APPENDIX C
Plan for a Short-term Evaluation of PEPFAR Implementation
Letter Report # 1
Committee on the President’s Emergency Plan for AIDS Relief (PEPFAR) Implementation Evaluation
Board on Global Health
Board on Children, Youth, and Families
INSTITUTE OF MEDICINE OF THE NATIONAL ACADEMIES
THE NATIONAL ACADEMIES PRESS
Washington, D.C.
THE NATIONAL ACADEMIES PRESS 500 Fifth Street, N.W. Washington, DC 20001
NOTICE: The project that is the subject of this report was approved by the Governing Board of the National Research Council, whose members are drawn from the councils of the National Academy of Sciences, the National Academy of Engineering, and the Institute of Medicine. The members of the committee responsible for the report were chosen for their special competences and with regard for appropriate balance.
This study was supported by Contract No. SAQMPD04C1166 between the National Academy of Sciences and the U.S. Department of State. Any opinions, findings, conclusions, or recommendations expressed in this publication are those of the author(s) and do not necessarily reflect the view of the organizations or agencies that provided support for this project.
Additional copies of this report are available in limited quantities from the Committee for the Evaluation of PEPFAR Implementation, Board on Global Health, Institute of Medicine, 500 Fifth Street, N.W., Washington, DC 20001. The full text is available online at http://www.nap.edu.
For more information about the Institute of Medicine, visit the IOM home page at: www.iom.edu.
Copyright 2006 by the National Academy of Sciences. All rights reserved.
Printed in the United States of America.
The serpent has been a symbol of long life, healing, and knowledge among almost all cultures and religions since the beginning of recorded history. The serpent adopted as a logotype by the Institute of Medicine is a relief carving from ancient Greece, now held by the Staatliche Museen in Berlin.
THE NATIONAL ACADEMIES
Advisers to the Nation on Science, Engineering, and Medicine
The National Academy of Sciences is a private, nonprofit, self-perpetuating society of distinguished scholars engaged in scientific and engineering research, dedicated to the furtherance of science and technology and to their use for the general welfare. Upon the authority of the charter granted to it by the Congress in 1863, the Academy has a mandate that requires it to advise the federal government on scientific and technical matters. Dr. Ralph J. Cicerone is president of the National Academy of Sciences.
The National Academy of Engineering was established in 1964, under the charter of the National Academy of Sciences, as a parallel organization of outstanding engineers. It is autonomous in its administration and in the selection of its members, sharing with the National Academy of Sciences the responsibility for advising the federal government. The National Academy of Engineering also sponsors engineering programs aimed at meeting national needs, encourages education and research, and recognizes the superior achievements of engineers. Dr. Wm. A. Wulf is president of the National Academy of Engineering.
The Institute of Medicine was established in 1970 by the National Academy of Sciences to secure the services of eminent members of appropriate professions in the examination of policy matters pertaining to the health of the public. The Institute acts under the responsibility given to the National Academy of Sciences by its congressional charter to be an adviser to the federal government and, upon its own initiative, to identify issues of medical care, research, and education. Dr. Harvey V. Fineberg is president of the Institute of Medicine.
The National Research Council was organized by the National Academy of Sciences in 1916 to associate the broad community of science and technology with the Academy’s purposes of furthering knowledge and advising the federal government. Functioning in accordance with general policies determined by the Academy, the Council has become the principal operating agency of both the National Academy of Sciences and the National Academy of Engineering in providing services to the government, the public, and the scientific and engineering communities. The Council is administered jointly by both Academies and the Institute of Medicine. Dr. Ralph J. Cicerone, and Dr. Wm. A. Wulf are chair and vice chair, respectively, of the National Research Council.
COMMITTEE FOR THE EVALUATION OF PEPFAR IMPLEMENTATION
JAIME SEPÜLVEDA AMOR (Chair), Director General, National Institutes of Health, Mexico
HELEN SMITS (Vice Chair), Former Faculty of Medicine, Eduardo Mondlane University, Mozambique
CHARLES CARPENTER (Treatment Subcommittee Chair), Professor of Medicine, Bio Med Medicine, Miriam Hospital, Brown University, Providence, Rhode Island
JAMES CURRAN (Prevention Subcommittee Chair), Dean, Professor of Epidemiology, Rollins School of Public Health, Emory University, Atlanta, Georgia
WILLIAM L. HOLZEMER (Care Subcommittee Chair), Professor of Nursing and Associate Dean, International Programs, School of Nursing, University of California, San Francisco
STEFANO BERTOZZI, Director of Health Economics, National Institutes of Health, Mexico
GEOFF GARNETT, Professor of Microparasite Epidemiology, Faculty of Medicine, Imperial College, London, United Kingdom
RUTH MACKLIN, Head, Division of Philosophy and History of Medicine, Department of Epidemiology and Population Health, Albert Einstein College of Medicine, New York
AFFETTE McCAW-BINNS, Professor, Reproductive Health Epidemiology, Section of Community Health, University of the West Indies, Jamaica
A. DAVID PALTIEL, Associate Professor and Head, Division of Health Policy and Administration, Yale University, New Haven, Connecticut
PRISCILLA REDDY, Director, Health Promotion Research and Development, National Health Promotion & Behavioural Intervention Research Unit, Medical Research Council of South Africa
DAVID ROSS, Director, Public Health Informatics Institute, Decatur, Georgia
HEATHER WEISS, Director, Harvard Family Research Project, Harvard University, Boston, Massachusetts
Subcommittee Members, Liaisons, and Study Consultants
MAUREEN BLACK, John A. Scholl Professor of Pediatrics, University of Maryland School of Medicine, Baltimore
HOOSEN COOVADIA, Victor Daitz Professor of HIV/AIDS Research, Centre for the AIDS Programme of Research, South Africa
HENRY FOMUNDAM, Acting Director, National HIV and AIDS Programme, MEDUNSA Pharmacovigilance Centre, South Africa
PAUL GERTLER, Chief Economist, Human Development Network, World Bank, Washington, D.C.
CARL LATKIN, Professor, Department of Health, Behavior, and Society, Johns Hopkins University Bloomberg School of Public Health, Baltimore, Maryland
JAMES NTOZI, Professor, Department of Population Studies, Makerere University, Uganda
JAMES SHERRY, Vice President, Policy, Research and Advocacy, Global Health Council, Washington, D.C.
