditions that existed before the continent was covered by ice.
The information in long sedimentary cores that may be recovered from deep environments such as Lake Vostok may provide a mechanism to constrain the preglacial to subglacial transition (SCAR SALE 2006). If we are to understand how life is expressed over time in these environments (SCAR SCALE 2006), it will be important to assess whether these environments persist during both glacial and interglacial periods or whether they are unstable and drain, creating “cessation” events. These records may help fill gaps in the history of Antarctica’s interior and the evolution of ice sheets and help us understand the role that the Antarctic interior played in the glacial and
paleoclimatic history of the Antarctic continental margins and the Earth throughout the Cenozoic (65 million to 2 million years ago). From a biological standpoint, these cores may provide information about the response of microbiological systems to climate change and illuminate the telltale signs of microbial evolution in icy environments, which could be applicable to the search for life in the solar system (SCAR SALE 2006).
GASES
Subglacial water is derived from glacial ice, which contains air trapped as the ice was being formed. As ice gets progressively buried, pressure in the ice increases proportional to the weight of the overburden. At depths of about ~1000 m, the trapped air and ice form a solid substance known as air clathrate hydrate. However, air hydrate is only stable in lakes below 1.5 km due the differences between its stability with respect to ice and bubbles and its stability with respect to water and dissolved gas (Lipenkov and Istomin 2001).
Results from the Vostok ice core show gas levels on the order of 0.8 to 1.0 L kg−1 of ice in glacial ice above Lake Vostok and levels near zero in the accretion ice (Jouzel et al. 1999; Souchez et al. 2003). A possible explanation for this lack of gas is that it is excluded during accretion ice formation. Given a closed system where glacial ice forms subglacial meltwater and contributes its full gas load, while accretion ice excludes gases, we would expect gases to build in the lake over time. McKay et al. (2003) show that this buildup would create a hypercharged water column with respect to atmospheric gases. For lakes at depths less than 1.5 km, the gas pressure builds up to the hydrostatic pressure and further gas goes into bubbles. For lakes deeper than 1.5 km, clathrate forms and the dissolved gas levels off at a concentration of ~2.5 L of gas per kilogram of lake water regardless of depth (Figure 2.13). At this level, any further input of gas would accumulate as clathrate. By way of comparison, the gas concentration in an unopened can of soda is about 3 L kg−1, and the gas content of Lake Nyos, which in 1986 catastrophically degassed killing 1700 people, is 2-4 L kg−1 (Kling et al. 1987). For Lake Vostok, the most significant impact of such a high gas pressure may be that this will create a hyperoxic environment, with oxygen levels ~50 times saturation (McKay et al. 2003).
Some subglacial lake sediments may contain air clathrate hydrates that provide a long-term record of atmospheric gas. As the glacier ice melts, air clathrate hydrate should remain stable. Air hydrate has an equilibrium density between 0.980 and 1.025 g cm−3 (Uchida and Hondoh 2000), and Lake Vostok is estimated to have an average water density of 1.016 ± 0.001 g m cm−3 (Wuest and Carmack 2000). Given these densities we would expect some hydrates to float and some to sink, but circulation may also cause hydrates to be kept in suspension (Lipenkov and Istomin 2001). If conditions are suitable, natural gas hydrates could also accumulate in situ.
The implications of potential high gas pressures on access to subglacial aquatic environments are largely dependent on the depth of the water body. Gas pressure cannot exceed hydrostatic pressure in subglacial lakes. However, if water is allowed to rise in a hole to depths shallower than 1500 m, or if lakes shallower than these depths are sampled, bubble formation can occur, potentially causing rapid degassing of these lakes.
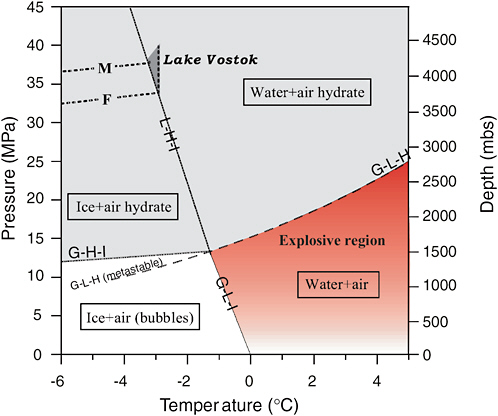
FIGURE 2.13 Phase diagram for air clathrate hydrate near 0°C. Dotted curves show the relations between temperature and pressure (depth) in the Antarctic ice sheet and sub-ice water at the reference sites M and F located in the zones of subglacial melting and freezing, respectively. Gray regions show fields of air hydrate stability. Small darkened triangle within this field covers the range of in situ conditions in Lake Vostok. The red shaded area is the area where if liquid water is present, explosive degassing could occur upon drilling into the water. Water from below allowed to rise into this zone could also explosively degas. The intensity of the red indicates the level of degassing risk. SOURCE: Lipenkov, V. Y. and Istomin, V. A., On the stability of air clathrate-hydrate crystals in subglacial Lake Vostok, Antarctica, Materialy Glyatsiologicheskikh Issledovanii, 91: 138-149.
