3
Ecological Effects of WindEnergy Development
CHAPTER OVERVIEW
At regional to global scales, the effects of wind energy on the environment often are considered to be positive, through the production of renewable energy and the potential displacement of mining activities, air pollution, and greenhouse gas emissions associated with nonrenewable energy sources (see Chapter 2). However, wind-energy facilities have been demonstrated to kill birds and bats and there is evidence that wind-energy development also can result in the loss of habitat for some species. To the extent that we understand how, when, and where wind-energy development most adversely affects organisms and their habitat, it will be possible to mitigate future impacts through careful siting decisions. In this chapter, we review the effects of wind-energy development on ecosystem structure and functioning, through direct effects of turbines on organisms, and on landscapes through alteration and displacement. We recommend a research and monitoring framework for reducing these impacts. Although the focus of our analysis is the Mid-Atlantic Highlands, we use all available information to assess general impacts. Although other sources of development on sites that are suitable for wind-energy development affect wildlife and their habitats (e.g., mineral extraction, cutting of timber), and there are other sources of anthropogenic mortality to animals, as stated previously, this committee was charged to focus on wind energy, and therefore did not conduct a comprehensive comparative analysis of impacts from other sources of development.
Wind turbines cause fatalities of birds and bats through collision, most
likely with the turbine blades. Species differ in their vulnerability to collision, in the likelihood that fatalities will have large-scale cumulative impacts on biotic communities, and in the extent to which their fatalities are discovered and publicized. This chapter reviews information on the probabilities of fatalities, which are affected by both abundance and behavioral characteristics of each species.
Factors such as the type, location, and operational schedules of turbines that may influence bird and bat fatalities are reviewed in this chapter. The overall importance of turbine-related deaths for bird populations is unclear. Collisions with wind turbines represent one element of the cumulative anthropogenic impacts on bird populations; other impacts include collisions with tall buildings, communications towers, other structures, and vehicles, as well as other sources of mortality such as predation by house cats (Erickson et al. 2001, 2005). While estimation of avian fatalities caused by wind-power generation is possible, the data on total bird deaths caused by most anthropogenic sources, including wind turbines, are sparse and less reliable than one would wish, and therefore it is not possible to provide an accurate estimate of the incremental contribution of wind-powered generation to cumulative bird deaths in time and space at current levels of development.
Data on bat fatalities are even sparser. While there have been a few reports of bat kills from other anthropogenic sources (e.g., through collisions with buildings and communications towers), the recent bat fatalities from wind turbines appear to be unprecedentedly high. More data on direct comparisons of turbine types are needed to establish whether and why migratory bats appear to be at the greatest risk of being killed. Clearly, a better understanding of the biology of the populations at risk and analysis of the cumulative effects of wind turbines and other anthropogenic sources on bird and bat mortality are needed.
The construction and maintenance of wind-energy facilities alter ecosystem structure, through vegetation clearing, soil disruption, and potential for erosion, and this is particularly problematic in areas that are difficult to reclaim, such as desert, shrub-steppe, and forested areas. In the MidAtlantic Highlands forest clearing represents perhaps the most significant potential change through fragmentation and loss of habitat for forest-dependent species. Changes in forest structure and the creation of openings alter microclimate and increase the amount of forest edge. There may also be important interactions between habitat alteration and the risk of fatalities, such as bat foraging behavior near turbines.
The recommendations in this chapter address the types of studies that need to be conducted prior to siting and prior to and following construction of wind-energy facilities to evaluate the potential and realized ecological impacts of wind-energy development. The recommendations also address
assessing the degree to which a particular site is acceptable for wind-energy development and the types of research and monitoring needed to help inform decision makers.
INTRODUCTION
There are two major ways that wind-energy development may influence ecosystem structure and functioning—through direct impacts on individual organisms and through impacts on habitat structure and functioning. Environmental influences of wind-energy facilities can propagate across a wide range of spatial scales, from the location of a single turbine to landscapes, regions, and the planet, and a range of temporal scales from short-term noise to long-term influences on habitat structure and influences on presence of species. In this chapter, we review the documented and potential influences of wind-energy development on ecosystem structure and functioning, focusing on scales of relevance to siting decisions and on influences on birds, bats, and other vertebrates.
Construction and operation of wind-energy facilities directly influence ecosystem structure. Site preparation activities, large machinery, transportation of turbine elements, and “feeder lines,” transmission lines that lead from the wind-energy facility to the electricity grid, all can lead to removal of vegetation, disturbance, and compaction of soil, soil erosion, and changes in hydrologic features. Although many of these activities are relatively local and short-term in practice (e.g., construction), there may be substantial effects on habitat quality for a variety of organisms. These changes will likely be detrimental to some species and beneficial to others. Wind-energy development that is focused on specific topographic features (e.g., ridgelines) that represent key habitat features for some species may have disproportionately detrimental impacts on those species that depend on or are closely associated with these habitats.
Recent reviews of available literature have clearly documented direct impacts of wind turbines on birds and bats (GAO 2005; Barclay and Kurta 2007; Kunz et al. 2007), including death from colliding with turbine blades. As discussed below, little is known about the circumstances contributing to fatalities, but issues such as turbine height and design, rotor velocity, number and dispersion of turbines, location of the turbine on the landscape, and the abundance, migration, and behavioral characteristics of each species present are likely to influence fatality rates. In addition, non-flying organisms may be affected by turbine construction and operation, because of alteration of habitat and behavioral avoidance, possibly due to noise, vibration, motion of turbines, or their mere presence in the landscape.
We can make three general predictions about the large-scale and longterm impacts of individual fatalities. First, life-history theory predicts that
characteristics of populations of affected species determine the consequences of increased mortality: organisms whose populations are characterized by low birth rate, long life span, naturally low mortality rates, a high trophic level, and small geographic ranges are likely to be most susceptible to cumulative, long-term impacts on population size, genetic diversity, and ultimately, population viability (e.g., McKinney 1997; Purvis et al. 2000). Bats are unusual among mammals with respect to their life-histories, because they typically have small body sizes but long life spans (Barclay and Harder 2003), and the probability of extinction in bats has been linked to several of these characteristics (Jones et al. 2003). Second, the effects of a decline in one species on entire biotic communities is determined by the role of the species in the larger context: losses of keystone species, organisms that have a disproportionately high impact on ecosystem functioning (Power et al. 1996), and those that provide important ecosystem services (Daily et al. 1997) are of most concern. Species that are important predators and perform critical top-down control over communities, and species that are important prey sources can be keystone species in both natural and human-altered ecosystems (Cleveland et al. 2006). Notably, many raptors and insectivorous bats fill these roles. Finally, we do not know how the migration patterns of affected species will influence regional-scale mortality; we also do not understand the consequences of deaths of individuals of these migrating species to the local populations they originate from. Unfortunately this type of information is nearly impossible to obtain.
The ecological influences of wind-energy facilities are complex, and can vary with spatial and temporal scale, location, season, weather, ecosystem type, species, and other factors. Moreover, many of the influences are likely cumulative, and ecological influences can interact in complex ways at wind-energy facilities and at other sites associated with changed land-use practices and other anthropogenic disturbances. Because of this complexity, evaluating ecological influences of wind-energy development is challenging and relies on understanding factors that are inadequately studied. Despite this, several patterns are beginning to emerge from the information currently available. Increased research using rigorous scientific methods will be critical to filling existing information gaps and improving reliability of predictions.
In this chapter, we review the literature on the ecological effects of wind-energy development, focusing on wildlife and their habitats. We then provide an assessment of projected impacts of future development in the Mid-Atlantic Highland region based on the limited information currently available. Finally, we provide an overview of current methods and metrics for monitoring ecological impacts of wind-energy facilities, and propose research and monitoring priorities.
BIRD DEATHS IN CONTEXT
A primary question that arises from considerations of current and projected cumulative bird deaths from wind turbines is whether and to what degree they are ecologically significant. A related (but nonetheless different) question is how the number of turbine-caused bird deaths compares with the number of all anthropogenically caused bird deaths in the United States. The committee approaches the answer to the latter question with great hesitation, for four reasons. First, the accuracy and precision of data available to answer the question are poor. Although it is clear that more birds are killed by other human activities than by wind turbines, both natural mortality rates for many species and fatalities resulting from many types of human activities are poorly documented. In addition, different sources of human-caused fatalities do not affect all bird species to the same degree. Second, the demographic consequences of various mortality rates are poorly understood for most bird species, as are factors such as the timing of fatalities and sex or age bias in fatalities resulting from different anthropogenic causes, which could have a variety of demographic impacts. Moreover, the demographic and ecological importance of any given mortality rate being considered is relative to population size, which is poorly known for most species. Third, grouping all species together in any estimate provides information that is not ecologically relevant. For example, the ecological consequences and conservation implications of the deaths of 10,000 starlings (Sturnus vulgaris) are far different from those of the deaths of 10,000 bald eagles (Haliaeetus leucocephalus). Finally, consideration of aggregate bird fatalities across the United States from any cause—including those caused by wind-energy installations—is not the appropriate spatial scale to address the question of interest. Region-specific information about the demographic effects of any cause of mortality on species of interest would be much more informative. Thus, for example, it is more important to know how many raptors of a particular species are killed by turbines and other human mortality sources in a particular region than it is to know how many raptors are killed nationwide.
Having said the above, we provide here estimates summarized by Erickson et al. (2005) and estimates reported by the U.S. Fish and Wildlife Service (USFWS 2002a). Those sources emphasize the uncertainty in the estimates, but the numbers are so large that they are not obscured even by the uncertainty. Collisions with buildings kill 97 to 976 million birds annually; collisions with high-tension lines kill at least 130 million birds, perhaps more than 1 billion; collisions with communications towers kill between 4 and 5 million based on “conservative estimates,” but could be as high as 50 million; cars may kill 80 million birds per year; and collisions with wind turbines killed an estimated 20,000 to 37,000 birds per year in
2003, with all but 9,200 of those deaths occurring in California. Toxic chemicals, including pesticides, kill more than 72 million birds each year, while domestic cats are estimated to kill hundreds of millions of songbirds and other species each year. Erickson et al. (2005) estimate that total cumulative bird mortality in the United States “may easily approach 1 billion birds per year.”
Clearly, bird deaths caused by wind turbines are a minute fraction of the total anthropogenic bird deaths—less than 0.003% in 2003 based on the estimates of Erickson et al. (2005). However, the committee re-emphasizes the importance of local and temporal factors in evaluating the effects of wind turbines on bird populations, including a consideration of local geography, seasonal bird abundances, and the species at risk. In addition, it is necessary to consider the possible cumulative bird deaths that can be expected if the use of wind energy increases according to recent projections (see Chapter 2).
TURBINES CAUSE FATALITIES TO BIRDS AND BATS
Information on fatalities of birds and bats associated with wind-energy facilities in the Mid-Atlantic Highlands is limited, largely because of the relatively small amount of wind-energy development in the region to date, the modest investments in monitoring and data collection, and in some cases, restricted access to wind-energy facilities for research and monitoring. This lack of information requires the use of information from other parts of the United States (and elsewhere). The following discussion summarizes what is known regarding bird and bat fatalities caused by windenergy facilities throughout the United States. National and regional results are related to the potential for fatalities in the Mid-Atlantic Highlands where appropriate.
Early industrial wind-energy facilities, most of which were developed in California in the early 1980s, were planned, permitted, constructed, and operated with little consideration for the potential impacts to birds or bats (Anderson et al. 1999). Discoveries of raptor fatalities at the Altamont Pass Wind Resource Area (APWRA) (Anderson and Estep 1988; Estep 1989; Orloff and Flannery 1992) triggered concern about possible impacts to birds from wind-energy development on the part of regulatory agencies, environmental groups, wildlife resource agencies, and wind- and electricutility industries throughout the country.
Initial discoveries of bird fatalities resulted from chance encounters by industry maintenance personnel with raptor carcasses at wind-energy facilities. Although fatalities of many bird species have since been documented at wind-energy facilities, raptors have received the most attention (Anderson and Estep 1988; Estep 1989; Howell and Noone 1992; Orloff and Flannery
1992, 1996; Howell 1995; Martí 1995; Anderson et al. 1996a,b, 1997, 1999, 2000; Johnson et al. 2000a,b; Thelander and Rugge 2000; Hunt 2002; Smallwood and Thelander 2004, 2005; Hoover and Morrison 2005). This attention is likely because raptors are lower in abundance than many other bird species, have symbolic and emotional value to many Americans, and are protected by federal and state laws. Raptor carcasses also remain much longer than carcasses of small birds, making fatalities of raptors more conspicuous to observers. Raptors occur in most areas with potential for wind-facility development, although raptor species appear to differ from one another in their susceptibility to collisions.
Early studies of wind-energy facility impacts on birds were based on the carcasses discovered during planned searches. However, fatality estimates did not account for potential survey biases, most importantly biases in searcher efficiency and carcass “life expectancy” or persistence. Most current estimates of fatalities include estimates for all species and are based on extrapolation of the number of observed fatalities at surveyed turbines to the entire wind-energy facility, although not all studies adequately correct for observer-detection bias and carcass persistence, the latter usually referred to as scavenger-removal bias (e.g., Erickson et al. 2004).
Until relatively recently, little attention has been given to bat fatalities at wind-energy installations. This is largely because few bat fatalities have been reported at most wind-energy facilities (Johnson 2005). While some bat fatalities were reported beginning in the early 1990s, few of the earliest studies of fatalities at wind-energy facilities were designed to look for or evaluate bat fatalities, and thus did not use systematic search protocols or account for observer bias or scavenging. The scarcity of reported fatalities also may be due in part to the rarity of post-construction studies designed specifically to detect bat fatalities at wind-energy facilities. Recent surveys indicate that some wind-energy facilities have killed large numbers of bats in the United States (Arnett 2005; Johnson 2005), Europe (Dürr and Bach 2004; Hötker et al. 2004; UNEP/EUROBATS 2006), and Canada (R.M.R. Barclay, University of Calgary, personal communication 2006).
BIRD AND BAT FATALITIES
In the following discussion, fatality rate is presented as fatalities/ turbine/year or fatalities/MW/year. Because turbine size, and presumably risk, varies from facility to facility, we have chosen to make comparisons of fatalities among turbines using the metric fatalities/MW/year. The MW used in this metric represents the nameplate capacity for the turbines and does not represent the actual amount of MW produced by a turbine or wind-energy plant. The reader is referred to Chapter 2 for a more general discussion of nameplate capacity. A more accurate measure of MW pro-
duction for individual turbines would provide a much better metric for comparison purposes. For example, two turbines with the same nameplate capacity may operate a much greater percentage of time at a Class 5 wind site than in a Class 4 wind site.
Bird Species Prone to Collisions with Wind Turbines
Songbirds (order Passeriformes) are by far the most abundant bird group in most terrestrial ecosystems, and also the most often reported as fatalities at wind-energy facilities. The number of fatalities reported by individual studies in the eastern United States ranges from 0 during a five-month study at the Searsburg, Vermont facility (Kerlinger 1997) to 11.7 birds per MW during a one-year study at Buffalo Mountain, Tennessee (Nicholson 2003). In a review of bird collisions reported in 31 studies at wind-energy facilities, Erickson et al. (2001) reported that 78% of the carcasses found at facilities outside of California were protected passerines (i.e., songbirds protected by the Migratory Bird Treaty Reform Act of 2005). The remainder of the fatalities included waterfowl (5.3%), waterbirds (3.3%), shorebirds (0.7%), diurnal raptors (2.7%), owls (0.5%), fowl-like (galliform) birds (4.0%), other (2.7%), and non-protected birds (e.g., starling, house sparrow, and rock dove or feral pigeon; 3.3%). Despite the relatively high proportion of passerines recorded, actual fatalities of passerines probably are underrepresented in most studies, because small birds are more difficult to detect and scavenging of small birds can be expected to be higher (e.g., Johnson et al. 2000b). Moreover, given the episodic nature of bird migration, it is possible that many previous studies with relatively long search intervals failed to detect some fatalities of small birds during the migration season, and thus existing estimates of fatalities could be underestimates.
Data allowing accurate estimates of bird fatalities at wind-energy facilities in the United States are limited, particularly in the Mid-Atlantic Highlands region. Of the studies reviewed for this report, 14 were conducted using a survey protocol for all seasons of occupancy for a one-year period (Table 3-1) and incorporated scavenging and searcher-efficiency biases into estimates (Erickson et al. 2000, 2004; Young et al. 2001, 2003a,b; Howe et al. 2002; Johnson et al. 2002, 2003b; Nicholson 2003; Kerns and Kerlinger 2004; Koford et al. 2004). Protocols used in these 14 studies varied considerably, but all generally followed the guidance in Anderson et al. (1999). The wind-energy facilities included in these studies contain turbines that range in size from 600 kW to 1.8 MW. Passerines make up 75% of the fatalities at these facilities and 76% of the fatalities at the two forested facilities in the eastern United States (Table 3-2, Figure 3-1). The greatest difference between fatalities at wind-energy facilities in the eastern United States and those in other regions is the relative abundance of doves,
pigeons, and “other” species (e.g., swifts and hummingbirds, cuckoos, woodpeckers) in the east.
Total annual bird fatalities per turbine and per MW are similar for all regions examined in these studies, although data from the two sites evaluated in the eastern United States suggest that more birds may be killed at windenergy facilities on forested ridge tops than in other regions. It is not known whether this is due to higher risk of collisions at these sites, or higher abundance of birds in the region. Most studies report that passerine fatalities occur throughout facilities, with no identified relationship to site characteristics (e.g., vegetation, topography, turbine density). The relatively high proportion of passerines probably reflects the fact that this group is by far the most abundant of all birds at the facilities where these fatalities occurred. Relative exposure is difficult to measure and there are no data suggesting that fatalities expressed as percentages are proportional to abundance. As discussed below, behavior appears to be important in determining the risk of collision.
The combined average raptor fatality rate for the 14 studies (Table 3-2) is 0.03 birds per turbine/year and 0.04 per MW/year. The regional raptor fatalities per MW/year are similar, ranging from 0.07 in the Pacific Northwest region to 0.02 in the eastern United States. With the exception of the two eastern facilities, Mountaineer and Buffalo Mountain, which are in forest (68 MW combined), the land use/land cover is similar in all regions (Table 3-1). Most of the wind-energy facilities occur in agricultural areas (333 MW combined) and agriculture/grassland/Conservation Reserve Program lands (438 MW combined), and the remainder occur in short-grass prairie (68 MW combined). Landscapes vary from mountains, plateaus, and ridges, to areas of low relief. Aside from the size of the rotor-swept area, each of these facilities used similar technologies. Bird abundance may be an important factor in fatalities (discussed in more detail below), although standard estimates of bird use are not available for all 14 studies.
