6
BioWatch and Enhanced National Biosurveillance Resources
The previous chapters describe aspects of the current and planned environmental monitoring technology of the Department of Homeland Security’s (DHS’s) BioWatch program and current and potentially enhanced surveillance practices in the public health and health care systems. Here the committee compares the BioWatch program to enhancing current surveillance practices in the public health and health care systems.1
EXPLORING THE EFFECTIVENESS OF BIOSURVEILLANCE RESOURCES
Evaluating the effectiveness of the BioWatch program is problematic for several reasons.
-
Definitive information about the likelihood of a catastrophic bioterrorist attack of the kind that BioWatch is intended to detect and about the specific nature, costs, and effectiveness of an enhanced surveillance system implemented through the public health and health care systems is not available.
-
No bioterrorism events have tested the currently deployed Generation 2 BioWatch system, and risk-management analyses do not appear to be available for all of the pathogens currently included in BioWatch testing.
|
1 |
As noted in Chapter 1, the public health and health care “systems” of the United States are highly decentralized. |
-
The Generation 3 BioWatch system has not yet been selected, so its assessment can be based only on the proposed operational requirements (DHS, 2008, 2009).
-
The public health and health care systems regularly face the need to recognize and respond to naturally occurring disease outbreaks and seasonal illness and are also expected to be able to detect and respond to bioterrorism events. Assessments of the effectiveness of surveillance systems in detecting disease outbreaks are limited, especially for newer syndromic surveillance techniques (Bravata et al., 2004; CDC, 2004; Buckeridge, 2007). In trying to determine their effectiveness in the event of bioterrorism, it is necessary to rely on simulations because actual data are not available.
-
Biosurveillance within the public health system is composed of numerous separate systems, varying in maturity, whose integrated cost and performance is difficult to assess for detection of either natural disease outbreaks or bioterrorist events, whether alone or in conjunction with BioWatch.
-
Natural outbreaks are heterogeneous, differing in size, location, agent, and mechanism of spread, as are the capabilities of state and local public health departments to recognize and respond to outbreaks and the availability of health care resources to provide treatment. Assessing the effectiveness of a potentially enhanced surveillance system is even more challenging.
-
The digitalization of health information that could improve the speed and ease of disease surveillance remains incomplete.
Despite these challenges, it is possible to make a basic but informative comparison of BioWatch with surveillance through the public health and health care systems.
Performance of the BioWatch System
A fundamental question is whether BioWatch can perform in a useful way. Answering this question requires considering several interrelated events that are necessary for BioWatch to contribute to the reduction of illness and loss of life (see Table 6-1). The official estimates of the probabilities of some of these events, especially that a bioterrorism attack will occur, that it will take place in a BioWatch jurisdiction, and that it will be delivered in a manner that BioWatch has the potential to detect, have not been made available to the committee. With the understanding that judgments about these probabilities will be crucial to policy decisions that must be made about the refinement and further deployment of BioWatch, the committee looked at the system’s potential performance assuming there is a detectable attack, that is, a large-scale airborne release of one of the
TABLE 6-1 Steps Necessary for BioWatch to Help Reduce Illness and Loss of Life
|
Necessary Step |
Likelihood |
Evidence |
|
Large airborne release of a bioagent |
Uncertain* |
|
|
Release occurs in BioWatch jurisdiction |
Uncertain* |
|
|
Bioagent among those detectable by BioWatch |
Uncertain* |
|
|
Bioagent remains viable and is of suitable particle size for infection |
Depends on agent processing method, dissemination method, agent vulnerability to ultraviolet light, and other environmental factors |
Military testing |
|
Bioagent plume reaches a BioWatch detector |
Depends on size and location of release relative to location and density of detectors; and on meteorological conditions that control dispersion (e.g., wind velocity, stability) |
Military testing, modeling |
|
Analysis of BioWatch sample produces positive reading when target agent is present (result of many substeps) |
Likely (?) |
Genetic material from naturally occurring organisms successfully identified from Generation 2 filters |
|
Decision makers receive positive BioWatch result and other information sufficient to promptly recognize a BioWatch signal as indication of a true bioterror event |
Variable, by jurisdiction; in general, uncertain |
No experience yet with a BAR later determined to result from a bioterror event Testimony from public health officials |
|
Effective prophylaxis or treatment exists |
Depends on the agent |
Medical literature |
|
Decision makers initiate prophylaxis or treatment |
Depends on the detected agent, other available information, and circumstances |
Experience from multiple BARs, but response to a BAR for anthrax may differ Testimony from public health officials |
|
Prophylaxis or treatment is carried out in time to reduce mortality and morbidity |
Depends on the agent, dose, time elapsed since human exposures, ability to define and target prophylaxis to the population exposed, and dispensing capabilities |
Modeling Exercises |
|
NOTE: BAR, BioWatch Actionable Result. *Information about this step may be available to those conducting the threat analysis but was not part of this committee’s review. |
||
pathogens included in the BioWatch assays in a location where a BioWatch device can obtain a sample for analysis.
The BioWatch system currently operates in more than 30 major metropolitan areas, with most of the collectors deployed in outdoor locations. Given the relatively modest number of devices available for BioWatch jurisdictions, their placement is a major factor in determining whether at least one collector would be in the path of an aerosol release. But their allocations were based upon dispersion models of urban environments that are especially challenging and sensitive to assumptions about parameter values (GAO, 2008b). Operational testing will be important to better understand collector efficacy and aerosol behavior in the complex microclimates of the urban environments of BioWatch jurisdictions, and the probability of plume detection.
On a few dozen occasions since implementation the BioWatch system, samples have produced positive findings that were interpreted as being genetic material from an organism among those currently being monitored. These BioWatch Actionable Results (BARs) have demonstrated that the current BioWatch technology can collect analyzable genetic material and that the current laboratory assays can detect this genetic material. In each BAR, BioWatch jurisdictions concluded that no terrorist release had occurred and that there was no indication of increased human illness. (The committee notes that it heard testimony from public health officials in some of these jurisdictions, but it did not have the opportunity to review the information available to the public health authorities in each case.)
However, as discussed in Chapter 3, several concerns about the BioWatch system’s technical performance remain, including questions about sample collection, laboratory analysis, siting practices, and program priorities.
One concern is the usefulness of the analyses of the BioWatch air samples. The information provided to the committee about BARs does not indicate whether viable organisms were collected. In addition, more needs to be learned about the genetic near neighbors of the pathogens that BioWatch targets and about the microbial ecology of the areas where BioWatch operates.
All of the BARs to date appear to be the result of procedurally accurate analyses of BioWatch samples. Although none of them has been determined to be due to bioterrorism, these BARs have been unplanned opportunities to test the BioWatch program’s plans and procedures. Nevertheless, they amount to false alarms that are costly to evaluate. Repeated false alarms may eventually create a sense of skepticism or complacency that could delay or hinder an appropriate response to a true bioterrorism event. The opposite, but related concern is determining the likelihood that the BioWatch collection and analysis process may fail to detect the presence of targeted organisms.
Realizing Benefits from the BioWatch System
The expected benefits from the BioWatch system derive primarily from the anticipation that it will decrease the time that elapses between a large-scale airborne release of a bioterrorism agent and the distribution and use of post-exposure prophylaxis and post-infection treatment. For BioWatch Generation 2, the time from the release of a biological agent to confirmation of positive results—the declaration of a BAR—would typically be 10 to 36 hours (depending on when an event occurs during the 24-hour collection cycle and allowing for filter recovery, primary screening, and agent-specific testing). As discussed in Chapter 3, plans for BioWatch Generation 3 include a 4- to 6-hour time to detect a pathogen.
The committee is most confident about the potential for early detection via BioWatch to reduce morbidity or mortality in the event of a massive aerosol attack using Bacillus anthracis spores, assuming an effective public health response capability is in place. This conclusion is based on evidence that includes the environmental stability of B. anthracis spores; empirical findings about infection and mortality rates from natural outbreaks, the Sverdlovsk release, and the 2001 anthrax letters; the relatively rapid incubation period of anthrax (during which victims have no symptoms); the potential for effective post-exposure antibiotic prophylaxis; and the rapidly decreasing efficacy of treatment after the onset of symptoms (e.g., Jernigan et al., 2001; Inglesby et al., 2002; Holty et al., 2006; Wilkening, 2006, 2008).
