5
Viral Hepatitis Services
Hepatitis B virus (HBV) and hepatitis C virus (HCV) infections cause substantial morbidity and mortality despite being preventable and treatable. Deficiencies in the implementation of established guidelines for the prevention, diagnosis, and medical management of chronic HBV and HCV infections perpetuate personal and economic burdens. This chapter reviews the current status of services to prevent and manage chronic hepatitis B and chronic hepatitis C. It then discusses the general components of viral hepatitis services. The chapter ends with an assessment of gaps in existing services, including a description of some models for services and committee recommendations to improve viral hepatitis prevention and management and to fill research needs. Services for the general US population are considered first and then services for special populations and service venues that have unique opportunities for interventions. Hepatitis B immunization is covered in Chapter 4 and so is not discussed in detail here.
The recommendations offered by the committee here are presented in the context of the current health-care system in the United States. The committee believes strongly that if the system changes as a result of health-care reform efforts, viral hepatitis services should have high priority in components of the reformed system that deal with prevention, chronic disease, and primary-care delivery. The committee’s recommendations regarding viral hepatitis services are summarized in Box 5-1.
|
BOX 5-1 Summary of Recommendations Regarding Viral Hepatitis Services General Population
Foreign-Born Populations
Illicit Drug Users
|
CURRENT STATUS
Health services related to viral hepatitis prevention, screening, and medical management are both limited and fragmented among entities at the federal, state, and local levels. Numerous federal agencies administer or fund some viral hepatitis–related services, including the Centers for Disease Control and Prevention (CDC), the Health Resources and Services
Pregnant Women
Incarcerated Populations
Community Health Facilities
High Impact Settings
|
Administration (HRSA), the Office of Minority Health, the Agency for Healthcare Quality and Research, the Centers for Medicare and Medicaid Services (CMS), the Substance Abuse and Mental Health Services Administration (SAMHSA), and the National Institutes of Health. Because there is no coordinated federal strategy for HBV and HCV prevention and control, those efforts are uneven in their application and funding. States, communi-
ties, and nongovernment organizations (NGOs) also provide viral hepatitis services, often with funding from federal agencies.
Most viral hepatitis–related activities in CDC are administered by the Division of Viral Hepatitis (DVH), which is part of the National Center for HIV/AIDS, Viral Hepatitis, Sexually Transmitted Disease, and Tuberculosis Prevention (NCHHSTP). The activities of the DVH, shown in Box 5-2, include surveillance and epidemiologic studies and clinical and laboratory research related to viral hepatitis. It supports viral hepatitis programs at the national, state, and community levels; disseminates hepatitis-related information to the public; and develops guidelines for prevention and control. In FY 2008, the DVH received $17.6 million, 2% of the NCHHSTP
|
BOX 5-2 Mission Statement of Centers for Disease Control and Prevention Division of Viral Hepatitis The Division of Viral Hepatitis (DVH) is the Public Health Service component that provides the scientific and programmatic foundation for the prevention, control, and elimination of hepatitis virus infections in the United States, and assists the international public health community in these activities. To achieve its mission, DVH:
|
budget (Ward, 2008a). In contrast, domestic HIV activities received 69%, sexually transmitted diseases (STDs) received 15%, and tuberculosis received 14% of the NCHHSTP FY 2008 budget. In FY 2009, the amount of NCHHSTP funding received by the DVH was not much greater, at $18.3 million (NASTAD, 2009) (personal communication, J. Efird, CDC, July 9, 2009). That low level of funding for the DVH has been relatively flat for the last 5 years.
HRSA, part of the US Department of Health and Human Services (HHS), is charged with increasing access to health care for people who are medically underserved. Several HRSA programs provide some direct services for viral hepatitis, including the Bureau of Primary Health Care, the
SOURCE: CDC, 2009a. |
Healthcare Systems Bureau, the HIV/AIDS Bureau, the Maternal and Child Health Bureau, the Office of Minority Health and Health Disparities, the Office of Planning and Evaluation, the Office of Rural Health Policy, and the Center for Quality (Raggio Ashley, 2009). In addition, viral hepatitis education and training activities are administered by the Bureau of Health Professions. HRSA funding supports federally qualified health centers that serve migrant, rural, tribal, and homeless populations. It also provides funding for Ryan White Care Act services and maternal and child health programs, such as Title V and Healthy Start, which provides some hepatitis B vaccination, testing, and counseling for HBV and HCV infections. Many people in HRSA-funded programs are foreign-born, including people from countries that have a high prevalence of hepatitis B or have behavior risk factors for HBV and HCV infection.
CMS, also a part of DHHS, provides health insurance through Medicare and Medicaid programs. Medicare covers people 65 years old or older, people under 65 years old who have specified disabilities, and people who have end-stage renal disease. Hepatitis B vaccination and its administration costs are covered by Part B of Medicare for people at high or intermediate risk for HBV infection (Rogers, 2009). People at low risk for HBV infection can receive the vaccine under Part D with a copayment that depends on their income level. Medicare will cover laboratory testing for HBV and HCV and treatment for chronic hepatitis B or hepatitis C. Medicaid is a state-administered program available to low-income individuals and families who fit into an eligibility group that is recognized by federal and state law. Eligibility for Medicaid and coverage for viral hepatitis services vary from state to state.
State and local (county and city) health departments obtain funds for viral hepatitis prevention and control activities from a variety of sources, including CDC, HRSA, SAMHSA, states, counties, cities, and private foundations. CDC funding supports adult viral hepatitis prevention coordinator (AVHPC) positions in 49 states and five cities (Ward, 2008a). The total funding level is about $5 million per year, and the average award is $90,000. CDC also funds perinatal hepatitis B coordinators in 64 states, cities, and territories at a total program cost of $7.5 million per year (CDC, 2009d). Funding for the AVHPC and perinatal hepatitis B coordinator positions covers only the coordinators’ salaries but not programmatic activities. CDC provides viral hepatitis program support—about $900,000 per year—in the form of grants for viral hepatitis training and education at the state and local levels.
A number of states have developed viral hepatitis prevention plans. At the committee’s request, the Institute of Medicine asked CDC to survey the 55 AVHPCs about the status of their jurisdiction’s plans (CDC, 2009g). All coordinators responded to the questionnaire. Of the 55, 32 (58.2%) indi-
cated that their states had a viral hepatitis prevention plan in place, half of which were completed in the last 5 years. Just over half of the plans include all the components in Table 5-1. All plans address hepatitis C prevention, and two-thirds (65.6%) address hepatitis B prevention. About 78% of the plans include hepatitis B vaccinations whether or not other hepatitis B prevention services are included. Some coordinators indicated that the CDC Section 317 vaccination initiative resulted in substantial progress toward implementing hepatitis B vaccination services in their jurisdictions. The medical management component is included in the smallest percentage of plans (62.5%) and just one-quarter of those plans have acted on this component. Overall, the coordinator survey revealed that over 40% of jurisdictions do not have plans; of the states that do have plans, only half have all the components, and only 20.7% of these reported that they had made progress in all the components. The primary barrier to plan implementation was financial constraints on overall funding and staffing (96.9%).
A number of NGOs have been established to address the prevention and control of HBV and HCV infections. Most of them focus on advocacy efforts, such as raising public awareness about viral hepatitis and encouraging people, especially in high-risk populations, to be vaccinated for hepatitis B, to undergo risk-factor screening for hepatitis B and hepatitis C, and to determine whether laboratory testing and medical management are needed. Many organizations target specific populations. For example, the Jade Ribbon Campaign targets Asians and Pacific Islanders to reduce the
TABLE 5-1 Summary of Adult Viral Hepatitis Prevention Coordinators Survey
prevalence of chronic HBV infection and HBV-related liver cancer (Asian Liver Center, 2009). The Harm Reduction Coalition is an example of an organization that develops and disseminates hepatitis C information among illicit-drug users (Harm Reduction Coalition, 2009). Information regarding the activities and programs supported by NGOs are presented primarily in Chapter 3.
Health services provided by federal agencies, state and local governments, and NGOs do not form part of a coordinated national campaign. Existing efforts at interagency information exchange, intermittent meetings to share plans and results, and joint administration of funds for some grants are not sufficient for the scale of the health burden presented by hepatitis B and hepatitis C. The lack of an accountable entity to lead a coordinated national effort has led to missed opportunities for prevention and identification of and treatment for chronic HBV and HCV infections.
COMPONENTS OF VIRAL HEPATITIS SERVICES
The committee has identified five core functions for comprehensive viral hepatitis services—(1) community outreach, (2) prevention, (3) identification of infected persons, (4) social and peer support, and (5) medical management (Box 5-3). Community outreach and immunization for primary prevention are discussed in depth in Chapters 3 and 4, respectively. Identification of infected persons, harm reduction, and medical management are reviewed below.
Identification of Infected Persons
There are two goals for identifying people chronically infected with HBV and HCV: to prevent transmission to close contacts (for example, through sharing of needles and other paraphernalia and through household and sexual contacts) and to reduce the risk of chronic liver disease through medical treatment and support. The identification of HBV-infected and HCV-infected people requires engagement of at-risk people and activism by the health-care–provider community. As discussed in Chapter 3, culturally relevant, accessible, and trusted sources of communication are required to increase awareness and promote use of appropriate services. Health-care and social-service providers, particularly primary-care providers, should be knowledgeable about chronic HBV and HCV infection and identify patients who are at risk because of their behavior or previous potential exposure to HBV or HCV. Programs and venues that serve at-risk populations—such as foreign-born people from HBV-endemic countries, the uninsured and underinsured, illicit-drug users, and homeless people—should also be knowl-
|
BOX 5-3 Components of Comprehensive Viral Hepatitis Services Community Outreach
Prevention
Identification of Infected Persons
Social and Peer Support
Medical Management
|
edgeable about viral hepatitis and should have mechanisms for identifying infected people and referring them to followup medical management.
The committee has defined a two-step process for identifying infected people:
-
Risk-factor screening. Risk-factor screening is the process of determining whether a person is at risk for being chronically infected or becoming infected with HBV or HCV. Risk factors include being born in a country where the disease is prevalent, and behavior such as illicit-drug use and having multiple sexual partners.
-
Serologic testing. Serologic testing is laboratory testing of blood specimens for biomarker confirmation of HBV or HCV infection.
Risk-Factor Screening
Hepatitis B Risk-Factor Screening CDC has identified risk factors for becoming infected or chronically infected with HBV (see Box 5-4). As discussed in Chapter 3, improved provider awareness about risk factors is critical for ensuring that people at risk for chronic HBV infection are identified and that those at risk for becoming infected with HBV are vaccinated. Providers should review patients’ backgrounds (for example, country of birth) and discuss relevant behaviors to determine what services they need.
Figure 5-1 illustrates the pathway of services and care for people depending on their risk factors identified. People who have HIV infection or other sexually transmitted infections, men who have sex with men, injection-drug users (IDUs), and institutionalized and incarcerated persons
|
BOX 5-4 Summary of CDC At-Risk Populations for Hepatitis B Virus Infection
SOURCE: Mast et al., 2005, 2006. |
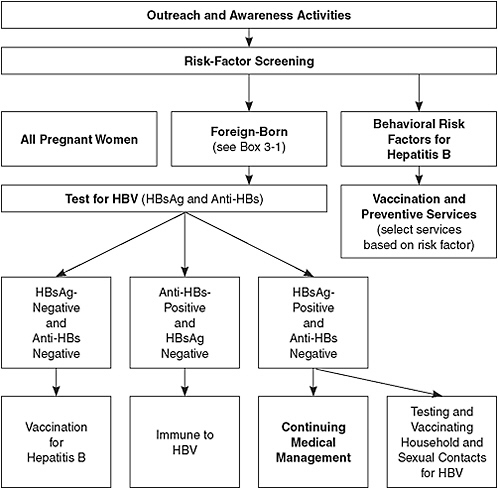
FIGURE 5-1 Hepatitis B services model.
Abbreviations: HBV, hepatitis B virus; HBsAg, hepatitis B surface antigen; anti-HBs, antibody to hepatitis B surface antigen.
are at increased risk for HBV infection. CDC recommends that all those populations be tested and given a first dose of vaccine at the time of testing. However, the committee believes that an acceptable alternative is hepatitis B vaccination without testing for all the populations except HIV-infected persons. This approach may facilitate increased vaccination rates. All persons found to have risk factors for HBV infection should receive counseling about prevention.
Hepatitis C Risk-Factor Screening CDC has identified risk factors for people at risk for being infected with HCV or becoming infected with HCV (see Box 5-5). Risk-factor screening has been tested by using question-
|
BOX 5-5 Summary of CDC At-Risk Populations for Hepatitis C Virus Infection
Abbreviation: HCV, hepatitis C virus. SOURCE: CDC, 2001. |
naires to assess a person’s potential exposure to HCV infection. It has been found to correlate with infection status and is an effective mechanism for identifying candidates for testing (Armstrong et al., 2006; McGinn et al., 2008; Zuniga et al., 2006). Armstrong et al. (2006) reported that 85% of HCV-infected people could be identified on the basis of three risk factors: injection-drug use, blood transfusion before 1992, and abnormal serum alanine aminotransferase levels. Additional studies have also found that questioning patients about exposures to known risk factors for hepatitis C is predictive of HCV infection in US veterans (Zuniga et al., 2006). People who are current or past users of illicit drugs may not fit the stereotype of an IDU, so all patients should be questioned about any past episode of illicit-drug injection. It has also been suggested that people who have tattoos and body piercings should be tested for HCV (Carey, 2003). Researchers who were evaluating hepatitis C incidence along the Texas–Mexico border found tattooing to be an independent risk factor for infection in their majority-Hispanic population (Hand and Vasquez, 2005). However, it is
unclear whether tattooing and body piercing are risk factors for infection or surrogates for other etiologic factors. As mentioned in Chapter 1, there is a high prevalence of HCV infection in Egypt, so Egyptian immigrants to the United States should be considered for serologic testing.
The issue of risk-factor screening and testing for HCV is controversial. In 1998, the US Public Health Service (USPHS) recommended a process of screening persons for HCV risk factors followed by laboratory testing for those potentially exposed to HCV (CDC, 1998). The 1998 USPHS recommendation to screen for risk factors among adults in the general population and to test those at risk was endorsed by a number of organizations, including the American Association for the Study of Liver Diseases (AASLD), the Infectious Diseases Society of America, and the American College of Physicians (Alter et al., 2004; Ghany et al., 2009). CDC recommends that all patients be evaluated for risk factors for HCV infection (Alter et al., 2004). In 2004, a separate group, the US Preventive Services Task Force (USPSTF), concluded that there was no direct evidence of the benefit of serologic testing for HCV infection in the general adult population and that there were inadequate data for determining accurately the benefits and risks associated with serological testing for HCV infection in otherwise healthy asymptomatic at-risk adults (Chou et al., 2004). As outcomes of treatment for chronic HCV infection improve, the controversy regarding screening and testing may diminish. An example of how improvements in treatment can change the value of identifying infected people can be seen in the advances in treatment for HIV. As effective antiretroviral therapies emerged, recommendations for screening and testing were expanded (Myers et al., 2009; Paltiel et al., 2005; Sanders et al., 2005).
Serologic Testing for Hepatitis B Virus and Hepatitis C Virus
Serologic tests to detect a history of exposure or to ascertain infection or immune status with respect to HBV and HCV use virus-specific antigens and antibodies, recombinant immunoblot assays (RIBAs), and viral nucleic acid (DNA and RNA) tests.
Rapid HBV and HCV detection tests are not available in the United States although they are available in other countries (Randrianirina et al., 2008). Rapid tests for HBsAg are available in developing countries and have high sensitivity and specificity (Randrianirina et al., 2008). Rapid te-Rapid testing in HIV interventions has been demonstrated to add substantial value in engaging hard-to-reach populations (Begley et al., 2008; Bowles et al., 2008; Clark et al., 2008; Kassler et al., 1997; Keenan and Keenan, 2001; Liang et al., 2005; Molitor et al., 1999; Reynolds et al., 2008; Schulden et al., 2008; Shrestha et al., 2008; Smith et al., 2006; Spielberg et al., 2001, 2003, 2005; Sullivan et al., 2004). The availability of rapid tests in the
|
BOX 5-6 Hepatitis B Virus-Specific Antigens and Antibodies Used for Testing Hepatitis B surface antigen (HBsAg): A protein on the surface of hepatitis B virus; it can be detected at high levels in serum during acute or chronic HBV infection. The presence of HBsAg indicates that a person is infected and infectious. The body normally produces antibodies to HBsAg as part of the normal immune response to infection. HBsAg is the antigen used to make hepatitis B vaccine. Hepatitis B surface antibody (anti-HBs): The presence of anti-HBs is generally interpreted as indicating recovery and immunity from HBV infection. Anti-HBs also develops in a person who has been successfully vaccinated against hepatitis B. Total hepatitis B core antibody (anti-HBc): This appears at the onset of symptoms in acute hepatitis B and generally persists for life. The presence of anti-HBc indicates previous or current infection with HBV in an undefined time frame. IgM antibody to hepatitis B core antigen (IgM anti-HBc): Positivity indicates recent infection with HBV (less than 6 months). Its presence indicates acute infection. SOURCE: CDC, 2009c. |
United States could enhance HBV and HCV detection and help to close gaps in care, particularly in hard-to-reach populations.
Hepatitis B Virus Laboratory Testing Serologic markers can be used to identify the different phases of HBV infection (Box 5-6). The preferred laboratory test for detecting current HBV infection is for hepatitis B surface antigen (HBsAg), and the principal test for detecting recovery from HBV infection is for anti-HB surface antibody (anti-HBs). An alternative is to test initially for anti-HB core antibody (anti-HBc)—which is present during acute, chronic, and resolved HBV infection—and, if the result is positive, to conduct followup testing for HBsAg and anti-HBs. HBV markers can be misinterpreted by clinicians, and this can lead to clinical errors in patient evaluations, counseling, or treatment. For example, anti-HBc and anti-HBs can be misinterpreted as indicators of active infection (Weinbaum, 2008).
