5
Availability of Radionuclides for Nuclear Medicine Research
This chapter addresses part of the fourth charge of the statement of task. It examines whether a shortage of radionuclides for nuclear medicine research exists, and if so, what impact it is having on basic and translational research, drug discovery, and patient care, and what short- and long-term strategies can be implemented to alleviate such shortages. The chapter is divided into the following sections:
5.1
BACKGROUND
At the very heart of all nuclear medicine procedures is the need for year-round, reliable availability of radionuclides. Currently, more than 70 percent of all procedures in nuclear medicine are based on technetium-99m (Nuclear Energy Agency 2000), a radionuclide produced by individual generators that use material produced in reactors outside of the United States.1 Growing use of positron emission tomography (PET) and targeted
radionuclide therapy has created the need for steady supplies of a variety of other radionuclides, and the demand is expected to increase (Wagner et al. 1999).
The production of radionuclides in the United States can be traced to the graphite reactor at Oak Ridge National Laboratory (ORNL) shortly after World War II. In its first year of operation, hundreds of shipments of 60 different radionuclides were made. Production of radionuclides for biomedical research continued until the reactor was shut down in 1963. Based on the successes achieved and the interest created by this early work, radionuclides were produced throughout the 1960s and 1970s at universities and national laboratories that had reactors, cyclotrons, or other accelerators available (Sidebar 5.1).
Commercial producers and distributors have played an important role in supplying radionuclides such as molybdenum-99/technetium-99m, thallium-201, gallium-67, indium-111, and iodine-123. With the advent of PET technology, beginning in the late 1970s, the need for a more reliable supply of radionuclides with short half-lives drove industry to develop small cyclotrons for supplying the primary radiopharmaceutical, fluorine-18-fluorodeoxyglucose (FDG). However, the market for radionuclides such as copper-67 and astatine-211 has never been large enough to encourage industry to produce them,2 and they are not readily available from low-energy PET cyclotrons. The issue of such “exotic” radionuclides, or radionuclides requested by a fairly small number of investigators for their research studies, has plagued the field for years. Many of these radionuclides will never be in high demand but could be important for advancing the understanding of fundamental biology or therapeutic efficacy (e.g., bromine-76 and copper-67).
5.2
SIGNIFICANT DISCOVERIES
Many of the discoveries associated with radionuclides were made possible by government research funding, particularly DOE research funding. The following examples indicate the variety and complexity of the types of investigations and discoveries that were made possible by these investments:
Molybdenum-99/Technetium-99m Generator
As mentioned earlier, technetium-99m is the most widely used radionuclide for nuclear medicine procedures in the world, accounting for more than 70 percent of all nuclear medicine procedures (Nuclear Energy Agency
|
2 |
A list of commercially available radiopharmaceuticals is provided in Appendix C. |
2000). The molybdenum-99/technetium-99m generator was invented at Brookhaven National Laboratory (BNL). This generator system is popular because the parent radionuclide (molybdenum-99) has a half-life of 66 hours while its decay product (technetium-99m) has a half-life of 6 hours. The differences in the half-lives and chemical properties of molybdenum and technetium are exploited to separate them in the generator (Sidebar 5.2). This separation can be repeated many times, and this system provides a nearly continuous supply of radionuclides at a low cost. Major efforts have been expended on developing the chemistry to incorporate technetium-99m into useful biological molecules. The results have included radiopharmaceuticals that assess cardiac function, blood flow, and bone metastases.
Carbon-11 Hot Atom Chemistry
The work of Alfred Wolf and co-workers throughout the 1960s and early 1970s at BNL laid the foundation for the production and labeling of carbon-11 in a variety of biologically active molecules. As shown in Sidebar 5.1, a carbon-11 atom produced in a particle accelerator will have a large amount of kinetic energy, more than enough to break ordinary chemical bonds. These particles are called hot atoms. Most of the science of radiotracers/radiopharmaceuticals, including radionuclide therapy, has its roots in hot atom chemistry.
