4
Consumer Involvement in Reporting Adverse Events
Recent Food and Drug Administration (FDA) recalls have adversely affected public perception of the pharmaceutical industry and the system for regulating drug safety. According to a 2005 poll of consumers, 43 percent of surveyed adults felt that the pharmaceutical industry did a “bad job” serving its consumers (Supermarkets, 2005). Although consumers have a great incentive to report adverse events, there are few efforts to engage them more actively in the process, and there is no comprehensive system in place for consumer reporting of adverse events in an outpatient setting. Workshop participants emphasized the need for increased consumer and advocate involvement in reporting events and ways in which this can be achieved. According to Forum member Michael Katz, who presented on behalf of the International Myeloma Foundation (IMF), advocacy organizations can take the initiative to quickly deliver meaningful drug safety data. The IMF began receiving anecdotal reports through its hotlines and e-mail list about elevated creatine levels associated with the use of zoledromic acid. Zoledromic acid had been approved for use in the prevention of bone mineral loss in a variety of specific clinical settings when administered by a 15-minute infusion; there was no warning about potential kidney toxicity in the label, but experience suggested that this could exist if proper infusion approaches were not used. The IMF helped advise patients about safe use of the medication based on clinical experience and the problem diminished. “In fact, the manufacturer is now publishing studies showing that there is now no elevated
kidney toxicity risk. In all likelihood, this is a direct result of the change in clinical practice,” Mr. Katz said.
In 2004, IMF again became involved in patient education about potential adverse events associated with zoledromic acid after receiving notice of a high incidence of osteonecrosis of the jaw in patients taking the drug. A web-based survey of myeloma and breast cancer patients conducted in July 2004 by the IMF identified a time-dependent risk related to the drug (Durie et al., 2005).
Public education programs that raise public awareness and communicate tangible public benefit of improved adverse reporting systems are ways in which patient advocacy groups and even patients themselves can play a role in reducing adverse drug events (ADEs). The development of an improved infrastructure for consumer reporting will benefit from the increased input of those that the system was designed to serve.
CONSUMER INVOLVEMENT IN THE CURRENT MEDWATCH SYSTEM
MedWatch captures only a fraction of the adverse events that occur, leaving the total burden unknown. Alison Rein of the National Consumers League concluded that this is due, in part, to a lack of meaningful consumer engagement in this process and the fact that reporting mechanisms are divorced from routine practice. Dr. Marvin Lipman of Consumers Union stated: “For consumers to play a role, they need to be made aware of the importance of reporting adverse drug effects, not only to their physician and pharmacist, but also to a central body, the FDA.” Ms. Rein reported that MedWatch is not on the radar of most consumers and is not integrated into the health-care delivery system. She compared patient reporting within the current MedWatch system to the United Kingdom’s new yellow card system (see Table 4-1). This system is managed by the Medicines and Healthcare Products Regulatory Agency (MHRA) and performs safety surveillance. Each report, which is actually a yellow card, is acknowledged and registered upon receipt and then entered into MHRA’s Adverse Drug Reactions Database. Reports are assessed by health-care professionals in the Pharmacovigilance Group of the MHRA Post Licensing Division. This assessment includes the use of data from other sources such as pre- and postclinical trials, case reports in the medical literature, data from other drug regulatory agencies, epidemiological studies, and record linkage databases. The Committee on Safety of Medicines and its Subcommittee on Pharmacovigilance advise MHRA on potential safety issues and appropriate regulatory actions.
The yellow card system allows consumers to report online, by prepaid mail, and by phone. Translation services are available for those who are
TABLE 4-1 Comparison of Consumer Reporting Systems
not proficient in English and thus may have difficulty completing the forms. Although MedWatch also can be accessed by the Internet, mail upon request, or telephone, Ms. Rein reported that when she tried to use the 1-800 number provided to consumers, she found the service difficult to navigate. She noted in particular that the terminology used on written forms and by the telephone service is above the level of health literacy of the average consumer with no medical background.
The yellow card system actively seeks reports of events associated with over-the-counter medications, vitamins, and herbal supplements in addition to prescribed medications, while the MedWatch system deals only with prescription drugs. Ms. Rein pointed out that the yellow card form is designed for consumers, not just for health-care professionals. However, the MHRA recommends that consumer reports be validated by a health-care professional (MHRA, 2005). Ms. Rein reported that the system can be accessed in some form in almost any relevant care delivery setting, such as pharmacies and physician offices. She viewed the yellow card’s user-friendliness and wide availability as encouraging patients to engage in safety surveillance by completing the form on their own. With MedWatch, there is no consumer interface, and all users receive the same form. The system encourages physicians to complete the written forms by instructing patients to fill the form out with their doctor. Ms. Rein sees this as discouraging patients from completing the forms themselves.
