1
Allylamine1
Acute Exposure Guideline Levels
PREFACE
Under the authority of the Federal Advisory Committee Act (P.L. 92-463) of 1972, the National Advisory Committee for Acute Exposure Guideline Levels for Hazardous Substances has been established to identify, review, and interpret relevant toxicologic and other scientific data and develop acute exposure guideline levels (AEGLs) for high-priority, acutely toxic chemicals.
AEGLs represent threshold exposure limits for the general public and are applicable to emergency exposure periods ranging from 10 minutes (min) to 8 hours (h). Three levels—AEGL-1, AEGL-2, and AEGL-3—are developed for each of five exposure periods (10 min, 30 min, 1 h, 4 h, and 8 h) and are distinguished by varying degrees of severity of toxic effects. The three AEGLs are defined as follows:
AEGL-1 is the airborne concentration (expressed as parts per million [ppm] or milligrams per cubic meter [mg/m3]) of a substance above which it is predicted that the general population, including susceptible individuals, could
experience notable discomfort, irritation, or certain asymptomatic nonsensory effects. However, the effects are not disabling and are transient and reversible upon cessation of exposure.
AEGL-2 is the airborne concentration (expressed as ppm or mg/m3) of a substance above which it is predicted that the general population, including susceptible individuals, could experience irreversible or other serious, long-lasting adverse health effects or an impaired ability to escape.
AEGL-3 is the airborne concentration (expressed as ppm or mg/m3) of a substance above which it is predicted that the general population, including susceptible individuals, could experience life-threatening health effects or death.
Airborne concentrations below the AEGL-1 represent exposure levels that can produce mild and progressively increasing but transient and nondisabling odor, taste, and sensory irritation or certain asymptomatic nonsensory effects. With increasing airborne concentrations above each AEGL, there is a progressive increase in the likelihood of occurrence and the severity of effects described for each corresponding AEGL. Although the AEGL values represent threshold levels for the general public, including susceptible subpopulations, such as infants, children, the elderly, persons with asthma, and those with other illnesses, it is recognized that individuals, subject to unique or idiosyncratic responses, could experience the effects described at concentrations below the corresponding AEGL.
SUMMARY
Allylamine is a colorless or yellowish volatile liquid with a very sharp ammonia-like odor that is irritating to mucous membranes. It is highly flammable and moderately reactive with oxidizing materials. Industrially, it is used in the vulcanization of rubber and in the synthesis of pharmaceuticals. In addition to being a severe respiratory, eye, and skin irritant, allylamine is a cardiovascular toxin when administered at high doses orally, by injection, or by inhalation. Allylamine cardiotoxicity is proposed to be related to its metabolism to acrolein and hydrogen peroxide.
AEGL-1 values were based on a study in which 35 young adult human volunteers were exposed for 5 min to 2.5, 5, or 10 ppm allylamine (10-14 per concentration; sex and age not specified; Hine et al. 1960). A group was also exposed briefly to 14 ppm, which was reported as intolerable and exposure was almost immediately terminated. The subjects graded their sensory responses for eye irritation, nose irritation, pulmonary discomfort, central nervous system (CNS) effects (headache, nausea), and olfactory cognition on a five-point scale (0 = absent; 1 = slight; 2 = moderate; 3 = severe; 4 = extreme or intolerable). All subjects detected the odor of allylamine, and there were dose-related increases in the incidence of slight or moderate eye irritation (21%, 15%, 50%), nose irritation (50%, 54%, 100%), and pulmonary discomfort (29%, 46%, 50%) at 2.5, 5,
and 10 ppm, respectively. CNS effects were not dose related. The same AEGL-1 value was used for 10 min to 8 h because mild sensory irritation or discomfort does not generally vary greatly with time. The AEGL-1 point of departure was 1.25 ppm, which was obtained by applying a modifying factor (MF) of 2-2.5 ppm, which was the lowest effect level. The MF was used because exposure was for only 5 min, and it is unclear whether “moderate” irritation or discomfort is comparable to “notable” irritation or discomfort, which exceeds the scope of AEGL-1. An intraspecies uncertainty factor of 3 was applied because allylamine acts as a contact irritant, and the severity of its effects is not expected to vary greatly among humans. Also, use of a greater uncertainty factor would yield a concentration below 0.2 ppm, which was a no-effect level for workers exposed for up to 4 h (Shell Oil Co. 1992). The derived AEGL-1 value of 0.42 ppm for 10 min to 8 h is also consistent with two mouse respiratory irritation studies (Gagnaire et al. 1989, 1993), from which it is predicted that exposure for a few hours to 0.9 ppm would cause sensory irritation in humans but that 0.09 ppm would not (Alarie 1981).
AEGL-2 values were based on two studies. The 10-, 30-, and 60-min AEGLs were developed from the Hine et al. (1960) human 5-min exposure study that was used to derive AEGL-1 values but using 10 ppm as the point of departure. Ten ppm caused slight or moderate eye and nose irritation and pulmonary discomfort and was the no-observed-adverse-effect level (NOAEL) for “intolerable” irritation that occurred at 14 ppm. The same value was adopted for 10-60 min because the degree of irritation or discomfort resulting from exposure to 10 ppm was not expected to increase over a 1-h period beyond the scope of AEGL-2. An intraspecies uncertainty factor of 3 was used because allylamine acts as a contact irritant and the severity of its effects is not expected to vary greatly among humans. The resulting AEGL-2 value of 3.3 ppm was not adopted for 4 or 8 h, however, because a rat study (Guzman et al. 1961) indicated that exposure to 3.3 ppm for 4 or 8 h may cause cardiotoxicity. In the latter study, exposure to 40 ppm for 16 h was a NOAEL for cardiovascular lesions, which were seen from exposure to 60 ppm for 14 h (myofibril fragment damage, perivascular edema, and cellular infiltration). Time-concentration scaling was performed using the ten Berge et al. (1986) equation Cn × t = k, where n = 1.7 was calculated from a linear regression of the Guzman et al. (1961) rat cardiotoxicity data. An interspecies uncertainty factor of 5 was applied because the mechanism of toxicity is similar among several mammalian species (and humans) but differences in susceptibility are unknown, and an uncertainty factor of 3 yields values approaching the no-observed-effect level (NOEL) for lethality from pulmonary lesions for a 4- or 8-h exposure. An intraspecies uncertainty factor of 10 was used because the variability of the cardiotoxic response to allylamine among humans is undefined, and potentially sensitive populations exist (diabetics, persons with congestive heart failure). This yields 4- and 8-h AEGLs of 1.8 and 1.2 ppm, respectively, indicating that for these longer exposure durations, cardiotoxicity is a more sensitive end point than eye and respiratory irritation.
AEGL-3 values were derived from a study on rat inhalation with a lethal concentration in 50% of the sample (LC50) in which exposures were for 1, 4, or 8 h (Hine et al. 1960). All treated rats showed signs of eye and respiratory tract irritation, and some had lacrimation and red nasal discharge. Rats that died had stomachs distended with air, fluid-filled lungs, alveolar hemorrhage, and pulmonary edema. The NOEL for lethality, as represented by LC01 (1% lethality) values calculated using probit analysis, was the AEGL-3 end point. The 1-h, 4-h, and 8-h AEGLs were obtained using the respective LC01 values. The 10- and 30-min AEGLs were derived from the 1-h LC01 using the relationship Cn × t = k , where n = 0.85 was calculated from the Hine et al.LC50 data. An uncertainty factor of 30 was applied: 10 to account for interspecies variability (lack of acute toxicity studies from other species with AEGL-3 level end points) and 3 for human variability (the steep dose-response (~2-fold increase in concentration caused mortality to increase from 0 to 100%) indicates that the NOEL for lethality due to direct destruction of lung tissue is not likely to vary greatly among humans). The derived AEGL-3 values, as well as the AEGL-1 and AEGL-2 values, are shown in Table 1-1.
1.
INTRODUCTION
Allylamine is a colorless or yellowish volatile liquid that is highly flammable and moderately reactive with oxidizing materials (HSDB 2003). It is completely soluble in water with a pKa of 9.7 and has a very sharp ammonia-like odor that is irritating to mucous membranes (Budavari et al. 1996; HSDB 2003; Boor and Hysmith 1987).2 Industrially, it is used in the vulcanization of rubber and in the synthesis of commercial products, including mercurial diuretics, sedatives, and antiseptics (Benya and Harbison 1994). Allylamine is manufactured by the amination of alkyl halides (e.g., allyl chloride and ammonia) and is also a natural constituent of foodstuffs (Budavari et al. 1996; HSDB 2003).
In addition to being a severe respiratory, eye, and skin irritant, allylamine is cardiotoxic when administered at high doses orally, by inhalation, or by injection. It has been used to induce cardiac and vascular lesions in laboratory animals to model human cardiovascular disease. Allylamine cardiotoxicity is proposed to be related to its metabolism to acrolein and hydrogen peroxide in cardiac and vascular tissues (Boor and Hysmith 1987; Ramos et al. 1988). Allylamine lethal inhalation toxicity has been examined in rats and mice and its nonlethal inhalation toxicity in single- and multiple-exposure studies using monkeys, rats, mice, and rabbits. Studies with human volunteers and exposures in the workplace have yielded limited information about the irritant and toxic effects of short-term inhalation exposure. The allylamine odor threshold is
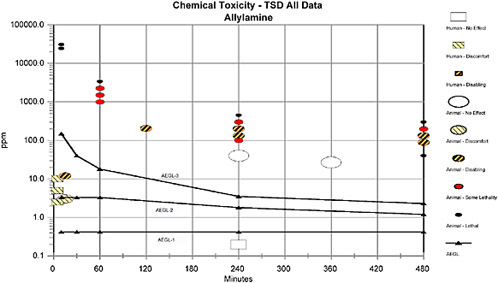
TABLE 1-1 Summary of AEGL Values for Allylamine
|
Classification |
10 min |
30 min |
1 h |
4 h |
8 h |
End Point (Reference) |
|
AEGL-1a (nondisabling) |
0.42 ppm (0.98 mg/m3) |
0.42 ppm (0.98 mg/m3) |
0.42 ppm (0.98 mg/m3) |
0.42 ppm (0.98 mg/m3) |
0.42 ppm (0.98 mg/m3) |
Mild human irritation or discomfort (Hine et al. 1960) |
|
AEGL-2 (disabling) |
3.3 ppm (7.7 mg/m3) |
3.3 ppm (7.7 mg/m3) |
3.3 ppm (7.7 mg/m3) |
1.8 ppm (4.2 mg/m3) |
1.2 ppm (2.8 mg/m3) |
Human eye and respiratory irritation and NOAEL for severe irritation (≤1 h; Hine et al. 1960); NOAEL for cardiovascular lesions in rats (≥4 h; Guzman et al. 1961) |
|
AEGL-3 (lethal) |
150 ppm (350 mg/m3) |
40 ppm (93 mg/m3) |
18 ppm (42 mg/m3) |
3.5 ppm (8.2 mg/m3) |
2.3 ppm (5.4 mg/m3) |
Lethality NOEL in rats (Hine et al. 1960) |
|
aOdor threshold is ≤2.5 ppm. |
||||||
<2.5ppm based on the human study of Hine et al. (1960) and is reported as 6.2 ppm by Summer (1971). Allylamine chemical and physical properties are listed in Table 1-2.
2.
HUMAN TOXICITY DATA
2.1.
Acute Lethality
No quantitative data were located regarding lethal allylamine exposure in humans. It was reported that allylamine inhalation may cause irregular respiration, cyanosis, excitement, convulsions, and death, although neither further details nor the source of this information was provided (HSDB 2003).
2.2.
Nonlethal Toxicity
2.2.1.
Odor Threshold/Odor Awareness
A published odor threshold was not found for allylamine. An unpublished source (van Doorn et al. 2002) reported 3.7 ppm as the odor detection threshold (OT50; that is, the concentration at which 50% of the odor panel observed an odor without necessarily recognizing it). A value of 3.7 conflicts with a sensory
TABLE 1-2 Chemical and Physical Data
|
Property |
Descriptor or Value |
Reference |
|
Synonyms |
Monoallylamine; 2-propenamine; 3-aminopropylene |
Budavari et al. 1996 |
|
Chemical formula |
CH2 = CHCH2 NH2 |
Budavari et al. 1996 |
|
Molecular weight |
57.10 |
Budavari et al. 1996 |
|
CAS registry number |
107-11-9 |
Benya and Harbison 1994 |
|
Physical state |
Liquid |
Budavari et al. 1996 |
|
Color |
Colorless or yellowish |
HSDB 2003 |
|
Solubility in water |
Completely miscible |
Budavari et al. 1996 |
|
Acid ionization constant, pKa |
9.7 |
HSDB 2003 |
|
Vapor pressure |
242 mm Hg at 25°C |
HSDB 2003 |
|
Vapor density (air = 1) |
1.97 |
Benya and Harbison 1994 |
|
Liquid density (water = 1) |
0.76 at 20/4°C |
Verschueren 1996 |
|
Melting point |
−88°C |
HSDB 2003 |
|
Boiling point |
55-58°C |
Budavari et al. 1996 |
|
Flammability/explosive limits |
2.2-22% |
HSDB 2003 |
|
Conversion factors |
1 mg/m3 = 0.428 ppm 1 ppm = 2.33 mg/m3 |
Verschueren 1996 |
threshold experimental study, in which all 36 volunteers exposed to allylamine for 5 min reported “olfactory cognition” at the lowest concentration tested of 2.5 ppm (Hine et al. 1960; see Section 2.2.2). Additionally, if the methodology of van Doorn et al. (2002) is used to calculate an LOA (level of distinct odor awareness; see Appendix B), a value of 58 ppm is calculated, which exceeds a concentration (i.e., 14 ppm) found to be intolerable by humans (Hine et al. 1960). The method used to determine the 3.7-ppm odor threshold was not reported, which may explain its discrepancy with the Hine et al. study (e.g., a higher concentration may be needed for detection by sniffing for a few seconds than by inhalation for 5 min).
