4
Detecting Biological Threats Through the Public Health and Health Care Systems: Current Status
Part of the charge to the committee was to “[d]escribe the characteristics of an ‘enhanced national surveillance system’ that relies on U.S. hospitals and the U.S. public health system.” To provide a snapshot of the nation’s current capability to detect outbreaks of infectious disease, this chapter focuses on local, state, and federal public health agencies, which have the lead responsibility for disease surveillance, investigation, and response. This chapter also describes the role of health care providers, hospitals, and other health care organizations in disease surveillance. Chapter 5 examines areas where surveillance for bioterrorism and infectious diseases might be enhanced.
In practice, the same surveillance capacities would support detection and monitoring of epidemics, regardless of whether they arise from an act of bioterrorism, from natural factors, or from the consequences of actions such as a failure to observe safe food handling or processing procedures. The committee did not review all of public health surveillance, which includes monitoring a wide spectrum of infectious and noninfectious diseases, injuries, behavioral risk factors, and other conditions; nor did it review all of biosurveillance, which includes monitoring for human diseases and animal or plant diseases and other biosphere conditions that may affect human health.
FRAMEWORK FOR INFECTIOUS DISEASE SURVEILLANCE
In the United States, state and local public health agencies have the authority and responsibility for carrying out most public health actions. Their responsibilities include disease surveillance, and they are the lead re-
sponders to public health emergencies in their jurisdictions. This allocation of responsibility reflects the fact that protection of public health was not established as a federal function in the Constitution and is therefore reserved to the states. However, the federal government has acquired public health responsibilities over the years, including acting in support of state and local public health agencies. A bioterrorism event, or other significant health emergency, will likely be met with a multiagency and multilevel response that includes participation by law enforcement and emergency management in addition to public health agencies. Federal roles and responsibilities in working with states and localities in the event of a major disaster or public health emergency are outlined annexes to the National Response Framework (FEMA, 2008a,b).
To ensure the broadest surveillance capabilities, health departments depend on receiving information from health care, occupational settings, environmental monitoring, and other sources, with the health care sector being the most critical source. Accordingly, a major determinant of the effectiveness of disease surveillance is the capacity of health care providers in various settings—hospitals, outpatient practices, and laboratories—to collect and report relevant information to public health authorities. Equally important is the capacity of public health agencies to receive and compile the data, conduct analyses, interpret the results, report the findings to constituents, and mount a timely and appropriate response when the data indicate a need for further investigation or public health intervention. Figure 4-1 illustrates in a generic fashion the basic flow of information for surveillance for significant infectious disease threats.
Especially in the event of an outbreak associated with a highly pathogenic organism or a bioterrorist attack, the capacity to act promptly in response to surveillance alerts is crucial to mitigate morbidity and mortality. But surveillance systems aimed at detecting outbreaks quickly must be calibrated in a way that effectively balances the inherently competing demands for timely recognition of outbreaks that merit public health intervention and for avoidance of excessive false alarms that may consume public health and health care resources for investigations, or even lead to an inappropriate and potentially dangerous response.
SURVEILLANCE OF INFECTIOUS DISEASES
Surveillance refers to an ongoing process of systematic collection, analysis, interpretation, and dissemination of health data that can be used to plan, implement, and evaluate appropriate medical and public health interventions (Thacker and Berkelman, 1988). Surveillance for the health effects of bioterrorism is generally integrated with other public health surveillance systems aimed at detecting the full range of infectious disease threats.
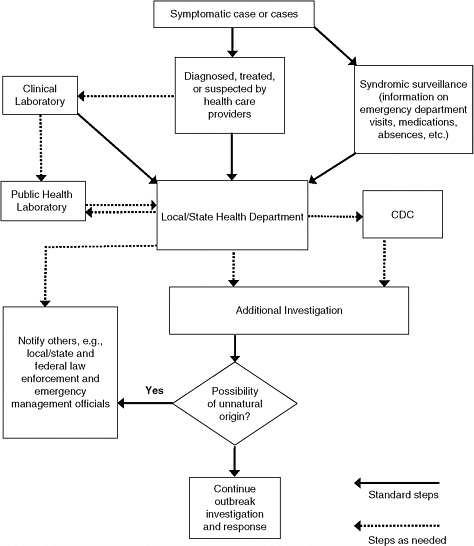
FIGURE 4-1 Simplified depiction of information flow in outbreak detection and reporting through the public health and health care systems. As a generic diagram, this figure shows core elements of the generation, capture, and analysis of and response to information on human health and tests of human specimens that are used to detect disease outbreaks. It does not attempt to convey all aspects of the information capture, analysis, and reporting or possible variations in the depicted pathways that may be used in this process or that are described in the text. This figure represents information flow stemming from human cases only; Figure 6-1 illustrates other important sources of surveillance information (e.g., monitoring environmental hazards or animal health) that may be used to detect bioterrorism or infectious disease outbreaks.
Traditionally, case reporting by health care professionals has been the mainstay of public health surveillance for the infectious diseases that states designate as reportable and other indications of disease outbreaks of concern. Because the criteria for confirming cases of reportable diseases typically include evidence of illness combined with laboratory-based documentation of specific infections, timely laboratory reporting of positive test results or cultures is also important and required by state health departments. Clinical and laboratory information may be combined with additional epidemiologic data, such as demographic data or information on possible exposures, to complete the case reporting process.
In the past decade, a number of local, state, and federal efforts to detect outbreaks through the collection and analysis of data on relatively nonspecific disease-related indicators, often called syndromic surveillance, have been launched (Buehler et al., 2008). Syndromic surveillance looks toward statistical signals of increases in the incidence of symptom complexes (e.g., diarrhea), use of health care services, or sales of over-the-counter medications (e.g., antidiarrheals) as indicators of potential disease epidemics. Another area of recent effort is combining and comparing data streams from different sectors to detect or interpret indications of a potential health emergency, or what is called biosurveillance data integration or information fusion.
Disease Reporting Requirements
Every state and territory has laws or regulations, or both, requiring the reporting of suspect or confirmed cases of specific diseases and other conditions to public health authorities. In addition, these laws typically mandate reporting of recognized disease outbreaks, regardless of whether they are the result of conditions listed as notifiable. Once disease reports are received by local health officials, actions may include verifying the diagnosis, investigating the sources of infection, ensuring appropriate treatment and infection control, identifying and providing appropriate care to contacts, and sometimes additional active case finding to assess the presence of additional cases in the community.