OLAITAN SOYANNWO, Professor of Anesthesia and Consultant Anesthetist, College of Medicine, University of Ibadan and University College Hospital, Ibadan, Nigeria
BURTON WILCKE, Jr., Chair and Associate Professor, Department of Biomedical Technologies, University of Vermont, Burlington
MICHAEL MERSON, (Board on Global Health Liaison), Anna M. R. Lauder Professor of Public Health, Yale School of Medicine, New Haven, Connecticut
ELENA O. NIGHTINGALE (Board on Children, Youth, and Families Liaison), Scholar-in-Residence, Institute of Medicine, The National Academies, Washington, D.C.
JULIA COFFMAN (Consultant), Independent Evaluation Consultant, Alexandria, Virginia
THOMAS N. DENNY (Consultant), Associate Professor Pathology, Laboratory Medicine, Associate Professor of Pediatrics, Associate Professor of Preventive Medicine and Community Health and Assistant Dean for Research in Health Policy UMD—New Jersey Medical School, New Jersey
FLORENCIA ZULBERTI (Consultant), Assistant Director for Global Health, National Institutes of Health, Mexico
Staff
PATRICK KELLEY, Director, Board on Global Health
ROSEMARY CHALK, Director, Board on Children, Youth, and Families
MICHELE ORZA, Study Director
ALICIA GABLE, Senior Program Officer (until June 2005)
KIMBERLY SCOTT, Senior Program Officer (from September 2005)
HEATHER COLVIN, Program Officer (until July 2005)
J. ALICE NIXON, Program Officer (from July 2005)
WEZI MUNTHALI, Research Associate (until October 2005)
ALYSON SCHWABER, Research Associate (until September 2005)
DIANNE STARE, Research Assistant (until September 2005)
ANGELA MENSAH, Senior Program Assistant (from June 2005)
KIMBERLY WEINGARTEN, Senior Program Assistant
ELIZABETH SHARP, Science and Technology Policy Fellow (January 2005 through April 2005)
SHARLENE BAGGA, Science and Technology Policy Fellow (June 2005 through August 2005)
CLAUDIA GROSSMAN, Science and Technology Policy Fellow (June 2005 through August 2005)
Reviewers
This report has been reviewed in draft form by individuals chosen for their diverse perspectives and technical expertise, in accordance with procedures approved by the NRC’s Report Review Committee. The purpose of this independent review is to provide candid and critical comments that will assist the institution in making its published report as sound as possible and to ensure that the report meets institutional standards for objectivity, evidence, and responsiveness to the study charge. The review comments and draft manuscript remain confidential to protect the integrity of the deliberative process. We wish to thank the following individuals for their review of this report:
Solomon R. Benatar, Professor of Medicine and Director, Bioethics Centre, Department of Medicine, Faculty of Health Sciences, University of Cape Town, Cape Town, Republic of South Africa
Thomas J. Coates, Professor, Department of Medicine, Division of Infectious Diseases, Prevention and Policy Research, David Geffen School of Medicine, University of California, Los Angeles, Los Angeles
Nils Daulaire, President and CEO, Global Health Council, White River Junction, Vermont
Anne Mills, Professor of Health Economics and Policy, London School of Hygiene and Tropical Medicine, London, United Kingdom
Philip Onyebujoh, Special Programme for Research and Training in Tropical Diseases, World Health Organization, Geneva, Switzerland
Mauro Schechter, Professor of Infectious Diseases, and Head, AIDS Research Laboratory, Hospital Universitario Clementino Fraga Filho Universidade, Rio de Janeiro, Brazil
Although the reviewers listed above have provided many constructive comments and suggestions, they were not asked to endorse the conclusions or recommendations nor did they see the final draft of the report before its release. The review of this report was overseen by Neal A. Vanselow. Appointed by the National Research Council and Institute of Medicine, he was responsible for making certain that an independent examination of this report was carried out in accordance with institutional procedures and that all review comments were carefully considered. Responsibility for the final content of this report rests entirely with the authoring committee and the institution.
Preface
Concern for the over 40 million people infected with HIV and others at risk of infection or otherwise affected through the impact on their families and communities moved the U.S. Congress on behalf of the American people to pass in May 2003 an unprecedented $15 billion international public health initiative—the U.S. Leadership against HIV/AIDS, Tuberculosis, and Malaria Act. With so much at stake from both a human and a fiscal perspective, Congress mandated that the Institute of Medicine review the groundbreaking initiative created by the legislation—the President’s Emergency Plan for AIDS Relief (PEPFAR). An independent, rigorous, multidisciplinary expert review of PEPFAR is in the best interests of the taxpayer, the scientific community, program implementers, and—most importantly—the people whose lives are in the balance.
The IOM’s legislative mandate to conduct an evaluation of PEPFAR is a complex challenge, in part because PEPFAR is effectively many programs in one. PEPFAR seeks to prevent seven million HIV infections, provide two million HIV-infected people with antiretroviral therapy, and care for ten million people affected by HIV/AIDS. These people live in fifteen different “focus countries,” most with limited health care system capacity for scale-up of HIV/AIDS-related services. Thus, our evaluation is of a multiplicity of programs that assume the characteristics and complexities of each of the focus countries.
The legislative mandate calls for our study of PEPFAR to be delivered at the three-year mark and in time to inform reauthorization discussions. This report outlines our plan for the mandated study, to be published next fall.
Due to delays between passage of the legislation, appropriation of funds, and initiation of programs, we are required to evaluate PEPFAR very early in its implementation when many of its programs are relatively immature. Our short-term evaluation can provide insights into whether PEPFAR is making reasonable progress toward its goals and can suggest ways in which the program can be improved to ensure that it ultimately meets its goals. However, it cannot adequately measure what matters most—the impact on the lives of the people the legislation seeks to serve. In recognition of this, in addition to providing the short-term evaluation that will be responsive to the legislative mandate, the IOM Committee was charged to plan a long-term evaluation to determine whether PEPFAR has ultimately succeeded in improving the lives of the people in the focus countries by preventing infections, treating patients, and caring for people. We plan to publish the plan for a long-term study shortly after publication of the mandated report.
Our collective responsibility in caring for those in need around the world demands that the challenge of the global HIV/AIDS pandemic be met in a way that is ethically, scientifically, and fiscally sound. We have been humbled by the myriad of questions raised by this global pandemic about how to most effectively prevent the spread of this disease and care for those affected by it. Given limited resources, there is an obligation to match the will to help others with the will to learn how best to help them. This international IOM committee has taken on the challenge of evaluating PEPFAR with determination and humility and is passionately committed to contributing to the effectiveness of PEPFAR in confronting the HIV/AIDS pandemic. We appreciate the help received from so many to date in developing this plan and look forward to a collaborative process of learning together as it is implemented.
