STEMMING THE TIDE OF MULTIDRUG-RESISTANT TUBERCULOSIS:
MAJOR BARRIERS TO ADDRESSING THE GROWING EPIDEMIC
A WHITE PAPER FOR THE INSTITUTE OF MEDICINE OF THE NATIONAL ACADEMIES
HARVARD MEDICAL SCHOOL
■
PARTNERS IN HEALTH
■
FRANÇOIS–XAVIER BAGNOUD CENTER FOR HEALTH AND HUMAN RIGHTS
■
BRIGHAM AND WOMEN’S HOSPITAL
November 2008
AUTHORS
Lead Authors
Salmaan Keshavjee
Department of Global Health and Social Medicine, Harvard Medical School/
Division of Global Health Equity, Brigham and Women’s Hospital/
Partners In Health
Kwonjune Seung
Division of Global Health Equity, Brigham and Women’s Hospital/
Partners In Health
Contributing Authors
Rajesh Gupta
Stanford University School of Medicine
Tom Nicholson
Partners In Health
Julie Rosenberg Talbot
Division of Global Health Equity, Brigham and Women’s Hospital
Chris Vanderwarker
University of Washington
Paul Zintl
Partners In Health/
Department of Global Health and Social Medicine, Harvard Medical School
Other Contributors:
Mercedes Becerra
Department of Global Health and Social Medicine, Harvard Medical School/
Division of Global Health Equity, Brigham and Women’s Hospital
Paul Farmer
Department of Global Health and Social Medicine, Harvard Medical School/
Division of Global Health Equity, Brigham and Women’s Hospital/
Partners In Health
Jennifer Furin
Department of Global Health and Social Medicine, Harvard Medical School/
Division of Global Health Equity, Brigham and Women’s Hospital/
Partners In Health
Stephen Hallisey
Department of Global Health Equity, Brigham and Women’s Hospital
Amy Judd
Division of Global Health Equity, Brigham and Women’s Hospital
Kathryn Kempton
Partners In Health
David Kim
Division of Global Health Equity, Brigham and Women’s Hospital
Jim Yong Kim
Department of Global Health and Social Medicine, Harvard Medical School/
Division of Global Health Equity, Brigham and Women’s Hospital/
Harvard School of Public Health/
François–Xavier Bagnoud Center For Health And Human Rights/
Partners In Health
Carole Mitnick
Department of Global Health and Social Medicine, Harvard Medical School/
Division of Global Health Equity, Brigham and Women’s Hospital
Joia Mukherjee
Department of Global Health and Social Medicine, Harvard Medical School/
Division of Global Health Equity, Brigham and Women’s Hospital/
Partners In Health
Edward Nardell
Harvard School of Public Health/
Division of Global Health Equity, Brigham and Women’s Hospital
Catherine Oettinger
Partners In Health
Sonya Shin
Department of Global Health and Social Medicine, Harvard Medical School/
Division of Global Health Equity, Brigham and Women’s Hospital
Valerie Varco
Division of Global Health Equity, Brigham and Women’s Hospital
Rebecca Weintraub
Department of Global Health and Social Medicine, Harvard Medical School/
Division of Global Health Equity, Brigham and Women’s Hospital
In-depth Interviews
Shalala Akhmedova
Coordinator, International Committee of Red Cross (Azerbaijan)
Peter Cegielski
Unites States Centers for Disease Control and Prevention
Agnes Gebhard
KNCV Tuberculosis Foundation
Ogtay Gozalov
Regional Tuberculosis Control Program, South Caucasus, Azerbaijan
Mark Harrington
Treatment Action Group
Phil Hopewell
University of California San Francisco
Vaira Leimane
WHO Collaborating Centre for Research and Training in Management of MDR-TB (Latvia)
Joël Keravec
Management Sciences for Health/Brazil
Laura Jacobus
Jacobus Pharmaceutical Co., Inc.
Moses Joloba
National Tuberculosis Program, Uganda
Fabienne Jouberton
Global Fund to fight AIDS, Tuberculosis and Malaria
Robert Matiru
Global Drug Facility, STOP TB Partnership
Fuad Mirzayev
World Health Organization
Pierre-Yves Norval
World Health Organization
Madhukar Pai
McGill University
C. N. Paramasivan
Foundation for Innovative New Diagnostics
Dmitri Pashkeevich
Office of the Special Representative of the WHO Director-General in Russia
Trevor Peters
Clinton Foundation
Alexei Prorekhin
Partners In Health, Russia
Steven Reynolds
Unites States Centers for Disease Control and Prevention--Uganda
John Ridderhof
Unites States Centers for Disease Control and Prevention
William Rodriguez
Brigham and Women’s Hospital/Harvard Medical School
Tamara Russell
Eli Lilly Global Manufacturing
Nina Schwalbe
Global Alliance for Vaccines and Immunization
Alex Sloutsky
Massachusetts State Laboratory Institute/University of Massachusetts
Thelma Tupasi
Tropical Disease Foundation, the Philippines
Karin Weyer
World Health Organization
Abigail Wright
World Health Organization
Acknowledgement of Support
Jaime Bayona
Socios En Salud
Ernesto Jaramillo
World Health Organization
Kitty Lambregts
KNCV Tuberculosis Foundation
Oksana Ponomarenko
Partners In Health, Russia
Mario Raviglione
World Health Organization
Peter Stephens
IMS Health
The authors would like to thank the many other colleagues who participated in numerous discussions on the topics covered in this document over the last year.
The views expressed in this document are solely those of the authors and are not meant to represent the position of any individual who was interviewed or gave support to this project, nor the Institute of Medicine, Harvard Medical School, Partners In Health, the François-Xavier Bagnoud Center for Health Human Rights or Brigham and Women’s Hospital.
Cover Photo: Open Society Institute/Pep Bonet
EXECUTIVE SUMMARY
Every year nearly 500,000 people worldwide fall ill from newly-acquired disease caused by multidrug-resistant tuberculosis (MDR-TB), adding to an estimated global burden of at least 1.5 million prevalent cases. This infectious disease is spread through the air and is caused by strains of Mycobacterium tuberculosis that are resistant to the two most effective first-line anti-tuberculosis drugs. Before they die from the disease, people infected with MDR-TB often transmit the mycobacterium to others. More ominously, tuberculosis strains now deemed extensively drug-resistant (XDR-TB) threaten the progress made to date in the treatment of resistant disease and necessitate an urgent call to action. Though aggressive treatment with second-line drugs has yielded a range of positive outcomes for patients with XDR-TB, the widespread emergence of totally drug-resistant strains (TDR-TB) would return us to the preantibiotic era.
Confronting MDR-TB is a core goal stated in the WHO’s Global Plan to Stop TB: 2006-2015. Under the original plan, at least 800,000 people with active MDR-TB were to be treated by 2015. A subsequent revision, reflecting the concern over XDR-TB, made a more ambitious call for universal access to treatment for all active MDR-TB patients; this will require the treatment of nearly 1.6 million patients by 2015. At present, only ten percent of new MDR-TB cases are treated each year, and less than two percent are receiving verifiable, quality-assured, second-line anti-TB drugs through WHO’s Green Light Committee (GLC) mechanism. Preventing the further emergence of strains of tuberculosis with broad-spectrum resistance—including those resistant to all first- and second-line anti-tuberculosis drugs—is dependent upon identifying and addressing barriers to effective diagnosis and treatment of drug-resistant tuberculosis without delay.
While multidrug-resistant strains of tuberculosis may have first emerged from inadequate treatment and control programs in the recent past, continued spread of this airborne pathogen is directly affected by the following barriers to large-scale, effective treatment delivery:
-
Exceedingly limited capacity to rapidly diagnose drug-resistant TB. True point-of-care testing is practically nonexistent, especially in the areas with the highest tuberculosis burden.
-
Limited supply of quality-assured second-line anti-tuberculosis drugs. The current supply is insufficient, even for the estimated two percent of MDR-TB patients being treated through the GLC mechanism. This is exacerbated by limited demand for quality-assured second-line anti-tuberculosis drugs in countries with high burdens of MDR-TB. These countries are using local manufacturers who often do not meet quality-assurance standards as defined by the WHO.
-
Ambiguous messaging about the importance of integration of MDR-TB into national tuberculosis control programs, perpetuated by a “pilot-program” mentality that has not been encouraging a push for universal access.
-
Inadequate mechanisms for delivering technical assistance to countries in a manner that sufficiently addresses the need and builds local capacity to effectively and safely treat and manage MDR-TB.
-
Lack of focus on interrupting transmission of the tuberculosis bacilli in congregate settings both in the community and in institutions such as hospitals, clinics, and prisons.
This paper provides several recommendations to facilitate the expansion of global treatment and prevention of multidrug-resistant tuberculosis. These include: promoting universal access to treatment as part of national tuberculosis control programs; improving and expanding laboratory capacity, including rapid point-of-service testing; reforming the current procurement system to ensure an adequate and accessible supply of quality-assured second-line drugs; providing ongoing, on-site technical assistance; and expanding the delivery of ambulatory-based MDR-TB treatment. It also includes recommendations concerning the development of effective transmission-control programs in resource-limited settings.
SPECIFIC RECOMMENDATIONS:
Diagnostics
-
Sustainable funding from bilateral and multilateral donors must be increased to support construction of in-country drug-sensitivity testing/rapid-testing laboratories and ongoing external quality assessments by supranational reference laboratories.
-
Creation of a system of long-term on-site technical assistance would help countries build and/or rapidly expand their capacity to perform mycobacterial culture, DST, and rapid molecular genetic tests for drug-resistant tuberculosis.
-
In-country laboratory networks for: specimen transport, data management, and certification and coordination of private laboratories need improvement.
-
Use of excess laboratory capacity for mycobacterial culture and drug-susceptibility testing in wealthy nations should be encouraged while laboratories are being built in poorer regions.
-
Priority must be given to research on—and funding for—the immediate development and rapid deployment of point-of-care testing for drug-susceptible and drug-resistant tuberculosis.
Drug Supply
-
The WHO and international partners should take immediate and rapid steps to increase the number of manufacturers of quality-assured second-line anti-tuberculosis drugs. A mechanism needs to be developed to make these drugs available at pre-negotiated prices to programs purchasing via the GDF and through direct-purchase by countries.
-
The GDF should create a tiered system of approval for manufacturers of second-line drugs—and purchase of product by the GLC mechanism—consistent with a manufacturer’s progress in the WHO’S Essential Drugs Monitoring (EDM) prequalification process. Large countries operating within the GLC mechanism should be allowed to purchase second-line anti-tuberculosis drugs from domestic manufacturers who have entered the EDM prequalification process.
-
The GLC mechanism should institute a transparent system for quantification of demand for second-line drugs.
-
The GDF should maintain a second-line anti-tuberculosis drug buffer stock (at minimum, enough to treat 5,000 patients) in order to facilitate rapid delivery of drugs to programs (less than one month).
-
There should be a global effort to increase the options available for treating MDR-TB and XDR-TB, by optimizing current regimens and by developing at least three new anti-TB drugs. Increased TB clinical trial capacity needs to be created, and mechanisms developed to fast-track new anti-TB drugs through the regulatory process.
Treatment Delivery
-
Universal treatment for drug-resistant tuberculosis within national TB control strategies—side by side with drug-susceptible disease—has to be clearly and actively promoted by multilateral and bilateral agencies, non-governmental organizations, and within countries. Universal TB treatment also must be well integrated with current HIV treatment initiatives.
-
The system of international technical assistance provision is currently inadequate. It must be transformed in order to better draw on the experience of successful regional MDR-TB-treatment programs, to include the provision of on-site, long-term technical assistance, and where necessary, to involve on-site implementation teams.
-
Community/Ambulatory-based MDR-TB treatment, and where appropriate, active collaboration with private-sector laboratories and tuberculosis treatment providers, should be actively promoted as a safe means of rapidly treating the largest number of patients. Delivery systems that support this will need to be strengthened and/or built.
-
Infection control to prevent transmission of TB strains has to be integrated fully into national TB-control strategies, with appropriate resources, training, implementation strategies, and monitoring.
-
Large global health initiatives—such as PEPFAR—and bilateral and institutional donors for global health should make improving the capacity to deliver MDR-TB treatment an important priority. The Global Fund and UNITAID have done so, and others should follow this lead with their influence and resources.
GLOSSARY OF TERMS:
|
Term |
Definition* |
|
Multidrug-resistant TB (MDR-TB) |
TB that is resistant to at least two of the best anti-TB drugs, isoniazid and rifampicin. These drugs are considered first-line drugs and are used to treat all persons with TB disease. |
|
Extensively drug-resistant TB (XDR-TB) |
TB that is resistant to isoniazid and rifampin, plus resistant to any fluoroquinolone and at least one of three injectable second-line drugs (i.e., amikacin, kanamycin, or capreomycin). |
|
First-line drugs |
The most common medicines used to treat newly diagnosed drug- susceptible TB are: isoniazid (INH); rifampin (RIF); ethambutol; and, pyrazinamide. |
|
Second-line drugs |
Drugs included in the treatment regimen for MDR TB are amikacin, capreomycin, ciprofloxacin, cycloserine, ethionamide, kanamycin, levofloxacin, ofloxacin, para-aminosalicylic acid, and prothionamide. |
|
* Source: US Centers for Disease Control |
|
SECTION I:
THE PROBLEM OF DRUG-RESISTANT TUBERCULOSIS
1
INTRODUCTION
Tuberculosis (TB) is one of the leading causes of death in the world today. The World Health Organization (WHO) estimates that Mycobacterium tuberculosis caused active disease in 9.15 million people across the globe, killing 1.6 million of them. More people carry the bacillus today—one-third of the world’s population—than at any other period in history.1
Known to medical science since earliest antiquity, TB has proven to be a remarkably hardy and resourceful foe. Its trademark symptoms—a hacking, productive cough, chest pain, fever—were accurately identified by Hippocrates in the fifth century BC; its contagiousness was established as early as the eleventh century AD; and the bacterium that causes it was isolated by Robert Koch in 1882. Antibiotics to treat the disease have been available for over half a century. But unlike earlier plagues that yielded readily to advances in medical science, TB has earned a fearsome reputation as one of the most tenacious and resilient threats to public health in recorded history.
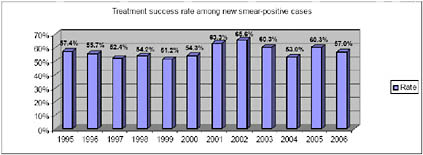
That resiliency arises in part from the bacterium’s ability to mutate and acquire drug resistance. In order to provide comprehensive TB care to some of the world’s poorest populations, in 1993 the WHO created the DOTS strategy—directly observed therapy, short-course—as a global programmatic strategy. Developed by the British Medical Research Council (MRC) and the International Union Against Tuberculosis and Lung Disease (IUATLD) in the 1970s and 1980s, DOTS was an attempt to provide effective tuberculosis (TB) treatment in the shortest possible time, and thereby prevent the development of drug-resistant TB and large numbers of “chronic cases.”2,3,4,5,6,7,8 DOTS was rolled out with great fanfare in 1993. For the first time, TB treatment was to be delivered to patients under uniform programmatic conditions, which involved the direct observation of therapy. Despite its huge success as a program, the early DOTS strategy had several key shortcomings that limited its effectiveness and necessitated a different approach. Firstly, DOTS was originally designed for settings and conditions in which resistance to first-line anti-TB drugs was minimal.9,10 However, in settings where a significant proportion of patients are infected with strains of M. tuberculosis that are already resistant to one or more of the first-line anti-TB drugs, short-course chemotherapy (the drug regimen in DOTS) is of limited utility.11,12,13,14,15,16,17,18In fact, in some places, use of the DOTS approach alone was contributing to poor outcomes and preventable mortality.19,20,21,22,23,24,25,26,27,28
According to the WHO, the amount of drug resistance has been trending upward in many parts of the world (at least one country in each of WHO’s six regions reports an MDR-TB incidence of greater
than 3 percent among new patients [see Figure 1]).29 Of particular concern are strains that are resistant to the two main first-line anti-TB drugs that form the back-bone of short-course chemotherapy, isoniazid and rifampin. Known as multi-drug resistant TB (MDR-TB), these strains have been found throughout the world,30,31 and are a significant cause of global TB morbidity and mortality.32,33,34,35,36,37 The total global burden of MDR-TB is estimated at almost 490,000 new cases per year, or over 4 percent of all TB cases; an estimated 120,000 of these patients die annually.38,39,40 MDR-TB has been implicated in institutional outbreaks in the United States, Europe, Asia and Latin America, outbreaks that produced high case fatality rates among immunosuppressed people, as well as high rates of transmission to other patients, caregivers, and family members.41,42,43,44,45,46,47,48,49 Because no new anti-TB drugs have been discovered or developed for decades, the antibiotic armamentarium with which to treat MDR-TB is surprisingly small. Patients who develop MDR-TB or XDR-TB require treatment for 18 to 24 months, sometimes hospitalization, and in some cases, surgical resection of infected lung tissue.
Figure 1: Countries and settings with MDR-TB prevalence higher than 5 percent (2002 to 2007)50
The problem of drug-resistance has become all the more frightening over the last half decade with the emergence of MDR-TB strains with broad-spectrum resistance to both first- and second-line anti-TB drugs. Some of these strains—known as extensively drug-resistant tuberculosis (XDR-TB)—have been found to be resistant to the most effective second-line anti-TB drugs: fluoroquinolones and parenteral anti-TB agents. A recent nosocomial outbreak of XDR-TB among HIV-positive patients in South Africa resulted in a case fatality rate of almost 100 percent.51 According to the World Health Organization, there are an estimated 40,000 cases of XDR-TB each year, half of whom die in short-order.
How drug-resistant TB emerges and spreads is best understood as two interlinked processes. Initially, a patient infected with drug-susceptible TB seeks treatment with standard, first-line medications. Under proper conditions—and assuming only quality-assured antibiotics are used—the patient will likely be cured and not relapse.52 However, if the patient is treated with an inadequate number of effective drugs for an appropriate length of time, does not complete her treatment regimen, or has problems absorbing the treatment regimen (as is often the case in patients with HIV), the treatment can fail.53 Although patients who fail treatment may have developed some drug resistance, most programs continue to prescribe multiple cycles of first-line anti-TB therapy. With each iteration of unsuccessful treatment, the number of drugs to which the patient becomes resistant increases (this process is called “amplification of resistance”).54,55 Newly infected patients are often not identified as having a drug-resistant strain of M. tuberculosis, and enter the same cycle as above, in which they are inadequately managed from the onset of treatment. Thus, in many ways, the recently recognized increase in global MDR-TB prevalence reflects serious deficiencies in both the programmatic approach to treating TB (drug-susceptible) and TB treatment delivery at the country-level. When MDR-TB strains appear in a given setting, the situation is exacerbated by: (1) constraints on the ability of local practitioners to diagnose drug-resistance, largely due to the absence of laboratory infrastructure; (2) the lack of a consistent and sufficient supply of quality-assured, second-line anti-TB drugs; and (3) programmatic challenges to delivering TB treatment for the requisite treatment length.56
Concern over the high burden of MDR-TB faced by many countries has recently led to major changes in the international TB community’s approach to the treatment of resistant strains in resource-poor settings. In 1998, global TB partners, including the WHO, created “DOTS-Plus,” which attempted to address the most glaring deficiencies of DOTS vis-à-vis treatment of drug-resistant TB. DOTS-Plus was greeted with skepticism by many TB experts and practitioners, who were concerned that the use of second-line anti-TB drugs would lead to expanded drug resistance. In 2000, to reassure those critics, the WHO and its partners established a multi-agency task force called the Green Light Committee (GLC) Housed at the WHO headquarters in Geneva, the GLC was assigned to improve access for programs to concessionary-priced second-line anti-TB drugs, while promoting the rational use of these drugs through appropriate programmatic management.57 The initial five projects approved by the GLC became known as DOTS-Plus pilot projects, and provided essential information for the development of the WHO’s global drug-resistant TB guidelines (Guidelines For The Programmatic Management Of Drug-Resistant Tuberculosis).58 Overall, using aggressive treatment regimens, direct-observation of therapy with incentives and enablers, and management of adverse events, the GLC pilot projects achieved cure-rates of 75to 80 percent for new cases of MDR-TB and between 65and 70 percent for previously treated
cases59,60,61,62,63,64 XDR-TB patients have been found to have cure rates of between 48 and 60 percent in program settings.65,66
Based on these results, as well as evidence that DOTS-Plus projects strengthen underlying TB control programs and reduce the reservoir of patients transmitting drug-resistant strains, the DOTS-Plus approach became the accepted management strategy for drug-resistant TB.67 In 2006, the Stop TB Strategy incorporated DOTS-Plus into an integrated strategy for TB control.68 Since 2000, the GLC has approved over 42,000 patients for treatment in 114 projects (see Figure 2).
Figure 2: GLC Projects and Patients as of August 2008

Alarmed over the surprising prevalence and growth of XDR-TB, policymakers from the WHO, key international partners, and affected countries met in the fall of 2006 to agree on a global strategy to combat MDR-TB and XDR-TB. They agreed on a two-year emergency plan— with a 2.15 billion USD budget—that called for aggressive revision of the 2015 targets to include “universal access” by 2015 (equating to nearly 1.6 million patients instead of the 800,000 patients covered in the original plan) and “universal access” by 2010.69 In addition, the plan calls for the treatment of 134,000 individuals by the end of 2008. Thus, although the gains of the GLC are impressive, they neither meet the WHO targets for MDR-TB treatment that were established in 2005, nor subsequent targets.70,71,72
The gap between the goals set by the WHO and the ability of health-workers on the ground to achieve them continues to vex policymakers throughout the TB community. For one thing, the most obvious explanation—inadequate funding—appears to be a fairly minor factor thanks to support from the Global Fund to Fight AIDS, Tuberculosis and Malaria (GFATM) and other funding sources. While
adequate financing is essential for successful implementation, evidence from the battle against other diseases (such as HIV, polio, and malaria) indicates that other issues must also be addressed by the global TB community.15
This White Paper, written for the Institute of Medicine (IOM), will attempt to delineate some of those other issues. The purpose of this document is not to provide an exhaustive inquiry into the complexities of MDR-TB care delivery. Rather, we aim to highlight and analyze the common difficulties that confront healthcare policymakers in resource-poor settings as they attempt to integrate MDR-TB treatment into their own national TB-control strategies and as they seek to expand treatment to all afflicted patients
2
A GENERAL FRAMEWORK FOR UNDERSTANDING BARRIERS TO MDR-TB SCALE-UP
Care delivery consists of myriad inter-connected activities. The care-delivery value chain (CDVC) model allows researchers to assign a value to every activity that occurs during the care of a patient for a specific medical condition. It identifies the discrete activities that are required to deliver care, illustrates their sequence and organization, and assesses the results in order to maximize the benefit to patients. Value is measured as a product of the many interdependent activities that make up the cycle. The value of any discrete activity can only be understood by considering its relation to other activities within the CDVC.
The CDVC for MDR-TB can be divided into four categories: population risk-stratification, diagnosis, intervention, and management. Each of these is an essential element, and together they constitute successful MDR-TB care delivery. As thorough as it is in assessing care, at the local level, the CDVC does not take into account macro-level barriers, such as the quality of imported pharmaceuticals or the lack of a point-of-care test for MDR-TB. For example, if we were to look at the category of “intervention” and ask why a certain country is not using quality-assured second-line anti-TB drugs for their MDR-TB patients, the answer—linked to many political and economic factors— lies outside the CDVC.
To bring these external factors into the equation, we have expanded the CDVC concept into the “implementation value chain” (IVC). The IVC is concerned with global variables that affect whether MDR-TB treatment can be successfully delivered to patients. The care cycle for an MDR-TB patient begins at the moment of infection and lasts until cure or death. In a country where MDR-TB is present, the implementation cycle begins at the moment when MDR-TB is acknowledged as a public-health concern and lasts until the creation of a management program within that country. Given the complexity of MDR-TB care— which involves everything from identifying infected patients and negotiating the
purchase of antibiotics on the open market to providing continuous treatment over a two-year period—the CDVC is inextricably bound to the IVC. Although the focus of this paper will be on the IVC, the ultimate aim is to improve conditions at the level of the patient so that treatment can be delivered effectively.
