2
Cases in Point: Learning from Experience
INTRODUCTION
Media accounts of medical research breakthroughs are full of examples of trial and study results that make headlines because of their potential to improve patient health or even save lives—but those headlines are sometimes misleading or limited in relevance to real-world care. This cycle has caused confusion and distrust among patients and consumers of health care. At the other end of the spectrum, researchers discount the value of some methodologies used to evaluate clinical effectiveness. In part these findings reflect the constantly evolving nature of scientific inquiry; but as illustrated in this chapter, these experiences offer lessons on the improvements needed in the design and interpretation of clinical effectiveness studies.
By reviewing examples of high-profile studies and trials that evaluated the effectiveness of hormone replacement therapy, drug-eluting coronary stents, bariatric surgery, antipsychotic medications, and lung cancer screening, this chapter illustrates the range of issues facing current effectiveness research. Examples of these issues include capturing important health outcomes throughout the lifecycle of an intervention; contending with the biologic complexity of disease and disease progression and rapid evolution of devices or surgical procedures or rapid uptake and application in broader patient populations. This chapter also illustrates the variety of questions that are vital to ensuring effective use of medical interventions and how these issues might require trials with ever-increasing sample sizes that can be completed in a reasonable time period.
The strengths and weaknesses of observational studies and randomized trials are reviewed. Also reviewed are well-recognized limitations of observational studies due to the potential for confounding by a variety of factors as well as their limited capacity to assess short-term or acute risks. Although randomized controlled trials (RCTs) have the advantage of minimizing confounding, RCTs are often constrained by higher costs, shorter duration of follow-up, and limited applicability to populations of greatest clinical relevance. However, mixed experiences with different investigative approaches do not argue for total cessation of any one approach in favor of another. Rather, as the authors in this chapter suggest, the research community needs to be more receptive to the use of alternative methodologies to generate insights into clinical effectiveness, and we need to determine which approach we use for a given question with full recognition of what is right for particular research circumstances. Collectively these experiences suggest the availability of a powerful array of methods, and when results are combined they produce more nuanced information needed to guide treatment decisions. Opportunities to strengthen these methods are discussed and, overall, greater attention is needed to define state-of-the-art methods so the quality of research is readily discernible regardless of study approach. In addition to methods, data and data system improvements are needed. Electronic health records and data registry approaches offer the opportunity to better systematically capture, track, and report outcomes. Moreover, there is the suggestion that a mix of research approaches, using the best advantages of particular designs, offers untapped promise and that researchers should be more open to adopting such approaches. Greater engagement by the healthcare system is imperative in the evaluation of effectiveness.
JoAnn E. Manson from Harvard Medical School reviews the divergent results of observational studies and RCTs, evaluating the effect of menopausal hormone replacement therapy (HRT) on coronary heart disease (CHD). Despite this divergence, both have contributed critically important information on the therapies’ effectiveness and implications for healthcare decision making. Building on this experience, Manson discusses factors that might have contributed to the different findings. She suggests that because the short- and long-term effects of a clinical intervention may differ, both observational studies and clinical trial design must have benefits to offer researchers. Perhaps, she says, we should consider research findings in the context of all of the available evidence and design studies to complement and extend existing data. Large-scale studies involving networks of electronic databases could facilitate evidence development. Due to the high cost and generally short duration of clinical trials, information about long-term risk may rely heavily on observational sources.
The Food and Drug Administration’s (FDA’s) Ashley B. Boam recounts
the differences in findings between initial pivotal clinical studies of drug-eluting coronary stents and subsequent studies using other methodology. She observes that further understanding of drug-eluting stents (DES) will likely come from a mix of randomized trials and observational registries, conducted both premarket and postmarket and involving a collaborative effort among regulators, industry, and academia. The next author, David R. Flum, a surgeon from the University of Washington, discusses the dichotomy between “effectiveness” and “efficacy” and the applicability of case series in the context of bariatric surgical interventions. He concludes that population-based registries—appropriately funded and constructed with clinician engagement—offer a compromise of strengths and limitations and may be the most effective tool for evaluating emerging healthcare technology.
Philip S. Wang from the National Institute of Mental Health (NIMH) discusses the recently completed, NIMH-sponsored comparative effectiveness trials of antipsychotic medications in patients with schizophrenia (the CATIE trial) as a model for a hybrid approach to study design that blends advantageous features of efficacy studies and large, simple trials. Wang reviews new data resources that may offer important opportunities to effectiveness research, practical clinical trials, adaptive designs, and cluster randomization when trials are not feasible, affordable, or in some cases, ethical.
In the context of cancer research, Peter B. Bach from Memorial Sloan-Kettering Cancer Center discusses issues in the evaluation of screening tests, particularly the use of surrogate measures of benefit. He reviews the results of a computer simulation model to determine the value of lung cancer screening tests to illustrate some of the key challenges and the need for better approaches to ensure the consistent evaluation of the effectiveness of screening tests prior to widespread adoption. In particular, he suggests the use of coverage and payment as effective means to generate population-based longitudinal data on outcomes among screened groups.
HORMONE REPLACEMENT THERAPY
JoAnn E. Manson, M.D., Dr.P.H.
Harvard Medical School
Observational studies and randomized clinical trials of menopausal hormone therapy (HT) and coronary heart disease have produced widely divergent results. In aggregate, observational studies indicate that women who take estrogen after menopause are 35–50 percent less likely to develop CHD than women who do not take estrogen (Grodstein and Stampfer, 2002), whereas randomized trials suggest a neutral or even elevated risk
of coronary events with menopausal HT (Anderson et al., 2004; Hulley et al., 1998; Manson et al., 2003; Rossouw et al., 2002). The cardiovascular findings from the two HT trials (estrogen plus progestin and estrogen-alone) in the Women’s Health Initiative (WHI) are presented in Table 2-1. Understanding the basis for the discordant findings may provide important lessons for the design of future studies and may suggest strategies for improving the reliability and quality of clinical research. Detailed analyses from observational studies and randomized clinical trials have elucidated both methodological and biological explanations for the divergent findings, suggesting avenues for additional research to advance evidence development and improve clinical decision making (Grodstein et al., 2000, 2003; Manson and Bassuk, 2007b; Manson et al., 2006; Michels and Manson, 2003; Prentice et al., 2006). It is hoped that lessons learned from the discrepant results, which have provided insights into the strengths and weaknesses of different sources of evidence, will serve as a springboard to the development of more reliable, efficient, and innovative designs for evaluating clinical interventions.
Methodological Factors That Contribute to the Divergent Findings
The potential role of methodologic factors must be considered in understanding the more favorable findings for HT in relation to CHD risk in observational studies than in clinical trials (Table 2-2). Well-recognized limitations of observational studies, including the potential for confounding by lifestyle practices, socioeconomic status, education, and access to medical care, as well as selection factors related to “indications for use,” can explain some—but not all—of the discrepancies (Grodstein et al., 2003; Manson et al., 2006; Michels and Manson, 2003; Prentice et al., 2006).
TABLE 2-1 Hazard Ratios and 95 Percent Confidence Intervals for Cardiovascular Outcomes and Total Mortality in the Overall Study Population of Women Aged 50–79 in the Women’s Health Initiative (WHI) Trials of Menopausal Hormone Therapy
|
WHI Hormone Therapy Trials |
Estrogen + Progestin (N = 16,608) |
Estrogen alone (N = 10,739) |
|
Coronary heart disease |
1.24 (1.00–1.54) |
0.95 (0.78–1.16) |
|
Stroke |
1.31 (1.03–1.68) |
1.33 (1.05–1.68) |
|
All-cause mortality |
1.00 (0.83–1.19) |
1.04 (0.88–1.22) |
|
SOURCES: Derived from Manson, J. E., J. Hsia, K. C. Johnson, et al. 2003. Estrogen plus progestin and the risk of coronary heart disease. New England Journal of Medicine 349:523-534; Rossouw, J. E., R. L. Prentice, J. E. Manson, et al. 2007. Postmenopausal hormone therapy and risk of cardiovascular disease by age and years since menopause. Journal of the American Medical Association 297:1465-1477. |
||
TABLE 2-2 Postmenopausal Hormone Therapy and CHD: Potential Explanations for Divergent Findings from Clinical Trials and Observational Studies
|
Potential Explanations for the Divergent Findings |
|
|
|
SOURCE: Derived from Grodstein, F., T. B. Clarkson, and J. E. Manson. 2003. Understanding the divergent data on postmenopausal hormone therapy. New England Journal of Medicine 348:645-650. |
Confounding by healthful lifestyle practices and “healthy user bias” among women taking HT may have led to an overestimation of the CHD benefits, but most studies examining this issue suggest that careful adjustments for these factors (such as smoking, other CHD risk factors, body mass index, physical activity, and diet) attenuate—but do not eliminate—the inverse associations between HT and CHD risk (Grodstein et al., 2000, 2003; Manson et al., 2006). Moreover, the Nurses’ Health Study, a large-scale cohort relatively homogeneous for educational attainment, occupation, and access to medical care, showed substantial reductions in CHD risk among HT users, compared to nonusers (Grodstein et al., 2000).
Another methodologic factor that has received less attention is the limitation of most observational studies in assessing short-term or acute risks (due to infrequent updates of exposures), leading to incomplete capture of early clinical events after initiation of therapy and the predominance of follow-up time among compliant long-term users of HT (Grodstein et al., 2003; Manson and Bassuk, 2007b). In contrast to the greater weighting of long-term use in observational studies, clinical trial results tend to reflect shorter term use. Given that CHD risks related to HT are greatest soon after initiation of therapy (Hulley et al., 1998; Manson et al., 2003) and reductions in risk may emerge with longer term use (discussed below) (Michels and Manson, 2003; Prentice et al., 2006; Rossouw et al., 2002), these differences may contribute to the discrepancies observed. Indeed, comparative analyses of HT and CHD in the observational and clinical trial components of the Women’s Health Initiative, with stratification by duration of treatment (comparing short-term versus long-term users), indicated greater convergence of study results when examining similar durations of
use (Prentice et al., 2006). For example, both the observational and clinical trial cohorts in the WHI suggested an increased risk of CHD during the first several years of HT use but a reduced risk with longer duration (>5 years) of use (Table 2-3).
Moreover, observational studies that have utilized electronic health and pharmacy records, which provide frequent updating of exposure (HT use) information via prescription records and facilitate the capture of both short- and long-term health outcomes, have tended to show less pronounced reductions in CHD risk related to HT use (Heckbert et al., 2001; Lemaitre et al., 2006). In a study utilizing computerized pharmacy records and outcomes databases (Group Health Cooperative), the associations between HT and CHD risk were similar to those observed in the WHI for women of comparable age and health status (Heckbert et al., 2001). However, substantial reductions in mortality among women with long-term use of HT have been observed even in studies using electronic pharmacy and health records (Ettinger et al., 1996).
Thus, methodologic differences between observational studies and clinical trials may not fully elucidate the basis for the discrepancies observed. Although large-scale randomized trials, the gold standard of clinical research, have the advantage of minimizing confounding by lifestyle practices, socioeconomic status, and other factors, they are often constrained by higher costs, shorter duration of follow-up, and, at times, limited applicability to populations of greatest clinical relevance. Furthermore, the findings of observational studies and clinical trials of HT are remarkably concordant
TABLE 2-3 Hazard Ratios (HR) and 95 Percent Confidence Intervals (CIs) in the Women’s Health Initative (WHI), According to Duration of HT Use
|
Comparison of Results from the WHI Clinical Trial (CT) and Observational Study (OS) for HT and CHD, According to Duration of Use |
||||
|
Years since HT Initiation |
Estrogen + Progestin HR (95% CI) |
Estrogen Alone HR (95% CI) |
||
|
CT |
OS |
CT |
OS |
|
|
<2 |
1.68 |
1.12 |
1.07 |
1.20 |
|
|
(1.15–2.45) |
(0.46–2.74) |
(0.68–1.68) |
(0.49–2.94) |
|
2–5 |
1.25 |
1.05 |
1.13 |
1.09 |
|
|
(0.87–1.79) |
(0.70–1.58) |
(0.70–1.58) |
(0.75–1.60) |
|
>5 |
0.66 |
0.83 |
0.80 |
0.73 |
|
|
(0.36–1.21) |
(0.67–1.01) |
(0.57–1.12) |
(0.61–0.84) |
|
SOURCE: Derived from Prentice, R., R. D. Langer, M. L. Stefanick, et al. 2006. Combined analysis of Women’s Health Initiative observational and clinical trial data on postmenopausal hormone treatment and cardiovascular disease. American Journal of Epidemiology 163(7):589-599. |
||||
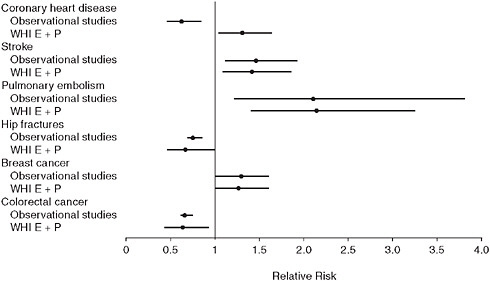
FIGURE 2-1 Relative risks and 95 percent confidence intervals for observational and clinical trial findings on hormone therapy (estrogen + progestin).
SOURCE: Michels, K. B., and J. E. Manson. 2003. Postmenopausal hormone therapy: A reversal of fortune. Circulation 107:1830-1833. Reprinted with permission from Michels and Manson, Circulation, 2003.
for non-CHD health outcomes, including stroke, venous thromboembolism, breast cancer, colorectal cancer, and fracture (Figure 2-1)—results that should also be affected by confounding and selection biases (Grodstein et al., 2003; Manson et al., 2006; Michels and Manson, 2003).
An emerging body of evidence supports the hypothesis that age or time since menopause critically influences the relationship between HT and CHD outcomes (Estrogen and progestogen use in peri- and postmenopausal women: March 2007 position statement of the North American Menopause Society, 2007; Grodstein et al., 2003; Manson et al., 2006). Women who participate in observational studies tend to be younger and closer to onset of menopause at the time of HT initiation than women in randomized trials (the latter are, on average, more than a decade past menopause onset at randomization). Thus, women in HT clinical trials tend to have later stages of atherosclerosis than their counterparts in observational studies and a possibly greater vulnerability to the adverse vascular effects of HT (Estrogen and progestogen use in peri- and postmenopausal women: March 2007 position statement of the North American Menopause Society, 2007; Manson et al., 2006). In contrast, if estrogen slows early stages of athero-sclerosis, as suggested by basic research, animal studies, and imaging findings, recently menopausal women with healthy vascular endothelium may be more likely to have a favorable coronary outcome than women more
distant from menopause (Manson et al., 2007; Mendelsohn and Karas, 2005; Mikkola and Clarkson, 2002). Moreover, absolute rates of adverse events and risks attributable to HT are lower in younger than older women, suggesting that the risk:benefit ratio may vary substantially by age and proximity to menopause onset (Estrogen and progestogen use in peri- and postmenopausal women: March 2007 position statement of the North American Menopause Society, 2007; Manson and Bassuk, 2007b). It is important to emphasize that the implication of the “timing hypothesis” is not that recently menopausal women be given HT for CHD prevention but rather that clinicians can be reassured about cardiac risks when considering short-term use of HT for vasomotor symptom management in such women. The theory that the influence of estrogen on atherosclerosis and coronary events may vary according to the underlying health of the vasculature and the evidence that a woman’s age and time since menopause onset may modulate CHD outcomes with HT, as well as implications for future research, are discussed below.
Biological Factors That May Contribute to the Divergent Findings
As noted above, randomized trials testing the effect of HT on clinical coronary outcomes have not confirmed the cardioprotective effect suggested by most observational studies. In the Heart and Estrogen/progestin Replacement Study (HERS), the 4-year incidence of major coronary events among women with a mean age of 67 years and with preexisting CHD was similar in the HT (oral conjugated equine estrogens [CEE] and medroxy-progesterone acetate [MPA]) and the placebo groups (Hulley et al., 1998). The HT group had a 50 percent increase in risk of CHD events during the first year of the trial, although this elevation was offset by a decreased risk in later years (Grady et al., 2002; Hulley et al., 1998). The Women’s Health Initiative examined the effects of oral CEE with or without MPA in healthy postmenopausal women aged 50–79 (mean age 63) (Anderson et al., 2004; Rossouw et al., 2002); participants had either an intact uterus (N = 16,608) or prior hysterectomy (N = 10,739), respectively. Women assigned to CEE + MPA for an average of 5.6 years were more likely to experience a CHD event than those assigned to placebo (relative risk [RR] = 1.24; 95 percent confidence interval [CI]: 1.00, 1.54), with the highest risk during the first year (Manson et al., 2003). Women assigned to CEE alone for an average of 6.8 years also experienced no overall reduction in CHD risk (RR = 0.95; 95 percent CI: 0.78, 1.16) (Prentice et al., 2006). Both WHI trials were stopped early—the CEE + MPA trial because of an increased risk of breast cancer and an unfavorable benefit–risk balance (Rossouw et al., 2002) and the CEE-alone trial because of an increased stroke risk that was not offset by a reduced CHD risk (Anderson et al., 2004). Although most randomized
clinical trials have tested the commonly used HT formulations of CEE + MPA or CEE alone, clinical trials using estradiol and other formulations of estrogen and/or progestin have also failed to demonstrate cardioprotection (Grodstein et al., 2003; Manson et al., 2006; Michels and Manson, 2003).
A key difference between participants in observational studies and those in clinical trials of HT is the timing of initiation of treatment in relation to menopause onset, which occurs on average at age 51 in the United States. Hormone users in observational studies typically start therapy in early menopause, whereas trial participants are often randomized to hormones more than a decade after cessation of menses. For example, in the Nurses’ Health Study cohort, about 80 percent of women who used HT began treatment within 2–3 years of menopause onset (Grodstein et al., 2003; Manson and Bassuk, 2007b). In contrast, WHI participants, with a mean baseline age of 63, were an average of at least 12 years past menopause at the time of trial enrollment and likely had more extensive atherosclerosis than newly menopausal women. In HERS, the mean age was 67 at baseline, and all participants had been previously diagnosed with CHD. It has been hypothesized that estrogen has diverse and opposing actions, slowing the earlier stages of atherosclerosis through favorable effects on the lipid profile and endothelial function, but triggering acute coronary events through prothrombotic and inflammatory mechanisms and plaque destabilization when advanced lesions are present (Estrogen and progestogen use in peri-and postmenopausal women: March 2007 position statement of the North American Menopause Society, 2007; Grodstein et al., 2003; Manson et al., 2006; Mendelsohn and Karas, 2005) (Figure 2-2).
This hypothesis is supported by several lines of evidence from basic and clinical studies. First, trials in humans show complex effects of exogenous estrogen on cardiovascular biomarkers (Estrogen and progestogen use in peri- and postmenopausal women: March 2007 position statement of the North American Menopause Society, 2007; Manson et al., 2006; Mendelsohn and Karas, 2005). Oral estrogen lowers low-density lipoprotein (LDL) cholesterol, lipoprotein(a), glucose, insulin, and homocysteine levels; inhibits oxidation of LDL cholesterol; increases high-density lipoprotein cholesterol; reverses postmenopausal increases in fibrinogen and plasminogen-activator inhibitor type 1; and improves endothelial function—all effects expected to lower coronary risk. However, oral estrogen also increases triglycerides, coagulation factors (factor VII, prothrombin fragments 1 and 2, and fibrinopeptide A), C-reactive protein, and matrix metalloproteinases—effects expected to raise coronary risk. Additionally, certain progestogens may offset some of estrogen’s benefits.
Data from controlled experiments in nonhuman primates also support the theory that the coronary effects of HT depend on the initial health of the vasculature. Conjugated estrogen (with or without a progestin) did not
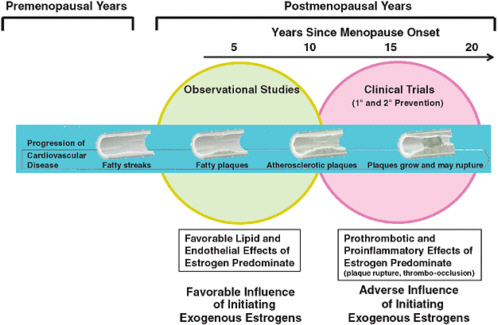
FIGURE 2-2 Schematic illustration of the interrelationships of timing of initiation of hormone therapy, vascular health, and clinical CHD outcomes.