Interpreting fatalities of breeding and migrating passerines is challenging because of inadequate estimation of exposure of different species to risk. The most common fatalities reported at wind-energy facilities in the western and middle United States are relatively common species, such as horned lark (Eremophila alpestris), vesper sparrow (Pooecetes gramineus), and bobolink (Dolichonyx oryzivorus). These species perform aerial courtship displays that frequently take them high enough to enter the rotor-swept area of a turbine (Kerlinger and Dowdell 2003). The western meadowlark (Sturnella neglecta), on the other hand, is quite common and is frequently reported in fatality records, yet is not often seen flying as high as the rotorswept area of wind turbines. By contrast, crows, ravens, and vultures are among the most common species seen flying within the rotor-swept area of turbines (e.g., Orloff and Flannery 1992; Erickson et al. 2004; Smallwood and Thelander 2004, 2005), yet they seldom are found during carcass
TABLE 3-1 Description of Wind-Energy Facilities Based on Data Collected During the Period of Bird Occupancy over a Minimum Period of One Year and Where Standardized Bird Mortality Studies Conducted, Including Scavenging and Searcher Efficiency Biases. Vegetation Categories Include Agriculture (AG), Grass Land (Grass), Conservation Reserve Program (CRP) Grasslands, Short-Grass Steppe, and Forest. Seasons Include Spring (S), Summer (Su), Fall (F), and Winter (W)
|
Wind Facility |
Vegetation |
Dates of Study |
|
Vansycle, OR |
Ag/Grass/CRP |
1/99-12/99 |
|
Nine Canyon, WA |
Ag/Grass/CRP |
9/02-8/03 |
|
Stateline, OR/WA |
Ag/Grass/CRP |
1/02-12/03 |
|
Combine Hills, OR |
Ag/Grass/CRP |
2/04-2/05 |
|
Klondike, OR |
Ag/Grass/CRP |
2/02-2/03 |
|
Foote Creek Rim, WY Phase I |
Short-grass Steppe |
11/98-12/00 |
|
Foote Creek Rim, WY Phase II |
Short-grass Steppe |
7/99-12/00 |
|
Wisconsin |
Agriculture |
Spring 98-12/00 |
|
Buffalo Ridge, MN Phase I |
Agriculture |
4/94-12/95 |
|
|
|
3/96-11/99 |
|
Buffalo Ridge, MN Phase II |
Agriculture |
3/98-11/99 |
|
Buffalo Ridge, MN Phase III |
Agriculture |
3/99-11/99 |
|
Top of Iowa, IW |
Agriculture |
4/03-12/03 |
|
Buffalo Mountain, TN |
Forest |
10/01-9/02 |
|
Mountaineer, WV |
Forest |
4/03-11/03 |
surveys. Clearly, abundance and behavior interact to influence exposure of breeding passerines and other birds to the risk of collisions.
While estimated bird fatalities for these 14 wind-energy facilities are relatively low when compared to other sources of bird fatalities (Erickson et al. 2001), the lack of multiyear estimates of density and other population characteristics at most wind-energy facilities makes it difficult to draw general conclusions about their effects on populations of bird fatalities. In addition, lack of replication of studies among facilities and years makes it impossible to evaluate natural variability and the likelihood of unusual episodic events in relation to bird fatalities.
Influences of Turbine Design on Bird Fatalities
The structure and design of existing wind turbines vary considerably, and it is likely that additional modifications will occur over time. Changes in turbine design result from technological improvements, differences in
|
Search Interval |
Number of Turbines in Facility |
Number of Turbines Searched |
Reference |
|
28 days |
38 |
38 |
Erickson et al. (2000) |
|
14 days S, Su, F |
37 |
37 |
Erickson et al. (2003b) |
|
28 days W |
|
|
|
|
14 days |
454 |
124-153 |
Erickson et al. (2004) |
|
28 |
41 |
41 |
Young et al. (2005) |
|
28 days |
16 |
16 |
Johnson et al. (2003b) |
|
28 days |
69 |
69 |
Young et al. (2001) |
|
28 days |
36 |
36 |
Young et al. (2003b) |
|
Daily-weekly |
31 |
31 |
Howe et al. (2002) |
|
7 days |
73 |
50 |
Johnson et al. (2002) |
|
14 days |
73 |
21 |
|
|
14 days |
143 |
40 |
Johnson et al. (2002) |
|
14 days |
138 |
30 |
Johnson et al. (2002) |
|
2-3 days |
89 |
26 |
Koford et al. (2004) |
|
2/week-weekly |
3 |
3 |
Nicholson (2003) |
|
S-11 days |
44 |
44 |
Kerns and Kerlinger |
|
Su-28 days |
|
|
(2004) |
|
F-7 days |
|
|
|
generation capacity, and in some cases, modifications to meet site-specific needs (such as modification of height because of Federal Aviation Administration [FAA] constraints). Differences in design of turbines could affect fatality rates of birds. For example, as turbine heights increase, nocturnally migrating passerines could be increasingly affected because they tend to migrate at levels above 400 feet (see Appendix C for further discussion).
Much of the early work on fatalities at wind-energy facilities occurred in California, because most wind energy was produced at three windresource areas: APWRA, San Gorgonio, and Tehachapi. Not coincidently, some of the existing concern regarding the impact of wind-energy facilities on birds is rooted in the fatalities that have occurred at the APWRA, and thus although many of the characteristics of APWRA differ from those of the Mid-Atlantic Highlands region, the history of APWRA provides important background and context.
The APWRA currently has between 5,000 and 5,400 turbines of various types and sizes, with an installed capacity of approximately 550 MW
TABLE 3-2 Regional and Overall Bird and Raptor Mortalitya at WindEnergy Facilities Based on Data Collected During the Period of Bird Occupancy over a Minimum Period of One Year and Where Standardized Bird Mortality Studies Were Conducted, Including Scavenging and Searcher Efficiency Biases Were Incorporated into the Estimates (additional metadata for these facilities contained in Table 3-1)
|
|
Project Size |
Turbine Characteristics |
|||
|
Wind Project |
Number of Turbines |
Number of MW |
Rotor Diameter (m) |
RotorSwept Area (m2) |
MW |
|
Pacific Northwest |
|
|
|
|
|
|
Stateline, OR/WAb |
454 |
300 |
47 |
1735 |
0.66 |
|
Vansycle, ORb |
38 |
25 |
47 |
1735 |
0.66 |
|
Combine Hills, ORb |
41 |
41 |
61 |
2961 |
1.00 |
|
Klondike, ORb |
16 |
24 |
65 |
3318 |
1.50 |
|
Nine Canyon, WAb |
37 |
48 |
62 |
3019 |
1.30 |
|
Totals or simple averages |
586 |
438 |
56 |
2554 |
1.02 |
|
Weighted averages |
586 |
438 |
49 |
1945 |
0.808 |
|
Rocky Mountain |
|
|
|
|
|
|
Foote Creek Rim, WY Phase Ic |
72 |
43 |
42 |
1385 |
0.60 |
|
Foote Creek Rim, WY Phase IIc |
33 |
25 |
44 |
1521 |
0.75 |
|
Totals or simple averages |
105 |
68 |
43 |
1453 |
0.675 |
|
Totals or weighted averages |
105 |
68 |
43 |
1428 |
0.655 |
|
Upper Midwest |
|
|
|
|
|
|
Wisconsin |
31 |
20 |
47 |
1735 |
0.66 |
|
Buffalo Ridge Phase Id |
73 |
22 |
33 |
855 |
0.30 |
|
Buffalo Ridge Phase Id |
143 |
107 |
48 |
1810 |
0.75 |
|
Buffalo Ridge, MN Phase IId |
139 |
104 |
48 |
1810 |
0.75 |
|
Top of Iowad |
89 |
80 |
52 |
2124 |
0.90 |
|
Totals or simple averages |
475 |
333.96 |
46 |
1667 |
0.67 |
|
Totals or weighted averages |
475 |
333.96 |
46 |
1717 |
0.53 |
|
East |
|
|
|
|
|
|
Buffalo Mountain, TNe |
3 |
2 |
47 |
1735 |
0.66 |
|
Mountaineer, WVe |
44 |
66 |
72 |
4072 |
1.50 |
|
Totals or simple averages |
47 |
68 |
60 |
2903 |
1.08 |
|
Overall (weighted average)f |
47 |
68 |
70 |
3922 |
1.45 |
|
aMortality rates are on a per year basis. bAgriculture/grassland/Conservation Reserve Program (CRP) lands. cShortgrass prairie. dAgricultural. eForest. fWeighted averages are by megawatt and turbine number. |
|||||
|
Raptor Mortality |
All Bird Mortality |
|
||
|
Number per Turbine per Year |
Number per MW per Year |
Number per Turbine per Year |
Number per MW per Year |
Source |
|
0.06 |
0.09 |
1.93 |
2.92 |
Erickson et al. (2004) |
|
0.00 |
0.00 |
0.63 |
0.95 |
Erickson et al. (2000) |
|
0.00 |
0.00 |
2.56 |
2.56 |
Young et al. (2005) |
|
0.00 |
0.00 |
1.42 |
0.95 |
Johnson et al. (2003b) |
|
0.07 |
0.05 |
3.59 |
2.76 |
Erickson et al. (2003b) |
|
0.03 |
0.03 |
2.03 |
2.03 |
|
|
0.05 |
0.07 |
1.98 |
2.65 |
|
|
0.03 |
0.05 |
1.50 |
2.50 |
Young et al. (2001) |
|
0.04 |
0.06 |
1.49 |
1.99 |
Young et al. (2003b) |
|
0.04 |
0.05 |
1.50 |
2.24 |
|
|
0.03 |
0.05 |
1.50 |
2.31 |
|
|
0.00 |
0.00 |
1.30 |
1.97 |
Howe et al. (2002) |
|
0.01 |
0.04 |
0.98 |
3.27 |
Johnson et al. (2002) |
|
0.00 |
0.00 |
2.27 |
3.03 |
Johnson et al. (2002) |
|
0.00 |
0.00 |
4.45 |
5.93 |
Johnson et al. (2002) |
|
0.01 |
0.01 |
1.29 |
1.44 |
Koford et al. (2004) |
|
0.00 |
0.01 |
2.06 |
3.13 |
|
|
0.00 |
0.00 |
2.22 |
3.50 |
|
|
0.00 |
0.00 |
7.70 |
11.67 |
Nicholson (2003) |
|
0.03 |
0.02 |
4.04 |
2.69 |
Kerns and Kerlinger (2004) |
|
0.02 |
0.01 |
5.87 |
7.18 |
|
|
0.03 |
0.02 |
4.27 |
2.96 |
|
|
|
|
|
|
|
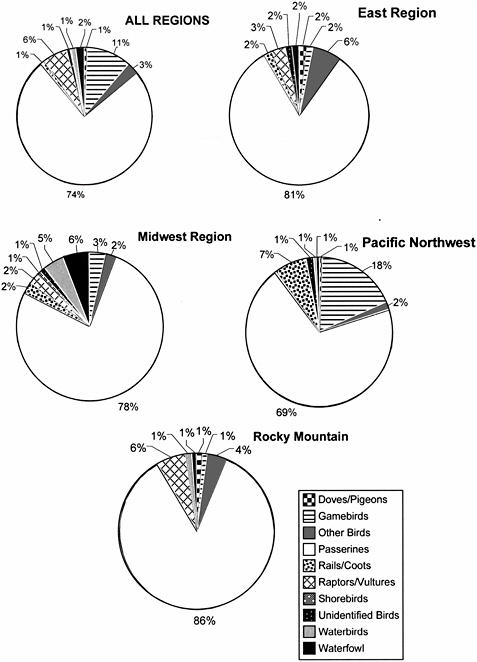
FIGURE 3-1 Composition of bird fatalities at 14 wind-energy facilities in the United States.
SOURCES: Compiled by committee from Erickson et al. 2000, 2003b, 2004; Young et al. 2001, 2003b, 2005; Howe et al. 2002; Johnson et al. 2002, 2003b; Nicholson 2003; Kerns and Kerlinger 2004; Koford et al. 2004.
(~102 kW/turbine); San Gorgonio consists of approximately 3,000 turbines of various types and sizes with an installed capacity of approximately 615 MW (~205 kW/turbine); and Tehachapi Pass has approximately 3,700 turbines with an installed capacity of approximately 600 MW (~162 kW/turbine). The following discussion generally refers to these facilities as “older generation” wind-energy facilities.
While replacement of some smaller turbines with modern turbines has occurred (through repowering), these three wind-resource areas primarily consist of relatively small turbines ranging from 40 to 200-300 kW, with the most common turbine rated at approximately 100 kW. Most of the higher-resource wind sites within each area have a high density of turbines, and the support structures for older turbines are both lattice and tubular, all with abundant perching locations for birds on the tower and nacelle (Figures 3-2a and b). (Figure 3-3 shows a more modern installation, Mountaineer, West Virginia, for comparison.) Additionally, all three areas have above-ground transmission lines. Perching sites for raptors are ubiquitous within all three areas, but particularly at the APWRA. There are different vegetation communities at all three sites, with San Gorgonio being the most arid, and Tehachapi the most montane and with some forest.
McCrary et al. (1986) conducted one of the earliest studies of the impact of wind-energy facilities on birds at San Gorgonio. However, the widely publicized report of bird fatalities at APWRA by Orloff and Flannery

FIGURE 3-2a Turbines at San Gorgonio showing lattice and monopole towers.
SOURCE: Photograph by David Policansky.

FIGURE 3-2b Turbines at San Gorgonio showing high density and diversity of types.
SOURCE: Photograph by David Policansky.
(1992) promoted the most scrutiny of the problem. In spite of subsequent industry attempts to reduce raptor fatalities, they remain relatively high at the APWRA and reduction of fatalities was the focus of a recent decision by the Alameda County Board of Supervisors to issue conditional permits for the continued operation of the facility.
Smallwood and Thelander (2004, 2005) investigated the impacts of approximately 1,500 turbines for 4 years and 2,500 turbines for 6 months; the turbines ranged from 40 to 330 kW. While the Smallwood and Thelander (2004, 2005) studies are the most comprehensive to date, due to small sample sizes for turbines greater than 150 kW, extrapolation of fatality rates to all turbines in the AWPRA may not be appropriate. Hunt (2002) completed a four-year radiotelemetry study of golden eagles at the APWRA and concluded that while the population is self-sustaining, fatalities resulting from wind-energy production were of concern because the population apparently depends on immigration of eagles from other subpopulations to fill vacant territories. A follow-up survey was conducted in 2005 (Hunt and Hunt 2006) to determine the proportion of occupied breeding golden-eagle

FIGURE 3-3 Mountaineer Wind Energy Center, West Virginia. The five turbines in this photograph are at the southwest end of the array of 1.5 MW turbines; they are at the lower left of the aerial view in Figure 3-7.
SOURCE: Photograph by David Policansky.
territories in the APWRA. Within a sample of 58 territories all territories occupied by eagle pairs in 2000 were also occupied in 2005.
Contemporary utility-scale wind-energy facilities use different turbines from those at the older wind-energy facilities discussed above. The turbines are larger, with lower rotational rates (~15-27 rpm), although they retain a relatively high tip speed (~80 m/sec); tubular towers; primarily underground electrical service; lighting following FAA guidelines; few perching opportunities; and the rotor-swept area is higher above ground level (agl). In addition, many of the developments have occurred in areas with a different land use than the earlier California wind-energy facilities. Nonetheless, the potential cumulative impacts of these turbines should not be overlooked, especially for resident species.
The estimated fatality rates for raptors at the older California turbines (e.g., Orloff and Flannery 1992; Anderson et al. 2004, 2005; Smallwood and Thelander 2004, 2005) are generally greater than for newer turbines (Figure 3-4), although most of the sites for the newer turbines have much
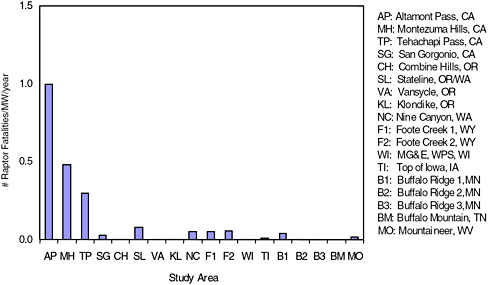
FIGURE 3-4 Fatality rates for raptors at 4 older wind-energy facilities (AP, MH, TP, SG) unadjusted for searcher efficiency, carcass-removal bias, and raptor abundances at the sites, and raptor fatality rates at 14 newer facilities (CH, SL, VA, KL, NC, F1, F2, WI, TI, B1, B2, B3, BM, MO) adjusted for searcher efficiency and carcassremoval bias.
SOURCES: Howell 1997; Erickson et al. 2000, 2003a,b, 2004; Howe et al. 2002; Johnson et al. 2002, 2003b; Nicholson 2003; Young et al. 2003a,c, 2005; Anderson et al. 2004, 2005; Kerns and Kerlinger 2004; Koford et al. 2004; Smallwood and Thelander 2004, 2005.
lower raptor abundance, there are relatively few studies of new wind-energy facilities, and there are major geographic gaps in the available data. Even though the raptor fatalities appear higher at wind-resource areas with the older technology, there is a marked difference among the older facilities. For example, raptor fatalities at the APWRA were higher than at Montezuma Hills, somewhat lower at Tehachapi (Anderson et al. 2004), and very low at the San Gorgonio facility (Anderson et al. 2005). Because the four facilities use similar technology, this difference may be influenced by other factors, most likely raptor abundance and prey availability.
The relationship of raptor abundance and technology will be better addressed when it is possible to study old and new turbines together in areas of varying raptor density. The three wind-energy facilities in northern California—High Winds and Diablo Winds in Solano County and the APWRA in Alameda County—may present such an opportunity when estimates of fatalities are published. The Solano County sites have newer turbines, and with the exception of golden eagles, higher raptor use than the APWRA (Orloff and Flannery 1992; Smallwood and Thelander 2004, 2005). Preliminary data from High Winds (Kerlinger et al. 2006) and Diablo Winds (WEST 2006) indicate they have higher raptor use, and higher raptor mortality than do projects in the Pacific Northwest (e.g., Erickson et al. 2004) and midwest (e.g., Johnson et al. 2000a,b). Alameda County, California, has permitted repowering of a small portion of the APWRA, replacing the MW production of smaller turbines with a smaller number of large newer turbines; fatality data from the APWRA collected before and after repowering can be used in a before/after control/impact (BACI) study, the preferred study design for observational studies (Anderson et al. 1999). Results from this and other repowering efforts in California will help evaluate the relative role of technology in bird fatalities, as would studies of fatalities at wind-energy facilities with large turbines in other areas of the country with relatively high raptor densities (e.g., eastern mountain ridges, coastal areas).
Most bird fatalities at wind-energy facilities are assumed to be caused by collisions with wind turbine blades. Even though there is no evidence indicating that passerines collide with turbine-support structures, numerous studies have documented passerine collisions with other solid structures (Erickson et al. 2001). Several studies have reported fatalities from buildings, and similar structures such as smokestacks and communications towers (Erickson et al. 2001). Bird fatalities associated with communications towers generally increase with height of the tower and lighting, with larger fatality events occurring at towers greater than 152 m (500 feet) in height. (Kerlinger 2000; Longcore et al. 2005). Nevertheless, shorter guyed towers1 (< 152 m)
may also present a risk for birds (Longcore et al. 2005). In a study of bird fatalities associated with 69 turbines and 5 guyed meteorological towers at a wind-energy facility in Carbon County, Wyoming, Johnson et al. (2001) reported that fatalities associated with the 40-m meteorological towers were three times greater than those associated with the 61-m wind turbines.
Although the steady red lights commonly recommended by the FAA have been shown to attract night-migrating birds and have been associated with an increase in bird fatalities at communications towers and other tall structures (Erickson et al. 2001; Manville 2001; Longcore et al. 2005; Gauthreaux and Belser 2006), there is no evidence to suggest a lighting effect on passerine fatalities at wind-energy facilities, with the exception of the Mountaineer Wind Energy Center in West Virginia. Kerns and Kerlinger (2004) reported the largest bird fatality event ever recorded at a wind-energy facility, with 33 documented passerine fatalities discovered on May 23, 2002. These fatalities apparently occurred during heavy fog conditions. All of the carcasses were located at a substation and at three adjacent turbines. The substation was brightly lit with sodium vapor lights. Following the discovery of the fatalities, the bright lights were turned off and no further large events were reported at the site. The second-largest fatality event documented involved 14 warblers, vireos, and flycatchers found during a May 17 carcass search of two adjacent turbines at the Buffalo Ridge, Minnesota wind-energy facility (Johnson et al. 2002). Like the West Virginia example, the event appeared to follow inclement weather, although only one of the turbines was lighted and lighting was not considered important (Johnson et al. 2002).
Influences of Site Characteristics on Bird Fatalities
Site characteristics may influence risk of fatality for birds, including location relative to key habitat resources (such as nesting sites, prey, water, and other resources) or concentration areas during migration, vegetative community in which the turbines are constructed, topographic position, and other factors. Relatively little is known about many of these relationships, but evidence for the importance of some of these variables is becoming clearer. Better understanding of these relationships will likely be helpful in siting decisions for future wind-facility development.