The states’ lists of reportable diseases vary, reflecting differences in the geographic distribution and importance of some conditions (e.g., certain insect-borne diseases). The combined total includes approximately 90 diseases.1 In terms of detecting cases of potential bioterrorism, all states require reporting of any cases of anthrax, botulism, tularemia, and brucel-
|
1 |
The composite list of diseases reportable to state health departments is available at http://www.cdc.gov/ncphi/disss/nndss/phs/files/NNDSS_event_code_list_January_2009_CLEARED.pdf. |
losis. All but one requires reporting of any cases of plague, and all but five any cases of smallpox.2
The authority for mandating disease reporting resides at the state and local level, but the state-based systems are harmonized at the national level by a voluntary set of reporting criteria and case definitions established by the Council of State and Territorial Epidemiologists (CSTE) and the federal Centers for Disease Control and Prevention (CDC). These arrangements allow CDC to collect reports of cases of specified diseases (without identifying information for individual patients) via the National Notifiable Diseases Surveillance System (NNDSS) for use in tracking and investigating regional- and national-level trends. The NNDSS encompasses the listing of nationally notifiable diseases, case definitions, and a system for transmitting case reports from local to state public health agencies to CDC. Currently, approximately 70 infectious diseases are designated as nationally notifiable.3 The list of these diseases is maintained and updated annually by CSTE in collaboration with CDC.
The National Electronic Telecommunications System for Surveillance (NETSS) is the current CDC mechanism that the 50 state health departments, New York City, the District of Columbia, and five U.S. Territories use to submit weekly reports of cases of nationally notifiable diseases.4 Since 1999, CDC has been developing and deploying the National Electronic Disease Surveillance System (NEDSS), an Internet-based system for automatically capturing and analyzing surveillance data that are already in electronic form, such as reports from many clinical laboratories (CDC, 2008b, 2009e). NEDSS is expected to replace NETSS and several other CDC surveillance systems.5 NEDSS seeks to facilitate the collection and interoperable use of case information from providers through vocabulary and transmission standards. A 2007 survey found that states are using a mix of software systems, with 13 states having established some interoperability across at least two surveillance modules (CSTE, 2008). At the time of the survey, 27 states were still acquiring necessary hardware and software. Funding was cited as an important obstacle to progress in implementing NEDSS (CSTE, 2008).
Case Reporting from the Health Care System
Physicians, nurses, and other health care workers see individuals seeking care for a health problem. The clinician provides a diagnosis based on
|
2 |
The list of notifiable diseases reported by state is at http://www.cdc.gov/ncphi/disss/nndss/phs/files/SRCA_FINAL_REPORT_2007.xls. |
|
3 |
The list of nationally notifiable infectious diseases is available at http://www.cdc.gov/ncphi/disss/nndss/phs/infdis2009.htm. |
|
4 |
|
|
5 |
history, physical examination, general observations, laboratory and radiologic tests, and other information. If the suspected or confirmed diagnosis is of a legally reportable disease, the health care provider is required to report it to the local or state health department. Similarly, if a laboratory performs a test diagnostic of a reportable condition, it is required to make a report. (The role of the laboratory is discussed below.)
Diagnosis and Reporting by Health Care Providers
Reporting by health care providers is one of the essential sources of information used to recognize or determine that a disease outbreak is occurring. An analysis of 43 disease outbreaks reported in Morbidity and Mortality Weekly Report in 1999 and 2000 found that information generated by the clinical health care system (defined in the study as nongovernment hospitals, physicians, pharmacists, and laboratories) was used in detecting 72 percent of the outbreaks, while detection of several others resulted from reports from other settings, including schools, prisons, and STD clinics (Dato et al., 2004).6 The same researchers drew from other sources to examine the initial detection of outbreaks of Lyme disease, Legionnaires’ disease, AIDS, hantavirus pulmonary syndrome, and SARS. The recognition of each of these newly emerging diseases involved reports from “astute” clinicians or other individuals who noted an unusual manifestation or clustering of disease (Dato et al., 2004).
Reporting of certain diseases is relatively prompt and complete, but it is well established that clinicians do not always remember to report the required diseases or to report them in a timely fashion (Doyle et al., 2002; Jajosky and Groseclose, 2004). They may simply be too busy to stop what they are doing, not know that the condition they have just diagnosed is reportable, or assume that a laboratory or infection control professional will report the case. In hospitals, infection control professionals usually report notifiable diseases, and with their targeted responsibility, their reporting tends to be more complete. However, most outpatient and ancillary health settings lack these focused professionals.
Several factors limit the effectiveness of provider reporting for early detection of bioterrorism. Reporting cannot occur until an infected patient becomes ill and seeks medical attention, and some individuals may not seek care. For those who do, arriving at a clinical facility involves a period of examination, testing, and diagnosis. If a patient is one of the first cases in a community, an atypical disease may be more difficult to diagnose,
and assessment and testing may take longer than usual. Clinicians may misdiagnose the patient, especially when the disease in question is rare, the clinical setting is extremely busy, the treating clinician typically manages a wide range of clinical problems, or the clinical staff are subject to frequent interruptions. These characteristics are commonly encountered in hospital emergency departments (EDs), which are increasingly used by the public for same-day or after-hours acute care. A diagnosis may also be missed or delayed if diagnostic laboratory tests are not done.
CDC and many state and local health departments have produced pocket cards and posters about case reporting for distribution to clinicians, and several professional organizations have developed short courses and other educational programs to maintain awareness of the clinical signs and symptoms of diseases caused by potential bioterrorism agents. Although such offerings may transiently increase practitioner knowledge, it is not clear that they have a sustained impact on the knowledge or behavior of health care providers.
In many jurisdictions, case reporting is still done with paper forms that are mailed or faxed. But reporting by telephone may be specified for diseases requiring the most rapid investigation or response. Regardless of how it is reported, a single report of a disease of special concern, such as inhalation anthrax or other infection associated with bioterrorism, is likely to trigger an immediate investigation. The advances in information technology are making it possible for health departments to provide Internet-based reporting systems to replace paper forms, often making it easier and faster to file a report.
Generally, however, scant resources are being directed towards strengthening the completeness, sensitivity, timeliness, and accuracy of provider reporting. Because traditional public health surveillance is heavily dependent on timely diagnosis and reporting by front-line health care providers and laboratory personnel, additional efforts are required to facilitate the performance of this essential task. For example, the future availability of well-tested clinical decision support systems may improve timeliness and accuracy of diagnoses, especially in busy EDs and other inpatient services, which are likely to first encounter the seriously ill. Increased educational outreach by health departments may make clinicians more aware of reporting requirements and reportable diseases.
In addition to improving health care providers’ awareness and compliance with reporting requirements, it is equally important that every health department have trained public health professionals designated to receive reports by telephone at all times. These individuals should be capable of handling queries about disease recognition, confirmatory diagnosis, infection control, and public health management, and they should also be able to quickly identify a subject matter expert if necessary. In a 2006 study,
however, less than a third of a sample of local health departments were able to connect a caller with an urgent case report to an appropriate public health professional within 30 minutes (Dausey et al., 2008).
