16
Medically Complex Patients
INTRODUCTION
Since 48 percent of Medicare beneficiaries have at least three chronic conditions and 21 percent have five or more conditions, it has been estimated that approximately 60 million Americans have multiple morbidities, a number that is expected to increase to 81 million by 2020 (Anderson and Horvath, 2002). Additionally, projections place levels of obesity at 41 percent by 2015 (Wang and Beydoun, 2007), with consequences for diabetes, heart disease, hypertension, cancer, and osteoarthritis. To explore the solutions needed to face these mounting challenges, presenters in this session discuss policy initiatives to facilitate care of the growing population of medically complex patients, emphasizing patient-centeredness, payment redesign, quality and consistency in treatment, streamlined and harmonized health insurance regulation, and prevention at personal and population levels.
Arnold Milstein of the Pacific Business Group on Health opens this session by reviewing promising initiatives to lower per capita healthcare spending and improve clinical outcomes for medically complex patients. He reports that two areas of reform have yielded the largest impacts: (1) multidisciplinary primary care teams providing ongoing care to patients with particular attention to preventing unplanned inpatient admissions, and (2) standardization of inpatient care to maximize quality and efficiency. In order to scale these practices to a national level, Milstein proposes a focus on substantial payment incentives, technical assistance, and additional research and evaluation.
R. Sean Morrison of Mount Sinai Medical Center agrees that care for patients with serious illness and their families is in much need of improvement. He explains that palliative care provides interdisciplinary care coordination and team-driven continuity of care that best responds to the episodic and long-term nature of chronic, complex disease. While the prevalence of these programs in hospitals grew from 5 percent in 2000 to 50 percent in 2008, palliative care still falls far short of being accessible to all who need it. For palliative care to be accessible to all patients with serious illness and their families, he urges consideration of a number of key policy initiatives, including education of patients, families, and healthcare professionals about the benefits of palliative care; additional resources for workforce development to train sufficient numbers of specialists to effectively provide palliative care to patients and families in need; patient-oriented and health services research; and reimbursement structures that promote team-based care.
Ronald A. Paulus of Geisinger Health System suggests that value-based payment models must move beyond payment for units of work or effort and instead reward demonstrated patient- and population-level clinical impact and outcomes. Paulus explains that care gaps are evidence- or consensus-based patient clinical needs as informed by age, gender, comorbidities, physiological parameters, and other factors. Primary care teams of practitioners, nurses, and specialists at Geisinger Health System work in closing these gaps for their patients. When supplemented by an electronic health record with enhanced decision support, population-level data, and integrated analytics, he explains, this approach can produce marked progress in patient and population outcomes. For example, among diabetic patients, this clinical care-based process has resulted in continuous stepwise improvement in the percentage of patients who have completed all nine care bundle components of evidence-based care, producing a fourfold percentage increase over 24 months for a group of more than 22,000 patients. He suggests that this model could also serve as a point of reference for those seeking to develop value-based payment models structured to encourage innovation, enhance patient experience, improve clinical quality, and contain costs.
Lastly, Anand K. Parekh of the U.S. Department of Health and Human Services identifies several policy areas that could further support tertiary prevention in individuals with multiple concurrent chronic conditions. Since medically complex patients have often been excluded from participation in randomized controlled clinical trials, he suggests that the external validity and generalizability of these studies to this population are limited. While identifying the importance of health professional training in the care of medically complex patients, he explains that many current evidence-based
guidelines focus on individual chronic diseases, thus disregarding the coexistence of other chronic conditions in patients and putting patients at risk of drug-drug or drug-disease adverse interactions. He additionally discusses patient engagement as playing a central role in patients’ management of their own care and provider payment reform as essential to incentivizing enhanced care coordination and care management.
PAYMENT POLICIES AND MEDICALLY COMPLEX PATIENTS
Arnold Milstein, M.D., M.P.H.
Pacific Business Group on Health and Mercer Health & Benefits
Public and private sector policy makers seek provider payment reforms that will both improve clinical outcomes and reduce healthcare spending substantially. Such improvement has been demonstrated for medically complex patients in scattered locations throughout the country via comparisons either with local peers or with the provider’s own prior year’s performance. One or both of two instrumental changes in care delivery were required.
-
Multidisciplinary primary care teams judiciously intensified care during and/or between office visits (Milstein and Gilbertson, 2009; Paulus et al., 2008) for patients at highest risk of near-term emergency room (ER) visits and unplanned inpatient admissions.
-
Inpatient care teams standardized and then iteratively refined an increasing portion of inpatient care, via either use of systematic process engineering tools such as the Toyota Production System (Bohmer and Ferlins, 2005) or adoption of externally or internally sourced checklists, standing order sets, and/or clinical implementation pathways (Pronovost et al., 2002).
Since current provider payment methods sometimes inadvertently penalize these changes, they were often led by exceptional innovators who persevered nonetheless. In addition to such fundamental redesign of care processes and exceptional provider leadership, what payment policies were required to enable these exceptional results? Intensification of primary care via multidisciplinary teams required either full transfer of health insurance risk from insurers to care providers via global capitation or substantial shared savings payments by insurers. Greater standardization of inpatient care via iterative clinical process reengineering required that a significant share of hospital revenues be paid by Medicare and/or another large source of fixed payment to hospitals for the inpatient portion of an episode of acute care.
