6
Crosscutting Issues and Synthesis
The preceding four chapters each focused on a priority pollutant class and identified a wide array of requirements and opportunities for advancing our understanding of long-range transport of pollution. Many of the issues discussed are unique to particular pollutants, but there are also a number of issues that are clearly important to a wide range of pollutants. This chapter highlights a series of crosscutting issues that the committee identified for focused discussion. We then present the committee’s vision of an integrated observational and modeling system to advance our understanding of long-range transport of pollution (Section 6.2), followed by an overview of key conclusions from this study (Section 6.3). This chapter is intended to build upon the findings and recommendations presented in preceding chapters, with the goal of maximizing the effectiveness of future efforts by addressing multiple pollutant classes simultaneously.
CROSSCUTTING ISSUES
New Pollutant Fingerprinting Techniques The high degree of complexity involved in identifying and quantifying long-range pollution transport, particularly in identifying and characterizing contributions from individual pollution sources, will require new developments in analytical and observational techniques. The difficulty in resolving sources is a limiting factor in determining the contributions of long-range transport for almost all pollutants. It is particularly difficult in the case of PM since their heterogeneous composition and their origins in both primary emission and secondary formation mean that chemical transformations need to be understood as
well. In the past decade there have been major advances in PM measurement techniques, but further advances will be needed to better characterize long-range transport.
Isotopic Signature Studies High-precision, stable isotope ratio measurements are increasingly being applied to both gaseous species and aerosol PM elemental source identification. With this technique the primary identification factor resides at the submolecular level. This technique can be highly sensitive because isotopic species are reduced in abundance and therefore small variations can be detected. Using traditional analytical methods, one often cannot precisely measure the primary species of interest when its background concentration is large (e.g., carbon dioxide). Isotopic measurements do not face such limitations, and are increasingly used to study atmospheric sources and sinks of compounds such as methane, nitrous oxide, carbon dioxide, carbon monoxide, ozone, and aerosol sulfate and nitrate.Recent simultaneous development of methods to measure stable isotopes at a high precision in micro- to nanogram-size samples, and the development of new quantum mechanical theories for the mechanisms that alter stable isotope ratios, have greatly advanced isotopic techniques. A review of the application of stable isotopes for atmospheric composition studies is available (Thiemens, 2006). Precise characterizations of isotopic compositions provide a new fingerprinting technique. This is a particularly powerful application for long-range transport studies because it is one of few measurements that provides information about the chemical processes that have occurred during transit as well as their bulk properties at arrival.
For example, applications of isotopic techniques provide a means by which ship emissions can be recognized in regions with multiple pollution sources that cannot be separated by traditional concentration measurements (Dominguez et al., 2008). Long-range transport of aerosols from Asia to the United States may also be recognized from their characteristic isotopic signature, and their secondary transformations during transit may be resolved (Patris et al., 2007). Isotope ratio measurements can also play a significant role in advancing our understanding of the biogeochemistry of mercury. As discussed in Chapter 4, many important aspects of mercury chemistry are presently unresolved, such as source identification, transformation, and storage in various environmental reservoirs. Isotope measurements can be an effective way to gain new insights on these issues, if used in combination with traditional concentration and chemical speciation measurements. The measurement of mercury isotopes is challenging, and further analytical developments are needed to fully exploit this technique. In the case of POPs there have thus far been few studies at the isotope level. Future developments of carbon and deuterium isotope ratio measurements
may be useful but limited, given the nonisotope specificity of sources and transformational processes.
Given recent advances in understanding the fundamental physical chemistry of isotope effects and the development of new isotope measurements (e.g., multi-isotope measurements of individual molecules; positional-specific isotopic measurements; and new measurement techniques, including in situ, real-time laser-based isotopic measurements and satellite remote sensing isotope measurements), the use of stable isotope ratio measurements could play an increasingly significant role in all aspects of understanding international pollutant transport.
Molecular Chirality Measurements A recently proposed technique involves the study of enantiomeric signatures of chiral organochlorine pesticides (OCPs). Chiral OCPs are manufactured in racemic form,1 but selective biodegradation processes can result in preferential loss of one enantiomer over the other and a shift to a nonracemic signature (i.e., unequal amounts of left- and right-handed molecules). In these cases a racemic signature may indicate recent use of the pesticide, while a nonracemic signature may indicate past use, with selective biodegradation of certain enantiomers and revolatilization from soils. For example, in a study by Genualdi et al. (2009) racemic alpha-hexachlorocyclohexane (HCH) was measured in Asian, transpacific, and free tropospheric air masses, while nonracemic alpha-HCH was measured in regional U.S air masses, thus illustrating the potential value of such techniques for distinguishing between transpacific and regional U.S. air masses.
Single-Particle Measurements A major advancement in aerosol science has been the development and continued enhancement of the ability to do real-time measurements of single aerosol particle composition and size. Conventional techniques typically require collection and subsequent analysis of the particles, which may alter the physical and chemical characteristics. With techniques such as aerosol-time-of-flight mass spectrometry (ATOFMS) the chemical composition and size of particles can be determined in real time and without collection. Such measurements are invaluable in chemically fingerprinting aerosols and their sources with a high degree of specificity. For episodic transport episodes the capability for fast timescale resolution allows for a more detailed modeling of transport in general and chemistry in particular. New developments in this area will be increasingly important for addressing the issue of secondary surface chemical reactions.
Online mass spectrometry methods can also be used for the accurate apportionment of aerosols by comparing the fingerprints of individual atmospheric particles to known source signatures. Such methodologies are particularly important in source identification and quantification. They have been used to study long range transport over India and determine the major source of the Atmospheric Brown Cloud (Spencer et al., 2008), and to identify the impacts from many other sources including incineration in Mexico City and China (Moffet et al., 2008; Zhang et al., 2009b), wildfires (Mühle et al., 2007), gasoline and diesel emissions, and ship emissions (Toner et al., 2008; Ault et al., 2009). These instruments are also beginning to be used to study the climatic impacts of specific pollution sources, including impacts on the optical properties (Moffet et al., 2008) and cloud-forming potential of atmospheric particles (Cubison et al., 2008; Furutani et al., 2008). Such studies are critical for determining what pollution sources are having the largest impact on our climate, so they can be properly regulated.
