8.B.1 In-Laboratory Hazard Reduction
8.B.2 Characterization of Waste
8.B.2.1 Characterization for Off-Site Management
8.B.2.2 Identification Responsibilities of All Laboratory Personnel
8.B.2.3 Characterization of Unknowns
8.B.2.4 In-Laboratory Test Procedures for Unknowns
8.B.3 Regulated Chemical Hazardous Waste
8.B.3.1 Definition of Characteristic Waste
8.B.3.2 Definition of Listed Waste
8.B.3.3 Determining the Regulatory Status of a Waste
8.B.4 Collection and Storage of Waste
8.B.4.1 Accumulation of Waste at the Location of Generation
8.B.4.2 Accumulation of Waste in a Central Area
8.B.4.3 Special Regulations for Laboratories at Academic Institutions
8.B.5 Disposal of Nonhazardous and Nonregulated Waste
8.B.6 Treatment and Disposal Options
8.B.6.1 Treatment and Recycling
8.B.6.2 Disposal in the Sanitary Sewer
8.B.6.3 Release to the Atmosphere
8.B.7 Monitoring Waste Services, Transport, and Off-Site Treatment and Disposal
8.B.7.1 Preparation for Off-Site Treatment or Disposal of Waste
8.B.7.2 Choice of Transporter and Disposal Facility
8.B.9 Manifesting Hazardous Wastes
8.B.10 Records and Record Keeping
8.C.1 Chemical–Radioactive (Mixed) Waste
8.C.1.1 Minimization of Mixed Waste
8.C.1.2 Safe Storage of Mixed Waste
8.C.1.3 Hazard Reduction of Mixed Waste
8.C.1.4 Commercial Disposal Services for Mixed Waste
8.C.2 Chemical–Biological Waste
8.C.2.3 Disinfection and Autoclaving of Contaminated Labware
8.C.2.4 Disposal of Chemically Contaminated Medical Waste and Sharps
8.C.2.5 Minimization Methods for Chemical–Biological Waste
8.C.3 Radioactive–Biological Waste
8.C.3.1 Off-Site Management of Low-Level Radioactive Waste
8.C.3.2 Disposal of Radioactive Animal Carcasses and Tissue
8.C.3.3 Disposal of Radioactive–Biological Contaminated Labware
8.C.3.4 Sewer Disposal of Radioactive–Biological Liquids
8.C.4 Chemical–Radioactive–Biological Waste
8.D PROCEDURES FOR THE LABORATORY-SCALE TREATMENT OF SURPLUS AND WASTE CHEMICALS
This chapter presents methods for the management and ultimate disposal of laboratory waste that may present chemical hazards, as well as those multihazardous wastes that contain some combination of chemical, radioactive, and biological hazards. The best strategy for managing laboratory waste aims to maximize safety and minimize environmental impact, and considers these objectives from the time of purchase. As suggested in previous chapters, there is a strategic hierarchy for managing chemicals and waste to accomplish these objectives.
The initial responsibility for implementing this hierarchy rests with trained laboratory personnel. These individuals are in the best position to know the chemical and physical properties of the materials they have used or synthesized. They are responsible for evaluating hazards, providing information necessary to make an accurate waste determination, and assisting in the evaluation of appropriate strategies for management, minimization, and disposal.
The overriding principle governing the prudent handling of laboratory waste is that no activity should begin unless a plan for the disposal of nonhazardous and hazardous waste has been formulated. Application of this simple principle ensures that the numerous state and federal regulatory requirements for waste handling are met and avoids unexpected difficulties, such as the generation of a form of waste (e.g., chemical, radioactive, biological) that the institution is not prepared to deal with.
There are four tiers to waste management to reduce its environmental impact: pollution prevention and source reduction; reuse or redistribution of unwanted, surplus materials; treatment, reclamation, and recycling of materials within the waste; and disposal through incineration, treatment, or land burial. The first tier of this strategic hierarchy incorporates the principles of green chemistry (see Chapter 5, section 5.B): pollution prevention and source reduction. Clearly, the best approach to laboratory waste is preventing its generation. Examples include reducing the scale of laboratory operations, reducing the formation of waste during laboratory operations, and substituting nonhazardous or less hazardous chemicals in chemical procedures.
The second strategic tier is to reuse unwanted material, redistribute surplus chemicals, and reduce hazards. Practices that implement this strategy include purchasing only what is needed, keeping chemical inventories to prevent the purchase of duplicates, and reusing excess materials. Sanitary sewer disposal of certain aqueous liquids is considered within this tier, although there are many restrictions (see section 8.B.6.2, below). At this tier it is important for laboratory personnel and environmental health and safety (EHS) staff to work cooperatively to determine the point at which the chemical becomes regulated as a waste and to ensure that requirements are met. In general terms, waste is defined as material that is discarded, is intended to be discarded, or is no longer useful for its intended purpose. This point may occur after the chemical has left the laboratory, however, if the organization has a way to reuse or redistribute the material or to use it in another procedure. Note that regulators may consider a material to be a waste if it is abandoned or is inherently wastelike (e.g., spilled materials). The determination of whether a waste is regulated as hazardous is usually made either by the institution’s EHS staff or by employees of the waste disposal firm.
While the first two tiers are the preferred ways of managing chemical waste, the third strategic tier also provides safety and environmental benefits (see Section 8.B.6). If waste cannot be prevented or minimized, the organization should consider recycling chemicals that can be recovered safely from the waste and the potential for recovering energy from the waste (e.g., using solvent as a fuel). Although some laboratories distill waste solvents for reuse, these strategies are most commonly accomplished by sending the waste to a commercial recycling or reclamation facility or to a fuel blender. These strategies are described later in this chapter.
The fourth and final strategic tier for managing laboratory waste includes incineration, other treatment methods, and land disposal. Decisions within this tier consider the environmental fate of the waste and its constituents and process byproducts after it leaves the institution or firm. As with other tiers, the goal is to minimize risk to health and the environment. Land disposal is the least desirable disposal method. Although modern hazardous waste landfills can contain waste for many decades, there is always a future risk of leaking, contaminated runoff or other harmful releases to the environment. Laboratories that ship chemical waste off-site must address land disposal restrictions and treatability standards, which were put in place to discourage landfilling. Other reasons to consider environmental fate include exhibiting good environmental stewardship, teaching students and employees responsible waste management practices, and maintaining a good public image.
Of course, all laboratories wish to avoid fines and sanctions from federal, state, and local regulators. Because these potential penalties can be significant, this laboratory waste management guidance includes information on laws, regulations, rules, and ordinances that are likely to be most important to people who work in laboratories and support laboratory operations.
Please note, however, that this book is not a compliance manual, and as such, its compliance information is incomplete. In particular, this chapter focuses on federal rules that apply to laboratory waste but not the many different requirements particular to each state or locale. Chapter 11 contains additional information on the institutional regulation of laboratory waste (as well as other environmental requirements) to complement this chapter’s details of laboratory waste regulation. There are many good compliance references to augment this book, and regulatory agencies should not be overlooked as another source of helpful information. Do not hesitate to seek legal advice when needed.
8.B.1 In-Laboratory Hazard Reduction
The first and second tiers of waste management broadly describe methods of reducing quantity and level of hazard of laboratory waste. Hazard reduction is part of the broad theme of pollution prevention that is encouraged throughout this book. From a chemist’s point of view, it is feasible to reduce the volume or the hazardous characteristics of many chemicals by conducting reactions and other hazard reduction procedures in the laboratory. It is becoming increasingly common to include such reactions as the final steps in an experimental sequence. Such procedures, as part of an academic or industrial experiment, usually involve small amounts of materials which can be handled easily and safely by laboratory personnel. Performing a hazard reduction procedure as part of an experiment has considerable economic advantages by eliminating the necessity to accumulate, handle, store, transport, and treat hazardous waste after the experiment. Furthermore, the laboratory professional who generates the potential waste often has the expertise and knowledge to safely handle the materials and perform hazard reduction procedures.
Conducting laboratory hazard reduction procedures for chemical hazardous waste makes most sense for hard-to-dispose-of waste, such as multihazardous waste, or for small or remote laboratories that generate very small quantities of easily treatable hazardous waste. In some cases, a simple procedure can make waste suitable for sewer disposal. When it can be done safely, knowledgeable laboratory staff may treat very small amounts of reactives that would otherwise pose a storage or transport risk. In some cases, waste is stabilized or encapsulated to enable safe storage and transport. More details can be found in section 8.B.6, below.
Keeping up-to-date chemical inventories can also reduce the in-laboratory hazards by simply reducing the quantity of hazardous material on-site. Ordering the smallest quantity of hazardous material required and reusing materials are also effective means of minimizing generation of hazardous waste.
Before beginning a detailed discussion of the handling of waste once it has been generated, it is important to understand the definition of waste, how it is characterized, and the regulations that govern it.
8.B.2 Characterization of Waste
Waste must be categorized as to its identity, constituents, and hazards so that it may be safely handled and managed. Categorization is necessary to determine a waste’s regulatory status, hazardous waste ID number, and treatability group, and to determine its proper U.S. Department of Transportation (DOT) shipping name, and to meet other transport, treatment, and disposal requirements.
The great variety of laboratory waste makes waste categorization challenging. Transport and waste regulations are written for commercially available high-volume chemicals, which may make it difficult to categorize some laboratory chemicals, such as experimental or newly synthesized materials. Categorization procedures must account for the common laboratory waste management practices of placing small containers of waste chemicals into a larger overpack drum, and combining of many solvents and solutes into a single drum of flammable liquids.
There are several acceptable information sources for waste characterization, including the identity of the source or raw materials, in-laboratory test procedures (such as those described below), and analysis by an environmental laboratory. Generator knowledge can be used for waste characterization, such as the knowledge of waste characteristics and constituents by laboratory personnel who conducted the process, procedure, or experiment.
8.B.2.1 Characterization for Off-Site Management
When waste is to be shipped off-site for recycling, reclamation, treatment, or disposal, the waste characterization information needed depends on the waste management facility’s requirements and its permit. Analytical methods have been established by the U.S. Environmental Protection Agency (EPA), and environmental laboratories that use EPA methods are often certified or accredited. Most of these methods are for commercially available chemicals, and so approved analytical procedures may not be available for some laboratory chemicals. It is important to work with your waste disposal firm to determine how laboratory waste is to be categorized. To avoid redundant analysis for recurring waste streams (e.g., chlorinated solvents,
labpacks of organic solids), waste disposal firms and off-site facilities often establish a waste-stream profile. In some cases, detailed analytical information is not necessary if waste containers fall within the profile’s hazard classification.
8.B.2.2 Identification Responsibilities of All Laboratory Personnel
Because proper management and disposal of laboratory waste requires information about its properties, it is very important that laboratory personnel accurately and completely identify and clearly label all chemical and waste containers in their laboratory, as well as maintain the integrity of source material labels. It is recommended that supplementary information be kept in a separate, readily available record (e.g., laboratory information system, lab notebook), especially for very small containers or collections. In academic laboratories where student turnover is frequent, identification is particularly important for the materials used or generated. This practice is as important for small quantities as it is for large quantities.
8.B.2.3 Characterization of Unknowns
Establishing the hazardous characteristics and evaluating the potential listing of clearly identified waste is usually quite simple. Unidentified materials (unknowns) present a problem, however, because recycling, treatment, and disposal facilities need to know characteristics and hazards to manage waste safely. All chemicals must be characterized sufficiently for safe transportation off-site.
Analysis of laboratory unknowns is expensive, especially if EPA methods must be used, or the presence of a constituent must be ruled out, and handling unknowns is risky due to the possible presence of unstable, reactive, or highly toxic chemicals or byproducts. Although expensive, some waste disposal firms offer on-site services to categorize unknown laboratory waste to prepare it for shipment to their treatment facility.
8.B.2.4 In-Laboratory Test Procedures for Unknowns
When the identity of the material is not known, simple in-laboratory test procedures can be carried out to determine the hazard class into which the material should be categorized. Because the generator may be able to supply some general information, it may be beneficial to carry out the test procedures before the materials are removed from the laboratory. Perform these tests only if they can be done safely, and only if they facilitate the characterization of the waste required by your hazardous waste disposal firm. Understand that the following test procedures are only to provide additional information, and do not meet EPA regulatory requirements for waste analysis.