Several modeling analyses suggest that the timing of the start and completion of a prophylaxis effort following detection of an anthrax release are critical factors in determining subsequent morbidity and mortality (e.g., Hupert et al., 2002; Buckeridge et al., 2005, 2006; Wein and Craft, 2005; Yang et al., 2006; Baccam and Boechler, 2007; Zaric et al., 2008). Early notice of a potentially catastrophic release of anthrax may speed a range of actions up to and potentially including initial distribution of prophylaxis from local supplies while further investigation proceeds and dispatch of prophylactic and therapeutic medications from the national stockpile is arranged. But such presumptive actions have to be weighed against the potential for adverse reactions if antibiotics or vaccines are administered and other negative consequences should the BioWatch signal ultimately prove to be a false alarm.
For agents other than B. anthracis, however, there is less information and less confidence that the BioWatch system’s early detection potential will result in decreasing morbidity and mortality. Better evidence and thoughtful consequence analysis are needed on other agents’ environmental stability in an aerosol attack, the effectiveness of post-exposure prophylaxis, and the time course and virulence of infection.
Although a BAR may present an opportunity to hasten the delivery of prophylactic or therapeutic care, it does not automatically trigger a public health response. The type and timing of a response are likely to depend on public health officials’ judicious interpretation of not only the BAR but also other information that may include the number of the BioWatch collectors affected, the strength of the real-time PCR signal in affected collectors, the availability of corroborating information (including follow-up environmental sampling or information from law enforcement or intelligence sources), the effectiveness of public notification, and the resources available to set up distribution centers. Testimony to the committee suggests that, depending on the circumstances, some public health officials may be hesitant to launch a high-regret action that may cause unnecessary alarm or even harm, such as initiating the widespread distribution of prophylaxis or treatment, without confirmed cases of disease in humans or animals. This may reduce but does not necessarily negate the value of BioWatch environmental detection. A BioWatch alert may trigger outreach to health care providers and help them identify early cases of illness that might otherwise be missed or ascribed to other causes. It could also trigger preparation for the delivery of medical countermeasures and shorten the interval from later confirmation to chemoprophylaxis. Plus, a BAR in one jurisdiction may be important context for the interpretation of a closely timed BAR in another location. Thus, a BAR may enhance response even if it does not automatically trigger distribution of prophylaxis. In addition, a BAR can spur investigation and collection of evidence needed in the national security and law enforcement response to bioterrorism.
Qualitative Assessment of Enhanced Surveillance Through Public Health and Health Care Systems
Detection of bioterrorism or a disease outbreak through the public health and health care systems is, by definition, the detection of symptoms or infection (or behavior related to the onset of illness). Because almost all infectious agents require an incubation period of hours to weeks before signs or symptoms of illness appear, none of the potential enhancements discussed in Chapter 5 can be expected to provide earlier notice of the potential for exposure than rapid environmental detection can. However, to varying degrees, many of these enhancements may facilitate earlier recognition of an outbreak that BioWatch cannot or does not detect, or provide confirmatory information after a BAR, and thus they may allow for earlier mobilization of a response than is possible now. These enhancements may also improve the effectiveness of the response by improving decision makers’ situational awareness.
The most important enhancements are those that will facilitate rapid diagnosis of infections, and that enable public health authorities to detect
incidence patterns consistent with an outbreak. For example, enhancement of public health laboratories’ capability and capacity to conduct molecular subtyping to detect clusters of genetically similar pathogens isolated from cases is used to link cases that might otherwise not be connected to a point source, such as accidental or intentional contamination of food products. Another desirable enhancement is the development and use of effective clinical decision support tools for diagnosis, reporting, and case management, because these may enable rapid identification and reporting of the index case in an outbreak. As is true with BioWatch, the benefits of such earlier detection are contingent on informed interpretation and decision making by health care providers, public health personnel, and other officials.
CDC has described attributes that should be considered in an evaluation of surveillance systems generally (CDC, 2001) and has recommended a framework specifically for evaluating surveillance systems for early detection of outbreaks (CDC, 2004). The overall attributes include a surveillance system’s usefulness, simplicity, flexibility, data quality, acceptability to data contributors, sensitivity, representativeness, predictive value, timeliness, and stability. The expectations for, and the importance of, any given attribute may vary, depending on the purpose of the surveillance system. Sensitivity, timeliness, and predictive value are of particular significance for early detection of outbreaks.
With the specific challenges of rapid detection of bioterrorism and other serious infectious disease threats in mind, the committee framed 10 related performance features that could be used to evaluate the potential contributions of BioWatch or enhanced surveillance tools that may be integrated into the public health and health care systems. These performance features are:
-
Facilitates early detection of an attack or the onset of an outbreak
-
Minimizes “false positive” alerts for an attack or outbreak
-
Improves recognition of an index case
-
Facilitates validation of signals of a possible bioterrorist attack or other outbreak and facilitates initial response decisions (e.g., whether to initiate mass prophylaxis)
-
Improves situational awareness during an event (e.g., the extent of exposure, disease trends and characteristics, available health care capacity) and allows characterization of the scale and scope of the incident so that mitigation can be effectively targeted
-
Improves communication among public health personnel and clinicians (e.g., event or case detection, event status, patient evaluation or treatment, infection control)
-
Improves communication among public health laboratories, clinical laboratories, and public health epidemiologists (e.g., event or case
-
detection, event status, specimen management, test procedures, quality assurance)
-
Detects bioterrorism attacks unlikely to be detected by existing environmental surveillance
-
Detects emerging infectious disease and other natural (unintentional) outbreaks
-
Aids surveillance of noninfectious health problems (e.g., injuries, chronic disease, intoxication)
Specific measures for enhancing surveillance in the public health and health care systems are discussed in Chapter 5 and listed in Box 6-1. Because formal evidence for the benefits of each proposed enhancement is limited and varies depending upon the enhancement, this qualitative assessment is largely based on expert opinion on the potential for improvement.
Note that the merits of expanding BioWatch to smaller localities need to be explored in risk-based analyses that account for many factors, including the probability of an attack in additional localities and the size of the potentially exposed population. With the current deployment in large urban areas, the BioWatch program has had the advantage of working with larger health departments that tend to have greater expertise and response capability than smaller health departments. Expanding BioWatch to more localities would require DHS to work with local health departments that are likely to need more federal interaction and support to be capable of analyzing and responding to a BAR.
EXAMINING THE POTENTIAL FOR EARLIER DETECTION TO IMPROVE OUTCOMES
To explore the impact of the timeliness of BioWatch detection and compare it with other surveillance approaches, the committee commissioned a simulation model to represent the timing of events in the detection of an aerosolized release of B. anthracis. In addition, a committee member (Stephen Pollock) developed an analytic approach (using the same model assumptions and parameters) as an alternative method to address these issues.
An anthrax attack was selected as a case study because timely response to inhalation anthrax is critical and because concerns about the threat from anthrax have driven much of the planning and action for bioterrorism preparedness. The model approaches were not designed to determine the likelihood that an anthrax attack would be detected by environmental sampling, but rather to assess the potential benefit from environmental detection, should an airborne attack occur and be detected. The model
|
BOX 6-1 Potential Enhancements to Surveillance Through Public Health and Health Care Legally Mandated Disease Reporting Enhance electronic laboratory reporting systems Enhance notifiable disease reporting by clinicians (outreach, electronic reporting procedures, 24/7 call lines) Enhance electronic death reporting systems Automated Health Care Information Systems and Public Health Linkages Enhance/use clinical decision support tools for diagnosis, reporting, and management (e.g., triage, infection control, and treatment) Enhance use of information from EMRs and other electronic health information sources to detect reportable conditions, unusual cases or trends (public health collects and collates information from multiple institutions) Enhance use of regional health information exchanges to detect reportable conditions, unusual cases or trends (public health interfaces with area-wide exchange that involves multiple institutions) Enhance public health capacity to electronically alert health care sector of important public health events (e.g. inform evaluation, triage, infection control, treatment) Laboratory and Diagnostic Testing Expand development and use of rapid multiplexed and point-of-care diagnostic tests for both common pathogens and bioterrorism agents Extend capacities to characterize pathogens and collate reports from geographically dispersed sources to identify related cases of infectious disease (e.g., PulseNet model for enteric pathogens) Expand/enhance capacity to collect and test clinical specimens as part of public health surveillance systems, either ongoing or in response to possible alerts Information Integration and Knowledge Sharing Improve integration and analysis of public health information within and across jurisdictions Enhance integration and analysis of public health information with other types of information (e.g., intelligence, law enforcement) |
evaluation does not address the potential merits of environmental sampling for pathogens other than anthrax.
