CHAPTER 1
Methane Hydrate Research in the United States
Ensuring reliable sources of natural gas is of significant strategic interest to the United States. Natural gas is the cleanest of all the fossil fuels, emitting from 25 to 50 percent less carbon dioxide than either oil or coal for each unit of energy produced.1 In recent years, natural gas has supplied approximately 20-25 percent of all energy consumed in the United States. In 2008, for example, a total of about 23 trillion cubic feet (TCF)2 of natural gas was used to supply heat and electrical power to various sectors of the economy, with domestic natural gas providing approximately 85 percent of this volume (EIA, 2009a,b). The relatively clean environmental footprint for combustion, the potential for securing significant domestic supplies, and the compatibility with existing infrastructure indicate that natural gas can be a cornerstone of an environmentally and economically sound domestic energy portfolio.
Accumulations of methane hydrate, a solid form of natural gas, may represent an enormous source of methane. Methane hydrate occurs in sediments within and below thick permafrost in Arctic regions and in the subsurface of most continental margins where water depths are greater than
|
1 |
http://www.eia.doe.gov/bookshelf/brochures/greenhouse/Chapter1.htm. |
|
2 |
651 × 109 m3. The available literature on methane hydrate employs a mix of metric and English units, appropriately reflecting international and domestic contributions to this field of study. This report uses the original measurement unit of the cited reference, whether metric or English, followed by a conversion to the other unit of measure. For the reader’s interest, Appendix D contains a comparison of units of measurement of amounts of methane by volume and by weight. |
about 1,500 feet (about 500 meters) (Figure 1.1; Box 1.1). Although the estimated total global volume of methane in methane hydrate is still debated, generally acknowledged estimates yield figures between 2 and 10 times greater than those of technically recoverable conventional natural gas resources (see Chapter 2). The existence of such a large and as-yet untapped methane hydrate resource has provided a strong global research incentive to determine how methane from methane hydrate might be produced as a technically safe, environmentally compatible, and economically competitive energy resource (e.g., Council of Canadian Academies, 2008).
Although methane is a cleaner-burning energy source than other fossil fuels, it is itself a significant greenhouse gas, about 25 times more potent per molecule than carbon dioxide on a 100-year basis (International Energy Administration, 2009). Thus, understanding the potential environmental impacts of methane hydrate degassing3 and the seafloor hazard (“geohazard”) potential resulting from methane hydrate dissociation, whether through natural processes or through oil and gas drilling and production, is also important as its potential for commercial production is considered and tested.
NATIONAL APPROACH TO METHANE HYDRATE RESEARCH AND DEVELOPMENT
The Department of Energy (DOE), through congressional authorization in the Methane Hydrate Research and Development Act of 2000 (P.L. 106-193), and as reauthorized in the Energy Policy Act of 2005 (P.L. 109-58) (Appendix A), has led a national research effort to understand (1) the physical nature of methane hydrate occurrences in sedimentary rock layers in offshore and in permafrost areas, (2) methods to quantify and explore for methane hydrate accumulations in nature, (3) the stability and behavior of methane hydrate when disturbed by drilling and production, (4) the technological requirements to produce methane from methane hydrate, and (5) the potential environmental impacts of methane
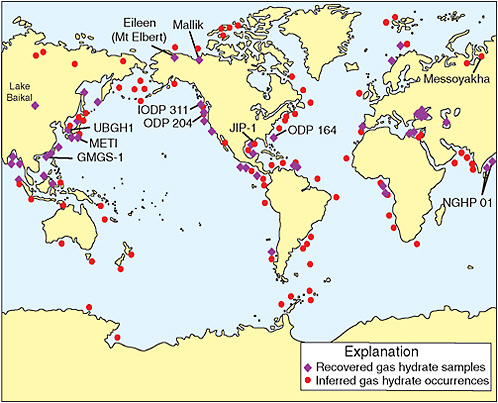
FIGURE 1.1 Worldwide locations of methane hydrate occurrences show the location of sampled and inferred methane hydrate in oceanic sediment of outer continental margins and permafrost regions. Many of the recovered methane hydrate samples have been obtained during deep coring projects or seafloor sampling operations. Most of the inferred methane hydrate occurrences are marine sites at which bottom-simulating reflectors have been observed on available seismic profiles. The methane hydrate occurrences reviewed in this report have also been highlighted on this map. Numbers adjacent to abbreviated site locality names identify project or drilling legs. GMGS = Guangzhou Marine Geological Survey; IODP = Integrated Ocean Drilling Program; JIP = joint industry project (Department of Energy Methane Hydrate Program supported); METI = Ministry of International Trade and Industry of Japan; NGHP = National Gas Hydrate Program of India; ODP = Ocean Drilling Program; UBGH = Ulleung Basin Gas Hydrate. Modified from Keith Kvenvolden and others from the U.S. Geological Survey.