2.2.2.
Experimental Studies
The sensory threshold for detecting inhaled allylamine was examined in 35 young adult human volunteers exposed for 5 min to 2.5, 5, 10, or 14 ppm (10-14 per concentration; sex and age not specified; Hine et al. 1960). It was not specified whether the same persons were exposed to more than one concentration. To compensate for the loss of allylamine upon the entrance of subjects into the 16,680-L chamber, the initial concentration of allylamine in the chamber
was about 10% greater than the target air allylamine concentration for the low concentrations and about 1% greater at the high allylamine concentrations (not specified which concentrations were considered low or high). The air allylamine concentration was continuously monitored by a recording infrared spectrophotometer. The subjects graded their sensory responses for eye irritation, nose irritation, pulmonary discomfort, CNS effects, and olfactory cognition on a five-point scale, ranging from “absent” to “extreme” (intolerable); results are shown in Table 1-3.
All test subjects detected the odor of allylamine at the lowest concentration tested (2.5 ppm). At 2.5, 5, and 10 ppm, respectively, there were dose-related increases in the incidence of slight or moderate eye irritation (21%, 15%, and 50%), nose irritation (50%, 54%, 100%), and pulmonary discomfort (29%, 46%, 50%). The incidence of slight or mild CNS effects, such as slight headache or nausea, was not dose related (21%, 0%, and 10% at 2.5, 5.0, and 10 ppm). At 14 ppm “irritation of [the] eyes, nose, and throat and pulmonary discomfort were considered intolerable, and exposure was terminated almost at once.” The number of subjects exposed to 14 ppm and their individual sensory evaluations were not given.
Summer (1971) reported 6.2 ppm as the odor threshold for allylamine and 80 ppm as the concentration at which it becomes irritating to humans. It was not described in detail how these values were obtained (i.e. exposure duration, range of concentrations tested), although it appears that they may have been obtained by a panel of “sniffers,” and thus exposure was for a few seconds. These values are much higher than the concentration (2.5 ppm) found to be detected by all exposed human subjects (13/13) within 5 min, with some subjects even experiencing mild respiratory irritation (Hine et al. 1960). The reason for the discrepancy between the two studies is unknown; it may be due to the different exposure durations.
TABLE 1-3 Sensory Responses of Human Subjects to a 5-Min Allylamine Inhalation Exposurea
2.2.3.
Case Reports
Workers at a chemical manufacturing company exposed to mono-, di-, and tri-allylamine (simultaneously) occasionally reported symptoms, including tightness, congestion, and pain in the chest (especially on breathing or coughing), sore throat, runny nose, nausea, vomiting, red eyeballs, tightness in the jaw and behind the ears, and hurting teeth but had normal cardiac creatine phosphokinase levels and electrocardiograms (EKGs) (Shell Oil Co. 1992). The symptoms were ameliorated by drinking Coca-Cola. Neither the exposure time (typically several minutes) nor air allylamine concentration was measured. Plant supervisors and the company’s industrial hygienist stated that symptoms were reported only when spills and leaks occurred, and 18 of 22 questionnaires filled out by workers (September-October 1981) checked “yes” for a question asking, “Was there was a spill or other unusual exposure at the time symptoms began?” The four people who checked “no” had numerous exposures and did not identify discrete incidents. To test a new stationary air sampler, ambient samples (no spills, etc.) were collected in five areas of the chemical plant (in September 1981) where workers could be present for up to 4 h on a typical day. In April 1982, personal monitoring was conducted by the company industrial hygienist. Workers were not evaluated. The air concentration was <0.1 to 0.2 ppm for monoallylamine, <0.01 to 0.3 ppm for diallylamine, and <0.01 to 0.6 ppm for triallylamine, all potential sources of product line leaks and of a maintenance procedure requiring opening of a product line. All air samples were below the limit of quantitation (LOQ) (0.5 or 5 ppm) for all three amines. People working in these areas wore protective clothing and/or respirators and were not examined. It is unlikely that symptoms would have been experienced by workers in either monitoring situation since there were no unintentional spills or leaks.
Guzman et al. (1961) carefully examined operators working with allylamine and did not find any cardiovascular effects, despite occasional complaints of irritation of the mucous membranes, nausea, and disagreeable odor. No experimental details or quantitative results were provided.
2.2.4.
Accidents
During the course of an acute inhalation study in mice, leaks developed in the apparatus and the workers fixing the leaks were exposed to an unknown concentration of allylamine vapors (Hart 1939). [The mice were exposed to 24,437-31,035 ppm allylamine in a 5-L bell jar connected to a flowmeter, and airflow was 750 mL/min.] The vapors initially caused severe irritation of the mucous membranes of the nose, mouth, and eyes, which developed into an intense burning with lacrimation, coryza, and sneezing. The symptoms disappeared quickly (not specified) after exposure ceased.
2.3.
Neurotoxicity
No human neurotoxicity studies were located with allylamine exposure by any route.
2.4.
Developmental/Reproductive Toxicity
No studies on the developmental or reproductive effects of allylamine in humans were located.
2.5.
Genotoxicity
No studies on the genotoxicity of allylamine in humans were located.
2.6.
Carcinogenicity
No studies on the carcinogenicity of allylamine in humans were located (nor of its proposed metabolite, acrolein). Neither the U.S. Environmental Protection Agency (EPA) nor the International Agency for Research on Cancer (IARC) has classified allylamine as to its carcinogenic potential.
2.7.
Summary
No human data were located involving acute lethal exposure to allylamine. Sensory irritation was experienced by volunteers exposed to 2.5-10 ppm for 5 min, whereas exposure to about 14 ppm was immediately intolerable (Hine et al. 1960). There were several case reports and accidents involving occupational exposure to allylamine, in which workers experienced chest pain and respiratory irritation, although neither exposure durations nor concentrations were available. No studies were located describing developmental, reproductive, genotoxic, or carcinogenic effects in humans.
3.
ANIMAL TOXICITY DATA
3.1.
Acute Lethality
Lethality as a toxic end point was described in several rat and mouse acute inhalation exposure studies. Kulagina (1975) reported an LC50 of 320 mg/m3 (137 ppm) for mammals, although no further experimental details were provided.
3.1.1.
Rats
Hine et al. (1960) determined allylamine LC50 values using groups of five male Long-Evans (Princeton strain) rats exposed for 1, 4, or 8 h. Exposure to evaporated liquid allylamine was in a 19.5-L cylindrical glass chamber. Air concentrations of allylamine were calculated using the standard gas concentration formula of Jacobs (1949); the actual concentration of allylamine in the chamber was not measured. The rats were observed for signs of toxicity during the exposure and for the ensuing 10 days. The resulting mortality and LC50 values as given by Hine et al. for the rats are shown in Table 1-4. At all concentrations tested, allylamine was irritating to the mucous membranes of the eyes and respiratory tract (as indicated by face-washing motions) and the rats appeared “depressed.” At higher concentrations (not specified), there was lacrimation and nasal discharge, which became tinged with blood at the end of the exposure (exposure durations were not specified). Rats that died from allylamine exposure had stomachs distended with air, fluid-filled lungs with hemorrhage in the alveolar spaces, and pulmonary edema; those surviving the 10-day observation period had no notable gross or microscopic pathology.
Guzman et al. (1961) attempted to define the allylamine inhalation exposure required to produce heart lesions in male Long-Evans rats from a single exposure of allylamine. The allylamine concentrations tested were 20-100 ppm, and the exposure duration was 4-48 h (1-20 rats per exposure scenario; see Table 1-5). The rats (120-180 g) were killed periodically for histologic examination of the heart (i.e., 8 h to 14 days after the start of exposure). Heart lesions were found at most exposure concentrations, but in some cases lesions were not induced regardless of the exposure scenario. Rats exposed for ≤24 h generally had mild lesions. Two rats died spontaneously, one after 4 h of exposure to 100 ppm and one after 8 h of exposure to 40 ppm; only the latter had heart lesions (a fibrinoid degenerative thrombus, a vessel change, and diffuse cellular infiltrate). It is possible that lethality would have resulted in some of these cases were the animals not killed so quickly after cessation of exposure. The results are summarized in Table 1-5.
Guzman et al. (1961) also conducted a series of multiple-exposure allylamine inhalation studies to examine the cardiotoxic effects of prolonged exposure. Male Long-Evans rats (15/concentration) were given 50 7-h exposures to 0, 5, 10, 20, or 40 ppm (5 days/week; Hine et al. 1960; Guzman et al. 1961). Exposure to vaporized liquid allylamine was in 200-L stainless steel chambers. Air allylamine concentrations were sampled periodically, and allylamine was measured using a method designed to measure ammonia (Goldman and Jacobs 1953). No effects were seen in rats exposed to 5 ppm, with the exception of one rat that was considered an outlier (it had heart lesions, extensive abdominal tumors, hepatic abscess, and lung atelectasis). Rats exposed to 10 ppm and higher had lowered weight gain, which was correlated at 20 ppm with depletion of body fat. Rats exposed to 40 ppm became emaciated and had dull fur and,
TABLE 1-4 Lethality of Rats Exposed to Allylamine Vapor
|
Nominal Exposure Concentration (ppm) |
Hours of Exposure |
Mortality |
Time of Death (from Start of Exposure) |
Observations |
LC50 (ppm) |
LC01 (ppm)a |
|
1,000 |
1 |
1/5 |
4 h |
Eye and respiratory irritation, bloody nasal discharge, “depressed” appearance. Rats that died had stomachs distended with air and fluid-filled hemorrhagic lungs and pulmonary edema. |
|
|
|
1,500 |
|
1/5 |
4 h |
|
|
|
|
2,250 |
|
3/5 |
2-4 h |
|
|
|
|
3,380 |
|
5/5 |
2-4 h |
286 |
104 |
|
|
133 |
4 |
0/5 |
— |
|
|
|
|
200 |
|
0/5 |
— |
|
|
|
|
300 |
|
3/5 |
2-4 h |
|
|
|
|
450 |
|
5/5 |
2-4 h |
|
|
|
|
89 |
8 |
0/5 |
— |
177 |
69.2 |
|
|
133 |
|
0/5 |
— |
|
|
|
|
200 |
|
4/5 |
8-24 h |
|
|
|
|
300 |
|
5/5 |
8-24 h |
|
|
|
|
aCalculated by probit analysis from LC50 data. Source: Data from Hine et al. 1960. Reprinted with permission; copyright 1960, American Medical Association. |
||||||
enlarged hearts and 5/15 died (not reported how long after beginning of treatment). One-third of the 40-ppm rats had pneumonia with cyanosis, a thorax full of clear liquid, rust-colored lungs, and a small spleen. Liver weights were increased at all allylamine concentrations and kidney weight at 20 ppm; however, there was no relationship to concentration in either case. Lesions in the heart and blood vessels were induced in 1/9 animals examined that were exposed to 20 ppm and 8/8 at 40 ppm. The most common heart lesions were interstitial fibrosis with areas of necrosis, muscle bundle polymorphonuclear cell infiltration, and inflammation of the smaller blood vessels. This study is summarized with all other multiple-exposure inhalation animal studies (described in the following sections) in Table 1-6.
