4
Power Train Technologies for Reducing Load-Specific Fuel Consumption
Technologies for reducing fuel consumption of medium-and heavy-duty vehicles depend on the power train type. For instance, replacing gasoline engines with diesel engines in medium-duty trucks is a very effective technology, but heavy-duty trucks are already more than 99 percent dieselized. This chapter discusses the energy balance for a typical diesel engine that leads to a resulting brake power or brake thermal efficiency. It presents technologies for improving the efficiency of diesel and gasoline engines (including fuels and emission systems) as well as technologies for transmissions and drive axles. It also discusses the role of hybrid power trains (electric and hydraulic) in reducing fuel consumption.
DIESEL ENGINE TECHNOLOGIES
Diesel engines use the high gas temperatures generated by compression as the ignition source. The timing of ignition is determined mainly by when the fuel is injected. These engines operate on the four-stroke-cycle principle and are arranged either in-line or in a “vee” configuration. Displacements range from 3.0 to 16.0 liters. These engines typically burn diesel fuel, and also some kerosene and some biodiesel blends. Some engines that were originally designed as diesel engines are converted to use spark ignition to take advantage of alternative fuels. These engines typically burn gaseous fuels such as compressed natural gas (CNG), liquefied natural gas (LNG), or propane, but other spark ignition fuels can also be used. Essentially all of the diesel engines used today in medium- and heavy-duty vehicles are turbocharged, direct fuel injected, and electronically controlled; most are intercooled or after cooled. In addition, they use exhaust gas recirculation (EGR) to limit in-cylinder formation of nitrogen oxides (NOx) and some form of exhaust aftertreatment (diesel oxidation catalyst [DOC] diesel particulate filter [DPF], or other system) to control particulate matter (PM) emissions. Starting in 2010, most diesel engines will add selective catalytic reduction systems (SCR) as a form of NOx aftertreatment to meet 2010 requirements. A typical diesel engine energy audit is shown in Figure 4-1, where the fuel energy is converted to brake power and the efficiency associated with the output power will be referred to as brake thermal efficiency. The accessory losses are for engine-driven pumps that are necessary to run the engine on a dynamometer or on the road (fuel, lubricating oil, cooling water). Auxiliary loads such as alternator, air compressor, and power steering pump will use a portion of the brake power.
The following material summarizes various technologies for reducing fuel consumption from diesel engines. Some of the engine technologies listed here are the products of participants in the multiagency, multicompany 21st Century Truck Partnership. The partnership’s goals for engines are to achieve 50 percent thermal efficiency, while meeting 2010 emissions standards, by 2010 and to develop technologies to achieve 55 percent thermal efficiency by 2013 (NRC, 2008).
Turbochargers
In a turbocharger the radial exhaust-driven turbine drives the radial compressor to increase the air density going into the engine. The turbochargers can have a fixed geometry or more commonly a variable geometry turbine, or they can have a “wastegated” turbine (a bypass). Improved efficiency of the compressor or turbine will improve fuel consumption. Higher pressure ratio radial compressors or axial compressors are emerging technologies. Improvements in compressor efficiency and/or turbine efficiency can contribute to improved fuel consumption. A presentation1 to the committee on Japan’s Top Runner fuel efficiency regulation estimated 0.3 to 0.5 percent improvement from increased supercharging efficiency. The TIAX investigation, by contract to the committee, put the improvement at up to 2 percent (TIAX,
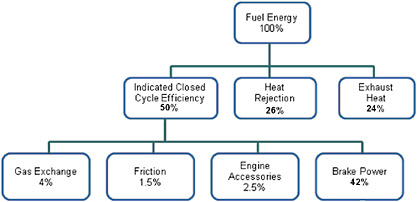
FIGURE 4-1 Energy audit for a typical diesel engine. SOURCE: Adapted from Vinod Duggal, Cummins, Inc., “Industrial Perspectives of the 21st Century Truck Partnership,” presentation to the committee, Dearborn, Mich., April 6, 2009, Slide 14 (and TIAX (2009), p. 4-3, Table 4-1).
2009, pp. 3-5 and 4-14). NESCCAF/ICCT (2009, p. 83) estimates the fuel savings of an improved-efficiency single-stage turbocharger at 1 percent. Another source projects that a high-pressure-ratio axial compressor will reduce fuel consumption by 1.1 to 3.6 percent.2
Almost all heavy-duty diesel engines sold in North America today use high-pressure loop EGR for control of engine-out NOx levels. To get EGR to flow from the exhaust manifold to the intake manifold, the pressure in the exhaust manifold must be higher than the pressure in the intake manifold. When the exhaust manifold pressure is higher than the intake manifold pressure, this is called having a negative ∆p, where ∆p refers to the difference in pressure between the intake and exhaust manifolds. High-efficiency turbochargers naturally produce a positive ∆p over much of their operating range, so turbocharger efficiency must be intentionally compromised in order to facilitate EGR flow. If it is possible to produce adequate EGR flow without reducing turbo efficiency, the overall engine efficiency will increase.
Dual-Stage Turbocharging with Intercooling
Modern engines use high-pressure ratios, which limit the efficiency of turbochargers. Using two turbochargers in series with intercooling would allow higher turbocharger efficiency, but this adds cost and packaging complexity and requires an EGR pump or other device such as a turbocompound system to facilitate EGR flow. Air-to-water intercooling is used after the first-stage compressor, in some applications, and air-to-air aftercooling is used after the second-stage compressor.
Conventional two-stage turbocharging employs two turbochargers working in series at all times. True sequential turbocharging switches turbochargers in and out of use as required, but they are normally connected in parallel. A modulated two-stage system brings some of the benefits of each of these two approaches. At low engine speeds it works as a two-stage system, delivering high-boost pressure despite the low engine speed. At high engine speeds it bypasses the small high-pressure turbocharger, allowing the bigger, low pressure turbocharger to work on its own and produce the higher flows at high engine speeds. Modulated two-stage systems offer the benefits of both high-boost pressure and wide-flow range, mainly due to the fact that two different-sized compressors are used. Using two compressors replicates the effect of a variable compressor without the need for a complex housing. The modulated two-stage system can have a high-pressure turbocharger far smaller than that of a conventional two-stage system, improving transient performance by reducing the turbo lag that affects both drivability and emissions.3
Dual-stage turbocharging is used in production by Navistar, Daimler Trucks, and Caterpillar in the United States and by MAN and Mercedes in Europe. Ford has announced that the 2011 diesel engine used in its Class 2b to 7 trucks will use a twin-compressor turbocharger (back-to-back compressors on the same shaft). Another source estimates a 2 to 5 percent reduction in fuel consumption.4 These benefits are only available if a way to provide the required EGR flow is available.
Mechanical Turbocompound
The base turbocharged engine remains unchanged and a power turbine is added to the exhaust stream to extract additional energy from the exhaust. The power turbine is connected to the crankshaft to supply additional power (NESCCAF/ICCT, 2009, p. 81). Typically, the attachment includes a fluid coupling (to allow for speed variation and to protect the power turbine from engine torsional vibration) and a gear set to match power turbine speed to crankshaft speed. Published information on the fuel consumption reduction from mechanical turbocompounding varies, as evidenced by the following: 3 percent, according to the Detroit Diesel Corporation,5 which has a turbocompound engine in production; 2.5 to 3 percent (NESCCAF/ICCT, 2009, p. 54); 3 percent (K.G. Duleep, Energy and Environmental Analysis)6 and 4 to 5 percent (Kruiswyk, 2008, pp. 212-214); TIAX (2009, pp. 4-17) used 2.5 to 3 percent. Some of these differences may depend on the operating condition or duty cycle that was considered by the different researchers. The performance of a turbocompound system tends to be highest at full load and much less or even zero at light load.
Electric Turbocompound
This approach is similar in concept to mechanical turbocompound, except that the power turbine drives an electrical generator (NESCCAF/ICCT, 2009, p. 29). The electricity produced can be used to power an electrical motor supplementing the engine output, to power electrified accessories, or to charge a hybrid system battery. Electric turbocompound is a technology that fits particularly well with a hybrid electric power train for long-haul applications where regenerative braking opportunities are limited. The benefits of electric turbocompound and an electric hybrid power train can be additive. Energy and Environmenal Analyis7 has said that “electric turbo-compound is more efficient and possible as part of hybrid packages.” Fuel consumption reduction benefits as large as 10 percent are claimed. The NESCCAF/ICCT study (p. 54) modeled an electric turbocompound system and estimated benefits at 4.2 percent, including electrification of accessories. Caterpillar, Inc., as part of Department of Energy (DOE) funded work, modeled a system that showed 3 to 5 percent improvement, while John Deere investigated a system (off-highway) that offered 10 percent improvement (Vuk, 2006; TIAX, 2009, p. A-10). None of these systems have been demonstrated commercially. TIAX (2009, pp. 3-5) used a range of 4 to 5 percent for its estimates, which included the benefits of electric accessories. Achieving the full benefit of electric turbocompound requires the electrification of vehicle accessories, the addition of an electric motor to apply turbocompound energy to supplement engine output, and an electric storage system (battery) to store any energy from the power turbine that is not immediately required. Making all of these changes to the vehicle will pose significant development and cost challenges.
Variable Valve Actuation
Variable valve actuation (VVA), also called variable valve timing or discrete variable valve lift, allows the valve actuation to be adjusted independently from the crankshaft angle. There are many implementations of VVA. Some are hydromechanical, such as the system used on some BMW passenger car engines. Other designs use electromagnets or high-pressure hydraulic systems. Some versions offer “full authority,” or unlimited, control of valve timing and lift, while other implementations offer limited control, such as variable duration only, variable lift only, or even more limited control, such as with the system used on some Caterpillar engines to permit a Miller cycle to be used under some operating conditions. VVA technology can also be used for cylinder deactivation. One of the primary drivers for introducing VVA in diesel engines is to facilitate the use of nonconventional combustion modes. According to several sources, variable valve timing can improve fuel consumption by about 1 percent when standard diesel combustion is used (NESCCAF/ICCT, 2009, p. 55).
Low-Temperature Exhaust Gas Recirculation (Also Called Advanced EGR Cooling)
Most medium- and heavy-duty vehicle diesel engines sold in the U.S. market today use cooled EGR, in which part of the exhaust gas is routed through a cooler (rejecting energy to the engine coolant) before being returned to the engine intake manifold. EGR is a technology employed to reduce peak combustion temperatures and thus NOx. Low-temperature EGR uses a larger or secondary EGR cooler to achieve lower intake charge temperatures, which tend to further reduce NOx formation. If the NOx requirement is unchanged, low-temperature EGR can allow changes such as more advanced injection timing that will increase engine efficiency slightly more than 1 percent (NESCCAF/ICCT, 2009, p. 62). Because low-temperature EGR reduces the engine’s exhaust temperature, it may not be compatible with exhaust energy recovery systems such as turbocompound or a bottoming cycle.
Electrification of Engine-Driven Accessories
Accessories that are traditionally gear or belt driven by a vehicle’s engine can be converted to electric power. Ex-
amples include the engine water pump, the air compressor, the power-steering pump, cooling fans, and the vehicle’s air-conditioning system. In many cases this can result in a reduction in power demand, because electrically powered accessories (such as the air compressor or power steering) operate only when needed if they are electrically powered, but they impose a parasitic demand all the time if they are engine driven. In other cases, such as cooling fans or an engine’s water pump, electric power allows the accessory to run at speeds independent of engine speed, which can reduce power consumption. Electrification of accessories can individually improve fuel consumption, but as a package on a hybrid vehicle it is estimated that 3 to 5 percent fuel consumption reduction is possible.8 The TIAX (2009, pp. 3-5) study used 2 to 4 percent fuel consumption improvement for accessory electrification, with the understanding that electrification of accessories will have more effect in short-haul/urban applications and less benefit in line-haul applications.
Engine Friction Reduction
Reduced friction in bearings, valve trains, and the piston-to-liner interface will improve efficiency. Any friction reduction must be carefully developed to avoid issues with durability or performance capability. An example would be to develop heavy-duty diesel engines to run on 10W-30 oil instead of the current standard of 15W-40. The lower viscosity oil would reduce friction, at the expense of bearing capability. Fuel consumption improvement from one source9 was 2 percent, whereas another source10 claims 1 to 1.5 percent. The use of a thermatic oil cooler (thermostatically controlled oil cooler) in conjunction with lower viscosity lubricating oils could yield 1.5 percent improvement.11 The effect of friction reduction and oil temperature control will be greatest during cold starts and under light load operation, where friction accounts for a larger portion of total energy consumption.
Alternative Combustion Cycles
Alternatives to the standard diesel combustion cycle are available, such as low-temperature combustion (LTC), homogeneous charge compression ignition (HCCI), and premix charge compression ignition (PCCI). These combustion modes can be more efficient than standard diesel combustion under some conditions, particularly when very low NOx is a requirement. There are significant control requirements to make these alternative combustion modes work, and these modes cannot generally be used over the whole operating range of the engine, nor have they demonstrated inherent fuel consumption advantages (NRC, 2008, Finding 3-8, p. 42). The primary purpose of alternative combustion cycles is to lower engine-out emissions. This can lead to either lower overall emissions or lower cost for exhaust aftertreatment.
Effects of DPF and SCR on Engine Efficiency
The use of emissions control devices has an influence on engine efficiency. This is true whether the emissions are controlled on an in-cylinder basis or via the aftertreatment. In most cases, the effect of adding an emissions control device increases fuel consumption, either directly by reducing the efficiency of energy extraction from the combustion process or indirectly by requiring the use of additional fuel to maintain the performance of an aftertreatment system.
Improved SCR Conversion Efficiency
NOx is formed in a reaction that occurs naturally whenever nitrogen and oxygen are heated above a certain temperature. The higher the temperature, the more rapid the NOx-forming reaction occurs. In-cylinder technologies to control NOx formation in diesel engines are aimed at reducing the maximum temperature reached by the gases in the combustion chamber. The approaches used include retarded injection timing, multiple injection events and injection rate shaping, EGR, charge air cooling, and alternative combustion modes (such as HCCI, PCCI, LTC). Some of these approaches leads to a decrease in work output of the engine due to exhaust emissions control (NRC, 2008), except charge air cooling.
The DPF is used to eliminate PM on an aftertreatment basis. A DPF requires energy for regenerating the filter on a periodic basis. This energy most commonly comes from injecting diesel fuel into the exhaust stream. By definition, fuel injected into the exhaust stream will not contribute to crankshaft power and thus represents a decrease in efficiency. A DOC or other device oxidizes the fuel in the exhaust stream, providing the heat required for DPF regeneration and increasing the fuel consumption of the vehicle. Another method to provide the heat required for DPF regeneration is to revise the air/fuel ratio of the engine to produce exhaust constituents and heat that are used to regenerate the DPF. This approach also increases the fuel consumption of the vehicle.