Challenge of Replication and Scale
Despite these exemplary pockets of success, the Centers for Medicare & Medicaid Services (CMS) has recently struggled to scale equivalently robust improvements by sharing with providers the savings from various forms of intensified ambulatory care for chronically ill Medicare beneficiaries. When improvements were attained, the effect sizes have been small relative to those reported by innovators (Brown et al., 2007). In addition, Medicare Payment Advisory Commission (MedPAC) analyses have shown that despite Medicare’s diagnosis-related group (DRG)-based fixed-price hospital payments, 88 percent of U.S. hospitals have levels of clinical outcomes and total cost per admission that are approximately 15 percent less favorable than the highest-performing 12 percent of hospitals. Many speculate that, like publicly available comparisons of provider performance, provider payment incentives may be a necessary but not sufficient condition for scaling benchmark performance on both quality and cost efficiency nationwide. Other evidence points to the importance of three cofactors: (1) the size of performance-based payment incentives; (2) the intensity of competition faced by providers, especially in the commercially insured market (MedPAC, 2009); and (3) the provision of effective technical assistance to providers by successful care innovators.
The Implied Public Policy Prescription
Success in improving the care of medically complex patients and lowering total per capita healthcare spending is likely to require addressing these three pivotal cofactors. The following prescription illustrates how federal policy could accomplish this.
-
Ensure adequate size of performance-based provider payment incentives: How large must performance-contingent payment be to motivate provider prioritization of benchmark performance attainment? The Institute of Medicine’s (IOM’s) review of available evidence on provider payment incentives suggests that they must be much larger than current incentives. The IOM concluded that performance-based payments must equal or exceed 10 percent of annual provider income (IOM, 2007). Current U.S. provider performance-based payments do not come close to this target. Federal policy should require that U.S. health plan beneficiaries be enrolled in plans that make at least 10 percent of provider payments “value contingent.”
Value-contingent payments encompass three principal changes in U.S. provider payment policies, each of which would have to be
-
conditioned on benchmark or improved quality of care: (1) payments that are bundled for all services delivered during acute care episodes for conditions that commonly require hospitalization, including any post-hospital recovery period; (2) payments that are globally capitated annually for all healthcare services; and/or (3) payments that share with providers the savings they produce for payers and patients. To eliminate the gap between average and benchmark performance, such performance-contingent payment should eventually be calibrated primarily to reward the attainment of benchmark performance rather than to reward performance improvement.
-
Ensure adequate intensity of provider competition: Federal policy should require that all U.S. health plan beneficiaries who are at high risk for ER use or unplanned hospitalization, whether enrolled in fee-for-service or managed care plans, be strongly incentivized to use providers who are performing above local average levels of measurable quality and low total combined cost of care to payers and patients.
In view of evidence that the intensity of competition for commercially insured patients motivates providers to deliver more value, and to impact providers who derive little to no income from federally funded health benefits plans, reform in health benefits tax policy or other incentives should be used to motivate private sector plans to adopt parallel policies. In addition, for providers whose market dominance enables them to neutralize such value-contingent payment and patient incentives, all-payer pricing systems or strengthened federal antitrust regulations may be required to prevent provider evasion of accountability for attaining benchmark value.
-
Ensure adequate technical assistance to non-benchmark providers: Another IOM review found that many U.S. providers lack the skills to rapidly improve clinical performance (Reid et al., 2005). To address this deficiency, new federal policy should require (1) that public and private payers share pro rata in the expense of efficiently providing technical assistance to support both the successful adoption by providers of multidisciplinary primary care teams for the severely ill and the standardization of inpatient care processes using models developed by benchmark providers; and (2) that a substantial portion of federal comparative effectiveness research funds be dedicated to comparison of options for accelerating providers’ rate of adoption of innovations demonstrated to deliver better clinical outcomes and lower per capita spending for medically complex patients.
Conclusion
Local exemplars show that it is possible both to reduce total per capita spending and to improve clinical outcomes for medically complex patients. However, replicating and scaling these local innovations to a national level will likely require three policy changes: (1) much larger performance-based provider payment incentives, (2) patient incentives to use higher-value providers, especially when they are severely ill, and (3) coordinated technical assistance funded pro rata by all payers to spread benchmark clinical performance rapidly. Initiating these three policies for medically complex patients will help propel improved outcomes and lower real per capita U.S. healthcare spending by more than 10 percent by 2019.
PALLIATIVE CARE, ACCESS, QUALITY, AND COSTS
R. Sean Morrison, M.D., Diane E. Meier, M.D., and Melissa Carlson, Ph.D.
Mount Sinai School of Medicine
Considerable data suggest that care for patients living with serious illness and their families is in need of improvement. Abundant studies document a high prevalence of pain and other symptoms, unmet family needs, poor communication between patients and healthcare providers, and rising costs of health care (Morrison and Meier, 2004). Palliative care provides a solution to the difficult challenges posed by medically complex patients. Palliative care’s interdisciplinary care coordination and team-driven continuity of care best respond to the episodic and long-term nature of chronic, multifaceted illnesses (National Quality Forum, 2006). Recent studies have demonstrated that these care programs improve clinical quality and patient and family satisfaction and, at the same time, reduce hospital expenditures (Morrison and Meier, 2004; Morrison et al., 2008). Whereas the number of hospital palliative care programs has grown dramatically over the past decade (Goldsmith et al., 2008), several key initiatives need to be undertaken for palliative care to be accessible to all patients with serious illness. First, there need to be educational initiatives to increase awareness of the benefits of this care in the setting of a serious illness and of the difference between palliative care and end-of-life care. Second, there need to be workforce initiatives to ensure sufficient numbers of specialists to effectively provide high-quality palliative care. Third, there need to be research initiatives to augment the currently inadequate evidence base. Finally, there need to be legislative changes to modify existing reimbursement structures to cover the team-based approach that is necessary to support high-quality palliative care services. This paper outlines a series of policy initiatives that address these issues.
