The Possibility of Life Elsewhere in the Universe
Christopher F. Chyba
Department of Astrophysical Sciences
Princeton University
One characteristic of the scientific investigations undertaken for the International Geophysical Year (IGY) in 1957–1958 was an emphasis on global measurements. Studies of Earth’s ionosphere, for example—crucial for the theory of short-wave radio communication—required data from all over the globe. Coordinated international studies certainly long predated the IGY, but the use of globalized data collection to support improvements in a world-spanning communications technology was a harbinger of today’s “globalization,” a term that is now, 50 years later, nearly a cliché.
But the IGY required an even larger context. Upper atmospheric studies also needed an understanding of the interaction of Earth with the Sun. Understanding Earth required placing Earth in its solar system context. In this sense the IGY can also be seen as a harbinger of what is now called “astrobiology.” Writing in 1974, shortly after the Moon landings, Carl Sagan asserted that we could, for the first time, try to understand life on Earth in its cosmic context. Space travel revealed that this was not just a metaphor, but literally true: we could only hope to understand the origin and evolution of life on Earth by placing Earth in the context of its solar system and galactic environments. Moreover, our understanding of the prospects for life elsewhere is in turn strongly shaped by our expanding knowledge of life on Earth.
THE LIFE WE KNOW
A discussion of life elsewhere therefore naturally begins with a review of some key aspects of the biosphere here on Earth. On Earth’s surface, there is something like a thousand trillion kilograms of carbon locked up in the living things that we see easily with our naked eye—plants, animals, and fungi. Most of this “biomass” is in trees. But in the past couple of decades, we’ve also learned that there seems to be a similar biomass of microscopic organisms living in the oceans, and another comparable biomass—this learned from deep-Earth drilling projects—of microscopic organisms living underground, down to depths of at least several kilometers. It appears that at least a small fraction of this subsurface biosphere is independent of surface conditions—that is, there are microorganisms living underground today that would likely continue to thrive even if the Sun were to go out, and photosynthesis shut down, tomorrow. This is not true for a great deal of subsurface life, much of which directly or indirectly depends on the energy harvested from sunlight at Earth’s surface—e.g., because it depends on the organic molecules produced by photosynthesis, or depends on the oxidized molecules resulting from the oxygen liberated by photosynthesis. But it appears that some microorganisms—such as those that make their living by combining hydrogen (produced from subsurface water weathering rocks) with carbon dioxide dissolved—might really represent ecosystems that are independent of the surface. As long as liquid water would persist in Earth’s interior—and this will be the case as long as there is enough internal geothermal heating to sustain some layer in Earth’s rocks where liquid water exists—it seems likely that there will be a subsurface biosphere.
The elucidation of Earth’s subsurface biosphere changes the way we think about the prospects for life
elsewhere. If deep biospheres are possible, even in the face of harsh surface conditions, then the prospects for subsurface life on Mars, Europa, or elsewhere seem greater. But we must remember that the requirements for habitability are not necessarily the same as the requirements for the origin of life. On Mars, it is at least possible that life originated at the surface, where it could take advantage of the tremendous available energy from the Sun, and then migrated to the subsurface as the surface became a freeze-dried desert. In the case of Jupiter’s moon Europa, which likely harbors a subsurface ocean of liquid water, it seems unlikely that there were hospitable surface conditions for more than a fleeting moment, if that, early in solar system history. For there to be life in Europa’s ocean, it would likely have to have originated in the subsurface. We do not understand the origin of life well enough to assess the plausibility of this scenario.
In both of these cases—Mars and Europa—life seems at least possible because of the likelihood of the presence of subsurface liquid water. It is fair to ask: must life depend on liquid water? How many of the apparently universal characteristics of life on Earth are requirements for life everywhere? Life on Earth is carbon-based; is this a general requirement or simply one of many possible alternatives?
Of course, we can not answer this question with confidence until we know more and have explored farther. But we are already getting some hints to the answer. Consider alternatives to carbon. Speculation has often focused on silicon-based life as an alternative to the carbon-based life we know. The theoretical reason for this can be seen by glancing at the periodic table of elements; silicon sits directly beneath carbon in this table, which is a short-hand way of saying that its chemical properties are similar. Since silicon, like carbon, is also an abundant element in the universe, it might seem to provide a good alternative. But in fact, silicon’s chemistry is more limited; except under extraordinary laboratory conditions, silicon atoms will not form double bonds with themselves, as carbon atoms do, so silicon chemistry is substantially more restricted than carbon chemistry. This is a consequence of the fact that the silicon atoms are simply bigger than carbon atoms, making double bonds much more difficult.