SOURCE: Manson, J. E., S. S. Bassuk, S. M. Harman, et al. 2006. Postmenopausal hormone therapy: New questions and the case for new clinical trials. Menopause 13(1):139-147.
affect the extent of coronary artery plaque in cynomolgus monkeys started on this treatment at 2 years (~6 human years) after oophorectomy and well after the establishment of atherosclerosis, but such therapy reduced plaque by 70 percent when initiated immediately after oophorectomy during the early stages of atherosclerosis (Mikkola and Clarkson, 2002) (Figure 2-3).
Similarly, imaging trials in women with significant coronary lesions at baseline have found estrogen to be ineffective in slowing the rate of arterial narrowing (Angerer et al., 2001; Herrington et al., 2000; Hodis et al., 2003; Waters et al., 2002). However, in an imaging trial that did not require participants to have significant vascular disease at entry, estrogen impeded progression of carotid atherosclerosis (Hodis et al., 2001).
When the WHI trials were initiated in the early 1990s, it was not well recognized that age or vascular health might be an important determinant of the effect of HT on coronary or other outcomes; thus, focused subgroup analyses were not emphasized at the outset, nor were the trials powered to detect potential interactions. However, given the striking discrepancies between findings from earlier observational studies and more recent randomized trials (including data from the large trials with hard clinical endpoints, smaller imaging studies, and experimental studies in animals), WHI investigators pursued more detailed analyses of the data to examine whether the timing hypothesis might account for the seemingly contradictory evidence on coronary effects of HT.
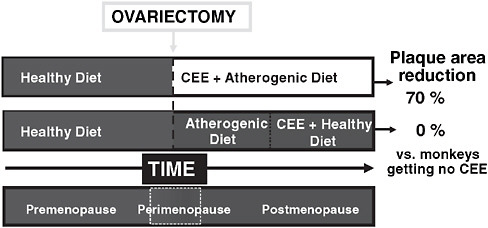
FIGURE 2-3 Role of timing of conjugated equine estrogen (CEE) initiation in relationship to ovariectomy in nonhuman primates.
NOTE: Modified and reprinted with permission from the European Society of Cardiology, Copyright © 2002.
SOURCE: Mikkola, T. S., and T. B. Clarkson. 2002. Estrogen replacement therapy, atherosclerosis, and vascular function. Cardiovasc Res 53:605-619.
The results of subgroup analyses of WHI data are consistent with the possibility that age or time since menopause influences the HT-CHD association. Subgroup analyses have been reported for the CEE + MPA (Manson et al., 2003) and CEE-alone (Hsia et al., 2006) trials individually and for a combined analysis of the two trials (Rossouw et al., 2007). The following section focuses primarily on the joint analysis that combined data from both trials, resulting in a large number of confirmed CHD end-points and increased statistical power (Rossouw et al., 2007). However, all of the above reports showed similar patterns.
In the WHI, the HT-associated risk of CHD (defined as myocardial infarction [MI] or coronary death) steadily increased with years since menopause.
In analyses that combined data from both trials, RRs were 0.76, 1.10, and 1.28 for women who were <10, 10–19, and ≥20 years past menopause at study entry, respectively (p, trend = 0.02) (Rossouw et al., 2007). Indeed, a pattern of increasing RRs with greater distance from menopause onset was apparent in both the estrogen-alone (E-alone) and estrogen-progestin (E + P) trials, and a similar gradient of relative risks was seen with increasing age (Rossouw et al., 2007) (Table 2-4). Among women aged 50–59,
TABLE 2-4 Hazard Ratios and 95 Percent Confidence Intervals for Selected Outcomes in the Women’s Health Initiative (WHI) Trials of Menopausal Hormone Therapy (joint analysis of the E + P and E-alone trials)
|
Combined Trials (Joint Analysis of the Two HT Trials in the WHI) |
||||
|
|
Years Since Menopause |
|||
|
|
<10 |
10–19 |
≥20 |
p, trend |
|
Coronary heart disease |
0.76 (0.50–1.16) |
1.10 (0.84–1.45) |
1.28 (1.03–1.58) |
0.02 |
|
Total mortality |
0.76 (0.53–1.09) |
0.98 (0.78–1.24) |
1.14 (0.96–1.36) |
0.51 |
|
Global indexa |
1.05 (0.86–1.27) |
1.12 (0.98–1.27) |
1.09 (0.98–1.22) |
0.82 |
|
|
Age (years) |
|||
|
|
50–59 |
60–69 |
70–79 |
p, trend |
|
Coronary heart disease |
0.93 (0.65–1.33) |
0.98 (0.79–1.21) |
1.26 (1.00–1.59) |
0.16 |
|
Total mortality |
0.70 (0.51–0.96) |
1.05 (0.87–1.26) |
1.14 (0.94–1.37) |
0.06 |
|
Global indexa |
0.96 (0.81–1.14) |
1.08 (0.97–1.20) |
1.14 (1.02–1.29) |
0.09 |
|
aThe global index is a composite outcome of coronary heart disease, stroke, pulmonary embolism, breast cancer, colorectal cancer, endometrial cancer, hip fracture, and mortality. SOURCE: Derived from Rossouw, J. E., R. L. Prentice, J. E. Manson, et al. 2007. Post-menopausal hormone therapy and risk of cardiovascular disease by age and years since menopause. JAMA 297:1465-1477. |
||||
TABLE 2-5 Hazard Ratios and 95 Percent Confidence Intervals for CHD Outcomes in the Women’s Health Initiative (WHI) Estrogen-Alone Trial, According to Age
assignment to estrogen alone was associated with significant reductions in the secondary end-point of coronary revascularization (RR = 0.55; 95 percent CI: 0.35, 0.86) and a composite end-point of MI, coronary death, or coronary revascularization (RR = 0.66; 95 percent CI: 0.44, 0.97) (Hsia et al., 2006) (Table 2-5). Taken together, the pattern of WHI results suggests a beneficial or neutral effect of HT on CHD risk among women closer to menopause (who are likely to have less atherosclerosis) but an adverse impact in later years. Similar results according to age or time since menopause have been obtained in observational studies and small clinical trials (Brownley et al., 2004; Grodstein et al., 2006; Lobo, 2004).
Salpeter et al. combined data from 22 smaller randomized trials with data from the WHI to provide the most comprehensive assessment to date of the influence of age on the relation between HT and CHD (Salpeter et al., 2006). Their analysis showed that in trials that enrolled predominantly younger participants (women aged <60 or within 10 years of menopause), HT was associated with a 30–40 percent reduction in CHD risk. In contrast, in trials with predominantly older participants, HT had little effect on such risk. A previous meta-analysis had not explicitly examined the effect of age (Hemminki and McPherson, 1997).
In the WHI, age influenced not only the relation between HT and CHD but also appeared to modulate the effect of HT on all-cause mortality and a composite outcome (“global index”) (Table 2-4). In an analysis that combined data from the two HT trials in the WHI, HT was associated with a significant reduction in mortality (RR = 0.70; 95 percent CI: 0.51, 0.96) among
women in their 50s but not among women aged 60 or older (Rossouw et al., 2007). A 2003 meta-analysis of 30 randomized trials, including the WHI CEE + MPA trial, found that HT was associated with a nearly 40 percent reduction in mortality in trials in which the mean age of participants was <60 but had no effect on mortality in other trials (Salpeter et al., 2004).
In an ancillary study of coronary artery calcium (CAC) measurements in the WHI CEE trial, conducted among women who were aged 50–59 at WHI enrollment, levels of CAC following trial completion were lower among women randomized to estrogen than those randomized to placebo (Manson et al., 2007). Odds ratios for the prevalence of high CAC (scores ≥100) were 0.69 (95 percent CI: 0.48, 0.98) overall and 0.46 (0.29, 0.73) among women with ≥80 percent adherence to study pills. High CAC correlates with a greater atherosclerotic plaque burden and has been shown to predict risk of future coronary events (Hecht et al., 2006). These findings further support the hypothesis that estrogen therapy reduces progression of atherosclerosis and subclinical coronary artery disease in younger women who are closer to the onset of menopause.
Thus, the existing evidence in support of the timing hypothesis is compelling, although the data are not yet conclusive and would not justify the use of HT for cardioprotection. However, even if the hypothesis is ultimately disproved and HT-associated RRs for CHD are shown to be similar across groups defined by age or time since menopause, the much lower absolute baseline risks of CHD and other events in younger or recently menopausal women translate to much lower absolute excess risks associated with HT use in these women as compared with women who are older or further past menopause. Estimates of such risks based on WHI data (for CHD, total mortality, and the global index) are provided in Table 2-6.
New trials are in progress to assess the possible differential effects of HT on the progression of atherosclerosis according to age at initiation (Hodis, 2007) and type of therapy (Harman et al., 2005).
Implications of the Timing Hypothesis for Clinical Decision Making
There is a clear consensus among mainstream health organizations and most healthcare providers that the use of HT should be limited to management of moderate-to-severe menopausal symptoms. Most of the current guidelines recommend against the use of HT at any age to prevent CHD and other chronic diseases (Estrogen and progestogen use in peri- and post-menopausal women: March 2007 position statement of the North American Menopause Society, 2007; Executive summary. Hormone therapy, 2004; Hormone therapy for the prevention of chronic conditions in post-menopausal women: Recommendations from the U.S. Preventive Services
TABLE 2-6 Attributable Risks (Cases per 10,000 Women Per Year) for Selected Outcomes in a Combined Analysis of the Women’s Health Initiative (WHI) E + P and E-alone Trials
|
Absolute Excess Risks (cases per 10,000 person years) by Age and Years Since Menopause in the Combined Trials (E + P and E-Alone) of the WHI |
||||||
|
Outcome |
Age (years) |
Years Since Menopause |
||||
|
50–59 |
60–69 |
70–79 |
<10 |
10–19 |
≥20 |
|
|
CHD |
–2 |
–1 |
+19a |
–6 |
+4 |
+17a |
|
Total mortality |
–10 |
–4 |
+16a |
–7 |
–1 |
+14 |
|
Global indexb |
–4 |
+15 |
+43 |
+5 |
+20 |
+23 |
|
aP = 0.03 compared with age 50–59 years or <10 years since menopause. bGlobal index is a composite outcome of CHD, stroke, pulmonary embolism, breast cancer, colorectal cancer, endometrial cancer, hip fracture, and mortality. SOURCE: Derived from Rossouw, J. E., R. L. Prentice, J. E. Manson, et al. 2007. Post-menopausal hormone therapy and risk of cardiovascular disease by age and years since menopause. Journal of the American Medical Association 297:1465-1477. |
||||||
Task Force, 2005; Mosca et al., 2007; Wathen et al., 2004), due to other known risks of HT. Although HT should never be prescribed specifically for coronary protection, the timing hypothesis can—and should—inform clinical decision making regarding the use of systemic HT for treatment of hot flashes and night sweats that are severe or frequent enough to disrupt sleep or quality of life—the classic and currently only compelling indications for such therapy (Estrogen and progestogen use in peri- and postmenopausal women: March 2007 position statement of the North American Menopause Society, 2007; Manson and Bassuk, 2007a). The timing hypothesis suggests that women in early menopause and at low baseline risk of CHD are unlikely to experience HT-associated coronary events and would have a more favorable benefit–risk profile on HT than older women.
Lessons Learned from the Hormone Therapy Controversies
The divergent findings on hormone therapy underscore the strengths and limitations of both observational and clinical trial research and suggest important strategies for improving the design of future studies. Understanding the basis for the discrepancies, and the relative convergence of findings after accounting for methodological and biological factors, provides lessons for improving the reliability and quality of research on clinical interventions. The key lessons and their implications for study design are as follows:
-
Short- and long-term effects of a clinical intervention may differ and clinical studies must be designed to capture time-varying effects. A strength of clinical trials is the ability to pinpoint the onset of an exposure/intervention and to capture early events, whereas observational studies often miss acute or short-term effects unless exposure information is updated frequently. Observational studies that utilize computerized health records and electronic pharmacy databases, however, may avoid these limitations due to their ability to capture prescription/medication data on a regular basis. Moreover, results of studies that use electronic health records tend to be largely convergent with results of clinical trials, supporting the advantages of this study design for medication-related research. Large-scale studies involving networks of electronic databases could facilitate evidence development in this area.
-
Regardless of the study design, analyses must consider time-varying effects when comparing results across studies. Duration of treatment may have an important influence on health outcomes. For HT, some risks tend to increase shortly after treatment initiation (e.g., venous thromboembolism, myocardial infarction) while other risks may be delayed (e.g., breast cancer). Conversely, some benefits may occur quickly (e.g., reduction in vasomotor symptoms), and others may require longer duration of treatment (reduction in osteoporotic fractures or slowing of atherogenesis in younger women). Comparisons across studies should account for duration of treatment. Clinical trials and observational studies of HT that initially appeared divergent showed similar results when analyses were stratified by duration of treatment (e.g., both the clinical trial and observational components of the WHI indicate a short-term increase in risk of CHD with HT followed by a declining risk with longer duration of treatment). Due to the high cost of clinical trials and generally short duration, information about long-term risks may rely heavily on observational sources. Thus, the totality of evidence from all available sources must be considered.
-
Clinical trials have the advantage of minimizing confounding and selection biases through the process of randomization, which works particularly well when the sample size is large. Observational studies can reduce these biases by careful adjustment for lifestyle factors, disease-related risk factors, socioeconomic factors, and access to medical care. Although these biases have contributed to the discrepancies between observational studies and clinical trials, they appear to be less important than the methodologic factors addressed above or the biological differences in the populations studied. For example, HT results in observational studies
-
and clinical trials are similar after accounting for differences in treatment duration (as discussed above) and differences in the age distribution of the study populations (as described below).
-
Biological factors, particularly a woman’s age, time since menopause, and underlying stage of atherosclerosis, may modulate her health outcomes on HT, particularly her risk of CHD. Estrogen is complex and has both favorable and adverse effects. Biological differences in study populations in observational studies and clinical trials may be primarily responsible for the discrepancies between these studies, although study design and methodologic factors have also contributed. Experimental studies in nonhuman primates also support a role of biological factors, such as time since menopause and underlying health of the vasculature, as modifying factors. Moreover, it is critically important to consider absolute rates, as well as relative risks, of health outcomes when evaluating the risk: benefit profile of a treatment in different populations. In the case of HT, the much lower absolute rate of cardiovascular disease (CVD) and other chronic diseases in younger women would suggest lower attributable risks in this population. Thus, the generalizability and applicability of findings to relevant clinical populations must be considered. The WHI was tremendously important in halting the growing practice of initiating HT in older women, and women at high risk of CHD, for the purpose of CVD prevention—this was demonstrated to be a harmful practice. However, the WHI could not provide conclusive answers about the risk–benefit profile of HT in recently menopausal women. Finally, the possibility of differences in health outcomes related to medication dose, formulation, and route of delivery warrants consideration and further study.
-
Surrogate markers, such as intermediate biomarkers (lipoproteins, thrombotic and inflammatory markers), noninvasive imaging studies (coronary artery calcium measurements or carotid ultrasound, mammographic density studies) may provide important insights about the health effects of different HT formulations and dosages. However, due to the complexity of HT’s effects and difficulty in predicting the net effect on risk, surrogate markers cannot substitute for the assessment of clinical events.
Conclusions
Observational studies, clinical trials, and basic research all have contributed critically important information to elucidate the health effects of HT and to inform decision making. Recent analyses have elucidated both methodological and biological explanations for the divergent findings and
suggested avenues for additional research to advance evidence development. The primary lesson is that we should consider research findings in the context of the totality of available evidence and design studies to complement and extend the existing data. Importantly, observational studies should be designed to capture both short- and long-term risks and should have frequent updating of exposure variables of interest (electronic health records and pharmacy databases may be useful). Clinical trials must be adequately powered to assess clinically relevant subgroups and to address the possibility of a modulating effect of key clinical variables. Consideration of absolute risks in research presentation and interpretation is critically important. Finally, it may be helpful to incorporate intermediate and surrogate markers (such as from imaging studies) into research designs, although such markers can never fully replace clinical event ascertainment. For HT and CHD, the emerging evidence to support the “timing hypothesis” does not imply that recently menopausal women should be prescribed HT for cardiac protection; rather it suggests that healthcare providers should avoid initiating HT in older women who are distant from menopause but need not be unduly concerned about CHD risks when considering short-term treatment to relieve vasomotor symptoms in recently menopausal women. This new information should aid clinical practice, suggest avenues for future research, and improve the quality of medical care and clinical decision making.
DRUG-ELUTING CORONARY STENTS
Ashley B. Boam, M.S.B.E.
Andrew Farb, M.D.
Food and Drug Administration, Center for Devices and Radiological Health
Each year approximately one million patients in the United States undergo percutaneous coronary intervention (PCI) for the treatment of symptomatic coronary atherosclerosis, of which 80 percent undergo placement of a coronary stent as part of this procedure. It is estimated that 650,000 patients annually are treated with drug-eluting stents, which reduce the need for repeat procedures due to restenosis compared with bare metal stents. Currently available DES consist of a metal stent platform, which acts as a mechanical scaffold, with a polymer and drug mixture coated on the surface of the stent platform. The polymer controls the elution of the drug from the stent into the artery wall with the objective of reducing restenosis (in-stent tissue regrowth following implantation). The coated stent is mounted on a balloon catheter used to deploy the stent within a coronary atherosclerotic lesion (site of luminal narrowing).
Development of DES
Clinical restenosis after arterial balloon injury occurs as a result of luminal re-narrowing secondary to (1) development of a neointima (consisting of vascular smooth muscle cells with an extracellular soft tissue matrix) and (2) adventitial fibrosis-induced arterial constriction (negative remodeling). The semi-rigid scaffold afforded by metal stents prevents arterial constriction that occurs post-balloon angioplasty, so that restenosis occurs as a consequence of neointimal growth alone. Compared to balloon angioplasty, bare metal stents improve arterial patency rates via (1) a reduction in rates of acute vessel closure and recoil and (2) a modest reduction in long-term restenosis rates by preventing negative remodeling. Implanted bare metal stents are foreign bodies and present an early thrombosis risk. In human clinical use, this risk has been minimized by (1) deployment techniques that directly oppose the stent struts to the subjacent arterial wall and (2) the use of two adjunctive antiplatelet drugs (aspirin and clopidogrel) until the stent is covered by an endothelialized neointima.
Residual high restenosis rates after bare metal stenting in higher risk lesions combined with an understanding of the pathogenesis of restenosis lead to investigations of interventions aimed to inhibit neointimal growth. Success in inhibiting neointimal thickening was achieved via the local delivery of agents that specifically targeted the cell cycle; these agents inhibit cellular proliferation and have anti-inflammatory properties. Preclinical studies demonstrated that antimitogenic agents (such as sirolimus and paclitaxel) eluted over time from a polymer coating reduced in-stent stenosis at 28 days (Farb et al., 2001; Suzuki et al., 2001).
Regulation of DES
Coronary DES are regulated by the Food and Drug Administration (FDA) as combination products (FDA, 2008f) because they are comprised of two or more regulated components, i.e., a drug and a device. In response to a Request for Determination of jurisdiction, the FDA determined that the primary mode of action was that of the device component (the mechanical support of the metal stent), and primary review responsibilities were assigned to the Center for Devices and Radiological Health (CDRH), with substantial consultative review by the Center for Drug Evaluation and Research (CDER) (FDA, 2008d). Given this framework, when a manufacturer wishes to evaluate new DES, approval of an Investigational Device Exemption (IDE) by the FDA and approval of the investigational plan by local Institutional Review Boards (IRBs) are required prior to beginning clinical studies in the United States. Approval
to market a DES in the United States requires approval of a premarket approval application (PMA) (FDA, 2008b).
