5
Enhancing Postmarket Safety Monitoring
|
Recommendations for Enhancement of the Postmarket Surveillance System from the IOM Report The Future of Drug Safety: Promoting and Protecting the Health of the Public Recommendation 4.1 The committee recommends that in order to improve the generation of new safety signals and hypotheses, CDER [Center for Drug Evaluation and Research] (a) conduct a systematic, scientific review of the AERS [Adverse Event Reporting System], (b) identify and implement changes in key factors that could lead to a more efficient system, and (c) systematically implement statistical-surveillance methods on a regular and routine basis for the automated generation of new safety signals. Recommendation 4.2 The committee recommends that in order to facilitate the formulation and testing of drug safety hypotheses, CDER (a) increase their intramural and extramural programs that access and study data from large automated healthcare databases and (b) include in these programs studies on drug utilization patterns and background incidence rates for adverse events of interest, and (c) develop and implement active surveillance of specific drugs and diseases as needed in a variety of settings. Recommendation 4.6 The committee recommends that CDER build internal epidemiological and informatics capacity in order to improve the postmarket assessment of drugs. |
A conclusion of the IOM report was that the FDA’s current postmarket surveillance system is neither as comprehensive nor as systematic as it needs to be to detect, interpret, and analyze safety signals effectively and efficiently. The current system relies primarily on data collected through passive surveillance. These data are housed in the
Adverse Event Reporting System (AERS) database, which comprises three datasets: adverse event data reported voluntarily through MedWatch (generally by physicians, other health care practitioners, and consumers), mandatory periodically reported data from product manufacturers, and mandatory 7- and 15-day expedited report data from manufacturers following notification of a serious and unexpected adverse event. While this passive surveillance system may be capable of detecting rare serious adverse events, it has several limitations, including profound underreporting, biased reporting, and difficulties in attributing an adverse event to a specific drug. Additionally, when analyzing postmarket epidemiological data collected through passive surveillance, it is difficult to know just how many people have taken a drug (i.e., to determine a denominator), it is difficult to know how many events occurred (i.e., to determine the numerator) because of underreporting, and therefore it is difficult to conclude the rate at which an event would take place (e.g., event x would occur in 1 of every 100,000 persons).
Discussion at the symposium focused on how to enhance the current postmarket safety monitoring system by implementing the recommendations of the IOM report listed above, including upgrading AERS, developing an active surveillance system based on automated health care databases, and building internal epidemiological and informatics capacity at the FDA. Multiple panelists expressed the view that rejuvenating the passive surveillance system and augmenting it with an active system would be a specific feasible next step toward a stronger and more effective drug safety system.
FDA INITIATIVES FOR IMPROVING DRUG SAFETY MONITORING1
Revamping the AERS System
Dr. Dal Pan described several initiatives to enhance the FDA’s current postmarket safety surveillance system. In response to Recommendation 4.1 of the IOM report (improving the generation of new safety signals and hypotheses), the Center for Drug Evaluation and Research (CDER) plans to upgrade to AERS II by adding functionalities that will allow for signal tracking, signal management, and data mining capability. Although the project is currently unfunded in the 2007 budget, the agency is evaluating system requirements and estimates the system could be operational in about 2 years once funded.
Enhancing the Agency’s Use of Observational Data
In fiscal year 2008, in addition to initiating the development of guidance on best practices for observational pharmacoepidemiological studies, CDER plans to expand its intramural capabilities to use observational data by hiring additional epidemiologists trained in the methods of analytical observational epidemiology, statistical programmers, and properly trained statisticians capable of working on complex statistical and epidemiological issues. Pilot programs are currently being conducted with the Centers for Medicare and Medicaid Services (CMS) and the Agency for Healthcare Research and Quality (AHRQ) to examine the feasibility of using CMS data and to identify the practical barriers involved. Expanding this effort would require increased resources. Additionally, the FDA plans on issuing a request for proposals for evaluation of adverse event reporting and inviting outside groups to study such reporting so as to determine how to maximize its public health impact.