Public and Professional Misperceptions
A major barrier to the continued growth of palliative care is the perception that palliative care is synonymous with hospice, “end-of-life care,” care of the dying, or the alternative to curative or life-prolonging treatments (Meier, 2003; Morrison and Meier, 2004). Palliative care differs from hospice in terms of both timing and reimbursement. It is available to patients who continue to benefit from curative or life-prolonging treatment and is not dependent on prognosis. Reimbursement for hospice care under the Medicare Hospice Benefit requires that an individual be certified by two physicians as terminal (defined as a prognosis of 6 months or less) and agree to give up Medicare coverage for potentially life-prolonging therapies. Palliative care’s independence from prognosis is especially important in the context of chronic debilitating diseases such as heart disease, stroke, or dementia for which prognostication is particularly difficult. This unfortunate misperception inhibits access to non-hospice palliative care early in the course of illness when patients and families can benefit greatly from the services palliative care provides. Furthermore, focusing on the end of life or care of the dying is politically problematic. Whereas explanations of humans’ long-standing fear of death have ranged from evolutionary to societal (Becker, 1973; Darwin, 2003; Hofmann et al., 1997; O’Gorman, 1998), the practical result is that efforts to focus healthcare reform on end-of-life care have met with resistance and have been relatively ineffective (Hanckock, 2009).
A national social marketing campaign to increase public and professional awareness of palliative care is critically needed. Such a campaign would define palliative care as appropriate care for persons with serious and life-limiting illness throughout the course of their disease, encourage patients and families to seek high-quality palliative care early in the course of illness, and educate healthcare professionals about the appropriate role of palliative care in the care of their patients. This campaign, similar to initiatives centered on smoking cessation and childhood obesity, would considerably facilitate the key policy initiatives outlined below. Table 16-1 details ease of policy implementation and potential barriers.
Workforce
A second major barrier facing the expansion of palliative care services is the lack of palliative medicine physicians. Whereas there is one cardiologist for every 71 persons experiencing a myocardial infarction and one oncologist for every 141 newly diagnosed cancer patients, there is only one palliative medicine physician for every 31,000 persons living with a serious and life-threatening illness (Center to Advance Palliative Care and the National Palliative Care Research Center, 2008). Furthermore, despite the
TABLE 16-1 Strategies for Policy Implementation
|
Policy Area |
Recommendation |
Ease of Implementation |
Potential Barriers |
|
Misperceptions |
Public–private social marketing campaign |
Straightforward |
Financial—securing public or private sector funding to initiate. |
|
Workforce |
Redistribute unused and create new GME training slots |
Moderate |
Resistance to adjusting established training levels, competition for slots from other medical specialties. Requires Congressional action to approve but could integrate into efforts currently under way with respect to primary care. |
|
|
Loan forgiveness programs |
Moderate |
Competition from other specialties. Requires congressional action, but existing models are available. |
|
|
Academic career awards |
Straightforward |
Expansion of HRSA budget for Title VII program beyond geriatrics; perceived failure of geriatrics program to increase the number of new trainees entering geriatrics training programs. Existing legislation exists, and plans to reintroduce legislation are under way. |
|
|
Midcareer training awards |
Moderate |
No current existing program to serve as an example; clinical infrastructure to support training programs would need to be developed. American Association of Medical Colleges is highly supportive and currently within American Academy of Hospice and Palliative Medicine’s strategic plan. |
|
|
CME training prior to relicensure |
Highly complex |
Requires new legislation in 50 states and the District of Columbia. |
|
Access and quality |
Bonus payments or penalties linked to palliative care delivery |
Straightforward |
Requires measures of palliative care availability and penetration of services. Could be readily integrated into CMS’s current reporting requirements for hospitals. |
|
Policy Area |
Recommendation |
Ease of Implementation |
Potential Barriers |
|
|
Link palliative care to bundled payments |
Complex |
Effectiveness of bundled payments has yet to be fully evaluated and tested; some difficulty in describing nature and scope of palliative care services; uncertainty whether hospice benefits should be included in bundle and difficulties in redefining hospice benefit. |
|
|
Increase M.D. reimbursement |
Moderate |
Requires development of time-based coding systems for non-ICU physicians; competition from other specialties (e.g., geriatrics, hospital medicine) for similar provisions; requires upfront investment in physician salaries to achieve longer-term cost savings. |
|
|
Establish a palliative care Resource Utilization Group (RUG) for nursing home reimbursement |
Moderate |
Reimbursement level would need to be comparable to restorative focused RUGs; distinction between indications of normal decline (e.g., weight loss, loss of function) and quality lapses for residents receiving palliative care need to be understood. |
|
|
Inclusion of palliative care in medical home models |
Moderate |
Requires existing models to develop partnerships with hospice and non-hospice palliative care programs; access to palliative care may not be uniformly available. |
|
|
Include palliative care in accreditation requirements |
Moderate |
Guidelines for palliative care service components at smaller hospitals and long-term care facilities need to be developed; resistance from Joint Commission given sizable number of hospitals and nursing homes that currently lack programs. |
|
Evidence base |
Develop Office of Palliative Care Research |
Complex |
Requires Congressional act akin to Office of AIDS Research. Existing model in place; does not require the development of new center or institute. |
recognition of palliative medicine as an official American Board of Medical Specialties (ABMS) subspecialty, only 24 states have fellowship training programs approved by the Accreditation Council for Graduate Medical Education (ACGME) (American Academy of Hospice and Palliative Medicine, 2008). Finally, because of the cap on GME residency training slots, the majority of current fellowship slots are supported by tenuous philanthropic dollars and not by Medicare funding.
