3
Particulate Matter
THE COMPLEX NATURE OF PARTICULATE MATTER
Unlike most other pollutants, particulate matter (PM) cannot be characterized by the space- and time-variations of the mass concentrations of a single compound. Important factors influencing PM transport and its environmental and health effects include the following:
Size and Morphology Environmental effects and lifetimes of particles vary with their size; thus, PM is classified by aerodynamic diameter. “PM10” is the mass of particulate matter with diameters smaller than 10 μm, and “PM2.5” or “fine PM” designates the fraction with aerodynamic diameters smaller than 2.5 μm; the “coarse” fraction is PM10 to PM2.5. Most attention has been focused on the fine fraction because it affects health, visibility, and radiative forcing. With lifetimes on the order of days to weeks, fine particulate matter can undergo long-range transport, producing global and regional in addition to local impacts. While particles larger than 10 μm are also found in the atmosphere, rapid removal generally limits their lifetime to the order of hours, and as they are too large to be respirable, their health impacts are considered of minor importance. Particles are often assumed to be spherical, whereas this is only the case for nonaggregated, liquid particles. Although not generally characterized, even in intensive measurement campaigns, particle shape and phase can influence radiative properties and health impacts.
Chemical Composition The varying chemical and physical nature of PM complicates the assessment of its impacts. Its composition depends on
the emitting sources or particle precursors and also on atmospheric conditions. Some PM components—Including nitrate species, organic species, and water—are semivolatile and repartition between the gas and particle phase depending on environmental factors such as temperature, relative humidity, or the composition of the PM. For modeling and monitoring purposes the composition of dry atmospheric PM is generally reduced to a few major categories. Commonly identified components include sulfates, nitrates, organic carbon, elemental or black carbon, sea salt, soil or crustal material, and specific elements of interest, such as Pb. The health effects associated with exposure to these individual components are not well characterized and are likely to vary signficantly.
Primary and Secondary Sources PM is emitted from both natural and anthropogenic sources, and its components are both primary (directly emitted) and secondary (formed in the atmosphere). Direct natural emissions come from wildfires, sea spray, and resuspension of organic matter such as leaf litter. The first of these produces primarily PM2.5, while the latter two are mainly PM10. Mineral dust has both natural and anthropogenic origins: it is lofted from arid and semiarid regions and can be mobilized by agricultural or construction activities. Its emission rates are especially susceptible to climate conditions. Combustion of fossil fuels and biofuels is a large primary anthropogenic source. Combustion processes are the only sources of black carbon, which together with “brown” carbon1 has an important role in PM light absorption. Sources of secondary PM precursors (gases leading to particulate matter through atmospheric reactions) include gaseous vegetative emissions, motor vehicle emissions, and wood-smoke emissions. Reduced sulfur and nitrogen compounds are oxidized to the particulate components sulfate and nitrate, respectively. Ammonium is a common cation (positively charged ion) incorporated from the gas phase into the particle phase to neutralize these acid secondary species, although sodium, calcium, and other cations derived from sea salt or minerals are also often present.
Mass or Number? Regulations and many measurement strategies have focused on characterizing the mass concentrations of PM, but for some health and welfare effects size or number concentrations may be the more relevant characteristic. This question is still unresolved. As discussed above PM differs from gaseous pollutants because it is a complex mixture with
wide variations in chemical composition and particle sizes. Their chemical composition reflects the particles’ origin as well as processing that has occurred during their lifetimes in the atmosphere. This report focuses mainly on particles with aerodynamic diameters below 2.5 micrometers (PM2.5) because they can be transported over much longer distances than larger particles, and because they have stronger effects on the health and environmental impacts of interest. Further, we focus on the regulated and therefore most available observed properties, mass concentrations, and bulk chemical compositions of PM, noting that other properties may assume more importance in the future as understanding of PM effects increases and regulations are accordingly revised. For example, PM acts as a vehicle for the atmospheric transport and inhalation of toxic substances such as heavy metals (Murphy et al., 2007) and PAHs (see Chapter 5).
The chemical composition of PM2.5 offers clues to its sources and atmospheric chemistry, and chemical characterization is critical to understanding the environmental impacts of present and future sources. Numerous measurements of PM composition have been published (Kim et al., 2000; Edgerton et al., 2006; Ondov et al., 2006). These are complemented by measurements of various components of PM emitted by specific sources (Andreae and Merlet, 2001; Watson et al., 2001; EPA, 2006b), including advanced measurements that characterize individual particles (Sodeman et al., 2005).
Mass concentrations of each species and size fraction are measured and reported using operational definitions and techniques (generally filter based) (e.g., Pitchford et al., 2007), which give an idea of the overall dry composition of the sampled size fraction. It is important to bear in mind that water can be the dominant (and unmeasured) PM constituent above ~ 90 percent relative humidity and sampling and analysis difficulties are known to be present for elemental carbon, organic carbon, and nitrate species, making their routine quantification less certain. More sophisticated measurements that are applied in intensive field studies are able to provide better characterization of the PM than that possible from filters, such as the mixing state of species within individual particles (Murphy et al., 2006) and the presence of trace quantities of toxic species (Moffet et al., 2008).
In this chapter we favor the use of the word “baseline” to indicate a potentially observable quantity that represents the cleanest 20 percent of daily surface observations. The word “background” implies an atmospheric concentration that occurs in the absence of modern anthropogenic sources; while models may be used to provide such a distinction, measurements are presently indifferent to this fractionation.
SOURCES OF PARTICULATE MATTER
Figure 3.1 shows the major sources of primary emissions of PM2.5 in the United States. Major components of these particles include sulfate, nitrate, black carbon, organic carbon, and mineral dust; the latter two are themselves complex mixtures. Figure 3.2 summarizes the global sources of major non dust PM components, including sources leading to secondary production of sulfate, nitrogen, and organic PM components. Secondary organic aerosol (SOA) precursor gases may originate naturally from plants (biogenic emissions) (Guenther et al., 2000), from open burning of biomass (Grieshop et al., 2008), or from energy-related sources (Robinson et al., 2007). Neither the reactions nor the exact compounds leading to formation of SOA are fully understood (Donahue et al., 2009), as demonstrated in recent comparisons of modeling and observations (e.g., Heald et al., 2006).
We note that the commonly measured major components included in Figure 3.2 do not fully characterize the often complex chemical nature of PM, and some of the minor species present are known to be toxic to humans or the environment. For example, the organic carbon fraction may contain POPs (see Chapter 5), a category that encompasses pesticides and polycyclic aromatic hydrocarbons, among other toxic species. Many metals have been detected in PM, including lead, zinc, mercury, and vanadium (e.g., Moffet et al., 2008). While these elements generally contribute only minimally to total
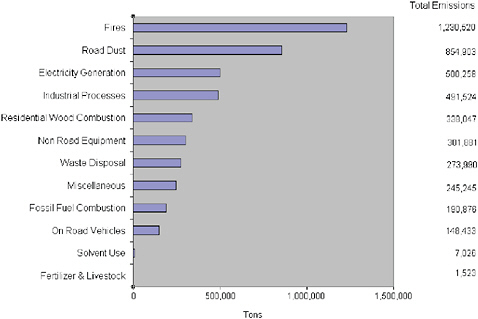
FIGURE 3.1 National primary emissions of PM2.5 by sector.
