3
Experimental Studies Relevant to the Pathophysiology of Secondhand Smoke
This chapter discusses pathophysiologic experiments that have investigated the cardiovascular effects of mainstream and sidestream tobacco smoke in cells, in animals, and in humans. It addresses the association between secondhand-smoke exposure and acute coronary events. Specifically it provides information on the biological plausibility of a causal relationship between secondhand smoke exposure and acute coronary events (Question 2, see Box 1-1) and information on the duration of exposure and time following cessation of exposure within which effects might be observed (Questions 3 and 5, see Box 1-1).
The studies reviewed include those with exposure to secondhand smoke and exposure to specific constituents of secondhand smoke. When secondhand smoke was used, the studies were conducted with cigarette smoke, not smoke from cigars, pipes, or hookahs. Typically, reference cigarettes (cigarettes that are manufactured according to a standard formula for research purposes to provide researchers a consistent and uniform test item) or Marlboro cigarettes are used. Studies have not demonstrated much variation in constituents among cigarette brands and types (HHS, 2001), nor in the concentrations of constituents in secondhand smoke (Daisey, 1999).
As discussed by Hatsukami et al. (2006), “several physiological changes involving potential mechanisms of smoking-induced cardiovascular disease have been observed in cigarette smokers compared with nonsmokers” who have not been exposed to secondhand smoke.
Cigarette smoke, either mainstream or secondhand smoke, could produce cardiovascular disease by a number of interrelated modes of action, including oxidative stress, hemodyamic and autonomic effects, endothelial
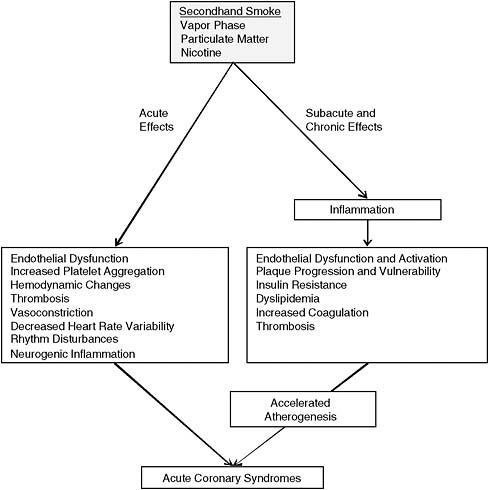
FIGURE 3-1 Potential mode of action of secondhand smoke.
NOTE: Schematic showing cardiovascular effects of secondhand smoke and how they might lead to acute myocardial infarction.
dysfunction, thrombosis, inflammation, hyperlipidemia or other effects (see Figure 3-1). Evidence related to those potential actions is discussed below, followed by a discussion of the effects of the individual constituents of secondhand smoke. Although those physiological changes have been observed and used to assess possible modes of action of secondhand smoke, to date most have not been formally validated as clinical tests and there is not a consensus within the scientific community that they are predictive of actual clinical disease (Ledford, 2008; Wang, 2008; WHO, 2007). Furthermore, a lack of specificity of exposure to secondhand smoke for those markers precludes their use as biomarkers that indicate a given case of cardiovascu-
lar disease is caused by exposure to secondhand smoke (Hatsukami et al., 2006). In this section, however, the committee uses those effects to examine whether secondhand-smoke exposure causes pathophysiologic changes that would contribute to the biological plausibility that decreasing secondhand-smoke exposure could lead to a decrease in acute myocardial infarctions (MIs).
EFFECTS OF CIGARETTE SMOKE
Oxidative Stress
As discussed in several review articles (for examples, see Armani et al., 2009; Burke and FitzGerald, 2003), oxidative stress could mediate many of the effects of smoke on the cardiovascular system. Such stress, during which endogenous antioxidants are overwhelmed by oxidants such as reactive oxygen species and free radicals, results in impaired cellular function. The exact mechanisms whereby oxidative stress leads to cardiovascular disease, such as atherosclerosis, are not clear, but it appears that oxidative stress may play a role in cardiovascular pathophysiology (Ballinger et al., 2002), and it could account for many effects of tobacco-smoke exposure, such as endothelial dysfunction, thrombosis, and inflammation (Raupach et al., 2006; Thomas et al., 2008).
Many constituents of mainstream and sidestream smoke are or produce free radicals capable of producing oxidative stress. Those constituents include vapor-phase carbonyl compounds (such as acrolein), oxides of nitrogen, metabolites of polycyclic aromatic hydrocarbons (PAHs), metals, and particulate matter (PM) (NRC, 1986). Mainstream cigarette smoke increases the concentrations of markers of oxidative stress, lipid peroxidation, protein oxidation, and DNA modification. Isoprostanes, indicators of lipid peroxidation and in vivo oxidation injury, are higher in smokers than in nonsmokers, and their concentrations decreased after smoking cessation for 2 weeks (Morrow et al., 1995). Smokers admitted to a cardiac outpatient center who then quit smoking had decreases in isoprostanes a few days after quitting, and the decreases continued until a steady state was reached 4 weeks after quiting (Pilz et al., 2000). Pignatelli et al. (2001) demonstrated that oxidized plasma proteins, another marker of oxidative stress, are higher in smokers than in nonsmokers.
Ahmadzadehfar et al. (2006) reported that exposure to secondhand smoke significantly increased isoprostane 8-epi-PGF2α in nonsmokers. After repeated secondhand-smoke exposure, isoprostane 8-epi-PGF2α in non-smokers reached nearly the same values as in smokers.
Probst-Hensch (2008) investigated whether the effects of secondhand smoke on the cardiovascular system are mediated by oxidative stress in a
sample of 1,122 nonsmoking subjects enrolled in an air-pollution study. Secondhand-smoke exposure was measured based on self-report during an interview as to “how many hours per day they were exposed to other people’s tobacco smoke (a) at home, (b) at the workplace, (c) in bars and restaurants, and (d) elsewhere.” Exposures were categorized as less than or equal to two hours per day, or more than two hours per day. The role of oxidative stress was assessed by looking at the interactions between glutathione S-transferase (GST) deficiency, which exhibits antioxidative properties, and the effects of secondhand smoke exposure on heart rate variability (HRV), a measure reflecting autonomic cardiac function. HRV was assessed from a 24-hour electrocardiogram recording, and subjects were genotyped for GSTM1, GSTT1, and GSTP1, GSTM1, GSTT1, and GSTP1 polymorphisms interacted with secondhand-smoke exposure to affect HRV. For example, the decrease in HRV in people exposed to secondhand smoke for more than two hours per day was greater when the GSTM1 genotype was deleted as compared to not deleted. That suggests a role of oxidative stress in secondhand smoke’s effects on HRV.
Furthermore, animal data reviewed in the surgeon general’s report (HHS, 2006) indicate that exposure to secondhand smoke worsens ischemic heart-event outcomes through free-radical activity. Animal data showed that a 30-min exposure to secondhand smoke resulted in oxidative DNA damage in the myocardium as measured by increases in 8-hydroxydeoxy-guanosine. Secondhand-smoke exposure also activates neutrophils, which leads to oxidation and tissue damage.
Data in animals also show oxidative effects. Secondhand-smoke exposure (30 mg/m3 total suspended particles from cigarette smoke, or equivalent to about two cigarettes every 15 minutes, for 6 hours per day, 5 days per week for 3 or 8 weeks) increased mitochondrial DNA damage in aortic tissue, which can be caused by increased reactive oxygen and nitrogen species, of apoE -/- mice (mice that lack a high-affinity ligand for lipoprotein receptors that result in them developing atherosclerotic plaques similar to those in humans) (Knight-Lozano et al., 2002). Data on apoE -/- mouse and human tissue indicate that mitochondrial DNA damage might be an early event in atherosclerosis (Ballinger et al., 2002). Eaton et al. (2006) examined the effect of acute tobacco-smoke exposure on mitochondrial function and calcium handling of cardiac cells in rats. Mitochondria were isolated after 6 h of secondhand smoke exposure (about 60 mg/m3, with an average nicotine concentration of 6.95 ± 0.62 mg/m3). Mitochondria from smoke-exposed rats had significantly higher adenosine diphosphate–stimulated production of adenosine triphosphate, had a more reduced redox state (nicotinamide adenine dinucleotide [NADH] ratio), showed more rapid membrane depolarization in response to calcium, and had significantly
increased cyclosporin A–sensitive Ca2+ release, although net Ca2+ uptake was unchanged.
Autonomic Effects
Cigarette smoke could affect the cardiovascular system through the autonomic nervous system, associated hemodynamic effects, or both. Heart rate is regulated by the interaction between the sympathetic and parasympathetic nervous systems. Sympathetic nervous system activation reduces heart-rate variability, and decreased heart-rate variability is associated with higher risk of cardiac death and of arrhythmic events after an acute MI (Buccelletti et al., 2009).
Smoking can have direct effects on heart rate, and those effects are thought to be mediated by actions on the sympathetic component of the autonomic nervous system. Nicotine acts on nicotinic cholinergic receptors in the brain and adrenal glands to activate the sympathetic nervous system, and this leads to epinephrine release. Nicotine thus acts as a sympathomimetic drug in increasing heart rate, blood pressure, and cardiac contractility and constricting some blood vessels (Haass and Kubler, 1997). Studies show that cigarette smoking increases a person’s heart rate (Benowitz et al., 1984; Minami et al., 1999). In the study by Minami et al. (1999) heart rate was higher by an average of 7 beats per minute while smoking compared to when not smoking, and smoking cessation for a week decreased heart rate.
Although nicotine from cigarette smoke transiently increases blood pressure, cigarette smoke has not been associated with hypertension in epidemiologic studies (HHS, 2004). Nicotine constricts coronary arteries via alpha-adrenergic effects (Winniford et al., 1986), and the coronary vasoconstriction is greater in diseased than in healthy coronary arteries (Nicod et al., 1984). In healthy smokers, coronary blood flow (CBF) increases in response to the cigarette smoking- or nicotine-mediated increase in myocardial work. In the absence of nicotine, however, the magnitude of the increase in myocardial work is less in healthy smokers. In people with coronary arterial disease, nicotine and cigarette smoke decrease CBF. Cigarette smoking is a strong risk factor for coronary vasospasm and for inadequacy of response to vasodilator medication (Caralis et al., 1992).
Secondhand smoke has been shown to affect heart-rate variability. Dietrich et al. (2007) examined the relationship between exposure to secondhand smoke and reduction in heart-rate variability. The study examined 1,218 nonsmokers ages 50 years and older who were participating in the Swiss Cohort Study on Air Pollution and Lung Disease in Adults (SAPALDIA) in 2001–2003. Those exposed to secondhand smoke for more than 2 h/day had lower heart-rate variability and a 2.7% higher heart rate
(95% CI, −0.01 to 5.34%) than those not exposed. The effects of secondhand smoke on heart-rate variability are similar to those observed after exposure to PM (Dietrich et al., 2007).
Argacha et al. (2008) further examined the vascular effects of secondhand smoke exposure to assess whether the effects are mediated by a non-specific reaction to smoke, or are more unique to tobacco smoke, the role of nicotine in the effects, the persistence of the effects following cessation of exposure, and the effect of secondhand smoke on microvascular function measured by skin blood flow. Using a cross-over design, the researchers exposed 11 healthy men to secondhand smoke, smoke from herbal cigarettes, or air (1 h exposure using a hermetic, 1-m3 Plexiglass box over the head of the subject, with a total of 6 cigarettes lit one every 10 minutes). Heart rate and aortic wave reflection increased and transit time decreased following exposure to secondhand-tobacco smoke, but not smoke from herbal cigarettes or air. None of the exposures affected blood pressure. Skin blood flow at normal temperature was unchanged by any of the treatments but was decreased in response to heating after exposure to secondhand-tobacco smoke. None of the effects of secondhand smoke persisted 20 minutes after exposure. A separate group of 14 men received 2 mg nicotine via a sublingual tablet; in those subjects, the effects on aortic wave reflection were related to the serum nicotine concentrations, indicating a possible role of nicotine in these effects seen after exposure to secondhand smoke.
Endothelial Dysfunction
The vascular endothelium, which lines the arteries, is a semipermeable layer of cells that are involved in the modulation of platelet activation, leukocyte adhesion, thrombosis, and regulation of vascular tone. It plays an important role in the regulation of blood flow, controlling the dilation and constriction of arteries (Hadi et al., 2005). Part of that regulation is through the production of vasoactive substances by the endothelial cells, including nitric oxide (NO), endothelin, prostacyclin, and angiotensinogen (Al-Qaisi et al., 2008). Endothelial dysfunction is one of the key early steps in the pathway to atherosclerosis (Hadi et al., 2005).