Several policy initiatives are likely to have a major impact on improving care for persons with serious illness. First, the GME cap should be lifted to allow the expansion of palliative care fellowship training programs, and currently unused GME slots should be redistributed to support ACGME-approved palliative medicine fellowship training. Second, loan forgiveness programs for palliative care physicians similar to those available to researchers through the National Institutes of Health (NIH) (U.S. Department of Health and Human Services, 2009) should be established at the Health Resources and Services Administration (HRSA) in order to promote palliative care as a viable career path for young physicians. Third, HRSA Title VII-supported career development awards (similar to Geriatric Health Professions Training Programs) should be established to support clinician educators who can integrate palliative care into medical school and residency training curricula (Reynolds, 2008). Fourth, HRSA should establish
mid-career training awards to support retraining of the current workforce into this new specialty. Finally, mandatory continuing medical education (CME) training in primary-level palliative care prior to state relicensing, similar to California’s provision for training in pain management (Medical Board of California, 2009), would ensure that all physicians were familiar with the core competencies of palliative medicine.
Access and Quality
Despite linear growth in the development of non-hospice palliative care programs, 47 percent of hospitals still lack palliative care programs, and palliative care in nursing homes and ambulatory settings outside of hospice is rare (Goldsmith et al., 2008). One barrier is that the current business model for palliative care is based on cost avoidance rather than on revenue generation. This model is unusual in health care, requires sophisticated analytical methods to employ successfully, and is thus difficult to integrate into hospitals’ current operating metrics. Additionally, accreditation requirements for hospitals and nursing homes do not yet include palliative care despite publication of consensus standards for quality palliative care by the National Quality Forum (National Quality Forum, 2006). Furthermore, existing palliative care programs are not mandated to observe these guidelines.
Near-term policy solutions that could address the barriers outlined above are fivefold. First, the reimbursement structure for palliative care should enable hospitals and nursing homes to generate revenue from providing palliative care services. For example, providing hospitals and nursing homes with bonus payments linked to palliative care delivery with a transition over 5 years to penalties for institutions not providing palliative care, requiring palliative care services as a condition of bundled payments, and adjustment of current physician reimbursement pay scales to support time-intensive goals of care discussions and care coordination would lead to a rapid growth in palliative care services and lower costs. Similarly, a new Resource Utilization Group category for palliative care reimbursement to nursing homes through Medicare would help counter the misperception that palliative care is incompatible with the restorative focus of nursing homes and increase access to palliative care for nursing home residents. Third, given the high costs associated with patients with serious and life-threatening illnesses (Dartmouth Atlas, 2008), medical home models should include non-hospice palliative care as a core component. Fourth, accreditation requirements for hospitals and nursing homes (e.g., the Joint Commission) should include published guidelines for palliative care to ensure that quality is consistent across programs (National Quality Forum, 2006).
Evidence Base
Unlike other areas of medicine, the knowledge base to support core elements of palliative care clinical practice (i.e., pain and symptom management, communication skills, care coordination) is inadequate. Reports from the IOM in 1997, 2001, and 2003 (Field and Behrman, 2003; Field and Cassel, 1997; IOM, 2001) and NIH in 2002 and 2004 (NIH, 2002, 2004) have called for major investments in research on palliative care. Yet, as of 2005, less than 0.1 percent of all NIH-awarded grants supported palliative care research. Policy initiatives to address this knowledge gap are straightforward and easily integrated within current biomedical research funding structures. NIH and the Agency for Healthcare Research and Quality (AHRQ) should reallocate 2 percent of their current budgets to focus on symptom relief, communication in the setting of serious illness, and comparative effectiveness research focused on patients with serious and advanced illness. An Office of Palliative Care Research modeled after the Office of AIDS Research should be established to oversee distribution of research funding. Finally, existing NIH career development award mechanisms could be utilized to support junior investigators and midcareer palliative care investigators in order to address the lack of established palliative care researchers.
Conclusion
Research over the past 20 years has conclusively demonstrated that too many seriously ill Americans who experience treatable suffering are impoverished because of uncompensated medical care (Himmelstein et al., 2005). At the same time, rising government healthcare expenditures threaten to bankrupt current Medicare savings (Siska et al., 2009). Palliative care offers an attractive solution to this problem by improving quality while substantially reducing costs for the most expensive and vulnerable patient population. This paper has outlined policy initiatives in four key areas that would rapidly bring palliative care to scale in the United States and help address the quality and costs issues outlined in this report.
PAYMENT AND BETTER CARE OF COMPLEX PATIENTS
Ronald A. Paulus, M.D., Jonathan Darer, M.D., and Walter F. Stewart, Ph.D.
Geisinger Health System
Novel, value-based payment models that will both “bend the cost curve” and improve population health are required to address the macro
trends of aging and chronic disease prevalence (Schoen et al., 2007). New models must move beyond payment for units of work and resource-based productivity measures and instead reward demonstrated patient- and population-level clinical impacts and outcomes. We describe a new approach at Geisinger Health System that seeks to optimize patients’ “care gap closure rate” and facilitate effective teamwork among primary care physicians, nurses, and specialists. Until formal, validated outcome measures are widely available, we believe that a revised payment model based, in part, on care gap closure rates would be a significant improvement from our current fee-for-service model.
Care Gaps—An Alternative Approach
Care gaps are defined as evidence- or consensus-based patient clinical needs as informed by age, gender, comorbidities, physiological parameters, and other factors. In general, each care gap is known to be directly associated with improved intermediate or clinical outcomes. Patients who fail to have their evidence-based care needs met have one or more care gaps, a common finding among chronically ill, multiple comorbid patients. Rather than narrowly focusing on disease-specific registries, care gaps exist in the broader context of a relevant population and its associated care needs and goals. Examples include prevention (e.g., screening mammography), chronic disease management (e.g., targeted LDL [low-density lipoprotein] level), unclosed loops (e.g., positive pap smear without intervention), medication safety (e.g., anticonvulsant monitoring), and other end points.