In an experiment to evaluate the effect of inhaled allylamine on EKGs, young adult Fischer 344 rats (5/sex) were exposed to 100 or 150 ppm for 6 h/day for 10 days over a 3-week-period (Lynch et al. 1983). Allylamine vapor was generated by metering liquid allylamine into the tangential air-feed manifold air stream at the top of the inhalation chamber; the concentration was monitored with an infrared analyzer. The EKGs were obtained prior to and on the day after the last allylamine exposure. Rats were killed and necropsied immediately following their EKGs; premature decedents were refrigerated and necropsied within 12 h of death if possible. During exposure, rats were seen sneezing and tearing, and they kept their eyes closed and their noses buried in their fur. Three of five males and three of five females in the 150-ppm group did not survive the 10-day exposure; all 100-ppm rats survived the 10-day treatment. Body weights
TABLE 1-5 Cardiotoxic Effects in Rats After a Single Allylamine Inhalation Exposure
|
Exposure Time (h) |
Concentration (ppm) |
Total No. of Rats Exposed |
No. of Rats Killed at Given Timea |
Lesion |
Histologic Heart Changes |
|
0 |
0 |
5 |
All at 14 days |
0 |
Occasional suggestive areas of round-cell infiltration |
|
4 |
100 |
8 |
2 at 4 days 1 death at 5 days 2 at 5 days 3 at 7 days |
0 0 ? + |
Slight perivascular edema; possible cellular infiltrate One definite myocardial lesion |
|
8 |
40 |
1 |
Died at 8 h |
+ |
Fibrinoid degenerative thrombus, one decisive vessel change, diffuse cellular infiltrate |
|
14 |
60 |
4 |
1 at 18 h |
+ |
Scattered myofibril fragments with loss of striation |
|
1 at 2 days |
+ + |
Scattered myofibril fragments with loss of striation |
|||
|
2 at 8 days |
|
Perivascular edema, cellular infiltration |
|||
|
16 |
40 |
20 |
11 at 8-17 h 4 at 7 days 5 at 14 days |
0 0 0 |
Occasional suggestive areas of round-cell infiltration, edema of some small vessel walls |
|
20 |
50 |
3 |
1 at <20 h 2 at 8 days |
+ + |
Scattered myofibril fragments with loss of striation Widespread endothelial lesion |
|
24 |
100/60b (6 h/18 h) |
3 |
2 at <24 h |
+ |
Scattered myofibril fragments with loss of striation, suggested perivascular lymphocytic cuffing Same, more pronounced |
|
1 at 2 days |
+ |
||||
|
32 |
40 |
5 |
All at 3 days |
+c |
Well-established heart lesions in 2/5 rats |
|
48 |
20 |
18 |
2 at 2 days 6 at 4 days 6 at 7 days 4 at 13 days |
+ + + 0 |
Several small areas of typical “infarcted cardiopathy” Several small areas of typical “infarcted cardiopathy” Several small areas of typical “infarcted cardiopathy” |
|
aCalculated from the beginning of the exposure period. All animals were killed except as noted. bExposure was to 100 ppm for 6 h followed by 60 ppm for 18 h. cThis field was left blank in the study report but should be as shown here. Source: Data from Guzman et al. 1961. Reprinted with permission; copyright 1961, American Medical Association. |
|||||
TABLE 1-6 Allylamine Multiple-Exposure Rat Lethality Studies
|
Animal |
Exposure Description |
Concentration (ppm) |
Effect |
Reference |
|
Long-Evans rats |
7 h/day for 50 days (5 days/week) |
5 |
No effect (one outlier) |
Hine et al. 1960; Guzman et al. 1961 |
|
10 |
Lower weight gain |
|||
|
20 |
Fat depletion; heart lesions (1/8) |
|||
|
40 |
Emaciation, heart lesions, pneumonia, 5/15 died (not specified when) |
|||
|
F-344 rats |
6 h/day for 10 days (5 days/week) |
100 |
Eye and nasal irritation, cardiotoxicity, lower body weight (bw) |
Lynch et al. 1983 |
|
150 |
Eye and nasal irritation, cardiotoxicity, lower bw, 6/10 died |
|||
|
F-344 rats |
6 h/day, 5 days/week for ≤24 weeks |
4 |
Lower bw starting at 2 weeks |
Lynch et al. 1989 |
|
Killed after 30, 60, 120 days |
40 |
Lower bw; higher heart/bw ratio after 120 days |
||
|
Killed after 30, 60, 120 days Killed after 30, 60, 90 days |
80 |
Lower bw; higher heart/bw ratio and cardiotoxicity after ≥30 days, 22/50 died |
were depressed for the 100- and 150-ppm animals, but they returned to control levels for the 100-ppm rats by the end of exposure. At both 100 and 150 ppm, heart weights and heart/body-weight ratios were increased and liver weights were decreased (statistical significance not specified), although the liver/body-weight ratios were unaffected. All 10 150-ppm rats had gross necrotic ventricular lesions. The EKGs of the survivors (while anesthetized) had an axis shift, complete inversion of the QRS complex, and attenuation of QRS amplitudes. The 100-ppm rats had less severe cardiac lesions and EKG changes.
In a follow-up study, Lynch et al. (1989) assessed cardiac toxicity in male and female F-344 rats (50 rats/group) exposed to 0, 4, or 40 ppm of allylamine for up to 24 weeks (Study I) and 0 or 80 ppm for up to 20 weeks (Study II). Exposure was for 6 h/day, 5 days/week. Rats were weighed every 2 weeks and were killed following 30, 60, or 120 days of exposure in Study I and 30, 60, or 90 days of exposure in Study II, at which time histopathology was performed. Clinical chemistry was evaluated at 30, 90, and 120 days and hematology and electrophysiology when the rats were put to death at 90 and 120 days. No treatment-related effects were seen in hematology or clinical chemistry parameters.
All treated animals had lowered body-weight gains throughout the study (values not given). The heart/body-weight ratio was increased in the 40-ppm rats after 120 days and in the 80-ppm rats at all time points but was accompanied by histopathologic changes (cardiac necrosis and fibrosis) in only the 80-ppm groups (all time points). Some of the 80-ppm rats (22/50) had moderate cardiac necrosis and died before being put to death, although the time of death was not given. Heart electrophysiology (PQ and QT intervals increased) was affected in only the 80-ppm males put to death at 90-days. This study is summarized in Table 1-6. (The description of this study was incomplete; only body weights were reported for 24 weeks for Group I and 20 weeks for Group II; other parameters were reported for only the 30-, 60-, 90-, and/or 120-day periods when rats were killed.)
3.1.2.
Mice
During a 10-min inhalation exposure to 1.27 mM/L allylamine (31,035 ppm), 28 of 30 white mice died; the two survivors died within 48 h (Hart 1939). Mice exposed to 1.10 or 1.00 mM/L (26,881 and 24,437 ppm) had mortality rates of 8/30 and 14/30, respectively, after the 10-min treatment; all survivors died by 48 h after exposure. Clinical signs, in order of appearance, were nasooral irritation, ear flushing, irregular respiration, cyanosis, delirium, convulsions, coma, and death. Exposures were in a 5-L bell jar connected to a flowmeter; a flow rate of 750 cc/min was maintained through the jar. No other details of the allylamine concentration analysis were given.
3.2.
Nonlethal Toxicity
Inhalation studies in which no lethality occurred were conducted using rats, mice, rabbits, and monkeys. The results are summarized in Table 1-7.
3.2.1.
Nonhuman Primates
Three male rhesus monkeys and one female (2.4-3.7 kg) were administered 73 exposures of 40 ppm allylamine for 4 h/day, 5 days/week, and were subsequently examined for cardiac effects (Guzman et al. 1961). Exposure was in a 200-L steel chamber into which allylamine was delivered by a constant-drive syringe and vaporized; the air allylamine concentrations were measured periodically. The airflow in the chamber was not described; however, in another study conducted by the same group in which a 200-L chamber was used, the dynamic airflow ranged from 10 to 15 L/min (Hine et al. 1960). It was not reported whether there were any control animals. EKGs were performed on the monkeys at the beginning and at the end of the experimental period. None of
TABLE 1-7 Multiple-Exposure Nonlethal Animal Studies
|
Animal |
Concentration (ppm) |
Exposure Description |
Effect |
Reference |
|
Long-Evans rats |
40 |
7 h/day for 10 days (5 day/week) |
Acute arteriole inflammation, focal muscle bundle necrosis, EKG changes. |
Guzman et al. 1961 |
|
7 h/day for 20 days |
As for 10 days but more severe; “healing” seen in some areas. |
|||
|
7 h/day for 40 days |
Fragmentation of muscle bundles, edematous arterioles (non-acute). |
|||
|
Long-Evans rats Rhesus monkeys Albino rabbits |
40 |
4 h/day for 73 days (5 day/week) |
No detectable (gross or microscopic) heart lesions |
Guzman et al. 1961 |
|
Swiss mice |
27 |
6 h/day for 4-14 days |
No histologic changes in nose, trachea, lungs |
Zissu 1995 |
the animals died prematurely, and there were no gross or microscopic heart lesions, alterations in heart/body-weight ratios, or changes in EKGs after the 73 exposures.
3.2.2.
Rats
Guzman et al. (1961) conducted an extensive series of both single-exposure and multiple-exposure studies to define the conditions that cause cardiotoxicity in male Long-Evans rats. In the single-exposure study, rats received 20-100 ppm of allylamine for 4-48 h. In the multiple-exposure study the rats received 50 (5 days/week) 7-h exposures to 5-40 ppm allylamine. Since mortality occurred at the highest doses tested in each study, they are described in Section 3.1.1 and summarized in Table 1-5. Guzman et al. also conducted two multiple-exposure experiments in which mortality did not occur; these are described below.
To determine the rate of induction of heart lesions, Guzman et al. subjected groups of 9-11 male Long-Evans rats to 10, 20, or 40 7-h exposures to 40 ppm allylamine (one rat had 17 exposures). Rats were killed immediately after the final exposure for gross and microscopic examination. EKGs were performed on the rats at the beginning and at the end of the experimental period. After 10 exposures, pathologic changes seen in the heart varied from inflammation of the smaller arteriole walls to focal necrosis of large areas of muscle bun-
dles. Similar but more severe changes occurred after 20 exposures, and “stages of healing” were seen in some areas. Heart lesions seen after 40 exposures appeared different: They were not “acute” and consisted of fragmentation of muscle bundles with replacement by loose edematous fibrous tissue, as well as edema in the arteriole outer coat. Abnormal EKGs (mainly elevation of the ST segment) were seen in some of the rats with heart lesions.
In another experiment, Guzman et al. examined species, differences in susceptibility to the cardiotoxic effect of allylamine. Rats (10 males) were exposed to 40 ppm of allylamine for 4 h/day for 73 days (5 days/week). Rabbits and monkeys were similarly treated, as described in the following sections. The prolonged treatment resulted in no detectable gross or microscopic heart lesions or deaths in the rats; other effects (such as irritation) were not addressed.
In an inhalation exposure study for which there were incomplete experimental data (e.g., number of animals exposed, exposure duration labeled as “variable,” individual animal results; Research Pathology Associates 1984), F-344 rats exposed to 100 or 150 ppm of allylamine for 5 or 10 days (hours/day not specified) had moderate myocardial necrosis. In another part of the study, nearly all rats of both sexes exposed to 80 ppm for 30, 60, 90, or a “variable” number of days had slight or moderate myocardial necrosis and fibrosis. Thymic atrophy and hepatocellular necrosis were found in two to four animals/sex in the “variable” -days exposure group, although no controls were available for comparison of incidences.
3.2.3.
Mice
The RD50 (i.e., concentration of allylamine causing a 50% decrease in the breathing rate; Gagnaire et al. 1989) of male OF1 Swiss mice was determined to be 9 ppm in two oronasal exposure studies. In one study, exposure was for a total of 15 min to 3-12 ppm (Gagnaire et al. 1989), and in the other total exposure was for 60 min to 6.3, 16.4, 23.6, or 43.3 ppm (Gagnaire et al. 1993). Mice were exposed by enclosing the head in a 200-L stainless steel exposure chamber into which allylamine vapor was delivered by bubbling air through the liquid amine. The effect on breathing rate was maximal 10-15 min after exposure in both studies; recovery after the 15-min exposure occurred within 1 min; recovery after the 60-min exposure was slower.
Pulmonary toxicity was analogously assessed in anesthetized, tracheally cannulated (TC) mice exposed to 38, 59, 79, or 205 ppm of allylamine for 120 min and an RD50TC of 157 ppm was determined (RD50TC is the concentration of allylamine causing a 50% decrease in the breathing rate of tracheally cannulated mice; Gagnaire et al. 1993). The maximal decrease in the breathing rate was seen after 15-60 min of exposure; recovery after the 120-min exposure was incomplete at 79 and 205 ppm during the ensuing 30-min observation period. The higher value of the RD50TC compared to the RD50 indicated that the respira-
tory toxicity of allylamine was primarily related to its upper-airway irritant effects.
Zissu (1995) exposed male OF1 Swiss mice by inhalation of 27 ppm allylamine (target concentration based on 3 times the RD50) for 6 h/day for 4, 9, or 14 days. No mortality occurred and no histologic lesions were seen in any part of the nasal passages (respiratory or olfactory epithelium) or in the trachea or lungs. Zissu concluded that respiratory histopathologic changes were not a function of sensory irritation for allylamine.
3.2.4.
Rabbits
No gross or microscopic heart lesions were detected in five male albino rabbits exposed 73 times to 40 ppm allylamine for 4 h/day (5 days/week; there were three controls; Guzman et al. 1961). The animals’ heart/body-weight ratios were normal, there were no mortalities, and no clinical observations (e.g., irritation) were reported. Exposure was in a 200-L steel chamber into which allylamine was delivered by a constant-drive syringe and vaporized; the chamber concentrations were measured periodically.
The method of Draize was used to evaluate the degree of eye irritation in the rabbits’ eyes to which 0.05 mL of undiluted compound (38 mg) was applied, followed by a 20-second (s) wash with distilled water (Hine et al. 1960). Readings were made after 1, 24, 48, and 72 h; allylamine proved to be too irritating to the rabbits’ eyes to permit differential measurements.
3.3.
Neurotoxicity
No studies were located that assessed the neurotoxicity of allylamine exposure on animals.
3.4.
Developmental/Reproductive Toxicity
No studies were located that assessed in vivo effects of allylamine exposure on animals. The embryotoxic potential of allylamine was estimated using the in vitro Chick Embryotoxicity Screening Test (CHEST) and fertilized eggs from White Leghorn fowl (Jelinek et al. 1985). The beginning of the embryotoxicity range on day 1.5 (defined as a shortening of the embryo trunk length after a 24-h exposure) was between 3 and 30 µg/embryo (i.e., highest ineffective concentration to lowest effective concentration). Application of 3-30 µg allylamine to 2- to 4-day-old embryos until day 8 did not result in body malformations; the mortality rate was 48%.
3.5.
