2
A Sketch of the Chemistry Behind Known Carbon-based Life on Earth
To support an informed analysis of the possibilities for life in the cosmos, this chapter summarizes chemical concepts needed to evaluate the plausibility of a potential exotic life form and shows how they relate to the chemistry of the life that we know. This summary is necessarily selective. Students of chemistry and related areas develop their understanding of molecular behavior and chemical reactivity through years of study that cannot be condensed into a few pages. The committee has chosen topics for emphasis to permit a discussion of terran life to support its later discussion of exotic life.
2.1
MOLECULAR STRUCTURE AND PHYSICAL PROPERTIES
2.1.1
Pairs of Electrons Form Bonds Between Atoms
Covalent bonds between two atoms are formed when the two atoms share a pair of electrons. In the simplest of molecules, dihydrogen (often written as H—H, or H2), a single line represents the pair of electrons that forms a single bond between the two hydrogen atoms. In water (H—O—H), the lines connecting each hydrogen atom to the oxygen atom each represent a pair of electrons that form a single bond holding the hydrogen atoms to the oxygen. In formaldehyde (H2C=O), the double line between the carbon atom and the oxygen atom represents two pairs of electrons, four electrons in total, joining the carbon atom to the oxygen atom.
A complete structural model for a molecule also shows the positions of electrons not involved in covalent bonding. For example, in the Lewis structures of formaldehyde and water (Figure 2.1), the oxygen atom in each carries two pairs of unshared electrons from the outer valence shell. Each of these electrons, not involved in a covalent bond, is represented by a dot. The oxygen atom in water has four nonbonding electrons, and the oxygen atom in formaldehyde carries two pairs of unshared electrons, represented by four dots on the oxygen atoms of the two molecules in the Lewis structure.
2.1.2
Distribution of Charge Is Key to the Physical Properties of Molecules
An electron carries a negative charge; a proton in a nucleus carries a positive charge. In many molecular species, the number of electrons is not equal to the number of protons. In this case, the molecule carries a charge and is called an ion. In sodium chloride (table salt), the sodium ion carries a positive charge and the chloride ion carries a negative charge (Figure 2.2).

FIGURE 2.1 Lewis structures for formaldehyde (left) and water (right) show the presence of four electrons (each represented by a dot) on the oxygen atoms of the two molecules.These electrons are not involved in the formation of any bond. The pairs of electrons involved in holding the molecule together are represented by lines.
Molecules with equal numbers of protons and electrons are not charged. But the distribution of electrons in three dimensions need not be uniform in the molecule with respect to the distribution of the nuclei. In this case, the distribution of negative charge in a molecule may be different from the distribution of positive charge, and the molecule has one or more bond dipole moments, the magnitude of which depends on these distributions. The more uneven the distributions of charge in space, the more polar the molecule is.a
In general, atoms with similar electronegativities share electrons equally. Carbon and hydrogen, as atoms, have similar electronegativities. Hence, the carbon-hydrogen bond is not generally associated with a large bond dipole moment, and molecules that contain carbon-hydrogen units are not particularly polar. In contrast,bonds joining carbon and hydrogen atoms to heteroatoms (atoms other than carbon or hydrogen)are often quite polar. For example, the oxygen-hydrogen bond, a bond between atoms with very different electronegativities, is generally associated with a large dipole moment. As a consequence,molecules that contain — OH groups are generally polar. Molecules that contain many unshared pairs of electrons are also more polar.
The nature and distribution of its charge are the dominant features in determining a molecular structure’s bulk physical properties. Knowing that a molecule is an ion is generally more important than almost any other piece of information for understanding the physical behavior of the molecule. If a molecule lacks a charge, then a statement about the magnitude of the dipole moment is most informative about its physical properties.
One of the more prominent features of polar molecules and ionic species is their ability to dissolve in polar solvents. Water, in turn, is one of the more polar solvents,because the distribution of electrons is quite different from the spatial arrangement of protons; the oxygen atom of H2O carries more negative charge, while the hydrogen atoms carry more positive charge (Figure 2.3).
As a consequence, water dissolves many salts and many molecules that have large dipole moments. Water does not dissolve molecules that lack a charge or a substantial dipole moment. Nonpolar molecules that contain many carbon-hydrogen and carbon-carbon units are called hydrocarbons and are well known as oils and fats.Their nonpolarity is the general reason that oil (petroleum oil and vegetable oil alike) and water do not mix.
2.1.3
Distribution of Charge Can Be Inferred from Molecular Structures
The polarities of molecules can be estimated from their molecular structures. The electronegativities of the constituent atoms are the key to making those estimates. The structure of the organic molecule is scanned, polar bonds between atoms with different electronegativities are identified, and the number of those polar bonds present in the molecule relative to the nonpolar bonds between carbon and hydrogen, or between
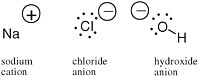
FIGURE 2.2 The sodium cation,the chloride anion, and the hydroxide anion, with their charges represented by the sign in a circle, have different numbers of protons and electrons.
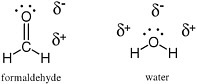
FIGURE 2.3 The oxygen atom is more electronegative than either carbon or hydrogen. Therefore,the bonds between oxygen and these atoms are polarized to place a fractional negative charge (indicated by δ−) on the oxygen atom and a fractional positive charge (indicated by δ+) on the carbon or hydrogen atoms.
carbon and another carbon, is determined. For example, it can be predicted that glucose, whose molecular structure (Figure 2.4) is easily seen to have many carbon-oxygen and oxygen-hydrogen polar bonds, is polar and easily dissolved in water, even though it is not an ion. Such molecules are called hydrophilic (water loving).
Conversely, the molecule octane, a component of gasoline, has only carbon and hydrogen atoms, 8 and 18, respectively, and thus only nonpolar carbon-carbon and carbon-hydrogen bonds. Octane is therefore predicted to be nonpolar and insoluble in water, but soluble in other oils. Such molecules are called hydrophobic (averse to water).
