PREFACE
Under the authority of the Federal Advisory Committee Act (P.L. 92-463) of 1972, the National Advisory Committee for Acute Exposure Guideline Levels for Hazardous Substances has been established to identify, review, and interpret relevant toxicologic and other scientific data and develop Acute Exposure Guideline Levels (AEGLs) for high-priority, acutely toxic chemicals.
AEGLs represent threshold exposure limits for the general public and are applicable to emergency exposure periods ranging from 10 min to 8 h. AEGL-2 and AEGL-3, and AEGL-1 levels as appropriate, will be developed for each of five exposure periods (10 min, 30 min, 1 h, 4 h, and 8 h) and will be distinguished by varying degrees of severity of toxic effects. It is believed that the recommended exposure levels are applicable to the general population, including infants and children and other individuals who may be susceptible. The three AEGLs have been defined as follows:
|
1 |
This document was prepared by AEGL Development Team member Cheryl Bast of Oak Ridge National Laboratory and Ernest Falke (Chemical Manager) of the National Advisory Committee on Acute Exposure Guideline Levels for Hazardous Substances (NAC). The NAC reviewed and revised the document, which was then reviewed by the National Research Council (NRC) Committee on Acute Exposure Guideline Levels. The NRC Committee concludes that the AEGLs developed in this document are scientifically valid conclusions based on data reviewed by the NRC and are consistent with the NRC guideline reports (NRC 1993, 2001). |
|
2 |
After an earlier version of this document was released in 2007, the committee evaluated AEGLs that were developed for eight metal phosphides: aluminum phosphide, potassium phosphide, sodium phosphide, zinc phosphide, calcium phosphide, magnesium phosphide, strontium phosphide, and magnesium aluminum phosphide. Because their acute toxicity results from the phosphine generated from hydrolysis of the metal phosphides, their AEGL values are likewise based upon phosphine AEGLs. AEGL values for the eight metal phosphides have been added as Appendix D of this document, Appendix 10. |
AEGL-1 is the airborne concentration [expressed as parts per million (ppm) or milligrams per cubic meter (mg/m3)] of a substance above which it is predicted that the general population, including susceptible individuals, could experience notable discomfort, irritation, or certain asymptomatic nonsensory effects. However, the effects are not disabling and are transient and reversible upon cessation of exposure.
AEGL-2 is the airborne concentration (expressed as ppm or mg/m3) of a substance above which it is predicted that the general population, including susceptible individuals, could experience irreversible or other serious, long-lasting adverse health effects or an impaired ability to escape.
AEGL-3 is the airborne concentration (expressed as ppm or mg/m3) of a substance above which it is predicted that the general population, including susceptible individuals, could experience life-threatening health effects or death.
Airborne concentrations below the AEGL-1 represent exposure levels that could produce mild and progressively increasing but transient and nondisabling odor, taste, and sensory irritation or certain asymptomatic nonsensory effects. With increasing airborne concentrations above each AEGL, there is a progressive increase in the likelihood of occurrence and the severity of effects described for each corresponding AEGL. Although the AEGL values represent threshold levels for the general public, including susceptible subpopulations, such as infants, children, the elderly, persons with asthma, and those with other illnesses, it is recognized that individuals, subject to unique or idiosyncratic responses, could experience the effects described at concentrations below the corresponding AEGL.
SUMMARY
Phosphine is a colorless gas used as a fumigant against insects and rodents in stored grain. The pesticide is usually applied as a metal phosphide and reacts with moisture to liberate phosphine gas. Phosphine is also used in the semiconductor industry. Information concerning human exposure to phosphine is of limited use in the derivation of AEGL values since exposure durations and concentrations are not precisely reported. Appropriate animal data are more abundant; however, data consistent with the definition of AEGL-1 values are not available. Therefore, due to insufficient data, AEGL-1 values were not derived.
The AEGL-2 was based on red mucoid nasal discharge in Fischer 344 rats exposed to 10 ppm phosphine for 6 h (Newton et al. 1993). An uncertainty factor (UF) of 3 was applied to account for interspecies variability since time-to-death lethality data from 45 min to 30 h for rats, mice, rabbits, and guinea pigs suggest little species variability (see Figure 10-2). A UF of 10 was applied to account for intraspecies variability since the human data suggest that children may be more sensitive than adults when exposed to presumably similar phosphine concentrations (total UF = 30). The concentration-exposure time rela-
tionship for many irritant and systemically acting vapors and gases may be described by Cn × t = k, where the exponent n ranges from 0.8 to 3.5 (ten Berge et al. 1986). For scaling the AEGL values for phosphine for the 30-min, 1-, 4-, and 8-h time points, the empirically derived value of 1 was used as the exponent n. The exponent n was derived from rat lethality data ranging from 1 to 6 h. The 30-min AEGL-2 was also adopted as the 10-min value due to the added uncertainty of extrapolating from a 6-h time point to 10 min.
The AEGL-3 was based on a concentration causing no deaths (18 ppm) in Sprague Dawley rats exposed to phosphine for 6 h. An uncertainty factor of 3 was applied to account for interspecies variability since lethality data from rats, mice, rabbits, and guinea pigs suggest little species variability. An uncertainty factor of 10 was applied to account for intraspecies variability since human data suggest that children may be more sensitive than adults when exposed to presumably similar phosphine concentrations (total UF = 30). The concentrationexposure time relationship for many irritant and systemically acting vapors and gases may be described by Cn × t = k, where the exponent n ranges from 0.8 to 3.5 (ten Berge et al. 1986). For scaling the AEGL values for phosphine for the 30-min, 1-, 4-, and 8-h time points, the empirically derived value of 1 was used as the exponent n. The exponent n was derived from rat lethality data ranging from 1 to 6 h. The 30-min AEGL-3 was also adopted as the 10-min value due to the added uncertainty of extrapolating from a 6-h time point to 10-min. The calculated values are listed in the Table 10-1.
AEGL values for the eight metal phosphides are presented in Appendix D.
1.
INTRODUCTION
Phosphine is a colorless gas used as a fumigant against insects and rodents in stored grain. Paper sachets containing aluminum phosphide are added to grain and the grain is then sealed. The aluminum phosphide reacts with moisture in the grain to produce the phosphine gas. Phosphine is also used as a doping agent to treat silicon crystals in the semiconductor industry and is a byproduct of metallurgical reactions (Hryhorczuk et al. 1992). Pure phosphine is odorless at concentrations up to 200 ppm (IPCS 1988); however, technical-grade phosphine contains impurities (up to 5% higher phosphines and substituted phosphines) that may be responsible for a garlic-like odor that can be detected at 1.5-3 ppm (Hryhorczuk et al. 1992). A concentration of 7.58 ppm has been reported as “irritating” to humans; however, no data to support this claim were provided (Ruth 1986). Naturally occurring phosphine is rare. It may occur transiently in marsh gas and other areas of anaerobic degradation of materials containing phosphorus. Phosphine is produced by the hydrolysis of aluminum phosphide or the electrolysis of phosphorus in the presence of hydrogen. The physicochemical data for phosphine are given in Table 10-2.
TABLE 10-1 Summary of AEGL Values for Phosphine
TABLE 10-2 Physical and Chemical Data
|
Property |
Descriptor or Value |
Reference |
|
Synonyms |
Hydrogen phosphide, phosphorus trihydride, phosphoretted hydrogen, phosphane |
|
|
CAS Registry No. |
7803-51-2 |
IPCS 1989 |
|
Chemical formula |
PH3 |
IPCS 1989 |
|
Molecular weight |
34.00 |
Budavari et al. 1989 |
|
Physical state |
Gas |
IPCS 1988 |
|
Vapor pressure |
41.3 atm at 20°C |
Braker and Mossman 1980 |
|
Vapor density |
1.17 |
IPCS 1988 |
|
Melting/boiling point |
−133.5°C/−87.4°C |
IPCS 1988 |
|
Solubility in water |
2.5% v/v at 20°C |
IPCS 1989 |
|
Conversion factors in air |
1 mg/m3 = 0.71 ppm 1 ppm = 1.41 mg/m3 |
IPCS 1989 |
2.
HUMAN TOXICITY DATA
2.1.
Acute Lethality
2.1.1.
Case Reports
Numerous case reports concerning human lethality from phosphine exposure were located; however, exposure time and concentrations were not specified in most of the reports. Two siblings, 2 and 4 year old, weighing 11 and 17 kg, respectively, died within 18 h of playing on fumigated wheat for 1 h (Heyndrickx et al. 1976). The wheat had been treated with pyrethrum and malathion (120 kg/2,000 tons of grain) and aluminum phosphide tablets (1 kg/2,000 tons of grain). Analysis of wheat samples taken the morning after the children were exposed yielded 0.95 mg/100 g to 1.6 mg/100 g malathion (1.3 mg/100 g average) and 0.02-0.5 ppm phosphine (0.2 ppm average). Two days after the children played on the wheat 1 ppm of phosphine was detected in several places just above the grain. Postmortem examinations suggested that the deaths were due to acute intoxication, most likely from phosphine, although malathion exposure may have been a contributing factor. The lungs of both children were congested and had atelectatical areas. Ethanol, commonly formed postmortem in both blood and tissues, also was detected in both children.
In another report, two deaths were reported in fumigated boxcars (MMWR 1994). Four males—12, 35, 39, and 52 years old—were discovered in a boxcar containing loose bulk lima beans that had been fumigated with aluminum phosphide. The men had been in the car for approximately 16 h and had periodically opened the hatch for fresh air as needed. When discovered, the 12-year-old was dead and the three men were ill, suggesting that children may be more susceptible than adults. The three survivors reported nausea, vomiting, headache, and abdominal discomfort. In another incident the body of a 23-year-old man was discovered in a rice-filled boxcar during unloading. The rice had been fumigated with aluminum phosphide 12 days prior to unloading, and autopsy results showed phosphine in tissue samples. No phosphine concentrations were reported in either incident.
Aluminum phosphide fumigation aboard a grain freighter on September 24, 1978, resulted in acute illness in two female children and 29 of 31 crew members (Wilson et al. 1980). Phosphine gas escaped from the holds of the ship through a cable housing located near the ship’s ventilation intake and around hatch covers on the forward deck; illness was associated with living or working on the forward deck. Phosphine concentrations were measured on September 30, 1978, 1-4 days after the onset of illness. The highest concentration of 20-30 ppm was detected in a void space of the main deck adjacent to the air ventilation intake. Concentrations between 7.5 and 10 ppm were measured around a hatch on the forward deck, while 0.5 ppm of phosphine was measured in some living quarters. Concentrations were measured with a Drager tube and are estimates of phosphine levels at that time. Wilson et al. stated that phosphine levels reach
peak concentrations in 4-5 days. They note that there was an association between severity of illness and living or working amid ships on the forward deck (phosphine was not detected in the rear crew cabins). Measurements were made shortly after peak exposures are likely to have occurred. Phosphine levels and exposure durations to those peak levels cannot be estimated from the data provided. Exposures were probably continuous for a period of days to levels above a range of 0.5-20 or 30 ppm. One of the two exposed children died (age 2), and postmortem examination revealed congestive heart failure, focal myocardial necrosis with mononuclear infiltrates and fragmented fibers, inflamed mitral and aortic valves, pulmonary edema, pleural effusion, desquamated respiratory epithelium with alveoli thickened by hemolyzed red cells, congested capillaries, an enlarged spleen, and aspirated gastrointestinal contents. The surviving child (age 4 years, 9 months) exhibited nausea, vomiting, dizziness, epigastric pain, and fatigue. An electrocardiogram (ECG) revealed tachycardia with ST depression, and an echocardiogram 24 h later showed dilation and poor function of the left ventricle. A transient increase in the myocardial band (MB) fraction of creatinine phosphokinase (CPK) was also observed. Clinical signs and symptoms resolved within 18 h, and ECG, echo, and CPK abnormalities resolved within 72 h. Crew members exhibited shortness of breath, cough, sputum production, nasal drainage, nausea, jaundice, vomiting, diarrhea, fatigue, headache, drowsiness, paresthesias, tremor, and weakness. Abnormal urinalyses and increased liver enzyme activities were observed in approximately 10% of exposed crew members compared to normal human values.
