6
Radiotracer and Radiopharmaceutical Chemistry
This chapter examines the “future needs for radiopharmaceutical development for the diagnosis and treatment of human disease,” the “national impediments to the efficient entry of promising new radiopharmaceutical compounds into clinical feasibility studies and strategies to overcome them,” and the “impacts of shortages of isotopes on nuclear medicine basic and translational research, drug discovery, and patient care, and short- and long-term strategies to alleviate these shortages if they exist” (charges 1, 3, and part of charge 4 of the statement of task). The content of this chapter, particularly the sections delineating needs and impediments, is derived largely from discussions with and presentations from chemists and other researchers working in the nuclear medicine field. The impact of the shortage of radionuclides was discussed in detail in Chapter 5.
The chapter is divided into the following sections:
-
Background (6.1),
-
Current Needs and Impediments (6.4), and
6.1
BACKGROUND
The history of nuclear medicine over the past 50 years highlights the strong link between investments in chemistry and the development of ra-
dionuclides and radiolabeled compounds. In fact, one can trace the major advances in nuclear medicine directly to research in chemistry. These advances have had a major impact on the practice of health care. According to the Society of Nuclear Medicine, 20 million nuclear medicine procedures using radiopharmaceuticals and imaging instruments are carried out in hospitals in the United States alone each year to diagnose disease and to deliver targeted treatments. These techniques have also been adopted by basic and clinical scientists in dozens of fields (e.g., cardiology, oncology, neurology, psychiatry) for diagnosis and as scientific tools. For example, many pharmaceutical companies are now developing radiopharmaceuticals as biomarkers for new drug targets to facilitate the entry of their new drugs into the practice of health care and to objectively examine drug efficacy at a particular target relative to clinical outcome (Erondu et al. 2006). This has created a demand for new radiopharmaceuticals and a corresponding need for chemists and other imaging scientists who are trained to develop them.
6.2
SIGNIFICANT DISCOVERIES
Government investments in chemistry have facilitated the advancement of nuclear medicine, molecular imaging,1 and targeted radionuclide therapy. For example, research in nuclear chemistry and radiochemistry (Sidebar 6.1), coupled with accelerator technology and engineering, has enabled the introduction of new radionuclides into the practice of medicine. Similarly, progress in synthetic organic and inorganic chemistry laid the groundwork for dozens of compounds labeled with positron emitters or single photon emitters, which are now used in many clinical specialties. These discoveries have resulted from the collaborative efforts of multi-disciplinary teams of scientists and clinician-scientists, ultimately translating new concepts into clinical practice. Three examples are provided in the following sections.
FDG-PET
Tumors and some organs, such as the brain, use glucose as a source of energy. FDG (Sidebar 2.2) is a fluorine-18-labeled derivative of glucose (fluorodeoxyglucose) which is used with positron emission tomography (PET) to provide a map of where glucose is metabolized in the body. Because tumors, as well as the brain and the heart, all use glucose as a source of energy, FDG is widely used in cancer diagnosis and in cardiology, neurology, and
|
SIDEBAR 6.1 Disciplines and Specialties within Chemistry Organic chemistry studies the synthesis, structure and properties of carbon compounds. Inorganic chemistry studies the chemistry of all elements of the periodic table. Of particular relevance to radiopharmaceutical chemistry is bioinorganic chemistry, which includes coordination chemistry and the incorporation of radiometals into targeted radiopharmaceuticals. Radiopharmaceutical chemistry designs, synthesizes, and evaluates chemical compounds that are labeled with a radionuclide. These radiopharmaceutical compounds are used for molecular imaging or for targeted radionuclide therapy (Chapter 4). Synthetic organic chemistry is one sub-area that is of particular relevance and deals with the design and synthesis of complex molecules. The second is medicinal chemistry, which seeks to design and synthesize new organic compounds to serve as drug candidates, as well as to understand how the compounds interact within living organisms. Nuclear and radiochemistry studies the chemical properties of radioactive elements to practical applications of radioactivity and nuclear technology. Combinatorial chemistry is used to generate different combinations of chemicals starting with a subset of compounds. The building blocks may be peptides, nucleic acids or small molecules. The libraries of compounds formed by this methodology are generally used in drug development. |
psychiatry. FDG is now widely available to hospitals throughout the United States and the world from a network of regional commercial cyclotron/FDG distribution centers (Figure 6.1). With the current large infrastructure of commercial cyclotron/FDG distribution centers, many chemists are developing other highly targeted fluorine-18-labeled compounds to take advantage of this unique network to broaden the use of PET for making health care decisions. The translation of FDG from the chemistry laboratory into a practical clinical tool had its roots in government-supported research in hot atom chemistry (see Chapter 5), cyclotron targetry, biochemistry, synthetic chemistry, nuclear chemistry, and radiochemistry that was integrated with engineering and automation (Fowler and Ido 2002).
