1
Surveillance Strategies
OVERVIEW
This chapter includes workshop presentations that illustrate a variety of goals, approaches, and methodologies for disease surveillance in humans, animals, and plants. As noted in the chapter’s first paper by keynote speaker Patrick Kelley, director of the Institute of Medicine’s Board on Global Health, current concepts of public health surveillance, inspired by approaches to military intelligence data gathering, originated in the 1950s. Today, traditional surveillance practices of disease reporting (by physicians, veterinarians, infection control practitioners, laboratorians, and medical examiners), followed by epidemiological and laboratory investigation, constitute the mainstay of local infectious disease surveillance where such expensive methods are feasible (mainly in developed countries). However, a range of nontraditional strategies including syndromic surveillance (the topic of Kelley’s paper, and another in this chapter by Michael Stoto) and electronic surveillance (the subject of Chapter 2), may prove well suited to settings where clinicians, laboratories, and hospitals are in short supply.
Local Surveillance: New York City
Although New York City’s size, diversity, and significance to international transportation create considerable opportunities for infectious outbreaks, local approaches to surveillance resemble those of many communities around the world, according to presenter Marci Layton of the New York City Department of Health and Mental Hygiene (DOHMH). New York health codes mandate disease reporting for more than 70 infectious diseases, ranging from common
pathogens such as Salmonella to the potentially disastrous, such as smallpox and anthrax. The health department receives reports by traditional phone, mail, and fax and—following a significant recent investment—by electronic and web-based methods as well. Participation in an electronic clinical laboratory reporting system, a secure network that allows DOHMH to receive laboratory-confirmed diagnoses in a timely manner, is mandated for all laboratories that diagnose New York City residents. This system enables DOHMH to spot citywide and neighborhood disease trends in routinely reported data that an individual physician would not be able to recognize, Layton said.
Upon receiving a report, DOHMH initiates an investigation to examine risk factors for infection in order to determine disease transmission routes, and, if appropriate, to arrange prophylaxis. “The most important thing we try to do is to make sure that every health care provider knows who and how to call to make a report,” Layton said.
In the event of an apparent or actual public health emergency, New York City’s health alert system quickly disseminates information to providers on the nature of the emergency and instructions on preparing and delivering diagnostic specimens. Because New York City is at high risk for receiving imported disease, DOHMH stays attuned to global infectious disease issues via surveillance networks such as ProMED-mail (see Morse in Chapter 2) and responds to reports of significant disease activity abroad by ramping up surveillance and alerting health-care providers in New York City to look for signs of an outbreak. After an outbreak of West Nile virus in 1999, and in light of increasing concern regarding the potential use of zoonotic diseases as bioterrorism agents, animal diseases were made reportable in New York City in 2000.
DOHMH has invested considerable hospital-preparedness funding to improve the ability of triage systems to recognize patients with significant risk factors for infectious disease, particularly patients with fever and respiratory illness who have traveled recently. This is crucial because, in Layton’s words, “New York City could be the next Toronto, with an unrecognized imported outbreak of severe acute respiratory syndrome (SARS)—or of bioterrorism, E. coli, or most worrisome of all, avian influenza.”
The realization that many unreported, hospitalized cases of viral encephalitis (a reportable disease) manifested during the West Nile virus outbreak caused DOHMH to adopt procedures to monitor similar nonspecific clinical syndromes. In 1998, the city began syndromic surveillance based on ambulance dispatch data; the system was expanded to monitor the entire emergency department in the wake of the 2001 World Trade Center attack, then further to monitor pharmacy sales, employee health, school absenteeism, and primary care visits. One of the most challenging aspects of responding to a syndromic signal is getting specimens to a lab for diagnostic testing, Layton observed, particularly specimens from the acutely ill patients typically seen in emergency departments. Rapid diagnostic testing is performed for a variety of pathogens at a single New
York City hospital, but only limited information is obtained from this proof-of-concept project.
To better balance time spent investigating syndromic surveillance signals versus outbreaks detected through traditional means, DOHMH is developing a protocol to reduce time wasted on false positives while ensuring the prompt investigation of real outbreaks. Syndromic surveillance systems have proven to be most useful for monitoring citywide seasonal outbreaks of infectious diseases (e.g., norovirus, influenza, respiratory syncytial virus [RSV]), Layton said, and less useful for detecting localized outbreaks.
“In my view, syndromic surveillance will never replace traditional surveillance, which is where most surveillance resources should continue to be invested,” she concluded. “The real public health challenge lies in creating the necessary infrastructure to analyze surveillance data, set priorities, and conduct investigations. I am concerned that increased investment in syndromic surveillance may occur at the expense of state and local public health infrastructure. More generally, if current funding patterns continue, whereby national programs addressing emerging infections and bioterrorism receive more and public health at the state and local levels receive less, our ability to make use of surveillance information will suffer.”
Toward Earlier Warning
Through the use of prediagnostic data, syndromic surveillance aims to provide timelier identification of disease outbreaks than can be attained through traditional surveillance methods, Kelley writes. After reviewing the theoretical underpinnings and historical development of syndromic surveillance, he discusses its potential applications in developing countries and its promise as a vehicle for achieving global disease surveillance as mandated in recent revisions of the International Health Regulations (IHRs). Unfortunately, “hasty, opportunistic implementations of syndromic surveillance,” including some U.S. projects, “have not allowed the theoretical power of the method a fair test,” he observes. In their stead, Kelley advocates the creation of surveillance systems, including syndromic components, designed to answer clear and specific questions. He also considers how syndromic surveillance could be applied to detect serious but low-frequency threats such as bioterror attacks, SARS, or avian influenza in time to contain their further spread.
Following Kelley’s paper, with its focus on the design of syndromic surveillance systems, Stoto’s essay considers their evaluation. He defines and applies a framework for gauging the usefulness of syndromic surveillance in public health practice, then uses it to identify a number of statistical and practical challenges to using such surveillance for detecting bioterrorist events. By contrast, he finds promise in using syndromic surveillance to detect natural disease outbreaks (including seasonal and pandemic influenza), and in monitoring public health
response to disease outbreaks. Realizing this potential will require designing systems that focus on these uses rather than being optimized for timely detection of large-scale bioterrorist attacks, Stoto concludes.
The next paper, by Joseph Lombardo of the Johns Hopkins Applied Physics Laboratory, addresses another aspect of timeliness in surveillance: the implications of “real-time” versus “batch reporting” of surveillance information. Noting that confusion has arisen around the use of these terms, Lombardo carefully defines them and provides illustrative examples. He concludes by describing the possible combination of both modes in surveillance systems that use efficient “batched” surveillance processes for the routine monitoring of public health, and more resource-intensive “real-time” processes to examine specific threats as they arise.
Surveillance of Animal and Plant Diseases
Recognizing that “the health of people, animals, plants, and the environment in which we all live are inextricably linked,” in the words of workshop presenter William Karesh, surveillance must encompass far more than human diseases. Karesh’s contribution to this chapter describes initial efforts toward this goal, focusing on projects undertaken by his own organization, the Wildlife Conservation Society (WCS). He describes the threat spectrum, origins, risk factors, and consequences of infectious disease in wild animals, and he observes that “the immediate effects of the diseases themselves are often the least of the worries. Infectious diseases of people and animals are drivers of poverty and associated civil unrest, disrupt ‘free’ ecosystem services such as drinking water and plant pollination, and can ruin otherwise well-planned and sustainable economic development efforts.”
In two papers that conclude the chapter, plant pathologists Jacqueline Fletcher of Oklahoma State University and James Stack of Kansas State University define threats (both natural and intentional) to U.S. crops and provide examples of high-consequence plant diseases. The first paper outlines components of a strong plant biosecurity strategy, discusses progress toward its achievement, and notes opportunities for further improvement. In the second paper, the authors evaluate each component of the biosecurity strategy (prevention, surveillance, detection, diagnosis, response, and recovery) and suggest specific actions the United States could take to support each area.
SYNDROMIC SURVEILLANCE: MOVING FROM THEORY TO PRACTICE
Patrick W. Kelley, M.D., Dr.P.H.1
The National Academies
Assessing the health of a community has similarities to assessing the health of a person. A variety of detectors of ill health can be brought to bear in ways that range from passive monitoring that depends on those affected to raise a concern to active and aggressive monitoring of those apparently without complaint to identify the earliest manifestations of a problem. The desire for earlier detection of acute health problems at either the individual or community level has in recent years stimulated the search for better “detector” mechanisms. Syndromic surveillance is one of these now in vogue as a solution to the growing challenge of early disease detection in communities and management of consequent public health interventions.
Though infectious disease reporting started in Europe and the United States in the late 1800s, it was not until 1925 that all U.S. states participated in national morbidity reporting. Only after Alex Langmuir went to the Centers for Disease Control and Prevention (CDC) in 1950 did the term “surveillance” become conceptualized beyond the monitoring contacts of persons with contagious diseases. At CDC Langmuir developed a concept of surveillance inspired by military intelligence data gathering and incorporated the approach into daily public health practice. Soon CDC had national systems for malaria, polio, and influenza. In more recent times, advances in laboratory and mathematical methods and technologies have pushed horizons farther and stretched academic definitions. These cutting-edge approaches to disease detection at the community level encompass networks for surveillance using molecular fingerprinting and exciting, web-based methods of information capture and assessment such as the Program for Monitoring Emerging Diseases (ProMED) and the Canadian-World Health Organization (WHO) Global Public Health Intelligence Network (GPHIN). In this more demanding context, we now have the evolution of automated syndromic surveillance.
The elaboration of more sophisticated approaches to surveillance has been stimulated by the recognition over the past 30 years of at least 30 “new” emerging infectious diseases. These encompass infections of plants, animals, and human beings. Of course, an acute concern is the threat of bioterrorism but many naturally occurring emerging disease outbreaks have highlighted the need for rapid detection and characterization. Perhaps the greatest concern now is the need to promptly recognize the syndromic pattern of an H5N1 influenza outbreak, here
or in remote parts of Asia or Africa, so that aggressive attempts to eliminate it can be instituted before it becomes uncontainable. Similar urgency arose during the 2003 severe acute respiratory syndrome (SARS) epidemic. For some of these emerging infections, it was months before an agent was isolated, and thus timely and sensitive public health surveillance and response was syndromic to a great degree. The tragedies of HIV in Africa and the slow recognition of SARS in China are reminders of the consequences of slow responses and motivate the question of what surveillance system designs could have made a difference. With bioterrorism a rapid assessment and response is even more critical.
“Syndromic surveillance” is defined by CDC as the collection and analysis of “health-related data that precede diagnosis and signal with sufficient probability of a case or an outbreak to warrant further public health response” (CDC, 2006a). This differs from more traditional surveillance in several ways but primarily the objective is that by using prediagnostic data, syndromic surveillance aims to be timelier in identifying emerging problems. The phenomena of emerging infections and all the associated aspects of globalization that accompany them, as well as the specter of bioterrorism, drive the need to be more cognizant of public health events and to act despite limited information. Timeliness is not the only advantage of the method, though. An additional goal is that syndromic surveillance should be more sensitive at detecting aberrations in normal patterns because it does not depend on confirmed diagnoses, something that can be an expensive proposition, especially in developing countries.
Some advocates have great enthusiasm for transitioning syndromic surveillance from the epidemiologic laboratory into routine practice, but others are skeptical, preferring to put their confidence in traditional approaches and the “astute clinicians” who have risen to the occasion so often in this country. Unfortunately, while developed countries have a fair number of clinicians who are astute at least much of the time, the developing world, where so many disease problems emerge, is a different case. A system of complementary systems—including clinicians, traditional methods, and well-designed syndromic surveillance tailored to the setting of a particular community—may ultimately yield the wide range of perspectives needed to meet the demanding public health challenges of emerging infections and globalization. The best mix of surveillance interventions will vary from community to community. A challenge now is to do the operations research to adapt academic surveillance concepts to unique community circumstances. This is important not only in communities with strong health systems, but also in developing countries, where nontraditional approaches may be more essential and affordable than in places with a relative abundance of astute clinicians, laboratories, and hospitals, such as the United States.
Some observers seem frustrated by syndromic surveillance because it has detected few outbreaks, as implemented in the United States over the past few years. Many doubt that it will perform better than alternative mechanisms to alert the public health community to a problem. Perhaps though hasty, opportunistic
implementations of syndromic surveillance have not allowed the theoretical power of the method a fair test. Also, the purposes of syndromic surveillance go beyond earlier detection and provide situational awareness across a community, something that individual clinicians can rarely provide. Though other mechanisms, to include astute clinicians, may help recognize a problem, an effective surveillance system, syndromic or otherwise, should also rapidly characterize a problem epidemiologically because this is essential to efficiently target what are invariably limited response assets. A system should enable civic leaders to establish the boundaries of the problem and allay some unjustified fears through more credible risk communication.
In tabletop exercises of public health crises, the value of information for management has been highlighted both as being in short supply and as being something that a properly constructed syndromic surveillance system should help develop. In one important biodefense tabletop simulation exercise, “Dark Winter,” Frank Keating, former governor of Oklahoma, said:
You can’t respond and make decisions unless you have the crispest, most current, and best information. And that’s what strikes me as a civil leader … that is … clearly missing (O’Toole et al., 2002).
Central to effective surveillance is beginning with a clear appreciation for the capabilities sought. Precisely what phenomena need detection, in precisely what populations is the detection needed, and what data would be most effective for that purpose? Much work has been accomplished in developing syndromic definitions and analytic algorithms but before syndromic surveillance is seen as the solution, the full range of scenarios that need to be detected must be considered as well as how best to build epidemiologic “detectors” for demographically different communities in both rich and poor countries.
Although in the United States there is a tendency to associate syndromic surveillance with the specter of bioterrorism, WHO has come to recognize that the protection of global health against emerging infections was poorly served by the last version of the International Health Regulations (IHRs), which mandated reporting to WHO only three specific diseases: yellow fever, plague, and cholera. Realizing that some of the most critical recent global public health threats—such as AIDS, SARS, Ebola, pandemic influenza, and Nipah virus—initially were ill-defined syndromes, a new version of the IHRs has been adopted by member states and is set to go into effect in 2007. This document calls on countries to maintain, at the local level, capabilities to detect and assess not only well-defined diseases and established causes of death, but also to report any significant levels of morbidity of potential international public health importance. So, the mandate for general global public health surveillance is moving beyond defined diseases to encompassing a global responsibility to detect and report, in a timely manner, internationally important disease events whether they are well or ill defined and
whether they are individual cases or clusters. A capability for syndromic detection seems central to the new paradigm, especially in countries that lack the resources for extensive use of more specific approaches.
Although the term “syndromic surveillance” has only been in vogue for about a decade and is thought to represent somewhat of a frontier in surveillance, the potential contributions of “prediagnostic surveillance” have been long established. In tracking down the last cases of smallpox and polio in developing countries, syndromic monitoring has been central. For decades, the military has also used syndromic approaches to monitor unit health on deployments and in training because it was the most cost-effective, rapid, and reliable way to monitor the health of the force, especially in austere conditions. The military often operates in settings with limited laboratory support, but with a critical need to detect health threats in a timely manner. For example, Figure 1-1 illustrates the tracking of diarrheal syndromes in a U.S. Marine force during the first Gulf War of 1990–1991. With regular syndromic tracking of morbidity seen in sick call, outbreaks were routinely recognized quickly by competent epidemiologists against normal background rates. Investigations were launched rapidly to contain problems that could debilitate unit combat effectiveness.
In U.S. military basic training camps, where respiratory syndromes are particularly devastating, for decades there has been well-developed, centrally monitored syndromic surveillance for acute respiratory syndromes (Gray, 2005; Gunzenhauser, 2003). Syndromic surveillance in the basic training setting has been used routinely to guide the use of mass antibiotic prophylaxis to prevent outbreaks of rheumatic fever when syndromically associated thresholds are crossed.
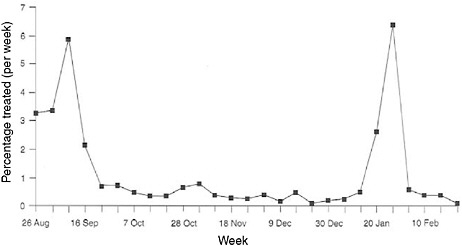
FIGURE 1-1 Syndromic surveillance of U.S. marines for treated diarrheal syndromes during the lead-up to the Persian Gulf War, 1990–1991.
SOURCE: Hanson (2005).
All of these practical implementations of syndromic surveillance reflect movement from theory and simple systems to complex systems. Moving from theory to practice involves a larger context where pieces must be made to work together and adapted to the locality.
Reflecting all the elements to be integrated, one might define a surveillance system, as distinct from surveillance, as follows:
A system for public health surveillance is a group of integrated and quality-assured, cost-effective, and legally and professionally acceptable processes, designed for the purpose of identifying in an ongoing, flexible, standardized, timely, simple, sensitive, and predictive manner the emergence of meaningful epidemiologic phenomena and their specific associations. These processes include human, laboratory, and informatics activities to skillfully manage information derived from an entire defined community (or a subgroup thereof that is sufficiently representative and large) and to disseminate that information in a timely and useful manner to those able to implement appropriate public health interventions.