SOURCE: EPA, http://epa.gov/air/emissions/pm.htm.
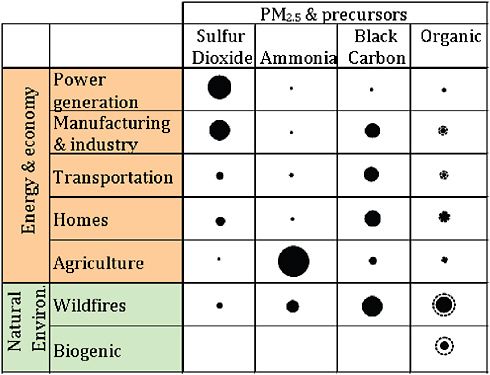
FIGURE 3.2 Global sources of PM2.5 components and precursors. Areas of black dots are proportional to fraction of each compound originating from individual sources; that is, dots in each column sum to the same total area. Sulfur dioxide (a precursor) leads to sulfate, while PM nitrogen species result from complex reactions involving ammonia and other nitrogen compounds, including organics. In the column for organic carbon, dashed circles show the approximate contribution of secondary organic aerosol formed from precursors. Values are from Bond et al. (2004), Cofala et al. (2007), and Heald and Spracklen (2009); secondary organic aerosol values are adapted from de Gouw and Jimenez (2009).
PM mass, the trace amounts carried by particulate matter can represent biologically significant levels and their presence in fine particulate matter means they may be able to lodge deeply in the lungs if inhaled.
PARTICULATE MATTER IMPACTS
Human Health Impacts Exposure to elevated levels of PM has long been associated with adverse human health impacts, and is a major source of morbidity and mortality worldwide. A European study using exposure-response functions for a 10 μg m–3 increase in PM10 found that this increase
accounted for 6 percent of total mortality, as well as more than 25,000 new cases of chronic bronchitis in adults, more than 290,000 episodes of bronchitis in children, more than 500,000 asthma attacks, and more than 16 million person-days of restricted activities in Austria, France, and Switzerland (Künzli et al., 2000). Health effects have been observed at all exposure concentrations, indicating a wide range of susceptibility and that some people are at risk even at the lower end of observed concentrations. There is convincing and consistent evidence that short- and long-term exposures to particulate matter can cause a wide range of adverse health effects. Effects related to short-term exposure include lung inflammatory reactions, respiratory symptoms, adverse effects on the cardiovascular system, increase in medication usage, and increase in mortality. Effects related to long-term exposure include increase in lower respiratory symptoms, reduction in lung function in children and adults, increase in chronic obstructive pulmonary disease, and reduction in life expectancy due mainly to cardiopulmonary mortality but also to lung cancer (WHO, 2006).
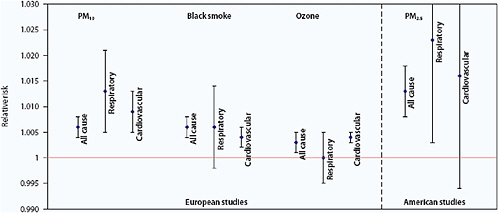
Figure 3.3 shows summary estimates for relative risks for mortality caused by different air pollutants. Particulate mass, particularly the mass in the smaller particles (e.g., PM2.5), show the highest relative risk for mortality. It remains unclear what components or characteristics are responsible for observed adverse health effects, with hypotheses including the presence of metals, sulfates, and other acidic species, or simply size (smaller than 0.1 μm) regardless of composition. Studies are inconsistent in attributing the health effects, whether to gaseous co-pollutants, particles themselves, trace elements, or toxic substances such as benzene and manganese (Samet and Krewski, 2007).
Recent studies estimated a 0.21 percent increase in deaths per day per 10 μg m−3 increase in PM10 exposure, and an increase of approximately 4-8 percent in long-term risk of death for each 10 μg m−3 rise in annual PM2.5 concentrations (Kaiser, 2005; Krewski et al., 2009). Comparing data on life expectancy, socioeconomic status, and demographic characteristics for 51 U.S. metropolitan areas with data on PM2.5 concentrations for the late 1970s to early 1980s with the late 1990s to early 2000s, Pope III et al. (2009) concluded that a decrease of 10 μg m−3 in the concentration of PM2.5 was associated with an estimated increase in mean life expectancy of 0.61 ± 0.20 year. A similar analysis in Europe, using a concentration-response function of an increase in risk of all-cause mortality by 6 percent per 10 μg m−3 of PM2.5, found that current exposure to PM from anthropogenic sources leads to an average loss of 8.6 months of life expectancy (WHO, 2006). The total number of premature deaths attributed to PM exposure was about 348,000 in 25 countries. Effects other than mortality, including some 100,000 annual hospital admissions, were also attributed to exposure. The study further concluded that PM from long-range transport
of pollutants contributes significantly to these effects. Recently Corbett et al. (2007) estimated that shipping-related PM emissions contribute ~ 60,000 excess deaths annually worldwide, primarily in coastal regions, which face greater exposure to emissions from port areas, and generally have higher population density (see Chapter 6).
Ecosystem Impacts Wet and dry deposition of particulate-phase sulfate and nitrate species constitute major contributions to the atmospheric inputs of sulfur, nitrogen, and acidity to ecosystems. Particles can also serve as vehicles for the atmospheric transport and ultimately deposition of other pollutants, such as heavy metals and POPs. The interactions of atmospheric aerosols with solar radiation can affect rates of photosynthesis in complex ways (Cohan et al., 2002).
Visibility and Radiative Forcing Impacts Submicron particulate matter is especially efficient at extinction of visible and ultraviolet radiation and represent the major contribution to visibility reduction in both rural and urban settings. This same mechanism is the basis for PM direct radiative forcing effects, that is, their impact on global radiation budget. The impacts of PM on radiative forcing, and the response of that forcing to future changes in PM emissions and lifetimes, are fundamentally different from those of greenhouse gases (GHG). First, while most GHGs are long-lived, PM has a short lifetime, so its influence on radiative budgets is more variable in space and time. Second, the direct radiative impacts of PM can be either warming or cooling, depending on the optical properties.
Global average impacts The Intergovernmental Panel on Climate Change (Forster et al., 2007a) produces regular assessments of radiative forcing (RF) and climatic impact. Aerosols generally have a net negative climate forcing because they reflect sunlight away from Earth. Exceptions with positive RF are strongly absorbing aerosols such as black carbon, or aerosols with even a small amount of absorption that are located above clouds. Because the sign of forcing (positive or negative) varies with different types of aerosol particles, mitigation (or increased emissions) of these different aerosols will have very different impacts on climate.
IPCC (2007) most recently estimated the direct RF of all anthropogenic aerosols as − 0.5 W/m2. Compared with greenhouse gas forcings of + 2.6 W/m2, this value is smaller but not insignificant. If recent findings regarding the ubiquity of organic aerosol (Heald et al., 2005) were linked with rapid secondary organic aerosol formation from anthropogenic sources (e.g. Robinson et al., 2007), the RF estimates would become slightly more negative. Several indirect links between PMs and climate have been postulated, because particles serve as sites for the formation of
liquid and ice-cloud particles. These include PM-induced modifications to cloud frequency, altitude, lifetime, microphysical and radiative properties, and effects on precipitation. Pollution aerosols are estimated to modify clouds to have smaller, more reflective droplets, with an additional negative forcing estimated at about −0.7 W/m2 (IPCC, 2007) with a ~ 50 percent uncertainty.