Automated Surveillance Using Electronic Medical Records
As electronic medical records and electronic health information exchange become more common and more robust, these resources are also being used to support identification of reportable conditions. For example, Klompas and colleagues have piloted a system for automated detection and reporting of notifiable diseases using electronic medical records (EMRs) (Klompas et al., 2008; Lazarus et al., 2009). They report that EMR-based detection and notification of the four notifiable diseases they examined is more timely, complete, and clinically detailed than traditional reporting. The program scans the EMR for combinations of diagnoses, symptoms, physical signs, and laboratory results to detect likely cases of reportable conditions and alerts and assists clinical staff to report these if warranted.
CSTE, under contract with CDC, is now identifying standardized codes from ICD-9 and ICD-10, SNOMED, and LOINC that correspond to each of the required elements of a case report.7 Although many challenges remain in operationalizing automated record and reporting systems, this work should facilitate faster and more complete detection and transmission of case reports based on information from EMRs, laboratory information systems, and regional health information exchanges.
Laboratory Reporting
In the process of establishing diagnoses, clinicians often have laboratory tests performed. These tests are performed by a mix of clinical, commercial, and public health laboratories. Most states mandate that definitive test results that are diagnostic of a reportable disease be reported directly from laboratories to public health authorities. In fact, laboratories are responsible for a much larger share of mandated case reporting than clinicians.
The generation of complete, accurate, reliable, and timely laboratory data is very important in surveillance. With laboratories required to report certain results directly to state (and some local) health departments, public health decision makers may have access to the information before the
clinician is able to use it to make a diagnosis and report it. However, since some the most common conditions, such as acute diarrhea, are often treated symptomatically, laboratory diagnosis is not always pursued, particularly as rising health care costs have resulted in efforts to limit unneeded tests.
Many laboratories use electronic laboratory information systems (LISs), which create the potential for electronic laboratory reporting (ELR) automatically between LISs and public health disease surveillance systems. ELR systems have improved both the timeliness and completeness of reporting for some diseases (e.g., Nguyen et al., 2007; CDC, 2008d; Moore et al., 2008; Overhage et al., 2008). Currently, at least 44 states have the capability for electronic laboratory reporting to the health department (TFAH, 2008).
ELR typically involves the creation of a unique interface between each laboratory and each public health surveillance program, which is often a costly and time-consuming barrier. As laboratory results are increasingly delivered to multiple users through community-wide electronic health information exchanges, the need for multiple interfaces is reduced and new opportunities are created to facilitate the speed and completeness of ELR transactions. For example, the Indiana Health Information Exchange has documented that the exchange could produce a major increase in the volume of laboratory reporting over that performed spontaneously by laboratory personnel (Overhage et al., 2008).
ELR has been found to be more timely and complete than paper reporting (Effler et al., 1999; Overhage et al., 2008), but this may not necessarily hold true for all conditions (Nguyen et al., 2007; CDC, 2008d). Electronic laboratory reports generally increase the ascertainment of conditions (and thus number of reports) at health departments, but these reports often lack demographic and clinical information. The end result can be a greater burden on public health agency personnel for case follow-up. Some intentional reductions in system sensitivity have been made to reduce false alarms (CDC, 2008a,d). In addition, ELR may not simplify reporting for diseases for which multiple tests must be evaluated, and it may generate misleading reports because of coding problems and inconsistencies (Nguyen et al., 2007).
Public Health Reference Laboratories and Molecular Epidemiology
State public health laboratories, and some local public health laboratories, often serve as reference laboratories for definitive characterization of microbial pathogens. Increasingly clinical specimens also now undergo phenotypic analysis or genetic “fingerprinting” using tools such as pulsed-field gel electrophoresis, insertion segment analysis, and nucleotide sequencing, the results of which can be compared to databases of other isolates around
the nation or world. This has allowed cases of illness to be linked to common sources that would otherwise have gone unnoticed (e.g., Malakmadze et al., 2005), and it underlies the detection and characterizations of several, recent national and international outbreaks of food-related disease (e.g., CDC, 2008c, 2009d). Similar tools were used to trace the October 2001 anthrax attack strains to a strain of anthrax stored in one particular federal facility.
Given the importance of prompt and capable laboratory diagnostics for conditions of public health and bioterrorism concern, the Laboratory Response Network (LRN) was established in 1999 to improve and sustain diagnostically and biosafety-proficient reference laboratories across the nation. The LRN resulted from the collaborative effort of the CDC, the Association of Public Health Laboratories, and the Federal Bureau of Investigation. More than 160 laboratories, at least one in each state, are part of the LRN, and they include hospital- and community-based “sentinel” laboratories, state and local public health reference laboratories, and national laboratories for highly specialized reference needs (see Box 4-1).
Syndromic Surveillance
Syndromic surveillance refers to surveillance methods that monitor disease syndromes, such as influenza-like or diarrheal symptom complexes, or other illness-associated behavior using data from ED or clinic visits, medication purchases, 9-1-1 calls, and work or school absenteeism (Mandl et al., 2004; Buehler et al., 2008). Another approach described recently involves assessing the frequency and nature of Internet searches for health-related information (Polgreen et al., 2008; Ginsberg et al., 2009). The rationale for syndromic surveillance is that it may provide warning of intentional or natural disease outbreaks earlier than traditional methods of surveillance that rely on disease diagnosis. Syndromic surveillance has been established rapidly and across large geographies for detection and tracking of rapidly moving emerging diseases (Foldy et al., 2004). Such systems can also be used to monitor a spectrum of infectious diseases, including seasonal respiratory and gastrointestinal viral illnesses, and health concerns other than infectious diseases (e.g., to examine changes in smoking cessation [Henning, 2004], falls [Dey et al., 2008], or respiratory illness associated with smoke exposure from wildfires). Thus, syndromic surveillance offers agility and multifunctionality often lacking in other surveillance methods (Olson et al., 2007; Buehler et al., 2009).
