2
Ozone
GENERAL INTRODUCTION ON OZONE
Air Quality Ozone (O3) is ubiquitous in the air we breathe. It is a health hazard to sensitive individuals, with a threshold (i.e. an ambient atmospheric concentration above which adverse health effects may occur), if one exists, that is below current U.S. and international standards (Bell et al., 2006). O3 reduces lung function and thus contributes to premature deaths, emergency room visits, and exacerbation of asthma symptoms; it damages food crops and ecosystems; and it deteriorates materials. Nations have developed Air Quality Standards (AQS) for ground-level O3 abundances to protect people and crops (e.g., the U.S. NAAQS, the Canada Wide Standard, EU standards, and WHO standards, see Figure 2.1). AQS are evolving, and in Europe have been enhanced to include protection for agricultural crops. The U.S. secondary (crops) and primary (human health) standards are the same, and but are becoming stricter as a result of increasing knowledge about the damage caused by O3.
For each year over the period 2000–2002, between 36 and 56 percent of ozone monitors in the United States failed to meet the then current ozone standard of 0.08 ppm (Figure 2.1) for the fourth-highest annual maximum 8-hr ozone concentration (Hubbell et al., 2005). Using health impact functions derived from the published literature, they estimate that if the standard had been attained, roughly 800 premature deaths, 4500 hospital and emergency department admissions, 900,000 school absences, and > 1 million minor restricted-activity days (per year averaged over the three years studied) would have been prevented. The simple average of benefits
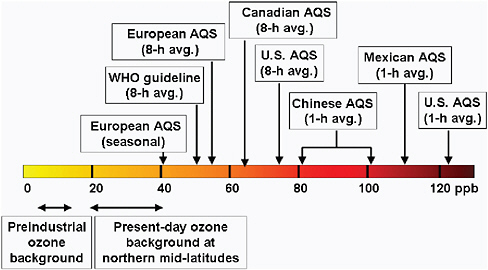
FIGURE 2.1 Ozone Air Quality Standards (AQS) in ppb (nanomoles of O3 per mole of dry air). Different national and international standards are noted as well as estimates for northern midlatitudes of the preindustrial background (i.e., O3 abundances with all anthropogenic emissions of NOx, CO, VOC, and CH4 cut off, and before current climate and stratospheric O3 change) and the present-day baseline abundances (i.e., the statistically defined lowest abundances of O3 in air flowing into the continents, typical of clean-air, remote marine sites). The pre-2008 U.S. AQS was 0.08 ppm, which through numerical roundoff meant that an AQS violation was 85 ppb or greater (D.J. Jacob, personal communication, 2009).
(including premature mortality) across the three years was estimated to be $4.9-$5.7 billion. If the analysis used the highest annual maximum 8-hr concentration, impacts would have increased by a factor of two to three. While highly uncertain (see Hubbell et al., 2005 for details), such estimates give a sense of the magnitude of impacts associated with ozone pollution.
In most metropolitan airsheds O3 has a daily cycle peaking in the late afternoon. It also varies with weather patterns and over seasons. An example of the variability of surface O3 abundance is shown is Figure 2.2 for a site in Michigan. This result is typical (i.e., violation of the U.S. NAAQS occurs episodically throughout the summer months) often for a few days in a row.
Atmospheric Chemistry and Dynamics Ozone is a highly reactive gas that is constantly being produced and destroyed by the natural cycles of atmospheric chemistry throughout the troposphere and stratosphere. About 90 percent of the column of O3, which protects living organisms from
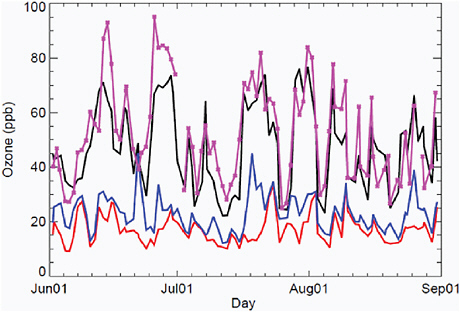
FIGURE 2.2 Time series of the daily, 8-hr, maximum O3 abundance (ppb) for June-August 2001 at Unionville, Michigan. Observations (magenta line) are compared with model results (black line). The model attribution of O3 sources shows the U.S. EPA policy relevant background (PRB, lowest red line) with all North American emissions cut off, and the background (blue line) with only U.S. emissions cut off. These calculations are short term and do not include the impact of CH4 emissions on O3, nor the impact of O3 precursor emissions on CH4.
SOURCE: Reprinted from Atmospheric Environment Vol. 43, H. Wang et al., Surface ozone background in the United States: Canadian and Mexican pollution influences, 2009, with permission from Elsevier.
damaging solar ultra-violet (UV) radiation, lies in the stratosphere generally more than 14 km above the surface. Even the worst pollution episodes with high O3 abundances near the surface have little impact on the total O3 column and do little to protect us from solar UV.
Tropospheric O3 is a secondary pollutant; it is not emitted directly but is instead produced photochemically in the troposphere by its precursors: reactive nitrogen oxides (NOx), carbon monoxide (CO), methane (CH4), and other volatile organic compounds (VOCs). (Secondary organic aerosols PM is also produced under these conditions; see Chapter 3.) These precursors exist naturally but are also emitted by human activities ranging from fossil fuel use to agriculture. Open burning of biomass, either natural or caused by humans, is a large source of O3 precursors. Another natural source of
tropospheric O3 is stratosphere-troposphere exchange. In such cases intrusions of stratospheric air (with O3 abundances initially in excess of 100 ppb in the free troposphere, before being diluted) can contribute tens of ppb to the baseline ozone levels at the surface (Wild et al., 2003).
In addition to emission of precursors, O3 production and loss is controlled by meteorological factors such as winds and convection that distribute precursors, clouds that control photochemistry, lightning generated NOx, and the temperatures and water vapor that control key chemical reactions. The lifetime of an O3 molecule in the troposphere varies according to the photochemical activity. Stevenson et al. (2006) estimate a mean tropospheric lifetime of 22 days, and Hsu and Prather (2009) derive a value of 30-35 days.
To determine the cause of elevated O3 abundances, atmospheric chemical transport models (CTMs) allow us to separate the O3 precursor sources with numerical experiments that turn on and off the different sources. In the case study shown in Figure 2.2, Wang et al. (2009a) calculate the different sources of surface O3 at the Unionville, Michigan site. A slowly varying baseline level of about 20 ppb is present when all North American (NA) emissions of NOx, CO, and VOC (but not CH4) are stopped (lowest red line). When emissions from Canada and Mexico are added (next lowest blue line), the baseline levels are increased by a few ppb, along with some much higher episodes (+ 20 ppb) presumably due to the nearby Canadian emissions. With the addition of U.S. emissions (black line), the model reproduces much of the observed episodic behavior, including the NAAQS violations, which in some cases are clearly enhanced by non-U.S. emissions.
Defining Background and Baseline Ozone The ultimate background level for tropospheric ozone existed before humans began to alter the atmosphere. In this preindustrial atmosphere tropospheric O3 sources included stratospheric intrusions; lightning NOx; natural surface emissions of CH4, CO, and VOCs; and wildfires. This preindustrial O3 can be defined but is not well determined by measurements (see below) or models. In EPA terms, the lowest curve in Figure 2.2 is defined as the “policy relevant background” (PRB) (i.e., without NA emissions of the short-lived precursors but with those of CH4). It is unclear whether the PRB always includes the increase in tropospheric O3 coming from NA sources that have already traveled around the globe, as it was in the Wang et al. global calculation cited above, since PRB can be calculated with nonglobal models. Thus, the term “background O3” is ambiguous and will always be used here with a qualifier. The background as defined here is hypothetical and not directly measurable.