On top of this theoretical caution, there is an empirical discovery that comes from radio-wavelength investigations of the space between the stars, the so-called interstellar medium (ISM). Probing the ISM at radio frequencies reveals that there is a rich carbon chemistry throughout our galaxy; to date there are nearly a hundred carbon-based molecules observed in the ISM. There is no comparable suite of silicon-based molecules seen. Now, the ISM was not investigated primarily to test the hypothesis of silicon-based life. Rather, scientists simply wanted to learn what was out there—this was largely exploratory science, not hypothesis-testing science. But as a result of exploration, it seems more likely that carbon will be the basis for chemical life elsewhere in the universe, should any exist. Of course, this is at most an implication, not a strong conclusion.
WHAT IS LIFE?
All life we know on Earth is carbon-based, but it shares many more commonalities as well. Its basic biochem-

FIGURE 5.1 The Andromeda Galaxy, M31. SOURCE: Image from Robert Gendler. Copyright 2005 Robert Gendler, www.robgendlerastropics.com.
istry is also the same: life on Earth stores its genetic information as deoxyribonucleic acid, DNA, and uses proteins to do most of the business of metabolizing, motility, and other tasks. A molecule closely related to DNA, called ribonucleic acid, or RNA, is used to mediate between the genetic information in DNA and the construction of proteins according to the genetic plans (Figure 5.2). There are certain viruses that store their genetic information in RNA, but to reproduce, this RNA must be converted to DNA within a host cell, and the DNA-protein reproductive machinery of that cell must be brought to bear. It is possible—though there is so far no good evidence for this—that there are single-celled organisms on Earth that are unlike the DNA-protein life that we know and that remain undetected. Certainly such life would be invisible to DNA probes. But in the absence of any evidence, it’s difficult to speculate much further along these lines. So far, the life we know on Earth is DNA-protein life.
Life on Earth builds its proteins by stringing together, like boxcars on a train, different sequences of amino acids among 20 that are coded for in the genetic sequence of DNA. A small number of other amino acids are also occasionally used. But from a large list of possible amino acids that could exist—some 70 different types have been found, for example, in certain meteorites—life on Earth uses only a small subset.
Most impressive is that DNA similarities can be used to construct a “phylogenetic tree”—a tree of evolutionary relationships—for all known life on Earth (Figure 5.3). These trees make it clear that all known life on Earth is related and, in fact, can be traced back to a “last common ancestor.” The exact nature of this last common ancestor is debated, but the relatedness of Earth life is not. There is only one known form of life on Earth, with a common origin.
Some laboratories are getting close to making forms of life (by some definitions of “life”) other than DNA-protein life, and of course it is possible that altogether
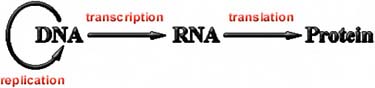
FIGURE 5.2 Ribonucleic acid (RNA) mediates between deoxyribonucleic acid (DNA) and proteins.
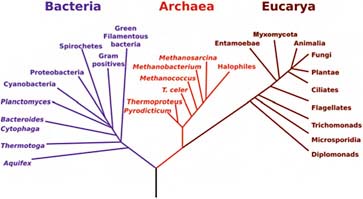
FIGURE 5.3 The phylogenetic tree of life based on comparative ssrRNA sequencing. SOURCE: Courtesy of NASA Astrobiology Institute.
different forms of life might be discovered elsewhere in the solar system or beyond. One might imagine that it would be convenient to have a general definition of what life is, apart from any particular details of life on Earth. At least since Aristotle, there have been efforts to define what life is, or to provide lists of its essential characteristics. Many definitions have been proposed. Their one common characteristic is that they all fail.
For example, there have been metabolic definitions, which try to define life as something that takes in energy, uses it to perform work, and then excretes wastes. But fire—which most would not want to call “alive”—also seems to do these things. In fact, the chemical reaction that powers fire is essentially the same as the one that we ourselves use. Thermodynamic definitions claim that life is characterized by a use of energy to create local order, but mineral crystals do the same, and most scientists would not want them to count crystals as “life.” This is a common problem: proposed definitions either include things that do not seem to be alive, or exclude things that we do consider living. Even the popular genetic or Darwinian definitions for life seem to exclude certain entities that are unambiguously alive, but that are not capable of Darwinian evolution.