It is with this framework in mind that we have approached the challenges of barriers to the implementation of appropriate MDR-TB care by dividing this discussion document into the following sections: (1) diagnosis of MDR-TB; (2) MDR-TB drug supply and access; and (3) strengthening the delivery of MDR-TB treatment. The authors believe that by focusing on these three areas healthcare providers and policymakers stand the best chance of attaining universal access to drug-resistant TB treatment and care for patients with MDR-TB.
SECTION II:
DIAGNOSIS OF MDR-TB
-
INTRODUCTION
-
THE ANATOMY OF A LABORATORY NETWORK
-
LABORATORY CAPACITY BUILDING
-
NEW TB TECHNOLOGIES AND THE NEED FOR POINT–OF-CARE TESTING
-
RECOMMENDATIONS
1
INTRODUCTION
1.1
The inadequacies of sputum smear microscopy
Laboratory services for tuberculosis have traditionally emphasized smear microscopy for the diagnosis of active pulmonary TB.73 Smear microscopy is a decentralized service conducted at or near the point of care (POC). The test, which is from the 19th century, has significant technical limitations (e.g. low sensitivity and problems with specificity in areas with a high prevalence of Mycobacteria other than TB),74,75,76 Nevertheless, it remains an important part of TB control because it is widely available and because it targets patients with bacilli in their sputum (who are the most infectious).
Two important developments in the epidemiology of TB have called into question the over-reliance on sputum smear microscopy as the main modality for TB diagnosis. The first is the increasing incidence of HIV-TB co-infection, which although deadly, often manifests itself as paucibacillary and/or extra-pulmonary disease, which is often smear-negative. The second development is the rise of drug-resistant TB, including MDR-TB and XDR-TB. These strains of TB cannot be distinguished from drug-susceptible strains through microscopy alone, but must be subjected to drug-sensitivity testing (DST) either by mycobacterial culture or genetic analysis. Therefore, although sputum smear microscopy remains a vital service,77 it does not provide the information required to reliably diagnose TB, identify drug-resistant cases, or monitor resistance in settings with high tuberculosis drug-resistance.
The success of the global response to drug-resistant tuberculosis hinges on the ability of the healthcare system to find and manage MDR- and XDR-TB cases. Mycobacterial culture and drug-
sensitivity testing are the foundations of current laboratory services for MDR/XDR-TB diagnosis and control, but they require more resources than smear microscopy does. Incubators, refrigeration, and biosafety hoods, in addition to laboratory consumables, are all needed for these techniques to be performed properly. In addition, the tests require improved biosafety containment facilities, which carry more intense design, engineering, construction, and maintenance costs. The turnaround time for culture-based DST, even for automated tests, is at least two to four weeks, and samples must contain viable TB bacilli.
New technologies to diagnose drug-resistance and/or smear negative TB are being developed that overcome some of the basic limitations of culture systems.78,79 Some of these alternatives have recently been validated and their use is being expanded.80 Even when new tests are implemented, they will still require the basic infrastructure of quality assured facilities, transport, and data management systems to make an impact. In the short term, culture and DST remains the mainstay of disease control and the infrastructure to support these techniques will assist in the roll out of future technologies.
1.2
Expanding Laboratory Capacity
Public health experts agree that controlling the MDR-TB epidemic and providing prompt curative services for those with TB disease is an essential public health function. As a result, significant attention has been drawn to the state of laboratory networks serving those missions. Observers have concluded that dramatic improvements in baseline capacity are necessary to meet anticipated surveillance and treatment targets.81,82,83,84,85 In 2005, the World Health Assembly passed a resolution requesting the Director-General “to implement and strengthen strategies for the effective control of, and management of persons drug-resistant tuberculosis.” The 2006 Global Plan to Stop TB stresses the importance of laboratory services, stating that “every country should have a well-resourced and fully functioning national reference laboratory.”86 The MDR-TB working group identified culture and DST services as indispensable components of the TB control effort.”87 The WHO Global Taskforce on XDR-TB echoed these recommendations in The Global MDR-TB and XDR-TB Response Plan. The plan called attention to three core priorities for TB laboratory infrastructure: accelerating access to laboratory services, improving infection control, and expanding surveillance.88,89 Smear microscopy is still the foundation for TB control, but a broad consensus among public health officials now supports increased use of culture and DST services.90,91
To design an effective strategy to improve laboratory networks, policymakers must first have a clear understanding of the gap. The WHO’s Global Tuberculosis Control report for 2007 (Global Tuberculosis Control 2008: Surveillance, Planning, Financing) succinctly summarizes the current state of laboratory services: “[National Tuberculosis Programs] in all WHO regions reported…too few laboratories, weak quality control, and limited facilities to carry out culture and drug susceptibility testing.” The report concluded, “Facilities to diagnose and treat MDR-TB, including extensively drug-resistant TB (XDR-TB), are not yet widely available; the scale of the XDR-TB problem globally is not yet known.“92 To address this problem, the World Health Organization’s Stop TB Department created the Global Laboratory Initiative (GLI), whose mandate is laboratory capacity development and coordination. According to material on the GLI website, in order to adequately diagnose MDR-TB in the general population, countries will need one culture facility per 5 million population and one DST facility per 10 million population. The GLFs calculations reflect the importance of tracking down difficult-to-diagnose categories of TB, including pediatric, extra-pulmonary, and sputum smear negative TB, as well as treatment failures and patients requiring retreatment for TB. Current global coverage is short of the GLI goals (see Table 1 on the next page).
The formation of the GLI is a major step forward. Recently the organization received funding to work with global partners, such as the Foundation for Innovative and New Diagnostics (FIND), to expand its well-regarded model of accelerated laboratory development in the Kingdom of Lesotho. FIND designed the model in conjunction with the Lesotho Ministry of Health and the international non-profit Partners In Health.”93 Despite achieving phenomenal gains, which will be discussed below, the GLI and its partnerships still face several important challenges, most notably in the development and deployment of true point-of-care testing for pulmonary and extra-pulmonary forms of TB—including drug-resistant TB.
Table 1: Coverage of laboratory services in select high-MDR-TB burden countries (2006)
|
|
|
Population |
National reference laboratory (NRL) |
Access to diagnostic services |
|||||
|
Sputum smear |
Culture |
DST |
|||||||
|
thousands |
number of labs |
per 100000 pop |
number of labs |
per 5 million pop |
number of labs |
per 10 million pop |
|||
|
1 |
India |
1,151,751 |
Y |
11,968 |
1.0 |
8 |
0.03 |
8 |
0.07 |
|
2 |
China |
1,320,864 |
Y |
3,010 |
0.2 |
360 |
1.4 |
90 |
2.7 |
|
3 |
Indonesia |
228,864 |
N |
4,855 |
2.1 |
41 |
0.9 |
11 |
1.8 |
|
4 |
South Africa |
48,282 |
Y |
143 |
0.3 |
13 |
1.3 |
8 |
2.7 |
|
5 |
Nigeria |
144,720 |
N |
694 |
0.5 |
0 |
0.0 |
0 |
0.0 |
|
6 |
Bangladesh |
155,991 |
Y |
687 |
0.4 |
3 |
0.1 |
0 |
0.2 |
|
7 |
Ethiopia |
81,021 |
Y |
713 |
0.9 |
1 |
0.1 |
1 |
0.1 |
|
8 |
Pakistan |
160,943 |
N |
982 |
0.6 |
3 |
0.1 |
1 |
0.2 |
|
9 |
Philippines |
86,264 |
Y |
2,374 |
2.8 |
3 |
0.2 |
3 |
0.3 |
|
10 |
DR Congo |
60,644 |
Y |
1,069 |
1.8 |
1 |
0.1 |
1 |
0.2 |
|
11 |
Russian |
143,221 |
N |
4,953 |
3.5 |
978 |
34 |
302 |
68 |
|
Federation |
|||||||||
|
12 |
Viet Nam |
86,206 |
Y |
874 |
1.0 |
18 |
1.0 |
2 |
2.1 |
|
13 |
Kenya |
36,553 |
Y |
770 |
2.1 |
2 |
0.3 |
2 |
0.5 |
|
14 |
UR Tanzania |
39,459 |
Y |
690 |
1.7 |
3 |
0.4 |
1 |
0.8 |
|
15 |
Uganda |
29,899 |
Y |
726 |
2.4 |
3 |
0.5 |
2 |
1.0 |
|
16 |
Brazil |
189,323 |
Y |
4,044 |
2.1 |
193 |
5.1 |
38 |
10 |
|
17 |
Mozambique |
20,971 |
Y |
250 |
1.2 |
1 |
0.2 |
1 |
0.5 |
|
18 |
Thailand |
63,444 |
Y |
937 |
1.5 |
65 |
5.1 |
18 |
10 |
|
19 |
Myanmar |
48,379 |
Y |
391 |
0.8 |
2 |
0.2 |
1 |
0.4 |
|
20 |
Zimbabwe |
13,228 |
Y |
180 |
1.4 |
1 |
0.4 |
1 |
0.8 |
|
21 |
Cambodia |
14,197 |
Y |
186 |
1.3 |
3 |
1.1 |
1 |
2.1 |
|
22 |
Afghanistan |
26,088 |
N |
500 |
1.9 |
1 |
0.2 |
1 |
0.4 |
|
Source: Global Laboratory Initiative, Stop TB Department, World Health Organization |
|||||||||
2
THE ANATOMY OF A LABORATORY NETWORK
2.1
TB laboratory networks
A laboratory network coordinates the shipment of specimens from peripheral sites to central laboratories, and provides for the reporting of results. Though commonplace in the developed world, such networks are relatively new to developing nations, which in the past have relied on simpler, on-site testing. Performing culture-based diagnosis requires more advanced mycobacteriology laboratories than sputum smear microscopy. Therefore, such facilities are not likely to be universally available at the district level, let alone at health centers, due to the technical demands of building and operating culture laboratories. Expanding access to currently available TB diagnostics will require extending laboratory networks, including the referral and data management systems, in order to get samples to testing nodes.
The global TB laboratory network today is a four-tiered system, as described below. National public health officials determine the priorities of labs within their borders, as well as the volume, quality, and timeliness of services provided. Meanwhile, supra-national laboratories provide quality assurance, technical assistance, and research sites to develop improved techniques.
-
Level IV Labs: There are 26 Level IV laboratories around the world. These labs, which maintain the highest standards and share responsibility for external quality control, together make up the Supra-national Reference Laboratories Network (SRLN).
-
Level III Labs: These national reference laboratories provide services, such as culture and DST, which are appropriate for a referral facility. They are often located in the national capital, or in large provincial centers.
-
Level II Labs: These regional facilities (sometimes called state or provincial labs) can often handle moderately sophisticated testing procedures—such as culture or DST—depending on such factors as factors as geography and the size of the district.
-
Level I labs: Clinic or district labs, located in towns and villages or in rural areas. These labs focus on such basic tasks as sputum smear microscopy.
Responsibility over the different levels varies by region and country. Some nations have dedicated national tuberculosis reference laboratories, while others maintain facilities that are shared among a number of disease-control units. Many Level II laboratories fall under the direct authority of national laboratories, but others are formally controlled by state or regional health departments and receive advice from national laboratories. Oversight of microscopy programs ranges from district laboratories to local health centers primarily operated by the National TB Program (NTP).
At the international level, Level III and Level IV laboratories interact with one another in a variety of ways. SRLNs engage in pioneering TB research, assist in capacity building, and support their national counterparts. The Level III laboratories operated by each individual nation handle the culture and DST needs of the domestic population. Level III and IV laboratories frequently collaborate on more ambitious projects, efforts that are normally funded by outside sources. Such collaborations are by their nature sporadic and are not available to all countries suffering from TB epidemics.
2.2
Third-party laboratories
Many nations have significant potential laboratory capacity located in for-profit facilities, universities, or within non-governmental organizations. It is not known how effectively this capacity has been tapped for diagnosing and treating TB. One report from India estimates that 60 to 88 percent of patients with “cough” were initially evaluated outside of the public sector, and up to 50 percent of TB cases were treated outside the NTP.94 Whether dedicated to research or service provision, these third-party providers and laboratories represent an important source of in-country talent and capacity that could potentially be tapped. Currently, their impact on TB treatment is decidedly mixed. For example, Zambia has at least five organizations with advanced mycobacterial culture systems using liquid-media, and yet national drug resistance data is not universally available and there is no national MDR-TB treatment program or algorithm to access DST results. Private, third-party facilities frequently draw the best-qualified laboratory administrators and technicians from the less well-funded public and non-profit sectors, exacerbating already severe shortages in reliable talent. Even in those instances when privately owned facilities do provide services to TB patients, the quality of those services is difficult to gauge without a global accreditation system and sufficient monitoring by a member of the SNRL network.
In the United States, over 80 percent of mycobacterial sputum smear and culture tests and over 50 percent of DSTs are conducted by the private sector.95 Tapping these types of private resources is not without difficulty. For example, researchers in developed countries have discovered persistent flaws in the coordination of services, ranging from transportation delays to inadequate adherence to testing protocols.96 It is difficult to extrapolate those findings to resource-poor settings, but basic problems in service coordination are likely to be exacerbated. Furthermore, when NTP collaborate with third-party laboratory systems, there is considerable risk of parallelism and wasteful duplication of services. Any program that seeks to accredit and utilize third-party laboratories—as will soon be the case in the Philippines as the move toward universal access to MDR-TB treatment—will need to ensure quality levels, equitable access, and close coordination with the public network established by the country’s National TB Reference Laboratory.
2.3
Drug resistance surveillance (DRS)
Close monitoring of drug resistance is key to the success of any country’s TB-control strategy.97,98,99 DRS data are used to create epidemiological profiles of countries and regions, to guide empiric treatment, and to respond to focal disease outbreaks and resistance trends. The WHO has completed four sequential surveys of global drug resistance.100,101,102,103 Unfortunately, in many highly
burdened nations, capacity does not exist for continuous TB surveillance. As a result, there is widespread concern that the limited DRS coverage underestimates the global burden of drug-resistance.104 Drug resistance surveillance gaps reflect the state of the global laboratory situation: just 11 of 22 high-burden countries have conducted recent DRS surveys, and 11 of 25 high-priority MDR-TB countries had conducted DRS as of 2006.105 The global community still relies primarily on modeling and extrapolation to understand the true extent of the MDR/XDR-TB crisis.106 In the past, these models have been dangerously wrong and have had a detrimental effect on global policy.107
It is considerably less burdensome to conduct periodic surveys of drug resistance than it is to sustain a national program of patient support. Surveillance data is usually based on representative samples, and in a significant number of cases, patients with active disease have not received treatment. In the future, this type of data collection needs to be linked with national TB treatment programs and local clinical teams.
|
Example: Global Polio Laboratory Network (GPLN) The Global Polio Laboratory Network (GPLN) is a centrally coordinated laboratory system created to manage the diagnostic needs of the global eradication campaign. Seven supra-national reference laboratories, 15 regional laboratories, and 123 national laboratories operate the polio surveillance safety net. Under this structure, individual laboratories can serve the needs of multiple countries. Testing is conducted according to a hierarchy of technical sophistication: molecular biology, the most complex testing regimen, is reserved for supra-national laboratories; less complex testing is done at regional and national laboratories. The WHO coordinates an accreditation system for laboratories and works to assure the quality assurance mechanisms, standardized reagents, standardized methods, and testing algorithms. Between 2004 and 2008, the network required $27.5 million in funding for laboratory operations and $12.5 million in staff costs, in addition to the contributions of national laboratories. The total cost was estimated by one source to reach $125 million annually.108 The GPLN laboratory network processes an estimated 80,000 samples annually—just a small fraction of the volume generated by the global TB community. The key features of the network include centralized standards and funding with strong coordination. Polio and measles laboratory facilities operate as regional centers of excellence: they are repositories of skills and they serve as training sites. They also provide additional capacity when other labs in the network become overwhelmed. There are clear service level benchmarks; measles laboratories are required to report results within 7 days of receiving a sample, and test results and data are documented on a central database.109 The outcome is a network which can |
|
manage the collection, processing, and reporting for a disease with relatively low-incidence requiring rapid laboratory responses. With its high incidence, prevalence and its need for sustained local service provision, the nature of global TB calls for a different, more decentralized laboratory structure. Nevertheless, the general systems developed to monitor polio—including the establishment of laboratories as centers of excellence, the sharing of capacity and funding, and the coordination of activities—can certainly inform the development of MDR-TB laboratory services at the local, regional and global levels. |
2.4
Capacity gap
One proxy for laboratory capacity is total laboratory volume reported compared against total estimated need. Culture capacity is believed to be significantly more developed than DST. In 2005, 12 million requests for mycobacterial culture were issued in developing nations, 8.6 million of those from high-burden countries. Russia, South Africa and India accounted for 92 percent of the high-burden requests; Russia alone had 6.6 million requests. An estimated 1.5 million liquid culture requests were performed in developing countries, although the number for high-burden countries is not known. While this figure seems impressive, based on epidemiological modeling the Stop TB Partnership’s Sub-group on Laboratory Capacity Strengthening (SLCS) estimates that 60 million annual cultures will be needed by 2015 to meet targets. While there has been some growth in access to mycobacterial cultures,110 the gap between need and capacity is quite considerable.
With respect to drug sensitivity testing (DST), the situation is even more worrisome. In 2006 developing countries ran approximately 630,000 DSTs as reported by FIND,111 of which an estimated 512,000 occurred in high burden countries. The WHO reported that 100,000 MDR-TB cases received DST support during that same year. Mathematical modeling conducted by FIND and the SLCS projects that 5 million annual DSTs will be required to meet basic treatment goals. This is a shortfall of almost 4.5 million DSTs compared to current capacity.
In 2005 18,000 new, laboratory-confirmed cases of MDR-TB were reported, at a time when epidemiological models predicted approximately 424,000 new cases of MDR-TB per year. Thus, only 4.3 percent of the disease burden was captured by the laboratory system. If we look only at high-burden countries, the numbers improve slightly, but the result is still well below what was predicted: 6.1 percent of predicted cases were captured by official reporting. These statistics are aggregate and difficult to
interpret, particularly in settings like China and India where significant private treatment alternatives exist. However, the size of the gap demonstrates the fundamental challenge facing the laboratory network.
Poor distribution of global laboratory resources may indicate that the shortage of testing capacity in high-burden countries is even more severe than at first glance. The recommended density of culture and DST laboratories globally is 1 culture lab per 5 million population and one DST lab per 10 million population. Actual global ratios are 1 per 1.2 million and 1 per 4.95 million, respectively, figures that may be skewed by the heavy concentration of such resources in developed countries. Sub-set analysis shows significant inequalities in distribution. Among high priority nations, the ratios are 1 culture laboratory per 7.8 million people and 1 DST laboratory per 14.2 million people. This means that laboratory services are hardest to find in precisely those settings that need them the most.
Measuring the strength of laboratory networks is a sufficiently complex task that different agencies have come up with widely divergent estimates of capacity. For example, FIND came up with a culture-laboratory density figure three times higher than the one arrived at by WHO’s 2007 report (Global Tuberculosis Control 2008: Surveillance, Planning, Financing), and twice as high for DST facility density. FIND included private facilities in key countries, a decision that accounts for part of this discrepancy. Regardless of methodology, both reports confirm that current laboratory infrastructure does not meet basic density requirements in high-priority or high-burden countries. Significant work remains to be done to determine if integrating private sector capacity with public programs is realistic in resource-poor settings. What is also abundantly clear is that excess capacity in developed nations is clearly not being utilized sufficiently. According to Dr. Alex Sloutsky of the Massachusetts State Laboratory Institute (MSLI), a supra-national reference laboratory, facilities in developed countries could use their excess capacity to help diagnose MDR-TB for programs where infrastructure is currently inadequate (and is being developed), or in places where disease and population dynamics would likely never warrant the creation of a dedicated laboratory.
2.5
Financing gap
In 2006, researchers drafting the Stop TB Strategy projected an enormous shortfall in the financing of efforts to combat the MDR-TB.112 Between 2006 and 2015, the authors warned, funding would lag behind target amounts by $31 billion. The SLCS estimated the gap in laboratory funding to be at least $2.5 billion between 2007 and 2015. By 2015 the infrastructure and capital expenditures for
laboratory expansion are estimated to require $700 million in funding and 800 new facilities.113
3
Laboratory Capacity Building
|
Country Example: Peru In the early 1990s, Peru’s culture and DST laboratory system had key deficiencies in policy, physical and biosafety infrastructure, and data management. Since Peru lacked a national policy for when to perform drug sensitivity testing (DST), local physicians requested testing on a case-by-case basis. Some physicians waited until a patient’s disease was quite advanced and others never requested DST at all. Technicians had inadequate equipment and training that endangered the integrity of the results, as well as their own safety. Mycobacterial cell culture occurred at the district level labs, and positive samples were sent to the National Reference Laboratory (NRL) for DST. DST results typically took almost six months to reach health centers, delaying treatment significantly. In 1996, an international research team1 began looking into ways to help Peru’s National TB Program improve access to culture and DST. In 2000, through the support of the Bill & Melinda Gates Foundation, the coalition expanded, and the goal became to expand laboratory capacity to support MDR-TB treatment throughout Peru. The Laboratory Improvement Project set the following goals: improving infection control and biosafety, setting national standards for ordering culture and DST, establishing systems for specimen transport and data management, streamlining culture and DST testing through conventional and rapid methods, and guaranteeing quality assurance through external monitoring and assistance. To establish biosafety and sound laboratory infrastructure, the project team had to make structural modifications and import equipment. Initially, the team could not find local experts with crucial technical skills in airflow engineering required for the construction of new facilities, and the lack of inspectors complicated routine maintenance. To solve these problems, project personnel sought funding from external grant and training programs to develop in-country capacity. They then formed teams of engineers and architects to improve individual laboratories. |
|
Project participants collaborated closely with the NTP to agree upon programmatic standards for laboratory utilization. On-site trainings allowed staff to tailor these norms to meet specific program conditions. Through an iterative process and facilitation of a close working relationship between laboratory personnel and the clinical teams, efforts to improve diagnostic standards throughout Peru were improved. Additionally, because efficient data collection and management are essential aspects of a strong laboratory network and good clinical care, the team devoted considerable effort and funding to improving these capacities. For example, in the city of Lima, which has a high density of MDR-TB, the program purchased two trucks that were used exclusively to transport specimens to laboratories. The team also analyzed every aspect of the system, from the collection of patient data to the reporting of lab results, to identify and improve areas where delays could be reduced and to monitor process improvement. Adequate funding was given to clinics and labs to ensure that they did not try to charge patients for lab or transport fees, deterring use. A real-time, web-based system was set up to simplify data management and provide access to staff members at all levels. Team members made a strategic decision to expand the capacity of district laboratories in areas with high MDR-TB rates, rather than expect these localities to rely on the national laboratory in Lima. The national lab was tasked with assisting local and regional facilities in monitoring quality control, upgrading data-management systems to facilitate the sharing of data, and other forms of problem-solving. While approximately 48,000 cultures and 1,000 DSTs were performed in Peru in 1996, a decade after the program’s launch, these figures had increased to approximately 101,000 cultures and 8,300 DSTs annually. Preliminary data from the first district indicates 96.4 percent concordance (used to assess testing accuracy) for rifampicin DST and 99.5 percent concordance for low-level INH resistance, with a median turn-around-time of 28 days to receive these results. The 28-day response time fulfills U.S. standards established in 1993, which suggest that initial DST results be reported within 30 days. Thanks to a collaborative approach, external technical assistance and funding, from 1996 to 2007, the NRL expanded laboratory capacity and quality in culture, first-line DST (by BACTEC 460), first-line conventional testing, first-line rapid DST in two districts, and second-line DST. |
3.1
Fragmented organization and a poorly defined role in TB control
Despite their critical role in TB control, laboratories have a poorly defined role in the overall strategy and mission of TB control in countries. The NTP—responsible for TB treatment, establishing norms and standards, writing large grants to multilateral institutions, and providing myriad other essential functions—is often managed separately from the laboratories on which they rely for essential and timely diagnostics. Central or national laboratories are often distinct from district laboratories, despite operating in a common network. Further, the degree of collaboration between laboratories and treatment providers is highly variable.114,115Because resources and planning frequently flow through the NTP, laboratory integration is critically important, but often lacking.116 TB laboratory directors need to have formal input in the design and management of TB strategy to ensure that the laboratory component is developed along with rest of the treatment strategy. Most importantly, they need partners at the level of clinical implementation to ensure the overall network functions smoothly, and that samples and ultimately, results, are transferred without problems. Greater collaboration is a basic requirement to establish service levels, drive appropriate utilization, and improve laboratory and clinical information systems.117
The complexity of MDR-TB treatment requires an additional level of integration of laboratory services since the management of the disease requires hematologic and biochemical monitoring of patients. This requires integration of laboratory services that goes beyond mycobacteriology, and in many settings, requires substantial strengthening of health systems.
