3
An Examination of Current and Potential Nutritional Risk Assessment Methods
Information gained from classical and nutritional risk assessments has led to new questions and possible new approaches to nutritional risk assessment. This chapter covers the perspectives of several nutritional scientists and risk assessment experts on various aspects of risk assessment. Robert Russell, an expert in human metabolism of retinoids and carotenoids, addressed how different groups have used risk assessment methods to establish upper intake levels for nutrients and identifies data gaps that impede the assessment of risk related to excessive nutrient intakes. Suzanne Murphy, an expert in dietary assessment methodology, covered potential connections between the establishment of nutrient requirements and Tolerable Upper Intake Levels (ULs). Christine Taylor, an expert in nutrition policy, pointed out lessons learned from an international workshop on the topic of upper levels for nutrients. Barbara Petersen, a risk assessment expert, provided background information on nutritional risk assessment and then introduced a method that could be used to consider both the risks and the benefits of nutrient intake as a function of that intake. Gregory Paoli, another risk assessment expert, distinguished safety-based standard setting from risk assessment and reviewed the rationale for formal risk assessment and the elements for risk-based advice. The chapter ends with a brief summary of the points raised during the commentary by Sanford Miller, whose expertise includes food safety.
THE INSTITUTE OF MEDICINE PROCESS TO ESTABLISH TOLERABLE UPPER INTAKE LEVELS: MODEL AND DATA NEEDS
Presenter: Robert M. Russell
The Institute of Medicine’s (IOM’s) conceptual model for the UL (see Figure O-1 in the Overview) involves determination of the highest level of daily nutrient intake that is likely to pose no risk of adverse health effects to almost all individuals in the general population. Robert Russell provided a brief review of the derivation of a UL and the sources of uncertainty and then focused on how three different expert groups arrived at different conclusions when they determined the upper levels1 for vitamin A, beta carotene, vitamin B6, and zinc. He concluded his presentation with a list of the kinds of data needed to improve the determination of upper levels for these four nutrients.
Brief Overview
The process of deriving an upper level typically involves the identification of a critical data set; the identification of a critical toxic effect (the endpoint, a no-observed-adverse-effect level [NOAEL] or a lowest-observed-adverse-effect level [LOAEL]); and the derivation of an uncertainty factor, which typically is less than 10 for nutritional risk assessment. By using this approach, the upper level is obtained by the following formula:
Therefore, the larger that the uncertainty factor is, the lower that the value of the upper level is.
The process of assigning an uncertainty factor has been somewhat subjective. Factors that have contributed to assigning a larger uncertainty factor include extrapolation of the findings of studies with animals to
humans, evaluation of the effects of short-term rather than long-term exposures, the use of small numbers of subjects in experimental studies, the greater severity of the hazard, and the use of a LOAEL rather than a NOAEL.
Selected Results from Different Expert Groups That Set Upper Levels for Nutrients
In preparation for this workshop, Russell examined reports by expert groups from three parts of the world: (1) IOM for the United States and Canada, (2) Expert Group on Vitamins and Minerals for the United Kingdom, and (3) Scientific Committee on Food for the European Union. He focused on the establishment of upper levels for vitamin A (EVM, 2003; IOM, 2001; SCF, 2002), beta carotene (EVM, 2003; IOM, 2000a; Rothman et al., 1995), vitamin B6 (EVM, 2003; IOM, 1998a; SCF, 2000), and zinc (EVM, 2003; IOM 2001; SCF, 2003). Russell found that the expert groups came to different conclusions, largely because of differences in the adverse effect identified or differences in the uncertainty factor used, as briefly described below.
Vitamin A
IOM set the UL for women of reproductive age by using a NOAEL for teratogenicity and an uncertainty factor of 1.5 (for a resultant UL of 3,000 micrograms per day [µg/day]). For all other adults, IOM set the UL by using a LOAEL for liver toxicity of 14,000 µg/day, based on case reports, and an uncertainty factor of 5. Among the other adverse effects considered were risks of bone mineral density changes, hip fracture, and bulging fontanel (for infants). The available data on the risk of hip fracture were not used because the findings were inconsistent (Freudenheim et al., 1986; HoutKooper et al., 1995; Melhus et al., 1998; Feskanich et al., 2002). More recent studies on the association of vitamin A with the risk of low bone mineral density (Ballew et al., 2001; Promislow et al., 2002; Rejnmark et al., 2004), hip fracture (Opotansky and Bilezikian, 2004; Rejnmark et al., 2004), and osteoporosis (Maggio et al., 2003; Penniston et al., 2006) remain inconclusive.
