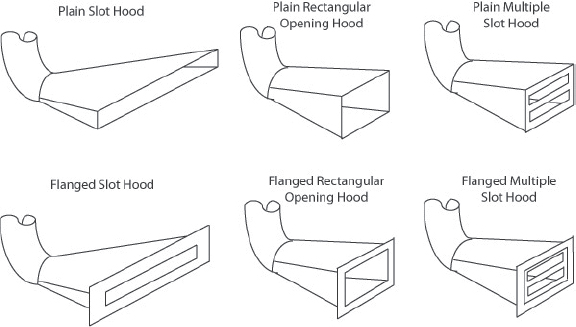
9.B GENERAL LABORATORY DESIGN CONSIDERATIONS
9.B.1 Relationship Between Wet Laboratory Spaces and Other Spaces
9.B.1.1 Relationship Between Laboratory and Office Spaces
9.B.2.1 Considerations for Open Laboratory Design
9.B.3 Closed Laboratories and Access
9.B.4 Equivalent Linear Feet of Workspace
9.B.5 Laboratory Layout and Furnishing
9.B.5.2 Casework, Furnishings, and Fixtures
9.B.5.5 Doors, Windows, and Walls
9.B.6 Noise and Vibration Issues
9.B.7 Safety Equipment and Utilities
9.B.8 Americans with Disability Act: Accessibility Issues Within the Laboratory
9.C.2 Laboratory Chemical Hoods
9.C.2.1 Laboratory Chemical Hood Face Velocity
9.C.2.2 Factors That Affect Laboratory Chemical Hood Performance
9.C.2.3 Prevention of Intentional Release of Hazardous Substances into Chemical Hoods
9.C.2.4 Laboratory Chemical Hood Performance Checks
9.C.2.7 Constant Operation of Laboratory Chemical Hoods
9.C.2.8 Testing and Verification
9.C.2.9 Laboratory Chemical Hood Design and Construction
9.C.2.10 Laboratory Chemical Hood Configurations
9.C.2.11 Laboratory Chemical Hood Exhaust Treatment
9.C.3 Other Local Exhaust Systems
9.C.3.1 Elephant Trunks, Snorkels, or Extractors
9.C.3.5 Clean Benches or Laminar Flow Hoods
9.C.3.8 Flammable-Liquid Storage Cabinets
9.C.4 General Laboratory Ventilation and Environmental Control Systems
9.C.4.1 Constant Air Volume (CAV) Systems
9.C.4.2 Variable Air Volume (VAV) Systems
9.C.6.1 Individual Laboratory Chemical Hood Fans
9.C.6.2 Manifolded (Common Header) Systems
9.C.6.3 Hybrid Exhaust Systems
9.D ROOM PRESSURE CONTROL SYSTEMS
9.E.2.1 Clean Room Classification
9.E.2.3 Laboratory Chemical Hoods and Laboratory Furniture in Clean Rooms
9.E.3 Environmental Rooms and Special Testing Laboratories
9.E.3.1 Alternatives to Environmental Rooms
9.E.4 Biological Safety Cabinets and Biosafety Facilities
9.E.4.2 Using a Biosafety Cabinet for Biological Materials
9.E.5 Nanoparticles and Nanomaterials
9.E.6 Explosion-Proof Chemical Hoods
9.F MAINTENANCE OF VENTILATION SYSTEMS
9.G VENTILATION SYSTEM MANAGEMENT PROGRAM
9.G.3 Inspection and Maintenance
9.G.4 Goals Performance Measurement
9.H.1 Low-Flow or High-Performance Laboratory Chemical Hoods
9.H.3 Variable Air Volume (VAV) Systems with Setback Controls
9.H.4 Variable Air Volume Systems, Diversity Factors
9.H.5 Lower General Ventilation Rates
9.H.6 Laboratory Chemical Hood Alternatives
9.H.8 Components of Heating, Ventilation, and Air-Conditioning (HVAC)
9.H.9 How to Choose a Ventilation System
9.I LABORATORY DECOMMISSIONING
Trained laboratory personnel must understand how chemical laboratory facilities operate. Given the chance, they should provide input to the laboratory designers to ensure that the facilities meet the needs of the functions of the laboratory. Laboratory personnel need to understand the capabilities and limitations of the ventilation systems, environmental controls, laboratory chemical hoods, and other exhaust devices associated with such equipment and how to use them properly. To ensure safety and efficiency, the experimental work should be viewed in the context of the entire laboratory and its facilities.
9.B GENERAL LABORATORY DESIGN CONSIDERATIONS
9.B.1 Relationship Between Wet Laboratory Spaces and Other Spaces
Modern laboratories, particularly in academia, often have contiguous spaces that include wet laboratories, computer laboratories, instruments, write-up spaces, office areas, and other spaces with varying degrees of chemical use and hazards. Maintaining a positive safety culture and at the same time meeting the safety and comfort needs of laboratory personnel are challenging under these circumstances.
• Wherever possible, separate wet chemical areas or those with a higher degree of hazard from other areas with a physical barrier, such as a wall, divider, or control device. The objective is to protect the computer laboratory or otherwise low-hazard area from the risk of the higher hazard, and thus eliminate the need to use protective equipment in the low hazard area.
• When such areas cannot be physically separated, or where the risk cannot be eliminated completely, individuals working at the computer or in the write-up area need to evaluate what level of protection may be needed to control the risk of exposure to the hazards in the other areas. For example, all individuals in a computer laboratory must wear eye protection if there is a risk of eye injury from operations in a contiguous area.
9.B.1.1 Relationship Between Laboratory and Office Spaces
Almost all laboratory personnel require both laboratory and office support space. Their desire to be aware of procedures and to have a constant presence in the laboratory usually demands that office space be located near the laboratory. The need for personnel safety, evolutionary technology allowing for computer-based research and data monitoring outside of the laboratory, as well as a desire to foster better interaction between researchers has driven the offices outside the laboratory proper.
Locating all offices outside the laboratory environment allows for a safer workspace where food can be consumed, quiet work can be done, and more paper and books can be stored. Locating the office zone very close to or adjacent to the laboratory for easy access and communication is desirable.
Some laboratories have office spaces within research areas. In this design, it is best to have an obvious separation between the laboratory area and the office area using partitions or, at a minimum, aisle space, but preferably using a wall and a door that can be closed. Occupants should not have to walk through laboratory areas to exit from their office space. Visitors and students should not have to walk through laboratories to get to researchers’ offices, because those persons do not have personal protective equipment (PPE). (See Vignette 9.1.)
9.B.2 Open Laboratory Design
Traditionally, laboratories were designed for individual research groups with walls separating the laboratories and support spaces. Group sizes ranged from 2 to 10 people, and most groups were completely self-contained, each with its own equipment and facilities (Figure 9.1).
Since the 1990s, the trend has been for researchers to collaborate in a cross-disciplinary nature; chemists, biologists, physicists, engineers, and computer scientists work together on a common goal. At the same time, laboratory designers have moved to open multiple-module laboratories that allow a wide variety of configurations for casework and equipment setups. These laboratories often support large or multiple teams and are configured with relocatable furnishings.
Even when not using a multidiscipline approach, many facilities have moved toward larger, more open laboratories with the belief that working in teams raises overall productivity, promote open communication, and facilitates resource sharing. Team sizes, in some disciplines, have risen and are frequently as high as 12 to 20 individuals.
9.B.2.1 Considerations for Open Laboratory Design
There are advantages and disadvantages to open laboratory design.
VIGNETTE 9.1
Appropriate use of personal protective equipment in shared spaces
In both these incidents, the research laboratories contained writing spaces with computer workstations and desks that were separated from the working part of the laboratory by only an aisle.
In one laboratory, a person was holding a 250-mL glass flask when it overpressurized and burst, spraying shards of glass across the laboratory. Not only did the person holding the flask receive multiple lacerations, but another person not involved in the procedure, sitting 3 m away at a desk, was hit by flying glass and received lacerations that required sutures.
In another laboratory, a container of nitric acid and methanol sitting in a chemical fume hood overpressurized and burst, spraying shards of glass and nitric acid over every surface of the laboratory. A person sitting 3 m away at a desk received some nitric acid and glass on the laboratory coat, but nowhere else.
In both cases, the potential for eye injuries, chemical burns, and physical injury to a person not involved in the experiment existed. Both incidents illustrate the importance of wearing eye protection and other protective equipment, as appropriate, whenever a risk is present.
Advantages include
• visibility among researchers;
• better communication and collaboration;
• easy to share resources, including equipment, space, and support staff;
• flexibility for future needs because of open floor plan with adaptable furnishings;
• significant space savings compared with smaller, enclosed laboratories; and
• cost savings (first building/renovation costs and ongoing operating costs) compared with smaller, enclosed laboratories.
Disadvantages and limitations include
• for large spaces, challenging to balance the ventilation system;
• limitations to the size or placement of the laboratory (e.g., the floor of the building, the type of research) because of chemical storage code limitations for flammable and other materials;
• need for isolated spaces because of specific types of work being conducted, such as cell or tissue work where cross-contamination is an issue, use of certain radioactive materials, lasers, materials requiring special security measures, glass-washing facilities (see section 9.B.3 for more information);
• challenge of storing chemicals and supplies when there is a lack of natural spaces created by walls and other fixtures;
• noise from people and equipment may be higher than in a closed laboratory; and
• inability of some researchers to work effectively in an open laboratory environment.
Design teams should work with the research teams to find solutions that accommodate the needs of the researchers as much as possible. A combination of open laboratory spaces with smaller areas dedicated to special functions is often necessary.
9.B.3 Closed Laboratories and Access
Closed or separate laboratory spaces are often necessary for certain functions because of the nature of the operation, equipment needs, or security concerns. These areas may or may not be separated with a door. The need for a door and access control should be examined carefully for code requirements, safety protocol, and containment concerns.
The following issues should be considered:
• Do the exits require doors by code?
• Must the corridor walls, doors, and frames be fire-rated by code?
• Is containment of spills or smoke an issue that demands doors?
• Is noise an issue that demands separation and attenuation?
• Does the need for room air pressure control necessitate a door closing the laboratory space off from other areas?
• Does the work present a hazard that requires that access by untrained personnel be controlled?
• Do some materials or equipment present a security risk?
• Do the materials require compliance with bio-safety guidelines?
Examples of operations or activities that may require separation from the main laboratory are in Table 9.1.
The use of unusually hazardous materials may re-
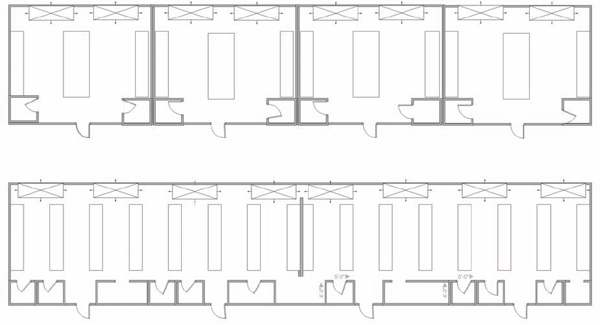
FIGURE 9.1 Open versus closed laboratory design. The top figure is an example of a typical closed laboratory design with four separate laboratories. The three walls separate the space and extend from floor to ceiling, with no shared spaces. The bottom figure is an example of an open laboratory in the same space. The wall extends from floor to ceiling but not from wall to wall (although in some designs, it could). Smaller working rooms with permanent or movable walls are set up for storage or activities that require closed spaces.
quire a dedicated area for such work to most efficiently manage security, safety, and environmental risk.
9.B.4 Equivalent Linear Feet of Workspace
When designing new laboratory spaces, consider the equivalent linear feet (ELF) of work surface within the laboratory. ELF can be divided into two categories: bench and equipment. Bench ELF is the required length of benchtop on which instruments can be set and where preparatory work takes place, as well as the length of laboratory chemical hoods. Equipment ELF includes the length of floor space for equipment that does not fit on a bench. Typically, every two laboratory personnel whose work mostly involves hazardous chemicals should have at least one chemical hood, and these should be large enough to provide each person with a minimum of 3 linear ft, but it could be 8 ft or more depending on the planned activities and type of chemistry.
|
|
|
|
Autoclaves |
Animal Handling Areas |
|
|
|
|
Darkrooms |
Electron microscopes |
|
Glasswashing facilities |
High-powered lasers |
|
Some radioactive materials |
Tissue culture work |
|
Exceptionally toxic materials |
High-pressure equipment |
|
|
|
Typical chemistry laboratories are designed to provide from 28 to 30 ELF per person. Quality control, biology, and analytical laboratories range from 20 to 28 ELF per person. Quality control and production laboratories tend toward the low end of this range, whereas research laboratories are at or above the high end of the range. This number includes the support space outside the laboratory that is needed. These values can vary widely and must be addressed carefully for each project.
9.B.5 Laboratory Layout and Furnishing
9.B.5.1 Adaptability
The frequency of change in laboratory use has made it desirable to provide furnishings and services that can be moved and adapted quickly. Although some services and surfaces will be fixed elements in any laboratory, such as sinks and chemical hoods, there are several options available to meet the adaptable needs for various types of research.
Current design practice is to locate fixed elements
such as laboratory chemical hoods and sinks at the perimeter of the laboratory, ensuring maximum mobility of interior equipment and furniture. Although fixed casework is common at the perimeters, moveable pieces are at the center to maximize flexibility. The central parts of the laboratory are configured with sturdy mobile carts, adjustable tables, and equipment racks.
Another trend for new laboratory buildings is to design interstitial spaces between the floors and to have all the utilities above the ceiling. The interstitial spaces are large enough to allow maintenance workers to access these utilities from above the ceiling for both routine servicing and to move plumbing and other utilities as research demands change.
Where interstitial spaces are not possible, overhead service carriers may be hung from the underside of the structural floor system. These service carriers may have quick connects to various utilities, such as local exhaust ventilation, computer cables, light fixtures, and electrical outlets.
9.B.5.2 Casework, Furnishings, and Fixtures
Casework should be durable and designed and constructed in a way that provides for long-term use, reuse, and relocation. Some materials may not hold up well to intensive chemistry or laboratory reconfiguration. Materials should be easy to clean and repair. For clean rooms, polypropylene or stainless steel may be preferable.
Work surfaces should be chemical resistant, smooth, and easy to clean. Benchwork areas should have knee space to allow for chairs near fixed instruments or for procedures requiring prolonged operation.
Work areas, including computers, should incorporate ergonomic features, such as adjustability, task lighting, and convenient equipment layout. Allow adequate space for ventilation and cooling of computers and other electronics.
Handwashing sinks for particularly hazardous materials may require elbow, foot, or electronic controls. Do not install more cupsinks than are needed. Unused sinks may develop dry traps that result in odor complaints.
9.B.5.3 Shared Spaces
Many facilities encourage sharing of some pieces of equipment. Locating the equipment in a space that is not defined as part of an individual’s work zone facilitates sharing. Some examples of equipment that can be shared are in Table 9.2.
In an open laboratory setting, duplication of much of this equipment can be avoided. Often, if the equipment is centrally located near a laboratory, it can be walled off to reduce noise.
The team needs to carefully address the need for alarms on specific pieces of equipment such as freezers and incubators that contain valuable samples.
Care must be taken, however, not to assume that sharing is always effective. There are certain pieces of equipment that must be dedicated to specific users.
9.B.5.4 Flooring
Wet laboratories should have chemically resistant covered flooring. Sheet goods are usually preferable to floor tiles, because floor tiles may loosen or degrade over time, particularly near laboratory chemical hoods and sinks. Rubberized materials or flooring with a small amount of grit may be more slip-resistant, which is desirable in chemical laboratories. Coved flooring that allows 4 to 8 in. of flooring material secured to the wall to form a wall base is also desirable.
Floors above areas with sensitive equipment, such as lasers, should be sealed to prevent leaks.
9.B.5.5 Doors, Windows, and Walls
Walls should be finished with material that is easy to clean and maintain. Fire code may require certain doors, frames, and walls to be fire-rated.
Doors should have view panels to prevent accidents caused by opening the door into a person on the other side and to allow individuals to see into the laboratory in case of an accident or injury. Doors should open in the direction of egress.
Laboratories should not have operable windows, particularly if there are chemical hoods or other local ventilation systems in the lab.
9.B.6 Noise and Vibration Issues
Many laboratories utilize equipment that may emit significant noise, require a stable structural environment, or both. During early planning stages, all equip-
ment should be discussed regarding any unique noise or vibration sensitivity in order to locate the equipment properly.
TABLE 9.2 Examples of Equipment That Can Be Shared Between Researchers and Research Groups
|
|
|
|
Balances |
Centrifuges |
|
|
|
|
Gas chromatographs |
High-performance liquid chromatographs |
|
Ice machines |
Incubators |
|
Mass spectrometers |
NMRs |
|
Ovens |
pH meters |
|
Refrigerators/freezers |
Weigh enclosures |
|
|
|
Large equipment such as centrifuges, shakers, and water baths often work best in separate equipment rooms. Pumps for older mass spectrometer units are both hot and noisy and are often located in either a small room or a hall. If in a closet, the area must have extra exhaust to remove heat, or else equipment may fail from overheating. With smaller and newer mass spectrometers, the pumps are often small and can fit into cabinets specifically designed for them. These pumps work especially well when water cooling is not required. Very few researchers need to hear their instrumentation running, but many want to see the equipment.
Another consideration crucial to equipment-intensive areas is the allowable vibration tolerance. Most analytical equipment such as NMRs, sensitive microscopes, mass spectrometers, and equipment utilizing light amplification (laser) require either vibration isolation tables or an area that is structurally designed to allow for very little vibration. Clarify the tolerance requirements with the user and equipment manufacturer during the equipment-programming phase, or early design process, so that the appropriate structure can be designed and the construction cost can be estimated more accurately.
9.B.7 Safety Equipment and Utilities
Each laboratory should have an adequate number and placement of safety showers, eyewash units, and fire extinguishers for its operations. (See Chapter 6, section 6.C.10, for more information.) The American National Standards Institute (ANSI) Z358.1-2004 standard provides guidance for safety shower and eyewash installation. The 2004 version recommends provision of tepid water, which can be complicated from an engineering standpoint. Although this standard does not address wastewater, most designers agree that emergency eyewash and shower units should be connected to drain piping. It is prudent to have floor drains near the units, preferably sloped to the drain to prevent excessive flooding and potential slip hazards. Consider choosing barrier-free safety showers and eyewash units that can accommodate individuals with disabilities. The maximum reach height for the activation control for safety showers is 48 in.
