Initial Guidance for an Update of the National Vaccine Plan
A Letter Report to the National Vaccine Program Office
Committee on the Review of Priorities in the National Vaccine Plan
Board on Population Health and Public Health Practice
INSTITUTE OF MEDICINE OF THE NATIONAL ACADEMIES
THE NATIONAL ACADEMIES PRESS
Washington, D.C.
THE NATIONAL ACADEMIES PRESS 500 Fifth Street, N.W. Washington, DC 20001
NOTICE: The project that is the subject of this report was approved by the Governing Board of the National Research Council, whose members are drawn from the councils of the National Academy of Sciences, the National Academy of Engineering, and the Institute of Medicine. The members of the committee responsible for the report were chosen for their special competences and with regard for appropriate balance.
This study was supported by Contract No. HHSP23320042509XI, TO #15 between the National Academy of Sciences and the Department of Health and Human Services. Any opinions, findings, conclusions, or recommendations expressed in this publication are those of the author(s) and do not necessarily reflect the view of the organizations or agencies that provided support for this project.
Additional copies of this report are available from the National Academies Press, 500 Fifth Street, N.W., Lockbox 285, Washington, DC 20055; (800) 624-6242 or (202) 334-3313 (in the Washington metropolitan area); Internet, http://www.nap.edu.
For more information about the Institute of Medicine, visit the IOM home page at: www.iom.edu.
Copyright 2008 by the National Academy of Sciences. All rights reserved.
Printed in the United States of America
The serpent has been a symbol of long life, healing, and knowledge among almost all cultures and religions since the beginning of recorded history. The serpent adopted as a logotype by the Institute of Medicine is a relief carving from ancient Greece, now held by the Staatliche Museen in Berlin.
Suggested citation: IOM (Institute of Medicine). 2008. Initial guidance for an update of the national vaccine plan: A letter report to the national vaccine program office. Washington, DC: The National Academies Press.
THE NATIONAL ACADEMIES
Advisers to the Nation on Science, Engineering, and Medicine
The National Academy of Sciences is a private, nonprofit, self-perpetuating society of distinguished scholars engaged in scientific and engineering research, dedicated to the furtherance of science and technology and to their use for the general welfare. Upon the authority of the charter granted to it by the Congress in 1863, the Academy has a mandate that requires it to advise the federal government on scientific and technical matters. Dr. Ralph J. Cicerone is president of the National Academy of Sciences.
The National Academy of Engineering was established in 1964, under the charter of the National Academy of Sciences, as a parallel organization of outstanding engineers. It is autonomous in its administration and in the selection of its members, sharing with the National Academy of Sciences the responsibility for advising the federal government. The National Academy of Engineering also sponsors engineering programs aimed at meeting national needs, encourages education and research, and recognizes the superior achievements of engineers. Dr. Charles M. Vest is president of the National Academy of Engineering.
The Institute of Medicine was established in 1970 by the National Academy of Sciences to secure the services of eminent members of appropriate professions in the examination of policy matters pertaining to the health of the public. The Institute acts under the responsibility given to the National Academy of Sciences by its congressional charter to be an adviser to the federal government and, upon its own initiative, to identify issues of medical care, research, and education. Dr. Harvey V. Fineberg is president of the Institute of Medicine.
The National Research Council was organized by the National Academy of Sciences in 1916 to associate the broad community of science and technology with the Academy’s purposes of furthering knowledge and advising the federal government. Functioning in accordance with general policies determined by the Academy, the Council has become the principal operating agency of both the National Academy of Sciences and the National Academy of Engineering in providing services to the government, the public, and the scientific and engineering communities. The Council is administered jointly by both Academies and the Institute of Medicine. Dr. Ralph J. Cicerone and Dr. Charles M. Vest are chair and vice chair, respectively, of the National Research Council.
COMMITTEE ON THE REVIEW OF PRIORITIES IN THE NATIONAL VACCINE PLAN
CLAIRE V. BROOME (Chair), Adjunct Professor, Division of Global Health, Rollins School of Public Health, Emory University
ÈLAINE CHATIGNY, Director General of Communications, Public Health Agency of Canada
TIMOTHY J. HOFF, Associate Professor of Health Policy and Management, Department of Health Policy, Management and Behavior, University at Albany, State University of New York
GRACE M. LEE, Assistant Professor of Pediatrics, Children’s Hospital, Harvard University School of Medicine
RICHARD MANDSAGER, Executive Director, The Children’s Hospital at Providence, Anchorage, Alaska
EDGAR K. MARCUSE, Professor of Pediatrics, Children’s Hospital and Regional Medical Center, University of Washington School of Medicine
A. DAVID PALTIEL, Professor and Acting Head, Division of Health Policy and Administration, Yale School of Public Health, Yale University
ARTHUR REINGOLD, Professor and Head, Division of Epidemiology, School of Public Health, University of California, Berkeley
DAVID B. REUBEN, Chief, Geriatric Medicine, and Director, Multicampus Program in Geriatric Medicine and Gerontology, University of California, Los Angeles
SARA ROSENBAUM, Hirsh Professor and Chair, Department of Health Policy, The George Washington University School of Public Health and Health Services
MILAGRITOS D. TAPIA, Assistant Professor, Pediatric Infectious Diseases, University of Maryland
Study Staff
ALINA BACIU, Study Director
AMY GELLER, Senior Health Policy Associate
LOUISE JORDAN, Senior Project Assistant
ROSE MARIE MARTINEZ, Director, Board on Population Health and Public Health Practice
REVIEWERS
This report has been reviewed in draft form by individuals chosen for their diverse perspectives and technical expertise, in accordance with procedures approved by the National Research Council’s Report Review Committee. The purpose of this independent review is to provide candid and critical comments that will assist the institution in making its published report as sound as possible and to ensure that the report meets institutional standards for objectivity, evidence, and responsiveness to the study charge. The review comments and draft manuscript remain confidential to protect the integrity of the deliberative process. We wish to thank the following individuals for their review of this report:
Jo Ivey Boufford, New York Academy of Medicine
Julie S. Downs, Department of Social and Decision Science, Carnegie Mellon University
Barton F. Haynes, Duke Human Vaccine Institute
Lisa Jackson, Group Health Center for Health Studies
Samuel L. Katz, Department of Pediatrics, Duke University Medical Center
Adel A.F. Mahmoud, Woodrow Wilson School and Department of Molecular Biology Princeton University
Joshua P. Metlay, Center for Clinical Epidemiology and Biostatistics, University of Pennsylvania
Thomas W. Valente, Department of Preventive Medicine, Keck School of Medicine, University of Southern California
Although the reviewers listed above have provided many constructive comments and suggestions, they were not asked to endorse the conclusions or recommendations nor did they see the final draft of the report before its release. The review of this report was overseen by Floyd E. Bloom, The Scripps Research Institute. Appointed by the National Research Council, he was responsible for making certain that an independent examination of this report was carried out in accordance with institutional procedures and that all review comments were carefully considered. Responsibility for the final content of this report rests entirely with the authoring committee and the institution.
June 10, 2008
Dr. Bruce Gellin, MD, MPH
Director
National Vaccine Program Office
U.S. Department of Health and Human Services
Hubert H. Humphrey Building 200 Independence Avenue, SW Room 715H Washington, DC 20201-0004
Dear Dr. Gellin:
The Committee on Review of Priorities in the National Vaccine Plan is pleased to offer you its letter report, Initial Guidance for an Update to the National Vaccine Plan. The committee has been given a statement of task in two parts (see Appendix B). The second part tasks the committee with reviewing priorities in the update to the National Vaccine Plan, which is currently under development by an interagency group led by the National Vaccine Program Office (NVPO). The first part of the statement of task asks the committee to review the 1994 National Vaccine Plan1 and then provide guidance on the development of the update to the plan.2 This letter report responds to the first part of the statement of task.