A brief overview of the approach and results of the models is presented here.
Modeling Approach
The models evaluated three approaches for the detection of an anthrax release: clinical case finding, syndromic surveillance, and environmental air sampling. These approaches are defined as follows:
-
Clinical case finding: Diagnosis may be made each time a patient seeks clinical care following the onset of symptoms. Blood cultures may be ordered as part of routine testing. The time to detection is the time from exposure until the first positive blood culture among all people who seek care.
-
Syndromic surveillance: Centralized collection and processing of data from primary care settings and emergency departments related to visits for respiratory conditions. For these models, detection is defined as occurring when an alert from a statistical algorithm indicates a higher than expected number of visits. (In practice, such an alert would have to be investigated, and an outbreak identified, to be considered a detection.)
-
Environmental air sampling: Fixed aerosol monitoring devices (e.g., BioWatch air samplers) may be “hit” by airborne spores. Detection occurs when the presence of B. anthracis is confirmed through polymerase chain reaction (PCR) testing of samples collected by one or more monitors.
The specific objectives of the modeling were:
-
To estimate, for each approach to detection, the average time (and, in the analytical model, the distribution of time) to detection and the time to response, defined as the initiation of mass antibiotic prophylaxis.
-
If all three approaches operate in parallel, to estimate the average time to response and the contribution of each approach to early detection.
-
If only clinical case finding operates, to estimate the incremental benefit of adding syndromic surveillance, environmental sampling, or both.
Detection through any approach results in a subsequent investigation and preparation to initiate mass antibiotic prophylaxis. Two scenarios are defined for these investigations and preparations: (1) following a “strong” hit and (2) following a “weak” hit. An example of a strong environmental hit would be BARs from multiple environmental monitoring devices; a strong clinical hit
might be numerous positive blood cultures. A weak hit, in contrast, might be a BAR from a single monitoring device or a single positive blood culture. Clinical case finding and environmental sampling are assumed to have either a weak or strong hit while syndromic surveillance is assumed to have only a weak hit. For this simulation, a hit is assumed to trigger prophylaxis after a defined interval, and the disease is assumed to respond to prophylaxis or treatment, with subsequent reduction in morbidity and mortality.
The discrete-event Monte Carlo simulation model produces many replications of output variables of interest (e.g., time to first detection, time to prophylaxis). Model input parameters and probability distributions for various random times are based on the literature and expert opinion provided by committee members. The analytic model uses identical inputs to provide a direct, numerical computation of probability distributions for the output variables. The simulation model approach has the advantage of flexibility and ability to represent complex probabilistic events. The analytic approach provides exact values of outputs of interest and facilitates the use of sensitivity analyses to understand how assumptions and input parameters affect these outputs.
Results of the Modeling Analyses
The analyses indicate that, under highly favorable scenarios, BioWatch environmental sampling has the potential to lead to dispensing of antibiotics 2 to 3 days sooner after an aerosolized anthrax release than does syndromic surveillance or clinical case finding. Such an advantage in the time to dispensing may have a substantial impact on mortality from an anthrax attack, assuming that a prompt and effective mass dispensing program can be implemented. A critical uncertainty in these scenarios is whether an airborne attack would be detected by environmental sampling. Although environmental monitoring need not detect a release with certainty to provide a population benefit comparable to other means of detection, the probability of detection must be substantial to provide such a benefit.
The benefit from including syndromic surveillance in addition to clinical case finding depends on the sensitivity and specificity of the syndromic surveillance system, and on the time between exposure and patients’ presenting with symptoms. Based on prior research that indicated that syndromic surveillance would detect an anthrax attack in a mean of 84 hours (Buckeridge et al., 2006), the current analysis found that the probability that an attack would be identified by syndromic surveillance before clinical case finding ranged from 30 percent (strong hit for clinical case finding) to 50 percent (weak hit for clinical case finding) when the specificity of syndromic surveillance was 0.9. This level of specificity resulted in a false alarm every 10 days. The average time to dispensing was improved only
modestly by the addition of syndromic surveillance to clinical case finding, by a half day or less. Depending on how long it takes to confirm a signal from syndromic surveillance, this modest advantage in detection may not lead to faster antibiotic dispensing.
Recent analyses suggest that the incubation period of anthrax is dose dependent, and that people with massive exposures may be ill within 24 to 48 hours (Wilkening, 2008). Preliminary analyses performed for the committee, involving reanalysis of a previous study (Buckeridge et al., 2006) with the addition of a dose-dependent incubation period, suggest that the addition of syndromic surveillance to clinical case finding may have the potential to result in dispensing of antibiotics up to a day earlier than with case finding alone. These results require confirmation with additional analyses, but they suggest that there may be circumstances in which syndromic surveillance provides an important early indication of an aerosol release of B. anthracis.
The effect of earlier initiation of antibiotic dispensing on mortality is complex, depending on not only the reduction in time until dispensing but also when the reduction occurs and assumptions about the efficiency of the dispensing process. For example, a 24-hour reduction in time until initiating dispensing will reduce mortality to a different extent if it occurs at day 2 after exposure versus day 5 after exposure. Different authors have used different assumptions about the timing of antibiotic dispensing and other factors to estimate different mortality curves for anthrax (e.g., Kaufman et al., 1997; Wilkening, 2006, 2008; Yang et al., 2006; Baccam and Bechler, 2007). As reflected in the discussions in previous chapters, an important component of this interval is likely to be the time public health officials need from the detection of a threat (from whatever source) to determine that distribution of antibiotic prophylaxis is an appropriate response. It is also uncertain whether public health officials would be able to provide antibiotic prophylaxis to hundreds of thousands, if not several million, people within 48 hours of determining that a large-scale aerosol attack with anthrax spores has occurred—the time frame that CDC has recommended as the planning target in its Cities Readiness Initiative.
Despite the uncertainty surrounding the effect of earlier dispensing on mortality, the mortality models can still be used to qualitatively illustrate the explicit trade-offs in mortality reduction between reducing the detection time and increasing the likelihood of detection by environmental sampling.
Modeling Conclusions
These model-based analyses indicate that environmental sampling may have, with two important caveats, the potential to provide an advantage
in early response to an anthrax release. The advantage is only realized if the release is detected and if a relatively rapid and effective response subsequently occurs. Thus, in the restricted scenario discussed above, aerosolized anthrax spores released in a jurisdiction with deployed BioWatch detectors that generate a BAR provides a “best case” scenario for the performance of the BioWatch system in terms of gaining time to make decisions and deploy antibiotic prophylaxis. However, other scenarios may lead to different conclusions about the value of the BioWatch system. For example, BioWatch may show greater benefit if a pathogen causing a contagious disease is detected and effective quarantine and isolation procedures can be implemented to limit the spread of the disease. With pathogens having longer incubation periods, the interval between detection by environmental sampling and clinical case finding or syndromic surveillance could be greater than it is for anthrax, giving public health officials more time to implement their response. For noncontagious illnesses that may respond well to treatment even after diagnosis (unlike anthrax), the opportunity for earlier detection with BioWatch may contribute relatively little to improvements in survival because waiting until clinical recognition may still result in high survival rates, but a BioWatch detection may have other benefits.
Finally, the analyses suggest that, in some circumstances, syndromic surveillance may provide an important warning of an anthrax attack earlier than clinical case finding. Quantifying the magnitude of this benefit requires analyses that were not possible within the time frame of this study. A major limitation of these analyses is that they do not account for potential synergies between the different detection approaches; signals from separate systems may be used to corroborate the occurrence of an outbreak.