TABLE 5-2 Interpretation of Hepatitis B Serologic Diagnostic Test Results
|
Antigen or Antibody Test |
Result |
Interpretation |
|
HBsAg Anti-HBc Anti-HBs |
All negative |
Susceptible |
|
HBsAg Anti-HBc Anti-HBs |
Negative Positive Positive |
Immune because of natural infection |
|
HBsAg Anti-HBc Anti-HBs |
Negative Negative Positive |
Immune because of hepatitis B vaccination |
|
HBsAg Anti-HBc IgM anti-HBc Anti-HBs |
Positive Positive Positive Negative |
Acutely infected |
|
HBsAg Anti-HBc IgM anti-HBc Anti-HBs |
Positive Positive Negative Negative |
Chronically infected |
|
HBsAg Anti-HBc Anti-HBs |
Negative Positive Negative |
Interpretation unclear; could be due to
|
|
Abbreviations: HBsAg, hepatitis B surface antigen; anti-HBc, total hepatitis B core antibody; anti-HBs, hepatitis B surface antibody; IgM anti-HBc, IgM antibody to hepatitis B core antigen. SOURCE: CDC, 2009c. |
||
Clinicians also may not know which tests to order to test for chronic vs acute viral HBV infection. Table 5-2 provides guidance on the interpretation of hepatitis B serologic test results.
Cost-effectiveness data on the use of laboratory testing in particular at-risk populations are available. As mentioned above, people born in foreign countries that have high rates of HBV (2% or more) are at the highest risk for chronic HBV infection and constitute the largest pool of undiagnosed persons (see Box 3-1). Laboratory testing of adult Asian and Pacific Islanders for HBV infection (10% prevalence of chronic HBV infection), monitoring and treating people who are found to be chronically infected, and
ring vaccination of their close contacts have been shown to be cost-effective (Hutton et al., 2007). That suggests that foreign-born persons from countries that have chronic HBV rates of 2% or greater should be screened for HBV infection. If they are negative for HBV seromarkers, they should be offered vaccination.
Other populations to be tested for HBV include IDUs, pregnant women, infants born to HBsAg-positive mothers, household contacts and sex partners of HBV-infected persons, men who have sex with men, and people infected with HIV. People scheduled for immunosuppressive therapy should also be tested for HBV infection because studies have clearly shown that persons who are HBsAg-positive have a risk of 20–50% of developing flares of hepatitis when undergoing cancer chemotherapy (Lok et al., 1991; Yeo and Johnson, 2006; Yeo et al., 2000). Reactivations have also been reported to occur with other types of immunosuppressives, notably anti–tumor-necrosis factor therapy for rheumatoid arthritis and inflammatory bowel disease (Esteve et al., 2004; Ostuni et al., 2003). Most flares of hepatitis in HBsAg-positive persons in this setting are asymptomatic, but icteric flares, hepatic decompensation, and death have been reported (Lok et al., 1991; Yeo et al., 2000). It has also been demonstrated that lamivudine prophylaxis can cause a substantial reduction in the incidence and severity of hepatitis flares in HBsAg-positive persons who are undergoing cancer chemotherapy (Hsu et al., 2008; Lau et al., 2002, 2003; Li et al., 2006; Rossi et al., 2001). Therefore, all persons scheduled to undergo cancer chemotherapy or immunosuppressive treatments should be screened for hepatitis B risk factors, and followup testing for HBsAg should be performed if warranted. Persons who are HBsAg-positive should be treated as recommended by established practice guidelines (Lok and McMahon, 2009).
Hepatitis C Virus Laboratory Testing Persons at risk for hepatitis C should be tested for antibodies to HCV with a licensed enzyme immunoassay (EIA), which has a high sensitivity (Alter et al., 2003). As with all screening tests, the predictive value of a positive test varies with the population prevalence, which for HCV is lowest in volunteer blood donors and highest in IDUs. False-negative EIA tests may occur in immunosuppressed populations, such as patients on hemodialysis or infected with HIV (Rahnavardi et al., 2008; Thio et al., 2000). In those settings, EIA-negative persons suspected of having HCV infection should also be tested for HCV RNA (Ghany et al., 2009). Table 5-3 provides guidance on the interpretation of hepatitis C test results.
HCV EIA-positive results should be confirmed with a second test, ideally before the information is presented to the patient to minimize the unnecessary harm of a false-positive result. There are two separate goals
of confirming a positive EIA: to make sure that the antibodies detected in the EIA were truly to HCV and not cross-reactive and to assess persons with antibodies to HCV to ascertain whether HCV infection is current or cleared spontaneously. The likelihood that a positive HCV EIA represents antibodies to HCV (as opposed to cross-reactive antibodies) depends on the strength of the EIA reaction. Values that exceed a particular threshold (for example, 3.8 for tests commonly used in the United States) are likely to be true HCV infections and unlikely to be false-positive (Alter et al., 2003; Pawlotsky et al., 1998). Thus, some laboratories report the ratio of the test result to the cutoff value, and ratios above that threshold can be assumed to represent HCV antibodies. Another way to establish whether a positive EIA reflects HCV antibodies is to run a supplemental antibody test, such as a RIBA recombinant immunoblot assay. By separating the antigens that are grouped in the screening EIA, the supplemental antibody test provides better specificity (Damen et al., 1995). However, supplemental antibody testing does not achieve the second goal of determining whether a person has HCV infection. Thus, most authorities recommend use of HCV-RNA testing as the next step after detection of HCV antibodies with EIA in all settings in which HCV testing is done in at-risk persons (Alter et al., 2003; Ghany et al., 2009). A positive result in both EIA and RNA tests means that a person needs further counseling and medical evaluation for chronic or acute HCV infection.
HCV-RNA testing is more expensive than antibody testing with EIA and may require more sophisticated laboratory capability and a longer reporting interval (Alter et al., 2003). Thus, although all reasonable efforts should be made to confirm positive EIA HCV results before presenting them, HCV-RNA testing may not be feasible in some settings. Lack of available HCV-RNA testing should not be an impediment to EIA testing, but counseling must reflect the uncertainty and the urgency of followup in another venue for further assessment.
Laboratory testing of at-risk populations to identify HCV-infected people has been found to be cost-effective when combined with proper medical-management and harm-reduction strategies (Tramarin et al., 2008). In particular, studies have found that HCV laboratory testing among current and former IDUs is cost-effective. A 2006 study of former IDUs in a prison in the United Kingdom found that laboratory testing and later treatment of inmates cost £16,514 (about $25,000) per quality adjusted life year (Castelnuovo et al., 2006). A 2008 study in Italy found that laboratory testing (followed by appropriate medical management) of IDUs resulted in a substantial difference in the incidence of premature death. In contrast, HCV laboratory testing for people who are in the hospital for surgery but have no other risk factors is unlikely to be cost-effective (Tramarin et al., 2008).
TABLE 5-3 Interpretation of Hepatitis C Virus Diagnostic Test Results
|
If HCV Test Result Is: |
Interpretation |
Action |
||
|
Anti-HCV Screening Test (EIA or CIA) |
Anti-HCV Supplemental Test: RIBA |
Anti-HCV Supplemental Test: HCV RNA |
HCV Status |
Additional Testing or Evaluation Required |
|
Positive |
Not done |
Not done |
Not known |
HCV RNA; RIBA if RNA negative |
|
Positive |
Not done |
Negative |
Probably not currently HCV infected; further testing requireda |
Obtain RIBA; if RIBA positive, repeat RNA |
|
Positive (high s/co ratio) |
Not done |
Not done |
Antibody probably true positive; need to distinguish past from current infection |
HCV RNA |
|
Positive |
Negative |
Not done |
False positive anti-HCV |
None |
|
Positive |
Positive |
Not done |
Past or current HCV infection |
HCV RNA; if RNA positive, evaluate for liver disease |
|
Positive |
Positive |
Negative |
Probable past HCV infection with recoverya |
Repeat HCV RNA to rule out active infectiona |
|
Positive |
Positive or not done |
Positive |
Current acute or chronic HCV infection |
Evaluate for chronic infection and liver disease |
|
Positive |
Indeterminate |
Not done |
Not known; possible false-positive anti-HCV or recovery from past HCV infection |
Test for HCV RNA or repeat anti-HCV testing |
|
Positive |
Indeterminate |
Positive |
Current acute or chronic HCV infection |
Evaluate for chronic infection and liver disease |
|
Positive |
Indeterminate |
Negative |
Probably not currently infected;a possible false-positive anti-HCV or recovery from past HCV infection |
Repeat HCV RNA or repeat anti-HCV testing |
|
aA single negative HCV-RNA result cannot determine infection status, inasmuch as a person might have intermittent viremia. Abbreviations: anti-HCV, antibody to HCV; EIA, enzyme immunoassay; CIA, enhanced chemiluminescence immunoassay; RIBA, recombinant immunoblot assay; RNA, ribonucleic acid; s/co ratio, signal-to-cutoff ratio. SOURCE: Adapted from Ghany et al., 2009. Diagnosis, management, and treatment of Hepatitis C: An update. Hepatology 49(4):1335-1374. Copyright 2009. Reprinted with permission of John Wiley & Sons, Inc.; CDC, 2009e. |
||||
Prevention
Vaccination
A vaccine for hepatitis B has been available since the 1980s. Research to develop a vaccine for hepatitis C continues although it is unlikely that a vaccine will be developed and licensed in the near future. Given the complexity of the issues surrounding vaccination of children and adults, this report devotes a separate chapter (Chapter 4) to immunization.
Harm Reduction
Harm reduction refers to programs and policies that seek to reduce the medical, social, and economic harms associated with illicit-drug use (IHRA, 2009). Support for abstinence is an element of harm reduction but is not a requirement for participation in harm-reduction programs. Harm reduction focuses on providing information about safer practices (for example, how to inject without exposing oneself to contaminated blood), providing materials for engaging in safer practices (such as needle syringes and condoms), and offering hepatitis B vaccination. Because harm reduction does not condemn illicit-drug use and instead seeks practical solutions to mitigate its harmful consequences, these programs can be controversial (Des Jarlais et al., 2009).
Medical Management
Evidence-based practice guidelines for both chronic hepatitis B and chronic hepatitis C have been published by the American Association for the Study of Liver Diseases (AASLD) and other organizations (Ghany et al., 2009; Lok and McMahon, 2009). The guidelines are updated regularly to reflect advances in care and should be referred to as the basis of appropriate medical management. For the purposes of this report, the committee specifies that the goals of medical management of chronically infected people are to decrease the risk of developing cirrhosis, to prevent hepatic decompensation, to decrease the risk of hepatocellular carcinoma in people chronically infected with HBV or HCV, and to effect secondary prevention of virus transmission.
The AASLD guidelines include recommendations for selection of patients who have chronic hepatitis B or hepatitis C for referral to specialists and for treatment with medications (Ghany et al., 2009; Lok and McMahon, 2009). Persons who are identified as HBsAg-positive should have a history taken and a physical examination performed by a primary-care provider with an emphasis on symptoms and signs of liver disease
(Lok and McMahon, 2009). Initial laboratory evaluation should include a full liver panel, complete blood count (CBC), and hepatitis B e antigen (HBeAg), anti-HB e antibody (anti-HBe), and HBV DNA tests. Further management will depend on the results. Persons who are HBeAg-positive and have increased alanine aminotransferase (ALT) should be referred for evaluation for possible liver biopsy and treatment. Likewise, persons who are HBeAg-negative and anti-HBe-positive, have increased HBV DNA (over 2,000 IU/mL), and have increased ALT should be referred to a specialist. People who have normal ALT, are HBeAg-negative and anti-HBe-positive, and have HBV DNA below 2,000 IU/mL can be followed with ALT and aspartate aminotransferase (AST) tests every 6 months by a primary care provider. If ALT increases above the normal limit, HBV DNA should be tested again; if it is above 2,000 IU/mL, the patient should be referred to a specialist. If a patient is HBeAg-positive and has normal ALT (that is, in the immune tolerant phase), tests for ALT, AST, HBeAg, and anti-HBe should be repeated every 6 months. If ALT rises above the normal range, the patient should be referred to a specialist. In patients who have increased ALT and HBV DNA, liver biopsy is often appropriate to determine the best candidates for treatment because it is recommended that patients who have more than mild inflammation and fibrosis on biopsy receive treatment, and laboratory tests are often unable to distinguish degrees of liver involvement (Lok and McMahon, 2004; NIH, 2008). In addition, any patient who has stigmata of liver disease—ascites, enlarged spleen, jaundice, or encephalopathy—or a platelet count below 100,000 (which is a sign of possible splenomegaly) should be referred immediately to a specialist. The AASLD provides hepatitis B–specific treatment guidelines, including how to select appropriate candidates for treatment, guidance on which antiviral medications to use, and how to address antiviral resistance (Lok and McMahon, 2009). All chronically HBV-infected patients, regardless of their ALT and HBV DNA status, must be followed on a regular basis, every 3–12 months depending on the activity of their disease.
Persons who are identified as anti-HCV-positive and who have HCV RNA present in their serum may initially be evaluated by a primary care provider (Ghany et al., 2009). The primary care provider should take a history and perform a physical examination with emphasis on symptoms and signs of liver disease. The initial laboratory evaluation should include a full liver panel, CBC, and HCV genotype tests. Patients found to have signs or symptoms of liver disease or a low platelet count (below 100,000) should be referred to a specialist who has experience in managing persons with advanced hepatitis C. Patients who are infected with HCV genotype 2 or 3 and who are interested in receiving treatment can be referred immediately for treatment consideration. A primary care provider should discuss with a patient who is infected with HCV genotype 1 the possibility of receiving
treatment and should emphasize that the treatment is successful in only about 50% of cases and that the side effects can be severe. For genotype 1 patients, it may be preferable first to do a liver biopsy to determine the degree of liver involvement and scarring before making a decision about whether treatment should be considered sooner rather than later. The AASLD provides hepatitis C–specific treatment guidelines, including how to select appropriate candidates for treatment and guidance on which antiviral medications to use (Ghany et al., 2009). Patients who do not want immediate referral or treatment should be followed every 6 months with a full liver panel and yearly CBC tests. Finally, primary care providers should counsel patients to abstain from, or at least limit, alcohol consumption because heavy alcohol use is the greatest contributor to the rate of progressive liver fibrosis. Patients who have a history of heavy alcohol intake should receive counseling.
Studies have found racial and ethnic disparities in the evaluation of and treatment for HCV infection (Butt et al., 2007; Rousseau et al., 2008). One study of veterans reported similar rates of referral and liver biopsy for HCV-infected persons of various racial populations but found that blacks were less likely than whites to have complete laboratory evaluations, including viral genotyping, and to receive antiviral treatment (Rousseau et al., 2008). Because patient characteristics that are associated with not responding to treatment generally are associated with not receiving treatment, it is difficult to ascertain from available research findings the degree to which lower uptake into treatment represents discrimination against minority populations or appropriate implementation of treatment guidelines. For example, in another study of veterans, less treatment was received by minority-groups members and by persons who were older, who had a history of drug and alcohol use, or who had comorbid illnesses (Butt et al., 2007).
Chronic HCV infection has been found to be an important cause of liver-related death in Alaska Natives (Wise et al., 2008). The federal government is responsible by treaty laws to provide medical care at no cost to American Indians and Alaska Natives, but the amount spent per person is far less than that spent for Medicare and Medicaid recipients or for incarcerated persons, and is not enough to pay for treatment for HCV infection in many tribal health-care systems. There is evidence that not all patients who initiate therapy complete it. Over 80% of participants in clinical trials completed the HCV antiviral therapy. However, researchers found that in a large national cohort of veterans less than one-fourth of the patients who began treatment for chronic hepatitis C completed a 48-week course. The major predictors of treatment noncompletion were pretreatment anemia and depression (Butt et al., 2009). Treatment completion rates appear to vary among ethnic and racial populations. For example, a study found that Hispanic patients were more likely to be candidates for treatment but were
less likely to initiate it; they were also more likely to discontinue treatment early, and discontinuation of treatment was associated with alcohol use (Cheung et al., 2005).
The risk of developing hepatocellular carcinoma (HCC) is a serious concern for patients who are infected with HBV or HCV, and providers should initiate regular monitoring for HCC (Bruix and Sherman, 2005). Patients who have chronic HBV infection and are at the highest risk for HCC include those who have first-degree relatives who developed HCC, persons who have cirrhosis, men 40 years old and older, and women 50 years old and older. Of patients who have chronic HCV infection, only those who have cirrhosis or advanced liver fibrosis (that is, bridging fibrosis) should be monitored for HCC. Monitoring of patients at high risk for HCC should be performed every 6 months.
Studies have found ethnic disparities in HCC treatment rates and mortality (Davila and El-Serag, 2006; Siegel et al., 2008; Sonnenday et al., 2007). Blacks and Hispanics had significantly higher HCC-related mortality than other racial and ethnic populations. Even after adjustment for stage of HCC and other demographic characteristics, blacks were 40% less likely than whites to receive local or surgical therapy. Another study found that blacks and Hispanics were 24–27% less likely than whites to receive surgical therapy (Sonnenday et al., 2007). A study that looked at liver transplants necessitated by HCC found that in 1998–2002, black and Asian patients were significantly less likely than white patients to receive a liver transplant (Siegel et al., 2008). Once researchers controlled for receipt of treatment, the difference in mortality in black patients was no longer significant (Davila and El-Serag, 2006). Those data on racial and ethnic disparities in the outcomes of and treatments for chronic hepatitis underscore the need for additional research to understand the biologic and societal basis of the disparities. They also indicate the urgency of new policies that ensure that optimal medical care is given to all without regard to race or ethnicity.
The economic costs of chronic hepatitis B and hepatitis C are high. In 2004, the average annual medical-care costs of chronic HBV infection and its complications per infected person in the United States were as follows: chronic HBV infection, $761; compensated cirrhosis, $227; decompensated cirrhosis, $11,459; liver transplantation, $86,552; transplantation care more than 12 months after transplantation, $12,560; and hepatocellular carcinoma, $7,533 (Lee et al., 2004). Medication costs were the largest proportion of the chronic HBV infection and compensated cirrhosis states and hospitalization costs made up the largest proportion of the other health states. In the same year, Chesson et al. (2004) estimated the annual net cost per case of chronic liver disease at $32,837 in the United States.
Although treatment costs are high, some studies have found that treatment can be cost-effective. In particular, several studies compared the costs
of various treatments for chronic HBV infection (for example, interferon, pegylated interferon, lamivudine, and adefovir) and found them to be cost-effective (Kanwal et al., 2005; Rajendra and Wong, 2007). Treatments for HCV infection with interferon or pegylated interferon plus ribavirin have also been shown to be cost-effective (Campos et al., 2007; Lidgren et al., 2007; Rajendra and Wong, 2007; Salomon et al., 2003).
