Page 99
5—
Extent and Distribution of Chronic Disease: An Overview
There are subtle, but important, differences between health and the absence of disease and between health promotion and disease prevention. The Constitution of the World Health Organization states in its preamble that "health is a state of complete physical, mental, and social well-being and not merely the absence of disease or infirmity." Definitions of health and of disease prevention promulgated by the Office of Disease Prevention and Health Promotion of the U.S. Department of Health and Human Services (DHHS) stress the differences in terms of personal behavior, level of prevention, and sense of well-being:
Health promotion = personal, environmental, or social interventions that facilitate behavioral adaptations conducive to improved health, level of function, and sense of well-being.
Disease prevention = personal, environmental, or social interventions that impede the occurrence of disease, injury, disability, or death—or the progression of detectable but asymptomatic disease (J. Michael McGinnis, DHHS, personal communication, 1988).
The committee carefully considered these definitions and decided that any attempt to address the association between diet and health in its broadest sense would necessarily be superficial, incomplete, and so diffuse as to be ineffective. Thus, it opted to focus on specific diet-related chronic diseases and conditions related to them.
The definition of diet is straightforward: Diet comprises all food and drink consumed by people. It is characterized by the average and the distribution of nutrients and foods consumed by an individual or by a defined group.
The committee gave special attention to major diet-related chronic diseases and conditions of adulthood, including atherosclerotic cardiovascular diseases (i.e., coronary heart disease, stroke, and peripheral arterial diseases), hypertension and related diseases, obesity and related diseases, cancers, osteoporosis, diabetes mellitus, hepatobiliary disease, and dental caries. It selected these because, according to current evidence, they are the most common diet-associated causes of death and disability among U.S. adults.
This chapter presents the descriptive epidemiologic features of those eight chronic diseases and conditions.
Atherosclerotic Cardiovascular Diseases
Coronary Heart Disease
Coronary heart disease (CHD) affects the cardiac muscle, mainly as a result of atherosclerotic disease of the coronary arteries and its complications. Atheromas and thrombosis combine to interrupt blood flow to the heart, producing clinical
Page 100
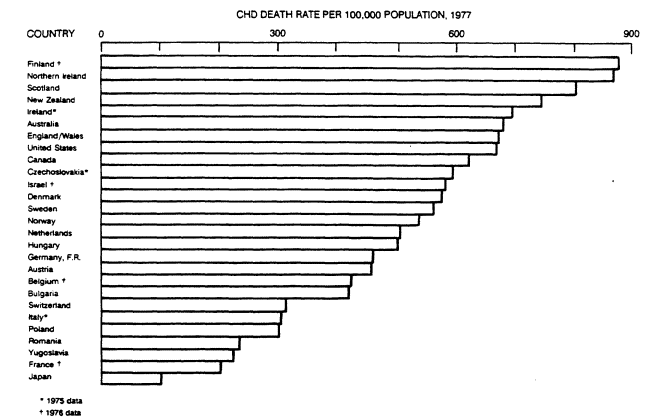
FIGURE 5-1
Rate of deaths from CHD per 100,000 population among 35- to 74-year-old
men in 1977 (except as otherwise noted) by country. Adapted from the report
of the Inter-Society Commission for Heart Disease Resources (1984).
events that may include sudden death. Morbidity from CHD, measured by population-based surveys and hospital records, includes clinical syndromes of myocardial infarction with damage to and scarring of the myocardium; coronary insufficiency, including angina pectoris and other symptoms of inadequate blood supply; complex arrhythmias, which may lead to sudden coronary death; and chronic heart disease characterized by heart failure or arrhythmias. Mortality from CHD is defined as the number or proportion of death certificates that are coded as International Classification of Disease (ICD) categories 410-411 (myocardial infarction) or 412-414 (angina pectoris and other chronic heart disease manifestations).
Population Differences In Frequency
Figure 5-1 depicts wide differences among countries in the vital statistics on CHD death among 35- to 74-year-old men in the 1970s. The highest reported CHD death rates occurred in Finland and the English-speaking countries, including the United States; the lowest rates were in Japan (Inter-Society Commission for Heart Disease Resources, 1984).
CHD death rates for women in the same year (not shown) were highest in Northern Ireland, Scotland, and the United States and lowest in Japan (Inter-Society Commission for Heart Disease Resources, 1984). These large geographic differences in CHD death rates were confirmed by studies comparing geographic differences in CHD incidence rates, such as the Seven Countries Study, in which the 10-year incidence rate among men 40 to 59 years old at the beginning of the study was about 200 per thousand in Finland as compared to about 40 per thousand in Japan and the Greek Islands (Keys, 1980).
Geographic differences in trends of reported deaths from CHD have been equally dramatic (see Figure 5-2). The largest decline among men from 35 to 74 years old occurred in the United States, followed closely by Australia and Canada, whereas rates rose strongly in Northern Ireland, Poland, and Bulgaria. Similar changes in death rates were also reported for women (not shown)—the largest
Page 101
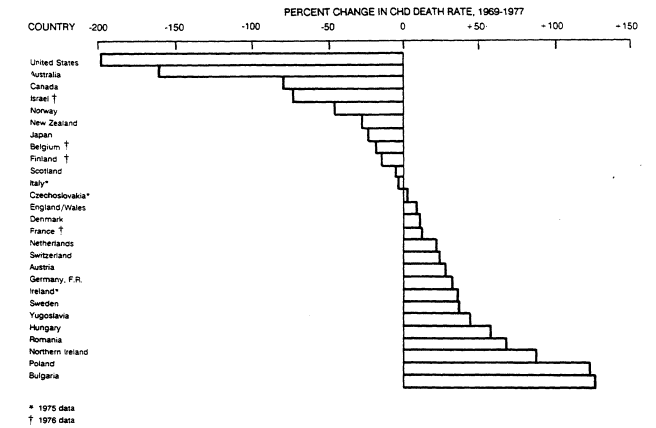
FIGURE 5-2
Percent change in rates of death from CHD from 1969 through 1977 among 35- to 74-year old men by
country. Adapted from the report of the Inter-Society Commission for Heart Disease Resources (1984).
declines again occurring in the United States and Australia, smaller decreases in other countries, and increases in several Eastern European countries (Inter-Society Commission for Heart Disease Resources, 1984).
In the United States, there are substantial regional differences in CHD death rates. In 1980, rates were highest in the East and Southeast (290-305 deaths per 100,000), intermediate (210-249) in the Midwest, and lowest (149-209) in the West and Southwest (DHHS, 1987).
Time Trends in the United States
From the 1950s to the mid-1960s, CHD rates in the United States rose 1 to 2% per year among men and women, whites and nonwhites. Subsequently, death rates declined by 2 to 3% per year. This decline has affected all ages, but has been especially evident in younger groups and was observed a few years earlier in women than in men.
From 1973 through 1979, CHD death rates again increased, but since 1980, they have returned to the 1965-1973 rate of decline. Unadjusted data suggest that the decline has continued through 1986 and early 1987, but with a slowing of rate (DHHS, 1987). This decline was greater in better educated, more affluent groups (DHHS, 1987) and is characterized by fewer out-of-hospital deaths, sudden unexpected deaths, and acute nonfatal myocardial infarction (Anastasiou-Nana et al., 1982; Elveback et al., 1986; Folsom et al., 1987; Gillum et al., 1984; Goldberg et al., 1986; Goldman et al., 1982; Gomez-Marin et al., 1987; Kuller et al., 1986; Pell and Fayerweather, 1985). These findings have been attributed to the increased availability of more sophisticated cardiac care and more effective emergency services (Gillum et al., 1984; Goldman and Cook, 1984). In addition, the decline in CHD deaths was generally accompanied by, and probably preceded by, decreased CHD incidence and by lowered average levels of factors associated with increased CHD risk in the population (e.g., cigarette smoking, hypertension, and high serum cholesterol levels) (Inter-Society Commission for Heart Disease Resources, 1984;
Page 102
Pell and Fayerweather, 1985). A decline in severity of atherosclerotic lesions at autopsy has also been noted (Solberg and Strong, 1983), although current evidence is insufficient to link this to the concomitant decrease in CHD mortality rates.
Similarly, in long-term cohort studies of CHD, decreases in sudden and out-of-hospital deaths, in-hospital proportion of cases dying (case fatality), and discharge diagnoses of CHD have been reported (Inter-Society Commission for Heart Disease Resources, 1984). The decrease in CHD case fatality rates may reflect improved medical care. With the exception of an increased incidence of nonfatal myocardial infarction noted in a study of women in Rochester, Minnesota (Elveback et al., 1981), there is much evidence that the incidence of first coronary events is declining (Kuller et al., 1986; Pell and Fayerweather, 1985). The evidence for improved survival from myocardial infarction. over the short term (30 days) is strong (Folsom et al., 1987; Goldberg et al., 1986; Pell and Fayerweather, 1985), but not consistent (Elveback et al., 1986; Goldman et al., 1982). One study has shown an improvement in 4-year survival after myocardial infarction, but much of that was due to improved survival in hospital (Gomez-Marin et al., 1987).
In the United States, there have been large regional differences in the trends of CHD death rates between 1960 and 1980 (DHHS, 1987). For example, age- and sex-adjusted CHD mortality rates (ages 35 to 74) declined 48 to 53% in Maryland, Delaware, Illinois, and Nevada and 40 to 44% in Connecticut, Washington, and California. Smaller declines have been noted throughout the western, midwestern, southern, and south-central states including Appalachia. The smallest changes were observed in Oklahoma, Mississippi, Tennessee, and Kentucky, where there were declines of only 12 to 21%.
Associations Between Risk Factors in Individuals and CHD
CHD prevalence, incidence, and mortality rates rise steeply with age, approximately doubling in each 5-year age class past age 24. The decline in age-specific CHD death rates between 1968 and 1978 in the United States occurred across all age groups; it was 40 to 50% for those in the 35- to 44-year-old age group and 15 to 20% among those over age 70 (DHHS, 1987).
CHD death rates in men are three times greater than in women in such high-incidence countries as the United States, the United Kingdom, northern European countries, New Zealand, and Australia (Inter-Society Commission for Heart Disease Resources, 1984). These sex differences are smaller after women pass menopause and in such low-CHD-incidence countries as France and Japan. In countries where CHD deaths have declined; percentage declines have generally been steeper among women than among men.
Ethnic and racial differences in CHD deaths within countries are closely associated with socioeconomic level. In the United States, CHD death rates are highest for white males, followed in descending order by black males, other nonwhite males, black females, white females, and nonwhite (other than black) females. The greatest rate of decline in CHD deaths between 1968 and 1978—almost 50%—occurred among nonwhite (but not black) women, followed by white women. Black women had the smallest decline (DHHS, 1987).
Differences among migrants were systematically documented in the Ni-Hon-San study conducted among Japanese living in Japan, Honolulu, and San Francisco. In that study, investigators observed that CHD prevalence and incidence rates tripled among Japanese within a generation of their migration to the West Coast of the United States and doubled in Japanese who migrated to Hawaii (Marmot et al., 1975; Robertson et al., 1977). This change was generally paralleled by changes in average levels of risk factors, including saturated fat in the diet and serum cholesterol levels. Similar changes occurred in Boston men who were born in Ireland compared to their brothers who stayed in Ireland (Kushi et al., 1985) and in South Pacific islanders who settled in New Zealand (Beaglehole et al., 1977; Prior and Stanhope, 1980). In all these groups, rates among migrants were near those of the newly adopted culture within 10 to 20 years.
The relationship of socioeconomic class and occupation to CHD risk differs among cultures. For example, a social classification based primarily on occupation was positively associated with CHD
Page 103
mortality in the United Kingdom in the 1950s and 1960s. Since 1960, this picture has changed; the social classification is now inversely related to CHD deaths in England and Wales (Marmot et al., 1978). In the United States, hourly wage earners now have greater CHD mortality and incidence rates than do salaried employees, although there appear to be no specific occupational exposures that exacerbate CHD risk (Pell and Fayerweather, 1985).
Other relationships are found between psychosocial factors and CHD risk. Generally, better educated people are nonsmokers and have healthier patterns of eating, lower serum cholesterol levels, leaner body mass index, and greater physical activity. Numerous studies have indicated slight to moderately strong associations between CHD risk and other psychosocial and behavioral characteristics, but the existence of coronary-prone behavior and personality has been increasingly questioned because few of those studies controlled for social networks, physical activity, diet, and alcohol intake (Inter-Society Commission for Heart Disease Resources, 1984; Jenkins, 1983).
Family history of early-onset CHD is an independent risk indicator in high-risk populations (Inter-Society Commission for Heart Disease Resources, 1984); however, familial hypercholesterolemia accounts for only a small percentage of the people with relatively high serum cholesterol levels in affluent cultures.
It is likely that individual differences in biologic risk factors such as serum lipoprotein levels and in responses to dietary factors (e.g., dietary cholesterol) are determined in large part by genetic factors. However, the absence of specific genetic markers limits the power of family studies to separate the genetic components. New findings concerning cell receptors for lipoproteins hold promise for improving understanding of the differences in individual responses to diet and susceptibility to CHD.
The relationship of specific dietary components to serum lipoprotein levels is discussed throughout this report (e.g., Chapters 6-10, 14, 16, 17, and 19), as are relative risk, attributable risk, and the population risk attributable to elevated blood lipoproteins.
CHD is a classic model of a disease with multiple causes. Evidence indicates that a diet high in saturated fatty acids and cholesterol along with relatively high levels of serum cholesterol are the greatest contributors to elevated population risk of atherosclerosis and CHD; population risk differences are largely explained by these related factors. However, within populations with a significant burden of atherosclerosis, several factors interact to determine an individual's risk. The most prominent of these, after age, sex, diet, and blood lipoproteins, are the level of arterial blood pressure and the number of cigarettes smoked. Peripheral arterial disease is one of the strongest independent predictors of CHD, stroke, and all causes of death (Criqui et al., 1985a). Numerous other factors, including lack of habitual physical activity, relative body weight (see section below on Obesity and Related Diseases), and diabetes, contribute to CHD (Inter-Society Commission for Heart Disease Resources, 1984).
Stroke
Stroke is the abrupt onset of neurological disability due to infarction of the area of brain served by an artery that is clogged by atherosclerotic plaque, blocked by an embolus, or ruptured, with destruction of brain tissue by hemorrhage. Transient ischemic attacks (TIAs) are episodes of temporary neurological disability due to insufficient arterial blood supply to the brain. In the United States, most strokes result from cerebral infarction, followed in frequency by the two major forms of cerebral hemorrhage—intracerebral and subarachnoid.
Stroke is a major cause of death among adults worldwide. In industrial countries, it is usually third among causes of death, following heart diseases and cancer.
Population Differences in Frequency
There are large differences in reported mortality rates for stroke. For example, age-adjusted 1970 estimates of prevalence rates for stroke per 100,000 people were 556 in Rochester, Minnesota, and 363 in the United Kingdom (Kurtzke, 1976). Studies in Japan suggest that the distribution of the types of stroke (e.g., infarction and hemorrhage) also varies widely (Omae et al., 1976). In populations, death rates from cerebral infarction generally parallel those from atherosclerosis and CHD, whereas rates of intracerebral hemorrhage usually parallel the frequency of hypertension. For example, stroke
Page 104
is the leading cause of deaths among adults in Japan, where hypertension is prevalent but CHD is uncommon (Komachi, 1977).
In past years, accurate assessment of stroke incidence and mortality has been hampered by frequent misdiagnosis. The descriptive epidemiology of stroke should improve, however, as diagnosis is enhanced by computed tomography (CT), which allows differentiation among the several basic causes of the stroke syndrome.
Strokes of all types were reported as the cause of death for approximately 182,000 persons in the United States in 1977; limitations in death certification do not permit reliable estimates by type of stroke. The American Heart Association (AHA, 1983) estimated the 1981 prevalence of residual damage from strokes at 1.87 million and of coronary disease at 4.6 million. Fatality shortly after the stroke occurs in about 15% of those affected, another 16% require institutional care, and 50% are permanently disabled (Kannel and Wolf, 1983). The average annual incidence of stroke in a 20-year follow-up of 45- to 74-year-old subjects in Framingham, Massachusetts, was 340 per 100,000 among men and 290 among women. Two-thirds of these cases had cerebral infarction (Kannel and Wolf, 1983). Rates were about equal for men and women from age 65 onward, and the rates climbed precipitously with age—1 per thousand at ages 45 to 54, 3.5 at ages 55 to 64, 9.0 at ages 65 to 74, 20.0 at ages 75 to 84, and 40.0 at age 85 and over.
In the United States, approximately 50% of the strokes are attributed to atherosclerosis and thrombosis, 12% to cerebral hemorrhage, 8% to subarachnoid hemorrhage, and 8% to embolism. The remainder are ill-defined (Kurtzke, 1976).
Time Trends in Mortality and Morbidity
Stroke deaths have declined for the past 50 years. In the 1940s and 1950s the rate of decline was 1% per year. Since 1972, the rate of decline has increased to 5% per year (Levy, 1979; Soltero et al., 1978). Between 1968 and 1981, the age-adjusted death rate from stroke fell 46% (Inter-Society Commission for Heart Disease Resources, 1984). The decline has been especially notable among nonwhites, especially nonwhite females. Population-based data on stroke incidence in the United States are rare, but in Rochester, Minnesota, the average annual incidence for all types of stroke declined from 190 per 100,000 during 1945-1949 to 104 during 1970-1974 (Garraway and Whisnant, 1987; Garraway et al., 1979). The Rochester data also documented that the decline in death rates ascribed to intracerebral hemorrhage began well before better diagnoses became possible through the use of more widely available CT scans and continued to 1979.
There is evidence that fatality among hospitalized stroke patients has not declined much. This indicates that the drop in deaths overall is attributable to out-of-hospital stroke deaths and suggests strongly that incidence must therefore have declined (Gillum et al., 1985).
The underlying causes of the long-term decline in stroke deaths are not well established. It is clear, however, that there has been improved control of the major risk factor—hypertension—as well as effective prevention of stroke in cases experiencing TIA. Nevertheless, computer modelling by Bonita and Beaglehole (1986) suggests that drug treatment of hypertension contributed less to reduced stroke mortality than reduction in levels of population risk factors (e.g., serum cholesterol).
Associations Between Risk Factors in Individuals and Stroke
Stroke deaths and incidence rates are very low until age 45. After that, they rise precipitously, more than doubling for each decade (Kannel and Wolf, 1983).
Stroke deaths, prevalence, and incidence rates are generally similar for men and women after age 55, in contrast to the much larger and more persistent sex differential for CHD. Cerebral atherosclerosis and stroke begin later in life. In postmenopausal women this is presumed to be due to the loss of protection afforded by their hormonal status (Kannel and Wolf, 1983). The downward trend in stroke deaths, which started earlier and was greater among women, can be explained in part by more effective hypertension control (Garraway and Whisnant, 1987).
In the United States, stroke deaths and incidence rates are higher among blacks than among whites (Kannel and Wolf, 1983). However, Ni-Hon-San data suggest that environment exerts an even greater influence, since stroke rates were substantially greater among Japanese living in Japan than among Japanese migrants living in Ha-
Page 105
waii and California (Kagan et al., 1976, 1980). Because there is little difference in the prevalence of hypertension in migrant Japanese populations, the difference in stroke rates was an early clue to the possibility that habitual diet and serum cholesterol levels play a role in hemorrhagic stroke (Kagan et al., 1976; Komachi, 1977; Takeya et al., 1984; Ueshima et al., 1980). Other studies confirm the role of acculturation in the rise and fall of stroke risk (Bonita and Beaglehole, 1982).
