1
Introduction
INTERNATIONAL IMPACTS ON LOCAL AND REGIONAL AIR POLLUTION
Human activities produce or enhance the release of a wide range of airborne substances that affect air quality; and poor air quality has long been recognized as an undesirable side effect of urban population concentrations and intensive industrial and agricultural activities. By the 1950s it was widely recognized that emissions of combustion-generated exhaust gases and smoke particles, malodorous (and sometimes toxic) emissions from factory and farm operations, and windblown dust from unpaved roads, construction sites, and degraded agricultural fields could all directly degrade local air quality. By the 1960s secondary gaseous and particulate smog pollutants, formed by atmospheric reactions triggered by photochemical processing of directly emitted pollutants, were confirmed as important components of local air pollution. During the last quarter of the 20th century it became increasingly clear that emissions and associated secondary pollutants could have serious effects well beyond the local community, impacting air quality, public health, and ecosystem viability on scales of hundreds to thousands of kilometers downwind. Recently atmospheric studies have demonstrated that near-surface pollutants can be lofted to high altitudes where strong winds can transport high concentrations across oceans to other continents.
We now know that atmospheric pollutants often have long-distance impacts on regional and continental scales (acid and nutrient deposition, atmospheric haze, particulate matter climate impact, persistent toxic
organic pollutant contamination) as well as hemispheric and global scales (greenhouse gas warming, mercury contamination, and stratospheric ozone depletion) (NRC, 1998). As we begin the 21st century, strong links between climate change and global air quality are becoming apparent, and the possibility that intercontinental transport of air pollutants can have a significant impact on local and regional air quality is more widely recognized (NRC, 2001; HTAP-TF, 2007; Stohl, 2004).
Air pollution, once thought of as purely a local issue, now is recognized as a complex problem that is also subject to regional, hemispheric, and even global influences. Although domestic sources are the primary contributors to most of our nation’s air quality problems, the United States is both a source and a receptor for pollutants transported great distances. Pollutants not only flow across our borders with Canada and Mexico but also travel between North America and Asia, Africa, and Europe. These pollutants contribute to public health threats, degraded visibility, agricultural and native vegetation injury, decreased domestic and wild animal viability, infrastructure materials damage, poorer water quality, degraded aquatic ecosystems, and climate change.
Long-range transport of air pollution from international sources is receiving increased attention in both the scientific literature and popular media. It is also an increasing concern for managers charged with meeting air quality standards. Air quality management stakeholders have advanced differing positions on how localities should address the impact of international pollutant transport in air quality planning. There also is concern about international pollution transport as a source of continuing exposure to chemicals that have been banned in the United States and other countries (e.g., certain persistent organic pollutants, POPs). The U.S. government is under increasing pressure from European countries to mitigate the transport of pollution across the Atlantic, and to address concerns about the contamination of pristine Arctic environments. Although there is now qualitative evidence of long-range transport of pollution, we need a more quantitative understanding of the true magnitude and dynamics of these flows, in order to assess their impacts on human health, ecosystem viability, and other concerns, and to design effective cooperative international control strategies. Some basic concepts needed to discuss the long-range transport of air pollutants are defined in Box 1.1.
To help address these issues the National Research Council (NRC) of the U.S. National Academies was asked to convene an ad hoc study committee to assess and summarize current understanding of the long-range transport of four key classes of pollutants: ozone (O3) and its precursors, particulate matter (PM) and its precursors, persistent organic pollutants
|
BOX 1.1 Definitions of Key Terms Because this report focuses on the impact of polluting substances from distant sources on local pollution concentrations, it is important to define some key environmental concepts and terms. An air pollutant is any airborne substance that has the capacity to adversely impact human health or other components of Earth’s biosphere. Most air pollutants have some natural background level (discussed below) although a few, such as some POPs, are entirely anthropogenic in origin. Air pollutants can be gaseous substances dispersed as individual molecules or very small condensed-phase liquid droplets or solid particles. The condensed airborne material is often collectively termed aerosol particles or particulate matter (PM). Air pollutants also are characterized by their creation mechanism. Primary pollutants are directly emitted into the atmosphere from pollution sources, while secondary pollutants are formed in the atmosphere from pollutant precursor species or other pollutants by chemical reaction. Secondary pollutants can be either gases or particles. Precursors for specific secondary pollutants are airborne substances with the capacity to be transformed into the pollutant of interest; they may or may not be pollutants in their own right. For example, nearly all volatile organic compounds (VOCs) are precursors for the formation of ozone, an important secondary pollutant, but some VOCs like benzene or formaldehyde are also highly toxic substances, while other VOCs like ethane or propane are not dangerous at normal atmospheric concentrations. Pollutants and pollutant precursors may have sources that are classified as either anthropogenic (caused by human activity) or natural (produced in the absence of human activity). Windborne dust from desert regions is an example of a natural primary pollutant; although humans can stimulate excess dust by driving vehicles over desert soil or engaging in unsustainable agricultural practices that enhance desert formation. Formaldehyde is an example of a gaseous pollutant with multiple sources. It can be a primary pollutant emitted in motor vehicle exhaust or a secondary pollutant formed from the atmospheric oxidation of VOCs. This report focuses on ozone (O3), a gaseous secondary pollutant, and its precursors; particulate matter (PM), which has important primary and secondary sources; mercury (Hg), which is found in both gaseous and condensed phase (PM) forms; and a number of persistent organic pollutants (POPs), most of which, like mercury, are partly gaseous and partly PM species. |
|