The effect of topography on fatality rates of birds is unclear. Of the 14 studies referred to in Table 3-1, most occurred in agricultural or grassland communities and in a variety of landscapes. Without more data from different plant communities and landscapes it is not possible to evaluate their influence of bird fatalities.
It is generally assumed that nocturnal migrating passerines move in broad fronts, as opposed to following specific and well-defined migration
pathways, and rarely respond to topography (Lowery and Newman 1966; Richardson 1972; Williams et al. 1977), but this topic needs further study. A continent-wide study of nocturnal bird migration based on birds crossing the disc of the moon during four nights in October in 1952 (Lowery and Newman 1966) found little or no evidence that migrating birds were influenced by major rivers or mountain ranges in the eastern United States. However, the rugged mountains in the western United States did appear to affect the patterns of migration. Flight responses of migrants to the Great Lakes and the Gulf of Mexico were mixed. Some species flew parallel to the shoreline and appeared to be avoiding a crossing while others were observed departing across the large bodies of water. Bingman et al. (1982) found that on most nights during autumn migration in eastern New York State passerines showed a preferred migration track toward the southwest and in strong winds from the west and northwest the migrants drifted. On reaching the Hudson River some of the migrants changed their headings and followed a track direction that closely paralleled the river, and in doing so partially compensated for the effects of wind drift.
Schüz et al. (1971) and Berthold (2001, pp. 57-60) concluded that most migratory species in Europe show broad-front migration for at least a portion of their journey and suggested that species that have broad breeding ranges (E-W) tend to have broad-front migration pathways that cross all geomorphological features (such as mountains, river valleys, lakes). Hüppop et al. (2006) noted that the migration of birds over the waters of the German Bight also is broad-front. Recent radar studies of migration in the continental United States also support the conclusion that many species of migratory birds show broad-front migration (Gauthreaux et al. 2003). Gauthreaux et al. (2003) used a network of NEXRAD weather radars to quantify nocturnal bird migration over the United States, and the migration maps produced from the study clearly show that large geographical-scale migratory movements occur in response to weather favorable to migration. No evidence of specific flyways can be seen in the migration maps at the scale of surveillance of the radars (240 km range), and the results are in keeping with the findings of Lowery and Newman (1966).
Weather surveillance NEXRAD radar has rather coarse resolution (1 km × 1.0°) and consequently may not detect deviations in migration patterns at smaller spatial scales. Moreover, migrants flying at low altitudes may be missed by Doppler weather surveillance radars. Low-flying migrants could respond to topographic features more readily than migrants flying at higher altitudes. This would explain some of the conflicting findings regarding flight paths reported for migratory birds. Williams et al. (2001) cite work in Europe suggesting migrating birds respond to coastlines, river systems, and the Alps (e.g., Eastwood 1967; Bruderer 1978, 1999; Bruderer and Jenni 1988). While responses of birds to coastlines and major rivers has
been noted in North America (e.g., Richardson 1978, Bingman et al. 1982), evidence is limited on the response to major changes in topography (McCrary et al. 1983). Williams et al. (2001) used radar, ceilometers, and daily censuses in a study of passerine migration in the area of Franconia Notch, New Hampshire, a major pass in the northern Appalachian Mountains. They report that what they assumed to be migrating passerines surveyed by marine X-band radar appeared to react to topography in the Franconia Notch area. However, the study design and X-band radar equipment used in the study focused on localized and relatively low-altitude target movements and did not allow assessment of a broader area for movement patterns, and some of the detected targets may have been bats. However, Mabee et al. (2006) reported that for 952 flight paths of targets approaching a high mountain ridge along the Allegheny Front in West Virginia, the vast majority (90.5%) did not alter their flight direction while crossing the ridge. The remaining targets either shifted their flight direction by at least 10 degrees (8.9%) while crossing the ridge or turned and did not cross the ridge (0.6%)—both considered reactions to the ridgeline.
There is considerable agreement that migration patterns of most birds are species-specific. Species with limited breeding and wintering ranges generally have restricted migration pathways, but species with widely dispersed breeding ranges tend to show broad-front migration. A recent discussion of the flyway versus broad-front migration patterns in the United States is in Lincoln et al. (1998, pp. 53-72).
Many of the mountain ridgelines, and in particular those along the eastern edge of the Appalachian Mountains, appear to provide migratory pathways for diurnal fall migrants such as raptors (Bednarz et al. 1990). Raptors concentrate along ridges during migration and during daily hunting flights, presumably to take advantage of rising thermals and favorable winds used for soaring. This relationship was quantified at the Foote Creek Rim (FCR) wind-energy facility in Wyoming (Johnson et al. 2000a). Approximately 85% of the golden eagles, ferruginous hawks, and Swainson’s hawks observed flying at the height of the rotor-swept area for the proposed turbines were within 50 m of the edge of the north to south trending mesa. Thus, raptors are likely more at risk when turbines are placed in areas where favorable winds exist for soaring.
Although high raptor fatalities have been documented at the APWRA, studies conducted at San Gorgonio and Tehachapi Pass (Anderson et al. 2004) documented relatively low raptor mortality (McCrary et al. 1983, 1984, 1986; Anderson et al. 2005) in comparison to the APWRA. The unadjusted per-turbine and per-MW raptor fatality rates reported for these sites are 0.006 and 0.03 for San Gorgonio, 0.04 and 0.20 for Tehachapi, and 0.1 and 1-1.23 for the APWRA. The primary difference among the three sites appears to be the abundance of raptors (Erickson et al. 2002).
The APWRA has the most raptors, presumably because of the abundance of prey, particularly small mammals (Smallwood and Thelander 2004, 2005). San Gorgonio has the fewest raptors, while raptor densities at Tehachapi Pass are intermediate (Anderson et al. 2004, 2005). The West Ridge within the Tehachapi Pass study area had the highest raptor use observed during the study, approximately half the estimated use of the APWRA (Anderson et al. 2004). The West Ridge also had the highest reported raptor fatalities among the three geographic subdivisions of Tehachapi Pass studied. These data suggest that differences in site quality, resulting in differences in abundance and exposure to turbines, may play an important role in determining mortality of some species. Smallwood and Thelander (2004, 2005) and Orloff and Flannery (1992) reported more raptor fatalities at wind turbines constructed in canyons at APWRA than at other locations within the area.
It also is usually assumed that nocturnally migrating passerines migrate relatively high agl. In a review of radar studies in the eastern United States, Kerlinger (1995) concluded that three-quarters of passerines (assumed passerines because bats were not considered) migrate at altitudes between 91 and 610 m. Recent marine radar studies conducted with modern X-band equipment capable of estimating target altitude from ~10 m to 1.5 km agl suggest that most nocturnal migrants fly above 125 m agl, the upper reach of most modern wind turbines. For example, using X-band marine radar in a vertical configuration, Mabee and Cooper (2002) for two study areas in the Pacific Northwest reported 3 and 9% of targets were below 125 m agl, while Mabee et al. (2004), also using vertical X-band marine radar, estimated that 13% of targets (birds and bats were not distinguished) detected on a mountain ridge in West Virginia were below 125 m agl. Nevertheless, X-band marine radar studies suggest there is a large amount of nighttime variation in flight altitudes (e.g., Cooper et al. 1995a,b), with targets averaging different altitudes on different nights and at different times during each night. Some of the intra-night variation is due to birds landing at dawn and taking flight at dusk, or bats emerging at dusk or returning at dawn. Kerlinger and Moore (1989) and Bruderer et al. (1995) concluded that atmospheric structure is the primary factor affecting flight direction and height of targets assumed to be migrating passerines. For example, Gauthreaux (1991) found that birds (and possibly bats) crossing the Gulf of Mexico appear to fly at altitudes where favorable winds exist.
In summary, it appears likely that nocturnally migrating passerines fly in broad fronts given the limit of resolution of current methods of detection, and that during migration the vast majority fly at altitudes well above the rotor-swept area of wind turbines. However, when weather conditions (e.g., low ceiling, light precipitation) compress bird migration closer to the surface, migrants may deviate their flights in response to topographi-
cal changes and could be at risk of collisions with wind turbines along ridgelines. Under favorable weather conditions migrant birds landing at night or beginning flight at dusk are potentially at risk of collision. This is particularly so if turbines are located adjacent to migratory stopover areas where migrants may be concentrated. Raptors often concentrate along topographic features when updrafts exist that facilitate soaring and may be at greater risk of collision when wind turbines are constructed in these locations. Nevertheless, prey abundance may also strongly influence raptor abundance and thus risk of collisions.
Temporal Pattern of Bird Fatalities at Wind-Energy Facilities
Although additional research is needed for more complete understanding of temporal patterns of fatalities at wind-energy facilities, a number of patterns emerge and it is clear that risk of fatality differs with location, meteorological condition, time of night, and time of year for both birds and bats.
Based on the available data, fatalities of passerines occurred in all months surveyed (Table 3-2). Bird fatalities along the Appalachian ridge have been most common from April through October (Nicholson 2003; Kerns and Kerlinger 2004), although the seasonal timing of fatalities varies somewhat among sites. For example, peak passerine fatalities occurred during spring migration at Buffalo Ridge, Minnesota (Johnson et al. 2002), and during fall migration at Stateline in Washington and Oregon (Erickson et al. 2004). This seasonal pattern suggests that both migrating and breeding resident bird species are being killed at wind-energy facilities (Howe et al. 2002; Johnson et al. 2002, 2003b; Young et al. 2003a, 2005; Koford et al. 2004).
Estimating the importance of fatalities to local populations requires that fatalities be assigned to a source population. However, allocation of fatalities to migrating and non-migrating passerines is problematic. It seems clear that some fatalities occur during migration. For example, a dead bird generally is considered a migrant if the species is not detected during bird surveys conducted during the breeding season and the habitat is unsuitable for nesting or brood rearing for the species. In many cases, however, the species may be present during the breeding season, but may be discovered as a fatality only, or more often during the migration season. Previous studies have not been able to distinguish resident breeders from migrants, although Erickson et al. (2001) provisionally reported a range of 34.4 to 59.9% of the fatalities as nocturnal migrants. Based on the available data, it appears that approximately half the reported fatalities at new wind-energy facilities are nocturnal migrating birds, primarily passerines, and the other half are resident birds. There is some evidence that young
birds disperse during the nighttime (Mukhin 2004), and this may account for some “breeding season” mortality.
For example, in a four-year study of summer movements of juvenile reed warblers (Acrocephalus scirpaceus) marked as nestlings in Europe, captures by song playback suggest the existence of nocturnal post-fledging movements in this species. The uncertainty as to the geographic source of birds (and bats) killed at wind turbines could possibly be reduced if feather or other tissue samples were taken from carcasses and examined for stable hydrogen isotopes (see Appendix C).
Inclement weather has been identified as an important factor contributing to bird collisions with other obstacles, including power lines, buildings, and communications towers (Estep 1989; Howe et al. 1995), although the effect of weather on fatalities at communications towers is confounded by the height of the tower, type of lighting, and whether the tower is guyed or unguyed. Johnson et al. (2002) estimated that as many as 51 of the 55 bird fatalities discovered at the Buffalo Ridge wind-energy facility in southwestern Minnesota may have occurred in association with thunderstorms, fog, and gusty winds. Estimating the effect of weather is problematic because it is difficult to observe migration in poor visibility and precipitation. Nonetheless, the association of fatalities with episodic weather events recorded at wind-energy facilities (e.g., Johnson et al. 2002) and communications towers (Erickson et al. 2001) suggests that weather could be a factor contributing to bird fatalities at these sites.
Bat Species Are Prone to Collision with Wind Turbines
Data allowing reliable assessments of bat fatalities at wind-energy facilities in the United States are limited. Only six of the studies that we reviewed were conducted using a systematic survey protocol for all seasons of occupancy for a one-year period (Table 3-3) and had scavenging and searcher-efficiency biases incorporated into estimates (Figure 3-4; Arnett 2005; Johnson 2005; Arnett et al. in press). In contrast, protocols for assessing bat fatalities varied considerably and thus make actual fatality rates difficult to compare (Arnett 2005). The wind-energy facilities included in these studies contain turbines that range in size from 600 kW to 1.8 MW. Bat fatalities at wind-energy facilities in the eastern United States are much higher than those in western states.
Of the 45 bat species known from North America (north of Mexico), 11 have been recovered in ground searches at wind-energy facilities (Johnson 2005; Kunz et al. 2007; Arnett et al. in press). Among these, nearly 75% have been foliage-roosting eastern red bats (Lasiurus borealis), hoary bats (Lasiurus cinereus), and tree-cavity-dwelling silver-haired bats (Lasionycteris noctivagans), each of which migrate long distances
TABLE 3-3 Regional Comparison of Characteristics of Monitoring Studies and Factors Influencing the Estimates of Bat Fatalities at 11 Wind-Energy Facilities in the United States
|
Region |
Facility |
Landscapea |
Estimated Fatalities/ MW/Yearb |
|
Pacific Northwest |
Klondike, OR |
CROP, GR |
0.8 |
|
|
Stateline, OR/WA |
SH, CROP |
1.7 |
|
|
Vansycle, OR |
CROP, GR |
1.1 |
|
|
Nine Canyon, WA |
GR, SH, CROP |
2.5 |
|
|
High Winds, CA |
GR, CROP |
2.0 |
|
Rocky Mountains |
Foote Creek Rim, WY |
SGP |
2.0 |
|
South Central |
Oklahoma Wind Energy Center, OK |
CROP, SH, GR |
0.8 |
|
Upper Midwest |
Buffalo Ridge, MN-I |
CROP, CRP, GR |
0.8 |
|
|
Buffalo Ridge, MN-II (1996-1999) |
CROP, CRP, GR |
2.5 |
|
|
Buffalo Ridge, MN-II (2001-2002) |
CROP, CRP, GR |
2.9 |
|
|
Lincoln, WI |
CROP |
6.5 |
|
|
Top of Iowa, IA |
CROP |
8.6 |
|
East |
Meyersdale, PAi |
DFR |
15.3 |
|
|
Mountaineer, WV (2003) |
DFR |
32.0 |
|
|
Mountaineer, WV (2004)i |
DFR |
25.3 |
|
|
Buffalo Mountain, TN-I |
DFR |
31.5 |
|
|
Buffalo Mountain, TN-II |
DFR |
41.1j |
|
aCROP = agricultural cropland, CRP = Conservation Reserve Program grassland, DFR = deciduous forested ridge, GR = grazed pasture or grassland, SGP = short grass prairie, SH = shrubland. bEstimated number of fatalities, corrected for searcher efficiency and carcass removal, per turbine divided by the number of megawatts (MW) of installed capacity. cOverall estimated percent searcher efficiency using bat or bird (*) carcasses during bias correction trials to correct fatality estimates. dNumber of bats or birds (*) used during bias correction trials and mean number of days that carcasses lasted during trials, the metric used to correct fatality estimates. eProportion of eight trial bats not scavenged after 7 days were used to adjust fatality estimates. |
|||
(Table 3-4). Other bat species killed by wind turbines in the United States include the western red bat (Lasiurus blossivilli), Seminole bat (L. seminolus), eastern pipistrelle (Pipistrellus subflavus), little brown myotis (Myotis lucifugus), northern long-eared bat (M. septentrionalis), long-eared myotis (M. evotis), big brown bat (Eptesicus fuscus), and Brazilian free-tailed bat (Tadarida brasiliensis).
To date, no fatalities of federally listed bat species have been reported (Johnson 2005), although it is possible that some of the bats that were
|
Search Interval |
Percent Search Efficiencyc |
Carcass Removal Bats/Dayd |
Reference |
|
28 days |
75* |
32*/14.2 |
Johnson et al. (2003a) |
|
14 days |
42* |
171* + 7 / 16.5 |
Erickson et al. (2003a) |
|
28 days |
50* |
40*/23.3 |
Erickson et al. (2000) |
|
14 days |
44* |
32*/11 |
Erickson et al. (2003b) |
|
14 days |
50* |
8e |
Kerlinger et al. (2006) |
|
14 days |
63 |
10 / 20 |
Young et al. (2003c), Gruver (2002) |
|
8 surveysf |
67g |
|
Piorkowski (2006) |
|
14 days |
29* |
40/10.4 |
Johnson et al. (2003b, 2004) |
|
14 days |
29* |
40/10.4 |
Johnson et al. (2003b) |
|
14 days |
53.4 |
48/10.4 |
Johnson et al. (2004) |
|
1-4 days |
70* |
50*/~10 |
Howe et al. (2002) |
|
2 days |
72* |
156*h |
Jain (2005) |
|
Daily |
25 |
153/18 |
Kerns et al. (2005) |
|
7-27 days |
28* |
30*/6.7 |
Kerns and Kerlinger (2004) |
|
Daily |
42 |
228/2.8 |
Kerns et al. (2005) |
|
3 days |
37 |
42/6.3 |
Fiedler (2004) |
|
7 days |
41 |
48/5.3 |
Fiedler et al. (2007) |
|
fTwo searches (one each in late May and late June) conducted at each turbine in 2004, and four searches every 14 days conducted at each turbine between May 15 and July 15 in 2005. gAuthor used a hypothetical range of carcass-removal rates derived from other studies (079%) to adjust fatality estimates. hNumber of birds used during six trials. The mean number of days that carcasses lasted was not available; on average 88% of bird carcasses remained 2 days after placement. iSix-week study period from August 1 to September 13, 2004. jWeighted mean number of bat fatalities/MW with weights equal to the proportion of 0.66 MW (n=3 of 18) and 1.8 MW (n=15 of 18) turbines. SOURCE: Modified from Arnett et al. in press. |
|||
overlooked by observers during surveys or taken by scavengers included endangered and threatened species, or in other years not sampled where conditions were conducive to use by listed species. Some wind-energy facilities may be constructed where it would be highly unlikely for endangered species to occur at the site. Search efficiency at these sites ranged from 25 to 75%, suggesting that many of the bats that were killed were never found (Arnett 2005; Johnson 2005; Arnett et al. in press) and that many of the bats that were killed were taken by scavengers. Nonetheless, the dominance
TABLE 3-4 Species Composition of Annual Bat Fatalities Reported for Wind-Energy Facilities in the United States
|
Speciesa |
Pacific Northwestb |
Rocky Mountains |
South Central |
Upper Midwest |
East |
Total |
|
Hoary bat |
153 (49.8%) |
155 (89.1%) |
10 (9.0%) |
309 (59.1%) |
396 (28.9%) |
1,023 (41.1%) |
|
Eastern red bat |
— |
— |
3 (2.7%) |
106 (20.3%) |
471 (34.4%) |
580 (23.3%) |
|
Western red bat |
4 (1.3%) |
— |
— |
— |
— |
4 (0.2%) |
|
Seminole bat |
— |
— |
— |
— |
1 (0.1%) |
1 (0.1%) |
|
Silver-haired bat |
94 (30.6%) |
7 (4.1%) |
1 (0.9%) |
35 (6.7%) |
72 (5.2%) |
209 (8.4%) |
|
Eastern pipistrelle |
— |
— |
1 (0.9%) |
7 (1.3%) |
253 (18.5%) |
261 (10.5%) |
|
Little brown myotis |
2 (0.7%) |
6 (3.5%) |
— |
17 (3.3%) |
120 (8.7%) |
145 (5.8%) |
|
Northern long-eared myotis |
— |
— |
— |
— |
8 (0.6%) |
8 (0.4%) |
|
Big brown bat |
2 (0.7%) |
2 (1.1%) |
1 (0.9%) |
19 (3.6%) |
35 (2.5%) |
59 (2.4%) |
|
Brazilian free-tailed bat |
48 (15.6%) |
— |
95 (85,5%) |
— |
— |
143 (5.7%) |
|
Unknown |
4 (1.3%) |
4 (2.2%) |
— |
30 (5.7) |
15 (1.1%) |
53 (2.1%) |
|
Total |
307 |
174 |
111 |
523 |
1,371 |
2,486 |
|
aOne confirmed anecdotal observation of a western long-eared myotis (Myotis evotis) has been reported in California, but is not included in this table. bPacific Northwest data from one wind-energy facility in California, three in eastern Oregon, and one in Washington; Rocky Mountain data from one facility each in Wyoming and Colorado; Upper Midwest data from one facility each in Minnesota, Wisconsin, and Iowa; South-Central data from one facility in Oklahoma; East data from one facility each in Pennsylvania, West Virginia, and Tennessee. SOURCES: Kunz et al. 2007; modified from Johnson 2005. Reprinted with permission; copyright 2007, Ecological Society of America. |
||||||
of the hoary bat in the reported fatalities appears to be a consistent theme in most studies in the United States to date, whereas fatalities of eastern red bats are highest in the east, and fatalities of silver-haired bats appear to be highest in the Pacific Northwest (Table 3-4).