As part of its information-gathering activities, the committee held a meeting that included presentations from representatives of NVPO and several Department of Health and Human Services (DHHS) agencies on the development of the 1994 plan, on accomplishments since 1994, and on early thinking about the update to the plan (see Appendix C for the meeting agenda). The committee also reviewed relevant literature, and study staff along with one or two committee members held informal conversations with several individuals familiar with the 1994 plan and its development.
BACKGROUND
NVPO was established by the enactment of Title XXI of the Public Health Service Act (Public Law 99-660), which also called for the preparation of the National Vaccine Plan. The language of the 1994 plan provides the following description of NVPO’s role:
|
1 |
The 1994 National Vaccine Plan is available at http://www.hhs.gov/nvpo/vacc_plan/. |
|
2 |
The committee’s other tasks include holding five workshops with national expert stakeholders in medicine, public health, industry, and vaccinology to review publicly available, draft planning documents from the Department of Health and Human Services, and then preparing a report with conclusions and recommendations about priority actions within the major components of the draft update to the new National Vaccine Plan. |
[T]he principal coordinating organization for the NVP is the National Vaccine Program Office (NVPO), within the Public Health Service (PHS). The NVPO’s responsibilities include providing overall leadership for the collaborative effort and monitoring the progress being made in achieving the plan’s goals. Within the PHS, the NVPO has the task of reviewing all budget requests associated with vaccine development and immunization programs to ensure that all major priorities are adequately covered and that there is no duplication of effort. (NVPO, 1994)
The legislation represented a response to several different developments. These developments included problems of vaccine safety; the reemergence of vaccine-preventable diseases, especially pertussis and measles, in the United States and other developed countries; the persistence of these and other vaccine-preventable diseases in developing countries; and vaccine industry concern regarding financial and liability-related impediments to the development of new vaccines. The legislation also contained provisions aimed at improved monitoring of the safety of recommended vaccines and at reducing industry concern about liability risks. The Vaccine Adverse Events Reporting System (VAERS) and the National Vaccine Injury Compensation Program both became operational in 1988.
The release of the 1994 National Vaccine Plan coincided with other federal action to expand immunization coverage among children and adults. Such actions included increased federal appropriations for state immunization efforts and passage of the Vaccines for Children (VFC) amendments to Medicaid (Public Law 103-66). VFC, building on the existing entitlement to immunizations for children enrolled in Medicaid, strengthened federal immunization coverage standards while extending the immunization entitlement to uninsured children, children served by American Indian and Alaska Native health programs, and underinsured children served through Federally Qualified Health Centers (FQHCs). In 1990, DHHS released Healthy People 2000, which set forth 19 objectives related to reducing infectious disease and improving immunization coverage among children and adults.
Various nonprofit organizations interested in children’s health and welfare also were part of efforts in the early 1990s to improve immunization services. Every Child By Two, for example, sought to draw family and community attention to the need to ensure that young children received vaccines according to the recommended schedule, not simply in response to school entry requirements. The Children’s Vaccine Initiative, begun in 1990 under the auspices of United Nations agencies, focused on delivery of vaccines to children in developing countries.
Features of the 1994 National Vaccine Plan
The plan was to “establish priorities in research and the development, testing, licensing, production, procurement, distribution, and effective use of vaccines, describe an optimal use of resources to carry out such priorities, and describe how each of the various departments and agencies will carry out their functions in consultation and coordination with the [National Vaccine] Program and in conformity with such priorities.” The 1994 plan’s aims included reducing “the incidence of infectious diseases through vaccine development and immunization” and integrating all U.S. efforts on vaccine development and immunization, whether their focus was domestic or global (NVPO, 1994: p. 13). The plan had four goals3: (1) to develop new and improved vaccines; (2) to ensure the optimal safety and effectiveness of vaccines and immunization; (3) to better educate the public and members of the health professions on the benefits and risks of immunizations; and (4) to achieve better use of existing vaccines to prevent disease, disability, and death. The plan also offered 26 objectives along with more than 70 strategies for achieving those objectives. In addition, 14 anticipated outcomes were offered as a basis for judging the success of the plan (see Appendix D).
The Committee’s Approach to Reviewing the Plan
The committee reviewed the goals, objectives, strategies, and anticipated outcomes presented in the plan. In the interest of time and in recognition of the statement of task and the plan’s acknowledged limitations (notably, the lack of measurable objectives), the committee did not undertake a point-by-point evaluation of what the plan has or has not achieved. Instead, in the first section of this letter report, the committee examines what has changed in the broader social, policy, and economic context of vaccine development and immunization, and highlights several areas where noteworthy progress has been made, particularly by federal agencies. The committee acknowledges that progress in developing and delivering vaccines has benefited from essential contributions by other stakeholders, including researchers, manufacturers, state and local public health agencies, and health care providers. In the second section of this letter report, the committee uses what it learned from reviewing the 1994 plan and the process of preparing it to distill key elements. Based on these elements, the committee offers guidance to NVPO and its partners on developing the update to the national vaccine plan.
CHANGES SINCE 1994
Important changes in the world, in American society, and in the delivery and financing of health care have occurred or have grown in prominence since 1994. For example, several key changes have been made in how the U.S. health care delivery system is organized. More elderly and underserved populations receive health care, including immunizations, through private health care delivery systems under the auspices of Medicaid and Medicare managed care programs.4 With significant government-
financed immunization activity now occurring through private entities that have a role in and the ability to influence coverage and financing decisions, there is growing dependence on the private sector to ensure that immunization goals for senior and underserved populations are met.
Like other medical products, vaccines have benefits and risks, and in recent decades vaccine safety has emerged as an important topic both for the public health and medical communities and for the public. Research on vaccine safety has increased and regulatory attention to safety has intensified. Milestones include the withdrawal in 1999 of the first licensed rotavirus vaccine after cases of intussusception were reported to VAERS and subsequent research by CDC showed that this type of bowel obstruction occurred with significantly increased frequency after rotavirus vaccine administration, and the replacement of older pertussis and polio vaccines with safer products (see below). Multiple factors converged to facilitate the emergence of an increasingly organized and vocal movement that questions the need for vaccines and their safety in general and alleges that specific vaccines, features of vaccines, or the expansion of the pediatric immunization schedule in the past 15 years have caused health problems in some children. These factors include the decline in the incidence of vaccine-preventable diseases in the United States, the greater interest in complementary and alternative medicine, an increase in consumerism, broader public concern about the varied risks inherent in modern life, a growing public mistrust of government agencies, and the proliferation of electronic communication (Clements and Ratzan, 2003; Clements et al., 1999; Colgrove and Bayer, 2005).
Major transformation also has occurred in the area of funding for vaccine research, both globally and domestically. In the United States, the federal government has a greater role in funding and guiding the development and evaluation of vaccines, particularly those directed against pandemic influenza and potential agents of bioterrorism. Globally, the Bill & Melinda Gates Foundation and international partnerships such as the GAVI Alliance5 fund new vaccine purchase, support strengthening immunization infrastructure in developing countries, and foster vaccine research and development.
PROGRESS SINCE 1994
As noted above, at its March 2008 meeting the committee heard a series of presentations from DHHS agencies, including the Centers for Disease Control and Prevention (CDC), the Food and Drug Administration (FDA), the Health Resources and Services Administration (HRSA), the National Institutes of Health (NIH), and the Centers for Medicare and Medicaid Services (CMS) (see Appendix C for the complete agenda).
