5
Origin of Life
To search for additional constraints on the limits of life, the committee considered topics related to the origin of life. There is a clear distinction between environments that are habitable and environments that might support the emergence of animate matter from inanimate matter. Indeed, many observers believe that although the surface of modern Earth is a habitable environment, life could not emerge here now. According to that thinking, the dioxygen that is present in today’s terran atmosphere would be toxic to any primitive life form that might emerge spontaneously.
It is conceivable that if we understood the processes by which life arises, we might constrain the existence of life to a small number of locales, to a similar array of organic species, or to a smaller number of liquid phases than the more general thermodynamic-structure-solvent trichotomy, discussed above, would tolerate. That would, in turn, be considered alongside emerging models of planetary formation to provide better direction for the targets of National Aeronautics and Space Administration (NASA) missions to the solar system.
Fifty years’ worth of effort has shown that it is difficult to model how life might have originated in any specific environment. The committee recognized that it is still more difficult to consider how life might have arisen in a generic environment. The details of an environment almost certainly determine how life might emerge.
The environment on early Earth is not well defined. The committee recommends that more information be obtained from missions, especially to comets, and that models of planetary formation continue to be developed. It is not certain even that terran life originated on Earth. On the basis of various considerations—including the abundance of water, the paucity of some minerals, and the nature of the atmosphere modeled for early Earth—various authors have suggested that life on Earth originated elsewhere, including locales as nearby as Mars and as distant as galactic nebulas. The geological record is considered to be intact for 4.5 Ga on Mars and Ceres, so there may still be mineralogical and isotopic signatures indicating past life. However, the detection of signs of past life would not be evidence that Mars or Ceres had an origin of life separate from Earth’s origin, nor would it provide evidence that panspermia occurred. In the absence of firm information concerning the requirements for the origin of life as we know it and the mechanisms of its formation, such speculation seems premature. Our knowledge of the environments in those remote locales several billion years ago is even less complete than our understanding of early Earth.
It is also not clear that terran life originated on Earth in the chemical form that we know now. Respectable hypotheses suggest, for example, that the three-biopolymer (DNA-RNA-proteins) system that characterizes all life that we know on Earth, a system in which nucleic acids serve roles predominantly in genetics and information transfer and proteins serve roles predominantly in catalysis, may not have been characteristic of life as it first originated. Only one of these complex chemical entities may have been represented in primitive life, or perhaps none at all.
Several hypotheses argue that RNA was the only genetically encoded component of biological catalysis during an earlier episode of life on Earth. Some others view that statement as true for the very first form of life on Earth (the RNA-first hypothesis). Others have argued that the first form of life on Earth was supported by genetic molecules that had structures quite different from the structure of DNA or RNA.
Some have even argued that the original genetic material was mineral, not organic.1,2 They suggest that a truly primitive replicator might have been a layered inorganic mineral, crystallizing from solution and in the process amplifying some particular permutation of stacking: either identical layers stacked on top of each other in different orientations or stacks of two or more chemically different layers. The “information” would be the particular stacking sequence of a crystal displayed like a bar code on its edges and maintained and extended through crystal growth with ions, or small molecular units, adding only to the edges. The stacking sequences would also specify particular phenotypic properties that would allow Darwinian competition.
As is evident in Section 5.7, a case can be made that the earliest forms of life on Earth contained no macromolecules at all and that heredity was carried by monomers3—still another route for future exploration.
5.1
LABORATORY SYNTHESIS OF ORGANIC MONOMERS
It has been more than 50 years since Stanley Miller first explored electrically induced chemical reactions that might convert simple gases into small organic molecules.4 The production of amino acids was especially easily demonstrated. More recently, the highly reducing atmosphere used by Miller has fallen out of favor as representative of the likely atmosphere on early Earth (although Kasting has shown that the impact of a large asteroid with iron causes a transient reducing atmosphere5). Even with more contemporary models of early planetary atmospheres, however, electrical discharge, ultraviolet radiation, and other sources of energy are suitable for creating organic species. For example, Box 5.1 lists compounds, called “tholins,” produced from relatively oxidizing environments under these conditions.
TABLE 5.1 Carbon in the Murchison Meteorite
|
Total carbon |
2.12%, 1.96% |
|
Carbon as interstellar grains |
|
|
Diamond |
400 ppm |
|
Silicon carbide |
7 ppm |
|
Graphite |
<2 ppm |
|
Carbonate minerals |
2-10% of total carbon |
|
Macromolecular carbon |
70-80% of total carbon |
|
SOURCE: Modified after J.R. Cronin, “Clues from the Origin of the Solar System: Meteorites,” pp. 119-146 in The Molecular Origins of Life: Assembling Pieces of the Puzzle, A. Brack A. (ed.), Cambridge University Press, Cambridge, U.K., 1998. |
|
Similar experiments have generated nonbiological routes for the synthesis of other organic molecules, including some molecules that are used in our own biochemistry. For example, the Oró-Orgel synthesis exploits the reactivity of HCN to make adenine (C5H5N5), one of the five nucleobases used to store information in DNA and RNA. Analogous synthesis generates adenine from formamide.
The complexity of the products of adding energy to simple organic mixtures, including the complexity of tholins, has a disadvantage. The diversity of products is so great in such experiments in prebiotic chemistry that they do not greatly limit the inventory of organic species that might have been present on early Earth.
5.2
NATURAL AVAILABILITY OF BIOLOGICAL-LIKE MOLECULES
5.2.1
Biological-like Molecules from the Cosmos
There is little doubt that natural processes generate organic molecules analogous to those generated by the laboratory experiments described above. Amino acids are found in natural specimens, including meteorites, that are almost certainly not influenced by biological processes. They include many amino acids that are not part of the human-like standard collection of encoded amino acids.
Some chemical fragments of DNA and RNA can also be found in meteorites (Tables 5.1 and 5.2). For example, some meteorites have been reported to contain small amounts of adenine, one of the nucleobases found in RNA and DNA. The current view is that the Murchison meteorite contained adenine, guanine, their hydrolysis products hypoxanthine and xanthine, and uracil. The reported concentration of all those substances, however, is low, about 1.3 ppm. The Murchison and other meteorites may also contain ribitol and ribonic acid, the reduced and oxidized forms of ribose, respectively, but ribose itself has not been found.6
TABLE 5.2 Organic Compounds in the Murchison Meteorite
|
Amino acids |
60 ppm |
Purines and pyrimidines |
1.3 ppm |
|
Aliphatic hydrocarbons |
>35 ppm |
Basic N-heterocycles |
7 ppm |
|
Aromatic hydrocarbons |
15-28 ppm |
Amines |
8 ppm |
|
Carboxylic acids |
>300 ppm |
Amides |
55-70 ppm |
|
Dicarboxylic acids |
>30 ppm |
Alcohols |
11 ppm |
|
Hydroxycarboxylic acids |
15 ppm |
Aldehydes and ketones |
27 ppm |
|
SOURCE: Data from Cronin, J.R., and Pizzarello, S. 1986. Amino acids of the Murchison meteorite. III. Seven carbon acyclic primary alpha-amino alkanoic acids. Geochim. Cosmochim. Acta 50:2419-2427. |
|||
TABLE 5.3 The Organic Content of the Tagish Lake Meteorite
|
Aliphatic hydrocarbons |
5 ppm |
Dicarboximides |
5.5 ppm |
|
Aromatic hydrocarbons |
≥1 ppm |
Sulfonic acids |
20.0 ppm |
|
Dicarboxylic acids |
17.5 ppm |
Amino acids |
≥0.1 ppm |
|
Carboxylic acids |
40 ppm |
Amines |
<0.1 ppm |
|
Pyridine carboxylic acids |
7.5 ppm |
Amides |
<0.1 ppm |
|
SOURCE: Data from Pizzarello, S., Huang, Y.S., Becker, L., Poreda, R.J., Nieman, R.A., Cooper, G., and Williams, M. 2001. The organic content of the Tagish Lake meteorite. Science 293:2236. |
|||
We do not know the extent to which the Murchison organics reflect what was available on early Earth before life emerged. The rich inventory of amino acids does not appear to be universal in carbonaceous chondrites (although the number that have been examined in detail is very small). For example, only a few amino acids (glycine, alanine, α-aminoisobutyric acid, α-amino-n-butyric acid, γ-aminobutyric acid) are found in the meteorite that fell in 2000 on Tagish Lake, Canada (Table 5.3).7 The near absence of complex amino acids is significant, inasmuch as the meteorite was captured in a pristine condition soon after it fell.