A 16-year-old male who was employed as an acetylene generator operator for approximately 1 month died from apparent phosphine poisoning (Harger and Spolyar 1958). The subject had experienced periods of dizziness while filling generator hoppers with calcium carbide and became unconscious on two occasions. On a third occasion he was found with his face near an open hopper, where the measured phosphine concentration was 75-95 ppm. On this occasion he could not be aroused and later died. Autopsy revealed lividity of the head and shoulder, bloody exudate from the nose and mouth, edematous lungs, and frothy exudate in the bronchioles and bronchi. Samples of air over the generators contained concentrations of phosphine between 1 and 14 ppm and <3 ppm arsine. The cause of death was reported to be acute pulmonary edema from inhalation of phosphine gas.
Garry et al. (1993) reported the accidental death of a 24-year-old woman who was 7 months pregnant. The victim’s home was located approximately 30 yards from a large bunker-type grain storage facility. The storage facility was topographically more than 5 feet higher than the home and was routinely fumigated with 49,000-82,950 aluminum phosphide pellets. Residents in the area complained of odor and dust coming from the grain, particularly in the evenings. On the afternoon of the fatal exposure there was a rain shower, followed by clear skies and wind of 5 mph in the direction of the patient’s home. She went outside between 8 and 9 p.m. and commented that the odor was “real strong.” At approximately 10:30 p.m. she was tachycardiac and vomiting, and clear frothy
sputum was coming from her nose and mouth. She suffered cardiac arrest shortly after midnight and died. The autopsy revealed pulmonary edema and aluminum concentrations of 713 ng/mL in the blood and >200 ppm in alveolar macrophages. The tachycardia and pulmonary edema noted are consistent with phosphine poisoning. The authors stated that because the phosphine generated from the metal phosphide is highly reactive and unstable, quantitative analysis of aluminum in blood and tissues was used as a marker of exposure to test for possible fumigant intoxication.
Shadnia et al. (2008) reported the accidental poisoning of a woman (age 35 years), her daughter (age 18 years), and son (age 6 years) from phosphine inhalation. The phosphine was released from 20 aluminum phosphide tablets stored in 15 bags of rice. The boy died 2-days postexposure; he had received no medical attention. The other two victims were admitted to the hospital 48 h postexposure and presented with metabolic acidosis, electrocardiogram abnormalities, and hypotension. The patients were discharged after 3 days.
Poisoning from ingestion of aluminum phosphide tablets for attempted suicides has also been reported (Misra et al. 1988a). Eight people (three females, five males; ages 14-25 years) ingested 0.5-20 aluminum phosphide tablets each. Gastritis, drowsiness/dizziness/coma, and peripheral vascular failure were observed in all patients, while cardiac arrhythmia was seen in three cases, and jaundice and kidney failure were observed in one case each. Six of the patients died, and autopsies of two revealed pulmonary edema, gastrointestinal mucosal congestion, petechial hemorrhage on liver and brain surfaces, desquamation of bronchiole epithelium, vacuolar degeneration of hepatocytes, and dilation and engorgement of hepatic central veins and sinusoids.
2.2.
Nonlethal Toxicity
2.2.1.
Case Reports
Case reports concerning human phosphine exposure were available; however, exposure times and concentrations were not specified in most of the reports.
Sixty-seven male grain fumigators in New South Wales were occupationally exposed to phosphine concentrations ranging from 0.4 to 35 ppm (measured in the breathing zone; Jones et al. 1964). Exposure durations were described as “intermittent.” Symptoms occurred immediately in some workers, while the onset of symptoms were delayed from several hours to 2 days in others. Symptoms included diarrhea (82%), nausea (73%), epigastric pain (65%), vomiting (29%), chest tightness (52%), breathlessness (34%), chest pain (29%), palpitations (27%), severe retrosternal pain (6%), headache (83%), dizziness (35%), and staggering gait (12%). There was no evidence of cumulative effects and no tendency to develop immunity.
Zaebst et al. (1988) reported odor but no adverse effects in grain fumigant workers exposed to >50 ppm phosphine for 2-5 min.
Verma et al. (2007) reported acute pancreatitis and myocarditis in a man after ingestion of aluminum phosphide pellets.
Twenty-two fumigation workers 24 to 60 years old (mean age 48) were evaluated for possible phosphine-related toxicity (Misra et al. 1988b). The mean duration of employment using aluminum phosphide was 11.1 years (range 0.5-29 years) and phosphine concentrations ranged from 0.17 to 2.11 ppm. The workers had also been exposed to malathion, hexachlorocyclohexane, and ethylene dibromide; however, during the study and for 4 weeks preceding the study, the workers were exposed only to phosphine. The following effects were reported: cough (18.2%); dyspnea (31.8%); chest tightness (27.3%); headache (31.8%); giddiness, numbness, lethargy (13.6% each); irritability (9.1%); anorexia and epigastric pain (18.2%); nausea (9.1%); and dry mouth (13.6%). Sensory and motor nerve conduction velocities were normal.
In another case, a 53-year-old man used a pesticide powder containing 28% calcium phosphide to poison moles in his garden (Schoonbroodt et al. 1992). He worked for approximately 2 h in rainy weather, and 18 h later experienced fever (40°C), dry cough, weakness, myalgia, headache, dizziness and nausea. Upon hospital admission he was anxious and cyanotic and complained of severe nasal obstruction. He had interstitial infiltrates of the left upper and right lower lobes of the lungs, sinus tachycardia, left anterior fascicular block, leucocyturia, and necrosis of the nasal mucosa. Despite antibiotic treatment, symptoms progressed and the patient developed pulmonary edema, left pleural effusion, and pericardial effusion. He was placed on a ventilator for 18 days and was discharged on day 32; one month after discharge his clinical profile was unremarkable.
Five workers were reportedly exposed to 0.01-0.001 ppm of phosphine during corn fumigation (Modrzejewski and Myslak 1967). However, no validation of analytical methods and no exposure times were reported, and there was no mention of probable occupational exposure to other pesticides. Vertigo, headache, nausea, and vomiting were observed in all subjects. Four workers exhibited dyspnea and bronchial inflammation; three had fever; and one had an enlarged liver, bilirubinemia, and jaundice. In another occupational exposure, three grain inspectors were instantaneously exposed to approximately 159-2,029 ppm of phosphine (no analytical validation provided) while inspecting railroad cars (Feldstein et al. 1991). Immediately after the exposures they experienced facial numbness and tingling, dizziness, nausea, shortness of breath, headache, disorientation, diaphoresis, despondency, and a “sense of doom.”
2.2.2.
Epidemiologic Studies
Epidemiologic studies regarding human exposure to phosphine were not available.
2.3.
Developmental/Reproductive Toxicity
No developmental/reproductive toxicity data concerning phosphine were identified in the available literature.
2.4.
Genetic Toxicology
Barbosa and Bonin (1994) observed no increase in clastogenicity or aneuploidy in lymphocytes of 31 long-term phosphine fumigators compared to a group of 21 matched controls. Sporadic phosphine exposure ranged from 0.1 to 2 ppm for an average of 11.6 years. Specific chromosome aberrations associated with occupational pesticide exposure are discussed in Section 2.5.
2.5.
Carcinogenicity
Epidemiologic data suggest that farm and grain industry workers have an increased incidence of non-Hodgkin’s lymphoma; however, because these workers are generally exposed to myriad pesticides and solvents throughout their working lives, it is difficult to use the data to determine whether there is an association between increased cancer incidence and exposure to a particular pesticide (Garry et al. 1992).
Several studies of chromosomal analysis of male pesticide applicators suggest that occupational exposure to phosphine or mixed exposure to phosphine and other pesticides may contribute to an increased incidence of aberrations (Garry et al. 1989, 1990, 1992). Although dose-related stable (translocations) and unstable (gaps and chromatid deletions) aberrations have been identified in the lymphocytes of phosphine applicators (Garry et al. 1989, 1990), the most convincing link between phosphine exposure and non-Hodgkin’s lymphoma is observed in molecular analyses of the stable aberrations (Garry et al. 1992). A group of 18 pesticide appliers exposed to phosphine or to phosphine and a mixture of other chemicals had an increased incidence of stable aberrations (1.3% of cells with aberrations) compared to five appliers who had ceased phosphine application for 8 months or 26 control subjects (0.2% cells with aberrations). There were four bands with an excess of breaks (over what would be expected on band length alone) in the exposed group and no breaks in the control group. These breaks were also not observed in the group that had ceased phosphine application. Each of these breaks is in a protooncogene region associated with non-Hodgkin’s lymphoma as follows: 1p13 (NRAS), 2p23 (NMYC), 14q32 (ELK2), and 21q22 (ETS-2). More breaks than expected were also observed in 1q32, 3p14, 7p15, and 14q11; however, these breaks are likely not due to pesticide exposure since increases were observed in both exposed and control subjects.
2.6.
Summary
Reports of occupational exposures and attempted suicides are numerous; however, data on exposure durations and concentrations are limited. Common clinical signs are headache, nausea, vomiting, coughing, shortness of breath, paresthesia, weakness, tremors, and jaundice. Pulmonary congestion, pleural effusion, and congestive heart failure may be observed upon postmortem examination. Fumigation workers exposed long term to phosphine have a higher incidence of both stable and less stable chromosomal aberrations. Molecular analysis of these lesions suggests that the breakpoints are near protooncogenes involved in non-Hodgkin’s lymphoma, possibly contributing to the increased incidence of lymphomas in pesticide workers. No reproductive or developmental data were available.
3.
ANIMAL TOXICITY DATA
3.1.
Acute Lethality
3.1.1.
Rats
A 4-h LC50 of 11 ppm (15.5 mg/m3) phosphine was reported for male Charles River-CD rats (Waritz and Brown 1975). The phosphine was analyzed by scrubbing samples of the chamber atmosphere through H2SO4 and analyzing the resulting solution for phosphorus. One sample was taken during each exposure. Signs of respiratory irritation, including salivation, lacrimation, face pawing, and dyspnea, were observed. Hyperemia of the ears also was observed. No effects attributable to phosphine exposure were observed at necropsy.
Newton (1991) reported a combined 6-h LC50 of 28 ppm (39.5 mg/m3) for male and female Sprague-Dawley rats. No deaths occurred in rats exposed to 18 ppm of phosphine for 6 h, suggesting a steep concentration-response curve. This study is discussed in further detail in Section 3.2.1.
Muthu et al. (1980) exposed groups of six adult female albino rats to varying concentrations of phosphine for 1, 4, 6, or 8 h and observed the animals for up to 4 weeks. The gas was generated by dropping aluminum phosphide pellets into distilled water in a beaker in the middle of the exposure chamber. By varying the number of aluminum phosphide pellets and the exposure times, different concentration-time products were obtained. Rats were exposed in a wire mesh cage that was placed in an “insulated aluminum paneled gas-tight atmospheric vault” with a volume of 5,943 L. Phosphine concentrations were measured every hour during the exposure periods with a “phosphine detector tube” developed by the study authors. No other study details were reported. A 1-h LC50 of 134 ppm and a 5.2-h LC50 of 28 ppm were calculated. LC95 values of 45 ppm and 33.3 ppm were calculated for 6.2-h and 8.8 h, respectively.
Groups of four male F344 rats were exposed to 0, 1, 5, or 10 ppm of phosphine for 6 h/day for up to 4 days. All rats died by the end of the third exposure to 10 ppm (Morgan et al. 1995). Deaths were not observed in rats exposed to 1 or 5 ppm, suggesting a steep concentration-response curve.
Neubert and Hoffmeister (1960) exposed groups of eight rats constantly to phosphine concentrations ranging from 71 to 3,294 ppm. Survival times ranged from 16 through 600 min. No further data were provided.
3.1.2.