Since the 2001 anthrax attacks, syndromic surveillance has become widespread, with use reported by more than 80 percent of the respondents to a survey of the health departments in the states, territories, the District of Columbia, and three large metropolitan jurisdictions (Buehler et al., 2008).
|
BOX 4-1 National Biosurveillance System: Laboratory Response Network 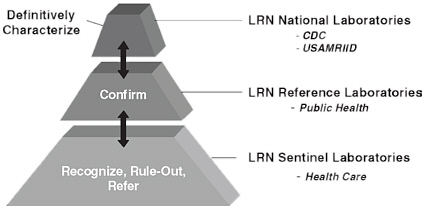 Local health care laboratories in hospitals and clinics and large commercial laboratories serve as LRN Sentinel Laboratories. All state public health laboratories and several large local public health laboratories serve as LRN Reference Laboratories. Both sentinel laboratories and reference laboratories work closely with CDC laboratories and programs to conduct standardized testing for the detection and confirmation of pathogens that threaten public health, including bioterrorism agents. Sentinel laboratories are at the front line for detecting infectious disease threats. They are trained by LRN Reference Laboratories to use standardized rule-out tests. If a sentinel laboratory suspects that a biological isolate from a patient may be an agent of bioterrorism (because the possibility cannot be ruled out), the laboratory immediately contacts the nearest LRN Reference Laboratory and transfers the agent to the reference facility for identification. If the LRN Reference Laboratory is unable to identify the isolate, it or the original specimen is sent to the CDC for definitive testing. Currently, more than 150 LRN laboratories are capable of submitting messages to CDC for all LRN assays using the LRN Results Messenger (CDC, 2009c). As described in Chapter 2, certain LRN Reference Laboratories are part of the BioWatch system. NOTE: USAMRIID, U.S. Army Medical Research Institute of Infectious Diseases. |
Syndromic surveillance is typically built on existing electronic streams of health information, including ED registration, medical record, pharmacy, and billing data. Once mechanisms are established for identifying data of interest and transmitting it to a health department or other designated recipient, the costs of collecting such electronic data are modest and decreasing per unit of information.
A number of states and local jurisdictions use such systems as Real-time Outbreak and Disease Surveillance (RODS),8 the Electronic Surveillance System for the Early Notification of Community-based Epidemics (ESSENCE) (Lombardo et al., 2003), or a system they have developed themselves (GAO, 2008b). In the absence of bioterrorism attacks, these systems are being used to track the onset, relative impact, and sweep of annual seasonal influenza and norovirus outbreaks.
RODS, developed at the University of Pittsburgh Department of Biomedical Informatics, is a system that receives data in real time from patient registrations at hospitals and acute care clinics and classifies chief complaints into one of seven syndrome categories. Statistical detection algorithms are applied to the aggregated data to identify anomalous patterns in the syndrome counts (Tsui et al., 2003). The University of Pittsburgh group also maintains data on sales of over-the-counter health care products that are available to public health officials for use in conjunction with other surveillance resources. ESSENCE was initially developed to monitor clusters of symptoms from ambulatory care visits by military personnel and their families. The data collection and analysis system has been implemented at local, state, and regional surveillance programs.
Syndromic surveillance is still a young science, with much of its potential for success and problems yet to be determined. These surveillance systems present a growing challenge of sifting through and interpreting large quantities of data of uneven precision and accuracy to identify statistical evidence of disease clustering or increasing trends that might signify a potential disease outbreak. This has led to active development and analysis of algorithms to simplify the identification of significant spatial and temporal aberrations in the occurrence of symptoms and other health data.
But no consensus has been reached on the most appropriate data or methods. Model-based analyses show relatively substantial false-negative rates with syndromic surveillance, and some analyses of real data have shown that it may either generate mistaken signals or miss outbreaks (e.g., Steiner-Sichel et al., 2004; Balter et al., 2005). A review of studies of outbreak detection found that characteristics of both the system (representativeness, detection algorithm, and the specificity) and the outbreak
|
8 |
Information about RODS is available at https://www.rods.pitt.edu/site/component/option,com_frontpage/Itemid,76/. |
(magnitude, distribution, and timing) influenced detection (Buckeridge, 2007). The review also identified inconsistencies in the evidence currently available.
Nevertheless, two-thirds of the states and territories with syndromic surveillance systems have found them sufficiently valuable that they are likely to expand their use of syndromic surveillance over the next 2 years (Buehler et al., 2008). The committee heard testimony that some public health officials have used their syndromic systems to provide reassurance that outbreaks are not occurring, including the use of syndromic surveillance data as part of assessments they have conducted following actionable alerts from the BioWatch system.
Syndromic Detection of Bioterrorism
The effectiveness of syndromic surveillance for detection of a bioterrorist attack remains uncertain. Modeling studies have focused mainly on the threat of a large-scale airborne attack with anthrax spores released in a major metropolitan area. For example, Buckeridge and colleagues (2005, 2006) used a model-based analysis to compare syndromic surveillance to traditional diagnosis for detection of an aerosolized anthrax release. This analysis indicated that syndromic surveillance would detect an increase in febrile respiratory syndromes sooner than traditional diagnostic methods for 30 percent to 60 percent of large-scale releases, depending on the specificity of the detection algorithm. The syndromic signal preceded detection by traditional diagnosis by 8 to 24 hours, a benefit that is modest, although potentially important for a disease with a short incubation period and high fatality rate. However, the system generated frequent false alarms (one every 10 days) when it was sufficiently sensitive to detect a substantial proportion of outbreaks before clinical case finding.
The challenge is finding an appropriate balance between timeliness and completeness of epidemic detection and the potential for generating unacceptable numbers of false alarms. The ability of public health staff to mobilize an effective epidemiologic and laboratory investigation to confirm that a signal represents an outbreak that merits a public health investigation and response is an additional crucial determinant of the usefulness of syndromic surveillance. Health departments have processes to evaluate alerts and dismiss many that are unlikely to represent a disease outbreak. When a full-scale investigation is called for, it is challenging to accomplish because the patients whose conditions generated the statistical signal may not be available for testing, clinical records may have insufficient information, and microbiologic testing is rarely done, particularly for patients with respiratory syndromes who are not hospitalized.
Even if syndromic surveillance does not provide early warning that a bioterrorism event has occurred, it may provide public health officials with better situational awareness, helping them to track trends and assess the extent and geographic location of illness in their community. However, it is likely that once an event has been made public, visits to EDs and clinical testing will increase even in the absence of disease; this will have to be accounted for in the interpretation and use of the data.
The value of syndromic surveillance for detection of bioterrorism has not been evaluated comprehensively and remains unproved. In the absence of actual bioterrorist attacks, particularly large-scale attacks, its effectiveness for providing early epidemic detection relative to other epidemic detection methods will remain untested and unproved. Further modeling studies and insights from practical experience with the detection of seasonal illness and larger community outbreaks unrelated to bioterrorism are needed. In jurisdictions using syndromic surveillance, evaluation of experiences with detecting natural outbreaks unrelated to bioterrorism, such as the 2009 novel influenza A (H1N1) outbreak, can be expected to provide valuable insights into the effectiveness and limitations of syndromic surveillance versus traditional outbreak detection methods.
