3
Composition and Components of Gestational Weight Gain: Physiology and Metabolism
Gestational weight gain (GWG) is a unique and complex biological phenomenon that supports the functions of growth and development of the fetus. Gestational weight gain is influenced not only by changes in maternal physiology and metabolism, but also by placental metabolism (Figure 3-1). The placenta functions as an endocrine organ, a barrier, and a transporter of substances between maternal and fetal circulation. Changes in maternal homeostasis can modify placental structure and function and thus impact fetal growth rate. Conversely, placental function may influence maternal metabolism through alterations in insulin sensitivity and systemic inflammation and thus influence GWG.
This chapter provides relevant background material on normal physiologic and metabolic changes that occur during pregnancy and are related to GWG. The discussion begins with a review of total and pattern of GWG in singleton, twin, and triplet pregnancies. Next, the unique chemi-
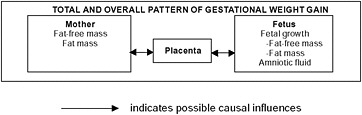
FIGURE 3-1 Schematic summary of components of gestational weight gain.
cal composition and accretion rates of maternal, placental, and fetal components of GWG are presented, followed by discussions of the maternal and fetal-placental physiology underlying weight gain in pregnancy. Lastly, pathophysiologic conditions that may adversely affect GWG are reviewed to provide a foundation for understanding changes in body weight and composition during pregnancy.
TOTAL AND PATTERN OF GESTATIONAL WEIGHT GAIN
Total Gestational Weight Gain
The total amount of weight gained in normal-term pregnancies varies considerably among women. Nevertheless, some generalizations can be made regarding tendencies and patterns of GWG in singleton and multiple pregnancies.
Singleton Pregnancies
An examination of studies published in the United States from 1985 to the present indicate that the mean total GWG of normal weight adult women giving birth to term infants ranged from a low of 10.0 to a high of 16.7 kg (Appendix C [Tables C-1A and C-1B] contains a tabular summary of the studies examined by the committee). Among adolescents, in general, GWG tended to be higher compared with adult women (means ranged from 14.6 to 18.0 kg in the studies examined). A consistent finding across studies was an inverse relationship between GWG and pregravid body mass index (BMI). Figure 3-2 illustrates a similar relationship with data derived from Abrams et al. (1986).
Since the release of the report Nutrition During Pregnancy (IOM, 1990) and its guidelines for GWG, a number of studies have examined GWG among overweight and obese women. Bianco et al. (1998) found that the mean GWG for 613 obese (BMI > 35) women averaged 9.1 ± 7.4 kg. Thirteen percent of the women, however, gained more than 16 kg, and 9 percent either lost or failed to gain weight. In a cohort study using birth certificate data from 120,251 obese women in Missouri, 18, 30, and 40 percent of the women gained < 6.8 kg in obese classes I, II, and III, respectively. The amount of total gain associated with minimal risk for preeclampsia, caesarean delivery, large-for-gestational age (LGA), and small-for-gestational age (SGA) outcomes was 4.6-11.4 and 0-4.1 for class I and II obesity, respectively; and weight loss of 0-4.1 kg for class III obesity (Kiel et al., 2007) (see Chapter 2 for definition of obesity classes).
A prospective study of a cohort of 245,526 Swedish women confirmed that GWG among obese women (BMI = 30-34.9) and very obese women
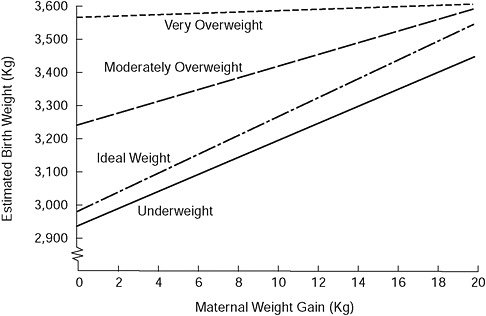
FIGURE 3-2 Birth weight as a function of maternal weight gain and prepregnancy weight for height.
SOURCE: Modified from Abrams and Laros (1986). This article was published in the American Journal of Obstetrics and Gynecology 154(3), Prepregnancy weight, weight gain, and birth weight, pp. 503-509. Copyright Elsevier (1986).
(BMI ≥ 35) was lower (11.1 and 8.7 kg, respectively) than among non-obese women (Cedergren, 2006). Low GWG (< 8 kg) occurred in 30.2 and 44.6 percent of the obese and very obese women, respectively. Among the 62,167 women in the Danish National Birth Cohort with data on GWG, about 36 percent of the obese women exhibited low rates of gain (0.28 kg per week). Fifty percent gained between 0.28 and 0.68 kg per week, and 14 percent gained > 0.68 kg per week (Nohr et al., 2007).
Obese women (BMI = 30-40) participating in a prenatal intervention gained less weight (adjusted GWG = 7.52 kg) than controls (adjusted GWG = 9.78 kg) and experienced no difference in pregnancy outcome (Claesson et al., 2008). In summary, from a population perspective, obese women as a group gain less weight than non-obese women, nevertheless GWG can vary widely.
Twin Pregnancies
Total GWG in twin pregnancies is generally higher than in singleton pregnancies with means ranging from 15 to 22 kg (Appendix C, Table C-2).
The cumulative weight gain stratified by pregravid BMI for mothers of twins born at 37-42 weeks of gestation and with an average twin birth weight ≥ 2,500 g is shown in Table 3-1. Cumulative and rates of weight gain by trimester are presented in Appendix C, Tables C-3A and C-3B.
Outcomes associated with GWG in twin pregnancies, as with single-ton pregnancies, are a function of pregravid BMI. Several studies have shown that, when stratified by pregravid BMI, increased GWG is associated with increased twin birth weight among underweight, normal weight, and overweight, but not obese, women (Brown and Schloesser, 1990; Luke et al., 1992; Lantz et al., 1996). Yeh and Shelton (2007) found that mean twin birth weights in the population studied increased incrementally from 2,237 g to 2,753 g for total GWG < 35, 35-45, 46-55, and > 55 pounds, respectively. The odds of having a twin delivery at ≥ 36 weeks gestation and birth weight ≥ 2,500 g were significantly lower among women who gained < 35 pounds (adjusted odds ratio [AOR] 0.49, 95% confidence interval
TABLE 3-1 Summary of Adjusted and Unadjusted* Cumulative Weight Gain, by Pregravid BMI Status for Mothers of Twins at Gestational Ages 37-42 Weeks, and with Average Twin Birth Weight > 2,500 g
|
Pregravid BMI |
Cumulative Weight Gain (To 37-42 weeks) |
Interquartile 25th-75th Percentile Ranges of Cumulative Weight Gain (To 37-42 weeks) |
||
|
kg |
lbs |
kg |
lbs |
|
|
Normal Weighta (n = 409) |
20.9 ± 0.3 |
45.9 ± 0.7 |
16.8-24.5 |
37-54 |
|
|
(21.0 ± 6.1)* |
(46.2 ± 13.4)* |
|
|
|
Overweightb (n = 154) |
18.9 ± 0.5 |
41.6 ± 1.1 |
14.1-22.7 |
31-50 |
|
|
(18.7 ± 7.0)* |
(41.1 ± 15.5)* |
|
|
|
Obesec (n = 143) |
15.7 ± 0.5 |
34.6 ± 1.2 |
11.4-19.1 |
25-42 |
|
|
(15.4 ± 7.2)* |
(34.0 ± 15.9)* |
|
|
|
NOTES: Results are presented as least square means ± standard error of mean (SEM) from models controlling for diabetes and gestational diabetes, preeclampsia, smoking during pregnancy, primiparity, and placental membranes (monochorionicity and missing chorionicity). Total cumulative gain is also adjusted for length of gestation. Results in parentheses are the unadjusted means ± standard deviation (SD) (also see Appendix C, Tables C-3A through C-3D). aBMI = 18.5-24.9 kg/m2. bBMI = 25.0-29.9 kg/m2. cBMI = ≥ 30 kg/m2. SOURCE: Historical cohort of twin births delivered at Johns Hopkins Hospital, Baltimore, Jackson Memorial Hospital, Miami, Medical University of South Carolina, Charleston, and University of Michigan, Ann Arbor, provided by Barbara Luke, Sc.D., M.P.H., R.D., and Mary L. Hediger, Ph.D. For more details on this historical cohort, see Luke et al. (2003). |
||||
[CI]: 0.37-0.65) and significantly higher among women who gained > 55 pounds (AOR 2.24, 95% CI: 1.51-3.33) compared to those who gained 35-45 pounds. Interestingly, GWG > 55 pounds was associated with an approximate 1.5 times greater likelihood of having a maternal complication (cumulative of gestational diabetes mellitus [GDM], pregnancy-induced hypertension, preeclampsia, and anemia [AOR 1.63, 95% CI: 1.02-2.60] or cesarean delivery [AOR 1.85, 95% CI: 1.20-2.87]).
In summary, GWG in twin gestations mirrors that in singleton pregnancies, i.e., there is an inverse relationship between maternal GWG and maternal prepregnancy BMI. These results suggest that a balance is needed between optimal GWG for maternal and twin outcomes.
Triplet and Quadruplet Pregnancies
Fewer studies are available on triplet and quadruplet pregnancies (Appendix C, Table C-2). Reported GWG among mothers carrying triplets ranged from 20.5 to 23.0 kg at 32-34 weeks and for quadruplets from 20.8 to 31.0 kg at 31-32 weeks (Luke, 1998). Total GWG in 38 triplet pregnancies was 20.2 kg at 33.4 weeks (Luke et al., 1995). The rate of gain was 0.48 kg per week before 24 weeks’ gestation and 0.96 kg per week after 24 weeks (Luke et al., 1995). Again, as with singleton and twin pregnancies, GWG is a function of BMI category; median gains were 15.5, 21.8, and 15 kg for low-, normal-, and high-BMI categories, respectively (Eddib et al., 2007).
Pattern of Gestational Weight Gain
The pattern of GWG is most commonly described as sigmoidal (Hytten and Chamberlain, 1991), but linear, concave, and convex patterns of weight gain have been observed as well (Villamor et al., 1998). The following discussion summarizes the committee’s review of the evidence on rate of GWG in singleton and twin pregnancies; observed relationships between GWG pattern and prepregnancy BMI; and birth weight outcomes associated with varying patterns of GWG in twin pregnancies.
Singleton Pregnancies
In the report Nutrition During Pregnancy (IOM, 1990) mean rates of GWG for well-nourished women with uncomplicated singleton pregnancies were reported as approximately 0.45 kg per week during the second trimester and 0.40 kg per week during the third trimester. Several studies, published since then indicate higher rates of weight gain in the second and third trimesters among American women with BMI values in the normal
range (Appendix C, Tables C-1A and C-1B). For example, the pattern of GWG by maternal BMI category was examined in a large cohort of women visiting the University of California, San Francisco clinics (Abrams and Selvin, 1995; Carmichael et al., 1997). Mean rate of gain was 0.169 kg per week in the first trimester. Mean weight gains were higher in the second (0.563 kg per week) than the third trimester (0.518 kg per week) in all groups except for obese women; and mean gains in the second and third trimester were higher in underweight and normal weight women than in overweight and obese women. Birth weight was correlated most strongly with gain in the second trimester (32.8 g/kg GWG versus 18 and 17 g/kg in the first and third trimesters, respectively).
In another study, mean rates of GWG in non-obese, low-income black and white women were 2.48 kg in the first trimester and 0.49 and 0.45 kg per week in the second and third trimesters, respectively (Hickey et al., 1995). In contrast, GWG rates among predominantly Hispanic women (n = 7,589) participating in the Prematurity Prevention Project were similar in the second (0.52 kg per week) and third trimesters (0.53 kg per week) (Siega-Riz et al., 1996); although the third-trimester gain was slightly lower in women who delivered preterm (0.50 vs. 0.53 kg per week). A similar GWG pattern has been observed in adolescents, although the median gain and rate of gain were higher throughout gestation; from mid-pregnancy to term, the rate of gain was 0.51 kg per week (Hediger et al., 1990).
In summary, the pattern of GWG is generally higher in the second trimester and is related to maternal pregravid BMI. However, pattern of GWG can vary depending on maternal ethnicity and age.
Twin Pregnancies
Luke and colleagues (1992) conducted a series of observational studies on outcomes associated with the rate of GWG in women with twin pregnancies who delivered infants at 37-42 weeks’ gestation and with mean birth weights exceeding 2,500 g. They (1992) found that low rate of GWG, defined as < 1.0 pound/week, was associated with a significant decrease in mean birth weight for twins compared to singletons (β, −0.137; p = 0.001). Significantly higher rates of GWG in the third trimester were observed among women whose mean birth weights for twins were ≥ 2,500 g compared to women with birth weights for twins that were < 2,500 g, regardless of BMI category; and no significant differences were seen for first and second trimester GWG rates.
