Personalized Cancer Medicine Technology
Several speakers illustrated both the accomplishments of personalized cancer medicine and the challenges that remain ahead, using examples in the treatment of leukemia, breast, colon, and lung cancer. These speakers discussed a number of tests that predict patient response to specific cancer treatments, including tests for the following:
-
HER2, which predicts a patient with breast cancer’s response to Herceptin.
-
Estrogen receptors, which predict a patient with breast cancer’s response to tamoxifen and aromatase inhibitors.
-
Mutations in the epidermal growth factor receptor (EGFR), which are predictive of a patient with lung cancer’s response to drugs such as gefitinib or erlotinib. The mutations also predict response when drugs that target EGFR are used in combination with other cytotoxic chemotherapies.
-
Mutations in the KRAS protein that play an important role in EGFR signaling, and predict an individual’s response to colon cancer drugs that act on this receptor, such as cetuximab.
-
Mutations in the tyrosine kinase receptor FLT3, which confer resistance to drugs that target the receptor in patients with leukemia.
-
Gene expression variations in tumors that predict breast cancer recurrence (Oncotype DX, MammaPrint).
-
Drug metabolism genetic variants that predict adverse reactions to the cancer drug irinotecan.
Many of the tests that are predictive of a therapeutic response (hereinafter, in this report, “predictive tests”) have regulatory approval and are the standard of care for certain cancer treatments. The breast cancer drug Herceptin, as well as the tests that indicate patients likely to respond to it, has been on the market since 1998 and has been used to treat half a million patients (Roche, 2008). More than 100,000 Oncotype Dx tests, a gene expression test that predicts a patient’s benefit from chemotherapy as well as breast cancer recurrence, have also been used to determine treatment planning since the test came on the market in 2004 (Genomic Health, 2009). About half of all estrogen-positive breast tumors in the United States are being evaluated with this preditive test, estimated Dr. Steven Shak of Genomic Health, the test’s developer. In addition, the UGT1A1 molecular assay has Food and Drug Administration (FDA) clearance for patients with colorectal cancer who are considering taking Camptosar (irinotecan), and tests for KRAS are approved by the European Medicines Agency (EMEA) to predict patients’ response to panitumumab and cetuximab therapy in colorectal cancer.1 Phase III clinical trials have recently confirmed the predictive value of EGFR mutations for response to gefitinib (Iressa) and erlotinib (Tarveva), leading the EMEA to announce its approval of gefitinib as a treatment for lung tumors that have activating EGFR mutations (AstraZeneca, 2009).
Predictive tests can be useful in health care because they often calculate an individual’s response to treatment better than other clinical indicators, said Dr. Bruce E. Johnson of the Dana-Farber Cancer Institute. For example, non-smoking women with a particular type of lung cancer are more likely to respond to erlotinib or gefitinib than other patients with lung cancer. Patients meeting these clinical characteristics have a median progression-free survival (PFS) of about 6 months, compared to a median PFS of less than 3 months in individuals without these clinical features. However, median PFS was nearly 15 months in individuals with EGFR mutations that predict response to erlotinib, versus only about 2 months in individuals without these mutations (see Figures 1a and 1b). Dr. Johnson and Dr. Rafael Amado of GlaxoSmithKline noted the importance of showing, with appropriately
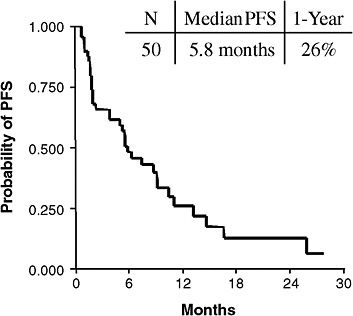
FIGURE 1a Clinically enriched patients. Non-smoking women with a particular type of lung cancer are more likely to respond to erlotinib or gefitinib than other patients with lung cancer. Patients meeting these clinical characteristics have a median progression-free survival (PFS) of about 6 months.
SOURCES: Johnson presentation (June 8, 2009); Bruce Johnson and David Jackman, Dana-Farber Cancer Institute.
designed clinical trials, that a predictive test truly predicts response to treatment, rather than indicating a prognosis independent of treatment.
A potential benefit of predictive tests is that they limit the number of individuals who will have an adverse or ineffective response to a therapeutic treatment. For example, the use of Oncotype DX reduces overall chemotherapy use by at least 20 percent (Shak, 2009). “There are a number of patients who are no longer receiving therapy uselessly, and there has been a lot of money saved,” said Dr. Amado. However, Dr. Mark Ratain of the University of Chicago Hospitals said that “the more we learn, the more we know we don’t know.” Deciphering the clinical implications of predictive tests can be challenging, even when they assess the function of just one key protein. Genetic assessments are likely to become more complex in the future. As a result, it will become necessary for researchers to develop multiple predictive tests that indicate the function of many, if not all, the nodes on those pathways that play crucial roles in the development or progression
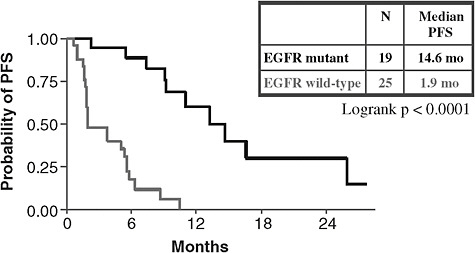
FIGURE 1b Genomically defined patients. Median progression-free survival (PFS) was nearly 15 months in individuals with lung cancer and epidermal growth factor receptor (EGFR) mutations that predict response to erlotinib, versus only about 2 months in individuals without these mutations.