The expert group in the United Kingdom considered the evidence base to be inadequate to establish an upper level, indicating that the study
on teratogenicity by Rothman and colleagues (1995), which IOM used to establish the UL for women of reproductive age, was biased. The report did indicate, however, that vitamin A intakes greater than 1,500 µg/day may be inappropriate, mainly on the basis of studies by Melhus et al. (1998) and Feskanich et al. (2002), and advised women not to take vitamin A supplements if they were pregnant.
The Scientific Committee on Food used the study by Rothman et al. (1995), identified a LOAEL rather than a NOAEL, used no uncertainty factor, and set an upper level identical to that set by IOM. This expert group reported that it did not use an uncertainty factor because other studies show that the true threshold for risk is probably higher. The upper level set by the Scientific Committee on Food was considered to cover the risk of hepatotoxicity.
The vitamin A UL of 600 retinol activity equivalents (RAE)/day that IOM set for infants (IOM, 2001) is based on the vitamin A content of breast milk. An intake of 600 RAE/day is lower than the estimated amount of vitamin A consumed by 56 percent of the 4- to 5-month-old infants participating in the Supplemental Nutrition Program for Women, Infants, and Children (WIC), suggesting that the UL value for infants may need to be reexamined.
Beta-Carotene
The example of beta-carotene also illustrates that different groups examining the same data may come to very different conclusions. Neither IOM nor the EU Scientific Committee on Food set an upper level for beta-carotene. The reasons that both expert groups gave for not setting an upper level were similar (IOM, 2000b; EC, 2000): no dose–response data were available, the data were conflicting, or different formulations had been used in different studies. By contrast, the United Kingdom’s expert committee set an upper level of 7 milligrams per day (mg/day) (EVM, 2003), an amount that is close to what some vegetarians ordinarily ingest. They used data from the Alpha-Tocopherol Beta-Carotene Study to establish a NOAEL of 20 mg (that study had also been reviewed by IOM and the group from the European Union). The United Kingdom’s expert committee used supportive data from a study of ferrets exposed to a dose of beta-carotene that would be comparable to the dose for humans. Even when the ferrets that were exposed to the beta-carotene were not exposed to cigarette smoke, they still developed squamous metaplasias. Further
investigation has demonstrated that dose matters: giving ferrets that had been exposed to cigarette smoke a beta-carotene dose comparable to 6 mg/day in humans did not result in metaplasia.
Vitamin B6
The expert groups from North America, the United Kingdom, and the European Union relied on data from different studies and set very different upper levels for vitamin B6. IOM used the study by Bernstein and Lobitz (1988) to set the NOAEL for vitamin B6 and used a small amount of data on doses under 200 mg to set a UF of 2 (IOM, 1998b). This resulted in a UL of 100 mg/day when vitamin B6 is consumed as pyridoxine. In the United Kingdom, the upper level of 10 mg/day was set by using data on ataxia in dogs (Phillips et al., 1978) and using a large uncertainty factor (300) based on the use of a LOAEL, interspecies variation, and interindividual variation (EVM, 2003). In the European Union, the upper level of 25 mg/day was set by using data from Dalton and Dalton (1987), and an uncertainty factor of 4 was selected because of deficiencies of the data in the research database (SCF, 2000).
Zinc
The three expert groups differed in their establishment of upper levels for zinc as well. IOM and the Expert Group on Vitamins and Minerals used the same key study (Yadrick et al., 1989), but they derived different LOAELs and used different uncertainty factors. The Scientific Committee on Vitamins and Minerals disregarded the study by Yadrick et al. (1989) and used two different studies instead: those of Davis et al. (2000) and Milne et al. (2001). The resulting upper levels were as follows:
-
IOM = 40 mg/day
-
United Kingdom = 25 mg/day
-
European Union = 25 mg/day
Despite the use of very different methods in the United Kingdom and the European Union, the result was the establishment of identical upper levels.
Concluding Remarks
To provide a more reliable and valid basis for establishing upper levels for the four nutrients discussed here, there is a need to obtain more complete data on the following:
-
defined critical endpoints associated with nutritional status (for vitamin A),
-
the biomarkers to be used to define chronic disease (for beta-carotene),
-
vitamin B6 intakes from long-term studies,
-
systems that dysfunction with nutrient excess (for zinc), and
-
dose–response (for all four nutrients).
