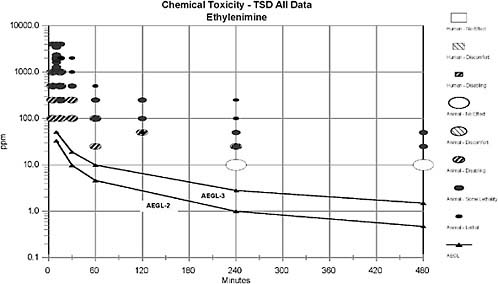
4
Ethylenimine1
Acute Exposure Guideline Levels
PREFACE
Under the authority of the Federal Advisory Committee Act (FACA) P.L. 92-463 of 1972, the National Advisory Committee for Acute Exposure Guideline Levels for Hazardous Substances (NAC/AEGL Committee) has been established to identify, review and interpret relevant toxicologic and other scientific data and develop AEGLs for high priority, acutely toxic chemicals.
AEGLs represent threshold exposure limits for the general public and are applicable to emergency exposure periods ranging from 10 min (min) to 8 h (h). Three levels—AEGL-1 and AEGL-2, and AEGL-3—will be developed for each of five exposure periods (10 and 30 min, 1 h, 4 h, and 8 h) and will be distinguished by varying degrees of severity of toxic effects. It is believed that the recommended exposure levels are applicable to the general population including infants and children, and other individuals who may be susceptible. The three AEGLs have been defined as follows:
AEGL-1 is the airborne concentration (expressed as parts per million or milligrams per cubic meter [ppm or mg/m3]) of a substance above which it is predicted that the general population, including susceptible individuals, could
experience notable discomfort, irritation, or certain asymptomatic, non-sensory effects. However, the effects are not disabling and are transient and reversible upon cessation of exposure.
AEGL-2 is the airborne concentration (expressed as ppm or mg/m3) of a substance above which it is predicted that the general population, including susceptible individuals, could experience irreversible or other serious, long-lasting adverse health effects or an impaired ability to escape.
AEGL-3 is the airborne concentration (expressed as ppm or mg/m3) of a substance above which it is predicted that the general population, including susceptible individuals, could experience life-threatening health effects or death.
Airborne concentrations below the AEGL-1 represent exposure levels that could produce mild and progressively increasing but transient and nondisabling odor, taste, and sensory irritation, or certain asymptomatic, non-sensory effects. With increasing airborne concentrations above each AEGL, there is a progressive increase in the likelihood of occurrence and the severity of effects described for each corresponding AEGL. Although the AEGL values represent threshold levels for the general public, including susceptible subpopulations, such as infants, children, the elderly, persons with asthma, and those with other illnesses, it is recognized that individuals, subject to idiosyncratic responses, could experience the effects described at concentrations below the corresponding AEGL.
SUMMARY
Ethylenimine is a volatile, clear, colorless, flammable explosive liquid that has an odor similar to that of ammonia and an odor threshold reported as 2 ppm in air; however an odor detection (OD50) was reported as 0.698. It is a very reactive direct-acting alkylating agent, the activity of which is similar to that of nitrogen and sulfur mustards. It is also very caustic, attacking numerous substances including plastics, metals, and glass that does not contain carbonate or borax. Estimates of annual U.S. production of ethylenimine range between 1.65 and 4.85 million pounds prior to 1994. Ethylenimine is used in the manufacture of products such as triethylenemelamine, paper, textile chemicals, adhesive binders, and petroleum refining chemicals. Ethylenimine is stored in 320-pound cylinders, but shipping quantities are unknown.
Relevant data on ethylenimine consisted of only a few case reports in humans and acute inhalation lethality studies in laboratory animals. Toxicity due to exposure to ethylenimine is generally delayed and includes irritation to contact organs (skin, eyes, oral cavity, and upper and lower respiratory tract), systemic toxicity, and death depending upon the concentration. At extremely high concentrations, however, irritation to contact organs may occur during or soon after exposure. The time course of irritation caused by ethylenimine is different from that caused by primary irritants such as ammonia, which causes an immediate response upon exposure regardless of concentration.
One person died after a brief exposure to a high, but unknown concentration of ethylenimine. Soon after exposure he experienced eye irritation, salivation, vomiting, and breathlessness. Pulmonary edema was diagnosed but was not considered the cause of death. Several people exposed to ethylenimine and N-ethylethylenimine for 1½ to 2 h suffered severe eye and respiratory tract irritation and vomiting that were delayed 3 to 7½ h after exposure, followed by hemoconcentration (increased in hemoglobin concentration), eosinophilia, and albuminuria. Occupational exposure to ethylenimine has produced skin sensitization, slow-healing dermatitis, rapidly reversible irritation to the eyes and respiratory tract, and blistering, reddening, and edema of the scrotum. Direct contact of liquid ethylenimine with the tongue caused delayed inflammation and edematous swelling in the mouth and inflammation of the eyes, and direct contact of liquid ethylenimine with the skin caused necrotizing painless burns. Ethylenimine is genotoxic in all test systems investigated including bacteria, fungi, plants, insects, and cultured mammalian cells. It is clastogenic in cultured human cells. Repeated subcutaneous injections of rats with ethylenimine produced sarcomas at the injection site, chronic oral administration produced pulmonary and liver tumors in mice, and a single subcutaneous injection of 7-day old mice produced pulmonary tumors.
Acute inhalation LC50 values were 2558, 1407, 545, 268, 259, 58, and 35 ppm for rats exposed to ethylenimine for 5, 10, 15, 60, 120, 240, or 480 min, respectively; LC50 values were 2906, 2824, 1283, 364, 235, 158, 45, and 27 ppm for guinea pigs exposed for 5, 10, 15, 30, 60, 120, 240, or 480 min, respectively (Carpenter et al. 1948); and the LC50 value was 2236 ppm for mice exposed for 10 min. In all studies, the time to death and other signs of toxicity were delayed depending on exposure concentration. Signs of toxicity in these animals included eye irritation, respiratory tract irritation, respiratory difficulty, prostration, complete loss of muscular coordination (mouse only), and convulsions (mouse only). Systemic effects included lung damage, congestion in lungs and all internal organs, damage to the kidney tubules, and albuminuria in rats and guinea pigs.
Data were not available for deriving AEGL-1 values for ethylenimine. The absence of AEGL-1 values does not imply that exposure below the AEGL-2 is without adverse health effects. The level of distinct odor awareness (LOA) for ethylenimine is 10.9 ppm. The LOA represents the concentration above which it is predicted that more than one-half the exposed population will experience at least a distinct odor intensity and about 10% of the population will experience a strong odor intensity. The LOA should help chemical emergency responders in assessing the public awareness of the exposure due to odor perception. Ethylenimine may not have distinct AEGL-1 warning properties.
No animal studies designed specifically to examine nonlethal toxicity due to acute inhalation exposure to ethylenimine were located in the search, and the human case report involved exposure to other substances that could have contributed to the observed toxic effects. Although the logical point of departure
(POD) for deriving AEGL-2 values is the no-observed-effect level (NOEL) for extreme respiratory difficulty in guinea pigs exposed to 10 ppm ethylenimine for 480 min (Carpenter et al. 1948), this derivation would lead to AEGL-2 values close to or exceeding the life-threatening AEGL-3 concentrations. Therefore, AEGL-2 values were derived using the next shorter duration of 240 min, which also is a clear NOEL for respiratory difficulty in guinea pigs exposed to 10 ppm. The total uncertainty factor was 10. An uncertainty factor of 3 was applied for interspecies differences, because ethylenimine is a very reactive direct-acting alkylating agent, and the AEGL-2 effects would most likely be confined to the respiratory tract. Respiratory tract damage appears to be due to direct effect of an alkylating agent on the respiratory epithelium, and this mechanism is not expected to be different among species. Humans and animals exhibit delays between the time of exposure and the onset of symptoms, and the eyes and respiratory tract were the most sensitive targets in rat, guinea pigs, and humans. An uncertainty factor of 3 was applied for intraspecies variability because the effects appear to involve direct contact of the eyes or respiratory epithelium with a very reactive alkylating agent and the alkylating activity is not expected to vary appreciably among individuals in the population. Five male students responded similarly to an exposure to ethylenimine with respect to the time of onset of symptoms and the intensity of effects. Studies have shown that DNA damage is likely the initiating step in a cascade of events leading to cell damage and DNA damage can persist in lungs and systemic organs following inhalation exposure to alkylating agents. This mechanism is unlikely to be different among individuals in the human population or among species. Scaling across the pertinent time frames was based on the equation C0.91 × t = k, where n = 0.91 was derived from the LC50 data for guinea pigs. The AEGL-2 values do not account for the potential carcinogenicity of ethylenimine, because quantitative data were not available for deriving a unit risk value.
AEGL-3 values were based on an acute inhalation study in rats (Carpenter et al. 1948). The LC01 (lethality threshold) of 15 ppm for the 8-h exposure duration was estimated by probit analysis. The 8-h LC01 was selected because it had the smallest standard error. A total uncertainty factor of 10 was applied to the LC01. An uncertainty factor of 3 was applied for interspecies differences based on the same rationale as described for AEGL-2 derivation. In addition, the LC50 values for three test animal species were within a factor of 2 of each other, and like other effects of ethylenimine, death was delayed in all three species. Similarly, humans experienced a delay in the onset of life-threatening and/or very serious effects after exposure to ethylenimine. An uncertainty factor of 3, instead of the default of 10, was applied for intraspecies variability based on the same rationale described for the AEGL-2 derivation. Scaling across the pertinent time frames was based on the equation C1.1 × t = k, where n = 1.1 was derived from LC50 data for rats.
AEGL values derived for ethylenimine are presented in Table 4-1.
TABLE 4-1 Summary of AEGL Values for Ethyleniminea,b [ppm (mg/m3)]
|
Classification |
10- min |
30-min |
1-h |
4-h |
8-h |
End Point (Reference) |
|
AEGL-1 (Nondisabling) |
Not recommendedc |
|
|
|
|
|
|
AEGL-2 (Disabling) |
33 (59) |
9.8 (18) |
4.6 (8.2) |
1.0 (1.8) |
0.47 (0.84 ) |
NOEL for extreme respiratory difficulty (Carpenter et al. 1948) |
|
AEGL-3 (Lethal) |
51 (91) |
19 (34) |
9.9 (18) |
2.8 (5.0) |
1.5 (2.7) |
Threshold for lethality (Carpenter et al. 1948) |
|
aAEGL-2 and -3 values do not account for the potential cancer risk associated with exposure to ethylenimine, because quantitative data were not available for deriving a unit risk value. bEffects at these concentrations may be delayed following exposure. cThe absence of AEGL-1 values does not imply that exposure below the AEGL-2 is without adverse health effects. |
||||||
1.
INTRODUCTION
Ethylenimine is a volatile, clear, colorless, flammable, and explosive liquid. It readily polymerizes, and it behaves like a secondary amine (Trochimowicz et al. 1994). Ethylenimine is highly caustic, attacking materials such as cork, rubber, many plastics, metals, and glass except those without carbonate or borax (Gresham and West 1975), and it polymerizes explosively on contact with silver, aluminum, or acid (IARC 1999). Ethylenimine has a high vapor pressure; therefore, it readily vaporizes at room temperature. It has a strong ammonia-like odor detectable by humans at 1.5 to 2 ppm (Carpenter et al. 1948, Santodonato 1985). Van Doorn et al. (2002) reported an odor detection (OD50) of 0.698 ppm. Physical and chemical properties of ethylenimine are presented in Table 4-2.
Ethylenimine is a direct-acting monofunctional alkylating agent. The alkylating property is dependent on formation of an ethylenimonium ion, and the free base can be transported across the cell membrane (Ramel 1981). About 91% of ethylenimine is present as the imonium ion at pH 7. The activity of ethylenimine is similar to that of nitrogen and sulfur mustards.
Ethylenimine is used as an intermediate in the production of triethylene-melamine; polymerized ethylenimine is used in paper, textile chemicals, adhesive binders, petroleum refining chemicals, fuels, lubricants, coating resins, varnishes, lacquers, agricultural chemicals, cosmetics, ion-exchange resins, photographic chemicals, colloid flocculants, and surfactants (Trochimowicz et al. 1994). In 1964, 750 metric tons (1.65 million pounds) of ethylenimine were being produced annually in the United States and twice that rate was produced by 1978 (Ham 1981). Santodonato (1985) reported production in the United States as greater than 3.3 million pounds in 1978. World production in 1981 was about 12,500 tons (Ham 1981). In 1994, Trochimowicz et al. noted that there was only one domestic producer of ethylenimine, which had a production capac-
ity of 2.2 million kilograms (4.85 million pounds). Cordova Chemical Co. Muskegon, MI produced ethylenimine in the early 1980s, and announced that they would no longer permit shipping of the material off site as of January 1983 (Santodonato 1985), and are no longer producing the chemical (personal communication, March 25, 1997). Current producer(s) of ethylenimine are unknown, and no current information on shipping quantities was found in the literature. According to a NIOSH report, ethylenimine is stored in 320-pound cylinders (Ruhe 1982).