Oxidant chemicals produce endothelial dysfunction both by injuring endothelial cells and by degrading NO, the latter of which normally has vasodilator and antiplatelet activity (Heiss et al., 2008; Zhang et al., 2006). Impaired endothelial function in smokers, as measured by flow-mediated dilation of the brachial artery, can be reversed, at least in part, by antioxidants (de Sousa et al., 2005; Neunteufl et al., 2000; Raitakari et al., 2000; Takase et al., 2004; Young et al., 2006). Nicotine was also reported to impair endothelial function acutely in human smokers. Smokers also have increased markers of endothelial dysfunction (Rocchi et al., 2007).
As discussed in the surgeon general’s report on secondhand smoke (HHS, 2006), data from experiments in animals and humans demonstrate that secondhand smoke also disrupts endothelial function by reducing NO. Endothelium-dependent vasodilation in nonsmokers is affected by chronic and acute exposures to secondhand smoke.
Mack et al. (2003) examined the effect of chronic exposure to secondhand smoke on arterial wall stiffness in baseline data from 227 never smokers (102 men, 125 women) enrolled in a clinical trial looking at Vitamin E treatment. Ultrasound images were used to measure arterial diameter and carotid artery intima-media thickness (IMT). A carotid stiffness index beta, computed using the change in arterial diameter between maximum and minimum dilation, was used as an indicator of arterial wall stiffness. Smoking and secondhand-smoke exposures (number of smokers and hours per day exposed at home, number of daily exposures at work and outside the home and work) were ascertained through a questionnaire. The stiffness index was associated with body mass index, fasting glucose, and IMT. The stiffness index was not related to exposure to secondhand smoke in the overall study population, but did increase with increased number and daily sources of exposure to secondhand smoke in those subjects with a body mass index of 27.1 kg/m2 or higher, ages 55 years or older, or with an IMT of 0.707 mm or higher. No other associations were statistically significant, including separate analyses by sex and age.
Heiss et al. (2008) exposed healthy nonsmokers to smoke-free air or secondhand smoke for 30 min on two nonconsecutive days and measured markers of endothelial dysfunction (Heiss et al., 2008). Plasma cotinine concentrations were unchanged after exposure to smoke-free air and reached about 0.3 ng/mL, a level “commonly observed in passive smokers” after exposure to secondhand smoke. The secondhand-smoke exposure increased endothelial progenitor cells (EPCs) and plasma vascular endothelial growth factor but eliminated EPC chemotaxis and decreased endothelial function as measured by flow-mediated dilation (FMD). The effects on FMD returned to normal after 2.5 h, but the effects on endothelial growth factors were still increased after 24 h. The detection of endothelial-cell damage in the blood as a result of short-term exposure to secondhand smoke suggests endothelial damage.
A 30-min exposure to secondhand smoke in a smoking room significantly reduced the coronary flow-velocity reserve in nonsmokers to a level similar to that seen in smokers before and after exposure to secondhand smoke (Otsuka et al., 2001). A 5-min exposure to secondhand smoke (mean carbon monoxide level in the exposure chamber, 30 parts per million) significantly reduced aortic distensibility in nonsmokers and smokers (Stefanadis et al., 1998).
Arterial stiffness can result in the impairment of the elasticity of the
aorta. Mahmud and Feely (2004) used wave reflection in the aorta as a marker of arterial stiffness to study the effect of exposure to secondhand smoke (15 cigarettes lit in an unventilated room over the course of 1 hour) on healthy nonsmokers (10 men, 11 women). No baseline differences were seen between the controls and treated groups. Following exposure to secondhand smoke brachial and aortic systolic blood pressure increased in males but not females, and an abnormality was observed in the radial and aortic pressure waveforms; no changes were seen in brachial or aortic diastolic blood pressure, heart rate, or left ventricular ejection duration in either sex. No changes were seen in a control group (6 men, 6 women) exposed to air only.
Kato et al. (2006) examined FMD in the bronchial artery and 8-isoprostane levels as indicators of vascular endothelial function and oxidative stress, respectively, in 30 male subjects (15 smokers who had abstained from smoking for at least 12 hours, 15 nonsmokers) exposed to secondhand smoke from 15 cigarettes (in a room 3 meters by 4 meters with a 2.5 meter ceiling with ventilation) for 30 minutes. FMD was lower and the levels of 8-isoprostane were higher at baseline in smokers than nonsmokers; neither changed in smokers. In nonsmokers, however, FMD decreased and the levels of 8-isoprostane increased following exposure to secondhand smoke.
Giannini et al. (2007) studied the effects of exposure to secondhand smoke (20 minutes in a 60 cubic meter enclosed space with 15 to 20 cigarettes smoked, achieving 30–35 ppm carbon monoxide) on vascular reactivity of the brachial artery (measured by FMD) in 18 healthy, nonsmoking volunteers. Carboxyhemaglobin was elevated after exposure to secondhand smoke. FMD was decreased following the exposure, but nitroglycerin-induced vasodilation was not changed significantly.
In contrast, in a study of 12 healthy nonsmokers (9 men, 3 women) exposed acutely to secondhand smoke (smoke from three cigarettes for 15 minutes with a clear plastic hood over the participant’s head; air was mixed with the smoke to maintain a carbon monoxide concentration of 20–40 ppm) no effects on vasodilation were seen (Kato et al., 1999). Carboxyhemoglobin concentrations increased from 0.53 ± 0.05% at baseline to 0.79 ± 0.05% after 30 minutes of exposure and plasma nicotine concentrations increased from 0.46 ± 0.12 ng/ml at baseline to 1.38 ± 0.47 ng/ml after exposure. Forearm vascular resistance, either baseline or its response to an endolethium-dependent vasodilator (acetylcholine) or an endothelium-independent vasodilator (sodium nitroprusside), was not changed by exposure to secondhand smoke.
Hausberg and Somers (2008) also saw no changes in forearm blood flow following exposure of 16 healthy nonsmokers beyond the changes seen in response to administration of vehicle. A significant increase was seen in muscle sympathetic nerve activity following the exposure to secondhand
smoke, but changes were not seen in blood pressure, except for the response to the cold pressor test, heart rate, and plasma concentrations of epinephrine and norepinephrine.
Data from animal studies demonstrate that some components of secondhand smoke—1,3-butadiene and PAHs that include 7,12-dimethylbenz [a,h]anthracene and benz[a]pyrene—speed up atherosclerosis development, which results from cell injury and hyperplasia (HHS, 2006). In addition, animal experiments have shown that exposure to secondhand smoke for a few weeks significantly accelerates the atherosclerotic process. Constituents of smoke increase low-density lipoprotein (LDL) cholesterol in the artery lining and bind it to the vessel wall (Roberts et al., 1996).
Platelets interact with subendothelial connective tissue, and damaged endothelial cells also play a role in plaque formation. Secondhand-smoke exposure is associated with the build up of glycoaminoglycan and glycoprotein in animal models, which results in atherogenesis (Latha et al., 1991).
Thrombosis
Platelets (thrombocytes) are cell derivatives that circulate in the blood and play a role in clot formation. When platelets are activated, they become sticky and adhere to each other (coagulate); platelets also can adhere to damaged vascular endothelium. Adherence of platelets increases thrombus formation, disrupts the coronary artery lining, speeds progression of atherosclerotic lesions, and is associated with increased risk of ischemic heart disease (Law and Wald, 2003). The acute cardiovascular effects of cigarette smoke result to a substantial degree from thrombosis-related events (Rahman and Laher, 2007).
In humans, platelet activation has been studied by measuring urinary excretion of thromboxane (TxM), a metabolite of thromboxane A2, which is released when platelets aggregate in vivo. Smokers have higher concentrations of TxM than nonsmokers (Modesti et al., 1989). One study found that the decline in TxM after smoking cessation was not found when smokers used nicotine patches but was found in those who did not use patches (Saareks et al., 2001). In another study, however, smoking cessation yielded similar decreases in TxM excretion regardless of the use of nicotine patches (Benowitz et al., 1993; Ramachandran et al., 2004). The role of nicotine in that effect, therefore, remains unclear.
Experimental research indicates that secondhand-smoke exposure results in increased platelet activation and aggregation. Researchers assayed platelet sensitivity, an indication of platelet aggregation, in human subjects (smokers and nonsmokers). Platelet sensitivity in nonsmokers increased after subjects sat for 20 min in a room where cigarettes had just been smoked (Burghuber et al., 1986) or in a corridor where others were smoking (Davis
et al., 1989). In addition, data on rabbits receiving a high-cholesterol diet (Sun et al., 1994; Zhu et al., 1993) and rats (Zhu et al., 1994) demonstrate that bleeding time, a measure of platelet aggregation, is shortened on exposure to secondhand smoke. Some studies have reported that nicotine in high doses activates platelets in animals (McDonald et al., 1973; Nemr et al., 2003).
Inflammation
Cigarette smoke produces systemic inflammatory effects. Although those biological effects have not been validated as predicting differences in tobacco-related injury or disease risk in randomized clinical trials, they have been predictors of future cardiovascular events in observational epidemiologic studies (Lindahl et al., 2000; Packard et al., 2000). High concentrations of activated oxygen species found in tobacco smoke could potentially damage heart muscle cells and lead to inflammation, which can result in additional organ injury.
Smoking is associated with higher polymorphonuclear (PMN) leukocyte counts, fibrinogen, C-reactive protein (CRP), and other inflammatory markers (HHS, 2004). Some in vitro and animal studies report that nicotine is a chemoattractant, enhances leukocyte adhesion, and increases release of some proinflammatory cytokines (Di Luozzo et al., 2005; Heeschen et al., 2003; Lau et al., 2006). Studies of smokers switching to nicotine medications, however, have found that inflammatory biomarkers decline as in those who quit smoking and do not take nicotine; this suggests that the nicotine in smoke is not responsible for the inflammation (Benowitz and Gourlay, 1997).
Venn and Britton (2007) examined the relationship between secondhand-smoke exposure, measured as plasma cotinine, and biomarkers of heart-disease risk—including CRP, homocysteine, fibrinogen, and white-cell count—in 7,599 never-smokers in the third National Health and Nutrition Examination Survey (NHANES III). Subjects with detectable but low serum cotinine concentrations (0.05–0.215 ng/mL) had significantly higher concentrations of fibrinogen (adjusted mean difference, 8.9 mg/dL; 95% CI, 0.9–17.0) and homocysteine (0.8 μmol/L; 95% CI, 0.4–1.1), but not CRP or white-cell count, than subjects with no detectable cotinine. Similar effects were observed in those with high serum cotinine concentrations (more than 0.215 ng/mL). The increased concentrations of fibrinogen and homocysteine observed in subjects exposed to secondhand smoke were about 30–45% of the concentrations in smokers.
Similarly, Wilkinson et al. (2007) used the NHANES III data to examine the relationship between secondhand-smoke exposure and CRP, focusing on never-smokers ages 6–18 years. An increase in serum cotinine
of 0.5 ng/mL was associated with an increase in CRP of 0.96 mg/dL (95% CI, 0.93–1.00).
Clark et al. (2008) used serum cotinine concentrations and the NHANES data (1999–2002) to examine the relationship between secondhand-smoke exposure and markers of inflammation in adult workers. Inflammatory markers analyzed included CRP, fibrinogen, homocysteine, and white cells. Serum cotinine concentrations were categorized as below the detection limit, low (above the detection limit but below 0.2 ng/mL), or high (0.2–15 ng/mL). Workers exposed with low and high levels of cotinine had significantly higher concentrations of homocysteine than unexposed workers. No significant differences were seen in concentrations of CRP, fibrinogen, and white cells.
Flouris et al. (2008) explored the sex-specific secondhand-smoke effects on gonadal and thyroid hormones, inflammatory cytokines, and vascular function. After exposing 28 nonsmoking adults (14 men and 14 women) to a simulated bar-restaurant environment for 1 hour, the study found interleukin-1β and systolic blood pressure significantly increased in men but not women. Gonadal hormones, however, were decreased following secondhand smoke exposure in both men and women.
Hyperlipidemia
Cigarette-smoking is associated with low high-density lipoprotein cholesterol (HDL-C), which is a risk factor for atherogenesis. Smoking is believed to exert effects on lipids, at least in part, by the sympathomimetic effects of nicotine (Woodward et al., 2006). Nicotine increases lipolysis and increases free fatty acid concentrations (Hellerstein et al., 1994). Increased fatty acid turnover is associated with overproduction of very-low-density-lipoprotein (VLDL) cholesterol, increased LD cholesterol, and decreased HDL cholesterol. One study reported that nicotine-patch administration prevented the expected normalization of HDL cholesterol after smoking cessation (Moffatt et al., 2000). Studies of smokeless tobacco users have been used to separate effects of nicotine (similar exposure from cigarette smoking and smokeless tobacco use) from the effects of combustion products (cigarette smoke only). The data on lipid abnormalities comparing smokeless tobacco to nontobacco users is conflicting, making it difficult to ascertain the role of nicotine (Tucker, 1989; Wallenfeldt et al., 2001).