The Sentinel Network
Another agency-wide initiative being considered is the formation of a Sentinel Network.2 In March 2007, the FDA sponsored a meeting of experts from all sectors involved in postmarket safety monitoring to discuss how the public and private sectors could work together to generate and use safety data most productively. Panelists noted the difficult informatics and methodological challenges to integrating public- and private-sector postmarket safety monitoring systems. Among the multitude of ways postmarket safety data could be used, the panelists agreed on three areas appropriate for active surveillance: (1) monitoring for specific adverse events related to a particular drug (based on pharmacological data and possible signals from clinical trial data); (2) monitoring for adverse events of concern with any drug, especially those events that have a low background rate and are often drug-induced, such as aplastic anemia or acute liver failure; and (3) monitoring for unexpected adverse events.
PUBLIC–PRIVATE PARTNERSHIP FOR DEVELOPING A NATIONAL ACTIVE SURVEILLANCE SYSTEM3
The FDA is planning initiatives aimed at responding to Recommendation 4.6 of the IOM report (developing an active surveillance system based on automated health care databases). These initiatives are aimed at the development of an active surveillance system that could be used to access data that already exist, looking for patterns of adverse events that may be related to specific drugs that would not be noticed through passive surveillance. However, Dr. Dal Pan noted that this effort will require additional funding, including significant resources for developing, testing, and validating such a system.
Dr. Dal Pan discussed CDER’s pilot work with CMS, in conjunction with AHRQ, to gain experience with large databases that could potentially be useful for an active surveillance system and identify practical barriers to gaining access to such databases. While this work is currently focused on epidemiological analysis of specific drug safety questions (and not on active surveillance), FDA epidemiologists have learned much about the CMS system. Although CDER already works with four outside organizations, the FDA is interested in increasing this number (adding, for example, other federal agencies such as AHRQ, the Department of Veterans Affairs [VA], and the Department of Defense [DoD]), as well expanding the funding allocated to each organization. Dr. Dal Pan noted that this expansion would require increasing FDA staffing resources, as well as financial resources for contracts to manage the programs. The FDA is also interested in gaining broader access to drug utilization data (CDER already accesses a large number of such databases but does not have access to some types of drug use data, such as data on drugs administered in outpatient clinics).
Considerations for Creating an Active Surveillance System
Dr. McClellan suggested that, while many of the FDA-proposed changes to the postmarket safety monitoring system that were discussed by Dr. Dal Pan and that are included in current legislative proposals may be beneficial for improving the U.S drug safety system, they are still based primarily on drug-by-drug or manufacturer-by-manufacturer approaches.
The proposed changes do not involve the type of routine, systematic collection and analysis of use and outcome data necessary for the system to progress. For example, pending legislation supports the additional use of existing electronic population-based databases, but there is no comprehensive infrastructure in place to link the various databases. In short, while steps are being taken in the right direction, they are not enough.
Dr. McClellan pointed to several key questions that should be taken into account in planning a national active surveillance system to monitor drug safety. Will the proposed system have the greatest impact on reducing the likelihood of the recurrence of a drug-induced serious adverse event (for example, a Vioxx-induced myocardial infarction or increased risk of suicide due to a selective serotonin reuptake inhibitor [SSRI])? In particular, will the new system have greater capacity to identify potential risks and areas in which heightened surveillance might be warranted? Will it have greater capacity to identify safety signals and affected patients more quickly and reliably than the current system? Will it utilize existing capacity to do a much better job of identifying circumstances under which drugs are used differently than they were in clinical trials? Will it aid in targeting efforts toward the type of postmarket clinical studies needed when a safety signal is unclear? Will it accomplish these tasks without unnecessary costs?
Dr. Krall stressed that, although it is important for the FDA to play a role in the development of a national system for active surveillance of drug safety, the agency should not have sole responsibility for the effort. Moreover, it is important that each health plan, each company with an approved drug, and each regulatory authority not build its own system, a strategy that would be unnecessarily costly and inevitably increase disputes over whose findings were valid. Consequently, Dr. Krall and other symposium panelists proposed that such a system be built as a public–private partnership.