The use of experimental animal models could be helpful in obtaining much of this information. In addition, uniform rules are needed for the application of uncertainty factors (for all four nutrients), and the use of a common approach to the establishment of upper levels could be helpful.
POTENTIAL CONNECTIONS BETWEEN ESTABLISHING NUTRIENT REQUIREMENTS AND TOLERABLE UPPER INTAKE LEVELS
Presenter: Suzanne P. Murphy
Although adverse health effects are associated with both nutrient inadequacy and nutrient excess, expert groups use very different approaches to set nutrient requirements and upper levels. Suzanne Murphy suggested that the term “nutritional risk assessment” can apply equally to the processes of determining requirements for nutrients and the establishment of upper levels for nutrients. Murphy described the two approaches, examined why different approaches are used, and explored whether risk assessment methods are applicable to both approaches and how the use of different approaches limits the use of the Dietary Reference Intakes (DRIs).
Why Are Different Approaches Used to Set Nutrient Requirements and Upper Levels?
Overview of the Two Approaches
To set nutrient requirements, the DRI expert panels were asked to estimate the average requirement (the Estimated Average Requirement [EAR]) and its standard deviation. They computed the Recommended Dietary Allowance (RDA) as the EAR plus two standard deviations of the requirement (IOM, 2006a). To set a UL, the UL expert group was asked to use a risk assessment approach that involves hazard identification, dose–response assessment, intake (exposure) assessment, and risk characterization (refer to Figure O-1 in the Overview). In both cases, the expert groups were asked to identify a functional outcome and to conduct a dose–response assessment, an intake assessment, and a risk characterization. The approaches and the terminology used differed, however.
Possible Reasons to Question the Use of Different Approaches
One might view too little of a nutrient to be a hazard, in the same way that too much of the nutrient would be a hazard. By the definition of nutrient, long-term inadequacy leads to the deterioration of health and, eventually, death. The concepts appear to be comparable: both can be viewed as distributions. The EAR concept describes a distribution of requirements. On the other hand, the UL concept describes a threshold, that is, the beginning of the risk of an adverse effect. Figure 3-1 depicts hypothetical distributions for requirements and adverse effects. If a person’s usual intake were at the amount indicated, the person would be at some risk from excessive intake. Indeed, Murphy argued that there could be a very low (nearly zero) risk of excessive intake in the homeostasis area of intake (see “Applying Risk Assessment Methods to Nutritional Risk Assessment” in Chapter 2), just as there could be a very low risk of inadequate intake in that area.
Because the UL is not defined with a mean and a distribution, Murphy noted that a complete description of the risk is problematic.
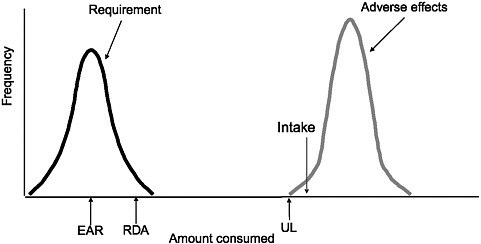
FIGURE 3-1 Hypothetical distributions of nutrient requirements and adverse effects. Intake at the amount indicated by the intake arrow would pose some risk, but without knowing the actual distribution, further interpretation would be problematic.
NOTE: EAR = Estimated Average Requirement; RDA = Recommended Dietary Allowance; UL = Tolerable Upper Intake Level.
Without a distribution, no method is available that can be used to interpret the prevalence of intakes above the UL. It would be desirable to be able to provide a quantification of the risk of an adverse effect rather than being limited to a statement that a certain percentage of the population has intakes above the UL.
Are Some Risk Assessment Methods Applicable to Both Nutrient Requirements and Upper Levels?
Murphy posed several questions about risk assessment methods that might be applicable to both nutrient requirements and upper levels:
-
Does the concept of a NOAEL apply to nutrient requirements? If so, the value would need to be at an intake higher than the RDA, which has an estimated 2 to 3 percent risk of inadequacy.
-
Are there similarities between a NOAEL and an Adequate Intake (AI)? For example, since the AI serves as a threshold, in some
-
cases it might be comparable to a NOAEL; that is, intakes above this threshold would not pose a risk of inadequacy.
