2
The BioWatch System
BioWatch is an early warning system for detection of certain pathogens that have been intentionally released into the air. The decision to field a “network of sensors to detect biological attack” was announced in January 2003 in the State of the Union address (The White House, 2003). Implementing this decision required rapid deployment of air sampling equipment and tools for laboratory analysis and development of procedures for operation and management of the system. By the end of 2003, BioWatch air samplers were operating on a continuous basis in more than 30 major metropolitan areas.1 The BioWatch system currently tests for the presence of airborne DNA from certain pathogens. As of mid-2008, millions of laboratory assays had been carried out on collected samples (Hooks, 2008a). With the current technology and practices, the laboratory results indicating a potential biological aerosol release are available 10 to 34 hours after sample collection. The program is working toward a transition in technology to reduce the interval from sample collection to completion of initial analysis to 4 to 6 hours.
This chapter describes the current features of the BioWatch system, including the technology and deployment of the air samplers, the laboratory analysis, the interpretation of data from BioWatch as a basis for public health action, and the plans for new technology for air sampling and analysis, organized in terms of planning and management, operations, and response. The chapter also presents a discussion of the costs of the BioWatch
program in its current form and with proposed changes in technology and scale of operation. Chapter 3 presents the committee’s evaluation of the effectiveness of the BioWatch program and system and provides recommendations for improvements.
BIOWATCH PROGRAM PLANNING AND MANAGEMENT
DHS states that the goal of the BioWatch program “is to establish and operate a bio-aerosol monitoring capability to accurately detect the release of biological threat agents of greatest concern to the [n]ation in locations that are at greatest risk of catastrophic consequences and to enable timely response and mitigation” (DHS, 2008c). The rationale for the BioWatch program is that early detection of an aerosolized bioterrorist attack could allow for mass distribution of prophylactic medications or other medical countermeasures in time to prevent widespread illness or deaths. For example, under a DHS “worst case” planning scenario involving an aerosolized anthrax attack in a large urban area and exposure of a few million people, hundreds of thousands of people would develop life-threatening disease requiring intensive medical care. Under this planning scenario, providing antibiotic prophylaxis to the exposed population within 3 days of the attack is projected to prevent nearly all of the deaths that would otherwise occur (Hooks, 2008a).
The program’s scope includes management and operation of the day-to-day air sampling and laboratory testing as well as planning and preparation for a bioterrorist attack and coordination and management tasks when DNA (or nucleic acid evidence) of a targeted pathogen is detected. The BioWatch system generates information that feeds into public health decision making and response, and DHS looks to state and local health agencies and the Department of Health and Human Services (HHS) at the federal level to prepare for and manage the public health response to BioWatch results.
Participants and Responsibilities
BioWatch is a federal program led by DHS and designed to operate in collaboration with other federal partners and with the states and localities where air samplers are deployed. The program is currently managed by the DHS Office of Health Affairs (OHA). Until 2007, the program was the responsibility of the DHS Directorate for Science and Technology (S&T). OHA continues to rely on S&T for technology development. S&T is also responsible for analyses to characterize biological threats and assess the risk they pose, including preparing the biennial Bioterrorism Risk Assessment (BTRA) and an integrated chemical, biological, radiological, and nuclear (CBRN) risk assessment.
Other federal partners in BioWatch include the Centers for Disease Control and Prevention (CDC) in HHS, the Environmental Protection Agency (EPA), the Federal Bureau of Investigation (FBI) in the Department of Justice, and the Department of Defense (DoD). The program also works closely with components of several of the Department of Energy’s (DOE’s) National Laboratories. These agencies participate in federal interagency activities that contribute to development of program planning and guidance for BioWatch, and some have operational responsibilities. CDC’s primary roles are the development of the BioWatch laboratory analyses, oversight of the quality assurance and quality control for those analyses, and consultation with state and local agencies on the public health response to an alert from the BioWatch system. EPA’s operational role has transitioned from having major responsibilities for routine field operations to advising on and participating in post-detection environmental sampling upon invitation from local jurisdictions. The FBI contributes intelligence and leads any resulting criminal investigation. DoD has separate biological monitoring systems for military bases and is beginning to coordinate its monitoring activities with the civilian BioWatch system. DOE National Laboratories are involved primarily in the development of technologies and analytic tools, but Lawrence Livermore National Laboratory (LLNL) also provides routine laboratory analysis for some BioWatch jurisdictions.