Characteristics of the 1994 plan make it difficult to attribute specific activities to plan objectives, and accordingly, the presentations given to the committee in general did not attempt to link accomplishments to the plan, other than noting their relevance to the pertinent goal in the plan. These presentations described many remarkable achievements, both in process (e.g., enhanced regulatory tools) and substance (e.g., approval of safe and effective new vaccines), of federal agencies working in collaboration with other
stakeholders in the U.S. vaccine system. Below, the committee highlights several examples of these achievements, as well as other areas of progress, and also notes the 1994 plan’s anticipated outcomes in areas that coincide with areas of progress (see Appendix D for a complete list of the outcomes). However, the committee does not attempt to assess the extent to which each of the 14 anticipated outcomes was realized,6 or to illustrate achievements related to all of the outcomes under each goal. Also, the committee did not undertake a systematic evaluation of achievements or failures to achieve plan objectives. The focus on progress in the field is intended to provide some context for the current environment for vaccine development and delivery, with the understanding that gaps and challenges remain in this complex domain of science, public health, and health care.
Goal 1:
Develop new and improved vaccines.
Four of the 14 anticipated outcomes in the 1994 plan are associated with this goal and include: improved vaccines, vaccines for diseases without vaccines, and regulatory improvements to facilitate vaccine Hcensure. Much progress has been made in the area of vaccine development.
Since 1994, more than 20 new vaccine products resulting from the collaborative efforts of NIH, academic, and industry researchers were approved by FDA (IOM, 2008). Novel vaccines introduced include vaccines against pediatric pneumococcal disease, meningococcal disease, and human papilloma virus. Also, vaccines with improved safety profiles received regulatory approval. For example, the introduction of a new acellular pertussis vaccine led to a reduction in reports of adverse events compared with the older, whole-cell vaccine (Braun et al., 2000). Similarly, the 1996 recommendation by the Advisory Committee on Immunization Practices (ACIP) to begin replacing oral polio vaccine with inactivated polio vaccine,7 and 2000 ACIP recommendation to replace all OPV with IPV led to the disappearance in the United States of vaccine-associated paralytic poliomyelitis (CDC, 2000; Wattigney et al., 2001).
NIH plays a crucial role in conducting and supporting both basic and applied vaccine research. In recent years, the agency has been involved in supporting a number of Investigational New Drug applications for vaccines, playing a role in the Hcensure of 17 different vaccines between 1994 and 2006, and has collaborated with the World Health Organization and nongovernmental organizations on vaccines of importance to developing countries. Most recently, NIH has been engaged in research related to vaccines for potential agents of bioterrorism and pandemic influenza (e.g., H5N1 inactivated vaccine).
Goal 2:
Ensure the optimal safety and effectiveness of vaccines and immunization.
One of the 14 anticipated outcomes in the 1994 plan is associated with this goal; it refers to continuous monitoring of vaccine efficacy and safety. There have been several notable activities in this area.
Since 1994, the FDA Center for Biologies Evaluation and Research (CBER), which regulates vaccines, has had an expanding array of regulatory tools and legislative requirements that facilitate the review and approval of safe and efficacious vaccines. For example, CBER has become better equipped to monitor manufacturer commitments to study the safety of vaccines after they are licensed.
In the past fourteen years, FDA and CDC have collaborated on surveillance for and evaluation of adverse events through their joint operation of VAERS. VAERS reporting procedures have been improved and simplified and better methods for monitoring and analyzing the data collected have been developed. Efforts have also been made to increase collaboration with CMS, the Department of Defense, and the Department of Veterans Affairs to improve surveillance and reporting of adverse events following immunization in the adult populations these agencies serve.
In addition, the Vaccine Safety Datalink (VSD) is a collaborative effort between CDC’s Immunization Safety Office and several large managed care organizations to monitor immunization safety and address the “gaps in scientific knowledge about rare and serious side effects following immunization.” Unlike VAERS, VSD permits systematic case finding and analysis of control data to assess potential adverse events, testing hypotheses concerning relationships between receipt of specific vaccines and the occurrence of specific adverse events. The VSD project, which has expanded from 4 to 8 participating managed care organization sites, not only conducts traditional epidemiologic studies on vaccine safety, but also has developed the capacity to conduct near real-time surveillance for adverse events after vaccination using Rapid Cycle Analysis methods.
In 2001, the Clinical Immunization Safety Assessment (CISA) Network was established. CISA is a network of six medical research centers with expertise in immunization safety. CISA sites focus on pathophysiologic mechanisms and identify biologic risks of adverse events following immunization (Iskander, 2007). Examples of CISA studies include research on possible genetic risk factors for post-vaccination Guillain-Barre syndrome and research on vaccine-associated encephalitis.
One of the objectives under Goal 2 in the 1994 plan was to “continue to ensure fair and efficient compensation to individuals injured by vaccines,” in reference to the National Vaccine Injury Compensation Program (VICP), which is based at HRSA and has operated since 1988 (see Appendix D for a list of goals and objectives). The VICP is a no-fault mechanism through which compensation can be awarded for claims of vaccine-related injury or death. Since 1994, nine vaccines have been added to the program and the list of compensable injuries has been updated periodically to incorporate new findings on vaccine safety, including those from Institute of Medicine (IOM) reviews and CDC studies.8
Goal 3:
Better educate the public and members of the health professions about the benefits and risks of immunizations.
Two of the 14 anticipated outcomes in the 1994 plan are associated with this goal, and they include the establishment of educational communication networks to inform all potential audiences about vaccine risks and benefits, and providing information to the public on the costs and benefits of the plan. There have been several developments in this area.
Since 1999, the American Academy of Pediatrics (AAP) has received funding through a cooperative agreement with CDC for its Childhood Immunization Support Program (CISP). CISP has been providing educational resources on immunization and immunization-related issues to health care providers and parents.
In 2000, DHHS, CDC, and the American Medical Association co-sponsored the first National Influenza Vaccine Summit, a group that meets annually and has members representing 100 public and private organizations interested in preventing influenza. Major aims of this activity include finding new ways to communicate with and to the public and health care providers.
Between 2002 and 2003, NVPO, CDC, IOM, and the Keystone Center9 collaborated on a proposal to stimulate public engagement in vaccine policy development. A National Vaccine Advisory Committee working group discussed the proposal and other models of public engagement during a 2004 workshop (NVAC, 2004). The collaboration among these organizations continued in 2005 in the form of a demonstration, or proof of principle, that vaccine policymaking could be well-informed by a substantive engagement of stakeholders and the public (The Keystone Center, 2005). The demonstration topic was pandemic influenza vaccine prioritization.
In 2007, FDA formed a risk communication advisory committee that will advise the agency on communication of risk and benefit information about the products the agency regulates.
Goal 4:
Achieve better use of existing vaccines to prevent disease, disability, and death.
Seven of the 14 anticipated outcomes in the 1994 plan are associated with this goal and include extending age-appropriate immunization with recommended vaccines to at least 90 percent of infants and children (the only measurable outcome or objective provided in the plan), and eliminating childhood diseases (e.g., diphtheria, Haemophilus influenzae Type b) as significant causes of death. Below, the committee highlights examples of progress the use of vaccines.
For each birth cohort (the approximately 4 million children born each year), routine childhood immunization has been estimated to prevent approximately 33,500 premature deaths and 14.3 million cases of vaccine-preventable illnesses (Zhou et al., 2005). The introduction of Haemophilus influenzae Type b (Hib) and pertussis vaccines
|
Safety Review Committee were published between 2001 through 2004 and may be viewed at www.nap.edu. |
|
9 |
The Keystone Center is a nonprofit that facilitates consensus-building for science-based public policy decisions (www.keystone.org). |
illustrates the impact of immunization. Hib once affected 1 out of 200 children under age 5 and killed 600 U.S. children each year. One-quarter of children surviving Hib meningitis had neurologic damage. Conjugate Hib vaccine was recommended by ACIP for all infants in 1991. Between 1994 and 1998, fewer than 10 fatal cases of invasive Hib disease were reported (CDC, 2007a), and rates of the disease fell by 99 percent overall (Adams et al., 1993). Before pertussis vaccine became available in the 1940s, the disease caused between 150,000 and 260,000 cases and from 5,000 to a peak of 9,000 deaths annually (CDC, 2006, 2007a). Between 1990 and 1996, there were 57 pertussis deaths, most in infants under 6 months of age (CDC, 2007a).
