5.B GREEN CHEMISTRY FOR EVERY LABORATORY
5.B.2 Microscale Work and Wet Chemistry Elimination
5.B.2.1 Design Less Hazardous Laboratory Processes and Reaction Conditions
5.B.3 Use Safer Solvents and Other Materials
5.B.4 Design Experimental Products for Degradation After Use
5.B.5 Include Real-Time Controls to Prevent Pollution
5.B.6 Minimize the Potential for Accidents
5.B.7 Green Chemistry Principles Avoid Multihazardous Waste Generation
5.B.8 Mercury Replacements in the Laboratory
5.B.8.3 Differential Manometers
5.D INVENTORY AND TRACKING OF CHEMICALS
5.D.2 Exchange of Chemicals Between Laboratories and Stockrooms
5.D.3 Recycling of Chemicals and Laboratory Materials
5.D.3.1 General Considerations
5.D.3.3 Recycling Containers, Packaging, and Labware
5.D.4 Labeling Commercially Packaged Chemicals
5.D.5 Labeling Other Chemical Containers
5.D.6 Labeling Experimental Materials
5.D.7 Use of Inventory and Tracking Systems in Emergency Planning
5.E STORAGE OF CHEMICALS IN STOCKROOMS AND LABORATORIES
5.E.2 Storage According to Compatibility
5.E.3 Containers and Equipment
5.E.5 Storing Flammable and Combustible Liquids
5.E.7 Storing Highly Reactive Substances
5.E.8 Storing Highly Toxic Substances
5.F TRANSFER, TRANSPORT, AND SHIPMENT OF CHEMICALS
5.F.1 Materials of Trade Exemption
5.F.2 Transfer, Transport, and Shipment of Nanomaterials
This chapter organizes the discussion of managing laboratory chemicals into six main topics: reducing and eliminating the use and generation of hazardous substances (green chemistry); acquisition; inventory and tracking; storage in stockrooms and laboratories; recycling of chemicals and laboratory materials; and transfer, transport, and shipment of chemicals. As Chapter 1 makes clear, prudence in these areas requires knowledge of the hazards posed by laboratory chemicals and the formulation of reasonable measures to control and minimize the risks associated with their handling and disposal. Not all risk can be eliminated, but through informed risk assessment and careful risk management, laboratory safety is greatly enhanced.
Trained laboratory personnel, laboratory supervisors, and individuals who handle chemicals will find essential information in this chapter. Each person has an important role to play in a chemical’s life cycle at an institution, and each one of them should be aware that the wise management of that life cycle not only minimizes risks to humans and to the environment but also decreases costs. Acknowledging this role and giving it due consideration is one element of the culture of safety within a laboratory.
5.B GREEN CHEMISTRY FOR EVERY LABORATORY
Green chemistry is the philosophy of designing products and processes that reduce or eliminate the use and generation of hazardous substances, which fts well with the overall goals of a culture of safety. The 12 principles of green chemistry (Anastas and Warner, 1998) can be applied in the laboratory as guidelines for prudent experimental design and execution. Some of the principles are explained in more detail below, with examples of their broader application. A wealth of green chemistry resources exists online in the form of reports, databases, and other Web applications and tools. These resources assist the development of green synthetic methods by providing information about the redesign of processes at the molecular level, the reduction or elimination of the use of hazardous materials, and the modification of chemical substances to make them safer.
5.B.1 Prevent Waste
Prudent laboratory chemical management begins with adopting the first green chemistry principle of waste prevention, which is considered before the ordering of the chemicals. When experiments have been carefully planned, trained laboratory personnel can be confident that they have chosen procedures that minimize the quantities of chemicals to be used and minimize the disposal of hazardous materials.
Experiment planning in the culture of laboratory safety includes minimization of the material used at each step of an experiment. Consider two simple examples: (1) Transferring a liquid reaction mixture or other solution from one flask to another container usually requires the use of a solvent to rinse out the flask. During this procedure, laboratory personnel should use the smallest amount of solvent possible that enables a complete transfer. (2) Celite is often used during filtrations to keep the pores of filter papers or filter frits from becoming clogged. When positioning the Celite, carefully determine the minimum amount needed to be effective. Other examples of such strategies include
• considering how a reaction product will be used and making only the amount needed for that use;
• appreciating the cost of making and storing unneeded material;
• thinking about minimization of material used in each step of an experiment;
• searching for ways to reduce the number of steps in an experiment;
• improving yields;
• recycling and reusing materials when possible;
• coordinating work with co-workers who may be using some of the same chemicals (section 5.D.2);
• considering the amount of reagents, solvents, and hazardous materials used by automated laboratory equipment when purchasing a new system;
• isolating nonhazardous waste from hazardous waste; and
• using a column purification system for recycling of used solvent (section 5.D.3)
These steps are increasingly important because of the changing requirements and economics of laboratory management.
5.B.2 Microscale Work and Wet Chemistry Elimination
One successful method of reducing hazards is to carry out chemical reactions and other laboratory procedures on a smaller scale (i.e., microscale) when feasible. In microscale chemistry the amounts of materials used are reduced to 25 to 100 mg for solids and 100 to 200 µL for liquids, compared with the usual 10 to 50 g for solids or 100 to 500 mL for liquids. Smaller scale synthetic methods save money because they require less reagent and result in less waste. Of course, not all laboratory procedures can be scaled down. Multigram laboratory preparation is often required to provide
sufficient material for further work. Whether large or small scale, exercise precaution appropriate to the scale, as well as the inherent hazards, of the procedure.
Similarly, in many cases instrumental analyses—which require little reagent and generate very little waste in themselves—can be substituted for wet chemistry. Consider the waste reduction inherent in spectroscopic organic analysis versus chemical derivatization. And, hazardous waste reduction also reduces both compliance and disposal costs. When purchasing equipment to automate laboratory processes, choose equipment that is efficacious for the job at hand, but uses the least amount of reagents or solvents, or uses materials that are least hazardous. (See Vignette 5.1.)
5.B.2.1 Design Less Hazardous Laboratory Processes and Reaction Conditions
The third principle of green chemistry suggests that, where possible, syntheses should be designed using less toxic reagents. Although the use of a toxic reagent does not necessarily imply generation of a toxic waste, in line with the first principle, chemists should evaluate potential sources of hazardous waste expected from the proposed synthesis and incorporate strategies to minimize them.
VIGNETTE 5.1
Pollution prevention reduces solvent waste
A pollution prevention assessment of one organic chemistry research laboratory at a university revealed that each of the 25 researchers in the group used 1 L of solvent, usually acetone, every week to clean and/or rinse glassware, spatulas, and other items used in their procedures. For example, a researcher might rinse a spatula with acetone at the end of a procedure or use a solvent to speed the drying process after cleaning with soap and water. The excuses for using the solvent ranged from not having enough glassware available (thus the need to expedite drying) to lack of good brushes for cleaning residue to simply taking a shortcut to the cleaning process.
The lab purchased more glassware, better brushes, and an ultrasonicator that uses a mild detergent. The savings in solvent purchase and disposal paid back the price of the new purchases within 3 months. Later, the lab installed under-the-bench lab dishwashers, which resulted in even further reductions in solvent use for cleaning.
5.B.3 Use Safer Solvents and Other Materials
Traditionally, chemists have chosen reagents and materials to meet scientific criteria without always giving careful consideration to waste minimization or environmental objectives. In synthetic procedures, overall yield and purity of the desired product are important factors, because better yield implies lower cost. On the other hand, material substitution can be an important consideration in manufacturing process design because of the large quantity, and potential cost, of chemicals involved. The following questions should be considered when choosing a material to be used as a reagent or solvent in an experimental procedure:
• Can this material be replaced by one that will expose the experimenter, and others who handle it, to less potential hazard?
• Can this material be replaced by one that will reduce or eliminate the hazardous waste and the resulting cost of waste disposal?
• Can these steps be taken in conjunction with yield maximization and minimization of overall waste and cost?
All things being equal, laboratories are safer when they substitute nonhazardous, or less hazardous, chemicals where possible by considering alternative synthetic routes and alternative procedures for working up reaction mixtures. The following additional examples illustrate the application of this principle to common laboratory procedures:
• To reduce the amount of copper released to the sewer, use iron complexes rather than copper when studying spectrophotometry in general chemistry.
• In liquid scintillation counting of low-level radioactive samples, where possible, use nonflammable, lower toxicity, water-miscible solvents rather than xylene, toluene, or dioxane, so as to eliminate fire hazard and waste that must be incinerated.
• Substitute solid or liquid reagents for hazardous gases that must be used at elevated pressure. As an example, phosgene is a highly toxic gas occasionally used as a reagent in organic transformations. Its use requires proper precautions to contain the gas and handle and dispose of cylinders. Commercially available products such as diphosgene (trichloromethyl) chloroformate, a liquid, or triphosgene bis(trichloromethyl) carbonate, a low-melting solid, are often substituted for phosgene by appropriate adjustment of experimental conditions or are used to generate
phosgene only on demand. Both chemicals are highly toxic themselves, and their use in any event should be considered carefully, but solids avoid the problems associated with handling a toxic gas.
• Consider carefully the use of reagents containing toxic heavy metals. For example, proprietary detergents for glassware (used, if necessary, with ultrasonic baths) are a safer substitute for chromic acid cleaning solutions. Various chromium(VI) and other metal oxidants have been important in synthetic organic chemistry, but other oxidants are possible substitutes. When planning a reaction, consider the cost of disposal of heavy metal waste in addition to its utility. Search the literature for other oxidation reagents tailored to the specific needs of a given transformation. (For information about reducing the use of mercury in laboratory equipment, see section 5.B.8.)