At the local level, some of the BioWatch jurisdictions have DHS-funded personnel responsible for coordinating the routine operation and maintenance of the air samplers and the collection and transport of the filters. Elsewhere these duties are carried out by local partners. State and local public health laboratories typically provide the facilities to house BioWatch laboratory operations for analysis of the filters, but the BioWatch program currently provides contract personnel to perform the analyses within these laboratories.
Each jurisdiction is expected to have a BioWatch Advisory Committee (BAC), or similar mechanism, that brings together critical decision makers who can establish local plans and procedures for the use of and response to BioWatch results. The BAC also serves as the focal point for coordination with federal officials in evaluating BioWatch data and determining the appropriate actions. Because BioWatch jurisdictions are major metropolitan areas, the planning and decision-making mechanisms may span multiple cities, counties, and states. In the event of a bioterrorist attack, public health officials will play an important role in decision making, but a unified response involving law enforcement, emergency management, environmental protection, and others will take place.2
BIOWATCH OPERATIONS
This section describes the operation of the BioWatch system, from the deployment of air samplers to the declaration of a BioWatch Actionable Result (BAR). A BAR occurs when analysis of a filter from a BioWatch air sampler confirms the presence of targeted signature nucleic acid sequences associated with a pathogenic organism.
Performance attributes of the BioWatch detection system can be described (or specified) in terms of two critical parameters:
-
Sensitivity refers to a system’s ability to detect a pathogen when it is present (a true positive event). This is commonly defined as the probability the system will produce a positive result for a particular pathogen, given that this pathogen is in fact present (in some predesignated amount or concentration) in the area being monitored.
-
Specificity refers to the system’s ability to detect a pathogen only when it is present and not to detect other “non-target” biological agents that are present (detection of a non-target is a “false positive” event). Defining the false alarm probability to be the probability that a system will produce a positive result for a particular pathogen given that pathogen is not present, specificity is seen to be equal to [1 − false alarm probability]. A system with a given specificity s and sampling rate r will have a false alarm rate = s•r (events per unit time).
For BioWatch, as in virtually any detection system, there is an inherent trade-off between sensitivity and specificity. Moreover, these parameters must be considered on two levels: (1) detecting the presence of a pathogen the system is intended to detect, and (2) the fraction of these detections that also exhibit evidence of a bioterrorist attack (see Box 2-1). The BioWatch system must exhibit, in testing and in practice, high enough values of sensitivity and specificity that public health decision makers will have confidence in the system’s performance and consequently in determining the course of action in response to a positive result from analysis of a BioWatch sample.
DHS has reported that among the millions of BioWatch assays that have been conducted, none have produced “false positives,” which reflects the narrow perspective of air sampling and laboratory testing (Hooks, 2008a; DHS, 2009). Indeed, the laboratory assays have never indicated the presence of a biological agent when it was not present, although several BARs have been declared that have been attributed to the detection of ambient DNA that was naturally present in the local environment. From the wider perspective of public health authorities responsible for determining whether a confirmed positive laboratory test (a BAR) represents a plausible indication of a bioterrorist attack meriting initiation of mass dispensing of prophylaxis, the committee concluded that all BARs to date have been
“BAR false positives,” meaning they have signaled the potential occurrence of a terrorist attack when none has occurred.
In addition to the performance of the BioWatch system’s technologies for sample collection and analysis, detection also depends on the numbers and distribution of air samplers, weather conditions (e.g., wind speed and direction), and the effects of the natural and man-made physical environments on dispersion. No finite array of even “perfect” collectors can detect all conceivable terrorist releases, even large-volume releases, under all circumstances. Thus the overall sensitivity of the BioWatch system will always be imperfect (less than 1).