Since 1994, the number of vaccines recommended for children and adolescents has increased from 9 to 16, including vaccines against varicella, pneumococcal disease, influenza, meningococcal disease, hepatitis A, rotavirus, and human papilloma virus (HPV). In 2006, immunization coverage for children aged 19-35 months exceeded 90 percent for several individual vaccines.10 However, 77 percent of children in this age group had received all doses of a series of recommended vaccines11 (CDC, 2007b).
GUIDANCE FOR DEVELOPING A NEW NATIONAL VACCINE PLAN
The committee learned from presentations at its March 2008 meeting and from conversations with individuals knowledgeable about the development of the 1994 National Vaccine Plan that its development served as (1) a tool to foster interagency dialogue, and (2) a mechanism for cataloguing activities and listing policy and research aspirations and prominent concerns that existed at that time (IOM, 2008; IOM Staff, 2008). However, there is little evidence that the plan served to guide or motivate activity that occurred after its preparation. As a result, it is difficult to attribute to the plan any changes that have occurred since 1994.
On the basis of its review of the 1994 plan and information gathered about its development, the committee has indentified several process and content areas that deserve particular attention as the update to the plan is developed.
Process Issues
The committee identified several limitations of the process of developing the 1994 plan that provide useful lessons in drafting the update to the National Vaccine Plan. These limitations include: the federal, rather than national, scope of the 1994 plan; the absence of a framework for evaluating and updating the plan; and the lack of explicit roles in the plan for stakeholders beyond the federal government (Figure 1 offers an illustration of the immunization system, which, despite being an incomplete representation, depicts the system’s complexity). Also, NVPO and agencies involved in
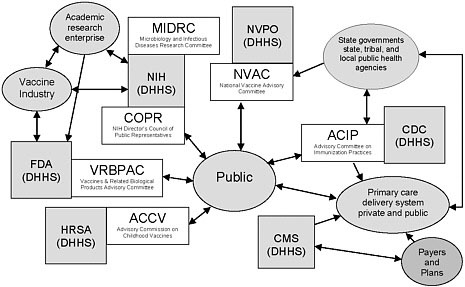
Figure 1 This figure is intended to illustrate some aspects of the immunization system’s complexity, not to be a complete description of the system. A number of federal advisory committees exist to provide advice and guidance to agencies in the Department of Health and Human Services (DHHS). Several of these committees are associated with vaccine- or immunization-specific programs. Four such committees, as well as two additional relevant committees are depicted in the figure.
Legend: Gray boxes represent federal agencies in the Department of Health and Human Services (DHHS) (other departments, such as the Departments of Defense, Veterans Affairs, and Homeland Security, also play important roles in the immunization system); white boxes represent federal advisory committees associated with DHHS agencies, and gray ovals represent other stakeholders. Acronyms: CDC = Centers for Disease Control and Prevention; CMS = Centers for Medicare & Medicaid Services; FDA = Food and Drug Administration; HRSA = Health Resources and Services Administration; NIH = National Institutes of Health; NVPO = National Vaccine Program Office. Notes about the federal advisory committees above: ACCV includes attorneys for injured children and for industry; NVAC Includes public, industry, state public health, and health care (AHIP) representation; ACIP includes public and state and local public health representation and liaisons to the vaccine industry and professional associations; COPR includes patients, family members of patients, health care and education professionals and members of the general public who advise the Director of the NIH on “matters of public interest, outreach and participation in NIH’s research-related activities”; VRBPAC includes public and nonvoting industry representation.
the development of the 1994 plan acknowledged that it did not serve as a central document for strategic planning among federal agencies.
To help avoid important limitations of the 1994 plan, the committee urges NVPO to give special attention to the following points as it coordinates the development of update to the plan.
1.
A Plan of National Scope
A National Vaccine Plan provides a mechanism for defining national, state, and local vaccine and immunization priorities and potentially for coordinating the activities of multiple federal agencies with the private sector to achieve them. NVPO has stated its intention and the commitment of the interagency group involved in drafting the new National Vaccine Plan to prepare a national plan and not merely a federal plan.
|
Box 1: On the Coordinating Role of NVPO (excerpt from the 1994 plan) Two formal mechanisms exist for coordinating Federal activity. The NVP Interagency Group includes those agencies with major vaccine-related responsibilities specifically mentioned in Public Law. 99-660, and the Interagency Committee on Immunization (ICI) includes all those Federal departments and agencies involved in immunization…. Each of these groups meets regularly to supplement day-to-day information exchange, and coordination, cooperation, and planning that is facilitated by the NVPO staff. In addition, the NVPO coordinates special cross-agency initiatives that are undertaken from time to time on specific topics of importance or other identified needs. (Source: NVPO, 1994: p. 49.) |
The committee is aware that the 1986 legislation for the National Vaccine Plan called for a plan to “describe how each of the various departments and agencies will carry out their functions in consultation and coordination” with NVPO and “in conformity” with priorities in the plan (NVPO, 1994: p. 60); see Box 1 for a description of NVPO’s coordinating role as provided in the 1994 plan.
The relationship between NVPO and NVAC in the development of the National Vaccine Plan in 1994 and the current update is important to understand. The 1994 plan stated that “[v]arious entities participate in the process of guiding and coordinating NVP activities. For example, the National Vaccine Advisory Committee (NVAC) (composed of nongovernmental experts in vaccine development and immunization) provides overall advice on vaccine development and immunization, as specified under P.L. 99-660” (NVPO, 1994: p. 49). It is the committee’s understanding that NVAC will play an important role in the development of the update to the plan, by reviewing early drafts and contributing white papers developed by NVAC subcommittees or working groups (e.g., on vaccine finance, on vaccine safety).
The statute does not mention the involvement of non-federal stakeholders, including the broad array described above. At the committee’s March 2008 meeting, however, NVPO described the vision of a national plan involving broad stakeholder input. The committee believes this vision is consistent with NVPO’s charge to “achieve optimal prevention of human infectious diseases through immunization and to achieve optimal prevention against adverse reactions to vaccines” (Public Law 99-660, §2103 [300aa-3]), a charge that can only be met through the efforts of multiple stakeholders, in addition to those of the federal government.
To develop a national plan, it will be important to give essential partners beyond federal agencies an early and meaningful role in framing the plan’s scope, goals, and objectives. Essential stakeholders in the U.S. immunization system include not only federal agencies but also the pharmaceutical industry, insurers, purchasers of health care services, health care providers, researchers in areas ranging from the basic sciences through health economics and health services research, state and local public health agencies responsible for vaccine delivery, schools and day care centers, foundations and other not-for-profit organizations, the mass media, and very importantly, a spectrum of the public, reflecting varying perspectives on the value of immunization (some, but not all relevant stakeholders are included in Figure 1).
This IOM committee has been asked to engage a broad range of expert stakeholders around each of the four goals in the national vaccine plan at a series of workshops. However, the committee underscores the importance of including the full
array of interested and relevant stakeholders as early as possible in the process of developing the plan. The committee recognizes the role of NVAC, which includes stakeholder representatives, in contributing to and reviewing the draft update to the plan. Other options for involving stakeholders early in the process include obtaining advance input from all relevant federal advisory committees (most of which include consumer, state public health agency, and industry representation), and notifying participants at the IOM committee’s stakeholder meetings that the draft plan is fully open to stakeholder input.12
At the federal level, NVPO has already engaged many agencies within the DHHS, as well as the Department of Defense, the Department of Veterans Affairs, and the State Department (the U.S. Agency for International Development). Additional consideration, could be (if it has not already been) given to involving the Department of Homeland Security, the Department of Agriculture, and perhaps others.