Examples of Collaboration in Surveillance
The link between health care laboratories and providers in hospitals and clinics is already established, as is the link between veterinary laboratories and veterinarians within academia and clinical practice. What must now be significantly improved nationwide are the working relationships between health care, public health, and veterinary laboratories, and the practice relationships between health care providers, public health epidemiologists, and veterinarians (Foldy, 2004). In addition, and of partcular importance, is the working partnership between the public health laboratory and the public health epidemiologists (Figure 4-2).
Not only must this partnership be strong, the workforce must be adequate and interdependent. A robust, near-real-time surveillance system requires effective laboratory and epidemiology personnel whose skills are reinforced not just by responding to atypical cases, but also through ongoing mutual efforts. Minnesota is an example of such real-time testing and investigation in practice. As isolates of infectious agents are routinely received from sentinel laboratories throughout the state, the agents are immediately identified and subtyped to detect clusters of genetically similar pathogens that may indicate the presence of an outbreak, either naturally occurring or as a potential act of terrorism. Daily, the laboratory provides the state health department’s epidemiology staff with a report of all the
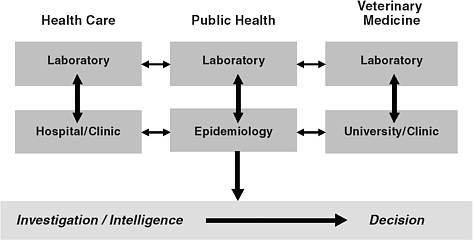
FIGURE 4-2 A schematic depiction of the relationships between functional components of biosurveillance and the associated flows of information. Information must flow effectively from the clinical and analytic elements of biosurveillance (the boxes in the upper portion of the figure) to the investigative and decision-making components of the public health system (the bar in the lower portion of the figure).
isolates tested. The report includes the genetic subtypes found linked to the patient’s name and location, as well as a historical perspective for each subtype (i.e., whether or not it has been seen previously).
DATA INTEGRATION AND SITUATIONAL AWARENESS
The data needed to detect an infectious disease outbreak or bioterrorism may come from a variety of sources, and being able to aggregate data across these sources may be necessary to recognize the nature of a disease event or understand its scope (e.g., Proctor et al., 1998). One hope is that being able to effectively monitor multiple data sources may also increase the possibility of detecting warning signs that could allow for advance preparation or even, perhaps, preventive action. For example, the presence of avian influenza may initially be recognized in the veterinary community, whereas an indication of a potential bioterrorist attack may first come to the attention of the intelligence community. Access to multiple data sources is also seen as an important resource for monitoring the course of a disease outbreak (natural or intentional) and guiding the response. Building an integrated, near-real-time repository of information presents many challenges, however. At the national level, both HHS and DHS are pursuing data integration activities.
BioSense
CDC’s BioSense program was initiated in 2002 in response to the threat of bioterrorism and other emerging diseases. It is being developed and administered by CDC’s public health informatics program. The program’s current aim is to serve as a resource for surveillance and situational awareness by assembling and analyzing health-related data from health care organizations, syndromic surveillance systems, and laboratories across the country and by providing access to that information to public health officials at federal, state, and local levels (CDC, 2009c). It is emphasizing secure and timely transmission of surveillance data, statistical analysis of surveillance data, reporting and display of information, and access to data for health departments to support investigation and response to disease outbreaks.
Currently, the program receives near-real-time clinical data from more than 590 health care facilities, mostly private or municipal hospital EDs (the majority via ED-based syndromic surveillance systems established by state or local health departments), ambulatory clinics, and clinical laboratories; more than 1,200 Department of Defense and Department of Veterans Affairs hospitals and clinics; and other sources, including major commercial diagnostic laboratories. At least some data are going to BioSense from the 50 largest metropolitan areas and all BioWatch jurisdictions (CDC, 2009c).
In general, state and local public health and hospital personnel have reported mixed views about the usefulness of BioSense, especially for early event detection, because of limited data relevant to their specific jurisdiction (Buehler et al., 2008, 2009; GAO, 2008b). Since late 2007, BioSense has been undergoing major changes in response to concerns of Congress and state and local health agencies about data protection and access, utility, and duplication of effort (TFAH, 2008). Rather than having hospitals report data directly to CDC, bypassing state and local health departments, the program is trying to achieve national coverage by fostering and integrating existing state and local syndromic surveillance systems (TFAH, 2008; CDC, 2009a,c). The goal is to have all levels of public health with jurisdiction over the 50 most populous metropolitan areas using the BioSense application for biosurveillance and situational awareness by accessing data directly from their health care facilities. This is to be accomplished by offering state and local public health agencies technical and financial support for developing and maintaining real-time surveillance systems; supporting formation of Regional Health Information Organizations and Health Information Exchanges to enable regional coordination of surveillance; developing a system of federated databases in which the data are stored locally but can be accessed by CDC and other authorized users; and developing better detection technologies (CDC, 2009a,c).
NBIS and NBIC
The National Biosurveillance Integration System (NBIS) was established in response to Homeland Security Presidential Directive 10, Biodefense for the 21st Century (The White House, 2004), which directed the Secretary of Homeland Security to create a national bioawareness system to detect a biological attack at the earliest possible moment and enable an effective response to reduce loss of life, economic impact, and social disruption. The purpose of NBIS is to acquire, integrate, analyze, and disseminate information from human disease, food, agricultural, water, meteorological, and environmental surveillance systems and relevant threat and intelligence information to provide continuous situational awareness, early warning of a possible attack, and a decision support system for outbreak and event response in the event of a biological incident, either intentional or naturally occurring (DHS, 2009b).
Establishing an effective NBIS has been difficult and time consuming (DHS, 2007; GAO, 2008a), and the National Biological Information Center (NBIC) was created to manage it. DHS opened NBIC in September 2008, but the program is still evolving. Currently, NBIC has permanent staff, it is implementing a new information technology system (NBIS 2.0), and it is developing a biological common operating picture in coordination with the other relevant federal agencies. Progress in building an interagency team has been slow. DHS identified 11 agencies to support NBIC operations but has memoranda of understanding with only 7 (DHS, 2009a,b). CDC has a detailee working in the center (DHS, 2009a), and NBIS has access to CDC’s BioSense (CDC, 2009b). Most of the anticipated information exchange awaits interagency security agreements that had not been signed as of March 2009.
PUBLIC HEALTH RESOURCES FOR DISEASE SURVEILLANCE
Every state has a public health agency, and there are approximately 2,900 health departments serving “local” jurisdictions (e.g., counties, townships) (NACCHO, 2006). More than 60 percent of the local health departments serve populations of fewer than 50,000 people. In 2005, local health departments had a total of about 160,000 full-time equivalent (FTE) workers (NACCHO, 2006), and state health departments had another 100,000 workers (TFAH, 2009).