New identification and characterization developments are also key in the general area of organics, especially particulate matter (Canagaratna et al., 2007), but also volatile and semivolatile species. Organic compounds may constitute between 10 and 75 percent of atmospheric aerosols by mass. Though these particles play an important role in radiative forcing and human health issues, understanding of their sources and atmospheric chemistry is poorly established. Recent advances in FTIR and X-ray scanning transmission spectroscopy have significantly improved our capacity to study such issues, especially with identifying functional groups and morphology of single particles, which may be source dependent. Two dimensional mapping of aerosol organic functional groups is emerging as a valuable technique for studying sources and transport of organics. The use of nondestructive soft X-ray beams (NEXAFS-STM) is another important emerging technique.
All these various single particle measurement techniques are important for the study of long-range transport of particulate matter. They help improve our understanding of aerosol chemistry, formation and growth processes, and subsequent impacts on atmospheric lifetime and transport properties.
Other Methods An array of other innovative pollution tracing or fingerprinting methods are currently being developed and tested. For instance, gas and aerosol ratios have also been used to give information on emissions and chemical processing (e.g., Parrish et al., 1998). This includes methods to quantify Asian emissions of Hg (0) (Jaffe et al., 2005a; Weiss-Penzias et al., 2007), emissions of particulate mercury (Finley et al., 2009), and ozone processing during transport (Parrish et al., 1998; Price et al., 2004).
Finding. Informed decision making about long-range pollution transport requires sophisticated abilities to identify and quantify specific pollution sources. Past studies have suffered from analytical limitations and an inability to make sufficiently frequent and precise measurements. Breakthroughs in analytical fingerprinting techniques (e.g., isotopic or enantiomeric signature measurements, online measurement of single particles, aerosol or gas ratios) expand our ability to define sources and their strengths, and to allow high-resolution characterization of transport.
Recommendation. We recommend continued investment in advancing these cutting-edge fingerprinting techniques and the widespread deployment of such techniques in both ground- and air-based studies, aimed at refining our assessment of pollution sources, transport, and chemical transformation. Particular emphasis should be placed on using such techniques to advance understanding of the complex reactions of organic species (in both the gas and particle phase) and the complexities of the mercury biogeochemical cycle.
Emission Sources, Inventories, and Projections Modeled estimates of long-range transport depend on the magnitude of present and future emissions in upwind regions. Emission fields are a limiting factor in the fidelity of these models. Some general emission inventory needs include the following:
Spatial resolution Presently most global inventories are available at 1° × 1° resolution. This has been sufficient for most global modeling studies, as it matches or exceeds the spatial resolution of most models. However, this resolution is insufficient for determining the initial evolution of continental plumes, especially for highly reactive pollutants. Both nested emission inventories and nested models, with higher resolution in high-concentration areas, are needed to more accurately represent initial plume processing and transport.
Emission projections Understanding the effects of a changing climate and an evolving economy on future natural or anthropogenic emissions requires mechanistic representations of the factors that affect those emissions. A good match between observations and models provides confidence that an inventory is reasonably accurate, but the underlying mechanisms and driving factors must also be well represented.
Emission Inventory Evaluation Inventory compilations have been produced to support modeling activities such as AEROCOM (Dentener et al., 2006). The utility of these collections lies in providing a common
format, common inputs for model intercomparion studies, and some comparison tools. Still missing and needed are quantitative assessments of inventory quality. First, the basic assumptions in different inventories need examination. This endeavor needs to include comparison and validation of the underlying driving factors: activity levels, technology mix, and emission rates. Second, work is needed to ensure that an inventory-model combination reproduces atmospheric observations reliably. Such a match is sought in intensive measurement campaigns (Huebert et al., 2003; Warneke et al., 2007), and diagnosing the causes of mismatches is the goal of inverse modeling techniques (Mendoza-Dominguez and Russell, 2000; Kasibhatla et al., 2002). The use of satellite data for selected tropospheric pollutants, including CO and NO2 and PM, can also be used to identify emission hot spots and evaluate regional emission inventories. Sophisticated inverse modeling, most recently utilizing adjoint methods with advanced CTMs, show great promise as top-down checks on bottom-up emission inventories (Kopacz et al., 2009; Kurokawa et al., 2009). Methods are also needed for rapidly updating emission inventories to incorporate the findings of these studies as soon as they become available.
Emission Inventory Diversity Atmospheric models have benefited from comparisons, and ensembles of such models are typically more robust than individual model results. The same diversity is frequently lacking in emission models. Despite the complexity of the responsible processes that produce emissions, single estimates of combustion, wildfire, dust, and biogenic emissions are typically used by all chemical and transport models. When separate estimates exist, they can identify discrepancies (Ma and van Aardenne, 2004). An increase in the number of contributors to emission estimates and projections may result in greater confidence in these critical inputs. At the same time, measurements need to be designed to test the causes of discrepancies.
Each emission source affects multiple pollutants and needs to be represented consistently to produce holistic estimates of changes in air quality. As discussed in Chapter 1, we separate emission sources into three categories: (i) those related to energy and economy, (ii) those associated with the natural environment, and (iii) legacy sources, or environmental reservoirs storing pollutants that can be re-emitted. There is some debate about these divisions; for example, dust lofted from disturbed soils is neither completely natural nor wholly attributable to economic activity; but we chose the classifications listed here because they correspond to broad differences between procedures in both estimating and mitigating emissions.
-
Energy and economy Energy use and other economic activity include the sectors of electricity generation, manufacturing, consumer
-
products, household energy, transportation, and agriculture. In turn, each sector may contain many source types that have very different emission rates. For example, household energy needs may be met with natural gas, which produces few emissions except NOx, or with unprocessed solid fuels, which have high emissions of particulate matter, carbon monoxide, and volatile organic compounds. Relative contributions and technological makeup of each sector vary between regions. HTAP-TF (2007) provides a comprehensive summary of emission inventories that represent energy and economy. Rather than reprising that review here, we describe general methods of producing those inventories and identify directions required to advance them further.