Traditional healthcare financial incentives, practices, and limited analytic functions collectively foster a care delivery system that is both inefficient and unreliable (McGlynn et al., 2003). Geisinger has implemented a primary care-based medical home model that relies on the use of an integrated platform consisting of an electronic health record (EHR), an advanced analytics platform, and a tailored communication process. Aggregated patient data along with associated analytical models are the foundation of the Clinical Decision Intelligence System (CDIS; see Figure 16-1). Care gaps are identified within CDIS and acted upon via the EHR through various effectors including order sets, reminders, flags, care plans, and direct patient communication.
Primary-Specialty Care Collaboration
The act of closing care gaps is a team-based, collaborative process among primary care practitioners, nurses, and specialists (Bach, 2007). To build successful primary-specialty care partnerships, expectations and roles must be clarified to identify accountable providers who are managing specific aspects
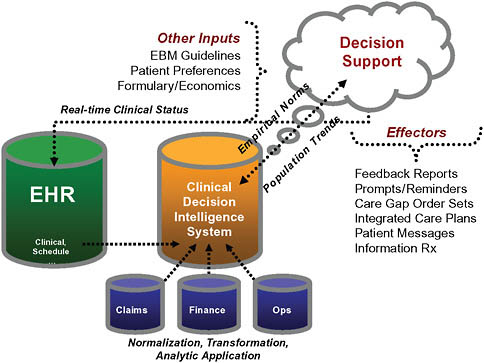
FIGURE 16-1 Geisinger transformation architecture.
of care. Potent collaboration examples include patients with hypertension > 140/90 mm Hg on three or more medications, patients with a hemoglobin A1c (HgbA1c) level persistently >9 percent, patients with abdominal aortic aneurysms >4 cm, and patients with Stage D heart failure. Key principles of the heart failure collaborative model are depicted in Figure 16-2 as an example.
The model establishes a defined set of activities that must be accomplished by the healthcare team, with an initial team focus on improving health status. If not possible, the dominant focus shifts to slowing disease progression, and a “primary specialist” is recruited who becomes actively engaged with increasing intensity if the underlying disease progresses despite best efforts. Throughout, the primary care provider and other team members ensure continuity of care and serve as the patient advocate. This commonsense model addresses gaps in the typical fragmented care model experienced by many Americans today.
Geisinger Care Gap Management Example
Geisinger has launched a series of initiatives focused on closing care gaps; here we specifically describe the management of patients with diabetes.
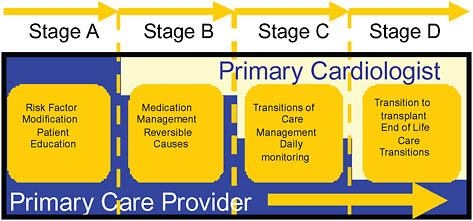
FIGURE 16-2 Primary care-specialist partnership in heart failure.
Clinicians defined a diabetic “care bundle” that includes nine evidence-based components of care (Weber et al., 2008). First, care gaps are identified for each individual patient within CDIS by comparing her electronic information against evidence-based care needs as defined in the bundle. Second, nurses are provided with an automated rooming tool that, among other things, enables the nurse to verify the presence or absence of a given care gap. Post-rooming, an automated, patient-specific, care gap-informed order set is generated at the point of care; the physician can “accept all” with one click or can add, delete, or edit any given order. Importantly, this same care gap information is sent directly to patients via the MyGeisinger consumer Web portal. Patients are encouraged to address needs virtually with their providers, or they can schedule care gap interventions (e.g., a laboratory draw, a foot exam) directly online with a few clicks.
This clinical care-based process has resulted in continuous stepwise improvement in the percentage of patients with all nine care bundle components complete, producing a fourfold percentage increase over 24 months for a group of more than 22,000 persons with diabetes. However, despite this significant progress, approximately 8 percent of all persons with diabetes continued to exhibit a persistently elevated HgbA1c >9 percent. To address the needs of this population with difficult-to-manage diabetes, a semiautomated process was implemented to temporarily transfer care from primary care to endocrinology. This process included (sequentially, as needed) a joint letter from primary care and endocrinology physicians explaining the program and their combined recommendation that the patient should attend, up to three calls from the scheduling office, and a final call from the primary care practice itself. Colleagues at Geisinger
facilitated more than 50 endocrinology and diabetes educator evaluations in a single practice location. While the numbers are small, the average HgbA1c levels declined 1.6 and 1.8 percent, respectively, at 8 and 15 weeks’ post-referral. To address concerns about care continuity and satisfaction, pre- and post-intervention satisfaction was measured for both primary care providers and patients; all were either constant or improved from baseline post-intervention.
Looking Forward—Possible Implications?
When we incentivize volume, procedures, and high-cost care, that’s exactly what we get. Incentives for true value-based productivity and related measurement are rare or nonexistent. In fact, the very scale used to determine most productivity performance and “value” (the resource-based relative value scale [RBRVS]) was instead created specifically to approximate resource use, not value. This notion of healthcare “productivity” is analogous to rewarding Dell for the resource intensity of its PC production, rather than for the features, performance, and reliability—the value—of its products. Although we are at a nascent stage in our goal to create a true value-based productivity model at Geisinger, we believe that measures incorporating concepts relevant to the rate of care gap closures or care gap closures per RBRVU (resource-based relative value unit) would be a significant step forward in reorienting the incentive system to promote wellness, disease containment, and disease prevention. If combined with a low-value utilization ratio incorporating both regular care “failures” (e.g., heart failure exacerbations) and low-value resource consumption (e.g., low-utility imaging), it could also serve to bend the curve on costs.