Sprinkler systems may be required by the building code and are almost always recommended. For areas with water-sensitive equipment or materials, consider preaction systems. Most dry or alternative systems do not function in a laboratory environment with chemical hoods and other ventilation. There may be resistance to the idea of installing sprinkler systems in laboratories, particularly laboratories that use water-sensitive chemicals or equipment. The following facts may be helpful:
• Each sprinkler head is individually and directly activated by the heat of the fire, not by smoke or an alarm system. Thus, small fires are not likely to activate the sprinkler and moderate-size fires will likely activate only one or two heads. Indeed, more than 95% of fires are extinguished by one or two sprinkler heads.
• Statistics show that the sprinkler head failure rate is 1 in 16 million.
• In the event that the water from the sprinkler system reacts with water-sensitive materials, ensuing fires would be quenched once the reaction stopped. Damage is likely to be less severe than if a fire was not suppressed and was allowed to reach other flammable or combustible materials in the laboratory.
• Laboratory equipment, including lasers, is just as likely to be harmed by the fire as by the water. Without the sprinkler system, a fire that is large enough to activate the sprinkler system would result in response by the fire department. The sprinkler heads are designed to release water at a rate of 10–15 gallons per minute (gpm), whereas a firefighter’s hose delivers 250–500 gpm.
• Dry chemical systems can seriously damage electronic and other laboratory equipment and are impractical in a building-wide system. Alternative agents are impractical because of the amount of space required for the cylinders and are most effective in rooms or areas that are sealed, which is not how laboratories are designed. These systems are most practical for an individual application, such as a piece of equipment or a “sealed” room.
• Locate utility shutoff switches outside or at the exit of the laboratory. The purpose of the switch is to shut down potentially hazardous operations quickly in the event of an emergency.
• Locate room purge buttons at the exits in laboratories with chemical hoods. For most laboratory buildings, activating the room purge button shuts down or minimizes supply air while increasing exhaust ventilation. In the event of a chemical spill, activating the purge system will help ventilate the resulting chemical vapors more quickly.
• Laboratories should have abundant electrical supply outlets to eliminate the need for extension cords and multiplug adapters. Place electrical panels in an accessible area not likely to be ob-
structed. Install ground-fault circuit interrupters near sinks and wet areas.
• Assess and provide for emergency power needs.
• Where possible, install chilled water loops for equipment requiring cooling. Chilled water loops save energy, water, and sewer costs.
9.B.8 Americans with Disability Act: Accessibility Issues Within the Laboratory
Title 1 of the Americans with Disabilities Act (ADA) of 1990 requires an employer to provide reasonable accommodation for qualified individuals with disabilities who are employees or applicants for employment, unless doing so would cause undue hardship. The design team and the owner are responsible for identifying what reasonable accommodations should and can be made to meet ADA guidelines or requirements.
In addition, some school systems and municipalities require a minimum number or percentage of accessible work areas in teaching laboratories. Accessible furniture, including laboratory chemical hoods, are readily available from most suppliers. The American Chemical Society has an excellent resource available online or in print, Teaching Chemistry to Students with Disabilities: A Manual for High Schools, Colleges, and Graduate Programs (ACS, 2001).
It is prudent to provide barrier-free safety showers and eyewash units for all laboratories. Figure 9.2 illustrates the specifications for barrier-free emergency equipment, according to ANSI 117.1-1992, “Accessible and Usable Building Facilities.”
Additional accommodations will likely need to be made individually, depending on the special needs of the researcher. Partnering with the researcher, supervisor, and a laboratory safety professional will help determine the extent of the accommodations.
For wet laboratories, service animals should either have a place outside the lab or an area within the laboratory that is accessible without the animal having to traverse areas where chemicals or other hazardous materials could be present at floor level, including spills.
9.B.9 Older Facilities
Aging facilities can present multiple challenges. As materials of construction begin to degrade, the safety and environmental provisions of the facility often degrade as well. Although some equipment and materials may continue to function well for many years, modern alternatives may offer better safety and environmental sustainability features.
For older facilities, it is important to have a strong operations and maintenance program that monitors and maintains plumbing, ventilation, and structural components. Nonetheless, as individual laboratories or spaces are renovated for new uses or upgrades, there are opportunities for improving and modernizing building systems.
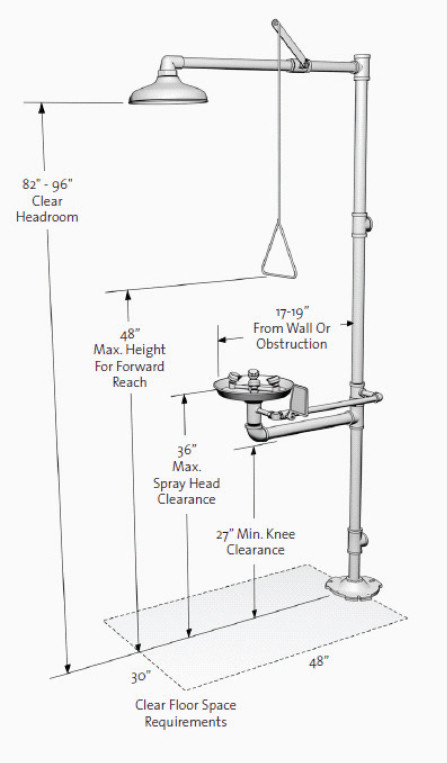
FIGURE 9.2 Specifications for barrier-free safety showers and eyewash units.
Depending on the location of the laboratory building, there may be requirements for bringing the entire building up to current building codes and standards once a certain percentage of the building is under renovation. These code requirements may include fire protection systems, accessibility, plumbing, ventilation, alarm systems, chemical storage restrictions, and egress issues.
With rising interest in energy conservation, there have been numerous studies and instances of retrocommissioning of laboratories. The focus is generally
on the laboratory ventilation system, with the goal of managing airflow and temperature control to eliminate waste and reduce overall energy use. In “Laboratories for the 21st Century” the U.S. Environmental Protection Agency (EPA/DOE, 2006), reports that in most studied cases, retro-commissioning, when planned and executed well, resulted in reductions of at least 30% of overall facility energy use with a payback period of less than 3 years.
The typical retro-commissioning process proceeds in five major steps:
1. Planning. Bring facility and EHS staff, design engineers, and users together to discuss goals. Gather information about the current system, including the original plans, as-built plans, major alterations, and current function, including ventilation rates. Develop the retro-commissioning plan.
2. Preinvestigation. Verify all systems including the direct digital control or building automation systems, evaluate all components that affect energy use, and verify monitoring systems.
3. Investigation. Benchmark utility and energy use data, analyze trends, and test all equipment. Testing should include functional testing of chemical hoods and related components, including face velocity tests, containment tests, etc.
4. Implementation. Select which improvements will be made and prioritize them. Implement the improvements and test performance.
5. Handoff. Clearly document information and provide training to laboratory personnel and maintenance personnel.
Common conditions that lead to energy waste include
• overabundance of laboratory chemical hoods,
• laboratory chemical hoods with large bypass openings,
• dampers in fixed positions,
• overventilated laboratory spaces,
• excessive duct pressure,
• fans set to override position,
• fans that are no longer operating efficiently,
• constant volume systems with no setback for temperature or airflow when unoccupied, and
• high face velocities.
Whether retro-commissioning for energy efficiency or for safety, ensure that all stakeholders are involved in the process. Once the work is complete, continue to monitor efficiency and safety. It is important to include trained laboratory personnel in the feedback process. If systems are not used correctly or if they are bypassed, the retro-commissioning efficiency may deteriorate.
The laboratory ventilation system, whether it is the general ventilation, a chemical hood, or a specialized exhaust system, is a critical means to control airborne chemicals in the laboratory.
At a minimum, a well-designed laboratory ventilation system should include the following:
• Heating and cooling should be adequate for the comfort of laboratory occupants and operation of laboratory equipment.
• A differential should exist between the amount of air exhausted from the laboratory and the amount supplied to the laboratory to maintain a negative pressure between the laboratory and adjacent nonlaboratory spaces. This pressure differential prevents uncontrolled chemical vapors from leaving the laboratory. Clean rooms may require a slightly positive pressure differential. There should be separation between common spaces and the clean room to prevent migration of airborne contaminants.
• Exhaust ventilation devices should be appropriate to materials and operations in the laboratory.
Many devices are used to control emissions of hazardous materials in the laboratory. A risk assessment helps to determine the best choice for a particular operation or material (Table 9.3).
NOTE: Clean benches are not designed for use with hazardous materials. These are appropriate for use in work with materials that necessitate clean work conditions and should only be used for materials or chemicals that one could safety use on a benchtop.
9.C.1 Risk Assessment
For all materials, the objective is to keep airborne concentrations below established exposure limits (see Chapter 4, section 4.C.2.1). Where there is no established exposure limit, where mixtures are present, or where reactions may result in products that are not completely characterized, prudent practice keeps exposures ALARA (as low as reasonably achievable).
For chemicals, determine whether the material is flammable or reactive or if it poses a health hazard from inhalation. If no significant risk exists, the work does not likely require any special ventilation. If potential risk does exist, look at the physical properties of the chemical, specifically its vapor pressure and vapor density.
TABLE 9.3 Laboratory Engineering Controls for Personal Protection
|
|
||
|
Type of Ventilation |
Typical Number of Air Changes or Face Velocity in Linear Feet per Minute (fpm) as Appropriate |
Examples of Use |
|
|
||
|
General laboratory ventilation |
6–12 air changes/hour, depending on laboratory design and system operation |
• Nonvolatile chemicals • Nonhazardous materials |
|
Environmental rooms |
0 air changes |
• Materials that require special environmental controls • Nonhazardous amounts of flammable, toxic, or reactive chemicals. |
|
Laboratory chemical hoods |
10–15 air changes/minute or 60-100 fpm depending on hood type |
• Flammable, toxic, or reactive materials • Products or mixtures with uncharacterized hazards |
|
Unventilated storage cabinets |
0 air changes |
• Flammable liquids • Corrosives • Moderately toxic chemicals |
|
Ventilated storage cabinets |
1–2 air changes/minute |
• Highly toxic, hazardous, or odiferous chemicals (if equipped with flame arrestors) |
|
Recirculating biosafety cabinets |
A1: 75 fpm A2: 100 fpm B1: 100 fpm |
• Biological materials • Nanoparticles, as of the date of publication • Biological materials • Nanoparticles, as of the date of publication • Minute amounts of volatile chemicals |
|
Total exhaust biosafety cabinet |
B2: 100 fpm |
• Biological materials • Nanoparticles, as of the date of publication • Minute amounts of volatile chemicals |
|
Glovebox |
Varies from no change to very high rate of change, depending on the glovebox and the application |
• Positive pressure for specialty environments • Negative pressure for highly toxic materials |
|
Downdraft table |
150–250 fpm depending on design |
• Perfusions with paraformaldehyde, work with volatile, low to moderately hazardous materials with higher vapor density where access from more than one side is necessary |
|
Elephant trunk |
150–200 fpm at opening |
• Local ventilation of a tabletop • Discharge from equipment such as a gas chromatograph |
|
Canopy |
N/A |
• Ventilation of heat, steam, low or nontoxic materials with low vapor density |
|
Ductless laboratory chemical hood |
10–15 air changes/minute |
• Materials that are compatible with the filtration system, in controlled quantities and under controlled conditions • Not suitable for particularly hazardous substances |
|
Slot hood |
Varies with application |
• Local ventilation of higher density materials at the source, such as an acid bath |
|
Ventilated balance enclosure |
5–10 air changes/minute |
• Weighing and initial dissolution of highly toxic or potent materials |
|
Benchtop ventilated enclosures |
Variable per the needs of the materials |
• Benchtop equipment, such as rotovaps |
|
|
||
Vapor pressure is usually measured in millimeters of mercury. A low vapor pressure (<10 mmHg) indicates that the chemical does not readily form vapors at room temperature. General laboratory ventilation or an alternative such as the elephant trunk or snorkel may be appropriate, unless the material is heated or in a higher temperature room that might promote vapor formation. High vapor pressure indicates that the material easily forms vapors and may require use of a ventilated enclosure, such as a chemical hood.
Vapor density is compared to that of air, which is 1. A chemical having a vapor density greater than 1 is heavier than air. If the vapors need to be controlled, a chemical hood or a ventilation device that draws air from below, such as a downdraft table or a slot hood or elephant trunk with the exhaust aimed low may be
appropriate. Conversely, a chemical with a vapor density less than 1 is lighter than air. Besides a chemical hood, a ventilation device that draws air from above, such as an elephant trunk or snorkel with the exhaust positioned above the source, may work best.
For radioactive or biological materials, consider whether the operations might cause the materials to aerosolize or become airborne and whether inhalation poses a risk to health or the environment. Determine whether filtration or trapping is required or recommended.
For manipulating solid particulates, a chemical hood and similar equipment with higher airflow may be too turbulent. Weighing boxes or ventilated balance enclosures may be a better fit for such work.
For nanomaterials, a laboratory chemical hood might be too turbulent for manipulating the materials. Also, consider whether the exhaust containing these tiny particles should be filtered. Studies have shown that high-efficiency particulate air (HEPA) filters are very effective for nanosize particles. Containment tests for chemical hoods allow for a very minor amount of leakage into the breathing zone of the user. For chemical vapors, such an amount may be insignificant, but in the same volume of nanoparticles, the number of particles may be quite large, and biosafety cabinets, gloveboxes or filtering hoods would be better. (See section 9.E.5 for more information.)
More specialized ventilation systems, such as biosafety cabinets and gloveboxes, may be necessary to control specific types of hazards, as discussed later in this chapter.
9.C.2 Laboratory Chemical Hoods
Laboratory chemical hoods are the most important components used to protect laboratory personnel from exposure to hazardous chemicals and agents. Functionally, a standard chemical hood is a fire-and chemical-resistant enclosure with one opening (face) in the front with a movable window (sash) to allow user access to the interior. Large volumes of air are drawn through the face and out the top into an exhaust duct to contain and remove contaminants from the laboratory. Note that because a substantial amount of energy is required to supply tempered supply air to even a small hood, the use of hoods to store bottles of toxic or corrosive chemicals is a very wasteful practice, which can seriously impair the effectiveness of the hood as a local ventilation device. Thus, it is preferable to provide separate vented cabinets for the storage of toxic or corrosive chemicals. The amount of air exhausted by such cabinets is much less than that exhausted by a properly operating hood.
A well-designed hood, when properly installed and maintained, offers a substantial degree of protection to the user if it is used appropriately and its limitations are understood. Chemical hoods are the best choice, particularly when mixtures or uncharacterized products are present and any time there is a need to manage chemicals using the ALARA principle.
9.C.2.1 Laboratory Chemical Hood Face Velocity
The average velocity of air drawn through the face of the laboratory chemical hood is called the face velocity. The face velocity greatly influences the ability to contain hazardous substances, that is, its containment efficiency. Face velocities that are too low or too high reduce the containment efficiency.
Face velocity is only one indicator of hood performance and one should not rely on it as a sole basis for determining the containment ability of the chemical hood. There are no regulations that specify acceptable face velocity. Indeed, modern hood designs incorporate interior configurations that affect the airflow patterns and are effective at different ranges of face velocity.
For traditional chemical hoods, several professional organizations have recommended that the chemical hood maintain a face velocity between 80 and 100 feet per minute (fpm). Face velocities between 100 and 120 fpm have been recommended in the past for substances of very high toxicity or where outside influences adversely affect hood performance. However, energy costs to operate the chemical hood are directly proportional to the face velocity and there is no consistent evidence that the higher face velocity results in better containment. Face velocities approaching or exceeding 150 fpm should not be used; they may cause turbulence around the periphery of the sash opening and actually reduce the capture efficiency, and may reentrain settled particles into the air.
With the desire for more sustainable laboratory ventilation design, manufacturers are producing high-performance hoods, also known as low-flow hoods, that achieve the same level of containment as traditional ones, but at a lower face velocity. These chemical hoods are designed to operate at 60 or 80 fpm and in some cases even lower. (See section 9.C.2.9.3.6.)
Average face velocity is determined by measuring individual points across the plane of the sash opening and calculating their average. A more robust measure of containment uses tracer gases to provide quantitative data and smoke testing to visualize airflow patterns. ASHRAE/ANSI 110 testing is an example of this technique (see section 9.C.2.8 for more information). This type of testing should be conducted at the time the chemical hood is installed, when substantial changes are made to the ventilation system, including rebalanc-
ing and periodically as part of a recommissioning or maintenance program.
Once a chemical hood is tested and determined to be acceptable via the ASHRAE/ANSI 110 method or an equivalent means, the face velocity should be noted and used as the reference point for routine testing. Each chemical hood, laboratory, facility, or site must define the acceptable average face velocity, minimum acceptable point velocity, and maximum standard deviation of velocities, as well as when ASHRAE/ANSI 110 or visualization testing is required. These requirements should be incorporated into the laboratory’s Chemical Hygiene Plan and ventilation system management plans (see section 9.H).
When first installed and balanced, a laboratory chemical hood must be subjected to the ASHRAE/ANSI 110 or equivalent test before it is commissioned. When multiple similar chemical hoods are installed at the same time, at least half should be tested, provided the design is standardized relative to location of doors and traffic, and to location and type of air supply diffusers.
9.C.2.2 Factors That Affect Laboratory Chemical Hood Performance
Tracer gas containment testing of chemical hoods reveals that air currents impinging on the face at a velocity exceeding 30 to 50% of the face velocity reduce the containment efficiency by causing turbulence and interfering with the laminar flow of the air entering the chemical hood. Thirty to fifty percent of a face velocity of 100 fpm, for example, is 30 to 50 fpm, which represents a very low velocity that can be produced in many ways. The rate of 20 fpm is considered to be still air because that is the velocity at which most people first begin to sense air movement.
9.C.2.2.1 Proximity to Traffic
Most people walk at approximately 250 fpm (approximately 3 mph [4.8 kph]) and as they walk, vortices exceeding 250 fpm form behind them. If a person walks in front of an open chemical hood, the vortices can overcome the face velocity and pull contaminants into the vortex, and into the laboratory. Therefore, laboratory chemical hoods should not be located on heavily traveled aisles, and those that are should be kept closed when not in use. Foot traffic near these chemical hoods should be avoided when work is being performed.