Long-range transport is a term that must be defined in context. In the United States, long-range pollutant transport is usually discussed in terms of multistate pollution episodes where emissions in upwind states lead to high pollutant levels in downwind states. In the context of this report long-range transport around the Northern Hemisphere generally refers to transpacific or transatlantic flows to or from North America or flows between Europe and Asia. North/south long-range transport in this report refers to pollution movement between semitropical regions and midlatitudes or midlatitudes and northern/arctic regions. A key issue for this report is to discern when significant concentrations of pollutants from distant international sources contribute to local pollution levels. Such events can appear as pollution-rich plumes that are sufficiently discrete to be recognized by available measurement systems or by a general diffuse enhancement of relevant ambient pollutants (i.e., persistent transport). In either case the contribution from long-range transport must be defined relative to background concentrations. From an observational perspective, background (or baseline) concentrations are often estimated as the average weakly varying concentrations against which pollution plumes (events of enhanced pollutant concentrations) can be referenced. This provides a simple way to quantify the pollutant enhancements associated with plumes, although the background can still contain a contribution from a particular source diluted over the larger scale. An alternative observational definition of background is the lower envelope of the frequency distribution of concentrations, reflecting conditions of minimum pollutant source influence. This approach removes the influence of diluted pollution sources, although the definition of the lower envelope of concentrations may not be robust. Another method used to estimate background is to conduct a sensitivity simulation in a global model, in which the pollution sources that are not considered to be part of the background are inactivated for longer than the lifetime of the pollutants being studied. This removes ambiguity in defining the background but cannot be directly verified by observations, so the validity of the model must be carefully evaluated. Finally, the U.S. Environmental Protection Agency (EPA) has defined a policy relevant background (PRB) as the level of a specific pollutant that would exist in the absence of North American emissions of that pollutant and its precursors. The PRB cannot be measured directly but can be estimated by modeling studies. Discussions in Chapters 2-5 will indicate which type of background is meant in the context of the pollutant species being discussed. |
(POPs), and mercury (Hg).1 Considerably more research effort has been expended to investigate photochemically-derived air pollutants like O3 and PM than airborne toxic chemicals like Hg and POPs; as a result, the emission sources, atmospheric transformation, and removal processes are better characterized for the former. This is also because atmospheric concentrations of O3 and PM are much higher than those of airborne Hg and individual POPs, thus making measurements easier to perform. Accurately quantifying the local impacts of distant emissions is difficult for all pollutants, but the less complete knowledge base available for Hg and POPs results in a greater level of uncertainty in the evaluation of long-range transport impacts on health and the environment.
This study is aimed at aiding U.S. decision makers engaged in developing domestic and international air quality management policies, as well as those engaged in planning and funding atmospheric and environmental research. We hope our findings and recommendations will help to shape both near-term policy responses and the research investments that are needed as a foundation for designing effective longer-term policy responses. It also is intended to help inform the final assessment by the Long Range Transport of Air Pollutants (LRTAP) Task Force on Hemispheric Transport of Air Pollution (HTAP-TF). The HTAP efforts are not focused specifically on pollution flow into out of the United States but rather on examining pollutant flows throughout the Northern Hemisphere. We anticipate that our assessment effort, focused specifically on the information needs of U.S. policy makers, will be a valuable complement to the HTAP assessment efforts. The NRC study was designed to be independent of the HTAP process, but several steps were taken to ensure effective coordination and communication between the two efforts. For example, several of the NRC committee members are directly involved in HTAP analysis and assessment activities, the first NRC committee meeting was held in conjunction with a workshop of the HTAP task force, and several of the speakers invited to subsequent NRC meetings were active in the HTAP process.
MOTIVATIONS FOR CONCERN
Despite increasing growth and urbanization, air quality in developed countries generally has improved over the past 25 years because of serious efforts to set and meet air quality standards. However, air quality in much of the less developed world has declined due to increasing emissions from rapidly expanding and poorly regulated motor vehicle fleets, growing industrial and power generation activities, and domestic coal and biomass
|
1 |
Information on the committee’s sponsors, its schedule, and its full statement of task are presented in Appendix A. |
burning. Widespread overuse and degradation of crop, grazing, and woodlot lands also have led to greater levels of airborne dust; more intensive agricultural activities have led to increasing levels of fixed nitrogen and persistent organic pesticides.
Maintaining or improving living standards for a rapidly growing and urbanizing world population tends to increase global pollutant emissions from the industrial, transportation, agricultural, and energy sectors, as well as domestic and commercial heating, cooling, lighting, and cooking systems. Increased emissions push ambient concentrations of both primary and secondary air pollutants higher. Scientific studies have quantified the adverse impacts of atmospheric pollutants on human health, agricultural yields, and natural ecosystems. These studies often reveal serious impacts at relatively low pollutant concentrations, putting pressure on air quality managers to decrease the allowable concentrations of many air pollutants.
The conflict between increasing air pollutant levels in some parts of the world, and more stringent air quality standards in other parts of the world, leads to concerns that if even small fractions of the pollutants from Nation 1 reach Nation 2, Nation 2 may find it significantly more difficult to meet its mandated air quality goals. The cost of not meeting air quality standards on human health, agricultural production, and ecosystem viabilty can be high, so accepting higher pollution levels is an unattractive option. A brief overview of some of the costs associated with poor air quality follows.
Human Health Impacts Alleviating the impact of air pollution on human health is the primary motivation of air quality management programs around the world. Some air pollutants can affect human health directly by inhalation, introducing pollutants that directly harm the throat and lungs and reach other internal organs after entering the pulmonary blood flow. Air pollutants also can be deposited to the ecosystems that provide humans with food and drinking water, including agricultural soils, fresh surface and ground water, and marine waters, where they enter the food chain or drinking water supplies, leading to human exposure by ingestion. Of the four classes of pollutants considered in this report, human exposure to O3 and PM is dominated by inhalation, while exposures to POPs and Hg occur primarily by ingestion.