Of course, this approach also requires fundamentally reengineered care processes. At Geisinger we seek to reengineer care processes by hard-wiring evidence-based care, eliminating waste, automating care processes as feasible and appropriate, delegating care to the least-cost competent caregiver, and seeking to engage and activate the consumer-patient and her family as team members in the care journey—all supported by our EHR, analytic databases, and intelligent decision support. Taken collectively, we believe that similar realigned financial incentives and large-scale care reengineering can bend the curve on cost and create enhanced value for all Americans.
CARE OF PATIENTS WITH MULTIPLE CHRONIC CONDITIONS
Anand K. Parekh,1 M.D., M.P.H.
U.S. Department of Health and Human Services
It remains to be seen whether care coordination and chronic care management can indeed bend the cost curve while improving quality either individually or synergistically with other delivery system reforms. Attention to five specific policy levers could help achieve this for the most high need population: (1) improving the external validity of clinical trials; (2) incorporating the concept of multimorbidity into clinical guidelines; (3) integrating care of patients with multiple chronic conditions (MCCs) into health professions education; (4) designing payment incentives to support care coordination; and (5) integrating self-care management with structured case management.
Who Is the Target Population?
With the aim of reducing uncoordinated care expected to lead to excessive healthcare costs, “medically complex patients” are those exhibiting patterns of use of care demonstrating lack of coordination (Owens, 2009). Such individuals are likely those, for example, who are hospitalized for ambulatory care-sensitive conditions, who are readmitted within 30 days to hospitals for preventable conditions, who access emergency rooms for primary care, or who have inconsistent drug use and adherence patterns along with a host of different prescribers and pharmacies. Ideally, all payers, public and private, need to identify individuals whose patterns of care demonstrate a lack of coordination and analyze their care provision. More commonly, payers have selected specific groups of beneficiaries as proxies for the uncoordinated care population, such as beneficiaries associated with high-cost care, specific chronic diseases, or severe disease.
One broad group increasingly considered to represent the uncoordinated care population includes beneficiaries with multiple concurrent chronic conditions. A robust body of literature has shown that as individuals accrue more chronic conditions, significant health outcomes worsen, including increased mortality, poorer functional status, more unnecessary hospitalizations for ambulatory care-sensitive conditions, additional adverse drug events, increased episodes of conflicting medical advice, and augmented duplicative tests (Johns Hopkins University and Partnership for Solutions, 2008; Lee et al., 2007; Vogeli et al., 2007; Warshaw, 2006;
Wolff et al., 2002). In addition, total healthcare spending, prescription drug spending, and out-of-pocket spending also rise with the number of chronic conditions.
This paper focuses on the population with multiple chronic conditions as a group at high risk for uncoordinated care. In addition, the MCC population serves the purpose of introducing several policy areas that could be explored to support care coordination and chronic care management.
Provider-Focused Policy Levers
Improving the External Validity of Clinical Trials
Many randomized controlled clinical trials (RCTs) exclude patients with multiple chronic conditions to ensure the internal validity of the findings; in other cases, investigators do not report the comorbidities of patients enrolled in RCTs (Fortin et al., 2006). Unfortunately, once approved, the subject drugs or devices are often prescribed for patients with multiple chronic conditions without safety or efficacy data. Not surprisingly, these MCC patients are at higher risk for adverse events and ineffective treatments. From a public health perspective, although a more robust postmarketing surveillance system would be helpful to better characterize these events, a more proactive and preventive strategy to address safety and efficacy would ensure that RCTs include these patients in the first place. The potential costs of recruiting more participants to take into account individuals with multiple chronic conditions need to be weighed against the potential back end costs of dealing with major adverse events in an increasingly large population with multiple chronic conditions that has not been adequately included in studies.
Incorporating Multimorbidity in Clinical Guidelines
A review of clinical practice guidelines for the treatment of older patients with various chronic conditions has demonstrated that many guidelines do not contain specific recommendations or modifications for patients with multiple comorbid conditions (Boyd et al., 2005). Thus, healthcare providers currently have little guidance on how to apply guidelines when treating patients with multimorbidities. In lieu of this, clinicians taking care of patients with multiple chronic conditions likely follow numerous single-disease guidelines for the specific conditions of their MCC patients. However, the benefits or harms of combining the recommendations in each of several guidelines for an individual are unknown (Tinetti et al., 2004). Theoretically, many drug-drug and drug-disease interactions might occur, particularly in the multiple chronic condition population. Health services
and comparative effectiveness research are needed to prioritize prevention, treatment, and management decisions for this population and to develop best practices to guide providers in quality care.
Integrating Care of Patients with Multiple Chronic Conditions in Health Professions Education
Physicians have deemed their medical training for chronic illness care as inadequate for a variety of competencies such as geriatric syndromes, chronic pain, nutrition, patient education, coordination of services, and interdisciplinary teamwork (Darer et al., 2004). To prepare for an increasingly medically complex population, all health professional students likely to care for patients with MCCs need to learn how to prioritize treatment of chronic conditions in individuals, deal with drug-disease interactions, and consider patient preferences when making care plans. Modification of existing undergraduate and graduate health professional school curriculums should be undertaken to address this point. In addition, use of GME funding as a policy lever to focus training on chronic care management and coordination should be considered. For example, as a condition to receiving full GME funding, teaching hospitals could be required to ensure that their trainees in certain applicable residency programs receive training in chronic care competencies.