Technetium-99m
Technetium-99m is a radionuclide that emits a photon, and this energy is ideally matched to the Anger camera, a device used in nuclear medicine worldwide. Not only is technetium-99m valuable clinically, but it is also practical for routine use, because it is extracted from molybdenum-99,
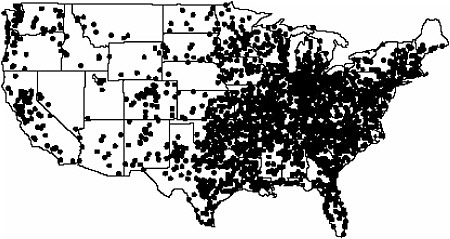
FIGURE 6.1 Map of commercial cyclotron/FDG/radiopharmacies in the United States. SOURCE: Courtesy of Michael Phelps, University of California at Los Angeles.
which has a 66-hour half-life. Greater understanding of the properties of technetium through research spanning over a number of decades (Nicolini and Mazzi 1999) laid the groundwork for developing many technetium-99m labeled radiopharmaceuticals. In parallel to these advances in chemistry, nuclear medicine kits were developed and refined, facilitating the preparation and commercialization of technetium-99m-radiopharmaceuticals (Eckelman and Richards 1970, 1971). These radiopharmaceuticals allow physicians to diagnose life-threatening diseases with great accuracy. The availability of technetium-99m and the radiopharmaceuticals derived from it exemplify the advances in patient care that can result from collaborative efforts among chemists, physicists, and biologists from around the world. Today, technetium-99m is the most widely used radionuclide in nuclear medicine, accounting for more than 70 percent of all procedures (Nuclear Energy Agency 2000).
Targeted Radionuclide Therapy
Targeted radionuclide therapy (see Sidebar 2.3) is a form of treatment that delivers therapeutic doses of radiation to malignant tumors by administering a molecule that is labeled with a radionuclide. For example, alpha particle emitters such as astatine-211 have great appeal for targeted radionuclide therapy because of their high toxicity to the cell and their abil-
ity to irradiate tumor volumes in the cellular range (i.e., 50–80 microns). Translation of alpha-particle emitters into the clinical domain has now been accomplished. This could not have occurred without advances in several areas of radiochemistry, including radionuclide production, separations chemistry, and labeling methods that circumvent the problem related to high radiation levels (i.e., radiolysis) generated by therapeutic levels of radionuclide (Zalutsky 2003). Targeted radionuclide therapy is discussed in further detail in Chapter 4.
6.3
CURRENT STATE OF THE FIELD AND EMERGING PRIORITIES
In the pharmaceutical industry and in many clinical specialties—particularly oncology, cardiology, neurology, and psychiatry—there is a demand for new radiopharmaceuticals to advance our knowledge of human biochemistry and physiology and to improve the ability to diagnose and treat diseases. The committee reviewed the current state and trends in radiopharmaceutical research and development (R&D), which are discussed in the following two sections. The first section (6.3.1) summarizes five priority areas with broad public health impact where radiopharmaceuticals could serve as scientific and clinical tools leading to major breakthroughs in health care and basic understanding of human biology. The second section (6.3.2) describes technologies and methods currently being explored that could enable innovations in radiopharmaceutical development and advances in these five priority areas.
6.3.1
Broad Public Health Priorities Enabled by Radiopharmaceutical Technology
-
Cancer Biology and Targeted Radionuclide Therapy. Greater understanding of the abnormal biology of tumor cells will allow cancer treatments to be developed that target these features (rather than non-specifically targeting rapidly dividing cells, which is the approach of most chemotherapeutics). Research is needed to develop the following: radiopharmaceuticals that enable an understanding and characterization of abnormal cellular biology to predict the most effective therapy in a particular patient; labeled anti-cancer drugs to determine whether the drug targets the tumor; radiotherapeutic agents to deliver the radionuclide to the tumor; and diagnostic radiopharmaceuticals to monitor response to treatment (Webber 2005).