Jaime Sepúlveda Amor, MD, Dr.Sc.
Chair
INTRODUCTION
HIV/AIDS has evolved into the world’s greatest public health crisis. Forty million people are estimated to be living with HIV/AIDS, 64 percent of them in sub-Saharan Africa (UNAIDS, 2004a). HIV prevalence among adults 15-49 years of age now exceeds 15 percent in many countries and has approached nearly 30 percent in Botswana, Lesotho, Swaziland, and Zimbabwe. In 2004 alone, almost 5 million people are thought to have become infected with HIV, including 2 million women and 800,000 children. UNAIDS estimated that 3.1 million people died of AIDS worldwide in 2004, and that AIDS has reversed the gains in life expectancy that had been achieved by Africa over the past 50 years (UNAIDS, 2004a).
By 2003, an estimated 12 million children had been orphaned in sub-Saharan Africa as a result of HIV/AIDS, half of whom are between the ages of 10 and 14 (UNAIDS, 2004a). Girls and women are especially vulnerable to HIV, and now account for 50 percent of people living with HIV worldwide and 57 percent of those in sub-Saharan Africa (UNAIDS, 2004a). In addition, HIV/AIDS has severely strained national economies and contributed to political instability in many of the countries experiencing an epidemic.
The U.S. Leadership against HIV/AIDS, Tuberculosis, and Malaria Act
Global funding in response to HIV/AIDS has increased dramatically since 2001. On May 27, 2003, the U.S. Congress passed Public Law 108-25: the United States Leadership against HIV/AIDS, Tuberculosis, and Malaria Act of 2003 (the Act). Provisions of this legislation 1) required the President to establish a comprehensive, integrated 5-year strategy to combat global HIV/AIDS, including specific objectives, strategies, and approaches related to HIV/AIDS prevention, treatment, and care; 2) assigned priorities for relevant executive branch agencies; 3) required improved coordination among such agencies; and 4) projected general levels of resources needed to achieve the stated goals. The legislation emphasized the establishment of programs that focus on national HIV/AIDS strategies of recipient countries, women and children, strengthening of health care infrastructure and workforce, and effective monitoring and evaluation to assess programmatic success. This legislation also required the President to establish the Office of the Global AIDS Coordinator (OGAC) within the U.S. Department of State, which would have primary responsibility for oversight and coordination of all U.S. international activities to combat the HIV/AIDS pandemic. On October 6, 2003, Randall Tobias was sworn in as the first Global AIDS Coordinator, with the rank of Ambassador. On February 23, 2004,
Ambassador Tobias presented the U.S. 5-year Global HIV/AIDS Strategy to Congress.
President’s Emergency Plan for AIDS Relief (PEPFAR)
The initiative is commonly known by the title given to the U.S. 5-year Global HIV/AIDS Strategy: “The President’s Emergency Plan for AIDS Relief”, or PEPFAR. In order to measure the progress of the initiative, the PEPFAR strategy establishes three overarching goals:
-
To encourage bold leadership at every level to fight HIV/AIDS;
-
To apply best practices within bilateral HIV/AIDS prevention, treatment, and care programs, in concert with the objectives and policies of host governments’ national HIV/AIDS strategies; and
-
To encourage partners, including multilateral organizations and other host governments, to coordinate at all levels to strengthen response efforts, to embrace best practices, to adhere to principles of sound management, and to harmonize monitoring and evaluation efforts to ensure the most effective and efficient use of resources (OGAC, 2004).
The PEPFAR strategy also describes the principles according to which it will achieve its mission and goals, including responding with urgency to the crisis; seeking new approaches; coordinating the U.S. Government oversight and direction of PEPFAR activities; drawing on the scientific evidence base in developing interventions; establishing measurable goals for programs; harmonizing program development and implementation with the host countries; integrating prevention, treatment and care programs; building national capacity; encouraging national leadership; and coordinating with other partners (OGAC, 2004).
PEPFAR, while encompassing activities in over 120 countries, is focused on the development of comprehensive and integrated prevention, treatment, and care programs in 15 countries selected largely because they are heavily affected by HIV/AIDS (OGAC, 2005b). With regard to funding, $10 billion of the $15 billion authorized under the Act is to be allocated to efforts in these 15 countries over five years (OGAC, 2005b). Currently, the 15 PEPFAR focus countries are Botswana, Cote d’Ivoire, Ethiopia, Guyana, Haiti, Kenya, Mozambique, Namibia, Nigeria, Rwanda, South Africa, Tanzania, Uganda, Zambia, and Vietnam. PEPFAR has established additional goals for its prevention, treatment and care programs in these countries, specifically: to support prevention of 7 million new HIV infections, treatment of
2 million HIV-infected people with antiretroviral therapy (ART), and care of 10 million people infected and affected by HIV/AIDS.1
OGAC is responsible for maintaining the focus of PEPFAR by leading policy development, program oversight, and coordination both among U.S. government departments and agencies and with other donors and governments. Additionally, OGAC is responsible for the allocation of funds which are distributed through a number of U.S. government departments and agencies including the Departments of State, Defense (DoD), Commerce, Labor (DoL), and Health and Human Services (HHS); the U.S. Agency for International Development (USAID); and the Peace Corps (OGAC, 2005a). The two largest implementing entities are USAID and HHS which includes the Centers for Disease Control and Prevention (CDC), Food and Drug Administration, National Institute of Health (NIH), and Health Resources and Services Administration (HRSA). Within OGAC, staffing is organized into several groups, all of which include OGAC staff as well as representatives from the other U.S. government departments and agencies coordinated by OGAC. These groups include the Policy Group, incorporating representation from USAID, HHS, the White House and the National Security Council; the Deputy Principals Group, handling program management and logistics with representation from agencies including the Peace Corps, HHS, USAID, DoD and DoL; and a Scientific Steering Committee, consisting of representatives from CDC, NIH, USAID, and DoD (Moloney-Kitts, 2005). Finally, Core and Technical Teams, whose members are drawn from a wide range of U.S. government agencies, are responsible for supporting programs in PEPFAR countries with specific technical and implementation issues.