It is also significant that no discovery of a dipeptide in meteorites has yet been reported. Joining two amino acids is the first step toward the synthesis of proteins, such as those found in contemporary terran life. If the meteorite organics analyzed to date are representative of planetary processing of primitive organic compounds, the process of assembling amino acids into polypeptides (short strings) may have been carried out first within living cells.
5.2.2
Biological-like Molecules from Planetary Processes
Current research is showing the interactions between organic molecules and a wide array of minerals. These include the formation of carboxylic acids in thermal vent chemistry and the formation of reduced chemical species through photochemistry involving semiconducting minerals.8
5.2.3
The Origin of Phosphorus
Phosphorus is an important component of terran life, but its synthesis in stars is not simple. It is produced as phosphorus-31 in stars with 15 protons and 16 neutrons and hence is an “odd-Z” element. Odd-Z elements are more difficult to produce than “even-Z” elements having an equal number of protons and neutrons; these can be produced from other even-Z elements via an “alpha chain” from helium. Odd-Z elements like P-31 are generally produced in abundance only when there is an excess of neutrons to protons. This excess emerges only as the universe ages, implying that life based on phosphorus cannot have emerged early in the life of the universe. Odd-Z elements are also less abundant in the Sun than common elements, such as carbon and oxygen, by factors in excess of 100, although current models with sophisticated stellar nucleosynthesis account rather well for the observed phosphorus abundance in the Sun.9
Phosphorus is abundant on Earth, both as an element (the 11th-most abundant atom in Earth’s crust) and as phosphate. Meteorites hold a variety of phosphate-containing minerals and some phosphide minerals.10 Scientists at the University of Arizona have recently suggested that Fe3P, the mineral schreibersite, leads to the formation of phosphate and phosphite when corroded in water. Although phosphorylation of alcohols was not demonstrated, mechanistic considerations suggest that it should be possible. It is noteworthy that a clear prebiotic pathway for the chemical incorporation of phosphate into RNA or DNA has not been found. No nucleosides (nucleobases joined to sugars) have been reported from meteorites. Nor has evidence been found in any meteorite of the presence of nucleosides or nucleotides (nucleosides attached to phosphates). That suggests that nucleic acids were first formed as products of metabolism.
5.2.4
The Origin of Metabolism
Considerations of the emergence of life on Earth have often focused on the spontaneous abiotic formation of RNA, which is both a genetic polymer and a catalytic polymer. The chemical complexity of this molecule suggests that the probability of such an event, although not zero, is extremely small, given the absence on present-day Earth of conditions that would favor its formation.11
Catalysts may have played an important role in establishing the early metabolism that ultimately led to the biosynthesis of RNA. An intriguing possibility is that modern metabolic pathways emerged by a stepwise process of recruitment of ever more effective catalysts to catalyze steps in a primordial chemical-reaction network. Transitionmetal sulfides and mineral surfaces are known to be able to catalyze the formation of simple organic compounds. Later, small molecules—such as amino acids, short peptides, and cofactors—may have catalyzed reactions required to produce more complicated organic compounds. Although their catalytic abilities are known to be limited in both acceleration and specificity compared with later macromolecular RNA or protein catalysts, some small molecules are remarkably effective catalysts. For example, pyridoxal phosphate catalyzes the rate of decarboxylation of amino acids by 10 orders of magnitude.12 That cofactor has been retained at the active site of many modern enzymes. Iron-sulfur clusters are also found in many modern enzymes and may be relics of a time in which they catalyzed similar reactions but without the context of the protein. A stage in which RNA provided the best function, either alone or in combination with peptides that helped to stabilize folded RNA structures, may have come next.13 What is clear is that of the synthesis of polypeptides by the translation of encoded RNA eventually became a focus of natural selection, in which the fitness of organisms depended critically on the catalytic abilities of enzymes involved in metabolic processes. In that transformation, protein enzymes replaced most RNA enzymes. An alternative possibility is that RNA catalysis never exceeded the extent to which it is present in modern biochemistry and that short peptides and cofactors carried the catalytic burden until the development of translation.
That diversity of possibilities creates ample opportunities for Earth-based experimental work in the origin of life, and the committee recommends enhanced efforts to exploit them. Such Earth-based research is critical for informing the design of planetary missions whose payloads can detect the conditions for the emergence of life and also detect primitive life, especially life that has not yet evolved to the point where it synthesizes peptides by translation.
5.3
THERMODYNAMIC EQUILIBRIA
Given a source of organic precursors, the question remains, Which reactions might have occurred with and between the precursors on early Earth, and in what quantities would they have been found? To address that question, the committee looked at the thermodynamic properties of molecules.
First, it considered the concept of the reduction-oxidation (redox) state, which is often used to describe organic and other molecules. The rules used to calculate an oxidation state are different between inorganic species and organic species. For example, Fe++ and Fe+++ have different redox states; the second lacks an electron that the first has. For organic molecules, however, the redox state is generally described with the ratio of the number of hydrogen atoms in a molecule to the number of heteroatoms.
Because its carbon is bonded to two oxygen atoms and no hydrogen atoms, carbon dioxide is as oxidized as a carbon atom can be. Methane, in which carbon is bonded only to hydrogen, is as reduced as a carbon atom can be. Formaldehyde is at the same oxidation level as elemental carbon (because it has an equal number of bonds to hydrogen and oxygen). Viewed alternatively,the ratio of hydrogen atoms (2) to oxygen atoms (1) is the same in formaldehyde as in water. Thus, compounds of the formula Cn(H2O)n can be converted to elemental carbon by heating, which extrudes water without a net change in the redox state of the carbons.
At one level, understanding the thermodynamics of carbon-containing molecules with respect to oxidation or reduction is as simple as asking whether hydrogen or oxygen is more abundant in the environment. In the modern terran atmosphere, which contains abundant dioxygen, essentially all compounds that contain reduced carbon are thermodynamically unstable with respect to oxidation to carbon dioxide. From a thermodynamic perspective, virtually all organic matter placed in today’s atmosphere will eventually “burn” and yield carbon dioxide
and water. The rate of the burning, however, can be very low at 20-40°C and at today’s atmospheric oxygen partial pressure.