There is evidence that people’s ability to pay affects whether they seek and receive appropriate medical care for chronic hepatitis B and hepatitis C. For example, among people who tested positive for HCV antibody at public STD clinics in San Diego and an HIV test-site screening program, the presence or absence of health insurance was strongly associated with whether later medical care was received for HCV (Mark et al., 2007).
MAJOR GAPS IN SERVICES
The lack of comprehensive case management (that is, initial clinical evaluation and laboratory testing, regularly scheduled clinical and laboratory monitoring, appropriate referral and treatment, and monitoring for HCC) for people who have chronic hepatitis B or hepatitis C and who do not have access to private health insurance and care is an important gap in control of chronic viral hepatitis. The committee believes that people who are living with chronic HBV or HCV infection should receive the health-care services outlined in Box 5-3. The Ryan White Care program for people who are living with HIV/AIDS is a federal approach that could be replicated to fill the void in health-care services for patients who have HBV or HCV infection. The committee recognizes that uncertainties in funding and health-care reform may make implementation of such a program challenging.
General Population
Various factors can lead to difficulties in accessing screening, prevention, testing, and care related to viral hepatitis. Obstacles to obtaining such services may be limitations in private or public insurance coverage and cost-sharing, lack of access to public health insurance, lack of public funding to support implementation of state viral hepatitis plans, lack of hepatitis awareness and health literacy, inadequacy of sites or practice settings where health-care services are received, transportation needs, social stigmas, fear of legal prosecution related to drug use and immigration, and such cultural factors as religious beliefs, beliefs about biologic products, health perceptions, and language. Among those, however, the most important barriers to receipt of existing services are inadequacy of health-insurance coverage and lack of money to pay for services.
Having insurance, either privately or publicly funded, has a positive association with laboratory testing for HBV infection, and those who have private insurance have the highest testing rates. For example, Choe et al. (2006) reported a strong relation between having ever been tested for HBV and insurance coverage in Vietnamese American men in Seattle, Washington. In their study, 70% of privately insured people and 51% of people insured by the Washington State Basic Health Insurance Plan were ever tested for HBV. As discussed in Chapter 4, health insurance must provide strong coverage for immunization, counseling services, medical treatment, and prescription drugs, or the insurance’s cost-sharing features will prevent use of services. High deductibles (amounts to be paid out of pocket before coverage begins) or benefit limits are common in insurance policies that are provided by medium and small employers or in-network plans (which provide different coverage in network from out of network). That is not the case with more comprehensive insurance coverage typically seen in integrated delivery systems and health maintenance organizations (HMOs). As of 2007, 21% of workers who had employer-sponsored health insurance were covered under HMOs (Claxton et al., 2007). In publicly funded venues—where services for the poor or special risk populations, such as STD clinics, or for IDUs are provided—inadequacies in funding for hepatitis-related services may limit testing or other services (Boutwell et al., 2005; Brown et al., 2007; Heseltine and McFarlane, 2007; McIntyre et al., 2008).
The current fragmentation of viral hepatitis services involving vaccination, risk-factor screening, laboratory testing, and medical management is a major obstacle to the effective delivery of needed services and makes compliance more difficult. The lack of coordination between services can inhibit use by requiring people to travel to multiple sites to obtain care, impairs the development of trusting relationships among multiple providers, and taxes a health system’s ability to transfer information where and when it is needed for good clinical care. Studies have examined program integration for HIV, STD, and viral hepatitis services and found that integration brings more at-risk people into the system (Birkhead et al., 2007; Gilbert et al., 2005; Gunn et al., 2007; Hennessy et al., 2007; Kresina et al., 2008; Stopka et al., 2007; Zimmerman et al., 2007).
One important consequence of the fragmentation of viral-hepatitis services is inconsistency in referral of people who have chronic viral hepatitis for appropriate medical care. That gap reflects deficiencies primary-care providers’ knowledge, and it can be substantial when there are barriers, such as physical barriers (that is, screening and testing services in a different location from medical-management services), economic barriers, and cultural barriers. As discussed below, the Department of Veterans Affairs (VA) is notable for having bridged the gap integrating health services.
The VA Medical Center in Minnesota developed a hospital-based model that could serve as a template for health-care providers in integrated delivery systems, accountable care organizations, and HMOs (Groom et al., 2008). The medical center established a method to screen patients for HCV risk factors, to initiate appropriate viral testing, to counsel patients, and to refer them to a dedicated hepatitis clinic for medical evaluations, liver biopsies, and appropriate antiviral therapy. In addition, it traced the outcome of therapy and continued to follow those who did not respond. VA performed risk-assessment screening of 36,422 patients for HCV infection (Groom et al., 2008). The screening identified 12,485 patients (34%) who had risk indications for anti-HCV testing. Anti-HCV antibodies were identified in 681 (5.4% of those at risk) and HCV RNA was detected in 520 (4.2% of those at risk and 76% of those who were anti-HCV positive). Of those who tested positive for HCV RNA, 430 (83%) were referred to the hepatitis clinic, of whom 382 (73%) attended. A relatively large percentage of patients (45%) were evaluated in the clinic and underwent liver biopsy. On the basis of the extent of fibrosis on biopsy, 124 patients received anti-viral therapy—32% of the patients referred to the clinic and 24% of those who had viremia. A sustained virologic response occurred in 37% of the treated patients. Thus, 46 patients could be considered cured, and 17 had stage 3 or 4 fibrosis, which could potentially result in end-stage liver disease and possibly HCC.
Closed systems, such as HMOs, and integrated delivery systems have the potential to replicate the VA model. Those sources of health care share the VA advantages of control of the various providers and care settings, comprehensive coverage that reduces financial barriers to compliance, administrative and information systems to track and share information, team-based processes of care, and the ability to enforce standards of performance.
The federal government is the largest purchaser of health insurance nationally, with about 8 million people covered through the Federal Employees Health Benefits Program and those covered through Medicare, Medicaid, and the Children’s Health Insurance Program. Given its tremendous purchasing power, the federal government is well positioned to be the leader in the development and enforcement of guidelines to ensure that the people for whom it provides health care have access to risk-factor screening, serologic testing for HBV and HCV, and appropriate medical management.
Recommendation 5-1. Federally funded health-insurance programs—such as Medicare, Medicaid, and the Federal Employees Health Benefits Program—should incorporate guidelines for risk-factor screening for hepatitis B and hepatitis C as a required core component of pre-
ventive care so that at-risk people receive serologic testing for hepatitis B virus and hepatitis C virus and chronically infected patients receive appropriate medical management.
The committee has included recommendations regarding coverage of vaccination for infants, children, and adults in Chapter 4.
Foreign-Born People
There are over 37 million foreign-born residents in the United States; they represent about 12% of the nation’s population (U.S. Census Bureau, 2008). Of the foreign-born population, 27% were born in Asia, 4% in Africa, and roughly 7% in other regions that have intermediate or high HBV endemicity (see Box 3-1). Nearly half the US foreign-born population (6% of the total population) originated in HBV-endemic countries (U.S. Census Bureau, 2008), and 40,000–45,000 legal immigrants from these countries enter the United States each year (U.S. Department of Homeland Security, 2009; Weinbaum, 2008). It is increasingly urgent that culturally appropriate programs provide hepatitis B screening and related services to this high-risk population.
Efforts to deliver hepatitis B–related services to the foreign-born population have been sparse. At the federal level, there are limited and fragmented resources to track and fund such services. On the local and regional levels, some culturally tailored community-based or faith-based screening programs target foreign-born people, such as those involving Asian and Pacific Islander populations in San Francisco, Maryland, and New York City (CDC, 2006; Chao et al., 2009a; Hsu et al., 2007). However, few of the independent programs have been replicated in other communities of at-risk foreign-born populations, so many regions in the United States that have at-risk foreign-born populations lack community-based hepatitis B screening (Rein et al., 2009). Few HBV screening programs are designed for other high-risk foreign-born populations, including Africans, Middle Easterners, eastern Europeans, and others from HBV-endemic regions. It is unknown whether the model programs developed for Asians and Pacific Islanders could be adapted for some of those populations or whether new culturally tailored programs would need to be created.
The key to eliminating HBV transmission is identification of people who are living with chronic HBV infection. As described in Chapter 3, there is a pervasive ignorance about hepatitis B among Asians and Pacific Islanders, and it can be assumed that other foreign-born populations in the United States are similarly uninformed about HBV risks, prevention, testing, and management. That contributes to the observation that up to two-thirds of those who are chronically infected with HBV are unaware of their infection
status (Lin et al., 2007). The lack of awareness in foreign-born populations from HBV-endemic countries is compounded by gaps in knowledge and preventive practice among health-care providers, particularly if they are serving a large number of foreign-born, high-risk patients (see Chapter 3).
Cultural and institutional impediments are particularly important for the foreign-born. For example, culture-specific stigmas may be attached to a diagnosis of chronic hepatitis B. In China, there is discrimination against people who are chronically infected with HBV, and such people reportedly have been expelled from schools, fired from jobs, and shunned by other community members despite the recent passage of national antidiscrimination laws (China Digital Times, 2009). Such social stigma and discrimination may contribute to the reluctance of immigrants from HBV-endemic countries to undergo HBsAg testing or to seek medical attention for a positive test result after settling in the United States.
Institutional barriers include administrative procedures and the absence of culturally responsive support services. For example, a recent survey of hospitals in the San Francisco Bay area—a region where 29% of the population is foreign-born—found that fewer than half routinely collect information on patients’ birthplaces (Gomez et al., 2003). The collection of information on the birthplace of patients’ parents is even rarer—but relevant for risk assessment. English-language proficiency and cultural preferences of foreign-born patients may pose additional challenges to institutions that are not prepared to work with these patient factors. Non-English-speaking patients report that physicians are intolerant and impatient toward them and fail to use interpreter services, even when available, to facilitate communication (Barr and Wanat, 2005; Giordano and Cooper, 2009; Giordano et al., 2009). As a result of patient–physician language discordance and impaired communication, such patients have poorer comprehension of medical conditions, testing, and treatment; have low compliance; and are more likely to miss followup appointments (Giordano and Cooper, 2009; Giordano et al., 2009; Jacobs et al., 2006; Manson, 1988; Zickmund et al., 2004).
There is a need for evidence-based strategies and programs to disseminate information about hepatitis B transmission, infection, and treatment to culturally and demographically diverse populations. A community-based participatory research approach, in which communities are actively engaged in equal partnership with scientists, is needed to ensure that the programs are acceptable, accessible, and sustainable in the communities where they are based. Such programs should also be flexible and scalable so that other communities can tailor them to their own needs. The committee believes that these tasks are best accomplished with the approach outlined in Recommendations 3-1 and 3-2 in Chapter 3. The community-based approach as outlined in Recommendation 3-2 would be strengthened by additional
resources to provide screening, testing, and vaccination services. Therefore, the committee offers the following recommendation:
Recommendation 5-2. The Centers for Disease Control and Prevention, in conjunction with other federal agencies and state agencies, should provide resources for the expansion of community-based programs that provide hepatitis B screening, testing, and vaccination services that target foreign-born populations.
Illicit-Drug Users
Preventing bloodborne infectious diseases, particularly hepatitis C, in illicit-drug users is an important public-health challenge. Hepatitis C incidence in IDUs has been reported to be 2–40 per 100 person-years (PY) of observation, with most rates in the range of 15–30 per 100 PY (Maher et al., 2006; Mathei et al., 2005; van den Berg et al., 2007b). HCV prevalence in IDUs is typically 35–70%, depending on geographic location and duration of exposure to injection-drug use (Hagan et al., 2008). The early years after onset of drug injection are high-risk periods when HCV seroconversion rates are particularly high (Maher et al., 2006).
Non-injection-drug users (NIDUs) who sniff or snort heroin, cocaine, and other drugs also have a high risk of HCV infection. A meta-analysis of 26 studies showed that HCV prevalence in NIDUs was 2–35%, with a median of 14% (Scheinmann et al., 2007). Whether drug practices, sexual exposures, or both are the sources of HCV transmission is unclear (Scheinmann et al., 2007). Low rates of HCV seroconversion have been reported in NIDUs—0.4–2.7 per 100 PY—rates that are similar to those observed in sex partners of HCV-RNA–positive persons (1.2 per 100 PY) (Fuller et al., 2004; Neaigus et al., 2007; Rooney and Gilson, 1998). Studies have shown that HCV RNA can be detected on the surface of crack pipes, so it is biologically plausible that drug-use practices are a route of transmission in these people (Fischer et al., 2008). Research is needed to explicate the etiology of HCV infection in NIDUs so that effective prevention strategies can be designed.
To understand the development and opportunities for control of this hyperendemic state of HCV infection in IDUs, it is important to consider multiple features of the disease agent, the human host, and the environment that determine the occurrence of infection (Lillienfeld and Lillienfeld, 1980). HCV is efficiently transmitted via bloodborne exposure, and several studies have shown that transmission can occur via the shared use of syringes, drug cookers, and filtration cotton (Hagan et al., 2001; Hahn et al., 2002; Thorpe et al., 2002). It takes only a very small amount of infectious blood on injection equipment to result in infection. Awareness of risk
of HCV infection associated with the shared use of cookers and cotton is not widespread, and about 40% of HCV transmission may be attributable to the sharing of these items (Hagan et al., 2010). Injection often takes place in settings that are chaotic, rushed, or otherwise not conducive to safe practices, thereby increasing the risk of disease transmission (Rhodes and Treloar, 2008). The persistence of moderate levels of unsafe injection behaviors seems to be sufficient to maintain relatively high rates of new infections (Thiede et al., 2007). The high prevalence of infectious carriers also means that there is a high probability that one or more IDUs present in the injection setting may be capable of transmitting HCV.
HBV infection rates in both IDUs and NIDUs are high. Seroincidence in IDUs has been reported to be 10–12% per year (Hagan et al., 1999; Ruan et al., 2007). A study that looked at evidence of past or present HBV infections found rates of 37% in IDUs and 19% in NIDUs (Kuo et al., 2004). HBV transmission in these populations generally occurs as a result of drug-related and sexual exposures to infected people. A study of more than 800 young IDUs (up to 30 years old) found low hepatitis B vaccination coverage (22%) and a high prevalence of HBV infection (21%) (Lum et al., 2008).
Although drug use is associated with many serious acute and chronic medical conditions, health-care utilization among drug users is low compared with persons who do not use illicit drugs (Chitwood et al., 1999; Contoreggi et al., 1998; O’Toole et al., 2007, 2008). Health care for both IDUs and NIDUs is sporadic and generally received in hospital emergency rooms, correction facilities, and STD clinics (Chitwood et al., 1999; Huckans et al., 2005). Given this population’s limited access to health care and services, it is important to have prevention and care services in settings that IDUs and NIDUs are likely to frequent or to develop programs that will draw them into care.
Program Venues
Because of its similarity to HIV in transmission routes, public-health practitioners expected that strategies that were working for HIV would work similarly in the case of HCV. The two major public-health interventions that have been shown to reduce HIV risk in IDUs are drug-treatment programs and syringe-exchange programs (SEPs) (Des Jarlais et al., 1996; Metzger et al., 1998).
Drug-treatment programs offer few services related to hepatitis B and hepatitis C and are constrained by lack of funding (Stanley, 1999). A nationwide study of drug-treatment clinics found that although most clinics educated patients about the importance of testing for HCV, only 7% tested all clients for HCV and 22% tested none (Astone et al., 2003; Strauss et al., 2002, 2004).
Starting in the 1980s with the introduction of HIV into IDU populations, there were mass awareness and safe-injection campaigns that resulted in substantial reductions in syringe-sharing (Des Jarlais and Semaan, 2008). In the United States, SEPs are now available in 31 states, the District of Columbia, and Puerto Rico (Des Jarlais et al., 2009). Several characteristics support the use of SEPs as sites of care for and prevention of hepatitis B and hepatitis C. SEPs, in addition to providing safe injection materials and counseling services, are key access points for screening and referral to followup medical care (Des Jarlais et al., 2009). SEPs appear to attract and retain high-risk injectors, in particular those at highest risk for HIV or HCV seroconversion (Hagan et al., 2001; Schechter et al., 1999). SEPs can be referral pathways to other programs. Demand for drug treatment is high among exchange users and SEPs are important venues for drug-treatment referral (Kidorf et al., 2009; Strauss et al., 2003a). About 92% of US SEPs offer referrals to substance-abuse treatment programs (Des Jarlais et al., 2009), and 33% offer on-site medical care (Des Jarlais et al., 2009), although the services offered vary and there is great geographic variability in their distribution. Syringe coverage rates—the number of syringes available via SEPs per 100 injections—are also highly variable, ranging between 0.03 and 20 per 100 injections, and there are vast regions of the United States where SEPs are not available (Tempalski et al., 2008). Those factors limit the impact of the programs for HCV control (Tempalski et al., 2007, 2008). In addition, SEPs have not been as successful in reducing the shared use of other injection equipment, such as cookers and cottons, as they have been in reducing syringe-sharing (Hagan and Thiede, 2000).
Prevention Strategies
Several strategies to reduce HCV transmission in IDUs have been evaluated. A number of studies have examined opiate-substitution treatment and HCV seroconversion (summarized in Table 5-4). Results of those studies suggest that retention in drug treatment is likely to be protective against HCV seroconversion (Dolan et al., 2005; Rezza et al., 1996; Smyth et al., 2000; Thiede et al., 2000). Retention in drug treatment may also be associated with personal characteristics that are related to lower risk of HCV infection, but in any case it appears that risk is reduced in persons who do remain in treatment. It is plausible for drug treatment to reduce the risk of HCV infection inasmuch as it reduces the frequency of injection, and some IDUs stop injecting altogether. One limitation of drug-treatment programs is that only a relatively small proportion of IDUs (about one-sixth) are in treatment at any given time. In addition, the studies were limited to opiate-substitution programs; cocaine injectors and other non-opiate injectors may not experience similar benefits.