Each of the focus countries has a U.S. Government Mission team that is intended to provide a unified strategy and voice and is responsible for coordination of PEPFAR-sponsored programs in the country. The team is led by the U.S. ambassador and includes representatives from all of the implementing departments and agencies. It is supported by a core team based in OGAC which serves as a liaison between the field and headquarters. In many countries, high-level PEPFAR advisory committees have been formed to ensure a close working relationship between the U.S. government and host-country counterparts (OGAC, 2005a). In an effort to create self-sustaining, lasting systems to address HIV/AIDS, PEPFAR emphasizes the development of national leadership, human resources, and other capacities through collaboration of Mission teams with existing country partners, as well as with other donors.
Study Goals and Approach
The Act mandates that the Institute of Medicine (IOM) evaluate PEPFAR and directs the President to consider IOM’s findings. Specifically Sec. 101 (c) (1) of the Act states:
“Not later than 3 years after the date of the enactment of this Act, the Institute of Medicine shall publish findings comparing the success rates of the various programs and methods used under the [PEPFAR] strategy.
In prioritizing the distribution of resources under the [PEPFAR] strategy, the President shall consider the findings published by the Institute of Medicine under this subsection.”
Thus, the current task of the IOM is to be responsive to this mandate and provide to Congress in time for reauthorization discussions an assessment of PEPFAR implementation at the three-year mark. The IOM has undertaken an independent, expert consensus committee process to plan, conduct, and report on this short-term evaluation, the scope of which is limited to the implementation of PEPFAR in the focus countries and does not include the U.S. contribution to the Global Fund to Fight AIDS, Tuberculosis, and Malaria (Global Fund) which is also coordinated by OGAC. The IOM Committee for the Evaluation of PEPFAR Implementation (the Committee) has three advisory Subcommittees focused on prevention, treatment, and care (see Appendix 1). This letter report outlines the Committee’s plan for the short-term evaluation.
In addition to the size and complexity of PEPFAR, two additional features make the Committee’s task an especially challenging one: PEPFAR is of necessity a dynamic, evolving program and it is still relatively early in its implementation.
As its name implies, OGAC has implemented PEPFAR on an emergency basis and, as the Act acknowledged, has had to “maintain sufficient flexibility and remain responsive to the ever-changing nature of the HIV/AIDS pandemic.” Thus PEPFAR is a rapidly-moving, continuously-evolving target for evaluation. The Committee is prepared to find considerable changes in PEPFAR throughout its evaluation, and has developed an approach to the evaluation that will allow it to adapt not only to changes in PEPFAR implementation, but also to what the Committee learns as the evaluation proceeds. The Committee’s evaluation plan should be viewed as a work-in-progress that will be modified to reflect both the dynamic nature of PEPFAR and what the Committee learns—particularly as it visits the PEPFAR focus countries to directly observe implementation activities.
Ultimately the “success” of PEPFAR will be judged by whether it has achieved its near-term goals of effectively supporting the prevention of 7 million HIV infections, treatment for 2 million people with HIV/AIDS
with ART, and care of 10 million people infected with and affected by HIV/AIDS, as well as its longer-term goal of sustainable gains against the HIV/AIDS epidemics in the focus countries. However, although the Act was passed in May 2003, funds were not appropriated until January 2004, and the majority of the first year’s funding was not obligated until September 2004 (see Appendix 2). Thus, at the time of the Committee’s evaluation, PEPFAR will have been supporting the implementation of prevention, treatment and care programs in the focus countries for less than two years—less time than perhaps Congress had envisioned when they wrote the mandate for the IOM study. It would not be reasonable or feasible to evaluate PEPFAR solely against these goals so early in implementation, and therefore in this short-term evaluation the Committee plans instead to evaluate how PEPFAR is progressing toward 1) achieving these goals and 2) building the monitoring and evaluation capacity to demonstrate that it has achieved them.
The short-term evaluation can provide insights into whether PEPFAR is making reasonable progress toward its goals and can suggest ways in which the program can be improved to ensure that it ultimately meets its goals. However, it cannot adequately measure what matters most—the impact on the lives of the people the legislation seeks to serve. In recognition of this, in addition to providing the short-term evaluation that will be responsive to the legislative mandate, the IOM Committee is planning a long-term evaluation to determine whether PEPFAR has ultimately succeeded in improving the lives of the people in the focus countries by preventing infections, treating patients, and caring for people. We plan to publish the plan for a long-term study shortly after publication of the mandated report. This long-term evaluation plan will be informed by the Committee’s fuller understanding of PEPFAR implementation and the challenging experience of evaluating it.
In the development of its plan, the Committee has consulted widely and remains open to receiving input from the broad range of parties interested in and affected by PEPFAR. To develop the plan, the Committee (and the additional subcommittee members) convened first in February 2005 and then again in April 2005 when the Committee was fully-formed to review documentation of and hear testimony about PEPFAR. The questions highlighted in the plan reflect the multiple data sources that the committee believes capture the primary components of PEPFAR implementation. The Committee has considered peer-reviewed literature on global efforts to combat HIV/AIDS in resource-limited settings, documentation provided by OGAC, and other program-related materials. The Committee has heard testimony from OGAC officials and PEPFAR Mission staff; global organizations such as the World Health Organization, UNAIDS, Global Fund, and World Bank; and numerous PEPFAR grantees and partners. In addition, one Committee member and one staff attended the PEPFAR Annual Meeting
in Addis Ababa, Ethiopia in May 2005. Following these initial information–gathering activities and several phone conferences to further explore information specific to prevention, treatment, and care, the Committee met in July 2005 to outline the short-term evaluation plan (see Figure 1).
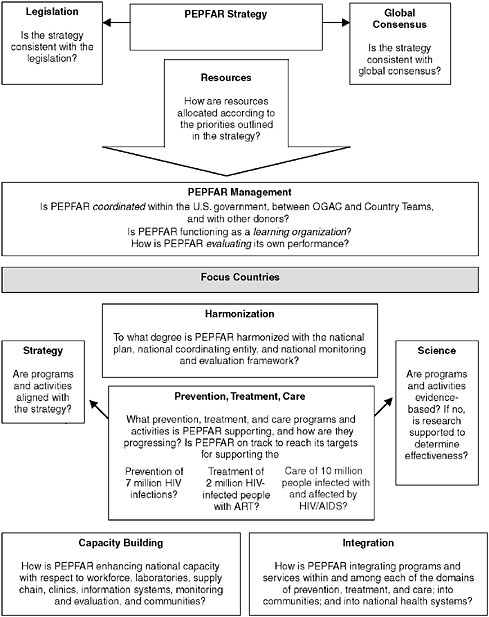
FIGURE 1-1 Short-term PEPFAR evaluation plan.
