5
New Developments in and Challenges to Nutritional Risk Assessment
A number of new developments can have an impact on nutritional risk assessment. In this session, moderated by Robert L. Buchanan, John Milner addressed the roles of nutrigenomics and population variability in nutritional risk assessment and challenges that these factors present; Amy Subar reviewed dietary intake assessment methods, and described innovative methods to collect and analyze food and nutrient intake data; and Joanne Holden described the major U.S. source of food composition data, the expansion of these data, and uncertainties and data needs. No open discussion occurred at the end of the session.
ROLES OF NUTRIGENOMICS AND POPULATION VARIABILITY IN NUTRITIONAL RISK ASSESSMENT
Presenter: John Milner1
John Milner described nutrigenomics as a three-phased prong that involves nutrigenetics, nutritional epigenetics, and nutritional transcriptomics (Figure 5-1). In his presentation, Milner focused on genomics, the very wide variability among individuals seen in study results, other complicating factors, and the potential value of genetic information.
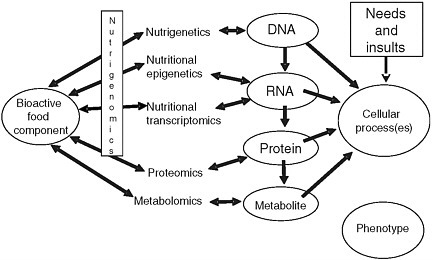
FIGURE 5-1 Interrelationships of bioactive food components, nutrigenomics, and other factors that influence cellular processes.
Genomics and Variability
Genomics is an exceedingly complex topic, in part because there are approximately 30,000 genes and 8 million to 10 million single-nucleotide polymorphisms (SNPs). Few intervention studies, however, have truly tested the importance of SNPs to gene expression. Most of the evidence about the effects of diet as an environmental stimulus on gene expression comes from observational studies of differences in the patterns of deoxyribonucleic acid (DNA) methylation in response to folate intake. Issa et al. (2001), for example, showed a 300-fold variation in the methylation pattern among individuals.
An individual’s response to dietary interventions will depend in part on his or her genetic background (nutrigenetic effects), the cumulative effects of food components on genetic expression profiles (nutritional transcriptomic and nutritional epigenomic effects), the occurrence and activities of proteins (nutritional proteomic effects), and the dose and temporal changes in cellular low-molecular-weight compounds (metabolomic effects).
The examination of epidemiological data from studies on the relationships of food or food components to health outcomes reveals much variability in outcomes both among studies and within single studies. Examples include studies that have addressed the influence of soy on the
relative risk of breast cancer, the influence of tomato intake or plasma lycopene concentration (a potentially useful biomarker) on the relative risk of prostate cancer, and the influence of tomato and tomato product intake on serum lycopene concentrations. The wide variability in outcomes suggests that a number of factors may be involved, such as the region of the world where the study was conducted, the inclusion of both premenopausal and postmenopausal women, the form in which the product was consumed, and genetic differences among the subjects. Milner suggested that a solution may result from an increased understanding of genomics and of the associated genetic processes and how they affect the overall phenotype of an individual.
Other Complicating Factors
The Complex Nature of Food
Approximately 25,000 bioactive food components exist in the overall food supply. This leads to the question, “Which of the many dietary components are the most important for evaluating nutritional risk?” Candidates include essential nutrients but also many types of nonessential food components:
-
phytochemicals, such as carotenoids and isothiocyanates;
-
zoochemicals, such as conjugated linoleic acid and omega-3 fatty acids;
-
fungochemicals, especially compounds with biological activity that are found in mushrooms; and
-
bacteriochemicals, which are products formed during fermentation and compounds resulting from the action of the intestinal flora.
The prioritization of dietary variables poses a substantial challenge.
Microbes
Microbes account for about 9 percent of the cells in the human body, but ordinarily they are overlooked in studies. Mammalian genes can af-
fect the actions of some microbes that grow in the gastrointestinal tract. Among a large percentage of individuals of Asian descent, for example, gastrointestinal microbes metabolize soybeans to form equol, a substance that possesses anticancer properties (Decroos et al., 2005, 2006); but the occurrence of equol production varies widely among individuals and populations (Setchell and Cole, 2006).
Amounts and Times of Administration of a Food Component
In an intervention trial, Duffield-Lillico and colleagues (2002) found that the response to supplemental selenium differed by baseline selenium status. If the time of dietary exposure differs—before or after age 55 years, for example, in the study by Limburg et al. (2005)—the dietary exposure may create a different response among individuals. Moreover, a study by Swami and colleagues (2005) suggests that dynamic interactions among different food components may influence the quantity of a substance needed for a response.
