Summary and Assessment
GLOBAL INFECTIOUS DISEASE SURVEILLANCE AND DETECTION: ASSESSING THE CHALLENGES—FINDING SOLUTIONS
Early detection is essential to the control of emerging, reemerging, and novel infectious diseases, whether naturally occurring or intentionally introduced. Containing the spread of such diseases in a profoundly interconnected world requires active vigilance for signs of an outbreak, rapid recognition of its presence, and diagnosis of its microbial cause, in addition to strategies and resources for an appropriate and efficient response. Although these actions are often viewed in terms of human public health, they also challenge the plant and animal health communities.
Surveillance, defined as “the continual scrutiny of all aspects of occurrence and spread of a disease that are pertinent to effective control” (IOM, 2003; Last, 1995; WHO, 2000), involves the “systematic collection, analysis, interpretation, and dissemination of health data” (WHO, 2000). Disease detection and diagnosis is the act of discovering a novel, emerging, or reemerging disease or disease event and identifying its cause. Diagnosis is “the cornerstone of effective disease control and prevention efforts, including surveillance” (IOM, 2003).
Disease surveillance and detection relies heavily on the astute individual: the clinician, veterinarian, plant pathologist, farmer, livestock manager, or agricultural extension agent who notices something unusual, atypical, or suspicious and brings this discovery in a timely way to the attention of an appropriate representative of human public health, veterinary medicine, or agriculture. Most developed countries have the ability to detect and diagnose human, animal, and plant diseases
The Forum’s role was limited to planning the workshop, and the workshop summary has been prepared by the workshop rapporteurs as a factual summary of what occurred at the workshop.
and have some type of active or passive surveillance for many well-characterized agents. However, many developing countries—where most of the global population resides—lack the resources or infrastructure to support such activities.
One way to close this gap in infectious disease surveillance and detection may lie with the dispersion of technological advances such as regional syndromic surveillance, bioinformatics, and rapid diagnostic methods. Such tools and approaches have already made important contributions to infectious disease control and prevention efforts, albeit mainly in the developed world. Further improvements are expected to result from ongoing progress in infectious disease awareness and reporting, and from the continued development and deployment of efficient, low-cost diagnostic platforms. A major challenge to global disease surveillance and detection, and to this workshop, is not only the detection and reporting of well-characterized “known” infectious diseases, but also the ability to detect novel, emerging, or reemerging infectious diseases in relatively low-tech environments. There is a corresponding need to also develop redundant/ complimentary systems for infectious disease detection that go beyond the yield of the more traditional surveillance systems and approaches.
The Institute of Medicine’s (IOM’s) Forum on Microbial Threats convened a workshop addressing Global Infectious Disease Surveillance and Detection: Assessing the Challenges—Finding Solutions on December 12 and 13, 2006, to consider these and other scientific and policy issues relevant to the practice of disease surveillance and detection. To adequately cover a broad range of topics related to global infectious disease surveillance and detection, the Forum had to be selective in prioritizing the challenges and exploring solutions to disease detection and surveillance.
While the workshop did explore a variety of conventional and novel approaches for disease surveillance and detection, the workshop organizers did not attempt to critique standard domestic disease detection approaches nor did the workshop make recommendations about what an “optimal” or “desirable” disease surveillance and detection system would look like. Workshop participants examined current and emerging methods and strategies for the surveillance, detection, and diagnosis of human, animal, and plant diseases in order to assess resource needs and opportunities for improving and coordinating global infectious disease surveillance, detection, and reporting.
Organization of Workshop Summary
This workshop summary was prepared for the Forum membership in the name of the rapporteurs and includes a collection of individually authored papers and commentary.1 Sections of the workshop summary not specifically attributed
to an individual reflect the views of the rapporteurs and not those of the Forum on Microbial Threats, its sponsors, or the IOM. The contents of the unattributed sections are based on the presentations and discussions at the workshop.
The workshop summary is organized into chapters as a topic-by-topic description of the presentations and discussions that took place at the workshop. Its purpose is to present lessons from relevant experience, to delineate a range of pivotal issues and their respective problems, and to offer potential responses as described by workshop participants.
Although this workshop summary provides an account of the individual presentations, it also reflects an important aspect of the Forum philosophy. The workshop functions as a dialogue among representatives from different sectors and presents their beliefs about which areas may merit further attention. The reader should be aware, however, that the material presented here expresses the views and opinions of the individuals participating in the workshop and not the deliberations and conclusions of a formally constituted IOM study committee. These proceedings summarize only what participants stated in the workshop and are not intended to be an exhaustive exploration of the subject matter or a representation of consensus evaluation.
Surveillance Strategies
The practice of infectious disease surveillance is no longer restricted to its original role in recognizing outbreaks of feared human diseases. Workshop presentations reflected diverse goals, approaches, and methodologies for disease surveillance in humans, animals, and plants. To place these presentations and ensuing discussions in context, we begin by briefly describing the multiple purposes served by public health surveillance, as well as current disease surveillance practices in animals and plants.
Surveillance Purposes and Practices
Public Health Surveillance
In the United States, public health surveillance for infectious disease is conducted through a variety of state and federal programs (GAO, 2004). Health-care providers and others report cases of “notifiable” infectious disease (as defined by local and state health codes) to health departments; health department officials verify disease reports, monitor disease incidence, identify possible outbreaks, and forward their findings to the Centers for Disease Control and Prevention (CDC). CDC and other federal agencies, including the Food and Drug Administration (FDA), the U.S. Department of Agriculture (USDA), and the Department of Defense (DoD), independently gather and analyze information for disease surveillance. In addition, these agencies fund domestic and international networks of disease surveillance laboratories that develop diagnostic tests and conduct disease
diagnostic research. Although the CDC has provided guidelines for surveillance systems funded by the federal government, evaluation is generally lacking. Furthermore, as noted by Forum member Edward McSweegan, little evidence has been provided on the cost-effectiveness of massive federal public health surveillance investments (see also Eban, 2007).
Early Warning
Some surveillance systems are designed to provide early warning of a disease threat by detecting the mere presence of potentially infectious microorganisms. The federal BioWatch program, for example, uses a network of aerosol sampling stations to monitor major U.S. population centers for a range of potential biological agents, such as anthrax, plague, and tularemia (the entire list of pathogens is not publicly available) (Shea and Lister, 2003; OIG, 2005). The goal of this program is to detect biological agents within 36 hours of release, allowing federal, state, and local officials to organize a timely response (OIG, 2005).
Surveillance also extends to symptoms indicative of infectious disease. Syndromic surveillance2—the real time monitoring of nonspecific, prediagnostic indicators for disease outbreaks—has been widely adopted by cities, states, and the federal government as a means to provide early warning of infectious disease outbreaks (Sosin, 2003; Stoto, 2005). Several syndromic surveillance systems are currently operational. The Real Time Outbreak and Disease Surveillance System (RODS) is used by several states to gather data on the symptoms of emergency room patients (GAO, 2004). The RODS laboratory at the University of Pittsburgh also created the National Retail Data Monitor (NRDM) to examine sales of over-the-counter health-care products.3 The Electronic Surveillance System for the Early Notification of Community-Based Epidemics (ESSENCE), operated by DoD, allows epidemiologists to track, in real-time, syndromes reported in daily data feeds from regional hospitals and clinics in the National Capital area (GAO, 2004). The federal BioSense program—in which the United States has invested an estimated $230 million since its 2004 inception—aggregates data relevant to bioterrorism and other public health threats from numerous electronic sources
|
2 |
The term “syndromic surveillance” applies to surveillance using health-related data that precede diagnosis and signal a sufficient probability of a case or an outbreak to warrant further public health response. Though historically syndromic surveillance has been used to target investigation of potential cases, its utility for detecting outbreaks associated with bioterrorism is increasingly being explored by public health officials (CDC, 2006a). |
|
3 |
The National Retail Data Monitor (NRDM) is a public health surveillance tool that collects and analyzes daily sales data for over-the-counter health-care products. NRDM collects sales data for selected over-the-counter health-care products in near-real time from more than 15,000 retail stores and makes them available to public health officials. NRDM is one of the first examples of a national data utility for public health surveillance that collects, redistributes, and analyzes daily sales-volume data of selected health-care products, thereby reducing the effort for both data providers and health departments. For further information on the NRDM, see Wagner et al., 2004. |
(GAO, 2004; Eban, 2007). Despite the considerable investments that have been made in domestic syndromic surveillance systems, many workshop participants noted, their promise remains largely unproven (Descenclos, 2006; Bravata et al., 2004; Reingold, 2003; RAND Corporation, 2004; Stoto, 2005; Sosin, 2003).
Situational Awareness
Surveillance approaches are also used to monitor the progress and outcome of an intervention to mitigate or stop the progression of a communicable disease, as during the recent severe acute respiratory syndrome (SARS) pandemic (IOM, 2004; Heymann and Rodier, 2004) and in the campaigns to eradicate smallpox (Henderson, 1999) and polio (WHO, 2006). The broad and multifaceted use of surveillance to describe and inform response over the entire course of an outbreak, known as “situational awareness,” was a central topic of workshop discussion, as noted below.
Animals
The practice of surveillance is not limited to human diseases. Some surveillance systems protect economically and ecologically important animal or plant species; others are designed to detect transmission of a zoonotic disease among animal and human populations over space and time, and to predict future transmission patterns.
Within the complex network of federal agencies that govern animal health, separate—and in some cases, parallel—surveillance programs are conducted by USDA, Department of Homeland Security (DHS), DoD, Department of Health and Human Services (HHS), the Department of the Interior (DoI), and Department of Commerce (NRC, 2005). As noted in the recent National Research Council report, Animal Health at the Crossroads, “whether due to historic structures or functions of … related federal, state, and local governments, or because of changes and challenges in funding and resources, there is an apparent disconnect between [animal health] agencies that should function in partnership” (NRC, 2005). A further element of disintegration is introduced through the practice of disease-specific surveillance at both federal and state levels.
Technological advances in disease detection that have benefited public health surveillance—such as rapid, automated, sensitive, and portable sampling and assay systems and DNA-based diagnostic tools—remain to be adapted to track animal diseases (NRC, 2005). Such tools could have significantly reduced the severe burden of recent outbreaks such as exotic Newcastle disease (END) among chickens in the United States and foot-and-mouth disease (FMD) among cattle in the United Kingdom; a recent analysis supports the use of polymerase chain reaction (PCR) to screen bulk milk for the FMD virus (Thurmond and Perez, 2006). Other early warning technologies with potential application to animal
disease surveillance include embedded monitoring chips to measure temperature and other physiological states, gene-based pathogen assays, and biosensors.
Plants
Plant disease surveillance occurs at several levels: through growers, who monitor crops for signs of disease; at the local and regional levels, by private crop consultants and USDA cooperative extension agents who diagnose disease and provide advice to growers on outbreak management; at the national level, through programs such as the National Plant Diagnostic Network (NPDN; see subsequent discussion) and BioWatch; and at the international level through collaborative research organizations such as the Consultative Group on International Agriculture Research (CGIAR) (Fletcher, 2005; Stack et al., 2006).
In recent years, a broad range of molecular techniques, including PCR-based and immunological assays and DNA arrays, have been adapted to detect and track the spread of plant pathogens (Alvarez, 2004; Schaad et al., 2003). Although routine diagnosis of many crop diseases can now be made within a day by real-time PCR, there is further need to develop same-day, onsite protocols for identifying plant pathogens, as well as standardized procedures to validate diagnostic protocols (Schaad et al., 2003). In theory, earlier detection of plant pathogens could be achieved through the capture of molecular signals from pathogens in situ; however, this and related technologies are likely to be first applied to detect animal and human pathogens (Cook, 2005; Schaad et al., 2003).