To address the issue of how to create a comprehensive rather than a piecemeal system, Dr. Platt argued that health plans—which serve large, defined populations and share many priorities with public health agencies—could play a substantial role in forming such a public–private partnership. Specifically, health plans’ administrative and claims databases, enhanced by other data, such as laboratory test results, and with access to full-text medical records (especially to electronic medical records, which are becoming increasingly available), could serve as an important resource for active surveillance. Drs. Platt and McClellan both indicated that it should be feasible to create a system with access to information on 100 million persons, a number that would provide enough statistical power to answer important safety questions quickly. They further suggested that a database system of this size could have detected a signal of
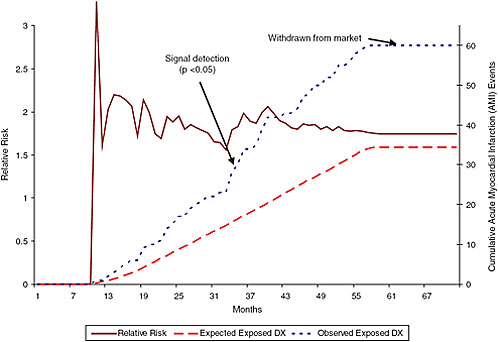
FIGURE 5-1 Monthly incidence of acute myocardial infarction (AMI) for Vioxx users.
Using data from the Health Maintenance Organization Research Network (HMORN) CERTs (Centers for Education and Research on Therapeutics) for a population of 7 million, researchers performed a retrospective month-by-month analysis of relative risk and occurrence of cumulative AMI events for new users of Vioxx in comparison with new users of naproxen. With a population of 7 million, signal detection for AMI occurred at 34 months. Dr. Platt argued that using a database of 100 million people would have enabled signal detection after 2 to 3 months.
NOTE: Comparator = naproxen. Adjusted for age, sex, health plan. DX = drug reaction.
SOURCE: Platt, 2007.
myocardial infarction risk among Vioxx recipients within a few months (Figure 5-1).
In addition to allowing for earlier detection, a large, defined population of 100 million (spread across multiple databases) could serve a critical role in follow-up. Dr. Platt used a recent public health example to demonstrate the limitations of an existing surveillance system, the Centers for Disease Control and Prevention’s (CDC) Vaccine Safety Datalink database.
The question has arisen as to whether a meningococcal conjugate vaccine (Menactra),4 approved in 2005, causes Guillain-Barre Syndrome (GBS), an inflammation of the peripheral nerves. Within 15 months following the vaccine’s approval, there were 15 spontaneous reports of GBS occurring soon after immunization. However, the Vaccine Safety Datalink database has limited capacity and has data on only about 100,000 of an estimated 5.7 million distributed vaccine doses, while the background rate of GBS is only about 1.5 events per 100,000 person-years. Therefore, it would take several more years before the Vaccine Safety Datalink would be expected to detect a signal unless the excess risk were very great. A substantially larger population would make it possible to detect safety signals much sooner. Dr. McClellan proposed a postmarket system for detecting drug risks that would have four major components, all with FDA oversight: better data, public–private collaboration, a systematic strategy for analysis, and supplemental clinical studies.
Better Data
Dr. McClellan explained that the system needs a mechanism for pooling relevant data from public and private databases on prescription use and health outcomes in a much more systematic and ongoing way than is currently done or proposed. He suggested that the infrastructure built for this purpose should make use of private health plan, Medicare, and VA/DoD data. Dr. Platt noted that data sources should include health plans’ claims data, which would exist in standard format files and be preprocessed to allow rapid queries. The system should allow access to full-text medical records, as needed, and other data, such as laboratory test results, as they become available. Dr. Walker added that everything that is paid for through the health insurance industry is recorded in its databases. These data could be organized into a chronological file and enhanced and linked with other databases, including those containing pharmacy claims data, physician and facility claims data, laboratory test results, and demographic and other consumer information (Figure 5-2). The data could then be accessed in real time and in a web-based, interactive manner. Multiple panelists agreed that using enhanced claims data, including links to full-text medical records, would be an efficient way to conduct postmarket monitoring on a real-time basis.
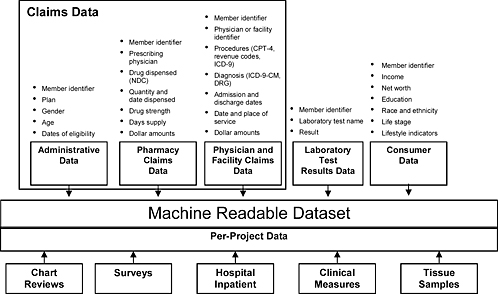
FIGURE 5-2 Proposed composition of an enhanced claims database.