BioWatch Air Samplers and Their Deployment
DHS is responsible for selecting the devices that are used for air sampling, and it funds their purchase and deployment. Changes in types of detector technology and deployment within the BioWatch program are described as different BioWatch “Generations.” Table 2-1 describes the characteristics of Generations 1 and 2, and the planned Generation 3.
TABLE 2-1 Features of BioWatch Generations 1, 2, and 3
|
BioWatch Generation |
Year Deployed |
Deployment Characteristics |
|
Generation 1 |
2003 |
Jurisdictions: >30 Multiple air samplers per jurisdiction |
|
Generation 2 |
2005 |
Jurisdictions: Same as Generation 1 More air sampling units deployed outdoors in selected jurisdictions; indoor facility monitoring. Multiple units per jurisdiction; a few hundred units deployed in total. |
|
Generation 3 (as proposed) |
2012–2013 |
Jurisdictions: Some increase over Generation 2 Detectors: Some increase over Generation 2 |
|
NOTES: BASIS, Biological Aerosol Sentry and Information System; DHS, Department of Homeland Security; LANL, Los Alamos National Laboratory; LRN, Laboratory Response Network; OHA, Office of Health Affairs; PCR, polymerase chain reaction; S&T, Science and Technology Directorate. SOURCES: DHS (2008a, 2009); Gordon (2008); Hooks (2008b); Johns (2008). |
||
The initial deployment of BioWatch air samplers took place in 2003 (Generation 1), and a revised, expanded deployment using the same sampler technology was carried out in 2005 (Generation 2). A Generation 3 deployment is being planned. It will differ from Generation 2 in its planned use of new, automated devices that will combine sample collection, preparation, and analysis within the device and that will send results electronically to the local public health laboratory director for interpretation. DHS had also planned (and subsequently cancelled) deployment of a Generation 2.5 to provide an interim automated indoor detection capability before the deployment of Generation 3.
BioWatch Air Samplers
The BioWatch air samplers are designed for continuous routine air monitoring in urban environments. Air is mechanically pulled into the instrument and particles are collected onto a dry filter housed within a removable filter unit. The filter units are collected and taken to a laboratory where the filters are removed and the particles on the filters are extracted and characterized by genomic analysis. Under routine conditions, the filter units are collected at 24-hour intervals; filters may be collected more frequently during high-profile events. Up to 10 hours may elapse from the time
|
Technical Features |
Developed by |
Managed by |
|
Air sampler: Portable Sampling Unit (PSU) and Dry Filter Unit (DFU) Sample collection: Manual sample collection typically every 24 hours, followed by manual laboratory analysis Analytic Methods: Manual sample analysis using real-time PCR |
Adapted from BASIS technology by DHS S&T |
DHS S&T |
|
Air sampler: Same as Generation 1 Sample collection: Same as Generation 1 Analytic methods: Similar to Generation 1 |
Same as Generation 1 |
2005–2007: DHS S&T 2007–present: DHS OHA |
|
Detector: Unknown Sample collection: Automated sample collection and preparation Analytic methods: Automated systems |
Not determined as of this writing |
DHS OHA |
a filter unit is collected until the results of the initial testing of the sample are available (see Figure 2-1).
The current BioWatch air samplers were adapted from the Biological Aerosol Sentry and Information System (BASIS), which was developed by Los Alamos National Laboratory (LANL) and LLNL (DHS, 2008b). BASIS monitors had been deployed in some locations after the 9/11 attacks and were used at the 2002 Winter Olympics in Salt Lake City, Utah (Heller, 2003). They were designed and developed for short-term biological threat aerosol monitoring at special events (Heller, 2003).
Deployment of Air Samplers
Each BioWatch jurisdiction currently has multiple samplers (DHS, 2009). DHS also provides additional air samplers for special events. Originally, the BioWatch samplers were mounted on existing outdoor EPA air quality monitoring sites (EPA, 2005). The siting of the air samplers has been modified over time.