Three general approaches, or a combination thereof, can be used to develop regional or global emission fields. The first method requires data from individual facilities, which are either measured by continuous emission monitors or self-reported. These data have the greatest potential for accuracy, although challenges certainly exist in both monitoring and reporting. Such data are available only from large point sources in regions with strong regulations, including North America, Europe, Japan, and increasingly, China.
In a second approach the emission estimate is based on the technological makeup within each emitting sector, including abatement technologies. This approach is used by many global and regional inventories, including the RAINS (Regional Air Pollution Information and Simulation) model based at International Institute for Applied Systems Analysis (IIASA) (Kupiainen and Klimont, 2007), inventories for TRACE-P (Streets et al., 2003) and individual countries (Reddy and Venkataraman, 2002), and some global models of individual pollutants (Bond et al., 2004). The supporting technology descriptions may be obtained from records, from expert judgment, or by extrapolation from other regions. These estimates benefit greatly from involvement of country experts.
The last method requires the least detail: applying a single emission factor to an entire emitting sector. These emission factors may depend on region, with more polluting activities assumed in regions with less development or lower technology. This was the approach used in EDGAR 2.0 (Emissions Database for Global Atmospheric Research) (van Aardenne et al., 2001) and many other global inventories. EDGAR is currently being updated to a technology-based approach.
Because future emissions entail both activity changes and technology changes, treatment of single sectors provides only a qualitative sense of future emissions. Nevertheless, it is the method presently used by many integrated assessment models (van Vuuren et al., 2006). Studies consider technology change in a simple fashion by applying principles of the “Environmental Kuznets Curve,” in which cleaner activity is assumed to follow development. However, some studies have questioned the support for this
-
simple relationship (Deacon and Norman, 2006), as development may occur more quickly than expected based on history (Stern, 2004). Thus, hybrid models that link technological change and economic growth are desirable.
Detailed emission projections in which technological change is linked with economic conditions are well under way for large point sources and for on-road transportation. Such projections are available in regions with experience in environmental regulations, such as the United States, Europe, and China. Modeling is less advanced for sources that are more dispersed, including off-road vehicles, agricultural emissions, waste burning, and residential combustion.
In all cases current inventories and future projections are less developed and more uncertain when a history of regulation is lacking. International working groups such as those convened under NARSTO and the United Nations Environment Program (UNEP) “Atmospheric Brown Cloud” are needed to share knowledge about driving factors and to build capacity for inventory estimation in developing countries. Emissions of air pollutants differ from those of greenhouse gases, particularly CO2, because they may be highly dependent on the combustion or manufacturing process. The sectoral divisions used for reporting inventories for the United Nations Framework Convention on Climate Change (UNFCCC) tend to be inadequate for air quality purposes because they do not identify these individual processes.
The evolving trajectories of global and regional economies are one of the greatest uncertainties in projecting air pollutant emissions. Inherent uncertainties are likely to persist even with additional understanding of the factors that cause and mitigate emissions. Modeling and regulatory approaches need to acknowledge and account for such future uncertainties.
-
Natural environment Contributions from the natural environment are observed in both episodic transport and in regional and transported background concentrations. These emissions originate from dust storms, forest fires, vegetation, soil, freshwater, and ocean surfaces, all of which depend greatly on meteorology, land use, and climate. Because of the latter two factors, anthropogenic activity plays a role in these emissions.
Estimating emissions from this assortment of natural sources requires representation of biogeochemical connections between the emission source and the environment. Both atmospheric variables and human interactions govern the emitting capacity—for example, whether barren soil has the potential to emit dust, the amount of biomass in grassland that can be burned, or the prevalence of broadleaf trees that may emit terpenes. Meteorology also affects whether and when the emission occurs—for example, dust lofting with increased wind speed, increased terpene emissions at
-
higher temperatures, the correlation of Hg soil emissions with temperature, solar radiation, and soil moisture. Such fundamental relationships are used to predict both present-day and future emissions.
Challenges in modeling emissions include large seasonal variability and the need to simulate sources in remote environments with little available data. Onsets of episodic events, such as fire ignition, are nearly impossible to predict. Thus, models of present-day emissions combine process modeling with inputs from remote sensing (Reid et al., 2004; Guenther et al., 2006; van der Werf et al., 2006; Kalashnikova and Kahn, 2008). Examples of satellite data incorporated include burned area and new fire and dust plume events.
Progress in projecting future emissions from the natural environment will require challenging and confirming the fundamental relationships in emission models. This evaluation should occur both at large scales, using remote sensing data and intensive field experiments, and at microphysical scales, for example, with flux chamber, eddy correlation, and relaxed eddy accumulation measurements. Statistical characterization that covers magnitudes and frequencies of emission events is needed to assess future impacts on both background and episodic exceedances. Unusual events such as volcano eruptions also affect atmospheric chemistry, and no effort is made to predict these.
-
Legacy emissions Legacy emissions result when pollutants are reemitted from environmental reservoirs. Predicting these sources is the most complex of all, as it requires modeling not only the re-emission process but also the history of inputs to the reservoir and their removal or re-emission over time. Despite the importance of these emissions for toxic and environmentally stable pollutants, modeling of legacy emissions is still in its infancy (Bergan et al., 1999; Lohmann et al., 2007).
Finding. Consistency in modeling long-range transport requires a source-based, multi-pollutant treatment of emission inventories. These inventories are fundamental to the fidelity of models that estimate present-day and future long-range transport. High spatial resolution is needed to represent reactive plumes at their origins.
Finding. Improvements in present-day emission inventories will be possible only by representing the factors that modulate those emissions. These factors are as diverse as the response of the natural environment to changing climate and shifts in the mix of technology caused by tightened emission standards.
Finding. Understanding of global cycling for very long-lived pollutants, such as mercury or POPs, is limited in part by the lack of
emission inventories that estimate re-emission from environmental reservoirs (legacy emissions).