2.2
MOLECULAR REACTIVITY
Molecules that contain only carbon-carbon and carbon-hydrogen covalent bonds are relatively unreactive at standard temperatures.b Even terran life does not generally break unactivated carbon-carbon bonds directly and requires highly reactive species when it does so. The issue is slightly complicated because bonds can break in different ways.A bond strength is usually reported as a homolytic disassociation, in which one of the two electrons forming the bond remains on each of the atoms. Bond dissociation energies describe a bond’s strength after the bond has been broken and no new bonds are being formed. Reactivity is a different concept not unrelated to bond strength but often dependent on environment, because atoms that are no longer bonded can form bonds elsewhere.
The key to chemical reactions, including terran biochemical reactions,at standard temperatures and pressures is the reactivity of carbon-carbon and carbon-hydrogen bonds in molecules that also contain carbon-heteroatom (any atom other than carbon or hydrogen) bonds. Bonds to heteroatoms are often said to activate carbon-carbon and carbon-hydrogen bonds. In terran metabolism, the most important heteroatoms are oxygen and nitrogen, although sulfur is also important, and other heteroatoms such as phosphorus occasionally play a role.
The influence of these heteroatoms on the reactivity of carbon-scaffolded molecules is briefly reviewed in Section 2.4.1. As one unifying principle,hydrogen-heteroatom bonds break and re-form dynamically in water. In contrast, carbon-heteroatom bonds are rarely broken at standard temperatures unless another bond is formed at the same time. In general, for a chemical reaction to proceed under standard conditions at a rate metabolically useful for terran organisms, new bonds must be formed as old bonds are broken, or soon thereafter.
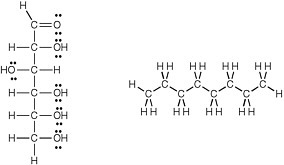
FIGURE 2.4 Structures of glucose (left) a and octane (right). Given the large number of dipolar C-O bonds and the large number of nonbonding electrons (the dots in the structure), one expects glucose to be a hydrophilic molecule that dissolves well in water.In contrast, octane can be seen, by its structure, to contain only nondipolar C-C and C-H bonds, and no unshared electron pairs. Therefore, octane is expected to be a hydrophobic compound insoluble in water.
2.2.1
Reactive Centers in the Structure of a Molecule
The movement of pairs of electrons is key to understanding chemical reactions that involve the making and breaking of bonds (Box 2.1). Nucleophilic and electrophilic centers in a molecular structure are recognized by examining that structure. Nucleophilic centers (Box 2.2) that bear an electron pair prominently in a Lewis structure are easy to spot. Electrophilic centers are frequently less so, especially when the electrophilic center is a carbon atom. For example, the carbon of formaldehyde (H2CO) has all four of its valences occupied and does not seem to have a valence available to form a new bond with anything.
If, however, one of the two bonds between carbon and oxygen breaks, with the electron pair moving from a position between the carbon and the oxygen to a new position on the oxygen, then the carbon center has a valence free. It then welcomes nucleophilic attack from an oxygen atom, such as from any nearby H2O molecule. This process is shown in Figure 2.5.
The nucleophilic and electrophilic centers in a molecule are defined with respect to a specific reaction. Many molecules contain both nucleophilic and electrophilic centers; which centers react under any given circumstances depends on those circumstances and the availability of reaction partners. Only through experience are chemists able to predict, under any given circumstances, which nucleophilic centers in a system will react with which electrophilic centers.
|
BOX 2.1 The Movement of Pairs of Electrons in Reactions Covalent bonds are formed by pairs of electrons. Therefore, the movement of electron pairs is key to understanding chemical reactions that involve the making and breaking of covalent bonds. Any pair of electrons not already involved in a bond is available (at least in principle) to form a new bond. And a pair of electrons originally occupied as part of a bond can form a new bond if the original bond is broken. Organic chemists use curved arrows to describe reactions between nucleophilic and electrophilic centers that produce a new bond. The curved arrow begins with the pair of electrons that will form a new bond in the product. The arrow is drawn to end at a position (on the structures of the reactants) where the electron pair will be after the bond is formed. Figure 2.1.1 shows the reaction of the unshared pair of electrons on the oxygen atom of water (in red) with H+ (a proton) to give H3O+ (the hydronium ion). This simple bond-forming reaction represents the process by which protons move freely in water. 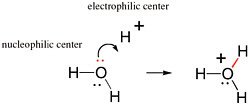 FIGURE 2.1.1 The formation of a new bond between the oxygen atom of a water molecule and a proton to give the hydronium ion. The red pair of electrons on the oxygen is originally unshared and is the pair of electrons used to form the new bond, which is also shown in red. |
|
BOX 2.2 Nucleophilic Centers and the Formation of New Bonds In a chemical reaction, the molecule that contributes the pair of electrons that forms a new bond is called the nucleophile. To form a bond, the electron pair on the nucleophile must find an atom that lacks a bond in its reaction partner, or will soon lack a bond through breakage. This partner is called the electrophile. Thus, in the reaction shown in Figure 2.5, the oxygen in the water molecule was the nucleophilic center, while the hydrogen in H was the electrophilic center. A more complex reaction with water that leads to the motion of protons involves the formation of a new bond between the oxygen atom of water, acting as the nucleophile, and a hydrogen atom of another water molecule acting as the electrophile (Figure 2.2.1). Here, the hydrogen that is acting as an electrophile is originally bonded; to form a new bond to the incoming nucleophile, it must lose the original bond. 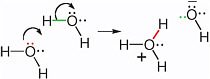 FIGURE 2.2.1 Two curved arrows can be written to show the transfer of a proton from one water molecule to another to give the hydronium ion (H3O+) and the hydroxide ion (HO−). The oxygen carrying the red electrons is the nucleophilic center. It brings the electron pair that is to form the new bond (red). The hydrogen attached to the oxygen in the second water molecule via the green bond is the electrophilic center. |
2.2.2
The Reactivity of Water
As suggested by its structure and the discussion above, water is itself reactive, presenting both a nucleophilic oxygen atom and an acidic hydrogen atom. This has both advantages and disadvantages for a biosolvent. First, because of the availability of an acidic hydrogen, reactions in water never lack for this species. Thus, reactions that require the equivalent of an H+ can always find one from water, so easily that many chemists ignore the movement of protons when they consider reactivity in biology. Protons can always move easily in water. They can always be gained, and they can always be lost.