In general, precisely determining the molecular structure of the unknown material is not necessary. Hazard classification usually satisfies the regulatory requirements and those of the treatment disposal facility. However, it is important to establish which analytical data are required by the disposal facility.
Trained laboratory personnel who carry out the analytical procedures should be familiar with the characteristics of the waste and any necessary precautions. Because the hazards of the materials being tested are unknown, the use of proper personal protection and safety devices such as chemical hoods and shields is imperative. Older samples are particularly dangerous because they may have changed in composition, for example, through the formation of peroxides. (See Chapter 4, section 4.D.3.2, for information on the formation and identification of peroxides. See Chapter 6, section 6.G.3, for information on testing and disposal of peroxides.)
The following information is commonly required by treatment and disposal facilities before they agree to handle unknown materials:
• physical description,
• water reactivity,
• water solubility,
• pH,
• ignitability (flammability),
• presence of oxidizer,
• presence of sulfides or cyanides,
• presence of halogens,
• presence of radioactive materials,
• presence of biohazardous materials,
• presence of toxic constituents,
• presence of polychlorinated biphenyls (PCBs), and
• presence of high-odor compounds.
The following test procedures are readily accomplished by trained laboratory personnel. The overall sequence for testing is depicted in Figure 8.1 for liquid and solid materials.
• Physical description. Include the state of the material (solid, liquid), the color, and the consistency (for solids) or viscosity (for liquids). For liquid materials, describe the clarity of the solution (transparent, translucent, or opaque). If an unknown material is a bi- or tri-layered liquid, describe each layer separately, giving an approximate percentage of the total for each layer. After
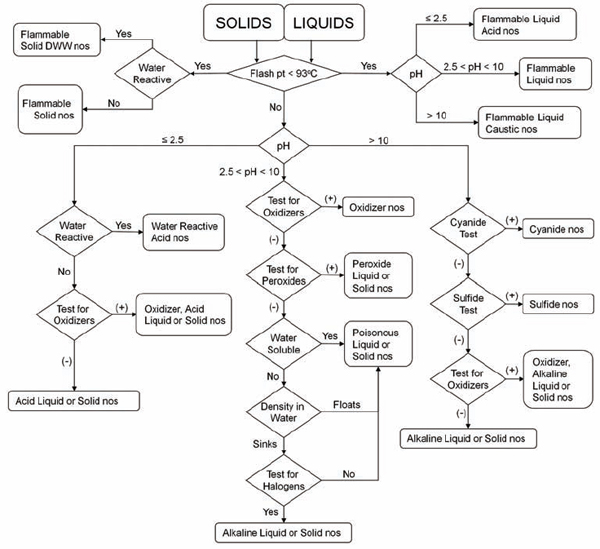
FIGURE 8.1 Flowchart for categorizing unknown chemicals for waste disposal. This decision tree shows the sequence of tests to be performed to determine the appropriate hazard category of an unknown chemical. DWW, dangerous when wet; nos, not otherwise specified. Following categorization, select a hazard reduction procedure (section 8.D) or disposal option (section 8.B.6).
taking appropriate safety precautions for handling the unknown, including the use of personal protection devices, remove a small sample for use in the following tests.
• Water reactivity. Carefully add a small quantity of the unknown to a few milliliters of water. Observe any changes, including heat evolution, gas evolution, and flame generation.
• Water solubility. Observe the solubility of the unknown in water. If it is an insoluble liquid, note whether it is less or more dense than water (i.e., does it float or sink?). Most nonhalogenated organic liquids are less dense than water.
• pH. Test the material with multirange pH paper. If the sample is water-soluble, test the pH of a 10% aqueous solution. Carrying out a neutralization titration may also be desirable or even required.
• Ignitability (flammability). Place a small sample of the material (<5 mL) in an aluminum test tray. Apply an ignition source, typically a propane torch, to the test sample for 0.5 second. If the material supports its own combustion, it is a flammable liquid with a flash point of less than 60 °C (140 °F). If the sample does not ignite, apply the ignition source again for 1 second. If the material burns, it is combustible. Combustible materials have a flash point between 60 and 93 °C (140 and 200 °F).
• Presence of oxidizer. Wet commercially available starch-iodide paper with 1 N hydrochloric acid, and place a small portion of the unknown on the wetted paper. A change in color of the paper to dark purple is a positive test for an oxidizer. The test can also be carried out by adding 0.1 to 0.2 g of sodium or potassium iodide to 1 mL of an acidic 10% solution of the unknown. Development of a yellow-brown color indicates an oxidizer. To test for hydroperoxides in water-insoluble organic solvents, dip the starch-iodine test paper into the solvent, and let it dry. Add a drop of water to the same section of the paper. Development of a dark color indicates the presence of hydroperoxides.
• Presence of peroxides. The following tests detect
most (but not all) peroxy compounds, including all hydroperoxides. Please take care. (See Chapter 4, section 4.D.3.2, and Chapter 6, section 6.G.3, for information on the hazards of peroxides.)
ο Peroxide test strips, which turn to an indicative color in the presence of peroxides, are available commercially. Note that these strips must be air dried until the solvent evaporates and exposed to moisture for proper operation.
ο Add 1 to 3 mL of the liquid to be tested to an equal volume of acetic acid, add a few drops of 5% aqueous potassium iodide solution, and shake. The appearance of a yellow to brown color indicates the presence of peroxides. Alternatively, addition of 1 mL of a freshly prepared 10% solution of potassium iodide to 10 mL of an organic liquid in a 25-mL glass cylinder produces a yellow color if peroxides are present.
ο Add 0.5 mL of the liquid to be tested to a mixture of 1 mL of 10% aqueous potassium iodide solution and 0.5 mL of dilute hydrochloric acid to which has been added a few drops of starch solution just prior to the test. The appearance of a blue or blue-black color within 1 minute indicates the presence of peroxides.
None of these tests should be applied to materials (such as metallic potassium) that may be contaminated with inorganic peroxides. (See Chapter 6, section 6.G.3, for more information about peroxide testing.)
• Presence of sulfide. Commercial test strips for the presence of sulfide are available, and their use is recommended. If the test strips are not available in the laboratory, the following test can be performed. Warning: This test produces hazardous and odiferous vapors. Use only small quantities of solution for the test and use appropriate ventilation. The test for inorganic sulfides is carried out only when the pH of an aqueous solution of the unknown is greater than 10. Add a few drops of concentrated hydrochloric acid to a sample of the unknown while holding a piece of commercial lead acetate paper, wet with distilled water, over the sample. Development of a brown-black color on the paper indicates generation of hydrogen sulfide.
• Presence of cyanide. Commercial test strips for the presence of cyanide are available, and their use is strongly recommended.
• Presence of halogen. Heat a piece of copper wire until red in a flame. Cool the wire in distilled or deionized water, and dip it into the unknown. Again heat the wire in the flame. The presence of halogen is indicated by a green color around the wire in the flame.
8.B.3 Regulated Chemical Hazardous Waste
An important question for planning within the laboratory is whether a waste is regulated as a hazardous waste, because regulated hazardous waste must be handled and disposed of in specific ways. This determination has important implications that can lead to significant differences in disposal cost. Regulatory definitions often differ from common definitions. EPA defines chemical hazardous waste under the Resource Conservation and Recovery Act of 1978 (RCRA 40 CFR Parts 260-272). EPA and RCRA establish the federal standards for chemical hazardous waste. The U.S. Nuclear Regulatory Commission (USNRC) defines radioactive waste. Hazardous biological waste is regulated less stringently under federal law, but its management is addressed in the Occupational Safety and Health Administration (OSHA) bloodborne pathogens standards and in Biosafety in Microbiological and Biomedical Laboratories (BMBL; HHS/CDC/NIH, 2007a).
Note that, although close attention must be paid to the regulatory definitions and procedures that govern the handling and disposal of waste, primary importance must be given to the safe and prudent handling of all laboratory wastes. Evaluate unregulated wastes and consider special handling if they pose occupational, environmental, or unknown risks.
Chemical waste that is regulated as “hazardous waste” is defined by EPA in either of two ways: (1) waste that has certain hazardous characteristics and (2) waste that is on certain lists of chemicals. The first category is based on properties of materials that should be familiar to all trained laboratory personnel. The second category comprises lists, established by EPA, of certain common hazardous chemicals and chemical wastes. These lists generally include materials that are widely used and recognized as hazardous. Chemicals are placed on these RCRA lists primarily on the basis of their toxicity. See below to determine if waste is hazardous or not.
Regardless of the regulatory definitions of hazard, understanding chemical characteristics that pose potential hazards is a fundamental part of the education and training of laboratory personnel. These characteristics may be derived from knowledge of the properties or precursors of the waste. The characteristics may also be established by specific tests cited in the regulations.
(Regulatory issues, specifically RCRA, are discussed further in Chapter 11, section 11.E.1.)
8.B.3.1 Definition of Characteristic Waste
According to federal law, the properties of chemical waste that pose hazards are as follows. Note that
these definitions are unique, especially the definition of waste having the characteristic of toxicity.
1. Ignitability. Ignitable materials are defined as having one or more of the following characteristics:
(a) liquids that have a flash point of less than 60 °C (140 °F) or some other characteristic that has the potential to cause fire;
(b) materials other than liquids that are capable, under standard temperature and pressure, of causing fire by friction, adsorption of moisture, or spontaneous chemical changes and, when ignited, burn so vigorously and persistently that they create a hazard;
(c) flammable compressed gases, including those that form flammable mixtures;
(d) oxidizers that stimulate combustion of organic materials.
Ignitable materials include most common organic solvents, gases such as hydrogen and hydrocarbons, and certain nitrate salts.
2. Corrosivity. Corrosive liquids have a pH ≤ 2 or pH ≥ 12.5 or corrode certain grades of steel. Most common laboratory acids and bases are corrosive. Solid corrosives, such as sodium hydroxide pellets and powders, are not legally considered by RCRA to be corrosive. However, trained laboratory personnel must recognize that such materials are extremely dangerous to skin and eyes and must be handled accordingly.
3. Reactivity. The reactivity classification includes substances that are unstable, react violently with water, detonate if exposed to some initiating source, or produce toxic gases. Alkali metals, peroxides and compounds that have peroxidized, and cyanide or sulfide compounds are classed as reactive.
4. Toxicity. Toxicity is established through the toxicity characteristic leaching procedure (TCLP) test, which measures the tendency of certain toxic materials to be leached (extracted) from the waste material under circumstances assumed to reproduce conditions of a landfill. The TCLP list includes a relatively small number of industrially important toxic chemicals and is based on the leachate concentration, above which a waste is considered hazardous. Failure to pass the TCLP results in classification of a material as a toxic waste. The TCLP test is primarily for solid materials; liquids are typically evaluated on a straight concentration basis. TCLP analyses are usually performed by environmental testing laboratories.
8.B.3.2 Definition of Listed Waste
A chemical waste that does not exhibit one of the above characteristics may still be regulated if it is a listed waste. Although EPA has developed several lists of hazardous waste, three regulatory lists are of most interest to trained laboratory personnel:
• F list: waste from nonspecific sources (e.g., spent solvents and process or reaction waste);
• U list: hazardous waste (e.g., toxic laboratory chemicals); and
• P list: acutely hazardous waste [e.g., highly toxic laboratory chemicals, that is, chemicals having a lethal dose (LD50) of <50 mg/kg (oral, rat)].
Of the listed wastes, the most common for laboratories are the F wastes, which include many laboratory solvents. These include halogenated solvents (methylene chloride, tetrachloroethylene, and chlorinated fluorocarbons) and nonhalogenated solvents (xylene, acetone, ethyl acetate, ethyl benzene, ethyl ether, methyl isobutyl ketone, methanol, and n-butyl alcohol). Note that these are regulated under this listing only if they have been used (spent).