The MedWatch system collects data in fewer fields than the yellow card system. Ms. Rein believes that it would be helpful in MedWatch to have more data initially available and have the ability to narrow down
as analysis dictates, rather than collecting too few data to be able to run a desired analysis.
The MedWatch program in its current form has several challenges as also noted by Dr. Lipman. Its minimal staff is responsible for capturing events associated not only with drugs, but with devices as well. MedWatch is also set up to focus on potentially lethal events, not the more frequent minor events that can still significantly decrease patients’ quality of life. Minor events are not dismissed, but they are not the focus of analysis. A key to making the system more functional will be to define which data are most useful and to determine how best to engage consumers.
OUTREACH PROGRAMS AND TOOLS
Dr. Lipman reported that adverse events are estimated to occur in as many as 20 percent of patients taking medications, totaling more than 4 million events per year. Many adverse events are not life threatening, but they may cause serious physical or mental distress. Dr. Lipman noted that consumers are the individuals who are most motivated to report adverse events, because they have the most at stake. Engaging consumers to work with their health-care providers would be an important way to “connect the dots” and make sure all necessary information is transmitted to the FDA. As Mr. Katz pointed out, patients have as much to lose by misinformation as by oversensitivity to drug safety. “It’s very scary to think about adverse events that could happen to you as a patient. But when you’re in a space with an incurable disease … it’s even scarier to think that drugs will be taken off the market on a wholesale basis, or that it will be harder to gain approval for new drugs that could help people,” he added.
Engaging patients can result in a much richer understanding of drug effects. The cause of a poor health outcome is difficult to determine. Where does the course of the disease end, and where do the effects of the drug begin? The system for detecting adverse events is full of noise, and sometimes a true signal is difficult to discern. Patient advocates and the FDA could work together to eliminate some of this noise. A role for advocacy groups would be to provide reporting assistance and help patients understand what should be reported, said Karen Cox of University of Missouri Health Care. Dr. Cox discussed how consumers interact with the University of Missouri Health Care system. She indicated that consumers have been successful in using the online health system web page to enter their compliments or complaints.
Mr. Katz called for the development of outreach programs for patients to share their collective experiences with each other and with those who develop health-care policy. Consumer groups and patient advocacy organizations have begun to take steps toward providing consumers with
more information about drugs and their potential adverse effects. Dr. Lipman cited a public outreach project launched by Consumers Union in 2004 as a step toward providing consumers with reliable information about drug safety. Consumers Union initiated the Best Buy Drugs website (www.crbestbuydrugs.org) to educate consumers about their medications, specifically the drugs that give the best value. The website consists of monographs examining ACE (angiotensin-converting enzyme) inhibitors, antidepressants, antihistamines, drugs for attention deficit hyperactivity disorder, beta-blockers, calcium channel blockers, proton pump inhibitors, and nonsteroidal anti-inflammatories. The monographs follow the methodology of the Oregon state program—the Drug Effectiveness Review Project. Fourteen state Medicaid programs have adopted these reviews. The website also provides information about diseases and disorders and common side effects for drugs in each class.
The Internet has provided a very powerful tool for consumers to find and share information via e-mail lists, support groups, and networking. The Consumer Reports website (www.consumerreports.org) alone has more than 2 million active subscribers, said Dr. Lipman. The greatest strength of the Internet as a communication vehicle is that it is a free medium that many people can access easily. Dr. Cox reported that University of Missouri Health Care receives 24 percent of its patient compliments and complaints via the Internet (with no advertising) (see Figure 4-1). In addition to the Internet, patient-initiated reports come from a variety of different sources such as phone calls, letters, e-mails, and patient satisfaction surveys.
POSTMARKETING SURVEILLANCE AND DIRECT-TO-CONSUMER ADVERTISING
Mr. Katz provided a description of the drug approval system (see Figure 4-2). In the preapproval stage, drugs are assumed to be harmful until they are proven safe at reasonable dosages. However, there is a paradigm shift in the way drug safety is considered after approval: drugs are assumed safe once approved. Drug companies have limited incentives to conduct postmarketing safety studies because evidence against a product would necessitate labeling changes or even withdrawal from the market. In Mr. Katz’s opinion, the current spontaneous reporting-based system is too slow to be relied upon for accurate, up-to-date postmarketing safety surveillance.
Direct-to-consumer (DTC) advertising continues to play an influential role in consumers’ medication use. It can stimulate consumers’ discussion with physicians regarding medical conditions, but it can also elevate the use of products in circumstances that could be inappropriate. There
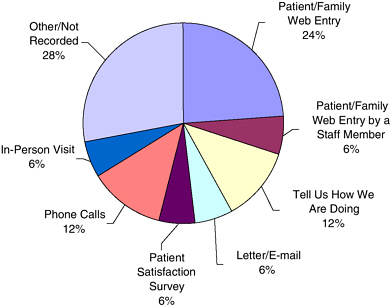
FIGURE 4-1 Patient-initiated comments (compliments and complaints) by source in University of Missouri Health Care (n = 6,661 over 45 months).