Mice
Omae et al. (1996) exposed groups of 10 male ICR mice to 17.2, 25.1, 31.7, 41.6, or 59.2 ppm of phosphine for 1-h or to 22.5, 26.5, 33.4, 45.5, or 66.9 ppm for 4 h. The source gas was 99.995% pure phosphine (used in semiconductor manufacturing) diluted with purified nitrogen. It was supplied at a constant rate, mixed with filtered room air, and introduced into the whole-body 550-L exposure chamber. Phosphine concentrations were measured via gas chromatography every 12 min during exposure. The oxygen concentration in the chamber was measured simultaneously with a digital oximeter. The 1-h LC50 was >59.2 ppm, whereas the 4-h LC50 was between 26.5 and 33.4 ppm. In all of the 1-h exposed animals, the mice exhibited face-washing movements and were very active in the initial exposure period. No adverse signs were noted during the 3-day observation period after exposure. In the 4-h exposed mice, the initial observations were similar to those of the 1-h group. However, 3 h after the start of exposure at 33.4 ppm and above, the mice became slower in response to tapping on the chamber glass and supine posture was observed. After the completion of exposure at 22.5 ppm and above, slight tremor and piloerection were observed. At 33.4 ppm and above, mild loss of spontaneous motor activity and piloerection were observed, and at 45.4 ppm and above, complete loss of motor activity, ocular cloudiness, and moribundity were noted.
A 2-h LCL0 of 270 ppm (380 mg/m3) phosphine was reported for an unspecified strain of mice (NIOSH 1994, 2005). No further experimental details were provided.
3.1.3.
Guinea Pigs
A 4-h LCL0 of 100 ppm (141 mg/m3) phosphine was reported for an unspecified strain of guinea pigs (NIOSH 1994, 2005). No further experimental details were provided.
3.1.4.
Cats
A 2-h LCL0 of 50 ppm (70.5 mg/m3) of phosphine was reported for an unspecified strain of cat (NIOSH 1994, 2005). In another report an unspecified
strain of cats survived exposure to 170 ppm (240 mg/m3) for 2 h (IPCS 1988). No further experimental details were provided for either report.
3.1.5.
Rabbits
A 20-min LCL0 of 2,500 ppm (3,525 mg/m3) phosphine was reported for an unspecified strain of rabbit (NIOSH 1994, 2005). In another report an unspecified strain of rabbits survived exposure to 50 ppm (70 mg/m3) for 10 min; however, exposure to 100 ppm (140 mg/m3) was fatal to all exposed animals in 2.5-3 h, and 500 ppm (700 mg/m3) was fatal in 25-30 min (IPCS 1988). No further experimental details were provided for either study.
3.2.
Nonlethal Toxicity
3.2.1.
Rats
Groups of five Sprague-Dawley rats/sex were exposed to target phosphine concentrations of 0, 1, 5, or 26 ppm (actual concentrations 0, 1.3, 6.0, or 28 ppm, respectively) for 6 h, while additional groups of 10 male Sprague-Dawley rats were exposed to target concentrations of 0, 3, 12, or 18 ppm (actual concentrations 0, 3.1, 10, or 18 ppm, respectively) for 6 h (Newton 1991). Actual exposure concentrations were determined hourly by gas chromatography, and particle size distribution for any possible background aerosol was measured with a TSI aerodynamic particle sizer. Actual exposure concentrations and mortality data are presented in Table 10-3. Postexposure observations were unremarkable except for the surviving 26-ppm animals. Hunched appearance, coarse tremors, decreased activity, and cold to touch were observed and resolved by the following day. Mean body-weight data were generally unremarkable. Body-weight
TABLE 10-3 Exposure Concentration and Mortality of Rats Exposed to Phosphine for 6 h
|
Target Exposure Concentration (ppm) |
Actual Mean Concentration (ppm) |
Mortality |
||
|
Male |
Female |
Total |
||
|
0 |
0 |
0/5 |
0/5 |
0/10 |
|
1.0 |
1.3 |
0/5 |
0/5 |
0/10 |
|
5.4 |
6.0 |
0/5 |
0/5 |
0/10 |
|
26 |
28 |
3/5 |
2/5 |
5/10 |
|
0 |
0 |
0/10 |
– |
0/10 |
|
3.0 |
3.1 |
0/10 |
– |
0/10 |
|
12 |
10 |
0/10 |
– |
0/10 |
|
18 |
18 |
0/10 |
– |
0/10 |
|
Source: Newton 1991. Reprinted with permission; copyright 1991, Huntingdon Life Sciences. |
||||
decreases of approximately 10% were observed on day 2 in surviving animals from the 12-, 18-, and 2-6-ppm groups. The decreases were not statistically significant.
Dose-related statistically significant increases in mean hemoglobin, hematocrit, and red blood cell levels were observed in the 10- and 18-ppm males (hematology values were measured only in the 0-, 3.1-, 10-, and 18-ppm males). However, these increases, possibly the result of dehydration, are of questionable biologic significance since they are generally within normal historical ranges for Sprague-Dawley rats (Charles River Laboratories 1984). No Heinz Bodies were observed in control or treated animals. Hematology data are presented in Table 10-4.
Results from chromosomal analyses of exposed rats are given in a separate report (Hazleton Laboratories 1991). Statistically significant increases in stable chromosomal aberrations were observed only in male rats exposed to 3.1, 6.0, and 18 ppm of phosphine. Although some aberrations were observed in treated females and in males treated with 10 or 28 ppm, the incidence was not statistically significant. Due to the lack of a clear dose response in males and the small magnitude of response in females, the increase in aberrations is of questionable toxicologic significance.
In another study, Newton et al. (1993) exposed 15 Fischer 344 rats/sex/group to 0, 2.5, 5, or 10 ppm (0, 3.5, 7, or 14 mg/m3) phosphine for 6 h. No animals died during the study. During the exposure period, red mucoid nasal discharges were observed at all exposure concentrations; however, these effects resolved during the 14-day recovery period. There was no effect on body weight, and there were no abnormal findings during postmortem examinations.
Newton et al. also conducted a subchronic study, exposing groups of 30 male and 30 female Fischer 344 rats to 0, 0.37, 1.0, or 3.1 ppm of phosphine 6 h/day, 5 days/week, for 13 weeks. Ten animals/sex/group were killed after 4 and 13 weeks of exposure and after 4 weeks of recovery. A satellite group of 10 animals/sex/concentration (0 or 10-ppm exposure) was added after animals were killed after the 4-weeks of exposure because no effects were observed in the 3.1-ppm group. However, 4/10 females (10 ppm) died after three exposures. Thus, the 10-ppm exposures were stopped and satellite groups at target concentrations of 0 or 5 ppm were added; all of these animals survived until they were killed 13 days later. Statistically significant decreases in body weights, accompanied by significant decreases in feed consumption, were observed in males and females exposed to 1 ppm and higher. Statistically significant (p < .05), although biologically questionable, hematologic and clinical chemistry parameters were affected only in male animals at 3.1 ppm and above. All previously described effects were reversible. Treatment-related lung congestion and kidney lesions (coagulative necrosis of proximal convoluted tubular epithelia) were observed in both males and females exposed to 10 ppm. The kidney pathology was more severe in males than in females. No pathology was observed at 3.1 or 1 ppm.
TABLE 10-4 Mean Hematology Values for Male Rats Exposed to Phosphine for 6 h
|
Target Exposure Concentration (ppm) |
Actual Mean Concentration (ppm) |
Hematology Values (mean ± SD) |
||
|
HBG (g/dl) |
HCT (%) |
RBC (106/µl) |
||
|
Historical control rangea |
|
13-21 |
39-51 |
4.2-6.2 |
|
0 |
0 |
13.1 ± 0.6 |
39 ± 1 |
6.47 ± 0.24 |
|
3.0 |
3.1 |
13.2 ± 0.4 |
39 ± 1 |
6.45 ± 0.3 |
|
12 |
10 |
14.2 ± 0.6b |
42 ± 2b |
6.88 ± 0.34b |
|
18 |
18 |
14.7 ± 0.7b |
43 ± 2b |
7.11 ± 0.4b |
|
aCharles River Laboratories 1984. bp < .05. Source: Newton 1991. Reprinted with permission; copyright 1991, Huntingdon Life Sciences. |
||||
Morgan et al. (1995) exposed 18 F344 rats/sex/group to 0, 1.25, 2.5, or 5 ppm of phosphine 6 h/day, 5 days/week, for 2 weeks. None of the rats died as a result of phosphine exposure. Male rats exposed to 5 ppm had decreased (21-29%) lung weights after 2 weeks, and female rats exposed to 5 ppm had increased (16-27%) heart weights after 2 weeks. The significance of these data appear somewhat equivocal in that no microscopic evidence of treatment-related effects were found in any of the tissues examined from rats or mice exposed to 5 ppm for 2 weeks.
3.2.2.
Mice
Groups of four male B6C3F1 mice were exposed to 0, 1, 5, or 10 ppm of phosphine for 6 h/day for 4 days (Morgan et al. 1995). All mice were killed moribund after the fourth exposure. Pathology data were collected, and there were no treatment-related effects observed in mice exposed to 1 or 5 ppm. Anemia, decreased leukocyte counts, increased serum ALT and SDH activities, increased urine nitrogen, degeneration and necrosis of renal tubule epithelium, myocardial degeneration, and liver foci and degeneration were observed in the 10-ppm group. In a follow-up study, Morgan et al. exposed 18 mice/sex/group to 0, 1.25, 2.5, or 5 ppm of phosphine for 6 h/day, 5 days/week, for 2 weeks. None of the mice died as a result of the phosphine exposure. Male mice exposed to 5 ppm had decreased (21-29%) lung weights after 2 weeks, and female mice exposed to 5 ppm had increased (16-27%) heart weights after 2 weeks.
3.3.
Developmental/Reproductive Toxicity
Groups of 18 pregnant F344 rats were exposed to 0, 0.03, 3.0, 5.0, or 7.5 ppm of phosphine for 6 h/day during days 6-15 of gestation (Newton et al.
1993). Fourteen females exposed to 7.5 ppm died after 3-10 days of exposure. No maternal deaths were observed at lower concentrations, suggesting a steep concentration response with regard to lethality. Due to excessive the mortality, the remaining animals in the 7.5-ppm group were terminated. No adverse treatment-related effects were observed in maternal body weight and feed consumption, maternal physical observations, or uterine implantation. There was a statistically significant increase in the mean number of resorption sites and the mean resorption/implantation ratio in the 0.03-ppm group. However, in the absence of similar observations at the higher concentrations of phosphine, these effects are not considered toxicologically relevant. No treatment-related effects were observed during maternal postmortem examinations. No treatment-related effects were observed on mean fetal weights or fetal sex ratio. No external, visceral, or skeletal malformations were observed in the fetuses.
Kligerman et al. (1994a) exposed 50 male B6C3F1 mice to 5 ppm of phosphine for 6 h/day for 10 days over a 12-day period. The treated males were then mated to groups of untreated females (two females/male) on each of six consecutive 4-day mating intervals. None of the females had a significant increase in percent resorptions, and there was no difference in pregnancy rates between control and treated animals.
3.4.
Genetic Toxicology
Kligerman et al. (1994b) exposed male CD-1 mice to 0, 5, 10, or 15 ppm of phosphine for 6 h. No increase in the incidence of chromosome aberrations was observed in cultured splenocytes, and no increase in micronuclei incidence was observed in bone marrow smears. In another study, Kligerman et al. (1994a) exposed groups of male F344/N rats and male B6C3F1 mice to 0, 1.25, 2.5, or 5 ppm of phosphine for 6 h/day for 9 days over an 11-day period. No increase in cytogenetic end points was observed over controls in cultured lymphocytes or in bone marrow smears. Barbosa et al. (1994) observed an increased incidence of micronuclei in mice exposed to 4.5 ppm of phosphine for 6 h/day, 5 days/week, for 13 weeks, but no increase was seen in mice exposed similarly to 0.3 or 1.0 ppm.
3.5.
Carcinogenicity
No data concerning the carcinogenicity of phosphine from inhalation exposure were identified in the available literature.
3.6.