Finding. Traditional bottom-up emission inventories need to be periodically validated by top-down inverse CTM modeling of measured atmospheric concentration fields. Airborne multipollutant concentration measurements are ideal when available; satellite measurements are also becoming important and offer data for some key pollutants that are frequently updated, allowing assessment of temporal variations in emissions. The use of adjoint methods with state-of-the-science CTMs shows great promise for improving the accuracy of inverse model results.
Recommendation. Enhance the ability to understand, forecast, and manage changing emission sources by designing field experiments that not only confirm emission totals but also link them to the fundamental sources and processes that generate them. These connections will decrease uncertainty in projections of emissions from both economic activity and the natural environment.
Recommendation. Promote national and international efforts to improve Northern Hemisphere regional and national emission inventories’ accuracy, timeliness, spatial and temporal resolution, multipollutant coverage, and intercomparability. Also stimulate the collection, evaluation, and use of airborne and satellite multipollutant concentration data and its analysis using state-of-the-science inverse CTM modeling techniques, to independently evaluate the accuracy, completeness, and temporal and spatial fidelity of available emission inventories.
Recommendation. Enhance the understanding of legacy emissions of Hg and POPs by modeling environmental flows and reservoirs, in conjunction with historical emission inventories that cover several decades of human activity.
Meteorological Processes Chemical transport models as well as trajectory calculations and particle dispersion models rely on meteorological conditions to simulate pollutant transport. Meteorological processes lift pollutants from the planetary boundary layer into the free troposphere, can transport them for great distances, and determine where and when they will sink back into the boundary layer to influence local air quality. If meteorological conditions are not properly represented in a chemical
transport model, no amount of sophisticated chemistry can overcome the errors in pollutant transport that will result. Therefore, future advances in understanding and quantifying long-range transport of pollution will be closely linked to advances in meteorology and will require the collaborative efforts of meteorologists and atmospheric chemists.
Additional data are needed to accurately describe global meteorological conditions at even the synoptic scale, as well as the mesoscale phenomena that are embedded therein. Relatively little data are available over the oceans and less developed countries. As a result circulation centers, wind patterns, and transport pathways such as warm conveyor belts can be incorrectly located; these errors compound in time as trajectories and other tracer parameters are calculated. Meteorological observations are used for diagnostic studies and to initialize meteorological models. Initializing models with accurate meteorological data is a fundamentally important step in making numerical forecasts. Further modeling accuracy can be achieved, particularly in retrospective simulations, if four-dimensional data assimilation (FDDA) is used. FDDA is a process by which gridded or point observations are ingested into a running simulation to nudge the simulated variables toward the observed values.
Additional vertical profiles of temperature, humidity, and winds are needed over large areas of the globe. Although various satellite sensors and retrieval algorithms have been developed to provide this information, their major limitation is crude vertical resolution and often their dependence on first-guess information. Further advances in satellite sensor technology and algorithm development are sorely needed to help fill the existing data gaps. Unmanned aerial vehicles are another potential source of this needed information.
In addition to needing meteorological data, the models meant for chemistry transport studies need to be run at high resolution in an attempt to incorporate the myriad of circulations (see Appendix B) that affect long-range pollutant transport. For example, to represent the effects of topography, horizontal resolutions of < 10 km may be necessary. Variations in topography can block pollutant transport, lift the pollution to higher altitudes, or channel it through valleys. Mesoscale thermally induced mountain and valley circulations can greatly influence transport by modifying the depth of the PBL. Thus, pollutants released in areas of complex terrain may be transported quite differently than if released over flat terrain. High-resolution topographic data are available, but the meteorological models need sufficient resolution to fully utilize the data. Accurately representing these processes on an intercontinental scale will require either horizontal grid resolutions of 10 km or less (especially near the release areas) through the use of embedded high-resolution grids within coarser domains [nested grids] or through the use of parameterization schemes.
The PBL is the source of most pollutants, and even at high resolution (1-10 km) the complex, turbulent meteorological processes occurring within it (even over flat terrain) cannot be explicitly resolved by numerical models; they need to be parameterized based on larger-scale meteorological variables. Although current procedures produce reasonable results in many cases, models of pollutants sometimes represent release into the free troposphere inaccurately in both space and time. Even if the sign of this process is correctly parameterized, its magnitude may not be.
Finding. Major improvements in current abilities to model several aspects of pollution transport are needed, including
-
A more complete understanding of PBL physics to allow the development of parameterization schemes that are sufficiently robust to perform in a range of locations. Although many PBL physics schemes exist, many are based on midlatitude atmospheric dynamics and may not be suitable in either polar regions or the tropics. The goal of a PBL scheme in a numerical model is to represent the transfers of momentum, moisture, and heat in the PBL as well as the free atmosphere. Thus, inadequate PBL schemes can quickly degrade meteorological and air quality forecasts. Since these schemes rely heavily on a land surface model (LSM) to represent heat and moisture transfer from the land surface, the LSMs also affect turbulent mixing within the PBL. Therefore, we have to understand land surface dynamics around the world to aid the PBL schemes. LSMs require high-resolution land use and vegetation data from satellites in order to be effective. Advances in this area will improve PBL and LSM procedures, thereby preventing the degradation of model performance.
-
Models need to better represent the processes occurring at horizontal resolutions of 5 to 10 km. Convective transport, whether dry or associated with thunderstorms, is a prime example that directly affects pollutant transport. Many convective parameterization schemes have been designed for grid resolutions greater than 10 km. Since none of these is uniformly best in every situation, it often is difficult to decide which is best in a given situation. At grid resolutions less than 10 km, parameterization schemes either may aid or hinder air quality forecasts depending on their treatment of the convective vertical motions and the subsequent vertical redistribution of atmospheric pollutants. Very high-resolution cloud-resolving models only can be used only over very limited areas because of their computational expense. It is vital that meteorological models be able to properly depict convection
-
since it is a major source of pollutant transport out of the PBL and into the free troposphere where stronger winds are located.