The philosopher Carol Cleland and I have argued that this general problem should not surprise us. We have analogized the current situation to that facing Leonardo da Vinci when, five centuries ago, he grappled with what “water” is. There is a page in his Arundel Codex on which he lists the contradictory characteristics of water—he considers only liquid water—noting that sometimes it’s yellow, sometimes green, sometimes muddy; sometimes bitter, sometimes
sweet, and so on. It’s just very hard for Leonardo to say what the fundamental nature of water is. In retrospect, this should not surprise us. Leonardo was trying to understand “water” at a time before there was any theory of atoms and molecules. Once such a theory exists, it is easy to say what water is—water is H2O—full stop, end of story. This clarity comes not from a “definition” of water, but rather a theoretical identity statement. In the context of molecular theory, water can be precisely identified, and there is no ambiguity. Water is H2O, and that tells us what we mean, even if there are impurities that make a liquid solution sweet, or green, and even if the water is frozen as a solid or boiled into a vapor. But this precision is only possible in the context of an appropriate theory.
But currently, we have nothing analogous to molecular theory in our efforts to understand life. We do not even know if such a general theory of life is possible. In its absence, it’s hard to see how a definition of life will answer any scientific questions for us. Definitions do not answer scientific questions about the world. On the other hand, it may be impossible to devise a general theory without the perspective that will come from discovering other forms of life—should other forms, in fact, exist, and should we be able to recognize them.
THE STUDY OF LIFE IN THE UNIVERSE
The study of life beyond that we know on Earth was famously given the name “exobiology” in a groundbreaking paper published in Science in 1960, titled “Exobiology: Approaches to Life Beyond Earth,” written by the Nobel prize-winning biologist Joshua Lederberg. In 1964, another biologist, George Gaylord Simpson, published something of a reply paper in Science titled “The Nonprevalence of Humanoids,” in which he famously scorned exobiology as a science whose subject matter may not exist. This is rhetorically powerful at first glance, but in fact puzzling from the point of view of an astrophysicist: in fact, much cutting-edge work in astrophysics, in physics, and even in fields such as materials science concerns entities or phenomena that may not exist. The Higgs boson, higher dimensions of spacetime, room-temperature superconductors—all could turn out not to exist. It is a strange view of science that this means that their investigation is somehow risible.
Since Lederberg’s landmark paper, other words meant to encompass the field have been proposed. “Cosmobiology”—the biology of the cosmos—is one that particularly appeals to me, but is seldom used. “Bioastronomy” is also used, but the most prevalent term now in the United States is “astrobiology,” defined to mean the study of life in the universe. With this definition, there is no artificial—and scientifically unwise—division between the study of life on Earth and the study of possible life elsewhere.
ASTROBIOLOGY IN THE SOLAR SYSTEM
The past half-century of solar system exploration has reinforced the lesson that no arbitrary division should be placed between life on Earth and astrobiology. Consider what has been learned about Earth’s Moon. It may be true that the primary drivers for lunar exploration were political rather than scientific, but the scientific payoff of lunar samples returned to Earth—primarily by the Apollo missions but also by Soviet robotic Luna missions—has been huge. Much of what we now understand about early solar system history, and therefore early Earth history, begins with the Moon missions. This is because the surface of Earth is young, even though Earth is not. Earth is 4.6 billion years old,

FIGURE 5.4 The Moon. SOURCE: Courtesy of P.-M. Heden of Vallentuna, Sweden.
but there are nearly no rocks left on its surface—due to destruction by plate tectonics and erosion—to tell the tale of early conditions on our own planet. Yet the ancient sedimentary rocks we do have hint that life was established very early on, probably by 3.5 billions years ago, and possibly by 3.8 billion years ago. The Moon, however, died geologically billions of years ago, so preserves much of its record from these early dates. This history, built upon the dating of lunar samples correlated with crater counts on the lunar surface, reveals that the Moon was once subject to an intense bombardment of comets and asteroids—a bombardment exponentially higher prior to 3.8 billion years ago than is the case today. Comparison of the lunar cratering record to that of Mercury and ancient Mars suggests that the entire inner solar system was subject to this same bombardment. Therefore the origin of life on Earth must have taken place in the midst of this bombardment, with important implications both for destruction and delivery of carbon-bearing (so-called organic) molecules of use for the origin of life. To learn this about the conditions for early life on Earth, we had to visit the Moon and planets.