As shown in Figure 1-2, a surveillance system needs to be seen in the context in which it works and as reflecting a hierarchy of elements that depend on each other. One needs a clear and specific idea of what questions the system should address. Who should be under surveillance and for what are most critical. Developers of syndromic surveillance systems often start to conceptualize
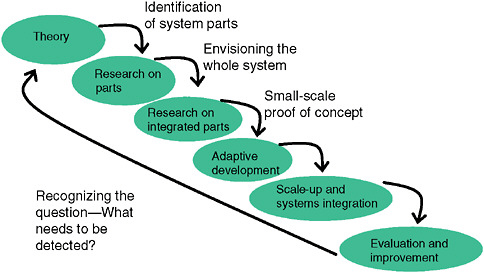
FIGURE 1-2 Conceptual steps in development and implementation of a syndromic surveillance system in a community.
SOURCE: Kelley (2006).
a system with opportunistically available data rather than a clear definition of the range of scenarios that their surveillance system must be able to recognize as priorities. Typical “opportunistic” data might be routinely collected for other purposes during an emergency room consultation or from “convenient” sources such as government clinics regardless of how well they sample the community of interest. Opportunistic datasets are rarely the strongest cornerstone on which to build and can handicap an otherwise rigorous implementation.
Different epidemiologic scenarios will affect populations in different ways. Key though is that if one wants to detect any epidemiologic scenario, the population under surveillance should include the one likely affected. If space and time separate these populations, as may be the case with the most easily available “opportunistic” datasets, little signal will be generated. If demographic misclassification affects the description with respect to person, place, and time, associations may be missed. If one lets the surveillance question drive the development of the database used, there is a better chance that the population under surveillance will generate a strong signal because it will include a substantial fraction of those exposed. Resources should be invested into negotiating for and developing data with the richest “veins of ore” rather than focusing it proportionately on the mining of poorly conceived data sources with ever more complex analytic methods. An example of this became obvious in looking at convenient outpatient data in the Department of Defense (DoD) Electronic Surveillance System for the Early Notification of Community-based Epidemics (ESSENCE), developed in the late 1990s for use for surveillance in the National Capital Region.
Like syndromic surveillance systems, the datasets initially available to ESSENCE routinely classified patients experiencing morbidity by the ZIP code of residence. The problem is that one could reasonably assume that most exposures, natural or manmade, would occur away from home in places such as the Pentagon, the Capitol, a sports venue, or the subway. As became evident in a geographic analysis, the bulk of military health-care beneficiaries tracked through ESSENCE did not live where many exposures would most likely occur, in the District of Columbia, but rather had homes scattered over a hundred ZIP codes throughout the region. This residence-based misclassification, stemming from the use of “opportunistic data” easily at hand, would have greatly diluted syndromic signals arising from exposures at the workplace. This misclassification produces what might be termed the “donut-hole effect” (Figure 1-3).
As exposed persons migrate from a center city worksite of exposure, where they might be classified most effectively as an “exposed” population, they disperse into the suburbs, where they blend with unexposed populations so completely that any signal is greatly damped out. Overcoming this depends on not settling for datasets of convenience. Populations in which those under medical surveillance have limited geographic mobility can help correct for the donut-hole effect. Students at universities might be one example. Residents of nursing homes and prisons may be other populations where there is less risk that place of
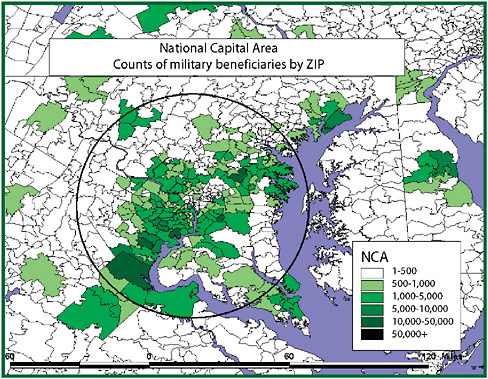
FIGURE 1-3 The donut-hole effect.
SOURCE: Kelley (2006).
exposure and place of residence differ. Another setting is military basic training. However, a limitation of many of these populations is that they may not be near the locations where surveillance is most critically needed, making their ability to serve as sentinels less than ideal.
With the DoD ESSENCE, some of the most impressive syndromic signals have come from basic training outbreaks, where the exposed population lived and worked in the same location. This meant there was no problem with the migration phenomena causing people exposed in one place to be classified geographically in another. The strength of the signal and its rapid detection was also greatly facilitated by the ability to attribute morbidity to a well-defined denominator population that included most cases. For populations on the move, if they work in high-value targets such as centers of government, it may be a high-yield investment to develop a way to ensure that they can be classified by both their primary residence and primary workplace.
In moving from syndromic surveillance theory to practice, the first step is appreciating not what data are at hand, but what are the “who, what, and when” questions that need to be answered. The most effective surveillance systems will likely be systems of systems because the questions to be answered will reflect
multiple scenarios, each of which presents a different challenge. The classic incident is an exposure to a whole community. In the bioterrorism scenario, this might be a regionwide aerosol plume, but many other scenarios may be even more likely and successful. Potential exposure scenarios include the following:
-
Regionwide aerosol plume
-
Seeding of a focal or traveling population with contagious (suicidal) persons (e.g., smallpox)
-
Contaminated food distribution (e.g., Salmonella spp., hepatitis, E. coli, or bovine spongiform encephalopathy)
-
Contaminated water supply (focal or general)
-
Focused attack against high-value worksite or event (e.g., letters to Congress)
-
Generalized aerosol plume against high-value site
-
Focused aerosol attack against general population (e.g., mass transit)
The classic image is of a region-wide aerosol plume that distributes kilograms of an agent upwind from a population center with the idea of causing tens of thousands of deaths and incapacitations. This is perhaps the worst case, but likely the easiest to detect because it could affect large numbers of people across a wide geographic swath. Perhaps a more likely challenge for public health would be the seeding of a focal or traveling population with an infectious agent, such as SARS or pandemic influenza. Debate is needed on the question of how best to apply syndromic surveillance methods to detect serious, but lower frequency, events in time to contain their further spread. Beyond the astute clinician, who may be an uncommon commodity especially in some developing countries, what is the most sensitive mechanism to detect aberrancy at the population level when only a handful of nondescript cases are initially involved, as might be the case with an early human pandemic influenza scenario? Could the initial hands full of cases of SARS in Viet Nam or China have been better contained if alerts had been raised earlier and if communications to those who could have acted had been more rapid? How could syndromic surveillance have been adapted to supplement the astute clinician in the scenarios in Hanoi, Hong Kong, Singapore, or Toronto? Does syndromic surveillance have a role in scenarios such as these or in identifying clusters of avian flu in Indonesia or Cambodia?
In considering rare but important low-frequency emergences of a new infectious disease, the example of West Nile further illustrates the fact that the questions asked of a surveillance system differ based on the agent and the scenario to be detected. For West Nile encephalitis, tracking infrequent and not highly unique human syndromes across a large general human population may not be the most effective way to achieve the rapid recognition envisioned in the new IHRs. Figure 1-4 shows the estimated sensitivity for West Nile virus by different surveillance methods. A system of systems that includes animals that
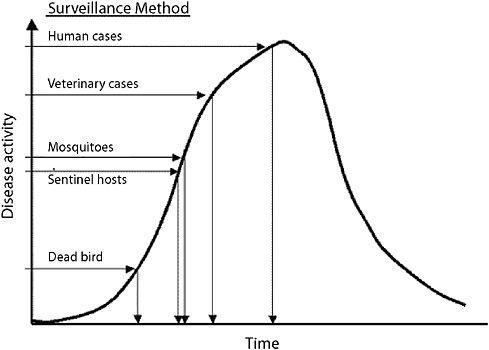
FIGURE 1-4 Estimated sensitivity for West Nile virus by different surveillance methods.
SOURCE: CDC (2003).
manifest aberrations earlier in time would be preferable to waiting until larger numbers of people develop encephalitis and are admitted to intensive care units.
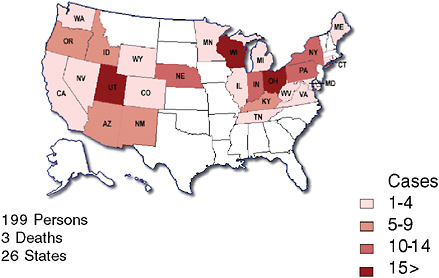
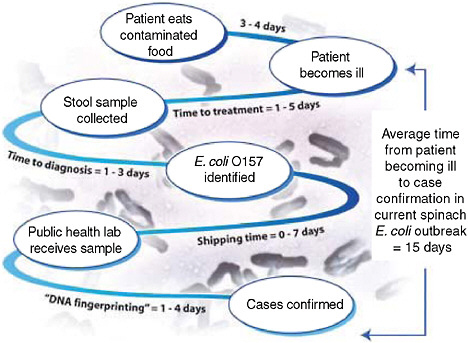
One of the more recent national public health concerns in the United States has been the outbreak of E. coli O157:H7 associated with consumption of raw spinach. Could a configuration of syndromic surveillance detect a focal or a dispersed outbreak from contaminated food? The E. coli outbreak involved a few hundred cases across the country (Figure 1-5) (FDA, 2006). Would a focus on unexplained hemolytic uremic syndrome be a way to complement the impressive but slow molecular fingerprinting approaches that ultimately carried the day? The molecular approaches to DNA fingerprinting for outbreak identification were certainly valuable, but more than 10 days could easily pass between when a patient develops symptoms and when a case is confirmed and linked with other cases with the same fingerprint (Figure 1-6). Syndromic surveillance seeks to narrow the gap.
Another important outbreak scenario to detect is the contaminated water supply. The infamous Milwaukee cryptosporidiosis outbreak caused hundreds of thousands of cases of diarrhea, but its nature was such that recognition of
cryptosporidiosis as the specific cause was quite delayed (MacKenzie et al., 1994). Most sick people did not seek care. Labs were not testing for the agent routinely, and many cases were just diagnosed as viral gastroenteritis. Could a thoughtfully designed syndromic system of systems have led to more prompt recognition and mitigation of the outbreak? The epidemiology of this significant public health event should make operators of syndromic surveillance systems consider how well their systems and the datasets used would pick up a problem with a municipal water system. For example, this outbreak pointed out how small a fraction of those affected may actually seek medical care (6.5 percent here), much less go to an emergency room. How can the morbidity represented by these individuals not be lost for surveillance purposes? Furthermore, as noted earlier, many syndromic systems analyze data routinely by residential ZIP code, but how many routinely group residences based on an appreciation for how water flows through the municipal water distribution system in their city? In Milwaukee it was clear that the map of the distribution system would have correlated powerfully with a pattern of attack. The sparing of special populations such as nursing home residents was reminiscent of John Snow’s observations on the sparing of the Whatney’s Brewery workers from the cholera outbreak in London in the late 19th century.
Another important scenario to think about is the focused attack against a high-value site such as the 2001 anthrax letter attacks. Tragically in this attack a number of people died, but some lives were probably saved by the action of the hoped for astute clinician. Beyond the astute clinicians, however, what system configuration would pick up those low-frequency cases that may reflect serious morbidity as a harbinger of a more widespread exposure? Individual cases were identified in emergency rooms in this attack. Some were not so quickly recognized and may have taken on a different characterization if appreciated in a larger epidemiologic context rather than counting on an individual astute clinician to sense a “big picture” beyond his field of vision. Perhaps rigorous surveillance of intensive care units (ICUs) for epidemiologically unexpected admissions may be a critical underdeveloped element of syndromic surveillance for problems such as this anthrax episode and outbreaks of problems such as West Nile or SARS. ICU surveillance may permit the time for more detailed epidemiologic characterization of epidemiologically suspect cases, that is, cases that are admitted with no obvious predisposing reason. Pooling across a municipal region may allow appreciation of patterns that no single astute clinician could be counted on to detect, much the same way that unexplained death surveillance may be helpful, if not too late.
Perhaps the most important scenario to detect is the “failed scenario.” We know that the worst case scenario of a biological attack would not be easy for most perpetrators, but that does not necessarily discourage them from trying. Being able to detect a modest trial run outcome would be a much more useful capability than designing a system for a more obvious worst case scenario.
The first generation of an avian influenza cluster would also be the best time to appreciate a problem. A goal of surveillance systems should be to not only detect the classic worst case attack early or the widespread deaths of chickens, but also to detect what may more often be a botched attempt that falls far short of the perpetrators’ hopes or the earliest generation avian flu outbreaks. The unsuccessful 1993 attempts by Aum Shinrikyo to spray anthrax over the city of Tokyo illustrate this point (Takahashi et al., 1994). Fortunately this incompetent attempt did not cause a single case, but if it had, even one case could have been valuable to recognize as a harbinger of future threats. Perhaps the complete failure of this anthrax attempt caused Aum Shinrikyo to move on and use sarin in the Tokyo subway. A lesson is that motivated enemies will keep trying and could get better with practice. A comprehensive surveillance system should set its sights on detecting a wide range of scenarios to include trial runs or largely botched low-yield events that may indicate that more effective efforts are in the offing.
A recent review of abstracts accepted for presentation at the October 2006 International Disease Surveillance Conference in Baltimore, Maryland, showed that more states than not have started to explore syndromic approaches to disease detection and management. In addition to the United States, seven foreign jurisdictions also came to the meeting to present systems for syndromic surveillance. In comparing the datasets represented in systems described at the 2003 meeting with the 2006 abstracts, implemented systems are still overwhelmingly focused on emergency rooms and hospital diagnoses—81 percent in 2006 (Figure 1-7 and Table 1-1). Although these data sources are obviously relevant for many scenarios mentioned and may be the most convenient, they are not necessarily the answer to all challenges. Other populations and venues may lend themselves to better classification with respect to person, place of exposure, and time. To get the most power out of the analytical methodologies being developed, there may be justification to put the focus on other datasets to illuminate different aspects of the clinical continuum and work so they contain the most informative fields.
Each of these varied data sources in Table 1-2 may provide a unique perspective on a particular epidemiologic scenario, especially if public health practitioners help shape the characteristics of the data rather than just settling for what data are readily available. If public health practitioners are on the alert for emerging infections, including bioterrorism, the aim should be to do more than detect only large unexplained outbreaks, but also to have the ability to detect isolated, unexpected cases with unusual age, gender, or occupational characteristics. The need to do this is driven not only by American concerns over bioterrorism, but is also reflected in visions of the new IHRs. If public health officials are to detect and contain pandemic influenza, it is doubtful that they will be very successful if they fail to recognize emerging patterns until there is a large unexplained outbreak.
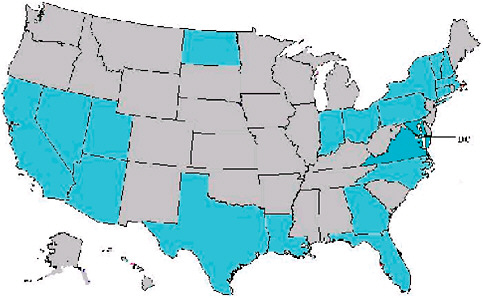
FIGURE 1-7 Locations of surveillance systems in abstracts for the 2006 International Society for Disease Surveillance (ISDS) meeting. Countries represented include United States, Canada, Netherlands, Taiwan, Hong Kong, France, Scotland, and Greece. States represented include AZ, CA, CT, DC, FL, GA, IN, LA, MA, MD, NC, ND, NH, NV, NY, OH, PA, TX, UT, VA, VT.
SOURCE: Kelley (2006).
To summarize, as demonstrated by Figure 1-8, public health surveillance begins with understanding the questions “who, what, and when” that need to be asked, and then it seeks the most effective data sources.
A system for public health surveillance, which is what needs to be built in the move from academic theory to practice, is built on that data foundation, but it also needs a set of powerful analytic tools and skillful people to use them and interpret the findings. The skill sets of local public health staff to interpret data of this type need expansion. Because this is a complex science still under development, perhaps academic partnerships need to be sought for all serious adaptations of these concepts to specific localities. Few approaches can be just “dropped in” without an appreciation for local epidemiologic and demographic peculiarities. Perhaps most in short supply are the resources to do something promptly to respond to findings. Budgets for surveillance systems should be accompanied by budgets for a serious response capability. Finally, the underlying population demographic structures and exposure likelihoods of some localities may make syndromic surveillance a low-yield, cost-inefficient activity. This may not be the destination for every community. Guidelines for where performance is expected to be lower are needed as well as insights into where value is likely to be added.
TABLE 1-1 Sources for Syndromic Surveillance, 2003 and 2006 Annual Meeting Abstracts
|
|
2003 |
2006 |
|||
|
Data Sources |
# of Abstracts |
% of Abstracts |
# of Abstracts |
% of Abstracts |
|
|
Emergency departments |
29 |
48 |
38 |
56 |
|
|
Hospital diagnosis |
7 |
12 |
17 |
25 |
↑ |
|
Office/clinic visits |
13 |
22 |
11 |
16 |
↓ |
|
Over-the-counter drugs |
5 |
8 |
7 |
10 |
↑ |
|
911/emergency medical service runs |
6 |
10 |
6 |
9 |
↓ |
|
Laboratory results |
2 |
3 |
5 |
7 |
↑ |
|
Nurse advice lines |
4 |
7 |
3 |
4 |
↓ |
|
Laboratory orders |
1 |
2 |
1 |
1 |
|
|
School nurse records |
— |
— |
1 |
1 |
|
|
Poison control center |
5 |
8 |
1 |
1 |
↓ |
|
Veterinary diagnosis |
3 |
5 |
1 |
1 |
↓ |
|
Health-care employee absenteeism |
— |
— |
1 |
1 |
|
|
School absenteeism |
7 |
12 |
1 |
1 |
↓ |
|
School perception of an outbreak |
1 |
2 |
— |
— |
|
|
Medical examiners |
2 |
3 |
— |
— |
|
|
Thermometer sales |
— |
— |
1 |
1 |
|
|
Evacuation shelter primary reports |
— |
— |
1 |
1 |
|
|
Local/regional news sources |
— |
— |
1 |
1 |
|
|
Web logs |
— |
— |
1 |
1 |
|
|
Online obituaries |
1 |
2 |
— |
— |
|
|
Medical center parking lot volume |
1 |
2 |
— |
— |
|
|
SOURCES: Sosin and DeThomasis (2004) and Kelley (2006). |
|||||
TABLE 1-2 Potential Sources of Data for Syndromic Surveillance
|
|
SOURCE: Kelley (2006). |
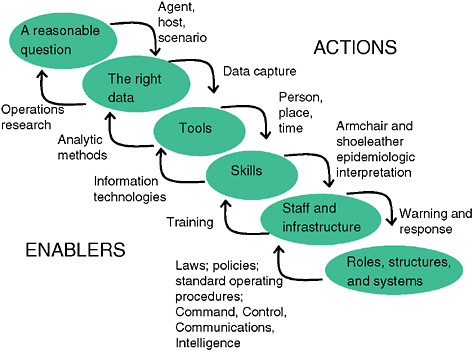
FIGURE 1-8 System requirements for public health surveillance.