The SCR aftertreatment system for reducing NOx also requires a fluid, which is urea mixed with water (called Adblue in Europe and DEF [Diesel Exhaust Fluid] in the United States), to supply the reducing agent. The urea is made from natural gas. The energy use of this fluid and/or its cost must be accounted for in the calculation of energy consumption. The use of SCR can allow a higher engine-out NOx level, which in turn can be used to reduce fuel consumption, but
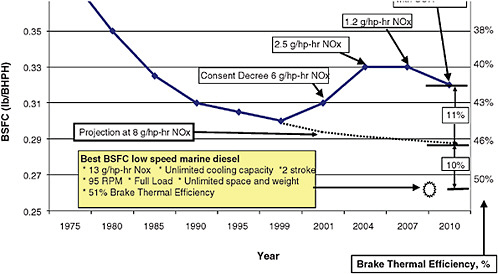
FIGURE 4-2 Historical trend of heavy-duty truck engine fuel consumption as a function of NOx requirement. SOURCE: Tony Greszler, Volvo, October 2009.
this improvement must be weighed against the urea consumption of the SCR aftertreatment system. The upcoming 2010 heavy-duty emissions standards reduce the allowable NOx level by a factor of 6 from 1.2 g/hp-hr to 0.2 g/hp-hr, which limits the ability of some manufacturers to use SCR to increase engine-out emissions.
There is a close relationship between emissions control requirements and fuel consumption. In particular, certain technologies that are used to control NOx emissions have the effect of increasing fuel consumption. See Figure 4-2 for an example of this trend. Figure 4-2 also compares truck engines with the most efficient large marine engines, which so far do not face any emissions constraints. The efficiency of large marine engines is due to several factors that cannot be reproduced in vehicle applications. Marine engines are very large and heavy, they run at very low speeds, they have a source of unlimited cooling capacity (seawater), and they face (for the time being) no emissions constraints. All four of these factors contribute to the high efficiency of marine engines. According to information provided by Volvo,12 the fuel consumption of truck diesel engines is about 10 percent higher than for marine engines due to the size, weight, and cooling factors that are limits faced in vehicle applications. The 2010 model truck engines will suffer an 11 percent fuel consumption penalty for NOx control (compared with an 8 g/hp-hr NOx engine, see Figure 4.2), which is less than the penalty of 2002 and 2007 engines. This penalty does not include the energy content of DEF (urea) for the SCR system.
Most 2010 engines will use SCR aftertreatment to reduce NOx emissions. Daimler’s Detroit Diesel estimates that, with the installation of SCR in 2010, fuel consumption will be reduced 3 to 4 percent by a combination of higher engine-out NOx, controls and fuel system improvements, reduced DPF regeneration frequency, and other efficiency gains, while Volvo estimates the potential improvement of its 2010 engines at 2 percent. As the SCR system conversion efficiency improves, it allows higher engine-out NOx emissions. Engine manufacturers can take advantage of this by making changes in fuel injection timing or by using less EGR, in order to reduce fuel consumption.
Thermal Insulation of Ports and Manifolds
Thermal insulation would reduce heat rejection to the engine coolant (from exhaust ports) or to the ambient air (from manifolds). The energy retained in the exhaust can
be used by downstream devices such as a turbocompound system or bottoming cycle. Caterpillar Inc. made components, such as air gap pistons and exhaust port liners as part of the 21st Century Truck Partnership, but reported results are not available (NRC, 2008, p. 30). The anticipated benefit is small.
Improved Work Extraction from Combustion Process
The compression ratio, expansion ratio, combustion chamber shape, injection spray pattern, injection pressure, injection timing, injection rate shaping, air/fuel mixing, peak cylinder pressure limit, air/fuel ratio, and EGR rate are all parameters that can be modified in an effort to reduce fuel consumption. Improved combustion chamber design allows for improved air management and mixing. Improved materials and structural design enable higher cylinder pressures. These enhancements allow more precise control of the timing and rate of heat release (combustion) as well as higher combustion temperatures, both of which can improve thermal efficiency. Unfortunately, higher combustion temperatures also lead to higher NOx. Combustion chamber design enhancements may require more advanced materials and a more complex machining process. More complex and expensive fuel systems allow greater control of injection pressure, timing, and rate shaping. In addition, because higher cylinder pressures must not result in higher NOx, measures must be taken to allow the improved fuel consumption without creating an increase in emissions. These measures may include improved NOx conversion efficiency by the aftertreatment, advanced fuel injection techniques (which enable more detailed control of combustion), and improved engine controls. The efficiency benefit of these improvements is estimated at 1 to 3 percent.13
Finely controlled, high-pressure fuel injection is a key enabler for more fuel efficient combustion and a cleaner, more consistent fuel burn. Current state-of-the-art systems planned for deployment in 2010 engines include very high pressure (2,000 to 2,400 bar) common rail injection systems with advanced nozzle designs that are capable of finely shaped and controlled spray, along with multiple injection events per cycle.
Potential future improvements will continue to improve control, allow more accurate timing and metering of injection with combustion events, and further increase fuel injection pressure. Improved material properties and controls could enable pressures of up to 3,000 bar in the 2015 time frame and perhaps 4,000 bar by 2020. Future systems will also utilize increasingly sophisticated injection techniques such as variable-spray nozzles, piezo-electric nozzles, or supercritical fuel injection (fuel changing instantaneously from liquid state to supercritical gaseous state at injection based on site visits). These advances may be possible in the 2013 to 2015 time frame (TIAX, 2009). Fuel injection systems were estimated on site visits to offer between 1 and 4 percent improvement in fuel consumption; Vyas et al. (2002) estimates fuel injection systems have the potential to improve fuel consumption by 6 percent, although this estimate is now several years old, and considerable improvement in fuel systems has already been made since 2002. Real-time combustion control with start of combustion sensors can also yield a fuel consumption reduction of 1 percent to 4 percent.14
Engine Electronic Controller Calibration Management
Advanced engine controls will be enabled in part by the onboard diagnostic systems that are mandated for medium and heavy trucks beginning in 2010 on one family and across the board in 2013. Increasingly sophisticated engine controls, particularly a transition to closed-loop control approaches, will enable engine efficiency improvements. Closed-loop controls will feed information about the engine’s operating regime and emissions back to the system controls. This improved feedback will allow manufacturers to optimize emissions and fuel consumption within the constraints of emissions requirements across a variety of operating conditions.
Better use of calibration tools to improve control of EGR, injection rate shapes, multiple injection events, and increased injection pressure can yield 1 to 4 percent fuel consumption reduction. These benefits are redundant with those described above for improved work extraction from the combustion process. Another feature already in use on some long-haul trucks is adding 200 lb-ft of torque in the top two transmission gears, which manufacturers claim can give 2 percent reduction in fuel consumption by reducing the need for downshifting on modest grades. With the next generation electronic controller, using model-based controls, it is predicted that another 1 to 4 percent fuel consumption reduction will be achieved.15 Note that the reductions listed in here may be repeats of reductions from previous sections, and there may be some redundancy in the percentages quoted, but the concepts and percentages presented here came from committee site visits where engineers talked about these reductions. The overall approach of more sophisticated control of the combustion process tends to include several building blocks, such as upgraded fuel system capabilities, sophisticated control algorithms, additional sensor inputs for feedback control, and technologies such as model-based controls. The benefits of this approach are often claimed by each of the individual building blocks, leading to redundant claims on the same fuel consumption benefit.
Bottoming Cycle
A bottoming cycle is basically a secondary engine that uses exhaust energy or other heat sources from the primary engine to develop additional power without using additional fuel. The energy sources used by the bottoming cycle are sources that normally go to waste in a conventional engine. A typical bottoming cycle includes the following components: a feed pump to drive the working fluid from the condenser to the evaporator (or boiler); the evaporator, which transfers waste heat energy from the primary engine to the working fluid; an expander, which takes energy from the working fluid to make mechanical power; and a condenser that rejects unused heat energy from the bottoming cycle working fluid before starting a new cycle. The power generated by the expander can be used to make electricity, which in turn can power an electric motor supplementing the engine output, power electrified accessories, or charge a hybrid system battery. Sources of energy to power a bottoming cycle can include the EGR stream, exhaust stream, charge air stream, and engine coolant circuit (NESCCAF/ICCT, 2009, pp. 85-88). Cummins, Inc. has shown a projected increase of thermal efficiency from 49.1 to 52.9 percent (7.2 percent decrease in fuel consumption) using an organic Rankine cycle. Cummins also lists turbocompounding and a Brayton cycle as alternative methods of extracting work from unused energy in the exhaust stream. Cummins reports recovering 2.5 thermal efficiency points from the exhaust and 1.3 thermal efficiency points from the coolant and EGR stream.16 The NESCCAF/ICCT report (2009, pp. 55-56) showed the effect of a steam bottoming cycle to reduce fuel consumption by up to 10 percent.
Other Technologies
Other technologies for reducing the fuel consumption of diesel engines are discussed in the press almost every day (e.g., Automotive News, Transport Topics, Diesel Fuel News, DieselNet.com). Some are emerging technologies and may not become production feasible, including new diesel engines of two-stroke-cycle design, split-cycle design, free-piston design, rotary design and camless engines with digital valve control such as the Sturman Industries concept. The list of potential technologies also includes oxygen injection into the intake air, hydrogen injection into the intake air, air injection from the air compressor to overcome turbo lag, or the use of fuel-borne catalysts such as platinum and cerium.
Diesel Engine Summary
In summary, to add up all these individual potential reductions to arrive at an overall potential fuel consumption reduction would not be correct because there would be double counting of some effects. The best recent attempts at packaging fuel-saving technologies for engines were in the DOE programs with a goal of demonstrating 50 percent thermal efficiency while meeting 2010 emissions. The National Research Council (2008) review of that program showed a baseline thermal efficiency of 42 percent with a goal of 50 percent, or a 19 percent improvement in thermal efficiency and a 16 percent fuel consumption reduction. Three engine manufacturers—Caterpillar, Cummins, and Detroit Diesel—were funded at a level exceeding $116 million over five years with the following result, according to the report: “These results show that none of the industry partners achieved the goal of measuring 50 percent thermal efficiency at 2010 emissions from a complete engine system.”
Cummins has supplied the committee with the following research roadmaps for achieving 49.1 percent thermal efficiency and 52.9 percent thermal efficiency. Figure 4-3 is a research roadmap for 49.1 percent thermal efficiency by 2016, which is an improvement of 17 percent from the 42 percent baseline (14.5 percent reduction in fuel consumption). Figure 4-4 is a research roadmap for 52.9 percent thermal efficiency by 2019, which is an improvement of 26 percent from the baseline 42 percent (20.6 percent reduction in fuel consumption). These roadmaps can be compared to the baseline shown in Figure 4-1. Note that these are plans and goals, not actual development results. Actual results that will be achieved in development may vary from the planned benefits.
In its report for the committee, TIAX (2009, Tables 5-8 and Table 5-9) summarized the diesel engine fuel consumption potential reductions by range of years and by application as shown in Table 4.1.
GASOLINE ENGINE TECHNOLOGIES
Gasoline engines operate with a premixed charge of fuel and air and use spark ignition to start the combustion process. They are used in many Class 2b applications as well as Class 3 to 6 applications. Within the medium-duty truck sector, all gasoline engines operate on the four-stroke-cycle principle and are of an in-line or “vee” configuration. Displacements of these engines typically range from 6 to 8 liters. These engines normally burn gasoline, but with slight changes they can burn natural gas (compressed [CNG] or liquefied [LNG]), propane, hydrogen, ethanol, methanol, and so forth.
The fundamental operating principle for gasoline engines used today relies on creating a well-mixed charge of gasoline and air at the time the spark plug fires. After the combustion process is over, a catalyst in the exhaust system is used to perform the final emissions cleanup. Emissions of NOx, carbon monoxide, and unburned hydrocarbons are the principal species being treated by the catalyst in the exhaust. The three-way catalyst treats all three of these emissions simultaneously; however, the three-way catalyst will function
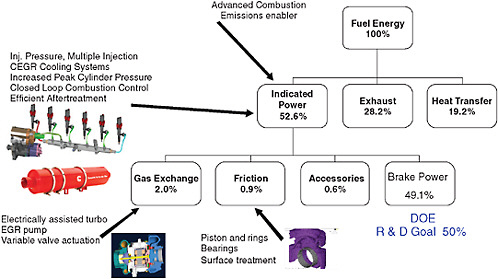
FIGURE 4-3 Research roadmap for 49.1 percent thermal efficiency by 2016. SOURCE: Provided under license by Cummins Inc. Copyright 2009 Cummins Inc. All rights reserved.
properly only if the air/fuel ratio is carefully controlled to be chemically correct, or stoichiometric.17
Heavy-duty gasoline engines, and most heavy-duty engines using spark-ignited alternative fuels such as natural gas, use a relatively simple emissions control strategy. The engine operates at a stoichiometric air/fuel ratio across most of the operating range, with relatively high engine-out emissions levels. A three-way catalyst is used both to oxidize hydrocarbon and carbon monoxide emissions and to reduce NOx. The three-way catalyst can function properly only if the air/fuel ratio is carefully controlled in order to meet current and future emissions requirements. Additional considerations for gasoline engine emissions control include achieving rapid catalyst light-off on startup and controlling evaporative emissions, but the basic emissions control technology for spark-ignited engines is the relatively simple and inexpensive three-way catalyst.
There is a fuel consumption penalty that comes with the three-way catalyst used on gasoline engines. Because the air/fuel ratio must be maintained at stoichiometric all the way down to idle, the pumping losses from throttling are large. Lean operation could provide significant fuel savings but would not allow the NOx reduction function of the three-way catalyst to work. Many technologies that could be applied to gasoline engines to reduce fuel consumption are not used, primarily because of the need to maintain low NOx emissions. For example, lean gasoline direct injection (GDI) has the potential to provide double-digit percentage reductions in fuel consumption, but it is not used, because it would result in higher NOx emissions. The NOx emissions of a lean GDI engine could be much lower than those of an unregulated engine, but engine makers have not been able to make lean GDI reach the very stringent U.S. NOx standards applied to new cars and trucks today. GDI has been used in Europe, where NOx is less stringently regulated.
One consequence of requiring a stoichiometric mixture of air and fuel is that the intake airflow needs to be throttled for lighter load operation. Lighter loads necessitate a lower fuel flow rate into the engine, and since the air/fuel mixture is to be maintained in stoichiometric proportions, the airflow rate needs to be reduced in proportion to the fuel flow rate. The process of throttling the intake airflow results in significant pumping losses that are not present in diesel engines that operate using traditional diesel combustion. This pumping loss is one of the main reasons spark ignition engines are less efficient than diesel engines. The magnitude of the pumping loss depends on the operating duty cycle of the engine. If the engine spends most of its time in light load operation, its throttling losses will be higher than for an engine that spends most of its operation under heavier load. Also, the pumping work will depend on the engine size relative to the vehicle. A smaller engine size in a given vehicle application will spend a higher portion of its operation at a higher load, relative to a
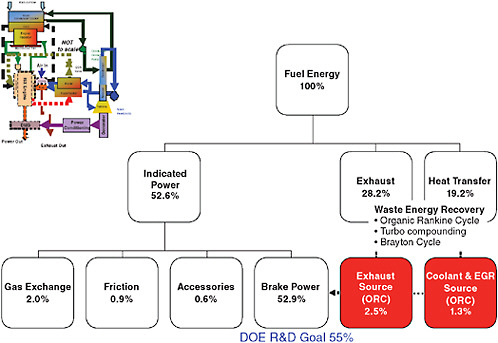
FIGURE 4-4 Research roadmap for 52.9 percent thermal efficiency by 2019. SOURCE: Provided under license by Cummins Inc. Copyright 2009 Cummins Inc. All rights reserved.