Migratory tree bats are the commonest reported bat fatalities at windenergy facilities in the United States. The numbers of bats killed in the eastern United States at wind-energy facilities installed along forested ridge tops have ranged from 15.3 to 41.1 bats/MW/year of installed capacity (Table 3-4). Bat fatalities reported from other regions of the western and midwestern United States have been lower, ranging from 0.8 to 8.6 bats/ MW/year. Nonetheless, a recent study designed to assess bat fatalities in southwestern Alberta (Canada) found that fatalities were comparable to those found at wind-energy facilities located in forested ridges of the eastern United States (R.M.R. Barclay and E. Baerwald, University of Calgary, personal communication 2006).
There are, however, geographic differences in fatalities/MW of installed capacity among bat species. Bat fatalities at wind-energy facilities appear to be highest along forested ridge tops in the eastern United States and lowest in relatively open landscapes in the midwestern and in western states (Fiedler 2004; Johnson 2005; Fiedler et al. 2007; Arnett et al. in press), although relatively large numbers of fatalities have been reported in agricultural regions from northern Iowa (Jain 2005) and southwestern Alberta, Canada (R.M.R. Barclay and E. Baerwald, University of Calgary, personal communication 2006). Additionally, in a recent study conducted in mixed-grass prairie with wooded ravines in Woodward County, northcentral Oklahoma, Piorkowski (2006) found 111 dead bats beneath wind turbines, 86% of which were pregnant or lactating Brazilian free-tailed bats. Western red bats, hoary bats, silver-haired bats, and Brazilian freetailed bats also have been reported at wind-energy facilities in northern California (Kerlinger et al. 2006). To date, no assessments of bat fatalities have been reported at wind-energy facilities in the southwestern United States, a region where large numbers of migratory Brazilian free-tailed bats are resident during the warm months (McCracken 2003; Russell and McCracken 2006), and where this species provides important ecosystem services to agriculture (Cleveland et al. 2006). High fatality rates also can be expected for other species in the southwestern United States, where bat fatalities have not been monitored, and at wind-energy facilities in western states where rigorous monitoring for bat fatalities has been limited (Kunz et al. in press a). Despite the relatively high proportion of fatalities of migratory tree-roosting bats in each of the five regions summarized in Table 3-4, the eastern pipistrelle, a non-migratory species, accounted for 18.8% of the fatalities in the eastern United States.
Evaluations of the four sites in the Mid-Atlantic Highlands and else-
where, where search efficiencies have been assessed, represent the best available data, but even those evaluations are limited because of the highly variable search efforts and carcass-removal studies. Studies where search efficiency and carcass removals are assessed daily provide the best data set for interpreting fatality rates (Mountaineer Wind Energy Center in 2004, Table 3-3). It is not known whether the high fatalities in the Mid-Atlantic Highlands wind-energy facilities and other areas in the eastern United States actually differ from those reported in other regions, or whether instead they reflect higher risk, higher abundance of migratory bats in the region, or limited search efforts in other regions. Most studies report that fatalities occur throughout the facilities, with no identified relationship to site characteristics (e.g., vegetation, topography, or turbine density) (Arnett 2005; Arnett et al. in press). The relatively high proportion of migratory bats may be influenced by the fact that these bats often forage along topographically uniform linear landscapes (i.e., ridgelines, forest edges). Given that there are no reliable abundance data for migratory tree species or, in fact, most other species of bats, it is impossible to determine at this time whether regional differences in fatalities are proportional to abundance. Given the apparent episodic nature of bat migration (Arnett et al. in press), it is possible that many previous studies with relatively long search intervals failed to detect some fatality events involving bats during migration, and thus existing estimates of fatalities may be too low. As discussed further below, the foraging and roosting behavior of migratory tree-roosting bats may provide important insight for estimating risk of collision.
The lack of multiyear studies and previous, possibly biased estimates of fatalities at most existing wind-energy facilities makes it difficult to draw general conclusions about the long-term effects of bat deaths on bat populations. This is partly due to the lack of efforts to look for bats in early studies, since bat fatalities were not recognized as a problem.
In particular, lack of replication of studies to assess bat activity and fatalities among different wind-energy facilities and years makes it impossible to evaluate natural variation, in particular episodic migration events, changing weather conditions, and other stochastic events as they relate to fatalities.
Influences of Turbine Design on Bat Fatalities
Relatively little is known about the influence of wind-turbine design on bat fatalities. To date, most large numbers of turbine-related bat fatalities have been reported from large, onshore utility-scale wind-energy facilities, in which 1 to 1.5 MW turbines are mounted on cylindrical monopoles. Few if any fatalities were reported from older, lattice-tower turbines that were the source of high raptor fatalities at the facilities in California, although
search protocols were designed primarily for the detection of raptors (e.g., ≥ 30-day search intervals), and thus bat fatalities were most likely underestimated. Most modern wind turbines are tall and white, extending well above the forest canopies in the eastern United States, and quite likely are visually (if not acoustically) detectable to bats on cloudless nights. These large turbines stand in sharp contrast to the surrounding vegetation, and one hypothesis is that they may function as a visual beacon to bats and their insect prey (many insects are attracted to large white objects [Kunz et al. 2007]), especially during nights with sufficient moonlight.
All wind turbines produce sound that can be detected by most humans, and presumably by bats as well. Some turbines also produce broadband ultrasound (a range of frequencies above 20 kHz, approximately the upper limit of human hearing) as well as infrasound (defined as frequencies below 20 Hz, approximately the lower limit of human hearing). The ears of echolocating insectivorous bats are primarily tuned to a range of ultrasonic frequencies, which they use while navigating and capturing insect prey, although many species also produce and respond to frequencies below 20 kHz. Thus, sounds produced by modern wind turbines, which include audible and ultrasonic frequencies (some sounds are generated by the gear box in the nacelle, whereas others are produced by the rotation of the blades through air—often producing a “swishing” sound), may either attract bats—given their curiosity about novel objects in the environment—or confuse them upon detection. Additional research is needed to quantify the responses of bats to these sounds.
Although FAA lighting is not mandatory, the FAA does make recommendations to developers, which usually are followed. Recent observations summarized by Horn et al. (in press) suggest that bats are not attracted to FAA lights installed on wind turbines, although these blinking lights produce broadband pulsed ultrasonic frequencies (T.H. Kunz and S. Gauthreaux, personal observation 2006) that could function as an attractant to bats if they are used on wind turbines. Nonetheless, because ultrasonic frequencies are highly attenuated, especially in moist air (Griffin 1971; Lawrence and Simmons 1982), it is not likely that these sounds would function as a long-distance beacon that would either attract or repel bats. The functional range of echolocation for insectivorous bats that emit frequencies between 25 and 125 kHz can be as short as 5 m (Stilz and Schnitzler 2005).
Lighting on associated maintenance buildings or power stations at wind-energy facilities appears to attract insects. However, given that some insects are attracted to different types of lighting and light-colored objects, wind-turbine monopoles and blades may attract insects that bats feed on. Moreover, the large numbers of insects struck by moving turbine blades suggest that nocturnally flying insects are common at the height of the rotor-swept area (Corten and Veldkamp 2001). Accumulations of dead or
moribund insects on the blades of wind turbines can reduce the efficiency of turbines by up to 50%, at least in some regions. Flying insects may also be attracted to the heat produced by nacelles of wind turbines (Ahlén 2002, 2003; Hensen 2004), and if bats respond to high densities of flying insects near wind turbines, their chances of being struck by turbine blades probably are increased (Kunz et al. 2007).
Wind turbines also produce obvious blade-tip vortices (Figure 3-5), and if bats get temporarily trapped in these moving air masses it may be difficult for them to escape. Rapid pressure changes associated with these conditions may lead to internal injuries, disorientation, and death of bats (Dürr and Bach 2004; Hensen 2004; Kunz et al. 2007).

FIGURE 3-5 Blade-tip vortices created by moving rotor blades in a wind tunnel illustrate the swirling wake that trails downwind from an operating wind turbine.
SOURCE: Robert W. Thresher, National Renewable Energy Laboratory.
The causal factors and patterns of bat fatalities at wind turbines remain uncertain. Observations using thermal infrared imaging suggest that sometimes bats are killed by direct impact with turbine blades (Horn et al. in press). However, there are many unanswered questions. Are bats unable to detect rotating wind-turbine blades during migration and when they forage? When blade tips of large wind turbines rotate at speeds up to 80 m/sec (180 mph), a bat flying at speeds ranging from 2 to 27 m/sec (Neuweiler 2000) would not be able to react fast enough to avoid collision in the rotor-swept area. Are bats attracted to moving turbine blades? The turbine and blades produce audible sounds, ultrasound, and infrasonic vibrations, and because some bat species are known to orient to distant sounds (Buchler and Childs 1981), it is possible that bats are attracted to sounds produced by turbines or become disoriented and when they are migrating or feeding in the vicinity of wind turbines (Kunz et al. 2007).
Alternatively, it is conceivable that bats are visually attracted to wind turbines (Kunz et al. 2007). Migratory hoary bats reportedly seek the nearest available trees when daylight approaches (Dalquest 1943; Cryan and Brown in press), thus bats may mistake the large, conspicuous monopoles of wind turbines for roost trees (Kunz and Lumsden 2003). Because bats are curious animals, they may be killed as they explore novel objects in their environment. Observations of bat activity at wind turbines in Iowa (Jain 2005) and in Sweden (Ahlén 2002) suggest that bats were not attracted to turbines. However, if bats were simply colliding with random objects, bat fatalities also would be expected at meteorological towers. To date, no bat carcasses have been found near meteorological towers, even though these towers have been searched in several monitoring projects (Johnson 2005; Arnett et al. in press).
Will major developments of wind-energy facilities pose increased risks to bats in areas where they migrate or commute nightly to and from roosts? Can migratory species sustain high fatality rates, insofar as eastern red bats already appear to be in decline in New York (Mearns 1898) and in three Midwestern states (Whitaker et al. 2002; Carter et al. 2003; Winhold et al. 2005)? Bats are relatively long-lived (Wilkinson and South 2002; BrunetRossini and Austad 2004) and have low reproductive rates compared to many other mammals (Barclay and Harder 2003). For example, on average, the maximum recorded life span of a bat is 3.5 times greater than a non-flying placental mammal of similar size. Records now exist for individuals of at least five bat species in the wild surviving more than 30 years (Wilkinson and South 2002). Moreover, bats of the family Vespertilionidae (the family of most bats killed by wind turbines in North America) have average litter sizes of between 1.11 and 1.38 litters per year (Barclay and Harder 2003). These traits may seriously limit their ability to recover from persistent or repeated fatality events.
Given our current knowledge and the projected development of wind-energy facilities in the United States and elsewhere, the potential for biologically significant, cumulative impacts is a major concern (Kunz et al. 2007).
Independent of wind turbines and other anthropogenic structures, the migration period probably is a time of high mortality in bats, mostly during adverse weather and other stochastic events (Griffin 1970; Tuttle and Stevenson 1977; Fenton and Thomas 1985; Fleming and Eby 2003). There are enormous gaps in knowledge about migration in bats and the underlying evolutionary forces that have led to this behavior. If migratory tree bats experience naturally high mortality during migration from such factors as inclement weather, predation, and reduced food supplies, it is possible that with their low reproductive rates they will not be able to adjust to the expected cumulative affects resulting from the development of wind-energy facilities proposed in the United States and elsewhere (Kunz et al. 2007).
Influence of Site Characteristics on Bat Fatalities
Recent studies suggest a geographic pattern to bat fatalities at wind-energy facilities (Table 3-3). The unexpectedly high fatalities of migratory tree bats (Lasionyceris and Lasiurus) might reflect a risk to their populations, given that large numbers of these bats have been reported from these regions of North America (Cryan 2003; Kunz et al. 2007). While most evidence suggests that bats may be most vulnerable during the migration period, the observations of fatalities of Brazilian free-tailed bats in Oklahoma suggests that some species, in particular those that form large colonies and disperse and feed nightly at high altitudes (Williams et al. 1973; Cleveland et al. 2006), also may be at considerable risk. With relatively recent development of large wind-energy facilities in west Texas in the expected migratory route of Brazilian free-tailed bats from Carlsbad Caverns National Park, and more wind-energy facilities being proposed for west Texas and along the border with Mexico, migrating Brazilian freetailed bats may be at risk. Regions of the United States where large numbers of bats are believed to concentrate in roosts and disperse and forage nightly at altitudes within the rotor-swept zone of modern wind turbines should be high priorities for investigation.
Temporal Patterns of Bat Fatalities at Wind-Energy Facilities
Much of the uncertainty about spatial and temporal factors responsible for high fatalities, especially those experienced by migratory tree-roosting species, reflects the scarcity of intensive and long-term studies conducted on these species, especially at wind-energy facilities during the maternity
periods from May through July, and during migratory periods and when resident bats feed in the vicinity of wind-energy facilities (Kunz et al. 2007). Available data suggest that most bat fatalities at wind-energy facilities occur during fall migration (Table 3-3). However, these observations may be biased because of reduced effort in collection during the spring and summer migration periods, with reduced effort during the intervening periods. For example, spring migration of eastern red bats, hoary bats, and silverhaired bats in North America generally occurs from early April through mid-June, and autumn migration from mid-July through November (Cryan 2003). Moreover, other species killed by wind turbines in the eastern United States—the eastern pipistrelle, big brown bat, little brown myotis, and northern long-eared bats—are resident throughout much of their geographic range from mid-April to mid-October (Barbour and Davis 1969). Tracking with aircraft indicates that migrating Indiana bats (Myotis sodalis) usually are traveling directly towards their summer destination shortly after they leave their hibernacula (A. Hicks, New York Department of Environmental Conservation, personal communication 2006) (Figure 3-6).
While most bats in North America migrate from winter to summer roosts (e.g., Myotis species), the distances traveled are not comparable to the long-distance movements made by migratory tree-roosting species (Griffin 1970; Fleming and Eby 2003). Wind-energy facilities on mountain ridges in the Mid-Atlantic Highlands and elsewhere in the eastern United States have resulted in the highest reported bat fatalities for tree-roosting species (Nicholson 2003; Fiedler 2004; Arnett 2005; Arnett et al. in press). Thus, seasonal migrations, social behavior, orientation cues, and roosting habits differ markedly among hibernating and long-distance migrating species. However, higher bat fatalities are not confined to forested mountain ridges such as the mid-Atlantic region and elsewhere in the eastern United States. If this is the case, migratory bats could be vulnerable to high mortality from expanded wind-energy development in other regions of North America.
Preliminary observations suggest a strong association of bat fatalities with thermal inversions following frontal passage (Arnett 2005). Thermal inversions create cool, foggy conditions in the valleys with warmer air rising to the ridge tops that remain clear. These conditions could provide strong inducement for both insects and bats, whether migrating or not, to concentrate their activities along ridge tops (Kunz et al. 2007).
Although almost nothing is known about weather conditions that stimulate bat migration, one reasonable assumption is that conditions that are favorable for bird migration would also be favorable for bat migration. According to a review of studies on the timing of bird migration in relation to weather (Richardson 1990), the greatest density of migration occurs with following winds relative to the preferred direction of migration,
FIGURE 3-6 Migration route of an Indiana bat over forested ridge tops in eastern Pennsylvania (immediately south of Wilkes Barre, Luzerne County). This bat was captured and released at an abandoned coal mine at 00:04 h on April 14, 2006. It was tracked by aircraft traveling in a southeasterly direction, settling in a dead maple snag at 04:45 h. In the early evening of April 14, it foraged briefly and returned to its roost at 20:00 h (due to heavy fog). It emerged from its roost tree at 20:15 on night of April 15, but at 20:40 it was temporarily lost heading south (near Kutztown, Berks County). On April 16, it was located roosting in a shagbark hickory tree in forested wetland 90 km (56 miles) from its release site.
SOURCE: C.M. Butchkoski and G. Turner, Pennsylvania Game Commission, personal communication 2006. Reprinted with permission; copyright 2006, C.M. Butchkoski and G. Turner.
but some migration in headwinds has been recorded for some species and when migrants are flying over extensive bodies of water and cannot land. Because of co-variation among weather variables there is also correlation of peak numbers of migrants with other weather variables (e.g., falling temperatures and rising barometric pressure after a cold front passage in fall), but it is difficult to tell whether the relationships are coincidental or causative. Clearly birds do not typically initiate migration when weather
conditions are poor (poor visibility, rain, very low cloud ceiling), but on rare occasions migrants aloft may move into locations with such conditions and either land or continue to fly at low altitudes.
WIND-ENERGY PROJECTS ALTER ECOSYSTEM STRUCTURE
The effects of wind-energy projects on ecosystem structure, and in particular habitats for various species, depend upon the vegetation and other landscape components for resident and migratory species that exist prior to construction. For example, influences of a project on a previously logged and subsequently surface-mined site typically differ from influences at a previously undisturbed forest site. An aerial photograph (Figure 3-7) provides an example of this variation on the Mountaineer Wind Energy Center in Tucker County, West Virginia. The turbines on the northeast end of the turbine string appear to have been constructed in a relatively undisturbed portion of the ridge, while the turbines near the center of the turbine string are constructed in an area of coal- and gravel-mining activity.

FIGURE 3-7 Aerial view of Mountaineer Wind-Energy Facility, which includes 44 1.5 MW turbines. SOURCE: Photograph by David Policansky.
Disturbance is likely dependent on individual site differences with respect to topography, type of vegetation, amount of existing roads, historic land use, and size and dispersion of turbines.
Estimates of direct surface disturbance per turbine vary by source and geographic location. The Bureau of Land Management (BLM 2005a) estimates the potential surface disturbance per turbine to be approximately 3 acres on land administered by the Bureau of Land Management, whereas Nicholson (2003) estimated surface disturbance at 1 acre per turbine for the 16-turbine Buffalo Mountain, Tennessee wind-energy facility. From aerial photography Boone et al. (2005) estimated that disturbance resulting from the construction of eight of the turbines at the Mountaineer Wind Energy Center ranged from 3.9 to 7.1 acres per turbine, not including forest removal for road construction and associated maintenance facilities. However, the sample of turbines was arbitrary and could not be extrapolated to the entire wind-energy facility.
Creating open areas in contiguous forest changes microclimate, by increasing light and wind in newly opened areas (Marsh et al. 2005). This results in increased temperature and reduced relative humidity and soil moisture of affected area (Kapos et al. 1997; Turton and Freiburger 1997), and can lead to elevated rates of wind throw resulting in modified forest structure (Laurance 1997). The intensity of effect varies with topographic features such as slope and elevation, but the fact that wind turbines are often placed on ridge tops, locations of high sustained winds, likely exacerbates the potential for structural damage to vegetation at some sites.