SOURCES: Johnson presentation (June 8, 2009); Bruce Johnson and David Jackman, Dana-Farber Cancer Institute.
of various cancers. Dr. Stephen Friend of Sage Bionetworks suggested that because of redundant backup pathways and feedback loops, scientists need to model and consider entire pathway networks when developing predictive tests.
DECIPHERING THE CLINICAL IMPLICATIONS
Dr. Donald Small of the Sidney Kimmel Comprehensive Cancer Center illustrated some of the difficulties of making treatment decisions based on the results of predictive tests. For example, treatment decisions for patients with acute myelogenous leukemia (AML) are often based on the results of tests for mutations on the tyrosine kinase receptor FLT3. This receptor plays a role in stimulating the proliferation of blood stem cells and dendritic cells of the immune system. Researchers have discovered a number of mutations on this gene, as well as in the DNA stretch that controls its activation, which affect the responsiveness of patients with AML to FLT3 inhibitor drugs. However, the mere presence of specific mutations does not determine responsiveness to anti-FLT3 treatment. Rather, the ratio of the mutant gene to the wild-type allele predicts responsiveness (Smith et al., 2004). Patients
with the lowest ratio of the mutant gene to the wild-type allele have the best clinical prognosis (Figure 2) (Meshinchi et al., 2006). Complicating the clinical decision making, however, is evidence that patients with FLT3 mutations who receive a bone marrow transplant have similar outcomes to those patients without mutations. As a result, some clinicians are inclined to treat patients with AML with a bone marrow transplant, rather than treating them with a FLT3 inhibitor.
Another example of how the development of predictive tests may out-pace the clinical understanding of these tests is in the use of Oncotype DX. A high recurrence score from an Oncotype DX test indicates those women with estrogen receptor-positive (ER-positive), node-negative breast cancer who are at high risk for relapse and most likely to benefit from adjuvant chemotherapy. A low recurrence score indicates women who should only receive hormonal therapy (Paik et al., 2006). However, the test does not provide useful information on how women whose scores are in the middle range should be treated. The clinical study, TailoRx, is currently assessing the predictive value of these mid-range scores (NCI, 2009b), but in the meantime clinicians are unsure what the best treatment is for women with these intermediate scores.
“I recently tried to help a woman who had been diagnosed with a small ER-positive breast cancer with no lymph node involvement,” said Amy Bonoff of the National Breast Cancer Coalition. “But she had a gene assay test that showed she was in the high middle range for risk of recurrence. What should she do? No one has the answer to that. She now has a piece of information that will keep her awake at night, and she really can’t make medical decisions” based on it. Ms. Bonoff stressed that “for a biomarker to be clinically meaningful it must improve patient outcomes in a meaningful way, and predict disease outcome in the absence of treatment or guide the use of therapy targeted to the marker.” Dr. Richard Schilsky of the University of Chicago and the Cancer and Leukemia Group B (CALGB), added, “Biomarker development needs to start off by defining the intended use of the test. If we can’t define what it’s going to be used for, why develop it?” However, Dr. Shak noted that personalized medicine requires the integration of other prognostic factors, such as tumor size and grade, with genetic factors. “These factors all need to be taken into account. Oncotype DX is not a recipe,” he said.
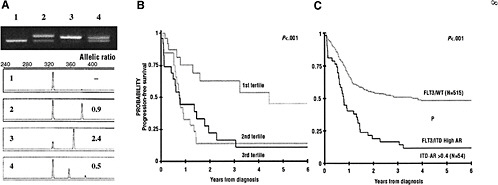
FIGURE 2 Allelic ratio (mutant to wild-type FLT3 allele) affects the prognostic significance of FLT3/ITD mutations. (A) Example of ITD-AR determination by Genescan analysis. The top panel is the agarose gel resolution of PCR product from a normal marrow (lane 1) and specimens from 3 patients with FLT3/ITD (lanes 2-4). The lower panels show the result of the Genescan analysis and ITD-AR determination. (B) Actuarial progression-free survival (PFS) from study entry for patients with FLT3/ITD based on allelic ratio by tertiles. (C) Actuarial PFS from study entry for patients with high ITD-AR (ITD-AR > 0.4) compared with those with FLT3/WT. Patients were from a Children’s Oncology Group acute myelogenous leukemia trial.
SOURCES: Small presentation (June 8, 2009); Meshinchi et al. (2006). This research was originally published in Blood. Meshinchi, S., T. A. Alonzo, D. L. Stirewalt, M. Zwaan, M. Zimmerman, D. Reinhardt, G. J. Kaspers, N. A. Heerema, R. Gerbing, B. J. Lange, and J. P. Radich. impliof FLT3 mutations in pediatric AML. 2006; Vol 108(12):3654–3661. © the American Society of Hematology.