Among a large multiethnic population of 646 twin pregnancies at ≥ 28 weeks’ gestation, birth weight increased by 14, 20, and 17 g for each pound of weight gained between 0 and 20 weeks’ gestation, 20 and 28 weeks’ gestation, and 28 weeks to birth, respectively (Luke et al., 1997). Mean total GWG
was 17.4 kg in a larger cohort of 1,564 twin births of > 28 weeks’ gestation from the same population (Luke et al., 1998). In a similar study, Luke et al. (2003) found that rates of GWG associated with optimal outcomes were greater for underweight and normal weight women than for overweight and obese women. These results are similar to those of singleton pregnancies.
COMPONENTS OF GESTATIONAL WEIGHT GAIN
As pregnancy progresses, protein, fat, water, and minerals are deposited in the fetus, placenta, amniotic fluid, uterus, mammary gland, blood, and adipose tissue (Figure 3-3). The products of conception (placenta, fetus, amniotic fluid) comprise approximately 35 percent of the total GWG (Pitkin, 1976). The extent to which these changes in body composition are critical for normal fetal development or are incidental to pregnancy is not completely understood.
Maternal Components of Gestational Weight Gain
The committee reviewed evidence on maternal total body water (TBW) accretion, fat-free mass (FFM) accretion (i.e., protein accretion), and fat mass (FM) accretion. Each of these maternal components of GWG exhibit
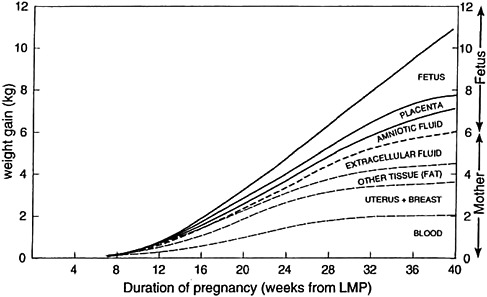
FIGURE 3-3 Components of gestational weight gain.
NOTE: LMP = last menstrual period.
SOURCE: Pitkin, 1976. Nutritional support in obstetrics and gynecology. Clinical Obstetrics and Gynecology 19(3): 489-513. Reprinted with permission.
unique patterns of accretion during pregnancy, with varying effects on outcome.
Total Body Water Accretion
Total body water accretion is largely under hormonal control and is highly variable during pregnancy. Across several studies, TBW accretion measured by deuterium or antipyrine tracers averaged about 7-8 liters (L) in healthy pregnancies (Hytten and Chamberlain, 1991). Expansion of the extracellular fluid (ECF) measured using the tracer sodium thiocyanate is estimated to be about 6-7 L. For a reference 12.5-kg GWG, total water gain at term is distributed in the fetus (2,414 g), placenta (540 g), amniotic fluid (792 g), blood-free uterus (800 g), mammary gland (304 g), blood (1,267 g), and ECF (1,496 g) with no edema or leg edema and ECF (4,697 g) with generalized edema (Hytten and Chamberlain, 1991). Maternal age, parity, and height did not affect the incidence of edema, but overweight women had greater generalized edema than underweight women. As pregnancy advances, plasma volume expansion measured using Evans blue dye increases up to 45 percent (Rosso, 1990); maternal plasma volume expansion correlates positively with birth weight. Monthly bioimpedance analysis (BIA) measurements in 170 healthy pregnant women confirmed the progressive expansion of TBW, intracellular water (ICW), and ECF during pregnancy (Larciprete et al., 2003). Larciprete et al. (2003) also found that total body water accretion was positively correlated with birth weight, in agreement with other investigations (Langhoof-Roos et al., 1987; Lederman et al., 1997; Mardones-Santander et al., 1998; Butte et al., 2003).
Fat-Free Mass: Protein Accretion
Protein is accrued predominantly in the fetus (42 percent), but also in the uterus (17 percent), blood (14 percent), placenta (10 percent), and breasts (8 percent) (Hytten and Chamberlain, 1991). Protein accrual occurs predominantly in late pregnancy. Protein deposition has been estimated from measurements of total body potassium (TBK) accretion derived by whole-body counting in a number of studies of pregnant women (King et al., 1973; Emerson et al., 1975; Pipe et al., 1979; Forsum et al., 1988; Butte et al., 2003). King et al. (1973) observed a rate of TBK accretion of 24 milliequivalents (meq) per week between 26 and 40 weeks’ gestation. Pipe et al. (1979) found a 312 meq potassium (K) increase. Lower increments of 110 and 187 meq at 36 weeks were found over pregravid values in two other studies (Forsum et al., 1988; Butte et al., 2003). Based on a potassium-nitrogen ratio in fetal tissues of 2.15 meq potassium/g nitrogen, the total protein deposition estimated from the longitudinal studies
of King et al. (1973), Pipe et al. (1979), Forsum et al. (1988), and Butte et al. (2003) is 686 g. A study of 108 black adolescents showed a mean rate of TBK accretion of 21 meq per week between 16 and 35 weeks’ gestation, consistent with adult studies (Stevens-Simon et al., 1997). In summary, these recent studies suggest that protein accretion may be less than the approximate (~1 kg) estimates of the earlier findings of Hytten and Chamberlin (1991).
Fat Mass: Fat Accretion
Based on serial measurements of skinfold thickness at seven sites made in 84 healthy, pregnant women, fat appears to be deposited preferentially over the hips, back, and upper thighs up to about 30 weeks’ gestation (Figure 3-4; Taggart et al., 1967). This pattern of fat deposition is unique to pregnancy.
Sohlstrom and Forsum (1995) used magnetic resonance imaging to show that the majority of fat deposited during pregnancy is subcutaneous. Based on estimates of fat deposition and distribution both before and after
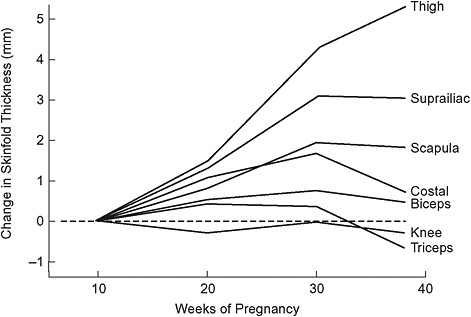
FIGURE 3-4 Longitudinal changes in skinfold thickness throughout pregnancy.
SOURCE: Taggart et al., 1967. Changes in skinfolds during pregnancy. British Journal of Nutrition 21(2): 439-451. Reprinted with the permission of Cambridge University Press.
pregnancy, they found that of the adipose tissue gained during pregnancy, 76 percent was deposited subcutaneously, similar to the fat distribution before pregnancy. Of the total fat deposition, 46 percent was in the lower trunk, 32 percent in the upper trunk, 16 percent in the thighs, 1 percent in the calves, 4 percent in the upper arms, and 1 percent in the forearms. Postpartum, fat was mobilized more completely from the thighs than the trunk, and non-subcutaneous fat in the upper trunk actually increased postpartum. Evidence obtained with computer tomography from 14 women suggests that childbearing may be associated with acquisition of visceral fat (Gunderson et al., 2008).
Measurement of fat mass during pregnancy is technically challenging because the usual methodology is imprecise, invalid, or not applicable to pregnancy. Skinfold measurements lack the precision necessary to estimate changes in fat mass accurately. Two-component body composition methods based on TBW, body density, and TBK are invalid during pregnancy because of the increased hydration of FFM that occurs during pregnancy; the constants for hydration, density, and K content of FFM used in two-compartment models are not applicable to pregnant women and would lead to erroneous estimations of FFM and FM. However, two-component models that use corrected constants for the hydration, density, and K content of FFM in pregnancy, as determined by van Raaij et al. (1988) and Hopkinson et al. (1997) are satisfactory for use with pregnant women, as are three-or four-component models (Fuller et al., 1992) in which the hydration or density of FFM is measured. Fat accretion models estimated in pregnant women using corrected two-component models or three- and four-component body composition are summarized in Appendix C, Table C-4.
Figure 3-5 shows a four-compartment body composition model of FM, TBW, protein, bone mineral, and non-osseous mineral measured by hydrodensitometry, deuterium dilution, and densitometry (dual energy X-ray absorptometry, DXA) (Lederman et al., 1997). When applied (after pregnancy) to 200 healthy women at 14 and 37 weeks of gestation, the model showed that obese women gained significantly less fat than underweight and normal weight women (8.7 vs. 12.6 and 12.2 kg, respectively). There were no differences in the amount of TBW gained among the underweight, normal weight or obese women. The majority of women studied did not conform to the recommendations of the Institute of Medicine (IOM) (1990). Sixty-seven percent of underweight, 61 percent of normal weight, 69 percent of overweight, and 78 percent of obese women gained outside the recommended ranges. Fat accretion paralleled GWG; FM gain was positively correlated with GWG (r = 0.81) and inversely correlated (r = −0.25) with pregravid weight. For those that gained within the IOM (1990) recommended ranges, FM gain was highest among the underweight (6.0 kg), followed by the normal weight (3.8 kg), overweight (2.8 kg), and obese
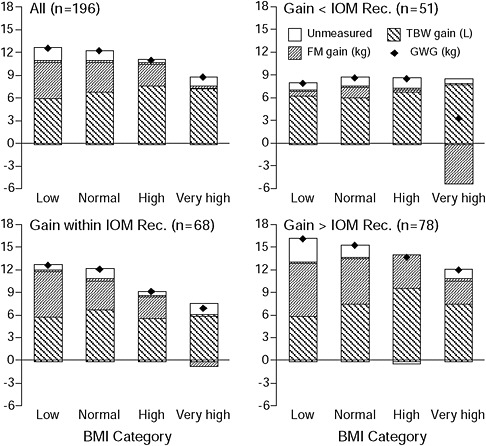
FIGURE 3-5 Body weight and composition changes in 196 women are presented by pregravid BMI category (low n = 21, normal n = 118, high n = 29, and very high n = 28). Gains in total body water and fat mass and gestational weight gain also are presented by compliance with the IOM 1990 recommendations for weight gain: women gaining less than (n = 51), within (n = 68), and more than (n = 78) the recommendations from IOM (1990).
SOURCE: Lederman et al., 1997.
(−0.6 kg). For those who gained less than the recommendations, FM gain was 0.6 kg in the underweight, 1.3 kg in the normal weight, 0.3 kg in the overweight, and −5.2 kg in the obese. For those that gained more than the recommendations, FM was highest in the underweight (6.9 kg), followed by the normal weight (6.0 kg), overweight (4.2 kg), and obese (3.1 kg).
Butte et al. (2003) used a four-compartment body composition model based on TBK, TBW, body volume, and bone mineral content measured by whole-body counting, deuterium dilution, hydrodensitometry, bone, and DXA (pre- and postgravid only) before pregnancy; at 9, 22, and 36 weeks Low Normal High er high
of gestation; and at 2, 6, and 27 weeks after delivery (see Figure 3-6). They also estimated protein accretion using prompt-gamma activation measurements of total body nitrogen (TBN) taken before and after pregnancy. They found total body K and TBN did not differ before and immediately after pregnancy but did decline postpartum. On average, weight gain was 42
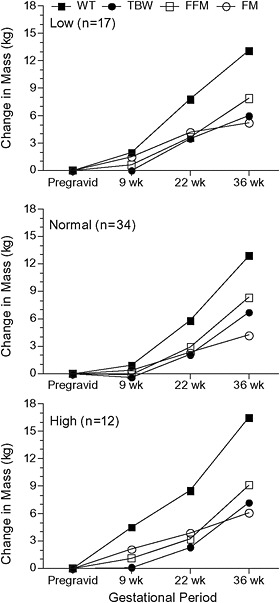
FIGURE 3-6 Changes in body weight and composition of 63 women (low pregravid BMI n = 17; normal pregravid BMI n = 34; high pregravid BMI n = 12) measured at 9, 22, and 36 weeks’ gestation.