At the local level, a third of health departments employed fewer than 10 FTE workers. The median number of employees among departments serving a population of a million or more was about 500. Nearly all (98 percent) of these large health departments have epidemiologists (scientists trained in disease surveillance and control), and a similar percentage (96 percent)
have an emergency preparedness coordinator (NACCHO, 2006). But only 50 percent of health departments serving populations of 100,000–249,999 reported having an epidemiologist on staff. Overall, states employed about 2,500 epidemiologists in 2006 (CSTE, 2007). About 42 percent of the epidemiologists had assignments related to infectious diseases, and 14 percent were assigned to bioterrorism and emergency preparedness programs.
For 2005, a third of local health departments reported expenditures of less than $500,000 a year, and a fifth spent more than $5 million (NACCHO, 2006). Local health departments are typically funded by a mix of local, state, and federal funds. Overall, they received 29 percent of their funding from local sources, 23 percent from the state, and 20 percent from federal funds (either directly or through the state). Much of the remainder of their funding was derived from sources such as Medicare, Medicaid, and fees for services. A survey in late 2008 found that 44 percent of local health departments expected their new budgets to be smaller than the current one (NACCHO, 2008). Staff are also being lost, with 53 percent of health departments having lost staff in 2008 and 46 percent expecting to do so during 2009.
At the state level, public health agencies’ budgets for fiscal year (FY) 2007–2008 ranged from $8.6 million to $3.0 billion (TFAH, 2009). On average, state health departments received approximately 49 percent of their funding from the federal government (ASTHO, 2009). In 2009, state health departments are facing substantial budget pressures. Nearly 30 percent had smaller budgets in FY 2008 than in FY 2007, and more than two-thirds expected their budgets to be reduced from FY 2008 to FY 2009, some by 10 percent or more (ASTHO, 2009). Forty percent of states reported expecting to lose staff in FY 2009.
The current financial problems at the state and local level reflect both the consequences of the current recession and declines in federal funding for emergency preparedness after a substantial infusion following the events of 2001. The Public Health Emergency Preparedness (PHEP) cooperative agreement program, for which states and territories as well as the New York City, Chicago, Los Angeles, and Washington, DC, health departments are eligible, increased from about $50 million a year to more than $900 million in FY 2002. The FY 2008 appropriation was $700 million, and the FY 2010 budget request is $715 million (CDC, 2009c). During this period, states also had access to funds from a total of $600 million appropriated for planning and preparedness for pandemic influenza. These federal funds have helped state and local health departments improve their capacity in disease surveillance and other preparedness areas over the past several years. Among other things, these increases in federal funding helped health departments hire additional staff, including epidemiologists to support improved surveillance capacity, but some of these positions may be lost as state and local budgets are reduced.
CONCLUDING REMARKS
Many novel and promising surveillance techniques and programs have been developed at the local, state, and federal levels in recent years, spurred in part by funding for bioterrorism and public health emergency preparedness since 2001. However, the nation’s resources for surveillance have shortcomings.
-
There is insufficient evidence regarding the public health utility of novel surveillance techniques, and funding for evaluation of surveillance approaches is insufficient.
-
Surveillance capacities are unevenly distributed among states and localities.
-
National standards for surveillance data and for interoperability between surveillance systems are incompletely developed and unevenly implemented; the important efforts underway to resolve this suffer from inadequate funding and focus.
-
Insufficient attention has been paid to the timeliness and accuracy of case reporting, or how this process can be strengthened.
-
Insufficient attention has been paid to effective methods for linking, integrating, analyzing, and displaying multiple surveillance platforms for optimal situational awareness, decision making, and response.
-
Federal funding, while substantial after 2001, has been year-to-year, which disrupts orderly program planning and discourages recruitment of qualified personnel; more recently this funding has been declining. Meanwhile, the economic conditions in 2009 make it difficult for many state and local governments to support their current activities, let alone make investments in new systems or make up the loss in federal funding.
FINDING: Inconsistent local and state public health capabilities, combined with inadequate systems for information exchange and situational awareness spanning jurisdictions up to the national level, leave the nation with many holes in the ability to promptly detect, confirm, and respond to disease clusters or bioagent attack, as well as providing the opportunity for duplication of effort. The gaps are likely to worsen with recent reductions of federal funding for these functions in the face of a recession affecting local and state public health budgets.
REFERENCES
Ashford, D.A., R.M. Kaiser, M.E. Bales, K. Shutt, A. Patrawalla, A. McShan, J.W. Tappero, B.A. Perkins, and A.L. Dannenberg. 2003. Planning against biological terrorism: Lessons from outbreak investigations. Emerging Infectious Diseases 9(5):515–519. http://www.cdc.gov/ncidod/EID/vol9no5/pdfs/02-0388.pdf (accessed March 9, 2009).
ASTHO (Association of State and Territorial Health Officials). 2009. Impact of budget cuts on state public health. http://www.astho.org/Advocacy/2009-Advocacy-Materials/Impact-of-Budget-Cuts-on-State-Public-Health/ (accessed June 28, 2009).
Balter, S., D. Weiss, H. Hanson, V. Reddy, D. Das, and R. Heffernan. 2005. Three years of emergency department gastrointestinal syndromic surveillance in New York City: What have we found? Morbidity and Mortality Weekly Report 54(Suppl.):175–180.
Buckeridge, D.L. 2007. Outbreak detection through automated surveillance: A review of the determinants of detection. Journal of Biomedical Informatics 40(4):370–379.
Buckeridge, D.L., P. Switzer, D. Owens, D. Siegrist, J. Pavlin, and M. Musen. 2005. An2005. An evaluation model for syndromic surveillance: Assessing the performance of a temporal algorithm. Morbidity and Mortality Weekly Report 54(Suppl.):109–115.
Buckeridge, D.L., D.K. Owens, P. Switzer, J. Frank, and M.A. Musen. 2006. Evaluating detection of an inhalational anthrax outbreak. Emerging Infectious Diseases 12(12):1942–1949.
Buehler, J.W., A. Sonricker, M. Paladini, P. Soper, and F. Mostashari. 2008. Syndromic surveillance practice in the U.S.: Findings from a survey of state, territorial, and selected local health departments. Advances in Disease Surveillance 6:3. http://www.isdsjournal.org/article/view/2618/2517 (accessed March 20, 2009).
Buehler, J.W., E.A. Whitney, D. Smith, M.J. Prietula, S.H. Stanton, and A.P. Isakov. 2009. Situational uses of syndromic surveillance. Biosecurity and Bioterrorism: Biodefense Strategy, Practice, and Science 7(2):165–177. http://www.liebertonline.com/doi/pdf/10.1089/bsp.2009.0013
CDC (Centers for Disease Control and Prevention). 2008a. Effect of electronic laboratory reporting on the burden of Lyme disease surveillance—New Jersey, 2001–2006. Morbidity and Mortality Weekly Report 57(2):42–45. http://www.cdc.gov/mmwr/preview/mmwrhtml/mm5702a4.htm (accessed March 9, 2009).
