Ultraviolet Irradiation: An Age-Old Emerging Technology for Water Treatment
KARL G. LINDEN
University of Colorado at Boulder
Ultraviolet (UV) irradiation is considered an emerging technology for public health protection even though it is an ageless process that was instrumental in bringing about life on Earth and was harnessed into a technology for human use in treating water in the late 1800s (Downes and Blunt, 1877). This paper will review why there is now such an emerging interest in UV, the basics of UV technology, why UV works, the use of UV for disinfection and oxidation of pollutants in water, and the frontiers of UV technologies.
TRADITIONAL USE OF UV TECHNOLOGIES IN WATER TREATMENT
Since the 1970s, there has been a growing understanding that chlorine application for disinfection of water has adverse effects in both natural waters and drinking water. In wastewater, chlorine was used to disinfect the sewage before it was discharged into natural water bodies. In drinking water, chlorine has been used for over 100 years to disinfect water for potable uses. In North America, UV first found wide application for treatment of wastewater. Because chlorine residual is toxic to aquatic life, the discharge of wastewater into waterways had to cease by either dechlorinating the water or changing the disinfectant. UV was a natural application because it was effective, does not require any storage or extended contact time, and does not leave any residual. Until recently UV was not acceptable for disinfection of drinking water for the very reason it was accepted for
wastewater: no residual. However, growing concern over chlorinated disinfection by-products associated with more stringent regulations (EPA, 1996), coupled with the ineffectiveness of chlorine against hardy protozoan pathogens, has led to a search for disinfection alternatives since the 1970s. In 1998 and soon thereafter it was discovered that UV was in fact very effective against many chlorine-resistant pathogens, including Cryptosporidium (Clancy et al., 1998) and Giardia (Linden et al., 2002), which propelled UV into the forefront of disinfection alternatives and allowed the U.S. Environment Protection Agency (EPA) to establish more stringent regulations for the control of Cryptosporidium and Giardia in drinking water. Now UV is a fast-growing disinfection process being incorporated all over the world and is encouraged as an effective water disinfection process in the most recent EPA drinking water regulations (EPA, 2006).
HOW DOES UV WORK FOR DISINFECTION AND OXIDATION?
UV is a physical process, harnessing the power and energy of photons to effect destruction of pathogenic microorganisms and break apart chemical bonds of pollutants. In order for UV to be effective, the photons both have to be absorbed by the target pathogen or chemical (first law of photochemistry) and pack enough energy to cause a lasting photochemical effect.
In disinfection applications the photon target is the DNA of a microorganism. The absorbance spectrum of the target is one determinant of the wavelengths that will be most effective. UV irradiation covers the electromagnetic spectrum from 100 nm to 400 nm (100-200 nm is the vacuum UV range), where the most important wavelengths for disinfection are between 240 nm and 280 nm, the peak wavelengths of DNA absorbance (Figure 1). In chemical destruction applications, the important factors are the absorbance features of the target pollutant and the efficiency of the photonic transformation of chemical bonds (known as the quantum yield). Two examples of target pollutants are also presented in Figure 1.
Engineered UV systems were first developed at the turn of the 20th century with the mercury arc lamp. Current conventional UV lamps include mercury vapor lamps of the low-pressure (LP) and medium-pressure (MP) variety, indicating the internal mercury vapor pressure, which dictates the emission spectrum illustrated in Figure 2. LP lamps are low in power, high in UV efficiency, and generate near monochromatic emission at 253.7 nm, near the peak DNA absorbance. MP lamps are higher in power, lower in UV efficiency, and generate a broadband polychromatic emission with characteristic peaks between 200 nm and 400 nm. These differences translate into engineering decisions and opportunities for each lamp, specific to the application considered. On the frontiers of UV lamp technology are nonmercury-based sources including high-energy, pulsed UV-based lamps, often with xenon gas, and UV-based light-emitting diodes (LEDs) currently under development. An example of the types of spectra emitted from a pulsed UV source is presented in Figure 2. These new UV sources may soon radically
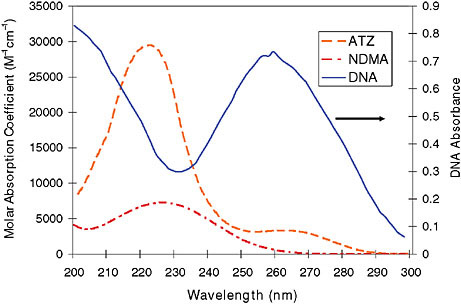
FIGURE 1 Absorbance spectra of chemical pollutants atrazine (ATZ) and N-nitrosodimethylamine (NDMA), compared with the absorbance features of DNA.