SOURCE: Butte et al., 2003.
percent FM and 58 percent FFM. GWG was correlated linearly with gains in TBW (r = 0.39), TBK (r = 0.49), protein (r = 0.49), FFM (r = 0.50), and FM (r = 0.76). Gains in TBW, TBK, protein, and FFM did not differ among low-, normal- and high-BMI groups; only FM gain was higher in the high-BMI group who also gained more weight. The body composition changes in those women who gained (mean 14.4 kg) within IOM (1990) recommendations were TBW (7.1 kg), TBK (5.0 g), protein (370 g), FFM (8.4 kg), and FM (4.1 kg). Postpartum weight retention positively correlated with GWG and FM gain, but not with total TBW, TBK, or FFM gain. Postpartum FM retention positively correlated with GWG and FM gain. FM retention at 27 weeks’ postpartum was higher in those who gained above the recommendations (5.3 kg) than those that gained within (2.3 kg) and below (−0.5 kg) them. Birth weight was positively correlated with gains in weight, TBW, TBK, protein, and FFM, but not FM gain. Lederman et al. (1997) also found that maternal weight and FFM, but not FM, at term related to birth weight.
In summary, much of the variance in GWG is accounted for by the increase in fat mass, because that much of an increase in FFM also represents an increase in water. Similar to what was observed in GWG, the increase in fat mass during gestation is inversely proportional to pregravid obesity.
The relationships between accretion of maternal fat mass as a function of pregravid obesity may relate to pregravid maternal metabolic function. Catalano et al. (1998) measured for body composition, basal oxygen consumption (VO2), and insulin sensitivity in 16 healthy lean women before pregnancy and at 12-14 weeks and 34-36 weeks of gestation. In early pregnancy, women with abnormal glucose tolerance had smaller increases in FM (1.3 kg) and percentage FM (1.6 percent) compared to those with normal glucose tolerance (2.0 kg, 3.6 percent). Fat accretion did not differ from early to late gestation but changes in maternal insulin sensitivity were inversely correlated with changes in energy expenditure and FM accretion in early but not late pregnancy.
Placenta
The following discussion describes the committee’s review of the evidence on weight, compositional, and functional changes that occur during placental development and whether and how prepregnant BMI and obesity impact these changes.
Placental Weight
Molteni et al. (1978) demonstrated a linear relationship between fetal growth and placental mass, fetal weight, and placental growth in both early and late gestation; and a significant increase in the mean placental weight
and the fetal-placental weight ratio with advancing gestation in pregnancies that are appropriate-for-gestational age (AGA) and LGA. In infants that were born SGA, placental weight showed no increase after 36 weeks, but the fetal-placental weight ratio continued to increase. Therefore, although there may be further growth of the fetus, albeit not optimal, there is a lack of placental growth commonly referred to as placental insufficiency. The basis for altered placental growth and function may be related to a variety of pathologies such as nutritional, vascular (e.g., hypertension, diabetic vasculopathy), or anatomic disorders.
There are a limited number of cases of higher-order placental weights in higher multiples, but Pinar et al. (2002) published a series of reference weights from triplet pregnancies. See Table C-5 in Appendix C for normative criteria for placental weight in singleton, twin, and triplet pregnancies.
Placental Growth
Normal placental growth using human tissue is difficult to ascertain because placentas obtained from early pregnancy are often the result of an abnormal pregnancy outcome. Prior to 20 weeks, most placentas are obtained at the time of either spontaneous or elective termination. In mid-pregnancy, placentas are obtained after either a preterm delivery or placental dysfunction such as placenta previa or abruptio placenta. Abramovich (1969) was able to obtain placental weights at the time of abdominal hysterectomy with an intact amniotic sac. The average weight of the placenta at 10-12 weeks was 51 g, 12-14 weeks 66 g, 14-16 weeks 85 g, 16-18 weeks 110 g, and 18-20 weeks 141 g.
Because of the intrinsic problem of using cross-sectional data to determine normal placental growth, there developed an interest in the use of ultrasound to estimate placental growth using various volumetric measures. Bleker and Hoogland (1981) estimated placental volumes using longitudinal ultrasonographic techniques. Placental volume was 200 cm2 at 21 weeks’ gestation, 300 cm2 at 28 weeks, and 500 cm2 at term. The placental area was found to increase linearly until 24 weeks. There was a decreasing growth rate in the last trimester, although 15 percent of placentas showed a continuous increase through pregnancy.
Placental Development
Several specific structural and functional changes in placental development occur with advancing gestation. Teasdale (1980) described these changes in placentas delivered in healthy pregnancies between 22 and 40 weeks’ gestation. The first stage of placental growth, which lasts through 36 weeks, is characterized by increases in both the parenchymal and non-parenchymal tissue. The parenchyma is composed primarily of intravillous
space, the trophoblast tissue (i.e., cytotrophoblast, syncytiotrophoblast), and fetal capillaries of peripheral and stem villi. The non-parenchymal tissue is composed of the decidual and chorionic plates, intercotyledonary septa, fetal vessels, connective tissue, and fibrin deposits. The second phase of placental development, lasting from 36 weeks until term, is the maturation phase. The maturation phase of placental growth is characterized by an increase in fetal growth but without an increase in placental functional or parenchymal tissueI; only the non-parenchymal (i.e., nonfunctional) placental tissue increases. These changes are consistent with the early placental growth and development that occurs and is necessary for rapid fetal growth in the last trimester of pregnancy, when fetal weight increases from a mean of 1,000 g to 3,400 g (in the general U.S. population).
In addition to these changes, there also may be differences in placental function as a consequence of a women’s pregravid BMI. In general, obese women are more likely to have larger placentas and neonates in comparison to average-weight women. Alterations in maternal metabolic function during pregnancy are most likely mediated through placental hormone and cytokine production, which in turn affect maternal fat accretion and nutrient availability. Recently, Challier et al. (2008) reported that the placentas of obese women (pregravid BMI > 30 kg/m2) had a two- to three-fold increase in the number of macrophages in comparison with placentas of average weight (pregravid BMI < 25 kg/m2) women. There was also increased expression of the proinflammatory cytokines interleukin (IL-1), tumor necrosis factor-alpha (TNF-α), and IL-6. Hence, the chronic inflammation associated with obesity may affect placental growth and function, thereby altering maternal metabolic function and resulting in the women with pregravid obesity having decreased maternal pregravid maternal insulin resistance and decreased maternal fat accretion but increased placental and fetal growth.
Placental Composition
The composition of the placenta varies with gestational age as well as maternal metabolic status. Approximately 88 percent of placental weight is water. In comparison, the fetus at term has approximately 80 percent water in its fat-free mass. In studies of Widdowson and Spray (1951), the composition of placentas ranging from 17 to 40 weeks’ gestation was analyzed. The mean percentage of water was 88 percent, protein 11 percent, and fat 1 percent. Garrow and Hawes (1971) similarly reported that in more than 700 placentas, the blood-free placenta had approximately 10 percent protein. In a further analysis of the effect of maternal diabetes on placental composition, Diamant et al. (1982) described increased placental mass, amount of DNA, glycogen, and lipids in the placentas of women with diabetes compared to a normal glucose-tolerant control group. The
relative changes in glycogen and fat exceeded the changes in amount (mg) of DNA, suggesting that a true increase in glycogen and fat per placental cell may have occurred. The increase in lipids in the placenta of the women with diabetes consisted primarily of triglycerides and phospholipids but not cholesterol (see Table C-6 in Appendix C for placental lipid content).
Fetus
The optimal weight for a term infant is difficult to define. Not only are available methods for measuring fetal growth rate limited and prone to error, but fetal growth is impacted by a wide range of maternal physiological, lifestyle, and other factors. The following discussion summarizes the committee’s review of the evidence on patterns of fetal growth in singleton and multiple pregnancies and factors that alter those patterns. This information provides a foundation for understanding some of the physiological determinants of GWG identified and discussed in Chapter 4.
Patterns of Fetal Growth for Singletons, Twins, and Triplets
Singletons With the exception of longitudinal studies using methods such as ultrasound, all measures of fetal growth are cross-sectional by definition (i.e., each fetus having been measured only once) (Hytten and Chamberlain, 1991). The criteria that are commonly used are to classify fetal growth are:
-
SGA (i.e., birth weight less than the 10th percentile for gestational age);
-
AGA (i.e., birth weight between the 10th and 90th percentile for gestational age); and
-
LGA (i.e., birth weight greater than the 90th percentile for gestational age).
These criteria were arbitrarily chosen to help assess the neonatal risk for both short-term and, more recently, long-term morbidity. Since that time there have been numerous other publications relative to fetal growth rates.
For the fetus that is deemed viable, fetal weight, as a measure of fetal growth, is usually determined at the time of delivery. The gestational age of viability has decreased steadily over the years, and the fetus is now considered potentially viable at 23-24 weeks. Therefore, most of the fetal growth curves relating to viable fetuses rely on clinical data starting from the mid-second trimester. Although the numbers are small, there appears to be minimal variation in fetal growth through 25 weeks’ gestation (Archie et al., 2006).
Recently, Thomas et al. (2000) compared gestation-specific growth parameters derived from data on 27,229 neonates from 85 nurseries with parameters developed in the late 1960s. For neonates at < 30 weeks’ gestation, there were smaller variances and lower average weights, lengths, and head circumferences than previously published norms. For neonates > 36 weeks’ gestation, the variance was similar, but the neonates were larger and heavier. The authors concluded that using older growth curves resulted in misclassification of gender- and race-specific criteria for SGA and LGA. Since then, many investigators have observed an increase in birth weight at term (Orskou et al., 2001; Ananth and Wen, 2002; Surkan, 2004; Catalano et al., 2007). Hence, the use of current birth weight curves is important in the assessment of fetal growth. Oken et al. (2003) published U.S. birth weight curves based on the 1999 and 2000 United States Natality datasets from 22 through 44 weeks’ gestation.
Although gestational age is an important factor related to fetal growth, other factors affect not only fetal growth but also the pattern of growth. These include gender, with males growing more rapidly from the mid-third trimester through term (Figure 3-7); and maternal age, height, weight, GWG, obesity, and parity (Catalano et al., 2007). Paternal factors can also affect fetal growth, although they explain much less of the variance than maternal factors do (Klebanoff et al., 1998). High altitude results in decreased fetal growth, as does maternal hypoxia. Maternal medical problems, e.g., hypertensive disorders, autoimmune disease, and smoking can also result in decreased fetal growth. In contrast, maternal diabetes without evidence of vascular involvement often results in increased fetal growth (see Chapter 4 for detailed discussion).
The question of ethnic differences in fetal growth and implications for neonatal health has become more relevant recently. Kierans et al. (2008) evaluated all births in British Columbia from 1981 through 2000 and examined fetal growth and perinatal mortality in Chinese, South Asian, First Nation (Native American Indian), and other (primarily Caucasian) populations. They concluded that the ethnic differences in fetal growth rates were physiologic, not pathologic.
The rate of premature delivery (i.e., before 37 weeks’ gestation) in the United States is approximately 12.5 percent. As such, birth weight tables that rely on actual neonatal weights for preterm infants represent a much smaller percentage of all births. Furthermore, there is evidence that infants born prematurely are smaller than infants of the same gestational age who remain in utero (Weiner et al., 1985).
In summary, normal fetal growth is relatively uniform until mid-second trimester. At term there is much greater variation in fetal weight as a result of varying determinants of GWG and other maternal factors (see Chapter 4 for complete discussion). Lastly, there has been an increase in term birth
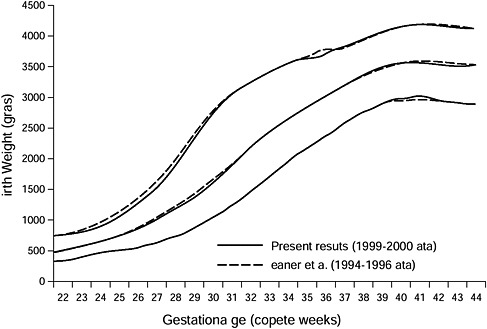
FIGURE 3-7 Select reference percentiles for birth weight at each gestational age from 22 to 44 completed weeks for all singleton infants.
SOURCE: Oken E., K. P. Kleinman, J. Rich-Edwards and M. W. Gillman. 2003. Reprinted with permission from BMC Pediatrics 3: 6, by BioMed Central.
weight in developed countries over the past two decades, most likely because of the increased prevalence of obesity.
Twins and triplets Fetal growth in multiple gestations is very similar to singleton growth until the third trimester. Although there is a tendency to consider multiple gestations as being growth restricted, Blickstein (2002) described the fetal mass of a multiple pregnancy as “growth promoted” and the smaller size of the fetus as “growth adapted.” In addition to previously discussed variables that may affect fetal growth, such as gender and parity, in twin gestations chorionicity may also affect fetal growth. Ananth et al. (1998) reported that twins from monochorionic gestations weigh on average 66 g less than those from dichorionic gestations after correction for gestational age.