TABLE 4-1 Diesel Engine Fuel Consumption (percentage) by Years and Applications
|
Application |
2013-2015 |
2015-2020 |
|
Tractor trailer |
10.5 |
20 |
|
Class 6 box truck |
9 |
14 |
|
Class 6 bucket truck |
7.2 |
11.2 |
|
Refuse truck |
10.5 |
14 |
|
Urban bus |
9 |
14 |
|
Motor coach |
10.5 |
20 |
|
Class 2b pickup and van |
14 |
23 |
|
SOURCE: TIAX (2009). |
||
larger engine in the same vehicle, and consequently will have a lower pumping loss than the corresponding larger engine. As an approximate guide, pumping losses might range from 2 to 5 percent of the fuel energy (Patton et al., 2002).
Compared to diesel engines, spark ignition engines are generally simpler and less expensive, they have more effective and lower cost exhaust emissions aftertreatment systems, and they have higher fuel consumption.
The current emphasis in the development of spark ignition engines is on reducing fuel consumption. Figure 4-5 gives a qualitative partitioning of the fuel energy for a typical gasoline-fueled vehicle. This is analogous to Figure 4-1, which gives an energy partitioning for diesel-powered vehicles. Figure 4-5 is illustrative in describing the technologies being considered to reduce gasoline engine fuel consumption.
The proportion of the fuel energy that gets converted into indicated work is a direct measure of the engine’s fuel conversion efficiency. Factors that affect an engine’s fuel conversion efficiency include irreversibilities18 in the combustion process, the amount of energy leaving the engine cylinder as heat transfer, and the energy remaining in the exhaust at the end of the expansion process. These losses represent fuel energy that did not get converted into useful shaft work. Not all of the energy that was converted into work in the combustion process makes it to the final shaft output.
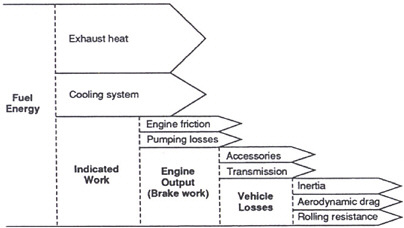
FIGURE 4-5 Partitioning of the fuel energy in a gasoline-fueled engine (proportions vary with vehicle design, type of engine, and operating conditions). SOURCE: NRC (1992).
Some gets used to overcome friction, some is used to pump air and fuel into the engine, and the exhaust gases out of the engine, and some is used to power accessories. The work that makes it to the drive wheels is used to overcome vehicle inertia, aerodynamic drag, and rolling resistance. The relative ranking of these energy uses is highly dependent on the vehicle and the application to which it is being applied.
As indicated in the discussion of the pumping work above, the magnitude of these energy partitions is highly dependent on the engine size, its application in the vehicle, and the duty cycle under which the vehicle is operating. The best way to quantify the partitioning shown in Figure 4-5 would be to take the specific data for the application of interest or through the application of a verified system simulation program.
Opportunities to reduce the fuel consumption of gasoline engines include improving engine efficiency (trying to reduce the proportion of the fuel energy leaving as heat transfer, exhaust energy, and pumping work), reducing the energy lost to friction, and reducing the power required for running accessories. Brief descriptions of different technologies for reducing fuel consumption are given below.
Variable Valve Actuation and Cylinder Deactivation
There are many approaches to VVA. These include cam phasers, variable lift mechanisms, fully flexible valve trains, and cylinder deactivation. The primary loss that the VVA systems are trying to reduce is the pumping, or throttling, loss. A variable compression ratio allows the engine to operate at different compression ratios for different loads in order to maximize the engine efficiency over the widest load range possible. The combination of VVA and variable compression ratio keeps the engine operation closer to its maximum efficiency point, with minimal pumping work, over larger portions of the duty cycle.
Cylinder deactivation is an approach that minimizes pumping losses by varying the total working displacement of the engine. The “smaller” engine operates closer to wide-open throttle at lower loads, which reduces the pumping work.
Cam phasers allow the valve timing to be changed with engine operating conditions. For example, the timing can be shifted with engine speed to optimize the engine breathing with engine speed. VVT can also be used in place of the intake throttle. Either opening the intake valve late or closing it late can regulate the amount of air/fuel mixture captured in the cylinder. This can be done with lower throttling losses than would occur with the conventional intake throttle.
Different engine designs will lend themselves more easily to different valve control technologies. For example, overhead valve systems with the cam in the block versus a single overhead cam versus a double overhead cam design will have to invoke different VVA approaches, which may favor different valve manipulation strategies for the different engine configurations. So decisions as to which valve technology to invoke would be based on the engine configuration, the application’s duty cycle, and the incremental cost of implementation. Estimates for the fuel consumption reduction achievable through variable valve lift and/timing range from 1 to 3.5 percent (TIAX, 2009, p. 4-33).
Gasoline Direct Injection Engines
GDI engines refer to an embodiment in which the fuel injector is mounted so that the fuel is directly injected into the cylinder, as opposed to the more common port fuel injection,
where the fuel injector is mounted in the intake port. There are two philosophies of engine operation that fall under the classification of GDI engines.
In one case the engine is still operated with a stoichiometric mixture of air and fuel. This approach enables a three-way catalyst to be used in the exhaust, so emissions standards can be met. However, by mandating operation with a stoichiometric mixture, the engine still needs to be throttled. For a direct-injected stoichiometric engine the efficiency improvements come from less fuel being used during transient engine responses and from internal cooling of the cylinder charge from the fuel vaporization. This leads to a higher knock margin, which allows a higher compression ratio to be used. These engines are also more tolerant of EGR, so higher compression ratios can be used without an NOx penalty. There will be an attendant efficiency improvement with the higher compression ratio. The reduction in fuel consumption achieved via stiochiometric direct injection will be dependent on the extent to which this technology is combined with other technology packages. Based on estimates from a light-duty study (NHTSA, 2009), referenced by TIAX, a fuel consumption reduction with stoichiometric direct injection engines relative to a port-injected engine with VVT described above would range from 2 to 3 percent (TIAX, 2009, p. 4-33).
The other approach to GDI is to attempt to replicate the breathing characteristics of the diesel engine by minimizing the throttling of the intake air and controlling the load by varying the air/fuel ratio. Reduction in fuel consumption with this approach to direct injection comes from reduced pumping losses and higher efficiency from the lean-burning mixture, as well as the potential to increase the compression ratio as with the stoichiometric direct injection. The drawback to this approach is that the three-way catalyst is no longer effective, so the engine has the same aftertreatment challenges and expense as the diesel engine. The application of the lean burn direct injection technology will almost certainly be coupled with using turbocharging (described below) as well, so one must consider the combination of technology packages. Referencing the same NHTSA report referred to above, TIAX reported a potential reduction in fuel consumption by applying turbocharged lean burn GDI technology relative to the VVA nonturbocharged stoichiometric engine of 10 to 14 percent (TIAX, 2009, p. 4-33).
Different Combustion Modes
VVA mechanisms and fuel injection systems open up the possibility of incorporating advanced combustion regimes into the engine operating map. For example LTC, a general classification for auto-igniting combustion modes such as HCCI, partially PCCI, and compression-aided ignition, offers potential for lean low-emissions combustion in which throttling losses are minimized and catalytic converters are not needed (Zhao et al., 2003). Incorporating these alternative modes of combustion into the engine’s operating map will require much higher levels of sensing and control than are currently in use in today’s engines. For example, it is likely that real-time cylinder pressure sensing would be necessary to activate and control transitions between conventional combustion and LTC-type combustion during engine operation. If these combustion regimes can be incorporated into the operating map of gasoline engines, fuel consumption could be reduced via lean combustion at light loads, with minimal pumping losses, without the need for lean exhaust aftertreatment. Then during higher load operation, where throttling requirements are low, the engine could revert back to stoichiometric operation where the three-way catalyst is effective. It has been estimated that incorporation of LTC operation into the engine could reduce fuel consumption by 10 to 12 percent (TIAX, 2009). See Table 4-2.
Turbocharging and Downsizing
Turbocharging a gasoline engine is similar to turbocharging a diesel engine in that it is motivated by the desire to redirect energy that was leaving the engine in the exhaust gases back into the engine. The turbocharger converts exhaust energy into higher pressure and temperature intake gases using a turbine in the exhaust gas stream and a compressor in the intake air stream. Because of the differences in the fuels and the combustion processes between the two types of engines, there are different constraints that limit the application of turbocharging in a spark ignition engine relative to a compression ignition engine. Because the air and fuel are premixed in a spark ignition engine, as the pressure and temperature of the mixture at the start of compression is increased via turbocharging, the possibility of knocking combustion increases. Consequently it is common for the compression ratio to be decreased when the spark ignition engine is turbocharged. This tends to decrease the thermal efficiency of the engine. It is also likely that more exhaust gas will be recirculated when the engine is turbocharged. However, if successfully implemented, the turbocharged engine will have a higher power density than the nonturbocharged engine so it can be smaller and lighter for the same power output. This reduction of weight and potential reduction in frontal area of the vehicle has an attendant fuel consumption reduction benefit.
Turbocharging can be combined with other technologies, like in-cylinder direct injection and/or VVA to compound the benefits of the various technologies. The direct injection of fuel results in in-cylinder evaporative cooling, which helps counter the increased tendency toward knocking attributable to the turbocharging. Consequently the compression ratio may not need to be lowered to avoid knocking combustion to the same extent it would with turbocharging without direct injection. This coupled with the increased EGR tolerance of the direct injection and turbocharged engines helps keep NOx emissions from rising. In addition, because the power
density of the engine has been increased, it may be possible to run at lower speeds and still generate the power required to drive the vehicle. The lower engine speeds result in lower friction losses, which is beneficial to fuel consumption. So it is possible to combine many of the technologies to compound their benefits. The technology review by TIAX found that a turbocharged downsized engine offers a fuel consumption reduction potential of approximately 2 percent, while a turbocharged downsized direct injection engine with VVA offers a potential 10 to 14 percent reduction in fuel consumption (TIAX, 2009).
Once such technologies as turbocharging, direct injection, and variable valve actuation, and so forth, have been incorporated into the power train, the engine becomes quite flexible. For example, with such flexibility it is possible to use multiple fuels which that are carried separately onboard the vehicle. Approaches such as using gasoline and E85 (Stein et al., 2009) or varying mixtures of gasoline and diesel (Hanson et al., 2010) have been demonstrated. In these demonstrations, fuel consumption levels comparable, or superior to, that of conventional diesel engines have been achieved. The fundamental concept is one of optimizing the fuel characteristics and engine operating conditions for best performance—for example, the lowest fuel consumption and emissions, at each operating point. Such an engine would have characteristics of both gasoline and diesel engines. The duel-fuel concept would require infrastructure changes to address dispensing two separate fuels to a vehicle.
Accessory Loads
As with diesel-powered vehicles, decoupling the direct drive of the accessories from the engine offers the potential to optimally match the accessory use to the duty cycle. Examples of this technology that offer potential to reduce fuel consumption by reducing the amount of engine shaft work that was originally going to the driveline but was tapped to run the accessories instead include electrically driven engine oil and cooling pumps, mechanically clutched or electrically driven radiator cooling fans, electrically driven air-conditioner compressors, and high-efficiency alternators. One benefit of accessory load reduction is that the work saved through accessory optimization goes directly to the vehicle. The fuel-savings potential is highly dependent on the duty cycle of the engine.
Integrated Technologies
It is important to realize that the technologies described above may not be discrete. For example, using VVA for intake throttling reduction along with cylinder deactivation for engine displacement reduction are technologies motivated by the desire to reduce pumping work. Using them together will not result in fuel consumption reductions that are additive. Also, some technologies are facilitators for addressing multiple losses. For example, electric hybrids enable one to downsize the engine, operate the engine over a narrower and optimal portion of it operational map, and reduce accessory load on the engine through accessory electrification.
As discussed above, the fuel savings that comes from reducing any of the losses described above will be highly dependent on the vehicle itself and its duty cycle. To evaluate the potential for the various technologies to reduce the vehicle’s fuel consumption, it is best to evaluate the technologies as integrated packages applied to a specific vehicle operating on a representative duty cycle. For example, consider the package cited above of a downsized turbocharged direct injection engine with VVA. Engine and vehicle simulation packages exist that are capable of giving good comparative rankings of the fuel consumption reduction potential of different combinations of technologies.
One such example of a technology assessment program is HEDGE (High Efficiency Dilute Gasoline Engines) at SwRI.19 In this program a combination of technologies is being evaluated, including a downsized, turbocharged, direct injection, high-EGR, VVA engine, which is fueled with either gasoline or gasoline and diesel (dual fuel), with an emphasis on lower speed operation. Results presented to the committee were encouraging. Laboratory tests showed combinations of technologies that demonstrated gasoline engine thermal efficiencies greater than 40 percent, with fuel consumption reductions on the order of 20 percent, with 35 and 45 percent reductions in particulate matter and NOx emissions, respectively. It is evident from this work that, as stated above, it is the synergistic combination of various technologies, matched to engine duty cycle, that are critical for achieving reduced fuel consumption with low emissions. Note that introducing such a technology package may require a significant redesign of the engine. An ignition system tolerant of a high-EGR rate is required, and cylinder pressures are higher than in typical gasoline engines.
Shifting to Diesel Engines
Shifting from gasoline to diesel engines offers significant fuel efficiency benefits. This is primarily due to higher compression ratios and reduced gas exchange losses. However, it should be noted that, due to emissions regulations (which have degraded the efficiency of diesels), and advances in spark ignition technology (e.g., HCCI, turbocharging, direct injection), the gap between gasoline and diesels has narrowed considerably. The committee compares diesel and gasoline engines in more detail in the next section.
TABLE 4-2 Technologies for Fuel Consumption Reduction Applicable to Gasoline-Powered Engines for the Medium-Duty Vehicle Class and Estimated Fuel Consumption Reduction and Incremental Costs
|
Technology |
Incremental to… |
Incremental Cost and Benefit |
|
|
Percent Fuel Consumption Benefit |
Cost ($) |
||
|
Friction reduction |
— |
0.5-2.5 |
110-500a |
|
Variable valve timing (VVT) |
Friction |
1-3 |
122 |
|
Variable valve lift (VVL)b |
VVT |
1-3.5 |
400-750 |
|
Cylinder deactivationc |
VVL |
2.5-3 |
75 |
|
Stoichiometric GDId |
Cylinder deactivation |
2-3 |
512-930 |
|
Turbocharging and downsizing |
S-GDI |
2.1-2.2 |
1,229 |
|
Diesele |
— |
19-24 |
7,900-9,400 |
|
Lean-burn GDI |
S-GDI |
10-14 |
750f |
|
Gasoline HCCI |
10-12 |
S-GDI |
685 |
|
Accessory electrification |
Applicable to any package |
2-4 |
1,000-2,000 (current) 500 (high volume) |
|
NOTES AND ASSUMPTIONS: Baselines are explained in the text in the section “Summary of Technologies for Gasoline Engines.” The duty cycle is the typical duty cycle of a medium-duty vehicle with a gasoline engine. The diesel fuel consumption benefit includes the 10 percent higher heating value of diesel fuel. “Friction reduction” includes mechanical accessory improvements. Values assume (roughly) constant performance. The time frame covered is 2015 to 2020. a$13 to $49 per cylinder + $5 for lubricants. b$51 per cylinder for discrete VVL; $70-$75 per cylinder for continuous VVL. cOffers marginal benefit on DOHC engines; these benefits reflect SOHC. d$64 to $93 per cylinder. eDiesel fuel consumption incremental to baseline gasoline engine. fIncremental cost over direct injection stoichiometric engine. SOURCE: TIAX (2009), p. 4-33. |
|||
Summary of Technologies for Gasoline Engines
As part of the committee’s activities, TIAX was contracted to assemble and categorize different engine technologies, their potential fuel consumption benefit, and their incremental costs. Table 4-2 is a tabulation of the technologies discussed above, their potential fuel consumption benefits, and their incremental costs; the baseline engine on which the estimates of fuel consumption are based is a port fuel-injected, naturally aspirated, fixed-valve-timing engine with 8 to 10 cylinders.