INCREASING COMPLEXITY OF PREDICTIVE TESTS
The use of the KRAS test in patients with colorectal cancer demonstrates the need for more complex predictive testing, and a better understanding of how predictive tests work. It is standard practice to only treat colorectal cancer patients with EGFR-targeting drugs if they have the KRAS genetic profile that is likely to render them responsive to such treatment. The use of KRAS genotyping results in a near doubling of response rate and progression-free survival of patients with colorectal cancer treated with these medicines, compared to an unselected patient population, Dr. Amado said (Jonker et al., 2007). However, these are marginal results because the response rate is still only about 20 percent in patients with the correct KRAS genetic profile. “Clearly there’s more beyond KRAS,” he said.
KRAS is a node on one of two pathways thought to be essential for EGFR signaling. A key node on the other pathway is P13K (Figure 3) (Scaltriti and Baselga, 2006). Recent data reveal that mutations in KRAS do not affect an individual’s sensitivity to anti-EGFR treatments. Instead, mutations in an effector protein downstream from KRAS, called B-Raf, predicts response to anti-EGFR treatment independent of KRAS mutations (Di Nicolantonio et al., 2008). About 10 percent of colorectal patients
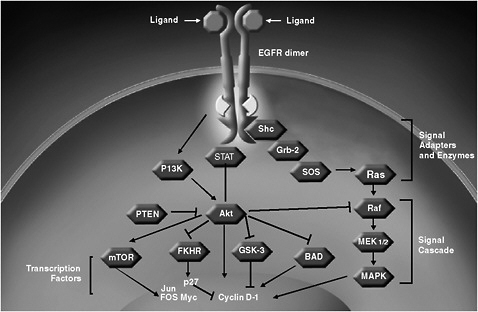
FIGURE 3 EGFR signal transduction.
SOURCE: Amado presentation (June 8, 2009).
have B-Raf mutations, 30 percent have wild-type KRAS with B-Raf, and 60 percent have B-Raf mutations and wild-type KRAS. Mutations in either of these two genes predicts lack of response to cetuximab (Di Nicolantonio et al., 2008). Preliminary data also suggest that levels of expression of certain ligand proteins (AREG or EREG) predict responsiveness to anti-EGFR treatment in colorectal cancer patients independent of KRAS status. One study found that a “combimarker” (i.e., detecting KRAS mutations and expression levels of these ligand proteins) could select a population with an overall survival ratio of .43, compared to a ratio of .7 if no markers are used to select patients (Jonker et al., 2009). “What these data are suggesting is that it’s not really about a single node in the pathway, but rather about the pathway itself,” said Dr. Amado. “If we’re looking at genes in isolation, we may make incremental movement forward, but ideally in the future, we should have techniques that are really looking down that pathway that’s activated for individual tumors. Hopefully our predictive test capability will evolve in that direction.”
Aiding that evolution are genomics technologies, which give researchers the opportunity to assay large sets of genetic markers simultaneously to determine the “genetic signatures” that correlate with prognosis and/or responsiveness to treatment. Dr. Friend described several predictive tests that examine large sets of genetic markers that use this technology, including an FDA-cleared, 70-gene expression test called MammaPrint, which predicts women likely to experience a recurrence of their breast cancer, and the Oncotype DX test (Paik et al., 2004; van’t Veer et al., 2002). He pointed out that genetic signatures can distinguish between tumors that are ER positive and negative and those that are HER2 positive and negative, suggesting that the signatures correlate well with the underlying biology of the tumors.
Dr. Friend also described research that used cells in culture or tumor cells in mice to discern the groups of genes that are upregulated or down-regulated by RAS or RAS inhibitors (Bild et al., 2006; Blum et al., 2007; Sweet-Cordero et al., 2005). This work revealed that whole sets of genes can act like switches—turn on or off—in response to certain drugs or proteins. He suggested that research should focus on identifying genetic signatures in patients’ tumors that indicate whether their cancer-promoting pathways are likely to be blocked by treatment. For example, Dr. Friend and his colleagues developed a 147-gene signature that assesses the RAS pathway as a whole, and identifies, with greater than 90 percent sensitivity, KRAS-mutant lung tumors and cancer cell lines (Friend, 2009).
Interestingly, there is an overlap of only one gene in the MammaPrint
and Oncotype DX genetic signature, and an overlap of 14 genes in the Merck RAS genetic signature and another RAS signature (Friend, 2009). Dr. Friend stressed the importance of ascertaining why there is not more overlap between the various genetic signatures that predict the same outcomes, and noted that as more signatures are developed, it will be difficult to decide which ones are the best ones to put into practice.
Dr. Friend also called for a better understanding of the pathways being tested. More insight is needed into the overarching causal mechanisms that are driving the cancer, including an awareness of redundant feedback loops he called networks, which become active when the pathways are blocked. “Not only do you have to have the markers, but you also have to understand the pathway and the network that’s sitting behind it,” he said. “If you look at the data that are coming, the data are miniscule compared to what’s going to happen in the next 5 or 10 years. We’ll have the ability to have a DNA sequence across the entire tumor on most patients and then look also at expression profiling, because you can do it at the same time.” Dr. Ratain concurred, stating that “our current strategy in pharmacogenomics is to collect DNA samples in conjunction with large clinical trials and to perform genome-wide typing to identify candidates associated with both toxicity and efficacy. Then we can conduct replication studies using samples from other similar studies, and perform mechanistic studies to confirm function.” A recent study used such a strategy to show a genomic basis for an adverse reaction to statin treatment (statin myopathy) (Search Collaborative Group et al., 2008). “This shows the power of genome-wide association for discovery of functional variants,” Dr. Ratain said.