In the absence of oxygen and in the presence of H2, reduced carbon is thermodynamically preferred. That is certainly true deep in the ocean, for example, near hydrothermal vents, where the synthesis of reduced organic compounds is thermodynamically favored. Shock, Cody, and others have exploited that fact to propose net synthesis of organic molecules in anoxic environments.14,15
A reaction that is thermodynamically “uphill” (not energetically favored) in one direction can become “downhill” in the same direction if the environmental conditions are changed. If (A + B) ![]() (C + D), the reaction can be pulled to the right if D is removed, converting all (A + B) to C. Conversely, if excess D is added, C will be driven to (A + B). That behavior of equilibria often appears in textbooks as Le Chatelier’s principle.
(C + D), the reaction can be pulled to the right if D is removed, converting all (A + B) to C. Conversely, if excess D is added, C will be driven to (A + B). That behavior of equilibria often appears in textbooks as Le Chatelier’s principle.
It is important to note that no biological compound can ever be said to be universally “high in energy.” Each reaction has a free energy, or ΔG0, which is measured at a standard, arbitrarily defined, concentration. ΔG0 does not determine whether the corresponding chemical reaction runs in the forward or reverse direction, however. This is determined as well by the concentrations of the reactants and the products, and the direction in which the state is out of equilibrium. This is captured by ΔG, which reflects both ΔG0 as well as the concentration of the reactants and products.
For this reason, it is not useful to speak of the “energy” of any particular compound. Rather, the free energy ΔG of a system, which makes a statement about whether it can do work, is determined by the degree to which the system is out of equilibrium. This, in turn, is defined by the equation ΔG = ΔG0 + RT ln [product]/[reactant].
In that context, adenosine triphosphate (ATP), the currency of energy in all cells, is viewed as “high energy” only because at equilibrium the reaction ATP + water ![]() ADP + inorganic phosphate contains more ADP and inorganic phosphate than ATP. If, however, the initial state contains ADP + inorganic phosphate and no ATP, the process spontaneously proceeds in the direction of the synthesis of ATP from ADP and inorganic phosphate. In that case, ADP and inorganic phosphate are the “high-energy” compounds.
ADP + inorganic phosphate contains more ADP and inorganic phosphate than ATP. If, however, the initial state contains ADP + inorganic phosphate and no ATP, the process spontaneously proceeds in the direction of the synthesis of ATP from ADP and inorganic phosphate. In that case, ADP and inorganic phosphate are the “high-energy” compounds.
Other generalizations concerning reactivity are based on the principles of thermodynamics. For example, organic molecules contain hydrogen atoms, which, given an appropriate catalyst or source of energy (ultraviolet light, for example), might generate H2. Because H2 molecules have a lower mass than other molecules, they move faster on average and therefore preferentially escape from planetary bodies, especially those with low mass and, consequentially, weak gravitational attraction. Although both the formation and the loss of H2 may be slow, cosmic processes have time. A collection of organic molecules slowly becomes more oxidized through loss of H2.
That is presumably what is occurring today on the surface of Mars. Above Mars, water is dissociated by ultraviolet radiation to yield H· and ·OH, the hydrogen radical and the hydroxyl radical. Two H· units can combine to give H2. The H2 then escapes from Mars, leaving behind HOOH, hydrogen peroxide. Under typical conditions on Earth, hydrogen peroxide might be viewed as a high-energy compound;on Mars, escape of H2 leads to its formation over time. On an aqueous body, such as Europa, the hydrogen peroxide formed by radiation will decompose into water and oxygen. The oxygen would then be available for the biological oxidation of other organic compounds formed by radiation or water-rock reactions, such as methane and formaldehyde. The concentrations of formaldehyde and oxygen from radiation were considered sufficient to support a microbial ecosystem on Europa.16
Carbon is likely to congeal to high-molecular-weight polymers as H2 distills off. In extraterrestrial environments, we expect lower hydrocarbons eventually to transform into pure carbon, either diamond (in which all the carbons are singly bonded to other carbons), fullerenes and graphite (in which each interaction between a pair of carbons is the approximate equivalent of 1.5 bonds), or carbon bonded to other elements that cannot be converted to a volatile form.
Polycyclic aromatic hydrocarbons can be viewed as “carbon on the way to forming graphite.” They are common in extraterrestrial environments. Their central structures are fragments of graphite with bonding to hydrogen atoms at the edges. They become larger and larger, and more and more like graphite, as more hydrogen distills away.
5.4
PROBLEMS IN ORIGINS
Chemists’ objection to the notion that life is a natural consequence of organic reactivity is simple and comes from broadly based empirical experience in organic-chemistry laboratories. Addition of energy to mixtures of
organic species makes the mixtures more complex and less likely to support life. Shapiro has provided a thoughtful and detailed discussion of the difficulties.17-22 Briefly summarized, it suggests that existing prebiotic chemistry experiments do not offer plausible hypotheses for routes to complex biomolecules. In the complex chemical mixtures generated under prebiotic conditions, one may be able to find trace amounts of amino acids and perhaps nucleobases. Some might indeed catalyze reactions that have some utility. But other compounds may well inhibit catalysis or catalyze undesired reactions. For example, Joyce and Orgel pointed out that the clay-catalyzed condensation of nucleotides to yield small chains performed best, under the conditions that they considered, if only one enantiomer of the starting material was present. If both were present, the desired reaction with the desired enantiomer might be inhibited by the other enantiomer.23 Furthermore, the combination of any bifunctional molecule into an information-bearing polymer would be expected to be terminated at an early stage by the presence of an excess of molecules that bear only one functionality.24
Even crystallization, a well-documented method of obtaining order through self-organization, is not a particularly powerful way to separate mixtures of organic chemicals into their constituents. Normally, an organic compound must be relatively pure before crystallization occurs. That salts crystallize better may explain why crystals are more common in the mineral world than in the organic world. Even organic salts can have problems in crystallizing from an impure mixture.
Those facts generate the central problem in prebiotic chemistry. Spontaneous self-organization is not known to be an intrinsic property of most organic matter, at least as observed in the laboratory. It can be driven only by an external source of free energy that is coupled to the organic system.
5.4.1
Nucleophilic and Electrophilic Reactions Can Destroy as Well as Create
As described above, formidable chemical obstacles oppose the abiotic synthesis of such biopolymers as RNA, DNA, and protein despite their prominence in life today. Numerous degradative processes would also hinder any event of that kind. The same inherent reactivities that generate organic molecules can convert them into complex mixtures. An example can be seen in the processes that might have generated the sugar ribose, a key component of RNA and DNA, under prebiotic conditions. A reaction called the formose reaction is known to produce ribose by converting formaldehyde in the presence of calcium hydroxide into several sugars, including ribose.25-27
The formose reaction exploits the natural electrophilicity of formaldehyde and the natural nucleophilicity of the enediolate of glycolaldehyde, a carbohydrate that has been detected in interstellar clouds.28 That species reacts as a nucleophile with formaldehyde (acting as an electrophile) to yield glyceraldehyde. Reaction of glyceraldehyde with a second equivalent of the enediolate generates a pentose sugar (ribose, arabinose, xylose, or lyxose, depending on stereochemistry). A curved-arrow mechanism describes this process in Figure 5.1.
