3
Biosignatures and Abiotic Chemistry
Life as we know it (i.e., terran life, as discussed in Chapter 1) is based on organic chemistry and is constructed of carbonaceous compounds. These organic materials are pervasive in Earth’s crust and constitute an extensive chemical and isotopic record of past life that far exceeds what is recorded by visible fossils.1 The ubiquity of coal, organic-rich black shales, and petroleum hydrocarbons, for example, is one manifestation of life’s activities that extends deep into the geological record and can be used to observe past biological activity and events.2 In fact, biogenic organic matter is so ubiquitous and overwhelming in its abundance that it is exceedingly difficult to identify organic compounds and organic matter of unambiguously nonbiological origin. The notable exceptions are organic compounds in meteorites and synthetics.3
Experience with studies of terrestrial materials suggests that of all the various life-detection techniques available, analysis of carbon chemistry is the first among equals. Imaging and other life-detection techniques are important and will always be part and parcel of planetary exploration, but few would assert that any single methodology provides a more robust way to find extraterrestrial life than organic analysis. Accordingly, the prime emphasis here is on chemical methods for life detection. However, organic analysis alone is insufficient to detect life. The results from an ensemble of all of the relevant methodologies, combined with considerations of geological and environmental plausibility, will likely provide the best evidence for the presence or absence of life in a sample.
Although all of the assumed characteristics of hypothetical martian life forms discussed in Chapter 1 can inform and guide the overall search for biosignatures, the assumption concerning the key role likely to be played by organic chemistry will prove to be particularly important. This assumption implies that martian organisms would produce and use a wide range of small molecules and organic polymers that could serve as chemical biosignatures in their intact or fragmentary states. But to apply this knowledge for remote sensing experiments on Mars or other planetary bodies, astrobologists need to distinguish reliably between biological molecules and those that are nonbiological in origin. The following discussion identifies specific features that distinguish abiotic compounds from compounds or patterns produced by present-day life on Earth. To address the past geocentric focus, the discussion
considers some generic features that could not be generated abiologically and that would be the foundation of a sound approach to the recognition of nonterran life.
ABIOTIC CHEMISTRY
Abiotic chemistry, both organic and inorganic, provides important information about the pathways that might have led toward an origin of life. Unfortunately, there is in origin-of-life scenarios no consensus about the synthesis of organics on early Earth or elsewhere, and so astrobiologists cannot search for a specific chemistry. Among the models suggested as possibly relevant for the origin of life are atmospheric electric discharges, as proposed by Miller and Urey,4 which have been shown to synthesize a range of organic compounds, including amino acids, from mixtures of methane, ammonia, and water. Discharge experiments yield few organic compounds when carried out in the kinds of oxidized gas mixtures of carbon dioxide thought to have predominated on early Mars. Additional processes that might have contributed to the inventory of organic compounds on early Mars include those associated with the transient effects of bolide impacts5 and, more importantly, a variety of mineral-catalyzed chemical reactions including water-rock reactions (e.g., serpentinization) and Strecker, Fischer-Tropsch, and FeS-driven organic synthesis.6 Water-rock reactions produce copious amounts of hydrogen that could lead to the subsurface formation of hydrocarbons from carbon dioxide and have also been shown to reduce nitrogen to ammonia,7 both of which could make their way to planetary surfaces. Strecker synthesis is the reaction of ammonia, hydrogen cyanide, and aldehydes to give amino acids and related products. Fischer-Tropsch chemistry is the mineral-catalyzed high-temperature reaction of carbon monoxide and hydrogen to give hydrocarbons. FeS-driven organic synthesis, first proposed by Wächtershäuser,8,9 has been experimentally demonstrated for only a relatively limited set of syntheses.
It is safe to assume that organic compounds that might have contributed to the prebiotic potential of the planet could have been synthesized elsewhere in the solar system or in interstellar space and then carried to the surface of Mars via carbonaceous chondrites and interplanetary dust particles. Since there is no consensus about the past history of prebiotic processes on Mars, it is more constructive to first consider the availability of the elements that constitute organic matter.
-
Carbon. C is found as gaseous carbon dioxide in the martian atmosphere, as carbon dioxide ice, and as carbonate minerals. Carbonates have been found in small amounts in martian meteorites but have not been detected in significant quantities by orbital remote sensing techniques or in chemical analyses of the martian regolith by landers.
-
Hydrogen. H is present as water ice and vapor and in hydrated minerals, and may be present within the crust as liquid water. The high D/H ratios of martian water show that Mars has lost a fraction of its water to space from the upper atmosphere. Because of the low atmospheric pressure, liquid water is not stable at the surface of modern Mars. The polar ice caps are thought to contain significant quantities of water ice, and the Gamma Ray Spectrometer on the Mars Odyssey spacecraft has detected significant quantities of subsurface hydrogen, presumably in the form of water ice.10 Thus, the abundance of hydrogen would not have hindered life on Mars at any time in its history.
-
Nitrogen. N is poorly retained by the inner planets owing to its volatility and stability as N2 and also to the relative instability and solubility of its involatile forms. Currently, 2.7 percent of the martian atmosphere is nitrogen. Although nitrogen is crucial for life, it may be rare on Mars.11 The observed ratio of 15N/14N suggests that a large fraction of the planet’s nitrogen inventory has been lost to space. No measurements have yet identified nitrogen stored in surface or subsurface minerals.
-
Oxygen. O is present in H2O and CO2, in oxides and sulfate minerals on the highly oxidized surface, and in silicates and other minerals within the crust.
-
Phosphorus. Phosphate minerals are actually more abundant in meteorites than in most igneous rocks on Earth. Volatile compounds of phosphorus (phosphorus pentoxide and phosphine) are rare, making phosphate minerals more valuable as sources of phosphorus for organisms than other biotic elements with common volatile forms.