3.2
Laboratory technical assistance
Increasing laboratory capacity rapidly requires the input of experienced (senior) laboratory personnel, the allocation of appropriate human and financial resources by laboratory leadership, and the mentoring and training of local laboratory staff. Discussions with individuals involved in the laboratory capacity-building efforts in Lesotho, Peru, and Uganda noted that the ability to scale up laboratory efforts was directly related to the skill and experience of their supervising director, the mentorship provided by those providing technical assistance, and financial, technical, and logistical assistance from a reliable nongovernmental partner. Technical assistance needs to be provided by personnel (national or international) who have the capacity to establish routines, maintain quality assurance, train technicians, and control the supply chain, while working within the framework of the national laboratory. This individual must also
liaise with the rest of the tuberculosis control infrastructure including the NTP and the primary treatment teams.
Through our discussions with those who have participated in successful laboratory capacity-building endeavors we have identified some definable characteristics of effective technical assistance:
-
The technical assistance is on-site, in-country, and conducted by a person or team with a skill set that includes an understanding of both the scientific functions of the laboratory and laboratory management.
-
The technical assistance is long-term (not just one or two short visits), and can range from one or two months to an entire year, depending on the needs of the laboratory.
-
Laboratory staff members have dedicated time to interact and work with the technical assistance provider.
-
The technical assistance is closely tied to a capacity building plan for sustainable local leadership.
-
The technical assistance provider must have the authority and channels to work closely with partners in the clinical system, and must have a clear mandate with resources to execute their tasks.
How such a system is orchestrated is critical to its success. Possible structures range from a centrally administered program at a multilateral institution to a completely decentralized system based at regional supra-national reference laboratories or at regional MDR-TB technical assistance centers. Other alternatives include the establishment of fellowship programs modeled on the US Center for Disease Control and Preventions Epidemic Intelligence Service in which developing professionals are engaged with an extensive network of peers and mentors.
Laboratory functions are a specialized domain of knowledge and mobilizing the right people with the right competencies to address the problem will likely require a unique solution. As new rounds of funding from multilateral and bilateral initiatives expand access to technical assistance funds for countries, the laboratory community must be able to meet demand with qualified professionals. Locating the appropriate supply will be a key challenge.
3.3
Human resources
There has been significant global attention paid to human resources and health, both within TB control and more broadly. 118,119,120,121Universal themes of inadequate pre-service and in-service training programs, poor skill distribution, poor compensation, low motivation, and insufficient resources are consistent problems. Critical human resources for laboratory scale-up fall into three basic categories: laboratory management, laboratory technicians, and biosafety support staff.
3.3.1
Laboratory Management
Laboratories require management teams capable of logistics and forecasting, planning for staff turnover and sustaining quality. Updating standard operating procedures, ensuring that protocols are adhered to, and adapting program guidelines to changing conditions requires more advanced training and there is an acute shortage of staff with the required competencies. Laboratory directors in Uganda, Peru, Lesotho, Botswana, and Zambia all indicated that training staff in the technical aspects of mycobacteriological control may be relatively easy, but the crucial role of laboratory leadership faces challenging shortages because the training to acquire the necessary management skills is resource and time intensive.
Discussions with the 10-year laboratory capacity building project in Peru noted that the greatest impediment to improving the speed of laboratory improvements was the lack of a dedicated, on-site, external (to the laboratory), experienced, technical assistance provider that could work with laboratory management (e.g. laboratory director) to build laboratory leadership capacity. This theme is not unique to Peru: for example, a laboratory in Zambia recently purchased MGIT technology and found that implementation was slower than desired. Ultimately, a consultant from the United States was required and an experienced MDR-TB laboratory director from Eastern Europe was hired to lead the project. Program administrators from Lesotho identified strong laboratory technical assistance provided by an individual based in-country and working closely with the TB laboratory director and leadership as a key driver to their rapid expansion. Anecdotal experiences from case studies reflect the broader evidence base for improving quality and capacity in health systems: appropriate technical knowledge and supervision with feedback is the most validated technique for expanding quality services. Country experiences also noted that common problems with laboratory capacity building included technical assistance provided during brief visits without hands-on interactions with all staff, supervisors who had insufficient time in their job descriptions to provide daily support, staff absences (due to salary supplementation by per diems, hence the desire to travel for work), and poor accountability structures due to insufficient resources.
3.3.2
Technicians
The requirements for mycobacteriology necessitate rigorous laboratory technique, attention to detail, and quality assurance protocols for laboratory technicians. Significant debate exists about the formal educational requirements for staff in these roles,122 but emerging experience suggests that with strong leadership, secondary-school graduates with appropriate and sufficient training can work as basic laboratory technicians. The individual competencies to provide services are not exceedingly complex and training does not require exorbitant capital expenditures. Critical to success are clear standardized operating procedures and their consistent application under supervision with feedback. Significant debate still exists about what level of accreditation and pre-service training is adequate for laboratory operations and how government hiring regulations should be adapted123
3.3.3
Biosafety personnel
Another critical barrier to expanding laboratory capacity is the physical plant to support culture and DST services in the setting of an airborne infectious disease with high mortality. Rehabilitation or construction of new facilities demands scarce resources beyond mere financing, including advanced engineering and construction skills. The physical requirements for mycobacterial culture include biosafety hoods, the preparation of media and reagent supply, proficiency in sterile laboratory technique, incubators, refrigeration and machine service contracts if automated systems are utilized. Successful facilities have negative air flow systems which also require appropriate maintenance support. The equipment can be purchased and imported easily; the staff required to design the facility, the biosafety protocols, and to maintain standards are more difficult to access.
Laboratory officials in Southern Africa speculated that South Africa is the only regional country with sufficient supply of biosafety personnel capable of designing facilities and developing the necessary protocols. Neighboring nations reported having to import experts for elements ranging from design to construction to maintenance. Estimates vary, but design and approval processes can take up to six times as long as construction, frequently due to insufficient local resources. Further, key stakeholder interviews revealed that shortages in infection control personnel are also prevalent in multilateral institutions. Yet, this gap was unrecognized in the most recent Global Plan to Stop TB and the GLC process still has no formal guidelines for infection control planning.
3.4
The referral network
A study of laboratory function in Peru documented that over 50 percent of the total turnaround time is occupied by factors related to the referral and data management components of laboratory operations,124 highlighting the critical role these areas play in laboratory service provision. Improvements in referral network operations and data management that previously required less attention due to local services are now critical as samples and data routinely flow from institution to institution. For wide-spread access, caregivers need to know when culture or DST is indicated, how to get the sample, where to send it, and when they expect a quality assured result to inform clinical decision making. Each of these steps are relatively simple, but taken together it creates a system with multiple parts that must function together. To be successful, laboratory business plans focusing on maximizing network function need to be financed and encouraged.
Laboratory services start at the point-of-care where a treatment team decides to request services, guided by indications for testing. Patient information is then collected. A sample must be procured containing live bacilli. That sample must then be stored securely and transported to the appropriate culture facility. Indications for testing and transport logistics are critical barriers to expanding services. Solutions to sample transport are readily available but take time and money to implement in a considered fashion. Some countries, like Botswana, have invested in contracts with commercial carriers such as DHL. The United States has provided block grants to states to hire couriers, and a variant of that system was used in Georgia via a central dispatch mechanism. Papua New Guinea has experimented with international courier service to Australia for sample processing. Uganda and Malawi have experimented with utilizing local bus companies,125 while Peru bought trucks and hired drivers. Many countries reported that currently the indications for testing, the collection and storage systems, and transport are often ad hoc events. Samples frequently travel with whatever form of transport is available and testing is often initiated through informal requests and peer networks. What is important is that for any system to be successful for TB patients, the majority of whom tend to be poor, the system of sample-transportation has to fall squarely under the aegis of the NTP and laboratory system, so that the burden (and cost) does not fall upon the patient. Secondly, once samples actually reach the laboratory, systems of internal transportation (within the laboratory itself and between laboratories) has to be highly organized so as not to result in samples piling up unanalyzed, or samples waiting for transfer to another laboratory within the system.
Making referral networks operational will require highly individualized solutions depending on local conditions; it is unlikely that any single solution exists. The key component to overcoming the barrier is
dedicated resources to support expanded services and a requirement to invest and develop timely, sustained referral networks with clear operating procedures supported by the lab and the clinical providers.
3.5
Data management
Accurate information about the sample type, patient demographics, and TB history must be accurately transmitted to the laboratory for drug resistance surveillance, while routine management requires more basic patient identifiers. The laboratory must perform the tests and document results. Results then must go to national registry and to the treatment team. Historically, laboratory systems in developing countries relied on on-site testing and paper documentation. Because the monitoring regimen for MDR-TB requires multiple follow-up tests, the volume of information for each patient is large. As laboratories expand to networks and samples and data move geographically the complexity expands.
Many commercial culture systems and DST allow for easy digital documentation, but getting electronic results to the local clinical information systems and central data repositories at the national reference lab is significantly challenged by highly variable resource levels. Direct web-access has facilitated clinical information access in Peru,126,127 but other sites have limited access to the web-based resources. Automated or manual faxes have been utilized but maintenance of peripheral machines (paper and ink shortages) complicates sustainability, though the basic infrastructure of telephone lines may be available. Final solutions maybe as simple as dedicated manual systems based on paper hard-copies and clear protocols. While there is no single recommendation for all nations, solutions to these problems are readily available. What is needed are country-level resources, dedicated planning to account for crucial systems components, and teams to design and implement them.
3.6
Quality assurance
Multiple strategies exist for quality assurance, but the ability to produce consistently accurate results regularly supported by quality control is fundamental to laboratory operations. Quality initiatives generate confidence among the treatment community to expand reliance on services. Quality also establishes a platform or foundation for the expansion of services and integration of new, more advanced testing. Those involved in laboratory scale up in Uganda, Botswana, Peru, and Lesotho all noted that sustaining initial laboratory quality was a significant challenge. The reasons for this are that many laboratories in resource-limited settings have limited human and financial resources to devote to quality assurance, and many laboratory directors have neither the time nor training to guide the process appropriately. Furthermore, in order to maintain quality standard of operations laboratories must have
managers who can anticipate stock needs and supply reagents and supplies regularly, service for machinery to keep the system working, reliable access to engineers who can address air-flow needs, coordination with treatment sites to predict volume and deliver at mutually determined service levels. Finding and supporting these systems over time is an issue of training, financing and political will.
Our case study of Peru showed that while making quality assurance a routine component of good laboratory practice was critical, the program faced two important challenges: (1) having sufficient preparatory training prior to laboratory expansion; and (2) sustaining quality over time. Quality assurance activities for culture systems create the need for global operations; samples or staff must flow from national reference laboratories to supra-national reference laboratories for proficiency checking, while foreign talent is often necessary to provide initial guidance on facility maintenance and to build sufficient local capacity. For instance, laboratories in Botswana had little trouble accessing talent from South Africa to build their reference laboratories but had more trouble maintaining the facility—both getting the right people and ensuring the political support to fund the activities.
As a result of these difficulties, it is no surprise that the availability of external quality assurance among high burden/high priority countries is still quite variable. Most countries had laboratory supervision plans in place (a key measure in quality control of smear-microscopy), but only 50 percent of these plans were implemented. The Global Tuberculosis Control Report 2007 concludes that: “Most countries had neither national policies to expand culture and DST services nor the technical capacity to implement and support such services.”128 The culprits identified include problems with infrastructure, transport, human resources, and funding. Despite the significant improvements many countries still do not have the foundation of successful quality assurance programs.
|
The case of rapid laboratory capacity building in Lesotho A laboratory improvement initiative in Lesotho was initiated following the outcome of a WHO Laboratory Assessment mission in November 2006. Key partners from the Ministry of Health, Partners In Health (PIH), the Foundation for Innovative New Diagnostics (FIND) and WHO, determined that laboratory capacity improvement was a necessary component of Lesotho’s MDR-TB control strategy Coalition partners felt that technical consultants operating on periodic assessments would be insufficient to drive the process at the required rate. PIH was delegated responsibility for logistics and coordinating the referral network, WHO contributed the needs assessment, FIND seconded experienced laboratory consultants to provide on-site long-term technical assistance, and the Government of Lesotho committed sustained resources. |
|
A technical consultant with advanced laboratory training was on-site by May 2007. The early stages were characterized by improvisation; at one point, offices were based out of trailers to ensure adequate space for laboratory renovations. Because of the rural/urban mix, Lesotho focused on building centralized capacity at the National TB Reference Laboratory. Engineering controls, such as a continuous negative air pressure system with a HEPA filtered air source to supply more than 10 air exchanges per hour, and an on-site sterilization system were established. The process was complete by July 2007 – less than 1 year after the initial assessment – and supported by funding from PIH, the Open Society Institute, FIND, and GFATM. Prior to the initiation of this collaboration, the National TB Reference Laboratory was able to process 150 cultures and 30 DST to 1st-line anti-TB agents per year. Clinicians referred samples for testing through informal channels with no systematic indications for testing. When the initiative began, set indications for referral were established as well as systems to get samples to the laboratory in timely fashion. SOP and quality assurance mechanisms were firmly embedded as staff training continued. The South African Medical Research Council (SAMRC) became an integral partner in establishing proficiency testing and technical assistance. Initial procedures focused on the use of solid culture via solid medium. Capacity was improved to 160 specimens per week and 20 DSTs per week. By August 2007 the first cultures were processed and proficiency testing began. This was later expanded to a more rapid, automated liquid-medium (MGIT) culture system. In collaboration with the MSLI, a rapid survey was done to establish baseline epidemic knowledge in the Kingdom of Lesotho. A nationwide DRS is now in process. Long-term challenges will be transitioning leadership from technical advisors to local leadership, supporting the MDR-TB treatment program, and rolling out rapid screening of isoniazid and rifampin resistance with molecular diagnostic techniques. Critical challenges for Lesotho include sustaining initial quality. The rapid improvements were credited to on-site leadership by a strong technical assistance provider with experience in developing countries, complete government support, and access to resources for appropriate staffing. Training local talent in laboratory procedures was easy to accomplish but required strong commitment from technical partners, the laboratory system and the Government of Lesotho. |
3.7
Lessons learned from the experiences of Peru and Lesotho
Despite different population sizes, resource levels, and disease dynamics there were six important themes to be taken from the work in Lesotho and Peru, themes that have resonated strongly with laboratory leaders in multiple settings.
-
Political Will: Collaborative leadership across multiple groups. Both Peru and Lesotho had strong relationships with local NTPs and SNRLs to guide optimal program decision making and link laboratory and clinical systems. Both programs also noted that initially, those relationships were not robust and significant work was done to strengthen and develop communication.
-
Technical leadership: Lesotho succeeded in less than one year because of on-site technical assistance. The number one barrier to improved speed in the Peruvian initiative was lack of a full time technical expert on-site in Lima to oversee the project.
-
Physical Plant: Both projects required expatriate teams for engineering, architectural, construction, and maintenance needs. Finding and utilizing these teams were significant barriers, ultimately taking more time than construction and training.
-
Quality Assurance: Lesotho credited strong quality assurance protocols at the initial training as a key success factor in rapid capacity development. Peruvian leaders noted that a more rigorous focus on quality assurance would have delayed initial capacity but would have ultimately led to faster, more robust service. Both sites emphasized that sustained political commitment to quality was essential to success, and a significant future challenge.
-
Referral and Data Management: Lesotho has yet to scale up primary treatment nationally, however solving transport and programmatic concerns surrounding indications for testing, results reporting, data management, and service levels were critical to success in Peru.
-
Local conditions create vastly different solution – flexibility is paramount: In Lesotho centralized laboratory services was the preferred approach because of the geographic considerations and estimated volumes. In Peru, a centralized solution would quickly have been insufficient and decentralized strategies were required. Lesotho has insufficient resources and a stronger rural service network while Peru has concentrated levels of MDR-TB in major cities. For Peru, purchasing laboratory-based trucks for
-
transport made sense while in Lesotho new solutions will be needed. Both countries had shortages in technical capabilities – in Peru training local experts in new competencies was the solution for long-term sustainability, while Lesotho can likely use its proximity to South Africa and relationship with the South African Medical Research Council (MRC) to provide for maintenance and structural needs.
It is clear that the provision of sufficient on-going technical assistance has a bearing on the ability of the laboratory to build capacity rapidly. This is not the only requirement—others, as discussed above, include sufficient staffing, funding, and infrastructure—but it is one that has repeatedly emerged from our discussions with sites that have undertaken capacity building. In order to facilitate long-term sustainability, any technical assistance has to involve laboratory management, training-of-trainers, and partnership with national TB programs.
4
NEW TB TECHNOLOGIES AND THE NEED FOR POINT-OF-CARE TESTING
In analyzing risk factors for delay in the diagnosis of pulmonary tuberculosis, a study from Thailand in 2006 divided delays into patient factors and physician factors.129 Interestingly, they found that having health insurance was not associated with shorter patient delay (in fact, it was associated with an increase in delays). Rather, some TB suspects reported not seeking treatment because they had to pay for different tests, including x-rays, and could not afford them. Others, who did not have to pay for tests, reported inconvenience of transportation, lengthy queues, and lack of confidence in the quality of the public health care system as their reason for not coming in quickly. Even when a qualified provider was consulted, TB suspects had to make an average of 3.3 visits before they were given a final diagnosis. Only 8.4 percent of patients were diagnosed at the first visit; only 36.6 percent were treated within one week after seeing a qualified provider. Similar findings have been seen elsewhere.130,131,132,133
It is startling to see these delays in patients who have no obvious risk factors for paucibacillary disease or extra-pulmonary tuberculosis. In a recent study from Rwanda (where the rate of TB-HIV co-infection was 62 percent), smear-negative pulmonary tuberculosis, extra-pulmonary tuberculosis, and the use of an antibiotic trial (in the absence of a TB diagnosis; recommended by WHO) was associated with significant delays in the initiation of therapy.134 When analyzing the distribution of time delays before initiation of TB treatment, the study found that patient delays constituted 44 percent of the delay; 56 percent was due to health service delays and treatment delays. In the end, only 18 percent of patients were started on TB therapy within one month; only 56 percent were started on therapy within two months. Although the
authors of the study cited rural residence as a risk factor to later initiation of therapy, they attribute the bulk of the health care system to difficulty of diagnosis.
Both of these studies underscore the difficulty patients face getting to health facilities, and once they are there, having their TB disease properly and rapidly diagnosed and treatment initiated. For patients with drug-resistant tuberculosis disease, the problem is exacerbated by the fact that simply diagnosing tuberculosis is not enough; the drug-resistant phenotype has to also be identified. It is toward addressing this problem that the GLI and FIND have been working to scale-up the ability for countries to rapidly test for drug resistance using rapid molecular tests (e.g. one produced by Hain Lifesciences). Such tests identify Mycobacteria tuberculosis (versus other mycobacteria) as well as probe for resistance to isoniazid and rifampin resistance and provide results within 48 hours. This allows for more rapid initiation of appropriate therapy, of paramount importance in high HIV settings where TB mortality is rapid and high.
While these steps are significant, they do not address the problems described by the Thai and Rwandan studies discussed above, where part of the problem was that patients had to repeatedly return to the physician in order to get a diagnosis. If their TB was not pulmonary or not captured from their sputum, they had an even longer wait before therapy would begin. In order to address this problem, Treatment Action Group (TAG) and the AIDS and Rights Alliance for Southern Africa (ARASA) organized a meeting in Cambridge, United Kingdom, to develop an agenda for expediting research and development of point-of-care assays for diagnosing active TB in resource poor settings through an analysis of the gaps in current efforts, challenges to test development and unanswered scientific questions. The meeting brought together research and technical partners, many of whom had been involved in the development of a dip-stick test for HIV. The meeting concluded that such a test is possible for TB (using sputum, urine, and/or blood), and is actually within reach, but will require significant resources and political commitment.
5
RECOMMENDATIONS
5.1 Sustainable funding from bilateral and multilateral donors must be increased to support construction of in-country drug-sensitivity testing/rapid-testing laboratories and ongoing external quality assessments by supranational reference laboratories. Construction of laboratories capable of performing reliable mycobacterial culture and drug-sensitivity testing to important first- and second-line anti-TB drugs is the cornerstone of the current diagnostic strategy for drug-resistant TB. These laboratories will require external quality control by SNRLs. Laboratories also need protected staff, salaries, budgets, and time to execute quality assurance responsibilities.
5.2. Creation of a system of long-term on-site technical assistance would help countries build and/or rapidly expand their capacity to perform mycobacterial culture, DST, and rapid molecular genetic tests for drug-resistant tuberculosis. Building TB laboratory capacity requires sustained technical assistance by experienced individuals with experience in laboratory management and high technical proficiency. Case studies of laboratory scale up and literature reviews support the hypothesis that on-site, long-term technical assistance with strong feedback is one of the strongest mechanisms to improve system performance. Systems are needed to develop, fund, and allocate scarce technical assistance talent to accelerate laboratory scale up.
5.3. In-country laboratory networks for: specimen transport, data management, and certification and coordination of private laboratories need improvement. Ad hoc indications for testing, transport of specimens to central laboratories, and poor data management have been longstanding barriers to successful treatment programs. Country level resources and action plans targeting referral networks and data management, the processes of getting samples in and data out, are essential to expanding laboratory capacity. Because many countries have private laboratories with mycobacterial culture and DST capabilities, attention needs to be given to helping these countries certify and coordinate the work of these laboratories so that they can make better use of this capacity.
5.4 Use of excess laboratory capacity for mycobacterium culture and drug-susceptibility testing in wealthy nations should be encouraged while laboratories are being built in poorer regions. Data from the GLI show that rich-nation mycobacterial laboratories possess unused capacity to perform mycobacterial culture and DST. While capacity is being built up in countries lacking laboratories, a consortium of laboratories with excess capacity should be developed and utilized so that patients can begin drug-resistant TB treatment regardless of their country’s current laboratory capabilities.
5.5. Priority must be given to research on—and funding for—the immediate development and rapid deployment of point-of-care testing for drug-susceptible and drug-resistant tuberculosis. Current approaches to laboratory capacity-building are aimed at ensuring that existing diagnostics are available to countries. Efforts need to be expanded on the development of rapid point-of-care testing for TB as a means of ensuring timely and accurate diagnosis of TB and drug-resistant TB. This is critical for high-HIV settings, for pediatric tuberculosis, and for patients with extra-pulmonary drug-resistant TB.
SECTION III:
MDR-TB DRUG SUPPLY
-
INTRODUCTION
-
THE GLC INITIATIVE: ACTORS AND RESPONSIBILITIES
-
THE GLC INITIATIVE: INSTITUTIONAL BARRIERS
-
DRUG SUPPLY AND ENGAGEMENT OF DRUG MANUFACTURERS IN MDR-TB RESPONSE
-
REDEFINING THE PARADIGM OF THE GLC MECHANISM
-
RECOMMENDATIONS
1
INTRODUCTION
The World Health Organization (WHO) estimated in 2008 that approximately 490,000 new cases of MDR-TB emerged in 2006.135 However, less then 10 percent of these patients will receive any care (with drugs of unknown quality and under varying programmatic conditions) and approximately 2 percent will receive care using quality-assured second-line anti-TB drugs in programs complying with WHO’s Guidelines for the programmatic management of drug-resistant tuberculosis (see Figure 3).