Production Excitation Functions3 for Fluorine-18, Carbon-11, and Oxygen-15
At the very heart of radiotracer research is the ability to produce sufficient quantities of radionuclide to be incorporated into biologically useful molecules. During the late 1970s, a group of researchers under the guidance of Alfred Wolf at BNL examined a number of excitation functions to demonstrate that a simple, low-energy, proton-only accelerator could produce the requisite quantities of the most widely used radionuclides for PET. This work encouraged a commercial company to design and build a small cyclotron dedicated to providing large quantities of fluorine-18, carbon-11, and oxygen-15 to PET centers. There are now nearly 200 of these cyclotrons positioned around the world providing an infrastructure for supply of FDG and other PET tracers (see Figure 6.1 for the geographic distribution of cyclotrons in the United States).
|
SIDEBAR 5.1 Types of Machines that Produce Radionuclides and How Radionuclides Are Created There are two main waysa of producing radionuclides with a nuclear reactor or with a particle accelerator. The two methods are complementary in providing a wide variety of radionuclides for research and patient care. A nuclear reactor is a device in which nuclear chain reactions are initiated, controlled, and sustained at a steady rate. Nuclear reactors are most commonly used to generate electricity, but are also used as a neutron source to produce radionuclides (Figure 1). 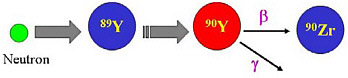 FIGURE 1 Schematic of yttrium-90 (Y-90) generation via neutron capture of the stable element yttrium-89 (Y-89). Yttrium-90 is a radionuclide used in targeted radionuclide therapy which decays with a half-life of 2.7 days (see Chapter 7). SOURCE: Courtesy of Thomas Ruth, TRIUMF. A particle accelerator is a device that uses electric fields to propel electrically charged particles to high speeds, which then collide with targets. Out of this collision, many subatomic particles are produced (http://www2.slac.stanford.edu/wc/accelerator.html). Particle accelerators can be found in everyday use (e.g., the cathode ray in a television set), but are also used in other settings, such as to produce medical or research radionuclides (Figure 2). Broadly, there are two types of accelerators: linear (linac) and circular (cyclotron) (Figure 3). In a linear accelerator, particles are accelerated in a straight line, whereas in a circular accelerator, the particles move in a circular path. |
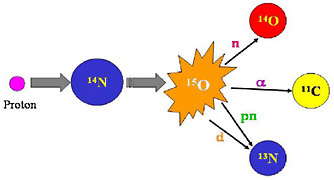 FIGURE 2 In radionuclide production, one element is transmuted into another. The above schematic illustrates how an atom of nitrogen is bombarded by a proton producing an excited oxygen atom (O-15) which then can emit any one of several particles to leave the new atom. In this case the new atoms are all positron-emitting radionuclides. These radionuclides can be separated by physical and/or chemical means. SOURCE: Courtesy of Thomas Ruth, TRIUMF.  FIGURE 3 Photograph of the interior of a cyclotron shows the copper dees, the accelerating component of a cyclotron and the 4 “hills” of the magnet. This cavity is enclosed with a plate so that a chamber capable of sustaining a vacuum is formed. Ions of a light particle such as hydrogen or helium are injected into the center of the cyclotron where they are accelerated by the electrically charged dees. The dees are high-voltage cavities that change polarity (electrical charge) at a high frequency (radiofrequency = tens of megahertz). The magnet forces the charged particles to move in a circular path. As the particle gains energy the circular path increases in radius until it reaches the energy desired, whereupon it is extracted and directed to a target material where a nuclear reaction forms the radionuclide of choice (see Figure 1). SOURCE: Courtesy of Thomas Ruth, TRIUMF. |
|
SIDEBAR 5.2 Generators A generator is a device that is used to extract one nuclide from another. For example, technetium-99m is recovered from technetium generators, which are shielded cartridges that contain molybdenum-99. Saline solutions can be passed through these generators (a process known as “milking”) to recover the technetium-99m. |
Development of Practical Generator Systems
The ability to access PET radionuclides without the use of onsite accelerators or reactors depends upon the availability of generator-produced radionuclides. Lawrence Berkeley National Laboratory developed the first practical generators for the germanium-68/gallium-68 and the strontium-82/rubidium-82 pairs. Strontium-82 is now being used with increasing frequency for clinical cardiac studies. It is presently supplied through a consortium of accelerators throughout the world that run parasitically and is not under the control of the user community. With the increasing demand for the strontium-82 generator, the current sources may not be sufficient in sustaining availability of this radionuclide.