Regulatory History
The first two DES to be approved for marketing in the United States were the CYPHER Sirolimus-Eluting Coronary Stent (Cordis Corporation, a Johnson & Johnson company, Miami Lakes, Florida) in April 2003 (FDA, 2008a) and the TAXUS Express2 Paclitaxel-Eluting Coronary Stent (Boston Scientific, Natick, Massachusetts) in March 2004 (FDA, 2008e). The PMAs for these products included substantial laboratory testing and animal studies in addition to chemistry and manufacturing information. Pharmacokinetic (PK) assessments of drug elution were performed using in vitro methods, and in vivo PK studies were completed in animals and humans. DES were implanted in coronary arteries of animal models to evaluate device handling performance and histologic changes within arteries, myocardium, and other body organs. Both DES were evaluated in clinical trials that compared the DES to the identical bare metal (uncoated) stent in patients with symptomatic coronary artery disease undergoing PCI of a single lesion. In this patient population, both the CYPHER and TAXUS DES demonstrated clinically significant reductions in the incidence of repeat procedures needed to treat restenosis, without any apparent differences in the rates of death or myocardial infarction. This substantial improvement in effectiveness led to widespread adoption of these products, with DES used in up to 80 percent of PCI patients treated with stents. In anticipation of use of DES in a large number of patients, including use outside of the labeled indication, the FDA required both manufacturers to conduct postapproval studies in which 2,000 consecutive patients receiving the DES were enrolled into a single-arm registry study and followed for at least 1 year. The consecutive nature of these postapproval studies reduced the influence of selection bias but allowed for the enrollment of large numbers of “off-label” patients.
The Search for Potential Surrogate Endpoints
As rates of reintervention to treat restenosis fell from double to single digits, the size of a trial needed to show either non-inferiority or superiority of a new DES to one of the two approved DES grew rapidly in comparison to the initial trials in which superiority to a bare metal stent was the objective. Following approval and rapid clinical adoption of the first two DES, researchers turned their attention to the development of surrogate markers for effectiveness.
Angiographic evaluation of patients undergoing PCI has been a standard component of stent trials for many years. Such imaging studies provide insight into the mechanistic action of the stent by providing quantitative assessments of the amount of neointimal growth within the stent and the 5 mm margin proximal and distal to the stent. Angiographic end-points such as late lumen loss (the difference between minimal lumen diameter measured immediately post stent implantation and follow-up angiography, typically performed 9 months post stent implantation) and percent arterial diameter stenosis have been identified as potential surrogate markers for the clinical end-point of target lesion revascularization, or the need for reintervention to treat restenosis in the stented area (Mauri et al., 2005; Pocock et al., 2008). Imaging end-points are commonly measured as continuous variables, and this powerful discriminatory advantage can be utilized to design trials with sample sizes considerably smaller than typically needed for standard binary clinical end-points (e.g., target lesion or target vessel revascularization). Further, angiographic evaluations are objective measures evaluated by core laboratories, which helps to minimize potential bias.
Clinical Trials Utilizing Angiographic End-points
The third DES to be FDA-approved was the Endeavor Zotarolimus Drug-Eluting Coronary Stent (Medtronic, Santa Clara, California) (FDA, 2008c). The Endeavor stent was the first DES to incorporate a drug not previously approved for systemic use. Zotarolimus (Abbott Laboratories, Abbott Park, Illinois) was developed specifically for use on a DES; therefore, the DES manufacturer, Medtronic, provided safety information on the drug alone in addition to the stent-based drug delivery evaluation as performed for the CYPHER and TAXUS stents. The first major clinical study conducted by Medtronic was a randomized trial comparing Endeavor to the identical bare metal stent. This study was conducted outside of the United States as the manufacturer believed that a trial in which a DES was randomized to a bare metal stent could not be conducted in the United States due to the widespread adoption of the CYPHER stent by U.S. interventional cardiologists. A second study randomizing the Endeavor stent to the CYPHER stent was conducted in the United States, with a primary end-point based on angiographic evaluation. Finally, a third study was completed in the United States to evaluate the performance of the Endeavor stent versus the approved TAXUS stent. This third study utilized a clinical measure as the primary end-point, with a powered secondary end-point based on angiographic measurements. The Endeavor stent proved to have superior clinical performance (a composite of cardiac death, MI, and target
vessel revascularization) in its comparison to the bare metal stent and to be non-inferior in clinical performance to the TAXUS stent. However, in both trials assessing angiographic outcomes, the Endeavor stent failed its non-inferiority comparison to both the CYPHER and TAXUS stents (see further discussion below regarding use of angiographic end-points). In the FDA’s final evaluation, the strength of the clinical assessments and the absence of any safety concerns were found to outweigh the less favorable angiographic results, and the Endeavor stent was approved in February 2008.
Emergence of a Safety Concern
Following the approval of the first two coronary DES, data were presented at the American College of Cardiology Scientific Sessions in Atlanta, Georgia, in March 2006 and at the European Society of Cardiology Annual Meeting/World Congress of Cardiology Meeting in Barcelona, Spain, in September 2006 that suggested a small but significant increase in the rate of stent thrombosis associated with DES compared to bare metal stents, occurring after the first year of implantation. Such a finding was of significant concern to physicians, manufacturers, and the FDA, as stent thrombosis is associated with high rates of acute MI and mortality.
Stent thrombosis was a known safety concern that had been observed with use of bare metal stents prior to the introduction of DES. Experience with bare metal stents revealed that the appropriate use of dual oral antiplatelet medications (aspirin plus a thienopyridine such as clopidogrel1) minimized the occurrence of stent thrombosis (which was typically observed within the first 30 days post stenting) until an endothelial lining was regenerated over the stent surface. Based on the antiproliferative actions of the drugs released from DES, the recommended duration of dual antiplatelet therapy following DES implantation was extended to 3 to 6 months, in recognition that inhibition of restenosis may also inhibit re-endothelialization of injured arterial surfaces and prolong the window of risk for stent thrombosis. Several reports noted that premature discontinuation of clopidogrel was an independent risk factor for stent thrombosis (Iakovou et al., 2005; Kuchulakanti et al., 2006). Moreover, meta-analyses of available randomized trials of the CYPHER stent and the TAXUS stent showed a numerical increase in the rates of stent thrombosis for both DES compared to their respective bare metal stent controls after 1 year. Further, an ongoing risk of approximately 0.6 percent per year was reported in patients receiving DES in two large European institutions (Daemen et al., 2007). Such data questioned whether 3 to 6 months of clopidogrel was sufficient, and raised
the possibility that a longer administration may be prudent. However, other publications reported cases of stent thrombosis despite the continued use of clopidogrel (Airoldi et al., 2007; de la Torre-Hernandez et al., 2008). Finally, the risk of bleeding associated with extended use of clopidogrel (as reported in the CREDO [Steinhubl et al., 2002], CURE [Fox et al., 2004], and CHARISMA [Bhatt et al., 2006] studies) has not been well characterized in comparison to a presumed reduction in risk of stent thrombosis. While appropriate use of clopidogrel is certainly important, other issues observed in cases of DES thrombosis include lesion factors (e.g., arterial bifurcations and long lesions requiring overlapping stents), hypersensitivity reactions to the DES polymer coating, and stent strut malapposition to the underlying arterial wall (Finn et al., 2007; Virmani et al., 2004). Thus, the occurrence of stent thrombosis is multifactorial, and in some cases clopidogrel use may not influence stent thrombosis rates.
FDA convened an Advisory Panel meeting on December 7 and 8, 2006, in an effort to fully characterize the risks, timing, and incidence of DES thrombosis. Three topics were discussed by the experts on the panel, DES manufacturers, and clinical investigators: (1) the rates of stent thrombosis and associated clinical sequelae (death and MI) when DES are used in accordance with their labeled indications; (2) the rates of stent thrombosis and associated clinical sequelae (death and MI) when DES are used in a broader, more complex population of patients and lesions; and (3) the optimal duration of dual antiplatelet therapy in patients who receive DES. The Panel concluded that both the CYPHER and TAXUS DES are associated with a small increase in stent thrombosis compared to bare metal stents that emerges 1 year post stent implantation. However, based on the data available, this increased risk of stent thrombosis was not associated with an increased risk of death or MI compared to bare metal stents. The Panel also found that off-label use of DES is associated with an increased risk of stent thrombosis, death, or MI compared to on-label use; however, with more complex patients, an increased risk in adverse events is not unexpected. Data on off-label use are limited, and additional studies are needed to determine optimal treatment strategies for more complex patients. Finally, the Panel concluded that the optimal duration of antiplatelet therapy, specifically clopidogrel, is unknown and DES thrombosis may still occur despite continued therapy. However, it recommended that the labeling for both approved DES should include reference to the American College of Cardiology/America Hospital Assocation/Society for Cardiovascular Angiography and Interventions PCI Practice Guidelines, which recommend that patients receive aspirin indefinitely plus a minimum of 3 months (for CYPHER patients) or 6 months (for TAXUS patients) of clopidogrel, with therapy extended to 12 months in patients at a low risk of bleeding. More
specific information about the meeting and the conclusions reached are available on the FDA’s website.2
Lessons Learned
Experience from the development, review, and marketing of the first two DES has provided several important lessons. First, the application of knowledge gained from past devices, such as bare metal stents, is not always appropriate or predictive for DES. As noted above, while stent thrombosis was known to occur with bare metal stents, simply extending the duration of antiplatelet therapy until a point beyond when drug elution is presumably complete (and theoretically, arterial healing would be complete with re-establishment of a functional endothelial covering on the stent) does not appear to have been sufficient to eliminate the risk of DES thrombosis. Second, although the FDA anticipated that the overwhelming efficacy of DES in the prevention of restenosis would lead to extensive use of DES in higher risk patients and lesions beyond those studied for initial approval (which were reflected in the labeled indication), the extent of this off-label use was surprising. The “all-comers” postapproval studies conducted by the manufacturers of CYPHER and TAXUS indicated that approximately 60 percent of patients who received DES after FDA approval had indications for stenting beyond those in the labeled indication, including patients with multiple vessel disease, with disease in the left main artery or in a bifurcated artery, and those patients being treated for an acute MI. In this setting, the data previously collected to describe the safety profile of these stents were only applicable to 40 percent of the population actually receiving them, compelling the FDA and manufacturers to find reasonable approaches to development of additional data to understand the risks and benefits in treatment of patients treated with DES outside of their approved indication of use. Third, the postmarket experience gained with the CYPHER and TAXUS stents indicated a need for longer follow-up in postmarket studies. With the emergence of stent thrombosis events occurring after 1 year post implantation, longer studies are needed to understand whether these events continue to accrue at the same rate or at an increasing or decreasing frequency after the first year and to understand the impact of these events on the incidence of cardiac death and MI during this time period. Fourth, more recently, the clinical trials of the Endeavor stent have enhanced our understanding of the relationship of angiographic imaging measurements (such as late lumen loss) to clinical end-points (such as target lesion or target vessel revascular-
|
2 |
FDA statements available at http://www.fda.gov/cdrh/news/091406.html and http://www.fda.gov/cdrh/news/010407.html. Panel summary and transcript available at http://www.fda.gov/ohrms/dockets/ac/cdrh06.html#circulatory. |
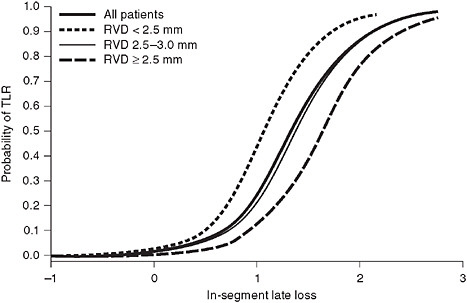
FIGURE 2-4 The relationship between late loss and TLR, evaluated using logistic regression.
SOURCE: Reprinted from the Journal of the American College of Cardiology, Vol 51, Pocock, SJ, et al., Angiographic surrogate endpoints in drug-eluting stent trials: A systematic evaluation based on individual patient data from 11 randomized, controlled trials. Pages 23-32. Copyright 2008, with permission from Elsevier.
ization [TLR or TVR, respectively]). As noted above, the Endeavor stent failed to meet non-inferiority for the angiographic endpoint comparing its late loss measurements to those of either CYPHER or TAXUS; however, a comparison of clinical measures (cardiac death, MI, and TVR) demonstrated that the Endeavor stent was non-inferior to the TAXUS stent. Why the apparent discrepancy? As observed in Figure 2-4, when the relationship between late loss and TLR is evaluated using logistic regression, the resulting model is curvilinear. In the case of the comparison of Endeavor to TAXUS, the observed late loss values (0.36 mm for Endeavor and 0.23 mm for TAXUS) are located on the flat portion of the late loss/TLR curve, a point at which a statistically significant difference in late loss between stents (or failure to achieve statistical non-inferiority) may not translate into important differences in a clinical end-point such as TLR.
Issues Needing Resolution
The issue of late stent thrombosis continues to be a critical issue for currently approved DES and the next generation of devices. Further study
is needed to better define etiologies and their individual contributions to the overall risk of stent thrombosis. At present, there is no animal model that can predict stent thrombosis in patients, meaning that assessment of this clinically important event can only be conducted in human trials. Additionally, the FDA and the clinical community await further data to define the optimal duration of antiplatelet therapy that would appropriately balance a reduction in stent thrombosis with the risk of significant bleeding. Currently, the low event rates and the long term nature of the DES thrombosis question lead to the need for large trials of long duration. FDA currently requests that postmarket studies be designed and appropriately sized to define the incidence of stent thrombosis through at least 5 years of follow-up.
Patients who present with coronary artery disease and undergo stenting represent a heterogeneous population with diverse clinical features and atherosclerotic plaque morphology, which presents a challenge to manufacturers, investigators, and the FDA in the design of clinical studies. For certain patients, such as those with stable coronary artery disease involving discrete lesions in one or two coronary arteries, use of DES is the current standard of care, and as such, an approved DES can be used as a control. However, to evaluate DES in patients such as those who are treated in the course of an acute MI, or those with three-vessel disease, use of bare metal stents or coronary artery bypass graft surgery, respectively, may be an appropriate control. An efficient approach to study patients across this diverse population could lead to DES approval for extended indications, with more relevant clinical data available for physicians and patients weighing treatment options.
Issues for the Future
Looking ahead to development of new DES, a number of issues require consideration. With clinical event rates in the single digits and multiple DES on the market, manufacturers and investigators will face challenges in developing clinical trials that do not require ever-increasing sample sizes and that can be completed in a reasonable time period.
New technologies are also likely to pose additional issues for manufacturers and the FDA. By eliminating one or all of its components over time, degradable stent coatings and even fully degradable stents have the potential to reduce the risk of stent thrombosis compared to a permanent intraarterial implant. However, standard test methods used for stents of durable materials will likely have to be altered and time periods for evaluation, such as in animal studies, lengthened to fully characterize the degradation process and the fate of the degradation products. Whether clinical studies will
also require a longer follow-up duration, past the time of full degradation, to adequately assess safety and effectiveness remains to be determined.
With the development of new stent platform designs and drug delivery mechanisms, future DES may elute more than one drug or be covered with biological substances such as cells or antibodies. Evaluation of novel DES, especially in the development of quality control measures, will require enhanced scientific methods and creativity on the part of the manufacturers and the FDA scientists tasked with assessment of these products.
Potential Solutions
Given the focus on safety concerns and the desire for clinical endpoints, one might question whether angiographic studies still retain relevance. The FDA believes that these data remain important for several reasons. First, angiographic measurements provide mechanistic information about the performance of the stent. Second, because they enable comparative assessments of effectiveness in relatively small populations, angiographic end-points provide important information early in DES development when different drug doses or elution profiles are being considered. Such early studies may not only focus a manufacturer’s efforts and resources on a DES candidate with a higher chance of success, they also may prevent ineffective stents from reaching large numbers of patients in a pivotal trial. Third, collection of intensive imaging assessments in early development may help to determine whether extended follow-up is necessary for degradable stents or durable stents with degradable coatings. In addition to angiography, other imaging modalities such as intravascular ultrasound (IVUS) provide important information on stent/arterial wall apposition and arterial remodeling.
As the FDA has moved to strengthen its available sources of information on products after they are approved for marketing, CDRH has also turned to the collection of postmarket information as a mechanism to augment premarket trial results, which by their nature are limited in scope and duration. As an example, an ideal safety end-point for a DES premarket trial would be a combination of cardiac death and MI; however, due to the low rates of these events, in a non-inferiority comparison to an approved DES that employs a clinically relevant non-inferiority margin, sample sizes would exceed 10,000. Therefore, the FDA recommends use of a composite that also contains an effectiveness measure (usually TLR) in premarket trials, but requests that a prespecified secondary hypothesis be established to compare rates of cardiac death and MI to the control, with a plan to collect additional data in postmarket studies to increase the precision of this comparison. Postmarket studies also allow for the evaluation of clinically
significant adverse events that occur at very low rates, such as stent thrombosis. Such trials, especially if conducted as “all-comers” studies in which all consenting patients are entered, can enroll large numbers of patients rapidly, with extended follow-up to provide an improved assessment of the risk of stent thrombosis compared to relatively smaller and shorter premarket trials. In general, use of a combination of premarket trials and postmarket studies will provide the most efficient way to bring promising new products to market without compromising safety.
Postmarket studies also may offer a more efficient mechanism to gain certain expanded indications following initial marketing approval. The FDA is open to such an approach if hypotheses are established prospectively, appropriate performance goals or historical data can be identified to serve as controls, and the postmarket study is conducted under an IDE.
The lack of data to establish the optimal duration of dual antiplatelet therapy for patients receiving DES has left healthcare providers wondering how best to treat their patients, especially those who need to interrupt their therapy for an invasive surgical procedure. As noted above, however, given the low rates of stent thrombosis, to compare different durations of dual antiplatelet therapy would require studies of more than 10,000 patients for each individual DES. Based on available data, the FDA currently believes that this issue equally applies to all DES, which presents an opportunity to study this issue in DES as a class, rather than as separate products. With this in mind, as part of its Critical Path initiative,3 the FDA is collaborating with device and drug manufacturers and academic physicians to develop a large study involving multiple DES in which clarity on this issue might finally be achieved.
In summary, a number of challenges face the FDA, manufacturers, and the clinical community in designing clinical trials to support marketing approval of future DES, in assuring that critical safety issues are answered, and in achieving these goals without stifling innovation. A mixture of premarket and postmarket data, thoughtful approaches to the design of clinical programs, and continued collaboration among manufacturers, healthcare providers, and the FDA will lead to potential solutions.
|
3 |
More information available at http://www.fda.gov/oc/initiatives/criticalpath/. |
BARIATRIC SURGERY
David R. Flum, M.D., M.P.H.
University of Washington
A Safer, Higher Quality, Learning Healthcare System in Surgery: The Role of Regional Collaboratives
One characteristic of a learning healthcare system is its capacity to generate the evidence needed to judge the effectiveness and cost-effectiveness of delivered care and to employ this evidence to deliver optimal care. Innovative surgical procedures and other invasive healthcare interventions represent a unique aspect of healthcare delivery that challenges a developing healthcare system. This review describes the challenges of evaluating interventions, the current state of evaluation of interventions and novel regional collaboratives that are more effectively evaluating the utility of procedures and should be an important component of our future healthcare system.
The Challenge of Evaluating Interventions
When considering the value of interventions the potential for harm caused by the intervention must be balanced by the harms relieved by addressing the condition. This is particularly the case with implantable devices, new procedures and techniques where there is no formalized process for training or postmarket surveillance of procedural harms. Since procedures are performed by individuals and often in nonstandardized fashion, the associated risk also may be considerably less predictable than the risk of more standardized interventions such as the administration of medications. Variation in outcome based on technical and technician factors is a well- established phenomenon, and variation in training, education, and practice is the hallmark of the surgical profession. For example, the adoption of new technologies into the operating room such as laparoscopic approaches to cholecystectomy, antireflux procedures and bariatric interventions did not follow rigorous animal testing or established training programs and at most hospitals required no proof of competency before introduction into practice. When first disseminated most practicing surgeons learned laparoscopy at weekend courses and then refined the skill in their patients. The surge in serious, common bile duct injury (CBDI) after the introduction of laparoscopic cholecystectomy (A prospective analysis of 1518 laparoscopic cholecystectomies. The Southern Surgeons Club, 1991) suggests in an extreme the potential of technician-related adverse outcome. In this case increasing rates of
CBDI (and variability in the occurrence of CBDI) were linked to surgeon inexperience and the inevitable outcome of a “technique in development” or the learning curve. Complicating issues of the learning curve is that there is no surveillance system for the detection of adverse outcomes. The regulatory environment places few limits on technologic innovation in the operating room and other areas where invasive treatments are performed. While pharmaceutical agents require rigorous pre- and sometimes post-market testing to demonstrate safety and efficacy, the regulatory burden for approval of new devices is much less stringent. For devices/procedures, regulatory systems focus on proof of concept rather than comparative effectiveness or even safety once the technology is no longer exclusively “in the hands” of experts. Furthermore, there is no regulatory requirement for innovative surgical interventions that do not involve a device but rather evolve from techniques and equipment already being used. Surgeons innovate on a daily basis using tools and techniques approved for other purposes with varying levels of success. Successful innovations are a staple of the profession and have undoubtedly resulted in improved procedures, but the failed innovations (and sometimes even the successful innovations) are neither systematically tracked nor reported to other surgeons. Moreover, industry stakeholders and thought leaders continually refine new techniques and devices making the study of any intervention a “moving target” and last year’s research no longer applicable. Lastly, our healthcare culture includes doctors responding to marketing campaigns directed to patients and clinician-industry partnerships that make the control of new interventions and their evaluation deeply problematic.