SOURCE: Karen Cox, workshop presentation.
has been widespread discussion about tighter regulation of the content of these ads. Currently, the United States is the only country other than New Zealand that allows them. Mr. Katz noted that DTC ads should contain reference to a consumer portal, such as MedWatch, for adverse event reporting.
ROLE OF ADVOCACY GROUPS
Advocacy groups can represent an intermediary between the public and the research community. They can work with clinicians and scientists to identify drug risks and preemptively address them by promoting safer drugs. Advocates can also use their networking capabilities to get information out to the public. To improve consumer involvement in reporting adverse events, advocacy groups could direct their resources to consumer education. Dr. Lipman recommended that mechanisms for the delivery of educational content could consist of point-of-sale drug information leaflets, consumer representatives or FDA advisory panels, and a clinical trials registry system that reports both positive and negative results of
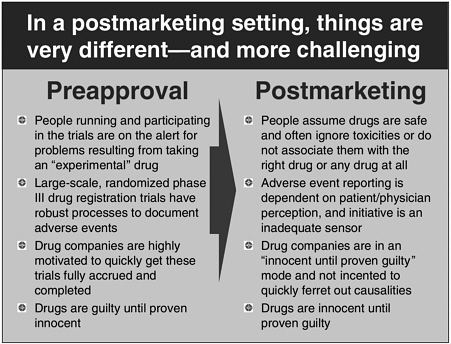
FIGURE 4-2 Comparing pre- and postmarketing paradigms.
SOURCE: Michael Katz, workshop presentation.
drug studies. Marketing success stories of change driven by consumer reports may boost consumer confidence in the regulatory and safety surveillance system.
Advocacy group participation would help protect particularly vulnerable patient populations, such as young children and the elderly. More than twice as many prescriptions were filled for those 65 and older (23.5 prescriptions per year) than for those younger than 65 (10.1 prescriptions per year) (Stagnitti, 2004). A greater number of medications taken by a patient increases the likelihood of an adverse event occurring. There has been concern that drugs for seniors are often not appropriately prescribed. One study found that as many as 21.3 percent of community-dwelling patients 65 years or older were using at least one inappropriately prescribed drug (Liu and Christensen, 2002). Inappropriate prescribing of drugs in this age group, specifically for women, has led to concern for patient safety and appropriate utilization of health-care resources. In light of the recent concern about an increased risk of cardiovascular events associated with the cyclooxygenase-2 (COX-2) inhibitor drugs, studies showing an increase in the prescribing of COX-2 drugs for patients who would be appropri-
ate candidates for nonsteroidal anti-inflammatory drugs are especially relevant (Goulding, 2004). The issue of over- or underprescribing medications for the elderly requires greater scrutiny in order to prevent potential adverse events and to promote better health and appropriate utilization of health-care resources.
POTENTIAL SOLUTIONS AND NEXT STEPS
Solutions to reporting issues may not necessarily have to be high tech and can build upon existing mechanisms for reporting. Ms. Rein suggested looking to successful reporting programs, such as the United Kingdom’s yellow card system, for models of how to provide consumers with multiple avenues for reporting. Ms. Rein believed that the MedWatch system needs to be improved but is still a valuable surveillance tool. “We need to work to improve visibility of MedWatch by integrating reporting into the health-care delivery system,” she said. Ms. Rein suggested public service announcements and direct-to-consumer advertising as possible ways to increase MedWatch visibility, as well as distributing the form (and/or access information) to patients in relevant clinical care settings. User accessibility could be improved by establishing separate web and telephone interfaces to provide consumers with multiple avenues for reporting events. Beyond MedWatch, Ms. Rein indicated that adverse event reporting should be a central element in electronic prescribing and medication management systems. Other avenues include technical support for training in ADE recognition and reporting and reimbursement policies that create incentives for ADE reporting; communication channels between doctors and patients and between patients and the FDA could be enhanced. Electronic resources could be further developed for disseminating safety and reporting information.
Dr. Lipman suggested next steps that would benefit consumers. The first is the formation of a drug safety oversight board with its own regulatory power, comprising consumer representatives and scientists with no industry ties or involvement in the approval process. Second, a clinical trials registry should be established and monitored. Both positive and negative trial results should be posted in a public forum. Third, DTC ads could be regulated with respect to content and subject to a two- to three-year moratorium after a drug is marketed. “To counter pharmaceutical ads, the FDA itself could launch a program of public service advertisements about drug safety, adverse drug reaction reporting, and the importance of postmarketing surveillance,” said Dr. Lipman.