TABLE 5-4 Studies of Association Between Opiate Substitution Treatment and Hepatitis C Virus Seroconversion
|
Reference |
Location |
Design |
Results |
|
Rezza et al., 1996 |
Italy |
Case-control |
MMTP protective against HCV seroconversion (OR, 0.34; 95% CI, 0.1–1.1) |
|
Crofts et al., 1997 |
Melbourne, Australia |
Cohort |
HCV incidence in people Continuously in MMTP: 36.9/100 PY (95% CI 19.1-70.9) Interrupted MMTP: 14.2/100 PY (95% CI 6.3-31.6) No MMTP: 21.4 (95% CI 8.0-57.0) |
|
Thiede et al., 2000 |
Seattle, WA |
Cohort in MMTP at enrollment |
Reduced incidence in those who continued treatment vs those who left treatment (adjusted OR, 0.4; 95% CI, 0–4.2) |
|
Patrick et al., 2001 |
Vancouver, Canada |
Cohort |
Cumulative HCV incidence 25% in those in MMTP vs 42% in others (p = 0.20) |
|
Smyth et al., 2003 |
Dublin, Ireland |
Cohort |
Lower incidence in those in MMTP over 3 months vs others (52 vs. 75 per 100 PY; p = 0.06) |
|
Dolan et al., 2005 |
Sydney, Australia |
Cohort of incarcerated IDUs |
Lowest incidence in those in continuous MMTP (8/100 PY) vs. those in for less than 5 months (23/100 PY) (p = 0.01) |
|
Maher et al., 2006 |
Sydney, Australia |
Cohort |
Being in treatment during the follow-up period had no effect on HCV seroconversion (OR = 0.83, 95% CI 0.51-1.35) |
|
Abbreviations: CI, confidence interval; HCV, hepatitis C virus; IDU, injection-drug user; MMTP, methadone maintenance therapy program; NS, not significant; OR, odds ratio; PY, person-years. |
|||
Research results suggest that multicomponent risk reduction may be needed to control HCV in active injectors. Some studies have shown that the incidence of HCV infection continues to be high in IDUs who participate in SEPs (Hagan et al., 1999; Holtzman et al., 2009; Mansson et al., 2000; Patrick et al., 2001). However, other studies have found associations between SEPs and reductions in HCV infection rates. A case-control study showed that use of a SEP in Tacoma, Washington, was associated with an 88% lower risk of HCV infection and an 82% lower risk of HBV infection (Hagan et al., 1995). A study in Amsterdam showed that IDUs who had “full participation in harm reduction”—they obtained all syringes from a syringe exchange or received at least 60 mg of methadone per day—had substantially and significantly lower rates of HCV seroconversion: fewer than 5 per 100 PY versus more than 25 per 100 PY in others who did not participate fully (van den Berg et al., 2007a).
A recent study of long-term IDUs who remained HIV- and HCV-seronegative showed that they relied on a number of strategies to avoid infection, including maintaining a regular supply of syringes and drug-preparation equipment, managing their addiction by entering drug treatment as needed to reduce their dosage, and maintaining social support to provide stability (Mateu-Gelabert et al., 2007). Drug users who are successful in avoiding infection have developed strategies to maintain control over their chaotic lives. It is clear that HCV prevention is more challenging than HIV prevention for IDUs and will require greater efforts and resources (Hagan et al., 2008).
Research related to the practice of disinfecting syringes with bleach indicates that it has no effect on HCV seroconversion (Hagan and Thiede, 2003; Kapadia et al., 2002). Development of new disinfecting agents that are effective in drug-injection settings may contribute to prevention of HCV infection in IDUs.
Other potentially useful prevention strategies focus on HCV education, testing, and counseling. A large multicity randomized controlled trial of young HIV-negative and HCV-negative IDUs showed that participation in a six-session peer-education training program led to significant reductions in unsafe injections (Garfein et al., 2007). A randomized controlled trial of a similar intervention for HCV-positive young injectors also showed reductions in behavior that may transmit HCV (Latka et al., 2008).
Recommendations
Given the large set of factors that favor HCV transmission in IDUs, it is not surprising that interventions that address individual aspects of risk have not been shown to reduce incidence in individual injectors. In light of the biology of HCV transmission—exposure to very small doses of in-
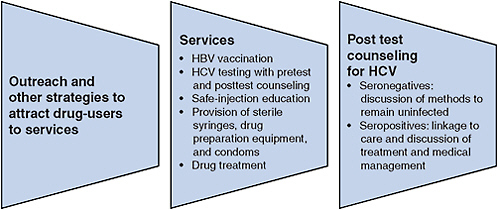
FIGURE 5-2 Essential viral hepatitis services for illicit-drug users.
Abbreviations: HBV, hepatitis B virus; HCV, hepatitis C virus.
fectious blood on injection equipment can result in infection—methods to promote safe injection can be considered essential for HCV control. Safe-injection strategies require access to sterile syringes and other equipment and education to promote adoption and maintenance of safe behavior. HCV testing and counseling to increase awareness of infection status will also support safe practices. Access to sterile syringes and other equipment can be increased through a combination of SEPs, pharmacy sales, and other methods, such as the use of syringe-vending machines (Islam et al., 2008; McDonald, 2009; Moatti et al., 2001). Drug treatment will reduce injection frequency and assist a modest proportion of injectors to achieve abstinence. Research has shown that none of those approaches by itself is sufficient to eliminate HCV transmission. Because HCV prevention is a function of multiple factors—safe-injection strategies, education, testing, and drug treatment—an integrated program that includes all these essential elements is more likely to be effective in preventing hepatitis C (see Figure 5-2).
Recommendation 5-3. Federal, state, and local agencies should expand programs to reduce the risk of hepatitis C virus infection through injection-drug use by providing comprehensive hepatitis C virus prevention programs. At a minimum, the programs should include access to sterile needle syringes and drug-preparation equipment because the shared use of these materials has been shown to lead to transmission of hepatitis C virus.
Recommendation 5-4. Federal and state governments should expand services to reduce the harm caused by chronic hepatitis B and hepati-
tis C. The services should include testing to detect infection, counseling to reduce alcohol use and secondary transmission, hepatitis B vaccination, and referral for or provision of medical management.
On the basis of current knowledge of the etiology and prevention of HCV in IDUs, prevention strategies should include access to sterile injection equipment, safe-injection education, HCV testing and counseling, and access to drug-treatment programs. Programs should include education about safe drug use (avoiding the shared use of implements to administer drugs by smoking or inhalation) and reduction in sex-related risks, and all participants in the programs should be offered the hepatitis B vaccine. The programs should be studied to elucidate the etiology of HCV infection in IDUs and to guide the design of prevention programs. As mentioned above, studies have shown that the first few years after onset of injection-drug use constitute a high-risk period in which the rate of HCV infection can exceed 40%. Preventing the transition from non-injection-drug use to injection-drug use will probably avert many HCV infections. The committee therefore offers the following research recommendation.
Recommendation 5-5. Innovative, effective, multicomponent hepatitis C virus prevention strategies for injection-drug users and non-injection-drug users should be developed and evaluated to achieve greater control of hepatitis C virus transmission. In particular,
-
Hepatitis C prevention programs for persons who smoke or sniff heroin, cocaine, and other drugs should be developed and tested.
-
Programs to prevent the transition from noninjection use of illicit drugs to injection should be developed and implemented.
Pregnant Women
The Advisory Committee on Immunization Practices has recommended routine screening of all pregnant women for HBsAg since 1988 (see Chapter 4). The value and benefits of routine screening of pregnant women for HBsAg were reaffirmed by the USPSTF in 2009 (U.S. Preventive Services Task Force, 2009). Today, 27 states have maternal HBsAg-screening laws, and 24 have specific maternal-HBsAg regulations that require health providers to report all cases of positive HBsAg blood tests to the local health department (CDC, 2007).
More than 95% of pregnant women in the United States are tested prenatally for HBsAg (Mast et al., 2005). States and large metropolitan areas are eligible to receive federal funding to support perinatal hepatitis B prevention programs through CDC’s National Center for Immunization
and Respiratory Disease (Jacques-Carroll et al., 2007). The programs are administered by state and local public-health departments and vary in reach and intensity. As mentioned in Chapter 2, many programs simply provide surveillance, and others provide comprehensive case management that even includes client home visits by local coordinators. That variability accounts for the wide variation (17–59%) in rates of vaccination of household contacts of HBsAg-positive pregnant women (Euler et al., 2003a).
Adequately funded perinatal hepatitis B programs are effective. Among women enrolled in such programs for case management, the rate of administration of the birth dose of hepatitis B vaccine and HBIG was as high as 94%, with a three-dose completion rate of 71% (Jacques-Carroll, 2008). Perinatal hepatitis B programs identify twice as many household and sexual contacts per infant as was reported to the national database, with high rates of programmatic compliance in households of foreign-born people (Euler et al., 2003a). Most US programs are understaffed and underfunded, however, making adequate case management difficult. This gap has a two-fold effect in that chronically infected women do not receive the appropriate medical management and referral and perinatal transmission continues to occur. CDC estimates that only 50% of HBsAg-positive pregnant women are identified for case management (CDC, 2005). It has been estimated that failure of perinatal HBV-prevention efforts result in about 1,000 cases of chronic HBV infection in newborns each year (Ward, 2008b).
Hepatitis B Medical Management of Pregnant Women
An estimated 20,000 infants a year are born to women who test positive for HBV (Euler et al., 2003b). Those women require followup services to ensure that they are knowledgeable about risks posed by their chronic infection and that they receive appropriate referral for long-term medical management. Their close contacts at home should be tested for HBV infection, those who are uninfected should be vaccinated, and medical referral should be provided to those found to have chronic HBV infection. Cases among household contacts are not uncommon when this risk group is pursued aggressively for testing. Data reported by Euler et al. (2003a) showed that 7–35% of household contacts of HBsAg-positive pregnant women were HBsAg-positive.
Deficiencies in health-care providers’ knowledge or appropriate followup of HBsAg-positive pregnant women are noteworthy and require special emphasis in HBV prevention and control strategies. Obstetricians’ knowledge and preventive practices are suboptimal. In a 1997 study of San Francisco obstetricians, over 90% of respondents acknowledged the public-health importance of HBV infection and believed that HBV education was feasible, but only 53% of the responding obstetricians offered
HBV information to their patients (Zola et al., 1997). In a more recent California study of obstetrical practices in 2008, only 62% referred patients who had new diagnoses of HBV to internists or specialists for followup of their chronic HBV infection (Chao et al., 2009b).
Hepatitis B services for foreign-born pregnant women are in need of improved resources that are more culturally and linguistically appropriate. Some 73% of HBsAg-positive pregnant women in the United States were born in East Asia or Southeast Asia (Din, 2009). Among them, Asian and Pacific Islanders, who account for only 5.7% of all births in the United States (CDC, 2009b), account for over two-thirds of births to mothers who have chronic HBV infection. CDC-funded perinatal HBV prevention coordinators are responsible for educating HBsAg-positive mothers and for referring them for appropriate medical management. The coordinators are restricted in their ability to fulfill that responsibility in culturally relevant ways, because of inadequate training and resources (Chao et al., 2009a). To strengthen the capacity and capabilities of the perinatal-HBV coordinators, the committee offers the following recommendation:
Recommendation 5-6. The Centers for Disease Control and Prevention should provide additional resources and guidance to perinatal hepatitis B prevention program coordinators to expand and enhance the capacity to identify chronically infected pregnant women and provide case-management services, including referral for appropriate medical management.
Preventing Perinatal Transmission
Practice guidelines and additional recommendations focused on vaccination to prevent perinatal transmission are detailed in Chapter 4. There is a need to fund research to guide the effective use of antiviral medications late in pregnancy to prevent maternofetal HBV transmission, particularly by high-risk pregnant women who are positive for HBeAg or who have high HBV loads. Results of a few small studies have suggested that the use of lamivudine in the last trimester of pregnancy reduces the rate of perinatal transmission from mothers with high HBV DNA (Li et al., 2003; van Nunen et al., 2000; van Zonneveld et al., 2003). Xu et al. (2009) reported the results of a small randomized, double-blind, placebo-controlled trial involving 155 participants divided into treatment groups; the purpose of the trial was to see whether lamivudine given during late pregnancy could reduce perinatal transmission of HBV. Results suggested that lamivudine in late pregnancy was safe and could reduce HBV transmission from mothers who had high viral loads to their infants who also received HBIG passive immunization. However, the study was small, and large randomized,
double-blind, placebo-controlled trials are needed to evaluate the efficacy and safety of oral hepatitis B antiviral therapy in eliminating perinatal HBV transmission from women at high risk for perinatal transmission. Although an increasing number of effective HBV antiviral suppressive medications have become available for the management of chronic HBV infection, very little research has been done on the use of these medications during the last trimester of pregnancy to eliminate the risk of perinatal transmission, particularly in the high-risk population of women who test positive for HBeAg or have a high HBV load.
Recommendation 5-7. The National Institutes of Health should support a study of the effectiveness and safety of peripartum antiviral therapy to reduce and possibly eliminate perinatal hepatitis B virus transmission from women at high risk for perinatal transmission.
Correctional Settings
Incarcerated populations have higher rates of both HBV infection and HCV infection than the general population. Correctional facilities present a unique opportunity to bring viral hepatitis services to at-risk populations. The period of incarceration is opportune for education about hepatitis B and hepatitis C (see Chapter 3). Inmate peer-education programs have been particularly effective for HIV/AIDS education and have also been used for hepatitis education (Simmons, 2004), but relatively few prison systems provide such programs (Collica, 2007).
Correctional facilities include both jails and prisons. Jails are operated by county and local jurisdictions and house people who have been arrested and are awaiting trial, people who have been convicted of misdemeanor crimes, and people who have been convicted of felony crimes with short-term sentences (usually less than one year). The length of stay in jail can be a short as a few hours or longer than a year. Prisons are operated by states and the federal government. They house people who have been convicted of felony crimes with sentences generally of one year or longer. A few states have combined systems that include both jail and prison inmates.
The prevalence of chronic HBV infection in correctional settings (prisons and jails) is estimated to be 1–4%, and that of chronic HCV infection has been reported to vary from 12% to 35% (Boutwell et al., 2005; Weinbaum et al., 2003). The high prevalence in this population is not primarily a result of incarceration but rather indicative of people who engage in risky behavior and were in risky settings before incarceration.
Hennessey et al. (2009) looked at HBV-infection and HCV-infection prevalence in jail inmates. They found evidence of past HBV infections in 31% of Asian inmates, 21% of black inmates, 14% of white inmates, and
11% of Hispanic inmates. Asian inmates had the highest percentage of chronic HBV infection (4.7%), and Hispanic inmates had the second highest percentage (3.6%). White inmates had the highest prevalence of HCV infection (24%). People who had previously been incarcerated had higher HCV prevalence in all age groups.
Correctional systems are constitutionally required to provide necessary health care to inmates that is consistent with the community standard of care. Screening of all incarcerated people for risk factors can identify those for whom blood tests for infection are indicated, and the high prevalence of HCV infection in prisons justifies such screening so that appropriate treatment can be provided to inmates whose blood tests are positive. Although screening, testing, and treatment could impose an economic burden (Spaulding et al., 2006), a number of correctional systems have successfully implemented medical management programs for viral hepatitis (Allen et al., 2003; Chew et al., 2009; Farley et al., 2005; Maru et al., 2008; Sabbatani et al., 2006; Sterling et al., 2004).
Hepatitis B vaccination in prisons is highly cost-effective; it was estimated in 2002 to cost $415 per HBV infection averted (Pisu et al., 2002). When made available, vaccinations in prisons have high uptake rates. Texas and Michigan inmate vaccination uptake rates have been reportedly been 60–80% (Vallabhaneni et al., 2004). Vallabhaneni et al. (2004) found that 93% of 153 male inmates who were asked said that they would agree to hepatitis B vaccination while incarcerated. Such prevention interventions save society money because they reduce postincarceration morbidity and mortality (Pisu et al., 2002). However, prison budgets have often not been sufficient to provide hepatitis B vaccinations, or other HBV and HCV services.
To capitalize on inmate readiness to participate in hepatitis prevention and control activities, correctional systems and public-health departments need to collaborate to provide targeted testing, appropriate standard-of-care medical management during incarceration, and followup medical services after release into the community. However, there are several barriers to such collaboration. Health departments and correctional facilities do not always exchange health information, and it can be difficult to track prisoners once they are released. State registries for hepatitis B and hepatitis C cases are needed so that incarcerated persons with these diseases can be quickly identified and properly managed once returned into local communities. The primary barrier to such collaboration is funding (McIntyre et al., 2008). Most correctional systems do not initiate treatment for chronic HCV infection unless an incarcerated person has sufficient time remaining on his or her sentence to complete treatment, which generally takes 6–12 months (Spaulding et al., 2006).
Obstacles to collaboration between correctional systems and government health institutions can be overcome. For example, in New York State,
an effort known as the Hepatitis C Continuity Program, which has been in place since 2006, involves collaboration among the state Department of Correctional Services, the state Department of Health, the state Division of Parole, the New York City Health and Hospitals Corporation, medical centers throughout the state, and manufacturers of medications for hepatitis C (Klein et al., 2007). Such collaborative programs should serve as a model nationally.
Recommendation 5-8. The Centers for Disease Control and Prevention and the Department of Justice should create an initiative to foster partnerships between health departments and corrections systems to ensure the availability of comprehensive viral hepatitis services for incarcerated people.
The initiative should include at least the following:
-
All incarcerated people should be offered screening and testing for hepatitis B and hepatitis C.
-
All susceptible incarcerated people should be offered hepatitis B vaccine.
-
Educational programs, including peer education, should include emphasis on hepatitis B and hepatitis C.
-
Systems should be developed to ensure the continuity of medical management for hepatitis B and hepatitis C once infected persons are released from incarceration.
Community Health Facilities
There is a great deal of variation in the types of viral hepatitis services available within the United States. Several states—including Florida, California, Massachusetts, and Texas—have attempted to introduce some hepatitis services into publicly funded settings because of a lack of adequate federal funding for hepatitis B and hepatitis C services. Florida has been offering laboratory testing and vaccination through county health departments since 1999 by using a Hepatitis Prevention Program established and funded by the state legislature (Baldy et al., 2007). The program has expanded services and coordination between the county health departments, the Bureau of HIV/AIDS, the Bureau of Epidemiology, the Bureau of Immunization, and the Bureau of Tuberculosis and Refugee Health. Texas funded a program from 2000 to 2005 to support statewide HCV counseling and testing among high-risk adults (Heseltine and McFarlane, 2007). The program also included a Web-based data-tracking system for monitoring hepatitis C testing and counseling across the state. Of the al-
most 40,000 tests performed, 23.2% were HCV-positive. Because funding was inadequate, the number of tests administered dropped from almost 12,000 in 2003 to about 1,200 in 2004. As is apparent in the examples above, a state-by-state approach of providing publicly funded viral-hepatitis screening, testing, and care leads to wide variability in the type and quality of services available in different regions and leaves many regions in need without the necessary services.