9.C.2.2.2 Proximity to Supply Air Diffusers
Air is supplied continuously to laboratories to replace the air exhausted through laboratory chemical hoods and other exhaust sources and to provide ventilation and temperature/humidity control. This air usually enters the laboratory through devices called supply air diffusers located in the ceiling. Velocities that exceed 800 fpm are frequently encountered at the face of these diffusers. If air currents from these diffusers reach the face of a chemical hood before they decay to 30 to 50% of the face velocity, they cause the same effect as air currents produced by a person walking in front of the chemical hood. Normally, the effect is not as pronounced as the traffic effect, but it occurs constantly, whereas the traffic effect is transient. Relocating the diffuser, replacing it with another type, or rebalancing the diffuser air volumes in the laboratory can alleviate this problem.
9.C.2.2.3 Proximity to Windows and Doors
Exterior windows with movable sashes are not recommended in laboratories. Wind blowing through the windows and high-velocity vortices caused when doors open can strip contaminants out of the chemical hoods and interfere with laboratory static pressure controls. Place hoods away from doors and heavy traffic aisles to reduce the chance of turbulence reducing the effectiveness of the hood.
9.C.2.3 Prevention of Intentional Release of Hazardous Substances into Chemical Hoods
Laboratory chemical hoods should be regarded as safety devices that can contain and exhaust toxic, offensive, or flammable materials that form as a result of laboratory procedures. Just as you should never flush laboratory waste down a drain, never intentionally send waste up the chemical hood. Do not use the chemical hood as a means of treating or disposing of chemical waste, including intentionally emptying hazardous gases from compressed gas cylinders or allowing waste solvent to evaporate.
For some operations, condensers, traps, and/or scrubbers are recommended or necessary to contain and collect vapors or dusts to prevent the release of harmful concentrations of hazardous materials from the chemical hood exhaust.
9.C.2.4 Laboratory Chemical Hood Performance Checks
When checking if laboratory chemical hoods are performing properly, observe the following guidelines:
• Evaluate each hood before initial use and on a regular basis (at least once a year) to visualize airflow and to verify that the face velocity meets the criteria specified for it in the laboratory’s Chemical Hygiene Plan or laboratory ventilation plan.
• Verify the absence of excessive turbulence (see section 9.C.2.6, below).
• Make sure that a continuous performance monitoring device is present, and check it every time the chemical hood is used. (For further information, see section 9.C.2.8 on testing and verification.)
Box 9.1 provides a list of things to do to maximize chemical hood efficiency.
9.C.2.5 Housekeeping
Keep laboratory chemical hoods and adjacent work areas clean and free of debris at all times. Keep solid objects and materials (such as paper) from entering the exhaust ducts, because they can lodge in the ducts or fans and adversely affect their operation. The chemical hood will have better airflow across its work surface if it contains a minimal number of bottles, beakers, and laboratory apparatus; therefore, prudent practice keeps unnecessary equipment and glassware outside the chemical hood at all times and stores all chemicals in approved storage cans, containers, or cabinets. Furthermore, keep the workspace neat and clean in all laboratory operations, particularly those involving the use of chemical hoods, so that any procedure or experiment can be undertaken without the possibility of disturbing, or even destroying, what is being done.
9.C.2.6 Sash Operation
Except when adjustments to the apparatus are being made, keep the chemical hood closed, with vertical sashes down and horizontal sashes closed, to help prevent the spread of a fire, spill, or other hazard into the laboratory. Horizontal sliding sashes should not be removed. The face opening should be kept small to improve the overall performance of the hood. If the face velocity becomes excessive, the facility engineers should make adjustments or corrections.
For chemical hoods without face velocity controls (see section 9.C.4.1), the sash should be positioned to produce the recommended face velocity, which often occurs only over a limited range of sash positions. This range should be determined and marked during laboratory chemical hood testing. Do not raise the sash above the working height for which it has been tested to maintain adequate face velocity. Doing so may allow the release of contaminants from the chemical hood into the laboratory environment.
Chemical hood sashes may move vertically (sash moves up and down), horizontally (sash is divided in panes that move side to side to provide the opening to the hood interior), or a combination of both. Although both types of sash offer protection from the materials within the hood and help control or maintain airflow, consider the following:
BOX 9.1
Quick Guide for Maximizing Efficiency of Laboratory Chemical Hoods
Many factors can compromise the efficiency of chemical hood operation, and most are avoidable. Be aware of all behavior that can, in some way, modify the chemical hood and its capabilities. Always consider the following:
• Keep chemical fume hood exhaust fans on at all times.
• If possible, position the chemical hood sash so that work is performed by extending the arms under or around the sash, placing the head in front of the sash, and keeping the sash between the person and the chemical source. View the procedure through the sash, which acts as a primary barrier if a spill, splash, or explosion should occur.
• Avoid opening and closing the sash rapidly, and avoid swift arm and body movements in front of or inside the chemical hood. These actions may increase turbulence and reduce the containment efficiency.
• Place chemical sources and apparatus at least 6 in. behind the face. Paint a colored stripe or apply tape to the work surface 6 in. back from the face to serve as a reminder Quantitative chemical hood containment tests reveal that the concentration of contaminant in the breathing zone can be 300 times higher from a source located at the front of the face than from a source placed at least 6 in. back. This concentration continues to decline as the source is moved farther toward the back.
• Place equipment as far to the back of the chemical hood as practical without blocking the bottom baffle.
• Separate and elevate each instrument by using blocks or racks; air should flow easily around all apparatus.
• Do not use large pieces of equipment in a chemical hood, because they tend to cause dead spaces in the airflow and reduce the efficiency.
• If a large piece of equipment emits fumes or heat outside a chemical hood, have a special-purpose hood designed and installed to ventilate that particular device. This method of ventilation is much more efficient than placing the equipment in a chemical fume hood, and it will consume much less air.
• Do not modify chemical hoods in any way that adversely affects performance. This includes adding, removing, or changing any of the components, such as baffles, sashes, airfoils, liners, and exhaust connections.
• Make sure all highly toxic or offensive vapors are scrubbed or adsorbed before the exit gases are released into the chemical hood exhaust system (see section 9.C.2.11.1 on chemical hood scrubbers).
• Keep the sash closed whenever the chemical hood is not actively in use or is unattended.
• Some experimentation requires the lab personnel to access equipment or materials toward the upper portion of the chemical hood. If the chemical hood is equipped with a vertical sash, it may be necessary to raise the sash completely in order to conduct the procedure.
ο The laboratory chemical hood must provide adequate containment at that sash height. Thus, the chemical hood must be tested in that position.
ο With the sash completely raised, it no longer provides a barrier between the chemical hood user and the materials within the hood.
ο If the only way to keep the sash in a fully raised position requires the use of a sash stop, the laboratory personnel may get into the habit of leaving the sash in this position, potentially reducing the safety and energy efficiency of the chemical hood.
• The standard operating position for the vertical sash may be comfortable for the majority of users. However, shorter laboratory personnel may find that this position does not provide an adequate barrier from the materials within the chemical hood and may need to adjust downward. Taller laboratory personnel may need to raise the sash more in order to comfortably work in the chemical hood.
For chemical hoods with horizontal sashes, the intended operating configuration is to open the panes in such a way that at least one pane is between both arms, providing a barrier between the user and the contents of the chemical hood. In addition,
• Do not remove panes. Permanently removing panes may decrease the safety afforded by the sash barrier and negatively affect containment and waste energy.
• Working with all panes moved to one side or through an opening in the center of the laboratory chemical hood provides no barrier between the user and the materials within the chemical hood. The chemical hood is not intended to be used in this configuration.
Sash panes should be equal width with a maximum of 15 in. (375 mm) to accommodate use of the sash pane as a protective barrier with operator arm on either side.
Conventional glass or plastic sashes are not designed to provide explosion protection per ANSI/NFPA (ANSI, 2004; NFPA, 2004). Sash panes and viewing panes constructed of composite material (safety glass backed by polycarbonate, with the safety glass toward the explosion hazard) are recommended for chemical hoods used when there is the possibility of explosion or violent overpressurization (e.g., hydrogenation, perchloric acid).
For all laboratory chemical hoods, the sash should be kept closed when the hood is not actively attended. Lowering or closing the sash not only provides additional personal protection but also results in significant energy conservation. Some chemical hoods may be equipped with automatic sash-positioning systems with counterweighting or electronic controls (see section 9.H.2).
9.C.2.7 Constant Operation of Laboratory Chemical Hoods
Although turning laboratory chemical hoods off when not in use saves energy, keeping them on at all times is safer, especially if they are connected directly to a single fan. Because most laboratory facilities are under negative pressure, air may be drawn backward through the nonoperating fan, down the duct, and into the laboratory unless an ultralow-leakage backdraft damper is used in the duct. If the air is cold, it may freeze liquids in the hood. The ducts are rarely insulated; therefore, condensation and ice may form in cold weather. When the chemical hood is turned on again and the duct temperature rises, the ice will melt, and water will run down the ductwork, drip into the hood, and possibly react with chemicals in the hood.
Chemical hoods connected to a common exhaust manifold offer the advantage that the main exhaust system is rarely shut down. Hence, positive ventilation is available on the system at all times. In a constant air volume (CAV) system (see section 9.C.4.1), install shut-off dampers to each chemical hood, allowing passage of enough air to prevent fumes from leaking into the laboratory when the sash is closed. Prudent practice allows 10 to 20% of the full volume of flow to be drawn through the laboratory chemical hood in the off position to prevent excessive corrosion.
Some laboratory chemical hoods on variable air volume (VAV) systems (see section 9.C.4.2) have automatic setback controls that adjust the airflow to a lower face velocity when not in use. The setback may be triggered by occupancy sensors, a light switch, or a timer or a completely lowered sash. Understand what triggers the setback and ensure that the chemical hood is not used for hazardous operations when in setback mode.
Some chemical hoods do have on/off switches and may be turned off for energy conservation reasons. They should only be turned off when they are empty of hazardous materials. An example of an acceptable operation would be a teaching laboratory where the
empty chemical hoods are turned off when the laboratory is not in use.
9.C.2.8 Testing and Verification
The OSHA lab standard includes a provision regarding laboratory chemical hoods, including a requirement for some type of continuous monitoring device on each chemical hood to allow the user to verify performance and routine testing of the hood. It does not specify a test protocol.
Laboratory chemical hoods should be tested at least as follows:
• containment test by manufacturer;
• containment test after installation and prior to initial use (commissioning);
• annual or more frequent face velocity and airflow visualization;
• performance test any time a potential problem is reported; and
• containment test after significant changes to the ventilation system, including rebalancing or recommissioning.
9.C.2.8.1 Initial Testing
All laboratory chemical hoods should be tested before they leave the manufacturer according to ANSI/ASHRAE Standard 110-1995 or equivalent, Methods of Testing Performance of Laboratory Fume Hoods (ANSI, 1995). They should pass the low- and high-volume smoke challenges with no leakage or flow reversals and have a control level of 0.05 ppm or less on the tracer gas test. It is highly recommended that chemical hoods be retested by trained personnel after installation in their final location, using ANSI/ASHRAE 110-1995 or equivalent testing. The control level of tracer gas for an “as installed” or “as used” test via the ANSI/ASHRAE 110-1995 method should not exceed 0.1 ppm.
The ANSI/ASHRAE 110-1995 test is the most practical way to determine chemical hood capture efficiency quantitatively. The test includes several components, which may be used together or separately, including face velocity testing, flow visualization, face velocity controller response testing, and tracer gas containment testing. These tests are much more accurate than face velocity and smoke testing alone. Respectively, ASHRAE and ANSI found that 28% or 38% of chemical hoods tested using this method did not meet the pass criteria, even though face velocity testing alone found them to be in an acceptable face velocity range.
Performance should be evaluated against the design Specifications for uniform airflow across the chemical hood face as well as for the total exhaust air volume. Equally important is the evaluation of operator exposure. The first step in the evaluation of hood performance is the use of a smoke tube or similar device to determine that the laboratory chemical hood is on and exhausting air. The second step is to measure the velocity of the airflow at the face of the hood. The third step is to determine the uniformity of air delivery to the hood face by making a series of face velocity measurements taken in a grid pattern.
Leak testing is normally conducted using a mannequin equipped with sensors for the test gas. As an alternative, a person wearing the sensors or collectors may follow a sequence of movements to simulate common activities, such as transferring chemicals. It is most accurate to perform the in-place tests with the chemical hood at least partially loaded with common materials (e.g., chemical containers filled with water, equipment normally used in the chemical hood), in order to be more representative of operating conditions.
For the ASHRAE 110-1995 leak testing, the method calls for a release rate for the test gas of 4 liters per minute (Lpm), but suggests that higher rates may be used. One-liter per minute release rate approximates pouring a volatile solvent from one beaker to another. Eight liters per minute approximates boiling water on a 500-W hot plate. The 4-Lpm rate is an intermediate of these two conditions. If there is a possibility that the chemical hood will be used for volatile materials under heating conditions, consider a higher release rate of up to 8 Lpm for worst-case conditions.
The total volume of air exhausted by a laboratory chemical hood is the sum of the face volume (average face velocity times face area of the hood) plus air leakage, which averages about 5 to 15% of the face volume. If the laboratory chemical hood and the general ventilating system are properly designed, face velocities in the range of the design criteria will provide a laminar flow of air over the work surface and sides of the hood. Higher face velocities (150 fpm or more), which exhaust the general laboratory air at a greater rate, waste energy and are likely to degrade hood performance by creating air turbulence at the face and within the chemical hood, causing vapors to spill out into the laboratory (Figure 9.3).
An additional method for containment testing is the EN 14175, which is the standard adopted by the European Union and replaces several other procedures that were in place for individual countries. Parts 3 (Type tests) and 4 (On-site tests) of this standard address methods for “as manufactured” and “as installed/used” systems, respectively.
9.C.2.8.2 Routine Testing
At least annually, the following test procedures should be conducted for all chemical hoods:
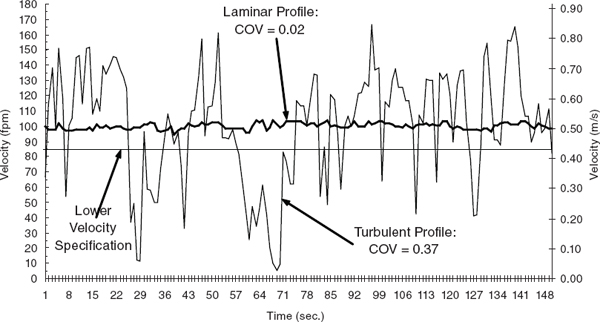
FIGURE 9.3 Laminar versus turbulent velocity profile. Velocity data are from a single traverse point on two separate hoods. The light line represents a hood where supply air interference caused large variations in velocity, a “typical” turbulent profile. Eddy currents and flow reversals caused by a turbulent airflow pattern may cause spillage and leakage of contaminants from the hood into the laboratory environment. In contrast, the bold line represents a hood having an almost ideal velocity profile, indicative of a laminar airflow pattern. The coefficient of variation (COV) is used as a predictor of the level of turbulence experienced at the face of a hood. A high COV indicates a turbulent air profile and most likely is a strong indicator of poor containment; a low COV indicates a laminar flow profile and likely good containment.
SOURCE: Maupins and Hitchings (1998). Reprinted by permission of Taylor & Francis.
• Analyze face velocity using the method and criteria described in section 9.C.2.8.4.
• Visualize airflow using smoke tubes, bombs, or fog generators.
• Verify that continuous flow monitoring devices are working properly.
• Verify that other controls, including automatic sash positioners, alarm systems, etc. are functioning properly.
• Check the sash to ensure that it is in good condition, moves easily, is unobstructed, and has adequate clarity to see inside the laboratory chemical hood.
• Ensure that the laboratory chemical hood is being used as intended (e.g., no evidence of perchloric acid in a chemical hood not designed for it, not using it as a chemical storage device).
• Note any conditions that could affect laboratory chemical hood performance, such as large equipment, excessive storage, etc.
• Take corrective actions where necessary and retest.
Provide information and test results to the chemical hood users and/or supervisors. Document the results in order to maintain a log showing the history of chemical hood performance.
9.C.2.8.3 Additional Testing
Laboratory personnel should request a chemical hood performance evaluation any time there is a change in any aspect of the ventilation system. Thus, changes in the total volume of supply air, changes in the locations of supply air diffusers, or the addition of other auxiliary local ventilation devices (e.g., more chemical hoods, vented cabinets, and snorkels) all call for reevaluation of the performance of all chemical hoods in the laboratory.
9.C.2.8.4 Face Velocity Testing
Visually divide the face opening of a laboratory chemical hood into an imaginary grid, with each grid space being approximately 1 ft2 in area. Using an anemometer, velometer, or similar device, take a measurement at the center of each grid space. Face velocity readings should be integrated for at least 10 seconds (20 is preferable) because of the fluctuations in flow. The measured velocity will likely fluctuate for several seconds; record the reading once it has stabilized. Calculate the average of the velocity for every
grid space. The resulting number is the average face velocity. Analyze the results to determine if any one measurement is 20% or more above or below the average. Such readings indicate the possibility of turbulent or nonlaminar airflow. Smoke tests will help confirm whether this is problematic.
Traditional handheld instruments are subject to probe movement and positioning errors as well as reading errors owing to the optimistic bias of the investigator. Also, the traditional method yields only a snapshot of the velocity data, and no measure of variation over time is possible. To overcome this limitation, take velocity data while using a velocity transducer connected to a data acquisition system and read continuously by a computer for approximately 30 seconds at each traverse point. If the transducer is fixed in place, using a ring stand or similar apparatus, and is properly positioned and oriented, this method overcomes the errors and drawbacks associated with the traditional method. The variation in data for a traverse point can be used as an indicator of turbulence, an important additional performance indicator that has been almost completely overlooked in the past.
If the standard deviation of the average velocity profile at each point exceeds 20% of the mean, or the average standard deviation of velocities at each traverse point (turbulence) exceeds 15% of the mean face velocity, corrections should be made by adjusting the interior baffles and, if necessary, by altering the path of the supply air flowing into the room (see Figure 9.4). Most laboratory chemical hoods are equipped with a baffle that has movable slot openings at both the top and the bottom, which should be moved until the airflow is essentially uniform. Larger chemical hoods may require additional slots in the baffle to achieve uniform airflow across the face. These adjustments should be made by an experienced laboratory ventilation engineer or technician using proper instrumentation.
9.C.2.8.5 Testing Criteria
Prior to the initial tests, determine the acceptance criteria for the ANSI/ASHRAE 110-1995 leak test, face velocity (based on the results of the ANSI/ASHRAE testing and the design of the laboratory chemical hood), and visual airflow tests.