NOTE: CPT-4 = Current Procedural Terminology, 4th Edition; DRG = Diagnosis Related Grouping; ICD-9 =International Classification of Diseases, 9th Revision; ICD-9-CM = International Classification of Diseases, 9th Revision, Clinical Modification; NDC = National Drug Code.
SOURCE: Walker, 2007. Copyright 2007 i3, used with permission.
Public–Private Collaboration
While the FDA is very interested in having available the type of enhanced infrastructure for postmarket safety data described above, the agency has neither the resources nor a plan to implement it. Therefore, Dr. McClellan suggested that the acquisition of better data and the implementation of a systematic strategy for analysis will require additional support for postmarket surveillance, the development of a comprehensive implementation plan, and effective public–private collaboration to carry it out. Dr. Platt described the developing Centers for Education and Research on Therapeutics (CERTs) Health Plan Consortium for Public Health, whose goal is to “improve the safety and safe use of marketed vaccines and prescription drugs by studying their use in large populations of health plan members.” The consortium could be an important component of a national active surveillance system that would include other data sources. As Dr. Tilson noted, the CERTs were created under congressional mandate and have robust processes that already make them good hosts for a public–private partnership. Plans are for partners in the consortium to include health plans, federal agencies, industry, foundations, the academic community, and the public. Federal public health agencies (e.g., CMS, AHRQ) are already collaborating with the FDA on stimulating analyses of this type, the private sector has expressed willingness to help, and academia must be engaged. The FDA currently has postmarket surveillance contracts with medical record–linked claims databases from the Health Maintenance Organization Research Network (HMORN), United Healthcare, Tennessee and Washington State Medicaid, and the VA, which collectively cover about 26 million persons. However, Dr. Platt argued that these systems are insufficient and should also include databases from Medicare (Parts A, B, and D), Medicaid from most large states, and private health plans.
As envisioned by Dr. Krall, the public–private partnership would (1) have the mandate to carry out surveillance on behalf of all stakeholders (health systems, companies, and the FDA); (2) acquire data, develop analysis methodology, and conduct and report the results of analyses, with the regulatory authority and companies interpreting the results as they do today; and (3) operate transparently, with the FDA having the right of first call on the partnership’s analytical capability. Drs. Platt and McClellan both agreed that transparency and confidentiality are critical to establishing a public–private database for use in active surveillance of drug safety. Dr. McClellan added that if non-FDA partners were to help in identifying priority questions and mechanisms for answering those questions, this would have to be done through a transparent process.
Systematic Strategy for Analysis
Once the necessary infrastructure has been established, the new system will need a strategy for conducting analyses. While this strategy could be based on expert guidance, Dr. McClellan suggested that the FDA should continue to have a central role in the effort as it will be making ultimate judgments about risks and benefits for labeling and other purposes. Dr. Krall proposed that the system should have the capability to (1) focus on the period of uncertainty following approval; (2) detect classic “drug list” events, drug- or class-specific events, increases in events with large public health consequences, and unsuspected events; (3) confirm benefit or effectiveness; (4) serve as a source of hypothesis-driven studies to validate signals; and (5) provide quantitative, real-time output. Dr. Platt asserted that prospective evaluation for “anticipated” adverse outcomes should be the primary objective of the system. “Anticipated” would include the FDA’s list of Designated Medical Events, which has been responsible for a large fraction of product withdrawals, plus other outcomes of concern because of the chemical class involved or events observed during preclinical evaluation. When asked whether more could be gained from developing and refining data mining methods that would point in unexpected directions and help in formulating the right questions, Dr. Platt agreed that this should be one of the goals of postmarket surveillance. However, the science here is much less well developed, and he maintained that the first investment should be in building a system that can deal rapidly with problems there is reason to expect.
Another question was raised about the extent to which the system would be used to go beyond signal detection and enable the quantitative evaluation of benefit. Dr. Krall replied that initially, the new system would complement existing systems by providing crucial information that is currently lacking; eventually, however, it would enable an enhanced understanding of benefit and risk. Dr. Walker added that, although epidemiologists traditionally avoid benefit studies because of their greater potential for confounding, the data needed for the purpose do exist.