Despite its support for efforts to generate a more truly national plan, the committee recognizes that there are formidable barriers to achieving meaningful collaboration in this complex field where public health, medical, ethical, economic, societal, and individual objectives collide. Participation of all potential stakeholders would involve a wide array of potentially conflicting agendas, accountabilities, as well as regulatory, legal, and other limitations. Effective collaboration will be challenging to achieve even among federal agencies such as CDC, NIH, FDA, and HRSA, each of which has its own priorities, resource constraints, and culture.
2.
A Plan that Is Used, Evaluated, and Updated
Ideally, a national vaccine plan would serve as a critical reference and coordinating mechanism for federal agency strategic planning. It would also enable and sustain greater coordination among all stakeholders with a role in vaccine development, delivery, and policy. Empirical research in management shows that systematic evaluation leads to a higher likelihood of success in implementing strategic plans (Armstrong, 1982; Kaplan and Norton, 1992). The 1994 plan lacked activity milestones and specific role designations for initiatives, and it did not differentiate longer- and shorter-term outcomes to help measure plan success. The update to the National Vaccine Plan should contain appropriate evaluative mechanisms, objective measures, and milestones, if the plan is to fulfill its potential as a blueprint for action on national priorities in vaccine development and immunization.
Recommendation 1: The committee recommends that NVPO and its partners include for each strategic initiative listed under the four plan goals the following details:
-
The primary responsible party (government agency or other stakeholder)
-
Secondary participant(s) (government agency or other stakeholder)
-
Measurable short, mid, and longer term outcomes to assess success of the initiative
-
Identification of costs and potential funding sources (e.g., professional judgment budgets) to support pursuit of the initiative
The plan also should include a timetable and process for regular updates that reflect the dynamic nature of the field.
3.
Facilitating and Sustaining Stakeholder Participation in Plan Implementation
The 1994 plan does not appear to have been coordinated with related efforts that were already under way when it was being developed, such as Healthy People 2000, or to have been a reference point for subsequent efforts, including Healthy People 2010. An exception appears to be the federally-sponsored Task Force on Safer Childhood Vaccines, which issued a report in 1998 and noted that its recommendations were consistent with the goals of the National Vaccine Plan (NIAID, 1998).
Obtaining input from a broad range of stakeholders, including the public, as described in (1) above, should be followed by finding ways to encourage and motivate continued involvement of those stakeholders. One way to accomplish this is to link the plan with other national plans, and the committee understands that an effort has begun to consider ways to coordinate with the Healthy People 2020 process.
Recommendation 2: The committee recommends that NVPO and its partners identify specific and creative strategies (not limited to funding) that federal agencies and programs could use to motivate stakeholders to implement objectives in the national vaccine plan.
Examples include
-
linking various types of grant programs to the plan (e.g., in announcing vaccine-related research grants, require applicants to explain how the proposed research relates to or advances a goal or objective of the plan); and
-
asking recipients of other types of federal funding, such as vaccine funding for states, federal health financing programs (Medicare and Medicaid) or health care delivery programs (Federally Qualified Health Centers), to demonstrate that their activities promote plan objectives.
4.
Making Explicit What Was Important in Developing the Plan
A plan cannot and should not offer to take every possible action to achieve a goal, and it should not be simply a wide-ranging collection of planned activities or a list of desired activities. The committee believes it is important that NVPO and its planning partners explain in the draft update to the plan the process by which priorities and objectives in the plan were selected. Ideally, a plan will aim to address a well-targeted set of major strategic issues, such as considering the relative opportunity cost and cost-benefit of pursuing one type of objective compared to another. A second important aspect of the process would involve identifying cross-cutting issues that require the attention and engagement of multiple federal agencies, as well as multiple stakeholders.
Recommendation 3: The committee recommends that NVPO and its partners explain in the draft update to the National Vaccine Plan 13 what was important to include and why, and the process by which items were selected for inclusion or discarded.
Content Areas
The committee noted some important omissions in the 1994 plan and identified several emerging areas and changes in context that will require attention in a major national document on the future of vaccine development and immunization. NVPO’s initial work on the update to the National Vaccine Plan identified 13 topics of interest (Orenstein, 2008). These topics include enhancing vaccine research and development, developing specific vaccines, adult immunization, adolescent immunization, childhood vaccination, financial barriers, vaccine supply, vaccine safety, vaccine injury/compensation, communication and education, surveillance, preparedness, and global health. The committee agrees that many of these topics deserve attention in the updated plan, but is not commenting on all of them here. Specific guidance is, however, offered on two topics that NVPO has already identified: vaccine finance and communication. On the pages that follow, the committee highlights these and four additional topical areas it believes are important to consider in developing the update to the plan.
1.
A Flexible Immunization System Capable of Responding to Innovation in the Development and Use of Vaccines
In the last several years, the use and purpose of vaccines has broadened to include new populations, new applications (e.g., exploration of therapeutic vaccines), and new technologies (e.g., introduction of new modes of delivery or combination vaccines, research on adjuvants to extend available vaccine doses). The science, technology, and use of vaccines continue to evolve. These changes will require flexibility and adaptability in the existing mechanisms for vaccine delivery, finance, communication about vaccines, and so on.
For example, implementation of the ACIP recommendation for universal annual influenza immunization in children exceeds the current capacity of the health care system to administer the vaccine to all relevant populations. Another example is found in the introduction of an HPV vaccine as a means for cervical cancer prevention, which has presented new communication and coverage challenges.
Recommendation 4: The committee recommends that NVPO and its partners include in the update to the National Vaccine Plan mechanisms to assess the “horizon” of innovation and new developments in vaccines, and explore strategic objectives or initiatives that enable timely consideration of and decision making to address emerging opportunities and challenges.
2.
Vaccine Financing
The mechanisms that the nation employs to finance the purchase of vaccines, and their deployment to and administration in clinical settings are central to national planning efforts, particularly for more costly new vaccines. In light of the nation’s complex approach to health care financing, which rests on a patchwork of public and private health insurance arrangements, supplemented by various federal and state direct public investments in the purchase, distribution, and administration of vaccines, the updated National Vaccine Plan needs to consider the issue of financing in a more substantive manner than the 1994 plan. This consideration may take into account that because vaccines are administered by health professionals in various practice settings, addressing the issue of financing requires more than deciding whether a particular type of vaccine will be covered, but also how administration costs will be financed and the manner in which coverage will be effectuated and payment made and even what types of professionals are authorized to administer vaccines. As one example, no mechanism currently exists for ensuring that new adult vaccines recommended by ACIP will be accounted for in existing public funding sources in a timely way to ensure use of these vaccines on the large scale needed to support national disease prevention goals.
Another recent example, which points to the importance of a national strategic focus on the intricacies of vaccine financing and how best to structure an effective payment approach, is the case of Medicare beneficiaries’ experience with the varicella-zoster vaccine. Medicare vaccine coverage now spans both Medicare Part B (medical care) and Part D (outpatient prescription drugs), which employ different approaches to coverage and payment. Part B treats payment of covered vaccines as an ancillary clinical service. This means that the treating clinician can accept assignment of the benefit and bill directly for the vaccine and its administration fee.
However, Medicare Part B covers only certain specified vaccines (against hepatitis B, influenza, and pneumococcal pneumonia). The Part D prescription drug program remedies this shortcoming by entitling enrolled beneficiaries to coverage of recommended vaccines not covered under Part B (Whitman, 2008). At the same time, however, Part D is not structured to operate as a means of financing provider-administered drugs and biologies; indeed, providers are barred from billing for services. As a result, a Medicare beneficiary enrolled in Part D must go through an unusually complicated series of steps to gain access to vaccine coverage for a new and important vaccine such as the varicella-zoster vaccine. The physician must prescribe varicella-zoster vaccine before the patient’s visit, and the patient then must procure the vaccine and bring it to the physician’s office to be administered.