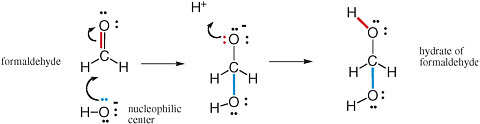
FIGURE 2.5 Reaction of the electrophilic carbon of formaldehyde with the nucleophilic oxygen of hydroxide gives the hydrate of formaldehyde. Note the concerted formation of a new bond (blue) using the unshared pair of electrons (also blue) on the oxygen nucleophilic center with the breaking of a bond (red). This bond breaking delivers an unshared pair of electrons (red) to the oxygen intermediate. These electrons can form a new bond (red) to an electrophilic proton.
The reactivity of water creates problems as well. In particular, many molecules are unstable in water. This generalization applies to many molecules important in terran metabolism, catalysis, and genetics. In some cases, molecules simply decompose through reaction in water, and require another round of metabolism for replacement. For genetic molecules, damage by water must be repaired.
2.3
MOLECULAR STABILITY
2.3.1
Chemical Bonds Have Different Strengths
The formalism outlined above that describes chemical bonding concerns covalent bonds. Table 2.1 lists the strengths of typical covalent bonds found frequently in terran biochemistry. The precise value of the energies associated with bond breaking depends on the context of the molecule.
To place the numbers in Table 2.1 in perspective, at one atmosphere of pressure at room temperature in a balloon filled with dihydrogen gas, the volume must be on the order of the size of the Milky Way Galaxy for there to be a 50 percent chance that it will contain one dissociated molecule. In human terms, the covalent bond between two hydrogen atoms is thus very strong. At reduced pressures, dihydrogen is more easily dissociated into two constituent atoms at equilibrium. Thus, the equilibrium H2=H· + H· (where · represents a single electron, and H· represents a hydrogen atom) is equally distributed between species on each side of the equation at concentrations of 10−71 molar.
Molecules do not need to have formal covalent bonds to associate. For example, solids are associates of molecules held together by weaker bonds. Liquids, including water, also arise through weak association of molecules that do not involve intermolecular covalent bonding. Some interactions, such as between Na+ and Cl− in table salt, are strong until the compound comes into contact with a solvent such as water, which interacts strongly with the separated ions.
Chemists have a variety of names to describe these weaker bonds. Bonds between Na+ and Cl− in table salt are often referred to as ionic bonds. Weaker interactions between the positively and negatively charged ends of dipolar molecules are also possible. In particular, a hydrogen atom attached to a heteroatom (in terran biochemistry, the nitrogen-hydrogen and oxygen-hydrogen bonds are particularly important) generally carries a significant fraction of a positive charge. Therefore, the hydrogen interacts strongly with the negatively charged ends of dipolar molecules (such as the oxygen atom of water). This type of interaction is often called a hydrogen bond. Hydrogen bonds are typically 5 percent as strong as a typical covalent bond. Hydrogen bonds between the hydrogen-oxygen and oxygen units of different water molecules account for the ability of water to form a liquid at standard terran temperatures and pressures (Figure 2.6).
TABLE 2.1 Bond Energies of Typical Covalent Bonds Found Frequently in Terran Biochemistry
|
Covalent Bond |
Energy (kilojoules/mol) |
|
H—H |
436 |
|
H—C |
413 |
|
C—C |
348 |
|
H—N |
391 |
|
N—N |
170 |
|
H—O |
366 |
|
O—O |
145 |
|
C=C |
614 |
|
C—S |
272 |
|
N≡N |
945 |
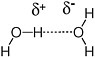
FIGURE 2.6 A hydrogen bond between two water molecules accounts for the liquid phase of water at standard temperatures and pressures.
Even the concept of covalent bonding has a degree of anthropocentricity. A covalent bond is, operationally, a bond that is largely stable at temperatures and pressures where humans live. At lower temperatures, a single hydrogen bond can hold together a molecular complex for as long a time as a covalent bond holds together a complex at room temperature.
2.3.2
Temperature Limits on Organic Molecular Stability
The strength of bonds between atoms is undoubtedly a universal feature, the same on Earth as elsewhere in the solar system, in other systems in the galaxy, and indeed in other galaxies throughout the universe. This implies a universal range for the temperature at which life based on carbon, hydrogen, oxygen, and nitrogen is possible.
Bond strengths make it difficult to imagine life based on carbon-backboned chemistry at temperatures above 600 K, which corresponds to 327°C, or 620°F—not dramatically higher than the temperature of a typical kitchen oven set at its highest setting. This expectation applies strictly to life at terran sea-level pressure, given that some decomposition reactions are significantly slower at higher pressure. But presumably it is possible to rule out carbon-based life at temperatures over 800 K at any pressure.
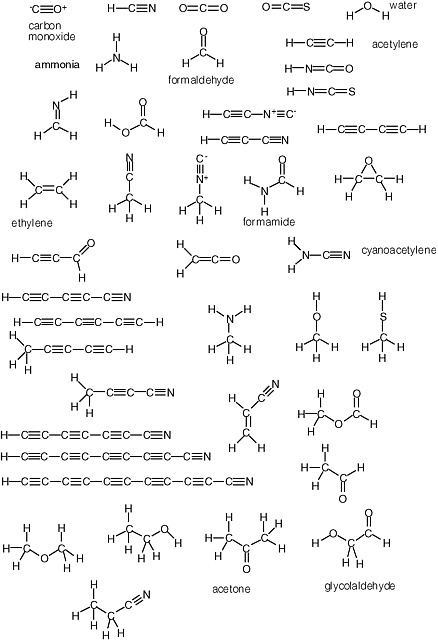
Carbohydrates are especially unstable, even at temperatures well below the boiling point of water. This instability arises from the fact that they contain a C=O unit. Because of this instability, some have proposed that carbohydrates could not have been part of genetic molecules in early life, as they are in modern life, at least until advanced metabolic repair, and sequestration became available to manage their reactivity. Indeed, until recently, no nonbiological process was well established that would yield carbohydrates under plausibly prebiotic conditions and in sufficient concentrations before the carbohydrates were then destroyed under the same conditions in which they were formed.1 The simplest carbohydrate that has been observed in the interstellar medium is formaldehyde; the most complex, glycolaldehyde (Figure 2.7).