• F-TEDA-BF4, or 1-chloromethyl-4-fluoro-1,4-diazoniabicyclo[2.2.2]octane bis(tetrafluoroborate), substitutes for more hazardous reagents in many fluorination procedures. To reduce the reactivity and toxicity risks associated with perchloryl fluoride, fluorine, and other fluorinating reagents, search the literature for appropriate substitutes.
• Avoid solvents listed as select carcinogens (for a definition of select carcinogens, see Chapter 4, section 4.C.3.4), reproductive toxins, or hazardous air pollutants. Choose solvents with relatively high American Conference of Governmental Industrial Hygienists threshold limit values. Recognizing that not all hazards can always be reduced simultaneously, the best substitute solvent meets needed experimental constraints but has physiochemical properties, such as boiling point, flash point, and dielectric constant that are similar to the original solvent. Although cost can be a factor, consider the benefits of safety, health, and the environment as well. For example, heptane is more costly than hexane, but is very similar physiochemically and is not listed by the U.S. Environmental Protection Agency (EPA) as a hazardous air pollutant. Toluene usually can substitute for the carcinogen benzene. Chemical suppliers now highlight solvents with lower hazards including reduced flammability and potential for peroxide formation.
• Supercritical fluids present an interesting case in conflicting green chemistry principles. Supercritical CO2 as a solvent involves a chemically relatively benign material, carbon dioxide. Reaction workup requires only ambient heat, and there is no hazardous waste. On the other hand, it requires elevated pressure. Supercritical solvents for chromatography and synthesis require specialized equipment for handling, but because of the ubiquity of chromatography methods operating at elevated pressure and the common nature of the pumps and vessels necessary, much of the hazard has been mitigated. The technology for using supercritical fluids has developed rapidly in recent years. Consider use of these materials, but with appropriate precaution and dedicated permanent equipment.
5.B.4 Design Experimental Products for Degradation After Use
Green chemistry practitioners plan synthesis and other processes so that, as part of the experiment, the products and byproducts are rendered safe or less hazardous. For example, they include in the experimental plan reaction workup steps that deactivate hazardous materials or reduce their toxicity.
5.B.5 Include Real-Time Controls to Prevent Pollution
To cut costs, firms are increasingly asking for justin-time delivery of raw materials and using other real-time controls. Green chemistry laboratories can borrow this strategy. A quantity of hazardous chemical not ordered is one to which trained laboratory personnel are not exposed, for which appropriate storage need not be found, which need not be tracked in an inventory control system, and which will not end up requiring costly disposal when it becomes a waste.
Part of acquiring a chemical is a life-cycle analysis. All costs associated with the presence of each chemical at an institution must be considered. The purchase cost is only the beginning; the handling costs, human as well as financial, and the disposal costs must be taken into account. Without close attention to these aspects of managing chemicals in a laboratory, orders are not likely to be minimized, and unused chemicals become a significant fraction of the laboratory’s hazardous waste.
The American Chemical Society’s booklet Less Is Better: Laboratory Chemical Management for Waste Reduction (Task Force on Laboratory Waste Management, 1993) gives several reasons for ordering chemicals in smaller containers, even if that means using several containers of a material for a single experiment:
• Consequence of breakage is substantially reduced for small package sizes.
• Risk of accident and exposure to hazardous material is less when handling smaller containers.
• Storeroom space needs are reduced when only a single size is inventoried.
• Containers are emptied faster, resulting in less chance for decomposition of reactive compounds.
• Use of the so-called “economy size” often dictates a need for other equipment, such as transfer containers, funnels, pumps, and labels. Added labor to subdivide the larger quantities into smaller containers, as well as additional personal protective equipment for the hazards involved, also may be needed. In most cases, it is safer, and may be less costly, to allow commercial providers to break bulk rather than “doing it yourself.”
• If unused hazardous material must be disposed of, the disposal cost per container is less for smaller containers.
An institution should also minimize the amount of chemical accepted as a gift or as part of a research contract. More than one laboratory has been burdened with the cost of disposing of a donated chemical that was not needed.
Donated material can easily become a liability. A chemical engineering researcher accepted a 55-gallon drum of an experimental diisocyanate as part of a research contract. The ensuing research project used less than 1 gallon of the material, and the grantor would not take the material back for disposal. No commercial incinerator would handle the material in its bulk form. The remaining material had to be transferred to 1-liter containers and sent as lab packs for disposal, at significant cost.
In section 5.D.2, the exchange or transfer of chemicals to other trained laboratory personnel is discussed. Smaller containers increase the chance that chemicals to be transferred are in sealed containers, which increases the receiver’s confidence that the chemicals are pure.
5.B.6 Minimize the Potential for Accidents
Green chemistry also means designing to reduce accidents, injuries, and exposures to laboratory, storeroom, and receiving personnel. Chapters 4 and 6 explain planning and risk assessment for laboratory personnel. Be sure that hazardous properties are understood before a material is purchased, synthesized, or otherwise acquired. Search references and the literature to be cognizant of the properties of explosivity, water and air reactivity, instability, age-related degradation, and pressurization when contained. Searches of historical laboratory accident data reveal risks associated with experimental setups, procedures, equipment, facilities, inadequate training, and noncompliance with safety rules. Trained laboratory personnel with this knowledge should communicate it to co-workers and material handling personnel. New laboratory personnel deserve a special orientation.
5.B.7 Green Chemistry Principles Avoid Multihazardous Waste Generation
Because the management of multihazardous waste is often difficult, prudent green chemistry principles minimize its generation. Chapter 8, sections 8.C.2 and 8.C.3, provides information on eliminating or minimizing the components of waste that are biological or radioactive hazards, respectively. For chemical– biological waste, the primary strategy for minimizing the multihazardous waste is to maintain segregation of chemical and biological waste streams as much as possible. For reduction of radioactive hazards, the strategies discussed include substituting nonradioactive materials for radioactive materials, substituting radioisotopes having shorter decay times (e.g., when radioactive iodine is specified, using iodine-131, with a half-life of 8 days, instead of iodine-125, with a half-life of 60 days), and carrying out procedures with smaller amounts of materials.
5.B.8 Mercury Replacements in the Laboratory
Chronic exposure to mercury (Chemical Abstracts Service [CAS] No. 7439-97-6) through any route can produce central nervous system damage (Mallinkrodt Baker, Inc., 2008). Common exposure routes include inhalation, ingestion, and skin or eye contact. Thermometers and manometers are the most common laboratory uses of elementary mercury, and in many cases, there are suitable nonmercury alternatives available. Broken thermometers and manometers create a health hazard in the laboratory and, where possible, should be replaced with mercury-free substitutes.
The consequences of broken mercury-filled equipment (thermometers, manometers, diffusion pumps, bubblers, etc.) can include personnel exposure, laboratory and environmental contamination, mercury spill cleanup, and disposal of mercury and mercury-contaminated debris. Mercury spills are challenging to clean up completely and require training and special spill control materials (see Chapter 6, section 6.C.10.8, for more information about mercury spill cleanup). Elemental mercury is very heavy and can be expensive to dispose as waste (Foster, 2005a). Replacing mercury-filled equipment in the laboratory ensures compliance with 2 of the 12 principles of green chemistry: No. 1, “Prevent Waste: Design chemical syntheses to prevent waste, leaving no waste to treat or clean up”; and No. 12, “Minimize the potential for accidents: Design chemicals and their forms (solid, liquid, or gas) to minimize the potential for chemical accidents including explosions, fires, and releases to the environment” (Anastas and Warner, 1998).
5.B.8.1 Thermometers
Design a mercury thermometer replacement program to provide safe, suitable substitutes for use in laboratories. Factors that should be considered during the mercury replacement process are various applications in the laboratories, required temperature range, thermometer length, immersion depth, scale divisions, cost, accuracy in relation to application, and durability upon exposure to corrosive solutions. In some cases, these alternative thermometers have a more limited temperature range than a mercury thermometer. Perform tests for accuracy in the laboratory prior to a total replacement program to ensure that the mercury substitutes will be suitable for the methods that will be employed in that particular laboratory. To ensure accuracy, thermometers must be calibrated using approved methods such as ASTM E 77 (ASTM International, 2007a) and must be traceable to the National Institute of Standards and Technology (NIST).
There is a wide selection of mercury-free liquid-filled thermometers available, including spirit thermometers (filled with biodegradable petroleum-based mineral spirits and dyes) and alcohol-based thermometers. When broken, these thermometers present no hazardous material disposal problems. Some spirit thermometers had a history of the thread breaking more easily than a mercury thermometer, but many of the newer formulations have overcome this problem. In the event that the thread breaks, the simplest and safest method to reunite the liquid is to use a centrifuge. Carefully insert the thermometer, bulb down, in the centrifuge. Use cotton wadding at the bottom of the cup to prevent any damage to the bulb. Turn on the centrifuge and in just a few seconds all the liquid will be forced past the separation. Note that if the cup is not deep enough, and all the centrifuge force is not below the column, the column will split, forcing half the liquid in the bulb and half the liquid in the expansion chamber (Izzo, 2002).