The other categories of listed waste common to laboratories are the U and P lists, which include many chemicals frequently found in laboratories. U and P lists pertain to
• waste chemicals that have not been used, because once used, the U or P listing does not apply;
• spills and spill cleanup material from U- or P-listed compounds; and
• rinsate from triple rinsing of empty containers of P compounds (described below), which is collected and handled as hazardous.
8.B.3.3 Determining the Regulatory Status of a Waste
The EPA regulations place the burden of determining whether a waste is regulated as hazardous and in what hazard classification it falls on the waste generator. Most laboratories rely on their EHS staff or their waste disposal firm to determine EPA and DOT regulatory categories (such as EPA ID numbers and transportation classes), as well as waste characterization information needed by the recycling, treatment, or disposal facility.
Testing is not necessarily required, and in most cases trained laboratory personnel are able to provide sufficient information about the waste to categorize it by general hazard categories. If the waste is not a common chemical with known characteristics, enough information about it must be supplied to satisfy the regulatory
requirements and to ensure that it can be handled and disposed of safely. The information needed to characterize a waste also depends on the method of ultimate disposal. (See the discussion of disposal methods in sections 8.B.6 to 8.B.7, below.)
8.B.3.4 Empty Containers
The rules for disposal of empty hazardous waste containers, and cleaning the empty containers, are complex. A container or inner liner of a container that contained hazardous waste is “empty” under federal regulations if all waste has been removed by standard practice and no more than 2.5 cm (1 in.) of residue, or 3% by weight of containers less than 110 gal, remains. If the container held acute hazardous waste, triple rinsing or equivalent measures are required before the container is “empty” within the federal regulations. The rinsate must be collected and handled as acutely hazardous waste. “Empty” containers are no longer subject to federal regulation.
These are minimum standards. If empty containers are to be recycled or disposed of in the normal trash, it is recommended that labels be removed from empty hazardous waste containers, and that they be emptied as much as possible. Consider rinsing emptied containers with water or a detergent solution. Resulting rinsate from containers previously holding acutely hazardous waste are hazardous waste and must be disposed of accordingly. Rinsate resulting from cleaning of other hazardous waste containers is hazardous waste if it exhibits EPA’s hazardous waste characteristics of ignitability, corrosivity, reactivity, or toxicity. It is prudent to follow these guidelines for disposing of empty containers of nonhazardous and nonregulated laboratory chemicals.
Properly cleaning containers as described above, and recycling or disposing of them with the normal trash, reduces costs as well as the volume of hazardous waste generated. Alternatively, some firms and institutions decide that it is more convenient to handle all empty chemical containers from laboratories as hazardous waste and dispose of them accordingly. This especially makes sense if the rinsate is hazardous.
8.B.4 Collection and Storage of Waste
8.B.4.1 Accumulation of Waste at the Location of Generation
Laboratory experiments generate a great variety of waste, including used disposable laboratory ware, filter media and similar materials, aqueous solutions, and hazardous and nonhazardous chemicals. As stated in the introduction to this chapter, begin no activity unless a plan for disposal of all waste, hazardous and nonhazardous, has been formulated.
The accumulation and temporary storage of waste in the laboratory is called satellite accumulation. The legal standards for satellite accumulation are included in this section; they are also good practices for the management of nonregulated waste. To ensure security and management oversight, chemical waste should be accumulated at or near the point of generation, and under control of laboratory personnel. Note that there is an optional alternative federal standard for the accumulation of waste within laboratories of colleges, universities, teaching hospitals, and certain nonprofit research facilities associated with colleges or universities. This is described in section 8.B.4.3, below.
Each category of waste has certain precautions and appropriate disposal methods. Below is a list of requirements and good practices for accumulating chemical waste in the laboratory:
• Collect hazardous or flammable waste solvents in an appropriate container pending transfer to the institution’s central facility or satellite site for chemical waste handling or pickup by commercial disposal firm. Often, different kinds of waste are accumulated within a common container.
• Take care not to mix incompatible waste. This is a special concern with commingled waste solvents, which must be chemically compatible to ensure that heat generation, gas evolution, or another reaction does not occur. (See the discussion of commingling in section 8.B.4.2, below.) For example, waste solvents can usually be mixed for disposal, with due regard for the compatibility of the components.
• Keep wastes segregated by how they will be managed. For example, because nonhalogenated solvents are more suitable for fuel blending, many laboratories collect halogenated and nonhalogenated solvent wastes separately.
• Collect waste in dependable containers that are compatible with their contents. Keep containers closed except when adding or subtracting waste. Separate containers of incompatible materials physically or otherwise stored in a protective manner. (See Chapter 5, section 5.E.2, for storing chemicals according to their compatibility.)
• Use an appropriate container for the collection of liquid waste. Glass bottles are impervious to most chemicals but present a breakage hazard, and narrow-neck bottles are difficult to empty. The use of plastic (e.g., polyethylene jerry cans) or metal (galvanized or stainless steel) safety containers for the collection of liquid waste is strongly encouraged. Note that flame arresters in safety
containers can easily become plugged if there is sediment and may need to be cleaned occasionally. Do not store amines or corrosive materials in metal containers.
• Do not use galvanized steel safety cans for halogenated waste solvents because they tend to corrode and leak.
• As detailed below, clearly and securely label waste containers with their contents.
• Securely cap waste containers when not in immediate use. To minimize releases to the atmosphere, when a funnel is used either immediately reclose the container or use a capped waste funnel. Do not use the same funnel for containers containing incompatible waste types.
• Collect aqueous waste separately from organic solvent waste. Some laboratories may be served by a wastewater treatment facility that allows the disposal of aqueous waste to the sanitary sewer if it falls within a narrow range of acceptable waste types. Thus, solutions of nonhazardous salts or water-miscible organic materials may be acceptable in some localities. Solutions containing flammable or hazardous waste, even if water-miscible, are almost never allowed, and water-immiscible substances must never be put down the drain. Collect aqueous waste for nonsewer disposal in a container selected for resistance to corrosion. Do not use glass for aqueous waste if there is danger of freezing. Depending on the requirements of the disposal facility, adjustment of the pH of aqueous waste may be required. Such adjustment requires consideration of the possible consequences of the neutralization reaction that might take place: gas evolution, heat generation, or precipitation.
• Place solid chemical waste, such as reaction byproducts or contaminated filter or chromatography media, in an appropriately labeled container to await disposal or pickup. Segregate unwanted reagents for disposal in their original containers, if possible. If original containers are used, labels should be intact and fully legible. Make every effort to use, share, or recycle unwanted reagents rather than commit them to disposal. (See Chapter 5, sections 5.D and 5.E, for a discussion of labeling alternatives.)
• Consider how to dispose of nonhazardous solid waste in laboratory trash or segregate it for recycling. Check the laboratory chemical safety summary, material safety data sheet, or other appropriate reference to determine toxicity. Consult institutional policy on nonhazardous solid waste disposal.
Trained laboratory personnel, who are most familiar with the waste and its generation, need to be actively involved in waste identification and management decisions, so that the waste is managed safely and efficiently. Often the appropriate time to decide to recycle or reuse surplus materials is shortly after the waste is generated, rather than when they are sent for disposal. Once combined with other waste materials, recycling or reuse may be more difficult. Evaluate all the costs and benefits of either decision at this time.
Safety considerations must be of primary concern. Store waste in clearly labeled containers in a designated location that does not interfere with normal laboratory operations. Ventilated storage may be appropriate. Use secondary containment such as trays, for spills or leakage from the primary containers. Many states require the use of secondary containment for wastes in satellite accumulation areas.
Federal regulations allow the indefinite accumulation of up to 55 gal of hazardous waste or 1 qt of acutely hazardous waste at or near the point of generation. However, prudence dictates that the quantities accumulated are consistent with good safety practices. Furthermore, satellite accumulation time must be consistent with the stability of the material. The general recommendation is that waste not be held for more than 1 year; some states specifically set this limit for satellite accumulation time. Within 3 days of the time that the amount of waste exceeds the 55-gal (or 1-qt) limit, manage it under the storage and accumulation time limits required at a central accumulation area, as described below.
Packaging and labeling are key parts of this initial in-laboratory operation. Label every container of hazardous waste with the material’s identity and its hazard (e.g., flammable, corrosive) and the words “hazardous waste.” Although the identity need not be a complete listing of all chemical constituents, knowledgeable laboratory professionals or waste handlers should be able to evaluate the hazard. However, when compatible wastes are collected in a common container, keep a list of the components to aid in later disposal decisions. Labeling must be clear and permanent. Although federal regulations do not require posting the date when satellite accumulation begins, some states do require this. The institution may suggest that this information be recorded as part of its chemical management plan.
8.B.4.2 Accumulation of Waste in a Central Area
The central accumulation area is an important component in the organization’s chemical management plan. In addition to being the primary location where waste management occurs, it may also be the location where excess chemicals are held for possible redistribution. Along with the laboratory, the central accumula-
tion area is often where hazard reduction of waste takes place through allowable on-site treatment processes.
The central accumulation area is often the appropriate place to accomplish considerable cost savings by commingling (i.e., combining) similar waste materials. This is the process where compatible wastes from various sources are combined prior to disposal. Commingling is particularly suitable for waste solvents because disposal of liquid in a 55-gal drum is generally much less expensive than disposal of the same volume of liquid in small containers. Because mixing waste requires transfer of waste between containers, the identity of all materials must be known and their compatibility understood. Although these procedures are very cost-effective, they require additional safety precautions, including the use of personal protective equipment and special and engineering controls. In addition to the facility needs described below, commingling areas require non-explosive electrical systems, grounding and bonding between floors and containers, nonsparking conductive floors and containers, and specialized ventilation systems. A walk-in fume hood is often used for both solvent commingling and the storage of commingling equipment. It is important to design the process to minimize lifting, awkward procedures that may cause injury, and the handling of heavy drums and equipment.
In some cases the disposal method and ultimate fate of the waste require that different wastes not be accumulated together. For example, if commingled waste contains significant amounts of halogenated solvents (usually >1%), disposing of the mixture can be considerably more expensive. In such cases segregation of halogenated and nonhalogenated solvents is economically favorable.
According to federal regulations, storage at a central accumulation area is normally limited to 90 days, although more time is allowed for small-quantity generators or other special situations (180 or 270 days). The count begins when the waste is brought to the central accumulation area from the laboratory or satellite accumulation area. A special permit is required for storage beyond the above limits. Obtaining such a permit is usually too expensive and too time-consuming for most laboratory operations. (See RCRA and Chapter 11, section 11.E.1, for more information.)
Store waste materials within a central accumulation area in appropriate and clearly labeled containers and separate them according to chemical compatibility as noted in Chapter 5, section 5.E.2. The label must include the accumulation start date and the words “hazardous waste.”
Central accumulation areas should have fire suppression systems, ventilation, and dikes to avoid sewer contamination in case of a spill. Employees must be trained in correct handling of the materials as well as contingency planning and emergency response. The area should be secure, and employees should be encouraged to report any suspicious activity. Employees should know how to activate alarms, how to use fire extinguishers and other emergency response equipment, how to exit, and the location of the exterior assembly point. Be sure to document training and provide periodic refreshers.
Transportation of waste from laboratories (satellite accumulation areas) to the central accumulation area also requires specific attention to safety. Transport materials in appropriate and clearly labeled containers. Make provision for spill control in case of an accident during transportation and handling. Larger institutions are advised to have an internal tracking system to follow the movement of waste. If public roads are used to transport laboratory waste, additional DOT packaging, marking, labeling, and manifesting regulations may apply, as described below.
Final preparations for off-site disposal usually occur at the central accumulation area. Decisions on disposal options are best made here as larger quantities of waste are gathered. Identification of unknown materials not carried out within the laboratory must be completed at this point because unidentified waste cannot be shipped to a disposal site.
Your hazardous waste disposal firm is frequently involved with this phase of waste management. The decision of whether to involve a hazardous waste disposal firm, how, and when is largely based on logistics and economics. Table 8.1 describes the tasks involved in initiating off-site disposal and provides recommendations for what should be done in-house by staff and what should be contracted to professional service companies.
