2
Batteries and Battery Packs for PHEVs
Battery cells, and the packs into which they are assembled, are the key component that will largely determine the viability of PHEVs. The battery packs must be affordable, durable, and safe. No commercially available battery meets all these requirements. Rechargeable lithium-ion (Li-ion) batteries, made by the billions for small electronic devices, are the most promising technology for automotive propulsion and will be used in the first-generation PHEVs soon to be rolled out. This section reviews the relevant technologies and estimates how their characteristics may evolve over the coming years.
TYPES OF PHEVS
Several configurations are possible for PHEV drive trains. The two considered here represent those that may be introduced, as shown in Figure 2.1. A PHEV differs from a hybrid electric vehicle (HEV) in that the battery can be charged from the electrical grid and operate the vehicle independently of the internal combustion engine (ICE) for a limited all-electric range (AER). This is the charge-depleting mode of operation. The ICE starts when the battery reaches its minimum state of charge and operates the generator to charge the battery. This charge-sustaining mode of operation prevents the battery from being discharged too deeply.
The PHEV-10 is designed for an AER of 10 miles before the ICE must start. It is similar to the Toyota Prius but has a larger battery and modified control electronics. Its split-power blended (or parallel-drive) configuration can drive the car either with only the electric motor powered by the battery or with the gasoline engine. When the battery is discharged to its minimum allowable level, the engine starts and the vehicle operates in a charge-sustaining mode, as in a conventional HEV. The engine will also start and assist in driving the wheels when more power is needed than can be delivered by the electric motor for rapid acceleration or heavy-load hill climbing. The PHEV-10 requires a more robust battery than an HEV because it must operate over a wide state of charge (SOC) range, enduring many deep charge/discharge cycles.1
The PHEV-40 has its engine, battery, and electric motor in series. The engine only charges the battery, and all propulsion comes from the electric motor. Thus it has a larger battery and motor than the PHEV-10 but a longer AER. It is conceptually similar to the General Motors Volt in design.
The size of the battery required to provide propulsion depends on the size and weight of the vehicle and the AER desired. For simplicity, this report considers just one vehicle, a midsize car, as representative of the fleet of light-duty vehicles, as was done in the 2008 Hydrogen Report. While midsize cars may not perfectly represent the fleet, they are adequate to illustrate the critical issues. Various recent studies have reported a range of energy requirements for midsize cars: in a study of Prius conversions to PHEVs, on average the vehicles required 238 watt-hours (Wh) per mile (Francfort, 2009). A simulated driving analysis calculated 170-200 Wh per mile energy consumption.2 In addition, the GM Volt is expected to reach 40 miles on 8 kWh from its batteries, or 200 Wh per mile.3 The committee assumed that the vehicles it analyzed would initially require 200 Wh per mile in its calculations. Larger, heavier vehicles would require substantially more energy per mile and bigger, more-expensive batteries, but those are not considered in this report. All vehicles are expected to become more efficient over time, and PHEVs will require less gasoline and less electricity, as discussed in Chapter 4.
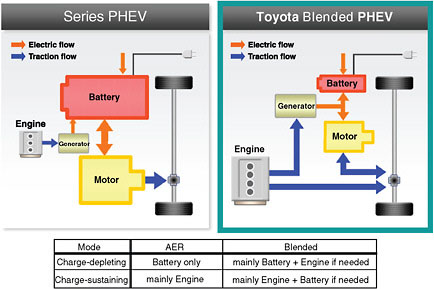
FIGURE 2.1 Plug-in hybrid electric vehicle concepts. SOURCE: Toyota.
Thus, the PHEV-10 requires 2.0 kWh of battery energy (actually used) to drive its 10-mile AER. The PHEV-40 draws 8 kWh of battery-stored energy to meet its 40-mile AER in charge-depletion mode before the engine starts and begins supplying power to operate the vehicle in charge-sustaining mode.4 For the 50 percent SOC assumed in this report for the first generation of vehicles, the nameplate capacities are 4 kWh for the PHEV-10 and 16 kWh for the PHEV-40.