-
Should uncertainty factors be used when nutrient requirements are set? The distribution around the EAR expresses uncertainty; but, perhaps, other uncertainties should be addressed more explicitly, such as extrapolations of requirements from one age or sex group to another.
How Do the Different Approaches Limit the Uses of the DRIs?
Knowing the distribution of requirements for a nutrient allows the estimation of the prevalence of inadequacy. The situation is somewhat different for adverse effects. In particular, knowing a threshold for adverse effects allows estimation of the proportion of the population at risk of adverse effects, not the proportion experiencing adverse effects.
The WIC population provides an example of differences in the usefulness of the EAR and the UL. In a study conducted to evaluate the potential benefits and risks of revising the WIC food packages (IOM, 2006b), benefit was defined as a reduction in the prevalence of inadequate nutrient intake (intakes less than the EAR), or a reduction in the prevalence of excessive nutrient intake (intakes greater than the UL), or both. Risk was defined as an increase in either of these. The analysis predicted that the revised food package would lead to a reduction in the prevalence of inadequate iron intakes, which should be measurable as a decrease in the prevalence of women with poor iron status. In contrast, despite the predicted substantial reduction in the prevalence of excessive vitamin A intakes by formula-fed infants ages 6 to 12 months, it is unclear whether a reduction in adverse effects (anorexia, hyperirritability, skin lesions) would be expected as a result of the changes in the food package.
Concluding Remarks
To identify commonalities and to align the methods better, Murphy recommended a review of the models that have been used to set nutrient requirements and ULs. She suggested that a more consistent approach to the various methods for the establishment of the DRIs could increase
their scientific credibility and their usefulness in assessing and planning intakes.
LESSONS ABOUT NUTRITIONAL RISK ASSESSMENT LEARNED FROM THE FOOD AND AGRICULTURE ORGANIZATION/WORLD HEALTH ORGANIZATION TECHNICAL WORKSHOP
Presenter: Christine L. Taylor
In her presentation, Christine Taylor provided an overview of the work carried out during a technical workshop on nutritional risk assessment from the perspective of lessons that may be relevant to this Food Forum Workshop on Nutritional Risk Assessment. She also identified special considerations that call for multidisciplinary expertise and made closing remarks.
Overview of the Workshop in Geneva
In May 2005, 18 international scientists with a range of expertise met in Geneva, Switzerland, for the Food and Agriculture Organization/World Health Organization (FAO/WHO) Technical Workshop on Nutrient Risk Assessment. The participants examined the long-standing scientific principles of risk assessment that had been established for nonnutrient substances, and taking into account the principles of nutrition science, they worked to adapt them for nutrient substances. The resulting publication, A Model for Establishing Upper Levels of Intake for Nutrients and Related Substances: Report of a Joint FAO/WHO Technical Workshop on Nutrient Risk Assessment (FAO/WHO, 2005), is available at http://www.who.int/ipcs/methods/nra/en/index.html.
Nutritional Risk Assessment Requires Adaptations of Classical Risk Assessment
From the beginning of that project, it was clear that the classical risk assessment approaches, which have been well established for nonnutrients, are useful for nutritional risk assessment but are not always directly
applicable to nutrients. They must be adapted to take into consideration a range of factors. These factors include the specific homeostatic mechanisms unique to nutrients; the metabolic and physiological differences among nutrients by age, sex, and life stage; and the need to provide nutritional risk assessment in the face of limited data. Another very important factor that distinguishes nutrients from nonnutrients is that nutrients are associated with two types of risk (dual risks) rather than just one type of risk. That is, nutrients may demonstrate a risk because of too little intake as well as because of too much intake, as shown in Figure 3-2. The interest is in estimating and characterizing both types of risk. Different data sets are used to produce the two risk curves. Despite the appearance of Figure 3-2, the curves are unlikely to be symmetrical.
Nutritional Risk Assessment Needs a Multidisciplinary Perspective
During the FAO/WHO technical workshop, it was noted that a range of expertise is essential to adapt risk assessment approaches to nutrient substances and to carry out the assessments appropriately. Bailar and
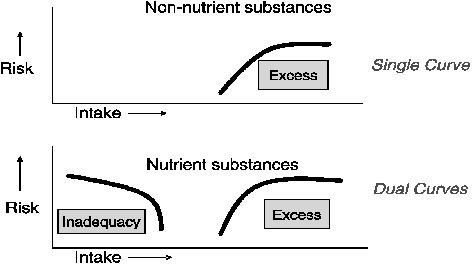
FIGURE 3-2 Comparison of the relationship between intake and risk for nonnutrient and nutrient substances. Nutrients differ from nonnutrients in that nutrients present dual risk curves (that is, risk curves for both inadequacy and excess).