SHORT-TERM EVALUATION QUESTIONS
Strategy
Is the PEPFAR Strategy consistent with the Act and relevant global consensus?
The same section of the Act that mandates the IOM study also specifies the major elements that should be contained in the PEPFAR Strategy. In addition, the legislation calls for the U.S. to take a strong leadership position with respect to the global effort to combat HIV/AIDS and also to coordinate its efforts with the international donor community. The global HIV/AIDS community, led by organizations such as those of UNAIDS, has published guidelines outlining international consensus strategies for the development and implementation of prevention, treatment, and care programs.
-
Does the PEPFAR strategy address the major elements of the Act such as:
-
Developing specific objectives, approaches and strategies for activities related to HIV/AIDS prevention, treatment and care? (Sec. 101. a.1 & Sec.101.b.3.C)
-
Focusing prevention funding, educational messages and activities on the reduction of HIV/AIDS behavioral risks? (Sec. 101.a.4 & Sec.101. b.3.C)
-
Linking resources to program objectives and establishing of priorities for the distribution of resources based on factors such as population characteristics and needs as well as the existing infrastructure or funding levels? (Sec. 101.a.6 & 9 & Sec.101.b.3.M)
-
Maximizing United States capabilities in the areas of technical assistance and training? (Sec. 101.a.8 & Sec.101.b.3.H)
-
Improving coordination and reducing duplication among relevant executive branch agencies, foreign governments, and international organizations? (Sec. 101.a.3 & Sec.101.b.3.K & L)
-
Focusing on strategies developed to meet the needs of women, orphans and families? (Sec. 101.b.3.E, G, R, & S)
-
Promoting the development and implementation of strategies and programs designed to enhance the development of health care infrastructure, delivery systems and leadership capacity? (Sec.101.a.2 & Sec.101. b.3.C & D)
-
Establishing monitoring and evaluating programs in order to measure success of the strategies, promoting successful models, and terminating unsuccessful programs? (Sec.101.b.3.B & N)
-
-
Is the PEPFAR strategy consistent with best practices outlined in global consensus documents related to prevention, treatment and care of HIV/AIDS including:
-
The Global Strategy Framework on HIV/AIDS (UNAIDS, 2001)
-
Intensifying HIV Prevention (UNAIDS, 2005)
-
A Public Health Approach for Scaling Up Antiretroviral (ARV) Treatment: A Toolkit for Programme Managers (WHO, 2003)
-
The Framework for the Protection, Care and Support of Orphans and Vulnerable Children Living in a World with HIV and AIDS (UNAIDS and UNICEF, 2004)
-
Joint ILO/WHO guidelines on health services and HIV/AIDS (ILO/WHO, 2005)
-
-
Has OGAC modified the PEPFAR strategy to reflect insights gained and lessons learned during implementation?
Resources
How is PEPFAR allocating resources and what is the effect on implementation?
Both the Act and the PEPFAR strategy include guidance on the allocation of resources under the program. The legislation authorizes an annual appropriation for PEPFAR and outlines prioritization for resources in terms of program type (e.g., 20% for prevention, 55% for treatment, 15% for care, 10% for orphans and vulnerable children), and population and/or country characteristics (e.g., size and demographics of the HIV positive population, existing infrastructure). The PEPFAR strategy outlines allocation mechanisms including country allocations based on each country’s five-year strategic plan and performance assessments related to reaching annual prevention, treatment, and care targets, as well as a central funding mechanism for regional activities. Although there are a number of constraints on resource allocation, OGAC leadership and country missions have discretion in the allocation of resources amongst agencies, countries, programs, and activities.
-
How is PEPFAR allocating resources according to the priorities outlined in its strategy?
-
How is PEPFAR managing congressional allocations in the context of harmonization?
-
How do PEPFAR’s funding mechanisms affect operations of programs?
-
How is PEPFAR addressing the coordination of regulations for procurement among and within cooperating agencies?
-
How is the Central Procurement Contract functioning?
-
What kinds of technical assistance is PEPFAR providing and how is it distributed?
PEPFAR Management
How is PEPFAR coordinating its efforts, evaluating it progress, and improving its programs?
Coordination
Coordination is one of the key elements of PEPFAR implementation identified by the Act. More specifically, the stated objectives within the legislation include strengthening coordination among U.S. government agencies “to ensure effective and efficient use of financial and technical resources” and to foster greater dialogue and synchronization of efforts with foreign governments and international organizations. OGAC has emphasized its “new way of doing business” and has focused a great deal of effort on its role as “Coordinator” across U.S. government agencies and with other donors.
-
How well are U.S. government agencies coordinating under the auspices of the Office of the Global AIDS Coordinator?
-
How well coordinated are PEPFAR operations between headquarters and missions?
-
How well coordinated is PEPFAR with other HIV/AIDS donors at all levels?
Monitoring and Evaluation
Measurement to assess progress, to improve programs, and for accountability is critical to the success of PEPFAR. With some unavoidable overlap, questions related to PEPFAR’s support of the focus countries’ monitoring and evaluation frameworks and capacity are emphasized in the harmonization and capacity building sections respectively. The questions emphasized in this section pertain primarily to PEPFAR’s efforts to monitor and evaluate itself and demonstrate that it has achieved its goals.
-
How is PEPFAR monitoring progress on the major components of its program?
-
Coordination
-
-
-
Harmonization
-
Intermediate Milestones for:
-
Prevention of 7 million HIV infections
-
Treatment of 2 million HIV-infected people with ART
-
Care for 10 million people infected with and affected by HIV/AIDS
-
-
Capacity Building
-
Integration
-
-
How is PEPFAR planning to evaluate its success in the long-term?
-
Prevention of 7 Million HIV Infections
-
Treatment of 2 Million HIV-infected People with ART
-
Care for 10 Million People Infected with and Affected by HIV/AIDS
-
Reduction of the HIV/AIDS Epidemic in the Focus Countries
-
Improved Survival and Quality of Life for People in the Focus Countries
-
Sustainable Programs and Systems
-
Enhanced Knowledge Base
-
-
How does PEPFAR manage difficult monitoring and evaluation issues such as measurement of infections prevented and attribution of people treated or cared for?
-
How does PEPFAR assure the quality of programs and services?
-
What steps is PEPFAR taking to ensure that the necessary epidemiologic data and other information are available from the focus countries to assess the initiative’s short-, as well as long-term impact?