Baseline O3 is used here (as baseline PM is used in Chapter 3) to describe a measurable quantity, the statistically defined lowest abundances
of O3 in the air flowing into a country, which is typical of clean-air, remote marine sites at the same latitude. Baseline air thus includes upwind pollution that contributes to the diffuse, uniform increase in O3 but not the episodic events. Baseline O3 will vary with location and season in the Northern Hemisphere and it can change over time. For example, the global emissions of CH4 over the last several decades are responsible for the increased CH4 abundances relative to preindustrial levels and have caused an increase in baseline O3 of about 5 ppb (Fiore et al., 2008). Emissions of NOx, CO, and VOC from neighboring states or the other side of the continent will also contribute to an increasingly diffuse background that is indistinguishable from baseline O3 in most urban airsheds and beyond local control. Defining the baseline level of O3 entering an Air Quality Management District, and how it might change, is of critical importance as it defines a minimum O3 exposure before local pollution is added. A key point to recognize, however, is that production of O3 is not necessarily linear, and hence the peak O3 during a NAAQS violation does not simply shift linearly with the baseline level.
Preindustrial Ozone The change in tropospheric O3 since the preindustrial era is difficult to evaluate. Surface measurements at several sites in both hemispheres from the 19th and early 20th centuries require careful evaluation and calibration of the early instrumentation (Kley et al., 1988; Volz and Kley, 1988; Marenco et al., 1994; Harris et al., 1997; Staehelin et al., 1998; Pavelin et al., 1999). The best estimates are extremely low O3 abundances (on the order of 10 ppb), which cannot be explained with current models by merely removing anthropogenic emissions of NOx, CO, VOC, and CH4 but rather, require large reductions in the natural sources of O3 (Mickley et al., 2001). Multimodel studies, including most global CTMs (Prather, 2001; Gauss et al., 2006), give a range of results when the known anthropogenic emissions of O3 precursors are removed, but they converge on a best estimate of about 33 percent increase in global tropospheric O3 over preindustrial levels, with a greater fractional increase in the Northern Hemisphere (40-100 percent). There remains uncertainty in this value, yet when we combine the limited historical observations with our proven chemical knowledge of the formation of O3 and the model studies, we have high confidence that current baseline levels of tropospheric O3 in the Northern Hemisphere are much greater than the natural preindustrial levels. This elevated baseline is maintained by emissions of precursors worldwide.
Ozone the Greenhouse Gas In terms of driving 20th-century climate change, tropospheric O3 is the third most important anthropogenic greenhouse gas after carbon dioxide (CO2) and CH4. Best current estimates give a radiative forcing (year 2005 minus year 1750) of + 0.35 W m−2, which
can be compared with + 1.66 W m−2 for CO2 (see Gauss et al., 2006; Forster et al., 2007b). Ozone in the free troposphere, not in the boundary layer over polluted regions, is responsible for most of this forcing, yet the O3 abundances throughout the troposphere are increased by these urban and industrial emissions. Anthropogenic radiative forcing for both tropospheric O3 and aerosols occurs primarily in the Northern Hemisphere, and there remains some uncertainty (e.g., Mickley et al., 2004; Shindell et al., 2006) as to whether these changes have disproportionate climate impact on regional scales in the northern midlatitudes.
Finding. Combining the evidence from observations and models, and including our basic knowledge of atmospheric chemistry, there is high confidence that human activities have raised the baseline levels of tropospheric O3 in the Northern Hemisphere by 40-100 percent above preindustrial levels. Much of this increase can be directly attributed to anthropogenic emissions of ozone precursor species (CH4, NOx, CO, VOCs).
Finding. U.S. NAAQS violations (e.g., 8-hr average greater than 75 ppb) are caused primarily by a combination of regional emissions and unfavorable meteorology. These are augmented by a changing baseline and episodic events caused, for example, by wildfires, lightning NOx, occasional stratospheric intrusions, as well as distant anthropogenic emissions. Most violations are only a few ppb above the standard, and thus the increase in baseline O3 since the preindustrial era driven by global pollution has contributed to these violations.
CHANGING BASELINE O3AND IMPACT ON AIR QUALITY STANDARDS
Detecting trends in baseline O3 is complicated by the natural variability in tropospheric O3 due to weather patterns, seasons, and short-term climate variations. Regular measurements need to be maintained for a decade or longer, thus intensive aircraft field campaign data are not directly useful for trend analyses. Surface O3 is routinely measured at many locations using continuous UV absorption instrumentation, a well-documented method. Vertical profiles of O3 in the free troposphere are measured at many locations by balloon-borne sensors (ozonesondes), typically launched weekly as part of the World Meteorological Organization’s Global Atmospheric Watch (WMO GAW) program. The ozonesonde data have been analyzed for trends (e.g., Oltmans et al., 2008) although the lower sampling frequency of the sondes, together with difficulties related to long-term calibra-
tion, limit the statistical detection of trends using these data (Deshler et al., 2008). For the last 15 years vertical O3 profile measurements have been made on board civil aircraft as part of the MOZAIC program (Marenco et al., 1998), which provide the more frequent measurements needed for trend detection at certain airports.
Given that tropospheric O3 increased sometime over the 20th century due to increasing emissions of precursors including CH4, the present rate of increase is a key uncertainty. Comprehensive reviews of Northern Hemisphere O3 trends have been presented by Vingarzan (2004), Cape (2008) and Royal Society (2008). Ozone trends vary substantially from place to place. The majority of sites with long-term observations indicate an increasing trend in O3 over the last two to three decades. The trends are in the range of 0.5-2 percent/yr (approximately 0.2-0.8 ppb/yr). Over the most recent decade the positive trend appears to have leveled off at some sites (e.g., Oltmans et al., 2006), while at others, such as the Atlantic coastal site at Mace Head, Ireland, and numerous rural sites in the western United States, O3 continues to increase. A statistically robust trend in baseline O3 of 0.2-0.3 ppb/yr has been reported at Mace Head for the period of 1987-2007 (Derwent et al., 2007). The baseline O3 abundances at Mace Head (e.g., 55 ppb in March 1999) are high compared with similar sites on the U.S. west coast.
Along the west coast of the United States a number of remote sites have been used to derive trends in O3 (Jaffe et al., 2003; Parrish et al., 2004b, 2008). Farther inland, Jaffe and Ray (2007) analyzed data from rural CASTNET sites. While a variety of methods have been used by the different groups, the results are consistent in suggesting an increase in baseline O3 of 0.3-0.4 ppb/yr. These trends are present in all seasons, and Figure 2.3 shows data from eight rural CASTNET sites in the western U.S. (Jaffe and Ray, 2007). These results are consistent with earlier work that suggests an increasing contribution to surface O3 in the United States from external sources during spring (e.g., Lin et al., 2000) and possibly other seasons as well. In England an average trend of 0.14 ppb/yr was reported for 13 rural sites over the period 1991–2006 (Jenkin, 2008).
In the free troposphere O3 data records are much more limited. Analysis by Logan et al. (1999) and Prather Figure 4.8 (2001) shows an unsteady but clear increase in O3 in the middle troposphere of the northern mid-latitudes of about 0.2 ppb/yr for the period 1970–1997. At several mountain stations in Europe a positive trend in O3 of 0.4-0.5 ppb/yr has been reported for 1991–2002, with the most statistically robust trends reported for fall, winter, and spring (Royal Society, 2008). Using a 10-year record of ozonesondes from a U.S. west coast location Oltmans et al. (2008) reported no significant trends. Oltmans et al. (2006) report significant regional variations in the trends, compared the more consistent patterns seen in
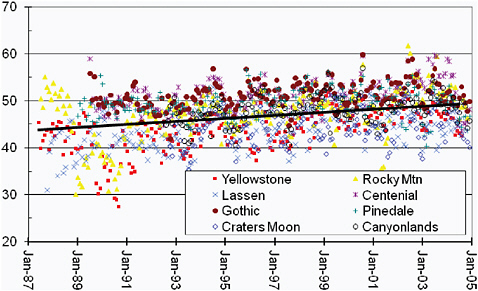
FIGURE 2.3 Monthly mean O3 abundances (ppb) from 1987 to 2005 from eight rural CASTNET sites in the western U.S. The average seasonal pattern at each site has been removed and a linear regression fit to the data: +0.34 ppb/yr.