There is no similar low-temperature limit for carbon-based life. The temperature throughout most of the cosmos is much colder than standard temperature, and organic species are expected to be abundant. Indeed, microwave spectroscopy has established the presence of a large number of these in interstellar gases (Figure 2.8).
2.4
MOLECULAR REACTIVITY IN TERRAN LIFE: METABOLISM
Metabolism includes the set of all chemical reactions that occur within a living system. It is often formally limited to those reactions that are controlled by catalysts encoded by the genome of that system. These reactions result in the extraction of energy and raw materials from the environment. They also lead to synthesis of macro-
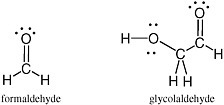
FIGURE 2.7 Formaldehyde and glycolaldehyde.
molecules required for cellular function and to synthesis of compounds that allow an organism to manipulate its environment and interact effectively with other organisms in the environment.
2.4.1
Heteroatoms Confer Reactivity to Hydrocarbons to Enable Metabolism
The stability of carbon-carbon and carbon-hydrogen bonds at standard temperature and pressure means that stable organic compounds are easily available to forms of life in such environments. It also means that compounds built exclusively from carbon and hydrogen do not easily react and—in biological terms—that organic compounds containing only carbon and hydrogen atoms are not easily metabolized.
Heteroatoms create opportunities for reactivity by activating carbon-carbon and carbon-hydrogen bonds, in general by offering a place for electrons to go during a reaction sequence. Oxygen and nitrogen are frequently used in terran life for this purpose.Indeed, virtually every pathway in central metabolism exploits the electrophilicity of carbon doubly bonded to nitrogen or oxygen (C=N or C=O), or the electrophilicity of phosphorus doubly bonded to oxygen (the core of phosphate metabolism and terran bioenergetics).
Many reactions in terran metabolism are simple variants of a few basic reactions of carbon compounds containing a carbon atom doubly bonded to oxygen. For example,glycolaldehyde has a proton attached to a carbon next to its C=O carbonyl group. This proton can be removed by a base (calcium hydroxide is useful) in a reaction represented by curved arrows indicating delivery of electrons to the electronegative oxygen atom (Figure 2.9).
The curved arrows in Figure 2.10 show how electrons move in this process,which is commonly called enolization.In the figure, green electrons originally used to form the bond between the proton and the rest of the glycolaldehyde form a second bond between the two carbons of glycolaldehyde. The red pair of electrons, which originally formed the second bond between carbon and oxygen in the glycolaldehyde, goes to the oxygen atom,giving it a negative charge. To remove the proton, a pair of electrons (blue in Figure 2.10) from a hydroxide forms a new bond to the moving proton and generates a water molecule. This process is the enolization of glycolaldehyde.
The carbon that lost a proton is now a nucleophilic center and can therefore react with formaldehyde. As mentioned above, formaldehyde has an electrophilic center on carbon. Therefore,the carbon of formaldehyde can react with the nucleophilic carbon on the enolate of glycolaldehyde to form a new compound containing three carbon atoms, a three-carbon carbohydrate called glyceraldehyde. The overall reaction sequence is often called an aldol addition reaction, here of formaldehyde and glycolaldehyde.
Much of terran metabolism includes variants of these basic processes.
2.4.2
The Energetic Requirements for Metabolism
Metabolism cannot occur in a system that is at thermodynamic equilibrium.The synthesis of molecules and the construction of cellular structures require energy that an organism must obtain from the environment and that
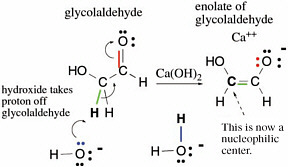
FIGURE 2.9 An archetypal set of reactions in terran metabolism,illustrating how the presence of bonds to heteroatoms (here, a double bond to oxygen)activates a C—H bond for breakage, a bond that would not break under standard conditions without that activation.
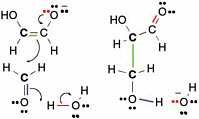
FIGURE 2.10 The enolate of glycolaldehyde, containing a new nucleophilic center, can react with an electrophilic center on formaldehyde to form a new C—C bond. This is another archetypal reaction in terran metabolism.
must be coupled to processes within the organism, rather than dissipated as heat or used to stimulate the formation of unreactive substances. One useful form of coupling leads to the formation of more-reactive compounds.
Except in the unusual case where the energetic compounds are used immediately, that energy must be stored in a chemical or physical form. Energy is stored in two general ways in terran organisms, either as a gradient of concentrations of species across a physical structure (e.g., a biological membrane) or in a molecule that has a high free energy of reaction under the conditions that prevail inside the organism. Adenosine triphosphate (ATP) is a well-known example of the latter.
Terran life uses photons from the Sun and thermal energy from the chemical disequilibria generated by Earth’s internal heat sources as its primary sources of external energy. Photosynthesis is the primary process for obtaining energy from the Sun. Moving along the food chain, nonphotosynthetic systems must obtain their energy from high-energy compounds that they consume.
Often, this energy is transferred along the food chain via high-energy species. Whether obtained directly or from the environment, the energy in those species is used by their transformation to lower-energy species. These transformations often involve the movement of atoms and electrons to other organic or inorganic acceptors. The energy can also be transferred to a new molecule by metabolic coupling with a synthetic process that requires energy.
