5
Crotonaldehyde, trans and cis + trans1
Acute Exposure Guideline Levels
PREFACE
Under the authority of the Federal Advisory Committee Act (P.L. 92-463) of 1972, the National Advisory Committee for Acute Exposure Guideline Levels for Hazardous Substances has been established to identify, review, and interpret relevant toxicologic and other scientific data and develop acute exposure guideline levels (AEGLs) for high-priority, acutely toxic chemicals.
AEGLs represent threshold exposure limits for the general public and are applicable to emergency exposure periods ranging from 10 minutes (min) to 8 hours (h). Three levels—AEGL-1, AEGL-2, and AEGL-3—are developed for each of five exposure periods (10 min, 30 min, 1 h, 4 h, and 8 h) and are distinguished by varying degrees of severity of toxic effects. The three AEGLs are defined as follows:
AEGL-1 is the airborne concentration (expressed as parts per million [ppm] or milligrams per cubic meter [mg/m3]) of a substance above which it is predicted that the general population, including susceptible individuals, could experience notable discomfort, irritation, or certain asymptomatic nonsensory effects. However, the effects are not disabling and are transient and reversible upon cessation of exposure.
AEGL-2 is the airborne concentration (expressed as ppm or mg/m3) of a substance above which it is predicted that the general population, including susceptible individuals, could experience irreversible or other serious, long-lasting adverse health effects or an impaired ability to escape.
AEGL-3 is the airborne concentration (expressed as ppm or mg/m3) of a substance above which it is predicted that the general population, including susceptible individuals, could experience life-threatening health effects or death.
Airborne concentrations below the AEGL-1 represent exposure levels that can produce mild and progressively increasing but transient and nondisabling odor, taste, and sensory irritation or certain asymptomatic nonsensory effects. With increasing airborne concentrations above each AEGL, there is a progressive increase in the likelihood of occurrence and the severity of effects described for each corresponding AEGL. Although the AEGL values represent threshold levels for the general public, including susceptible subpopulations, such as infants, children, the elderly, persons with asthma, and those with other illnesses, it is recognized that individuals, subject to unique or idiosyncratic responses, could experience the effects described at concentrations below the corresponding AEGL.
SUMMARY
Crotonaldehyde is a colorless, flammable liquid and a potent eye, skin, and respiratory irritant. Inhaled crotonaldehyde can cause a burning sensation in the nasal and upper respiratory tract, lacrimation, coughing, bronchoconstriction, pulmonary edema, and deep lung damage. Crotonaldehyde is used primarily for the manufacture of sorbic acid and other organic chemicals. It is found in tobacco smoke and is a combustion product of diesel engines and wood but also occurs naturally in meat, fish, and many fruits and vegetables.
Crotonaldehyde exists as the cis and the trans isomer; commercial crotonaldehyde is a mixture of the two isomers consisting of >95% trans isomer. Because no in vivo exposure studies were located for the individual isomers (information was for the commercial mixture), the AEGL values in this document apply to both trans-crotonaldehyde (123-73-9) and the cis-trans mixture (4170-30-3).
AEGL-1 values were derived from a Health Hazard Evaluation conducted by National Institute for Occupational Safety and Health (NIOSH) in which workers exposed to approximately 0.56 ppm of crotonaldehyde for <8h reported occasional minor eye irritation (Fannick 1982). The same exposure concentration was adopted for 10 min to 8 h because the critical end point (minor eye irritation in humans) was mild and mild irritant effects do not vary greatly over time. A total uncertainty factor of 3 was applied to account for intraspecies variability, because the eye irritation is a direct surface-contact effect not subject to pharmacokinetic differences between individuals.
AEGL-2 values were based on a pulmonary function study in which rats were exposed for 5-240 min to 10-580 ppm of crotonaldehyde; individual exposure concentrations and durations were not given (Rinehart 1967). Rats had reduced pulmonary gas uptake ability and, above 8,000 ppm-min, proliferative lesions of the respiratory bronchioles. Exposures above 16,000 ppm-min induced pulmonary edema and death. AEGL-2 values were calculated by dividing 8,000 ppm-min by 10, 30, 60, 240, or 480 min because concentration and time appeared to be equally important factors in altering the pulmonary uptake of CO and ether (supported by n = 1.2 derived from an LC50 study [a lethal concentration in 50% of the rats] by Rinehart [1967]). A total uncertainty factor of 30 was used: 10 for interspecies uncertainty (because the actual exposure concentration and time were not known for the key study and there was a lack of supporting animal studies) and 3 for intraspecies uncertainty (although human variability to crotonaldehyde toxicity is not well-defined, a greater uncertainty factor was judged inappropriate because it yields 4- and 8-h AEGL-2 concentrations that caused only mild irritation in workers exposed for up to 8 h; Fannick 1982).
The AEGL-3 was based on an LC50 study in which rats were exposed to crotonaldehyde vapor for 5 min to 4 h (Rinehart 1967). Most deaths occurred by 4 days after exposure. The animals had clear or slightly blood-tinged nasal exudate; the rats that died within 1 day also had terminal convulsions. Necropsy showed that a few rats had pulmonary congestion. The 10-min, 30-min, 1-h, and 4-h AEGLs were obtained using the respective LC01 values calculated by probit analysis from the mortality data. The 8-h AEGLs were derived from the 4-h LC01 using the relationship Cn × t = k, where n = 1.2 was derived by ten Berge et al. (1986) from this study LC50 data. A total uncertainty factor of 10 was applied: 3 for interspecies uncertainty because interspecies variability was small (LC50 values for rats, mice, and guinea pigs were within a factor of 2.5, and these studies yield similar or higher AEGL-3 values) and 3 for intraspecies uncertainty because great human variability is unlikely given the homogeneity of the animal data and a larger uncertainty factor yields 8-h AEGL-3 concentrations that caused only mild irritation in workers exposed for up to 8 h (Fannick 1982). A summary of AEGL values is shown in Table 5-1.
A cancer inhalation slope factor was derived for crotonaldehyde and used to estimate the 10−4 excess cancer risk from a single 30-min to 8-h exposure, as shown in Appendix D. Crotonaldehyde concentrations associated with a 10−4 excess cancer risk were 25-fold greater than the toxicity-based AEGL-2 values for 30 to 480 min. The noncarcinogenic end points were considered to be more appropriate for AEGL-2 derivation because (1) there is insufficient evidence that inhalation is a route that results in crotonaldehyde-induced liver lesions or neoplasia at concentrations comparable to the AEGL-2 values; (2) the data used to derive the cancer slope factor were very weak (the key study had only one dose group and one control group; the high dose was excluded due to lack of fit), and most of the neoplastic changes were benign; (3) AEGL values are applicable to rare events or single, once-in-a-lifetime exposures, and the data indicate that
TABLE 5-1 Summary of AEGL Values for Crotonaldehyde
|
Classification |
10 min |
30 min |
1 h |
4 h |
8 h |
End Point (Reference) |
|
AEGL-1a (nondisabling) |
0.19 ppm (0.55 mg/m3) |
0.19 ppm (0.55 mg/m3) |
0.19 ppm (0.55 mg/m3) |
0.19 ppm (0.55 mg/m3) |
0.19 ppm (0.55 mg/m3) |
Mild eye irritation in humans (Fannick 1982) |
|
AEGL-2 (disabling) |
27 ppm (77 mg/m3) |
8.9 ppm (26 mg/m3) |
4.4 ppm (13 mg/m3) |
1.1 ppm (3.2 mg/m3) |
0.56 ppm (1.6 mg/m3) |
Impaired pulmonary function, NOAEL for bronchiole lesions (Rinehart 1967) |
|
AEGL-3 (lethal) |
44 ppm (130 mg/m3) |
27 ppm (77 mg/m3) |
14 ppm (40 mg/m3) |
2.6 ppm (7.4 mg/m3) |
1.5 ppm (4.3 mg/m3) |
Lethality NOEL (Rinehart 1967). |
|
aOdor threshold has been reported as 0.035-1.05 ppm. |
||||||
TNM neoplasms resulted from lifetime treatment; and (4) a direct comparison of estimated TNM cancer risk and AEGL values is not appropriate due to large differences in the methodologies used to obtain these numbers.
1.
INTRODUCTION
Crotonaldehyde (CH3CH = CHCHO) exists as a cis isomer (15798-64-8) and a trans isomer (123-73-9) or as a mixture of the two isomers (4170-30-3). Commercial crotonaldehyde (4170-30-3) consists of >95% trans isomer and <5% cis isomer (Budavari et al. 1996; IARC 1995). With the exception of one reported odor detection level, no physical or chemical data or human or animal studies were located for the cis or trans isomers individually; all available information was for the commercial (cis-trans) mixture. Therefore, the AEGL values prepared in this document will apply to both trans-crotonaldehyde (123-73-9) and the cis-trans mixture (4170-30-3). The Occupational Safety and Health Administration (OSHA), NIOSH, and the American Conference of Governmental Industrial Hygienists (ACGIH) have adopted the same occupational exposure limits (permissible exposure limit, recommended exposure limit, Threshold Limit Value) for both isomers.
Crotonaldehyde is a potent lacrimator and an extreme eye, respiratory, and skin irritant. Exposures to sufficiently high concentrations have produced choking, coughing, and a burning sensation on the face, in the nasal and oral passages, and in the upper respiratory tract as well as bronchoconstriction and pulmonary edema (HSDB 2005). Its odor threshold has been reported as 0.035-0.2
ppm (Verschueren 1996), 0.037-1.05 ppm (Ruth 1986), 0.038 ppm (Tepikina et al. 1997), and 0.12 ppm (trans isomer; Amoore and Hautala 1983).
Human exposure to crotonaldehyde occurs from both man-made and natural sources. Crotonaldehyde has been identified in exhaust from jet, gasoline; and diesel engines; from tobacco smoke; and from the combustion of polymers and wood (IARC 1995). Crotonaldehyde occurs naturally in meat, fish, many fruits (apples, grapes, strawberries, tomatoes) and vegetables (cabbage, cauliflower, Brussel sprouts, carrots), bread, cheese, milk, beer, wine, and liquors (IARC 1995). It is emitted from volcanoes, from the Chinese arbor vitae plant, and from pine and deciduous forests (IARC 1995; HSDB 2005). Crotonaldehyde has been detected in drinking water, wastewater, human milk, and expired air from nonsmokers.
Crotonaldehyde is a very flammable liquid (Budavari et al. 1996). It is manufactured commercially by adding aldol to a boiling dilute acid solution and removing the crotonaldehyde by distillation. Crotonaldehyde is used primarily for the production of sorbic acid; it is also used for the synthesis of butyl alcohol, butyraldehyde, quinaldine, thiophenes, pyridenes, dyes, pesticides, pharmaceuticals, rubber antioxidants, and chemical warfare agents and as a warning agent in locating breaks and leaks in pipes (IARC 1995, Budavari et al. 1996; Verschueren 1996). Crotonaldehyde degrades in the atmosphere by reacting with photochemically produced hydroxyl radicals (half-life of about 11 h) or ozone (half-life of about 15.5 days; HSDB 2005).
U.S. production of crotonaldehyde in 1975 was >2,000 pounds, and about 463 pounds was imported into the United States in 1984 (HSDB 2005). The chemical and physical properties of crotonaldehyde are listed in Table 5-2; discrete information was not available for the trans isomer of crotonaldehyde, and the information given is for the cis-trans mixture (except for synonyms and the CAS registry numbers).
2.
HUMAN TOXICITY DATA
2.1.
Acute Lethality
Crotonaldehyde vapor “may be fatal” if inhaled or absorbed through the skin; no further information was provided (Eastman Chemical Co. 1998).
2.2.
Nonlethal Toxicity
2.2.1.
Odor Threshold and Odor Awareness
A wide range of concentrations have been reported for the human odor detection and irritation thresholds for crotonaldehyde, perhaps in some cases due
TABLE 5-2 Chemical and Physical Data
|
Property |
Descriptor or Value |
Reference |
|
Synonyms |
4170-30-3: 2-butenal, crotonal, crotonic aldehyde, 1-formyl-propene, β-methylacrolein 123-73-9: (E)-2-butenal, (E)-crotonaldehyde, trans-2-butenal, trans-crotonaldehyde |
IARC 1995 |
|
Chemical formula |
CH3CH = CH − CHO |
Budavari et al. 1996 |
|
Molecular weight |
70.09 |
Budavari et al. 1996 |
|
CAS registry number |
4170-30-3 (mixture of cis and trans isomers) 123-73-9 (trans isomer) |
IARC 1995 |
|
Physical state |
Liquid |
Budavari et al. 1996 |
|
Color |
White liquid; yellows on contact with air |
NIOSH 2002 |
|
Solubility in water |
18.1 g/100 g at 20°C |
Budavari et al. 1996 |
|
Vapor pressure |
19 mmHg at 20°C |
Verschueren 1996 |
|
Vapor density (air = 1) |
2.41 |
Budavari et al. 1996 |
|
Liquid density (water = 1) |
0.853 at 20/20°C |
Budavari et al. 1996 |
|
Melting point |
−76.5°C |
Budavari et al. 1996 |
|
Boiling point |
104.0°C at 760 mm |
Budavari et al. 1996 |
|
Flammability/explosion limits |
2.1-15.5% |
NIOSH 2002 |
|
Conversion factors |
1 mg/m3 = 0.349 ppm; 1 ppm = 2.87 mg/m3 |
Verschueren 1996, IARC 1995 |
to analytical measurement errors (Steinhagen and Barrow 1984). Amoore and Hautala (1983) reported the odor threshold to be 0.12 ppm for trans-crotonaldehyde, whereas the irritation threshold was 14 ppm and 19 ppm for the nose and eyes, respectively. In several secondary sources, the odor detection threshold for crotonaldehyde was given as 0.035-1.05 ppm and the irritation threshold was 8.0 ppm (Ruth 1986; Verschueren 1996). In a study in which 25 volunteers were exposed to 0.02-2.3 mg/m3 (0.007-0.8 ppm) of crotonaldehyde, the odor was detected by several persons at the lowest concentration tested, and roughly half the people were able to detect the odor at 0.11 mg/m3 (0.038 ppm; Tepikina et al. 1997). The test subjects were exposed to each concentration repeatedly (about 2-4 times) to eliminate guessing and also to “pure air” to give a point of reference (i.e., incidence of false positives). An unpublished source (van Doorn et al. 2002) reported 0.069 ppm and 0.063-0.2 ppm as the trans-crotonaldehyde and cis-crotonaldehyde odor detection thresholds, respectively (OT50; i.e., concentration at which 50% of the odor panel observed an odor without necessarily recognizing it).
2.2.2.
Experimental Studies
Twelve healthy males ages 18-45 were exposed for 10 or 15 min to 12 mg/m3 (about 4.1 ppm) in a 100-m3 chamber at 20-25°C with a wind velocity of 1 mph (exposure duration was unclear from the study text; Sim and Pattle 1957). Crotonaldehyde vapor was produced by bubbling air through a known volume of liquid until all of the liquid evaporated; air samples were analyzed for concentration by using a bubbler containing hydroxylamine hydrochloride solution at pH 4.5 and noting the pH change. The men reported the crotonaldehyde vapor to be highly irritating to all mucosal surfaces, particularly the nose and upper respiratory tract (Sim and Pattle 1957). Lacrimation occurred after an average of 30 s, but eye irritation “did not increase after onset of lacrimation.” A confounding factor in the experiment was that there were no restrictions on the men’s activities, and they were allowed to smoke tobacco during exposure; smoking or activity levels were not provided.