Public Health Surveillance: A Local Perspective
The traditional model of infectious disease surveillance remains essential to public health practice, particularly at the local level. Speaker Marci Layton, of the New York City Department of Health and Mental Hygiene (DOHMH), emphasized the importance of reports—of both routine and unusual findings—by health-care providers to local health departments. The interpretation and investigation of such reports by DOHMH officials supports the identification and control of infectious disease in one of the world’s largest and most cosmopolitan cities (see Chapter 1 overview). These efforts have been boosted in recent years by the introduction of electronic reporting for laboratory results and web-based reporting by health-care providers. An alert system has also been established to inform area health-care providers of public health emergencies.
Because of the high risk for disease importation into New York City, DOHMH officials stay abreast of international infectious disease trends, ramping up surveillance and alerting health-care providers in response to threatened outbreaks. The city has also invested federal funding to improve the ability of hospital triage systems to identify and appropriately treat patients who show symptoms associated with an emerging infectious disease. This is crucial, Layton observed,
because New York City “could be the next Toronto, with an unrecognized SARS outbreak from overseas.”
Syndromic Surveillance
Layton noted that many infectious disease threats (e.g., influenza, SARS, and viral encephalitis, as well as potential bioterrorism agents such as anthrax and smallpox) manifest as syndromes with nonspecific symptoms (“influenza-like symptoms”). In the case of a rapidly spreading, emerging infectious disease, laboratory diagnosis may be impossible. Under these circumstances, she said, syndromic surveillance systems can alert public health authorities to an outbreak before it is revealed in reports from health-care providers.
Keynote speaker Patrick Kelley, director of the Institute of Medicine’s Board on Global Health, and presenter Michael Stoto, of the Georgetown University’s School of Nursing and Health Studies, reviewed the theoretical underpinnings and historical development of syndromic surveillance (see Kelley, Stoto in Chapter 1). When people first develop symptoms, following an exposure or first contact with a novel or rapidly emerging infectious disease, they may be much more likely to attempt to treat themselves and stay home from work or school rather than seeking care from a health-care provider to obtain a clinical or laboratory diagnosis (Stoto, 2005). Syndromic surveillance systems monitor existing descriptive data of these behaviors (e.g., school and work absenteeism, sales of over-the-counter medications, illness-related 911 calls, emergency room admissions for symptoms indicative of infectious disease) for patterns or clusters of behaviors suggestive of an illness outbreak. The concept of syndromic surveillance is doubly attractive because in addition to its potential to increase the speed and effectiveness of the public health response to natural or deliberate disease outbreaks, it costs far less to implement than traditional, labor-intensive approaches to disease surveillance (Stoto, 2005). However, the ability of syndromic surveillance to reduce disease-related morbidity and mortality remains to be demonstrated, as does its cost-effectiveness (Bravata et al., 2004; Reingold, 2003; RAND Corporation, 2004; Stoto, 2005; Sosin, 2003). Although rigorous evaluations of syndromic surveillance in general may be impossible, individual systems can be assessed under a variety of circumstances (Reingold, 2003). Moreover, because syndromic surveillance systems are warning devices, it will be critical to determine their utility within the context of health systems that respond to both “true” and “false” alarms (Pavlin, 2003; RAND Corporation, 2004).
Global Syndromic Surveillance
In parts of the world where clinicians are in short supply, syndromic surveillance offers a promising model for disease detection, Kelley observed (see
Chapter 1). Infectious disease is a major cause of morbidity and mortality in low-resource populations, and such environments frequently provide amplifying conditions for emerging pathogens. Recognition of this threat has spurred the World Health Organization (WHO) to revise the International Health Regulations (IHRs)—the legal framework for international cooperation on infectious disease surveillance. Once limited to a trio of internationally notifiable diseases (plague, cholera, and yellow fever), as of June 15, 2007, the revised IHRs became the “world’s first legally binding agreement in the fight against public health emergencies of international concern” (WHO, 2007). Reporting of new and reemerging diseases with epidemic or pandemic potential, as well as diseases associated with acute chemical or radionuclear events, will be mandatory regardless of their origin or source (WHO, 2007).
“The mandate for general global public health surveillance is moving beyond named diseases to encompass a global responsibility to detect and report in a timely manner internationally important disease events, whether they are individual cases or clusters, whether they are well-defined diseases or ill-defined diseases,” Kelley explained. Syndrome detection is central to this new paradigm, and should be viewed as one of a collection of approaches to global surveillance for infectious diseases, he said. However, he also noted considerable challenges in moving syndromic surveillance from theory to practice.
Syndromic Surveillance by Design
Kelley emphasized that a key step in developing effective syndromic surveillance systems—and one that has frequently been overlooked—is the precise definition of system capabilities. While considerable effort has been applied to the development of syndromic definitions (e.g., for flu-like illnesses that may indicate bioterrorism), far less attention has been paid to identifying robust detectors of those conditions, he said. Moreover, rather than formulate clear and specific questions and design systems to answer them, he observed that developers of syndromic surveillance systems have too often created systems based on opportunistic datasets.
In addition to appropriate data to answer essential questions, a system for public health surveillance requires powerful analytical tools, as well as technically proficient analysts to use them and accurately interpret the findings, Kelley said. He added that these considerations are equally applicable to domestic surveillance programs that, due to their complexity, might be fruitfully developed through academic partnerships with individual communities. Kelley also advocated strengthening the epidemiological capacity at the local level in order to inform the interpretation of syndromic findings in light of “local epidemiological peculiarities,” as well as to ensure a rapid response to syndromic alerts.
From Syndromic Surveillance to “Situational Awareness”
Syndromic surveillance systems are handicapped by their very nature. Not only must they obtain relevant and accurate data quickly and from a variety of sources, but they must also be tuned to recognize unusual trends against a highly variable background; otherwise, syndromic surveillance systems may either miss an important event or generate unacceptable levels of false positives (see contributions by Kelley, Stoto in Chapter 1). Indeed, Stoto explained, according to the syndromic detection algorithm, it is impossible to increase the sensitivity, specificity, or timeliness of syndromic detection without reducing the other two attributes. This point is illustrated by a recent model of outbreak detection for inhalational anthrax by Buckeridge and colleagues (2006), who concluded that “when syndromic surveillance was sufficiently sensitive to detect a substantial proportion of outbreaks before clinical case finding, it generated frequent false alarms” (Buckeridge et al., 2006).
Stoto explored several additional examples of this dilemma, all of which support his contention that traditional, statistics-based syndromic surveillance systems are unsuited to the detection of rare, small-scale events such as a localized biological attack or the initial cases of a newly imported or emerging disease. He suggested, rather, that syndromic surveillance was most likely to be valuable in detecting potentially large-scale, natural disease outbreaks (e.g., seasonal and pandemic influenza, foodborne disease) for which the useful “detection window” is relatively broad.
Case-Finding by Syndrome
Instead of bypassing health-care providers, Stoto said that syndromic surveillance technology could be used to “arm astute physicians and health departments with modern approaches to finding small numbers of cases” and allow health professionals to identify them before they are formally diagnosed. Such “case-finding” surveillance systems currently in operation include the Syndromic Reporting Information System (SYRIS) (ARES, 2007; Mandl et al., 2004; CDC, 2006b), Rapid Syndrome Validation Project (RSVP) (Zelicoff et al., 2001), and Lightweight Epidemiological Advanced Detection Emergency Response System (LEADERS) (Green and Kaufman, 2002). Because case-finding syndromic surveillance requires early reporting of symptoms, it can only succeed in “an atmosphere that doesn’t penalize people for getting it wrong,” Stoto said (and, as other participants noted, for getting it right, that is, for being the bearer of bad news). Under enabling conditions, he said, case-finding syndromic surveillance could build the kind of strong relationships between public health and health-care providers that are critical to effective outbreak response.
“Like any alarm system, [syndromic surveillance is] only as good as what happens when the bell rings,” Stoto concluded. “It must be followed with active
surveillance and epidemiological investigation, and with policy decisions regarding intervention.” Speaker Joseph Lombardo, of the Johns Hopkins University Applied Physics Laboratory, further advised that syndromic surveillance systems be designed to meet the specific needs of epidemiologists and public health analysts. “The tools need to be built to support those individuals, and I believe public health informatics has a tremendous role in doing that,” he said.
Situational Awareness
Several workshop participants described the use of syndromic surveillance data beyond the mere detection of behavioral “signals” of an outbreak. Kelley noted that syndromic data could support efforts to characterize infectious diseases, help target outbreak response, and inform risk communication. Lombardo distinguished between syndromic surveillance, which he defined as an automated detection and alarm system, and “situational awareness,” a term long used by DoD that encompasses disease classification, tracking, response, and outcome monitoring, in addition to detection. Viewed through the lens of situational awareness, syndromic surveillance provides a rapid means to obtain descriptive data throughout the course of an infectious disease outbreak. Epidemiologists and others who monitor surveillance findings represent “the most important component of an advanced disease surveillance system,” Lombardo insisted. “They cannot be replaced by statistics.”
Real-Time and Batched Reporting
In addition to collecting strategic data, well-designed public health surveillance systems incorporate appropriate mechanisms to process information and deliver it to users. The computational performance of these tasks may occur in “real time” or it may be “batched,” according to Lombardo, who explained the implications of these descriptions for infectious disease surveillance (see Chapter 1).
Real-time computing methods (presently used in video games and automotive safety systems) permit an immediate response to surveillance data, Lombardo said. Batching may occur at any of several junctures along the path from data collection to reporting, he explained; the term “batched reporting” may therefore reflect the simultaneous collection of multiple data points, or the contemporaneous processing of data collected at different times, or the reporting, at regular intervals, of the outcome of sequentially processed data. Batched health data may be reported to users as soon as it is processed, at regular intervals, or accessed on demand.
Breaking the electronic surveillance process into a series of steps, Lombardo compared the potential and consequences for real-time and batched reporting. Only certain syndromic surveillance data are available in real time, he noted.
For example, while cash registers transmit medication purchases immediately, schools report absenteeism on a daily basis. Moreover, he observed, “the benefits of real-time data collection are only realized if the other components of a surveillance system are real-time as well.”
Data may be continuously communicated for processing via a virtual private network (used in some hospitals), or it may be sent by file transfer protocol as batched files in intervals of seconds to hours. At the data processing step, the distinction between real time and batched may not be meaningful if computation is complex and therefore time consuming, Lombardo observed. For example, a spatial analysis of disease phenomena across a series of ZIP codes could take a long time to process; however, surveillance systems can allow analysts selectively to invoke certain processes in real time in order to monitor a potentially urgent situation.
Surveillance reports can be delivered in real time, in the form of automatic alerts, but Lombardo described considerable problems with this feature. As previously noted, many reports that are based on syndromic data represent false positives and will therefore require an epidemiologist’s attention and expertise to discern a true signal among considerable background noise. This can take time.
Unless surveillance reports are subject to continuous analysis, it makes no sense to invest resources in providing them on a real-time basis, Lombardo concluded. “Getting information several times an hour should be more than adequate for public health needs,” he said. To provide for public health emergencies, he envisioned two modes of operation for advanced disease surveillance systems: batched reporting for routine analysis, and real-time reporting, which would be based on case definition and used for more focused surveillance during a crisis.
Animal Disease Surveillance
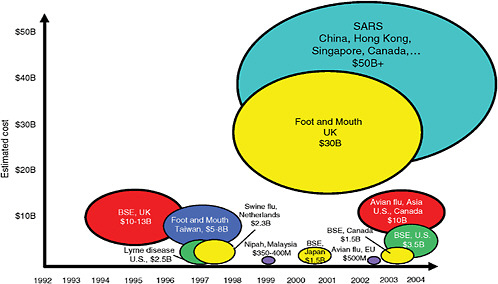
Two important factors contribute to the proliferation of zoonotic diseases: the explosive growth of human and domestic animal populations, and the increasingly close physical proximity within which humans and domestic and wild animals live (Karesh and Cook, 2005; NRC, 2005). Infectious diseases primarily affecting animals can have both direct and indirect impacts on humans (Table SA-1), including significant economic consequences (Figure SA-1). Therefore it is widely acknowledged that the timely identification of future emerging microbial threats (on the order of SARS, West Nile virus, or H5N1 avian influenza) will require an integrated international approach to disease surveillance. Progress toward this goal has been hampered by a variety of economic and political factors, most notably the threat of trade embargoes against countries that voluntarily report livestock or wildlife disease outbreaks.