Despite the reactivity inherent in glycolaldehyde and formaldehyde, the formose reaction does not offer a compelling source of prebiotic ribose. Under typical formose reaction conditions, ribose not only forms but also decomposes. In the presence of calcium hydroxide, ribose is rapidly converted to a mixture of organic species; this mixture has never been thoroughly characterized, but it does not appear to contain much ribose, and it is not an auspicious precursor of life. The further reaction of ribose in the presence of calcium hydroxide occurs because ribose itself has both electrophilic and nucleophilic sites, respectively, at the aldehyde carbon and at the carbon directly bonded to the aldehyde (after enolization—see Figure 5.1). Molecules having both reactivities tend, as expected, to polymerize as the nucleophilic sites and electrophilic sites react with each other, with more formaldehyde, with water, or with other electrophiles in the increasingly complex mixture. Those reactivities undoubtedly cause the rapid destruction of the ribose formed under formose conditions. On the basis of those reactivities, Larralde, Robertson, and Miller concluded that “ribose and other sugars were not components of the first genetic material.”29
A solution to the instability of ribose has been offered by Eschenmoser, Arrhenius, and others. It has focused on the generation of sugar phosphates, which have long been known to be more stable to degradation under alkaline conditions. A possible mechanism for forming them is shown in Figure 5.2.
For those reasons, some have suggested that life may have begun with an alternative organic compound as a genetic material, not RNA, but have been based on molecules that are less fragile.30 They are commonly suggested to be molecules that do not have carbohydrates in their backbones. Underlying that concept is the notion
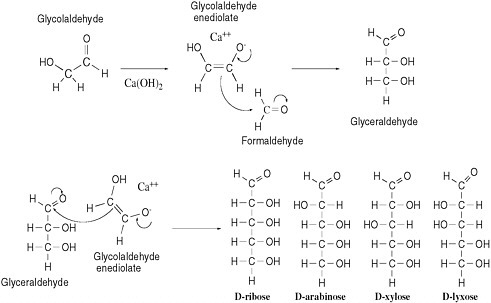
FIGURE 5.1 The aldol addition reaction, called an addition because the reaction adds one molecule to another. This addition reaction yields D,L-pentoses (only D-pentoses shown; note that the L-pentoses arise from reaction of L-glyceraldehyde and that all the species formed are formed as racemic mixtures) by combination of the enediolate of glycolaldehyde and glyceraldehyde.
of a “genetic takeover,” in which delicate RNA or DNA molecules arose rather late in the development of life, supplanting a hardier genetic molecule on which life was founded.31
The reactivity of nucleophilic and electrophilic centers can convert molecules that were plausibly present on early Earth into biologically interesting products. But the products themselves often have nucleophilic and electrophilic centers and will therefore react further and yield less interesting products. That is the central paradox associated with theories positing that the origin of life involved a polymeric replicator, even given plausible mechanisms for the creation of its components.
5.4.2
The Reactivity of Water Constrains Routes to Origins
As noted above, the nucleophilicity of water allows it to enter into reactions that cause the degradation of biological macromolecules, including DNA and proteins. Analogous problems are associated with the assembly of biopolymers. In water, the assembly of nucleosides from component sugars and nucleobases, the assembly of nucleotides from nucleosides and phosphate, and the assembly of oligonucleotides from nucleotides are all thermodynamically uphill in water.
That is also true for polypeptide chains that join amino acids. Two amino acids do not spontaneously join in water. Rather, the opposite reaction is thermodynamically favored at any plausible concentrations: polypeptide chains spontaneously hydrolyze in water, yielding their constituent amino acids. Those obstacles can be avoided if we adopt an alternative explanation: that life began with a mixture of small molecules rather than with a biopolymer.
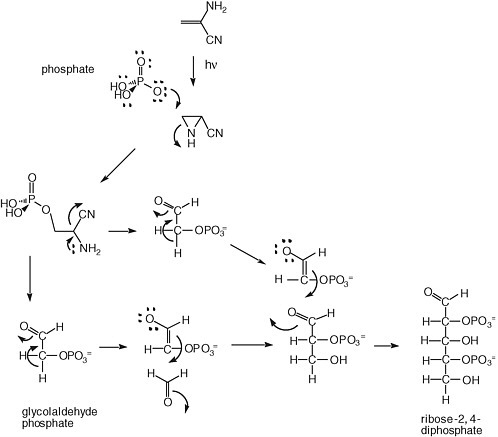
FIGURE 5.2 Synthesis of ribose-2,4-diphosphate from glycolaldehyde phosphate, as proposed by Eschenmoser.
5.5
MINERALS AS A POSSIBLE SOLUTION TO THE INSTABILITY OF RIBOSE
Despite the difficulties outlined above, the intellectual elegance of the “RNA world” theory of the origin of life32 has led many scientists to seek abiotic routes to the components of RNA. That can be illustrated with the chemical instability of ribose.
One approach to stabilize ribose exploits the fact that borate forms complexes with 1,2-dihydroxy units in organic molecules. The borate complex carries a negative charge. The anionic nature of the complex should prevent glyceraldehyde from losing a proton to create a nucleophilic enolate but not prevent glyceraldehyde from reacting as an electrophile with the enediolate of glycolaldehyde to generate pentoses.
Furthermore, the 1,2-dihydroxy unit of the cyclic form of ribose should form a stable complex with borate. That would stabilize the cyclic form of ribose at the expense of the aldehyde form. It should render ribose largely
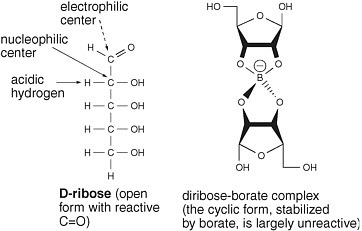
FIGURE 5.3 Borate minerals stabilize ribose under conditions in which it is formed from glycolaldehyde and glyceraldehyde. Wedges are used to describe bonds that project above and below the plane of the paper.
unreactive as either a nucleophile or an electrophile, in that the cyclic form lacks a C=O carbonyl group, which is the center of this molecule’s electrophilicity (Figure 5.3).
Experiments confirm that reasoning. In the presence of Ca(OH)2 under formose conditions at temperatures of 25-85°C, a solution of glycolaldehyde and glyceraldehyde rapidly turns brown. The brown mixture holds little ribose. When the same incubation takes place in the presence of the borate-containing mineral colemanite (Ca2B6O11·5H2O), however, the solution does not turn brown, and ribose, with other five-carbon carbohydrates, is found.33 Borate has evidently constrained the intrinsic reactivity of glyceraldehyde to form tar, causing it to enter a productive reaction with glycolaldehyde.
Boron is not abundant in the solar system, although it is known in carbonaceous chondrites, where it is almost certainly present as borate.34 Borate is, however, excluded from many mineral-forming processes, appearing in tourmalines, minerals best known as gemstones. Tourmaline weathers to generate borates, which have appreciable solubility in water. As a consequence, colemanite and other borate-containing minerals are delivered to deserts by runoff from the weathering mountains and are crystallized from water as it evaporates to yield evaporites. Such conditions are close to what is needed to generate ribose and related sugars.