SOURCE: Reprinted from Atmospheric Environment Vol. 41, D. Jaffe and J. Ray, Increase in surface ozone at rural sites in the western U.S., 2007, with permission from Elsevier.
surface observations. For example, they report significant increases in tropospheric O3 over Europe and Japan in the 1970s and 1980s, but a much smaller increase over the United States. Tanimoto et al. (2008) identified a significant increasing trend in free tropospheric O3 downwind of China for 1998-2006, a period of rapid growth in Chinese NOx emissions. Likewise, Bortz et al. (2006) report a large trend, 1.1 ppb/yr, in the northern tropics for the MOZAIC flight-level data for 1994-2003. While evidence generally shows that more regions of the Northern Hemisphere have increasing O3 baselines than have constant or decreasing ones, the magnitudes and specific patterns are not fully understood or simulated in chemical-transport models. Changes in natural variability, which can influence, for example, the stratospheric flux of O3 into the troposphere, could be a contributing factor.
Impact on Air Quality Standards Long-range transport of O3 in pollution plumes can be seen as distinct episodes of elevated O3 (e.g., Figure 1.3). Plumes of Asian origin with O3 abundances exceeding 80 ppb are observed
from aircraft in the free troposphere over the United States (Nowak et al., 2004; Liang et al., 2007). Observations at remote surface sites do not show detectable long-range pollution events for ozone, and this likely reflects the dilution during entrainment into the boundary layer (Hudman et al., 2004). Direct boundary layer transport from Asia is not a major contributor to the North American baseline because of the unfavorable circulation and the fast chemical loss of ozone under moist, sunlit conditions. Such transport does occur between North America and Europe and may raise the baseline O3 observed coming into Europe.
Models and observations do, however, indicate that the intercontinental pollution influence on U.S. surface O3 manifests itself as a large-scale, diffuse increase in baseline O3, which is due to O3 and its precursors being transported around the globe by fast winds in the free troposphere and then subsiding and mixing into the surface layer. One estimate of the increase in U.S. baseline O3 due to all non-U.S. emissions is 5-10 ppb with relatively little variability (Fiore et al., 2003a). This baseline enhancement can be characterized as an increase in the frequency of AQS violations (Fiore et al., 2002), but this may be misleading in that violations determined by a threshold can be caused by any small contribution to the total. A better measure of the ability of Air Quality Management Districts to control their AQS violations is the relative importance of domestic vs. distant emissions in contributing to local surface O3 (Jacob et al., 1999; Fiore et al., 2008).
In 2008 the U.S. NAAQS O3 standard was tightened from an 8-hr average of 0.08 ppm (effectively 85 ppb) to 75 ppb, thus increasing the importance of the baseline O3 abundances controlled by global emissions of O3 precursors. The O3 standard is evaluated relative to the PRB, which is presumably not amenable to North American regulation. The argument that tighter ozone standards are unachievable is based on some observations of O3 abundances at remote sites that are often in excess of 60 ppb (Lefohn et al., 2001), and presumably represent the PRB. Recent modeling and analysis refutes this, finding a 20-40 ppb PRB for the United States and noting that those larger abundances are associated either with high-elevation sites or with more distant North American pollution (Fiore et al., 2003b).
Finding. Baseline tropospheric O3 abundances at many remote locations in the Northern Hemisphere have changed over the last few decades at rates ranging from hardly at all to as much as 1 ppb/yr. The causes of these changes are not clear.
Recommendation. The measurements documenting changes in baseline O3 over the last few decades need to be systematically and collectively reviewed using consistent methods of analysis. Obser-
vations of O3 and related trace species should be placed in perspective through global chemical-transport modeling that includes the history of key factors controlling tropospheric O3: anthropogenic and natural emissions of precursors, stratospheric O3, and climate. An analysis of the key uncertainties should be undertaken to ensure that future changes can be attributed with greater confidence.
DIRECT OBSERVATION OF LONG-RANGE TRANSPORT OF O3AND PRECURSORS
A variety of observational tools are used to identify long-range transport of O3 and its precursors. Satellite datasets provide a continuous large-scale view and can sometimes track large plumes originating from intense pollution events as they cross the hemisphere (Edwards et al., 2004; McMillan et al., 2008; Zhang et al., 2008a). These observations are generally limited, however, to very intense pollution plumes and a few species. A spaceborne lidar (CALIPSO) has recently provided useful information on vertical structure by measuring the aerosols in plumes. As noted above, ozonesondes, the MOZAIC data (Thouret et al., 2006), and ground-based O3 lidars (Colette and Ancellet, 2005), also provide high-resolution vertical profiles that can be used to identify pollution plumes, but not map their extent or transport in the same way as satellites. Brief, intensive field campaigns involving a range of in situ measurements and mobile lidars are often able to map out tropospheric ozone and provide important detail on tropospheric O3 plumes, including precursors, vertical profiles, and their dilution into the baseline levels. Figure 2.4 shows an aircraft-measured curtain of tropospheric O3 on a transect from Japan to Hawaii during TRACE-P (Wild et al., 2004b). The corresponding CTM simulation is able to broadly match the pollution plumes from Asia, the stratospheric intrusions, and the sharp gradients across a frontal system. Such intensive field campaigns involving coordinated flights from several locations have been successful in providing data on transport pathways and provide an integrating step between the measurements from satellite and distant ground-based monitoring sites located in source and receptor regions.
Long-range tropospheric ozone transport can potentially be detected by satellite observation of tropospheric O3 columns that are derived using residual techniques (i.e., subtract a stratospheric component, which accounts for about 90 percent of the total, from the total column measurement with the aid of other measurement or model information). Unfortunately, there is often a strong coincidence of extra-tropical tropospheric ozone column anomalies with probable troposphere-stratosphere exchange events or folds (Schoeberl et al., 2007; Zhang et al., 2008a), and these may be mistaken for pollution events since folds often occur in the Atlantic and Pacific pollu-
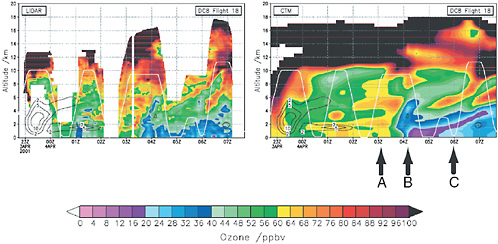
FIGURE 2.4 Vertical profile of tropospheric O3 (ppb) from Pacific transit (April 3, 2001) from Tokyo to Hawaii during TRACE-P. The DC-8 lidar observations on the left show (A) an O3 pollution plume from Asia (02Z-04Z, 1-6 km), (B) a strong frontal passage (04Z, 0-4 km), and (C) stratospheric intrusions (05Z-07Z, 4-9 km) that are reasonably well simulated by the model results, shown on the right (Wild et al., 2004b).
tion corridors. Nevertheless, monthly averaged tropospheric column ozone (TCO) derived from the Aura satellite (MLS and OMI) indicate significant O3 enhancements in the regions of greatest precursor emission (i.e. eastern U.S., Europe, East Asia, and a broad enhanced region in the midlatitudes associated with pollution export from the continents (Figure 2.5) (Ziemke et al., 2006). Seasonally, the TCO follows the Northern Hemisphere industrial pollution in the July and the Southern Hemisphere biomass burning source of O3 in November.