-
Sulfur. S is very abundant as sulfates at the martian surface, and sulfides are common accessory minerals in martian meteorites and, presumably, the martian crust. Isotopic measurements suggest that sulfur species are also present in the martian atmosphere.12
-
Other metals. Metal ions such as are required by biological systems—Mg, Ca, Na, K, and transition elements—are abundant in martian surface rocks and, presumably, in subsurface rocks as well.
TERRAN BIOSIGNATURES AND POTENTIAL MARTIAN BIOSIGNATURES
Molecular Biosignatures
The carbon chemistry of terran organisms is well understood. Researchers have detailed knowledge of the metabolic and reproductive machinery of many living organisms and can recognize the residual chemicals long after life has expired. Chemistry provides many tools for identifying extant and fossil carbon-based life on Earth and, potentially, throughout the universe.
At the most basic level, researchers can examine the elemental composition of bulk organic matter preserved on Mars or in returned Mars samples as an indicator of biogenicity. On Earth, all organisms are composed largely of the six elements—C, H, N, O, P, and S—whose abundances are discussed above and in Chapter 2. Their proportions vary between organisms and ecosystems.13 Mechanisms and pathways involved in preservation can change these ratios; for example, N and P decline significantly during fossilization. Nevertheless, the discovery in a Mars sediment sample of organic matter with significant abundances of N, O, P, and S would indicate a similarity to biological material on Earth. The relative scarcity of N (see previous section) combined with the key role it plays in biological processes suggests that organic nitrogen compounds would be an important potential biosignature.14
Organic geochemists coined the term “biological marker compound” or “biomarker” to describe individual organic compounds that serve as molecular biosignatures.15–17 Biomarkers comprise a spectrum of biomolecules spanning those that are present in living systems (biomarkers for extant life), structurally-related fossil derivatives that have been preserved in sediments (biomarkers for past life), or complex chemicals that have generic traits characteristic of biology but for which no precursor organism is known (sometimes called orphan biomarkers). The last set could include molecules derived from unrecognized terran life (present or past) or extraterrestrial life.
Biomolecules commonly show a huge diversity of chemical structures. However, unambiguous identification of something as chemically complex and biology-specific as DNA, a protein, a phospholipid, a steroid, or even a select set of small molecules would be difficult to refute as a successful life-detection experiment. Such a set of select small molecules might include some of the 20 protein amino acids in large excess over their nonprotein counterparts, some sugars, or a select group of fatty acids such as might be found in the polar lipids of contemporary organisms. While nucleic acids, proteins, carbohydrates, and intermediary metabolites are essential components of life, and obviously potential molecular biosignatures, compounds in these classes are rapidly recycled by other living systems and are chemically fragile. On Earth, they are not known for their ability to survive intact over geological timescales.
Lipids and structural biopolymers are biologically essential classes of compounds renowned for their stability under harsh environmental conditions.18 Hydrocarbons, for example, are a class of lipid known to be stable on Earth over billion-year timescales.19,20 Furthermore, their chemical structures can be as diagnostic for biology as those of amino acids or other biomolecules. Thermodynamic arguments suggest that the lower temperatures on Mars would aid in the preservation of hydrocarbons. The specific empirical evidence for this comes from observations of petroleum deposits on Earth: high-temperature reservoirs show enhanced hydrocarbon cracking (i.e., more gas and gasoline-grade hydrocarbons) compared to equivalent low-temperature reservoirs.
Several important molecular biosignatures result from the propensity of molecules containing just a few carbon atoms to exist in different chemical and structural configurations, known as isomers. In other words, isomers are molecules having the same number of atoms of each element (i.e., their chemical formulas are the same), but exhibiting different connectivities between, and/or spatial arrangements of, their constituent atoms. In the simplest of cases, isomers of the same compound might be chemically identical but differ in their ability to rotate polarized light (e.g., the chirality of amino acids, as described in Box 3.1). In more complex examples, the connectivity and
spatial arrangements of atoms in organic molecules might give rise to compounds with very different chemical and physical characteristics (e.g., the diastereoisomers and structural isomers described in Boxes 3.2 and 3.3, respectively). All of these properties can unambiguously indicate biological origins because living systems frequently make use of just one of the multiple isomers that can exist for any given molecule.21,22
Another important set of molecular biosignatures can be identified, based on the observation that all known organisms utilize a universal subset of small metabolites as generic building blocks for constructing biomass and more complex biomolecules.23 The 20 amino acids of proteins, the four nucleotides of DNA, and the acetate precursor of most lipids are prime examples of generic building blocks. This simple fact, so fundamental to life on Earth, leads to patterns in the molecules of life and in the molecular remains of past life. This is in stark contrast to organic compounds produced in abiotic processes, which have structures and distributions with distinctly different patterns more likely to reflect thermodynamic controls. For any class of organic compounds, biosynthesis results in recurring patterns, readily recognizable to organic chemists. Detection of particular patterns (e.g., biomolecules with a preference for even or odd numbers of carbon atoms, as described in Box 3.4) and recurring themes (e.g., families of related molecules with a limited subset of all the possible numbers of carbon atoms, as described in Box 3.5) in small to moderate-sized organic molecules could lead to the validation of biosignatures for both terran and, possibly, nonterran life.
Taken together, these various chemical characteristics have led researchers to identify the following generic molecular biosignatures for carbon-based life:
-
Chirality (see Box 3.1),
-
Diastereoisomeric preference (see Box 3.2),
-
Structural isomer preference (see Box 3.3),
-
Repeating structural subunits or atomic ratios (see Box 3.4), and
-
Uneven distribution patterns or clusters of structurally related compounds (see Box 3.5).
In summary, any family of organic molecules common to Earthly life (e.g., lipids) if discovered on Mars would be important biological markers. However, at a more basic level, patterns of carbon number, or limited isomer distributions, or, isotopic composition (see next section), consistent with synthesis from small, repeating precursor molecules may point the way to the detection of extraterrestrial life be it terran or non-terran in its biological architecture.