Moffat et al. (2004) assessed the effect of secondhand smoke on blood lipids. Exposure of 12 healthy, male nonsmokers to secondhand smoke (6-hour continuous exposure in a smoking chamber with a volunteer smoker smoking six cigarettes at a rate of one cigarette per hour plus nine other cigarettes burned to attain mean air concentrations of carbon monoxide of 12 ppm, and nicotine of 16 μg/m3) reduced HDL-C, increased the
total cholesterol to HDL-C ratio, and decreased the HDL2-C to HDL3-C ratio by 18%, 14%, and 13% at 8, 16, and 24 hours after exposure. No effects were seen on total cholesterol.
Yuan et al. (2007) developed a smoking system that simulated secondhand-smoke exposure and a mouse model to examine effects related to atherogenesis. They found that exposure to secondhand smoke (6 hours per day consisting of 10 minutes of smoking with a 5 minute break for 5 days per week; particle concentration was maintained at 25 ± 2 mg/m3) decreased plasma HDL-C in the blood and decreased the ratios of HDL-C to LDL-C, of HDL-C to triglyceride, and of HDL-C to total cholesterol. Those changes lead to lipid accumulation in the aorta and lipid deposition in heart vessels and hepatocytes. Smoke-exposed mice also had increased monocyte chemoattractant protein (MCP) in the circulation and heart tissues, increased macrophages in arterial walls, and decreased adiponectin (adiponectin protects endothelial cells).
Other Effects
Research has examined the relationship between secondhand-smoke exposure and metabolic syndrome, a clinical diagnosis whose characteristics include central obesity, dyslipidemia, hyperglycemia, and hypertension, as well as glucose intolerance and diabetes. Weitzman et al. (2005) used data from 3,211 adolescents (12 to 19 years of age) in NHANES III, 1988–1994) and found that exposure to secondhand smoke, as assessed by self-report or by serum cotinine concentrations, was associated with the metabolic syndrome. Houston et al. (2006) compared secondhand-smoke exposure, ascertained by a questionnaire administered by an interviewer and serum cotinine concentrations, and time to develop glucose intolerance in the Coronary Artery Risk Development in Young Adults (CARDIA) study. Never smoking subjects exposed to secondhand smoke had a greater risk of developing glucose intolerance than those nonsmokers not exposed to secondhand smoke (no detectable serum cotinine).
Metsios et al. (2007) exposed 18 healthy, nonsmoking adults (9 females, 9 males) to secondhand smoke (generated by combustion of a variety of brands of cigarettes adjusted to a carbon monoxide concentration of 23 ± 1 ppm) for 1 hour inside an environmental chamber and examined resting energy expenditure, as an indicator of metabolism, and thyroid hormones, both of which have previously been shown to be affected by smoking. Secondhand-smoke exposure increased resting energy expenditure, and T3 and fT4 thyroid hormone concentrations.
EFFECTS OF CONSTITUENTS OF CIGARETTE SMOKE
The constituents of secondhand smoke are discussed in Chapter 2. The following section describes the cardiovascular effects of some of those constituents. These data are from experimental studies in cells or animals or, in some cases, intentional human-dosing studies. Some of the effects in cell systems or animals are seen with exposures above those seen in humans following secondhand-smoke exposure. Also, because of the differences in the experiments and the overlapping end points discussed across the different chemicals, it is not possible to parse out or attribute a specific effect to a specific component of secondhand smoke (Smith and Fischer, 2001a). These pathophysiologic data, however, are important for investigating the potential modes of action of secondhand smoke, as well as contributing to the plausibility that secondhand smoke could have cardiovascular effects. Table 3-1 summarizes the effects of these compounds.
Carbonyls
Vapor-phase carbonyls—mainly acrolein, acetaldehyde, butyraldehyde, formaldehyde, and propionaldehyde—are some of the most reactive and abundant constituents of cigarette smoke and as a group are emitted at a rate of about 1,000 μg/cigarette in mainstream smoke (Dong and Moldoveanu, 2004). Because of their reactivity, carbonyls are more difficult to measure and conduct experiments with and so are not as well characterized as other smoke constituents, but they are likely to have toxic effects, including oxidative stress, as a result of their reactivity. Among the carbonyls, the α,β compounds—such as acrolein, crotonaldehyde, and 3-vinylpyridine—are most reactive and therefore more likely to be cardiotoxic than less reactive carbonyls, such as acetaldehyde, butyraldehyde, and formaldehyde. The concentration of acrolein and other carbonyls in indoor air may exceed outdoor concentrations by a factor of 2–20. Concentrations of 20–300 μg/m3 have been reported in smoky indoor environments, such as bars, restaurants, automobiles, and trains (Badre et al., 1978).
Because of efficient electron delocalization, acrolein and related α,β-unsaturated carbonyls are highly electrophilic and react avidly with nucleophilic cell constituents, such as glutathione; lysine, histidine, and arginine side chains of proteins; guanosine in nucleic acids; and amino phospholipids (Esterbauer et al., 1991). Their high cardiovascular toxicity has been demonstrated in a variety of in vivo and in vitro systems (Bhatnagar, 2004).
Isolated rat hearts perfused with 10 μM acrolein become arrhythmic and stop contracting within 15 minutes (Sklar et al., 1991). Low doses of such aldehydes as acrolein and formaldehyde have vasopressor effects (Green and Egle, 1983), which suggest a potential mechanism for increased
TABLE 3-1 Known Cardiovascular Toxicity of Cigarette Smoke Constituentsa
|
Compound |
Cardiovascular Toxicity |
Risk Categoryb |
|
Carbon monoxide |
Moderate |
Suppression of cardiac function, S-T depression in patients with stable CAD |
|
Nicotine |
High |
Hemodynamic changes |
|
Acetaldehyde |
Low |
|
|
Acetic acid |
Low |
|
|
Nitrogen oxides |
Low |
|
|
Formaldehyde |
Medium |
Hypertension, atherosclerosis |
|
Benzene |
Moderate |
Tachycardia, arrhythmia, arterial hypertension |
|
Acetone |
Low |
|
|
Catechol |
Low |
|
|
1,3-butadiene |
Moderate |
Atherosclerosis |
|
Toluene |
Low |
|
|
Methanol |
Low |
|
|
Hydroquinone |
Low |
|
|
Phenol |
Low |
|
|
Acrolein |
High |
Hypertension; atherogenesis, decreased plaque stability, increased thrombosis; suppression of coronary flow and cardiac contractility |
|
Methylethylketone |
Low |
|
|
Propionaldehyde |
Low |
|
|
Pyridine |
Moderate |
|
|
Carbon disulfide |
Moderate |
Hypertension, ischemic heart disease, thrombosis, hypercholesterolemia, arrhythmias, decreased cardiac output |
|
3-vinylpyridine |
Moderate |
Atherosclerosis |
|
Cholesterol |
Low |
|
|
Crotonaldehyde |
High |
Hypertension, atherogenesis, decrease in plaque stability, increased thrombosis; suppression of coronary flow and cardiac contractility |
|
Butyraldehyde |
Moderate |
Hypertension |
|
Compound |
Cardiovascular Toxicity |
Risk Categoryb |
|
Cadmium |
High |
Endothelial dysfunction, inflammation, atherosclerosis |
|
Lead |
High |
Hypertension |
|
Benzo[a]pyrene |
Moderate |
Ischemic heart disease, atherosclerosis |
|
1,3 butadiene |
High |
Increased CVD risk and atherogenesis |
|
Particulate matter |
High |
Arrhythmias, atherosclerosis, ischemic heart disease, hypertension, heart failure, stroke, insulin resistance |
|
NOTE: CAD, coronary artery disease; CVD, cardiovascular disease. a The cardiovascular toxicity of most secondhand-smoke constituents is unknown. bData are compiled from Bhatnagar, 2006; HHS, 2006; O’Toole et al., 2009; and Smith and Fischer, 2001. |
||
systolic blood pressure. Acrolein can form protein adducts and can oxidize thioreduxins in endothelial cells—effects that promote atherogenesis in vitro. Epidemiologic data indicate that occupational exposure to aldehydes increases the risk of cardiovascular disease. The increased risk of atherosclerotic heart disease in workers in plants that produce formaldehyde (Stewart et al., 1990) and the higher incidence of heart disease in undertakers (Levine et al., 1984), embalmers (Walrath and Fraumeni, 1984), and perfumery workers (Guberan and Raymond, 1985) have been linked to aldehyde exposure.
Mechanistic studies show that exposure to aldehydes decreases cardiac contractility (Luo et al., 2007), increases thrombosis, and leads to dyslipidemia and lipoprotein modification (Bhatnagar, 2004). Those changes could acutely and chronically increase cardiovascular disease risk. Like secondhand smoke, acrolein induces endoplasmic reticulum stress and triggers the unfolded-protein response (Haberzettl et al., 2009). Acute exposure to acrolein activates matrix metalloproteases in advanced plaques of apoE-null mice (O’Toole et al., 2009); this indicates that exposure to acrolein in secondhand smoke could destabilize arterial lesions and trigger coronary events and acute MI. Inhalation exposure to acrolein at concentrations found in secondhand smoke can induce endothelial dysfunction in mice similar to that observed on exposure to secondhand smoke (Conklin et al., 2009); this dysfunction was exaggerated on deletion of glutathione S-transferase P (GST-P), indicating that differences in metabolic disposition of acrolein due to polymorphic variations in the human GST-P gene may be a significant modulator of human cardiovascular disease risk due to
secondhand-smoke exposure. Aqueous extracts of cigarette smoke, acrolein and crotonaldehyde, each induce neurogenic inflammation by stimulating the excitatory ion-channel transient receptor potential type A1 (TRPA1) (Andre et al., 2008). Those observations suggest that unsaturated aldehydes may be the main causative agents in the activation of airway sensory neurons, which results in neurogenic inflammation and respiratory hypersensitivity. It remains unclear, however, whether respiratory or inflammatory changes secondary to aldehyde-induced activation of TRPA1 could account for the cardiovascular effects of secondhand smoke.
Other aldehydes generated in secondhand smoke—such as formaldehyde, butyrlaldehyde, and acetaldehyde—are less toxic, but they could increase the toxicity of acrolein and crotonaldehyde. It has been shown that coexposure to acrolein with other aldehydes, such as formaldehyde and acetaldehyde, results in a more pronounced decrease in respiratory rate in male Wistar rats than exposure to acrolein only (Cassee et al., 1996). Moreover, such aldehydes as acrolein and formaldehyde are adsorbed on carbon, and this could further facilitate their pulmonary deposition and systemic delivery. That is supported by the observation that acrolein or formaldehyde delivered adsorbed on carbon or simultaneously with carbon is chemotactic for PMNs (Kilburn and McKenzie, 1978) and that coadministration of acrolein with carbon black, but not either agent alone, has a combined effect on the innate and acquired defenses of the lung (Jakab, 1993). Therefore, aldehydes delivered in cigarette smoke if carried on PM are likely to be more toxic and penetrate more deeply than those present in volatile gases.
Butadiene
Butadiene is a reactive component of the vapor phase of secondhand smoke. It is generated at about 400 μg/cigarette (Cal EPA, 1991). Sampling in indoor bars where there is smoking and measurements of personal exposure in workplaces where there is smoking indicate 1,3-butadiene concentrations of 1–4 μg/m3 (Brunnemann et al., 1990; Heavner et al., 1996). A smoking ban in an Irish pub has been shown to result in a 95% reduction in 1,3-butadiene concentrations (McNabola et al., 2006). Butadiene has known carcinogenic activity (Jackson et al., 2000), and chronic exposure to 1,3-butadiene has also been linked to an increase in risk of cardiovascular disease. In a case–control cohort study of workers in a styrene-butadiene manufacturing plant in the United States from 1943 to 1982, black workers had a significantly increased standardized mortality ratio (SMR) for cardiovascular disease risk (1.47; 95% CI, 1.17–1.77); the SMR for cardiovascular disease was not increased in white workers (Matanoski and Tao, 2002). The atherogenic potential of butadiene has been documented in experimen-
tal animals. The studies showed that exposure at 20 ppm accelerates the speed at which plaque development occurs in cockerels (Penn and Snyder, 1996) although the incidence of plaque development was not significantly different between the exposed and unexposed groups. Acute effects of butadiene on endothelial function or hemodynamics have not been reported, and the cardiovascular disease risk posed by butadiene at concentrations present in secondhand smoke has not been assessed directly.