SOURCE: Kelley (2006).
SYNDROMIC SURVEILLANCE IN PUBLIC HEALTH PRACTICE
Michael A. Stoto, Ph.D.2
Georgetown University
Heightened awareness of the risks of bioterrorism since 9/11, coupled with a growing concern about naturally emerging and reemerging diseases such as West Nile, severe acute respiratory syndrome (SARS), and pandemic influenza, have led public health policy makers to realize the need for early warning systems and, more generally, improved surveillance. The sooner health officials know about an attack or a natural disease outbreak, for example, the sooner they can treat those who have already been exposed to the pathogen to minimize the health consequences, vaccinate some or all of the population to prevent further infection, and identify and isolate cases to prevent further transmission. In addition, improved surveillance systems should allow for better “situational awareness” and thus help to manage the response to public health emergencies.
Traditional public health surveillance approaches monitor disease using pre-specified case definitions and employ manual data collection, human decision making, and manual data entry. In contrast, newly developed syndromic surveillance systems employ sophisticated information technology (IT) and statistical methods to gather, process, and analyze large amounts of data and display the information for decision makers in a timely way. For example, syndromic surveillance systems assume that during an attack or a disease outbreak, people will first develop symptoms, then stay home from work or school, attempt to self-treat with over-the-counter products, and eventually see a physician with nonspecific symptoms days before they are formally diagnosed and reported to the health department. To identify such behaviors, syndromic surveillance systems regularly monitor existing data for sudden changes or anomalies that might signal a disease outbreak. Syndromic surveillance systems have been developed to include data on school and work absenteeism, sales of over-the-counter products, calls to nurse hotlines, and counts of hospital emergency room (ER) admissions or reports from primary physicians for certain symptoms or complaints (Mandl et al., 2004).
Recognizing that the “ability to gather and analyze information quickly and accurately would improve the nation’s ability to recognize natural disease outbreaks, track emerging infections, identify intentional biological attacks, and monitor disease trends,” the Institute of Medicine (IOM) recently called for more research on syndromic surveillance and other “innovative systems of surveillance that capitalize on advances in information technology.” However, because surveillance systems in the United States “remain fragmented and have not evolved at the same rate as … electronic technological advances,” the IOM calls for these systems to be “carefully evaluated for their usefulness in detection of infectious disease epidemics, including their potential for detection of major biothreat agents, their ability to monitor the spread of epidemics, and their cost effective-ness” before widespread implementation (IOM, 2003).
To address the issues identified by the IOM, this paper begins by describing a framework for evaluating the usefulness of syndromic surveillance in public health practice. Application of this framework to existing systems identifies a number of statistical and practical concerns when syndromic surveillance is used to detect bioterrorist events. The analysis suggests, however, that these systems may be more useful in detecting natural disease outbreaks (including seasonal and pandemic influenza) and in the public health response to known disease outbreaks.
Evaluation of Syndromic Surveillance Systems’ Usefulness
Asking whether syndromic surveillance “works” or not is not particularly helpful. Rather, just as clinicians need to know the performance characteristics of screening and diagnostic tests, public health epidemiologists need to charac-
terize the performance of syndromic surveillance detection systems in terms of the kinds of events that can be detected as a function of the responsible antigen, outbreak size, timing, and other characteristics. Thus, evaluation of syndromic surveillance systems’ usefulness involves a number of dimensions.
Evaluations of data accuracy and use, for example, include studies of the accuracy of electronic records that form the basis of the systems compared with an independent source, the accuracy of use of standard codes, the accuracy of data preprocessing, and similar issues. This aspect of evaluation also includes studies of the appropriateness of methods and protocols for data analysis, data display, monitoring, and reporting, as well as how these methods are applied and how they lead to action.
Evaluations of system utility include studies of the costs and benefits of day-to-day use of syndromic surveillance, relative to existing systems, to identify communicable or reportable diseases, to increase situational awareness, or to assist in investigation and management of a disease outbreak. These studies also assess the costs and benefits to users of identifying and evaluating data anomalies using the system, as well as flexibility, acceptability, and stability. Finally, evaluation studies characterize statistical properties such as sensitivity, false-positive rates, and timeliness. As illustrated below, statistical evaluations can be based on simulation studies and comparisons of syndromic surveillance findings with known actual events.
Concerns About Syndromic Surveillance in Public Health Practice
Despite the generally recognized promise of syndromic surveillance systems, there are many practical concerns about the use of these systems in state and local public health practice. The possibility of earlier detection and more rapid response to a bioterrorist event has tremendous intuitive appeal, but its success depends on local health departments’ ability to respond effectively. When a syndromic surveillance system sounds an alarm, health departments typically wait a day or two to see if the number of cases continues to remain high or if a similar signal is found in other data sources. Doing so, of course, reduces both the timeliness and sensitivity of the original system. If the health department decides that an epidemiological investigation is warranted, it may begin by identifying those who are ill and talking to their physicians. If this does not resolve the matter, additional tests must be ordered and clinical specimens gathered for laboratory analysis. Health departments might also choose to initiate active surveillance by contacting physicians to find out if they have seen similar cases.
The detection of a sudden increase in cases of influenza-like illness (ILI)—the kind of condition that syndromic surveillance can detect—can mean many things. It could mean a bioterrorist attack, but is more likely a natural occurrence, perhaps even the beginning of the annual flu season. An increase in sales of flu medication might simply mean that pharmacies are having a promotion. A surge
in absenteeism could reflect natural causes, or even a period of particularly pleasant spring weather. Similar problems can occur when changes in local hospital systems, or even in coding practices, can result in substantial changes that could raise concern if they are not understood.
Additionally, a syndromic surveillance system that says only that “there have been five excess cases of ILI at hospital X” is not of much use unless the five cases can be identified and reported to health officials. For example, if there are 65 cases rather than the 60 expected, syndromic surveillance systems cannot say which 5 are the “excess” ones, and all 65 must be investigated.
Like all alarm systems, syndromic surveillance detection algorithms have intrinsic statistical tradeoffs. The most well known is between sensitivity, the ability to detect an attack when it occurs, and the false-positive rate, the probability of sounding an alarm when in fact there is no attack. The costs of excessive false alarms are both monetary, in terms of resources needed to respond to phantom events, and operational, as too many false events desensitize responders to real events. Taking into account the different data types and multiple jurisdictions, thousands of syndromic surveillance systems soon will be running simultaneously in cities and counties throughout the United States. If 1,000 data streams are being monitored, each with a 0.1 percent false-positive rate (which is very low), there will be approximately one false alarm per day.
The timeliness of a surveillance system depends on the time it takes to generate and acquire data, analyze it, and take action (Buehler et al., 2003). Even when the cause and route of exposure are known, the available control strategies—quarantine of suspected cases, mass vaccination, and so on—are expensive and controversial, and often their efficacy is unknown. Coupled with the confusion that is likely during a terrorist attack or even a natural disease outbreak, deciding what to do could take days to weeks.
With syndromic surveillance, an additional component is the time required to accumulate enough evidence of an outbreak to trigger a detection algorithm. To illustrate this point, Stoto and colleagues used a simulation approach to analyze ILI emergency department admissions data from a typical urban hospital. A hypothetical number of extra cases spread over a number of days were added to actual baseline data to mimic the pattern of a potential bioterror attack. Figure 1-9 (A and B) indicates the size and speed that outbreaks must attain before they are detectable, according to four statistical detection algorithms. The solid bar represents an algorithm that uses only one day’s data. The other three detection algorithms, shown with shared bars, average cases over several days. These results are sobering: Even with an excess of nine cases over two days (the first two days of the “fast” outbreak), three times the daily average, there was only about a 50 percent chance that the alarm would go off. When 18 cases were spread over nine days, chances were still no better than 50-50 that the alarm would sound by the ninth day (Stoto et al., 2004).
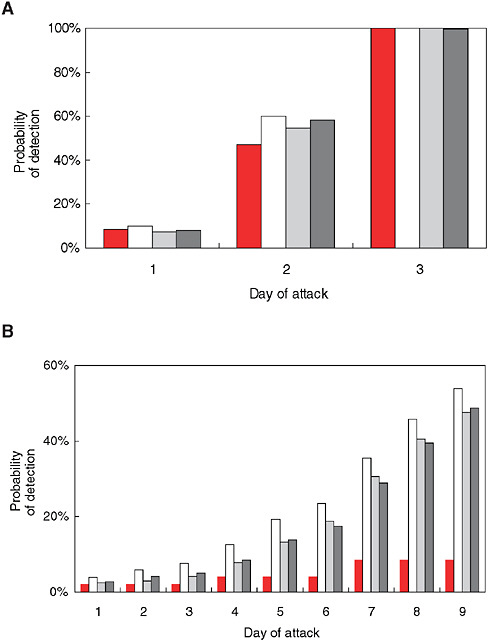
FIGURE 1-9 Sensitivity of syndromic surveillance (probability of detection by day) for influenza-like illness at a typical urban hospital emergency room using four detection algorithms, as indicated by shading pattern (see text). A) fast outbreak: 18 cases over three days, B) slow outbreak: 18 extra cases over nine days.
SOURCE: Stoto et al. (2004). Reprinted with permission from Chance. Copyright 2004 by the American Statistical Association. All rights reserved.
Can Performance Be Improved?
Simulation studies such as the one summarized in Figure 1-9 (A and B) has shown that unless a bioterrorism outlook is exceptionally large, syndromic surveillance detection algorithms take days to be detected (Stoto et al., 2004; Jackson et al., 2006; Buckeridge et al., 2006; Stoto et al., 2007). Results like this naturally lead one to ask whether this performance can be improved. Indeed, there are a number of approaches; however, although these approaches may lead to better performance for some outbreak types, they are less able to detect others.
Syndromes other than ILI, for example, might be more easily detected because they are less common, but this only works if a terrorist—or nature—chooses to use an agent that caused those symptoms. Systems can and typically are set up to monitor eight or more separate sets of symptoms. Doing so increases sensitivity simply because more conditions are monitored, but as discussed above, increasing the number of syndromes monitored will also increase the number of false positives.
Another possibility is to pool data over multiple data streams, perhaps from all hospitals in a metropolitan area or state. A number of cities are currently doing this. If this results in both the signal and the background increasing proportionally, it will result in a more effective system. If, however, there were 18 extra cases of ILI in a city, but they all appeared at one hospital, this signal would be lost in the noise of the entire city’s cases. Moreover, such an increase would be clear without any sophisticated surveillance system. One can analyze the data for the entire city and for each hospital individually, but with 10 separate analyses, the number of false positives would also increase.
Finally, the data can be analyzed geographically. If there were 18 extra cases of ILI in a city, and all lived in the same neighborhood, that would be more informative than 18 cases scattered throughout the city—it would suggest a biological agent released in that area. This is only effective, however, for a geographically focused bioattack, and would not work if terrorists chose to expose people in an office building or at an airport. It is also less likely to detect seasonal or pandemic influenza, which spreads rapidly before symptoms appear
Alternative Applications of Syndromic Surveillance
Since 9/11, the focus of syndromic surveillance efforts has been on early detection of bioterrorist events. The most value, however, may ultimately come from its use in the detection of natural disease outbreaks. More generally, if 21st century syndromic surveillance means effective use of health information technology in identifying cases before they are formally diagnosed, it can supplement traditional public health approaches and improve their effectiveness.
One potential use is in detecting influenza outbreaks. In an “ordinary” year, influenza results in 36,000 or more deaths and more than 200,000 hospitalizations
in the United States alone. In addition to this human toll, influenza-related costs are more $10 billion a year. A pandemic, or worldwide outbreak of a new influenza virus, perhaps evolving from the H5N1 avian flu virus circulating in Asia, could dwarf this impact by overwhelming our health and medical capabilities, potentially resulting in hundreds of thousands of deaths, millions of hospitalizations, and hundreds of billions of dollars in direct and indirect costs. Syndromic surveillance systems feature prominently in federal, state, and local plans to prepare the United States for pandemic flu (Homeland Security Council, 2005).
The Centers for Disease Control and Prevention (CDC) has a number of influenza surveillance systems in place (CDC, 2007), yet they do not provide population-based rates of incidence or prevalence rates on a national level because many infected persons are asymptomatic or experience only mild illness and do not seek medical care. Also, laboratory testing is not common and test results become available late in the course of the illness. Epidemiological characteristics of both seasonal and pandemic influenza, however, suggest that syndromic surveillance and other surveillance systems are likely to make an important contribution beyond the capabilities of existing surveillance systems, and thus enable a more effective public health response. Simulation studies have shown that unless a bioterrorism outlook is exceptionally large, syndromic surveillance detection algorithms take days to be detected (Stoto et al., 2004; Jackson et al., 2006; Buckeridge et al., 2006; Stoto et al., 2006). This time frame is longer than some proponents of syndromic surveillance as a tool to detect bioterrorism suggest is needed (Wagner et al., 2001). Compared to the current influenza surveillance systems, however, a one-week lead time would provide valuable information, and this is likely to be achievable for syndromic surveillance.
Furthermore, a number of studies have demonstrated the potential that syndromic surveillance of ILI offers at the national, state, and local levels. Sebastiani and colleagues (2006) have shown that children and infants presenting to the pediatric emergency department (ED) with respiratory syndromes are an early indicator of impending influenza morbidity and mortality, sometimes by as much as three weeks. Using data from New York City, Lu and colleagues (2006) have shown that monitoring both outpatient and ED data can enhance detection of ILI outbreaks. With similar data, Olson and colleagues (2005) note that age-stratified analyses of ED visits for fever and respiratory complaints offer the potential for more precise quantification of the burden of illness, earlier warning of the arrival of epidemic influenza, and greater sensitivity for detecting the characteristic age shift of pandemic influenza. Comparing unspecified infection cases in Washington, DC, hospitals using optimal detection algorithms to CDC’s sentinel physician data for the South Atlantic states for four years in which there was a discernable influenza outbreak, Stoto and colleagues (2007) found that in two of those years, the DC syndromic surveillance based on hospital emergency room data outperformed the other two systems, and in one year it flagged only two days
after the CDC system. Given a built-in delay of about two weeks in the CDC system, this is a substantial advantage.
In normal flu seasons, laboratory analysis to determine whether a case is truly influenza, or to identify the viral strain, is rarely done. Testing, however, is critical for identifying pandemic influenza, in which an antigenic shift results in a new viral strain to which few people are immune by virtue of previous exposure. Syndromic surveillance of flu-like symptoms might trigger more laboratory analysis than is typically done and in this way hasten the public health response. In a normal flu season, Labus (2005) has reported that early identification of the start of the influenza season using syndromic surveillance in Clark County, Nevada, enabled the notification of the medical community. Physicians were encouraged to submit specimens for culture, and the county health department provided kits to help them do this, which allowed for rapid identification of the major circulating strain. In 2003–2004 (a period with a marked increase of early season influenza and deaths in children in other parts of the country) this syndromic surveillance system allowed for better tracking, and provided data for daily reports to decision makers and the media.
Because of their focus on the early detection of bioterrorist events, most syndromic surveillance systems are designed to detect large increases in the number of people with common symptoms such as ILI. As a result, they cannot be expected to detect small numbers of cases, even if very unusual. One reason is that in a small disease outbreak or the early stages of a larger one, each case will be seen by only one physician. The natural tendency of physicians who see only one case, however suspicious it may be, is to discount it. After all, physicians are appropriately taught “when you hear hoofbeats, think horse, not zebra.” Some may fear the embarrassment of reporting a case that may turn out to be a false alarm.
Modern health informatics systems provide the potential to identify the presence of small numbers of cases of concern before they are formally diagnosed. For example, automated systems can aggregate data for a metropolitan area, spanning local reporting jurisdictions, to identify, say, cases of rash and fever, which would suggest smallpox. Systems can also be set up to enable and encourage early reporting of cases based on symptoms only. For example, the Syndrome Reporting Information System (SYRIS) system, now operating in Lubbock, Texas, and elsewhere, enables physicians to report suspicious cases to the local health department without waiting for laboratory confirmation, and encourages them to do so by providing feedback in the form of information about practice guidelines and other similar cases (Lindley and Ward, 2007). This can be thought of as a kind of “active syndromic surveillance” or as IT support for astute physicians.