Bailer (1999) referred to risk assessment as “the mother of all uncertainties.” Uncertainty is inherent because of the limited nature of the data, an incomplete understanding of the process, and the different ways of viewing the questions. An expert’s disciplinary background is likely to influence the way in which that expert views and discusses uncertainty, including variability.
Thus, nutritional risk assessment needs to include and to give mutual respect and equal weight to a wide range of experts: risk assessors, nutritionists, clinicians, physiologists, pathologists, epidemiologists, biochemists, food scientists, statisticians, and, perhaps, others. Furthermore, trained experts in risk assessment inform and facilitate the general decision-making process. As the discipline of risk assessment gains experience, the models and approaches available may evolve. For instance, in nonnutritional risk assessment, there is now some movement away from cut-point, qualitative, or policy models and toward probabilistic or quantitative models. Quantitative models portray risk as a distribution (such models require considerable data). Nutritional risk assessors may need to consider these newer models in the future.
Key Aspects of Nutritional Risk Assessment That Call for Multidisciplinary Expertise
Specific Homeostatic Mechanisms
Specific homeostatic mechanisms allow the maintenance of normal body functions in the presence of various intakes of essential nutrients. Examples include the following:
-
changes in iron stores lead to increases or decreases in the absorption of iron, as appropriate;
-
the blood calcium concentration acts in the regulation of the renal conversion of vitamin D to the active vitamin D hormone;
-
the renal excretion of calcium occurs when the concentration of the mineral in blood exceeds a threshold;
-
the liver stores excess vitamin C until the capacity of the liver is exceeded.
Appropriate multidisciplinary expertise allows careful consideration of such mechanisms in nutritional risk assessment.
Age, Sex, and Life Stage
Risk assessment for nonnutrients often considers lifetime exposures, and the risk is expressed on the basis of body weight. When nutritional risk is assessed, special consideration must be given to a person’s age, sex, and life stage. The nutrient-related metabolic and physiological states differ depending upon such factors, and these factors can result in different intake–response relationships and different adverse effects. Multidisciplinary expertise helps make it possible to address such factors appropriately.
Extrapolation or Scaling
When data on the relationship between intake and response are lacking for some age and sex groups or for people at certain life stages, extrapolation or scaling is an approach that uses data from one group to make estimates for another group. Extrapolation for nonnutrients often uses body weight as the basis; but for nutrients this approach may have drawbacks because it does not take into account intermediary metabolic rates, energy intake, or the basal metabolic rate. More appropriate approaches may use surface area or energy requirements as the basis for extrapolation. The basis used for extrapolation can have substantial effects on the estimates that are made. Multidisciplinary expertise may help with the selection of the best approach to extrapolation and scaling.
Intake Assessments
Uncertainties associated with intake assessments often play a minor role in classical risk assessment. To be conservative on the side of safety, classical risk assessment may use large correction factors (called default options in Chapter 2). When the correction factors are large, the biases or errors associated with intake estimates are less relevant. In the case of nutrients, however, the use of large safety or correction factors is seldom an option because they could lead to an upper level that is too low to ensure an adequate intake. Therefore, in nutritional risk assessment, careful
attention should be directed to the nonsystematic bias, errors, and variability associated with intake estimates. Moreover, to obtain an estimate of total intake, it may be necessary to combine intake data from different sources, thereby introducing additional challenges. Such challenges call for the nutritional risk assessment team to include individuals with various types of expertise.
Other
In general, data have not been systematically generated for nutritional risk assessment. Instead, nutritional risk assessors must usually rely on secondary data obtained from studies of the benefits or mechanisms of action of nutrients. The guidance and criteria needed for decision making in the face of limited data call for the use of a multidisciplinary approach to nutritional risk assessment. Because concerns about protection of the public’s health may drive the final decisions that are made about nutritional risk, the inclusion of individuals with a diversity of expertise helps ensure that the most appropriate questions will form the basis for a systematic review of the data.
Terminology also presents a challenge. Terms such as “hazard,” “dose,” and “exposure” have different meanings or may be troubling or perplexing to experts from different disciplines. The milieu of a multidisciplinary approach helps overcome this challenge.