LITHIUM-ION BATTERY CELL CHEMISTRIES
For PHEVs to be widely accepted by consumers, batteries must be significantly cheaper than they are now, durable enough to have a long life, and safe. In addition, they will have to meet performance goals, which will require
-
High power density to deliver the current needed for demanding driving conditions;
-
High energy density for storing the needed energy for an extended all-electric range; and
-
Wide range of SOC while maintaining a long cycle life.
Li-ion batteries currently are the only serious option for PHEVs. They are smaller and lighter than other batteries, and they promise to withstand multiple large SOC swings while maintaining their performance. They have more than twice the energy density and about three times the power density of the nickel-metal-hydride (NiMH) batteries used in current HEVs, and four times the energy density of the lead-acid batteries used in most vehicles today.
What no Li-ion battery can do—yet—is simultaneously deliver both high power density and high energy density at a reasonable cost. To meet this challenge, several promising Li-ion chemistries are being vigorously pursued by companies, research institutions, and governments. The technology is advancing rapidly, but there is no guarantee that any Li-ion battery will be developed that meets all goals for vehicle use. Table 2.1 compares the attributes of four of the more promising Li-ion battery chemistries.
Li-ion battery manufacturing technology is essentially the same for all battery chemistries. Typically the electrodes of Li-ion batteries are coated on metal foils, usually copper foil for the negative electrode and aluminum foil for the positive electrode, separated by an electrolyte (Nelson et al., 2009). Typical electrolytes are derived from solutions of LiPF6 salt in a solvent blend of ethylene carbonate and various linear carbonates, such as dimethyl carbonate (Tikhonov and Koch, 2009; Zhang et al., 2002).
TABLE 2.1 Characteristics of Li-Ion Batteries Involving Different Chemistries
While the power density (W/kg) of the cell is fixed by the surface area of the electrode foil, the energy density (Wh/kg) can be varied over a limited, but significant, range simply by increasing or decreasing coating thickness. HEV batteries, which require high power more than high energy storage, have thin electrode coatings. By contrast, electric vehicle (EV) batteries require high energy density and have thicker electrode coatings. Research has yielded new concepts for better electrodes and electrolytes. For example, raising the cell voltage to 5 V would increase the battery’s energy density. Could this lead to a better PHEV battery? It is simply too early to tell.
In this report overall properties such as energy density, power density, and total energy available refer to the full range from 100 percent to 0 percent SOC. Energy and power density are intrinsic properties, and total available energy is the nameplate capacity of the cell or battery. For batteries in battery packs for vehicle operation, this report refers to the energy (kWh) actually used—that is, the nameplate capacity of all the cells in the pack multiplied by the allowable SOC.
LITHIUM-ION BATTERY PACKS
For PHEV applications, about 100 Li-ion cells are connected in series to provide the design voltage to operate the electrical propulsion motors. These cell groups are then installed in parallel, as needed, to provide the energy to drive the motor for the distance desired. Battery packs consist of these groups of cells, the supporting frame, electronic controls, and cooling systems to protect the cells. The current focus is on improving battery durability, safety, and cost competitiveness.
Battery Durability
Auto manufacturers have indicated that they intend to offer an 8-year warranty in 49 states and a 10-year warranty in California on PHEV battery systems as part of the drive-train warranty. Current commercial Li-ion batteries typically last 3 to 4 years, which is a function of both the number of charge/discharge cycles and calendar life (Howell, 2009). Some degradation is inevitable; for the purposes of this report, about 20 percent over the warranty period is assumed. If the PHEV-40 is expected to still have its required 8 kWh (actually used) of energy needed for an AER of 40 miles with the same 50 percent SOC range in 10 years, it could be sized to provide 10 kWh (actually used) energy initially. The other option is to assume that the SOC range is increased over time to account for battery degradation, which could be adjusted every year when the vehicle is brought in for servicing. This is the approach that the committee chose for the estimations that follow. If degradation is not too large or does not accelerate with larger SOC range, this should be satisfactory, but until demonstrated it remains a concern.5
Figure 2.2 compares the SOC variation for PHEV and HEV batteries. In a PHEV, batteries must undergo multiple large SOC range cycles without significant degradation. A 10-year life would require the batteries to undergo at least 2,000 cycles and still stay within the prescribed performance range. At present, this requires limiting the SOC range. The right-hand figure shows the much narrower (and less demanding) SOC range of an HEV.
