7
Instrumentation and Computational Sciences
This chapter addresses the second charge to the committee to provide findings and recommendations regarding “future needs for computational and instrument development for more precise localization of radiotracers in normal and aberrant cell physiologies.” The content of this chapter, particularly of the sections delineating future needs, includes information and opinions derived from discussions with physicists, engineers, and mathematicians working in both industry and academia.
The chapter is divided into the following six sections:
-
Background (7.1),
-
Future Needs (7.4),
7.1
BACKGROUND
As discussed in other chapters of this report, the use of nuclear medicine technology for both diagnostic and therapeutic applications is central to the goal of personalized medicine. A key component of nuclear medicine is the quantitative imaging of radiopharmaceutical distributions. Imaging scientists often classify imaging tasks into two main categories: the detection task and the estimation task. The goal of detection is to see if some-
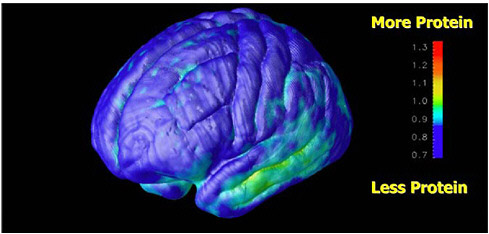
FIGURE 7.1 Parametric image of brain that shows the progression of abnormality of proteins in patients with Alzheimer’s disease. SOURCE: Courtesy of Henry Huang, UCLA.
thing is present (i.e., reading an image and making a diagnosis based on where the activity is located and the relative accumulation of the radiotracer to other tissues), and the goal of the estimation task is to determine how much of it is present (e.g., to measure the rate of glucose metabolism from a fluorine-18 fluorodeoxyglucose (FDG)-positron emission tomography (PET) study). The first step to achieve these goals is to acquire the data by accurately measuring the activity of the radioactive tracer in the patient. Once the data are acquired, image reconstruction algorithms are required to generate tomographic image sets of the spatial distribution of radiotracer within the body.
Recent developments involve modeling the physical characteristics of the camera into the iterative reconstruction process to improve image quality and radionuclide quantification. Finally, in addition to basic image display and analysis tools, advanced compartmental modeling tools are needed for those applications in which it is necessary to relate tracer uptake kinetics to physiologic or biochemical measures such as perfusion or receptor concentration, etc. (see Figure 7.1). Thus nuclear medicine imaging will gain from a continuum of improvements. These range from advances in solid-state materials for radiation detectors to increase sensitivity,1 faster
scintillators for higher count rate performance and time-of-flight (TOF) applications, and higher light output for improved aperture, and maximum image quality and tracer quantification with the integration of tracer kinetic models into the workstation toolkit.
To meet these goals, the development of imaging instruments and computational tools requires collaborative teams of investigators in physics, engineering, and mathematics who understand the entire process of nuclear medicine image generation. This ranges from understanding radionuclide decay (from simple single photon and positron emitters such as technetium-99m and fluorine-18 to complex isotope decays such as iodine-124 with positrons and prompt gamma emissions, which complicate signal acquisition in the 511-keV window), to the attenuation and scatter properties in the patient, to single photon and PET detector characteristics, to gamma camera and PET scanner performance, image reconstruction, processing, and finally image registration and display tools.
Starting with basic detector technologies, there has been (and continues to be) a natural synergy between the nuclear medicine detector/scanner development teams and basic nuclear and high-energy physics groups. As is discussed in Section 7.2, many of the nuclear medicine instruments used in the clinic today had their roots in the nuclear and high-energy physics laboratories that were developing advanced detectors to investigate the nucleus and structure of matter. For example, the most important annual meeting for the science and engineering of nuclear medicine instrumentation and imaging techniques is the Institute of Electrical and Electronics Engineers (IEEE) Nuclear Science Symposium and Medical Imaging Conference, which is a joint conference between nuclear medicine physics and instrumentation development teams and high-energy physics groups. It is at this meeting that the leading-edge technologies in radiation detectors are presented, frequently providing the impetus for innovations in nuclear medicine equipment. Those in attendance include physicists, engineers, and scientists from universities, national laboratories, and industry, and the atmosphere is one of multidisciplinary collaboration to bring new technologies into improved clinical instrumentation.