Genotoxicity
Allylamine was not mutagenic in the Salmonella/microsome preincubation assay when tested at concentrations of 0, 1, 3, 10, 33, 100, 333, 1,000, or 3,333 µg/plate using strains TA98, TA100, TA1535, and TA1537. Testing was in the presence or absence of Aroclor-induced rat or hamster liver S9 (Zeiger et al. 1987). Negative results were also obtained by Lijinsky and Andrews (1980) with the Salmonella/microsome preincubation assay using strains TA98, TA100, TA1535, TA1537, and TA1538 (1-1,000 µg/plate) and by McMahon et al. (1979) using 10 Salmonella and E. coli tester strains and agar plates with gradients of 0.1-1,000 µg/mL allylamine, with or without Aroclor-induced rat or hamster liver S9.
3.6.
Carcinogenicity
No studies on the carcinogenicity of allylamine in animals were located. Neither EPA nor IARC has classified allylamine as to carcinogenicity. The allylamine metabolite acrolein EPA weight-of-evidence characterization, under the 1999 Draft Revised Guidelines for Carcinogen Risk Assessment, is that the potential carcinogenicity of acrolein cannot be determined because the existing “data are inadequate for an assessment of human carcinogenic potential for either the oral or inhalation route of exposure” (EPA 2004).
3.7.
Summary
Allylamine inhalation caused cardiotoxicity in rats in several single- and multiple-exposure studies, which, unfortunately, did not record any accompanying sensory irritation. Cardiovascular lesions were not found following inhalation exposure in species other than rats but were induced in a variety of animal species by the oral, parenteral, inhalation, and intravenous routes (Boor et al. 1979; Boor and Hysmith 1987). The single-exposure data from Guzman et al. (1961) suggest that exposure concentration is a relatively greater factor than time in inducing cardiotoxicity. For example, 0/20 rats developed lesions from exposure to 40 ppm for 16 h, but 4/4 had cardiac lesions from exposure to 60 ppm for 14 h. Consistent with this result, the concentration-time relationship as defined by the ten Berge et al. (1986) equation Cn × t = k yields 1.7 as the exponent n using this study’s data.
No in vivo developmental, reproductive, or carcinogenicity studies were located. Allylamine was not mutagenic in any conducted Salmonella/microsome preincubation assays.
4.
SPECIAL CONSIDERATIONS
4.1.
Metabolism and Disposition
No information was located regarding allylamine metabolism after inhalation exposure, but some animal toxicokinetic data were found for oral and intravenous administration.
In a toxicokinetics study conducted by Boor (1985), allylamine was absorbed by male Sprague-Dawley rats within minutes of gavage administration of 150 mg/kg radiolabeled allylamine. Radioactivity was found in numerous organs, the greatest amount being in the aorta and coronary arteries, where levels were about 5- to 10-fold greater than in most other organs, including the myocardium. A fraction (30-40%) of the animals, however, had counts in the aorta that were 10- to 20-fold lower than those of the majority of the animals at all time points. The liver and kidney had the next highest amounts of radioactivity. Radioactivity was quickly eliminated: Liver counts dropped to low levels by 45 min and the half-life was ≤1 h in other organs, including the adrenals, aorta, coronaries, heart, kidneys, and lung. Half-lives were not determined for some organs due to low and irregular levels of label in the postabsorptive phase (brain, blood, liver, pancreas, skeletal muscle, spleen, and fat). About 60% of the given radioactivity was excreted in the urine by 24 h, after which time there was little additional excretion, possibly due to retention in blood and other organs. No radioactivity was found in the feces at any time (up to 96 h after gavage).
Orally administered allylamine was shown to be metabolized to acrolein and hydrogen peroxide, which may both be responsible for the observed toxic effects (Boor et al. 1987). The metabolite acrolein has been detected in both rat and human aorta, myocardium, and liver homogenates incubated with allylamine (Boor and Nelson 1982). The acrolein is believed to be subsequently conjugated with glutathione to form 3-hydroxypropylmercapturic acid, which was the sole metabolite in the urine of Sprague-Dawley rats collected 24 and 48 h after gavage with 150 mg/kg radiolabeled allylamine (2 µCi/kg 14C-labeled; Boor et al. 1987). Male Sprague-Dawley rats given 5-150 mg/kg of allylamine by gavage excreted 44-48% of the given dose as 3-hydroxypropylmercapturic acid over 0-24 h, and only 3% during 24-48 h, whereas 75% of a given dose of acrolein (13 mg/kg) was metabolized to 3-hydroxypropylmercapturic acid after 24 h (Sanduja et al. 1989). Consistent with glutathione involvement in allylamine metabolism, a depletion of reduced glutathione in the aorta, blood, and lungs was shown to occur and be maximal 1-6 h after gavage treatment with allylamine (Awasthi and Boor 1994).
It is proposed that the metabolism of allylamine to acrolein and hydrogen peroxide occurs via benzylamine oxidase, which is a form of amine oxidase with high activity in vascular tissue, especially the aorta (Boor et al. 1987). Consistent with this, the benzylamine oxidase inhibitor, semicarbazide, protected myocytes from toxic effects of allylamine in vitro, whereas the monoamine oxidase inhibitors clorgyline and pargyline were ineffective (Ramos et al. 1988).
4.2.
Mechanism of Toxicity
Allylamine has been shown to cause severe myocardial damage and vascular smooth muscle lesions in a variety of animal species upon acute exposure (Boor and Hysmith 1987). It has been used to cause lesions (proliferation of smooth muscle cells and fibrosis) that mimic human atherosclerosis by the oral, parenteral, inhalation, and intravenous routes (Boor et al. 1979; Boor and Hysmith 1987). Allylamine cardiovascular toxicity was shown in many mammalian species to be dependent on metabolism of allylamine by semicarbazide-sensitive amine oxidase (SSAO) to acrolein, hydrogen peroxide, and ammonia (Lyles 1996). SSAO is found in many tissues in mammals. Its activity in human and rat tissue homogenates was shown to be the highest in the aorta, followed by the lungs and digestive system, but very little activity was found in cardiac endothelial cells or myocytes (Lewinsohn et al. 1978; Lyles 1996). (SSAO activity was measured as nanomoles of benzylamine hydrochloride metabolized/milligrams of protein/30 min.)
Mechanisms proposed for allylamine-induced cardiovascular toxicity implicate the metabolite acrolein as the major toxicant. One mechanism proposes that the cardiac and vascular damage is caused by lipid peroxidation by acrolein, modulation of the cellular glutathione status, and damage of the mitochondrial membranes by acrolein (or another unknown metabolite) and hydrogen peroxide (Awasthi and Boor 1994; Ramos et al. 1994). Consistent with mitochondrial membrane injury, 20 min after intravenous injection of allylamine, aortic mitochondrial malate dehydrogenase activity decreased, whereas cytosolic malate dehydrogenase activity increased (Hysmith and Boor 1985). Examination of Sprague-Dawley rats 1, 3, and 5 h after gavage with 150 mg of allylamine/kg showed a marked depletion in free-sulfhydryl (SH) content in the aorta, epicardium, and endocardium; a marked increase in the formation of thiobarbiturate-reactive substance by aortic mitochondria; and increased capacity to generate hydroxyl radicals (deoxyribose degradation method) in the aorta (Awasthi and Boor 1994).
More recently, Conklin et al. (2001) proposed a two-step model for allylamine-induced cardiotoxicity: (1) metabolism of allylamine by SSAO to acrolein, hydrogen peroxide, and ammonia and (2) injury of the coronary artery vascular smooth muscle cells by acrolein and possibly the other metabolites, which causes its hypercontraction and vasospasm and results in ischemia and subendocardial necrosis. This model was supported by a study in which isolated rings of rat coronary artery and thoracic aorta incubated with 100-1,000 µM allylamine or acrolein exhibited increased basal tension and vasospasm (increased contraction and slow-wave vasomotion) and irreversibly inhibited vessel contractility (Conklin et al. 2001). There was no effect, however, on endothelium-dependent acetylcholine-induced relaxation of either vessel. Pretreatment with the SSAO inhibitor semicarbazide reduced or eliminated most effects in both tissues. Effects for the two compounds were similar, with some qualitative and quantitative differences. Conklin et al. also showed that SSAO activity was
comparable in human homogenized coronary arteries and aorta, and both were inhibited by semicarbazide.
4.3.
Structure-Activity Relationships
The structurally related secondary amines di(β-methyl-allyl)amine, diallylamine, and β-methylallylamine caused a greater incidence of mortality than allylamine (on a molar basis) during a 10-min exposure of white mice (Hart 1939). The mice exhibited the same symptoms as those produced by allylamine during the 10 min (e.g., nasooral irritation followed by vasodilation of the extremities, arrhythmic respiration, cyanosis, convulsions, coma, and death). Inhalation of allyl chloride and allyl alcohol generally appeared to have effects similar to that of allylamine.
A comparison of the LC50 values derived by Hine et al. (1960) indicated that allylamine was about twice as toxic as triallylamine and about 10 times as toxic as diallylamine. Saturation of the double bond decreased toxicity, as n-propylamine was considerably less toxic in both acute and chronic vapor exposure than allylamine.
The concentration of allylamine that lowered the breathing rate of male OF1 Swiss mice by 50% after 15 min of exposure (the RD50),—9 ppm—was comparable to the RD50 values of other allylic respiratory tract irritants: diallylamine (4 ppm), allyl glycidyl ether (5.7 ppm), allyl acetate (2.9 ppm), allyl alcohol (3.9 ppm), allyl ether (5 ppm), and acrolein (2.9 ppm), although it was much lower than that of other nonallylic amines (RD50 values of 51-202 ppm; Gagnaire et al. 1989).
4.4.
Other Relevant Information
4.4.1.
Species Variability
Allylamine has been used experimentally to induce and study cardiovascular lesions in rats, dogs, calves, monkeys, and rabbits when administered intravenously, intradermally, orally, and/or by intraarterial injection (Boor et al. 1979; Boor and Hysmith 1987). The lesions were shown to be dependent on the metabolism of allylamine by SSAO, of which tissue levels were greatest in the aorta and coronary arteries (Lewinsohn et al. 1978; Conklin et al. 2001; Boor and Hysmith 1987; Lyles 1996). SSAO specificity for allylamine as a substrate has not been determined, and Lyles (1996) has shown that substrate specificity of plasma and tissue SSAO varied considerably among species for a number of aromatic and aliphatic amines. Inhalation exposure was also capable of inducing cardiovascular lesions, although this was shown only in rats. The data thus suggest that a similar mechanism is responsible for cardiovascular injury in many
mammalian species (also humans), but the susceptibility of different species by the inhalation route is unknown.
Data were scant regarding species variability from acute lethal exposures. Although the oral lethal dose in 50% (LD50) of the rats and mice was within a factor of 2 (Boor and Hysmith 1987), LC50 was determined only for rats.
4.4.2.
Susceptible Populations
Two populations exist that may be susceptible to allylamine toxicity due to their increased levels of plasma SSAO activity. This enzyme metabolizes allylamine to acrolein, hydrogen peroxide, and ammonia, which was shown to be a key step in allylamine-induced cardiovascular damage in several animal studies. Elevated levels of SSAO were found in patients with insulin-dependent diabetes mellitus (Boomsma et al. 1995) and congestive heart failure (Boomsma et al. 1997). SSAO levels increased with the severity of the disease, and patients with both maladies had higher SSAO levels than those with either malady alone. Since endothelial dysfunction is present in both diseases, Boomsma et al. speculated that SSAO may be involved in the pathogenesis of vascular endothelial damage.
4.4.3.
Concentration-Exposure Duration Relationship
The AEGL-1 was based on mild irritation experienced by human volunteers from exposure to 2.5 ppm of allylamine for 5 min (Hine et al. 1960). No concentration-time scaling was performed because mild irritant effects do not generally vary greatly with time and the same AEGL-1 value was used for 10 min to 8 h.
The 10, 30, and 60-min AEGL-2 values were based on human exposure to 10 ppm for 5 min, which caused slight or moderate eye and nose irritation, pulmonary discomfort, and severe olfactory cognition and which was the NOAEL for “extreme or intolerable” irritation (Hine et al. 1960). The same AEGL-2 value was adopted for 10-60 min because the degree of irritation from exposure for 5 min was not expected to increase over a 1-h period beyond the scope of AEGL-2. The 4- and 8-h AEGL-2 values were derived from the Guzman et al. (1961) rat cardiotoxicity study, which was also used to determine the exponent n = 1.7 in ten Berge et al. (1986) concentration-time relationship equation Cn × t = k. In the Guzman et al. study, male Long-Evans rats were exposed to 20-100 ppm of allylamine for 4-48 h (shown in Table 1-5), and the responses designated as “+” for histologic heart changes were used in the regression analysis to obtain n. An outlier that died after an 8-h exposure to 40 ppm was excluded (a very similar value is obtained for n if this outlier is included, although R2 decreases from 0.89 to 0.86). The regression output and graph obtained from the Guzman et al. data are shown in Appendix B.
The Guzman et al. rat study would have yielded 4.1 ppm for the 1-h AEGL-2. The same value would be appropriate for 10 and 30 min because scaling from 16 h to <1 h involves unacceptably high inherent uncertainty (analogous to scaling from ≥4 h to 10 min) and the 1-h value would be adopted to be protective of human health. However, the human irritation study (Hine et al. 1960) yielded AEGL-2 values of 3.3 ppm for 10-60 min, providing a more sensitive end point for this time period than the rat cardiotoxicity study.
For allylamine AEGL-3 derivation, the exponent n was determined by regression analysis to be 0.85 (rounded from 0.8458), based on the rat LC50 study of Hine et al. (1960; shown in Table 1-4). In this study, male Long-Evans rats were exposed for 1, 4, or 8 h, each time to four different concentrations of allylamine (air allylamine concentrations were calculated and not measured). Rats that died from exposure had stomachs distended with air, fluid-filled lungs, alveolar hemorrhage, and pulmonary edema. The regression output graph obtained from the Hine et al. data is shown in Appendix B.