-
Is PEPFAR supporting programs to generate the information they need for on-going program improvement?
Learning Organization
PEPFAR is an acknowledged “emergency” response. By design, the initiative moved rapidly into implementation before all of the arguably necessary pieces were in place, and many foundational elements remain to be developed—for example, OGAC has yet to issue the majority of guidance documents that it has said are under development. In recognition of this, however, PEPFAR has committed to “learning-by-doing.”
-
Is PEPFAR functioning as a learning organization (Senge, 1990)?
-
Does PEPFAR have an approach to learning by doing that allows them to generate and share evidence to improve programs?
Harmonization
How is PEPFAR harmonizing its efforts in the focus countries?
In April 2004, UNAIDS, the United Kingdom, and the United States co-hosted a high-level meeting at which donors reaffirmed their commitment to strengthening national AIDS responses to be led by the affected countries themselves. They endorsed the “Three Ones” as guiding principles to improve the country-level response (UNAIDS, 2004b):
-
One agreed upon HIV/AIDS Action Framework that provides the basis for coordinating the work of all partners
-
One National AIDS Coordinating Authority, with a broad-based multi-sectoral mandate
-
One agreed upon country-level Monitoring and Evaluation System.
PEPFAR has expressed full commitment to the Three Ones, and although these principles were not formally in place at the time of the passage of the Act, the legislation calls for the U.S. to be coordinated with other donors, and thus PEPFAR’s commitment to the Three Ones is consistent with the legislation. All of the PEPFAR focus countries have national AIDS authorities, and thus with this commitment, “Harmonization” with the Three Ones of each focus country became the centerpiece of the structure of PEPFAR. As such, it is central to the structure of the IOM evaluation.
-
To what degree is PEPFAR harmonized with and what are the explanations for varying degrees of harmonization with the national plan, the national coordinating entity, and the national monitoring and evaluation framework?
-
How do the goals that PEPFAR assigned to each country for number of infections prevented, number of people receiving treatment, and number of people cared for align with the country’s goals?
-
How does PEPFAR manage differences between its plan and the national plan?
-
How has PEPFAR affected the development of national plans?
-
How does PEPFAR manage varying degrees of participation in the national coordinating entity?
-
How does PEPFAR manage differences between its data collection and reporting requirements and those of the national monitoring and evaluation framework?
Prevention, Treatment & Care
Are PEPFAR prevention, treatment and care activities aligned with its strategy and the best available science and how is implementation progressing?
Prevention, treatment and care activities are key components of an effective and comprehensive response to HIV/AIDS; helping to decrease risk, vulnerability, and impact of the epidemic (UNAIDS, 2001). The PEPFAR strategy identifies prevention, treatment and care programs as critical interventions, around which other PEPFAR strategies, such as strengthening health care systems, building capacity for long term sustainability of programs and the collection of strategic information, are structured (OGAC, 2004). The targets for PEPFAR-funded prevention, treatment and care programs—to support prevention of 7 million new HIV infections, treatment of 2 million HIV-infected people with ART, and care of 10 million people infected and affected by HIV/AIDS—were introduced in the State of the Union address given by President Bush in 2003, referenced in the Act, and later included in the PEPFAR strategy (OGAC, 2004).
-
What programs and activities is PEPFAR supporting to address aspects of Prevention such as Behavioral Change, Blood Safety, Counseling and Testing, Post Exposure Prophylaxis, Prevention of Maternal to Child Transmission, and Safe Medical Practice?
-
What programs and activities is PEPFAR supporting to address aspects of Treatment such as ARV Therapy, Clinical Laboratory Testing, and Prevention of Maternal to Child Transmission Plus?
-
What programs and activities is PEPFAR supporting to address aspects of Care, particularly for Orphans and Vulnerable Children, such as Health Services and Social Support Services, Pain Management, and Prevention and Treatment of Opportunistic Infections?
-
Are the programs and activities evidence-based?
-
If evidence-base is incomplete or unclear, is PEPFAR supporting research to determine effectiveness?
-
What programs and activities are focused on women and girls?
-
How do programs and activities address stigmatization of and discrimination against people living with HIV/AIDS?
-
How do programs and activities address issues of equity and human rights?
-
How are prevention, treatment, and care programs and activities progressing?
-
Has PEPFAR achieved the intermediate milestones established to measure progress in reaching its goals of supporting the prevention of 7
-
million HIV infections, treatment of 2 million HIV-infected people with ART, and care for 10 million people infected and affected by HIV/AIDS?
Integration
PEPFAR seeks to address prevention, treatment, and care in an integrated approach. The availability of these three interventions and effective linkages among them is expected to strengthen the effect of each intervention and have an impact that is greater than the sum of its parts. Integration of PEPFAR programs into the community and of HIV/AIDS programs into national health systems is intended to strengthen and enhance indigenous ownership and sustainability of these programs.
-
How is PEPFAR integrating its programs and services and supporting integration with existing programs and services:
-
Within the domains of prevention, treatment, and care?
-
Among the domains of prevention, treatment, and care?
-
With communities?
-
With national health systems?
-
Capacity Building
In order to meet the goals of expanding and sustaining HIV/AIDS prevention, treatment, and care, PEPFAR must address the common barriers that affect progress in many countries: namely the scarcity of human resources and institutional capacity compounded by fragile health care systems (OGAC, 2005a). According to OGAC “supporting capacity development is the heart of the [PEPFAR] initiative” and more than $200 million of PEPFAR funding in 2005 is dedicated to capacity building efforts (Dybul, 2005). PEPFAR is committed to working predominantly with indigenous partners and believes that building capacity is necessary in order to enable national programs to achieve results, monitor and evaluate their activities, and sustain their programs over the long-term. (OGAC, 2004).
-
How is PEPFAR enhancing the capacity necessary for the focus countries to have sustainable HIV/AIDS prevention, treatment, and care programs?
-
Specifically, what is PEPFAR doing to support the building and strengthening of:
-
Human Resources?
-
Institutions?
-
Health Systems (including laboratories, clinics, supply chain)?
-
Communities?
-
-
-
Information Systems?
-
Monitoring and Evaluation?
-
-
How does PEPFAR-funded technical assistance to national governments for activities such as policy development and dissemination of national strategies and guidelines contribute to the enhancement of national capacity?