Potential Value of Genetic Information
Genetic information may assist the nutritional risk assessment process in a number of ways, including the following:
-
the identification of people who must achieve minimum nutrient intakes to reduce their risk (e.g., see Wong et al., 2003);
-
the identification of those people who would benefit the most and those who would benefit the least from dietary change (e.g., see Ordovas et al., 2002);
-
the identification of those at risk of a specific adverse effect (e.g., see Rapuri et al., 2001);
-
the formulation of appropriate interventions; and
-
improved interpretation of study results (e.g., see Yang et al., 2001).
For people with specific genetic polymorphisms, a study by Ahn and colleagues (2004) suggests that the benefits of the intake of fruits and vegetables may appear once a threshold intake is exceeded. Ordovas and
coworkers (2002) reported that setting an intake goal may decrease the risk in some individuals but increase the risk in others.
Concluding Remarks
In conclusion, Milner stressed that (1) understanding nutrigenomics is fundamental to establishing sensitive and reliable biomarkers that will be useful in assessing risk, (2) effective communication is needed so that consumers can understand the relevance of their own genetics to protecting their health, and (3) a bioethical framework must be upheld to prevent discrimination.
MEASURING USUAL DIETARY INTAKE FOR RISK ASSESSMENT
Presenter: Amy F. Subar
The precision of any dietary risk assessment depends on the strength of the intake assessment. Amy Subar addressed the strengths and the limitations of major dietary intake assessment methods and introduced some innovative methods for the collection and analysis of data.
Major Intake Assessment Methods Used in Population Studies and Their Strengths and Limitations
The major intake assessment methods include food records or diaries, 24-hour dietary recalls, and food frequency questionnaires (FFQs). Subar also mentioned methods that use biomarkers.
Food Records or Diaries
Food records or diaries require the subject to record all foods that he or she consumed, including their amounts. The methods used to obtain food records or diaries vary considerably. For example, investigators may or may not train the respondents, conduct a detailed review of the
records, or use highly standardized coding rules. Some investigators have developed electronic methods involving personal digital assistants or cell phones.
Strengths Food records and diaries provide detailed, quantified information about intake that could be relatively accurate. The data are rich with regard to information about nutrient intake, cooking practices, and meal and eating frequencies.
Limitations A major limitation of food records or diaries is that the process of recording food intakes influences the diet and may thus introduce bias. Moreover, the method requires literacy, imposes a large burden on both the respondent and the investigator, and is subject to sample selection bias. Recording becomes even less complete over time, and underreporting is typical, especially for overweight or obese respondents.
Twenty-Four-Hour Dietary Recalls
The 24-hour dietary recall method is widely used in dietary surveillance. The interviewer asks the respondent questions to obtain information on the types and the amounts of all foods that the respondent consumed during a defined 24-hour period. Variations in the approach used to obtain 24-hour recalls include the extent of the training of the interviewers, the standardization of probing questions, the method of administration, and the use of aids to estimate portion size.
Strengths The strengths of 24-hour recalls are similar to those of food records or diaries, with two additions: the recalls (1) do not affect eating behavior and (2) are subject to less sample selection bias than are food records or diaries.
Limitations A key limitation of the 24-hour recall method is that subjects have imperfect knowledge and memory of their food consumption. Recalls are costly to administer, and a single day’s recall is insufficient to estimate one’s usual intake (the theoretical long-run average daily intake) of a dietary component. As with food records, reporting may be less complete with subsequent recalls, and underreporting is typical.
Food Frequency Questionnaires
FFQs address the need for a feasible method to collect dietary intake data in large studies. FFQs vary in the foods that they include, the methods of addressing portion size and food preparation, the period covered, and other characteristics. Different methods are used to develop the food list and the corresponding nutrient database. The food list and database may be developed with a specific population subgroup in mind.
Strengths The strengths of FFQs include their low respondent burden, their attention to the usual individual intake of foods with one administration of the FFQ, the low cost of administration and processing of the FFQ, and the lack of an effect on eating behavior.
Limitations FFQs lack detail, require literacy, are cognitively complex, and are subject to severe measurement error. Different FFQs may perform differently, and different populations may respond differently to the same FFQ.