DIESEL ENGINES VERSUS GASOLINE ENGINES
Diesel engines offer significant fuel savings over gasoline engines, when measured on a fuel consumption (gallon per mile) or load-specific fuel consumption (gallons per ton-mile) basis. Depending on the engines and operating conditions, diesel engines can provide 19 to 24 percent lower fuel consumption than gasoline engines. Two factors account for this advantage:
-
Diesel fuel has approximately 12 percent higher energy content than gasoline, which allows diesel engines to extract more energy per gallon from the fuel.
-
Diesel engines have a higher thermal efficiency than gasoline engines, which allows diesel engines to provide more shaft power for a given amount of fuel energy released.
Several factors contribute to the higher thermal efficiency of diesel engines. One is the lack of a throttle on traditional diesel engines. Pulling air across a closed throttle imposes significant pumping losses, so diesel engines enjoy a significant advantage in pumping loss, especially at light load. Another factor that contributes to the higher thermal efficiency of diesel engines is high compression ratio. This is made possible by the compression ignition cycle of the diesel. The higher expansion ratio that comes with a higher compression ratio allows the diesel to extract more of the combustion energy before the exhaust valve opens near the end of the power stroke. Because of the high cylinder pressures encountered as a result of high compression ratio, turbocharging, lean air/fuel ratios, and the use of EGR, the diesel engine has the disadvantage of being heavier (pounds/cubic inch) and more costly to manufacture. The fuel system of diesel engines also contributes significantly to the cost penalty of diesel engines.
The most common fuels for medium- and heavy-duty engines are gasoline and No. 2 ultralow-sulfur diesel fuel. Both spark-ignited engines and compression ignition engines can burn “other” fuel types as discussed earlier, but most com-
mon are renewable fuels (as required by the Renewable Fuels Standard of the Energy Policy Act of 2007), such as ethanol for spark-ignited engines and biodiesel for compression ignition engines. Both of these renewable fuels have lower heating values (Btu/gallon) than their counterpart gasoline and diesel fuel, resulting in higher fuel consumption when measured on a volume basis (liters or gallons). Natural gas is also used in gasoline and converted diesel engines but is low in heating value and used in gaseous form, requiring the expression of fuel consumption data on an equivalent energy basis.
Another factor that may arise if diesel engines are substituted for gasoline engines, or renewable fuels play a bigger role in the mix of fuels used for medium- and heavy-duty vehicles, is the impact on petroleum refineries. Each barrel of petroleum produces various proportions of fuels, such as diesel, gasoline, kerosene, fuel oil, and others, to supply the demand for these fuels. If the fuel market were to shift significantly in the demand for diesel fuel versus gasoline, e.g., refineries would need to modify their processes to change the mix of fuels produced from each barrel of petroleum refined.
Both gasoline and diesel engines incorporate exhaust emissions control systems for hydrocarbons HCs, CO, and NOx. Gasoline engines also control evaporative emissions and diesel engines control PM. These emissions are controlled by both the engine combustion process (engine out) and the use of catalytic converters, DOCs, DPFs, lean NOx catalysts, and SCR.
The emissions control approach used with heavy duty diesel engines has been quite different from spark-ignited engines. All strategies used for on-highway trucks through 2009 relied on in-cylinder controls, including EGR, to limit NOx, rather than on aftertreatment. The 2007 U.S. Environmental Protection Agency (EPA) heavy-duty on-highway standards forced the use of DPFs to control PM. DPFs are the first widely used aftertreatment system on diesel engines, although some diesel engines have also used oxidation catalysts to control HC emissions. For 2010 most diesel truck manufacturers plan to add SCR aftertreatment to meet the new 2010 NOx requirements.
Volvo is the first company to publicly price heavy-duty diesel vehicles with 2010 emissions control systems. The company plans to charge $9,600 for the SCR system on 2010 model heavy-duty trucks (Fleet Owner Web magazine, March 3, 2009). Volvo’s surcharge on 2007 emissions levels is $7,500, which covers the cost of a DPF system for PM control and a cooled EGR system for in-cylinder NOx control. The total emissions control surcharge of $17,100 for 2010 is not far below the cost of a complete heavy-duty diesel engine. Navistar, maker of international trucks, announced a $6,000 emissions surcharge for 2010 medium-duty trucks, and an $8,000 surcharge for heavy-duty trucks (Reuters, July 28, 2009). Navistar is the only major truck maker that does not plan to use SCR to comply with the 2010 NOx requirement. The 2010 emissions surcharges are on top of surcharges of $5,000 to $6,000 for medium-duty trucks and $7,000 to $10,000 for heavy-duty trucks that Navistar charged for 2007 emissions (Navistar press release, November 8, 2005). Daimler Trucks North America, makers of Freightliner and Western Star trucks, announced increases of $6,700 to $7,300 for medium-duty trucks and $9,000 for 2010 heavy-duty trucks, compared to 2007 (Transport Topics, August 8, 2009).
The cost of meeting new emissions standards with gasoline engines is typically measured in hundreds, rather than thousands, of dollars. Diesel engines start with a significant cost disadvantage compared to gasoline engines, because of their greater strength (to withstand the high-cylinder pressures of compression ignition) and their far more sophisticated fuel systems. Diesel fuel systems have injection pressures of 1,600 to 3,000 bar, while even the expensive (by gasoline engine standards) GDI fuel systems require only 100 to 200 bar. Port injection systems for gasoline engines typically use injection pressures of only a few bar. The need to create and control extreme pressures has a major effect on diesel fuel system cost.
When the higher cost of diesel engines is added to the far higher cost of diesel emissions control aftertreatment, there is a powerful market incentive to move toward gasoline engines, except where the durability of the diesel engine is required. Over the period from 2004 to 2008, diesel engines lost market penetration to gasoline engines in Class 3, 5, and 7 trucks while increasing market penetration in Class 2 and 4 trucks (see Table 4-3).
This trend can be expected to accelerate in 2010 and beyond, when medium-duty diesel engines with aftertreatment may cost $10,000 more than a gasoline engine option. For any operation with relatively low average vehicle miles per year, gasoline engines will make more economic sense. Since gasoline engines are significantly less fuel efficient than diesel engines, this means that fuel consumption of the medium-duty truck fleet will increase as a result of falling diesel engine market share.
TABLE 4-3 Diesel Truck Sales as a Percentage of Total Truck Sales
|
Class |
2004 |
2005 |
2006 |
2007 |
2008 |
|
1 |
0.10 |
0.1 |
0.0 |
0.0 |
0.0 |
|
2 |
9.2 |
9.5 |
10.1 |
10.4 |
12.9 |
|
3 |
68.6 |
68.6 |
68.6 |
42.5 |
44.1 |
|
4 |
70.6 |
73.8 |
75.7 |
78.5 |
80.9 |
|
5 |
91.7 |
92.2 |
91.6 |
91.8 |
92.3 |
|
6 |
75.8 |
73.4 |
75.3 |
52.4 |
58.0 |
|
7 |
53.6 |
55.8 |
58.5 |
50.4 |
50.3 |
|
8 |
100.0 |
100.0 |
100.0 |
100.0 |
99.7 |
|
Total |
9.1 |
10.3 |
11.6 |
9.3 |
10.8 |
|
SOURCE: DOE, EERE (2009), based on Ward’s Motor Vehicle Facts and Figures. |
|||||
TRANSMISSION AND DRIVELINE TECHNOLOGIES
“Transmission and driveline” together refer to the system that connects the propulsion system to the wheels. It includes the transmission, the final drive, and the axle. Options for realizing fuel consumption benefits by optimizing the transmission and driveline generally fall into one of two categories:
-
Improved driveline efficiency. Strategies that increase the efficiency of the power transfer from the propulsion system to the wheels.
-
Improved system integration. Strategies that enable the engine to operate at higher average drive cycle efficiency.
Improved integration of the driveline with the power train in a tractor trailer can raise the average efficiency of the engine over an actual real-world drive cycle. The easiest form of this approach entails appropriately matching system gearing to the specific application. This process entails selecting a top gear and rear axle ratio that matches typical cruise speed (ensuring that the engine is in its peak efficiency window). These specifications vary from fleet to fleet; to the extent that a vehicle purchaser knows the specific roads on which a truck will travel, systems can be highly optimized to match their application. Conversely, some purchasers simply use standard specifications, or specify vehicles based on what they purchased in the past. As such, all major original equipment manufacturers (OEMs) make a concerted effort to work with purchasing agents to properly manage the vehicle specification process.
Transmissions
Class 3 through 8 vehicles use three basic types of transmission. The most common transmission type is the manual transmission (MT). Class 3 to 7 trucks typically use 5- to 8-speed transmissions, many of which are synchronized. Class 8 vehicles typically use 9-18 speed transmissions, most of which are not synchronized. Synchronizers are universally used in passenger car transmissions to make shifting easier. The synchronizer is a small clutch that matches the transmission input shaft speed to the speed required on shaft of the gear being engaged. Synchronizers are not used in the main box of heavy-duty transmissions both to reduce cost and to eliminate an expensive wear item. They are used in heavy-duty transmissions to synchronize the main box with the auxiliary box. The auxiliary box is used to multiply the number of speeds of the main box of the transmission. For example, a 5-speed main box can be combined with a 2-speed auxiliary box to form a 10-speed transmission. Another example is the 18-speed transmission, which consists of a 5-speed main box with two 2-speed auxiliary boxes. This makes for a total of 20 available ratios, but two of these are redundant.
With MTs, having more ratios can generally lead to a better match between engine speed and road speed, which reduces fuel consumption. However, there are drawbacks to transmissions with more ratios. They require more work on the part of the driver, and they cost more. They are often larger and heavier than transmissions with fewer ratios, and they may be less efficient because more gears are in mesh at any given time. One additional drawback of a high gear count is the frequent power interruptions caused by the need to shift through a large number of gears.
MTs have the highest market share in long-haul truck applications. According to information supplied by Daimler Trucks North America (DTNA) (Freightliner),20 the share of MTs in line-haul trucks declined from 90 percent in 2004 to a still dominant 82 percent in 2008. At the other extreme, MTs are rarely used in urban applications such as transit buses and refuse haulers. For long-haul Class 8 trucks, the most common transmission types are 10-speed, 13-speed, and 18-speed. The 13- and 18-speed transmissions have smaller-ratio steps, which allows the driver to better match the engine rpm to road conditions. However, these transmissions also have more gears in mesh at any given time than 10-speed transmissions do, so there is an efficiency penalty for having the additional ratios available. There is also a penalty involved in the more frequent power interruptions for shifting that occur with a higher gear count transmission. The 13- and 18-speed transmissions are most often used by heavy haulers (operators that run over 80,000 pounds gross vehicle weight) and by on/off highway operators. These operators need the flexibility provided by a larger number of gear ratios. Most standard long-haul operators use 10-speed MTs to achieve the best balance between vehicle performance and fuel consumption. In this situation the market has gravitated toward the most fuel-efficient MT available.
The automated manual transmission (AMT) has been gradually gaining market share over the past 10 years. According to DTNA figures, AMTs represented 10 percent of the line-haul transmissions in 2004, increasing to 18 percent in 2008. The AMT is typically based on the platform of a standard MT. Additional actuators and controls are added to allow the transmission control module to take over the shifting activities of the driver. Actuators perform both the shifting and clutch actuation for the driver. The AMT controller can match the shift performance (shift time and smoothness) of a skilled driver, provided the controls are well designed and carefully tuned. The AMT offers several advantages over a conventional manual transmission:
-
The requirement for driver skill is lower.
-
There is less driver distraction, improving vehicle safety and productivity.
-
The control module decides when to shift, which can be used to reduce fuel consumption compared to an average driver.
-
Shifts are always performed smoothly, which can improve transmission durability.
The downsides of the AMT are higher cost and more complexity—more parts that can fail. TIAX (2009, p. 4-70) estimates the cost of AMTs at $4,000 to $5,700 over a comparable MT. There is also a slight weight increase. Donnie Stover, fleet manager for Averitt, reported on December 16, 2008 a 3-percent improvement in average fuel consumption when using AMTs in place of standard MTs in sleeper cab team tractors. These are trucks that use two drivers working together to allow nearly 24-hour operation. This fuel consumption reduction comes from allowing the transmission controller to determine shift points rather than the driver. The best drivers can beat the fuel consumption of an AMT, but average drivers cannot match the results of the AMT.
Other features being added to new AMTs include microprocessor technology to continuously monitor changes in road grade, vehicle speed, acceleration, torque demands, weight, and air resistance.21 This technology allows the vehicle to select the best gear and fuel setting while minimizing fuel consumption. It also allows the engine and transmission to know when to go into freewheeling when neither power nor engine braking is needed. In these situations, the engine goes back to idle and the transmission slips into neutral when power is not needed. TIAX estimated a 4 to 8 percent reduction in fuel consumption based on site visits with the committee. The fuel savings potential for AMT will vary based on duty cycle and driver training. Long-haul cycles on level ground require little shifting and thus offer little potential for improvement, while duty cycles involving hills, congestion, and urban driving have much more potential for fuel savings, both with AMT and driver training.
The third transmission type seen in Class 3 to 8 vehicles is the traditional torque converter automatic transmission (AT). These transmissions typically have five to seven gear ratios, with a torque converter and a lockup clutch. Many ATs use the torque converter only at low road speed and run in lockup mode in all the higher gears, a feature reduces fuel consumption. Fully automatic transmissions are most popular in urban applications such as transit buses and refuse haulers, but they are also widely used in other applications, including some very heavy on/off highway vehicle applications. Standard 80,000 lb long-haul trucks are one application where ATs are very rare. The DTNA data show that the share of MTs in Class 8 non line-haul applications is lower than for line-haul applications, at 67 percent in 2008. AMT transmissions have about 9 percent share in these non-line haul trucks, while torque converter automatics enjoy a 24 percent share, up from just over 10 percent in 2004. The share of ATs is probably higher in the Class 2b to 7 range.
ATs share the driver skill, productivity, and safety advantages of AMTs. They also offer the ability to complete upshifts under full engine power, something that cannot be done with manual or automated manual transmissions. This can be a significant productivity (trip time) factor in applications with frequent large changes in vehicle speed, such as urban or suburban driving. With an MT or AMT, the engine fueling is shut off during each upshift. This interrupts power generation during the shift, which typically takes about 1 second in lower gears and up to 2 seconds in higher gears. However, after the shift is completed, the engine still requires some time (typically 2 to 3 seconds) to return to full power once the shift is completed.22 In the future, if the development of heavy-duty dual-clutch transmissions progresses as it has for light-duty vehicles, a dual-clutch transmission will remove the problem of interrupting the power during shifting. There can be a fuel consumption advantage as well as a productivity advantage in performing full-power upshifts, because the engine can continue to operate at an efficient point during and after shifts.
ATs are slightly heavier than MTs, but the use of an AT allows the clutch and flywheel to be replaced by a flexplate and torque convertor. Overall truck weight with an AT is slightly lower than with an MT. TIAX reports the cost of heavy-duty ATs at $15,000 over an MT.