Gielen et al. (2007) reported on customized birth weight charts in more than 4,277 twin pairs in Flanders from 1964 through 2002. In their study, birth weight was affected by maternal parity and age. Zygosity, fetal gender, chorionicity, fusion of the placentas, placental weight, and site of
umbilical cord insertion all influenced twin birth weights. These variables can account for as much as a 1,000 g difference in weight at term. After 40 weeks’ gestation, there is a decrease in weight of twins with a monochorionic monozygotic placentation, while dichorionic dizygotic twins continue to grow. Min et al. (2000) estimated growth in 1,831 twin pregnancies using ultrasound at 2-week intervals from 20 through 40 weeks’ gestation. The weight difference between twins and singleton pregnancies at their respective 50th percentiles was 147 g (10 percent) at 30 weeks’ gestation, 242 g (14 percent) at 32 weeks’ gestation, 347 g (17 percent) at 34 weeks’ gestation, 450 g (19 percent) at 36 weeks’ gestation, 579 g (22 percent) at 38 weeks’ gestation, and 772 g (27 percent) at 40 weeks’ gestation.
Lastly, Glinianaia et al. (2000) reported on 690 triplets born in Norway from 1967 through 1995. The birth weight by gestational age curves of the triplets were almost identical to those of singleton and twin gestations before 30 weeks. From 31 weeks of gestation onward, the median birth weight of triplets consistently diverged from that of twins. At 38 and 39 weeks’ gestation the difference reached 478 and 541 g, respectively, with a weight difference between twins and triplets of 650 g in the 10th percentile at 39 weeks.
In summary, the growth rate in multiple gestations is similar to growth rate in singleton gestations up to approximately 30 weeks’ gestation. In the third trimester, there is a decrease in individual fetal growth, more so in triplets than in twins, which may be related to placental function.
Fetal Body Composition
The human fetus at term has a significantly different body composition than most other mammalian species. At birth the human fetus has approximately 12-16 percent body fat. In contrast, laboratory animals have 1-2 percent body fat at birth (Widdowson, 1950). Using DXA, Koo et al. (2000) found that among the 214 singletons studied, neonates whose birth weight was < 2,500 g had 6 to 14 percent body fat. Neonates whose birth weight was > 2,500 g had 8 to 20 percent body fat. The mean percentage of body fat for a 3,500-g infant was 16.2 percent. Using total body electrical conductivity, Catalano et al. (2003) reported that body fat was 10.4 ± 4.6 percent in 220 term healthy singleton neonates. The difference in results between the two studies primarily represents differences in methodologies. Theoretically, the accrual of fetal fat has two possible sources: one is from the transfer of free fatty acids from the mother, and the second is de novo synthesis of fatty acids from substrates such as glucose, lactate, and acetate provided by the mother (Girard and Ferre, 1982). Regardless of the substrate source, fetal insulin is required for the fetus to increase adipose tissue stores.
The remaining tissue in the human fetus is lean body mass or FFM, which consists primarily of glycogen, protein, and water. At birth the human fetus has approximately 40 g of glycogen, primarily in muscle and liver tissue (Girard and Ferre, 1982). The protein content of the term fetus is approximately 12.8 percent of total body weight, or 15 percent of FFM (i.e., about 500 g; Fomon et al., 1982; Spady, 1989). The remainder is water. In the human fetus at term, approximately 80 percent of FFM is water (Fomon et al., 1982).
With respect to temporal changes in fetal growth rates, generally the human fetus weighs approximately 1 kg at 28 weeks and then, over the next 12 weeks, gains approximately 2.5 kg. In the mid-second semester, fetal fat tissue begins to accrue and FFM as a percentage of total body weight begins decreasing. The gold standard for estimating fetal body composition is carcass analysis, although investigators have also used ultrasound to characterize the changes in composition that occur during gestation. Sparks (1984), reviewed data from 169 carcass analyses of fetuses and concluded that the differences in FFM are less variable than fat content at each gestational age. Changes in fetal FM may reflect changes in the intrauterine environment, while changes in FFM may be more representative of genetic factors. Bernstein et al. (1997) found that, although the rate of fetal FFM accretion appeared linear when considered in aggregate, the compartments of FFM changed differentially. Specifically, peripheral muscle growth accelerated and head circumference decelerated in late gestation; fetal fat deposition accelerated as a quadratic function. Hence, fetal growth of FM and FFM follow unique patterns and offer an additional means to assess normal and abnormal growth.
With respect to any observed association between neonate body composition and changes in maternal body composition, Butte et al. (2003) found that infant body composition at 2 weeks of age (FFM, FM, or percent FM) was not correlated with maternal body composition before or after pregnancy or with maternal gains in TBW, TBK, FFM, and FM during pregnancy. The investigators used DXA to assess body composition in 63 term singletons and related these changes to maternal body composition measured using a multi-component model. While neonate body composition bore no association with any other measured factor, birth weight correlated positively with prepregnancy weight (r = 0.34), prepregnancy FM (r = 0.32), GWG (r = 0.35), net GWG (r = 0.26), rate of weight gain (r = 0.28), gestational age (r = 0.49), gestational gains in TBW (r = 0.37), TBK (r = 0.35), and FFM (r = 0.39), but not FM. The investigators used multiple regression analysis to show that maternal FFM gains in the first, second, and third trimesters each independently contributed to birth weight, as did maternal TBW gains during the second and third trimesters and maternal TBK gain in the third trimester.
As with fetal growth patterns, multiple factors are associated with alterations in fetal body composition, including:
-
genetic (e.g., at birth, male fetuses have greater lean body mass than females, and as a consequence, females have a higher percentage of body fat (Catalano et al., 1995; Ibanez et al., 2008);
-
maternal parity, which is positively correlated with neonatal adiposity (Harvey et al., 2007);
-
prepregnancy BMI, with birth weight significantly greater in neonates of overweight and obese women than underweight or normal weight women because of increased FM, not FFM (Sewell et al., 2006; Hull et al., 2008);
-
maternal weight gain, which is associated with both increased fetal FFM and increased FM (and maternal pregravid BMI [Catalano and Ehrenberg, 2006]);
-
maternal medical problems, such as gestational diabetes mellitus (GDM), that are associated with an increase in birth weight (again because of increased FM, and in the macrosomic neonate a relative decrease in FFM) (Catalano et al., 2003; Durnwald et al., 2004);
-
environmental factors (see Chapter 4) such as maternal smoking which has a negative effect on fetal growth on the order of 150 g, which primarily decreases fetal FFM (Lindsay et al., 1997); and
-
increased altitude, which has been reported to be associated with a 339-g decrease in birth weight (Ballew and Haas, 1986, showed that crown-head length was reduced by 1 cm, although the sum of five skinfolds was 5 mm greater, in those born at high altitude compared to those born at sea level).
In their study of > 400 newborns using total body electrical conductivity, Catalano and Ehrenberg (2006) found that maternal pregravid BMI had a stronger correlation with fetal adiposity than maternal weight gain and GDM did.
In summary, the human fetus has a high percentage of body fat (12-16 percent) at birth compared to most mammalian species. Fetal fat mass contributes the greatest percentage of variance in birth weight, is affected by the in utero environment, and is more strongly correlated with maternal pregravid BMI than GWG.
Amniotic Fluid
The committee reviewed evidence on amniotic fluid volumetric changes in gestation and determined that amniotic fluid is an important component of GWG. There are four major sources of volume flow into and out of
the amniotic sac in late gestation (Ross and Brace, 2001). The two major inflow sources are fetal urine and lung liquid secretions. The two major outflows are fetal swallowing and intra-membranous absorption. Brace and Wolf (1989) reported on a series of 705 published amniotic fluid volumes derived from either direct collection or dye dilution techniques. At 8 weeks of gestation, amniotic volume increases at a rate of 10 mL per week, and at 13 weeks the rate increases to 25 mL per week. The maximal increase in amniotic fluid of 60 mL per week occurs at 21 weeks’ gestation. The weekly volume increment then decreases and reaches zero at 33 weeks’ gestation (i.e., the time at which maximal volume is reached).
There is wide variation in the amount of amniotic fluid in a normal pregnancy. Decreased amniotic fluid (i.e., oligohydramnios) occurs in approximately 8.2 percent of pregnancies, and increased amniotic fluid (i.e., polyhydramnios) occurs in approximately 1.6 percent of pregnancies (Ross and Brace, 2001). Oligohydramnios may occur as a consequence of fetal renal obstruction or dysplasia and may be associated with fetal growth restriction. Polyhydramnios is associated with various fetal structural anomalies such as congenital esophageal atresia, fetal anemia, congenital infections, and maternal diabetes. Given the wide range of normal amniotic fluid volume at term, this compartment may affect maternal GWG by as much as 1 kg.
MATERNAL PHYSIOLOGY
Understanding the unique physiologic, metabolic, and endocrine milieu of the pregnant woman is crucial to understanding the mechanisms underlying GWG. The pregnant woman undergoes dramatic physiologic changes in anticipation and in support of fetal growth. Changes in many of the obligatory components of GWG (for example, TBW) are directly related to the alterations in maternal physiology that must occur for a healthy fetus and placenta to grow and develop. When the evidence permitted, the committee considered how physiological changes impact GWG and neonatal outcome. As with other information in the chapter, the findings summarized here provide a foundation for understanding the physiological predictors of GWG and identifying ways to intervene.
Cardiovascular Changes
In early pregnancy, cardiac output increases about 30-50 percent as a result of an increase in heart rate—primarily stroke volume—and remains elevated until term (Hytten and Chamberlain, 1991). As pregnancy progresses, blood flow increases to the uterus, kidney, skin, and probably the alimentary tract. Arterial blood pressure may decrease in mid-pregnancy as a result of increased peripheral vasodilatation and in order to maintain
perfusion; this results in an increase in cardiac output and a relatively small decrease in mid-gestational blood pressure. Venous blood pressure rises in the lower limbs due to mechanical and hydrostatic pressure in the pelvis, causing edema in the lower limbs. Because of these cardiovascular changes, it is possible to have reduced exercise tolerance and dyspnea.
Physiological changes in circulation during pregnancy are marked and variable (Gabbe et al., 1991; Hytten and Chamberlain, 1991). Plasma volume increases progressively to 50 percent by 30-34 weeks of gestation. Importantly, plasma volume expansion is correlated with clinical performance and birth weight. Poor plasma volume expansion is associated with a poorly growing fetus and poor reproductive performance. The increases in maternal plasma volume account for a significant portion of the increase in total body water during pregnancy.
Red blood cell mass also increases about 18 percent by term without iron supplementation and 30 percent with iron supplementation. Minute ventilation increases 30-40 percent by late pregnancy due to increased tidal volume. Oxygen consumption increases only 15-20 percent, resulting in an increase in alveolar and arterial PAO2 (partial pressure of oxygen) and a fall in PACO2 (partial pressure of carbon dioxide) levels (Gabbe et al., 1991).
Renal Changes
Renal plasma flow increases 70 percent over pregravid levels by 16 weeks of gestation and is maintained until late pregnancy when it falls slightly (Gabbe et al., 1991). Glomerular filtration rate (GFR) increases early in pregnancy, up to 50 percent by term. As a result of the increased GFR, serum levels of urea and creatinine decline. Plasma osmolarity declines early in pregnancy due to a reduction in serum sodium and associated anions. There is a net accumulation of approximately 900-1,000 meq of sodium in the fetus, placenta, and intravascular and interstitial fluids. There is a large increase in tubular sodium reabsorption during pregnancy, promoted by increased aldosterone, estrogen, and deoxycorticosterone. Plasma renin activity, renin substrate, and angiotensin levels increase five-to tenfold above the pregravid values. The adaptations in maternal renal physiology during gestation are among the primary mechanisms accounting for the increase in plasma volume and hence total body water during gestation.
Endocrine Changes
The plasma concentration of corticosteroid-binding globulin (CBG) increases significantly, reflecting increased hepatic synthesis (Gabbe et al., 1991). Estrogen-induced increases in CBG lead to an elevated plasma cortisol concentration, with a three-fold increase occurring by the end of the
third trimester. The concentration of the metabolically active free cortisol also progressively increases through gestation due to increased production and decreased clearance. Adrenocorticotropic hormone (ACTH) level is suppressed during pregnancy due to the action of estrogen and progesterone. The plasma concentration of dehydroepiandrosterone sulfate (DHEAS) declines during pregnancy due to an increase in metabolic clearance by the placenta and maternal liver.
The renin-angiotensin system changes dramatically during pregnancy. The adrenal gland remains responsive to the trophic action of angiotensin II, even though a refractory effect of pressors to angiotensin II develops early in pregnancy. This provides a probable explanation for the expansion of plasma volume during pregnancy. The secretion of prolactin from the pituitary and uterine decidua increases steadily during pregnancy. In contrast, luteinizing hormone and follicle-stimulating hormone are suppressed to levels similar to the luteal phase of ovulation. Growth hormone secretion is inhibited presumably by placental growth hormone production.