Figure 3: MDR-TB patients scheduled to receive treatment in WHO/GLC-approved projects and non-GLC projects (1000s of patients; 2004 to 2008; Source: WHO 2008)
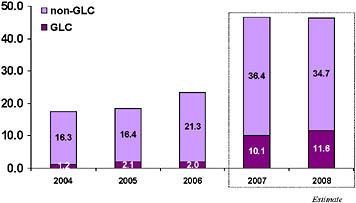
MDR-TB treatment projects currently have two options for procurement of second-line drugs:
-
Procuring quality-assured drugs from the Global Drug Facility (GDF) under the auspices of WHO’s Green Light Committee (GLC initiative), often at concessionary prices; this is the required option for projects with financing from the Global Fund to fight AIDS, Tuberculosis and Malaria (GFATM) or UNITAID.
-
Procuring drugs of unknown quality through state procurement mechanisms and/or the open market.
The Stop TB Partnership’s MDR-TB Working Group met in Tbilisi, Georgia in September 2007 to assess the state of MDR-TB management internationally, measured against the standards of the World Health Organization Global Plan to Stop TB (2006-2015) and the Global MDR/XDR-TB Response plan (2007-2008).136,137 Worldwide shortages of quality-assured second-line drugs (most notably shortages of PAS in 2006 and Capreomycin in 2007) and delays in delivery of quality-assured drugs by the GDF to GLC-approved projects were topics of urgent concern.
In light of the increased numbers of programs applying to the GLC and the projected increase in patients to be enrolled for MDR-TB treatment in GLC-approved projects, it was clear to participants in Tbilisi that failure to resolve these shortages and delays would result in many treatment projects’ circumventing the GLC/GDF mechanisms and would thereby undermine efforts to ensure the increasing use of quality-assured drugs. There was even evidence to suggest that the shortages and delays would encourage some large high-MDR-burden countries to seek exemption from the GFATM to the requirement for GLC-approval of the MDR-TB component of GFATM-supported projects.
In response, the MDR-TB Working Group of the Stop TB Partnership formed a Drug Management Subgroup (DMSG) to address the XDR-TB emergency and to foster effective communication with all relevant institutions and organizations.
2
The GLC Initiative: Actors and Responsibilities
2.1
The Green Light Committee (GLC)
The mandate of the GLC is: (1) to mobilize an effective response to inaccessibly high prices and questionable quality of second-line drugs on the international market; (2) to prevent development of resistance through monitoring and evaluation of GLC-approved MDR-TB pilot projects; and (3) to act as
an advisory body to WHO on MDR-TB policy. Since the GLC’s launch in 2000, the GLC has approved over 40,000 patients for MDR-TB/XDR-TB treatment in 114 projects. Evidence has demonstrated that the integration of MDR-TB treatment into national TB control strategies is both clinically appropriate and cost-effective; as a result, the committee’s responsibilities have expanded beyond small, “pilot” projects, and include programs of increasing size and complexity.138
The system of affiliated institutions that has collectively accepted responsibility for the practical implementation of this growing mandate is now known as the GLC initiative, with the GLC itself assuming specific duties within that group. Accordingly, the GLC is responsible for MDR-TB project approval, which allows the release of GFATM or UNITAID monies to a project on condition of continuing compliance with programmatic standards and provides access to concessionary-priced quality-assured second-line anti-TB drugs. The GLC mechanism provides technical assistance to projects through WHO’s Stop TB Department (TB/HIV and Drug Resistance, THD) to facilitate effective program management (see Figure 4). This includes pre-application planning and, if necessary, pre-application site visits.
Figure 4: Areas of Practical Responsibility for the GLC Initiative
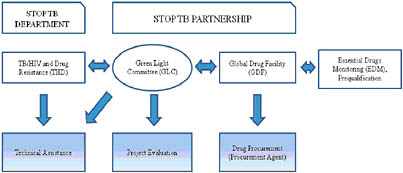
Institutions currently represented on the GLC are the United States Centers for Disease Control (Atlanta, USA), Hospital Muniz (Buenos Aires, Argentina), International Union Against Tuberculosis and Lung Disease (Paris, France), KNCV Tuberculosis Foundation (Den Haag, Netherlands), Medecins Sans Frontieres (Paris, France), Partners In Health/Harvard Medical School (Boston, USA), National TB
Program (Riga, Latvia), the World Health Organization (Geneva, Switzerland), and the World Care Council.
Programs apply to the GLC using a standard application form available on the GLC website. Often the number of patients approved by the GLC for treatment can be modified from the project’s original request; GLC members’ institutional experience informs this modification based on perceived project capacity. Regular missions carried out by a trained cadre of GLC consultants evaluate project performance and attempt to catalyze program scale-up (to achieve universal access) as appropriate.
It is important to note that the GLC itself is not responsible for drug procurement. The GLC is responsible for ensuring that GLC-approved projects use only quality-assured drugs and deliver these drugs under optimal program conditions as described in WHO’s Guidelines for the programmatic management of drug-resistant tuberculosis. The procurement of these drugs is the responsibility of the GDF and its contracted procurement agent—currently the International Dispensary Association (IDA).
2.2
The Global Drug Facility (GDF)
Founded in 2001 under the Stop TB Partnership and hosted by the WHO, the Global Drug Facility (GDF) originally was mandated to oversee procurement of first-line TB medications. In May 2007, the GDF announced that it had provided anti-TB drug treatments for 10 million people to 78 countries since its inception.139
In 2006, the Stop TB Partnership Coordinating Board, the Stop TB Department of WHO, and the Working Group on DOTS-Plus for MDR-TB assigned to the GDF responsibilities for procurement of second-line anti-TB drugs for GLC projects. The GDF gradually assumed these responsibilities, taking them up fully in 2007. The GDF has contracted with its procurement agent, IDA, to supply drugs to all GLC projects. Some projects place orders through the GDF, which then forwards the orders to IDA. Other projects place their orders directly with IDA. The GDF tracks orders, monitors the performance of the procurement agent, compiles forecasts of future drug needs, and negotiates with suppliers interested in being added to the GDF’s approved suppliers list.
In November 2007, the GDF began purchasing a buffer stock of second-line drugs for up to 800 patients. When this buffer stock is in place, it will ensure that a ready supply of MDR-TB drugs is available for projects needing immediate assistance. They have recently received additional funding from UNITAID to expand the buffer stock to include enough drugs for 5000 patients. When the buffer stock is eventually built—and this has been difficult because of global shortages of quality-assured second-line drugs—drugs
from this stockpile will be used to avoid stock-outs with existing projects and to expedite the launch of new sites.140
2.3
Procurement agent
The procurement agent’s contract is currently negotiated with the GDF for a period of 24 months, with the option of a further extension of 12 months.141 At the time of the GLC’s inception in 2000, the procurement agent was the Belgium-based logistic and supply division of the international nongovernmental organization Médecins Sans Frontières (MSF), Transfer (now called MSF Supply). Since 2001, the role of procurement agent has been filled by the International Dispensary Association (IDA) of the Netherlands. Upon transfer of the procurement responsibilities from the GLC Secretariat to the GDF, IDA won a competitive tender for contract as procurement agent in 2007; its current contract is in place through 2009.
The procurement agent is responsible for overseeing second-line drug purchases, identifying potential suppliers for each medication, and soliciting agreements with the manufacturers for reduced prices for GLC-approved projects. Such agreements may include the establishment of a maximum quantity or volume of reduced-price drugs. The agent communicates directly with suppliers to inform them of the expected need for a particular drug, and then arranges delivery to its facility (currently in Amsterdam). Following this, the agent allocates those drugs to GLC treatment sites per the orders received from the respective projects. Before placing an order with IDA, all GLC-approved projects must submit the total expected drug needs for a full 2-year course of treatment for their patient cohort. These quantities are approved by the GLC and shared with the GDF, which procures the second-line drugs for approved projects by working in partnership with the procurement agent. The GDF sends an authorization letter to IDA indicating the drug needs for the project in question. Once IDA has received the letter of authorization from the GDF they are able to sell the project the drugs required, up to the approved quantity.
The agent receives the quotation request from a project site and responds with pricing information and expected delivery dates. Once an order is confirmed by a project site and full payment for the drugs made to IDA, the agent communicates with the manufacturers and production begins. The project site is kept informed of delivery status and any expected delays. The procurement agent contacts the project site when delivery is arranged, and at that time provides the project with the shipping date and relevant paperwork.
2.4
The WHO Essential Drugs Monitoring (EDM) Prequalification Program
The GLC requires that all manufacturers of second-line drugs wishing to participate in the GLC initiative be approved by the WHO’s Essential Drugs Monitoring Prequalification Program. The program was launched in 2001 to facilitate approval of high-quality medicines for HIV/AIDS, malaria, and tuberculosis and has already approved a number of fixed-dose combination antiretroviral medicines. It is operated in close cooperation with UNAIDS and UNICEF, and draws its financial support from the World Bank, GFATM, UNITAID, the Gates Foundation, and contributions from several national governments. The program’s focus initially targeted medicines to treat HIV/AIDS. A system for assessing and increasing access to pharmaceutical products for the treatment of tuberculosis was adopted by the program in 2002.
The prequalification program’s two priorities are: (1) to evaluate the compliance of pharmaceutical products with WHO standards for generic products; and (2) to certify that these products are manufactured according to good manufacturing practices (GMP). Although the WHO Prequalification Program has approved a large number of drugs for HIV/AIDS, it has approved many fewer anti-TB medications and only two second-line anti-TB drugs (cycloserine and ethionamide, both from Macleods Pharmaceuticals Ltd of India). The GDF also accepts as quality-assured those second-line anti-TB drugs that have documented approval from a “stringent national drug regulatory authority” (SNRA), such as the US Food and Drug Administration (FDA) or European Medicines Agency (EMEA). However, even with this waiver of WHO Prequalification, the GDF offers access to only one quality-assured supplier for most second-line drugs. The reliance on single sources of supply offers little leverage for reducing prices of the drugs. It also subjects orders to logistical delays and supply disruptions that delay patient treatment and enrollment in existing projects and could significantly impede the launch of newly-approved projects in the next 12 to 18 months, if there are no newly-approved quality-assured suppliers.
2.5
UNITAID
UNITAID is a new source of funding for second-line anti- TB drugs. Officially launched in September 2006, UNITAID is a consortium of five countries (France, Brazil, Chile, Norway and the United Kingdom) that has created an international drug purchase facility. The goal of UNITAID is “to provide long-term, sustainable and predictable funding to increase access and reduce prices of quality drugs and diagnostics for the treatment of HIV/AIDS, malaria and tuberculosis in developing countries.”142 Funding for UNITAID is raised by levying a tax on airline tickets in its participating countries.
UNITAID is funding the purchase of second-line anti-TB drugs for seventeen low-income countries over a 5-year period.143 Recipient countries will transmit all orders through the GDF and UNITAID will
prepay the procurement agent (IDA) for drug orders. UNITAID and the GFATM have recently reached an agreement that all GFATM-grantees will have their second-line anti-TB drug orders paid for by UNITAID through this mechanism.144
|
The procurement experience of the Philippines145 The Tropical Disease Foundation (TDF) started treating MDR-TB at the Makati Medical Center in Manila in 2003. They found early on that they were unable to budget properly for medications because the prices offered through the GLC mechanism varied by year for each individual drug, especially ofloxacin, cycloserine, and capreomycin (see Figure 5 and Figure 6). Figure 5: Price variation for ofloxacin, prothionamide, kanamycin, and cycloserine (2003-2007) 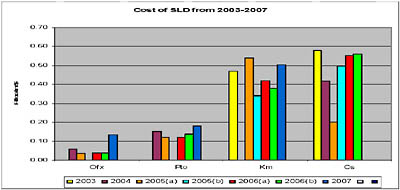 Figure 6: Price variation for capreomycin and PAS (2003-2007) 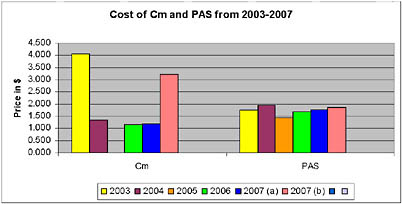 |
|
They also found that the average shelf-lives of drugs from time of receipt in-country varied. For example, as shown in the graph below, capreomycin, cycloserine, pyrazinamide and para-aminosalicylic acid (PAS) were received by the project with expiry dates within less than 2 years (See Figure 7). Some of this was due to the shelf-lives of the drugs themselves, others were due to the delivery of short-life stock. In any case, the natural time constraints inherent in these drugs are exacerbated by late delivery times, to the detriment of the recipient programs and their patients. Figure 7: Average shelf-life of drugs from date of delivery, TDF, Manila, Philippines 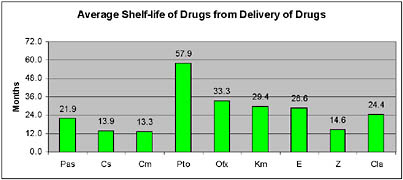 For various technical reasons, TDF initially used an ordering system that involved the local WHO office. In 2007, they began ordering second-line medications directly from the GDF’s procurement agent, IDA. They calculated the average time between placing an order directly with IDA and receiving the drugs in the country (see Figure 8). Figure 8: Average time to delivery for second-line medications ordered directly through IDA (2007; Philippines) 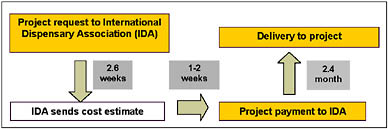 They found that the mean time required to receive second-line medications was 3.6 months. Despite this mean, they have had situations in which they have not received certain essential second-line drugs (e.g. kanamycin) for more than 6 months. |
3
THE GLC INITIATIVE: INSTITUTIONAL BARRIERS
The GLC initiative has approved life-saving therapy for more than 40,000 patients since its establishment in 2000. More than 10,000 patients per year are now being approved for treatment and that number is expected to grow. It is clear that the GLC “pilot program” phase—a phase characterized by the small projects that helped establish the GLC protocols and helped lay the foundation for the creation of a functional mechanism to assure that patients were receiving quality-assured drugs under sound programmatic conditions—is now over. Given the large burden of MDR-TB in many settings, MDR-TB treatment has to become standard of care in all TB programs. If treatment projects around the world are to be initiated and expanded using quality-assured drugs under WHO program protocols, countries will need access to an expanded supply of second-line drugs. If there are not adequate supplies and smooth, effective mechanisms to procure them, there will be little incentive for projects to seek GLC endorsement. At the moment, there is only one quality-assured supplier available for most second-line anti-TB drugs purchased through the GLC mechanism. Projects are experiencing delays due to inadequate drug supplies and logistical problems, resulting in significant complaints about the GLC initiative and significant pressure from large MDR-TB-burdened countries to circumvent the GLC. If the GLC is to play a meaningful role in the next decade of MDR-TB expansion, it will certainly have to improve the procurement mechanism and the availability of quality-assured second-line drugs.
3.1
Single procurement agent, the GDF, and transparency
At the moment, the GDF has a single procurement agent—currently IDA—for all the second-line anti-TB drugs it offers. It holds a monopoly on all GLC procurement, which interferes with legal requirements in some countries that all purchasing be open to transparent tender. As there are increasingly more GLC projects and patients, the GLC and the GDF are likely to find it increasingly difficult to maintain their insistence on a single procurement agent for projects around the world.
Equity and transparency in the allocation of concessionary-price drugs are additional challenges for the GDF/IDA as they respond to increasing numbers of projects and orders for drugs. The allocation of scarce, reliable supply presents obvious budgetary and scheduling challenges as projects often cannot predict which product they will be allocated, at what price, and when they will ultimately receive delivery. For example, Eli Lilly offers the GDF a fixed annual quantity of capreomycin at a reduced price of roughly $1 per vial and a larger quantity of the product at around $3 per vial. Once these quantities are consumed, subsequent orders are filled by the GDF/IDA at higher prices Projects paying higher costs struggle to understand the basis on which the lower-cost supplies of capreomycin are distributed. For
example, some projects question whether priority is given to large projects over small projects or to existing patients over new (or expansion) cohorts. A similar situation exists with cycloserine: a fixed quantity from Eli Lilly is available at a discounted cost of $0.14 per capsule; once consumed, the remaining supply is sourced from MacLeods Pharmaceuticals at a cost of $0.50 per capsule. This dramatic price variance, coupled with the inability to predict which supplier’s product they will receive, can cause a project site to face a severe budget shortfall. Information gathered from multiple projects indicates that it is not unusual to submit a request to IDA assuming that the Lilly cycloserine will be available, only to receive a quotation that includes MacLeod’s cycloserine and is significantly costlier than anticipated; in the case of larger projects the difference can range in the hundreds of thousands of dollars for sizable shipments. Projects routinely submit large annual orders to IDA along with a proposed delivery schedule; however, product availability, quality-assurance procedures, national registration and customs issues (discussed further below), packing, and document preparation often result in delivery delays. Projects routinely operate with minimal supplies and are often forced to alter or suspend patient enrollment to correspond to the available supply of drugs.146
3.2
Prequalification of second-line anti-TB drugs has been slow at WHO
There are only two second-line anti-TB drugs that are prequalified by the WHO Prequalification Program and only 17 products for TB in total. In contrast, there are 62 antiretroviral agents and 33 medicines prequalified for HIV/AIDS-related diseases.147 This discrepancy likely results from the attractiveness of the high-volume HIV/AIDS-drug market to suppliers. In addition, the international commitment to funding for HIV treatment in developing countries has been more robust than that for TB, at least until recently. More recently, the Prequalification Program has reported having made an effort to prequalify second-line anti-TB drugs, but the response from both EDM and potential suppliers has been disappointing, for reasons that are somewhat unclear. Some suppliers complain that the prequalification program is slow and bureaucratic, and that it does not effectively engage with manufacturers on the level required to encourage improvement of production standards to international levels. There may be evidence to support this complaint. All GDF-approved second-line drugs have been approved by other Stringent National Regulatory Authorities, such as the FDA, yet only two of these products are WHO-prequalified. With financial support from The Bill & Melinda Gates Foundation, the Prequalification Program has recently added professional staff, but it will be a challenge for this approval process to keep up with the rapidly increasing demand for second-line drugs in projects approved by the GLC.
Second-line drugs are widely available in MDR-TB-priority countries and only a small proportion of these drugs are quality-assured. A Russian case study provided by the authors of Pathways to Patients, a
2007 publication from the TB Alliance, estimates the value of the second-line drug market in Russia to be $56 million. Only $6 million of this was to be financed through Global Fund grants and purchased through the GDF. The remainder was to be financed by the federal budget in Russia and likely sourced from domestic Russian pharmaceutical firms, none of which are prequalified by the WHO. Pathways to Patients places a rough estimate of the size of the second-line drug market in China at $25 million. Although the authors stress the difficulty of accurately estimating second-line drug sales in Russia and China, the combined total for these two countries of more than $75 million represents roughly 8 to 10 times the value of quality-assured drugs sold to GLC projects in 2007. It will take a concerted effort by international partners and national regulatory authorities in large, high-MDR-TB-burden countries to facilitate and increasingly insist upon quality-assured products. These products typically will be more expensive2 and their introduction will threaten vested economic interests of local non-quality-assured producers, but the patient outcomes will surely be improved.
Figure 9: WHO’s twenty-five priority MDR-TB and XDR-TB countries148
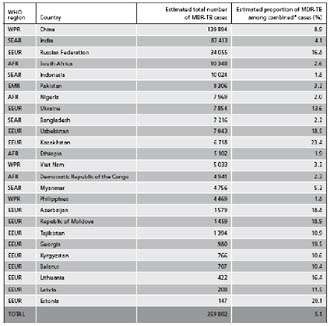
|
National regulatory and legal barriers to procurement: the Case of Russia As of 2003, the WHO determined that 62 percent of MDR-TB patients resided in three countries: Russia, India, and China (see Figure 10).149 This reality necessitates that the international TB community institutionalize effective cooperation and compliance practices with national customs services of recipient countries. The case of Russia is emblematic of the problems faced in importing quality-assured second-line anti-TB drugs into individual national markets and warrants an in-depth analysis. Figure 10: MDR-TB infections by Country, WHO Estimate (with confidence interval) 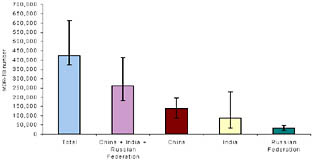 Source: WHO 2003 Significant improvements in the economy of the Russian Federation in recent years have resulted in its reassignment as an upper-middle-income country by the World Bank. This designation preceded the announcement by World Bank president Robert Zoellick in October 2007 that Russia had changed its World Bank status from that of borrower to donor, effective immediately. Despite the upward trajectory of the Russian economy and increasing political willingness to contribute to, rather than receive money from, international aid institutions, public health systems throughout the country continue to be deeply flawed and underfunded. Of particular concern in Russia is the rise of TB in the post-Soviet period (closely associated with economic crises and poverty) and the increase in incidence of MDR-TB (see Figure 11 and Figure 12). |
|
Figure 11: TB notification rate and unemployment rate, Russian Federation, 1985-2006 (all jurisdictional entities)150  In 2004, the province of Tomsk, located in western Siberia, began a five-year, USD 10.8 million GFATM grant for management of TB and MDR-TB. One year later, the Russian Health Care Foundation (RHCF) began its primary phase as principal recipient of a $88.1 million GFATM grant for the treatment of MDR-TB in 20 provinces/territories. GLC approval was required for the disbursement of both grants, as mandated by the GFATM grant agreements.  In 2006, the Russian Federation changed the requirements for the importation of pharmaceutical products, primarily in the sphere of registration procedures. Along with submission of additional documentation, all manufacturing companies were required to have a physical representative office in the Russian Federation. Despite the compliance of providers within required timelines, several factors turned these procedural changes into life-threatening crises for hundreds of patients. Of particular concern was the cessation of importation of quality-assured PAS sodium and PASER® (a gradual release formulation of |
|
para-aminosalicylic acid) for GLC-approved projects. This resulted in the interruption of treatment regimens for hundreds of TB patients throughout the Russian Federation. One factor contributing to this crisis was the lack of effective, timely communication between the federal government, regional TB services, and international/national institutions. Earlier announcement of the change in registration procedures (e.g. six months in advance), as well as higher drug ceilings for importation as permitted by the Russian Federation’s Humanitarian commission, could have allowed for projects to establish buffer stocks at their projects to prepare for potential cases of prolonged procedural or legal obstacles to the importation of GLC-approved second-line drugs. Because the government of the Russian Federation has not designated the MDR-TB crisis as an “emergency situation,” fast-track importation of second-line medications has not been possible. Another challenge faced in the Russian Federation is the importation quota established by the country’s Humanitarian Commission (a semi-governmental organization, which must determine which organizations seeking tax exemptions for importation qualify as “humanitarian” versus “technical”). The quantities of drugs allowed to be imported are limited and must be determined at the start of each year, when a project submits a dossier that is approved by the Humanitarian Commission. This prevents projects from establishing a substantial buffer stock. More importantly, it also limits flexibility in response to occasional donations or accelerated delivery dates. Russian projects have had to decline offers of short-dated capreomycin because of quota limitations. Although Russia has many second-line anti-TB drug suppliers (there are 10 suppliers of amikacin, eight of kanamycin, five of capreomycin, 24 of ofloxacin, six of levofloxacin, one of moxifloxacin, 12 of prothionamide, four of ethionamide, and four of cycloserine), none are prequalified by WHO.152 Russian registration and importation regulations are strict, and consequently it is difficult to find suppliers that are both approved by the GDF and registered in Russia. This often limits Russian procurement to a single supplier and in some cases, a single production facility. For example, Eli Lilly’s capreomycin is registered in Russia, but only the product produced at the company’s German facility is approved; capreomycin produced by Eli Lilly in the United States or Hungary cannot be imported into Russia. Matters are often further complicated by the fact that drug importations by organizations not designated as “humanitarian” (e.g. the Russian Health Care Foundation) face even stricter rules and are often not able to import non-Russian-labeled or short-dated drug stocks. Shipments from IDA have been returned for these reasons and other paper-work issues. |
|
The lesson drawn from the experience in Russia is that some of the larger nations have well-developed regulatory institutions that govern the importation of medicines and the use of medicines within their borders. It is crucial that the GDF and the procurement agent are extremely well-informed regarding customs documentation and shipment preparation requirements for each country. Since the role of both entities is to ensure that drugs are successfully imported by TB programs, mechanisms must be created so that predictable delays and complications in the customs service are avoided, and that changes to national policies are monitored and anticipated. Also, sustained political action will be required to encourage Russia-based producers to enter the WHO/EDM prequalification system and thereby ensure a long-term in-country supply of quality-assured second-line drugs. |
4
DRUG SUPPLY AND ENGAGEMENT OF DRUG MANUFACTURERS IN MDR-TB RESPONSE
4.1
MDR-TB projects working outside the GLC initiative
As described above, a large global proportion of patients receiving MDR-TB treatment do so outside of the GLC mechanism. Countries reported to the WHO that they treated an estimated 36.4 thousand patients outside of the GLC system in 2007 and that they will similarly treat 34.7 thousand patients in 2008. Countries like Brazil and South Africa have treatment policies that differ from WHO guidelines and strict policies about second-line anti-TB drug purchasing that require them to use local manufacturers. The case of Brazil is illustrative of the drugs-related issues faced in these countries.