High concentrations of ozone and particulate matter increase the risk of cardiovascular and respiratory diseases, as well as a wide range of other adverse health outcomes (Cohen et al., 2004; EPA, 2004, 2006a; WHO, 2006; NRC, 2008). The total health burden caused by these pollutants can be considerable; the World Health Organization estimates that 41,200 Americans die prematurely each year due to exposure to elevated concentrations of PM that is less than 10 microns in diameter (WHO, 2002). Efforts to reduce airborne particulate matter can produce dramatic results;
a recent correlation of mortality data in 51 metropolitan areas with reductions in fine PM (less than 2.5 micrometers in diameter) between the late 1970s and early 1980s and the late 1990s and early 2000s estimated that reducing the average fine PM ambient concentrations by 10μg/m3 resulted in increasing mean average lifetimes by 0.61 ± 0.20 years (Pope III et al., 2009). A number of time-series and cohort studies have found the concentration–response relationship between PM exposure and mortality response to be close to linear with no evidence of a threshold below which no adverse health impacts are observed (Daniels et al., 2000; Pope, 2000; Schwartz et al., 2002, 2008; Pope and Dockery, 2006).
The impact of airborne ozone on human mortality is not as large as that of fine PM, but a recent NRC review confirmed that short-term exposure to high-ambient ozone levels does produce significant premature mortality, and that the risk of mortality is not limited to those already at a high risk of death (NRC, 2008). The total health burden of PM and ozone pollution is considerably larger than their impacts on premature mortality alone and is dominated by subclinical and symptomatic events. The risk of suffering an adverse health event due to exposure to air pollution is small, but widespread exposures result in large numbers of affected individuals. The extent of these health impacts depends on dose, with susceptibility varying with age, health status, diet, and genetics.
Mercury is a neurotoxin that can cause hand tremors, increases in memory disturbance, and other adverse health impacts (EPA, 1997). There is evidence of adverse human health effects from environmental exposures to POPs, and significant concern about elevated concentrations of these chemicals in a range of tissues, including venous and cord blood, adipose tissue, and breast milk (Li et al., 2006a).
Environmental Impacts Although impacts on human health may be the dominant reason for concern about air pollution (coming from both local and nonlocal sources), there are a variety of other adverse impacts that underlie interest in air pollution dynamics. These include impacts on ecosystem health, including impacts on agriculture productivity and wild fish populations, which in turn have serious implications for human well-being; reduced visibility due to pollution-induced haze, which obscures views in scenic locations around the world; and degradation of materials in buildings, monuments, vehicles, and other infrastructure components. The effects of short-lived air pollutant species on regional and global climate, through both direct interaction with atmospheric radiation and indirect effects related to changes in cloud properties are a growing concern. More information about specific air pollutants and other environmental impacts is presented in the following four chapters.
LONG-RANGE TRANSPORT OF POLLUTION
The fact that pollutants emitted near the surface of one continent can influence human health or crop yields on another continent many thousands of miles away seems unlikely to many people. One might suppose that as polluted air mixes with relatively clean air over the oceans, the pollution concentration would be diluted to inconsequential levels. In some instances, however, surprisingly intense pollution plumes can travel across broad oceans, delivering significant pollutant concentrations to a down-wind continent. Under other conditions (for some pollutants) diffuse export can lead to an overall increase in tropospheric abundance, thus increasing pollution in background clean air. In all cases the ultimate impacts will depend on how pollutants being transported aloft are mixed down to the surface. A brief overview of the dynamics of atmospheric air masses and their associated time and distance scales is presented below, and a more detailed explanation is provided in Appendix B.
Atmospheric Dynamics of Long-Range Transport Most meteorological phenomena that affect long-range pollutant transport occur in the troposphere, the lowest portion of the atmosphere, which extends from the surface to ~ 18 km in the tropics and ~ 8 km in polar regions. The tropopause is the boundary between the troposphere and the stratosphere, the next higher atmospheric layer. The troposphere can be subdivided into two regions—the planetary boundary layer (PBL) and the free troposphere. The PBL typically is confined to the lowest 1-2 km of the troposphere. Although most pollutants are released in this layer, their horizontal transport usually is quite slow due to relatively weak winds near Earth’s surface. Conversely, pollutants that are transported from the PBL into the free troposphere often travel great distances because of the stronger winds aloft, including the jet streams, the strongest rivers of atmospheric flow that encircle Earth. The mechanisms producing upward transport into the free atmosphere (e.g., thunderstorms and middle latitude low-pressure systems) play important roles in determining whether or not long-range pollutant transport will occur. The weather phenomena that affect the long-range transport of pollution range in size from small, short-lived turbulent eddies to systems that span continents and can last weeks or months. Thunderstorms, sea breezes, and high and low-pressure systems all play a role in transporting pollutants both horizontally and vertically.
It is convenient to think of intercontinental transport in terms of two different mechanisms. In the first, transport occurs in well-defined puffs of pollution that are lifted out of the polluted PBL of the source region. Once in the free troposphere relatively strong and persistent winds move the polluted air mass great distances with minimal dilution and removal of its
constituents (except during rain events) although the pollutants eventually become diluted into the background. This type of transport has been documented frequently. The air mass then can be mixed down to the surface, where it contributes to local air quality degradation. Because the polluted air mass remains relatively distinct, it is possible to identify its impacts from observations, even at great distances. In the second transport mechanism, pollutants from the source region are rapidly mixed into the background air. When this occurs, the source of the pollutants is more difficult to trace, and the exact contribution from each region is more difficult to determine. These two transport mechanisms occur simultaneously to varying degrees. Chemical transport models (CTMs) are important tools to quantify the contributions for both types of transport.