In normal pregnancy, thyroxine-binding globulin concentration is increased and the circulating pool of extrathyroidal iodide is decreased due to increased renal clearance. These changes cause the thyroid to enlarge and to synthesize and secrete the thyroid hormones T4 (thyroxine) and T3 (triiodothyronine) more actively. Despite elevated total T4 and T3, the concentrations of active hormones (free T4 and free T3) are unchanged during normal pregnancy, with the exception of a transient increase in the first trimester in some women (Gabbe et al., 1991; Glinoer, 2004).
Adipose tissue produces an array of adipokines known to have profound effects on metabolism and fertility, but their role in reproductive performance is yet to be fully understood. In addition to adipose tissue, leptin and its receptor, TNF-α, and resistin also are expressed in the placenta (Mitchell et al., 2005). Serum adiponectin is lower in the third trimester, a change that correlates with a decrease in insulin sensitivity (Catalano et al., 2006). Increases in maternal fat mass most likely are related to the decreases in circulating adiponectin concentrations.
Metabolic Changes
Many of the metabolic adjustments of pregnancy are well established in early pregnancy, when fetal nutrient demands are still minor. Minimal nutrient balances are usually positive, reflecting the anabolic state of the fetus and the mother. In the absence of nausea or “morning sickness,” most women experience an increase in appetite in the beginning of pregnancy (Gabbe et al., 1991). Several gastrointestinal changes occur during pregnancy, including decreased tone and motility of the stomach, reduced gastric acid secretion, delayed gastric emptying, and increased gastric mucous
secretion as a function of increased progesterone. Motility of the small intestine is also reduced during gestation; however, except for enhanced iron absorption, nutrient absorption is unchanged. These physiologic changes may affect the pattern of gestational weight gain in early gestation.
Changes in protein and nitrogen metabolism occur in early pregnancy, presumably in response to pregnancy-related hormones (Kalhan, 2000). Serum total α-amino nitrogen deceases, as does the rate of urea synthesis and the rate of transamination of branched-chain amino acids, which are aimed at conservation of nitrogen and protein accretion in pregnancy. Protein turnover on a weight basis, however, does not change (Kalhan, 2000). Serum total protein and albumin fall progressively and by term are 30 percent lower than nonpregnant values (Hytten and Chamberlain, 1991). The concentrations of binding proteins for corticosteroids, sex steroids, thyroid hormones, and vitamin D also increase.
Changes in carbohydrate and lipid metabolism occur during pregnancy to ensure a continuous supply of nutrients to the growing fetus (Butte, 2000). In early pregnancy, glucose tolerance is normal or improved slightly, and peripheral (muscle) sensitivity to insulin and hepatic basal glucose production are normal or increase by as much as 15 percent (Catalano et al., 1991, 1992, 1993). As pregnancy advances, nutrient-stimulated insulin responses increase progressively despite only minor deterioration in glucose tolerance, which is consistent with progressive insulin resistance (Kühl, 1991). In late pregnancy, insulin action is 50-60 percent lower than in nonpregnant state (Ryan et al., 1985; Buchanan et al., 1990; Catalano et al., 1991, 1992, 1993). By the third trimester, basal and 24-hour mean insulin concentrations may double (Lesser and Carpenter, 1994). The first and second phases of insulin release increase threefold by late pregnancy (Catalano et al., 1991). These alterations in maternal insulin sensitivity affect not only glucose metabolism but also lipid metabolism, resulting in a decreased ability of insulin to suppress lipolysis (Catalano et al., 2002).
Alterations in maternal physiology during pregnancy are mediated by placental factors, as evidenced by the significant increase in maternal insulin sensitivity that occurs within days after delivery of the fetus and placenta (Ryan et al., 1985). Alterations in maternal metabolism have generally been ascribed to placental hormones, such as hPL, progesterone, and estrogen (Kalkhoff et al., 1979; Ryan and Enns, 1988). Recently, Kirwan et al. (2002) reported that circulating cytokines (i.e., TNF-α concentration) were inversely correlated with insulin sensitivity.
The metabolic changes in insulin sensitivity that occur during pregnancy are modified by inflammatory factors (Friedman et al., 1999, 2008). In women with normal glucose tolerance during pregnancy who lose significant weight postpartum, there is a return to normal metabolic function. However, in women with GDM, particularly if there is no decrease in post-
partum weight or adiposity, there remains a significant inflammatory milieu that results in chronic insulin resistance, increasing the risk of diabetes and the metabolic syndrome.
Depending on the pregravid insulin sensitivity status of the woman, insulin sensitivity may increase or decrease during early pregnancy. In the very insulin-sensitive woman, insulin sensitivity most often decreases and is accompanied by an increase in adipose tissue and basal metabolic rate (Catalano et al., 1998). In contrast, in the more insulin-resistant women (e.g., those who are obese or have GDM), insulin sensitivity often increases and is accompanied by a decrease in basal metabolic rate and potential loss of adipose tissue (Okereke et al., 2004) (Figure 3-8). These physiologic changes may help to explain in part the relative decrease in weight gain in
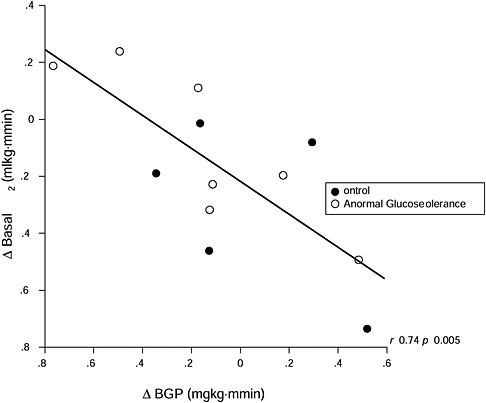
FIGURE 3-8 Alterations in basal VO2 per kilogram of FFM per minute in relation to changes in basal endogenous glucose production
SOURCE: Catalano et al., 1998. Reprinted from American Journal of Obstetrics and Gynecology, Volume 179, Issue 1, Catalano P. M., N. M. Roman-Drago, S. B. Amini and E. A. Sims, Longitudinal changes in body composition and energy balance in lean women with normal and abnormal glucose tolerance during pregnancy, pp. 156-165. Copyright (2008), with permission from Elsevier.
obese insulin-resistant women compared to the greater increases in weight in lean insulin-sensitive women in early gestation. The placental factors related to these alterations in insulin sensitivity, energy expenditure, and adipose tissue are not well understood relative to metabolic alterations in late pregnancy. Although there is a significant increase in maternal leptin concentrations in early pregnancy (Hauguel-de Mouzon et al., 2006), most likely related to placental production, the increased leptin concentrations do not appear to be associated differently with energy expenditure or fat accretion between lean and obese women.
FETAL-PLACENTAL PHYSIOLOGY
Three primary functions of the placenta are to serve as a barrier or filter, to transport substances between maternal and fetal circulation, and to mediate a large spectrum of endocrine activity. Changes in maternal homeostasis and associated changes in placental structure or function can result in changes in fetal growth rate in both normal and non-normal pregnancies (Thame et al., 2004; Desoye and Kaufman, 2005; MacLaughlin et al., 2005; Swanson and Bewtra, 2008).
Transport Function
Changes in the maternal environment have been shown to have an impact on specific steps of placental transport of the major energy substrates (i.e., glucose, lipids, amino acids; Hauguel de-Mouzon and Shafrir, 2001). For example, maternal diabetes results in increased availability of glucose, which is transported directly across the placenta for fetal utilization (Baumann et al., 2002). In contrast to glucose, which is transported along a concentration gradient, regulation of lipid transfer from maternal to fetal circulation is more complex. The placenta has the capacity to regulate the uptake, storage, and release of maternal lipids through multiple regulatory mechanisms and thus control fetal plasma lipid composition (Haggarty, 2002).
Changes in the maternal environment may also modify placental endocrine function. For example, changes in maternal circulating cholesterol affect lipid metabolism in human term placenta (Marseille-Tremblay et al., 2008). Higher cholesterol uptake may subsequently impact steroidogenesis because cholesterol is the primary precursor for progesterone synthesis (Pasqualini, 2005).
Interaction of Maternal and Placental Metabolism
The question of whether or how placental function(s) may have an impact on maternal metabolism has received little attention. Besides the
uterus, the feto-placental unit, intra- and extravascular fluids, and mammary gland, most of the weight gain that occurs over the course of a pregnancy lies in changes in maternal adipose tissue mass. In this context, the placental contribution to weight changes through the action of systemic factors that control the pathways of lipid synthesis and storage within the adipocyte must be taken into consideration. The placenta does not release adipogenic substrates into the maternal circulation. Hence, the most probable routes by which placental function would alter the regulation of lipogenic pathways are modulation of maternal insulin sensitivity and inflammation, as discussed previously.
Placental Hormone Production
The sex steroids and human placental lactogen (hPL), which best reflect the endocrine function of the placenta have been considered primary candidates for regulation of maternal insulin sensitivity (Leturque et al., 1989). Although estrogens certainly have insulin sensitizing properties, the action of progesterone is clearly linked to diminishing insulin sensitivity and weight gain (Kalkhoff, 1982; Gonzalez et al., 2000; Xiang et al., 2007). Hence, an imbalance in placental progesterone production may be a contributing factor to maternal weight regulation. Human placental lactogen is the most abundant polypeptide hormone produced by the placenta with strong anabolic and lipolytic properties. Inasmuch as hPL enhances maternal nitrogen accrual, there has been speculation that this process could contribute to weight regulation (Florini et al., 1966). However, the lipolytic action of hPL on adipose tissue has received more experimental support. One consequence of the lipolytic effect of hPL is the re-orientation of maternal metabolism toward lipid rather than glucose utilization, favoring glucose sparing for the fetus. Interestingly, the ability of hPL to mediate pregnancy-induced insulin resistance, as suggested by Grumbach et al. (1968), was never fully established. Thus, the exact contribution of hPL to the regulation of maternal homeostasis remains to be established. Further, whether hPL synthesis is modified in pathologic pregnancies also has not been confirmed (Stewart et al., 1989).
Just as occurs in white adipose tissue, the placenta also synthesizes a large array of cytokines (Hauguel-de Mouzon and Guerre-Millo, 2006; Desoye and Hauguel-de Mouzon, 2007). All placenta-derived cytokines except leptin, which is released in large amounts in maternal circulation, likely act in either a paracrine or autocrine manner. Obesity and diabetes are associated with increased placental leptin production and maternal hyperleptinemia, but the consequences of high systemic leptin are unclear at this time (Hauguel-de Mouzon et al., 2006). One possible consequence is resistance to the central satiety effect of leptin during pregnancy (Grattan et al., 2007).
Another potential contribution of the placenta to the regulation of maternal metabolism and subsequent alteration in maternal weight gain is systemic inflammatory priming by circulating syncytiotrophoblast microparticles (STBMs). Syncytiotrophoblast microparticles bind to monocytes and stimulate the production of inflammatory cytokines (Germain et al., 2007; Rovere-Querini et al., 2007). In addition to local placental inflammation, these microparticles are potential contributors to the altered systemic inflammatory response in pregnancy (Challier et al., 2008). Consequently, increased macrophage infiltration into maternal adipose tissue in combination with increased insulin resistance may contribute to the regulation of adipose mass during pregnancy (Xu et al., 2003).
Taken together, there is little direct evidence that placental hormonal factors directly regulate maternal homeostasis and, particularly, quantitative changes in adipose tissue mass. The role of progesterone, hPL, and leptin in maternal insulin sensitivity and energy homeostasis remains to be established; inflammatory mechanisms are novel potential regulatory pathways that will also have to be examined.
ABNORMAL MATERNAL METABOLISM
Weight Loss During Pregnancy
Weight loss or no GWG as a result of dietary caloric insufficiency should induce certain maternal hormonal and metabolic responses. Given the obligatory weight gain in the maternal tissues (uterus, breast, blood) and the fetal-placental unit, a weight gain less than ~7.5-8.5 kg would imply mobilization of maternal adipose tissue and possibly protein stores. Metabolic profile, dietary patterns, and eating behaviors of pregnant women undergoing weight loss or no weight gain have not been studied, but expected changes in fuel homeostasis can be deduced from studies conducted in pregnant women subjected to fasting.