4.4.4.
Concurrent Exposure Issues
Inhalation exposure (whole-body) can also result in inadvertent exposure of the skin, which can potentially add significantly to allylamine toxicity. Several studies indicate that allylamine is absorbed through the skin and can cause acute lethality. Application of 0.05 or 0.10 mL allylamine (39 and 76 mg, respectively, based on a density of 0.76 g/mL) on a 1-cm2 piece of toweling to the shaved abdominal skin of albino rats caused skin necrosis and death after 5-18 h (animal weight not given; Hart 1939). Application of 0.025 mL (19 mg) of allylamine caused slight skin irritation. In a skin corrosivity study conducted by Springborn Life Sciences (1989), one of three New Zealand female albino rabbits died from 3 min of skin exposure to 0.5 mL (about 176 mg/kg) allylamine applied on a gauze patch. The animal died between 1 and 24 h after exposure and had dark red lungs on necropsy. The two surviving rabbits had labored breathing and decreased activity as well as edema and hair loss directly below the area of the test article application. All three rabbits had skin corrosion (necrosis) immediately after the 3-min exposure, and eschar formation was evident from 24 h through the 7-day observation period.
Hine et al. (1960) determined a dermal LD50 of 35 mg/kg from an occluded exposure of New Zealand male rabbits for several hours to 13, 25, 50, or 100 mg of allylamine/kg (using undiluted allylamine, 0.76 g/mL). No deaths resulted from the application of 13 or 25 mg/kg of allylamine (observation period unclear, perhaps several weeks); 3/3 rabbits died 3-24 h after treatment with 50 mg allylamine/kg, and 3/3 died 3-4 h after application of 100 mg. There was considerable local erythema and eschar formation at the application site. Rabbits that died usually had fluid in the pleural cavity and dilation of the gastroenteric veins. All had severely congested lungs. Liver lesions were seen in one rabbit that died 8 h after exposure and in one rabbit killed 10 days after exposure.
5.
DATA ANALYSIS FOR AEGL-1
5.1.
Summary of Human Data Relevant to AEGL-1
Two human studies were considered potentially useful for AEGL-1 derivation. Hine et al. (1960) conducted a quantitative experiment in which 35 volunteers were exposed to 2.5, 5, or 10 ppm of allylamine for 5 min and to 14 ppm for <1 min. All subjects detected the odor, and there were dose-related increases in the incidence of slight or moderate eye irritation (21%, 15%, 50%), nose irritation (50%, 54%, 100%), and pulmonary discomfort (29%, 46%, 50%) at 2.5, 5, and 10 ppm, respectively. Those exposed briefly to 14 ppm reported it to be intolerable. CNS effects (i.e., slight headache and nausea) were reported but were not dose related. In the other human study, stationary air samplings at various areas of a chemical manufacturing plant showed ambient concentrations of allylamine ranging from <0.1 to 0.2 ppm, as well as di- and tri-allylamine at roughly similar air concentrations (each <0.1 to 0.6 ppm; Shell Oil Co. 1992). Worker exposure to these concentrations was for up to 4 h/day over an undefined period of days/years, but workers were not examined when these air concentrations were measured. Workers experienced chest tightness/congestion/pain, sore throat, runny nose, nausea, vomiting, red eyeballs, tightness in the jaw and behind the ears, and hurting teeth only when spills or leaks occurred, at which time allylamine concentrations were not measured.
5.2.
Summary of Animal Data Relevant to AEGL-1
There were no short-term animal studies appropriate for an AEGL-1 determination. In many studies the focus was on a cardiotoxicity end point, and observations regarding irritation or other reversible effects were not reported (e.g., Guzman et al. 1961; see Section 6.2).
5.3.
Derivation of AEGL-1
AEGL-1 values were based on the Hine et al. (1960) a study in which 35 young adult human volunteers were exposed for 5 min to 2.5, 5, or 10 ppm of allylamine, which caused dose-related increases in the incidence of slight or moderate eye irritation, nose irritation, and pulmonary discomfort but not CNS effects. The same AEGL-1 value was used for 10 min to 8 h because mild sensory irritation or discomfort does not generally vary greatly with time. The AEGL-1 point of departure was 1.25 ppm, which was obtained by applying a modifying factor of 2 to 2.5 ppm, which was the lowest effect level. The MF was used because exposure was for only 5 min and it is unclear whether “moderate” irritation or discomfort is comparable to “notable” irritation or discomfort, which exceeds the scope of AEGL-1. An intraspecies uncertainty factor of
3 was applied because allylamine acts as a contact irritant, and the severity of its effects is not expected to vary greatly among humans. Also, use of a greater uncertainty factor would yield a concentration below 0.2 ppm, which was a no-effect level for workers exposed for up to 4 h (Shell Oil Co. 1992). The derived AEGL-1 value of 0.42 ppm for 10 min to 8 h is also consistent with two mouse respiratory irritation studies (Gagnaire et al. 1989, 1993), from which it is predicted that exposure for a few hours to 0.9 ppm would cause sensory irritation in humans but that 0.09 ppm would not (Alarie 1981). According to Alarie, exposure to 0.1 of the RD50 (i.e., 0.9 ppm) for several hours to days should result in some sensory irritation in humans, whereas 0.01 × RD50 (0.09 ppm) should cause no sensory irritation. The results are summarized in Table 1-8.
6.
DATA ANALYSIS FOR AEGL-2
6.1.
Summary of Human Data Relevant to AEGL-2
The only human study useful for AEGL-2 derivation is that of Hine et al. (1960), which was used to derive AEGL-1 values. Human volunteers exposed for 5 min to 2.5-10 ppm allylamine had slight to moderate eye and nose irritation and pulmonary discomfort, whereas at the next higher concentration tested, ~14 ppm, exposure was “intolerable” (extreme pulmonary discomfort and irritation of the eyes, nose, and throat) and was almost immediately terminated.
6.2.
Summary of Animal Data Relevant to AEGL-2
The only relevant single-exposure study evaluated male Long-Evans rats exposed to 20-100 ppm for 4-48 h and examined histologically 8 h to 14 days after the start of exposure (Guzman et al. 1961). Heart lesions included scattered myofibril fragments with loss of striation, perivascular edema, and cellular infiltration.
AEGL-2 values can also be derived from two multiple-exposure rat studies conducted by Guzman et al. (1961), using a single exposure as a conservative estimate of exposure duration. Male Long-Evans rats given 50 7-h exposures to 0, 5, 10, 20, or 40 ppm of allylamine (5 days/week) had no effects at 5 ppm; lowered weight gains at 10 ppm; heart and blood vessel lesions and depletion of body fat at 20 ppm; and cardiotoxicity, lung congestion, emaciation, and
TABLE 1-8 AEGL-1 Values for Allylamine
|
10 min |
30 min |
1 h |
4 h |
8 h |
|
0.42 ppm (0.98 mg/m3) |
0.42 ppm (0.98 mg/m3) |
0.42 ppm (0.98 mg/m3) |
0.42 ppm (0.98 mg/m3) |
0.42 ppm (0.98 mg/m3) |
5/15 mortality at 40 ppm (Hine et al. 1960; Guzman et al. 1961). AEGL-2 values could be derived using a single exposure to 10 ppm (20 ppm would be a NOEL for lethality). In a second experiment, rats were examined after 10, 20, or 40 7-h exposures to 40 ppm, revealing extensive heart and blood vessel histologic changes and altered EKGs after 10 exposures (Guzman et al. 1961); a single 7-h exposure to 40 ppm could be used to derive AEGL-2 values.
6.3.
Derivation of AEGL-2
AEGL-2 values were based on two studies: the 10-, 30-, and 60-min values were based on sensory irritation in human volunteers (Hine et al. 1960), and the 4- and 8-h values were based on cardiotoxicity in rats (Guzman et al. 1961) because cardiotoxicity was a more sensitive end point than sensory irritation when exposure was for 4 or 8 h.
The 10-, 30-, and 60-min AEGLs were developed from the Hine et al. human 5-min exposure study that was used to derive AEGL-1 values, but using 10 ppm as the point of departure. Ten ppm caused slight or moderate eye and nose irritation and pulmonary discomfort and was the NOAEL for “intolerable” irritation that occurred at 14 ppm. The same value was adopted for 10-60 min because the degree of irritation or discomfort resulting from exposure to 10 ppm was not expected to increase over a 1-h period beyond the scope of AEGL-2. An intraspecies uncertainty factor of 3 was used because allylamine is acting as a contact irritant, and the severity of its effects is not expected to vary greatly among humans. The resulting AEGL-2 values of 3.3 ppm were not adopted for 4 or 8 h, however, because a rat study (Guzman et al. 1961) indicated that exposure to 3.3 ppm for 4 or 8 h may cause cardiotoxicity. In the latter study, exposure to 40 ppm for 16 h was a NOAEL for cardiovascular lesions, which were seen from exposure to 60 ppm for 14 h (myofibril fragment damage, perivascular edema, and cellular infiltration). Time-concentration scaling was performed using the ten Berge et al. (1986) equation Cn × t = k, where n = 1.7 was calculated from a linear regression of the Guzman et al. (1961) rat cardiotoxicity data. An interspecies uncertainty factor of 5 was applied because the mechanism of toxicity is similar among several mammalian species (and humans), but differences in susceptibility are unknown, and an uncertainty factor of 3 yields values approaching the NOEL for lethality from pulmonary lesions for a 4- or an 8-h exposure. An intraspecies uncertainty factor of 10 was used because the variability of the cardiotoxic response to allylamine among humans is undefined, and potentially sensitive populations exist (diabetics, persons with congestive heart failure). This yields 4- and 8-h AEGLs of 1.8 and 1.2 ppm, respectively, indicating that for these longer exposure durations, cardiotoxicity is a more sensitive end point than eye and respiratory irritation. The AEGL-2 values for allylamine are summarized in Table 1-9; the calculations are detailed in Appendix A.
TABLE 1-9 AEGL-2 Values for Allylamine
|
10 min |
30 min |
1 h |
4 h |
8 h |
|
3.3 ppm (7.7 mg/m3) |
3.3 ppm (7.7 mg/m3) |
3.3 ppm (7.7 mg/m3) |
1.8 ppm (4.2 mg/m3) |
1.2 ppm (2.8 mg/m3) |
7.
DATA ANALYSIS FOR AEGL-3
7.1.
Summary of Human Data Relevant to AEGL-3
Although it was noted that acute allylamine exposure may lead to death (HSDB 2003), no quantitative human data were located for AEGL-3 derivation.
7.2.
Summary of Animal Data Relevant to AEGL-3
There were two single-exposure rat studies and one multiple-exposure rat study that were considered for AEGL-3 derivation. In one single-exposure study, Long-Evans rats were exposed for 1, 4, or 8 h to determine the LC50 values for allylamine (Hine et al. 1960). Rats dying from exposure had stomachs distended with air, fluid-filled lungs, alveolar hemorrhage, and pulmonary edema. In a single-exposure study conducted by Guzman et al. (1961), male Long-Evans rats were administered 20-100 ppm for 4-48 h, and were examined histologically 8 h to 14 days after the start of dosing. One rat (of eight tested) died after exposure to 100 ppm for 4 h and had cardiovascular lesions.
In a multiple-exposure study conducted by Lynch et al. (1983), Fischer 344 rats (5/sex) exposed to 100 or 150 ppm of allylamine for 6 h/day for 10 days all survived exposure to 100 ppm, but 3/5 males and 3/5 females died at 150 ppm. Both dose groups had increased heart weights and heart lesions, and the 150-ppm rats had altered EKGs; a single 6-h exposure to 100 ppm could be considered a NOEL for lethality.
7.3.
Derivation of AEGL-3
The Hine et al. (1960) rat LC50 study was used to derive AEGL-3 values. Rats that died had stomachs distended with air, fluid-filled lungs, alveolar hemorrhage, and pulmonary edema. The 1-h, 4-h, and 8-h AEGLs were obtained directly from the 1-h, 4-h, and 8-h LC01 values (533.2, 104.1, and 69.2 ppm, respectively) calculated by probit analysis from the mortality data. The 10-min and 30-min AEGLs were derived from the 1-h LC01 using the relationship Cn × t = k, where n = 0.85 was calculated from this study LC50 data. An uncertainty factor of 30 was applied: 10 to account for interspecies variability (lack of acute toxicity studies from other species with AEGL-3 level end points) and 3 for hu-
man variability [the steep dose-response (~2-fold increase in concentration caused mortality to increase from 0 to 100%) indicates that the NOEL for lethality due to direct destruction of lung tissue is not likely to vary greatly among humans]. The Hine et al. study had a high level of confidence because all of the three LC01 values were obtained from the mortality data of 20 animals (four concentrations for each exposure time, five rats at each concentration), and extrapolation was only required for the 10- and 30-min time points. The derived AEGL-3 values are shown in Table 1-10; calculations are detailed in Appendix A.
8.
SUMMARY OF AEGLs
8.1.
AEGL Values and Toxicity End Points
A summary of the AEGL values for allylamine and their relationship to one another is shown in Table 1-11. Extrapolation across time was performed by exponential scaling (ten Berge et al. 1986) using the relationship Cn × t = k, where n = 1.7 for 4- and 8-h AEGL-2 derivations and n = 0.85 was used for AEGL-3 derivation. Concentration-time scaling was not performed for developing AEGL-1 values or the 10 to 60 min AEGL-2 values.