As mentioned earlier in this chapter, the Health Resources and Services Administration (HRSA) administers grant programs across the country to deliver care to uninsured or underinsured people in community health centers, migrant health centers, homeless programs, and public-housing primary-care programs. The role of federally funded community health facilities is to provide critical and timely access to comprehensive primary-care services to medically underserved communities. Data from HRSA’s Health Center Program’s Uniform Data System showed that those facilities served about 16.1 million patients in 2007 at over 7,000 service sites. Of the patients seeking care, 91% were below the poverty level, 39% were uninsured, 930,589 were homeless, and 826,977 were migrant or seasonal farm workers. The facilities also serve a high percentage of foreign-born people (for example, refugee and immigrants). Such facilities often provide the only health-care services available to disadvantaged populations, particularly in rural areas. Although 20% of the US population lives in rural areas, just 11% of the nation’s physicians work there. People who live in rural areas tend to have lower incomes and lower rates of health insurance, and they are in poorer health than their urban counterparts (Ricketts, 2000). Community health centers are the sole source of primary care in many rural areas (Regan et al., 2003; Ricketts, 2000; Rust et al., 2009; Wright, 2009). For people who reside in urban areas, the barriers to health care are related principally to health insurance, transportation, and information about affordable care (Ahmed et al., 2001).
About one-third of patients who seek care at community health facilities are uninsured, and uninsured patients who seek care at these facilities are likely to use them as a medical home for primary care (Carlson et al., 2001). For the most part, the types of services sought at these facilities are similar to those sought by the general population and consist principally of primary care (Henning et al., 2008). Patients at community health facilities are also more likely to discuss health-promotion strategies than patients in other primary-care settings (Carlson et al., 2001). The availability of these facilities has also been shown to decrease the hospitalization rates in the areas that they service (Probst et al., 2009).
The committee did not find published information on viral-hepatitis services in community health facilities, but several studies have looked at the quality of care for other chronic conditions and for preventive services,
such as immunizations. Those studies have found that despite serving disadvantaged populations, community health centers are able to offer high-quality preventive and chronic health-care services at costs comparable with those of facilities used by the general population (Appel et al., 2006; Carlson et al., 2001; Christman et al., 2004; Eisert et al., 2008; Falik et al., 2001; Hicks et al., 2006). Community health facilities have also been found to mitigate racial and ethnic disparities in health-care delivery and services (Appel et al., 2006; Christman et al., 2004; Eisert et al., 2008).
HRSA has oversight over its grantees and has the authority to implement health-care interventions on a national scale. HRSA facilities are well positioned to develop and implement a national strategy to expand viral-hepatitis services to medically underserved and often at-risk populations. HRSA has no centralized viral-hepatitis prevention or control program and is unable to determine the burden of hepatitis B and hepatitis C infections in the patients served in its programs (Raggio Ashley, 2009). Many community health facilities already offer some viral-hepatitis services that include prevention (such as immunizations), screening, testing and medical management. However, there is little published information about these programs.
Although there are no HRSA programs at a national level that focus on viral hepatitis, there are programs for other health concerns that could be used as models. For example, the Health Disparities Collaborative is a national effort to eliminate health disparities and improve health-care delivery in HRSA service-delivery organizations, including community health facilities. The initiative includes intervention to improve health-care delivery processes and chronic health conditions, such as asthma and diabetes (Chin et al., 2004; HRSA, 2009b). It has improved the quality of care in community health facilities for specific conditions (Landon et al., 2007). Viral hepatitis is not one of the diseases included in the program, but this type of program could be expanded to include viral-hepatitis services.
HRSA’s Uniform Data Systems (UDS) tracks a variety of information at the national, state, and individual-grantee levels, such as community health centers, migrant health centers, health-care programs for the homeless, and public-housing primary-care programs. The information collected includes patient demographics, services, staffing, clinical indicators, use rates, and associated costs (HRSA, 2009a). Such data systems could potentially be modified to include collection of data on viral-hepatitis services.
On the basis of those findings, the committee offers the following recommendation to expand the provision of viral hepatitis services:
Recommendation 5-9. The Health Resources and Services Administration should provide adequate resources to federally funded community health facilities for provision of comprehensive viral-hepatitis services.
Targeting Settings That Serve At-Risk Populations
Integrating viral hepatitis services in a broad array of settings creates more opportunities to identify at-risk clients and to get them other services that they need (Hoffman et al., 2004). STD/HIV clinics, shelter-based programs, and mobile health units are settings that serve populations that are at risk for hepatitis B and hepatitis C.
STD–HIV Clinics
Clinical venues that provide screening, identification, and care for people at risk for or infected with STDs and HIV present critical opportunities to provide similar viral hepatitis services. CDC has estimated that almost 30% of people who have received a diagnosis of acute hepatitis B have previously been treated for an STD (Goldstein et al., 2002). Among HIV-infected people, rates of chronic hepatitis B are about 6–14%, and rates of chronic hepatitis C about 33% (Alter, 2006; Sherman et al., 2002; Sulkowski, 2008; Thio et al., 2002). In 2001, the National Alliance of State and Territorial AIDS Directors recommended that state health programs integrate HIV, STD, and viral hepatitis prevention services and that programs offer hepatitis A and hepatitis B vaccination; counseling and testing for HIV, STDs, hepatitis B, and hepatitis C; and partner services and referrals to additional prevention and health-care services (NASTAD, 2001). CDC’s 2006 STD Treatment Guidelines recommend that all unvaccinated persons attending STD clinics receive the hepatitis B vaccine (Workowski and Berman, 2006). The concept of integrating hepatitis B vaccination into STD clinics has been accepted and needs to be expanded to all STD clinic venues.
Some progress has been made in the integration of viral hepatitis services into health-care settings, such as STD or HIV clinics, that serve high-risk populations. A study by Gilbert et al. (2005) showed that many STD clinics have effectively introduced a policy and a plan for hepatitis B prevention; 55% of STD clinics had come to consider hepatitis B vaccination a program responsibility, and 78% had established a vaccination program. From 1997 to 2001, there was a marked increase in the proportion of clinics that offered hepatitis B vaccine (from 61% to 82%), provided hepatitis B educational materials (from 49% to 84%), and accessed federal vaccination programs (from 48% to 84%). In areas where a state STD program had distributed a hepatitis B prevention plan, 88% of STD clinics offered hepatitis B vaccination compared with 50% in areas where a prevention plan had not been developed. The main obstacles cited were the lack of resources for services and low patient compliance. The need for and effect of hepatitis B vaccination was underscored in a study of an urban STD clinic
in San Diego that began to offer risk-factor screening, laboratory testing, and immunizations services in 1998 (Gunn et al., 2007); the program included risk-factor screening of 21,631 people and found that about 69% of patients offered the hepatitis B vaccine accepted it.
A study of risk factors for hepatitis C and laboratory testing of people who sought care at an STD clinic found that 4.9% of the 3,367 attendees who were tested for HCV infection were positive (Gunn et al., 2003). Almost 85% of those who tested positive learned of their infection for the first time through this screening process.
Subiadur et al. (2007) found that viral hepatitis prevention services can be incorporated into a busy STD clinic if staff and resources are available. Similarly, an evaluation of Texas’s HCV program found that staff did not find it difficult to integrate hepatitis C services if sufficient resources were available, such as access to laboratory testing and adequate staffing levels (Heseltine and McFarlane, 2007).
Integrating viral hepatitis services into existing programs increases the opportunity for people to identify other unmet health needs or conditions. A study that assessed the integration of viral hepatitis services (vaccination and screening) into a New York City STD clinic found that the services attracted at-risk people to the clinic and that they benefited from the other services offered (Hennessy et al., 2007). Of 8,778 people in the STD clinic who received hepatitis services, 279 (3%) were self-reported IDUs and 161 (58% of these) reported that the availability of hepatitis services was the primary reason for their clinic visit. Among the 161, 12 new STDs and two HIV infections were diagnosed. IDUs made up only a small proportion of those who attended STD clinics in this demonstration project, but it seems clear that some IDUs will seek hepatitis services if they are offered without charge.
As with STD clinics, there are data that indicate that viral hepatitis prevention and care can be integrated into HIV clinics. The USPHS guidelines for management of opportunistic infections in HIV-infected persons include guidance for detection and management of chronic viral hepatitis (CDC, 2002). The guidelines call for testing of all HIV-infected persons for chronic hepatitis B and hepatitis C and for provision of hepatitis A and hepatitis B vaccination to those who are susceptible. In addition, there are guidelines for medical treatment of those who are chronically infected. There are data that suggest that a much lower proportion of patients actually receive treatment for chronic viral hepatitis. A study of 845 HIV–HCV coinfected patients who attended the Johns Hopkins HIV Clinic in Baltimore found 277 were referred for hepatitis C care. Of those patients referred to care, only 185 of these came for more than one appointment, 125 completed a pretreatment assessment, and 29 started HCV treatment (Mehta et al., 2006).
Shelter-Based Programs
People who are temporarily or consistently homeless are at increased risk for infectious diseases, including hepatitis B and hepatitis C, because of poor living conditions, poor access to health care, high prevalence of drug use, sexual contact with multiple partners, and sharing of personal-hygiene equipment, such as razors (Badiaga et al., 2008; Boyce et al., 2009). The prevalence of HIV, HBV, and HCV among drug-involved street sex workers in Miami, Florida, was 22.4%, 53.4%, and 29.7%, respectively; and 42% of participants were homeless (Inciardi et al., 2006). Homeless adolescents and runaways are at particular risk because they are less likely than their peers to be vaccinated for HBV and to have access to health care and are more likely to engage in risky behaviors, such as drug use and sex work (Sneller et al., 2008).
The current literature suggests that public-health programs for the homeless should address issues related to unsafe sex, drug abuse, homelessness, and other lifestyle factors that contribute to adverse health outcomes. Reaching that population is difficult, and appropriate street-based and shelter-based interventions are potentially effective in doing so. Collaboration among providers of services to the homeless will be needed to provide counseling, education, testing, and such interventions as condom distribution, syringe-access programs, and vaccination against HBV (Badiaga et al., 2008; Boyce et al., 2009; Inciardi et al., 2006; Rosenheck et al., 2003; Roy et al., 2007; Sneller et al., 2008). All homeless persons should be offered the hepatitis B vaccine. A study of vaccination of homeless adults found that reducing HBV-related disease through vaccinations in this population is cost-effective and is associated with substantial improvements in quality of life (Greengold et al., 2009).
Mobile Health Units
Community-based mobile services, such as the use of mobile health vans, can mitigate some access issues. Programs that use mobile health-care vans have been successful in providing HIV prevention and testing services to at-risk people who might not seek health-care services in other settings. Street outreach programs have been successful in reaching marginalized populations in HIV/AIDS prevention programs (Valentine and Wright-De Aguero, 1996). Kahn et al. (2003) found that mobile vans are a feasible approach to community-based STD screening and treatment, are accepted by the community, and are capable of identifying people who have STDs. A study of polysubstance abuse and HIV/STD risk behaviors in men who have sex with men used a mobile van as the service access point and found that polysubstance users had high rates of uninsurance (21%) and that 96%
were first-time users of mobile health-van services (Mimiaga et al., 2008). Mobile vans have some drawbacks. Shrestha et al. (2008) reviewed the cost effectiveness of clinic-based versus mobile outreach efforts to identify HIV cases. They found that the cost of providing a new HIV diagnosis was considerably higher in the outreach settings than in the clinic (Clark et al., 2008). However, a clinic setting is effective only if clients are drawn to the facility. Hence, innovative approaches of this type should be considered for hard-to-reach populations.
Recommendation
Integration of viral hepatitis services into venues such as STD-HIV clinics, shelters, and mobile health units, is likely to have long-term benefits because most of the people who use these types of clinics engage in high-risk behaviors or are in high-risk settings. Therefore, the committee offers the following recommendation:
Recommendation 5-10. The Health Resources and Services Administration and the Centers for Disease Control and Prevention should provide resources and guidance to integrate comprehensive viral hepatitis services into settings that serve high-risk populations such as STD clinics, sites for HIV services and care, homeless shelters, and mobile health units.
REFERENCES
Ahmed, S. M., J. P. Lemkau, N. Nealeigh, and B. Mann. 2001. Barriers to healthcare access in a non-elderly urban poor American population. Health & Social Care in the Community 9(6):445-453.
Allen, S. A., A. C. Spaulding, A. M. Osei, L. E. Taylor, A. M. Cabral, and J. D. Rich. 2003. Treatment of chronic hepatitis C in a state correctional facility. Annals of Internal Medicine 138(3):187-190.
Alter, M. J. 2006. Epidemiology of viral hepatitis and HIV co-infection. Journal of Hepatology 44(Suppl 1):S6-S9.
Alter, M. J., W. L. Kuhnert, and L. Finelli. 2003. Guidelines for laboratory testing and result reporting of antibody to hepatitis C virus. Centers for Disease Control and Prevention. Morbidity and Morality Weekly: Recommendations and Reports 52(RR-3):1-13, 15; quiz CE11-CE14.
Alter, M. J., L. B. Seeff, B. R. Bacon, D. L. Thomas, M. O. Rigsby, and A. M. Di Bisceglie. 2004. Testing for hepatitis C virus infection should be routine for persons at increased risk for infection. Annals of Internal Medicine 141(9):715-717.
Appel, A., R. Everhart, P. S. Mehler, and T. D. MacKenzie. 2006. Lack of ethnic disparities in adult immunization rates among underserved older patients in an urban public health system. Medical Care 44(11):1054-1058.
Armstrong, G. L., A. Wasley, E. P. Simard, G. M. McQuillan, W. L. Kuhnert, and M. J. Alter. 2006. The prevalence of hepatitis C virus infection in the United States, 1999 through 2002. Annals of Internal Medicine 144(10):705-714.
Asian Liver Center. 2009. Jade Ribbon Campaign. http://liver.stanford.edu/Outreach/JRC.html (accessed August 29, 2009).
Astone, J., S. M. Strauss, Z. P. Vassilev, and D. C. Des Jarlais. 2003. Provision of hepatitis C education in a nationwide sample of drug treatment programs. Journal of Drug Education 33(1):107-117.
Badiaga, S., D. Raoult, and P. Brouqui. 2008. Preventing and controlling emerging and reemerging transmissible diseases in the homeless. Emerging Infectious Diseases 14(9): 1353-1359.
Baldy, L. M., C. Urbas, J. L. Harris, T. S. Jones, and P. E. Reichert. 2007. Establishing a viral hepatitis prevention and control program: Florida’s experience. Public Health Reports 122(Suppl 2):24-30.
Barr, D. A., and S. F. Wanat. 2005. Listening to patients: Cultural and linguistic barriers to health care access. Family Medicine 37(3):199-204.
Begley, E. B., A. M. Oster, B. Song, L. Lesondak, K. Voorhees, M. Esquivel, R. L. Merrick, J. Carrel, D. Sebesta, J. Vergeront, D. Shrestha, and J. D. Heffelfinger. 2008. Incorporating rapid HIV testing into partner counseling and referral services. Public Health Reports 123(Suppl 3):126-135.
Birkhead, G. S., S. J. Klein, A. R. Candelas, D. A. O’Connell, J. R. Rothman, I. S. Feldman, D. S. Tsui, R. A. Cotroneo, and C. A. Flanigan. 2007. Integrating multiple programme and policy approaches to hepatitis C prevention and care for injection drug users: A comprehensive approach. International Journal of Drug Policy 18(5):417-425.
Boutwell, A. E., S. A. Allen, and J. D. Rich. 2005. Opportunities to address the hepatitis C epidemic in the correctional setting. Clinical Infectious Diseases 40(s5):S367-S372.
Bowles, K. E., H. A. Clark, E. Tai, P. S. Sullivan, B. Song, J. Tsang, C. A. Dietz, J. Mir, A. Mares-DelGrasso, C. Calhoun, D. Aguirre, C. Emerson, and J. D. Heffelfinger. 2008. Implementing rapid HIV testing in outreach and community settings: Results from an advancing HIV prevention demonstration project conducted in seven U.S. Cities. Public Health Reports 123(Suppl 3):78-85.
Boyce, D. E., A. D. Tice, F. V. Ona, K. T. Akinaka, and H. Lusk. 2009. Viral hepatitis in a homeless shelter in Hawai’i. Hawaii Medical Journal 68(5):113-115.
Brown, L. S., S. Kritz, R. J. Goldsmith, E. J. Bini, J. Robinson, D. Alderson, and J. Rotrosen. 2007. Health services for HIV/AIDS, HCV, and sexually transmitted infections in substance abuse treatment programs. Public Health Reports 122(4):441-451.
Bruix, J., and M. Sherman. 2005. Management of hepatocellular carcinoma. Hepatology 42(5):1208-1236.
Butt, A. A., A. C. Justice, M. Skanderson, M. O. Rigsby, C. B. Good, and C. K. Kwoh. 2007. Rate and predictors of treatment prescription for hepatitis C. Gut 56(3):385-389.
Butt, A. A., K. A. McGinnis, M. Skanderson, and A. C. Justice. 2009. Hepatitis C treatment completion rates in routine clinical care. Liver International 1478-3223.
Campos, N. G., J. A. Salomon, J. C. Servoss, D. P. Nunes, J. H. Samet, K. A. Freedberg, and S. J. Goldie. 2007. Cost-effectiveness of treatment for hepatitis C in an urban cohort co-infected with HIV. American Journal of Medicine 120(3):272-279.
Carey, W. 2003. Tests and screening strategies for the diagnosis of hepatitis C. Cleveland Clinic Journal of Medicine 70(Suppl 4):S7-S13.
Carlson, B. L., J. Eden, D. O’Connor, and J. Regan. 2001. Primary care of patients without insurance by community health centers. Journal of Ambulatory Care Management 24(2):47-59.