Curiously, those conditions are also found in the most recent explorations on Mars. Although the instrument package delivered to Mars was not configured to detect either boron or ribose, the ratio of chloride to bromide salts is consistent with the formation of evaporite minerals on Mars. Given the appropriate source rocks, these should include alkaline borate minerals, analogous to those found in, for example, Death Valley. (The key mechanism for obtaining alkaline water on Earth is the conversion of olivine minerals to brucite in a process known as serpentinization; this is the process that raises the pH of Lake Mono, for example.) These are exactly the conditions described above that generate ribose. Indeed, noting that such conditions may not have been present on early Earth, Kirschvink has suggested that terran life originated from ribose formed on Mars.35
Solutions of that type are improbable. In exchange for a simplification mixture of the formose reaction, we must specify the presence of glycolaldehyde, glyceraldehyde, and borate minerals (and the absence of many potentially interfering substances, such as cyanide) and a route by which the protective borate will be removed so that the route to oligonucleotide formation remains open. Ribose is only one of the six components present in RNA. An extended number of steps involving the synthesis, purification, transportation, and highly specific
connection of the six components would be required to generate even a short RNA molecule, let alone one with biological function.
5.6
MINERALS INVOLVED IN THE CONSTRUCTION OF BIOMOLECULES
Minerals can participate in many ways in the synthesis and interconversion of organic species. For example, Martin and colleagues at Harvard have noted that sphalerite can convert photochemical energy into chemical energy.36 Minerals can also provide a direct source of redox chemical energy, such as in the conversion of sulfides to disulfides and vice versa. In addition, they may catalyze reactions and provide compartments to house evolving chemical systems.
5.7
SMALL-MOLECULE (“METABOLISM FIRST”) THEORIES OF LIFE’S ORIGIN
5.7.1
Life Without a Replicator
Replicator theories, which state that life began with the spontaneous formation of RNA or another information-rich genetic polymer that could direct its own replication, face difficulties in fundamental chemistry. These obstacles have long been thought to place them in the category of extremely improbable events. The appearance of such a replicator appears to require the combination of many chemicals in a long reaction sequence in a specific order, interspersed with a number of complicated separations, purifications, and changes of location.37 Physical law does not forbid such a process, but if a replicator initiated life and no natural environments can be found that make its generation favored, life may be very rare in the universe, and life that we encounter elsewhere is likely to be a result of panspermia.
It is reasonable to consider the assumption that life began, somehow, among one of the mixtures of small organic molecules that are produced by abiotic processes. The only natural examples in hand today are the components of meteorites that have fallen to Earth (see Section 5.2.1) and particles returned by the Stardust mission. Spectroscopy has also yielded partial lists of the organic molecules in interstellar space and interplanetary dust clouds.
For such a mixture to move in the direction of life, self-organization would be necessary. That process would increase the concentration of some components of the mixture either at the expense of others or by new synthesis from raw materials, such as carbon monoxide or carbon dioxide. An external source of free energy would be needed to drive the changes, which otherwise would involve an overall negative change in entropy.
That view of the origin of life has commonly been called “metabolism first”; the absence of a genetic polymer has been equated with the lack of any mechanism for heredity. As we have seen, replicator theories center on the spontaneous formation of large, information-bearing organic polymers endowed with the ability to copy themselves. The hereditary information carried in the sequence of such a polymer is called a genome.
In the words of Lancet and colleagues, a “fundamentally different approach has envisaged primordial self-replication as the collective property of ensembles of relatively simple molecules, interconnected by networks of mutually catalytic interactions.”38 The hereditary information in this case would be represented by the identity and concentration of its components. The term compositional genome has been used to describe this system, in which genetic information is not stored in a list, as in DNA, but is represented by the presence or absence of organic components.39,40 As an analogy, consider DNA to be the equivalent of a class list that records the full possible enrollment in a course. The information in a compositional genome would be represented by the presence of students who have turned up on a particular day.
A molecular assembly with a compositional genome would adapt to changes in the surrounding environment by altering the composition of the system and in the reactions used to sustain it. Growth of the system would take place through the acquisition or synthesis of additional quantities of the key components, and reproduction would occur when physical forces split the enlarged system into two or more fragments. For successful reproduction, each “daughter” fragment resulting from the division should contain a sufficient quantity of the key molecules to enable the networks of mutually catalytic interactions to continue. Such networks have been demonstrated in computer simulations,41,42 and “an experimental demonstration that amphiphilic assemblies display self-replication behavior” has been carried out.43 According to Lancet and colleagues, when competition for material and
energy was established between the fragments, Darwinian natural selection would then provide a driving force for further evolution.44
Those concepts are not new. They were initiated in the ideas of Alexander Oparin during the first half of the 20th century, and a limited but accurate scheme of this type was presented by Eakin.45 A summary of the ideas of a number of contributors—including Harold Morowitz, Christian de Duve, Gunther Wächtershäuser, Stuart Kauffmann, Freeman Dyson, Michael Russell, and Doron Lancet—can be found in a review by Fry.46
The experts do not agree, however, on specific details of the type of energy to be used, the source of the organic raw materials, the identity of the responsive chemical system, and the most suitable location on the early Earth for chemical self-organization. A consensus exists that some barrier is needed to protect the evolving entity from dispersal by diffusion, but the experts differ on the nature of the barrier. The committee can provide only a brief account of the more prominent suggestions here, and the listed references (and in some cases other sections of this report) should be consulted for more details. Some of the key variables are summarized below.
-
The energy source. A variety of possibilities were available on early Earth.47,48 Morowitz has considered solar radiation and chemical redox energy to be the most significant and favored the latter because of the difficulty of effectively harnessing solar radiation.49 The redox energy is derived primarily from encounters between the effluents of a reduced mantle and the more oxidized atmosphere and lithosphere. The oxidized regions are produced by the photochemical decomposition of water and the loss of hydrogen to space. Reduced and oxidized species are brought into contact by volcanism and other geological processes, which produce a supply of available free energy.50 Section 2.4.2 provides an overview of the most prominent energy sources currently supporting terran life.
-
The location. In the past, the suggestions have varied from Darwin’s “warm little pond” to the global ocean as a gigantic “prebiotic soup.” Three sites have received special attention recently: hydrothermal deep-sea vents51 and mounds52 and the ocean-atmosphere interface.53,54
-
The diffusion barrier. Much attention has been directed toward primitive amphiphile vesicles, inasmuch as they self-assemble from simple components and have an obvious ancestral connection with the more complex membranes that enclose modern cells. A review has been provided by Monnard and Deamer.55 The papers by Segre et al. and Hanczyc et al. contain additional discussion.56,57 Other prominent alternatives that would limit loss by diffusion have been electrostatic forces at mineral surfaces,58 iron sulfide membranes,59 and aerosols at the ocean-atmosphere interface.60 Section 2.7.1 discusses the function of compartmentalization in Earth life today.