CDC. 2008b. Justification of estimates for appropriation committees: FY 2009. http://www.cdc.gov/fmo/topic/Budget%20Information/appropriations_budget_form_pdf/FY09_CDC_CJ_Final.pdf (accessed March 25, 2009).
CDC. 2008c. Outbreak of Salmonella serotype Saintpaul infections, associated with multiple raw produce items—United States, 2008. Morbidity and Mortality Weekly Report 57(34):929–934.
CDC. 2008d. Potential effects of electronic laboratory reporting on improving timeliness of infectious disease notification—Florida, 2002–2006. Morbidity and Mortality Weekly Report 57(49):1325–1328. http://www.cdc.gov/mmwr/preview/mmwrhtml/mm5749a2.htm?s_cid=mm5749a2_e (accessed December 22, 2008).
CDC. 2009a. FY 2008 CDC annual performance report. Atlanta, GA: CDC. http://www.cdc.gov/fmo/PDFs/FY_2008_CDC_Annual_Performance_Report.pdf (accessed March 20, 2009).
CDC. 2009b. IOM review. Document submitted to the Committee on Effectiveness of National Biosurveillance Systems: BioWatch and the Public Health System, January 13, Washington, DC.
CDC. 2009c. Justification of estimates for appropriation committees: FY 2010. Atlanta, GA: CDC. http://www.cdc.gov/fmo/topic/Budget%20Information/appropriations_budget_form_pdf/FY2010_CDC_CJ_Final.pdf (accessed July 21, 2009).
CDC. 2009d. Multistate outbreak of Salmonella infections, associated with peanut butter and peanut butter-containing products—United States, 2008–2009. Morbidity and Mortality Weekly Report 58(4):85–90.
CDC. 2009e. Status of state electronic disease surveillance systems—United States, 2007. Morbidity and Mortality Weekly Report 58(29):804–807.
CSTE (Council of State and Territorial Epidemiologists). 2007. 2006 National assessment of epidemiologic capacity: Findings and recommendations. Version 1.0. Atlanta, GA: CSTE. http://www.cste.org/pdffiles/2007/2006CSTEECAFINALFullDocument.pdf (accessed July 28, 2009).
CSTE. 2008. National Electronic Disease Surveillance System: A status report on implementation. Atlanta, GA: CSTE. http://www.cste.org/dnn/LinkClick.aspx?fileticket=BJJMGWMITb8%3d&tabid=173&mid=712 (accessed July 14, 2009).
Dato, V., M.M. Wagner, and A. Fapohunda. 2004. How outbreaks of infectious disease are detected: A review of surveillance systems and outbreaks. Public Health Reports 119(5):464–471. http://www.pubmedcentral.nih.gov/picrender.fcgi?artid=1497658&blobtype=pdf (accessed March 9, 2009).
Dausey, D.J., A. Chandra, A.G. Schaefer, B. Bahney, A. Haviland, S. Zakowski, and N. Lurie. 2008. Measuring the performance of telephone-based disease surveillance systems in local health departments. American Journal of Public Health 98(9):1706–1711. http://www.ajph.org/cgi/reprint/AJPH.2007.114710v1 (accessed March 24, 2009).
Dey, A.N., J.I. Tokars, P. Hicks, M. Miller, and R. English. 2008. Automated monitoring of injuries due to falls using the BioSense system. Public Health Informatics Conference 2008. http://www.cdc.gov/biosense/files/Automated_Monitoring.pdf (accessed July 14, 2009).
DHS (Department of Homeland Security). 2007. Better management needed for the National Bio-Surveillance Integration System program. OIG-07-61. Washington, DC: Office of Inspector General, DHS. http://www.dhs.gov/xoig/assets/mgmtrpts/OIG_07-61_Jul07.pdf (accessed March 25, 2009).
DHS. 2009a. Department of Homeland Security annual performance report: Fiscal years 2008–2010. Washington, DC: DHS http://www.dhs.gov/xlibrary/assets/cfo_apr_fy2008.pdf (accessed March 26, 2009).
DHS. 2009b. Department of Homeland Security FY 2010 congressional justification budget. http://www.dhs.gov/xlibrary/assets/dhs_congressional_budget_justification_fy2010.pdf (accessed July 28, 2009).
Doyle, T.J., M.K. Glynn, and S.L. Groseclose. 2002. Completeness of notifiable infectious disease reporting in the United States: An analytical literature review. American Journal of Epidemiology 155(9):866–874.
Effler, P., M. Ching-Lee, A. Bogard, M.C. Ieong, T. Nekomoto, and D. Jernigan. 1999. Statewide system of electronic notifiable disease reporting from clinical laboratories: Comparing automated reporting with conventional methods. Journal of the American Medical Association 282(19):1845–1850. http://jama.ama-assn.org/cgi/reprint/282/19/1845 (accessed March 9, 2009).
FEMA (Federal Emergency Management Agency). 2008a. Biological incident annex. http://www.fema.gov/pdf/emergency/nrf/nrf_BiologicalIncidentAnnex.pdf (July 24, 2009).
FEMA. 2008b. Emergency support function #8—Public health and medical services annex. http://www.fema.gov/pdf/emergency/nrf/nrf-esf-08.pdf (accessed July 13, 2009).
Foldy, S.L. 2004. Linking better surveillance to better outcomes. Morbidity and Mortality Weekly Report 53(Suppl.):12–16. http://www.cdc.gov/mmwr/PDF/wk/mm53SU01.pdf (accessed March 23, 2009).
Foldy, S., E.N. Barthell, J.C. Silva, P. Biedrzycki, D. Howe, M. Erme, B. Keaton, C. Hamilton, L. Brewer, G. Miller, E. Eby, R. Coles, K. Pemble, and C. Felton. 2004. SARS surveillance project: Internet-enabled multi-region syndromic surveillance for rapidly emerging disease. Morbidity and Mortality Weekly Report 53(Suppl.):215–220. http://www.cdc.gov/mmwr/preview/ind2004_su.html (accessed July 14, 2009).
GAO (Government Accountability Office). 2008a. Biosurveillance: Preliminary observations on Department of Homeland Security’s biosurveillance initiatives. GAO-08-960T. http://www.gao.gov/new.items/d08960t.pdf (accessed March 26, 2009).
GAO. 2008b. Health information technology: More detailed plans needed for the Centers for Disease Control and Prevention’s redesigned BioSense program. GAO-09-100. Washington, DC. http://www.gao.gov/new.items/d09100.pdf (accessed March 20, 2009).