|
Country Profile: Brazil The Brazilian National TB program has no formal ties to the GLC initiative for procurement. Rather, Brazil has implemented a largely self-contained system of drug production, allocation, and tracking for second-line drugs. The program currently receives institutional support and financing from the Brazilian government and USAID. A network of public research and production facilities produces a majority of drugs, though on occasion public labs have experienced delays up to 6 months in providing product to the system. Private Brazilian anti-MDR-TB drug manufacturers often provide quicker emergency responses to drug shortages, as they are largely unencumbered by procedural government restrictions. By law, the Brazilian TB system maintains a buffer stock of 25 percent of total anti-TB drugs in circulation at the central Helio Fraga facilities, which are solely responsible for second-line drug storage. |
|
Strict purchasing regulations apply in Brazil, resulting in a TB system that only utilizes the international drug market as a last resort, after all public and private domestic options have been exhausted. In 2007, the Brazilian TB community commissioned quality screening for dozens of samples of state-produced anti-MDR-TB medications. According to data from this evaluation, 36 samples representing 14 different products, 10 different active ingredients, and 11 different producers were collected during the first phase of the National Program for Quality Testing of Essential Medicines (Proveme). In total, 35 samples were analyzed, of which 22 were approved (63 percent) and 13 were considered non-satisfactory (37 percent). Of these 13 samples, seven were found with labeling non-conformities (20 percent) and six did not meet product quality standards (17 percent).153 This investigation suggests that quality control is an crucial issue, even in a highly-developed, largely self-sufficent national MDR-TB program. The fact that Brazil is actively investigating the quality of the medications in its system is an encouraging sign, though other national programs may not be so transparent. |
4.2
Available drug supply through GLC initiative
All second-line anti-TB drugs approved for use at GLC sites are currently off patent.3 The following chart displays prices for available second-line drugs through the Global Drug Facility (GDF), available to GLC-approved projects:
Table 2: Second-line anti-TB drug (with pricing) available through the GLC mechanism (2007)
|
Product |
Description |
Pills/Vials/Kits |
Unit Price (US$) |
Avg. cost per Pill/Vial/Kit |
GDF-approved manufacturers |
WHO Prequal.4 |
|
Amikacin |
500 mg / 2 mL injectable vial |
10 |
$15.10 |
$1.51 |
Medochimie Pharmaceuticals |
NO |
|
Capreomycin (A) |
Powder for injection - 1 gram vial |
1 |
$3.21 |
$3.21 |
Eli Lilly |
NO |
|
Capreomycin (B) |
Powder for injection - 1 gram vial |
1 |
$1.07 |
$1.07 |
Eli Lilly |
NO |
|
Cycloserine (A) |
250 mg capsule |
100 |
$50.96 |
$0.51 |
MacLeods Pharmaceuticals Ltd. |
YES |
|
Cycloserine (B) |
250 mg capsule |
100 |
$14.12 |
$0.14 |
Eli Lilly |
NO |
|
Ethionamide |
250 mg tablet |
100 |
$10.21 |
$0.10 |
MacLeods Pharmaceuticals Ltd. |
YES |
|
Kanamycin |
Powder for injection - 1 gram vial |
50 |
$26.50 |
$0.53 |
Panpharma |
NO |
|
Levofloxacin 250 |
250 mg tablet |
100 |
$4.00 |
$0.05 |
MacLeods Pharmaceuticals Ltd. |
NO |
|
Levofloxacin 500 |
500 mg tablet |
100 |
$6.98 |
$0.07 |
MacLeods Pharmaceuticals Ltd. |
NO |
|
Ofloxacin |
200 mg tablet |
100 |
$3.49 |
$0.03 |
MacLeods Pharmaceuticals Ltd. |
NO |
|
PASER |
4 gram granules sachet |
30 |
$59.10 |
$1.97 |
Jacobus Pharma Company Ltd. |
NO |
|
Prothionamide |
250 mg tablet |
100 |
$16.00 |
$0.16 |
Fatol Arzneimitel |
NO |
|
Moxifloxacin154 |
400 mg tablet |
1 |
$5.93 |
$5.93 |
Bayer Pharmaceuticals |
NO |
|
Source: Stop TB155 |
||||||
4.3
Incentives and disincentives for entry into the second-line anti-TB drug market
Shortly after the GLC’s launch in 2001, authors Gupta, Kim, Raviglione, et al. argued in Science that there were four distinct advantages for manufacturers who would enter the pooled GLC procurement system:156
-
Involvement with the GLC process represented a chance for a manufacturer to display commitment to increasing access to critical therapies in developing countries. It also can build a unique, high-profile international relationship for smaller manufacturers.
-
Participation in the GLC initiative would assure proper use of a manufacturer’s drugs and would not result in the creation of significant further resistance.
-
The GLC and the GDF would provide institutional support for the registration and importation of drugs; since they would be provided through a WHO mechanism, it would not require the payment of national tariffs and duties.
-
Industry benefits from single-source demand, which allows for manufacturers to adjust facilities and production capacity for scheduled long-term production, rather than responding to irregular orders from particular projects.
Advantages (1) and (2) are difficult to measure (and therefore difficult to dispute) though they appear self-evident. Advantage (3) has generally proven true with regards to tariffs and duties, although some programs have experienced difficulties with tariffs and duties and there have been problems that persist on the part of the procurement agent, the GDF, and national governments in coordinating timely preparation of required documentation. Advantage (4), which predicts stable, reliable forecasting for manufacturers through the GLC initiative, proves to be the least true of the four advantages over the past seven years; only now are more sophisticated forecasting systems being designed at the GDF to strengthen the existing ad hoc ordering system inherited from the early days of the GLC initiative.157
Many manufacturers of anti-MDR-TB drugs based outside of North America and Western Europe have not been overly eager to participate in the GLC Initiative, as they have existing, often lucrative contracts with their national TB programs. It is fair to assume that some of these manufacturers are not subject to quality control or quality assurance at the level required for prequalification by the WHO. The issue is exacerbated by the fact that with the small number of patients being treated under the GLC initiative, there is little financial incentive for manufacturers to undergo the arduous task of WHO Prequalification. Currently, due to high production costs, inaccurate forecasting, and concessional pricing, even companies
who are already providing drugs to the GLC Initiative report having limited motivation to stay involved purely for the purposes of profit generation. The companies involved further report that regardless of a second-line anti-TB drug’s profitability (which if it exists, is often modest, according to industry interviews),158 use of company facilities for second-line drug production typically does not maximize profit generation. Furthermore, no entity exists to assume risk and absorb financial losses from incorrect projections. For this reason, expansion of the number of patients treated under the aegis of the GLC initiative and expansion of the GDF buffer stock to 5000 patients are particularly important to convince manufacturers to stay in the market.
As discussed earlier, another disincentive to participating in the GLC Imitative and WHO Prequalification is that some countries (e.g. Brazil, China, Korea, India, Russia, and South Africa) have local pharmaceutical industries and robust national markets for second-line drugs. Private market sales of second-line drugs are significant in most countries and have increased substantially in the last three years. For example, preliminary survey data on private-sector sales in MDR-TB priority countries show that sales of second-line drugs used solely for TB, such as prothionamide, rose dramatically in China (up 73 percent) and Russia (up 338 percent).159 In China, seven different suppliers, none of whom were quality-assured, accounted for these private-market sales of prothionamide; 12 separate suppliers sold the drug in Russia, one of whom sold quality-assured product but accounted for only two percent of total volume. Quantities of second-line drugs sold in the private sector were sufficient to treat many more MDR-TB patients than the countries had enrolled in GLC projects, by significant factors: sales of prothionamide in China were adequate for more than 3,500 MDR-TB patients and the country has a GLC project that is projected to enroll 354 patients; sales of the drug in India would have treated more than 1,800 patients, nine times more than the 200 patient in India’s GLC project. Data on sales of second-line drugs used for indications other than TB are still more impressive. Private-sector sales of ofloxacin in India, for example, would have treated more than a million MDR-TB patients last year; there were more than 100 suppliers selling the drug, few of whom are quality-assured. So there are substantial and efficient markets throughout the world for second-line drugs, but the market for quality-assured second-line anti-TB drugs appears not to be one of them.
If a majority of the manufacturers of second-line anti-TB medications in key high-burden MDR-TB countries could be brought in to the prequalification program, the shortfall of drugs for GLC projects could be addressed while simultaneously guaranteeing that the drugs supplied to NTP in high-burden countries would be of demonstrably superior quality. Pharmaceutical company executives and governmental health authorities are both positioned to make decisions regarding this possibility.
The dynamics of the second-line anti-TB drug market could change in coming years. From all indications, the sales of second-line drugs—quality-assured or not—will continue to grow considerably. Evidence from GLC applications and reports to WHO of patients already on treatment indicate that public market purchases of second-line drugs will continue to increase rapidly and it is likely that private market demand will as well. Demand for second-line drugs will expand with the increasing availability of international financing for poorer countries that have significant MDR-TB burdens (e.g. through GFATM, UNITAID, and possibly PEPFAR). Very recently, demand was also bolstered by UNITAID financing for a buffer stock of second-line drugs for up to 5,000 patients, to be purchased by the GDF. This last development is particularly significant in the short term, in that it creates well-characterized, firm, and immediate demand for quality-assured second-line drugs (for more than half as many patient regimens than the market supplied all of last year), subject to none of the delays and uncertainties that have always characterized this small, peculiar, and idiosyncratic market in the past.
These changes in the market for quality-assured drugs will take some time to be understood by national and international market participants because up until now, the market for these drugs has been so small, so strictly controlled, and limited to such a small number of approved suppliers. The sooner suppliers of second-line drugs understand the changing dynamics of the market, the more inclined they will be to incur the upfront expense of having their products quality-assured. The more readily national governments and other purchasers of second-line drugs are able to access quality-assured drugs, the more likely they will be to insist upon them for their patients. WHO, GLC, and the GDF could take some steps to facilitate mechanisms and improve incentives for suppliers and purchasers of second-line drugs. In so doing, they could catalyze a virtuous cycle wherein the supply and the demand for quality-assured drugs both increase as the overall market continues to expand.
4.4
New therapies for MDR-TB
Given the market realities surrounding the second-line anti-TB drug market, it should come as no surprise that the emergence of drug-resistant disease as a public health concern has not resulted in a proportional response from pharmaceutical companies in research and development. No new treatment breakthroughs have been made available to patients in decades, despite progress in the laboratory. According to the Treatment Action Group, $120 million was spent for anti-TB (including MDR-TB) drug development in 2005 worldwide yet the total invested in clinical trials was no more than $20 to $30 million.160 If successful, human trials currently underway which aim to shorten the duration of first-line therapy could eventually decrease incidence of drug-resistant disease, though the likelihood of any of these compounds reaching the market by 2010 has been estimated at less than 5%.161
Compared to first-line drugs, the development of second-line therapies offer comparative advantages and disadvantages. As Glickman et al. assert in Seminars in Respiratory and Critical Care Medicine, “for MDR- and XDR-TB, the target product profile might pose a somewhat lower ‘bar’ because the currently available drugs are less effective, have more associated adverse effects, and are significantly more expensive.”162 While this is promising for patients with MDR-TB, various factors including the extremely long treatment period for MDR-TB patients, the multi-year follow-up that is required, and the concurrent medications given in the current standard of care that can obscure the effect of new therapies all make testing new drugs on MDR-TB patients less attractive.163
However, the relatively large number of MDR-TB patients being treated by GLC-approved projects creates a new possibility for clinical trials where none existed previously. As Mitnick et al. points out, “for the first time in 30 years, several new drug classes that hold promise for MDR-TB treatment are under development,” and “the expansion of MDR-TB treatment programs provides the settings in which trials can be implemented… Four elements are needed to make MDR-TB treatment trials a reality: money; additional work on the drug pipeline; rigorous, interdisciplinary preclinical work on individual agents and regimens; and an understanding that TB clinical trials need not be a zero–sum endeavor.”164
Aside from the availability of sites for clinical trials, a debate is currently underway regarding the cost and likelihood of development of major new TB therapies. Glickman et al. assert that “achieving 95% confidence of at least one new tuberculosis drug will take 12 years and costs will approach $400 million.”165 Andrew Farlow of Oxford University challenged this claim, putting the figure (using Glickman’s transition probabilities) at $136.75 million.166 Regardless, the outlook for new therapies is a complicated picture which demands continuing commitment from policy-makers, researchers, research funding agencies, and the pharmaceutical industry.
Recently there has been some consensus among stakeholders to marshal resources and political will to provide capacity for clinical trials of new MDR-TB therapies. In June 2008, representatives from NGOs, governments, donors, the pharmaceutical industry, and academia met in Cambridge, Massachusetts167 and declared the formation of a new initiative called RESIST-TB, whose aims are: to conduct priority clinical trials that test strategies in adults and children to prevent drug-resistant TB; to shorten and improve treatment for drug-resistant TB; to mobilize the resources needed for these trials; to build the capacity of trial sites; and to ensure that these efforts complement those of other groups.
4.5
Governmental health authorities and high quality second-line drugs
As of summer 2008, the majority of high-burden countries are working outside the GLC initiative to provide most or all MDR-TB treatment to their citizens. The spectrum of engagement varies from very limited involvement (e.g. South Africa), to some involvement (e.g. China and India), to significant involvement (e.g. Russia). Regardless of the level of involvement, drugs of unverifiable quality produced by local industries should surely be a major focus of concern as MDR-TB continues to spread apace in those nations. The financial benefit of buying drugs of questionable quality will be outweighed by the problems associated with a growing population of MDR-TB patients and of those with higher-spectrum resistance (e.g. XDR-TB). This could result in governmental health authorities urging or financing their national manufacturers to go through WHO Prequalification for the sake of its citizens’ health, or initiating the process to have its own regulatory authority deemed stringent (as has been done in South Africa-MCC). The outcome in either case would be higher-quality drugs manufactured in high-burden areas. The GLC initiative/GDF and WHO Prequalification could assure that its capacity to provide second-line anti-TB drugs to participating projects are increased by assisting manufacturers through the process early on in return for agreements of supply at a reduced price for some period of time after approval.
4.6
Manufacturers in high-burden countries
There are reasons to expect that manufacturers of second-line drugs in high-burden countries may become more amenable to GLC involvement in the future. Producers in these countries operate in fundamentally different business environments than their North American and Western European counterparts, but significant state intervention in the pharmaceutical industry does not necessarily result in security for these companies; often the opposite is true. Profit-margin regulations and price caps in countries like China and India change regularly, and as a result many of their companies have expressed interest in selling their products internationally to diversify their revenue streams.168,169 Indeed, China and India are presently working together to streamline the production of raw materials for ailing pharmaceutical companies in India that are languishing under the price-control regime.170
|
Public-Public Partnership: Eli Lilly Technology Transfer for Capreomycin and Cycloserine American pharmaceutical company Eli Lilly and Co. developed capreomycin (Capastat®) and cycloserine (Seromycin®) in the 1950s and early 1960s, and has been the leading global producer of these drugs. Since the inception of the GLC in 2000, Eli Lilly has undertaken a philanthropic effort to provide concessionary-priced second-line drugs to GLC-approved programs. In 2006 alone, 1.2 million capsules of cycloserine and 280,000 vials of capreomycin were provided (a large proportion at a discount) to the GLC initiative.171 Additionally, Lilly introduced another innovative philanthropic initiative in the form of production technology transfer to high-burden countries.172 This was done in the context of Lilly’s desire to eventually withdraw from the MDR-TB market.173 At the time of transfer, capreomycin was a non-patented monopoly drug. Cycloserine had been approved by the WHO Prequalification Program for production by Macleod’s of India, though it is sold at a significantly higher price by that producer. The transfer provided manufacturers with the necessary knowledge and manufacturing technology required to produce the active pharmaceutical ingredients (APIs) and final products. Lilly committed to purchase equipment, upgrade facilities, and provide training in business management and Good Manufacturing Practices (GMP) for selected partners. A pharmaceutical company in each of the four highest-burdened countries was selected based primarily on its willingness to supply the drugs to the GLC at a negotiated rate. SIA International (Russia), Aspen Pharmaceutical (South Africa), Hisun Pharmaceutical (China), and Shasun Chemicals and Drugs (India) were selected to take part in the process. All partners contributed financially to the initiative and agreed to supply the drugs to the GLC with a maximum of 20 percent profit margin.174 Aspen sold its first batch of cycloserine to Botswana in 2005, while the new facility was under construction. Currently, the factory is at full capacity (it is designed to manufacture 4 billion capsules annually) and is assuming Lilly’s share of cycloserine production for the GLC initiative. Its production of cycloserine had been approved by South Africa’s regulatory body, Medicines Control Council (MCC), now considered a stringent drug authority (though it is not a retroactive consideration and Aspen must still complete its WHO Prequalification application). It has additionally received GMP certification from the WHO and all associated parties predict that full-scale production of capreomycin by Aspen should be possible by the second quarter of 2009.175 |
|
According to Lilly representatives, the difficulties arising from the complex production of cycloserine and capreomycin were exacerbated by poor forecasting of need, both by the WHO at the partnership’s inception in 2003 and by the GLC initiative in the years following.176 |
5
Redefining the Paradigm of the GLC Mechanism
Although the GLC “pilot project” era officially ended with the change in WHO treatment guidelines for MDR-TB patients in 2006, the GLC initiative is still functioning with “pilot project” procurement mechanisms and policies. Changes in both operating procedure and logistics could significantly enhance the supply and demand of quality-assured second-line drugs.
In the pilot project era of the GLC, the demand for quality-assured drugs was small; this is clearly no longer the case. Drugs could only be accessed through a single procurement agent, which is still the case: projects purchasing second-line drugs with financing from GFATM or UNITAID are required, by the terms of the grant, to have GLC approval and to use only the GDF and its procurement agent. As the market has expanded rapidly, it has outgrown the capacity of a single procurement agent and for many second-line anti-TB drugs, a single quality-approved manufacturer. Experience has shown that it is no longer practical to require all GLC projects to purchase second-line drugs only through one procurement agent and indeed some countries are prohibited by law from purchasing from a sole, pre-determined source. The GFATM and UNITAID will certainly continue to insist that their grant funds be used only for the purchase of second-line drugs that are quality-assured, but they are not likely to long maintain their requirement that recipients use a single procurement agent, especially if the procurement agent is unable to procure and deliver the second-line drugs in a timely and consistent manner over an extended period of time.
Through some modification in the way it operates, the GDF could strive to become the most attractive supplier of second-line drugs in the market—on pricing and supply logistics—becoming the option that GLC projects want to use. A first step would be for the GDF to move as expeditiously as possible to retain more than one procurement agent to act on its behalf. This would provide the GDF with a choice of procurement agents; it could award contracts based on the ability of agents to fill orders most expeditiously and to take advantage of regional capabilities and relationships. Through this approach, another major advantage will have been secured: the GDF will become the largest and most consistent purchaser of second-line drugs. If it can maintain a buffer stock of second-line drugs for up to 5,000
patients, the procurement of those drugs will give it pooled procurement advantages and market pricing power over and above discounted prices it obtains through its role as the GLC-procurement mechanism.
In order to open the market to alternative procurement strategies, the GLC could take the additional step of separating GLC approval from the requirement to purchase only through the GDF and its procurement agent; instead, it could require that all GLC-approved projects purchase quality-assured drugs. This would open the door for GDF and other international partners to create criteria for “quality-assurance” (drawing from WHO Prequalification requirements as well as those of Stringent National Regulatory Authorities and agreements governing their operations) that could be used by countries who choose to open drug purchases to tender.
By both expanding the number of its own procurement agents and expanding the ability of countries to purchase quality-assured second-line drugs on their own, the GDF would strengthen the following areas:
-
Logistics. The GLC/GDF will have responded to the logistical problems, which now cause so much concern, by establishing a limited number of procurement agents to operate in various regions of the world and who will likely respond more efficiently to increasing demand and be better equipped to resolve the types of registration and customs obstacles which delay the delivery of second-line drugs.
-
Pooled Procurement. The GDF will retain access to information on all purchases of second-line drugs for GLC projects, can assist regional procurement agents in pooling purchases, and can increase the size of orders for projects as it fills its own UNITAID-funded buffer stock.
-
Negotiations with quality-assured suppliers for preferred pricing. The GDF can keep the responsibility of negotiating standard, preferred pricing for all regional GDF procurement agents, while allowing the procurement agents to purchase quality-assured second-line drugs on better terms if available.
-
Preferred pricing. If the GDF is the major high-volume purchaser of quality-assured second-line drugs (through its procurement agents) it would be unlikely that many countries (except perhaps the largest) would garner better terms from manufacturers. For countries that require multiple bid-procurement processes, GDF’s regional agent could submit a bid as one of many potential suppliers to the country. It is likely, backed-up by access to the GDF buffer stock and pre-negotiated GLC prices, that the GDF agent would win the majority of contracts.
6
RECOMMENDATIONS
6.1 The WHO and international partners should take immediate and rapid steps to increase the number of manufacturers of quality-assured second-line anti-TB drugs. A mechanism needs to be developed to make these drugs available at pre-negotiated prices to programs purchasing via the GDFD and through direct-purchase by countries. Specifically, this means having the GLC uncouple the important emphasis on quality-assured drugs from the mode of purchase. Countries should be able to purchase second-line drugs however they choose, as long as they use quality-assured products as specified on a list of GDF-approved suppliers. In order to remain competitive and address some of the shortfalls in the current system, the GDF should increase the number of procurement agents available to countries participating in the GLC initiative. This would allow the system to take advantage of regional competencies such as knowledge of local manufacturers and understanding of national customs/regulatory rules. Lastly, WHO should define their quality-assurance criteria along the lines likely to be adopted next month by the GFATM and create a system to monitor that the criteria are being followed.
6.2 The GDF should create a tiered system of approval for manufacturers of second-line drugs who are in the WHO Prequalification Program. Large countries operating within the GLC initative should be allowed to purchase second-line anti-TB drugs from domestic manufacturers who have entered the WHO Prequalification process. In the current system, manufacturers are unable to sell their products unless they are prequalified by WHO or have prequalification from a Stringent Regulatory Authority. Manufacturers who are in the process of WHO Prequalification are unable to sell their products even though they may be very close to full approval. A tiered purchasing approach—where manufacturers who commit to completing the WHO process can sell their products under certain circumstances and with stringent batch testing—would act as an incentive to manufacturers to enter the WHO process, increase the number of qualified manufacturers who can sell drugs through the GLC initative, and alleviate the global shortages experienced with some second-line drugs. This would increase competition and lower price (as mandated by UNITAID funding). Criteria for participation in such a system would have to be developed along the lines of the tiered system that already exists for first-line TB drugs.