One important factor to consider is acceptable sash position. It is common to set the acceptance criteria as an acceptable level of containment and/or face velocity range at the standard operating position of the sash, often 18 in. However, one must understand how the chemical hood will be used to determine the range of
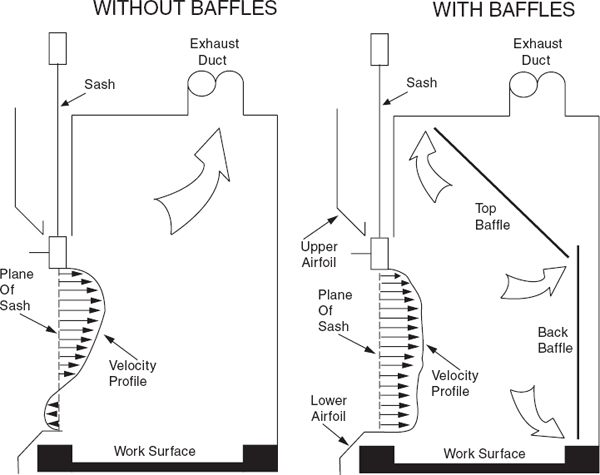
FIGURE 9.4 Effect of baffles on face velocity profile in a laboratory chemical hood.
sash positions needed. For example, if the users will need to sometimes use the hood with vertical sash fully open, then the test criteria should be for 100% sash opening.
It may be prudent to set the acceptance criteria with the sash 100% open and 80% open, ensuring adequate containment at both of these positions.
9.C.2.8.6 Instrumentation
Anemometers and other instruments used to measure face velocity must be accurate in order to supply meaningful data. Instruments should be calibrated at least once a year and the calibration should be National Institute of Standards and Technology traceable.
9.C.2.8.7 Additional Exposure Monitoring
If there is any concern that a laboratory chemical hood or other ventilation device may not provide enough protection to the trained laboratory personnel, it is prudent to measure worker exposure while the hood is being used for its intended purpose. By conducting personal air-sampling using traditional industrial hygiene techniques, worker exposure (both excursion peak and time-weighted average) can be measured. The criterion for evaluating the hood should be the desired performance (i.e., does the hood contain vapors and gases at the desired worker-exposure level?). A sufficient number of measurements should be made to define a statistically significant maximum exposure based on worst-case operating conditions. Direct-reading instruments may be available for determining the short-term concentration excursions that may occur in chemical hood use.
9.C.2.9 Laboratory Chemical Hood Design and Construction
When specifying a laboratory chemical hood for use in a particular activity, laboratory personnel should be aware of the design features. Assistance from an industrial hygienist, ventilation engineer, or laboratory consultant is recommended when deciding to purchase a chemical hood.
9.C.2.9.1 General Design Recommendations
Construct laboratory chemical hoods and the associated exhaust ducts of nonflammable materials. Equip them with vertical, horizontal, or combination vertical/horizontal sashes that can be closed. For the glass within the sash, use either laminated safety glass that is at least 7/32-in. thick or other equally safe material that will not shatter if there is an explosion inside. Locate the utility control valves, electrical receptacles, and other fixtures outside the chemical hood to minimize the need to reach within the chemical hood proper. Other specifications regarding the construction materials, plumbing requirements, and interior design vary, depending on the intended use. (See Chapter 7, sections 7.C.1.1 and 7.C.1.2) Information regarding the minimum flow rate through hoods can be found in ANSI Z9.5.
Although chemical hoods are most commonly used to control concentrations of toxic vapors, they can also serve to dilute and exhaust flammable vapors. Although theoretically possible, it is extremely unlikely (even under worst-case scenarios) that the concentration of flammable vapors will reach the lower explosive limit (LEL) in the exhaust duct. However, somewhere between the source and the exhaust outlet of the chemical hood, the concentration will pass through the upper explosive limit and the LEL before being fully diluted at the outlet. Both the designer and the user should recognize this hazard and eliminate possible sources of ignition within the chemical hood and its ductwork if there is a potential for explosion. The use of duct sprinklers or other suppression methods in laboratory hood ductwork is not necessary or desirable.
9.C.2.9.2 Special Design Features
Since the invention of the chemical hood, two major improvements have been made in the design—airfoils and baffles. Include both features on any new purchases.
Airfoils built into the bottom and sides of the sash opening significantly reduce boundary turbulence and improve capture performance. Fit new chemical hoods with airfoils and retrofit any hoods without airfoils
When air is drawn through a laboratory chemical hood without a baffle (see Figure 9.4), most of the air is drawn through the upper part of the opening, producing an uneven velocity distribution across the face opening. All chemical hoods should have baffles. When baffles are installed, the velocity distribution is greatly improved. Adjustable baffles can improve hood performance and are desirable if the adjustments are made by an experienced industrial hygienist, consultant, or technician.
9.C.2.9.3 Laboratory Chemical Hood Airflow Types
The first chemical hoods were simply boxes that were open on one side and connected to an exhaust duct. Since they were first introduced, many variations on this basic design have been made. Six of the major variants in airflow design are listed below with their characteristics. Conventional laboratory chemical hoods are the most common and include benchtop, distillation, and walk-in hoods of the CAV, CAV bypass, nonbypass, and VAV, with or without airfoils. Auxiliary air hoods and ductless chemical hoods are not considered conventional and are used less often.
Trained laboratory personnel should know what kind they are using and what its advantages and limitations are. In general, the initial cost of a CAV system may be less than VAV, but the life-cycle cost of the VAV will almost always be lower than a CAV system.
9.C.2.9.3.1 Constant Air Volume Laboratory Chemical Hoods
A CAV chemical hood draws a constant exhaust volume regardless of sash position. Because the volume is constant, the face velocity varies inversely with the sash position. The laboratory chemical hood volume should be adjusted to achieve the proper face velocity at the desired working height of the sash, and the chemical hood should be operated at this height. (See section 9.C.4.)
9.C.2.9.3.2 Constant Air Volume Nonbypass Laboratory Chemical Hoods
A nonbypass chemical hood has only one major opening through which the air may pass, that is, the sash opening. The airflow pattern is shown in Figure 9.5. A CAV nonbypass chemical hood has the undesirable characteristic of producing very large face velocities at small sash openings. As the sash is lowered, face velocities may exceed 1,000 fpm near the bottom. Face velocities are limited by the leakage through cracks and under the airfoil and by the increasing pressure drop as the sash is closed.
A common misconception is that the volume of air exhausted by this type of chemical hood decreases when the sash is closed. Although the pressure drop increases slightly as the sash is closed, no appreciable change in volume occurs. All chemical hoods should be closed when not in use, because they provide a primary barrier to the spread of a fire or chemical release.
Many trained laboratory personnel are reluctant to close their CAV nonbypass chemical hoods because of the increase in air velocity and noise that occurs when the sash is lowered. This high-velocity air jet sweeping over the work surface often disturbs gravimetric measurements, causes undesired cooling of heated vessels and glassware, and can blow sample trays, gloves, and paper towels to the back of the laboratory chemical hood, where they may be drawn into the exhaust system. Exercise care to prevent materials from entering the exhaust system where they can lodge in the ductwork, reducing airflow, or can be conveyed through the system and drawn into the exhaust fan and damage the fan or cause sparks.
Because of numerous operational problems with the design of nonbypass hoods, their installation in new
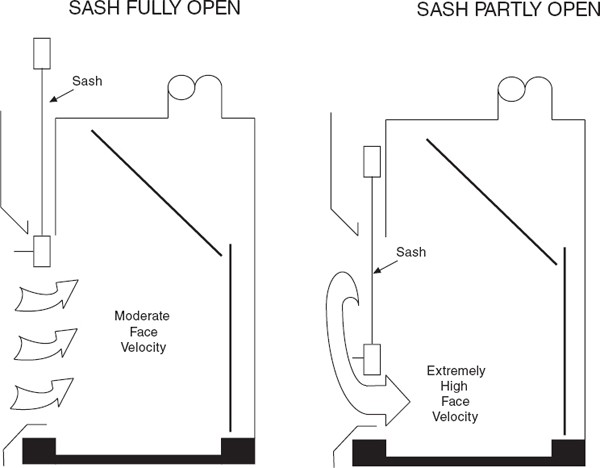
FIGURE 9.5 Effect of sash placement on airflow in a nonbypass laboratory chemical hood.
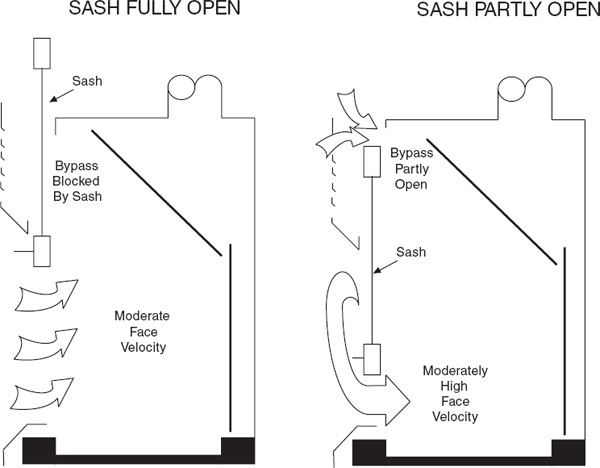
FIGURE 9.6 Effect of sash placement on airflow in a bypass laboratory chemical hood.
facilities is discouraged. If present in existing facilities, their replacement should be considered. In many instances, the cost of replacement can be recouped from the resulting reduction in energy costs.
9.C.2.9.3.3 Bypass Laboratory Chemical Hoods
A bypass chemical hood is shown in Figure 9.6. It is similar to the nonbypass design but has an opening above the sash through which air may pass at low sash positions. Because the opening is usually 20 to 30% of the maximum open area of the sash, this hood will still exhibit the increasing velocity characteristic of the nonbypass chemical hood as the sash is lowered. But the face velocity stops increasing as the sash is lowered to the position where the bypass opening is exposed by the falling sash. The terminal face velocity of these types of hoods depends on the bypass area but is usually in the range of 300 to 500 fpm—significantly higher than the recommended operating face velocity. Therefore, the air volume for bypass laboratory chemical hoods should also be adjusted to achieve the desired face velocity at the desired sash height, and the hood should be operated at this position. This arrangement is usually found in combination with a vertical sash, because this is the simplest arrangement for opening the bypass. Varieties are available for horizontal sashes, but the bypass mechanisms are complicated and may cause maintenance problems. For a well-designed bypass hood, the face velocity will stay relatively constant until open about 12 in., then increases rapidly.
9.C.2.9.3.4 Variable Air Volume Laboratory Chemical Hoods
A VAV chemical hood, also known as a constant velocity hood, is one that has been fitted with a face velocity control, which varies the amount of air exhausted from the chemical hood in response to the sash opening to maintain a constant face velocity. In addition to providing an acceptable face velocity over a relatively large sash opening (compared to a CAV hood), VAV hoods also provide significant energy savings by reducing the flow rate when it is closed. These types of hoods are usually of the nonbypass design to reduce air volume (see below). Even though the face velocity responds to the position of the sash, the face velocity may drop off as the sash height increases, depending on the design. As a result, there is a maximum sash height above which the chemical hood becomes less effective.
9.C.2.9.3.5 Auxiliary Air Laboratory Chemical Hoods
Quantitative tracer gas testing of many auxiliary air laboratory chemical hoods has revealed that, even
when adjusted properly and with the supply air properly conditioned, significantly higher personnel exposure to the materials used may occur than with conventional (non-auxiliary air) chemical hoods. They should not be purchased for new installations, and existing ones should be replaced or modified to eliminate the supply air feature. This feature causes a disturbance of the velocity profile and leakage of fumes into the personnel breathing zone.
The auxiliary air chemical hood was developed in the 1970s primarily to reduce laboratory energy consumption and is a combination of a bypass hood and a supply air diffuser located at the top of the sash. They were intended to introduce unconditioned or tempered air, as much as 70% of the air exhausted, directly to the front of the chemical hood. Ideally, this unconditioned air bypasses the laboratory and significantly reduces air-conditioning and heating costs. In practice, however, many problems are caused by introducing unconditioned or slightly conditioned air above the sash, all of which may produce a loss of containment.
9.C.2.9.3.6 Low-Flow or High-Performance Laboratory Chemical Hoods
With rising energy costs and high interest in sustainable laboratory design, manufacturers are producing low-flow, “high-performance” hoods that are able to meet the performance criteria of the ANSI/ASHRAE 110-1995 tests at a lower face velocity. They tend to be deeper than the traditional laboratory chemical hood and some have altered air front airfoils, internal or external auxiliary air devices, and/or automatic baffle controls. There are other design differences from a traditional chemical hood; thus, it is usually not possible to simply reduce the flow of a traditional hood to a lower face velocity and expect it to meet the same performance criteria as these specially designed hoods.
Like any other chemical hood, the design criteria and limitations need to be fully understood before one is selected for the laboratory. For example, if the chemical hood is designed to meet performance criteria at a sash height of 18 in., but users must operate it at a sash height of 24 in., the hood may not be effective at 24 in., creating a potentially hazardous situation.
Reviews by users have been mixed. For best results, be sure that the engineers, trained laboratory personnel, and the vendors understand how the chemical hoods are intended to be used. Their design and function continue to improve.
9.C.2.9.3.7 Ductless Laboratory Chemical Hoods
Ductless laboratory chemical hoods are ventilated enclosures that have their own fan, which draws air out and through filters and ultimately recirculates it into the laboratory. The filters are designed to trap vapors generated in the chemical hood and exhaust clean air back into the laboratory. They frequently use activated carbon filters, HEPA filters, or a combination of the two. Newer filter materials on the market claim that they capture a larger variety of chemicals.
These ventilated enclosures do not necessarily achieve the same level of capture and containment as a chemical hood. Unlike a conventional laboratory chemical hood, it is not possible to conduct tracer gas studies to measure containment even with the newer technology ductless hoods. Because the collection efficiency of the filters decreases over time, the filters must be monitored and replaced routinely. Depending on the materials and the laboratory environment, chemicals can desorb from the filter and reenter the laboratory over time. They do not control fire hazards and National Fire Protection Association standard 45 states “Ductless chemical fume hoods that pass air from the hood interior through an absorption filter and then discharge the air into the laboratory are only applicable for use with nuisance vapors and dusts that do not present a fire or toxicity hazard” (NFPA, 2004).
Ductless chemical hoods have extremely limited applications and should be used only where the hazard is very low, where the access to the hood and the chemicals used in it are carefully controlled, and under the supervision of a laboratory supervisor who is familiar with its serious limitations. If these limitations cannot be accommodated, do not use this type of device.
The benefits of recirculating chemical hoods are that they are much more energy efficient than a ducted chemical hood and they do not require a ventilation system that relies on a fan on the roof or upper levels. Some urban buildings retrofitted with laboratories on lower floors, buildings with limitations on the ventilation system or laboratories with minor chemical use have successfully used these ductless hoods, under the limited conditions cited above and with rigorous filter maintenance programs. They can also be used for control of particulate material where a chemical hood or even Class 1 or 2 biosafety cabinets provide too much turbulent air (see section 9.E.4.1).
To determine whether recirculating hoods are appropriate, an industrial hygienist or safety professional should conduct a risk assessment that includes
• an analysis of the chemicals that will be used, the hazards they pose, and the materials they generate as byproducts;
• the frequency and duration of use of these chemicals; and
• the nature of the materials that must be controlled compared to the filter media provided with the recirculating hood.
Individuals using recirculating hoods need training on the use and limitations of the recirculating hood. Each ductless chemical hood should have signage explaining the limitations, how to detect whether the filter media are working, and the filter maintenance schedule.
9.C.2.10 Laboratory Chemical Hood Configurations
9.C.2.1 0.1 Benchtop Laboratory Chemical Hoods
As the name implies, a benchtop chemical hood sits on a laboratory bench with the work surface at bench height. It can be of the CAV or VAV variety and can have a bypass or nonbypass design. The sash can be a vertical-rising or a horizontal-sliding type or a combination of the two. Normally, the work surface is dished or has a raised lip around the periphery to contain spills. Sinks in chemical hoods are not recommended because they encourage laboratory personnel to dispose of chemicals in them. If they must be used, to drain cooling water from a condenser, for instance, they should be fitted with a standpipe to prevent chemical spills from entering the drain. The condenser water drain can be run into the standpipe. Spills will be caught in the cupsink by the standpipe for later cleanup and disposal. A lip on the cupsink could be used as an alternative to a standpipe to prevent spills from getting into the sink. A typical benchtop chemical hood is shown in Figure 9.7.
9.C.2.10.2 Distillation (Knee-High or Low-Boy) Chemical Fume Hoods
The distillation hood is similar to the benchtop hood except that the work surface is closer to the floor to allow more vertical space inside for tall apparatuses such as distillation columns. A typical distillation hood is shown in Figure 9.8.
9.C.2.10.3 Walk-In Laboratory Chemical Hoods
A walk-in hood stands on the floor of the laboratory and is used for very tall or large apparatus. The sash can be either horizontal or double- or triple-hung vertical. These hoods are usually of the nonbypass type. The word “walk-in” is a misnomer; one should never actually walk into a chemical hood when it is operating and contains hazardous chemicals. Once past the plane of the sash, the personnel are inside with the chemicals. If the personnel are required to enter the hood during operations where hazardous chemicals are present,
FIGURE 9.7 Diagram of a typical benchtop laboratory chemical hood.
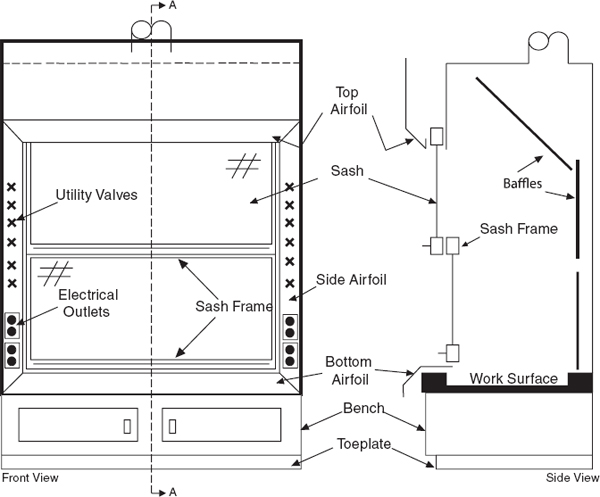
FIGURE 9.8 Diagram of a typical distillation hood.
they should wear PPE appropriate for the hazard. It may include respirators, chemical splash goggles, rubber gloves, boots, suits, and self-contained breathing apparatus. A typical walk-in chemical hood is shown in Figure 9.9.