The database on inhalation exposure to ethylenimine is limited to only a few human case reports and acute inhalation studies in animals.
TABLE 4-2 Physical and Chemical Data for Ethylenimine
|
Parameter |
Data |
Reference |
|
Chemical Name |
Ethylenimine |
|
|
Synonyms |
Ethyleneimine, aziridine, dimethylenimine, azacyclopropane, azirane, ENT-50324 |
RTECS 2008 |
|
CAS Reg. No. |
151-56-4 |
RTECS 2008 |
|
Chemical Formula |
C2H5N |
RTECS 2008 |
|
Molecular Weight |
43.08 |
RTECS 2008 |
|
Physical State |
Clear, colorless liquid mobile, colorless, very volatile fluid |
Lewis 1993 Trochimowicz et al. 1994 |
|
Odor |
Ammonia-like |
Carpenter et al. 1948 |
|
Melting Point |
−73.96°C −71°C |
O’Neil et al. 2001 Verschueren 1996 |
|
Boiling Point |
56 to 57°C at 760 mm Hg 55 to 56°C |
O’Neil et al. 2001 Verschueren 1996 |
|
Freezing Point |
−78°C |
Lewis 1993 |
|
Flash Point |
−11.1°C |
Lewis 1993 |
|
Density |
0.8321 (24°C /4°C) |
O’Neil et al. 2001 |
|
Solubility |
Completely miscible in water Soluble in alcohol |
Verschueren 1996 O’Neil et al. 2001 |
|
Vapor Pressure |
13.3 kPa (100 mm Hg) at 9.7°C 160 mm Hg at 20 °C 250 mm Hg at 30°C |
Ham 1981 O’Neil et al. 2001 Verschueren 1996 |
|
Vapor density |
1.5 |
Verschueren 1996 |
|
Autoignition Temperature |
322°C |
Lewis 1993 |
|
Flammability Limits in air |
3.6 to 46% |
Lewis 1993 |
|
Explosive limit |
3.6% (lower) to 4.6% (upper) |
Trochimowicz et al. 1994 |
|
Saturated conc. in air |
375 g/m3 at 2°C 567 g/m3 at 30°C |
Verschueren 1996 |
|
Alkalinity |
Strongly alkaline |
O’Neil et al. 2001 |
|
Conversion |
1 mg/m3 = 0.56 ppm; 1 ppm = 1.79 mg/m3 |
Verschueren 1996 |
2.
HUMAN TOXICITY DATA
2.1.
Acute Lethality
Gresham and West (1975) described an accident in which a 57-year-old man was exposed to ethylenimine vapor for “probably not more than five minutes” while dispensing the chemical into small containers. He showed signs of eye, nasal, and laryngeal irritation along with salivation, vomiting, and acute breathlessness. Clinical examination revealed that he had pulmonary edema. He required assisted respiration for several weeks and was dismissed from the hospital 5 weeks after admission. Three weeks later, he was readmitted to the hospital as his condition suddenly deteriorated as he developed shortness of breath, a wheezy cough, bronchospasm, tracheal ulcerations, and stenosis. He died 2 weeks later (10 weeks after exposure). The autopsy showed collapsed and flabby trachea and bronchi and pulmonary edema. Histopathologic examination showed destruction of the cartilaginous structure of the tracheobronchial tree, i.e. the mucosa was replaced by granulation or occasionally fibrous tissue. Granulomatous polyps were found in the smaller bronchi along with emphysema and bronchopneumonia. There was some debate whether tracheal destruction was a direct effect of inhaling ethylenimine or if it was caused by the extensive delay in granulation resulting from the aggressive steroid therapy in the early stages of treatment. The authors concluded that the tracheal damage was likely due to delayed granulation of the trachea caused by steroid therapy; consequently, there was doubt that his death resulted from inhaling ethylenimine. The concentration of ethylenimine to which this worker was exposed was not known, but the severity of the initial symptoms suggested that exposure was intense.
2.2.
Nonlethal Toxicity
2.2.1.
Odor Threshold
Carpenter et al. (1948) reported that 2 ppm was the lowest concentration at which eight subjects detected the odor of ethylenimine upon entering a room. The odor of ethylenimine is described as being similar to that of ammonia; therefore, the odor does not serve as a specific warning for the presence of ethylenimine in the air (Carpenter et al. 1948).
The level of distinct odor awareness (LOA) for ethylenimine calculated based on an odor threshold (OT50) of 0.698 ppm and using the guidance provided by van Doorn et al. (2002) is 10.9 ppm. The LOA calculation is presented in Appendix C.
2.2.2.
Experimental Studies, Case Reports, and Anecdotal Data
Carpenter et al. (1948) reported that eye and nose irritation does not occur in humans exposed to concentrations of ethylenimine vapor less than 100 ppm,
and that the onset of irritation is not “prompt” at this concentration. [This type of irritation appears to be different from that caused by a primary sensory irritant, which usually occurs immediately upon exposure.] No additional information was provided in this report to support the authors’conclusion. These are the only data reported by Carpenter et al. (1948), and no additional inferences or conclusions can be drawn from these data.
Exposure to ethylenimine was associated with skin sensitization in two laboratory workers. Severe slow-healing dermatitis of the hand in one worker engaged in production of ethylenimine and conjunctivitis, nasal irritation, and throat irritation that persisted for about 1 day in two or three workers was reported by Carpenter et al. (1948). No additional information was provided in this report.
In an attempt to force five male college students from a room, they were exposed successively to ammonia, isopentane, ethylenimine, and N-ethylethylenimine during a 2- to 3-h period (Weightman and Hoyle 1964). The men were exposed in a 10 × 14-foot ventilated room at a temperature of about 7 to 16°C.
They were first exposed to 500 mL of household ammonia and 100 mL of isopentane; the odors were removed with a ventilation fan in about 5 to 10 min. One hour later, they were exposed to 20 g of ethylenimine and 100 g of N-ethylethylenimine. When the men left the room about 1½ to 2 h later, they were aware of lacrimation and smarting of the eyes. However, it was not until 3 to 7 h later that symptoms of lacrimation, eye inflammation, photophobia, nausea, vomiting, and inflammation of the respiratory tract caused the men to seek medical attention. Profound hacking cough with normal chest findings developed in the first 12 h. Clinical observations included fever, conjunctival irritation, evidence of liver inflammation, transitory increase in hemoglobin concentrations up to 20 g, eosinophilia (7 to 13%), mild albuminuria, and extensive respiratory irritation manifested by decreased respiratory (pulmonary) function. Ulceration of the posterior nasal cavity was reported for one student and an abnormal electrocardiogram was noted in another. The students were released from the hospital within 11 to 25 days. Three students recovered completely in about 3 months, whereas two continued to show residual conjunctival inflammation and reduced respiratory function. The effects experienced by the students cannot be attributed entirely to ethylenimine, because these exposures involved both ethylenimine and N-ethylethylenimine. However, some symptoms (lacrimation, eye inflammation, photophobia, nausea, vomiting and extreme respiratory tract irritation) are similar to those described after exposure to sulfur mustard, another alkylating agent (ATSDR 2003, NRC 2003).
Ammonia causes irritation immediately upon exposure; the delayed onset of irritation to the eyes and respiratory tract of the students suggests that the eye and respiratory tract inflammation was not caused by exposure to ammonia. Isopentane is unlikely to cause damage to the respiratory tract. Concentrations up to 500 ppm are without effect in humans, and the LC50 for isopentane is extremely high for the mouse (1,000,000 mg/m3 = 339,000 ppm) (Cavender 1994). Toxicity information on N-ethylethylenimine could not be found; it is possible
that N-ethylethylenimine could be a weak alkylating agent similar to ethylenimine, and it may have contributed to the effects observed in the students.
Five maintenance workers in an ethylenimine plant experienced irritation and swelling of the scrotum after exposure to ethylenimine fumes (vapor) or in one case liquid ethylenimine (Thiess et al. 1971). Symptoms did not become noticeable until several hours after exposure or until the day after exposure. A burning sensation in the scrotal area, reddening and blistering of the scrotal skin, and painful swelling of the scrotum occurred in one worker after liquid ethylenimine was spilled on his trousers. He recovered within 8 days. The other workers who wore protective clothing and respirators experienced no signs or symptoms suggestive of exposure to the eyes or respiratory tract. Exposure to the scrotal area was believed to have occurred via the bottom opening of the pant legs of the rubber suits, which conducted the vapors like a chimney. One worker experienced superficial erosion and intense reddening of the scrotal area, another experienced severe swelling and blister formation on the scrotum, and two experienced “considerable” scrotal swelling. The skin lesions healed within a few days except for one worker who had edema that extended to the penis and preputium and aggravated a preexisting condition. He required surgery (circumcision) and 4 weeks to recover. The swelling of the scrotum was caused by edema of the scrotal area. The authors noted that spermiogenesis was not affected suggesting that the effects were local involving only the skin.
Danehy and Pflaum (1938) described a worker who inadvertently spilled one drop (about 50 μL) of ethylenimine on his tongue; he immediately rinsed his mouth with water. Two hours after the incident, vomiting occurred several times at about 30-min intervals followed by inflammation and edema of the epithelium in the mouth and throat, swelling of the uvula, and inflammation of the eyes. The symptoms resolved within 2 days after the incident. Inhalation exposure from this incident was possible, but would have been negligible. Danehy and Pflaum (1938) also reported that vapors (no concentration reported) inhaled for a short period of time (23 min) were “definitely toxic” to humans.
2.2.3.
Epidemiologic Studies
No epidemiologic studies on the toxicity of ethylenimine were located in the literature searched.
2.3.
Developmental and Reproductive Toxicity
No developmental or reproductive toxicity studies were located in the available literature.
2.4.
Carcinogenicity
An unconfirmed report noted that no evidence of carcinogenicity was found among 144 ethylenimine workers after 40 years of experience (D.J. Kilian, Dow Chemical, personal communication, Oct. 17, 1973). No other information on the potential carcinogenicity of ethylenimine in humans was located in the available literature.
2.5.
Genotoxicity
There was no significant increase in chromosome aberrations in leukocytes of chemical workers exposed to <0.5 ppm of ethylenimine for a mean of 8 years (Gaeth and Thiess 1972). No other in vivo human genotoxicity data were located in the available literature.
Significantly increased frequencies of chromatid breaks/gaps and exchanges were induced in cultured human embryonic lung fibroblast cells (WI-38) and in human leukocytes incubated with 10−4 M ethylenimine. Increased frequencies in chromatid breaks/gaps were observed in human fibroblast cells incubated with 10−5 M ethylenimine (Chang and Elequin 1967).
2.6.
Occupational Exposure
Occupational exposure to ethylenimine can occur during its production or use. Because ethylenimine is a suspect carcinogen, OSHA (29 CFR 1910.1003 [1999]) require that workers be protected against contact with and exposure to ethylenimine; therefore, occupational exposure is expected to be very low. Ruhe (1982) reported air concentrations ranging from 0.01 to 0.03 mg/m3 in an area where workers connect and disconnect ethylenimine cylinders. The air samples were collected by six lapel samplers worn by workers who wore full protective equipment including airline respirators (a type of supply air respirator) while carrying out these operations. The four workers handling the ethylenimine cylinders reported no health problems. The exposure to the workers were probably lower than the measured air concentrations. No other data on occupational exposure were located in the available literature.
2.7.
Summary
Although the odor threshold for ethylenimine is reported to be about 2 ppm, there is no specific warning of the presence of ethylenimine in air because the odor is similar to that of ammonia. Because individuals would not be able to distinguish the odor of ammonia and ethylenimine, they would not take steps to
avoid or lessen exposure if they thought they were exposed to ammonia. In contrast to ammonia, eye and respiratory tract inflammation or irritation may not be noticed during the actual exposure to ethylenimine.
One human fatality occurred after a brief exposure to a high, but unknown concentration of ethylenimine. Irritation was noticed soon after exposure, probably because of the high exposure concentration. However, it is possible that the cause of death was due to the secondary effects of treatment (steroid therapy) rather than exposure to ethylenimine.
Nonlethal effects due to inhalation exposure to ethylenimine are characterized by irritation or damage to contact organs; the effects are delayed in onset depending on exposure concentration. Severe eye and respiratory tract inflammation, photophobia, nausea, vomiting, and coughing may develop several hours after exposure to ammonia, isopentane, ethylenimine, and N-ethylethylenimine in succession. Hemoconcentration (markedly increased hemoglobin concentration), eosinophilia, albuminuria, and evidence of liver inflammation were noted during clinical examination. Symptoms following exposure to ammonia or isopentane are different from those described in this report suggesting that these compounds did not cause the observed effects. Skin sensitization, severe slow-healing dermatitis, and rapidly reversible eye and respiratory tract irritation have been associated with occupational exposure to ethylenimine. Direct contact of liquid ethylenimine on the tongue resulted in delayed serious effects on the oral cavity and eyes, and direct contact of liquid on the skin caused necrotizing painless burns. No data were found on the developmental/reproductive toxicity of ethylenimine in humans. Ethylenimine is clastogenic in cultured human cells. An unconfirmed report noted that no evidence of carcinogenicity was found among 144 ethylenimine workers after 40 years of experience (D.J. Kilian, Dow Chemical, personal communication, 1973).