-
The source of organic materials. Possible sources cited have included mineral-catalyzed hydrothermal synthesis, atmospheric syntheses driven by radiation or electrical discharges (see Section 5.1), and delivery from outer space (see Section 5.2). Cleaves and Chalmers have summarized the alternatives.61
-
The reactive chemical system. Some scientists have attempted to specify key components of such a system but not the entire reactive system. See, for example, de Duve62,63 and Weber.64,65 Others have suggested complete chemical cycles. Modified versions of the reductive citric acid cycle, a carbon-fixation pathway that is used by several organisms today, have been proposed.66-70
5.7.2
Coupling to an Energy Source as a Driver of Chemical Self-organization
Some versions of “metabolism first” schemes have drawn criticism in the literature.71,72 The schemes have assumed that a self-sustaining metabolic network must be autotrophic;73 that is, it must obtain its carbon supply entirely from carbon dioxide, rather than using carbon compounds made by abiotic processes (Sections 5.2.1 and 5.2.2). The assumption that chemicals in proposed reactions in the central metabolic cycle will be catalyzed by other compounds that participate in the cycle has also been questioned.74 Such features are attractive but not essential to the idea of life with small molecules. It has been argued that the objections can be met in principle by introducing a small number of assumptions:75
-
A thermodynamically favorable, irreversible “driver” reaction is coupled directly to an external source of available free energy and can occur in a plausible abiotic setting. The term coupled indicates that one process cannot occur without the other. Many examples of coupled reactions occur in modern biochemistry. In an abiotic setting,
-
such coupling would make a particular reaction favorable from a thermodynamic point of view. Other reversible processes that could produce the starting material for the driver reaction would be shifted in that direction.
-
A multistep reversible pathway can convert the product of the driver reaction back to the starting material, completing a cycle. If such a pathway existed, the synthesis of its members would be favored at the expense of competing reactions to maximize the discharge of the free-energy source. Energy-driven self-organization would take place.
-
The cycle functions at a “profit” in its environment: the gain of carbon by the cycle exceeds its loss by all mechanisms. Some losses of material from the central cycle are inevitable, either by diffusion or by irreversible side reactions that lead to such products as unreactive molecules or insoluble tars. For the metabolic network (the central cycle and associated reactions that feed material into it) to grow, the losses must be compensated for by the absorption of carbon dioxide or organic substances provided by the environment.
The diversity of organic chemistry, with its harvest of competing, interconnected reactions,76,77 would become an asset rather than a liability in the case of the energy-driven system described above. The existence of side-reaction paths could provide the network with the capacity of reacting to changes in the environment, for example by providing an alternative path for closure of the cycle if one central step were hindered. If alternative pathways were explored, a variety of catalysts and substances that hinder loss of material might be encountered and added to the network, enhancing its capability.78
5.7.3
Significance and Implications for Astrobiology
The above discussion uses organic chemistry as a reference point, but no specific compounds are identified. Any abundant set of interconnected reactions that is compatible with a specific geochemical environment might qualify as a starting point for metabolism. Although it is logically possible that only a single solution exists, which corresponds to the start of life on Earth, there is no reason to believe that this must be the case. Some requirements for the small-molecule scenario have been specified, but they are far more permissive than those required by the polymeric-replicator theory. The proposal involving metabolism suggests a universe in which energy-driven self-organization may take place under a variety of circumstances and afford a harvest of “weird” life. The polymeric-replicator theory points to a cosmos that may be barren except for Earth.
5.8
OPPORTUNITIES FOR RESEARCH
5.8.1
Research on Earth
After a long hiatus, research in prebiotic chemistry once again has momentum. For the proposal that life originated in complex mixtures of small molecules (“metabolism first”), there is a framework but not a specific recipe to illustrate how a coupled free-energy source could initiate the process of self-organization. A detailed discussion of the possible further development of one system of this type79 can be found in Lindahl.80 In the long run, the question will be answered by space missions, but appropriate laboratory experiments may provide some guidance.
The first priority in such experiments should be to characterize any systems that will self-organize when coupled to appropriate free-energy sources. The principal initial task will be the identification of candidate driver reactions. There should be no need to specify the remainder of the system in advance once a plausible driver reaction has been found. If the materials and coupled energy source needed for the reaction were brought together, perhaps with an input of simple carbon-containing compounds to provide for growth, the metabolic network should establish itself, and its identity could be determined with simple analysis. Nature will be instructing us, rather than our attempting to impose our schemes onto it. The information that we would gather by observing such a system could enable us to identify the later steps, such as compartment formation, in the energy-driven self-organization process that leads to life.
Alternative opportunities await those who continue to explore replicator-based solutions for the origin of life. In the RNA-first tradition, a number of groups are continuing to search for plausible prebiotic syntheses for ribose
and other RNA components. Several involve mineral combinations that are now thought to be likely on Mars. Further research is needed to constrain the inventory of water on early Earth.
The largest remaining paradox in replicator-first theories concerns how to address the unfavorable interactions between water and biological molecules. Even if the heterocycles and sugars can be prepared and stabilized and can accumulate under prebiotic conditions, there are few good approaches to assembling them in water uphill against a thermodynamic gradient favoring hydrolysis. That paradox may be partially resolved by considering alternative solvents.
The possibility of life based entirely on minerals could also be explored on Earth, both in field work and in controlled laboratory studies. Attention could be given to the relationship between mineral morphology and the detailed sequence of layers in the mineral. Examples of edge catalysis of organic reactions by minerals could be sought, as well as edge adsorption of abnormal inorganic cations and organic molecules. Above all, it should be asked whether edge growth could perpetuate stacking irregularities that would afford the minerals that contained them an advantage in survival.
5.8.2
Research in Space
In the search for weird life in space, “replicator first” and “metabolism first” theories share a goal: “follow the carbon.” The goal is to identify organic mixtures that differ sharply in composition from the nearly random collections identified in meteorites. For “metabolism first” advocates, such collections would represent key components of metabolic cycles. For “replicator first” supporters, the compounds might include the building blocks of genetic polymers. Such a strategy would be highly preferable to one that assumes that replicator first (RNA in particular) is correct—for example, the inclusion in space missions of experiments specifically designed to detect nucleic acids. The committee recommends that missions planned for Mars should include instruments that detect lighter atoms, simple organic functional groups, and organic carbon. Such instruments were originally planned as part of the Athena payload to Mars but were later removed. A total-carbon analysis instrument was delivered by Beagle 2, but this mission was lost. As a consequence, the Opportunity and Spirit rovers were unable to confirm the presence of borates or simple organic materials even though the locales that they visited would theoretically be very likely to contain them. Similar considerations should guide small-organic-molecule detectors that could function on the surface of Europa and Titan.
That approach offers the opportunity for research that combines the exploration of potential metabolic cycles with the synthetic biology of unnatural nucleic acid analogues and their building blocks. The results of the studies would be used to guide the design of instruments. That may be one of the principal ways in which ground-based research in astrobiology can inform NASA missions of exploration in the cosmos. The committee recommends that NASA fund carefully constructed programs in fundamental organic chemistry and synthetic biology for that purpose and for the design of instruments that might detect weird life on Earth and elsewhere.
5.9
REFERENCES
1 Cairns-Smith, A.G. 1982. Genetic Takeover and the Mineral Origins of Life. Cambridge University Press, Cambridge, U.K.
2 Cairns-Smith, A.G. 2002. The origin of life: Clays. Pp. 169-192 in Frontiers of Life, Volume 1 (D. Baltimore, R. Dulbecco, F. Jacob, and R. Levi-Montalcini, eds.). Academic Press, San Diego, Calif.