Medicaid also deserves national attention because of its importance in closing the health gap between the richest and poorest Americans. Coverage of immunizations under Medicaid is an option in the case of beneficiaries ages 21 and older. A 2003 study conducted for CDC documented that immunization coverage at ACIP recommended levels is far less than universal for non-institutionalized adults, with only 32 states offering such coverage (Stewart et al., 2003). Adult immunization objectives would be reached more widely if state Medicaid agencies had available to them more active guidance on the value of adult immunization coverage, and tools for coverage and
payment options (including the use of replacement programs for the adult immunization supply).
3
Focus on Disparities in Access to Vaccines
Access to immunization is an issue closely linked with vaccine financing. The 1994 plan did not include a focus on disparities—whether socioeconomic or ethnic disparities—in access to vaccines, and the committee believes it is important to consider this area in drafting the update to the plan.
Innovations in vaccine research and development have led to the availability of several new vaccines (e.g., HPV vaccine, meningococcal conjugate vaccine, varicella-zoster vaccine). However, the growing number and cost of new vaccines in recent years have resulted in significant financial barriers and subsequently reduced access to newer vaccines now available for both children and adults. Although VFC (Vaccines for Children) has made vaccines available to the uninsured, there are still formidable barriers to access to care (including for Medicaid patients) and to some vaccines for the underinsured.
The VFC program has been remarkably successful in ensuring access to new vaccines for children who are uninsured, Medicaid insured, or Alaskan Native or American Indian. VFC also provides vaccines to underinsured children (i.e., those enrolled in health insurance plans that do not cover the cost of all recommended vaccines), but only if they are served at Federally Qualified Health Centers or Rural Health Centers, which are not readily accessible to all children. If underinsured children are seen in a private provider’s office, they must pay out-of-pocket for the cost of newer, more expensive vaccines or go to public health clinics to receive these vaccines. Of grave concern is the inability of some states to provide these vaccines even in public health clinics due to limitations in federal and state financing (Lee et al., 2007). This greatly limits timely access to new vaccines and perpetuates the personal, societal, and economic costs of these diseases.
4.
Communication as a Key Component of Vaccine Policies and Practices
A growing proportion of the public (and health care professionals) are uncertain about the benefits and the safety of vaccines and recommended immunization practices (Poland and Jacobson, 2001). Such concerns have resulted in underimmunization, disease outbreaks in the United States, and sustained transmissions of vaccine-preventable disease in other countries. These facts indicate that developing communication strategies to support immunization objectives requires understanding the beliefs and values of intended audiences.
To understand all the dimensions of the public’s decision making about vaccines, it is necessary to examine the gaps between what the experts perceive as risks and benefits, and what members of the public perceive to be the risks and benefits of vaccines. This work, informed by research in communication and the social sciences, is needed to (1) develop strategies to mitigate misinformation and to communicate messages relevant to the prevalent concerns, and (2) provide people with the information they need in language they understand to help them make informed decisions.
Recommendation 5: The committee recommends that the update to the National Vaccine Plan include a comprehensive framework for communicating with the public and other key stakeholders such as health care providers about the benefits (both individual and community) and risks of vaccination. Communication strategies that are implemented should be evaluated for their effect on knowledge and behavior.
Such a framework could include strategies to communicate at every stage of a vaccine’s lifecycle (i.e., not only at the time of FDA approval and ACIP recommendation), an emphasis on two-way communication with the public and health care providers, and strategies to incorporate the best available scientific evidence (e.g., on human behavior and decision making) and a range of communication approaches (social marketing techniques, use of targeted strategies to provide information to people who search the World Wide Web for immunization or vaccine information, etc.). Other strategies could include training key spokespersons (e.g., top scientists and others who are not communication professionals) on effectively communicating with the media regarding vaccines and immunization.
5.
Vaccine Supply Issues as a Barrier to Achieving Optimal Coverage
In the year after the 2000 FDA approval of the 7-valent pneumococcal conjugate vaccine, and subsequent ACIP recommendation for universal childhood use of the vaccine, the supplier announced a shortfall in supplies (CDC, 2001). CDC published interim recommendations that called for reserving the vaccine for certain groups of children. This example illustrates one of several possible causes of inadequate vaccine supply, which also may be caused by “companies leaving the vaccine market, manufacturing or production problems, and insufficient stockpiles” (CDC, 2008).
New state-of-the-art vaccine production and inventory management techniques have greatly increased the efficiency and profitability of vaccine manufacture in the United States, but they have also exacerbated the nation’s vulnerability to vaccine supply shortages. For example, “just-in-time” business practices (i.e., deliberately reducing inventory levels and delivering products only on an as-needed basis) discourage stockpiling (Wysocki and Lueck, 2006). They may create the incentive to under-produce (which could potentially lead to shortages), and they lead manufacturers to move production facilities to locations outside the country (potentially raising concerns about supply and complicating FDA oversight).
In presentations at the committee’s March 2008 meeting, NVPO identified supply issues as a priority area. The committee believes strategic initiatives to consider the factors that contribute to vaccine shortages and possible solutions can be pursued as part of the update to the National Vaccine Plan.
Recommendation 6: The committee recommends that NVPO and its partners consider ways the update to the National Vaccine Plan could spur research for creative solutions to vaccine supply problems.
Exploring the costs and benefits of shifting from a just-in-time to a just-in-case approach (Wysocki and Lueck, 2006), and the use of incentives, cost-sharing contracts, accounting rule modifications, and other mechanisms to align societal public health objectives with private manufacturing choices are among many areas that warrant more research and innovation.
6.
Changes in the Global Context
As noted above, it has become nearly impossible to neatly separate domestic and global vaccine issues because of porous borders and emerging infectious diseases on the one hand, and the global vaccine marketplace on the other hand.
The committee believes it is important that drafters of the update to the National Vaccine Plan pay special attention to the evolving global vaccine and immunization issues, in particular to industry views of the global marketplace as a more viable market for their vaccine products than the United States (Milstien et al., 2006). There is current tension between developing products for the U.S. market and focusing on global needs. For example, different serotypes of a disease-causing agent may be prevalent in different geographic areas, and some vaccines are developed to target serotypes found in the United States and exclude those that affect developing countries (Cutts et al., 2005; Klugman et al., 2003; Milstien et al., 2006).
CONCLUDING REMARKS
This letter report contains the committee’s initial guidance to the National Vaccine Program Office and its partners as they draft the update to the National Vaccine Plan. Based on the committee’s review of the 1994 plan and the process to develop it, and our knowledge about changes since 1994, we identified four process and six content areas to bring to NVPO’s attention. The committee also made six recommendations. The committee underscores the preliminary nature of the guidance provided in this letter report. The committee’s continuing work, including reviewing the evidence and receiving the input of national stakeholders, will form the basis for more detailed recommendations on priorities in the update to the National Vaccine Plan.
The committee thanks you for the opportunity to assist the National Vaccine Program Office as it coordinates the drafting of the update to the National Vaccine Plan.
Claire V. Broome,
Chair
Committee on the Review of Priorities in the National Vaccine Plan
Appendix A
References
Adams, W. G., K. A. Deaver, S. L. Cochi, B. D. Plikaytis, E. R. Zell, C. V. Broome, and J. D. Wenger. 1993. Decline of childhood haemophilus influenzae type b (hib) disease in the hib vaccine era. Journal of the American Medical Association 269(2):221-226.
Armstrong, J. 1982. The value of formal planning for strategic decisions: Review of empirical research. Strategic Management Journal 3(3): 197-211.
Braun, M. M., G. T. Mootrey, M. E. Salive, R. T. Chen, S. S. Ellenberg, and V. W. Group. 2000. Infant immunization with acellular pertussis vaccines in the United States: Assessment of the first two years’ data from the Vaccine Adverse Event Reporting System (VAERS). Pediatrics 106(4).