Metals
Sidestream tobacco smoke contains traces of metals including cadmium, chromium, lead, and nickel (Cal EPA, 2005a). The cardiovascular toxicity of trace metals has not been well studied. However, because of their ability to inhibit the electron transport chain and to increase the generation of reactive oxygen species, they could induce cardiovascular dysfunction even at low exposures. Bernhard et al. (2006) examined serum concentrations of metals in young nonsmokers, passive smokers, and smokers. No significant differences were seen in serum concentrations of aluminum, cobalt, copper, iron, manganese, nickel, lead, or zinc in smokers compared with nonsmokers. However, serum concentrations of cadmium and strontium were significantly higher in smokers compared with nonsmokers.
Cadmium, in particular, has been reported to be highly toxic to cardiovascular tissue. At concentrations found in smokers it dysregulates transcription, exerts stress, and damages the structural integrity of the vascular endothelium (Bernhard et al., 2006). Measurements of antioxidant enzymes indicated that the heart is more vulnerable to dietary cadmium than are the kidneys (Jamall and Roque, 1989). Cadmium compounds stress and may deregulate transcription, damage the vascular endothelium, and have proinflammatory properties. Cadmium has also been linked to high risk of peripheral arterial disease (PAD) in smokers (Navas-Acien et al., 2004). It appears to be an important mediator of smoking-induced PAD in that it has been reported that adjustment for cadmium decreased the strength of association between PAD and smoking (Navas-Acien et al., 2004). That cadmium has been shown to increase atherosclerosis in susceptible animal models (Revis et al., 1981; Subramanyam et al., 1992) suggests that it could also contribute to the chronic atherogenic effects of secondhand smoke.
Exposure to lead at low concentrations has been linked to hypertension. A meta-analysis of more than 30 epidemiologic studies, however, found only a weak association between increased blood pressure and increased blood lead in humans (Nawrot et al., 2002). Chronic exposure of rats to lead in drinking water at low concentrations has been reported to increase blood pressure in rats, and the increase was associated with an increase in
the abundance of markers of oxidative stress; hence, lead might increase the production of reactive oxygen species and decrease NO bioavailability (Gonick et al., 1997; Marques et al., 2001; Nowack et al., 1993). A similar weak association has been reported between blood lead and all-causes circulatory and cardiovascular mortality (Lustberg and Silbergeld, 2002). Whether exposure to secondhand smoke results in an increase in blood-lead levels sufficient to induce cardiovascular toxicity has not been established. Also, the cardiovascular toxicity of low concentrations of chromium and nickel has not been reported.
Carbon Disulfide
Chronic occupational exposure to carbon disulfide (CS2) has been associated with an increased prevalence of high cholesterol concentrations, atherosclerosis, and ischemic heart disease. Several studies of workers in the viscose-rayon industry have reported significant excesses in mortality due to coronary arterial disease and cardiovascular mortality (Balcarova and Halik, 1991; Omae et al., 1998; Partanen et al., 1970). Occupational exposure to CS2 (between 3 and 65 ppm) has been found to be significantly associated with an increase in LDL cholesterol and with systolic and diastolic blood pressure (Chang et al., 2007; Egeland et al., 1992; Kotseva and De Bacquer, 2000). Exposed workers are at high risk for electrocardiogram (ECG) abnormalities (Kuo et al., 1997). Animals exposed to high concentrations of CS2 (225 ppm for 6 h for 14 weeks) had increased blood pressure and decreased cardiac output (Morvai et al., 2005). The rapid reversibility of the effect of CS2 on cardiovascular disease indicates that the effect is directly cardiotoxic or thrombotic (Sweetnam et al., 1987). The reported cardiovascular effects of CS2, however, seem to appear only after long exposure (5–10 years) at high concentrations. It has been estimated, for example, that it may take a cumulative exposure index of 58–220 year–ppm for viscose-rayon workers to develop hypertension (Chang et al., 2007). Although CS2 has been detected in secondhand smoke, the concentration measured was several orders of magnitude lower than its permissible exposure limit of 10 ppm. Nevertheless, the effects of low-dose human or animal exposure to CS2 in tobacco smoke on cardiovascular disease have not been examined.
Benzene
Tobacco smoke contains relatively high concentrations of benzene. Approximately 30 μg/cigarette are in mainstream smoke (Smith and Fischer, 2001b) and 163–353 μg/cigarette are emitted into sidestream smoke. Workers occupationally exposed to high concentrations of benzene have an
increased prevalence of arterial hypertension and pathologic ECG changes related to conduction defects and repolarization disturbances (Kotseva and Popov, 1998). Excessive cardiovascular disease risk in commercial press workers (Zoloth et al., 1986) and perfume-industry employees (Guberan and Raymond, 1985) has also been linked to exposure to solvents that include benzene. Subacute poisoning with benzene causes disorders in repolarization and arrhythmia as measured by ECG (Morvai et al., 1976). In rats, benzene increases ventricular tachycardia induced by epinephrine (Juhasz and Bodor, 2000). Benzene also increases the number of ectopic ventricular beats after induction of arrhythmia produced by coronary ligation or aconitine (Magos et al., 1990). Rats and guinea pigs inhaling benzene vapor develop ventricular tachycardia (Tripathi and Thomas, 1986).
The effects of exposure of humans or animals to doses of benzene relevant to those derived from secondhand smoke are unknown.
Nicotine
Nicotine has the potential to have adverse effects on cardiovascular function, although the magnitude of its contribution to cardiovascular disease caused by smoking or exposure to secondhand smoke is uncertain. A contribution of nicotine to cardiovascular events due to secondhand smoke is less likely because the amount of nicotine absorbed in the systemic circulation from secondhand smoke is extremely small. Nonetheless, because we cannot definitively exclude any contribution of nicotine, we briefly review here some of the concerns about nicotine and cardiovascular toxicity.
Studies of users of smokeless tobacco suggest that nicotine is not a major contributor to cardiovascular disease (Arabi, 2006). Users of smokeless tobacco are chronically exposed to as much nicotine as smokers. However, epidemiologic studies have found no increase or small increases in cardiovascular risk in smokeless-tobacco users compared with nonusers of tobacco; in studies that did find some risk, the risk was much lower in smokeless-tobacco users than in cigarette smokers (Arabi, 2006; Hergens et al., 2008; Lee, 2007).
In smokers, nicotine is believed to contribute to abnormalities in lipid profiles. Nicotine, in part by systemic release of catecholamines, increases lipolysis and increases free fatty acid concentrations (Andersson and Arner, 2001; Andersson et al., 1993; Hellerstein et al., 1994; Sztalryd et al., 1996). Increased free fatty acid turnover is associated with the overproduction of cholesterol VLDL, which results in lowering of HDL-C (Therond, 2009). Nicotine is also believed to contribute to insulin resistance via effects of the release of catecholamines (Chelland Campbell et al., 2008); such an effect is unlikely to contribute to insulin resistance in people exposed to secondhand smoke, however, because the nicotine exposure is so low.
Nicotine in amounts delivered in cigarette smoke acts as a sympathomimetic drug in increasing heart rate, blood pressure, and cardiac contractility and in constricting some blood vessels (Benowitz, 2003). Nicotine infusion impairs endothelial function in people (Chalon et al., 2000). Studies in cell systems have reported that nicotine can down-regulate the expression of endothelial nitric oxide synthase, an enzyme involved in the generation of NO, which mediates vasodilation (Zhang et al., 2001). Nicotine also is reported to up-regulate asymmetric dimethylarginine, which would further impair the release of NO (Jiang et al., 2006). Animal studies comparing effects of secondhand-smoke exposure on vascular function found no difference in the extent of impairment of endothelial function between smoke generated from cigarettes with nicotine and without nicotine, and this suggests that the contribution of nicotine was minor at most.
Nicotine might contribute to inflammation by increasing concentrations of intracellular adhesion molecules and vascular cell adhesion molecules, which would result in greater adhesion of leukocytes to blood vessels and thus could promote inflammation and atherogenesis. Nicotine increases secretion of the proinflammatory cytokine interleukin-12 in cultured dendritic cells (Aicher et al., 2003). It is reported to promote release of growth factors, and this could enhance vascular cell proliferation and contribute to atherogenesis (Cucina et al., 2000a,b,c). It has also been reported to promote angiogenesis, which could contribute to progression of atherosclerotic plaques. Nicotine has been shown in experimental systems to release growth factors, including NO, prostacyclin, vascular endothelial growth factor, and fibroblast growth factor (Heeschen et al., 2001; Lane et al., 2005). The relevance of the animal models of effects of nicotine on vascular function to human responses to secondhand smoke is not clear (Hanna, 2006). The effects of nicotine reported in some in vitro and animal studies are opposite the effects of cigarette-smoke exposure. Furthermore, the doses of nicotine administered in many experimental studies are much higher than those seen in smokers and far higher than those exposed to secondhand smoke. Most mechanistic studies involve acute administration of nicotine, whereas tolerance of nicotine develops in chronic exposure, as might be the case with long-term secondhand-smoke exposure (Hanna, 2006). Acute exposures could occur, for example in people who are not routinely exposed to secondhand smoke but periodically visit a smoky bar or restaurant. The contributions of small doses of nicotine seen in secondhand-smoke exposure to human cardiovascular disease, however, are difficult to predict.
Polycyclic Aromatic Hydrocarbons
Several PAHs present in tobacco smoke—including heterocyclines, heterocyclic aromatic amines, and nitro compounds—have been shown
to be potent locally acting carcinogens in laboratory animals, but their cardiovascular effects are not well understood. Several epidemiologic and toxicologic studies have provided evidence that occupational exposure to PAHs is a risk factor for ischemic heart disease. In a cohort of male asphalt workers, indexes of exposure to benzo[a]pyrene were positively associated with mortality from ischemic heart disease (Burstyn et al., 2005). The highest relative risk (RR) of fatal ischemic heart disease (1.64) was observed in connection with average benzo[a]pyrene exposures at 273 ng/m3 or higher. Ramos and Moorthy (2005) reviewed evidence for a role and potential modes-of-action of PAHs in inducing vascular injury and atherosclerosis, presenting data on the formation of PAH-DNA adducts within vessel walls following bioactivation of PAHs. Acute effects of PAHs on thrombosis, endothelial dysfunction, and arrhythmias, however, have not been reported in the literature. The cardiotoxicity of many PAHs and how they might interact with benzo[a]pyrene have not been evaluated, and this adds to the uncertainty in the pathophysiology of PAHs in secondhand smoke.
Particulate Matter
Indoor particles due to secondhand smoke have been categorized as respirable, or “fine” particles that can be inhaled into the lungs and pose health concerns. Sidestream smoke particle size has been reported to range from 0.01 to 1.0 micrometers, with both the mean and median particle diameter in the submicrometer size range (Cal EPA, 2005a). The particles, therefore, are included when sampling is conducted for particles that are less than 2.5 μm in diameter (referred to as PM2.5, the so-called fine fraction). In contrast, the particulate phase of mainstream smoke is a concentrated aerosol with more than 5 × 1025 particles per cubic centimeter (Ingebrethsen, 1986), which ranges in particle size from 0.1 to 1 micrometers. Studies consistently indicate that the range, mean, and median diameter of particles in sidestream smoke are smaller than those in mainstream smoke (Cal EPA, 2005a). Secondhand smoke also contains particles that are much smaller (mass median diameter) than the particles in mainstream smoke; however, the characteristics of PM in secondhand smoke change over time due to the “aging process,” which includes coagulation, hygroscopic growth, evaporation, condensation, and other reactions (Cal EPA, 2005a).
Many studies cited in Chapter 2 indicate that smoking generates high levels of PM2.5 and that tobacco smoke is a significant source of indoor air PM2.5 levels in areas with smoking activity. Thus, from those data it can be inferred that typical concentrations encountered where tobacco smoke is present would be up to approximately 100 μg/m3. Concentrations of 100–300 μg/m3 should be considered high, and above 300 μg/m3 would be very high, but observed in some bars and discos. Further data on the
effect of smoking bans on indoor air concentrations of PM are presented in Chapter 2.
Mechanistically, a part of the toxicity of secondhand smoke could be viewed as a special case of toxicity that is due to an increase in ambient PM. It has been shown that chronic exposure to environments rich in respirable particles increases noninjury mortality and decreases life expectancy (Bhatnagar, 2006; Brook et al., 2004; Chow et al., 2006).
Time-series data collected from more than 100 million people in 119 cities in the United States and Europe show that for each 10-μg/m3 increase in PM10 there is a 0.3–0.7% increase in cardiovascular mortality (Bhatnagar, 2006). The effects of chronic exposure appear to be larger. On average each 10-μg/m3 chronic increase in PM2.5 has been reported to be associated with an 8–18% increased risk of cardiovascular causes of mortality (ischemic heart disease, dysrhythmia, heart failure, and cardiac arrest) (Pope et al., 2004).