For liquid-filled thermometers used to measure the temperature of liquids, accuracy will also depend on choosing the correct immersion depth. This is less of an issue for mercury thermometers because mercury generally has better thermoconductivity. A total immersion thermometer is designed to indicate temperatures correctly when the bulb and all but 12 mm of the liquid column are immersed in the bath medium. The top 12 mm of the liquid column should be above the bath medium so that the thermometer can be read and the material does not distill at high temperatures. Thermometers that have been graduated for total immersion usually have no markings on the back pertaining to immersion. A partial immersion thermometer is designed to indicate temperatures correctly when the bulb and a specified portion of the stem are exposed to the temperature being measured and the remainder of the stem is exposed to the ambient temperature. Partial immersion thermometers are clearly marked with a permanently placed line on the stem to indicate the proper immersion depth (ASTM International, 2007a).
In addition to thermometers that are filled with mercury-alternative liquids, long-stem digital thermometers are available with probes that are resistant to most laboratory chemicals, including acids, bases, and solvents. The bright displays, usually ¼ in. high, are easy to read and display the temperature in both degrees Fahrenheit and degrees Celsius, with ranges from -58 to 302 °F and –50 to 150 °C. Long-stem thermometers are constructed of plastic and stainless steel and do not contain glass or mercury, which make them ideal thermometers for use in academic laboratories. The stems are generally 8 inches long with an overall thermometer length of 11 in.
5.B.8.2 Digital Thermometers
Where a mercury thermometer is the only option, armor cases, which protect against breakage without affecting accuracy, or Teflon-coated mercury thermometers are recommended. These are particularly useful in high-temperature ovens, oil baths, and autoclaves, where cleaning up a mercury spill can be challenging and the spill creates a serious health hazard.
5.B.8.3 Differential Manometers
Depending on the measurement range, labs can substitute water or calibrated oils for mercury. Pressure transducers or electronic pressure gauges may also be an alternative to a conventional manometer.
5.C.1 Ordering Chemicals
Authority to place orders for chemicals may be centralized in one purchasing office or may be dispersed to varying degrees throughout the institution. The advent of highly computerized purchasing systems, and even online ordering, has made it feasible to allow ordering at the departmental or research group level. However, the ability to control ordering of certain types of materials through a central purchasing system (e.g., prohibiting flammables in containers over a certain size or ensuring appropriate licensing of radioactive material users) is almost completely lost when the purchasing function is decentralized. In these cases, other creative ways of exercising control need to be found.
Before purchasing a chemical, prudent laboratory personnel ask several questions:
• Is the material already available from another laboratory within the institution or from a surplus-chemical stockroom? If so, waste is reduced, and the purchase price is saved. The tendency to use only new chemicals because of their purity should be scrutinized, and that tendency should be carefully justified to ensure that materials already on hand are used whenever possible.
• What is the minimum quantity that will suffice for current use? Chemical purchases should not be determined by the cheaper unit price basis of large quantities but rather by the amount needed for the experiment. The cost of disposing of the excess is likely to exceed any potential savings gained in a bulk purchase (i.e., the cost of getting rid of a chemical may exceed its acquisition cost). If a quantity smaller than the minimum offered by a supplier is needed, the supplier should be contacted and repackaging requested. Compressed gas cylinders, including lecture bottles, should normally be purchased from suppliers who accept return of empty cylinders. If paying demurrage charges, the laboratory may want to return partially filled cylinders that will not be used in the near future.
• What is the maximum size container allowed in the areas where the material will be used and stored? Fire codes and institutional policies regulate quantities of certain chemicals, most notably flammables and combustibles. For these materials, a maximum allowable quantity for laboratory storage has been established (see also sections 5.E.5 and 5.E.6).
• Can the chemical be managed safely when it arrives? Does it require special storage, such as in a drybox, refrigerator, or freezer? Do receiving personnel need to be notified of the order and given special instructions for receipt? Will any special equipment necessary to use the chemical be ready when it arrives? An effort should be made to order chemicals for just-in-time delivery by purchasing all unstable or extremely reactive materials from the same supplier with a request for one delivery at the best time for performing an experiment.
• Does the chemical present any unique security risks? Is it a controlled substance? Is there a risk of potential intentional misuse of the chemical? Will the quantity ordered affect compliance with the U.S. Department of Homeland Security (DHS) Chemical Facility Anti-Terrorism Standard (CFATS)? (See Chapter 10 for a discussion of laboratory security.)
• Is the chemical unstable? Inherently unstable materials may have very short storage times and should be purchased just before use to avoid losing a reagent and creating an unnecessary waste of material and time. Some materials may require express or overnight delivery and will not tolerate being held in transit over a weekend or holiday.
• Can the waste be managed satisfactorily? A chemical that produces a new category of waste may cause problems for the waste management program. An appropriate waste characterization and method for proper disposal should be identified before the chemical is ordered.
Within an institution or organization, one of the advantages of computerization of ordering is that information about deliveries of chemicals can be retrieved from the chemical supplier, which provides a clear picture of the purchasing history and distribution of chemicals across buildings. Some institutions include in their annual contracts with suppliers a requirement to report on a monthly, a quarterly, or an annual basis the quantity of each type of chemical purchased and the location to which it was delivered. This information can be helpful in preparing the various annual reports on chemical use that may be required by federal, state, or local agencies. For example, centralized ordering may assist the institution in complying with the Controlled Substances Act and with CFATS. In addition, such a system is also useful for tracking the use of flammables, locations of Food and Drug Administration drug precursors, and DHS chemicals of interest. [See Handbook of Chemical Health and Safety (Alaimo, 2001); Code of Federal Regulations, 1998.]
A purchase order for a chemical should include a request for a material safety data sheet (MSDS). However, many of the larger laboratory chemical suppliers send each MSDS only when an organization first orders the chemical. Subsequent orders of the same chemical are not accompanied by the MSDS. Therefore, a central network of accessible MSDSs should be established. This collection of MSDSs can be electronic if computer access is available to all employees at all times.
5.C.2 Receiving Chemicals
Chemicals arrive at institutions in a variety of ways, including U.S. mail, commercial package delivery, express mail services, and direct delivery from chemical warehouses. Deliveries of chemicals should be confined to areas that are equipped to handle them, usually a loading dock, receiving room, or laboratory. Proper equipment for receipt of chemicals includes chains for temporarily holding cylinders and carts designed to safely move various types of chemical containers. Shelves, tables, or caged areas should be designated for packages to avoid damage by receiving room vehicles. Chemical deliveries should not be made to depart-
mental offices because, in general, offices are unlikely to be equipped to receive these packages. However, if delivery to such an office is the only option, a separate undisturbed location, such as a table or shelf, should be identified for chemical deliveries, and the person ordering the material should be notified immediately on its arrival.
Receiving room, loading dock, and clerical personnel should to be trained adequately to recognize hazards that may be associated with chemicals coming into the facility. They need to know what to do if a package is leaking or if there is a spill in the receiving facility, and they need to know who to call for assistance when a problem develops. They should also be trained to identify activity that could suggest a security risk, such as unauthorized personnel near the loading dock or unwarranted interest in their activities. The Department of Transportation (DOT) requires training for anyone involved in the movement of hazardous materials, including individuals who have been designated to receive hazardous materials on behalf of the organization (see Chapter 11, sections 11.E.1.5, and 11.F.1).
Your firm or institution should decide if stockroom or laboratory personnel are responsible for unpacking incoming chemicals. Incoming packages should be promptly opened and inspected to ensure that containers are sealed in good condition and to confirm what was ordered. The unpacked chemicals should be stored safely. In particular, reactive chemicals shipped in metal containers (e.g., lithium aluminum hydride, sodium peroxide, phosphorus)—which are often sealed—must be promptly unpacked and stored to prevent degradation and corrosion and to be available for periodic inspection.
Transportation of chemicals within the facility, whether by internal staff or outside delivery personnel, must be done safely. Single boxes of chemicals in their original packaging can be hand carried to their destination if they are light enough to manage easily. Groups of packages or heavy packages should be transported on a cart that is stable, has straps or sides to contain packages securely, and has wheels large enough to negotiate uneven surfaces easily. Suitable carriers (e.g., secondary containment) should be used when transporting individual containers of liquids.
Cylinders of compressed gases should always be secured on specially designed carts and never be dragged or rolled. The cap should always be securely in place. Whenever possible, chemicals and gas cylinders should be moved on freight elevators that are not used for public occupancy, especially when moving toxic, cryogenic, or asphyxiating gases.
If outside delivery personnel do not handle materials according to the receiving facility’s standards, immediate correction should be sought, or other carriers or suppliers should be used. Original purchase order should specify delivery criteria. Some examples of delivery criteria would be that the gas cylinder must have a cap and the cap must not be stuck, and damaged containers may not be accepted without the inspection and approval of a technically qualified individual on-site.
When packages are opened in the laboratory, laboratory personnel should verify that the container is intact and is labeled, at a minimum, with an accurate name on a well-adhered label. For unstable materials, and preferably for all materials, the date of receipt should be on the label. Labels placed by the manufacturer should remain intact. New chemicals should be entered into the laboratory’s inventory promptly and moved to the appropriate storage area.
5.D INVENTORY AND TRACKING OF CHEMICALS
5.D.1 General Considerations
Prudent management of chemicals in any laboratory is greatly facilitated by keeping an accurate inventory of the chemicals stored. An inventory is a record (usually a database) that lists the chemicals in the laboratory, along with information essential for their proper management. Chemical inventories are also a vital tool, and in some cases are required, for maintaining regulatory compliance. An organization cannot adequately manage safety, security, emergency planning, waste disposal, and the like without knowing what chemicals are on-site and where they are stored. Without an up-to-date inventory of chemicals, many important questions pertinent to prudent management of chemicals can be answered only by visually scanning container labels. A well-managed inventory system promotes economical use of chemicals by making it possible to determine immediately what chemicals are on hand. An inventory is not limited to materials obtained from commercial sources but includes chemicals synthesized in a laboratory. If a chemical is on hand, the time and expense of procuring new material are avoided. Information on chemicals that present particular storage or disposal problems facilitates appropriate planning for their handling. Although a detailed list of hundreds or thousands of chemicals stored in a particular location may not be directly useful to emergency responders, it can be used to prepare a summary of the types of chemicals stored and the hazards that might be encountered. In larger organizations where chemicals are stored in multiple locations, the inventory system should include the storage location for each container of each chemical. An inventory system is also of use when considering laboratory security concerns. It can assist in ensuring compliance with regulations, such
as CFATS (see Chapter 10), tracking of materials to ensure that they are not intercepted en route, and in identification of unusual orders within the department or organization.