The threshold for crotonaldehyde irritation in humans was reported as 0.0005 mg/liter (L) (0.17 ppm; Trofimov 1962). In this experiment, volunteers inhaled crotonaldehyde vapor through a mask for 1 min; it was not specified how the vapor was generated or how the concentrations were measured. Factors taken into account were odor detection and irritation of the eyes and mucous membranes of the nose and trachea; it was not specified on which of these end points the estimated irritation threshold was actually based. Trofimov suggested that the maximum permissible concentration of crotonaldehyde in air should be limited to 0.0005-0.0007 mg/L (0.17-0.24 ppm) to prevent irritation.
2.2.3.
Occupational and Other Exposures
Laboratory personnel (two or three people) who “sniffed” 15 ppm of crotonaldehyde vapor for a few seconds (<30 s) during brief openings of animal chambers reported that the odor was very strong but not intolerable and that there was no eye discomfort. The personnel who “sniffed” 45-50 ppm of crotonaldehyde vapor only momentarily noted that the odor was “very strong, pungent, and disagreeable, but not particularly biting to nasal passages” (Rinehart 1967, 1998). Lacrimation was not induced in the subjects, although they experienced a burning sensation of the conjunctivae and a strong desire to blink repeatedly.
NIOSH conducted a Health Hazard Evaluation in a chemical plant (Sandoz Colors and Chemicals) in East Hanover, New Jersey, at the request of workers at the plant, some of whom complained of occasional minor eye irritation (Fannick 1982). NIOSH measured crotonaldehyde air concentrations using midget impingers; analysis was performed using gas chromatography with flame ionization detection. Eight air samplers were placed near the vats of chemicals and two were worn by the NIOSH industrial hygienist, who was near the vats most of the time. These measurements likely overestimated the actual exposure concentrations because workers were allowed to move about and were not near
the vats during an entire 8-h work shift. NIOSH determined that the average crotonaldehyde concentration of general air samples was 1.6 mg/m3 (0.56 ppm; range, <0.35 to 1.1 ppm; 0.35 ppm was the limit of quantitation). The two personal samples were 0.66 and 0.73 ppm. These workers were also simultaneously exposed to acetic acid and small amounts of acetaldehyde (which occasionally caused a perceptible sweet odor), 3-hydroxybutyraldehyde, and dimethoxane. Crotonaldehyde was probably the most potent irritant among these chemicals, based on its greater quantity and its much lower RD50 (reference dose—the concentration that decreases the respiration rate of mice by 50% due to respiratory irritation [Schaper, 1993; Fannick 1982]).
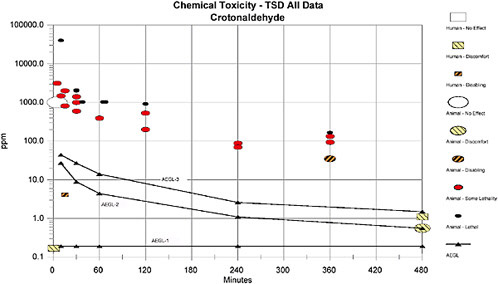
Fieldner et al. (1954) reported that inhalation exposure to crotonaldehyde at 3.5-14 ppm was sufficiently irritating to wake a sleeping person and that 3.8 ppm was irritating within 10 s. Dalla Vale and Dudley (1939) compiled a list of “threshold values” that produce a noticeable odor in the air. The list included crotonaldehyde at 7.3 ppm, which the authors characterized as an eye and a nose irritant. (Experimental details for these two studies were not available.) A summary of the human studies is presented in Table 5-3.
2.3.
Neurotoxicity
No human neurotoxicity studies were located for crotonaldehyde exposure by any route.
2.4.
Developmental and Reproductive Toxicity
No human studies were located that described developmental or reproductive effects resulting from acute exposure to crotonaldehyde.
2.5.
Genotoxicity
Crotonaldehyde (5-250 µM) induced sister chromatid exchanges, structural (but not numerical) chromosome aberrations, and micronuclei in cultured human lymphocytes and Namalva cells (a permanent lymphoblastoid cell line; Dittberner et al. 1995). The micronuclei were centromere-negative by fluorescence in situ hybridization using a human centromere-specific DNA probe, indicating crotonaldehyde was acting by a clastogenic mechanism.
Nath et al. (1998) compared the levels of crotonaldehyde adducts in gingival tissue DNA from human smokers and nonsmokers using a 32P-postlabeling high-performance liquid chromatography method. Smokers had significantly higher levels of the DNA adducts than the nonsmokers (5.5- to 8.8-fold increase). Crotonaldehyde (without exogenous activation) also was shown to bind
TABLE 5-3 Human Crotonaldehyde Exposure Data
|
Exposure Concentration |
Exposure Time |
End Point and Confounding Factors |
Reference |
|
0.035-0.2 ppm 0.037-1.05 ppm 0.12 ppm |
Undefined (a few seconds) |
Odor thresholds from secondary sources; descriptions of most of the original studies were unavailable. |
Verschueren 1996; Ruth 1986; Amoore and Hautala 1983 |
|
0.038 ppm |
Undefined (few seconds) |
Subjects were exposed multiple times. Roughly half detected odor at this air concentration. |
Tepikina et al. 1997 |
|
0.17 ppm |
1 min |
Odor detection and/or irritation; exposure through mask; undefined analytical method. |
Trofimov 1962 |
|
0.56 ppm (up to 1.1 ppm) |
<8 h |
Occasional eye irritation; concentration up to 1.1 ppm; co-exposure to other chemicals. |
Fannick 1982 |
|
4.1 ppm |
15 min (10 min) |
Marked respiratory irritation; lacrimation in ~30 s; co-exposure to cigarette smoke. |
Sim and Pattle 1957 |
|
3.5-14 ppm 3.8 ppm |
Undefined 10 s |
Irritation sufficient to wake a sleeping person “Irritating within 10 s; no further details. |
Fieldner et al. 1954 |
|
7.3 ppm |
Undefined (seconds?) |
Very sharp odor and strong irritation to the eye and nose; no experimental details. |
Dalla Vale and Dudley 1939 |
|
8 ppm 14 ppm (nose) 19 ppm (eyes) |
Undefined (a few seconds) |
Irritation threshold; methods used to determine or define “irritation” were not given. |
Ruth 1986; Amoore and Hautala 1983; Amoore and Hautala 1983 |
|
15 ppm |
<30 s |
Lab workers “sniffed” crotonaldehyde. Odor strong but not intolerable; no eye discomfort. |
Rinehart 1967 |
|
45-50 ppm |
<30 s |
Lab workers “sniffed” crotonaldehyde. Odor strong, pungent, and disagreeable; burning sensation of conjunctivae but no lacrimation. |
Rinehart 1967 |
the DNA of human fibroblasts in vitro (Wilson et al. 1991). Hecht et al. (2001) showed that deoxyguanosine and DNA Schiff-base adducts that formed after crotonaldehyde exposure were unstable at the nucleoside level but stable in DNA.
2.6.
Carcinogenicity
No human data were located that described carcinogenicity associated with crotonaldehyde exposure. In 1991 the U.S. Environmental Protection Agency (EPA) classified crotonaldehyde as in group C (a possible human carcinogen; EPA 2002) based on limited animal data (Chung et al. 1986; see Section 3.6). The International Agency for Research on Cancer (IARC) concluded that there was inadequate evidence for humans and in experimental animals to establish the carcinogenicity of crotonaldehyde and placed it in group 3 (not classifiable as to its carcinogenicity to humans; IARC 1995).
2.7.
Summary
No information concerning acute lethal human exposure to crotonaldehyde was located. Values reported for the odor detection and irritation thresholds in humans were quite variable, ranging from 0.035 to 1.05 ppm and 0.17 to 14 ppm, respectively. The variation may be due to differences in exposure conditions or analytical measurements of concentration, which were often not reported. For example, laboratory workers who intentionally “sniffed” crotonaldehyde for a few seconds found 15 ppm strong but not intolerable, whereas in other studies 3.5-14 ppm (duration unknown) was sufficiently irritating to wake up a sleeping person, and volunteers exposed to 4.1 ppm for 15 min (and also possibly to tobacco smoke) experienced respiratory irritation and lacrimation after an average of 30 s. Workers exposed occupationally to concentrations up to 1.1 ppm crotonaldehyde (along with several other chemicals) reported occasional mild eye irritation. There are no data to indicate that crotonaldehyde is neurotoxic or a human carcinogen by any route of exposure. Crotonaldehyde was clastogenic in cultured human cells. Crotonaldehyde DNA adducts were detected in human buccal cells, in higher levels in smokers than nonsmokers. The chemistry of crotonaldehyde and its direct reactions with DNA and deoxyguanosine have been characterized.
3.
ANIMAL TOXICITY DATA
3.1.
Acute Lethality
Death resulting from acute inhalation exposure to crotonaldehyde has been reported in rats, mice, guinea pigs, and rabbits. The available studies are summarized in Table 5-4.
TABLE 5-4 Acute Lethality of Crotonaldehyde Inhalation Exposure in Animals
|
Species |
Exposure Time |
Concentration (ppm) |
End Point; Reference |
|
|
Rat |
30 min |
35-2450 |
LC50= 1400 ppm. Gasping, eyes tightly shut, lacrimation, nose secretion during treatment; hyperemia in lungs, heart, kidneys, liver, spleen, and brain (Skog 1950). |
|
|
Rat |
1 min 10 min |
“Saturated” (~40,000) |
LC0; no other effects described. LC100; no other effects described. (Smyth and Carpenter 1944; Smyth 1966; Union Carbide Corp. 1992) |
|
|
Rat |
6 h |
35-98 |
LC0; rats had pink extremities, nasal irritation, and labored breathing |
|
|
|
6 h on days 1,2,4 |
94-108 |
LC≥75; rats gasped, had pink extremities, one death after day 1, two after day 4 (other killed on day 5). Lungs were congested. |
|
|
|
6 h |
133; 166; 359 |
LC100; all died within 2 days except for 1 rat inhaling 166 ppm; rats gasped, had nasal irritation, pink extremities, and weight loss. |
|
|
|
30-43 min; 2 h |
2,094-16,229 907; 1,256 |
LC100; death within 2 hours; gasping, pink extremities, tremors, convulsions, salivation, and prostration (Eastman Kodak Corp. 1992). |
|
|
Rat |
5 min 10 min 15 min 30 min 60 min 4 h |
1,920-4,640 800-2,050 550-1,290 370-890 370-640 50-200 |
LC50 = 3132 LC50 = 1480 LC50 = 809 LC50 = 593 LC50 = 391 LC50 = 88 |
All rats gasped, had lowered respiratory rate, lost weight; excitatory stage was seen at ≥1000 ppm; most deaths by day 4, some had clear or blood-stained nasal discharge; few rats had pulmonary congestion (Rinehart 1967; see Table 5-5). |
|
Rat |
4 h |
~70 (not stated) |
LC50 = 70 ppm; no other effects described (Voronii et al. 1982). |
|
|
Species |
Exposure Time |
Concentration (ppm) |
End Point; Reference |
|
Mouse |
2 h |
~530 (not stated) |
LC50 = 530; face rubbing, respiratory distress, excitation, convulsions, lung hemorrhage, edema in lungs and brain, glomerular capillary damage (Trofimov 1962). |
|
Mouse |
2 h |
~200 (not stated) |
LC50 = 200 ppm; no other effects described (Voronii et al. 1982). |
|
Guinea pig |
5 min 30 min 15 min 30 min |
1,000 1,000 2,000 2,000 |
LC0; no other effects described. LC50; no other effects described. LC50; no other effects described. LC100; no other effects described (Smyth 1966). |
|
Mouse |
38 min 64 min |
1,021 ppm vapor 2,663 mg/m3 aerosol |
LC100 exposures. Animals blinked, closed their eyes, and rubbed their faces with their paws, then settled down and breathed deeply and slowly until they convulsed just prior to death. All animals had fluid in the pleural cavity and expanded, edematous, and hemorrhagic lungs with distended alveoli and ruptured alveolar septa due to bronchial constriction; livers appeared enlarged and there was fluid in the peritoneal cavity (Salem and Cullumbine 1960). |
|
Guinea pig |
68 min 86 min |
1,021 ppm vapor 2,663 mg/m3 aerosol |
|
|
Rabbit |
65 79min |
1,021 ppm vapor 2,663 mg/m3 aerosol |
3.1.1.
Rats
Skog (1950) obtained a 30-min LC50 of 4,000 mg/m3 (1,400 ppm) for 48 white rats exposed to 100-7,000 mg/m3 (35-2,450 ppm) of crotonaldehyde vapor (sex, individual concentrations tested, and rats per concentration were not given). Exposure concentrations were not measured analytically but were calculated from the amount of air used to vaporize a measured amount of liquid crotonaldehyde to achieve the target concentration. During treatment the rats gasped and jerked their heads backward at each breath, shut their eyes, lacrimated, and had heavy nose secretion. Exposure was followed by a 3-week observation period; all rats that died did so on or before the second day after treatment. The surviving animals breathed with a “snuffling” sound for 4-5 days after cessation of exposure. Histological examination of the lungs, heart, kidneys, liver, spleen, and brain from at least four rats revealed hyperemia and hemorrhage in the lungs, heart, liver, and kidneys; no edema was evident in the lungs.
Rinehart (1967) conducted an extensive series of experiments to assess the acute toxicity of crotonaldehyde in male Wistar rats. The rats were exposed for 5 min to 4 h and observed for 2 weeks; exposure concentrations and durations are given in Table 5-5. Crotonaldehyde vapors were generated by bubbling nitrogen gas through liquid crotonaldehyde (90% pure) and mixing this with air; the oxygen concentration was maintained at ≥17.8%. Exposure was in either a 20-L glass chamber or a 1,700-L wooden chamber (the latter was used for lower concentrations; which were not specified). Crotonaldehyde concentrations were measured two to five times over the exposure period using a colorimetric reaction with modified Schiff-Elvove reagent; the analytical concentrations were about 42% of the nominal concentration (range: 29-61%). Rinehart suggested that the discrepancy between the nominal and analytical concentrations was due to crotonaldehyde absorption on chamber walls, oxidation, and/or polymerization. The 30-min LC50 obtained by Rinehart (600 ppm) was about 2-fold lower than that obtained by Skog; 1950; 1,400 ppm). Rinehart suggested this difference may have been due to a loss of crotonaldehyde between the point of vapor generation and the animal breathing zone.
During exposure, rats inhaling ≥ 1,000 ppm developed an excitatory stage, and all treated animals had signs of respiratory distress (gasping and lowered respiratory rate) that persisted for several days in some cases. Treated rats lost up to 25% of their body weight within the first 3 days, roughly in proportion to their exposure concentration. Most deaths occurred within 4 days after exposure; these animals had clear or slightly blood-stained nasal discharge; rats that died within a day had terminal convulsions. Death from days 5-14 were attributed to secondary infections. Necropsy showed that a few animals had pulmonary congestion but that other organs were grossly normal. Rinehart visually estimated LC50 values from log-probit plots and obtained values similar to those that can be obtained by probit analysis using the method of Litchfield and Wilcoxon (the estimated and calculated LC50 values are shown in Table 5-5).