|
BOX 1.1 The Basics of Gas Hydrate and the Importance of Methane Hydrate Gas hydrate is an ice-like substance that forms when gas, at high concentrations, and water come into contact at high pressures and low temperatures (e.g., 60 bars, 4°C). Gas hydrate is composed of water molecules that bind together by hydrogen bonds to form a network of cages of various sizes. Small gas molecules such as methane, propane, and carbon dioxide (“guest” molecules) initiate cage formation and may become trapped in these cages (see opposite page). Other cages may remain vacant. Typically, large hydrate cages are more than 95 percent full of guests, while small cages are around 50 percent full of guests. The most common, naturally occurring gas hydrate structure is known as structure l (“sl”; see opposite page), which contains methane “guest” molecules. Therefore, gas hydrate occurring naturally in permafrost and marine sediments (see images on third page of box) is often referred to as methane hydrate.a Microbial methanogenesis (the decay of organic matter at shallow depths and low temperatures) is commonly the source of the methane stored in these hydrates. The formation of other gas hydrate structures (e.g., sll and sH, which are not discussed further because these structures are less common in nature than sl) requires additional components of heavier hydrocarbon gases, which are minimally formed during methanogenic gas production. The existence of these heavier components may indicate a thermogenic gas source. Thermogenic processes occur at higher temperatures and greater depths within sedimentary rocks where buried organic material is thermally altered into liquid and gaseous hydrocarbons. Although most of these hydrocarbons may remain at depth as “conventional” oil and natural gas accumulations, some of the gases, including methane, may also migrate to shallow depths and form methane hydrate if appropriate pressure and temperature conditions and sufficient free water exist. An important difference between methane hydrate deposits and those of “conventional” gas accumulations is the nature of the sedimentary rocks within which the gas is found: conventional natural gas fields trap gas in porous sedimentary beds, surrounded by impermeable rocks; methane hydrate deposits occur in relatively unconsolidated sediments where the ice-like hydrate structure itself serves as the trap for individual gas molecules. These characteristics add challenges to producing methane from methane hydrate—hence the description of methane hydrate as an “unconventional” gas resource. |
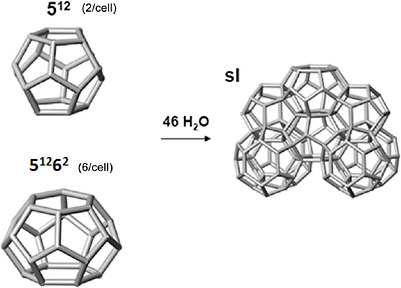 The structure l hydrate unit cell (unit cell = smallest repeating unit of the hydrate crystal) contains 46 water molecules and is composed of two small water cages (512) and six large water cages (51262). The water cages can trap gas molecules (not shown). SOURCE: Koh and Sloan (2008). Because methane is trapped within the hydrate crystal structure, methane gas in hydrate is greatly compressed. This results in an “energy density” for methane hydrate up to 164 volumes of gas per volume of hydrate (at standard temperature and pressure, or STP) which can be substantially higher than the energy density for conventional gas reservoirs at the same depth. Because the occurrence of methane hydrate is related to specific pressure-temperature conditions, increasing temperature and/or decreasing pressure cause the hydrate to become unstable and “dissociate,”b producing methane gas and water (see graphs on next facing page). This dissociation process can take place naturally, because of changing geologic conditions, or may be induced, for example, by drilling through methane hydrate to reach conventional oil and gas or methane hydrate deposits. These dissociation processes may also have environmental and drilling-safety impacts that need to be recognized and understood. |