Casting our view farther out from the Sun, the planet Mars is one of the most intriguing possible venues for ancient or even extant life in the solar system. Among such venues, it is also most easily accessible from Earth, with spacecraft travel times that are less than one year. Spacecraft flybys, orbiters, landers and rovers have made it clear than ancient Mars once had abundant liquid water at its surface, and there is strong evidence that, in specific locations at specific times today or in the geologically very recent past, liquid water still reaches and flows at the surface (Figure 5.5). The surface itself is now a freeze-dried desert where liquid water must either freeze or evaporate. But given what we’ve learned about the deep biosphere on Earth, the possibility that life on Mars exists in subsurface liquid water environments—environments that may occasionally reach the surface—must be taken seriously. Because of their proximity, Mars and Earth may exchange meteorites that are created as ejecta from large impacts, and it is not out of the question that whichever planet first originated life could than have inoculated the other. Only discovering and examining possible martian life could answer this question with certainty.
Beyond Mars, in orbit around the planet Jupiter,

FIGURE 5.5 Evidence for recent liquid water on Mars—the south-facing walls of Nirgal Vallis. SOURCE: NASA/JPL/Malin Space Science Systems. MGS MOC Release No. MOC2-240.
lies the moon Europa, just a bit smaller in size than Earth’s Moon. There is now strong evidence that Europa harbors an ocean of liquid water beneath its extremely cold outermost layer of ice (Figure 5.6). The volume of this ocean is about twice that of Earth’s oceans. At the floor of Europa’s ocean, as on Earth, liquid water is in contact with rock, raising the possibility of important water-mineral interactions in the presence of hydrothermal energy. Data from the magnetometer on the Galileo spacecraft not only supports the existence of the ocean, but suggests that it is very salty and that the overlying ice may be only 10 kilometers thick, or even thinner. Could there be life in this ocean? Speculative studies suggest that the energy sources needed to support life should be present. But whether the origin of life could have occurred in an ocean that was beneath kilometers of ice—so likely cutoff from sunlight—is an open question. It is much harder for Earth and Europa to successfully exchange microorganisms via meteorites than is the case for Earth and Mars, so if there is life on Europa, it is likely due to a separate origin from life on
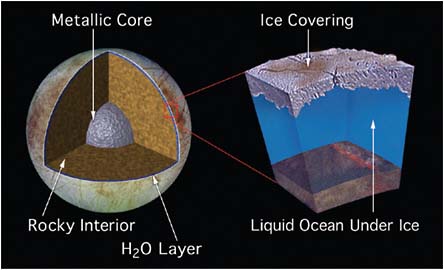
FIGURE 5.6 Cross-sectional diagram of Europa’s 80–150 km thick H2O layer assuming a metal core surrounded by rock mantle: An intermediate subsurface slush also remains a possibility. SOURCE: Courtesy of NASA/JPL. Available at http://photojournal.jpl.nasa.gov/catalog/PIA01669.
Earth. But because of the liquid water ocean, Europa may be the most intriguing site for extraterrestrial life in our solar system. It appears that Jupiter’s Mercury-sized moons, Ganymede and Callisto, harbor deeper subsurface liquid water oceans as well.
Still farther out from the Sun, the planet Saturn hosts at least two intriguing worlds. The Cassini spacecraft has revealed that tiny Enceladus has active geysers of ice crystals that may originate in a subsurface sea of liquid water, though the exact mechanism for the geysers and whether there is enough energy to sustain liquid water in Enceladus’ subsurface remains to be convincingly argued. Farther out from Saturn lies the Mercury-sized world Titan, with its dense atmosphere of nitrogen and methane. There is some evidence that Titan, too, may harbor a subsurface liquid water ocean. All of these worlds need much more exploration and should receive it later this century. Missions to the outer solar system take time (the travel time to Jupiter is 3 years from Earth) and are expensive. But a balanced program of solar system exploration, especially one emphasizing astrobiology, must systematically explore the Jovian and Saturnian systems as well as Mars.
PLANETARY PROTECTION
An important issue in planetary exploration is planetary protection. It was Lederberg who, during the IGY in 1957, wrote to the president of the National Academy of Sciences to raise this as an issue, and the Academy worked with the International Council of Scientific Unions to create an international study group on this question. The Outer Space Treaty, which entered into force in 1967 and is best known for forbidding the placement of “weapons of mass destruction” in outer space, requires space-faring nations to avoid the “harmful contamination” of other celestial bodies. Within a decade, then, Lederberg’s personal concern had given way to an international treaty requirement.