Laboratory waste typically leaves the generator’s facility commingled in drums as compatible wastes or within a labpack. Labpacks are containers, often 55-gal drums, in which small containers of waste are packed with an absorbent material. Labpacks had been used as the principal method for disposing of laboratory waste within a landfill. However, recent landfill disposal restrictions severely limit landfill disposal of hazardous materials. Thus, the labpack has become principally a shipping container. The labpack is taken to a permitted treatment, storage, and disposal facility (TSDF), where it is either incinerated or unpacked and the contents redistributed for safe, efficient, and legal treatment and disposal.
If chemical hazardous waste is being accumulated for recycling (e.g., waste lead, solvents for redistillation), federal law requires 75% or more of these materials to be recycled or disposed of in each calendar year.
TABLE 8.1 Assignment of Tasks for Waste Handling
|
|
||
|
Task |
Who Should Perform |
Why? |
|
|
||
|
Determine if waste is regulated as hazardous |
Staff |
Knowledge of the waste, liability considerations, and economics |
|
Segregate according to hazard class |
Staff |
Economics and safety in storage |
|
Determine if the material will be recycled or reused |
Staff |
Economics, knowledge of in-house requirements and capabilities |
|
Commingling if appropriate |
Staff |
Economics, safety, liability, storage space; waste disposal firm could be consulted for advice |
|
Determine appropriate disposal method |
Staff and employees of the waste disposal firm |
Waste disposal firm is aware of options for Specific waste streams; staff should be involved because of liability and cost |
|
Determine packing protocol for labpacks |
Waste disposal firm |
The waste disposal firm is aware of what is required by the treatment, storage, and disposal facility |
|
Labpacking |
Waste disposal firm |
The waste disposal firm is generally required to do labpacking |
|
Manifest preparation |
Waste disposal firm; review by Staff |
The waste disposal firm typically has more experience and will prepare the manifest; staff should be properly trained in how to review a manifest because of liability and cost considerations |
|
|
||
This fgure does not apply to surplus chemicals held for redistribution to other laboratories.
8.B.4.3 Special Regulations for Laboratories at Academic Institutions
Although laboratories are generally required to comply with the same regulations as industrial facilities, regulations promulgated in 2008 provide limited relief for academic institutions from some of the requirements associated with on-site management. When adopted by the state in which the laboratory is located, these alternative standards are available to colleges, universities, teaching hospitals, and certain nonprofit research facilities associated with colleges or universities. These standards are completely optional, at the discretion of the educational or research institution. To take advantage of these provisions, academic facilities must implement a performance-oriented laboratory management plan. This facility-specific plan must provide for seven required elements:
1. labeling standards,
2. container standards,
3. training requirements,
4. removal frequency of unwanted chemicals,
5. hazardous waste determinations,
6. laboratory cleanouts, and
7. prevention of emergencies.
The provisions allow academic facilities additional time to move waste from laboratories to central accumulation areas and additional time and flexibility in making waste determinations, and encourage laboratory cleanouts by providing relief from some time limits and generator classification provisions. However, these alternative standards require semiannual removal of all laboratory hazardous waste, whereas the standard satellite accumulation rule has no time limit for the accumulation of laboratory waste in unfilled containers smaller than 55 gal.
The academic waste rule does not apply in states with primacy over RCRA regulations until promulgated by the state. Check with your state environmental agency to see if the rule has been implemented. The entire text of the rule is available at the EPA Web site, www.epa.gov.
8.B.5 Disposal of Nonhazardous and Nonregulated Waste
Some nonregulated laboratory waste is hazardous and should be safely managed. There are more waste management options for nonregulated waste, especially with regard to hazard reduction procedures.
Some laboratories have policies that require all chemical waste to be handled as if it were regulated as hazardous. This recognizes the potential liabilities associated with misperceptions or the improper handling of nonregulated as well as regulated waste. For example, a trash hauler or landfill operator may become alarmed by a laboratory chemical container, even if it contains sucrose. Note that if different types of waste are comingled, though, then the mixture must be treated as hazardous waste, and the cost for disposal of the nonhazardous portion may increase. Also consider the possibility that a hazardous material may be improperly labeled or described as nonhazardous.
When safe and allowed by regulation, disposal of nonhazardous waste via the normal trash or sewer can substantially reduce disposal costs. Many state and local regulations restrict or prohibit the disposal of waste in municipal landfills or sewer systems, and so it is wise to check the rules and requirements of the local solid waste management authority and develop a list of materials that can be disposed of safely and legally in the normal trash. The common wastes usually not regulated as hazardous include certain salts (e.g., potassium chloride and sodium carbonate), many biochemicals, nutrients, and natural products (e.g., sugars and amino acids), and inert materials used in a laboratory (e.g., noncontaminated chromatography resins and gels). In some places, the laboratory’s hazardous waste disposal firm may assist with disposal of nonregulated materials.
8.B.6 Treatment and Disposal Options
As described in the introduction to this chapter, the third tier of waste management entails reclamation and recycling of materials from the waste. These methods should be considered in conjunction with the fourth tier, disposal. Reclamation, recycling, and disposal methods for chemical hazardous waste are described in this section.
The question of what forms of treatment are allowed under federal regulations poses a dilemma for laboratory professionals. Federal regulations define treatment as “any method … designed to change the physical, chemical, or biological character or composition of any hazardous waste so as to neutralize such waste, or so as to recover energy or material resources from the waste, or so as to render the waste nonhazardous or less hazardous ….” In most cases, treatment requires a state or federal permit. The regulatory procedures and costs to obtain a permit for treatment are beyond the resources of most laboratories. Under federal law, laboratory treatment of chemical hazardous waste without a permit is allowed in the following instances:
• small-scale “treatment” that is part of a laboratory procedure, such as the last step of a chemical procedure;
• a state that allows “permit-by-rule,” treatment, that is, by allowing categorical or blanket permitting of certain small-scale treatment methods;
• elementary acid-base neutralization; and
• treatment in the waste collection container (see section 8.D for regulatory information).
Of course, treatment restrictions apply only to chemical hazardous wastes that are regulated by EPA. Some biological toxins not listed by EPA can be easily denatured without a permit by heat or an appropriate solvent. No permit is required to irretrievably mix small amounts of controlled substances (not regulated by EPA) into bulk waste flammable solvents. Because illegal waste treatment can lead to fines, it is most important that, before carrying out any processes that could be considered treatment, the responsible laboratory personnel or the institution’s EHS office check with the local, state, or regional EPA to clarify its interpretation of the rules. Some states do allow small-scale treatment of waste, but many do not.
(Section 8.D, below, provides methods for small-scale treatment of common chemicals.)
To minimize costs and manage laboratory waste most efficiently, it is important to consider treatment and disposal options as early as possible, and plan ahead. For example, the method of waste collection impacts how waste will be stored, as well as its efficient transfer to a treatment or disposal facility. In addition to the hazard reduction procedures described above, laboratories utilize several treatment and disposal options because of the great variety of waste generated, and because each option (described below) has its own advantages for Specific wastes, and so planning can be difficult. Although landfill disposal is not described separately below, it is often the disposal method for encapsulated waste, treatment residues, and ash from incineration. Note that disposal options change as technology and environmental concerns change. When feasible, waste minimization is always a best practice. (See Chapter 5, section 5.B, for step-by-step instructions on source reduction, and section 8.C, below, for general information on minimizing hazardous waste.)
8.B.6.1 Treatment and Recycling
There are various methods for physical and chemical treatment of hazardous wastes, as well as methods for recycling, reclamation, and recovery of valuable materials contained in the waste. These methods include neutralization, oxidation-reduction, distillation, digestion, encapsulation, and several forms of thermal treatment. While the expense and practicality of these technologies is largely based on the specific nature and volume of the material, treatment or recycling is preferable to incineration for some hazardous wastes. For example, high- and low-pH wastes may be neutralized, resulting in treatable wastewater and salts. Incineration of mercury and other toxic metals is restricted; recycling, recovery, or encapsulation is environmentally preferred. Filtration of aqueous-based wastes may also significantly decrease volumes and result in wastewaters suitable for treatment in a sewage treatment facility. Note that recycling and reclamation extend to reclamation of energy as well as materials,
and flammable waste liquids from laboratory operations are almost universally consolidated and used in fuel blending operations, typically to power cement plants. These liquids may also be used as a fuel source for rotary kilns.
8.B.6.2 Disposal in the Sanitary Sewer
Disposal in the sewer system (down the drain) had been a common method of waste disposal until recent years. However, environmental concerns, the viability of publicly owned treatment works (POTW), and a changing disposal culture have changed that custom markedly. In fact, many industrial and academic laboratory facilities have completely eliminated sewer disposal. Most sewer disposal is controlled locally, and it is therefore advisable to consult with the POTW to determine what is allowed. Yet, if permitted by the sewer facility, it is often reasonable to consider disposal of some chemical waste materials in the sanitary sewer. These include substances that are water-soluble and those that do not violate the federal prohibitions on disposal of waste materials that interfere with POTW operations or pose a hazard.
Chemicals that may be permissible for sewer disposal include aqueous solutions that readily biode-grade and low-toxicity solutions of inorganic substances. When allowed by law, liquid laboratory wastes that are commonly disposed of in the sanitary sewer include spent buffer solutions, neutralized mineral acids and caustics, and very dilute aqueous solutions of water-soluble organic solvents (e.g., methanol, ethanol). After checking with authorities, some laboratories flush small amounts of water-soluble nontoxic solids into the sanitary sewer with excess water. Examples of potentially sewer-disposable solids include sodium or potassium chloride, nutrients, and other chemicals generally regarded as safe. Disposal of water-miscible flammable liquids in the sewer system is usually severely limited. Water-immiscible chemicals should never go down the drain.
Under federal, state, and local law, there are various exemptions, exclusions, effluent limits, and permitting requirements that may apply to laboratory wastewaters. For most labs, there are allowances for disposing of aqueous waste, rinsate, and certain hazardous and other laboratory wastes (within limits) via the sanitary sewer. Requirements vary by state, locale, and the individual laboratory’s plumbing and sewer system, as well as other facility discharges and treatment systems that the laboratory is part of. Be aware that there are notification requirements for sewer discharges of any acute hazardous waste to a POTW (and more than 15 kg per month of other hazardous waste), and a one-time notification requirement for discharges that fall within the federal domestic sewage exclusion.
In general, if laboratory wastes are discharged via a sanitary sewer to a POTW, follow the advice above to contact your POTW as to permitting and notification requirements and effluent limits. If not, contact your state water pollution control office to determine permitting and notification requirements and effluent limits.
Waste approved for drain disposal should be disposed of only in drains that flow to a POTW, never into a storm drain or septic system. Waste should be flushed with at least a 100-fold excess of water, and the facility’s wastewater effluent should be checked periodically to ensure that concentration limits are not being exceeded.
8.B.6.3 Release to the Atmosphere
The release of vapors to the atmosphere, via, for example, open evaporation or laboratory chemical hood effluent, is not an acceptable disposal method. Apparatus for operations expected to release vapors should be equipped with appropriate trapping devices. Although laboratory emissions are not considered a major source under the Clean Air Act, deliberate disposal of materials via evaporation of vapors is strictly prohibited under RCRA.
Chemical hoods, the most common source of laboratory releases to the atmosphere, are designed as safety devices to transport vapors away from laboratory personnel, not as a routine means for volatile waste disposal. Units containing absorbent filters have been introduced into some laboratories, but have limited absorbing capacity. Redirection of hood vapors to a common trapping device can completely eliminate discharge into the atmosphere. (See Chapter 9, for more details.)
8.B.6.4 Incineration
Incineration is the most common disposal method for laboratory wastes. Incineration is normally performed in rotary kilns at high temperatures (1200–1400 °F). This technology provides for complete destruction of most organic materials and significantly reduces the volume of residual material which must be disposed of by landfill. However, it is an expensive option, generally requiring the use of significant volumes of fuel to generate the required temperatures. Also, some materials, such as mercury and mercury salts, may not be incinerated because of regulations and limitations of the destruction capability. (For information about reducing the use of mercury in laboratories, see Chapter 5, section 5.B.8.)