Real-time access to prediagnostic data can also help health authorities respond to public health threats. If person-to-person transmission of avian flu virus is documented in Asia, for example, health departments in Europe and the United States might want to identify and follow up on local cases of people hos-
pitalized with flu-like symptoms, and syndromic surveillance systems could be designed to identify them. If an environmental sensor detects signs of the terrorist agent tularemia, syndromic surveillance systems can be checked for cases with appropriate symptoms. This actually occurred in Washington, DC in 2005, and the lack of cases in area emergency rooms reassured local officials that the alarm was false. Syndromic surveillance systems can also be queried to determine background rates when it is not clear whether a reported cluster of cases is unusual.
The E. coli O157:H57 outbreak in the New York City area in late 2006 provides an example of how syndromic surveillance could have been used for case finding. The outbreak came to light on November 17 when the first case was reported to a local health department in New Jersey. By November 27, 11 cases were reported in that jurisdiction. Three days later the Taco Bell restaurant, where people in 9 of the 11 cases had eaten closed voluntarily. On December 1, a similar case (originally attributed to another cause) was reported to a local health department in New York state, and it turned out that this person and three others in that jurisdiction had eaten at a different Taco Bell restaurant. By December 4, all Taco Bells in the New York metropolitan area were closed, and two days later a particular food item, green onions, was identified as the likely source of contamination. By December 9, more than 61 E. coli O157:H57 cases in at least four states were reported (CDC, 2006d).
Although a number of syndromic surveillance systems were operating at this time in New York City and the surrounding jurisdictions, there were too few cases in any location to detect. However, once the outbreak was identified in New Jersey, an advanced syndromic surveillance system could have searched emergency department admissions for cases of bloody diarrhea and abdominal cramps in the entire metropolitan area. Cases so identified could have been interviewed to take a food history, and lab samples obtained to test for E. coli O157:H57. In addition, health departments could have initiated active surveillance by physicians in the area, searched data from surrounding states to identify additional cases for follow-up and to confirm lack of cases elsewhere. If these steps had been taken, it is possible the restaurant chain and green onions could have been identified and remedial steps taken earlier—either closing the restaurant or removing the green onions. It is also likely that the additional data from syndromic surveillance systems could have resolved the uncertainty about what was happening and thus diminished public concerns.
Using syndromic surveillance—essentially, prediagnostic health information in existing electronic databases—as these examples suggest requires flexible and easily accessible IT systems, as well as a relationship between data providers and health departments that enables the systems to be used when needed. A benefit of developing these relationships may be improved communications between health-care providers and public health, which is essential to responding to any health emergency.
Conclusions
Any careful review of the development of syndromic surveillance in the past five years would have to conclude that much impressive work has been done with respect to information technology, including the real-time integration of many disparate data streams, and analysis—the development of statistical models, detection algorithms, and methods to visualize syndromic data. From a public health practice point of view, however, the value of syndromic surveillance for detecting bioterrorist attacks has not yet been demonstrated. There are two major reasons for this conclusion. First, in statistical terms, there is a relatively narrow window between what can be detected in the first few days and what is obvious. Second, better integration with public health systems is needed before information generated is useful in guiding a public health response. The analysis in this paper, however, suggests that the most important contribution of syndromic surveillance to public health practice may be for natural disease outbreaks, such as seasonal and pandemic flu, and as a tool to monitor outbreaks and guide the public health response. Realizing this potential will require designing systems that focus on these uses rather than being optimized for timely detection of large-scale bioterrorist attacks. Instead of automating the process of detecting outbreaks with statistical detection algorithms, it might be more useful to build flexible analytical tools into syndromic surveillance systems so they can monitor ongoing bioevents and facilitate epidemiological analysis.
IMPLICATIONS OF “REAL TIME” VERSUS “BATCH REPORTING” FOR SURVEILLANCE
Joseph Lombardo, M.S.3
The Johns Hopkins University
Introduction
In the context of disease surveillance, there has been confusion promulgated by vendors of systems on the requirement for “real-time” data feeds. The Institute of Medicine requested the author to present material addressing the subject, “Real Time” Versus “Batched” Reporting for Surveillance. The following discussion is based on the author’s career of 37-plus years in developing, evaluating, operating, and improving surveillance systems in different domains. Ten of these years have been spent on developing and improving the Electronic Surveillance System for the Early Notification of Community-Based Epidemics (ESSENCE), a disease
surveillance system being used globally and locally by public health organizations (Lombardo and Buckeridge, 2007).
Definition
The terms “real time” and “batched” for disease surveillance can be used to mean different things by different authors. Any discussion must begin with some formal definition of these terms. The Institute of Electrical and Electronics Engineers’ (IEEE’s) Computer Society Technical Committee defines real-time systems as those “in which its temporal properties are essential for reliability and correctness; the example applications include embedded systems, control systems, monitoring systems, and multimedia systems” (IEEE-TCRTS, 2007).
Real-time computing systems are required for time-critical applications where the result of a computing process is time critical. Examples with which most everyone is familiar are video games where a split-second delay could change the result of an outcome, or the use of antilock brake systems in cars to provide immediate feedback and response to avoid a collision.
The term “batch” is used in computing much as it is in baking: a set of programs or jobs processed on a computer at one time, like baking a batch of cookies in the oven. The Encarta (Microsoft Encarta, 2007) definition includes:
-
Process items as batch: To process or assemble items as a batch or in batches.
-
Computer programs processed together: A set of programs or jobs processed on a computer at one time.
Batched reporting of surveillance data, however, can mean a variety of things. The following are just a few:
-
Batched collection of health indicator data;
-
Batched processing of indicator data;
-
Reporting to health surveillance monitors that one or more rules have been triggered at a periodic time interval; and
-
Sending reports for reportable diseases in a group at some specific reporting interval.
The term “reporting” is used when the provider of the data (e.g., hospital, pharmacy, laboratory) sends data to the site where surveillance is being conducted. This is usually the first step in the surveillance process. The term “batched processing” is the processing of several files by applying mathematical algorithms to derive information from the data. These algorithms can be used to convert unstructured text data into structured data, for the identification of abnormal trends in the data, or for transforming data and information to be viewed in
a manner that would permit easy interpretation by a variety of users. Batched reporting is also used to refer to the actions needed to present data and algorithm outputs to the users of surveillance systems. Collection and processing of data do not occur at the same time as when data and results are being made available to the user. Batched health data may be reported to users as soon as it is processed, or it may be delivered at regular intervals, or accessed on demand.
The term “batched reporting” also has been used in the context of providing notification of reportable disease to a higher public health authority. Reports of animal diseases occurred monthly in some jurisdictions for those diseases that are reportable, but do not pose an immediate threat.
Surveillance Context
Data Acquisition and Archiving
Figure 1-10 presents an example of a generic disease surveillance system. Data acquisition occurs on the left of the figure. User interfaces are on the right, and archiving and analytic processes are in the center. Possible sources of early indicators of population health include 911 calls, emergency medical services, emergency department chief complaints, over-the-counter self-medications, etc. Some of the indicator data can be made available in real time while others can not.
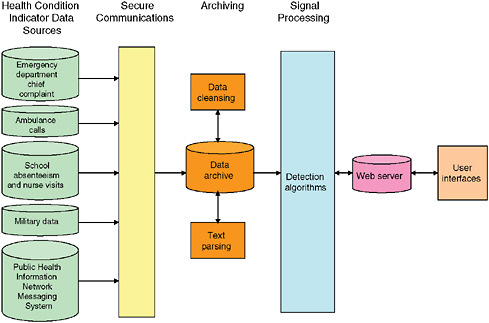
FIGURE 1-10 Electronic health monitoring components.
SOURCE: Lombardo (2006).
Only data that is captured in real time can be made available for surveillance in real time. When a cashier in a large retail chain scans an item the transaction can be captured and transmitted to the company’s distribution center. Several large retailers of over-the-counter medications capture their sales in real time so they can keep track of inventory in each store. Schools track absenteeism on a daily basis and not throughout the school day. School nurses could potentially track every student visit as it occurs. Many hospitals now have automated information systems based on the Health Level Seven (HL-7) format. These systems provide a comprehensive framework for the exchange, integration, sharing, and retrieval of electronic health information. Such information includes the instruction of orders; clinical observations and data, including test results; admission, transfer and discharge records; and billing information. HL-7 has become a standard for the interfacing of clinical data for many large hospitals (Health Data Standards: The Players, 2007). Monitoring a hospital’s HL-7 message traffic creates a record of activities within the hospital as information is entered and archived. Monitoring an HL-7 data stream provides hospital record data as close to the time they are created as possible.
To preserve the timeliness of HL-7 records, many developers and surveillance system users believe the records need to be transmitted to the automated surveillance system as quickly as they are created. One method for preserving this timeliness is to provide continuous transmission of HL-7 records between the hospital and the surveillance system. The use of a virtual private network (VPN) permits HL-7 records to be transmitted as soon as they appear on the hospital’s network.
Another popular mechanism for data transmission uses the File Transfer Protocol (FTP). Records are accumulated and “batched” over some time interval, then sent at a specific time to the FTP site, where they are picked up by the surveillance system for archiving and processing. The Center for Disease Control and Prevention’s (CDC’s) BioSense program aggregates HL-7 hospital records every 15 minutes, and transfers them to CDC using the Public Health Information Network (PHIN) Messaging System.
Most state and local health departments have varying requirements for the timeliness in which data are provided for surveillance. Many health departments believe that receiving data once a day may be sufficient, while others believe that real time is mandatory. The Department of Health and Human Services for Montgomery County, Maryland, has implemented its data collection surveillance component so it can acquire data at higher rates during times when the department is concerned about a possible health risk.
Data Processing
Once the data are acquired and archived by the surveillance system several processing steps could occur. Initial processing is needed to reduce entry and
transmission errors. The term used in Figure 1-10 to describe these processes is “data cleansing.” Separate processing algorithms are needed to convert text data, such as chief complaint, clinical notes, and radiology reports, into a structured data for use in signal analysis. These processes are referred to as “text parsing” in Figure 1-10.
Automated surveillance systems employ a variety of algorithms4 to process data for early detection of a health event. “Signal processing” is a term frequently used for these processes. If the datasets are large or diverse, or come from many different sources, the signal processing steps can require several minutes to hours of computing time. Certain algorithms, such as those for spatial analysis (e.g., attempting to form spatial clusters across hundreds of ZIP codes) are particularly time consuming; as a result, such cases tend to be processed as batches because they simply cannot be performed in anything resembling real time. Processing is initiated and results are provided after well-defined periods, such as every four hours.
Some surveillance systems are interactive and allow the user to invoke specific processes to get an immediate result. These systems permit the user to analyze and view data as they are being received. ESSENCE provides both options. Data are processed at regular intervals and results available for display, but they are also available for user-defined analysis as soon as they are received, archived, and preliminary processes are completed.
User Interfaces
Many advanced disease surveillance systems take advantage of modern Internet technology. Typically, a user/analyst views a website once a day, but in the event of an emergent health threat, more frequent or ongoing analysis is possible if data are available.
Most modern disease surveillance systems provide outputs to users as soon as the signal processing phase is complete. Users log on to the surveillance system and view the alerts or data. The alerts may be in the form of “flags” indicating that a predetermined “threshold” has been exceeded or an anomalous condition detected; temporal and spatial data displays; or lists of cases that contributed to the alert.
“Real Time” Versus “Batched”
Most modern disease surveillance systems have multiple processes that must be completed before the data are provided to users. Collecting data in real time while processing it in batch due to the constraints in computing time does not make
for a real time system. Going through the extra expense of maintaining a VPN to collect HL-7 hospitals as they are being created makes little sense unless these data can be processed and made available to the analyst also in real time However, the question remains whether real time is even needed by public health.
It is hard to conceive of any public health need for the more timely collection of data than that provided by CDC’s BioSense program. This program has implemented the collection of “batched” HL-7 hospital records every 15 minutes. The total throughput or time delay of the current BioSense processing steps is not known to the author, but it can safely be estimated to be greater than 15 minutes. The BioSense data feed is batched, but more timely than systems claiming to be real time.
Given constraints on time and resources, one could envision two modes of operation for electronic surveillance systems: one for the routine monitoring of public health, and the other to examine a specific threat based on case definition. For routine monitoring purposes, it will be of paramount importance to keep alert rates to a manageable level. The focused monitoring of perceived threats should be a rare occurrence, but essential information should be obtainable in sufficient time to mount an effective response to an emerging crisis.
Summary
The term “real time” as defined by the IEEE’s Computer Society Technical Committee is not appropriate for use in describing modern automated disease surveillance systems. The benefits of real-time data collection are only realized if all other components of a surveillance system satisfy the real-time criteria. Receiving and processing health indicator data several times an hour should be more than adequate for public health needs, even during public health emergencies. The use of the term “real time” is often confused by vendors who misuse the term in an effort to distinguish their product as being better than someone else’s. Consumers should attempt to understand the actual system characteristics rather than relaying the misuse of terms by vendors of surveillance systems.
ONE WORLD—ONE HEALTH: WILDLIFE AND EMERGING DISEASE SURVEILLANCE
William B. Karesh, D.V.M.5
Wildlife Conservation Society
Outbreaks of avian influenza, severe acute respiratory syndrome (SARS), Ebola hemorrhagic fever, bovine spongiform encephalopathy (mad cow disease),
and other emerging diseases are surprising the public, disrupting globalization, resulting in massive economic losses, and jeopardizing business and diplomatic relations. These diseases, which are able to cross the Darwinian divide between animals and people, do not depend on humans for their survival and easily live far from the reaches of most medical interventions. Their competitive advantage in this regard demands that we revisit basic strategies for disease control, including the assumptions from the 1950s declaring the chapter on the threat of infectious diseases closed. Not only was this narrow, urban human health point of view premature, but it diverted resources away from preparedness for dealing with the modern-day world of rapid travel and transportation of both goods and people, higher human population densities, and a growing dependence on intensified livestock production.
Although many in the developed world would hardly recognize meat not wrapped in clear plastic, the vast majority of humans still live in a world like our great-grandparents’, buying their food fresh, salted, or smoked in open-air markets, or gathering it themselves. For much of the world, there are no systems of inspections for these markets, and few people have access to good health care, education on hygiene, common vaccinations, or antibiotics. The global transport of animals and animal products, which includes hundreds of species of wildlife (Karesh et al., 2005), also provides safe passage for their bacteria, viruses, fungi, and even the prion proteins that cause insidious illnesses such as mad cow disease and chronic wasting disease of deer and elk. Surveillance of infectious diseases is most useful when it occurs as close to the source as possible, rather than waiting to measure morbidity and mortality in distant lands. This requires a new approach, one that engages people around the world to work together in earnest and share findings in a timely manner.
Currently, no government agency is responsible for, or capable of, the surveillance and prevention of the myriad diseases residing around the world. None are given the responsibility for robustly pursuing the simplest of concepts—the health of people, animals, plants, and the environment in which we all live are inextricably linked. The great gains from specialization in the fields of human health, public health, livestock health, and wildlife health have unfortunately resulted in academic hubris and reduced communication across disciplines by the end of the 20th century. Avian influenza serves as the most recent reminder that, in fact, there is only “one health.” Over the last decade, the Wildlife Conservation Society (WCS) has been working to engage stakeholders in this concept with projects and a series of symposia utilizing the One World—One Health theme in Durban in 2003 (Osofsky et al., 2005a, b), New York in 2004, Bangkok and Beijing in 2005 (Karesh and Cook, 2005), and Brasilia in 2007. The products of these meetings as well as guidelines for future efforts such as the “Manhattan Principles” are openly available.6 This one health concept is gaining wide
acceptance and most recently has been endorsed by both the American Medical Association and the American Veterinary Medical Association. However, putting words into action presents the biggest challenge, and the world’s agencies and academies devoted to human and animal health were built to support separate sectors rather than to facilitate collaboration.
The U.S. Department of Agriculture (USDA) is mandated and funded to protect the U.S. livestock industry. Radar screens are set to blink when livestock are threatened. Even the more recent concerns of agroterrorism have not done enough to support the global outreach necessary to understanding and reducing diseases overseas before they reach U.S. shores. The wildlife services branch of USDA traditionally was focused on wildlife control and eradication in order to protect livestock. It is rapidly trying to remake itself in a modern world that is recognizing the cultural, ecosystem, and economic value of wildlife itself. But developing an effective program, building a reputation and trust among the wildlife community, and developing expertise in wildlife surveillance will take a long-term commitment that may or may not be on the horizon (or appropriate, in all fairness) for a federal agency focused on agricultural production and markets.
The United Nations’ Food and Agriculture Organization’s (FAO’s) priorities are the production of livestock and crops, with a focus on the urgent needs of developing countries. Traditionally, few resources were devoted to exploring the linkages of the health of wild plants and animals with their domesticated cousins. This has changed since 2005, and a small program was begun in collaboration with the WCS to coordinate responses and investigations of highly pathogenic avian influenza virus in wild birds.
The World Organization for Animal Health (OIE)7 has a volunteer committee composed of six people who meet for three days per year to address all of the world’s wildlife-related disease issues. In the past two years, they have formed a parallel committee to address zoonotic and emerging diseases but the two committees are not linked to one another. The World Health Organization (WHO) is directed at human health, but until the change in the International Health Regulations (IHRs) that took effect this year, they could only respond on official invitation from a country that may or may not know about, or want to reveal, the presence of a disease. The changes in scope will allow for gathering of information without going through official channels. This could help significantly in global response time, but the IHRs are still institutionally entrenched in a world of human disease. The U.S. Centers for Disease Control and Prevention (CDC) has the responsibility to prevent human diseases in the United States, and extend their reach around the world, but also only when invited.