Several panelists discussed the important need to study off-label use of prescription drugs. When the FDA approves a drug, it does so based upon the risk and benefit data collected on the drug when it was tested in the indicated population. Thus if a drug is used for other than what is specified on the label (i.e., off-label), the drug may not have been tested for safety and efficacy in that situation. Dr. Alving stated that off-label use should not necessarily be prohibited. However, she stressed that if drugs are to be used off-label, it is important to have available a public database that can be used to report and track those data. Dr. Walker added that such a database should also collect data on concomitant medicines, as
they may have an impact on drug reactions. A public–private partnership could play a large role in capturing and analyzing data for off-label use.
A final question was related to psychiatric medications and why investigation of their safety lags so far behind that of other, nonpsychiatric drugs. Dr. McClellan responded that this is just such a problem that a large-scale effort could address. Psychiatric drugs are often used in patients with comorbid conditions, for off-label conditions, and over long periods of time. These factors are difficult to address in premarket studies. The postmarket monitoring effort discussed here would provide a way to learn more systematically about these types of drugs.
Supplemental Clinical Studies
Dr. McClellen suggested that even if the system has access to good population-based data, not all questions would be answerable with observational data alone. Consequently, resources would still be required for postmarket clinical studies focused on cases in which detection of safety signals is insufficient for resolving whether a drug causes an elevated risk of an adverse event.
Active Surveillance Prototypes
According to Dr. Walker, a prototype of the kind of surveillance system envisioned, though on a much smaller scale, already exists. This prototype, developed by i3 Drug Safety using United Healthcare’s population of 12 million, is “a general-purpose medical data warehouse built on a health insurance transaction platform” and incorporates diverse data sources. The prototype was up and running about 6 months after its development. While it covers only 12 million people, it is scalable to datasets of any size. The retrieval time is only about 1 or 2 minutes, and a database of 100 million persons would probably not take much longer. Dr. Walker emphasized that, while i3’s prototype is not the last word in automated prescription surveillance, it demonstrates that the principal obstacles to developing such a system are not technological.
Dr. Krall described in some detail a prototype system validated by GlaxoSmithKline. The system uses two health care databases (totaling approximately 45 million persons with more than 22 months of exposure) and a methodology for comparing terminology between the two databases. While the analyses are not simple, each requiring substantial custom design, Dr. Krall argued that it should be possible (at least for classic drug-related events) to develop a methodology that would be applicable to most drugs, would be repeatable over time, and could serve as the foundation for a systemwide surveillance system.
Drs. Walker and Krall were asked to comment on what they thought would be necessary to scale up the efforts they described in their presentations. Dr. Krall replied that the greatest need is a mechanism for drawing the various stakeholders together to coalesce their common interests, ideas, and approaches.
Implementation and Funding
Dr. Platt suggested that remarkably few resources would be needed to establish the proposed public–private partnership for surveillance of drug safety. Dr. McClellan also stated that only limited additional funding would be needed, noting that all the required building blocks are in place for a postmarket infrastructure to be feasible now. Dr. Platt pointed to AHRQ, the FDA, and CDC as being in a position to initiate the necessary dialogue, with CMS being a principal partner. Dr. McClellan also identified the CERTs, subject to FDA guidance, as a possible convener and a good home for the public–private partnership as they are already conducting analyses on some of the salient data, and there is considerable interest in expanding those efforts. With regard to time, Dr. Platt said that it is not unreasonable to expect to have at least a rudimentary system covering 100 million people up and running within a year. Electronic databases of private plans already collectively handle well over 100 million people annually. Additionally, the FDA is using electronic Medicare data (Parts A and B) on a pilot basis, and some state Medicaid programs are contributing data to similar efforts. Together, these various private and public databases encompass more than 100 million persons that could form the base for a single, comprehensive surveillance network.
Dr. Walker suggested that the concerns of stakeholders accustomed to controlling the flow of data are the main obstacle to the implementation of the system. Dr. Platt noted, however, that in his experience, the owners of the data are willing to have the data used for important public health purposes as long as they can be confident that these are the only uses involved. He emphasized that the system would be a federated one and that no single entity would have ownership of it. He pointed to CDC’s Vaccine Safety Datalink as a pioneering effort in this regard: all the databases that contribute to that system reside separately but contribute to unified analyses.