CDC (Centers for Disease Control and Prevention). 2000 (May 19). Poliomyelitis Prevention in the United States. Updated Recommendations of the Advisory Committee on Immunization Practices (ACIP). Morbidity and Morality Weekly Report 49(KR05):1-22.
CDC. 2001. Vaccines and preventable diseases: Decreased availability of pneumococcal conjugate vaccine (PCV-7). http://www.cdc.gov/vaccines/vacgen/shortages/past/pneumo-2001.htm (accessed June 5, 2008).
CDC. 2006 (December 15). Immunization information systems progress—United States, 2005. Morbidity and Morality Weekly Report 55(49): 1327-1329.
CDC. 2007a. What would happen if we stopped vaccinations? http://www.cdc.gov/vaccines/vacgen/whatifstop.htm (accessed April 29, 2008).
CDC. 2007b (August 31). National, state, and local area vaccination coverage among children aged 19—35 months—United States, 2006. Morbidity and Morality Weekly Report 56(34):880-885.
CDC 2008. Current vaccine shortages and delays, http://www.cdc.gov/vaccines/vacgen/shortages/default.htm#why (accessed June 5,2008).
Clements, C. J., and S. Ratzan. 2003. Misled and confused? Telling the public about MMR vaccine safety. Journal of Medical Ethics 29:22-26.
Clements, C., G. Evans, S. Dittman, and A. Reeler. 1999. Vaccine safety concerns everyone. Vaccine October 29(17 Suppl 3):S90-S94.
CMS (Centers for Medicare and Medicaid Services). 2006. Medicaid managed care enrollment report. http://www.cms.hhs.gov/MedicaidDataSourcesGenInfo/Downloads/mmcer06.pdf (accessed April 21, 2008).
Colgrove, J., and R. Bayer. 2005. Could it happen here? Vaccine risk controversies and the specter of derailment. Health Affairs 24(3):729-739.
Cutts, F. T., S. M. A. Zaman, G. Enwere, S. Jaffar, O. S. Levine, J. B. Okoko, C. Oluwalana, A. Vaughan, S. K. Obaro, A. Leach, K. P. McAdam, E. Biney, M. Saaka, U. Onwuchekwa, F. Yallop, N. F. Pierce, B. M. Greenwood, and R. A. Adegbola. Efficacy of nine-valent pneumococcal conjugate vaccine against pneumonia and invasive pneumococcal disease in the Gambia: Randomised, double-blind, placebo-controlled trial. The Lancet 365(9465): 1139-1146.
IOM (Institute of Medicine). 2008. Transcript of meeting one of the Committee on the Review of Priorities in the National Vaccine Plan. Washington, DC.
IOM Staff. 2008. IOM staff notes: Major themes from conversations with key individuals knowledgeable about the process of preparing the 1994 National Vaccine Plan. Washington, DC.
Iskander, J. 2007. Current CDC vaccine safety activities. Presentation at Vaccine Safety Evaluation: Post Marketing Surveillance Conference. Bethesda, MD.
Kaplan, R. S., and D. P. Norton. 1992. The balanced scorecard: Measures that drive performance. Harvard Business Review 70(1):71-79.
The Keystone Center. 2005. Citizen voices on pandemic flu choices: A report of the public engagement pilot project on pandemic influenza. http://www.keystone.org/spp/documents/FINALREPORT_PEPPPI_DEC_2005.pdf (accessed June 3,2008).
Klugman, K. P., S. A. Madhi, R. E. Huebner, R. Kohberger, N. Mbelle, N. Pierce, and the Vaccine Trialists Group. 2003. A trial of a 9-valent pneumococcal conjugate vaccine in children with and those without HIV infection. New England Journal of Medicine 349(14):1341-1348.
Lee, G. M., J. M. Santoli, and C. Hannan. 2007. Gaps in vaccine financing for underinsured children in the United States. Journal of the American Medical Association 298(6):638-643.
Milstien, J. B., M. Kaddar, and M. P. Kieny. 2006. The impact of globalization on vaccine development and availability. Health Affairs 25(4):1061-1069.
NIAID (National Institute of Allergy and Infectious Diseases). 1998. Task force on safer childhood vaccines: Final report and recommendations. Bethesda, MD: National Institues of Health.
NVAC (National Vaccine Advisory Committee). 2004. Public participation working group meeting on models for enhancing public involvement in vaccine decision-making meeting summary. http://www.hhs.gOv/nvpo/public_participation/NVAC_Public_Participcompleteminutes.html (accessed May 22, 2008).
NVPO (National Vaccine Program Office). 1994. Disease prevention through vaccine development and immunization: The U.S. National Vaccine Plan—1994. Washington, DC: Department of Health and Human Services.
NVPO. 1997. NVPO Interagency group workshop agenda book. St. Michaels, MD: NVPO.
Orenstein, W. A. 2008. Draft priorities of the national vaccine plan. Presentation at Meeting One of the Committee on Review of Priorities in the National Vaccine Plan. Washington, DC.
Poland, G. A., and R. M. Jacobson. 2001. Unde rstanding those who do not understand: A brief review of the anti-vaccine movement. Vaccine 19:2440-2445.
Stewart, A., S. Rosenbaum, M. Cox, and A. Lee. 2003. Medicaid coverage of immunizations for non-institutionalized adults. Washington, DC: The George Washington University. http://www.gwumc.edu/sphhs/departments/healthpolicy/chsrp/downloads/Medicaid_Immunization_Study.pdf (accessed June 5, 2008).
Wattigney, W. A., G. T. Mootrey, M. M. Braun, and R. T. Chen. 2001. Surveillance for poliovirus vaccine adverse events, 1991 to 1998: Impact of a sequential vaccination schedule of inactivated poliovirus vaccine followed by oral poliovirus vaccine. Pediatrics 107(83).
Whitman, B. 2008. Clearing up confusion over Medicare coverage for vaccines. ACP Internist March 2008:8. http://www.acponline.org/clinical_information/journals_publications/acp_internist/mar08/nine.htm (accessed May 15, 2008).
Wysocki, B., and S. Lueck. 2006. Margin of safety: Just-in-time inventories make U.S. vulnerable in a pandemic. Wall Street Journal, January 12, 2006.
Zhou, F., J. Santoli, M. L. Messonnier, H. R. Yusuf, A. Shefer, S. Y. Chu, L. Rodewald, and R. Harpaz. 2005. Economic evaluation of the 7-vaccine routine childhood immunization schedule in the United States, 2001. Archives of Pediatric & Adolescent Medicine 159(12):1136-1144.
Appendix B
Statement of Task
The federal government issued “Disease Prevention through Vaccine Development and Immunization, The US National Vaccine Plan” in 1994. The Institute of Medicine will convene an ad hoc committee to review the 1994 National Vaccine Plan and then provide guidance on the development of the update to the National Vaccine Plan. This will be delineated in a letter report to the National Vaccine Program Office.
The paragraph above constitutes the statement of task for the first part of the committee’s work. A short description of the second part of the committee’s work is provided below.
The committee will hold five meetings, each of which will involve a significant portion of time in open session with expert stakeholders to explore areas of the developing plan. Verbatim, uncorrected transcripts of the open sessions will be delivered to NVPO within a month after each meeting. Commissioned papers will be presented on less-well explored areas of the Plan. A final consensus report about priorities for the updated National Vaccine Plan will be delivered and publicly released no later than six months after the final meeting.