Plans for Generation 3 Samplers and Deployment
DHS has been pursuing an automated detection capability for the BioWatch system since 2003 (HSARPA, 2003) to reduce the time needed to
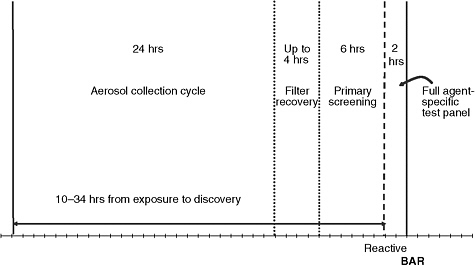
FIGURE 2-1 Event-to-detection time line for BioWatch Generations 1 and 2. Filter recovery and transport can take up to 4 hours, and primary laboratory screening takes about 6 hours. If the primary screening indicates a positive result, confirmatory testing requires an additional 2 hours.
SOURCE: Adapted from Runge (2008).
obtain test results to within 4 to 6 hours of sample collection. The Generation 3 plans include increasing the number of jurisdictions participating in the BioWatch program, the number of monitoring devices in the participating jurisdictions, the number of devices in indoor locations, and potentially the number of biological agents being monitored. Chapter 3 discusses the objectives and planning for Generation 3 in detail.
BioWatch Laboratories
With the current Generation 2 BioWatch system, filters from the air samplers are collected daily for analysis in designated laboratories. In most BioWatch jurisdictions, the laboratory facilities are housed in state public health laboratories that are reference facilities in the national Laboratory Response Network (LRN).3 In some large jurisdictions, the LRN reference
|
3 |
The Laboratory Response Network (LRN) is an integrated network of state and local public health, federal, military, and international laboratories that can respond to terrorism and other public health emergencies. In the United States, the LRN relies on three tiers of laboratories. The sentinel laboratories perform routine diagnostic and identification testing. Reference laboratories are able to perform more advanced testing and pursue investigation of specimens. A small number of national laboratories are responsible for highly specialized testing and have facilities suitable for handling highly infectious biological agents. The LRN is described further in Chapter 3 and at http://www.bt.cdc.gov/lrn/. |
laboratories are located in the local public health department. CDC provides oversight in the development of the laboratory assays for the BioWatch system and approves and validates their use. The personnel who perform the BioWatch analyses currently work under contract with DHS. The state or local host laboratory provides support such as safety training and security but does not have direct authority over the BioWatch personnel.
RESPONSE TO A BIOWATCH ACTIONABLE RESULT
Declaring a BAR
When a positive result from tests of a BioWatch sample is deemed to warrant it, a BAR is declared. Local, state, and federal officials assess relevant information and determine the course of action to pursue.
The committee considers the term “BioWatch Actionable Result,” or BAR, misleading because the term implies that action can be taken immediately, but further investigation and deliberation are generally needed. A BAR indicates only that genetic material consistent with a target pathogen was present on a BioWatch filter. A BAR does not confirm that a terrorist attack has occurred, that a viable pathogen was detected, or that people have actually been exposed. Thus, the committee sees a BAR alone as unlikely to be a sufficient basis to automatically trigger a specific response by public health authorities. Instead, the response will be specific to the situation. So far, BARs have been attributed to the detection of ambient DNA that was naturally present in the local environment, and no evidence of increased rates of human disease has been noted.
Critical Information Needed to Respond to a BAR
The declaration of a BAR begins a process of information gathering and assessment to interpret the significance of the BAR and determine what response, if any, is appropriate. A primary goal of the immediate post-BAR steps is to collect, analyze, and interpret available data to decide whether they indicate that a bioterrorist attack has occurred. The decision to interpret a BAR as evidence that a bioterrorist attack has occurred would lead to predictable strategic responses that would be difficult to roll back once started. These actions may have a massive impact on public perception of and confidence in the public health system if they are initiated in response to what proves to be a false alarm.
There is no simple algorithm to guide decision makers on the public health response to a major biological threat from the release of a bioterror agent. The decisions made will hinge on a variety of inputs and depend heavily on whether the information is sufficient to determine that an effective release of a bioterrorism agent is likely to have occurred. Critical
inputs for decision making in response to a BAR are reviewed in the sections that follow.