Regional impacts Global average forcing is only a partial measure of aerosol-climate interactions. Aerosol RF near source regions can be greater than global average RF by an order of magnitude, and RF reflects changes at the top of the atmosphere, ignoring redistribution of energy within the atmosphere (Satheesh and Ramanathan, 2000). Regional impacts of aerosols may include shifts in rainfall distribution (Rotstayn and Lohmann, 2002; Wang, 2007) or energy available to drive the hydrologic cycle (Meehl et al., 2008). Thus, the net aerosol impact on climate is not neatly represented by radiative forcing (NRC, 2005).
Climatic impacts of transported PM The source-receptor approach described by HTAP-TF (2007) might also be used to indicate the impact of emissions in one region on RF, optical depth, or column burden in another region. Aerosol optical depths above downwind continents can be ~ 10-20 percent of those in source regions (Koch et al., 2007). Model calculations of Reddy and Boucher (2007) predicted that 20-30 percent of the black carbon column loading in East Asia, North America, and Europe was imported from other regions. RF by aerosol and gaseous emissions from isolated regions and sectors can be of the order of 100-400 mW/m2 (Shindell et al., 2008a). These perturbations are small but not insignificant; radiative impacts of one region on another region are of the same magnitude as global average aerosol forcing.
Despite the fact that aerosol PM concentrations vary regionally, recent studies on the impact of aerosol distributions on climate suggest that this response should be considered in a global context. Levy et al. (2008) and Kirkevåg et al. (2008) have found that climate simulations using aerosol loadings projected to the year 2100 using the SRES A2 scenario produce regional patterns of surface temperature warming that do not follow the regional patterns of changes in aerosol emissions, tropospheric loadings, or radiative forcing. Rather, the regional patterns of warming from aerosols are more similar to the patterns for well-mixed greenhouse gases.
Sensitive regions For regions with no internal sources of pollution, intercontinental transport from industrialized regions represents the only anthropogenic impact. For example, transported aerosols and other pollutants contribute to haze in the Arctic (Law and Stohl, 2007), which has both
warming and cooling components (Quinn et al., 2008). Absorbing aerosols in the haze when deposited on snow may hasten the onset of spring melt (Flanner et al., 2007). The connection between transported pollution and impacts on sensitive regions is an area of active research. Transport from lower latitudes to the Arctic is particularly uncertain in comparison with other source-receptor relationships (Shindell et al., 2008b).
THE REGULATORY CONTEXT FOR CONTROL OF PM
The import of PM into the United States has the potential to affect the ability to achieve compliance with U.S. regulations, which are aimed at both limiting ambient levels of PM to protect human health and welfare and at controlling emissions from new and existing sources. At the present time welfare-based standards for pollutants apply only to visibility impairment and potential crop damage and not to direct or indirect radiative forcing impacts.
Surface Concentrations: Primary Standards Current U.S. health-based (primary) NAAQS for ambient concentrations of particulate matter focus on dry, unspeciated PM mass concentrations and exist for both PM10 and PM2.5. The most recent revision of the U.S. particulate matter standard, promulgated in 2006, tightened the 24-hr fine particle (PM2.5) standard from 65 μg m−3 to 35 μg m−3 and retained the annual fine particle standard at 15 μg m−3. To be in compliance with the annual standard the 3-yr average of the weighted annual mean PM2.5 concentrations from single or multiple community-oriented monitors must not exceed 15 μg m−3, while to attain the 24-hr standard, the 3-yr average of the 98th percentile of 24-hr concentrations at each population-oriented monitor within an area must not exceed 35 μg m−3. The existing 24-hr PM10 standard of 150 μg m−3 was retained, but the annual PM10 standard was revoked due to the lack of clear evidence linking long-term exposure to PM10 with adverse health impacts. Figure 3.4 compares the 24-hr PM2.5 ambient standards for the United States, Canada, Mexico, and the World Health Organization. U.S. standards are higher than WHO-recommended levels, and may undergo further downward revision as epidemiological data continue to be collected and analyzed; the relative role of non-U.S. sources will assume more importance as standards are tightened.
We note here that even relatively small fractional contributions of PM, as may occur from long-range transport of PM, can be significant in national control strategies. For example, the U.S. Prevention of Significant Deterioration (PSD) Act and Clean Air Interstate Rule (CAIR) specify allowable incremental emissions for new U.S. sources. Figure 3.4 shows the proposed allowable 24-hr PM2.5 emissions increment (2 μg m−3) for
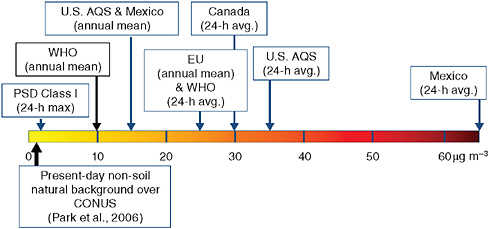
FIGURE 3.4 Comparison of current 24-hr health-based PM2.5 standards for the indicated countries, and U.S. allowable 24-hr emissions increment for Class I areas under the Prevention of Significant Deterioration rule.
new sources impacting Class I areas under PSD; the annual mean allowable increment is 1 μg m–3. Under CAIR, EPA considers an upwind state to contribute significantly to a downwind nonattainment area if the state’s maximum contribution to PM2.5 in the area is ≥ 0.2 μg m3.
Surface Concentrations: Secondary Standards U.S. secondary standards are set to protect human welfare, including visibility. In contrast to the NAAQS approach, which requires all areas of the nation to demonstrate that ambient pollutant concentrations are below a specified standard, the regional haze rule (passed in 1999) addresses only national parks and wilderness areas. State and federal agencies are required to improve visibility in these areas toward “natural background conditions” by 2064. Background conditions are those unaffected by anthropogenic emissions, so these may include impacts from sources such as fires and dust, including PM that has undergone long-range transport. Rising anthropogenic PM emissions from other countries that impact U.S. PM concentrations could present significant roadblocks to the achievement of designated background visibility conditions (Park et al., 2006), a complication not clearly addressed in the existing U.S. regulatory framework.
Emissions Standards Primary PM emissions from on-road and nonroad mobile sources have been specifically targeted for reductions in the United States. The EPA established progressively more stringent mobile-source PM emission standards beginning in the mid-1970s for on-road
vehicles and in the early 1990s for non-road engines and equipment. With the designation of diesel exhaust as a likely human carcinogen, a particular focus in recent years has been establishing emission standards for diesel engines. Diesel PM is found primarily in the PM2.5 fraction, and studies show that as sulfur is removed from diesel fuel, the sizes of emitted particles become smaller. Black carbon is a main components of diesel exhaust and its ability to absorb shortwave radiation contributes a positive radiative forcing.