SPECT and PET cameras are highly integrated pieces of equipment that rely on the optimization of multiple subsystems to achieve peak performance. In SPECT, this requires high-quality crystal manufacture, collimator design, high-quantum-efficiency photomultiplier tubes, fast signal processing electronics, integrated gating for cardiac applications, advanced image processing tools, and so on. Weakness in any one of these components substantially affects the final image quality. As the field moves to earlier detection of cancer and neurological and cardiac diseases, pressure is exerted on scientists and equipment manufacturers to improve the limits of detectability of nuclear imaging devices (through improvements in both
resolution and sensitivity), and to incorporate new technology that will achieve this objective in the clinical imaging equipment of today, so that it is reliable, practical, and easy to use.
7.2
SIGNIFICANT DISCOVERIES
Federal investments in instrumentation and computational development have included infrastructure support at the national laboratories as well as direct grants to universities and national laboratories from the Department of Energy (DOE), the National Science Foundation, and the National Institutes of Health (NIH). Almost all of the core technologies in instrumentation and computation used in nuclear medicine have been developed as a result of the Atomic Energy Commission (AEC) and the DOE funding (see Chapter 2). Some of the key developments include the Anger camera (the basic foundation for all single photon imaging systems currently in use), SPECT, PET, cyclotron targetry and radionuclide generator systems, basic image reconstruction algorithms, and kinetic modeling applied to PET and SPECT studies.
The state-of-the-art SPECT and PET scanners used today in the clinic illustrate how critical it is to have the infrastructure and funding to support the flow of technology from the nuclear and high-energy laboratories that develop new detectors and electronics to the nuclear medicine instrumentation laboratories that develop new imaging tools. Often these basic science experiments are large efforts that utilize the expertise of many individuals at the national laboratories with contributions from university-based basic research groups. There are many examples of projects that were promising but high risk, and development of these projects was undertaken by DOE-funded laboratories which had the time and expertise to work on the new technologies over many years. DOE’s mission to develop new technologies for imaging allowed for long-range (i.e., more than 5-year), high-risk projects with an emphasis on basic instrumentation research. In contrast, NIH’s mission is to carry out more short-term (2 to 5 years), lower-risk (i.e., more preliminary data) projects that emphasize the integration or evaluation of new technologies for clinical application. The long-range view of DOE support allowed time for the investigator to pursue alternative approaches, some of which failed, in search of the most practical solution.
To illustrate this flow of technology, we note two of the many examples of technology development in PET instrumentation that were funded by DOE, as neither was a good candidate for NIH funding, and that were later commercialized. The first is the University of Pennsylvania’s PennPET scanner project. The PennPET project developed scanners designed for clinical use that were based on Anger-logic detectors (similar in concept to the Anger camera developed earlier at UC Radiation Laboratory with AEC
funding) and fully three-dimensional data acquisition and image reconstruction. This technology was commercialized by UGM Medical Systems in the early 1990s, and later marketed by General Electric, ADAC Laboratories, and Philips Medical Systems.
The second example is the University of California at Los Angeles (UCLA) microPET scanner project. The UCLA microPET scanner was the first PET scanner to utilize lutetium oxyorthosilicate (LSO), a material that has since become the scintillator of choice for many clinical scanners. Moreover, it set a precedent for dedicated, high-resolution, small animal imaging scanners (see Figure 7.2) that was commercialized by Concorde Microsystems in the late 1990s. This has helped initiate the recent growth of preclinical research with imaging of small animals (e.g., mice and rats) to study disease and develop new pharmaceuticals for monitoring and treatment (see Figure 7.3). Today there are at least 200 scanners installed by a variety of vendors, half of which can be found in pharmaceutical industrial laboratories.