Certain patterns of metabolism have emerged on Earth. Multicellular organisms, without exception, use O2 as a terminal electron acceptor. Plants obtain energy from light and carbon from CO2, and animals obtain both energy and carbon from metabolizing ingested organic compounds. Microbes display a much greater diversity in metabolic strategies. Table 2.2, which shows examples of the range of electron donors and acceptors used by microbes, illustrates the range of possible strategies for harnessing energy available from redox couples in the environment.
2.4.3
Terran Life Has a Common Set of Reactions That Form a Core Metabolism
A century of study of metabolism in terran molecules has revealed a common core of metabolic processes through terran life. This commonality exists, in part, because of common features throughout terran genetics and biocatalysis. The core includes the tricarboxylic acid cycle, glycolysis, and synthetic pathways for construction of simple amino acids, sugars, purines, and pyrimidines.
This core was presumably required for primitive terran organisms to conduct their fundamental metabolism. A subset of the core processes furnished the building blocks of cellular macromolecules. Many organisms have shed pieces of the universal core as a consequence of adaptation to particular ecological niches in which certain metabolic abilities are dispensable.
Some variations on the universal core are seen. For example, there are five pathways for biosynthesis of the key amino acid lysine, three beginning with aspartate and two beginning with -ketoglutarate. In both cases, the early steps in the pathway are identical, and variations occur in the final steps.
Nevertheless, the universality of this metabolic core that is still distinguishable 3.8 billion years after the emergence of life on Earth is consistent with the ribosomal RNA evidence that all known forms of life on Earth have descended from a common progenitor.
TABLE 2.2 Examples of Electron Donors and Acceptors Used by Microbes
|
Electron Donor |
Electron Acceptor |
Example Organisms |
|
Phosphite (HPO3−2) |
Sulfate (SO4−2) |
Desulfotignum phosphitoxidans |
|
Hydrogen |
O2 |
Ralstonia eutrophus |
|
Hydrogen |
Fe+3 |
Acidothiobacillus ferrooxidans |
|
Sulfide |
O2 |
Sulfolobus acidocaldarius |
|
Sulfide |
Nitrate |
Thiobacillus denitrificans |
|
Sulfur |
O2 |
Acidianus ambivalens |
|
Ammonium (NH4+) |
O2 |
Nitrosomonas sp. |
|
Nitrite |
O2 |
Nitrobacter sp. |
|
Fe+2 |
O2, nitrate (NO3−) |
Acidothiobacillus ferrooxidans |
|
Organic compounds |
Fe+3 |
Acidiphilium cryptum |
|
Organic compounds |
Nitrate (NO3−) |
Paracoccus denitrificans |
|
Organic compounds |
Arsenate |
Desulfosporosinus sp. strain Y5 |
|
Organic compounds |
Mn+4 |
Shewanella putrefaciens |
|
H2, organic compounds |
Sulfate |
Desulfovibrio sp. |
|
H2, formate, CO, alcohols |
CO2 |
Methanogens |
2.5
CATALYSIS
Even when organic molecules are activated by heteroatoms, chemical reactions that form and break carbon-carbon bonds are slow at standard temperature and pressure. Thus there is a need for catalysis, a process involving the addition of an exogenous substance (the catalyst) that speeds the rate of chemical reaction, but survives the reaction unchanged.
Catalysts are central to life. First, the catalysts can be derived from inherited information. This permits genetics and, ultimately, Darwinian processes, to determine what reactions occur within the confines of a living system. Conversely, reactions that are sufficiently fast that they do not require catalysis are not under the control of the living system. Therefore, they cannot be part of a Darwinian process that leads to improved adaptation of the organism.
Many of the chemical reactions that are at the core of terran metabolism are extremely slow in the absence of catalysis (see Table 2.3). Simple monomers can generate rate enhancements of 1,000-fold.2 Highly evolved protein catalysts accelerate reactions by 7 to 19 orders of magnitude.3
In addition to simply accelerating chemical reactions, catalysts play an important role in channeling molecules toward formation of particular products. Typical abiotic reactions yield a mix of products. Reactions under the control of biological catalysts generally yield a single product, because the catalysts accelerate the rate of a particular reaction far beyond the rates of competing, uncatalyzed reactions. Indeed, catalysts can foster reactions that simply would not occur in the absence of catalysis because of competing reactions. In terran biocatalysts, this selectivity is achieved by sequestration of reactive intermediates at an active site in which solvent has been excluded, and by orientation of reacting centers to promote interactions between particular atoms.
Polymeric biological catalysts are called enzymes. Terran enzymes are, in most cases, protein molecules. Typical bacteria have on the order of 2,000 protein enzymes. Proteins are excellent catalysts because they fold to give stable yet dynamic conformations, and deliver functionality from the side chains of the 20 amino acids into regions of space where they are most useful for catalysis. The 20 amino acids that make up proteins provide structural diversity that can be used to develop binding pockets that are highly specific and bind substrates with high affinity. Further, many of these amino acids provide functional groups that participate in catalysis (see Figure 2.11). Many enzymes also contain cofactors that expand the range of chemical reactions that can be catalyzed. These include metal ions and metal ion clusters, as well as a range of organic cofactors.
TABLE 2.3 Bond Energies of Typical Covalent Bonds Found Frequently in Terran Biochemistry
|
Reaction |
Half-life for Spontaneous Reaction in Water at 25°C |
|
Hydration of fumarate |
7 × 105 years |
|
Isomerization of triose phosphate |
2 days |
|
Decarboxylation of orotidine 5'-monophosphate |
8 × 107 years |
|
Peptide bond hydrolysis |
450 years |
|
Phosphodiester bond hydrolysis |
>13 × 106 years |
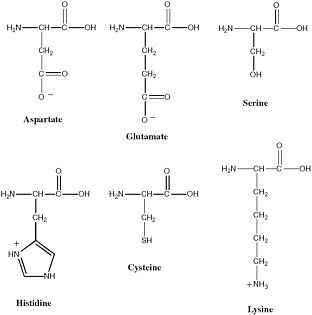
FIGURE 2.11 Amino acids whose side chains are most important in catalysis at enzyme active sites.