Castelnuovo, E., J. Thompson-Coon, M. Pitt, M. Cramp, U. Siebert, A. Price, and K. Stein. 2006. The cost-effectiveness of testing for hepatitis C in former injecting drug users. Health Technology Assessment 10(32): III-IV, IX-XII, 1-93.
CDC (Centers for Disease Control and Prevention). 1998. Recommendations for prevention and control of hepatitis C virus (HCV) infection and HCV-related chronic disease. Centers for Disease Control and Prevention. Morbidity and Morality Weekly: Recommendations and Reports 47(RR-19):1-39.
———. 2001. National hepatitis C prevention strategy: A comprehensive strategy for the prevention and control of hepatitis C virus infection and its consequences. Atlanta, GA: CDC. http://www.cdc.gov/hepatitis/HCV/Strategy/PDFs/NatHepCPrevStrategy.pdf (accessed August 21, 2009)
———. 2002. 2001 USPHS/IDSA guidelines for the prevention of opportunistic infections in persons infected with human immunodeficiency virus. Infectious Diseases in Obstetrics and Gynecology 10(1):3-64.
———. 2005. A comprehensive immunization strategy to eliminate transmission of hepatitis B virus in the United States. Part 1: Immunization of infants, children, and adolescents. Morbidity and Mortality Weekly Report 54(RR16):1-23.
———. 2006. Screening for chronic hepatitis B among Asian/Pacific Islander populations—New York City, 2005. Morbidity and Mortality Weekly Report 55(18):505-509.
———. 2007. National immunization survey: Hepatitis B vaccine birth-dose rates. http://www.cdc.gov/Hepatitis/Partners/PeriHepBCoord.htm (accessed June 28, 2009).
———. 2009a. About the Division of Viral Hepatitis. http://www.cdc.gov/hepatitis/AboutUs.htm (accessed August 21, 2009).
———. 2009b. Births: Final data for 2006. National Vital Statistics Reports 57(7). http://www.cdc.gov/nchs/data/nvsr/nvsr57/nvsr57_07.pdf (accessed August 21, 2009).
———. 2009c. Interpretation of hepatitis B serologic test results. http://www.cdc.gov/hepatitis/HBV/PDFs/SerologicChartv8.pdf (accessed August 21, 2009).
———. 2009d. Perinatal hepatitis B coordinator list. http://www.cdc.gov/vaccines/vpd-vac/hepb/perinatal-contacts.htm (accessed August 18, 2009).
———. 2009e. Reference for the interpretation of hepatitis C virus (HCV) test results. http://www.cdc.gov/hepatitis/HCV/PDFs/hcv_graph.pdf (accessed August 21, 2009).
———. 2009f. Report on the status of state viral hepatitis plans for the Institute of Medicine executive summary of responses (n=55). National Hepatitis TA Center, New York State Department of Health.
———. 2009g. Status of state electronic disease surveillance systems—United States, 2007. Morbidity and Mortality Weekly Report 58(29):804-807.
Chao, S. D., E. T. Chang, and S. K. So. 2009a. Eliminating the threat of chronic hepatitis B in the Asian and Pacific Islander community: A call to action. Asian Pacific Journal of Cancer Prevention 10(3):497-512.
Chao, S. D., C. Cheung, A. Yue, and S. K. So. 2009b. Low hepatitis B knowledge among perinatal healthcare providers serving county with nation’s highest rate of births to mothers chronically infected with hepatitis B. Paper presented at Poster presentation at the 13th Internationational Symposium on Viral Hepatitis and Liver Disease, Washington, DC. March 20-24, 2009.
Chesson, H. W., J. M. Blandford, T. L. Gift, G. Tao, and K. L. Irwin. 2004. The estimated direct medical cost of sexually transmitted diseases among American youth, 2000. Perspectives on Sexual and Reproductive Health 36(1):11-19.
Cheung, R. C., S. Currie, H. Shen, S. B. Ho, E. J. Bini, B. S. Anand, N. Brau, and T. L. Wright. 2005. Chronic hepatitis C in latinos: Natural history, treatment eligibility, acceptance, and outcomes. American Journal of Gastroenterology 100(10):2186-2193.
Chew, K. W., S. A. Allen, L. E. Taylor, J. D. Rich, and E. Feller. 2009. Treatment outcomes with pegylated interferon and ribavirin for male prisoners with chronic hepatitis C. Journal of Clinical Gastroenterology 43(7):686-691.
Chin, M. H., S. Cook, M. L. Drum, L. Jin, M. Guillen, C. A. Humikowski, J. Koppert, J. F. Harrison, S. Lippold, and C. T. Schaefer. 2004. Improving diabetes care in mid-west community health centers with the health disparities collaborative. Diabetes Care 27(1):2-8.
China Digital Times. 2009. China news tagged with: hepatitis B. http://chinadigitaltimes.net/china/hepatitis-b/ (accessed August 21, 2009).
Chitwood, D. D., D. C. McBride, M. T. French, and M. Comerford. 1999. Health care need and utilization: A preliminary comparison of injection drug users, other illicit drug users, and nonusers. Substance Use and Misuse 34(4-5):727-746.
Choe, J. H., V. M. Taylor, Y. Yasui, N. Burke, T. Nguyen, E. Acorda, and J. C. Jackson. 2006. Health care access and sociodemographic factors associated with hepatitis B testing in Vietnamese American men. Journal of Immigrant and Minority Health 8(3):193-201.
Chou, R., E. C. Clark, and M. Helfand. 2004. Screening for hepatitis C virus infection: A review of the evidence for the U.S. Preventive Services Task Force. Annals of Internal Medicine 140(6):465-479.
Christman, L. K., R. Abdulla, P. B. Jacobsen, A. B. Cantor, D. Y. Mayhew, K. S. Thompson, J. P. Krischer, and R. G. Roetzheim. 2004. Colorectal cancer screening among a sample of community health center attendees. Journal of Health Care for the Poor and Underserved 15(2):281-293.
Clark, H. A., K. E. Bowles, B. Song, and J. D. Heffelfinger. 2008. Implementation of rapid HIV testing programs in community and outreach settings: Perspectives from staff at eight community-based organizations in seven U.S. Cities. Public Health Reports 123(Suppl 3):86-93.
Claxton, G., B. DiJulio, B. Finder, E. Becker, S. Hawkins, J. Pickreign, H. Whitmore, and J. Gabel. 2007. Kaiser and the Health Research and Education Trust survey of employer-sponsored health benefits, 1999-2007. Chicago, IL.
Collica, K. 2007. The prevalence of HIV peer programming in American prisons: An opportunity wasted. Journal of Correctional Health Care 13:278.
Contoreggi, C., V. E. Rexroad, and W. R. Lange. 1998. Current management of infectious complications in the injecting drug user. Journal of Substance Abuse Treatment 15(2):95-106.
Crofts, N., R. Louie, and B. Loff. 1997. The next plague: Stigmatization and discrimination related to hepatitis C virus infection in Australia. Health and Human Rights 2(2):87-97.
Damen, M., H. Zaaijer, H. Cuypers, H. Vrielink, C. Poel, H. Reesink, and P. Lelie. 1995. Reliability of the third-generation recombinant immunoblot assay for hepatitis C virus. Transfusion 35(9):745-749.
Davila, J. A., and H. B. El-Serag. 2006. Racial differences in survival of hepatocellular car-cinoma in the United States: A population-based study. Clinical Gastroentorology and Hepatology 4(1):104-110; quiz 104-105.
Des Jarlais, D. C., and S. Semaan. 2008. HIV prevention for injecting drug users: The first 25 years and counting. Psychosomatic Medicine 70(5):606-611.
Des Jarlais, D. C., M. Marmor, D. Paone, S. Titus, Q. Shi, T. Perlis, B. Jose, and S. R. Friedman. 1996. HIV incidence among injecting drug users in New York city syringe-exchange programmes. Lancet 348(9033):987-991.
Des Jarlais, D. C., C. McKnight, C. Goldblatt, and D. Purchase. 2009. Doing harm reduction better: Syringe exchange in the United States. Addiction 104(9):1441-1446.
Din, E. 2009. Estimating the number of births to hepatitis B surface antigen-positive women in select US states, 2004. Paper presented at 13th International Symposium of Viral Hepatitis and Liver Disease, Washington, DC. March 20-24, 2009.
Dolan, K. A., J. Shearer, B. White, J. Zhou, J. Kaldor, and A. D. Wodak. 2005. Four-year follow-up of imprisoned male heroin users and methadone treatment: Mortality, reincarceration and hepatitis C infection. Addiction 100(6):820-828.
Eisert, S. L., P. S. Mehler, and P. A. Gabow. 2008. Can America’s urban safety net systems be a solution to unequal treatment? Journal of Urban Health 85(5):766-778.
Esteve, M., C. Saro, F. Gonzalez-Huix, F. Suarez, M. Forne, and J. M. Viver. 2004. Chronic hepatitis B reactivation following infliximab therapy in Crohn’s disease patients: Need for primary prophylaxis. Gut 53(9):1363-1365.
Euler, G. L., J. Copeland, and W. W. Williams. 2003a. Impact of four urban perinatal hepatitis B prevention programs on screening and vaccination of infants and household members. American Journal of Epidemiology 157(8):747-753.
Euler, G. L., J. R. Copeland, M. C. Rangel, and W. W. Williams. 2003b. Antibody response to postexposure prophylaxis in infants born to hepatitis B surface antigen-positive women. Pediatric Infectious Disease Journal 22(2):123-129.
Falik, M., J. Needleman, B. L. Wells, and J. Korb. 2001. Ambulatory care sensitive hospitalizations and emergency visits: Experiences of Medicaid patients using federally qualified health centers. Medical Care 39(6):551-561.
Farley, J., S. Vasdev, B. Fischer, E. Haydon, J. Rehm, and T. A. Farley. 2005. Feasibility and outcome of HCV treatment in a Canadian federal prison population. American Journal of Public Health 95(10):1737-1739.
Fischer, B., J. Powis, M. Firestone Cruz, K. Rudzinski, and J. Rehm. 2008. Hepatitis C virus transmission among oral crack users: Viral detection on crack paraphernalia. European Journal of Gastroenterology and Hepatology 20(1):29-32.
Fuller, C. M., D. C. Ompad, S. Galea, Y. Wu, B. Koblin, and D. Vlahov. 2004. Hepatitis C incidence—a comparison between injection and noninjection drug users in New York city. Journal of Urban Health 81(1):20-24.
Garfein, R. S., E. T. Golub, A. E. Greenberg, H. Hagan, D. L. Hanson, S. M. Hudson, F. Kapadia, M. H. Latka, L. J. Ouellet, D. W. Purcell, S. A. Strathdee, and H. Thiede. 2007. A peer-education intervention to reduce injection risk behaviors for HIV and hepatitis C virus infection in young injection drug users. AIDS 21(14):1923-1932.
Ghany, M. G., D. B. Strader, D. L. Thomas, and L. Seeff. 2009. Diagnosis, management, and treatment of hepatitis C: An update. Hepatology 49(4):1335-1374.
Gilbert, L. K., J. Bulger, K. Scanlon, K. Ford, D. Bergmire-Sweat, and C. Weinbaum. 2005. Integrating hepatitis B prevention into sexually transmitted disease services: U.S. sexually transmitted disease program and clinic trends—1997 and 2001. Sexually Transmitted Diseases 32(6):346-350.
Giordano, C., and C. Cooper. 2009. The influence of race and language on chronic hepatitis C virus infection management. European Journal of Gastroenterology and Hepatology 21(2):131-136.
Giordano, C., E. F. Druyts, G. Garber, and C. Cooper. 2009. Evaluation of immigration status, race and language barriers on chronic hepatitis C virus infection management and treatment outcomes. European Journal of Gastroenterology and Hepatology 21(9): 963-968.
Goldstein, S. T., M. J. Alter, I. T. Williams, L. A. Moyer, F. N. Judson, K. Mottram, M. Fleenor, P. L. Ryder, and H. S. Margolis. 2002. Incidence and risk factors for acute hepatitis B in the United States, 1982-1998: Implications for vaccination programs. Journal of Infectious Diseases 185(6):713-719.
Gomez, S. L., G. M. Le, D. W. West, W. A. Satariano, and L. O’Connor. 2003. Hospital policy and practice regarding the collection of data on race, ethnicity, and birthplace. American Journal of Public Health 93(10):1685-1688.
Greengold, B., A. Nyamathi, G. Kominski, D. Wiley, M. A. Lewis, F. Hodge, M. Singer, and B. Spiegel. 2009. Cost-effectiveness analysis of behavioral interventions to improve vaccination compliance in homeless adults. Vaccine 27(5):718-725.
Groom, H., E. Dieperink, D. B. Nelson, J. Garrard, J. R. Johnson, S. L. Ewing, H. Stockley, J. Durfee, Y. Jonk, M. L. Willenbring, and S. B. Ho. 2008. Outcomes of a hepatitis C screening program at a large urban VA medical center. Journal of Clinical Gastroenterology 42(1):97-106.
Gunn, R. A., M. A. Lee, P. J. Murray, R. A. Gilchick, and H. S. Margolis. 2007. Hepatitis B vaccination of men who have sex with men attending an urban STD clinic: Impact of an ongoing vaccination program, 1998-2003. Sexually Transmitted Diseases 34(9):663-668.
Gunn, R. A., P. J. Murray, C. H. Brennan, D. B. Callahan, M. J. Alter, and H. S. Margolis. 2003. Evaluation of screening criteria to identify persons with hepatitis C virus infection among sexually transmitted disease clinic clients: Results from the San Diego viral hepatitis integration project. Sexually Transmitted Diseases 30(4):340-344.
Hagan, H., and H. Thiede. 2000. Changes in injection risk behavior associated with participation in the Seattle needle-exchange program. Journal of Urban Health 77(3):369-382.
———. 2003. Does bleach disinfection of syringes help prevent hepatitis C virus transmission? Epidemiology 14(5):628-629; author reply 629.
Hagan, H., D. C. Jarlais, S. R. Friedman, D. Purchase, and M. J. Alter. 1995. Reduced risk of hepatitis B and hepatitis C among injection drug users in the Tacoma syringe exchange program. American Journal of Public Health 85(11):1531-1537.
Hagan, H., J. P. McGough, H. Thiede, N. S. Weiss, S. Hopkins, and E. R. Alexander. 1999. Syringe exchange and risk of infection with hepatitis B and C viruses. American Journal of Epidemiology 149(3):203-213.
Hagan, H., E. R. Pouget, D. C. Des Jarlais, and C. Lelutiu-Weinberger. 2008. Meta-regression of hepatitis C virus infection in relation to time since onset of illicit drug injection: The influence of time and place. American Journal of Epidemiology 168(10):1099-1109.
Hagan, H., E. R. Pouget, I. T. Williams, R. L. Garfein, S. A. Strathdee, S. M. Hudson, M. Latka, and L. Ouellet. 2010. Attribution of HCV seroconversion risk in young injection drug users in five U.S. cities. Journal of Infectious Diseases 201(3):328-385.
Hagan, H., H. Thiede, N. S. Weiss, S. G. Hopkins, J. S. Duchin, and E. R. Alexander. 2001. Sharing of drug preparation equipment as a risk factor for hepatitis C. American Journal of Public Health 91(1):42-46.
Hahn, J. A., K. Page-Shafer, P. J. Lum, P. Bourgois, E. Stein, J. L. Evans, M. P. Busch, L. H. Tobler, B. Phelps, and A. R. Moss. 2002. Hepatitis C virus seroconversion among young injection drug users: Relationships and risks. Journal of Infectious Diseases 186(11):1558-1564.
Hand, W. L., and Y. Vasquez. 2005. Risk factors for hepatitis C on the Texas–Mexico border. American Journal of Gastroenterology 100:2180–2185.
Harm Reduction Coalition. 2009. Hepatitis C. http://www.harmreduction.org/article.php?list=type&type=50 (accessed August 21, 2009).
Hennessey, K. A., A. A. Kim, V. Griffin, N. T. Collins, C. M. Weinbaum, and K. Sabin. 2009. Prevalence of infection with hepatitis B and C viruses and co-infection with HIV in three jails: A case for viral hepatitis prevention in jails in the United States. Journal of Urban Health 86(1):93-105.
Hennessy, R. R., I. B. Weisfuse, and K. Schlanger. 2007. Does integrating viral hepatitis services into a public STD clinic attract injection drug users for care? Public Health Reports 122(Suppl 2):31-35.
Henning, G. F., M. Graybill, and J. George. 2008. Reason for visit: Is migrant health care that different? Journal of Rural Health 24(2):219-220.
Heseltine, G., and J. McFarlane. 2007. Texas statewide hepatitis C counseling and testing, 2000-2005. Public Health Reports 122(Suppl 2):6-11.
Hicks, L. S., A. J. O’Malley, T. A. Lieu, T. Keegan, N. L. Cook, B. J. McNeil, B. E. Landon, and E. Guadagnoli. 2006. The quality of chronic disease care in U.S. community health centers. Health Affairs 25(6):1712-1723.
Hoffman, H. L., C. A. Castro-Donlan, V. M. Johnson, and D. R. Church. 2004. The Massachusetts HIV, hepatitis, addiction services integration (HHASI) experience: Responding to the comprehensive needs of individuals with co-occurring risks and conditions. Public Health Reports 119(1):25-31.
Holtzman, D., V. Barry, L. J. Ouellet, D. C. Jarlais, D. Vlahov, E. T. Golub, S. M. Hudson, and R. S. Garfein. 2009. The influence of needle exchange programs on injection risk behaviors and infection with hepatitis C virus among young injection drug users in select cities in the United States, 1994-2004. Preventive Medicine 49(1):68-73.
HRSA (Health Resources and Services Administration). 2009a. The health center program: Uniform data system (UDS). http://bphc.hrsa.gov/uds/ (accessed November, 13, 2009).
———. 2009b. Quality healthcare collaboration. http://www.healthdisparities.net/hdc/html/collaborativesOverview.aspx (accessed November 12, 2009).