Although they share comparable objectives, the U.S. animal health community lags far behind its public health counterpart in terms of surveillance infrastructure and technology (NRC, 2005). These deficits were raised in several
TABLE SA-1 Animal Diseases Associated with Direct and Indirect Human Impacts
|
Infectious Disease |
Affects Wildlife |
Affects Domestic Animals |
Affects Humans Directly |
Affects Humans Indirectly |
|
Brucellosis |
X |
X |
X |
X |
|
Canine Parvovirus |
X |
X |
|
|
|
Chagas |
X |
X |
X |
|
|
Distemper |
X |
X |
|
|
|
Foot-and-mouth disease |
X |
X |
|
X |
|
Leishmania |
X |
|
X |
|
|
Leptospirosis |
X |
X |
X |
|
|
Rabies |
X |
X |
X |
X |
|
Scabies |
X |
X |
X |
|
|
Toxoplasmosis |
X |
X |
X |
|
|
SOURCE: WCS (2007). |
||||
workshop discussions, and particularly in comments from veterinary pathologist Tracey McNamara of the Wildlife Conservation Society (WCS). In 1999, McNamara linked the presence of dead birds on the grounds of two New York City zoos with the first human cases of West Nile encephalitis in the United States. Thereafter, she led the effort to create a national surveillance network for the disease involving more than 35 zoos (Watanabe, 2002). A far more comprehensive and integrated strategy is needed for the surveillance of zoonoses, McNamara said. She noted that there is no provision for veterinarians who routinely diagnose infectious disease in zoo animals and wildlife to report unusual findings or send samples to public health authorities for testing, as physicians are required to do. “Zoos are the most overlooked long-term epidemiological monitoring stations in the United States and in the world today,” she concluded.
As part of their overall mission, WCS conducts routine surveillance for a wide variety of infectious diseases in animals around the world, including the 20,000 residents of zoological parks in the New York City area. In his presentation, William Karesh, director of the Society’s Field Veterinary Program, described ongoing programs to monitor two important zoonoses: Ebola virus and avian influenza (see Karesh in Chapter 1).
Ebola Virus Surveillance in Central Africa
In a reversal of standard public health thinking, WCS views humans as a worrisome source of diseases that infect great apes. To protect endangered gorillas in central Africa from the Ebola virus, WCS has supported human disease surveillance among the underserved populations that live in close contact with the gorillas by training local people in simple data collection, syndromic surveil-
lance, and basic laboratory diagnosis. These efforts, which helped to identify the link between human outbreaks of Ebola virus and the consumption of gorilla and chimpanzee meat (Leroy et al., 2004), now enable community members to avoid infection with the virus by providing early warning of outbreaks in animals. Current surveillance is also directed toward evaluating the effectiveness of human Ebola vaccine candidates in wildlife.
In addition to their efforts on behalf of gorillas, WCS has sought to teach people in central Africa how to avoid getting Ebola through basic hygiene measures such as hand washing and cooking meat thoroughly. These lessons have afforded opportunities to improve overall food safety in communities, Karesh observed.
Global Surveillance for Avian Influenza
Over the course of several years, WCS has worked with individual governments to conduct surveillance for avian influenza in wild birds. This typically involves basic epidemiology and viral sample collection and characterization (by CDC); in some instances, birds are tracked with radio transmitters. In Mongolia, such a program has provided a candidate virus for development of a human influenza vaccine, Karesh said. More recently, these individual efforts have been combined into the Global Avian Influenza Network for Surveillance (GAINS). The program seeks to expand international surveillance for influenza in wild birds and promote the dissemination of surveillance information to governments, international organizations, the private sector, and the public.4 With support from USDA, the U.S. Agency for International Development (USAID), and the Food and Agriculture Organization of the United Nations (FAO), GAINS trains individuals and organizations to collect samples for analysis by a network of diagnostic labs, the results of which are disseminated through a common, open-access database. Participants in the program, which currently reaches 24 countries, include hunters, birdwatchers, and other members of the public, as well as animal health professionals. Karesh acknowledged that GAINS raises privacy concerns in the United States. (“Who wants to say they have a sick bird on their property?”), but he also observed, “the rest of the world doesn’t seem to have that problem.”
Indeed, as Karesh notes in Chapter 1, the early success of GAINS has led to an expansion of the program to address a broader range of infectious diseases and species. The Wildlife Global Animal Information Network for Surveillance (Wildlife GAINS) aims to establish “a comprehensive worldwide wildlife health surveillance system to enhance preparedness for and awareness of emerging infectious diseases,” he reports.
|
4 |
See http://www.gains.org. |
Plant Disease Surveillance and Detection
While plant health programs address many of the same challenges (e.g., globalization, biosecurity) and use similar tools and approaches as their animal and public health counterparts, the near impossibility of preventing the global spread of plant pathogens orients surveillance and detection toward preparedness for disease. Agricultural production in the United States is especially vulnerable because it encompasses vast areas, observed speaker Jacqueline Fletcher of the University of Oklahoma (see Fletcher and Stack in Chapter 1).
Considerable time often elapses between the introduction of an agricultural pathogen and its detection; therefore, federal programs such as the aforementioned NPDN focus on the early detection of plant diseases to minimize economic losses. Because it would be too expensive to eradicate the more than 50,000 plant diseases currently in the United States, the typical strategy is to minimize the economic impact of each disease, Fletcher explained. However, given sufficient warning prior to the introduction of a new plant disease threat, researchers can reduce the impact of disease by identifying chemical control measures or by breeding resistant crop varieties.
National Plant Diagnostic Network
Early detection, aimed at reducing the economic impact of plant diseases, is the central mission of NPDN, according to speaker James Stack of Kansas State University (see Stack and Fletcher in Chapter 1). Created in 2002 by the U.S. Secretary of Agriculture, NPDN links plant disease and pest diagnostic facilities at land grant universities in order to provide rapid detection and accurate diagnosis of important plant pathogens and pests (Stack et al., 2006). These efforts received further federal support in 2004, under a Homeland Security Presidential Directive (HSPD-9), which “establishes a national policy to defend the agriculture and food system against terrorist attacks, major disasters, and other emergencies.” HSPD-9 mandates the development of a national agricultural biosecurity initiative that would expand capacity for disease detection and diagnosis (White House, 2004).
According to Stack, NPDN is currently pursuing a range of passive and active disease surveillance strategies. These include sentinel surveillance for specific pathogens (e.g., soybean rust, introduced to the United States by hurricanes in 2004); random surveys for plant disease conducted by specialists throughout the country; strategic and bidirectional surveillance, which attempt to locate the source of disease outbreaks; syndromic surveillance (also for soybean rust); and biosensors (for toxin-producing pathogens in stored grains and seeds). While mandatory reporting of high-consequence pathogens and pests has been instituted in some circumstances, Stack noted that the considerable disincentives to do so probably lead to high rates of noncompliance.
NPDN has also undertaken several projects intended to maximize the productivity of shrinking numbers of plant scientists trained in diagnosing disease, Stack said. These include the creation of a curriculum to teach agricultural workers to recognize signs of important plant diseases, and the development of diagnostic databases and a “telemedicine” system that links state agricultural labs to diagnostic experts.
National Center for Plant Biosecurity
Fletcher discussed a proposal for a comprehensive National Center for Plant Biosecurity that has been endorsed by a broad coalition of scientific societies with a common interest in crop protection. It is envisioned that the center, modeled on CDC, would coordinate plant disease information at the national level and collect and disseminate knowledge on plant-disease management and agricultural biosecurity. The center’s mission would be fundamentally different from that of USDA, which is driven by the needs of agribusiness and focused on near-term profitability, Stack explained. “We need another group that can step back from that and look at our plant-based systems from a strategic standpoint,” he said.
Although the threat of agricultural bioterrorism provided the impetus for proposing the center’s creation, Fletcher said, its benefits would be substantial in the absence of such crises. This is particularly true because former USDA functions, such as the Animal and Plant Health Inspection Service (APHIS) and the Plum Island Animal Disease Center, were subsumed by DHS, Stack observed. That move has diminished attention to the specific missions of these agencies, which represent “a first pair of eyes for what is coming into the country,” he said. “If they don’t know what they are looking for, then that’s wasted time.”
Surveillance Networks
In traditional public health surveillance (based on reports from medical practitioners, as described in the previous section), information travels up or down the public health hierarchy, from the local to the international level and vice versa. According to Forum member Stephen Morse, this seemingly orderly scenario tends to produce surveillance that is patchy and erratic due to differing priorities at various levels of the public health system, and information that is too often focused on “diseases of the moment.” By contrast, electronic network surveillance systems have the potential to recognize any outbreak, including that of an emerging or otherwise unexpected disease, on a global scale.
The presentations and discussions summarized below demonstrate the power of electronic networks to collect and integrate information on infectious disease from a variety of sources and the ability of networks to disseminate such intelligence widely and rapidly to the user community. Participants also noted several
limitations of disease surveillance networks and—much as they had done for syndromic surveillance systems—urged the creation of network surveillance technologies that address specific public health needs and strengthen connections between “astute clinicians” and public health practitioners.
ProMED-Mail
The first disease surveillance network, ProMED-mail (PMM), began in 1994 as a project of the Federation of American Scientists’ Program for Monitoring Emerging Diseases (ProMED) (Madoff and Woodall, 2005). Morse, a founding member of both ProMED and PMM, recounted that the network was initially intended to enhance communication between working group members; however, the listserv’s potential as an outbreak reporting system was quickly recognized and expanded (see Morse in Chapter 2). Now sponsored by the International Society for Infectious Diseases, PMM is a free, nonprofit, noncommercial, moderated e-mail list that serves in excess of 37,000 subscribers in more than 150 countries, as well as anyone with access to the website.5
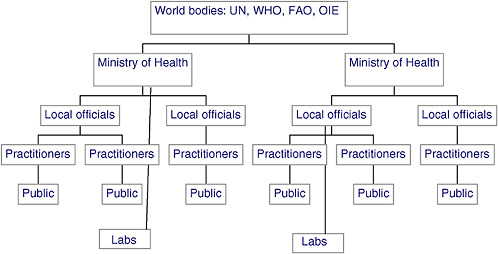
As illustrated in Figure SA-2, traditional public health reporting follows a linear “bottom-up” process, beginning with an ill person presenting to a local doctor, where they may receive medical tests. If the doctor or laboratorian finds evidence of a “reportable” disease, or merely something unusual, he or she reports the discovery to local health officials. If the apparent threat is severe, local health officials report it to the national ministry or department of health, which forwards the report to international health organizations (“world bodies”) if the case is of global concern.
Figure SA-3 depicts the nonhierarchical ProMED network, which fosters the exchange of information on reported diseases among a variety of sources.
In addition to volunteer “rapporteurs,” who provide information on possible infectious disease outbreaks specific to their geographic area, PMM receives information from subscribers (who may report firsthand or from other sources) and from staff-conducted searches of the Internet, media, and various official and unofficial websites (Madoff, 2004). Moderators assess these reports for plausibility (via established rumor verification protocols and private query to experts), edit them as necessary, and often add comments or context before posting. Furthermore, because PMM aggregates reports from various locations, it can reveal the geographical extent of an outbreak. Morse noted that this system has resulted in several emerging disease reporting “firsts,” including outbreaks of Ebola virus in Zaire (1995), West Nile virus in the United States (1999), SARS in China (2002), and H5N1 avian influenza in Indonesia (2003).