TABLE 5-5 Mortality of Rats Exposed to Crotonaldehyde Vapor for 5-240 Minutes
|
Exposure Time (min) |
Analytical Concentration (ppm) |
Cumulative Mortality at Selected Times After Exposure (days) |
LC50 Calculated (estimated)a |
||||
|
1 |
2 |
4 |
7 |
14 |
|||
|
5 |
1,920 |
0/5 |
0/5 |
0/5 |
0/5 |
0/5 |
3,132 ppm (3,150 ppm) |
|
2,420 |
0/5 |
0/5 |
0/5 |
0/5 |
1/5 |
||
|
2,680 |
0/5 |
1/5 |
1/5 |
1/5 |
1/5 |
||
|
3,180 |
3/5 |
3/5 |
3/5 |
3/5 |
3/5 |
||
|
4,160 |
4/5 |
4/5 |
4/5 |
4/5 |
4/5 |
||
|
4,640 |
4/5 |
5/5 |
5/5 |
5/5 |
5/5 |
||
|
10 |
800 |
0/12 |
0/12 |
0/12 |
1/12 |
1/12 |
1,480 ppm (1,380 ppm) |
|
1,110 |
0/12 |
0/12 |
0/12 |
1/12 |
4/12 |
||
|
1,380 |
3/12 |
4/12 |
4/12 |
4/12 |
6/12 |
||
|
1,820 |
6/12 |
7/12 |
7/12 |
7/12 |
7/12 |
||
|
2050 |
8/12 |
8/12 |
9/12 |
9/12 |
9/12 |
||
|
15 |
550 |
0/10 |
0/10 |
0/10 |
0/10 |
0/10 |
809 ppm (750 ppm) |
|
680 |
0/10 |
2/10 |
2/10 |
2/10 |
2/10 |
||
|
750 |
2/10 |
4/10 |
4/10 |
4/10 |
5/10 |
||
|
850 |
2/10 |
3/10 |
5/10 |
5/10 |
7/10 |
||
|
980 |
3/10 |
6/10 |
6/10 |
7/10 |
7/10 |
||
|
1,090 |
3/10 |
5/10 |
7/10 |
8/10 |
8/10 |
||
|
1,290 |
5/10 |
7/10 |
10/10 |
10/10 |
10/10 |
||
|
30 |
370 |
0/10 |
0/10 |
0/10 |
0/10 |
0/10 |
593 ppm (600 ppm) |
|
420 |
1/10 |
2/10 |
2/10 |
2/10 |
2/10 |
||
|
530 |
2/10 |
4/10 |
4/10 |
4/10 |
4/10 |
||
|
675 |
4/10 |
6/10 |
6/10 |
6/10 |
6/10 |
||
|
800 |
5/10 |
7/10 |
7/10 |
7/10 |
8/10 |
||
|
890 |
6/10 |
9/10 |
9/10 |
9/10 |
9/10 |
||
|
60 |
370 |
1/10 |
1/10 |
2/10 |
3/10 |
4/10 |
391 ppm (380 ppm) |
|
400 |
3/10 |
4/10 |
5/10 |
5/10 |
6/10 |
||
|
490 |
3/10 |
5/10 |
6/10 |
6/10 |
7/10 |
||
|
590 |
4/10 |
6/10 |
7/10 |
7/10 |
7/10 |
||
|
640 |
8/10 |
9/10 |
10/10 |
10/10 |
10/10 |
||
|
240 |
50 |
0/10 |
0/10 |
1/10 |
1/10 |
1/10 |
88 ppm (85 ppm) |
|
60 |
0/10 |
0/10 |
2/10 |
2/10 |
2/10 |
||
|
70 |
0/10 |
1/10 |
3/10 |
3/10 |
4/10 |
||
|
100 |
4/10 |
5/10 |
5/10 |
5/10 |
6/10 |
||
|
120 |
5/10 |
5/10 |
8/10 |
8/10 |
8/10 |
||
|
200 |
6/10 |
6/10 |
9/10 |
9/10 |
9/10 |
||
|
aLC50 for the 14-day mortality data were calculated by probit analysis in May 1998; values in parentheses are the LC50 estimates given by Rinehart (1967). Source: Adapted from Rinehart 1967. Reprinted with permission; copyright 1967, American Industrial Hygiene Association Journal. |
|||||||
Several of the rat acute lethality studies summarized in Table 5-4 were sparsely described and omitted significant details of the experimental procedure and/or results. In related studies described by Smyth and Carpenter (1944), Smyth (1966), and Union Carbide Corp. (1992), six male albino rats exposed to a flowing stream of air saturated with crotonaldehyde vapor (about 40,000 ppm) for 1 min had 0 deaths, whereas exposure for 10 min killed the six rats in the ensuing 2-week observation period. Voronii et al. (1982) reported a 4-h LC50 of 200 mg/m3 (70 ppm) for white rats during an observation period of 2 weeks. In preliminary acute toxicity studies, groups of three or four rats (sex and strain not specified) were exposed to nominal crotonaldehyde concentrations of 2,094-16,229 ppm for 30-43 min, 907 or 1,256 ppm for 2 h, 133-359 ppm for 6 h, or 94-108 ppm for 6 h/day on days 1, 2, and 4 (Eastman Kodak Corp. 1992). Many animals died, as shown in Table 5-4. Symptoms included gasping, labored breathing, pink extremities, tremors, convulsions, salivation, and prostration. Microscopic examination of unspecified animals revealed lung congestion.
3.1.2.
Mice
Salem and Cullumbine (1960) exposed groups of 50 mice to a mean concentration of 2,925 mg/m3 (1,021 ppm) of crotonaldehyde vapor or to 2,663 mg/m3 of crotonaldehyde aerosol in a 1-m3 plate-glass exposure chamber. The aerosol particle size was estimated to be 0.7 µm in diameter. Upon exposure, the mice initially blinked, closed their eyes, and rubbed their faces with their paws but then settled down and breathed deeply and slowly until they convulsed just prior to death. The mice died after an average exposure of 38 min for the vapor and 64 min for the aerosol. All animals had fluid in the pleural cavity and expanded, edematous, and hemorrhagic lungs with distended alveoli and ruptured alveolar septa due to bronchial constriction. The livers appeared enlarged, and there was fluid in the peritoneal cavity.
The mean lethal concentration or LC50 for white mice exposed to crotonaldehyde for 2 h was stated to be 530 ppm by Trofimov (1962) and 200 ppm by Voronii et al. (1982). Trofimov reported that the animals rubbed their faces with their paws and displayed respiratory distress and that microscopic examination showed lung hemorrhage, edema in the lungs and brain, and disintegration of renal glomerular capillaries.
3.1.3.
Guinea Pigs
Three of six guinea pigs exposed to 2,000 ppm of crotonaldehyde vapor (nominal) for 15 min or 1,000 ppm for 30 min died. Exposure to 1,000 ppm (nominal) for 5 min resulted in 0 deaths, whereas six of the died from a 30-min exposure to 2,000 ppm (further details not provided; Smyth 1966).
All 20 guinea pigs died following exposure for an average of 68 min to crotonaldehyde vapor at 2,925 mg/m3 (1,021 ppm) or for 86 min to crotonaldehyde aerosol at 2,663 mg/m3 (0.7 µ in diameter) (Salem and Cullumbine 1960). Initially, exposed animals blinked, closed their eyes, and rubbed their faces with their paws but then settled down and breathed deeply and slowly until they convulsed just prior to death. All animals had fluid in the pleural cavity and expanded, edematous, and hemorrhagic lungs with distended alveoli and ruptured alveolar septa due to bronchial constriction. The livers appeared enlarged, and there was fluid in the peritoneal cavity.
3.1.4.
Rabbits
Death ensued in five rabbits exposed for an average of 65 min to crotonaldehyde vapor at 2,925 mg/m3 (1,021 ppm) or for 79 min to crotonaldehyde aerosol at 2,663 mg/m3 (0.7 µ in diameter) (Salem and Cullumbine 1960). Initially, exposed animals blinked, closed their eyes, and rubbed their faces with their paws but then settled down and breathed deeply and slowly until they convulsed just prior to death. All animals had fluid in the pleural cavity and expanded, edematous, and hemorrhagic lungs with distended alveoli and ruptured alveolar septa due to bronchial constriction. The livers appeared enlarged, and there was fluid in the peritoneal cavity.
3.2.
Nonlethal Toxicity
3.2.1.
Rats
Alterations in pulmonary performance caused by exposure to 10-580 ppm of crotonaldehyde for 5 min to 4 h were investigated using Wistar rats (Rinehart 1967). Pulmonary performance was evaluated by measuring the rates of ether and CO absorption over a 24-h period following crotonaldehyde exposure; typical evaluations were at 1, 2, 6, 10, and 24 h postexposure (Rinehart 1998). A parallel drop in CO and ether uptake implies that the pulmonary ventilation rate was reduced (compared to preexposure levels); a greater drop in CO than ether absorption suggests that the diffusion rate of oxygen from air in the lungs into the blood was reduced (Rinehart and Hatch 1964). The individual concentrations and exposure times were not given; rather test responses were presented for five ranges of concentration times time (Ct) due to variations found among animals within any given exposure scenario. Twelve rats were tested in each exposure range, as shown in Table 5-6. Crotonaldehyde caused a parallel dose-dependent decrease in CO and ether uptake rates that were significant at the 5% or 10% level (for CO and ether, respectively) for Ct of ≥2,000 ppm-min. Death occurred in four animals before 24 h (time not specified) treated with 16,000-32,000 ppm-
TABLE 5-6 Pulmonary Responses of Rats That Inhaled 10-580 ppm of Crotonaldehyde for 5-240 min
|
Concentration × Time Range (ppm-min) |
Geometric Mean Concentration × Time |
Number of Animals |
CO Uptake Rate (% of preexposure ± 1 SD) |
Ether Uptake Rate (% of preexposure ± 1 SD) |
|
Controls |
0 |
12 |
99.5 ± 12.5 |
103.1 ± 12.8 |
|
1,000-2,000 |
1,330 |
12 |
92.9 ± 9.0 |
94.8 ± 9.4 |
|
2,000-4,000 |
2,730 |
12 |
89.9 ± 5.6** |
92.8 ± 5.7* |
|
4,000-8,000 |
5,390 |
12 |
86.7 ± 11.3** |
91.0 ± 14.9* |
|
8,000-16,000 |
10,940 |
12 |
73.3 ± 12.8** |
81.2 ± 9.6** |
|
16,000-32,000 |
21,430 |
10 |
58.3 ± 10.8** |
67.0 ± 9.2** |
|
16,000-32,000 (animals died) |
28,900 |
4 |
<40 |
<40 |
|
Significantly different from controls: *p ≤ .10, **p <.05. Source: Rinehart 1967. Reprinted with permission; copyright 1967, American Industrial Hygiene Association Journal. |
||||
min (geometric mean = 28,900 ppm-min). Concentration and time were stated to be roughly equally important in determining toxicity. The maximal depression in the uptake of the gases occurred 6-10 h after treatment, with subsequent recovery taking 24-72 h. Animals exposed to >8,000 ppm-min and autopsied 3 days after exposure had proliferative lesions of the respiratory bronchioles. Edema was evident only at high Ct values (>16,000 ppm-min), where death occurred within 24 h. Based on these results, Rinehart (1967) concluded that “crotonaldehyde is predominantly a typical deep lung irritant,” with the point of attack being the bronchiole and not the alveolus itself.
The concentration of crotonaldehyde calculated to reduce the respiration rate of male F344 rats by 50% upon exposure for 10 min (RD50) was 23.2 ppm (Babiuk et al. 1985). Rats (four per concentration) were exposed to five to eight different concentrations (not specified). Crotonaldehyde vapor was generated in a modified impinger and was carried to the inlet of a head-only exposure chamber by a nitrogen stream; chamber concentrations were continuously monitored with an infrared gas spectrophotometer. Rats that were exposed 6 h/day for 9 days to 15 ppm of formaldehyde, followed by challenge on day 10 with crotonaldehyde, had a similar RD50 (20.5 ppm), indicating desensitization was not caused by prior formaldehyde inhalation (Babiuk et al. 1985).
Rats (sex and strain not specified) were exposed for 30 min to 12.7, 1.3, 0.28, 0.14, or 0.02 mg/m3 of crotonaldehyde vapor (Tepikina et al. 1997). After 72 h, some animals were necropsied (exposure concentration not specified), and changes were seen in the morphology of the lung and liver tissues of rats exposed to 12.7 or 1.3 mg/m3. The nature of the changes and the analytical technique used to measure crotonaldehyde in air were not described.
3.2.2.
Mice
The RD50 (i.e., 50% reduction in respiration rate) values for crotonaldehyde vapor in male Swiss-Webster mice and B6C3F1 mice were 3.53 and 4.88 ppm, respectively (Steinhagen and Barrow 1984). Mice were exposed to crotonaldehyde for 10 min in a head-only exposure chamber, and their breathing rates were measured using plethysmographic techniques (Alarie 1966). The crotonaldehyde chamber concentrations were continuously monitored with an infrared gas spectrophotometer (Steinhagen and Barrow 1984).
3.2.3.
Rabbits
The threshold concentration of crotonaldehyde in air that was irritating to the mucosa of rabbits was reported as 0.05 mg/L (17.5 ppm; Trofimov 1962).
Respiration and heart rate were significantly decreased in male rabbits that inhaled 5 ppm of crotonaldehyde for <10 min (Ikeda et al. 1980).
3.2.4.
Cats
The threshold concentration of crotonaldehyde in air that was irritating to the mucosa of cats was 0.009 mg/L (3.15 ppm; Trofimov 1962).
3.3.
Neurotoxicity
No neurotoxicity animal studies were located with crotonaldehyde exposure by any route.
3.4.
Developmental and Reproductive Toxicity
No mammalian developmental or reproductive toxicity studies were located with crotonaldehyde exposure by any route.
3.5.
Genotoxicity
Crotonaldehyde (≤0.5 µL/assay) was mutagenic in Salmonella typhimurium TA100 when tested using a modified liquid suspension protocol, with or without metabolic activation (Lijinsky and Andrews 1980; Neudecker et al. 1981, 1989; Lutz et al. 1982; Zeng et al. 1986; Eder et al. 1992, 1993). There was no evidence for mutagenicity using the standard Ames plate-incorporation assay (Simmon et al. 1977; Florin et al. 1980; Cooper et al. 1987). The Salmo-
nella tester strains TA1535, TA1537, TA1538, and TA98 did not show an increase in the number of revertants when using either the liquid suspension or plate incorporation methods (Florin et al. 1980; Lijinsky and Andrews 1980; Neudecker et al. 1981). The high cytotoxicity of crotonaldehyde and formation of pinpoint colonies confounded the assay (Eder et al. 1993).
Crotonaldehyde was not genotoxic in the SOS chromotest using E. Coli PQ37 and PQ243. In this test the sfi A gene-linked β-galactosidase activity is determined as a measure of the induction of the SOS repair system by xenobiotics. The lack of a response may have been a result of inadequate exposure concentration, which was intended to prevent crotonaldehyde bacteriotoxicity (Eder et al. 1992). When ethanol was used as the crotonaldehyde solvent instead of DMSO, a positive response was obtained with E. Coli PQ37 (Eder et al. 1993). A weak SOS response was seen in Salmonella typhimurium TA1535/pSK1002 without metabolic activation (Benamira and Marnett 1992).
Crotonaldehyde did not induce mitotic recombination in Saccharomyces cerevisiae D3 (Simmon et al. 1977).