Hsu, C., C. A. Hsiung, I. J. Su, W. S. Hwang, M. C. Wang, S. F. Lin, T. H. Lin, H. H. Hsiao, J. H. Young, M. C. Chang, Y. M. Liao, C. C. Li, H. B. Wu, H. F. Tien, T. Y. Chao, T. W. Liu, A. L. Cheng, and P. J. Chen. 2008. A revisit of prophylactic lamivudine for chemotherapy-associated hepatitis B reactivation in non-Hodgkin’s lymphoma: A randomized trial. Hepatology 47(3):844-853.
Hsu, C. E., L. C. Liu, H. S. Juon, Y. W. Chiu, J. Bawa, U. Tillman, M. Li, J. Miller, and M. Wang. 2007. Reducing liver cancer disparities: A community-based hepatitis-B prevention program for Asian-American communities. Journal of the National Medical Association 99(8):900-907.
Huckans, M. S., A. D. Blackwell, T. A. Harms, D. W. Indest, and P. Hauser. 2005. Integrated hepatitis C virus treatment: Addressing comorbid substance use disorders and HIV infection. AIDS 19(Suppl 3):S106-S115.
Hutton, D. W., D. Tan, S. K. So, and M. L. Brandeau. 2007. Cost-effectiveness of screening and vaccinating Asian and Pacific Islander adults for hepatitis B. Annals of Internal Medicine 147(7):460-469.
IHRA (International Harm Reduction Association). 2009. What is harm reduction? http://www.ihra.net/Whatisharmreduction (accessed September 14, 2009).
Inciardi, J. A., H. L. Surratt, and S. P. Kurtz. 2006. HIV, HBV, and HCV infections among drug-involved, inner-city, street sex workers in Miami, Florida. AIDS Behav 10(2):139-147.
Islam, M., A. Wodak, and K. M. Conigrave. 2008. The effectiveness and safety of syringe vending machines as a component of needle syringe programmes in community settings. International Journal on Drug Policy 19(6):436-441.
Jacobs, E., A. H. Chen, L. S. Karliner, N. Agger-Gupta, and S. Mutha. 2006. The need for more research on language barriers in health care: A proposed research agenda. Milbank Quarterly 84(1):111-133.
Jacques-Carroll, L. 2008. Essentials of perinatal hepatitis B prevention: A training series for coordinators and case managers—assessment and evaluation. http://www2.cdc.gov/vaccines/ed/hepbtraining (accessed June 28, 2009).
Jacques-Carroll, L., E. E. Mast, and S. Wang. 2007. Managing a perinatal hepatitis B prevention program: A guide to life as a program coordinator. http://www.cdc.gov/Hepatitis/Partners/PeriHepBCoord.htm (accessed August 18, 2009).
Kahn, R. H., K. E. Moseley, J. N. Thilges, G. Johnson, and T. A. Farley. 2003. Community-based screening and treatment for STDs: Results from a mobile clinic initiative. Sexually Transmitted Diseases 30(8):654-658.
Kanwal, F., I. M. Gralnek, P. Martin, G. S. Dulai, M. Farid, and B. M. Spiegel. 2005. Treatment alternatives for chronic hepatitis B virus infection: A cost-effectiveness analysis. Annals of Internal Medicine 142(10):821-831.
Kapadia, F., D. Vlahov, D. C. Des Jarlais, S. A. Strathdee, L. Ouellet, P. Kerndt, E. E. Morse, I. Williams, and R. S. Garfein. 2002. Does bleach disinfection of syringes protect against hepatitis C infection among young adult injection drug users? Epidemiology 13(6):738-741.
Kassler, W. J., B. A. Dillon, C. Haley, W. K. Jones, and A. Goldman. 1997. On-site, rapid HIV testing with same-day results and counseling. AIDS 11(8):1045-1051.
Keenan, P. A., and J. M. Keenan. 2001. Rapid HIV testing in urban outreach: A strategy for improving posttest counseling rates. AIDS Education and Prevention 13(6):541-550.
Kidorf, M., V. L. King, K. Neufeld, J. Peirce, K. Kolodner, and R. K. Brooner. 2009. Improving substance abuse treatment enrollment in community syringe exchangers. Addiction 104(5):786-795.
Klein, S. J., L. N. Wright, G. S. Birkhead, B. A. Mojica, L. C. Klopf, L. A. Klein, E. L. Tanner, I. S. Feldman, and E. J. Fraley. 2007. Promoting HCV treatment completion for prison inmates: New York state’s hepatitis C continuity program. Public Health Reports 122(Suppl 2):83-88.
Kresina, T. F., R. D. Bruce, R. Lubran, and H. W. Clark. 2008. Integration of viral hepatitis services into opioid treatment programs. Journal of Opioid Management 4(6):369-381.
Kuo, I., S. G. Sherman, D. L. Thomas, and S. A. Strathdee. 2004. Hepatitis B virus infection and vaccination among young injection and non-injection drug users: Missed opportunities to prevent infection. Drug and Alcohol Dependence 73(1):69-78.
Landon, B. E., L. S. Hicks, A. J. O’Malley, T. A. Lieu, T. Keegan, B. J. McNeil, and E. Guadagnoli. 2007. Improving the management of chronic disease at community health centers. New England Journal of Medicine 356(9):921-934.
Latka, M. H., H. Hagan, F. Kapadia, E. T. Golub, S. Bonner, J. V. Campbell, M. H. Coady, R. S. Garfein, M. Pu, D. L. Thomas, T. K. Thiel, and S. A. Strathdee. 2008. A randomized intervention trial to reduce the lending of used injection equipment among injection drug users infected with hepatitis C. American Journal of Public Health 98(5):853-861.
Lau, G. K., M. L. He, D. Y. Fong, A. Bartholomeusz, W. Y. Au, A. K. Lie, S. Locarnini, and R. Liang. 2002. Preemptive use of lamivudine reduces hepatitis B exacerbation after allogeneic hematopoietic cell transplantation. Hepatology 36(3):702-709.
Lau, G. K., H. H. Yiu, D. Y. Fong, H. C. Cheng, W. Y. Au, L. S. Lai, M. Cheung, H. Y. Zhang, A. Lie, R. Ngan, and R. Liang. 2003. Early is superior to deferred preemptive lamivudine therapy for hepatitis B patients undergoing chemotherapy. Gastroenterology 125(6):1742-1749.
Lee, T. A., D. L. Veenstra, U. H. Iloeje, and S. D. Sullivan. 2004. Cost of chronic hepatitis B infection in the United States. Journal of Clinical Gastroenterology 38(10 Suppl 3): S144-S147.
Li, X. M., Y. B. Yang, H. Y. Hou, Z. J. Shi, H. M. Shen, B. Q. Teng, A. M. Li, M. F. Shi, and L. Zou. 2003. Interruption of HBV intrauterine transmission: A clinical study. World Journal of Gastroenterology 9(7):1501-1503.
Li, Y.-H., Y.-F. He, W.-Q. Jiang, F.-H. Wang, X.-B. Lin, L. Zhang, Z.-J. Xia, X.-F. Sun, H.-Q. Huang, T.-Y. Lin, Y.-J. He, and Z.-Z. Guan. 2006. Lamivudine prophylaxis reduces the incidence and severity of hepatitis in hepatitis B virus carriers who receive chemotherapy for lymphoma. Cancer 106(6):1320-1325.
Liang, T. S., E. Erbelding, C. A. Jacob, H. Wicker, C. Christmyer, S. Brunson, D. Richardson, and J. M. Ellen. 2005. Rapid HIV testing of clients of a mobile STD/HIV clinic. AIDS Patient Care and STDs 19(4):253-257.
Lidgren, M., A. Hollander, O. Weiland, and B. Jonsson. 2007. Productivity improvements in hepatitis C treatment: Impact on efficacy, cost, cost-effectiveness and quality of life. Scandinavian Journal of Gastroenterology 42(7):867-877.
Lillienfeld, A. M., and D. E. Lillienfeld. 1980. Foundations of epidemiology. New York: Oxford University Press.
Lin, S. Y., E. T. Chang, and S. K. So. 2007. Why we should routinely screen Asian American adults for hepatitis B: A cross-sectional study of Asians in California. Hepatology 46(4):1034-1040.
Lok, A. S., and B. J. McMahon. 2004. Chronic hepatitis B: Update of recommendations. Hepatology 39(3):857-861.
———. 2009. Chronic hepatitis B: Update 2009. Hepatology 50(3):661-662.
Lok, A. S., R. H. Liang, E. K. Chiu, K. L. Wong, T. K. Chan, and D. Todd. 1991. Reactivation of hepatitis B virus replication in patients receiving cytotoxic therapy. Report of a prospective study. Gastroenterology 100(1):182-188.
Lum, P. J., J. A. Hahn, K. P. Shafer, J. L. Evans, P. J. Davidson, E. Stein, and A. R. Moss. 2008. Hepatitis B virus infection and immunization status in a new generation of injection drug users in San Francisco. Journal of Viral Hepatitis 15(3):229-236.
Maher, L., B. Jalaludin, K. G. Chant, R. Jayasuriya, T. Sladden, J. M. Kaldor, and P. L. Sargent. 2006. Incidence and risk factors for hepatitis C seroconversion in injecting drug users in Australia. Addiction 101(10):1499-1508.
Manson, A. 1988. Language concordance as a determinant of patient compliance and emergency room use in patients with asthma. Medical Care 26(12):1119-1128.
Mansson, A. S., T. Moestrup, E. Nordenfelt, and A. Widell. 2000. Continued transmission of hepatitis B and C viruses, but no transmission of human immunodeficiency virus among intravenous drug users participating in a syringe/needle exchange program. Scandinavian Journal of Infectious Diseases 32(3):253-258.
Mark, K. E., P. J. Murray, D. B. Callahan, and R. A. Gunn. 2007. Medical care and alcohol use after testing hepatitis C antibody positive at STD clinic and HIV test site screening programs. Public Health Reports 122(1):37-43.
Maru, D. S., R. D. Bruce, S. Basu, and F. L. Altice. 2008. Clinical outcomes of hepatitis C treatment in a prison setting: Feasibility and effectiveness for challenging treatment populations. Clinical Infectious Diseases 47(7):952-961.
Mast, E. E., H. S. Margolis, A. E. Fiore, E. W. Brink, S. T. Goldstein, S. A. Wang, L. A. Moyer, B. P. Bell, and M. J. Alter. 2005. A comprehensive immunization strategy to eliminate transmission of hepatitis B virus infection in the United States: Recommendations of the advisory committee on immunization practices (ACIP) part 1: Immunization of infants, children, and adolescents. Morbidity and Morality Weekly: Recommendations and Reports 54(RR-16):1-31.
Mast, E. E., C. M. Weinbaum, A. E. Fiore, M. J. Alter, B. P. Bell, L. Finelli, L. E. Rodewald, J. M. Douglas, Jr., R. S. Janssen, and J. W. Ward. 2006. A comprehensive immunization strategy to eliminate transmission of hepatitis B virus infection in the United States: Recommendations of the advisory committee on immunization practices (ACIP) part II: Immunization of adults. Morbidity and Morality Weekly: Recommendations and Reports 55(RR-16):1-33.
Mateu-Gelabert, P., C. Treloar, V. A. Calatayud, M. Sandoval, J. C. V. Zurián, L. Maher, T. Rhodes, and S. R. Friedman. 2007. How can hepatitis C be prevented in the long term? International Journal of Drug Policy 18(5):338-340.
Mathei, C., G. Robaeys, P. van Damme, F. Buntinx, and R. Verrando. 2005. Prevalence of hepatitis C in drug users in Flanders: Determinants and geographic differences. Epidemiology and Infection 133(1):127-136.
McDonald, D. 2009. The evaluation of a trial of syringe vending machines in Canberra, Australia. International Journal on Drug Policy 20(4):336-339.
McGinn, T., N. O’Connor-Moore, D. Alfandre, D. Gardenier, and J. Wisnivesky. 2008. Validation of a hepatitis C screening tool in primary care. Archives of Internal Medicine 168(18):2009-2013.
McIntyre, A. F., A. Studzinski, H. A. Beidinger, and C. Rabins. 2008. STD, HIV/AIDS, and hepatitis services in Illinois county jails. Sexually Transmitted Diseases 36(2 Suppl):36(2 Suppl): S37-S40.
Mehta, S. H., G. M. Lucas, L. B. Mirel, M. Torbenson, Y. Higgins, R. D. Moore, D. L. Thomas, and M. S. Sulkowski. 2006. Limited effectiveness of antiviral treatment for hepatitis C in an urban HIV clinic. AIDS 20(18):2361-2369.
Metzger, D. S., H. Navaline, and G. E. Woody. 1998. Drug abuse treatment as AIDS prevention. Public Health Reports 113(Suppl 1):97-106.
Mimiaga, M. J., S. L. Reisner, R. Vanderwarker, M. J. Gaucher, C. A. O’Connor, M. S. Medeiros, and S. A. Safren. 2008. Polysubstance use and HIV/STD risk behavior among Massachusetts men who have sex with men accessing department of public health mobile van services: Implications for intervention development. AIDS Patient Care and STDs 22(9):745-751.
Moatti, J. P., D. Vlahov, I. Feroni, V. Perrin, and Y. Obadia. 2001. Multiple access to sterile syringes for injection drug users: Vending machines, needle exchange programs and legal pharmacy sales in Marseille, France. European Addiction Research 7(1):40-45.
Molitor, F., R. A. Bell, S. R. Truax, J. D. Ruiz, and R. K. Sun. 1999. Predictors of failure to return for HIV test result and counseling by test site type. AIDS Education and Prevention 11(1):1-13.
Myers, J. J., C. Modica, M. S. Dufour, C. Bernstein, and K. McNamara. 2009. Routine rapid HIV screening in six community health centers serving populations at risk. Journal of General Internal Medicine 24(12):1269-1274.24(12):1269-1274.
NASTAD (National Alliance of State and Territorial AIDS Directors). 2001. Integrating viral hepatitis into HIV/AIDS/STD programs. HIV Prevention Bulletin September.
———. 2009. Fact sheet describing need for FY2010 appropriations for hepatitis prevention. National Alliance for State and Territorial AIDS Directors.
Neaigus, A., V. A. Gyarmathy, M. Zhao, M. Miller, S. R. Friedman, and D. C. Des Jarlais. 2007. Sexual and other noninjection risks for HBV and HCV seroconversions among noninjecting heroin users. Journal of Infectious Diseases 195(7):1052-1061.
NIH (National Institutes of Health). 2008.2008. NIH consensus development conference statement on the management of Hepatitis B. http://consensus.nih.gov/2008/hebB%20draft%20statement%20102208_FINAL.pdf (accessed August 21, 2009)..
Ostuni, P., C. Botsios, L. Punzi, P. Sfriso, and S. Todesco. 2003. Hepatitis B reactivation in a chronic hepatitis B surface antigen carrier with rheumatoid arthritis treated with inflix-imab and low dose methotrexate. Annals of the Rheumatic Diseases 62(7):686-687.
O’Toole, T. P., R. A. Pollini, D. E. Ford, and G. Bigelow. 2008. The health encounter as a treatable moment for homeless substance-using adults: The role of homelessness, health seeking behavior, readiness for behavior change and motivation for treatment. Addictive Behaviors 33(9):1239-1243.
O’Toole, T. P., R. Pollini, P. Gray, T. Jones, G. Bigelow, and D. E. Ford. 2007. Factors identifying high-frequency and low-frequency health service utilization among substance-using adults. Journal of Substance Abuse Treatment 33(1):51-59.
Paltiel, A. D., M. C. Weinstein, A. D. Kimmel, G. R. Seage, 3rd, E. Losina, H. Zhang, K. A. Freedberg, and R. P. Walensky. 2005. Expanded screening for HIV in the United States—an analysis of cost-effectiveness. New England Journal of Medicine 352(6):586-595.
Patrick, D. M., M. W. Tyndall, P. G. Cornelisse, K. Li, C. H. Sherlock, M. L. Rekart, S. A. Strathdee, S. L. Currie, M. T. Schechter, and M. V. O’Shaughnessy. 2001. Incidence of hepatitis C virus infection among injection drug users during an outbreak of HIV infection. Canadian Medical Association Journal 165(7):889-895.
Pawlotsky, J.-M., I. Lonjon, C. Hezode, B. Raynard, F. Darthuy, J. Remire, C.-J. Soussy, and D. Dhumeaux. 1998. What strategy should be used for diagnosis of hepatitis C virus infection in clinical laboratories? Hepatology 27(6):1700-1702.
Pisu, M., M. I. Meltzer, and R. Lyerla. 2002. Cost-effectiveness of hepatitis B vaccination of prison inmates. Vaccine 21(3-4):312-321.
Probst, J., J. Laditka, and S. Laditka. 2009. Association between community health center and rural health clinic presence and county-level hospitalization rates for ambulatory care sensitive conditions: An analysis across eight US states. BMC Health Services Research 9(1):134.
Raggio Ashley, T. P. 2009. Community health centers, the federal perspective: Hepatitis B and C activities at HRSA. Presentation to the committee, March 3, 2009.
Rahnavardi, M., S. M. Hosseini Moghaddam, and S. M. Alavian. 2008. Hepatitis C in hemo-dialysis patients: Current global magnitude, natural history, diagnostic difficulties, and preventive measures. American Journal of Nephrology 28(4):628-640.
Rajendra, A., and J. B. Wong. 2007. Economics of chronic hepatitis B and hepatitis C. Journal of Hepatology 47(4):608-617.
Randrianirina, F., J. F. Carod, E. Ratsima, J. B. Chretien, V. Richard, and A. Talarmin. 2008. Evaluation of the performance of four rapid tests for detection of hepatitis B surface antigen in Antananarivo, Madagascar. Journal of Virological Methods 151(2):294-297.
Regan, J., A. H. Schempf, J. Yoon, and R. M. Politzer. 2003. The role of federally funded health centers in serving the rural population. Journal of Rural Health 19(2):117-124; discussion 115-116.
Rein, D. B., S. B. Lesesne, P. J. Leese, and C. M. Weinbaum. 2009. Community-based hepatitis B screening programs in the United States in 2008. Journal of Viral Hepatitis 17(1):28-33.
Reynolds, G. L., D. G. Fisher, L. E. Napper, K. A. Marsh, C. Willey, and R. Brooks. 2008. Results from a multiple morbidities testing program offering rapid HIV testing bundled with hepatitis and sexually transmitted infection testing. Public Health Reports 123(Suppl 3):63-69.