Isotopic Biosignatures
The elements that are most important in organic chemistry all have multiple isotopes. The isotopic patterns of these elements and, increasingly, of transition metals can constitute biosignatures in terran samples. This is the case because kinetically controlled isotopic fractionations are common in biology and can be significant and dominant over equilibrium fractionation. Although geological processes fractionate these isotopes, biological processes tend to produce different, and sometimes diagnostic, effects. For example, enzymes involved in carbon fixation, methanogenesis, methane oxidation, sulfate reduction, and denitrification impose significant fractionations between precursor and product for carbon, hydrogen, sulfur, and nitrogen. Depletions or enrichments of certain isotopes from expected values can be used as biosignatures. However, such fractionations can reveal biological activity only if all the various components of a system are available for measurement and open system behavior has operated.
No fractionations will be observed if all of a precursor is converted to a product, regardless of whether equilibrium or kinetic fractionations operate. Furthermore, for an isotopic biosignature to be sound, the components of the system must be preserved intact without subsequent fractionation by physical or chemical processes. A myth commonly perpetuated is that a C-isotopic signature in organic carbon compounds of −20‰ to −80‰ is diagnostic of biology irrespective of any other factor. The 13C-composition in organic compounds can be a biosignature only if the isotopic composition of the precursor carbon source is also known and, importantly, if the pedigree of the materials is also consistent with biological processes. These issues have made biological interpretations of
|
BOX 3.1 Chirality An important property of carbon compounds is that the same atoms can bond to each other in the same manner while assuming different configurations in space. The different three-dimensional arrangements of organic molecules having the same chemical and structural formulas can lead to a number of important properties relevant to the study of biomarkers. One of these properties is chirality. That is, some molecules have their component atoms arranged in two different spatial configurations that are mirror images of each other. If the mirror images are not superimposable one upon the other, then the molecule is said to be chiral and its two structural forms are called enantiomers (Figure 3.1.1). The vast preponderance of biologically formed chiral compounds are synthesized exclusively as one or the other enantiomer; for example, right-handed sugars and left-handed amino acids are the norm in biological systems. This phenomenon is known as homochirality. Some organisms, bacteria for example, may synthesize the same chiral compound in different enantiomeric forms. Once the organism dies, and its biochemicals are released into the environment, their chiral purity may or may not persist depending on the relative stability of the chemical bonds in the enantiomers. Various natural chemical processes can lead to racemization, the formation of mixtures of the two enantiomers. Although racemization may result in loss or corruption of a biological signature, the rate at which it happens can also have a practical application, such as in the dating of fossil organic matter using the degree of amino acid racemization. Amino acids with a slight chiral excess of, presumably, abiotic origin occur in meteorites.1,2 Nevertheless, biology is the most likely source of compounds that occur purely or predominantly as one enantiomer. Enantiomeric excess can be detected in a number of ways. Chiral compounds are optically active. That is, they rotate the plane of polarized light passing through them when in solution. Direct observation of optical activity is cumbersome. Biochemical detection of enantiomeric excess is possible, but the methodologies are generally specific to individual compounds or compound types. The most widely applicable and sensitive techniques involve indirect measurement through gas chromatography or gas chromatography-mass spectrometry. 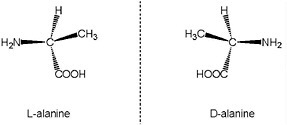
FIGURE 3.1.1 The atoms in the α-amino acid alanine can assume two different configurations in three-dimensional space. The two forms, L-alanine and D-alanine, are called enantiomers because they are non-superimposable mirror images of each other. Abiotic processes produce equal mixtures of both L and D enantiomers, but terran life preferentially uses the L or D form. For example, most organisms on Earth make exclusive use of the L form of α-amino acids. Chemical bonds oriented out of and into the plane of the page are shown as solid or dashed wedges, respectively. Courtesy of Roger E. Summons, Massachusetts Institute of Technology. |
|
BOX 3.2 Diastereomeric Preference Diastereomeric preference is another manifestation of the ability of atoms in certain molecules to assume different orientations in space. If the two spatial arrangements of atoms are not mirror images of each other, then the different molecular forms are known as diastereomers or diastereoisomers (Figure 3.2.1). Unlike enantiomers, diastereoisomers have different physical and chemical properties and can be separated by chromatography or other processes that exploit subtle differences in polarity. Simple sugars are good examples of diastereoisomers and the more complex the molecule, the more possibilities there are to form diastereomers. Thus, for example, the steroid cholesterol (see Figure 3.2.2) can exist in 256 different structural configurations, but living systems make use of only one of them.1
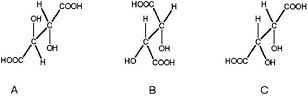
FIGURE 3.2.1 The ability of atoms in organic molecules to assume multiple configurations in three-dimensional space is demonstrated by these three forms of tartaric acid. Structures A and B and A and C are superimposable mirror images of each other and so are termed diastereomers. Structures B and C are non-superimposable mirror images of each other and are, thus, enantiomers (see Box 3.1). Courtesy of Roger E. Summons, Massachusetts Institute of Technology. 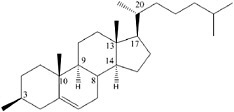
FIGURE 3.2.2 Structure of cholesterol with its eight asymmetric carbon atoms identified with their position number. Theoretically, this compound could exist in as many as 256 (28) possible stereoisomers, and yet biosynthesis produces only the one illustrated. |
carbon, nitrogen, or sulfur isotopic data in Archean sediments, for example, subject to debate.24–27 Although not likely to yield unambiguous biosignatures in the near future, isotopic analyses of martian sediments and atmospheric gases will be important for discerning their evolution and for establishing comparative data, as they do on Earth. Identification of a suite of supporting isotopic data in a reaction pathway, and its environmental context, is the most effective approach to identifying an isotopic biosignature. Elucidation of the isotopic systematics of
|
BOX 3.3 Structural Isomers The propensity of carbon compounds to exist with multiple ring systems and unsaturations means that the generic organic compound CpHqNrOsPtSu, can assume an enormous variety of possible structures, known as structural isomers.1 Despite the potential for variety, researchers observe that naturally synthesized biochemicals fall into patterns, and the number of known compounds is but a small subset of what is chemically feasible. Moreover, the biomolecule may be the thermodynamically least favored structure within a set of possible isomers if this aspect enhances its functional capacity. Structural isomers are readily separated using chromatography. In many, but not all cases, their mass spectra are also distinctive. As with other forms of isomerism, combinatorial instruments such as gas chromatographs-mass spectrometers and liquid chromatographs-mass spectrometers provide the most sensitive and diagnostic tools for trace analysis. |
the C-cycle on Earth has been underway for more than 50 years, and much remains to be understood.28,29 An added complication for studies of Mars is the unknown degree to which nonbiological atmospheric processes fractionate isotopes.