Appendix C
Meeting One Agenda14
Meeting One, March 3,2008
Committee on Review of Priorities in the National Vaccine Plan
AGENDA
National Academy of Sciences Building
2101 Constitution Avenue NW, Washington, DC
Lecture Room
|
1:00 - 1:10 pm |
Welcome and Committee Introductions |
|
|
Claire V. Broome Committee Chair |
|
1:10-1:20 pm |
Presentation |
|
|
Anand Parekh Acting Deputy Assistant Secretary for Health (Science and Medicine) Office of the Assistant Secretary for Health Department of Health and Human Services |
|
14 |
A Website (http://www.iom.edu/vaccineplan) and listserv were created to provide information to the public about the committee’s work and to facilitate communication with the committee. Materials from the committee’s March 2008 meeting are available in electronic form on the website. Further, a list of materials reviewed by the committee (in the form in which they were reviewed) including all submissions of information from the public and many items not cited in this report, can be found in the study’s public access file, obtained from the National Academies Public Access Records Office at (202)334-3543 or http://www8.nationalacademies.org/cp/ManageRequest.aspx?key=48905. |
|
1:20 – 1:50 pm |
Charge to the IOM Committee |
|
|
CAPT Raymond A. Strikas Medical Officer U.S. Public Health Service National Vaccine Program Office Department of Health and Human Services |
|
1:50 – 2:05 pm |
Questions from the Committee |
|
2:05 – 3:05 pm |
Key Dimensions of the National Vaccine Plan: Since 1994 and Future |
|
|
Melinda Wharton Deputy Director National Center for Immunizations and Respiratory Diseases Centers for Disease Control and Prevention |
|
|
Norman Baylor Director Office of Vaccines Research and Review Center for Biologies Evaluation Food and Drug Administration |
|
|
Carole A. Heilman Director Division of Microbiology and Infectious Diseases National Institute of Allergy and Infectious Diseases National Institutes of Health |
|
3:05 – 3:20 pm |
Questions from the Committee |
|
3:20 – 3:30 pm |
Break |
|
3:30 – 4:10 pm |
Key Dimensions of the National Vaccine Plan, continued |
|
|
Jeffrey Kelman Chief Medical Officer Center for Beneficiary Choices Centers for Medicare and Medicaid Services |
|
|
Geoffrey Evans Director Division of Vaccine Injury Compensation Healthcare Systems Bureau Health Resources and Services Administration |
|
|
Jerome Donlon Chief Scientist Advisor & Medical Officer, Office of the Assistant Secretary for Public Health Emergency Preparedness Department of Health and Human Services |
|
4:10 – 4:30 pm |
Questions from the Committee |
|
4:30 – 4:45 pm |
Relationship Between the National Vaccine Plan and Healthy People 2020 |
|
|
RADM Penelope Slade Royall Director Office of Disease Prevention and Health Promotion Office of Public Health and Science, Office of the Secretary Department of Health and Human Service |
|
4:45 – 5:15 pm |
Status of the New National Vaccine Plan |
|
|
Draft priorities for the National Vaccine Plan Walter Orenstein Professor of Medicine and Pediatrics Emory University School of Medicine Deputy Director, Emory Vaccine Center Consultant to the National Vaccine Plan |
|
|
Results of the first focus groups for public engagement Richard Tardif Oak Ridge Institute for Science and Education (ORISE) Consultant to the National Vaccine Plan |
|
|
Future plans for public engagement Roger Bernier Centers for Disease Control and Prevention |
|
5:15 – 5:30 pm |
Questions from the Committee |
|
5:30 – 5:45 pm |
Public Comments |
|
5:45 pm |
Adjourn |
Appendix D
1994 National Vaccine Plan Goals, Objectives, and Anticipated Outcomes
GOALS
|
1. Develop new and improved vaccines |
2. Ensure the optimal safety and effectiveness of vaccines and immunizations |
3. Better educate the public and members of the health professions on the benefits and risks of immunizations |
4. Achieve better use of existing vaccines to prevent disease, disability, and death |
OBJECTIVES
|
1.1 Develop new and improved vaccines for priority diseases |
2.1 Enhance the ability to evaluate the safety and effectiveness of vaccines |
3.1 Increase public demand for immunization, especially among populations at risk of underimmunization |
4.1 Ensure an adequate supply of vaccines |
|
1.2 Ensure the Nation’s capability to detect and respond effectively to new and emerging diseases in the United States and abroad |
2.2 Improve the surveillance and evaluation of adverse events following vaccination |
3.2 Improve the immunization practices of all health care providers |
4.2 Increase immunization coverage levels for infants and children |
|
1.3 Enhance the process of translating technologic innovations into new |
2.3 Ensure the optimal use of vaccines |
3.3. Increase the awareness of the benefits of immunization among special target audiences (third-party payers, employers, legislators, community leaders, hospital administrators, etc.) |
4.3 Maintain immunization coverage levels for school-aged children |
|
1.4 Ensure the Nation’s capability to evaluate new vaccines, and to conduct prompt reviews of new and improved candidate |
2.4 Continue to ensure fair and efficient compensation to individuals injured by vaccines |
3.4 Develop more effective methods of communicating the benefits and risks of immunization to health care providers, patients, and parents/guardians |
4.4 Increase immunization coverage levels among older adolescents, adults, and the elderly |
|
1.5 Promote the improvement of existing vaccines and development of new vaccines ad vaccine-related technologies for other diseases of importance in developing countries |
2.5 Promote and support the efforts of the World Health Organization to develop and harmonize international standards and improve regulatory capabilities in countries involved in vaccine production |
3.5 Continue to evaluate the benefits and impact of immunization through the use of cost-effectiveness studies |
4.5 Improve the surveillance of vaccine preventable diseases to assess the impact of immunization programs |
|
|
|
|
4.6 Establish registry and immunization tracking systems 4.7 Enhance immunization coverage to strengthen national defense 4.8 Enhance immunization coverage of international travelers who are of highest risk of acquiring vaccine-preventable diseases 4.9 Eradicate poliomyelitis globally 4.10 Promote better control of neonatal tetanus and measles, worldwide 4.11 Promote the self-sustaining capacity of immunization programs in developing countries |
ANTICIPATED15 OUTCOMES
Provision of adequate resources to make possible the vigorous and comprehensive pursuit of the wide range of activities outlined in the National Vaccine Plan could result in substantial health benefits for the American people by the year 2000. These benefits are expected to be realized as the following outcomes:
-
Age-appropriate immunization with all recommended vaccines will be extended to at least 90 percent of infants and children, and access to affordable vaccination services will be made available for every person in the United States.
-
Diphtheria, tetanus, poliomyelitis, measles, rubella, mumps, some forms of hepatitis, pertussis (whooping cough), and bacterial meningitis (from Haemophilus influenzae type b) will be essentially eliminated as significant causes of death, disease, and disability in the United States.
-
Educational communication networks will be in place that will inform all health care providers, communities, and families of the benefits and risks of vaccination.
-
In a global context, polio will be drastically reduced, if not eliminated, and neonatal tetanus and measles will be better controlled.
-
Pneumococcal pneumonia and influenza in American adults over the age of 65 will be significantly reduced.
-
A nationwide system will monitor the vaccines that children receive, and will remind parents when individual infants and children should be vaccinated.
-
A nationwide surveillance system will report and investigate cases of vaccine-preventable diseases.
-
Vaccine safety and efficacy will be continuously monitored, and adverse events following immunization will be reported and carefully analyzed.
-
Improved vaccines will replace some of the vaccines in current use.
-
Some vaccines requiring multiple doses and multiple contacts with the health care system will be replaced by more cost-effective ones that will improve people’s access to immunization.
-
Many new vaccines will be developed, or be much closer to licensure, for diseases for which effective vaccines do not now exist.
-
New mechanisms for the more rapid assessment of vaccines proposed for licensure will be in place.
-
A reliable supply of all recommended vaccines and a capability to respond to emergencies and emergent threats to public health will be achieved and sustained.
-
Information on the cost and benefits of the National Vaccine Plan will be made available on an ongoing basis to the American people.









