 Images showing different types of methane hydrate occurrences: (left) disseminated within pore-space of sand deposits (from Mount Elbert, Alaska North Slope), (right) layered methane hydrate occurrence from drillcore on Southern Hydrate Ridge (ODP Leg 204); sample about 1 centimeter in thickness. SOURCES: (a) Mount Elbert Science Team, photo by E. Rosenbaum (http://energy.usgs.gov/images/gashydrates/MtElbert_coresample2LG.jpg); (b) Tréhu et al. (2003) ODP Leg 204 volume (http://www-odp.tamu.edu/publications/204_IR/chap_02/c2_f11.htm). Facing page caption: Diagrams showing the depths within and below the permafrost or below sea level at which methane hydrate is stable. The geothermal (or hydrothermal) gradient (red dashed lines) is the change in temperature with depth. Methane hydrate can occur in the yellow envelope where the pressure (related to depth) and temperature are favorable for methane hydrate stability. In permafrost areas (left), the zone (yellow envelope) in which methane hydrate can exist in sediments lies between depths of about 200 and 1,100 meters (about 650-3,600 feet). In continental margins offshore (right), methane hydrate can occur, in this example, to a sediment depth of about 1,500 meters (about 4,900 feet). Although the methane hydrate stability zone extends above the seafloor, methane hydrate generally does not occur in the water column above the seafloor because the methane concentrations |
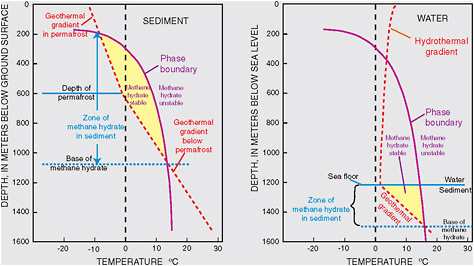 are typically too low to form methane hydrate and methane hydrate is buoyant in seawater. In both permafrost and continental margin cases hydrostatic pressure dominates the pressure regime and accounts for the similar shapes of the phase boundaries. SOURCE: Kvenvolden (1988).
|
degassing. Public Law 109-58 specified that DOE establish a National Research Council (NRC) study to assess the progress made by the DOE Methane Hydrate Research and Development Program (hereafter referred to as “the Program”) through the year 2009, with focus on the period since the last review of the Program by the NRC (2004). In response to DOE’s request, the NRC established the Committee on Assessment of the Department of Energy’s Methane Hydrate Research and Development Program to address the issues outlined in the study’s statement of task (Box 1.2). The committee consists of nine experts who contributed
|
BOX 1.2 Statement of Task The Energy Policy Act of 2005, Section 968, calls for the Secretary of Energy to enter into an agreement with the National Research Council to (1) conduct a study of the progress made under the Methane Hydrate Research and Development (R&D) Program, and (2) make recommendations for future methane hydrate R&D needs. Specifically, the study will
|
their professional expertise in areas of biogeochemistry; organic, environmental, and experimental geochemistry; geomechanics; geophysics; marine geology; oceanography; oil and gas exploration and production, including drilling in methane hydrate–bearing targets on land and at sea; petroleum engineering; and risk analysis (Appendix B).
This report constitutes this committee’s response to the study charge. This chapter provides the framework in which the committee examined the Program by reviewing briefly the Program highlights in the period from fiscal years 2000 to 2005 and some of the primary activities the Program has
|
undertaken since the 2004 NRC report was issued. Chapter 2 discusses the current state of methane hydrate research domestically and internationally through the description of recent, important experimental, theoretical, and field-based discoveries that have significantly advanced understanding of methane hydrate as well as some of the key remaining research challenges. Importantly, these discoveries and challenges have helped raise the level of research awareness given to methane hydrate from one of general scientific importance with respect to environmental and geohazard concerns to one of focused research interest in methane hydrate as a potentially viable energy resource. Chapter 3 specifically examines the research portfolio of the Program, and Chapter 4 describes the organizational processes the Program employs to coordinate methane hydrate research and development in the United States. Chapter 5 presents the committee’s conclusions and recommendations regarding the Program’s future research directions.