An example of an isotopic biomarker that might be used in the search for life on Mars is the 18O/16O ratio in phosphates.30 Phosphorus in the form of phosphates (PO43–) is utilized in genetic material and cell membranes, and as a cofactor and energy-transporting molecule in terran biology. On Earth, the ultimate source of PO43– is apatite that is dissolved, biologically processed, and redeposited as various sedimentary PO43– phases and as biogenic calcium phosphate deposits (phosphorites). Biologically processed PO43– on Earth has a strong biotic O-isotopic signature that is highly evolved from abiotic apatite baseline values. On Mars, evolution of the 18O/16O ratios in phosphates from this abiotic baseline could be used as a biomarker. Furthermore, the 18O/16O ratio of PO43– records temperature and high-temperature exchange reactions with water, also making PO43– a potential indicator of past hydrothermal activity on Mars.31
An additional example of an isotopic effect concerns the tendency in biological processes for large molecules to be synthesized by the repeated addition of subunits of two or five carbon atoms (see Box 3.4). The lipid building blocks acetate (C2) and isopentenyl pyrolphosphate (C5) are, for example, isotopically inhomogeneous. Acetate provides one of the best examples because it shows very significant differences in the 13C contents of its methyl and carboxyl carbons.32 The most overt consequences are isotopic ordering in fatty acids and a major isotopic difference between acetogenic and polyisoprenoid lipids. In a single organism, the isotopic differences between acetogenic and polyisoprenoid lipids depend on how many of the polyisoprenoid carbon atoms arise from acetate versus carbohydrate metabolism.33
Morphological Biosignatures
Morphological biosignatures represent the class of objects that can be interpreted as indicative of life based on their size, shape distribution, and provenance. Features of interest occur at both the macroscopic (e.g., stromatolites and microbially induced sedimentary structures) and the microscopic (e.g., microfossils) scale. If they were discovered on Mars, macroscale morphological features such as stromatolites, although being the subject of some contention as a definitive indicator of biogenicity,34 would prove to be highly desirable targets for further study and/or sample return.35–37
|
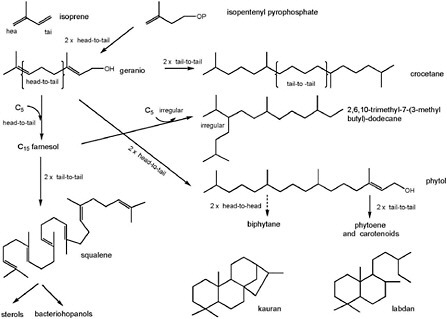
BOX 3.4 Subunits and Building Blocks of Complex Organic Molecules Virtually all biomolecules are constructed from a limited number of generic subunits or building blocks, the best-known examples being proteins and nucleic acids. Lipids, which are formed from only two basic building blocks, are polymers of either acetate or isopentenyldiphosphate precursors. The final products lack a hydrolyzable functionality (e.g., peptide linkages) at the point where subunits join, and, unlike other proteins and nucleic acids, lipids cannot be depolymerized. A classic example of lipids are those that are found in membrane lipid bilayers of bacteria and eukarya and are made up of fatty acids esterified to glycerol. The most common fatty acids are all-acetate products and thus have even carbon numbers (e.g., C14, C16, C18, and C20). Odd-carbon-numbered members, generally synthesized from a non-acetyl starter, exist but are less abundant. Extension of fatty acid chain length proceeds by the addition of further acetate units. Terminating and modifying reactions such as desaturation, reduction, or decarboxylation yield common intermediate-molecular-weight series of products such as the plant and algal waxes made up of even-numbered alcohols (e.g., C26, C28, C30, C32) and odd-numbered hydrocarbons (e.g., C25, C27, C29, C31). An additional illustration of the building-block principle is displayed by the terpenoids. These polymers of Δ3-isopentenyldiphosphate have somewhat more complex origins and much more complex structures (Figure 3.4.1). As a result of isoprenoid biosynthesis and its evolution over geological time, terran life contains an enormous array of complex molecules related through their C5 architecture. The multiplicity of isoprenoid biosynthetic pathways, their distribution across different phylogenetic groups, their requirement, or otherwise, for molecular oxygen, and the types of post-synthesis modification are generally held to provide a powerful biosignature of evolutionary origins. For example, the molecules resulting from the pathway shown in Figure 3.4.1 are highly diagnostic of biosynthesis because, individually, they exhibit many features of biosynthesis (e.g., carbon number, chirality, and subsets of isomers). Crocetane, 2,6,10-trimethyl-7-(3-methylbutyl)-dodecane, squalene, and biphytane are irregularly branched compounds, whereas phytane, labdane, and kaurane are regular and are constructed from four head-tail linked isoprene units. These compounds also illustrate how different structures can be diagnostic for specific physiologies (phytol and farnesol for photosynthesis, phytane for various archaea, crocetane for methanotrophy) or specific organisms (2,6,10-trimethyl-7-(3-methylbutyl)-dodecane for diatoms; biphytane for crenarchaeota; labdane and kaurane for conifers). |
Cameras and spectral imagers on previous, continuing, and planned life-detection missions to Mars are capable of identifying structures and objects ranging from the macroscopic to the minuscule that, on Earth, are considered visible signatures for past or present biological activity. Such objects and structures include intact microbes, metazoa and metaphytes, stromatolites, microbial mats, and other large-scale structures composed of aggregates of cells, as well as component parts of multicellular organisms such as cysts, pollen, embryos, organs, and so on. On Earth, these objects are pervasive in surface environments and in the deep subsurface and leave no doubt about how abundant and tenacious life is. Researchers can also, to a degree, visually identify in Earth’s sediments a rich fossil life extending in age to more than 2 billion years. So far, no such visible “biological” objects have been convincingly identified on Mars or in martian meteorites. If life exists, or existed in the past, on Mars or other
planetary bodies, the evidence has not been forthcoming. In many respects, the search for martian life mirrors the search for the earliest life on Earth and faces similar obstacles. Attempting to reconstruct terran life’s history back into deep time, researchers are confronted by the problem of a record made increasingly cryptic by the geochemical and geological processes that continually re-surface Earth and modify the rock record.