These models are first-cut simplifications, but nevertheless useful in identifying critical aspects of, and parameters governing, the response to a release. A more thorough modeling endeavor would have considered including more fidelity in the response modeling—in particular the probabilistic dependence between and among the random variables characterizing detection delays and response times, the size of an attack, and the state of readiness of the responders (a function of previous experience with false alarms, or alerts from other sources, etc.).
In summary, models can indicate certain circumstances, such as the anthrax scenario presented here, in which enhancement of the BioWatch system appears to provide benefits in terms of lives saved and illness avoided compared with traditional means of outbreak detection. The challenge, however, is to value this potential capability, given the uncertainties about (1) the likelihood of an attack, (2) the identity of the hazardous pathogens (either natural or intentionally released) that may be encountered, (3) the placement of detectors compared with the path of the release, (4) the performance of the various existing and proposed detection systems and methods, (5) the
capability of the public health and health care systems to respond rapidly and effectively to detection of a pathogen or the resulting illness, and (6) the probabilistic dependence among the critical variables involved in the response and in modeling the response, most critically the size of the attack and the consequent sensor detection times, and system response times.
Need for Formal Modeling and Evaluation of the BioWatch System
As emphasized in Chapter 3, there is a critical need for a more formal, detailed, and systematic approach to assessing various aspects of the effectiveness of BioWatch (see Recommendation 6). A definitive analysis was not performed during the course of this study, but the modeling framework presented here has the potential, in conjunction with information from the DHS Bioterrorism Risk Assessment (BTRA), to aid in understanding and comparing the relative benefits of environmental sampling, syndromic surveillance, and improved clinical case finding.2
Although rapid implementation of the BioWatch program was judged necessary in 2003, at this stage in the life of the program, DHS should be using available information and analytic tools to shape the program in a way that maximizes its potential benefits. This includes developing models that are more detailed to address the questions assessed in the modeling exercise described here. Such analyses should be performed for all pathogens that BioWatch is intended to detect. The value of including a particular pathogen in the BioWatch portfolio should reflect clear evidence of health benefits from earlier detection and minimal risk of detecting DNA fragments from organisms that are naturally present in the environment of BioWatch jurisdictions, as well as evidence of the probability of attack and detection sufficient to support the additional cost of surveillance for the pathogen. The results of these analyses should be used as part of the documentation presented for justifying enhancements for BioWatch or other approaches to achieving early detection of biothreats.
DHS should conduct these assessments in close coordination with HHS and state and local public health partners, and with the advice of the recommended advisory panel, because these partners are crucial in realizing the potential benefits of BioWatch. The scenarios contained in the BTRA provide a foundation upon which to base an evaluation of the contribution of the BioWatch program to its public health goals. With several years of operational experience in hand, the models can now
include realistic assumptions about the use of BioWatch results by public health authorities.
AN ECONOMIC PERSPECTIVE ON COSTS AND BENEFITS OF BIOWATCH
The committee was also asked to examine the costs of surveillance through BioWatch and through the public health and health care systems. The explicit federal program costs for BioWatch since 2003 can be tracked in the aggregate through DHS budget documents, but as discussed in previous chapters, the costs of other surveillance activities across federal, state, and local levels and the public and private sectors are much harder to document (Hebert et al., 2007; GAO, 2008a; Franco, 2009).
To gain some perspective on the costs of the BioWatch program, the committee used the framework of a “break even analysis.” The reasonableness of the costs can be considered from three points of view: (1) whether the expected benefits from BioWatch are likely to be greater than its costs for the country as a whole, (2) how the economic and logistical burdens of operating BioWatch are distributed, and (3) whether this distribution is acceptable to the affected parties. It is particularly important to understand the impact of BioWatch on state and local governments because they have the front-line responsibility for making most public health response decisions. Their perceptions of the reasonableness of the costs associated with BioWatch can be expected to strongly influence the acceptability and usefulness of the program.
National Perspective on Costs and Benefits of BioWatch
The most direct benefits expected from BioWatch are marginal reductions in the risk of loss of life or morbidity from early detection of a bioterrorism event. This is the life-saving contribution of a BioWatch alert over and above the normal functioning of public health and health care system surveillance and response. The modeling analysis presented earlier in this chapter offers some information on potential benefit in the form of an estimate of the expected reduction in time between attack and treatment that could result from BioWatch deployment, given a detectable attack. To make some assessment of the value of achieving the potential benefits of BioWatch, the committee considered the program’s costs, as discussed in Chapter 2, and estimates of the public’s willingness to pay for reduction in mortality risks. This is a widely accepted practice in evaluation of proposed health and safety regulations (The White House, 1993; EPA, 2000). Formally, estimates of willingness to pay for reductions in mortality risk are called “value of statistical life” (VSL) measures.
The Value of a Statistical Life
A “statistical life” is a population-level measure of mortality risk. It aggregates changes in individual, annual mortality risk to a population level. To illustrate, an action that reduces the annual mortality risk by 1/100,000 for each member of a population of 100,000 saves 1 statistical life over a year (Robinson, 2008). This change in mortality risk can be valued by looking at trade-offs people regularly make between expenditure of time or money and risk to safety and health. For example, many people trade off the benefit from saving the time it would take to walk to the corner to use a crosswalk against the increased risk of injury or death from jay-walking. Time can be given a monetary value based on wage rates and on a growing literature on the value people attach to time spent in nonwork activities. VSL estimates measure the magnitude of these trade-offs. For example, if on average people in a population of 100,000 are each willing to pay $60 to reduce mortality risk by 1/100,000, the implied VSL is $6 million [$60 ÷ (1/100,000)].
Under guidance from the Office of Management and Budget (OMB), agencies have leeway to develop their own criteria for regulatory economic analysis, including the choice of VSL estimates, but the choice must be based on a review of available research. OMB recognizes that best practices will therefore change with further research. Federal agencies currently use VSL estimates with midpoints ranging from $5 million to $7.6 million in their regulatory impact assessments (Robinson, 2008). In 2008, a component of DHS adopted use of a VSL of $6.3 million (Coast Guard, 2008; Robinson, 2008). These VSL values are used to monetize the value of benefits of government programs and are then compared to the expected costs of the program to society as means of determining the reasonableness of programs in regulatory impact analysis.
A significant body of research, in both the risk perception literature and the health valuation literature, strongly suggests that people are willing to pay more to reduce risks that they cannot control and risks that they dread (Chilton et al., 2006; Jenkins, 2006). An example of this might be the risk of illness and death from exposure to radiation from a nuclear accident. Evaluations of this literature conclude that VSLs for these types of risk are twice as high as those for commonplace risks that may be higher but over which individuals feel that they have some control, such as driving a car (Robinson, 2008).
Assessing the “Value” of the BioWatch Program
The committee used the VSL adopted by DHS to explore the costs of the BioWatch program in relation to the number of lives the program would
need to save over a specified period of time—10 years in this case—to “break even” if the only benefit were an incremental reduction in mortality risk. Making this calculation is complicated by not knowing the probability of an attack, the timing of an attack, the scale of an attack, the likelihood that an attack is in a form detectable by BioWatch, or the number of people exposed in an attack. If, for example, the probability of an attack over the 10-year interval is very low, a decision maker may have expectations that for a given program cost, the benefit in additional lives saved would need to be greater than if the probability of an attack during that period were high. Alternatively, if the dread of a bioterrorist event means that a higher VSL is acceptable, the number of additional lives that BioWatch would be expected to save to break even would be perhaps half as high as the standard VSL suggests.
Because of the fundamental unknowns, the committee chose to make simplifying assumptions that (1) the likelihood of attack is the same each year over the next 10 years and (2) the annual probability of a single, BioWatch-detectable attack is .1, .01, or .001. These assumptions are used solely because they are transparent and easily understood. The calculations also assume $6.3 million for a VSL, a 7 percent real discount rate as used in the cost analysis in Chapter 2, and income growth of 3 percent per year. To “break even” with an annual probability of attack of .1 per year, or 1 attack expected in 10 years, BioWatch Generation 2 would need to save 134 additional lives if an attack occurs; Generation 3 (configured according to the proposed acquisition and deployment plans) would have to save an additional 447 lives. With an annual probability of attack of .001 (a .01 chance of an attack in 10 years), BioWatch would need to save 13,398 additional lives with Generation 2 and 44,661 additional lives with the full acquisition and deployment of Generation 3. Table 6-2 shows the results of this simplified break even analysis.