Summary
A 4-h LC50 of 11 ppm of phosphine was reported for male Charles River-CD rats (Waritz and Brown 1975). Newton (1991) defined a 6-h LC50 of 28 ppm
for male and female Sprague-Dawley rats. Muthu et al. (1980) calculated a 5.2-h LC50 of 28 ppm and LC95 values of 45 ppm and 33.3 ppm for 6.2-h and 8.8 h, respectively, in female albino rats. Signs of respiratory irritation, including hyperemia of the ears, salivation, lacrimation, face pawing, and dyspnea, were observed in rats. Omae et al. (1996) determined a 1-h LC50 >59.2 ppm (the highest concentration tested), and a 4-h LC50 was between 26.5 and 33.4 ppm in male Institute for Cancer Research (ICR) mice. Clinical signs in mice included face-washing movements, increased activity in the initial exposure period, followed by a slowing in response to tapping on the chamber glass, supine posture, slight tremor, piloerection, mild loss of spontaneous motor activity progressing to complete loss of motor activity, ocular cloudiness, and moribundity. Selected mortality data are presented in Table 10-5. The concentration × time products from these data are relatively constant across species except for the Waritz and Brown (1975) data, which appear to be an outlier.
Exposure to phosphine affected multiple end points in rats and mice. Rats exposed for 6 h exhibited increased hematology values at 12 and 18 ppm; decreased body weight at 12, 18, and 26 ppm; and tremors, hunched appearance, decreased activity, and death at 26 ppm (Newton 1991). Rats exposed to 2.5, 5.0, or 10 ppm of phosphine for 6 h exhibited a red nasal discharge (Newton et al. 1993). Repeated exposures resulted in decreased lung weight and increased heart weight in rats and mice (Morgan et al. 1995) and reversible body-weight and feed-consumption decreases and lung and kidney histopathology in rats (Newton et al. 1993). Mice repeatedly exposed to 10 ppm exhibited anemia; decreased white-blood-cell counts; increased serum liver enzymes; and kidney, heart, and liver pathology (Morgan et al. 1995). Phosphine was not a developmental or reproductive toxicant in rats (Newton et al. 1993) or mice (Kligerman et al. 1994a). Carcinogenicity data were not available. Although several genotoxicity studies reported negative results, the results concerning micronuclei incidence were equivocal.
TABLE 10-5 Selected Lethality Data from Animals Exposed to Phosphine
|
End Point |
Species |
Concentration (ppm)a |
Time (h) |
C × T (ppm-h) |
Reference |
|
LC50 |
Rat |
11 |
4 |
44 |
Waritz and Brown 1975 |
|
LC50 |
Rat |
134 |
1 |
134 |
Muthu et al. 1980 |
|
LC50 |
Rat |
28 |
5.2 |
146 |
Muthu et al. 1980 |
|
LC50 |
Rat |
28 |
6 |
168 |
Newton 1991 |
|
LC50 |
Mouse |
26.5-33.4 |
4 |
106-134 |
Omae et al. 1996 |
|
LC95 |
Rat |
45 |
6.2 |
279 |
Muthu et al. 1980 |
|
LC95 |
Rat |
33.3 |
8.8 |
293 |
Muthu et al. 1980 |
|
a1 ppm = 1.41 mg/m3. |
|||||
4.
SPECIAL CONSIDERATIONS
4.1.
Metabolism and Disposition
A report of the International Programme on Chemical Safety indicated that inhaled phosphine is readily absorbed through the lungs and is primarily distributed to the blood, nervous system, and liver (IPCS 1988). The report also indicated that phosphide has been detected in the kidneys of fatal poisoning cases. In rats, phosphine not excreted in expired air is oxidized and is excreted in urine as hypophosphite and phosphite. The fact that phosphine is incompletely oxidized and the fact that the proportion of an administered dose that is eliminated as expired phosphine increases with dose suggest that the oxidative pathway is slow (IPCS 1988).
4.2.
Mechanism of Toxicity
In vitro, phosphine reacts with cytochrome c and cytochrome c oxidase, thereby inhibiting mitochondrial oxygen uptake (Chefurka et al. 1976; IPCS 1988). In vitro studies have shown that phosphine can react with the heme moiety of hemoglobin in the presence of oxygen (Chefurka et al. 1976). In rabbits treated with zinc phosphide, increases in serum glutamic-pyruvic and glutamicoxaloacetic transaminases, leucine aminopeptidase, aldolase, alkaline phosphatase, and albumin were observed. Hepatic fat metabolism was also disturbed. Cell death and loss of cell membrane integrity accounted for the increased liver enzymes, bronchiolytic effects, cloudy swelling of renal tubular epithelia, and hemorrhagic myocardial lesions (IPCS 1988). Phosphine and arsine are often described as chemically similar. However, no explanation exists as to why hemolysis does not occur as a result of phosphine poisoning.
4.3.
Structure-Activity Relationships
Phosphine may be successively alkylated, oxygenated, and esterified to yield phosphates or phosphites, phosphine oxide, phosphinic acid esters or phosphinates, and phosphoric acid esters or phosphonates. These compounds may contain alkyl or aryl groups (Bisesi 1994). Toxicologic properties of these compounds vary as follows: (1) central nervous system (CNS) damage with secondary paralysis, (2) CNS stimulation or convulsion with anesthetic action, (3) true or pseudocholinesterase inhibition, (4) irritation of dermal or respiratory system, and (5) no effect. Alkyl and aryl phosphates exhibit relatively high toxicity, and phosphonates are moderately to highly toxic, with toxicity lowest for alkyl derivatives and rising with increasing aromatization and further increasing with halogenation or nitro- group substitution. The toxicologic properties of organophosphites are similar to those of the phosphonates (Bisesi 1994). Since
these metabolites cause primarily neurologic symptoms, as opposed to the symptoms seen from phosphine toxicity, they are likely not the cause of death from phosphine exposure.
Four-hour LC50 values for Charles River-CD male rats were 11 ppm for phosphine, 38 ppm for phenylphosphine, and 8,865 ppm for triphenylphosphine (Waritz and Brown 1975). The dose-response curves were parallel, and all caused clinical signs associated with mild respiratory irritation. Additionally, triphenylphosphine caused severe weight loss immediately following exposure, followed by a normal rate of weight gain. Phosphine and phenylphosphine caused mild weight loss, followed by a normal rate of weight gain.
4.4.
Other Relevant Information
4.4.1.
Species Variability
Klimmer (1969) exposed groups of rats, rabbits, guinea pigs, and rabbits to phosphine concentrations ranging from 5.3 to 400 ppm for over 800 h. When Gehring et al. (1991) plotted exposure concentration against average time to death for these data, the values for all four species fell along the same log concentration-log time curve, suggesting little species variability (see Figure 10-1).
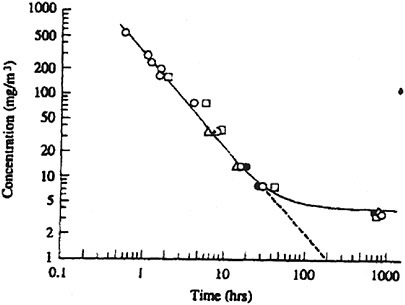
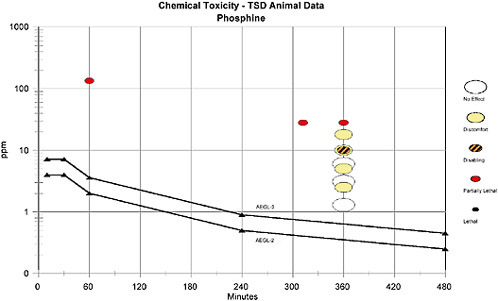
FIGURE 10-1 Phosphine concentration versus average time to death of rats (○), rabbits (∆), guinea pigs (●), and cats. Source: Gehring et al. (1991) from analysis of the data of Klimmer (1969).
4.4.2.
Concurrent Exposure Issues
In an occupational setting, phosphine is likely to be encountered in conjunction with other pesticides. No definitive data were located concerning the potential synergy of combinations of pesticides or of the relative toxic potential of phosphine in mixtures.
4.4.3.
Derivation of the Exponent n
The concentration-exposure time relationship for many irritant and systemically acting vapors and gases has been described by the relationship Cn × t = k, where the exponent n ranges from 0.8 to 3.5 (ten Berge et al. 1986). When rat LC50 data (from Table 10-5) ranging from 1- to 6-h exposure duration are utilized, an n value of 0.98 is derived (see Figure 10-2).
5.
RATIONALE FOR AEGL-1
5.1.
Summary of Human Data Relevant to AEGL-1
No human data are available for the derivation of AEGL-1 for phosphine. Nonlethal effects observed were more severe than those defined by AEGL-1; furthermore, no reliable exposure parameters were available.

FIGURE 10-2 Best-fit concentration (ppm phosphine) × time (exposure duration in hours) curve. Linear regression of rat lethality data.
5.2.
Summary of Animal Data Relevant to AEGL-1
Animal data from exposure to phosphine relevant to the derivation of AEGL-1 are not available.
5.3.
Derivation of AEGL-1
Appropriate data were not available for derivation of AEGL-1 values for phosphine. No human or animal data are consistent with the effects defined by AEGL-1. The fact that lethality has been observed in animals exposed to phosphine concentrations below the odor threshold (1.5-200 ppm, dependent on impurities) also supports this recommendation. Therefore, AEGL-1 values for phosphine are not recommended (see Table 10-6.)
6.
RATIONALE FOR AEGL-2
6.1.
Summary of Human Data Relevant to AEGL-2
No human data are available for the derivation of AEGL-2 for phosphine. Although effects such as headache, nausea, vomiting, coughing, shortness of breath, weakness, and paresthesia have been observed, the studies are not appropriate for the derivation since the descriptions of concentration, exposure time, and effects are not well defined.
6.2.
Summary of Animal Data Relevant to AEGL-2
A red mucoid discharge was observed in rats exposed to 10 ppm of phosphine for 6 h (Newton et al. 1993). In another experiment no pulmonary lesions or renal congestion were observed in rats exposed to 3.1 ppm for 6 h/day, 5 days/week, for 13 weeks.
6.3.
Derivation of AEGL-2
The red mucoid nasal discharge observed in rats exposed to 10 ppm of phosphine for 6 h (Newton et al. 1993) is used as the basis for the calculation of the AEGL-2. Although this effect does not meet the definition of AEGL-2, it is being utilized since no other relevant effect is observed in a single-exposure study. Since red mucoid nasal discharge by itself is less severe than the effects defined by AEGL-2 (irreversible or severe long-lasting effects), the resulting values should be protective. An uncertainty factor of 3 was applied to account
TABLE 10-6 AEGL-1 for Phosphine
|
AEGL Level |
10 min |
30 min |
1 h |
4 h |
8 h |
|
AEGL-1 |
NR |
NR |
NR |
NR |
NR |
|
NR: not recommended. Absence of an AEGL-1 does not imply that exposure below the AEGL-2 is without adverse effects. |
|||||
for interspecies variability since time-to-death lethality data from 45 min to 30 h for rats, mice, rabbits, and guinea pigs suggest little species variability (see Figure 10-1). An uncertainty factor of 10 will be applied to account for intraspecies variability since the human data suggest that children may be more sensitive than adults when exposed to presumably similar phosphine concentrations. For example, in both the MMWR (1994) and Wilson et al. (1980) reports, exposed children died, but exposed adults survived. The concentration-exposure time relationship for many irritant and systemically acting vapors and gases may be described by Cn × t = k, where the exponent n ranges from 0.8 to 3.5 (ten Berge et al. 1986). When rat lethality data of 1-6-h are utilized in the derivation of the exponent for phosphine, a value of 1 is obtained. For scaling the AEGL values for phosphine for the 30-min, 1-, 4-, and 8-h time points, this empirically derived value of 1 was used as the exponent n. The 30-min AEGL-2 was also adopted as the 10-min value due to the added uncertainty of extrapolating from a 6-h time point to 10 min. The values for AEGL-2 are given in Table 10-7.