AEGL-1 values were based on a study in which 35 young adult human volunteers were exposed for 5 min to 2.5, 5, or 10 ppm of allylamine (Hine et al. 1960). A group was also exposed briefly to 14 ppm, which was reported as intolerable, and exposure was almost immediately terminated. All subjects detected the odor of allylamine, and there were dose-related increases in the incidence of slight or moderate eye irritation (21%, 15%, 50%), nose irritation
TABLE 1-10 AEGL-3 Values for Allylamine
|
10 min |
30 min |
1 h |
4 h |
8 h |
|
150 ppm (350 mg/m3) |
40 ppm (93 mg/m3) |
18 ppm (42 mg/m3) |
3.5 ppm (8.2 mg/m3) |
2.3 ppm (5.4 mg/m3) |
TABLE 1-11 Summary of AEGL Values for Allylamine
|
Classification |
10 min |
30 min |
1 h |
4 h |
8 h |
|
AEGL-1 (nondisabling) |
0.42 ppm (0.98 mg/m3) |
0.42 ppm (0.98 mg/m3) |
0.42 ppm (0.98 mg/m3) |
0.42 ppm (0.98 mg/m3) |
0.42 ppm (0.98 mg/m3) |
|
AEGL-2 (disabling) |
3.3 ppm (7.7 mg/m3) |
3.3 ppm (7.7 mg/m3) |
3.3 ppm (7.7 mg/m3) |
1.8 ppm (4.2 mg/m3) |
1.2 ppm (2.8 mg/m3) |
|
AEGL-3 (lethal) |
150 ppm (350 mg/m3) |
40 ppm (93 mg/m3) |
18 ppm (42 mg/m3) |
3.5 ppm (8.2 mg/m3) |
2.3 ppm (5.4 mg/m3) |
(50%, 54%, 100%), and pulmonary discomfort (29%, 46%, 50%) at 2.5, 5, and 10 ppm, respectively. The incidence of CNS effects was not dose related. The same AEGL-1 value was used for 10 min to 8 h because mild sensory irritation or discomfort does not generally vary greatly with time. The AEGL-1 point of departure was 1.25 ppm, which was obtained by applying a modifying factor of 2-2.5 ppm, which was the lowest effect level. The MF was used because exposure was for only 5 min and it is unclear whether “moderate” irritation or discomfort is comparable to “notable” irritation or discomfort, which exceeds the scope of AEGL-1. An intraspecies uncertainty factor of 3 was applied because allylamine acts as a contact irritant, and the severity of its effects is not expected to vary greatly among humans. Also, use of a greater uncertainty factor would yield a concentration below 0.2 ppm, which was a no-effect level for workers exposed for up to 4 h (Shell Oil Co. 1992). The derived AEGL-1 value of 0.42 ppm for 10 min to 8 h is also consistent with two mouse respiratory irritation studies (Gagnaire et al. 1989, 1993), from which it is predicted that exposure for a few hours to 0.9 ppm would cause sensory irritation in humans but that 0.09 ppm would not (Alarie 1981).
AEGL-2 values were based on two studies. The 10-, 30-, and 60-min AEGLs were developed from the Hine et al. (1960) human 5-min exposure study that was used to derive AEGL-1 values, but using 10 ppm as the point of departure. Ten ppm caused slight or moderate eye and nose irritation and pulmonary discomfort and was the NOAEL for “intolerable” irritation that occurred at 14 ppm. The same value was adopted for 10-60 min because the degree of irritation or discomfort resulting from exposure to 10 ppm was not expected to increase over a 1-h period beyond the scope of AEGL-2. An intraspecies uncertainty factor of 3 was used because allylamine acts as a contact irritant, and the severity of its effects is not expected to vary greatly among humans. The resulting AEGL-2 values of 3.3 ppm were not adopted for 4 or 8 h, however, because a rat study (Guzman et al. 1961) indicated that exposure to 3.3 ppm for 4 or 8 h may cause cardiotoxicity. In the latter study, exposure to 40 ppm for 16 h was a NOAEL for cardiovascular lesions, which were seen from exposure to 60 ppm for 14 h (myofibril fragment damage, perivascular edema, and cellular infiltration). Time-concentration scaling was performed using the ten Berge et al. (1986) equation Cn × t = k, where n = 1.7 was calculated from a linear regression of the Guzman et al. rat cardiotoxicity data. An interspecies uncertainty factor of 5 was applied because the mechanism of toxicity is similar among several mammalian species (and humans), but differences in susceptibility are unknown, and an uncertainty factor of 3 yields values approaching the NOEL for lethality from pulmonary lesions for a 4- or an 8-h exposure. An intraspecies uncertainty factor of 10 was used because the variability of the cardiotoxic response to allylamine among humans is undefined, and potentially sensitive populations exist (diabetics, persons with congestive heart failure). This yields 4- and 8-h AEGLs of 1.8 and 1.2 ppm, respectively, indicating that for these longer exposure durations, cardiotoxicity is a more sensitive end point than eye and respiratory irritation.
AEGL-3 values were derived from a rat inhalation LC50 study in which exposures were for 1, 4, or 8 h (Hine et al. 1960). All treated rats showed signs of eye and respiratory tract irritation, and some had lacrimation and red nasal discharge. Rats that died had stomachs distended with air, fluid-filled lungs, alveolar hemorrhage, and pulmonary edema. The NOEL for lethality, as represented by LC01 values calculated using probit analysis, was the AEGL-3 toxicity end point. The 1-h, 4-h, and 8-h AEGLs were obtained using the respective LC01 values. The 10-min and 30-min AEGLs were derived from the 1-h LC01 using the relationship Cn × t = k, where n = 0.85 was calculated from this study’s LC50 data. An uncertainty factor of 30 was applied: 10 to account for interspecies variability (lack of acute toxicity studies from other species with AEGL-3 level end points) and 3 for human variability [the steep dose-response (~2-fold increase in concentration caused mortality to increase from 0 to 100%) indicates that the NOEL for lethality due to direct destruction of lung tissue is not likely to vary greatly among humans].
8.2.
Comparison with Other Standards and Guidelines
The existing standards and guidelines for allylamine are shown in Table 1-12. There are currently no established U.S. standards for exposure to allylamine. In a preliminary criteria document that has not been accepted, the National Institute for Occupational Safety and Health recommended an occupational exposure limit of 0.6 ppm as the ceiling limit for any 15-min period in a 10-h work shift (NIOSH 1979; cited in Boor and Hysmith 1987); this document is out of print and was not available for use in the present AEGL report). In the former Soviet Union the maximum allowed daily (8-h) concentration in work room air was 0.5 mg/m3 (0.2 ppm; ILO-CIS 1991).
TABLE 1-12 Extant Standards and Guidelines for Allylamine (Values in ppm)
|
Guideline |
10 min |
30 min |
1 h |
4 h |
8 h |
|
AEGL-1 |
0.42 |
0.42 |
0.42 |
0.42 |
0.42 |
|
AEGL-2 |
3.3 |
3.3 |
3.3 |
1.8 |
1.2 |
|
AEGL-3 |
150 |
40 |
18 |
3.5 |
2.3 |
|
LLV (Sweden)a |
|
|
|
|
2 |
|
STV (Sweden)b |
6 (15 min) |
|
|
|
|
|
aLLV (level limit value), Swedish Occupational Exposure Limit Values, by Ordinance of the Swedish National Board of Occupational Safety and Health, adopted July 28, 2000; defined analogous to the ACGIH TLV-TWA. bSTV (short-term value), Swedish Occupational Exposure Limit Values, by Ordinance of the Swedish National Board of Occupational Safety and Health, adopted July 28, 2000; defined as a recommended value consisting of a time-weighted average for exposure during a reference period of 15 min. |
|||||
Guzman et al. (1961) recommended that under normal operating circumstances air allylamine concentrations should not be greater than 5 ppm, presumably based on examination of operators working with allylamine and on results obtained in an earlier study where humans were exposed to 2.5-14 ppm of allylamine for 5 min (Hine et al. 1960).
Gagnaire et al. (1989, 1993) derived an RD50 value of 9 ppm for allylamine (concentration in air causing a 50% reduction in the breathing rate of mice from a 15-min inhalation exposure) and based on 0.1 × RD50, proposed <1 ppm as the highest permitted industrial exposure. At this concentration, humans are expected to experience slight discomfort; 0.01 × RD50 would be expected to produce no sensory irritation; 0.03 × RD50 (the geometric mean of 0.1 and 0.01), that is, 0.27 ppm, is suggested to be a convenient Threshold Limit Value (Gagnaire et al. 1989).
8.3.
Data Adequacy and Research Needs
The data available for AEGL-1 derivation were adequate. Two human exposure studies were available with consistent results, and the developed AEGL values were supported by a mouse RD50 study. An odor threshold was not definitively established to confirm that the 0.42-ppm concentration that was derived for AEGL-1 would be detectable by humans.
The database for AEGL-2 and AEGL-3 was moderate. An AEGL-2 data gap was the lack of acute exposure studies in which cardiotoxicity occurred with animals other than rats. Although there were data indicating that the mechanism of allylamine toxicity was similar among mammals and between rats and humans, there was no information regarding the relative species susceptibilities. There were no AEGL-3 level end point acute toxicity studies with species other than rats, and further studies are needed. The key study for AEGL-3 derivation was based on calculated air allylamine concentrations, and it was not stated whether these were confirmed experimentally. This study, however, was well conducted.
9.
REFERENCES
Alarie, Y. 1981. Dose-response analysis in animal studies: Prediction of human responses. Environ. Health Perspect. 42:9-13.
Awasthi, S., and P.J. Boor. 1994. Lipid peroxide and oxidative stress during acute allylamine-induced cardiovascular toxicity. J. Vasc. Res. 31(1):33-41.
Benya, T.J., and R.D. Harbison. 1994. Allylamines. Pp. 1132-1171 in Patty’s Industrial Hygiene and Toxicology, 4th Ed., Vol. 2B, G.D. Clayton, and F.E. Clayton, eds. New York: John Wiley & Sons.
Boomsma, F., F. Derkx, A.H. van den Meiracker, A.J. Man in’t Veld, and M.A. Schalekamp. 1995. Plasma semicarbazide-sensitive amine oxidase activity is ele-
vated in diabetes mellitus and correlates with glycosylated haemoglobin. Clin. Sci. 88(6):675-679.
Boomsma, F., D.J. van Veldhuisen, and P.J. de Kam, A.J. Man in’t Veld, A. Mosterd, K.I. Lie, and Schalekamp. 1997. Plasma semicarbazide-sensitive amine oxidase is elevated in patients with congestive heart failure. Cardiovasc. Res. 33(2):387-391.
Boor, P.J. 1985. Allylamine cardiovascular toxicity: V. Tissue distribution and toxicokinetics after oral administration. Toxicology 35(3):167-177.
Boor, P.J., and R.M. Hysmith. 1987. Allylamine cardiovascular toxicity: A review. Toxicology 44(2):129-145.
Boor, P.J., and T.J. Nelson. 1982. Biotransformation of the cardiovascular toxic, allylamine, by rat and human cardiovascular tissue. J. Mol. Cell. Cardiol. 14(11):679-682.
Boor, P.J., M.T. Moslen, and E.S. Reynolds. 1979. Allylamine cardiotoxicity: I. Sequence of pathologic events. Toxicol. Appl. Pharmacol. 50(3):581-592.
Boor, P.J., R. Sanduja, T.J. Nelson, and G.A. Ansari. 1987. In vivo metabolism of the cardiovascular toxin, allylamine. Biochem. Pharmacol. 36(24):4347-4353.
Budavari, S., M.J. O’Neil, A. Smith, P.E. Heckelman, and J.F. Kinneary, eds. 1996. P. 53 in the Merck Index, 12th Ed. Whitehouse Station, NJ: Merck & Co.
Conklin, D.J., C.L. Boyce, M.B. Trent, and P.J. Poor. 2001. Amine metabolism: A novel path to coronary artery vasospasm. Toxicol. Appl. Pharmacol. 175(2):149-159.
EPA (U.S. Environmental Protection Agency). 2004. Acrolein (CASRN 107-02-8). Integrated Risk Information System (IRIS), U.S. Environmental Protection Agency [online]. Available: http://www.epa.gov/iris/subst/0364.htm [accessed July 20, 2007].
Gagnaire, F., S. Azim, P. Bonnet, P. Simon, J.P. Guenier, and J. De Ceaurriz. 1989. Nasal irritation and pulmonary toxicity of aliphatic amines in mice. J. Appl. Toxicol. 9(5):301-304.
Gagnaire, F., S. Azim, P. Simon, B. Cossec, P. Bonnet, and J. De Ceaurriz. 1993. Sensory and pulmonary irritation of aliphatic amines in mice: A structure-activity relationship study. J. Appl. Toxicol. 13(2):129-135.
Goldman, F.H., and M.B. Jacobs. 1953. Chemical Methods in Industrial Hygiene. New York: Interscience Publishers.
Guzman, R.J., G.S. Loquvam, J.K. Kodama, and C.H. Hine. 1961. Myocarditis produced by allylamines. Arch. Environ. Health 2:62-73.
Hart, E.R. 1939. The toxicity of certain allylamines. Univ. Calif. Publ. Pharmacol. 1(1938-1941):213-219.
Hine, C.H., J.K. Kodama, R.J. Guzman, and G.S. Loquvam. 1960. The toxicity of allylamines. Arch. Environ. Health 1:343-352.