Fasting in Pregnant Women
Felig (1973) reported ketonemia, increased urinary nitrogen excretion, and exaggerated reduction in gluconeogenic amino acids in pregnant women after 84 hours of fasting prior to elective termination of pregnancy at 16-20 weeks’ gestation. Glucose and insulin were lower, and acetoacetate and β-hydroxybutyrate were two to three times higher in pregnant than nonpregnant women after 12-60 hours but not 84 hours of fasting (Felig and Lynch, 1970). Weight loss averaged 3.1 kg in nonpregnant women and 3.2 kg in pregnant women. Metzger et al. (1982) subjected lean (n = 11) and obese (n = 10) pregnant women and lean (n = 14) and obese (n = 13) non-pregnant controls to an 18-hour fast. At 12 hours, there were no significant
differences between groups, but by 16 and 18 hours, the pregnant women had substantial increases in free fatty acid (FFA) and β-hydroxybutyrate (βHA), both of which were inversely correlated with glucose levels. There was a significant difference in FFA concentrations between obese and lean pregnant women only at 16 hours of fasting. In contrast, there were no significant differences in βHA levels at any time point between lean and obese women.
Ketonuria and Ketonemia in Pregnancy
As first described by Freinkel (1980), pregnancy can be considered a condition of “accelerated starvation” because of the changes in maternal metabolism that occur because of the increase in insulin resistance. As discussed previously, the accelerated starvation occurs as a result of increased insulin resistance, particularly related to lipid metabolism. There is an increased risk of developing ketonuria and ketonemia in pregnancy even among women with normal glucose tolerance. Chez and Curcio (1987) reported that eight of nine women with clinically normal pregnancies developed ketonuria at various times during their pregnancy. Using a portable capillary meter, Gin et al. (2006) measured capillary blood ketones and βHA in women with normal glucose tolerance (controls) and those with GDM three times a day from 25 to 37 weeks’ gestation. Fasting ketonuria was strongly correlated with ketonemia in controls but not in women with GDM. There was a chronic increase in ketonemia levels in 12 percent of the controls and 47 percent of the women with GDM.
Pregnant women develop ketonemia much earlier than nonpregnant women during prolonged fasting because of the accelerated starvation. Felig (1973) studied women between 16 and 22 weeks’ gestation who elected termination of pregnancy and were willing to undergo prolonged fasting and compared them with a nonpregnant control group. After an overnight fast of at least 12 hours and for the first 36 to 60 hours of starvation, blood βHA and acetoacetate concentrations were two- to threefold higher in the pregnant group than in the nonpregnant group. The increase in lipolysis among the pregnant women was attributed to increases in hPL. The ketone concentrations in maternal blood were equivalent to those in amniotic fluid and were fortyfold above levels in fed subjects. The assumption is that amniotic fluid levels represent maternal-to-fetal transport. Felig (1973) also hypothesized that ketones become an important metabolic fuel for the fetal brain once glucose concentrations decrease, because the human fetal brain has the enzymes necessary for ketone oxidation.
Coetzee et al. (1980) reported that 19 percent of obese, insulin-dependent diabetic women on 1,000-kilocalorie (kcal) diets developed ketonuria. In contrast, in diabetic women eating higher-energy diets, only 14 percent had
ketonuria, and in pregnant nondiabetic women, only 7 percent developed ketonuria. Measurement of blood ketones was never positive if the urine measure was ≤ 2 plus and acetoacetate levels were always less than 1 mmol/L. There was no difference in neonatal outcomes among the three groups.
In summary, pregnant women are more likely to develop elevated measures of blood βHA and acetoacetate during prolonged fasting (after 12-18 hours) as a result of the metabolic and hormonal changes that occur during pregnancy. Although pregnant women with diabetes are more likely to develop elevated blood ketones than women with normal glucose tolerance, a substantial proportion of pregnant women with normal glucose tolerance have elevated blood ketone levels at some time during gestation. Although the evidence is based on associations and does not demonstrate causality, caution should be exercised regarding weight loss during pregnancy or no GWG, given the propensity to develop ketonemia, increased urinary nitrogen excretion, and decreased gluconeogenic amino acids. As discussed in Chapter 6, there are significant consequences of caloric insufficiency, low GWG, and poorly controlled diabetes for the child, and these are discussed in Chapter 6.
FINDINGS AND RECOMMENDATIONS
Findings
-
Total GWG in normal-term pregnancies displays considerable variability; nevertheless, some generalizations can be made regarding mean tendencies and patterns of GWG:
-
A consistent inverse relationship is observed between GWG and pregravid BMI category.
-
Mean GWG ranges from 10.0 to 16.7 kg in normal weight adults and 14.6 to 18.0 kg in adolescents giving birth to term infants.
-
The pattern of GWG is most commonly described as sigmoidal, with mean weight gains higher in the second than the third trimester across BMI categories, except for obese women.
-
Lower GWGs, on the order of 11 kg and 9 kg, have been confirmed in large cohorts of obese women and very obese women, respectively.
-
-
In its evaluation of GWG in multiple pregnancies, the committee relied on observational GWG data of women giving birth to twins born at 37-42 weeks of gestation and with an average twin birth weight ≥ 2,500 g:
-
-
Mean GWG of normal weight women with twin births ranges from 15.5 to 21.8 kg.
-
GWG for triplets ranges from 20.5 to 23.0 kg at 32-34 weeks and for quadruplets from 20.8 to 31.0 kg at 31-32 weeks.
-
-
When stratified by the World Health Organization (WHO) prepregnancy BMI categories, sample sizes from data on twins are insufficient to designate a range for underweight women with pregravid BMI < 18.5 kg/m2.
-
The extent to which fat mass accretion is critical rather than incidental to pregnancy is not clear, but unrestrained weight gain leads to postpartum weight retention.
-
Placental size is strongly correlated with fetal growth, averaging approximately 500 g in singleton pregnancies.
-
Amniotic fluid weight may affect maternal gestational weight gain by as much as 1 kg at term.
-
Gestational gains in weight, total body water, total body potassium, protein, and FFM, but not FM, are positively correlated with birth weight across all BMI categories.
-
Poor plasma volume expansion is associated with a poorly growing fetus and poor reproductive performance.
-
Pregnancy is a condition of systemic inflammation that also influences maternal and fetal nutrient utilization.
-
During prolonged fasting, i.e., 16-18 hours, pregnant women are more likely to develop elevated measures of blood βHA and acetoacetate. In women with diabetes, plasma FFA and βHA are inversely associated with intellectual development of the offspring at 3-5 years of age. Therefore, caution is warranted regarding periods of prolonged fasting and weight loss during pregnancy and the development of ketonuria.
Research Recommendations
Research Recommendation 3-1: The committee recommends that the National Institutes of Health and other relevant agencies should provide support to researchers to conduct studies in all classes of obese women, stratified by the severity of obesity, on the determinants and impact of GWG, pattern of weight gain, and its composition on maternal and child outcomes.
Research Recommendation 3-2: The committee recommends that the National Institutes of Health and other relevant agencies should provide support to researchers to conduct studies on the eating behaviors, patterns of dietary intake and physical activity, and metabolic profiles of
pregnant women, especially obese women, who experience low gain or weight loss during pregnancy. In addition, the committee recommends that researchers should conduct studies on the effects of weight loss or low GWG, including periods of prolonged fasting and the development of ketonuria/ketonemia during gestation, on growth and on development and long-term neurocognitive function in the offspring.
Areas for Additional Investigation
The committee identified the following areas for further investigation to support its research recommendation. The research community should conduct studies on:
-
Potential effects of maternal weight loss on components of maternal body composition for both the mother and the fetus, particularly in obese women; and
-
Mechanisms by which placental hormonal factors and systemic inflammation impact the regulation of maternal metabolism during pregnancy.
REFERENCES
Abramovich D. R.1969. The weight of placenta and membranes in early pregnancy. Journal of Obstetrics and Gynaecology of the British Commonwealth 76(6): 523-526.
Abrams B. F. and R. K. Laros, Jr. 1986. Prepregnancy weight, weight gain, and birth weight. American Journal of Obstetrics and Gynecology 154(3): 503-509.
Abrams B. and S. Selvin. 1995. Maternal weight gain pattern and birth weight. Obstetrics and Gynecology 86(2): 163-169.
Ananth C. V. and S. W. Wen. 2002. Trends in fetal growth among singleton gestations in the United States and Canada, 1985 through 1998. Seminars in Perinatology 26(4): 260-267.
Ananth C. V., A. M. Vintzileos, S. Shen-Schwarz, J. C. Smulian and Y. L. Lai. 1998. Standards of birth weight in twin gestations stratified by placental chorionicity. Obstetrics and Gynecology 91(6): 917-924.
Archie J. G., J. S. Collins and R. R. Lebel. 2006. Quantitative standards for fetal and neonatal autopsy. American Journal of Clinical Pathology 126(2): 256-265.
Ballew C. and J. D. Haas. 1986. Altitude differences in body composition among Bolivian newborns. Human Biology 58(6): 871-882.
Baumann M. U., S. Deborde and N. P. Illsley. 2002. Placental glucose transfer and fetal growth. Endocrine 19(1): 13-22.
Bernstein I. M., M. I. Goran, S. B. Amini and P. M. Catalano. 1997. Differential growth of fetal tissues during the second half of pregnancy. American Journal of Obstetrics and Gynecology 176(1 Pt 1): 28-32.
Bianco A. T., S. W. Smilen, Y. Davis, S. Lopez, R. Lapinski and C. J. Lockwood. 1998. Pregnancy outcome and weight gain recommendations for the morbidly obese woman. Obstetrics and Gynecology 91(1): 97-102.
Bleker O. P. and H. J. Hoogland. 1981. Short review: ultrasound in the estimation of human intrauterine placental growth. Placenta2(3): 275-278.
Blickstein I. 2002. Normal and abnormal growth of multiples. Seminars in Neonatology 7(3): 177-185.
Brace R. A. and E. J. Wolf. 1989. Normal amniotic fluid volume changes throughout pregnancy. American Journal of Obstetrics and Gynecology 161(2): 382-388.
Brown J. E. and P. T. Schloesser. 1990. Prepregnancy weight status, prenatal weight gain, and the outcome of term twin gestations. American Journal of Obstetrics and Gynecology 162(1): 182-186.
Buchanan T. A., B. E. Metzger, N. Freinkel and R. N. Bergman. 1990. Insulin sensitivity and B-cell responsiveness to glucose during late pregnancy in lean and moderately obese women with normal glucose tolerance or mild gestational diabetes. American Journal of Obstetrics and Gynecology 162(4): 1008-1014.
Butte N. F.2000. Carbohydrate and lipid metabolism in pregnancy: normal compared with gestational diabetes mellitus. American Journal of Clinical Nutrition 71(5 Suppl): 1256S-1261S.
Butte N. F., K. J. Ellis, W. W. Wong, J. M. Hopkinson and E. O. Smith. 2003. Composition of gestational weight gain impacts maternal fat retention and infant birth weight. American Journal of Obstetrics and Gynecology 189(5): 1423-1432.
Carmichael S., B. Abrams and S. Selvin. 1997. The association of pattern of maternal weight gain with length of gestation and risk of spontaneous preterm delivery. Paediatric and Perinatal Epidemiology 11(4): 392-406.
Catalano P. M. and H. M. Ehrenberg. 2006. The short- and long-term implications of maternal obesity on the mother and her offspring. British Journal of Obstetrics and Gynaecology 113(10): 1126-1133.
Catalano P. M., E. D. Tyzbir, N. M. Roman, S. B. Amini and E. A. Sims. 1991. Longitudinal changes in insulin release and insulin resistance in nonobese pregnant women. American Journal of Obstetrics and Gynecology 165(6 Pt 1): 1667-1672.
Catalano P. M., E. D. Tyzbir, R. R. Wolfe, N. M. Roman, S. B. Amini and E. A. Sims. 1992. Longitudinal changes in basal hepatic glucose production and suppression during insulin infusion in normal pregnant women. American Journal of Obstetrics and Gynecology 167(4 Pt 1): 913-919.
Catalano P. M., E. D. Tyzbir, R. R. Wolfe, J. Calles, N. M. Roman, S. B. Amini and E. A. Sims. 1993. Carbohydrate metabolism during pregnancy in control subjects and women with gestational diabetes. American Journal of Physiology264(1 Pt 1): E60-E67.
Catalano P. M., N. M. Drago and S. B. Amini. 1995. Factors affecting fetal growth and body composition. American Journal of Obstetrics and Gynecology 172(5): 1459-1463.
Catalano P. M., N. M. Roman-Drago, S. B. Amini and E. A. Sims. 1998. Longitudinal changes in body composition and energy balance in lean women with normal and abnormal glucose tolerance during pregnancy. American Journal of Obstetrics and Gynecology 179(1): 156-165.