Poor preservation and ambiguity about what constitutes a biosignature have confounded the search for visible evidence of early microbial life on Earth38–45 and in the martian meteorite ALH 84001 in particular.46 Related reports, and some of the controversies stemming from them, teach researchers that drawing an inference of biogenicity based on morphology is fraught with difficulties. If the feature being observed is demonstrably syngenetic with the host rock and displays a limited size (length and width) distribution, shows evidence of cellular
|
BOX 3.5 Clusters and Uneven Distribution Patterns of Structurally Related Compounds The biosynthesis of large organic molecules from smaller molecules, as discussed in Box 3.4, leads to wider consequences, evidence of which can, in principle, be used as biomarkers. The synthesis of lipids by organisms, for example, from C2 or C5 building blocks creates clusters of compounds that differ by n C2 (acetogenic lipids) or n C5 (polyisoprenoids) units, where n is a positive interger. In a typical sample of terrestrial lipids, researchers find, for example, a predominance of even-carbon-numbered fatty acids; odd-carbon-numbered hydrocarbons in leaf wax; C15, C20, and C25 acyclic isoprenoids; C20 and C30 cyclic terpenoids including steroids; and C40 carotenoids. Subsets of these traits are even identifiable in highly altered or processed materials such as petroleum, where n-alkanes may exhibit preferences for odd-over-even or even-over-odd carbon numbers. Clusters of carbon numbers have the potential to be biosignatures because they indicate biosynthesis from universal building blocks. In addition to obvious patterns of related compounds differing by two or five carbon atoms, the action of repeated addition of C2 or C5 subunits leads to an additional important biosignature. Functional biochemicals, such as lipids, have a tendency to show clusterings of related compounds at discrete molecular weight ranges. Examples of clusters seen include the following:
An additional biomarker related to clustering and isotopic fractionation is described in the subsection “Isotopic Biosignatures.” A factor complicating the use of these biosignatures is the fact that most samples of biologically produced organic matter come from organisms that exist in complex ecosystems. The volatile components of a microbial mat, for example, will show compound classes with carbon numbers distributed roughly as described above and in Box 3.4. Similarly, the lipids in biofilms from hydrothermal vents display an uneven-carbon-number distribution.1 The geological record is replete with additional examples.2 Moreover, the C25-C30 fraction might contain more material than the C15-C20 fraction. This “lumpiness” is in stark contrast to what is seen in assemblages of molecules made abiotically.3,4 The Fischer-Tropsch process used to synthesize hydrocarbons, for example, creates molecules with an exponential distribution of sizes, with C1 > C2 > C3 > C4, and so on, falling away to almost zero by C30. Similarly, the amino acids seen in meteorites exhibit more C1 than C2 than C3 than C4 and so on.5-8 |
degradation, or is part of a discernable population that occurs in discrete phases within the samples on Earth that are relevant to the context of the sample, then further investigation is warranted.47 The debates on early life and ALH 84001 (see Chapter 2) have shown that morphology must be combined with both chemistry and context to enable unambiguous detection of life. However, morphology is extremely valuable for detecting targets of interest for further investigation, particularly macroscopic structures such as stromatolites, microbial mats, and other large-scale aggregates created by communities of microorganisms.
Mineralogical and Inorganic Chemical Biosignatures
The mineralogy and chemistry of Earth materials can constitute a biosignature in some systems where organisms either accelerate or inhibit reactions that are thermodynamically possible. In addition, organisms can change the chemistry of rocks, fluids, and gases through the processes of secretion, assimilation, and electron transfer, sometimes creating mineralogical or chemical gradients that differ from those that would be established in an abiotic environment. Although there are a few examples of mineralogical biosignatures on Earth that unambiguously identify a biotic origin (e.g., coccoliths and diatoms), these are not likely to be applicable to Mars.48 Most other types of inorganic chemical biosignatures can provide only indirect evidence of the presence of life and would thus most likely constitute supporting evidence accompanying other more diagnostic criteria. Examples of inorganic biosignatures are discussed below.
Biota can affect the identity of phases manifested in the rock record. For example, some bacteria transform mackinawite to greigite (sulfides),49 and some fungi promote the formation of weddellite (Ca oxalate) in soils. These effects are related to the biological ability to nucleate minerals onto organic templates, or to the production of organic ligands that solubilize elements, affect growth mechanisms, or precipitate as salts. The inclusion of organic molecules or micronutrient impurities in mineral precipitates could also conceivably be indicative of biological activity.