This study assumes that SOC varies at most between 30 and 80 percent, or 50 percent of the total charge. The 30 percent lower limit is near the minimum and serves to maintain power and energy during charge-sustaining mode. The upper limit allows charging from regenerative braking while preventing overcharging and the resultant rapid battery degradation. A 50 percent range in SOC does, however, come at a price: The battery must have a nameplate capacity twice as high as the amount of energy actually needed and delivered to meet performance targets. In other words, the PHEV-40 will need a nameplate battery rating of 16 kWh to supply 8 kWh of the energy actually used for its 40 miles of charge-
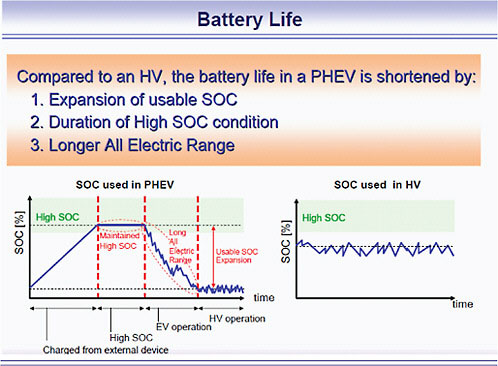
FIGURE 2.2 Differences in SOC requirements for PHEV batteries and HEV batteries. The PHEV is charged from an external source until it reaches its maximum state of charge, as shown on the left side of the figure. Its charge-depleting mode in AER takes it down to its minimum state of charge. The jagged portion of this curve is from regenerative braking, which partially recharges the battery. The level portion is charge-sustaining operation with the engine maintaining the battery charge around its lower SOC. The HEV also recharges from regenerative braking but operates in a much narrower SOC range. SOURCE: Toyota, presentation to the committee, May 18, 2009.
depleting driving (or 20 kWh if it is oversized to account for 20 percent degradation). Energy used is the product of the nameplate energy and the SOC range. Increasing the SOC range will increase the fraction of the nameplate energy used from a given battery pack size if that can be done without compromising durability.6 Generally, however, the industry believes that for the first 5 years or so, battery durability issues will require conservative battery management—that is, keeping the SOC range at about 50 percent.
New technology may help meet the durability challenge. One type of battery is claimed to have lasted 7,000 cycles in accelerated aging tests covering a wide (70 to 90 percent) range in SOC with little reduction in performance under controlled test conditions.7 However, life prediction is difficult, and actual performance will not be known until many vehicles are in service for many years. In the absence of this operational information, accelerated-age tests are used to estimate the expected performance, but they may not capture the full effect of actual aging.
In addition, higher temperatures and other excursions outside the design envelope (e.g., SOC limits and rate of charging) detract from durability and battery life. Accordingly, cooling and temperature control systems will have to be included in the battery pack, and operation-control strategies must avoid excursions in operational performance.
Battery-Pack Safety
Safety has been a concern with Li-ion batteries, which can overheat and catch fire or even explode, emitting burning gases. There appear to be two separate causes for these thermal runaways: contaminants and overcharging.
Contaminants, particularly small metal particles, can enter the cell during manufacturing, causing a short circuit between the anode and the cathode, resulting in a fire. Improved manufacturing techniques and rigorous quality control should manage this issue, albeit at an increased cost. Overcharging the cells or charging them too rapidly can lead to overheating, which can degrade the battery and limit its service life. With some Li-ion chemistries, over-charging can result in thermal runaway and catastrophic failure. Control systems to prevent this are discussed in the following section.