An important difference between the emphasis of this report relative to that of the last NRC evaluation (NRC, 2004) is the fact that the Program has matured significantly in both the number and progress of its sponsored research projects in the past 5 years. At the time the last review was conducted, only a small number of research projects sponsored by the Program were at advanced enough stages to provide published results that could be used to evaluate and gauge the direction of the Program. The present report thus places significant emphasis on the Program’s currently active research areas, progress with active research projects and their results, and impacts of the Program’s sponsored research activities to draw meaningful conclusions and recommendations that might further advance the Program. Because this present report is timed to coincide with the final year of the Program’s current authorization period, the report results are intended to inform decisions regarding the Program’s future directions and resources, particularly with regard to the suitability of methane hydrate to make a contribution to domestic natural gas supply by 2025. The year 2025, as assigned to the committee in its statement of task and also cited in several of the Program’s described aims, is viewed by this committee as a convenient longer-term mark against which the Program’s achievements can be planned and evaluated, rather than as an absolute determinant of the
Program’s overall success. The committee’s recommendations thus address research that will assess whether methane from methane hydrate can be technically produced without specific consideration of the years by which specific results will be achieved. The committee acknowledges that commercial production of methane from methane hydrate in the future will depend not only on technical feasibility but also on economic, regulatory, and other issues. The committee did not address these latter factors in the course of this study because they are not part of the Program’s current technical research mandate.
THE DOE4 METHANE HYDRATE RESEARCH AND DEVELOPMENT PROGRAM, 2000-PRESENT
2000 Through 2004
Although DOE has sponsored some research on methane hydrate since at least 1982, the 2000 Methane Hydrate Research and Development Act authorized DOE to establish a focused, 5-year national program, the broad purposes of which were (1) to improve coordination in methane hydrate research among various public and private agencies and science and engineering disciplines in the United States and (2) to support basic and applied research that identified, explored, assessed, and developed methane hydrate as an energy resource. Authorized initially with about $3 million in 2000, the funding levels for the program were authorized to increase to $12 million annually by 2004 (NRC, 2004; Appendix E).
In addition to serving a constructive, coordinating role regarding interagency methane hydrate research, the Program used relatively modest resources in these initial years to solicit proposals and provide partial support for three cooperative agreements with industry, one in the Gulf of Mexico (with Chevron in the management role) and two on the Alaska North
Slope (with BP Exploration Alaska [BPXA] and Maurer/Anadarko in the management roles). Twenty-nine smaller-scale projects were supported by the Program and performed by university, institute, and national laboratory researchers; two additional projects undertaken as part of the federal collaboration effort were managed by and received primary support from the U.S. Geological Survey (Appendix F). The Program also participated in an international drilling consortium managed and coordinated by the Geological Survey of Canada (GSC) and on cruises of the Ocean Drilling Program (ODP) and cruises sponsored by industry. The project coordinated by the GSC focused on drilling and testing a methane hydrate well (the Mallik well) in Arctic Canada (see Chapter 2 for discussion), and the ODP and industry cruises were organized to drill and log methane hydrate core samples.
The three large industry projects in this initial phase of the Program were designed around strong field-based components with an aim to drill exploration- and/or production-test wells in permafrost and offshore regions. Key early, long-term goals included development of exploration and drilling techniques appropriate for methane hydrate, characterization of the physical and chemical properties of methane hydrate from drill cores, and understanding methane hydrate as a potential geohazard. These goals were necessary for both the projects and the Program to generate results that could eventually be applied by industry in a commercial production setting. Two of these cooperative projects with industry (the Chevron- and BPXA-managed projects) continue to the present time (see below, and also Chapters 2 and 3). Exploration drilling was only conducted in one project (the “Hot Ice” well of the Maurer/Anadarko project) at the time of the NRC (2004) report. Unfortunately, no methane hydrate was found in this well. Inadequate site survey planning led to the failure of that endeavor.
The other 26 projects initiated in this period used laboratory experiments, modeling, sample (drill-core) analysis, geophysical research, and technology development to address a number of the Program mandates including understanding the physical and chemical characteristics of methane hydrate in place, the behavior of methane hydrate during changes
in pressure and temperature, the development of remote-sensing5 methods to detect and quantify methane hydrate, and development of new tools to collect and analyze methane hydrate core samples (NRC, 2004). Seventeen of these projects had been completed (by design) as of the time of the present assessment (Appendix F).