The proposed public–private partnership would require funding, including both core funding to build the necessary infrastructure and develop routine postmarket surveillance, and separate funding for individual projects to follow up on and confirm potential safety signals. Dr. Krall and Gretchen Dieck, Senior Vice President, Safety and Risk Management, Pfizer, Inc., proposed that the partnership should accept both public
and private funding. Earlier in the symposium, Dr. Henney had alluded to decreased public confidence in the FDA because of its reliance on industry user fees. While multiple panelists discussed the need for mixed funding for the public–private partnership, several stressed the importance of a transparent process that could help build pubic confidence despite the reliance on private financing.
While such an endeavor would be costly, Dr. McClellan stressed that the alternative would be more costly still. Once the infrastructure was in place, the incremental cost of conducting further studies and the value of having a more comprehensive population database available for conducting analyses would translate into a much higher return on investment than would be obtained if the system continued to collect and analyze adverse event data on a drug-by-drug and manufacturer-by-manufacturer basis. Not only would a comprehensive approach be less expensive, but it would also generate better information and better-targeted and timelier postmarket clinical studies. And while statistical methods need to be refined to handle the complexity of the dataset and analyses, Dr. McClellan argued that if additional support were provided, existing resources could be leveraged so that work using the system could begin with relatively little delay.
Dr. Walker argued that cost is relative. For example, the cost of implementing the envisioned system pales in comparison with the billion dollars being spent to renovate the Hard Rock Café in Las Vegas. Moreover, as a joint public–private effort, the system could draw on the infrastructure already in place. When asked directly about ongoing costs for maintaining the system, Dr. McClellan said, “I think the low tens of millions is probably a good figure.” He noted that this money is already being spent and that the enactment of the Prescription Drug User Fee Act (PDUFA) IV and pending drug safety legislation would result in more funding for postmarket surveillance activities. He stressed that just about every major stakeholder group has expressed the desire to see the further development of such a system, that the necessary technical capabilities exist, and that Congress is interested in addressing the problem. Drs. Platt and Krall stated that while the precise amount of funding required is unclear, they believe it is modest, agreeing that it is along the lines suggested by Dr. McClellan.
Governance
In addition to quality data, an accepted methodology, qualified expertise, and funding, a national surveillance system would require a governance mechanism. Because the system would be based on health claims data and medical record information, it would need to provide continued
assurance of patient confidentiality and appropriate use of the data. Panelists voiced concerns about issues of privacy, confidentiality, and informed consent with respect to data from clinical trials. Dr. Platt responded that this type of work has been conducted for several years now, and always with the full approval of Health Insurance Portability and Accountability Act (HIPAA) privacy boards and institutional review boards (IRBs). Dr. Walker added that there is more to be done, however, with respect to reframing the work in a public health context rather than as a research activity, in a way that engages the public and health care providers and familiarizes them with the idea that information derived from routine health care delivery can and should be used to improve health care itself. Additionally, he asserted that HIPAA works partly because it operates according to a clear set of rules. The new system would need a new set of rules for the use and interpretation of claims data so people would not be left wondering, for example, whether something is reportable. Once those rules had been formulated, there would likely be greater acceptance of the proposition.
Limitations of Automated Databases as a Resource for Drug Safety Studies5
Dr. Dieck discussed the benefits as well as the limitations of automated databases. She agreed with some of the earlier presenters that rapid epidemiological studies involving real-world data on large samples are the cornerstone of postmarket safety monitoring, and that they could also serve as an important resource for estimating background rates and drug effects. She cautioned, however, that there are issues to consider when thinking about using automated databases as a resource for drug safety studies:
-
Scientific safety rather than available data should drive the process. Health care claims data are not collected for research purposes and therefore may be limited. They are collected for such purposes as billing and reimbursement, and the potential lack of specificity in the coding of public-use data could make them less sensitive and consequently less useful for addressing some safety issues.
-
The data may not be collected uniformly across sites (although this is a technical issue that can be resolved), and some diagnostic or procedural codes may be inconsistent or rarely used. This point was also mentioned by Dr. Krall, who when discussing GlaxoSmithKline’s active surveillance databases said that the company had devised a way to com-
-
pare terminology used in two different databases. It is important to note that while this issue of developing common data standards and controlled vocabularies was not discussed in depth during the symposium, combining databases without standardization is nearly impossible and is a major challenge that must be overcome.
-
Public-use data may derive from skewed populations that make the data problematic for answering certain types of safety questions. For example, VA data, as rich as they may be, are for a military population, Medicare data for the elderly, Medicaid data for those on government assistance, and heath maintenance organization (HMO) data for the working healthy.