The use of suitable habitat by some forest-dwelling species (e.g., cerulean warbler [Dendroica cerulean] and redback salamander [Plethodon cinereus]) is influenced by the distance to the forest edge (i.e., the interface of forest and open areas). This “depth of edge influence” is sometimes referred to as the functional edge (Wood et al. 2006). Such an impact may radiate outside of the area actually disturbed by turbine development for some species to a distance of 100 m in all directions from the forest edge of the “footprint” (Reed et al. 1996; Haskell 2000). For certain taxa, however, the edge influence may continue to greater depths (e.g., over 200 m for invertebrates; Didham 1997) or greater than 340 m for cerulean warblers (Wood et al. 2006), resulting in much larger estimates of habitat loss for some species. Thus, the total short-term (i.e., during construction activities) loss of habitat for forest-dependent species is likely greater than that of the actual cleared area (Reed et al. 1996; Boone et al. 2005). The long-term impacts of a created opening will likely vary depending on the sensitivity of a species to depth-of-edge influence and the amount of activity in the open area.
The mechanism causing the loss of habitat due to the depth-of-edge influence may also differ among taxa. For example, some species appear
to avoid the edge because the habitat has been modified (e.g., for invertebrates) while other species may avoid the area due to disturbance (i.e., displacement) even though the habitat is not substantially modified. In the case of displacement the impact may be shorter-term if the disturbance is removed (e.g., construction) or the animals become habituated to the disturbance. However, if the effect is due to modification of the habitat so that it becomes less suitable, the impact is expected to be of longer duration.
Forested landscapes in the eastern United States are fragmented over broad geographic regions and species associated with edges generally have not experienced declines (e.g., Bell and Whitmore 1997). Habitat for some species actually has increased with increasing amount of edge, leading to increases in the populations of species in eastern forests such as white-tailed deer (Odocoileus virginianus), brown thrasher (Toxostoma rufum), northern cardinal (Cardenalis cardenalis), northern mockingbird (Mimus polyglottos), ruffed grouse (Bonasa umbellus), and wild turkey (Meleagris gallopavo). Creation of additional habitat for edge-associated species may place some of these species (including some bat species) at higher risk than if the turbines were not present at these sites. Some wildlife-management agencies (e.g., West Virginia Department of Natural Resources) have concluded that a goal of “creating edge” to benefit populations of harvested species may have unintended negative consequences. For example, the overabundance of edge-tolerant species such as white-tailed deer can have detrimental effects on forest productivity and wildlife species richness (Rossel et al. 2005).
Habitat fragmentation can be defined as the breaking up of large contiguous tracts of suitable habitat for a species into increasingly smaller patches that are isolated from each other by barriers consisting of unsuitable or less suitable habitat. There is a substantial literature that examines the effects of fragmentation on the ecology of forest ecosystems (e.g., Laurance and Cochrane 2001; Fahrig 2003), although much of this literature focuses on a larger spatial scale than that represented by the extent of most wind-energy projects. Wind-energy projects in the central Appalachian Mountains can fragment previously contiguous tracks of forest at some scale by road construction, turbine installation, and the presence of ancillary structures.
Habitats for forest species are linearly divided by turbine-maintenance roads paralleling the ridge. Such internal fragmentation may subdivide populations of some species (Goosem 1997); the magnitude and importance of these effects are influenced by the natural history of the individual taxa and the scale of the fragmentation. The effect of forest roads on aquatic and terrestrial communities has been documented and synthesized elsewhere (Trombulak and Frissell 2000; Forman et al. 2003; NRC 2004, 2005). Trombulak and Frissell summarize seven general effects:
-
Direct mortality can result from road construction. The effect is most significant for sessile or slow-moving organisms. Coupled with increased compaction, increased soil temperature beneath the road can adversely affect communities of soil organisms.
-
Mortality from collision with vehicles using roads may be significant on large, frequently traveled roads. Because vehicular traffic on wind-energy sites typically is infrequent, it is unlikely that collision with vehicles will be a significant source of mortality resulting from wind-energy development at most sites, including the Mid-Atlantic Highlands.
-
Forest roads may result in a modification of animal behavior. Some species (e.g., black bears [Ursus americanus]) avoid roads of high traffic volume, and forest roads in areas where they are hunted (Brody and Pelton 1989), while turkey vultures (Cathartes aura) are common along forest roads. Typically the roads and the surrounding surfaces at wind-energy facilities are maintained to 15-20 m wide, and are usually lightly traveled. However, roads prove to be barriers for such diverse taxa as land snails (even roads that are unpaved and < 3 m in width) and small mammals (Merriam et al. 1989; Baur and Baur 1990). Moreover, forest roads as small as 5-8 m in width can be barriers to salamander dispersal and gene flow (deMaynadier and Hunter 2000; Marsh and Beckman 2004; Marsh et al. 2005). Such effects are exacerbated by the grade of road verges. Steeper verges tend to decrease the dispersal ability of salamanders (Marsh et al. 2005). In contrast, some species use linear features such as roads as travel corridors or feeding habitat. For example, some species of bats forage along linear landscapes created by road cuts in forested habitats, where they forage mostly on aerial insects (Krusic et al. 1996; Menzel et al. 2002). Even species such as black bears that may avoid roads with high traffic may use forest roads with low traffic as travel lanes (Brody and Pelton 1989).
-
Forest roads disrupt the physical environment of the road bed as well as the adjacent edge. Soil density, even on closed roads, increases over time and can persist for periods in excess of 40 years. In addition to soil density, road-induced transformations can include changes in temperature, soil water content, light, dust, surface water flow, pattern of run-off, and sedimentation of downslope aquatic habitats, although sedimentation should be avoided through following the requirements of each facility’s National Pollutant Discharge Elimination System (NPDES) permit (EPA 2006d).
-
Forest roads can alter the chemical environment of the road bed and adjacent edge habitats. Edges along roads serve as concentrators of both nutrients (nitrogen compounds) and pollutants (sulfur compounds) (Weathers et al. 2001). This in turn can alter basic trophic processes such as food-web relationships between plants, insects, and the predators of insects (Valladares et al. 2006).
-
The presence of forest roads increases the spread of invasive species. Three mechanisms have been proposed for the establishment of invasives: the presence of altered habitat, increased stress to or removal of native species, and easier access to disturbed habitats by wild or human vectors (Turton and Freiburger 1997). In addition, poor reclamation practices may lead to lack of germination of desirable plants leaving the unvegetated disturbed site available for the establishment of invasives.
-
Forest roads can change humans’ use of land and water by increasing access to those resources, or by providing access where none previously was available, allowing increased hunting, fishing, recreational driving, and other activities (e.g., NRC 2003, 2005).
In summary, maintenance roads and areas cleared for turbine installation may result in a diversity of influences on forest-dwelling species. Unfortunately, there are no empirical studies that have investigated impacts of roads associated with wind-energy facilities on ecological processes in the area, and relatively few studies have examined ecological impacts of roads in the central Appalachian Highlands. As a result, the extent to which these impacts are manifested at any particular site are not known, and the population-level consequences also are uncertain.
Influences of Habitat Alteration on Birds
Effects of wind-energy development on habitats used by birds can be divided into two general categories: loss of habitat (including avoidance of disturbed and adjacent areas), and fragmentation effects to remaining habitat. Moreover, for a complete understanding of impacts, effects must be assessed relative to the state of the habitat suitable for individual species prior to the construction of a wind-energy facility. For example, a project located on a reclaimed surface mine would not have the same impact on forest birds as one located in a forest 100 times larger. In general, aerial photographs (e.g., Figure 3-7) indicate that the disturbance caused by wind-energy projects is linear along ridgelines, and that habitat for forest-dependent birds has been removed. Habitat loss has large and consistently negative effects on biodiversity (Fahrig 2003). In addition, many forest-dependent bird species respond to direct habitat loss and to changes in the configuration of habitat (fragmentation) resulting from that forest loss (Villard et al. 1999). Thus, assessments of the effects of wind-energy facilities on bird habitat should not be confined to simple measurement of the area of vegetation removed, but also should include analysis of habitat fragmentation and edge effects.
Impacts of wind-energy facilities on habitat are considered to be greater than collision-related fatalities on birds in Europe (Gill et al. 1996). Studies
of both onshore and offshore wind-energy facilities in Europe have reported disturbance effects ranging from 75 m to as far as 800 m from turbines for waterfowl, shorebirds, waders, and passerines (Peterson and Nohr 1989; Winkelman 1989, 1990, 1992a; Vauk 1990; Pedersen and Poulsen 1991; Larsen and Madsen 2000). Avoidance of wind-energy facilities varies among species and depends on site, season, tide, and whether the facility was in operation. Disturbance tends to be greatest for migrating birds while feeding and resting (Crockford 1992); disturbance to breeding birds appears to be negligible and was documented only in one study (Pedersen and Poulsen 1991). In terms of the layout of turbines at wind-energy facilities, Larsen and Madsen (2000) found that in the case of wintering pink-footed geese (Anser brachyrhynchus), avoidance distances from wind turbines that are constructed in lines were 100 m; they were 200 m when the turbines were clustered. For other bird groups or species at other European wind-energy facilities, no displacement effects were observed (Karlsson 1983; Winkelman 1989, 1990; Phillips 1994). It is likely that there is a gradient of avoidance, with extent of impact being a function of distance from the facility, although Winkelman (1995) reported reductions in use of up to 95% out to 500 m away from turbines. A recent radar study of bird movements at a wind-energy development off the coast of Denmark (Desholm and Kahlert 2005) found that the percentage of flocks of common eiders (Somateria mollissima) and geese entering an offshore wind-energy facility area decreased by a factor of 4 from pre-construction to initial operation. At night, migrating flocks were more prone to enter the wind-energy facility but counteracted the higher risk of collision in the dark by increasing their distance from individual turbines and flying in the corridors between turbines. Desholm and Kahlert (2005) estimated that less than 1% of the ducks and geese migrated close enough to the turbines to be at any risk of collision. However, there is no assessment of the issue of potential interference from turbines on the radar signal, potentially biasing study results.
Bird displacement associated with wind-energy development has received little attention in the United States. Howell and Noone (1992) found similar numbers of raptor nests before and after construction of Phase 1 of the Montezuma Hills, California wind-energy facility. A pair of golden eagles successfully nested 0.8 km from the FCR, Wyoming wind-energy plant for three different years after it became operational (Johnson et al. 2000a), and a Swainson’s hawk nested within 0.8 km of a small wind-energy plant in Oregon (Johnson et al. 2003b). Anecdotal evidence indicates that raptor use of the APWRA in California may have increased since installation of wind turbines (Orloff and Flannery 1992; AWEA 1995). Results of more than two years of raptor nest monitoring at the Stateline Wind Project showed no measurable change in raptor-nest density within two miles of the facilities. In a survey of breeding golden eagle territories
in the APWRA, Hunt and Hunt (2006) found that within a sample of 58 territories sampled, all territories occupied by eagle pairs in 2000 were also occupied in 2005.
The only case interpreted as avoidance of wind-energy plants by raptors occurred at the Buffalo Ridge facility, Minnesota, where raptor-nest density on 261 km2 of land surrounding the facility was 5.94/100 km2, yet no nests were present in the 32 km2 facility, even though habitat was similar (Usgaard et al. 1997). However, more information would be needed to conclude with confidence that the observed distribution of nests was due to raptor avoidance of turbines, and not due to chance or other factors. Osborn et al. (1998) reported that fewer birds and fewer species were within the Buffalo Ridge wind-energy facility in turbine plots than at reference plots, and concluded that birds avoided flying in areas with turbines. Also at the Buffalo Ridge facility, Leddy et al. (1999), using the impact gradient sampling design and linear regression methods, found that species-specific densities of male songbirds were significantly lower within 180 m of turbine locations in CRP grasslands than in CRP grasslands without turbines. Grasslands without turbines, as well as portions of grasslands located at least 180 m from turbines, had bird densities four times greater than grasslands located near turbines. In a 4-year study designed to evaluate displacement of breeding birds at the Buffalo Ridge site, Johnson et al. (2000b) used a BACI sampling design and linear regression models to assess displacement impacts. Their results indicated that the facility of 354 wind turbines displaced some groups and species of birds, and that the area of displacement was limited primarily to areas ≤ 100 m from turbines.
While similar avoidance of wind turbines has not been documented for other prairie species of conservation concern, such as many prairie-grouse species, studies of the impacts of other human disturbances on prairie chickens and sage grouse indicate that birds do avoid disturbed areas. It is likely that these species will be displaced by wind-power development, although the magnitude of the displacement is unknown. The relationship between wind-energy development and the habitats used by birds in the Mid-Atlantic Highlands has not been investigated, and information from other geographic locations and non-forest vegetation associations provide limited insight into how forest-dwelling birds respond to such habitat perturbation. However, the response of bird species to habitat alterations caused by changes in vegetation associated with timber management, mining, and insect outbreaks have been widely studied in the Mid-Atlantic Highlands (e.g., Duguay 1997; Bell and Whitmore 2000; Duguay et al. 2000, 2001; Hagan and Meehan 2002; Weakland and Wood 2005; Wood et al. 2005, 2006) and these studies provide some insight to the potential effects of wind-energy development. While changes in forest cover from a single wind-energy facility may not be of the same magnitude as those from
timber management or an insect outbreak, the total area disturbed by a wind-energy project, including roads and ancillary structures, as well as the depth of edge influence, would likely cover hundreds of hectares.
The response of birds to changes in vegetation structure varies with species, and changes that adversely affect some species may be positive for others. For example, in the Mid-Atlantic Highlands, removal of the forest canopy and subsequent understory release can benefit shrub-nesting species such as the eastern towhee (Pipilo erythrophthalmus), which responds positively in both gypsy-moth-defoliated forest tracts (Bell and Whitmore 1997) and timber-managed tracts (Duguay 1997; Duguay et al. 2000, 2001). Conversely, habitat for ovenbirds (Seiurus aurocapillus) and Blackburnian warblers (Dendroica fusca) is negatively correlated with understory density and positively correlated with the size and density of hardwood trees (Hagan and Meehan 2002). Moreover, data from Breeding Bird Surveys indicate that populations of edge species such as eastern towhee, indigo bunting (Passerina cyanea), and song sparrow (Melospiza melodea) generally are increasing within the Mid-Atlantic Highlands (Sauer et al. 2005). However, forest-interior species, including ovenbirds, Kentucky warblers (Oporornis formosus), and worm-eating warblers (Helmitheros vermivorus), are declining (Freemark and Collins 1992; Wenny et al. 1993).
In the Mid-Atlantic Highlands, three species of warbler—cerulean warbler, worm-eating warbler and ovenbird—are of conservation concern and thus are of particular interest with respect to wind-energy development in this region (USFWS 2002b). For example, the cerulean warbler appears to be declining precipitously (Robbins et al. 1992), and is experiencing approximately a 3% annual decrease in abundance (Link and Sauer 2002; Wood et al. 2006). This rate of decline, however, needs to be re-evaluated because cerulean warblers extensively use ridge tops in some areas of the Mid Atlantic Highlands, and these areas are not sampled as much as midslopes or valley floors (Wood et al. 2006); as a result, estimates of declines may be biased. Mid-Atlantic Highlands populations of worm-eating warblers are likewise declining, showing a 20% drop between 1996 and 2001 in the Monongahela and George Washington National Forests (Cooper et al. 2005a).
Ovenbirds are declining in eastern forests (Robbins et al. 1989; Sauer et al. 2005) and appear to be particularly sensitive to forest fragmentation, showing decreases in density adjacent to narrow, unpaved, interior forest roads and trails (Ortega and Capen 1999, 2002). Factors implicated in this decline are loss of insect-prey biomass in small forest fragments (Burke and Nol 1998), increased predation (Mattsson and Niemi 2006), and brood parasitism (Lloyd et al. 2005). In addition, both density and fecundity of ovenbirds were lower in large (> 2,000 ha) habitat patches than in unfragmented reference plots (located in > 2 million ha) (Porneluzi
and Faaborg 1999). Small forest fragments may act as population sinks that rely on continual re-supply from adjacent large forest tracts for ovenbirds (Nol et al. 2005). Nesting ovenbirds and five other species have recently been reported to decline in habitats altered by a wind-energy project near Searsburg, Vermont (Kerlinger 2002). Openings created for turbines and roads were hypothesized to be the likely cause of this decline (Kerlinger 2002). These are the only before and after data for a wind-energy development in forested habitats in the eastern United States.
Several additional bird species of concern have statutory protection and may occur in habitats impacted by wind-energy development (Table C-6 of Appendix C). All states in the Mid-Atlantic Highlands except West Virginia have State Endangered, Threatened, or Species of Conservation Concern legislation and have published lists of protected species, in addition to those protected under the U.S. Endangered Species Act (ESA). Most of these state-listed species occur at peripheral locations in their historic range (e.g., mourning warbler [Oporornis philadelphia]) and may not be at risk from a global perspective. Nonetheless, they do have protected status at the state level and need to be considered in siting assessments.
Long-term trend analysis by Sauer et al. (2005) using Breeding Bird Survey data for North American bird species that winter in the tropics (neotropical migrants) shows that populations of 45 species are declining (Appendix C, Table C-5). Most of these species either nest in Mid-Atlantic Highland habitats or migrate through the region seasonally. All of these species are protected under the Migratory Bird Treaty Reform Act of 2005 and should be included in siting studies as well as in long-term monitoring of existing wind-energy facilities.
Although habitat alteration resulting from wind-energy development often occurs at a relatively small scale, the cumulative effects of wind-energy development, in conjunction with changes in habitat from a variety of other past and present anthropogenic activities, could result in negative impacts on bird populations.
Influences of Habitat Alteration on Bats
Changes in habitat associated with wind-energy facilities can be relatively minor in some situations, such as may be the case in agricultural settings. In forested environments, however, habitat alteration at wind-energy facilities may be considerable. In addition to changes resulting from presence of the turbine itself, alteration of bat habitat results from road construction and maintenance, buildings and structures associated with turbines, and power lines associated with wind-energy facilities. Manipulation of vegetation, including creating and maintaining clearings around turbines, along roadsides, and along power line rights-of-way probably are the most
important form of bat habitat alteration associated with wind-energy facilities—alteration that may increase the activity of bats at these sites.
Alteration of vegetation associated with wind-energy facilities could influence bats in two ways. First, changes in vegetation associated with wind-energy facilities could influence the quality of habitat for bats, thereby influencing carrying capacity of the area, and ultimately influencing population abundance. Alternatively, changes in vegetation could alter the behavior of bats, thereby changing the risk of collision with turbine blades. The overall influence of habitat alteration on bats (and birds) at wind-energy facilities is thus a function of the relative influences of changes in population abundance and behavior (Figure 3-8).
Although some studies are under way to evaluate the influence of wind-energy facilities on bats, no studies have been published that directly examine influences of vegetation change associated with wind-energy facilities on bats. However, inference from studies that have examined the ecology and the influences of forest management practices on forest-dwelling bats can provide insight into potential influences of wind-energy facilities. Here we summarize likely influences of vegetation alteration associated with wind-energy facilities on roosts and roosting ecology, habitat use, and vertical patterns of activity of bats.
FIGURE 3-8 The influence of habitat alteration associated with wind-energy facilities on bats is a function of the combined influences of the ways that habitat alteration influences abundance and risk of collision with turbine blades.
Influences of Habitat Alteration on Roosts and Roosting of Bats
Bats use roosts as sites for resting, protection from weather and predators, rearing young, hibernation, digestion of food, mating, and social interactions (Kunz 1982a,b,c; Kunz and Lumsden 2003). Roosts have been postulated as limiting factors that influence distribution and abundance of bats (Humphrey 1975; Ports and Bradley 1996; West and Swain 1999). Bats use a variety of structures for roosting, including buildings, caves, bridges, hollow logs, foliage, leaf litter, and hollows, cavities, and crevices in trees, snags, and rock crevices. Of these, wind-energy development in the Mid-Atlantic Highlands and in other forested regions is most likely to influence availability of roosts in trees and snags. The geographic distribution of bats is also influenced by elevation, with males of several species being more common at higher elevations, especially in western states (Cryan 2003).