The overall transport of pollutants in the free atmosphere of the middle latitudes is from west to east due to the prevailing westerly winds (Figure 1.1). This easterly transport is greatly influenced by transient low-pressure systems. The east coast of Asia is an area of enhanced low-pressure development where systems form every few days and travel eastward toward North America. These transient lows contain several major transport channels, with the warm conveyor belt being the best defined. This conveyor belt begins near the surface in advance of cold fronts. It can transport surface-based pollution from the major industrialized areas of eastern Asia northward and then eastward. Air slowly rises out of the boundary layer, often
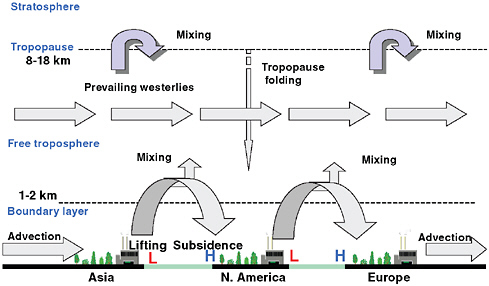
FIGURE 1.1 Schematic of the dominant dynamical processes involved in long-range midlatitude pollution transport. Ground level H and L symbols represent high- and low-pressure systems.
reaching the upper troposphere where the pollution is rapidly transported eastward toward North America by the strong winds aloft. West and southwest of the low in the advancing cooler air mass, dry air from the upper troposphere and lower stratosphere subsides toward the lower levels. This area often contains relatively small regions of a lowered tropopause (folds) and other mechanisms that mix stratospheric air into the troposphere.
The second major area of low-pressure development is the east coast of North America. The structure of these systems is similar to those forming near eastern Asia. However, these eastward moving storms transport surface-based pollution from the industrialized North American east coast to higher altitudes and then toward Europe. Europe generally is not an area of frequent low-pressure storm development. Winds within both the boundary layer and free troposphere climatologically are from the west, thereby completing the global transport cycle. At lower latitudes the prevailing winds typically flow from east to west, while the prevailing midlatitude flows are from west to east; winds from northern Africa can bring Saharan dust to the Caribbean, Florida, and other Gulf Coast states. Figure 1.2 illustrates a number of relevant long-range transport paths and representative time scales. Note that timescales may differ for concentrated pollution plumes and general transport of background air; for instance, Liu and
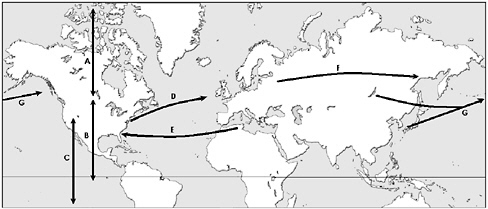
FIGURE 1.2 Major atmospheric transport pathways affecting North America. The general timescales of transport estimated by the committee from trajectory studies and other sources are: (A) Midlatitudes–Arctic exchange: 1-4 weeks. (B) Midlatitudes–Tropics exchange: 1-2 months. (C) Northern Hemisphere–Southern Hemisphere exchange: ~ 1 year. (D) North America to Western Europe: 3-13 days. (E) Northern Africa to North America: 1-2 weeks. (F) Eastern Europe to Asia: 1-2 weeks. (G) Eastern Asia to North America: 4-17 days. For (A), (B), and (C), transport occurs in both directions, depending on altitude (see Appendix B for details).
Mauzerall (2005) estimate that intercontinental transport times are, on average, 1-2 weeks longer than rapid transport times observed in plumes. Appendix B provides further discussion and illustration of these concepts.
Long-Range Pollutant Transport Example Figure 1.3 shows an example of transport from Asia to the Mt. Bachelor Observatory in central Oregon. On April 25, 2004, observations at Mt. Bachelor detected enhanced concentrations of carbon monoxide (a long-lived pollution plume tracer), ozone, fine submicron particulate matter, elemental mercury, as well as particulate-phase polycyclic aromatic hydrocarbons (PAHs) and hexachlorocyclohexane, which are persistent organic pollutants. The enhancements in the observed pollutants occured nearly simultaneously. The source for these pollutants can unambiguously be attributed to Asia based on meteorological data, the chemical pattern of pollutants and the absence of any short-lived tracer pollutants (e.g. NOx with lifetime τ of less than 1 day) that would indicate North American sources (see Jaffe et al., 2005a; Weiss-Penzias et al., 2006, 2007; Primbs et al., 2008a,b).
Meteorological analyses indicate that the polluted air mass detected at Mt. Bachelor on April 25 and 26 took approximately eight days to travel from East Asia to central Oregon.
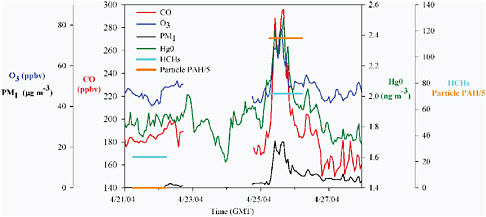
FIGURE 1.3 April 2004 observations from the Mt. Bachelor Observatory in central Oregon (2.7 km above sea level). On April 25, an air mass containing a variety of pollutants emitted in Asia was identified at the station. Note that measurements of HCH and PAHs occur on discrete filter samples, whereas the other pollutants are measured continuously. Units are pg/m3 for the 24-hr integrated sample measurements for April 21-22 and 25-26 of particulate phase PAHs and HCH (values shown on right axis).