Table 4-3 summarizes the lethal and nonlethal effects of ethylenimine in humans.
3.
ANIMAL TOXICITY DATA
3.1.
Acute Lethality
3.1.1.
Rats
Carpenter et al. (1948) conducted acute toxicity studies in Wistar rats exposed to ethylenimine by inhalation at concentrations ranging from 25 to 4000 ppm and exposure durations ranging from 5 to 480 min (8 h) (see Table 4-3). Each group consisted of five or six male rats. The concentration of ethylenimine in the exposure chamber was not determined analytically, but was verified by delivery rate, total volume of test material delivered, and consistent mortality response with increasing concentration. The age of the rats was not reported, but they weighed between 60 and 180 g at the time of exposure. The animals were
TABLE 4-3 Summary of Lethal and Nonlethal Effects on Ethylenimine in Humans
|
Concentration (ppm) |
Exposure Time |
Effects |
Comments |
Reference |
|
2 ppm |
NA |
Odor detection |
|
Carpenter et al. 1948 |
|
Unknown |
<5 min |
Vomiting, eye irritation, severe respiratory effects including pulmonary edema and destruction of tracheobronchial tree, death 10 weeks after exposure |
Death may have been caused by aggressive steroid therapy |
Gresham and West 1975 |
|
Unknown |
1½ to 2 h |
Severe vomiting, eye inflammation, photophobia, hemoconcentration, eosinophilia, coughing, extensive respiratory irritation |
Lacrimation and smarting of the eyes were noted at the end of exposure; other effects were delayed for several hours, the individuals were hospitalized 11 to 25 days; residual eye and respiratory effects remained 3 months after exposure; exposure to ammonia and isopentane ruled out as causative agents; N-ethylethylenimine could not be ruled out |
Weightman and Hoyle 1964 |
|
Unknown |
Not reported |
Damage to scrotal skin (reddening, blistering, swelling); no evidence of testicular effects |
Protective equipment prevented exposure to the eyes and respiratory tract; delayed onset (~12-24 h) |
Thiess et al. 1971 |
|
Unknown |
Unknown |
Skin sensitization |
Occurred in a laboratory worker |
Carpenter et al. 1948 |
|
Unknown |
Unknown |
Severe dermatitis, conjunctivitis, nose and throat irritation |
Effects on eyes, nose, and throat were transient |
Carpenter et al. 1948 |
|
NA (one drop on tongue, ca. 50 μL) |
Not reported, but probably only seconds |
Inflammation and edema of the epithelium of the oral cavity, inflammation of the eyes |
Inhalation exposure was possible, but negligible; study showed insidious nature of ethylenimine |
Danehy and Pflaum 1938 |
|
Spill of liquid on skin |
Not reported |
Necrotizing painless burns to hand; no other effects |
This study involved skin contact with liquid ethylenimine |
Weightman and Hoyle 1964 |
|
Concentration (ppm) |
Exposure Time |
Effects |
Comments |
Reference |
|
<0.5 ppm |
8 yr |
No chromosome aberrations detected |
This is an occupational exposure study |
Gaeth and Thiess 1972 |
|
10−5 to 10−4 M |
8 h |
Chromosome aberrations in human leukocytes and embryonic lung fibroblast cells |
In vitro study |
Chang and Elequin 1967 |
observed for 14 days after exposure. Clinical signs of toxicity consisted primarily of eye and respiratory tract irritation. Eye and nasal irritation occurred at concentrations of 100 ppm or higher, after 60 min at 100 ppm and immediately at concentrations greater than 100 ppm. No eye irritation was evident at concentrations of 10 to 50 ppm. Extreme respiratory difficulty was evident at all concentrations ≥25 ppm, but was not observed in less than 3 h at 25 ppm. No other details were presented concerning respiratory difficulty. Prostration was observed after exposure to 250 ppm for 3 h and after exposure to 500 ppm for 2 h. Prostration was not observed at other concentrations; at the higher concentration, death occurred without prostration. The individual exposure concentration, duration of exposure, mortality response at each concentration, and LC50 values are presented in Table 4-4. Deaths were generally delayed; one-half the deaths occurred before the end of day 3 and 9% occurred after day 10. Gross examination showed congestion and hemorrhage in the lungs and congestion in all internal organs. Microscopic examination showed pulmonary congestion and leakage of fluid and red blood cells into the bronchioles. Tubular epithelial necrosis and various degrees of cloudy swelling were seen in the kidneys. Clinical pathology showed albuminuria and transient leukopenia in rats the second day after exposure to 100 ppm for 120 min.
3.1.2.
Mice
Silver and McGrath (1948) exposed groups of 20 mice (strain and sex not specified) to nine concentrations of ethylenimine ranging from 2.1 to 6.1 mg/L (1176 to 3416 ppm) for 10 min and observed the surviving animals for 10 days. Exposures were based on measured concentrations of ethylenimine in the chamber atmosphere. The mortality response for each group is presented in Table 4-5. The mouse LC50 values may be slightly underestimated, because the mice were observed for only 10 days instead of 14 days after exposure. In the rat and guinea pig studies, 9% of the animals died more than 10 days after exposure (Carpenter et al. 1948). During exposure, the animals showed signs of eye and nose irritation within 2 min of initiating exposure and became hyperactive. Normal behavior resumed upon termination of exposure. Deaths occurred between 24 h postexposure to the end of the observation period. Prior to death, extreme prostration, complete loss of muscular coordination, and occasional violent convulsions were noted. The LC50 for a 10-min exposure was 2236 ppm.
Groups of mice were exposed to ethylenimine vapor at six concentrations ranging from 0.2 to 6.0 mg/L (114 to 3414 ppm) for 10 min and observed for ≤10 days (Todd and Taugher 1918). All mice died after exposure to concentrations of ≥3.5 mg/L (1960 ppm), whereas all mice survived after exposure to 1.0 mg/L (560 ppm). The deaths were delayed, but occurred within 48 h after exposure. No other details were available.
TABLE 4-4 Effects of Acute Exposure to Ethylenimine in Wistar Rats
|
Exposure Duration (min) |
Exposure Concentration (ppm) |
Mortality Response |
LC50a (ppm) |
|
5 |
100, 250, 500, 1000, 4000 |
0/6, 0/6, 1/6, 1/5, 4/6 |
2558 |
|
10 |
500, 1000, 2000, 4000 |
2/6, 4/6, 1/6, 5/6 |
1407 |
|
15 |
100, 250, 500, 1000, 2000, 4000 |
0/6, 1/6, 3/6, 5/6, 5/6, 6/6 |
545 |
|
30 |
500, 1000, 2000 |
5/6, 6/6, 5/5 |
Could not be determined |
|
60 |
100, 250, 500 |
0/6, 2/6, 6/6 |
268 |
|
120 |
50, 100, 250 |
0/6, 1/6, 3/6 |
259 |
|
240 |
25, 50, 100, 250 |
0/6, 2/5, 6/6, 6/6 |
58 |
|
480 |
25, 50 |
1/6, 5/6 |
35 |
|
aLC50 values calculated by probit analysis. Source: Carpenter et al. 1948. |
|||
TABLE 4-5 Mortality in Mice Exposed to Ethylenimine Vapor for 10 Minutes
|
Concentration |
||
|
mg/L |
ppm |
Mortality (%) |
|
2.1 |
1176 |
3/20 (15) |
|
2.3 |
1288 |
3/20 (15) |
|
2.9 |
1624 |
7/20 (35) |
|
3.3 |
1848 |
3/20 (15) |
|
3.4 |
1904 |
10/20 (50) |
|
3.5 |
1960 |
4/20 (20) |
|
3.7 |
2072 |
9/20 (45) |
|
4.2 |
2352 |
13/20 (65) |
|
6.1 |
3416 |
18/20 (90) |
|
LC50 value = 2236 ppm |
||
|
Source: Silver and McGrath 1948. Reprinted with permission; copyright 1948, Journal of Industrial Hygiene and Toxicology. |
||
3.1.3.
Guinea Pig
Carpenter et al. (1948) conducted acute toxicity studies in guinea pigs exposed to ethylenimine at concentrations ranging from 10 to 4000 ppm for durations ranging from 5 to 480 min (8 h). Each group consisted of 5, 6, or 12 guinea
pigs of both sexes. The concentration of ethylenimine in the exposure chamber was not determined by analytical measurement, but was verified by delivery rate, total volume of test material delivered, and consistent mortality response with increasing concentration. The age of the guinea pigs was not reported, but most weighed between 250 and 300 g when exposed to ethylenimine. The animals were observed for 14 days after exposure. The individual exposure concentrations, durations of exposure, mortality responses at individual concentrations, and LC50 values are presented in Table 4-6. Deaths were generally delayed with 50% dying by the end of day 3 and another 9% dying after day 10. Clinical signs of toxicity consisted primarily of eye and respiratory tract irritation. Eye irritation occurred 60 min after exposure to 100 ppm and immediately after exposure to concentrations greater than 100 ppm. No signs of eye irritation were observed at 10 to 50 ppm. Extreme respiratory difficulty was evident at all concentrations ≥25 ppm; it was not observed in less than 3 h at 25 ppm. Respiratory difficulty was not observed in guinea pigs exposed to 10 ppm for any duration. Prostration was observed 3 h after exposure to 250 ppm and 2 h after exposure to 500 ppm. Prostration was not evident at other concentrations; at the higher concentrations, death occurred without a preceding period of prostration. Gross pathologic examination showed congestion and hemorrhage in the lungs and congestion in all internal organs. Microscopic examination showed evidence of pulmonary congestion and leakage of fluid and red blood cells into the bronchioles. Tubular epithelial necrosis and various degrees of cloudy swelling were seen in the kidneys. Clinical pathology studies showed transient leukopenia, neutrophilia, and lymphopenia in guinea pigs exposed to 100 ppm of ethylenimine for 120 min.
3.2.
Nonlethal Toxicity
Six guinea pigs were shaved and exposed (except for the head) to ethylenimine vapor at a concentration of 4000 ppm for 4 h (Carpenter et al. 1948). No signs of toxicity were observed during the 14-day observation period, and no toxic effects on the skin were reported. There also was no effect on body weight gain. In contrast to inhalation exposure, no microscopic abnormalities were seen in the kidneys in guinea pigs that did not inhale ethylenimine. This study suggests that ethylenimine vapor was not absorbed into the systemic circulation through the skin or it is not absorbed at levels sufficient to cause systemic effects in the guinea pig. Studies on subcutaneous administration of ethylenimine showed that single or repeated injections of doses ≥1.25 mg/kg produced a characteristic lesion in the renal medulla, renal papillary necrosis (Axelsen 1978).
3.3.
Developmental and Reproductive Toxicity
No data were available on potential developmental or reproductive toxicity in laboratory animals exposed to ethylenimine by any route.
TABLE 4-6 Effects of Acute Exposure to Ethylenimine in Guinea Pigs
|
Exposure Duration (min) |
Exposure Concentration (ppm) |
Mortality Response |
LC50a (ppm) |
|
5 |
250, 500, 1000, 4000 |
0/6, 0/6, 0/6, 4/6 |
2906 |
|
10 |
2000, 4000 |
1/12, 6/6 |
2824 |
|
15 |
250, 500, 1000, 2000 |
0/6, 0/6, 0/6, 6/6 |
1283 |
|
30 |
100, 250, 500, 1000 |
0/6, 0/6, 5/6, 6/6 |
364 |
|
60 |
25, 100, 250, 500 |
0/12, 1/6, 2/6, 6/6 |
235 |
|
120 |
50, 100, 250, 500 |
0/6, 1/6, 5/6, 6/6 |
158 |
|
240 |
10, 25, 50, 100, 250 |
0/6, 2/5, 2/6, 6/6, 6/6 |
45 |
|
480 |
10, 25, 50 |
0/6, 2/6, 6/6 |
27 |
|
aLC50 values calculated using probit analysis. Source: Carpenter et al. 1948. Reprinted with permission; copyright 1948, Journal of Industrial Hygiene and Toxicology. |
|||
3.4.
Carcinogenicity
No data were available on the potential carcinogenicity of ethylenimine in laboratory animals exposed by inhalation. Oral and subcutaneous injection studies were available.
Walpole et al. (1954) studied the carcinogenicity of ethylenimine in rats injected subcutaneously with ethylenimine. Six male and six female albino rats per group were injected twice weekly with ethylenimine in arachis oil such that a total dose of 2.0 mg was administered in a total volume of 2.0 mL. Another six males and six females were treated similarly with a total dose of 1.0 to 1.2 mg in 1.0 to 1.2 mL of water. Controls were injected with similar volumes of arachis oil or water alone. Sarcomas developed at the injection site in 6/12 rats receiving ethylenimine in arachis oil compared with 0/24 receiving a comparable volume of arachis oil alone and in 2/12 rats injected with ethylenimine in water. A control for the latter groups injected with water was not described. The investigators did not explain the different responses after injecting ethylenimine in arachis oil and water.