APPROACH
In carrying out this evaluation plan, the Committee will use several approaches to examine PEPFAR’s strategic development and programmatic implementation. In order to answer questions related to PEPFAR’s strategy, the committee will compare the 5-year strategy with the authorizing legislation (P.L. 108-25) and relevant global consensus documents. To answer questions related to PEPFAR resource allocation, the committee will review and analyze budgetary and programmatic data provided by OGAC, PEPFAR Missions, and others. The Committee will examine the major aspects of program implementation including PEPFAR management; harmonization; prevention, treatment, and care programs and activities; capacity building; and integration through a wide variety of approaches including review of the scientific literature; analysis of PEPFAR guidance and other documents, national plans, and focus country reports; analysis of data from PEPFAR and other programs and donors; and discussions with OGAC staff, Mission staff, focus country officials, partners, program officials, community groups, and officials from other donor organizations.
Many of the aspects of program implementation that the Committee will be reviewing have a significant qualitative component, for example, Coordination. Thus, for example, in addition to examining whether there are structures and processes in place to facilitate coordination, the Committee will solicit the perspectives of all the major parties that are intended to be coordinated. We are attempting to create an environment in which individuals can speak frankly and toward that end have requested of OGAC that no OGAC staff be present when we hold discussions with Mission staff and that no PEPFAR staff be present when we visit sites that PEPFAR is supporting. The Committee will not attribute statements to individuals by name.
In order to facilitate consistency across discussions, the Committee is developing generic guides for each type of meeting or site visit to be conducted and plans to share them in advance with the parties involved so that they may be better prepared. These guides outline a standard set of issues to be addressed and also allow for additional issues to be explored, depending on the circumstance. These guides are intended to be tailored to the extent
possible based on what the Committee is able to learn in advance about the particular people or program involved.
The Committee respects global efforts at harmonization of monitoring and evaluation—in which PEPFAR is a participant (Rugg et al. 2004)—and intends to rely on existing indicators and data sources to the greatest extent possible (OGAC 2005c, UNAIDS 2002, WHO et al. 2004). The Committee continues to review global monitoring and evaluation efforts already underway, systems/processes already in place, and existing data. The indicators developed by PEPFAR and others continue to evolve; the Committee plans to follow this evolution and also expects to contribute to it.
Focus Country Visits
As a result of PEPFAR’s structure and commitment to harmonization, the majority of implementation activities are occurring in the focus countries. Thus, visits to the focus countries to directly observe implementation activities are a critical part of the Committee’s evaluation plan. The Committee anticipates that these country visits will provide insight into the programmatic successes and challenges through concrete examples and first-hand accounts of how PEPFAR is working on the ground. In recognition of the unique nature of the HIV/AIDS epidemic and the country response to it in each of the 15 focus countries, PEPFAR has been designed to support national leadership and to adapt to the specific needs of each country. It is therefore important to observe how the conditions prevailing in each focus country affect the implementation of PEPFAR. The Committee—in small delegations—plans to visit all of the focus countries, although some visits may be precluded due to security concerns. The generic agenda that has been developed for the country visits will be tailored to the particular circumstances of each focus country in concert with PEPFAR staff, PEPFAR partners, national officials, other donors, community leaders, and others to provide as comprehensive on overview of PEPFAR implementation as possible in a short time. The Committee anticipates that follow-up by phone and email will be needed, not only because it will not be possible to accomplish everything in one visit, but also because it expects to learn from each visit and to discover themes emerging after several visits that will need to be explored further. The Committee is developing the country visits as a continuous learning process. Toward this end, it is creating flexible agendas to allow the visiting teams to adapt to what they learn as the visit progresses; a mechanism for the team that is currently on a country visit to provide feedback to the next team that is about to begin a country visit; and a cumulative, cross-visit analysis after each set of trips.
Limitations
A key and detailed source of information about PEPFAR programs and activities is the Country Operational Plan (COP) that each focus country Mission is required to develop each year. Because OGAC considers some of the information in the COPs to be sensitive and therefore not appropriate for the public domain, the Committee has been allowed access only to versions of the COPs that have been redacted. The Committee’s work has been hampered by delays obtaining redacted versions of the fiscal year 2005 COPs, and we may encounter similar delays in obtaining the fiscal year 2006 COPs. Also, it may not be possible for the Committee to know the precise nature of the redacted information or its impact on our analysis. The Committee may encounter similar difficulties with respect to unpublished monitoring and evaluation data that PEPFAR has collected.
REFERENCES
Dybul M. 2005 (May 24). Integrated Prevention, Treatment and Care: Network Systems. Presentation at the PEPFAR Second Annual Field Meeting, Addis Ababa, Ethiopia.
ILO (International Labour Organization) and WHO (World Health Organization). 2005. Joint ILO/WHO guidelines on health services and HIV/AIDS: ILO.
Moloney-Kitts M. 2005 (April 19). OGAC Presentation. Presentation at the Second Meeting of the Institute of Medicine Committee for the Evaluation of PEPFAR Implementation, Washington, DC.
OGAC (Office of the Global AIDS Coordinator). 2004. The President’s Emergency Plan for AIDS Relief (PEPFAR): U.S. Five-Year Global HIV/AIDS Strategy. Washington, DC: OGAC.
OGAC. 2005a. PEPFAR First Annual Report. Washington, DC: OGAC.
OGAC. 2005b. Basic Requirements Under the President’s Emergency Plan for AIDS Relief for All Bilateral Programs. Washington, DC: OGAC.
OGAC. 2005c. The President’s Emergency Plan for AIDS Relief: Indicators, Reporting Requirements, and Guidelines for Focus Countries (Revised for FY2006 Reporting) July 29, 2005
Rugg D, Peersman G, Carael M. 2004. Global Advances in HIV/AIDS Monitoring and Evaluation. Minneapolis, MN: Wiley Periodicals, Inc.
Senge P. 1990. The Fifth Discipline: The Art and Practice of The Learning Organization. [Online]. Available: http://www.infed.org/thinkers/senge.htm [accessed October 9, 2005].
UNAIDS (Joint United Nations Program on HIV/AIDS). 2001. The Global Strategy Framework on HIV/AIDS. Geneva, Switzerland: UNAIDS.
UNAIDS. 2002. National AIDS Council’s Monitoring and Evaluation Operations Manual, Joint United Nations Programme on HIV/AIDS (UNAIDS) 2002.
UNAIDS. 2004a. 2004 Report on the Global AIDS Epidemic: 4th Global Report. Geneva, Switzerland: UNAIDS.