Most (more than 70%) PM-related deaths have cardiopulmonary causes. Specific associations have been reported between exposure to ambient air pollution and ischemic heart disease, congestive heart failure, and arrhythmias. Heart-failure deaths make up 10% of all cardiovascular deaths but account for 30% of cardiovascular deaths related to PM exposure (Bhatnagar, 2006). The Natural Resources Defense Council estimates that 60,000 of the 350,000 cases of sudden cardiac death in the United States each year are related to PM air pollution (Stone and Godleski, 1999). The majority of excessive mortality due to PM exposure attributed to cardiac deaths is similar in scale compared to the risk estimates of exposure associated with secondhand smoke. It has been estimated that exposure to secondhand smoke causes 46,000 (range, 22,700–69,600) excess cardiac deaths in the United States each year (Cal EPA, 2005b). The data highlight the high vulnerability of the cardiovascular system to environmental pollutants and lend indirect support to the notion that both secondhand tobacco smoke and ambient PM contain toxicants with high cardiovascular toxicity.
Further evidence that some of the secondhand-smoke cardiotoxicity is derived from or propagates through a process related to PM comes from several recent studies on PM toxicity. The studies show that PM exposure diminishes heart-rate variability, increases vasoconstriction and thrombosis, induces arrhythmias and endothelial dysfunction, and exacerbates the formation of atherosclerotic lesions in animals and humans (Bhatnagar, 2006; Brook et al., 2004). Similar modes of action have been invoked to explain the cardiovascular toxicity of secondhand smoke (see below). Early work by Aronow (1978) demonstrated that patients exposed to secondhand smoke from 15 cigarettes in 2 hours had elevated venous carboxyhemoglobin, as well as increased resting heart rate, systolic and diastolic blood pressure,
and decreased heart rate and systolic blood pressure at angina. A comparison of the exposure profiles of ambient PM and PM from secondhand smoke could also provide some estimate of the magnitude of the increased risk of cardiovascular death, as the committee has done in Chapter 7. One caveat, however, is that because of important differences in composition, secondhand tobacco smoke cannot be viewed as entirely particulate air pollution. It is possible that particles in secondhand smoke are less toxic than ambient PM; however, given that, as discussed in Chapter 2, tobacco smoke contains many reactive components at higher concentrations than in the ambient atmosphere (such as reactive carbonyls, nicotine, and carbon monxide [CO]), secondhand smoke probably is more toxic and probably has a higher associated risk of cardiovascular death than outdoor PM2.5.
Carbon Monoxide
Acute cardiovascular effects of CO in low concentrations are mild, and most data indicate that concentrations present in secondhand smoke do not affect cardiovascular function in healthy young adults (Smith et al., 2000a,b). Early work by Aronow (1978) however demonstrated that patients exposed to secondhand smoke from 15 cigarettes in 2 hours had elevated venous carboxyhemoglobin, as well as increased resting heart rate and systolic and diastolic blood pressure, and decreased heart rate and systolic blood pressure at angina. In addition, children exposed to secondhand smoke have been reported to have increased concentrations of 2,3-diphosphoglycerate (Moskowitz et al., 1990), a compound that is increased in hypoxic red cells; this indicates that exposure to secondhand smoke could decrease oxygen availability and induce tissue hypoxia. In agreement with that view, it has been shown that exposure to secondhand smoke lowered oxygen uptake and increased blood lactate in women engaged in exercise (McMurray et al., 1985). Moreover, atmospheric CO concentration has been shown to be associated with hospital admissions for ischemic heart disease (von Klot et al., 2005) and with increased risk of ST-segment depression during repeated exercise tests performed by patients with stable coronary artery disease exposed to carbon monoxide to result in carboxyhemoglobin levels of 2–3.9% (Allred et al., 1989, 1991). Overall, the data indicate that CO at concentrations present in secondhand smoke is unlikely to initiate atherogenesis or to affect plasma lipoproteins. It also appears unlikely that CO is an important cause of the acute vasoconstriction or increased thrombosis observed in humans and animals exposed to secondhand smoke (Smith and Fischer, 2001b), but it may be important in exacerbating ischemic changes in patients with pre-existing heart disease.
SUMMARY OF POTENTIAL MODES OF ACTION OF ACUTE CORONARY EVENTS DUE TO SECONDHAND-TOBACCO SMOKE
Exposure to secondhand smoke is likely to precipitate acute coronary events in two general ways. First, long-term exposure to secondhand smoke could predispose an individual for an event by promoting inflammation, oxidant-induced injury to blood vessels, activation of platelets, and possibly adverse effects on lipids, subsequently accelerating coronary artherosclerosis. Supporting the idea that secondhand-smoke exposure accelerates atherogenesis are human studies showing an increase in carotid artery intima–media thickness, an index of systemic atherosclerosis associated with secondhand smoke exposure (Diaz-Roux et al., 1995; Howard et al., 1998). Effects of secondhand smoke on atherogenesis would probably be promoted in the presence of other risk factors, such as family history of coronary heart disease (CHD), hypertension, diabetes, and genetic or diet-induced hyperlipidemia.
Second, in the presence of coronary atherosclerosis and coronary plaque, secondhand smoke is likely to produce acute MI by changing the balance between the demand for myocardial oxygen and nutrients and the demand for myocardial blood supply. Increase in demand for oxygen may be a consequence of sympathetic nervous stimulation seen in response to secondhand-smoke exposure (Hausberg et al., 1997), which could result in an increase in blood pressure and heart rate, as reported in some studies of PM exposure (Brook, 2005; Brook et al., 2003; Delfino et al., 2005), although the study by Hausberg and Somers (2008) did not see changes in those parameters.
An increase in myocardial work usually results in a compensatory increase in coronary blood flow mediated by a release of vasodilators, such as NO, from endothelial cells. Secondhand-smoke exposure reduces the ability to increase coronary blood flow by inducing endothelial dysfunction. That effect has been confirmed in human studies that used coronary angiography to assess coronary artery dilation after administration of acetylcholine and showed artery impairment by secondhand-smoke exposure (Sumieda et al., 1998) and in studies of reduced coronary-flow velocity reserve (Otsuka et al., 2001) after secondhand-smoke exposure. The induction of the chronic inflammatory state on exposure to oxidants in secondhand smoke can result in acute plaque rupture, which can precipitate local thrombosis and acute MI. Sluggish coronary blood flow and a prothrombotic state induced by secondhand smoke may trigger coronary thrombosis with acute MI or sudden death (Figure 3-1).
The pathophysiology of induction of cardiovascular disease by cigarette-smoking is complex and undoubtedly involves multiple chemical agents that are present in tobacco smoke. PM and oxidants such as acrolein
are believed to be agents that contribute most to smoking-induced cardiovascular disease. Results of a number of in vitro studies, animal studies, and human experimental studies suggest that nicotine may contribute to cardiovascular disease by a variety of modes of action but results of human studies involving administration of medicinal nicotine indicate that nicotine is not a major factor.
CONCLUSIONS
-
Several components of secondhand smoke, including carbonyls and PM, have been shown to exert significant cardiovascular toxicity. Acute and chronic effects of those chemicals have been identified. The effects appear at concentrations expected to be reached in the secondhand smoke to which people are exposed.
-
There is evidence from experimental studies that acute exposure to secondhand smoke induces endothelial dysfunction, increases thrombosis, causes inflammation, and potentially affects plaque stability adversely.
-
Indirect evidence obtained from studies of exposure to ambient PM support the notion that exposure to PM in secondhand smoke can trigger acute coronary events or initiate arrhthymogesis in vulnerable myocardium.
-
Overall, data on the pathophysiology of secondhand smoke exposure in humans, animals, and cells are consistent with a role as a potential causative trigger for acute coronary events.
REFERENCES
Ahmadzadehfar, H., A. Oguogho, Y. Efthimiou, H. Kritz, and H. Sinzinger. 2006. Passive cigarette smoking increases isoprostane formation. Life Sciences 78(8):894-897.
Aicher, A., C. Heeschen, M. Mohaupt, J. P. Cooke, A. M. Zeiher, and S. Dimmeler. 2003. Nicotine strongly activates dendritic cell-mediated adaptive immunity: Potential role for progression of atherosclerotic lesions. Circulation 107(4):604-611.
Allred, E. N., E. R. Bleecker, B. R. Chaitman, T. E. Dahms, S. O. Gottlieb, J. D. Hackney, D. Hayes, M. Pagano, R. H. Selvester, S. M. Walden, and et al. 1989. Acute effects of carbon monoxide exposure on individuals with coronary artery disease. Research Report—Health Effects Institute (25):1-79.
Allred, E. N., E. R. Bleecker, B. R. Chaitman, T. E. Dahms, S. O. Gottlieb, J. D. Hackney, M. Pagano, R. H. Selvester, S. M. Walden, and J. Warren. 1991. Effects of carbon monoxide on myocardial ischemia. Environmental Health Perspectives 91:89-132.
Al-Qaisi, M., R. K. Kharbanda, T. K. Mittal, and A. E. Donald. 2008. Measurement of endothelial function and its clinical utility for cardiovascular risk. Vascular Health and Risk Management 4(3):647-652.
Andersson, K., and P. Arner. 2001. Systemic nicotine stimulates human adipose tissue lipolysis through local cholinergic and catecholaminergic receptors. International Journal of Obesity & Related Metabolic Disorders: Journal of the International Association for the Study of Obesity 25(8):1225-1232.
Andersson, K., P. Eneroth, and P. Arner. 1993. Changes in circulating lipid and carbohydrate metabolites following systemic nicotine treatment in healthy men. International Journal of Obesity & Related Metabolic Disorders: Journal of the International Association for the Study of Obesity 17(12):675-680.
Andre, E., B. Campi, S. Materazzi, M. Trevisani, S. Amadesi, D. Massi, C. Creminon, N. Vaksman, R. Nassini, M. Civelli, P. G. Baraldi, D. P. Poole, N. W. Bunnett, P. Geppetti, and R. Patacchini. 2008. Cigarette smoke-induced neurogenic inflammation is mediated by alpha, beta-unsaturated aldehydes and the TRPA1 receptor in rodents. Journal of Clinical Investigation 118(7):2574-2582.
Arabi, Z. 2006. Metabolic and cardiovascular effects of smokeless tobacco. Journal of the Cardiometabolic Syndrome 1(5):345-350.
Argacha, J.-F., D. Adamopoulos, M. Gujic, D. Fontaine, N. Amyai, G. Berkenboom, and P. van de Borne. 2008. Acute effects of passive smoking on peripheral vascular function. Hypertension 51(6):1506-1511.
Armani, C., L. Landini, Jr., and A. Leone. 2009. Molecular and biochemical changes of the cardiovascular system due to smoking exposure. Current Pharmaceutical Design 15(10):1038-1053.
Aronow, W. S. 1978. Effect of passive smoking on angina pectoris. New England Journal of Medicine 299(1):21-24.
Badre, R., R. Guillerm, N. Abran, M. Bourdin, and C. Dumas. 1978. [Atmospheric pollution by smoking (author’s transl)]. Annales Pharmaceutiques Françaises 36(9-10):443-452.
Balcarova, O., and J. Halik. 1991. Ten-year epidemiological study of ischaemic heart disease (IHD) in workers exposed to carbon disulphide. Science of the Total Environment 101(1-2):97-99.
Ballinger, S. W., C. Patterson, C. A. Knight-Lozano, D. L. Burow, C. A. Conklin, Z. Hu, J. Reuf, C. Horaist, R. Lebovitz, G. C. Hunter, K. McIntyre, and M. S. Runge. 2002. Mitochondrial integrity and function in atherogenesis. Circulation 106(5):544-549.
Benowitz, N. L. 2003. Cigarette smoking and cardiovascular disease: Pathophysiology and implications for treatment. Progress in Cardiovascular Diseases 46(1):91-111.
Benowitz, N. L., and S. G. Gourlay. 1997. Cardiovascular toxicity of nicotine: Implications for nicotine replacement therapy. Journal of the American College of Cardiology 29(7):1422-1431.
Benowitz, N. L., F. Kuyt, and P. Jacob, 3rd. 1984. Influence of nicotine on cardiovascular and hormonal effects of cigarette smoking. Clinical Pharmacology & Therapeutics 36(1):74-81.
Benowitz, N. L., G. A. Fitzgerald, M. Wilson, and Q. Zhang. 1993. Nicotine effects on eicosanoid formation and hemostatic function: Comparison of transdermal nicotine and cigarette smoking. Journal of the American College of Cardiology 22(4):1159-1167.