Chemical and Microphysical Transformations During Long-Range Transport Pollutant species have finite residence times in the atmosphere before they are chemically transformed or deposited to the surface. In both the atmospheric boundary layer and the free troposphere, photochemical reactions can transform many pollutants into other chemical species. Microphysical processes such as particle nucleation, vapor condensation, and semivolatile evaporation transfer species between the gas phase and particulate condensed phases. Wet deposition can remove pollutants absorbed into cloud droplets or entrained into falling precipitation. Dry deposition (of both gaseous and particulate pollution) to vegetation, soil, water and humanmade material surfaces can occur for pollutants in the near-surface boundary layer. All of these transformation and removal processes (some of which are depicted in Figure 1.4) can operate during long-range trans-
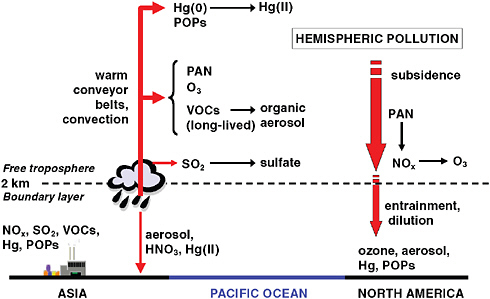
FIGURE 1.4 Some basic chemical and microphysical mechanisms involved in long-range (transpacific) pollution transport. Chemical transformation of Hg(0) to Hg(II) allows reactive Hg to be absorbed by aerosol particles and cloud droplets, peroxy-acetylnitrate (PAN) can sequester reactive nitrogen oxides (NOx) in the cold free troposphere but releases it to form ozone when air masses subside and warm. SO2 is oxidized to sulfate, and volatile organic compounds (VOCs) are oxidized to less volatile organic species by both gas phase and cloud droplet reactions, forming additional particulate matter. Wet deposition scavenges aerosol PM, nitric acid formed by oxidizing NOx, and Hg(II), while dry deposition removes a wide range of gaseous species as well as particle-bound species. The diagram depicts processes occurring during transpacific transport; these same processes occur during transatlantic transport.
port of air masses, with chemical transformations significantly reducing many reactive species with atmospheric chemical lifetimes of less than a week (Finlayson-Pitts and Pitts, 2006). In general, long-range transport can be significant when pollutant transformation rates are slow relative to long-distance transport times. More detailed examples of chemical transformation and removal processes are presented in subsequent chapters that discuss the production and transport of specific pollutant classes.
Tools for Understanding Long-Range Transport It is challenging to detect and quantify the impact of long-range transport on local air quality that is dominated by local sources. Clear examples of the impact of well-organized long-range transport of plumes like that described above are most common for free tropospheric observations; boundary layer examples are less common. In general, combinations of sophisticated measurement methods and advanced CTMs are required to recognize and quantify the boundary layer and surface impacts of long-range transport events. Models are also used to predict how future emission levels in various geographic source regions will impact pollutant concentrations in distant receptor regions.
Some of the currently utilized measurement and modeling tools are described in Boxes 1.2 and 1.3, respectively. Specific results obtained by utilizing these measurement and modeling tools are presented in subsequent chapters describing long-range transport of relevant pollutant classes. A prospective advanced integrated measurement and modeling system is presented in Chapter 6.
Emission Inventories Requirements Long-range transport of pollution is directly dependent on the magnitudes of emission fluxes of primary pollutants and precursors in upwind source regions. Accurate, up-to-date knowledge of the magnitudes of primary emissions, as well as their temporal and spatial distributions, are required to evaluate the range of impacts on air quality, climate, ecosystem viability, and human health for downwind receptor regions. Some basic concepts necessary to classify and quantify pollutant and pollutant precursor emissions are presented in Box 1.4.
Adequate emission inventories (EIs) are critical inputs for the chemical transport models that are traditionally used to quantify receptor region concentrations of both primary and secondary pollutants. These pollutants have a wide range of atmospheric lifetimes; high spatial and temporal fidelity predictions of impacts for species with lifetimes shorter than or approximately equivalent to transport times require similarly resolved emission data.
A recent three-nation assessment of North American emission inventories identified a wide range of deficiencies in EIs that are inputs to CTMs designed to predict photochemical oxidant and fine particulate concentra-
|
BOX 1.2 Observational Tools Used In the Study of Long-Range Transport of Pollution The study of long-range transport requires an integrated observing strategy encompassing techniques that span a large range of spatial and temporal scales. Satellite observations provide large-scale multiyear records of a limited number of key atmospheric trace gas and aerosol parameters. They produce important but limited resolution data in the horizontal, vertical, and temporal domains. However, these measurements provide critical context for more localized observations, and help extend local measurements to regional and global scales. Local and regional-scale atmospheric chemistry and dynamics are readily observable from aircraft with integrated chemical and meteorological instrumentation. Intensive airborne campaigns can provide a wide range of measurements, a detailed view of the important atmospheric processes, and good spatial and temporal resolution pollutant concentration measurements. Ship, train, and van-borne mobile laboratory instrument suites can produce similar data for the lower boundary layer, but these studies generally have limited temporal and spatial coverage. Surface monitoring sites and vertical profiling instruments yield datasets that are specific to a single location. These datasets are particularly useful for discerning trends as they are the only long-term, semicontinuous measurement of atmospheric pollution that we have. |
tion fields (NARSTO, 2005). These deficiencies are present even in the national and regional EIs produced by relatively experienced air quality management agencies in the three North American nations. This same study noted that assessing the adverse impact of long-range transport of pollution on North American air quality would require much improved emission inventories for the entire Northern Hemisphere.