The committee’s calculation is a conservative assessment of the potential benefits of the BioWatch system because it accounts only for reducing mortality in the event of a bioterrorism attack. Other benefits may include illnesses avoided and social and economic disruption averted. However, even successful detection of an attack would not completely prevent economic and social disruption. One estimate of the global costs in 2003 of the SARS outbreak is at least $40 billion (Lee and McKibbin, 2004). Another possibility not reflected in these calculations is the possible deterrent effect of BioWatch, which might lead a terrorist to choose another location or method of attack that puts fewer lives at risk. The committee did not undertake the complex task of estimating the potential impact of BioWatch on social and economic consequences of a biological attack, but such analyses should be part of the work that the committee recommends DHS perform in evaluating the BioWatch system (see Chapter 3, Recommendation 6).
TABLE 6-2 Implied Number of Additional Lives That BioWatch Would Have to Save in the Event of an Attack to “Break Even” on Program Costs
State and Local Impact
As noted in Chapter 2, the committee was able to obtain only limited information on the unreimbursed costs to states and localities where BioWatch operates. The data that were obtained cover only two types of in-kind contributions: (1) for laboratory support and analysis and (2) for public health support and response planning (e.g., participation on the BioWatch Advisory Committee, planning for responses to detection of true bioterrorism events, and planning and conducting exercises and responses to BARs). In each case, the costs were small relative to the jurisdictions’ total public health budgets.
Yet the committee heard representatives from multiple local and state jurisdictions raise concern about the burden placed on them by BioWatch. This concern does not seem to be based solely on the actual costs of BioWatch, but rather on a sense of skepticism about the public health value of BioWatch. Two factors appear to be fueling this skepticism. First, local and state public health departments are under increasing budgetary and staffing pressure. When confronted with a trade-off between expending time and energy to meet a steady stream of immediate public health needs, such as surveillance, treatment, and prevention of sexually transmitted diseases or detection of outbreaks of foodborne illness, and planning for high-impact but very rare, and perhaps hypothetical, events, such as a catastrophic airborne bioterrorism attack, the value of meeting the immediate need may seem greater.
Second, officials in some local and state public health jurisdictions do not appear confident that BioWatch would enhance their ability to reduce illnesses and death if an attack were to occur. Because interpretation of BARs is not straightforward, gathering sufficient information to determine an appropriate course of action may consume valuable lead time and staff resources. DHS may be able to boost state and local confidence in the value of BioWatch through thorough analyses and field testing of the technology and operating plans, along with informed participation by the users of BioWatch information in planning and decisions about BioWatch system operations.
COMPLEMENTARY SURVEILLANCE ROLES FOR BIOWATCH AND PUBLIC HEALTH AND HEALTH CARE
With certain reservations, the committee concluded that the BioWatch system in its current form (Generation 2) can fill a unique and complementary functional niche in the nation’s resources for detecting a potentially high-consequence biological risk, especially from a large-scale release of aerosolized anthrax spores. BioWatch has the potential to provide an earlier warning of the airborne presence of specific pathogens before the onset of human illness than surveillance tools based on clinical findings. By the same token, because BioWatch is so narrowly focused as a detection tool, it does not eliminate the need for the broad-based surveillance activities that can detect bioterrorism or naturally occurring disease outbreaks that BioWatch (enhanced or not) cannot or does not detect.
The Generation 3 deployment of BioWatch, as described to the committee, is expected to analyze air samples more frequently than the current system and therefore should be able to produce more timely alerts. But determining the appropriate public health response will still require expert synthesis of additional information to interpret the significance of the BAR. Such information may include which pathogen is detected, the number of BioWatch collectors producing alerts, whether the pathogen detected is known to be endemic to the area, or whether there is relevant intelligence information indicating the potential for an attack. Although the absence of a signal from BioWatch collectors cannot be taken as firm evidence that no release has occurred, positive signals from multiple collectors in a single jurisdiction, or information that collectors in other jurisdictions have produced BARs, could influence interpretation of the significance of a signal and decisions regarding the speed and scope of the public health response.
Enhancements to surveillance carried out through the public health and health care systems may also improve the timeliness of recognition of disease or even prodromal symptoms, whether from a bioterrorist event or
from a naturally occurring outbreak. But because these surveillance tools depend on detecting infections that follow exposure by hours or days, they cannot match the potential for earlier awareness provided by the BioWatch system.
With or without BioWatch, the public health and health care systems need to be capable of rapid and thoughtful review of clinical, animal, environmental, and law enforcement data by local experts with knowledge of the specific features of the area and its population. Figure 6-1 illustrates the variety of information sources that contribute to detection of significant public health threats. At best, BioWatch will only be one of these sources. Experience over the past few years with the emergence of the mosquito-borne West Nile virus, the international spread of SARS, repeated episodes of multistate foodborne illness, and the 2009 influenza pandemic demonstrates the importance of having many other surveillance resources to facilitate quick understanding of the nature and extent of the health threat.
FINDING: BioWatch and surveillance through the public health and health care systems are complementary. If a large-scale aerosol attack using certain pathogens were to occur in specific localities, BioWatch has the potential to provide a more timely alert than the public health
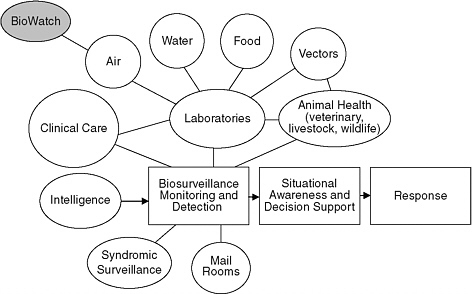
FIGURE 6-1 A schematic illustration of the relation between the BioWatch program and other sources of information needed for infectious disease surveillance in the public health and health care systems.
SOURCE: Adapted from Hooks (2008).
and health care systems. Surveillance through the public health and health care systems is broader and more flexible and is an essential element of daily activities at local, state, and federal levels to fully meet the public’s health needs.
Table 6-3 provides a broad-brush summary of key features of the narrowly targeted BioWatch and the wide scope of more traditional surveillance systems. It is challenging to make this kind of summary comparison because of the vast differences in the purpose and scope of the BioWatch system and infectious disease surveillance activities through the public health and health care systems.
The cost comparison is especially problematic. The BioWatch program is a relatively well-defined federal program, with some unidentified but limited costs incurred by the states and localities where it operates. On the other hand, the committee was unable to obtain information that would allow a determination of the costs of infectious disease surveillance for significant biological threats, or specifically for bioterrorism threats. These costs cannot be readily separated from other health surveillance programs, and as discussed in Chapter 5, the costs of the broader surveillance activities in the public health and health care systems are also difficult to estimate because current budgeting and accounting systems at the local, state, and federal levels do not use classifications that provide this information. The surveillance costs incurred by hospitals, outpatient services, laboratories, and the other private-sector components of the health care system are even less readily captured. In addition, the development costs and longer-term financial impact of enhancements to infectious disease surveillance, such as those discussed in Chapter 5, are unclear at the national level, and the costs may vary considerably for individual states and localities.
The only readily available reference point is a federal government estimate that total expenditures for federal, state, and local government public health activities amounted to $64.1 billion for 2007 (Hartman et al., 2009). This includes all types of public health activities, ranging from public health education to restaurant inspection as well as disease surveillance. The committee urges further examination of the costs of developing, operating, and modifying surveillance systems and related public health activities. Better cost information will be essential for officials at the federal, state, and local levels to assess the effectiveness of current and future approaches. In addition, new analytic approaches may be needed to study the cost-effectiveness of the broader disease surveillance infrastructure and its infectious disease components.