While DOE funding is responsible for many of the basic technologies used in PET and SPECT instrumentation today, NIH has also played a critical role. The funding expended by NIH for research has demonstrated the clinical utility and application of these technologies. A prime example is the recent development of PET/computed tomography (CT) scanners. Although the principles are based on PET technologies developed at DOE-funded laboratories, the first prototype PET/CT scanner was developed in the late 1990s as a result of an NIH grant at the University of Pittsburgh in collaboration with CTI PET Systems. That work demonstrated the immediate
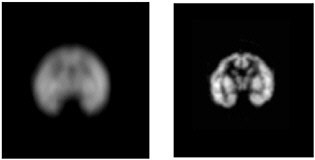
FIGURE 7.2 Comparison of images of a baby rhesus monkey brain phantom obtained with a clinical scanner (left) and a microPET scanner (right) illustrates the improvement in spatial resolution possible with a dedicated small animal scanner. SOURCE: Courtesy of Arion Chatziioannou, UCLA Crump Institute for Molecular Imaging.
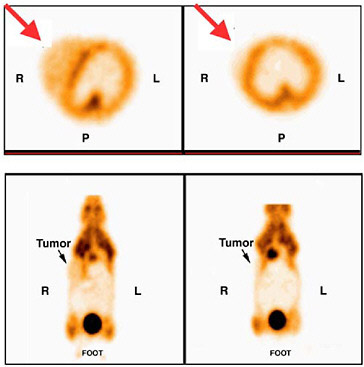
FIGURE 7.3 Images of a mammary tumor-bearing mouse imaged with fluorine-18-FDG following oncogene induction. The mouse was imaged first while doxycycline was being administered (left) and then 2 days later after doxycycline was turned off (right). Note decreased activity in tumor (at arrow) in second study. SOURCE: Courtesy of Lewis Chodosh, University of Pennsylvania.
impact of PET/CT in clinical oncology and the commercial sector rapidly developed these imaging instruments for clinical practice. In fact, all PET scanners today are marketed as PET/CT scanners because the combination of anatomic information (CT) and physiological information (PET) has proved to be essential for clinical diagnosis.
A similar story holds true for single photon emission computed tomography (SPECT)/CT scanners. The basic technical principles of SPECT were developed as a result of DOE funding at the University of Pennsylvania and Duke University. However, it was funding from NIH in the 1990s to the University of California at San Francisco that supported the research that integrated SPECT with CT (see Figure 7.4).
TOF PET is another example of a technology that is now available commercially as a result of funding from DOE through the 1980s and 1990s, and, more recently, funding from the National Institute of Bio-
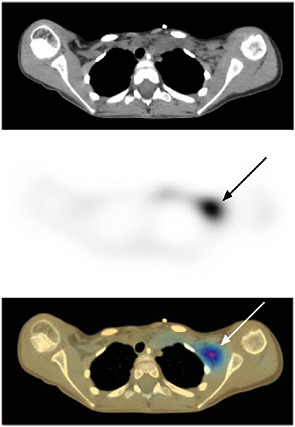
FIGURE 7.4 Neuroblastoma imaged with 131-I-meta-iodobenzylguanidine (MIBG) on a combined SPECT/CT instrument illustrates the benefit of correlating the functional data with the anatomical data. Top image is CT, middle image is SPECT, bottom image is combined SPECT/CT showing SPECT and CT in different colors. SOURCE: Reprinted by permission of the Society of Nuclear Medicine from Tang et al. 2001.
medical Imaging and Bioengineering. Its development took over 25 years to transition from the laboratory to the clinic since it required the combination of new developments of scintillators, electronics, and image reconstruction, which illustrates the need for long-term funding and involvement of different funding agencies. In 2006, TOF finally was introduced in a commercial product by Philips Medical Systems. Its advantage in cancer detection and staging has been demonstrated in terms of superior image quality (higher contrast with lower noise for the same number of events detected) and better detection of lesions compared to conventional PET (see Figure 7.5), particularly in heavy patients.
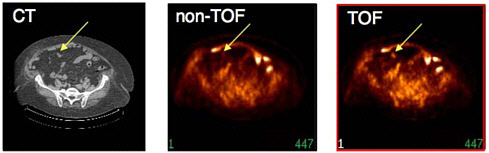
FIGURE 7.5 Colon cancer patient (119 kg) imaged with fluorine-18-FDG illustrating improvement in lesion detectability with TOF compared to conventional (non-TOF) PET for the same number of detected events and the same number of iterations in the reconstruction algorithm. The data were reconstructed without TOF information (middle) and with TOF information (right) and compared to low-dose CT image (left) acquired immediately beforehand on combined PET/CT instrument. Note the TOF reconstruction shows higher uptake and better definition of the lesion (at arrow). SOURCE: Courtesy of Joel Karp, University of Pennsylvania.