Proper choice of Li-ion chemistry, controlled manufacturing procedures, and onboard monitoring and temperature control should assure safe batteries and safe battery pack operation. Another safety concern, crashes, also must be resolved. Passengers and emergency workers must be safe from shocks and fumes. As with durability, battery and automotive manufacturers are confident that the safety issues can be overcome and managed. The consequences of catastrophic failures would be too great for manufacturers to market PHEVs that do not meet very high safety standards.
Battery Pack Cooling and Control Electronics
The battery pack, in addition to containing a hundred or so interconnected Li-ion cells, includes two control systems essential for the safety and durability of the batteries. Both of these systems include significant electronics and other equipment, located separate from, but connected to, the battery pack.
One of these systems monitors and controls the temperature of the battery cells. With the current state of Li-ion battery cell technology, the individual cell temperature should not exceed 60°C (140°F) because the batteries deteriorate at higher temperature.8 The electrically driven temperature-control unit uses cooling fluid to maintain battery temperature. Liquid cooling is assumed to be required for larger battery packs; smaller battery packs, such as for a PHEV-10, may allow air cooling.
The other system measures the voltage of each cell and ensures that it does not exceed an upper limit during charging or regenerative braking, which could lead to thermal runaway (overheating). The voltage of each Li-ion cell should not exceed its specified value by more than a few tens of millivolts. This balancing of charge is important to battery life and battery safety.
The non-cell portion of the battery pack (i.e., the structure and control systems) could account for around 50 percent of the pack’s cost and is less likely to produce large future cost reductions. However, with improved battery technology, operational experience, and better quality control in cell manufacture, it might be possible to monitor some of the cells rather than all of them, as is done now, which would help reduce battery pack cost. In addition to adding to costs, cooling and monitoring of the cells add significantly to the weight of the battery pack, reducing the power and energy density of the battery pack.
Battery-Pack Performance and Cost
Tables 2.2 and 2.3 summarize the committee’s estimates of Li-ion battery and battery-pack performance, and costs for the two PHEV types examined in three time periods, 2010 (current technology), 2020, and 2030. Additional detail on the committee’s analysis of battery-pack cost can be found in Appendix F. These estimates were arrived at after literature searches and discussions with industry experts. The values in the tables represent the judgment of the committee of the most probable rate of anticipated progress based on the entirety of the data available to it.9 Future battery and battery-pack costs are quite uncertain at this point. For that reason the committee feels that it will be important to reevaluate these costs in several years, when significant data on the first production cycle of PHEVs is available, which should allow better projections.
Optimistic and conservative estimates also were made for the production costs expected in 2010, 2020, and 2030. “Optimistic” means progress is faster than expected. “Conservative” means partial rather than “probable” success but could also mean that additional battery capacity (and thus cost) was necessary to account for degradation. The “probable” and “optimistic” estimates form the basis for the projections that the committee modeled, as discussed in the next section. Box 2.1 lists DOE’s battery targets for comparison.10 It is the committee’s opinion that these PHEV battery goals are extremely aggressive and are unlikely to be reached by the target date or even for a significant time beyond.