2.6
MACROMOLECULAR STRUCTURE IN TERRAN LIFE
Many functions critical for terran organisms are provided by macromolecules. Biological catalysis is performed largely by proteins. Hereditary information is generally stored in DNA. Compartmentalization is usually achieved using lipid bilayer membranes. Structural integrity is provided by polysaccharides and/or proteins. Movement on the nanometer to macroscopic scale is provided by special proteins that undergo specific and directional conformational changes.
Although specific macromolecules have evolved to provide these functions in life on Earth, it is possible that some or all of these functions might be provided by monomers or by different macromolecules in other forms of life. However, all things are not possible, as the physicochemical characteristics of macromolecules conferred by their molecular structures inherently limit their functional properties. Consideration of alternative macromolecules to serve these functions must be realistic in terms of structure-function relationships of particular macromolecules.
For example, polysaccharides are not likely to provide motor molecules, and proteins are not well suited to carry genetic information. Particular features of nucleic acids and proteins make them well suited for the functions they serve in life on Earth. An understanding of these features provides an intellectual framework for thinking about alternative macromolecules in other forms of life.
DNA is well suited to serve as the repository of hereditary information for many reasons. First, it is double-stranded, and the double strands are complementary. This permits a simple mechanism for replication, as noted by Watson and Crick. Further, the resulting redundancy provides correct sequence information in case of damage to one strand of DNA. The poly-anionic backbone causes DNA to adopt an extended structure that facilitates replication. Importantly, this extended structure is quite insensitive to the exact sequence of bases in the DNA.4,5 Finally, the interaction between the two complementary strands that is mediated by hydrogen-bonding interactions between the Watson-Crick-model faces of the bases is strong enough to provide molecular recognition and structural integrity, but not so strong that the strands cannot be easily separated to allow replication.
The connection between DNA and proteins involves the use of RNA as an intermediate message, as well as a triplet genetic code. Codes that utilize a different number of bases or different sizes of codons have been considered and are conceivable. However, if we assume that approximately 20 amino acids are required to create good protein structures, and the encoding molecule (on Earth, DNA) is built from four alphabetic letters, then most other coding strategies have either too little coding capacity or far too much. Only a triplet code using four nucleobases and a doublet code using six nucleobases have coding capacities in a range that might encode 20 amino acids. Of course, larger genetic alphabets, including those with six or more nucleobases, would allow other possibilities.
The life that we know also uses proteins for the majority of structural and catalytic functions. Proteins are particularly suited for these functions because of the structural properties of polymers of amino acids. The polyamide backbone of proteins is neutral, unlike that of nucleic acids. Further, the backbone has a repeating dipole able to make hydrogen bonds. These structural features are exploited as proteins fold into globular structures, as they promote the formation of stable secondary structures such as alpha helices and beta sheets.
The planarity of the amide linkers in the protein backbone restricts rotation around the carbon-nitrogen bond. This provides some restrictions on the number of conformers that can be adopted. The linkage joining amino acids in a polymer is quite stable, but not infinitely so, and it can be relatively easily hydrolyzed by enzymes to allow turnover of proteins within cells. This propitious combination of properties is conferred by the amide bonds linking the amino acids in the polymer; polymers linked by ester, thioester, ether, or carbon-carbon bonds lack one or more these properties.
Life as we know it builds its proteins primarily from the same 20 amino acids, although there are many other amino acids that might have been utilized. While it is important that the collection of amino acids used in proteins includes a sufficient number of small, large, hydrophilic, hydrophobic, and charged amino acids, the exact identities of the amino acids in each of these classes may not be critical. Moreover, the amino acids utilized for protein synthesis by familiar life are all L-amino acids, and there is no reason to think that D-amino acids could not have been utilized instead.
2.7
SUPRAMOLECULAR STRUCTURE IN TERRAN LIFE
Supramolecular structures include aggregates of biomolecules held together by noncovalent bonds. These enable many functions that are key to terran life and are presumed to be key to life generally.
2.7.1
Compartmentalization Arises from Supramolecular Structures
Compartmentalization is one of these key functions. Compartmentalization is global in terran life forms, which are built from cells that span a wide range of sizes. Cellular structure is so widespread on Earth that a central theory in biology is known as cell theory. An unexpected form of life that would be considered “weird” would be a life form that does not exploit cells but achieves a distinction between “self” and “non-self” that is not “cellular” as we define the term.
2.7.1.1
Advantages of Compartmentalization
Compartmentalization allows the concentration of molecules so that the rates of second-order chemical reactions can be increased over what could be achieved in, for example, a bulk ocean. Modern cells concentrate molecules in the cytoplasm to an amazing level; the concentrations of many metabolites are in the high micromolar or even millimolar range.
Compartmentalization also allows protection of sensitive molecules, both small metabolites and the macromolecules that serve structural, catalytic, and genetic functions, from harsh external conditions. This is especially evident for terran organisms that live in environments with a high or a low pH. Organisms that live in such environments maintain their internal environment at nearly neutral pH. The compartment walls allow them to do so.
Further, compartmentalization allows cells to control the import of nutrients and the export of products that manipulate the environment, or excrete waste. These include siderophores that enhance uptake of metal ions, quorum-sensing molecules that inform cells of the presence of others of their kind, molecules that form biofilms that allow attachment to surfaces and protection from mechanical and chemical stress, and toxins that inhibit the growth of competitors.
Important properties of a boundary between inside and outside can be identified in terran cells. Most importantly, the permeability of the boundary must be low enough to prevent loss of valuable metabolites, but high enough to allow the import of nutrients and the export of waste. In terran extant life, the permeability of cell boundaries is low, and transport processes are carried out by proteins embedded in the boundary. The boundary must be fluid rather than solid, to facilitate both transport and incorporation of new components during enlargement prior to cell division. Furthermore, a fluid boundary allows cells to engulf objects in their environments. This ability is important for predation in terran life.