-
Considerable pollution is released in urban settings that contain street canyon flows, radiational heating due to buildings, and obstacle effects that can temporarily trap pollutants within the PBL. In addition, nonlocal pollutants passing over urban landscapes can encounter enhanced mixing due to the rough surface. These effects may require grid resolutions of tens of meters in order to avoid the need for parameterization. At a typical mesoscale grid length (i.e., 10-20 km), the urban landscape is not explicitly resolved, requiring sophisticated schemes to account for the subgrid-scale processes. Such schemes currently are under development, but few have been implemented in operational forecast models.
Recommendation. Develop a better understanding of the basic submesoscale dynamic processes involved in the entrainment or detrainment, long-range transport, and deposition of pollutants. Utilize focused field studies, advances in satellite technology and improved data assimilation methods to continue to enhance meteorological and transport modeling capabilities. To be most effective meteorologists and atmospheric chemists, including both modelers and measurement specialists, should collaborate on efforts to obtain the phenomenological insights and data required to develop the better measurement techniques and improved numerical models that will enable us to adequately quantify the role of distant sources on local air quality.
International Pollutant Transport from Maritime Shipping and Aviation Sources Pollutant emissions from international ship and air transport are of direct interest to those studying long-range atmospheric pollutant transport because they represent a unique class of international pollutant transport, which is growing rapidly and may in some cases mimic long-range atmospheric transport. At a minimum, receptor sites (especially those near the coast) attempting to identify and characterize intercontinental atmospheric transport need to be able to distinguish between ship and aircraft emissions and emissions from distant sources.
Ship Emissions It is well recognized that marine vessels that consume sulfur-rich bunker fuels are significant sources of carbonaceous (soot) PM and gaseous and particulate sulfur (Dominguez et al., 2008; Dalsøren et al., 2009; Lack et al., 2009). The regulation of these sources at present is not stringent, although significant efforts are under way to strengthen international regulatory controls. The particulate emissions from ships using high
sulfur fuels are predominantly submicron-size sulfate and carbonaceous materials; recent measurements in the Atlantic and Gulf of Mexico reported average PM concentrations of 46 percent sulfate, 39 percent organics, and 15 percent black carbon (Lack et al., 2009). Ship PM emissions directly influence Earth’s albedo by reflection and absorption of sunlight, as well as by nucleating ship-track clouds; but their effect on global climate is thought to be small, relative to other aerosol sources. The most significant aspect of maritime emissions is the impact upon human health. Recent estimates suggest that as many as 60,000 deaths per year worldwide may be linked to ship emissions (Corbett et al., 2007). The economic cost of these health impacts to the United States alone may surpass $500 million annually (Gallagher and Taylor, 2003).
Ship PM emissions are capable of long-range transport and, coupled with the international aspects of maritime commerce and the predicted increase in global maritime activity, the consequences for health and economics of global coastal community is significant. As discussed in Dominguez et al. (2008) there are significant challenges associated with the resolution and quantification of local vs. long-range transported aerosols from ships. The issue is unique because it involves nonpoint sources and is subject to international regulations. As a consequence, better understanding of the nature, composition, and transport of ship emissions is highly desirable.
In addition to PM, ships emit significant quantities of CO2, SO2, CO, NOx, and VOCs (Dalsøren et al., 2009). Sulfur dioxide, nitrogen oxides, and volatile organic compounds all contribute to secondary aerosol PM formation; the latter two are also tropospheric ozone precursors. While ship emissions are not a major source of mercury or manufactured POPs, ship-emitted soot and gaseous emissions are a significant source of PAH, an important class of POPs.
Recent measurements of particulate and gaseous emissions from large diesel ships have further characterized ship emissions. It is directly observed that three morphological species of varying chemical composition are emitted. These include soot, which may be metal infused, mineral dust, sulfate, and a variety of carbonaceous particulate matter (Moldanová et al., 2009). This recent work also illustrated the extraordinary chemical complexity of ship emissions. Of particular importance is the observed linkage between PAH and PM. The PM composition was largely organic carbon, with trace amounts of elemental carbon, but analyses also revealed the presence of a complex mixture of PAHs. This work demonstrates that not only are ship emissions significant sources of both PM and PAHs but also may be distinct from other transportation emissions.
Aircraft Emissions As discussed in IPCC reports global aviation transport involves direct emissions of fine particles into the upper troposphere
and lower stratosphere (IPCC, 1999, 2007). In addition, volatile PM and ozone precursors, including SO2, CO, NOx, and VOCs, are emitted during both takeoff, landing, and cruise modes (IPCC, 1999; Royal Society, 2008). Both PM and selected gaseous aircraft emissions near airports are subject to regulation by individual nations, using standards set by the International Civil Aviation Organization. Emissions during cruise phases of aircraft however currently are not subject to regulation.
In general, aerosol number densities are elevated in areas of active aircraft commerce, and the major impact is their influence on the formation of persistent contrails and associated cirrus clouds. The mechanism and magnitude of the associated effects, however, are not well resolved. The consequences of the ground-level addition of sulfate and soot particles from jet fuel combustion appears to be modest in comparison to local background levels (IPCC, 2007). Ice nucleation in the upper troposphere and lower stratosphere, with subsequent transport and chemical reaction on ice surfaces is thought to be one of the largest concerns, although there are no estimates of the direct impact on cloud ice particle formation rates at present.
Finding. Emissions from ocean shipping and passenger and cargo aircraft are a source of long-range pollution transport that can complicate the detection and characterization of long-range atmospheric pollutant transport from historical land-based sources.
Recommendation. Studies of long-range atmospheric transport of pollution should be coordinated with studies of ship and aircraft cruise emissions, with the goal of determining methods to distinguish among these pollutant sources in source attribution studies.
STRENGTHENING INTEGRATED SYSTEMS FOR TRACKING AND ATTRIBUTION OF LONG-RANGE TRANSPORT OF POLLUTION
From an air quality management perspective there is a fundamental need to measure the ambient levels of pollutants discussed in this report and how their values change over time. There is a further need to relate pollution concentrations and their trends to emission sources and their changes. This information is needed, for example, to assess the effectiveness of current emission control strategies such as fuel desulfurization to reduce ambient levels of SO2 and acid deposition fluxes, and NOx emission reductions under the Clean Air Interstate Rule (CAIR) to reduce O3 and PM.