Catalano P. M., S. E. Nizielski, J. Shao, L. Preston, L. Qiao and J. E. Friedman. 2002. Down-regulated IRS-1 and PPARgamma in obese women with gestational diabetes: relationship to FFA during pregnancy. American Journal of Physiology Endocrinology and Metabolism 282(3): E522-E533.
Catalano P. M., A. Thomas, L. Huston-Presley and S. B. Amini. 2003. Increased fetal adiposity: a very sensitive marker of abnormal in utero development. American Journal of Obstetrics and Gynecology 189(6): 1698-1704.
Catalano P. M., M. Hoegh, J. Minium, L. Huston-Presley, S. Bernard, S. Kalhan and S. Hauguel-De Mouzon. 2006. Adiponectin in human pregnancy: implications for regulation of glucose and lipid metabolism. Diabetologia 49(7): 1677-1685.
Catalano P. M., A. Thomas, L. Huston-Presley and S. B. Amini. 2007. Phenotype of infants of mothers with gestational diabetes. Diabetes Care 30(Suppl 2): S156-S160.
Cedergren M. 2006. Effects of gestational weight gain and body mass index on obstetric outcome in Sweden. International Journal of Gynaecology and Obstetrics 93(3): 269-274.
Challier J. C., S. Basu, T. Bintein, J. Minium, K. Hotmire, P. M. Catalano and S. Hauguelde Mouzon. 2008. Obesity in pregnancy stimulates macrophage accumulation and inflammation in the placenta. Placenta 29(3): 274-281.
Chez R. A. and F. D. Curcio, 3rd. 1987. Ketonuria in normal pregnancy. Obstetrics and Gynecology 69(2): 272-274.
Claesson I. M., G. Sydsjo, J. Brynhildsen, M. Cedergren, A. Jeppsson, F. Nystrom, A. Sydsjo and A. Josefsson. 2008. Weight gain restriction for obese pregnant women: a case-control intervention study. British Journal of Obstetrics and Gynaecology 115(1): 44-50.
Coetzee E. J., W. P. Jackson and P. A. Berman. 1980. Ketonuria in pregnancy—with special reference to calorie-restricted food intake in obese diabetics. Diabetes 29(3): 177-181.
Desoye G. and S. Hauguel-de Mouzon. 2007. The human placenta in gestational diabetes mellitus. The insulin and cytokine network. Diabetes Care 30(Suppl 2): S120-S126.
Desoye G. and P. Kaufman. 2005. The human placenta in diabetes. In Diabetology of Pregnancy (Frontiers in Diabetes), Vol 17. J. Djelmis, G. Desoye and M. Ivasinevic. Basel, Switzerland: Karger; pp. 94-109.
Diamant Y. Z., B. E. Metzger, N. Freinkel and E. Shafrir. 1982. Placental lipid and glycogen content in human and experimental diabetes mellitus. American Journal of Obstetrics and Gynecology 144(1): 5-11.
Durnwald C., L. Huston-Presley, S. Amini and P. Catalano. 2004. Evaluation of body composition of large-for-gestational-age infants of women with gestational diabetes mellitus compared with women with normal glucose tolerance levels. American Journal of Obstetrics and Gynecology 191(3): 804-808.
Eddib A., J. Penvose-Yi, J. A. Shelton and J. Yeh. 2007. Triplet gestation outcomes in relation to maternal prepregnancy body mass index and weight gain. Journal of Maternal-Fetal & Neonatal Medicine 20(7): 515-519.
Emerson K., Jr., E. L. Poindexter and M. Kothari. 1975. Changes in total body composition during normal and diabetic pregnancy. Relation to oxygen consumption. Obstetrics and Gynecology 45(5): 505-511.
Felig P.1973. Maternal and fetal fuel homeostasis in human pregnancy. American Journal of Clinical Nutrition 26(9): 998-1005.
Felig P. and V. Lynch. 1970. Starvation in human pregnancy: hypoglycemia, hypoinsulinemia, and hyperketonemia. Science 170(961): 990-992.
Florini J. R., G. Tonelli, C. B. Breuer, J. Coppola, I. Ringler and P. H. Bell. 1966. Characterization and biological effects of purified placental protein (human). Endocrinology 79(4): 692-708.
Fomon S. J., F. Haschke, E. E. Ziegler and S. E. Nelson. 1982. Body composition of reference children from birth to age 10 years. American Journal of Clinical Nutrition 35(5 Suppl): 1169-1175.
Forsum E., A. Sadurskis and J. Wager. 1988. Resting metabolic rate and body composition of healthy Swedish women during pregnancy. American Journal of Clinical Nutrition 47(6): 942-947.
Freinkel N. 1980. Banting Lecture 1980. Of pregnancy and progeny. Diabetes 29(12): 1023-1035.
Friedman J. E., T. Ishizuka, J. Shao, L. Huston, T. Highman and P. Catalano. 1999. Impaired glucose transport and insulin receptor tyrosine phosphorylation in skeletal muscle from obese women with gestational diabetes. Diabetes 48(9): 1807-1814.
Friedman J. E., J. P. Kirwan, M. Jing, L. Presley and P. M. Catalano. 2008. Increased skeletal muscle tumor necrosis factor-alpha and impaired insulin signaling persist in obese women with gestational diabetes mellitus 1 year postpartum. Diabetes 57(3): 606-613.
Fuller N. J., S. A. Jebb, M. A. Laskey, W. A. Coward and M. Elia. 1992. Four-component model for the assessment of body composition in humans: comparison with alternative methods, and evaluation of the density and hydration of fat-free mass. Clinical Science (London) 82(6): 687-693.
Gabbe S., J. Niebyl and J. Simpson, Eds. (1991). Obstetrics Normal & Problem Pregnancies. New York: Churchill Livingstone.
Garrow J. S. and S. F. Hawes. 1971. The relationship of the size and composition of the human placenta to its functional capacity. Journal of Obstetrics and Gynaecology of the British Commonwealth 78(1): 22-28.
Germain S. J., G. P. Sacks, S. R. Sooranna, I. L. Sargent and C. W. Redman. 2007. Systemic inflammatory priming in normal pregnancy and preeclampsia: the role of circulating syncytiotrophoblast microparticles. Journal of Immunology 178(9): 5949-5956.
Gielen M., P. J. Lindsey, C. Derom, R. J. Loos, R. Derom, J. G. Nijhuis and R. Vlietinck. 2007. Twin birth weight standards. Neonatology 92(3): 164-173.
Gin H., A. Vambergue, C. Vasseur, V. Rigalleau, P. Dufour, A. Roques, M. Romon, D. Millet, P. Hincker and P. Fontaine. 2006. Blood ketone monitoring: a comparison between gestational diabetes and non-diabetic pregnant women. Diabetes and Metabolism 32(6): 592-597.
Girard J. and P. Ferre. 1982. Metabolic and hormonal changes around birth. In Biochemical Development of the Fetus and Neonate. C. T. Jones. New York: Elsevier Biomedical Press; p. 517.
Glinianaia S. V., R. Skjaerven and P. Magnus. 2000. Birthweight percentiles by gestational age in multiple births. A population-based study of Norwegian twins and triplets. Acta Obstetricia et Gynecologica Scandinavica 79(6): 450-458.
Glinoer D. 2004. Increased TBG during pregnancy and increased hormonal requirements. Thyroid 14(6): 479-480; author reply 479-480.
Gonzalez C., A. Alonso, N. Alvarez, F. Diaz, M. Martinez, S. Fernandez and A. M. Patterson. 2000. Role of 17beta-estradiol and/or progesterone on insulin sensitivity in the rat: implications during pregnancy. Journal of Endocrinology 166(2): 283-291.
Grattan D. R., S. R. Ladyman and R. A. Augustine. 2007. Hormonal induction of leptin resistance during pregnancy. Physiology & Behavior 91(4): 366-374.
Grumbach M. M., S. L. Kaplan, J. J. Sciarra and I. M. Burr. 1968. Chorionic growth hormoneprolactin (CGP): secretion, disposition, biologic activity in man, and postulated function as the “growth hormone” of the 2d half of pregnancy. Annals of the New York Academy of Sciences 148(2): 501-531.
Gunderson E. P., B. Sternfeld, M. F. Wellons, R. A. Whitmer, V. Chiang, C. P. Quesenberry, Jr., C. E. Lewis and S. Sidney. 2008. Childbearing may increase visceral adipose tissue independent of overall increase in body fat. Obesity (Silver Spring) 16(5): 1078-1084.
Haggarty P. 2002. Placental regulation of fatty acid delivery and its effect on fetal growth—a review. Placenta 23(Suppl A): S28-S38.
Harvey N. C., J. R. Poole, M. K. Javaid, E. M. Dennison, S. Robinson, H. M. Inskip, K. M. Godfrey, C. Cooper and A. A. Sayer. 2007. Parental determinants of neonatal body composition. Journal of Clinical Endocrinology and Metabolism 92(2): 523-526.
Hauguel-de Mouzon S. and M. Guerre-Millo. 2006. The placenta cytokine network and inflammatory signals. Placenta 27(8): 794-798.
Hauguel-de Mouzon S. and E. Shafrir. 2001. Carbohydrate and fat metabolism and related hormonal regulation in normal and diabetic placenta. Placenta 22(7): 619-627.
Hauguel-de Mouzon S., J. Lepercq and P. Catalano. 2006. The known and unknown of leptin in pregnancy. American Journal of Obstetrics and Gynecology 194(6): 1537-1545.
Hediger M. L., T. O. Scholl, I. G. Ances, D. H. Belsky and R. W. Salmon. 1990. Rate and amount of weight gain during adolescent pregnancy: associations with maternal weight-for-height and birth weight. American Journal of Clinical Nutrition 52(5): 793-799.
Hickey C. A., S. P. Cliver, S. F. McNeal, H. J. Hoffman and R. L. Goldenberg. 1995. Prenatal weight gain patterns and spontaneous preterm birth among nonobese black and white women. Obstetrics and Gynecology 85(6): 909-914.
Hopkinson J. M., N. F. Butte, K. J. Ellis, W. W. Wong, M. R. Puyau and E. O. Smith. 1997. Body fat estimation in late pregnancy and early postpartum: comparison of two-, three-, and four-component models. American Journal of Clinical Nutrition 65(2): 432-438.
Hull H. R., M. K. Dinger, A. W. Knehans, D. M. Thompson and D. A. Fields. 2008. Impact of maternal body mass index on neonate birthweight and body composition. American Journal of Obstetrics and Gynecology 198(4): 416 e411-e416.
Hytten F. and G. Chamberlain. 1991. Clinical Physiology in Obstetrics. Oxford: Blackwell Scientific Publications.
Ibanez L., G. Sebastiani, A. Lopez-Bermejo, M. Diaz, M. D. Gomez-Roig and F. de Zegher. 2008. Gender specificity of body adiposity and circulating adiponectin, visfatin, insulin, and insulin growth factor-I at term birth: relation to prenatal growth. Journal of Clinical Endocrinology and Metabolism 93(7): 2774-2778.
IOM (Institute of Medicine). 1990. Nutrition During Pregnancy. Washington, DC: National Academy Press.
Kalhan S. C. 2000. Protein metabolism in pregnancy. American Journal of Clinical Nutrition 71(5 Suppl): 1249S-1255S.
Kalkhoff R. K. 1982. Metabolic effects of progesterone. American Journal of Obstetrics and Gynecology 142(6 Pt 2): 735-738.
Kalkhoff R., A. Kissebah and H. Kim. 1979. Carbohydrate and lipid metabolism during normal pregnancy: relationship to gestational hormone action. In The Diabetic Pregnancy: A Perinatal Perspective. I. Merkatz and P. Adam. New York: Grune & Straton; pp. 3-21.
Kiel D. W., E. A. Dodson, R. Artal, T. K. Boehmer and T. L. Leet. 2007. Gestational weight gain and pregnancy outcomes in obese women: how much is enough? Obstetrics and Gynecology 110(4): 752-758.
Kierans W. J., K. S. Joseph, Z. C. Luo, R. Platt, R. Wilkins and M. S. Kramer. 2008. Does one size fit all? The case for ethnic-specific standards of fetal growth. BMC Pregnancy and Childbirth 8(1): 1.
King J. C., D. H. Calloway and S. Margen. 1973. Nitrogen retention, total body 40 K and weight gain in teenage pregnant girls. Journal of Nutrition 103(5): 772-785.
Kirwan J. P., S. Hauguel-De Mouzon, J. Lepercq, J. C. Challier, L. Huston-Presley, J. E. Friedman, S. C. Kalhan and P. M. Catalano. 2002. TNF-alpha is a predictor of insulin resistance in human pregnancy. Diabetes 51(7): 2207-2213.