There are few systematic data on the relationship of socioeconomic, occupational, or psychosocial characteristics to stroke death or incidence.
Close relatives of a stroke patient are at slightly greater risk of stroke than genetically unrelated persons (Heyden et al., 1969). Results of a study conducted in Göteborg, Sweden (Welin et al., 1987), suggest that maternal history is more important than paternal history in determining risk.
Hypertension has been consistently, strongly, continuously, and independently related to risk of stroke in individuals within populations at high risk for stroke (Dyken, 1984; Kannel and Wolf, 1983; Welin et al., 1987). Because of the great prevalence of hypertension in the United States, the population risk of stroke attributable to hypertension, as well as the risk for stroke for individuals, is high. The risk associated with elevated blood pressure increases continuously with increases in diastolic and systolic levels. It increases with increases in systolic blood pressure levels even when the diastolic level remains constant. In the Framingham 24-year follow-up study, for example, the 2-year incidence of stroke among men and women ages 50 to 79 was progressively greater as systolic blood pressure levels increased from below 140 to above 160 mm Hg, regardless of level of diastolic pressure. This finding suggests that an elevated systolic blood pressure even in the presence of normal diastolic blood pressure (i.e., isolated systolic hypertension) constitutes a risk for stroke (Kannel and Wolf, 1983).
Age-adjusted annual incidence of cerebral infarction was more than twice as great in people with diabetes as in those without the disease (Kannel and Wolf, 1983). This differential applied to both sexes and was independent of the associated risk factors: blood pressure, serum cholesterol, cigarette smoking, and electrocardiographic findings.
Cigarette smoking was positively and independently associated with cerebral infarction in men below age 65 in the Honolulu and Framingham studies (Abbott et al., 1986; Kannel and Wolf, 1983), but only a weak relationship was found in the Chicago Stroke Study (Ostfeld et al., 1974).
In the Framingham Study, stroke incidence was negatively related to relative body weight in men over age 65, but positively related under age 65 (Kannel and Wolf, 1983). Excessive abdominal fat was positively related in Göteborg men (Welin et al., 1987).
Several surveys link self-reported alcohol intake to risk of stroke, including cerebral hemorrhage and cerebral infarction. Age-adjusted incidence rose steadily from the level of nondrinkers to those drinking more than 30 ounces of alcohol per month in populations followed in Japan (Omae et al., 1976), Honolulu (Kagan et .al., 1980), Alabama (Peacock et al., 1972), Framingham (Marshall, 1971), and Chicago (Gill et al., 1986).
The presence of CHD, peripheral arterial disease (PAD), or hypertensive heart disease is strongly associated with stroke risk (Ostfeld et al., 1974).
Peripheral Arterial Disease (PAD)
The term PAD is used here to refer to a specific entity of peripheral vascular disease (PVD) and includes clinical syndromes of arterial insufficiency in the lower extremities. These syndromes are characterized by pain, inflammation, and ischemic damage to soft tissues from occlusion of the arteries. The characteristic syndrome of PAD is intermittent claudication—i.e., cramping, aching, and numbness of the extremities—which is induced by exercise but resolves promptly when exercise ceases. Arterial aneurysms, an advanced form of PAD, lead to swelling, tissue separation, or rupture of major arteries in the abdomen or pelvis. Arteriosclerosis obliterans is the most common clinical form, constituting the majority of cases of public health concern. Its basic pathology is obliteration of the arterial lumen due to the formation of atherosclerotic plaques with or without thrombosis.
PAD is diagnosed by a combination of clinical history (e.g., history of pain in the extremities upon exertion) and changes in the color, temperature, and appearance of the skin; arterial pulsations or abnormalities of blood flow; and blood pressure or pulse transmission or reappearance
Page 106
time. Angiography is the reference method for assessing type and severity of the arterial disease, but ultrasound promises to provide effective, noninvasive diagnoses.
There are few systematic data for comparing frequency of PAD among or within populations, and few studies of risk characteristics have been conducted within populations. In addition, mortality data for PAD are unreliable because of the variety of syndromes involved, their low relative frequency as a direct cause of death, and uncertainties pertaining to cause of death, which is usually ascribed to associated cardiac, brain, or kidney disease. Furthermore, there is no reliable information on trends in prevalence, incidence, or deaths from PAD. An exception is one population-based study, predominantly of whites with an average age of 66, which provided evidence that large-vessel PAD was present in 11.7% of the subjects (Criqui et al., 1985b).
Peripheral venous disease is not considered here because of its remote relationship to diet and nutrition.
Associations Between Risk Factors in Individuals and PAD
PAD is characteristically, but not exclusively, a disease of older age. In a California study, there was a dramatic increase in PAD prevalence by age (Criqui et al., 1985b). Framingham data also showed a steady rise in the average annual incidence of intermittent claudication by age, mainly from age 55 onward (Kannel and McGee, 1985). Clinical onset may be delayed to older ages by the large caliber of the arteries involved and, thus, the extreme degree of obstructive atherothrombotic disease that is required before blood flow is sufficiently impaired to produce symptoms.
There are few systematic data on the distribution of PAD by sex. An older Mayo Clinic series conducted in the 1940s indicated that the prevalence of PAD was six times higher in males than in females (Allen et al., 1946). In California, the prevalence of intermittent claudication was greater in men (2.2%) than in women (1.7%) (Criqui et al., 1985b). In Framingham, the incidence of intermittent claudication in men was approximately twice that in women up to age 65 (11.6 vs 5.3%); at older ages, the incidence was similar (Kannel and McGee, 1985).
There are no systematic population data on ethnic, racial, and migrant differences in PAD, but a clinical series in the United States suggest no large ethnic or racial differences (Juergens et al., 1959).
Systematic data are not available.
There is apparently no strong familial or genetic role in PAD. However, diabetes mellitus, which is associated with PAD as described below, does aggregate in families and could thus lead to a similar familial association for PAD.
Clinical observations suggest a strong concentration of PAD risk among people with diabetes (Schadt et al., 1961). This was confirmed by the greater annual incidence of intermittent claudication in diabetic men and women in the Framingham Study. The attributable fraction—i.e., the excess cases of intermittent claudication due to diabetes (at ages 45 to 74)—affects more men (13.6%) than women (2.7%) (Kannel and McGee, 1985).
Elevation of blood sugar, urinary sugar, or clinical diabetes increases the risk for intermittent claudication about four- or fivefold. In a population-based cohort of diabetics in Rochester, Minnesota, the cumulative incidence of PAD was 15% at 10 years and 45% at 20 years after diagnosis of diabetes (Melton et al., 1980). Diabetes and glucose intolerance are consistently associated with PAD in these and other studies (Bothig et al., 1976; Keen et al., 1965; Reunanen et al., 1982).
The incidence of intermittent claudication increases steadily and steeply at all ages with the number of cigarettes smoked (Kannel and McGee, 1985). Even beyond age 65, women smokers in Framingham were at greater risk. Some clinical series some years ago indicated that 90% of all PAD cases were cigarette smokers (Juergens et al., 1960). The strong relationship of PAD to smoking was confirmed by autopsy studies in which the incidence of aortic atheromatous plaque in white males was much greater in smokers than in nonsmokers (Strong and Richards, 1976).
Page 107
The relationship between systolic blood pressure and annual incidence of intermittent claudication in the Framingham 14-year followup was positive in both sexes. Ratios between hypertensives and normotensives were 2 or 3 to 1 (Kannel and Sorlie, 1979).
In the Framingham study, there was an inverse relationship between physical activity and intermittent claudication, but it was not statistically significant (Kannel and Sorlie, 1979). In Framingham, there was also a strong curvilinear relationship between the incidence of intermittent claudication and a coronary risk index of CHD risk characteristics including age, blood cholesterol level, electrocardiographic (ECG) findings, systolic blood pressure, relative weight, hemoglobin, and cigarette use (Kannel and McGee, 1985). The strongest relationships were observed for cigarette smoking and hypertension for both sexes and for impaired glucose tolerance, which was stronger for women than for men. Serum total cholesterol and relative weight were only weakly significant risk factors. Clustering of intermittent claudication with other atherosclerotic and cardiovascular diseases was also pronounced in the Framingham Study. Overt coronary disease, cerebrovascular disease, or congestive heart failure was found in one out of three cases of intermittent claudication at the time it was initially diagnosed, suggesting common risk characteristics. The relative risk of developing intermittent claudication in Framingham patients with CHD was four times the standard risk in the cohort, whereas people with angina pectoris had three times greater risk (Kannel and Shurtleff, 1971).
In the most recent systematic study of multiple risk factors for PAD, Criqui used objective diagnostic criteria for large- and small-vessel diseases (Michael Criqui, University of California, San Diego, personal communication, 1988). For large-vessel disease, they showed that age, smoking, fasting plasma glucose, and systolic blood pressure were strongly associated with the prevalence of PAD and that obesity, LDL, and HDL were marginally related. Small-vessel PAD was only weakly related to atherosclerotic cardiovascular disease risk factors.
In general, the risk factors for PAD are similar to those for CHD and stroke, although their relative importance differs. For example, diabetes and smoking are the preeminent risk factors for PAD but not for CHD, followed by triglycerides, VLDL, and blood glucose tolerance, and to a lesser extent LDL, HDL, and serum total cholesterol, which strongly predict CHD.
Hypertension and Hypertension-Related Diseases
Hypertension is defined as elevated arterial blood pressure measured indirectly by an inflatable cuff and pressure manometer. Blood pressure is a continuous or graded phenomenon, and .the risk of hypertension increases steadily with blood pressure level—either systolic (SBP) or diastolic (DBP). Therefore, any definition of elevated blood pressure is arbitrary and is done primarily to facilitate decisions regarding pharmacological therapy.
Traditionally, physicians diagnose hypertension using DBP, which is the lower value found during the resting phase of the cardiac cycle. DBP levels have been closely associated in clinical studies with manifest diseases or complications associated with high blood pressure (e.g., stroke, hypertensive heart disease, CHD, and kidney disease) and are used as the basis of most treatment decisions. Systematic population studies indicate, however, that systolic blood pressure, the higher value recorded during cardiac systole, is not only a more reliable measurement but also a more precise indicator of the risk of future complications in hypertension.
For practical treatment purposes and for population comparisons, the following criteria developed by the World Health Organization (WHO, 1978) are often used: normotension, systolic £ 140 and diastolic £ 90 mm Hg; borderline hypertension, systolic 141-159 and diastolic 91-94 mm Hg; and hypertension, systolic ³160 and diastolic ³ 95 mm Hg. Severe hypertension is often defined as diastolic levels of 115 mm Hg and above. The Joint National Committee on Detection, Evaluation, and Treatment of High Blood Pressure (NC, 1988) added classifications for high normal, mild, moderate, severe, and isolated systolic hypertension (see Table 20-1, Chapter 20).
Deaths related to hypertension have been variously classified over recent years. They have either been considered as a separate entity or combined with such classes of atherosclerotic cardiovascular diseases as CHD and stroke. Thus, it is not useful to consider vital statistics alone in discussing the epidemiology of hypertension. Hypertension is treated here primarily as a risk characteristic for atherosclerotic cardiovascular diseases rather than as a disease entity in itself.
Page 108
Population Differences in Blood Pressure and Hypertension
Surveys comparable to those of the U.S. National Center for Health Statistics (DHEW, 1963) have not been conducted outside the United States. However, many smaller-scale surveys attest to the fact that hypertension is prevalent in industrialized nations throughout the world. The prevalence in England (Hamilton et al., 1954), Western Europe (Keys, 1980), and Australia (MacMahon and Leeder, 1984) is similar to that in the United States. Data from China (Wu et al., 1982), Japan (Komachi and Shimamoto, 1980), and Korea (Kesteloot et al., 1976) show prevalence rates in urban populations equal to or greater than those in United States.
A low prevalence of hypertension has often been found in studies of traditional rural populations outside Western culture. More than 20 societies, representing a variety of races, diets, habitats, and modes of life, have been found to have virtually no hypertension and little or no tendency for blood pressure to rise with age. Social and anthropological characteristics of these populations are similar: they are relatively isolated, subsistence cultures; their salt intake is less than 4.5 g daily; there is an absence of obesity; and they remain physically active throughout life (Page, 1979). For more detailed data on the geographic distribution of blood pressure, see Epstein and Eckoff (1967).
There have been reports of regional differences in the frequency of hypertension and in mean blood pressure values. People in the southeastern United States tend to have higher pressures (DHHS, 1986) as do people in the northern provinces of Japan (Komachi, 1977; Omae et al., 1976) and in the French-speaking areas of Belgium (Kesteloot et al., 1980). Regional differences are not well explained, but they appear to be independent of race and socioeconomic status.
Time Trends in Frequency and Mortality
In the United States, average trends in blood pressure for people between the ages of 25 and 74 have been examined in surveys conducted by the National Center for Health Statistics—the Nationwide Health Examination Survey (NHES I) of 1960-1962 (DHEW, 1963) and the Nationwide Health and Nutrition Examination Surveys of 1971-1975 (NHANES I) (Abraham et al., 1983) and 1976-1980 (NHANES II) (Carroll et al., 1983). Assuming comparability of methods and design (DHEW, 1963; DHHS, 1986), one can see no significant trend in the population means or the distribution of average blood pressures for given ages between the first two surveys. However, the third survey, NHANES II, shows markedly lower average systolic blood pressures at all ages above 30. A much lower prevalence of hypertension is also apparent in people over 40 in that survey compared to those in the earlier two. When NHANES II data (DHHS, 1986) are used, the prevalence of definite hypertension is 17.7% in adults as defined by the World Health Organization (WHO, 1978) or 29.7% as defined by the criteria of the third Joint National Committee on Detection, Evaluation, and Treatment of High Blood Pressure (JNC, 1988).
Concurrent with the decline in hypertension prevalence and in mean blood pressure indicated in these U.S. national surveys, there has been an improvement in the status of medical detection and control of high blood pressure. Previously undetected hypertension (i.e., cases newly diagnosed at screening) was halved—from 51.1% during 1960-1962 to 26.6% during 1976-1980. The proportion of hypertension controlled by medication doubled—from 16% during 1960-1962 to 34.1% during 1976-1980.
Data are insufficient to attribute these changes with certainty to medical detection and control or to primary prevention in the general population. Both have occurred as modelled by Bonita and Beaglehole (1986), but there is as yet no direct evidence of a decrease in mean population blood pressure independent of medical treatment. There is also evidence that trends in high blood pressure are not an indirect result of changes in average relative weight or alcohol intake—both of which have risen in recent years in the United States while mean blood pressure has fallen.
The increased risk of morbidity and mortality from cardiovascular diseases associated with hypertension has been repeatedly demonstrated in epidemiologic studies throughout the industrialized world (Stamler et al., 1975). The risk of hypertension varies markedly with age, sex, race, and the total burden imposed by associated risk factors, including socioeconomic status, occupation, obesity, family history, psychosocial stresses, and other factors (as discussed below). The American Heart Association (AHA, 1973) has published multiple risk calculations for different age and sex
Page 109
groups based on major risk factors using Framingham study data. In general, the risk for major coronary disease events increases by 30% for each 10 mm Hg increase in systolic blood pressure in both men and women of all ages (Dawber and Kannel, 1972). Interaction of hypertension with other risk factors may alter their risk as much as sixfold.
A striking feature of the epidemiology of hypertension is the remarkable decrease in hypertension-related deaths, i.e., from all cardiovascular disease, cerebrovascular disease, renal disease, hypertensive heart disease, and CHD (DHHS, 1987). This trend is so consistent and strong that it must represent a real change, independent of improved classification in death certification. The beginning of the decline in hypertension-related mortality clearly antedated the widespread use of effective antihypertensive drugs, but it accelerated in the early 1970s when such products came into wide use. Population-based surveys in the U.S. Southwest (Franco et al., 1985) and in Minnesota (Folsom et al., 1983) indicate that 85 to 90% of high blood pressure cases are now detected and under therapeutic control in urban areas.
Estimating the proportion of cases of hypertension in the population attributed to a known risk factor (the population-attributable fraction) indicates the potential impact that effective reduction in the risk factor could have in reducing hypertension-associated mortality in the population. Calculation of the attributable fraction for all known risk related to hypertension suggests that effective control of hypertension in the population could result in a 20% reduction in all-cause mortality among whites along with a 30% reduction among black men and a 45% reduction among black women based on mortality rates among nonhypertensives. The estimated decline in death rates is similar to the observed decline in the age-adjusted U.S. mortality from 1968 to 1975. It is therefore likely that part of the decline in cardiovascular deaths as well as in all-cause deaths since 1972 is attributable to the improved medical control of hypertension. However, the actual contribution of control may be less than this estimation (Bonita and Beaglehole, 1986).
Associations Between Risk Factors in Individuals and Hypertension
As shown in Figure 5-3, systolic blood pressure rises steeply from infancy to adulthood (Voors et al., 1978) and levels off once adult height is reached. The age-related upward trend in mean systolic pressure occurs across most adult populations. Cross-sectional and cohort data from the Framingham Study (Kannel, 1980) give a somewhat different picture of therelationship of systolic and diastolic blood pressure to age in adults (Figure
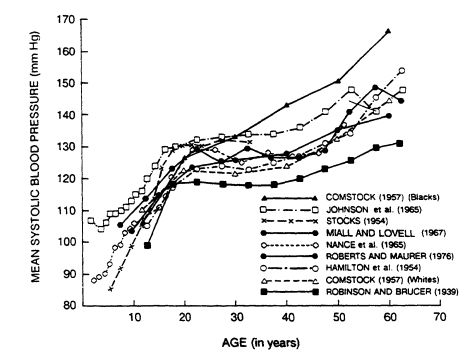
FIGURE 5-3
Changes in mean systolic blood pressures from infancy to age 70 in nine studies—
Comstock, 1957; Hamilton et al., 1954; Johnson et al., 1965; Miall and Lovell, 1967;
Nance et al., 1965; Roberts and Maurer, 1976; Robinson and Brucer, 1939;
Stocks, 1954. From Voors et al. (1978).
Page 110
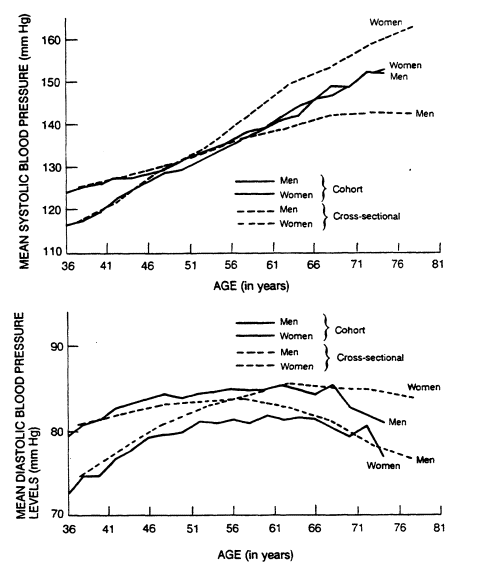
FIGURE 5-4
Cohort and cross-sectional age trends in systolic and
diastolic blood pressures by sex. From Kannel (1980).