|
8 |
Damage can start at 50°C, but deterioration is slow. Vehicles are unlikely to be exposed to such high ambient temperatures, but the heat given off by charging and discharging can lead to high temperatures inside the pack. Thus cooling is necessary, particularly in hot climates. |
|
9 |
The performance and cost numbers in Tables 2.2 and 2.3 are less optimistic than some others that have been claimed. Lithium-ion battery manufacturing is a well-developed technology. Worldwide over a billion Li-ion cells are currently produced every year. They are made by coating large sheets that are then cut up in small pieces for cell phones and other electronic devices. Vehicle batteries will be conceptually similar, but the sheets will be cut in larger pieces. Also, a large part of the cost of automotive batteries is the packaging, which involves electronics for monitoring the cell voltage and state of charge the SOC, cooling systems and, their mechanical supports and sheet metal. These components are not expected to decline greatly in cost. |
|
10 |
D. Howell, DOE, DOE targets for battery performance, presentation to the committee, June 2009. |
TABLE 2.2 Estimates of Li-Ion Battery Performance Parameters for a PHEV-40
|
Characteristic |
|
2010a |
2020 |
2030 |
|
Energy density at nameplate cell level, Wh/kg |
Probable |
150 |
200 |
200 |
|
Power density at nameplate cell level, W/kg for 12 sec |
Probable |
1,400 |
1,600 |
1,750 |
|
Energy density at nameplate battery pack level,b Wh/kg |
Probable |
120 |
150 |
150 |
|
Power density at nameplate battery pack level,c W/kg for 12 sec |
Probable |
1,150 |
1,250 |
1,400 |
|
Cycle life over SOC at 40°C ambient |
Probable |
3,000 |
5,000 |
7,500 |
|
Battery pack cost per kWh over SOC variation (8 kWh actually used), $/kWhd |
Conservative |
2,000 |
1,275 |
1,150 |
|
|
Probable |
1,750 |
1,120 |
1,000 |
|
|
Optimistic |
1,250 |
800 |
720 |
|
Battery pack cost per kWh for nameplate energy level (16 kWh), $/kWh |
Conservative |
1,000 |
638 |
575 |
|
|
Probable |
875 |
560 |
500 |
|
|
Optimistic |
625 |
400 |
360 |
|
Battery calendar life, yr |
Conservative |
3 |
7 |
9 |
|
|
Probable |
5 |
10 |
10 |
|
|
Optimistic |
8 |
12 |
15 |
|
NOTE: PHEV-40 nameplate battery rating 16 kWh (8 kWh usable); SOC variation range, 80-30 percent; 100+ kW peak power. aFirst production cycle. bBattery pack means the entire system, including packaging, cooling, and monitoring and control electronics. cPower density numbers for PHEVs are still variable since developers are engineering their cells to give optimum life and energy. dAs applied to SOC range actually used. Cost per kWh based on nameplate capacity would be half these. Additional information on the committee’s analysis of these costs is in Appendix F. |
||||
TABLE 2.3 Estimated Battery Performance Properties for a PHEV-10
|
Characteristic |
|
2010a |
2020 |
2030 |
|
Energy density at nameplate cell level, Wh/kg |
Probable |
100 |
150 |
150 |
|
Power density at nameplate cell level, W/kg for 12 sec |
Probable |
1,500 |
1,600 |
1,750 |
|
Energy density at nameplate battery pack level, Wh/kgb |
Probable |
80 |
110 |
125 |
|
Power density at nameplate battery pack level,c W/kg for 12 sec |
Probable |
1,250 |
1,350 |
1,400 |
|
Cycle life over SOC at 40°C ambient |
Probable |
3,000 |
5,000 |
7,500 |
|
Battery pack cost per kWh over SOC variation (2 kWh actually used), $/kWhd |
Conservative |
2500 |
1,600 |
1,450 |
|
|
Probable |
1,650 |
1,050 |
950 |
|
|
Optimistic |
1,250 |
800 |
725 |
|
Battery pack cost per kWh for nameplate energy level (4 kWh), $/kWh |
Conservative |
1,250 |
800 |
725 |
|
|
Probable |
825 |
525 |
475 |
|
|
Optimistic |
625 |
400 |
363 |
|
Battery calendar life, yr |
Conservative |
3 |
7 |
9 |
|
|
Probable |
5 |
10 |
10 |
|
|
Optimistic |
8 |
10 |
15 |
|
NOTE: PHEV-10 nameplate battery rating 4.0 kWh (2 kWh usable); SOC variation range, 80-30 percent; 50+ kW peak power. aFirst production cycle. bBattery pack means the entire system, including packaging, cooling, and monitoring and control electronics. cPower density numbers for PHEVs are still highly variable since developers are engineering their cells to give optimum life and energy. dAs applied to SOC range actually used. Cost per kWh based on nameplate capacity would be half these. Additional information on the committee’s analysis of these costs is in Appendix F. |
||||
PROJECTED PHEV INCREMENTAL COSTS
Tables 2.4 and 2.5 compare the current incremental cost of components for a PHEV-40 and a PHEV-10 with those of a conventional (nonhybrid) car. Savings from eliminating components or reducing size are shown as negative numbers; for example, the automatic transmission can be eliminated when the drive is electric.11 These incremental numbers are for the first round of PHEV production, including the estimated cost of the battery pack, the least well defined of the costs. Initially, the PHEV-40 is expected to cost the vehicle manufacturer about $18,000 more than an equivalent conventional car and the PHEV-10 to cost about $6,300 more. The price to the customer, before government subsidies, is likely to be significantly higher once manufacturers’ additional expenses and profit and dealers’ markup are added in.