Biological Agent Detected
The particular biological agent detected has major implications for the interpretation of a BAR and for shaping the potential response if bioterrorism is likely or suspected. The detected agent also has implications for the selection of medical countermeasures and guidance to be given to health care providers and the public. Different agents are mitigated with different drugs or vaccines and are different in their incubation periods, symptoms and effects, and transmissibility. Thus, the pathogen detected will influence the amount of time decision makers may have for additional information gathering and the potential consequences (positive and negative) of the decisions that must be made. Knowledge of the detected agent will help in specifying clinical syndromes warranting heightened surveillance and targeting guidance to emergency and other health care providers regarding the isolation, evaluation, treatment, and disposition of patients with illness that may arise from exposure.
Information from Air Samplers
Information from the network of BioWatch air samplers in a jurisdiction may provide additional context that is important in interpreting the significance of a BAR. The number of filters testing positive, their collection times, the duration of the collection process, and the locations of the air samplers that the filters came from may provide important information regarding the potential scale of population exposure and environmental contamination. Determining whether any other BioWatch jurisdictions have reported BARs or whether there are any suspected bioterrorist attacks reported elsewhere will contribute to situational awareness. Systems to rule out tampering and spoofing, such as closed circuit television or physical surveillance, may also provide valuable information.
Information from Law Enforcement
Local law enforcement agencies, the FBI, and intelligence agencies may have intelligence (e.g., observed behaviors, threats, intent, capabilities; or diversion of microbial stocks) that will help in assessing the likelihood that a BAR is the result of a bioterrorist attack. Other concerns may include whether any high-profile events took place in the area during the time that the filter that tested positive was in place. If a BAR is the result of bio-
terrorism or other deliberate action, law enforcement personnel will lead subsequent criminal and forensic investigations.
Disease Surveillance Data
As described in Chapter 4, data from a variety of public health and health care sources can be used in surveillance for infectious disease outbreaks. To inform the interpretation of a BAR, public health officials can take several steps to heighten surveillance for syndromes or diagnoses of concern. These steps may include implementing enhanced surveillance for both human and animal illness that targets the clinical signs and symptoms characteristic of illness caused by the detected pathogen. This may include heightened scrutiny or modifications of existing surveillance systems that monitor trends in specific diseases or trends in nonspecific manifestations of disease, or it may include outreach to hospitals, laboratories, or other health care sites to actively solicit reports of suspect illness. Local officials’ understanding of baseline rates of disease in their jurisdictions is important in interpreting the data that may be collected.
Post-BAR Environmental Testing and Incident Characterization
Environmental sampling may be conducted following declaration of a BAR. The information obtained may be used for various purposes, including aiding in interpretation of the laboratory finding, in an assessment of the nature and extent of the distribution of a detected pathogen, and in guiding the response to the BAR and other subsequent actions that may be needed. However, a lack of data validating the usefulness of environmental sampling and a lack of validated methods for carrying it out are concerns (GAO, 2008) and are discussed further in Chapter 3. In addition, laboratory capacity is a critical consideration following a BAR because of the needs for more frequent testing of BioWatch filters and post-BAR environmental sampling analyses.
Event Reconstruction Models
Event reconstruction models can be used to estimate the location of an aerosol release, bounds on the size of the release, and the time of release. Such models may also be used to suggest sites for additional environmental sampling following a BAR (Brown et al., 2006). Plume models can estimate the downwind zone of potential exposure, but essential information about the location, time, amount, and duration of a release is unlikely to be readily available immediately following a BAR, except in special circumstances.
Chapter 3 further discusses the limitations of event reconstruction models in public health decision making following a BAR.