Production of Tungsten-188/Rhenium-188 Generator
ORNL developed the tungsten-188/carrier-free rhenium-188 perrhenic acid generator system. Rhenium isotopes have chemistry similar to that of technetium and thus are of interest for adapting the extensive labeling tools created for technetium-99m. Rhenium-188 in particular is attractive for certain therapy applications because it emits a high-energy beta particle and has a relatively short half-life.
5.3
CURRENT STATE OF RADIONUCLIDE AVAILABILITY IN THE UNITED STATES
The Department of Energy’s (DOE) national laboratories remain the primary source of less commonly used or exotic radionuclides, produced from their large reactor and accelerator facilities. These facilities include the High Flux Isotope Reactor (HFIR) at ORNL, Brookhaven Linac Isotope Producer (BLIP) at BNL, the Isotope Production Facility at Los Alamos Nuclear Science Center (LANSCE) at Los Alamos National Laboratory
(LANL), and the Advanced Test Reactor (ATR)4 at Idaho National Laboratory (INL). The facilities at the national laboratories were designed and operated to fulfill their missions in physics, materials science, and other research programs. In addition to performing their primary missions, these reactors and accelerators were made available for the production of radionuclides to be carried out in parasitic mode (i.e., while accelerators are in operation for other purposes). However, as the interest in exotic radionuclides has grown, the national laboratories have not been able to meet the demands of the research community for regular and continuous availability of these radionuclides. Not only have the operating schedules been dictated by the primary users, but radionuclide production has been limited by age-related degradation of the facilities and extended shutdowns for facility maintenance. Of the major operational facilities that support radionuclide production, HFIR at ORNL was first operated in 1965, ATR at INL in 1970, the ORNL calutrons in 1944, BLIP at BNL in 1972, and LANSCE at LANL in 1974. There are currently no plans to replace these facilities.5
Medium-sized research reactors located on university campuses have also complemented the large DOE facilities by providing research quantities of medical isotopes. One successful example is the radiochemical and radiopharmaceutical research, production, and education program at the Missouri University Research Reactor (MURR). MURR was evaluated and identified as the best program in the United States by the Nuclear Energy Research Advisory Committee (NERAC) subcommittee (Reba et al. 2000), and the National Radionuclide Production Enhancement Program recently recommended that the MURR receive federal support of $7 million to upgrade its facility to increase the quality and quantity of radionuclide production for research and clinical applications (SNM 2005). However, the number of university research reactors in the United States has been in steady decline since the early 1970s. Of the 25 university research reactors currently in operation, 11 are licensed at or higher than 1 MW; the other 14 reactors are low-power reactors suitable only for training purposes (Bernard and Hu 2000, Rogers 2002). Most of these reactors were built in the late 1950s or 1960s and require continued facility upgrades and maintenance in order to fulfill their missions in research and education. Table 5.1 lists the reactor and accelerator facilities in the United States that have medical radionuclide production capability.