FINDING: The annualized direct costs of the current BioWatch Generation 2 program over the next 10 years will be approximately $80 million;
TABLE 6-3 Capabilities and Costs of the BioWatch System and Surveillance Through the Public Health and Health Care Systems
|
System |
Detection Capability |
Coverage |
Sensitivity and Specificity |
|
BioWatch System |
|||
|
Generation 2 |
Certain biological agents; environmental presence of airborne genetic material |
> 30 major metropolitan areas (i.e., BioWatch jurisdictions) |
Not publicly available Dozens of BARs to date; none linked to bioterrorism |
|
Generation 3 (proposed) |
Biological agents covered by Generation 2, with goal of including additional agents; environmental presence of airborne pathogens |
Expanded coverage |
System not yet selected |
|
Public Health and Health Care Systems |
|||
|
Disease recognition and reporting by health care providers and laboratories to health departments |
All human health hazards (e.g., biologic, chemical, environmental) that result in clinically recognized disease or injury |
Entire country, reports of uneven quality submitted to nearly 3,000 local and state health departments |
Not readily quantified; varies by disease, provider, location, reporting system, epidemiologic expertise, and other resources |
|
Timeliness |
Benefits |
Annual Costs |
Other Considerations |
|
Typical 24-hour sample collection cycle; 10- to 36-hour window from release to confirmation of screening test |
If aerosolized pathogen is detected, potential reduction in casualties if distribution of prophylaxis and treatment can be started sooner; potential interruption of contagion or environmental spread* |
$80 million (10-year average) Costs of recommended program changes not included |
Need for testing and evaluation of technology, holistic evaluation of goals, better tools to aid public health response, and assessment of environmental risk after a BAR |
|
Proposed 4- to 6-hour time to detect; automated sample processing and testing and reporting of results |
Potential for reduction in casualties may be greater than with Generation 2 because of plans for more frequent testing* |
$200 million (10-year average) for acquisition, deployment, and operation Costs of recommended program changes not included |
System not yet selected; actual performance may differ from proposed specifications; significant technological hurdles must be overcome to achieve desired system capabilities |
|
May depend on disease, skill of health care provider, availability of appropriate analysis tools, scale of pathogen exposure, reporting system; depends on evidence of infection, so not likely to detect before environmental surveillance |
Provide ongoing detection of intentional and naturally occurring outbreaks for prevention or treatment |
Unknown; data necessary to estimate costs of disease surveillance systems or marginal cost of surveillance for significant infectious disease threats not available |
Need for additional integration and information sharing across federal, state, and local levels; need for evaluation and incorporation of new techniques |
|
System |
Detection Capability |
Coverage |
Sensitivity and Specificity |
|
Syndromic surveillance |
Varies by system design and application |
Currently > 80% of state, tribal, large local jurisdictions have some form |
Varies by system design and application, scale of outbreak |
|
NOTE: BAR, BioWatch Actionable Result. *As described in the text, achieving a reduction in mortality with the BioWatch system depends not only on the BioWatch technology and communication of a BioWatch Actionable Result, but also on expeditious information gathering to confirm a bioterrorist event and having the capability to distribute mass prophylaxis or treatment to prevent or reduce illness and mortality. Also see Table 6-1. |
|||
over the same 10 years, the annualized direct costs for acquiring and operating the planned Generation 3 enhancement are projected to be roughly $200 million. The committee was unable to obtain information that would allow a determination of the costs attributable to infectious disease surveillance in the public health and health care systems. Better data are needed on these costs as part of any effort to assess the cost-effectiveness of infectious disease surveillance and measure improvements. In addition, innovative methods may be needed for assessing the impacts of a multifaceted and multipurpose public health infrastructure such as infectious disease surveillance.
INCORPORATING BIOWATCH INTO AN ENHANCED NATIONAL SURVEILLANCE SYSTEM
The nation lacks a clear, overarching architecture of interlocking interagency goals, metrics, and accountability to support a seamless process from detection of biological threats through response and recovery. This architecture will require strong, high-level leadership along with strong and consistent engagement of on-the-ground experts in federal, state, and local agencies. This architecture is both critical to, and must also be supported by, robust operational capabilities and information systems that allow for merging data from multiple sources and providing critical emergency planning and decision support to biodefense partners across local, state, and federal levels.
|
Timeliness |
Benefits |
Annual Costs |
Other Considerations |
|
May depend on scale of pathogen exposure, but not likely to detect before environmental surveillance |
May help detect and track sporadic and recurring infectious disease outbreaks |
Would be included in costs of disease surveillance; cost of developing and operating individual systems expected to vary widely |
Requires further testing and evaluation to assess strengths and limitations as tool to aid detection of infectious disease outbreaks |
Improving the Integration of BioWatch into Biosurveillance
BioWatch is a federally developed, implemented, and directed program to detect certain airborne biological pathogens, but it depends on state and local health departments to act on the information it produces. This configuration invites tensions from competing interests. Some of the challenges of integrating BioWatch functions into surveillance and decision making carried out by the public health community may stem in part from its superimposition onto existing systems that serve other important priorities. Local and state public health departments have diverse responsibilities that include both routine and outbreak surveillance activities, independent of the responsibilities that come with participation in the BioWatch program. As Figure 6-1 illustrates, BioWatch is just one of the many sources of information that health departments must monitor and evaluate on a continuing basis.
The imposition of new responsibilities for low probability events may be seen by local jurisdictions as detracting from their daily duties and responsibilities, and therefore may potentially be perceived as inappropriately burdensome. The occurrence to date of numerous BARs, all of which were determined to be unrelated to bioterrorism and each of which required an official and time-consuming response by local and state officials, has served to increase skepticism of the value of the program. Moreover, some communities may have been reluctant to participate in BioWatch because of its low perceived value but could not readily decline.
The apparently top-down approach that DHS has taken in its interaction with public health departments may be symptomatic of a departmental mindset. DHS has been urged before in other arenas to improve its partnerships with state and local stakeholders (e.g., GAO, 2008c). DHS has noted its plans to increase the involvement of state and local officials in their BioWatch planning, and it has taken some steps in this direction through a contract with the Center for Infectious Disease Research and Policy (CIDRAP) to promote communication among epidemiologists in BioWatch jurisdictions and between the epidemiologists and the BioWatch program (CIDRAP, no date).
Differences in priorities between local and federal entities are natural and are explicitly acknowledged in the National Response Framework: “Planning for low-probability, high-consequence scenarios is a [f]ederal focus and complements a [s]tate, tribal, and local focus on more likely and frequently experienced smaller-scale events” (FEMA, 2008, p. 71). The committee also recognizes that the BioWatch system was fielded rapidly and had to meet numerous technological, operational, and organizational challenges. Although the deployment of the BioWatch system has been somewhat rocky in terms of coordination and integration with local public health officials, there is a continuing national effort to achieve a more integrated system from the multitude of local and state systems for infectious disease surveillance. All told, the BioWatch system needs to be better integrated into local surveillance systems that themselves are ultimately better integrated into a whole that resembles more of a national biosurveillance system. With multiple demands on the attention and resources of public health authorities, such integration may be vital to the sustainability of the BioWatch system and its counterterrorism mission. This challenge is only made more urgent under the current conditions of increasing economic constraint.
An Enhanced National Biosurveillance System
A major challenge to meeting the goal of a “national” biosurveillance system is that authority for public health action resides primarily at the state and local levels, where a multitude of systems and approaches make for a fragmented and difficult-to-coordinate national picture. The federal government has operational responsibilities in certain limited areas of public health protection, but it wields considerable influence through leadership in developing tools and guidance and especially through funding programs that reflect federal priorities.
How can progress be made toward a national system when a top-down approach is not possible? National priorities should include ensuring that state and local health departments can meet and sustain basic performance
standards for surveillance and analysis, in conjunction with essential capabilities to respond both to the often unpredictable demands of public health emergencies and to the array of ongoing obligations to protect the public’s health. It is also important that BioWatch be factored into this view. But with programmatic and budgetary responsibility for BioWatch resting with DHS, the challenges of coordinating related responsibilities and systems that are dispersed across other federal departments only adds to the complexity of coordinating biosurveillance across federal, state, and local levels.