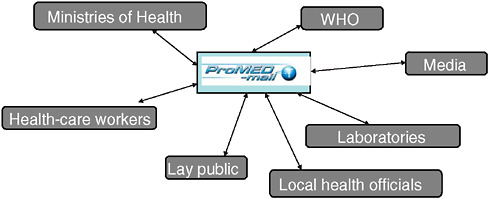
FIGURE SA-3 The power of the ProMED surveillance network.
SOURCE: Figure courtesy of Dr. Larry Madoff.
Global Outbreak Alert and Response Network
To connect the growing number of surveillance networks that followed PMM in terms of capacity for infectious disease diagnosis and response, WHO established the Global Outbreak Alert and Response Network (GOARN) in 2000.6 Conceived as a “network of networks,” GOARN pools human and technical resources from more than 100 institutions around the world (WHO, 2005) in order to rapidly identify, confirm, and respond to outbreaks of international importance. In 2002, after receiving worrisome reports from the Global Public Health Intelligence Network (GPHIN; see below) and the U.S. Global Emerging Infection Surveillance and Response System (GEIS), GOARN initiated the global response to an outbreak of a disease that would be named SARS (IOM, 2004; Heymann and Rodier, 2004).
The Global Public Health Intelligence Network (GPHIN)
Harnessing the power of automated Internet searching for disease surveillance, GPHIN scans thousands of websites in eight languages—including those identified by two “news aggregators,” who monitor thousands of news sources in dozens of languages—for early signs of infectious disease outbreaks in humans, animals, and plants, as well as for chemical incidents and disease threats associated with natural disasters (Mykhalovskiy and Weir, 2006). Abla Mawudeku, manager of GPHIN within the Public Health Agency of Canada, described the network’s creation by that agency in partnership with WHO in 1998, its operation and ongoing development, and its possible future as part of a planned open-access
surveillance program under the auspices of a yet-to-be-named nongovernmental organization (see Mawudeku et al. in Chapter 2).
After a scoring system sorts some 2,000 articles retrieved by GPHIN daily, a team of multilingual, multidisciplinary, and multicultural analysts review those articles deemed most relevant, Mawudeku explained. Several analysts work in staggered shifts to provide round-the-clock coverage. Upon receiving a report of concern, they follow a decision tree, based on IHR criteria (which may lead analysts to corroborate reports with other surveillance networks, such as ProMED-mail), to determine whether to post an alert. GPHIN does not systematically validate the information it posts, but relies on WHO to verify outbreak alerts through its country contacts (Mykhalovskiy and Weir, 2006). Figure SA-4 shows the source of initial reporting of events of potential public health concern by WHO in 2001–2002.
Subscribers to GPHIN receive alerts by e-mail or when they log on to the system’s website. In addition to WHO and Canadian governmental agencies (e.g., food inspection, defense, police), GPHIN’s audience includes ministries of health and departments of agriculture from several nations, as well as FAO, the World Organization for Animal Health (OIE),7 and the North Atlantic Treaty Organization (NATO). Depending on the services provided, GPHIN subscriptions (which in part reflect the expense of subscribing to news aggregators) cost 30,000 to 200,000 Canadian dollars per year, according to Mawudeku (Public Health Agency of Canada, 2007).
To continue to improve its service, GPHIN has begun to evaluate its own effectiveness. Criteria are based on the number of users; the timeliness, sensitivity, and specificity of alerts; the stability of the system in terms of limited downtime; and the flexibility of the system in terms of accommodating new technologies and in modifying search criteria to gather information on a situation of interest, Mawudeku said. The cost of operating and upgrading the system is a continual challenge, she observed, as well as a barrier to use by low-resource countries and agencies. However, recent events may portend a change in this situation. Having received the Technology, Entertainment and Design (TED) prize in 2006, epidemiologist Larry Brilliant (who played a key role in WHO’s campaign to eliminate smallpox, and who currently serves as Google’s chief of philanthropy) is currently marshalling an effort by the influential TED community to expand and enhance the GPHIN model (Zetter, 2006; Google, 2006; Hempel, 2006). With GPHIN as a starting point, Brilliant hopes to create a freely available, internationally independent system for the early detection of infectious disease outbreaks.
Due to potential conflicts of interest with the Canadian government, GPHIN’s staff cannot participate in the negotiations to make this service independent, Mawudeku explained. “I can’t tell you what will happen to the GPHIN system, whether we will continue to be within the [Public Health Agency of Canada] or not,” she said, adding that Google’s attention to GPHIN had at least prompted
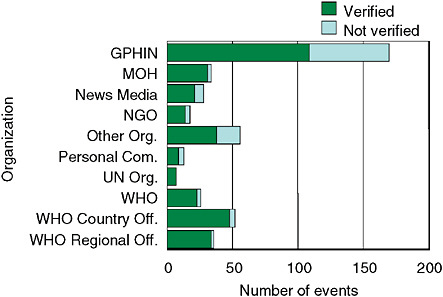
FIGURE SA-4 Source of initial reporting of potential events of public health concern by WHO between January 1, 2001, and December 31, 2002. Legend: Global Public Health Intelligence Network (GPHIN); Ministry of Health (MOH); Nongovernmental Organization (NGO); United Nations (UN); World Health Organization (WHO).
SOURCE: Mawudeku et al. (2002).
recognition that the network would benefit from greater financial support than the Canadian government currently provides. It would also satisfy the concerns of Forum member Gerald Keusch, who observed that government-operated sources of surveillance information raise “serious issues of credibility.”
HealthMap
HealthMap,8 a freely available, web-based surveillance network operating since September 2006, provides a global view of infectious disease outbreaks as reported by the WHO,9 PMM, Google News,10 and Eurosurveillance.11 John Brownstein, of the Children’s Hospital Informatics Program, Harvard–
|
8 |
|
|
9 |
|
|
10 |
|
|
11 |
Eurosurveillance, a free and open-access multiformat journal, publishes peer-reviewed information on communicable diseases from a European perspective. In March 2007, Eurosurveillance became the independent scientific in-house journal of the European Centre for Disease Control and Prevention (ECDC) in Stockholm, Sweden. For more information, see http://www.eurosurveillance.org. |
Massachusetts Institute of Technology (MIT) Division of Health Sciences and Technology, and the Harvard Medical School Center for Medical Bioinformatics, described the design of the system and efforts to evaluate its data sources (see Brownstein et al. in Chapter 2).
There is an abundance of open-source electronic surveillance networks for infectious disease, Brownstein said, but none provide a truly global perspective due to gaps in geographic or population coverage and expertise. HealthMap attempts to bridge these gaps by aggregating and integrating information from several surveillance networks to produce a graphic, continually updated model of global disease outbreaks over space and time. Alerts are displayed on a global map that can be viewed at a wide range of resolutions and they are linked to source sites that provide news of the outbreak and information on the disease.
Recognizing the tradeoffs between alert sensitivity and specificity, HealthMap’s creators are conducting an ongoing evaluation of their data sources with respect to these criteria (see Brownstein et al. in Chapter 2). Brownstein reported that, based on their first two months’ of data, PMM provided slightly greater timeliness and better coverage of rare infections as compared with Google News. On the other hand, he noted, news feeds provide a larger volume of data, making them more useful for describing the temporal and spatial distribution of large-scale seasonal infections (Figure SA-5). Brownstein believes “there is a real value in integrating these data sources … to get a much better picture of a global state of infectious diseases.”
HealthMap’s creators plan to expand their data sources to include CDC, the private sector, laboratories, the military, and blog searches. They also plan to incorporate information on animal infections and biotic and abiotic factors that influence disease emergence and transmission, Brownstein said. In addition to addressing reporting biases in current datasets, he noted that these additional sources should support the assessment of population risk, disease severity, and pathogen transmission within the HealthMap model. Data verification remains a challenge, he added; with only two employees, HealthMap must rely on data that have been validated by others—such as ProMED-mail reports.
Several participants recognized potential synergies between HealthMap and GPHIN, with each network offering important elements (HealthMap’s openness; GPHIN’s reach and verification capacity) of a long desired and sought-after “system of systems” approach to infectious disease surveillance. GPHIN’s current status within Health Canada and its reliance on commercial news aggregation services would likely prohibit the network from supplying data to HealthMap, Mawudeku explained; however, such collaboration could be possible if GPHIN evolves into an open, nongovernmental network as envisioned by Brilliant.
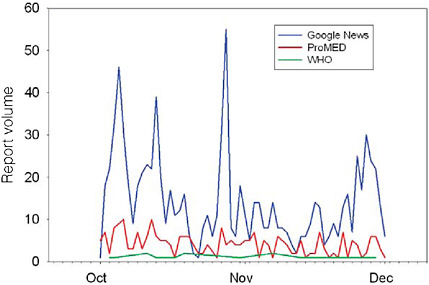
FIGURE SA-5 HealthMap alert volume by source. Google News: 899 (14.2 per day; 95 percent CI 11.8-17.2); ProMED: 257 (4.2 per day; 95 percent CI 3.5–4.7); World Health Organization: 15.
SOURCE: Brownstein (2006).
The Voxiva Model for Resource-Constrained Environments
As several workshop participants observed, a global infectious disease surveillance system capable of early detection and response must identify outbreaks where they most often arise: in the world’s most impoverished communities. Many developing countries, however, lack adequate disease surveillance systems capable of finding, diagnosing, and responding to diseases of global concern. These countries must also detect and control outbreaks of common diseases, such as measles, within their own borders. This is the void Voxiva12 attempts to fill, according to speaker Pamela Johnson, the company’s cofounder (see Johnson and Blazes in Chapter 2). Although projects have been launched to enable disease reporting in low-resource settings via the Internet, personal digital assistants (PDAs), and satellite dishes, she noted that none of these technologies directly reached the inhabitants of remote communities at risk for infectious disease outbreaks. By contrast, she reported that cellular phones have begun to connect even the most isolated villages as their usage rate grows far faster than that of the
Internet. Figure SA-6 illustrates the rapid growth of cell phone usage in Africa between 1994 and 2004.
Like other electronic surveillance networks, Voxiva is ultimately web-based, Johnson said. The network receives input from a variety of sources, including cellular and fixed-line telephones, personal computers, PDAs, and paper-based communications to “optimize the use of existing infrastructure to create multiple-channel, redundant systems” for data collection, she explained. Information is captured in a database that can then be shared with those who contribute reports, along with tools for data analysis and visualization. Gaining access to surveillance information and tools—in addition to acknowledgment for their participation—gives inhabitants of outbreak communities a powerful incentive for reporting, Johnson said.
Voxiva conducts both syndromic and traditional surveillance for human disease in a variety of settings in Asia, Africa, South America, and the United States, as well as systems to monitor animal health and adverse events. In Peru, Voxiva created a system to replace the paper-based monitoring of illness among members of its navy, a project described by David Blazes of the U.S. Naval Medical Research Center in Lima (see Johnson and Blazes in Chapter 2). When naval personnel or their family members present with disease symptoms at remote clinics in the Amazon jungle, for example, nurses or physicians enter data via cellular or toll-free public phones, or if necessary, relay it via radio to another site with telephone access. The data are captured, displayed in real time on a private web-based platform, and used to generate messages and alerts that feed back to
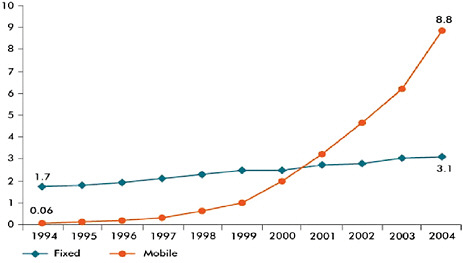
FIGURE SA-6 Telephone subscribers per 100 inhabitants, Africa 1995–2004.
SOURCE: Reproduced with the kind permission of the International Telecommunication Union (2006).
the reporting clinics. “The Peruvian Navy had no idea how many cases of any disease were occurring over a period of time,” Blazes observed. “Just setting baseline trends was very important.”