Physical properties of minerals might also yield indirect, albeit ambiguous, evidence of biological processes. For example, the size distribution of precipitates might indirectly suggest a biotic origin, given that many mineralogical by-products of metabolism are nanocrystalline because they are formed under conditions of high oversaturation.50 Surface etching or crystal habit, which can be affected by biological exudates or biofilm formation, might also be indirect indicators of biota. Biological phenomena can also be inferred in some cases from the characteristics of aggregations of minerals. Of possible interest for Mars is aggregation characteristic of Fe minerals precipitated by bacteria. For example, both the size distribution and the aggregation of magnetite crystals have been posited as biosignatures,51,52 although these characteristics have also been attributed to abiotic processes,53 thus pointing out the ambiguous nature of mineralogical properties as biosignatures.
Gradients in the concentration of elements recorded in Earth materials can also be diagnostic of biological phenomena. A well-known manifestation of elemental gradients driven by biological processes is certain soil horizons in which the exudation of organic complexants mobilizes elements and produces patterns indicative of the presence of biota.54 The formation of gradients in the concentration of elements at the meter scale in soil horizons and at the micron scale on mineral surfaces or in endolithic communities might thus be important.55–57 The assimilation of trace elements at a low concentration by microorganisms or the sequestration of toxic elements into biologically mediated precipitates could also create distributions of trace elements that record the prior presence of biota in regolith or sedimentary environments.
Anomalies in the concentration of phosphorus have also been suggested as possible biomarkers that could be used in the search for life on Mars.58 Phosphorus as PO43– is utilized in a wide variety of biological processes and material. The ultimate source of PO43– on Earth is igneous apatite, which is biologically processed and redeposited as biogenic calcium phosphates (phosphorites). On Earth, PO43– is adsorbed strongly to iron- and aluminum-oxides and oxyhydroxides under aqueous conditions. Phosphorus phases found in martian soils, sedimentary environments, and in association with the abundant iron oxides on Mars might be a good target in a search for phosphorus as a biosignature. Additionally, patterns of phosphorous concentration could be used to guide the search for potential PO43– biosignatures and other kinds of fossils.
Based on such considerations, past and present approaches to Mars astrobiological exploration have heavily emphasized instrument packages capable of detecting the chemical signatures of life, especially carbon compounds, isotopic signatures, and various other products of metabolism. The 2001 workshop on biosignatures organized by the NASA Biomarker Task Force established comprehensive objectives for developing a better understanding of biosignatures. Unfortunately, though, the results of the task group’s deliberations were never published in full.59 Because they represent an important starting point for future discussions, those objectives are reproduced in Appendix C.
REFERENCES
1. J.J. Brocks and R.E. Summons, “Sedimentary Hydrocarbons, Biomarkers for Early Life,” pp. 65-115 in Treatise in Geochemistry (H.D. Holland and K. Turekian, eds.), 2003; K.E. Peters, J.M. Moldowan, and C.C. Walters, The Biomarker Guide, Cambridge University Press, Cambridge, 2004.
2. See, for example, A.H. Knoll, R.E. Summons, J.R. Waldbauer and J.E. Zumberge, “Successions in Biological Primary Productivity in the Oceans” in The Evolution of Photosynthetic Organisms in the Oceans (P. Falkwoski and A.H. Knoll eds), in press; K.E. Peters, J.M. Moldowan and C.C Walters, The Biomarker Guide, Cambridge University Press, Cambridge, 2004.
3. See, for example, A.I. Rushdi and B.R.T. Simoneit, “Lipid Formation by Aqueous Fischer-Tropsch-Type Synthesis over a Temperature Range of 100 to 400°C,” Origins of Life and Evolution of Biospheres 31:103-118, 2004; J.D. Pasteris and B. Wopenka, “Laser–Raman Spectroscoy (Communication Arising): Images of the Earth’s Earliest Fossils?” Nature 420:476-477, 2002; B. Sherwood Lollar, T.D. Westgate, J.A. Ward, G.F. Slater, and G. Lacrampe-Couloume, “Abiogenic Formation of Alkanes in the Earth’s Crust as a Minor Source for Global Hydrocarbon Reservoirs,” Nature 416:522-524, 2002; T.M. McCollom, and J.S. Seewald, “Carbon Isotope Composition of Organic Compounds Produced by Abiotic Synthesis under Hydrothermal Conditions,” Earth and Planetary Science Letters 243:74-84, 2006.
4. S.L. Miller, “Production of Some Organic Compounds under Possible Primitive Earth Conditions, Journal of the American Chemical Society 7:2351, 1955.
5. J.A. Kasting, “Bolide Impacts and the Oxidation State of Carbon in the Earth’s Early Atmosphere,” Origins of Life and Evolution of the Biosphere 20:199-231, 1990.
6. See, for example, R.M. Hazen “Life’s Rocky Start,” Scientific American 284(4):76-85, 2001.
7. J.A. Brandes, N.Z. Boctor, G.D. Cody, B.A. Cooper, R.M. Hazen, and H.S. Yoder, “Abiotic Nitrogen Reduction on the Early Earth,” Nature 395:365-367, 1998.
8. G. Wächtershäuser, “Before Enzymes and Templates: Theory of Surface Metabolism,” Microbiology Review 52:452-484, 1988.
9. G. Wächtershäuser, “Evolution of the First Metabolic Cycles,” Proceedings of the National Academy of Sciences 87:200-204, 1990.