Other emission-based U.S. regulations include acidic deposition regulations, which are tied to PM concentration levels because the regulated pollutants—sulfur dioxide and nitrogen oxides—are precursors of secondary PM. Reductions in these species achieved as a result of acid deposition regulations have already resulted in improvements in ambient PM concentrations in many parts of the United States (see Chapter 1). International shipping activity is an example of currently unregulated emissions that have the potential to significantly affect PM in some regions. For example, auxiliary diesel engines operating within 24 nautical miles of the ports of Long Beach and Los Angeles are believed to be an important source of PM, nitrogen oxides, and sulfur to the southern California region (Minguillón et al., 2008), accounting for 10-44 percent of the non-sea-salt sulfate found in fine PM in coastal California (Dominguez et al., 2008). Corbett et al. (2007) estimated annual average contributions to PM2.5 from international shipping operations as high as 2 μg m−3 in some heavily traveled coastal regions (see Chapter 6 for further discussion of the role of shipping emissions in U.S. air quality).
Current Non-U.S. PM Policies Future trends in PM emissions in various regions around the globe are tied to the air quality standards in those regions. This linkage occurs because not only the levels of emissions but also the choices of pollutants and sources to target for controls in national emission plans are driven by air quality standards. The PM standard is a good example and as shown in Figure 3.4 the PM2.5 standard varies by country. Furthermore, the United States is the only country in the world with a visibility standard, and many countries, including most in Asia, do not have a PM2.5 standard. This is important, as smaller particles (such as PM2.5) can be transported over longer distances than larger particles, yet PM10 standards may emphasize sources that emit primarily coarse particles.
KEY PM TRANSPORT PATTERNS AND EFFECTS ON SURFACE CONCENTRATIONS
Local and regional emissions are responsible for most PM concentrations that exceed air quality standards, but it is now recognized that emis-
sions and transport at the intercontinental and global scales can lead to episodic spikes in concentrations that cannot be attributed solely to U.S. emissions. Seasonal transport patterns are discussed in Chapter 1, and Appendix B discusses the various mechanisms that influence the vertical mixing of pollutants. Although there are many parallels between the patterns of transport of ozone and PM into and out of the United States, one important difference is that emissions of PM are efficiently depleted by deposition processes, especially wet deposition (e.g., Zhao et al., 2008 and numerous earlier references), resulting in low and highly variable baseline concentrations. Depositional losses are lowest along transport pathways occurring at higher altitudes and having faster windspeeds, and thus such air masses are responsible for transporting PM mass to the United States in concentrations that clearly exceed expected baseline conditions. This transport has been unambiguously identified primarily in large-scale dust plumes but also occasionally in plumes from wildfires or industrial pollutants. Although relatively infrequent, such extreme events can nonetheless be highly policy relevant, since current U.S. standards allow only a limited number of exceedances to be omitted from averages used to determine compliance with the NAAQS. These events also highlight the possibility that more persistent but less readily discerned transport may be occurring at other times, contributing small but potentially important incremental increases to surface PM concentrations.
Based on analyses of transport patterns and of the nature and composition of PM, extreme events clearly attributable to non-U.S. sources have been documented. Here we summarize present knowledge of transport pathways and patterns of PM into the United States, as discussed extensively in HTAP-TF (2007).
Asian Dust and Pollution Plumes containing PM originating from Asian sources primarily affect the western United States. Transport that is rapid and dry enough to retain PM in transit across the Pacific Ocean occurs in the midtroposphere (see Figure 1.2). In the continental United States the enhanced PM from these plumes is most clearly observed at higher-elevation sites (e.g., VanCuren and Cahill, 2002). Episodic high-concentration dust events are most frequently observed at the surface in springtime because of increased Asian dust mobilization and favorable transport pathways during that season (Wells et al., 2007), although some studies suggest that Asian emissions are transported annually to elevated sites across the United States year-round (VanCuren and Cahill, 2002; VanCuren, 2003; VanCuren et al., 2005). A few extreme events of Asian dust transport have been documented, including the large April 1998 plume (Husar et al., 2001) that was tracked across the United States and an even larger plume that was observed in April 2001 and was responsible for sig-
nificant increments in PM10 (Jaffe et al., 2003c). Even a dust cloud from China, lofted to the upper troposphere, was transported more than one full circuit around the globe (Uno et al., 2009).
A recent analysis of surface PM data in the western United States, combined with satellite data, has identified significant interannual variations in the amount of transported Asian dust and pollution (Fischer et al., 2009). This analysis indicates that variations in transport and dust emissions could explain ~ 50 percent of the interannual variations in PM2.5 concentrations at IMPROVE sites in the western United States. In high dust years average PM2.5 concentrations at these sites were 3.7 μg m−3 (spring average), compared with 2.2 μg m−3 in low-dust years (Fischer et al., 2009). Some instances of elevated nondust PM have also been reported at West Coast sites (e.g., Jaffe et al., 2005b) and attributed to sulfur and other Asian industrial emissions (Bertschi and Jaffe, 2005; Weiss-Penzias et al., 2006, 2007; Hadley et al., 2007). Results from spring 2006 showed that episodic long-range transport events from Asia can contribute more than 1.5 μg m−3 of aerosol sulfate to coastal western Canada surface concentrations. The springtime mean contribution of the long-range transport of sulfate from Asia to surface PM levels in western Canada is estimated to be ~ 0.3 μg m−3 (van Donkelaar et al., 2008; Heald et al., 2006).
North African Dust Dust from North African sources is mobilized year-round (Prospero et al., 2002), but transport pathways vary seasonally. The direction of westward North African dust transport is tied to the shifts in position of the Bermuda high, with plumes affecting Amazonia in the Northern Hemisphere winter, and transport shifting northward during the summer months. Summertime dust events, with transported dust concentrations generally greater than 10 μg m−3, have been documented in the southeastern United States, occasionally extending into Texas and the mid-Atlantic states (e.g., Perry et al., 1997). NAAQS exceedances that may have occurred because of the incremental input of North African dust have been documented in the southeastern United States (Prospero, 1999a,b; Prospero et al., 2001). Liu et al (2009) uses source-receptor relationships to quantify the health impacts of the intercontinental transport of dust.
Asian and Canadian Wildfires The role of CO emissions from Siberian wildfires in influencing interannual variations in the Northern Hemispheric CO budget was demonstrated by Wotawa et al. (2001). This work highlighted the large potential perturbations to tropospheric chemistry, including PM concentrations, that are driven by high-latitude wildfires (Wotawa and Trainer, 2000; Morris et al., 2006). PM from Canadian wildfires has been observed in the Great Lakes region and linked to air quality degradation (Al-Saadi et al., 2005). A recent extreme event of smoke transport
from Canadian fires into Washington, DC, has been documented (Colarco et al., 2004). PM2.5 NAAQS were close to being exceeded as a result of this transport (Bein et al., 2008). Emissions from the large 2003 Siberian fires were transported to the U.S. Pacific Northwest and contributed significantly to surface PM concentrations and to an 8-hr O3 average greater than 90 ppbv (Jaffe et al., 2004). These fires also resulted in significant aerosol loading at the surface, with submicron aerosol scattering coefficients of up to 80 Mm−1, or approximately 27 μg m3 (Bertschi and Jaffe, 2005).