Emissions from regions with less experience in developing EIs are widely recognized to have even more serious problems, caused both by lack of basic emission data and inadequate institutional support for gathering and processing required data. EIs for regions with rapidly changing economies are particularly problematic. In addition to uncertainties in current emissions, high rates of economic growth in these regions are accompanied by significant population shifts and rapid changes in technology and infrastructure, both of which produce rapid changes in emission quantities and distributions. Thus, even if EIs are relatively accurate when compiled, they
|
BOX 1.3 Modeling Tools Used In the Study of Long-Range Transport of Pollution Chemical transport models (CTMs) are important tools that are used to explore pollution transport pathways and to assess the impact of long-range transport on ambient pollution levels. Identification of effective measures to reduce pollution loads at a specific place requires quantification of contributions from local and distant sources, distinguishing between natural vs. humanmade sources as well as among local, regional, transboundary, and intercontinental sources. We are seldom confronted with a situation where the observed concentration or deposition fields at a particular location are due solely to emissions from a particular source. The further we are from source regions and the longer the atmospheric lifetime of the pollutant, the more likely it is that multiple sources contribute to observed concentrations. Since observations generally cannot answer such questions on their own, models are widely used to provide the required information. CTMs are computer models that link the emissions of pollutants to their ambient distributions. They integrate the meteorological, chemical, and physical processes that control the fate and transport of pollutants in the atmosphere. Over the last decade our ability to predict air quality has improved significantly due to advancements in our ability to measure and model atmospheric chemical, transport, and removal processes. CTMs now track scores of chemical species in various physical phases interacting with hundreds of chemical reactions. In addition, the transport components of CTMs now are run in close interaction with dynamic meteorological models. Computational power and efficiencies have advanced to the point where CTMs can simulate pollution distributions in an urban airshed with a spatial resolution of less than one kilometer, and can cover the entire globe with horizontal resolution of less than 100 kilometers. Assessing the impacts of distant sources on local air quality requires methods to track the contributions of specific source regions to pollution levels over regions of interest (i.e., the source-receptor relationships). A source-receptor (S-R) relationship quantifies the contribution of an emission source to the concentration or deposition of a specific pollutant at a receptor point elsewhere. While CTMs have been used to calculate S-R relationships over relatively short distances (hundreds of kilometers), their application in global S-R analysis is recent (HTAP-TF, 2007). Calculations of S-R relationships can be classified into source- and receptor-oriented approaches. Most commonly used is a source-oriented |
|
approach, wherein emissions from individual source regions are perturbed (or tagged), and these perturbations are propagated forward throughout the modeling domain to future times (Wang et al., 1998; Wild et al., 2001; Derwent et al., 2004; Auvray and Bey, 2005; Sudo and Akimoto, 2007; Liu et al., 2007, 2008, 2009; Fiore et al., 2009; Saikawa et al., 2009). Calculated concentrations from a tagged source region provide that source region’s footprint, which predicts the concentration from that source at all possible receptor regions. In this approach the perturbations from each source region of interest are calculated individually, allowing estimation of the individual source contributions to the concentrations at a receptor region of interest. In the receptor-oriented approach the perturbation in the concentrations at the receptor location due to specified emissions are traced backward in time. Receptor-oriented source-receptor calculations can be performed using adjoint sensitivities as demonstrated in Hakami et al. (2006) and Henze et al. (2008), or by running air-parcel models backward in time (see Stohl et al., 2002). A key source-receptor calculation consideration is its sensitivity to the size of the perturbation. Since CTMs generally represent nonlinear systems, the response in principle should be dependent on the size of the perturbation. S-R relationships are model constructs, currently with no simple means of verification or validation. The accuracy and validity of the S-R relationships depend on the adequacy and completeness of the atmospheric models from which they have been derived. The capabilities of the current generation of CTMs are evaluated through direct comparisons of the predicted concentration fields with observations and through multimodel intercomparison studies (Fiore et al., 2009). Models are thus indespensible tools for advancing our knowledge of long-range transport; however, our confidence in model predictions is constrained by numerous sources of uncertainty in the basic inputs and assumptions that drive the models. This includes uncertainties in the emissions of pollutants, the meteorological process that drives pollutant transport and deposition, and the chemical and kinetic processes that drive pollutant formation and transformation. More specific details about the nature of these different uncertainties are discussed in the following chapters. Current CTMs are generally not configured to produce quantitative uncertainty estimates based on the uncertainties in these various inputs and assumptions; unfortunately, our ability to report quantitative uncertainty estimates is quite limited. |
|
BOX 1.4 Pollutant Emissions Overview Emissions of the pollutants and pollutant precursors described in this report may be associated with both natural and anthropogenic (human-caused) sources. Energy and Economy. We use the term “energy and economy” to describe sources related to energy consumption and economic activity. These sources, which are the most directly controllable, include emissions from energy production, industry and consumer products, homes, and agriculture. International ships and aircraft are a rapidly growing portion of these emissions; regulatory standards for these emissions are established by international management activities rather than by local air quality agencies. In the future, international economic activity will probably increase, but new and cleaner technology can decrease the amount of emissions per activity. Emission standards that drive adoption of clean technology may be implemented, usually after a country has enough capital to invest in its environment. In the interim, air quality in developing countries can be extremely poor (Gurjar et al., 2008). The net result of growth vs. cleanup might result in an inverted U shape for emissions: an initial increase followed by a decrease (Selden and Song, 1994), but it is not known whether improvements in technology will always counteract growth. These factors lead to uncertainty in predicting the future magnitude of air pollutant emissions. Energy and economy sources are responsible for large fractions of the ozone, mercury, and particulate matter budget, and most POP emissions. Natural environment. Another major source of pollutants is the natural environment, a blanket term for a variety of disparate sources. Open fires are large sources of ozone precursors, PM, and some combustion-derived POPs. They may be caused by deforestation, natural causes, prescribed fires for land management, and seasonal fires for agricultural land clearing. Dust emissions contribute PM, and are affected by wind speed and the nature of the land surface. Emissions from vegetation are termed “biogenic”; complex biogenic organic gases promote ozone formation and lead to secondary organic PM, while organic biogenic particles contribute to PM loadings. Other environmental sources affect individual species, such as mercury emissions from volcanic activity and ozone incursions from the stratosphere. Many of these sources are episodic, seasonally variable, and dependent on climate. Because some of these emissions, especially biomass fires and airborne dust, are enhanced by human interactions with the natural environment, we choose not to identify these as either fully anthropogenic or fully natural. |
|
Legacy emissions. Once released to the environment mercury can cycle between elemental and molecular forms, but its natural removal requires geological time scales. Persistent organic pollutants may break down very slowly, even after they are removed from the atmosphere. For these long-lived species relofting of accumulated material can contribute a significant fraction of the atmospheric burden. Legacy emissions can result either from continuing processes such as soil resuspension or from large, episodic perturbations like forest fires. Current and future emissions of legacy material therefore depend on the history of emissions (sometimes over decades); the potential for degradation over time; the rate of exchange between terrestrial, aquatic, and biotic surfaces with the atmosphere; and their potential for long-term sequestration in soils and sediments. These emissions may be considered either anthropogenic or natural, depending on the original source. |
may be badly outdated by the time they are updated, which may occur only at intervals of five to ten years.