These AEGL-2 values are considered protective since no effects defined by AEGL-2 were observed in rats repeatedly exposed to 3.1 ppm of phosphine for 6 h/day, 5 days/week, for 13 weeks (Newton et al. 1993) and only a 10% decrease in body weight was observed in rats exposed to 10 ppm for 6 h (Newton 1991).
7.
RATIONALE FOR AEGL-3
7.1.
Summary of Human Data Relevant to AEGL-3
Human data from exposure to phosphine are not appropriate for the derivation of AEGL-3, since the descriptions of concentration and exposure times are not well documented.
7.2.
Summary of Animal Data Relevant to AEGL-3
Waritz and Brown (1975) reported a 4-h LC50 for phosphine of 11 ppm in Charles River-CD rats, and Newton (1991) observed hunched appearance, coarse tremors, decreased activity, and death in Sprague-Dawley rats exposed to 28 ppm for 6 h. This study also points to a steep concentration-response curve
TABLE 10-7 AEGL-2 for Phosphine (ppm [mg/m3])
|
AEGL Level |
10 min |
30 min |
1 h |
4 h |
8 h |
|
AEGL-2 |
4.0 (5.6) |
4.0 (5.6) |
2.0 (2.8) |
0.50 (0.71) |
0.25 (0.35) |
since no deaths were seen in groups of 10 rats exposed to 18 or 10 ppm of phosphine for 6 h. Muthu et al. (1980) calculated a 5.2-h LC50 of 28 ppm and LC95 values of 45 ppm and 33.3 ppm for 6.2-h and 8.8-h exposures, respectively, in female albino rats. Omae et al. (1996) determined a 1-h LC50 >59.2 ppm, and a 4-h LC50 was between 26.5 and 33.4 ppm in male ICR mice.
7.3.
Derivation of AEGL-3
The highest concentration yielding no deaths in rats (18 ppm) for 6 h is used as the basis for the calculation of the AEGL-3 (Newton 1991). This study is selected because 10 animals per dose group were used and data were for exposures over a range of phosphine concentrations. The Waritz and Brown (1975) study included a small number of animals per dose group and were incompletely reported. Additionally, the Waritz and Brown data seem to be an outlier to the database (see Table 10-5). A rat study was chosen instead of mouse data since the exponent n was derived from rat data. An uncertainty factor of 3 was applied to account for interspecies variability since time-to-death lethality data from 45 min to 30 h for rats, mice, rabbits, and guinea pigs suggest little species variability (see Figure 10-1). An uncertainty factor of 10 is applied to account for intraspecies variability since the human data suggest that children may be more sensitive than adults when exposed to presumably similar phosphine concentrations. For example, in both the MMWR (1994) and Wilson et al. (1980) reports, exposed children died, but exposed adults survived. The concentration-exposure time relationship for many irritant and systemically acting vapors and gases may be described by Cn × t = k, where the exponent n ranges from 0.8 to 3.5 (ten Berge et al. 1986). When rat lethality data of 1-6-h are utilized in the derivation of the exponent for phosphine, a value of 1 is obtained. For scaling the AEGL values for phosphine for the 30-min, 1-, 4-, and 8-h time points, this empirically derived value of 1 was used as the exponent n. The 30-min AEGL-3 was also adopted as the 10-min value due to the added uncertainty of extrapolating from a 6-h time point to 10 min. The values for AEGL-3 are given in Table 10-8.
These values are considered protective since workers were repeatedly exposed for “brief” periods of time to phosphine concentrations up to 35 ppm with no life-threatening effects (Jones et al. 1964) and workers exposed to >50 ppm for 2-5 min experienced only odor (Zaebst et al. 1988).
8.
SUMMARY OF AEGL VALUES
8.1.
AEGL Values and Toxicity End Points
The derived AEGL values for various levels of effects and durations of exposure are summarized in Table 10-9. Appropriate data are not available for derivation of AEGL-1. AEGL-2 values were based on a red mucoid nasal discharge in rats exposed to phosphine for 6 h. A concentration causing no deaths in rats exposed to phosphine for 6 h was used for AEGL-3 calculation. It should be noted that the AEGL-2 and AEGL-3 values for phosphine are very close (AEGL-2; AEGL-3 ratio = 1.8). This reflects an extremely steep concentration-response curve for phosphine; there is little margin between nonlethal effects and death.
8.2.
Comparison with Other Standards and Criteria
Several organizations have developed standards and criteria for phosphine (see Table 10-10).
8.3.
Data Adequacy and Research Needs
Appropriate data from human studies are not available for phosphine. There appears to be little species variability with regard to lethality. With regard to nonlethal end points, data are equivocal and in many cases lack a concentration response-relationship.
TABLE 10-8 AEGL-3 for Phosphine (ppm [mg/m3])
|
AEGL Level |
10 min |
30 min |
1 h |
4 h |
8 h |
|
AEGL-3 |
7.2 (10) |
7.2 (10) |
3.6 (5.1) |
0.90 (1.3) |
0.45 (0.63) |
TABLE 10-9 Relational Comparison of AEGL Values fzor Phosphine (ppm [mg/m3])a
TABLE 10-10 Extant Standards and Guidelines for Phosphine
|
Guideline |
Exposure Duration |
||||
|
10 min |
30 min |
1 h |
4 h |
8 h |
|
|
AEGL-1 |
NR |
NR |
NR |
NR |
NR |
|
AEGL-2 |
4.0 ppm |
4.0 ppm |
2.0 ppm |
0.50 ppm |
0.25 ppm |
|
AEGL-3 |
7.2 ppm |
7.2 ppm |
3.6 ppm |
0.90 ppm |
0.45 ppm |
|
ERPG-1a |
|
|
NR |
|
|
|
ERPG-2a |
|
|
0.5 ppm |
|
|
|
ERPG-3a |
|
|
5 ppm |
|
|
|
NIOSH IDLHb |
50 ppm |
|
|
|
|
|
NIOSH RELc |
0.3 ppm |
|
|
|
|
|
NIOSH STELc |
1.0 ppm |
|
|
|
|
|
OSHA PEL-TWAd |
|
|
|
|
0.28 ppm |
|
ACGIH TLV-TWAe |
|
|
|
|
0.3 ppm |
|
ACGIH TLV-STELe |
1.0 ppm |
|
|
|
|
|
German MAK-TWAf |
|
|
|
|
0.1 ppm |
|
German MAK-Peakf |
|
|
|
|
1 ppm |
|
Dutch MACg |
|
1.0 ppm (15 min) |
|
|
0.3 ppm (8 hr) |
|
aERPG (Emergency Response Planning Guidelines, American Industrial Hygiene Association) (AIHA 2002). The ERPG-2 is the maximum airborne concentration below which it is believed nearly all individuals could be exposed for up to 1 h without experiencing or developing irreversible or other serious health effects or symptoms that could impair an individual’s ability to take protection action. The ERPG-2 for phosphine is based on human experience. The ERPG-3 is the maximum airborne concentration below which it is believed nearly all individuals could be exposed for up to 1 h without experiencing or developing life-threatening health effects. The ERPG-3 for phosphine is based on animal lethality data and human experience. bIDLH (Immediately Dangerous to Life and Health, National Institute of Occupational Safety and Health) (NIOSH 2002, 2005) represents the maximum concentration from which one could escape within 30 min without any escape-impairing symptoms or any irreversible health effects. The IDLH for phosphine is based on acute inhalation toxicity data in humans. cNIOSH REL-STEL (Recommended Exposure Limits–Short-Term Exposure Limit) (NIOSH 2002, 2005) is defined analogous to the ACGIH TLV-TWA. dOSHA PEL-TWA (Occupational Health and Safety Administration, Permissible Exposure Limits–Time-Weighted Average) (29 CFR 1910.1000 [2002]) is defined analogous to the ACGIH-TLV-TWA but is for exposures of no more than 10 h/day, 40 h/week. eACGIH TLV-TWA (American Conference of Governmental Industrial Hygienists, Threshold Limit Value–Time Weighted Average) (ACGIH 2002) is the time-weighted average concentration for a normal 8-h workday and a 40-h workweek, to which nearly all workers may be repeatedly exposed, day after day, without adverse effect. |
|||||
9.
REFERENCES
ACGIH (American Conference of Governmental Industrial Hygienists). 2002. Documentation of the Threshold Limit Values and Biological Exposure Indices, 7th Ed. American Conference of Governmental Industrial Hygienists, Cincinnati, OH.
AIHA (American Industrial Hygiene Association). 2002. Emergency Response Planning Guidelines for Phosphine. Fairfax, VA: AIHA Press.
Anger, F., F. Paysant, F. Brousse, I. Le Normand, P. Develay, Y. Gaillard, A. Baert, M.A. Le Gueut, G. Pepin, and J.P. Anger. 2000. Fatal aluminum phosphide poisoning. J. Anal. Toxicol. 24(2):90-92.
Bajaj, R., H.S. Wasir, R. Agarwal, A. Malhotra, P. Chopra, and M.L. Bhatia. 1988. Aluminum phosphide poisoning: Clinical toxicity and outcome in eleven intensively monitored patients. Natl. Med. J. India. 1(6):270-274.
Barbosa, A., and A.M. Bonin. 1994. Evaluation of phosphine genotoxicity at occupational levels of exposure in New South Wales, Australia. Occup. Environ. Med. 51(10):700- 705.
Barbosa, A., E. Rosinova, J. Dempsey, and A.M. Bonin. 1994. Determination of genotoxic and other effects in mice following short term repeated-dose and subchronic inhalation exposure to phosphine. Environ. Mol. Mutagen. 24(2):81-88.
Bisesi, M.S. 1994. Esters of organic phosphorus compounds. Pp. 3063-3118 in Patty’s Industrial Hygiene and Toxicology, 4th Ed., Vol. II, Part D., G.D. Clayton, and F.E. Clayton, eds. New York: John Wiley & Sons.
Braker, W., and A.L. Mossman. 1980. Matheson Gas Data Book, 6th Ed. Lyndhurst, NJ: Matheson.
Budavari, S., M.J. O’Neil, A. Smith, and P.E. Heckelman, eds. 1989. Phosphine. P. 1165 in The Merck Index: An Encyclopedia of Chemicals, Drugs, and Biologicals, 11th Ed. Rahway, NJ: Merck.
Chan, L.T., R.J. Crowley, D. Delliou, and R. Geyer. 1983. Phosphine analysis in postmortem specimens following ingestion of aluminum phosphide. J. Anal. Toxicol. 7(4):165-167.
Charles River Laboratories. 1984. Baseline Hematology and Clinical Chemistry Values for Charles River CD [Crl:CD(SD)BR] Rats as a Function of Sex and Age. Charles River Technical Bulletin 3(2).
Chefurka, W., K.P. Kashi, and E.J. Bond. 1976. The effect of phosphine on electron transport in mitochondria. Pestic. Biochem. Physiol. 6:65-84.
ChemIDplus. 2005a. Potassium phosphide. ChemIDplus, Specialized Information System, U.S. National Library of Medicine, Bethesda, MD [online]. Available: http://toxnet.nlm.nih.gov/cgi-bin/sis/htmlgen?CHEM [accessed 4-15-2005].
ChemIDplus. 2005b. Sodium phosphide. ChemIDplus, Specialized Information System, U.S. National Library of Medicine, Bethesda, MD [online]. Available: http://toxnet.nlm.nih.gov/cgi-bin/sis/htmlgen?CHEM [accessed 4-15-2005].
ChemIDplus. 2005c. Magnesium aluminum phosphide. ChemIDplus, Specialized Information System, U.S. National Library of Medicine, Bethesda, MD [online]. Available: http://toxnet.nlm.nih.gov/cgi-bin/sis/htmlgen?CHEM [accessed 4-15-2005].
Chugh, S.N., R. Pal, V. Singh, and S. Seth. 1996. Serial blood phosphine levels in acute aluminum phosphide poisoning. J. Assoc. Physicians India 44(3):841-842.
DFG (Deutsche Forschungsgemeinschaft). 2000. List of MAK and BAT Values 2000: Maximum Concentrations and Biological Tolerance Values at the Workplace. Commission for the Investigation of Health Hazards of Chemical Compounds in the Work Area Report No. 36. Weinheim, Federal Republic of Germany: Wiley-VCH.