The Program employed various mechanisms to oversee its research portfolio during this period. Establishing a Methane Hydrate Advisory Committee (MHAC), an Interagency Coordinating Committee, and selection and evaluation criteria for research proposals and projects were among the more encompassing of these organizational activities. Many of the findings and recommendations of the NRC (2004) report addressed these types of procedural aspects of the Program and indicated areas for improvement, and the report also underscored the critical role played by the Program in providing a national incentive to produce energy from and understand the implications of drilling through methane hydrate. The report went further to indicate that no obvious technical or engineering barriers were apparent that would deter the production of methane from methane hydrate in the future, given sufficient in-place reserves (NRC, 2004). The projects established during this initial period of the Program’s existence also established a precedent for collaboration among researchers from academia, federal agencies, research institutions, and industry, with industry and federal agencies in particular participating in cost-sharing agreements (Appendix F). The collaborations with industry are considered integral to enable future commercial-scale applications to be implemented.
2005 Through Present Day
Much of the congressional reauthorization language for the Program in the 2005 Energy Policy Act was similar to the 2000 Program authorization. Consistent themes between the two Acts included a focus on (1) basic and
applied research to develop methane hydrate as a commercial resource in an efficient and environmentally sound manner, (2) conducting exploratory drilling, (3) technology development to reduce the risk of drilling through methane hydrate, (4) mitigating the environmental impact of natural methane hydrate degassing and degassing associated with development, and (5) education and training. Procedurally, both pieces of legislation also placed importance on interagency coordination and DOE’s collaboration with other institutes, effective transfer and communication of knowledge and information, and establishment of an advisory panel of external experts (Appendix A).
Several new additions to the Program focus were also included in the 2005 language: (1) the descriptions of the research and development priorities were more nuanced, with new emphasis on remote-sensing techniques, including acquisition and processing of seismic data, to identify and characterize methane hydrate accumulations; and (2) specific exploratory drilling goals included one or more full-scale production tests in permafrost and nonpermafrost areas. The 2005 language also addressed the Program’s management and organization through identification of new graduate fellowships to support education and training, the establishment of external scientific competitive peer review as part of the proposal and grant process, and emphasis on ensuring greater participation by DOE in international cooperative projects. The role of the MHAC was also made more inclusive by indicating that the body would provide scientific oversight for the program, assess progress toward Program goals, and provide recommendations to increase the Program’s quality. The authorized appropriations over each of the fiscal years 2006-2010 were also indicated to increase above levels authorized for 2000-2004 (Appendix E).
With modest annual budgets, the DOE Program made specific efforts during 2005-2009 to enact programmatic and procedural changes to improve management and success of its sponsored research projects. These changes were implemented on DOE’s own initiative, on the basis of advice from the MHAC, and in response to recommendations in the previous NRC report. Operative visions and rationale for methane hydrate research in the nation and the role of the Program in coordinating this re-
search were also articulated in this period through several public documents (e.g., Boswell et al. [2006]; and MHAC [2007], which included the Interagency Five-Year Plan for Methane Hydrate Research and Development; see Chapter 4 for details). Two key goals articulated by the program include (1) providing by 2015 an initial assessment of the scale of the potentially commercially viable gas hydrate resource on the Alaska North Slope, and (2) demonstrating the technical recoverability and assessing the economic recoverability of marine gas hydrate–bearing sand reservoirs by 2025.6
Simultaneously with these programmatic efforts, DOE increased the number and scope of its smaller-scale research projects, established two new cooperative-agreement projects with industry, and supported the continuation of the Gulf of Mexico joint industry project managed by Chevron and the cooperative agreement on the Alaska North Slope with BPXA into more intricate phases in their planned research (Appendix F). Federal agencies and national laboratories also deepened their involvement in various collaborative research endeavors (details in Chapters 2, 3, and 4). Very broadly, then, the Program has taken specific actions in the past 5 years to increase the level and productivity of the national methane hydrate research and development that it helps to support.
COMMITTEE PROCESS
To address the study charge and establish conclusions and recommendations, the committee, in addition to its own expertise, reviewed (a) relevant DOE reports, (b) reports and other public documents from federal agencies involved in interagency methane hydrate research collaborations, (c) peer-reviewed literature on methane hydrate conducted both within and outside the auspices of the DOE program, (d) information from the DOE Web site,7 and (e) information submitted by and requested from external sources, including three public meetings (Appendix C). Public meetings included
dialogue with the study sponsors, other federal agencies, university and national laboratory researchers with projects supported by the Program, industry representatives from the large field projects, and importantly also, researchers from the Japanese methane hydrate program. In addition to discussion of research methods and results, information was also provided on the organizational and administrative process employed by the DOE program. Throughout the study process, the committee also received valuable input through informal interviews with various professionals associated with methane hydrate research and/or with various aspects of the Program, such as the MHAC members and participants in the interagency coordinating groups.