8.B.7 Monitoring Waste Services, Transport, and Off-Site Treatment and Disposal
The ultimate destination of waste is usually a treatment, recycling, and/or disposal facility, which is sometimes called a permitted TSDF. Here waste is treated (typically via chemical action or incineration), recycled, reclaimed, or disposed of in a landfill. Although the waste has left the generator’s facility, the generator retains the final responsibility for the long-term fate of the waste. As explained below, it is important that the generator verify that the waste transporter and TSDF operate in a way that is safe, compliant, and environmentally sound, and minimizes long-term liability. The procedures for preparing and transporting the waste to such a facility are similar to those described above. (See section 8.B.3.)
Waste is rarely transported from a generator’s site directly to the ultimate disposal facility. Because of the economics of transportation, only truckload quantities (usually at least 55- to 80-gal drums or a full tanker) are shipped directly. Most laboratory waste is transported to a transfer storage facility where it is consolidated with wastes from other generators and stored until truckload quantities are accumulated. Flammable solvents and other compatible materials are often blended to make them amenable for fuel recovery.
Generators are legally responsible for all aspects of hazardous waste management, including proper disposal, packaging, labeling, shipping, manifest preparation, recordkeeping, accumulation area operations and maintenance, accumulation time limit compliance, and contingency planning associated with hazardous waste management. However, generators may choose to handle some or all of these responsibilities using in-house personnel, or they may contract with professional waste management firms. The generator’s responsibilities are summarized below:
• inspecting waste containers to ensure complete and legal labeling, and container dating when required;
• waste accumulation, and managing accumulation areas for safety, security, aisle width, and separation of incompatibles;
• inspecting waste containers to ensure that they are always closed and in good condition, including repackaging of leaking containers;
• preparation and updating of waste management and contingency plans;
• sampling and characterization of routine wastes;
• sampling and characterization of “unusual” or new wastes;
• preparation of transportation documents;
• identification of disposal sites; and
• performance of periodic waste management audits to ensure compliance with regulations.
Regardless of which activities a generator decides to conduct in-house, it is imperative that well-trained, qualified staff be available to conduct the waste management activities. It is also important that these persons be given the independence, authority, and resources to properly manage the facility’s wastes and maintain regulatory compliance.
The selection of which activities to perform in-house and which services to handle through firms that specialize in waste disposal is dependent on the number, qualifications, and availability of in-house staff, organizational philosophy, and budgetary constraints. This is summarized in Table 8.1. It is very important to recognize that in the long term, it is the generator who bears the major liability to ensure proper handling and disposal of hazardous waste. Thus the choice of any outside waste disposal firm to participate in the process is extremely important.
When a generator designs or evaluates the effectiveness of its waste management program, it is important to know the types of outside services that are available and to determine if the use of such services is necessary or beneficial. Once a decision has been reached to hire a waste disposal firm for such services, it is important to know how to select, monitor, and work with such firms.
Waste disposal firms differ in the types of service they provide. Some firms furnish consulting services or directly provide transportation or disposal services. Other waste disposal firms provide both types of services, but usually specialize in one or the other.
Some waste disposal firms, referred to as brokers, generally provide few, if any, direct services. They coordinate, directly or through other specialty firms, the selection of disposal sites, acquisition of disposal approvals, and subsequent transportation and disposal. Brokers typically provide only limited direct consulting or waste management support services, which are often limited to waste sampling, packaging, preparation for shipment, and some regulatory compliance support.
Transportation services are designed to move the waste from the point of generation to the chosen TSDF. For laboratories, this typically involves transport of drums or other small containers by truck. For transport on public roads, federal regulations require laboratory waste to be contained in approved packages, such as drums approved by DOT. The packages must be marked and labeled according to DOT rules, and accompanied by a manifest, as described below. Preparers and drivers must receive DOT training. Drivers
need a special license. In many cases, vehicles must be placarded. Generators depend on waste dispsosal firms and transportation services for compliance with these rules. Many transportation firms also provide waste characterization and preparation, disposal site selection, and disposal approval services. Transport services can be contracted directly by a generator or through a broker.
Waste disposal firms also include companies that operate permitted treatment facilities, disposal sites, and recycling/reclamation facilities. The appropriate TSDF should be selected by the generator, although often this decision is heavily influenced by the waste disposal firm, which usually has ties to selected TSDFs or will make recommendations. The generator should always be involved in this decision, because it can affect long-term liability as well as short-term cost.
8.B.7.1 Preparation for Off-Site Treatment or Disposal of Waste
Transportation and packaging requirements dictate how waste is prepared for shipment to a transfer, treatment, or disposal facility. Laboratory waste is often placed into DOT-compliant 1- to 55-gal overpacks. Reactives, gases, and highly incompatible and some highly toxic chemicals must be packaged separately.
Using labpacks is quite simple. As described above, small containers of compatible waste materials are placed in a larger container, usually a 30- to 55-gal drum, along with appropriate packing materials, as they are collected. When a drum is filled and ready for shipping, an inventory list of the contents of the labpack is prepared.
For certain similar and compatible liquid wastes, however, collecting containers of waste in a labpack is usually much more expensive than commingling (i.e., mixing) the wastes, partly because a 55-gal lab-pack only holds about 16 gal of waste in its individual containers. Most often, nonhalogenated solvents are ideally suited for commingling in bulk, because the best disposal strategies for flammable liquids are fuel blending or recycling. Commingling requires opening of containers and transferring their contents from the smaller laboratory containers to a larger drum. Furthermore, the containers should be rinsed before they are considered nonhazardous, and the rinsate must be treated as a hazardous waste.
Safety precautions and facility requirements for commingling are described above. See section 8.B.3.4, above, for information on rinsing and disposal of empty containers. Drums of commingled waste usually require an analysis or listing of their contents.
Commingling does not make sense if safe facilities are not available, or when insufficient amounts of compatible waste are generated within storage time limits.
8.B.7.2 Choice of Transporter and Disposal Facility
Because the long-term liability for the waste remains with the generator, it is imperative that the generator be thoroughly familiar with the experience and record of the transporter and TSDF. Economic factors alone should not govern choices, because the long-term consequences can be significant. The generator must obtain assurance, in terms of documentation, permits, records, insurance and liability coverage, and regulatory compliance history, that the chosen service provider is reliable.
There is often an advantage, particularly for smaller facilities, to contracting for all of the hazardous waste disposal operations. These include the packing and appropriate labeling of waste for off-site transportation and disposal, preparation of the shipping manifest, and arranging for the transporter and disposal facility. Again, the liability remains with the generator, and so the choice of such a waste disposal firm is critical.
In some states, Minnesota and Montana, for example, arrangements have been developed with local regulators to allow a large laboratory waste generator to handle the waste from very small laboratories such as those at small colleges and public schools. This plan results in informed assistance and cost savings for the smaller units. In Wisconsin, a statewide commercial contract that can be accessed by all state educational systems has been arranged. There is usually significant advantage to working with local and state agencies to develop acceptable plans for disposal methods that are environmentally and economically favorable for both large and small generators.
8.B.8 Liability Concerns
Generators are liable for proper disposal regardless of who is involved once waste leaves the facility. Long-term liability usually refers to Superfund liability. Superfund, the federal environmental policy initiative officially known as the Comprehensive Environmental Response, Compensation and Liability Act (CERCLA), provides EPA with broad authority to initiate the cleanup of hazardous waste sites. Originally funded primarily by taxes on chemicals and petroleum, the trust fund was fully depleted in 2003. All cleanups are now paid for by organizations found by EPA to have contributed to the contamination. Referred to as PRPs (potentially responsible parties), these organizations include four classes of parties:
• current owner or operator of the site [CERCLA § 107(a)(1)];
• owner or operator of a site at the time that disposal of a hazardous substance, pollutant, or contaminant occurred [CERCLA § 107(a)(2)];
• person who arranged for the disposal of a hazardous substance, pollutant, or contaminant at a site [CERCLA § 107(a)(3)]; and
• person who transported a hazardous substance, pollutant, or contaminant to a site; that transporter must have also selected that site for the disposal of the hazardous substances, pollutants, or contaminants [CERCLA § 107(a)(4)]. (See 42 U.S.C. § 9607.)
Sites may become Superfund sites through bankruptcy or other financial stress, illegal or improper disposal, act of nature (flooding, tornado, earthquake), or fire or explosion. For these reasons, generators should be concerned about the ultimate disposal of their wastes; many choose to pay higher initial costs to have wastes incinerated so as to reduce their long-term liability. Generators may perform other actions to ensure proper disposal, such as facility audits, credit checks, inspections, and/or regulatory compliance reviews.
8.B.9 Manifesting Hazardous Wastes
Hazardous wastes are shipped off-site using a special hazardous materials bill of lading known as a Uniform Hazardous Waste Manifest (see Figure 8.2). Transporters typically complete the manifest, but the shipper (generator) is legally responsible for its accuracy and completeness. Generators are also responsible for keeping a copy of the manifest, as well as the returned manifest that documents the waste’s receipt at its destination. For this reason, it is important that generators have at least a basic understanding of the DOT and EPA requirements for proper completion of the form. Although instructions are provided with each manifest (printed on the back of the form), not all the information necessary to complete the manifest is provided.
Each container listed on the form must have a proper shipping name, hazard class, and EPA number. Shipping names are found in the Hazardous Materials Table (49 CFR § 172.101). For many chemical compounds, the name of the chemical is the proper shipping name. If the chemical name is not listed, then a generic hazard class (flammable liquid, corrosive solid, poisonous liquid, etc.) may be used. Once the proper shipping name has been identified, the columns to the right on the Hazardous Materials Table provide the hazard class, division, packaging requirements, and exemptions for the material.
EPA numbers must be provided for each line item on the manifest. These numbers are from the hazardous waste lists and characteristics associated with each waste. For instance, flammable liquids have a D001 hazardous waste ID number; corrosives are D002, and reactive wastes are D003. The toxic characteristics all have their own individual numbers. For example, arsenic wastes are D004. U- and P-listed chemicals each have their own number, beginning with either a U or a P, respectively. Should the waste carry more than one hazardous waste code, each should be listed on the manifest.
Other information on the manifest that must be provided (and completed correctly) are the generator’s EPA ID number; 24-hour emergency response number; generator address; name, address, and EPA number of the transporter; name, address and EPA number of the designated TSDF; and the generator certification.
Each manifest that includes labpacked material should also include inventory sheets for each container within each labpack. These forms help to meet the regulatory requirement for description of hazardous materials for regulated transportation; they also provide the permitted TSDF with Specific information regarding container contents. Other paperwork typically required for disposal includes a Land Disposal Restriction form, which provides information regarding proper disposal of each waste category on the manifest.
8.B.10 Records and Record Keeping
Records are needed both to meet regulatory requirements and to help monitor the success of the hazardous waste management program. Because the central accumulation area is usually the last place where waste is dealt with before it leaves the facility, it is often the most suitable place for ensuring that all appropriate and required records have been generated.
For regulatory purposes, the facility needs to keep records for on-site activities that include
• quantities and identification of waste generated and shipped;
• documentation of analyses of unknown materials if required;
• manifests for waste shipping as well as verification of disposal;
• inspection records, training records, contingency plans; and
• any other information required to ensure compliance to prevent long-term liability.
Although not required, a good practice is to request and keep certificates of final disposal or final disposi-
tion. Records of costs, internal tracking, and so forth can provide information on the success of the hazardous waste management program.
Multihazardous waste is a waste that presents any combination of chemical, radioactive, or biological hazards. This array of waste constituent hazards makes the management of multihazardous wastes difficult and complex. For example, low-level mixed waste (LLMW) is a multihazardous waste that contains both RCRA hazardous wastes that EPA regulates and low-level radioactive wastes (LLW or LLRW) that the USNRC regulates. The hazardous characteristics, treatment methods, and disposal requirements for these wastes are different and often incompatible. Other factors that further complicate the management of multihazardous wastes include a complex federal, state, and local regulatory framework; limited disposal options; and high disposal costs. Commercial treatment or disposal facilities for multihazardous waste from laboratories are scarce. There is little incentive for the development of a commercial market to treat and dispose of laboratory multihazardous waste because most of the waste that laboratories generate is unique to laboratories and small in volume. The management of multihazardous waste is particularly challenging for research laboratories where there are frequent changes in protocols, procedures, materials, and waste generating processes. These difficult and complex management issues can also make it difficult to promote and sustain prudent pollution prevention practices.