Laparoscopic bariatric surgery—a set of interventions intended to help patients lose weight and address obesity-related comorbid conditions—are an example of a relatively newly developed procedure that demonstrates all of these pitfalls. While performed only occasionally in the early 1990s, by 2002 this was one of the fastest growing segments of the surgical marketplace. The growth was more than 10-fold in a decade (Santry et al., 2005) and laparoscopic roux-en-y gastric bypass is one of the most technically advanced and demanding. Training for these procedures was and remains highly variable; minimal credentialing requirements evolved only after the procedure grew in popularity, laparoscopic skills, techniques, and devices used for other operations were simply adapted to the bariatric field; and an entire field’s experience grew through increasing practice. Absent a learning healthcare system, none of this growth or its expected outcomes was monitored in real time. Instead, scientific publications by the fields’ experienced practitioners were taken to represent the community’s experience with the procedures (Buchwald et al., 2004).
Effectiveness Versus Efficacy
A central problem in evaluating the value of interventions and devices is in distinguishing effectiveness (the extent to which it “works” in general practice) from efficacy (the extent to which it “works” in the controlled environment [i.e., among experts, research centers, trials, etc.]). It is very unusual for a new device or intervention to be tested in these real world environments, but effectiveness evaluations are really the only way to accommodate the varied training, experience, skill, and unselected populations that are found in the “average community.” Case series predominate in the reporting of new devices and interventions, and these are most often the product of clinicians reporting their best results and journals more willing to publish studies with better outcomes and larger numbers of patients. This is a form of publication bias that is difficult to track but assuredly occurs. Even when consecutive cohort studies are considered they are most often the product of expert clinicians and referral centers. These doctors undoubtedly have learned how to select patients well and have developed nonintervention-related success strategies (i.e., post-procedural care, pre-procedural interventions like smoking cessation—subtle, nonreported exclusions) that may interact with the effects of the intervention. This form of selection bias (selected clinicians and selected patients) makes it very hard to estimate the anticipated effects of the procedure when applied in the general community. Though this efficacy versus effectiveness conundrum is critical when evaluating intended treatment effects, it may be even more important when considering unintended effects such as safety problems. Most studies of devices and interventions are aimed at demonstrating therapeutic effect and so are relatively underpowered to identify important procedure-related harms (i.e., laparoscopic cholecystectomy and bile duct injury). It may take many years for individual clinician case reports focused on efficacy to reveal a safety outcome of concern as the technique is diffusing. The healthcare community has occasionally anticipated such problems and controlled the diffusion of new techniques. One example is laparoscopic colon resection for cancer where the American Society of Colon and Rectal Surgeons (ASCRS) applied a virtual moratorium on this technique (The American Society of Colon and Rectal Surgeons, 1994) while several studies were being conducted to assess its safety. Perhaps learning from the bile duct injury experience, ASCRS applied professional “peer pressure” through its journals and meetings to essentially restrict the application of the technique until concerns about port site recurrence and other safety/ efficacy outcomes could be resolved. Relying on professional societies to restrict the diffusion of new and emerging techniques and devices has by no means been the norm so it has fallen to evaluators of the healthcare system to assess a procedure’s value after its introduction.
In bariatric surgery no such moratorium took hold and rapid diffusion among a group of laparoscopic surgeons with highly variable skillsets took place. There was the proliferation of many case series suggesting high levels of safety and efficacy (Buchwald et al., 2004), but equal reports from the media of high-profile adverse outcomes and deaths that stimulated some state agencies to close down bariatric programs and prompted radical responses from the academic and public health communities (Commonwealth of Massachusetts Betsy Lehman Center for Patient Safety and Medical Error Reduction Expert Panel on Weight Loss Surgery: Executive Report, 2005).
Population-Level Research
Usually the product of academic research projects, our current healthcare system employs several techniques to assess effectiveness and safety. Most of these strategies require a post-hoc approach that assesses the impact of interventions years after they have occurred. This is often accomplished by studying medical claims data that has been submitted to billing agencies, state or federal repositories. These claims include virtually no clinical data, rely on often crude codes to describe procedures, lack codes to match updated procedures and have limited outcome information beyond discharge disposition (alive, dead, skilled nursing facility, etc.). These datasets do not include interventions performed in the outpatient environment, most are cross-sectional and miss outcome (including death) after discharge, and the ones with more subtle coding schemes (CPT codes instead of ICD9 codes) are for limited populations (Medicaid, Medicare, individual insurers). The timeliness of the data is problematic (often available years after the events) as is the granularity and accuracy of the coding schemes for risk adjustment. Despite these considerable limitations, when used for specific purposes they can help inform the healthcare system about the impact of procedures in the real world—the average community, average patient, average clinicians—without concern of publication or selection bias. Our group has used a longitudinal dataset based on Washington State hospital discharge abstracts (CHARS) to demonstrate (1) higher than expected rates of bile duct injury when less experienced surgeons fail to use an intraoperative x-ray (cholangiogram) to confirm anatomy (Flum et al., 2001a), (2) serious injury with laparoscopic anti-reflux surgery (Flum et al., 2002), (3) recurrence rates after modern incisional hernia repairs (Flum et al., 2003), (4) unnecessary appendectomy despite the availability of better diagnostic testing (Flum et al., 2001b), and (5) perioperative deaths and complications after bariatric surgery (Flum and Dellinger, 2004). In each case, these rates were “higher than expected” because the expected was derived from case series provided mostly by highly experienced experts. These studies may be used to help frame a
discussion about a procedures value through the context of effectiveness and can be important for insurance coverage decisions. For example, an evaluation showing relatively high perioperative mortality rates after bariatric surgery in a high-risk population—the Medicare disabled (Flum et al., 2005b)—was incorporated into a Centers for Medicare & Medicaid Services (CMS) coverage decision restricting the care of these patients to experienced centers. This was a public health policy intervention aimed at reducing procedural harm based on administrative data. Undoubtedly, our healthcare system “learns” from these research-based initiatives but the learning happens late, depends on interested investigators with narrow focus, and is at best uncoordinated.
Power of the Purchaser to Determine Effectiveness
Relying on the research community for these “after the fact” analyses, however, is a poor and not particularly sustainable way to build a safe, effective healthcare system. Large healthcare purchasers have been able to exert some positive influence in directing the system towards assessment of safety and effectiveness by crafting coverage decisions that generate evidence. On at least a few occasions, CMS has required that as a condition of coverage, patients be placed and followed on registry after novel interventions were introduced. Carotid stenting (Gray et al., 2007) and implantable defibrillators (Hammill et al., 2007) are two examples where this registry-based approach has undoubtedly limited the diffusion of the technique. For carotid stenting this registry approach has already identified a subgroup with a prohibitive risk for which stenting is not appropriate. More “coverage with registry” may be the single most effective way to determine the effectiveness of new interventions before their broad dissemination, but the lack of surveillance systems and reliable registry infrastructure may limit their development. Medicare’s decision regarding bariatric surgery was like a “coverage on registry” approach in that it approved the procedures only at accredited centers and a component of accreditation is participation in a registry-like activity aimed at monitoring and improving outcomes.
A different approach to gathering real-world evidence using a randomized intervention was helpful in determining the true value of lung volume reduction surgery (LVRS) for patients with chronic obstructive pulmonary disease (COPD) (Fishman et al., 2003). After years of being touted as life-saving treatment compared to selected or historic nonoperative cohorts, CMS restricted its payments of the procedures to patients enrolled in a randomized trial. The study, though conducted at a limited number of centers, demonstrated that LVRS was effective for only a small subset of patients and was contraindicated in another. The study found that LVRS was not particularly cost effective, and taken together these findings have dramatically curtailed its use.
The LVRS example included safety, efficacy/effectiveness, and cost-effective-ness in a nearly real-time fashion to inform the system about the true value of LVRS, and it accelerated our understanding of its role in our healthcare system. That is the hallmark of a learning healthcare system but one that is hard to replicate.
Regional Collaboratives
Though perhaps ideal, the active evidence generation of the LVRS trial has not been repeated for other controversial procedures and devices. LVRS required tremendous political and administrative leadership, was quite controversial and expensive for both the National Institutes of Health (NIH) and CMS, and required academics with an interest in the topic to come together despite their opinions. For these reasons, such studies are not likely to be the mechanism for the future “learning healthcare system” to use and evaluate new procedures and devices contemporaneously. A novel approach to contemporaneous use/evaluation of new procedures is coming from regional clinical collaboratives aimed at improving healthcare delivery. These collaboratives acknowledge that the health system evaluations performed by academic researchers may not be the most effective way to correct the lapses they identify. Academics are rewarded for work that identifies outcome variation and lapses in quality through grants, media recognition, and promotions, but lack the incentive or the skillsets to be involved in the correction of these lapses. The latter is work that typically requires community engagement, is not well funded, and may take years to accomplish.
These collaboratives include larger groups of clinicians who are working in the field and taking it in their own hands to create the types of learning systems that will correct themselves and deliver more optimal care. They are often limited in scope and resources but can be very effective. In Kentucky (Shively et al., 2004) general surgeons organized in the late 1990s to gather data from their own cases, to define optimal care delivery for commonly performed procedures, and to create systems that accomplished their delivery. In New England, vascular surgeons from over 10 institutions have recently partnered in a peer-to-peer network called The Vascular Study Group of Northern New England16 that is evaluating performance of certain novel vascular surgical procedures. In Washington State, surgeons from over 25 hospitals representing three-quarters of all the surgical care in the state have organized the Surgical Care and Outcomes Assessment Program (SCOAP) (Flum et al., 2005a).4 These collaboratives represent a growing movement that may be the most effective way to track
|
4 |
See www.surgicalCOAP.org. |
the use of interventions and their outcomes while simultaneously working to improve their quality.
SCOAP is a regional collaborative improving the quality and safety of surgical care and delivering more appropriate and cost-effective care. SCOAP has two components—a surveillance system gathering data on process of care and outcome of consecutive procedures at all participating hospitals, and an active correction function that engages surgeons to correct lapses in care delivery. The surveillance system relies on information technology infrastructures of varying sophistication (from paper based to full electronic medical record [EMR]) and joins surgeons at hospitals from all over the state—in rural and urban environments and in hospitals big and small—in a data-sharing/feedback network. The corrective function of SCOAP works through education, peer support/pressure, and effective use of checklists. Now in its second year, SCOAP has been continuously assessing the processes and outcomes associated with emerging procedures (i.e., laparoscopic gastric bypass, endovascular procedures of the aorta, minimally invasive pediatric surgery, and many others) and helping clinicians redirect care when lapses have been identified. SCOAP allows for innovation on the surgeon level but engages innovating surgeons in a clinician-led management committee so the variables that account for innovation/variation are included in SCOAP data collection. In this way, new procedures and techniques are simultaneously used and evaluated, and a learning healthcare system can be driven toward better performance. For example, using SCOAP data and the SCOAP quality improvement (QI) platform, bariatric surgeons (like other general, colorectal, pediatric, and vascular surgeons) are tracking quarter by quarter for changes in outcome and helping direct local QI activities to improve care delivery.
Another program aiming to assess the impact of procedures is the National Surgical Quality Improvement Program (NSQIP). NSQIP is supported by the American College of Surgeons and measures morbidity and mortality from surgical care at nearly 200 hospitals across the United States. Other national initiatives to study procedural effectiveness come from specialty societies such as The Society for Thoracic Surgery (STS), American College of Cardiology, and the Association for Thoracic Surgery. Strengths of these programs are that participation has become a cultural norm and in some cases a requirement for procedural payment. But all of these national datasets function at the level of surveillance, and because they carry a heavy administrative burden may not change quickly to accommodate innovation particularly well. Only variably do these surveillance systems draw communities of clinicians together to respond to problems in performance, and they may not be as focused on creating the learning/improving aspects of the developing healthcare system. An interesting hybrid exists in Washington State where a regional collaborative called Clinical Outcomes
Assessment Program (COAP) (Maynard et al., 2003) has been working for a decade to draw all hospitals in the state that deliver cardiac care together in programs that respond to variation found through the STS data such as reducing the use of transfusions to make surgical care safer.
Barriers and Policy Opportunities for Creating a Learning Healthcare System
Regional activities like SCOAP face tremendous obstacles because they are both hard to develop and sustain. Linking clinicians and hospitals across regions requires a sense of community among these groups that may not exist, and in many geographic areas these relationships have been strained through competition and other forces. Reconnecting as a clinical community and developing trust and mutual interest requires genuine engagement, leveling of hierarchies, and some fence mending. There is also no financial incentive for the volunteered time, team building, and development work that are a component of these initiatives and better performance through collaborative work is not specifically reimbursed. In some regions, the public health importance of an activity like SCOAP may not be enough to overcome the lack of incentives and broken relationships and unless a large payer with dominance in the marketplace compels this activity it may not even be possible. When a large payer does step in, these payer-led programs usually become pay for performance (P4P) initiatives such as the Surgical Care Improvement Program (SCIP) (MedQIC, 2008). Because P4P involves reimbursement and by default “winners and losers,” initiatives with heavy payer involvement focus on metrics that have the highest levels of evidence (and there are few procedure-based metrics that have high impact), require careful risk adjustment so that hospitals are not unfairly penalized (and there are not great risk-adjustment strategies), and come with a “top-down” feel that may not achieve the potential of peer-to-peer regional network. A variation on this theme that may work best has occurred in Michigan where a single payer aligned with clinical leaders at the University of Michigan and together they were able to build engagement across all hospitals and accomplish surveillance (using National Surgical Quality Improvement Program [NSQIP]) while hospitals benefited from millions of dollars worth of corporate investment and defrayed data collection costs (Birkmeyer et al., 2005). We have not found that other insurers in more plural markets have the willingness to commit to regional collaborative development on this level. Legislators also may want to compel this type of surveillance and QI activity, and while that may ensure that all hospitals participate and that at least certain metrics are measured, initiatives that come from legislative mandate have a punitive component. As a result, these may also fail to engage innovating clinicians in the most productive
manner. Large healthcare providers and health maintenance organizations may be best suited to facilitate these activities. Because they both employ clinicians and insure patients, they have alignment of financial interest and direct oversight of care delivery that is unheard of in more fractured systems. These groups (i.e., Kaiser Permanente, Group Health Cooperative) usually have better information technology (IT) allowing surveillance without as much chart abstraction that is required at hospitals without EMRs. This linkage of financial accountability and IT systems effectively compels clinicians to participate in surveillance and correction, but will only affect the members of those care networks and has limited applicability to the broader healthcare system. For example, in Washington State, 88 percent of patients do not receive care in such a system.
There are several policy opportunities that would allow a regional collaborative to flourish and should be considered in the development of a more effective, learning healthcare system. The U.S. Department of Health and Human Services and the Agency for Healthcare Research and Quality have developed the Chartered Healthcare Value Exchange program (AHRQ, 2008) as an attempt to support regional quality improvement. While acute care has not been the focus of the program, including surgical/ intervention care within its mandate and approving the ones (like SCOAP) that exist as test cases would clearly encourage the development of these systems to monitor and improve acute care. Medicare and other large insurers should consider preferential contracting to hospitals and clinicians that participate in such regional collaboratives as both demonstration projects and when considering “coverage with registry” decisions. This would obviate the need to recreate the wheel of valid and secure registries each time a new procedure was considered, would assure broad geographic application (i.e., not just major medical centers), and would assure that care delivered in these registries was being optimized by the engaged clinicians. Medicolegal protection of collaboratives such as SCOAP is also essential. SCOAP operates under the aegis of a state statute protecting these data from discovery (because they are being used for quality improvement), but not all states have such provisions, and the lack of such protection may be a barrier to further collaborative development. The limits of the current information technology infrastructure may be the greatest barrier to effective surveillance, and there are several congressional leaders working on this issue. We still appear to be many years away from the types of systems that would make monitoring of interventions with the “push of a button” a possibility at all hospitals across a region. For many years to come we will still rely on human abstraction of chart data, and while that will cost more, at least it should inform the design of the ultimately successful EMR. Lastly, a learning healthcare system has to recognize that it may not “get it right” the first time and that correcting lapses in care require both carrot
and stick approaches. There are few carrots (i.e., financial incentives) for volunteering the time, energy and effort for clinicians and their hospitals to deliver optimal care. As outlined above, preferential contracting to SCOAP hospitals and surgeons, for example, would be the most effective carrot for their development but may limit their effectiveness if overly prescriptive. “Stick approaches” include public exposure of underperformance, and that is often a motivator for those who believe that all data from such collaboratives should be given to the public. It is well accepted that the public has a right to know how the health system is performing, and transparency of data will be a touchstone in developing our new, learning healthcare system. Complete transparency, however may not be compatible with the development of these voluntary, self-correcting collaboratives. We should recognize these nuances of transparency and acknowledge that a limited, “reporting” dataset that grows with time should be the aim while clinicians are working to understand and then improve using a more restricted “developmental” dataset. Only in this way will the healthcare system continue to innovate at the same time it evaluates and improves performance.
Summary
-
Health system evaluation of the utility of new interventions, procedures, and devices is challenging because of differences in efficacy and effectiveness and in distinguishing the effects of the technology from the clinicians who apply the technology.
-
The current system encourages innovation but has not effectively monitored safety/effectiveness or created ways to optimize interventions.
-
A learning healthcare system should allow for the development/use of novel interventions while they are contemporaneously evaluated and then modified as needed to be maximally safe and effective.
-
Common approaches to evaluate interventions are ineffective because they are not contemporaneous or sensitive enough (i.e., administrative database research) or because they are too costly and challenging to organize (large-scale randomized trials linked to coverage), but coverage on registry may be an effective tool especially if linked to regional collaboratives of engaged clinicians.
-
Regional collaboratives that create a surveillance system assessing interventions and incorporating self-correction to improve performance offer the best elements of a learning healthcare system and should be encouraged.
-
Policy opportunities to encourage regional collaboratives:
-
Medicare and other payer demonstration projects providing payer-based incentives for participating members and hospitals.
-
-
-
Recognizing acute care delivery in current initiatives rewarding regional quality improvement (Chartered Healthcare Value Exchange).
-
State-level protections of data from medicolegal exposure if used only for the purposes of quality improvement and recognizing the importance of reasonable, rather than complete, transparency of the data these collaboratives generate.
-
ANTIPSYCHOTIC THERAPEUTICS
Philip S. Wang, M.D., Dr.P.H., National Institute of Mental Health and Harvard Medical School; M. Alan Brookhart, Ph.D., Harvard Medical School; Soko Setoguchi, M.D., Dr.P.H., Harvard Medical School; Christine Ulbricht, B.A., National Institute of Mental Health; Sebastian Schneeweiss, M.D., Sc.D., Harvard Medical School
Abstract
Antipsychotic medications are now widely utilized by patients and account for a large proportion of pharmaceutical spending, particularly in public healthcare programs. In spite of this, there is a paucity of evidence on the clinical effectiveness of antipsychotic regimens to help guide clinical, purchasing, and policy decisions. Fortunately, there have been advances in the populations, databases, study designs, and analytic methods that investigators can employ to help ensure that antipsychotic medication use is clinically effective. Several studies are raised to illustrate the potential of these developments. Findings from the recently completed NIMH-sponsored comparative effectiveness trials of antipsychotic medications in patients with schizophrenia (the CATIE schizophrenia trial) and Alzheimer’s dementia (the CATIE-AD trial) are described. An example of using clinical epidemiologic data and methods when trial data are not available—in this instance to determine if conventional and atypical antipsychotics share the same mortality risks in elderly patients—is also covered. Likewise, a study of the impact of limiting psychotropic prescriptions on patients with schizophrenia using quasi-experimental methods illustrates their utility when actual trials may not be feasible. Finally, simulation methods are raised as a means to answer questions concerning antipsychotic effectiveness when trials, clinical epidemiologic and even quasi-experimental studies may not be possible—in this case to shed light on the clinical effectiveness of a hypothetical strategy of using clozapine as a first-line antipsychotic agent.