7.3
CURRENT STATE OF THE FIELD AND EMERGING PRIORITIES
Emerging goals for nuclear medicine include early detection, which will require improvements in equipment sensitivity; the accurate quantification of biomarker uptake in disease for the evaluation of treatment response; and the quantification of radiotracer heterogeneity, which may be of potential utility for dose-painting applications of intensity-modulated radiotherapy. These limits may be tested in preclinical equipment such as microSPECT and microPET scanners, which operate at the cutting edge of the technology, with volumetric resolutions that are approximately 10-fold higher than their counterpart systems in the clinic. For example, the spatial resolution requirements to conduct meaningful preclinical research in mice are much more stringent than those required to conduct clinical studies in patients. To illustrate, a simple argument can be made that since the mouse brain volume is about 1/1,700th the volume of the human brain, we should scale the linear spatial resolution of a human scanner by a factor of 12 in each of the three dimensions to achieve a comparable resolution for mouse brain imaging. With current state-of-the-art technology for human brain imaging, 2.4-mm linear spatial resolution can be achieved with a dedicated brain scanner. To be able to achieve a similar linear spatial resolution in mice, the target is to reach a linear spatial resolution of 0.2 mm. To date, the best linear spatial resolution achieved for small-animal imaging in PET with a commercial instrument is 1.2 mm, which is still a factor of 6 too high (i.e., a factor of 216 in terms of volumetric resolution). Some university-based
developmental systems have achieved resolutions of 0.7 mm, but the goal of reaching less than 0.5 mm will require considerable technological development both in detector technology and image reconstruction algorithms. Such improvements in the spatial resolution of microPET systems are not impossible, and are governed by the minification of detector elements, light collection, and image processing software. In addition, methods to overcome depth-of-interaction (DOI) effects, which reduce spatial resolution of small bore scanners for points off central axis, are under development using phoswich and other detector technologies.
The sensitivity of state-of–the-art animal PET scanners is currently 5 percent (Myers and Hume 2002). However, to measure kinetics of tracers with low specific activity requires the development of new techniques than can increase sensitivity to 10 to 20 percent. Detector designs utilizing nonradioactive crystalline materials are advantageous because the high background signal associated with the current LSO crystals places limits on the detection of micrometastatic disease. Furthermore, improved algorithms that can stably reconstruct the activity distribution from low-count statistics will be important.
One advantage of PET compared to SPECT is that the spatial resolution and sensitivity are decoupled. It is very challenging to improve spatial resolution in SPECT without reducing sensitivity because of the basic need for collimation. Although superior spatial resolution has been achieved in SPECT for small-animal imaging using pinhole collimation (<0.5 mm), this has been reached at the cost of sensitivity and field-of-view. Although this trade-off is well suited for brain imaging of a mouse, current technology does not enable this level of performance for body imaging, or for larger animals.
Although great advances have been made in basic nuclear medicine imaging in both the detection and estimation tasks, personalized medicine (Sidebar 2.5) is a challenging goal. It requires the ability to detect many different signals that are specific to a patient’s disease. That requirement has led to the increasing development of hybrid imaging systems. Combining a high-spatial-resolution anatomical modality, such as CT, with a high sensitivity molecular imaging modality, such as PET or SPECT, has had a major impact on the current practice of medicine. For example, PET/CT scanners have been rapidly adopted in oncology in diagnosis and treatment planning. Some laboratories and equipment vendors have active research programs to develop PET/MRI scanners (see Figure 7.6).