To assess the extent to which national emission control strategies will be effective in meeting environmental targets, related to species of inter-
est in this report (i.e., O3, PM, Hg, and POPs), it is necessary to partition the concentration observed at a specific location into the part arising from national emissions (both local and regional), the part attributable to international sources, and the part coming from natural sources. As this analysis is carried out for more distant sources attribution becomes more difficult, since their contributions typically are weaker and more difficult to distinguish from local or regional components. As discussed throughout this report both observation- and model-based approaches exist for source attribution. These techniques have been developed and applied largely for source attribution applications at local and, more recently, regional scales. These techniques are now being extended for applications at the hemispheric and global scales. The model-based approaches at these scales suffer from large computational uncertainties (e.g., those related to global emissions and transport processes over long distances). Larger-scale observation-based approaches are limited due a variety of factors, including the loss of source signal over long distances, small numbers of relevant and representative observation sites devoted to source attribution, and the lack of a comprehensive suite of source detection and characterization measurements at available sites.
Improving our capability to assess the impacts of long-range transport of pollution on the effectiveness of national control strategies requires better quantification of the global distribution of pollutants and their trends, and an increased ability to perform source attribution analyses at hemispheric and global scales. The importance and required attributes of a global air quality observational strategy were articulated in an earlier NRC report Global Air Quality: An Imperative for Long-Term Observational Strategies (NRC, 2001), which found that current observational systems were not adequate for characterizing many important medium- and long-term global air quality changes. That report made the following recommendations.
-
Maintain and strengthen the existing measurement programs that are essential for detecting and understanding global air quality changes. High priority should be given to programs that aid in assessing long-term trends of background ozone and PM.
-
Establish new capabilities to provide long-term measurements and vertical profiles of reactive compounds and PM that will allow meaningful examination of long-range transport and trends in background concentrations.
These two recommendations are just as valid today as they were when issued in 2001. Some important surface-based trend measurements for ozone, PM and their precursors have continued; with some additional capacity developed (e.g., with the U.S. EPA’s NCORE network [http://www.epa.gov/ttn/amtic/ncore/],
as discussed in NRC [2008]). Satellite-borne instruments, especially from NASA’s Earth Observing System (EOS), have become active and produce multiyear tropospheric vertical column density measurements for some key pollutants. U.S. federal funding for new or improved pollutant trend and transport measurements was sparse during the period between 2001 and 2009, so little new capability has been deployed, or even planned, over that time frame. There is now concern that the current satellite pollutant observation capability is declining as many of the EOS instruments reach and pass their nominal life spans with no replacements in sight.
Improving our capabilities to quantify the contributions from specific source regions and sectors over the geographical regions of concern (e.g., EPA-designated air management regions, the Arctic) requires capabilities beyond those identified above for characterizing global air quality. It requires a strategy that both improves the individual components needed to perform source attributions and more closely integrates these components. As stated throughout this report there remain large uncertainties in the calculation of ambient pollution distributions and source-receptor relationships. Emissions, transport, chemical transformation, and removal processes are all major sources of uncertainty, and the relative contribution of these processes to overall uncertainty varies by pollutant. Reducing the uncertainty in predictions requires a better understanding of these processes. In addition, more critical tests of the ability of models to reproduce the processes governing intercontinental transport and the source-receptor relationships are needed. Reducing these uncertainties requires combining model and measurement techniques in an integrated analysis of data from an enhanced observing system.
Figure 6.1 depicts the major components of the needed system, including: emission inventories; chemical transport modeling; long term ground based monitoring; satellites; and intensive field studies. The specific capabilities and needs for improving the various components have been articulated earlier in this report. Here we focus on the overall system and in particular the benefits to be derived from closer integration of the different parts of this system, for instance,
-
closer integration of satellite observations with emissions and modeling components (through data assimilation and inverse modeling) will lead to better emission estimates in terms of spatial distribution and absolute magnitude;
-
closer integration of satellite- and ground-based observations will reduce uncertainties in observation-based spatial distributions;
-
closer integration of long-term, ground-based observations with emissions elements will produce a better understanding of geographical
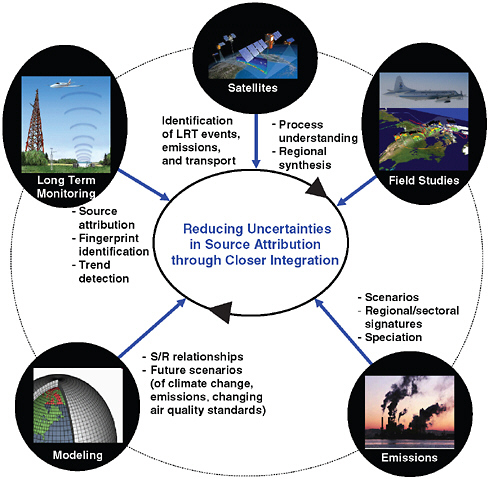
FIGURE 6.1 Major components of an integrated approach for source attribution. The circles denote the major components, the accompanying text indicates the major source and attribution-related outputs. Model and measurement elements refer to both meteorological and chemical components.
-
source signals, which can be exploited in fingerprint and observation-based source attribution analyses;
-
closer integration of ground-based observations and models will lead to reduced uncertainties as a result of more critical evaluation of model predictions, better calibration and evaluation of observation- and model-based attributions, and improved measurement strategies;
-
closer integration of field experiments with elements focused on source attribution will lead to improved understanding of processes affecting the source-receptor relationships, better techniques to follow targeted air masses (e.g., from specific source sectors or regions) over long
-
distances;closer integration of all these components will reduce the uncertainties in current estimates of the contribution of international sources to local air quality and more accurate assessments of the impacts of imported pollution on human health and agriculture.