Klebanoff M. A., B. R. Mednick, C. Schulsinger, N. J. Secher and P. H. Shiono. 1998. Father’s effect on infant birth weight. American Journal of Obstetrics and Gynecology 178(5): 1022-1026.
Koo W. W., J. C. Walters and E. M. Hockman. 2000. Body composition in human infants at birth and postnatally. Journal of Nutrition 130(9): 2188-2194.
Kühl C. 1991. Aetiology of gestational diabetes. Baillieres Clinical Obstetrics and Gynaecology 5(2): 279-292.
Langhoff-Roos J., G. Lindmark and M. Gebre-Medhin. 1987. Maternal fat stores and fat accretion during pregnancy in relation to infant birthweight. British Journal of Obstetrics and Gynaecology 94(12): 1170-1177.
Lantz M. E., R. A. Chez, A. Rodriguez and K. B. Porter. 1996. Maternal weight gain patterns and birth weight outcome in twin gestation. Obstetrics and Gynecology 87(4): 551-556.
Larciprete G., H. Valensise, B. Vasapollo, F. Altomare, R. Sorge, B. Casalino, A. De Lorenzo and D. Arduini. 2003. Body composition during normal pregnancy: reference ranges. Acta Diabetologica 40(Suppl 1): S225-S232.
Lederman S. A., A. Paxton, S. B. Heymsfield, J. Wang, J. Thornton and R. N. Pierson, Jr. 1997. Body fat and water changes during pregnancy in women with different body weight and weight gain. Obstetrics and Gynecology 90(4 Pt 1): 483-488.
Lesser K. B. and M. W. Carpenter. 1994. Metabolic changes associated with normal pregnancy and pregnancy complicated by diabetes mellitus. Seminars in Perinatology 18(5): 399-406.
Leturque A., S. Hauguel, M. T. Sutter Dub, P. Maulard and J. Girard. 1989. Effects of placental lactogen and progesterone on insulin stimulated glucose metabolism in rat muscles in vitro. Diabetes & Metabolism 15(4): 176-181.
Lindsay C. A., A. J. Thomas and P. M. Catalano. 1997. The effect of smoking tobacco on neonatal body composition. American Journal of Obstetrics and Gynecology 177(5): 1124-1128.
Luke B. 1998. What is the influence of maternal weight gain on the fetal growth of twins? Clinical Obstetrics and Gynecology 41(1): 56-64.
Luke B., J. Minogue and H. Abbey. 1992. The association between maternal weight gain and the birth weight of twins. The Journal of Maternal-Fetal Medicine 1: 267-276.
Luke B., E. Bryan, C. Sweetland, S. Leurgans and L. Keith. 1995. Prenatal weight gain and the birthweight of triplets. Acta Geneticae Medicae et Gemellologiae 44(2): 93-101.
Luke B., B. Gillespie, S. J. Min, M. Avni, F. R. Witter and M. J. O’Sullivan. 1997. Critical periods of maternal weight gain: effect on twin birth weight. American Journal of Obstetrics and Gynecology 177(5): 1055-1062.
Luke B., S. J. Min, B. Gillespie, M. Avni, F. R. Witter, R. B. Newman, J. G. Mauldin, F. A. Salman and M. J. O’Sullivan. 1998. The importance of early weight gain in the intrauterine growth and birth weight of twins. American Journal of Obstetrics and Gynecology 179(5): 1155-1161.
Luke B., M. L. Hediger, C. Nugent, R. B. Newman, J. G. Mauldin, F. R. Witter and M. J. O’Sullivan. 2003. Body mass index—specific weight gains associated with optimal birth weights in twin pregnancies. Journal of Reproductive Medicine 48(4): 217-224.
MacLaughlin S. M., S. K. Walker, C. T. Roberts, D. O. Kleemann and I. C. McMillen. 2005. Periconceptional nutrition and the relationship between maternal body weight changes in the periconceptional period and feto-placental growth in the sheep. The Journal of Physiology 565(Pt 1): 111-124.
Mardones-Santander F., G. Salazar, P. Rosso and L. Villarroel. 1998. Maternal body composition near term and birth weight. Obstetrics and Gynecology 91(6): 873-877.
Marseille-Tremblay C., M. Ethier-Chiasson, J. C. Forest, Y. Giguere, A. Masse, C. Mounier and J. Lafond. 2008. Impact of maternal circulating cholesterol and gestational diabetes mellitus on lipid metabolism in human term placenta. Molecular Reproduction and Development 75(6): 1054-1062.
Metzger B. E., V. Ravnikar, R. A. Vileisis and N. Freinkel. 1982. “Accelerated starvation” and the skipped breakfast in late normal pregnancy. Lancet 1(8272): 588-592.
Min S. J., B. Luke, B. Gillespie, L. Min, R. B. Newman, J. G. Mauldin, F. R. Witter, F. A. Salman and M. J. O’Sullivan. 2000. Birth weight references for twins. American Journal of Obstetrics and Gynecology 182(5): 1250-1257.
Mitchell M., D. T. Armstrong, R. L. Robker and R. J. Norman. 2005. Adipokines: implications for female fertility and obesity. Reproduction 130(5): 583-597.
Molteni R. A., S. J. Stys and F. C. Battaglia. 1978. Relationship of fetal and placental weight in human beings: fetal/placental weight ratios at various gestational ages and birth weight distributions. Journal of Reproductive Medicine 21(5): 327-334.
Nohr E. A., B. H. Bech, M. Vaeth, K. M. Rasmussen, T. B. Henriksen and J. Olsen. 2007. Obesity, gestational weight gain and preterm birth: a study within the Danish National Birth Cohort. Paediatric and Perinatal Epidemiology 21(1): 5-14.
Oken E., K. P. Kleinman, J. Rich-Edwards and M. W. Gillman. 2003. A nearly continuous measure of birth weight for gestational age using a United States national reference. BMC Pediatrics 3: 6.
Okereke N. C., L. Huston-Presley, S. B. Amini, S. Kalhan and P. M. Catalano. 2004. Longitudinal changes in energy expenditure and body composition in obese women with normal and impaired glucose tolerance. American Journal of Physiology Endocrinology and Metabolism 287(3): E472-E479.
Orskou J., U. Kesmodel, T. B. Henriksen and N. J. Secher. 2001. An increasing proportion of infants weigh more than 4000 grams at birth. Acta Obstetricia et Gynecologica Scandinavica 80(10): 931-936.
Pasqualini J. R. 2005. Enzymes involved in the formation and transformation of steroid hormones in the fetal and placental compartments. Journal of Steroid Biochemistry and Molecular Biology 97(5): 401-415.
Pinar H., M. Stephens, D. B. Singer, T. K. Boyd, S. M. Pflueger, D. L. Gang, D. J. Roberts and C. J. Sung. 2002. Triplet placentas: reference values for weights. Pediatric and Developmental Pathology 5(5): 495-498.
Pipe N. G., T. Smith, D. Halliday, C. J. Edmonds, C. Williams and T. M. Coltart. 1979. Changes in fat, fat-free mass and body water in human normal pregnancy. British Journal of Obstetrics and Gynaecology 86(12): 929-940.
Pitkin R. M. 1976. Nutritional support in obstetrics and gynecology. Clinical Obstetrics and Gynecology 19(3): 489-513.
Ross M. G. and R. A. Brace. 2001. National Institute of Child Health and Development Conference summary: amniotic fluid biology—basic and clinical aspects. Journal of Maternal-Fetal Medicine 10(1): 2-19.
Rosso P. 1990. Nutrition and Metabolism in Pregnancy: Mother and Fetus. New York: Oxford University Press.
Rovere-Querini P., M. T. Castiglioni, M. G. Sabbadini and A. A. Manfredi. 2007. Signals of cell death and tissue turnover during physiological pregnancy, pre-eclampsia, and autoimmunity. Autoimmunity 40(4): 290-294.
Ryan E. A. and L. Enns. 1988. Role of gestational hormones in the induction of insulin resistance. Journal of Clinical Endocrinology and Metabolism 67(2): 341-347.
Ryan E. A., M. J. O’Sullivan and J. S. Skyler. 1985. Insulin action during pregnancy. Studies with the euglycemic clamp technique. Diabetes 34(4): 380-389.
Sewell M. F., L. Huston-Presley, D. M. Super and P. Catalano. 2006. Increased neonatal fat mass, not lean body mass, is associated with maternal obesity. American Journal of Obstetrics and Gynecology 195(4): 1100-1103.
Siega-Riz A. M., L. S. Adair and C. J. Hobel. 1996. Maternal underweight status and inadequate rate of weight gain during the third trimester of pregnancy increases the risk of preterm delivery. Journal of Nutrition 126(1): 146-153.
Sohlstrom A. and E. Forsum. 1995. Changes in adipose tissue volume and distribution during reproduction in Swedish women as assessed by magnetic resonance imaging. American Journal of Clinical Nutrition 61(2): 287-295.
Spady D. W. 1989. Normal body composition of infants and children. In Report of the 98th Ross Conference on Pediatric Research. Body composition measurements in infants and children, Ross Laboratories; p. 67.
Sparks J. W. 1984. Human intrauterine growth and nutrient accretion. Seminars in Perinatology 8(2): 74-93.
Stevens-Simon C., E. R. McAnarney, K. J. Roghmann and G. B. Forbes. 1997. Composition of gestational weight gain in adolescent pregnancy. Journal of Maternal-Fetal Medicine 6(2): 79-86.
Stewart M. O., P. G. Whittaker, B. Persson, U. Hanson and T. Lind. 1989. A longitudinal study of circulating progesterone, oestradiol, hCG and hPL during pregnancy in type 1 diabetic mothers. British Journal of Obstetrics and Gynaecology 96(4): 415-423.
Surkan P. J., C. C. Hsieh, A. L. Johansson, P. W. Dickman and S. Cnattingius. 2004. Reasons for increasing trends in large for gestational age births. Obstetrics and Gynecology 104(4): 720-726.
Swanson L. D. and C. Bewtra. 2008. Increase in normal placental weights related to increase in maternal body mass index. Journal of Maternal-Fetal & Neonatal Medicine 21(2): 111-113.
Taggart N. R., R. M. Holliday, W. Z. Billewicz, F. E. Hytten and A. M. Thomson. 1967. Changes in skinfolds during pregnancy. British Journal of Nutrition 21(2): 439-451.
Teasdale F. 1980. Gestational changes in the functional structure of the human placenta in relation to fetal growth: a morphometric study. American Journal of Obstetrics and Gynecology 137(5): 560-568.
Thame M., C. Osmond, F. Bennett, R. Wilks and T. Forrester. 2004. Fetal growth is directly related to maternal anthropometry and placental volume. European Journal of Clinical Nutrition 58(6): 894-900.
Thomas P., J. Peabody, V. Turnier and R. H. Clark. 2000. A new look at intrauterine growth and the impact of race, altitude, and gender. Pediatrics 106(2): E21.
van Raaij J. M., M. E. Peek, S. H. Vermaat-Miedema, C. M. Schonk and J. G. Hautvast. 1988. New equations for estimating body fat mass in pregnancy from body density or total body water. American Journal of Clinical Nutrition 48(1): 24-29.
Villamor E., R. Gofin and B. Adler. 1998. Maternal anthropometry and pregnancy outcome among Jerusalem women. Annals of Human Biology 25(4): 331-343.
Weiner C. P., R. E. Sabbagha, N. Vaisrub and R. Depp. 1985. A hypothetical model suggesting suboptimal intrauterine growth in infants delivered preterm. Obstetrics and Gynecology 65(3): 323-326.
Widdowson E. M. 1950. Chemical composition of newly born mammals. Nature166(4224): 626-628.
Widdowson E. M. and C. M. Spray. 1951. Chemical development in utero. Archives of Disease in Childhood 26(127): 205-214.
Xiang A. H., M. Kawakubo, T. A. Buchanan and S. L. Kjos. 2007. A longitudinal study of lipids and blood pressure in relation to method of contraception in Latino women with prior gestational diabetes mellitus. Diabetes Care 30(8): 1952-1958.
Xu H., G. T. Barnes, Q. Yang, G. Tan, D. Yang, C. J. Chou, J. Sole, A. Nichols, J. S. Ross, L. A. Tartaglia and H. Chen. 2003. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. Journal of Clinical Investigation 112(12): 1821-1830.
Yeh J. and J. A. Shelton. 2007. Association of pre-pregnancy maternal body mass and maternal weight gain to newborn outcomes in twin pregnancies. Acta Obstetricia et Gynecologica Scandinavica 86(9): 1051-1057.








