9.C.2.10.4 California Laboratory Chemical Fume Hoods and Ventilated Enclosures
The California chemical fume hood is a ventilated enclosure with a movable sash on more than one side. They are usually accessed through a horizontal sliding sash from the front and rear. They may also have a sash on the ends. Because their configuration precludes the use of baffles and airfoils, they may not provide a suitable face velocity distribution across their many openings.
A ventilated enclosure is any site-fabricated chemical hood designed primarily for containing processes such as scale-up or pilot plant equipment. Most do not have baffles or airfoils, and most designs have not had the rigorous testing and design refinement that conventional mass-produced chemical hoods enjoy. Working at the opening of the devices, even when the plane of the opening has not been broken, may expose personnel to higher concentrations of hazardous materials than if a conventional hood were used.
9.C.2.10.5 Perchloric Acid Laboratory Chemical Hoods
The perchloric acid laboratory chemical hood, with its associated ductwork, exhaust fan, and support systems, is designed especially for use with perchloric acid and other materials that can deposit shock-sensitive crystalline materials in the hood and exhaust system. These materials become pyrophoric when they dry or dehydrate (see also Chapter 6, section 6.G.6). Special water spray systems are used to wash down all interior surfaces of the hood, duct, fan, and stack, and special drains are necessary to handle the effluent from the washdown. The liner and work surface are usually stainless steel with welded seams. Perchloric acid hoods have drains in their work surface. Water spray heads are usually installed in the top, behind the baffles, and in the interior. The water spray should be turned on whenever perchloric acid is being heated in the chemical fume hood. The ductwork should be fabricated of plastic, glass, or stainless steel and fitted with spray heads approximately every 10 ft on vertical runs and at each change in direction. The fan and stack should be fabricated of plastic, fiberglass, or stainless steel. Welded or flanged and gasketed fittings to provide airtight and watertight connections are recommended. Avoid horizontal runs because they
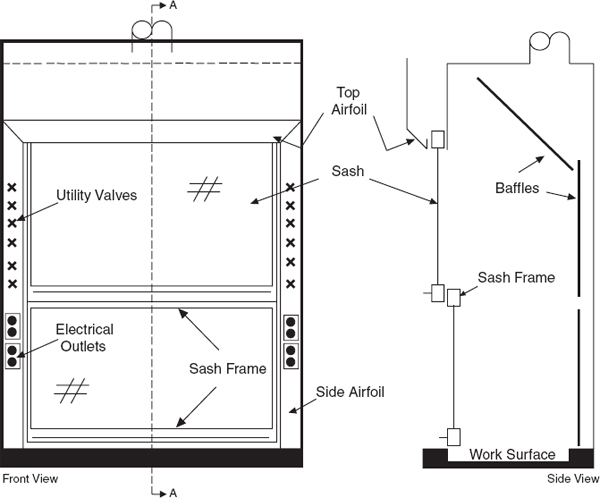
FIGURE 9.9 Diagram of a typical walk-in laboratory chemical hood.
inhibit drainage, and the spray action is not as effective on the top and sides of the duct. Any washdown piping, which is located outside must be protected from freezing. A drain and waste valve on the water supply piping that allows it to drain when not in use is helpful. Route the drain lines carefully to prevent the creation of traps that retain water. Write special operating procedures to cover the washdown procedure for these types of hoods. The exhaust from a perchloric acid hood should not be manifolded with that from other types of chemical hoods.
9.C.2.10.6 Radioisotope Laboratory Chemical Hoods
Design chemical hoods used for work with radioactive sources or materials so that they can be decontaminated completely on a regular basis. A usual feature is a one-piece stainless steel welded liner with smooth curved corners that can be cleaned easily and completely. The superstructure of radioisotope hoods is usually made stronger than that of a conventional hood to support lead bricks and other shielding that may be required. Special treatment of the exhaust from radioisotope hoods may be required by government regulations to prevent the release of radioactive material into the environment. This treatment usually involves the use of HEPA filters (see section 9.C.4.2).
Another practical way to handle radioactive materials that require special exhaust treatment is to use a containment chamber within a traditional chemical hood. Several safety supply companies offer portable disposable glovebag containment chambers with sufficient space to conduct the work and then dispose of them in accordance with applicable nuclear regulatory standards.
9.C.2.10.7 Clean Room Laboratory Chemical Hoods
Chemical hoods in clean rooms are generally no different than traditional chemical hoods, except that they are usually made of polypropylene or thermoplastics. Some have hinged sashes rather than sliding sashes. Most require separate chemical hoods for acid work and solvent work.
Polypropylene hoods burn easily, melt quickly, and may become fully involved in a fre. There are fre-retardant polypropylene and other thermoplastics available, but they cost more. Alternatively, an automatic fire extinguisher may be installed inside.
9.C.2.11 Laboratory Chemical Hood Exhaust Treatment
Until recently, treatment of laboratory chemical hood exhausts has been limited. Because effluent quantities and concentrations are relatively low compared
to those of other industrial air emission sources, their removal is technologically challenging. And the chemistry for a given chemical hood effluent can be difficult to predict and may change over time.
Nevertheless, legislation and regulations increasingly recognize that certain materials in laboratory chemical hoods may be sufficiently hazardous that they can no longer be expelled directly into the air. Therefore, the practice of removing these materials from exhaust streams will become increasingly more prevalent.
9.C.2.1 1.1 Laboratory Chemical Hood Scrubbers and Contaminant Removal Systems
A number of technologies are evolving for treating chemical hood exhaust by means of scrubbers and containment removal systems. Whenever possible, experiments involving toxic materials should be designed so that they are collected in traps or scrubbers rather than released. If for some reason collection is impossible, HEPA filters are recommended for highly toxic particulates. Liquid scrubbers may also be used to remove particulates, vapors, and gases from the exhaust system. None of these methods, however, is completely effective, and all trade an air pollution problem for a solid or liquid waste disposal problem. Incineration may be the ultimate method for destroying combustible compounds in exhaust air, but adequate temperature and dwell time are required to ensure complete combustion.
Incinerators require considerable capital to build and energy to operate; hence, other methods should be studied before resorting to their use. Determine the optimal system for collecting or destroying toxic materials in exhaust air on a case-by-case basis. Treatment of exhaust air should be considered only if it is not practical to pass the gases or vapors through a scrubber or adsorption train before they enter the exhaust air-stream. Also, if an exhaust system treatment device is added to an existing chemical hood, carefully evaluate the impact on the fan and other exhaust system components. These devices require significant additional energy to overcome the pressure drop they add to the system. (See also Chapter 8, section 8.B.6.1)
9.C.2.11.2 Liquid Scrubbers
A laboratory chemical hood scrubber is a laboratory-scale version of a typical packed-bed liquid scrubber used for industrial air pollution control. Figure 9.10 shows a schematic of a typical chemical hood scrubber.
Contaminated air from the chemical hood enters the unit and passes through the packed-bed, liquid spray section, and mist eliminator and into the exhaust system for release up the stack. The air and the
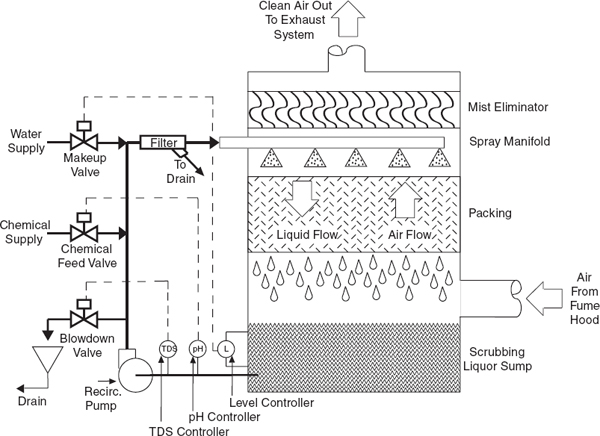
FIGURE 9.10 Schematic of a typical laboratory chemical hood scrubber.
scrubbing liquor pass in a countercurrent fashion for efficient gas-liquid contact. The scrubbing liquor is recirculated from the sump and back to the top of the system using a pump. Water-soluble gases, vapors, and aerosols are dissolved into the scrubbing liquor. Particulates are also captured quite effectively by this type of scrubber. Removal efficiencies for most water-soluble acid- and base-laden airstreams are usually between 95 and 98%.
Scrubber units are typically configured vertically and are located next to the chemical hood as shown in Figure 9.10. They are also produced in a top-mount version, in which the packing, spray manifold, and mist eliminator sections are located on top of the chemical hood and the sump and liquid-handling portion are underneath for a compact arrangement taking up no more floor area than the hood itself. Most hoods do not require a scrubber unit, assuming the exhaust stack is designed properly and chemical quantities of volatile materials are low.
9.C.2.11.3 Other Gas-Phase Filters
Another basic type of gas-phase filtration is available for chemical hoods in addition to liquid scrubbers. These are inert adsorbents and chemically active adsorbents. The inert variety includes activated carbon, activated alumina, and molecular sieves. These substances typically come in bulk form for use in a deep bed and are available also as cartridges and as panels for use in housings similar to particulate filter housings. They are usually manufactured in the form of beads, but they may take many forms. The beads are porous and have extremely large surface areas with sites onto which gas and vapor molecules are trapped or adsorbed as they pass through. Chemically active adsorbents are simply inert adsorbents impregnated with a strong oxidizer, such as potassium permanganate (purple media), which reacts with and destroys the organic vapors. Although there are other oxidizers targeted to specific compounds, the permanganates are the most popular. Adsorbents can handle hundreds of compounds, including most volatile organic components but also have an affinity for harmless species such as water vapor.
As the air passes through the adsorbent bed, gases are removed in a section of the bed. (For this discussion, gas means gases and vapors.) As the bed loads with gases, and if the adsorbent is not regenerated or replaced, eventually contaminants will break through the end of the bed. After breakthrough occurs, gases will pass through the bed at higher and higher concentrations at a steady state until the upstream and downstream levels are almost identical. To prevent breakthrough, the adsorbent must be either changed or regenerated on a regular basis. Downstream monitoring to detect breakthrough or sampling of the media to determine the remaining capacity of the bed should be performed regularly.
An undesirable characteristic of these types of scrubbers is that if high concentrations of organics or hydrocarbons are carried into the bed, as would occur if a liquid were spilled inside the hood, a large exotherm occurs in the reaction zone of the bed. This exotherm may cause a fire in the scrubber. Place these scrubbers and other downstream devices such as particulate filters in locations where the effects of a fire would be minimized. Fires can start in these devices at surprisingly low temperatures because of the catalytic action of the adsorbent matrix. Therefore, use and operate such devices with care.
9.C.2.11.4 High-Efficiency Filters
Air from laboratory chemical hoods and biological safety cabinets (BSCs) in which some radioactive or biologically active particulates are used should be properly filtered to remove these agents and prevent their release into the atmosphere. Other hazardous particulates may require this type of treatment as well. The most popular method of removal is a HEPA filter. These HEPA filters trap 99.97% of all particulates greater than 0.3 µm in diameter and may be just as effective with smaller particle sizes. Studies have shown that HEPA filters can be quite effective at trapping nanoparticles, due to Brownian motion and electrostatic capture. Before any filtration system is installed, a risk assessment should be performed to determine the need and the appropriate level of filtration required.
Ultra-low penetration air (ULPA) filters are an alternative to HEPA filters. These filters are 99.9995% efficient in removing particles greater than 0.12 µm. However, ULPA filters are more expensive than HEPA filters, and they increase the system static pressure. Note that any system designed to provide protection against radioactive particles can be expected to be effective against nanoparticles, and studies have confirmed that HEPA filters provide sufficient capture for nanoparticles (HHS/CDC/NIOSH, 2009a) making ULPA unnecessary.
These systems must be specified, purchased, and installed so that the filters can be changed without exposing the personnel or the environment to the agents trapped in the filter. Sterilizing the filter bank is prudent before changing filters that may contain etiologic agents.
The bag-in, bag-out method of replacing filters is a popular way to prevent personnel exposure. This method separates the contaminated filter and housing from the personnel and the environment by using a special plastic barrier bag and special procedures to prevent exposure to or release of the hazardous agent.
9.C.2.1 1.5 Thermal Oxidizers and Incinerators
Thermal oxidizers and incinerators are extremely expensive to purchase, install, operate, and maintain. However, they are one of the most effective methods of handling toxic and etiologic agents. The operational aspects of these devices are beyond the scope of this book. Also, their application to chemical hoods has historically been rare. When considering this method of pollution control, call an expert for assistance.
9.C.3 Other Local Exhaust Systems
Many types of laboratory equipment and apparatus that generate vapors and gases should not be used in a conventional laboratory chemical hood. Some examples are gas chromatographs, atomic absorption spectrophotometers, mixers, vacuum pumps, and ovens. If the vapors or gases emitted by these types of equipment are hazardous or noxious, or if it is undesirable to release them into the laboratory because of odor or heat, contain and remove them using local exhaust equipment. Local capture equipment and systems should be designed only by an experienced engineer or industrial hygienist. Also, users of these devices must have appropriate training.
Whether the emission source is a vacuum-pump discharge vent, a gas chromatograph exit port, or the top of a fractional distillation column, the local exhaust requirements are similar. The total airflow should be high enough to transport the volume of gases or vapors being emitted, and the capture velocity should be sufficient to collect the gases or vapors.
Despite limitations, specific ventilation capture systems provide effective control of emissions of toxic vapors or dusts if installed and used correctly and, in some cases, can result in energy savings. A separate dedicated exhaust system is recommended. Do not attach the capture system to an existing laboratory chemical hood duct unless fan capacity is increased and airflow to both hoods is properly balanced. One important consideration is the effect that such added local exhaust systems will have on the ventilation for the rest of the laboratory. Each additional capture hood will be a new exhaust port in the laboratory and will compete with the existing exhaust sources for air supply.
Downdraft ventilation has been used effectively to contain dusts and other dense particulates and high concentrations of heavy vapors that, because of their density, tend to fall. Such systems require special engineering considerations to ensure that the particulates are transported in the airstream. Here again, consult a ventilation engineer or industrial hygienist if this type of system is deemed suitable for a particular laboratory operation.
9.C.3.1 Elephant Trunks, Snorkels, or Extractors
An elephant trunk, or snorkel, is a piece of flexible duct or hose connected to an exhaust system. To capture contaminants effectively, it must be closer than approximately one-half a diameter of the hood from the end of the hose. An elephant trunk is particularly effective for capturing discharges from gas chromatographs, pipe nipples, and pieces of tubing if the hose is placed directly on top of the discharge with the end of the discharge protruding to the hose. Note that unless the intake for the snorkel is placed very close to the point source, it will be susceptible to inefficient capture. Newer designs mount the intake on an articulated arm, which tends to make the systems more effective and convenient to use. (See Figure 9.11.) The volume flow rate of the hose must be at least 110 to 150% of the flow rate of the discharge.
The face velocity for a snorkel or elephant trunk is usually 150–200 fpm. The velocity and the capture efficiency drop sharply with distance from the intake. As a result, efficient capture of contaminants is generally adequate when the discharge source is 2 in. away, but inadequate if it is 3 in. away. In cases where there is a question about efficacy of capture, perform a smoke test to determine if the flow rate is adequate (ACGIH, 2004).
9.C.3.2 Slot Hoods
Slot hoods are local exhaust ventilation hoods specially designed to capture contaminants generated according to a specific rate, distance in front of the hood, and release velocity for specific ambient airflow. In general, if designed properly, these hoods are more effective and operate using much less air than either elephant trunks or canopy hoods. To be effective, however, the geometry, flow rate, and static pressure must all be correct.
Typical slot hoods are shown in Figure 9.12. Each type has different capture characteristics and applications. If laboratory personnel believe that one of these devices is necessary, a qualified ventilation engineer should design the hood and exhaust system.
9.C.3.3 Canopy Hoods
The canopy hood is not only the most common local exhaust system but also probably the most misunderstood piece of industrial ventilation equipment. Industrial ventilation experts estimate that as many as 95% of the canopy hoods in use (other than in homes and restaurants) are misapplied and ineffective. The capture range of a canopy hood is extremely limited, and a large volume of air is needed for it to operate
effectively. Thus, a canopy hood works best when thermal or buoyant forces exist that move the contaminant up to the hood capture zone (a few inches below the opening). However, because canopy hoods are generally placed well above a contaminant source so that laboratory personnel can operate underneath them, they draw contaminants past the breathing zone and into the exhaust system. If a canopy hood exists in a laboratory, use it only for nonhazardous service, such as capturing heated air or water vapor from ovens or autoclaves. For design advice, consult the American Conference of Governmental Industrial Hygienists ventilation manual (ACGIH, 2004) and ANSI Z9.2.
9.C.3.4 Downdraft Hoods
Downdraft hoods or necropsy tables are specially designed work areas with ventilation slots on the sides of the work area. This type of system is useful for animal perfusions, gross anatomy laboratories, and other uses of chemicals where there is a need to have full access over and around the materials (which would be obstructed by the three sides of a chemical hood) and the chemicals in use have vapor densities that are heavier than air.
9.C.3.5 Clean Benches or Laminar Flow Hoods
A clean bench or laminar flow hood resembles a chemical hood but is not intended to provide protection to the user. A clean bench is generally closed on three sides and either is fully open in the front or has a partial opening. Some have hinged or sliding sashes. On the top or back of the clean bench, HEPA filters pull room air through the filters and pass that air across the work surface, providing clean air. The clean bench is for product protection, not personal protection, and is not connected to the ventilation system. Mark such equipment “not for use with hazardous materials” to remind laboratory personnel not to use anything in it that they would not use on the benchtop.
9.C.3.6 Ventilated Balance Enclosures
Ventilated balance enclosures are commonly used in laboratories to weigh toxic particulates. These devices are installed with different specifications for face velocity than the standard laboratory chemical hood and are well suited for locating sensitive balances that might be disturbed if placed in a laboratory chemical hood. The average face velocity is specified at 75 fpm plus or minus 10 fpm (0.40 ± 0.05 m/s). Individual face velocity at each grid point should be within a tolerance of plus or minus 20 fpm (0.10 m/s). Ventilated balance enclosures are typically equipped with HEPA filters to remove hazardous particulates captured within the device prior to exhaust. They can be either the recirculating type or 100% exhausted to the exterior.