Adult male Drosophila melanogaster injected with 3,500 ppm of crotonaldehyde (0.2-0.3 µL) 24-48 h before mating had a significant increase in sex-linked recessive lethals and in reciprocal (heritable) translocations (Woodruff et al. 1985). Males fed 4,000 ppm of crotonaldehyde for 3 days, however, failed to exhibit increased sex-linked recessive lethality. Chromosome breakage and reciprocal translocations were both detected.
Crotonaldehyde inhibited DNA synthesis in HeLa cells (Zeng et al. 1986) and induced chromosome aberrations and sister chromatid exchanges in CHO cells (Galloway et al. 1987). Unscheduled DNA synthesis was not induced by incubation of rat hepatocyte primary cell cultures with up to 7 mM crotonaldehyde (Williams et al. 1989).
Crotonaldehyde (without exogenous activation) was shown to bind to calf thymus DNA in vitro (Chung et al. 1984). In binding studies with nucleosides and 5-mononucleotides, crotonaldehyde formed three types of adducts with deoxyguanine and 2-deoxyguanosine 5-monophosphate (1,N2 and 7,8 adducts, and 1,N2 /7,8 bis-adducts), but there were no detectable products with the other nucleosides or 5-mononucleotides (Eder and Hoffman 1992). Crotonaldehyde DNA adducts were formed in CHO cells treated in culture (Foiles et al. 1990).
A 32P-postlabeling method has detected the cyclic 1,N2-propanedeoxyguanosine adduct (0.24 µmol/mol guanine) in the skin of mice treated topically with 1.4 mmol crotonaldehyde (Chung et al. 1989). Small amounts of this cyclic adduct have also been detected in the livers of untreated rats, mice, and humans (1.0-1.7, 0.2-1.0, and 0.3-2.0 adducts per 106 guanine residues, respectively; Nath and Chung 1994). DNA adducts were detected in the livers, lungs, kidneys, and large intestine (~3, 2, 1, and 0.5 adducts per 108 guanines) of 8-week old female F344 rats 20 h after receiving 300 mg/kg of crotonaldehyde in 1-mL corn oil by gavage (Eder et al. 1997). Most adducts were in the liver. No adducts were detected in untreated females in the same study.
Crotonaldehyde caused DNA-protein crosslinks in vitro, assayed using a filter-binding assay based on the precipitation of 3H-labeled plasmid DNA (pUC13) bound to calf-thymus histones (Kuykendall and Bogdanffy 1992). A 2-h treatment of the shuttle vector plasmid pZ189 with crotonaldehyde caused DNA damage including point mutations, deletions, insertions, and inversions; the vector was transfected into the human lymphoblastoid cell line GM0621 (Czerny et al. 1998).
Oral (2 g/L for 50 days) or intraperitoneal administration of crotonaldehyde to strain Q mice caused production of polyploid cells at all stages of spermatogenesis, degenerated spermatogenic cells in the seminiferous tubules, and abnormal pairing of sex chromosomes at diakinesis or metaphase I (Moutschen-Dahmen et al. 1976; Auerbach et al. 1977).
3.6.
Carcinogenicity
No inhalation exposure studies were located. One chronic oral bioassay was located in which male F344 rats were given 0, 0.6, or 6.0 mM of crotonaldehyde in drinking water for 113 weeks (Chung et al. 1986). This is equivalent to inhalation exposure to 0, 7.2, and 72 ppm, respectively, by route-to-route extrapolation, as described in Appendix D. The high-dose group had approximately 10% lower body weight gain starting at week 8, and 10 of 23 rats developed moderate to severe liver damage (fatty metamorphosis, focal necrosis, fibrosis, cholestasis, mononuclear cell infiltration). The incidence of hepatic neoplastic nodules and hepatocellular carcinomas combined was 0 of 23, 11 of 27 (p < .01), and 1 of 23 at 0, 0.6, and 6.0 mM, respectively (carcinoma: 0 of 23, 2 of 27, 0 of 23, respectively). The incidence of enzyme-altered liver foci, considered to be precursors to neoplasms, was 1 of 23, 23 of 27 (p < .01), and 13 of 23 (p < .01) at 0, 0.6 and 6.0 mM, respectively. No explanation was offered for the lack of a neoplastic dose-response. Interestingly, the 10 high-dose animals that had severe liver toxicity had no liver neoplasms, but the remaining 13 high-dose rats were found to have hepatocellular carcinomas. The authors state “it is worth noting” that two low-dose rats had urinary bladder papillomas (none in controls or high-dose group) but did not indicate whether they considered these tumors to be treatment related.
In 1991 EPA classified crotonaldehyde as a weight-of-evidence group C (possible human) carcinogen, although a quantitative estimate of the carcinogenic risk from oral exposure was not developed (EPA 2002). EPA classification was based on the increased incidence of hepatic neoplastic nodules and hepatocellular carcinomas (combined) in rats in the Chung et al. (1986) study (despite the lack of a dose-response), a lack of human data, crotonaldehyde genotoxic activity in some of the short-term tests, the anticipated reactivity of croton oil (a known tumor promoter) and aldehyde with DNA, and the fact that crotonaldehyde is a suspected metabolite of the probable human carcinogen N-nitrosopyrrolidine (EPA weight-of-evidence classification B2). Based on the
EPA’s 1999 Draft Revised Guidelines, the most appropriate cancer classification descriptor for crotonaldehyde would be “suggestive evidence of carcinogenicity, but not sufficient to assess human carcinogenic potential” (EPA 1999). The ACGIH (1998) has assigned crotonaldehyde to the A3, animal carcinogen, classification. This was based on positive genotoxicity data (caused mutations, clastogenicity, and DNA adducts) and on the Chung et al. (1986) carcinogenicity study in which crotonaldehyde-treated rats developed liver neoplastic lesions and hepatocellular carcinomas.
The IARC (1995), however, noted that the increased incidences of hepatic neoplastic nodules and altered liver-cell foci in rats in the Chung et al. study were not seen at the high dose. IARC therefore concluded that there was inadequate evidence in both humans and experimental animals to establish the carcinogenicity of crotonaldehyde and placed it in group 3 (not classifiable as to its carcinogenicity to humans).
In addition to being a possible metabolite of N-nitrosopyrrolidine (Wang et al. 1988), crotonaldehyde is a metabolite of the suspected human carcinogen 1,3-butadiene (Cheng and Ruth 1993; Filser et al. 2001; EPA 2002).
3.7.
Summary
In acute lethality studies, rats, mice, guinea pigs, and rabbits were exposed for 1 min to 6 h with crotonaldehyde concentrations ranging from 50 ppm to “saturated” vapor (about 40,000 ppm). Rat LC50 values for a given exposure period were about 2-fold lower than those for mice and guinea pigs, although in a second study the rat LC50 was comparable to that for the other two species. The differences in response may have been due to the use of nominal versus analytical concentrations. The animals in the acute lethality studies had breathing difficulties, lacrimation, blood-stained nose secretions, pink extremities, and body weight loss. Histological examination revealed ruptured alveolar septa and hemorrhage in the lungs, heart, liver, and kidneys. In a pulmonary function study, animals treated with >16,000 ppm-min died and some had lung edema, and rats exposed to >8,000 ppm-min developed proliferative lesions of the respiratory bronchioles. The respiration rate was reduced by 50% (i.e., RD50) in rats exposed head only for 10 min to 23.2 ppm and in mice exposed head only to 3.53-4.88 ppm.
Crotonaldehyde was mutagenic in Salmonella typhimurium TA100 (± metabolic activation), caused induction of the SOS response in E. Coli PQ37, induced sex-linked recessive lethals and reciprocal translocations in Drosophila melanogaster, inhibited DNA synthesis, induced chromosome aberrations and sister chromatid exchanges, and was shown to bind to DNA in vitro and in vivo. Male rats given 0.6 or 6.0 mM of crotonaldehyde in their drinking water for 113 weeks developed hepatic neoplastic nodules, hepatocellular carcinomas, and altered liver foci, although the incidence was not dose related.
4.
SPECIAL CONSIDERATIONS
4.1.
Metabolism and Disposition
Little information was available regarding the metabolism and disposition of crotonaldehyde following inhalation exposure. One route of human crotonaldehyde excretion is milk: Crotonaldehyde was detected qualitatively in the milk of 1 of 12 lactating women who lived in an urban environment for ≥1 year, although the atmospheric crotonaldehyde levels were not reported (Pellizzari et al. 1982).
Male F344 rats given 2.8 mg/kg of [14C]-crotonaldehyde intravenously excreted 31% of the administered radioactivity as 14CO2 and 37% as urinary metabolites within 6 h of dosing. Elimination of crotonaldehyde increased to 40% in expired air and 50% in the urine after 72 h (NTP 1985). Essentially all the crotonaldehyde was metabolized, as <1% of the 14C in the urine was parent compound. There was no significant radioactivity in any tissues or in the feces, suggesting that neither the parent compound nor its metabolites accumulated in the body.
[14C]-Crotonaldehyde administered by gavage to adult male F344 rats at 0.7, 3, or 35 mg/kg was largely absorbed from the gastrointestinal tract: 60-78% was excreted in the breath and urine within 12 h of dosing, and after 72 h, this increased to 82-86% (NTP 1985). Approximately 7% of the administered radioactivity was eliminated in the feces.
Crotonaldehyde can be conjugated with glutathione with or without glutathione S-transferase activity (Esterbauer et al. 1991). Male albino and black-hooded rats injected subcutaneously with 0.75 mmol/kg (53 mg/kg) of crotonaldehyde in olive oil had 3-hydroxyl-1-methylpropyl and 2-carboxyl-1-methylpropyl-mercapturic acids in their urine (collected over 24 h), which represented 6-15% of the given dose (Gray and Barnsley 1971). Smaller amounts of 2-carboxy-1-methylethylmercapturic acid also were occasionally detected. Because crotonaldehyde caused rapid sulfhydryl depletion in an in vitro reaction with glutathione in buffer, Gray and Barnsley (1971) proposed that the thiol group of glutathione was adding to the double bond of crotonaldehyde, which was then hydrolyzed to form these metabolites in the rat.
4.2.
Mechanism of Toxicity
Crotonaldehyde is a well-recognized severe eye and respiratory irritant, although little information regarding its mechanism of toxicity was available. It appears to be primarily a locally acting irritant; systemic effects were seen only after exposure to extremely high doses (i.e., which caused death within 2 h). Crotonaldehyde is a deep lung irritant, apparently acting at the level of the bronchioles (Rinehart 1967).
It has been suggested that depletion of reduced glutathione in cells is involved in cellular toxicity caused by crotonaldehyde (reviewed in ACGIH 1998). Human polymorphonuclear leukocytes (PMNLs) had a dose-related decrease in surface sulfhydryls and soluble sulfhydryls after in vitro treatment with crotonaldehyde (Witz et al. 1987) and a dose-dependent inhibition of PMNL adherence (assayed with nylon fiber columns) and chemotaxis (Bridges et al. 1980).
Crotonaldehyde caused ciliostasis in chicken tracheal organ cultures incubated for 5 min with 5 mM of crotonaldehyde (Pettersson et al. 1982). Since the basic mechanism of ciliated epithelia are likely similar in all organisms, including humans, Pettersson et al. suggested that the respiratory toxicity of inhaled crotonaldehyde may be due in part to its inhibition of ciliary movement.
4.3.
Structure-Activity Relationships
Steinhagen and Barrow (1984) evaluated the sensory irritation potential of inhaled aldehydes in B6C3F1 and Swiss-Webster mice by comparing the concentrations that caused a 50% reduction in the respiration rate (RD50). Saturated aliphatic aldehydes with ≥ 2 carbons (acetaldehyde, propionaldehyde, butyraldehyde, isobutyraldehyde, valeraldehyde, isovaleraldehyde, caproaldehyde, and 2-ethylbutyraldehyde) were the least irritating, with RD50 values of 750-4,200 ppm. Cyclic aldehydes (2-furaldehyde, cyclohexane carboxaldehyde, 3-cyclohexane-1-carboxaldehyde, and benzaldehyde) had RD50 values of 60-400 ppm. Unsaturated aliphatic aldehydes (formaldehyde, acrolein, and crotonaldehyde) were the most irritating, having RD50 values of 3.2/4.90, 1.03/1.41, and 3.53/4.88 ppm, respectively (in Swiss-Webster/B6C3F1 mice). The two strains of mice had similar RD50 values for any given chemical. Crotonaldehyde was thus shown to be a far more potent irritant than the cyclic or saturated aliphatic aldehydes, a less potent than acrolein, and a similarly potent irritant as formaldehyde in Swiss-Webster and B6C3F1 mice.
Skog (1950) compared the inhalation LC50 of several aldehydes, including formaldehyde, acetaldehyde, propionaldehyde, and butyraldehyde, acrolein, and crotonaldehyde. He found that for the saturated hydrocarbon aldehydes studied, the toxicity decreased with increased molecular weight (this also held true when administration was by injection). The unsaturated aldehydes—acrolein (the most toxic) and crotonaldehyde—were more acutely toxic than their saturated analogs propionaldehyde and butyraldehyde and were the most acutely toxic of the aldehydes tested. Crotonaldehyde, formaldehyde, and acrolein primarily caused lung and respiratory tract irritation and lung injury and had a mild narcotic effect, whereas the narcotic effect was the primary sign resulting from acetaldehyde, propionaldehyde, and butyraldehyde exposure.
Groups of 50 mice, 20 guinea pigs, and five rabbits were exposed to vapor and/or aerosols of acrolein, crotonaldehyde, formaldehyde, acetaldehyde, propi-
onaldehyde, and isomers of butyraldehyde until death ensued or up to 10 h (Salem and Cullumbine 1960). The results indicated that the unsaturated aldehydes (acrolein and crotonaldehyde) were more potent (in terms of mean fatal dose) than the saturated aldehydes and that increased chain length was associated with decreased toxicity. At necropsy all animals displayed severe alveolar lung damage: hemorrhage, distended alveoli, ruptured alveolar septa, and pleural edema. Toxicity of the compounds was similar whether they were in aerosol or vapor form.
4.4.
Other Relevant Information
4.4.1.
Species Variability
LC50 values for several species varied by a factor of ≤ 2.5 for several exposure durations, indicating that interspecies variability was minor. For example, a 15-min LC50 of 809 ppm (analytical) was obtained for rats by Rinehart (1967), whereas Smyth (1966) obtained a 15-min LC50 of 2,000 ppm (nominal). For 30-min exposures, LC50 values of 1,400 ppm (nominal) and 593 ppm (analytical) were obtained for rats (Skog 1950; Rinehart 1967) and an LC50 of 1,000 ppm (nominal) was obtained for guinea pigs (Smyth 1966). Mouse 2-h LC50 values of 200 ppm (unknown if nominal) and 530 ppm (analytical) are reported (Voronii et al. 1982; Trofimov 1962), and although rat 2-h LC50 values are not available, the mouse LC50 values are roughly consistent with rat 1-h LC50 values of 391 ppm (analytical; Rinehart 1967) and 4-h LC50 values of 70 ppm (unknown if nominal) and 88 ppm (analytical; Voronii et al. 1982; Rinehart 1967).
4.4.2.
Susceptible Populations
No populations uniquely susceptible to crotonaldehyde exposure were identified.
4.4.3.
Concentration-Exposure Duration Relationship
ten Berge et al. (1986) determined that the concentration-time relationship for many irritant and systemically acting vapors and gases may be described by Cn × t = k, where the exponent n ranged from 0.8 to 3.5, and n ranged from 1 to 3 for 90% of the chemicals examined. The value of n = 1.2 was determined by ten Berge et al. by linear regression analysis of the Rinehart (1967) rat LC50 data and was used to perform scaling across time for AEGL-3 values.