-
Neuroscience, Neurology and Psychiatry. A large fraction of the efforts in radiopharmaceutical chemistry over the past 30 years has been dedicated to understanding the relationship between brain chemistry, behavior, and disease. Although substantial progress has been made in many
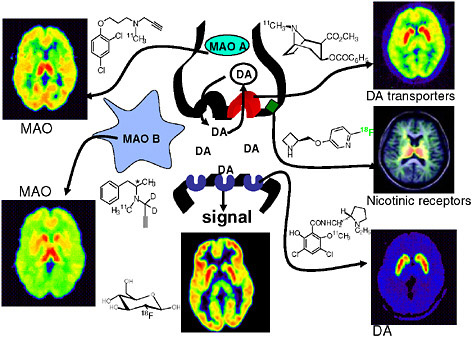
FIGURE 6.2 Radiotracers for imaging neurotransmitter function, as exemplified in the brain dopamine system. A simplified diagram of a dopamine (DA) synapse shows the dopamine transporter (red), dopamine receptors (blue), and monoamine oxidase (MAO) A and B, a nicotine binding site (green), and brain glucose metabolism along with radiotracer structures and human brain images corresponding to each of these molecular targets. SOURCE: Courtesy of Joanna Fowler, Brookhaven National Laboratory.
-
areas, only a handful of the hundreds of neurotransmitters2 and metabolic processes that drive brain function can be imaged and quantified with high specificity (Fowler et al. 2003, Kung et al. 2003) (see Figure 6.2). Many more highly targeted radiopharmaceuticals are required to identify the molecular abnormalities of neurodegenerative disorders, such as Alzheimer’s and Parkinson’s diseases, and psychiatric illnesses, such as schizophrenia and depression, so that better treatments can be developed. In addition, understanding addictive behaviors, such as cigarette smoking and overeating, is essential for the prevention of chronic diseases, such as diabetes, heart disease, and cancer (Bergen and Caporaso 1999, NIMH 2006). These diseases account for much of the morbidity and mortality in the United States and are a major public health burden. The intellectual and techni-
-
cal hurdles of developing the radiotracers of the future are enormous and depend on the stimulation of the flow of new ideas and the development of new technologies (see Section 6.3.2). However, the opportunities that molecular imaging offers to expand our knowledge of the human brain and to integrate this into the development of treatments for mental illness are unprecedented.
-
Drug Development. Radiolabeled compounds and imaging technologies are now being used in drug research and development both to measure drug pharmacokinetics and drug pharmacodynamics (Collins and Wahl 2002; also see Chapter 2). This has been stimulated in part by the increasingly prohibitive costs of drug development and the high rate of failure of new drugs entering clinical trials. Radiolabeled compounds can serve as scientific tools for early identification of problems such as poor bioavailability and non-target interactions which can lead to failure later on. In this way molecular imaging offers the potential for accelerating the process of drug discovery while also reducing costs. All large pharmaceutical companies today either have small-animal (microPET) and human PET scanners or relationships with academic and other PET programs that provide these unique scientific tools.
-
Cardiovascular Disease. A large fraction of the nuclear medicine tests conducted in the United States is used for cardiology. Single photon emission computed tomography tracers are now widely employed for estimating the severity of heart disease and for defining a patient’s future risk of heart attacks and cardiac death. PET tracers have also provided additional gains in accurately diagnosing coronary artery disease, reducing the need for invasive diagnostic procedures, such as coronary angiography. One research priority in cardiology includes identifying techniques for characterizing the functional and biological processes associated with structural alterations in the vessel wall that play a central role in the development of coronary artery disease. Further priorities include developing image-based radionuclide approaches for aiding in the design, implementation, and efficient assessment of gene- and cell-based treatment strategies in cardiovascular disease.
-
Genetics and Personalized Medicine. The sequencing of the human genome and new knowledge in proteomics, systems biology,3 and pathogenesis of human disease offer unprecedented opportunity for the development of new radiopharmaceuticals to image and quantify phenotypic expression of genetic pathology (e.g., upregulated EGF receptors in HER2 mutations). The development of these radiopharmaceuticals will allow scientists to better understand the relationship between genes and normal and abnormal
-
physiology, and to plan, deliver, and monitor treatment at the level of an individual patient. The identification of new genes and new protein products and their links to specific diseases will continue to generate the need for chemists to create new radiopharmaceuticals.