Medical, clinical, forensic, and environmental laboratories, and biomedical, biochemical, radiological, and other types of research laboratories generate multihazardous waste. Prudent management of these wastes is necessary to protect the health and safety of all laboratory personnel who handle, process, and store the waste for disposal, and to minimize the potential of harm to public health and the environment. A further objective of prudent management of multihazardous waste is to promote excellence in environmental stewardship. The Congress established a federal initiative for preventing or reducing pollution in the Pollution Prevention Act of 1990. This initiative can serve as a guide for developing prudent practices for managing multihazardous wastes.
The Pollution Prevention Act of 1990 established a national policy that emphasizes source reduction as the most desirable approach for preventing or reducing pollution. The policy created a new hierarchy for the management of hazardous wastes. The elements of that hierarchy are listed in order of priority and importance for accomplishing the objectives of the Pollution Prevention Act.
• Source reduction. Pollution should be prevented or reduced at the source whenever feasible.
• Recycling. Pollution that cannot be prevented should be recycled in an environmentally safe manner whenever feasible.
• Treatment. Pollution that cannot be prevented or recycled should be treated in an environmentally safe manner whenever feasible.
• Disposal. Disposal or other release into the environment should be employed only as a last resort and should be conducted in an environmentally safe manner.
Federal agencies are required to promote programs to advance this policy within their agencies and nationwide. The major research agencies within the federal government, and particularly the Department of Energy, National Institutes of Health (NIH), and EPA, are providing leadership in implementing the nation’s pollution prevention policy, and are achieving results in source reduction. For example, NIH’s low-level mixed waste (LLMW) minimization program demonstrated that a significant amount of mixed waste currently being generated can be reduced or eliminated. References found on the accompanying CD provide more detail about the source reduction and pollution prevention initiatives of those agencies, the achievements of which have encouraged academic research universities and corporate research facilities to focus their pollution prevention programs on source reduction. EHS programs at these institutions often share information on their Web sites regarding source reduction, recycling, treatment, and disposal.
Prudent waste management methods include a commitment by senior management to develop and support a waste minimization program. The program development should involve experienced laboratory personnel in planning waste minimization strategies and identifying source reduction options, such as incorporating pollution prevention goals into project proposals. Training of laboratory personnel to recognize opportunities for source reduction, reviewing research proposals to ensure adoption of available source reduction strategies, improving compliance with regulatory requirements, and institutional policy are among the new management initiatives at research institutions promoting pollution prevention. Multihazardous waste requires complex attention because of its combination of hazards and regulatory controls, as detailed in the following guidelines:
Assess the risk posed by the hazardous characteristics of the waste. A primary purpose of the risk assessment is to determine which hazardous constituent of the multihazardous waste presents the greatest risk. This knowledge can help identify source reduction and treatment possibilities to reduce the risk of the waste. An assessment that determines that a waste constituent does not present a significant risk may provide an opportunity for regulatory flexibility. For example, the USNRC or state authority may allow a licensee to manage a chemical–radioactive waste as a chemical waste without regard to radioactivity when the radioactive constituent concentration is less than what the USNRC specifies for an unrestricted area.
Minimize the hazardous constituents in the waste. Consider applying the waste minimization methods specific to each hazardous constituent of the waste. This strategy could result in reducing or eliminating one hazardous constituent from the waste stream and managing the waste as a single-hazard waste. For example, the substitution of nonignitable liquid scintillation fluid (LSF) for toluene-based LSF reduces a chemical–radioactive waste to a radioactive waste.
Determine options for managing the multihazardous waste. Waste management options include recycling, laboratory methods, management at institutional waste facilities, and treatment and disposal at commercial sites. Options can vary considerably between laboratories depending upon institutional capabilities and state and local laws. It may be appropriate to manage the waste in order of risk priority, from high to low risk. Options must be compatible with all hazards, and combinations of waste management methods may be limited by their order of application. Reject any combination or sequence of methods that may create an unreasonable risk to waste handlers or the environment, or that might increase the overall risk. If an option has a clear advantage in efficiency and safety, it should have highest priority. For example, if safe facilities are available on-site, hold short-half-life radioactive waste for decay before managing it as a chemical or biological waste. The EPA Final Rule on the storage, treatment, transportation, and disposal of low-level mixed waste will allow holding the waste for longer than 90 days.
Select a single management option when possible. Some waste management methods are appropriate for more than one waste hazard. Some multihazardous waste can be disposed of safely in the sanitary sewer when allowed by the local POTW.
8.C.1 Chemical–Radioactive (Mixed) Waste
LLMW is the most common form of multihazardous waste generated in laboratories and the most problematic for waste management. “Mixed waste” is the regulatory term for multihazardous waste that exhibits both chemical and radioactive hazards (40 CFR § 266.210). Mixed wastes are defined by EPA as “wastes that contain a chemical hazardous waste component regulated under Subtitle C of RCRA and a radioactive component consisting of source, special nuclear, or byproduct material regulated under the Atomic Energy Act of 1946 (AEA)” (EPA, 1986). The complex challenge of managing waste controlled by two federal agencies was reduced by the EPA Final Rule on the storage, treatment, transportation, and disposal of low-level mixed waste of 2001 (40 CFR Part 266, Subpart N). The rule conditionally exempts the hazardous waste constituents of LLMW from RCRA during storage and treatment. This change provides more opportunities for treatment of the chemical constituents in LLMW, enabling disposal as a single hazard constituent LLRW. State regulations may continue to inhibit laboratory and on-site minimization, storage, and treatment of mixed waste. The rule applies only to LLMW that meets the specified conditions and is generated under a single USNRC license or USNRC Agreement State license. The rule also exempts LLMW and hazardous naturally occurring or accelerator-produced radioactive materials (NARM) waste from RCRA manifest, transportation, and disposal requirements that adhere to the specified conditions. Under this conditional exemption, the waste remains subject to manifest, transport, and disposal requirements under the USNRC (or USNRC Agreement State) regulations for LLW or eligible NARM. This flexibility allows on-site storage of LLMW for periods longer than 90 days. The management opportunity to treat the hazardous waste constituents of LLMW on-site can reduce the dependence on services provided by commercial treatment and disposal facilities.
Examples of laboratory mixed waste include
• used flammable (e.g., toluene) liquid scintillation cocktails,
• phenol–chloroform mixtures from extraction of nucleic acids from radiolabeled cell components,
• aqueous solutions containing radioactive material and chloroform that occur in solutions generated by the neutralization of radioactive trichloroacetic acid solutions,
• certain gel electrophoresis waste (e.g., methanol or acetic acid containing radionuclides), and
• lead contaminated with radioactive materials.
Mixed waste produced at university, clinical, and medical research laboratories is typically a mixture of a LLRW and chemical hazardous waste. Mixed waste from nuclear and energy research laboratories can
include both low- and high-level (e.g., spent nuclear fuels) radioactive materials combined with chemical hazardous waste. Common laboratory waste management methods for radioactive constituents in waste include storage for decay and indefinite on-site storage, burial at a LLRW site, incineration, and sanitary sewer disposal. Disposal options for mixed waste are usually very expensive, and for many types of mixed waste, there are no management options other than indefinite storage on-site.
8.C.1.1 Minimization of Mixed Waste
Rigorous application of waste minimization principles can often solve the problems of managing mixed waste. Such efforts are most successful when scientists and EHS staff work together to evaluate laboratory processes. A successful collaborative minimization initiative undertaken by the NIH Mixed Waste Minimization Program demonstrated that the ultraviolet peroxidation treatment of aqueous mixed waste could reduce or eliminate a large portion of the mixed waste generated in the NIH research laboratories. This treatment method degrades hazardous organic compounds in high-volume aqueous mixed waste streams. The removal efficiency for a number of volatile and semi-volatile compounds is in excess of 99.99%. The treated waste can be discharged to the sanitary sewer (Rau, 1997).
Modifying laboratory processes, improving operations, or using substitute materials are approaches that can achieve minimization of mixed waste. Examples of these approaches include the following:
• Use 2.5-mL scintillation vials (“minivials”) rather than 10-mL vials. Adapters are available for scintillation counters with 10-mL vial racks.
• Count phosphorus-32 (32P) without scintillation fluid by the Cerenkov method on the tritium (3H) setting of a liquid scintillation counter (approximately 40% efficiency); iodine-125 (125I) can be counted without scintillation fluid in a gamma counter.
• Use microscale chemistry techniques.
• Eliminate the methanol/acetic acid (chemical) and radioactive mixed hazards in gel electrophoresis work by skipping the gel-fixing step if it is not required.
• Line lead containers with disposable plastic or use alternative shielding materials to prevent lead contamination by radioactivity.
• Reduce the volume of dry waste by compaction of contaminated waste gloves, absorbent pads, and glassware.
Some simple operational improvements can help minimize mixed waste. Purchase chemicals and radioactive materials in quantities necessary for a planned experiment to avoid creating surplus materials that may end up as waste. Establish procedures that will prevent commingling radioactive waste with noncontaminated materials and trash.
Consider substituting a less-hazardous constituent for either the chemical or the radioactive source of the mixed waste. The experimenter should use the minimum activity necessary and select the radionuclide with the most appropriate decay characteristics. Examples include the following:
• Use nonignitable scintillation fluid (e.g., phenyl-xylylethane, linear alkylbenzenes, and diisopro-pylnaphthalene) instead of flammable scintillation fluid (e.g., toluene, xylene, and pseudocumene). LSF that is sold as being “biodegradable” or “sewer disposable” is more appropriately labeled as “nonignitable” because biodegradability in the sanitary sewer can vary considerably with the local treatment facility.
• Use nonradioactive substitutes such as scintillation proximity assays for phosphorus-32 (32P) or sulfur-35 (35S) sequencing studies or 3H cation assays, and enhanced chemiluminescence as a substitute for 32P and 35S DNA probe labeling and Southern blot analysis.
• Substitute enriched stable isotopes for radionuclides in some cases. Mass spectrometry (MS) techniques, such as inductively coupled plasma-MS, are beginning to rival the sensitivity of some counting methods. Examples include use of oxy-gen-18 (18O) and deuterium (2H) with mass spectrometry detection as substitutes for oxygen-19 (19O) and 3H.
• Substitution of shorter-half-life radionuclides such as 32P (t1/2 = 14 days) for phosphorus-33 (33P) (t1/2 = 25 days) in orthophosphate studies, or 33P or 32P for 35S (t1/2 = 87 days) in nucleotides and deoxynucleotides. In many uses, iodine-131 (131I) (t1/2 = 8 days) can be substituted for 125I (t1/2 = 60 days). Additional exposure precautions may be required.
8.C.1.2 Safe Storage of Mixed Waste
Store waste containing short-half-life radionuclides for decay prior to subsequent waste management processing and disposal. On-site decay-in-storage of LLW is very efficient and minimizes handling and transportation risks. Most institutions designate a room or facility equipped with good ventilation, effluent trapping, and fire suppression to contain and manage on-site
decay-in-storage. Laboratory decay-in-storage space can provide safe storage for low-risk mixed wastes. Laboratory storage is not appropriate for storage of putrescent or reactive materials.
The specific USNRC requirements for decay-in-storage of radioactive waste are usually detailed in the institution’s license. Decay-in-storage is usually limited to half-lives of less than 65 days. When the short-half-life radionuclides have decayed to background levels (the length of time depending on the initial radioactivity level but typically defined as a storage period of at least 10 half-lives), the chemical–radioactive waste can be managed as a chemical waste. After the decay period, USNRC licenses usually require that the mixed waste be surveyed for external radiation prior to releasing it to the chemical waste stream.
Storage of mixed waste for decay for more than 90 days may require the approval of the state chemical hazardous waste authority. In permitted storage facilities, storage may be limited to 1 year for some types of mixed waste. Workers should contact their institution’s EHS staff or local hazardous waste agency to determine their regulatory status and requirements for storing mixed waste for decay.