For the calculation of AEGL-2 values, the end point was impaired pulmonary function and the NOAEL for proliferative lesions of the respiratory bronchioles at 8,000 ppm-min. A value of n = 1 was used to scale across time, based
on the fact that in this study there was a general dose response for increasing exposure levels, but individual concentrations and exposure times were not given (exposure was 5 min to 4 h for 10-580 ppm). Concentration and time were roughly equally important for toxicity. AEGL-2 values were therefore calculated by dividing 8,000 ppm-min by 10, 30, 60, 240, or 480 min.
No data were available from which to determine the concentration-time relationship for crotonaldehyde AEGL-1 effects (mild eye irritation). The n values used for AEGL-2 and AEGL-3 effects (n = 1.2 or n = 1) did not appear to be appropriate for predicting human sensory irritation based on a comparison of two human studies (Sim and Pattle 1957; Fannick 1982). In these studies, irritation was much greater for shorter exposure durations than for longer exposure durations yielding comparable Ct (concentration × time) values: 4.1-ppm exposure for 10 min (C1 × t = 41 ppm-min) was highly irritating to the upper respiratory tract and caused lacrimation, whereas exposure to 0.56 ppm for (up to) 8 h (C1 × t = 269 ppm-min) caused only mild eye irritation. It was thus considered more appropriate to use the same exposure concentration (0.56 ppm) for 10 min to 8 h since mild irritant effects generally do not vary greatly over time.
5.
DATA ANALYSIS FOR AEGL-1
5.1.
Summary of Human Data Relevant to AEGL-1
Two human studies were located in which concentrations of crotonaldehyde were measured and exposure durations were of appropriate length for deriving AEGL-1 values. In one study, 12 healthy males were exposed for 15 min to 4.1 ppm of crotonaldehyde vapor (and cigarette smoke) in a 100-m3 chamber. The men found it to be highly irritating to the nose and upper respiratory tract and lacrimated after about 30 s (Sim and Pattle 1957). In a second study, workers in a chemical plant who were exposed to a mean of 0.56 ppm for <8 h/day complained of occasional minor eye irritation (Fannick 1982). In the Fannick (1982) study, <0.35-1.1 ppm (mean = 0.56 ppm) was measured in eight stationary area samples and two personal samples worn by the hygienists were 0.66 and 0.73 ppm (limit of quantitation [LOQ] = 0.35 ppm). Both studies had the drawback that the subjects were likely exposed simultaneously to other chemicals (although crotonaldehyde was likely the most irritating). These concentrations (0.56 and 4.1 ppm) are above the generally reported odor detection threshold of 0.035-0.2 ppm (Amoore and Hautala 1983; Verschueren 1996).
Other studies in which humans were exposed to crotonaldehyde were not useful for AEGL derivation because the exposure time was too brief (≤1 min) or was not specified. These studies were compromised in that insufficient descriptions of the analytical method of crotonaldehyde concentration measurement were given.
5.2.
Summary of Animal Data Relevant to AEGL-1
The threshold concentrations of crotonaldehyde that were irritating to the mucosa of rabbits and cats were reported as 17.5 ppm and 3.15 ppm, respectively (Trofimov 1962).
5.3.
Derivation of AEGL-1
AEGL-1 values were derived from a Health Hazard Evaluation conducted by NIOSH at a U.S. chemical plant where some workers who were exposed to approximately 0.56 ppm of crotonaldehyde reported occasional minor eye irritation (Fannick 1982). It is possible that some of the workers had become adapted (inurred) to crotonaldehyde, but there was insufficient information to quantitate the effect of this phenomenon (which is commonly experienced with other aldehydes, e.g., formaldehyde). The workers were co-exposed to several other airborne chemicals, although available mouse (RD50) irritation data and occupational reports indicated that crotonaldehyde was the most irritating. Exponential scaling across time was not performed (see Section 4.4.2 for discussion); rather, it was considered more appropriate to adopt the same exposure concentration for 10 min to 8 h since the critical end point (ocular irritation) generally does not vary greatly over time. A total uncertainty factor of 3 was applied to account for intraspecies variability, because the eye irritation is a direct surface-contact effect not subject to pharmacokinetic differences between individuals. The resulting AEGL-1 values are shown in Table 5-7; calculations are detailed in Appendix A.
The AEGL-1 values are consistent with the RD50 values of 3.53 and 4.88 ppm that were obtained for crotonaldehyde using male Swiss-Webster and B6C3F1 mice, respectively (Steinhagen and Barrow 1984). According to Alarie (1981), 0.1 of the RD50 (i.e., 0.35 or 0.49 ppm) for several hours to days should result in some sensory irritation in humans, whereas 0.01 × RD50 (0.035 or 0.049 ppm) should cause no sensory irritation.
6.
DATA ANALYSIS FOR AEGL-2
6.1.
Summary of Human Data Relevant to AEGL-2
No human data were located that were appropriate for derivation of AEGL-2 levels.
TABLE 5-7 AEGL-1 Values for Crotonaldehyde
|
10 min |
30 min |
1 h |
4 h |
8 h |
|
0.19 ppm (0.55 mg/m3) |
0.19 ppm (0.55 mg/m3) |
0.19 ppm (0.55 mg/m3) |
0.19 ppm (0.55 mg/m3) |
0.19 ppm (0.55 mg/m3) |
6.2.
Summary of Animal Data Relevant to AEGL-2
Only one animal study presented an end point consistent with the AEGL-2 definition and provided sufficient experimental details for AEGL derivation. In this pulmonary performance study, rats displayed concentration-related reductions in the rates of ether and CO absorption compared to preexposure levels (see Table 5-6). Rats exposed to >8,000 ppm-min (product of concentration and time, individual values not provided) developed proliferative lesions of the respiratory bronchioles, but exposures above 16,000 ppm-min induced pulmonary edema and the animals died (Rinehart 1967).
Several animal studies described end points potentially within the scope of the AEGL-2 definition, although sufficient experimental detail was not provided for the studies to be useful for AEGL derivation. In one study the respiration rate and heart rate of male rabbits were significantly decreased after inhalation of 5 ppm of crotonaldehyde for <10 min (Ikeda et al. 1980). Nasopharyngeal mucosal morphological changes were found in rats exposed for 30 min to ≥0.45 ppm, although the nature of the changes and the analytical methods used were not described (Tepikina et al. 1997).
6.3.
Derivation of AEGL-2
AEGL-2 values were derived from the pulmonary performance study in which rats exposed to 8,000 ppm-min had reduced rates of gas absorption. This exposure was near the threshold for developing proliferative lesions of the respiratory bronchioles. Because the individual concentrations and exposure times were not given (exposure was 5 min to 4 h to 10-580 ppm), only the concentration × time (Ct) values, and it appeared from the overall data that concentration and time were roughly equally important for toxicity [this is also supported by n = 1.2 derived from the LC50 study by Rinehart (1967)], AEGL-2 values were calculated by dividing 8,000 ppm-min by 10, 30, 60, 240, or 480 min. A total uncertainty factor of 30 was used: 10 for interspecies uncertainty (because the actual exposure concentration and time were not known for the key study and there was a lack of supporting animal studies) and 3 for intraspecies uncertainty [although human variability to crotonaldehyde toxicity is not well defined, a greater uncertainty factor was judged inappropriate because it yields 4- and 8-h AEGL-2 concentrations that caused only mild irritation in workers exposed for up to 8 h (Fannick 1982)]. The resulting AEGL-2 values are shown in Table 5-8; calculations are shown in Appendix A.
A cancer inhalation slope factor was derived for crotonaldehyde and used to estimate the 10−4 excess cancer risk from a single 30-min to 8-h exposure, as shown in Appendix D. Crotonaldehyde concentrations associated with a 10−4 excess cancer risk were 25-fold greater than the toxicity-based AEGL-2 values
TABLE 5-8 AEGL-2 Values for Crotonaldehyde
|
10 min |
30 min |
1 h |
4 h |
8 h |
|
27 ppm (77 mg/m3) |
8.9 ppm (26 mg/m3) |
4.4 ppm (13 mg/m3) |
1.1 ppm (3.2 mg/m3) |
0.56 ppm (1.6 mg/m3) |
for 30 to 480 min. The noncarcinogenic end points were considered more appropriate for AEGL-2 derivation because (1) there is insufficient evidence that inhalation is a route that results in crotonaldehyde-induced liver lesions or neoplasia at concentrations comparable to the AEGL-2 values; (2) the data used to derive the cancer slope factor were very weak (the key study had only one dose and one control group; the high dose was excluded due to lack of fit), and most of the neoplastic changes were benign; (3) AEGL values are applicable to rare events or single once-in-a-lifetime exposures, and the data indicate that TNM neoplasms resulted from lifetime treatment; and (4) a direct comparison of estimated TNM cancer risk and AEGL values is not appropriate due to large differences in the methodologies used to obtain these numbers.
7.
DATA ANALYSIS FOR AEGL-3
7.1.
Summary of Human Data Relevant to AEGL-3
No quantitative information on lethal crotonaldehyde exposure in humans was located.
7.2.
Summary of Animal Data Relevant to AEGL-3
The most comprehensive lethality study was conducted by Rinehart (1967), where LC50 values were obtained for male Wistar rats exposed for 5, 10, 15, 30, 60, or 240 min. The mortality incidences were clearly concentration related for each exposure duration. The rats displayed obvious respiratory distress and a lowered respiratory rate during exposure and lost up to 25% of their body weight within the first 3 days. These animals had clear or slightly blood-stained nasal discharge; the rats that died within a day had terminal convulsions. Necropsy showed that a few animals had pulmonary congestion; other organs were grossly normal. Chamber exposure concentrations of crotonaldehyde were measured analytically.
A number of animal studies in which LC50 values were determined for a single exposure time can potentially be used to calculate AEGL-3 values, extrapolating to the necessary exposure times and applying appropriate uncertainty factors. These studies include (1) a 30-min exposure of rats in which an LC50 of 1,400 ppm (nominal) was obtained (Skog 1950); the rats gasped and had closed eyes, lacrimation, heavy nose secretion, hyperemia, and hemorrhage in the
lungs, heart, liver, and kidneys; no edema was evident in the lungs; (2) an LC50 of 70 ppm was reported for a 4-h exposure of white rats (no other details reported; Voronii et al. 1982); (3) white mice exposed to crotonaldehyde for 2 h had an LC50 of 530 ppm (measured); the animals rubbed their faces with their paws and displayed respiratory distress, a period of intense excitation, convulsions, and lung hemorrhage, and edema in the lungs and brain (Trofimov 1962); (4) a 2-h LC50 of 200 ppm was obtained for white mice (no other details given; Voronii et al. 1982); (5) guinea pigs exposed to 1,000 ppm for 30 min had 50% mortality (further experimental details not provided; Smyth 1966).
7.3.
Derivation of AEGL-3
The rat study conducted by Rinehart, in which LC50 values were obtained for exposures from 5 min to 4 h, was considered the most relevant for derivation of AEGL-3 values. The Rinehart protocol was an extensive study in which air crotonaldehyde concentrations were measured and 30-60 animals were used for each of the six exposure periods. The Rinehart study was used by ten Berge et al. (1986) to develop the value of n = 1.2 for scaling across time in the relationship Cn × t = k.
The AEGL-3s for 10 min, 30 min, 1 h, and 4 h were obtained directly from the 10-min, 30-min, 1-h, and 4-h LC01 values (440, 268, 138, and 26 ppm, respectively) calculated by probit analysis from the mortality data. The 8-h AEGL-3 values were extrapolated from the 4-h LC01 (26 ppm) using the relationship C1.2 × t = k. A total uncertainty factor of 10 was applied: 3 for interspecies uncertainty because interspecies variability was small (LC50 values for rats, mice, and guinea pigs were within a factor of 2.5, and these studies yield similar or higher AEGL-3 values) and 3 for intraspecies uncertainty because great human variability in unlikely given the homogeneity of the animal data, and a larger uncertainty factor yields 8-h AEGL-3 concentrations that caused only mild irritation in workers exposed for up to 8 h (Fannick 1982). The AEGL-3 values are shown in Table 5-9; calculations are shown in Appendix A.
8.
SUMMARY OF AEGLs
8.1.
AEGL Values and Toxicity End Points
A summary of the AEGL values for crotonaldehyde (trans isomer and commercial cis-trans mixture) and their relationships are shown is Table 5-10.
AEGL-1 values were derived from a Health Hazard Evaluation conducted by NIOSH in which workers who were exposed to approximately 0.56 ppm of crotonaldehyde for <8 h reported occasional minor eye irritation (Fannick 1982). Exponential scaling across time was not performed (see Section 4.4.2 for discus-
TABLE 5-9 AEGL-3 Values for Crotonaldehyde
|
10 min |
30 min |
1 h |
4 h |
8 h |
|
44 ppm (130 mg/m3) |
27 ppm (77 mg/m3) |
14 ppm (40 mg/m3) |
2.6 ppm (7.4 mg/m3) |
1.5 ppm (4.3 mg/m3) |
TABLE 5-10 Summary of AEGL Values for Crotonaldehyde
|
Classification |
10 min |
30 min |
1 h |
4 h |
8 h |
|
AEGL-1 (nondisabling) |
0.19 ppm (0.55 mg/m3) |
0.19 ppm (0.55 mg/m3) |
0.19 ppm (0.55 mg/m3) |
0.19 ppm (0.55 mg/m3) |
0.19 ppm (0.55 mg/m3) |
|
AEGL-2 (disabling) |
27 ppm (77 mg/m3) |
8.9 ppm (26 mg/m3) |
4.4 ppm (13 mg/m3) |
1.1 ppm (3.2 mg/m3) |
0.56 ppm (1.6 mg/m3) |
|
AEGL-3 (lethal) |
44 ppm (130 mg/m3) |
27 ppm (77 mg/m3) |
14 ppm (40 mg/m3) |
2.6 ppm (7.4 mg/m3) |
1.5 ppm (4.3 mg/m3) |
sion); rather, it was considered more appropriate to adopt the same exposure concentration for 10 min to 8 h since the critical end point (eye irritation) was mild and mild irritant effects generally do not vary greatly over time. A total uncertainty factor of 3 was applied to account for intraspecies variability because the eye irritation is a direct surface-contact effect not subject to pharmacokinetic differences between individuals.
AEGL-2 values were derived from the pulmonary performance study in which rats exposed to >8,000 ppm-min (individual concentrations and exposure times were not given) had lower rates of ether and CO absorption and were a NOAEL for proliferative lesions of the respiratory bronchioles. Because the available data suggested that concentration and time were roughly equal contributors to crotonaldehyde toxicity [this is also supported by n = 1.2 derived from the LC50 study by Rinehart (1967)], AEGL-2 values were calculated by dividing 8,000 ppm-min by 10, 30, 60, 240, or 480 min. A total uncertainty factor of 30 was used: 10 for interspecies uncertainty because the actual exposure concentration and time were not known for the key study and there was a lack of supporting animal studies and 3 for intraspecies uncertainty because, although human variability to crotonaldehyde toxicity is not well defined, a greater uncertainty factor was judged inappropriate because it yields 4- and 8-h AEGL-2 concentrations that caused only mild irritation in workers exposed for up to 8 h (Fannick 1982).