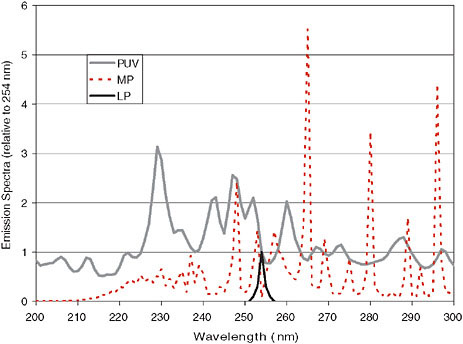
FIGURE 2 Emission spectra of conventional low-pressure (LP) and medium-pressureEmission Spectra (relative to 254 nm) (MP) mercury vapor lamps, compared with a new surface discharge pulsed-UV source. (Pulsed source developed by Phoenix Science and Technology, Chelmsford, MA.)
change the engineering design of UV systems, offering more flexibility in UV reactor configuration.
UV FOR DISINFECTION OF PATHOGENS IN WATER
Interestingly, the efficacy of UV against pathogens varies greatly, despite the ubiquitous nature of the nucleic acid target. UV doses are measured in units of millijoules per centimeter squared (mJ/cm2) or energy per unit area. The UV dose required for 99.99 percent inactivation of various pathogens is displayed in Figure 3. UV disinfection systems are commonly designed around a UV dose of 40 mJ/cm2. Clearly, both bacterial and protozoan pathogens are very easy to inactivate compared with viruses and adenoviruses in particular. However, recent research shows that MP UV is much more effective than LP UV, specifically for some hard-to-disinfect viruses (Linden et al., 2007). It is hypothesized that MP can cause a diversity of damage to the viral pathogen, and because of the complexity of the infection process for this virus, multiple types of damage are required for inactivation.
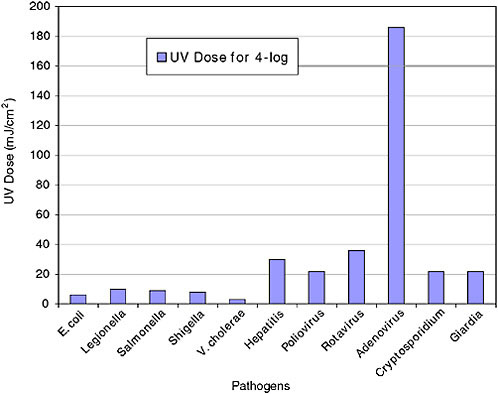
FIGURE 3 UV doses required for 4-log (99.99 percent) disinfection of various pathogens in water.
One caveat with disinfection is that many microbes have the ability to repair their UV-induced DNA damage through both light- and dark-based repair processes (Harm, 1980). Light-based repair involves an enzyme (photolyase) that gets activated during exposure to near UV and visible light wavelengths (350-450 nm). Dark repair typically involves excision repair and does not need light activation. These repair processes attack the pyrimidine dimers formed from UV exposure that leads to inactivation. At high enough doses (two to three times that needed for disinfection) the repair processes are not effective, and under typical drinking water conditions light-based repair is not a concern thanks to covered finished water reservoirs and the water distribution network.
UV FOR OXIDATION OF POLLUTANTS IN WATER
Another application for UV irradiation is the destruction of chemical pollutants in water (Oppenländer, 2003). This chemical destruction can take place through direct absorption of UV photons (photolysis) or indirectly by formation of highly reactive oxidative radicals. UV photolysis requires very high levels of UV energy, up to 100 times higher than what is required for disinfection. When hydrogen peroxide (H2O2) is added to water in the presence of UV photons in the germicidal range, the H2O2 is split into two hydroxyl radicals (•OH) by the photons and the radicals react rapidly (less than microseconds) with organic molecules in the water, thus degrading pollutants.
The UV-H2O2 process is termed an advanced oxidation process (AOP) because it accelerates the natural oxidation that occurs in the environment. It is a rapidly growing treatment technology and uses either LP or MP UV technologies or other UV sources with emissions overlapping that of H2O2 for destroying a variety of pollutants in water, including taste and odor-causing compounds, endocrine-disrupting compounds, pharmaceuticals, personal care products, and agricultural chemicals. Advanced oxidation processes for water treatment are a growing area of research because the public is getting more concerned about these emerging contaminants.