8.C.1.3 Hazard Reduction of Mixed Waste
Chemical hazards can be reduced by carrying out various common chemical reactions with the waste in the laboratory. However, “treatment” of chemical hazardous waste has regulatory implications that must be considered. Many of the same considerations apply to treatment of mixed waste.
Nevertheless, there are still justifiable and legal reasons to carry out such operations in the laboratory when hazards can be minimized safely. Neutralization, oxidation, reduction, and various other chemical conversions as well as physical methods of separation and concentration can be applied prudently to many laboratory-scale mixed wastes. However, the dual character of the hazard, chemical and radioactive, requires that additional precautions be exercised. Treatment for the chemical hazard must not create a radioactivity risk for personnel or the environment. For example, vapors or aerosols from a reaction, distillation, or evaporation must not lead to escape of unsafe levels of radioactive materials into the atmosphere. Laboratory chemical hoods appropriate for such operations should be designed to trap any radioactive effluent. When mixed waste is made chemically safe for disposal into the sanitary sewer, the laboratory must ensure that the radioactivity hazard is below the standards set by the POTW. Several examples for reducing the hazard of mixed waste are described below:
• The worker can reduce the chemical hazard to a safe level and then handle the material as only a radioactive hazardous waste. Many low-level radiation materials can then be allowed to decay to a safe level, following which simple disposal is allowable.
• Some radioactive methanol–acetic acid solutions from gel electrophoresis can be recycled via distillation and the methanol reused. The solution is neutralized prior to distillation to protect the distillation equipment from corrosion and to reduce the level of methyl acetate formed during the process.
• The volume of waste phenol, chloroform, methanol, and water containing radionuclides can be reduced by separating the nonaqueous portion using a separatory funnel. After separation, the organic phase can be distilled to produce chloroform waste, which may contain levels of radioactivity below license limits for radioactive waste. The still bottom and aqueous phase must be handled as a mixed waste.
• High-performance liquid chromatography, used to purify radiolabeled proteins and lipids, can generate a waste radioactive solution of acetonitrile, water, methanol, acetic acid, and often a small amount of dimethylformamide. When the solution is distilled by rotary flash evaporation, the distillate of acetonitrile, methanol, and water is nonradioactive and can be handled as a chemical hazardous waste. The radioactive still bottom, containing 1 to 5% methanol and acetic acid, can usually be neutralized, diluted, and disposed of in the sanitary sewer.
• Aqueous solutions containing uranyl or thorium compounds can be evaporated to dryness and the residues disposed of as radioactive waste. Because of their toxicity, solidification may be necessary prior to burial at a LLRW site.
• Activated carbon, Molecular Sieves®, synthetic resins, and ion-exchange resins have been used with varying success in the separation of chemical and radioactive waste constituents. Activated carbon has been used to remove low concentrations of chloroform (less than 150 ppm) from aqueous mixed waste solutions. However, activated carbon is not suitable for high concentrations of phenol–chloroform or acetonitrile–water mixed waste. Amberlite® XAD resin, a series of Amberlite® polymeric absorbent resins used in chromatography, has been shown to be effective in removing the organic constituents from aqueous phenol, chloroform, and methanol solutions, leaving an aqueous solution that can be managed as
a radioactive waste. Chemical constituents can be separated from mixed waste by using supercritical fluid extraction (e.g., carbon dioxide), which is now available commercially.
• Surface contamination from radioactively contaminated lead can be removed by dipping the contaminated lead into a solution of 1 M hydrochloric acid. After rinsing the lead with water, it usually can be documented as nonradioactive. The acidic wash and rinse solutions contain radio-nuclides and lead and must be handled as mixed waste. However, decontaminating the lead results in a smaller mass of mixed waste and allows the decontaminated lead to be reused or recycled. Commercial rinse products are also available for this purpose.
• Incineration is advantageous as a treatment for many types of chemical–radioactive waste, especially those that contain toxic or flammable organic chemicals. Incineration can destroy oxidizable organic chemicals in the waste. To comply with radionuclide release limits, USNRC licensees need to control emissions and may need to restrict the incinerator’s waste feed. Radioactive ash is typically managed as a radioactive waste. It is important to keep toxic metals (e.g., lead, mercury) out of the incinerable waste so that the ash is not chemically hazardous according to the TCLP test. On-site incineration minimizes handling and transportation risks; however, incineration of chemical waste is regulated by EPA and requires a permit, which is beyond the resources of most laboratory waste generators.
8.C.1.4 Commercial Disposal Services for Mixed Waste
Because of the great variety of laboratory mixed waste, it is often difficult to find a facility that can manage both the radioactive and the chemical hazards of the waste. In general, existing commercial disposal facilities are in business to manage mixed waste from the nuclear power industry, not waste from laboratories. Several commercial disposal facilities that accept mixed waste from off-site generators do exist in the United States. These sites have the capacity to manage LSF, halogenated organics, and other organic waste. Treatment capacity exists for stabilization, neutralization, decontamination/macroencapsulation of lead, and reduction of chromium waste.
In spite of this capacity, many types of laboratory mixed waste have no commercial repository. No commercial mixed-waste disposal facilities exist for waste contaminated with most toxic metals (such as mercury) or for lead-contaminated oils. Commercial disposal capacity likewise does not exist for high concentrations of halogen-containing organics and other TCLP waste, such as waste that contains chloroform.
8.C.2 Chemical–Biological Waste
Medical, clinical, and biomedical research laboratories generate waste that contains potentially infectious materials and viable agents that are capable of causing human disease. Biohazardous wastes can include tissues and carcasses of experimental animals involved in infectious disease studies; cell cultures of infectious agents; contaminated sharps, gloves, gowns, glassware, and instruments; and blood and other clinical specimens. Biohazardous waste presents a hazard to persons who handle the waste within the generating facility. Waste decontamination is the treatment method to control or eliminate the exposure hazard prior to waste handling and disposal. The OSHA Occupational Exposure to Bloodborne Pathogens rule (29 CFR § 1910.1030) established federal requirements for the collection and containment of certain laboratory wastes that contain human blood or body fluids for the purpose of preventing exposure of personnel to bloodborne pathogens. This rule promotes the use of standard microbiological practices including safe practices for handling biohazardous wastes. The rule also requires the treatment of all contaminated waste from research laboratories handling human immuno-deficiency virus (HIV) and hepatitis B virus (HBV) and other bloodborne pathogens by incineration or decontamination by a method known to destroy the pathogens within the waste materials. Federal regulations regarding transport and incineration may apply to the off-site management of nonlaboratory biohazardous waste, such as waste generated in medical or health care settings. Several states and local jurisdictions regulate the treatment and disposal of biohazardous wastes.
The Centers for Disease Control and Prevention (CDC) of the U.S. Department of Health and Human Services and the Animal and Plant Health Inspection Service of the U.S. Department of Agriculture promulgated rules under the Public Health Security and Bioterrorism Preparedness and Response Act of 2002 for the possession, use, and transfer of select agents and toxins. The rules require the destruction of select agents that are contaminants in any waste by validated laboratory decontamination methods, such as chemical decontamination or autoclave sterilization, before disposal.
Special procedures are required in disposing of multihazardous waste that includes both hazardous chemicals and materials contaminated with microor-
ganisms that are potentially pathogenic. The purpose of the special procedures is to prevent the release of infectious agents to the environment. The procedures involve decontaminating the mixed hazardous chemical and biohazardous waste to eliminate the biohazardous characteristics of the waste prior to disposal. Autoclaving and chemical decontamination are the methods of choice for decontaminating biohazardous waste. Autoclaving volatile chemicals is not appropriate because this practice could cause the release of the chemical to the environment.
Disposal is most difficult for the very small amount of chemical–biological waste that is EPA-regulated as chemically hazardous or contains a chemical, such as lead, that is inappropriate for an animal or medical waste incinerator. Disposal of tissue specimens preserved in ethanol or another flammable solvent is also difficult. In most cases, storage of this waste is limited to 90 days and must be managed at an EPA-permitted chemical waste facility. However, few chemical waste facilities are prepared to handle waste that is putrescible, infectious, or biohazardous.
8.C.2.1 Disposal of Chemically Contaminated Animal Tissue
Animal carcasses and tissues that contain a toxic chemical may be the most prominent chemical–biological laboratory waste. Such waste includes biological specimens preserved in formalin and rodents that have been fed lead, mercury, or PCBs in toxicity studies. If storage of such putrescible waste is necessary, refrigeration is usually advisable. Infectious waste should be stored separately in a secure area.
Incineration, which destroys potential infectious agents, is the most appropriate disposal method for putrescible waste. Large research institutions are likely to have an on-site animal incinerator. Medical waste incineration is also available through commercial waste haulers.
(If animal or commercial incineration is unavailable, methods in section 8.C.3.3, below, may be adaptable to chemical–biological waste.)
8.C.2.2 Sewer Disposal of Chemical–Biological Liquids
Laboratories that manipulate infectious agents, blood, or body fluids may generate waste that is contaminated with these materials and toxic chemicals. In most cases, blood and body fluids that contain toxic chemicals can be disposed of safely in a sanitary sewer, which is designed to accept biological waste. Approval for such disposal should be requested from the local wastewater treatment works. Chemical concentrations in such waste are typically low enough to be accepted by a local treatment works. OSHA recommends that a separate sink be used exclusively for disposal of human blood, body fluids, and infectious waste. It may be prudent to treat blood and body fluids with bleach (usually a 1:10 aqueous dilution of household bleach) prior to disposal in the sanitary sewer. Laboratory personnel should take care to prevent personal exposure while waste is being discharged into the sewer.
8.C.2.3 Disinfection and Autoclaving of Contaminated Labware
Contaminated labware may include cultures, stocks, petri plates, and other disposable laboratory items (e.g., gloves, pipettes, and tips). In many cases, the small quantities of infectious waste on labware can be disinfected safely with bleach or other chemical disinfectant (e.g., by soaking overnight). Once disinfected, the labware can be treated as a chemical waste. Laboratory personnel must check with the state or regional EPA office to determine if a treatment permit is required for chemical disinfection of chemical–biological waste.
Autoclaves can be used to steam-sterilize infectious waste but should be tested routinely for efficacy. Autoclaving does not require an EPA permit. Care must be taken because autoclaving of chemical–biological waste at 120 to 130 °C may result in the volatilization or release of the chemical constituent. Additional waste containment may be needed to minimize chemical releases, but it can interfere with steam penetration into the waste load and sterilization. Before autoclaving, evaluate the waste to verify that the heat and pressure of autoclaving do not create unsafe conditions.
Autoclaving waste containing flammable liquids may result in a fire or explosion. Note also that steam sterilization of waste that contains bleach may harm an autoclave. To autoclave voluminous chemical– biological waste streams, it may be appropriate to dedicate an autoclave room with ample ventilation and to restrict access.
8.C.2.4 Disposal of Chemically Contaminated Medical Waste and Sharps
Laboratories that work with human blood must adhere to OSHA’s Standard for Occupational Exposure to Bloodborne Pathogens (29 CFR § 1910.1030), which requires waste containment, marking, and labeling. The OSHA standard also regulates waste disposal from laboratories that manipulate HIV or HBV. In general, such waste that has chemical contamination can be incinerated with other medical waste.
Waste hypodermic needles and other “sharps” (e.g.,
scalpels and razor blades) need to be contained in a puncture-resistant waste collection container. Sharps should be destroyed by incineration or by grinding as part of the disinfection treatment. Incineration of chemical- or drug-contaminated needles in a medical waste incinerator is appropriate if the waste is not an EPA-regulated chemical waste and if the chemical’s toxicity or contamination is low. Needles and other sharps that are contaminated with toxic chemicals and infectious agents or blood can be autoclaved or disinfected on-site (see the precautions above), and then managed as a chemical waste. The waste container’s biohazard symbol and markings should be defaced after autoclaving or disinfection to indicate that the waste has been sterilized. Noninfectious needles and sharps with high chemical toxicity or contamination are accepted by chemical incinerators.