5-4). The cross-sectional data show systolic pressure starting lower in women than in men but rising more steeply, crossing over in the fifth decade, and continuing upward with age into the 80s, whereas pressures in men level off after age 70. Cohort data show systolic pressure starting lower in women, but rising to meet that of men by age 60, after which systolic pressure rises in men and women at the same rate. With respect to diastolic blood pressure, the cross-sectional data show a higher level in men than in women in their early decades and the reverse during the middle 50s when levels decrease in men and further increase in women. The cohort data show that diastolic pressures are consistently lower in women than in men of all ages, increase in both sexes in the early decades, flatten in the 50s, and decline after age 65.
On average, longitudinal studies show that systolic pressure increases about 20 mm Hg between ages 20 and 60 and an additional 20 mm between ages 60 and 80. Diastolic blood pressure, on the other hand, rises approximately 10 mm between the ages of 20 and 60 and gradually declines thereafter (Kannel, 1980).
The dramatic age trends in blood pressure observed in the Framingham data are characteristic of those seen in most Western societies. A notable feature of this age-related rise is the phenomenon called tracking, i.e., where elevated blood pressures tend to persist from youth to adulthood (Berenson et al., 1980).
A consistent finding among industrialized societies is a higher mean blood pressure and higher prevalence of hypertension among males from
Page 111
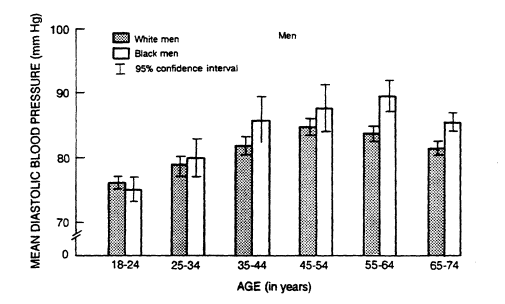
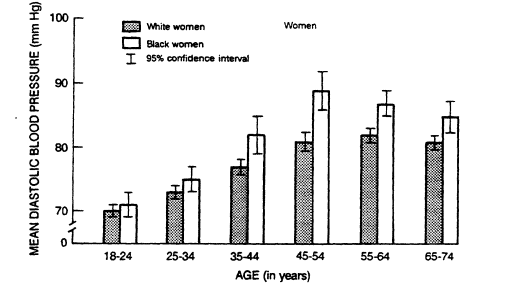
FIGURE 5-5
Mean diastolic blood pressure in U.S. whites and blacks by age and
sex from data collected from 1976 to 1980. From DHHS (1986).
adolescence through age 45. After age 45, mean blood pressure values are higher in women (DHHS, 1986).
An excess of hypertension and its complications has been found in black adults in the U.S. population (DHHS, 1986) and in most other societies that have been systematically studied. Among U.S. blacks there is a stronger upward age trend than among whites and a higher prevalence of hypertension at all ages (Figure 5-5). The point at which the average blood pressure of women crosses that of men (the cut point) is about 5 years earlier for blacks than for whites. In the Hypertension Detection and Follow-Up Program (HDFP, 1979) and in the Evans County Study (Heyman et al., 1971), blacks also had a higher prevalence of hypertension-related complications (such as cerebrovascular, cardiovascular, and renal events) than whites at all blood pressure levels (HDFP, 1979). Data on young people are inconsistent, but in Bogalusa, Louisiana, where automated instruments were used to reduce observer bias, higher blood pressure levels were demonstrated in blacks as early as age 5 (Berenson, 1980).
Migrant studies emphasize the effects of cultural and environmental factors on the expression of
Page 112
hypertension in individuals, and hence on population differences. Average blood pressure and the frequency of hypertension and its complications rise rapidly when people move from rural to urban settings. The effects of migration are evident as early as 1 month after changing location, and the proportionate increase in blood pressure is greater among older adults. These findings suggest that trends in blood pressure with age as well as mean levels of blood pressure are very sensitive to diet and other environmental influences (see Chapter 20).
The prevalence of hypertension varies inversely with educational attainment and with occupational, social, and economic status for blacks as well as whites. However, the higher blood pressure levels in blacks persist after control for socioeconomic variables (HDFP, 1979).
In the few occupational studies conducted, data on blood pressure differences are confounded by relative weight and alcohol intake, among other factors, and no specific occupations are known to be associated with excess hypertension. Physical activity associated with occupation is not consistently related to average blood pressure level (Leon and Blackburn, 1982).
Migrant population studies confirm that blood pressure is affected by the transition from traditional to urban societies (see Chapter 20), but it is difficult to separate the effects of psychosocial change from the changed physiological exposures that accompany migration. Life events, social behaviors, cultural dissonance, and related factors have been associated with blood pressure differences, but these studies have rarely controlled for physiological or nutritional confounders.
Within cultures where average blood pressure is elevated and the prevalence of hypertension is substantial, studies of families show a genetic effect relatively stronger than environmental variables, including relative body weight and sodium intake. First-degree genetic relatives have similar systolic and diastolic pressures. Relatives in the same household may share environmental factors as well as genes that may influence blood pressure; however, the similarity of blood pressure among genetic relatives is greater than among nongenetic relatives, greater for monozygotic than dizygotic twins, greater for first-degree genetic relatives, and greater among natural than among adopted children (Folkow, 1982). All these point to an influence of genetic factors on blood pressure. But these are weak predictors, and genetic susceptibility of individuals is not now measurable.
Blood pressure is a result of interaction between environmental and host factors. Phenotypic expression is modified by changing the environment, but this does not negate the overall importance of genes in etiology. The large variation in the frequency of hypertension among populations, the rapid changes in blood pressure levels and frequency of hypertension in migrant populations, and the ability to modify blood pressure levels experimentally without drugs all suggest that susceptibility is widespread and that the environment is the prominent factor in the expression of hypertension (Prineas and Blackburn, 1985).
Associations of Blood Pressure with Relative Body Weight and Obesity
The evidence associating dietary factors with hypertension is summarized in Chapter 20. Obesity has also been associated with hypertension, but in one study when body build was taken into consideration, total fatness was no longer related to blood pressure (Weinsier et al., 1985). Thus, hypertension may be affected by body build as well as by fatness. Weight reduction has also been associated with a decrease in blood pressure, independent of a decrease in sodium intake (Reisin et al., 1978). Evans County data show that overweight and a weight gain of 10 pounds or more are associated with the occurrence of new hypertension (³ 95 mm Hg), and that blood pressure rises stepwise according to the level of overweight or weight gain (Tyroler et al., 1975). Those overweight at the outset of the study, and those who gained 10 pounds or more, were 8 to 10 times more likely to develop new hypertension than those who were neither overweight nor had gained weight. The absolute effect was somewhat greater among blacks, but the gradient of relative risk was somewhat steeper among whites.
Associations of Blood Pressure and Hypertension
with Atherosclerotic Cardiovascular Diseases and Death
Blood pressure is a strong and independent risk predictor for CHD—a relationship that was thoroughly reviewed in the report of Inter-Society Commission for Heart Disease Resources (1984). CHD patients have higher average blood pressure than controls. Atherosclerosis in laboratory ani-
Page 113
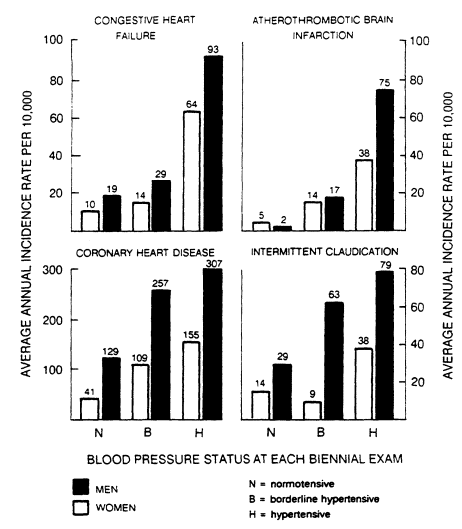
FIGURE 5-6
Average annual incidence of cardiovascular disease according to blood pressure status
at each biennial examination for men and women between the ages of 55 and 64, as observed
during the 16-year follow-up of a population in Framingham, Massachusetts. From Kannel (1974).
mals is directly related to pressure within the arterial system. Short-term and long-term follow-up studies indicate that both systolic and diastolic blood pressure predict CHD risk; this observation was strongest for systolic blood pressure, but it is not clear whether this resulted from more precise measurement or whether systolic pressure is closer in the chain of causation leading to atherosclerosis and CHD. The relationship between elevated blood pressure and risk of cerebral vascular accidents (CVA) and congestive heart failure (CHF) is even stronger than that between blood pressure and CHD. These relationships are consistent and widely confirmed (Inter-Society Commission for Heart Disease Resources, 1984).
Despite the strong individual correlations between blood pressure level and CHD risk within populations, the frequency of hypertension does not account for much of the variation of CHD incidence between populations. Nevertheless, estimates of the number of population events attributable to blood pressure suggest that changes in CHD incidence rates related to relatively small average blood pressure differences (reductions) hold a great potential for the prevention of CHD (Rose, 1981).
For men and for women, there is a strong and consistent relationship between systolic and diastolic blood pressure levels and death from all causes, including CHD and stroke, and the incidence of first coronary events.
More relevant to the public health importance of hypertension are the Framingham data on 55- to 64-year-old men and women (see Figure 5-6). These data show a doubling of risk for coronary and peripheral vascular disease, a quintupling of risk for heart failure, and a 10- to 20-fold higher risk of cerebral infarction in hypertensives (Kannel, 1974). The proportion of increased risk attributable to elevated blood pressure ranges from 57% (coronary events) to 89% (brain infarction). Thus, hypertension has a large impact on two leading causes of death—CHD and brain infarction.
Page 114
Obesity and Related Diseases
Obesity is defined as excessively high body fat in relation to lean body mass. Overweight refers to a deviation in body weight above some standard of acceptable weight, which is usually defined in relation to height. Because obesity and overweight are both continuous variables, any cut point is arbitrary. A rational basis for a cut point is the relative weight causally associated with the lowest mortality (see Chapter 21).
Techniques for measuring body fat are needed to distinguish between obesity and overweight resulting from increases in lean body mass. For most people, overweight is associated with increases in body fat stores. Professional athletes and body builders may be overweight despite low body fat stores. Overweight can be expressed in several ways, as shown below, and the varied use of these measurements confounds interpretation among studies. Relative weight refers to the ratio or percentage of actual weight to some standard or desirable weight. The most widely used standards are the Metropolitan Life Insurance Tables; actual weights-for-height compared to them are called Metropolitan Relative Weights. Weight and height can also be related by ratios such as weight divided by height, height divided by the cube root of weight (ponderal index), or weight divided by height to some power. When this power is 2, the ratio (weight/height2) is called the body mass index (BMI) or Quetelet Index (QI). Degrees of excess weight can also be defined in terms of the Broca Index, in which desirable weight in kilograms equals height in centimeters minus 100. Weights 20% higher than the Broca desirable weight are considered excessive in most European studies. Relative weight and the BMI are used most frequently in the United States and are becoming widely used internationally.
Obesity is measured by a number of techniques. Skinfold measurements are widely used in epidemiologic studies; however, comparisons of skinfold measurements to BMI by the National Center for Health Statistics (Abraham et al., 1983) indicated that a substantial proportion of people in the top 15% of BMI are not included in the top 15% of skinfolds, and vice versa. When only the top 5% for each measurement were compared, however, there was almost complete congruence, indicating that overweight (BMI) and obesity (skinfolds) are synonymous at this level. Thus, definitions of obesity can be based on either skinfold measurements or body weight in relation to height (BMI) as follows: (1) a BMI above 30 kg/m2, (2) a triceps plus subscapular skinfold above 45 mm in males and 65 mm in females, or (3) body fat more than 25% of body weight in males or 30% in females. Overweight, on the other hand, has been defined as follows: (1) a BMI between 25 and 30 kg/m2, (2) body weight 20 to 40% above the median weight for normal frame size according to the Metropolitan Life Insurance Tables, or (3) weight 20%. above the calculated Broca Index.
In addition to definitions of total body fat, considerable evidence suggests that regional fat distribution influences the risk of mortality from atherosclerotic cardiovascular diseases and diabetes mellitus. The most widely used techniques for assessing regional fat distribution involve determining the ratio of abdominal (waist) circumference to gluteal region (hip) circumference, measuring subscapular skinfolds, or determining the ratio of skinfolds on the trunk to those on the extremities (see Chapter 21).
Population Differences in Frequency
In a survey of selected European countries, Kluthe and Schubert (1985) showed that the prevalence of obesity among females ranged from 2 to more than 50%, and among males, from 2 to more than 40%. These differences were partly related to the different measurements used.
Bray (1985) compared the percent of overweight (BMI between 25 and 30) and obese (BMI over 30) people in Australia, the United States, Canada, Great Britain, and the Netherlands (Table 5-1). The overall percentage of males and females with a BMI between 25 and 30 is similar in the five countries. The percentages for those with a BMI above 30 kg/m2 is similar in Australia and Britain, but nearly twice as great in the United States and Canada.
On the basis of the evidence described above, approximately 34 million adults in the United States were overweight between 1976 and 1980. Of these, about 12.4 million were severely overweight (Abraham et al., 1983).
Japanese migrants living in Hawaii or California have a higher relative body weight compared to
Page 115
| TABLE 5-1 Percentage of People with a Given Body Mass Index in Several Affluent Countriesa | |||||
| Overweight (BMI 25-30 kg/m2) | Obese (BMI >30 kg/m2) | ||||
| Country | Age | M | F | M | F |
| United States | 20-74 | 31 | 24 | 12 | 12 |
| Canada | 20-69 | 40 | 28 | 9 | 12 |
| Great Britain | 16-65 | 34 | 24 | 6 | 8 |
| Netherlands | 20+ | 34 | 24 | 4 | 6 |
| Australia | 25-64 | 34 | 24 | 7 | 7 |
| a Adapted from Bray (1985). Data Sources: Abraham et al. (1983); Black et al. (1983); Millar and Stephens (1987); National Heart Foundation of Australia (1981); Seidell et al. (1986) | |||||
native Japanese living in Japan. Similarly, Irish immigrants living in Boston tend to be heavier than brothers of the same height and age remaining in Ireland (Brown et al., 1970).
Time Trends in Frequency
Several studies suggest that there has been a progressive increase in relative weight throughout the past hundred years in industrial societies (Abraham et al., 1983; Bray, 1979). For example, the weight of men 5 feet, 8 inches tall inducted into U.S. military service rose from 147 pounds in 1863 to 168 pounds in 1962. Life insurance data and NCHS data also show a small age-related increase in average relative body weight. Increases in the percentage of overweight men and women are greatest in the younger age groups. A comparison of the three sets of NHANES data (19601962, 1971-1974, 1976-1980) (Harper, 1987) based on the same criteria for overweight in men is shown in Figure 5-7. Overall, there was no change over the three surveys. In the 25- to 54-year age groups, however, there was an increase in the percentage of overweight men and women. Beyond age 54, the percentage of overweight men and women decreased.
Associations Between Risk Factors in Individuals and Obesity
Relative body weight increases with age in both men and women, but there is a greater proportional increase among women (Figure 5-8). The percentage of overweight among black women is higher than among white women at all ages. The percentage of overweight among white men is higher than among black men until age 35 when the proportion of overweight black men begins to exceed that of white men. After age 55, the percentage of overweight among white and black men is similar (Abraham et al., 1983).
Women tend to weigh more than their male counterparts. A higher percentage of women are obese in almost all reported studies (Kluthe and Schubert, 1985; Van Itallie, 1985) (see Figure 5-8).
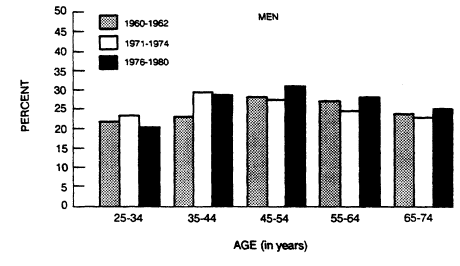
FIGURE 5-7
Percentage of overweight men in the United States, by age,
1960-1962, 1971-1974, and 1976-1980. From Harper (1987).
Page 116
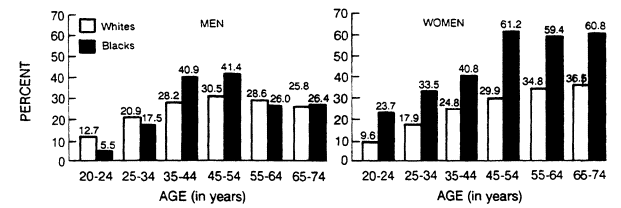
FIGURE 5-8
Percentage of overweight men and nonpregnant women in the United States,
by age and race, during 1976-1980. From Van Itallie (1985).
The prevalence of obesity differs among races (Figure 5-8). For men between 20 and 34 years of age, more whites than blacks are obese, but between the ages of 35 and 54, the frequency of obesity is higher among black men than among white men. After age 55, these race differences for men vanish. The data for women are more uniform: a higher percentage of black women (solid bars) are overweight between ages 20 and 74. Between the ages of 45 and 54, for example, 29.9% of white women compared to 61.2% of black women are obese.
Studies of obesity have almost uniformly demonstrated the importance of socioeconomic status in females and, to a much smaller degree, in males (Moore et al., 1962). As Figure 5-9 indicates, the percentage of overweight women above age 25 was highest in the poverty group. The differences among males were small and not statistically significant. There is no clear evidence that occupation plays an important role in the development of obesity. Although a number of studies have purported to show personality or behavioral patterns associated with obesity, other more methodologically rigorous studies have, in general, shown no association. Thus, no psychological risk factors for the development of obesity have been identified (Wadden and Stunkard, 1985).
There is a high familial association with obesity. If both parents are overweight, approximately 80% of the offspring are overweight. When neither parent is overweight, fewer than 10% of the children are overweight (Bray, 1987). The separation of genetic from environmental factors has relied on studies of twins and adopted children. Using path analysis to evaluate data on identical twins, nonidentical twins, and various parental and sibling relation-
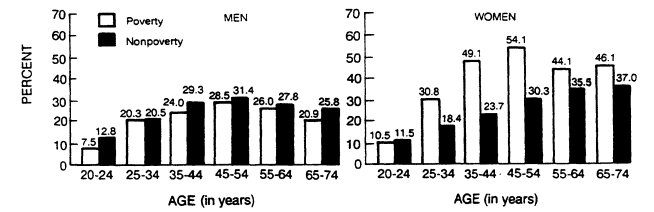
FIGURE 5-9
Percentage of overweight men and nonpregnant women in the United States,
by age and poverty status, during 1976-1980. From Van Itallie (1985).
Page 117
ships, Bouchard et al. (1988) concluded that approximately 50% of the variation in body fatness is transmissible to offspring, and of this amount, approximately half is under genetic control. This is lower than the estimates of heritability published by Stunkard et al. (1986), who compared BMI among identical and nonidentical twins and estimated heritability of body weight to be between 50 and 80%.
From these studies in affluent countries, it can be concluded that there is an important genetic component in the development of obesity. This genetic component interacts with environmental agents, primarily diet and level of physical activity, to produce obesity in susceptible people.