Concluding Remarks
In her closing comments, Taylor pointed out that the need to view nutritional risk assessment through a number of perspectives is underscored by the Hindu parable about six blind men who went to see the elephant. Each man was certain that he knew the nature of the elephant, but in fact, each had touched only a part of the animal. Therefore, each man was partly correct and partly incorrect in his assessment. One of the most important lessons learned during the Geneva workshop was that nutritional risk assessment requires the involvement of experts from many fields and the establishment of an environment that respects and gives equal weight to each one.
EXPANDING METHODS USED IN NUTRITIONAL RISK ASSESSMENTS
Presenter: Barbara Petersen2
Nutrient intake by consumers is undergoing change. In the past, huge swings in the intakes of some nutrients occurred seasonally, diets may not have been adequate for optimal health, and the focus of assessment was on deficient intakes of a relatively small number of nutrients. Today, however, there is reason for concern about both excess and deficient intakes, interest in the wide array of beneficial components of food has grown, there are fewer seasonal differences in food patterns, consumers want improved foods without adverse effects on the taste of the foods or on health, and food processors can readily modify foods. Such changes call for a review of assessment methods. Barbara Petersen provided an overview of current assessment methods, described a tool (the Beneficial Utility Index) that is being designed to quantify the relationships between benefits and risks at different intake levels, and summarized the reasons for the use of such an index.
Overview of Current Assessment Methods
Some background information is helpful in understanding the extent to which classical risk assessment paradigms are useful in nutritional and functional food evaluations, whether they are holding back progress (and if so, how), and unique aspects of safety assessments for nutrients.
Classical Risk Assessment Methods
Classical risk assessment methods incorporate many variables and assumptions to quantify exposures and risks. The methods, with some modification, should be useful for evaluating nutrients. A quick look at the differences in methods used for toxic substances and pharmaceuticals will help guide the selection of the best method for nutrients.
Toxic substances Because toxic substances are presumed to offer no benefit to consumers, interest in the public health outcomes of exposure to toxic substances calls for the substances to pose no risk to the consumer. The results of tests conducted in animals predominate in assessments of the risk of potentially toxic substances. Thus, the risk assessment process is designed to be very conservative (erring on the side of safety) and to use worst-case scenarios for potential toxic responses. In addition, standardization of the assessment methods is important so that policy decisions are consistent.
Pharmaceuticals Because pharmaceuticals are presumed to provide consumer benefit, some adverse effects may be acceptable. Testing of the substance is done in both animals and humans. Human studies are essential for assessment of the potential risks of pharmaceuticals. Risk assessments are less conservative, and information about the benefits and the risks is communicated to consumers.
Safety Assessments
Typically, safety assessments involve a simple comparison of an Acceptable Daily Intake (ADI), which is estimated using animal toxicology or human studies and expressed per kilogram of body weight per day, with an Estimated Daily Intake (EDI), which is determined per kilogram of body weight per day. If the ADI is greater than the EDI, the substance is acceptable. If the ADI is less than the EDI, either the substance is unacceptable or further research is required.
Nutritional Assessments
Nutritional assessments are more complicated than classical risk assessments because they try to balance benefits and risks. Various mechanisms affect the absorption, metabolism, storage, and excretion of nutrients. The risks and benefits may differ according to the underlying biochemical mechanisms, the time frames of exposure, and the subpopulations who consume the nutrient. Because of potential adverse outcomes to the health of the public, attention must be directed to the quality of nutrient intake estimates: What are the effects of overestimating or underestimating intakes? How can the estimates be improved? How can
descriptions of the quality of the intake estimates be improved? Other questions related to nutritional risk assessments include whether nutrients should be treated more like toxic compounds or like pharmaceuticals and how to consider both the benefits and risks that nutrient intake may pose.
Many, if not all, nutrients are essential and also potentially toxic; they pose risks (e.g., nutrient deficiency) at low intake as well as at high intakes (e.g., nutrient toxicity). Typical plots of these dual risks, as in Figure 3-2, have a number of limitations. In particular, they
-
do not address the degree of variability in dietary intakes,
-
do not balance the severity of the adverse effects of selected intakes of a nutrient against the biological benefits of the nutrient, and
-
do not help describe the benefits and adverse effects that operate over very different time scales or by different biochemical mechanisms.