The concern is scientifically well founded. Investigations with NASA’s Long-Duration Exposure Facility (LDEF) and European Retrievable Carrier (EURECA) experiments reveal that certain microorganisms survive 6 years in space at the 1 percent level—i.e., one out of a hundred Bacillus subtilis spores survive for this long—whereas 25 percent survive a year in space. In both cases, survival requires that the organisms are shielded from the Sun’s ultraviolet light, but any organism inside a spacecraft would be. The organisms freeze-dry, or lyophilize, in the cold vacuum, but when introduced to liquid water they revive. Most NASA Mars mission spacecraft are constructed in class-100,000 clean rooms, which means they have thousands of viable sporulating bacteria present per square meter of spacecraft surface, and probably ten or more times as many other types of bacteria. Since it takes less than a year to get to Mars, this means that Mars spacecraft carry a viable bioload of microorganisms with them to the Red Planet. The first question becomes, then, whether any of these organisms could find their way from the martian surface into habitable niches with liquid water in the subsurface, and if so, whether they could grow in that new environment. The odds are long, but not impossible. The second

FIGURE 5.7 An overall side view of the Long Duration Exposure Facility grappled by remote manipulator system during STS-32 retrieval. SOURCE: NASA Langley Research Center. Image # EL-1994-00078.
question is what level of additional measures to reduce the bioload of Mars spacecraft should be taken during spacecraft construction. A recent report that I chaired for the National Research Council (NRC), Preventing the Forward Contamination of Mars, looked at these questions and concluded that NASA needs to better understand the numbers and types of microorganisms that currently fly on its spacecraft and take more stringent steps to reduce spacecraft bioload.
The current international interpretation of the Outer Space Treaty requirement is that microorganisms carried to other planets must not be allowed to take hold on that world in a way that would render it difficult or impossible to determine if a truly alien biosphere might be present. That is, planetary protection as it stands is really about “protecting the science” from contamination, not about protecting any possible alien biosphere from potential ecological assault. Our NRC report urged that it was time for an international meeting to reconsider whether planetary protection should be reinterpreted to be about “protecting the planet,” and not just “protecting the science.”
FOUR WAYS TO LOOK FOR LIFE
So far we have discussed in situ investigation of the solar system, in which spacecraft land on other bodies and conduct experiments at the surface to look for life. Closely related is the biological examination, in terrestrial laboratories, of samples from other worlds. These samples could arrive on Earth in an uncontrolled way, via meteorites that originated as debris blown off another world by a big impact, or in a controlled way, as samples brought back by a dedicated spacecraft. But both cases involve hands-on investigations for the presence of life in the solar system.
A third way to search for life is to examine the light coming from the atmospheres of other worlds—i.e., spectroscopy—to determine the chemical composition of those worlds’ atmospheres in the hope of finding the chemical signatures of another biosphere. This has been done for Mars and other planets in our solar system for decades and has just become possible for certain giant exoplanets—planets in orbit around a star other than our Sun.

FIGURE 5.9 NASA’s first mission capable of finding Earth-size and smaller planets. SOURCE: Courtesy of NASA.
With the Kepler mission that will launch in the next few years, we should soon know the statistics of the presence of Earth-sized planets around other stars. [Figure 5.9] Kepler will allow us to determine the orbits of these planets (assuming that there are any) and therefore their distances from their stars. With knowledge of the stars, we will know which, if any, of these worlds lie in the right range of distances for liquid water oceans to be possible on their surfaces. In a few years’ time, we will go from almost no knowledge of whether other Earth-like planets exist to knowing their statistics and potential surface habitability. This is an extraordinary moment. Humans have speculated for millennia about whether other planets like ours could exist—for example, Aristotle asked (and answered, on theoretical grounds) this question in his book On the Heavens. In a few years we will no longer have to speculate. We should not let human civilization sleepwalk through this remarkable transition in our knowledge about our place in the universe.
Some decades further on we will be able to observe these planets from dedicated satellites in space, and determine the composition of their atmospheres. The hope is that we might detect some combination of gases in some atmospheres that equilibrium chemistry would seem to forbid, but which biology might just generate. This could imply that there are biospheres on these worlds.
Or maybe not. The evidence would be circumstantial, and as soon as such data were reported, scientists would rightly, and conservatively, search for non-biological explanations. Indeed, we have seen this already at Mars: it is now clear that the martian atmosphere, a highly oxidizing atmosphere flooded with ultraviolet light that should not permit organics to exist for long, contains patches of the simple organic molecule methane—at about the 10 parts per billion level. The methane must be produced by localized sources at the surface; it is well out of equilibrium with the existing atmosphere. It could be the product of a martian version of the methanogenic bacteria we know on Earth. But already there have also been published papers suggesting explanations in terms of martian geochemistry. Atmospheric chemistry consistent with biological sources may provide hints of life, but it evidently it does not in itself provide decisive arguments for the existence of life.