CO is the only long-lived O3 precursor readily measured from satellite that can provide a good indication of long-range transport. Intense pollution sources can produce plumes with enhancements of > 100 percent over background values. See Figure 2.6 for an example of CO pollution plumes observed with the Terra/MOPITT satellite instrument. Correlations of enhanced O3 and CO within pollution plumes measured by Aura/TES suggest initial export of O3 in the plumes from Asia along with continued production of O3 during transpacific transport (Zhang et al., 2008a). These correlations are also used to analyze in situ measurements to identify sources of plumes (forest fires vs. fossil fuel combustion) and to try to track them during long-range transport (Parrish et al., 1993; Stohl et al., 2002).
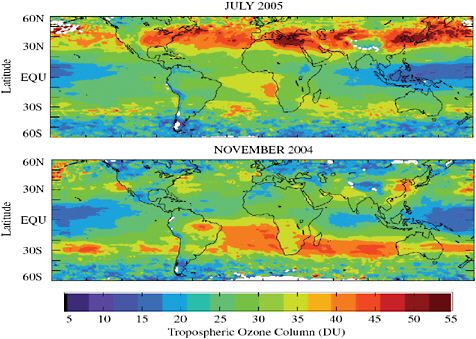
FIGURE 2.5 Monthly averaged OMI/MLS TCO (in Dobson units) for July 2005 and November 2004 (Ziemke et al., 2006).
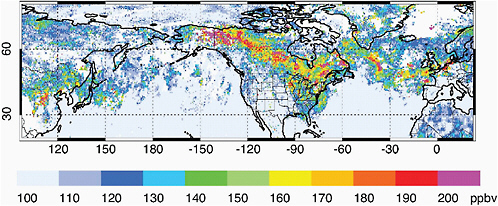
FIGURE 2.6 Average CO abundance (ppb) in the free troposphere, as observed by the satellite Terra/MOPITT for the 8-day period July 15-23, 2004. Plumes of anthropogenic CO pollution can be seen leaving Asia and crossing the Pacific Ocean, and those of Alaskan and Canadian forest fires can be seen crossing North America and the Atlantic Ocean towards Europe.
SOURCE: Provided by D. Edwards, National Center For Atmospheric Research.
Such correlations are used to deduce photochemical production and loss rates for CO and O3 during transit, but other factors such as the large-scale gradients along the plume and in background abundances complicate these analyses (Chin et al., 1994; Pfister et al., 2006; Real et al., 2008).
The availability of nearly a decade of satellite CO observations has led to the derivation of multiyear regional CO inventories through inverse modeling (Pétron et al., 2004; Kopacz et al., 2009) that has increased our emission estimates for CO from fossil and biofuel use in Asia by a large factor, and improved our understanding of the emission patterns. CO can also be used as a proxy for inferring emissions and distributions of other species that are readily measured to correlate with CO using in situ measurements but are not readily measured directly from satellite. Although satellite measurement of nitrogen dioxide (NO2) and formaldehyde (HCHO) clearly indicate source regions for these O3 precursors and provide emissions estimates (Fu et al., 2007; Wang, 2007), long-range transport of these species is not expected nor readily observed because of their short lifetimes (Martin et al., 2006).
Aircraft measurement campaigns feature a diversity of well-calibrated instruments that can measure multiple species and allow diagnosis of the chemical and dynamical processes influencing O3 during long-range transport, providing a critical test of chemistry and transport models. A recent example is the ICARTT campaign that included components from North America (INTEX-NA, NEAQS) and Europe (ITOP), as well as a Lagrangian experiment (IGAC Lagrangian 2K4) with dedicated flights using multiple aircraft to sample the same air masses several times as they crossed the North Atlantic between North America and Europe. Analysis of an anthropogenic plume transported at low levels and a forest fire plume that descended into Europe showed O3 destruction in the former and production in the latter (Real et al., 2007).
Spring import of enhanced O3 levels into western North America has been identified in analysis of surface data and aircraft measurements during various campaigns focused on quantifying transport of polluted air masses from Asia to North America (e.g. TRACE-P, INTEX-B, ITCT-2K2). For example, analysis of INTEX-B data suggests that Asian pollution plumes contributed 9 ± 3 ppb to an O3 abundance of 54 ppb at Mount Bachelor, Oregon (2.7 km altitude) during April-May 2006 (Zhang et al., 2008a). Results based on the GEOS-CHEM model suggest that about half of the pollution O3 was produced in the emission region and half during transport across the Pacific, largely as a result of the decomposition of PAN (peroxyacetyl nitrate, an urban air pollutant formed in the air as part of photochemical smog) (Heald et al., 2003). Long-range transport of pollutants from Europe to North America is expected but remains much less well quantified.
Transoceanic transport of pollution O3 takes place mostly in the free troposphere where meteorological conditions occasionally favor the preservation of distinct plumes as compared with the marine boundary layer. In addition, O3 has a longer atmospheric lifetime in the free troposphere than in the warmer boundary layer (Heald et al., 2003; Price et al., 2004), thus allowing individual Asian plumes to be observed at mountain sites in the western United States (Jaffe et al., 2005b; Reidmiller et al., 2009b). Corresponding plumes are not generally observed at the surface (Goldstein et al., 2004; Hudman et al., 2004), presumably because of the dilution into large air masses in the boundary layer, or because of loss during boundary layer transport. Substantial production of O3 in boreal forest fire plumes may also lead to pollution events in downwind regions (Real et al., 2007). Better understanding and modeling of the entrainment of freetropospheric O3 plumes into the boundary layer in the impacted region is needed to improve our estimates of the impact of long-range transport of pollution O3 on AQS.
Finding. Plumes containing high levels of O3 and its precursors (NOx, CO, VOCs) can be transported between continents and are observed downwind of the major industrial regions and large wildfires in North America, Europe, and Asia. They are observed in the free troposphere over impacted regions but rarely at the surface due to dilution in the boundary layer.
Recommendation. Conduct focused research efforts that couple measurements with models to quantify the process of air exchange between boundary layer and free troposphere, in order to better understand how free tropospheric O3 enhanced by long-range transport is mixed to surface.
MODELING AND ATTRIBUTION OF O3FROM GLOBAL SOURCES
HTAP and Other Model Results In this section we focus our attention primarily on estimates of ozone import and export into and out of North America (NA). Attribution of the amount of O3 produced from emissions from different regions or sources can be derived either by keeping track or tagging the O3 formed over or downwind from the particular region (e.g., Derwent et al., 2004; Sudo and Akimoto, 2007) or by reducing or increasing particular emissions over the region of interest (e.g., Yienger et al., 2000; Wild and Akimoto, 2001; Auvray and Bey, 2005). Chemistry-transport models (CTMs) or chemistry-climate models (CCMs) are also used to perform budget studies that estimate the contributions from different sources over a particular region. For example, Pfister et al. (2008) used the global MOZART CTM to estimate that the fraction of summertime
U.S. O3 in 2004 originating from stratosphere (26 ± 6 percent) was comparable to that from U.S. emissions (25 ± 9 percent) with smaller contributions from Eurasian sources (13 ± 5 percent), lightning (10 ± 2 percent), and boreal forest fires (3 ± 2 percent) that were very active in Alaska during this particular year. Other studies also highlight the importance of lightning NOx over North America (Hudman et al., 2006; Singh et al., 2007). These results illustrate the complexity of the natural and anthropogenic sources influencing ozone distributions over the United States.
As part of HTAP-TF (2007) a thorough analysis of results from many different (ensemble) model simulations has been performed with the aim to reduce uncertainties by combining results from models with different representations of emissions, transport, and chemical processes. A consistent set of analysis metrics was also used (see http://htap.icg.fz-juelich.de/data/FrontPage for details). Of particular relevance to this report are results from 21 global and hemispheric CTMs that were used to estimate the change in surface ozone resulting from reduced emissions over East Asia (EA), North America (NA), Europe (EU), and South Asia (SA) (Fiore et al., 2009). Note that NA, EA and EU regions have slightly different areas and SA is a factor of two lower. About half the models had a horizontal resolution of 3 × 3 degrees or finer. For each region, anthropogenic emissions of NOx, CO, and VOCs from all sectors, including shipping, were reduced by 20 percent (first for each individual sector, then for all of them together) for model simulations of the year 2001. The 20 percent changes in ozone precursor emissions used in HTAP are comparable with recent changes: U.S. total NOx emissions decreased about 3 percent/yr between 2000 and 2006, mostly in the eastern U.S. (EPA-TTN; http://www.epa.gov/ttnchie1/trends/); whereas East Asian emissions increased approximately 7 percent/yr for the years 2000-2005 (Ohara et al., 2007).