Another new and exciting technology under development is the Silicon Photomultiplier (SiPM) device. SiPM devices have the potential to offer a low-cost, high-performance photon sensor for use in a wide variety of gamma-ray imaging systems. These devices were originally developed for high-energy physics experiments but are now being tested in both physics
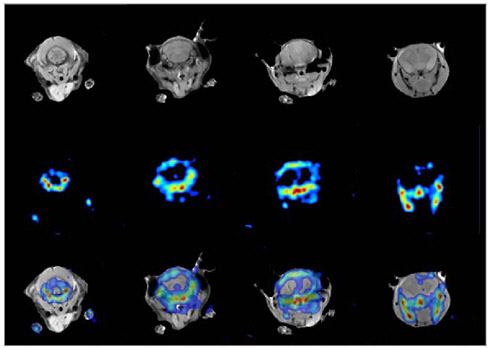
FIGURE 7.6 Simultaneous PET/MR images of mouse brain. The MR image (top row) shows both bone and tissue in the head of the mouse, whereas the PET image (middle row) for this particular study highlights mainly the bony structures of the jaw and skull of the mouse since the data were acquired using fluorine-18-fluoride. An overlay of images and correlation of both modalities is shown in the bottom row, with PET and MR in different colors. SOURCE: Reprinted by permission of the Society of Nuclear Medicine from Cantana et al. 2006.
and nuclear medicine imaging research laboratories for future imaging instruments. Although the potential is already apparent, the use of these devices in imaging instruments has many challenges and risks, and long-term research funding will be required to bring this technology to the clinic. This is also an example of a technology that requires early collaboration between academia and industry during the development phase, since the eventual solution for medical imaging detectors will require input into the design of the photon sensor. Development of SiPMs is ongoing at both large companies (e.g., Hamamatsu) and small companies (e.g., Zecotek, SensL, and Photonique), but it is currently difficult to fund this type of research at universities or national laboratories.
Hand-in-hand with the development of detectors, electronics, and imaging instruments goes the development of image reconstruction algorithms,
simulation tools, and techniques for kinetic model analysis. Development of these software tools is essential to accurately model the data and thereby quantify the radiotracer uptake in nuclear medicine studies. The ability to perform this task in practice has benefited from the increased availability of powerful computing resources. For example, an iterative image reconstruction algorithm with data corrections built into the system model was considered to be impractical a decade ago. Yet, this type of algorithm can now be used to generate images in a practical amount of time in both the research laboratory and the clinic (Figures 7.7 and 7.8).
7.4
FUTURE NEEDS
Leaders in instrumentation and computational development in nuclear medicine from universities, national laboratories, and industry were solicited for commentary and analysis. Based on discussions with these experts
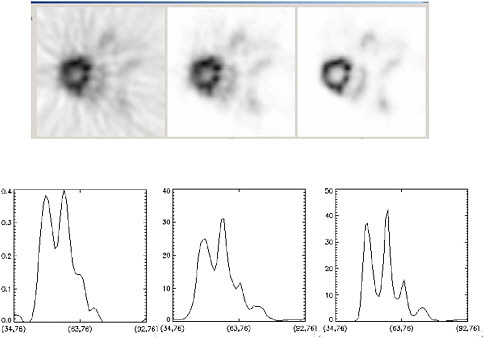
FIGURE 7.7 Images of mouse heart illustrate improvements due to image reconstruction: (left) filtered backprojection algorithm, (middle) iterative ordered-set expectation maximization (OSEM) algorithm, (right) OSEM with detector response modeling. Note that the OSEM reconstruction with detector response modeling has the lowest noise and the best definition of myocardial uptake. SOURCE: Courtesy of Thomas Lewellen, University of Washington.
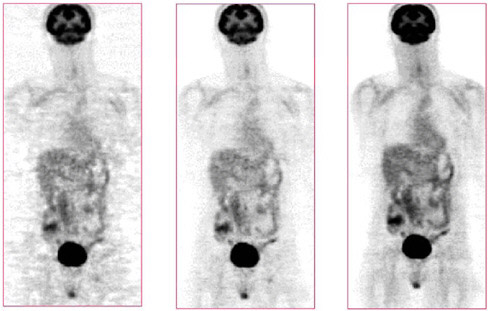
FIGURE 7.8 Images of clinical patient illustrate improvements due to image reconstruction: (left) filtered backprojection algorithm, (middle) iterative OSEM algorithm, (right) OSEM with detector response modeling. Note that the OSEM reconstruction with detector response modeling has the lowest noise and overall best image quality. SOURCE: Courtesy of Paul Kinahan, University of Washington.
as well as committee members’ own experiences, the committee concluded that improvements in instrumentation and computation are necessary to lead to advances in quantitative imaging and, in turn, nuclear medicine. These improvements depend on the following:
-
better spatial resolution;
-
higher sensitivity;
-
further integration of instruments to provide multimodality (multisignal) imaging;
-
increased coupling of detector and electronics design with image reconstruction; and
-
development of clinically robust software tools for data processing and analysis.