Designing Payment Incentives to Support Care Coordination
Initial results from Medicare demonstration projects designed to support payment incentives to providers for care coordination and chronic disease management have been sobering. Lessons learned include the importance of frequent in-person contact with patients, a focus on transitional care, promoting self-care management and adherence to care, and clear communication channels between care coordinators and primary care providers (Bott et al., 2009; Peikes et al., 2009). Many previous disease management efforts focused on individual illnesses and, regrettably, ignored the interplay between multiple chronic conditions. All of these lessons need to be applied to newer models of care coordination currently being considered in health reform legislation, including the medical home, community health teams, accountable care organizations, and transitional care strategies. Selecting a narrow target population, testing competing models of care, overcoming implementation challenges including recruitment of patients and providers, and closely monitoring real-time data are needed for more rapid acceptance of payment models that support care coordination into the Medicare program.
Patient-Focused Policy Levers
Integrating Self-Care Management with Structured Case Management
As chronic conditions increase, the number of prescribed medications, adverse drug events, and episodes of medication noncompliance also rises. Self-care management is essential to ensure medication adherence and overall symptom awareness and management. Providers can reinforce good health behaviors through education, particularly through novel patient encounter strategies such as group visits and secure messaging. Payers should develop pilot programs to confirm that chronic disease self-care management and novel patient encounter strategies reduce health costs by decreasing hospitalizations and utilization of care. In addition, patient incentives to adopt healthy lifestyle choices, such as reduced cost sharing, should be studied to see if they reduce the burden of disease and lower healthcare costs.
Looking Forward
By addressing these policy areas, providers and patients will have better information and incentives to coordinate and manage the care of complex patients, such as those with multiple chronic conditions. Defining the target population for these efforts is critically important to realizing any potential cost savings or quality enhancement.
REFERENCES
American Academy of Hospice and Palliative Medicine. 2008. Fellowship program directory. Chicago, IL.
Anderson, G., and J. Horvath. 2002. Making the case for ongoing care. Princeton, NJ: Robert Wood Johnson’s Partnership for Solutions.
Bach, P. B. 2007. How many doctors does it take to treat a patient? http://online.wsj.com/articles/SB118238673713942795.html (accessed June 21, 2007).
Becker, E. 1973. The denial of death. New York: Free Press Paperbacks.
Bohmer, R. M. J., and E. M. Ferlins. 2005. Harvard business school case 606-044.2005. Virginia Mason Medical Center. Virginia.
Bott, D. M., M. C. Kapp, L. B. Johnson, and L. M. Magno. 2009. Disease management for chronically ill beneficiaries in traditional Medicare. Health Affairs (Millwood) 28(1):86-98.
Boyd, C. M., J. Darer, C. Boult, F. L.P., L. Boult, and A. W. Wu. 2005. Clinical practice guidelines and quality of care for older patients with multiple comorbid diseases: Implications for pay for performance. Journal of the American Medical Association 294(6):716-724.
Brown, R., D. Peikes, A. Chen, J. Ng, J. Schore, and C. Soh. 2007. The evaluation of the Medicare coordinated care demonstrations: Findings for the first two years. New Jersey: Mathematica Policy Research, Inc.
Center to Advance Palliative Care and the National Palliative Care Research Center. 2008. America’s care of serious illness: A state-by-state report card on access to palliative care in our nation’s hospitals. New York.
Darer, J., D. H. Hwang, H. H. Pham, E. B. Bass, and G. Anderson. 2004. More training needed in chronic care: A survey of U.S. physicians. Academic Medicine 79(6):541-548.
Dartmouth Atlas. 2008. Chronic care atlas. http://www.dartmouthatlas.org/atlases/2008_Chronic_Care_Atlas.pdf (accessed August 1, 2009).
Darwin, C. 2003. The origin of species. New York: Signet Classics.
Field, M. J., and R. E. Behrman, eds. 2003. When children die: Improving palliative and end-of-life care for children and their families. Washington, DC: The National Academies Press.
Field, M. J., and C. K. Cassel, eds. 1997. Approaching death: Improving care at the end of life. Washington, DC: National Academy Press.
Fortin, M., J. Dionne, G. Pinho, J. Gignac, J. Almirall, and L. Lapointe. 2006. Randomized controlled trials: Do they have external validity for patients with multiple comorbidities? Annals of Family Medicine 4:104-108.
Goldsmith, B., J. Dietrich, Q. Du, and R. S. Morrison. 2008. Variability in access to hospital palliative care in the United States. Journal of Palliative Medicine 11(8):1094-1102.
Hanckock, J. 2009. Grassley: Government shouldn’t “decide when to pull the plug on grandma.” Iowa Independent, August 12.
Himmelstein, D. U., E. Warren, D. Thorne, and S. Woolhandler. 2005. Illness and injury as contributors to bankruptcy. Health Affairs (Millwood) Suppl Web Exclusives: W5-63-W65-73.
Hofmann, J. C., N. S. Wenger, R. B. Davis, J. Teno, A. F. Connors, Jr., N. Desbiens, J. Lynn, and R. S. Phillips. 1997. Patient preferences for communication with physicians about end-of-life decisions. Support investigators. Study to understand prognoses and preference for outcomes and risks of treatment. Annals of Internal Medicine 127(1):1-12.
IOM (Institute of Medicine). 2001. Improving palliative care for cancer. Washington, DC: National Academy Press.
——. 2007. Rewarding provider performance: Aligning incentives in Medicare (Pathways to Quality Health Care Series). Washington, DC: The National Academies Press.
Johns Hopkins University and Partnership for Solutions. 2008. Chronic conditions: Making the case for ongoing care. http://www.fightchronicdisease.com/news/pfcd/documents/ChronicCareChartboo,_FINAL.pfd (accessed June 1, 2008).
Lee, T., A. E. Shield, C. Vogeli, T. B. Gibson, M. Woong-Sohn, W. Marder, D. Blumenthal, and K. B. Weiss. 2007. Mortality rate in veterans with multiple chronic conditions. Journal of General Internal Medicine 22(3):403-407.