Responses to reductions in foreign emissions (not including methane) were found to be larger in spring and fall, with largest responses to reductions in NOx emissions. Import sensitivities (defined as the ratio in surface O3 response to a 20 percent decrease in the three foreign source regions vs. a 20 percent decrease in the domestic emissions) peak during the winter and spring or late fall (0.5 to 1.1) when the response of O3 to domestic emissions is small (Figure 7 in Fiore et al., 2009). Note that “domestic” as used here does not mean local emissions but includes all interstate and even international emissions within the NA region. During the summer months when domestic O3 production is at a maximum, import sensitivities for all regions are small (0.2-0.3). The number of days with 8-hr average O3 abundances greater than 60 ppb are much more sensitive to reduction in domestic emissions than in foreign (Reidmiller et al., 2009a).
Figure 2.7 shows the modeled change in 24-hr average surface O3 over NA resulting from a 20 percent change in anthropogenic precursor
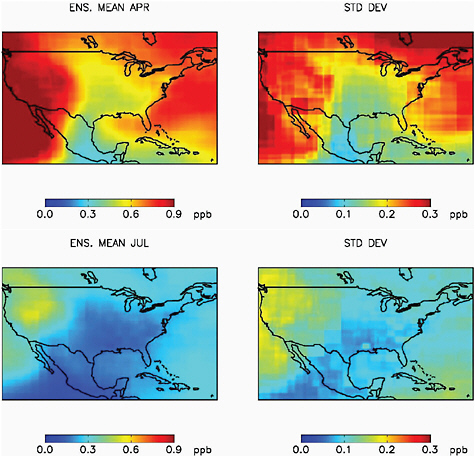
FIGURE 2.7 Model mean surface O3 (ppb) for April and July over North America that is attributable to 20 percent of the anthropogenic emissions of O3 precursors (NOx, CO, VOC) from the other three major industrial regions of the NH (EA, EU, SA). The standard deviation of the ensemble of 14 HTAP models is also shown (Fiore, personal communiation. See Fiore et al., 2009 for details).
emissions (NOx, CO, VOC) from the combined foreign source regions (EA + SA + EU), where the O3 change results from emissions being adjusted from 80 to 100 percent of current levels. The April and July monthly means from the ensemble of 14 HTAP models along with the standard deviation of the ensemble are plotted. Peak levels of imported O3 can be seen over the west coast of North America, with higher values in spring than in summer. Smaller increases are also seen over eastern Canada and off the U.S. east coast. Interestingly, there is also predicted import into Florida in November, arising from transport of emissions from Europe around the Azores High
during the winter months. EU and EA sources (not shown separately) are dominant, contributing about equally (Figure 5a in Fiore et al., 2009).
The HTAP analysis also considered the impact of NA on other regions. For example, while EA is mainly influenced by EU emissions (particularly in spring), there is also a non-negligible contribution from NA emissions. Their results also confirmed previous studies showing that NA is the main external contributor to surface EU O3 due to its relatively close proximity. In those studies (summarized in Table 5.2 in HTAP-TF, 2007) ozone enhancements varied between 1 to 5 ppb but sometimes as high as 10 ppb for particular events.
The recent HTAP results based on ensemble model results show less variability and lower enhancements since these estimates provide an estimation of average increases in baseline O3 due to different source regions and not due to particular events. Derwent et al. (2008) also examined regional influences in source-receptor relationships affecting European O3 by conducting a series of increased NOx pulse experiments over 10 × 10 degree grids covering East Asia and North America. Largest responses to NA emissions were found at sites in Western Europe. In winter modeled O3 responses at Mace Head were more sensitive to emissions in northern NA whereas in summer emissions from eastern NA produced higher responses. Since O3 has a shorter lifetime in summer, closer pollution sources have a greater influence. This location showed positive responses in the short term (up to several months) followed by smaller negative responses in the longer term (1 year or more) due to feedbacks between NOx, OH, O3, and CH4 chemistry. This resulted at certain locations in an integrated net negative response, especially in summer (e.g., to changes in southern U.S. emissions).
Changing NOx, CO, or VOC perturbs OH and thus the CH4 lifetime that in turn changes CH4 and baseline O3 abundances over decades (Wild et al., 2001). Fiore et al. (2009) also tried to quantify some of these short-versus long-term feedbacks in O3 responses; reducing NOx emissions over all regions lowers OH and increases CH4, leading to reductions in the short-term decrease in surface O3 by 15-20 percent; reducing CO or nonmethane VOCs by 20 percent has the opposite effect and leads to additional increases in surface O3 of around 35 percent and 10 percent, respectively. Combined emission reductions offset each other leading to long-term effects that were always less than 3 percent.
This concept of different timescales influencing the impact of O3 responses in downwind receptor regions has also been examined using the adjoint modeling approach. For example, Figure 2.8 shows the sensitivity of the mean surface O3 concentrations at Trinidad Head, California, to production over upwind source regions (Hakami et al., 2007). Local O3 sources have the greatest impact, although the Asian contribution from eastern China and Japan and O3 produced during transport over the North
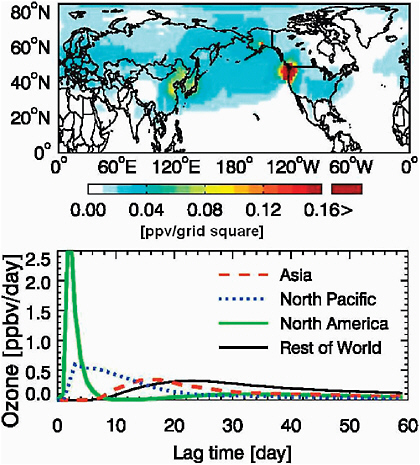
FIGURE 2.8 Sensitivity of surface O3 abundance at Trinidad Head, California, to ozone production in different regions of the world, as inferred from the GEOS-Chem adjoint model for the period April 17 - May 15, 2006 (Zhang et al., 2009a). Left: sensitivities integrated in time over the depth of the tropospheric column. Right: time-dependent sensitivities (going back in time) to O3 production over Asia, the North Pacific, North America, and the Rest of World. These results help demonstrate that production over any one region (e.g., North America) can include long-range transport of distant sources of O3 precursors as well as local emissions (Kotchenruther et al., 2001).
Pacific plume are significant. The time lag in the response (impact) at Trinidad Head is about 16 days.
Emissions of O3 precursors in Asia have been increasing rapidly (Irie et al., 2005; Richter et al., 2005; Ohara et al., 2007), and this has resulted in increased production and outflow of O3, especially during spring (Tanimoto et al., 2008; Xu et al., 2008; Zhang and Tao, 2009). As a result some
researchers have suggested that the increasing Asian emissions are responsible for the trends in spring O3 along the west coast of the United States (Jaffe et al., 2003b; Parrish et al., 2004). The HTAP assessment (Fiore et al., 2009; Reidmiller et al., 2009a) can be used to evaluate the significance of increasing Asian emissions and the consistency with observations. According to Reidmiller et al. (2009b) a 20 percent reduction in East Asian emissions would reduce the spring average maximum daily 8-hr average in the western United States by approximately 0.4 ppb. Assuming linearity, a 10 percent/yr increase in Asian precursor emissions would correspond to an increase in MDA8 of ~ 0.2 ppb/yr, which is similar in magnitude to the 0.34 ppb/yr increase in mean daytime O3 reported by Jaffe and Ray (2007). Although the observed increase in O3 was found in all seasons, the HTAP assessment suggests that only during winter-spring is the increase consistent with the increasing Asian emissions. During summer, an increase in wildfires across the western United States is the most likely cause for the increase in O3 (Jaffe et al., 2008).