SETI1
Besides the three techniques for searching for extraterrestrial life so far discussed—in situ investigations, examination of samples delivered to Earth, and remote sensing of planetary atmospheres—there is one other
approach to the search for life that human civilization currently has underway. This is the search for extraterrestrial intelligence (or, rather, technology), or SETI. SETI need make no assumptions about the biochemical or other makeup of extraterrestrial life. It must, on the other hand, rely on the existence of technology capable of communicating across interstellar distances.
The most powerful targeted search to date has been the SETI Institute’s Project Phoenix, which observed roughly the thousand nearest Sun-like stars for radio-frequency broadcasts. Phoenix completed its search at the Arecibo radiotelescope in Puerto Rico, the world’s largest, and therefore most sensitive, radio receiver (Figure 5.10). Radio frequencies are the natural frequency to use for interstellar communications, because of the so-called microwave window where galactic background noise is lowest. For each target star, Project Phoenix examined billions of frequencies. Algorithms assumed that the frequency would drift, as a real transmission certainly would due to the motion of the source of the transmission relative to Earth. To be a credible detection, any signal received had to stand up against multiple tests, including a check against all known confounding signals (e.g., from Earth-orbiting satellites or interplanetary probes), a requirement that the frequency be so well defined (i.e., that the bandwidth be narrow) as to only be possible artificially, and a demonstration that the source was detected not only at Arecibo but on a follow-up radiotelescope in Britain as well. No source has ever passed through all of these filters.
It is sometimes said that humanity has looked and looked and looked for extraterrestrial radio transmissions without finding any, so it must be that we are alone. Superficially, this might seem to follow from the fact that SETI radio searches have been carried out since the first search was conducted by Frank Drake nearly 50 years ago. But in fact, even Project Phoenix has only scratched the surface. The nearly 1,000 stars it has searched account for just a ten-millionth of the stars in our galaxy. The SETI Institute and the University of California are now constructing the Allen Telescope Array (ATA) in northern California using almost entirely private funds (Figure 5.11). This array will

FIGURE 5.10 The 1,000-foot (305-meter) dish at Arecibo, Puerto Rico, is the most sensitive radio telescope in the world. It was used by Projects Phoenix and SERENDIP, and it’s currently feeding huge volumes of data to SETI@home. SOURCE: NAIC Arecibo Observatory, a facility of the National Science Foundation.
FIGURE 5.11 Artist rendering of completed ATA-350. SOURCE: Courtesy of Isaac Gary.
perform SETI searches all day every day (rather than the few weeks per year that were possible at Arecibo), using the most recent technology. Once completed, the ATA should examine around a million stars in a decade of observing. But even this will represent just a hundred-thousandth of the stars in our galaxy. If technical civilizations beaming signals across interstellar distances are more rare than one in every hundred thousand stars, even the ATA will not be successful anytime soon. But in the absence of any mature theory about the prevalence of intelligent life and technology, the search is the best that we can do.
Nevertheless, arguments have been put forward regarding the likelihood of extraterrestrial intelligence. Perhaps the most common and intuitive is the simple comment that with so many stars—hundreds of billions in our galaxy alone—it just can not be that we’re the only civilization. On the face of it, this assertion also seems consistent with the Copernican principle, the idea that Earth has no unique status in the universe. But in fact, this line of reasoning does not hold up. The reason is that we do not know the probability of the origin of life, and then intelligence, and then technology, on an Earth-like world. If this probability were extremely small—say, less than one in a hundred billion—then Earth could be the only planet in the galaxy harboring an intelligent civilization. There could still be a hundred million other Earth-like worlds, but only one would have hit the jackpot. This would be like rolling six identical dice and having only one came up with a six. There is nothing special about that particular die; any one of them could have rolled a six but statistically most of them would not. The Copernican principle is not violated, but Earth could still be unique.
The Drake equation summarizes this way of looking at the problem. Frank Drake wrote down his equation as a meeting agenda for a workshop on SETI in 1961. The Drake equation reads: N=R*fpneflfifcL, where N is the number of technically communicative civilizations in our galaxy, R* is the galaxy’s rate of star formation, fp is the fraction of those stars around which planets form, ne is the number of planets in such systems suitable for the origin of life, fl is the fraction of those planets on which life originates, fi is the fraction of those on which life evolves intelligence, fc is the fraction of those intelligent species that become communicative across interstellar distances, and L is the average lifetime of a communicative civilization.