Bernhard, D., A. Rossmann, B. Henderson, M. Kind, A. Seubert, and G. Wick. 2006. Increased serum cadmium and strontium levels in young smokers: Effects on arterial endothelial cell gene transcription. Arteriosclerosis, Thrombosis & Vascular Biology 26(4):833-838.
Bhatnagar, A. 2004. Cardiovascular pathophysiology of environmental pollutants. American Journal of Physiology—Heart & Circulatory Physiology 286(2):H479-H485.
———. 2006. Environmental cardiology: Studying mechanistic links between pollution and heart disease. Circulation Research 99(7):692-705.
Brook, R. D. 2005. You are what you breathe: Evidence linking air pollution and blood pressure. Current Hypertension Reports 7(6):427-434.
Brook, R. D., J. R. Brook, and S. Rajagopalan. 2003. Air pollution: The “heart” of the problem. Current Hypertension Reports 5(1):32-39.
Brook, R. D., B. Franklin, W. Cascio, Y. Hong, G. Howard, M. Lipsett, R. Luepker, M. Mittleman, J. Samet, S. C. Smith, Jr., and I. Tager. Expert Panel on Population and Prevention Science of the American Heart Association. 2004. Air pollution and cardiovascular disease: A statement for healthcare professionals from the expert panel on population and prevention science of the American Heart Association. Circulation 109(21):2655-2671.
Brunnemann, K. D., M. R. Kagan, J. E. Cox, and D. Hoffmann. 1990. Analysis of 1,3-butadiene and other selected gas-phase components in cigarette mainstream and sidestream smoke by gas chromatography-mass selective detection. Carcinogenesis 11(10):1863-1868.
Buccelletti, E., E. Gilardi, E. Scaini, L. Galiuto, R. Persiani, A. Biondi, F. Basile, and N. G. Silveri. 2009. Heart rate variability and myocardial infarction: Systematic literature review and metanalysis. European Review for Medical and Pharmacological Sciences 13(4):299-307.
Burghuber, O. C., C. Punzengruber, H. Sinzinger, P. Haber, and K. Silberbauer. 1986. Platelet sensitivity to prostacyclin in smokers and non-smokers. Chest 90(1):34-38.
Burke, A., and G. A. FitzGerald. 2003. Oxidative stress and smoking-induced vascular injury. Progress in Cardiovascular Diseases 46(1):79-90.
Burstyn, I., H. Kromhout, T. Partanen, O. Svane, S. Langard, W. Ahrens, T. Kauppinen, I. Stucker, J. Shaham, D. Heederik, G. Ferro, P. Heikkila, M. Hooiveld, C. Johansen, B. G. Randem, and P. Boffetta. 2005. Polycyclic aromatic hydrocarbons and fatal ischemic heart disease. Epidemiology 16(6):744-750.
Cal EPA (California Environmental Protection Agency). 1991. Proposed identification of 1,3 butadiene as a toxic air contaminant. Sacramento: California Environmental Protection Agency.
———. 2005a. Proposed identification of environmental tobacco smoke as a toxic air contaminant. Part A: Exposure assessment. Sacramento: California Environmental Protection Agency.
———. 2005b. Proposed identification of environmental tobacco smoke as a toxic air contaminant. Part B: Health effects. Sacramento: California Environmental Protection Agency.
Caralis, D. G., U. Deligonul, M. J. Kern, and J. D. Cohen. 1992. Smoking is a risk factor for coronary spasm in young women. Circulation 85(3):905-909.
Cassee, F. R., J. H. Arts, J. P. Groten, and V. J. Feron. 1996. Sensory irritation to mixtures of formaldehyde, acrolein, and acetaldehyde in rats. Archives of Toxicology 70(6):329-337.
Chalon, S., H. Moreno, Jr., N. L. Benowitz, B. B. Hoffman, and T. F. Blaschke. 2000. Nicotine impairs endothelium-dependent dilatation in human veins in vivo. Clinical Pharmacology & Therapeutics 67(4):391-397.
Chang, S. J., C. J. Chen, T. S. Shih, T. C. Chou, and F. C. Sung. 2007. Risk for hypertension in workers exposed to carbon disulfide in the viscose rayon industry. American Journal of Industrial Medicine 50(1):22-27.
Chelland Campbell, S., R. J. Moffatt, and B. A. Stamford. 2008. Smoking and smoking cessation—the relationship between cardiovascular disease and lipoprotein metabolism: A review. Atherosclerosis 201(2):225-235.
Chow, J. C., J. G. Watson, J. L. Mauderly, D. L. Costa, R. E. Wyzga, S. Vedal, G. M. Hidy, S. L. Altshuler, D. Marrack, J. M. Heuss, G. T. Wolff, C. A. Pope, and D.W. Dockery. 2006. Health effects of fine particulate matter air pollution: Lines that connect. Journal of the Air & Waste Management Association 56:1368-1380.
Clark, J. D., 3rd, J. D. Wilkinson, W. G. LeBlanc, N. A. Dietz, K. L. Arheart, L. E. Fleming, and D. J. Lee. 2008. Inflammatory markers and secondhand tobacco smoke exposure among U.S. Workers. American Journal of Industrial Medicine 51(8):626-632.
Conklin, D. J., P. Haberzettl, R. A. Prough, and A. Bhatnagar. 2009. Glutathione S-transferase P protects against endothelial dysfunction induced by exposure to tobacco smoke. American Journal of Physiology—Heart & Circulatory Physiology.
Cucina, A., P. Sapienza, V. Borrelli, V. Corvino, G. Foresi, B. Randone, A. Cavallaro, and L. Santoro-D’Angelo. 2000a. Nicotine reorganizes cytoskeleton of vascular endothelial cell through platelet-derived growth factor BB. Journal of Surgical Research 92(2):233-238.
Cucina, A., P. Sapienza, V. Corvino, V. Borrelli, V. Mariani, B. Randone, L. Santoro D’Angelo, and A. Cavallaro. 2000b. Nicotine-induced smooth muscle cell proliferation is mediated through bFGF and TGF-beta 1. Surgery 127(3):316-322.
Cucina, A., P. Sapienza, V. Corvino, V. Borrelli, B. Randone, L. Santoro-D’Angelo, and A. Cavallaro. 2000c. Nicotine induces platelet-derived growth factor release and cytoskeletal alteration in aortic smooth muscle cells. Surgery 127(1):72-78.
Daisey, J. M. 1999. Tracers for assessing exposure to environmental tobacco smoke: What are they tracing? Environmental Health Perspectives 107 Suppl 2:319-327.
Davis, J. W., L. Shelton, I. S. Watanabe, and J. Arnold. 1989. Passive smoking affects endothelium and platelets. Archives of Internal Medicine 149(2):386-389.
de Sousa, M. G., J. C. Yugar-Toledo, M. Rubira, S. E. Ferreira-Melo, R. Plentz, D. Barbieri, F. Consolim-Colombo, M. C. Irigoyen, and H. Moreno, Jr. 2005. Ascorbic acid improves impaired venous and arterial endothelium-dependent dilation in smokers. Acta Pharmacologica Sinica 26(4):447-452.
Delfino, R. J., C. Sioutas, and S. Malik. 2005. Potential role of ultrafine particles in associations between airborne particle mass and cardiovascular health. Environmental Health Perspectives 113(8):934-946.
Di Luozzo, G., S. Pradhan, A. K. Dhadwal, A. Chen, H. Ueno, and B. E. Sumpio. 2005. Nicotine induces mitogen-activated protein kinase dependent vascular smooth muscle cell migration. Atherosclerosis 178(2):271-277.
Dietrich, D. F., J. Schwartz, C. Schindler, J.-M. Gaspoz, J.-C. Barthelemy, J.-M. Tschopp, F. Roche, A. von Eckardstein, O. Brandli, P. Leuenberger, D. R. Gold, U. Ackermann-Liebrich, and S. Team. 2007. Effects of passive smoking on heart rate variability, heart rate and blood pressure: An observational study. International Journal of Epidemiology 36(4):834-840.
Dong, J. Z., and S. C. Moldoveanu. 2004. Gas chromatography-mass spectrometry of carbonyl compounds in cigarette mainstream smoke after derivatization with 2,4-dinitrophenylhydrazine. Journal of Chromatography A 1027(1-2):25-35.
Eaton, M., H. Gursahani, Y. Arieli, K. Pinkerton, and S. Schaefer. 2006. Acute tobacco smoke exposure promotes mitochondrial permeability transition in rat heart. Journal of Toxicology and Environmental Health—Part A: Current Issues 69(15):1497-1510.
Egeland, G. M., G. A. Burkhart, T. M. Schnorr, R. W. Hornung, J. M. Fajen, and S. T. Lee. 1992. Effects of exposure to carbon disulphide on low density lipoprotein cholesterol concentration and diastolic blood pressure. British Journal of Industrial Medicine 49(4):287-293.
Esterbauer, H., R. J. Schaur, and H. Zollner. 1991. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radical Biology & Medicine 11(1):81-128.
Flouris, A. D., G. S. Metsios, A. Z. Jamurtas, and Y. Koutedakis. 2008. Sexual dimorphism in the acute effects of secondhand smoke on thyroid hormone secretion, inflammatory markers and vascular function. American Journal of Physiology—Endocrinology and Metabolism 294(2).
Giannini, D., A. Leone, D. Di Bisceglie, M. Nuti, G. Strata, F. Buttitta, L. Masserini, and A. Balbarini. 2007. The effects of acute passive smoke exposure on endothelium-dependent brachial artery dilation in healthy individuals. Angiology 58(2):211-217.
Gonick, H. C., Y. Ding, S. C. Bondy, Z. Ni, and N. D. Vaziri. 1997. Lead-induced hypertension: Interplay of nitric oxide and reactive oxygen species. Hypertension 30(6):1487-1492.
Green, M. A., and J. L. Egle, Jr. 1983. Effects of intravenous acetaldehyde, acrolein, formaldehyde and propionaldehyde on arterial blood pressure following acute guanethidine treatment. Research Communications in Chemical Pathology & Pharmacology 40(2):337-340.
Guberan, E., and L. Raymond. 1985. Mortality and cancer incidence in the perfumery and flavour industry of geneva. British Journal of Industrial Medicine 42(4):240-245.
Haass, M., and W. Kubler. 1997. Nicotine and sympathetic neurotransmission. Cardiovascular Drugs and Therapy 10(6):657-665.
Haberzettl, P., E. Vladykovskaya, S. Srivastava, and A. Bhatnagar. 2009. Role of endoplasmic reticulum stress in acrolein-induced endothelial activation. Toxicology & Applied Pharmacology 234(1):14-24.
Hadi, H. A., C. S. Carr, and J. Al Suwaidi. 2005. Endothelial dysfunction: Cardiovascular risk factors, therapy, and outcome. Vascular Health and Risk Management 1(3):183-198.
Hanna, S. T. 2006. Nicotine effect on cardiovascular system and ion channels. Journal of Cardiovascular Pharmacology 47(3):348-358.
Hatsukami, D. K., N. L. Benowitz, S. I. Rennard, C. Oncken, and S. S. Hecht. 2006. Biomarkers to assess the utility of potential reduced exposure tobacco products. Nicotine & Tobacco Research 8(2):169-191.
Hausberg, M., and V. K. Somers. 2008. Environmental smoke exposure: A complex cardiovascular challenge. Hypertension 51(6):1468-1469.
Hausberg, M., A. L. Mark, M. D. Winniford, R. E. Brown, and V. K. Somers. 1997. Sympathetic and vascular effects of short-term passive smoke exposure in healthy nonsmokers. Circulation 96(1):282-287.
Heavner, D., W. T. Morgan, and M. W. Odgen. 1996. Determination of volatile organic compounds and respirable suspended particulate matter in New Jersey and Pennsylvania homes and workplaces. Environment International 22:159-183.
Heeschen, C., J. J. Jang, M. Weis, A. Pathak, S. Kaji, R. S. Hu, P. S. Tsao, F. L. Johnson, and J. P. Cooke. 2001. Nicotine stimulates angiogenesis and promotes tumor growth and atherosclerosis. Nature Medicine 7(7):833-839.
Heeschen, C., M. Weis, and J. P. Cooke. 2003. Nicotine promotes arteriogenesis. Journal of the American College of Cardiology 41(3):489-496.
Heiss, C., N. Amabile, A. C. Lee, W. M. Real, S. F. Schick, D. Lao, M. L. Wong, S. Jahn, F. S. Angeli, P. Minasi, M. L. Springer, S. K. Hammond, S. A. Glantz, W. Grossman, J. R. Balmes, and Y. Yeghiazarians. 2008. Brief secondhand smoke exposure depresses endothelial progenitor cells activity and endothelial function: Sustained vascular injury and blunted nitric oxide production. Journal of the American College of Cardiology 51(18):1760-1771.