The AT has some fuel consumption penalties compared to a conventional transmission as well. The hydraulic pump required to fill the torque converter and actuate the shift events draws power from the engine. When the transmission operates in converter mode, a significant percentage of the input power from the engine is lost as heat in the torque converter. The AT will operate with an open converter (lockup clutch disengaged) at low engine and vehicle speeds, whereas the MT and AMT use a more efficient closed clutch. The shift schedule of medium- and heavy-duty ATs has evolved over the years to minimize the time spent in converter mode.
The primary disadvantages of the AT compared to other transmission types are much higher cost and more complexity. The warranty period for ATs is much shorter than for MTs, and warranty repairs must be made by the transmission dealership. These factors discourage the use of ATs except in applications where the advantages of using an AT in the vehicle operating cycle are very great. AT applications are typically in urban and suburban operations. There are a number of features under development to improve the efficiency of ATs, including lower friction and lower parasitic hardware, more elaborate shift strategies, a reduced load on the engine during stops, and automatic shift into neutral when the parking brake is applied.23 TIAX (2009, 4-70) estimated a 0 to 5
percent reduction in fuel consumption from the use of ATs, in tractor-trailer applications, in its report to the committee.
Research has been done on other transmission types, such as continuously variable transmissions (CVTs) and dual-clutch transmissions. Several CVT designs have been proposed for heavy-duty vehicles, and Allison Transmission has signed an agreement with Torotrak to develop its CVT design for heavy-duty vehicle applications. It is not yet clear if any of these alternative transmission types will reach volume production. CVTs tend to have lower mechanical efficiency than MTs or AMTs, but they make up for this by allowing an optimum match between engine speed and road speed under all operating conditions. The main challenges facing the use of CVTs for truck and bus applications are mechanical efficiency, reliability, durability, and cost.
A comparison of transmission fuel consumption performance can be made by considering four driving-cycle components: idle, acceleration, steady-speed cruise, and deceleration. At idle the AT has a disadvantage due to torque converter loss and hydraulic pump parasitic power. The addition of an “auto neutral” function greatly reduces this disadvantage. Under acceleration the AT has an advantage over the other types due to power shifting. Shifts are completed without changing the fueling command, so boost pressure is maintained and engine operation is more efficient. The AMT has an advantage over an average driver with an MT because of computer-controlled shift points. At cruise the MT and AMT have a slight advantage because they do not require a hydraulic pump. It should be noted that cruise fuel consumption is very strongly dependent on speed and the final drive gearing, and these selections are independent of the transmission type. Under deceleration the AT has a slight advantage over other types because there is no need to blip the engine fueling for downshifts. This blip is necessary with both manual and automated manual transmissions to get the engine speed to match the speed of the transmission gears.
Overall, the selection of transmission type has only a relatively small impact on vehicle fuel consumption. The exception is in urban and suburban operation, where the AT may offer a modest reduction in fuel consumption, combined with significantly greater productivity (average trip speed).24 The higher productivity is a result of avoiding power interruptions during acceleration. Fuel consumption differences due to transmission selection are normally a few percent or less. The shift calibration schedule for AMTs and ATs can have a modest impact on fuel consumption. A driver’s shift behavior with MTs can have a significant effect on fuel consumption; a driver with poor habits may suffer a 10 to 20 percent fuel consumption penalty in urban and suburban driving.25 The line-haul market is likely to move more in the direction of AMTs as costs fall and reliability is proven. This move will provide a modest fuel consumption benefit. A move to ATs for stop-and-go-type operations may also provide a modest benefit. Just as the engine market is very competitive in terms of fuel consumption, so is the transmission market. Long-haul operators will often change transmission type to gain a 1 or 2 percent reduction in fuel consumption, as long as the cost is reasonable and the reliability of the technology is solid.
Rear Axle Ratio
The selection of rear axle ratio is one of the most important decisions in specifying a truck. The axle ratio determines the engine rpm at the vehicle’s cruise speed, which is a very important fuel consumption parameter. The axle ratio also determines the grade capability and acceleration performance of the vehicle. A tall (numerically low) axle ratio is typically good for fuel consumption but bad for acceleration performance and grade capability. This means that the choice of axle ratio involves a trade-off between fuel consumption and vehicle performance. Operators select axle ratios based on the type of loads and routes they expect to operate with. Most engine and vehicle OEMs have sophisticated software that can look at specific trucking operations and recommend the best axle ratio for a given application.
Great care must be taken in developing any fuel consumption requirements to avoid a situation where operators are forced to select an axle ratio that is not appropriate for their operation. A logging operation, for example, must use a shorter (numerically higher) axle ratio than a standard long-haul tractor, in order to get adequate off-road performance.
Low-Friction Transmission, Axle, and Wheel Bearing Lubricants
Special lubricants can be used to reduce friction in the transmission and axles. Typically synthetic lubricants are specified to reduce viscosity, especially in low-temperature conditions. Many tests conducted by fleets have documented fuel savings of at least 1 percent when using low-friction lubricants. Because of the relatively low cost, synthetic lubricants are becoming widespread in the industry. Eaton and Dana have recently made low-friction synthetic lubricants standard on all their heavy-duty transmissions and axles.26 Truck OEMs typically charge $35 to $55 for synthetic lube in tandem-drive axles. Synthetic lube and grease for wheel bearings is also available at relatively low cost.
Rear Axle Types
For Class 3 vehicles several types of axles are used. The standard single-reduction axle with an open differential has the lowest friction and is best suited for highway use. Some
applications need improved traction for operation in lowfriction environments such as snow or off-road conditions. The limited-slip differential uses a clutch pack to provide a limited amount of torque to both ends of the axle when there is a significant speed difference between the two wheels. This is the preferred differential for the front axle of a fourwheel-drive vehicle, since the clutch pack allows some speed differential for easier steering. Some limited-slip axles use electronic actuators to engage the limited-slip clutches.
A range of axle designs and configurations is available for medium- and heavy-duty vehicles. The designs vary based on the weight of the vehicle and the intended application. For drive axles, single, tandem, and tridem (three drive axles) configurations are available. The axle may have no differential, a single-speed differential, a two-speed differential, or a double-reduction differential (two gear sets in series to get a numerically high axle ratio). The most common axle type is the single-speed differential, and more than 17 different ratios are available for this design. The same range of ratios is available in tandem and tridem axles. Axle prices range from $2,600 to $15,000, depending on the type selected, the weight rating, and other features.
In general, axle design is selected to match the intended operation of the truck. The selection of axle ratio is the most important factor in determining driveline-related fuel consumption. A ratio that matches proper engine speed at cruise is critical. Other axle features have only a minor effect on fuel consumption. In general, to get the lowest fuel consumption, a vehicle should use the minimum number of drive axles required for the intended application. Each additional drive axle adds friction and weight to the driveline and thus has a fuel consumption penalty. For example, the 6 × 2 arrangement for tandem axle trucks is estimated to provide about a 1 percent fuel consumption reduction compared to the standard 6 × 4 arrangement, at the expense of limited traction (TIAX, 2009, p. 4-69). A 6 × 4 arrangement refers to a truck with six wheels (dual wheels count as one), with one steer axle and two drive axles. This is the standard arrangement for long-haul tractors. A 6 × 2 tractor has only one drive axle, with the second rear axle used only to carry load. To make a 6 × 2 design practical, a method of unloading the nondriven axle must be used for situations where traction is critical. The 6 × 2 layout is popular in Europe, but not in the U.S. market. Resale value is a major factor keeping fleets from specifying 6 × 2 tractors. Buyers are concerned about running into low- or no-traction difficulties due to the driven axle being lifted to the point of no traction when crossing uneven road or ground. This issue can be avoided by using air bags and a driver control valve to lower the drive axle back onto the road surface, but this adds cost and complexity to the vehicle.
Transmission and Driveline Summary
In its report for the committee, TIAX (2009) summarized the transmission and driveline fuel consumption potential reduction by range of years and by application as shown in Table 4-4.
TABLE 4-4 TIAX Summary of Transmission and Driveline Potential Fuel Consumption Reduction (percentage) by Range of Years and by Application
|
|
2013-2015 |
2015-2020 |
|
Tractor trailer |
5.0 |
7 |
|
Class 6 box truck |
1.5 |
4 |
|
Class 6 bucket truck |
1.2 |
3.2 |
|
Refuse truck |
1.5 |
4 |
|
Urban bus |
1.5 |
4 |
|
Motor coach |
2.0 |
4.5 |
|
Class 2b pickup and van |
4.5 |
7.5 |
The committee believes that the claims for reduced fuel consumption of tractor trailers would apply only to those not properly specified today. Many tractors are already well specified for their application, and the savings is likely to be less than shown above.
HYBRID POWER TRAINS
A hybrid vehicle (HV) combines at least two energy converters, such as internal combustion engines (ICEs), electric drives, and hydraulic drives. The ultimate goal of the HV is to provide the equivalent power, range, and safety as a conventional vehicle while reducing fuel consumption and harmful emissions. HVs have the potential to realize several advantages, including the following:
-
Regenerative braking. A regenerative brake is an energy mechanism that reduces vehicle speed by converting some of its kinetic energy into a storable form for future use instead of dissipating it as heat as with a conventional brake. The significance of regeneration becomes apparent when one considers that approximately 60 percent of the total energy spent in the U.S. Federal Urban Driving Schedule is used to overcome the effect of inertia and that, theoretically, up to 50 percent of this energy could be recovered. This “maximum theoretical percentage of recoverable energy” will vary with duty cycle and vehicle characteristics, since it depends on the deceleration level and vehicle aerodynamic drag and rolling resistance. Regenerative braking can also reduce brake wear and the resulting fine particulate dust.
-
Higher electric machine efficiency. In comparison with the ICE, the electric machine is a simpler and more efficient machine. For instance, the moving parts of an electrical machine consist primarily of the armature (DC motor) or rotor (AC motor) and bearings.
-
Improved torque characteristics. Electric machines are more suited to vehicle applications, with high torque at low speed and less torque at cruising speed. When the electric machine torque dips, the ICE must be engaged to harness torque.
-
Reduced emissions. Reduced emissions occur through smoothening of transients and idle elimination.
-
Operate at best efficiency. For selected configurations, optimal engine operation. operate the engine in its “sweet spot,” staying close to its best efficiency line (also called E-line).
-
Downsize engine. Engine downsizing might be possible to accommodate average load (not peak load) and consequently reduce engine and power train weight.
-
Engine shutoff. Engine shutoff is possible, thereby reducing fuel consumption, emissions and noise vibration and harshness. In the case of line-haul vehicles, engine shutoff can be achieved through electrification of overnight hotel load; and in the case of service trucks such as utility vehicles, the power take-off can be electrified.
-
Accessory electrification. Accessory electrification allows parasitic loads to run on an as-needed basis. Electrified accessories are often more efficient than belt-driven ones.
-
Better drivability. An electric machine reacts faster to a throttle input than an ICE; furthermore, torque from the ICE and the electric drive train can be added up whenever needed in certain configurations.
-
Robustness. For some configurations, such as the parallel, the vehicle may be operable with either of the power sources when one fails.
-
Plug-in hybrids. Plug-in hybrids can help absorb excess electricity from the grid at night and improve energy security and diversity.
-
Electrification. Electrification can enable waste heat recovery, thus bringing on board systems to generate and store electricity, and use it when needed.
HV disadvantages include the following:
-
Increased power train and electronic complexity.
-
Increased vehicle mass due to addition of components.
-
Increased cost due to additional components and complexity of the power management.
-
Overall system reliability can be lower due to increased complexity.
-
If not optimized for the appropriate drive cycle, benefits may not be fully realized, or fuel consumption may even increase.
The two major types of hybrids are electrical and hydraulic:
-
A hybrid electric vehicle (HEV) combines electric and mechanical power devices. The main components of an HEV that differentiate it from a standard ICE vehicle are the electric machine (motor and generator), energy storage (e.g., battery or ultracapacitors), and power electronics. The electric machine absorbs braking energy, stores it in the energy storage system, and uses it to meet acceleration and peak power demands. HEVs are widely used in almost all vehicle weight classes—light- medium- and heavy-duty vehicles.
-
A hydraulic hybrid vehicle (HHV) combines hydraulic and mechanical components. The four main components of a hydraulic hybrid power train are the working fluid, fluid reservoir, hydraulic pump/motor (in a parallel hybrid system) or in-wheel motors and pumps (in a series hybrid system), and an accumulator. The hydraulic accumulator stores the energy (as highly compressed nitrogen gas) and a variable displacement pump acts as a motor while driving the wheels and as a generator while absorbing regenerative braking energy. This system suits medium- and heavy-duty vehicles operating with high-power, low-energy requirements, including stop-and-go driving profiles (e.g., refuse vehicles, inner-city buses, and delivery vans).
The task of achieving fuel savings using a hybrid architecture depends on the type of power train selected as well as the component sizes and technology, the vehicle control strategy, and the driving cycle. When approached as a system, an HV is an integrated propulsion system. Fuel consumption reductions can be realized only after sensible optimization of power management based on a suitable driving cycle. The vehicle’s power demand is met by the different power sources on board. For instance, a simple acceleration, cruising, and braking cycle for an HEV demonstrates the best use of different power sources based on the vehicle’s power demand: during small accelerations, only the energy storage power is used (electric vehicle mode), and during braking some of the energy is absorbed and stored. The ICE does not start to operate during low-power demands due to its poor efficiency compared to the electric system. The ICE is used only during medium- and high-power demands where its efficiency is higher. More discussion on vehicle-level management strategies is given in a subsequent section.
Comparison of Energy Storage Devices
Generally light-duty vehicles, small trucks, and transit buses typically make use of electric systems, whereas heavy-duty vehicles make use of both electrical and hydraulic systems (except transit buses). Truck applications enable regenerating and reusing significant amounts of braking energy. Consequently, power flows through the hybrid subsystem can be very high. This makes both ultracapacitors and hydraulic
FIGURE 4-6 Power density versus energy density of various technologies. SOURCE: Baseley et al. (2007). Reprinted with permission from SAE Paper 2007-10-4150. © 2007 SAE International.
storage very attractive since they are characterized by very high power density levels. However, the energy density of both ultracapacitors and hydraulic systems is lower than that of batteries and, hence, energy cannot be supplied over a long duration. The high-power systems are well suited for a driving cycle with several start-stops, as energy can be captured and released quickly. A battery, on the other hand, has greater energy density and can be used for long energy storage and supply. The Ragone diagrams shown in Figure 4-6 compare the power density versus the energy density of different energy storage systems. Note that the ultracapacitors, hydraulic accumulators, and advanced flywheels have the highest power density among present-day storage systems, but their energy density is limited significantly.
Hybrid Vehicle Architecture
A number of different system architectures are being considered to meet different applications. They are broadly classified as series, parallel, and power split. The selection of system architecture depends mainly on the application. The following sections describe some of the possible power train configurations under each architecture.