These costs are likely to decline over time. Table 2.6 summarizes projections of cost reductions for the different components for the two PHEV types for 2015, 2020, and 2030. Reduction estimates are posited on technology improvements, on experience gained over time through several cycles of technology evolution, and from increased economies of scale. The committee held discussions with various experts who provided valuable input to this table. There was good agreement on the expected rate of improvements, particularly for the non-battery components, where there is considerable experience. There also was general agreement that battery pack costs would decline significantly, but not dramatically, for the first 10-15 years of commercial experience and would later slow.
The reductions expected mirror the experience with NiMH batteries for HEVs, where costs came down significantly at first but then decreased much more slowly. The NiMH battery pack for HEVs saw a cost reduction of about 11 percent from 2000 to 2006 but since has seen much less change. Li-ion battery cost decreased by about 35 percent from 2000 to 2008, but most of that was at the beginning of that period, with only about 5 percent after 2004 (Howell, 2009). Manufacturers of Li-ion batteries with technology similar to consumer batteries are already considerably further along the learning curve than were manufacturers of NiMH batteries when HEVs were introduced, so steep cost reductions seem unlikely. Nor does it seem likely that the cost of materials will decline greatly. Indeed, some materials, including lithium, may increase in cost with additional demand, but the committee believes that the supply of lithium will be adequate for any plausible number of PHEVs manufactured worldwide.
It is likely that much of the reductions in Li-ion cell costs will come from technology innovations, with smaller reductions from manufacturing improvements and volume
|
11 |
The PHEV-10 will require a transmission because the engine is connected directly to the wheels. However, the committee assumed that manufacturers would use a small, electronically controlled continuously variable transmission (ECVT) such as used in the 2010 Prius. This cost is included under power electronics in Table 2.5. |
TABLE 2.4 Projected Incremental Costa of Components for PHEV-40 for Production in 2010 Using Current Technology Compared with an Equivalent Current Nonhybrid Vehicle
|
Component |
|
Price That a Supplier Charges the Vehicle Manufacturer for the Technology |
Cost Reductions in Components due to Vehicle Changes in Going to PHEV-40 |
Incremental Cost of PHEV-40 Vehicle vs. Modern, Comparable ICE Vehicle |
|
Motor/generator |
Probable |
1,800 |
|
1,800 |
|
Power electronics, DC/DC converter (1.2 kW), and inverter |
Probable |
2,500 |
|
2,500 |
|
Li-ion battery pack |
Conservative |
16,000 |
|
16,000 |
|
8 kWh actually used |
Probable |
14,000 |
|
14,000 |
|
16 kWh nameplate capacityb |
Optimistic |
10,000 |
|
10,000 |
|
Electrical accessories |
Probable |
100 |
|
100 |
|
Electric air conditioning |
Probable |
400 |
|
400 |
|
Regenerative brakes |
Probable |
180 |
|
180 |
|
Electric power steering/water pump |
Probable |
200 |
|
200 |
|
Body/chassis/special components |
Probable |
200 |
|
200 |
|
Automatic transmission |
Probable |
|
850 |
−85 |
|
Starter and alternator |
Probable |
|
95 |
−9 |
|
Engine simplification |
Probable |
|
300 |
−30 |
|
Total |
Conservative |
21,380 |
|
20,135 |
|
|
Probable |
19,380 |
1,245 |
18,135 |
|
|
Optimistic |
15,380 |
|
14,135 |
|
aSeries plug-in hybrid 40-mile AER, 100+ kW peak power, 8 kWh usable; 16 kWh nameplate capacity. bSee Appendix F for further information on the committee’s analysis of costs. |
||||