It is important to realize that global source attribution has not been a major design consideration in current monitoring strategies. There are additional activities needed for source attribution beyond those needed for compliance and detection of long-term trends in ambient concentrations. These additional needs can largely be met by complementing existing activities. For example, the recent report Observing Weather and Climate from the Ground Up: A Nationwide Network of Networks (NRC, 2009) describes a national observational infrastructure that will improve capabilities to observe and predict meteorology and air quality at the national level. It calls for enhancing the vertical dimension of key meteorological and chemical observations, increasing the number of observation sites, and the inclusion of additional chemical parameters that will help in the integration of satellite and surface observations. This plan calls for improved measurement technologies in current observational platforms (such as ground-based air quality monitoring networks, commercial aircraft, and balloons or sondes), in an enhanced lidar network, in new instrument platforms such as supersites for measuring a comprehensive suite of chemical species in remote locations, and in unmanned aerial vehicles for long-duration sampling of the atmosphere over a wide range of altitudes. This network would provide a wealth of information needed for chemical data assimilation and improved prediction of transport and chemical processes, which are needed to reduce uncertainties in source attribution estimates.
For source attribution applications the above envisioned mesoscale network would need to be complemented by additional observations at designated source attribution sites. At these sites the additional measurements would include, for instance, all of the key pollutants for which source attribution is deemed important (e.g., O3, PM, Hg, and POPs), and a suite of observations with source attribution applications, including gas-phase PAN, speciated NMHCs, and other trace gases, selected isotopic ratios, and aerosol parameters, including composition as a function of size and single particle elemental analysis. This breadth of observations is needed to capture source signals over timescales from days to weeks and from specific source sectors (e.g., biomass burning, coal combustion). The source attribution network could be built upon exisiting long-term monitoring sites (such as Mauna Loa), with additional sites created in regions where the assessment of global contribution is needed, both within and outside the contiguous United States (e.g., Alaska). This would include elevated sites that can routinely sample free tropospheric air and are relatively free from local
influences. A few of these observation sites could be identified as source attribution supersites, where more detailed and exploratory observations are taken, and where there is an embedded research program dedicated to the integration of these observations with modeling, emissions, satellite, and field study components, all focused on source attribution applications. Further details on measurement parameters and locations need to be determined by an expert group charged with designing the source attribution network.
In a similar manner improvements in our satellite observing capabilities are also needed. Currently satellite observations can provide long-term global records of a limited number of key atmospheric trace gas and aerosol parameters; observations of long-range and intercontinental transport of pollution from continental source regions; column or partial column retrievals with very limited sensitivity to the lowermost troposphere; and top-down emissions estimates on fairly coarse scales. To improve their use in long-range transport studies the following are needed:
-
improvements in resolution
-
spatial coverage to facilitate the tracking of individual plume events.
-
spatial resolution in source and receptor regions to isolate city-scale features.
-
temporal resolution to capture diurnal variation of photochemistry and emissions especially in source and receptor regions. (The difficulty achieving this from polar orbit provides motivation for geostationary satellite placement).
-
vertical profile information to isolate surface sources; characterize PBL venting and entrainment of pollution; and track the transport of thin plumes. This requires multispectral and multiangle retrieval techniques and improved coverage from surface- and space-based lidar.
-
-
improved information on aerosol size distribution and chemical speciation.
-
improved retrievals over cold and bright surfaces.
Research into the closer integration of observations and models has increased over the last few years (Carmichael et al., 2008b). A greater number of models are engaged in advanced chemical data assimilation, and some are moving toward operational status (e.g., the European Union’s Global and Regional Earth-system Monitoring using Satellite and in situ data [GEMS project, http://gems.ecmwf.int/]). Further efforts are needed to evaluate and refine the application of these assimilation approaches for source attribution. These approaches also need to be applied in the design
of new observing systems, including new remote sensing platforms such as geostationary satellites. To accomplish these goals, model and emission inventory components need to be refined and aligned with observational strategies.
Ultimately a global network of source attribution observation sites is needed. A reasonable starting point for reaching this long-term goal is to better coordinate existing networks (see Appendix C). Programs such as the World Meteorological Organization’s Global Atmosphere Watch (WMO/GAW) have made substantial efforts to establish a global network of observational sites including sites in undersampled, remote regions around the world, supported by data centers and quality control programs to enhance integration of air quality measurements from different national and regional networks.
The source attribution analysis described here requires substantial international cooperation and open exchange of data from national emission inventories and air quality monitoring networks. This effort fits well within the international WMO/GAW Integrated Global Atmospheric Chemistry Observations (IGACO) component of the Integrated Global Observing Strategy (IGOS), a partnership of international organizations concerned with global environmental-change issues, which links research, long-term monitoring and operational programs (WMO, 2004).
Building bridges between experts in emissions, meteorological and chemical modeling, satellite remote sensing, in situ observing, and the different pollutant expert communities is critical for carrying out the integrated science envisioned in Figure 6.1. An important consideration in the design and execution of a source attribution analysis will be strategies to build and strengthen connections between these communities. Programs like the International Global Atmospheric Chemistry project can play important roles in coordinating international activities that address a variety of important issues related to global air quality.
Finding. Improving our capability to assess the effectiveness of national control strategies requires better quantification of the global distribution of pollutants and their trends (as changes in the global background will impact national strategies and their effectiveness) and an increased ability to perform source attribution analyses at global scales.
Finding. Improving our capabilities to quantify the contributions from specific source regions and sectors of concern requires a strategy that improves the individual components needed to perform source attributions, and that more closely integrates these components.
Finding. Global source attribution has not been a major design consideration in current monitoring strategies. Thus there are additional activities needed for source attribution beyond those for compliance and detection of long-term trends in ambient concentrations.
Finding: Source attribution analysis, which builds upon a global observing system with integration among other key elements, requires substantial international cooperation and an open exchange by countries of data from national emission inventories and air quality monitoring networks.
Finding. Building bridges among experts in emissions, meteorological and chemical modeling, satellite and in situ observing, and the different pollutant research communities is a critical requirement to carry out the integrated science envisioned.