Introduction
Both the Institute of Medicine’s Roundtable on Value & Science-Driven Health Care and the Congressional Budget Office (2007) have raised the importance of research to improve the clinical effectiveness of medical treatments. Nowhere is the need for such research greater than in the case of antipsychotic medication regimens used to treat schizophrenia spectrum and other psychotic disorders. The remainder of this chapter provides a brief description of the reasons why this clinical effectiveness research is necessary, recent advances in the research armamentarium available to conduct such investigations, and examples of how these research designs and methods have been applied in studies of antipsychotic medication effectiveness.
Clinical Effectiveness Research on Antipsychotic Medications to Improve Practice and Inform Policy
Synthesis of the first antipsychotic medication, chlorpromazine, in 1954 launched the modern era of pharmacotherapy for schizophrenia as well as other mental disorders (Schatzberg and Nemeroff, 2006). Thereafter, numerous related “first-generation” neuroleptic drugs were developed that all blocked dopamine-2 receptors in the central nervous system. In the late 1980s, a newer “second-generation” of antipsychotic medications was developed that promised to improve upon the earlier, conventional drugs—hence their designation as “atypical” agents. While most atypicals are distinguishable only by their side effect profiles, one agent, clozapine, has been found to not only possess superior efficacy for treating refractory schizophrenia but also increases the risk for agranulocytosis (Kane et al., 1988).
In spite of a half-century of experience with antipsychotic medications, data on their clinical effectiveness are urgently needed for several reasons. First, in the absence of information on the effectiveness of agents—especially compared to available alternatives—practice decisions are often made on the basis of efficacy and safety data that may not represent the outcomes achievable in typical patients or circumstances. For example, earlier randomized controlled clinical trials suggested that the newer second-generation atypical antipsychotics were less likely than the older conventional neuroleptics to cause side effects such as extrapyramidal symptoms and were possibly more efficacious for the negative symptoms of schizophrenia (Leucht et al., 1999). On the basis of such data as well as their promotion as being safer and more tolerable, atypical use increased rapidly in the second half of the 1990s and soon accounted for the majority of antipsychotic use (Wang et al., 2000)—many years before findings from
comparative effectiveness trials such as the NIMH CATIE study became available (Lieberman et al., 2005). When even RCT data do not exist, practice decisions are often made on the basis of anecdote or judgment. An analysis of a nationally generalizable sample of U.S. psychiatrists revealed that by the late 1990s over 1 in 6 patients with schizophrenia spectrum disorders were being given standing regimens of multiple concurrent anti-psychotics, despite the lack of data on the effectiveness or safety of this practice (Wang et al., 2000). In fact, some emerging evidence suggests the polypharmacy regimen of clozapine plus risperidone may not be superior to clozapine alone (Honer et al., 2006).
In part because of rapid adoption and diffusion of new regimens before sufficient clinical effectiveness data are available, antipsychotic medication costs have increased dramatically especially in public programs such as Medicare and Medicaid. According to the Congressional Budget Office, federal spending on Medicare and Medicaid as a share of gross domestic product has tripled over the past 30 years, rising from 1.3 percent in 1975 to 4 percent in 2007; this spending is projected to continue increasing to 12 percent of gross domestic product (GDP) by 2050 under current policies (Congressional Budget Office, 2007). Spending on just atypicals comprises nearly 30 percent of total drug expenditures for some Medicaid programs (Polinski et al., 2007). Without clear data on the effectiveness and safety of antipsychotic regimens, public and private payers are left uncertain if such costs are justified. These challenges were evident in one recent analysis of Medicaid prior authorization policies, a frequently applied means by which insurers attempt to control drug costs (Polinski et al., 2007). In this analysis there appeared to be no consistent relationship between the application of prior authorization policies and overall spending on atypical antipsychotic medications, suggesting that Medicaid programs may not have sufficient data on their comparative effectiveness to know whether their use should be promoted or deterred. This study also suggested that a paucity of data may leave payers ill-equipped to respond to new challenges such as emerging drug safety issues or regulatory advisories. In April 2005, the Food and Drug Administration (FDA) issued an advisory describing increased mortality among elderly patients with dementia taking atypical antipsychotics. More than a year later, no state Medicaid program changed its prior authorization policy in response, again suggesting that insufficient clinical effectiveness data exist for policy makers to weigh risks and benefits from regimens and tailor their decisions accordingly (Polinski et al., 2007).
A final reason why new clinical effectiveness research is needed is that in spite of large healthcare expenditures, many people with schizophrenia and other forms of serious mental illness experience unmet needs for effective treatment and poor health outcomes. Studies of the general U.S. popu-
lation have shown that the vast majority of people experiencing a serious psychotic disorder in the prior year fail to receive adequate care (Wang et al., 2002b). Examinations of geographic variation in health care spending and outcomes also suggest many of the treatments received are of poor quality and likely to be ineffective. For example, despite spending the greatest percentage of GDP on health care, there is little evidence that the United States achieves better outcomes; in fact, recent data from the World Health Organization’s World Mental Health Survey indicate the rate of receiving effective treatment in the United States lags behind other developed nations (Wang et al., 2007).
Advances in Clinical Effectiveness Research
How can investigators answer the many remaining questions concerning the clinical effectiveness of antipsychotic medications, enhance practice, and inform policy as well as purchasing decisions? As importantly, how can valid answers—both externally as well as internally—be generated feasibly, quickly, and affordably? Fortunately, there have been recent advances in populations, databases, study designs, and analytic methods that have expanded the armamentarium investigators can draw upon.
New effectiveness trials—called practical clinical trials—can be used to explicitly answer the pressing questions faced by clinicians and decisions makers (March et al., 2005; Tunis et al., 2003). Practical clinical trials compare clinically relevant alternative regimens on a broad range of outcomes in typical patients. Recruiting representative and adequate numbers of such patients from diverse settings has been greatly assisted by establishing practice-based clinical trial networks. For example, building practice-based clinical trial networks in schizophrenia, depression, and bipolar disorder was essential for allowing NIMH to conduct the CATIE, STAR-D, and STEP-BD practical clinical trials (Lieberman et al., 2005; Sachs et al., 2007; Trivedi et al., 2006). Other advances in clinical trial methodology—such as new adaptive designs and cluster randomization (Glynn et al., 2007; Murphy et al., 2007) also have been crucial and offer great promise for conducting new clinical effectiveness research in the future.
Clearly many pressing questions will not be amenable to study through comparative effectiveness trials, either because such trials are not feasible, affordable, or in some cases ethical. For this reason, developments in clinical epidemiology and other clinical effectiveness research methodologies have been important. New study populations and data sources are available for descriptive and analytic studies of mental health treatments. Nationally representative resources include the American Psychiatric Association’s Practice Research Network of psychiatrists and general population surveys such as the National Comorbidity Survey Replication (Wang et al.,
2000, 2005a). Available governmental administrative databases such as from Medicaid programs are often enriched with patients with psychiatric disorders due to the poverty and disability associated with mental illnesses; likewise, available HMO databases are an excellent resource for studying primary care, the most frequent setting for mental health treatments (Wang et al., 2002a). However, taking advantage of these resources for clinical effectiveness research has also required the development of new methodologies. For example, developing new analytic methods for observational studies that offer enhanced control for confounding, such as propensity scores and instrumental variable techniques, is crucial (Brookhart et al., 2007; Sturmer et al., 2007). Likewise, advances in the methodologies available for conducting quasi-experimental studies and simulation studies are critical (Gold et al., 1996; Schneeweiss et al., 2001).
Examples of Clinical Effectiveness Research on Antipsychotic Medications
The remainder of this article covers studies that illustrate how these developments in populations, databases, study designs, and analytic methods could be used to improve the clinical effectiveness of antipsychotic medications.
Comparative Effectiveness Trials of Antipsychotic Medications
The NIMH CATIE Schizophrenia Trial. As mentioned above, earlier randomized controlled efficacy trials had suggested that atypical agents might cause fewer extrapyramidal side effects and might possibly be better at treating negative symptoms than first-generation neuroleptics (Leucht et al., 1999). However, the largely industry-sponsored trials that were available presented an at times confusing view of the relative advantages and drawbacks of individual agents (Heres et al., 2006). Furthermore, head-to-head data on the comparative effectiveness of antipsychotic medications in real-world patient populations and typical practice conditions were lacking. For this reason, NIMH supported the Clinical Antipsychotic Trial of Intervention Effectiveness (CATIE) trials in schizophrenia. From the outset, the CATIE schizophrenia study was designed to be an effectiveness rather than an efficacy trial. It involved a large (1,460) number of patients with chronic schizophrenia drawn from 57 diverse practice sites in 24 states. Typical patients (e.g., those with comorbid conditions) and practice conditions (e.g., switching treatments and adjunctive medications) were included. Patients were randomized in Phase I to one of four atypical agents (olanzapine, quetiapine, risperidone, and ziprasidone) or an active comparator agent (the conventional drug perphenazine) and followed for 18 months. On the primary outcome of all-cause discontinuation, three-
quarters of all patients were unable to remain on their treatments due to inefficacy or intolerable side effects (Lieberman et al., 2005). Olanzapine was the most effective antipsychotic but this superiority appeared to come at the price of greater weight gain and increases in glucose and lipids. The conventional antipsychotic, perphenazine, was comparable in efficacy to the remaining atypical agents although it was associated with more discontinuation due to extrapyramidal side effects. In a subsequent cost-effectiveness analysis, (Rosenheck et al., 2006) treatment with perphenazine was associated with 20–30 percent lower healthcare costs (largely due to lower drugs costs from available generics) and comparable effectiveness as the second-generation medications.
The NIMH CATIE Alzheimer’s Dementia Trial. Antipsychotic medications are also prescribed to elderly patients with dementia to control behavioral disturbances. The frequency of neuroleptic prescribing led to federal legislation in the 1980s restricting such use (Schorr et al., 1994). However, the introduction of atypicals and some efficacy studies finding modest improvements in agitation with their use led to atypicals becoming the pharmacologic treatment of choice for behavioral disturbances in dementia patients (Alexopoulos et al., 2004; Jeste et al., 2005; Kindermann et al., 2002; Sink et al., 2005). One recent study estimated that one-quarter of Medicare beneficiaries in nursing homes are given atypical agents (Breisacher et al., 2005).
To shed light on the relative benefits and risks of such practices, NIMH also conducted a comparative effectiveness trial of atypical antipsychotic medications used in Alzheimer’s dementia (CATIE-AD). Outpatients (421) were drawn from 42 practice sites and randomized to olanzapine, quetiapine, risperidone, or placebo and followed for up to 36 weeks. No differences were found between arms on the time to all-cause discontinuation. Although olanzapine and risperidone appeared to possess greater efficacy than quetiapine or placebo, these advantages were offset by more adverse effects (Schneider et al., 2006). In a cost-effectiveness analysis, there were no differences in effectiveness between active treatments or placebo, but there were significantly lower healthcare costs for patients assigned to placebo (Rosenheck et al., 2007).
Clinical Epidemiology Studies of Antipsychotic Effectiveness
Mortality Risks from Conventional versus Atypical Antipsychotics in the Elderly. Strengths of the CATIE trials—including their large number of typical patients drawn from diverse practice sites and observed over long follow-up periods—also contributed to high costs, in excess of $50 million. While there continues to be a pressing need for data on the comparative effectiveness of antipsychotic regimens, such costs, the time required for completion, and
other challenges of conducting large practical clinical trials make it clear that additional means will be needed to answer urgent public health questions.
A study of the short-term mortality associated with conventional antipsychotic use by elderly patients illustrates how clinical epidemiology might be used when data from comparative effectiveness trials are not available or possible. In 2005, the FDA issued an advisory warning that the atypicals aripiprazole, olanzapine, quetiapine, and risperidone were associated with a 60–70 percent increased risk of death versus placebo in 17 short-term randomized placebo-controlled trials among elderly dementia patients (FDA, 2005). “Black box” warnings were added to the labels of all atypical antipsychotics describing these risks and advising that atypicals were not approved for behavioral symptoms from dementia in elderly patients. A meta-analysis by Schneider and colleagues (Schneider et al., 2005) of 15 short-term randomized controlled trials also found a statistically significant 54 percent increased relative risk of death and 1 percent absolute risk difference for atypical antipsychotics versus placebo.
Because of insufficient clinical trial data on the mortality associated with conventional antipsychotics in elderly dementia patients, the FDA did not include these agents in its advisory (FDA, 2005; Kuehn, 2005). For this reason, clinicians might have simply switched elderly patients to these older agents (Strong, 2005), particularly since their replacement by the newer drugs occurred relatively rapidly and recently (Dewa et al., 2002). However, based mainly on extrapolations from younger populations, some suggested that conventional antipsychotic medications could in theory pose risks equal to or greater than those of the newer drugs in older populations (Chan et al., 1999; Lawlor, 2004; Maixner et al., 1999; Tariot, 1999).
In a clinical epidemiologic study based upon data from the largest U.S. state pharmacy benefit program for the elderly, we found that those initiating conventional agents had a 37 percent greater dose-dependent risk of short-term mortality than those prescribed atypical antipsychotics (Wang et al., 2005b). These results were robust to alternative analytic methods to control for potential confounding, including multivariate Cox models, propensity-score adjustments, and an instrumental variable analysis employing the prescribing physician’s preference for conventional or atypical antipsychotics as the instrument (Brookhart et al., 2007). In spite of confirmatory analyses in other populations and databases (Schneeweiss et al., 2007), the risk of unmeasured or unadjusted confounding cannot be completely excluded in clinical epidemiologic studies. For this reason, a meta-analysis of randomized trials among elderly with dementia that found the conventional agent, haloperidol, increased short-term mortality versus placebo by 107 percent (a risk numerically greater than that seen for atypical agents) provides some additional reassurance concerning the internal validity of these clinical epidemiologic findings (Schneider et al., 2005).
Quasi-Experimental Studies of Antipsychotic Effectiveness
Impact of Limiting Psychotropic Prescription Benefits on Patients with Schizophrenia. Quasi-experimental studies are another promising means for improving the effectiveness of antipsychotic regimens, particularly by informing the design of sound public policies. One illustrative example examined the impacts of imposing a three-prescription-per-month cap for psychotropic drugs on Medicaid beneficiaries with schizophrenia (Soumerai et al., 1994). Interrupted time series regression analyses were employed to examine changes in the rates of medication and other healthcare utilization—from a baseline 14-month period prior to the prescription cap’s implementation, to the 11 months during its application, as well as to a 17-month period after the cap was discontinued. To control for background temporal trends in the use of psychotropic medications and other forms of health care, the investigators employed a comparison cohort from a state with no restrictions on drug reimbursement during the study periods.
Results from this study indicated that this form of limiting psychotropic prescription drug coverage significantly reduced antipsychotic medication consumption by 15 percent. Implementing the cap was also associated with a significant 57 percent increase in the frequency of visits to community mental health centers as well as a sharp increase in the use of emergency mental health services and partial hospitalizations. Use of antipsychotic and other psychotropic medications, as well as most mental health services, returned to their baseline levels after the psychotropic prescription cap policy was abandoned. An accompanying economic analysis indicated the increase in total mental healthcare costs per patient to Medicaid during the cap exceeded the savings in drug costs by a factor of 17.
Simulation Studies of Antipsychotic Effectiveness
The Cost-Effectiveness of Using Clozapine as a First-Line Versus Third-Line Antipsychotic. Clearly, clinical epidemiologic studies and quasi-experimental studies provide useful means for improving the effectiveness of antipsychotic medication practices and policies when comparative effectiveness trials may not be available, affordable, or feasible. However there also are many questions concerning the clinical effectiveness of antipsychotics that may not be answerable, even by clinical epidemiologic or quasi-experimental designs. This is especially true for hypothetical antipsychotic medication strategies or practices for which empirical data are absent. In such situations, simulation studies may be the only alternative available to shed light on the clinical effectiveness of regimens.
One such hypothetical regimen involves using the atypical clozapine as a first-line agent. Since its introduction in the late 1980s, clozapine has
been restricted to only patients who have failed at least two trials of other antipsychotic medications because of concerns that its use as a first-line agent would lead to greater mortality, mainly through agranulocytosis. Another requirement initially imposed because of this risk was that patients have their white blood cell (WBC) counts checked weekly prior to receiving each week’s prescription. Because of such requirements, clozapine has been underutilized even among treatment-resistant patients with schizophrenia (Conley and Buchanan, 1997).
However, since these restrictions on clozapine were imposed, additional evidence has emerged. A meta-analysis (Wahlbeck et al., 1999) and other RCT data (Lieberman et al., 2003) from treatment-sensitive as well as treatment-resistant patients, found that clozapine is significantly more likely than conventional antipsychotics to improve psychotic episodes and prevent relapse. Data from the Clozaril National Registry have shown that the incidence of agranulocytosis and fatality resulting from it are substantially lower than originally feared (Honigfeld et al., 1998). This has led to reductions in the requirements for WBC monitoring and costs associated with clozapine therapy. Clozapine has been shown to be relatively free of extrapyramidal side effects and may be a treatment for tardive dyskinesia (Lieberman et al., 1991). It also has been associated with lower rates of suicide attempts and completed suicides (Meltzer and Fatemi, 1995; Meltzer and Okayli, 1995; Meltzer et al., 2003; Walker et al., 1997). Finally, generic forms of clozapine have now become available, further lowering its cost.
Whether these potentially greater benefits as well as lower risks and costs for clozapine could justify its expanded use as a hypothetical first-line agent in treatment-sensitive patients remains a question for which empirical data are lacking. For that reason, we employed a simulation model to assess the effectiveness and costs of using clozapine as a potential first-line treatment for schizophrenia, relative to the current practice of restricting clozapine for only patients who have failed two trials of other antipsychotics (Wang et al., 2004). A Markov model was created based upon available RCTs and epidemiologic data and was used to track the clinical and economic outcomes of these two strategies in a hypothetical cohort of patients with schizophrenia undergoing an acute psychotic episode. Results of this simulation showed that using clozapine as a first agent would lead to modest gains in life expectancy as well as in quality-adjusted life expectancy, relative to restricting its use to patients who failed two other antipsychotics. The cost-effectiveness ratio of using clozapine first versus using clozapine third was $24,100 per quality-adjusted life year (QALY), well within conventional benchmarks used to determine whether healthcare interventions may be worth investing in. However, there is often great uncertainty with the inputs in such simulation models. For this reason, it was reassuring that
in both one-way and probabilistic sensitivity analyses, the base-case findings from this simulation study were robust to a wide variety of assumptions.
Conclusions
Throughout the IOM Roundtable on Value & Science-Driven Health Care workshop on Redesigning the Clinical Effectiveness Research Paradigm, the breadth, depth, and pressing nature of needs for new clinical effectiveness research on medical treatments was evident. As this chapter illustrates, the need for such research on antipsychotic medications is no exception. Such research is critical to enhance practice, inform policy and purchasing decisions, and ultimately improve the health outcomes experienced by extremely vulnerable populations like those with psychotic disorders. Advances in the armamentarium available to conduct such research provide some grounds for optimism that these needs can be met in the future.
CANCER SCREENING
Peter B. Bach, M.D., M.A.P.P.
Memorial Sloan-Kettering Cancer Center
New Cancer Screening Tests: Challenges for Evidence
In the context of clinical medicine or typical practice, clinical disease usually “presents.” That is, patients arrive with symptoms or signs—fever and night sweats with a cough, or a fractured limb—and thus the “afflicted population” is constituted of those individuals presenting with frank manifestations of their condition. However, a strong argument can be made for looking for preclinical conditions; in other words, the patient who should receive a vaccination against pneumonia before he/she develops fever and night sweats, or a patient who is at risk for falls before he/she breaks a limb. This is what screening is intended to do—essentially scan an unaffected population to look for people who are at risk for developing some condition. The underlying general rationale of screening is that we can decrease morbidity and mortality and other negative outcomes by looking for patients with preclinical conditions, as the interventions at that point reduce future negative health outcomes.