Obviously this equation is not an equation analogous to, say, the ideal gas law equation. The ideal gas law hypothesizes a relationship among the pressure, volume, and temperature of gases in the laboratory, so is subject to empirical test. The Drake equation does not pose this kind of testable hypothesis. Rather it is
a type of “Fermi problem,” an example of the sort of back-of-the-envelope thinking made famous by Enrico Fermi in his graduate examinations, by asking questions like “How many piano tuners are there in the city of Chicago?” At first glance, you either know the answer to this question or you do not, and if you do not, there is no easy way to figure it out. But in fact, by breaking the calculation down into a product of numbers that may be estimated (such as the population of Chicago, the number of people per family, the fraction of families that own pianos, how often pianos have to be tuned, and so on) one can make a reasonable estimate of the correct answer.
But this can not be done with the Drake equation. While the three factors R*, fp, and ne can be assigned credible estimates on the basis of what we already know, the remaining factors can only be guessed. L, in particular, moves us into the realm of extraterrestrial sociology and political science, which remain less developed fields. At its upper end we might imagine that L could be as long as the age of the galaxy, ~1010 years. At its lower end it could be as short as the interval between, say, the invention of radio and the mass production of thermonuclear weapons; based on our experience, this interval could be as short as decades. The average value of L in the galaxy might well be anywhere in this interval, although even a small number of very long-lived civilizations could make the average quite long indeed. In the face of the uncertainties the Drake equation reveals, the large-numbers argument cannot resolve questions about the frequency of civilizations in our galaxy.
INTELLIGENCE ON EARTH
Another way to assess the prospects for other intelligent life is to extrapolate from the history of life on Earth. There is a set of arguments bearing on this question that have been rehearsed for a full century, beginning in 1904 with Alfred Russel Wallace, the co-discoverer of the theory of evolution, and being revived at intervals since by a series of authors. The pessimists in this argument emphasize the contingency of evolution, for example how if one were to replay the evolution of animals, the results would likely be very different, and in particular “the chance becomes vanishingly small that anything like human intelligence would grace the replay” as Stephen J. Gould wrote in 1989. The evolution of human intelligence, after all, depended on a series of contingent factors, including the collision of a major asteroid with Earth 65 million years ago. The counterarguments are equally familiar: convergence is frequently observed in evolutionary history, and nature has evolved complex phenomena such as eyesight and flight many times, so that even though any given evolutionary line might be highly contingent, a large number of parallel paths may lead to the same functional outcome. To this the reply is made that technical intelligence has only evolved once on Earth, so evidently convergence was not operating in this particular case. But things are not this clear-cut; as the marine biologist Lori Marino’s work has emphasized, several species of marine mammals developed a level of intelligence that by quantifiable measures is in excess of that of chimpanzees and slightly in excess of that of homo habilis, one of modern humans’ tool-using ancestors.
Marino and her colleagues begin with a reproducible measurement that correlates with what is meant by intelligence, and that can be employed with the fossil record as well as contemporary organisms. There is at least one such measure, called encephalization. Encephalization is typically expressed as a quotient (hence, encephalization quotient, or EQ) that quantifies how much smaller or larger a particular animal’s brain is compared to the expected (via a regression over many animals) brain size for an animal of that body size. Animals with EQs above 1 are brainier than average; those with EQ values below 1 are less brainy than expected for their body size. There is strong evidence that EQ among primates correlates with the ability to innovate, social learning and tool use; among birds it correlates with behavioral flexibility. It seems, therefore, to provide a good measurable proxy for “intelligence.” Contemporary humans have the highest EQ on Earth at 7.1, meaning that our EQ is more than 7 times greater than expected for an animal of our body weight.
In well-controlled studies, dolphins have been shown to be capable of mirror self-recognition, an ability demonstrated only by a few other animals besides humans (Figure 5.12). The highest EQ values on Earth after modern humans are those of four dolphin species, with the highest of the four being about 4.5. Great apes have EQs lower than this, with a mean around 1.9. This is about the same as that of the human ancestor
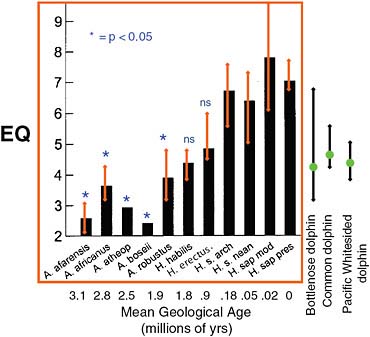
FIGURE 5.12 SOURCE: Courtesy of L. Marino.