It is equally essential that the linkages between the public health and health care systems be strengthened. Health care providers in the public and private sectors, along with the large community of clinical and commercial laboratories, are essential partners with public health. Because the surveillance needs of the public health system are not their first priority, it is essential that the public health perspective be effectively represented as health care and laboratory information systems evolve so that technologies are created that serve needs in both enhancing the care of individual patients and protecting the health of the public.
In the committee’s view, a national biosurveillance system should aid in protecting the nation from significant, time-critical biological threats of all types, whether intentionally released (i.e., bioterrorism), accidentally dispersed, or naturally arising (i.e., emerging or re-emerging infectious disease). This system would employ a layered approach that includes
-
judicious use of environmental surveillance tools, such as BioWatch, to detect a small number of current and potential future airborne threats that could harm a large number of people;
-
an enhanced capacity to recognize, isolate, and report index cases from clinical settings;
-
refined approaches to analyzing epidemiologic data to detect aberrant signals that may indicate a disease outbreak and track its spread;
-
improved and more rapid methods for use in the laboratory or at the point of care to detect, verify, and characterize a biological threat;
-
improved tools for information management and communication among all appropriate stakeholders (e.g., electronic laboratory reporting systems);
-
streamlined approaches at the federal, state, and local levels to (a) intelligence and information sharing, (b) decision making to minimize delay between the emergence of a threat and appropriate action to minimize its consequences, and (c) timely after-action analysis to identify strengths and weaknesses in policies and procedures; and
-
sustainable funding that encourages program integration (in contrast to categorical funding streams).
The committee sees an important federal role in at least three critical areas: (1) helping to develop and validate improved methods for infectious disease surveillance, (2) developing a mechanism to achieve and sustain national situational awareness of biological threats, and (3) building and sustaining critical public health workforce competencies.
Improved Methods for Infectious Disease Surveillance
Surveillance is an essential public health practice, but it has long rested on multiple independent data collection activities, often constrained by limited resources and narrow programmatic requirements. As information and communication technologies have evolved and become more accessible to health departments and health care providers, they are making it possible to improve the collection, integration, and analysis of surveillance data. Many novel and promising surveillance techniques and programs have been developed rapidly at the local, state, and federal levels, spurred in part by funding for bioterrorism and public health emergency preparedness.
At present, national standards for surveillance data and for interoperability between surveillance systems are incompletely developed and unevenly implemented because of limited funding and inconsistent direction. Insufficient attention has been paid to linking, analyzing, and displaying multiple surveillance platforms for optimal situational awareness, decision making, and response. In addition, many new systems and techniques have not yet received sufficient evaluation to ensure that they are effective and being used appropriately.
The committee believes that this complex series of problems must be addressed by a dedicated, strategic, integrated, and adequately funded program. If the federal government, working collaboratively with state and local officials, were able to strengthen and streamline and, where possible, automate core public health surveillance functions such as clinical case finding and laboratory reporting, this could reduce the daily operational stress felt by many local and state health departments and foster better working relationships.
Situational Awareness
In addition to new and improved surveillance tools, there is a critical need for a means to bring information together to provide situational awareness regarding potential or active threats to the public’s health. Most of the information that enables detection, characterization, and ongoing management and mitigation of both natural and bioterrorism-related outbreaks is generated at the local or regional level and typically assembled at a statewide level. Compartmentalization of this information based on geography,
agency, or professional subject area may impede detection and interpretation of events that are unfolding on a national or international scale.
Health departments also have increased access to electronic information on animal health, vector control, water and air quality, meteorology, and other information to aid in monitoring threats to human health. Resources for situational awareness also need to facilitate communication between public health officials and clinical providers. In addition, efforts are being made to increase information sharing between public health agencies and others with a role in emergency preparedness and response, including the intelligence, law enforcement, emergency management, and business communities.
At the federal level, activities in both DHS and HHS are aimed at more effective integration of information to improve situational awareness. In DHS, the National Biosurveillance Integration Center (NBIC) is intended to bring together national and international information that could enhance awareness of potential and active biological threats of all types. In HHS, the BioSense program is focusing on assembling and sharing data from public health and health care sources to support situational awareness at local, state, and federal levels. Another effort to promote information exchange and situational awareness is the development of state and local “fusion” centers, which facilitate access to and sharing of information between federal, state and local officials.
These information-sharing activities face several challenges. On the informatics and technology side, the challenges include reconciling data structures and vocabularies from independent information systems. Policy concerns include finding an appropriate balance between the national security concerns of DHS and the intelligence community, which have tended to favor limited access to the information, and the need for an effective partnership with state and local officials who need better access to information that will help them recognize and respond to bioterrorism and other significant biological threats. Another consideration is the need to ensure that the exchange of health-related information appropriately calibrates the need for personally identifiable information so that individual privacy is protected. In addition, there is a need to encourage local and state authorities to share information within a state, with other states, and with federal agencies. Their willingness to do so will typically be related to the net value they obtain from such exchanges.
Public Health Workforce Levels and Competencies
The committee has recommended (see Chapter 5, Recommendation 11) that HHS and DHS make bolstering the public health workforce a priority. Benefits from new methods or tools will be limited without sufficient, and adequately trained, personnel to employ and deploy them. Public
health surveillance to detect and investigate potential bioterrorism events or naturally occurring infectious disease outbreaks requires the continuous availability of well-trained laboratory and epidemiology staff in sufficient numbers to be able to conduct advanced laboratory analyses and epidemiologic investigations in real time.
State and local health departments have faced persistent difficulties in hiring and retaining personnel and now face new budget constraints that are forcing further staff reductions in many places. Moreover, as new technologies and analytic techniques become the norm, there is a need to ensure access to training in their use for the existing workforce and to ensure that academic programs are adequately preparing the workforce of the future to use them. Cooperation between academia and the public health practice community can help identify appropriate competencies and develop credentials that foster readiness to respond to biothreats.
CONCLUDING OBSERVATIONS
BioWatch is a federal program designed by DHS to be operated by the public health system at the state and local levels in order to serve national security interests. This is an awkward and organizationally challenging arrangement. Because the potential for BioWatch to help limit morbidity and mortality depends so heavily on the ability of health departments and the health care system to analyze the nature of the threat and take quick and decisive action, it is essential that the operation and management of BioWatch be well integrated with the jurisdictions in which it operates.
State and local authorities, whose knowledge of endemic health risks and available resources cannot be replicated at the federal level, need to be recognized as essential and valuable partners not only in the BioWatch program but also in broader national biosurveillance and biodefense efforts. Likewise, recognizing that a bioterror attack is a threat against the entire population of the United States, state and local officials need to remain open and willing to partner with the federal government to ensure that resources, expertise, and decisions are used in the most appropriate way to ensure our national security.
Finally, it is essential that policy makers recognize that the benefits of any form of infectious disease surveillance will not be realized if states and communities do not also have the capability to respond effectively to a public health emergency. Despite the substantial progress that many localities have made in advancing their mass dispensing capacity, having the ability to administer antibiotic prophylaxis to hundreds of thousands, if not several million, urban area residents within a few days following detection of a bioterrorist attack remains a challenge.
REFERENCES
Baccam, P., and M. Boechler. 2007. Public health response to an anthrax attack: An evaluation of vaccination policy options. Biosecurity and Bioterrorism: Biodefense Strategy, Practice, and Science 5(1):26–34.
Bravata, D.M., K.M. McDonald, H. Szeto, W.M. Smith, C. Rydzak, and D.K. Owens. 2004. A conceptual framework for evaluating information technologies and decision support systems for bioterrorism preparedness and response. Medical Decision Making 24(2):192–206.
Buckeridge, D.L. 2007. Outbreak detection through automated surveillance: A review of the determinants of detection. Journal of Biomedical Informatics 40(4):370–379.
Buckeridge, D.L., P. Switzer, D. Owens, D. Siegrist, J. Pavlin, and M. Musen. 2005. An evaluation model for syndromic surveillance: Assessing the performance of a temporal algorithm. Morbidity and Mortality Weekly Report 54(Suppl.):109–115.
Buckeridge, D.L., D.K. Owens, P. Switzer, J. Frank, and M.A. Musen. 2006. Evaluating detection of an inhalational anthrax outbreak. Emerging Infectious Diseases 12:1942–1949.