Rezza, G., L. Sagliocca, M. Zaccarelli, M. Nespoli, M. Siconolfi, and C. Baldassarre. 1996. Incidence rate and risk factors for HCV seroconversion among injecting drug users in an area with low HIV seroprevalence. Scandinavian Journal of Infectious Diseases 28(1):27-29.
Rhodes, T., and C. Treloar. 2008. The social production of hepatitis C risk among injecting drug users: A qualitative synthesis. Addiction 103(10):1593-1603.
Ricketts, T. C. 2000. The changing nature of rural health care. Annual Review of Public Health 21(1):639-657.
Rogers, W. 2009. Viral hepatitis prevention polices and programs, Centers for Medicare and Medicaid services. Presentation to the committee, March 3, 2009.
Rooney, G., and R. J. Gilson. 1998. Sexual transmission of hepatitis C virus infection. Sexually Transmitted Infections 74(6):399-404.
Rosenheck, R. A., S. G. Resnick, and J. P. Morrissey. 2003. Closing service system gaps for homeless clients with a dual diagnosis: Integrated teams and interagency cooperation. Journal of Mental Health Policy and Economics 6(2):77-87.
Rossi, G., A. Pelizzari, M. Motta, and M. Puoti. 2001. Primary prophylaxis with lamivudine of hepatitis B virus reactivation in chronic HBsAg carriers with lymphoid malignancies treated with chemotherapy. British Journal of Haematology 115(1):58-62.
Rousseau, C. M., G. N. Ioannou, J. A. Todd-Stenberg, K. L. Sloan, M. F. Larson, C. W. Forsberg, and J. A. Dominitz. 2008. Racial differences in the evaluation and treatment of hepatitis C among veterans: A retrospective cohort study. American Journal of Public Health 98(5):846-852.
Roy, E., E. Nonn, N. Haley, and J. Cox. 2007. Hepatitis C meanings and preventive strategies among street-involved young injection drug users in Montreal. International Journal on Drug Policy 18(5):397-405.
Ruan, Y., G. Qin, L. Yin, K. Chen, H. Z. Qian, C. Hao, S. Liang, J. Zhu, H. Xing, K. Hong, and Y. Shao. 2007. Incidence of HIV, hepatitis C and hepatitis B viruses among injection drug users in southwestern China: A 3-year follow-up study. AIDS 21(Suppl 8): S39-S46.
Rust, G., P. Baltrus, J. Ye, E. Daniels, A. Quarshie, P. Boumbulian, and H. Strothers. 2009. Presence of a community health center and uninsured emergency department visit rates in rural counties. Journal of Rural Health 25(1):8-16.
Sabbatani, S., R. Giuliani, and R. Manfredi. 2006. Combined pegylated interferon and ribavirin for the management of chronic hepatitis C in a prison setting. Brazilian Journal of Infectious Diseases 10(4):274-278.
Salomon, J. A., M. C. Weinstein, J. K. Hammitt, and S. J. Goldie. 2003. Cost-effectiveness of treatment for chronic hepatitis C infection in an evolving patient population. Journal of the American Medical Association 290(2):228-237.
Sanders, G. D., A. M. Bayoumi, V. Sundaram, S. P. Bilir, C. P. Neukermans, C. E. Rydzak, L. R. Douglass, L. C. Lazzeroni, M. Holodniy, and D. K. Owens. 2005. Cost-effectiveness of screening for HIV in the era of highly active antiretroviral therapy. New England Journal of Medicine 352(6):570-585.
Schechter, M. T., S. A. Strathdee, P. G. Cornelisse, S. Currie, D. M. Patrick, M. L. Rekart, and M. V. O’Shaughnessy. 1999. Do needle exchange programmes increase the spread of HIV among injection drug users?: An investigation of the Vancouver outbreak. AIDS 13(6):F45-F51.
Scheinmann, R., H. Hagan, C. Lelutiu-Weinberger, R. Stern, D. C. Des Jarlais, P. L. Flom, and S. Strauss. 2007. Non-injection drug use and hepatitis C virus: A systematic review. Drug and Alcohol Dependence 89(1):1-12.
Schulden, J. D., B. Song, A. Barros, A. Mares-DelGrasso, C. W. Martin, R. Ramirez, L. C. Smith, D. P. Wheeler, A. M. Oster, P. S. Sullivan, and J. D. Heffelfinger. 2008. Rapid HIV testing in transgender communities by community-based organizations in three cities. Public Health Reports 123(Suppl 3):101-114.
Sherman, K. E., S. D. Rouster, R. T. Chung, and N. Rajicic. 2002. Hepatitis C virus prevalence among patients infected with human immunodeficiency virus: A cross sectional analysis of the US adult AIDS clinical trials group. Clinical Infectious Diseases 34(6):831-837.
Shrestha, R. K., H. A. Clark, S. L. Sansom, B. Song, H. Buckendahl, C. B. Calhoun, A. B. Hutchinson, and J. D. Heffelfinger. 2008. Cost-effectiveness of finding new HIV diagnoses using rapid HIV testing in community-based organizations. Public Health Reports 123(Suppl 3):94-100.
Siegel, A. B., R. B. McBride, H. B. El-Serag, D. L. Hershman, R. S. Brown, Jr., J. F. Renz, J. Emond, and A. I. Neugut. 2008. Racial disparities in utilization of liver transplantation for hepatocellular carcinoma in the United States, 1998-2002. American Journal of Gastroenterology 103(1):120-127.
Simmons, T. M. 2004. Inmate peer education programs: 101. Infectious Diseases in Corrections Report. December 2004.
Smith, L. V., E. T. Rudy, M. Javanbakht, A. Uniyal, L. S. Sy, T. Horton, and P. R. Kerndt. 2006. Client satisfaction with rapid HIV testing: Comparison between an urban sexually transmitted disease clinic and a community-based testing center. AIDS Patient Care and STDs 20(10):693-700.
Smyth, B. P., E. Keenan, and J. J. O’Connor. 2000. Assessment of hepatitis C infection in injecting drug users attending an addiction treatment clinic. Irish Journal of Medical Science 169(2):129-132.
Smyth, B. P., J. J. O’Connor, J. Barry, and E. Keenan. 2003. Retrospective cohort study examining incidence of HIV and hepatitis C infection among injecting drug users in Dublin. Journal of Epidemiology and Community Health 57(4):310-311.
Sneller, V. P., D. B. Fishbein, C. M. Weinbaum, A. Lombard, P. Murray, J. A. McLaurin, and L. Friedman. 2008. Vaccinating adolescents in high-risk settings: Lessons learned from experiences with hepatitis B vaccine. Pediatrics 121(Suppl 1):S55-S62.
Sonnenday, C. J., J. B. Dimick, R. D. Schulick, and M. A. Choti. 2007. Racial and geographic disparities in the utilization of surgical therapy for hepatocellular carcinoma. Journal of Gastrointestinal Surgery 11(12):1636-1646; discussion 1646.
Spaulding, A. C., C. M. Weinbaum, D. T.-Y. Lau, R. Sterling, L. B. Seeff, H. S. Margolis, and J. H. Hoofnagle. 2006. A framework for management of hepatitis C in prisons. Annals of Internal Medicine 144(10):762-769.
Spielberg, F., B. M. Branson, G. M. Goldbaum, D. Lockhart, A. Kurth, C. L. Celum, A. Rossini, C. W. Critchlow, and R. W. Wood. 2003. Overcoming barriers to HIV testing: Preferences for new strategies among clients of a needle exchange, a sexually transmitted disease clinic, and sex venues for men who have sex with men. Journal of Acquired Immune Deficiency Syndromes 32(3):318-327.
Spielberg, F., B. M. Branson, G. M. Goldbaum, D. Lockhart, A. Kurth, A. Rossini, and R. W. Wood. 2005. Choosing HIV counseling and testing strategies for outreach settings: A randomized trial. Journal of Acquired Immune Deficiency Syndromes 38(3):348-355.
Spielberg, F., A. Kurth, P. M. Gorbach, and G. Goldbaum. 2001. Moving from apprehension to action: HIV counseling and testing preferences in three at-risk populations. AIDS Education and Prevention 13(6):524-540.
Stanley, A. H. 1999. Primary care and addiction treatment: Lessons learned from building bridges across traditions. Journal of Addictive Diseases 18(2):65-82.
Sterling, R. K., C. M. Hofmann, V. A. Luketic, A. J. Sanyal, M. J. Contos, A. S. Mills, and M. L. Shiffman. 2004. Treatment of chronic hepatitis C virus in the Virginia Department of Corrections: Can compliance overcome racial differences to response? American Journal of Gastroenterology 99(5):866-872.
Stopka, T. J., C. Marshall, R. N. Bluthenthal, D. S. Webb, and S. R. Truax. 2007. HCV and HIV counseling and testing integration in California: An innovative approach to increase HIV counseling and testing rates. Public Health Reports 122(Suppl 2):68-73.
Strauss, S. M., J. M. Astone, D. D. Jarlais, and H. Hagan. 2004. A comparison of HCV antibody testing in drug-free and methadone maintenance treatment programs in the United States. Drug and Alcohol Dependence 73(3):227-236.
Strauss, S. M., J. M. Astone, Z. P. Vassilev, D. C. Des Jarlais, and H. Hagan. 2003. Gaps in the drug-free and methadone treatment program response to Hepatitis C. Journal of Substance Abuse Treatment 24(4):291-297.
Strauss, S. M., G. P. Falkin, Z. Vassilev, D. C. Des Jarlais, and J. Astone. 2002. A nationwide survey of hepatitis C services provided by drug treatment programs. Journal of Substance Abuse Treatment 22(2):55-62.
Subiadur, J., J. L. Harris, and C. A. Rietmeijer. 2007. Integrating viral hepatitis prevention services into an urban STD clinic: Denver, Colorado. Public Health Reports 122(Suppl 2):12-17.
Sulkowski, M., S. 2008. Viral hepatitis and HIV coinfection. Journal of Hepatology 48(2): 353-367.
Sullivan, P. S., A. Lansky, and A. Drake. 2004. Failure to return for HIV test results among persons at high risk for HIV infection: Results from a multistate interview project. Journal of Acquired Immune Deficiency Syndromes 35(5):511-518.
Tempalski, B., H. L. Cooper, S. R. Friedman, D. C. Des Jarlais, J. Brady, and K. Gostnell. 2008. Correlates of syringe coverage for heroin injection in 35 large metropolitan areas in the US in which heroin is the dominant injected drug. International Journal on Drug Policy 19(Suppl 1):S47-S58.
Tempalski, B., P. L. Flom, S. R. Friedman, D. C. Des Jarlais, J. J. Friedman, C. McKnight, and R. Friedman. 2007. Social and political factors predicting the presence of syringe exchange programs in 96 US metropolitan areas. American Journal of Public Health 97(3):437-447.
Thiede, H., H. Hagan, and C. S. Murrill. 2000. Methadone treatment and HIV and hepatitis B and C risk reduction among injectors in the Seattle area. Journal of Urban Health 77(3):331-345.
Thiede, H., H. Hagan, J. V. Campbell, S. A. Strathdee, S. L. Bailey, S. M. Hudson, F. Kapadia, and R. S. Garfein. 2007. Prevalence and correlates of indirect sharing practices among young adult injection drug users in five U.S. Cities. Drug and Alcohol Dependence 91(Suppl 1):S39-S47.
Thio, C. L., K. R. Nolt, J. Astemborski, D. Vlahov, K. E. Nelson, and D. L. Thomas. 2000. Screening for hepatitis C virus in human immunodeficiency virus-infected individuals. Journal of Clinical Microbiology 38(2):575-577.
Thio, C. L., E. C. Seaberg, R. Skolasky, Jr., J. Phair, B. Visscher, A. Munoz, and D. L. Thomas. 2002. HIV-1, hepatitis B virus, and risk of liver-related mortality in the multicenter cohort study (MACS). Lancet 360(9349):1921-1926.
Thorpe, L. E., L. J. Ouellet, R. Hershow, S. L. Bailey, I. T. Williams, J. Williamson, E. R. Monterroso, and R. S. Garfein. 2002. Risk of hepatitis C virus infection among young adult injection drug users who share injection equipment. American Journal of Epidemiology 155(7):645-653.
Tramarin, A., N. Gennaro, F. A. Compostella, C. Gallo, L. J. Wendelaar Bonga, and M. J. Postma. 2008. HCV screening to enable early treatment of hepatitis C: A mathematical model to analyse costs and outcomes in two populations. Current Pharmaceutical Design 14(17):1655-1660.
U.S. Census Bureau. 2008. 2007 American community survey. http://factfinder.census.gov/servlet/STTable?_bm=y&-qr_name=ACS_2008_3YR_G00_S0502&-geo_id=01000US&-ds_name=ACS_2008_3YR_G00_&-_lang=en&-format=&-CONTEXT=st (accessed August 20, 2009).
U.S. Department of Homeland Security. 2009. Yearbook of immigration statistics: 2008. Table 3: Persons obtaining legal permanent resident status by region and country of birth: Fiscal years 1999 to 2008. http://www.dhs.gov/files/statistics/publications/yearbook.shtm (accessed August 21, 2009).
U.S. Preventive Services Task Force. 2009. Screening for hepatitis B virus infection in pregnancy: U.S. Preventive Services Task Force reaffirmation recommendation statement. Annals of Internal Medicine 150(12):869-873, W154.
Valentine, J., and L. Wright-De Aguero. 1996. Defining the components of street outreach for HIV prevention: The contact and the encounter. Public Health Reports 111(Suppl 1):69-74.
Vallabhaneni, S., G. E. Macalino, S. E. Reinert, B. Schwartzapfel, F. A. Wolf, and J. D. Rich. 2004. Prisoners’ attitudes toward hepatitis B vaccination. Preventive Medicine 38(6):828-833.
van den Berg, C. H., C. Smit, M. Bakker, R. B. Geskus, B. Berkhout, S. Jurriaans, R. A. Coutinho, K. C. Wolthers, and M. Prins. 2007b. Major decline of hepatitis C virus incidence rate over two decades in a cohort of drug users. European Journal of Epidemiology 22(3):183-193.
van den Berg, C., C. Smit, G. Van Brussel, R. Coutinho, and M. Prins. 2007a. Full participation in harm reduction programmes is associated with decreased risk for human immunodeficiency virus and hepatitis C virus: Evidence from the Amsterdam cohort studies among drug users. Addiction 102(9):1454-1462.
van Nunen, A. B., R. A. de Man, R. A. Heijtink, H. G. Niesters, and S. W. Schalm. 2000. Lamivudine in the last 4 weeks of pregnancy to prevent perinatal transmission in highly viremic chronic hepatitis B patients. Journal of Hepatology 32(6):1040-1041.
van Zonneveld, M., A. B. van Nunen, H. G. Niesters, R. A. de Man, S. W. Schalm, and H. L. Janssen. 2003. Lamivudine treatment during pregnancy to prevent perinatal transmission of hepatitis B virus infection. Journal of Viral Hepatitis 10(4):294-297.
Ward, J. 2008a. FY 2008 domestic enacted funds. Presentation to the committee: December 4, 2008.
Ward, J. W. 2008b. Time for renewed commitment to viral hepatitis prevention. American Journal of Public Health 98(5):779-781.
Weinbaum, C. M. 2008. Recommendations for identification and public health management of persons with chronic hepatitis b virus infection. Paper presented at NIH Consensus Development Conference: Management of Hepatitis B, Natcher Conference Center, National Institutes of Health (Bethesda, MD).
Weinbaum, C., R. Lyerla, and H. S. Margolis. 2003. Prevention and control of infections with hepatitis viruses in correctional settings. Centers for Disease Control and Prevention. Morbidity and Mortality Weekly Report Recommendations and Reports 52(RR-1):1-36; quiz CE31-CE34.
Wise, M., S. Bialek, L. Finelli, B. P. Bell, and F. Sorvillo. 2008. Changing trends in hepatitis C-related mortality in the United States, 1995-2004. Hepatology 47(4):1128-1135.
Workowski, K., and S. Berman. 2006. Sexually transmitted diseases: Treatment guidelines, 2006. Morbidity and Mortality Weekly Report 55(RR-11):1-94.
Wright, D. B. 2009. Care in the country: A historical case study of long-term sustainability in 4 rural health centers. American Journal of Public Health 99(9):1612-1618.
Xu, W. M., Y. T. Cui, L. Wang, H. Yang, Z. Q. Liang, X. M. Li, S. L. Zhang, F. Y. Qiao, F. Campbell, C. N. Chang, S. Gardner, and M. Atkins. 2009. Lamivudine in late pregnancy to prevent perinatal transmission of hepatitis B virus infection: A multicentre, randomized, double-blind, placebo-controlled study. Journal of Viral Hepatitis 16(2):94-103.
Yeo, W., and P. J. Johnson. 2006. Diagnosis, prevention and management of hepatitis B virus reactivation during anticancer therapy. Hepatology 43(2):209-220.
Yeo, W., P. K. Chan, S. Zhong, W. M. Ho, J. L. Steinberg, J. S. Tam, P. Hui, N. W. Leung, B. Zee, and P. J. Johnson. 2000. Frequency of hepatitis B virus reactivation in cancer patients undergoing cytotoxic chemotherapy: A prospective study of 626 patients with identification of risk factors. Journal of Medical Virology 62(3):299-307.
Zickmund, S., S. L. Hillis, M. J. Barnett, L. Ippolito, and D. R. LaBrecque. 2004. Hepatitis C virus-infected patients report communication problems with physicians. Hepatology 39(4):999-1007.
Zimmerman, R., C. Finley, C. Rabins, and K. McMahon. 2007. Integrating viral hepatitis prevention into STD clinics in Illinois (excluding Chicago), 1999-2005. Public Health Reports 122(Suppl 2):18-23.
Zola, J., N. Smith, S. Goldman, and B. A. Woodruff. 1997. Attitudes and educational practices of obstetric providers regarding infant hepatitis B vaccination. Obstetrics and Gynecology 89(1):61-64.
Zuniga, I. A., J. J. Chen, D. S. Lane, J. Allmer, and V. E. Jimenez-Lucho. 2006. Analysis of a hepatitis C screening programme for US veterans. Epidemiology and Infection 134(02):249-257.






























