If procedures for the facile updating of information on storage locations are developed, the system becomes a tracking system. Such a system promotes the sharing of chemicals originally purchased by different research groups or laboratories. The more laboratories in an organization agree to share chemicals, the greater the likelihood that items unneeded in one location will be used elsewhere. Tracking systems are more complex to establish than simple inventories and require more effort to maintain, but their favorable impact on the economics and efficiency of chemical use in a large organization often justify their use.
Each record in a chemical inventory database generally corresponds to a single container of a chemical rather than merely to the chemical itself. This approach allows for a more logical correspondence between the records in the database and the chemicals stored in the laboratory. The following data fields for each item are recommended for any system:
• name as printed on the container;
• molecular formula, for further identification and to provide a simple means of searching;
• CAS registry number, for unambiguous identification of chemicals despite the use of different naming conventions;
• source; and
• size of container or original quantity of chemical.
In addition, the following information may be useful:
• hazard classification, as a guide to safe storage, handling, and disposal;
• date of acquisition, to ensure that unstable chemicals are not stored beyond their useful life;
• storage location, in laboratories where multiple locations exist; and
• on-site owner or staff member responsible for the sample.
In a chemical tracking system, how the consumption of chemicals is tracked must be considered. The effort involved in maintaining data on the precise contents of each container must be weighed against the potential benefit such a system would provide. Many tracking systems omit this information and record only the container size.
A simple inventory system records the above information for each container on index cards, which are then kept in an accessible location in some logical order, such as by molecular formula. The ease of searching such a card file is limited by its size and the order in which it is sorted. This type of system has obvious advantages in terms of simplicity and low cost, but it suffers several limitations. Listings of chemicals must be prepared manually, and the integrity of the database depends on how well the card file is maintained.
For an inventory of more than a few hundred chemicals, a computer-based system offers advantages. Many spreadsheet and database programs maintain an effective chemical inventory system, cross-referenced by different scientific or common names. The integrity of the inventory system is enhanced by the ease of making backup copies of the database. Searches for desired chemicals are carried out in a number of ways, depending on the software. The ability to search and sort the database, for example, by hazard classification, acquisition date, owner, or other parameters, and to prepare lists of the results of such a sort contribute to efficiency in a variety of chemical management tasks. Section 5.C.1 notes the prudence of establishing a central network of MSDSs. Including MSDSs and laboratory chemical safety summaries (LCSSs) (see Chapter 4 and accompanying CD) in the inventory’s database is highly desirable. Alternatively, the inventory could be linked to other databases containing safety and environmental information about the chemicals. The quality of MSDSs varies significantly from one manufacturer to another. LCSSs, which are targeted to the needs of typical trained laboratory personnel, are a useful supplement to the information provided by MSDSs.
Having a fully capable chemical tracking system depends on careful selection of database software. Such a package should permit access from multiple terminals or networked computers and, most importantly, have a foolproof efficient method for rapidly recording the physical transfer of a chemical from one location to another. Bar-code labeling of chemical containers as they are received provides a means of rapid error-free entry of information for a chemical tracking system. If reagent chemical suppliers were to adopt a system in which chemical containers were labeled with bar codes providing essential information on their products, the maintenance of chemical tracking systems would be greatly facilitated. Proprietary software packages for tracking chemicals are available. Organizations operating under good laboratory practice regulations may even want to track the quantity of material in each container. The investment in hardware, software, and personnel to set up and maintain a chemical inventory tracking system is considerable but pays significant dividends in terms of economical and prudent management of chemicals.
As with any database, the usefulness of an inventory or chemical tracking system depends on the integrity of the information it contains. If an inventory system
is used to locate chemicals for use or sharing in the laboratory, even a moderate degree of inaccuracy erodes confidence in the system and discourages use. The need for high fidelity of data is greater for a tracking system, because trained laboratory personnel will rely on it to save time locating chemicals rather than physically searching. For these reasons, appropriate measures should be taken periodically to purge any inventory or tracking system of inaccurate data. A physical inventory of chemicals stored, verification of the data on each item, and reconciliation of differences are performed annually. This procedure coincides with an effort to identify unneeded, outdated, or deteriorated chemicals and to arrange for their disposal. The following guidelines for culling inventory may be helpful:
• Consider disposing of materials not expected to be used within a reasonable period, for example, 2 years. For stable, relatively nonhazardous substances with indefinite shelf lives, a decision to retain them in storage should take into account their economic value, scarcity, availability, and storage costs.
• Make sure that deteriorating containers or containers in which evidence of a chemical change in the contents is apparent are inspected and handled by someone experienced in the possible hazards inherent in such situations.
• Dispose of or recycle chemicals before the expiration date on the container.
• Replace deteriorating labels before information is obscured or lost to ensure traceability and appropriate storage and disposal of the chemicals.
• Because many odoriferous substances make their presence known despite all efforts to contain them, aggressively purge such items from storage and inventory.
• Aggressively cull the inventory of chemicals that require storage at reduced temperature in environmental rooms or refrigerators. Because these chemicals may include air- and moisture-sensitive materials, they are especially prone to problems that are exacerbated by the effects of condensation.
• Dispose of all hazardous chemicals at the completion of the laboratory professional’s tenure or transfer to another laboratory. The institution’s cleanup policy for departing laboratory researchers and students should be enforced strictly to avoid abandoned unknowns that pose unknown hazards to remaining personnel and have high disposal costs.
• Develop and enforce procedures for transfer or disposal of chemicals and other materials when decommissioning laboratories because of renovation or relocation. Try to avoid receiving entire chemical inventories from decommissioned laboratories and do not donate entire chemical inventories to schools or small businesses.
Chemical inventory challenges have not changed since the first use of index card files. The initial challenge is ensuring that every laboratory chemical gets entered into the inventory. This task often requires the concerted effort of many staff members. The second challenge is keeping the inventory current. Meeting this challenge usually requires designating one or more responsible individuals to enter new materials into the system; these individuals are the only personnel who should have write/edit access to the inventory. Facility procedures must make sure that notice of all new materials is presented to these designated individuals for entry into the inventory. Assuming that every staff member will faithfully enter new chemicals into the system results in an obsolete inventory. A third challenge is making sure that consumed chemicals, that is, empty containers, are removed from the active inventory.
Inventories are valuable to laboratory operations if everyone supports and contributes to the inventory. Managers with budgetary responsibilities appreciate the value of an established inventory system in reducing procurement and operating costs. Laboratory waste coordinators favor more efficient use of in-house materials resulting in reduced quantities of waste.
More information about chemical management systems can be found in Chapter 2, section 2.D.4.
5.D.2 Exchange of Chemicals Between Laboratories and Stockrooms
The exchange or transfer of chemicals between laboratories at an institution depends on the kind of inventory system and central stockroom facilities in place. Some institutions encourage laboratory personnel to return materials to the central stockroom for redistribution to others. The containers are sealed or open with a portion of the material used. Containers that have been opened are often of sufficient purity to be used as is in many procedures. If the purity is in doubt, the person who returned the material should be consulted. The stockroom personnel can update the central inventory periodically to indicate what is available for exchange or transfer. For an exchange program to be effective, all contributors to and users of the facility must reach a consensus on the standards to be followed concerning the labeling and purity of stored chemicals.
A word of caution is offered in regard to surplus-chemical stockrooms; they must be managed with the same degree of control as a new-chemical storage area.
The surplus-chemical stockroom is not a depository for any chemical that will not be wanted in the laboratory within a reasonable period (e.g., 2 to 3 years); such materials are to be disposed of properly. Rooms that are used as general depositories of unwanted chemicals become mini-Superfund sites because of lack of control.
Academic institutions could recycle common organic solvents from one research laboratory to another, or from research laboratories to teaching laboratories. For example, chromatography effluents such as toluene could be collected from research laboratories, distilled, and checked for purity before reuse. Commercial distillation systems are available for such purposes, but laboratory personnel performing the distillations or working in the immediate vicinity need appropriate training. (See Chapter 7 for hazards associated with distillation.)
Laboratory-to-laboratory exchange can be an effective alternative to a central surplus-chemical stockroom in organizations unwilling or unable to manage a central storeroom properly. In such a system, trained laboratory personnel retain responsibility for the storage of unwanted chemicals but notify colleagues periodically of available materials. A chemical tracking system as described above facilitates an exchange system greatly. If colleagues within the same laboratory are using the same hazardous material, particularly one that is susceptible to decomposition on contact with air or water, they should try to coordinate the timing of their experiments.
5.D.3 Recycling of Chemicals and Laboratory Materials
5.D.3.1 General Considerations
Chemical recycling takes many forms. In each case a material that is not quite clean enough to be used as is must be brought to a higher level of purity or changed to a different physical state.