Biomarkers
If the presence of a biological compound had a strong relationship to the intake of a nutrient, it could provide the basis for a useful method to assess the intake of that nutrient. A recovery biomarker is one that represents a one-to-one relationship. (Doubly labeled water and urinary nitrogen are two examples of a recovery biomarker.) A concentration biomarker reflects the biological response to the intake of a nutrient in terms of a complex metabolism. (Serum selenium and serum ferritin are examples of concentration biomarkers.) Concentration biomarkers cannot be used to assess the amount of a nutrient consumed, but they do correlate with the amount consumed. Some forms of nutrients in the body have no relationship to nutrient intake at all because their concentration is closely regulated by physiological processes.
Innovative Methods of Collecting and Analyzing Intake Data
Investigators are exploring the automation of instruments to simplify and reduce the costs of data collection. Such instruments include World Wide Web-based FFQs and 24-hour diet recalls. In addition, the National Cancer Institute (NCI) has developed an approach that combines dietary assessment methods to improve overall estimates of population intake distributions and individual intakes. With the NCI method of analyzing intake data, the strengths of the two methods may be complementary.
Automated Methods of Data Collection
Subar described the NCI’s prototype for an automated self-administered 24-hour recall (ASA24). It is intended to be user-friendly. For example, it allows the user to search and browse for foods, includes pictures for the identification of foods and the estimation of portion size, lists all the selections made, and asks questions. NCI’s vision is that ASA24 will be a complete system for probing, coding, and analysis that can be updated easily and that is publicly available on the Web. It would also be adaptable to other languages. Modeled after the dietary surveillance system in the National Health and Nutrition Examination Survey, it would allow the collection of multiple 24-hour recalls at a minimal cost. Among the next steps in the development of ASA24 are cognitive testing and validation against interviewer-administered recalls.
National Cancer Institute Method to Estimate Usual Intake
To estimate the proportion of the population with intakes above or below a particular cutoff point, one needs to estimate the usual intake distribution for the population. To do this, investigators have used data from 24-hour recalls or from food records and statistical modeling (typically, the National Research Council method [NRC, 1986] or the Iowa State University method [Nusser et al., 1996]). These statistical methods are well established for most nutrients and for foods consumed daily by nearly everyone. The methods are less useful for estimation of the usual intake of foods consumed episodically, especially because of the large number of zero intakes.
In response to the challenge, the NCI method decomposes usual intake as follows:
The relative frequency of nonzero 24-hour recalls provides information about the consumption probability. Because the amounts consumed on consumption days are always positive, standard statistical methods can provide the distribution of the usual intakes on the consumption day. Combining both distributions produces an estimate of the distribution of usual intake.
The NCI method for foods uses a two-part nonlinear mixed model to correlate the intake probability and the intake amount. The use of covariates allows direct evaluation of the effects of covariates on usual intake and helps correct for measurement error. The Food Propensity Questionnaire, an FFQ that asks about the long-term frequency of intake but not about portion sizes, could be used as a covariate in the NCI model. This use of the Food Propensity Questionnaire in conjunction with the 24-hour recall removes concerns about the bias introduced by FFQs when they are used to estimate absolute intake. Moreover, combining data from the 24-hour recalls and the Food Propensity Questionnaire may substantially improve the estimates. Further information on these methods is published in The Journal of the American Dietetic Association (Dodd et al., 2006; Subar et al., 2006; Tooze et al., 2006).
Concluding Remarks
Subar concluded by emphasizing that good estimates of usual intakes are necessary for risk assessment and that good models with which to make such estimates are available. Continued research on methods and analytical techniques for estimating intakes is needed to improve nutritional risk assessment.
NUTRIENTS AND FOOD COMPOSITION: DATA NEEDS
Presenter: Joanne M. Holden
The assessment of the intake of dietary components requires information on the composition of foods and of supplements. Sources of food composition data include the U.S. Department of Agriculture (USDA), the food industry, developers of secondary databases, databases in other nations and regions, the scientific literature, and limited food analyses conducted for specific studies. Joanne Holden described the work of USDA’s Agricultural Research Service in relation to food composition data, highlighted the collaborative nature of the National Food and Nutrient Analysis Program (NFNAP), and concluded by identifying data and resource needs.
Current Status of USDA’s Food Composition Data
As shown in Figure 5-2, data flow into the USDA nutrient databank from many sources. After the data that are received undergo review, evaluation, and estimation, USDA releases the information in a number of different forms. The most notable of these forms is the National Nutrient Database for Standard Reference (SR), which is the authoritative source of food composition data for the United States. This database covers a wide array of foods (more than 7,200), including agricultural commodities, processed and prepared foods, formulated foods, selected brand name foods and fast foods, candies, and beverages. The 19th edition of the database (SR19) includes both original analytical data and calculated data, giving values for up to 140 nutritional components. The nutrient composition of each food is presented for various weights and measures (e.g., 100 grams, one-half cup, one medium piece [of a specified weight] of the food). Recently, the nutrient units used (e.g., dietary folate equivalents) have been harmonized with those used for the Dietary Reference Intakes. The data are available at www.ars.usda.gov/nutrientdata.