Mexican and Central American Agricultural and Wild Fires Agricultural burning occurs each spring (April and May) in Mexico and Central America (Mendoza et al., 2005). Annual but variable impacts on PM concentrations in national parks across the southwestern United States have been documented (Gebhart et al., 2001; Park et al., 2003), but the impacts of these sources on NAAQS exceedances in the region are unknown (Choi and Fernando, 2007). In some years drought and weather conditions have caused burns to erupt into uncontrolled wildfires, and these extreme events are more readily identified in observations. For example, in 1998 shifting weather patterns clearly transported smoke from such wildfires into the United States (Kreidenweis et al., 2001; Park et al., 2003; In et al., 2007), where it was tracked northward and into the eastern United States (Peppler et al., 2000; Falke et al., 2001).
Transport and Deposition into U.S. Arctic Regions. Short-lived species such as PM have been implicated in the magnification of Arctic warming (Quinn et al., 2008) through their direct and indirect effects on the radiation budget and through deposition of absorbing PM (black carbon) to snow and ice surfaces, where they can accelerate melting (Shindell, 2007). Transport and deposition of PM to the Arctic depends on the season, with varying contributions from the transport of European, East Asian, South Asian, and North American pollution, as well as dust and wildfire emissions (Quinn et al., 2008). The results from a coordinated comparison of 17 models (Shindell et al., 2008b) suggested that European emissions dominate aerosol transport to the surface in the Arctic, whereas East Asian emissions dominate in the upper troposphere.
MODELING AND ATTRIBUTION OF PM TRANSPORT AND TRENDS
Trends in PM Concentrations Data from monitoring networks have been analyzed to attempt to discern trends in PM surface concentrations. Prospero et al. (2003) found that trends in non-sea-salt sulfate concentrations from a 20-year PM record from Midway Island in the North Pacific followed trends in SO2 emissions from China, suggesting a similar trend
might be expected in PM imported from Asia to the United States. In contrast, the analyses of Jaffe et al. (2005b) using PM data from four sites in the western United States showed no apparent long-term trend over a similar time period. In general, trend analyses of IMPROVE data (e.g., Malm et al., 2002) are either inconclusive or show trends that appear to be more closely tied with changing U.S. emissions (Schichtel et al., 2001). For example, DeBell et al. (2006) found 10-year (1989-1999) statistically significant decreasing trends in sulfate concentrations in Class I areas throughout much of the contiguous United States, linked to decreasing U.S. SO2 emissions. A recent study by Wang et al. (2009b) analyzed visibility datasets from around the globe and found evidence for a global increase in aerosol optical depth from 1973 to 2007, despite a net decrease over Europe over this same period. The conclusions of DeBell et al. (2006) for surface PM are also different from this global estimate: they reported statistically-significant improving trends from 1995 to 2004 for the best visibility (generally lowest-PM) days at 17 IMPROVE sites in the western United States, including Alaska, suggesting baseline PM concentrations in those locations were not increasing over that time period.
Space-Based Observations Satellite imagery has long been used to observe transport of PM such as pollution, smoke, and dust plumes from space, but only recently have attempts been made to use remote sensing products to obtain quantitative estimates of transported PM mass. Yu et al. (2008) combined MODIS aerosol retrievals with analyzed wind fields to estimate fluxes of pollution (nondust) PM leaving Asia and arriving in North America. They further showed that these estimates were very similar to those computed independently from a global model, and the imported fluxes are about 15 percent of local emissions from the United States and Canada, as also estimated by Chin et al. (2007). Yu et al. (2008) note large uncertainties on the order of a factor of 2 and stress that the estimates are column amounts and cannot be immediately applied to estimate effects on surface air quality. One complication is that water can represent the dominant mass fraction of PM2.5, and its concentration in PM varies strongly with aerosol composition and ambient relative humidity, making it difficult to account for its presence from space-based observations alone. There is also increasing interest in using satellite observations of aerosol optical depth to infer information regarding surface PM2.5 concentrations. Current experience suggests that PM2.5 surface concentrations are proportional to satellite aerosol observations for regions where aerosol composition and vertical profiles are relatively stable (e.g., eastern United States), but this relationship weakens where aerosol composition and vertical profile have large variations (e.g., western United States) (Al-Saadi et al., 2005). Martin (2008) discusses application of remote sensing to monitor surface
air quality, including a review of current techniques and criteria for future instruments aimed at this problem.
Modeling of PM Concentrations As indicated above, it remains difficult to evaluate the influence of non-U.S. sources on PM solely from present-day observations, particularly to discern those sources that might contribute relatively small increments to surface PM concentrations. Extreme events are the most readily identified and documented from observational datasets and exhibit large interannual variability due to large-scale influences, such as drought and general circulation changes (e.g., Prospero and Lamb, 2003; Fischer et al., 2009). Nevertheless, because monitoring sites are fixed and transport altitude and latitude can vary, observations are likely to miss even extreme events if monitoring sites are not located directly in the transport pathway. Modeling can help to fill in observational gaps and attribute PM increments to source regions. CTMs provide a means to estimate four-dimensional aerosol distributions based on an emission distribution and the governing meteorological fields. There are several recent activities to compare and evaluate PM predictions using CTMs, including the AEROCOM study designed to evaluate the main uncertainties in global aerosol models (Kinne et al., 2006; Schulz et al., 2006; Textor et al., 2006), the Model Intercomparison Study in Asia (MICS-Asia) (Carmichael et al., 2008a), and the HTAP study (HTAP-TF, 2007). There are also reviews focused on specific components of PM, for example, the review of secondary organic aerosol modeling by Kanakidou et al. (2005), and the intercomparison of dust predictions (Uno et al., 2006).
The treatment of PM in CTMs has become more complex in recent years (Kinne et al., 2006). Many models now describe PM as composed of several chemical species that are distributed among particles of diameters from nanometers to several microns. Most of the model comparisons have focused on model-to-model comparisons with limited in model-to-observation comparisons. This is due in large part to the limited number and sparse global coverage of PM composition measurements. The most extensive comparisons with measurement have focused on global datasets of aerosol optical depth from satellites and Sun photometers. Interestingly, the total aerosol optical depth in most models is comparable to observation-based estimates (Kinne et al., 2006), whereas the underlying aerosol fields of sulphate, organic matter, dust, and sea salt showed a much larger variation. Thus speciated PM observations are needed to better evaluate and constrain models.
In general, the predictive skill of CTMs for PM is poorer than that for ozone. For example, the skill of seven regional CTMs to predict surface PM2.5 and ozone was recently evaluated against observations collected during August and September 2006, through the AIRNow network
(Aerometric Information Retrieval Now) in eastern Texas and adjoining states (McKeen et al., 2009). Ensemble O3 and PM2.5 forecasts created by combining the seven separate forecasts with equal weighting and simple bias-corrected forecasts were also evaluated in terms of standard statistical measures, threshold statistics, and variance analysis. For O3 the models and ensemble generally show predictive skill relative to persistence for the entire region but fail to predict the highest O3 events in the Houston region. For PM2.5 none of the models nor their ensemble showed skill relative to persistence, and the models showed significant bias.