Hellerstein, M. K., N. L. Benowitz, R. A. Neese, J. M. Schwartz, R. Hoh, P. Jacob, 3rd, J. Hsieh, and D. Faix. 1994. Effects of cigarette smoking and its cessation on lipid metabolism and energy expenditure in heavy smokers. Journal of Clinical Investigation 93(1):265-272.
Hergens, M. P., M. Lambe, G. Pershagen, and W. Ye. 2008. Risk of hypertension amongst Swedish male snuff users: A prospective study. Journal of Internal Medicine 264(2):187-194.
HHS (U.S. Department of Health and Human Services). 2001. Risks associated with smoking cigarettes with low machine-measured yields of tar and nicotine. Bethesda, MD: National Institutes of Health, National Cancer Institute.
———. 2004. The health consequences of smoking: A report of the surgeon general. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Coordinating Center for Health Promotion, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health.
———. 2006. The health consequences of involuntary exposure to tobacco smoke: A report of the surgeon general. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Coordinating Center for Health Promotion, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health.
Houston, T. K., S. D. Person, M. J. Pletcher, K. Liu, C. Iribarren, and C. I. Kiefe. 2006. Active and passive smoking and development of glucose intolerance among young adults in a prospective cohort: CARDIA study. BMJ 332(7549):1064-1069.
Ingebrethsen, B. J. 1986. Aerosol studies of cigarette smoke. Recent Advances in Tobacco Science 12: 54-142.
Jackson, M. A., H. F. Stack, J. M. Rice, and M. D. Waters. 2000. A review of the genetic and related effects of 1,3-butadiene in rodents and humans. Mutation Research 463(3):181-213.
Jakab, G. J. 1993. The toxicologic interactions resulting from inhalation of carbon black and acrolein on pulmonary antibacterial and antiviral defenses. Toxicology & Applied Pharmacology 121(2):167-175.
Jamall, I. S., and H. Roque. 1989. Cadmium-induced alterations in ocular trace elements. Influence of dietary selenium and copper. Biological Trace Element Research 23:55-63.
Jiang, D. J., S. J. Jia, J. Yan, Z. Zhou, Q. Yuan, and Y. J. Li. 2006. Involvement of DDAH/ADMA/NOS pathway in nicotine-induced endothelial dysfunction. Biochemical & Biophysical Research Communications 349(2):683-693.
Juhasz, A., and N. Bodor. 2000. Cardiovascular studies on different classes of soft drugs. Pharmazie 55(3):228-238.
Kato, M., P. Roberts-Thomson, B. G. Phillips, K. Narkiewicz, W. G. Haynes, C. A. Pesek, and V. K. Somers. 1999. The effects of short-term passive smoke exposure on endothelium-dependent and independent vasodilation. Journal of Hypertension 17(10):1395-1401.
Kato, T., T. Inoue, T. Morooka, N. Yoshimoto, and K. Node. 2006. Short-term passive smoking causes endothelial dysfunction via oxidative stress in nonsmokers. Canadian Journal of Physiology & Pharmacology 84(5):523-529.
Kilburn, K. H., and W. N. McKenzie. 1978. Leukocyte recruitment to airways by aldehyde-carbon combinations that mimic cigarette smoke. Laboratory Investigation 38(2): 134-142.
Knight-Lozano, C. A., C. G. Young, D. L. Burow, Z. Y. Hu, D. Uyeminami, K. E. Pinkerton, H. Ischiropoulos, and S. W. Ballinger. 2002. Cigarette smoke exposure and hypercholesterolemia increase mitochondrial damage in cardiovascular tissues. Circulation 105(7):849-854.
Kotseva, K. P., and D. De Bacquer. 2000. Cardiovascular effects of occupational exposure to carbon disulphide. Occupational Medicine (London) 50(1):43-47.
Kotseva, K., and T. Popov. 1998. Study of the cardiovascular effects of occupational exposure to organic solvents. International Archives of Occupational & Environmental Health 71 Suppl:S87-S91.
Kuo, H. W., J. S. Lai, M. Lin, and E. S. Su. 1997. Effects of exposure to carbon disulfide (CS2) on electrocardiographic features of ischemic heart disease among viscose rayon factory workers. International Archives of Occupational & Environmental Health 70(1): 61-66.
Lane, D., E. A. Gray, R. S. Mathur, and S. P. Mathur. 2005. Up-regulation of vascular endothelial growth factor-C by nicotine in cervical cancer cell lines. American Journal of Reproduction Immunology 53(3):153-158.
Latha, M. S., P. L. Vijayammal, and P. A. Kurup. 1991. Changes in the glycosaminoglycans and glycoproteins in the tissues in rats exposed to cigarette smoke. Atherosclerosis 86(1):49-54.
Lau, P. P., L. Li, A. J. Merched, A. L. Zhang, K. W. Ko, and L. Chan. 2006. Nicotine induces proinflammatory responses in macrophages and the aorta leading to acceleration of atherosclerosis in low-density lipoprotein receptor(-/-) mice. Arteriosclerosis, Thrombosis & Vascular Biology 26(1):143-149.
Law, M. R., and N. J. Wald. 2003. Environmental tobacco smoke and ischemic heart disease. Progress in Cardiovascular Diseases 46(1):31-38.
Ledford, H. 2008. Drug markers questioned. Nature 452(7187):510-511.
Lee, P. N. 2007. Circulatory disease and smokeless tobacco in western populations: A review of the evidence. International Journal of Epidemiology 36(4):789-804.
Levine, R. J., D. A. Andjelkovich, and L. K. Shaw. 1984. The mortality of Ontario undertakers and a review of formaldehyde-related mortality studies. Journal of Occupational Medicine 26(10):740-746.
Lindahl, B., H. Toss, A. Siegbahn, P. Venge, and L. Wallentin. 2000. Markers of myocardial damage and inflammation in relation to long-term mortality in unstable coronary artery disease. New England Journal of Medicine 343(16):1139-1147.
Luo, J., B. G. Hill, Y. Gu, J. Cai, S. Srivastava, A. Bhatnagar, and S. D. Prabhu. 2007. Mechanisms of acrolein-induced myocardial dysfunction: Implications for environmental and endogenous aldehyde exposure. American Journal of Physiology—Heart & Circulatory Physiology 293(6):H3673-H3684.
Lustberg, M., and E. Silbergeld. 2002. Blood lead levels and mortality. Archives of Internal Medicine 162(21):2443-2449.
Mack, W. J., T. Islam, Z. Lee, R. H. Selzer, and H. N. Hodis. 2003. Environmental tobacco smoke and carotid arterial stiffness. Preventive Medicine 37(2):148-154.
Magos, G. A., M. Lorenzana-Jimenez, and H. Vidrio. 1990. Toluene and benzene inhalation influences on ventricular arrhythmias in the rat. Neurotoxicology & Teratology 12(2):119-124.
Mahmud, A., and J. Feely. 2004. Effects of passive smoking on blood pressure and aortic pressure waveform in healthy young adults—influence of gender. British Journal of Clinical Pharmacology 57(1):37-43.
Marques, M., I. Millas, A. Jimenez, E. Garcia-Colis, J. A. Rodriguez-Feo, S. Velasco, A. Barrientos, S. Casado, and A. Lopez-Farre. 2001. Alteration of the soluble guanylate cyclase system in the vascular wall of lead-induced hypertension in rats. Journal of the American Science of Nephrology 12(12):2594-2600.
Matanoski, G. M., and X. Tao. 2002. Case-cohort study of styrene exposure and ischemic heart disease. Research Report—Health Effects Institute (108):1-29.
McDonald, T. P., D. Woodard, and M. Cottrell. 1973. Effect of nicotine on clot retraction of rat blood platelets. Pharmacology 9(6):357-366.
McMurray, R. G., L. L. Hicks, and D. L. Thompson. 1985. The effects of passive inhalation of cigarette smoke on exercise performance. European Journal of Applied Physiology & Occupational Physiology 54(2):196-200.
McNabola, A., B. Broderick, P. Johnston, and L. Gill. 2006. Effects of the smoking ban on benzene and 1,3-butadiene levels in pubs in Dublin. Journal of Environmental Science and Health, Part A Toxic/Hazardous Substances and Environmental Engineering 41(5):799-810.
Metsios, G. S., A. D. Flouris, A. Z. Jamurtas, A. E. Carrillo, D. Kouretas, A. E. Germenis, K. Gourgoulianis, T. Kiropoulos, M. N. Tzatzarakis, A. M. Tsatsakis, and Y. Koutedakis. 2007. Brief report: A brief exposure to moderate passive smoke increases metabolism and thyroid hormone secretion. Journal of Clinical Endocrinology and Metabolism 92(1):208-211.
Minami, J., T. Ishimitsu, and H. Matsuoka. 1999. Effects of smoking cessation on blood pressure and heart rate variability in habitual smokers. Hypertension 33(1 Pt 2):586-590.
Modesti, P. A., R. Abbate, G. F. Gensini, A. Colella, and G. G. Neri Serneri. 1989. Platelet thromboxane A2 receptors in habitual smokers. Thrombosis Research 55(2):195-201.
Moffatt, R. J., K. D. Biggerstaff, and B. A. Stamford. 2000. Effects of the transdermal nicotine patch on normalization of HDL-C and its subfractions. Preventive Medicine 31(2 Pt 1):148-152.
Moffatt, R. J., S. A. Chelland, D. L. Pecott, and B. A. Stamford. 2004. Acute exposure to environmental tobacco smoke reduces HDL-C and HDL2-C. Preventive Medicine 38(5):637-641.
Morrow, J. D., B. Frei, A. W. Longmire, J. M. Gaziano, S. M. Lynch, Y. Shyr, W. E. Strauss, J. A. Oates, and L. J. Roberts, 2nd. 1995. Increase in circulating products of lipid peroxidation (F2-isoprostanes) in smokers. Smoking as a cause of oxidative damage. New England Journal of Medicine 332(18):1198-1203.
Morvai, V., A. Hudak, G. Ungvary, and B. Varga. 1976. ECG changes in benzene, toluene and xylene poisoned rats. Acta Medica Academiae Scientarium Hungaricae 33(3):275-286.
Morvai, V., E. Szakmary, and G. Ungvary. 2005. The effects of carbon disulfide and ethanol on the circulatory system of rats. Journal of Toxicology & Environmental Health Part A 68(10):797-809.
Moskowitz, W. B., M. Mosteller, R. M. Schieken, R. Bossano, J. K. Hewitt, J. N. Bodurtha, and J. P. Segrest. 1990. Lipoprotein and oxygen transport alterations in passive smoking preadolescent children. The MCV Twin Study. Circulation 81(2):586-592.
Navas-Acien, A., E. Selvin, A. R. Sharrett, E. Calderon-Aranda, E. Silbergeld, and E. Guallar. 2004. Lead, cadmium, smoking, and increased risk of peripheral arterial disease. Circulation 109(25):3196-3201.
Nawrot, T. S., L. Thijs, E. M. Den Hond, H. A. Roels, and J. A. Staessen. 2002. An epidemiological re-appraisal of the association between blood pressure and blood lead: A meta-analysis. Journal of Human Hypertension 16(2):123-131.
Nemr, R., B. Lasserre, and R. Chahine. 2003. Effects of nicotine on thromboxane/prostacyclin balance in myocardial ischemia. Prostaglandins Leukotrienes & Essential Fatty Acids 68(3):191-195.
Neunteufl, T., U. Priglinger, S. Heher, M. Zehetgruber, G. Soregi, S. Lehr, K. Huber, G. Maurer, F. Weidinger, and K. Kostner. 2000. Effects of vitamin E on chronic and acute endothelial dysfunction in smokers. Journal of the American College of Cardiology 35(2):277-283.
Nicod, P., R. Rehr, M. D. Winniford, W. B. Campbell, B. G. Firth, and L. D. Hillis. 1984. Acute systemic and coronary hemodynamic and serologic responses to cigarette smoking in long-term smokers with atherosclerotic coronary artery disease. Journal of the American College of Cardiology 4(5):964-971.
Nowack, R., D. Fliser, J. Richter, C. Horne, E. Mutschler, and E. Ritz. 1993. Effects of angiotensin-converting enzyme inhibition on renal sodium handling after furosemide injection. The Clinical Investigator 71(8):622-627.
NRC (National Research Council). 1986. Environmental tobacco smoke: Measuring exposures and assessing health effects. Washington, DC: National Academy Press.
Omae, K., T. Takebayashi, T. Nomiyama, C. Ishizuka, H. Nakashima, T. Uemura, S. Tanaka, T. Yamauchi, T. O’Uchi, Y. Horichi, and H. Sakurai. 1998. Cross sectional observation of the effects of carbon disulphide on arteriosclerosis in rayon manufacturing workers. Occupational & Environmental Medicine 55(7):468-472.