The comprehensive study conducted by Rinehart, in which rat LC50 values were obtained for exposures from 5 min to 4 h, was used to derive AEGL-3 values. The AEGL-3s for 10 min, 30 min, 1 h, and 4 h were obtained directly from the 10-min, 30-min, 1-h, and 4-h LC01 values (440, 268, 138, and 26 ppm, respectively) calculated by probit analysis from the mortality data. The 8-h AEGL-3 values were extrapolated from the 4-h LC01 using the relationship C1.2 × t = k. A total uncertainty factor of 10 was applied: 3 for interspecies uncer-
tainty because interspecies variability was small (LC50 values for rats, mice, and guinea pigs were within a factor of 2.5, and these studies yield similar or higher AEGL-3 values) and 3 for intraspecies uncertainty because great human variability is unlikely given the homogeneity of the animal data, and a larger uncertainty factor yields 8-h AEGL-3 concentrations that caused only mild irritation in workers exposed for up to 8 h (Fannick 1982).
A cancer inhalation slope factor was derived for crotonaldehyde and used to estimate the 10−4 excess cancer risk from a single 30-min to 8-h exposure. Crotonaldehyde concentrations associated with a 10−4 excess cancer risk were 25-fold greater than the toxicity-based AEGL-2 values for 30 to 480 min. The noncarcinogenic end points were considered more appropriate for AEGL-2 derivation, as detailed in Appendix D.
8.2.
Comparison with Other Standards and Guidelines
The existing standards and guidelines for crotonaldehyde (in all cases for both the cis and trans isomers) are summarized in Table 5-11.
TABLE 5-11 Extant Standards and Guidelines for cis- and trans-Crotonaldehyde (values in ppm)
|
Guideline |
Exposure Duration |
||||
|
10 min |
30 min |
1 h |
4 h |
8 h |
|
|
AEGL-1 |
0.19 |
0.19 |
0.19 |
0.19 |
0.19 |
|
AEGL-2 |
27 |
8.9 |
4.4 |
1.1 |
0.56 |
|
AEGL-3 |
44 |
27 |
14 |
2.6 |
1.5 |
|
ERPG-1 (AIHA)a |
|
|
2 |
|
|
|
ERPG-2 (AIHA) |
|
|
10 |
|
|
|
ERPG-3 (AIHA) |
|
|
50 |
|
|
|
PEL-TWA (OSHA)b |
|
|
|
|
2 |
|
IDLH (NIOSH)c |
|
50 |
|
|
|
|
REL-TWA (NIOSH)d |
|
|
|
|
2 |
|
TLV-Ceiling (ACGIH)e |
0.3 |
|
|
|
|
|
MAK (Germany)f |
|
|
|
|
—f |
|
MAC (The Netherlands)g |
|
|
|
|
2 |
|
aERPG (emergency response planning guidelines, American Industrial Hygiene Association (AIHA 2004; values under review; documented 9/1/87). ERPG-1 is the maximum airborne concentration below which it is believed nearly all individuals could be exposed for up to 1 h without experiencing other than mild, transient adverse health effects or without perceiving a clearly defined objectionable odor. The ERPG-1 for crotonaldehyde is based on odor threshold data (Amoore and Hautala 1983; Verschueren 1996) and human exposure studies (Sim and Pattle 1957; Rinehart 1967). ERPG-2 is the maximum airborne concentration below which it is believed nearly all individuals could be exposed |
|||||
The OSHA PEL and NIOSH REL (8-h TWA exposure limit) for crotonaldehyde is 2 ppm (6 mg/m3) to prevent eye and respiratory irritation (NIOSH 1994, 2002; OSHA 2005). The TLV applies to both the trans isomer (123-73-9) and the cis-trans mixture (4170-31-3) (IARC 1995; OSHA 2005). The same occupational exposure limits (2 ppm TLV-TWA) are used in Australia, Belgium, Denmark, Finland, France, Italy, the Philippines, Switzerland, and the United Kingdom (IARC 1995; RTECS 2005).
The ACGIH had recommended a TLV-TWA of 2 ppm (5.7 mg/m3) for both isomers (with certain defined permitted excursions above 2 ppm) from 1967 to 1997 but in 1998 omitted the TLV-TWA and adopted a TLV ceiling of 0.3 ppm (ACGIH 1998). This reduction was based on a reevaluation of the available human data of Sim and Pattle (1957; respiratory and eye irritation from 4.1 ppm crotonaldehyde; lacrimation within about 30 s), Rinehart (1967; 15 ppm for 15 min [Rinehart’s stated exposure ≤30 s] strong but not intolerable), and the mouse RD50 study of Steinhagen and Barrow (1984). The TLV committee concluded that the mouse RD50 data were consistent with the Sim and Pattle (1957) but not the Rinehart (1967) data and suggested that the discrepancy between the two sets of data was due to “analysis errors between the two methods used.” Additionally, since crotonaldehyde was a “rapidly acting irritant,” had an RD50 similar to that of formaldehyde (which is structurally and functionally related to crotonaldehyde), and an extensive body of evidence supported the TLV ceiling of 0.3 ppm for formaldehyde, the TLV committee concluded that the occupational exposure limit of crotonaldehyde should be consistent with that of formaldehyde (ACGIH 1998).
8.3.
Data Quality and Research Needs
The human and animal data available to derive AEGL-1 and AEGL-2 values were limited, and further investigations are warranted. In many of the LC50 studies, air crotonaldehyde concentrations were nominal and not measured, and comparisons of obtained LC50 values with other studies were not as meaningful.
Limited human data were available but were sufficient to develop AEGL-1 values. The key study (Fannick 1982) was from a site investigation conducted by NIOSH, and crotonaldehyde concentrations were measured analytically. The actual exposure time and the associated air crotonaldehyde concentrations were not provided, although this was not critical for the AEGL-1 calculations because the same value (based on ocular irritation) was adopted across all time points. A possible confounding factor was the simultaneous exposure of the workers to several other airborne chemicals, although it is likely that crotonaldehyde was the most acutely irritating of all the airborne chemicals used in the plant.
Only one rigorous animal study with an end point within the scope of the definition of AEGL-2 was located. The rat pulmonary function study of Rinehart (1967), from which the AEGL-2 values were derived, was well conducted and crotonaldehyde air concentrations were measured, although the actual concentrations and exposure times were not presented (C × t values were listed).
The database for AEGL-3 derivation was considered adequate, primarily due to the availability of the comprehensive single-exposure rat acute lethality study by Rinehart. In this study, 30-60 animals were tested at five to seven crotonaldehyde concentrations for six different exposure times, and a clear concentration response (for mortality) was seen for each exposure time. Similar or
higher AEGL-3 values could be derived from other rat LC50 studies as well as from mouse and guinea pig LC50 studies.
9.
REFERENCES
ACGIH (American Conference of Government Industrial Hygienists). 1998. Crotonaldehyde. In Documentation of the Threshold Limit Values and Biological Exposure Indices, 6th Ed. American Conference of Government Industrial Hygienists, Cincinnati, OH.
ACGIH (American Conference of Government Industrial Hygienists). 2004. Crotonaldehyde. P. 24 in Threshold Limit Values and Biological Exposure Indices for Chemical Substances and Physical Agents. American Conference of Government Industrial Hygienists, Cincinnati, OH.
AIHA (American Industrial Hygiene Association). 2004. Emergency Response Planning Guidelines: Crotonaldehyde. Fairfax, VA: AIHA Press.
Alarie, Y. 1966. Irritating properties of airborne materials to the upper respiratory tract. Arch. Environ. Health 13(4):433-449.
Alarie, Y. 1981. Dose-response analysis in animal studies: Prediction of human responses. Environ. Health Perspect. 42:9-13.
Amoore, J.E., and E. Hautala. 1983. Odor as an aid to chemical safety: Odor thresholds compared with threshold limit values and volatilities for 214 industrial chemicals in air and water dilution. J. Appl. Toxicol. 3(6):272-290.
Auerbach, C., M. Moutschen-Dahmen, and J. Moutschen. 1977. Genetic and cytogenetical effects of formaldehyde and related compounds. Mutat. Res. 39(3-4):317-361.
Babiuk, C., W.H. Steinhagen, and C.S. Barrow. 1985. Sensory irritation response to inhaled aldehydes after formaldehyde pretreatment. Toxicol. Appl. Pharmacol. 79(1):143-149.
Benamira, M., and L.J. Marnett. 1992. The lipid peroxidation product 4-hydroxynonenal is a potent inducer of the SOS response. Mutat. Res. 293(1):1-10.
Bridges, R.B., L. Hsieh, and D.G. Haack. 1980. Effects of cigarette smoke and its constituents on the adherence of polymorphonuclear leukocytes. Infect. Immun. 29(3):1096-1101.
Budavari, S., M.J. O'Neil, A. Smith, P.E. Heckelman, and J.F. Kinneary, eds. 1996. P. 439 in The Merck Index: An Encyclopedia of Chemicals, Drugs, and Biologicals, 12th Ed. Whitehouse Station, NJ: Merck.
Cheng, X., and J.A. Ruth. 1993. A simplified methodology for quantitation of butadiene metabolites. Application to the study of 1,3-butadiene metabolism by rat liver microsomes. Drug Metabol. Dispos. 21(1):121-124.
Chung, F., R. Young, and S.S. Hecht. 1984. Formation of cyclic 1,N2-propanodeoxyguanosine adducts in DNA upon reaction with acrolein or crotonaldehyde. Cancer Res. 44(3):990-995.
Chung, F., T. Tanaka, and S.S. Hecht. 1986. Induction of liver tumors in F344 rats by crotonaldehyde. Cancer Res. 46(3):1285-1289.
Chung, F., R. Young, and S.S. Hecht. 1989. Detection of cyclic 1,N2-propanodeoxyguanosine adducts in DNA of rats treated with N-nitropyrrolidine and mice treated with crotonaldehyde. Carcinogenesis 10(7):1291-1297.
Cooper, K.O., G. Witz, and C.M. Witmer. 1987. Mutagenicity and toxicity studies of several α,β-unsaturated aldehydes in the Salmonella typhimurium mutagenicity assay. Environ. Mutagen. 9(3):289-295.
Crump, K.S., and R.B. Howe. 1984. The multistage model with a time-dependent dose pattern: Applications to carcinogenic risk assessment. Risk Anal. 4(3):163-176.
Czerny, C., E. Eder, and T.M. Runger. 1998. Genotoxicity and mutagenicity of the alpha, beta-unsaturated carbonyl compound crotonaldehyde (butenal) on a plasmid shuttle vector. Mutat. Res. 407(2):125-134.
Dalla Vale, J.M., and H.C. Dudley. 1939. Evaluation of odor nuisance in the manufacture of kraft paper. Public Health Rep. 54:35-43.
DFG (Deutsche Forschungsgemeinschaft). 2002. List of MAK and BAT Values 2002. Maximum Concentrations and Biological Tolerance Values at the Workplace. Commission for the Investigation of Health Hazards of Chemical Compounds in the Work Area Report No. 38. Weinheim, Federal Republic of Germany: Wiley-VCH.
Dittberner, U., G. Eisenbrand, and H. Zankl. 1995. Genotoxic effects of the α, β-unsaturated aldehydes 2-trans-butenal, 2-trans-hexenal and 2-trans, 6-cis-nonadienal. Mutat. Res. 335(3):259-265.
Eastman Chemical Company. 1998. Material Safety Data Sheet—Crotonaldehyde (4170-30-3). Eastman Chemical Company, Kingsport, TN.
Eastman Kodak Company. 1992. Initial Submission: Acute inhalation Toxicity Study of 2-butenal (Crotonaldehyde) in Rats with Cover Letter Dated 09/21/92. Produced 04/26/61; EPA Doc. ID 88-920010705.
Eder, E., and C. Hoffman. 1992. Identification and characterization of deoxyguanosine-crotonaldehyde adducts. Formation of 7, 8 cyclic adducts and 1, N2,7,8 bis-cyclic adducts. Chem Res. Toxicol. 5(6):802-808.
Eder, E., C. Deininger, T. Neudecker, and D. Deininger. 1992. Mutagenicity of β-alkyl substituted acrolein congeners in the Salmonella typhimurium strain TA100 and genotoxicity testing in the SOS chromotest. Environ. Mol. Mutagen. 19(4):338-345.
Eder, E., S. Scheckenbach, C. Deininger, and C. Hoffman. 1993. The possible role of α, β-unsaturated carbonyl compounds in mutagenesis and carcinogenesis. Toxicol. Lett. 67(1-3):87-103.
Eder, E., T. Budiawan, D. Shuler, and M. Otteneder. 1997. Assessment of the tumorinitiating potential of α, β-unsaturated carbonyl compounds by 32P postlabeling quantification of DNA adducts in vivo. Recent Results Cancer Res. 143:65-75.
Elfarra, A.A., R.J. Duescher, and C.M. Pasch. 1991. Mechanisms of 1,3-butadiene oxidation to butadiene monoxide and crotonaldehyde by mouse liver microsomes and chloroperoxidase. Arch. Biochem. Biophys. 286(1):244-251.
EPA (U.S. Environmental Protection Agency). 1999. Guidelines for Carcinogen Risk Assessment. Review Draft. NCEA-F-0644. Risk Assessment Forum, U.S. Environmental Protection Agency, Washington, DC. July 1999 [online]. Available: http://www.epa.gov/iris/cancer_gls.pdf [accessed July 25, 2007].
EPA (U.S. Environmental Protection Agency). 2005. Crotonaldehyde [CAS No. 123-73-9]. Integrated Risk Information System (IRIS), U.S. Environmental Protection Agency [online]. Available: http://www.epa.gov/iris/subst/0464.htm [accessed July 25, 2007].
Esterbauer, H., R.J. Schaur, and H. Zollner. 1991. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic. Biol. Med. 11(1):81-128.
Fannick, N. 1982. Health Hazard Evaluation Report: Sandoz Colors and Chemicals, East Hanover, New Jersey. HETA-81-102-1244. Cincinnati, OH: National Institute for Occupational Safety and Health.
Fieldner, Sayers, Yant, et al. 1954. P. 397 in Vrednye vetschestva v promyshlennosti (as cited in Trofimov 1962).
Filser, J.G., T.H. Faller, S. Bhowmik, A. Schuster, W. Kessler, C. Pütz and G.A. Csanády. 2001. First-pass metabolism on once-through perfused livers of rats and mice. Chem. Biol. Interact. 135/136: 249-265.
Florin, I., L. Rutberg, M. Curvall, and C.R. Enzell. 1980. Screening of tobacco smoke constituents for mutagenicity using the Ames’ test. Toxicology 15(3):219-232.
Foiles, P.G., S.A. Akerkar, L.M. Miglietta, and F.L. Chung. 1990. Formation of cyclic deoxyguanosine adducts in Chinese hamster ovary cells by acrolein and crotonaldehyde. Carcinogenesis 11(11):2059-2061.
Galloway, S.M., M.J. Armstrong, C. Reuben, S. Colman; B. Brown, C. Cannon, A.D. Bloom, F. Nakamura, M. Ahmed, S. Duk, J. Rimpo, B.H. Margolin, M.A. Resnick, B. Anderson, and E. Zeiger. 1987. Chromosome aberrations and sister chromatid exchanges in Chinese hamster ovary cells: Evaluation of 108 chemicals. Environ. Mol. Mutagen. 10 (Suppl. 10):1-175.