Beneficial Utility Index
The Beneficial Utility Index (BUI) is a tool proposed for use for quantification of the relationship between the benefits and the risks of nutrient intake under different intake scenarios.3 The tool is intended to be useful in addressing two desired public health outcomes both in the short term and over a lifetime: (1) maximizing efficacy (benefits) and (2) minimizing toxicity (harm). The tool would also help provide an understanding of situations that affect the ratio of benefit to harm.
Data and Models Used
The BUI is designed for use with populations and not individuals. It incorporates dose–response curves for benefits (for example, the impact of either reducing a nutrient deficiency or improving nutritional status), dose–response curves for harm, and weighting factors. The most important or the most sensitive effects (markers) to be included in the model must be prioritized. Examples of markers for the benefits of increasing
the intake of a food component include the concentrations of nutrients in the blood, enzyme activities, antioxidant function, and platelet status.
The BUI considers sources of variability, such as short-term and long-term dietary practices (including those affecting the bioavailability of the nutrient) and differences in individual requirements for the nutrient.
Beneficial Utility Index Curves
Two curves are used to develop the BUI: (1) the benefit–efficacy distribution curve, which is a distribution of the probability of the occurrence of the defined benefit at different intake levels, and (2) the toxicity distribution curve, which shows the distribution of the probability of the occurrence of negative consequences. These curves are combined mathematically to compute the BUI, which is depicted in Figure 3-3.
The next step is weighting the BUI to reflect the severity of the outcomes that result from a deficiency of a nutrient (that is, the degree of potential benefit of improving nutritional status), the severity of the toxic effects from the nutrient, the amount of uncertainty in the data, the biological variability in the population, and the variability in intake of the nutrient.
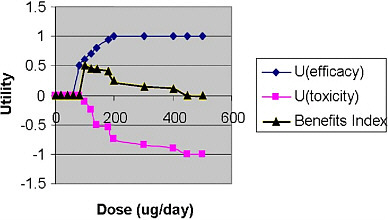
FIGURE 3-3 Beneficial Utility Index. U = utility.
Hypothetical Example Using a Beneficial Utility Index
Petersen presented an example in which hypothetical nutrient GYX had been shown to improve brain development in children and to be essentially free of adverse effects except for an occasional skin rash that is reversible with a decreased intake of GYX. In this example, a literature review revealed that a similar hypothetical compound, GYZ, causes diarrhea at elevated doses, which is a reason for concern. Data regarding GYZ could be used to derive weighting factors at different doses, which could be applied to the index. Methods can be used to incorporate uncertainty into the efficacy and toxicity curves and, thus, into the BUI, as depicted in Figure 3-4.
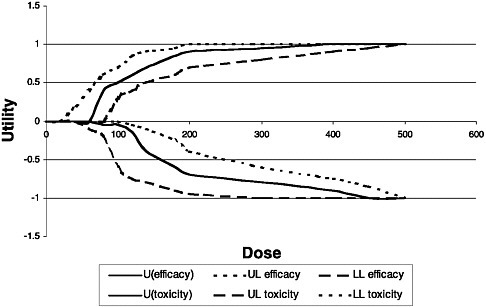
FIGURE 3-4 Impact of the BUI: Incorporating the uncertainty into the efficacy and toxicity curves: example of the impact on the BUI by using hypothetical nutrient GYX. U = utility; LL = lower limit; UL = upper limit.
Concluding Remarks
Petersen took the position that nutrients and other beneficial components of food need their own tool for use in the evaluation of their risks and benefits. The tool needs to include both benefits and risks (likelihood and severity) and to allow an understanding of the dose–response, the timing of consumption, differential mechanisms of action, and consumer compliance with dietary guidance. The potential advantages of a utility index include the ability to (1) quantify the relationship between benefits and risks, (2) understand uncertainty and variability more completely, and (3) incorporate additional factors to weight the results.
KEY CHALLENGES IN RISK-BASED APPROACHES TO NUTRITION POLICY
Presenter: Gregory Paoli
Risk assessment is a processing tool. Risk assessors process evidence to generate statements of the probability of individual events. They combine these probabilities to determine the probability of an adverse outcome of interest. According to Gregory Paoli, the primary value of risk assessment lies in its ability to infer the probability of adverse outcomes by appropriately combining a formal representation of the risk-generating system with the rules of inferring probability. He also noted that the approach used to set the EAR, which uses distributions of inadequacy, involves elements of risk assessment.
Risk-Based Advice
Risk-based advice may be based on a formal risk assessment or on a safety assessment.