BRL (1968) reported the results of carcinogenicity studies in which ethylenimine was given to mice by subcutaneous injection or by gavage/dietary administration. A single dose of 4.64 mg/kg was injected subcutaneously (distilled water vehicle) into 18 male and 18 female B6C3F1 or B6AKF1 mice. The same number of animals were given 4.64 mg/kg by gavage (gelatin vehicle) from 7 to 28 days of age inclusive; the compound was then administered at a dietary concentration delivering approximately the same dosage level until sacrificed 18 months after initiating the study. The dietary concentration was not adjusted to maintain the same dosage with changing body weight. There was no statistically significant increase in the incidence of tumors at any site in animals
injected with ethylenimine. Oral administration resulted in significant increases in incidences of pulmonary adenomas and hepatomas in male and female B6C3F1 mice and male B6AKF1 males; the incidence of pulmonary adenomas was significantly increased in B6AKF1 females.
Groups of 18 male and 18 female 7-day old (C57BL/6 × C3H/Anf)F1 and the same number of 7-day old (C57Bl/6 × AKR)F1 mice were injected subcutaneously with a 4.64-mg/kg body weight dose of ethylenimine and observed for 80 weeks (BRL 1968). The male mice of both strains developed lung tumors (5 or 6 of 18 mice), and two mice of the (C57BL/6 × C3H/Anf)F1 developed hepatomas and lymphomas. The total tumor incidence for both male strains was significantly greater than that of the controls. Only one female of each strain developed a lung tumor.
IARC (1999) recently evaluated the potential carcinogenicity of ethylenimine and concluded that ethylenimine is possibly carcinogenic to humans (Group 2B). This conclusion took into consideration that ethylenimine is a direct-acting alkylating agent that is mutagenic in a large number of test systems, including bacteria, insects, mammalian cells in culture, and mice (assessed in vivo by the dominant lethality test). IARC (1999) also noted that ethylenimine forms DNA adducts that are promutagenic.
3.5.
Genotoxicity
Ethylenimine is a very reactive monofunctional alkylating agent; the formation of an ethylenimonium ion accounts for its alkylating activity (Verschaeve and Kirsch-Volders 1990). Ethylenimine is a direct-acting genotoxic agent requiring no metabolic activation for its genotoxic activity. Ramel (1981) reported that ethylenimine had been tested for genetic toxicity in about 150 species and concluded that it is a very potent direct-acting mutagen, producing point mutations and chromosome aberrations. This chemical “is very mutagenic in all test systems investigated;” only a few negative results have been published and these were attributed to the use of low doses (Verschaeve and Kirsch-Volders 1990).
Ethylenimine is mutagenic in the Ames test in Salmonella typhimurium strains TA100 and TA1535 (Haroun and Ames 1981; Ramel 1981; Verschaeve and Kirsch-Volders 1990). It induces mitotic recombinations, gene conversions, and forward mutations in yeast and other fungi (Brockman et al. 1981; Loprieno 1981; Zimmermann 1981; Verschaeve and Kirsch-Volders 1990). Ethylenimine has been tested extensively in a variety of plant species and has been shown to be mutagenic and/or clastogenic in barley, wheat, Crepis capillaris, cotton, lettuce, cucumber, tobacco, rice, maize, and other plants. Ethylenimine induces high frequencies of dominant lethal mutations, sex-linked and autosomal recessive lethal mutations, and translocations in Drosophila melanogaster (Vogel et al. 1981). Ethylenimine is genotoxic in Bracon hebetor (parasitic wasp) and silkworm caterpillars and pupae (Verschaeve and Kirsch-Volders 1990). Chi-
nese hamster ovary cells had chromosome breaks after in vitro incubation with ethylenimine (Velazquez et al. 1973).
No studies were found on potential genotoxicity in laboratory animals exposed to ethylenimine by the inhalation route. Dominant lethality was evidenced by postimplantation loss in female mice mated with male C57BL/6 mice that received intraperitoneal injections of 5-6 mg/kg ethylenimine. The dominant lethality studies showed that postmeiotic spermatozoa were the target of ethylenimine (Malashenko 1968, Malashenko and Egorov 1968).
3.6.
Summary
The LC50 values for mice, rats, and guinea pigs are summarized in Table 4-7. All deaths occurred after exposure was terminated. In rats and guinea pigs, eye irritation was delayed at 100 ppm but occurred immediately upon initiating exposure to higher concentrations. Respiratory difficulty occurred at concentrations of 25 ppm and above, but at 25 ppm, respiratory difficulty was not observed in animals exposed for less than 3 h. No deaths or signs of toxicity occurred in guinea pigs exposed to 10 ppm of ethylenimine for 4 or 8 h; rats were not exposed to 10 ppm (Carpenter et al. 1948). All groups of mice were exposed to high concentrations (>1100 ppm), and the signs of toxicity (eye and respiratory irritation) occurred during the 10-min exposure. Death, however, was delayed and was preceded by prostration (seen at two concentrations in rats and guinea pigs), loss of muscular coordination, and convulsions (not observed in rats and guinea pigs) (Silver and McGrath 1948). Gross and histopathologic examination showed damage to the lungs, congestion in all internal organs, and damage to the kidney manifested by tubular necrosis and albuminuria in rats. The response to inhaled ethylenimine in rats and guinea pigs were similar, suggesting a similar mode of action, and mice responded similarly to rats and guinea pigs, with some minor differences. A quantitative difference may exist for the three species as evidenced by a twofold difference in the LC50 values for 10-min exposures to ethylenimine.
No inhalation studies specifically designed to examine effects of inhaling nonlethal concentrations of ethylenimine were located in the available literature. One study showed that dermal only exposure to ethylenimine vapor at a concentration of 4000 ppm for 4 h failed to elicit any signs of toxicity in guinea pigs (Carpenter et al. 1948).
Ethylenimine is a very reactive direct-acting alkylating agent that is genotoxic in all test systems investigated including bacteria, yeast and other fungi, various plant species, insects, and mammalian cells in vitro. An in vivo oral study showed dominant lethality in mice after intraperitoneal injection of ethylenimine (Malashenko and Egorov 1968). Ethylenimine has not been tested for carcinogenicity by the inhalation route, but it is carcinogenic in mice at a
TABLE 4-7 Summary of Lethality Data for Experimental Animalsa
|
Species/Sex |
LC50a |
Exposure Time (min) |
Comments |
|
|
ppm |
mg/m3 |
|||
|
Rat |
2558 |
4579 |
5 |
1/6 Died at lowest lethal concentration, 500 ppm |
|
Guinea pig |
2906 |
5202 |
5 |
None died at second highest concentration tested, 1000 ppm |
|
Rat |
1407 |
2519 |
10 |
2/6 Died at lowest concentration tested, 500 ppm |
|
Mouse |
2236 |
4002 |
10 |
3/20 died at lowest concentration tested, 1176 ppm |
|
Guinea pig |
2824 |
5055 |
10 |
1/12 Died at lowest concentration tested, 2000 ppm |
|
Rat |
545 |
976 |
15 |
Lowest lethal concentration, 250 ppm (1/6 died) |
|
Guinea pig |
1283 |
2279 |
15 |
No deaths at second highest concentration, 1000 ppm |
|
Rat |
Could not be determined |
— |
30 |
5/6 Died at lowest concentration tested, 500 ppm |
|
Guinea pig |
364 |
652 |
30 |
5/6 Deaths at 500 ppm; no deaths at 250 ppm |
|
Rat |
268 |
480 |
60 |
Lowest lethal concentration, 250 ppm (2/6 died) |
|
Guinea pig |
235 |
421 |
60 |
Lowest lethal concentration, 100 ppm (1/6 died) |
|
Rat |
259 |
464 |
120 |
Lowest lethal concentration, 100 ppm, (1/6 died) |
|
Guinea pig |
158 |
283 |
120 |
Lowest lethal concentration, 100 ppm (1/6 died) |
|
Rat |
58 |
104 |
240 |
Lowest lethal concentration, 50 ppm (2/5 died) |
|
Guinea pig |
45 |
81 |
240 |
Lowest lethal concentration, 25 ppm (2/5 died) |
|
Rat |
35 |
63 |
480 |
1/6 Died at lowest concentration tested, 25 ppm |
|
Guinea pig |
27 |
48 |
480 |
Lowest lethal concentration, 25 ppm (2/6 died) |
|
aLC50 values of lowest tested concentration causing death. Sources: Carpenter et al. 1948 (rat and guinea pig data); Silver and McGrath 1948 (mouse data). |
||||
distant site (lung tumors) after a single subcutaneous injection, in rats at the injection site (sarcomas) in rats after multiple subcutaneous injections (Walpole et al. 1954; BRL 1968), and after oral administration (hepatomas and pulmonary tumors) (BRL 1968).
4.
SPECIAL CONSIDERATIONS
4.1.
Metabolism, Disposition, and Kinetics
Data on absorption, distribution, and metabolism after inhalation exposure to ethylenimine were not located in the available literature. Wright and Rowe (1967) studied the distribution, metabolism, and excretion of ethylenimine in male Dow-Wistar rats after intraperitoneal injection of 80 μg of ethylenimine-[14C] (0.30 to 0.42 mg/kg) and found that 3.37 to 4.85% of the dose was expired as 14CO2 during the first 24 or 96 h after dosing. In addition, a volatile basic radioactive substance tentatively identified as unmetabolized ethylenimine was also eliminated in expired air. These levels ranged from 0.95 to 2.75% of the dose within 24 to 96 h. The major route of excretion was urine, which contained between 45.8 and 58.6% of the dose between 24 and 96 h. A small amount of unmetabolized ethylenimine was excreted in urine, but the largest fraction was converted to unidentified metabolites. By comparison, Jackson and James (1965) reported that rats injected with 1.0 to 2.9 mg/kg of ethylenimine excreted 10 to 38% of the dose in urine within 6 h and mice injected with 1.0 to 5.0 mg/kg excreted 7 to 28% in urine. Wright and Rowe (1967) also noted that ethylenimine was not eliminated in feces. They reported a 6-h half time of excretion as CO2 and a 2-h half time of elimination as ethylenimine in expired air. Overall elimination of ethylenimine showed two compartments; one with an elimination half time of 16 h and one with a half time of 56 days. The authors noted that the long half time of elimination was due to the binding of the radio-active material to tissue components and that the material incorporated into tissues was the parent compound and not a metabolite.
Tissue distribution studies showed that all tissues accumulated radioactive material to some degree within the first 24 h, but it was markedly reduced by 96 h (Wright and Rowe 1967). The highest specific activity was found in the liver followed in decreasing order by cecum, spleen, kidneys, intestines, and bone marrow. The long half-life suggests that the material accumulating in tissues turned over very slowly and was not available for further metabolism.
The metabolites of ethylenimine were not identified. Wright and Rowe (1967) concluded that a portion of the ethylenimine is converted to a substance that can be converted to CO2, but the major portion proceeded by a route that did not involve oxidation. They further noted that ethylenimine or a metabolite retaining the aziridine ring reacted with tissues components.
4.2.
Mechanism of Toxicity
Ethylenimine causes extreme inflammation and blistering upon contact with skin, eyes, and respiratory tract. The effects are delayed relative to the time of exposure. Ethylenimine is a very reactive alkylating agent and its toxicity may be related to its alkylating properties. Ethylenimine forms ethylenimonium ions (Ramel 1981) that readily alkylate macromolecules via amino, sulfhydro, and carboxyl groups on proteins and phosphate and amino groups on nucleic acids (Hemminki 1994; Trochimowicz et al. 1994).
Death and other effects occurring after inhalation exposure to ethylenimine are delayed, i.e. depending on the concentration, effects may not become apparent until long after exposure started or was terminated (insidious nature of ethylenimine). The delayed effects suggest that biologic accumulation may be a factor in initiating overt toxic responses.
Although ethylenimine is a potent irritant that causes inflammation and blistering upon direct contact with tissue, there is no evidence indicating that it is a sensory irritant via stimulation of the trigeminal nerve. Silver and McGrath (1948) compared acute toxicity in groups of mice exposed to ethylenimine with mice exposed to ammonia for 10 min. The concentrations of both compounds were in the lethal range. Mice exposed to ammonia (primary irritant) exhibited immediate signs (within 1 min) of initiating exposure, closing of eyes, gasping, and pawing and scratching of the nose. Then the mice became quiet and death, which was preceded by convulsions, occurred about 5 min after exposure started; almost all mice that died did so during exposure. The surviving animals recovered rapidly after cessation of exposure. The effect of ethylenimine on mice was described in Section 3.1.2. Mice exposed to ethylenimine also showed evidence of eye and nose irritation; however, no deaths occurred during the first 24 h after exposure. Silver and McGrath (1948) stated that the physiological actions of ammonia and ethylenimine are quite different. They also acknowledged the insidious nature of ethylenimine and stated that the secondary peak of deaths 4-5 days after exposure resembles that of nitrogen mustards.