Series Hybrids
Series Hybrid Electric
In a series HEV, as illustrated in Figure 4-7, an electric generator, coupled to an ICE, supplies electricity to the electric machine to propel the truck and to the energy storage system when it needs to be recharged. Generally for batteries the engine/generator set keeps the energy storage system charged between 50 and 70 percent for charge sustaining HEVs.27 (For PHEVs, the energy storage system is used in a wider operating range (e.g., 80 to 30 percent for the GM Volt). In this configuration the ICE and vehicle speeds are decoupled and only the electric machine is connected to the wheels. The ICE does not need to speed up or slow down as
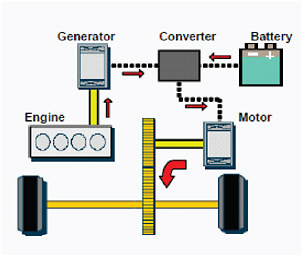
FIGURE 4-7 Series hybrid electric vehicle. Courtesy of University of Michigan.
the load varies. As a consequence, the engine can run at its optimum performance (best engine efficiency zones), greatly reducing fuel consumption. Moreover, the ICE never idles, thus reducing overall emissions. However, because the electric machine is the only one connected to the wheels and the engine/generator set is sized for sustained grade ability, this configuration requires a large energy storage system pack, electric machine, and engine. For this system to be viable, it must possess an overall high efficiency in total power processing. While the added mass and the component inefficiencies make that configuration unlikely for small trucks, it is more viable for large vehicles, such as buses, which are less sensitive to increases in overall weight. It should be pointed out that the elimination of the mechanical driveshaft can be an important advantage of adopting the series configuration. One example is the transit buses where elimination of the mechanical driveshaft makes it possible to lower the bus floor for improved wheelchair access.
Several variations of the series configurations have been considered. One of the important considerations in the design of a series HEV is related to the use of a single-gear ratio versus a two-speed transmission. Using a single-gear ratio usually leads to low maximum vehicle speed and poor performance at high speed due to the low electric machine torque at that regime. When applications require better performance at high speeds, a two-speed transmission is considered. If electric machines are used at each of the wheels, instead of a single electric machine, torque vectoring is possible, improving vehicle stability.
Torque vectoring can be defined as distributing the majority of the power to the wheels that have traction. If the front wheels have better traction than the rear wheels (rear wheels might be running on icy patches for some particular time), the power is transmitted to the front wheels and not the rear wheels. Torque vectoring can also be done between the front left and front right wheels. As each wheel can be powered independently, the number of degrees of freedom to control the vehicle traction is increased, and in turn vehicle stability can be improved. Torque vectoring is achieved by using redesigned differentials, which means that wheels don’t need to be stopped, and even better, the vehicle won’t suffer from a sudden loss of power as it is negotiating an unexpected loss in traction.
Series Hybrid Hydraulic
HHVs employ the same basic architecture as HEVs. However, in an HHV the battery is replaced with a hydraulic accumulator, and electric machines are replaced with hydraulic motors/pumps. As in the HEV architecture, kinetic energy from braking can be recovered and stored: in an HHV, braking energy is used to drive a hydraulic motor that pumps fluid from a low-pressure fluid reservoir to a high-pressure accumulator. This energy can then be used to supplement engine power by releasing the fluid in the high-pressure accumulator back to the low-pressure reservoir, driving the motor in the process. The series configuration of the HHV can be implemented using a high-pressure accumulator along with a low-pressure reservoir, as shown in Figure 4-8.
Current hydraulic hybrids are capable of capturing on the order of 70 percent of kinetic energy from heavy-duty vehicles. This is due to both the high rates at which power can be recovered and the fact that the energy storage system is virtually lossless, i.e., there is very little energy lost (Gotting, 2007). Hydraulic hybrids are targeted for power-driven applications—that is, duty cycles that have high regenerative braking requirements but relatively low energy requirements, such as refuse trucks and house-to-house delivery vehicles. Series hydraulic hybrids are still in the prototype stage. They are being evaluated for pickup and delivery vehicles and for refuse haulers, for which they have demonstrated fuel consumption reductions on the order of 50 percent (J. Kargul, U.S. EPA, presentation to the committee, April 6-7, 2009).
Parallel Hybrids
Parallel Hybrid Electric
Parallel hybrids have mechanical connections to the wheels from both the electric machine and the engine, as il-
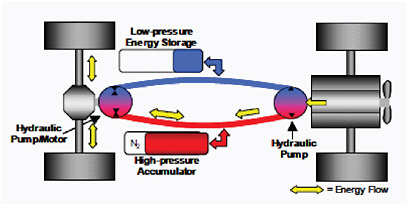
FIGURE 4-8 Series engine hybrid hydraulic vehicle. SOURCE: Eaton-HTUF (2009). Courtesy of Eaton.
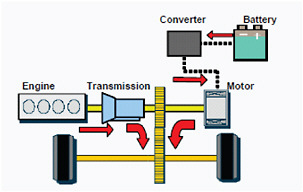
FIGURE 4-9. Parallel hybrid electric vehicle. Courtesy of University of Michigan.
lustrated in Figure 4-9. The electric machine can be located anywhere between the output engine shaft and the wheels. These vehicles do not need a dedicated generator; the electric machine can be used as a generator to recharge the batteries. In a parallel HEV the electric machine can assist the engine during startup and acceleration.
Because the electric machine and the engine are both coupled directly to the wheels, they can share the power during acceleration. Therefore, it is possible to downsize both the engine and the electric machine compared to series hybrids (the vehicle mass is then reduced compared to the series hybrid architecture). It is also possible to increase the hybridization degree by downsizing the engine and upsizing the electric machine; note, though, that downsizing the ICE is not practical in applications that require extended high-power operation, such as long-haul trucks. For some configurations the ICE can operate close to its best efficiency curve, with the electric machine assisting it or recharging the battery. However, it should be noted that the ICE speed is not independent of the vehicle speed even though there is some degree of freedom over the engine load.
Numerous options have been studied for parallel architectures, ranging from micro or mild versions of the starter-alternator type to full-featured pre- and posttransmission hybrids, as described below.
Starter-Alternator Type
In a starter-alternator configuration the electric machine is connected to the engine crankshaft directly on the shaft, as shown in Figure 4-9, or coupled through a belt, as shown in Figure 4-10. The main advantage of this configuration is the ability to turn the ICE off during idling. Since the electric machine speed is linked to the engine, the vehicle cannot operate in electric mode other than for extremely low speeds (e.g., creep). In addition, the electric machine is used to smooth the engine torque by providing power during high transient events to reduce emissions. Finally, some regenerative braking energy is recaptured but only a small amount due to the limitation in size of the electric machine. Two families are defined, based on the battery voltage: micro and mild HEV. This system requires minimal modifications and cost.
Pretransmission Type
For pretransmission parallel HEVs, the electric machine is located in between the clutch and the transmission, as illustrated in Figure 4-11. This configuration allows operation in electric mode during low- and medium-power demands, in addition to the ICE on/off operation. The location of the electric machine allows torque multiplication through the transmission, allowing a small maximum speed and providing good assist during high-power demands.
Post-transmission Type
In a post-transmission configuration, shown in Figure 4-12, the electric machine does not have the benefit of gear ratio changes. As a result, it must operate on a very broad vehicle speed range, requiring high torque over a wide speed range. The main disadvantage of the configuration is the need for high torque requirements for electric machines,
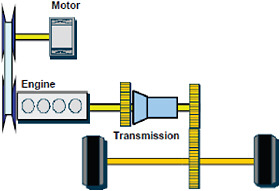
FIGURE 4-10 Example of integrated starter generator configuration coupled through a belt. Courtesy of University of Michigan.
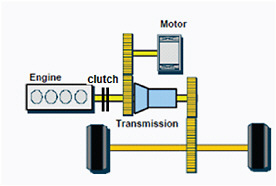
FIGURE 4-11 Example of pre-transmission parallel configuration. Courtesy of University of Michigan.
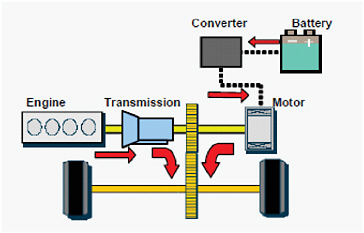
FIGURE 4-12 Example of post-transmission configuration. Courtesy of University of Michigan.
leading to an electric machine that is both larger and heavier than the corresponding machine in the pre-transmission configuration. However, compared to the pre-transmission type, it allows a higher efficiency path from the energy storage to the wheel, increasing the amount of regenerative braking captured at the energy storage system.
Parallel Hydraulic Hybrid
A parallel hydraulic hybrid utilizes two power sources, the ICE and the hydraulic motor for launch assist (HLA) to improve acceleration and reduce fuel consumption. The hydraulic pump/motor is located behind the transmission for more effective regeneration during braking. The hydraulic pump/motor is coupled to a propeller shaft via a transfer case, as shown in Figure 4-13. The HLA power source is an axial piston pump/motor with variable displacement. The hydraulic displacement per revolution can be adjusted via inclination of the swash plate to absorb or to produce desired torque. When pumping, hydraulic fluid flows from the low-pressure reservoir to the high-pressure accumulator; when motoring, hydraulic fluid flows in the reverse direction. The accumulator contains the hydraulic fluid and inert gas such as nitrogen, separated by a piston. When hydraulic fluid flows in, the gas is compressed, and its internal energy is increased. When discharging, fluid flows out through the motor and into the reservoir. The reservoir can be regarded as an accumulator working at much lower pressure (e.g., 8.5 to 12.5 bar). The size of hydraulic components is configured to absorb sufficient braking energy.
Power Split Hybrids
Power Split Hybrid Electric
Power split hybrids combine the best aspects of both series and parallel hybrids to create an extremely efficient system. As shown in Figure 4-14, this system divides the engine power along two paths: one goes to the generator to produce electricity, and one goes through a mechanical gear system to drive the wheels. In addition, a regenerative system uses the kinetic energy of deceleration and braking to produce electricity, which is stored in the energy storage system.
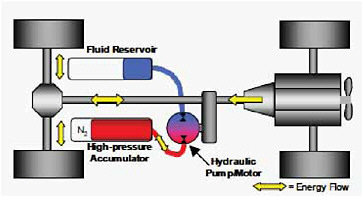
FIGURE 4-13 Parallel hydraulic launch assist hybrid architecture. SOURCE: Eaton-HTUF (2009). Courtesy of Eaton.
The most common configuration, called an input split, is composed of the following components: a power-split device (transmission), two electric machines, and an engine. According to the situation, all these elements operate differently. Indeed, the engine is not always on and the electricity from the generator may go directly to the wheel to help propel the truck or through an inverter to be stored in the battery. The different possibilities of an input split configuration are as follows:
-
When starting out, when moving slowly, or when the state of the battery charge is high enough, the ICE is not as efficient as electric drive, so the ICE is turned off and the electric machine alone propels the truck.
-
During normal operation, the ICE power is split, with part going to drive the vehicle and part being used to generate electricity. The electricity goes to the electric machine, which assists in propelling the truck. The generator acts as a starter to activate the engine.
-
During full-throttle acceleration, the energy storage (e.g., battery) provides extra energy.
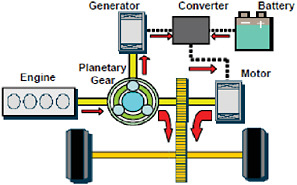
FIGURE 4-14 Power-split hybrid electric vehicle. Courtesy of University of Michigan.
-
During deceleration or braking, the electric machine acts as a generator, transforming the kinetic energy of the wheels into electricity.
Note that the two electric machines and the planetary gear behave as a continuously variable transmission (CVT) whose instantaneous gear ration depends on the amount of power fed from the generator to the motor.
Several variations of the power split have been implemented, each providing different advantages. In a single-mode power-split hybrid, the first electric machine is used to control the engine speed while the second one provides the remainder of the power required to follow the vehicle trace. In a dual-mode power system, composed of a compound mode in addition to the input mode, the size of the electric machine can be minimized as each motor is used to control the engine speed in different conditions. A dual-mode power system with several fixed gears minimizes the electric machine power requirements, and the system efficiency can be further improved by reducing the energy recirculation through the use of the fixed gears. The dual-split typically requires at least two planetary gears and one or more clutches, in contrast to the single-split, which requires only one planetary gear and no internal clutches.
The multimode power-split effects are as follows:
-
Smaller electric machine peak power and sizes.
-
Addition of clutches to transmission increases spin and pump losses.
-
ICE may not be at its optimum point during the fixed-gear mode.
-
Higher tractive capability during fixed-gear mode.
Power-Split Hybrid Hydraulic
In principle, hydraulic power-split architectures similar to the electric power-split architectures can be implemented as well. For instance, in Tavares et al. (2009), a hydraulic power-split system similar to the Toyota hybrid system in a Prius was chosen. Instead of using electric components, the proposed system is a hydraulic hybrid, comprising of two pump/motors coupled to a planetary gear set and a hydropneumatic accumulator for energy storage.
Plug-in Hybrids
Plug-in hybrids differ from HEVs by their ability to recharge the energy storage system through the electric grid. Since the vehicle is designed with high energy storage, batteries are usually used for this application. All the HEV configurations described above can be used for plug-in hybrids. In most cases, due to the electric energy focus, the electric machine power is increased compared to an HEV.
The vehicles can be recharged during the night when electricity prices are low in terms of demand and cost. Commercial vehicles, made by Smith Newton, are being delivered in the United States to AT&T, Frito-Lay, Coca-Cola, Staples, Kansas City Power & Light, and Pacific Gas and Electric Company. Odyne develops and manufactures plug-in hybrid electric drive systems for medium- and heavy-duty trucks. Odyne has developed three major systems: 10 kWh, 18 kWh, and 35 kWh. These systems can be used on a wide variety of truck applications, including bucket trucks, digger derricks, and underground utility vehicles. Eaton Corporation, Ford Motor Company, and the Electric Power Research Institute (EPRI) have developed both diesel and gasoline versions of a plug-in hybrid system for trucks (EPRI, 2008; Eaton, 2009). The system has been demonstrated on the medium duty, Ford F550, “trouble” truck platform used to repair and maintain the transmission and distribution infrastructure of utilities. By using grid electricity stored in batteries for part of the vehicle’s daily duty cycle, the plug-in vehicle can operate at the job site for several hours continuously, running the bucket, power tools, lights, and accessories without the need to run the gasoline or diesel engine. The plug-in hybrid truck, which is estimated to deliver fuel economy improvements of up to 70 percent compared with a conventionally powered truck, with corresponding reductions in harmful emissions, was developed for Southern California Edison. Eaton’s current hybrid systems used widely in work trucks and delivery vehicles, can reduce fuel consumption by 30 to 60 percent, with similar percentages in emission reductions, extended brake life, and idle time reductions up to 87 percent during work-site operations.
Plug-in hybrids will benefit considerably from intelligent vehicle technologies, especially if the algorithm knows how much farther the vehicle is going to be used and at what rate. In addition to drive-cycle characteristics, such as vehicle speed or acceleration, distance is a critical parameter to minimize plug-in vehicles fuel consumption (Karbowski, 2007).
Knowing what each type of hybrid demands in terms of electric power will give an immediate picture of the size and type of battery needed and in turn an idea about the weight and cost of implementing it.
For heavy-duty applications where the driver sleeps in the vehicle with the engine running while it is parked, a significant source of fuel consumption is the approximately 8 hours of engine idling time used a day to operate the air conditioning, heat, or on-board appliances (such as a television or microwave) and also to keep the fuel warm in cold weather. While hybrids in general aim for idle elimination, the ICE in a plug-in or conventional hybrid vehicle can be run at a specified and efficient power range to store energy in the battery packs. The ICE can be switched on and off as the state-of-charge requires. For plug-in hybrid vehicles, these so-called hotel loads can be powered with grid electricity at rest stops, if the required infrastructure is in place.