6.3 The GLC initiative and the GDF should institute a reliable and transparent system for quantification of demand for second-line drugs. The GDF should expedite its ongoing efforts to develop a comprehensive system of needs projection that takes into account projects’ patient enrollment, capacity, and GFATM grant disbursements. The expertise needed for this effort is available; private industry experts, logistics consulting firms and non-governmental organizations (e.g. Management Sciences for Health) are qualified to assist in needs projection and in creating systems to track medications from the point of production to the point of consumption by the patient. This will not only help countries know when they are going to receive their drug orders, but will allow manufacturers to estimate the future market.
6.4 The GDF should maintain a second-line anti-TB drug buffer stock (at minimum, enough to treat 5,000 patients) in order to facilitate rapid delivery of drugs to programs (less than one month). The GDF has recently received funding from UNITAID for a 5,000-patient buffer stock of second-line drugs. Orders for this stock should be placed independent of orders from projects and should be specifically targeted at encouraging new manufacturers to enter the market. The presence of a buffer stock will also reduce waiting time for drugs to less than two weeks rather than the current three to six months.
6.5 There should be a global effort to increase the options available for treating MDR-TB and XDR-TB, by optimizing current regimens and by developing at least three new anti-TB drugs. Increased TB clinical trial capacity needs to be created, and mechanisms developed to fast-track new anti-TB drugs through the regulatory process. Current therapies for MDR-TB and XDR-TB are woefully inadequate: the treatment takes two years, throughout which patients face numerous medication-related adverse events. These regimens need to be optimized so that adverse events are minimized. New therapies targeted specifically to M. tuberculosis need to be developed and mechanisms for fast-tracking regulatory approval of promising agents need to be worked out with regulatory agencies. Increased clinical trials capacity for novel TB treatments must be developed simultaneously.
SECTION IV:
MDR-TB TREATMENT DELIVERY
-
INTRODUCTION
-
SHIFTING THE PARADIGM FROM “PILOT” PROJECTS TO AN INTEGRATED STRATEGY
-
ADDRESSING THE MDR-TB TREATMENT IMPLEMENTATION GAP
-
EXPANDING MODELS OF CARE
-
RECOMMENDATIONS
1
INTRODUCTION
According to the recent WHO/IUATLD survey of global drug resistance, the global burden of drug-resistant TB is significant and growing very quickly. Although early intervention with appropriate and aggressive second-line drug regimens can result in cure rates over 75 percent, MDR-TB diagnosis and treatment programs have not even begun to keep pace with the epidemic.177,178,179,180
At the country level, the growing problem of drug-resistance is unwelcome by most NTPs, many of which are still struggling to control drug-sensitive TB. Spurred by the rapid increase in MDR-TB and XDR-TB observed globally, programs are trying to come to terms with their drug-resistant TB epidemics. Treatment of drug-resistant TB, however, is expensive and complex: patients require 18 to 24 months of therapy with four to eight medications, including daily injections for at least six months; treatment is fraught with numerous adverse events which require additional management. Most national TB programs are ill-equipped to provide the necessary services for management of MDR-TB/XDR-TB.181 In some cases, the required clinical and laboratory expertise may not even exist within the public sector; in many countries, human resources for the actual delivery of care and systems of care delivery themselves are lacking. All of this is exacerbated by weak health systems in many settings182,183,184,185and by the challenges of delivering treatment to poor and marginalized patients who often face many social and economic barriers to receiving adequate care.186,187,188,189,190,191,192
The area of treatment delivery is complex and broad, and this paper does not fully address all relevant aspects of care delivery. Rather, the purpose of what follows is to highlight important factors that may have an effect on the rate and nature of MDR-TB treatment scale-up, and to offer some targeted solutions.
2
SHIFTING THE PARADIGM FROM “PILOT” PROJECTS TO AN INTEGRATED STRATEGY
When drug-resistance was identified as a major global problem in the mid- to late-1990s, it was initially considered an additional intervention that countries could choose to implement, if needed, in their TB-control strategies. Because of the belief that drug-resistant TB would disappear as DOTS programs improved and the worry that MDR-TB treatment (at that time called “DOTS-Plus”) would draw important financial and human resources away from DOTS programs, a false dichotomy emerged between the programmatic management of drug-susceptible and drug-resistant TB. For a long time, countries were advised by the WHO and other international partners to focus primarily on drug-susceptible TB. This resulted in a lack of integration of drug-resistant TB treatment into national programs and, as evidenced by findings from the recent WHO/IUATLD global drug-resistance survey, had profound effects on the epidemiology of TB.193 In 2006, the Stop TB framework called for the integration of drug-resistant TB treatment as part of national TB-control strategies.
As discussed previously, the GLC initiative was formed in 2000 with the aim of providing concessionary-priced quality-assured second-line drugs to MDR-TB treatment pilot projects.194 The idea was to maintain a high standard of programmatic vigilance in order to prevent the emergence of super-drug-resistant strains of TB. Initial pilot projects in Estonia, Latvia, Peru, the Philippines, and Tomsk (Russian Fedration) were quite successful and yielded cure rates of 77 percent among new cases of MDR-TB and 69 percent among previously treated cases of MDR-TB patients.195,196,197,198 Recent data suggest that the MDR-TB epidemics in Estonia and Latvia—both of whose pilot projects offered universal access to MDR-TB treatment—might in fact be leveling off.199
The WHO’s Global Plan to Stop TB: 2006-2015 established a set of treatment targets for 2015, including the treatment of 800,000 patients with MDR-TB.200 With the XDR-TB outbreak in the Republic of South Africa in 2006, a two-year emergency plan called for an aggressive revision of the 2015 targets to include “universal access” by 2015 (equating to nearly 1.6 million patients) and for the treatment of 134,000 individuals by the end of 2008. However, because just over 40,000 patients have been approved for treatment in GLC projects to date and a smaller number have actually received treatment, it is unlikely that this goal will be met.
One of the main problems has been the integration of MDR-TB care into national TB-control strategies. The dichotomy between drug-susceptible and drug-resistant TB created by early policies, coupled with the current approach to drug-susceptible TB—using Category I then Category II, etc.—has resulted in lukewarm commitment on the part of some global partners to MDR-TB scale-up and mixed messages reaching programs, leaving some countries confused as to how to proceed. The XDR-TB outbreak changed some of these dynamics, but still many countries report being advised not to include MDR-TB treatment until they have a fully functioning DOTS program. Because the disease is airborne, this approach has resulted in a growth of MDR-TB in many settings and the unnecessary deaths of countless patients.
Since the GLC is the gatekeeper both to concessionally-priced second-line drugs and to the release of GFATM and/or UNITAID funding, programs are required to use the mechanism. Although the GLC is not charged with the task of global scale-up of MDR-TB treatment, many observers perceive the GLC as part of the scale-up bottleneck; in some countries, it is referred to as the “red-light committee.” This, however, is not the case: projects are rarely rejected as the process is designed to assist any interested project in improving its program to the point of GLC approval. Because of the iterative process that the GLC undertakes with each application, approval time (from time of submission to full approval) can take months even for a few patients. Eventually a small cohort of patients is permitted to begin treatment while the TB program increases its capacity for program expansion. GLC consultants visit programs regularly for monitoring, evaluation, and the provision of technical assistance. It is during these visits, often conducted yearly, that further program expansion can be recommended. Therefore, the “bottleneck” lies in the very process of ensuring the integrity of projects and their ability to safely deliver MDR-TB treatment.
Because many countries lack the necessary infrastructure for MDR-TB treatment, nearly all projects are approved as part of a limited effort in a country—a “GLC pilot project.” The justification for pilot projects is that they allow clinical and programmatic experience to be gained and epidemiological data to be collected in preparation for scale-up of a larger, national program. While the reason for the pilot project approach is obvious—to prevent the emergence of broad-spectrum anti-TB drug resistance resulting from poorly delivered MDR-TB care—its most serious flaw is that it does not address the epidemiological reality of a rapidly spreading, airborne illness. Early diagnosis and effective treatment of MDR-TB patients is not just good clinical care—it is also a public health intervention that prevents many new cases. As the recent global MDR-TB surveillance report has shown, treating a small fraction of known MDR-TB cases does nothing but ensure that the number of MDR-TB cases will continue to increase. Sadly, only five GLC projects—Estonia, Latvia, Lesotho, Nepal, and Peru—currently offer
universal access to MDR-TB treatment, a far cry from what one would expect given the current Stop TB recommendations.
One of the reasons that WHO’s DOTS strategy for the treatment of drug-susceptible TB has been so successful is that countries were encouraged to rapidly scale-up implementation and were given ample assistance to do so. Despite the extra staffing requirements for the direct observation of therapy, the need for quality-assured smear microscopy, and the management required for maintaining drugs stocks, the project has been a resounding success. The same needs to be accomplished for MDR-TB. In order to do so, the approach of the GLC toward projects has to shift from a “pilot project” mentality to one where full integration of MDR-TB treatment in national TB-control strategies is the immediate and desired outcome. At a time when GFATM money is available to countries facing MDR-TB/XDR-TB epidemics, waiting for countries to have perfect DOTS programs before they can expand MDR-TB treatment risks losing an important opportunity for program strengthening. MDR-TB treatment integration can bring additional resources into cash-strapped NTPs, encourage renewed political commitment, and strengthen the capacity of diagnostic services, clinical management, and case management.201 With the current funding streams, NTPs are now in a position to innovate beyond their current models of care, specifically addressing barriers such as poor nutrition, lack of transportation, adverse event management, and social isolation, all of which can have a bearing on improved management of both drug-susceptible and drug-resistant TB disease.
In order to achieve the goal of full integration and universal access to MDR-TB care, the role of the GLC initiative as a whole needs to shift away from a “pilot project” approach to one which encourages projects to scale-up MDR-TB treatment as rapidly as possible, by facilitating solutions to implementation barriers. This will require both a concerted effort on the part of the entire GLC initiative, the WHO, and international partners, innovative approaches to the provision of technical and material assistance, and in some cases, long-term on-site teams that can aid ministries of health with actual program implementation.
3
ADDRESSING THE MDR-TB TREATMENT IMPLEMENTATION GAP
Countries as diverse as Azerbaijan, Peru, Latvia, and Lesotho all emphasize the important role that technical assistance played in the initial stages of their MDR-TB programs. They also note problems with the technical assistance that they received, and report that the most useful assistance tended to be on-site and long-term.
International consultants often spend limited time in each country, leaving a list of recommendations specific to their narrow areas of expertise. Despite plenty of resources and trained field workers, some
programs are unable to move forward: organizations disagree on how to collaborate or share facilities; people disagree on who gets what role; groups disagree about how to divide the territory. These are issues that consultants are not always prepared to mediate; even for those who are willing to mediate, it is almost impossible to do so from outside the country after a technical assistance mission is completed.
The case of Lesotho, described below (p. 73), is illustrative. Because of local will and sufficient on-site expert assistance, Lesotho was able to rapidly launch an MDR-TB treatment program that included a safe, well-though-out plan to care for very ill patients in a hospital facility. Without the guidance and hands-on interaction of technical partners, it is likely this would not have happened so rapidly. Similar experiences were described in interviews with programs in Azerbaijan, Latvia, Tomsk, the Philippines, and Peru. The major lesson from all these examples is that having intensive technical assistance— a very hands-on form of technical assistance that we could perhaps call technical accompaniment—helps countries achieve integration of drug-resistant TB management into their national TB-control strategies and programs.
While the GLC initiative has done its utmost to provide technical assistance to projects before and during MDR-TB treatment implementation, there is limited capacity to engage with projects with the depth of commitment required to fuel rapid scale-up. The GLC’s mandate is to approve projects based on their applications and to provide guidance as to how they may improve their projects if needed. In many poor countries, projects need ongoing training, help with their GLC application, help with their GFATM application (to fund the project), help with program implementation, and ultimately, help in program operations. Yearly visits from the GLC and other international advisory/monitoring boards have not appeared to be enough to help countries expand programs and integrate MDR-TB treatment into their TB-control strategies.
Given the profound gap in the estimated number of patients with MDR-TB and those who receive any treatment—it is estimated that less than 50,000 of the almost half a million new annual cases receive any treatment—solutions are urgently needed (see Figure 12). What is required is a mechanism for the delivery of technical assistance and technical accompaniment based on a country’s need. An example of this is a global health initiative similar to PEPFAR. Since 2004, PEPFAR has provided treatment to over 1.6 million patients in 15 focus countries, including 367,000 patients co-infected with HIV and TB (see Figure 13). The example of PEPFAR suggests that appropriate diagnostic technology and access to quality assured medications is not enough to ensure project implementation; rather, in order for a complex health intervention to be successful in a short period of time, it requires: (1) sufficient resources; (2) an implementation strategy; and (3) an on-site implementation mechanism.
Figure 12: MDR-TB patients scheduled to receive treatment in WHO/GLC-approved projects and non-GLC projects compared to the estimated number of patients who require treatment (1000s of patients; 2004 to 2006; Source: WHO 2008)
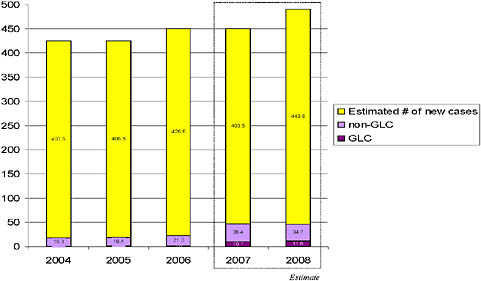
Figure 13: The number of individuals receiving antiretroviral treatment in PEPFAR’s 15 focus-countries202
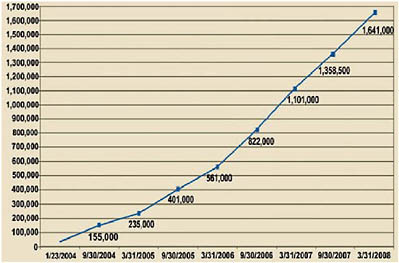
Countries included: Botswana, Cote d’Ivoire, Ethiopia, Guyana, Haiti, Kenya, Mozambique, Namibia, Nigeria, Rwanda, South Africa, Tanzania, Uganda, Vietnam (added in 2004), Zambia
Addressing the MDR-TB program implementation gap is a complex problem because it requires health system strengthening and the integration of often-vertical TB programs into broader health services. Much knowledge on how to achieve this goal exists in some of the earlier GLC pilot sites, many of which are now national programs. Achieving universal access to MDR-TB treatment will require a considerable increase in the pace of patient enrollment in high-burden MDR-TB settings; in order to achieve this, the TB community will need to look at its own achievements (e.g. GLC programs that have achieved nationwide expansion), as well as draw from the experience of other global health initiatives (e.g. PEPFAR, Guinea Worm eradication, etc.). At the very minimum, the following are needed: (1) a mechanism for using the lessons learned at the early GLC pilot sites and drawing on regional expertise to assist programs/countries in rapid MDR-TB treatment expansion; (2) a system to provide long-term on-site laboratory, programmatic, and clinical assistance and mentorship to national TB-control programs through implementation agencies; (3) the participation of global health initiatives, such as PEPFAR, in MDR-TB treatment expansion; and (4) the prioritization of MDR-TB treatment delivery (program implementation) by large bilateral donors—such as the Canadian International Development Agency (CIDA) and the United Kingdom Department for International Development (DFID)—and large global health foundations such as the Bill & Melinda Gates Foundation.
4
EXPANDING MODELS OF CARE
4.1
Community-based models for MDR-TB treatment
There are significant differences in the way that countries treat patients with MDR-TB. One of the most important differences is varying use of inpatient care.
When designing MDR-TB treatment programs, countries often turn to inpatient care for two reasons. Firstly, many chest specialists are more comfortable with treating MDR-TB patients in the hospital, where complicated regimens can be monitored closely for adverse events. This is particularly true in countries of the former Soviet Union, which have a history of hospitalizing even drug-sensitive TB patients, but also in many other countries, such as South Africa (which uses an ambulatory model for patients in treatment for drug-sensitive TB but not MDR-TB). Secondly, as discussed above, many countries lack sufficient ambulatory infrastructure—human and physical—to provide the complex treatment required for MDR-TB, and therefore find it easier to launch hospital-based programs.
In countries with a rapidly increasing numbers of drug-resistant TB patients, an emphasis on hospitalization can become a serious bottleneck to scale-up. As hospital beds run out, clinicians create waiting lists of patients who are already diagnosed with MDR-TB but cannot start treatment. While waiting for a hospital bed, infected patients can transmit their disease to others and by the time they are finally admitted, they may be seriously ill and at higher risk for treatment failure and death. Furthermore, hospitalization at the beginning of treatment does not guarantee adherence until the end of treatment. Patients who are discharged from the hospital may immediately default if adherence support is not provided.
The advantages of an ambulatory model of care for MDR-TB are much the same as for drug-sensitive TB. Ambulatory care allows patients to integrate themselves into community and family life and rejoin the workforce. Many GLC-approved MDR-TB treatment programs have a strong ambulatory care component using trained community-based workers, and some of the most successful have initiated MDR-TB treatment on an outpatient basis in all but the most severely ill patients. Outpatient models of care also decrease the problem of nosocomial transmission to other patients and staff within overcrowded, poorly ventilated hospital wards.
At the Stop-TB Partnership’s MDR-TB Working Group meeting in Tbilisi, Georgia, in September 2007, members endorsed a community-based approach for MDR-TB management as a way forward for NTPs. As the next case illustrates, the challenges to achieving this goal are great and much needs to be done to help countries make the important transition from inpatient to outpatient MDR-TB care.
|
Country Profile: Azerbaijan Many countries are faced with difficulties in changing their models of care to allow for universal access to MDR-TB management. Azerbaijan is an interesting example of a country primed for MDR-TB treatment expansion, but which has faced many challenges along the way. In 1995, it was estimated that drug-resistance among new and retreatment patients was not a significant problem; by the time the 2008 WHO Drug Resistance Survey data was published, Azerbaijan was found to have 22.3 percent MDR-TB among new patients and 55.8 percent among retreatment patients. Azerbaijan is an oil-rich country located in Southwestern Asia, bordering the Caspian Sea on its east and sandwiched between Iran and Russia with European borders. It is home to 8.4 million people, evenly divided between rural and urban areas. The country gained independence from the Soviet Union in 1991, but it has been struggling with territorial conflicts, displaced people, and corruption since that time, with |
|
accusations of authoritarian rule. Gross Domestic Product is USD 6,476 (PPP) per capita and literacy rates are almost at 100 percent. The most recent prevalence of HIV/AIDS is 0.1 percent and prevalence of TB is 85 per 100,000 population per year, as of a 2005 estimate.203 After the fall of the Soviet Union, the health care system remained a function of the state, but some private institutions and providers emerged. Pharmaceuticals became privatized and unregulated, with 70 percent of the drugs on the market today being imported and only 5 to 10 percent is free of charge. In 1998 fee-for-service and informal payments began, accounting for 57 percent of healthcare costs. The new payment schemes created a barrier to care for many. A 2001 survey found one third of households could not access necessary health care services.204 In 1991, Azerbaijan faced a recurrence of TB. At the time, the country also faced a shortage of TB medications, did not have a national reference laboratory, had limited managerial capacity, and no longer offered tuberculosis-treatment training previously mandated by the former Soviet Union. In 1994 TB prevalence in the prisons was almost 50 times the national rate and 24 percent of cases died. There were about 25,000 people in prisons, where overcrowding, poor health, and other risk factors contributed to the rapid spread and the high prevalence. In the civilian population, treatment was mostly provided by private practitioners, many of whom were paid by pharmaceutical companies for each second-line drug prescription they wrote.205 During this time, patients turned to self-treatment with intermittent supplies of first- and second-line drugs from family members or other outside sources when possible. The International Committee of the Red Cross (ICRC) helped set up the first DOTS program in the prison system in 1995, treating over 300 patients in the central prison hospital. Despite strict compliance and 100 percent DOTS coverage in the penitentiary sector by 1998, many patients were found to be failing treatment.206 Studies later revealed that these patients were suffering from drug-resistant disease.207,208 DOTS was adopted as the national strategy by the Ministry of Health in 2005, after which the country reported 100 percent coverage. Despite this coverage, treatment success rates among DOTS patients were quite low because of increasing drug resistance. |
4.2
Participation of the private sector
In many countries (e.g. India and the Philippines) the private sector plays a significant and important role in the diagnosis and treatment of TB.209,210,211,212,213 While the private sector does offer the opportunity for treatment to many patients, the impact on TB control is mixed for a number of reasons: (1) private practitioners may deviate from international standards and use non-standard treatment regimens, which may contribute to treatment failure (with possible amplification of drug resistance) and death; (2) private practitioners often do not have the resources to provide adherence support (incentives and enablers) between clinic visits; and (3) patients often have to pay for follow-up sputum examinations and clinic visits, which is a disincentive to completing the entire treatment (6 months for drug-susceptible TB and 18 to 24 months for drug-resistant TB).
The relative proportion of MDR-TB treated by the private sector is not known because it often takes place outside of the reporting structures of NTPs. It is likely, however, to be quite substantial, simply because the capacity to diagnose and treat MDR-TB does not exist within the public sector in many countries. However, as in the treatment of drug-sensitive TB, the epidemiological impact of treatment in the private sector can be mixed. Even in middle-income countries where pulmonologists and chest specialists exist, they may not have expertise or experience with MDR-TB. The high cost of MDR-TB treatment is generally passed on to the patients, who cannot possibly afford to complete a full course of treatment (not to mention the ancillary care required to manage adverse events). MDR-TB treatment in the private sector is notoriously irregular; high default and failures rates are likely to be quite common and have been blamed in some settings for the creation of XDR-TB strains.
While technical assistance for the programmatic management of MDR-TB has been largely focused on NTPs, there are important potential lessons in the experience of PPM-DOTS (public-private mix DOTS). PPM-DOTS has been successful in several countries by pioneering collaboration between the NTP and private practitioners. In Manila, Philippines, the Tropical Disease Foundation and the Philippines National Tuberculosis Program are working to provide private practitioners with training about MDR-TB and current treatment guidelines, are providing assistance with the provision of DOT and patient supports, and are registering and following patients who initiate therapy outside of the national system. They have also begun to enlist private laboratories in MDR-TB-treatment expansion through training and supervision and the provision of external quality assurance.
Given that few countries have treatment programs that are run solely by NTPs, it is clear that engaging with the private sector is integral to ensuring proper treatment for both drug-susceptible and drug-resistant TB.