Ethylenimine is toxicologically similar to the mustard compounds. Papirmeister et al. (1985) proposed a mechanism by which mustard compounds induce vesicant activity in the skin. The initial step in the proposed mechanism is the rapid alkylation of DNA followed by depurination of DNA, DNA strand breaks, activation of poly(ADP-ribose polymerase), depletion of NAD+, inhibition of glycolysis, activation of the hexose monophosphate shunt, release of protease, and cell damage leading to blistering. Ethylenimine reacts with guanosine and deoxyguanosine to form 7-aminoethylguanine derivatives in vitro, which readily undergo depurination (Hemminki 1994). Rao et al. (1999) showed that sulfur mustard damages DNA in lungs, liver, spleen, and thymus of mice after exposure by inhalation or dermal contact, and that the damage persists for at least 7 days depending on the tissue and concentration of sulfur mustard.
Ethylenimine is a blistering agent and is likely to produce damage in the eyes, skin, and respiratory tract in a manner similar to that of the mustards. A more detailed discussion of the mechanism of toxicity of sulfur mustard was presented by Watson and Griffin (1992) and NRC (2003).
4.3.
Structure–Activity Relationship
Carpenter et al. (1948) conducted acute inhalation studies with three compounds structurally similar to ethylenimine: ethylenediamine, propylenimine, and triethylamine. No deaths occurred in groups of rats or guinea pigs exposed to 1000 ppm of ethylene diamine for up to 8 h and observed for 14 days. Exposure to 500 ppm of propylenimine for 240 min caused the death of 5/6 rats; exposure for 1, 2, or 4 h caused the death of 1/6, 3/5, and 6/6 guinea pigs, respectively. No deaths occurred among six guinea pigs per group exposed to triethylamine at concentrations of 250 or 500 ppm for 4 h; 2/6 deaths occurred at 1000 ppm for 4 h, and 4/6 deaths occurred at 2000 ppm for 2 h. Based on the lethality data, ethylenimine is more acutely toxic than either of the latter substances.
4.4.
Other Relevant Information
4.4.1.
Species Variability
The LC50 values showed only small differences in sensitivity to ethylenimine between the rat and guinea pig at specific exposure concentrations and durations. The differences in exposure concentrations selected for the experiments made it difficult to compare the mouse with the rat and guinea pig. The LC50 for a 10-min exposure is similar for the mouse (2236 ppm) and guinea pig (2824 ppm). At individual exposure concentrations, however, rats were more sensitive than mice followed by guinea pigs for a 10-min exposure. Rats were more sensitive than guinea pigs for a 15-min exposure and equally sensitive to guinea pigs for a 60-min exposure, but guinea pigs were slightly more sensitive than rats for exposure times greater than 60 min. Since only five or six animals per group were exposed at most concentrations and times, the detection level of the experiment was very low. The selection of exposure concentrations for each exposure duration could account for some of the difference between species, and variations in exposure concentrations, which were not measured, also could have contributed to the species differences.
All species showed the characteristic delayed mortality after inhalation exposure to ethylenimine and showed similar clinical signs of toxicity. Eye and respiratory tract irritation and characteristic delayed response also have been observed in humans exposed to ethylenimine.
4.4.2.
Susceptible Subpopulations
No data are available on susceptible subpopulations exposed to ethylenimine. Ethylenimine is a potent respiratory irritant, but there is no evidence that it is a primary irritant that could trigger a response in asthmatics at concentrations lower than those causing effects in the general population.
4.4.3.
Concentration-Exposure Duration Relationship
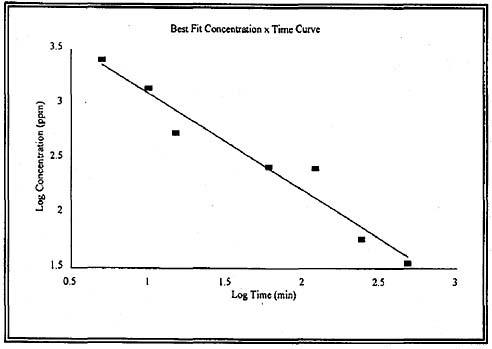
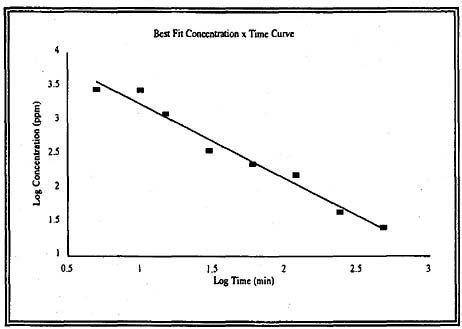
The LC50 data for rats and guinea pigs can be used to determine the relationship between the concentration of ethylenimine and the exposure duration. A linear relationship was observed for LC50 concentration and exposure durations ranging from 5 to 480 min (8 h) when plotted as a log-log relationship. These data for the rat (Figure 4-1) and guinea pig (Figure 4-2) are presented below. The calculated values of n are 1.1 for the rat data and 0.91 for the guinea pig data.
5.
DATA ANALYSIS AND AEGL-1
5.1.
Summary of Human Data Relevant to AEGL-1
Ethylenimine has an ammonia-like odor and an odor detection threshold of 2 ppm (Carpenter et al. 1948). Odor will not serve as a specific warning to the presence of ethylenimine, because its odor is similar to that of ammonia. No other data pertinent to deriving AEGL-1 values for ethylenimine were available.
5.2.
Summary of Animal Data Relevant to AEGL-1
No animal data were available for deriving AEGL-1 values.
5.3.
Derivation of AEGL-1
Data were not available for deriving AEGL-1 values for ethylenimine; therefore, no values are recommended (Table 4-8). The absence of AEGL-1 values does not imply that exposures below the AEGL-2 are without adverse health effects.
6.
DATA ANALYSIS AND AEGL-2
6.1.
Summary of Human Data Relevant to AEGL-2
Human data relevant to AEGL-2 were discussed in Section 2.2. Ethylenimine exposure causes skin sensitization, severe slow-healing dermatitis, blister-
TABLE 4-8 AEGL-1 Values for Ethylenimine [ppm (mg/m3)]
|
10 min |
30 min |
1 h |
4 h |
8 h |
|
NR |
NR |
NR |
NR |
NR |
|
NR: Not recommended. The absence of AEGL-1 values does not imply that exposure below the AEGL-2 is without adverse health effects. |
||||
ing, reddening, and edema of the scrotum, nasal irritation, and throat irritation. Carpenter et al. (1948) reported that irritation does not occur in humans exposed to less than 100 ppm; these investigators did not describe the subjects or exposure protocol.
Five male students exposed successively to ammonia, isopentane, ethylenimine, and N-ethylethylenimine over a period of 2- to 3-h, experienced lacrimation, eye inflammation, coughing, respiratory tract inflammation, nausea, and vomiting; clinical findings included hemoconcentration, eosinophilia, albuminuria, and liver inflammation (Weightman and Hoyle 1964). Most of the effects were delayed in onset or the attainment of maximum severity. Ammonia and isopentane can be ruled out as causative agents; however, no data were found on the toxicity of N-ethylethylenimine and it cannot be ruled out as a contributing factor to the observed effects (see section 2.2.1). Exposure concentrations were not reported for ammonia, isopentane, ethylenimine, or N-ethylethylenimine. Another case report described scrotal skin irritation with no testicular involvement in several men exposed to ethylenimine vapor; no exposure concentrations or durations were reported (Thiess et al. 1971).
6.2.
Summary of Animal Data Relevant to AEGL-2
No animal studies specifically designed to examine the nonlethal effects of exposure to ethylenimine were available for deriving AEGL-2 values. An acute lethality study showed that exposure to 10 ppm for 240 or 480 min was not lethal and did not affect the eyes or respiratory tract of guinea pigs. A concentration of 25 ppm did not cause death or respiratory difficulty in guinea pigs exposed for 60 min, but caused death after exposure for 240 min. No deaths occurred among rats exposed to 25 ppm for 240 min, but this concentration caused extreme respiratory difficulty after 3 h of exposure to both species. Although no deaths occurred in either rats or guinea pigs exposed to 50 ppm for 120 min, extreme respiratory difficulty was observed at this concentration.
6.3.
Derivation of AEGL-2
Data are sparse for deriving AEGL-2 values. The acute inhalation studies used to derive LC50 values for rats and guinea pigs were considered for deriving AEGL-2 values. Exposure concentrations of 25 ppm for durations greater than 3 h caused extreme respiratory and may impair escape. Therefore, the next lowest concentration of 10 ppm (240 min), which caused no respiratory difficulty in
guinea pigs, was used for deriving AEGL-2 values. Although the logical point of departure (POD) for deriving AEGL-2 values is the no-observed-effect level (NOEL) for extreme respiratory difficulty in guinea pigs exposed to 10 ppm ethylenimine for 480 min (Carpenter et al. 1948), this derivation would lead to AEGL-2 values close to or exceeding the life-threatening AEGL-3 concentrations. Therefore, AEGL-2 values were derived using the next shorter duration of 240 min, which also is a clear NOEL for respiratory difficulty in guinea pigs exposed to 10 ppm. The total uncertainty factor is 10. An uncertainty factor of 3 was applied for interspecies differences, because ethylenimine is a very reactive direct-acting alkylating agent, and the AEGL-2 effects would most likely be confined to the respiratory tract. Respiratory tract damage appears to be due to a direct effect of an alkylating agent on the respiratory epithelium, and this mechanism is not expected to be different among species (NRC 2003). Humans and animals exhibit delays between the time of exposure and the onset of symptoms and the eyes and respiratory tract are the most sensitive targets in rat, guinea pigs, and humans. An uncertainty factor of 3 was applied for intraspecies variability because the effects appear to involve direct contact of the eyes or respiratory epithelium with a very reactive alkylating agent, and the alkylating activity is not expected to vary considerably among individuals in the population. Five male students responded similarly to an exposure to ethylenimine with respect to the time of onset of symptoms and the intensity of effects. Studies have shown that DNA damage is probably the initiating step in a cascade of events leading to cell damage (Papirmeister et al. 1985) and DNA damage is persistent in respiratory and systemic organs following inhalation exposure to alkylating agents (Rao et al. 1999), such as ethylenimine. Extrapolation across the pertinent time frames was based on ten Berge’s equation Cn × t = k, where n = 0.91 (Figure 4-2). The value of n was derived by regression analysis of the LC50 data for guinea pigs exposed 5 to 480 min to ethylenimine. The resulting AEGL-2 values are presented in Table 4-9.
The AEGL-2 values are below the irritation threshold of 100 ppm reported by Carpenter et al. (1948). Quantitative data are not adequate for deriving cancer risk estimates (unit risk value) for inhaled ethylenimine (see Appendix B).
7.
DATA ANALYSIS AND AEGL-3
7.1.
Summary of Human Data Relevant to AEGL-3
Only one death due to exposure to ethylenimine was found in the literature. A worker died after an intense exposure to an unknown concentration of ethylenimine for 5 min or less (Gresham and West 1975). Detailed exposure conditions were not reported. Signs and symptoms of exposure included eye irritation, vomiting, severe respiratory irritation, and pulmonary edema. Because the patient was treated aggressively with steroids, death may have been due to secondary effects of treatment and not to exposure to ethylenimine.
TABLE 4-9 AEGL-2 Values for Ethylenimine [ppm (mg/m3)]
|
10 min |
30 min |
1 h |
4 h |
8 h |
|
33 (59) |
9.8 (18) |
4.6 (8.2) |
1.0 (1.8) |
0.47 (0.84) |
7.2.
Summary of Animal Data Relevant to AEGL-3
Acute lethality studies were conducted in rats and guinea pigs exposed to ethylenimine at concentrations ranging from 10 to 4000 ppm for 5 to 480 min (Carpenter et al. 1948) and in mice exposed to 1176 to 3416 ppm for 10 min (Silver and McGrath 1948). All deaths in all species and at all concentrations were delayed, occurring 24 h to more than 10 days after exposure. All species showed signs of eye and respiratory tract irritation; in addition, the mouse showed signs of muscular incoordination and developed convulsions. Prostration was observed before death of rats and guinea pigs. The quality of the studies was similar, except for calculated instead of analytically-determined exposure concentrations for rats and guinea pigs Carpenter et al. (1948). LC50 values for the rat and guinea pig were not reported by Carpenter et al. (1948), but were calculated by probit analysis. At the shorter exposure durations (5 to 15 min), LC50 values were slightly higher for the guinea pig than the rat. However, at the longer durations (360 min), the values for the guinea pig were slightly lower, but still comparable to those for the rat. The postexposure observation period was only 10 days for mice and the studies on rats and guinea pigs showed that 9% of the deaths occurred after day 10 of the observation period showing that an extended observation period is important in evaluating mortality after exposure to ethylenimine.