Feinstein, A., M. Heumann, and M. Barnett. 1991. Fumigant intoxication during transport of grain by railroad. J. Occup. Med. 33:64-65.
Garry, V.F., J. Griffith, T.J. Danzl, R.L. Nelson, E.B. Whorton, L.A. Krueger, and J. Cervenka. 1989. Human genotoxicity: Pesticide applicators and phosphine. Science 246(4927):251-255.
Garry, V.F., R.L. Nelson, T.J. Danzl, J. Cervenka, L.A. Krueger, J. Griffith, and E.B. Whorton. 1990. Human genotoxicity in phosphine-exposed fumigant applicators. Prog. Clin. Biol. Res. 340C: 367-376.
Garry, V.F., T.J. Danzl, R. Tarone, J. Griffith, J. Cervenka, L. Krueger, E.B. Whorton, and R.L. Nelson. 1992. Chromosome rearrangements in fumigant appliers: Possible relationship to non-Hodgkins lymphoma risk. Cancer Epidemiol. Biomarkers Prev. 1(4):287-291.
Garry, V.F., P.F. Good, J.C. Manivel, and D.P. Perl. 1993. Investigation of a fatality from nonoccupational aluminum phosphide exposure: Measurement of aluminum in tissue and body fluids as a marker of exposure. J. Lab. Clin. Med. 122(6):739-747.
Gehring, P.J., R.J. Nolan, P.G. Watanabe, and A.M. Schumann. 1991. Solvents, fumigants, and related compounds: Phosphine. Pp. 657-661 in Handbook of Pesticide Toxicology, Vol. 2, W.J. Hayes, Jr., and E.R. Laws, Jr., eds. San Diego: Academic Press.
Harger, R.N., and L.W. Spolyar. 1958. Toxicity of phosphine, with a possible fatality from this poison. AMA Arch. Ind. Health 18(6):497-504.
Hazleton. 1991. Mutagenicity Test on Phosphine: Measuring Chromosomal Aberration Frequencies in Cultured Rat Whole Blood Lymphocytes. Study No. 12256-0-444. Hazleton Laboratories America, Inc., Kensington, MD.
Heyndrickx, A., C. Van Peteqhem, M. Van Den Heede, and R. Lauwaert. 1976. A double fatality with children due to fumigated wheat. Eur. J. Toxicol. Environ. Hyg. 9(2):113-118.
Hryhorczuk, D.O., S.E. Aks, and J.W. Turk. 1992. Unusual occupational toxins. Occup. Med. 7(3):567-586.
HSDB (Hazardous Substances Data Bank). 2007a. Calcium Phosphide (CASRN: 1305-99-3). Specialized Information System, U.S. National Library of Medicine, Bethesda, MD [online]. Available: http://toxnet.nlm.nih.gov/cgi-bin/sis/search. [accessed Aug. 1, 2008].
HSDB (Hazardous Substances Data Bank). 2007b. Zinc Phosphide. (CASRN: 1314-84-7). Specialized Information System, U.S. National Library of Medicine, Bethesda, MD [online]. Available: http://toxnet.nlm.nih.gov/cgi-bin/sis/search.[accessed Aug. 1, 2008].
IPCS (International Programme on Chemical Safety). 1988. Phosphine and Selected Metal Phosphides. Environmental Health Criteria 73. Geneva: World Health Organization [online]. Available: http://www.inchem.org/documents/ehc/ehc/ehc73.htm [accessed Aug. 2, 2007].
IPCS (International Programme on Chemical Safety). 1989. Phosphine and Selected Metal Phosphides. Health and Safety Guide No. 28. Geneva: World Health Organization [online]. Available: http://www.inchem.org/documents/hsg/hsg/hsg028.htm [accessed Aug. 1, 2008].
Jones, A.T., R.C. Jones, and E.O. Longley. 1964. Environmental and clinical aspects of bulk wheat fumigation with aluminum phosphide. Am. Ind. Hyg. Assoc. J. 25:376-379.
Kligerman, A.D, J.B. Bishop, G.L. Erexson, H.C. Price, R.W. O’Connor, D.L. Morgan, and E. Zeiger. 1994a. Cytogenetic and germ cell effects of phosphine inhalation by rodents: II. Subacute exposures to rats and mice. Environ. Mol. Mutagen. 24(4):301-306.
Kligerman, A.D, M.F. Bryant, C.L. Doerr, G.L. Erexson, P. Kwanyuen, and J.K. McGee. 1994b. Cytogenetic effects of phosphine inhalation by rodents: I. Acute 6 h exposure of mice. Environ. Mol. Mutagen. 23(3):186-189.
Klimmer, O.R. 1969. Contribution to the study of the action of phosphine (PH3). On the question of so-called chronic phosphine poisoning [in German]. Arch. Toxikol. 24(2):164-187.
Lewis, R.J., Jr. 1996a. Sodium phosphide. P. 2990 in Sax’s Dangerous Properties of Industrial Materials, 9th Ed. New York: Van Nostrand Reinhold.
Lewis, R.J., Jr. 1996b. Strontium phosphide. P. 3024 in Sax’s Dangerous Properties of Industrial Materials, 9th Ed. New York: Van Nostrand Reinhold.
Misra, U.K., A.K. Tripathi, R. Pandey, and B. Bhargwa. 1988a. Acute phosphine poisoning following ingestion of aluminum phosphide. Hum. Toxicol. 7(4):343-345.
Misra, U.K., S.K. Bhargava, D. Nag, M.M. Kidwai, and M.M. Lal. 1988b. Occupational phosphine exposure in Indian workers. Toxicol. Lett. 42(3):257-263.
MMWR (Morbidity and Mortality Weekly Report). 1994. Deaths associated with exposure to fumigants in railroad cars-United States. MMWR 43(27):489-491.
Modrzejewski, J., and Z. Myslak. 1967. Phosphines poisoning during corn vermin fumigation in a port elevator [in Polish]. Med. Pr. 18(1):78-82.
Morgan, D.L., M.P. Moorman, M.R. Elwell, R.E. Wilson, S.M. Ward, M.B. Thompson, R.W. O’Connor, and H.C. Price. 1995. Inhalation toxicity of phosphine for Fischer 344 rats and B6C3F1 mice. Inhal. Toxicol. 7(2):225-238.
Muthu, M., M.K. Krishnakumari, V. Muralidhara, and S.K. Majumder. 1980. A study on the acute inhalation toxicity of phosphine to albino rats. Bull. Environ. Contam. Toxicol. 24(3):404-410.
Newton, P.E. 1991. Acute Inhalation Exposures of Rats to Phosphine. Project No. 90-8271. Bio/Dynamics, Inc. East Millstone, NJ.
Newton, P.E., R.E. Schroeder, J.B. Sullivan, W.M. Busey, and D.A. Banas. 1993. Inhalation toxicity of phosphine in the rat: Acute, subchronic, and developmental. Inhal. Toxicol. 5(2):223-239.
Neubert, D., and I. Hoffmeister. 1960. Changes in intermediary metabolism following exposure to hydrogen phosphide. Naunyn-Schmiedeberg’s Arch. Exp. Pathol. Pharmakol. 239: 219-233 (as cited in IPCS 1988).
NIOSH (National Institute for Occupational Safety and Health). 1994. NIOSH Pocket Guide to Chemical Hazards. U.S. Department of Human Services, Public Health Service, Centers for Disease Control and Prevention, NIOSH.
NIOSH (National Institute for Occupational Safety and Health). 2002. Documentation for Immediately Dangerous to Life of Health Concentrations (IDLHS). Phosphine. Washington, D.C: NIOSH.
NIOSH (National Institute for Occupational Safety and Health). 2005. Phosphine. In NIOSH Pocket Guide to Chemical Hazards. Publication No. 2005-149. National Institute for Occupational Safety and Health, Public Health Service, U.S. Department of Health, Education and Welfare, Cincinnati, OH [online]. Available: http://www.cdc.gov/niosh/npg/npgd0505.html [accessed Aug. 3, 2007].
NRC (National Research Council). 1993. Guidelines for Developing Community Emergency Exposure Levels for Hazardous Substances. Washington, DC: National Academy Press.
NRC (National Research Council). 2001. Standing Operating Procedures for Developing Acute Exposure Guideline Levels for Hazardous Chemicals. Washington, DC: National Academy Press.
OECD (Organisation for Economic Co-operation and Development). 2001. Examples of water-reactive substances that evolve large quantities of toxic gas(es) when in contact with water and their respective gas evolution rates. Table 2 in Harmonised System for the Classification of Substance which in Contact with Water, Emit Toxic Gases. ENV/JM/HCL(2001)5. Task Force on Harmonisation of Classification and Labelling, Organisation for Economic Co-operation and Development.
O'Neil, M.J. A. Smith, and P.E. Heckelman, eds. 2001. The Merck Index, 13th Ed. Whitehouse Station, NJ: Merck & Co., Inc.
Omae, K., C. Ishizuka, H. Nakashima, H. Sakurai, K. Yamazaki, K. Mori, T. Shibata, H. Kanoh, M. Kudo, and M. Tati. 1996. Acute and subacute inhalation toxicity of highly purified phosphine in male ICR mice. J. Occup. Health 38(1):36-42.
Ruth, J.H. 1986. Odor thresholds and irritation levels of several chemical substances. Am. Ind. Hyg. Assoc. J. 47(3):A142-A151.
Schoonbroodt, D., P. Guffens, P. Jousten, J. Ingels, and J. Grodos. 1992. Acute phosphine poisoning? A case report and review. Acta. Clin. Belg. 47(4):280-284.
SDU Uitgevers (Ministry of Social Affairs and Employment). 2000. National MAC (Maximum Allowable Concentration) List, 2000. Ministry of Social Affairs and Employment, The Hague, The Netherlands.
Shadnia, S., O. Mehrpour, and M. Abdollahi. 2008. Unintentional poisoning by phosphine released from aluminum phosphide. Hum. Exp. Toxicol. 27(1):87-89.
ten Berge, W.F., A. Zwart, and L.M. Appelman. 1986. Concentration-time mortality response relationship of irritant and systemically acting vapors and gases. J. Hazard. Mater. 13: 302-309.
Verma, S.K, S. Ahmad, N. Shirazi, S.P. Barthwal, D. Khurana, M. Chugh, and H.S. Gambhir. 2007. Acute pancreatitis: A lesser-known complication of aluminum phosphide poisoning. Hum. Exp. Toxicol. 26(12):979-981.
Waritz, R.S., and R.M. Brown. 1975. Acute and subacute inhalation toxicities of phosphine, phenylphosphine, and triphenylphosphine. Am. Ind. Hyg. J. 36(6):452458.
Wilson, R., F.H. Lovejoy, R.J. Jaeger, and P.L. Landrigan. 1980. Acute phosphine poisoning aboard a grain freighter. Epidemiologic, clinical, and pathologic findings. JAMA 244(2):148-150.
Zaebst, D.D., L.M. Blade, G.E. Burroughs, P. Morrelli-Schroth, and W.J. Woodfin. 1988. Phosphine exposures in grain elevators during fumigation with aluminum phosphide. Appl. Ind. Hyg. 3(5): 146-154.
APPENDIX A
Derivation of AEGL-1 Values
Quantitative data regarding responses consistent with the AEGL-1 definition were not available for acute inhalation exposure to phosphine. Because of the lack of appropriate data, AEGL-1 values could not be determined. Data showing lethality in animals exposed to phosphine at concentrations below the odor threshold (1.5-200 ppm, depending on impurities) supports this decision. Absence of an AEGL-1 value does not imply that exposure below the AEGL-2 concentration is without adverse effects.