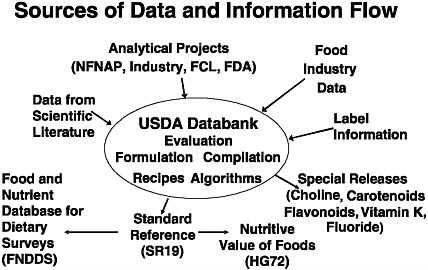
FIGURE 5-2 Sources of data and information flow for the U.S. Department of Agriculture’s nutrient databank. NOTE: FCL = Food Composition Laboratory, FDA = Food and Drug Administration, NFNAP = National Food and Nutrient Analysis Program, USDA = U.S. Department of Agriculture.
The databank is expanding to include nonnutrients, new sources of information about traditional nutrients, specific forms of food components (e.g., the carotenoid lutein), and compounds used in large amounts in foods or dietary supplements. Among the substances added over the past few years are five subclasses of flavonoids, trans-fatty acid, choline, proanthocyanidins, added sugars, and fluoride. Some of these substances appear in special-interest databases. Extensive product reformulations (such as in response to concerns and regulations related to trans-fatty acids) sometimes require time-consuming updates.
Efforts are under way to develop fast and accurate high-capacity nutrient profiling methods that use food extracts. Intended uses include the categorization of foods, the identification of food components, and the quantification of the amounts of selected components.
The goal is for the SR data to be representative of the nutrient profile of the national food supply and to be applicable to populations and various subpopulations and to clinical and metabolic studies. USDA makes the data available in several electronic forms, which provide numerous advantages over printed volumes.
National Food and Nutrient Analysis Program
The NFNAP is a cooperative effort that is supported by many institutes of the National Institutes of Health (NIH); the Food and Drug Administration; and a number of other partners from the U.S. Department of Health and Human Services, USDA, the food industry, and universities. The NFNAP effort includes the identification of key foods and nutrients for analysis, the evaluation of the quality of existing data, the development and implementation of a plan for the nationally based sampling of foods, the analysis of sampled foods using valid methods and rigorous quality control procedures, and the compilation and dissemination of representative estimates, including variability estimates.
Key foods are defined as the list of foods that provide about 75 percent of the dietary intake of a specific food component. Some foods are key foods for several nutrients. Examples of key foods include fluid milk (both milk with 3.25 percent milk fat and milk with 2 percent milk fat), whole chicken eggs, and pizza. The dynamic nature of the U.S. food supply calls for periodic updates of key foods. For example, recent work on the selenium content of ground beef found that the variation in selenium content by geographic region was less than expected. Currently, work is under way through NFNAP to sample and update the information on 105 foods.
Uncertainties Regarding the Composition of Foods and Supplements
The variance in the composition of foods is a function of the variance of components inherent to the food and of the variance associated with the method used to analyze the food. Important issues related to the compositions of foods and supplements include the statistical bias that occurs with changes in the food or supplement formulation, the variability in composition, the validity of the analytical methods, the actual fortification levels, and the specificities of the descriptions of the reported foods. The assessment of the composition of selected fortified foods has revealed some rather large discrepancies between label claims and the actual content determined on analysis.
USDA, the Office of Dietary Supplements at NIH, and others are conducting pilot research on the compositions of dietary supplements. They are developing a dietary supplements ingredients database with
selected analytical verification of the data in the database. It will become available on the Web and initially will include data on the ingredients in vitamin and mineral preparations, amino acid preparations, fortified foods, and beverages. Later, it will include data on the ingredients in herbal and other botanical products.
Concluding Remarks
Holden’s closing remarks focused on food composition data needs and the need for resources. Key data needs include the following:
-
new food composition data to match changing food consumption patterns,
-
estimates of the contents of food components that are important to the public’s health,
-
estimates of variability in foods (such as foods of different cultivars or from different geographical locations), and
-
more specific data for brand-name products.
In addition, there is a need for valid and robust analytical methods and for reference materials for quality control. Sustained federal funding, especially from USDA but also from NIH and other federal departments and agencies, is critical to support the acquisition, compilation, and analysis of food composition data. Continued collaboration with the food industry is needed to obtain current data about the compositions of products, support for sampling and analysis, and support for the dissemination of the data.