HSDB (Hazardous Substances Data Bank). 2003. Allylamine (CASRN: 107-11-9). TOXNET, Specialized Information Services, U.S. National Library of Medicine, Bethesda, MD [online]. Available: http://toxnet.nlm.nih.gov/cgi-bin/sis/htmlgen?HSDB [accessed July 20, 2007].
Hysmith, R.M., and P.J. Boor. 1985. Allylamine cardiovascular toxicity: VI. Subcellular distribution in rat aortas. Toxicology 35(3):179-187.
ILO-CIS (International Labour Organisation CIS database). 1991. Occupational Exposure Limits for Airborne Toxic Substances: Values of Selected Countries. International Labour Office, Geneva.
Jacobs, M.B. 1949. The Analytical Chemistry of Industrial Poisons, Hazards, and Solvents, 2nd Ed. New York: Interscience Publishers.
Jelinek, R., M. Peterka, and Z. Rychter. 1985. Chick embryotoxicity screening test—130 substances tested. Indian J. Exp. Biol. 23(10):588-595.
Kulagina, N.K. 1975. Dependence of the biological activity of aliphatic amines on chemical structure and physicochemical properties [in Russian]. Toksikol. Nov. Prom. Khim. Vesh. 14:80-90.
Lewinsohn, R., K.H. Bohm, V. Glover, and M. Sandler. 1978. A benzylamine oxidase distinct from monoamine oxidase B—widespread distribution in man and rat. Biochem. Pharmacol. 27(14):1857-1863.
Lijinsky, W., and A.W. Andrews. 1980. Mutagenicity of vinyl compounds in Salmonella typhimurium. Teratog. Carcinog. Mutagen. 1(3):259-267.
Lyles, G.A. 1996. Mammalian plasma and tissue-bound semicarbazide-sensitive amine oxidases: Biochemical, pharmacological and toxicological aspects. Int. J. Biochem. Cell Biol. 28(3):259-274.
Lynch, D.W., W.J. Moorman, J.C. Clark, P.L. Hamlin, and T.R. Lewis. 1983. Electrocardiographic changes in F-344 rats exposed to inhaled allylamine vapor. Toxicologist 3(1):59[Abstract No. 236].
Lynch, D.W., W.J. Moorman, T.R. Lewis, P. Stober, R.D. Hamlin, and R.L. Schueler. 1989. Cardiac toxicity in F-344 rats following exposure to inhaled allylamine (AA) vapor. Toxicologist 9:277 [Abstract].
McMahon, R.E., J.C. Cline, and C.Z. Thompson. 1979. Assay of 855 test chemicals in ten tester strains using a new modification of the Ames test for bacterial mutagens. Cancer Res. 39(3):682-693.
NIOSH (National Institute for Occupational Safety and Health). 1979. NIOSH Criteria Document: Standards for Occupational Exposure to Primary Aliphatic Monoamines (Preliminary). U.S. Department of Health, Education, and Welfare, Division of Criteria Documentation and Standards Development (as cited in Boor and Hysmith 1987).
NRC (National Research Council). 1993. Guidance for Developing Community Emergency Exposure Levels for Hazardous Substances. Washington, DC: National Academy Press.
NRC (National Research Council). 2001. Standing Operating Procedures for Developing Acute Exposure Guideline for Airborne Chemicals. Washington, DC: National Academy Press.
Ramos, K., S.L. Grossman, and L.R. Cox. 1988. Allylamine-induced vascular toxicity in vitro: Prevention by semicarbazide-sensitive amine oxidase inhibitors. Toxicol. Appl. Pharmacol. 95(1):61-71.
Ramos, K.S., R.C. Bowes III, X. Ou, and T.J. Weber. 1994. Responses of vascular smooth muscle cells to toxic insult: Cellular and molecular perspectives for environmental toxicants. J. Toxicol. Environ. Health 43(4):419-440.
Research Pathology Associates, Inc. 1984. Pathologic Findings in Fischer 344 Rats Exposed by Inhalation to Allylamine, Ethylamine, Diethylamine, and Triethylamine with Cover Letter Dated 04/24/84. EPA/OTS Doc #86-870000813.
Sanduja, R., G.A. Ansari, and P.J. Boor. 1989. 3-Hydroxypropylmercapturic acid: A biologic marker of exposure to allylic and related compounds. J. Appl. Toxicol. 9(4):235-238.
Shell Oil Company. 1992. Initial Submission: Letter Submitting Enclosed Information on Exposure of Workers to Mono-allylamine, Di-allylamine, and Tri-allylamine. EPA/OTS Doc #88-920002051.
Springborn Life Sciences, Inc. 1989. Skin Corrosivity Study of C-1323 Allylamine in Rabbits (IMO) with Cover Letter Dated 10/26/89. EPA/OTS Doc #89-900000035.
Summer, W. 1971. Odor Pollution in Air: Causes and Control. London: Leonard Hill.
Swedish National Board of Occupational Safety and Health. 2000. Swedish Occupational Exposure Limits: LLV (Level Limit Values), CLV (Ceiling Limit Values), and STV (Short-Term Values). Adopted July 28, 2000, by Ordinance of the Swedish National Board of Occupational Safety and Health.
ten Berge, W.F., A. Zwart, and L.M. Appelman. 1986. Concentration-time mortality response relationship of irritant and systemically acting vapors and gases. J. Hazard. Mater. 13(3):301-309.
van Doorn, R., M. Ruijten, and T. Van Harreveld. 2002. Guidance for the Application of Odor in 22 Chemical Emergency Response, Version 2.1. August 29, 2002.
Verschueren, K., ed. 1996. Allylamine. Pp. 159-160 in Handbook of Environmental Data on Organic Chemicals, 3rd Ed. New York: Van Nostrand Reinhold.
Zeiger, E., B. Anderson, S. Haworth, T. Lawlor, K. Mortelmans, and W. Speck. 1987. Salmonella mutagenicity tests: III. Results from the testing of 255 chemicals. Environ. Mutagen. 9(Suppl.9):1-109.
Zissu, D. 1995. Histopathological changes in the respiratory tract of mice exposed to ten families of airborne chemicals. J. Appl. Toxicol. 15(3):207-213.
APPENDIX A
Derivation of AEGL-1 Values
|
Key study: |
Hine et al. (1960). Thirty five young adult human volunteers were exposed for 5 min to 2.5, 5, or 10 ppm of allylamine (10-14/concentration; sex and age not specified). A group was also exposed briefly to 14 ppm, which was reported as intolerable, and exposure was almost immediately terminated. All subjects detected the odor of allylamine, and there were dose-related increases in the incidence of slight or moderate eye irritation (21%, 15%, 50%), nose irritation (50%, 54%, 100%), and pulmonary discomfort (29%, 46%, 50%) at 2.5, 5, and 10 ppm, respectively. The AEGL-1 point of departure was 1.25 ppm, which was obtained by applying a modifying factor of 2 to the lowest effect level of 2.5 ppm. |
|
Toxicity end point: |
Mild sensory irritation or discomfort in humans exposed to 1.25 ppm for 10 min to 8 h. |
|
Scaling: |
None: The same AEGL-1 value was used for 10 min to 8 h because mild sensory irritation or discomfort does not vary greatly with time. |
|
Uncertainty Factors: |
Total uncertainty factor: 3. |
|
Interspecies: |
Not applicable. |
|
Intraspecies: |
3; allylamine acts as a contact irritant, and the severity of its effects is not expected to vary greatly among humans. Also, use of a greater uncertainty factor would yield a concentration below 0.2 ppm, which was a no-effect level for workers exposed for up to 4 h (Shell Oil Co. 1992). |
|
Modifying factor: |
2, because exposure was for only 5 min and it is unclear whether “moderate” irritation or discomfort is comparable to “notable” irritation or discomfort, which exceeds the scope of AEGL-1. |
|
Calculations: |
|
|
10-min AEGL-1: |
2.5 ppm/6 = 0.42 ppm (0.98 mg/m3) |
|
30-min AEGL-1: |
2.5 ppm/6 = 0.42 ppm (0.98 mg/m3) |
|
1-h AEGL-1: |
2.5 ppm/6 = 0.42 ppm (0.98 mg/m3) |
|
4-h AEGL-1: |
2.5 ppm/6 = 0.42 ppm (0.98 mg/m3) |
|
8-h AEGL-1: |
2.5 ppm/6 = 0.42 ppm (0.98 mg/m3) |
Derivation of AEGL-2 Values
10, 30, and 60 min
|
Key study: |
Hine et al. (1960); same study used to derive AEGL-1 values. Young adult human volunteers (35; sex and ages unknown) were exposed to 2.5, 5, 10, or 14 ppm. The AEGL-2 point of departure was 10 ppm, which caused slight or moderate eye and nose irritation and pulmonary discomfort and was the NOAEL for “intolerable” irritation seen at 14 ppm. |
|
Toxicity end point: |
Human sensory irritation or discomfort; NOAEL for “intolerable” irritation. |
|
Scaling: |
None; the degree of irritation or discomfort resulting from exposure to 10 ppm was not expected to increase over a 1-h period beyond the scope of AEGL-2. |
|
Uncertainty factors: |
Total uncertainty factor: 3. |
|
Interspecies: |
Not applicable. |
|
Intraspecies: |
3; used because allylamine acts as a contact irritant, and the severity of its effects is not expected to vary greatly among humans. |
|
Calculations for 10, 30, and 60 min: |
|
|
|
10 ppm/3 = 3.3 ppm (7.7 mg/m3) |
4 and 8 h
|
Key study: |
Guzman et al. (1961). Male Long-Evans rats exposed to 40 ppm for 16 h had heart morphology comparable to controls (NOAEL), but rats exposed to 60 ppm for 14 h had cardiovascular lesions consisting of scattered myofibril fragments with loss of striation, perivascular edema, and cellular infiltration (LOAEL). |
|
Toxicity end point: |
NOAEL for cardiovascular lesions. |
|
Scaling: |
C1.7 × t = k (ten Berge et al. 1986); n = 1.7 was calculated from a linear regression of the Guzman et al. (1961) rat cardiotoxicity data. |
|
Uncertainty factors: |
Total uncertainty factor: 50. |
|
Interspecies: |
5; the mechanism of toxicity is similar among several mammalian species (and humans), but differences in susceptibility are unknown; 3 yields values approaching NOEL for lethality from pulmonary lesions. |
|
Intraspecies: |
10; the variability of cardiotoxic response among humans is unknown, and potentially sensitive populations exist (diabetics, persons with congestive heart failure). |
|
Calculations for 4 and 8 h: |
|
|
|
|
|
4-h AEGL-2: |
C1.7 × 4 h = 10.95 ppm1.7-h 4-h AEGL-2 = 1.8 ppm (4.2 mg/m3) |
|
8-h AEGL-2: |
C1.7 × 8 h = 10.95 ppm1.7-h 8-h AEGL-2 = C = 1.2 ppm (2.8 mg/m3) |
Derivation of AEGL-3 Values
|
Key study: |
Hine et al. 1960. Rat inhalation LC50 study. All treated rats showed signs of eye and respiratory tract irritation, and some had lacrimation and red nasal discharge. Rats dying from exposure had stomachs distended with air, |
|
|
fluid-filled lungs, alveolar hemorrhage, and pulmonary edema. |
|
Toxicity end point: |
Lethality NOELs, estimated from LC01 values obtained by probit analysis: 1-h LC01 = 533 ppm 4-h LC01 = 104 ppm 8-h LC01 = 69.2 ppm |
|
Scaling: |
C0.85 × t = k (data from Hine et al. 1960; ten Berge et al. 1986). |
|
Note: |
Only the 10- and 30-min AEGL-3 values required scaling (used the 1-h LC01). |
|
Uncertainty factors: |
Total uncertainty factor: 30. |
|
Interspecies: |
10; to account for the lack of acute toxicity studies with AEGL-3 end points from other species. |
|
Intraspecies: |
3; steep dose-response (~2-fold increase in concentration caused mortality to increase from 0 to 100%) indicates the NOEL for lethality due to direct destruction of lung tissue is not likely to vary greatly among humans. |
|
Calculations for 10 and 30 min: |
|
|
|
|
|
10-min AEGL-3: |
C0.85 × 0.167 h = 11.54 ppm0.85-h 10-min AEGL-3 = 150 ppm (350 mg/m3) |
|
30-min AEGL-3: |
C0.85 × 0.5 h = 11.54 ppm0.85-h 30-min AEGL-3 = 40 ppm (93 mg/m3) |
|
Calculations for 1, 4, and 8 h: |
|
|
1-h AEGL-3: |
C = 533 ppm (= LC01) 1-h AEGL-3 = 533 ppm/30 = 18 ppm (42 mg/m3) |
|
4-h AEGL-3: |
C = 104 ppm (= LC01) 4-h AEGL-3 = 104 ppm/30 = 3.5 ppm (8.2 mg/m3) |
|
8-h AEGL-3: |
C = 69.2 ppm (= LC01) 8-h AEGL-3 = 69.2 ppm/30 = 2.3 ppm (5.4 mg/m3) |
APPENDIX B
Derivation of the Level of Distinct Odor Awareness
The level of distinct odor awareness (LOA) represents the concentration above which it is predicted that more than half of the exposed population will experience at least a distinct odor intensity; about 10% of the population will experience a strong odor intensity. The LOA should help chemical emergency responders in assessing public awareness of the exposure due to odor perception. The LOA derivation follows the guidance given by van Doorn et al. (2002).