Some biomedical research generates materials contaminated with blood and cytotoxic antineoplastic drugs or other highly potent drugs. Incineration of these materials as medical waste is appropriate. In some cases, chemical disinfection and treatment can be combined to destroy both infectious agents and the drug. Note that unemptied source containers of some drugs are EPA-listed hazardous waste and must be managed as a regulated chemical waste.
8.C.2.5 Minimization Methods for Chemical– Biological Waste
Waste minimization methods used for chemical waste can be used to reduce or eliminate the chemical hazard of chemical–biological waste. Some laboratories that generate biohazardous waste have replaced disposable items with reusable supplies, which are disinfected between uses.
For biological waste, waste minimization can be accomplished best through careful source separation of biological waste from other waste streams. When state guidelines for defining infectious waste do not exist, it is important for laboratories to define carefully those biological wastes that can be disposed of safely as non-infectious within the framework of the CDC and NIH guidelines (HHS/CDC/NIH, 2007a). Training workers to identify and separate biological waste will prevent its inadvertent mixing with other waste streams and normal trash.
8.C.3 Radioactive–Biological Waste
The management of radioactive–biological laboratory waste can be difficult because of limited on- and off-site disposal options. Basic principles for the management of radioactive–biological waste include the following:
• Risk associated with the waste should be assessed. It may be prudent to decontaminate highly bio-hazardous agents first to minimize handling risks. Appropriate containment, handling, and storage precautions should be taken prior to treatment.
• Radioactive–biological waste containing short-half-life radionuclides can be held for decay. After decay-in-storage, most USNRC licenses allow the waste to be managed as biological waste. If the waste supports the growth of an infectious agent that it contains, storage should be in a freezer to prevent the waste’s infectious load from increasing.
• Refrigerated storage facilities or other preservation methods are necessary for putrescible waste.
8.C.3.1 Off-Site Management of Low-Level Radioactive Waste
Many laboratories do not have an on-site incinerator for radioactive–biological waste. Communities tend to oppose waste incinerators, and on-site incineration is prohibitively costly for some radioactive–biological waste generators. Even institutions that have incinerators must usually rely on off-site disposal for some of their radioactive waste. For radioactive putrescible waste, off-site disposal requires special packaging, storage, and transport considerations.
8.C.3.2 Disposal of Radioactive Animal Carcasses and Tissue
Waste radioactive animal carcasses and tissue generated from biomedical research typically pose no significant infectious hazard, but they are putrescible. USNRC regulations allow animal carcasses and tissue with less than 1.85 kBq/g of 3H or 14C to be disposed of without regard to radioactivity. Thus animal carcasses and tissue below this limit need not be managed as a radioactive–biological waste but only as a biological waste.
Animal tissue with higher levels of activity or other radionuclides must be managed as a radioactive waste. As with all putrescible waste, waste should be refrigerated, frozen, or otherwise preserved during accumulation, transport, and storage.
Although on-site incineration is the preferred method of managing radioactive animal carcasses and tissue, several alternatives exist. Alkaline digestion of animal carcasses containing 3H, 14C, and formaldehyde, followed by neutralization, results in an aqueous radioactive stream that can usually be disposed of in the sanitary sewer. The process uses 1 M potassium hydroxide at 300 °C and pressures up to 150 psi. Commercial units are available for this process. Radioac-
tive animal carcasses may be accepted at a LLRW site when packed in lime.
Some institutions grind radioactive animal tissue for disposal in the sanitary sewer. USNRC requires that all such sewer-disposable waste be dispersible within the liquid effluent. Preventing contamination and exposure of waste handlers to dust or particles is an important safety measure in this operation.
Autoclaving of infectious animal carcasses is difficult because of the waste’s high heat capacity and poor heat conductivity, and often unproductive because treated waste remains putrescible.
8.C.3.3 Disposal of Radioactive–Biological Contaminated Labware
Radioactive–biological contaminated labware (e.g., gloves and disposable laboratory articles) is generated from biomedical research using radioactive materials with infectious agents, blood, and body fluids. On-site incineration, autoclaving, and off-site disposal are the management options for this waste. Chemical decontamination (e.g., soaking in bleach) may be appropriate if it can be done without risking personal exposure, increasing waste volumes, or creating a waste that is difficult to handle (e.g., wet waste). After disinfection, radioactive–biological waste can be managed as radioactive waste.
Infectious waste and sharps containers that contain radionuclides can be autoclaved safely if the following precautions are satisfied:
• Monitor the air emissions of a test load to determine if the release of radioactive material is in compliance with USNRC license limits.
• Wipe-test the autoclave interior for surface contamination regularly.
• For ongoing treatment of this waste, dedicate an autoclave or autoclave room for this purpose. The room should have ample ventilation.
• Restrict access during autoclaving.
• Test the autoclave efficacy regularly using biological and chemical indicators.
Radioactive needles contaminated with infectious agents or blood should be autoclaved as described above, and then incinerated on-site or shipped to a LLRW site. To prevent injuries, it is important that hypodermic needles and other sharps be kept in waste containers that are puncture-resistant, leakproof, and closable from the point of discard through ultimate disposal. To prevent generation of radioactive aerosols, destruction of needles by grinding or a similar means is not recommended.
8.C.3.4 Sewer Disposal of Radioactive–Biological Liquids
Radioactive blood, body fluids, and other sewer-compatible liquids may be disposed of in the sanitary sewer if quantities are within USNRC license and treatment work limits. Precautions must be taken to prevent exposure of waste handlers. OSHA recommends that disposal of human blood and body fluids be done in a dedicated sink.
8.C.4 Chemical–Radioactive–Biological Waste
Chemical–radioactive–biological laboratory waste is the most difficult multihazardous waste to manage. The strategies for managing the various other types of multihazardous waste described above are generally applicable to chemical–radioactive–biological waste. For example, toxicological research sometimes generates animal tissue that contains a radioactively labeled toxic chemical. However, the chemical toxicity of such waste is commonly inconsequential, both legally and in relation to the waste’s other characteristics. It could be appropriate to dispose of such animal tissue as a radioactive–biological waste, without regard to its low toxic chemical content.
Reduction or elimination of one of the waste hazards through waste management methods is often an efficient first step. Decay-in-storage is a simple, low-cost way to reduce the radioactivity hazard of a waste with short-lived radionuclides. After decay, most USNRC licenses allow the waste to be managed as a chemical–biological waste. Similarly, the use of a biological decontaminate can reduce a chemical–radioactive– biological waste to a chemical–radioactive waste. Autoclaves are readily available to most laboratories for destruction of infectious agents. Autoclaving or disinfection makes sense when any of the waste’s characteristics (e.g., nutrient value) could support the growth of an infectious agent it contains and thus could increase the waste’s risk.
Certain waste treatments reduce multiple hazards in one step. For example, incineration can destroy oxidizable organic chemicals and infectious agents, waste feed rates can be controlled to meet emission limits for volatile radionuclides, and radioactive ash can be disposed of as a dry radioactive waste. Likewise, some chemical treatment methods (e.g., those using bleach) both oxidize toxic chemicals and disinfect biological hazards. Such treatment could convert a chemical–radioactive–biological waste to a radioactive waste. The ultraviolet peroxidation treatment method may well demonstrate this capability.
8.D PROCEDURES FOR THE LABORATORY-SCALE TREATMENT OF SURPLUS AND WASTE CHEMICALS
As described above in section 8.B.5, there are many good reasons to perform in-laboratory-scale hazard reduction procedures. The pros and cons of many other waste management methods are discussed earlier in this book (see Chapter 5, section 5.B, and Chapter 6, section 6.B). The small-scale treatment, hazard reduction procedures, and deactivation of products (and byproducts) as part of the experiment plan make sense for certain wastes and certain situations at the level of the actual generator, the trained laboratory personnel. Beware that unless there is a significant reduction in risk by such action, there may be little benefit in carrying out a procedure that will simply produce another kind of waste with similar risks and challenges for disposal. Section 8.B.6 describes when federal law allows treatment of hazardous waste without permit. To recap, they are
• In certain states small-scale treatment is allowed within a laboratory, sometimes as part of a permit-by-rule allowance. Be sure to check with your state regulators.
• Treatment in an accumulation container is allowed.
• Elementary neutralization (see 8.D.1, below); the mixing of acidic and alkaline waste to form a salt solution, has long been encouraged as long as safety considerations are addressed. In particular, dilute solutions should be used to avoid rapid heat generation.
• Treatment is allowed as part of an experiment (or the last step) before it becomes a waste. Treatment of experimental byproducts assumes the material has not been declared a waste or handled in a wastelike manner. Such treatment cannot be performed anywhere other than the location where the byproduct was generated.
An explanation of the federal allowance to treat waste in an accumulation container has been published in the Federal Register (1986). For this allowance, the container must be kept closed except when adding or removing waste, and all standard time limits for accumulation and container management apply. Depending on the final disposition of treatment byproducts, federal Land Disposal Restrictions (40 CFR 268) treatability standards may apply.
To ensure compliance, be sure to check local and state regulations that may apply, and seek a legal review if any clarification is needed.
8.D.1 Treatment of Acids and Bases
Neutralization of acids and bases (corrosives) is generally exempt from a RCRA treatment permit. However, because the products of the reaction are often disposed of in the sanitary sewer, it is important to ensure that hazardous waste such as toxic metal ions is not a part of the effluent.
In most laboratories, both waste acids and waste bases are generated, and so it is most economical to collect them separately and then neutralize one with the other. If additional acid or base is required, sulfuric or hydrochloric acid and sodium or magnesium hydroxide, respectively, can be used.
Safety must be carefully considered before beginning any work. If the acid or base is highly concentrated, it is prudent to first dilute it with cold water (adding the acid or base to the water) to a concentration below 10%. Then the acid and base are mixed, and the additional water is slowly added when necessary to cool and dilute the neutralized product. The concentration of neutral salts disposed of in the sanitary sewer should generally be below 1%.
8.D.2 Treatment of Other Chemicals
The procedures listed below are for general use at the laboratory scale. Additional procedures can be found in the earlier editions of this book1 and other books listed on the accompanying CD. See Tables 8.2 and 8.3 for a list of types of chemicals that have known treatment methods. Specific procedures for laboratory treatment are increasingly being included in the experimental sections of chemical journals and in publications such as Organic Syntheses and Inorganic Syntheses.
Safety must be the first consideration before undertaking any of the procedures suggested. Procedures presented in this book are intended to be carried out only by, or under the direct supervision of, a trained scientist or technologist who understands the chemistry and hazards involved. Appropriate personal protection should be used. (See Chapter 7, section 7.F, for information on protective equipment and Chapter 6 for more information about working with chemicals.) With the exception of neutralization, procedures are generally intended for application only in small quantities, that is, not more than a few hundred grams. Because risks tend to increase exponentially with scale, larger quantities should be treated only in small batches unless a qualified chemist has demonstrated that the procedure can be scaled
_______________
1Prudent Practices for Disposal of Chemicals from Laboratories (NRC, 1983); Prudent Practices in the Laboratory: Handling and Disposal of Chemicals (NRC, 1995).
|
|
|
|
Aldehydes |
Hydroperoxides |
|
|
|
|
Amines |
Peroxides |
|
Anhydrides |
Sulfides |
|
Halides |
Thiols (mercaptans) |
|
|
|
up safely. The generator must ensure that the procedure eliminates the regulated hazard before the products are disposed of as nonhazardous waste. In addition, if the procedure suggests disposal of the product into the sanitary sewer, this strategy must comply with local regulations.
|
|
|
|
Alkali Metals |
Inorganic Peroxides and Hydroperoxides |
|
|
|
|
Bromates |
Iodates |
|
Cations (precipitation to their hydroxides) |
Metal azides |
|
Chemicals in which neither the cation nor the anion presents a significant hazard |
Metal catalysts |
|
Chlorates |
Metal hydrides |
|
Chromates |
Molybdates |
|
Halides and acid halides of nonmetals |
Periodates |
|
Hypochlorites |
Permanganates |
|
Inorganic cyanides |
Persulfates |
|
Inorganic ions |
Water-reactive metal halides |
|
|
|





