TABLE 2.5 Projected Incremental Costa of Components for PHEV-10 for Production in 2010 Using Current Technology Compared with an Equivalent Current Nonhybrid Vehicle
|
Component |
|
Price That a Supplier Charges the Vehicle Manufacturer for the Technology |
Cost Reductions in Components due to Vehicle Changes in Going to PHEV-10 |
Incremental Cost of PHEV-10 Vehicle vs. Modern, Comparable ICE Vehicle |
|
Motor/generator |
Probable |
1,500 |
|
1,500 |
|
Power electronics, DC/DC converter (1.2 kW), and inverter |
Probable |
1,500 |
|
1,500 |
|
Li-ion battery pack |
Conservative |
4,000 |
|
4,000 |
|
2.0 kWh actually used |
Probable |
3,300 |
|
3,300 |
|
(4 kWh nameplate capacity)b |
Optimistic |
2,500 |
|
2,500 |
|
Electrical accessories |
Probable |
100 |
|
100 |
|
Electrical air conditioning |
Probable |
400 |
|
400 |
|
Regenerative brakes |
Probable |
180 |
|
180 |
|
Electric power steering and water pump |
Probable |
200 |
|
200 |
|
Body/chassis/special parts |
Probable |
200 |
|
200 |
|
Automatic transmission |
Probable |
|
850 |
−85 |
|
Starter and alternator |
Probable |
|
95 |
−9 |
|
Engine simplification |
Probable |
|
120 |
−12 |
|
Total |
Conservative |
8,080 |
|
7,015 |
|
|
Probable |
7,380 |
1,065 |
6,315 |
|
|
Optimistic |
6,580 |
|
5,515 |
|
aSplit-power plug-in hybrid, 10-mile AER capacity, 50+ kW peak power, 2 kWh usable; 4 kWh nameplate capacity. bSee Appendix F for further information on the committee’s analysis of costs. |
||||
TABLE 2.6 Percent Projected Cost Reductions for Different Components with Increased Production and Learning by Doing
|
Component |
Year Reduction Achieved/Year Against Which Compared |
||
|
2015a/2010 |
|||
|
Motor/generator/gear set |
5 |
5 |
5 |
|
Power electronics, AC/DC converter |
10 |
15 |
5 |
|
Li-ion battery pack |
25 |
15 |
10 |
|
Electrical accessories |
5 |
5 |
5 |
|
Air conditioning |
10 |
5 |
5 |
|
Regenerative brakes |
5 |
5 |
5 |
|
Electric power steering + water pump |
5 |
5 |
5 |
|
Body/chassis/special components |
10 |
5 |
5 |
|
NOTE: Estimated cost reductions are based on increased production volumes and anticipated improvements in technology and production techniques. Unanticipated technology advances (breakthroughs) could lead to faster reductions. aAssumed production, 25,000 vehicles per year. bAssumed production, 1 million vehicles per year. cAssumed production, 1 million-plus vehicles per year. |
|||
production.12 Although it is hard to quantify, about half of the cell cost is estimated to be for materials, and the cells account for about half the battery pack cost, further reducing the impact of cell-only cost reductions.
The additional costs for changes in mechanical and electrical components in going from a conventional vehicle to a PHEV are considered quite predictable and have the expected impact on vehicle cost. These estimates (Table 2.4 and Table 2.5) are only for the cost of the components to the vehicle manufacturer and do not include the cost for vehicle engineering, R&D, or the automakers’ capital investments. These and other markups to the vehicle price, which is what the customer will see, are addressed in Chapter 4 of this report.
Overall, Li-ion battery-pack costs may decline by almost 50 percent, as shown in Table 2.2, from $1,750 per kWh energy actually used in 2010 to about $1,000 per kWh in 2030. Collectively, the reductions in component costs lead to future PHEV costs shown in Table 2.7. These estimates do not consider the possibility of technological breakthroughs, which, if they occur, could significantly reduce the costs and improve the viability of PHEVs. Table 2.7 and the scenarios that follow do not report the conservative estimates, for if costs remain that high, PHEVs are unlikely to achieve much success in the market.