Dr. Friend stressed the need to integrate different types of genomic information, and using Bayesian approaches, build up probabilistic causal models of disease that go beyond just looking at markers on a pathway. He and his colleagues used such an approach to build a model of obesity that indicated that nine genes were key players in the disorder (Schadt et al., 2005). A validation study then showed that eight of those nine genes modulate obesity when they are overexpressed, altered, or knocked out (Yang et al., 2009). “We can now build predictive, causal networks,” he said. “When you go to a tumor state, instead of ranking genes that are altered, we think it’s much better to actually look at the networks that are broken and reassociate them” (Figure 4).
However, such assessments require collaboration on a large scale. “No one company or institution should or could build these probabilistic causal maps,” Dr. Friend said. “It won’t work if we work in fiefdoms. We need to
FIGURE 4 Networks facilitate direct identification of genes that are causal for disease (obesity).
SOURCES: Friend presentation (June 8, 2009) and Schadt et al. (2005); Yang et al. (2009). Reprinted by permission from Macmillan Publishers Ltd: Nature Genetics (Yang, X., J. L. Deignan, H. Qi, J. Zhu, S. Qian, J. Zhong, G. Torosyan, S. Majid, B. Falkard, R. R. Kleinhanz, J. Karlsson, L. W. Castellani, S. Mumick, K. Wang, T. Xie, M. Coon, C. Zhang, D. Estrada-Smith, C. R. Farber, S. S. Wang, A. van Nas, A. Ghazalpour, B. Zhang, D. J. MacNeil, J. R. Lamb, K. M. Dipple, M. L. Reitman, M. Mehrabian, P. Y. Lum, E. E. Schadt, A. J. Lusis, and T. A. Drake. 2009. Validation of candidate causal genes for obesity that affect shared metabolic pathways and networks. Nature Genetics 41(4):415–423.), Copyright (2009).
create a commons where scientists can combine their datasets with others to build network models. Chemists and physicists have used structures and models of what they work on for decades. The irony is that doctors don’t. They really don’t have molecular, physiologic models of disease, but rather little pathway maps that have worked as examples.”
Dr. Friend recently formed a nonprofit organization called Sage Bionetworks. This organization will provide a commons for the creation of disease models based on the assembly of coherent biomedical data into probabilistic and integrative bionetworks models (Friend, 2009). These models evolve via modifications made by contributor scientists. The ultimate mission of Sage is to accelerate the elimination of human diseases.
Dr. Robert Mass of Genentech, Inc., agreed on the importance of going beyond gene expression data to understand the underlying tumor biology, but noted that even with that understanding, developing the appropriate predictive tests can be difficult. For example, examination of the HER2 tumor-promoting pathway led researchers at Genentech to discover that tumors responsive to Herceptin appeared to have dimerization of HER2, with either HER1 or HER3 (Mass, 2009). However, detecting HER2 dimerization in clinical samples is difficult to do because it requires detecting phosphorylated HER2 or activated HER2—modified forms of the proteins that are short-lived and difficult to detect in fresh tissue, and virtually impossible to reliably detect in formalin-fixed, paraffin-embedded tissue, according to Dr. Mass. As a result, researchers had to detect downstream surrogate markers, such as low levels of HER3 in ovarian cancer patients, as measured by quantitative reverse transcriptase polymerase chain reaction (PCR), and HER2 amplification in breast cancer patients. “It’s going to be complicated because we may be using different markers for different groups of patients, which is a challenge to a drug developer,” Dr. Mass said.
Adding to the complexity of developing personalized cancer medicine is individual variability in how much of a given drug reaches its target, Dr. Small pointed out. He noted that typically, the assays to test the effectiveness of drugs that target tyrosine kinase receptors, such as FLT3, are done in the absence of fetal calf serum or similar compounds that mimic the effects that bloodstream products have on the binding of a drug on its target. Human plasma has numerous proteins that can bind to drugs. A recent study indicated that binding can change the concentration of drugs in the bloodstream from the nanomolar range to the micromolar range, he said (Levis et al., 2006) (Figure 5). Different patients show different bind-
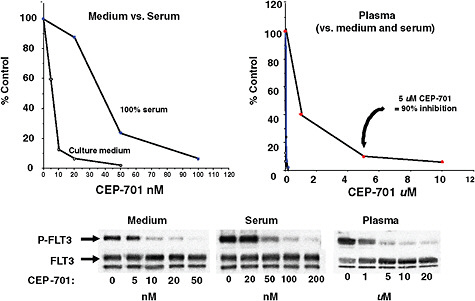
FIGURE 5 Drug binding: Inhibition of FLT3 autophosphorylation by CEP-701.