Model Uncertainties The results presented in the previous sections rely largely on simulations using global models. Even though results from many different models were used in the HTAP analysis, there are still significant uncertainties in individual model calculations. Comparison with observations is used as a means of evaluating model performance. For example, Fiore et al. (2009) attempted to assess model skill by comparing the HTAP ensemble model mean with surface O3 observations in the United States, Europe, and Asia. They found that modeled monthly mean surface O3 concentrations capture observed seasonal cycles at European sites reasonably well, but overestimated summertime concentrations over Japan and the eastern United States (more than 14 ppb in July). Biases were particularly large over the southeastern United States and may be related to uncertainties in the chemical reactions among isoprene, NOx, and O3. A similar but more detailed analysis focusing on the United States also reports a significant positive bias in the HTAP model ensemble results for summer in the eastern United States, along with a negative bias of surface O3 from the ensemble mean for the western United States during spring (Reidmiller et al., 2009a). Large uncertainties in the emissions may be significant sources of error in these model simulations. For example, in the HTAP exercise, emissions of nonmethane VOCs varied by a factor of 10 between models.
To evaluate long-range transport of pollutants, more rigorous validation of models is required where model performance is evaluated in terms of their ability to simulate pollutant plume transport and dilution (to baseline) and chemical and physical processing,including transport of plumes into downwind receptor regions where mixing into the boundary layer is important. Comparisons with data collected during dedicated aircraft campaigns are able
to pinpoint particular errors in global models related to emissions, dynamics, or wet deposition and thereby reduce uncertainties. For example, Hudman et al. (2008) used INTEX-NA data to conclude that EPA CO emissions are too high by 60 percent. In another study based on analysis of INTEX-B data, Zhang et al. (2008a) added weight to previous work (Palmer et al., 2003) suggesting that Asian emissions are too low by a factor of two. Analysis of ICARTT data collected in the same air mass during transport across the North Atlantic also showed that global models are unable to capture the evolution of, for example, forest fire plumes related to insufficient resolution or missing or inaccurate photochemistry (e.g., Real et al., 2007).
Global models need to be run at higher horizontal and vertical resolutions and combined with Lagrangian techniques or regional air quality models in order to capture plume transport between continents. The rate at which pollutant plumes mix with so-called background air determines whether a plume event will be detected over a receptor region or whether the signal will contribute to enhanced baseline concentrations. Recently, Pisso et al. (2009) estimated that global models need to be run with at least 40 km horizontal and 500 m vertical resolution in order to simulate long-range transport of pollutant plumes. The impact of resolution errors on modeled O3 production rates is less clear. While Wild and Prather (2000) found linearly decreasing errors ranging from 27 percent at 5.5 degrees to 5 percent at 1.1 degrees, Esler et al. (2004) suggested that errors might become nonlinear below 1 degree and possibly much higher (20-50 percent) in the sharp gradients at plume edges.
Finding. Baseline O3 levels in the Northern Hemisphere are elevated by current anthropogenic emissions of NOx, CO, and VOCs. Multimodel studies calculate that a 20 percent reduction in these emissions from any three of the four major industrial regions of the Northern Hemisphere will reduce surface O3 in the fourth region by about 1 ppb on average (i.e., a baseline enhancement) but with large spatial and seasonal variation. Results of similar magnitude are found for a 20 percent reduction in CH4 emissions. Unfortunately, the range of results across this multimodel ensemble is comparable to the average result. These calculations were conducted for 2001 and in the following eight years, emissions have changed by 20 percent or more in some regions (e.g., decreasing in the United States, increasing in East Asia).
Recommendation. As models provide the only practical method of attributing a fraction of surface O3 and possible AQS violations to different sources of pollution, a major effort must be made to calibrate, test, and improve these models with a wide
range of atmospheric chemistry measurements ranging from individual events to climate statistics of air pollution. These tests must span the range of necessary modeling from global transport and stratosphere-troposphere exchange down to the urban airshed. While improving the models is a priority, realistically quantifying their uncertainty is key. Development of adjoint and ensemble modeling approaches will be important in assessing NAAQS compliance and intracontinental pollution under rules such as the U.S. EPA Clean Air Interstate Rule.
HEALTH IMPACTS OF IMPORTED O3
Acute exposure to elevated O3 levels is associated with increased hospital admissions for pneumonia, chronic obstructive pulmonary disease, asthma, allergic rhinitis and other respiratory diseases, and with premature mortality (e.g., Mudway and Kelly, 2000; Gryparis et al., 2004; Bell et al., 2005; Ito et al., 2005; Levy et al., 2005; Bell et al., 2006; NRC, 2008; Jerrett et al., 2009). A 10-ppb increase in 1-hr daily maximum ozone is associated with a 0.41-0.66 percent increase in mortality (Gryparis et al., 2004; Ito et al., 2005; Levy et al., 2005). Outdoor ozone concentrations and activity patterns are the primary determinants of ozone exposure (Suh et al., 2000; Levy et al., 2005). A recent NRC committee concluded that “the association between short-term changes in ozone concentrations and mortality is generally linear throughout most of the concentration range.… If there is a threshold, it is probably at a concentration below the current ambient air standard.” (NRC, 2008). In addition, there is weak evidence that chronic exposure to ozone increases mortality; if confirmed, the total health burden of exposure to ozone would be much higher than current estimates (NRC, 2008).
Anenberg et al. (2009) estimated the impacts of intercontinental O3 transport on mortality using the multimodel ensemble mean surface O3 responses to perturbation scenarios produced by the HTAP analyses (HTAP-TF, 2007; Fiore et al., 2009) and health impact functions. A health impact function is used to calculate avoided deaths in a population, using baseline mortality rates, the modeled O3 changes, and a concentration-response factor.1 For cardiopulmonary deaths the concentration response function
was based on Bell et al. (2004): a 0.64 percent increase in cardiovascular and respiratory mortality for each 10 ppb increase in 24-hr average O3 with a 95 percent confidence interval of 0.31 to 0.98 percent. The avoided nonaccidental mortality is also evaluated and shown to be larger, since it includes cardiopulmonary mortality.
As shown in Tables 2.1 and 2.2, 20 percent reductions of anthropogenic NOx, nonmethane VOCs, and CO emissions in NA were estimated to avoid more deaths outside NA than within (68-76 percent of resulting avoided deaths in the Northern Hemisphere [NH] occur outside NA). Reductions in EU were also estimated to avoid more deaths outside EU than within when a low-concentration threshold was applied (55-58 percent of resulting avoided deaths in the NH occurred outside of EU). The opposite was true for EA and
TABLE 2.1 Annual avoided cardiopulmonary deaths (in hundreds) in each receptor region and in the entire NH, when anthropogenic NOx, NMVOC, and CO emissions are reduced by 20 percent within each region.