Gray, J.M., and E.A. Barnsley. 1971. The metabolism of crotyl phosphate, crotyl alcohol and crotonaldehyde. Xenobiotica 1(1):55-67.
Hecht, S.S., E.J. McIntee, and M. Wang. 2001. New DNA adducts of crotonaldehyde and acetaldehyde. Toxicology 166(1-2):31-36.
Howe, R.B., K.S. Crump, and C. Van Landingham. 1986. GLOBAL86: A Computer Program to Extrapolate Quantal Animal Toxicity Data to Low Doses. Prepared for U.S. Environmental Protection Agency, Washington, DC, by Clement Associates, Inc., Ruston, LA. Subcontract No. 2-251U-2745.
HSDB (Hazardous Substances Data Bank). 2005. Crotonaldehyde (CASRN 4170-30-3). TOXNET, Specialized Information Services, U.S. National Library of Medicine, Bethesda, MD [online]. Available: http://toxnet.nlm.nih.gov/cgibin/sis/htmlgen?HSDB [accessed July 25, 2007].
IARC (International Agency for Research on Cancer). 1995. Crotonaldehyde. Pp. 373-391in Dry Cleaning, Some Chlorinated Solvents and Other Industrial Chemicals. IARC Monographs on the Evaluation of Carcinogenic Risk of Chemicals to Humans Vol. 63. Lyon, France: International Agency for Research on Cancer.
Ikeda, A., U. Horiguchi, and K. Koyoshi. 1980. Research of the effect of air pollution. 2. Studies on biological effects of carbohydrates (on aldehydes) [in Japanese]. Kanagawa-ken Taiki Osen Chosa Kenkyu Hokoku 22:193-196.
Kuykendall, J.R., and M.S. Bogdanffy. 1992. Efficiency of DNA-histone crosslinking induced by saturated and unsaturated aldehydes in vitro. Mutat. Res. 283(2):131-136.
Lijinsky, W., and A.W. Andrews. 1980. Mutagenicity of vinyl compounds in Salmonella typhimurium. Teratogen. Carcinog. Mutagen. 1(3):259-267.
Lutz, D., E. Eder, T. Neudecker and D. Henschler. 1982. Structure-mutagenicity relationship in α,β-unsaturated carbonylic compounds and their corresponding allylic alcohols. Mutat. Res. 93:305-315.
Margineanu, D.G., E. Katona, and J. Popa. 1981. Effect of protein cross-linking aldehydes on nerve activity. Arch. Int. Physiol. Biochim. 89(2):159-165.
Moutschen, J., M. Moutschen-Dahmen, N. Houbrechts, and A. Colizzi. 1976. Cytotoxicity and mutagenicity of two aldehydes: Crotonaldehyde and butylaldehyde in the mouse. Bull. Soc. R. Sci. Liege. 45:58-72.
Nath, R.G., and F. Chung. 1994. Detection of exocyclic 1,N2-propanodeoxyguanosine adducts as common DNA lesions in rodents and humans. Proc. Natl. Acad. Sci. USA 91(16):7491-7495.
Nath, R.G., J.E. Ocando, J.B. Guttenplan, and F. Chung. 1998. 1,N2-propanodeoxyguanosine adducts: Potential new biomarkers of smoking-induced DNA damage in human oral tissue. Cancer Res. 58(4):581-584.
Neudecker, T., D. Lutz, E. Eder, and D. Henschler. 1981. Crotonaldehyde is mutagenic in a modified Salmonella typhimurium mutagenicity testing system. Mutat. Res. 91(1):27-31.
Neudecker, T., E. Eder, C. Deininger, and D. Henschler. 1989. Crotonaldehyde is mutagenic in Salmonella typhimurium TA100. Environ. Mol. Mutagen. 14(3):146-148.
NIOSH (National Institute for Occupational Safety and Health). 1994. Crotonaldehyde. In: Documentation for Immediately Dangerous to Life or Health Concentrations. NTIS PB-94-195047. National Institute for Occupational Safety and Health, Public Health Service, U.S. Department of Health, Education and Welfare, Cincinnati, OH. May 1994 [online]. Available: http://www.cdc.gov/niosh/idlh/idlhintr.html [accessed July 25, 2007].
NIOSH (National Institute for Occupational Safety and Health). 2002. Ethylenediamine. In: NIOSH Pocket Guide to Chemical Hazards. NIOSH 2002-140. National Institute for Occupational Safety and Health, Public Health Service, U.S. Department of Health, Education and Welfare, Cincinnati, OH.
NRC (National Research Council). 1985. Hydrazine. Pp. 5-21 in Emergency and Continuous Exposure Guidance Levels for Selected Airborne Contaminants, Vol. 5. Washington, DC: National Academy Press.
NRC (National Research Council). 1993. Guidelines for Developing Community Emergency Exposure Levels for Hazardous Substances. Washington, DC: National Academy Press.
NRC (National Research Council). 2001. Standing Operating Procedures for Developing Acute Exposure Guideline Levels for Hazardous Chemicals. Washington, DC: National Academy Press.
NTP (National Toxicology Program). 1985. Adsorption, Disposition, Metabolism and Excretion of Crotonaldehyde. Prepared by A.R. Jeffcoat, Chemistry and Life Sciences, for National Institute of Environmental Health Sciences, Research Triangle Park, NC.
Pellizzari, E.D., T.D. Hartwell, B.S. Harris, R.D. Waddell, D.A. Whitaker, and M.D. Erickson. 1982. Purgeable organic compounds in mother’s milk. Bull. Environ. Contam. Toxicol. 28(3):322-328.
Pettersson, B., M. Curvall, and C.R. Enzell. 1982. Effects of tobacco smoke compounds on the ciliary activity of the embryo chicken trachea in vitro. Toxicology 23(1):41-55.
Rinehart, W.E. 1967. The effect on rats of single exposures to crotonaldehyde vapor. Am. Ind. Hyg. Assoc. J. 28(6):561-566.
Rinehart, W.E., and R. Hatch. 1964. Concentration-time product (CT) as an expression of dose in sublethal exposures to phosgene. Am. Ind. Hyg. Assoc. J. 25:545-553.
RTECS (Registry of Toxic Effects of Chemical Substances). 2006. Crotonaldehyde. Specialized Information Services. National Institute for Occupational Safety and Health [online]. Available: http://www.cdc.gov/niosh/rtecs/gp90f178.html [accessed July 2005].
Ruth, J.H. 1986. Odor thresholds and irritation levels of several chemical substances: A review. Am. Ind. Hyg. Assoc. 47(3):142-151.
Salem, H., and H. Cullumbine. 1960. Inhalation toxicities of some aldehydes. Toxicol. Appl. Pharmacol. 2:183-187.
Schaper, M. 1993. Development of a database for sensory irritants and its use in establishing occupational exposure limits. Am. Ind. Hyg. Assoc. J. 54(9):488-544.
SDU Uitgevers (Ministry of Social Affairs and Employment). 2000. National MAC (Maximum Allowable Concentration) List, 2000. Ministry of Social Affairs and Employment, The Hague, The Netherlands.
Shrager, P.G., A. Strickholm and R.I. Macey. 1969. Chemical modification of crayfish axons by protein crosslinking aldehydes. J. Cell Physiol. 74(1):91-100.
Sim, V.M., and R.E. Pattle. 1957. Effect of possible smog irritants on human subjects. JAMA 165(15):1908-1913.
Simmon, V.F., K. Kauhanen and R.G. Tardiff. 1977. Mutagenic activity of chemicals identified in drinking water. Dev. Toxicol. Environ. Sci. 2:249-258.
Skog, E. 1950. A toxicological investigation of lower aliphatic aldehydes. I. Toxicity of formaldehyde, acetaldehyde, propionaldehyde and butyraldehyde as well as of acrolein and crotonaldehyde. Acta Pharmacol. Toxicol. 6(4):299-318.
Smyth, H.F., Jr., and C.P. Carpenter. 1944. The place of the range finding test in the industrial toxicology laboratory. J. Ind. Hyg. Toxicol. 26(8):269-273.
Steinhagen, W.H., and C.S. Barrow. 1984. Sensory irritation structure-activity study of inhaled aldehydes in B6C3F1 and Swiss-Webster mice. Toxicol. Appl. Pharmacol. 72(3):495-503.
ten Berge, W.F., A. Zwart, and L.M. Appelman. 1986. Concentration-time mortality response relationship of irritant and systemically acting vapors and gases. J. Hazard. Mater. 13: 302-309.
Tepikina, L.A., E.L. Skvortsova, Z.V. Shipulina, A.V. Kartashova, I.U.N. Mol'kov, and N.N. Sizova. 1997. Substantiation of MAC for crotonaldehyde in environmental air [in Russian]. Gig. Sanit. 3:3-5.
Teranishi, R., R.G. Buttery, and D.G. Guadagni. 1974. Odor quality and chemical structure in fruit and vegetable flavors. Ann. N.Y. Acad. Sci. 237:209-216.
Trofimov, L.V. 1962. Comparative toxic action of crotonaldehyde and butyraldehyde [in Russian]. Gig. Tr. Prof. Zabol. 6:34-40.
Union Carbide Corp. 1992. Initial Submission: Summary of Range-Finding Tests on Crotonaldehyde with Cover Letter Dated September 08, 1992. Produced March 11, 1942. Union Carbide Corp., Danbury, CT. EPA Doc ID 88-920009348.
van Doorn, R., M. Ruijten, and T. Van Harreveld. 2002. Guidance for the Application of Odor in 22 Chemical Emergency Response, Version 2.1. August 29, 2002.
Verschueren, K., ed. 1983. Crotonaldehyde. Pp. 410-411 in Handbook of Environmental Data on Organic Chemicals, 2nd Ed. New York: Van Nostrand Reinhold.
Verschueren, K., ed. 1996. Crotonaldehyde. Pp. 552-553 in Handbook of Environmental Data on Organic Chemicals, 3rd Ed. New York: Van Nostrand Reinhold.
Voronii, V.A., et al. 1982. Information from the Soviet Toxicology Center: Correction of crotonaldehyde toxicity data [in Russian]. Gig. Tr. Prof. Zabol. 26(8):54-55.
Wang, M.Y., F.L. Chung, and S.S. Hecht. 1988. Identification of crotonaldehyde as a hepatic microsomal metabolite formed by alpha-hydroxylation of the carcinogen N-nitrosopyrrolidine. Chem. Res. Toxicol. 1(1):28-31.
Williams, G.M., H. Mori, and C.A. McQueen. 1989. Structure-activity relationships in the rat hepatocyte DNA-repair test for 300 chemicals. Mutat. Res. 221(3):263-286.
Wilson, V.L., P.G. Foiles, F. Chung, A.C. Povey, A.A. Frank and C.C. Harris. 1991. Detection of acrolein and crotonaldehyde DNA adducts in cultured human cells and canine peripheral blood lymphocytes by 32P-postlabeling and nucleotide chromatography. Carcinogenesis 12(8):1483-1490.
Witz, G., N.J. Lawrie, M.A. Amoruso, and B.D. Goldstein. 1987. Inhibition by reactive aldehydes of superoxide anion radical production from stimulated polymorphonuclear leukocytes and pulmonary alveolar macrophages. Effects on cellular sulfhydryl groups and NADPH oxidase activity. Biochem. Pharmacol. 36(5):721-726.
Woodruff, R.C., J.M. Mason, R. Valencia, and S. Zimmering. 1985. Chemical mutagenesis testing in Drosophila. V. Results of 53 coded compounds tested for the National Toxicology Program. Environ. Mutagen. 7(5):677-702.
Zeng, X. 1985. The toxic interaction of acetaldehyde and crotonaldehyde [in Chinese]. Zhonghua Yu Fang Yi Xue Za Zhi. 19(5):278-280.
APPENDIX A
Derivation of AEGL-1 Values
|
Key study: |
Fannick 1982. Human occupational exposure to a mean concentration of 0.56 ppm crotonaldehyde during a workday caused occasional eye irritation; exposure time not given but was <8 h. |
|
Toxicity end point: |
Ocular irritation. |
|
Scaling: |
None: 0.56 ppm = k; the critical end point (eye irritation) was mild, and mild irritant effects generally do not vary greatly over time. |
|
Uncertainty factors: |
Total uncertainty factor: 3 |
|
Interspecies: |
Not applicable |
|
Intraspecies: |
3, for intraspecies variability because the eye irritation is a direct surface-contact effect not subject to pharmacokinetic differences between individuals. |
|
Calculations: |
|
|
10-min AEGL-1: |
0.56 ppm/3 = 0.19 ppm (0.55 mg/m3) |
|
30-min AEGL-1: |
0.56 ppm/3 = 0.19 ppm (0.55 mg/m3) |
|
1-h AEGL-1: |
0.56 ppm/3 = 0.19 ppm (0.55 mg/m3) |
|
4-h AEGL-1: |
0.56 ppm/3 = 0.19 ppm (0.55 mg/m3) |
|
8-h AEGL-1: |
0.56 ppm/3 = 0.19 ppm (0.55 mg/m3) |
Derivation of AEGL-2 Values
|
Key study: |
Rinehart 1967. Rat pulmonary function study. Rats had lower rates of ether and CO absorption and those exposed to >8,000 ppm-min (product of concentration and time; individual concentrations and exposure times were not given) developed proliferative respiratory bronchiole lesions. |
|
Toxicity end point: |
Moderate pulmonary impairment and NOAEL for proliferative lesions of the respiratory bronchioles. |
|
Scaling: |
C1 × t = k (concentration and time were approximately equally important for toxicity) |
|
Uncertainty factors: |
Total uncertainty factor: 30 |
|
Interspecies: |
10: The actual exposure concentration and time were not known for the key study, and there was a lack of supporting animal studies. |
|
Intraspecies: |
3: Although human variability to crotonaldehyde toxicity is not well defined, a greater uncertainty factor was judged inappropriate because it yields 4- and 8-h AEGL-2 concentrations that caused only mild irritation in workers exposed for up to 8 h (Fannick 1982). |
|
Calculations: |
(Concentration)1 (30-480 min) = k = 8,000 ppm-min Apply the total UF of 30-8,000 ppm-min and get k = 267 ppm-min |
|
10-min AEGL-2: |
C1 × 10 min = 267 ppm-min 10 min AEGL-2 = 267 ppm-min/10 min = 27 ppm (77 mg/m3) |
|
30-min AEGL-2: |
C1 × 30 min = 267 ppm-min 30-min AEGL-2 = 267 ppm-min/30 min = 8.9 ppm (26 mg/m3) |
|
1-h AEGL-2: |
C1 × 60 min = 267 ppm-min 1 h AEGL-2 = 267 ppm-min/60 min = 4.4 ppm (13 mg/m3) |
|
4-h AEGL-2: |
C1 × 240 min = 267 ppm-min 4-h AEGL-2 = 267 ppm-min/240 min = 1.1 ppm (3.2 mg/m3) |
|
8-h AEGL-2: |
C1 × 480 min = 267 ppm-min 8-h AEGL-2 = 267 ppm-min/480 min = 0.56 ppm (1.6 mg/m3) |
Derivation of AEGL-3 Values
|
Key study: |
Rinehart 1967. Rat 5-min to 4-h exposure inhalation LC50 study. Most deaths occurred by 4 days after exposure, and the animals had clear or slightly blood-tinged nasal exudate; rats that died within 1 day also had terminal convulsions. Autopsy showed that a few rats had pulmonary congestion. |
|
Toxicity end point: |
Lethality NOELs, estimated from LC01 values obtained by probit analysis: 10-min LC01 = 440 ppm (standard error = 153) 30-min LC01 = 268 ppm (standard error = 50) 1-h LC01 = 138 ppm (standard error = 71) 4-h LC01 = 26 ppm (standard error = 7.8); used to derive 8-h values |
|
Scaling: |
C1.2 × t = k (Rinehart 1967 LC50 data; ten Berge et al. 1986) |
|
Uncertainty factors: |
Total uncertainty factor: 10 |
|
Interspecies: |
3, Interspecies variability was small (LC50 values for rats, mice, and guinea pigs were within a factor of 2.5; these studies yield similar or higher AEGL-3 values). |
|
Intraspecies: |
3, Great human variability is unlikely given the homogeneity of the animal data, and a larger uncertainty factor yields 8-h AEGL-3 concentrations that caused only mild irritation in workers exposed for up to 8 h (Fannick 1982). |
|
Calculations for 10, 30, 60, and 240 min: |
|
|
10-min AEGL-3: |
10-min LC01 = 440 ppm 10-min AEGL-3 = 440/10 = 44 ppm (130 mg/m3) |
|
30-min AEGL-3: |
30-min LC01 = 268 ppm 30-min AEGL-3 = 268/10 = 27 ppm (77 mg/m3) |
|
1-h AEGL-3: |
1-h LC01 = 138 ppm 1-h AEGL-3 = 138/10 = 14 ppm (40 mg/m3) |
|
4-h AEGL-3: |
4-h LC01 = 26 ppm 4-h AEGL-3 = 26/10 = 2.6 ppm (7.4 mg/m3) |
|
Calculations for 8 h: |
|
|
|
|
|
8-h AEGL-3 |
C1.2 × 480 min = 755.4 ppm-min |
|
8-h AEGL-3 |
C = 1.5 ppm (4.3 mg/m3) |
APPENDIX B
Derivation of the Level of Distinct Odor Awareness (LOA)
The level of distinct odor awareness (LOA) represents the concentration above which it is predicted that more than half of the exposed population will experience at least a distinct odor intensity; about 10% of the population will experience a strong odor intensity. The LOA should help chemical emergency responders in assessing public awareness of exposure due to odor perception. The LOA derivation follows the guidance given by van Doorn et al. (2002).