6.3.2
Specific Technologies and Methods
The demand for new radiopharmaceuticals from many medical specialties particularly oncology, cardiology, neurology, and psychiatry, and especially the pharmaceutical industry, has placed a sense of urgency on stimulating the flow of new ideas and accelerating the pace of development. Currently, chemists working in the areas of molecular imaging and targeted radionuclide therapy are focused on designing and synthesizing radiopharmaceuticals with the required bioavailability and specificity to act as true tracers targeting specific cellular elements (e.g., receptors, enzymes, transporters, antigens, etc.) in healthy human subjects and in patients. Goals are to make labeling chemistry occur faster, more efficiently, and at smaller and smaller scales to give labeled compounds of very high specific activity that can act as true tracers.4
Specific activity is a particularly important consideration in the design of molecularly targeted imaging agents and therapeutics. The degree to which improving specific activity must be addressed depends on the nature of the molecular target; specific activity is critical for imaging receptors present at a copy number of 1,000 per cell, but less of an issue with receptors such as the epidermal growth factor receptor that are present at a concentration of millions per cell. Improving specific activity can also be essential for molecules that are exquisitely chemotoxic or can perturb biology at subnanomolar concentrations. We note that the specific activity values described in the literature are generally far below the theoretical values and are highly variable. Thus, identifying and removing sources of carrier in radionuclide production (in cases where the target and the radionuclide are different chemical elements) and in radiotracer synthesis remains one of the major challenges in radiopharmaceutical chemistry.
Two high research priorities that are under investigation are carbon-11 and fluorine-18 chemistry and peptide and antibody labeling. Research in these areas has been stimulated by the increased utilization of PET and the promise of targeted radionuclide therapy. Fluorine-18 radiopharmaceuticals and antibody and peptide radiopharmaceuticals each have their own specific sets of challenges and needs which are further described in Sidebars 6.2 and 6.3. In addition, radiopharmaceuticals labeled with gallium-68 (a
|
SIDEBAR 6.2 Fluorine-18 Radiopharmaceutical Chemistry Needs Fluorine-18 [F-18]-labeled compounds are expected to play a large role in the future of nuclear medicine because of the relatively long half-life of F-18 and the large network of cyclotron/radiopharmacy distribution centers operating throughout the United States from which they can be obtained (see Figure 6.1). To advance the development and translation of F-18-labeled compounds, the following are needed:
|
positron emitter that is available from the Ge-68/Ga-68 generator) are used for receptor imaging and research purposes in a large number of clinical diagnostic and therapeutic applications throughout Europe (Antunes et al. 2007). Although experience with Ga-68 labeled tracers in the United States is limited, this radionuclide and the radiopharmaceuticals labeled with it deserve attention, because the generator for Ga-68 is convenient to use and the resulting radiopharmaceuticals could be distributed to the large network of facilities with stand-alone PET cameras for clinical imaging.
In addition to performing research directed toward radiotracer synthesis, chemists also design radiotracers and investigate the mechanisms underlying the distribution and kinetics of labeled compounds in living systems. This type of work addresses a major obstacle in radiotracer R&D—namely, the lack of knowledge for predicting which radiolabeled compounds will have the bioavailability, specificity, and kinetics required to image and quantify specific molecular targets in vivo or to target tumor tissue while sparing healthy organs. In this regard, progress in understanding and reducing non-specific binding would be a major advance. Of particular importance is research on the design and development of radiotracers that are more broadly applicable to common pathophysiological processes, which may be more useful and more readily commercialized (e.g., targets involved
|
SIDEBAR 6.3 Antibody/Peptide Radiopharmaceutical Chemistry Needs A central issue in the advancement of targeted radionuclide therapy lies in the design and development of labeled antibodies and peptides that target the tumor and spare healthy tissues. Chemistry plays a major role in this process, and the research priorities identified by the committee with input from experts are as follows:
|
in inflammation and infection, angiogenesis, tissue hypoxia, mitochondrial targets, cell signaling targets, and targets associated with diabetes, obesity, metabolic syndrome, or liver disease).