If Lake Vostok contains air clathrate hydrate in equilibrium with water, why are clathrates not found in the accretion ice? Based on laboratory studies, air clathrate has been found to have an equilibrium density between 980 and 1025 kg m−3 depending on the cage filling (occupancy) and the gas composition (Uchida and Hondoh 2000). McKay et al. (2003) show theoretically how clathrate density could increase to 1080 kg m−3 with a clathrate composition of 10 percent CO2. In comparison, Lake Vostok water density is 1018 kg m−3 (Wuest and Carmack 2000), so we would expect
some clathrates to sink and others to float depending on gas composition. The absence of clathrates in the accretion ice may be related to a dominance of heavier clathrate through CO2 incorporation (McKay et al. 2003) and/or floating clathrates not collecting in areas where accretion ice forms.
This discussion does not consider gases that may be accumulating in subglacial sediments since we know nothing about these environments. However, on a continent the size of Antarctica there are bound to be areas where, for example, methane hydrates accumulate. Methane hydrate has a relatively low density but accumulates on the ocean bottom attached to sediments. In the ocean, any methane hydrate allowed to float free will dissociate en route to the surface. In a subglacial lake, besides accumulating in the sediment, methane hydrate could also accumulate at the underside of the ice as long as the pressure-temperature state remains below dissociation limits. The dissociation pressure of methane clathrate is about 26 atmospheres and the clathrate would remain stable in Lake Vostok (Miller 1974).
It is the refreezing process, not bottom melting, that leads to concentration of atmospheric gases in subglacial water. In situations where there is melting but not refreezing, the gas concentration in subglacial water will approximate that of glacial ice and hyperconcentration is not an issue. For Lake Vostok, there is strong evidence that freeze-on is an important process (e.g., Jouzel et al. 1999; Siegert et al. 2000; Bell et al. 2002; Tikku et al. 2004), but it does not necessarily follow that gases are hyperconcentrated in the lake. If the lake behaves as an open hydrologic system, receiving water from an upstream catchment and spilling it continuously or episodically to a downstream flow network, the residence time for water in the lake could be short enough to minimize the concentrating effects of refreezing. At present there is no information on through-flow of water for Lake Vostok, so the parsimonious—though not necessarily correct—assumption is that through-flow can be neglected.
CONCLUSIONS
The conditions of topographic slope that favor the formation of subglacial lakes are far more stringent than those that apply to subaerial lakes. Large lakes such as Lake Vostok lie in steep-sided physiographic basins that, unless recently formed, would have existed before the ice sheet and been even more effective as subaerial water traps. Thus, it is highly likely that large Antarctic subglacial lakes existed preglacially (some 35 million years ago) as subaerial lakes, that unless scoured during glaciation, should contain preglacial sediment. Continuous existence of these aquatic environments, from before the onset of glaciation to the present day, is much less likely than the presence of sediments. Seismic data have been used to estimate a 300-m thickness of sediments near Vostok Station. The lake sediments could contain an unparalleled record of Antarctic paleoenvironmental information, extending well beyond the limit of ice core records.
Most Antarctic subglacial lakes were detected by airborne radar sounding. The largest of these have a clear surface expression, while smaller lakes have a less obvious surface trace. A high-resolution digital elevation model, drawing on a variety of sources, has been produced for the entire ice sheet (Liu et al. 1999) and it seems very unlikely that any Vostok-sized lakes remain undiscovered.