-
The ability to adjust for important confounders, such as sociodemographic factors, health behaviors, and use of over-the-counter products, is limited.
-
Safety end-point data need to be validated with medical records. While some automated databases do this very well, others do not.
-
In some instances, a medicine may not be reimbursable (e.g., Viagra), or its use may be restricted (e.g., COX-2 inhibitors), with different types of patients taking different drugs (e.g., COX-2 inhibitors versus nonsteroidal anti-inflammatory drugs [NSAIDs]). Thus limited claims data will be available for these drugs.
-
The potential exists for channeling bias. For example, if people gained weight with their antischizophrenic medication, they may have been channeled toward Geodon because of its weight-neutral effects. However, because those individuals had previously gained weight, they would now generally be more susceptible to normal health risks as well as to the risks of Geodon.
-
Many postmarket safety studies involve specialized populations that would likely not fall under the purview of a public–private partnership that relied on an automated database as its cornerstone. Pfizer’s postmarket safety studies (some completed, others ongoing), for example, are each in some way specialized. One such effort is the Exubera VOLUME LST study, an 8-year trial that started in the third quarter of 2006. This study involves looking at abnormalities of lung function, something not normally included in electronic medical records or reimbursement codes since many such patients are asymptomatic.
Examples of Successful Public–Private Partnerships6
Dr. Alving described the public–private partnerships already being forged through Clinical and Translational Science Awards (CTSAs), which
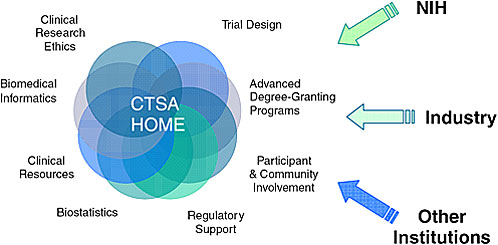
FIGURE 5-3 NIH’s Clinical and Translational Science Awards: A home for clinical and translational science.
SOURCE: Alving, 2007.
she identified as one of the most significant outcomes of the National Institutes of Health (NIH) Roadmap (Figure 5-3). The goal of the CTSA program is to accelerate and encourage the translation of basic biomedical discoveries into clinical science and medical practice. CTSA centers serve as a means of removing interdisciplinary barriers and encouraging creative, innovative approaches to solving complex medical problems. The centers offer advanced degree-granting programs in clinical and translational science; involve investigators from a wide range of medical, veterinary, and other biomedical disciplines; and interact with the FDA, industry, and other institutions.
The ultimate objective is to build a national consortium and forge new partnerships with public and private health care organizations. CTSAs will be distributed among more than 60 academic health centers by 2012, with the first awards having been granted in 2006. Already, interdisciplinary pilot programs are under way at, for example:
-
Duke University, where advanced informatics and health service delivery methods are being used to translate bench–bedside findings to populations;
-
University of California–San Francisco, where opportunities are being pursued in conjunction with the San Francisco VA and Kaiser Permanente;
-
Oregon Health and Science University, where investigators are developing new informatics capabilities to partner with Kaiser Permanente, the Northwest Center for Health Research, the Oregon Rural Practice Research Network, and the Portland VA Medical Center;
-
University of California-Davis, where new community research centers are being developed to expand efforts addressing minority and medically underserved populations; and
-
University of Pennsylvania, where robust efforts in cancer bioinformatics (CA BIG) are being led by the National Cancer Institute to improve the informatics capacity to report adverse events.
In addition to the partnerships cited above, NIH CTSA teams are being created to support efforts to develop new informatics capabilities. Additionally, the CTSA program has developed robust working relationships with the FDA and CMS. Updated information on the program is available at the CTSA website (http://ctsaweb.org).
Summary
Dr. McClellan summarized the discussion of a public–private partnership for the development of a national active surveillance network to monitor drug safety. He stated that it is “an issue whose time has come because . . . there is uniform agreement that this approach is feasible . . . and FDA reform is front and center in the legislative agenda and in the public agenda.” He cited interest at the highest levels within the Department of Health and Human Services in building a much more interoperative electronically based health care system, and suggested that creating a public–private postmarket drug safety monitoring system linking multiple existing databases is a leading edge of that effort.

