COSTS OF THE BIOWATCH SYSTEM
To evaluate the costs of BioWatch Generation 2 and Generation 3 and the relevant merits and capabilities of the system, the committee engaged Industrial Economics, Incorporated (IEc), to assist in collecting information and developing a model to forecast the costs of operating the current and future BioWatch monitoring systems. Under the committee’s guidance, IEc used data provided by DHS concerning the current costs of the BioWatch system to estimate expenditures necessary to operate the program, without improvement (Generation 2), for the next 10 years. It provided a similar forecast assuming DHS’s proposed improvements (Generation 3) are implemented. At the request of the committee, IEc also considered a third scenario in which the current air samplers are replaced with the improved technology (i.e., autonomous detection equipment); however, no new detection sites within existing jurisdictions or new jurisdictions are added to the program (Generation 3 without expansion).
DHS provided cost data in briefings to the committee and in response to a detailed request for cost information on development, maintenance, and other aspects of each BioWatch generation. According to IEc calculations, the majority of costs under each scenario (roughly 95 percent or more) are borne by the federal government. This information is supplemented by limited data obtained from committee members familiar with the funding and in-kind support provided by local jurisdictions. In-kind support consists of labor and laboratory materials contributed to BioWatch procedures by local jurisdictions without reimbursement by DHS.
As shown in Table 2-2, under a scenario in which the existing air samplers are used and replaced with similar technology when their useful life expires, the annualized direct cost of maintaining the current BioWatch system (Generation 2) is estimated to be $80 million. Annualized cost represents the total present value cost (total anticipated costs of the program over the 10-year period applying a discount rate), divided by 10. The specific funding requirements for the program will vary significantly from year to year, depending on when the existing instruments are replaced.
Upgrading the BioWatch system to Generation 3 involves improvements that include replacing the existing air samplers, which require manual retrieval and laboratory analysis of filters, with automated detectors capable of onsite sample analysis and an anticipated expansion of the BioWatch system’s coverage. Over the next decade, such upgrades will more than double the direct cost of the program to $200 million on an annualized basis. The higher cost is mainly driven by the expansion of coverage. As shown by comparing
TABLE 2-2 Forecasted Cost of Implementing Alternative BioWatch Scenarios (10-year forecast)
|
Scenario |
Total Present Value Costa |
Annualized Cost |
|
Generation 2 |
$0.6 billion |
$ 80 million |
|
Generation 3 |
$2.0 billion |
$200 million |
|
Generation 3 without expansionb |
$0.8 billion |
$100 million |
|
NOTE: The calculations assume a 7 percent real discount rate. If a 3 percent real discount rate is applied, total costs for Generation 2 and Generation 3 without expansion are $0.7 billion and $0.9 billion, respectively, and total costs for Generation 3 are $2 billion. Total annualized costs are unchanged. Because resources invested today are worth more than the same investments in the future, total present value (in 2009 dollars) of the stream of future costs over 10 years is calculated assuming a 7 percent discount rate. aTotal present value cost is the total anticipated cost of the program over a 10-year period, after applying a discount rate. b“Generation 3 without expansion” refers to only direct replacement of current Generation 2 air samplers with the automated detectors to be used in the Generation 3 deployment, without other changes proposed for Generation 3. SOURCE: IEc (2009). |
||
the Generation 2 scenario to a scenario in which the Generation 3 technology is used without expanding the number of jurisdictions or deployed detectors, the cost of acquiring and fielding new technology is largely offset by the cost savings associated with automated analysis of the detector samples.4
The estimate of the total cost of each of the scenarios is subject to considerable uncertainty because of limitations in the data available for the analysis. The estimates do not account for the financial and in-kind costs borne by the states and localities in which BioWatch is deployed. These states and localities must currently provide some support for day-to-day BioWatch operations as well as the costs for responding to periodic BARs. The automated testing anticipated with Generation 3 may tend to reduce the day-to-day demands on states and localities, but the implications of the new generation of BioWatch technology for the frequency of BARs are unknown.
REFERENCES
Brown, M.J., M.D. Williams, G.E. Streit, M. Nelson, and S. Linger. 2006. An operational event reconstruction tool: Source inversion for biological agent detectors. 86th American Meteorological Society Annual Meeting. Atlanta, GA. http://ams.confex.com/ams/pdfpapers/126686.pdf (accessed July 9, 2009).