Like GPHIN, Blazes said that Voxiva has begun to evaluate its surveillance systems, based on criteria such as simplicity, flexibility, stability, and sustainability, as defined by CDC (CDC, 2001). But Voxiva’s involvement in public health extends well beyond surveillance, he noted: Their systems are linked to diagnosis and response teams that can provide guidance or assistance in the event of an outbreak. In addition, Voxiva provides basic training in epidemiology and outbreak management to its clients, using freely available, web-based curricula.13 All these functions contribute to Voxiva’s overarching purpose, as defined by Johnson, to use informatics to build and support networks of astute clinicians.
Considerations for Surveillance Networks
In response to these presentations, workshop participants raised a series of general issues regarding the structure, function, and future of public health surveillance networks. Although the open-access PMM and HealthMap networks are well established and poised to expand, some Forum members noted that none of these networks features the sort of open editing made popular by the free online encyclopedia Wikipedia.14 Brownstein said that HealthMap may add a user editing function, but input would be limited to a group of experts. Keusch pointed out that a similar “portal” model has been used to collect other types of information; for example, to construct the Encyclopedia of Earth, a free, searchable collection of articles on earth science and ecology written by a diverse team of experts who collaborate and review each other’s work.15 By contrast, Forum member Gary Roselle expressed concern that HealthMap’s “beautiful maps of data” derive from potentially erroneous newspapers and websites, and worse, could be influenced to manipulate markets. In subsequent remarks, speaker Will Hueston (see next section and Chapter 4) asserted that the maps would “set back international development because it supports the idea that a country either has the disease or doesn’t have the disease and the country either is at zero risk or is at risk.” Furthermore, he predicted that the vast economic consequences (e.g., trade embargoes; decreased tourism and investment) of such labels would inhibit disease reporting.
Regarding limitations in network access, one Forum member wondered how public response to the SARS pandemic might have changed had GPHIN been
|
13 |
To date Voxiva has trained more than 1,300 epidemiologists on the basics of outbreak management. Their objectives and curricula are in Spanish and English and are freely available on the Web. For more information about the training provided by Voxiva, see http://www.nmrcd.med.navy.mil/outbreak/. |
|
14 |
|
|
15 |
freely accessible. Mawudeku speculated that the release of unverified information might have created unnecessary panic, and she advised that such information be accompanied by qualifying commentary if it were provided in an open version of GPHIN. Another barrier to network openness exists at the level of data acquisition. Countries do not share surveillance data without government approval, participants observed, and the IHRs do not presently impose sufficient consequences to overcome economic barriers to reporting disease. This necessarily limits information available to surveillance networks, except perhaps Voxiva, whose clients own their data and use the network’s information as they see fit. On the other hand, Johnson pointed out, clients sufficiently interested in collecting such data tend to have the greatest capacity to respond to an apparent outbreak.
Looking to the future, Forum member George Korch asked how surveillance data accumulated by networks might be analyzed further to tease out underlying factors and relationships, such as previously unknown societal or environmental influences on disease transmission. To the extent that they occur at all, such analyses are currently conducted on an ad hoc basis; however, both Brownstein and Mawudeku said their networks are discussing possibilities for deeper and more detailed surveillance studies, which are likely to proceed as collaborations with academia and research institutions. Brownstein also predicted that basic research in Internet-based surveillance would benefit from the recent “explosion of work” on syndromic surveillance systems; for example, by using previously developed methods to characterize datasets and reveal their hidden biases.
Finally, participants observed that surveillance networks, like other advanced technologies that have been integrated into the practice of public health, tend to be driven by innovation rather than designed to solve important problems. They urged greater involvement by the public health community in creating tools in response to pressing public health challenges, and noted that the development of a common lexicon by technologists and public health practitioners is crucial to advancing their collaboration.
Detection and Diagnostics
Current microbial detection and identification methods include microbiological culture, nucleic acid-based techniques such as gene amplification via PCR, and immunological (antibody-based) assays (Peruski and Peruski, 2003; Fredricks and Relman, 1999; Tang et al., 1997). Each of these platforms offers complementary advantages and disadvantages for infectious disease diagnosis:
-
Microbiological culture, with staining and microscopy, is the most widely used method for identifying pathogens, particularly in developing countries. Despite being slow and limited in sensitivity for some clinically relevant microbes, culture often provides the best means to assess complex microbial phenotypes, such as drug resistance.
-
PCR is a sensitive, specific, and rapid approach for identifying microbes, including those that are nonviable or inactivated (Peruski and Peruski, 2003; Gilbert, 2002). Hundreds of different microbe-specific nucleic acid amplification tests have been described, but only a few such tests are routinely used in a clinical setting to detect pathogens that include N. gonorrheae, C. trachomatis, herpes simplex virus, and HIV (Fredricks and Relman, 1999; Tang et al., 1997). PCR methods may also be used to detect drug resistance in pathogens (Fluit et al., 2001), but the diversity of genotypes and mechanisms associated with this phenotype, and the difficulty of predicting expression from simple gene detection, have hampered the universal adoption of this approach. Real-time quantitative PCR, which permits sample processing in minutes, powers environmental detection systems for infectious diseases and biological warfare agents, as well as innovative point-of-care diagnostic tests (Ivnitski et al., 2003; Peruski and Peruski, 2003; Raja et al., 2005).
-
Immunoassays are usually less sensitive and specific than culture and PCR (Peruski and Peruski, 2003). Solid-phase, “hand-held” immunoassays for specific pathogens are rapid, rugged, and easy to use. However, their application is generally limited to screening or confirming diagnoses.
Newer diagnostic platforms, still largely in development, include nucleic acid microarrays (“labs on chips” containing hundreds to thousands of oligonucleotide probes for signature sequences) and mass spectrometry techniques for sequence analysis (Briese et al., 2005; Palacios et al., 2006; Palacios et al., 2007; Anthony et al., 2001). These technologies permit detection of a wide range of known disease-causing organisms (not limited to microbes) and can often distinguish new pathogen species, strains, and genotypes. Additional innovations include methods for the simultaneous identification of complex mixtures of organisms (Ecker et al., 2005; Hofstadler et al., 2005). Potential applications of such multiplexed detection technologies include the characterization of polymicrobial infections common in epidemics of respiratory disease, and the creation of “universal biosensors” to permit the simultaneous identification of a broad range of infectious agents in an environmental or clinical sample.
The Diagnostic Landscape
Workshop presentations on infectious disease detection and diagnostics followed a metaphorical road as they surveyed the immediate landscape of capacity, needs, and challenges; anticipated developments around the next turn; and imagined a far horizon of disease diagnosis prior to the appearance of symptoms. In the course of this journey, participants examined a variety of approaches to infectious disease detection and diagnosis and raised significant considerations for continued development of this field.
Developing Countries
While threats posed by emerging diseases, pandemic influenza, and bioterrorism underscored workshop discussion, Mark Perkins of the Foundation for Innovative New Diagnostics (FIND) reminded participants of the severe burden presently imposed on the developing world by infectious diseases such as tuberculosis (TB) and malaria (see Perkins and Small in Chapter 3). Culture and microscopy are often the only diagnostic technologies available in developing countries, typically through a small number of facilities that cannot begin to meet national needs. This not only hinders treatment for infectious disease in developing countries, he noted, but surveillance as well. Perkins reported that half of the 22 countries with the highest burden of disease from TB have three or fewer laboratories that can perform drug-susceptibility testing, a key component of TB treatment and control; similar barriers also deter the detection and treatment of trypanosomaisis and malaria.
Developing countries’ needs for rapid, accurate, inexpensive, and robust diagnostics could be met by recent advances in genomics, proteomics, and materials science, but for the lack of a profitable market for such developments, Perkins observed. FIND therefore guides the development and adoption of novel diagnostic products for diseases of the developing world in much the same way as public–private partnerships have been established to produce drugs and vaccines for low-resource settings (Perkins and Small, 2006). With FIND’s support, companies that produce low-cost diagnostics for use in developing countries realize sufficient cost savings (in manufacturing, approval procedures, and marketing) to sustain profits. “So far it has been a very successful model,” Perkins reported. “We have about 18 different technologies in the pipeline.”
Diagnostics for TB figure prominently among the Foundation’s current projects. Perkins described a liquid culture system for TB that reduces detection time by several weeks; a phage replication assay and an automated detection system, both capable of detecting rifampicin-resistant TB in sputum (signaling a patient that will fail standard therapy); and an easy-to-use PCR diagnostic system that can be performed in clinics without laboratory support. He cautioned that many substandard rapid tests for TB (as well as other diseases, including malaria) have already appeared on the market. “Small companies, the kinds of companies that are making these tests, are not going to invest in going back to the genome and figuring out what the right targets are,” he observed. Nevertheless, Perkins concluded, “Our belief at FIND is that if you make the right technology, people will use it.”
On the Battlefield
Much like public health workers in developing countries, soldiers at risk of contracting infectious diseases, either from the natural environment or from
bioweapons, need diagnostics that are rugged, rapid, and easy to use. According to speaker Mark Wolcott of the Diagnostic Systems Division at the U.S. Army Medical Research Institute of Infectious Diseases (USAMRIID), diagnostic assays must satisfy additional criteria for use in battle. Accuracy is paramount, and tests must recognize a broad range of potential pathogens, he explained. “Negatives are problems,” he said, “and false negatives [which may result from a bioengineered pathogen] are of greater concern than false positives.” The military currently relies on a combination of PCR tests, immunoassays, and traditional microbiology to diagnose infectious diseases in the field, while pursuing a strategy to develop comprehensive diagnostic tools.
Animal Diseases
Recognizing the advantages of DNA-based diagnostic tools, Alex Ardans and colleagues have developed PCR-based assays to screen for pathogens associated with exotic Newcastle disease (END) in poultry and foot-and-mouth disease (FMD) in cattle (Crossley et al., 2005; Heller, 2006; Thurmond and Perez, 2006). Ardans, who directs the California Animal Health and Food Safety Laboratory System, also described how the discovery of TB among cattle in several of the state’s large dairies led to the development of a highly efficient testing program.
Although the state laboratory system spearheads surveillance for several important animal diseases (including zoonoses such as avian influenza, bovine spongiform encephalopathy, and West Nile encephalitis), Ardans suggested that its most crucial role is in recognizing unusual disease events. He noted, for example, that while END was “no stranger” to rural California, a recent outbreak in an urban setting—among fighting cocks, whose handlers worked in and spread the disease to commercial operations—took the state by surprise. The laboratory responded by optimizing an existing real-time PCR assay for END that was used to perform more than 85,000 tests (Crossley et al., 2005). “These emergency efforts are a real opportunity to develop some new knowledge,” Ardans said. “They are unique in what they will give us, and it’s a rare opportunity to improve the diagnostics. [How else] would you get a chance to validate an assay using 85,000 samples?”
Such situations, Ardans observed, also highlight the importance of meeting diagnostic needs with appropriate technologies. In pursuing the source of E. coli O157:H7 in a recent outbreak in spinach, laboratory researchers discovered that the use of a gauze swab to sample irrigation waters for contaminants performed better than newer concentration technologies. (As previously noted, surveillance for infectious plant diseases depends largely on available methods of disease detection and diagnosis; see also Chapter 1.)
The Road Ahead: Diagnostics in Development
Inspired in part by the image of the original Star Trek’s character “Bones”® diagnosing a patient with a wave of his medical tricorder (Figure SA-7), Wolcott and fellow DoD researchers are attempting to construct an “integrated diagnostic system” for field use that can detect viruses, bacteria, toxins, “and anything else that could possibly be thrown at us in the biological detection arena,” he said. The current prototype relies on automated real-time PCR, but DoD researchers are testing a wide range of diagnostic technologies (e.g., microarrays, handheld immunoassays, electrochemiluminescence) and targets (e.g., microbial toxins, as well as nucleic acids), according to Wolcott. “We have to have multiple platforms to give us the assurance that what we are reporting up the chain of command is actually there,” he said. The ultimate goal is to combine multiple platforms into a single, universal system for field diagnosis. While the time constraints and primitive conditions of battle present significant barriers to the use of microarrays, Wolcott speculated that chip technology eventually would be adapted to provide point-of-care diagnosis for soldiers in action.