To meet these intellectual and technical challenges, a new molecular imaging radiotracer discovery and development process needs to be developed based on modern genomics, proteomics, and systems biology and driven by the invention of new molecular technology platforms to synthesize, label, and biologically screen in vitro for translation from a good scientific base to animal models and patients. This process should focus on being a measurement science, not on clinical diagnostics, even though that is the end
objective through the measurements that result. Molecular diagnostics and molecular therapeutics are desperately in need of biochemical, biological, and pharmacological measurements of disease in vivo and in patients to guide the discovery and development of new generations of more effective and personalized drugs. To keep pace with these clinical and research demands, nuclear medicine researchers are also seeing innovation in automation. For example, technologies that will provide simple, inexpensive modules for making carbon-11 labeled precursors and for automating other routine operations (e.g., quality control) are being developed. Furthermore, as noted in Chapter 2, microfluidic and microchip technologies are expected to advance this field (Figure 6.3)5 (Lee et al. 2005). Emerging areas such as nanosciences,6 advanced materials sciences,7 and strategically designed combinatorial libraries8 will also play an integral role in driving both radiopharmaceutical design and automation.
Although there is a need to develop new radiopharmaceuticals for new molecular targets, it is important to note that there are many highly promising radionuclides, precursor molecules, and radiopharmaceuticals that are not readily available to institutions without an infrastructure for isotope production or radiopharmaceutical chemistry. These include fluorine-18-fluoro-L-dihydroxyphenylalanine (FDOPA), fluorine-18-fluoro-L-thymidine (FLT), copper-64-diacetyl-bis(N4-methyl-thiosemicarbazone (ATSM), iodine-123-meta-iodobenzylguanidine (MIBG), fluorine-18-fluorocholine, fluorine-18-fallypride, fluorine-18-fluoroprophyl-β-carbomethyoxy-3β-(4-iodophenyltropane) (FP-CIT), Quadramet®, Therasphere®, copper-64-DOTA peptides, copper-62-generator, fluorine-18-fluoride, iodine-124 labeled antibodies, peptides, and targeted therapy drugs that inhibit signal transduction molecules. Thus, there is a need to increase the availability of specialized radiopharmaceuticals both for clinical diagnosis and treatment and also as research tools in the exploration of novel applications (in the “bench-to-bedside-and-back trajectory”). For example, MIBG, used
|
5 |
Microfluidics is a multi-disciplinary field that studies how fluids behave at microliter and nanoliter volume and the design of systems in which small volumes of fluids will be used to provide automated sample processing, synthesis, separation, and measurements in devices commonly termed lab-on-a chip (see Chapter 2). For example, it is used in procedures such as in DNA analysis. |
|
6 |
Nanoscience is the study of atoms, molecules, and objects whose size is between 1 and 100 nanometers. |
|
7 |
Materials science is an inter-disciplinary field comprising applied physics, chemistry, and engineering that studies the physical properties of matter and its applications. |
|
8 |
Combinatorial libraries are sets of compounds prepared using combinatorial chemistry (see Sidebar 6.1). These libraries allow scientists to access a wide range of substances and to search for compounds that bind to specific biological and non-biological targets. For example, when a molecule is added to the library, some of the compounds in the library will bind to it, enabling the discovery of individual compounds that recognize that molecule. |
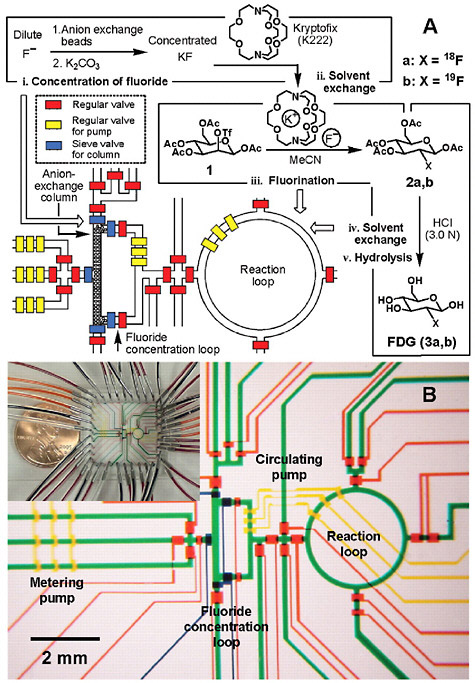
FIGURE 6.3 Integrated microfluidics for the synthesis of FDG. (A) Schematic representation of a chemical reaction circuit used in the production of FDG. (B) Optical micrograph of the central area of the circuit. SOURCE: Lee et al. 2005. Reprinted with permission from the American Association for the Advancement of Science.
initially mainly for assessment of neuroendocrine tumors, is now showing promise in early diagnosis of heart failure, a major health and economic issue in the United States. It is important to keep in mind that any new developments in targeted radionuclide therapy require access to research radionuclides (see Chapters 4 and 5).