4.3
Transmission control
A major factor behind the growth of MDR-TB globally is transmission, much of which is occurring in congregate settings. The classic example in recent years is that of Russia, where the role of the prison system in fueling the TB epidemic is clear. In 1997 notification rates among the 1.1 million incarcerated people were 4000 per 100,000 population and among civilians 81.3 per 100,000 people. The 300,000 incarcerated people who are released each year move through the vast prison system relatively quickly, spending anywhere from three months to three years in various sectors. Among patients in the civilian sector, about 25 to 30 percent of new cases report a history of prior incarceration. Prisons have served as an “epidemiological pump” for transmitting resistant strains of TB.214 A similar phenomenon may be taking place in hospitals and clinics throughout the world, which are commonly crowded, poorly ventilated, and filled with highly-infectious TB patients. In one study performed at a large public hospital in Lima, Peru, 13 percent of the 250 patients admitted to the general medical ward had TB and 20 percent of those with TB had multi-drug resistance; 75 percent of MDR-TB patients had not been suspected of having TB at all when they entered the hospital.215 In a study of DOTS patients in Tomsk, Russia, hospitalization was found to be the greatest risk factor for the acquisition of MDR-TB.216 Similar findings have been noted elsewhere.217 The situation is exacerbated by the HIV epidemic in many countries and the increased risk of nosocomial transmission in health facilities.218 The XDR-TB epidemic in KwaZulu Natal, South Africa took place largely among HIV-infected patients who had been in congregate settings.219
|
Transmission control is possible in poor settings: an example from Lesotho220 Lesotho is a mountainous country located entirely within the borders of South Africa, with a population of approximately two million people. It has one of the highest reported rates of TB incidence in the world: 602 per 100,000 population in 2005, translating to over 10,000 reported cases per year.221 Approximately 10 percent of these patients are believed to have MDR-TB and a further 20 percent mono-and poly-drug-resistant TB. At least 25 percent of the population is already infected with HIV,222 with a TB-HIV co-infection rate estimated at between 76 and 92 percent.223 In 2006, after the XDR-TB outbreak in the Republic of South Africa (where an estimated 70 percent of working-age men migrate for employment), the Government of Lesotho formed a partnership with Partners In Health (PIH), the |
|
Foundation for Innovative New Diagnostics (FIND), and the WHO to create a national MDR-TB-treatment program. Although this new MDR-TB-treatment program was envisioned as primarily outpatient, it became clear very early in the planning process that, given the high level of HIV co-infection, malnutrition, and advanced TB disease, some patients will require hospital-level care. Once this need was identified, the Ministry of Health and Social Welfare (MoHSW) approved as part of this initiative the refurbishment of an unused leprosy hospital at Bostabelo, Maseru (the capital city). Guidance for renovation was obtained from an international infection control and engineering consultant working in South Africa. PIH staff were on-site and worked closely with the MoHSW, the Ministry of Planning, contractors and the engineering consultant to create an appropriate renovation plan and see it to completion. Prior to renovation, the facility was in reasonable physical shape, but had no adequate infection control mechanisms in place. Additionally, it lacked appropriate toilet and shower facilities, family or visiting areas, and a functional nurses’ station. A sophisticated ventilation system that meets international standards was installed at Botsabelo MDR-TB hospital to minimize the risk of infection transmission and cross-infection among the medical staff and patients. The refurbishment also included the creation of a family room for patients, separation of the TB Unit from a nearby HIV Unit on the same hospital grounds, updated toilet and shower facilities, and creation of a pleasant and humane environment, including an outdoor veranda and sitting area, for patients undergoing long-term treatment. A multi-year maintenance contract was established with the company that installed the equipment. Patients are stabilized at Botsabelo Hospital before being discharged to community-level care. This care is delivered by paid and carefully-trained treatment supporters who visit patients in their homes twice a day. These workers are provided with respirators. Patients with very advanced disease, those who are living long distances from health centers, or those who live in very crowded conditions are provided with furnished temporary accommodations near a public health center. |
Transmission control should not only be limited to facilities. Where possible, patients should be treated outside of congregate settings; this way, more patients can be treated with less risk of cross-transmission. Health workers delivering care to peoples’ homes, and even family members, need to be protected with properly designed and fitted respirators. Families should receive necessary assistance to ensure that patients are not living in overcrowded rooms with insufficient ventilation.
The case of Lesotho demonstrates that even in very poor countries, it is possible to have appropriate infection control. In order for this to happen, infection control needs to be a priority and assistance should be given to countries to facilitate this. The Lesotho example entailed outside resources and a fairly technologically sophisticated solution which may not be possible is many high-risk settings. However, every congregate setting where both TB and HIV are prevalent should employ a sound triage strategy coupled with the use of thoughtfully designed or renovated buildings. One such triage strategy, in use for a decade in Haiti, is described below.
|
Administrative and simple engineering controls make a difference: an example from Haiti In the Partners In Health site in Cange, Haiti, most TB is treated in the community. However, when hospitalization is required, patients can be separated into one of three settings based on the status of two readily obtained tests: the AFB smear and the HIV serology. Patients who are AFB-smear negative can be hospitalized on the general medical ward regardless of HIV status. The rationale is that the TB risk for HIV patients will be low if all AFB-smear positive patients are carefully excluded. Patients who are AFB-smear positive but HIV-negative are hospitalized on an especially well-ventilated TB ward equipped with upper room ultraviolet germicidal air disinfection. Finally, patients who are both AFB-positive and HIV-positive, who cannot be reasonably hospitalized on either the general medical ward or the TB ward, are assigned to one of the few simple isolation rooms, equipped with an exhaust fan and upper room ultraviolet germicidal air disinfection. More of these simple isolation rooms provide greater flexibility and can accommodate MDR or XDR cases, but the Cange Hospital in Haiti has functioned well with just 6 isolation rooms. This is not an ideal transmission control program, since smear-negative TB patients are known to transmit and undiagnosed TB cases may be on the general medical ward, but it is a vast improvement over the chaotic conditions of hospitalization commonplace in many parts of the world. Implementation of such a program is not resource intensive and should be considered a minimum standard for transmission control in hospitals without the resources or expertise to do what Lesotho was able to do. However, some expertise is still required in the design of general medical wards, TB wards, and simple isolation rooms to ensure that conditions are as safe for patients as staff and resources allow. As additional resources become available, programs can aspire to solutions like that implemented in Lesotho. |
5
RECOMMENDATIONS
5.1 Universal treatment for drug-resistant TB within national TB control stragies—side by side with drug-susceptible disease—has to be clearly and actively promoted by multilateral and bilateral agencies, non-governmental organizations, and within countries. Universal TB treatment also must be well integrated with current HIV treatment initiatives. This will entail being more pro-active in providing technical assistance and advising countries to rapidly build capacity for MDR-TB treatment and management. The successful example of DOTS scale-up can provide guidance for this approach. Because of the high risk of TB infection in patients with HIV, TB control strategies have to be integrated with HIV treatment initiatives.
5.2 The system of international technical assistance provision is currently inadequate. It must be transformed in order to better draw on the experience of successful regional MDR-TB-treatment programs, to include the provision of on-site, long-term technical assistance, and where necessary, to involve on-site implementation teams. With appropriate funding, such an approach will ensure that countries receive timely and appropriate technical assistance that can have a direct bearing on their scale-up plans. The regional Technical Assistance Center (TAC) Consortium being developed by the Core Group of the Stop TB Partnership’s MDR-TB Working Group is an important initial step to addressing this problem, but will not be sufficient on its own.
5.3 The Community/Ambulatory-based MDR-TB treatment, and where appropriate, active collaboration with private-sector laboratories and tuberculosis treatment providers, should be actively promoted as a safe means of rapidly treating the largest number of patients. Delivery systems that support this will need to be strengthened and/or built. There has to be a greater overt push for sound approaches to ambulatory care so that more patients can receive treatment at home and avoid spending extended periods of time in congregate settings. Additionally, the private sector should be engaged in all aspects of diagnosis and treatment in order to leverage national resources and optimize patient care.
5.4 Infection control to prevent transmission of TB strains has to be integrated fully into national TB-control strategies, with appropriate resources, training, implementation strategies, and monitoring. This means programmatic integration of engineering and administrative strategies to reduce of transmission; developing active triage and separation strategies for all settings; and an emphasis on protecting health workers from infection. The WHO, other multi-lateral and bilateral agencies, and international partners must increase the provision of technical assistance to strengthen transmission control, and ensure that it is a part of all funded projects.
5.5 Large global health initiatives—such as PEPFAR—and bilateral and institutional donors for global health should make improving the capacity to deliver MDR-TB treatment an important priority. The GFATM and UNITAID have done so, and others should follow this lead with their influence and resources. Programs such as PEPFAR have been phenomenally successful in delivering treatment to large numbers of patients infected with HIV. In areas with high TB-HIV co-infection, MDR-TB treatment needs to be better integrated into existing programs. Similarly, large donors should include active MDR-TB treatment delivery as a program priority.
SECTION V:
REFERENCES
1 World Health Organization. Global Tuberculosis Control: Surveillance, Finance, Planning. Geneva, World Health Organization, 2007.
2 Fox W. Compliance of patients and physicians: Experience and lessons from tuberculosis—I. British Medical Journal, 1983; 287, 33-35.
3 Kochi A. Tuberculosis control—is DOTS the health breakthrough of the 1990s? World Health Forum 1997;18(3-4), 225-32.
4 Floyd K, Wilkinson D, Gilks C. Comparison of cost effectiveness of directly observed treatment (DOT) and conventionally delivered treatement for tuberculosis: Experience from rural South Africa. British Medical Journal, 1997; 315, 1407-1411.
5 Chaulk CP, Moore-Rice K, Rizzo R, Chaisson RE. Eleven years of community-based directly observed therapy for tuberculosis. The Journal of the American Medical Association, 1995; 274(12), 945-951.
6 Weis SE, Slocum PC, Blais FX, King B, Nunn M, Matney Badakhshan, Gomez E, Foresman BH. The effect of directly observed therapy on the rates of drug resistance an relapse of tuberculosis. New England Journal of Medicine, 1993; 118, 139-145.
7 World Health Organization. Treatment of Tuberculosis: Guidelines for National Programmes. 3rd ed. Geneva, World Health Organization, 2003. WHO/CDS/2003.313.
8 Fox W. Whither short-course chemotherapy. British Journal Diseases of the Chest 1981; 75: 331-357.
9 Gupta R, Raviglione MC, and MA Espinal. Tuberculosis as a major global health problem in the 21st century: a WHO perspective. Seminars in Respiratory and Critical Care Medicine. 2004; 25(3): 245-53.
10 Dye C, Williams BG, Espinal MA, and MC Raviglione. Erasing the world's slow stain: strategies to beat multidrug-resistant tuberculosis. Science. 2002; 295(5562): 2042-6.
11 World Health Organization/International Union Against Tuberculosis and Lung Disease. Global Project on Anti-Tuberculosis Drug Resistance Surveillance. Anti-tuberculosis drug resistance in the world: report no. 4. Geneva, Switzerland: World Health Organization; 2008.
12 Iseman MD, Goble M. Multidrug-resistant tuberculosis. New England Journal of Medicine, 1996; 334(4), 167.
13 Espinal MA, Kim SJ, Suarez PG, et el. Standard short-course chemotherapy for drug-resistant tuberculosis: treatment outcomes in 6 countries. JAMA 2000;283:2537-2545.
14 Frieden TR, Fujiwara E, Washko R, Hamburg M. Tuberculosis in New York City—turning the tide. New England Journal of Medicine, 1995;333(4), 229-33.
15 Frieden TR, Sherman LF, Maw KL, et al. A multi-institutional outbreak of highly drug-resistant tuberculosis: epidemiology and clinical outcomes. JAMA 1996;276:1229-35.
16 Neville, K., A. Bromberg, R. Bromberg, S. Bonk, B.A. Hanna, and W.N. Rom. 1994. The third epidemic -multi-drug resistant tuberculosis. Chest 105:45-48.
17 Raviglione MC. Rieder HL. Styblo K. Khomenko AG. Esteves K. Kochi A. Tuberculosis trends in eastern Europe and the former USSR. [Journal Article] Tubercle & Lung Disease. 75(6):400-16, 1994 Dec.
18 Pablos-Mendes A, Raviglione MC, Laszlo A, Binkin N, Rieder HL, Bustreo F, Cohn DL, Lambregts-van Weezenbeek CS, Kim SJ, Chaulet P, Nunn P. Global surveillance for antituberculosis-drug resistance, 1994-1997. World Health Organization—International Union against Tuberculosis and Lung Disease Working Group on anti-tuberculosis drug resistance surveillane. New England Journal of Medicine, 2003; 338(23), 1641-1649.
19 Farmer PE, Kononets AS, Borisov SE, Goldfarb A, Healing T, McKee M. Recrudescent tuberculosis in the Russian Federation. In: The Global Impact of Drug-Resistant Tuberculosis. Boston, MA: Harvard Medical School and Open Society Institute, 1999.
20 Coninx R, Pfiffer G, Mathieu C. Drug resistant tuberculosis in prisons in Azerbaijan: case study. BMJ 1998; 316:1423-1425.
21 Kimerling M, Kluge H, Vezhnina N, Lacovazzi T, Demeulenaere T, Portales F et al. Inadequacy of the current WHO re-treament regimen in a central Siberian prison: treatment failure and MDR-TB. Int J Tuberc Lung Dis 1999; 3(5):451-453.
22 Centers for Disease Control and Prevention. Primary multi-drug-resistant tuberculosis—Ivanova Oblast, Russia, 1999. MMWR 1999; 48(30): 661-663.
23 Kimerling ME. The Russian equation: an evolving paradigm in tuberculosis control. Int J Tuberc Lung Dis 2000; 4 (Suppl 2): S160-S167.
24 Seung, KJ, Gelmanova, IE, Peremitin GG, et al. The effect of initial drug resistance on treatment response and acquired drug resistance during standardized short-course chemotherapy for tuberculosis. Clin Infect Dis 2004; 39(9):1321-8.
25 Coninx R, Mathieu C, Debacker M, et al. First-line tuberculosis therapy and drug-resistant Mycobacterium tuberculosis in prisons. Lancet 1999; 353: 969-973.
26 Cox HS, Niemann S, Ismailov G, et al. Risk of acquired drug resistance during short-course directly observed treatment of tuberculosis in an area with high levels of drug resistance. Clinical Infectious Diseases 2007; 44(11): 1421-7.
27 Bonnet, M, Sizaire, V, Kebede Y, et al. Does one size fit all? Drug resistance and standard treatments: results of six tuberculosis programmes in former Soviet countries. International Journal of Tuberculosis and Lung Disease. 2005; 9(10): 1147-54.
28 Faustini A, Hall AJ and CA Perucci. Tuberculosis treatment outcomes in Europe: a systematic review. European Respiratory Journal. 2005; 26(3): 503-10.
29 World Health Organization/International Union Against Tuberculosis and Lung Disease. Global Project on Anti-Tuberculosis Drug Resistance Surveillance. Anti-tuberculosis drug resistance in the world: report no. 4. Geneva, Switzerland: World Health Organization; 2008.
30 World Health Organization/International Union Against Tuberculosis and Lung Disease. Global Project on Anti-Tuberculosis Drug Resistance Surveillance. Anti-tuberculosis drug resistance in the world: report no. 4. Geneva, Switzerland: World Health Organization; 2008.
31 Li X, Zhang Y, Shen X, et al. Transmission of drug-resistant tuberculosis among treated patients in Shanghai, China. J Infect Dis 2007; 195: 864-9.
32 Mitchison, DA and AJ Nunn. Influence of initial drug resistance on the response to short-course chemotherapy of pulmonary tuberculosis. Am Rev Respir Dis 1986; 122:423-30.
33 Neville K, Bromberg A, Bromberg R, et al. The third epidemic –multi-drug resistant tuberculosis. Chest. 1994; 105:45-48.
34 Blower S, Small P and P Hopewell. Control strategies for tuberculosis epidemics: new models for old problems. Science 1996; 273: 497-500.
35 Cohen T and M Murray. Modeling epidemics of multidrug-resistant M. tuberculosis of heterogeneous fitness. Nat Med 2004; 10(10):1117-21.
36 Blower SM and T Chou. Modeling the emergence of the 'hot zones': tuberculosis and the amplification dynamics of drug resistance. Nat Med 2004; 10(10):1111-6.
37 Migliori GB, Espinal M, Danilova ID, et al. Frequency of recurrence among MDR-TB cases ‘successfully’ treated with standardised short-course chemotherapy. Int J Tuberc Lung Dis.2000;6(10):858-864.
38 Zignol, M, Hosseini, MS, Wright A, et al. Global incidence of multidrug-resistant tuberculosis. J Infect Dis 2006; 194: 479-85.
39 Zignol M, Wright A, Jaramillo E, et al. Patients with previously treated tuberculosis no longer neglected. Clinical Infectious Disease 2007; 44(1): 61-64.
40 Sisodia RS, Wares DF, Sahu S, et al. Source of retreatment cases under the revised national TB control programme in Rajasthan, India, 2003. International Journal of Tuberculosis and Lung Disease 2006; 10(12): 1373-9.
41 Frieden, TR, Fujiwara, PI, Washko RM, et al. Tuberculosis in New York City—turning the tide. New Engl J Med 1995; 333(4): 229-33.
42 Frieden TR, Sherman LF, Maw KL, et al. A multi-institutional outbreak of highly drug-resistant tuberculosis: epidemiology and clinical outcomes. JAMA 1996; 276: 1229-35.
43 Vailway, SE, Greifinder, RB, Papania M, et al. Multidrug-resistant tuberculosis in the New York state prison system, 1990-1991. J Infect Dis 1994; 170: 151-6.
44 Nardell E, McInnis B and B Thomas. Exogenous reinfection with tuberculosis in a shelter for the homeless. N Engl J Med. 1986; 315: 1570-3.
45 Beck-Sague, C, Dooley SW, Hutton MD, et al. Hospital outbreak of multidrug-resistant Mycobacterium tuberculosis infections: Factors in transmission to staff and HIV-infected patients. J Am Med Assoc 1992; 268: 1280-6.
46 Barnes PF, el-Hajj H, Preston-Martin S, et al. Transmission of tuberculosis among the urban homeless. J Am Med Assoc. 1996; 275: 305-7.
47 Pablos-Mendez, A, Raviglione MC, Battan R, et al. Drug resistant tuberculosis among the homeless in New York City. N Y State J Med 1990; 90: 351-5.
48 Kritski AL, Marques MJ, Rabahi MF, et al. Transmission of tuberculosis to close contacts of patients with multidrug-resistant tuberculosis. Am J Respir Crit. 1996; 153: 331-5.
49 Rullán, J, Herrera D, Cano R, et al. Nosocomial transmission of multidrug-resistant tuberculosis in Spain. Emerg Infect Dis 1996; 2: 125-9.
50 World Health Organization/International Union Against Tuberculosis and Lung Disease. Global Project on Anti-Tuberculosis Drug Resistance Surveillance. Anti-tuberculosis drug resistance in the world: report no. 4. Geneva, Switzerland: World Health Organization; 2008.
51 Gandhi NR, Moll A, Sturm AW, et al. Extensively drug-resistant tuberculosis as a cause of death in patients co infected with tuberculosis and HIV in a rural area of South Africa. Lancet 2006; 368: 1575-80.
52 World Health Organization. Treatment of Tuberculosis: Guidelines for National Programmes. 3rd ed. Geneva, World Health Organization, 2003. WHO/CDS/2003.313.
53 Espinal MA, Laserson K, Camacho M, et al. Determinants of drug-resistant tuberculosis: analysis of 11 countries. International Journal of Tuberculosis and Lung Disease. 2001; 5(10): 887-93.
54 Farmer PE. Managerial successes, clinical failures. International Journal of Tuberculosis and Lung Disease 1999; 3: 365-367.
55 Seung KJ, Gelmanova IE, Peremitin GG, et al. The effect of initial drug resistance on treatment response and acquired drug resistance during standardized short-course chemotherapy for tuberculosis. Clinical Infectious Diseases 2004; 39: 1321-8.
56 Raviglione MC, Gupta R, Dye C and MA Espinal. The burden of drug-resistant tuberculosis and mechanisms for its control. Annals of the New York Academy of Sciences 2001; 953: 88-97.
57 Gupta R, Kim JY, Espinal MA, et al. Responding to market failures in tuberculosis control. Science 2001; 293: 1048-1051.
58 World Health Organization. Guidelines for the Programmatic Management of Drug Resistant Tuberculosis. Geneva, World Health Organization, 2007. WHO/HTM/TB/2006.361
59 Mukherjee JS, Rich ML, Socci AR, et al. Programmes and principles in treatment of multidrug-resistant tuberculosis. Lancet 2004; 363: 474-81.
60 Nathanson E, Lambregts van Weezenbeek CSW, Rich MR, et al. Multidrug-resistant tuberculosis management in resource limited settings. Emerging Infectious Diseases. 2006; 12(9) 1389-1397.
61 Leimane V, Riekstina V, Holtz TH, et al. Clinical outcome of individualized treatment of multidrug-resistant tuberculosis in Latvia: a retrospective cohort study. Lancet 2005; 365(9456): 318-326.
62 Mitnick C, Bayona J, Palacios E, et al. Community-based treatment for multidrug-resistant tuberculosis in Lima, Peru. New England Journal of Medicine. 2003; 348(2): 119-28.
63 Tupasi T, Gupta R, Quelapio M, et al. Feasibility and cost-effectiveness of treating multidrug-resistant tuberculosis: a cohort study in the Philippines. Public Library of Science – Medicine. 2006; 3(9): e352.
64 Shin SS, Furin JJ, Alacantra F, et al. Long-term follow up for multidrug-resistant tuberculosis. Emerging Infectious Diseases. 2006; 12(4): 687-688.
65 Mitnick CD, Shin SS, Seung KJ et al. Comprensive Treatment of Extensively Drug-Resistant Tuberculosis. NEJM, August 7, 2008; 359(6): 563-574.
66 Keshavjee S, Gelmanova IY, Kim JY, Mishustin SP, Strelis AK, Andreev YG, Mukherjee JS, Pasechnikov AD, Atwood S, Rich ML, Furin JJ, Nardell EA, Farmer PE, Shin SS. Extensively drug resistant tuberculosis: Lessons from MDR-TB treatment scale-up in Tomsk, Russia. Lancet. August 25, 2008; 372(9639):early on-line publication.
67 Keshavjee S, Gelmanova I, Pasechnikov A, Mushustin S, Andreev Y, Yedilbayev A, et al. Treating Multi-Drug Resistant Tuberculosis in Tomsk, Russia: Developing programs that address the linkage between poverty and disease. Ann N Y Acad Sci 2007 Oct 22.
68 Raviglione MC and MW Uplekar. WHO’s new Stop TB Strategy. Lancet 2006; 367:952-5.
69 World Health Organzation. The Global MDR-TB and XDR-TB Response Plan. Geneva, Switzerland: World Health Organization; 2007. WHO/HTM/STB/2007.387.
70 World Health Organzation/Stop TB Partnership. Global Plan to Stop TB: 2001-2005. Geneva, Switzerland: World Health Organization, 2003. WHO/HTM/STB/2003.23.
71 World Health Organzation/Stop TB Partnership. Progress Report on the Global Plan to Stop TB: 2001-2005. Geneva, Switzerland: World Health Organization, 2004. WHO/HTM/STB/2004.29.
72 World Health Organzation/Stop TB Partnership. Global Plan to Stop TB: 2006-2015. Geneva, Switzerland: World Health Organization, 2006. WHO/HTM/STB/2006.38.
73 Amadottir T, Reider H, and D Enarson. Tuberculosis Programs: Review, Planning, Technical Support. International Union Against Tuberculosis and Lung Disease: Paris, 1998.
74 Squire, SB, Belaye, AK, Kashoti A, et al. 'Lost' smear-positive pulmonary tuberculosis cases: where are they and why did we lose them? Int J Tuberc Lung Dis 2005. 9 (1): 25-31.
75 Improving the Diagnosis of Tuberculosis through the Optimization of Sputum Microscopy. World Health Organization: Geneva, 2005.
76 Improving the Diagnosis of Tuberculosis through the Optimization of Sputum Microscopy. World Health Organization: Geneva, 2005.
77 Hamid Salim A, Aung KJ, Hossain MA, et al. Early and rapid microscopy-based diagnosis of true treatment failure and MDR-TB. Int J Tuberc Lung Dis, 2006. 10(11): p. 1248-54.
78 Pai M, Kalantri S and K Dheda. New tools and emerging technologies for the diagnosis of tuberculosis: part II. Active tuberculosis and drug resistance. Expert Rev Mol Diagn 2006; 6(3): 423-32.
79 Palomino, J.C., Newer diagnostics for tuberculosis and multi-drug resistant tuberculosis. Curr Opin Pulm Med, 2006. 12(3): p. 172-8.
80 New Technologies for Tuberculosis Control: A Framework for their Adoption, Introduction, and Implementation. World Health Organization: Geneva, 2007.
81 Aziz M, Ryszewska K, Blanc L, et al. Expanding culture and drug susceptibility testing capacity in tuberculosis diagnostic services: the new challenge. Int J Tuberc Lung Dis 2007. 11(3): p. 247-50.
82 Ridderhof, JC, van Deum, A, Kam KM, et al. Roles of laboratories and laboratory systems in effective tuberculosis programmes. Bull World Health Organ 2007; 85(5): p. 354-9.
83 Strategic Approach for the Strengthening of Laboratory Services for Tuberculosis Control: 2006-2009. World Health Organization: Geneva, 2006.
84 Blondal K. Barriers to reaching the targets for tuberculosis control: multidrug-resistant tuberculosis. Bull World Health Organ, 2007; 85(5): 387-90; discussion 391-4.