FIGURE SA-7 Star Trek medical tricorder.16
SOURCE: Printed with permission from CBS Paramount.
Following a similar technological progression from PCR to microarrays, the Pandora’s Box Project, based at Columbia University’s Greene Infectious Disease Laboratory, employs a staged strategy for molecular pathogen surveillance and discovery (see Lipkin and Briese in Chapter 3) (Palacios et al., 2007). As described by speaker and Greene Laboratory director W. Ian Lipkin, the first stage consists of MassTag PCR, a technique that attaches reporter “tags” of distinct masses to the amplified sequences, permitting the simultaneous, highly sensitive detection of more than 20 different pathogens. This platform, which is both inexpensive (at approximately $10 per 20-plex assay) and rapid, has been used to distinguish among various viral hemorrhagic fevers, Lipkin said; it is currently being adapted for the diagnosis of gastroenteritis. MassTag PCR has also enabled the recent discovery of a virus responsible for a significant proportion of flu-like respiratory disease (Lamson et al., 2006).
A second stage of diagnosis becomes necessary when the first stage fails, or when a larger number of sequences must be screened, Lipkin continued. For this purpose, the researchers first designed a pair of extensive microarrays, called GreeneChips, for viruses and other respiratory pathogens, and then a panmicrobial array that incorporates more than 29,000 60-mer probes from filamentous fungi and yeasts, and parasites, as well as from viruses and bacteria that infect vertebrates (Palacios et al., 2007). Together, these arrays comprise a system for assaying nucleic acids extracted from clinical samples (e.g., nasopharyngeal aspirates, blood, urine) or cell culture. If the less expensive ($100 per assay) respiratory or viral arrays fail to detect a pathogen, he explained, “then we go to progressively more comprehensive chips that top out at approximately $350.”
“These are surveillance tools,” Lipkin said of both MassTag PCR and GreeneChips. “All they do is give you a plus/minus, presence-or-absence, sort of an answer, if they give you an answer at all. You ultimately have to come back to surveillance assays [such as] quantitation, with real-time PCR, [and] you have to do serology.” More importantly, he observed, because diagnosis requires the integration of various test results with other information, such as epidemiological data, the GreeneChip “is never really going to replace a seasoned, thoughtful clinician.”
Lipkin also noted that, despite the obvious advantages of multiplexed detection (and in anticipation of less expensive versions of microarrays), the widespread adoption of microarrays for disease detection would require a revision in regulatory standards based on the more sensitive single-agent model. “The gold standard, invariably, is single-agent [detection] with an identical match between template and probe as opposed to multiplex systems, which tolerate sequence divergence,” he explained. The need to obtain intellectual property licenses for each of potentially thousands of sequences restricts the development of array-based diagnostics, Lipkin added. However, both regulatory and intellectual property barriers could be minimized by constructing multiplex assays of 20 or so carefully chosen sequences, according to Forum member Patrick Fitch,
of Battelle Memorial Institute. “Defining the problem space goes a long way to getting optimization of the assay you want,” he concluded.
GreeneChips are tools for discovery, as well as for diagnosis. Because as many as 100 different regions of a genome may be represented on the chip, assays reveal enough sequence information to enable the rapid sequencing of novel pathogens, several of which have been characterized. Analyses of GreenChip data have led to the identification of a novel target for antiviral drugs, which Lipkin described as an “on/off switch in the human immune system.” He predicted that broad, unbiased pathogen-detection methods will continue to provoke unanticipated discoveries and enable researchers to explore the apparent link between infectious and chronic diseases.
The Far Horizon: Presymptomatic Diagnosis
Imagining a future in which bioterrorism agents are continually reengineered to evade standard detection and diagnostic methods, as well as therapeutics, speaker and Forum member Stephen Johnston proposed a model of diagnosis for exposure to a pathogen prior to the appearance of symptoms (see Johnston in Chapter 3). In this situation, he argued, specific defenses against threat agents (e.g., vaccines) will be useless. Instead, he envisions the creation of platforms for defense against the full range of potential bioweapons, such as the means to recognize and respond to the earliest possible signs of infectious disease in individual patients. Host-based, presymptomatic diagnosis could be accomplished by monitoring a person’s blood serum chemistry for changes suggestive of compromised health status, Johnston explained; he is currently involved in developing a device to perform such analyses. He noted that the noninvasive sampling of breath and saliva is attractive in theory, but that neither of these sources offered the diversity or concentration of metabolic components found in blood.
Monitoring the biological signatures of infectious disease will require making thousands to millions of simultaneous measurements and comparing them to well-established baselines, Johnston said. Ideally, this would occur through a continuous process. Therefore the monitoring device would need to be easily accessible (e.g., in the home), robust, inexpensive, and capable of quickly measuring thousands of variables—specifications that also apply to point-of-care devices for low-resource settings. Because the symptoms of respiratory viruses appear in as little as one day, Johnston hypothesized that presymptomatic detection would need to register changes in the nanomolar-to-picomolar range and would require clear baselines and the integration of multiple measurements to avoid an unacceptable level of false positives. In response, Forum member David Relman of Stanford University referred to an article by Kohane and colleagues (2006), who describe substantive risks inherent in the practice of personalized, genomic medicine, among them the imprudence of using “testing panels comprising a sizeable fraction of the genome for clinical care or screening” (Kohane et
al., 2006). Johnston acknowledged that scant evidence—most of it derived from early postinfection transcription patterns—suggests such measurements could actually anticipate the development of respiratory symptoms. He attributed this dearth of supporting data to the lack of funding for research in this area and to the yet-unsolved problem of what, exactly, to measure, and how. Johnston and coworkers are currently attempting to develop synthetic antibody techniques for monitoring infection-related changes in protein levels.
In addition to offering the best chance of treatment for known, emerging, or bioengineered pathogens, detecting infectious disease at the earliest possible moment would permit diagnosis-based triage and increase the effectiveness of quarantine or other social distancing measures, Johnston predicted. He anticipated that presymptomatic diagnosis will have an even greater impact on everyday medical care. “We have a healthcare system that can’t be sustained in terms of physical economy,” he said, adding that care for ill patients accounts for nearly 90 percent of health-care spending. “Why does it cost so much? Because we are diagnosing sick people, taking care of sick people; we even develop our drugs for sick people.” Therefore, he insisted, our society has no choice but to move from postsymptomatic to presymptomatic diagnosis.
Considerations for Detection and Diagnosis
Given the considerable interdependence of surveillance, detection, and diagnostic activities as they relate to infectious disease, it is not surprising that key challenges identified by workshop participants in their discussions of surveillance strategies would resurface as they explored current and future prospects for disease detection and diagnosis. Once again, participants stressed the importance of selecting and acquiring clinically relevant samples or specimens, the establishment of said relevance against a background of natural variation, and the need for standards to guide system design and evaluation.
Workshop participants also considered the status of infectious disease diagnostics, which they characterized as a largely unsupported area within the crowded field of medical diagnostics. Fitch observed that pharmaceutical companies tend to develop relatively low-margin diagnostic tests only when they can be linked to highly profitable therapeutics (e.g., for cardiac disease and cancer). Nevertheless, participants urged that the vast experience of commercial producers of medical diagnostics be brought to bear on public efforts to develop applications for infectious diseases. For example, Relman suggested that industry could participate in efforts to identify and evaluate common principles and platforms for sample processing, signal generation and detection, and data analysis. However, he added, these considerations are contingent on a more fundamental set of yet-to-be-determined specifications for any surveillance or detection program: the exact set of questions to be answered and the appropriate setting in which to ask them.
The Challenge of Coordination
Workshop participants, having considered a broad range of tools and strategies for infectious disease surveillance, detection, and diagnosis, turned to the difficult issue of effectively combining them. Hueston, director of the University of Minnesota’s Center for Animal Health and Food Safety, launched this discussion with his presentation on the coordination of disease surveillance, detection, diagnostics, and reporting. This topic is a frequent focus of Hueston’s work, which emphasizes risk communication and the facilitation of public–private partnerships (see Chapter 4).
Shifting the Public Health Paradigm
Certain powerful concepts and conditions that influence the practice of public health inhibit the coordination of infectious disease surveillance, detection, and diagnosis, according to Hueston. Table SA-2 summarizes these elements of the current public health paradigm, as defined by Hueston, and pairs them with his proposed alternatives.
Hueston identified several factors driving the current paradigm that specifically undermine public health coordination. Chief among them is high health
TABLE SA-2 Current Public Health Paradigm and Alternative World View
|
Current Paradigm |
Alternative World View |
|
Health focus is individual; benefits accrue primarily to the developed world |
Health focus is global society; benefits accrue to all |
|
Health is absence of disease |
Health is well-being (in mind, body, spirit) |
|
Infectious disease is all about the agent |
Infectious disease emerges at the convergence of agent, host, environment |
|
Zero risk is achievable |
Zero risk is unachievable; risk management is the goal |
|
Success is eradication/cure |
Success is homeostasis with microbes that are ubiquitous, constantly evolving, and adapting |
|
Public health function is to react |
Public health function is health promotion |
|
Reaction requires agent detection |
Risk management can be successful whether or not microbe is identified |
|
Urgency dictates priority |
Surveillance informs policy and guides action on basis of importance |
|
Answers lie solely in technology |
Answers involve people, politics, partners |
|
SOURCE: Hueston (2006). |
|
status in the United States, which reinforces the tendency of public health to focus on the urgent (i.e., the disease or agent du jour) rather than the important (i.e., emergency preparedness); this situation is exacerbated by a budget process that takes money from “important” programs to fund “urgent” ones. Parochialism influences our sense of urgency, he observed, causing us “to focus on those things about which we are most interested in the United States as opposed to looking at the true [global] public health priorities.” Hueston added that assigning blame for public health threats—and especially the tendency to “shoot the messengers” who identify them—suppresses essential collaboration in surveillance. For example, before the United States adopted a policy of “zero tolerance” for food contamination, companies monitored for more pathogens and kept records of their findings, a practice that supported scientific evaluation of the impact of new intervention strategies. Now, he said, “if they monitor and get positive reports, they are culpable and have self-incriminated, so they stopped monitoring.”
Coordinating the spectrum of public health activities associated with disease surveillance and detection is an inherently political task, and therefore strongly influenced by societal and organizational culture, Hueston asserted. “To be effective in politics over the long term and to build coordination and collaboration requires people skills,” he observed, and yet increasingly in educational fields relevant to public health, considerations of interpersonal and executive skills are largely ignored under the misguided assumption that science and technology can replace them. Rather, as Korch noted in the ensuing discussion, in a risk management model of public health, understanding and responding to specific social contexts is crucial to effective risk reduction and communication.
There is no “magic bullet to change paradigms,” Hueston stated, stressing that steady progress can be made through small successes. This progress, albeit slow, needs to be properly recognized and celebrated. Because the most effective engine for change is educating the next generation of leaders early in their careers, he urged educators to encourage greater global and transdisciplinary awareness in future public health professionals.
Optimal Surveillance for Risk Management
Clearing the way for true coordination and collaboration would enable optimal surveillance, as Hueston defined it: an integrated and dynamic system with ongoing data collection and real-time analysis to inform risk management, and thereby drive policy and action, with a feedback process to facilitate continuous evolution and adaptation. Information would be drawn from a broad range of disciplines relevant to physical and mental health, as well as domestic and wild animal health and plant health, through the complementary processes of agent surveillance and host and environmental monitoring.