Ginsberg, J., M.H. Mohebbit, R.S. Ptel, L. Brammer, M.S. Smolinski, and L. Brilliant. 2009. Detecting influenza epidemics using search engine query data. Nature 457:1012–1014.
Henning, K.J. 2004. Overview of syndromic surveillance: What is syndromic surveillance? Morbidity and Mortality Weekly Report 53(Suppl.):5–11. http://www.cdc.gov/mmwR/preview/mmwrhtml/su5301a3.htm (accessed July 14, 2009).
Jajosky, R.A., and S.L. Groseclose. 2004. Evaluation of reporting timeliness of public health surveillance systems for infectious diseases. BioMed Central Public Health 4:29. http://www.biomedcentral.com/content/pdf/1471-2458-4-29.pdf (accessed March 9, 2009).
Klompas, M., R. Lazarus, R. Platt, X. Hou, F. Campion, B. Kruskal, G. Haney, J. Daniel, A. DeMaria, and S.J.N. McNabb. 2008. Automated detection and reporting of notifiable diseases using electronic medical records versus passive surveillance—Massachusetts, June 2006–July 2007. Morbidity and Mortality Weekly Report 57(14):373–376. http://www.cdc.gov/mmwr/preview/mmwrhtml/mm5714a4.htm (accessed March 9, 2009).
Lazarus, R., M. Klompas, F.X. Campion, S. McNabb, X. Hou, J. Daniel, G. Haney, A. DeMaria, L. Lenart, and R. Platt. 2009. Electronic support for public health: Validated case finding and reporting for notifiable diseases using electronic medical data. Journal of the American Medical Informatics Association 16(1):18–24. http://www.jamia.org/cgi/reprint/16/1/18 (accessed February 28, 2009).
Lombardo, J., H. Burkom, E. Elbert, S. Macgruder, S.H. Lewis, W. Loschen, J. Sari, C. Sniegoski, R. Wojcik, and J. Pavlin. 2003. A systems overview of the Electronic Surveillance System for Early Notification of Community-based Epidemics (ESSENCE II). Journal of Urban Health 80(2 Suppl. 1):i32–i42.
Malakmadze, N., I.M. Gonzalez, T. Oemig, I. Isiadinso, D. Rembert, M.M. Mccauley, P. Wand, L. Diem, L. Cowan, G.J. Palumbo, M. Fraser, and K. Ijaz. 2005. Unsuspected recent transmission of tuberculosis among high-risk groups: Implications of universal tuberculosis genotyping in its detection. Clinical Infectious Diseases 40(3):366–373.
Mandl, K.D., J.M. Overhage, M.M. Wagner, W.B. Lober, P. Sebastiani, F. Mostashari, J.A. Pavlin, P.H. Gesteland, T. Treadwell, E. Koski, L. Hutwagner, D.L. Buckeridge, R.D. Aller, and S. Grannis. 2004. Implementing syndromic surveillance: A practical guide informed by the early experience. Journal of American Medical Informatics Association 11(2):141–150. http://www.pubmedcentral.nih.gov/picrender.fcgi?artid=353021&blobtype=pdf (accessed March 24, 2009).
Moore, K.M., V. Reddy, D. Kapell, and S. Balter. 2008. Impact of electronic laboratory reporting on hepatitis A surveillance in New York City. Journal of Public Health Management and Practice 14(5):437–441.
NACCHO (National Association of County and City Health Officials). 2006. 2005 national profile of local health departments. Washington, DC: NACCHO. http://www.naccho.org/topics/infrastructure/profile/upload/NACCHO_report_final_000.pdf (accessed December 31, 2008).
NACCHO. 2008. Preliminary findings: NACCHO survey of local health departments’ budget cuts and workforce reductions. December. Washington, DC: NACCHO. http://www.naccho.org/advocacy/upload/LHDbudgetsjobsDec08.pdf (accessed January 2, 2009).
Nguyen, T.Q., L. Thorpe, H.A. Makki, and F. Mostashari. 2007. Benefits and barriers to electronic laboratory results reporting for notifiable diseases: The New York City Department of Health and Mental Hygiene experience. American Journal of Public Health 97 (Suppl. 1):S142–S145.
Olson, D.R., R.T. Heffernan, M. Paladini, K. Konty, D. Weiss, and F. Mastashari. 2007. Monitoring the impact of influenza by age: Emergency department fever and respiratory complaint surveillance in New York City. PLoS Medicine 4(8):e247. http://www.plosmedicine.org/article/info%3Adoi%2F10.1371%2Fjournal.pmed.0040247 (accessed July 29, 2009).
Overhage, J.M., S. Grannis, and C.J. McDonald. 2008. A comparison of the completeness and timeliness of automated electronic laboratory reporting and spontaneous reporting of notifiable conditions. American Journal of Public Health 98(2):344–350.
Polgreen, P.M., Y. Chen, D.J. Pennock, and F.D. Nelson. 2008. Using internet searches for influenza surveillance. Clinical Infectious Diseases 47:1443–1448. http://www.journals.uchicago.edu/doi/abs/10.1086/593098 (accessed July 15, 2009).
Proctor, M.E., K.A. Blair, and J.P. Davis. 1998. Surveillance for waterborne illness detection: An assessment following a massive waterborne Cryptosporidium outbreak. Epidemiology and Infection 120(1):43–54.
Steiner-Sichel, L., J. Greenko, R. Heffernan, M. Layton, and D. Weiss. 2004. Field investigations of emergency department syndromic surveillance signals—New York City. Morbidity and Mortality Weekly Report 53(Suppl.): 184–189.
TFAH (Trust for America’s Health). 2008. Ready or not? Protecting the public’s health from diseases, disasters, and bioterrorism. Washington, DC: TFAH. http://healthyamericans.org/assets/files/bioterror-report-2008.pdf (accessed July 17, 2009).
TFAH. 2009. Shortchanging America’s health: A state-by-state look at how federal public health dollars are spent and key state health facts. Washington, DC: TFAH. http://healthyamericans.org/report/61/shortchanging09 (accessed July 26, 2009).
Thacker, S.B., and R.L. Berkelman. 1988. Public health surveillance in the United States. Epidemiologic Reviews 10:164–190.
Tsui, F.-C., J.U. Espino, V.M. Dato, P.H. Gesteland, J. Hutman, and M.M. Wagner. 2003. Technical description of RODS: A real-time public health surveillance system. Journal of the American Medical Informatics Association 10(5):399–408.
The White House. 2004. Homeland Security Presidential Directive (HSPD) 10: Biodefense for the 21st Century. Washington, DC. http://www.exercise.dhs.gov/xabout/laws/gc_1217605824325.shtm#1 (accessed July 24, 2009).