TABLE 2.2 Annual avoided nonaccidental deaths (in hundreds) in each region and in the entire Northern Hemisphere when anthropogenic NOx, NMVOC, and CO emissions are reduced by 20 percent within each region.
|
Source Region |
Receptor Regions |
||||
|
NA |
EA |
SA |
EU |
NH |
|
|
NA |
16 (1 , 30) |
10 (6 , 14) |
10 (6 , 15) |
17 (11, 23) |
60 (27 , 94) |
|
EA |
3 (2 , 5) |
65 (15, 120) |
10 (5 , 16) |
8 (5 , 11) |
93 (26 , 160) |
|
SA |
1 (0 , 2) |
6 (2 , 10) |
132 (80,180) |
3 (1 , 5) |
148 (89 , 210) |
|
EU |
3 (2 , 4) |
12 (6 , 18) |
11 (6 , 17) |
25 (-9 , 58) |
60 (9 , 110) |
|
Note: This assumes no low-concentration threshold for pollution impacts. Confidence intervals (68 percent) are derived from ± 1 standard deviation of the model ensemble’s O3 change. They do not reflect uncertainty in the concentration response function. Note that nonaccidental deaths is a larger class that includes cardiopulmonary deaths (from Table 2.1). SOURCE: Anenberg et al. (2009). |
|||||
SA, with 70 percent of the resulting NH avoided deaths occurring within the source region for EA and 90 percent for SA. Reducing emissions in any of the four regions resulted in many annual avoided deaths in EA and SA, due to large populations and high baseline mortality rates. Reducing anthropogenic CH4 emissions in each region by 20 percent gives about half the number of avoided mortalities in the NH as does the 20 percent reduction in NOx, CO, and VOC emissions. Reductions in CH4, however, will also reduce O3 and mortality in the tropics and SH (West et al, 2006).
The relative importance of source-receptor pairs for mortality was strongly influenced by the accuracy and consistency of the HTAP model ensemble, and this had disproportionately large standard deviations for O3 responses in the same region where emissions were reduced. None of the HTAP models used to generate the O3 response to emission reductions has the necessary resolution to simulate urban air quality; hence the domestic response is likely underestimated. The more distant response to emissions does depend on resolution (see discussion above) but less so. In general, the impact of ozone-modeling uncertainty was greater than that of concentration-response-function uncertainty.
Finding. With high confidence we can state that increases in O3 occur in populated regions due to distant pollution and such increases are detrimental at some level to human health, agriculture, and ecosystems. A preliminary study finds that about 500 premature cardiopulmonary deaths could be avoided annually in North America by a combined 20 percent reduction in NOx, CO, and VOC emissions from the other three major Northern Hemisphere industrial regions and, correspondingly, about 1800 in Europe. The uncertainty in these estimates is large, at least ± 50 percent, and reflects uncertainties in modeling both O3 change and health effects.
Recommendation. Perform additional research to (1) attribute quantitatively the surface O3 change to distant emissions; (2) specify the shape of the exposure-response curve (i.e., whether a threshold exists); (3) estimate avoided premature health burdens across a wider range of health outcomes; and (4) estimate avoided premature health burdens for particularly sensitive groups (i.e., children and others).
THE FUTURE—CHANGING CLIMATE AND EMISSIONS
Impact of Climate Change on Local Pollution Episodes Current observations and model projections show a warming climate. This climate
change leads to increased atmospheric water vapor that causes substantial O3 reductions especially in the tropical lower troposphere, and it also leads to enhanced stratosphere-troposphere exchange, which increases the flux of stratospheric O3 into the troposphere (Collins et al., 2003; Sudo et al., 2003; Shindell et al., 2006; Zeng et al., 2008). In addition, we expect that recovery of the stratospheric ozone depletion caused by anthropogenic halocarbons will also lead to increases in the stratospheric O3 flux (e.g., Fusco and Logan, 2003). These major climatic changes will drive baseline O3 in different directions depending on latitude and season. For example, photochemical reduction in O3 is greatest at low latitudes while stratospherie-driven increases will dominate high latitudes. Lightning-generated NOx, possibly the largest natural O3 source in the tropics, will increase if deep convection increases in a warmer climate (Toumi et al., 1996). Current model projections do not provide an adequate scientific consensus due to the net effect of 21st-century climate change.
Polluted U.S. sites show a strong correlation of high-ozone episodes with elevated temperature (Lin et al., 2001). This correlation is well reproduced in models and is driven in part by chemistry, biogenic VOC emissions, and the association of high temperatures with stagnation events that trap pollution (Jacob et al., 1993; Sillman and Samson, 1995). There appears to have been an increase in the frequency of stagnation events in the eastern United States over the past decades, compromising progress to achieve the ozone AQS (Leibernsperger et al., 2008). In one model this specific pattern is predicted as a consequence of current climate change due to northward shift of storm tracks (Mickley et al., 2004). Thus, a range of evidence projects that 21st-century climate change will increase the O3 AQS violations driven by local pollution.
Quantifying these effects requires 21st century projections using global climate models (for the O3 baseline) as well as extrapolation of climate change to regional scales relevant to AQS with either separate regional models or statistical methods to downscale the meteorology. A number of such studies have been conducted for the United States, starting with the work of Hogrefe et al. (2004), and these are reviewed by Jacob and Winner (2009). Results from ensembles of climate models are presented by Weaver et al. (2009). Consistently, models project increases for polluted U.S. regions ranging from 1 to 10 ppb in surface O3 as a result of climate change over the coming decades, with largest increases found in urban environments and during peak pollution episodes. All models find consistently large increases in the northeast, but there is more disagreement in the southeast and west (Weaver et al., 2009). The climate-change impact on AQS violations will lessen if NOx emissions decrease (Wu et al., 2008b), due in part to a decrease in the O3 baseline (Murazaki and Hess, 2006). These high O3 pollution events in the future climate are driven by
local meteorology and local emissions, since the baseline O3 changes are uncertain.
Impact of Non-Local Emissions Change on Local Air Quality Baseline O3 will change as anthropogenic emissions of precursors around the world shift as a result of population and energy trends, industrialization of the developing world, and environmental controls in the developed world. NOx emissions in China have doubled over the past decade (Zhang et al., 2008a), while emissions in the United States and Europe have decreased (van der A et al., 2008). If precursor emissions across North America and Europe continue to decrease in order to meet air quality goals, increasing emissions in the developing world could offset some of these benefits.
Model simulations based on the 2001 IPCC scenarios projected future increases in the surface O3 baseline over the Northern Hemisphere of 2-7 ppb by 2030 (Prather et al., 2003), but more recent work corrects some of the IPCC assumptions and reduces precursor emissions (Dentener et al., 2005; Stevenson et al., 2006). Additional model studies using the uncorrected IPCC scenarios project a 2-6 ppb increase in baseline O3 over the United States due to emissions outside North America by 2050 using one IPCC scenario (Wu et al., 2008a) and < 1 ppb increase with another lower-emissions IPCC scenario (Lin et al., 2008).
A more consistent prediction was that baseline O3 will increase due to the increasing abundance of CH4 (Prather, 2001). All of the 2001 IPCC scenarios predicted an increase in anthropogenic CH4 emissions over at least the next several decades and an increase in baseline O3 to accompany it, but this projection ran counter to the observations showing a leveling of CH4 abundances since 1998 (Dlugokencky et al., 2003). Various explanations have arisen regarding the CH4 trend (e.g., Bousquet et al., 2006; Chen and Prinn, 2006; Fiore et al., 2006), but there is no clear consensus as to the cause. In 2007, however, the annual CH4 increases returned (Rigby et al., 2008). Given that most inventories project increasing anthropogenic CH4 emissions (e.g., Wuebbles and Hayhoe, 2002), one must be prepared to accept parallel increases in baseline O3.
Finding. Projected climate change will lead to a warmer climate with shifts in atmospheric circulation. All of these changes have the potential to affect air quality, for better or worse:
-
higher water vapor abundances associated with a warmer climate drives down baseline O3 abundances due to the more rapid photochemical destruction of O3 in the marine boundary layer and the tropics;
-
projections of more rapid stratospheric circulation increases the flux of O3 into the troposphere, raising the baseline;
-
warmer temperatures generate higher O3 pollution levels from local emissions;
-
for some regions, projected increase in wildfires can increase O3;
-
changing tropospheric circulation, such as the northward shift of storm tracks at mid-latitudes, may increase/decrease the number of stagnation events that lead to the worst pollution episodes; and
-
increasing convective activity and lightning will enhance NOx and the O3 baseline.
In general these changes reduce the role of non-local emissions while enhancing that of local emissions in AQS violations.