7.3.
Derivation of AEGL-3
The acute lethality studies in rats and guinea pigs were adequate for deriving AEGL-3 values for ethylenimine, and both studies were used to estimate lethality thresholds (LC01). These values are presented in Table 4-10. A 30-min LC01 could not be determined from the rat data; the data were unsuitable for probit analysis because a dose-response relationship was not observed at the concentrations tested. The LC01 values show considerable variation for both species, but the standard error for the LC01 for the 480-min exposure to the rat is small compared with the standard errors for the other LC01 values. Therefore, the LC01 for the 480-min exposure to the rat (15 ppm) served as the basis for deriving AEGL-3 values.
A total uncertainty factor of 10 was applied to the LC01. An uncertainty factor of 3 was applied for interspecies differences, because ethylenimine is a very reactive direct-acting alkylating agent, and the AEGL-2 effects would most likely be confined to the respiratory tract. Respiratory tract damage appears to
TABLE 4-10 Estimates of the Threshold for Lethality (LC01) to Ethylenimine
|
Species |
Exposure Duration (min) |
LC50a (ppm) |
LC01a (ppm) |
|
Rat |
5 |
2558 ± 1792 |
37 ± 53 |
|
|
10 |
1407 ± 977 |
3 ± 16 |
|
|
15 |
545 ± 162 |
35 ± 30 |
|
|
30 |
Not determined |
Not determined |
|
|
60 |
268 ± 57 |
70 ± 38 |
|
|
120 |
259 ± 259 |
20 ± 24 |
|
|
240 |
58 ± 14 |
11 ± 7 |
|
|
480 |
35 ± 5 |
15 ± 6 |
|
Guinea pig |
5 |
2906 ± 1583 |
139 ± 146 |
|
|
10 |
2824 ± 319 |
1580 ± 292 |
|
|
15 |
1283 ± 326 |
231 ± 144 |
|
|
30 |
364 ± 79 |
77 ± 45 |
|
|
60 |
235 ± 77 |
19 ± 16 |
|
|
120 |
158 ± 37 |
30 ± 17 |
|
|
240 |
45 ± 13 |
4 ± 3 |
|
|
480 |
27 ± 6 |
7 ± 4 |
|
aLC50 and LC01 values (derived using probit analysis) ± standard error. |
|||
be due to a direct effect of an alkylating agent on the respiratory epithelium, and this mechanism is not expected to be different among species (NRC 2003). Humans and animals exhibit delays between the time of exposure and the onset of symptoms and the eyes and respiratory tract are the most sensitive targets in rat, guinea pigs, and humans. In addition, the LC50 values for three test animal species were within a factor of 2 of each other, and like other effects of ethylenimine, death and serious effects were delayed in all three species. Similarly, humans experienced a delay in the onset of life-threatening and/or very serious effects after exposure to ethylenimine. An uncertainty factor of 3 was applied for intraspecies variability because the effects appear to involve direct contact of the eyes or respiratory epithelium with a very reactive alkylating agent, and the alkylating activity is not expected to vary considerably among individuals in the population. Five male students responded similarly to an exposure to ethylenimine with respect to the time of onset of symptoms and the intensity of effects. Studies have shown that DNA damage is probably the initiating step in a cascade of events leading to cell damage (Papirmeister et al. 1985) and DNA damage is persistent in respiratory and systemic organs following inhalation exposure to alkylating agents (Rao et al. 1999), such as ethylenimine. An uncertainty factor of 3, instead of the default of 10, was applied for intraspecies variability
based on the same rationale described for AEGL-2 derivation. Time frame scaling was determined by linear regression of the LC50 values for the rat exposed for durations ranging from 10 to 480 min; the value of n is 1.1 (Figure 4-1). The ten Berge equation for time frame scaling is as follows: Cn × t = k, where C = concentration, n = 1.1, t = duration of exposure, and k = constant. The AEGL-3 values are presented in Table 4-11.
The AEGL-values presented in Table 4-11 are all below the 100 ppm that Carpenter et al. (1948) reported as the threshold for irritation in humans. The LC01 (15 ppm) for the 8-h exposure is less than the lowest concentration causing death in rats and guinea pigs. The AEGL-3 for a 60-min exposure is threefold lower than the concentration causing no deaths in rats and only one death in guinea pigs.
Ethylenimine has carcinogenic activity in rodents by oral and parenteral routes and is placed in a class of suspect human carcinogen by OSHA (29 CFR 1910.1003 [1999]) and is classified as possibly carcinogenic to humans by IARC (1999). Because quantitative data are not available for deriving a unit risk value, AEGL-3 values for ethylenimine do not take potential cancer risk into consideration.
8.
SUMMARY OF AEGL VALUES
8.1.
AEGL Values and Acute Toxicity End Points
Data were not available for deriving AEGL-1 values; therefore, no values are recommended. The absence of AEGL-1 values does not imply that exposures below the AEGL-2 are without adverse health effects. AEGL-2 values were based on a NOEL for extreme respiratory difficulty in guinea pigs exposed to ethylenimine for 240 min. Uncertainty factors of 3 for intraspecies variability and 3 for interspecies differences (total uncertainty factor was 10) were applied to the NOEL. Time scaling was based on the equation Cn × t = k, where n = 0.91 was derived from guinea pig data.
The human data were not adequate for deriving AEGL-3 values. AEGL-3 values were based on the estimated lethality threshold (LC01) for rats exposed to ethylenimine for 480 min. Uncertainty factors of 3 for intraspecies variability and 3 for interspecies differences (total uncertainty factor was 10) were applied to the LC01. Time scaling was based on the equation Cn × t = k, where n = 1.1 was derived from rat data. The AEGL values are summarized in Table 4-12.
8.2.
Comparison with Other Standards and Criteria
The ACGIH TLV-TWA is 0.5 ppm (0.88 mg/m3) (ACGIH 2001) and the IDLH is 100 ppm (NIOSH 1996). The ACGIH TLV has assigned a skin notation (ACGIH 2001); it causes dermatitis and sensitization. OSHA (29 CFR 1910.1003 [1999]) considers ethylenimine to be an occupational suspect car-
TABLE 4-11 AEGL-3 Values for Ethylenimine [ppm (mg/m3)]
|
10 min |
30 min |
1 h |
4 h |
8 h |
|
51 (91) |
19 (34) |
9.9 (18) |
2.8 (5.0) |
1.5 (2.7) |
TABLE 4-12 Summary of AEGL Values for Ethyleniminea,b [ppm (mg/m3)]
|
Classification |
10 min |
30 min |
1 h |
4 h |
8 h |
End Point (Reference) |
|
AEGL-1 (Nondisabling) |
Not recommendedc |
|
|
|
|
|
|
AEGL-2 (Disabling) |
33 (59) |
9.8 (18) |
4.6 (8.2) |
1.0 (1.8) |
0.47 (0.84) |
NOEL for extreme respiratory difficulty (Carpenter et al. 1948) |
|
AEGL-3 (Lethal) |
51 (91) |
19 (34) |
9.9 (18) |
2.8 (5.0) |
1.5 (2.7) |
Threshold for lethality (Carpenter et al. 1948) |
|
aAEGL-2 and -3 values do not take into consideration the potential cancer risk due to exposure to ethylenimine, because quantitative data were not available for deriving a unit risk value. bEffects at these concentrations may be delayed until after exposure. cThe absence of AEGL-1 values does not imply that exposure below the AEGL-2 level is without adverse health effects. |
||||||
cinogen; a permissible exposure level (PEL) was not established. OSHA (29 CFR 1910.1003 [1999]) requires that employees engaged in handling ethylenimine be protected against contact with and exposure to ethylenimine. When working with ethylenimine under certain conditions, OSHA regulations require that workers be supplied with full body protective clothing, half-face filter-type respirator with a filter for dusts, mists, and fumes, or air-purifying cartridges or canisters (29 CFR 1910.1003 [1999]). Table 4-13 compares existing standards and guidelines with the derived AEGL values. No other standards or guidelines are available for ethylenimine.
8.3.
Data Quality and Research Needs
There are significant data gaps concerning human and animal studies on ethylenimine exposure. Human studies are precluded because ethylenimine is a suspect carcinogen. Acute lethality studies were available in three laboratory species, two of which were exposed to varying concentration of ethylenimine for durations ranging from 5 to 480 min. The log concentration vs log time plot showed a linear relationship of the LC50 values for the entire range of exposure durations. Therefore, the data for deriving AEGL-3 values were robust and could be scaled over the full range of relevant time periods (10 to 480 min). The only studies available for deriving AEGL-2 values were the acute lethality studies in rats and guinea pigs. The AEGL-2 values were, therefore, derived from a no-effect-level for extreme respiratory difficulty determined from the guinea pig
TABLE 4-13 Extant Standards and Guidelines for Ethyleneimine
|
Guideline |
Exposure Duration |
||||
|
10 min |
30 min |
1 h |
4 h |
8 h |
|
|
AEGL-1 |
Not recommended |
|
|
|
|
|
AEGL-2 |
33 ppm |
9.8 ppm |
4.6 ppm |
1.0 ppm |
0.47 ppm |
|
AEGL-3 |
51 ppm |
19 ppm |
9.9 ppm |
2.8 ppm |
1.5 ppm |
|
IDLH (NIOSH)a |
NA |
100 ppm |
NA |
NA |
NA |
|
PEL-TWA (OSHA)b |
No values established; considered to be an occupational suspect carcinogen |
||||
|
TLV-TWA(ACGIH)c |
0.5 ppm (8-h), skin; Confirmed Animal Carcinogen with Unknown Relevance to Humans |
||||
|
MAK (Germany)d |
No value (carcinogenicity category 2: shown to cause cancer in animals; skin absorption; germ mutagen category 3A) |
||||
|
aIDLH (Immediately Dangerous to Life and Health, National Institute of Occupational Safety and Health) (NIOSH 1996) represents the maximum concentration from which one could escape within 30 min without any escape-impairing symptoms, or any irreversible health effects. bPEL-TWA (Permissible Exposure Limits - Time Weighted Average, Occupational Health and Safety Administration) (29 CFR 1910.1003 [1999]) is defined analogous to the ACGIH-TLV-TWA, but is for exposures of no more than 10 h/day, 40 h/week. cTLV-STEL (Threshold Limit Value – short-term exposure limit, American Conference of Governmental Industrial Hygienists) (ACGIH 2001) is defined as a 15 min TWA exposure which should not be exceeded at any time during the workday even if the 8-h TWA is within the TLV-TWA. Exposures above the TLV-TWA up to the STEL should not be longer than 15 min and should not occur more than 4 times per day. There should be at least 60 min between successive exposures in this range. dMAK (Maximale Arbeitsplatzkonzentration [Maximum Workplace Concentration] [German Research Association) (DFG 2000) is defined analogous to the ACGIH-TLV-TWA. |
|||||
no-effect-level for extreme respiratory difficulty determined from the guinea pig study. Guinea pigs but not rats were exposed to the lowest concentration not associated with a very serious effect. Therefore, AEGL-2 values were derived from one data point in one species. In addition, data are not available for deriving cancer risk values for ethylenimine; therefore, the AEGL values do not take into account potential carcinogenicity. Because it is a very reactive direct-acting alkylating agent, there is concern about potential carcinogenicity of ethylenimine after a single exposure as demonstrated in mice receiving a single subcutaneous injection (BRL 1968). A single-exposure inhalation carcinogenicity study would provide data for conducting a cancer risk assessment.
9.
REFERENCES
ACGIH (American Conference of Governmental Industrial Hygienists). 2001. TLVs and BEIs: Threshold Limit Values for Chemical Substances and Physical Agents
and Biological Exposure Indices. American Conference of Governmental Industrial Hygienists, Cincinnati, OH.
ATSDR (Agency for Toxic Substances Disease Registry). 2003. Toxicological Profile for Sulfur Mustard (Update). U.S. Department of Health and Human Services, Public Health Service, Agency for Toxic Substances and Disease Registry, Atlanta, GA [online]. Available: http://www.atsdr.cdc.gov/toxprofiles/tp49.pdf [accessed Oct. 29, 2008].
Axelsen, R.A. 1978. Experimental renal papillary necrosis in the rat: The selective vulnerability of medullary structures to injury. Virchows. Arch. A Pathol. Anat. Histol. 381(1):79-84.
BRL (Bionetics Research Labs). 1968. Evaluation of Carcinogenic, Teratogenic, and Mutagenic Activities of Selected Pesticides and Industrial Chemicals, Vol. 1. Carcinogenic Study. NCI-DCCP.CG-1973-1-1. NTIS PB-223-159. Prepared by Bionetics Research Labs, Bethesda, MD, for the National Cancer Institute, Bethesda, MD.