No government agency or multilateral organization is charged with uniting knowledge and efforts that span the diversity of disease threats to people, domestic animals, and wildlife. No one is ensuring that health solutions are based on the
input of expertise from human, domestic animal, and wildlife health professionals and equally important, communicated across disciplines in terms that effectively motivate all stakeholders and demonstrate common goals.
Clearly, there is an urgent need for a new health paradigm that not only integrates the efforts of disparate groups, but possibly more important, balances their respective influences to prevent both the gaps and the biases that we are now coming to recognize. The failure to recognize and aggressively address the broad range of diseases that have no respect for hundreds of years of earnest scientific classification, places animals and people in great danger. The immediate effects of the diseases themselves are often the least of the worries. Infectious diseases of people and animals are drivers of poverty and associated civil unrest, disrupt “free” ecosystem services such as drinking water and plant pollination, and can ruin otherwise well-planned and sustainable economic development efforts.
Analyses indicate that more than 60 percent of the over 1,400 infectious diseases currently known to modern medicine are shared between humans and animals (Taylor et al., 2001). From an anthropocentric point of view, most of these infectious agents are labeled zoonotic, or diseases of animals that infect people. Anthrax, Rift Valley fever, plague, Lyme disease, and monkeypox are just a few examples. Receiving less attention is the other group that moves across species boundaries, the anthropozoonotic diseases. These infectious diseases are typically found in humans but can, and do, infect animals. Human herpes virus, human tuberculosis, and human measles are all transmissible to a variety of animal species, with devastating consequences. This traditional division of infectious agents into two groups is convenient for teaching purposes, but lacks the broader and critically important concept that these diseases can move back and forth, and change characteristics in the process. Avian influenza is but one disease that is teaching the medical world about the need for a more holistic point of view.
The consensus of scientific opinion on the origin of HIV/AIDS links it to human consumption of nonhuman primates along with their simian immunodeficiency viruses, estimated to have taken place in Africa late in the first half of the 20th century (Feng et al., 1999). Recent Ebola hemorrhagic fever outbreaks in humans in Africa have a similar history. The disease was first recognized by the western world when it appeared in the Democratic Republic of Congo in 1976, around the Ebola River. The virus infects people, gorillas, chimpanzees, and monkeys (Leroy et al., 2004). It causes severe internal and external hemorrhaging, and can be extremely deadly, killing up to 90 percent of its human victims. Infection spreads quickly, especially via caregivers and by those who flee to escape the illness. Outbreaks have been recorded in Sudan, Gabon, Republic of Congo, Democratic Republic of Congo, Côte d’Ivoire, and Uganda. But it is clear that both people and nonhuman primates suffer equally from the disease. Outbreaks have caused declines in lowland gorillas and chimpanzees in Gabon and the Republic of Congo, and chimpanzees in western equatorial Africa. Other forest animals, such as duikers—small antelopes—and bush pigs may also be
affected. When subsistence hunters discover a sick or dead animal in the forest, they view it as good fortune and bring it home to feed their families and trade with neighbors. The Ebola virus then easily infects those handling the meat, and a chain of contacts and infections ensues. Each human outbreak in central Africa during the late 1990s and the first years of this century was traced to humans handling infected great apes.
The SARS coronavirus has been associated with the trade in small wild carnivores. This disease first appeared to the world in China’s Guangdong Province in late 2002. People began complaining of high fever, cough, and diarrhea, and eventually developed severe pneumonia. It was an unknown disease, and it was very contagious. Within a matter of weeks, it spread via a hotel visitor in Hong Kong to five continents. By July 2003, WHO had tallied 8,437 cases, with 813 deaths. Mostly because of a lack of understanding of this “new” disease, global travel and trade were disrupted as fear spread. A coronavirus (a family of viruses found in many animal species) was finally discovered to be the culprit, and it was also detected in masked palm civets that were farmed in the region and sold for human consumption. Later, evidence of the virus was also found in raccoon dogs, ferrets, and badgers in the wildlife markets, as well as domestic cats living in the city and a closely related coronavirus in bats commonly sold in the same markets. Epidemiological studies have concluded that the first human infections did indeed come through animal contact, though the exact species has not been definitively identified (Tu et al., 2004). In the weeks after SARS, the Chinese government responded by closing down live wildlife markets. Within 10 days, nearly a million animals were confiscated, many brought in from other parts of the world with their exotic viruses and bacteria, demonstrating that law enforcement can in fact be used to reduce or control the trade in wildlife and wildlife products. The animals were mixed and matched, exposed to each other’s wastes and even fed to each other. If a virus or bacteria was hoping to win the big lottery of jumping among species, going to the markets of Guangdong would be like buying a million lottery tickets. The profits from the wildlife trade in China pale in comparison with the estimated U.S. $50 billion global economic costs resulting from the brief SARS event of 2003 (Newcomb, 2003).
The inadvertent movement of infectious agents due to wildlife handling and trade, as well as domestic animal movement, is not limited to human pathogens, but also extends to those that can devastate native wildlife, which serve as biological linchpins for environmental integrity and provide a range of cultural and quantifiable economic values (Karesh et al., 2005). In 2005, H5N1 Type A influenza virus was isolated from two mountain hawk eagles illegally imported from Thailand in airline cabin carry-on baggage to Belgium (OIE, 2004). Tuberculosis originating from domestic cattle has now infected wild herds of bison in Canada, deer in Michigan and Wisconsin, and Cape buffalo and lions in South Africa. Surveillance of these wild populations is now needed not only to assess risk for humans and livestock, but for the wild animals themselves. In one swift
outbreak of rinderpest, a disease originally introduced to Africa by the importation of domestic cattle, more wild buffalo died in Kenya in 1999 than were killed by illegal poaching during the previous two decades.
Exact quantification of the global wildlife trade is impossible because it ranges in scale from extremely local to major international routes, and much is illegal, or through informal channels. WCS figures compiled from a variety of sources for just the live wildlife trade indicate that each year, roughly 40,000 live primates, 4 million live birds, and 640,000 live reptiles are traded globally (Karesh et al., 2005). Daily, wild mammals, birds, and reptiles flow through trading centers where they are in contact with humans and dozens of other species before being shipped to other markets, sold locally, and even freed back into the wild with new potential pathogens as part of religious customs such as merit release or because they become unwanted pets. Conservative estimates indicate that in east and southeast Asia, tens of millions of wild animals are shipped regionally and from around the world annually for food or use in traditional medicine. The estimate for trade and local and regional consumption of wild animal meat in Central Africa alone is more than 1 billion kg per year (Wilkie and Carpenter, 1999). In Central Africa, estimates of the number of animals consumed by humans annually vary, but a figure of 579 million has been proposed. Estimates for consumption in the Amazon Basin range from 67 to 164 million kg annually, comprising, for mammals alone, between 6.4 million and 15.8 million individuals (Peres, 2000).
Hunters, middle marketers, and consumers make some type of contact with each animal traded. Additionally, domestic animals and wild scavengers in villages and market areas consume the remnants and wastes from the traded and to-be-traded wildlife. These numbers combined suggest that at least some multiple of 1 billion direct and indirect contacts among wildlife, humans, and domestic animals result from the handling of wildlife and the wildlife trade annually.
In addition to the direct health effects of the pathogens on people and animals, animal-related disease outbreaks have caused hundreds of billions of dollars of economic damage globally, destabilizing trade, and resulting in devastating effects on human livelihoods. According to studies performed by Bio-Economic Research Associates, the rash of emerging or reemerging livestock disease outbreaks around the world since the mid-1990s—including mad cow disease, foot-and-mouth disease, avian influenza, swine fever, and other diseases—has been estimated to have cost the world’s economies more than $100 billion. The costs are rarely borne by the same individuals that profit from the movement of animals and their pathogens. As mentioned earlier, the cost of SARS alone to the global economy has been put at more than $50 billion (Newcomb, 2003). Wildlife market traders did not bear the costs of the SARS outbreak. The rodent importer in Texas did not reimburse government agencies for the millions of dollars spent on the response to monkeypox in the United States. Hundreds of
millions of public dollars will be spent in attempting to remove tuberculosis and brucellosis from wildlife populations infected by domestic animals.
In early 2003, FAO reported that more than one-third of all global meat trade was embargoed as a result of mad cow disease, avian influenza, and other livestock disease outbreaks. The projected growth of industrial livestock production in developing countries to meet rising global protein demand will increase both the economic and the food security impacts of future disease outbreaks, and the global economic impacts do not adequately reflect the local, direct effects.
Preventing and controlling infectious diseases in the modern world requires a far broader range of expertise than needed for previously isolated systems in highly developed countries. The challenges seen in controlling avian influenza in Asia and Africa are just one example of the multispecies disease dilemma. Most of these diseases threaten local people directly, as well as their livestock and their livelihoods. They decimate wildlife and undermine ecosystem stability and services, and with modern travel and transport, they can quickly pose a threat to any nation. Fear, understandably founded on a lack of information, can drive global responses and economic reactions far beyond the actual cost of disease control.
Currently, it appears that a few people in some of the most remote places on earth, many from nongovernmental organizations (NGOs) and many working at local government levels but unlinked to larger formal networks, are working to fill the intersectorial gaps in health care as they relate to emerging diseases and wildlife. WCS’s global health programs are an example of a private-sector effort linked to governmental and multilateral agencies that bring together stakeholders, from civil society and a variety of government sectors, to develop surveillance and information-sharing networks. The work is directed where rare infectious diseases are least understood and local institutions have the fewest capabilities to effect prevention and control. Our staff and partners routinely encounter diseases such as anthrax, avian influenza, monkeypox, and Ebola where they naturally occur. We build local capacity to conduct surveillance and reporting networks at very low costs. When attention was being misdirected at wild birds in efforts to control the current avian influenza outbreaks in Southeast and East Asia, these new, but informally recognized participants in health discussions, were the first to point out that migratory routes and seasonal timings did not correspond with the regional spread of the disease as posited by articles in prestigious scientific journals—it was the largely uncontrolled movements of domestic birds that were spreading this disease, not wildlife. Control efforts would be needlessly misdirected without this simple input to decision makers.
Building bridges across disciplines to solve health problems can have simple but significant synergistic effects. Studies in South America have shown that, contrary to common opinion, livestock diseases pose more threats to wildlife than the other way around. In much of the world, reducing disease in domestic animals would benefit several industries, improve human health and livelihoods, and help protect wild animals from livestock and other domestic animal diseases.
In Central Africa, gorillas and chimpanzees have little to no immunity to common human diseases. Local people and tourists threaten wild populations with these illnesses, which could be simply avoided by implementation of good preventive health programs and practices in villages. People and wildlife both benefit. WCS’s work with Ebola hemorrhagic fever in gorillas and chimpanzees has shown that when investments are made for working not just in the cities but in the forests, natural resource managers can help to detect the presence of the disease in wildlife months before the first human cases—providing the lead time to warn villagers not to hunt or handle the animals that are the source of infection.
Over the past two years, the WCS network of local villagers and hunters, park managers and staff, government public health officials, and regional laboratories has detected outbreaks of Ebola in great apes and notified local communities. For the first time, known human outbreaks resulting from the disease in animals have not occurred. This broader, one-health approach is much more effective and inexpensive than the traditional “quarantine and stamping out” efforts after an outbreak has begun. A set of guiding concepts on these themes, called the Manhattan Principles, was developed by human and animal health specialists in conjunction with wildlife conservation professionals.8
Another large-scale example of a worldwide private–public collaborative effort is the Global Avian Influenza Network for Surveillance (GAINS) of wild birds. The U.S. Agency for International Development (USAID), CDC, and USDA are providing support to WCS to develop and administer the wild bird GAINS program. GAINS is a smart and targeted investment in the U.S. government’s fight against avian influenza, because wild birds around the world can serve as sentinels for the early detection of the virus’s presence.
Awareness of and interest in the GAINS program continues to grow. Working relationships with local institutions are being built in more than 28 countries, with many more anticipated. This network of partners builds a “window on the world” and has helped GAINS bring timely and pertinent information that will help combat the threats posed by highly pathogenic avian influenza to both humans and animals.
The GAINS program has made significant progress in its global implementation since receiving start-up funding in summer 2006. Collaborations have been established between WCS and U.S.-based and international organizations—including governments, NGOs, and universities—to work together to improve our understanding of the dynamics of avian influenza, and to evaluate disease risks for people, biodiversity, and domestic poultry. WCS staff have been in active discussions with colleagues from USAID, CDC, Department of Homeland Security (DHS), Department of Defense (DoD), U.S. Geological Survey (USGS), USDA, National Institutes of Health (NIH), Department of State (DoS), WHO, FAO, as well as university and private-sector experts to address integrated approaches to
global disease information management issues. Together with FAO, WCS has conducted training efforts in Eastern Europe, Latin America, and the Caribbean, and a recent agreement with USDA will expand training and bird monitoring in Latin America. WCS is providing technical expertise related to health monitoring of wild birds and capacity-building activities around the world.9
GAINS fieldwork also enables the isolation of new viral strains, which can contribute to vaccine development and help guide preparedness in the United States and abroad. One of the primary purposes of GAINS is to share international disease information through an interactive, publicly accessible web-based database, a working prototype of which has already been made available on the GAINS website. The database is starting to map sample collection sites, fly-ways, and results of biological surveillance. The goal is to alert decision makers about disease occurrence rather than waiting for traditional scientific journal publication.
From Afghanistan to Zimbabwe, field surveillance for avian influenza is currently underway. Our work in Mongolia illustrates the field methodology being used in many sites. Mongolia has been a hot spot for avian influenza outbreaks in the past two years and is a country where wild birds appear to be of particular importance to the ecology of the disease. Last year WCS staff collected more than 3,500 samples at 42 sites across the country. WCS staff collected an H5N1 strain of avian influenza virus from wild birds that have been selected by WHO to be used in human pandemic vaccine development and testing. Working with WCS staff in Mongolia, USGS scientists fitted whooper swans from the region with satellite transmitters (supplied by FAO) in early August, and some have been tracked to China, Korea, and Russia. These types of data may shed light on possible viral transmission routes across Asia.
The early successes with the Wild Bird GAINS program has led to expansion of the program to a broader range of infectious diseases and species. Named the Wildlife Global Animal Information Network for Surveillance (Wildlife GAINS), the effort is designed to establish a comprehensive, worldwide wildlife health surveillance system to enhance preparedness for and awareness of emerging infectious diseases. This nongovernmentally managed network would connect a wide variety of U.S. government agency partners, multilateral agency partners, conservation organizations, veterinary and medical schools, and other national and international partners. The unique strengths and capacities that NGOs such as the Wildlife Conservation Society have to work with developing country governments and scientific colleagues must be harnessed to develop and enhance surveillance mechanisms that are of great importance to human security and well-being.
Workers in the fields of health and global governance need to find ways to focus skills and expand resources to make the entire world safer from infectious
|
9 |
See http://www.gains.org. |
disease. The financial costs of disease outbreaks are currently borne by the global economy and will only serve to slow economic development where it is needed the most. There is an obvious need to break down barriers among health disciplines to prevent any one of them from restricting funding to their area of interest, and there is an urgent need to build bridges among the government agencies and the privately operating individuals and organizations around the world that now take responsibility with only scarce resources. Immediately, before the next global pandemic, trade in wildlife needs to be dramatically reduced and, like the livestock industry, properly regulated. Finally, global health will not be achieved without a philosophical shift from the “expert dictates” paradigm inherent to both science and medicine, to a broader, multistakeholder approach, based on the understanding that there is only “one world and one health.”
AGRICULTURAL BIOSECURITY: THREATS AND IMPACTS FOR PLANT PATHOGENS
Jacqueline Fletcher, Ph.D.10
Oklahoma State University
James P. Stack, Ph.D.11
Kansas State University
Plant Vulnerability to Disease
Plant resources in the United States, including crops, rangelands, and forests, are vulnerable to endemic, introduced, and emerging pathogens (American Phytopathological Society Public Policy Board, 2002; Casagrande, 2000; Madden and Wheelis, 2003; Wheelis et al., 2002; Whitby, 2002). An estimated 65 percent of U.S. crop losses, valued at $137 billion, are attributed to introduced pathogens annually (Pimentel et al., 2000). Increasing globalization and international trade activities create a strong likelihood that many other exotic plant pathogens will arrive in the United States in the coming years.
The vulnerabilities of U.S. agricultural production to emerging diseases result from a number of factors. Huge acreages are planted with grains and forage crops, or are covered with grasslands or forests. Because it is impossible to regularly or frequently monitor such extensive areas for disease symptoms, long periods are likely to pass between the time a pathogen is introduced and when it is detected. A second source of vulnerability is the lack of genetic diversity in our plant resources; most of our nation’s production is centered on just three
crops: wheat, corn, and soybeans. Within these and other crop species, certain genotypes conferring attributes important for yield and quality are preferentially grown over large areas, increasing the chance that a pathogen detrimental to that cultivar will have serious impact.