85 Raviglione MC and IM Smith. XDR tuberculosis--implications for global public health. N Engl J Med 2007; 356 (7): 656-9.
86 The Stop TB Strategy. Building on DOTS to Meet the TB-Related Millenium Development Goals. Stop TB Partnership: Geneva, 2006.
87 The Global MDR-TB & XDR-TB Response Plan 2007-2008. Stop TB Partnership: Geneva, 2007.
88 Migliori GB, Loddenkemper R, Blasi F and MC Raviglione. 125 years after Robert Koch's discovery of the tubercle bacillus: the new XDR-TB threat. Is "science" enough to tackle the epidemic? European Respiratory Journal 2007; 29:423-427.
89 The Global MDR-TB & XDR-TB Response Plan 2007-2008. In. Geneva: Stop TB Partnership; 2007.
90 Garner P, Alejandria M, and MA Lansang. Is DOTS-plus a feasible and cost-effective strategy? PLoS Med 2006;3 (9): e350.
91 Portero JL and M Rubio. Cost-effective control of drug-resistant TB: listening to other voices. PLoS Med 2006; 3 (12): e542.
92 World Health Organization (2008). Global Tuberculosis Control 2008: Surveillance, Planning, Financing. Available at http://www.who.int/tb/publications/global_report/2008/en/index.html (accessed on 11 August 2008).
93 Keshavjee, S, Seung, K, Satti H, et al. Building capacity for multidrug-resistant tuberculosis treatment: health systems strengthening in Lesotho. Innovations. 2007 Fall; 2(4):87-106.
94 Uplekar M, Pathania V and M Raviglione. Private practitioners and public health: weak links in tuberculosis control. Lancet 2000; 358(9285): 912-6.
95 Centers for Disease Control and Prevention. National plan for reliable tuberculosis laboratory services using a systems approach: recommendations from CDC and the Association of Public Health Laboratories Task Force on Tuberculosis Laboratory Services. Morbidity and Mortality Weekly Report 2005; 54(RR-6).
96 Pascopella L, Kellam S, Ridderhof J, et al. Laboratory reporting of tuberculosis test results and patient treatment initiation in California. J Clin Microbiol, 2004. 42(9): p. 4209-13.
97 Interim Recommendations for the Surveillance of Drug Resistance in Tuberculosis. World Health Organization: Geneva, 2007.
98 The Public Health Service National Tuberculosis Reference Laboratory and the National Laboratory Network: Minimum Requirements, Role and Operation in a Low-Income Country. International Union Against Tuberculosis and Lung Disease: Paris, 1998.
99 Guidelines for surveillance of drug resistance in tuberculosis. World Health Organization: Geneva, 2003.
100 Anti-tuberculosis drug resistance in the world: the WHO/IUATLD Global Project on Anti-tuberculosis Drug Resistance Surveillance. World Health Organization: Geneva, 1997.
101 Anti-tuberculosis drug resistance in the world: the WHO/IUATLD Global Project on Anti-tuberculosis Drug Resistance Surveillance, Report No. 2. World Health Organization: Geneva, 2000.
102 Anti-tuberculosis drug resistance in the world, third global report. World Health Organization: Geneva, 2003.
103 World Health Organization/International Union Against Tuberculosis and Lung Disease. Global Project on Anti-Tuberculosis Drug Resistance Surveillance. Anti-tuberculosis drug resistance in the world: report no. 4. Geneva, Switzerland: World Health Organization, 2008.
104 Stop TB Working Group on DOTS-Plus for MDR-TB Strategic Plan 2006-2015. World Health Organization: Geneva, 2005.
105 Zignol M, Hosseini MS, Wright A, Weezenbeek CL, Nunn P, Watt CJ, et al. Global incidence of multidrug-resistant tuberculosis. J Infect Dis 2006;194:479-485.
106 Gopinath K, Manisankar M, Kumar S, et al. Controlling multidrug-resistant tuberculosis in India. Lancet 2007. 369(9563): p. 741-2; author reply 742.
107 Dye C, Williams BG, Espinal MA, Raviglione MC. Erasing the world's slow stain: strategies to beat multidrug-resistant tuberculosis. Science. 2002 Mar 15;295(5562):2042-6.
108 de Gourville, E, Duintjer Tebbens RJ, Sangrujee N, et al., Global surveillance and the value of information: the case of the global polio laboratory network. Risk Anal 2006; 26(6):1557-69.
109 Featherstone D, Brown, D and R Sanders. Development of the Global Measles Laboratory Network. J Infect Dis, 2003; 187 Suppl 1: S264-9.
110 Cohen GM. Access to diagnostics in support of HIV/AIDS and tuberculosis treatment in developing countries. Aids 2007; 21 Suppl 4: S81-7.
111 Diagnostics for Tuberculosis: Global Demand and Market Potential. World Health Organization: Geneva, 2007.
112 Maher D, Dye C, Floyd K, et al. Planning to improve global health: the next decade of tuberculosis control. Bull World Health Organ 2007. 85(5): p. 341-7.
113 Ridderhof, JC, van Deun, A, Kam, KM, et al., Roles of laboratories and laboratory systems in effective tuberculosis programmes. Bull World Health Organ 2007; 85(5): 354-9.
114 Bates I and K Maitland. Are laboratory services coming of age in sub-Saharan Africa? Clin Infect Dis 2006; 42 (3): 383-4.
115 Muula AS and FC Maseko. Medical laboratory services in Africa deserve more. Clin Infect Dis 2006; 42(10): 1503.
116 Martin R, Hearn TL, Ridderhof J, et al. Implementation of a quality systems approach for laboratory practice in resource-constrained countries. Aids 2005. 19 Suppl 2: p. S59-65.
117 Dukes Hamilton, C, Sterling, TR, Blumberg HM, et al., Extensively drug-resistant tuberculosis: are we learning from history or repeating it? Clin Infect Dis 2007; 45(3): 338-42.
118 Harries AD, Zachariah R, Bergstrom K, et al. Human resources for control of tuberculosis and HIV-associated tuberculosis. Int J Tuberc Lung Dis 2005. 9(2): p. 128-37.
119 Chen, L, Evans, T Anand S, et al. Human resources for health: overcoming the crisis. Lancet 2004; 364(9449): 1984-90.
120 Narasimhan V, Brown H, Pablos-Mendez, A, et al. Responding to the global human resources crisis. Lancet 2004. 363(9419): p. 1469-72.
121 Hanvoravongchai, P. Scaling up health workforces in response to critical shortages. Lancet 2007; 370(9605): 2080-1.
122 Mullan F and S Frehywot. Non-physician clinicians in 47 sub-Saharan African countries. Lancet 2007; 370(9605): 2158-63.
123 Hongoro C and B McPake. How to bridge the gap in human resources for health. Lancet 2004; 364(9443): 1451-6.
124 Yagui M, Perales MT, Asencios L, et al. Timely diagnosis of MDR-TB under program conditions: is rapid drug susceptibility testing sufficient? Int J Tuberc Lung Dis 2006. 10(8): p. 838-43.
125 Petti, CA, Polage CR, Quinn TC, et al. Laboratory medicine in Africa: a barrier to effective health care. Clin Infect Dis 2006; 42(3): 377-82.
126 Blaya JA and HS Fraser. Development, implementation and preliminary study of a PDA-based tuberculosis result collection system. AMIA Annu Symp Proc 2006: 41-5.
127 Blaya, JA, Shin SS, Yagui MJ, et al. A web-based laboratory information system to improve quality of care of tuberculosis patients in Peru: functional requirements, implementation and usage statistics. BMC Med Inform Decis Mak 2007; 7: 33.
128 Global Tuberculosis Control 2007. Surveillance, Planning, Financing. World Health Organization: Geneva, 2007.
129 Rojpibulstit M, Kanjanakiritamrong J and V Chongsuvivatwong. Patient and health system delays in the diagnosis of tuberculosis in Southern Thailand after health care reform. Int J Tuberc Lung Dis 2006; 10(4):422-428.
130 Lonnroth K, Thuong LM, Linh PD and VK Diwan. Delay and discontinuity—a survey of TB patients’ search of a diagnosis in a diversified health care system. Int J Tuberc Lung Dis 1999; 3:992-1000.
131 Liam CK and BG Tang. Delay in the diagnosis and treatment of pulmonary tuberculosis in patients attending a university teaching hospital. Int J Tuberc Lung Dis 1997; 1:326-332.
132 Rajeswari R, Chandrasekaran V, Suhadev M, Sivasubramaniam S, Sudha G and G Renu. Factors associated with patient and health system delays in the diagnosis of tuberculosis in South India. Int J Tuberc Lung Dis 2002; 6:789-795.
133 Cheng G, Tolhurst R, Li RZ, et al. Factors affecting delays in tuberculosis diagnosis in rural China: a case study in four counties in Shandong Province. Trop Med Int health 2005; 99:355-362.
134 Lorent N, Mugwaneza P, Mugabekazi J, Gasana M, Van Bastelaere S, Clerinx J, et al. Risk factors for delay in the diagnosis and treatment of tuberculosis at a referral hospital in Rwanda. Int J Tuberc Lung Dis 2008; 12(4):392-396.
135 World Health Organization/International Union Against Tuberculosis and Lung Disease. Global Project on Anti-Tuberculosis Drug Resistance Surveillance. Anti-tuberculosis drug resistance in the world: report no. 4. Geneva, Switzerland: World Health Organization; 2008. Pg 14
136 Summary: Strategic Plans 2006 - 2015 of the Partnership, Working Group and Secretariat (Stop TB) http://www.stoptb.org/globalplan/plan_p3main.asp?p=3
137 The Global MDR-TB & XDR-TB Response Plan 2007-2008. In. Geneva: Stop TB Partnership; 2007. www.stoptb.org/resource_center/assets/documents/Global%20MDR-TB_and_%20XDR-TB_Response%20Plan_2007-08.pdf
138 Mattelli, Migliori, Cirillo, Centis, Girardi, Raviglioni Multi-drug Resistant and Extensively-drug Resistant Mycobacterium Tuberculosis: Epidemiology and Control. Future Drugs, 2007. Pg 865
139 World Health Organization, “Stop TB Partnership delivers treatments for 10 million people in six years” http://www.who.int/mediacentre/news/releases/2007/pr25/en/index.html
140 Interview with GDF, November 2007
141 Request for Proposals for second-line anti-TB Drugs Procurement Agent(s), August, 2006 Available: http://www.stoptb.org/gdf/newsevents/newsarchive.asp
142 Unitaid Homepage http://www.unitaid.eu
143 Unitaid Homepage http://www.unitaid.eu
144 Interviews with GLC representatives
145 Tropical Disease Foundation, Manila, Philippines. Permission for use of this material was obtained from Dr. Thelma Tupasi.
146 Interviews with GLC-approved project procurement managers
147 WHO list of prequalified medicinal products. http://healthtech.who.int/pq/ Site accessed on August 12, 2008.
148 World Health Organzation. The Global MDR-TB and XDR-TB Response Plan. Geneva, Switzerland: World Health Organization; 2007. WHO/HTM/STB/2007.387.
149 TB infections by country, 2003, WHO estimate
150 Russian Ministry of Health and Social Development 2007
151 Russian Ministry of Health and Social Development 2007
152 From IMS Health, conveyed to the Drug Management Sub-Committee of the Stop TB Partnership, MDR-TB Working Group
153 Keravec J. Implementation of a National Program for TB Drugs Quality Assuaranc in Brazil, Projeto MSH/Rational Pharmaceutical Management Plus Program (RPM Plus) Rio de Janeiro, Brazil 2007.
154 Currently offered through GDF, though not listed on GDF 2006 chart of medications
155 Stop TB Partnership, Global Drug Facility Drugs, Diagnostics, and other TB supplies, list of 2nd line drugs. http://www.stoptb.org/gdf/drugsupply/drugs_available.asp#2nd%20Line%20Drugs
156 Gupta R, Kim JY, Espinal MA, et al. Responding to Market Failures in Tuberculosis Control. Science 2001 Aug 10;293(5532):1049-51.
157 Interview, GDF November 2007
158 Interviews with pharmaceutical industry representatives.
159 From IMS Health, conveyed to the Drug Management Sub-Committee of the Stop TB Partnership, MDR-TB Working Group. Cited with permission from IMS Health and the WHO.
160 Feuer C (2006) Tuberculosis research and development: A critical analysis. Treatment Action Group. Available: http://www.aidsinfonyc.org/tag/tbhiv/tbrandd.pdf. Accessed 7 October 2007
161 Glickman et al. “A Portfolio Model of Drug Development for Tuberculosis” Science. March 3, 2006. pg 1246.
162 Ginsberg, Ann. “Emerging Drugs for Active Tuberculosis” Seminars in Respiratory and Critical Care Medicine. 2008. 29(5)
163 Sacks, Leonard and Behrman, Rachel E. “Developing new drugs for the treatment of drug-resistant tuberculosis: a regulatory perspective” Tuberculosis (2008) 88 Suppl 1.
164 Mitnick CD et al. Randomized trials to optimize treatment of multidrug-resistant tuberculosis. PLoS Med 4(11): e292. doi:10.1371/journal.pmed.0040292
165 Glickman et al. “A Portfolio Model of Drug Development for Tuberculosis” Science. March 3, 2006. pg 1246.
166 Farlow, Letter to Science, February 23, 2007; in response to “A Portfolio Model of Drug Development for Tuberculosis” Science. March 3, 2006. pg 1246.
167 This workshop was convened in Cambridge, Massachusetts, USA on June 10 to 12, 2008, by partners of the MDR-TB Working Group of the Stop-TB Partnership. It was sponsored and organized by: Boston University School of Public Health, International Union Against TB & Lung Disease, KNCV Tuberculosis Foundation, MDR-TB Working Group of the Stop-TB Partnership, Médecins Sans Frontières, Partners In Health/Harvard Medical School, Potts Memorial Foundation, Treatment Action Group, World Health Organization.
168 Sun Q, Santoro MA, Meng Q, et al. Pharmaceutical Policy in China. Health Affairs 2008 Jul-Aug;27(4):1042-50.
169 PSU Pharma Companiess May be Kept Out of Price Control for Now http://economictimes.indiatimes.com/Economy/PSU_pharma_cos_out_of_price_control/articleshow/3266374.cms
170 Ibid
171 Lilly MDR-TB Partnership Facts http://lillymdr-tb.com/facts.html
172 Interviews with Eli Lilly, Aspen Pharmacare October 2007- January 2008
173 Ibid.
174 Ibid.
175 Ibid.
176 Interviews with Eli Lilly, October 2007
177 Park SK, CT Kim and SD Song. Outcome of chemotherapy in 107 patients with pulmonary tuberculosis resistant to isoniazid and rifampicin. Int J Tuberc Lung Dis 1998. 2:877-884.
178 Telzak EE, Sepkowitz K, Alpert P, et al. Multidrug-resistant tuberculosis in patients in patients without HIV infection. New Engl J Med 1995.333:907-903.
179 Farmer PE, Kim JY, Mitnick CD, et al. Responding to Outbreaks of Multidrug-resistant tuberculosis: Introducing DOTS-Plus. In: Tuberculosis: A comprehensive International Approach, 2nd edition. 2000. Reichman L. and Hershfield ES ed. 447-69 Marcel Dekker, Inc. New York, NY.
180 Leimane V, Riekstina V, Holtz TH, et al. 2005. Clinical outcome of individualised treatment of multidrug-resistant tuberculosis in Latvia: a retrospective cohort study. Lancet 365(9456):318-26.
181 Farmer PE, Furin JJ and SS Shin. Managing multidrug-resistant tuberculosis. Journal of Respiratory Diseases 2000. 21(1), 53-56.
182 World Health Organization (2006). “Opportunities for Global Health Initiatives in the Health System Action Agenda,” Department of Health Policy, Development and Services Evidence and Information for Policy, World Health Organization (WHO) Working Paper No. 4.
183 Coker RJ, Atun RA and M McKee. Health-care system frailties and public health control of communicable disease on the European Union's new eastern border. Lancet 2004. 363(9418):1389-92.
184 See: Global Fund, eleventh board meeting (28-30 September 2005). “Report of the Technical Review Panel and the Secretariat on Round Five Proposals,” <http://www.theglobalfund.org/en/files/about/technical/report/Round_5_TRP_Report.pdf > (accessed 2 Jan 2008).
185 Opportunities for Global Health Initiatives in the Health System Action Agenda, Department of Health Policy, Development and Services Evidence and Information for Policy, World Health Organization (WHO) Working Paper No. 4. 2006.
186 Keshavjee S, Gelmanova I, Pasechnikov A, Mushustin S, Andreev Y, et al. “Treating Multi-Drug Resistant Tuberculosis in Tomsk, Russia: Developing programs that address the linkage between poverty and disease,” Ann N Y Acad Sci. 2007 Oct 22; epub ahead of print.
187 Keshavjee S, Seung K, Satti H, Furin J, Farmer P, Kim JY, Becerra M. Building capacity for multidrug-resistant tuberculosis treatment: health systems strengthening in Lesotho. Innovations 2007 Fall; 2(4):87-106.
188 World Health Organization. 1999. Global Tuberculosis Control, WHO Report 1999. Geneva: World Health Organization.
189 Nardell E. Tuberculosis in homeless, residential care facilities, prisons, nursing homes, and other close communities. Semin Respir Infect 1989.4:206.
190 Moore M, McCray E and I Onorato. Cross matching TB and AIDS registries: TB patients with HIV coinfection, United States, 1993-1994. Publ Health Rep 1999.114:269-77.
191 Murray J. Tuberculosis and human immunodeficiency virus infections during the 1990s. Bull Int Union Tuberc Lung Dis 1991; 66:21-5.
192 Sumartojo, E. When tuberculosis treatment fails: a social behavioral account of patient adherence. Am Rev Respir Dis 1993. 147:1311-20.
193 World Health Organization/International Union Against Tuberculosis and Lung Disease. Global Project on Anti-Tuberculosis Drug Resistance Surveillance. Anti-tuberculosis drug resistance in the world: report no. 4. Geneva, Switzerland: World Health Organization; 2008.
194 Gupta R, Kim JY, Espinal MA, et al. Responding to market failures in tuberculosis control. Science. 2001; 293: 1048-1051.
195 Nathanson E, Lambregts van Weezenbeek CSW, Rich MR, et al. Multidrug-resistant tuberculosis management in resource limited settings. Emerging Infectious Diseases. 2006; 12(9)1389-1397.
196 Leimane V, Riekstina V, Holtz TH, et al. Clinical outcome of individualised treatment of multidrug-resistant tuberculosis in Latvia: a retrospective cohort study. Lancet. 2005; 365(9456): 318-326.
197 Mitnick C, Bayona J, Palacios E, et al. Community-based treatment for multidrug-resistant tuberculosis in Lima, Peru. New England Journal of Medicine. 2003; 348(2): 119-28.
198 Tupasi T, Gupta R, Quelapio M, et al. Feasibility and cost-effectiveness of treating multidrug-resistant tuberculosis: a cohort study in the Philippines. PLOS Med. 2006; 3(9): e352.
199 World Health Organization/International Union Against Tuberculosis and Lung Disease. Global Project on Anti-Tuberculosis Drug Resistance Surveillance. Anti-tuberculosis drug resistance in the world: report no. 4. Geneva, Switzerland: World Health Organization; 2008.
200 World Health Organzation/Stop TB Partnership. Global Plan to Stop TB: 2006-2015. Geneva, Switzerland: World Health Organization; 2006. WHO/HTM/STB/2006.38.
201 Keshavjee S., Gelmanova I., Pasechnikov A., Mushustin S., Andreev Y., et al. Treating Multi-Drug Resistant Tuberculosis in Tomsk, Russia: Developing programs that address the linkage between poverty and disease. Ann N Y Acad Sci. 2007 Oct 22; epub ahead of print.
202 PEPFAR Homepage. http://www.pepfar.gov/pepfar/press/81964.htm. Accessed November 20, 2008.
203 World Health Organization. Azerbaijan Tuberculosis Profile. In: WHO; 2007.
204 Holley J, Akhundov O, Nolte E. Health care systems in transition: Azerbaijan. In. Edited by WHO Regional Office for Europe on behalf of the European Observatory on Health Systems and Policies. Copenhagen; 2004.
205 Gozalov O. phone interview. In. phone interview ed: Rosenberg, Julie; 2007.
206 Holley J, Akhundov O, Nolte E. Health care systems in transition: Azerbaijan. In. Edited by WHO Regional Office for Europe on behalf of the European Observatory on Health Systems and Policies. Copenhagen; 2004.
207 Coninx R, Pfyffer GE, Mathieu C, Savina D, Debacker M, Jafarov F, et al. Drug-resistant tuberculosis in prisons in Azerbaijan: case study. Bmj 1998,316:1423-1425.
208 Pfyffer GE, Strassle A, van Gorkum T, Portaels F, Rigouts L, Mathieu C, et al. Multidrug-resistant tuberculosis in prison inmates, Azerbaijan. Emerg Infect Dis 2001,7:855-861.
209 World Health Organization. Involving Private Practitioners in Tuberculosis Control: Issues, Interventions, and Emerging Policy Framework. Geneva, World Health Organization, 2001. WHO/CDS/TB/2001.285.
210 Uplekar MW, Rangan S. Private doctors and tuberculosis control in India. Tubercle and Lung Disease. 1993; 74:332-337.
211 Uplekar MW, Juvekar SK, Parande DB, et al. Tuberculosis management in private practice and its implications. Indian Journal of Tuberculosis. 1996; 43: 19-22.
212 Uplekar M, Juvekar S, Morankar S, et al. Tuberculosis patients and practitioners in private clinics in India. International Journal of Tuberculosis and Lung Disease. 1998; 2: 324-329.
213 Singla N, Sharma PP, Singla R, Jain RC. Survey of knowledge, attitudes and practices for tuberculosis among general practitioners in Delhi, India. International Journal of Tuberculosis and Lung Disease. 1998; 2: 384-389.
214 Kimerling ME, The Russian equation: an evolving paradigm in tuberculosis control. Int J Tuberc Lung Dis 2000. 4(12 Suppl 2): p. S160-7.
215 Willingham FF, Schmitz TL, Contreras M, et al. Hospital control and multidrug-resistant pulmonary tuberculosis in female patients, Lima, Peru. Emerging Infectious Diseases 2001. 7(1): p. 123-127.
216 Gelmanova IY, Keshavjee S, Golubchikova VT, Berezina VI, Strelis AK, Yanova GV, Atwood S, Murray M. Barriers to successful tuberculosis treatment in Tomsk, Russia; non-adherence, default, and the acquisition of multi-drug resistance. Bull WHO Sep; 85(9): 703–711.
217 Li X, Zhang Y, Shen X, Shen G, Gui X, Sun B, Mei J, Deriemer K, Small PM, Gao Q. Transmission of drug-resistant tuberculosis among treated patients in Shanghai, China. J Infect Dis 2007 Mar 15: 195(6):864–9.
218 Wells CD, Cegielski JP, Nelson LJ, et al. HIV infection and mutlidrug-resistant tuberculosis--the perfect storm. The Journal of Infectious Diseases 2007. 196(supplement 1): p. S86-S107.
219 Basu S, Andrews JR, Poolman EM, et al. Prevention of nosocomial transmission of extensively drug-resistant tuberculosis in rural South African district hospitals: an epidemiological modeling study. Lancet, 2007. 370(9597): p. 1500-7.
220 Excerpted from: Keshavjee S, Seung K, Satti H, Furin J, Farmer P, Kim JY, Becerra M. Building capacity for multidrug-resistant tuberculosis treatment: health systems strengthening in Lesotho. Innovations. 2007 Fall; 2(4):87-106.
221 See: World Health Organization (2007) “WHO Report: Global Tuberculosis Control: Africa.” <http://www.who.int/tb/publications/global_report/2007/pdf/afr.pdf> (accessed 6 Jan 2008).
222 See: World Health Organization (September 2005). “Summary Country Profile for HIV/AIDS Treatment Scale-up: Lesotho.” http://www.who.int/hiv/HIVCP_LSO.pdf> (accessed 6 Jan 2008).
223 Ministry of Health and Social Welfare, Government of Lesotho, 2006