The opportunities afforded by improvements in instrumentation and computation could include the following:
-
The development of new technology platforms (e.g., integrated microfluidics chips and other automated chemistry and biological screening technologies and nanotechnologies) that would accelerate, diversify, and lower the cost of discovering and validating new molecular imaging probes, biomarkers, surrogate markers, and labeled drugs, as well as new radiotherapeutic agents.
-
The invention of new miniaturized particle-accelerator and associated technologies to develop small, low-cost electronic generators for producing short-lived radioisotopes for local use in research and clinical programs. DOE has the largest accelerator technology program in the world, including novel miniaturized accelerator technologies in the DOE weapons program.
-
The invention of new detector technologies for PET and SPECT that would enhance sensitivity as well as spatial and temporal resolution. All the successful detectors in PET and SPECT today came from the physics programs of DOE. New base detector materials and detection logic are needed to invent new generations of PET and SPECT imaging systems.
-
The development of new iterative algorithms and high-speed/high-capacity computational systems for rapid image reconstruction; this would allow image data to be converted to quantitative parametric images pertaining to biological and pharmacological processes in disease.
7.5
FINDINGS
-
Synergistic collaborations between national laboratories and universities have led to the successful transition of technology from the basic physics laboratory to both biological research and clinical settings. Furthermore, the collaborations between the DOE-funded laboratories and the NIH-funded laboratories have illustrated the value of funding from different agencies with different missions. However, with the loss of nuclear medicine funding from the DOE Office of Biological and Environmental Research (DOE-OBER) in FY 2006, the amount being spent on basic instrument development has fallen from $6.3 million to only $1.9 million (DOE 2006), limiting the ability to explore new and innovative technology solutions.
-
Developments in nuclear imaging instrumentation directly provide tangible benefits for the emerging field of molecular imaging. Three examples of these upcoming technologies include TOF PET, combined PET/MR machines, and SPECT/CT with the potential to allow quantification of single photon radiotracers for the first time; these three technologies will directly impact future patient management in the following ways:
-
TOF PET allows significant improvements in clinical image
-
-
quality, in particular for large patients (>250 pounds), where current three-dimensional PET scanners are frequently of borderline quality. Further advances in timing resolution beyond the current 600 picoseconds of the Philips Gemini TOF will be coupled to further improvements in signal-to-noise ratio, image contrast, and reduced partial volume corrections, allowing more accurate tracer quantification in small structures.
-
-
Advances in the technology of hybrid scanners will combine the benefits of the soft tissue anatomy, MR spectroscopy, and functional MR alongside the sensitivity of PET imaging. This has the potential to revolutionize imaging of the brain, and with it spur interest in body PET/MR systems for imaging the prostate where spectroscopy is well developed.
-
Advances in SPECT/CT instruments will directly facilitate quantitative SPECT studies, of vital importance in targeted radionuclide therapies. Software is under development to co-register serial SPECT/ CT exams and generate dosimetric maps for the radionuclide, of significant importance for patient-specific targeted therapy planning. The extensive portfolio of SPECT agents approved by the Food and Drug Administration coupled with the unique ability of SPECT to perform simultaneous multienergy window exams widens previously untapped opportunities in single photon nuclear medicine imaging, through advances in quantitative SPECT imaging.
-
The above examples represent only a portion of the advances that are likely to be seen in molecular imaging instrumentation over the next decade.
7.6
RECOMMENDATIONS
RECOMMENDATION: Encourage interdisciplinary collaboration. The DOE-OBER should continue to encourage collaborations between basic chemistry, physics, computer science, and imaging laboratories, as well as multi-disciplinary centers focused on nuclear medicine technology development and application, to stimulate the flow of new ideas for the development and translation of next-generation radiopharmaceuticals and imaging instrumentation. The role of industry should be considered and mechanisms developed that would hasten the technology development process.