One could argue that screening is in fact the dominant activity in much of primary care. For example, testing for serum glucose levels in patients to look for diabetes in asymptomatic individuals—largely those without polyuria, polydypsia, or any other presentation of diabetes. In such cases, blood tests are ordered for patients who feel fine in order to
scan for relevant preclinical conditions. Similarly, the pap smear looks primarily for predisease, such as dysplastic cells in the epithelium; it also looks for invasive and noninvasive cancers. A third example is that of the electrocardiogram (EKG). Included in the “Welcome to Medicare” visit, for example, an EKG performed in a patient without cardiac symptoms not only looks for a number of cardiac defects, including conduction abnormalities, but also screens for undiagnosed or silent coronary disease. Fundamentally, such screens look for a precondition in a patient who has no symptoms or “clinical presentation.”
Screening encompasses a large range of today’s medical activities. At one end are basic screening questions that physicians are to ask their patients. Such questions range from inquiries about gun ownership in the home and the presence of a swimming pool to questions about a family history of cancer. They all fall under the rubric of screening, in that they provide an avenue through which clinicians can gauge the risk for someone being shot in the home or drowning in the swimming pool or develop cancer—all with an expectation that those risks can be altered once they are known about.
At the other end are several different kinds of tests. Radiologic surveys, such as whole body computed tomography (CT) scans or magnetic resonance angiography of the cerebral circulation in a patient with no history of cerebral ischemia are one kind. Genetic profiles are another. In oncology, the test for the BRCA mutations has become increasingly popular, despite genuine uncertainty regarding the extent to which its presence predicts the development of either breast or ovarian cancer (Begg et al., 2008).
Value from Screening: Case Finding, Surrogate Markers, End-point Alteration
To determine the value of screening tests is difficult, whether the test is a questionnaire or an expensive diagnostic test being used off-label for screening. The problem begins with finding agreement on the intent of the screening evaluation: Is it intended to merely detect individuals at risk (i.e., find “cases”), or is its value predicated on its ability to alter the natural history of the condition that is being screened (i.e., “end-point alteration”). To be certain, screening tests are often evaluated purely for their ability to find cases, or individuals with preclinical disease. For example, the way one evaluates different questionnaires to screen for alcoholism is to determine how frequently, when applying this test, researchers are likely to find people who are alcoholics. While such information is vitally important to the process of care, it provides very little evidence that screening patients for alcoholism helps the patient, or is a good use of medical resources. What one aims to do, however, is to identify an individual at excess risk of
health consequences of alcohol use, such as liver failure, then intervene to decrease the amount drunk and hence the risk of these complication. Finding people who are alcoholics (i.e., “case finding”) does not ensure that the risk of liver failure is reduced.
Because case finding does not necessarily alter the end-point, screening tests are often evaluated for their ability to lead to an action—usually some kind of active prevention—that should then mitigate the risk of the actual end-point. This makes the “action” a surrogate marker of benefit. In the example of alcohol use, a surrogate marker of benefit might be that people found to have alcoholism through screening are fairly likely to enroll in Alcoholics Anonymous when their physician, who screened them, recommends it.
But, it has to be appreciated that enrollment in Alcoholics Anonymous is still a surrogate for a health benefit achieved by screening patients for alcoholism and then referring them to the program. To truly know if one of these screening evaluation yields a benefit, its use would have to be linked to reductions in liver failure or other alcohol-related complications. Enrollment in Alcoholics Anonymous is just a predictor (of uncertain correlation) with the benefit. In truth, one does not know whether or not the questionnaire detected alcoholics who really are as likely to develop liver failure and other complications as alcoholics who enroll in Alcoholics Anonymous after some other event, such as a traffic accident.
So, the most relevant approach to evaluating a screening test is precisely the same as the manner in which any medical intervention should be evaluated: Determine whether or not the intervention alters the clinical outcome. The ideal way to do this is by determining whether or not the use of the screen decreases, for instance, the frequency of the complication. In the case of alcoholism screening, the end-point would be the occurrence of a medical or social complication of alcoholism. In cancer screening, death due to the disease is typically the end-point of interest. Because the net effect of the screen, the actions taken as a result of the screen, and the end-point are assessed together, a comparator is needed, and so some sort of comparative study of screened and unscreened individuals must be run. In cancer screening trials, for example, determining if a screened subject’s risk of dying of cancer is reduced relative to a scenario where they had not been screened is the goal—most often achieved by a randomized comparative trial. Comparators can come from a variety of other sources, however, such as a parallel or historical population, but for screening approaches, the randomized comparator is the least fraught with bias.
Even though there is an established gold standard for the evaluation of screening tests, there is no guarantee that such comparative trials, be they randomized or historic, are done before screening tests are adopted. For instance, the PSA test for prostate cancer has yet to be evaluated in a
randomized trial where it can be determined if screening with PSA, finding prostate cancer through screening, and treating it reduces the risk a screened man will die of prostate cancer (there is one such study—the PLCO—ongoing).
Take the more dynamic example of lung cancer screening. The Princeton Longevity Center, for instance, advertises lung cancer screening. Its website includes some statements of fact about the lung cancer mortality rates in the United States. The website also cites, correctly, mortality rates from different cancers. It also quotes a prominent advocate of CT screening stating that “the current 5-year survival rate for lung cancer is only 14 percent, but that could soar to 80 percent if all smokers received annual CT exams and early treatment.” Beneath all this the website states that lung scans are recommended for cigarettes or cigar smokers, those with a history of tuberculosis or pneumonia, and for nonsmokers with exposure to secondhand smoke or exposure to asbestos or radon—in other words, a relatively large fraction of the adult population (www.theplc.net). The site notably omits the fact that lung cancer screening has been evaluated by numerous organizations, and not one recommends it (Table 2-7).
Why might this website promote CT screening while recommending bodies do not? The answer lies in the continuum of possible evaluations of screening. In lung cancer screening, particularly given the risks and costs, the appropriate end-point for benefit evaluation is disease-specific mortality and the appropriate comparator is an un-screened group. The randomized studies are still ongoing. Meanwhile, advocates of screening are accepting case finding and other weak surrogates of benefit, such as disease-specific survival of cases, as evidence of benefit.
The data on case finding are impressive, as are the surrogate measures of benefit. Several high-quality studies for instance have demonstrated that CT scanning identifies numerous small foci of lung cancer in smokers, averaging 1–2 percent of CT screenings (Swensen et al., 2003). In the pilot study of the National Lung Screening Trial, nearly half of all lung cancers found through screening were early stage (Gohagan et al., 2005). Most of these cancers are conventionally considered to be highly treatable: so-called “resectable” cancers that practitioners in cancer care would like to encounter more often than they typically do.
Several studies also have demonstrated an impressive surrogate endpoint—excellent survival after treatment of small cancers found by CT screening (Henschke, 2007). These studies have suggested that disease-specific survival is above 80 percent at 5 years for individuals with early stage cancer found by CT. This contrasts to a 15 percent 5-year survival probability in epidemiologic or cancer registry data, where more than 75 percent of cancers found are advanced and therefore incurable. So,
TABLE 2-7 Recommendations for Low-Dose CT (LDCT) Scans by Leading Medical Organizations
|
Recommending Body |
Recommendation |
|
National Cancer Institute (www.cancer.gov/cancertopics/pdq/screening/lung/healthprofessional) |
The evidence is inadequate to determine whether screening reduces mortality from lung cancer. On the basis of solid evidence, screening would lead to false-positive results and unnecessary invasive diagnostic procedures and treatments. |
|
American Cancer Society (Smith RA, Cokkinides V, Eyre HJ. American Cancer Society guidelines for early detection of cancer, 2005. CA Cancer J Clin 2005; 55:31-44) |
Lung cancer screening is not a routine practice for the general public or even for people who are at increased risk, such as smokers. |
|
U.S. Preventive Services Task Force (www.ahrq.gov/clinic/uspstf/uspslung.htm) |
The evidence is insufficient to recommend for or against screening asymptomatic individuals for lung cancer with LDCT, chest x-ray, sputum cytology, or a combination of these tests. |
|
Canadian Coordination Office for Health Technology Assessment (www.cadth.ca/media/pdf/213_ct_cetap_e.pdf) |
Evidence does not exist to suggest that detecting early-stage lung cancer reduces mortality. |
|
American College of Chest Physicians |
Not recommended outside of well-designed clinical trial. |
|
Society of Thoracic Radiology (Aberle D, Gamsu, Henschke C, et al. A consensus statement of the Society of Thoracic Radiology: screening for lung cancer with helical computed tomography. J Thorac Imaging 2001; 16:65-68) |
Mass screening for lung cancer is not currently advocated. Suitable subjects who wish to participate should be encouraged to do so in controlled trials so that the value of CT screening can be ascertained as soon as possible. |
|
NOTE: Modified and reprinted with permission from Copyright Clearance Center, Copyright © 2007. SOURCE: Bach, P. B., G. A. Silvestri, M. Hanger, and J. R. Jett. 2007b. Screening for lung cancer: American College of Chest Physicians evidence-based clinical practice guidelines (2nd edition). Chest 132(3 Suppl):69S-77S. |
|
these studies are good news, but they are nonetheless focusing on case finding and surrogate measures of benefit.
My colleagues and I published a paper in 2007 that examined the interrelationship between case finding, surrogate end-points of stage distribution and case survival, and an actual measure of screening’s benefits: the extent
to which it alters disease-specific mortality. Our findings emphasize just how misleading the intermediate measures of benefit can be. The paper, which was the first comparative assessment of CT screening for lung cancer, used a computer simulation model to estimate what would have happened in the absence of screening among 3,000 individuals who were screened (Bach et al., 2007a). The model’s predictions come from multi-variable models based on data from a large randomized trial conducted by the National Cancer Institute (NCI), and had hundreds of thousands of person-years and more than a thousand events. The predictors are age, smoking status (duration and intensity), and asbestos exposure. We validated these models in several studies before undertaking this analysis (Bach and Begg, 2006; Bach et al., 2003, 2004; Cronin et al., 2006). The validations demonstrated that the models predict within a few percentage points (worst case, plus or minus 8 percent) the number of events that would occur in current and former smokers in the relevant age over time in the absence of screening.
In the study, we documented that the prior assessments that had been done were correct—we revalidated that screening populations with CT locates a large proportion of early-stage cancers—in our case, 65 percent of cancer detected were early stage. We also reaffirmed that screen-detected cases have an excellent survival rate. When we considered only the early cancers, the survival rate was 94 percent. These are clearly spectacular outcomes, and they match all of the prior studies. The results were rather different, however, when we looked at end-points that screening is intended to affect—there was neither a reduction in advanced cancers nor in deaths from lung cancer. We found no evidence that CT screening intercepted early cases before they became advanced, and we did not find that screening and early treatment led to a reduction in deaths from lung cancer.
Figure 2-5 shows one of these results. The x-axis represents years, the solid lines are the observed counts, and the dotted lines are the models’ predictions. There is a marked increase in the number of lung cancer diagnoses relative to what would have been seen absent screening. But, there was no “stage shift” or substitution—the same number of advanced cancers were encountered as would have been seen in the absence of screening.
Of particular importance in this analysis is that the risk ratio for cases found by screening relative to what would occur in the absence of screening has continued to diverge over time, exceeding 2.5 in each year of followup. The overall risk ratio is about 3.2. This result can only be explained if screening is finding cancers that otherwise would not have appeared clinically, and so this is one of the fallacies of case finding as a metric of benefit: Screening tests can find cases that are not precursors of serious disease, and thus finding these cases cannot benefit the patient.
While occurrence of advanced cancer is a legitimate end-point, lung cancer death is the health outcome of greatest importance. Figure 2-6
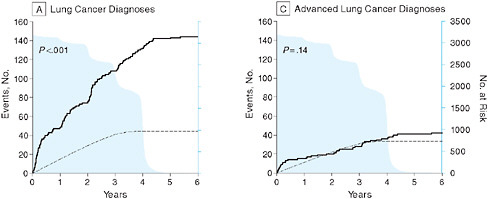
FIGURE 2-5 Lung cancer data indicating that a large increase in findings of cases through screening (A) did not lead to a reduction in advanced cancer diagnoses (C).
SOURCE: Bach, P. B., J. R. Jett, U. Pastorino, M. S. Tockman, S. J. Swensen, et al. 2007. Computed tomography screening and lung cancer outcomes. Journal of the American Medical Association 297(9):953-961. Copyright © 2007 American Medical Association. All rights reserved.
compares the number of lung cancer resections of early-stage cancers performed in this population relative to what was expected in the absence of screening. There was a 10-fold increase resulting from screening. These additional treatments are reasonably considered the action that should result in benefit. Thus, in a paradigm where screening is evaluated purely
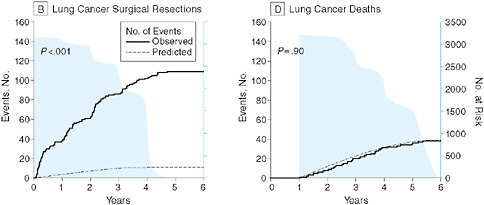
FIGURE 2-6 Treatment of early lung cancer cases (B) not averting death (D).
SOURCE: Bach, P. B., J. R. Jett, U. Pastorino, M. S. Tockman, S. J. Swensen, et al. 2007. Computed tomography screening and lung cancer outcomes. Journal of the American Medical Association 297(9):953-961. Copyright © 2007 American Medical Association. All rights reserved.
through surrogates, a finding that an increased number of patients were treated could be construed as a marker of screening’s benefits. Yet, in this same population, as can be seen in the figure, there was no reduction in the number of lung cancer deaths relative to what was predicted. The counts of predicted and observed deaths were essentially perfectly matched, at 38.8 predicted and 38.0 observed. So, even though there is clearly an uptick in the action that should lead to benefit, there is no evidence that added action results in better outcomes for the screened population.
We derive several concluding observations about screening. First, the paradoxical reality of surrogate end-points is that they are readily available but can be misleading. Simply stated, screening often picks up pseudodisease, disease that is often too quickly characterized as “early” or “curable,” rather than clinically unimportant or benign. In point of fact, lung cancer screening provides something of a textbook example, in that as one begins to look for conditions and apply tests that are imperfect or that rely on what might be characterized as amplifying weak and potentially uncertain signals, one will stumble upon abnormalities that have no clinical future. This appears to be happening in lung cancer screening to a very significant extent. There are similar examples where cancer screening has led to upticks in case finding, but the extent to which this leads to appropriate action that reduces disease-specific mortality is uncertain. In each case, as you begin to look, you find much more disease than you would expect, and more than can frankly progress to cause clinical conditions or death.
In other words, to evaluate screening methods, appropriate comparators are needed because they are illuminating, while surrogate end-points such as case finding are deceptive. To obtain an appropriate comparator, randomized controlled trials are the gold standard. But, alternatives, such as historical controls, parallel controls, or modeled controls (as in our example), can also be informative.
Doing comparative studies for screening tests is hard, however. In the case of lung cancer screening, the fact that there is broad equipoise made it possible for the NCI to launch a multicenter randomized trial comparing CT screening to chest-X-ray screening, and for several European countries to launch trials comparing CT to no screening. But even in lung screening, there has been resistance to the trial by pro-screening groups, and widespread advertising of CT screening as “proven” even though the trial is ongoing. In general terms, randomized trials will not always be done, and so it is important to construct approaches to the evaluation of screening tests that are sufficiently valid in the absence of randomization. As we showed in our study, which took advantage of the relatively unique linkage between lung cancer risk and reportable risk factors, we were able to assess the impact of CT screening without randomization to some extent, and at
least from that we could determine that there would at best be substantial trade-offs between harms and benefits if screening were undertaken.
The evaluation of neuroblastoma screening and of the Pap smear were assessed using comparisons between population mortality rates due to the disease between those who were and were not screened (using either historical or parallel populations)—something only achievable when widespread screening has already been adopted. In each case, the results were convincing. More commonly, there will be neither a feasible mechanism for randomizing patients nor sufficient adoption to gauge changes in population mortality rates. In these situations, single arm registry–based trials are conceivably a reasonable way to assess the consequences of screening. Such an approach could work as long as the expected rates of death from the disease are known in advance, and the entire population of screened individuals are followed successfully, to ascertain the rates. Other problems include the reality that only a handful of conditions lend themselves well to determining “expected rates,” and that few screening tests can really be disentangled from the cascade of events that follow them to a sufficient extent to determine whether or not the finding of additional cases through screening was responsible for the benefits or harms that occurred.
Whether or not payment policy or regulation can enhance learning about screening technologies prior to their wide adoption is also a challenging question. Payers will find that mandating registry participation or enrollment in a trial will promote the ire of screening advocates, who are particularly skilled at mobilizing advocacy groups. Moreover, many unproven screening tests are affordable to some, even though the procedures and tests they trigger can add up to great expense. For instance, phone calls to the various I-ELCAP lung cancer screening sites demonstrated that most of the centers ask for $400 to $500 for a single screening test, so individuals with high net worth will obtain these tests without reimbursement.
For most patients, such tests are out of reach financially, and so restriction of payment, or requirements for coverage with evidence development (CED) may be an effective way of ensuring that screening tests are assessed using appropriate endpoints at the time they are being introduced. For instance, CMS recently announced a proposed national coverage of computed tomography coronary arteriography under CED—the design of the assessment has not been articulated, but the notion that coverage of the technology may be coupled to an evaluation of it is very promising.
Regulatory routes are also something that could be considered, but policies would need to change. For instance, the FDA could hold screening assays and tests to a standard of demonstrated benefit, rather than the current lower standards. At best such an approach could limit the introduction of new tests that have no current usages until the right assessments are
done. However, this may constitute too high a bar to introduction for the makers of many of these tests, and thus may stifle enthusiasm for pursuing innovation in prediction and prognostication—an undesirable consequence. Moreover, many of the current screening approaches, such as CT screening of the lung, merely involve the off-label use of an approved device. In this case, the CT scanner is already approved for diagnosis, and the FDA’s ability to limit its use off label is essentially nonexistent. Organized medicine could play a more active role too, emphasizing the importance of following preventive services guidelines when offering screening examinations. But, there are bound to be outliers who are compelled by indirect measures of benefit, no matter what organized bodies conclude about the evidence.
In summary, evaluating screening tests is challenging, and surrogates such as case finding rates are deceptive and always biased in favor of the screening test. It is worth establishing paths for screening tests to be evaluated in a consistent manner before they are widely adopted. Doing so will be difficult. The desire to believe in the paradigm of early detection is strong. But, judicious use of coverage and payment, particularly towards the goal of generating population-based longitudinal data on outcomes among screened groups, compared to a relevant unscreened population, is an avenue that can and should be actively pursued.
REFERENCES
AHRQ (Agency for Healthcare Research and Quality). 2008. Chartered Value Exchanges: Local Networks with National Standards. http://www.ahrq.gov/qual/value/localnetworks.htm (accessed February 19, 2008).
Airoldi, F., A. Colombo, N. Morici, A. Latib, J. Cosgrave, L. Buellesfeld, E. Bonizzoni, M. Carlino, U. Gerckens, C. Godino, G. Melzi, I. Michev, M. Montorfano, G. M. Sangiorgi, A. Qasim, A. Chieffo, C. Briguori, and E. Grube. 2007. Incidence and predictors of drug-eluting stent thrombosis during and after discontinuation of thienopyridine treatment. Circulation 116(7):745-754.
Alexopoulos, G. S., J. Streim, D. Carpenter, and J. P. Docherty. 2004. Using antipsychotic agents in older patients. Journal of Clinical Psychiatry 65(Supl 2):5-99; discussion 100-102; quiz 103-104.
The American Society of Colon and Rectal Surgeons. 1994. Approved statement on laparoscopic colectomy. Diseases of the Colon & Rectum 37:8-12.
Anderson, G. L., M. Limacher, A. R. Assaf, T. Bassford, S. A. Beresford, H. Black, D. Bonds, R. Brunner, R. Brzyski, B. Caan, R. Chlebowski, D. Curb, M. Gass, J. Hays, G. Heiss, S. Hendrix, B. V. Howard, J. Hsia, A. Hubbell, R. Jackson, K. C. Johnson, H. Judd, J. M. Kotchen, L. Kuller, A. Z. LaCroix, D. Lane, R. D. Langer, N. Lasser, C. E. Lewis, J. Manson, K. Margolis, J. Ockene, M. J. O’Sullivan, L. Phillips, R. L. Prentice, C. Ritenbaugh, J. Robbins, J. E. Rossouw, G. Sarto, M. L. Stefanick, L. Van Horn, J. Wactawski-Wende, R. Wallace, and S. Wassertheil-Smoller. 2004. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: The women’s health initiative randomized controlled trial. Journal of the American Medical Association 291(14):1701-1712.