CRS (Congressional Research Service). 2003. The BioWatch program: Detection of bioterrorism. RL32152. Washington, DC: Library of Congress. http://digital.library.unt.edu/govdocs/crs/permalink/meta-crs-8189:1 (accessed April 21, 2009).
DHS (Department of Homeland Security). 2008a. Annual performance report, fiscal years 2007–2009. Washington, DC: DHS. http://www.dhs.gov/xlibrary/assets/cfo_apr_fy2007.pdf (accessed August 5, 2009).
DHS. 2008b. BioWatch outdoor program: Guidance documents for BioWatch jurisdictions. Washington, DC: DHS.
DHS. 2008c. NAS information request #2, DHS OHA written responses. Document submitted to the Committee on Effectiveness of National Biosurveillance Systems: BioWatch and the Public Health System, October 31. Washington, DC.
DHS. 2009. BioWatch Gen-3 industry day. https://www.fbo.gov/download/18d/18d10ae7b9c2106327715c7a5dc53c43/BiowatchGen3_Industry-Day_Slides.pdf (accessed March 24, 2009).
EPA (Environmental Protection Agency). 2005. EPA needs to fulfill its designated responsibilities to ensure effective BioWatch program. Inspector General Report No. 2005-P-00012, March 23. Washington, DC: EPA. http://www.epa.gov/oigearth/reports/2005/20050323-2005-P-00012.pdf (accessed July 9, 2009).
FEMA (Federal Emergency Management Agency). 2008. National Incident Management System. http://www.fema.gov/emergency/nims/ (accessed April 13, 2009).
GAO (Government Accountability Office). 2008. Homeland security: First responders’ ability to detect and model hazardous releases in urban areas is significantly limited. GAO-08-180. Washington, DC: GAO. http://www.gao.gov/products/GAO-08-180 (accessed July 9, 2009).
Gordon, R. 2008. Application of aerosol plume modeling in BioWatch program. Presentation to the Committee on Effectiveness of National Biosurveillance Systems: BioWatch and the Public Health System, Meeting 3, November 3–5, Washington, DC.
Heller, A. 2003. BASIS counters airborne counterterrorism. Science & Technology Review (October):6–7. Lawrence Livermore National Laboratory UCRL-52000-03-10. https://www.llnl.gov/str/October03/pdfs/10_03.pdf (accessed February 10, 2009).
Hooks, R. 2008a. BioWatch program. Presentation and public comments to the Committee on Effectiveness of National Biosurveillance Systems: BioWatch and the Public Health System, Meeting 1, July 30–31, Washington, DC.
Hooks, R. 2008b. BioWatch generation 3 deployment strategy. Presentation to the Committee on Effectiveness of National Biosurveillance Systems: BioWatch and the Public Health System, Meeting 2, September 22–24, Washington, DC.
HSARPA (Homeland Security Advanced Research Projects Agency). 2003. Detection systems for biological and chemical countermeasure (DSBCC). Research Announcement 03-01, September 23. Washington, DC: Department of Homeland Security.
IEc (Industrial Economics, Incorporated). 2009. Revised estimated cost of BioWatch generations 2 and 3. Commissioned paper. Committee on Effectiveness of National Biosurveillance Systems: BioWatch and the Public Health System, Washington, DC.
Johns, M. 2008. BioWatch past and projected costs. Presentation to the Committee on Effectiveness of National Biosurveillance Systems: BioWatch and the Public Health System, Meeting 3, November 3–5, Washington, DC.
NRC (National Research Council). 2005. Sensor systems for biological agent attacks: Protecting buildings and military bases. Washington, DC: The National Academies Press.
Runge, J.W. 2008. Effectiveness of national biosurveillance systems: BioWatch and the public health system. Presentation and public comments to the Committee on Effectiveness of National Biosurveillance Systems: BioWatch and the Public Health System, Meeting 1, July 30–31, Washington, DC.
The White House. 2003. State of the Union Address. January 28. http://www.gpoaccess.gov/sou/index.html (accessed March 4, 2009).