Recommendation. An integrated source attribution program should be established to help assess the contribution of distant sources to U.S. air quality and to evaluate the effectiveness of national control strategies to meet environmental targets. The program should focus on improving capabilities (and reducing uncertainties) within the areas of emission measurements and estimates, atmospheric chemical and meteorological modeling, long-term ground-based observations, satellite remote sensing, field experiments, and impact assessments—and integrating these components as effectively as possible to focus on attribution to particular sources and regions.
Recommendation. An expert group should be established to help design this source attribution network (e.g., suggesting parameters to be measured, identifying appropriate monitoring sites, developing an embedded research program). These efforts should take into consideration the need for international cooperation and the opportunities to collaborate within existing international efforts such as the WMO/GAW International Global Atmospheric Chemistry Observation program.
SUMMARY OF KEY MESSAGES
Strong evidence from both observations and modeling studies confirms that the pollutants considered in this study are transported over long ranges, both to and from North America, and that U.S. environmental
goals are affected to varying degrees by nondomestic sources of these pollutants. It is also clear that our ability to characterize such impacts is currently limited, due to uncertainties related to source strengths, chemical transformations during transport, transport mechanisms, rates of exchange between the boundary layer and free troposphere, and a number of other important issues.
Under present global socioeconomic scenarios nondomestic influences on air quality are expected to increase in the future and will likely be an issue of increasing concern. Enhancing observations, chemical transport models, trend analyses, studies of reaction mechanisms for relevant species, and emission inventories and projections will all be of critical importance to better quantify such effects.
The pollutants explored in this study do not represent all species of concern, but they do illustrate the variability of pollutant composition and behavior and provide a focused target for analyzing the phenomenon of long-range transport. To improve our quantitative understanding of such issues, we recommend that the United States strongly support more extensive international cooperation in research (observational and modeling) and assessment, and ultimately in emissions control efforts.
The four classes of pollutants analyzed in this study, the Committee found a number of crosscutting shortcomings in our capabilities for observation, analysis, emissions prediction, and transport dynamics.
Observations Ambient concentrations vary significantly for the different species of interest, but in all cases, their temporal and spatial variations are not sufficiently understood, and observations of a significantly greater resolution are needed. This includes both ground- and aircraft-based monitoring and satellite observations. Each pollutant species has significantly different characteristics, including chemical reactivity, atmospheric creation and loss processes, and phase change properties. In all cases, however, there is a critical need for measurements of sufficient temporal and spatial resolution to allow better quantification of long-range transport. There is also a consistent need for observational platforms that allow one to discern between episodic and nonepisodic events, and between remote contributions to background pollution vs. less frequent, large perturbation events. In many cases (e.g., for mercury) there are major gaps in our understanding of species’ chemical fate in the atmosphere and in other environmental reservoirs. Comprehensive measurements are the only viable means to fill these gaps and provide the quantitative data required for informed decision making.
Emission Inventories There is a significant variation in the adequacy of existing inventories. None could be described as fully adequate, but some
(such as mercury) are particularly poorly resolved, as a result of environmentally complex chemical interactions. Some pollutant sources, such as ships and aircraft, that are inherently international in nature require more sophisticated and extensive observations and analysis. Emission inventories may be improved in some cases by development of additional satellite observations, assuming continued development and launch of advanced sensors and suitable platforms designed to increase the vertical and horizontal resolution, increased species coverage, and higher sensitivities for important tropospheric pollutants. The geographic extent of long-range transport episodes precludes the option of relying solely on in situ (ground- or aircraft-based) observations. Long-range transport models and inventories at the continental and hemispheric scales will increasingly rely on remote sensing observations, coupled with inverse models that help to better define fluxes from specific source regions.
Modeling Capabilities While a number of sophisticated chemical transport models exist, expanded capabilities are crucial for understanding and analysis of current observations, developing accurate projections of future changes, and supporting informed decision making regarding long-range transport of pollution. For example, observed changes in baseline ozone concentrations and some episodic ozone pollution events have evaded systematic understanding and description. Models are needed that can better quantify the processes of boundary-layer exchange and plume dispersion for all species addressed in this report. The scales of such modeling analyses need to range from local to hemispheric, if the issue of long-range transport and the associated perturbations are to be fully understood. There also needs to be significant interaction between observational networks and modeling efforts, to help guide sampling and measurement protocols and for calibration and sensitivity analysis of new models. More pervasive and robust methods for estimating the uncertainties in model results need to be developed and implemented.
The various pollutants addressed in this report differ in their consequences for human health and the environment, but in each case there is potential for significant and growing adverse impacts. We see a clear need to enhance scientific understanding of these pollutants’ interactions with the environment, including their long-range transport, entrainment and deposition fluxes, and ultimate impacts on human health and ecosystems. Addressing issues of long-range pollutant transport requires a significant degree of international cooperation.
As a final note, the Committee wishes to emphasize the obvious fact that our planet shares one atmosphere. Pollution emissions within any one country affect populations, ecosystems, and climate properties well beyond
national borders. Measures taken to decrease emissions in any one region can have benefits that are distributed across the Northern Hemisphere. The United States, as both a source and receptor of this long-range pollution, has a responsibility to remain actively engaged in addressing this issue.
It is clear that local pollution can be affected by global sources, although in most cases air quality violations are driven by local emissions. But regardless of where the pollution originates, protecting human and ecological systems from dangerous levels of pollution should be the policymakers’ primary objective. Meeting this objective will require strengthening domestic pollution control efforts to whatever levels are required to ensure that a population’s total pollution exposure (from local, regional and distant sources) does not exceed safe levels. However, reducing the impacts of distant emissions on local air quality cannot be achieved by domestic efforts alone. Cooperative international action, to advance our understanding of long-range transport of pollution and its impacts, and to use that understanding to effectively control emissions from both domestic sources—needs to be vigorously pursued. The Committee hopes that the analyses and recommendations in this report will help stimulate and guide those actions.


