Angerer, P., S. Stork, W. Kothny, P. Schmitt, and C. von Schacky. 2001. Effect of oral post-menopausal hormone replacement on progression of atherosclerosis: A randomized, controlled trial. Arteriosclerosis, Thrombosis, and Vascular Biology 21(2):262-268.
Bach, P. B., and C. B. Begg. 2006. Further validation of lung cancer mortality model. Chest. Published online, March 18, 2005. No longer accessible.
Bach, P. B., M. W. Kattan, M. D. Thornquist, M. G. Kris, R. C. Tate, M. J. Barnett, L. J. Hsieh, and C. B. Begg. 2003. Variations in lung cancer risk among smokers. Journal of the National Cancer Institute 95(6):470-478.
Bach, P. B., E. B. Elkin, U. Pastorino, M. W. Kattan, A. I. Mushlin, C. B. Begg, and D. M. Parkin. 2004. Benchmarking lung cancer mortality rates in current and former smokers. Chest 126(6):1742-1749.
Bach, P. B., J. R. Jett, U. Pastorino, M. S. Tockman, S. J. Swensen, and C. B. Begg. 2007a. Computed tomography screening and lung cancer outcomes. Journal of the American Medical Association 297(9):953-961.297(9):953-961.
Bach, P. B., G. A. Silvestri, M. Hanger, and J. R. Jett. 2007b. Screening for lung cancer: ACCP evidence-based clinical practice guidelines (2nd edition). Chest 132(3 Supl):69S-77S.
Begg, C. B., R. W. Haile, A. Borg, K. E. Malone, P. Concannon, D. C. Thomas, B. Langholz, L. Bernstein, J. H. Olsen, C. F. Lynch, H. Anton-Culver, M. Capanu, X. Liang, A. J. Hummer, C. Sima, and J. L. Bernstein. 2008. Variation of breast cancer risk among BRCA1/2 carriers. Journal of the American Medical Association 299(2):194-201.
Bhatt, D. L., K. A. Fox, W. Hacke, P. B. Berger, H. R. Black, W. E. Boden, P. Cacoub, E. A. Cohen, M. A. Creager, J. D. Easton, M. D. Flather, S. M. Haffner, C. W. Hamm, G. J. Hankey, S. C. Johnston, K. H. Mak, J. L. Mas, G. Montalescot, T. A. Pearson, P. G. Steg, S. R. Steinhubl, M. A. Weber, D. M. Brennan, L. Fabry-Ribaudo, J. Booth, and E. J. Topol. 2006. Clopidogrel and aspirin versus aspirin alone for the prevention of atherothrombotic events. New England Journal of Medicine 354(16):1706-1717.
Birkmeyer, N. J., D. Share, D. A. Campbell, Jr., R. L. Prager, M. Moscucci, and J. D. Birkmeyer. 2005. Partnering with payers to improve surgical quality: The Michigan plan. Surgery 138(5):815-820.
Breisacher, B., R. Limcangco, and L. Simoni-Wastila. 2005. The quality of antipsychotic drug prescribing in nursing homes. Archives of Internal Medicine 165:1280-1285.
Brookhart, M. A., J. A. Rassen, P. S. Wang, C. Dormuth, H. Mogun, and S. Schneeweiss. 2007. Evaluating the validity of an instrumental variable study of neuroleptics: Can between-physician differences in prescribing patterns be used to estimate treatment effects? Medical Care 45(10 Supl 2):S116-S122.
Brownley, K. A., A. L. Hinderliter, S. G. West, K. M. Grewen, J. F. Steege, S. S. Girdler, and K. C. Light. 2004. Cardiovascular effects of 6 months of hormone replacement therapy versus placebo: Differences associated with years since menopause. American Journal of Obstetrics and Gynecology 190(4):1052-1058.
Buchwald, H., Y. Avidor, E. Braunwald, M. D. Jensen, W. Pories, K. Fahrbach, and K. Schoelles. 2004. Bariatric surgery: A systematic review and meta-analysis. American Medical Association 292(14):1724-1737.
Chan, Y. C., S. F. Pariser, and G. Neufeld. 1999. Atypical antipsychotics in older adults. Pharmacotherapy 19(7):811-822.
Commonwealth of Massachusetts Betsy Lehman Center for Patient Safety and Medical Error Reduction Expert Panel on Weight Loss Surgery: Executive report. 2005. Obesity Research 13(2):205-226.
Congressional Budget Office. 2007. Research on the comparative effectiveness of medical treatments: Issues and options for an expanded federal role. Washington, DC: Congress of the United States.
Conley, R. R., and R. W. Buchanan. 1997. Evaluation of treatment-resistant schizophrenia. Schizophrenia Bulletin 23(4):663-674.
Cronin, K., M. H. Gail, Z. Zou, P. B. Bach, J. Virtamo, and D. Albanes. 2006. Validation of a model of lung cancer risk prediction among smokers. Journal of the National Cancer Institute 98:1-4.
Daemen, J., P. Wenaweser, K. Tsuchida, L. Abrecht, S. Vaina, C. Morger, N. Kukreja, P. Juni, G. Sianos, G. Hellige, R. T. van Domburg, O. M. Hess, E. Boersma, B. Meier, S. Windecker, and P. W. Serruys. 2007. Early and late coronary stent thrombosis of sirolimus-eluting and paclitaxel-eluting stents in routine clinical practice: Data from a large two-institutional cohort study. Lancet 369(9562):667-678.
de la Torre-Hernandez, J. M., F. Alfonso, F. Hernandez, J. Elizaga, M. Sanmartin, E. Pinar, I. Lozano, J. M. Vazquez, J. Botas, A. P. de Prado, J. M. Hernandez, J. Sanchis, J. M. Nodar, A. Gomez-Jaume, M. Larman, J. A. Diarte, J. Rodriguez-Collado, J. R. Rumoroso, J. R. Lopez-Minguez, and J. Mauri. 2008. Drug-eluting stent thrombosis: Results from the multicenter spanish registry estrofa (estudio espanol sobre trombosis de stents farmacoactivos). Journal of the American College of Cardiology 51(10):986-990.
Dewa, C. S., G. Remington, N. Herrmann, J. Fearnley, and P. Goering. 2002. How much are atypical antipsychotic agents being used, and do they reach the populations who need them? A Canadian experience. Clinical Therapeutics 24(9):1466-1476.
Estrogen and progestogen use in peri- and postmenopausal women: March 2007 position statement of the North American Menopause Society. 2007. Menopause 14(2):168-182.
Ettinger, B., G. D. Friedman, T. Bush, and C. P. Quesenberry, Jr. 1996. Reduced mortality associated with long-term postmenopausal estrogen therapy. Obstetrics and Gynecology 87(1):6-12.
Executive summary. Hormone therapy. 2004. American Journal of Obstetrics and Gynecology 104(4 Supl):1S-4S.
Farb, A., P. F. Heller, S. Shroff, L. Cheng, F. D. Kolodgie, A. J. Carter, D. S. Scott, J. Froehlich, and R. Virmani. 2001. Pathological analysis of local delivery of paclitaxel via a polymercoated stent. Circulation 104(4):473-479.
FDA (Food and Drug Administration). 2005. FDA Public Health Advisory: Deaths with Antipsychotics in Elderly Patients with Behavioral Disturbances. http://www.fda.gov/cder/drug/advisory/antipsychotics.htm (accessed April 15, 2005).
———. 2008a. Center for Devices and Radiological Health: CYPHER™ Sirolimus-eluting Co-onary Stent on RAPTOR®Over-the-wire Delivery System or RAPTORRAIL®Rapid Exchange Delivery System–p020026. http://www.fda.gov/cdrh/pdf2/P020026.html (accessed May 20, 2008).
———. 2008b. Center for Devices and Radiological Health: Device Advice. http://www.fda.gov/cdrh/devadvice/ (accessed May 20, 2008).
———. 2008c. Endeavor®Zotarolimus-eluting Coronary Stent on the Over-the-wire (OTW), Rapid Exchange (RX), or Multi Exchange II (MX2) Stent Delivery Systems–p060033. http://www.fda.gov/cdrh/pdf6/P060033.html (accessed May 20, 2008).
———. 2008d. Jurisdictional update: Drug-eluting Cardiovascular Stents. http://www.fda.gov/oc/combination/stents.html (accessed May 20, 2008).
———. 2008e. TAXUS™ Express 2™ Paclitaxel-eluting Coronary Stent System (Monorai and Over-the-wire)–p030025 Name. http://www.fda.gov/cdrh/pdf3/P030025.html (accessed May 20, 2008).
———. 2008f. Title 21: Chapter 1—food and drugs, subchapter a—general, part 3—product jurisdiction. In Code of Federal Regulations. Washington, DC: Food and Drug Administration, Department of Health and Human Services.
Finn, A. V., G. Nakazawa, M. Joner, F. D. Kolodgie, E. K. Mont, H. K. Gold, and R. Virmani. 2007. Vascular responses to drug eluting stents: Importance of delayed healing. Arteriosclerosis, Thrombosis, and Vascular Biology 27(7):1500-1510.
Fishman, A., F. Martinez, K. Naunheim, S. Piantadosi, R. Wise, A. Ries, G. Weinmann, and D. E. Wood. 2003. A randomized trial comparing lung-volume-reduction surgery with medical therapy for severe emphysema. New England Journal of Medicine 348(21):2059-2073.
Flum, D. R., and E. P. Dellinger. 2004. Impact of gastric bypass operation on survival: A population-based analysis. Journal of the American College of Surgeons 199(4):543-551.
Flum, D. R., T. Koepsell, P. Heagerty, M. Sinanan, and E. P. Dellinger. 2001a. Common bile duct injury during laparoscopic cholecystectomy and the use of intraoperative cholangiography: Adverse outcome or preventable error? Archives of Surgery 136(11):1287-1292.
Flum, D. R., A. Morris, T. Koepsell, and E. P. Dellinger. 2001b. Has misdiagnosis of appendicitis decreased over time? A population-based analysis. Journal of the American Medical Association 286(14):1748-1753.
Flum, D. R., T. Koepsell, P. Heagerty, and C. A. Pellegrini. 2002. The nationwide frequency of major adverse outcomes in antireflux surgery and the role of surgeon experience, 1992-1997. Journal of the American College of Surgeons 195(5):611-618.
Flum, D. R., K. Horvath, and T. Koepsell. 2003. Have outcomes of incisional hernia repair improved with time? A population-based analysis. Annals of Surgery 237(1):129-135.
Flum, D. R., N. Fisher, J. Thompson, M. Marcus-Smith, M. Florence, and C. A. Pellegrini. 2005a. Washington state’s approach to variability in surgical processes/outcomes: Surgical clinical outcomes assessment program (SCOAP). Surgery 138(5):821-828.
Flum, D. R., L. Salem, J. A. Elrod, E. P. Dellinger, A. Cheadle, and L. Chan. 2005b. Early mortality among medicare beneficiaries undergoing bariatric surgical procedures. Journal of the American Medical Association 294(15):1903-1908.
Fox, K. A., S. R. Mehta, R. Peters, F. Zhao, N. Lakkis, B. J. Gersh, and S. Yusuf. 2004. Benefits and risks of the combination of clopidogrel and aspirin in patients undergoing surgical revascularization for non-st-elevation acute coronary syndrome: The clopidogrel in unstable angina to prevent recurrent ischemic events (cure) trial. Circulation 110(10):1202-1208.
Glynn, R. J., M. A. Brookhart, M. Stedman, J. Avorn, and D. H. Solomon. 2007. Design of cluster-randomized trials of quality improvement interventions aimed at medical care providers. Medical Care 45(10 Supl 2):S38-S43.
Gohagan, J. K., P. M. Marcus, R. M. Fagerstrom, P. F. Pinsky, B. S. Kramer, P. C. Prorok, S. Ascher, W. Bailey, B. Brewer, T. Church, D. Engelhard, M. Ford, M. Fouad, M. Freedman, E. Gelmann, D. Gierada, W. Hocking, S. Inampudi, B. Irons, C. C. Johnson, A. Jones, G. Kucera, P. Kvale, K. Lappe, W. Manor, A. Moore, H. Nath, S. Neff, M. Oken, M. Plunkett, H. Price, D. Reding, T. Riley, M. Schwartz, D. Spizarny, R. Yoffie, and C. Zylak. 2005. Final results of the lung screening study, a randomized feasibility study of spiral CT versus chest x-ray screening for lung cancer. Lung Cancer 47(1):9-15.
Gold, M., J. Siegel, L. Russell, and M. Weinstein, eds. 1996. Cost-effectiveness in Health and Medicine. New York: Oxford University Press.
Grady, D., D. Herrington, V. Bittner, R. Blumenthal, M. Davidson, M. Hlatky, J. Hsia, S. Hulley, A. Herd, S. Khan, L. K. Newby, D. Waters, E. Vittinghoff, and N. Wenger. 2002. Cardiovascular disease outcomes during 6.8 years of hormone therapy: Heart and estrogen/progestin replacement study follow-up (HERS II). Journal of the American Medical Association 288(1):49-57.
Gray, W. A., J. S. Yadav, P. Verta, A. Scicli, R. Fairman, M. Wholey, L. N. Hopkins, R. Atkinson, R. Raabe, S. Barnwell, and R. Green. 2007. The capture registry: Predictors of outcomes in carotid artery stenting with embolic protection for high surgical risk patients in the early post-approval setting. Catheterization and Cardiovascular Interventions 70(7):1025-1033.
Grodstein, F., and M. Stampfer. 2002. The epidemiology of postmenopausal hormone therapy and cardiovascular disease. In Thrombosis and Thromboembolism, edited by S. Z. Goldhaber and P. M. Ridker. New York: Marcel Dekker. Pp. 67-78.
Grodstein, F., J. E. Manson, G. A. Colditz, W. C. Willett, F. E. Speizer, and M. J. Stampfer. 2000. A prospective, observational study of postmenopausal hormone therapy and primary prevention of cardiovascular disease. Annals of Internal Medicine 133(12):933-941.
Grodstein, F., T. B. Clarkson, and J. E. Manson. 2003. Understanding the divergent data on postmenopausal hormone therapy. New England Journal of Medicine 348(7):645-650.
Grodstein, F., J. E. Manson, and M. J. Stampfer. 2006. Hormone therapy and coronary heart disease: The role of time since menopause and age at hormone initiation. Journal Women’s Health (Larchmt) 15(1):35-44.
Hammill, S. C., L. W. Stevenson, A. H. Kadish, M. S. Kremers, P. Heidenreich, B. D. Lindsay, M. J. Mirro, M. J. Radford, Y. Wang, C. M. Lang, J. C. Harder, and R. G. Brindis. 2007. Review of the registry’s first year, data collected, and future plans. Heart Rhythm 4(9):1260-1263.
Harman, S. M., E. A. Brinton, M. Cedars, R. Lobo, J. E. Manson, G. R. Merriam, V. M. Miller, F. Naftolin, and N. Santoro. 2005. KEEPS: The Kronos Early Estrogen Prevention Study. Climacteric 8(1):3-12.
Hecht, H. S., M. J. Budoff, D. S. Berman, J. Ehrlich, and J. A. Rumberger. 2006. Coronary artery calcium scanning: Clinical paradigms for cardiac risk assessment and treatment. American Heart Journal 151(6):1139-1146.
Heckbert, S. R., R. C. Kaplan, N. S. Weiss, B. M. Psaty, D. Lin, C. D. Furberg, J. R. Starr, G. D. Anderson, and A. Z. LaCroix. 2001. Risk of recurrent coronary events in relation to use and recent initiation of postmenopausal hormone therapy. Archives of Internal Medicine 161(14):1709-1713.
Hemminki, E., and K. McPherson. 1997. Impact of postmenopausal hormone therapy on cardiovascular events and cancer: Pooled data from clinical trials. British Medical Journal 315(7101):149-153.
Henschke, C. I. 2007. Survival of patients with clinical stage I lung cancer diagnosed by computed tomography screening for lung cancer. Clinical Cancer Research 13(17): 4949-4950.
Heres, S., J. Davis, K. Maino, E. Jetzinger, W. Kissling, and S. Leucht. 2006. Why olanzapine beats risperidone, risperidone beats quetiapine, and quetiapine beats olanzapine: An exploratory analysis of head-to-head comparison studies of second-generation antipsychotics. American Journal of Psychiatry 163(2):185-194.
Herrington, D. M., D. M. Reboussin, K. B. Brosnihan, P. C. Sharp, S. A. Shumaker, T. E. Snyder, C. D. Furberg, G. J. Kowalchuk, T. D. Stuckey, W. J. Rogers, D. H. Givens, and D. Waters. 2000. Effects of estrogen replacement on the progression of coronary-artery atherosclerosis. New England Journal of Medicine 343(8):522-529.
Hodis, H. N. 2007. Early versus Late Intervention Trial with Estrogen (ELITE) Website. http://clinicaltrials.gov/show/NCT00114517 (accessed May 20, 2008).
Hodis, H. N., W. J. Mack, R. A. Lobo, D. Shoupe, A. Sevanian, P. R. Mahrer, R. H. Selzer, C. R. Liu Cr, C. H. Liu Ch, and S. P. Azen. 2001. Estrogen in the prevention of atherosclerosis. A randomized, double-blind, placebo-controlled trial. Annals of Internal Medicine 135(11):939-953.
Hodis, H. N., W. J. Mack, S. P. Azen, R. A. Lobo, D. Shoupe, P. R. Mahrer, D. P. Faxon, L. Cashin-Hemphill, M. E. Sanmarco, W. J. French, T. L. Shook, T. D. Gaarder, A. O. Mehra, R. Rabbani, A. Sevanian, A. B. Shil, M. Torres, K. H. Vogelbach, and R. H. Selzer. 2003. Hormone therapy and the progression of coronary-artery atherosclerosis in postmenopausal women. New England Journal of Medicine 349(6):535-545.
Honer, W. G., A. E. Thornton, E. Y. Chen, R. C. Chan, J. O. Wong, A. Bergmann, P. Falkai, E. Pomarol-Clotet, P. J. McKenna, E. Stip, R. Williams, G. W. MacEwan, K. Wasan, and R. Procyshyn. 2006. Clozapine alone versus clozapine and risperidone with refractory schizophrenia. New England Journal of Medicine 354(5):472-482.
Honigfeld, G., F. Arellano, J. Sethi, A. Bianchini, and J. Schein. 1998. Reducing clozapine-related morbidity and mortality: 5 years of experience with the clozaril national registry. Journal of Clinical Psychiatry 59(Supl 3):3-7.
Hormone therapy for the prevention of chronic conditions in postmenopausal women: Recommendations from the U.S. Preventive Services Task Force. 2005. Annals of Internal Medicine 142(10):855-860.
Hsia, J., M. H. Criqui, D. M. Herrington, J. E. Manson, L. Wu, S. R. Heckbert, M. Allison, M. M. McDermott, J. Robinson, and K. Masaki. 2006. Conjugated equine estrogens and peripheral arterial disease risk: The Women’s Health Initiative. American Heart Journal 152(1):170-176.
Hulley, S., D. Grady, T. Bush, C. Furberg, D. Herrington, B. Riggs, and E. Vittinghoff. 1998. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. Heart and estrogen/progestin replacement study (HERS) research group. Journal of the American Medical Association 280(7):605-613.
Iakovou, I., T. Schmidt, E. Bonizzoni, L. Ge, G. M. Sangiorgi, G. Stankovic, F. Airoldi, A. Chieffo, M. Montorfano, M. Carlino, I. Michev, N. Corvaja, C. Briguori, U. Gerckens, E. Grube, and A. Colombo. 2005. Incidence, predictors, and outcome of thrombosis after successful implantation of drug-eluting stents. Journal of the American Medical Association 293(17):2126-2130.
Jeste, D., J. Sable, and C. Salzman. 2005. Treatment of late-life disordered behavior, agitation, and psychosis. In Clinical Geriatric Psychopharmacology. 4th ed., edited by C. Salzman. Philadelphia, PA: Lippincott, Williams & Wilkins. Pp. 129-195.
Kane, J., G. Honigfeld, J. Singer, and H. Meltzer. 1988. Clozapine for the treatment-resistant schizophrenic. A double-blind comparison with chlorpromazine. Archives of General Psychiatry 45(9):789-796.