Obesity as a Risk Factor for Chronic Diseases
There is evidence that obesity is an independent risk factor for the development of atherosclerotic cardiovascular diseases, hypertension, diabetes mellitus, gallbladder disease, and some cancers, but opinions about the relative importance of these associations differ. In five prospective studies, fat distribution was found to be more strongly related to risk of total deaths, stroke, heart disease, and diabetes mellitus than was BMI or total body fat. The risk doubled in people with an increased ratio of abdominal to hip fat and showed a graded relationship at higher levels (Donahue et al., 1987; Ducimetiere et al., 1986; Lapidus et al., 1984; Larsson et al., 1984; Stokes et al., 1985).
Using BMI or relative weight, studies with small populations or of short duration usually fail to find an independent association of overweight with cardiovascular deaths (Keys et al., 1972; Pooling Project Research Group, 1978). Some longer studies (Hubert et al., 1983; Rabkin et al., 1977) or studies with more subjects (Lew and Garfinkel, 1979; Society of Actuaries, 1980; Waaler, 1984) have led to the conclusion that overweight is an independent predictor of risk of atherosclerotic cardiovascular diseases. This may be due to the strong association of obesity with risk of hypertension, diabetes mellitus, and lowered levels of HDL cholesterol, which are in themselves important risk factors for atherosclerotic cardiovascular diseases (Manson et al., 1987).
Diabetes mellitus is the disease most strongly associated with obesity. Mortality among people with a BMI of 35 kg/m2 or greater is eightfold higher than that of people with a normal BMI (20 to 25 kg/m2) (Lew and Garfinkel, 1979; Waaler, 1983).
The next strongest association of obesity is with gallbladder disease. Those with a BMI of 33 kg/m2 had four times the death rate from gallbladder disease as those with normal BMI (Lew and Garfinkel, 1979).
Obesity and possibly body build are also related to risk of hypertension (Weinsier et al., 1985; see Chapter 20). Weight reduction is associated with reduced blood pressure levels (Reisen et al., 1978).
Obesity is a risk factor for endometrial cancer (Lew and Garfinkel, 1979) and for postmenopausal breast cancer (Lubin et al., 1985). Further considerations of these associations and of possible associations with other cancer sites (e.g., ovary and prostate) are discussed in Chapter 21.
Cancers
Cancers are populations of cells that have acquired the ability to multiply and spread without the usual biologic restraints (NRC, 1982). There are at least as many different cancers as there are tissues of the body. It is important to consider the distribution and determinants of cancers according to the individual cancer sites. This section focuses on cancers that are common in the United States today and are associated with dietary factors.
Cancers are diagnosed by many different procedures but are usually confirmed by histological examination of resected tissue. They are classified by the International Classification of Diseases (ICD) largely by site, such as lung cancer and breast cancer. A few cancers are classified according to their histologic characteristics, e.g., melanomas and leukemias.
The cancers discussed in this report fall within ICD-9 categories 140 through 208 for malignant tumors (ICD, 1977). ICD-9 categories 210 through 239 apply to tumors of uncertain nature or to those known to be benign (i.e., not infiltrating into tissues or spreading to other parts of the body).
There are major differences in the occurrence of cancers among countries. In general, rates are high in North America and low in parts of Africa and Asia. For certain cancer sites (e.g., esophagus, stomach, and liver), however, this general tendency is reversed. Within the United States, there tends to be a slightly higher incidence of all cancers in the industrial Northeast and slightly lower rates in rural areas. But for stomach cancers, the reverse is true. Most cancers occur with greater frequency as people age. Some tumors, however, are relatively frequent in children. These include
Page 118
leukemia—especially acute leukemia—bone tumors, and brain tumors.
Esophageal Cancer (ICD 150)
There is substantial international variation in incidence of esophageal cancer. High rates are found across most of middle Asia, from northern Iran and southern USSR around the Caspian Sea to northern China. In Europe, high rates occur in the Calvados region of France. Even within these areas, however, there is a large variation in incidence. For example, age-adjusted annual incidence rates in the Caspian region of Iran ranged from a high of 165.5 and 195.3 per 100,000 for males and females, respectively, in the northeast region of Gonbad to a low of 17.9 and 4.9 in the north-central region of Gilan (Mahboubi et al., 1973).
Incidence rates in the United States, a relatively low-risk area for esophageal cancer, vary considerably by race and sex. For example, rates among blacks in the San Francisco Bay Area are 11.7 for males and 3.6 for females; the corresponding rates for Chinese and whites in the same area are 6.9 and 2.1, and 4.0 and 1.9, respectively (Waterhouse et al., 1982). The higher incidence among males in all three groups has been observed in other U.S. populations as well and is a characteristic common to low-risk populations (Day and Munoz, 1982).
The disproportionately high incidence of esophageal cancer in Bay Area blacks relative to whites mirrors the higher rates of the disease seen in U.S. blacks in general. Annual age-adjusted esophageal cancer mortality rates for U.S. blacks from 1976 to 1980 exceeded those for whites by approximately threefold (14.0 and 4.2 per 100,000 among males; 3.4 and 1.2 among females). The excess mortality in blacks relative to whites was greater at younger ages; a more than sixfold difference in rates was noted in males under age 55. From 1950 to 1980, mortality rates for blacks nearly doubled, whereas rates among whites have remained fairly constant. Annual age-specific mortality rates among blacks have also risen sharply over time, but current data suggest that the rate of increase is less in people born after 1925 (Blot and Fraumeni, 1987).
The influence of migration on esophageal cancer rates is uncertain. Polish immigrants in the United States have been reported to have higher mortality rates than either U.S. whites or Poles living in Poland (Staszewski, 1974). Mortality rates among U.S. residents who migrated from Italy, Germany, Sweden, Norway, and Ireland have also been higher than those among natives of the host country or the country of origin (Haenszel, 1961).
Stomach Cancer (ICD 151)
In the United States, there has been a striking decrease in both incidence of and mortality from stomach cancer over the past 40 to 50 years (Howson et al., 1986). U.S. rates are now among the lowest in the world. Fifty years ago, stomach cancer was the most important cause of cancer death in both sexes and is now ranked fifth to sixth in importance among cancer deaths. This decline has occurred among all age groups, but stomach cancer remains more common in older people.
The mortality rate for stomach cancer is approximately twice as high for males as for females. This form of cancer tends to be more common among lower socioeconomic groups and people who migrate to the United States, compared to native-born Americans. Rates among immigrant populations tend to change to levels of the adopted country relatively slowly over one to two generations.
Stomach cancer is more frequent in Asia, particularly in Japan, but incidence rates in that country have also fallen substantially in recent years (Waterhouse et al., 1982).
Colorectal Cancer (ICD 153, 154)
Although there are differences between colon and rectal cancer, both cancers are often considered together. Colorectal cancers tend to be more common in countries where breast cancer is common and relatively rare in countries where breast cancer is also relatively rare (Waterhouse et al., 1982). There is also a strong international association between colorectal cancer rates and rates for endometrial, ovarian, and to a lesser extent prostate cancer. Within the United States, rates tend to be higher in the northern part of the country and in urban areas. The male-to-female ratio for colon cancer is close to 1; for rectal cancer, it is 1.4. Migrants to the United States from eastern Europe and Asia have an increased incidence, resembling that of the host country within 10 to 15 years. There are still differences in incidence among racial groups, but these are narrowing. There is familial aggregation of colon cancer, and the disease is common in those with certain rare genetic disorders, particularly familial polyposis and Gardner's syndrome.
Both incidence and mortality from colorectal cancer have been relatively stable for the past 30 to
Page 119
40 years. Recently, however, there has been an indication that mortality is decreasing among females in North America and, possibly, among males in the United States.
Liver Cancer (ICD 155.0)
Liver cancer is relatively rare in the United States but is common in Africa and Asia (Waterhouse et al., 1982), where it has been associated with hepatitis B infection as well as aflatoxin contamination of foods (see Chapter 22). Primary liver cancer is more common in men than in women (ratio, about 3 or 4 to 1), among persons of low socioeconomic groups and among blacks and Hispanics, compared to whites. Genetic or familial aggregation has not been noted. Like most other cancers, incidence increases progressively with age.
Pancreatic Cancer (ICD 157)
The incidence of pancreatic cancer in the United States has apparently increased in the past 20 to 30 years, but it has been relatively stable in the past 10 years. Some of these increases may have been due to better diagnosis. Pancreatic cancer is more common among men (ratio, approximately 1.5 to 1) and occurs more frequently in the United States than in Africa and Asia. High rates are also found among Polynesians in New Zealand and in Hawaii (Waterhouse et al., 1982). In the United States, rates seem to be higher for blacks than for whites. In general, pancreatic cancer occurs more commonly in higher socioeconomic groups and does not appear to have a familial association. Changes with migration have not been studied.
Lung Cancer (ICD 162)
Some of the highest lung cancer rates in the world are found in the United States-among whites living in Hawaii and blacks in Los Angeles (Waterhouse et al., 1982). In the past, rates were lower for blacks than for whites, but this has been reversed in some areas, especially among males at ages below 55. Rates are generally lower in other ethnic groups, particularly Hispanics and people of Asian origin, but are increasing rapidly. When the data within individual birth cohorts are considered, lung cancer is found to increase progressively with increasing age, particularly at older ages. In the United States, lung cancer began to increase for males early in this century and for females, shortly after World War II. The overall rates are beginning to stabilize for males and to fall for those under 55 years old. In 1983, the male age-standardized rate was lower than in any year since 1977 (Horm and Kessler, 1986). For females, however, rates are continuing to increase, and deaths from lung cancer currently surpass deaths from breast cancer in many states. If present trends continue, there will be a 1-to-1 sex ratio for lung cancer by about the year 2000 (in comparison to the current ratio of 3 or 4 to 1 and the ratio 20 years ago of 6 or 8 to 1). Historically, rates have tended to be higher in the highest socioeconomic group, but these groups now have rates below average (Devesa et al., 1987).
Breast Cancer (ICD 174)
Breast cancer incidence in females is much greater than in males (ratio, 100 to 1). For this reason, the following discussion concentrates on breast cancer in females.
Breast cancer is more common in the United States than in other parts of the world, but rates are beginning to rise in many other countries. In recent tabulations, the highest incidence rate in the world is found among Caucasians living in Hawaii. Other high rates in the United States are found in the northern parts of the mainland, particularly in affluent areas (Waterhouse et al., 1982).
Breast cancer risk increases with age, but the slope of the age-specific incidence curve is different before and after menopause. Thus, risk rises rapidly up to about the age of 50 to 55. There is then a slowing of the rate of increase, or even a reversal in some populations, followed by another rise in high-risk populations. Breast cancer tends to be more common among higher socioeconomic groups and among Caucasians. Recently, however, rates have been rising among blacks, Hispanics, and people of Asian origin. Women who migrate to the United States from Europe, particularly eastern Europe, and from Asia generally have a lower incidence of breast cancer, which over the next one or two generations generally rises to approximate that of the United States.
There is a clear familial aggregation for 10 to 25% of breast cancer cases. In a rare subset of these, breast cancer behaves as a dominantly inherited characteristic from either the maternal or paternal side. Most cases of breast cancer appear to be spontaneous, but if genetic variation is widely
Page 120
distributed and its effect weak, it would be extremely difficult to determine with current epidemiologic methods the percentage of genetically induced breast cancers. Attempts are under way to identify genetic markers of transmission. One marker, the enzyme glutamic-6-pyruvate transaminase, was found to be linked to a number of families (King et al., 1983), but further study has not shown this to be a general phenomenon. Mortality from breast cancer has been stable in the United States for the past 40 years. Recently, however, there appears to be a slight reduction of deaths among women under age 50 and a slight increase in deaths among women over age 50. The incidence of breast cancer as reported by most cancer registries over the past 20 to 30 years has been rising, first in premenopausal women and then in postmenopausal women, but this increase may be spurious. Some of these changes may be related to changes in reproductive practices and to earlier diagnoses prompted by an increased awareness of the importance of breast cancer. A particularly dramatic example of the latter occurred during the 1970s: After breast cancer was diagnosed in the wives of the President and Vice-President of the United States, reported incidence rates in the United States rose by more than 10% in a single year and then subsided, without a detectable effect on mortality rates. This reflects an increased awareness by women, their subsequent visits to physicians for breast examinations, and the diagnosis of very early lesions with good prognosis.
Endometrial Cancer (ICD 182.0)
Endometrial cancer is correlated internationally with the incidence of breast, colorectal, and ovarian cancers, but tends to be more common in the United States than in other parts of the world (Waterhouse et al., 1982) and is found most frequently in higher socioeconomic groups and among whites. U.S. incidence rates have increased in the past 10 to 15 years, particularly in the western part of the country, without a corresponding increase in mortality. This rise was followed by a fall after demonstration of an association between the occurrence of endometrial cancer and administration of noncontraceptive estrogens after menopause (Austin and Roe, 1982).
Ovarian Cancer (ICD 183.0)
Ovarian cancer is the major cause of death from cancer of the reproductive system in women in North America. It is relatively common in the United States and other western countries compared to Asia and tends to occur more frequently in countries with high rates for cancer of the breast, colon, and endometrium (Waterhouse et al., 1982). The age-specific curve shows an earlier increase in incidence than for many other cancer sites, with a tendency for incidence to stabilize in middle years and then possibly to decline at older ages. Ovarian cancer tends to be more common in higher socioeconomic groups and aggregates in some families, suggesting that it may have a genetic basis. In general, however, ovarian cancer has only a weak association with familial association.
Bladder Cancer (ICD 188)
Cancer of the urinary bladder is relatively frequent in the United States compared to other parts of the world (Waterhouse et al., 1982). In some areas in North Africa, however, bladder cancer associated with schistosomiasis is common. Bladder cancer occurs more frequently in men than in women (ratio, approximately 2 to 1) and in lower socioeconomic groups, but does not appear to aggregate in families. There are no major ethnic or racial variations in bladder cancer. Changes upon migration have not been studied.
Prostate Cancer (ICD 185)
Cancer of the prostate is common in the United States and in other western countries (Waterhouse et al., 1982). Among U.S. blacks, it is particularly common and is increasing. It is relatively rare in males under the age of 45; the highest incidence is found among men in their seventies and eighties. Although incidence is increasing, there seems to be no corresponding trend in mortality, perhaps because part of the increase is a diagnostic artifact from more frequent detection of early-stage prostate cancer.
Osteoporosis
Osteoporosis (see Chapter 23) is a multifactorial, complex disorder characterized by an absolute decrease in bone mass per unit volume. Defining osteoporosis is difficult, since for a specific age and sex there is a wide, continuously distributed amount of bone mass (Mazess, 1983; Newton-John and Morgan, 1970; Riggs et al., 1981). Studies based on radiological techniques have demon-
Page 121
strated unequal rates of bone loss in different parts of the skeleton (Genant et al., 1982; Riggs et al., 1981, 1982). Bone mass tends to decrease after the fourth or fifth decade of age. As it becomes too low, structural integrity and mechanical support are not maintained and fractures occur after minimal trauma. The major sites of fracture are the hip, vertebrae, distal forearm (Colles' fracture), humerus, and pelvis. In clinical research, the diagnosis of osteoporosis is frequently applied only to patients in whom one or more fractures have already occurred (NIH, 1984).
Population Differences in Frequency
Age- and sex-adjusted hip fracture incidence rates in the United States are higher than those in any other region on which data have been published (Lewinnek et al., 1980; Nordin, 1966). Residents of New Zealand and western Europe as well as Israelis from western Europe also have high rates. The lowest reported rates are found among the South African Bantu (Bloom and Pogrund, 1982; Lewinnek et al., 1980).
Osteoporosis afflicts 24 million Americans (half of the women over 45 years of age and 90% of the women over 75 years of age). It is associated with an estimated 1.3 million fractures of the vertebrae, hips, forearms, and other bones per year (NIAMS, 1988).
The committee found no data on osteoporosis in migrants.
In Nottingham in the United Kingdom, the number of patients with fractures of the proximal femur more than doubled between 1971 and 1981. From 1971 to 1977, the rate of increase in fracture incidence was approximately 6% per year; after 1977, it rose to 10% per year (Wallace, 1983). In this same population, the rate of femoral fractures in women increased from 8 per 1,000 in 1971 to 16 per 1,000 in 1981. Similar trends have been reported for Denmark Jensen and Tøndevald, 1980).
At the present rates of population change, by the year 2030 approximately 20% of the population in the United States will be 65 years of age or older (NIH, 1984). Thus, the proportion of individuals predisposed to fractures of the hip, femur, forearm, or vertebrae is expected to increase.
Associations Between Risk Factors in Individuals and Osteoporosis
The demographic characteristics associated with osteoporosis and osteoporosis-related fractures are age over 40, female sex, and Caucasian race. Loss of bone mass begins at approximately 40 years of age in men and women and in blacks and whites. The most rapid decrease in bone density occurs among white women, particularly around 50 years of age (Garn, 1975).
Osteoporotic fractures are more common in elderly women than in men of the same ages, but the degree of female predominance depends on the type of fracture. Less than 20% of the women between 45 and 49 years of age have x-ray evidence of osteoporosis in their dorsolumbar spines; however, almost all women age 75 years and older have evidence of osteoporosis in this region (Iskrant and Smith, 1969). Females are two to three times more likely than males to suffer hip fractures (Gallagher et al., 1980). Incidence rates for Colles' fractures and fractures of the proximal humerus, vertebrae, and pelvis are six to eight times higher in women than in men (Owen et al., 1982; Rose et al., 1982).
Because women tend to live longer than men, the absolute incidence is even higher. One-third of women 65 years of age and older have vertebral fractures—the most common break caused by osteoporosis. By extreme old age, one-third of all women and one-sixth of all men will have suffered hip fractures, leading to death in 12 to 20% of the cases. For half of those who survive, the fracture leads to long-term nursing care (Cummings et al., 1985). Studies of postmenopausal women suggest that the number of years since menopause may be more important than chronological age as a determinant of bone mass (Lindquist et al., 1979; Richelson et al., 1984).
Osteoporosis most commonly found in men and women after middle life may consist of two distinct syndromes called Type I and Type II. Type I osteoporosis occurs in a relatively small subset of postmenopausal women between 51 and 65 years of age. It is characterized by excessive and disproportionate trabecular bone loss and is associated with vertebral fractures and decreased sex hormone production. Type II occurs in a large proportion of women or men who are more than 75 years of age. This is characterized by a proportionate loss of both cortical and trabecular bone and is associated
Page 122
with hip or vertebral fractures. Osteoporosis occurring between 66 and 75 years of age may represent a combination of these types (Riggs et al., 1982).
White women are about twice as likely as black women to suffer hip fractures. Most studies, but not all, indicate that the risk for hip fractures is higher among white men than black men (Bollett et al., 1965; Farmer et al., 1984). The incidence of hip and other osteoporotic fractures in Asian or Hispanic populations in the United States has not been reported, but Asian-Americans appear to have somewhat less cortical bone mass than whites of similar age (Garn et al., 1964; Yano et al., 1984).
Socioeconomic status has not been associated with osteoporosis, except possibly through poor diet. Gross malnutrition stunts growth and reduces bone mass. No occupational, social, behavioral, or psychological factors have been associated with osteoporosis.
Genetically determined differences in bone mass are illustrated by differences between races. In comparison to whites, blacks in the United States have higher bone mass, greater bone density, thicker bone cortex, and greater vertebral density (Cohn et al., 1977; Garn et al., 1972; Smith and Rizek, 1966; Trotter et al., 1960).