In the discussion that followed his presentation, Hueston noted the potential economic benefits of surveillance systems for both developing and industrial-
ized countries, but also warned that too much openness could undermine such systems. “If our goal is to promote public health, and surveillance precipitates action to control the disease, do we always have to make the information public?” he wondered aloud. Forum member Johnston responded emphatically that such secrecy “has only gotten us in trouble, that it is elitist and that it is only going to come back and bite us in the long run. If we want to foster a further schism between the public and the scientific community, the best thing we can do for that is to withhold information.” Hueston responded that he agreed with Johnston’s position in principle, but insisted that under some circumstances, the unintended consequences of publicizing information outweighs the potential benefits, such as sharing of animal disease surveillance data in wildlife that precipitates unwarranted trade restrictions on commercially produced products. He concluded that the release of surveillance information should be evaluated on a case-by-case basis. Forum member Margaret Hamburg noted that timing is crucial to such communication and observed that public health officials “get into trouble if we provide information before we fully understand it and before we understand how we are going to respond.”
Because the definition of risk is individual and fueled by emotion, public health professionals must address the perception of risk, Hueston explained. Trust is not built merely by sharing data, but by helping people understand information by providing it in context, he said. But, he continued, this is only the first stage. The public must then be actively engaged to discuss their perception of risk and identify priorities for action.
Needs and Opportunities
This section recounts needs and opportunities for both research and policy derived from workshop discussions on infectious disease surveillance, detection, and diagnosis. Participants, including members of a concluding discussion panel (see Chapter 4 overview), identified a series of issues critical to the development and implementation of effective methods and strategies for the detection of infectious disease and described key challenges in responding to increasingly early disease alerts.
Critical Issues in Infectious Disease Surveillance and Detection
The following areas were the subject of extended discussion with reference to both surveillance and detection.
System Design and Development
Hueston captured a recurring theme in this workshop’s discussion when he quoted management guru Stephen Covey’s advice to “begin with the end in
mind” (Covey, 1989). As previously noted, many decried the evolution of surveillance and detection systems based on available technologies and databases, rather than in response to well-defined public health needs. Participants suggested the following actions to improve the design and development of surveillance and detection systems:
-
Develop a common design lexicon to improve communication and collaboration between public health practitioners and information technologists.
-
Devise methods to analyze surveillance data through time in order to understand factors and mechanisms that underlie apparent trends.
-
Create syndromic surveillance systems that can adapt to signals as they are received so that an increase in symptom prevalence prompts intensified testing.
-
Broaden the purview of surveillance to encompass social circumstances that affect public health.
-
Incorporate mechanisms to filter surveillance data to reduce false-positive (and panic-inducing) alarms.
-
Recognize and incorporate promising surveillance concepts from noninfectious disease applications.
-
Support basic research in disease surveillance, especially among plant and animal populations.
-
Develop incentives to promote the development of infectious disease diagnostics and to integrate academic and commercial efforts toward this goal.
-
Consider models of infectious disease beyond the replication of viruses or bacteria within organ systems. These would include toxin-producing microbes (e.g., Clostridium botulinum) and pathogens that affect the immune system or immune responses (including delayed or chronic effects, such as those associated with hepatitis C and human immunodeficiency virus [HIV]).
-
Do not overlook longstanding and effective elements of disease detection: pathology, microbiology, and of course, the astute clinician.
System Evaluation
Workshop participants encouraged critical analysis, comparison, and evaluation of the performance of existing surveillance and detection systems, and in particular, of the U.S. BioSense (syndromic surveillance) and BioWatch (specific threat detection) programs. Their suggestions include the following:
-
Identify the essential components of a global infectious disease surveillance system in order to prioritize funding.
-
Support operational research to evaluate and optimize informatic systems for processing epidemiological data, particularly when used in syndromic surveillance.
-
Develop methods to analyze and compare cost-effectiveness of surveillance and detection systems.
-
Design mechanisms for continuous feedback and improvement into surveillance and detection systems.
-
Reconsider the role of syndromic surveillance in disease control, given the lack of evidence for its effectiveness in early detection of biological attacks and its promise for tracking large-scale, natural disease outbreaks such as H5N1 avian influenza.
Integration of Information
Workshop participants also stressed the importance of integrating information on infectious diseases from diverse sources and methods to obtain a comprehensive view of disease risk and severity. In particular, they encouraged the development of mechanisms to connect local sources of surveillance data (including information on animal infections, insect vector distributions, climate, and vegetation) with global surveillance networks.
Information Transparency, Control, and Access
As noted in the previous section, workshop participants expressed divergent opinions concerning the risks and benefits associated with the public disclosure of surveillance findings. Most participants acknowledged a need to balance transparency—a foundation of both public and international trust—against the potential consequences associated with public misinterpretation and overreaction. Several participants urged consideration of political and economic factors, as well as timing (i.e., releasing surveillance information by public health authorities only after it is fully understood and a response is planned or underway), in making such decisions.
Reporting
Recognizing that the reporting of unusual findings by health practitioners (and subsequently by governments) is essential to infectious disease surveillance and detection, workshop participants considered a range of incentives to promote the affirmative reporting of human, animal, and plant health status at all levels, including the following:
-
Develop and broadly implement standards for infectious disease reporting and sample submission to public health laboratories.
-
Pay clinicians, especially those in developing countries, to report findings to national public health authorities.
-
Ensure the confidentiality of health practitioners who report infectious disease, while recognizing their contribution to public health. In the case of agricultural diseases, provide financial support for farmers who report disease and guard intellectual property rights of seed companies who assist in identifying vulnerable germplasm.
Participants also suggested a variety of mechanisms and tools to improve the collection and use of reported information, as follows:
-
Fund the procurement, storage, submission, and diagnostic testing of clinical and animal specimens from a broad spectrum of private and public sources.
-
Encourage data collection to support the characterization of natural variation and define baseline health status; reward the reporting of negative data. WHO’s Health Metrics Network, a global partnership to build capacity and expertise to provide better health information to decision makers at all levels, represents a potential source of baseline data.17
-
Support the development of global surveillance and laboratory capacity as mandated by recent revisions to the IHRs. Some suggested that this could be accomplished through increased funding to WHO; others argued that WHO must first be reformed and strengthened; others questioned whether a new intergovernmental entity would need to be invented to achieve the goals set by the IHRs.
-
Support parallel efforts by OIE, NATO, USDA, and the European Union to develop global surveillance capacity for animal and plant diseases.
From Alarm to Action
In the spirit of beginning with the end in mind, workshop participants also considered the fate of information derived from infectious disease surveillance and detection systems. Several participants observed that U.S. government investment in the detection of biological threats far outstrips its ability to respond to such crises. Some decried the shortsightedness of creating global surveillance networks for infectious disease without also providing for disease control and containment, as well as for public preparedness and risk communication. As Kelley acknowledged, far more than science will be required to help affected communities accept the uncertainty that characterizes the course of an infectious disease emergency and the ensuing public health response.
REFERENCES
Alvarez, A. M. 2004. Integrated approaches for detection of plant pathogenic bacteria and diagnosis of bacterial diseases. Annual Review of Phytopathology 42:339-366.
Anthony, R. M., T. J. Brown, and G. L. French. 2001. DNA array technology and diagnostic microbiology. Expert Review of Molecular Diagnostics 1(1):30-38.
ARES Corporation (Applied Research and Engineering Sciences). 2007. SYRIS™ Syndome Reporting Information System, http://www.arescorporation.com/about.aspx?style=5&pict_id=227&menu_id=117&id=630 (accessed June 4, 2007).
Bravata, D., K. M. McDonald, W. M. Smith, C. Rydzak, H. Szeto, D. L. Buckeridge, C. Haberland, and D. K. Owens. 2004. Systematic review: Surveillance systems for early detection of bioterrorism-related diseases. Annals of Internal Medicine 140(11):910-917.
Briese, T., G. Palacios, M. Kokoris, O. Jabado, Z. Liu, N. Renwick, V. Kapoor, I. Casas, F. Pozo, R. Limberger, P. Perez-Brena, J. Ju, and W. I. Lipkin. 2005. Diagnostic system for rapid and sensitive differential detection of pathogens. Emerging Infectious Diseases 11(2):310-313.
Brownstein, J. 2006. HealthMap: Global disease alert mapping. Presentation at the Institute of Medicine Forum on Microbial Threats, Washington, DC, December 12-13.
Buckeridge, D. L., D. K. Owens, P. Switzer, J. Frank, and M. A. Musen. 2006. Evaluating detection of an inhalational anthrax outbreak. Emerging Infectious Diseases 12(12):1942-1949, http://www.cdc.gov/ncidod/eid/vol12no12/06-0331.htm (accessed June 4, 2007).
CDC (Centers for Disease Control and Prevention). 2001. Updated guidelines for evaluating public health surveillance systems. Morbidity and Mortality Weekly Report 50(RR13):1-35, http://www.cdc.gov/mmwr/preview/mmwrhtml/rr5013a1.htm (accessed April 18, 2007).
CDC. 2006a. Syndromic surveillance: An applied approach to outbreak detection, http://www.cdc.gov/EPO/dphsi/syndromic.htm (accessed June 4, 2007).
CDC. 2006b. Annotated bibliography for syndromic surveillance, http://www.cdc.gov/epo/dphsi/syndromic/websites.htm (accessed June 4, 2007).
Cook, J. 2005. Food supply terrorism as a biosecurity issue: Assessing the problem. Presentation at the Institute of Medicine Forum on Microbial Threats, Washington, DC, June 28.
Covey, S. 1989. The seven habits of highly effective people. New York: Simon and Schuster.
Crossley, B. M., S. K. Hietala, L. M. Shih, L. Lee, E. W. Skowronski, and A. A. Ardans. 2005. High-throughput real-time PCR assay to detect the exotic Newcastle Disease Virus during the California 2002–2003 outbreak. Journal of Veterinary Diagnostic Investigation 17(2):124-132.
Desenclos, J.-C. 2006. Are there “new” and “old” ways to track infectious disease hazards and outbreaks? Eurosurveillance 11(12):206-207, http://www.eurosurveillance.org/em/v11n12/1112221.asp (accessed June 4, 2007).
Eban, K. 2007. Biosense or biononsense? The Scientist 21(4):32.
Ecker, D. J., R. Sampath, L. B. Blyn, M. W. Eshoo, C. Ivy, J. A. Ecker, B. Libby, V. Samant, K. A. Sannes-Lowry, R. E. Melton, K. Russell, N. Freed, C. Barrozo, J. Wu, K. Rudnick, A. Desai, E. Moradi, D. J. Knize, D. W. Robbins, J. C. Hannis, P. M. Harrell, C. Massire, T. A. Hall, Y. Jiang, R. Ranken, J. J. Drader, N. White, J. A. McNeil, S. T. Crooke, and S. A. Hofstadler. 2005. Rapid identification and strain typing of respiratory pathogens for epidemic surveillance. Proceedings of the National Academy of Sciences 102(22):8012-8017.
Fletcher, J. 2005. Food supply terrorism as a biosecurity issue: Assessing the problem. Presentation at the Institute of Medicine Forum on Microbial Threats, Washington, DC, June 28.
Fluit, A. C., M. R. Visser, and F. J. Schmitz. 2001. Molecular detection of antimicrobial resistance. Clinical Microbiology Reviews 14(4):836-871.
Fredricks, D. N., and D. A. Relman. 1999. Application of polymerase chain reaction to the diagnosis of infectious diseases. Clinical Infectious Diseases 29(3):475-486, quiz 487-488.
GAO (Government Accountability Office). 2004. Emerging infectious diseases: Review of state and federal disease surveillance efforts. Washington, DC: GAO.
Gilbert, G. L. 2002. Molecular diagnostics in infectious diseases and public health microbiology: Cottage industry to postgenomics. Trends in Molecular Medicine 8(6):280-287.
Google. 2006. Google names Larry Brilliant as executive director of Google.org, http://www.google.com/intl/en/press/pressrel/brilliant.html (accessed June 4, 2007).
Green, M. S., and Z. Kaufman. 2002. Surveillance systems for early detection and mapping of the spread of morbidity caused by bioterrorism. Harefuah 141(Spec No):31-33, 122.
Heller, A. 2006. Protecting the nation’s livestock. Science and Technology Review (May):11-17.