2.7.1.2
Compartmentalization Exploits the Low Polarity of C—C and C—H Bonds
All known life forms on Earth are surrounded by bilayer membranes that exploit the unique behavior of molecular structures that have, in one part, hydrophobic units built primarily from nonpolar carbon-carbon and carbon-hydrogen bonds and, in another part, polar bonds involving heteroatoms. The first part of the molecule is insoluble in water and therefore aggregates. The second part is soluble in water and presents itself to bulk water solvent, sequestering the hydrophobic parts of the molecule to the interior.
The detailed structure of membranes is not uniform in terran life (Figure 2.12). Membrane bilayers in bacteria arise from supramolecular organization of phospholipids, with hydrophobic fatty acids attached to a variety of polar head groups by ester linkages. Eukaryotic membranes also contain phospholipids, but they are distinctive in the inclusion of sterols (such as cholesterol), another class of hydrophobic molecules.
Archaeal membranes, in contrast, contain lipids in which the hydrophobic tails are linked to the head groups by ether linkages, rather than ester linkages. Since ether linkages are more stable to heat and extremes of pH, this feature is presumed to be important to the ability of Archaea to survive in extreme environments.
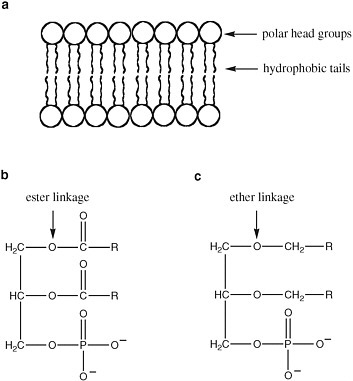
FIGURE 2.12 (a) The structure of a bilayer lipid membrane; (b) the structure of the glyceryl esters that are major components of bacterial and eukaryotic cell membranes; and (c) the structure of the glyceryl ethers that are major components of archaean cell membranes. R is a hydrophilic chain of CH and CH2 atoms.
Further diversity in terran membrane architecture is found in the presence of a second structure enclosing the cell. Some microbes have only a single membrane. However, some bacteria (referred to as gram-negative bacteria because of their staining properties) have both an inner and an outer membrane, between which is a layer of polymeric material (peptidoglycan) that provides structural rigidity. In contrast, eukaryotic cells contain a number of compartments, each of which is bounded by a membrane. These compartments include the nucleus, the mitochondria, the chloroplast, lysozomes, the Golgi apparatus, and the endoplasmic reticulum. Thus, eukaryotes use membranes to organize the structure and activities of larger and more complex cells. Some of the membranes are the vestiges of endosymbiosis.
2.7.1.3
Compartmentalization Assists in the Generation of High-energy Compounds
The membrane barrier surrounding cells is a key component in energy metabolism, because processes that harvest energy from either light or oxidative reactions result in storage of energy as an electrochemical gradient across the membrane. This gradient is subsequently used to drive energy-requiring processes such as synthesis of ATP or accumulation of nutrients against a concentration gradient. ATP is the fundamental unit of energy currency used by cells; its hydrolysis can be coupled to a large number of endergonic processes to result in an energetically favorable process.
Some, but not all, of the benefits conferred by compartmentalization inside lipid bilayer membranes can be achieved by surfaces. Surfaces allow concentration of metabolites, and, if the surface is a mineral that is not in redox equilibrium with its surroundings, a surface can provide a source of energy. Compartmentalization can also be achieved inside porous minerals. For example, the walls of hydrothermal vents are porous and trap organic material6 and may indeed have provided the first compartmentalization that allowed the emergence of metabolism and macromolecules in a protected environment.7 Also, there are tiny pores in rocks, including tubes in chroysotile, and there are microcracks in quartz, both of which could support diffusion and dispersion by currents.
2.7.2
Supramolecular Soluble Structures
Supramolecular structures are found throughout biology. These are assemblages of covalently bound molecules that form functional aggregates using noncovalent bonding.
One particularly important example is also an exception to the rule that catalysis in terran life is carried out by protein enzymes. This is the ribosome, the molecular machine that synthesizes proteins using the information provided by a messenger RNA. It is an ancient machine and a key component of every known form of life on Earth. The ribosome is a large complex of at least three RNA molecules and more than 50 proteins. The structure of the large subunit of the ribosome was solved in 2000,8 followed closely by the structure of the entire ribosome.9 Consistent with the “RNA world” model, RNA was present at the site in the ribosome where the peptide bond is synthesized.10 The active site is composed entirely of RNA. Nearly all catalytic functions in living organisms have been taken over by proteins, which are inherently more suited for catalysis than RNAs. However, at the heart of this centrally important and ancient molecular machine, catalysis is still being executed by the RNA.
2.8
THE RELATIONSHIP BETWEEN WATER AND BIOMOLECULES
2.8.1
Adaptation of Terran Biomolecules to Water
Terran life uses water as a solvent. As expected, terran biomolecules have multiple signatures of their compatibility with water as a solvent. Further, terran biochemistry exploits the distinction between polar molecules, which are soluble in water, and nonpolar molecules, which are not. This is exemplified in the use of hydrophobic interactions as a way to fold proteins and organize supramolecular structures, inter alia.
Water also constrains the structure of carbon-containing biomolecules involved in metabolism. At the very least, for life to exploit the dynamic behavior of species dissolved in a solvent, the biomolecules serving as metabolic intermediates must be soluble in that solvent at appropriate concentrations. This is the simplest explanation for the prevalence of hydroxyl groups in organic molecules central to biological metabolism. Glucose has five and dissolves well in water.
Charge also helps a molecule to dissolve in water. Accordingly, many intermediates in terran metabolism are charged. Most typically, an uncharged molecule acquires a charge through phosphorylation. Other metabolic intermediates are intrinsically charged. For example, the citric acid cycle is based on tri- and dicarboxylic acid intermediates. These are ionized at physiological pH, making them very soluble in water. Terran metabolism also exploits the metabolic reactivity of the C=O carbonyl group, which supports chemical reactions in water well.