McGlynn, E. A., S. M. Asch, J. Adams, J. Keesey, J. Hicks, A. DeCristofaro, and E. A. Kerr. 2003. The quality of health care delivered to adults in the United States. New England Journal of Medicine 348(26):2635-2645.
Medical Board of California. 2009. AB 487: Pain management and the appropriate care and treatment of the terminally ill. http://www.medbd.ca.gov/licensee/continuing_education_laws.html (accessed September 21, 2009).
MedPAC (Medicare Payment Advisory Commission). 2009. Report to the Congress: Improving incentives in the Medicare program. Washington, DC.
Meier, D. E. 2003. When pain and suffering do not require a prognosis: Working toward meaningful hospital-hospice partnership. Journal of Palliative Medicine 6(1):109-115.
Milstein, A., and B. Gilbertson. 2009. American medical home runs. Health Affairs (Millwood) 28(5):1317-1326.
Morrison, R. S., and D. E. Meier. 2004. Clinical practice: Palliative care. New England Journal of Medicine 350(25):2582-2590.
Morrison, R. S., J. D. Penrod, J. B. Cassel, M. Caust-Ellenbogen, A. Litke, L. Spragens, and D. E. Meier. 2008. Cost savings associated with U.S. hospital palliative care consultation programs. Archives of Internal Medicine 168(16):1783-1790.
NIH (National Institutes of Health). 2002. Symptom management in cancer: Pain, depression and fatigue. http://consensus.nih.gov/2002/2002CancerPainDepressionFatiguesos022html.htm (accessed June 23, 2006).
——. 2004. National Institutes of Health state-of-the-science conference statement on improving end-of-life care. http://consensus.nih.gov/2004/2004EndOfLifeCareSOS024htmlhtm. (accessed May 2009).
National Quality Forum. 2006. A national framework and preferred practices for palliative and hospice care quality. Washington, DC.
O’Gorman, S. M. 1998. Death and dying in contemporary society: An evaluation of current attitudes and the rituals associated with death and dying and their relevance to recent understandings of health and healing. Journal of Advanced Nursing 27(6):1127-1135.
Owens, M. 2009. Identifying and quantifying the cost of uncoordinated care: Opportunities for savings and improved outcomes. Paper read at The Healthcare Imperative: Lowering Costs and Improving Outcomes workshop, Washington, DC, July 16-18.
Paulus, R. A., K. Davis, and G. D. Steele. 2008. Continuous innovation in health care: Implications of the Geisinger experience. Health Affairs (Millwood) 27(5):1235-1245.
Peikes, D., A. Chen, J. Schore, and R. Brown. 2009. Effects of care coordination on hospitalization, quality of care, and health care expenditures among Medicare beneficiaries: 15 randomized trials. Journal of the American Medical Association 301(6):603-618.
Pronovost, P. J., D. C. Angus, T. Dorman, K. A. Robinson, T. T. Dremsizov, and T. L. Young. 2002. Physician staffing patterns and clinical outcomes in critically ill patients. Journal of the American Medical Association 288(17):2151-2162.
Reid, P., W. Compton, J. Grossman, and G. Fanjiang. 2005. Building a better delivery system: A new engineering/health care partnership. Washington, DC: The National Academies Press.
Reynolds, P. 2008. Title VII innovations in American medical and dental education: Responding to 21st century priorities for the health of the American public. Academic Medicine 83(11):1015-1020.
Schoen, C., S. Guterman, A. Shih, J. Lau, S. Kasimow, and A. Gauthier. 2007. Bending the curve: Options for the Commonwealth Fund Commission on a high performance health system. http://www.commonwealthfund.org/~/media/Files/Publications/Fund%20Report/2007/Dec/Bending%20the%20Curve%20%20Options%20for%20Achieving%20Savings%20and%20Improving%20Value%20in%20U%20S%20%20Health%20Spending/Schoen_bendingthecurve_1080%20pdf.pdf (accessed September 19, 2009).
Siska, A., et al. 2009. Health spending projections through 2018: Recession effects add uncertainty to the outlook. Health Affairs 28(2):w346-w357.
Tinetti, M. E., S. T. Bogardus, Jr., and J. V. Agostini. 2004. Potential pitfalls of disease-specific guidelines for patients with multiple conditions. New England Journal of Medicine 351(27):2870-2874.
U.S. Department of Health and Human Services. 2009. National Institutes of Health loan repayment program. http://www.lrp.nih.gov/ (accessed September 21, 2009).
Vogeli, C., A. E. Shields, T. A. Lee, T. B. Gibson, W. D. Marder, K. B. Weiss, and D. Blumenthal. 2007. Multiple chronic conditions: Prevalence, health consequences, and implications for quality, care management, and costs. Journal of General Internal Medicine 22(Suppl 3):391-395.
Wang, Y., and M. A. Beydoun. 2007. The obesity epidemic in the United States—Gender, age, socioeconomic, racial/ethnic, and geographic characteristics: A systematic review and meta-regression analysis. Epidemiologic Reviews 29:6-28.
Warshaw, G., ed. 2006. Introduction: Advances and challenges in care of older people with chronic illness. Generations 30(3):5-10.
Weber, V., F. Bloom, S. Pierdon, and C. Wood. 2008. Employing the electronic health record to improve diabetes care: A multifaceted intervention in an integrated delivery system. Journal of General Internal Medicine 23(4):379-382.
Wolff, J. L., B. Starfield, and G. Anderson. 2002. Prevalence, expenditures, and complications of multiple chronic conditions in the elderly. Archives of Internal Medicine 162(20):2269-2276.
