Brockman, H.E., C.Y. Hung, F.J. de Serres, and T.M. Ong. 1981. Mutagenicity of selected chemicals in Neurospora Crassa. Pp. 109-138 in Comparative Chemical Mutagenesis, F.J. De Serres, and M.D. Shelby, eds. Environmental Science Research Vol. 24. New York: Plenum Press.
Carpenter, C.P., H.F. Smyth, Jr., and C.B. Shaffer. 1948. The acute toxicity of ethylene imine to small animals. J. Ind. Hyg. Toxicol. 30(1):2-6.
Cavender, F. 1994. Aliphatic hydrocarbons. Pp. 1221-1266 in Patty’s Industrial Hygiene and Toxicology, Vol. II B, Toxicology, 4th Ed., G.D. Clayton, and F.E. Clayton, eds. New York: John Wiley and Sons.
Chang, T.H., and F.T. Elequin. 1967. Induction of chromosome aberrations in cultured human cells by ethylenimine and its relation to cell cycle. Mutat. Res. 4(1):83-89.
Danehy, J.P., and D.J. Pflaum. 1938. Toxicity of ethylene imine. Ind. Eng. Chem. 30(7):778.
DFG (Deutsche Forschungsgemeinschaft). 2000. List of MAK and BAT Values 2000. Maximum Concentrations and Biological Tolerance Values at the Workplace Report No. 36. Weinheim, Federal Republic of Germany: Wiley VCH.
Gaeth, V.J., and A.M. Thiess. 1972. Chromosome studies on chemical workers. Zbl. Arbeitsmed. Arbeischutz. 22:357-362 (as cited in Preston et al. 1981).
Gresham, G.A., and I.E. West. 1975. Injury and repair of tracheobronchial cartilage following accidental exposure to ethyleneimine. J. Clin. Pathol. 28(7):564-567.
Ham, G.E. 1981. Imines, cyclic. Pp. 142-166 in Kirk-Othmer Encyclopedia of Chemical Technology, Vol 13, 3rd Ed. New York: John Wiley & Son.
Haroun, L., and B.N. Ames. 1981. Mutagenicity of selected chemicals in the Salmonella/microsome mutagenicity test. Pp. 27-68 in Comparative Chemical Mutagenesis, F.J. De Serres, and M.D. Shelby, eds. Environmental Science Research Vol. 24. New York: Plenum Press.
Hemminki, K. 1994. DNA adducts of nitrogen mustard and ethylene imines. Pp. 313-321 in DNA Adducts: Identification and Biological Significance, K. Hemminki, A. Dipple, D.E.G. Shuker, F.F. Kadlubar, and H. Bartsch, eds. IARC Scientific Publication No. 125. Lyon, France: International Agency for Research on Cancer.
IARC (International Agency for Research on Cancer). 1999. Aziridine. Pp. 337-344 in Re-evaluation of Some Organic Chemicals, Hydrazine and Hydrogen Peroxide, Part II. IARC Monograph on the Evaluation of Carcinogenic Risk to Humans Vol. 71. Lyon, France: International Agency for Research on Cancer.
Jackson, H., and R.M.V. James. 1965. Metabolic studies with certain ethyleneimine derivatives in relation to diuresis. Br. J. Pharmacol. Chemother. 25(1):223-227.
Lewis, R.J., Jr. 1993. Pp. 490-491 in Hawley’s Condensed Chemical Dictionary, 12th Ed. New York: Van Nostrand Reinhold Co.
Loprieno, N. 1981. Mutagenicity of selected chemicals in yeast: Mutation-induction at specific loci. Pp. 139-150 in Comparative Chemical Mutagenesis, F.J. De Serres, and M.D. Shelby, eds. Environmental Science Research Vol. 24. New York: Plenum Press.
Malashenko, A.M. 1968. Chemical mutagenesis in laboratory mammals [in Russian]. Sov. Genet. 4:538-543.
Malashenko, A.M., and I.K. Egorov. 1968. Induction of dominant lethals in mice by ethylenimine and diethyl sulfate. Sov. Genet. 4:14-18.
NIOSH (National Institute of Occupational Safety and Health). 1996. Documentation for Immediately Dangerous to Life or Health Concentrations (IDLH): NIOSH Chemical Listing and Documentation of Revised IDLH Values (as of 3/1/95)-Ethylenimine. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Institute of Occupational Safety and Health [online]. Available: http://www.cdc.gov/niosh/idlh/151564.html [accessed Oct. 30, 2008].
NRC (National Research Council). 2003. Sulfur Mustard (Agent HD). Pp. 301-383 in Acute Exposure Guideline Levels for Selected Airborne Chemicals, Vol. 3. Washington, DC: National Academies Press.
NRC (National Resource Council). 2001. Standing Operating Procedures for Developing Acute Exposure Guideline Levels for Hazardous Chemicals. Washington, DC: National Academy Press.
NRC (National Research Council). 1993. Guidelines for Developing Community Emergency Exposure Levels for Hazardous Substances. Washington, DC: National Academy Press.
O’Neil, M.J., A. Smith, P.E. Heckelman, J.R. Obenchain, Jr., J. Gallipeau, and M.A. D’Arecca. 2001. Ethylenimine. Pp. 676-677 in The Merck Index: An Encyclopedia of Chemicals, Drugs, and Biologicals, 13th Ed. Whitehouse Station, NJ: Merck.
Papirmeister, B., C.L. Gross, H.L. Meier, J.P. Petrali, and J.B. Johnson. 1985. Molecular basis for mustard-induced vesication. Fundam. Appl. Toxicol. 5(6 Pt. 2):S134-S149.
Pierce, J.O. 1993. Alkaline materials. Pp. 755-782 in Patty’s Industrial Hygiene and Toxicology, Vol. IIA Toxicology, 4th Ed., G.D. Clayton, and F.E. Clayton, eds. New York: John Wiley & Sons.
Preston, R.J., I.D. Adler, A. Leonard, and M.F. Lyon. 1981. Mutagenicity of selected chemicals in in vivo cytogenetic assays. Pp. 549-631 in Comparative Chemical Mutagenesis, F.J. De Serres, and M.D. Shelby, eds. Environmental Science Research Vol. 24. New York: Plenum Press.
Ramel, C. 1981. Comparative mutagenicity of triethylenemelamine, trenimon, and ethylenimine. Pp. 943-976 in Comparative Chemical Mutagenesis, F.J. De Serres, and M.D. Shelby, eds. Environmental Science Research Vol. 24. New York: Plenum Press.
Rao, P.V.L., R. Vijayaraghavan, and A.S. Bhaskar. 1999. Sulphur mustard induced DNA damage in mice after dermal and inhalation exposure. Toxicology 139(1-2):39-51.
RTECS (Registry of Toxic Effects of Chemical Substances). 2008. Ethylenimine. RTECS No. KX5075000. National Institute for Occupational Safety and Health
[online]. Available: http://www.cdc.gov/niosh/rtecs/kx4d7038.html#L [accessed Oct. 31, 2008].
Ruhe, R.L. 1982. Health Hazard Evaluation Report: Hercules, Incorporated, Hopewell, Virginia. HETA 82-287-1240. PB84-172766. National Institute for Occupational Safety and Health, Cincinnati, OH.
Santodonato, J. 1985. Monograph on Human Exposure to Chemicals in the Workplace: Aziridine. Final report. SRC TR 84-740. PB86-136587. Prepared by Syracuse Research Corporation for the National Cancer Institute, Bethesda, MD.
Silver, S.D., and F.P. McGrath. 1948. A comparison of acute toxicities of ethylene imine and ammonia to mice. J. Ind. Hyg. Toxicol. 30(1):7-9.
Thiess, A.M., W. Hey, and H.J. Ludewigs. 1971. Case reports on scrotal dermatitis following exposure to ethylene imine vapors [in German]. Zentralbl. Arbeitsmed. 21(12):365-368.
Todd and Taugher. 1918. Report 246 of the Pharmacological and Toxicological Division of the Chemical Warfare Service (as cited in Silver and McGrath 1948).
Trochimowicz, H.J., G.L. Kennedy, Jr., and N.D. Krivanek. 1994. Heterocyclic and miscellaneous nitrogen compounds. Pp. 3285-3521 in Patty’s Industrial Hygiene and toxicology, Vol. IIB, Toxicology, 4th Ed., G.D. Clayton, and F.E. Clayton, eds. New York: John Wiley & Sons.
van Doorn, R., M. Ruijten and T. Van Harreveld. 2002. Guidance for the Application of Odor in 22 Chemical Emergency Response, Version 2.1, August 29, 2002. Public Health Service of Rotterdam, The Netherlands.
Velazquez, A., C. de Nava, R. Coutino, and I. Pulido. 1973. The relationship between gene and chromosome mutations in cultured chinese hamster cells exposed to aflatoxin B1. Mutat. Res. 21(4):241-242.
Verschaeve, L., and M. Kirsch-Volders. 1990. Mutagenicity of ethyleneimine. Mutat. Res. 238(1):39-56.
Verschueren, K. 1996. Pp. 975-978 in Handbook of Environmental Data on Organic Chemicals, 3rd Ed. New York: van Nostrand Reinhold.
Vogel, E., A. Schalet, W.R. Lee, and F. Wuergler. 1981. Mutagenicity of selected chemicals in Drosophila. Pp. 175-256 in Comparative Chemical Mutagenesis, F.J. De Serres, and M.D. Shelby, eds. Environmental Science Research Vol. 24. New York: Plenum Press.
Walpole, A.L., D.C. Roberts, F.L. Rose, J.A. Hendry, and R.F. Homer. 1954. Cytotoxic agents: IV. The carcinogenic actions of some monofunctional ethyleneimine derivatives. Br. J. Pharmacol. Chemother. 9(3):306-323.
Watson, A.P., and G.D. Griffin. 1992. Toxicity of vesicant agents scheduled for destruction by the chemical stockpile disposal program. Environ. Health Perspect. 98:259-280.
Weightman, J., and J.P. Hoyle. 1964. Accidental exposure to ethylenimine and N-ethylethylenimine vapors. J. Am. Med. Assoc. 189:543-545.
Wright, G.J., and V.K. Rowe. 1967. Ethylenimine: Studies of the distribution and metabolism in the rat using carbon-14. Toxicol. Appl. Pharmacol. 11(3):575-584.
Zimmermann, F.K. 1981. Mutagenicity of selected chemicals in yeast: Mitotic recombination, gene conversion, and nondisjunction. Pp. 151-174 in Comparative Chemical Mutagenesis, F.J. De Serres, and M.D. Shelby, eds. Environmental Science Research Vol. 24. New York: Plenum Press.
APPENDIX A
Derivation of AEGL Values for Ethylenimine
Derivation of AEGL-2
|
Key study: |
Carpenter et al. 1948 |
|
Toxicity End Point: |
NOEL for extreme respiratory difficulty; 10 ppm for a 240-min exposure to guinea pigs |
|
Time Scaling: |
Cn × t = k; n = 0.91 based on regression analysis of the guinea pig data C = 10 ppm/10 (uncertainty factor) = 1.0 ppm Cn × t = k; C = 1.0 ppm, t = 240 min, n = 0.91 k = 240 ppm min |
|
Uncertainty Factors: |
Total = 10: 3 for interspecies differences, because ethylenimine is a very reactive direct-acting alkylating agent, and the AEGL-2 effects would be confined to the respiratory tract. Respiratory tract damage appears to be due to direct effect of an alkylating agent on the respiratory epithelium, and this mechanism is not expected to be different among species (NRC 2003). Humans and animals exhibit delays between the time of exposure and the onset of symptoms and the eyes and respiratory tract are the most sensitive targets in both species. |
|
|
3 for intraspecies variability, because the effects appear to involve direct contact of the eyes or respiratory epithelium with a very reactive alkylating agent. Studies have shown that DNA damage is probably the initiating step in a cascade of events leading to cell damage and DNA damage is persistent in respiratory and systemic organs following inhalation exposure to alkylating agents. Alkylating activity of ethylenimine is not expected to vary appreciably among individuals in the population. |
|
Calculations: |
|
|
10-min AEGL-2 |
C = (k/t)1/0.91 = (240 ppm min/10 min)1/0.91 = 33 ppm |
|
30-min AEGL-2 |
C = (k/t)1/0.91 = (240 ppm min/30 min)1/0.91 = 9.8 ppm |
|
1-h AEGL-2 |
C =(k/t)1/0.91 = (240 ppm min/60 min)1/0.91 = 4.6 ppm |
|
4-h AEGL-2 |
C = (k/t)1/0.91 = (240 ppm min/240 min)1/0.91 = 1.0 ppm |
|
8-h AEGL-2 |
C =(k/t)1/0.91 = (240 ppm min/480 min)1/0.91 = 0.47 ppm |
Derivation of AEGL-3
|
Key Study: |
Carpenter et al. 1948 |
|
Toxicity End Point: |
Threshold for lethality: the LC50 for a 480 min exposure was 35 ppm, the data was extrapolated to a LC01 (15 ppm) |
|
Time Scaling: |
Cn × t = k; n = 1.1 based on regression analysis of the rat data C = 15 ppm/10 (total uncertainty factor) = 1.5 ppm Cn × t = k; C = 1.5 ppm, t = 480 min, n = 1.1 k = 749.7934 ppm min |
|
Uncertainty Factors: |
Total = 10: 3 for interspecies differences, because ethylenimine is a very reactive direct-acting alkylating agent, and the AEGL-2 effects would be confined to the respiratory tract. Respiratory tract damage appears to be due to direct effect of an alkylating agent on the respiratory epithelium, and this mechanism is not expected to be different among species (NRC 2003). Humans and animals exhibit delays between the time of exposure and the onset of symptoms and the eyes and respiratory tract are the most sensitive targets in both species. In addition, the LC50 values for three species are within a factor of 2 and signs of very serious or life-threatening toxicity appear to be similar between animals and humans. |
|
|
3 for intraspecies variability, because the effects appear to involve direct contact of the eyes or respiratory epithelium with a very reactive alkylating agent. Studies have shown that DNA damage is probably the initiating step in a cascade of events leading to cell damage and DNA damage is persistent in respiratory and systemic organs following inhalation exposure to alkylating agents. Alkylating activity of ethylenimine is not expected to vary appreciably among individuals in the population |
|
Calculations: |
|
|
10-min AEGL-3 |
C = (k/t)1/1.1 = (749.7934 ppm min/10 min)1/1.1 = 51 ppm |
|
30-min AEGL-3 |
C = (k/t)1/1.1 = (749.7934 ppm min/30 min)1/1.1 = 19 ppm |
|
1-h AEGL-3 |
C = (k/t)1/1.1 = (749.7934 ppm min/60 min)1/1.1 = 9.9 ppm |
|
4-h AEGL-3 |
C = (k/t)1/1.1 = (749.7934 ppm min/240 min)1/1.1 = 2.8 ppm |
|
8-h AEGL-3 |
C = (k/t)1/1.1 = (749.7934 ppm min/480 min)1/1.1 = 1.5 ppm |
APPENDIX B
Quantitative Cancer Assessment for Ethylenimine
Two reports were available for assessing the potential carcinogenicity of ethylenimine. In one study, 50% of rats injected subcutaneously with ethylenimine in arachis oil developed sarcomas at the injection site, whereas no rats injected with arachis oil alone and only 17% injected with ethylenimine in water developed sarcomas (Walpole et al. 1954). The route or exposure for this study precludes deriving a risk value for comparison with AEGL values.