TABLE 5.1 Reactor and Accelerator Facilities in the United States with Medical Radionuclide Production Capability
|
Location |
Facility |
Power |
Medical Radionuclides Currently Produced |
|
Reactors |
|
|
|
|
ORNL |
HFIR |
85 MW |
225Ac,252Cf, 43K, 103Pd, 188W, 117mSn, 147Pm, 177Lu, 186Re, 166Ho, 194Ir, 191mIr, and others |
|
University of Missouri |
MURRa |
10 MW |
32P, 166Ho, 192Ir, 35S, 186Re, 90Y, 51Cr, 103Pd, 177mLu, and others |
|
Massachusetts Institute of Technology |
MITR-IIa |
5 MW |
198Au, 90Y, 192Ir, and others (research quantities) |
|
University of California at Davis |
MNRCa |
2 MW |
125I and others (research quantities) |
|
Oregon State |
OSTRa |
1 MW |
Variety (research quantities) |
|
Accelerators |
|
|
|
|
LANL |
LANSCE |
800 MeV proton |
26Al, 67Cu, 68Ge, 82Sr, 86Y, 124I, and others |
|
BNL |
BLIP |
200 MeV proton |
67Cu, 82Sr, 68Ge, and others |
|
Washington University |
cyclotrons |
|
64Cu, 77Br, 66Ga, 124I, 94mTc |
|
Trace Life Sciencesb |
Various LINAC and cyclotrons |
|
64Cu, 67Cu, 111In, 123I, 201Tl |
|
aNon-DOE facilities: University research reactors. bCommercial production facility. SOURCE: DOE Isotope Program. |
|||
Compounding these infrastructure issues is concern about the availability of enriched stable isotopes6 that are used as target materials for the production of radionuclides, regardless of method. Nearly all enriched stable isotopes that are used in nuclear medicine are imported from foreign suppliers. The primary domestic source, calutrons7 at ORNL, has been on standby
since 1998 because of competitive pricing from foreign suppliers (Reba et al. 2000). According to a report produced by the NERAC (Wagner et al. 1999), ORNL had a substantial inventory of enriched stable isotopes. Although the supply is not seen as disappearing in the near term, there is a concern that without a clear plan to address future needs, researchers both in the United States and worldwide will face a shortage of enriched stable isotopes.
Research radionuclide distribution has also been affected by the Energy and Water Development Appropriations Act of 1990 (Public Law 101-101), which requires the DOE to operate on a full cost recovery8 model (Sidebar 5.3). A consequence of this law has been the competing demand between producing high-cost, non-commercial radionuclides for researchers and supplying high-volume, commercial-use radionuclides to the private sector. The requirement for full cost recovery has made access to novel radionuclides cost-prohibitive for the vast majority of laboratories and clinics and is one of the major impediments to progress in nuclear medicine research.
A number of studies by different organizations, including the Institute of Medicine, have investigated the isotope (i.e., radionuclides and stable isotopes) needs of the country (Sidebar 5.4 provides a list of references). All of these studies came to the same conclusion: a dedicated radionuclide production facility is urgently needed to foster and facilitate research and training in the use of radionuclides in the biosciences and to provide a domestic, year-round, continuous supply of radionuclides for nuclear medicine practice.
5.4
CURRENT AND FUTURE NEEDS
To determine the current and future radionuclide production needs for furthering nuclear medicine research, the committee solicited input from experts in the field. Table 5.2 is a list of the radionuclides most frequently described as being essential to nuclear medicine research. Several of these research radionuclides are not being produced in sufficient quantities to meet the research demand. The technical and nontechnical needs and impediments are summarized in Sections 5.4.1 and 5.4.2, respectively.