Diabetes Mellitus
Diabetes mellitus, a disorder of carbohydrate utilization secondary to a relative or absolute deficiency of insulin, is the seventh leading underlying cause of death in the United States. Diabetes mellitus is characterized by high blood glucose levels. It can be diagnosed by the presence of classical signs and symptoms, including elevated blood glucose levels, by a fasting plasma glucose >140 mg/dl, or by an abnormal oral glucose tolerance test (Harris, 1985). Two distinct primary forms of diabetes mellitus are Type I, or insulin-dependent (IDDM), and Type II, or noninsulin dependent (NIDDM). This classification replaces the older terminology, juvenile-onset and adult-onset diabetes. IDDM is usually the result of the destruction of the insulin-secreting b cells in the pancreatic islets of Langerhans. It is believed to be an immune-linked disorder, and there seems to be an increased risk of IDDM in subjects with certain traits associated with the HLA (human leukocyte group A) or histocompatibility immune response genes. In contrast, NIDDM is associated with aging and genetic traits and is closely linked to the insulin resistance associated with adiposity.
Other forms of diabetes include gestational diabetes. This transient condition occurs in approximately 2 to 5% of pregnancies and is believed to be a marker for future development of NIDDM. Diabetes mellitus can also be secondary to or associated with other conditions. This accounts for approximately 2% of all cases of diabetes (Melton et al., 1983; Merkatz et al., 1980). Although some data on the distribution of IDDM are presented for contrast, this report is concerned primarily with NIDDM, which is the only form of diabetes associated with diet as a risk factor. Except where indicated, the data on NIDDM (and IDDM) presented below are taken from two comprehensive reviews (Everhart et al., 1985; LaPorte and Cruickshanks, 1985), which appeared in a publication of the National Institutes of Health Diabetes in America (National Diabetes Data Group, 1985).
Population Differences in Frequency
Statistics on NIDDM are available for less than 2% of the world's population (LaPorte et al., 1985) and most probably underestimate the true magnitude of disease. Population surveys of blood glucose levels suggest that undiagnosed (occult) disease is common and increases with age. Data do suggest, however, that age-adjusted incidence rates of NIDDM vary considerably. Rates per 1,000 people age 40 and older range from 9.4 among male Israeli civil servants, to 16.8 and 11.3 among male and female Nauruans (in the South Pacific), and to 55.3 and 45.8 in U.S. males and females, respectively.
Approximately 6 million people in the United States have been diagnosed by a physician as being diabetic, and each year, approximately 500,000 new cases are diagnosed. An additional 4 million to 5 million people may have diabetes without knowing it. Since IDDM accounts for only 5% of known cases of diabetes and only an additional 2% of diabetes is secondary to or associated with other disorders, it is clear that the vast majority of diabetics have NIDDM.
Page 123
In 1982, diabetes was identified as the underlying cause of almost 35,000 deaths in the United States. It was the seventh leading cause of death that year and was listed as a contributing cause in an additional 95,000 deaths. Reliable mortality rates for NIDDM alone are not available; however, there are estimates that the 20-year survival for people with NIDDM is 50 to 80% of survival in the general adult population.
The prevalence of NIDDM in Japanese who migrated to Hawaii is higher (12.3 per 100 people) than in Japanese residing in Hiroshima, Japan (6.9 per 100 people) (Kawate et al., 1979). The age-adjusted prevalence rates in Japanese residents of Hawaii appear to be intermediate between those of Caucasians and native Hawaiians (Sloan, 1963). Asian-Indian migrants to high-risk countries also exhibit higher prevalence rates than their native counterparts and the same or even higher rates than those for natives of their adoptive country (Taylor and Zimmet, 1983). This increase in risk has also been observed in Yemenite migrants to Israel (Cohen et al., 1979).
Time Trends
NIDDM incidence rates in the United States have increased from 3.8 per 10,000 people in 1935-1936 (Spiegelman and Marks, 1946) to 22.7 per 10,000 people in 1979-1981. It is unlikely that improved screening and diagnosis alone can account for the increase in NIDDM.
Associations Between Risk Factors in Individuals and Diabetes Mellitus
The median age of people with diabetes (61) is higher than that of the general U.S. population (42). Approximately 9% of people 65 years old and older are believed to have NIDDM (Drury et al., 1985).
NIDDM mortality rates are difficult to ascertain, because this disease is a major contributor to cardiovascular and cerebrovascular deaths and is often a contributing cause (sometimes not even listed) rather than the certified underlying cause of death from these diseases. Approximately 76% of diabetes-related deaths (most presumed to be NIDDM) occur among people 65 years of age and older; 45% of those deaths occur after age 74 (Harris and Entmacher, 1985).
There are no clear sex differences in IDDM and NIDDM incidence rates. Rates are slightly higher among U.S. females, possibly reflecting more frequent use of physicians and, presumably, higher rates of NIDDM detection.
Prevalence rates of NIDDM among adult blacks, Hispanics, and Asian-Americans appear to be higher than those among whites. For blacks, the rates are 50% higher than those for whites. The incidence rates for diabetes in some Native American tribes, such as the Pimas, are among the highest recorded in the world, their age-sex-adjusted incidence rates being more than 40 times higher than comparable rates in Rochester, Minnesota (Knowler et al., 1978).
The U.S. data linking NIDDM rates to socioeconomic status are inconsistent with international data. Unlike rates in economically developing countries, which are positively associated with socioeconomic status, NIDDM rates in the United States are highest among the poor (Everhart et al., 1985).
There is some degree of familial aggregation for NIDDM. It is two to four times more common among the parents of children 20 years old or older with diabetes; nearly 33% of persons with diabetes compared to 4% of those without report having a sibling with the disease, and concordance rates of NIDDM are higher among monozygotic than among dizygotic twins. There are no known genetic markers to identify people at high risk of NIDDM. However, adiposity, a risk factor for NIDDM, appears to be at least partly determined by genetic predisposition.
Adiposity is the major risk factor for NIDDM. It is consistently correlated with NIDDM prevalence rates in intercountry, migrant, and other epidemiologic studies. A high waist-to-hip ratio, indicating a more central distribution of body fat, may also increase NIDDM risk in nondiabetics.
Diabetes as a Risk Factor for Other Chronic Diseases
The primary causes of death among diabetics are varied, since NIDDM is associated with a host of
Page 124
complications. These complications include ketoacidosis, kidney and renal disorders, accelerated arteriosclerosis leading to CHD, peripheral vascular and cerebrovascular diseases, blindness, hypertension, infections, complications of pregnancy, and neuropathy. Death among diabetics below age 20 (presumably with IDDM) is caused primarily by acute diabetic complications (e.g., ketoacidosis), but at older ages, renal disease accounts for nearly half of diabetic deaths and coronary atherosclerosis is responsible for most of the remainder.
Hepatobiliary Disease
Because of its many functions, the liver is subject to injury from a variety of causes, including nutritional imbalance. In industrialized Western countries, however, nutritional causes of chronic liver disease are uncommon and are related primarily to intake of alcoholic beverages. The prevalence of alcohol-induced liver disease, commonly called alcoholic cirrhosis, is quite high in most industrialized countries, including the United States. The major disease of the biliary system, gallstones (cholelithiasis), also is believed to be associated with nutritional factors, but the evidence is not as conclusive and the responsible nutrients have not been identified precisely.
Another liver disease is viral hepatitis—a frequent cause of acute hepatic inflammation and necrosis that occasionally becomes a chronic infection and produces one variety of cirrhosis. A few inherited disorders include hemochromatosis, which involves iron metabolism; Wilson's disease, which involves copper metabolism; and a1-antitrypsin deficiency. The liver is frequently injured by circulatory disorders, metastatic cancer, and obstruction of the biliary system by gallstones. These are not discussed here because nutrition is not a major contributor to their causes.
Effects of Alcohol on the Liver
Three distinct, but often overlapping, types of liver damage are found in chronic alcoholics: fatty liver, alcoholic hepatitis, and cirrhosis (Galambos, 1972; Mezey, 1980, 1982). Fatty liver occurs in most heavy drinkers (Bhathl et al., 1975) and is an immediate metabolic consequence of ethanol. It has been observed in animals (Lieber et al., 1963, 1975) as well as in humans (Rubin and Lieber, 1968) after ingestion of ethanol. Fatty liver, however, is not specific for alcohol ingestion; it may also result from a variety of other toxic, nutritional, and circulatory injuries. Alcoholic hepatitis (hepatic necrosis and inflammation) and cirrhosis occur less frequently than fatty liver among chronic alcoholics, but they are more advanced stages of liver disease. Alcoholic hepatitis, a life-threatening complication of heavy chronic alcohol consumption, may be a precursor to alcoholic cirrhosis (Rubin and Lieber, 1974). The metabolic processes involved in the effects of alcohol on the liver are described in detail in Chapter 16 of this report.
Both fatty liver and hepatitis due to alcohol ingestion are reversible when alcohol is withdrawn, and they leave no permanent stigmata. However, repeated episodes of fatty liver and hepatitis, and possibly other direct effects of alcohol on the liver, cause progressive scarring, which begins in the portal triads and extends to encircle the liver lobules. Some liver cells may regenerate in a disorganized pattern to form nodules, but the total liver mass is markedly decreased and the effective functioning liver mass is reduced even more.
There is no universally accepted definition of cirrhosis of the liver, but a committee of the World Health Organization defined it as a diffuse process characterized by fibrosis, loss of normal liver architecture, and the development of structurally abnormal nodules of liver tissue (Anthony et al., 1977). The essential features of cirrhosis also include fatty degeneration and necrosis of liver cells, regeneration of liver cells in abnormal patterns, proliferation of connective tissue, and alterations in the vascular supply. These changes eventually result in liver cell failure and portal hypertension (O'Brien et al., 1979).
Cirrhosis has been classified on the basis of anatomic characteristics, clinical history, and presumed etiology, with these various classifications differing in frequency according to age, sex, and geography. The most commonly encountered type in the United States is alcoholic cirrhosis, also referred to interchangeably as portal cirrhosis and Laennec's cirrhosis. Diagnoses of these cases are based on a history of chronic alcoholism together with the characteristic clinical manifestations and pathological findings. They must be differentiated from cirrhosis due to other causes, such as chronic viral hepatitis, hemochromatosis, obstructive lesions of the biliary tract, congestive heart failure, and congenital malformations (O'Brien et al., 1979). In their terminal stages, as in chronic renal disease, the different types of cirrhosis have many features in common, and clinical history often provides the most decisive information in deter-
Page 125
mining its etiology. Morbidity and mortality due to alcoholic cirrhosis are related principally to the loss of liver function, to derangements in the vascular system of the liver, or to both.
Cholelithiasis is often associated with cholecystitis. Gallstones form most frequently in the gallbladder but may infrequently occur in other portions of the biliary system. Most gallstones (80% or more) are composed predominantly of cholesterol combined with small amounts of calcium salts. Less frequently, gallstones are composed of calcium bilirubinate and related heme pigments derived from the degradation of hemoglobin. Gallstones may range from less than 1 mm up to several centimeters in diameter. Radiopacity is determined by their calcium content.
Because cholesterol-containing gallstones are more frequent, most research efforts have focused on their etiology and pathogenesis. The conditions that contribute to the formation of these gallstones are complex. The major factor is the ratio of cholesterol to bile acids and phospholipids, which stabilize cholesterol in an aqueous solution by forming micelles with a hydrophilic shell around a core of cholesterol. The proportions of cholesterol and solubilizing materials are determined by intake and absorption of dietary cholesterol, efficiency of evacuation of the gallbladder, endogenous synthesis of cholesterol, efficiency of the enterohepatic circulation of bile acids, rates of bile acid synthesis, drugs and hormones (particularly estrogens), and probably by many other factors. Nutrition is likely to affect many of these processes (reviewed by Bennion and Grundy, 1978a,b, and by Low-Beer, 1985).
Alcoholic Cirrhosis
Population Differences in Frequency
Rates of mortality from alcoholic cirrhosis vary greatly among countries. In the early 1970s, France, Italy, and Portugal had the highest rates, averaging between 50 and 60 deaths per 100,000 people 25 years and older. The United States ranked eighth out of 26 countries with an annual rate of approximately 29 deaths per 100,000 people over age 25. Similar rates were found in Japan, Switzerland, and Czechoslovakia. The United Kingdom had the lowest rate (5.7 deaths/ 100,000 over age 25) of the 26 countries (Schmidt, 1977). The rates of few other chronic diseases in the technologically developed Western countries vary as. widely from country to country as do rates of alcoholic cirrhosis of the liver.
Rates of mortality from alcoholic cirrhosis are approximately twice as high for men as for women in the United States and Canada (Schmidt, 1977), and the ratio of rates for men to those for women have increased gradually since World War II. The highest mortality is concentrated in the 25- to 64-year-old range, particularly in the 35- to 44-year-old group. Since alcoholic cirrhosis is associated most strongly and consistently with alcohol consumption, it shares the many socioeconomic and ethnic correlates of alcoholism.
There are few data on alcoholic cirrhosis in migrants. Rates of alcoholism vary greatly among Hispanics from various countries, and Asian-Americans tend to have lower frequencies of alcohol abuse than does the general population, but there are no specific data on alcoholic cirrhosis (NIAAA, 1985).
Time Trends in Mortality and Morbidity
Rates of death from alcoholic cirrhosis in the United States rose from 1959 to 1971 by an average of 3.8% per year in men and 3.4% per year in women (Schmidt, 1977). The rate of annual increase during the same period was much higher among men in Czechoslovakia (9.2%), Sweden (7.5%), and Italy (7.1%) and was somewhat higher in several other countries. In none of the 18 countries examined did the rate of change in men decrease. Since 1950, alcoholic cirrhosis has ranked consistently among the 10 leading causes of deaths in the U.S. population between the ages of 25 and 64. In some years, it has ranked much higher among middle-aged people. In 1977, for example, alcoholic cirrhosis was the fourth leading cause of death among 45- to 64-year-old people. At a rate of 39.2 per 100,000, it was just behind stroke (52.4) and ahead of motor vehicle accidents (25.5) (DHEW, 1979b). In general, consistent with the gradual but steady increase from year to year, alcoholic cirrhosis moved from seventh to fifth place among leading causes of death in the United States between 1950 and 1973 (Schmidt, 1977). However, mortality from alcoholic cirrhosis began to decline shortly thereafter and in 1983 reached the lowest level recorded since 1959. In that year, it was
Page 126
the ninth leading cause of death for all ages, accounting for 28,000 deaths (NIAAA, 1985).
In other countries, marked fluctuations in mortality from alcoholic cirrhosis have accompanied drastic changes in the availability of beverage alcohol. The most dramatic example occurred in France, where the rates fell from approximately 35 per 100,000 before World War II to about 6 per 100,000 during the war, and then increased to 35 or more per 100,000 after the war (Terris, 1967). Less dramatic but similar changes over time have been observed in other countries.
Alcoholic cirrhosis has consistently been correlated predominantly with the per-capita consumption of alcohol on a national basis (Gordis et al., 1983). In turn, alcohol consumption and alcoholic cirrhosis have been correlated positively with the availability and the ease of access to alcohol as stated above, and inversely with cost (Terris, 1967).
Rates of mortality from alcoholic cirrhosis are twofold higher in males than in females, are negligible under 25 years of age, are appreciable in the 25- to 44-year age group (8.6/10,000), peak from age 45 to 64 (39.2/100,000), and decline slightly after age 65 (36.7/100,000) (DHEW, 1979b).
The risk of alcoholic cirrhosis from lifetime alcohol consumption was expressed in a mathematical model by Skog (1984). Most alcoholics develop cirrhosis only after many years of alcohol abuse, and the risk increases as a function of any suitable measure of consumption. More than 50% of the risk is determined by current intake plus the preceding 5 years of consumption. Consumption rates during the preceding 6 to 25 years contribute to the balance of the risk, but the weight of the predictive power for each of those years is much less. This model of risk is consistent with observations that mortality due to most chronic progressive diseases decreases rapidly when the causative agent is removed.
Gallbladder Disease and Gallstones
Population Differences in Frequency
The prevalence of gallstones also varies widely among countries, but reliable data are difficult to obtain because gallstones are difficult to detect in population surveys. Furthermore, although they are frequent causes of morbidity, they do not often cause death. Thus, mortality rates cannot be used as indices of occurrence.
The prevalence of gallstones at autopsy is high (affecting 10% or more of all adults) in the industrialized countries—in the United States, Australia, and Europe. It is lower (5% or less) in the nonindustrialized countries of Asia and Africa (Brett and Barker, 1976). However, there have been no systematic population surveys using similar methods in different areas to compare gallstone prevalence among countries or morbidity related to gallstones.
In most countries, the prevalence of gallstones is two to three times higher in women than in men (Brett and Barker, 1976). In the United States, Native Americans and people descended from an admixture of New World natives (such as Latin Americans or Mexican Americans) have a much higher prevalence of gallstones than those with a West European heritage (Arevalo et al., 1987; Comess et al., 1967; Hesse, 1959; Sampliner et al., 1970; Weiss et al., 1984). Three-fourths of middle-aged Pima Indian women had evidence of gallbladder disease when surveyed by cholecystography, and approximately 80% of those with abnormal cholecystograms were found to have gallstones at surgery or at autopsy (Sampliner et al., 1970). Thus, the prevalence of gallstones in adult Pima women exceeds 50%, compared to estimates of 10 to 20% for comparable U.S. whites and blacks.
Information comparing the frequency of gallstones and gallbladder disease in migrants with that in their countries of origin is limited. Data do suggest, however, that environmental factors (e.g., diet) may influence risk. A survey of hospital admissions in Hawaii showed that Chinese had the highest admission rate for gallbladder disease. In contrast, gallbladder disease is reported to be rare among native Chinese living in China (Yamase and McNamara, 1972).
Time Trends in Mortality and Morbidity
There is no information regarding changes in prevalence of gallstones or gallbladder disease over time in the United States. In Japan, evidence suggests that the prevalence of gallstones at autopsy increased about threefold between 1950 and 1970 (Nagase et al., 1978).
Page 127
Associations Between Risk Factors in Individuals and Gallbladder Disease and Gallstones
Being a female and being obese are most consistently associated with gallstones (reviewed by Bennion and Grundy, 1978a,b, and by Low-Beer, 1985). Obesity is also related to supersaturation of gallbladder bile, and gallstones have been linked with high-calorie diets, but not with cholesterol intake, type of dietary fat, or dietary fiber (Bennion and Grundy, 1978a,b). In an analysis of the Framingham cohort after 10 years of observation, incidence of gallbladder disease (principally cholelithiasis) was twice as high in women as in men and was associated with an increase in weight and number of pregnancies, but was not related to serum cholesterol concentration, cigarette smoking, physical activity, CHD, or intakes of alcohol, cholesterol, fat, or protein (Friedman et al., 1966). In Japan, a review of surgical patients indicated that younger people had more cholesterol-containing gallstones (Nagase et al., 1978).
Dental Caries
Dental caries is localized demineralization of the tooth surface caused primarily by organic acid metabolites of oral microorganisms. The disease leads to a chronic progressive destruction of the teeth. In epidemiologic studies, caries prevalence is expressed as decayed, missing, and filled teeth (dmft) for primary dentition and as DMFT for permanent teeth.