Large-diameter living and dead trees provide important roosts for many species of forest-dwelling bats (Kunz and Lumsden 2003; Barclay and Kurta 2007). The roosting ecology of the Indiana bat is of particular concern throughout its range in the eastern United States, as this species is listed as endangered by the U.S. Fish and Wildlife Service; Indiana bats roost in cavities and crevices beneath the exfoliating bark of living and dead hardwoods and conifers during summer months (Kurta et al. 1996, 2002; Callahan et al. 1997; Gumbert et al. 2002). Indiana bats also have been reported to roost in buildings (Butchkoski and Hassinger 2002). The roosting ecology of bats of the genus Lasiurus also is of interest, as these bats appear to be particularly vulnerable to fatalities at wind-energy facilities. Eastern red bats and hoary bats generally roost in the foliage of several different species of trees and shrubs during the spring, summer, and fall (Constantine 1966; Menzel et al. 1995, 1998; Carter et al. 2003). The silver-haired bat typically roosts in tree cavities (Betts 1996; Vonhof 1996).
Clearing forests at and around wind-energy facilities could result in removal of actual or potential roost sites for Indiana bats, eastern red bats, hoary bats, and silver-haired bats, and several other species that occur in or migrate through the Mid-Atlantic Highlands. In Pennsylvania, the typical foraging habitat of Indiana bats is in upland forests (Butchkoski and Hassinger 2002). Moreover, removing dead trees that are adjacent to roadways developed for wind-energy facilities because of their potential hazards to safety or their risk of obstructing roadways can reduce the number of potential roosts for several species of bats.
Use and quality of roosts also may be influenced by the microclimatic changes resulting from habitat alteration. Microclimate appears to play an important role in determining quality and use of roosts in forest settings (Hayes 2003; Kunz and Lumsden 2003; Barclay and Kurta 2007; Hayes and Loeb 2007). For example, although the primary roosts of Indiana bats
are mostly in wooded riparian habitats that receive considerable solar radiation (Humphrey et al. 1977; Callahan et al. 1997; Britzke et al. 2003), more recent evidence suggests that some roost in forested areas (Kurta and Kennedy 2002). Thermal environment also is thought to influence use of roosts by foliage-roosting bats, although less is known about the influences of temperature on foliage-roosting bats or the scale at which it operates. In Kentucky, eastern red bats selected roosts in foliage with lower temperatures than in other points in the same tree (Hutchinson and Lacki 2001), possibly to minimize heat stress during high summer temperatures or to conserve energy by entering daily torpor.
Changes in forest structure and creation of openings are likely to alter microclimatic conditions in forested regions used by roosting bats (Kunz and Lumsden 2003). In general, these changes should increase roost temperatures in the affected area. When these changes are important enough, they may improve roosting conditions for crevice- and cavity-roosting species; however, these influences are difficult to predict with any degree of certainty, are likely to be site-specific, and may differ among species and at different times of the year.
Several species of bats also regularly roost in human-made structures (Kunz 1982a,b,c, 2004). However, we are unaware of records of bats roosting in structures associated with wind-energy facilities in the United States, although bats have gained access to and roosted in the nacelle in Europe (Hensen 2004). Nonetheless, bat species that appear to be most at risk of being killed by wind turbines in the Mid-Atlantic Highlands include eastern red bats, hoary bats, silver-haired bats, and eastern pipistrelles. The latter species typically roosts in foliage during the summer months (Veilleux and Veilleux 2004; Veilleux et al. 2004), although it also is known to roost in buildings (Fujita and Kunz 1984; Hoying and Kunz 1998; Whitaker 1998).
Establishment of artificial roosts (e.g., Burke 1999; Arnett and Hayes 2000; Brittingham and Williams 2000; Chambers et al. 2002; Kunz 2003) is sometimes proposed to mitigate loss of roosts resulting from changes in land-use practices. However, encouraging increased roosting sites at or near wind-energy facilities could increase use of areas and increase risk of fatalities by collisions with turbines. Thus, mitigating loss of natural roosts at or near wind-energy facilities by constructing artificial roosts at these sites may not be effective.
Influences of Habitat Alteration on Habitat Use by Bats
Construction of roadways, management of vegetation, and the selective clearing of forests associated with the development of some wind-energy facilities can influence use of the area by bats. These influences could be
manifested as changes in carrying capacity of an area or through influences of patterns of habitat use on risk of collision with turbines.
Many species of bats commonly use edges between forested and non-forested habitat and small forest gaps for commuting and foraging (Furlonger et al. 1987; Clark et al. 1993; Krusic et al. 1996; Walsh and Harris 1996; Wethington et al. 1996; Grindal and Brigham 1999; Zimmerman and Glanz 2000; Hogberg et al. 2002). For example, bat activity was greater along forest-clearcut edges than within clearcuts or uncut forests in British Columbia (Grindal and Brigham 1999), greater in forest clearings ranging from 0.5 to 1.5 ha in size than in intact forests in British Columbia (Grindal and Brigham 1998), greater along logging roads than in intact forest in South Carolina (Menzel et al. 2002), and greater along forest trails than in interior forests in New Hampshire (Krusic et al. 1996). Increased use of gaps, edges, and roadways is likely a consequence of reduced clutter (the number of obstacles a bat must detect and avoid in a given area [Fenton 1990]) along edges, increased availability of prey, or a combination of these factors. It is quite likely that construction of roads and clearings at wind-energy facilities in forested regions improves foraging habitats for several species of bats in the Mid-Atlantic Highlands, and elsewhere where similar habitat exists.
All bat species known to occur in the eastern United States, including the Mid-Atlantic Highlands, are insectivorous. These bats consume large quantities of nocturnal insects (Aubrey et al. 2003); both empirical evidence and anecdotal observations support the hypotheses that bats respond to prey availability and that prey availability is influenced by vegetation structure and to habitat alteration (e.g., agriculture). However, determining the relationship of distribution and abundance of insects to habitat use or population abundance of bats has been hampered by difficulties in determining abundance and availability of insects at appropriate spatial scales (Kunz 1988; Kunz and Lumsden 2003; Hayes and Loeb 2007). Thus, challenges lie ahead in estimating the influences of habitat changes on the prey base for insectivorous bats at wind-energy facilities. Changes that increase actual or relative abundance of insects preyed on by bats, or the vulnerability of insects to predation by bats at altitudes within the rotor-swept area of turbines could influence risk of bats to collisions with turbines. Clearly, large numbers of insects often are present in the vicinity of wind-turbine rotors, judging from insects that are known to accumulate on turbine blades in some regions (Corten and Veldkamp 2001).
Most of the studies of habitat use by bats have been conducted using recording devices. Only a few studies have evaluated vertical patterns of habitat use by insectivorous bats (e.g., Kurta 1982; Kalcounis et al. 1999; Hayes and Gruver 2000; Kunz 2004). Risk of collision with wind turbines is strongly influenced by vertical patterns of habitat use by bats, and is at
least partially a function of the altitudes at which bats commute, forage, and migrate. Some of the species-specific differences in fatalities at wind turbines could be related to variation in vertical patterns of nightly foraging or migratory activity, possibly in response to prey resources, although currently there are no data available to test this hypothesis. It is unclear if or how habitat alteration at wind-energy facilities influences vertical patterns of habitat use by bats, but changes in vertical activity in response to habitat alteration and insect resources at wind-energy facilities could strongly influence fatality risks to bats. Vertical activity of bats could be influenced by the vertical distribution and abundance of aerial insects. Typically, insects rise to high altitudes above the ground on daily thermals, and then drop to lower altitudes as the lower atmosphere cools throughout the night (Figure 3-9).
Although habitat alteration resulting from wind-energy development often occurs at a relatively small scale, it is likely that the cumulative effects of wind-energy development, in conjunction with changes in habitat from a variety of other activities, will result in negative impacts on bat populations. Given the distances that bats travel nightly and during migration, contribu-
FIGURE 3-9 Vertical distribution of airborne fauna, recorded using an X-band vertically pointing radar on April 15, 1994. Note that insect targets drop markedly in elevation from before sunset until 2400 h. Most of the larger targets (assumed to be migrating birds and bats) occur at higher altitudes.
SOURCE: McGill University 2000. Reprinted with permission; copyright 2000, McGill University.
tions of wind-energy development to changes in landscape characteristics could influence bat populations. Unfortunately, the influences of habitat characteristics on bats at large spatial scales are poorly understood. Some bats have been shown to respond negatively to forest fragmentation in a number of areas (e.g., Pavey 1998; Law et al. 1999; Schulze et al. 2000; Estrada and Coates-Estrada 2002), but there is little information available about responses of bats to characteristics at the landscape scale in North America (Hayes and Loeb 2007). Lack of information on influences of landscape-scale patterns on bats precludes assessment of the likely impacts of habitat alterations at wind-energy facilities at broad spatial scales.
The combined influences of changes in availability of roosts, microclimatic conditions at roosts, availability of prey, vertical patterns of use, and landscape structure on bat populations in the Mid-Atlantic Highlands are difficult to predict with any precision. Moreover, the magnitude of influence of these factors may be site-specific and depend on site characteristics prior to construction of wind-energy facilities and associated infrastructure. If these changes were considered in the absence of direct influences of turbines on fatalities of bats, it is likely that we would conclude that impacts were not significantly negative in light of other threats to bats in the region and habitat changes resulting from other land uses. However, even this provisional conclusion must be tempered by the scale of habitat alteration; broad-scale proliferation of wind-energy facilities in the Mid-Atlantic Highlands and in other regions of the United States could result in significant consequences for habitat for bats and other species. For bats, the interaction among habitat alteration, influences on bat activity patterns, and risk of collision with wind turbines could be an important factor in bat fatalities in the Mid-Atlantic Highlands. Gaining increased understanding of these interactions could help inform in pre-siting risk assessments for bats.
Influences of Habitat Alteration on Terrestrial Mammals
Historically, higher elevation ridges of the Mid-Atlantic Highlands consisted of forest stands dominated by red spruce (Picea rubens). Late 19th- and early 20th-century logging operations reduced these stands to scattered remnants of mixed hardwood and spruce composition (Brooks 1965; Mielke et al. 1986). The federally listed (endangered) subspecies of the northern flying squirrel, the West Virginia northern flying squirrel (Glaucomys sabrinus fuscus), sometimes referred to as the Virginia northern flying squirrel, is closely associated with this spruce habitat. Genetically distinct from other populations of the species (Arbogast et al. 2005), this subspecies has been found at more than 100 separate sites along the ridge tops of the Mid-Atlantic Highlands (USFWS 2006). Current populations of the squirrel can be found in mixed stands of red spruce, cherry (Prunus
serotina), and yellow poplar (Liriodendron tulipifera), although spruce is preferred (Menzel 2003; Menzel et al. 2006). Populations are locally expanding due to second-growth regeneration of upper-elevation forest tracts (USFWS 2006). The West Virginia northern flying squirrel is unique among squirrels in being active year-round and subsisting primarily on lichens, mushrooms, and mycorrhizal fungi, the latter of which are located by olfaction (Loeb et al. 2000; Mitchell 2001). There is an apparent symbiotic relationship between the squirrels and mycorrhizal fungi. The squirrels depend on fungi for food, while the fungi depend on the squirrels to disperse their spores as well as nitrogen-fixing bacteria, which are essential to the growth of red spruce (Mitchell 2001; USFWS 2006). Moreover, the overall condition of red-spruce forests appears to be strongly influenced by the presence of the squirrels (Mitchell 2001; USFWS 2006). Construction of wind turbines and associated roads can result in loss of mixed spruce/hardwood forest habitat and could lead to concomitant drops in squirrel population densities. The lack of quantitative data pertaining to the loss of spruce forest and squirrel habitat at wind-energy facilities limits our understanding of the potential impacts of wind-energy development.
Also of conservation interest is the Allegheny woodrat (Neotoma magister). Although not listed under the federal Endangered Species Act, this species is identified as endangered on state lists in New York, New Jersey, and Maryland; threatened in Pennsylvania; species of concern in North Carolina and Virginia; and a species “somewhat vulnerable to extirpation” in West Virginia. It is believed to be extinct in New York, New Jersey, and Connecticut. It is patchily distributed throughout the Mid-Atlantic Highlands in cliff lines and rock outcroppings, which provide their required nest locations (Castleberry 2000). Recent population declines have been dramatic and potential causal factors include anthropogenic disturbance near nest locations, increased predation by great horned owls (Bubo virginianus) and raccoons (Procyon lotor) directly linked to forest fragmentation, increased incidence of the parasitic raccoon roundworm (Baylisascaris procyonis), and diminished colonization of new locations because they need rock-outcrop habitats (Balcom and Yahner 1996; Castleberry et al. 2001, 2002; LoGiudice 2003; Hassinger 2005). A recent study based on 735 defined Allegheny woodrat “habitat sites” in higher-elevation forests in Pennsylvania showed that the occupancy rate of these sites increased with distance to non-forest edge (Hassinger et al. 2005). Moreover, habitat sites >2 km from a forest edge were 1.7-11.1 times more likely to be occupied than habitat sites within 1 km of a forest edge. Similarly, habitat sites 1-2 km from a forest edge were 1.7-3.8 times more likely to be occupied (Diefenbach et al. 2005). The lack of quantitative data pertaining to the loss of potential Allegheny woodrat habitat in the Mid-Atlantic Highlands is a data gap in the development of wind-energy projects.
Another mammalian species with unique habitat requirements in the Mid-Atlantic Highlands region is the snowshoe hare (Lepus americanus). Cyclically abundant in more northern habitats, this species reaches its southernmost distribution along the high ridges of Pennsylvania, Virginia, Maryland, and West Virginia (Brooks 1965). While this species is not protected under the U.S. Endangered Species Act, it is listed as “endangered/ extirpated” in Maryland (MDDNR 2003) and “extremely rare” in Virginia (Roble 2006). This species is legally hunted in West Virginia. Populations of snowshoe hares occupy boreal forests at the northern end of their range while “southern populations occur primarily in insular patches of suitable habitat set amidst less-preferred areas” (Wirsing et al. 2002, p. 170). Brushy undergrowth and tree saplings, often aspen (Populus tremuloides), cottonwood (P. deltoides), or birch (Betula spp.) are the preferred habitat in the Mid-Atlantic Highlands. Tree removals in conjunction with wind-energy development could alter habitat for hares, and given their protected status in Maryland and Virginia, accurate pre-siting surveys should be conducted. The isolated population in Garrett County, Maryland, occurs in a location suitable for wind-energy development.
In the Mid-Atlantic Highlands, managed populations of large game mammals include the black bear and white-tailed deer, while managed furbearers include raccoon, beaver (Castor canadensis), red fox (Vulpes vulpes), gray fox (Urocyon cinereoargenteus), mink (Mustela vison), and fisher (Martes pennanti). Generally, trading the forested habitats of these species for gravel roads and foundation pads is unlikely to be beneficial. For example, black bears rely on forest habitats for food, cover, and denning sites (Brody and Pelton 1989). Because their selected habitats include a variety of interspersed vegetation types ranging from dense old-growth forests to forest openings rich in berries, bears have been referred to as “landscape species” (Gaines et al. 2005). Thus, analysis of any one vegetation type may be inconclusive and broad spatial analysis of the cumulative effects of human activity are required for effective habitat management (Gaines et al. 2005). However, forest-management practices in the region, such as thinning, clearcutting, and the construction of forest roads generally increase the amount of available soft mast (berries, shrub, and regenerating tree saplings) but also decrease the amount of hard mast (acorns and other nuts) available to black bears (Mitchell and Powell 2003). Soft mast would be reduced by maintenance of wind-energy facility roads and tower pads in a gravel state. Moreover, black bears avoid high-traffic roads, such as interstate highways and other divided highways, as well as low-traffic forest roads that provide access to hunters and their dogs (Brody and Pelton 1989). However, bears can learn to use low-traffic roads to move within their home range (Brody and Pelton 1989). In summary, the effects of wind-energy development in the Mid-Atlantic Highlands on black bears needs to be assessed at the landscape level and
in conjunction with the cumulative aspects of all anthropogenic changes in forest structure. The relationship between wind-energy development and furbearer population biology also is unstudied at this time.
Small-mammal (e.g., Peromyscus sp., Microtus sp., and Blarina sp.) populations probably would not be affected by wind-energy development. Small-mammal populations may sometimes form demographic metapopulations under some conditions (Merriam et al. 1989). Even narrow (< 3 m), gravel roads can act as barriers to movements of prairie voles (Microtus ochrogaster) and white-footed mice (Peromyscus leucopus), and thus may isolate some populations genetically (Swihart and Slade 1984; Merriam et al. 1989). It is unclear what, if any, effect this isolation might have on small-mammal populations in the Mid-Atlantic Highlands. The lack of information on the effects of isolation is identified as a data gap in assessment of the ecological consequences of wind-energy development in the region.
Influences of Habitat Alternation on Amphibians and Reptiles
Amphibians play important roles in the functioning of forested ecosystems in the central Appalachians (Burton and Likens 1975a; Wyman 1998). It has been estimated that salamander biomass in eastern deciduous forests is 24 times that of birds (Greenberg 2001) and that it exceeds that of birds and mammals combined (Burton and Likens 1975b; Hairston 1987). Moreover, amphibians often are more sensitive to habitat alteration than birds and mammals (Marsh and Beckman 2004). Amphibians native to Mid-Atlantic Highland forest environments require aquatic or moist terrestrial habitats to complete their life cycles. Populations of both groups are influenced by the microclimate of forest floor habitats, specifically soil moisture and temperature, and species that lay eggs in aquatic systems also rely on free-standing water, even if it is ephemeral. Even without grading and construction of roads, slight removal of canopy vegetation may result in significant reduction of the amphibian fauna from forest tracts in some situations (Petranka et al. 1993; Ash 1997; Knapp et al. 2003). Knapp et al. (2003), for example, detected significant reduction in densities in Plethodon and Desgmognathus salamanders as a result of removal of canopy vegetation and almost all salamander taxa were adversely affected by timber removal (Petranka et al. 1993).
Amphibian species that require vernal pools for mating and egg-laying may be attracted to roadside ditches and ruts in maintenance roads by the presence of temporary water. However, if they become dry before the larvae become independent of water, such features may be “attractive sinks” (Delibes et al. 2001; Battin 2004), because animals that use them have reduced reproductive output that could contribute to the decline or loss of local populations. In a forest study of anthropogenic and natural
pools, both larval wood frogs (Rana sylvatica) and larval spotted salamanders (Ambystoma maculatum) suffered high mortality from premature drying in the anthropogenic pools (DiMauro and Hunter 2002). During “wet years” the larvae that metamorphosed were significantly smaller in anthropogenic ponds than in natural ones; the anthropogenic pools were subject to increased solar radiation and a more porous substrate, which resulted in elevated water temperatures and faster drying rates (DiMauro and Hunter 2002).
One species of amphibian in the Mid-Atlantic Highlands has is listed as threatened under the ESA. Cheat Mountain salamanders (Plethodon nettingi) occur in high forested landscapes in five West Virginia counties: Pocahontas, Pendleton, Grant, Tucker, and Randolph (Green and Pauley 1987, T. Pauley, Marshall University, personal communication 2006). The species was originally thought to occur only in spruce forests, but now is known also to occur in high mixed hardwood/conifer tracks (Pauley 1981). Removal of mixed hardwood/spruce trees and replacement with gravel roads and tower pads could be detrimental to this species.