10. W.V. Boynton, W.C. Feldman, S.W. Squyres, T.H. Prettyman, J. Brückner, L.G. Evans, R.C. Reedy, R. Starr, J.R. Arnold, D.M. Drake, P.A.J. Englert, A.E. Metzger, I. Mitrofanov, J.I. Trombka, C. d’Uston, H. Wänke, O. Gasnault, D.K. Hamara, D.M. Janes, R.L. Marcialis, S. Maurice, I. Mikheeva, G.J. Taylor, R. Tokar, and C. Shinohara, “Distribution of Hydrogen in the Near Surface of Mars: Evidence for Subsurface Ice Deposits,” Science 297:81-85, 2002.
11. D.G. Capone, R. Popa, B. Flood, K.H. Nealson, “Geochemistry. Follow the Nitrogen,”. Science 312:708-709, 2006.
12. J. Farquhar, J. Savarino, T.L. Jackson, M.H. Thiemens, “Evidence of Atmospheric Sulphur in the Martian Regolith from Sulphur Isotopes in Meteorites,” Nature 404:50-52, 2000.
13. P.G. Falkowski and C.S. Davis, “Natural Proportions,” Nature 431:131, 2004.
14. D.G. Capone, R. Popa, B. Flood, K.H. Nealson, “Geochemistry. Follow the Nitrogen,”. Science 312:708-709, 2006.
15. G. Eglinton and M. Calvin “Chemical Fossils,” Scientific American 261:32-43, 1967.
16. M.H. Engel and S.A. Macko, eds., Organic Geochemistry Principles and Applications, Plenum Press, New York, 1993.
17. K.E. Peters, J.M. Moldowan, and C.C. Walters,. The Biomarker Guide, Cambridge University Press, Cambridge, 2004.
18. M.H. Engel and S.A. Macko, eds., Organic Geochemistry Principles and Applications, Plenum Press, New York, 1993.
19. J.J. Brocks and R.E. Summons, “Sedimentary Hydrocarbons, Biomarkers for Early Life,” pp. 65-115 in Treatise in Geochemistry (H.D. Holland and K. Turekian, eds.), 2003.
20. K.E. Peters, J.M. Moldowan, and C.C. Walters,. The Biomarker Guide, Cambridge University Press, Cambridge, 2004.
21. K.E. Peters, J.M. Moldowan, and C.C. Walters, The Biomarker Guide, Cambridge University Press, Cambridge, 2004.
22. E.L. Eliel, S.H. Wilen, and L.N. Mander, Stereochemistry of Organic Compounds, Wiley, New York, 1994.
23. See, for example, N.A. Campbell and J.B. Reece, Biology (7th edition), Benjamin Cummings, 2004.
24. See, for example, S.J. Mojzsis, G. Arrhenius, K.D. McKeegan, T.M. Harrison, A.P. Nutman, and C.R. Friend, “Evidence for Life on Earth Before 3,800 Million Years Ago,” Nature 384:55-59, 1996.
25. M.A. van Zuilen, K. Mathew, B. Wopenka, A. Lepland, K. Marti, and G. Arrhenius, “Nitrogen and Argon Isotopic Signatures in Graphite from the 3.8-Ga-old Isua Supracrustal Belt, Southern West Greenland,” Geochimica et Cosmochimica Acta 69:1241-1252, 2005.
26. Y. Ueno, H. Yurimoto, H. Yoshioka, T. Komiya, and S. Maruyama, “Ion Microprobe Analysis of Graphite from ca. 3.8 Ga Metasediments, Isua Supracrustal Belt, West Greenland: Relationship between Metamorphism and Carbon Isotopic Composition,” Geochimica et Cosmochimica Acta 66:1257-1268, 2002.
27. Y. Shen, R. Buick and D.E. Canfield “Isotopic Evidence for Microbial Sulphate Reduction in the Early Archaean Era,” Nature 410:77-81, 2001.
28. H. Craig, “The Geochemistry of the Stable Carbon Isotopes of Carbon,” Geochimica et Cosmochimica Acta 3:53-92, 1953.
29. J.M. Hayes and J.R. Waldbauer, “The Carbon Cycle and Associated Redox Processes through Time,” Philosophical Transactions of the Royal Society B: Biological Science 361:931-950, 2006.
30. R.E. Blake, J.C. Alt, and A.M. Martini, “Oxygen Isotope Ratios of PO4–: An Inorganic Indicator of Enzymatic Activity and P Metabolism and a New Biomarker in the Search for Life,” Proceedings of the National Academy of Sciences, Astrobiology Special Feature 98:2148-2153, 2001.
31. R.E. Blake, J.C. Alt, and A.M. Martini, “Oxygen Isotope Ratios of PO4–: An Inorganic Indicator of Enzymatic Activity and P Metabolism and a New Biomarker in the Search for Life,” Proceedings of the National Academy of Sciences, Astrobiology Special Feature 98:2148-2153, 2001.
32. J.M. Hayes, “Fractionation of Carbon and Hydrogen Isotopes in Biosynthetic Processes,” Reviews in Mineralogy and Geochemistry 43:225-277, 2001.
33. J.M. Hayes, “Fractionation of Carbon and Hydrogen Isotopes in Biosynthetic Processes,” Reviews in Mineralogy and Geochemistry 43:225-277, 2001.
34. J.M. Garcia-Ruiz, S.T. Hyde, A.M. Carnerup, A.G. Christy, M.J. Van Kranendonk, and N.J. Welham, “Self-Assembled Silica-Carbonate Structures and Detection of Ancient Microfossils,” Science 302:1194-1197, 2003.
35. H.J. Hofmann, K. Grey, A.H. Hickman, and R. Thorpe, “Origin of 3.45 Ga Coniform Stromatolites in Warrawoona Group, Western Australia,” Geological Society of America Bulletin 111:1256-1262, 1999.
36. S.L. Cady, J.D. Farmer, J.P. Grotzinger, J.W. Schopf, and A. Steele, “Morphological Biosignatures and the Search for Life on Mars,” Astrobiology 3:351-368, 2003.