Recycling occurs on-site or off-site. On-site recycling occurs at the laboratory or at a central location that collects recyclables from several laboratories. Because on-site recycling can be very time and energy intensive, it may not be economically justifiable. In some cases, although the amount of waste may be quite small, it can require very expensive disposal if a commercial vendor must be used. Before a decision on recycling is made, the cost of avoided waste disposal should be calculated. Because of the difficulty of maintaining the needed level of cleanliness and safety, on-site recycling of mercury and other toxic metals is no longer recommended. Another significant issue is whether recycling activities require a waste treatment permit under the Resource Conservation and Recovery Act (RCRA). More information about this regulation can be found in Chapters 8 and 11. State and local regulations must also be considered.
Off-site commercial firms recycle, reclaim, purify, and stabilize vacuum pump oil, solvents, mercury, rare materials, and metals. Off-site recycling is preferable to disposal, and sometimes is less expensive. Another off-site option is to work with suppliers of laboratory chemicals who accept return of unopened chemicals, including highly reactive chemicals. Gas suppliers sometimes accept returns of partially used cylinders.
A general comment applicable to all recycling is that a recyclable waste stream needs to be kept as clean as possible. If a laboratory produces a large quantity of waste xylene, small quantities of other organic solvents should be collected in a separate container, because the distillation process gives a better product with fewer materials to separate. Steps should also be taken to avoid getting mercury into oils used in vacuum systems, and oil baths. Similarly, certain ions in a solution of waste metal salts have a serious negative impact on the recrystallization process. Identify users for a recycled product before time and energy are wasted on producing a product that must still be disposed of as a waste. Recycling some of the chemicals used in large undergraduate courses is especially cost-effective because the users are known well in advance.
Many recycling processes result in some residue that is not reusable and will probably have to be handled as a hazardous waste.
5.D.3.2 Solvent Recycling
Because the choice of a distillation unit for solvent recycling is controlled largely by the level of purity desired in the solvent, know the intended use of the redistilled solvent before equipment is purchased. A simple flask, column, and condenser setup may be adequate for a solvent that will be used for crude separations or for initial glassware cleaning. For a much higher level of purity, a spinning band column is probably required. Stills with automatic controls that shut down the system under conditions such as loss of cooling or overheating of the still pot are highly recommended, because they enhance the safety of the distillation operation greatly. Overall, distillation is likely to be most effective when fairly large quantities (roughly 5 L) of relatively clean single-solvent waste are accumulated before the distillation process is begun.
5.D.3.3 Recycling Containers, Packaging, and Labware
Laboratory materials other than chemicals, such as containers or packaging materials and parts of labora-
tory instruments, can also be recycled. Examples include certain clean glass and plastic containers, drums and pails, plastic and film scrap, cardboard, office paper, lightbulbs, circuit boards, other electronics, and metals such as steel and aluminum. Note that an empty container may still be subject to management requirements. See the following regulations: 40 CFR § 261.7 (EPA “empty”); 49 CFR § 173.29 (DOT “empty”); 49 CFR §§ 173.12(c) and 173.28 (DOT “reuse”).
5.D.4 Labeling Commercially Packaged Chemicals
Warning: Do not remove or deface any existing labels on incoming containers of chemicals and other materials.
Commercially packaged (by U.S. manufacturers) chemical containers received from 1986 onward generally meet current labeling requirements. The label usually includes the name of the chemical and any necessary handling and hazard information. Inadequate labels on older containers should be updated to meet current standards. To avoid ambiguity about chemical names, many labels carry the CAS registry number as an unambiguous identifier and this information should be added to any label that does not include it. On receipt of a chemical, the manufacturer’s label is supplemented by the date received and possibly the name and location of the individual responsible for purchasing the chemical. If chemicals from commercial sources are repackaged into transfer vessels, the new containers should be labeled with all essential information on the original container.
5.D.5 Labeling Other Chemical Containers
The overriding goal of prudent practice in the identification of laboratory chemicals is to avoid abandoned containers of unknown materials that may be expensive or dangerous to dispose of. The contents of all chemical containers and transfer vessels, including, but not limited to, beakers, flasks, reaction vessels, and process equipment, should be properly identified. The labels should be understandable to trained laboratory personnel and members of well-trained emergency response teams. Labels or tags should be resistant to fading from age, chemical exposure, temperature, humidity, and sunlight.
Chemical identification and hazard warning labels on containers used for storing chemicals should include the following information:
• identity of the owner,
• chemical identification and identity of hazard component(s), and
• appropriate hazard warnings.
Materials transferred from primary (labeled) bulk containers to transfer vessels (e.g., safety cans and squeeze bottles) should be labeled with chemical identification and synonyms, precautions, and first-aid information.
Label containers in immediate use, such as beakers and flasks, with the chemical contents. All reactants should be labeled with enough information to avoid confusion between them.
5.D.6 Labeling Experimental Materials
Labeling all containers of experimental chemical materials is prudent. Because the properties of an experimental material are generally not completely known, do not expect its label to provide all necessary information to ensure safe handling.
The most important information on the label of an experimental material is the name of the researcher responsible, as well as any other information, such as a laboratory notebook reference, that can readily lead to what is known about the material. For items that are to be stored and retained within a laboratory where the properties of materials are likely to be well understood, only the sample identification and name are needed.
(For information about labeling samples for transport and shipping, see section 5.F.)
5.D.7 Use of Inventory and Tracking Systems in Emergency Planning
The most important information to have in an emergency is how to access a researcher who is knowledgeable about the chemical(s) involved. In addition, an organization’s emergency preparedness plan should include what to do in the event of a hazardous material release. The inventory and tracking systems and the ability to access and make use of them are essential to proper functioning of the plan in an emergency. The care taken in labeling chemicals is also extremely important. (See Chapter 6, section 6.C.10, for a detailed discussion of what to do in laboratory emergencies.)
5.E STORAGE OF CHEMICALS IN STOCKROOMS AND LABORATORIES
The storage requirements and limitations for stockrooms and laboratories vary widely depending on
• level of expertise of the employees,
• level of safety features designed into the facility,
• level of security designed into the facility,
• location of the facility and neighboring homes or buildings,
• nature of the chemical operations,
• accessibility of the stockroom,
• local and state regulations,
• insurance requirements, and
• building and fire codes.
Many local, state, and federal regulations have specific requirements that affect the handling and storage of chemicals in laboratories and stockrooms. For example, radioactive materials, consumable alcohol, explosives, dual-use materials, and hazardous waste have requirements ranging from locked storage cabinets and controlled access to specified waste containers and regulated areas. Stringent requirements may also be placed on an institution by its insurance carriers.
Controlled substances (e.g., narcotics and other controlled prescription drugs) used in research or with research animals have special requirements. The laboratory director must first register with the U.S. Drug Enforcement Agency (DEA) and with the relevant state agency to purchase, possess, or use a Schedule 1–5 controlled substance. Schedule 1 and 2 drugs (e.g., morphine, pentobarbital) must be stored in a safe that is bolted to the floor or wall. Schedule 3–5 drugs (e.g., chloral hydrate, phenobarbital) must be stored in a locked drawer or cabinet. Access should be limited to the laboratory director and, if necessary, no more than the one or two laboratory members who will be using the substance. Detailed inventory records must be kept up-to-date, including amounts purchased, used, left on hand, and disposed of. Contact your local DEA office for disposal instructions. In some cases a DEA agent must witness disposal or packaging for shipment to a disposal facility.
5.E.1 General Considerations
In general, store materials and equipment in cabinets and on shelving designated for such storage:
• Avoid storing materials and equipment on top of cabinets. With all stored items, maintain a clearance of at least 18 inches from the sprinkler heads to allow proper functioning of the sprinkler system [see National Fire Protection Association Standard 13 (NFPA, 2010)].
• To make chemicals readily accessible and to reduce accidents caused by overreaching, do not store materials on shelves higher than 5 ft (~1.5 m). If retrieving materials stored above head level, use a step stool.
• Store heavy materials on lower shelves. While recommended for all laboratories, this is particularly important in areas where seismic activity is possible because items may fall during an earthquake.
• Keep exits, passageways, areas under tables or benches, and emergency equipment areas free of stored equipment and materials to allow for ease of egress and access in case of emergency.
Storing chemicals in stockrooms and laboratories requires consideration of a number of health and safety factors. In addition to the inventory control and storage area considerations discussed above, proper use of containers and equipment is crucial (see section 5.E.3).
In addition to the basic storage area guidelines above, follow these general guidelines when storing chemicals:
• Label all chemical containers appropriately to ensure that chemicals will be stored safely.
• Place the user’s name and the date received on all purchased materials to facilitate inventory control.
• To assist in maintaining a clean work environment and to ensure that segregation of incompatible chemicals is maintained, provide a definite storage place for each chemical and return the chemical to that location after each use.
• To avoid clutter, avoid storing chemicals on benchtops, except for those chemicals being used currently.
• To avoid clutter and to maintain adequate airflow, avoid storing chemicals in chemical hoods, except for those chemicals in current use.
• Store volatile toxic or odoriferous chemicals in a ventilated cabinet. Check with the institution’s environmental health and safety officer.
• Provide ventilated storage near laboratory chemical hoods.
• If a chemical does not require a ventilated cabinet, store it inside a closable cabinet or on a shelf that has a lip to prevent containers from sliding off in the event of a fire, serious accident, or earthquake.
• Do not expose stored chemicals to heat or direct sunlight.
• Observe all precautions regarding the storage of incompatible chemicals.
• Separate chemicals into compatible groups and store alphabetically within compatible groups. See Table 5.1 and Figure 5.1 for one suggested method for arranging chemicals. Because chemicals in storage are contained, their separation by compatibility groups can be simplified. The color-coded system described here allows for ease of storage. As explained in Chapter 6, compatibility precautions for mixing chemicals are far more complex.