Ecology and natural history of reptiles are poorly studied in forest communities potentially modified by wind-energy development in the Mid-Atlantic Highlands. Generally, reptiles respond differently to the creation of edge habitats than amphibians. Reptiles are more mobile than most amphibians and certain species patrol forest edges in search of prey. In addition, since reptiles are typically associated with warmer, drier environments than amphibians are, they may gain a positive thermoregulatory advantage by taking advantage of increased solar radiation associated with forest clearings (Greenberg 2001). One reptilian species of concern is the timber rattlesnake (Crotalus horridus), which has been extirpated from most of its historic range (Clark et al. 2003) and survives in isolated patches of forests, including locations on or near ridge tops in the central Appalachians (Green and Pauley 1987, F. Jernajic, West Virginia Division of Natural Resources, personal communication 2006). Winter dens also occur along Appalachian ridges and are shared by rattlesnakes, copperheads (Agkistrodon contortix), and black rat snakes (Elaphe obsoleta). Timber rattlesnakes are of conservation importance because they have low fecundity, long reproductive cycles (Brown 1993; Martin 1993), and are heavily persecuted by humans (Clark et al. 2003). Alteration of habitat related to wind-energy development could influence habitat suitability for this species, but we are unaware of any studies at wind-energy developments that have examined these effects.
Influences of Habitat Alteration on Fish and Other Aquatic Organisms
Aquatic habitats are not common along Mid-Atlantic Highland ridges. By the very nature of the terrain, establishment of permanent bodies of
water and associated wetland habitat is reduced when compared with nearby downstream valleys. Uncontrolled erosion caused by anthropogenic activities at wind-energy facilities could have far-reaching consequences for aquatic habitats. Since wind-energy facilities in the Mid-Atlantic Highlands are at or near the top of mountain ridges, and hence they are in areas that receive large amounts of rain (> 125 cm per year, see CPC 2004), the potential exists for run-off and erosion. Erosion and sedimentation are avoided through following the requirements of each wind-energy facility’s NPDES permit (EPA 2006d).
PROJECTED CUMULATIVE IMPACTS OF BIRD AND BAT FATALITIES: A WORKING HYPOTHESIS
Because we lack extensive data on the ecological influences of wind-energy facilities, projection of likely impacts in the Mid-Atlantic Highlands is challenging. Among the uncertainties that restrict our ability to assess impacts accurately are uncertainties in magnitude and pattern of future wind-energy development in the region, and lack of spatial and temporal replication in fatality assessments in the region. Nonetheless, it is valuable to prepare a preliminary assessment of potential cumulative impacts based on the limited information that is currently available. Here we estimate expected cumulative impacts on bats and birds based on current estimates of fatalities and projections of installed capacity of wind-energy facilities in the Mid-Atlantic Highlands.
Assumptions
Future development of wind-energy facilities in the Mid-Atlantic Highlands region, and elsewhere, depends on complex interactions among economic factors, technological development, regulatory changes, political forces, and other factors that cannot be predicted easily or accurately (Chapter 2). Here we provide a range of estimates of potential impacts for both birds and bats under the assumption that the National Renewable Energy Laboratory (NREL) Wind Energy Deployment System (WinDS) model and the PJM Interconnection queue (Table 3-5) estimates of projected installed capacity represent the range of potential wind-energy development that will occur in the Mid-Atlantic Highlands. The projections provide an upper and lower boundary, based on estimates of 2020 installed capacity (Table 3-5), and thus provide important hypotheses for testing. While it is conceivable that radically different fatality rates could occur in other locations in the eastern United States, using the information available from the few sites surveyed in the eastern United States to date (Tables 3-2 to 3-4) is the most realistic approach for evaluating potential cumulative impacts at this time.
TABLE 3-5 Estimates of Existing and Projected Installed Capacity for Wind-Energy Facilities in the Mid-Atlantic Highlands by 2020, and the Equivalent Number of 1.5 MW Wind Turbines That Would Generate This Capacity
|
Basis for Estimate |
Capacity (MW) |
Equivalent Number of 1.5 MW Turbines |
|
NREL estimate of total technical capacitya |
8015 |
5344 |
|
NREL WinDS model reference case projection for 2020b |
2158 |
1439 |
|
In-service, or approved by state regulatory authorityc |
1144 |
763 |
|
PJM (electricity grid operator) interconnection queued |
3856 |
2571 |
|
aWind-capacity potential for MD, PA, VA, and WV provided on March 16, 2006, by National Renewable Energy Laboratory (NREL), Golden, CO. Estimate limited to Class 3 and better wind areas above 1,000 feet elevation. Standard exclusions applied by NREL for defining available wind resource, including environmental, land-use, and other criteria. See Appendix B for description of the wind resource database and exclusion criteria. bModeled onshore capacity totals for MD, PA, VA, and WV provided on March 16, 2006, by NREL, Golden, CO, based on application of the Wind Deployment System (WinDS) model (for model information see NREL 2006b). As indicated in Table 2-3, the WinDS projections for U.S. wind-energy development are much larger than those provided by the Energy Information Agency (EIA 2006a). EIA projections for MAH development, however, are not available. cBased on assembled information for in-service wind projects and wind projects with state or local-level approval listed in the PJM interconnection queue (Boone 2006). dBased on assembled information for wind-energy projects listed in the PJM Interconnection queue in addition to in-service projects and projects with state or local-level approval (Boone 2006). |
||
We base our estimation of fatalities on the information available in the eastern United States for birds (Table 3-2) and for the Mid-Atlantic Highlands for bats (Table 3-4). Our estimates for the lowest and highest fatality rates reported for the Mid-Atlantic Highlands (Tables 3-1 to 3-4) are based on only two studies selected as bounds; thus they may not bracket the true extremes that might occur and thus provide estimates of cumulative impacts to be expected in 2020, based on stated assumptions. These assumptions are: (1) reported fatality estimates are representative of the range that could be expected (i.e., estimates based on more sites and improved bias corrections are not likely to increase the range of the numbers of birds and bats killed by wind turbines); (2) observed variation in fatality rates are representative of the Mid-Atlantic Highlands (i.e., as more wind-energy facilities are developed, minimum and maximum fatalities may change); (3) there will be no significant technological changes that reduce or increase fatalities (i.e., more and larger wind turbines than NREL- or PJM-based
projections will not be installed; (4) the numbers of resident and migrating bird and bat species will remain constant (i.e., no decline in populations from wind-turbine-related fatalities or other factors is expected); and (5) the relationship of installed capacity to operational hours and rotor-swept area will not change. Because our estimates are specific to the Mid-Atlantic Highlands, the number of reported fatalities and assumption might differ significantly for other geographic regions and should not be applied to them without additional study (Kunz et al. 2007).
Projected Cumulative Impacts
Based on the assumptions noted above for wind-energy development in the Mid-Atlantic Highlands, at projected levels of development by the NREL WinDS model for 2020 and the best available information (lowest and highest mean fatality rates; Table 3-2), we estimate that the projected avian fatalities in the mid-Atlantic regions could range from a mean minimum of approximately 5,805 birds per year (based on the fatality rate at the Mountaineer Wind Energy Center, West Virginia), to a maximum of approximately 25,183 birds per year (based on the fatality rate estimated for the Buffalo Mountain Wind Park in Tennessee). Using similar logic and the PJM-based projections for development, the projected range of avian fatalities increases to approximately 10,372 to 44,999 per year.
Under the assumption that the species composition of fatalities will be similar to the data presented above (Figure 3-1), we predict that these fatalities will primarily consist of passerines (Table 3-6). In the existing studies in this region at Mountaineer (Kerns and Kerlinger 2004) and Buffalo Mountain (Nicholson 2003), most individual passerine species made up a relatively small percentage of the passerine fatalities, up to 5%, resulting in the potential for approximately 200 to 1,000 individuals of any one species being killed per year using data from the NREL WinDS model projections and 400 to 1,800 killed per year using data from the PJM-based projections. However, at the Mountaineer site approximately 35% of the passerines killed were of the same species (red-eyed vireo, Vireo olivaceus). Thus, it is possible that from 1,600 to 7,000 individuals of a single species could be killed per year using NREL WinDS model projections and 2,900 to 12,700 per year using PJM-based projections.
The biological importance of these fatalities depends on the number of passerines in the affected population and whether the birds killed were migrant or resident in the areas of impact. Based on the existing data, it appears that approximately 50% of the passerines are migrant and losses to migrating and resident populations of passerines in this region would be approximately 2,400 to 10,000 each per year using NREL WinDS model projections and 4,200 to 18,000 per year using PJM-based projec-
TABLE 3-6 Projected Annual Number of Bird Fatalities from Wind Turbines Expected in 2020. Based on Estimates of Current Proportional Fatality Rates and Available Estimates of Installed Capacity for the Mid-Atlantic Highlands Region
|
|
Proportion of Total Fatalitiesb |
Projections Based on the NREL WinDS Model of Installed Capacitya |
|
|
|
Minimum Projected Number of Bird Fatalitiesc |
Maximum Projected Number of Bird Fatalitiesd |
|
|
Total |
|
5,805 (6,000) |
25,183 |
|
Species Groupb |
|
|
|
|
Doves/pigeons |
.02 |
116 503 |
|
|
Gamebirds |
.02 |
116 |
503 |
|
Other birds |
.06 |
348 |
1,510 |
|
Passerines |
.81 |
4,702 |
20,398 |
|
Rails/coots |
.02 |
116 |
503 |
|
Raptors/vultures |
.03 |
174 |
755 |
|
Unidentified birds |
.02 |
116 |
503 |
|
Waterfowl |
.02 |
116 |
503 |
|
|
|
Projections Based on the PJM Grid-Operator Queuee |
|
|
|
Proportion of Fatalities |
Minimum Projected Number of Bird Fatalitiesf |
Maximum Projected Number of Bird Fatalitiesg |
|
Total |
|
|
10,372 44,999 |
|
Species Groupb |
|
|
|
|
Doves/pigeons |
.02 |
207 |
899 |
|
Gamebirds |
.02 |
207 |
899 |
|
Other birds |
.06 |
622 |
2,699 |
|
Passerines |
.81 |
8401 |
36,449 |
|
Rails/coots |
.02 |
207 |
899 |
|
Raptors/vultures |
.03 |
311 |
1,349 |
|
Unidentified birds |
.02 |
207 |
899 |
|
Waterfowl |
.02 |
207 |
899 |
|
aEstimated installed capacity of 2,158 MW based on National Renewable Energy Laboratory (NREL) WinDS Model for the Mid-Atlantic Highlands for the year 2020 (NREL 2006b). bEstimated species-specific fatality rates are based on data collected in the eastern United States (Figure 3-1). cMinimum projected number of fatalities in 2020 is based on the product of 2.69 bird fatalities/MW reported from the Mountaineer Wind Energy Center, WV (from Table 3-2), and the estimated installed capacity (2,158 MW) = 5,805. The species group-specific annual minimum number of projected bird fatalities is the product of the minimum number of projected fatalities and the species group-specific proportional fatality rates (column 2). dMaximum projected number of fatalities in 2020 is based on the product of 11.67 bird fatalities/MW reported from the Buffalo Mountain Wind Energy Center, TN (from Table 3-2), and the estimated installed capacity (2,158 MW) = 25,183. The species group-specific annual maximum number of projected fatalities is the product of the maximum number of projected fatalities and the species group-specific proportional fatality rates (column 2). |
|||
|
eEstimated installed capacity of 3,856 MW based on PJM (electricity grid operator interconnection queue) for the Mid-Atlantic Highlands for the year 2020 (Boone 2006). fMinimum projected number of fatalities in 2020 is based on the product of 2.69 bird fatalities/MW reported from the Mountaineer Wind Energy Center, WV (from Table 3-2), and the estimated installed capacity (3,856 MW) = 10,372 (10,500). The species group-specific annual minimum number of projected bird fatalities is the product of the minimum number of projected fatalities and the species group-specific proportional fatality rates (column 2). gMaximum projected number of fatalities in 2020 is based on the product of 11.67 bird fatalities/MW reported from the Buffalo Mountain Wind Energy Center, TN (from Table 3-2), and the estimated installed capacity (3,856 MW) = 44,999. The species group-specific annual maximum number of projected fatalities is the product of the maximum number of projected fatalities and the species group-specific proportional fatality rates (column 2). |
tions. Estimating the fatalities for local populations based on projections for the year 2020 requires the assumption that several local populations are affected. On the assumption that the Mountaineer facility represents a typical development for the future (66 MW) in the region, and that a total of 2,158 to 3,856 MW of capacity will be installed by then, there would be 33 to 58 wind-energy facilities. Furthermore, the upper end of the range of projected fatalities for the two development scenarios would result in approximately 300 passerines killed per facility per year. Thus, if up to 5% of the birds killed locally are of the same species, one could expect that most local populations would suffer the loss of approximately 15 birds per year. Under the assumption that an individual species could be much more vulnerable than the average to collisions, and using the red-eyed vireo as an example, up to 35% of the birds killed locally could be of one species (105 birds per year) and presumably be from one local population.
Local populations of raptors and vultures are much smaller than passerine populations and thus potentially more at risk for population effects of fatalities from wind-energy generation. Using the same logic and data sources for raptors and vultures as were used for passerines, approximately 9-23 individuals per year of these species are projected to be killed at each of these sites using the lowest and highest range of projected wind-energy development. Some of the birds would be resident and some migrant.
Based on currently available information on bat fatalities in the eastern United States, projected cumulative impacts using estimates of installed capacity for the Mid-Atlantic Highlands in the year 2020, along with supporting data, assumptions, and calculations, are in Table 3-7. Minimum and maximum estimates of installed capacity for this region range from 2,158
TABLE 3-7 Projected Annual Number of Bat Fatalities from Wind Turbines Expected in 2020. Based on Projections of Installed Capacity for This Region and Current Proportional Fatality Rates Available from the Eastern United States
|
|
Fatality Ratec |
Projections Based on the NREL WinDS Model of Installed Capacitya |
|
|
Speciesb |
Minimumd |
Maximume |
|
|
Hoary bat |
0.289 |
9,542 |
17,899 |
|
Eastern red bat |
0.344 |
11,358 |
21,306 |
|
Silver-haired bat |
0.052 |
1,717 |
3,221 |
|
Eastern pipistrelle |
0.185 |
6,108 |
11,458 |
|
Little brown myotis |
0.087 |
2,873 |
5,388 |
|
Northern long-eared myotis |
0.006 |
198 |
372 |
|
Big brown bat |
0.025 |
825 |
1,548 |
|
Unknown/other |
0.012 |
396 |
743 |
|
TOTAL |
|
33,017 |
61,935 |
|
|
Fatality Ratec |
Projections Based on the PJM Grid Operator Interconnection Queuef |
|
|
|
Minimumg |
Maximumh |
|
|
Hoary bat |
0.289 |
17,050 |
31,983 |
|
Eastern red bat |
0.344 |
20,295 |
38,069 |
|
Silver-haired bat |
0.052 |
3,068 |
5,756 |
|
Eastern pipistrelle |
0.185 |
10,914 |
20,473 |
|
Little brown myotis |
0.087 |
5,133 |
9,628 |
|
Northern long-eared myotis |
0.006 |
354 |
664 |
|
Big brown bat |
0.025 |
1,475 |
2,767 |
|
Unknown/other |
0.012 |
708 |
1,328 |
|
TOTAL |
|
58,997 |
110,667 |
|
aEstimated installed capacity of 2,158 MW based on National Renewable Energy Laboratory (NREL) WinDS Model for the Mid-Atlantic Highlands for the year 2020 (Table 3-5). bEastern red bats, hoary bats, and silver-haired bats are the only species in the eastern United States known to undertake long-distance migrations (Barbour and Davis 1969). cEstimated species-specific fatality rates are based on data collected in the eastern United States (Table 3-4). dMinimum projected number of fatalities in 2020 is based on the product of 15.3 bat fatalities/ MW/year reported from the Meyersdale Wind Energy Center, PA (from Table 3-4), and the estimated installed capacity (2,158 MW) = 33,017. The species-specific annual minimum number of projected bat fatalities is the product of the species-specific fatality rates (column 2) and the minimum total number of fatalities (e.g., for the hoary bat, 0.289 * 33,017 = 9,542). eMaximum projected number of fatalities in 2020 is based on the product of 28.7 bat fatalities/MW/year (average for 2003 and 2004) reported from the Mountaineer Wind Energy Center, WV (from Table 3-4), and the projected installed capacity (2,158 MW) = 61,935. The species-specific annual maximum number of projected bat fatalities is the product of the species-specific fatality rates (column 2) and the total maximum number of fatalities. |
|||
|
fEstimated installed capacity of 3,856 MW based on PJM (electricity grid operator interconnection queue) for the Mid-Atlantic Highlands for the year 2020 (Table 3-5). gMinimum projected number of fatalities in 2020 is based on the product of 15.3 bat fatalities/MW/year reported from the Meyersdale Wind Energy Center, PA (from Table 3-4), and the projected installed capacity (3,856 MW) = 58,997. The species-specific annual minimum number of projected bat fatalities is the product of the species-specific fatality rates (column 2) and the total minimum projected number of fatalities. hMaximum projected number of fatalities in 2020 is based on the product of 28.7 bat fatalities/MW/year (average of year 2003 and 2004) reported from the Mountaineer Wind Energy Center, WV (from Table 3-4), and the projected installed capacity (3,856 MW) = 110,667. The species-specific annual maximum number of projected bat fatalities is the product of the species-specific fatality rates (column 2) and the total maximum projected number of fatalities. SOURCE: Kunz et al. 2007. |
MW (based on the NREL WinDS model) to 3,856 MW (from the PJM Interconnection queue), as was the case for the bird-fatality projections.
These cumulative fatality projections for bats based on fatality rates determined for this region should be regarded as provisional (Table 3-4). Although some of the empirical data for this region were not collected consistently, the data summarized in Table 3-3 are the best available data for assessing cumulative impacts.
Based on estimates of installed capacity and the limitations and assumptions regarding fatality rates noted above, the minimum and maximum projected fatalities of bats presented in Tables 3-4, 3-5, and 3-7 would range from 33,017 to 61,935 per year based on the NREL’s WinDS model and 58,997 to 110,667 per year based on the PJM Interconnection queue. These projected cumulative impacts in 2020 based on the WinDS model and PJM Interconnection queue would cause annual fatalities of 9,542 to 31,983 hoary bats, 11,358 to 38,069 eastern red bats, 1,717 to 5,755 silver-haired bats, and 6,108 to 20,473 eastern pipistrelles in the mid-Atlantic region. These projections should be considered as hypotheses, until improved estimates (or enumerations) of installed capacity and bat fatalities become available for this region (Kunz et al. 2007).
No projections were made for the endangered Indiana bat, Rafinesque’s big-eared bat (Corynorhinus rafinesquii), or the regionally listed small-footed myotis (Myotis leibii), because no fatalities for these three species have been reported at wind turbines in the Mid-Atlantic Highlands. This should not be interpreted as reflecting a judgment that no members of those species will be killed. It is possible that their behavior and distribution pre-
vent them from coming into contact with turbines, or it is possible that their rarity has not yet led to a recorded fatality of any of those species.
Ecological Implications of Projected Cumulative Impacts
These projections of cumulative bat and bird fatalities for the Mid-Atlantic Highlands by the year 2020 assume that bat and bird populations living in or migrating through the region each year would be constant. The latter assumption is likely to be violated given assorted caveats about expected inter-annual variability; however, given that we have presented both worst-case (maximum number of fatalities/year) and best-case (minimum number of fatalities/year) scenarios, our projected fatality rates in the Mid-Atlantic Highlands bracket expected extremes. These projected fatalities can best be considered as hypotheses to be tested with future data on fatalities from the Mid-Atlantic Highlands and other regions where bird and bat fatalities have been reported, and by adjusting monitoring protocols to minimize potentially confounding assumptions (Kunz et al. 2007).
A question that arises from these projections is whether they are of biological importance to bat and bird populations. The answer differs for birds and bats and for migratory and local populations. For birds, it is unlikely that this predicted level of fatalities would result in measurable impacts to migratory populations of most species. However, for rare species and local populations, the impacts, when combined with other sources of mortality such as large weather-related bird kills, could affect viability, and thereby affect overall risks to populations. A definitive conclusion on these predicted impacts requires more information on the demographics of rare and local populations of birds than is currently available.