Australopithecus. Among our more recent ancestors, the tool-users Homo erectus and the earlier Homo habilis had EQ values of about 5.3 and 4.3, respectively.
These results suggest that the evolution of human intelligence on Earth is not an entirely exceptional phenomenon. With a sufficiently large database of EQ measurements for fossil whale species, one can go further, and begin to test other long-standing assertions about intelligence, such as the claim that increases in encephalization should be pervasive because of the selective advantage that is conferred by bigger brains. Marino and her colleagues have done this analysis, applying statistical tests to data for modern and fossil whales going back 50 million years. They show that while the overall trend in encephalization has been increasing, at any given speciation event, the successor species was as statistically likely to have a lower EQ as a higher one. That is, encephalization was not pervasively advantageous; the increase in intelligence at the high end of encephalization seems better modeled as a random walk rather than a pervasive selection pressure favoring bigger brains. But it should be emphasized that the size of the data set here is so far very small, and nearly no funding is available for this kind of work.
These results are those of only a nascent research program, but they emphasize that there are reproducible, quantitative methods that can be applied to begin to address some long-standing assertions about the likelihood of the evolution of intelligence in the universe.
Just as studies of microscopic life on Earth inform thinking about the prospects for microorganisms elsewhere, so can rigorous exploration of the evolution of intelligence on Earth inform our thinking about the prospects for intelligence elsewhere. Treating “intelligence” as a property of the biological universe that can be quantitatively investigated should allow us to move beyond polemics and begin to push back the boundaries of our ignorance in a data-driven fashion.
ASTROBIOLOGY AND THE HUMAN FUTURE
Fermi posed his famous question “Don’t you ever wonder where everybody is?” to three colleagues at Los Alamos National Laboratory in 1950. In its modern version, the “Fermi paradox” maintains that if other civilizations exist in the Milky Way galaxy, some must be much older, perhaps billions of years older than ours; that such civilizations would long ago have developed interstellar travel; that they would then have explored or colonized the galaxy on a timescale that is short compared with the galaxy’s lifetime; and that they would therefore be here. But since they are not here, they must not exist! The paradox obviously does not hold in a strict logical sense, since each of its assertions is at best a claim of probability, but it has been a powerful force on thinking about the prospects for extraterrestrial intelligence.
Whatever the rigor of the Fermi paradox, there have been many solutions proposed for it. The challenge to most of these solutions is the large-number assertion: while this or that explanation might explain the failure of some, even most, civilizations to colonize the galaxy, the timescale for colonization is putatively so short that unless the total number of civilizations in galactic history were quite small, the galaxy would indeed have been colonized. These colonization scenarios have posited exponential reproduction and paid little attention to ecological factors, such as the evolution of predation or other behavior that could have the effect of reducing the rate of expansion of a space-faring population. What parameters does one choose in predator-prey modeling to depict accurately the expansion timescales of competing technical civilizations? It is hard to make such parameter choices with a feeling of confidence. And it is close to impossible to know whether such simple analogies from life on Earth are or are not applicable.
Various practical arguments against galactic spaceflight being commonplace have been countered by invoking either genetic engineering or artificial intelligence in the form of self-replicating and evolving machines. We should not exaggerate the ease or casualness with which substantial genetic manipulation of human beings will be done, but as Robert Carlson has shown, basic measures of human bioengineering power, such as the time or cost required to sequence or synthesize short sequences of DNA, show that biotechnology is exponentially advancing at a rate even faster than that of Moore’s law in computing. It is hard to know what comes after this exponential lift-off. It may prove generally true that there is only a brief interval during which a species is technically intelligent yet still retains its biologically evolved form. If so, we should expect that any civilization with which we make contact through SETI or otherwise is unlikely to resemble its biological predecessor species. If the question is “what will they look like?” the answer may be “whatever they want to.”
But well before biotechnology permits the reengineering of the human species, it will put great power for extremely dangerous manipulations of microorganisms into the hands of small groups of the technically competent. Indeed, it is doing so already. (The National Academies has already convened two committees to examine this issue.) We do not have adequate models from Cold War arms control or nuclear nonproliferation for how to manage this new world, gaining the benefits of biotechnology for public health and food security while preventing disaster. The same technological expertise that makes possible our increasingly sophisticated searches for life brings with it powerful new opportunities, if mishandled, for destruction. Astrobiology is defined as “the study of the living universe.” If so, then the discipline must also speak to the future of human civilization, a thing uniquely precious regardless of whether it is entirely alone or one of many in the galaxy.