3 Segré, D., and Lancet, D. 2000. Composing life. EMBO Reports 1:217-222.
4 Miller, S.L., 1953. Production of amino acids under possible primitive Earth conditions. Science 117:528.
5 Kasting, J.F. 1990. Bolide impacts and the oxidation state of carbon in the Earth’s early atmosphere. Origins Life 20:199-231. See also Schaefer, L., and Fegley Jr., B., 2007, Outgassing of ordinary chondritic material and some of its implications for the chemistry of asteroids, planets, and satellites, Icarus 186.2:462-483.
6 Cooper, G., Novelle, K., Belisle, W., Sarinana, J., Brabham, K., and Garrel, L. 2001. Carbonaceous meteorites as a source of sugar-related organic compounds for the early Earth. Nature 414:879-884.
7 Pizzarello, S., Huang, Y.S., Becker, L., Poreda, R.J., Nieman, R.A., Cooper, G., and Williams, M. 2001. The organic content of the Tagish Lake meteorite. Science 293:2236.
8 Zhang, X.V., Martin, S.T., Friend, C.M., Schoonen, M.A., and Holland, H.D., 2004, Mineral-assisted pathways in prebiotic synthesis: Photoelectrochemical reduction of carbon (+IV) by manganese sulfide, J. Am. Chem. Soc. 126(36):11247-11253; Zhang, X.V., and Martin, S.T., 2006, Driving parts of Krebs cycle in reverse through mineral photochemistry, J. Am. Chem. Soc. 128(50):16032-16033.
9 Maciá, E. 2005. The role of phosphorus in chemical evolution. Chem. Soc. Rev. 34:691-701.
10 Moore, C.B. 1971. Phosphorus. Pp.131-135 in Handbook of Elemental Abundances in Meteorites (B. Mason, ed.). Gordon and Breach, New York.
11 Joyce, G.F., and Orgel, L.E. 1999. Prospects for understanding the origin of the RNA world. Pp. 49-77 in The RNA World, Second Edition (R.F. Gesteland, T.R. Cech, and J.F. Atkins, eds.). Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
12 Zabinski, R.F., and Toney, M.D. 2001. Metal ion inhibition of nonenzymatic pyridoxal phosphate catalyzed decarboxylation and transamination.J. Am. Chem. Soc. 123:193-198.
13 Noller, H.F. 2004. The driving force for molecular evolution of translation. RNA 10:1833-1837.
14 Cody, G.D., Hazen, R.M., Brandes, J.A., Morowitz, H., and Yoder, H.S., Jr. 2001. Geochemical roots of autrotrophic carbon fixation: Hydrothermal experiments in the system citric acid, H2O-(±FeS)−(±NiS).Geo chim. Cosmochim. Acta 65(20):3557-3576.
15 Amend, J.P., and Shock, E.L. 1998. Energetics of amino acid synthesis in hydrothermal ecosystems. Science 281:1659-1662.
16 Chyba, C.F. 2000. Energy for microbial life on Europa. Nature 403:381-382.
17 Shapiro, R. 1984. The improbability of prebiotic nucleic acid synthesis. Origins Life 14:565-570.
18 Shapiro, R. 1988. Prebiotic ribose synthesis. A critical analysis. Origins Life Evol. Biosph. 18:71-85.
19 Shapiro, R. 1995. The prebiotic role of adenine: A critical analysis. Origins Life Evol. Biosph. 25:83-98.
20 Shapiro, R. 1999. Prebiotic cytosine synthesis: A critical analysis. Implications for the origin of life. Proc. Natl. Acad. Sci. U.S.A. 96:4396-4401.
21 Shapiro, R. 2000. A replicator was not involved in the origin of life. IUBMB Life 49:173-176.
22 Shapiro, R. 2006. Small molecule interactions were central to the origin of life. Q. Rev. Biol. 81:105-125.
23 Joyce, G.F., and Orgel, L.E. 1999. Prospects for understanding the origin of the RNA world. Pp. 49-77 in The RNA World, Second Edition (R.F. Gesteland, T.R. Cech, and J.F. Atkins, eds.). Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
24 Shapiro, R. 2000. A replicator was not involved in the origin of life. IUBMB Life 49:173-176.
25 Boutlerow, A. 1861. Formation of monosaccharides from formaldehyde [in French]. C.R. Séances Acad. Sci. Fr. 53:145-147.
26 Breslow, R. 1959. On the mechanism of the formose reaction. Tetrahedron Lett. 21:22-26.
27 Zubay, G. 1998. Studies on the lead-catalyzed synthesis of aldopentoses. Origins Life Evol. Biosph. 28:13-26.
28 Hollis, J.M., Vogel, S.N., Snyder, L.E., Jewell, P.R., and Lovas, F.J. 2001. The spatial scale of glycolaldehyde in the galactic center. Astrophys. J. 554:L81-L85.
29 Larralde, R., Robertson, M.P., and Miller, S.L. 1995. Rates of decomposition of ribose and other sugars. Implications for chemical evolution. Proc. Natl. Acad. Sci. U.S.A. 92:8158-8160.
30 Nielsen, P.E. 2004. PNA technology. Mol. Biotech. 26:233-248.
31 Cairns-Smith, A. 1982. Genetic Takeover and the Mineral Origins of Life. Cambridge University Press, Cambridge, U.K.
32 Gilbert, W. 1986. Origins of life: The RNA world. Nature 319:618.
33 Ricardo, A., Carrigan, M.A., Olcott, A.N., and Benner, S.A. 2004. Borate minerals stabilize ribose. Science 303:196.
34 Zhai, M., and Shaw, D.M. 1994. Boron cosmochemistry. Part I: Boron in meteorites. Meteoritics 29:607-615.
35 Kirschvink, J.L., Weiss, B.P., and Beukes, N.J. 2006. Boron, ribose, and a martian origin for terrestrial life. Geochim. Cosmochim. Acta Supplement 70(18):S320.
36 Zhang, X.V., Martin, S.T., Friend, C.M., Schoonen, M.A, and Holland. H.D., 2004, Mineral-assisted pathways in prebiotic synthesis: Photoelectrochemical reduction of carbon (+IV) by manganese sulfide, J. Am. Chem. Soc. 126 (36):11247-11253; Zhang, X.V., and Martin, S.T., 2006, Driving parts of Krebs cycle in reverse through mineral photochemistry, J. Am. Chem. Soc. 128(50):16032-16033.
37 Cairns-Smith, A. 1982. Genetic Takeover and the Mineral Origins of Life. Cambridge University Press, Cambridge, U.K.
38 Segré, D., Ben-Eli, D., and Lancet, D. 2000. Compositional genomes: Prebiotic information transfer in mutually catalytic noncovalent assemblies. Proc. Natl. Acad. Sci. U.S.A. 97:4112-4117, p. 4112.