For bats, the question draws attention to the almost complete lack of data for population estimates of any species considered here, either on a regional or continental scale (Kunz et al. 2007). A risk assessment of biological impacts typically requires knowledge of baseline populations. Nonetheless, the numbers of fatalities projected above for bats in the MidAtlantic Highlands suggest that bat populations might be at risk, because they reflect fatality rates as high as or higher than fatality rates that have been reported for bats from other measurable anthropogenic sources (Kunz et al. 2007).
CONCLUSIONS AND RECOMMENDATIONS
Our understanding of the ecological effects of wind-energy development in the Mid-Atlantic Highlands region and elsewhere is limited by minimal monitoring efforts at existing wind-energy facilities and by poor understanding of key aspects of species ecology, of causal mechanisms
underlying fatalities at wind-energy facilities, and of the reliability of our projections of fatalities at wind-energy facilities. This section contains the committee’s conclusions about the known and potential ecological effects of wind-energy projects, identification of information needs, and recommendations for research and monitoring.
Ecological Effects of Wind-Energy Projects
-
While research and monitoring studies admittedly are limited, a synthesis of the existing studies indicates that adverse effects of wind-energy facilities on ecosystem structure and functioning have occurred. This knowledge should be used to guide decisions on planning, siting, and operation.
-
Wind turbines cause fatalities of birds and bats through collision, most likely with the turbine blades.
-
Species differ in their vulnerability to collision. The probability of fatality is most likely a function of abundance, local concentrations, and the behavioral characteristics of species.
-
Migratory tree-roosting bat species appear to be most susceptible to direct impacts. To date, the highest fatality rates have been reported in the Mid-Atlantic Highlands, although recent evidence suggests that bats from grassland and agricultural landscapes may also experience high fatality rates. Migratory tree bats constitute over 78% of all fatalities reported at wind-energy facilities, and thus appear to be killed disproportionately to highly colonial species. To date, no endangered species have been reported being killed at existing wind-energy facilities, although only a few sites have been monitored. Increased risks are expected as more wind-energy facilities are developed. Risks of fatalities to bats in the southwestern United States, especially in Texas, where large wind-energy facilities exist and have been proposed, are largely unknown because data have not been reported for most of these facilities.
-
Abundance interacts with behavior to influence exposure of breeding passerines, raptors, and bats to the risk of collisions. Raptors appear to be the most vulnerable to collisions. On average raptors constitute 6% of the reported fatalities at wind-energy facilities, yet they are far less abundant than most other groups of birds (e.g., passerines). By contrast, crows, ravens, and vultures are among the most common species seen flying within the rotor-swept area of turbines, yet they are seldom found during carcass surveys. Nocturnally migrating passerines are the most abundant species at most wind-energy facilities and are the most commonly reported fatalities. Nonetheless, fatalities among passerines vary more than can be explained by abundance alone.
-
Species differ in the extent to which their fatalities are discovered
-
and publicized. Small birds and bats are more difficult to find than others during planned searches and incidentally. Large birds such as raptors are more easily seen, and are often more publicized because of their charismatic status and perceived importance in the environment.
-
The location of wind-energy facilities on the landscape (e.g., agricultural lands, ridge tops, canyons, grasslands) influences bird and bat fatalities. Available evidence suggests that fatalities are positively correlated with bird abundance. Landscape features influence density by concentrating prey or through providing favorable conditions for other activities such as nesting, feeding, and flying (e.g., updrafts for raptor soaring and linear landscapes for bats).
-
The characteristics (e.g., rotor-swept area, height, support structure, lighting, number of turbines) of wind-energy facilities may act synergistically to cause bird and bat fatalities. Newer, larger turbines installed on monopoles may cause fewer bird fatalities per MW than the smaller, older, lattice-style turbines, but the ability to determine the significance of these characteristics is limited by sparse data; in addition, other factors such as the local and regional abundances of birds and bats and landscape variation confound understanding of the effects of turbine characteristics noted above.
-
The lack of estimates of population sizes and other population parameters for birds and bats and the lack of multiyear studies at most existing wind-energy facilities make it difficult to draw general conclusions about how wind turbines and population characteristics interact to influence mortality of birds and bats. In addition, lack of replication of studies among facilities and years makes it impossible to evaluate natural variability, in particular unusual episodic events, in relation to fatalities and to predict the potential for future population effects. It is essential that the potential for population effects be evaluated as wind-energy facilities become more numerous.
-
Fatality rates of migratory tree bats appear to be high in some landscapes (e.g., forested ridge tops), although almost nothing is known about the population status of these species, and the biological significance of reported fatalities. Nonetheless, this lack of data on bat populations points to a critical need to evaluate the status of these and other species that may be at risk, especially as wind-energy facilities proliferate, and a need to evaluate where major cumulative impacts could be expected.
-
The construction and maintenance of wind turbines and associated infrastructure (e.g., roads) alters ecosystem structure through vegetation clearing, soil disruption, and potential for erosion and noise.
-
Based on similar types of construction and development, it is likely that wind-energy facilities will adversely alter ecosystems indirectly, especially through the following cumulative impacts:
-
Forest clearing resulting from road construction, transmission lines leading to the grid, and turbine placements represents perhaps the most significant potential change through habitat loss and fragmentation for forest-dependent species. This impact is particularly important in the Mid-Atlantic Highlands, because wind-energy projects there all have been constructed or proposed in forested areas.
-
Changes in forest structure and the creation of openings may alter microclimate and increase the amount of forest edge.
-
Plants and animals throughout the ecosystem respond differently to these changes, and particular attention should be paid to species listed under the ESA and species of concern (Appendix C) that are known to have narrow habitat requirements and whose niches are disproportionately altered.
Information Needs
Here we identify information needs related to understanding, predicting, and managing bird and bat fatalities and landscape and habitat alterations. For each of these categories we suggest important information needs that we judge should be given the highest priority for monitoring and research based on our collective understanding of the issues, weighed by tractability and best practices. The following recommendations are not meant to apply to every situation and should be modified given the characteristics of the site being developed, the species of concern, the results of pilot studies, and the amount of information applicable to that site. If wind-energy development continues in a region, research and monitoring protocols should evolve as more becomes known.
Research is needed to develop mitigation approaches for existing facilities and to aid in assessing risk at proposed facilities. The latter is particularly important in landscapes where unusually high bird and bat fatalities have already been reported and in regions where facilities are planned where little is known about migration, foraging, and fatalities associated with wind-energy facilities (e.g., the Mid-Atlantic Highlands and the south-western United States).
Following accepted scientific protocols, hypotheses should be developed to help address unanswered questions. Testing hypotheses promises to provide science-based answers that will help inform developers, decision makers, policy makers, and other stakeholders concerning actual and expected impacts of wind-energy development on bat and bird population and on landscapes and habitats of other animals that might be altered by construction.
Some of these information needs are beyond the scope of any individual developer (e.g., population status of affected species). Therefore, a collab-
orative effort by industry and agencies to fund the necessary research to address these overarching questions should be initiated. Other information could be developed as part of the permitting process. Decision makers could require owners and developers to fund research and monitoring studies by qualified researchers at the proposed wind-energy facilities; developers and operators should provide full access (subject to safety and proprietary concerns) to researchers at existing wind-energy facilities. The research should be conducted openly and the protocols and results should be subject to peer review.
-
Follow established scientific principles in conducting monitoring studies and experiments.
-
Follow established research methods and metrics (summarized in Appendix C).
-
Evaluate the efficacy of tools needed to make reliable predictions that would assess measures to reduce the risk of fatalities (e.g., evaluate potential mitigation measures).
-
Develop new quantitative tools to predict fatalities at proposed and existing wind-energy facilities.
-
Develop estimates of exposure for use in evaluating fatalities and for estimating risk (e.g., radar studies at existing facilities in combination with fatality data to develop stronger risk-assessment tools).
-
Improve tools and protocols that can discriminate migrating birds from migrating bats, operate in inclement weather, and provide cost-effective estimates of numbers and movements of flying birds and bats.
-
Develop models to predict risk based on geographic region, topography, season, weather, lunar cycles, and characteristics of different turbines.
-
Improve methods and metrics to determine the context of the number of fatalities related to the number of birds moving through the airspace (proportionality).
-
Identify potential biases associated with estimation of fatalities, including necessary search effort (plot size, frequency of search, methods of searching), the probability that a carcass will be detected if present, and the probability that a carcass will be removed so that its detection probability is zero.
-
-
Encourage and conduct studies to support impact assessments.
-
Assess effects of changing technologies (e.g., larger turbines) on bird and bat fatalities.
-
Identify impacts of different types of lighting on bat and bird fatalities.
-
-
-
Assess how different landscape features may affect bird and bat fatalities (mountain ridges, agriculture, grassland, canyons).
-
Assess how weather fronts influence bat and bird fatalities.
-
Identify bat and bird migratory patterns over space and time.
-
Determine whether migratory birds and bats adjust their migratory paths or exhibit other behaviors that may cause them to avoid turbines.
-
Determine whether fatalities from turbines reduce the breeding or stopover density and reproductive success of birds and bats.
-
Conduct studies to identify methods of mitigating impacts of wind turbines on bats, birds, and other wildlife.
-
Hypothesis-Based Research on Bats
Knowledge about bat fatalities at wind-energy plants is very limited, mainly because the large number of bats killed has been recognized only recently. Eleven hypotheses are listed below, as examples, to help address how, when, where, and why bats are being killed at wind-energy facilities (Kunz et al. 2007). These hypotheses are not mutually exclusive, as several postulated factors might act synergistically to produce the high fatalities that have been reported.
-
Linear-Corridor Hypothesis: Wind-energy facilities constructed along forested ridge tops create clearings with linear landscapes that are attractive to bats. Bats frequently use these linear landscapes during migration and while commuting and foraging (Limpens and Kapteyn 1991; Verboom and Spoelstra 1999; Hensen 2004; Menzel et al. 2005a), and thus may be placed at increased risk of being killed (Dürr and Bach 2004).
-
Roost-Attraction Hypothesis: Tree-roosting bats commonly seek roosts in tall trees (Pierson 1998; Kunz and Lumsden 2003; Barclay and Kurta 2007) and thus if wind turbines are perceived as potential roosts (Ahlén 2002, 2003; Hensen 2004), their presence could contribute to increased risks of being killed when bats search for night roosts or during migratory stopovers.
-
Landscape-Attraction Hypothesis: Modifications of landscapes needed to install wind-energy facilities, including the construction of wide power-access corridors and removal of trees to create clearings (usually 0.5-2 ha) around each turbine site, create conditions favorable for insects on which bats feed (Lewis 1970; Grindal and Brigham 1998; Hensen 2004). Thus, bats that are attracted to and feed on insects in these altered landscapes may be at an increased risk of being killed by wind turbines.
-
Low Wind-Velocity Hypothesis: Fatalities of aerial feeding and
-
migrating bats are highest on nights during periods of low wind velocity (Fiedler 2004; Hensen 2004; Arnett 2006), in part because flying insects are most active under these conditions (Ahlén 2002, 2003).
-
Heat-Attraction Hypothesis: Flying insects are attracted to the heat produced by nacelles of wind turbines (Corten and Veldkamp 2001; Ahlén 2002, 2003; Hensen 2004). As bats respond to high densities of flying insects near wind turbines, they may be at increased risk of being struck by turbine blades.
-
Acoustic-Attraction Hypothesis: Bats are attracted to audible and ultrasonic sound produced by wind turbines (Schmidt and Joermann 1986; Ahlén 2002, 2003). Sounds produced by the turbine generator and the swishing sounds of rotating turbine blades may attract bats, thus increasing risks of collision and fatality.
-
Visual-Attraction Hypothesis: Insects flying at night are visually attracted to wind turbines (von Hensen 2004). Inasmuch as bats may feed on those insects, they become vulnerable to collisions with the turbine blades.
-
Echolocation-Failure Hypothesis: Migrating and foraging bats fail to detect wind turbines by echolocation, or miscalculate rotor velocity (Ahlén 2002, 2003). If bats are unable to detect the moving turbine blades, they may be struck and killed directly.
-
Electromagnetic-Field Disorientation Hypothesis: If bats have receptors sensitive to magnetic fields (Buchler and Wasilewski 1985), and wind turbines produce complex electromagnetic fields in the vicinity of the nacelle, the flight behavior of bats may be altered by these fields and thus increases their risk of being killed by rotating turbine blades.
-
Decompression Hypothesis: Bats flying in the vicinity of turbines may experience rapid decompression (Dürr and Bach 2004; Hensen 2004). Rapid pressure change may cause internal injuries or disorientation, thus increasing risk of death.
-
Thermal-Inversion Hypothesis: The altitude at which bats migrate and/or feed may be influenced by thermal inversions, forcing them to the altitude of rotor-swept areas (Arnett 2005). The most likely impact of thermal inversions is to create dense fog in cool valleys, possibly concentrating both bats and insects on ridges, and thus encouraging bats to feed over the ridges on those nights, if for no other reason than to avoid the cool air and fog.
Research Recommendations
Research should focus on two general lines of inquiry, including methodological research addressing improved tools and monitoring protocols as necessary, and hypothesis-driven research to provide information that will help inform developers, decision makers, policy makers, and other
stakeholders to deal with actual and expected impacts of wind-energy development on populations and ecosystems.
At a national scale, it would be appropriate to identify multiyear research goals that place the impacts of wind-energy development into a broad environmental perspective. Research initiatives should be encouraged to identify biological impacts of wind-energy development, and compare these impacts and risks with those of competing power-generating technologies.
Research should focus on regions and sites where existing and new information suggest the greatest potential for biologically significant adverse impacts on birds and bats at proposed and existing wind-energy facilities. For example, while current evidence suggests that bat fatalities have been the highest at wind-energy facilities in forested mounted ridge tops in the Mid-Atlantic Highlands, recent monitoring studies in agricultural landscape in the Midwest and at wind-energy facilities in southwestern Alberta, Canada, suggest that fatality rates of migratory tree bats may be as high as those reported for the Mid-Atlantic Highlands. We also expect that high bat fatalities are occurring or will occur in the southwestern United States, where large numbers of Brazilian free-tailed bats form maternity colonies (McCracken 2003), and where there is high bat-species richness (O’Shea and Bogan 2003). However, to date, no appropriately designed fatality surveys have been reported at wind-energy facilities in this region. Given the observed geographic variation in fatality rates of both birds and bats, research is needed to evaluate where the risks or fatalities are high so that similar areas can be avoided. Improved assessments, with a focus on evaluation of causes and cumulative impacts, should be an urgent research priority. Proceeding with large-scale development of wind-energy facilities before identifying risks likely threatens both bats and the public acceptance of wind energy as an environmentally friendly form of energy (Kunz et al. 2007). Thus, the initial developments should be used as an opportunity to understand the risks before the full wind-energy potential of the Mid-Atlantic Highlands is developed.
The highest priority for avian habitat is the quantification and prediction of habitat impacts, including loss because of the spatial demands of wind-energy facilities (e.g., roads and turbine pads) and displacement impacts because of behavioral response or habitat degradation, particularly on forest-dwelling and shrub-steppe and grassland birds. In addition, the role of wind in large-scale fragmentation of habitat for species dependent on forests should be evaluated. Finally, the impact of habitat loss or modification should be evaluated in terms of the potential for demographic impacts on ground-nesting birds.
Clearly defined pre- and post-construction studies are needed to inform decision makers about the feasibility of constructing a new project and
mitigating the adverse effects of existing facilities. The studies should be replicable and compared with other studies conducted in areas with similar topography and habitat. Where appropriate, pre- and post-construction studies should be conducted as recommended below.
-
Pre-siting Studies
-
Conduct pre-siting studies that allow the comparison of multiple sites when making decisions about where to develop wind energy.
-
Identify species of special concern and their habitat needs; these include species listed under the federal ESA, such as the West Virginia northern flying squirrel, as well as species listed by the appropriate state, such as the Allegheny woodrat.
-
-
Pre-construction Studies
-
Conduct regional assessments to identify species of concern, including those vulnerable to direct impacts and those vulnerable to habitat loss.
-
Develop pre-construction estimates of potential biological significance of fatalities based on estimated fatality rates and demographics of the species of concern.
-
Conduct multiyear studies when appropriate to assess daily, seasonal and interannual variability of bird and bat populations.
-
Establish species-specific abundance, periods of use (both seasonally and within a day), and behavior in relation to proposed turbines placement locally, regionally, and nationally.
-
Identify habitat characteristics for birds, bats, and other animals, such as topography and types of vegetation at each proposed sites.
-
-
Post-construction Studies
-
Conduct full-season, multiyear, post-construction studies where appropriate to assess variability of bird and bat fatalities.
-
Identify number, species composition, and timing of fatalities.
-
Estimate the biological significance of bird and bat fatalities.
-
Clarify the relationship of small-scale (e.g., habitat disturbance and species displacement) versus large-scale impacts (e.g., landscape alteration and fragmentation) of development on bird and bat populations.
-
Conduct experiments to test alternative mitigation procedures (strategic shutdowns, feathering, blade painting and other potential deterrents, and lighting) that could avoid or reduce current fatality rates—independent of a meta-analysis to assess biological significance and adverse cumulative impacts.
-
-
General
-
Develop predictive and risk-assessment models of potential cumulative impacts of proposed wind-energy facilities, based on monitoring studies and hypothesis-based research.
-
Summary
More information is needed on the characteristics of bird and bat fatalities at wind facilities in all regions of the county, and in particular areas that are relatively unstudied such as the Mid-Atlantic Highlands, the arid southwest, and coastal areas. Turbine characteristics, turbine siting, and abundance appear to be important factors in determining the risk of raptor fatalities at wind-energy facilities. Compared to relatively high raptor fatalities at some older facilities in California, direct impacts of wind-energy development on passerines at the current level of development appear to be minimal. At current levels of development existing data suggest that new-generation turbines (e.g., fewer turbines mounted on monopoles with greater rotor-swept zones) may cause lower bird fatalities in agricultural and grassland areas than older smaller turbines have caused in California. Data on bird fatalities are absent for many existing wind-energy facilities, particularly in Texas and the southwestern United States. Additionally, new areas are being proposed for development where no previous data on bird and bat fatalities exist. It is important to assess impacts in existing and new areas to determine if trends are consistent with existing information. In particular, only two short-term post-construction studies have been conducted in the Mid-Atlantic Highlands and any new facilities should be used as learning opportunities.
Additional information also is needed to characterize bat fatalities in all regions of the country where wind-energy development has occurred or where it is expected. Most wind-turbine-related bat fatalities in the United States have been of migratory species. To date, no fatalities of federally listed bat species have been documented, although as wind-energy development increases geographically, some threatened and endangered species could be at risk. Among the studies that have been conducted, the highest bat fatality rates appear to occur episodically in late summer and early autumn during periods of relatively low wind speeds (< 6 m/sec), at times when wind-energy generation is low, especially following passing weather fronts. To date, few studies have evaluated fatalities during spring migration or during the summer maternity period. Moreover, among fatality surveys that have been conducted, few have consistently corrected results for observer bias and scavenger removal, protocols that are needed to provide reliable data on fatalities. While current evidence suggests that the highest fatality rates are of migratory tree-roosting species along ridge tops in
eastern deciduous forests, recent evidence suggests that similar fatality rates may occur in some agricultural and grassland regions. Bats in other regions of the country that have high wind capacity and are currently undergoing rapid wind-energy development (e.g., southwestern United States), where some of the largest bat colonies in North America are known, may be at considerable risk from wind-energy development during both migratory and maternity periods. Projected development of wind-energy facilities throughout the United States should be evaluated for cumulative impacts on different species considered at risk.









































