CDC (Centers for Disease Control and Prevention). 2001. Updated guidelines for evaluating public health surveillance systems: Recommendations from the Guidelines Working Group. Morbidity and Mortality Weekly Report 50(RR-13):1–29. http://www.cdc.gov/mmwr/PDF/rr/rr5013.pdf (accessed August 7, 2009).
CDC. 2004. Framework for evaluating public health surveillance systems for early detection of outbreaks: Recommendations from the CDC Working Group. Morbidity and Mortality Weekly Report 53(RR-5):1–16. http://www.cdc.gov/mmwr/PDF/rr/rr5305.pdf (accessed August 7, 2009).
Chilton, S., M. Jones-Lee, F. Kiraly, H. Metcalf, and W. Pang. 2006. Dread Risks. Journal of Risk and Uncertainty 33(3):165–182. http://www.springerlink.com/content/p02538767t15252r/fulltext.pdf (accessed April 10, 2009).
CIDRAP (Center for Infectious Disease Research and Policy). No date. Public health and BioWatch. http://www.cidrap.umn.edu/cidrap/center/mission/articles/biow.html (accessed April 2, 2009).
Coast Guard. 2008. Vessel Requirements for Notices of Arrival and Departure, and Automatic Identification System. Proposed Rule. Federal Register 73(242):76295–76318.
Craft, D.L., L.M. Wein, and A.H. Wilkins. 2005. Analyzing bioterror response logistics: The case of anthrax. Management Science 51(5):679–694.
DHS (Department of Homeland Security). 2008. BioWatch Gen-3 autonomous detection system: Operational requirements document (ORD). Version 1.0, December 11. Washington, DC: Office of Health Affairs.
DHS. 2009. BioWatch Gen-3 request for proposal: HSHQDC-09-R-00045. https://www.fbo.gov/download/54e/54e9ae25a4a9d2d39a9e9e836955001f/HSHQDC-09-R-00045_ (5-27-2009).pdf (accessed August 5, 2009).
EPA (Environmental Protection Agency). 2000. Guidelines for preparing economic analyses. EPA 240-R-00-003. Washington, DC: EPA. http://yosemite.epa.gov/ee/epa/eed.nsf/webpages/homepage (accessed July 16, 2009).
FEMA (Federal Emergency Management Agency). 2008. National response framework. Washington, DC: DHS. http://www.fema.gov/pdf/emergency/nrf/nrf-core.pdf (accessed August 7, 2009).
Franco, C. 2009. Funding biodefense. Biosecurity and Bioterrorism: Biodefense Strategy, Practice, and Science 7(1):1–2.
GAO (Government Accountability Office). 2008a. Health information technology: More detailed plans needed for the Centers for Disease Control and Prevention’s redesigned BioSense program. GAO-09-100. Washington, DC: GAO. http://www.gao.gov/new.items/d09100.pdf (accessed March 20, 2009).
GAO. 2008b. Homeland security: First responders’ ability to detect and model hazardous releases in urban areas is significantly limited. GAO-08-180. Washington, DC: GAO. http://www.gao.gov/products/GAO-08-180 (accessed July 16, 2009).
GAO. 2008c. National response framework: FEMA needs policies and procedures to better integrate non-federal stakeholders in the revision process. GAO-08-768. Washington, DC: GAO. http://www.gao.gov/products/GAO-08-768 (accessed July 16, 2009).
Hartman, M., A. Martin, P. McDonnell, and A. Catlin. 2009. National health spending in 2007: Slower drug spending contributes to lowest rate of overall growth since 1998. Health Affairs 28(1):246–261. http://content.healthaffairs.org/cgi/content/short/28/1/246 (accessed July 16, 2009).
Hebert, K., N. Henderson, and E.A. Gursky. 2007. Building preparedness by improving fiscal accountability. Journal of Public Health Management and Practice 13(2):200–201.
Holty, J.E., D.M. Bravata, H. Liu, R.A. Olshen, K.M. McDonald, and D.K. Owens. 2006. Systematic review: A century of inhalational anthrax cases from 1900 to 2005. Annals of Internal Medicine 144(4):270–280.
Hooks, R. 2008. Presentation and public comments to the Committee on Effectiveness of National Biosurveillance Systems: BioWatch and the Public Health System, Meeting 1, July 30–31, Washington, DC.
Hupert, N., A.I. Mushlin, and M.A. Callahan. 2002. Modeling the public health response to bioterrorism: Using discrete event simulation to design antibiotic distribution centers. Medical Decision Making 22(Suppl. 1):S17–S25. http://mdm.sagepub.com/cgi/reprint/22/suppl_1/s17 (accessed February 12, 2009).
Inglesby T.V., T. O’Toole, D.A. Henderson, J.G. Bartlett, M.S. Ascher, E. Eitzen, A.M. Friedlander, J. Gerberding, J. Hauer, J. Hughes, J. McDade, M.T. Osterholm, G. Parker, T.M. Perl, P.K. Russell, K. Tonat, and the Working Group on Biodefense. 2002. Anthrax as a biological weapon, 2002: Updated recommendations for management. Journal of the American Medical Association 287(17):2236–2252.
Jenkins, C.M. 2006. Risk perception and terrorism: Applying the psychometric paradigm. Homeland Security Affairs 2(2):1–14.
Jernigan, J.A., D.S. Stephens, D.A. Ashford, C. Omenaca, M.S. Topiel, M. Galbraith, M. Tapper, T.L. Fisk, S. Zaki, T. Popovic, R.F. Meyer, C.P. Quinn, S.A. Harper, S.K. Fridkin, J.J. Sejvar, C.W. Shepard, M. McConnell, J. Guarner, W.J. Shieh, J.M. Malecki, J.L. Gerberding, J.M. Hughes, B.A. Perkins, and members of the Anthrax Bioterrorism Investigation Team. 2001. Bioterrorism-related inhalational anthrax: The first 10 cases reported in the United States. Emerging Infectious Diseases 7(6):933–944.
Kaufmann, A.F., M.I. Meltzer, and G.P. Schmid. 1997. The economic impact of a bioterrorist attack: Are prevention and post-attack intervention programs justifiable? Emerging Infectious Diseases 3(2):83–94. ftp://ftp.cdc.gov/pub/EID/vol3no2/adobe/kaufman.pdf. (accessed November 2, 2008).
Lee, W., and J.W. McKibbin. 2004. Estimating the global economic costs of SARS. In Learning from SARS. Washington, DC: The National Academies Press. Pp. 92–109.
Robinson, L. 2008. Valuing mortality risk reductions in homeland security regulatory analyses. Final Report to U.S. Customs and Border Protection, Department of Homeland Security. June 2008. Cambridge, MA: Industrial Economics, Inc.
Wein, L.M., and D.L. Craft. 2005. Evaluation of public health interventions for anthrax: A report to the Secretary’s Council on Public Health Preparedness. Biosecurity and Bioterrorism: Biodefense Strategy, Practice, and Science 3(4):348–356.
The White House. 1993. Executive Order 12866 – Regulatory Planning and Review. http://www.whitehouse.gov/omb/inforeg/eo12866.pdf (accessed August 7, 2009).
Wilkening, D.A. 2006. Sverdlovsk revisited: Modeling human inhalational anthrax. Proceedings of the National Academy of Sciences 103(20):7589–7594.
Wilkening, D.A. 2008. Modeling the incubation period of inhalational anthrax. Medical Decision Making 28(4):593–605.
Yang, L., I. Chumfong, T. West, H. Ammerlahn, and A. Yoshimura. 2006. Earlier detection in emergency response to an anthrax attack. SAND2006-1740. Livermore, CA: Sandia National Laboratories. http://www.prod.sandia.gov/cgi-bin/techlib/access-control.pl/2006/061740.pdf (accessed February 12, 2009).
Zaric, G.S., D.M. Bravata, J.C. Holty, K.M. McDonald, D.K. Owens, and M.L. Brandeau. 2008. Modeling the logistics of response to anthrax bioterrorism. Medical Decision Making 28(3):332–350.


