5.4.1
Technical Needs and Impediments
There is no domestic (i.e., U.S.) source for most of the medical radionuclides used in day-to-day nuclear medicine practice. Furthermore, the lack of dedicated domestic accelerator and reactor facilities for year-round production of medical radionuclides for research is limiting the develop-
|
SIDEBAR 5.3 The Energy and Water Development Appropriations Act of 1990 (Public Law 101-101) P.L. 101-101 is one of two major laws that provide the authority to regulate radionuclide production and distribution in the United States. Unlike the Atomic Energy Act of 1954, which was passed to promote the production of radionuclides for research, the intended goal of P.L. 101-101 was to provide an incentive for cost-effectiveness by bringing the management of radionuclide production and distribution under one roof. Appropriations to the program are mainly applied to the maintenance and upgrade of production facilities. Under the DOE’s Isotope Programa radionuclides are sold to researchers at prices that recover the direct production cost, while commercial customers pay the full cost including allocated facility costs. Note that no provision for the production of radionuclides made exclusively for research was made (IOM 1995). The Isotope Program’s resources in millions of dollars are depicted in the figure below. 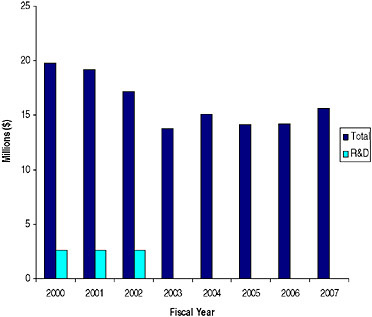 Annual Appropriations for the Department of Energy-Office of Nuclear Energy’s (DOE-NE) Isotope Program, 2000-2006 ($ in millions) SOURCE: Data provided by DOE-NE. |
|
SIDEBAR 5.4 Studies Reviewing Isotope Supply in the United States Separated Isotopes: Vital Tools for Science & Medicine, A Report of the National Research Council, National Academy Press, Washington, D.C., 1982. (NRC 1982) Adelstein SJ, Manning JF, eds. Isotopes for Medicine & the Life Sciences. Committee on Biomedical Isotopes. Division of Health Sciences Policy, National Academy Press, Washington, D.C., 1995. (IOM 1995) Ketchum LE, Green MA, Jurisson SS: Research Radionuclide Availability in North America. J. Nucl. Med. 38 (7): 15N-19N, and 38(8): 21N-48N, 1997. (Ketchum et al. 1997) Medical Isotope Workshop, Spicer KM, Baron S, Frey GD, O’Brien H, Gostic RC, Rowe RW, Spellman RMN, eds: Med Coll of South Carolina, Pub, 1998. (Spicer et al. 1998) Wagner HN Jr, Reba RC, Brown R, Coleman E, Knight L, Sullivan D, Caretta R, Babich JW, Carpenter A, Nichols D, Spicer K, Scott S, and Tenforde T. Expert Panel Forecast of Future Demand for Medical Isotopes. March, 1999, http://www.ne.doe.gov/nerac/isotopedemand.pdf (Wagner et al. 1999) Reba RC, Atcher RW, Bennett RG, Finn RD, Knight LC, Kramer HH, Mtingwa S, Ruth TJ, Sullivan DC, and Woodward JB. Final Report, NERAC Subcommittee for Isotope Research & Production Planning. April 2000, pp 1-32. Published on line by DOE and viewed at http://www.nuclear.gov/nerac/finalisotopereport.pdf (Reba et al. 2000) National Radionuclide Production Enhancement (NRPE) Program: Meeting Our Nation’s Need for Radionuclides. Society of Nuclear Medicine. May 2005. (SNM 2005) Audit Report: Management of the Department’s Isotope Program. DOE/IG-0709. November 2005. (DOE 2005) Rivard MJ, Bobek LM, Butler RA, Garland MA, Hill DJ, Krieger JK Muckerheide JB, Patton BD, Silberstein EB. The US national isotope program: Current status and strategy for future success. Applied Radiation and Isotopes 63 (2005) 157–178. (Rivard et al. 2005) |
ment and evaluation of new radiopharmaceuticals. The parasitic use of physics machines has failed to meet the radionuclide type, quantity, timeliness of production, and cost requirements of the medical research community. For example, copper-67 has shown great promise as a therapeutic radionuclide, but it is available only through the parasitic use of accelerators with missions other than radionuclide production.9 Another example is astatine-211, an alpha-emitting radionuclide that requires a medium-
TABLE 5.2 Therapeutic Radionuclides Used for Nuclear Medicine Research
|
Radionuclide |
Description |
Production |
|
Lutetium-177 |
Beta emitter, 6.7-d half-life |
Reactor |
|
Astatine-211 |
Alpha emitter, 7.2-h half-life |
Accelerator |
|
Yttrium-90 |
Beta emitter, 64-h half-life |
Reactor |
|
Rhenium-186 |
Beta emitter, 3.7-d half-life |
Reactor |
|
Rhenium-188 |
Beta emitter, 17-h half-life |
Reactor |
|
Holmium-166 |
Beta emitter, 27-h half-life |
Reactor |
|
Iodine-131 |
Beta emitter, 8.0-d half-life |
Reactor |
|
Samarium-153 |
Beta emitter, 46-h half-life |
Reactor |
|
Bromine-77 |
Beta emitter, 57-h half-life |
Accelerator |
|
Copper-67 |
Beta emitter, 62-h half-life |
Accelerator |
|
Actinium-225 |
Alpha emitter, 10.0-d half-life |
Accelerator |
|
Strontium-89 |
Beta emitter, 50.5-d half-life |
Reactor |
energy alpha-particle accelerator for its production. There are only a few accelerators remaining in the United States that are capable of producing astatine-211 and these are primarily used for clinical PET programs and for radiation therapy.