• Store flammable liquids in approved flammable-liquid storage cabinets.
• Consider the security needs for the materials.
TABLE 5.1 Examples of Compatible Storage Groups
|
|
|
|
A: Compatible Organic Bases Diethylamine Piperidine Triethanolamine Benzylamine Benzyltrimethylammonium hydroxide B: Compatible Pyrophoric & Water-Reactive Materials Sodium borohydride Benzoyl chloride Zinc dust Alkyl lithium solutions such as methyl lithium in tetrahydrofuran Methanesulfonyl chloride Lithium aluminum hydride C: Compatible Inorganic Bases Sodium hydroxide Ammonium hydroxide Lithium hydroxide Cesium hydroxide D: Compatible Organic Acids Acetic acid Citric acid Maleic acid Propionic acid Benzoic acid E: Compatible Oxidizers Including Peroxides Nitric acid Perchloric acid Sodium hypochlorite Hydrogen peroxide 3-Chloroperoxybenzoic acid |
F: Compatible Inorganic Acids not Including Oxidizers or Combustibles Hydrochloric acid Sulfuric acid Phosphoric acid Hydrogen fluoride solution J: Poison Compressed Gases Sulfur dioxide Hexafluoropropylene K: Compatible Explosives or Other Highly Unstable Materials Picric acid dry(<10% H2O) Nitroguanidine Tetrazole Urea nitrate L: Nonreactive Flammables and Combustibles, Including Solvents Benzene Methanol Toluene Tetrahydrofuran X: Incompatible with ALL Other Storage Groups Picric acid moist (10-40% H2O) Phosphorus Benzyl azide Sodium hydrogen sulfide |
|
|
|
NOTE: A larger list of examples can be found on the CD that accompanies this book.
SOURCE: Adapted from Stanford University’s Chem Tracker Storage System. Used with permission from Lawrence M. Gibbs, Stanford University.
Some chemicals are regulated by federal agencies and require locked cabinets or storage in secure areas.
5.E.2 Storage According to Compatibility
It is prudent to store containers of incompatible chemicals separately. Separation of incompatibles will reduce the risk of mixing in case of accidental breakage, fire, earthquake, or response to a laboratory emergency. Even when containers are tightly closed, fugitive vapors can cause deleterious incompatibility reactions that degrade labels, shelves, cabinets, and containers themselves. As discussed in Chapter 4, a far more detailed review of incompatibilities needs to be done when chemicals are deliberately mixed,
Figure 5.1 (also available on the CD accompanying this book) and Table 5.1 show an example of a detailed classification system for the storage of groups of chemicals by compatibility. The system classifies chemicals into 11 storage groups. Each group should be separated by secondary containment (e.g., plastic trays) or, ideally, stored in its own storage cabinet. According to this system, it is most important to separate storage groups B (compatible pyrophoric and water-reactive chemicals) and X (incompatible with all other storage groups). These two groups merit their own storage cabinets. The accompanying compact disc includes a spreadsheet of hundreds of chemicals listed according to these storage groups.
There are other good classification systems for storing chemicals according to compatibility. At a minimum, always store fuels away from oxidizers. In other systems, the following chemical groups are kept separate by using secondary containment, cabinets, or distance:
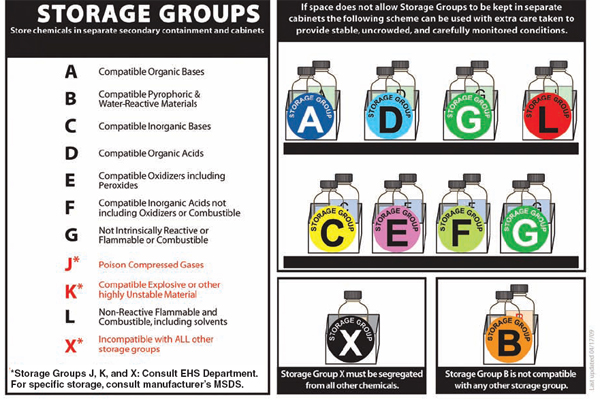
FIGURE 5.1 Compatible storage group classification system. This system should be used in conjunction with specific storage conditions taken from the manufacturer’s label and material safety data sheet. SOURCE: Adapted from Stanford University’s ChemTracker Storage System. Used with permission from Lawrence M. Gibbs, Stanford University. NOTE: Also available on the CD accompanying this book.
• oxidizers, including peroxides;
• corrosives—inorganic bases;
• corrosives—inorganic acids, not including oxidizers or combustibles;
• flammable materials;
• reproductive toxins;
• select carcinogens; and
• substances with a high degree of acute toxicity.
Depending on the chemicals, their amounts, and the activities of your laboratory, it may make sense to separate these alternative storage groups. Also be sure to follow any storage information on the container’s label or on the chemical’s MSDS.
In seismically active regions, storage of chemicals requires additional stabilization of shelving and containers. Shelving and other storage units should be secured and contain a front-edge lip to prevent containers from falling. Ideally, containers of liquids are placed on a metal or plastic tray that could hold the liquid if the container broke while on the shelf. All laboratories, not only those in seismically active regions, benefit from these additional storage precautions.
5.E.3 Containers and Equipment
Specific guidelines regarding containers and equipment to use in storing chemicals are as follows:
• Use of corrosion-resistant storage trays as secondary containment for spills, leaks, drips, or weeping is a good idea. Polypropylene trays are suitable for most purposes.
• Use secondary containment (i.e., an overpack) to retain materials if the primary container breaks or leaks.
• Provide vented cabinets beneath chemical hoods for storing hazardous materials. (This encourages the use of the hoods for transferring such materials.)
• Seal containers to minimize escape of corrosive, flammable, or toxic vapors.
5.E.4 Cold Storage
Safe storage of chemicals, biologicals, and radioactive materials in refrigerators, cold rooms, or freezers requires good labels, organization, and active manage-
ment. The laboratory director assigns responsibility for keeping these units safe, clean, and organized and monitors their proper operation. Extra care is required because frost and condensation not only obscure labels but also make containers hard to hold and easy to drop. Too often, research materials are stored haphazardly in cold storage areas. To ensure safety:
• Use chemical storage refrigerators only for storing chemicals.
• Use waterproof tape and markers to label laboratory refrigerators and freezers with the following:
NO FOOD—LAB CHEMICAL STORAGE ONLY
• Do not store flammable liquids in a refrigerator unless it is approved for such storage. Such refrigerators are designed not to spark inside the refrigerator. If refrigerated storage is needed inside a flammable-storage room, it is advisable to choose an explosion-proof refrigerator. Do not store oxidizers or highly reactive materials in the same unit as flammables.
• All containers must be closed and stable to reduce the risk of a spill. Round-bottom flasks need secondary containment.
• Label all materials in the refrigerator with contents, owner, date of acquisition or preparation, and nature of any potential hazard.
• Organize contents by owner but keep incompatibles separate. Organize by labeling shelves and posting the organization scheme on the outside of the unit.
• Secondary containment, such as plastic trays, is highly recommended for all containers. Secondary containment captures spills and leaks and facilitates organization and labeling.
• Every year, review the entire contents of each cold storage unit. Dispose of all unlabeled, unknown, or unwanted materials.
• When any trained laboratory personnel leaves, review the contents of each cold storage unit to identify that person’s material, so that it can be disposed of or reassigned.
5.E.5 Storing Flammable and Combustible Liquids
NFPA Standard 45 (NFPA, 2004) limits the quantity of flammable and combustible liquids in laboratories. (International, state, and local building codes and regulations should also be consulted.) The quantity allowed depends on a number of factors, including
• construction of the laboratory,
• number of fire control zones in the building,
• floor level where the laboratory is located,
• fire protection systems built into the laboratory,
• storage of flammable liquids in flammable-liquid storage cabinets or safety cans, and
• type of laboratory (i.e., instructional or research and development).
Many laboratories have a business (B) classification with sprinkler systems and a flammable and combustible liquid storage limitation, as shown in Table 5.2. Note that laboratory unit fire hazard classes are based on the quantities of flammable and combustible liquids in the space. This classification significantly affects the fire separation requirements for the laboratory. Most research laboratories fall under Class B, C, or D.
Note that some laboratories may be in jurisdictions that refer to the International Code Agency rather than NFPA, and state and local regulations may be more stringent than those cited here. Laboratory personnel and organization should be sure to check the requirements specific to their area.
The container size for storing flammable and combustible liquids is limited both by NFPA Standards 30 and 45 and by the Occupational Safety and Health Administration (OSHA). Limitations are based on the type of container and the flammability of the liquid, as shown in Table 5.3.
Label all chemical containers with the identity of the contents and hazard warning information. All chemical waste containers must have appropriate waste labels. Flammable liquids that are not stored in safety cans should be placed in storage cabinets rated for flammable storage. When space allows, store combustible liquids in flammable-storage cabinets. Otherwise, store combustible liquids in their original containers. Store 55-gal drums of flammable and combustible liquids in special storage rooms for flammable liquids. Keep flammable and combustible liquids away from strong oxidizing agents, such as nitric or chromic acid, permanganates, chlorates, perchlorates, and peroxides. Keep flammable and combustible liquids away from any ignition sources. Remember that many flammable vapors are heavier than air and can travel to ignition sources. Take the following additional precautions when storing flammable liquids:
• When possible, store quantities of flammable liquids greater than 1 L (approximately 1 qt, or 32 oz) in safety cans. Refer to Table 5.3.