Batteries for Hybrid Vehicles
With the increased use of electrified systems in vehicles for both drivelines and accessories, it is important to consider the choice of the right battery technology. The battery’s contribution to the vehicle’s overall power is growing at a rapid pace, and the choices of battery affects the cost, reliability and service life, packaging space and weight, recyclable and “green” issues of the vehicle itself. As a rule of thumb, the battery system represents one-third of the overall increase in the cost of hybridization (Alamgir and Sastry, 2008). While hybrids and electric vehicles technologies are very promising, batteries are the Achille’s heel. Some of the several battery technologies available include lead-acid, zinc bromine, nickel cadmium, nickel metal hydride, lithium ion, and lithium polymer. Lead-acid, zinc bromine and nickel metal hydride (Ni-MH) were used in the initial era of EVs and HVs, but current vehicles rely on Ni-MH and lithium ion (Li-ion) battery technologies, with the latter seemingly having superior attributes for a vehicle system.
Figure 4-15 compares the relative gravimetric energy and power capabilities for the battery types used or being considered for automotive applications. The figure shows (as rectangular domains) the approximate ranges of energy and power densities required for the batteries of the various advanced-technology vehicles, including full HEV, plug-in hybrid electric vehicle (PHEV), and full performance battery electric vehicle (FPBEV); for details on vehicle types, refer to Kalhammer et al. (2007). These so-called Ragone plots show that, for each type, batteries designed for high power densities have substantially lower energy densities than batteries optimized for high energy (FPBEV designs). Whenever the performance domain for a specific vehicle type is below and to the left of the Ragone performance characteristic for a particular battery type, properly engineered versions of that battery type can be expected to meet vehicle power and energy requirements. It appears that Li-ion has good potential to be configured for superior power and energy density to meet various applications.
Figure 4-16 depicts the Li-ion performance status versus the targets set by the DOE FreedomCAR program (NREL, 2004). While Li-ion meets most of the requirements, but concerns remain regarding cost and life, as well as abuse tolerance to extreme operating temperatures and rapid charging and discharging rates within a safety level to be used in cars. In particular, the performance of Li-ion batteries erodes drastically at extreme temperatures (above 65 °C or below 0 °C). Therefore, in order to maintain battery life and performance, expensive and complex thermal management systems might be required. Also, under abusive conditions such as inadvertent overcharge, short circuit, or over-heating, a Li-ion cell will generate gas and experience an increase of internal pressure (Snyder et al., 2009). Hence, Li-ion technology might present a number of new system design and validation challenges that ensures robustness and durability for vehicle applications compared to NiMH technology. Accordingly, there is a need to carefully consider the trade-offs between Li-ion and NiMH technology, in terms of various factors, including system design, validation implications, and performance, among others.
Li-ion developments with iron, manganese, and nickel instead of cobalt have made them cheaper and safer. Modification of the electrode nanostructure has increased charge and discharge rate and cycle life. Current prototypes built by SAFT, LG, and A123, among others, are demonstrating the potential for lower-cost, longer-life Li-ion battery systems with less need for complex thermal management.
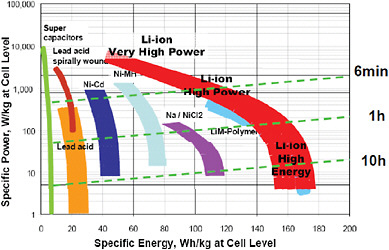
FIGURE 4-15 Battery type versus specific power and energy. SOURCE: Kalhammer et al. (2007).
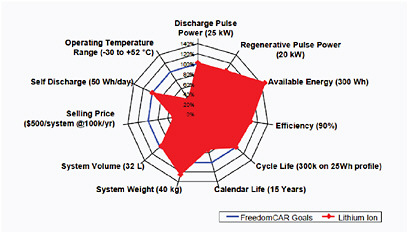
FIGURE 4-16 Li-ion status versus targets (for power-assist HEV). SOURCE: DOE (2005).
This is expected to help make HEVs more competitive in the marketplace and enable consumers to receive a faster payback. For instance, Ford has tested in the Escape hybrid model a Li-ion battery system that is said to be 20-30 percent smaller and 50 percent lighter than today’s Ni-MH technology, at 30 percent lower cost.28 Also, Argonne National Laboratory reports that the Enerdel/Argonne lithium-ion battery promises high reliability, light weight, and potential to meet the U.S. Advanced Battery Consortium’s $500 manufacturing price criterion for a 25-kilowatt battery (ANL, 2008). A recent NRC report has estimated costs for Li-ion batteries for light-duty plug-in electric hybrid vehicles, including projections of costs per kWh, which present a range for what such batteries may cost in the future (NRC, 2010).
Adding Ultracapacitors to Battery Packs (Dual Energy Storage)
Ultracapacitors (UCs) have their own merits and demerits compared to batteries. They can be charged and discharged very quickly, they have a longer life, and their performance does not degrade appreciably with use. On the other hand, for an equal size/weight, their energy-storing capacity (energy density) is extremely low. Despite this shortcoming, UCs can be supplemented by batteries. UCs offer a way to extend the life of a hybrid vehicle’s power source, reducing the need to oversize its battery packs. If UCs were paired with batteries, they could protect batteries from intense bursts of power such as those needed for acceleration, thereby reducing the need to oversize battery packs and extending the life of the batteries (NREL, 2004). UCs could also ensure that a vehicle can accelerate just as well at the end of its life as at the beginning. UCs are shown to improve the performance of batteries especially for start-stop ISA hybrids and mild hybrid systems. UCs can enable redesigning of batteries to hold more energy. Paired with UCs, batteries would not need to deliver bursts of power and so could be made with just a few layers of very thick electrodes, reducing the amount of supporting material needed. That could enable storing twice as much energy in the same space. The combined system has a better low-temperature performance and improved power and energy storage abilities. Disadvantages include increased energy storage cost and need for power converters.
Hybrid Technology Status
Table 4-5 presents a snapshot of the technology status and applications of the various hybrid system architectures currently available in the market.
Hybrid Electric Vehicles
Class 3 to 6 Straight Box Trucks
Hybrid type: Parallel heavy-duty hybrid system with no electric power takeoff (ePTO).
Fuel savings 20 to 25 percent were demonstrated depending on duty cycle. Table 4-6 shows the configuration used by three different manufacturers and the benefit obtained.
There are also a number of opportunities to continue to optimize the system. These include full electrification of accessories, which will allow engine shutdown at idle; integration of the hybrid system with emissions control;
|
28 |
“Ford’s Accelerated Battery Research Drives Development of Vehicle Electrification Plans.” Available on the Ford Motor Company website at http://media.ford.com/article_display.cfm?article_id30221. |
TABLE 4-5 Different Vehicle Architectures, Their Status as of Today and Primary Applications
|
Architecture |
Technology Status |
Primary Applications |
|
Medium-duty/heavy-duty parallel HEV |
Available now: Eaton, Azure, Volvo |
Refuse, urban pickup, and delivery (P&D) |
|
Medium-duty/heavy-duty parallel HEV w/e PTO |
Available now: Eaton, Volvo |
Bucket truck |
|
Parallel gasoline or diesel HEV bus |
Available now: ISE, Enova, BAE |
Transit bus |
|
Two-mode diesel HEV bus |
Allison |
Transit bus |
|
Series gasoline or diesel HEV bus |
Available now: ISE |
Transit bus |
|
Parallel hydraulic hybrid |
Introduced in 2009: Eaton, Parker Hannifin, Crane Carrier |
Refuse, urban P&D |
|
Series hydraulic hybrid |
Demo vehicles |
Refuse, urban P&D |
|
Parallel Class 2b |
Demo vehicles |
Class 2b pickups and vans |
|
Two-mode Class 2b |
Demo vehicles |
Class 2b pickups and vans |
|
Line-haul dual-mode HEV |
Demo vehicles |
Line-haul tractor trailer, |
|
Line-haul parallel HEV |
Demo vehicles |
Line-haul tractor trailer, motor coach |
|
SOURCE: TIAX (2009). |
||
TABLE 4-6 Production-Intent Medium-Duty and Heavy-Duty HEV Systems, No ePTO
|
System Attribute |
Eaton |
Volvo |
Azure |
|
Motor (peak) |
44 kW |
120 kW |
100 kW |
|
Battery |
Li-ion, 2 kWh |
Li-ion |
Ni-MH 2.4 kWH 288 V |
|
Percentage FC |
20-25 |
20 |
23-25 |
|
No idle? |
No |
Yes |
Yes |
|
Electric launch |
Yes |
Yes—12 mph |
Yes |
|
Applications |
Class 3-7 delivery; refuse soon |
Refuse; delivery soon |
Small delivery, shuttle |
|
SOURCE: TIAX (2009). |
|||
engine downsizing in certain applications;29 and improved integration and packaging. In combination these enhancements could improve fuel consumption benefits by another 5 to 10 percent (TIAX, 2009, page 4-81). Table 4-7 shows the predicted improvement that can be achieved with the parallel HEV of the future compared with the present.
TABLE 4-7 Hybrid Technology, Benefits and Added Weight for Class 3 to Class 6 Box Trucks
|
Architecture |
Percent Benefit (FC) |
Weight Added (lb) |
|
Parallel HEV |
20-25 |
450 |
|
Parallel HEV, future |
25-35 |
350 |
|
SOURCE: TIAX (2009). |
||
TABLE 4-8 Hybrid Technology, Benefits and Added Weight for Class 3 to Class 6 Bucket Trucks
|
Architecture |
Percent Benefit (FC) |
Weight Added (lb) |
|
Parallel HEV w/ePTO |
30-40 |
~650 |
|
Future Parallel w/ePTO |
35-45 |
~500 |
|
SOURCE: TIAX (2009). |
||
TABLE 4-9 Hybrid Technology, Benefits and Added Weight for Refuse Haulers
|
Architecture |
% Benefit (FC) |
Weight Added (lb) |
|
Parallel HEV |
20 |
450 |
|
Parallel HEV (future) |
25-30 |
~350 |
|
Parallel HEV, ePTO |
25 |
~650 |
|
Parallel HEV, ePTO (future) |
30-35 |
~500 |
|
Parallel HHV |
20-25 |
1,000 |
|
Series HHV |
40-50 |
~1,500 |
|
SOURCE: TIAX (2009). |
||
Class: 3-6 Bucket Trucks
Hybrid type: Parallel hybrid electric with ePTO.
HEVs with an ePTO system have demonstrated a 30 to 40 percent reduction in fuel consumption and a nearly 90 percent reduction in idle time.30 Table 4-8 compares this system with the predicted future of this type of vehicles.
Refuse Haulers
Hybrid type: Parallel hybrid electric with and without ePTO.
Fleet tests have demonstrated fuel consumption benefits on the order of 20 percent (TIAX, 2009, page 4-83) (see Table 4-9). These systems can also be tuned to maximize acceleration, which increases productivity by allowing a single vehicle to visit more houses per day. Systems tuned to maximize productivity have been shown to increase productivity by 11.5 percent while decreasing fuel use by 14 percent, for a total fuel savings of 26 percent. Both HEVs and HHVs also
|
29 |
Note that both the scope for engine downsizing and the benefits are very limited in heavy-duty applications and apply more to light-duty cases. For example, in-line haul fuel consumption with an 11- to 13-liter engine is the same as with a 15-liter engine. Since the durability of the larger engine is better, the market has gone mostly to the larger engine, except in very weight sensitive applications (bulk haulers, tankers, etc.). |
|
30 |
Freightliner, Run Smart, Business Class M2e Hybrid, AFVi Technology Showcase, May 2008. Available at www.oregonsae.org/Meetings/M2Hybrid/M2e_SAE.ppt. |
offer a significant operation and maintenance (O&M) savings by more than doubling brake life. The grid-charged compactor option (where the compactor used in a refuse hauler can be operated using electricity obtained from the grid) reduces fuel use by 25 percent.
Tractor Trailers
Hybrid type: Dual mode and parallel hybrid electric.
A line-haul duty cycle limits the amount of energy that can be recovered from regenerative braking. Rather, the advantages of hybridizing in this segment come from electrifying accessories and hotel loads, as well as limited gains from energy recovery and launch assist.
A dual-mode HEV (Arvin Meritor’s system; TIAX, 2009), and a parallel HEV (Eaton’s system; Coryell, 2008) are considered. Over-the-road benefits (i.e., not including idle reduction) are estimated to range from 5 to 7 percent for the parallel system (NESCCAF, 2009; Coryell, 2008) and 6 to 9 percent for the dual-mode system.
These on-road benefits are supplemented by overnight hotel road reduction, which reduces fuel use by an additional 5 to 8 percent, similar to that observed for auxiliary power unit idle reduction systems. Hotel loads are met by running the engine for a few minutes (4 minutes per hour, according to an Eaton/PACCAR demonstration) to recharge the battery (Carpenter, 2007).
Transit Buses
Hybrid type: Series, parallel, and dual-mode hybrid electric.
Fuel consumption savings range from 12 to 50 percent depending on the severity of the duty cycle (see FTA, 2005, page 2). The Central Business District cycle fuel consumption is decreased some 50 percent compared to the nonhybrid diesel baseline. The benefit decreases to 18 and 12 percent for the arterial and commuter cycles, respectively. This decrease is expected since the hybrid system’s benefit is increased for cycles with more stops and starts. In general, the fuel efficiency figures can be represented as shown in Table 4-10.
TABLE 4-10 Hybrid Technology, Benefits and Added Weight for Transit Buses
|
Architecture |
Percent Benefit (FC) |
Incremental Weight (lb) |
|
Gasoline series |
25-35a |
2,000 |
|
Diesel series |
30-40 |
2,600 |
|
Diesel parallel and dual-mode |
22-35b |
940-2,840 |
|
aSite visits. bFTA (2005). SOURCE: TIAX (2009). |
||
Motor Coaches
Hybrid type: Parallel hybrid electric.
The Northeast Advanced Vehicle Consortium showed on-road fuel savings for an MCI motor coach of 5 to 40 percent on a high-speed suburban duty cycle (FTA, 2005).
Class 2b Pickup Trucks
Hybrid type: Parallel and dual-mode hybrid electric.
The fuel consumption benefit for a Class 2b dual-mode hybrid is estimated to range from 20 to 30 percent, based on estimates from Vyas et al. (2002) and EPA (2008).
Simulation-Based Assessment and Interpretation of HEV Potential for Fuel Savings
The previous section demonstrates that reported fuel-saving benefits resulting from the adoption of various HEV architectures can vary over a considerable range, even for the same truck class. This is attributed to numerous factors, including differences in component characteristics and sizes, power management strategies, duty cycles, and driver behavior. High-fidelity modeling and simulation can play an important role in exploring potential benefits and assessing alternatives using common boundary conditions, as well as parametrically assessing the effects of various critical variables. It is crucial to stress the importance of accurate, real-world driving cycles to evaluate the potential performance of different hybrid vehicle architectures and their performance compared to traditional vehicles. As an illustration of the power of modeling and simulation when applied to HV fuel consumption assessments, select architectures are compared under prescribed driving cycles. Further, this section reports on the impact of using real-world driving cycles, including the effect of removing the breaks from the highway cycle to more accurately reflect real-world conditions and the effect of the grade of the driving cycle on fuel consumption.