Derivation of AEGL-2 Values
|
Key study: |
Newton et al. 1993 |
|
Toxicity end point: |
Red nasal mucoid discharge in rats exposed to 10 ppm phosphine for 6 h |
|
Scaling: |
C1 × t = k (30 min; 1, 4, and 8 h) 10 ppm × 6 h = 60 ppm·h |
|
Uncertainty factors: |
3 for interspecies variability 10 for intraspecies variability |
|
10-min AEGL-2: |
4.0 ppm (30-min value adopted as 10-min value) |
|
30-min AEGL-2: |
C × 0.5 h = 60 ppm·h C = 120 ppm 30-min AEGL-2 = 120 ppm/30 = 4.0 ppm |
|
1-h AEGL-2: |
C × 1 h = 60 ppm·h C = 60 ppm 1-h AEGL-2 = 60 ppm/30 = 2.0 ppm |
|
4-h AEGL-2: |
C × 4 h = 60 ppm·h C = 15 ppm 4-h AEGL-2 = 15 ppm/30 = 0.50 ppm |
|
8-h AEGL-2: |
C × 8 h = 60 ppm·h C = 7.5 ppm 8-h AEGL-2 = 7.5 ppm/30 = 0.25 ppm |
Derivation of AEGL-3 Values
|
Key study: |
Newton 1991 |
|
Toxicity end point: |
No-effect level for death (18 ppm) in rats exposed to phosphine for 6 h |
|
Scaling: |
C1 × t = k (30 min; 1, 4, and 8 h) 18 ppm × 6 h = 108 ppm·h |
|
Uncertainty factors: |
3 for interspecies variability 10 for intraspecies variability |
|
10-min AEGL-3: |
7.2 ppm (30-min value adopted as 10-min value) |
|
30-min AEGL-3: |
C × 0.5 h = 108 ppm·h C = 216 ppm 30-min AEGL-3 = 216 ppm/30 = 7.2 ppm |
|
1-h AEGL-3: |
C × 1 h = 108 ppm·h C = 108 ppm 1-h AEGL-3 = 108 ppm/30 = 3.6 ppm |
|
4-h AEGL-3: |
C × 4 h = 108 ppm·h C = 27 ppm 4-h AEGL-3 = 27 ppm/30 = 0.90 ppm |
|
8-h AEGL-3: |
C × 8 h = 108 ppm·h C = 13.5 ppm 8-h AEGL-3 = 13.5 ppm/30 = 0.45 ppm |
APPENDIX B
ACUTE EXPOSURE GUIDELINES FOR PHOSPHINE
Derivation Summary for Phosphine (CAS No. 7803-51-2)
AEGL-1 VALUES
|
10 min |
30 min |
1 h |
4 h |
8 h |
|
Not Recommended |
Not Recommended |
Not Recommended |
Not Recommended |
Not Recommended |
|
Reference: Data unavailable. |
||||
|
Test species/Strain/Number: Not applicable. |
||||
|
Exposure route/Concentrations/Durations: Not applicable. |
||||
|
Effects: Not applicable. |
||||
|
End point/Concentration/Rationale: Not applicable. |
||||
|
Uncertainty factors/Rationale: Not applicable. |
||||
|
Modifying factor: Not applicable. |
||||
|
Animal to human dosimetric adjustment: Not applicable. |
||||
|
Time scaling: Not applicable. |
||||
|
Data quality and research needs: Appropriate data were not available for derivation of AEGL-1 values. |
||||
AEGL-2 VALUES
|
10 min |
30 min |
1 h |
4 h |
8 h |
||
|
4.0 ppm |
4.0 ppm |
2.0 ppm |
0.50 ppm |
0.25 ppm |
||
|
Reference: Newton, P.E., R.E. Schroeder, J.B. Sullivan, W.M. Busey, and D.A. Banas. 1993. Inhalation toxicity of phosphine in the rat: Acute, subchronic, and developmental. Inhal. Toxicol. 5(2):223-239. |
||||||
|
Test species/Strain/Number: F344 rats/ 15/sex/concentration |
||||||
|
Exposure route/Concentrations/Durations: Inhalation: 0, 2.5, 5.0, or 10 ppm, 6 h/day |
||||||
|
Effects: |
||||||
|
2.5 ppm |
red mucoid nasal discharge |
|||||
|
5.0 ppm |
red mucoid nasal discharge |
|||||
|
10 ppm |
red mucoid nasal discharge (determinant for AEGL-2) |
|||||
|
End point/Concentration/Rationale: 10 ppm, exposure was for 6 h; red mucoid nasal discharge. |
||||||
|
Uncertainty factors/Rationale: |
||||||
|
Total uncertainty factor: 30 |
||||||
|
|
Interspecies: |
3—Lethality data (45 min to 30 h) for rats, mice, rabbits, and guinea pigs suggest little species variability. |
||||
|
10 min |
30 min |
1 h |
4 h |
8 h |
||
|
4.0 ppm |
4.0 ppm |
2.0 ppm |
0.50 ppm |
0.25 ppm |
||
|
Uncertainty factors/Rationale: |
||||||
|
|
Intraspecies: |
10—Children appear to be more sensitive than adults to the effects of phosphine. There were two case reports in which exposed children died, but adults exposed under similar conditions survived. |
||||
|
Modifying factor: Not applicable. |
||||||
|
Animal to human dosimetric adjustment: None; insufficient data. |
||||||
|
Time scaling: Cn × t = k, where n = 1; empirically derived from rat lethality data from 1 to 6 h. Time scaling was employed for the 30-min, 1-, 4-, and 8-h time points. The 30-min AEGL-2 was also adopted as the 10-min value due to the added uncertainty of extrapolating from a 6-h time point to 10 min. |
||||||
|
Data quality and research needs: Data for effects defined by AEGL-2 are limited. |
||||||
AEGL-3 VALUES
|
10 min |
30 min |
1 h |
4 h |
8 h |
|||
|
7.2 ppm |
7.2 ppm |
3.6 ppm |
0.90 ppm |
0.45 ppm |
|||
|
Reference: Newton, P.E. 1991. Acute Inhalation Exposures of Rats to Phosphine. Project No. 90-8271. East Millstone, NJ: Bio/Dynamics, Inc. |
|||||||
|
Test species/Strain/Sex/Number: Sprague-Dawley rats, 5/sex/concentration or 10 males/concentration. |
|||||||
|
Exposure route/Concentrations/Durations: Inhalation: 0, 1.3, 6.0, or 28 ppm for 6 h (5/sex/group); 0, 3.1, 10, or 18 ppm for 6 h (10 males/group). |
|||||||
|
Effects: Exposure was for 6 h |
|||||||
|
|
Concentration |
Mortality |
|||||
|
|
0 ppm |
0/10 |
|||||
|
|
1.3 ppm |
0/10 |
|||||
|
|
3.1 ppm |
0/10 |
|||||
|
|
6.0 ppm |
0/10 |
|||||
|
|
10 ppm |
0/10 |
|||||
|
|
18 ppm |
0/10 (determinant for AEGL-3) |
|||||
|
|
28 ppm |
5/10 |
|||||
|
|
LC50: 28 ppm |
|
|||||
|
End point/Concentration/Rationale: Threshold for lethality; 18 ppm, 6 h. This study was chosen because 10 animals per dose group were used and data were for exposures over a range of phosphine concentrations. |
|||||||
|
10 min |
30 min |
1 h |
4 h |
8 h |
||
|
7.2 ppm |
7.2 ppm |
3.6 ppm |
0.90 ppm |
0.45 ppm |
||
|
Uncertainty factors/Rationale: |
||||||
|
Total uncertainty factor: 30 |
||||||
|
|
Interspecies: |
3—Lethality data (45 min to 30 h) for rats, mice, rabbits, and guinea pigs suggest little species variability. |
||||
|
|
Intraspecies: |
10—Children appear to be more sensitive than adults to the effects of phosphine. There were two case reports in which exposed children died, but adults exposed under similar conditions survived. |
||||
|
Modifying factor: Not applicable. |
||||||
|
Animal to human dosimetric adjustment: Insufficient data |
||||||
|
Time scaling: Cn × t = k where n = 1; empirically derived from rat lethality data from 1 to 6 h. Time scaling was employed for the 30-min, 1-, 4-, and 8-h time points. The 30-min AEGL-3 was also adopted as the 10-min value due to the added uncertainty of extrapolating from a 6-h time point to 10 min. |
||||||
|
Data quality and research needs: Study is considered appropriate for AEGL-3 derivation since exposures are over a wide range of phosphine concentrations and utilize a sufficient number of animals. |
||||||
APPENDIX D
AEGL Values for Selected Metal Phosphides
Aluminum Phosphide (AlP)
Potassium Phosphide (K3P)
Sodium Phosphide (Na3P)
Zinc Phosphide (Zn3P2)
Calcium Phosphide (Ca3P2)
Magnesium Phosphide (Mg3P2)
Strontium Phosphide (Sr3P2)
Magnesium Aluminum Phosphide (Mg3AlP3)
SUMMARY
Metal phosphides are solids and are typically used as fumigants against insects and rodents in stored grain. The metal phosphides react rapidly with water and moisture in the air or stored grain to produce phosphine gas. It is the phosphine gas that is responsible for acute toxicity, and the rate of phosphine generation is dependent on ambient temperature and humidity and the chemical structure of the phosphide (Anger et al. 2000).
In the absence of appropriate chemical-specific data for the metal phosphides considered in this appendix, the AEGL-2 and AEGL-3 values for phosphine were used to obtain AEGL-2 and AEGL-3 values, respectively, for the metal phosphides. The use of phosphine as a surrogate for the metal phosphides is deemed appropriate because qualitative (clinical signs) and quantitative (phosphine blood level) data suggest that the phosphine hydrolysis product is responsible for acute toxicity from metal phosphides. The phosphine AEGL-2 and AEGL-3 values were used as target values for calculating the concentrations of metal phosphide needed to generate the phosphine AEGL values.
Because AEGL-1 values for phosphine are not recommended (due to insufficient data), AEGL-1 values for the metal phosphides considered in this appendix are also not recommended. The calculated values are listed in Table D-1 below.
D.I.
INTRODUCTION
Metal phosphides are solids and are typically used as fumigants against insects and rodents in stored grain. The metal phosphides react rapidly with water and moisture in the air or stored grain to produce phosphine gas. It is the phosphine gas which is responsible for acute toxicity, and the rate of phosphine generation is dependent on ambient temperature and humidity and the chemical structure of the phosphide (Anger et al. 2000).
TABLE D-1 AEGL Values for Metal Phosphidesa
Aluminum Phosphide (CAS No. 20859-73-8), Potassium Phosphide (CAS No. 20770-41-6), and Sodium Phosphide (CAS No. 12058-85-4): One mole of aluminum phosphide, potassium phosphide, or sodium phosphide will react rapidly with water or moisture to produce a maximum of 1 mole of phosphine gas as follows:
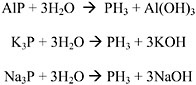
Zinc Phosphide (CAS No. 1314-84-7), Calcium Phosphide (CAS No. 1305-99-3), Magnesium Phosphide (CAS No. 10257-74-8), and Strontium Phosphide (CAS No. 12504-13-1): One mole of zinc phosphide, calcium phosphide, magnesium phosphide or strontium phosphide will react rapidly with water or moisture to produce a maximum of 2 moles of phosphine gas as follows:

Magnesium Aluminum Phosphide (CAS No. None): One mole of magnesium aluminum phosphide will react rapidly with water or moisture to produce a maximum of 3 moles of phosphine gas as follows:
Aluminum phosphide is a gray or yellow crystalline solid prepared from red phosphorus and aluminum powder (O’Neil et al. 2001). Commercial aluminum phosphide sachets contain 70% aluminum phosphide and 30% aluminum carbonate (Bajaj et al. 1988). Calcium phosphide is a red-brown or gray solid prepared by heating calcium phosphate with aluminum or carbon by passing phosphorus vapors over metallic calcium. In addition to its use as a rodenticide, calcium phosphide is also used in signal fires and pyrotechnics and in the purification of copper and copper alloys (HSDB 2007a). Zinc phosphide is a gray solid and may be produced by passing phosphine through a solution of zinc sulfate (HSDB 2007b). Manufacturing information on the other metal phosphides considered in this appendix was not located. Available physico-chemical data for the metal phosphides are shown in Tables D-2 through D-9.