Recommendation. Test and improve the simulation of air quality, including pollution episodes in both global and regional climate models. Ensure that the climate models include global atmospheric chemistry so that the model ensembles run for climate change assessments can also be used to evaluate changes in air quality. Analyze the climate statistics that are specific to air pollution: boundary layer height, stagnation episodes, intercontinental transport, stratosphere-troposphere exchange, lightning, wildfires, and other natural emissions of O3 precursors.
Finding. In East Asia and much of the developing world O3 precursor emissions are expected to increase rapidly over the next few decades and would likely raise the surface O3 baseline in the United States by a few ppb. Methane increases as projected and recently observed, if continued over the next few decades, would additionally raise the O3 baseline by a few ppb.
Recommendation. (1) Develop and improve the accuracy of emissions inventories for the current epoch as well as projections for the next decade. Include natural emissions and the development of interactive emissions models that depend on climate variables. (2) Establish a global international measurement strategy (e.g., through WMO/GAW) based on surface sites and airborne platforms that can identify changes in regional emissions of the ozone precursors: CH4, NOx, CO, VOCs, and relevant secondary compounds, such as PAN. (3) Merge these operational networks with satellite measurements and develop the modeling capability to pro-
vide reliable measures of emissions on a subcontinental scale. (See the discussion of integrated systems in Chapter 6.)
SUMMARY OF KEY FINDINGS AND RECOMMENDATIONS
Question: What is known about current long-range transport of ozone and its precursors?
Finding. Combining the evidence from observations and models and including our basic knowledge of atmospheric chemistry there is high confidence that human activities have raised the baseline levels of tropospheric O3 in the Northern Hemisphere by 40-100 percent above preindustrial levels. Much of this increase can be directly attributed to anthropogenic emissions of ozone precursor species (CH4, NOx, CO, VOCs).
Finding. Baseline tropospheric O3 abundances at many remote locations in the Northern Hemisphere have changed over the last few decades at rates ranging from hardly at all to as much as 1 ppb/yr. The cause of these changes is not clear.
Recommendation. The measurements documenting changes in baseline O3 over the last few decades need to be systematically and collectively reviewed using consistent methods of analysis. Observations of O3 and related trace species should be placed in perspective through global chemical-transport modeling that includes the history of key factors controlling tropospheric O3: anthropogenic and natural emissions of precursors, stratospheric O3, and climate. An analysis of the key uncertainties should be undertaken with an eye toward ensuring that future changes can be attributed with greater confidence.
Finding. Plumes containing high levels of O3 and its precursors (NOx, CO, VOCs) are observed downwind of the major industrial regions (and large wildfires) in North America, Europe, and Asia. They can be transported between continents and are observed in the free troposphere over affected regions but rarely at the surface due to dilution in the boundary layer.
Recommendation. Conduct focused research efforts that couple measurements with models to quantify the process of air exchange between boundary layer and free troposphere in order to better
understand how free tropospheric O3 enhanced by long-range transport is mixed to surface.
Finding. Baseline O3 levels in the Northern Hemisphere are elevated by current anthropogenic emissions of NOx, CO, and VOCs. Multimodel studies calculate that a 20 percent reduction in these emissions from any three of the four major industrial regions of the Northern Hemisphere will reduce surface O3 in the fourth region by about 1 ppb on average, but with large spatial and seasonal variation. Results of similar magnitude are found for a 20 percent reduction in CH4 emissions. Unfortunately, the range of results across this multimodel ensemble is comparable to the average result. These calculations were conducted for 2001 and in the following eight years these emissions have changed by 20 percent or more.
Recommendation. Since models provide the only practical method of attributing a fraction of surface O3, and possible AQS violations, to different sources of pollution, a major effort must be made to calibrate, test, and improve these models with a wide range of atmospheric chemistry measurements ranging from individual events to climate statistics of air pollution. These tests must span the range of necessary modeling from global transport and stratosphere-troposphere exchange down to the urban airshed. While improving the models is a priority, realistically quantifying their uncertainty is key. Development of adjoint and ensemble modeling approaches will be important in assessing NAAQS compliance and intracontinental pollution under rules such as the U.S. EPA Clean Air Interstate Rule.
Question: What are the potential implications of long-range O3transport for U.S. environmental goals?
Finding. U.S. NAAQS violations (e.g., 8-hr average greater than 75 ppb) are caused primarily by a combination of regional emissions and unfavorable meteorology. These are augmented by a changing baseline and episodic events caused, for example, by wildfires, lightning NOx, occasional stratospheric intrusions, and distant anthropogenic emissions. Most violations are only a few ppb above the standard; thus the increase in baseline O3 since the preindustrial era driven by global pollution has contributed to these.
Finding. With high confidence we can state that elevated levels of O3 occur over populated regions due to distant pollution and such increases are detrimental at some level to human health, agriculture, and ecosystems. A preliminary study finds that about 500 premature cardiopulmonary deaths could be avoided annually in North America by a combined 20 percent reduction in NOx, CO, and VOC emissions from the other three major Northern Hemisphere industrial regions and, correspondingly, about 1800 in Europe. The uncertainty in these estimates is large, at least ± 50 percent, and reflects uncertainties in modeling both O3 change and health effects.
Recommendation. Perform additional research to (1) attribute quantitatively the surface O3 change to distant emissions; (2) specify the shape of the exposure-response curve (i.e., whether a threshold exists); (3) estimate avoided premature health burdens across a wider range of health outcomes; and (4) estimate avoided premature health burdens for particularly sensitive groups (i.e., children and others).
Question: What factors might influence the relative importance of U.S. and foreign contributions to O3change in the future?
Finding. Projected climate change will lead to a warmer climate with shifts in atmospheric circulation. All of these changes have the potential to impact air quality for better or worse:
-
Higher water vapor abundances associated with a warmer climate drives baseline O3 abundances down due to the more rapid photochemical destruction of O3 in the marine boundary layer and the tropics.
-
Projections of more rapid stratospheric circulation increases the flux of O3 into the troposphere, raising the baseline.
-
Warmer temperatures generate higher O3 pollution levels from local emissions.
-
For some regions, projected increase in wildfires can increase O3.
-
Changing tropospheric circulation, such as the northward shift of storm tracks at midlatitudes, may increase or decrease the number of stagnation events that lead to the worst pollution episodes.
-
Increasing convective activity and lightning will enhance NOx and baseline O3.
In general these changes reduce the role of nonlocal emissions while enhancing that of local emissions in AQS violations.
Recommendation. Test and improve the simulation of air quality, including pollution episodes in both global and regional climate models. Ensure that the climate models include global atmospheric chemistry so that the model ensembles run for climate change assessments can also be used to evaluate changes in air quality. Analyze the climate statistics that are specific to air pollution: boundary layer height, stagnation episodes, intercontinental transport, stratosphere-troposphere exchange, lightning, wildfires, and other natural emissions of O3 precursors.
Finding. In East Asia and much of the developing world O3 precursor emissions are expected to increase rapidly over the next few decades and would likely raise the surface O3 baseline in the United States by a few ppb. Methane increases, as projected and recently observed, if continued over the next few decades would additionally raise the O3 baseline by a few ppb.
Recommendation. (1) Develop and improve the accuracy of emissions inventories for the current epoch as well as projections for the next decade. Include also natural emissions and the development of interactive emissions models that depend on climate variables. (2) Establish a global, international measurement strategy (e.g., through WMO/GAW) based on surface sites and airborne platforms that can identify changes in regional emissions of the ozone precursors: CH4, NOx, CO, VOCs, and relevant secondary compounds such as PAN. (3) Merge these operational networks with satellite measurements and develop the modeling capability to provide reliable measures of emissions on a subcontinental scale. (See the discussion of integrated systems in Chapter 6.)
