• Store combustible liquids either in their original (or other NFPA- and DOT-approved) containers or in safety cans. Refer to Table 5.3.
| Laboratory Unit Fire Hazard Class | Class of Liquid | Excluding Quantities in Rated Storage Cabinets/Safety Cans (max per 100 ft2) | Including Quantities in Rated Storage Cabinets/Safety Cans (max per 100 ft2) | ||
| gal | L | gal | L | ||
| A (high fire hazard) | Class I flammable (flash point <100 °F) | 10 | 38 | 20 | 76 |
| Combined Class I, II, IIIA (flash point <200 °F) | 20 | 76 | 40 | 150 | |
| B (moderate fire hazard) | Class I flammable (flash point <100 °F) | 5 | 20 | 10 | 38 |
| Combined Class I, II, IIIA (flash point <200 °F) | 10 | 38 | 20 | 76 | |
| C (low fire hazard) | Class I flammable (flash point <100 °F) | 2 | 7.5 | 4 | 15 |
| Combined Class I, II, IIIA (flash point <200 °F) | 4 | 15 | 8 | 30 | |
| D (minimal fire hazard) | Class I flammable (flash point <100 °F) | 1 | 4 | 2 | 7.5 |
| Combined Class I, II, IIIA (flash point <200 °F) | 1 | 4 | 2 | 7.5 | |
NOTE: Limits for laboratories in health care occupancies and in K-12 educational facilities may be significantly lower.
SOURCE: Reproduced with permission from NFPA 45, Fire Protection for Laboratories Using Chemicals, Copyright 2004, National Fire Protection Association. This reprinted material is not the complete and official position of NFPA on the referenced subject, which is represented only by the standard in its entirety.
TABLE 5.3 Container Size for Storage of Flammable and Combustible Liquids
| Container | Flammable Liquidsa | Combustible Liquidsb | ||||||||
| Class IA | Class IB | Class IC | Class II | Class IIIA | ||||||
| L | gal | L | gal | L | gal | L | gal | L | gal | |
| Glassc,d | 0.5 | 0.12 | 1 | 0.25 | 4 | 1 | 4 | 1 | 20 | 5 |
| Metal/approved plasticd | 4 | 1 | 20 | 5 | 20 | 5 | 20 | 5 | 20 | 5 |
| Safety cansd | 10 | 2.6 | 20 | 5 | 20 | 5 | 20 | 5 | 20 | 5 |
NOTE: Label safety cans with contents and hazard warning information. Safety cans containing flammable or combustible liquid waste must have appropriate waste labels. Place 20-L (5-gal) and smaller containers of flammable liquids that are not in safety cans in storage cabinets for flammable liquids. Do not vent these cabinets unless they also contain volatile toxics or odoriferous chemicals. Aerosol cans that contain 21% (by volume), or greater, alcohol or petroleum-based liquids are considered Class IA flammables. When space allows, store combustible liquids in storage cabinets for flammable liquids. Otherwise, store combustible liquids in their original (or other Department of Transportation–approved) containers according. Store 55-gal drums of flammable and combustible liquids in special storage rooms for flammable liquids. Keep flammable and combustible liquids away from strong oxidizing agents, such as nitric or chromic acid, permanganates, chlorates, perchlorates, and peroxides. Keep flammable and combustible liquids away from an ignition source. Remember that most flammable vapors are heavier than air and can travel to ignition sources.
aClass IA includes those flammable liquids having flash points <73 °F and having a boiling point <100 °F, Class IB includes those having flash points <73 °F and having a boiling point 100 °F, and Class IC includes those having flash points ≥73 °F and <100 °F. Aerosol cans that contain 21% (by volume), or greater, alcohol or petroleum-based liquids are considered Class IA flammables.
bClass II includes those combustible liquids having flash points at ≥100 °F and <140 °F, Class IIIA includes those having flash points ≥140 °F and <200 °F, and Class IIIB includes those having flash points ≥200 °F.
cGlass containers as large as 1 gal can be used if needed and if the required purity would be adversely affected by storage in a metal or approved plastic container, or if the liquid would cause excessive corrosion or degradation of a metal or approved plastic container.
dIn educational and institutional laboratory work areas, containers for Class I or Class II liquids should not exceed 8 L (32.1 gal) for safety cans or 4 L (1 gal) for other containers.
SOURCE: Reproduced with permission from NFPA 45, Fire Protection for Laboratories Using Chemicals, Copyright© 2004, National Fire Protection Association. This reprinted material is not the complete and official position of NFPA on the referenced subject, which is represented only by the standard in its entirety.
5.E.6 Storing Gas Cylinders
Check applicable international, regional, or local building and fire codes to determine the maximum amount of gas to be stored in a laboratory. These limits vary by storage conditions and type of chemical.
With toxic and reactive gases, or large quantities of asphyxiating gases, a special gas cabinet may be required. Gas cabinets are designed for leak detection, safe change-outs, ventilation, and emergency release.
The following general precautions should be taken when storing compressed gas cylinders or lecture bottles:
• Always label cylinders with their contents; do not depend on the manufacturer’s color code. They may vary across companies.
• Securely strap or chain gas cylinders to a wall or benchtop. In seismically active areas, use more than one strap or chain.
• When cylinders are no longer in use, shut the valves, relieve the pressure in the gas regulators, remove the regulators, and cap the cylinders.
• Segregate gas cylinder storage from the storage of other chemicals.
• Do not store corrosives near gas cylinders or lecture bottles. Corrosive vapors from mineral acids can deface markings and damage valves.
• Keep incompatible classes of gases stored separately. Keep flammables away from reactives, which include oxidizers and corrosives. (For more information on storage of flammable gases, see Chapter 7, section 7.D.3.3)
• Segregate empty cylinders from full cylinders.
• Keep in mind the physical state—compressed, cryogenic, or liquefied—of the gases.
• Do not abandon cylinders in the dock storage areas.
• Return cylinders to the supplier when you are finished with them.
For commonly used laboratory gases, consider the installation of in-house gas systems. Such systems remove the need for transport and in-laboratory handling of compressed gas cylinders. Chapter 6, section 6.H, provides additional information on working with compressed gases in the laboratory.
5.E.7 Storing Highly Reactive Substances
Check applicable international, regional, or local building and fire codes to determine the maximum amount of highly reactive chemicals that can be stored in a laboratory. These limits vary by storage conditions and type of chemical. Follow these additional guidelines when storing highly reactive substances:
• Consider the storage requirements of each highly reactive chemical prior to bringing it into the laboratory.
• Consult the MSDSs or other literature in making decisions about storage of highly reactive chemicals.
• Bring into the laboratory only the quantities of material needed for immediate purposes (<3- to 6-month supply, depending on the nature and sensitivity of the materials).
• Label, date, and inventory all highly reactive materials as soon as received. Make sure the label states
DANGER! HIGHLY REACTIVE MATERIAL!
• Do not open a container of highly reactive material that is past its expiration date. Call your institution’s hazardous waste coordinator for special instructions.
• Do not open a liquid organic peroxide or peroxide former if crystals or a precipitate are present. Call your institution’s hazardous waste coordinator for special instructions.
• For each highly reactive chemical, determine a review date to reevaluate its need and condition and to dispose of (or recycle) material that degrades over time.
• Segregate the following materials:
ο oxidizing agents from reducing agents and combustibles,
ο powerful reducing agents from readily reducible substrates,
ο pyrophoric compounds from flammables, and
ο perchloric acid from reducing agents.
• Store highly reactive liquids in trays large enough to hold the contents of the bottles.
• Store perchloric acid bottles in glass or ceramic trays.
• Store peroxidizable materials away from heat and light.
• Store materials that react vigorously with water away from possible contact with water.
• Store thermally unstable materials in a refrigerator. Use a refrigerator with these safety features:
ο all spark-producing controls on the outside,
ο a magnetically locked door,
ο an alarm to warn when the temperature is too high, and
ο a backup power supply.
• Store liquid organic peroxides at the lowest possible temperature consistent with the solubility or freezing point. Liquid peroxides are particularly sensitive during phase changes. Follow the manufacturer’s guidelines for storage of these
highly hazardous materials. (See Chapter 4, section 4.D.)
• Inspect and test peroxide-forming chemicals periodically (these should be labeled with an acquisition or expiration date), and dispose of chemicals that have exceeded their safe storage lifetime.
• Store particularly sensitive materials or larger amounts of explosive materials in explosion relief boxes.
• Restrict access to the storage facility.
• Assign responsibility for the storage facility and the above responsibilities to one primary person and a backup person. Review this responsibility at least yearly.
5.E.8 Storing Highly Toxic Substances
Take the following precautions when storing carcinogens, reproductive toxins, and chemicals with a high degree of acute toxicity:
• Store chemicals known to be highly toxic in ventilated storage in unbreakable, chemically resistant secondary containment.
• Keep quantities at a minimum working level.
• Label storage areas with appropriate warning signs, such as
CAUTION! REPRODUCTIVE TOXIN STORAGE
or
CAUTION! CANCER-SUSPECT AGENT STORAGE
• Limit access to storage areas.
• Maintain an inventory of all highly toxic chemicals. Keep records of acquisition, use, possession, and disposal. Some localities require that inventories be maintained of all hazardous chemicals in laboratories.
Note: Facilities covered by the OSHA Laboratory Standard must use and store carcinogens, reproductive toxins, and chemicals with a high degree of acute toxicity in designated areas.