There are many reasons for the difficulty in prediction of PM and the large diversity in the individual PM component fields predicted by different models. For example, there are large uncertainties in the emissions of particles and their precursors (as discussed already in this chapter). In addition, there remain gaps in our understanding of the chemistry of PM formation and the rates by which PM is removed by dry and wet processes, stemming in large part from the difficulty in modeling cloudiness and precipitation. While the relative sources of uncertainty vary from species to species, in general the uncertainties (normalized: uncertainty/mean) are large by factors of 2 to 6 (Bates et al., 2006).
Only a few studies have explored source-receptor relationships for aerosol emissions and burdens on continental scales. The HTAP report (HTAP-TF, 2007) summarizes this work and presents the most comprehensive multimodel analysis of source-receptor relationships on hemispheric scales. Figure 3.5 summarizes the results from the model calculations in terms of the extent to which 20 percent perturbations in local and distant anthropogenic emissions of aerosol precursors and aerosol primary emissions contribute to mean surface PM2.5, total sulfate deposition, and sulfate column loadings over several receptor source regions: North America (NA), Europe (EU), South Asia (SA), and East Asia (EA). It should be pointed out that these results are the annual and NA mean values. The results for a specific location and period of time may be significantly different from the mean. In Figure 3.6 these same data are presented as import sensitivities, defined as the sum of the changes in the quantity due to 20 percent perturbations in anthropogenic emissions in all other regions, divided by the change in that quantity due to 20 percent changes in domestic emissions. The +/− 1 standard deviation between the model results (10 models) are also shown and indicate that there is a large diversity among models in the attribution of the role of distant sources.
For North America the relative contribution from all distant sources (Europe, East Asia, and South Asia added together, divided by the contribution from NA sources) to surface PM2.5, total sulfate deposition, and sulfate column loadings is estimated as 5, 9, and 33 percent, respectively. Shown in Figure 3.7 is the relative contribution of NA emissions to surface PM2.5
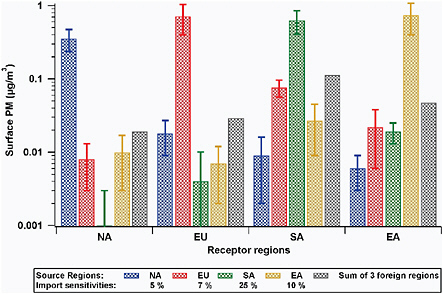
FIGURE 3.5 Increments in modeled surface PM2.5 concentrations in several regions (NA = North America, EU = Europe, SA = South Asia, EA = East Asia), computed for 20 percent increases in emissions from the indicated source regions (colored bars). The error bars indicate +/− 1 one standard deviation among the 10 participating models. Note the log scale on the y-axis.
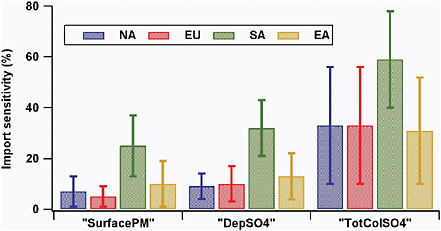
FIGURE 3.6 Import sensitivities for the various regions in Figure 3.5, defined as the sum of the changes in the quantity due to 20 percent perturbations in anthropogenic emissions in all other regions, divided by the change in that quantity due to 20 percent changes in domestic emissions, expressed as a percentage. “SurfacePM” = surface concentrations of PM2.5; “DepSO4” = sulfate deposited to the surface; “TotColSO4” = total column sulfate loading.
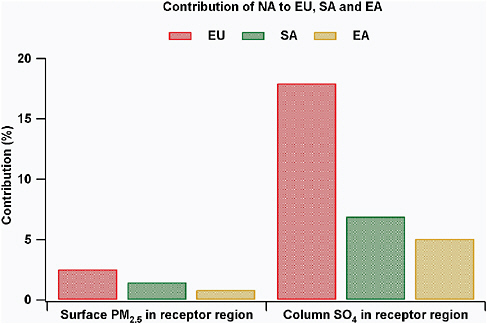
FIGURE 3.7 The contribution to surface PM2.5 and sulfate column amounts (expressed as a percentage) of North American emissions relative to domestic emissions in the receptor region (e.g., for EU = ΔNA/ΔEU).
and sulfate column amounts due to changes in domestic emissions (e.g., for EU = ΔNA/ΔEU). NA contributions range from 1 to 3 percent for surface PM and 5 to 17 percent for column sulfate, with the contributions to EU>SA>EA. The increase in import sensitivity from surface concentrations to column loadings indicates the substantial role of aerosol transport above the planetary boundary layer in the long-range transport of PM. The column aerosol loadings play an important role in radiative forcing. The fact that the import sensitivity of total column aerosol load is significantly larger than that for the surface concentrations means that regional aerosol radiative forcing and climate change are more greatly affected by distant sources than are air quality goals.
The HTAP comparison results provide insights into how the uncertainties in the models impact the source-receptor relationships. The chemical, removal, and emission processes all affect the lifetime of the species in the atmosphere. The impact of one region on another depends on the transport pathways, their length, and the intensity of the removal processes that the PM experiences along its way. The model-to-model diversity of sulfate lifetimes in absolute terms for a given region is considerable (a factor of
four), and reflects differences in the process level model formulations. In general, models that predict longer lifetimes predict larger contributions from distant sources. In addition, emissions from North America and East Asia appear to be slightly more efficiently removed than those from Europe and South Asia. These results suggest the need to develop observational techniques to constrain the lifetime of PM in the atmosphere, which would provide a strong metric for evaluating and improving models.
The use of the PM source attribution information in the assessment of impacts is just beginning. For example, Liu et al. (2009) use tagging to identify intercontinental source-receptor relationships, and estimate that approximately 90,000 premature deaths (for adults age 30 and up) globally and 1,100 in North America are associated with exposure to nondust PM2.5 of foreign origin. It is worth noting that this number of premature mortalities in North America is comparable to the reduction in premature mortalities expected to result from tightening the U.S. 8-hr O3 standard from 84 ppbv to 75 ppbv.
FUTURE PROJECTIONS OF FACTORS INFLUENCING PM SURFACE CONCENTRATIONS
A 20 percent increase in emissions in Europe, East Asia, and South Asia is estimated to change the annual PM2.5 concentrations at the surface in North America by ~ 0.02 μg m−3 (HTAP-TF, 2007). This import contribution amounts to 2 percent of the proposed annual average PM2.5 PSD increment (1 μg m−3), and 50 percent of the proposed significant impact level, 0.04 μg m−3, in Class I areas. The significant impact level is the level of ambient impact that is considered to represent a significant contribution to nonattainment. Larger increments are allowed in other areas, thus implying the biggest relative impact from imported emissions in national parks and wilderness areas. However, the estimated ~ 0.02 μg m−3 represents 10 percent of the allowed interstate impact under CAIR. The emissions of major PM components and precursors (BC and SO2) are estimated to have increased in Asia by ~ 50 percent from 2000 to 2006 (Zhang et al., 2009a), over two times the 20 percent assumed in the modeling experiment. Regional and seasonal contributions can be much higher. Although the most significant impacts on U.S. air quality from long-range transport of PM are likely to be related to compliance with the regional haze rule, the impacts extend beyond Class I areas.