SOURCES: Small presentation (June 8, 2009) and Levis et al., 2006. This work was originally published in Blood. Levis, M., P. Brown, B. D. Smith, A. Stine, R. Pham, R. Stone, D. DeAngelo, I. Galinsky, F. Giles, E. Estey, H. Kantarjian, P. Cohen, Y. Wang, J. Roesel, J. E. Karp, and D. Small. Plasma inhibitory activity (PIA): a pharmacodynamic assay reveals insights into the basis for cytotoxic response to FLT3 inhibitors. 2006; Vol 108(10):3477–3483. © the American Society of Hematology.
ing to FTL3 inhibitors, as determined by assays with FLT3 inhibition using patient serum. In leukemia cell lines, a drug could inhibit 80–90 percent of FLT3 receptor activity in the presence of some patients’ serum, but only achieve 60–70 percent inhibition in the presence of serum from other patients. In addition, this study found that clinical response to these drugs correlated with the degree of inhibition achieved in the assays (Smith et al., 2004). “Shouldn’t we be individualizing drug dosing to attain sufficient inhibition in all patients?” Dr. Small asked. “This is something that hasn’t really been occurring in typical tyrosine kinase inhibitor trials.”
Non-genetic sources of variability also need to be considered, Dr. Ratain pointed out. These include dose and schedule, disease severity, concomitant conditions and use of other drugs, liver and kidney function, and age. Because many new cancer treatments are oral drugs, the effect of diet on their action needs to be considered, he added. “Although I spend most of my life thinking about pharmacogenomics, particularly germ line, it all goes
to naught if we don’t also consider these non-genetic issues,” Dr. Ratain said. Dr. Fred Appelbaum of Fred Hutchinson Cancer Research Center concurred, saying, “In oncology, so many of our patients are elderly and have a litany of comorbidities that hugely affect their tolerance to drugs and their toxicities. It’s easier to look at genes and profiling. It’s very hard to get all the data necessary to list all the comorbidities that will influence toxicities.”
TEST VALIDATION
The characteristics of a reliable test is analytic validity (accuracy in detecting the specific entity it was designed to detect) and clinical validity (accuracy for a specific clinical purpose, e.g., predicting response to treatment). Predictive tests also should be useful in clinical decision making and in improving patient outcomes (clinical utility).
Determining the analytical validity of a predictive test is a long and arduous process, Dr. Shak said. “Just as the development of a drug cannot be achieved by performing a single study, the same thing is true with regard to the development of a predictive test and its validation.” Analytic validation requires showing assay performance, standardization and analytic performance, and whether the assay performs the same under different formats and conditions. To assess analytic validity, researchers must take into account variability in sample preparation. For example, in the real-world clinical setting, there can be variability in the time from when tumor tissue is harvested in an operating suite and is placed in formalin, as well as in the time a tissue sample remains in formalin. An assay has to perform consistently under all variations in sample preparation.
The development process for a predictive test also has to be standardized and reproducible. “Typically it takes us between 6 to 12 months to look at reproducibility, and to ensure that every aspect of the assay is going to be performed properly, and all the reagents are appropriately qualified and the specifications are set. One needs to be patient in that regard—these are critically important steps that can’t be avoided,” said Dr. Shak. His laboratory had to specify more than 150 standard operating procedures for its 5-step Oncotype DX test.
Determining the clinical validity and utility of a predictive test can also be time consuming and challenging. These qualifications require showing that the assay is “fit for purpose,” and ultimately provides some patient benefit. Typically, a retrospective/prospective study is done to clinically validate a predictive test and show its clinical utility, Dr. Schilsky explained.
Exploratory or correlative analyses are done on clinically annotated specimens that were collected prospectively. The assay methods are applied retrospectively after the clinical outcomes of the trial are known. Although prospective clinical trials are viewed as the gold standard for determining clinical utility, such retrospective/prospective trials suffice, as long as they are done in a rigorous manner (i.e., a different dataset is used for clinical utility than was used for validation, and the analyses are prespecified, robust, and show a large treatment effect), Dr. Amado said. A biologically plausible effect gives further support for the clinical utility, and may preclude the need for a prospective study, he added. Dr. Daniel Hayes of the University of Michigan Comprehensive Cancer Center concurred, saying, “If you’re going to use archived samples, you have to be as rigorous as if it was a prospective trial. You have to have a prospectively written protocol, and put down the statistical power you think you’re going to get. And you need more validated datasets if you’re using archive samples than you would for a prospective clinical trial.”
Dr. Friend cautioned that sometimes the dataset originally collected—and on which the retrospective/prospective analysis is done to show a biomarker’s clinical validity or utility—may have a skewed population or bias. He suggested making sure that such biomarker studies apply to a broad population. Dr. Ratain added that researchers and clinicians should be careful about overinterpreting nonreplicated findings. “Retrospective is fine as long as it’s well replicated. All too often you see findings presented at prestigious meetings that really are not well replicated.” Dr. Ratain also noted that randomized trials are often “a missing metric” in the assessment of predictive tests.
Risa Stack of Kleiner Perkins Caulfield and Byers stressed that the ability to use archived samples is key to innovation in personalized medicine. Traditionally, she said, such use of archived samples has not been allowed in the FDA approval process. Without this avenue of study, companies have to do prospective studies that may take as long as 10 years to complete. By that time, the therapies for which the predictive tests were developed may no longer be relevant. However, Dr. Mansfield of the FDA pointed out that the FDA has always allowed archive samples when it is appropriate to use them, and offers a guidance document about using leftover samples that are deidentified.