Population Differences in Prevalence
Sreenby (1982) compared the prevalence of dental caries in children ages 6 (in 23 countries) and 12 (in 47 countries) using data obtained from the World Health Organization's Global Oral Epidemiology Bank (Barmes and Sardo-Infirri, 1977). The dmft and DMFT values used by Sreenby (1982) are indices of the cumulative effect of dental caries over the life of the dentition. Thus, for the primary dentition in 6-year-olds, the exposure period was approximately 4 to 5 years; for permanent teeth in the 12-year-olds, the period was 4 to 6 years for the molars and anterior teeth.
Prevalence of DMFT in the 6-year-olds surveyed ranged from 0.9 per child in Cameroon to 9.3 in Japan. Prevalence was highest in Asian countries and lowest in the African countries surveyed except in South Africa, which had an intermediate level (4.4) similar to that of the United States (3.4) and the United Kingdom (4.5).
The prevalence of DMFT among 12-year-old children ranged from 0.1 in Zambia to 10.6 in Switzerland. Again, the mean DMFT tended to be lowest in African countries; however, unlike the prevalence for 6-year-olds, the DMFT prevalence for 12-year-olds was highest in Switzerland and in the northern European countries of Denmark and Finland. Rates for Asian countries tended to be intermediate like those of the United States (4.0) and the United Kingdom (5.5)
Striking differences in the prevalence of caries in the U.S. population by geographic area were noted in the 1980 national survey of caries in schoolchildren (NIDR, 1981). Caries prevalence was highest in the Northeast, lowest in the Southwest, and at intermediate levels elsewhere.
According to data derived from the the National Health Survey for 1971 to 1973 (NIDR, 1981), people of both sexes and all ages (1 to 74 years) had an average of 13 decayed, missing, and filled permanent teeth. A closer review of this figure indicates that there was an average of 1.3, 5.3, and 6.4 decayed, missing, and filled teeth, respectively. An estimated 14.7% of the adults (18 to 74 years old) had lost all their permanent teeth. In a study of the oral health of U.S. adults, completed in 1986, only 4% of people of ages 18 to 65 were edentulous (Miller et al., 1987).
No information on caries in migrants was found.
Time Trends
Until the 1970s, caries was most prevalent (affecting 95% of the population) in developed countries, especially in those with diets high in refined carbohydrates. The only exception occurred during and just after World War II, when the prevalence rates of caries dropped precipitously, although temporarily, in children living in Europe (Sognnaes, 1948; Toverud, 1957) and Japan (Takeuchi, 1961). During the 1970s, the prevalence of caries declined dramatically again among school-aged children not only in many countries of western Europe but also in North America (Glass, 1982). In contrast, the rate of caries is increasing in many Third World countries (Barmes and Sardo-Infirri, 1977).
Page 128
In the 1971-1973 National Health Survey, children between the ages of 5 and 17 had decay or fillings in an average of 7.06 teeth (NIDR, 1981). By 1981, this number had dropped to 4.77 (NIDR, 1981). Likewise, as noted above, the prevalence of edentulia dropped from 14.7% in 18- to 74-year-old people during 1971-1973 (NIDR, 1981) to 4% in the 18- to 65-year age group in 1986 (Miller et al., 1987). There are reports that root surface caries is becoming more prevalent among older people—a result perhaps of decreased edentulia (Seichter, 1987). This is discussed in the next section.
Associations Between Risk Factors in Individuals and Dental Caries
Caries of the tooth crown is predominantly a disease of children and adolescents. It begins as soon as teeth erupt; incidence remains high, and prevalence increases linearly up to ages 18 to 20. After age 25 or so, the incidence of coronal caries diminishes, but the disease may still occur. The epidemiologic study of caries in adults becomes increasingly complicated by the fact that it is often not possible to distinguish between teeth lost because of caries and those missing because of periodontal and other oral diseases. What is certain is that caries of the root surface of the teeth, secondary to exposure of the root by recession of the gingivae, becomes increasingly prevalent with advancing age. In a national survey of employed U.S. adults, completed in 1986 (Miller et al., 1987), root-surface caries had affected approximately 60% of the population by age 65; the average was about three lesions per person.
Caries prevalence is higher among females than among males at every age. This observation is only partly explained by the earlier eruption of teeth in females, leading to longer exposure to the oral environment. Root-surface caries is more common in males for reasons that are not yet clear (Carlos, 1984).
Chung et al. (1970) analyzed the relationship of race to caries prevalence in 9,912 12- to 18-year-old children in Hawaii. They noted that dental caries was most prevalent among children of Japanese origin, followed by children of Hawaiian origin. Children with Caucasian, Puerto Rican, or Filipino parentage had the lowest caries activity. In a subsequent survey of 910 eighth-grade students in Hawaii, Hankin et al. (1973) found that the prevalence of caries in Hawaiian children had surpassed that in children of Japanese ancestry. Caucasian children continued to enjoy the lower rates. A 1980 U.S. national survey (NIDR, 1981) showed no notable differences in caries prevalence with respect to color, ethnic group, or socioeconomic status. However, the HHANES (Hispanic Health and Nutrition Examination Survey) of 1982-1984 indicated that Mexican-American children residing in the Southwest had a dental caries distribution similar to that of other groups in the region, but that Mexican-American children from low-income families had nearly two times more decayed teeth than did Mexican-American children from high income families (Ismail et al., 1987).
No information on caries was found in these areas.
There is some evidence of genetic predisposition to caries (Witkop, 1962), but this appears to be a minor etiologic factor compared to the local influences of diet, bacterial deposits on teeth, and less than optimal ingestion of fluoride.
References
Abbott, R.D., Y. Yin, D.M. Reed, and K. Yano. 1986. Risk of stroke in male cigarette smokers. N. Engl. J. Med. 315: 717-720.
Abraham, S., M.D. Carroll, M.F. Najjar, and R. Fulwood. 1983. Obese and overweight adults in the United States. Vital and Health Statistics, Series 11, No. 230. DHHS Publ. No. (PHS) 83-1680. National Center for Health Statistics. Public Health Service, U.S. Department of Health and Human Services, Hyattsville, Md. 93 pp.
AHA (American Heart Association). 1973. Coronary Risk Handbook: Estimating Risk of Coronary Heart Disease in Daily Practice. American Heart Association, New York. 35 pp.
AHA (American Heart Association). 1983. 1984 Heart Facts. Office of Communications, Dallas. 25 pp.
Allen, E.V., N.W. Barker, and E.A. Hines, Jr. 1946. Peripheral Vascular Diseases. W.B. Saunders, Philadelphia. 871 pp.
Anastasiou-Nana, M., S. Nanas, J. Stamler, J. Marquardt, R. Stamler, H.A. Lindberg, D.M. Berkson, K. Liu, E. Stevens, M. Mansour, and T. Tokich. 1982. Changes in rates of sudden CHD death with first vs. recurrent events,
Page 129
Chicago Peoples Gas Co. Study, 1960-1980. Circulation, Suppl. 66:11-236.
Anthony, P.P., K.G. Ishak, N.C. Nayak, H.E. Poulsen, P.J. Scheuer, and L.H. Sobin. 1977. The morphology of cirrhosis: definition, nomenclature, and classification. Bull. W.H.O. 55:521-540.
Arevalo, J.A., A.O. Wollitzer, M.B. Corporon, M. Larios, D. Huante, and M.T. Ortiz. 1987. Ethnic variability in cholelithiasis: an autopsy study. West. J. Med. 147:44-47.
Austin, D.F., and K.M. Roe. 1982. The decreasing incidence of endometrial cancer: public health implications. Am. J. Public Health 72:65-68.
Barnes, D.E., and J. Sardo-Infirri. 1977. World Health Organization activities in oral epidemiology. Community Dent. Oral Epidemiol. 5:22-29.
Beaglehole, R., C.E. Salmond, A. Hooper, J. Huntsman, J.M. Stanhope, J.C. Cassel, and I.A. Prior. 1977. Blood pressure and social interaction in Tokelauan migrants in New Zealand. J. Chronic Dis. 30:803-812.
Bennion, L.J., and S.M. Grundy. 1978a. Risk factors for the development of cholelithiasis in man (first of two parts). N. Engl. J. Med. 229:1161-1167.
Bennion, L.J., and S.M. Grundy. 1978b. Risk factors for the development of cholelithiasis in man (second of two parts). N. Engl. J. Med. 229:1221-1227.
Berenson, G.S. 1980. Cardiovascular Risk Factors in Children: The Early Natural History of Atherosclerosis and Essential Hypertension. Oxford University Press, New York. 453 pp.
Berenson, G.S., A.W. Voors, and L.S. Webber. 1980. Importance of blood pressures in children: distribution and measurable determinants. Pp. 71-97 in H. Kesteloot and J.V. Joossens, eds. Epidemiology of Arterial Blood Pressure. Martinus Nijhoff, The Hague.
Bhathal, P.S., P. Wilkinson, S. Clifton, J.B. Rankin, and J.N. Santamaria. 1975. The spectrum of liver diseases in alcoholism. Aust. N.Z.J. Med. 5:49-57.
Black, D., W.P.T. James, G.M. Besser, C.G.D. Brook, D. Craddock, J.S. Garrow, T.D.R. Hockaday, B. Lewis, T.R.E. Pilkington, J.T. Silverstone, J.I. Mann, D.S. Miller, D.A. Pyke, D.G. Williams, and R.K. Skinner. 1983. A report of the Royal College of Physicians. J. R. Coll. Physicians London 17:5-65.
Bloom, R.A., and H. Pogrund. 1982. Humeral cortical thickness in female Bantu-its relationship to the incidence of femoral neck fracture. Skeletal Radiol. 8:59-62.
Blot, W.J., and J.F. Fraumeni, Jr. 1987. Trends in esophageal cancer mortality among U.S. blacks and whites. Am. J. Public Health 77:296-298.
Bollet, A.J., G. Engh, and W. Parson. 1965. Epidemiology of osteoporosis; sex and race incidence of hip fractures. Arch. Intern. Med. 116:191-194.
Bonita, R., and R. Beaglehole. 1982. Trends in cerebrovascular disease mortality in New Zealand. N.Z. Med. J. 95:411-414.
Bonita, R., and R. Beaglehole. 1986. Does treatment of hypertension explain the decline in mortality from stroke? Br. Med. J. 292:191-192.
Bothig, S., V.I. Metelitsa, W. Barth, A.A. Aleksandrov, I. Schneider, T.P. Ostrovskaya, E.V. Kokurina, I.I. Saposhinkov, I.P. Iliushina, and L.S. Gurevich. 1976. Prevalence of ischaemic heart disease, arterial hypertension and intermittent claudication, and distribution of risk factors among middle-aged men in Moscow and Berlin. Cor Vasa 18:104-118.
Bouchard, E., L Perusse, C.. Leblanc, A. Tremblay, and G. Theriault. 1988. Inheritance of the amount and distribution of human body fat. Int. J. Obesity 12:205-215.
Bray, G.A., ed. 1979. Obesity in America. NIH Publ. No. 79-359. National Institutes of Health, Public Health Service, U.S. Department of Health, Education, and Welfare, Bethesda, Md. 285 pp.
Bray, G.A. 1985. Obesity: definition, diagnosis and disadvantages. Med. J. Aust. 142:S2-S8.
Bray, G.A. 1987. Obesity and the heart. Modern Concepts Cardiovasc. Dis. 56:67-71.
Brett, M., and D.J. Barker. 1976. The world distribution of gallstones. Int. J. Epidemiol. 5:335-341.
Brown, J., G.J. Bourke, G.F. Gearty, A. Finnegan, M. Hill, F.C. Heffernan-Fox, D.E. Fitzgerald, J. Kennedy, R.W. Childers, W.J.E. Jessop, M.F. Trulson, M.C. Latham, S. Cronin, M.B. McCann, R.E. Clancy, I. Gore, H.W. Stroudt, D.M. Hegsted, and F.J. Stare. 1970. Nutritional and epidemiologic factors related to heart disease. World Rev. Nutr. Diet. 12:1-42.
Carlos, J.P. 1984. Epidemiologic trends in caries: impact on adults and the aged. Pp. 131-148 in B. Guggenheim, ed. Cariology Today. S. Karger, Basel.
Carroll, M.D., S. Abraham, and C.M. Dresser. 1983. Dietary Intake Source Data: United States, 1976-1980. Vital and Health Statistics, Series 11, No. 231. DHHS Publ. No. (PHS) 83-1681. National Center for Health Statistics, Public Health Service, U.S. Department of Health and Human Services, Hyattsville, Md. 483 pp.
Chung, C.S., D.W. Runck, J.D. Niswander, S.E. Bilben, and M.C.W. Kau. 1970. Genetic and epidemiologic studies of oral characteristics in Hawaii's schoolchildren. I. Caries and periodontal disease. J. Dent. Res. 49:1374-1385.
Cohen, A.M., J. Fidel, B. Cohen, A. Furst, and S. Eisenberg. 1979. Diabetes, blood lipids, lipoproteins, and change of environment: restudy of the ''new immigrant Yemenites" in Israel. Metabolism 28:716-728.
Cohn, S.H., C. Abesamis, S. Yasumura, J.F. Aloia, I. Zanzi, and K.J. Ellis. 1977. Comparative skeletal mass and radial bone mineral content in black and white women. Metabolism 26:171-178.
Comess, L.J., P.H. Bennet, and T.A. Burch. 1967. Clinical gallbladder disease in Pima Indians: its high prevalence in contrast to Framingham, Massachusetts. N. Engl. J. Med. 277:894-898.
Comstock, G.W. 1957. An epidemiologic study of blood pressure levels in a biracial community in the southern United States. Am. J. Hyg. 65:271-315.
Criqui, M.H., S.S. Coughlin, and A. Fronek. 1985a. Noninvasively diagnosed peripheral arterial disease as a predictor of mortality: results from a prospective study. Circulation 72:768-773.
Criqui, M.H., A. Fronek, E. Barrett-Connor, M.R. Klauber, S. Gabriel, and D. Goodman. 1985b. The prevalence of peripheral arterial disease in a defined population. Circulation 71:510-515.
Cummings, S.R., J.L. Kelsey, M.C. Nevitt, and K.J. O'Dowd. 1985. Epidemiology of osteoporosis and osteoporotic fractures. Epidemiol. Rev. 7:178-208.
Dawber, T.R., and W.B. Kannel. 1972. Current status of coronary prevention. Lessons from the Framingham Study. Prev. Med. 1:499-512.
Day, N.E., and N. Munoz. 1982. Esophagus. Pp. 596-623 in D. Schottenfeld and J.F. Fraumeni, Jr., eds. Cancer Epidemi-
Page 130
ology and Prevention. W.B. Saunders Co., Philadelphia.
Devesa, S.S., D.T. Silverman, J.L. Young, Jr., E.S. Pollack, C.C. Brown, J.W. Horm, C.L. Percy, M.H. Myers, F.W. McKay, and J.F. Fraumeni, Jr. 1987. Cancer incidence and mortality trends among whites in the United States, 1947-84. J. Natl. Cancer Inst. 79:701-770.
DHEW (U.S. Department of Health, Education, and Welfare). 1963. Origin, Program, and Operation of the U.S. National Health Survey. Vital and Health Statistics, Series 1, No. 1. PHS Publ. No. 1000. National Center for Health Statistics, Public Health Service, U.S. Department of Health, Education, and Welfare, Washington, D.C. 41 pp.
DHEW (U.S. Department of Health, Education, and Welfare). 1979a. Dietary Intake Source Data: United States, 1971-74. Vital and Health Statistics, DHEW Publ. No. (PHS) 79-1221. National Center for Health Statistics, Public Health Service, U.S. Department of Health, Education, and Welfare, Hyattsville, Md. 421 pp.
DHEW (U.S. Department of Health, Education, and Welfare). 1979b. Healthy People: The Surgeon General's Report on Health Promotion and Disease Prevention. DHEW (PHS) Publ. No. 79-55071, Office of the Assistant Secretary for Health and Surgeon General, Public Health Service. U.S. Government Printing Office, Washington, D.C. (various pagings).
DHHS (U.S. Department of Health and Human Services). 1986. Blood Pressure Levels in Persons 18-74 Years of Age in 1976-80, and Trends in Blood Pressure from 1960 to 1980 in the United States. Data from the National Health Survey, Series 11, No. 234. DHHS Publ. No. (PHS) 86-1684. National Center for Health Statistics, Public Health Service, U.S. Department of Health and Human Services, Hyattsville, Md. 68 pp.
DHHS (U.S. Department of Health and Human Services). 1987. Monthly Vital Statistics Report, Vol. 36: The Advance Report of Final Mortality Statistics, 1985. DHHS Publ. No. (PHS) 87-1120. National Center for Health Statistics, Public Health Service, U.S. Department of Health and Human Services, Hyattsville, Md. 48 pp.
Donahue, R.P., R.D. Abbott, E. Bloom, D.M. Reed, and K. Yano. 1987. Central obesity and coronary heart disease in men. Lancet 1:821-824.
Drury, T.F., K.M. Danchik, and M.I. Harris. 1985. Sociodemographic characteristics of adult diabetics. Pp. VII-I to VII-37 in M.I. Harris and R.F. Hamman, eds. Diabetes in America: Diabetes Data Compiled 1984. Report of the National Diabetes Data Group. NIH Publ. No. 85-1468. National Institute of Arthritis, Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Public Health Service. U.S. Department of Health and Human Services, Bethesda, Md.
Ducimetiere, P., J. Richard, and F. Cambien. 1986. The pattern of subcutaneous fat distribution in middle-aged men and the risk of coronary heart disease: the Paris Prospective Study. Int. J. Obes. 10:229-240.
Dyken, M.L, P.A. Wolf, H.J.M. Barnett, J.J. Bergan, W.K. Hass, W.B. Kannel, L. Kuller, J.F. Kurtke, and T.M. Thoralf. 1984. Risk factors in stroke: a statement for physicians by the subcommittee on risk factors and stroke of the Stroke Council. Stroke 15:1105-1111.
Elveback, LR., D.C. Connolly, and L.T. Kurland. 1981. Coronary heart disease in residents of Rochester, Minnesota. II. Mortality, incidence, and survivorship, 1950-1975. Mayo Clin. Proc. 56:665-672.
Elveback, L.R., D.C. Connolly, and L.J. Melton III. 1986. Coronary heart disease in residents of Rochester, Minnesota. VII. Incidence, 1950 through 1982. Mayo Clin. Proc. 61:896-900.
Epstein, F.H., and R.D. Eckoff. 1967. The epidemiology of high blood pressure-geographic distribution and etiologic factors. Pp. 155-161 in J. Stamler, R. Stamler, and T.M. Pullman, eds. Epidemiology of Hypertension. Grune and Stratton, New York.
Everhart, J., W.C. Knowler, and P.H. Bennett. 1985. Incidence and risk factors for noninsulin-dependent diabetes. Pp. IV-I to IV-35 in M.I. Harris and R.F. Hamman, eds. Diabetes in America: Diabetes Data Compiled 1984. Report of the National Diabetes Data Group. NIH Publ. No. 85-1468. National Institute of Arthritis, Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Public Health Service. U.S. Department of Health and Human Services, Bethesda, Md.
Farmer, M.E., L.R. White, J.A. Brody, and K.R. Bailey. 1984. Race and sex differences in hip fracture incidence. Am. J. Public Health 74:1374-1380.