Although changes in baseline PM concentrations currently appear insignificant in comparison with local contributions to NAAQS exceedances in urban areas, as standards tighten a rising baseline would represent an increasing percentage of the total that cannot be controlled by domestic regulations. Furthermore, imported PM may represent a disproportionately
higher fraction of specific aerosol components that may be targeted in future regulations. Thus, shifting future priorities may enhance the relative importance of non-U.S. contributions to PM. It should also be noted that changes in emissions from foreign sources may change the magnitude of extreme events, in addition to the baseline changes mentioned above.
In Table 3.1 we provide rough estimates of future emission changes based on the knowledge available today. The remainder of this section describes the sources of the values in the table. Large uncertainties in these estimates can be expected, due to imperfect understanding about the trajectories of economy and technology, and about the responses of forests and deserts. The values here are provided only to evaluate whether future changes in intercontinental transport could be expected to significantly affect PM budgets in the United States. Most models that examine changes in future emissions due to anthropogenic global change compare present-day conditions with a doubled-CO2 climate (expected to occur ~ 2050) or with conditions in 2100. This study is focusing on a shorter time horizon, roughly to 2030; to approximate the response in 2030 we use half the change projected for 2050 (doubled CO2), or one-quarter of the change projected for 2100 (recognizing that this approach may miss some nonlinearities in the actual response). We rely on model results summarized by the recent Intergovernmental Panel on Climate Change report (IPCC, 2007) when possible.
TABLE 3.1 Possible Future Changes in Emissions of PM (see text for explanation of how numbers were derived).
Energy and Economy We use the sum of the increases in sulfur dioxide, black carbon, nitrate, and organic carbon emissions to estimate PM increases for 2030. Our range of estimates is based on the maximum feasible reduction and current legislation scenarios produced by IIASA (Cofala et al., 2007). These changes are −45 to +50 percent for East Asia, −80 to −50 percent for Central America, and −60 to −80 percent for the United States. A wider range of economic and technological scenarios has been explored for shipping emissions (Eyring et al., 2005), and that range is also given in the table. The contribution of secondary organic particulate matter is not included in this analysis, but estimates of future increases are less than 10 percent by 2030, based on 2100 estimates (Heald et al., 2008).
Dust Desert dust is mobilized by wind from exposed surfaces. Dust emissions are affected by the nature of the land surface, which changes due to human activity and vegetation (McConnell et al., 2007; Neff et al., 2008), and by surface windspeed (Tegen, 2003; Engelstaedter and Washington, 2007). These governing factors result in significant annual variability (Prospero and Lamb, 2003; Qian et al., 2004) and sensitivity to climate, including emission damping by increased vegetation in a higher-CO2 world (Mahowald and Luo, 2003). IPCC (2007, Section 7.5.1.1) provides a range of changes by 2100, from −60 to 380 percent, and we use one-quarter of this value.
Open Fires Open fires occur because of deforestation, natural causes such as lightning strikes, prescribed fires for land management, and seasonal fires for agricultural land clearing. In our estimates of future changes we neglect two of these: deforestation, which is a minor factor in most source regions, and prescribed fires, which depend on management policies. A warmer climate is expected to increase fire frequency and quantity (IPCC, 2007, Section 7.3.3.1; Spracklen et al., 2007). This change is attributable to droughts and to other changes in climate regimes for both tropical areas and boreal forests. We infer, from estimates of changes in Canadian fires by 2100 (Flannigan et al., 2005; Girardin and Mudelsee, 2008), that an increase of 10-30 percent could be expected by 2030. However, many of these estimates do not account for all feedbacks, such as changes in vegetation or ignition frequency. Further, the burned area of Canadian forest fires has already doubled between the 1970s and 1990s (Kasischke and Turetsky, 2006). Thus, the value in Table 3.1 could be an underestimate.
Transport Pollutants arriving in either the United States or regions affected by the United States, may be altered due to future synoptic-scale dynamics, convection and transport at high altitudes, or climate modes such as El Niño or the North Atlantic Oscillation (HTAP-TF, 2007). If the
average climate changes, we may also expect differences in reaction rates, such as those leading to secondary organic particulate matter, and removal rates, such as rainout. While specific studies focused on the impact of climate change on the long-range transport of pollutants are just beginning, a number of robust changes in transport patterns under climate change scenarios have emerged from model, theoretical, and observational studies (HTAP-TF, 2007). These studies suggest less frequent large-scale venting of the boundary layer, which implies less export of pollution from the boundary layer. However, once exported from a source region, the extent of long-range transport depends upon the lifetime of the pollutant. For the case of particulate matter the lifetimes are heavily dependent on the wet removal processes (i.e., the lifetimes decrease as wet removal rates increase). Changes in cloud properties and precipitation patterns and quantity resulting from climate change will affect the transport distances of PM. Thus, if PM lifetimes are decreased, export from source regions may remain unchanged or even decrease despite increasing future emissions. We also know that pollution export is dependent upon location (HTAP-TF, 2007), with export from Southeast Asia being more efficient than from North America and export from Europe least efficient. This is due to the amount of convective activity over oceans (Stohl et al., 2002). Future emission increases are projected to be shifted toward the tropics (IPCC, 2007), and the efficiency of export from those regions experiencing increases need to be considered in order to evaluate their impact on long-range transport.
KEY FINDINGS AND RECOMMENDATIONS
Question: What do we know about the current import and export of PM?
Finding. Satellite, aircraft, and ground-based observations demonstrate that PM can undergo long-range transport. Episodic events associated with biomass burning plumes, dust storms, and fast transport of industrial pollution are most easily identifiable in observations from rural areas and high altitudes, where they represent large excursions above typical ambient concentrations. Some excursions in PM concentrations clearly attributable to long-range transport episodes have been recorded at the surface in urban areas, but in general, the contributions from long-range transport in such regions are difficult to isolate from PM of U.S. origin.
Finding. It remains difficult to quantify from observations alone the frequencies and magnitudes of contributions from persistent long-range transport of PM. Such transport occurs routinely, but
presumably brings much lower concentrations of PM than do episodic events, making it difficult to quantitatively distinguish the long-range transport signal from that due to local and regional PM sources.
Finding. Ensemble studies of chemical transport models estimate that PM sources in Europe, South Asia, and East Asia contribute on average 0.05–0.15 μg m−3 to the continental U.S. annual mean surface PM2.5 concentration (obtained by scaling the 20 percent perturbation results by a factor of 5 to account for 100 percent of the foreign influence, ignoring any nonlinear effects). Larger contributions can occur for particular seasons and geographical regions.
Finding. Chemical transport models can be used to estimate long-range transport contributions to atmospheric PM concentrations, as well as the contributions to deposition of sulfate, nitrate, metals, Hg, and POPs. However, large uncertainties in model estimates result from several factors, including lack of observational constraints, particularly in the free troposphere where much of the transport occurs; poorly constrained or unknown emissions of some primary particles and the emissions and conversion of PM precursors (especially for secondary organic PM); and poorly constrained wet and dry PM removal processes, as reflected in the large differences in PM lifetimes among models.