Dr. Shak pointed out that it can be statistically challenging to determine the clinical validity and utility of predictive tests that use genomic or genetic microarray technology. The multiple analyses done simultaneously
with these predictive tests increase the likelihood that an initial association detected as statistically significant will ultimately end up being an artifact. “The good news about looking at thousands of genes is the fact that you’ll always see positive results,” he said. “One of the obstacles of this field is human nature—when one sees a little bit of results in 70 patients, it’s really easy to get excited and feel you’re only 10 yards away from having the next best test. We need discipline and very close interaction with our statistical colleagues—both the clinical biostatisticians and the non-clinical biostatisticians—so you can identify artifacts and show reproducibility, outliers, and linearity,” Dr. Shak said. For example, recent evidence reviews and recommendations by the EGAPP working group suggests there is insufficient clinical utility for several predictive tests that are currently the standard of care, and that more studies are needed (EGAPP Working Group, 2009a, 2009b).
To truly confirm initial findings and clinically validate a biomarker, researchers often have to conduct studies using large number of patient tumor samples. Several speakers noted the difficulties in acquiring sufficient numbers of tumor samples. Dr. Shak said the clinical validation study done on the Oncotype DX test would have been impossible if the National Surgical Adjuvant Breast and Bowel Project, a clinical trials cooperative group, had not preserved tissue samples it collected in the 1990s to establish the benefit of chemotherapy in women with breast cancer (Paik et al., 2004). “There should be funding that would allow us to be able to collect and save tissue blocks so we can learn from our studies,” said Dr. Shak. Dr. Schilsky pointed out that the quality and variability in the biospecimens collected at various sites participating in clinical trials necessary to validate predictive tests can also be problematic. Dr. Mass called for having more repositories of frozen tumor tissue that is properly collected.
An alternative method to retrospective/prospective trials for validating a biomarker is to conduct prospective biomarker-drug codevelopment studies, in which patients are identified as biomarker positive or biomarker negative, and both groups are randomized to receive the new treatment versus standard treatment. However, accruing the large number of patients needed to validate a biomarker in this manner is a major hurdle, especially when the expected outcome is minimal, and the treatment being tested with the biomarker is already available clinically, Dr. Schilsky noted. “It’s far easier to just give the treatment to the patients,” he said, adding that the numbers of patients required for a biomarker validation study often far exceed the number of patients needed to assess the clinical efficacy of a
drug. Dr. Mass added that “it’s almost impossible to do prospective validation unless you go to some part of a developing country where no access to these drugs is available, but there are ethical challenges with doing that. These prospective validation studies are just not achievable.”
Ms. Bonoff and another participant suggested tapping the advocacy community to foster more patient outreach and education on biomarkers, with the intent of encouraging more patients to participate in clinical validation trials on biomarkers. She suggested using a strategy similar to that used by Dr. Susan Love, who used the Internet to create a “million-person army” of women with breast cancer; participating women are notified of clinical studies on breast cancer, including clinical trials on breast cancer drugs (Love/Avon Army of Women, 2009). Many of these women volunteer for such trials. “We need to figure out a way to get patients themselves to say, ‘I want these assays. I know they’re not sound yet, and I want to help build them,’ ” said an unidentified participant. Dr. Debra Leonard of the Weill Cornell Medical College suggested capturing data from the medical practices of early users of predictive tests. These data could be used to analyze the clinical value of those tests, perhaps with the aid of electronic medical records.
The low level of funding for validating biomarkers has also hampered their development, several speakers asserted. Federal grants and other incentives traditionally are geared toward individual accomplishments, but the translational research needed to further personalized medicine is a collaborative process, said Dr. Shak. “The biggest policy issue to me is how we can better align all of our incentives across the board to get us working together as a team in order to deliver on the promise of personalized medicine,” he said.
Dr. Schilsky raised the need for commercial partners in biomarker validation studies. Dr. Ratain said his experience was that corporate entities were uninterested in supporting his pharmacogenetic research on the metabolism of irinotecan, which led to tests that predict adverse reactions to the drug. Instead, he relied on the National Cancer Institute (NCI) for funding. Only when the FDA changed the drug label of irinotecan to include information that linked a specific genetic variant with a heightened risk of an adverse reaction to the drug did corporations show an interest in developing predictive tests for the variant, he said. The reluctance of drug companies to support the development of predictive tests is a major impediment to the transfer of this technology. “There is a lack of a corporate entity that has the financial wherewithal to really develop these tests,” he said.
Dr. Schilsky added that academic collaborations with industry partners to conduct these trials often results in legal tussles over who owns the data or specimens collected, and other intellectual property right issues. “We can spend years in negotiation over these types of issues,” he said.
Patent claims on predictive tests also can impede innovation if one has to acquire numerous patent licenses to develop a multigene test, and there are competing patent licenses on different sets of genes, Dr. Ratain pointed out. Dr. Mass commented that a use patent on Oncotype DX should prevent people from using the same 21 genes in the assay in the same way, but should not prevent investigators from striving to improve such assays using some of those genes or using the same genes, but in a different way or for a different purpose.