More than 50,000 plant diseases occur in the United States (Madden, 2001; Madden and Wheelis, 2003), caused by a variety of pathogens, including fungi, viruses, viroids, bacteria, nematodes, and parasitic plants. These organisms are disseminated by various means, including wind, water, agricultural equipment, seeds or propagative plant parts, insect vectors, animals, or farm workers. For any given region and crop, producers may deal with up to 10 to 15 serious plant diseases that can cause severe economic repercussions (Pinstrup-Andersen, 2001). About 65 percent of U.S. crop losses are due to nonindigenous (introduced) pathogens, amounting to an estimated cost of $137 billion annually (Pimentel et al., 2000). All crop pests (pathogens, arthropods, and weeds) combined caused preharvest losses of 42 percent and an additional 10 percent loss after harvest. Of these, 13 percent were due to plant pathogens, 15 percent to arthropods, and 13 percent to weeds. Worldwide, losses for the eight major crops that comprise half of the global croplands were estimated at $300 billion in 1988–1990 (Oerke et al., 1994).
A number of pathogens that occur elsewhere in the world are of significant concern to U.S. plant production, should they arrive. Most past introductions of plant pathogens to the United States have been unintentional. Many pathogens not yet in the United States would pose significant threats to our current crops. Because eradication of plant pathogens is rarely physically or financially feasible, the only effective approach is to manage the disease so that its impact falls below an economic threshold—the point at which management costs exceed the profits associated with production.
The use of microbes, such as the anthrax bacterium, against human targets is a highly visible act with immediate consequences. Directing pathogens toward agricultural targets may be less visible, and effects may not be apparent for some time. However, such actions, which effectively target the nation’s food supply—from its production in the field to its place on the plate—may have serious and long-range impacts (Adam, 2006). Many plant pathogens can be acquired readily by those wanting to use them intentionally for purposes of harm. Furthermore, they may be attractive agents for nefarious applications because they can be handled, grown, transported, and disseminated with little technical expertise or equipment, and pose little or no danger to the health of the handler. The Institute of Medicine (IOM)/National Research Council (NRC) Committee on Advances in Technology and the Prevention of Their Application to Next Generation Biowarfare Threats recently concluded (2006) that the increasing accessibility and simplicity of technological information related to pathogens increase the likelihood that rogue states or individuals may use such knowledge in a criminal manner.
History of Biological Warfare
Biological warfare has been targeted to agricultural systems in the past. Around the world, state-sponsored programs supported research to enhance the suitability of microorganisms for use as weapons (Casagrande, 2000; CIDRAP, 2003; Madden and Wheelis, 2003; Wheelis et al., 2002; Whitby, 2002). Before the Biological and Toxin Weapons Convention outlawed state programs on biological weapons in 1972 (IOM/NRC, 2006), U.S. research programs had focused on the pathogens causing anthrax, foot-and-mouth disease, and rice blast. Germany had bioweapons programs during World Wars I and II, the former Soviet Union during World War II and the Cold War, and Iraq at the time of the Iran–Iraq War. Islamic militants in Afghanistan were involved in weaponization of the fungus Puccinia graminis, causal agent of wheat rust. Canada, France, Japan, and the United Kingdom also considered the use of bioweapons against agricultural targets.
Despite knowledge of such research activity in multiple countries, no reports of the deliberate use of pathogens against crops or other plants have been published. Yet, indicators of increasing likelihood of such use point to the need for preparedness. The United States must develop the capabilities and knowledge to ensure the safety and security of our food at all levels, and at all points of production—the distribution pathway from farmers’ fields to the consumer. Rapid action is critical if we are to have these capabilities in place before they are needed for a devastating incident.
Impacts of Plant Diseases
Past incidences of the impacts of crop diseases on human health and society may be helpful in illustrating the potential damage of plant pathogens. The Irish potato famine (1845–1846), caused by the plant pathogen Phytophthora infestans, led to extensive famine and resulted in the deaths of a million people and the emigration of another 1.5 million Irish, many to the United States (Large, 1940; Carefoot and Sprott, 1969; Schumann, 1991). During the same timeframe, the severe impact of a rust fungus on coffee production in Ceylon (now Sri Lanka), the prime supplier of coffee to Great Britain, forced much of British society to turn to tea as their primary hot beverage (Large, 1940; Schumann, 1991). Weather conditions in both the United States and Europe during World War I were favorable for the development of plant diseases in wheat and potatoes, crops essential for nourishing the troops on both continents. The critical food shortages that ensued were factors in the movement and strength of the troops and changed the course of the war. Brown spot of rice contributed to the Great Bengal Famine of 1943, and in the United States, a 1970 epidemic of corn leaf blight destroyed about 20 percent of the $1 billion crop (Rogers et al., 1999).
U.S. agricultural infrastructure is strong, diverse, and resilient. Temporary unavailability or elimination of a certain food product because of plant disease
is unlikely to result in significant nutritional hardship for Americans. The same cannot be said for all countries, however; a serious rice disease in Southeast Asia, for example, could lead to malnutrition and hunger, destabilizing social infrastructures in affected areas.
Deliberate introductions of plant pathogens to crops and other plant resources in the United States could have serious non-nutritional impacts (Budowle et al., 2005a, b; Murch et al., 2003; Fletcher et al., 2006). Likely impacts of crop diseases in the United States include losses in the quality and quantity of our food, increases in consumer food prices, costs of growing crops that are less desirable, and costs of management strategies, both short term (crop destruction, pesticide application, or redirecting use of the crop) and long term (development of resistant varieties) (Casagrande, 2000; Madden and Wheelis, 2003; Wheelis et al., 2002; Whitby, 2001, 2002). Several plant pathogens can also infect humans; these are primarily opportunistic pathogens of greatest concern to immunocompromised patients, the very young, or the old. Some plant pathogenic fungi produce mycotoxins that can pose important health risks for humans, and other species produce spores that are allergenic. There also can be important indirect impacts on human nutrition, as well as on the agricultural community, if plant products used for livestock feed are lost.
The most significant impacts of deliberate plant pathogen introductions, however, are likely to be economic in nature. Imposition of quarantines and embargoes on U.S. agricultural products not only affect producers, but have downstream effects on the commercial enterprises that harvest, store, package, transport, add value, and market the commodity. Perhaps more importantly, there could be a loss of trading partners and markets worldwide. Furthermore, knowledge of intentional targeting of the food supply by those intending harm would lead to a loss of public trust in our food and in the ability of government to ensure its safety. Ultimately, rural communities that rely on agricultural production may be destabilized and grower livelihoods threatened.
At the other end of the food system continuum, there has been an alarming rise in the incidence of foodborne illnesses due to microbial contamination of fruits and vegetables. A recent survey by the U.S. Food and Drug Administration (FDA) of samples from major distributors showed that 1.6 percent of domestic produce was contaminated with human pathogens (FDA, 2001). Recent incidents of contamination of leafy greens and peanut butter with the human pathogens E. coli O157:H7 and Salmonella spp. demonstrate the devastating impact that may result from the introduction of microbes into fresh food plants. Today’s mass food production operations and national distribution systems have caused a significant increase in the scope of such foodborne illnesses (Maslanka et al., 2002). Once primarily local events, with local response, food contamination incidents are now far more widespread. In the fall 2006 E. coli outbreaks, 205 victims in 26 states suffered severe disease and 3 died. As a result, consumers changed their buying
habits, producers of the affected crops suffered significant economic losses, and downstream enterprises were negatively impacted (FDA, 2007).
High-Consequence Plant Pathogens and Diseases
In 2004, the U.S. Department of Agriculture (USDA) Animal and Plant Health Inspection Service (APHIS), as designated in 7 C.F.R. Part 331 of the Agricultural Bioterrorism Protection Act of 2002, first established a list of plant pathogens of high consequence to be designated as Select Agents12 (Table 1-3). Although this list is similar in nature to the Select Agent lists for human and zoonotic diseases, there is one important difference. Plant pathogen Select Agents are, at the time of their placement on the list, exotic microbes not endemic or established in the United States. This contrasts with the policy of listing indigenous human and animal pathogens on their respective lists. The fact that, by definition, plant Select Agents are not indigenous within the United States necessitates the imposition of strict regulations, registrations, restrictions, and security13 on any research or possession of these microbes.
Originally consisting of 10 plant pathogens, the recent removal of 2 pathogens (Plum pox virus and Phakopsora pachyrhizi, the causal agent of soybean rust) after their arrival and establishment in the United States has left the list with 8 agents. A mandated biannual review and possible revision of the plant Select Agent list is underway at the time of this writing.
Citrus Canker: A Recent Example of Significant Disease Impact
Florida produces about 80 percent of the citrus grown in the United States, and most of the state’s fruit is processed for juice. The industry is worth about $1.4 billion per year. Although Xanthomonas axopogonis pv. citri, the bacterium that causes the devastating citrus canker disease, is not on the Select Agent list, it has occurred in Florida citrus-growing areas several times since the turn of the century, each time causing brown, necrotic, raised scars or cankers on leaves, stems, and fruit.
Canker is a quarantine disease; fruit from affected areas cannot be moved across state lines or sold in the world market. Because the disease was not considered established in the United States, an eradication strategy has been in place for many years. This approach was successful in Florida in the early 1900s and again in the 1980s. APHIS and the Florida Department of Agriculture and Consumer Sciences adopted that same strategy for the most recent outbreak, which was first detected in 1995. However, the latter outbreak presented new challenges.
TABLE 1-3 U.S. Select Agent List for Plants
|
Citrus greening (African) |
|
Citrus greening (Asian) |
|
Potato bacterial wilt |
|
Rice bacterial leaf streak |
|
Citrus variegated chlorosis bacteria |
|
Philippine downy mildew of corn |
|
Brown stripe downy mildew of corn |
|
Potato wart fungus |
|
|
|
|
|
Soybean rust |
|
Pox of stone fruits |
|
*Also spelled pachyrhizae. SOURCE: Fletcher (2006). |
|
First, the initial eradication guidelines called for elimination of the diseased tree, plus any citrus trees within a 125-foot radius of the symptomatic plant. Although this policy was not popular, it was relatively well accepted. However, in the late 1990s, these measures failed to prevent disease spread. Further research led to an eradication strategy modification requiring the elimination of all citrus trees within a 1,900-foot radius of any infected tree. Complicating matters was the fact that the 1995 outbreak was not confined to commercial groves. It also was widespread in residential areas of Miami where landowners objected, some filing lawsuits to stop the eradication campaign. While the legal issues were debated in court, the disease continued to spread. The eradication plan eventually was upheld by the courts and the program was reinstated (Gottwald et al., 2002); overall, $200 million was spent and more than 10 million trees were destroyed (Brown, 2001). However, the occurrence of several hurricanes in Florida in 2005 spread the pathogen far beyond its previous locations and eliminated hope of eradication. In 2006, USDA APHIS revised its approach to focus on managing the disease.
Arrival of Two Plant Pathogen Select Agents
Two plant pathogens on the Select Agent list have arrived in the United States in the past two years. Were we ready for them?
Soybean rust, caused by the fungus Phakopsora pachyrhizi, affects a major U.S. crop that is grown over 75 million acres and is worth more than $18 billion a year (USDA-ERS, 2007). In areas where the rust is endemic, such as Asia and South America, yield losses commonly range from 10 to 30 percent, but can be much higher. U.S. producers, who grow 74 million acres of soybeans/year (accounting for about $15.7 billion), feared that the arrival of the fungus would
severely impact the industry. U.S. epidemiologists had been monitoring the global movement of the pathogen for several years in an effort to provide warning for its inevitable arrival to U.S. territory. The disease was first detected in the United States in fall 2004 (Schneider et al., 2005). The first diagnosis, in Louisiana, was quickly followed by detection in several other states, but because the disease arrived after the soybean crop had been harvested there was no impact on production that year. Indeed, the 2005 and 2006 growing seasons were characterized by weather patterns unsupportive of P. pachyrhizae infection and disease, so actual losses have not yet approached the damaging levels anticipated. This has been good news for producers, but ironically, has prompted a sense of security that may be unfounded in future years when conditions may be more conducive to pathogen establishment.
Regardless of the seriousness of soybean rust to date, the fact that the pathogen was distributed widely—again likely by hurricane winds in 2005 (Stokstad, 2004)—and the fact that it establishes easily and overwinters in a variety of hosts, including an extremely invasive vine called kudzu, means it is now considered to be established in the United States (Pivonia and Yang, 2004). Since the plant Select Agent list contains only exotic pathogens, APHIS removed P. pachyrhizae from the list in 2006. “Delisting” has several implications, both positive and negative. Federally mandated response to, and management of, a Select Agent is extremely expensive for both federal and state agencies. In addition, the extensive and expensive policies, certifications, permits, and containment requirements for scientific research on Select Agents are significant disincentives for plant pathologists to work on the pathogens of greatest concern. The removal of P. pachyrhizi from the list will facilitate research, but it also changes the responsibility of federal agencies in their response to the disease.
The bacterial pathogen Liberobacter asiaticus, a Select Agent that causes a disease officially known as “huanglongbing,” or citrus greening, was discovered in Florida in fall 2005 (APHIS, 2007). Its possible arrival had been a concern for at least two years, after it was learned that its insect vector—the citrus-feeding Asian citrus psyllid, Diaphorina citri—had arrived in the state and would be likely to spread the pathogen quickly should it arrive. Like citrus canker, huanglongbing was more damaging than anticipated because it, too, occurred during the 2005 hurricane season, when extensive wind dissemination of inoculative vector insects quickly resulted in the pathogen becoming endemic in the state. The question of whether L. asiaticus will or should be removed from the Select Agent list is complicated by the fact that, although the bacterium may be established in Florida, it is not yet known to occur in citrus-growing regions of Texas, California, and other southern states.
Components of a Strong Plant Biosecurity Strategy
A robust system of preparedness for threatening exotic or emerging plant diseases will require the following elements:
-
Early detection and diagnostic systems
-
Epidemiological models for predicting pathogen spread
-
Reasonable but effective strategies and policies for crop biosecurity
-
Distributed physical and administrative infrastructure
-
A national system for strategic planning and response coordination
-
Microbial forensic capability: validated technology and investigative capability
Before 2001, our national capability in plant disease diagnostics and recovery was fragmented, poorly supported. and of limited effectiveness due to declining resources. In the past five years, however, significant improvements in infrastructure will help to ensure preparedness for a serious plant pathogen introduction event. In 2004, President George Bush issued Homeland Security Presidential Directive 9 (HSPD-9), which mandated a National Plant Disease Recovery System (NPDRS). The USDA Office of Pest Management Policy, assigned by the Secretary of Agriculture to develop the NPDRS, has worked to develop specific Recovery Plans for each of the Select Agents as well as for several other plant diseases of high consequence. Their approach, which is well underway at this time, has been to bring key experts from federal agencies, private industry, and academia together for the development of each plan, and to partner with the American Phytopathological Society (a 5,000-member professional society dedicated to plant health) to ensure broad-based community input and participation. Another important initiative has been the establishment—by USDA-Cooperative State Research, Education, and Extension Service (CSREES)—of the National Plant Diagnostic Network, an interconnected system of diagnostic laboratories affiliated with land grant universities and/or state departments of agriculture in each state (Stack et al., 2006).
At this writing, it is clear that many individual improvements and initiatives have enhanced our nation’s ability to prevent and prepare for emerging plant diseases and pathogens. Individual efforts within federal agencies concerned with agricultural biosecurity (USDA APHIS, CSREES, and Agricultural Research Service [ARS]; Departments of Defense and Homeland Security; Environmental Protection Agency; and Food and Drug Administration) also have enhanced our preparedness. However, significant gaps remain.
Preparedness for events involving intentional introductions of plant pathogens, whether for purposes of bioterror or biocrime, must include a strong national security plan that encompasses microbial forensics and criminal attribution. However, U.S. crop producers, consultants, and agricultural scientists, unaccustomed to considering the possibility of intentional pathogen introduction, traditionally focus disease management strategies on prevention, rapid eradication, or long-term management. A recent study (Fletcher et al., 2006) assessed currently available information, technologies, and resources, developed for peaceful applications, which can be utilized for plant pathogen forensics. The authors also prioritized activities and resource expenditures needed to enhance our plant
pathogen forensics capabilities. Strategies needed for a comprehensive national microbial forensic capability, to determine the source of the pathogen and provide evidence for attribution, include (1) assuring high stringency of investigative technologies (validation, confidence, statistical significance, consistency); (2) tracing pathogen origin and movement; (3) identifying the timing and site of initial introduction; (4) identifying the perpetrators; (5) collecting evidence for criminal attribution; and (6) forming linkages with the law enforcement and security communities.
One of the most pressing gaps, because it impacts all the others, is the need for greater communication, cooperation, and coordination between and among federal agencies, academic institutions, and industry. Each of the agencies and entities contributing to national agricultural security has a unique mission and specific goals for which it is accountable to its stakeholders, and each is responsible for different elements of an outbreak response. Currently, there is no single entity, such as the Centers for Disease Control and Prevention (CDC) or a national center, to ensure strategic planning for future preparedness and the most effective and efficient response to a plant pathogen emergency. To be effective, this coordinating function should be established at a level above individual agencies. The coordinating entity would not duplicate or unnecessarily overlap the diverse elements of a robust national biosecurity plan because most of these responsibilities are charged to existing components of government. It would focus on strategic planning, program reviews, and coordination of activities among federal agencies, private entities, and academia; prioritization of research and education needs for allocation of limited resources; database and pathogen collections; and coordination of public relations.