TABLE D-2 Physicochemical Data for Aluminum Phosphide
|
Parameter |
Description/Value |
Reference |
|
Synonyms (commercial product) |
Celphos, Phostoxin, Quickphos |
O’Neil et al. 2001 |
|
CAS Registry No. |
20859-73-8 |
O’Neil et al. 2001 |
|
Chemical formula |
AlP |
O’Neil et al. 2001 |
|
Molecular weight |
57.96 |
O’Neil et al. 2001 |
|
Physical state |
Solid, gray of yellow crystals |
O’Neil et al. 2001 |
|
Parameter |
Description/Value |
Reference |
|
Relative density (water = 1) |
2.9 |
IPCS 1989 |
|
Melting point |
>1350°C |
IPCS 1989 |
|
Solubility in water |
Reactive produces phosphine gas |
IPCS 1989 |
TABLE D-3 Physicochemical Data for Potassium Phosphide
|
Parameter |
Description/Value |
Reference |
|
CAS Registry No. |
20770-41-6 |
ChemIDPlus 2005a |
|
Chemical formula |
K3P |
ChemIDPlus 2005a |
|
Molecular weight |
148.3 |
ChemIDPlus 2005a |
TABLE D-4 Physicochemical Data for Sodium Phosphide
|
Parameter |
Description/Value |
Reference |
|
Synonyms |
Trisodium phosphide |
ChemIDPlus 2005b |
|
CAS Registry No. |
12058-85-4 |
ChemIDPlus 2005b |
|
Chemical formula |
Na3P |
ChemIDPlus 2005b |
|
Molecular weight |
99.94 |
Lewis 1996a |
|
Physical state |
Solid, red crystals |
Lewis 1996a |
|
Melting point |
Decomposes |
Lewis 1996a |
|
Solubility in water |
Reacts violently |
Lewis 1996a |
TABLE D-5 Physicochemical Data for Zinc Phosphide
|
Parameter |
Description/Value |
Reference |
|
Synonym |
Trizinc diphosphide |
IPCS 1989 |
|
CAS Registry No. |
1314-84-7 |
IPCS 1989 |
|
Chemical formula |
Zn3P2 |
IPCS 1989 |
|
Molecular weight |
258.1 |
IPCS 1989 |
|
Physical state |
Solid, gray powder or crystals |
O’Neil et al. 2001 |
|
Relative density (water = 1) |
4.55 |
O’Neil et al.2001 |
|
Melting point |
Sublimes |
IPCS 1989 |
|
Solubility in water |
Insoluble, reacts |
IPCS 1989 |
TABLE D-6 Physicochemical Data for Calcium Phosphide
|
Parameter |
Description/Value |
Reference |
|
Synonyms (commercial product) |
Calcium photophor, photophor |
O’Neil et al. 2001 |
|
CAS Registry No. |
1305-99-3 |
O’Neil et al. 2001 |
|
Chemical formula |
Ca3P2 |
O’Neil et al. 2001 |
|
Parameter |
Description/Value |
Reference |
|
Molecular weight |
182.18 |
O’Neil et al. 2001 |
|
Physical state |
Solid, red-brown crystals or gray lumps |
O’Neil et al. 2001 |
|
Relative density (water = 1) |
2.51 |
O’Neil et al. 2001 |
|
Melting point |
1600°C |
O’Neil et al. 2001 |
|
Solubility in water |
Decomposes |
O’Neil et al. 2001 |
TABLE D-7 Physicochemical Data for Magnesium Phosphide
|
Parameter |
Description/Value |
Reference |
|
Synonyms |
Trimagnesium diphosphide |
IPCS 1989 |
|
CAS Registry No. |
12057-74-8 |
IPCS 1989 |
|
Chemical formula |
Mg3P2 |
IPCS 1989 |
|
Molecular weight |
134.87 |
IPCS 1989 |
|
Physical state |
Solid, gray or bright yellow crystals |
IPCS 1989 |
|
Relative density (water = 1) |
2.1 |
IPCS 1989 |
|
Melting point |
>750°C |
IPCS 1989 |
|
Solubility in water |
Moisture sensitive, reacts |
IPCS 1989 |
TABLE D-8 Physicochemical Data for Strontium Phosphide
|
Parameter |
Description/Value |
Reference |
|
CAS Registry No. |
12504-13-1 |
Lewis 1996b |
|
Chemical formula |
Sr3P2 |
Lewis 1996b |
|
Molecular weight |
324.9 |
Lewis 1996b |
|
Physical state |
Solid |
Lewis 1996b |
TABLE D-9 Physicochemical Data for Magnesium Aluminum Phosphide
|
Parameter |
Description/Value |
Reference |
|
CAS Registry No. |
None |
ChemIDPlus 2005c |
|
Chemical formula |
Mg3AlP3 |
ChemIDPlus 2005c |
|
Molecular weight |
192.8 |
— |
|
Physical state |
Solid |
ChemIDPlus 2005c |
D.II.
SPECIAL CONSIDERATIONS
Metabolism and Disposition
Solid metal phosphides deposited on moist respiratory tract surfaces may hydrolyze and release absorbable phosphine. However, a more likely scenario would involve atmospheric hydrolysis of metal phosphides to phosphine gas.
Chan et al. (1983) detected phosphine in postmortem stomach, blood, and liver specimens from a 27-year-old man who had ingested aluminum phosphide tablets. The phosphine was released from the samples after acid treatment. Similarly, Anger et al. (2000) identified phosphine in postmortem brain, liver, and kidneys of a 39-year-old man who had committed suicide by ingestion of aluminum phosphide tablets.
Chugh et al. (1996) measured blood phosphine levels in patients with severe (n = 30), mild (n = 10), or minimal (n = 5) toxicity due to aluminum phosphide ingestion. Patients with severe toxicity had ingested “fresh” aluminum phosphide compound and were in a state of shock. Those with mild toxicity had ingested “old” aluminum phosphide compound and presented with hypotension and gastrointestinal symptoms. Patients with minimal toxicity ingested some powder from the aluminum phosphide tablets and presented with only nausea and occasional vomiting. Blood phosphine levels were positively correlated with severity of clinical signs and to dose of pesticide. At admission, blood phosphine levels were 71% higher (p < 0.001) in patients in the severe toxicity group than in the mild toxicity group of patients; blood phosphine was not detected in the minimal toxicity group of patients. Blood phosphine levels were also correlated to mortality; patients having blood phosphine levels ≤ 1.067 ± 0.16 mg% survived, whereas those with blood phosphine above this apparent threshold died (6 of 30 in the severe toxicity group).
Garry et al. (1993) described a fatality from inhalation of aluminum phosphide aerosol In this case report, blood aluminum concentration was used as a marker of exposure (see Section 2.1.1).
Mechanism of Toxicity
Metal phosphides react rapidly with moisture in air to produce phosphine gas. It is the phosphine gas that is responsible for acute inhalation toxicity from metal phosphide exposure. The rate of phosphine generation is dependent on ambient temperature and humidity (Anger et al. 2000) in addition to the chemical structure of the metal phosphide. The hydrolysis reactions and phosphine evolution rates (OECD 2001) of the metal phosphides considered in this appendix are summarized in Table D-10.
D.III.
RATIONALE AND AEGL-1
Summary of Human Data Relevant to AEGL-1
No human data are available for the derivation of AEGL-1 for the metal phosphides considered in this appendix.
TABLE D-10 Hydrolysis of Metal Phosphides
|
Metal Phosphide |
Hydrolysis Reaction |
Maximum Number of moles of phosphine produced per mole of metal phosphide hydrolyzed |
Phosphine evolution rate at 20°C and 1 atm (mL/kg• min) |
|
Aluminum Phosphide |
AlP + 3H2O |
1 |
2069.7 |
|
Potassium Phosphide |
K3P + 3H2O |
1 |
807.6 |
|
Sodium Phosphide |
Na3P + 3H2O |
1 |
997.8 |
|
Zinc Phosphide |
Zn3P2 + 6H2O |
2 |
929.9 |
|
Calcium Phosphide |
Ca3P2 + 6H2O |
2 |
1274.6 |
|
Magnesium Phosphide |
Mg3P2 + 6H2O |
2 |
1781.4 |
|
Strontium Phosphide |
Sr3P2 + 6H2O |
2 |
737.1 |
|
Magnesium Aluminum Phosphide |
Mg3AlP3 + 9H2O |
3 |
1865.2 |
Summary of Animal Data Relevant to AEGL-1
No animal data are available for the derivation of AEGL-1 for the metal phosphides considered in this appendix.
Derivation of AEGL-1
No human or animal data are consistent with the effects defined by AEGL-1. Data were also insufficient for derivation of AEGL-1 values for phosphine; thus phosphine cannot be used as a surrogate. Therefore, AEGL-1 values for the metal phosphides considered in this appendix are not recommended (Table D-11).
D.IV.
RATIONALE AND AEGL-2
Summary of Human Data Relevant to AEGL-2
No human data are available for the derivation of AEGL-2 for the metal phosphides considered in this appendix.
TABLE D-11 AEGL-1 Values for Metal Phosphides
|
Compound |
10 min |
30 min |
1 h |
4 h |
8 h |
|
Aluminum Phosphide |
NR |
NR |
NR |
NR |
NR |
|
Potassium Phosphide |
NR |
NR |
NR |
NR |
NR |
|
Sodium Phosphide |
NR |
NR |
NR |
NR |
NR |
|
Zinc Phosphide |
NR |
NR |
NR |
NR |
NR |
|
Calcium Phosphide |
NR |
NR |
NR |
NR |
NR |
|
Magnesium Phosphide |
NR |
NR |
NR |
NR |
NR |
|
Strontium Phosphide |
NR |
NR |
NR |
NR |
NR |
|
Magnesium Aluminum Phosphide |
NR |
NR |
NR |
NR |
NR |
|
NR: not recommended. Absence of an AEGL-1 does not imply that exposure below the AEGL-2 is without adverse effects. |
|||||
Summary of Animal Data Relevant to AEGL-2
No animal data are available for the derivation of AEGL-2 for the metal phosphides considered in this appendix.
Derivation of AEGL-2
In the absence of appropriate chemical-specific data for the metal phosphides considered in this appendix, the AEGL-2 values for phosphine will be used to obtain AEGL-2 values for the metal phosphides. The use of phosphine as a surrogate for the metal phosphides is deemed appropriate because qualitative (clinical signs) and quantitative (phosphine blood level) data suggest that the phosphine hydrolysis product is responsible for acute toxicity from metal phosphides. The phosphine AEGL-2 values will be used as target values for calculating the concentrations of metal phosphide needed to generate the phosphine AEGL values. Calculations were done using the methodology in NRC (2001) and are for 25 degrees C and 760 mm Hg. The metal phosphide values for AEGL-2 are given in Table D-12.
D.V.
RATIONALE AND AEGL-3
Summary of Human Data Relevant to AEGL-3
No human data are available for the derivation of AEGL-3 for the metal phosphides considered in this appendix.
Summary of Animal Data Relevant to AEGL-3
No animal data are available for the derivation of AEGL-3 for the metal phosphides considered in this appendix.
TABLE D-12 AEGL-2 Values For Metal Phosphidesa
Derivation of AEGL-3
In the absence of appropriate chemical-specific data for the metal phosphides considered in this appendix, the AEGL-3 values for phosphine will be used to obtain AEGL-3 values for the metal phosphides. The use of phosphine as a surrogate for the metal phosphides is deemed appropriate because qualitative (clinical signs) and quantitative (phosphine blood level) data suggest that the phosphine hydrolysis product is responsible for acute toxicity from metal phosphides. The phosphine AEGL-3 values will be used as target values for calculating the concentrations of metal phosphide needed to generate the phosphine AEGL values. Calculations were done using the methodology in NRC (2001) and are for 25 degrees C and 760 mm Hg. The metal phosphide values for AEGL-3 are given in Table D-13.
D.VI.
Comparison with Other Standards and Criteria
No other exposure criteria or guidelines were located for the metal phosphides.
TABLE D-13 AEGL-3 Values for Metal Phosphidesa