Another factor that can hamper biomarker development and validation is the requirement that academic laboratories conducting predictive tests must achieve the Clinical Laboratory Improvement Amendments of 1988 (CLIA)2 certification, Dr. Schilsky said. “This is a huge issue in making the transition from moving an assay from an academic research lab into a more clinically informative setting,” he said. “We’ve had CALGB trials we have been doing for which we’ve had to find alternative laboratories in the middle of the trial because all of a sudden this stringency about using CLIA-certified laboratories has increased, and we’ve had to say to a research lab that’s been doing an assay for years, ‘You can’t do this assay anymore because you’re not CLIA certified.’ It’s a big obstacle.” Dr. Roy S. Herbst of the M.D. Anderson Cancer Center added that this could also pose a problem for researchers using adaptive trials to test predictive markers. This requires identifying the markers and then testing them in real time within the same trial, “so this whole idea of CLIA and how we’re going to do it and get paid for it when the assays are being developed in real time is a pressing issue,” he said.
TEST RELIABILITY
Even if all the obstacles above are overcome, and tests and clinical trials do reveal the analytical validity, clinical validity, and clinical utility of a predictive test, the reliability of test results can still be problematic due to
inaccuracies in how the test is performed in the laboratory. Emblematic of these issues are tests for HER2 amplification.
The breast cancer drug Herceptin is only effective in women with tumors that have excess copies of the HER2 gene. When Herceptin was ready for clinical testing, a technique used to detect gene amplification called fluorescent in situ hybridization (FISH) was in its infancy and was not appropriate to use to detect HER2 amplification, said Dr. Mass. Instead, researchers at Genentech developed a test that used an immunohistochemical technology to detect HER2 protein levels, which, when elevated, indicate gene amplification (Mass, 2009). When Herceptin first came out on the market, its label specified that it be used in conjunction with this “HercepTest” diagnostic.
Shortly afterward, further tests by Genentech suggested that the FISH test for HER2 amplication was more accurate and reliable than the HercepTest. Four years later the FISH test entered the market, and was also added to the Herceptin label as an option for discerning patients likely to respond to the drug. However, for reimbursement and other reasons, the FISH test is often only done when the HercepTest test gives an equivocal result, so many more HercepTests than FISH tests are conducted, Dr. Mass noted.
Despite the break throughs in HER2 testing, lab testing errors can be as high as 20 percent even in CLIA-certified labs, according to a study done by the College of American Pathologists (CAP) and ASCO (Table 1) (Wolff et al., 2007). This suggests the need for better quality control and standardiza-
TABLE 1 HER2 Diagnostic Test’s Error Rates (Concordance Central vs. Local Lab, Study N9831)
tion in HER2 testing, Dr. Shak said. Ms. Bonoff added, “There’s significant variation in the results of these commonly used HER2 tests in different laboratories, as well as different tests for the same marker, illustrating the crying need for standardization of testing parameters. As a patient advocate, I must point out how unnerving it is for patients when they face ambiguous and/or divergent results from predictive tests. We need the investment and policies that encourage bringing those technical innovations to standardized and practical implementation. The process of standardization is very important.”
Dr. Hayes added that “the best marker stinks unless the assay is done well.” He noted that the ASCO/CAP HER2 guidelines have led to CAP establishing proficiency requirements for HER2 testing. For a lab to achieve CAP accreditation for HER2 testing, it must achieve a 95 percent concordance with a central reading (CAP, 2007). He believes the FISH test’s accuracy has been overestimated in comparison to HercepTest. “I think FISH has been done well because Mike Press does it well, and the people at Mayo Clinic do it well. But there are just as many mistakes in FISH as there are in HercepTest,” he said.
TRANSLATION CHALLENGES
An additional technological hurdle to personalized medicine in oncology is implementing predictive tests into clinical practice. For example, an analysis by United Healthcare revealed that patients who are eligible for Herceptin often do not receive it, and those who are unlikely to respond to Herceptin are often treated with the drug (Phillips, 2008). This analysis estimates that as many as a third of patients may have received inappropriate treatment, Ms. Bonoff reported.
Ms. Bonoff was also critical of the shortcomings of Herceptin as a treatment for breast cancer. About a quarter of breast cancer patients overexpress the HER2 gene, and thus are eligible for treatment with Herceptin. Of those eligible, she said, about 5,000 U.S. patients receive Herceptin without any clinical benefit, and about 7,000 patients who could derive benefit are not being treated because of a false-negative test result (Phillips, 2008). Even patients who do respond to Herceptin eventually usually experience a recurrence of their breast cancer (Romond et al., 2005). “As patients, we have a tempered view of all the latest promises of breakthroughs of tests that will reduce our treatment, and rarely do; of new biomarkers that will make a real difference, and have not,” she said. “Don’t oversell personalized
medicine. We know that breast cancer is many different diseases, and treatment that is tailored to specific tumor characteristics seems like a logical research path to follow. But we must remember that an intervention in the lab is years away from clinical impact. We are going in the right direction, but we should not jump the gun before the evidence is in. I am concerned about promising new approaches to diagnoses that are hyped before they are adequately validated or don’t positively impact patients. The most elegant and innovative scientific research in the world means nothing if it can’t help any person to live longer or better.”