An odor detection threshold (OT50; i.e., the concentration at which 50% of the odor panel observed an odor without necessarily recognizing it) of 3.7 ppm was reported for allylamine by van Doorn et al (2002). This value conflicts with a sensory threshold experimental study in which all 36 volunteers exposed to allylamine for 5 min reported “olfactory cognition” at the lowest concentration tested of 2.5 ppm. Proceeding with the use of 3.7 ppm yields the following:
The concentration (C) leading to an odor intensity (I) of distinct odor detection (I = 3) is derived using the Fechner function:
For the Fechner coefficient the default of kw = 2.33 will be used due to the lack of chemical-specific data:
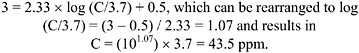
The resulting concentration is multiplied by an empirical field correction factor. It takes into account that in everyday life such factors as sex, age, sleep, smoking, upper-airway infections and allergies, and distraction increase the odor detection threshold by a factor of 4. In addition, it takes into account that odor perception is very fast (about 5 s), which leads to the perception of concentration peaks. Based on current knowledge, a factor of 1/3 is applied to adjust for peak exposure. Adjustment for distraction and peak exposure leads to a correction factor of 4/3 = 1.33.
The calculated LOA for allylamine is 58 ppm, which exceeds a concentration (i.e., 14 ppm) found to be intolerable by humans (Hine et al. 1960). Therefore, the calculated LOA conflicts with human empirical data, as did the OT50, and neither the LOA nor the OT50 is considered valid.
APPENDIX C
Time-Scaling Calculations
Allylamine: AEGL-2 n-Value Derivation
Derivation of n for Cn × t = k, Based on Rat Cardiotoxicity Data (Guzman et al. 1961)
|
Input Data: |
Regression Output: |
||||
|
Concentration |
Log Concentration |
Time (h) |
Log Time |
Intercept |
3.4443 |
|
100 |
2.0000 |
4 |
2.3802 |
Slope |
−0.5845 |
|
60 |
1.7782 |
14 |
2.9243 |
R Squared |
0.8922 |
|
50 |
1.6990 |
20 |
3.0792 |
Correlation |
−0.9446 |
|
40 |
1.6021 |
32 |
3.2833 |
Degrees of freedom |
3 |
|
20 |
1.3010 |
48 |
3.4594 |
Observations |
5 |
|
n = 1.71. k = 781244.19. |
|||||
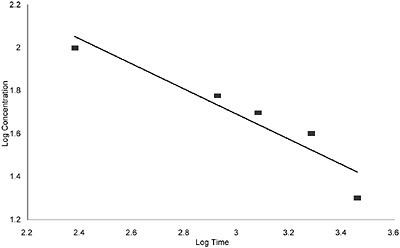
FIGURE 1-1 Best-fit concentration (ppm allylamine) × time (exposure duration in hours) curve. Linear progression of rat lethality data.
Allylamine: AEGL-3 n-Value Derivation
Derivation of n for Cn × t = k, Based on Rat LC50 Study Data of Hine et al. (1960)
|
Input Data: |
Regression Output: |
||||
|
Concentration |
Log Concentration |
Time (h) |
Log Time |
Intercept |
3.2567 |
|
1,933 |
3.2862 |
1 |
0.0000 |
Slope |
−1.1823 |
|
286 |
2.4564 |
4 |
0.6021 |
R 2 |
−0.9798 |
|
177 |
2.2480 |
8 |
0.9031 |
Correlation |
−0.9898 |
|
|
|
|
|
Degrees of freedom |
1 |
|
|
|
|
|
Observations |
3 |
|
n = 0.8457765 k = 568.14929. |
|||||
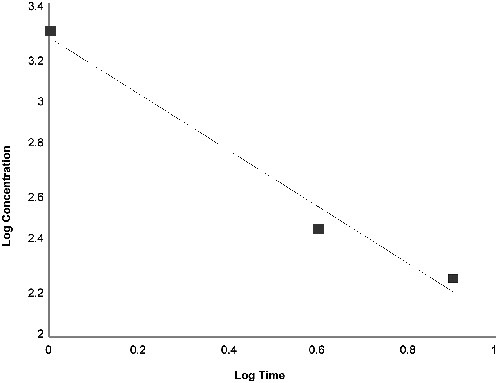
FIGURE 1-2 Best-fit concentration (ppm allylamine) × time (exposure duration in hours) curve. Linear progression of rat lethality data.
APPENDIX E
Acute Exposure Guideline Levels for Allylamine
Derivation Summary for Allylamine AEGLs (107-11-9)
AEGL-1 VALUES
|
10 min |
30 min |
1 h |
4 h |
8 h |
|
0.42 ppm |
0.42 ppm |
0.42 ppm |
0.42 ppm |
0.42 ppm |
|
Key Reference: Hine, C.H., J.K. Kodama, R.J. Guzman, and G.S. Loquvam. 1960. The toxicity of allylamines. Arch. Environ. Health 1:343-352. |
||||
|
Test Species/Strain/Number: Young adult human volunteers (age and sex not specified), 10-14/concentration |
||||
|
Exposure Route/Concentrations/Durations: Inhalation; 2.5, 5, or 10 ppm for 5 min; 14 ppm <1 min. |
||||
|
Effects: All subjects detected the odor of allylamine. At 2.5, 5, and 10 ppm, respectively, there were dose-related increases in the incidence of slight or moderate eye irritation (21%, 15%, 50%), nose irritation (50%, 54%, 100%), and pulmonary discomfort (29%, 46%, 50%). The incidence of CNS effects was not dose related. The AEGL-1 point of departure was 1.25 ppm, which was obtained by applying a modifying factor of 2 to the lowest effect level of 2.5 ppm, to ensure that effects do not exceed AEGL-1 severity. |
||||
|
End point/Concentration/Rationale: Mild sensory irritation or discomfort in humans exposed to 1.25 ppm for 10 min to 8 h. |
||||
|
Uncertainty Factors/Rationale: Uncertainty Factors: Total uncertainty factor: 3. Interspecies: Not applicable. Intraspecies: 3; allylamine acts as a contact irritant, and the severity of its effects is not expected to vary greatly among humans. Also, use of a greater uncertainty factor would yield a concentration below 0.2 ppm, which was a no-effect level for workers exposed for up to 4 h (Shell Oil Co. 1992). |
||||
|
Modifying Factor: 2; applied because exposure was for only 5 min and it is unclear whether “moderate” irritation or discomfort is comparable to “notable” irritation or discomfort, which exceeds the scope of AEGL-1. |
||||
|
Animal to Human Dosimetric Adjustment: None. |
||||
|
Time Scaling: None; same AEGL value is adopted for 10 min to 8 h because mild sensory irritation or discomfort is not expected to vary greatly over time. |
||||
|
Data Adequacy: Dataset was adequate. A human experimental study was used to develop AEGL-1 values, which are supported by an occupational monitoring study indicating 0.2 ppm was a no-effect level in workers (Shell Oil Co. 1992) and by two mouse RD50 studies (Gagnaire et al. 1989, 1993) from which it is predicted that 0.9 ppm should result in some sensory irritation in humans, whereas 0.09 ppm should cause no sensory irritation (Alarie 1981). |
||||
AEGL-2 VALUES
|
10 min |
30 min |
1 h |
4 h |
8 h |
|
3.3 ppm |
3.3 ppm |
3.3 ppm |
1.8 ppm |
1.2 ppm |
|
Key Reference: Hine, C.H., J.K. Kodama, R.J. Guzman, and G.S. Loquvam. 1960. The toxicity of allylamines. Arch. Environ. Health 1:343-352. |
Key Reference: Guzman, R.J., G.S. Loquvam, J.K. Kodama, and C.H. Hine. 1961. Myocarditis produced by allylamines. Arch. Environ. Health 2:62-73. |
|||
|
Test Species/Strain/Sex/Number: Young adult humans (age and sex unspecified), 10-14/concentration |
Test Species/Strain/Sex/Number: Male Long-Evans rats; 1-20/group, as shown in Table 1-5 of Section 3.1.1. |
|||
|
Exposure Route/Concentrations/Durations: Inhalation; 2.5, 5, or 10 ppm for 5 min; 14 ppm <1 min. |
Exposure Route/Concentrations/Durations: Inhalation; exposure to 20-100 ppm for 4-48 h, as shown in Table 1-5 of Section 3.1.1. Rats within dose groups were killed for analysis 8 h to 14 days after the start of exposure. |
|||
|
Effects: At 2.5, 5, and 10 ppm, respectively, there were dose-related increases in the incidence of slight or moderate eye irritation (21%, 15%, 50%), nose irritation (50%, 54%, 100%), and pulmonary discomfort (29%, 46%, 50%); the point of departure was 10 ppm, which was NOAEL for “intolerable” seen at 14 ppm. |
Effects: Depending on exposure scenario, ranged from no noted cardiovascular effects to severe myocardial and/or cardiovascular lesions; there were several deaths, as shown in Table 1-5 of Section 3.1.1. The LOAEL for cardiovascular lesions was exposure to 60 ppm for 14 h, and the NOAEL was exposure to 40 ppm for 16 h. |
|||
|
End point/Concentration/Rationale: Sensory irritation or discomfort in humans and the NOAEL for “intolerable” irritation. |
End point/Concentration/Rationale: Exposure to 40 ppm for 16 h was the NOAEL for cardiovascular lesions. |
|||
|
Uncertainty Factors/Rationale: Total uncertainty factor: 3. Interspecies: Not applicable. Intraspecies: 3: Used because allylamine is acting as a contact irritant, and the severity of its effects is not expected to vary greatly among humans. |
Uncertainty Factors/Rationale: Total uncertainty factor: 50. Interspecies: 5; mechanism of toxicity is similar among several mammalian species (and humans), but differences in susceptibility are unknown; 3 yielded values approaching the NOEL for lethality from pulmonary lesions. Intraspecies: 10; variability of cardiotoxic response among humans is unknown, and potentially sensitive populations exist (diabetics, persons with congestive heart failure). |
|||
|
Modifying Factor: None. |
Modifying Factor: None. |
|||
|
Animal to Human Dosimetric Adjustment: None. |
Animal to Human Dosimetric Adjustment: None. |
|||
|
10 min |
30 min |
1 h |
4 h |
8 h |
|
3.3 ppm |
3.3 ppm |
3.3 ppm |
1.8 ppm |
1.2 ppm |
|
Time Scaling: None, because the degree of irritation is expected to remain within the scope of AEGL-2 for 60 min. |
Time Scaling: Cn × t = k, where n = 1.7 [n is based on regression analysis of Guzman et al. (1961) cardiotoxicity data]. |
|||
|
Data Adequacy: Dataset was limited but adequate. All derived AEGL-2 values were well below 14 ppm, which was “intolerable” to human volunteers (Hine et al. 1960). |
||||
AEGL-3 VALUES
|
10 min |
30 min |
1 h |
4 h |
8 h |
|
|
150 ppm |
40 ppm |
18 ppm |
3.5 ppm |
2.3 ppm |
|
|
Reference: Hine, C.H., J.K. Kodama, R.J. Guzman, and G.S. Loquvam. 1960. The toxicity of allylamines. Arch. Environ. Health 1:343-352. |
|||||
|
Test Species/Strain/Sex/Number: Long-Evans rats, five/dose group. |
|||||
|
Exposure Route/Concentrations/Durations: Inhalation for 1, 4, or 8 h; see below: |
|||||
|
Effects: All treated rats showed signs of eye and respiratory tract irritation, and some had lacrimation and red nasal discharge. Rats that died had stomachs distended with air, fluid-filled lungs, alveolar hemorrhage, and pulmonary edema. Mortality rates and calculated LC50 and LC01 values are as follows: |
|||||
|
1-h exposure |
4-h exposure |
8-h exposure |
|||
|
Concentration |
Mortality |
Concentration |
Mortality |
Concentration |
Mortality |
|
1,000 ppm |
1/5 |
133 ppm |
0/5 |
89 ppm |
0/5 |
|
1,500 ppm |
1/5 |
200 ppm |
0/5 |
133 ppm |
0/5 |
|
2,250 ppm |
3/5 |
300 ppm |
3/5 |
200 ppm |
4/5 |
|
3,380 ppm |
5/5 |
450 ppm |
5/5 |
300 ppm |
5/5 |
|
LC50 = 1933 ppm |
LC50 = 286 ppm |
LC50 =177 ppm |
|||
|
LC01 = 533 ppm |
LC01 = 104 ppm |
LC01 = 69.2 ppm |
|||
|
End point/Concentration/Rationale: LC01 values, representing the lethality NOEL, were calculated by probit analysis using 1-, 4-, and 8-h exposure data from the key study. |
|||||
|
Uncertainty Factors/Rationale: Total uncertainty factor: 30. Uncertainty factors: Total uncertainty factor: 30. Interspecies: 10; to account for the lack of acute toxicity studies with AEGL-3 end points from other species. Intraspecies: 3; steep dose-response (~2-fold increase in concentration caused mortality to increase from 0 to 100%) indicates the NOEL for lethality due to direct destruction of lung tissue is not likely to vary greatly among humans. |
|||||
|
Modifying Factor: Not applicable. |
|||||
|
Animal to Human Dosimetric Adjustment: Not applied. |
|||||
|
Time Scaling: Cn × t = k, where n = 0.85, based on regression analysis of key study. Time scaling was used only for derivation of the 10- and 30-min values, using the 1-h LC01. |
|||||
|
Data Adequacy: The dataset was limited but adequate. The key rat study was well conducted, and the data were internally consistent. |
|||||