Formal Risk Assessment
Formal risk assessments may be conducted on the basis of two rationales: a practical rationale or a public policy rationale.
Practical rationale Two features provide a practical rationale for conducting formal risk assessment: the process (1) allows the management of problems of overwhelming complexity and (2) provides links to appropriate tools. The complexity relates to multiple hazards, multiple pathways, multiple agents, multiple outcomes, and cascading events, all in the face of much uncertainty. Probability provides a multidisciplinary language. This makes it possible to use the tested tools of the decision sciences and of the risk and reliability sciences to help address the problem.
On the other hand, risk assessment may require too much time and too many resources to solve a problem. It may prevent decisions from being made.
Public policy rationale In matters of public health, there is a desire for the use of a rationale that calls for decisions to meet a standard of reasonableness. What one is accountable for may determine whether a risk assessment or a safety assessment is the preferred approach.
Safety-Based Standard Setting
Safety-based standard setting differs substantially from risk assessment. The safety-based standard setting approach includes no estimate of the probability of harm and ordinarily includes no exposure assessment. One cannot use the safety assessment approach to predict risk, even when an exposure assessment has been completed. ULs represent the values set by using safety-based standard setting. The UL value (for example, 2 grams of a certain nutrient per day for adults) includes uncertainty, variability, uncertainty about the variability, and value judgments. It can be an adequate risk management tool, but it does not represent the outcome of a risk assessment.
Basic Elements for Risk-Based Advice
According to Paoli, three elements are necessary for use of the term risk-based advice:
-
Decisions are based on explicit knowledge and a description of the full risk-generating system, including hazards, pathways, and outcomes.
-
The process includes measurements on the dimensions of likelihood and the magnitude of consequences.
-
Decisions are based on their capacity to reduce risk at reasonable cost.
Such advice is needed for complex situations involving a variety of risks, for example, the multiple risks facing a certain group of people, such as the Inuits of northern Quebec, Canada.
Disability-Adjusted Life Years
The concept of disability-adjusted life years attempts to describe the burden of disease by considering the duration and the severity of health outcomes. This concept can be combined with data on the frequency of occurrence of the specific disease to become a useful part of risk assessment. Paoli encouraged the use of this approach.
Risk Communication
Risk management may result in a communication product (the analogy that Paoli gave was an air quality health index) that results in behavioral change. When risk is mitigated through a communication product, it would be useful to extend the risk assessment paradigm to include an estimate of the amount of risk reduction that results from communication of the risk.
Concluding Remarks
Reasonableness is a complex combination of the evidentiary, managerial, and obligatory aspects of decision-making processes. Risk assessment provides a structure for providing these elements in a defensible way. Risk assessment also opens a large multidisciplinary tool kit for analysis, clear and succinct presentation of the knowledge, and decision making.
COMMENTARY
Discussant: Sanford A. Miller
In his brief commentary, Sanford Miller highlighted a few of the challenges facing nutritional risk assessment and proposed several steps for addressing the challenges.
Challenges for Nutritional Risk Assessment
Classical risk assessors address the risks associated with single very small events and thus tend to think in terms of precision and to have models with high specificities. In contrast, those who assess the risks of nutrient substances must take into account the fact that the substance may affect multiple systems or organs. How can risk assessors factor the multiple effects into a risk assessment? In addition, how can they address both the risk and the benefit of the same substance? Many of the default decisions made in nutritional risk assessment have been made on an ad hoc basis, differing from substance to substance, dose to dose, and expert group to expert group.
Clearly, the inclusion of a variety of individuals with diverse skills is important in nutritional risk assessment. It can be challenging, however, to gather all the experts needed to conduct an appropriate risk assessment. Moreover, it often is difficult for the experts to understand each other.
Possible Steps to Address the Challenges
Among the steps that Miller proposed are the following:
-
Agree on a set of criteria for the selection of outcomes to be used in a nutritional risk assessment.
-
Agree on default options to be used to address uncertainties.
-
Develop training programs in the area of nutritional risk assessment. Such academic programs would need to cover all or most of the areas that are used in the risk assessment process.
OPEN DISCUSSION
Moderator: Molly Kretsch
The key points that were raised in the open discussion have been incorporated into Chapter 6, Perspectives on Challenges and Solutions: Summary Remarks and Suggested Next Steps.
