Ms. Bonoff also asked researchers not to neglect prevention in their efforts to develop personalized medicine. “Right now we have poor tools to determine who is at risk for developing disease, and end up applying a one-size-fits-all approach to most screening and prevention interventions. This results in overuse of medical resources and overdiagnoses,” she said.
Contributing to the misuse of predictive tests is also insufficient physician education, Dr. Ratain pointed out. “The average clinician knows very little pharmacology and genetics, so how is he or she supposed to use pharmacogenetics?” Mark Gorman of the National Coalition for Cancer Survivorship asked, stating that ultimately the decision to use predictive tests will be made by clinicians and their patients. “There are policy ways to try and address the knowledge and skill of the clinicians, decision support, and the time that clinicians have to spend with their patients trying to support and sort through complicated bodies of information,” he said. Ms. Bonoff also stressed the need to educate physicians about new developments in personalized medicine, questioning how quickly new treatment protocols are disseminated into the communities where most patients are treated. “Once we figure out which patients benefit from a specific treatment, when all the evidence is in, will we make the clinical changes necessary to make sure that only those patients receive treatment? How do we integrate new evidence into existing clinical practice?” she asked.
The Secretary’s Advisory Committee on Genetic Testing (SACGT) (the predecessor to the current Secretary’s Advisory Committee on Genetics, Health, and Society, or SACGHS) recognized that the clinical use of genetic testing could be improved by enhanced genetic education of healthcare providers, insurers, and patients (SACGT, 2000b). Clinical decision support tools, such as electronic medical records, might be able to fill in some of the gaps in that education, said SACGHS Chair Dr. Andrea Ferreira-Gonzalez of Virginia Commonwealth University. These tools can discern the information
from a patient’s record that will help physicians to make their clinical decisions. However, “it’s not [known] how that would play out as we continue to leverage the information technology of the electronic medical record to start mining the data to not only improve [health care], but also improve the education of healthcare providers,” Dr. Ferreira-Gonzalez said. SACGHS also recommended that the U.S. Department of Health and Human Services (HHS) allocate resources to the Centers for Disease Control and Prevention (CDC), Agency for Healthcare Research and Quality (AHRQ), Health Resources and Services Administration, and National Institutes of Health (NIH) for research and development of clinical decision support systems (SACGHS, 2008a). Dr. Ferreira-Gonzalez stressed that “you can do the testing, but if the clinician or the consumer doesn’t know how to interpret the test, you might as well have not done the quality testing.”
Dr. Ratain noted that clinicians will probably have to wrestle with data overload problems. The commercial software packages that clinicians typically use are not designed to reliably analyze and interpret the immense amount of data generated with genome-wide typing or sequencing. He also questioned the availability of these tests to clinicians at large. Dr. Johnson noted that there may also be limited availability of patient tumor tissue for such testing, especially for inaccessible tumors, such as lung cancers. Dr. Mass added that a biomarker study his company did on ovarian cancer required them to remove a large piece of tumor with a laparoscopic biopsy. It took a year to acquire the Institutional Review Board approvals for the protocol at the half-dozen sites in which they conducted the study.
CODEVELOPMENT CHALLENGES
Several speakers stressed the need to develop biomarkers concurrently with targeted drugs. Dr. Shak noted that it was not until Herceptin was in Phase III testing that a clinical assay was developed to identify people likely to be responsive to the drug, and “we scrambled over the last 9 to 12 months to find a commercial partner to work out what needed to be done in order to present data to the FDA regarding the HercepTest. An important lesson that I and many of us have learned is that you don’t want to think about that late,” but rather it is important to start developing a biomarker assay early on in the drug development process. Ms. Bonoff said, “Tamoxifen and Herceptin are perfect examples of how it’s so important that the discovery of predictive biomarkers must not exist in a void, and that the successful development of drugs depends on the parallel development of predictive biomarkers. If we
don’t want drugs to be developed in a void, we must ensure that the interdisciplinary work needed becomes standard practice or we’re just wasting time.”
Dr. Hayes noted that the chances of codevelopment of a tumor marker and a therapeutic occurring at the start of clinical testing are about 10 percent, because often what was originally thought to be a good marker for the therapeutic turns out to be ineffective, and a new tumor marker shows more promise. He suggested that the FDA should stipulate that no registry trial be accepted without a prospective codevelopment plan, or at least a prospective plan for a specimen bank, and a transparent system to access specimens that provides adequate protection for intellectual property rights. “The sin is that the large pharmaceutical companies have not collected and bagged and stored specimens so that we could ask questions from the trials that they’ve run,” he said.
“A lot of therapies are generic, like chemotherapy, that we apply right now based on prognostic factors,” Dr. Hayes noted, “but we could really come up with better predictive factors for these therapies.” He suggested that in addition to codevelopment of specific markers, testing of generic markers for existing chemotherapies should also be done. Dr. Leonard concurred, noting that “there is a tremendous amount of research on markers for the proper use of existing drugs. But if you’re going to fix the marker development, validation, and implementation system for the new drugs, please do it for existing ones too.” Dr. Bruce Quinn of Foley Hoag, LLP, added that biomarkers for generic drugs are just as important to develop as those for new branded drugs.






