Finding. There are growing capabilities for observing total column amounts of PM from ground-based and satellite observations, and this information is being used to detect global and regional column trends. Yet the linkages between column PM amounts and surface PM levels are complicated and not well understood; for example, there is little information about how PM aloft is mixed down to the surface. This limits our ability to quantify the effect of persistent transport on surface concentrations based on column observations. There is, however, ongoing work to improve our ability to model these relationships between column and surface PM.
Finding. There is not enough observational evidence to demonstrate clear trends in average baseline levels of surface PM on a global or hemispheric scale. Because of the relatively short lifetime of PM, observations must be gathered over long time periods (around a decade at least) before trends can be distinguished from short-term variability.
Recommendations. Improve our ability to characterize the import and export of PM by implementing
-
continuous aerosol observatories in key locations, to be defined as part of a comprehensive measurement strategy, including strategically located marine- and mountaintop-based sites. Key observational variables include those that can help with identifying source fingerprints (e.g., speciated and size-resolved aerosols) to permit quantification of the associated PM fractions with high time resolution. A minimum requirement is one sample per 24 hours, but shorter time resolution will yield even greater capability to distinguish between local and long-range transport. Long-term observations are needed to encompass the statistical variability of natural processes, as well as to separate trends in emissions from variability in transport and removal.
-
long-term, time-resolved data collection, both inside and outside urban areas, combined with meteorological modeling of horizontal and vertical transport. Such data are needed to elucidate the impact of long-range transport in regions that are near air quality standard compliance limits, where the contribution of long-range transport is generally most difficult to detect.
-
improvement and application of methods for quantifying import and export of PM using space-based observations. A particular focus should be on observations and methods that can be used to constrain vertical distributions (e.g., space-based and ground-based lidars) and those that can elucidate the relationships between column amounts of PM and surface PM concentrations.
-
advancements in modeling capabilities that include merging bottom-up with top-down emission estimates; validating combined emission and model systems with in situ and remotely sensed observations; constraining PM column amounts, including speciated PM when possible; and refining and applying fingerprinting techniques in observations to aid in verification of modeled source attributions. Modeling improvements that are particularly important in evaluation of long-range transport are the simulation of dust emissions, fire emissions, and production of secondary organic PM.
Question: What are potential implications of long-range PM transport for meeting environmental goals?
Finding. With the exception of occasional extreme episodes, most often attributable to dust and wildfires, long-range transport of PM
is estimated to represent a negligible contribution to PM NAAQS exceedances in most regions in the United States.
Finding. Increases in surface PM from episodic or persistent transport are significant relative to other sources in places such as the Arctic and portions of the western United States, which do not have large local pollution sources. In particular, strategies to achieve compliance with the regional haze rule across the United States may be complicated by imported PM because existing U.S. regulations prohibit increments in annual mean PM2.5 concentrations above 1 μg m−3 in Class I areas, and even tighter limits on incremental contributions may be imposed in future regulations.
Finding. Some initial studies have begun to estimate the premature mortalities attributable to long-range transport of PM, but they are very preliminary at this time due to the large uncertainties in estimates of imported and exported PM. These estimates are not only limited by uncertainties in quantitative estimates of the imported fraction of PM but also by lack of a mechanistic understanding of how the individual components of PM are linked to health. Current estimates indicate that domestic sources of PM are by far the larger risk to human health. Any import of PM from distant sources could add to the health burden, because there is thought to be no threshold for PM health impacts (and impacts rise linearly with increasing concentration).
Finding. Both longer-term sustained and episodic increases in column PM across the United States attributable to long-range transport may represent potentially significant perturbations to the regional radiative balance.
Finding. The ecological and agricultural impacts of long-range transport of PM have not been evaluated.
Recommendation. Improve abilities to estimate the potential implications of non-U.S. PM sources for the attainment of U.S. environmental goals by:
-
improving quantification of imported and exported PM fractions, which in turn requires coordinated modeling and observational strategies (see next question);
-
developing the capability to quantify the trends in PM surface concentrations and to attribute these trends to emissions from
-
specific sources. Because baseline PM concentrations are so low and variable, questions regarding trends in this baseline can be answered only through a long-term strategy that integrates observations and models. Baseline and background changes tend to be regional (e.g., Northern Hemisphere; Arctic, Pacific, and Atlantic regions), thus requiring corresponding regional strategies;
-
reevaluating the health burden resulting from imported PM, as better mechanistic understanding of PM-health links becomes available;
-
assessing the other impacts of imported PM, such as adverse consequences for ecological and agricultural systems, and consequences for regional and global radiative forcing.
Question: How might factors influencing the relative importance of U.S. and foreign contributions to PM change in the future?
Finding. A warming climate is expected to lead to shifts in emissions of PM associated with the natural environment and changes in the meteorology that affects pollution dispersion. Hypothesized impacts include:
-
increasing annual numbers of forest fires, which in turn would increase the frequency of extreme PM concentration events from long-range transport of fire emissions;
-
changes in dust particle sources (although even the sign of increase or decrease is currently uncertain);
-
changes (often reductions) in the rate at which surface and boundary layer air is vented to the upper atmosphere;
-
complex changes in cloud amount and lifetime and precipitation patterns, in turn affecting PM removal rates and spatial patterns of deposition;
-
changes in PM precursor emissions, particularly biogenic gases emitted from vegetation.
These changes would all have implications for how much PM (and precursors) is emitted from various source regions, how much of the emitted PM escapes from source areas, and the fraction of PM that survives transport to affect areas downwind. At present, it is not possible to predict the net impacts.
Finding. In the short term (e.g., next 10–20 years), emissions from developing countries will likely continue to increase. In the longer term it is not possible to predict how future emissions will change
and how these will in turn affect long-range transport of PM. As global economic and industrial activities expand, greater emissions of PM and precursor species might be expected, with projected growth varying widely, depending on economic scenario assumptions. The result could be a higher baseline of imported PM; however, introduction of standards, and cleaner technologies to address national standards moving toward WHO guidelines, may slow or reverse this trend.
Finding. A better understanding of the health effects of PM may lead to different or more stringent standards in the United States and elsewhere. For example, revised regulations may not focus solely on mandating lower acceptable concentrations of PM2.5 but may also (or instead) focus on particular fractions or characteristics of PM, such as number concentration, the presence of toxic metals, or the particles’ oxidative state. Identification of the PM characteristics responsible for health outcomes will also allow monitoring, modeling, and apportionment activities to be tailored toward those characteristics, which will in turn help advance our understanding of the true significance of long-range transport of PM.
Recommendation. Increase our ability to forecast the future significance of imported PM by:
-
increasing understanding of how future climate will affect patterns of dust mobilization, fire frequency and intensity, and biogenic emissions;
-
understanding how regional perturbations in aerosols affect climate;
-
developing multipollutant emission inventories with high spatial resolution that reflect economic growth, technological change, and incoming regulations;
-
improving the forecasting of changes in atmospheric dynamics that affect transport of pollution plumes, particularly boundary layer venting and precipitation, under changing climates;
-
designing effective observational networks that enable rigorous analysis of PM composition, trends, and other factors that relate to long-range transport.
Additional information will be needed to pinpoint the PM characteristics and monitoring strategies that are most useful for understanding impacts on human health.