Folkow, B. 1982. Physiological aspects of primary hypertension. Physiol. Rev. 62:347-504.
Folsom, A.R., R.V. Luepker, R.F. Gillum, D.R. Jacobs, R.J. Prineas, H.L. Taylor, and H. Blackburn. 1983. Improvement in hypertension detection and control from 19731974 to 1980-1981. The Minnesota Heart Survey experience. J. Am. Med. Assoc. 250:916-921.
Folsom, A.R., O. Gomez-Marin, R.F. Gillum, T.E. Kottke, W. Lohman, and D.R. Jacobs, Jr. 1987. Out-of-hospital coronary death in an urban population—validation of death certificate diagnosis. The Minnesota Heart Survey. Am. J. Epidemiol. 125:1012-1018.
Franco, L.J., M.P. Stem, M. Rosenthal, S.M. Haffner, H.P. Hazuda, and P.J. Cameaux. 1985. Prevalence, detection and control of hypertension in a biethnic community. The San Antonio Heart Study. Am. J. Epidemiol. 121:684-696.
Friedman, G.D., W.B. Kannel, and T.R. Dawber. 1966. The epidemiology of gallbladder disease: observations in the Framingham Study. J. Chron. Dis. 19:273-292.
Galambos, J.T. 1972. Alcoholic hepatitis: its therapy and prognosis. Prog. Liver Dis. 4:567-588.
Gallagher, J.C., L.J. Melton, B.L. Riggs, and E. Bergstrath. 1980. Epidemiology of fractures of the proximal femur in Rochester, Minnesota. Clin. Orthop. 150:163-171.
Garn, S.M. 1975. Bone-loss and aging. Pp. 39-57 in R. Goldman and M. Rockstein, eds. The Physiology and Pathology of Human Aging. Academic Press, New York.
Garn, S.M., E.M. Pao, and M.E. Rihl. 1964. Compact bone in Chinese and Japanese. Science 143:1439-1440.
Garn, S.M., S.T. Sandusky, J.M. Nagy, and M.B. McCann. 1972. Advanced skeletal development in low-income Negro children. J. Pediatr. 80:965-969.
Garraway, W.M., and J.P. Whisnant. 1987. The changing pattern of hypertension and the declining incidence of stroke. J. Am. Med. Assoc. 258:214-217.
Garraway, W.M., J.P. Whisnant, A.J. Furlan, LH. Phillips II, LT. Kurland, and W.M. O'Fallon. 1979. The declining incidence of stroke. N. Engl. J. Med. 300:449-452.
Genant, H.K., C.E. Cann, B. Ettinger, and G.S. Gordan. 1982. Quantitative computed tomography of vertebral spongiosa: a sensitive method for detecting early bone loss after oophorectomy. Ann. Intern. Med. 97:699-705.
Gill, J.S., A.V. Zezulka, M.J. Shipley, S.K. Gill, and D.G.
Page 131
Beevers. 1986. Stroke and alcohol consumption. N. Engl. J. Med. 315:1041-1046.
Gillum, R.F., P.). Hannan, R.J. Prineas, D.R. Jacobs, Jr., O. Gomez-Martin, R.V. Luepker, J. Baxter, T.E. Kottke, and H. Blackburn. 1984. Coronary heart disease mortality trends in Minnesota, 1960-1980: the Minnesota Heart Survey. Am. J. Public Health 74:360-362.
Gillum, R.F., O. Gomez-Marin, T.E. Kottke, D.R. Jacobs, Jr., R.J. Prineas, A.R. Folsom, R.V. Luepker, and H. Blackburn. 1985. Acute stroke in a metropolitan area, 1970 and 1980. The Minnesota Heart Survey. J. Chronic Dis. 38: 891-898.
Glass, R.L., ed. 1982. The First International Conference on the Declining Prevalence of Dental Caries. J. Dent. Res. (sp. iss.) 61:1301-1383.
Goldberg, R.J., J.M. Gore, J.S. Alpert, and J.E. Dalen. 1986. Recent changes in attack and survival rates of acute myocardial infarction (1975 through 1981). The Worcester Heart Attack Study. J. Am. Med. Assoc. 255:2774-2779.
Goldman, L., and E.F. Cook. 1984. The decline in ischemic heart disease mortality rates: an analysis of the comparative effects of medical interventions and changes in lifestyle. Ann. Intern. Med. 101:825-836.
Goldman, L, F. Cook, B. Hashimoto, P. Stone, J. Muller, and A. Loscalzo. 1982. Evidence that hospital care for acute myocardial infarction has not contributed to the decline in coronary mortality between 1973-1974 and 1978-1979. Circulation 65:936-942.
Gomez-Marin, O., A.R. Folsom, T.E. Kottke, S.C. Wu, D.R. Jacobs, Jr., R.F. Gillum, S.A. Edlavitch, and H. Blackburn. 1987. Improvement in long-term survival among patients hospitalized with acute myocardial infarction, 1970 to 1980. The Minnesota Heart Survey. N. Engl. J. Med. 316:1353-1359.
Gordis, E., V.P. Dole, and M.J. Ashley. 1983. Regulation of alcohol consumption: individual appetite and social policy. Am. J. Med. 74:322-334.
Haenszel, W. 1961. Cancer mortality among the foreign-born in the United States. J. Natl. Cancer Inst. 26:37-132.
Hamilton, M., G.W. Pickering, J.A. Roberts, and G.S. Sowry. 1954. Aetiology of essential hypertension; arterial pressure in the general population. Clin. Sci. 13:11-35.
Hankin, J.H., C.S. Chung, and M.C. Kau. 1973. Genetic and epidemiologic studies of oral characteristics in Hawaii's school children: dietary patterns and caries prevalence. J. Dent. Res. 52:1079-1086.
Harper, A.E. 1987. Transitions in health status: implications for dietary recommendations. Am. J. Clin. Nutr. 45:1094-1107.
Harris, M.I. 1985. Classification and diagnostic criteria for diabetes and other categories of glucose intolerance. Pp. II-1 to II-10 in M.I. Harris and R.F. Hamman, eds. Diabetes in America: Diabetes Data Compiled 1984. Report of the National Diabetes Data Group. NIH Publ. No. 85-1468. National Institute of Arthritis, Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Public Health Service. U.S. Department of Health and Human Services, Bethesda, Md.
Harris, M.I., and P.S. Entmacher. 1985. Mortality from diabetes. Pp. XXIX-1 to XXIX-48 in M.I. Harris and R.F. Hamman, eds. Diabetes in America: Diabetes Data Compiled 1984. Report of the National Diabetes Data Group.
NIH Publ. No. 85-1468. National Institute of Arthritis, Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Public Health Service. U.S. Department of Health and Human Services, Bethesda, Md.
HDFP (Hypertension Detection and Follow-up Program Cooperative Group). 1979. Five-year findings of the Hypertension Detection and Follow-up Program. II. Mortality by race-sex, and age. J. Am. Med. Assoc. 242:2572-2577.
Hesse, F.G. 1959. Incidence of cholecystitis and other diseases among Pima Indians of Southern Arizona. J. Am. Med. Assoc. 170:1789-1790.
Heyden, S., A. Heyman, and L. Camplong. 1969. Mortality patterns among parents of patients with atherosclerotic cerebrovascular disease. J. Chronic Dis. 22:105-110.
Heyman, A., H.R. Karp, S. Heyden, A. Bartel, J.C. Cassel, H.A. Tyroler, and C.G Hames. 1971. Cerebrovascular disease in the biracial population of Evans County, Georgia. Arch. Intern. Med. 128:949-955.
Horm, J.W., and L.G. Kessler. 1986. Falling rates of lung cancer in men in the United States. Lancet 1:425-426.
Howson, C.P., T. Hiyama, and E.L. Wynder. 1986. The decline in gastric cancer: epidemiology of an unplanned triumph. Epidemiol. Rev. 8:1-27.
Hubert, H.B., M. Feinleib, P.M. McNamara, and W.P. Castelli. 1983. Obesity as an independent risk factor for cardiovascular disease: a 26-year follow-up of participants in the Framingham Heart Study. Circulation 67:968-977.
ICD (International Classification of Diseases). 1977. Manual of the International Statistical Classification of Diseases, Injuries, and Causes of Death, Vol. 1. Based on the Recommendations of the Ninth Revision Conference, 1975, and Adopted by the Twenty-Ninth World Health Assembly. World Health Organization, Geneva. 773 pp.
Inter-Society Commission for Heart Disease Resources. 1984. Optimal resources for primary prevention of atherosclerotic diseases. Circulation 70:153A-205A.
Iskrant, A. P., and R.W. Smith, Jr. 1969. Osteoporosis in women 45 years and over related to subsequent fractures. Public Health Rep. 84:33-38.
Ismail, A.L., B.A. Burt, and J.A. Brunelle. 1987. Prevalence of dental caries and periodontal disease in Mexican American children aged 5 to 17 years: results from southwestern NHANES, 1982-83. Am. J. Public Health 77:967-970.
Jenkins, C.D. 1983. Psychosocial and Behavioral Factors. Pp. 98-112 in N.M. Kaplan and J. Stamler, eds. Prevention of Coronary Heart Disease. W.B. Saunders, Philadelphia.
Jensen, J.S., and E. Tøndevald. 1980. A prognostic evaluation of the hospital resources required for the treatment of hip fractures. Acta Orthop. Scand. 51:515-522.
JNC (Joint National Committee on Detection, Evaluation, and Treatment of High Blood Pressure). 1988. The 1988 Report of the Joint National Committee on Detection, Evaluation, and Treatment of High Blood Pressure. Arch. Intern. Med. 148:1023-1038.
Johnson, B.C., F.H. Epstein, and M.O. Kjelsberg. 1965. Distributions and family studies of blood pressure and serum cholesterol levels in a total community—Tecumseh, Michigan. J. Chronic Dis. 18:147-160.
Juergens, J.L, N.W. Barker, and E.A. Hines, Jr. 1959. Arteriosclerosis obliterans: review of 520 cases with special reference to pathogenic and prognostic factors. Circulation 21:188-195.
Kagan, A., J. Popper, G.G. Rhoads, Y. Takeya, H. Kato, G.B. Goode, and M. Marmot. 1976. Epidemiologic studies of coronary heart disease and stroke in Japanese men living in Japan, Hawaii and California: prevalence of stroke. Pp.
Page 132
267-277 in P. Scheinberg, ed. Cerebrovascular Diseases: Tenth Princeton Conference. Raven Press, New York.
Kagan, A., J.S. Popper, and G.G. Rhoads. 1980. Factors related to stroke incidence in Hawaiian Japanese men. The Honolulu Heart Study. Stroke 11:14-21.
Kannel, W.B. 1974. Role of blood pressure in cardiovascular morbidity and mortality. Prog. Cardiovasc. Dis. 17:5-24.
Kannel, W.B. 1980. Host and environmental determinants of hypertension: perspective from the Framingham Study. Pp. 265-295 in H. Kesteloot and J.V. Joossens, eds. Epidemiology of Arterial Blood Pressure. Martinus Nijhoff, The Hague.
Kannel, W.B., and D.L. McGee. 1985. Update on some epidemiologic features of intermittent claudication: the Framingham Study. J. Am. Geriatr. Soc. 33:13-18.
Kannel, W.B., and D. Shurtleff. 1971. The natural history of arteriosclerosis obliterans. Cardiovasc. Clin. 3:37-52.
Kannel, W.B., and P. Sorlie. 1979. Some health benefits of physical activity. The Framingham Study. Arch. Intern. Med. 139:857-861.
Kannel, W.B., and P.A. Wolf. 1983. Epidemiology of cerebrovascular disease. Pp. 1-24 in R.W.R. Russell, ed. Vascular Disease of the Central Nervous System. Churchill Livingstone, Edinburgh.
Kawate, R., M. Yamakido, Y. Nishimoto, P.H. Bennett, R.F. Hamman, and W.C. Knowler. 1979. Diabetes mellitus and its vascular complications in Japanese migrants on the Island of Hawaii. Diabetes Care 2:161-170.
Keen, H., G. Rose, D.A. Pyke, D. Boyns, C. Chlouverakis, and S. Mistry. 1965. Blood-sugar and arterial disease. Lancet 2:505-508.
Kesteloot, H., C.S. Song, J.S. Song, B.C. Park, E. Brems-Heyns, and J.V. Joossens. 1976. An epidemiological survey of arterial blood pressure in Korea using home reading. Pp. 141-148 in G. Rorive and H. Van Cauwenberge, eds. The Arterial Hypertensive Disease: A Symposium. Masson, New York.
Kesteloot, H., B.C. Park, C.S. Lee, E. Brems-Heyns, and J.V. Joossens. 1980. A comparative study of blood pressure and sodium intake in Belgium and in Korea. Pp. 453-470 in H. Kesteloot and J.V. Joossens, eds. Epidemiology of Arterial Blood Pressure. Martinus Nijhoff, The Hague.
Keys, A. 1980. Seven Countries: A Multivariate Analysis of Death and Coronary Heart Disease. Harvard University Press, Cambridge, Mass. 381 pp.
Keys, A., C. Arvanis, H. Blackburn, F.S.P. Van Buchem, R. Buzina, B.S. Djordjevic, F. Fidanza, M.J. Karvonen, A. Menotti, V. Puddi, and H.L. Taylor. 1972. Coronary heart disease: overweight and obesity as risk factors. Ann. Intern. Med. 77:15-27.
King, M.C., RC. Go, H.T. Lynch, R.C. Elston, P.I. Terasaki, N.L Petrakis, G.C. Rodgers, D. Lattanzio, and J. Bailey-Wilson. 1983. Genetic epidemiology of breast cancer and associated cancers in high-risk families. II. Linkage analysis. J. Natl. Cancer Inst. 71:463-467.
Kluthe, R., and A. Schubert. 1985. Obesity in Europe. Ann. Intern. Med. 103:1037-1042.
Knowler, W.C., P.H. Bennett, R.F. Hamman, and M. Miller. 1978. Diabetes incidence and prevalence in Pima Indians: a 19-fold greater incidence than in Rochester, Minnesota. Am. J. Epidemiol. 108:497-505.
Komachi, Y. 1977. Recent problems in cerebrovascular accidents: characteristics of stroke in Japan. Nippon Ronen Igakkai Zasshi 14:359-364.
Komachi, Y., and T. Shimamoto. 1980. Regional differences in blood pressure and its nutritional background in several Japanese populations. Pp. 379-400 in H. Kesteloot and J.V.
Joossens, eds. Epidemiology of Arterial Blood Pressure. Martinus Nijhoff, The Hague.
Kuller, L.H., J.A. Perper, W.S. Dai, G. Rutan, and N. Traven. 1986. Sudden death and the decline in coronary heart disease mortality. J. Chronic Dis. 39:1001-1019.
Kurtzke, J.F. 1976. An introduction to the epidemiology of cerebrovascular disease. Pp. 239-253 in P. Scheinberg, ed. Cerebrovascular Diseases: Tenth Princeton Conference. Raven Press, New York.
Kushi, L.H., R.A. Lew, F.J. Stare, C.R. Ellison, M. el Lozy, G. Bourke, L. Daly, I. Graham, N. Hickey, R. Mulcahy, and J. Kevaney. 1985. Diet and 20-year mortality from coronary heart disease. The Ireland-Boston Diet-Heart Study. N. Engl. J. Med. 312:811-818.
Lapidus, L., C. Bengtsson, B. Larsson, K. Pennert, E. Rybo, and L. Sjöström. 1984. Distribution of adipose tissue and risk of cardiovascular disease and death: a 12-year follow up of participants in the population study of women in Gothenburg, Sweden. Br. Med. J. 289:1257-1261.
LaPorte, R.E., and K.J. Cruickshanks. 1985. Incidence and risk factors for insulin-dependent diabetes. Pp. III-1 to III-12 in M.I. Harris and R.F. Hamman, eds. Diabetes in America: Diabetes Data Compiled 1984. Report of the National Diabetes Data Group. NIH Publ. No. 85-1468. National Institute of Arthritis, Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Public Health Service. U.S. Department of Health and Human Services, Bethesda, Md.
LaPorte, R.E., N. Tajima, H.K. Akerblom, N. Berlin, J. Brosseau, M. Christy, A.L. Drash, H. Fishbein, A. Green, R. Hamman, M. Harris, H. King, Z. Laron, and A. Neil. 1985. Geographic differences in the risk of insulin-dependent diabetes mellitus: the importance of registries. Diabetes Care 8 suppl. 1:101-107.
Larsson, B., K. Svärdsudd, L. Welin, L Wilhelmsen, P. Björntorp, and G. Tibblin. 1984. Abdominal adipose tissue distribution, obesity, and risk of cardiovascular disease and death: 13-year follow-up of participants in the study of men born in 1913. Br. Med. J. 288:1401-1404.
Leon, A.S., and H. Blackburn. 1982. Physical activity and hypertension. Pp. 14-36 in P. Sleight and E. Freis, eds. Hypertension: Cardiology, Vol. 1. Butterworth Scientific, London.
Levy, R.I. 1979. Stroke decline: implications and prospects. N. Engl. J. Med. 300:490-491.
Lew, E.A., and L. Garfinkel. 1979. Variations in mortality by weight among 750,000 men and women. J. Chronic Dis. 32: 563-576.
Lewinnek, G.E., J. Kelsey, A.A. White III, and N.J. Kreiger. 1980. The significance and a comparative analysis of the epidemiology of hip fractures. Clin. Orthop. (152):35-43.
Lieber, C.S., D.P. Jones, J. Mendelson, and L.M. DeCarli. 1963. Fatty liver, hyperlipemeia and hyperuricemia produced by prolonged alcohol consumption, despite adequate dietary intake. Trans. Assoc. Am. Physicians 76:289-301.
Lieber, C.S., L. DeCarli, and E. Rubin. 1975. Sequential production of fatty liver, hepatitis, and cirrhosis in subhuman primates fed ethanol with adequate diets. Proc. Natl. Acad. Sci. U.S.A. 72:437-441.
Lindquist, O., C. Bengtsson, T. Hansson, and B. Roos. 1979. Age at menopause and its relation to osteoporosis. Maturitas 1:175-181.
Page 133
Low-Beer, T.S. 1985. Nutrition and cholesterol gallstones. Proc. Nutr. Soc. 44:127-134.
Lubin, F., A.M. Ruder, Y. Wax, and B. Modan. 1985. Overweight and changes in weight throughout adult life in breast cancer etiology. A case-control study. Am. J. Epidemiol. 122:579-588.
MacMahon, S.W., and S.R. Leeder. 1984. Blood pressure levels and mortality from cerebrovascular disease in Australia and the United States. Am. J. Epidemiol. 120:865-875.
Mahboubi, E., J. Kmet, P.J. Cook, N.E. Day, P. Ghadirian, and S. Salmasizadeh. 1973. Oesophageal cancer studies in the Caspian Littoral of Iran: the Caspian cancer registry. Br. J. Cancer 28:197-214.
Manson, J.E., M.J. Stampfer, C.H. Hennekens, and W.C. Willett. 1987. Body weight and longevity. A reassessment. J. Am. Med. Assoc. 257:353-358.
Marmot, M.G., S.L. Syme, A. Kagan, H. Kato, J.B. Cohen, and J. Belsky. 1975. Epidemiologic studies of coronary heart disease and stroke in Japanese men living in Japan, Hawaii and California: prevalence of coronary and hypertensive heart disease and associated risk factors. Am. J. Epidemiol. 102:514-525.