O’Toole, T. E., Y. T. Zheng, J. Hellmann, D. J. Conklin, O. Barski, and A. Bhatnagar. 2009. Acrolein activates matrix metalloproteinases by increasing reactive oxygen species in macrophages. Toxicology and Applied Pharmacology 236(2):194-201.
Otsuka, R., H. Watanabe, K. Hirata, K. Tokai, T. Muro, M. Yoshiyama, K. Takeuchi, and J. Yoshikawa. 2001. Acute effects of passive smoking on the coronary circulation in healthy young adults. JAMA 286(4):436-441.
Packard, C. J., D. S. O’Reilly, M. J. Caslake, A. D. McMahon, I. Ford, J. Cooney, C. H. Macphee, K. E. Suckling, M. Krishna, F. E. Wilkinson, A. Rumley, and G. D. Lowe. 2000. Lipoprotein-associated phospholipase A2 as an independent predictor of coronary heart disease. West of Scotland coronary prevention study group. New England Journal of Medicine 343(16):1148-1155.
Partanen, T., S. Hernberg, C. H. Nordman, and P. Sumari. 1970. Coronary heart disease among workers exposed to carbon disulphide. British Journal of Industrial Medicine 27(4):313-325.
Penn, A., and C. A. Snyder. 1996. Butadiene inhalation accelerates arteriosclerotic plaque development in cockerels. Toxicology 113(1-3):351-354.
Pilz, H., A. Oguogho, F. Chehne, G. Lupattelli, B. Palumbo, and H. Sinzinger. 2000. Quitting cigarette smoking results in a fast improvement of in vivo oxidation injury (determined via plasma, serum and urinary isoprostane). Thrombosis Research 99(3):209-221.
Pope, C. A., 3rd, R. T. Burnett, G. D. Thurston, M. J. Thun, E. E. Calle, D. Krewski, and J. J. Godleski. 2004. Cardiovascular mortality and long-term exposure to particulate air pollution: Epidemiological evidence of general pathophysiological pathways of disease. Circulation 109(1):71-77.
Probst-Hensch, N. M., M. Imboden, D. Felber Dietrich, J. C. Barthelemy, U. Ackermann-Liebrich, W. Berger, J. M. Gaspoz, and J. Schwartz. 2008. Glutathione S-transferase polymorphisms, passive smoking, obesity, and heart rate variability in nonsmokers. Environmental Health Perspectives 116(11):1494-1499.
Rahman, M. M., and I. Laher. 2007. Structural and functional alteration of blood vessels caused by cigarette smoking: An overview of molecular mechanisms. Current Vascular Pharmacology 5(4):276-292.
Raitakari, O. T., M. R. Adams, R. J. McCredie, K. A. Griffiths, R. Stocker, and D. S. Celermajer. 2000. Oral vitamin C and endothelial function in smokers: Short-term improvement, but no sustained beneficial effect. Journal of the American College of Cardiology 35(6):1616-1621.
Ramachandran, J., D. Rubenstein, D. Bluestein, and J. Jesty. 2004. Activation of platelets exposed to shear stress in the presence of smoke extracts of low-nicotine and zero-nicotine cigarettes: The protective effect of nicotine. Nicotine & Tobacco Research 6(5):835-841.
Ramos, K. S., and B. Moorthy. 2005. Bioactivation of polycyclic aromatic hydrocarbon carcinogens within the vascular wall: Implications for human atherogenesis. Drug Metabolism Reviews 37(4):595-610.
Raupach, T., K. Schafer, S. Konstantinides, and S. Andreas. 2006. Secondhand smoke as an acute threat for the cardiovascular system: A change in paradigm. European Heart Journal 27(4):386-392.
Revis, N. W., A. R. Zinsmeister, and R. Bull. 1981. Atherosclerosis and hypertension induction by lead and cadmium ions: An effect prevented by calcium ion. Proceedings of the National Academy of Sciences of the United States of America 78(10):6494-6498.
Roberts, K. A., A. A. Rezai, K. E. Pinkerton, and J. C. Rutledge. 1996. Effect of environmental tobacco smoke on LDL accumulation in the artery wall. Circulation 94(9):2248-2253.
Rocchi, E., F. Bursi, P. Ventura, A. Ronzoni, C. Gozzi, G. Casalgrandi, L. Marri, R. Rossi, and M. G. Modena. 2007. Anti- and pro-oxidant factors and endothelial dysfunction in chronic cigarette smokers with coronary heart disease. European Journal of Internal Medicine 18(4):314-320.
Saareks, V., P. Ylitalo, J. Alanko, I. Mucha, and A. Riutta. 2001. Effects of smoking cessation and nicotine substitution on systemic eicosanoid production in man. Naunyn-Schmiedebergs Archives of Pharmacology 363(5):556-561.
Sklar, J. L., P. G. Anderson, and P. J. Boor. 1991. Allylamine and acrolein toxicity in perfused rat hearts. Toxicology & Applied Pharmacology 107(3):535-544.
Smith, C. J., and T. H. Fischer. 2001. Particulate and vapor phase constituents of cigarette mainstream smoke and risk of myocardial infarction. Atherosclerosis 158(2):257-267.
Smith, C. J., T. A. Perfetti, M. A. Mullens, A. Rodgman, and D. J. Doolittle. 2000a. “IARC Group 2B carcinogens” reported in cigarette mainstream smoke. Food & Chemical Toxicology 38(9):825-848.
Smith, C. J., T. A. Perfetti, M. A. Rumple, A. Rodgman, and D. J. Doolittle. 2000b. “IARC Group 2A carcinogens” reported in cigarette mainstream smoke. Food & Chemical Toxicology 38(4):371-383.
Stefanadis, C., C. Vlachopoulos, E. Tsiamis, L. Diamantopoulos, K. Toutouzas, N. Giatrakos, S. Vaina, D. Tsekoura, and P. Toutouzas. 1998. Unfavorable effects of passive smoking on aortic function in men. Annals of Internal Medicine 128(6):426-434.
Stewart, P. A., C. Schairer, and A. Blair. 1990. Comparison of jobs, exposures, and mortality risks for short-term and long-term workers. Journal of Occupational Medicine 32(8):703-708.
Stone, P. H., and J. J. Godleski. 1999. First steps toward understanding the pathophysiologic link between air pollution and cardiac mortality. American Heart Journal 138(5 Pt 1):804-807.
Subramanyam, G., M. Bhaskar, and S. Govindappa. 1992. The role of cadmium in induction of atherosclerosis in rabbits. Indian Heart Journal 44(3):177-180.
Sun, Y. P., B. Q. Zhu, R. E. Sievers, S. A. Glantz, and W. W. Parmley. 1994. Metoprolol does not attenuate atherosclerosis in lipid-fed rabbits exposed to environmental tobacco smoke. Circulation 89(5):2260-2265.
Sweetnam, P. M., S. W. Taylor, and P. C. Elwood. 1987. Exposure to carbon disulphide and ischaemic heart disease in a viscose rayon factory. British Journal of Industrial Medicine 44(4):220-227.
Sztalryd, C., J. Hamilton, B. A. Horwitz, P. Johnson, and F. B. Kraemer. 1996. Alterations of lipolysis and lipoprotein lipase in chronically nicotine-treated rats. American Journal of Physiology 270(2 Pt 1):E215-E223.
Takase, B., H. Etsuda, Y. Matsushima, M. Ayaori, H. Kusano, A. Hamabe, A. Uehata, F. Ohsuzu, M. Ishihara, and A. Kurita. 2004. Effect of chronic oral supplementation with vitamins on the endothelial function in chronic smokers. Angiology 55(6):653-660.
Therond, P. 2009. Catabolism of lipoproteins and metabolic syndrome. Current Opinion in Clinical Nutrition and Metabolic Care 12(4):366-371.
Thomas, S. R., P. K. Witting, and G. R. Drummond. 2008. Redox control of endothelial function and dysfunction: Molecular mechanisms and therapeutic opportunities. Antioxidants & Redox Signaling 10(10):1713-1765.
Tripathi, R. M., and G. P. Thomas. 1986. A simple method for the production of ventricular tachycardia in the rat and guinea pig. Journal of Pharmacological Methods 15(3):279-282.
Tucker, L. A. 1989. Use of smokeless tobacco, cigarette smoking, and hypercholesterolemia. American Journal of Public Health 79(8):1048-1050.
Venn, A., and J. Britton. 2007. Exposure to secondhand smoke and biomarkers of cardiovascular disease risk in never-smoking adults. Circulation 115(8):990-995.
von Klot, S., A. Peters, P. Aalto, T. Bellander, N. Berglind, D. D’Ippoliti, R. Elosua, A. Hormann, M. Kulmala, T. Lanki, H. Lowel, J. Pekkanen, S. Picciotto, J. Sunyer, and F. Forastiere. 2005. Ambient air pollution is associated with increased risk of hospital cardiac readmissions of myocardial infarction survivors in five european cities. Circulation 112(20):3073-3079.
Wallenfeldt, K., J. Hulthe, L. Bokemark, J. Wikstrand, and B. Fagerberg. 2001. Carotid and femoral atherosclerosis, cardiovascular risk factors and C-reactive protein in relation to smokeless tobacco use or smoking in 58-year-old men. Journal of Internal Medicine 250(6):492-501.
Walrath, J., and J. F. Fraumeni, Jr. 1984. Cancer and other causes of death among embalmers. Cancer Research 44(10):4638-4641.
Wang, T. J. 2008. New cardiovascular risk factors exist, but are they clinically useful? European Heart Journal 29(4):441-444.
Weitzman, M., S. Cook, P. Auinger, T. A. Florin, S. Daniels, M. Nguyen, and J. P. Winickoff. 2005. Tobacco smoke exposure is associated with the metabolic syndrome in adolescents. Circulation 112(6):862-869.
WHO (World Health Organization). 2007. The scientific basis of tobacco product regulation: Report of a WHO study group. Geneva, Switzerland: World Health Organization.
Wilkinson, J. D., D. J. Lee, and K. L. Arheart. 2007. Secondhand smoke exposure and C-reactive protein levels in youth. Nicotine and Tobacco Research 9(2):305-307.
Winniford, M. D., K. R. Wheelan, M. S. Kremers, V. Ugolini, E. van den Berg, Jr., E. H. Niggemann, D. E. Jansen, and L. D. Hillis. 1986. Smoking-induced coronary vasoconstriction in patients with atherosclerotic coronary artery disease: Evidence for adrenergically mediated alterations in coronary artery tone. Circulation 73(4):662-667.
Woodward, M., A. Rumley, C. Rumley, S. Lewington, C. E. Morrison, and G. D. Lowe. 2006. The association between homocysteine and myocardial infarction is independent of age, sex, blood pressure, cholesterol, smoking and markers of inflammation: The Glasgow Myocardial Infarction Study. Blood Coagulation & Fibrinolysis 17(1):1-5.
Young, J. M., B. I. Shand, P. M. McGregor, R. S. Scott, and C. M. Frampton. 2006. Comparative effects of enzogenol and vitamin C supplementation versus vitamin C alone on endothelial function and biochemical markers of oxidative stress and inflammation in chronic smokers. Free Radical Research 40(1):85-94.
Yuan, H., L. S. Wong, M. Bhattacharya, C. Ma, M. Zafarani, M. Yao, M. Schneider, R. E. Pitas, and M. Martins-Green. 2007. The effects of second-hand smoke on biological processes important in atherogenesis. BMC Cardiovascular Disorders 7(1).
Zhang, S., I. Day, and S. Ye. 2001. Nicotine induced changes in gene expression by human coronary artery endothelial cells. Atherosclerosis 154(2):277-283.
Zhang, W. Z., K. Venardos, J. Chin-Dusting, and D. M. Kaye. 2006. Adverse effects of cigarette smoke on no bioavailability: Role of arginine metabolism and oxidative stress. Hypertension 48(2):278-285.
Zhu, B. Q., Y. P. Sun, R. E. Sievers, W. M. Isenberg, S. A. Glantz, and W. W. Parmley. 1993. Passive smoking increases experimental atherosclerosis in cholesterol-fed rabbits. Journal of the American College of Cardiology 21(1):225-232.
Zhu, B. Q., Y. P. Sun, R. E. Sievers, S. A. Glantz, W. W. Parmley, and C. L. Wolfe. 1994. Exposure to environmental tobacco smoke increases myocardial infarct size in rats. Circulation 89(3):1282-1290.
Zoloth, S. R., D. M. Michaels, J. R. Villalbi, and M. Lacher. 1986. Patterns of mortality among commercial pressmen. Journal of the National Cancer Institute 76(6):1047-1051.




