Global emission inventories have been developed to model and assess large-scale air quality and climate processes and trends. Such inventories may also provide boundary conditions for urban or regional air quality modeling studies, but the quality of these EIs may be uneven. For example, Butler et al. (2008) assessed the emissions of CO, NOx and nonmethane VOCs in three leading global EIs for 32 large cities and concluded that these compilations often assign inconsistent emissions levels, frequently varying by a factor of two for individual cities.
Without accurate emission inventory data for both local and upwind emissions at required spatial and temporal resolutions, CTMs cannot be expected to correctly predict how pollutants will be transformed during long-range transport or to quantify either the absolute or relative impact of these pollutants on local air quality. Deficiencies in hemispheric and global emission inventories that affect assessments of long-range transport will be discussed in subsequent chapters.
LONG-RANGE TRANSPORT POLICY CONTEXT
To assess the significance of long-range pollution transport on the achievement of environmental goals, it is necessary to identify the specific
environmental goals of greatest concern and to understand the landscape of relevant national and international policies, statutes, and agreements. A general overview of this landscape is provided here. Specific policy contexts for each relevant pollutant class will be further discussed in Chapters 2-5.
International Policy Context International action on long-range pollutant transport has revolved largely around the U.N. Long Range Transport of Air Pollution (LRTAP) convention, which was signed in 1979 by 30 European countries, the United States, and Canada. The convention itself demands that the signatory parties attempt to reduce transboundary air pollution. It has been supplemented by eight successive protocols (see Box 1.5) that set reduction goals for specific compounds, and established a mechanism for supporting scientific data gathering to monitor compliance and progress. The LRTAP process has met success in achieving targeted reduction goals for specific pollutants, and has been able to adjust goals as scientific understanding evolves. LRTAP working groups continue to consider new protocols and investigate new subjects of concern.
|
BOX 1.5 LRTAP Protocols
|
One evolving issue is the global or hemispheric nature of air pollution transport. Because air quality gains made by the original LRTAP parties could be reversed as industrial and mobile emissions increase in nonmember countries, there is growing consensus that more effective agreements to address pollutant emissions on a global (or more specifically, on a northern hemispheric) scale are needed. LRTAP has established a Task Force on Hemispheric Transport of Air Pollutants (HTAP-TF) that is charged with advancing and assessing the state of knowledge with respect to the flows of air pollutants across the Northern Hemisphere to inform future policy negotiations under the Convention. A number of other existing bilateral, regional, and global agreements are potential vehicles for addressing the issue of long-range pollution transport, including
-
the U.S.-Mexico Border 2012 Program: LaPaz agreement on cooperation for the protection and improvement of the environment in the border area (addressing O3 and PM);
-
the U.S.-Canada air quality agreement (addressing O3 and PM) and strategy for the virtual elimination of persistent toxic substances in the Great Lakes (addressing Hg and POPs);
-
the Commission on Environmental Cooperation’s North American Regional action plan (addressing Hg and POPs); and
-
the UNEP Stockholm Convention on POPs and Global Hg Partnership.
The UN Framework Convention on Climate Change (UNFCCC) focuses on long-lived greenhouse gases like CO2, CH4, and N2O. It has long been recognized that shorter-lived radiatively active gases and particles (such as ozone and black carbon aerosols) also play important roles in climate change. As discussed in later chapters, because of the complex chemistry and physics involved, there are large uncertainties in quantifying these impacts. The Intergovernmental Panel on Climate Change and the U.S. Climate Change Science Program are making progress in including short-lived gases and particles in climate change assessment studies, but they are not presently included in the UNFCCC or other climate change mitigation agreements.
As discussed further in later chapters, studies of international pollution sources must account not only for the emissions from particular countries and regions but also from mobile sources that are not easily ascribed to any particular nation, like shipping and aviation. The International Maritime Organization recently passed a new international protocol for gradual reduction of allowable ship SOx and NOx emission that will enter into force in 2010. The International Civil Aviation Organization has technology development and assessment programs addressing aircraft NOx emissions,
but there are presently no international protocols or agreements for actually controlling these emissions.