An odor detection threshold (OT50; i.e., concentration at which 50% of the odor panel observed an odor without necessarily recognizing it) of 0.069 ppm was reported for the trans isomer and 0.063-0.20 ppm for the cis isomer of crotonaldehyde. The value of 0.069 was used for the LOA calculations because commercial crotonaldehyde (tested in the animal studies) is a mixture of the two isomers consisting of >95% trans isomer.
The concentration C leading to an odor intensity (I) of distinct odor detection (I = 3) is derived using the Fechner function:
For the Fechner coefficient, the default of kw = 2.33 will be used due to the lack of chemical-specific data:
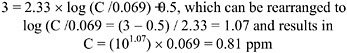
The resulting concentration is multiplied by an empirical field correction factor. It takes into account that in everyday life factors such as sex, age, sleep, smoking, upper-airway infections, and allergies, as well as distraction, increase the odor detection threshold by a factor of 4. In addition, it takes into account that odor perception is very fast (about 5 s), which leads to the perception of
APPENDIX D
CARCINOGENICITY ASSESSMENT
Preliminary Cancer Assessment of Crotonaldehyde
A preliminary cancer assessment of crotonaldehyde was performed using data from Chung et al. (1986). In this study, male F344 rats were treated with 0, 0.6, or 6.0 mM of crotonaldehyde in their drinking water for 113 weeks. The high-dose group had approximately 10% lower body weight gain starting at week 8. The incidence of hepatic neoplastic nodules and hepatocellular carcinomas (combined) was 0/23, 11/27*, and 1/23 at 0, 0.6, and 6.0 mM, respectively (*p < .01; carcinoma: 0/23, 2/27, 0/23, respectively).
The oral dose can be extrapolated to an air concentration that results in an equivalent human inhaled dose when assuming 100% lung absorption (NRC 1993). The extrapolation uses a rat intake of 2.06 mg of crotonaldehyde/day from the drinking water at the low dose (0.049 L/day (default) × 0.6 mmol/L × 70.09 g/mol crotonaldehyde), default body weights (BW) of 70 kg for humans and 0.35 kg for rats, and an inhalation rate of 20 m3/day for humans. The calculation is performed as follows:
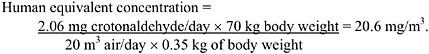
This yields air concentrations of 20.6 mg/m3 (7.2 ppm) and 206 mg/m3 (72 ppm), respectively, for 0.6 and 6.0 mM crotonaldehyde in water. Using the linearized multistage model (GLOBAL86 program; Howe et al. 1986), the inhalation unit risk (or slope factor; i.e., q1*) was calculated to be 0.0327 per (mg/m3). Note that the high dose was excluded from the unit risk calculation by the GLOBAL86 program due to lack of fit.
For a lifetime theoretical cancer risk of 10−4, crotonaldehyde air concentration is 10−4/0.0327 (mg/m3)−1 = 3.06 × 10−3 mg/m3. To convert a 70-year exposure to a 24-h exposure:
An additional adjustment factor of 6 is applied to account for uncertainty regarding the stages of the carcinogenic process at which TNM or its metabolites may act (Crump and Howe 1984):
For exposures of less than 24 h, the fractional exposure (f) becomes 1/f × 24 h (NRC 1985). (Extrapolation to 10 min was not calculated due to unacceptably large inherent uncertainty; see Section 4.4.3.)
|
Exposure Duration |
AEGL-2 Values (ppm) Based on Toxicity End Points |
Crotonaldehyde Exposure Concentrations (ppm) with an Excess Cancer Risk of |
||
|
10−4 |
10−5 |
10−6 |
||
|
½ h |
8.9 |
221 |
22 |
2.2 |
|
1 h |
4.4 |
110 |
11 |
1.1 |
|
4 h |
1.1 |
28 |
2.8 |
0.28 |
|
8 h |
0.56 |
14 |
1.4 |
0.14 |
Because animal doses were converted to an air concentration that results in an equivalent human inhaled dose for the derivation of the cancer slope factor, no reduction of exposure levels is applied to account for interspecies variability.
Crotonaldehyde concentrations associated with a 10−4 excess cancer risk for a single 30- to 480-min exposure were 25-fold greater than the toxicity-based AEGL-2 values for 30-480 min. The noncarcinogenic end points were considered to be more appropriate for AEGL-2 derivation because (1) there is insufficient evidence that inhalation is a route that results in crotonaldehyde-induced liver lesions or neoplasia at concentrations comparable to the AEGL-2 values (liver effects were mentioned in two inhalation studies: Skog (1950) reported hyperemia in multiple organs, including the liver, at unspecified exposure concentrations, and Salem and Cullumbine (1960) found that livers appeared enlarged in animals exposed to concentrations that killed all animals within 86 min); (2) the data used to derive the cancer slope factor were very weak (the key study had only one dose and one control group; the high dose was excluded due to lack of fit), and most of the neoplastic changes were benign; (3) multiple worst-case assumptions were made in extrapolating from the oral route to the inhalation route and in the derivation of the cancer slope factor; and (4) AEGL values are applicable to rare events or single, once-in-a-lifetime exposures and the neoplasms resulted from lifetime treatment.
APPENDIX E
ACUTE EXPOSURE GUIDELINE LEVELS FOR CROTONALDEHYDE
Derivation Summary for Crotonaldehyde AEGLS(CAS Nos. 123-73-9 and 4170-30-3)
AEGL-1 VALUES
|
10-min |
30-min |
1-h |
4-h |
8-h |
|
0.19 ppm |
0.19 ppm |
0.19 ppm |
0.19 ppm |
0.19 ppm |
|
Key reference: Fannick, N. 1982. Sandoz Colors and Chemicals, East Hanover, New Jersey. Health Hazard Evaluation Report No. HETA-81-102-1244. National Institute for Occupational Safety and Health, Hazard Evaluations and Technical Assistance Branch, Cincinnati, OH. |
||||
|
Test species/Strain/Sex/Number: Humans; number not specified but likely <10. |
||||
|
Exposure route/Concentrations/Durations: Inhalation for <8 h to 0.56 ppm; highest measured air concentration was 1.1 ppm. |
||||
|
Effects: Slight eye irritation. |
||||
|
End point/Concentration/Rationale: Workers exposed to 0.56 ppm for a portion of their 8-h work shift occasionally had mild eye irritation. |
||||
|
Uncertainty factors/Rationale: Uncertainty factors: Total uncertainty factor: 3 Interspecies: Not applicable Intraspecies: 3: for intraspecies variability because the eye irritation is a direct surface-contact effect not subject to pharmacokinetic differences between individuals. |
||||
|
Modifying factor: None. |
||||
|
Animal to human dosimetric adjustment: Not necessary |
||||
|
Time scaling: The same value is adopted for 10-min to 8-h exposures because the critical end point (eye irritation) was mild and mild irritant effects generally do not vary greatly over time. Human exposure studies suggested that scaling across time was not appropriate (the degree of irritation was much greater at shorter time periods than at longer time periods for the same Ct). |
||||
|
Data adequacy: Database of appropriate studies was limited but included human data. The key study was conducted by NIOSH, and crotonaldehyde concentrations were measured analytically. A possible confounding factor was co-exposure of the workers to several other airborne chemicals, although mouse irritation data indicate that crotonaldehyde was the most irritating of the chemicals present. |
||||
AEGL-2 VALUES
|
10-min |
30-min |
1-h |
4-h |
8-h |
|
27 ppm |
8.9 ppm |
4.4 ppm |
1.1 ppm |
0.56 ppm |
|
Key reference: Rinehart, W. 1967. The effect on rats of single exposures to crotonaldehyde vapor. Am. Ind. Hyg. Assoc. J. 28:561-566. |
||||
|
Test species/Strain/Sex/Number: Male Sprague-Dawley rats; 12-16 per Ct (concentration × time) range |
||||
|
Exposure route/Concentrations/Durations: Inhalation for 5 min to 4 h of 10-580 ppm; individual concentrations and exposure times were not given. |
||||
|
Effects: Decreased pulmonary function at ≥ 2,000 ppm-min, manifest as a 5-50% reduction in CO and ether uptake rates compared to preexposure values. Proliferative lesions of the respiratory bronchioles occurred at >8,000 ppm-min. |
||||
|
End point/Concentration/Rationale: Decreased pulmonary function and NOAEL for proliferative lesions of the respiratory bronchioles at 8,000 ppm-min. |
||||
|
Uncertainty factors/Rationale: Total uncertainty factor: 30 Interspecies: 10: The actual exposure concentration and time were not known for the key study, and there was a lack of supporting animal studies. |
||||
|
Intraspecies: 3: Although human variability to crotonaldehyde toxicity is not well defined, a greater uncertainty factor was judged inappropriate because it yields 4- and 8-h AEGL-2 concentrations that caused only mild irritation in workers exposed for up to 8 h (Fannick 1982). |
||||
|
Modifying factor: None. |
||||
|
Animal to human dosimetric adjustment: Not applied |
||||
|
Time scaling: Concentration and time appeared to be roughly equally important for toxicity; i.e., C1 × t = k, which is also supported by n = 1.2 derived from an LC50 study by Rinehart (1967). AEGL-2 values were calculated by dividing 8,000 ppm-min by 10, 30, 60, 240, or 480 min. |
||||
|
Data adequacy: The database of appropriate studies was small. The key study was well conducted and crotonaldehyde air concentrations were measured, although the actual concentrations and exposure times were not given (only Ct values). |
||||
AEGL-3 VALUES
|
10-min |
30-min |
1-h |
4-h |
8-h |
|
44 ppm |
27 ppm |
14 ppm |
2.6 ppm |
1.5 ppm |
|
Key reference: Rinehart, W. 1967. The effect on rats of single exposures to crotonaldehyde vapor. Am. Ind. Hyg. Assoc. J. 28:561-566. |
||||
|
Test species/Strain/Sex/Number: Male Sprague-Dawley rats; 5-12/concentration (see below) |
||||
|
10-min |
30-min |
1-h |
4-h |
8-h |
|
|
44 ppm |
27 ppm |
14 ppm |
2.6 ppm |
1.5 ppm |
|
|
Exposure route/Concentrations/Durations: Inhalation: see below for exposure times and concentrations. |
|||||
|
Effects: Most deaths occurred by 4 days after exposure, and the animals had clear or slightly blood-tinged nasal exudate (rats that died within 1 day also had terminal convulsions); some had pulmonary congestion. |
|||||
|
5-min ppm–mortality |
10-min ppm–mortality |
15-min ppm–mortality |
30-min ppm–mortality |
60 min ppm–mortality |
240-min ppm–mortality |
|
1,920 – 0/5 |
800 – 1/12 |
550 – 0/10 |
370 – 0/10 |
370 – 4/10 |
50 – 1/10 |
|
2,420 – 1/5 |
1,110 – 4/12 |
680 – 2/10 |
420 – 2/10 |
400 – 6/10 |
60 – 2/10 |
|
2,680 – 1/5 |
1,380 – 6/12 |
750 – 5/10 |
530 – 4/10 |
490 – 7/10 |
70 – 4/10 |
|
3,180 – 3/5 |
1,820 – 7/12 |
850 – 7/10 |
675 – 6/10 |
590 – 7/10 |
100 – 6/10 |
|
4,160 – 4/5 |
2,050 – 9/12 |
980 – 7/10 |
800 – 8/10 |
640 – 10/10 |
120 – 8/10 |
|
4,640 – 5/5 |
LC50 = 1480 |
1,090 – 8/10 |
890 – 9/10 |
LC50 = 391 |
200 – 9/10 |
|
LC50 = 3132 |
LC01 = 440 |
1,290 – 10/10 |
LC50 = 593 |
LC01 = 138 |
LC50 = 88 |
|
LC01 = 1492 |
|
LC50 = 809 |
LC01 = 268 |
|
LC01 = 26 |
|
|
|
LC01 = 419 |
|
|
|
|
End point/Concentration/Rationale: LC01 values, representing the NOEL for lethality, were obtained by probit analysis and used to obtain the 10-, 30-, 1-h, and 4-h AEGL-3 values. The 8-h values were derived from the 4-h LC01 by exponential time scaling and using n = 1.2. |
|||||
|
Uncertainty factors/Rationale: Total uncertainty factor: 10 Interspecies: 3: Interspecies variability was small (LC50 values for rats, mice, and guinea pigs were within a factor of 2.5, and these studies yielded similar or higher AEGL-3 values). Intraspecies: 3: Great human variability in unlikely given the homogeneity of the animal data, and a larger uncertainty factor yields 8-h AEGL-3 concentrations that caused only mild irritation in workers exposed for up to 8 h (Fannick 1982). |
|||||
|
Modifying factor: None. |
|||||
|
Animal to human dosimetric adjustment: Not applied |
|||||
|
Time scaling: Performed only for 8-h time point by exponential scaling; i.e., Cn × t = k, where n = 1.2 was derived by ten Berge et al. (1986) from the Rinehart (1967) rat LC50 data. |
|||||
|
Data adequacy: Database quality was considered adequate, and the key study was well conducted: 30-60 animals were tested per exposure time at five to seven crotonaldehyde concentrations, and a clear dose-response was obtained. Similar or higher AEGL-3 values could be obtained with mice, rats, and guinea pigs. |
|||||