Hempel, J. 2006 (February 22). Google’s brilliant philanthropist. Business Week, http://www.businessweek.com/technology/content/feb2006/tc20060222_088020.htm (accessed June 4, 2007).
Henderson, D. A. 1999. Eradication: Lessons from the past. Morbidity and Mortality Weekly Report 48(SU01):16-22.
Heymann, D., and G. Rodier. 2004. Global surveillance, national surveillance, and SARS. Emerging Infectious Diseases 10(2):173-175.
Hofstadler, S. A., R. Sampath, L. B. Blyn, M. W. Eshoo, T. A. Hall, Y. Jiang, J. J. Drader, J. C. Hannis, K. A. Sannes-Lowry, L. L. Cummins, B. Libby, D. J. Walcott, A. Schink, C. Massire, R. Ranken, J. Gutierrez, S. Manilili, C. Ivy, R. Melton, H. Levene, G. Barrett-Wilt, F. Li, V. Zapp, N. White, V. Samant, J. A. McNeil, D. Knize, D. Robbins, K. Rudnick, A. Desai, E. Moradi, and D. J. Ecker. 2005. TIGER: The universal biosensor. International Journal of Mass Spectrometry 242:23-41.
Hueston, W. 2006. Coordination of disease surveillance, detection, diagnostics, and reporting. Presentation at the Institute of Medicine Forum on Microbial Threats, Washington, DC, December 12-13.
International Telecommunication Union. 2006. Fixed versus mobile uptake in Africa, 1995-2004, http://www.itu.int/ITU-D/ict/statistics/ict/graphs/af5.jpg (accessed July 25, 2007).
IOM (Institute of Medicine). 2003. Microbial threats to health: Emergence, detection, and response. Washington, DC: The National Academies Press.
IOM. 2004. Learning from SARS. Washington, DC: The National Academies Press.
Ivnitski, D., D. J. O’Neil, A. Gattuso, R. Schlicht, M. Calidonna, and R. Fisher. 2003. Nucleic acid approaches for detection and identification of biological warfare and infectious disease agents. BioTechniques 35(4):862-869.
Johnson, P. 2006. Using cell phone technology for infectious disease surveillance: A model for low-resource environments. Presentation at the Institute of Medicine Forum on Microbial Threats, Washington, DC, December 12-13.
Karesh, W. B. 2006. Animal disease surveillance. Presentation at the Institute of Medicine Forum on Microbial Threats, Washington, DC, December 12-13.
Karesh, W. B., and R. A. Cook. 2005. The human-animal link. Foreign Affairs 84(4):38-50.
Kohane, I. S., D. R. Masys, and R. B. Altman. 2006. The incidentalome. Journal of the American Medical Association 2(296):212-215.
Lamson, D., N. Renwick, V. Kapoor, Z. Liu, G. Palacios, J. Ju, A. Dean, K. St. George, T. Briese, and W. I. Lipkin. 2006. MassTag polymerase-chain-reaction detection of respiratory pathogens, including a new rhinovirus genotype, that caused influenza-like illness in New York state during 2004-2005. Journal of Infectious Diseases 194(10):1398-1402.
Last, J. M. 1995. A dictionary of epidemiology. Oxford, UK: Oxford University Press.
Leroy, E. M., P. Rouquet, P. Formenty, S. Souquiere, A. Kilbourne, J. M. Froment, M. Bermejo, S. Smit, W. Karesh, R. Swanepoel, S. R. Zaki, and P. E. Rollin. 2004. Multiple Ebola virus transmission events and rapid decline of central African wildlife. Science 303(5656):387-390.
Madoff, L. C. 2004. ProMED-mail: An early warning system for emerging diseases. Clinical Infectious Diseases 39(2):227-232.
Madoff, L. C., and J. P. Woodall. 2005. The Internet and the global monitoring of emerging diseases: Lessons from the first 10 years of ProMED-mail. Archives of Medical Research 36(6): 724-730.
Mandl, K. D, J. M. Overhage, M. M. Wagner, W. B. Lober, P. Sebastiani, F. Mostashari, J. A. Pavlin, P. H. Gesteland, T. Treadwell, E. Koski, L. Hutwagner, D. L. Buckeridge, R. D. Aller, and S. Grannis. 2004. Implementing syndromic surveillance: A practical guide informed by early experience. Journal of the American Medical Informatics Association 11(12):141-150.
Mawudeku, A. 2006. Discussion of the Global Public Health Intelligence Network. Presentation at the Institute of Medicine Forum on Microbial Threats, Washington, DC, December 12-13.
Mawudeku, A., H. Molnar-Szakacs, D. H. Werker, R. Andraghetti, P. Uhthoff, G. Guerrero, and Q. Xu. 2002. The Global Public Health Intelligence Network: An “early warning” system. Joint WHO/Health Canada presentation at the International Epidemiological Conference, Montreal, Quebec, Canada, August 18-22.
Morse, S. S. 2006. ProMED and ProMED-mail: Prototype global early warning systems. Presentation at the Institute of Medicine Forum on Microbial Threats, Washington, DC, December 12-13.
Mykhalovskiy, E., and E. Weir. 2006. The Global Public Health Intelligence Network and early warning outbreak detection. Canadian Journal of Public Health 97(1):42-44.
NRC (National Research Council). 2005. Animal health at the crossroads: Preventing, detecting, and diagnosing animal diseases. Washington, DC: The National Academies Press.
OIG (Office of the Inspector General). 2005. EPA needs to fulfill its designated responsibility to ensure effective BioWatch program, http://www.epa.gov/oig/reports/2005/20050323-2005-P-00012.pdf (accessed June 1, 2007).
Palacios, G., T. Briese, V. Kapoor, O. Jabado, Z. Liu, M. Venter, J. Zhai, N. Renwick, A. Grolla, T. W. Geisbert, C. Drosten, J. Towner, J. Ju, J. Paweska, S. T. Nichol, R. Swanepoel, H. Feldmann, P. B. Jahrnling, and W. I. Lipkin. 2006. Mass tag polymerase chain reaction for differential diagnosis of viral hemorrhagic fevers. Emerging Infectious Diseases 12(4):692-695, http://www.cdc.gov/ncidod/eid/vol12no04/pdfs/05-1515.pdf (accessed June 4, 2007).
Palacios, G., P.-L. Quan, O. Jabado, S. Conlan, D. L. Hirschberg, Y. Liu, J. Zhai, N. Renwick, J. Hiu, H. Hegyi, A. Grolla, J. E. Strong, J. S. Towner, T. W. Geisbert, P. B. Jahrling, C. Büchen-Osmond, H. Ellerbrok, M. P. Sanchez-Seco, Y. Lussier, P. Formenty, S. T. Nichol, H. Feldmann, T. Briese, and W. I. Lipkin. 2007. Panmicrobial oligonucleotide array for diagnosis of infectious diseases. Emerging Infectious Diseases 13(1):73-81, http://www.cdc.gov/ncidod/eid/13/1/73.htm (accessed June 4, 2007).
Pavlin, J. A. 2003. Investigation of disease outbreaks detected by “syndromic” surveillance systems. Journal of Urban Health 80(2):i107-i114.
Perkins, M. D., and P. M. Small. 2006. Partnering for better microbial diagnostics. Nature Biotechnology 24(8):919-921.
Peruski, L. F., and A. H. Peruski. 2003. Rapid diagnostic assays in the genomic biology era: Detection and identification of infectious disease and biological weapons agents. BioTechniques 35(4):840-846.
Public Health Agency of Canada. 2007. The Global Public Health Intelligence Network (GPHIN), http://www.phac-aspc.gc.ca/gphin/index.html (accessed April 18, 2007).
Raja, S., J. Ching, L. Xi, S. J. Hughes, R. Chang, W. Wong, W. McMillan, W. E. Gooding, K. S. McCarty, Jr., M. Chestney, J. D. Luketich, and T. E. Godfrey. 2005. Technology for automated, rapid, and quantitative PCR or reverse transcription-PCR clinical testing. Clinical Chemistry 51(5):882-890.
RAND Corporation. 2004. Syndromic surveillance: An effective tool for detecting bioterrorism?, http://www.rand.org/pubs/research_briefs/2005/RB9042.pdf (accessed June 4, 2007).
Reingold, A. 2003. If syndromic surveillance is the answer, what is the question? Biosecurity and Bioterrorism: Biodefense Strategy, Practice, and Science 1(2):1-5.
Schaad, N. W., R. D. Frederick, J. Shaw, W. L. Schneider, R. Hickson, M. D. Petrillo, and D. G. Luster. 2003. Advances in molecular-based diagnostics in meeting crop biosecurity and phyto-sanitary issues. Annual Review of Phytopathology 41:305-324.
Shea, D. A., and S. A. Lister. 2003. The BioWatch program: Detection of bioterrorism. Washington, DC: Congressional Research Service.
Sosin, D. A. 2003. Syndromic surveillance: The case for skillful investment. Biosecurity and Bioterrorism: Biodefense Strategy, Practice, and Science 1(4):247-253.
Stack, J., K. Cardwell, R. Hammerschmidt, J. Byrne, R. Loria, K. Snover-Clift, W. Baldwin, G. Wisler, H. Beck, R. Bostock, C. Thomas, and E. Luke. 2006. The National Plant Diagnostic Network. Plant Disease 90:128-136.
Stoto, M. A. 2005. Syndromic surveillance. Issues in Science and Technology 21(3):49-56.
Tang, Y. W., G. W. Procop, and D. H. Persing. 1997. Molecular diagnostics of infectious diseases. Clinical Chemistry 43(11):2021-2038.
Thurmond, M. C., and A. M. Perez. 2006. Modeled detection time for surveillance for foot-and-mouth disease virus in bulk tank milk. American Journal of Veterinary Research 67(12):2017-2024.
Wagner, M. M., F. C. Tsui, J. Espino, W. Hogan, J. Hutman, J. Hersh, D. Neill, A. Moore, G. Parks, C. Lewis, and R. Aller. 2004. National retail data monitor for public health surveillance. Morbidity and Mortality Weekly Report 53(Suppl):40-42.
Watanabe, M. 2002. News profile: Tracey McNamara, veterinary pathologist. The Scientist 16(5):60.
WCS (Wildlife Conservation Society). 2007. Wildlife health hotspots, http://www.wcs.org/sw-high_tech_tools/wildlifehealthscience/fvp/168570/guidelinesandpapers/animalhealthmatters/168789/ (accessed May 31, 2007).
White House. 2004. Homeland Security Presidential Directive/HSPD-9, http://www.whitehouse.gov/news/releases/2004/02/20040203-2.html (accessed June 4, 2007).
WHO (World Health Organization). 2000. Report on global surveillance of epidemic-prone infectious diseases. Geneva, Switzerland: WHO.
WHO. 2005. Worldwide distribution of GOARN partner institutions and networks, http://www.who.int/csr/outbreaknetwork/GOARNMapenglish.pdf (accessed June 4, 2007).
WHO. 2006. Poliomyelitis, http://www.who.int/mediacentre/factsheets/fs114/en/ (accessed June 4, 2007).
WHO. 2007. International Health Regulations (IHR), http://www.who.int/csr/ihr/en/ (accessed June 4, 2007).
Wikipedia contributors. 2007. Tricorder. Wikipedia, The Free Encyclopedia, http://en.wikipedia.org/w/index.php?title=Tricorder&oldid=148276569 (accessed July 31, 2007).
Zelicoff, A., J. Brillman, D. W. Forslund, J. E. George, S. Zink, S. Koenig, T. Staab, G. Simpson, E. Umland, and K. Bersell. 2001. The Rapid Syndrome Validation Project (RSVP). Proceedings of the AMIA Symposium:771-775.
Zetter, K. 2006 (February 23). Brilliant’s wish: Disease alerts. Wired, http://www.wired.com/science/discoveries/news/2006/02/70280/ (accessed June 4, 2007).