5.F TRANSFER, TRANSPORT, AND SHIPMENT OF CHEMICALS
U.S. and international regulations apply to the movement of chemicals, samples, and other research materials on public roads, by airplane, or by mail or other carrier. When moving these materials on-site, anyone personally transporting regulated materials between adjacent or neighboring buildings within an institution should walk. (Secondary containment, such as a rubber bucket, should always be used for carrying bottled chemicals.) Organizations located in a larger campus setting should have guidelines indicating if special courier or designated vehicles are to be used to transport regulated materials according to applicable regulations.
Samples of experimental material to be transferred outside the laboratory, or that may be handled by individuals not generally familiar with the type of material involved, should be labeled as completely as possible. In addition, hazardous samples sent to individuals at another institution must be accompanied by appropriate labeling and an MSDS, according to OSHA’s Hazard Communication Standard amendments and OSHA’s Laboratory Standard hazard identification provision, including the name, address, and contact information of the sender and recipient for samples in transit. When available, the following information should accompany experimental materials:
• Originator: List the name of the owner or individual who first obtained the material. If sending the material to another facility, add contact information for the person who can provide safe handling information.
• Identification: Include, at least, the laboratory notebook reference.
• Hazardous components: List primary components that are known to be hazardous.
• Potential hazards: Indicate all known or potential hazards.
• Date: Note the date that the material was placed in the container and labeled.
• Ship to: Indicate the name, location, and telephone number of the person to whom the material is being transferred.
When transporting or shipping most chemicals, biological agents, and radioactive materials, even small amounts or samples preserved in solvents or alcohol, domestically or internationally, please note that the DOT or the International Air Transport Association (IATA) regulations may apply. Before preparing any packages for shipment, personnel must have documented evidence that they have complete DOT and IATA training. DOT controls shipment of chemicals by a specific set of hazardous materials regulations, 49 CFR Parts 100-199 (updated 2006). These regulations contain detailed instructions on how to identify, package, mark, label, document, and placard hazardous materials. Shipments not in compliance with the applicable regulations may not be offered or accepted for transportation. The regulations on training for safe transportation of hazardous materials are located in
49 CFR § 172.700–172.704 (updated Oct. 1, 2006). All individuals who are preparing hazardous materials for shipment must communicate with their institution’s transportation coordinators. Shipment of experimental materials is also discussed in Chapter 11, sections 11.F.1 and 11.F.2.
The use of personal vehicles, company or institutional vehicles (including airplanes), and customer vehicles for transporting regulated materials, which may be hazardous, is a major concern. In many cases, handling regulated materials in this manner is prohibited by DOT or will require shipping papers, placarding, and/or other conditions. Most businesses and academic institutions forbid the use of privately owned personal vehicles, because of the serious insurance consequences if an accident occurs. Most individuals will find that their personal vehicle insurance does not cover them when they are transporting hazardous materials.
Shipping chemicals by air is regulated by IATA. An individual who holds IATA certification must inspect the packaging, review the paperwork, and sign the shipping papers. For domestic shipping by ground or rail, DOT regulations apply and may require a bill of lading or manifest, placarding, special packaging, and other conditions.
Be aware that international transfer of chemicals and research materials is regulated by EPA, the Department of Commerce, and the U.S. Customs Bureau as imports and exports. Federal and international laws strictly regulate domestic and international transport of samples, specimens, drugs, and genetic elements, as well as research equipment, technologies, and supplies—even if the material is not hazardous, valuable, or uncommon. Mail, shipments, and luggage are being screened for these materials. Packages to or from research institutions receive additional scrutiny, as well as any package that appears to contain bottles or liquids.
Chapter 8 describes the requirements for shipping hazardous waste.
5.F.1 Materials of Trade Exemption
DOT has an exception to many requirements for transportation of hazardous materials, referred to as the “materials of trade” (MOT) exemption, which applies to the transportation of small quantities of hazardous materials that are part of your business. Examples include the following:
• facilities maintenance services (i.e., paints and paint thinners for painters and gasoline for groundskeepers),
• researchers (i.e., preservatives for field samples), and
• educational demonstrations (i.e., chemicals for public school outreach education programs).
Under this exemption, it is permissible to transport your own hazardous materials as long as certain conditions are met. These include proper packaging according to DOT requirements. The packaging must be the manufacturer’s original packaging or a package of equal or greater strength and integrity. The packaging must be marked with a common name or a proper shipping name from the Hazardous Materials Table. Other requirements are
• Packagings must be leaktight for liquids and gases, and siftproof for solids.
• Packages must be securely closed, secured against movement, and protected against damage.
• Outer packagings are not required for receptacles (such as cans or bottles) that are secured against movement in cages, bins, boxes, or compartments.
• Cylinders and pressure vessels must conform to DOT’s hazardous materials regulations (49 CFR Parts 171–180) except that outer packagings are not required. These cylinders must be marked with the proper shipping name and identification number and have a hazard class warning label.
• If the package contains a reportable quantity of a hazardous substance, it must be marked “RQ.” Reportable quantities are found in Appendix A of 49 CFR § 172.101.
5.F.2 Transfer, Transport, and Shipment of Nanomaterials
This guidance applies to the movement of material from a laboratory to and from off-site locations. Personnel who package and prepare nanomaterials for shipment off-site must be current on hazardous material employee training required by 49 CFR Part 172, Subpart H. Consult your institution’s shipping department for assistance and routing of your materials. Although the guidelines provided here are for nanomaterials, the procedures are worth considering for shipping any material.
Any nanomaterial that meets the definition of a hazardous material according to 49 CFR § 171.8 and is classified as a hazardous material in accordance with 49 CFR §§ 173.115–173.141 and 173.403–173.436 must be packaged and marked, and labeled shipping papers must be prepared. The package must be shipped in accordance with 49 CFR Parts 100–185 and all applicable regulations.
Any nanomaterial shipped by air that meets the definition of a dangerous good according to the In-
ternational Civil Aviation Organization (ICAO) must be packaged, marked, labeled, and shipped with an accompanying properly prepared dangerous goods declaration in accordance with the ICAO technical instructions.
Nanomaterials that are suspected to be hazardous (e.g., toxic, reactive, flammable) should be classified, labeled, marked, and manifested as though that hazard exists. These materials should be classified and shipped as samples according to 49 CFR § 172.101(c) (11) unless the material is specifically prohibited by sections 173.21, 173.54, 173.56(d), 173.56(e), 173.224(c), or 173.225(b). These suspect materials should be packaged in accordance with sections 5.F.2.1 and 5.F.2.2, below.
Nanomaterials that do not meet DOT’s criteria listed above may pose health and safety risks to personnel handling the materials if the materials are released during transport. Therefore, all shipments of nanomaterials, regardless of whether they meet the definition for hazardous materials, should be consistently packaged using the equivalent of a DOT-certified packing group I (PG I) container and labeled as described in section 5.F.2.1, below.
5.F.2.1 Off-Site Transport and Shipments of Nanomaterials
This section applies to nanomaterials that are sent to a laboratory and from a laboratory to off-site locations and that do not otherwise meet the DOT definition of hazardous material.
The outer and inner package should meet the definition of PG I–type package. The innermost container should be tightly sealed to prevent leakage of nanomaterials. It should have a secondary seal, such as a tape seal, or a wire tie to prevent a removable closure from inadvertently opening during transport.
The outer package should be filled with shock-absorbing material that can
• protect the inner sample container(s) from damage and
• absorb liquids that might leak from the inner container(s) during normal events in transport.
As depicted in Figure 5.2, the inner package should be labeled (not to be confused with DOT hazard labeling).
If the nanomaterial is in the form of dry dispersible particles, add the following line of text:
Nanomaterials can exhibit unusual reactivity and toxicity. Avoid breathing dust, ingestion, and skin contact.

FIGURE 5.2 Recommended inner packaging label for on-site transfer of nanomaterials.
Documentation and notifications for off-site transfer of nanomaterials should include the following:
• a signed and complete dangerous goods declaration or shipping papers prepared in accordance with ICAO and DOT regulations by certified/qualified hazardous material employees who are authorized to release materials from the site;
• available descriptions of the material (e.g., MSDSs) (researchers should prepare a document for the samples that describes known properties and other properties that are reasonably likely to be exhibited by samples); and
• a notification to the receiving facility of the incoming shipment.
All materials should be transported by a qualified carrier.
• Shipments of nanomaterials classified as other materials (neither recognized HazMat or suspected DOT HazMat) may be transported using the most expeditious method provided they are packaged according to the requirements in section 5.F.1.
• The driver must possess basic hazard information on the commodity being transported, that is, material name, quantity, form, and MSDS if available.
5.F.2.2 On-Site Transfer and Transport of Nanomaterials
The on-site transfer of nanomaterials should follow the site-Specific transportation safety document or other institutional document (i.e., Chemical Hygiene Plan); in lieu of such a document, the transfer should
fully comply with DOT requirements. The site’s transportation authority (e.g., transportation safety officer or equivalent) should be the authority having jurisdiction over the requirements for packaging, marking, and documenting necessary for on-site transfers. For nanomaterials, the following is suggested:
• Assess and record the hazards posed by the material(s) following a graded approach that takes into account the form of the material(s) (e.g., free particle versus fixed on substrate).
• Use packaging consistent with the recommendations for off-site shipment or that affords an equivalent level of safety.
• Mark the transfer containers in accordance with the recommendations for off-site shipments.
• Include the following documents in the package:
ο results of the safety assessment and
ο an MSDS, if available, or a similar form detailing possible hazards associated with the material.
• Notify the receiving facility of the incoming shipment.






