6.4
CURRENT NEEDS AND IMPEDIMENTS
Although the scientific opportunities and medical challenges have never been more exciting and the demand for new radiopharmaceuticals has never been greater, the nuclear medicine infrastructure on which future innovation and discovery depend hangs in the balance. Four major impediments—some of which are elaborated further in other chapters of the report—stand in the way of scientific and medical progress and the competitive edge that the United States has held for more than 50 years:
-
Lack of Support for Radiopharmaceutical R&D. The committee finds that as a result of the reduction in funding from the U.S. Department of Energy-Office of Biological and Environmental Research (DOE-OBER) has seen a substantial loss of support for basic radiopharmaceutical chemistry research. This includes methodological research in synthetic chemistry, yield optimization, purification strategies, structure-activity relationships, radionuclide and targetry research, and preclinical and clinical evaluation. In addition, there is no support for infrastructures (accelerators, imaging instruments) that are the underpinning of radiopharmaceutical development.
-
Shortage of Trained Chemists and Physician Scientists (see Chapter 8). One of the most enriching aspects of radiopharmaceutical research is that it is generally carried out in an interdisciplinary environment where chemists, physicists, engineers, biologists, and physicians work together sharing the excitement of solving important problems in medicine. However, there is a critical shortage of trained chemists (typically, synthetic chemists with expertise in nuclear chemistry and radiochemistry are needed) for radiotracer and radiopharmaceutical R&D. This is a major impediment that has been documented in multiple reports over the past 20 years (e.g., DOE 2002, NRC 2007). There is also a lack of trained physician-scientists who are able to provide the expertise to collaborate in the basic clinical feasibility studies required to translate promising radiopharmaceuticals to the clinic.
-
Inappropriate Regulatory Requirements (see Chapter 4). Because the ultimate goal in radiopharmaceutical R&D is to use radiopharmaceuticals as scientific and clinical tools to investigate the systems biology of disease in healthy human subjects and patients, obtaining approval to
-
evaluate promising new labeled compounds in humans is essential. Currently, this is a bottleneck, stifling innovation and driving many research groups to carry out their initial evaluations of new radiopharmaceuticals with collaborators in other countries. A regulatory framework specific for radiopharmaceuticals that will facilitate the rapid and safe translation of radiopharmaceuticals from animals to humans for clinical feasibility studies is clearly needed.
-
Limited Radionuclide Availability (see Chapter 5). There is no dedicated domestic high energy accelerator for R&D and training for the development of the radionuclides of the future and for year-round production of medical radionuclides. These are needed not only for developing and evaluating the targeted radiotherapeutic agents, but also for production of some largely unexplored PET tracers. Radionuclides that are available intermittently from DOE labs are expensive due to full cost recovery requirements preventing their development for nuclear medicine.
6.5
RECOMMENDATIONS
The committee formulated two recommendations to meet the future needs for radiopharmaceutical development for the diagnosis and treatment of human disease and to overcome national impediments to their entry into the practice of health care.
RECOMMENDATION 1: Enhance the federal commitment to nuclear medicine research. Given the somewhat different orientations of the DOE and the National Institutes of Health (NIH) toward nuclear medicine research, the two agencies should find some cooperative mechanism to support radionuclide production and distribution; basic research in radionuclide production, nuclear imaging, radiopharmaceutical/radiotracer and therapy development; and the transfer of these technologies into routine clinical use.
Implementation Action 1A1: A national nuclear medicine research program should be coordinated by the DOE and NIH, with the former emphasizing the general development of technology and the latter disease-specific applications.
Implementation Action 1A2: In developing their strategic plan, the agencies should avail themselves of advice from a broad range of authorities in academia, national laboratories and industry; these authorities should include experts in physics, engineering, chemistry, radiopharmaceutical science, commercial development, regulatory affairs, clinical trials, and radiation biology.
RECOMMENDATION 2: Encourage interdisciplinary collaboration. DOE-OBER should support collaborations between basic chemistry and physics laboratories, as well as multi-disciplinary centers focused on nuclear medicine technology development and application, to stimulate the flow of new ideas for the development of next-generation radiopharmaceuticals and imaging instrumentation.