TABLE 2.7 Estimated PHEV Incremental Costs
|
|
2011a |
2015 |
2020 |
2030 |
|
PHEV-40 |
14,100-18,100 |
11,200-14,200 |
9,600-12,200 |
8,800-11,000 |
|
PHEV-10 |
5,500-6,300 |
4,600-5,200 |
4,100-4,500 |
3,700-4,100 |
|
NOTE: These are the incremental costs to manufacture the vehicle itself, relative to a conventional (nonhybrid) vehicle. They do not include engineering, overhead, or other costs, or profit, and thus are not the total incremental prices to the customer. Ranges represent probable and optimistic assessments of battery technology progress. aCosts for 2011 are based on low battery production rates in response to contracts initiated about 2 years earlier. |
||||
OTHER TECHNOLOGY OPTIONS AND POTENTIAL BREAKTHROUGHS
The cost of Li-ion batteries is currently very high, making it difficult for PHEVs to be cost competitive when the cost of gasoline is less than $4 per gallon. Although considerable progress is expected in reducing battery costs, it is not clear that sufficient cost reductions can be achieved with Li-ion batteries or battery packs to make PHEVs cost competitive without substantial subsidies.
Announcements continue from researchers about improvements in Li-ion batteries, including better electrodes and electrolytes and, possibly, higher cell voltages (to 5 V), resulting in better energy density. Unfortunately, it is hard to evaluate the practicality of these concepts or to assess which, if any, will become commercial and when.
Other Li-ion battery cell chemistries may offer better performance than those currently projected for PHEV applications,13 but serious questions remain about their durability, safety, and costs. There appears to be little chance that any of these could become commercially cost competitive in the near future.
A breakthrough in battery technology would definitely improve the prospect of PHEVs becoming economically competitive. It is not possible to predict or schedule scientific and technical breakthroughs, but a continued, substantial scientific research effort is needed to increase the chances that this will occur. However, even if a breakthrough occurs, it will be decades before it has a great impact. Major battery developments will require considerable work and time prior to commercialization to confirm cost advantage, durability, and safety, and years more to achieve significant penetration into the fleet.
Options such as the lithium-air battery and solid polymer Li-ion electrolyte batteries are under study. Several large U.S. corporations are working on lithium-air technology, which could offer 5 to 10 times as much energy density as the Li-ion batteries discussed above. This battery is much
lighter, but there are issues of safety, primarily because of lithium’s reactivity with water, and regeneration or recharging needs to be developed. Solid polymer Li-ion electrolyte batteries offer higher energy densities and more stability than Li-ion batteries, but safety and operational challenges (such as achieving acceptable current density at ambient temperatures) will be difficult to meet. There do not appear to be any other radically new battery technologies on the horizon (the lithium metal-air battery concept has been around for many years) that could economically provide the enhanced performance needed, but the vibrant research and development programs world-wide may produce a technology that will overcome these barriers.
Also, totally different approaches are being considered. Swapping battery packs at stations that charge them for the next vehicle is one possibility, but it is not clear if pack and vehicle design will be sufficiently standardized to make this widely practical. Battery leasing is another proposal. Leasing could lower the initial cost to the consumer and perhaps provide some reassurance about durability, but it would not necessarily lower overall costs.
It should also be noted that higher CAFE standards or high oil prices will improve the competitiveness of PHEVs. Conversely, HEV cost and performance characteristics will continue to improve, reducing the fuel-saving advantage of PHEVs. Although HEVs will be more expensive than nonhybrid vehicles, PHEVs will be significantly more expensive than HEVs. However, the low fuel consumption of PHEVs, especially the PHEV-40 type, will be advantageous in helping the United States reduce its dependence on imported oil. Also, once the carbon intensity of grid electricity is reduced, PHEVs will be able to significantly reduce greenhouse gas (GHG) emissions from the light-duty vehicle sector.