Seven-day old male and female mice of two strains given a subcutaneous injection of ethylenimine (4.64 mg/kg body weight) and observed for 80 weeks caused an increase in the incidence of lung tumors in male mice of both strain and in the total incidence of tumors in male mice (BRL 1968). Although this is a single exposure study, it cannot be used to derive risk values for inhalation exposure, because the test material was administered subcutaneously. This study shows that ethylenimine can induce a carcinogenic response distant from the application site after only a single dose.
In another study, two strains of male and female mice were administered ethylenimine by gavage for 3 weeks followed by ethylenimine in feed for up to 18 months (BRL 1968). All groups exposed to ethylenimine developed neoplasms (pulmonary adenomas and/or hepatomas) by the end of the study. This study cannot be used to derive risk values because of the lack of an adequate dose term for the mouse. Ethylenimine was administered by gavage at a specific dose (4.64 mg/kg/day) from 7- to 28-days of age followed by administration in feed for up to 18 months at a concentration that delivered the gavage dose. The investigators stated that the concentration of ethylenimine in feed corresponded to the gavage dose, but the actual concentration of ethylenimine in the feed was not reported. The concentration was likely based on body weight and feed consumption of a 28-day old mouse, and this concentration was not adjusted during the course of the study to maintain a constant dose. Therefore, because body weight and food consumption change markedly with growth of the mouse, the dose received between day 28 of age until termination of the study also changes markedly and cannot be estimated. Consequently, without an estimate of the dose term, a quantitative assessment cannot be conducted for comparison with AEGL values. Further, only one experimental dose was used in this study, precluding an adequate dose-response assessment.
APPENDIX C
Derivation of the Level of Distinct Odor Awareness (LOA) for Ethylenimine
The level of distinct odor awareness (LOA) represents the concentration above which it is predicted that more than one-half of the exposed population will experience at least a distinct odor intensity and about 10% of the population will experience a strong odor intensity. The LOA should help chemical emergency responders in assessing the public awareness of the exposure due to odor perception. The LOA derivation follows the guidance given by van Doorn et al. (2002).
The odor detection threshold (OT50) for ethylenimine is 0.6980 ppm (van Doorn et al. 2002). The concentration (C) leading to an odor intensity (I) of distinct odor detection (I=3) is derived using the Fechner function:
For the Fechner coefficient, the default kw = 2.33 will be used because of the lack of chemical specific data:
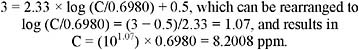
The resulting concentration is multiplied by an empirical field correction factor. It takes into account that in every day life, factors such as sex, age, sleep, smoking, upper airway infections, and allergy, as well as distraction increase the odor detection threshold by a factor of 4. In addition, it takes into account that odor perception is very fast (about 5 seconds), which leads to the perception of concentration peaks. Based on the current knowledge, a factor of 1/3 is applied to adjust for peak exposure. Adjustments for distraction and peak exposure lead to a correction factor of 4/3 = 1.33.
Therefore, the LOA for ethylenimine is 10.907 ppm.
APPENDIX D
Derivation Summary for Ethylenimine AEGL Values
AEGL -1 VALUES
|
10 min |
30 min |
1 h |
4 h |
8 h |
|
Not recommended |
||||
|
Key Reference: Not applicable. |
||||
|
Test Species/Strain/Number: Not applicable. |
||||
|
Exposure Route/Concentration/Durations: Not applicable. |
||||
|
Effects: Not applicable. |
||||
|
End Point/Concentration/Rationale: Not applicable. |
||||
|
Uncertainty Factors/Rationale: Not applicable. Total uncertainty factor: Interspecies: Not applicable. Intraspecies: Not applicable. |
||||
|
Modifying Factor: Not applicable. |
||||
|
Animal to Human Dosimetric Adjustment: Not applicable. |
||||
|
Time Scaling: Not applicable. |
||||
|
Data Adequacy: Data were not available for deriving AEGL-1 values. |
||||
AEGL -2 VALUES
|
10 min |
30 min |
1 h |
4 h |
8 h |
|
33 ppm |
9.8 ppm |
4.6 ppm |
1.0 ppm |
0.47 ppm |
|
Key Reference: Carpenter, C.P., H.F. Smyth, Jr., and C.B. Shaffer. 1948. The acute toxicity of ethylene imine to small animals. J. Ind. Hyg. Toxicol. 30(1):2-6. |
||||
|
Test Species/Strain/Number: male guinea pigs, 6 per group |
||||
|
Exposure Route/Concentration/Durations: Inhalation; 10, 25, 50, 100, or 250 ppm for 240 min |
||||
|
Effects: Guinea pigs were exposed for 240 min. Clinical signs: eye and respiratory irritation, and extreme respiratory difficulty at 25-250 ppm; prostration at 250 ppm; no effects at 10 ppm Gross pathologic effects: congestion and hemorrhage in the lungs, congestion in all internal organs at 25-250 ppm; no effects at 10 ppm Microscopic effects: lung congestion leakage of fluid and red blood cells into bronchioles, tubular necrosis and cloudy swelling in the kidneys at 25-250 ppm; no effects at 10 ppm Mortality: 10 ppm, (0/6), 25 ppm (2/6), 50 ppm (2/6), 100 ppm (6/6), and 250 ppm (6/6) |
||||
|
10 min |
30 min |
1 h |
4 h |
8 h |
|
33 ppm |
9.8 ppm |
4.6 ppm |
1.0 ppm |
0.47 ppm |
|
End Point/Concentration/Rationale: No-effect-level for lethality in the guinea pig: 10 ppm exposure for 4 h; effects at 25 ppm and higher were more severe than those defined for AEGL 2. The logical point of departure (POD) is 10 ppm for 480 min, but this POD would lead to AEGL-2 values close to or exceeding the life-threatening AEGL-3 concentrations. |
||||
|
Uncertainty Factors/Rationale: Total uncertainty factor: 10 Interspecies: 3 - Ethylenimine is a very reactive direct-acting alkylating agent, and the AEGL-2 effects would be confined to the respiratory tract. Respiratory tract damage appears to be due to direct effect of an alkylating agent on the respiratory epithelium, and this mechanism is not expected to be different among species. Humans and animals exhibit delays between the time of exposure and the onset of symptoms and the eyes and respiratory tract are the most sensitive targets in both species. Intraspecies: 3 - The effects appear to involve direct contact of the eyes or respiratory epithelium with a very reactive alkylating agent, and the alkylating activity of ethylenimine is not expected to vary appreciably among individuals in the population. Studies have shown that DNA damage is probably the initiating step in a cascade of events leading to cell damage and DNA damage is persistent in respiratory and systemic organs following inhalation exposure to alkylating agents. |
||||
|
Modifying Factor: 1 |
||||
|
Animal to Human Dosimetric Adjustment: 1 |
||||
|
Time Scaling: Cn × k = t, where n = 0.91 derived empirically from guinea pig LC50 data with exposure times ranging from 5 min to 480 min. |
||||
|
Data Adequacy: The only studies available for deriving AEGL-2 values were the acute lethality studies in rats and guinea pigs. The AEGL-2 values were, therefore, derived from a no-effect-level for extreme respiratory difficulty determined from the guinea pig study. Ethylenimine has carcinogenic activity; but these values do not take into consideration the potential excess lifetime cancer risk due to a single exposure. |
||||
AEGL -3 VALUES
|
10 min |
30 min |
1 h |
4 h |
8 h |
|
51 ppm |
19 ppm |
9.9 ppm |
2.8 ppm |
1.5 ppm |
|
Key Reference: Carpenter, C.P., H.F. Smyth, Jr., and C.B. Shaffer. 1948. The acute toxicity of ethylene imine to small animals. J. Ind. Hyg. Toxicol. 30(1):2-6. |
||||
|
Test Species/Strain/Number: Male Wistar rats, 6 per group. |
||||
|
Exposure Route/Concentration/Durations: Inhalation, 25 or 50 ppm for 480 min. |
||||
|
10 min |
30 min |
1 h |
4 h |
8 h |
|
51 ppm |
19 ppm |
9.9 ppm |
2.8 ppm |
1.5 ppm |
|
Effects: Exposure was for 480 min. Effects occurred at both concentrations. Clinical signs: eye and respiratory irritation, and extreme respiratory difficulty Gross pathologic effects: congestion and hemorrhage in the lungs, congestion in all internal organs. Microscopic effects: lung congestion leakage of fluid and red blood cells into bronchioles, tubular necrosis and cloudy swelling in the kidneys. Mortality: 25 ppm (1/6) and 50 ppm (5/6) |
||||
|
End Point/Concentration/Rationale: The threshold for lethality in rats exposed for 480 min exposure was 15 ppm (LC01), derived by probit analysis of the data. |
||||
|
Uncertainty Factors/Rationale: Total uncertainty factor: 10 Interspecies: 3 - ethylenimine is a very reactive direct-acting alkylating agent, and the AEGL-2 effects would be confined to the respiratory tract. Respiratory tract damage appears to be due to direct effect of an alkylating agent on the respiratory epithelium, and this mechanism is not expected to be different among species. Humans and animals exhibit delays between the time of exposure and the onset of symptoms and the eyes and respiratory tract are the most sensitive targets in both species. In addition, LC50 values for three species did not vary by more than twofold and signs of very serious or life-threatening toxicity appear to be similar between animals and humans. |
||||
|
Intraspecies: 3 - the effects appear to involve direct contact of the eyes or respiratory epithelium with a very reactive alkylating agent, and the alkylating activity of ethylenimine is not expected to vary appreciably among individuals in the population. Studies have shown that DNA damage is probably the initiating step in a cascade of events leading to cell damage, and DNA damage is persistent in respiratory and systemic organs following inhalation exposure to alkylating agents. |
||||
|
Modifying Factor: 1 |
||||
|
Animal to Human Dosimetric Adjustment:1 |
||||
|
Time Scaling: Cn × k = t, where n = 1.1 derived empirically from rat LC50 data for exposures from 5 min to 480 min. The 480 min value gave the LC01 with the smallest standard error. All other values were calculated from 480 min. |
||||
|
Data Adequacy: Acute lethality studies were available for three laboratory species, two of which were exposed to varying concentration of ethylenimine for duration ranging from 5 to 480 min. The log concentration versus log time plot showed a linear relationship of the LC50 values for the entire range of exposure durations. Therefore, the data set available for deriving AEGL-3 values was robust and it could be extrapolated over the full range of relevant time periods (10 to 480 min). Ethylenimine has potential carcinogenic activity; however, data were not available for deriving cancer risk values. The AEGL values do not take into consideration the potential excess lifetime cancer risk due to a single exposure. |
||||