Housings for ventilated balance enclosures are generally constructed of minimum 3/8-in.-thick (10-mm-thick) clear acrylic. Edges of the vertical sides are beveled, rounded, or otherwise aerodynamically designed to reduce turbulence at the perimeter of the face. Ventilated balance enclosures consist of an integrated dished base that facilitates cleaning at the interface of the vertical and horizontal surfaces. Airfoil sills have an ergonomic radius on the front edge. Sash configuration consists of a hinged single sash pane for cabinet widths and provides a full, clear, and unobstructed side-to-side view of the entire cabinet interior. Sash openings are usually at a fixed height of 8 to 12 in. (200 to 300 mm) above the work surface.
9.C.3.7 Gas Cabinets
Whenever possible, minimize use of highly toxic or hazardous gases and restrict them to lecture bottles that are placed on stands and used within the confines of a chemical hood.
Use and store containers for highly toxic or hazardous gases, such as diborane, phosgene, or arsine, that are too large to be used within a chemical hood in ventilated gas cabinets. In the event of a leak or rupture, a gas cabinet prevents the gas from contaminating the laboratory. Consult the standards developed by SEMI for specific, recommended exhaust rates for gas cabinets.
Connect gas cabinets to laboratory exhaust ventilation using metal ductwork, rather than flexible tubing, because such tubing is more apt to develop leaks. Use coaxial tubing for delivering gas from the cylinder to the apparatus. Coaxial tubing consists of an internal tube containing the toxic gas, inside another tube. Nitrogen, which is maintained at a pressure higher than the delivery pressure of the toxic gas, is between the two sets of tubing, ensuring that, in the event of a leak in the inner tubing, the gas will not leak into the room.
9.C.3.8 Flammable-Liquid Storage Cabinets
Store flammable and combustible liquids only in approved flammable-liquid storage cabinets, not in a chemical hood, on the bench, or in an unapproved storage cabinet. These cabinets are designed to prevent the temperature inside the cabinet from rising quickly in the event of a fire directly outside of the cabinet. These cabinets may be ventilated or unventi-
lated. Ventilating flammable-liquid storage cabinets is a matter for debate. One view is that all such cabinets should be vented by using an approved exhaust system, because it reduces the concentration of flammable vapors below the LEL inside the cabinet. A properly designed cabinet ventilation system does this under most circumstances and results in a situation in which no fuel is rich enough in vapor to support combustion. However, with liquid in the cabinet and a source of fresh air provided by the ventilation system, all that is needed is an ignition source. The other view is that in most circumstances flammable-liquid storage cabinets should not be ventilated.
Both opinions are valid, depending on the conditions. Ventilation is prudent when the liquids stored in the cabinet are highly toxic or extremely odoriferous. Particularly odoriferous substances such as mercaptans have such a low odor threshold that even with meticulous housekeeping the odors persist; and, ventilation may be desired. Local authorities may have specific regulations regarding the need for ventilation within the fire cabinet.
If a ventilated flammable-liquid storage cabinet is used under a chemical hood, do not vent it into the chemical hood above it. It should have a separate exhaust duct connected to the exhaust system. Fires occur most frequently in chemical hoods and may propagate into a flammable-liquid storage cabinet that is directly vented into it.
If a specially designed flammable storage cabinet ventilation system is installed, use an Air Movement Control Association C-type spark-resistant fan and an explosion-proof motor. Most fractional horsepower fans commonly used for this purpose do not meet this criterion and should not be used. If the building has a common laboratory chemical hood exhaust system, hook a flammable-liquid storage cabinet up to it for ventilation.
9.C.3.9 Benchtop Enclosures
Many laboratory ventilation system manufacturers offer ventilated enclosures that can be sized to fit equipment that would normally be placed in a chemical hood, such as rotovaps and microwave ovens. They can be made of metal or plastic and could have doors or sashes for access. The velocity of air will vary depending on the material being ventilated. The enclosure may be fitted with a filtration system for nanomaterials. By placing larger equipment in a ventilated enclosure rather than a hood, the amount of space in the hood in maximized and smaller hoods may be acceptable, resulting in energy and space savings.
9.C.4 General Laboratory Ventilation and Environmental Control Systems
General ventilation systems control the quantity and quality of the air supplied to and exhausted from the laboratory. The general ventilation system should ensure that the air is continuously replaced so that concentrations of odoriferous or toxic substances do not increase during the workday and are not recirculated from laboratory to laboratory.
Exhaust systems fall into two main categories: general and specific. General systems serve the whole laboratory and include devices such as chemical hoods and snorkels, as codes and good design practices allow. Specific systems serve isotope hoods, perchloric acid hoods, or other high-hazard sources that require isolation from the general laboratory exhaust systems.
General laboratory ventilation is typically set to provide 6 to 12 room air changes per hour. However, there is no specific requirement for ventilation rates. More airflow may be required to cool laboratories with high internal heat loads, such as those with analytical equipment, or to service laboratories with large specific exhaust system requirements or those with high densities of chemical hoods or other local exhaust ventilation devices. The ACGIH industrial ventilation manual states that “‘Air changes per hour’ or ‘air changes per minute’ is a poor basis for ventilation criteria where environmental control of hazards, heat and/or odors is required. The required ventilation depends on the problem, not the size of the room in which it occurs” (ACGIH, 2004). Where dilution ventilation will be the primary means of controlling exposure, the ventilation rate is dependent upon the materials in use. Standard industrial hygiene calculations may help to determine the required rate. Computational fluid dynamics models are often utilized to determine minimal rates when the lab is occupied and unoccupied.
Air should always flow from the offices, corridors, and support spaces into the laboratories. Exhaust all air from chemical laboratories outdoors and do not recirculate it. Thus, the air pressure in chemical laboratories should be negative with respect to the rest of the building unless the laboratory is also a clean room (see section 9.E.2). The outside air intakes for a laboratory building should be in a location that reduces the possibility of reentrainment of laboratory exhaust or contaminants from other sources such as waste disposal areas and loading docks.
Although the supply system provides dilution of toxic gases, vapors, aerosols, and dust, it gives only modest protection, especially if these impurities are released into the laboratory in any significant quantity. Perform operations that release these toxins, such as running reactions, heating or evaporating solvents, and
transfer of chemicals from one container to another, in a laboratory chemical hood where possible. Vent laboratory apparatus that may discharge toxic vapors, such as vacuum pump exhausts, gas chromatograph exit ports, liquid chromatographs, and distillation columns to an exhaust device such as an elephant trunk.
The steady increase in the cost of energy, coupled with a greater awareness of the risks associated with the use of chemicals in the laboratory, has caused a conflict between the desire to minimize the costs of heating, cooling, humidifying, and dehumidifying laboratory air and the need to provide laboratory personnel with adequate ventilation. However, cost considerations should never take precedence over ensuring that personnel are protected from hazardous concentrations of airborne toxic substances.
9.C.4.1 Constant Air Volume Systems
CAV air systems assume constant exhaust and supply airflow rates throughout the laboratory. Although such systems are the easiest to design, and sometimes are the easiest to operate, they have significant drawbacks due to their high energy consumption and limited flexibility. Classical CAV design assumes that all chemical hoods operate 24 hours per day, 7 days per week, and at constant maximum volume. Adding, changing, or removing chemical hoods or other exhaust sources for CAV systems requires rebalancing the entire system to accommodate the changes. Most CAV systems in operation today are unbalanced and operate under significant negative pressure. These conditions are caused by the inherent inflexibility of this design type, coupled with the addition of chemical hoods not originally in the plan.
9.C.4.2 Variable Air Volume Systems
VAV systems are based on laboratory chemical hoods with face velocity controls. As users operate the chemical hoods, the exhaust volume from the laboratory changes and the supply air volume must adapt to maintain a volume balance and room pressure control. Consult an experienced laboratory ventilation engineer to design these systems, because the systems and controls are complex and must be designed, sized, and matched to operate effectively together.
VAV systems provide many opportunities for increased safety and energy conservation that cannot be accomplished with a CAV system. Individual laboratory chemical hoods, groups, or all chemical hoods on the same system can be adjusted to a lower airflow when not in use through timers, occupancy sensors, or other means (see section 9.H.3). Similarly, exhaust may be automatically increased to purge the room in the event of a spill or release (see section 9.C.6.4).
9.C.5 Supply Systems
Well-designed laboratory air supply systems approach the ideal condition of laminar airflow, directing clean incoming air over laboratory personnel and sweeping contaminated air away from their breathing zone. Ventilation systems with well-designed diffusers that optimize complete mixing may also be satisfactory. Usually, several carefully selected supply-air diffusers are used in the laboratory. Ceiling plenums with perforated ceiling tiles have been used with some success, but can be difficult to design and maintain properly. In cases where high airflow rates are required, fabric diffusers may be a better option.
9.C.6 Exhaust Systems
9.C.6.1 Individual Laboratory Chemical Hood Fans
In some exhaust systems, particularly where there are just a few hoods in a building, each chemical hood has its own exhaust fan. This arrangement has both advantages and disadvantages.
Advantages include the following:
• The possibility of cross-contamination from one laboratory chemical hood discharge to another is eliminated.
• The potential to treat individual chemical hood exhaust (as opposed to treating all chemical hood exhaust) is excellent.
• A fan failure will affect only one chemical hood.
Disadvantages include the following:
• There is no way to dilute the laboratory chemical hood effluent before release.
• Providing redundancy and emergency power for this arrangement is difficult and expensive.
• The potential to use diversity (see section 9.C.6.2) is limited, as is the potential to use VAV controls.
• The potential to recover heat from individual fans is almost nonexistent.
• The maintenance requirement for these systems is considerable, because they contain many pieces of equipment and have many roof penetrations, which can cause leakage problems.
• The mechanical (shaft) space requirements, initial cost, and operating cost are higher than for alternative systems, such as manifolded systems.
9.C.6.2 Manifolded (Common Header) Systems
For compatible exhaust streams, providing a common manifolded exhaust system is an attractive design alternative to individual laboratory chemical hood fans. This design is chosen increasingly for new laboratory buildings and is compatible with VAV systems.
Manifolded VAV systems also allow design engineers to take advantage of diversity. Simply stated, diversity is an estimate of the actual expected peak airflow rate expressed as a percentage of the total exhaust capacity. The rationale is that it is not reasonable to expect that all chemical hoods would have all sashes open and laboratory personnel actively working at the same time. Thus, the exhaust system is designed for a maximum load of a lower percentage of the exhaust capacity, rather than 100%, which results in both installation cost savings and ongoing energy savings. If an aggressive diversity rate is desired, the customer may need to indemnify the designers. Engineering firms may be reluctant to design for aggressive diversity because of the potential for liability if the system capacity is later deemed inadequate. Additionally, understanding building usage patterns is critical to diversity calculation. For example, because of consistency in laboratory practices from day to day, an industrial research lab may be able to accommodate a much greater diversity than a chemistry instructional laboratory.
Manifolded systems have the following advantages and disadvantages:
• The potential for mixing and dilution of high concentrations of contaminants from a single chemical hood by the air exhaust from all the other chemical hoods on the system is excellent.
• The cross-contamination potential from one hood to another is minimal.
• The potential to provide redundancy of exhaust fans and emergency power to these systems is excellent.
• Conversely, the effects of a fan failure are widespread and serious; hence, redundancy is required in most cases.
• The potential to take advantage of VAV diversity and flow variation is also excellent, as is the ability to oversize the system for future expansion and flexibility.
• The ability to treat individual exhausts is retained by using new in-line liquid scrubber technologies.
• The maintenance, operating, and initial costs of these systems are all lower than for individual chemical hood fan systems, and these systems require fewer roof penetrations.
• The heat recovery potential for these systems is maximized by collecting all the exhaust sources into a common duct.
Some municipalities have adopted the International Mechanical Codes, including IMC 510, which poses some restrictions on manifolding exhaust ductwork. Check with your code compliance officials to determine how this affects your buildings. Most of this code includes exemptions for laboratory applications.
9.C.6.3 Hybrid Exhaust Systems
Certain types of laboratory chemical hoods and exhaust sources, such as perchloric acid hoods, should not be manifolded with other types of chemical hood exhausts. In large buildings where the designer wishes to take advantage of the benefits of manifolded exhaust systems but wishes to isolate a few exhaust streams, a combination, or hybrid, of these two types of systems is usually the most prudent and cost-effective alternative.
9.C.6.4 Room Purge Systems
A room purge system is useful in the event of a spill or release in the laboratory. These systems may be found in areas with NMR spectrometers or other large sources of cryogenic gases as well as other areas where a spill or release may cause an asphyxiation hazard. The system works when laboratory personnel depress the purge button located at the entrance to the laboratory. Air in the laboratory ceases to be recirculated and the exhaust is maximized with the goal of removing the dangerous materials. The button can be pulled to its original position to reset the ventilation system to normal operation or may require a key for reset. Note, however, that these systems should not be considered foolproof. In any case where there is a threat of asphyxiation, it is best to evacuate the area and follow emergency protocols as mandated by your organization. In areas with documented asphyxiation risks, flow restrictors should be put in place where feasible.
9.C.6.5 Exhaust Stacks
Proper stack design and placement are an extremely important aspect of good exhaust system design. Recirculation of contaminated air from the chemical hood exhaust system into the fresh air supply of the facility or adjacent facilities may occur if stacks are not provided or if they are not designed properly to force the contaminated exhaust air up and into the prevailing wind stream. Stack design should take into account building aerodynamics, local terrain, nearby structures, and local meteorological information. Consult an experienced laboratory consultant with expertise in atmospheric dispersion to design exhaust stacks for a laboratory facility. Typical exhaust discharge velocity is recommended to be 3,000–4,000 fpm, but velocities
can be lower with manifolded systems because of the benefit of dilution.
9.D ROOM PRESSURE CONTROL SYSTEMS
Laboratories and clean rooms usually require that a differential pressure be maintained between them and adjoining nonlaboratory spaces. This requirement for a pressure differential may come from code considerations or from the intended use of the space. For example, NFPA 45 states that “air pressure in the laboratory work areas shall be negative with respect to corridors and non-laboratory areas of the laboratory unit …” (NFPA, 2004). This rule helps prevent the migration of fire, smoke, and chemical releases from the laboratory space. Laboratories containing radiation hazards or biohazards may also be required by government agencies to maintain a negative pressure to contain these hazards. Clean rooms, on the other hand, are normally operated at a positive static pressure to prevent infiltration of particulates. (See sections 9.E.2 and 9.E.3, below, for further information.)
9.E.1 Gloveboxes
Unlike a chemical hood, gloveboxes are fully enclosed and are under negative or positive pressure. Gloveboxes are usually small units that have multiple openings in which arm-length rubber gloves are mounted. The operator works inside the box by using these gloves. Construction materials vary widely, depending on the intended use. Clear plastic is frequently used, because it allows visibility of the work area and is easily cleaned.
A glovebox operating under negative pressure is generally used for highly toxic materials, when a chemical hood might not offer adequate protection. A rule of thumb is that a chemical hood offers protection for up to 10,000 times the immediately dangerous concentration of a chemical. The airflow through the glovebox is relatively low, and the exhaust usually must be filtered or scrubbed before it is released into the exhaust system. Nanoparticles can also be used in a glovebox. A cautionary statement pertaining to devices such as gloveboxes: Because these devices are designed with very low airflow rates, the rate of contaminant dilution is minimal. Therefore, to ensure adequate protection to laboratory personnel, these devices must be routinely tested for leaks to ensure that enclosure integrity is sufficient. If leakage is detected, the source of contaminant release must be identified and repaired prior to any further work.
A glovebox operating under positive pressure may be used for experiments that require protection from moisture or oxygen or a high-purity inert atmosphere. Usually, the chamber is pressurized with argon or nitrogen. If this type of glovebox is to be used with hazardous chemicals, test the glovebox for leaks before each use. A method to monitor the integrity of the system (such as a shutoff valve or a pressure gauge designed into it) is required.
9.E.2 Clean Rooms
Clean rooms are special laboratories or workspaces in which large volumes of air are supplied through HEPA filters to reduce the particulates present in the room, in order to protect research materials. As nanotechnology becomes more prevalent in scientific research across many disciplines, clean rooms are becoming more and more in demand, not just in pharmaceutical, microbiological, optical, and microelectronics laboratories. Special construction materials and techniques, air-handling equipment, filters, garments, and procedures are required, depending on the cleanliness level of the facility. Consult a laboratory expert in clean room operation before a clean room is designed, built, or worked in.
9.E.2.1 Clean Room Classification
Clean room classifications refer to the number of particles larger than 0.5 µm/ft3 of volume. Unfiltered ambient air has approximately 500,000 to 1,000,000 particles/ft3.
Many laboratories still use the U.S. FED STD 209E classification system (GSA, 1992; see also ACGIH, 1998), although it was officially cancelled by the General Services Administration of the U.S. Department of Commerce on November 29, 2001 (http://www.iest.org/). This system denotes Class 10,000 to Class 1, with the class number referring to the maximum number of particles larger than 0.5 µm/ft3 of air. Thus, a Class 1000 clean room allows no more than 1,000 particles larger than 0.5 µm/ft3 of air (Table 9.4). Although not as widely used in the United States, the International Organization for Standardization (ISO) 14644-1 clean room standard classification system is becoming more common. This classification uses a logarithm of the maximum number of particles larger than 0.1 µm/m3 of volume. Thus, an ISO Class 3 clean room has a maximum of 103 = 1,000 particles of 0.1 µm/m3 of air (Table 9.5).
9.E.2.2 Clean Room Protocols
The main objective of a clean room is to protect the materials and equipment from particulates. Whereas
TABLE 9.4 US FED STD 209E Clean Room Classification
| Class | Maximum Particles/ft3 | ISO Equivalent | ||||
| >0.1 μm | >0.2 μm | >0.3 μm | >0.5 μm | >5 μm | ||
| 1 | 35 | 7 | 3 | 1 | – | ISO 3 |
| 10 | 350 | 75 | 30 | 10 | – | ISO 4 |
| 100 | – | 750 | 300 | 100 | – | ISO 5 |
| 1,000 | – | – | – | 1,000 | 7 | ISO 6 |
| 10,000 | – | – | – | 10,000 | 70 | ISO 7 |
| 100,000 | – | – | – | 100,000 | 700 | ISO 8 |
SOURCE : ANSI/IEST/ISO 14644-1:1999.
most laboratories maintain negative airflow with respect to adjacent nonlaboratory areas, clean rooms may be slightly positive. Thus, it is important to ensure that hazardous materials are stored in ventilated cabinets and work with volatile hazardous materials is done with proper ventilation.