Conclusions
Our nation’s agricultural industry is strong and our food supply is among the safest in the world, but vulnerabilities do exist. Recent initiatives in various branches of government, academia, and industry have enhanced the security of our plant resources, but gaps and needs remain. Fortunately, the actions needed to sustain and protect our plant resources from intentional pathogen introduction, and to recover from deliberate plant disease outbreaks, will also enhance the effectiveness and productivity of normal U.S. agricultural enterprises. For example, in addition to the threat of intentional introductions of exotic plant pathogens and pests, new pathogen species or races emerge naturally. Globalization of markets, unprecedented international travel, and changes in climate from various causes all contribute to an increased likelihood that pathogens will move across national borders and employ adaptive strategies in response to exposure to new environments. Let us use the opportunities provided by these challenges to strengthen our agricultural production systems, and ensure that our nation continues to lead the world in providing food that is abundant, reliable, nutritious and safe.
PLANT BIOSECURITY INFRASTRUCTURE FOR DISEASE SURVEILLANCE AND DIAGNOSTICS
James P. Stack, Ph.D.14
Kansas State University
Jacqueline Fletcher, Ph.D.15
Oklahoma State University
Introduction
The vital role of plants in society is not well understood by the general population or by most policy makers. Healthy plant systems are a prerequisite to the health and welfare of human and animal systems, and are essential to the economies of developed nations. Human, animal, and plant systems are intricately linked; the intersection of these three systems forms the basis of our economy, our culture, and our standard of living. The emerging one-medicine concept of holistic health that encompasses animal and human systems is rational and obvious when we consider the value inherent in these systems and the impact that zoonotic diseases have had over the past 50 years (Dudley, 2004; Karesh and Cook, 2005; Potter, 2004). However, we must expand that holistic one-medicine concept to include plant systems.
When we assess value within our primary living systems, it is appropriate that human systems have the most value, animal systems second, and plant systems third. However, plant systems are the foundation of all three. Plants generate the oxygen we breathe. They are the food we consume directly and the feed we provide to the animals we consume. They are the fibers that clothe us and the timber that shelters us, and they are becoming the fuels that power the technologies associated with our high standard of living. They stabilize our ecosystems and beautify our landscapes. Plants have great aesthetic value and great economic value. Healthy plant systems are vital to our national economy and consequently to our national security. The stability of societies and economies depends on the health of plant systems (Diamond, 2005). Therefore, we must protect our natural and agricultural plant systems to ensure the sustainability of our food production systems and ultimately our society.
A Biosecurity Framework
A national strategy for plant biosecurity must be comprehensive with respect to science and policy and must address issues of infrastructure, technology, and
education. One conceptual approach to the development of plant biosecurity infrastructure is based on a simple outbreak model. In its simplest form, this model includes the following components: the source of the outbreak agent; the introduction of the agent into some new environment; the detection of that agent at some point after the introduction event; the accurate diagnosis of the new agent at some point after the detection event; the response to the outbreak; and the resolution of the outbreak. Each component requires a unique strategy for preparedness: potential introductions require a prevention strategy; detection requires a surveillance strategy; diagnosis requires a technology strategy; response requires a communications and mitigation strategy; and resolution requires a recovery strategy.
Prevention
The U.S. Departments of Agriculture (USDA) and Homeland Security (DHS) share responsibility for preventing the introduction of new plant pathogens and insect pests that threaten our plant systems. This is accomplished through the activities and programs of Customs and Border Protection (CBP) and USDA’s Animal and Plant Health Inspection Service Plant Protection and Quarantine (USDA-APHIS-PPQ). Due to the extremely large and increasing volume of imports of plants and plant products, port and border inspections can never be 100 percent effective in preventing the accidental or intentional introduction of new agents. The increase in Internet-based commerce further adds to this challenge by providing a means to circumvent the inspection and quarantine process associated with interstate and international trade. Consequently, we must anticipate the introduction of agents that threaten our plant systems, whether accidental due to global trade, intentional due to terrorism or crime, or natural due to weather events (e.g., hurricanes).
A prevention strategy should include the capability to intercept those agents with a high probability of introduction and establishment. Several lists of high-consequence pathogens and pests have been generated by government agencies and scientific societies. One such list identified more than 500 plant pathogens and nematodes and over 700 insects and mites that pose threats to U.S. plant systems (Huber, 2002). We lack the resources necessary to develop specific plans for over 1,200 organisms. Because there is no defining set of characteristics to determine which threat agent will become established and cause significant damage, a prioritization process is needed to identify those high consequence agents with the greatest potential to cause persistent, wide-scale damage such that specific interception protocols are required. For example, if a new race of a pathogen emerged with the potential to destroy over 50 percent of the U.S. wheat crop, its characteristics indicate that the pathogen will establish and spread, there are no effective management tools, and pathways for pathogen introduction exist, then a comprehensive preparedness, response, and recovery strategy should be developed for that specific pathogen.
Surveillance, Detection, and Diagnosis
An Institute of Medicine study identified six critical elements necessary to a food safety system (IOM, 1998). These same six elements would provide the framework for biodefense against threats to national food security (King, 1999). Among those elements was a comprehensive surveillance and monitoring system. This element is as important for plant-based systems as it is for human and animal systems.
For the purposes of this paper, surveillance is the process of searching, detection is the process of finding, and diagnosis is the process of determining and/or verifying what is found. The National Plant Diagnostic Network (NPDN) was established by USDA in 2002 to provide the necessary critical infrastructure to facilitate early detection and rapid diagnosis of disease and pest outbreaks in natural and agricultural plant systems (Stack et al., 2006). This is accomplished through the primary mission areas of building infrastructure for diagnostics and communications and through training and education programs that target first detectors and diagnosticians.
Surveillance and detection We should assume that introductions will continue to occur as a result of global trade and the increasing threats of intentional introductions due to bioterrorism and biocrime. If the projections for increased trade and climate change are accurate, it is quite possible that the frequency and severity of introductions will increase.
Our current surveillance and detection systems vary significantly according to plant system, target pathogen or pest, and geographic region. Funding for surveillance of plant systems is most often allocated for specific target agents; consequently, those programs are executed only in areas at risk. Because of limited funding, general surveillance at the field level is minimal. For some plant systems, industry has implemented very effective surveillance programs, and the data are provided to APHIS. Mechanisms to share data are being explored.
Among the major limitations to an effective surveillance system is not having enough trained personnel in the field. Unlike human and animal systems, in which doctors and veterinarians are distributed throughout rural and agricultural areas, few plant doctors with diagnostic expertise operate at the local level with plant-based systems. NPDN, in collaboration with Cooperative State Research, Education, and Extension Service (CSREES), APHIS, the Extension Disaster Education Network, and the Regional Integrated Pest Management (IPM) Centers, has developed a training and education program targeting first detectors at the local level. Its registry of trained first detectors may serve as a resource for outbreak management.
Diagnosis NPDN was established to provide a triage system for the rapid and accurate diagnosis of introduced plant pathogens and insect pests. Because of a decline in national and local support for plant diagnostics over many years, state labs varied tremendously in diagnostic infrastructure and experience. With
funding from USDA, NPDN has rebuilt and enhanced much of that infrastructure and implemented programs to train diagnosticians in the latest diagnostic technologies (Stack et al., 2006).
Morse identified three elements for an effective early warning system; clinical recognition, epidemiological investigation capability, and laboratory capacity (Morse, 2002). NPDN has become an integral component for early warning and NPDN labs provide surge diagnostic support during outbreaks.
NPDN has created a national database for the diagnostic data collected at the network labs. An NPDN epidemiology group is developing data analysis tools that include syndromic analysis. Many of the issues and challenges associated with syndromic surveillance in human systems (Stoto, 2005; Stoto et al., 2004) also apply to plant systems. Because there are many natural introductions in plant systems, syndromic surveillance might prove to be a useful approach. Coordination and communication among all the disciplines will be important.
Response
Response to plant disease outbreaks resulting from new pathogen introductions is a responsibility of USDA APHIS. For most introductions, APHIS provides the leadership for a coordinated response that often includes APHIS-led rapid deployment teams, state departments of agriculture, industry, and in some cases, land grant university diagnostic labs. An elaborate structure exists within APHIS for the development of response plans to high-consequence pathogens and pests.
NPDN, in partnership with APHIS and state departments of agriculture, has developed and implemented a training exercise program to facilitate preparedness for outbreak response. All 50 states have participated in at least one exercise involving local, state, and federal governments, as well as state, regional, and national diagnostic labs. The exercise scenario makes clear the roles and responsibilities of all participants. After the exercise scenario, action reports are analyzed to identify areas in need of improvement.
Recovery (A Superficial Treatment)
Recovery, which follows response, is the strategy by which to return a system to the preevent mean or to a new, but stable, mean. An effective recovery strategy will be comprehensive in nature and include short-term plans that address the transition from response to the new system mean, while long-term plans will need to address prevention and recovery from subsequent introductions. The scope of recovery plans vary as a function of the scale of the outbreak and the ripple effects throughout the national and global economies. While response revolves around outbreak delineation, containment, eradication, and management, recovery is focused on local and system-level issues, including ecological impacts,
production shortfalls, effects on transportation systems, impacts on trade agreements, market reentry strategies, and replacement markets or systems.
Mandated by Homeland Security Presidential Directive 9 (HSPD-9), the National Plant Disease Recovery System (NPDRS) was established within the USDA Agricultural Research Service. NPDRS has involved other federal agencies (e.g., APHIS and CSREES), state departments of agriculture, scientific societies, and universities in the development of national response plans for the Select Agents and other high-consequence pathogens.
Among the challenges of an effective plant disease recovery strategy will be to find cost-effective solutions for low profit margin systems. Deriving a cost–benefit premium that achieves sustainable plant systems without significantly raising the percentage of the U.S. income spent on food or without causing irreversible ecosystem damage will be challenging. One goal for such a strategy would be establishing mechanisms for national cooperation among public and private sectors and international cooperation that facilitates collaboration without compromising trade. The true cost of risk reduction is not known. More effective predictive models for invasiveness, impacts, and recovery outcomes will be needed.
To date, NPDRS has focused on response plans. The challenge for NPDRS will be to transition into the development of recovery strategies in the face of increasing introductions that call for more response plans.
Challenges
The Select Agent Paradox
The Select Agent program includes a requirement for the identification of high-consequence plant pathogens and toxins having a reasonable potential to cause significant ecological or economic damage and the potential for deliberate introduction. Once a pathogen is designated as a Select Agent, strict laws regulate its possession, handling, and dissemination. Responsibility for managing a plant disease outbreak caused by a Select Agent resides with APHIS. If it is suspected or determined that the introduction was intentional, then the Federal Bureau of Investigation would share primary responsibility.
The original Select Agent list for plant pathogens included 10 pathogens (see Fletcher and Stack earlier in this chapter). Since its adoption, at least four of these agents have been introduced into the United States either accidentally as a result of trade (Ralstonia solanacearum, Liberobacter asiaticus, Plum pox virus) or naturally as a result of a weather event (Phakopsora polysora) (Stokstad, 2004). Two of those agents are now considered to be endemic and were removed from the Select Agent list. Once removed from the list, the management of the threat agent shifted from primarily a federal responsibility to primarily a state and local responsibility.
The utility and effectiveness of the Select Agent program should be reviewed. At best, it reduces the potential for an accidental escape from a domestic lab and impedes the illicit acquisition of a viable culture or toxin preparation from a domestic lab or commercial culture collection. At worst, it precludes achieving a state of preparedness at the state and local levels. Pathway analyses indicate that for most of the Select Agents, there is an equal or greater probability of being introduced accidentally or naturally than intentionally. If these agents are truly the organisms of greatest concern, we should be encouraging many of our scientists to conduct the research necessary to ensure that we can detect them quickly, diagnose them correctly, and respond effectively to minimize the potential negative impact. If working with these agents is too difficult for U.S. scientists then we will not be building the necessary expertise for the organisms that pose the greatest threat to the country. A reevaluation of the goals and effectiveness of the Select Agent Rule should be executed with specific reference to the unintended consequences that impair preparedness and response.
Animal and Plant Health Inspection Service
The authority for regulating high-consequence plant pathogens and insect pests resides within APHIS. Responsibilities include providing emergency response to outbreaks; issuing permits for interstate transport and international importation of pathogens and pests; coordinating national and regional pest surveys; providing training programs; and developing and validating diagnostic protocols. Most of these tasks are time sensitive and resource intensive, sometimes with significant legal ramifications. Yet, among the USDA agencies, APHIS has historically received the least funding. Its level of support seems disproportionate to its responsibility. If we are to develop and maintain a national state of preparedness in the face of increasing plant pathogen and pest introductions, increased support within USDA for APHIS and increased support within APHIS for plant programs will be necessary.
Sampling
Sampling underpins the successful implementation of every strategy on which a successful biosecurity program depends. A sampling protocol depends on the characteristics of the target agent, the environment in which it exists, and the matrix from which it is to be sampled. Consequently, much effort should be applied to the development and validation of the methods deployed. However, the extremely large number of potential threat agents in plant systems precludes implementation of a comprehensive sampling strategy for each agent. Therefore, more general sampling strategies are needed that increase the probability of interception for a wide array of agents.
Formulating and implementing a national strategy for recovery from single or multiple introductions to plant systems is a challenge beyond the mission of any single agency or department. It will require the coordination of several government departments at the local, state, and federal levels; public and private educational institutions; and the many industries that support plant systems in the United States. As has been identified for zoonotic disease surveillance (Dudley, 2004), a central body with responsibility for plant disease health that would develop a national strategy does not exist.
Summary
There are many challenges to achieving plant biosecurity within the United States and across the world. The success of U.S. agriculture has made possible a high standard of living with a safe, inexpensive, and dependable food supply system. But it has also left us complacent with respect to food production. Educational programs are needed to increase awareness among the general population and among policy makers regarding the interdependence of plant, animal, and human systems. Appropriately, human systems have the greatest value in society and require the greatest investment of our time and resources. Sustenance of healthy human and animal systems requires healthy plant systems. Having less value does not mean having little value.
The world at the beginning of the 21st century is vastly different than it was at the beginning of the 20th century. Among the challenges to sustainable living systems are globalization, climate change, population growth, and bioterrorism/biocrime. There is neither a single strategy nor a single technology that will ensure the security of our living systems. The benefits of globalization are tremendous, but so too are the risks if we do not prepare for the consequences with respect to emerging diseases of humans, animals, and plants. Consequently, all nations must be secure if any nation is to be secure. Through modern transportation systems and international commerce, some of the natural barriers (e.g., oceans) to the dispersal of pathogens have been circumvented or eliminated. Most plant pathogens once took decades to disperse naturally around the world. Through normal commerce it may now take only a few days to a few weeks. Two introductions of the Select Agent Ralstonia solanacearum r3b2 in 2003 and 2004 from Kenya and Guatemala, respectively, are good examples. The threat of intentional introduction could reduce that dispersal interval to one day.
Historically, pathogens have moved naturally and accidentally among nations around the world. However, the rate of their border crossings has increased dramatically, resulting in drastically reduced time to prepare for an introduction. International cooperation is essential to achieve plant biosecurity. The importance of global management of disease outbreaks to minimize large-scale impacts was justified effectively for animal and human diseases (Karesh and Cook, 2005).
The same case can be made for plant diseases. Many of the plant pathogens that have caused epidemics in North America over the past 150 years were introduced from Africa, Asia, Europe, and South America. Intuitively, the health and stability of plant production systems in the United States depends on good plant surveillance systems in other parts of the world. Improved cooperation among nations is required for prevention and rapid outbreak intervention.
REFERENCES
Adam, G.2006. Significant ways to spread plant virus diseases in agricultural ecosystems: Is agroterrorism possible? In Virus diseases and crop biosecurity, edited by J. I. Cooper, T. Kuene, and V. P. Polischuk. New York: Springer. Pp. 45-54.
American Phytopathological Society Public Policy Board. 2002. First line of defense, http://www.apsnet.org (accessed May 1, 2007).
APHIS (Animal and Plant Health Inspection Service). 2007. Citrus greening, http://www.aphis.usda.gov/plant_health/plant_pest_info/citrus_greening/index.shtml (accessed July 23, 2007).
Banner Engineering Corp. 2007. PresencePLUS glossary, http://www.baneng.com/literature_resources/reference/glossary_pplus.html (accessed April 30, 2007).
Brown, K. 2001. Florida fights to stop citrus canker. Science 292(5525):2275-2276.
Buckeridge, D. L., D. K. Owens, P. Switzer, J. Frank, and M. A. Musen. 2006. Evaluating detection of an inhalational anthrax outbreak. Emerging Infectious Diseases 12(12):1942-1949.
Budowle, B., J. Burans, R. G. Breeze, M. R. Wilson, and R. Chakraborty. 2005a. Microbial forensics. In Microbial forensics, edited by R. Breeze, B. Budowle, and S. Shutzer. Burlington, MA: Elsevier Academic Press. Pp. 1-26.
Budowle, B., M. D. Johnson, C. M. Fraser, T. J. Leighton, R. S. Murch, and R. Chakraborty. 2005b. Genetic analysis and attribution of microbial forensics evidence. Critical Reviews in Microbiology 31(4):233-254.
Budowle, B., S. E. Schutzer, M. S. Ascher, R. M. Atlas, J. P. Burans, R. Chakraborty, J. J. Dunn, C. M. Fraser, D. R. Franz, T. L. Leighton, S. A. Morse, R. S. Murch, J. Ravel, D. L. Rock, T. R. Slezak, S. P. Velsko, A. C. Walsh, and R. A. Walters. 2005c. Toward a system of microbial forensics: From sample collection to interpretation of evidence. Applied and Environmental Microbiology 71(5):2209-2213.
Buehler, J. W., R. L. Berkelman, D. M. Hartley, and C. J. Peters. 2003. Syndromic surveillance and bioterrorism-related epidemics. Emerging Infectious Diseases 9(10):1197-1204.