Kindermann, S. S., C. R. Dolder, A. Bailey, I. R. Katz, and D. V. Jeste. 2002. Pharmacological treatment of psychosis and agitation in elderly patients with dementia: Four decades of experience. Drugs Aging 19(4):257-276.
Kuchulakanti, P. K., W. W. Chu, R. Torguson, P. Ohlmann, S. W. Rha, L. C. Clavijo, S. W. Kim, A. Bui, N. Gevorkian, Z. Xue, K. Smith, J. Fournadjieva, W. O. Suddath, L. F. Satler, A. D. Pichard, K. M. Kent, and R. Waksman. 2006. Correlates and long-term outcomes of angiographically proven stent thrombosis with sirolimus- and paclitaxeleluting stents. Circulation 113(8):1108-1113.
Kuehn, B. M. 2005. FDA warns antipsychotic drugs may be risky for elderly. Journal of the American Medical Association 293(20):2462.
Lawlor, B. A. 2004. Behavioral and psychological symptoms in dementia: The role of atypical antipsychotics. Journal of Clinical Psychiatry 65(Supl 11):5-10.
Lemaitre, R. N., N. S. Weiss, N. L. Smith, B. M. Psaty, T. Lumley, E. B. Larson, and S. R. Heckbert. 2006. Esterified estrogen and conjugated equine estrogen and the risk of incident myocardial infarction and stroke. Archives of Internal Medicine 166(4):399-404.
Leucht, S., G. Pitschel-Walz, D. Abraham, and W. Kissling. 1999. Efficacy and extrapyramidal side-effects of the new antipsychotics olanzapine, quetiapine, risperidone, and sertindole compared to conventional antipsychotics and placebo. A meta-analysis of randomized controlled trials. Schizophrenia Research 35(1):51-68.
Lieberman, J. A., B. L. Saltz, C. A. Johns, S. Pollack, M. Borenstein, and J. Kane. 1991. The effects of clozapine on tardive dyskinesia. British Journal of Psychiatry 158:503-510.
Lieberman, J. A., M. Phillips, H. Gu, S. Stroup, P. Zhang, L. Kong, Z. Ji, G. Koch, and R. M. Hamer. 2003. Atypical and conventional antipsychotic drugs in treatment-naive first-episode schizophrenia: A 52-week randomized trial of clozapine vs chlorpromazine. Neuropsychopharmacology 28(5):995-1003.
Lieberman, J. A., T. S. Stroup, J. P. McEvoy, M. S. Swartz, R. A. Rosenheck, D. O. Perkins, R. S. Keefe, S. M. Davis, C. E. Davis, B. D. Lebowitz, J. Severe, and J. K. Hsiao. 2005. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. New England Journal of Medicine 353(12):1209-1223.
Lobo, R. A. 2004. Evaluation of cardiovascular event rates with hormone therapy in healthy, early postmenopausal women: Results from 2 large clinical trials. Archives of Internal Medicine 164(5):482-484.
Maixner, S. M., A. M. Mellow, and R. Tandon. 1999. The efficacy, safety, and tolerability of antipsychotics in the elderly. Journal of Clinical Psychiatry 60(Supl 8):29-41.
Manson, J. E., and S. S. Bassuk. 2007a. Hot Flashes, Hormones and Your Health. New York: McGraw-Hill.
———. 2007b. Invited commentary: Hormone therapy and risk of coronary heart disease why renew the focus on the early years of menopause? American Journal of Epidemiology 166(5):511-517.
Manson, J. E., J. Hsia, K. C. Johnson, J. E. Rossouw, A. R. Assaf, N. L. Lasser, M. Trevisan, H. R. Black, S. R. Heckbert, R. Detrano, O. L. Strickland, N. D. Wong, J. R. Crouse, E. Stein, and M. Cushman. 2003. Estrogen plus progestin and the risk of coronary heart disease. New England Journal of Medicine 349(6):523-534.
Manson, J. E., S. S. Bassuk, S. M. Harman, E. A. Brinton, M. I. Cedars, R. Lobo, G. R. Merriam, V. M. Miller, F. Naftolin, and N. Santoro. 2006. Postmenopausal hormone therapy: New questions and the case for new clinical trials. Menopause 13(1):139-147.
Manson, J. E., M. A. Allison, J. E. Rossouw, J. J. Carr, R. D. Langer, J. Hsia, L. H. Kuller, B. B. Cochrane, J. R. Hunt, S. E. Ludlam, M. B. Pettinger, M. Gass, K. L. Margolis, L. Nathan, J. K. Ockene, R. L. Prentice, J. Robbins, and M. L. Stefanick. 2007. Estrogen therapy and coronary-artery calcification. New England Journal of Medicine 356(25):2591-2602.
March, J. S., S. G. Silva, S. Compton, M. Shapiro, R. Califf, and R. Krishnan. 2005. The case for practical clinical trials in psychiatry. American Journal of Psychiatry 162(5): 836-846.
Mauri, L., E. J. Orav, and R. E. Kuntz. 2005. Late loss in lumen diameter and binary restenosis for drug-eluting stent comparison. Circulation 111(25):3435-3442.
Maynard, C., J. R. Goss, D. J. Malenka, and M. Reisman. 2003. Adjusting for patient differences in predicting hospital mortality for percutaneous coronary interventions in the clinical outcomes assessment program. American Heart Journal 145(4):658-664.
MedQIC. 2008. SCIP Project Information. http://www.medqic.org/scip (accessed February 19, 2008).
Meltzer, H., and H. Fatemi. 1995. Suicide in schizophrenia: The effect of clozapine. Clinical Neuro-pharmacology 18:S18-S24.
Meltzer, H. Y., and G. Okayli. 1995. Reduction of suicidality during clozapine treatment of neuroleptic-resistant schizophrenia: Impact on risk-benefit assessment. American Journal of Psychiatry 152(2):183-190.
Meltzer, H. Y., L. Alphs, A. I. Green, A. C. Altamura, R. Anand, A. Bertoldi, M. Bourgeois, G. Chouinard, M. Z. Islam, J. Kane, R. Krishnan, J. P. Lindenmayer, and S. Potkin. 2003. Clozapine treatment for suicidality in schizophrenia: International suicide prevention trial (intersept). Archives of Geneneral Psychiatry 60(1):82-91.
Mendelsohn, M. E., and R. H. Karas. 2005. Molecular and cellular basis of cardiovascular gender differences. Science 308(5728):1583-1587.
Michels, K. B., and J. E. Manson. 2003. Postmenopausal hormone therapy: A reversal of fortune. Circulation 107(14):1830-1833.
Mikkola, T. S., and T. B. Clarkson. 2002. Estrogen replacement therapy, atherosclerosis, and vascular function. Cardiovascular Research 53(3):605-619.
Mosca, L., C. L. Banka, E. J. Benjamin, K. Berra, C. Bushnell, R. J. Dolor, T. G. Ganiats, A. S. Gomes, H. L. Gornik, C. Gracia, M. Gulati, C. K. Haan, D. R. Judelson, N. Keenan, E. Kelepouris, E. D. Michos, L. K. Newby, S. Oparil, P. Ouyang, M. C. Oz, D. Petitti, V. W. Pinn, R. F. Redberg, R. Scott, K. Sherif, S. C. Smith, Jr., G. Sopko, R. H. Steinhorn, N. J. Stone, K. A. Taubert, B. A. Todd, E. Urbina, and N. K. Wenger. 2007. Evidence-based guidelines for cardiovascular disease prevention in women: 2007 update. Circulation 115(11):1481-1501.
Murphy, S. A., D. W. Oslin, A. J. Rush, and J. Zhu. 2007. Methodological challenges in constructing effective treatment sequences for chronic psychiatric disorders. Neuropsychopharmacology 32(2):257-262.
Pocock, S. J., A. J. Lansky, R. Mehran, J. J. Popma, M. P. Fahy, Y. Na, G. Dangas, J. W. Moses, T. Pucelikova, D. E. Kandzari, S. G. Ellis, M. B. Leon, and G. W. Stone. 2008. Angiographic surrogate end points in drug-eluting stent trials: A systematic evaluation based on individual patient data from 11 randomized, controlled trials. Journal of the American College of Cardiology 51(1):23-32.
Polinski, J. M., P. S. Wang, and M. A. Fischer. 2007. Medicaid’s prior authorization program and access to atypical antipsychotic medications. Health Affairs (Millwood) 26(3): 750-760.
Prentice, R. L., R. D. Langer, M. L. Stefanick, B. V. Howard, M. Pettinger, G. L. Anderson, D. Barad, J. D. Curb, J. Kotchen, L. Kuller, M. Limacher, and J. Wactawski-Wende. 2006. Combined analysis of Women’s Health Initiative observational and clinical trial data on postmenopausal hormone treatment and cardiovascular disease. American Journal of Epidemiology 163(7):589-599.
A prospective analysis of 1518 laparoscopic cholecystectomies. The Southern Surgeons Club. 1991. New England Journal of Medicine 324(16):1073-1078.
Roberts, T. G., Jr., and B. A. Chabner. 2004. Beyond fast track for drug approvals. New England Journal of Medicine 351(5):501-505.
Rosenheck, R. A., D. L. Leslie, J. Sindelar, E. A. Miller, H. Lin, T. S. Stroup, J. McEvoy, S. M. Davis, R. S. Keefe, M. Swartz, D. O. Perkins, J. K. Hsiao, and J. Lieberman. 2006. Cost-effectiveness of second-generation antipsychotics and perphenazine in a randomized trial of treatment for chronic schizophrenia. American Journal of Psychiatry 163(12):2080-2089.
Rosenheck, R. A., D. L. Leslie, J. L. Sindelar, E. A. Miller, P. N. Tariot, K. S. Dagerman, S. M. Davis, B. D. Lebowitz, P. Rabins, J. K. Hsiao, J. A. Lieberman, and L. S. Schneider. 2007. Cost-benefit analysis of second-generation antipsychotics and placebo in a randomized trial of the treatment of psychosis and aggression in alzheimer disease. Archives of General Psychiatry 64(11):1259-1268.
Rossouw, J. E., G. L. Anderson, R. L. Prentice, A. Z. LaCroix, C. Kooperberg, M. L. Stefanick, R. D. Jackson, S. A. Beresford, B. V. Howard, K. C. Johnson, J. M. Kotchen, and J. Ockene. 2002. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: Principal results from the Women’s Health Initiative randomized controlled trial. Journal of the American Medical Association 288(3):321-333.
Rossouw, J. E., R. L. Prentice, J. E. Manson, L. Wu, D. Barad, V. M. Barnabei, M. Ko, A. Z. LaCroix, K. L. Margolis, and M. L. Stefanick. 2007. Postmenopausal hormone therapy and risk of cardiovascular disease by age and years since menopause. Journal of the American Medical Association 297(13):1465-1477.
Sachs, G. S., A. A. Nierenberg, J. R. Calabrese, L. B. Marangell, S. R. Wisniewski, L. Gyulai, E. S. Friedman, C. L. Bowden, M. D. Fossey, M. J. Ostacher, T. A. Ketter, J. Patel, P. Hauser, D. Rapport, J. M. Martinez, M. H. Allen, D. J. Miklowitz, M. W. Otto, E. B. Dennehy, and M. E. Thase. 2007. Effectiveness of adjunctive antidepressant treatment for bipolar depression. New England Journal of Medicine 356(17):1711-1722.
Salpeter, S. R., J. M. Walsh, E. Greyber, T. M. Ormiston, and E. E. Salpeter. 2004. Mortality associated with hormone replacement therapy in younger and older women: A metaanalysis. Journal of General Internal Medicine 19(7):791-804.
Salpeter, S. R., J. M. Walsh, E. Greyber, and E. E. Salpeter. 2006. Brief report: Coronary heart disease events associated with hormone therapy in younger and older women. A metaanalysis. Journal of General Internal Medicine 21(4):363-366.
Santry, H. P., D. L. Gillen, and D. S. Lauderdale. 2005. Trends in bariatric surgical procedures. Journal of the American Medical Association 294(15):1909-1917.
Schatzberg, A. F., and C. B. Nemeroff. 2006. Essentials of Clinical Psychopharmacology. 2nd ed. Arlington, VA: American Psychiatric Publishing, Inc.
Schneeweiss, S., M. Maclure, A. M. Walker, P. Grootendorst, and S. B. Soumerai. 2001. On the evaluation of drug benefits policy changes with longitudinal claims data: The policy maker’s versus the clinician’s perspective. Health Policy 55(2):97-109.
Schneeweiss, S., S. Setoguchi, A. Brookhart, C. Dormuth, and P. S. Wang. 2007. Risk of death associated with the use of conventional versus atypical antipsychotic drugs among elderly patients. Canadian Medical Association Journal 176(5):627-632.
Schneider, L. S., K. S. Dagerman, and P. Insel. 2005. Risk of death with atypical antipsychotic drug treatment for dementia: Meta-analysis of randomized placebo-controlled trials. Journal of the American Medical Association 294(15):1934-1943.
Schneider, L. S., P. N. Tariot, K. S. Dagerman, S. M. Davis, J. K. Hsiao, M. S. Ismail, B. D. Lebowitz, C. G. Lyketsos, J. M. Ryan, T. S. Stroup, D. L. Sultzer, D. Weintraub, and J. A. Lieberman. 2006. Effectiveness of atypical antipsychotic drugs in patients with Alzheimer’s disease. New England Journal of Medicine 355(15):1525-1538.
Schorr, R., R. Fought, and W. Ray. 1994. Changes in antipsychotic drug use in nursing homes during implementation of the OBRA-87 regulations. Journal of the American Medical Association 271:358-362.
Shively, E. H., M. J. Heine, R. H. Schell, J. N. Sharpe, R. N. Garrison, S. R. Vallance, K. J. DeSimone, and H. C. Polk, Jr. 2004. Practicing surgeons lead in quality care, safety, and cost control. Annals of Surgery 239(6):752-760; discussion 760-752.
Sink, K. M., K. F. Holden, and K. Yaffe. 2005. Pharmacological treatment of neuropsychiatric symptoms of dementia: A review of the evidence. Journal of the American Medical Association 293(5):596-608.
Soumerai, S. B., T. J. McLaughlin, D. Ross-Degnan, C. S. Casteris, and P. Bollini. 1994. Effects of a limit on medicaid drug-reimbursement benefits on the use of psychotropic agents and acute mental health services by patients with schizophrenia. New England Journal of Medicine 331(10):650-655.
Steinhubl, S. R., P. B. Berger, J. T. Mann, 3rd, E. T. Fry, A. DeLago, C. Wilmer, and E. J. Topol. 2002. Early and sustained dual oral antiplatelet therapy following percutaneous coronary intervention: A randomized controlled trial. Journal of the American Medical Association 288(19):2411-2420.
Strong, C. 2005. Antipsychotic use in elderly patients with dementia prompts new FDA warning. Neuropsychiatry Review 6:1-17.
Sturmer, T., R. J. Glynn, K. J. Rothman, J. Avorn, and S. Schneeweiss. 2007. Adjustments for unmeasured confounders in pharmacoepidemiologic database studies using external information. Medical Care 45(10 Supl 2):S158-S165.
Suzuki, T., G. Kopia, S. Hayashi, L. R. Bailey, G. Llanos, R. Wilensky, B. D. Klugherz, G. Papandreou, P. Narayan, M. B. Leon, A. C. Yeung, F. Tio, P. S. Tsao, R. Falotico, and A. J. Carter. 2001. Stent-based delivery of sirolimus reduces neointimal formation in a porcine coronary model. Circulation 104(10):1188-1193.
Swensen, S. J., J. R. Jett, T. E. Hartman, D. E. Midthun, J. A. Sloan, A. M. Sykes, G. L. Aughenbaugh, and M. A. Clemens. 2003. Lung cancer screening with CT: Mayo clinic experience. Radiology 226(3):756-761.
Tariot, P. N. 1999. The older patient: The ongoing challenge of efficacy and tolerability. Journal of Clinical Psychiatry 60(Supl 23):29-33.
Trivedi, M. H., M. Fava, S. R. Wisniewski, M. E. Thase, F. Quitkin, D. Warden, L. Ritz, A. A. Nierenberg, B. D. Lebowitz, M. M. Biggs, J. F. Luther, K. Shores-Wilson, and A. J. Rush. 2006. Medication augmentation after the failure of SSRIS for depression. New England Journal of Medicine 354(12):1243-1252.
Tunis, S. R., D. B. Stryer, and C. M. Clancy. 2003. Practical clinical trials: Increasing the value of clinical research for decision making in clinical and health policy. Journal of the American Medical Association 290(12):1624-1632.
Virmani, R., G. Guagliumi, A. Farb, G. Musumeci, N. Grieco, T. Motta, L. Mihalcsik, M. Tespili, O. Valsecchi, and F. D. Kolodgie. 2004. Localized hypersensitivity and late coronary thrombosis secondary to a sirolimus-eluting stent: Should we be cautious? Circulation 109(6):701-705.
Wahlbeck, K., M. Cheine, A. Essali, and C. Adams. 1999. Evidence of clozapine’s effectiveness in schizophrenia: A systematic review and meta-analysis of randomized trials. American Journal of Psychiatry 156(7):990-999.
Walker, A. M., L. L. Lanza, F. Arellano, and K. J. Rothman. 1997. Mortality in current and former users of clozapine. Epidemiology 8(6):671-677.
Wang, P. S., J. C. West, T. Tanielian, and H. A. Pincus. 2000. Recent patterns and predictors of antipsychotic medication regimens used to treat schizophrenia and other psychotic disorders. Schizophrenia Bulletin 26(2):451-457.
Wang, P., A. Walker, and J. Avorn. 2002a. The pharmacoepidemiology of psychiatric medications. In Textbook in Psychiatric Epidemiology. 2nd ed., edited by M. T. Twang and M. Tohen. New York: John Wiley & Sons, Inc. Pp. 181-194.
Wang, P. S., O. Demler, and R. C. Kessler. 2002b. Adequacy of treatment for serious mental illness in the United States. American Journal of Public Health 92(1):92-98.
Wang, P. S., D. A. Ganz, J. S. Benner, R. J. Glynn, and J. Avorn. 2004. Should clozapine continue to be restricted to third-line status for schizophrenia?: A decision-analytic model. Jounal of Mental Health Policy and Economics 7(2):77-85.
Wang, P. S., M. Lane, M. Olfson, H. A. Pincus, K. B. Wells, and R. C. Kessler. 2005a. Twelve-month use of mental health services in the United States: Results from the national co-morbidity survey replication. Archives of General Psychiatry Psychiatry 62(6):629-640.
Wang, P. S., S. Schneeweiss, J. Avorn, M. A. Fischer, H. Mogun, D. H. Solomon, and M. A. Brookhart. 2005b. Risk of death in elderly users of conventional vs. atypical antipsychotic medications. New England Journal of Medicine 353(22):2335-2341.
Wang, P. S., S. Aguilar-Gaxiola, J. Alonso, M. C. Angermeyer, G. Borges, E. J. Bromet, R. Bruffaerts, G. de Girolamo, R. de Graaf, O. Gureje, J. M. Haro, E. G. Karam, R. C. Kessler, V. Kovess, M. C. Lane, S. Lee, D. Levinson, Y. Ono, M. Petukhova, J. Posada-Villa, S. Seedat, and J. E. Wells. 2007. Use of mental health services for anxiety, mood, and substance disorders in 17 countries in the WHO world mental health surveys. Lancet 370(9590):841-850.
Waters, D. D., E. L. Alderman, J. Hsia, B. V. Howard, F. R. Cobb, W. J. Rogers, P. Ouyang, P. Thompson, J. C. Tardif, L. Higginson, V. Bittner, M. Steffes, D. J. Gordon, M. Proschan, N. Younes, and J. I. Verter. 2002. Effects of hormone replacement therapy and antioxidant vitamin supplements on coronary atherosclerosis in postmenopausal women: A randomized controlled trial. Journal of the American Medical Association 288(19):2432-2440.
Wathen, C. N., D. S. Feig, J. W. Feightner, B. L. Abramson, and A. M. Cheung. 2004. Hormone replacement therapy for the primary prevention of chronic diseases: Recommendation statement from the Canadian Task Force on Preventive Health Care. Canadian Medical Association Journal 170(10):1535-1537.




































