Depending on the clean room level, laboratory personnel may need to follow special protocols to minimize generation of particulates, including some or all of the following:
• Wear special clothing ranging from shoe covers-only to shoe covers and special laboratory coats to fully encapsulating bunny suits with head cover and beard cover.
• Use an air shower before entering the clean room.
• Keep personal items out of the clean room.
• Use only specially made notebooks and paper in the clean room; no felt-tip pens (except permanent markers).
• Avoid bringing wood-pulp-based products into the clean room, such as magazines, books, regular tissues, and regular paper.
• Do not bring styrofoam or powders or any products that may produce dusts or aerosols into the clean room.
9.E.2.3 Laboratory Chemical Hoods and Laboratory Furniture in Clean Rooms
Laboratory chemical hoods and laboratory furniture in clean rooms must be easy to clean and not subject to rust or chalking. Most prefer not to use materials with painted surfaces, which may chalk or peel over time, or wood products that may form wood dusts. Stainless steel and thermoplastics are the most common materials.
Polypropylene chemical hoods are commonplace in clean rooms. The main concern is that this material burns and melts very easily. In the event of a fire, a polypropylene chemical hood may become fully involved. For this reason, it is prudent to choose either a fire-retardant polypropylene or another thermoplastic or to install an automatic fire extinguisher within the hood.
For nanomaterials, consider whether a chemical hood might be too turbulent for manipulating the materials. A biosafety cabinet, a ventilated enclosure with HEPA filtration, or a glovebox may be better alternatives. (See section 9.E.5 for more information.)
TABLE 9.5 ISO Classification of Air Cleanliness for Clean Rooms
| Class | Maximum Particles/m3 | FED STD 209E Equivalent | |||||
| >0.1 μm | >0.2 μm | >0.3 μm | >0.5 μm | >1 μm | >5 μm | ||
| ISO 1 | 10 | 2 | — | — | — | — | |
| ISO 2 | 100 | 24 | 10 | 4 | — | — | |
| ISO 3 | 1,000 | 237 | 102 | 35 | 8 | — | Class 1 |
| ISO 4 | 10,000 | 23,700 | 1,020 | 352 | 83 | — | Class 10 |
| ISO 5 | 100,000 | 237,000 | 10,200 | 3,520 | 832 | 29 | Class 100 |
| ISO 6 | 1,000,0000 | — | 102,000 | 35,200 | 8,320 | 293 | Class 1000 |
| ISO 7 | — | — | — | 352,000 | 83,200 | 2930 | Class 10,000 |
| ISO 8 | — | — | — | 3,520,000 | 832,000 | 29,300 | Class 100,000 |
| ISO 9 | — | — | — | 35,200,000 | 8,320,000 | 293,000 | Room air |
SOURCE: ACGIH (1998). Copyright 1998. Reprinted with permission.
9.E.3 Environmental Rooms and Special Testing Laboratories
Environmental rooms, either refrigeration cold rooms or warm rooms, for growth of organisms and cells, are designed and built to be closed air circulation systems. Thus, the release of any toxic substance into these rooms poses potential dangers. Their contained atmosphere creates significant potential for the formation of aerosols and for cross-contamination of research projects. Control for these problems by preventing the release of aerosols or gases into the room. Special ventilation systems can be designed, but they will almost always degrade the temperature and humidity stability of the room. Special environmentally controlled cabinets are available to condition or store smaller quantities of materials at a much lower cost than in an environmental room.
Because environmental rooms have contained atmospheres, personnel who work inside them must be able to escape rapidly. Doors for these rooms should have magnetic latches (preferable) or breakaway handles to allow easy escape. These rooms should have emergency lighting so that a person will not be confined in the dark if the main power fails. Because these rooms are often missed when evaluating building alarm systems, be sure that the fire alarm or other alarm systems are audible and/or visible from inside the room.
As is the case for other refrigerators, do not use volatile flammable solvents in cold rooms (see Chapter 7, section 7.C.3). The exposed motors for the circulation fans can serve as a source of ignition and initiate an explosion.
Avoid the use of volatile acids in these rooms, because such acids can corrode the cooling coils in the refrigeration system, which can lead to leaks of refrigerants. Also avoid other asphyxiants such as nitrogen gas in enclosed spaces. Oxygen monitors and flammable gas detectors are recommended when the possibility of a low oxygen or flammable atmosphere exists in the room.
Box 9.2 provides some basic guidelines for working in environmental rooms.
9.E.3.1 Alternatives to Environmental Rooms
Shaker boxes may be a viable alternative to environmental rooms. These boxes come in a variety of shapes and sizes and may be stackable. They use less electricity, take up much less space, and have just as much control over the environment.
A shaker box is a sealed cabinet with a pull-out work surface. The user may control the environment within the cabinet, including the temperature, humidity, carbon dioxide level, lighting, and vibration. Shaker boxes may be used as incubators or for cooling, giving a full range of options.
BOX 9.2
Quick Guide for Working in Environmental Rooms
Mold growth can cause problems for an experiment and affect personnel health. To avoid mold:
• Report any leaks or condensation to maintenance personnel for repair.
• Clean up spills immediately. Mold thrives on organic material.
• Do not keep papers or cardboard in the room. If such materials are needed, keep them in plastic bags.
• Do not use wood. Replace wood shelving with plastic or metal.
• Clean all surfaces with a hospital-grade disinfectant.
Be wary of using flammable materials in this room:
• There may be sources of ignition in the room, including fan motors.
• Do not store flammable liquids in the room.
Ventilation is limited:
• Chemical vapors may accumulate. Do not use materials that require local ventilation. Even materials that normally may be used on a benchtop may pose a risk in a closed environment. Do a full risk assessment.
• Limit the use of compressed gases in the event that they may displace oxygen and cause an oxygen-deficient atmosphere.
• Do not store dry ice or liquid nitrogen in an environmental room, because sublimation of the carbon dioxide may displace the air in the room, creating an asphyxiation hazard.
Do not store foods in an environmental room:
• Do not store alcoholic or nonalcoholic beverages.
• Foods may absorb chemical vapors. Do not store any food in these rooms.
9.E.4 Biological Safety Cabinets and Biosafety Facilities
BSCs are common containment and protection devices used in laboratories working with biological agents. BSCs and other facilities in which viable
organisms are handled require special construction and operating procedures to protect laboratory personnel and the environment. Conventional chemical hoods should never be used to contain biological hazards. Biosafety in Microbiological and Biomedical Laboratories (HHS/CDC/NIH, 2007a), Primary Containment for Biohazards: Selection, Installation, and Use of Biological Safety Cabinets ((HHS/CDC/NIH, 2007b), and Biosafety in the Laboratory: Prudent Practices for the Handling and Disposal of Infectious Materials (NRC, 1989) give detailed information on this subject.
9.E.4.1 Biosafety Cabinets
A biosafety cabinet is specially designed and constructed to offer protection to the laboratory personnel and clean filtered air to the materials within the workspace. A biosafety cabinet may also be effective for controlling nanoparticles.
The three classes of biosafety cabinets for work with biological agents are briefly described below. For more information, see the guide Primary Containment for Biohazards: Selection, Installation, and Use of Biological Safety Cabinets (HHS/CDC/NIH, 2007b).
• A Class I biosafety cabinet does not provide a clean work environment but does provide some protection to the user. Like a chemical hood, it draws air through the face of the cabinet away from the user, across the work surface, through a set of HEPA filters, and back into the laboratory.
• A Class II biosafety cabinet (Type A1, A2, B1, or B2) provides a clean work environment and protection to the user. Internal supply air passes through a HEPA filter in a downward laminar flow across the work surface, preventing cross-contamination. It works by drawing room air around laboratory personnel through slots in the work surface at the front of the cabinet, offering user protection. Air also is exhausted through a grill along the back of the cabinet and is either recirculated through HEPA filters to the internal workspace or passes through another set of filters to be exhausted to the room or through ductwork and out of the building. (See Figure 9.13.)
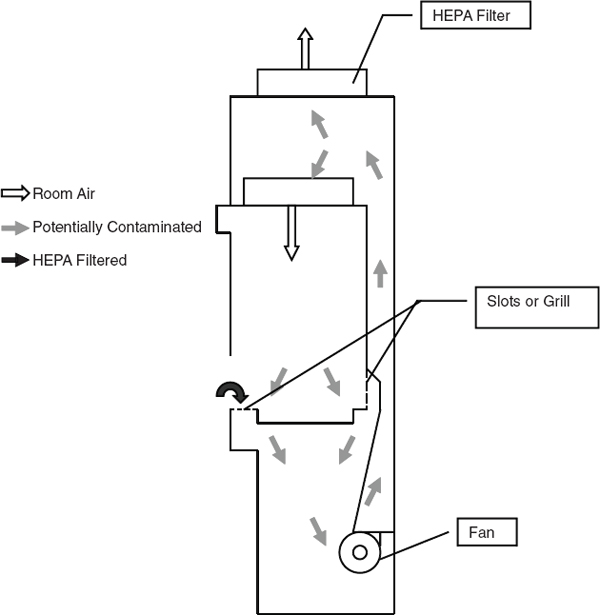
FIGURE 9.13 Example of a Class II biosafety cabinet. Room air passes around the user through the grill at the front of the cabinet. Filtered air passes into the cabinet over the materials, providing a clean environment for the materials in the cabinet. Potentially contaminated air moves through the grill and slots, across the cabinet, and passes through HEPA filters.
• A Class III biosafety cabinet provides maximum protection to laboratory personnel and the working environment. This type of cabinet is a glove-box with HEPA filter exhaust.
A biosafety cabinet is generally not suited for work with hazardous chemicals. Most biosafety cabinets exhaust the contaminated air through HEPA filters back into the laboratory. This type of filter will not contain most hazardous materials, particularly gases, fumes, or vapors. Even when connected to the laboratory exhaust system, a ducted biosafety cabinet may not provide enough containment for work with hazardous chemicals. For field testing of biosafety cabinets, consult NSF/ANSI Standard 49-2009.
Some Class II biosafety cabinets may be connected to the laboratory exhaust system and may be touted as a combination biosafety cabinet and chemical hood. However, even when ducted, a biosafety cabinet may not provide adequate containment for work with hazardous materials.
Table 9.6 provides an overview of the characteristics of different types of biosafety cabinets.
9.E.4.2 Using a Biosafety Cabinet for Biological Materials
The following protocol should be followed when using a biosafety cabinet for work with biological materials:
• Turn the cabinet on at least 10–l5 minutes prior to use, if the cabinet is not left running. Verify the cabinet is operating properly and has been certified within the dates recommended by your institution.
• Disinfect work surface with 70% alcohol or other suitable disinfectant.
• Place items into the cabinet so that they can be worked with efficiently without unnecessary disruption of the airflow, working with materials from the clean to the dirty side.
• Wear appropriate PPE. At a minimum, this will include a buttoned laboratory coat and gloves.
• Adjust the working height of the stool or stand so that the worker’s face is above the front opening.
• Delay manipulation of materials for approximately 1 minute after placing the hands/arms inside the cabinet.
• Minimize the frequency of moving hands in and out of the cabinet.
• Do not disturb the airflow by covering any of the grill or slots with materials.
• Work at a moderate pace to prevent airflow disruption that occurs with rapid movements.
• Wipe the bottom and sides of the cabinet surfaces with disinfectant when work is completed.
Unlike a chemical hood, a biosafety cabinet contains filters that must be changed on a regular basis. The biosafety cabinet must be decontaminated before replacing the filters and then recertified for use. Check
TABLE 9.6 Comparison of Biosafety Cabinet Characteristics
| BSC Class | Face Velocity | Airflow Pattern | Applications | |
| Nonvolatile Toxic Chemicals and Radionuclides | Volatile Toxic Chemicals and Radionuclides | |||
| I | 75 | In at front through HEPA to the outside or into the room through HEPA | Yes | When exhausted outdoorsa,b |
| II, A1 | 75 | 70% recirculated to the cabinet work area through HEPA; 30% balance can be exhausted through HEPA back into the room or to outside through a canopy unit; plenums are under negative pressure | Yes (minute amounts) | No |
| II, B1 | 100 | 30% recirculated, 70% exhausted; exhaust cabinet air must pass through a dedicated duct to the outside through a HEPA filter | Yes | Yes (minute amounts)a,b |
| II, B2 | 100 | No recirculation; total exhaust to the outside through a HEPA filter | Yes | Yes (small amounts)a,b |
| II, A2 | 100 | Similar to II, A1, but has 100 fpm intake air velocity and plenums are under negative pressure to room; exhaust air can be ducted to outside through a canopy unit | Yes | When exhausted outdoors (formerly “B3”) (minute amounts)a,b |
| III | N/A | Supply air is HEPA filtered; exhaust air passes through two HEPA filters in series and is exhausted to the outside via a hard connection | Yes | Yes (small amounts)a,b |
aInstallation may require a special duct to the outside, an in-line charcoal filter, and a sparkproof (explosion-proof) motor and other electrical components in the cabinet. Discharge of a Class I or Class II, Type A2 cabinet into a room should not occur if volatile chemicals are used.
bIn no instance should the chemical concentration approach the lower explosion limits of the compounds.
SOURCE: HHS/CDC/NIH (2007b); NSF/ANSI Standard 49-2009.
with your institutional biosafety officer for required frequency.
9.E.5 Nanoparticles and Nanomaterials
Engineering control techniques such as source enclosure (i.e., isolating the generation source from the worker) and local exhaust ventilation systems should be effective for capturing airborne nanomaterials, based on what is known of nanomaterial motion and behavior in air.
Though traditional chemical hoods may be used for research on nanoscale particles and materials, some researchers find it challenging to work with nanoparticles in hoods operating with a 100-fpm face velocity because of turbulent airflow. In addition, limited studies demonstrate that chemical hoods that operate at a 100-fpm face velocity, even those that pass the ANSI/ASRAE containment tests, may allow nanoparticles to escape in quantities that may pose a risk to health or the environment (Ellenbecker and Tsai, 2008). This is similar to the experience of pharmaceutical companies handling dry powder formulations research. Lower-flow, reduced-turbulence hoods may be warranted. Even at lower face velocities, dispersion of particles may result in loss of materials or contamination of surfaces or both. This active area of research should be carefully monitored by anyone working with nanoparticles in a laboratory.
Because the effect of nanomaterials on the environment is still a topic of research and debate, prudent practice ensures that they do not disperse into the environment through the ventilation system. HEPA filters, which are 99.99% efficient at removing 0.3-µm and larger particles, are also very effective in trapping nanoscale particles. Some vendors offer ULPA filters, which are 99.9995% efficient at removing 0.12-µm and larger particles. Although ULPA filters are more efficient than HEPA filters, HEPA filters are generally acceptable for nanoparticle work. HEPA filters should be properly seated in well-designed filter housings.
Ionizers that are placed in either a chemical hood or a cabinet or are integrated into a cabinet can help minimize dispersion of nanomaterials, reducing loss of materials and keeping the work surfaces cleaner. Exercise caution working with explosive or highly flammable chemicals near an ionizer.
Stainless steel is much easier to clean and may show areas where materials have dispersed. Enclosures with stainless steel work surfaces are good for nanomaterial work but are not necessary.
There are several alternatives for controlling nanomaterials in the laboratory, and many ventilation vendors are working on systems specifically designed for nanoparticles.
• A low-flow enclosure or chemical hood equipped with a HEPA filter on the exhaust side is effective at reducing turbulence, preventing nanomaterials from being released into the environment through the exhaust system, and providing good containment for both nanomaterials and hazardous chemicals. For laboratories that can only provide one type of containment, this is a good alternative.
• A negative-pressure glovebox is effective.
• Class I biosafety cabinets that exhaust air through HEPA filters into the room or those that are hard-ducted to the outdoors may provide good containment for nanoparticles. Class II biosafety cabinets that exhaust air through HEPA filters back into the room or those that are hard-ducted to the outdoors may be a good choice. A glovebox provides a high level of protection and can be equipped with HEPA filtration.
Some vendors have produced other alternatives, most of which are Class I biosafety cabinets equipped with an ionizer near the front edge.
For laboratories with both hazardous chemicals and nanoparticle work, one strategy is to handle the nanoparticles in a Class I or II biosafety cabinet or a low-flow enclosure (see above), transfer the particles into solution, and then continue work in a laboratory chemical hood.
Do not use horizontal laminar-flow hoods (clean benches) that direct a flow of HEPA-filtered air into the user’s face for any operations involving hazardous materials or engineered nanomaterials.
9.E.6 Explosion-Proof Chemical Hoods
For operations involving materials that could explode, protection aimed at preventing ignition and containing an explosion may be necessary. The sash should be composed of a composite material of safety glass backed by polycarbonate, with the safety glass on the interior side of the sash. In addition, all components of the hood, including the electrical supply, lighting, etc., must be explosion-proof.
9.F MAINTENANCE OF VENTILATION SYSTEMS
Even the best-engineered and most carefully installed ventilation system requires routine maintenance. Blocked or plugged air intakes and exhausts, as well as control system calibration and operation, alter the performance of the total ventilation system. Filters become loaded, belts loosen, bearings require lubrication, motors need attention, ducts corrode, and minor components fail. These malfunctions, individually or
collectively, affect overall ventilation performance. Some laboratory ventilation systems have become so complex that prudent practice requires a special team of facilities staff dedicated to the maintenance of the system.
Inspect and maintain facility-related environmental controls and safety systems, including chemical hoods and room pressure controls, fire and smoke alarms, and special alarms and monitors for gases, on a regular basis.
Evaluate each laboratory periodically for the quality and quantity of its general ventilation and anytime a change is made, either to the general ventilation system for the building or to some aspect of local ventilation within the laboratory. The size of a room and its geometry, coupled with the velocity and volume of supply air, determine its air patterns. Airflow paths into and within a room can be determined by observing smoke patterns. Convenient sources of smoke for this purpose are the commercial smoke tubes available from local safety and laboratory supply companies. If the general laboratory ventilation is satisfactory, the movement of supply air from corridors and other diffusers into the laboratory and out through laboratory chemical hoods and other exhaust sources should be relatively uniform. There should be no areas where air remains static or areas that have unusually high airflow velocities. If stagnant areas are found, consult a ventilation engineer, and make appropriate changes to supply or exhaust sources to correct the deficiencies.