The compatibility of a biomolecule with water is seen especially well in genetics. The DNA and RNA molecules that transfer genetic information have a repeating charge in their backbone, carried by the phosphate linkers. This repeating negative charge makes DNA and RNA extremely soluble in water. It also prevents their passing across hydrophobic membranes. Further, the nucleobases that encode the genetic information are, by comparison, hydrophobic. Therefore, they lie inside the double helix, away from water, in base pairs that are well known features of the Watson-Crick model for duplex nucleic acids.
2.8.2
Disadvantages of Water for Terran Biomolecules
Water, however, carries both nucleophilic and electrophilic centers. This means that water reacts with many biomolecules in a way that damages them. In the case of proteins, as noted above, water reacts with the amide backbone to degrade proteins, generating amino acids as hydrolysis products (see Figure 2.13). This can be disadvantageous if the protein is desired, as it requires that the protein be re-synthesized. The turnover of proteins is important, however, in any system living in a dynamic environment. Thus, the hydrolytic instability of proteins in water is key to maintaining life.
The disadvantageous reactivity of water is especially obvious when considering RNA and DNA (see Figure 2.14). Cytidine, for example, hydrolytically deaminates to give uridine with a half-life of ca. 70 years in water at 300 K.11 Adenosine and guanosine also hydrolytically deaminate in water at only slightly slower rates. A more recent study at the base level affords the following half-lives for the bases at 298 K: cytosine, 340 years; adenine and guanine, about 10,000 years.12 As a consequence, terran DNA in water is continuously damaged in a way that causes it to lose its genetic information. In modern terran life, this continuous water-generated damage is mitigated through continuous repair.
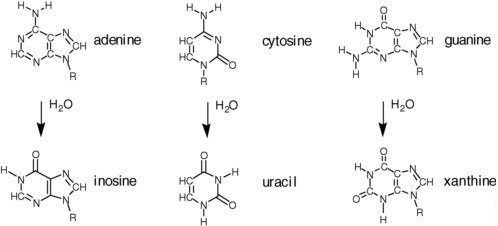
FIGURE 2.13 Hydrolytic degradation of the standard nucleobases.
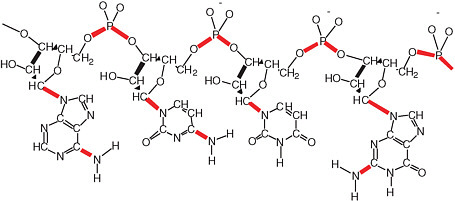
FIGURE 2.14 A generic RNA molecule. The bonds shown in red are thermodynamically unstable with respect to hydrolysis in water. Each of these bonds represents a problem for prebiotic synthesis, as well as the maintenance of the genetic information in RNA in modern life. Today, the aggressive reactivity of water with respect to molecules like RNA and DNA is mitigated by sophisticated repair systems. It is difficult to imagine such complex repair systems having been present at the dawn of life. This conundrum underlies the most significant paradox in the structure of genetic matter with respect to the origin of life. On the one hand, the repeating charge in the backbone suggests that the molecule worked in a hydrophilic solvent such as water. On the other hand, the abundance of easily hydrolyzable bonds suggests that RNA could not have been easily assembled in water.
2.9
REFERENCES
1. See, for example, Breslow R., 1959, On the mechanism of the formose reaction, Tetrahedron Lett. 21:222-226; Butlerow, A., 1861, Bildung einer zuckerartigen Substanz durch Synthese. Annalen 120:295-298; and Ricardo, A., Carrigan, M.A., Olcott, A.N., and Benner, S.A., 2004, orate minerals tabilize ibose, Science 03:1196.
2. Weber, A.L. 2001. The sugar model: Catalysis by amines and amino acid products. Orig. Life Evol. Biosph. 31:71-886.
3. Wolfenden, R., and Snider, R. 2001.The depth of chemical time and the power of enzymes as catalysts. Accounts Chem. Res. 34:938-945.
4. Benner, S.A., and Hutter, D. 2002. Phosphates, DNA, and the search for nonterran life: A second generation model for genetic molecules. Bioorg. Chem. 30:62-880.
5. Richert, C., Roughton, A.L., and Benner, S.A., 1996. Nonionic analogs of RNA with dimethylsulfone bridges. J. Am. Chem. Soc. 118:4518-4531.
6. Takano, Y., Marumo, K., Ebashi, T., Gupta, L.P., Kawahata, H., Kobayashi, K., Yamagishi, A., and Kuwubara, T. 2005. In situ ore formation experiment: Amino acids and amino sugars trapped in artificial chimneys on deep-ssea hydrothermal systems at Suiyo Seamount, Izu-Bonin Arc, Pacific Ocean. Bull. Chem. Soc. Jpn. 8:638-651.
7. Martin, W., and Russell, M. 2003. On the origin of cells. A hypothesis for the evolutionary transitions from abiotic geochemistry to chemoautotrophic prokaryotes, and from prokaryotes to nucleated cells. Phil. Trans. Roy. Soc. Lond. 58:559-885.
8. Ban, N., Nissen, P., Hansen, J., Moore, P.B., and Steitz, T.A. 2000. The complete atomic structure of the large ribosomal subunit at 2.4 Å resolution. Science 89:905-920.
9. Yusupov, M.M., Yusupova, G.Z., Baucom, A., Lieberman, K., Earnest, T.N., Cate, J.H.D., and Noller, H.F. 2001. Crystal structure of the ribosome at 5.5 Å resolution. Science 292:883-896.
10. Ban, N., Nissen, P., Hansen, J., Moore, P.B., and Steitz, T.A. 2000. The complete atomic structure of the large ribosomal subunit at 2.4 Å resolution. Science 89:905-920.
11. Frick, L., Mac Neela J.P., and Wolfenden, R. 1987. Transition state stabilization by deaminases. Rates of nonenzymic hydrolysis of adenosine and cytidine. Bioorg. Chem. 5:100-108.
12. Levy, M., and Miller, S.M. 1998. The stability of the RNA bases: Implications for the origin of life. Proc. Natl. Acad. Sci. U.S.A. 95:7933-7938.