OTHER UV APPLICATIONS
Another area of growing interest is the use of UV irradiation for treatment of water in lesser developed countries. A number of student groups working through Engineers Without Borders and other groups have installed LP UV systems in Africa, Latin America, and Asia. These systems can be constructed with local materials and run using a low-power solar panel. Solar UV disinfection, using the sun’s UV rays, is also an area of high interest among students: water is placed in
discarded plastic bottles and exposed to sunlight over 4 to 6 hours to disinfect it by light- and heat-based processes. These applications illustrate the practicality of UV technologies for providing safe water.
THE FRONTIERS OF UV
Although UV itself is an emerging technology on the frontier of water treatment, there are many new engineering and scientific advances that continue to push and improve the technology and help develop a better understanding of the fundamentals of how UV works, leading to improved process design. Advances in UV lamp technologies coupled with concerns about mercury in UV lamps have led to lamp development, including the new pulsed-UV lamps with instanton capabilities and radiation intensities up to 10,000 times greater than LP UV sources. UV-based LEDs are also emerging, as this technology is being pushed to lower wavelengths that are more germicidally effective. New technologies offer the possibility of radical changes in UV reactor design and improvements in effectiveness for water treatment, at lower energy costs, with safe materials for the environment. As UV technologies become accepted for drinking water, they are also being explored in water reuse applications, an important source of new water, as our freshwater resources are rapidly being either compromised or drained. Of great interest is the UV-AOP process in water reuse for combined disinfection and oxidation of contaminants. Advances in molecular biology and analytical chemistry have all contributed to furthering the knowledge of how UV works for improving water quality and have led to new developments in UV technologies. In summary, UV technology is another example of harnessing the power of nature for use by humans in our modern society. Natural UV from the sun has been harvested, engineered, and channeled to serve water-quality needs and produce safe water.
REFERENCES
Clancy, J. L., T. M. Hargy, M. Marshall, and J. Dyksen. 1998. Inactivation of oocysts of Cryptosporidium parvum in water using ultraviolet light. Journal of American Water Works Association 90:92-102.
Downes, A., and T. P. Blunt. 1977. Research on the effect of light upon bacteria and other organisms. Proceedings of the Royal Society of London 26:488-500.
EPA (U.S. Environmental Protection Agency). 1996. Fact Sheet-Key Provisions of Safe Drinking Water Act 1986 Amendments: Outlines Provisions of the 1986 Amendments to the 1974 Act, Safe Drinking Water Act Amendments of 1996—General Guide to Provisions [EPA 810-S-96-001]. Washington, DC: EPA.
EPA. 2006. Federal Register 71(3). Washington, DC: EPA.
Harm, W. 1980. Biological Effects of Ultraviolet Radiation. Cambridge, U.K.: Cambridge University Press.
Linden, K. G., J. Thurston, R. Schaefer, and J. P. Malley, Jr. 2007. Enhanced inactivation adenovirus types 2 and 40 under medium pressure and pulsed UV disinfection systems. Applied and Environmental Microbiology 73(23):7571-7574.
Linden, K. G., G. A. Shin, G. Faubert, W. Cairns, and M. D. Sobsey. 2002. UV disinfection of Giardia lamblia in water. Environmental Science and Technology 36:2519-2522.
Oppenländer, T. 2003. Photochemical Purification of Water and Air: Advanced Oxidation Processes (AOPs): Principles, Reaction Mechanisms, Reactor Concepts. New York: Wiley-VCH.
ADDITIONAL READING
Darby, J. L., K. E. Snider, and G. Tchobanoglous. 1993. Ultraviolet disinfection for wastewater reclamation and reuse subject to restrictive standards. Water Environment Research 65(2):169-180.
EPA (U.S. Environmental Protection Agency). 1986. Office of Research and Development. Design Manual: Municipal Wastewater Disinfection. EPA 625/1-86/021. Cincinnati, OH: EPA.
EPA. 2006. Ultraviolet Disinfection Guidance Manual. Office of Water, EPA 815-R-06-007. Washington, DC: EPA.
Jagger, J. H. 1967. Introduction to Research in UV Photobiology. Englewood Cliffs, NJ: PrenticeHall, Inc.
Linden, K. G., and J. L. Darby. 1997. Estimating effective germicidal dose from medium pressure UV lamps. ASCE Journal of Environmental Engineering 123(11):612-619.
Qualls, R. G., and J. D. Johnson. 1983. Bioassay and dose measurement in UV disinfection. Applied Environmental Microbiology 45:872-877.
Wolfe, R. L. 1990. Ultraviolet disinfection of potable water. Environmental Science and Technology 24(6):768-773.