Because of the dependence of ice melting temperature on pressure, the basal melting temperature under large areas of the ice sheet is at or below −2°C. Much of the base of the Antarctic ice sheet is at the melting point, and for the ice sheet as a whole, net
basal melting exceeds net basal freezing. One result of basal melting is excess meltwater production beneath the ice sheet. Of necessity, a drainage system must form to transfer excess water from the ice sheet interior toward the periphery to regions of water storage or removal. Evidence for subglacial water discharge events comes from satellite detection of rapid (month- to year-scale) changes in the ice surface elevation of West Antarctic ice streams and above a known subglacial lake in central East Antarctica. Streams could form in meltwater channels that occur irregularly at the bottom of the ice sheet or could flow within layers of rocks or fractured bedrock that resemble the hyporheic flow paths beneath the beds of most rivers and streams. Most subglacial aquatic environments are likely to be part of an extensive subglacial drainage system rather than being hydrologically isolated. The flow of subglacial water is guided by the topography of the ice sheet surface and, to a lesser extent, the topography of the subglacial bed; together these topographic influences subdivide the Antarctic ice sheet into discrete drainage basins. Although the subglacial water system is likely to be interconnected, either continuously or sporadically, not every part of the water system is connected to every other part. The most plausible conclusion is that subglacial aquatic environments are simply one component of a subglacial drainage system that is organized into hydrologic catchments, analogous to those that occur subaerially. However, a basin-scale analysis of hydrologic catchments is necessary before lake-to-lake interactions can be critically examined.
The circulation of subglacial lakes is driven by small gradients in the potential energy of lake water that can result from vertical or lateral gradients in water density. This density depends on the pressure, temperature, and salinity of water. Of the three density dependents, the salinity of the subglacial aquatic environments is least constrained. Measurements of Vostok accretion ice and modeling results provide evidence for low-salinity lake water. However, if one temperature extrapolation is correct, higher-salinity water is inferred because increased salinity depresses the temperature of freezing. The low-salinity hypothesis also raises questions about the residence time of lake water and how low salinity can be maintained. The question of the salinity of subglacial lakes has not yet been answered definitively.
An important contribution to the circulation of Lake Vostok is associated with the sloping ice ceiling of the lake. Although the ceiling slope is not great, it would be much less if ice flow were aligned with the long axis of the lake. For many subglacial lakes, the ceiling may be nearly flat and this contribution to lake circulation could become small or negligible.
Glacial ice is formed by compression of surface snowfall. During the transformation from snow to ice, air trapped in the snow pack becomes entombed as bubbles within solid ice. As the depth of burial increases, these bubbles disappear and atmospheric gases are stored in solid form as air hydrates. The concentration of air in Antarctic glacial ice is estimated at 90 cm3 (at standard temperature and pressure [STP]) per kilogram of ice (McKay 2003); this concentration of air far exceeds the concentration of air-saturated water under the same conditions. Thus, subglacial meltwater is more highly concentrated in the dominant air gases (N2 and O2) than subaerial water. Freezing of meltwater concentrates chemical impurities in the liquid phase and, in subglacial lakes, could result in hyperconcentration of gases such as oxygen. It is not clear whether microbes are affected by these high concentrations. Chemically, subglacial aquatic environments can be expected to vary widely from site to site, and the complete
absence of viable microbes cannot be excluded. Extreme oligotrophic environments are themselves unusual and scientifically interesting.
Ice at the base of the Antarctic ice sheet is not older than 1-2 million years. Thus the maximum separation time between ice deposited at the surface as snowfall and ice melting at the base of the ice sheet is 2 million years. Microbes deposited at the surface of the ice sheet 1-2 million years in the past are, at present, inoculating the subglacial aquatic environment. These aquatic systems are likely to contain materials, derived from the ice and possibly from bedrock and preglacial sediments that, despite the high pressure and low temperature, would allow slow growth of some microbes. A full discussion of the potential biological environment is presented in Chapter 3.
There is considerable uncertainty in the values of the coefficients that govern the partitioning of chemical and biological constituents of subglacial aquatic environments during freeze-on of ice accreting at the bottom of the ice sheet. This uncertainty makes it challenging to determine the chemical and microbial concentrations of water by analyzing chemical and microbial concentrations in ice accreted at the lake surface.
It is possible that contaminants will be introduced into subglacial aquatic environments during exploration and sampling. If these environments constitute a subglacial drainage system that is organized into hydrologic catchments, then potential downstream impacts must be taken into account; contamination introduced near the headwaters of a drainage system will have greater impact than the same level of contamination at sites farther downstream. However, the volume associated with each subglacial environment and the decreased residence times that result from subglacial connectivity may help reduce the impact of contamination.