Domestic Policy Context Most national air quality goals are defined in various provisions of the Clean Air Act (CAA). Of particular interest are the National Ambient Air Quality Standards (NAAQS) (in CAA§109) for O3, PM, and their precursors, SOx and NOx. The NAAQS are set by the EPA “to protect public health, allowing an adequate margin of safety, and to protect the public welfare from any known or anticipated adverse effects.” The NAAQS are periodically revised in response to new scientific understanding of these impacts. For example, the 8-hr ozone standard was recently tightened from 0.08 ppm to 0.075 ppm; and the 24-hour fine particle standards were tightened from 65 μg/m3 to 35 μg/m3.
Meeting these more stringent air quality standards will likely require reductions in a variety of emission sources in many U.S. regions. As emissions in many other parts of the world increase, both the relative and absolute contributions of international transport to air quality problems in the United States are expected to increase.2 Of particular interest is that incremental contributions of these flows to U.S. air quality degradation may be of the same order of magnitude as the incremental air quality improvements that are expected to result from some of the recent tightening of the NAAQS.
Improving our understanding of long-range pollution transport is particularly important for a few aspects of NAAQS implementation, for example, the concept of Policy Relevant Background (PRB), the ozone concentrations that would exist in the absence of North American anthropogenic emissions. The PRB (which cannot be directly measured but must be estimated in modeling studies) is used by EPA as a floor for estimating risk associated with alternative NAAQS levels.
Another example is CAA §179b (International Border Areas), which mandates that state, local, and regional authorities will not be penalized or otherwise burdened and held responsible for the impact of pollution emissions from foreign sources. The U.S. Chamber of Commerce and other interested parties have complained that EPA has provided no clear, consistent guidance to state, local, and regional authorities seeking to account for the impact of foreign emissions in calculating attainment of CAA standards. Also of interest is the CAA provision (319b) covering “exceptional events,” which are designated as unusual or naturally occurring events (often from major forest fires) that can affect air quality but are not reasonably controllable using techniques that local air agencies may implement. The EPA
issued a rule effective as of May 2007 that governs the review and handling of air quality data influenced by exceptional events. Other CAA provisions relevant to the issue of long-range pollution transport include
-
§115: International Air Pollution;
-
§160: Prevention of Significant Deterioration;
-
§169a: Visibility Protection for Federal Class I Areas;
-
§401: Acid Deposition Control;
-
§103(j): National Acid Precipitation Assessment Program;
-
§112f: Hazardous Air Pollutants “Residual Risk Standard”; and
-
§112m: Great Waters Program and Hazardous Air Pollutants.
Beyond the CAA the EPA recently has developed and implemented a number of more integrated multipollutant and multistate air quality control measures. This includes the NOx SIP Call, a market-based cap-and-trade program that has successfully reduced NOx emissions from power plants and other large combustion sources in the eastern United States. This also includes the Clean Air Interstate Rule (CAIR) issued in March 2005. CAIR was designed to further reduce emissions of sulfur dioxide (SO2) and nitrogen oxides (NOx) across 28 eastern states, using an emissions trading system, in order to address the effect of an upwind state’s emissions on a downwind state’s ability to meet air quality standards for O3 and PM. In February 2008 a U.S. Court of Appeals issued an order to vacate CAIR, stating that it violated the EPA’s statutory authority. However, in December 2008 the court withdrew this vacatur order and held that the rule could stay in effect at least until it is replaced by a rule consistent with the earlier opinion. EPA’s Clean Air Mercury Rule (CAMR), the first federally mandated requirement that coal-fired electric utilities reduce their emissions of mercury, was finalized in March 2005, but (as with CAIR) the rule was vacated by a U.S. Court of Appeals in February 2008. As of this writing, the rule has not been reinstated and its future is uncertain. By the end of 2007, 23 states had proposed or adopted Hg emission rules more stringent than those embodied in CAMR; and in mid-2008 nearly 20 states were planning to institute state-enforced Hg emissions control requirements (Milford and Pienciak, 2009).
The main exposure pathway for Hg and some POPs is through aquatic ecosystems (i.e., fish consumption rather than through direct atmospheric inhalation). Control of these species is addressed through a number of other national and state-level statutes, including the Clean Water Act; state-level Water Quality Standards and Total Maximum Daily Load (TMDL) standards; the Federal Insecticide, Fungicide, and Rodenticide Act; and the Toxic Substances Control Act. In addition, the EPA and the Food and Drug Administraiton issue consumption advisories related to Hg contamination.
The use of critical loads to assess and limit the deposition of acid and nutrient compounds also may be relevant. The concept of critical loads is currently used in Europe in the context of establishing LRTAP emission control standards for SOx and NOx. Although it is still a nascent concept in U.S. environmental policy, there are a number of state and provincial efforts to apply critical loads strategies for various regions of North America.
REPORT ORGANIZATION
This chapter has presented an overview of the basic phenomena controlling long-range pollution transport and the tools used to study this phenomenon. The policy context that frames our need to understand this issue also has been reviewed. Chapters 2-5 provide more detailed discussions about what is known and what needs to be learned for each of the main pollutant classes that we were asked to address in this study (ozone and its precursors, particulate matter and its precursors, gaseous and particulate mercury, and persistent organic pollutants). Chapter 6 addresses a number of crosscutting issues that affect all of these pollutants, and provides a synthesis of key findings and recommendation for future research and observational needs. Appendix A presents the committee’s statement of task and some details about its sponsorship and operations. Appendix B provides a more detailed discussion of the meteorological dynamics that affect long-range transport of pollution. Appendix C presents a summary of recent observational activities relevant to long-range transport.
