39 Segré, D., and Lancet D. 2000. Composing life. EMBO Reports 1:217-222.
40 Zhai, M., and Shaw, D.M. 1994. Boron cosmochemistry. Part I: Boron in meteorites. Meteoritics 29:607-615.
41 Segré, D., and Lancet, D. 2000. Composing life. EMBO Reports 1:217-222.
42 Zhai, M., and Shaw, D.M. 1994. Boron cosmochemistry. Part I: Boron in meteorites. Meteoritics 29:607-615.
43 Segré, D., Ben-Eli, D., and Lancet, D. 2000. Compositional genomes: Prebiotic information transfer in mutually catalytic noncovalent assemblies. Proc. Natl. Acad. Sci. U.S.A. 97:4112-4117, p. 4112.
44 Segré, D., Ben-Eli, D., and Lancet, D. 2000. Compositional genomes: Prebiotic information transfer in mutually catalytic noncovalent assemblies. Proc. Natl. Acad. Sci. U.S.A. 97:4112-4117.
45 Eakin, R.E. 1963. An approach to the evolution of metabolism. Proc. Natl. Acad. Sci. U.S.A. 40:360-366.
46 Fry, I. 2000. The Emergence of Life on Earth: A Scientific Overview. Rutgers University Press, New Brunswick, N.J.
47 Chang, S. 1993. Prebiotic synthesis in planetary environments. Pp. 259-299 in The Chemistry of Life’s Origins (J.M. Greenberg, C.X. Mendoza-Gómez, and V. Piranello, eds.). Kluwer Academic Publishers, Dordrecht, Netherlands.
48 Deamer, D.W. 1997. The first living systems: A bioenergetic perspective. Microbiol. Mol. Biol. Rev. 61:239-261.
49 Morowitz, H.J. 1999. A theory of biochemical organization, metabolic pathways, and evolution. Complexity 4:39-53.
50 Smith, E., and Morowitz, H. 2004. Universality in intermediary metabolism. Proc. Natl. Acad. Sci. U.S.A. 101:13168-13173.
51 Holm, N.G. 1992. Why are hydrothermal systems proposed as plausible environments for the origin of life? Origins Life Evol. Biosph. 22:5-14.
52 Martin, W., and Russell, M.J. 2002. On the origins of cells: A hypothesis for the evolutionary transitions from abiotic geochemistry to chemoautotrophic prokaryotes, and from prokaryotes to nucleated cells. Philos. Trans. R. Soc. Lond., B, Biol. Sci. 385:59-85.
53 Chang, S. 1993. Prebiotic synthesis in planetary environments. Pp. 259-299 in The Chemistry of Life’s Origins (J.M. Greenberg, C.X. Mendoza-Gómez, and V. Piranello, eds.). Kluwer Academic Publishers, Dordrecht, Netherlands.
54 Donaldson, D.J., Tervahattu, H., Tuck, A.F., and Vaida, V. 2004. Organic aerosols and the origin of life: An hypothesis. Origins Life Evol. Biosph. 34:57-67.
55 Monnard, P.A., and Deamer, D.W. 2002. Membrane self-assembly processes: Steps toward the first cellular life. Anat. Rec. 268:196-207.
56 Segré, D., Ben-Eli, D., Deamer, D.W, and Lancet, D. 2001. The lipid world. Origins Life Evol. Biosph. 31:119-145.
57 Hanczyc, M.M., Fujikawa, S.M., and Szostak, J.W. 2003. Experimental models of primitive cellular compartments: Encapsulation, growth, and division. Science 302:618-622.
58 Wächtershäuser, G. 1992. Groundworks for an evolutionary biochemistry: The iron-sulphur world. Prog. Biophys. Mol. Biol. 58:85-201.
59 Russell, M.J., Daniel, R.M., Hall, A.J., and Sherringham, J.A. 1994. A hydrothermically precipitated catalytic iron sulfide membrane as a first step toward life. J. Mol. Evol. 39:231-243.
60 Donaldson, D.J., Tervahattu, H., Tuck, A.F., and Vaida, V. 2004. Organic aerosols and the origin of life: An hypothesis. Origins Life Evol. Biosph. 34:57-67.
61 Cleaves II, H.J., and Chalmers, J.H. 2004. Extremophiles may be irrelevant to the origin of life. Astrobiology 4:1-9.
62 de Duve, C. 1991. Blueprint for a Cell: The Nature and Origin of Life. Neil Patterson Publishers, Burlington, N.C.
63 de Duve, C. 2003. A research proposal for the origin of life. Origins Life Evol. Biosph. 33:559-574.
64 Weber, A.L. 2001. The sugar model: Catalytic flow reactor dynamics of pyruvaldehyde synthesis from triose catalyzed by poly-l-lysine contained in a dialyzer. Origins Life Evol. Biosph. 31:231-240.
65 Weber, A.L. 2002. Chemical constraints governing the origin of metabolism: The thermodynamic landscape of carbon group transformations under mild aqueous conditions. Origins Life Evol. Biosph. 32:333-357.
66 Cody, G.D., Hazen, R.M., Brandes, J.A., Morowitz, H., and Yoder, H.S., Jr. 2001. Geochemical roots of autrotrophic carbon fixation: Hydrothermal experiments in the system citric acid, H2O-(±FeS)−(±NiS). Geochim. Cosmochim. Acta 65(20):3557-3576.
67 Morowitz, H.J. 1999. A theory of biochemical organization, metabolic pathways, and evolution. Complexity 4:39-53.
68 Wächtershäuser, G. 1992. Groundworks for an evolutionary biochemistry: The iron-sulphur world. Prog. Biophys. Mol. Biol. 58:85-201.
69 Lindahl, P.A. 2004. Stepwise evolution of nonliving to living chemical systems. Origins Life Evol. Biosph. 34:371-389.
70 Smith, E., and Morowitz, H. 2004. Universality in intermediary metabolism. Proc. Natl. Acad. Sci. U.S.A. 101:13168-13173.
71 Orgel, L.E. 2000. Self-organizing biochemical cycles. Proc. Natl. Acad. Sci. U.S.A. 97:12503-12507.
72 Pross, A. 2004. Causation and the origin of life: Metabolism or replication first? Origins Life Evol. Biosph. 34:307-321.
73 Wächtershäuser, G. 1992. Groundworks for an evolutionary biochemistry: The iron-sulphur world. Prog. Biophys. Mol. Biol. 58:85-201.
74 Orgel, L.E. 2000. Self-organizing biochemical cycles. Proc. Natl. Acad. Sci. U.S.A. 97:12503-12507.
75 Shapiro, R. 2006. Small molecule interactions were central to the origin of life. Q. Rev. Biol. 81:105-125.
76 Weber, A.L. 2001. The sugar model: Catalytic flow reactor dynamics of pyruvaldehyde synthesis from triose catalyzed by poly-l-lysine contained in a dialyzer. Origins Life Evol. Biosph. 31:231-240.
77 Weber, A.L. 2002. Chemical constraints governing the origin of metabolism: The thermodynamic landscape of carbon group transformations under mild aqueous conditions. Origins Life Evol. Biosph. 32:333-357.
78 Shapiro, R. 2006. Small molecule interactions were central to the origin of life. Q. Rev. Biol. 81:105-125.
79 Wächtershäuser, G. 1992. Groundworks for an evolutionary biochemistry: The iron-sulphur world. Prog. Biophys. Mol. Biol. 58:85-201.
80 Lindahl, P.A. 2004. Stepwise evolution of nonliving to living chemical systems. Origins Life Evol. Biosph. 34:371-389.
