Carefoot, G. L., and E. R. Sprott. 1969. Famine on the wind; plant disease and human history. London, England: Angus and Robertson.
Casagrande, R. 2000 (Fall/Winter). Biological terrorism targeted at agriculture: The threat to U.S. national security. The Nonproliferation Review: 92-105, http://cns.miis.edu/pubs/npr/vol07/73/73casa.pdf (accessed May 1, 2007).
CDC (Centers for Disease Control and Prevention). 2003. Estimated sensitivity of West Nile virus surveillance methods, http://www.cdc.gov/ncidod/dvbid/westnile/misc/slides/roehrig/slide27.htm (accessed May 31, 2007).
CDC. 2006a. Syndromic surveillance: An applied approach to outbreak detection, http://www.cdc.gov/epo/dphsi/syndromic.htm (accessed April 26, 2007).
CDC. 2006b. E. coli O157:H7 outbreak case counts by state, http://www.cdc.gov/foodborne/ecolispinach/case_count_us_map.htm (accessed May 10, 2007).
CDC. 2006c. Timeline for reporting of E. coli cases, http://www.cdc.gov/ecoli/reportingtimeline.htm (accessed April 26, 2007).
CDC. 2006d. Multistate outbreak of E. coli O157 infections linked to Taco Bell, http://www.cdc.gov/ecoli/2006/december/index.htm (accessed April 19, 2007).
CDC. 2007. Overview of influenza surveillance in the United States, http://www.cdc.gov/flu/weekly/pdf/flu-surveillance-overview.pdf (accessed April 19, 2007).
CIDRAP (Center for Infectious Disease Research and Policy). 2003. Overview of agricultural biosecurity, http://www.cidrap.umn.edu/cidrap/content/biosecurity/ag-biosec/biofacts/agbiooview.html (accessed May 31, 2007).
Diamond, J. 2005. Collapse: How societies choose to succeed or fail. New York: Viking Penguin.
Dudley, J. P. 2004. Global zoonotic disease surveillance: An emerging public health and biosecurity imperative. BioScience 54(11):982-983.
FDA (Food and Drug Administration). 2001. Survey of domestic fresh produce: Interim results, http://www.cfsan.fda.gov/~dms/prodsur9.html (accessed May 1, 2007).
FDA. 2006. Nationwide E. coli O157:H7 outbreak: Questions and answers, http://www.cfsan.fda.gov/~dms/spinacqa.html (accessed April 26, 2007).
FDA. 2007. FDA finalizes report on 2006 spinach outbreak, http://www.fda.gov/bbs/topics/NEWS/2007/NEW01593.html (accessed May 1, 2007).
Feng, G., E. Bailes, D. L. Robertson, Y. Chen, C. M. Rodenburg, S. F. Michael, L. B. Cummins, L. O. Arthur, M. Peeters, G. M. Shaw, P. M. Sharp, and B. H. Hahn. 1999. Origin of HIV-1 in the chimpanzee Pan troglodytes troglodytes. Nature 397(6718):436-441.
Fletcher, J. 2006. Plant disease surveillance and detection. Paper presented at the Institute of Medicine Forum on Microbial Threats, Washington, DC, December 12-13.
Fletcher, J., C. L. Bender, W. T. Cobb, S. E. Gold, C. A. Ishimaru, D. G. Luster, U. K. Melcher, R. L. Murch, H. Scherm, R. C. Seem, J. L. Sherwood, B. Sobral, and S. A. Tolin. 2006. Plant pathogen forensics: Capabilities, needs and recommendations. Microbiology and Molecular Biology Reviews 70(2):450-471, http://mmbr.asm.org/cgi/reprint/70/2/450/ (accessed May 1, 2007).
Gottwald, T. R., J. H. Graham, and T. S. Schubert. 2002. Citrus canker: The pathogen and its impact. Plant Health Progress, http://www.apsnet.org/online/feature/citruscanker/ (accessed May 30, 2007).
Gray, G. C., B. Feighner, D. H. Trump, S. W. Berg, M. J. Zajdowicz, and T. R. Zajdowicz. 2005. Diseases spread by close personal contact. In Military preventive medicine: Mobilization and deployment Vol. 2, edited by P. W. Kelley. Washington, DC: Office of the Surgeon General, U.S. Army. Pp. 1117-1211.
Gunzenhauser, J. D. 2003. Communicable disease control in basic training: Programmatic aspects. In Military preventive medicine: Mobilization and deployment Vol. 1, edited by P. W. Kelley. Washington, DC: Office of the Surgeon General, U.S. Army. Pp. 173-194.
Hanson, K. 2005. Disease and nonbattle injury surveillance: Outcome measure for force health protection. In Military preventive medicine: Mobilization and deployment Vol. 2, edited by P. W. Kelley. Washington, DC: Office of the Surgeon General, U.S. Army. P. 719.
Health Data Standards: The Players. 2007. http://faculty.washington.edu/ocarroll/infrmatc/database/data/players.htm (accessed April 30, 2007).
Homeland Security Council. 2005. National strategy for pandemic influenza. Washington, DC: Department of Health and Human Services, http://www.pandemicflu.gov/plan/federal/index.html (accessed April 19, 2007).
Huber, D. M. 2002 (March). Invasive pest species: Impacts on agricultural production, natural resources, and the environment. Council for Agricultural Science and Technology Issue Paper 20, http://downloads.heartland.org/04664i.pdf (accessed May 25, 2007).
IEEE-TCRTS (Institute of Electrical and Electronics Engineers, Inc. Technical Committee on Real-Time Systems). 2007. About IEEE-TCRTS, http://cs-mailman.bu.edu/mailman/listinfo/ieee-tcrts (accessed April 30, 2007).
IOM (Institute of Medicine). 1998. Ensuring safe food from production to consumption. Washington, DC: National Academy Press.
IOM. 2003. Microbial threats to health: Emergence, detection, and response. Washington, DC: The National Academies Press.
IOM/NRC (National Research Council). 2006. Globalization, biosecurity and the future of the life sciences. Washington, DC: The National Academies Press. Pp. 15-138.
Jackson, M. L., A. Baer, I. Painter, and J. Duchin. 2006. Systematic comparison of algorithms used in syndromic surveillance. Advances in Disease Surveillance 1:35.
Karesh, W. B., and R. A. Cook. 2005 (July/August). The human–animal link. Foreign Affairs: 38-50.
Karesh, W. B., R. A. Cook, E. L. Bennett, and J. Newcomb. 2005. Wildlife trade and global disease emergence. Emerging Infectious Diseases 11(7):1000-1002, http://www.cdc.gov/ncidod/EID/vol11no07/pdfs/05-0194.pdf (accessed May 25, 2007).
Kelley, P. 2006. Syndromic surveillance: Moving from theory to practice. Keynote address at the Institute of Medicine Forum on Microbial Threats, Washington, DC, December 12-13.
King, L. 1999. A domestic legislative agenda for improving food safety and safeguards from terrorist attacks on the U.S. food supplies and U.S. agricultural interests. Annals of the New York Academy of Sciences 894:228-232.
Labus, B. 2005. Practical applications of syndromic surveillance: 2003–2004 influenza season. Emergency Preparedness E-newsletter, http://bt.naccho.org/E-newsletter-archive/SS-Influenza.htm (accessed April 19, 2007).
Large, E. C. 1940. The advance of the fungi. New York: Dover Publications.
Leroy, E. M., P. Rouquet, P. Formenty, S. Souquière, A. Kilbourne, J.-M. Froment, M. Bermejo, S. Smit, W. Karesh, R. Swanepoel, S. R. Saki, and P. E. Rollin. 2004. Multiple Ebola virus transmission events and rapid decline of Central African wildlife. Science 303(5656):387-390, http://www.sciencemag.org/cgi/content/abstract/303/5656/387?etoc/ (accessed May 25, 2007).
Lindley, C., and T. Ward. 2007. Experience with clinician-based syndromic surveillance in West Texas. Advances in Disease Surveillance 2:108, http://www.isdsjournal.org/article/view/862/745 (accessed April 19, 2007).
Lombardo, J. S. 2006. Implications of “real time” versus “batch reporting” for surveillance. Presentation at the Institute of Medicine Forum on Microbial Threats, Washington, DC, December 12-13.
Lombardo, J. S., and D. L. Buckeridge. 2007. Disease surveillance: A public health informatics approach. Hoboken, NJ: John Wiley & Sons.
Lu, J., K. Konty, F. Mostashari, and K. Metzger. 2006. Could outpatient visits enhance our ability of early detecting influenza-like illness outbreaks? Advances in Disease Surveillance 1:44.
MacKenzie, W., N. Hoxie, M. Proctor, M. Gradus, K. A. Blair, D. E. Peterson, J. J. Kazmierczak, D. G. Addiss, K. R. Fox, and J. B. Rose. 1994. A massive outbreak in Milwaukee of cryptosporidium infection transmitted through the public water supply. New England Journal of Medicine 331(3):161-167.
Madden, L., and M. Wheelis. 2003. The threat of plant pathogens as weapons against U.S. crops. Annual Reviews of Phytopathology 41:155-176.
Mandl, K. D., J. M. Overhage, M. M. Wagner, W. B. Lober, P. Sebastiani, F. Mostashari, J. A. Pavlin, P. H. Gesteland, T. Treadwell, E. Koski, L. Hutwagner, D. L. Buckeridge, R. D. Aller, and S. Grannis. 2004. Syndromic surveillance: A guide informed by the early experience. Journal of the American Medical Informatics Association 11(2):141-150.
Maslanka, S. E., J. Sobel, and B. Swaminathan. 2002. Reducing the risk: Foodborne pathogen and toxin diagnostics. In Biological threats and terrorism: Assessing the science and response capabilities. Institute of Medicine. 2002. Washington, DC: The National Academies Press.
Microsoft Encarta. 2007. http://encarta.msn.com/encnet/refpages/search.aspx?q=batch&Submit2=Go (accessed April 30, 2007).
Morse, S. S. 2002. The vigilance defense. Scientific American 287(4):88-89.
Murch, R. S. 2003. Microbial forensics: Building a national capacity to investigate bioterrorism. Biosecurity and Bioterrorism: Biodefence Strategy, Practice, and Science 1(2):117-122.
Newcomb, J. 2003. Biology and borders: SARS and the new economics of bio-security. Cambridge, MA: Bio-Economics Research Associates.
Oerke, E. C., H. W. Dehne, F. Schonbeck, and A. Weber. 1994. Crop production and crop protection: Estimated losses in major food and cash crops. Amsterdam, The Netherlands: Elsevier Science BV.
OIE (Office International des Epizooties). 2004. Avian influenza—Belgium ex Thailand: Smuggled birds (02): OIE. ProMED http://www.promedmail.org, archive number: 20041027.2907 (accessed May 25, 2007).
Olson, D. R., R. T. Heffernan, and F. Mostashari. 2005. Age matters: Emergency department syndromic surveillance for epidemic influenza. Paper presented at the National Syndromic Surveillance Conference, Seattle, Washington, September 14-15, 2005.
Osofsky, S. A., S. Cleaveland, W. B. Karesh, M. D. Kock, P. J. Nyhus, L. Starr, and A. Yang, eds. 2005a. Conservation and development interventions at the wildlife/livestock interface: Implications for wildlife, livestock and human health. Gland, Switzerland and Cambridge, United Kingdom: IUCN. Pp. xxxiii and 220.
Osofsky, S. A., R. A. Kock, M. D. Kock, G. Kalema-Zikusoka, R. Grahn, T. Leyland, and W. B. Karesh. 2005b. Building support for protected areas using a ‘one health’ perspective. In Friends for life: New partners in support of protected areas, edited by J. A. McNeely. Gland, Switzerland and Cambridge, United Kingdom: IUCN. Pp. 65-79.
O’Toole, T., M. Mair, and T. Inglesby. 2002. Shining light on “Dark Winter.” Clinical Infectious Diseases 34(7):972-983.
Peres, C. A. 2000. Effects of subsistence hunting on vertebrate community structure in Amazonian forests. In Hunting for sustainability in tropical forests, edited by J. G. Robinson and E. L. Bennett. New York: Columbia University Press. Pp. 168-198.
Pimentel, D., L. Lach, R. Zuniga, and D. Morrison. 2000. Environmental and economic costs of nonindigenous species in the United States. Bioscience 50(1):53-65.
Pinstrup-Andersen, P. 2001. The future world food situation and the role of plant diseases. Plant Health Instructor, http://www.ifpri.org/pubs/articles/2001/pinstrup01_01.pdf (accessed May 1, 2007).
Pivonia, S., and X. B. Yang. 2004. Assessment of the potential year-round establishment of soybean rust throughout the world. Plant Disease 88(5):523-529.
Potter, P. 2004. “One medicine” for animal and human health [about the cover]. Emerging Infectious Diseases 10(12), http://www.cdc.gov/ncidod/EID/vol10no12/about_cover.htm (accessed May 2, 2007).
Rogers, P., S. Whitby, and M. Dando. 1999. Biological warfare against crops. Scientific American 280(6):70-75.
Schneider, R. W., C. A. Hollier, H. K. Whitam, M. E. Palm, J. M. McKemy, J. R. Hernández, L. Levy, and R. DeVries-Paterson. 2005. First report of soybean rust caused by Phakopsora pachyrhizi in the continental United States. Plant Disease 89:774.
Schumann, G. L. 1991. Plant diseases: Their biology and social impact. St. Paul, MN: APS Press.
Sebastiani, P., K. Mandl, P. Szolovits, I. S. Kohane, and M. F. Ramoni. 2006. A Bayesian dynamic model for influenza surveillance. Statistics in Medicine 25(11):1823-1825.
Sosin, D. M., and J. DeThomasis. 2004. Evaluation challenges for syndromic surveillance—making incremental progress. Morbidity and Mortality Weekly Report 53(Suppl):125-129, http://www.cdc.gov/mmwr/preview/mmwrhtml/su5301a25.htm (accessed May 9, 2007).
Stack, J., K. Cardwell, R. Hammerschmidt, J. Byrne, R. Loria, K. Snover-Clift, W. Baldwin, G. Wisler, H. Beck, R. Bostock, C. Thomas, and E. Luke. 2006. The National Plant Diagnostic Network. Plant Disease 90:128-136.
Stokstad, E. 2004. Plant pathologists gear up for battle with dread fungus. Science 306(5702): 1672-1673.
Stoto, M. A. 2005 (Spring). Syndromic surveillance. Issues in Science and Technology:49-56.
Stoto, M. A., M. Schonlau, and L. T. Mariano. 2004. Syndromic surveillance: Is it worth the effort? Chance 17(1):19-24.
Stoto, M. A., R. D. Fricker, A. J. Jain, A. Diamond, J. O. Davies-Cole, C. Glymph, G. Kidane, G. Lum, L. Jones, K. Dehan, and C. Yuan. 2006. Evaluating statistical methods for syndromic surveillance. In Statistical methods in counter-terrorism, edited by D. Olwell, A. G. Wilson, and G. Wilson. New York: Springer. Pp. 141-172.
Stoto, M. A., B. A. Griffin, A. Jain, J. O. Davies-Cole, G. Lum, G. Kidane, S. C. Washington. 2007. Fine-tuning and evaluation of detection algorithms for syndromic surveillance. Advances in Disease Surveillance 2:213.
Takahashi, H., P. Keim, A. F. Kaufmann, C. Keys, K. L. Smith, K. Taniguchi, S. Inouye, and T. Kurata. 1994. Bacillus anthracis incident, Kameido, Tokyo, 1993. Emerging Infectious Diseases 10(1):117-120, http://www.cdc.gov/ncidod/EID/vol10no1/pdfs/03-0238.pdf (accessed April 26, 2007).
Taylor, L. H., S. M. Latham, and M. E. J. Woolhouse. 2001. Risk factors for human disease emergence. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences 356(1411):983-989.
Tu, C., G. Crameri, X. Kong, J. Chen, Y. Sun, M. Yu, H. Xiang, X. Xia, S. Liu, T. Ren, Y. Yu, B. T. Eaton, H. Xuan, and L. F. Wang. 2004. Antibodies to SARS coronavirus in civets. Emerging Infectious Diseases 10(12):2244-2248, http://www.cdc.gov/ncidod/EID/vol10no12/04-0520.htm (accessed May 25, 2007).
USDA-ERS (U.S. Department of Agriculture, Economic Research Service). 2007. Soybeans and oil crops, http://www.ers.usda.gov/Briefing/SoybeansOilCrops/ (accessed May 1, 2007).
Wagner, M., F. C. Tsui, J. U. Espino, V. M. Dato, D. F. Sittig, R. A. Caruana, L. F. McGinnis, D. W. Deerfield, M. J. Druzdzel, and D. B. Fridsma. 2001. The emerging science of very early detection of disease outbreaks. Journal of Public Health Management and Practice 7(6):50-58.
Wheelis, M., R. Casagrande, and L. V. Madden. 2002. Biological attack on agriculture: Low-tech, high impact bioterrorism. Bioscience 52(7):569-576.
Whitby, S. M. 2001. The potential use of plant pathogens against crops. Microbes and Infection 3(1):73-80.
Whitby, S. M. 2002. Biological warfare against crops. Basingstoke, UK: Palgrave Macmillan.
Wilkie, D. S., and J. F. Carpenter. 1999. Bushmeat and hunting in the Congo Basin: An assessment of impacts and options for mitigation. Biodiversity and Conservation 8(7):927-955.