UNAIDS. 2004b. “Three Ones” Key Principles. Conference Paper 1, Washington Consultation of April 4, 2004. Geneva, Switzerland: UNAIDS. [Online]. Available: http://www.unaids.org/NetTools/Misc/DocInfo.aspx?LANG=en&href=http://gva-doc-owl/WEBcontent/Documents/pub/UNA-docs/Three-Ones_KeyPrinciples_en.pdf [accessed August 25, 2005].
UNAIDS and UNICEF (United Nations Children’s Fund). 2004. The Framework for the Protection, Care and Support of Orphans and Vulnerable Children Living in a World with HIV and AIDS. Geneva, Switzerland: UNAIDS.
WHO. 2003. A Public Health Approach for Scaling Up Antiretroviral (ARV) Treatment: A Toolkit for Programme Managers. [Online]. Available: http://www.who.int/hiv/toolkit/arv/en/index.jsp [accessed September 1, 2005].
WHO, UNAIDS, The Global Fund to Fight AIDS, Tuberculosis & Malaria, USAID, US Department of State, US Department of Health and Human Services, CDC, UNICEF and the World Bank. 2004. Monitoring and Evaluation Toolkit HIV/AIDS, Tuberculosis and Malaria.
APPENDIX 1
The Committee for the Evaluation of PEPFAR Implementation
The committee is composed of 13 members. Committee members were selected for their international experience in low- and middle-income countries, as well as their individual expertise in the following areas relevant to the committee’s charge: behavioral science, bioethics, biostatistics, community nursing, community development, economics, epidemiology, infectious disease (adult), informatics, maternal and child health, modeling, monitoring and evaluation, operations research, professional training/education, public health program management, quality of care, and social services. Three advisory subcommittees comprised of 6-7 members each, are focused on prevention, treatment, and care. Additional members who serve only on the subcommittees provide expanded expertise in the following areas: child psychology/psychiatry, child welfare/services, demography, health communication, health education, infectious disease (pediatric), laboratory quality, logistics, palliative care, and pharmacology.
APPENDIX 2
PEPFAR Chronology
|
May 2003 |
PEPFAR authorizing legislation passed: “United States Leadership Against HIV/AIDS, Tuberculosis, and Malaria Act of 2003” |
|
October 2003 |
Ambassador Tobias sworn in as first U.S. Global AIDS Coordinator |
|
January 2004 |
First appropriation under the United States Leadership Against HIV/AIDS, Tuberculosis, and Malaria Act of 2003 |
|
February 2004 |
U.S. 5-year Global HIV/AIDS Strategy published: “The President’s Emergency Plan for AIDS Relief (PEPFAR)” |
|
March 2004 |
First year funds became available for obligation; grant and contract awards commenced; $350 million was provided to initial awardees |
|
April 2004 |
The “Three Ones” endorsed as guiding principles to improve the global response to HIV/AIDS |
|
June 2004 |
First PEPFAR Annual Meeting |
|
September 2004 |
Majority of first year funding obligated |
|
March 2005 |
First PEPFAR Annual Report published “ABC” Prevention Guidance published Palliative Care Guidance published |
|
May 2005 |
Draft Guidance for Strategic Information published |
|
May 2005 |
Second PEPFAR Annual Meeting |
|
June 2005 |
Just over 50% of State Department authorized staff positions filled at OGAC. Additional staff on board included detailees from other US government agencies, contractors, fellows, and interns. |
|
August 2005 |
Basic Requirements Under the President’s Emergency Plan for AIDS Relief for all Bilateral Programs published |
|
May 2008 |
United States Leadership Against HIV/AIDS, Tuberculosis, and Malaria Act of 2003 expires |
|
2008 |
Goal of 2 million People with HIV/AIDS on Antiretroviral Therapy |
|
2008 |
Goal of 10 million People Affected by HIV/AIDS Receiving Care |
|
2010 |
Goal of Preventing 7 million HIV Infections |
APPENDIX 3
Abbreviations
AIDS Acquired Immunodeficiency Syndrome
ART Antiretroviral Therapy
ARV Antiretroviral Drug
CDC Centers for Disease Control and Prevention
COP Country Operational Plan
DoD Department of Defense
DoL Department of Labor
HHS Health and Human Services
HIV Human Immunodeficiency Virus
HRSA Health Resources and Services Administration
ILO International Labour Organization
NIH National Institutes of Health
OGAC Office of the Global AIDS Coordinator
PEPFAR The President’s Emergency Plan for AIDS Relief
UNAIDS Joint United Nations Programme on HIV/AIDS
UNICEF United Nations Children’s Fund
USAID U.S. Agency for International Development
WHO World Health Organization
Note: Individuals in italics serve only on the subcommittees.
Committee for the Evaluation of PEPFAR Implementation
Chair—Jaime Sepulveda, M.D., Dr.Sc.
Vice Chair—Helen Smits, M.D., M.A.C.P.
Stefano Bertozzi, Ph.D., M.D.
James Curran, M.D., M.P.H.
William Holzemer, R.N., Ph.D., FAAN
Affette McCaw-Binns, Ph.D.
Charles Carpenter, M.D.
Geoff Garnett, Ph.D.
Ruth Macklin, Ph.D.
David Paltiel, Ph.D.
Priscilla Reddy, M.P.H., Ph.D.
David Ross, Sc.D.
Heather Weiss, Ed.D.
Mike Merson, M.D. (BGH Liaison)
Treatment Subcommittee
Chair—Charles Carpenter, M.D.
Hoosen Coovadia, M.B.B.S., M.D
Henry Fomundam Pharm.D.
David Paltiel, Ph.D.
Helen Smits, M.D., M.A.C.P.
Olaitan Soyannwo, M.Med., D.A., F.W.A.C.S., F.I.C.S.
Burton Wilcke, Ph.D.
Prevention Subcommittee
Chair—James Curran, M.D., M.P.H.
Stefano Bertozzi, Ph.D., M.D.
Geoff Garnett, Ph.D.
Paul Gertler, Ph.D.
Carl Latkin, M.S., Ph.D.
Priscilla Reddy, M.P.H., Ph.D.
Care Subcommittee
Chair—William Holzemer, R.N., Ph.D., FAAN
Maureen Black, Ph.D.
Affette McCaw-Binns, Ph.D.
James Ntozi, M.Sc., Ph.D.
James Sherry, M.D., Ph.D.
Heather Weiss, Ed.D.
Elena Nightingale, M.D., Ph.D. (BCYF Liaison)

