Marmot, M.G., A.M. Adelstein, N. Robinson, and G.A. Rose. 1978. Changing social-class distribution of heart disease. Br. Med. J. 2:1109-1112.
Marshall, J. 1971. Familial incidence of cerebrovascular disease. J. Med. Genet. 8:84-89.
Mazess, R.B. 1983. The noninvasive measurement of skeletal mass. Pp. 223-279 in W.A. Peck, ed. Bone and Mineral Research, Annual 1: A Yearly Survey of Developments in the Field of Bone and Mineral Metabolism. Excerpta Medica, Amsterdam.
Melton, L.J., III, K.M. Macken, P.J. Palumbo, and L.R. Elveback. 1980. Incidence and prevalence of clinical peripheral vascular disease in a population-based cohort of diabetic patients. Diabetes Care 3:650-654.
Melton, L.J., III, P.J. Palumbo, and C.P. Chu. 1983. Incidence of diabetes mellitus by clinical type. Diabetes Care 6: 75-86.
Merkatz, I.R., M.A. Duchon, T.S. Yamashita, and H.B. Houser. 1980. A pilot community-based screening program for gestational diabetes. Diabetes Care 3:453-457.
Mezey, E. 1980. Alcoholic liver disease: roles of alcohol and malnutrition. Am. J. Clin. Nutr. 33:2709-2718.
Mezey, E. 1982. Alcoholic liver disease. Prog. Liver Dis. 7: 555-572.
Miall, W.E., and H.G. Lovell. 1967. Relation between change of blood pressure and age. Br. Med. J. 2:660-664.
Millar, W.J., and T. Stephens. 1987. The prevalence of overweight and obesity in Britain, Canada, and United States. Am. J. Public Health 77:38-41.
Miller, A.J., J.A. Brunelle, J.P. Carlos, L.J. Brown, and H. Löe. 1987. Oral Health of United States Adults. The National Survey of Oral Health in U.S. Employed Adults and Seniors: 1985-1986, National Findings. NIH Publ. No. 87-2868. Epidemiology and Oral Disease Prevention, National Institute of Dental Research, National Institutes of Health, Public Health Service, U.S. Department of Health and Human Services, Bethesda, Md. 168 pp.
Moore, M.E., A. Stunkard, and L. Srole. 1962. Obesity, social class, and mental illness. J. Am. Med. Assoc. 181:962-966.
Nagase, M., H. Tanimura, M. Setoyama, and Y. Hikasa. 1978. Present features of gallstones in Japan. A collective review of 2,144 cases. Am. J. Surg. 135:788-790.
Nance, W.E., H. Krieger, E. Azevedo, and M.P. Mi. 1965. Human blood pressure and the ABO blood group system: an apparent association. Hum. Biol. 37:238-244.
National Diabetes Data Group. 1985. Diabetes in America: Diabetes Data Compiled 1984. NIH Publ. No. 85-1468. National Institute of Arthritis, Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Public Health Service, U.S. Department of Health and Human Services, Bethesda, Md. (various pagings).
National Heart Foundation of Australia. 1981. Risk Factor Prevalence Study. No. 1. NHFA, Canberra, Australia. 136 pp.
Newton-John, H.F., and D.B. Morgan. 1970. The loss of bone with age, osteoporosis, and fractures. Clin. Orthop. 71:229-252.
NIAAA (National Institute on Alcohol Abuse and Alcoholism). 1985. Liver Cirrhosis Mortality in the United States: U.S. Alcohol Epidemiologic Data Reference Manual, Vol. II. Alcohol, Drug Abuse, and Mental Health Administration, Public Health Service, U.S. Department of Health and Human Services, Rockville. Md. 232 pp.
NIAMS (National Institute of Arthritis and Musculoskeletal and Skin Diseases). 1988. Arthritis, Rheumatic Diseases, and Related Disorders: Moyer Report. National Institutes of Health, Public Health Service, U.S. Department of Health and Human Services, Bethesda, Md. 23 pp.
NIDR (National Institute of Dental Research). 1981. The Prevalence of Dental Caries in United States Children, 1979-1980: The National Dental Caries Prevalence Survey.
NIH Publ. No. 82-2245. National Caries Program, National Institutes of Health, Public Health Service, U.S. Department of Health and Human Services, Bethesda, Md. 159 pp.
NIH (National Institutes of Health). 1984. Osteoporosis: NIH Consensus Development Conference. National Institute of Arthritis, Diabetes, and Digestive and Kidney Diseases and the Office of Medical Applications of Research, Bethesda, Md. 87 pp.
Nordin, B.E. 1966. International patterns of osteoporosis. Clin. Orthop. 45:17-30.
NRC (National Research Council). 1982. Diet, Nutrition, and Cancer. Report of the Committee on Diet, Nutrition, and Cancer, Assembly of Life Sciences. National Academy Press, Washington, D.C. 478 pp.
O'Brien, M.J., M.R.C. Path, and L.S. Gottlieb. 1979. The liver and biliary tract. Pp. 1009-1091 in S.L. Robbins and R.S. Cotran, eds. Pathologic Basis of Disease, 2nd ed. W.B. Saunders, Philadelphia.
Omae, T., M. Takeshita, and Y. Hirota. 1976. The Hisayama Study and Joint Study on cerebrovascular diseases in Japan. Pp. 255-265 in P. Scheinberg, ed. Cerebrovascular Diseases: Tenth Princeton Conference. Raven Press, New York.
Ostfeld, A.M., R.B. Shekelle, H. Klawans, and H.M. Tufo. 1974. Epidemiology of stroke in an elderly welfare population. Am. J. Public Health 64:450-458.
Owen, R.A., L.J. Melton III, K.A. Johnson, D.M. Ilstrup, and B.L. Riggs. 1982. Incidence of Colles' fracture in a North American community. Am. J. Public Health 72:605-607.
Page, L.B. 1979. Hypertension and atherosclerosis in primitive and acculturating societies. Pp. 1-12 in J.C. Hunt, ed. Hypertension Update, Vol. 1. Health Learning Systems, Lyndhurst, N.J.
Peacock, P.B., C.P. Riley, T.D. Lampton, S.S. Raffel, and
Page 134
J.S. Walker. 1972. The Birmingham Stroke, Epidemiology and Rehabilitation Study. Pp. 231-345 in G.T. Stewart, ed. Trends in Epidemiology: Application to Health Service Research and Training. C.C. Thomas, Springfield, Ill.
Pell, S., and W.E. Fayerweather. 1985. Trends in the incidence of myocardial infarction and in associated mortality and morbidity in a large employed population, 1957-1983. N. Engl. J. Med. 312:1005-1111.
Pooling Project Research Group. 1978. Relationship of blood pressure, serum cholesterol, smoking habit, relative weight and ECG abnormalities to incidence of major coronary events: final report of the Pooling Project. J. Chronic Dis. 31:201-306.
Prineas, R.J., and H. Blackburn. 1983. Clinical and epidemiologic relationships between electrolytes and hypertension. Pp. 63-85 in M.J. Horan, M. Blaustein, J.B. Dunbar, W. Kachadorian, N.M Kaplan, and A.P. Simopoulos, eds. NIH Workshop on Nutrition and Hypertension: Proceedings from a Symposium. Biomedical Information Corp., New York.
Prior, I.A.M., and J.M. Stanhope. 1980. Blood pressure patterns, salt use and migration in the Pacific. Pp. 243-262 in H. Kesteloot and J.V. Joossens, eds. Epidemiology of Arterial Blood Pressure. Martinus Nijhoff, The Hague.
Rabkin, S.W., F.A. Mathewson, and P.H. Hsu. 1977. Relation of body weight to development of ischemic heart disease in a cohort of young North American men after a 26 year observation period: the Manitoba Study. Am. J. Cardiol. 39:452-458.
Reisin, E., R. Abel, M. Modan, D.S. Silverberg, H.E., Eliahou, and B. Modan. 1978. Effect of weight loss without salt restriction on the reduction of blood pressure in overweight hypertensive patients. N. Engl. J. Med. 298:1-6.
Reunanen, A., H. Takkunen, and A. Aromaa. 1982. Prevalence of intermittent claudication and its effect on mortality. Acta Med. Scand. 211:249-256.
Richelson, L.S., H.W. Wahner, L. J. Melton III, and B.L. Riggs. 1984. Relative contributions of aging and estrogen deficiency to postmenopausal bone loss. N. Engl. J. Med. 311:1273-1275.
Riggs, B.L., H.W. Wahner, W.L. Dunn, R.B. Mazess, K.P. Offord, and L.J. Melton III. 1981. Differential changes in bone mineral density of the appendicular and axial skeleton with aging: relationship to spinal osteoporosis. J. Clin. Invest. 67:328-335.
Riggs, B.L, H.W. Wahner, E. Seeman, K.P. Offord, W.L. Dunn, R.B. Mazess, K.A. Johnson, and LJ. Melton III. 1982. Changes in bone mineral density of the proximal femur and spine with aging. Differences between the postmenopausal and senile osteoporosis syndromes. J. Clin. Invest. 70:716-723.
Roberts, J., and K. Maurer. 1976. Blood Pressure of Persons 6-74 Years of Age in the United States: Advance Data from Vital and Health Statistics of the National Center for Health Statistics, No. 1. Public Health Service, U.S. Department of Health, Education, and Welfare, Rockville, Md. 6 pp.
Robertson, T.L, H. Kato, G.G. Rhodes, A. Kagan, M. Marmot, S.L Syme, T. Gordon, R.M. Worth, J.L Belsky, D.S. Dock, M. Miyanishi, and S. Kawamoto. 1977. Epidemiologic studies of coronary heart disease and stroke in Japanese men living in Japan, Hawaii and California: incidence of myocardial infarction and death from coronary heart disease. Am. J. Cardiol. 39:239-243.
Robinson, S.C., and M. Brucer. 1939. Range of normal blood pressure: statistical and clinical study of 11,383 persons. Arch. Intern. Med. 64:409-444.
Rose, G. 1981. Strategy of prevention: lessons from cardiovascular disease. Br. Med. J. 282:1847-1851.
Rose, S.H., LJ. Melton III, B.F. Morrey, D.M. Ilstrup, and B.L. Riggs. 1982. Epidemiologic features of humeral fractures. Clin. Orthop. 168:24-30.
Rubin, E., and C.S. Lieber. 1968. Alcohol-induced hepatic injury in nonalcoholic volunteers. N. Engl. J. Med. 278: 869-76.
Rubin, E., and C.S. Lieber. 1974. Fatty liver, alcoholic hepatitis and cirrhosis produced by alcohol in primates: N Engl. J. Med. 290:128-135.
Sampliner, R.E., P.H. Bennett, L.J. Comess, F.A. Rose, and T.A. Burch. 1970. Gallbladder disease in Pima Indians. Demonstration of high prevalence and early onset by cholecystography. N. Engl. J. Med. 283:1358-1364.
Schadt, D.C., E.A. Hines, Jr., J.L. Juergens, and N.W. Barker. 1961. Chronic atherosclerotic occlusion of the femoral artery. J. Am. Med. Assoc. 175:937-940.
Schmidt, W. 1977. Cirrhosis and alcohol consumption: an epidemiological perspective. Pp. 15-47 in G. Edwards and M. Grant, eds. Alcoholism: New Knowledge and New Responses. University Park Press, Baltimore.
Seichter, U. 1987. Root surface caries: a critical literature review. J. Am. Dent. Assoc. 115:305-310.
Seidell, J.C., K.C. Bakx, P. Deurenberg, H.J. van den Hoogen, J.G. Hautvast, and T. Stijnen. 1986. Overweight and chronic illness-a retrospective cohort study, with a follow-up of 6-17 years, in men and women of initially 20-50 years of age. J. Chronic Dis. 39:585-593.
Skog, O.J. 1984. The risk function for liver cirrhosis from lifetime alcohol consumption. J. Stud. Alcohol 45:199-208.
Sloan, N.R. 1963. Ethnic distribution of diabetes mellitus in Hawaii. J. Am. Med. Assoc. 183:419-424.
Smith, R.W., and J. Rizek. 1966. Epidemiologic studies of osteoporosis in women of Puerto Rico and southeastern Michigan with special reference to age, race, national origin and to other related or associated findings. Clin. Orthop. 45:31-48.
Society of Actuaries. 1980. Build Study of 1979. Society of Actuaries and Association of Life Insurance Medical Directors of America, Chicago. 255 pp.
Sognnaes, R.F. 1948. Analysis in wartime reduction of dental caries in European children with special regard to observations in Norway. Am. J. Dis. Child. 75:792-821.
Solberg, L.A., and J.P. Strong. 1983. Risk factors and atherosclerotic lesions. A review of autopsy studies. Arteriosclerosis 3:187-198.
Soltero, I., K. Kiu, R. Cooper, J. Stamler, and D. Garside. 1978. Trends in mortality from cerebrovascular diseases in the United States, 1960 to 1975. Stroke 9:549-555.
Spiegelman, M., and H.H. Marks. 1946. Age and sex variations in the prevalence and onset of diabetes mellitus. Am. J. Public Health 36:26-33.
Sreenby, L.M. 1982. Sugar availability, sugar consumption and dental caries. Community Dent. Oral Epidemiol. 10: 1-7.
Stamler, J., D. Berkson, A. Dyer, M.H. Lepper, H.A. Lindberg, O. Paul, H. McKean, P. Rhomberg, J.A. Schoenberger, R.B. Shekelle, and R. Stamler. 1975. Relationship of multiple variables to blood pressure findings from four Chicago epidemiologic studies. Pp. 307-356 in O. Paul, ed.
Page 135
Epidemiology and Control of Hypertension. Stratton Intercontinental Medical Book Co., New York.
Staszewski, J. 1974. Cancer of the upper alimentary tract and larynx in Poland and in Polish-born Americans. Br. J. Cancer 29:389-399.
Stocks, R. 1954. The etiology of essential hypertension. I. The arterial pressure in the general population. Clin. Sci. 13:11-35.
Stokes, J., III, R.J. Garrison, and W.B. Kannel. 1985. The independent contributions of various indices of obesity to the 22-year incidence of coronary heart disease: the Framingham Heart Study. Pp. 49-57 in . Vague, P. Bjorntorp, B. Guy-Grand, and M. Rebuffe-Scrive, eds. Metabolic Complications of Human Obesities. Elsevier, New York.
Strong, J.P., and M.L. Richards. 1976. Cigarette smoking and atherosclerosis in autopsied men. Atherosclerosis 23:451-476.
Stunkard, A.J., T.I.A. Sorensen, C. Hanis, T.W. Teasdale, R. Chakraborty, W.J. Schull, and F. Schulsinger. 1986. An adoption study of human obesity. N. Engl. J. Med. 314:193-198.
Takeuchi, M. 1961. Epidemiologic study on dental caries in Japanese children, before, during and after World War II. Int. Dent. J. 11:443-457.
Takeya, Y., J.S. Popper, Y. Shimizu, H. Kato, G.G. Rhoads, and A. Kagan. 1984. Epidemiologic studies of coronary heart disease and stroke in Japanese men living in Japan, Hawaii and California: incidence of stroke in Japan and Hawaii. Stroke 15:15-23.
Taylor, R., and P. Zimmet. 1983. Migrant studies in diabetes epidemiology. Pp. 58-77 in J.I. Mann, K. Pyorala, and A. Teuscher, eds. Diabetes in Epidemiological Perspective. Churchill Livingstone, Edinburgh.
Terris, M. 1967. Epidemiology of cirrhosis of the liver: national mortality data. Am. J. Public Health 57:2076-2088.
Toverud, G. 1957. The influence of war and post-war conditions on the teeth of Norwegian school children. III. Discussion of food supply and dental condition in Norway and other European countries. Milbank Mem. Fund Q. 35:373-459.
Trotter, M., G.E. Broman, and R.R. Peterson. 1960. Densities of bones of white and negro skeletons. J. Bone Jt. Surg. Am. Vol. 42A:50-58.
Tyroler, H.A., S. Heyden, and C.G. Hames. 1975. Weight and hypertension: Evans County studies of blacks and whites. Pp. 177-204 in O. Paul, ed. Epidemiology and Control of Hypertension. Stratton Intercontinental Medical Book Co., New York.
Ueshima, H., M. Iida, T. Shimamoto, M. Konishi, K. Tsujioka, M. Tanigaki, N. Nakanishi, H. Ozawa, S. Kojima, and Y. Komachi. 1980. Multivariate analysis of risk factors for stroke. Eight-year follow-up study of farming villages in Akita, Japan. Prev. Med. 9:722-740.
Van Itallie, T.B. 1985. Health implications of overweight and obesity in the United States. Ann. Intern. Med. 103:983-988.
Voors, A.W., LS. Webber, and G.S. Berenson. 1978. Relationship of blood pressure levels to height and weight in children. Cardiovasc. Med. 3:911-918.
Waaler, H.T. 1984. Height, weight and mortality. The Norwegian experience. Acta Med. Scand. 679:1-56.
Wadden, T.A., and A.J. Stunkard. 1985. Social and psychological consequences of obesity. Ann. Intern. Med. 103: 1062-1067.
Wallace, W.A. 1983. The increasing incidence of fractures of the proximal femur: an orthopaedic epidemic. Lancet 1: 1413-1414.
Waterhouse, J., C. Muir, K. Shanmugaratnam, and J. Powell. 1982. Cancer Incidence in Five Continents, Vol. IV. IARC Scientific Publications No. 42. International Agency for Research on Cancer, Lyon, France. 811 pp.
Weinsier, RL., D.J. Norris, R. Birch, R.S. Bernstein, J. Wang, M.U. Yang, R.N. Pierson, Jr., and T.B. Van Itallie. 1985. The relative contribution of body fat and fat pattern to blood pressure level. Hypertension 7:578-585.
Weiss, K.M., R.E. Ferrell, C.L. Hanis, and P.N. Styne. 1984. Genetics and epidemiology of gallbladder disease in New World native peoples. Am. J. Hum. Genet. 36:1259-1278.
Welin, L., K. Svärdsudd, L Wilhelmsen, B. Larsson, and G. Tibblin. 1987. Analysis of risk factors for stroke in a cohort of men born in 1913. N. Engl. J. Med. 317:521-526.
WHO (World Health Organization). 1978. Arterial Hypertension: Report of a WHO Expert Committee. Technical Rep. Ser. 628. World Health Organization, Geneva. 58 pp.
Witkop, C.J., Jr., ed. 1962. Genetics and Dental Health: Proceedings of an International Symposium Held at the National Institutes of Health. McGraw-Hill, New York. 300 pp.
Wu, Y.K., C.Q. Lu, R.C. Gao, J.S. Yu, and G.C. Liu. 1982. Nation-wide hypertension screening in China during 1979-1980. Chin. Med. J. 95:101-108.
Yamase, H., and J.J. McNamara. 1972. Geographic differences in the incidence of gallbladder disease: influence of environment and ethnic background. Am. J. Surg. 123: 667-670.
Yano, K., R.D. Wasnich, J.M. Vogel, and L.K. Heilbrun. 1984. Bone mineral measurements among middle-aged and elderly Japanese residents in Hawaii. Am. J. Epidemiol. 119: 751-764.
There was a problem loading page 136.






