37. A.C. Allwood, M.R. Walter, B.S. Kamber, C.P. Marshall, and I.W. Burch, “Stromatolite Reef from the Early Archaean Era of Australia,” Nature 441:714-718, 2006.
38. See, for example, D.R. Lowe, “Abiological Origin of Described Stromatolites Older than 3.2 Ga,” Geology 22:387-390, 1994.
39. J.P. Grotzinger and A.H. Knoll, “Stromatolites in Precambrian Carbonates; Evolutionary Mileposts or Environmental Dipsticks,” Annual Reviews of Earth and Planetary Sciences 27:313-358, 1999.
40. H.J. Hofmann, K. Grey, A.H. Hickman, and R. Thorpe, “Origin of 3.45 Ga Coniform Stromatolites in Warrawoona. Group, Western Australia.” Geological Society of America Bulletin 111:1256-1262, 1999.
41. M.D. Brasier, O.R. Green, A.P. Jephcoat, A.K. Kleppe, M.J. Van Kranendonk, J.F. Lindsay, A. Steele, and N.V. Grassineau, “Questioning the Evidence for Earth’s Oldest Fossils,” Nature 416:76-81, 2002.
42. J.W. Schopf, “Microfossils of the Early Archaean Apex Chert: New Evidence of the Antiquity of Life,” Science 260:640-646, 1993.
43. J.W. Schopf, “Are the Oldest Fossils Cyanobacteria?,” pp. 23-61 in Evolution of Microbial Life Society for General Microbiology Symposium 54 (D. McL. Roberts, P. Sharp, G. Alderson, and M. Collins, eds.), Cambridge University Press, Cambridge, 1996.
44. J.W. Schopf, A.B. Kudryavtsev, D.G. Agresti, T.J. Wdowiak, and A.D. Czaja, “Laser Raman Imagery of Earth’s Earliest Fossils,” Nature 416:73-76, 2002.; J.M. Garcia-Ruiz, S.T. Hyde, A.M. Carnerup, V. Christy, M.J. Van Kranendonk, and N.J. Welham, “Self-Assembled Silica-Carbonate Structures and Detection of Ancient Microfossils,” Science 302:1194-1197, 2003.
45. S.M. Awramik and K. Grey, “Stromatolites: Biogenicity, Biosignatures, and Bioconfusion,” pp. 227-235 in Astrobiology and Planetary Missions (R. B. Hoover, G.V. Levin, A.Y. Rozanov, G.R. Gladstone, eds.), Proceedings of the SPIE, Volume 5906, 2005.
46. D.S. McKay, E.K. Gibson, Jr., K.L. Thomas-Keprt, H. Vali, C.S. Romanek, S.J. Clemett, X.D.F. Chillier, C.R. Maechling, and R.N. Zare, “Search for Past Life on Mars: Possible Relic Biogenic Activity in Martian Meteorite ALH 84001,” Science 273:924-930, 1996.
47. J.W. Schopf, “The Oldest Fossils and What they Mean,” pp. 29-63 in J.W. Schopf (ed.), Major Events in the History of Life, Jones and Bartlett Publishers, Boston, Mass., 1992.
48. J.F. Banfield, J.W. Moreau, C.S. Chan, S.A. Welch, and B. Little, “Mineralogical Biosignatures and the Search for Life on Mars,” Astrobiology 1:447-465, 2001.
49. M.B. McNeil and B. Little, “Mackinawite Formation during Microbial Corrosion,” Journal of Corrosion 46:599-600, 1990.
50. J.F. Banfield, J.W. Moreau, C.S. Chan, S.A. Welch, and B. Little, “Mineralogical Biosignatures and the Search for Life on Mars,” Astrobiology 1:447-465, 2001.
51. K.L Thomas-Keprta, D.A. Bazylinski, J.L. Kirschvink, S.J. Clemett, D.S. McKay, S.J. Wentworth, H. Vali, E.K. Gibson, and C.S. Romanek, “Elongated Prismatic Magnetite Crystals in ALH 84001 Carbonate Globules: Potential Martian Magnetofossils,” Geochimica et Cosmochimica Acta 64:4049-4081, 2000.
52. K.L. Thomas-Keprta, S.J. Clemett, D.A. Bazylinski, J.L. Kirschvink, D.S. McKay, S.J. Wentworth, H. Vali, E.K. Gibson, Jr., M.F. McKay, and C.S. Romanek, “Truncated Hexa-Octahedral Magnetite Crystals in ALH 84001: Presumptive Biosignatures,” Proceedings of the National Academy of Sciences 98:2164-2169, 2001.
53. See, for example, A.H. Treiman, “Submicron Magnetite Grains and Carbon Compounds in Martian Meteorite ALH 84001: Inorganic, Abiotic Formation by Shock and Thermal Metamorphism,” Astrobiology 3:369-392, 2003.
54. A. Neaman, J. Chorover, and S.L. Brantley, “Element Mobility Patterns Record Organic Ligands in Soils on Early Earth,” Geology 33(2):117-120, 2005.
55. B. Kalinowski, L. Liermann, S.L. Brantley, A. Barnes, and C.G. Pantano, “X Ray Photoelectron Evidence for Bacteria-Enhanced Dissolution of Hornblende,” Geochimica et Cosmochimica Acta 64:1331-1343, 2000.
56. A. Neaman, J. Chorover, and S.L. Brantley, “Element Mobility Patterns Record Organic Ligands in Soils on Early Earth,” Geology 33(2):117-120, 2005.
57. H.J. Sun and E.I. Friedmann, “Growth on Geological Time Scales in the Antarctic Cryptoendolithic Microbial Community,” Geomicrobiology Journal 16:193-202, 1999.
58. G. Weckwerth and M. Schidlowski, “Phosphorus as a Potential Guide in the Search for Extinct Life on Mars,” Advances in Space Research 15:185-191, 1995.
59. Michael Meyer, NASA Science Mission Directorate, personal communication, 2006.