CONCLUDING REMARKS
The future U.S. energy portfolio is evolving as energy demand, greenhouse gas emissions, the energy transmission infrastructure, and national energy security issues are considered nationally and locally. Informed planning to develop consistent energy and environmental programs requires consideration of existing and emerging energy sources. With global energy demand projected to increase, unconventional resources such as methane hydrate become important to consider as part of the future U.S. energy portfolio. Methane derived from methane hydrate is an emerging resource candidate that has captured domestic and international research attention but which also presents a number of technical and environmental challenges that require attention before commercial production can be realized. These challenges include developing the technology necessary to produce methane from this unconventional gas occurrence and understanding more about methane hydrate in terms of its potential to behave as a geohazard and how degassing of methane hydrate may affect the environment. Because most of the methane hydrate research presently conducted in the United States is supported by the DOE Program and its federal partners, this report is designed to give DOE, other agencies, and policy makers a framework in which to evaluate the goals of and to determine appropriate support for
the Program in the context of the nation’s future energy and environmental needs.
REFERENCES
Boswell, R., R. Amato, R. Coffin, T. Collett, G. Dellagiarino, R. Fisk, J. Gettrust, B. Haq, D. Hutchinson, K. Puglise, P. Ray, and K. Rose. 2006. An Interagency Roadmap for Methane Hydrate Research and Development. Available online at http://www.netl.doe.gov/technologies/oil-gas/publications/ Hydrates/pdf/TnteragencyRoadmap.pdf. Accessed October 15, 2009.
Council of Canadian Academies. 2008. Energy from Gas Hydrates: Assessing the Opportunities and Challenges for Canada. Ottawa, Ontario: Council of Canadian Academies. 206 pp.
ETA (Energy Information Administration). 2009a. Natural Gas Comsumption by End Use. Available online at http://tonto.eia.doe.gov/dnav/ng/ng_cons_sum_dcu_nus_a.htm. Accessed October 15, 2009.
ETA. 2009b. Natural Gas Gross Withdrawals and Production. Available online at http://tonto.eia.doe.gov/dnav/ng/ng_prod_sum_dcu_NUS_a.htm. Accessed October 15, 2009.
International Energy Administration. 2009. Energy Sector Methane Recovery and Use: The Importance of Policy. Available online at http://www.iea.org/papers/2009/methane_brochure.pdf. Accessed October 15, 2009.
Koh, C. A., and E. D. Sloan. 2008. Natural gas hydrates: Recent advances and challenges in energy and environmental applications. AIChE Journal 53(7):1636-1643.
Kvenvolden, K. A. 1988. Methane hydrate—a major reservoir of carbon in the shallow geosphere? Chemical Geology 71:41-51.
MHAC (Methane Hydrate Advisory Committee). 2007. Report to Congress: An Assessment of the Methane Hydrate Research Program and an Assessment of the 5-Year Research Plan of the Department of Energy. Available online at http://www.fe.doe.gov/programs/oilgas/hydrates/MHAC-07-ReportToCongress-final.pdf. Accessed October 15, 2009.
NRC (National Research Council). 2004. Charting the Future of Methane Hydrate Research in the United States. Washington, DC: The National Academies Press. 193 pp.
Tréhu, A. M., G. Bohrmann, F. R. Rack, M. E. Torres, N. L. Bangs, S. R. Barr, W. S. Borowski, G. E. Claypool, T. S. Collett, M. E. Delwiche, G. R. Dickens, D. S. Goldberg, E. Gràcia, G. Guèrin, M. Holland, J. E. Johnson, Y.-J. Lee, C.-S. Liu, P. E. Long, A. V. Milkov, M. Riedel, P. Schulteiss, X. Su, B. Teichert, H. Tomaru, M. Vanneste, M. Watanabe, and J. L. Weinberger. 2003. Proceedings of the Ocean Drilling Program, Initial Reports 204.


