The number of air changes per hour within a laboratory can be estimated by dividing the total volume of the laboratory (in cubic feet) by the rate at which exhaust air is removed (in cubic feet per minute) and multiplying the total by 60. For each exhaust port (e.g., laboratory chemical hoods), the product of the face area (in square feet) and the average face velocity (in linear feet per minute) gives the exhaust rate for that source (in cubic feet per minute). The sum of these rates for all exhaust sources yields the total rate at which air is exhausted from the laboratory. The rate at which air is exhausted from the laboratory should equal the rate at which supply air is introduced into the room. Thus, decreasing the flow rate of supply air (perhaps to conserve energy) decreases the number of air changes per hour in the laboratory, the face velocities of the chemical hoods, and the capture velocities of all other local ventilation systems.
Airflows are usually measured with thermal anemometers or velometers. These instruments are available from safety supply companies or laboratory supply houses. The proper calibration and use of these instruments and the evaluation of the data are a separate discipline. Consult an industrial hygienist or a ventilation engineer whenever serious ventilation problems are suspected or when decisions on appropriate changes to a ventilation system are needed to achieve a proper balance of supply and exhaust air.
All ventilation systems should have a device that readily permits the user to monitor whether the total system and its essential components are functioning properly. Manometer, pressure gauges, and other devices that measure the static pressure in the air ducts are sometimes used to reduce the need to manually measure airflow. Determine the need for and the type of monitoring device on a case-by-case basis. If the substance of interest has excellent warning properties and the consequence of overexposure is minimal, the system will need less stringent control than if the substance is highly toxic or has poor warning properties.
9.G VENTILATION SYSTEM MANAGEMENT PROGRAM
The laboratory ventilation system is one of the most important aspects of laboratory safety and, at the same time, is likely to be the highest consumer of energy in the laboratory building. Managing all facets of the ventilation system is crucial to maximize safety and energy conservation.
The AIHA/ANSI Z9.5-2003 Laboratory Ventilation Standard provides an outline for a ventilation management program and recommends appointing a responsible person to oversee the program. The Leadership in Energy and Environmental Design (LEED) Green Building Rating System™ of the U.S. Green Building Council uses this model as a consideration in its certification system for rating laboratory buildings.
Overall, there are four main aspects of a ventilation system management program: design criteria, training for laboratory personnel, system maintenance, and performance measurement.
9.G.1 Design Criteria
The institution should determine the criteria to use for all new installations of chemical hoods and other ventilation systems. This might include
• testing criteria as installed (e.g., all or a representative sampling of the hoods must pass ANSI/ASHRAE 110-1995 containment testing as installed);
• chemical hood design criteria (e.g., face velocity criteria at specific sash height and sash design);
• types of continuous monitoring systems preferred or required (e.g., face velocity reading, magnehelic gauge);
• acceptable diversity factors;
• energy conservation strategies;
• alarm systems;
• type of duct work;
• noise criteria;
• preference for VAV systems (designing one extra fan into each system); and
• backup power.
9.G.2 Training Program
No matter how well a system is designed or maintained, no matter what lengths an institution has gone to for the sake of safety and energy conservation, if laboratory personnel do not use the equipment properly, individual users can defeat these efforts with their own behaviors.
Laboratory personnel who insist on working at the edge of the laboratory chemical hood, raise the sash above its maximum operating height, defeat alarms, disable sash closures, do not move an elephant trunk close to the source, block baffles, use loose materials in the chemical hood and clog the ductwork, leave the sash open when not working at the chemical hood, fail to report that a filter needs to be changed reduce safety and sustainability efforts. Sometimes, these actions are due to lack of consideration; sometimes personnel may simply not understand the implications.
All laboratory personnel should receive training that includes
• how to use the ventilation equipment,
• consequences of improper use,
• what to do in the event of system failure,
• what to do in the event of a power outage,
• special considerations or rules for the equipment,
• significance of signage and postings.
Training may be one-on-one, classroom, Web-based, or whatever format fits the culture of the institution and the needs of the laboratory.
Many laboratories, particularly academic research laboratories, experience high turnover rates. Good signage and postings complement training and act as constant reminders (Figure 9.14).
Consider the following types of signs and postings:
• sash position for laboratory chemical hoods,
• telltales (ribbons or similar materials on chemical hood sashes with a key to good performance),
• meaning of any audible or visual alarms,
• function of occupancy sensors (e.g., setback mode tied to light switch),
• downtimes if the system has a setback mode that is on a timer, and
• reminder to lower the sash when not in active use.
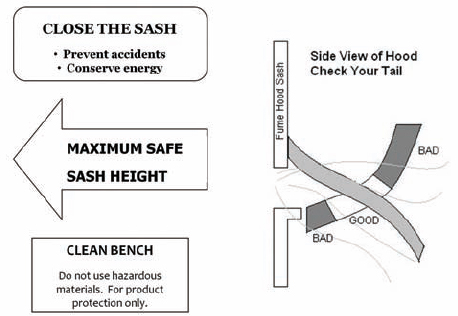
FIGURE 9.14 Examples of postings for laboratory chemical hoods. Clockwise from top left: reminder to close the chemical hood sash, guide to checking the telltale ribbon taped to the sash of the chemical hood, reminder that a clean bench is not for hazardous chemicals, indicator showing the safe maximum sash height.
9.G.3 Inspection and Maintenance
Maintenance is key to a ventilation system management program. The program should describe the elements of the inspection and maintenance program, including
• designation of who conducts inspections and how often;
• how inspections are recorded;
• inspection criteria for laboratory chemical hoods including
ο face velocity testing—equipment used, history,
ο how recorded,
ο how posted on the chemical hood, and
ο will maximum sash height be marked and how;
• criteria for working on roofs and around stacks;
• fan maintenance schedule;
• VAV system maintenance schedule;
• alarms and controls maintenance schedule; and
• schedule for recommissioning the ventilation system.
9.G.4 Goals Performance Measurement
The old adage that “you can’t manage what you don’t measure” rings true too with the ventilation management program. At least annually, evaluate the effectiveness of the program, including
• energy use and savings,
• emission issues,
• trends in chemical hood performance (signs of deterioration, etc.), and
• review of the life cycle of the ventilation system.
9.G.5 Commissioning
When a new ventilation system in installed, new components are installed, or any significant change to the ventilation system occurs, consider hiring a commissioning agent with experience with laboratory facilities. An outside commissioning agent will ensure that the system meets the criteria you have selected, note any design errors, handle problems, and facilitate testing, installation, etc. In-house staff or hired consultants will continue to maintain the equipment, but the startup issues can be overwhelming. Ensure that those who will be using and maintaining the system receive training.
Cost considerations should never take precedence over ensuring that laboratory personnel are protected from hazardous concentrations of airborne toxic substances. That sentiment bears repeating. However, since the 1980s, the chemical hood has become a fixture in a laboratory, sometimes whether it was needed or not. Many laboratory research buildings have several chemical hoods that remain unused, even as thousands of cubic feet of conditioned air passes through them every minute. In a typical laboratory building containing office space, meeting space, and laboratories, the labs constitute one-sixth of the floor space, yet consume a third of the energy.
One suburban university that is relatively typical of a research campus conducted a study of the origin of its carbon inventory and determined that 37% was from laboratory buildings, which constitute 15% of the total building area on campus (see Figure 9.15).
Typically, at any one time, fewer than half the hoods in a given laboratory are in active use. Chemical hoods are excellent, but they are not the only solution for reducing exposure to a safe level. Where laboratory chemical hoods are needed, the amount of energy they consume can be reduced. (See Vignette 9.2.)
Several options for energy conservation have been presented in previous sections of this chapter. More technologies are being developed and become available every year. Each deserves attention and scrutiny before using them in a research laboratory environment.
This section focuses on sustainability with respect to ventilation, but sustainability can be supported in other areas through water conservation, following appropriate waste disposal techniques, considering the principles of green chemistry when performing research, and investigating ways to reduce the energy needs of the building.
FIGURE 9.15 Carbon inventory of a research university campus.
9.H.1 Low-Flow or High-Performance Laboratory Chemical Hoods
Low-flow or high-performance hoods operate at a lower face velocity and save energy by reducing the amount of conditioned air that passes through them. They tend to be more expensive than traditional chemical hoods, but the energy savings generally result in a quick payback. They are deeper than a traditional chemical hood and may not occupy the same space in a retrofit situation. See section 9.C.2.9.3.6 for more information.
VIGNETTE 9.2
Sustainability considerations in laboratory ventilation design
In the initial design discussions for an academic research laboratory, the principal investigator called for six 8-ft chemical hoods plus two ventilated Class II biosafety cabinets. After discussions about how this equipment was to be used and the operations of the laboratory, the EHS staff and the engineers suggested alternatives, including ventilated equipment enclosures and snorkels. These changes resulted in significant savings in first costs, space, operating costs, and energy consumption, while better fitting the needs of the researchers.
9.H.2 Automatic Sash Closers
For most laboratory chemical hoods, especially those on VAV systems, when the sash is closed, they draw much less air, resulting in significant energy savings. Laboratory personnel do forget to close the sash or find it cumbersome to keep closing the sash every time they step away.
Modern automatic sash closers have a sensor technology that uses a proximity or motion detector to sense when there is no one in front of the chemical hood. The sensor has a timer that can be adjusted to a set time period; after that time, if no one appears to be working at the hood, the system gently closes the sash. Like a garage door closer, there is usually a sensor at the bottom edge of the sash, such that if anything, even a pipette, crosses the plane of the sash, the sash will stop closing to avoid breaking or bumping whatever is below the sash.
Some sash designs include counterweights that automatically lower the sash to a set level when the laboratory personnel step away. The sash does not close completely but does lower substantially.
Automatic sash closers can result in significant cost savings and add to the safety of laboratory personnel by keeping a barrier between the materials in the chemical hood and personnel and materials in the laboratory.
9.H.3 Variable Air Volume Systems with Setback Controls
Most chemical hoods are used only a portion of the day. An advantage of a VAV system is that individual chemical hoods or an entire system can be adjusted to a setback mode, a low flow that maintains negative pressure but conserves energy.
The setback mode may be activated in a number of ways, such as:
• a timer for an individual chemical hood or an entire system where work schedules are predictable;
• occupancy sensors, set back when sensors indicate that the laboratory or the chemical hood is not in use;
• sash position, set back when the sash is fully closed, especially useful in conjunction with automatic sash closers; and
• light switch, set back when lights are turned off, indicating that the laboratory is unoccupied.
9.H.4 Variable Air Volume Systems, Diversity Factors
Another advantage of a VAV system with manifolded exhaust is that the system could be designed for just a portion of that maximum airflow, rather than for a system that handles 100% of the hoods it serves. The rationale is that it is extremely unlikely that all the chemical hoods would be operating with the sash open at the same time. The diversity factor is the maximum percentage of airflow ever needed at once.
By designing the system to handle a smaller number of chemical hoods, the system takes advantage of smaller ductwork and fewer fans, resulting in both first-cost savings and ongoing energy cost savings. Prudent practice adds at least one extra fan to the system both for maintenance reasons (always able to have one fan down) and for future growth.
9.H.5 Lower General Ventilation Rates
As discussed in section 9.C.4, many laboratories have a minimum of 6 to 12 air changes per hour. Some laboratories have been able to lower these rates based on the materials and operations in the laboratory. Consultants experienced in computational fluid dynamics modeling are able to take information about the chemicals and processes and the ventilation system and predict how a lower air change rate might affect laboratory air quality.
Some laboratories have installed active chemical monitoring systems that sample for and provide real-time measurements of carbon dioxide and specific chemicals, adjusting the airflow in the room as needed to maintain an acceptable air quality. Limitations do exist for this method, but it may be useful in some situations.
9.H.6 Laboratory Chemical Hood Alternatives
The laboratory chemical hood is a fabulous engineering control, but it is not the only one. Perform a risk assessment and consider the other alternatives. Many of the alternatives will result in lower energy usage without compromising safety.
9.H.7 Retro-Commissioning
For facilities with ventilation systems that were not designed for energy efficiency, consider whether it makes sense to replace all or parts of the system with newer, more efficient alternatives. Retro-commissioning a laboratory ventilation system can result in large energy savings and a safer ventilation system and may have a relatively short payback period. See section 9.B.9 for additional information.
9.H.8 Components of Heating, Ventilation, and Air-Conditioning (HVAC)
There are many technologies aimed at energy conservation for ventilation systems. Examples include chilled beams for cooling labs and offices, reheat systems that cool or heat within zones rather than for all labs on the system, and enthalpy wheels for retaining latent and sensible heat, just to name a few.
Technologies continue to improve and new ideas are being tested constantly. The following resources, mostly available online, may be useful in identifying and evaluating these systems:
• EPA Laboratories in the 21st Century (Labs 21) (http://www.labs21century.gov/),
• US Green Building Council’s LEED (http://www.usgbc.org), and
• ASHRAE Laboratory Design Guide (http://ateam.lbl.gov/).
9.H.9 How to Choose a Ventilation System
There is no one choice that is right for every laboratory. The designers, the laboratory users, and the facilities staff must discuss the possibilities. EHS professionals and laboratory managers are helpful in these discussions as well. The individuals who decide which systems to install must understand the needs of the users, and the users must understand how the systems work, the capabilities and limitations of the systems, and what to expect from them. The facilities staff must understand how the systems need to be maintained, and those who are choosing the system need to know whether there is in-house expertise to maintain them.
Check local, state, and federal codes and regulations before choosing a new system. Only a few actual regulations cover ventilation systems, but more and more municipalities are adopting international building and mechanical codes. These codes impose limitations on manifolding ductwork and may require detection or sprinklers within ducts.
When considering a new technology, benchmarking is usually helpful. Find someone who is using a similar system and discuss their experience. Ask for samples. Visit laboratories that use similar products. Find the systems that work best for your applications. Continue communications between the users and the installers and the maintenance staff to ensure that the systems are working as intended.
Remember that even if all the chemical hoods are removed, ventilation is still needed in the laboratory.
9.I LABORATORY DECOMMISSIONING
A laboratory must be properly decommissioned prior to changing its use. Among other steps, decommissioning entails decontamination and the removal of hazards to ensure the safety of future occupants and others who may enter the space. Decommissioning must be done prior to renovation, even if the space is to be reused as a laboratory. Because laboratory operations differ, it is appropriate to decommission a laboratory whenever there is a significant change in occupancy. Areas outside of the laboratory, such as ventilation ductwork, coldrooms, hallway freezers and common storage areas, should also be decommissioned if they are concurrently subject to a significant change in use or occupancy. Decommissioning must also be done prior to the demolition of a laboratory.
Before decommissioning begins it is important to establish a level of cleanliness that meets the regulatory and institutional safety standards for the next occupancy. Detailed radiological assessment and decontamination guidelines are available in the Multi-Agency Radiation Survey and Site Investigation Manual (MARSSIM), available from the Nuclear Regulatory Commission and other government agencies (EPA/USNRC/DOE/DOD, 2000). Although a helpful Laboratory Decommissioning Standard is available from the American National Standards Institute (ANSI Z9.11, 2008), there are few standards for an acceptable level of residual chemical contamination. Even when environmental cleanup standards exist, it may be difficult to apply them to laboratory decommissioning.
Be sure to document the assessment, decontamination and removal activities, and to issue a final clearance statement. A Laboratory Closeout Checklist is included on the disc that accompanies this book. It may be appropriate to prepare a written Decommissioning Plan.
9.I.1 Assessment
The first step in laboratory decommissioning is to assess any hazards that may remain in the space. Review the known or likely historic uses of the space, as well as records of spills and accidents, laboratory manuals and notebooks, and published papers of research conducted in the lab. Ask former occupants what hazardous materials they used and if they know of any contaminated areas.
The assessment of radiological hazards is relatively straightforward and requires standard methods for handheld survey meters and wipe tests for removable contamination. Because it is easy to do, a radiological survey should be done unless it can be assured that no radioactive material had been used in the space.
Because many chemicals require a unique protocol for sampling and analysis, a chemical contamination assessment usually requires that the potential contaminants be well-defined. A field sampling plan should describe how wipe tests will be taken, the wetting solvent used, the protocol for grid sampling (or other sampling scheme), necessary analytical sensitivity, and the methodology that will be used to evaluate the results.
9.I.2 Removal, Cleaning, and Decontamination
The second step in decommissioning is to remove all hazards from the space. Be sure that all chemicals, radioactive materials, and biologicals have been removed from use and storage areas, including refrigerators and freezers. Movable equipment should be appropriately cleaned and/or disinfected, and removed from the lab.
Residual perchloric acid and mercury contamination are common concerns for laboratory decommissioning. If perchloric acid was used outside of a hood designed for that purpose, hoods and ductwork can become contaminated with explosive metal perchlorates. (See section 9.C.2.10.5 for information about the hazards of perchloric acid in laboratory hoods and ventilation.)
Mercury is used in most laboratories, and mercury spills are common. Unless it is certain that no mercury was used, laboratory decommissioning should include testing of floors, sinks, cupboards, and molding around furniture and walls. Be sure to check and clean sink p-traps. Visual inspection alone is inadequate as historic spills may reach beneath floor tiles and furniture, and behind walls. As described in the ANSI Laboratory Decommissioning Standard, modern mercury testing utilizes a portable atomic absorption spectrophotometer with a sensitivity of 2 ng/m3. Decommissioning clearance levels consider the U.S. Agency for Toxic Substances and Disease Registry’s Minimal Risk Level (MRL) of 200 ng/m3 for non-occupationally exposed individuals. Chapter 6, section 6.C.10.8, includes information on dealing with mercury contamination. Additional mercury testing may be necessary as furniture, floors, walls, and plumbing are removed during renovation.
After hazardous materials and movable equipment have been removed, areas known to be contaminated (e.g., stained floors and cupboards) should be cleaned appropriately, or destructively removed and disposed of. Chemical decontamination can be done using appropriate surfactant soaps, solvents, neutralizing agents, or other cleaners.
Unless is it known that no biological materials were used in the space, the furniture, equipment, and other surfaces should be cleaned with an appropriate disinfectant. Sophisticated biological decontamination technologies are available for areas where high-risk pathogens have been used.
As a precautionary measure, it is a standard practice to remove dusts and other settled particulates via a thorough final wet-cleaning of floors, vertical surfaces and furniture using commercial cleaning products.
9.I.3 Clearance
Final tests or survey results can be used to verify decontamination. In some cases regulatory authorities allow permanent marking of a porous floor or wall where a radioactive material or chemical has penetrated deeply, and destructive removal is impractical prior to the building’s demolition. When removal, decontamination, and cleaning meet planned decommissioning standards, a final area clearance statement can be issued, and renovation, demolition, or the new occupancy can commence.