Although procuring isotopes from foreign countries, such as Germany and Russia, and increasing international collaborations are worthwhile alternatives, relying solely on foreign sources has a number of drawbacks. These include increased transit time over international borders, which for radionuclides that decay during transport, is an important consideration; and possible changes in radionuclide production priorities that may adversely affect U.S. researchers. A number of studies that have reviewed this issue have concluded that the United States should have a dedicated radionuclide production facility to meet the needs of the research community (IOM 1995, Wagner et al. 1999, Reba et al. 2000). The operation of such a facility would have to be subsidized to allow researchers to explore new and novel uses of radionuclides.10
For research that uses short-lived radionuclides, it is essential to have an accelerator onsite to provide these radionuclides when needed. The
existing hospital-based cyclotrons are, in general, fully committed to their own programs and cannot be considered as a reliable resource for exotic research radionuclides. In addition, many of these radionuclides can only be made on accelerators with energy of 30 MeV or above or require particles other than protons, neither of which can be provided by current hospital-based cyclotrons.
5.4.2
Non-Technical Needs and Impediments
The DOE-NE Isotope Program is failing to meet the needs of the research community because the effort is not adequately coordinated with NIH activities or with the DOE-Office of Biological and Environmental Research. Additionally, P. L. 101-101 (Sidebar 5.3), which requires full cost recovery for DOE-supplied radionuclides, whether for clinical use or research, has stifled research radionuclide production and radiopharmaceutical research. As a consequence, few new radiotracers have become commercially available over the past decade and there is a lack of radiotracers in the commercial pipeline.
In terms of research, the user community is a single investigator or small number of investigators, for whom the cost of producing exotic radionuclides exceeds available budgets. It has been difficult to include such expenses in research grants because the dollar value is disproportionately higher than other research expenses. Therefore, unlike commercial vendors who can pass on the costs to a wider user community, investigators looking into new ways to use radionuclides for diagnosis and treatment cannot afford the full costs of radionuclides sold by the DOE. Such a barrier reduces the demands for novel radionuclides. It has also created the perception that the nuclear medicine community is not interested because it is not requesting the radionuclides. While it is true that there are no new radionuclides with the requisite physical and chemical properties for use in imaging and therapy, there will continue to be investigations into new applications of the known radionuclides. Thus, an argument can be made that the DOE radionuclide production facility, which might benefit from new uses, should bear all or at least some of the development costs. However, the production facility is not a research organization, and so, some mechanism would need to be set up to vet applications for subsidy.
5.5
RECOMMENDATIONS
RECOMMENDATION 1: Improve domestic medical radionuclide production. To alleviate the shortage of accelerator- and nuclear reactor-produced medical radionuclides needed for research, a dedicated accelerator and an upgrade to a nuclear reactor should be considered.
This recommendation is consistent with other studies that have reviewed medical isotope supply in the United States and have come to the same conclusions (IOM 1995, Wagner et al. 1999, Reba et al. 2000).
RECOMMENDATION 2: Review enriched stable isotope inventory and evaluate domestic supply options if needed. The current inventory of enriched stable isotopes is decreasing and there is growing concern that the aging calutrons cannot be operated cost-effectively to meet demand if reopened. The DOE should evaluate the option of a domestic enriched isotope supply source to ensure availability for medical research.