Argonne National Laboratory (ANL) has conducted a case study on mild- and full-hybrid versions of a parallel pre-transmission hybrid (see Figure 4-17) for a Class 8 long-haul truck and compared predicted fuel consumption with the conventional power train configuration. The “mild-hybrid” configuration augmented the baseline engine with a 50-kW motor and a 5-kWh battery, thus enabling engine idle off, torque assist, and regenerative braking. The “full-hybrid” configuration featured the baseline engine plus a 200-kW motor (traction), a 50-kW motor (starter + generator), and a 25-kWh battery, thus enabling all features of a mild hybrid plus operation in electric-only mode and long idle, (i.e., battery capable of meeting energy demands for 10 hours for full hotel stop).
The three versions of the truck (conventional, mild and full hybrid) were simulated on three highway cycles (HHDDT [Heavy Heavy-Duty Diesel Truck Schedule] 65, HHDDT Cruise, HHDDT High Speed) and two transient/urban cycles (HHDDT Transient, UDDS Truck). Tests were conducted
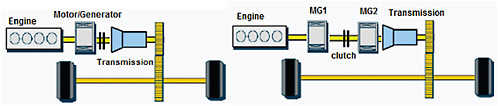
FIGURE 4-17 Hybrid configurations considered in ANL study. SOURCE: ANL (2009).
TABLE 4-11 Characteristics of Primary Drive Cycles
|
|
Average Speed (mph) |
Maximum Speed (mph) |
Maximum Acceleration (m/s2) |
Maximum Deceleration (m/s2) |
Distance (miles) |
Duration (s) |
Time Stopped (%) |
|
HHDT65 |
50 |
66.7 |
2 |
−2.8 |
26.5 |
1,904 |
5 |
|
HHDT Cruise |
39.9 |
59.1 |
0.42 |
−0.59 |
23.1 |
2,083 |
6 |
|
HHDDT High Speed |
50.2 |
66.1 |
0.69 |
−1.2 |
10.5 |
757 |
6 |
|
HHDDT Transient |
15.3 |
47.5 |
1.32 |
−2.4 |
2.8 |
668 |
17 |
|
UDDS Truck |
18.7 |
57.7 |
1.9 |
−2.1 |
5.5 |
1,060 |
33 |
|
SOURCE: ANL (2009). |
|||||||
at 50 percent load and full load. Table 4-11 gives important information about the drive cycles, which significantly affect the performance of the hybrid system, as will be shown in the results. The drive cycles used in the tests are shown in Table 4-12.
Figure 4-18 illustrates the predicted fuel savings of mild and full parallel hybrid configurations compared to a conventional power train for a Class 8 truck. For both configurations, the fuel savings are lower on the highway cycles, which is to be expected since the hybrid system does not help much at cruising speeds where the engine already operates efficiently. It should be noted that neither hybrid system has enough electrical storage to contribute to cruise power demand for any significant length of time. The mild-hybrid configuration shows fewer savings than the full hybrid, peaking at 11 percent, while the full hybrid can save up to 40 percent on an urban cycle. The fuel savings also tend to be lower with added mass, and this can be seen by the lower percentage benefit for the 100 percent load compared to the 50 percent load case.
Figure 4-19 shows the fraction of the total braking energy that is recovered at the wheel—meaning not including the driveline and electric machine losses involved in the channeling of that energy into the battery. The recovery rate depends on the cycle aggressiveness during deceleration. A heavier truck is more likely to reach its regenerative braking torque limitation sooner than a lighter one—hence the lower predictions for the fully loaded truck.
Figure 4-20 compares engine efficiencies for the conventional vehicle with the two hybrids over the drive cycles at two different loads. The mild hybrid does not show signifi-
TABLE 4-12 Profiles of Primary Drive Cycles
|
Drive Cycle |
Profile |
|
HHDDT 65 |
|
|
HHDDT Cruise |
|
|
HHDDT High Speed |
|
|
HHDDT Transient |
|
|
UDDS Truck |
|
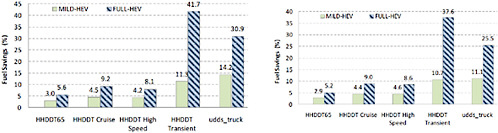
FIGURE 4-18 Fuel savings with respect to conventional cycles on standard drive cycles under (left) a 50 percent load and (right) a 100 percent load. SOURCE: ANL (2009).
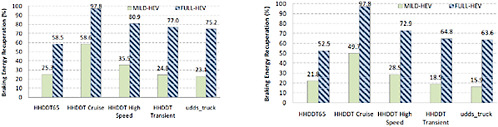
FIGURE 4-19 Percentage of braking energy recovered at the wheels under (left) a 50 percent load and (right) a 100 percent load. SOURCE: ANL (2009).
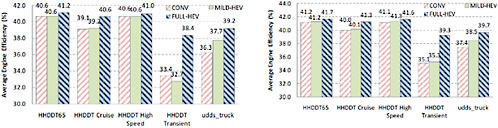
FIGURE 4-20 Percentage average engine efficiency of conventional and hybrid trucks for (left) a 50 percent load and (right) a 100 percent load on standard cycles. SOURCE: ANL (2009).
cant improvement since start-stop is the only main feature in it to aid in engine efficiency. The full hybrid gains in the transient and urban cycles as the engine can be completely switched off in electric-only mode.
Effect of Drive Cycle on Hybrid Performance
The drive cycle or duty cycle plays an important role in determining the following: type of hybrid technology to be used, level of hybridization and sizing of components, and power management strategy.
Effect of Removing Breaks from Highway Cycle
Since the HHDDT cycle is short and does not represent the real highway cycle, a new cycle was formulated with original acceleration followed by a cruising part and finally a deceleration part. Figure 4-21 shows how the new drive cycle was obtained from the HHDDT cycle. The results of removing stops from the HHDDT cycle have been grouped in Figure 4-22. In every other bar the hybrids are compared with the conventional vehicle, hence the values are greater.
When the breaks are removed from the highway cycle, there is a significant drop in fuel savings in the hybrid con-
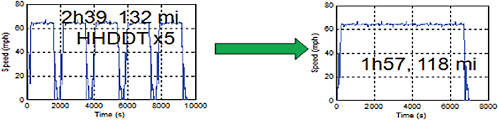
FIGURE 4-21 HHDDT 65 cycle repeated five times with stops (left) and without stops (right). SOURCE: ANL (2009).
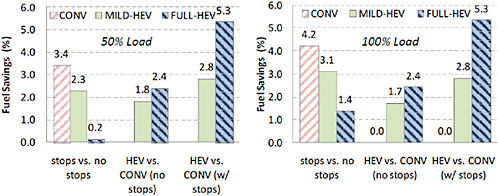
FIGURE 4-22 Fuel consumption reduction due to stop removal, with respect to conventional vehicles without stops, and with respect to conventional vehicles with stops (50 percent load on the left, 100 percent load on the right). SOURCE: ANL (2009).
figuration. However, the conventional configuration benefits the most when the stops are removed as the hybrids could have recovered part of the kinetic energy while braking. In the case of the full hybrid, the savings are more than halved (5.3 percent fuel saved on a cycle with stops, 2.4 percent fuel saved on a cycle without stops). In general, the hybrids still outperform the conventional vehicles in all cases as there are still some gains using the hybrid system even when the stops are removed.
Fuel Savings in Grades for Hybrid Configurations
Due to the lack of real-world drive cycles that include grades and to illustrate the potential benefits of hybridization in a “hilly” terrain, idealized sinusoidal road profiles were created. The elevation of such a road is a sinusoidal function of the horizontal distance, with a “hill” period varying between 1 and 3 km. Maximum grades also vary from 0 to 4 percent. All combinations of maximum grade and period were analyzed. Figure 4-23 shows the profile created.
For the mild-hybrid truck, the motor reaches its rated power when braking for grades 3 percent and higher when half-loaded and at or above 2.5 percent when fully loaded. The full hybrid hits its regenerative braking limit only when fully loaded at or above 3.5 percent grade. Thus, the full hybrid can capture more kinetic energy while braking, as expected.
The simulation results suggest that the mild hybrid has no advantage over the conventional vehicle when the grade is less than 2 percent, as there is not enough energy generated for accessories (see Figure 4-24).31 Charge balancing is hard to achieve, so the engine might be used to charge and hence may result in higher than expected fuel consumption. Furthermore, there is not much reduction in fuel consumption with grades greater than 3 percent, as the electric machine will reach its maximum potential.
The results also indicate that the available fuel savings with the full-HEV configuration can increase by as much as
14 percent for a 4 percent maximum grade with a hill period of 1 km. This suggests that the value of hybridization in tractor-trailer trucks may be significant in hilly terrains.
Hydraulic Hybrid Vehicles
Hydraulic hybrids have demonstrated fuel savings for medium- and heavy-duty applications. The energy savings can be attributed to optimization of engine operation and regenerative braking energy absorption.
EPA has been actively involved in vehicle-level demonstrations of this technology by using hydraulic launch assist in retrofitted urban delivery trucks. A hydraulic package of a reversible hydraulic pump/motor and accumulators was added to the vehicle to reduce fuel consumption, while keeping the vehicle’s conventional engine and transmission. Fuel savings of 25 and 45 percent were realized during city driving. For instance, Ford Motor Company, working jointly with EPA, demonstrated hydrualic power assist on a full-size sport utility vehicle platform fitted with a hydraulic pump/motor and valve block provided by infield technologies and carbon fiber accumulators developed by EPA. The pump/motor was connected to the vehicle driveshaft in parallel with the conventional power train. The vehicle demonstrated the ability to improve fuel economy by close to 24 percent on a start/stop, city-typical driving cycle (Kepner, 2002). Emissions were reduced by 20 to 30 percent. Better acceleration, reduced brake maintenance, and reduced operating costs (consumer payback of 4 to 5 years for city driving) were among other benefits (EPA, 2004). If the transmission and transfer case were replaced by a complete hydraulic drive train, larger fuel consumption benefits could be realized. In June 2006, EPA and United Parcel Service (UPS) demonstrated the world’s first full hydraulic hybrid delivery truck, which realized 60 to 70 percent reductions in fuel consumption under urban driving conditions and up to 40 percent reductions in greenhouse gas emissions, with an estimated payback shorter than 3 years.
Currently, EPA is focusing more on full-series hydraulic hybrids, along with improved vehicle aerodynamics, tires and advanced ICEs, including HCCI gasoline engines, free piston engines, completely variable displacement engines, alcohol engines, and exhaust heat recovery systems. Eaton Corporation, in collaboration with the EPA, has developed a series hydraulic hybrid power system that combines a high-efficiency diesel engine and a unique hydraulic propulsion system to replace the conventional drive train and transmission (Eaton, 2009). The engine operates at its “sweet spot facilitated by the continuously variable transmission (CVT) functionality of the series hybrid hydraulic system and by regenerative braking.” Fuel savings of 50 to 70 percent have been achieved, corresponding to a 40 percent reduction in greenhouse gases, 50 percent reduction in unburned hydrocarbons, and 60 percent reduction in particulate matter (EPA, 2009).
Simulation-Based Assessments of Hydraulic Hybrids
EPA predicted that the UPS prototype hydraulic hybrid vehicles would be able to capture and reuse 70 to 80 percent of the otherwise wasted braking energy (EPA, 2009).
Kim and Filipi (2007) from the University of Michigan conducted a simulation study on a series hydraulic hybrid light truck (mass = 5,112 kg). Approximately a 68 percent reduction in fuel consumption can be achieved in city driving and about 12 percent in highway conditions. The energy savings can be attributed to regeneration and engine shutdowns and a smaller fraction to optimization of engine operation. Design optimization over the complete driving cycle enabled right-sizing of all hydraulic pumps/motors, the accumulator volume, and the gear ratio of the two-step transmission. Downsizing the engine to roughly 75 percent of the baseline matched the acceleration of the conventional vehicle. In addition, it was found that having two smaller propulsion motors for each axle reduced fuel economy consumption by an additional 10 percent compared to a single propulsion motor architecture. The advantage is that the rear electric machine could be used mainly for acceleration and the front electric machine for regenerative braking, where both electric machines operate on high loads and in turn yield greater efficiency. Wu et al. (2004), also from the University of Michigan, developed a power management algorithm for a parallel hybrid and predicted fuel savings ranging from 28 to 48 percent depending on the types of pumps used.
Anderson et al. (2005) conducted a simulation study comparing hydraulic hybrid and electric hybrid vehicles. Fuel savings over a variety of driving cycles were found to be 39 percent for parallel hydraulic hybrids, compared to 31 and 34 percent improvements for parallel and series electric hybrids, respectively. Gotting (2007) estimated the fuel consumption benefit for the parallel HHV to range from 20 to 25 percent for Class 3 to 6 box trucks.
While indicative of the range of potential benefits, it should be noted that simulations are carried out under ideal conditions—hence results typically represent best-case scenarios. Real-world savings in fuel consumption are likely to be lower, because of off-design duty cycles and practical production vehicle constraints.
Power Management in Hybrid Vehicles
Power management is key in obtaining maximum performance and fuel savings from a hybrid vehicle. The objective of a power management algorithm in a hybrid vehicle is to compute the optimum operating point of the overall system for any amount of power demanded from the driver. The cost criteria are usually fuel economy and emissions. The way the power is managed will depend a lot on the sizing and characteristics of each of the components and their instantaneous state of operation. Mechanical efficiencies of the components (i.e., transmission, torque converter, differential), rolling resistance, aero drag coefficients, and instantaneous operating
point of components, among others, are of great importance with respect to fuel consumption but are considered as given. The hybrid power train architecture is also assumed to be given. There are three general power management algorithm types, as outlined below.
Heuristic Rule-Based
This type of control is constituted by heuristic rules—for example, if/then statements to split the power demanded from the driver into the electrical and mechanical subsystems. The majority of these rules are thermostatic—that is, actions are triggered when certain conditions are met. They are simple and easy to implement and less computationally expensive to develop, but they need a significant amount of tuning in order to achieve better results compared to a conventional vehicle. Their basic simplicity means the system will not operate at its best potential at all times and there is significant room for improvement. A rule-based algorithm for a particular vehicle can never be readily used for another. Nevertheless, this type of control is often used due to its ease of implementation and lower cost.
Table 4-13 shows the difference in fuel economy figures obtained during simulation of a hybrid electric transit bus using three different rule-based control algorithms.
Chu et al. (2003) developed a series HEV military bus (15,000 kg) prototype model and formulated an energy management strategy enabling the ICE to operate in its peak efficiency zones for reduced fuel consumption. A parametric design methodology also was established. Simulation results on four different driving cycles show that, on average, fuel savings improve by about 17 percent and, acceleration times by 25 percent, with top speed and gradability being the same compared to a conventional vehicle.
Real Time
Lately, there has been a growing research effort toward developing real-time power management algorithms of the power split between the thermal and electrical paths of HEVs. The main aspects of this approach are concerned with (1) the self-sustainability of the electrical path, which must be guaranteed for the entire driving cycle since the storage system cannot be expected to be recharged by an external source (fuel converter primarily, brake regeneration secondarily) and (2) the fact that no, or only limited, a priori knowledge of future driving conditions is available.
TABLE 4-12 Fuel Economy and Exhaust Emissions of Hybrid Electric Transit Bus with Various Control Strategies, Taipei City Bus Cycle
|
Hybrid Electric Bus |
Fuel Economy (km/L) |
CO (g/km) |
HC (g/km) |
NOx (g/km) |
PM (g/km) |
|
Speed control |
1.82 |
1.14 |
0.31 |
28.52 |
0.29 |
|
Torque control |
2.02 |
0.71 |
0.27 |
20.50 |
0.26 |
|
Power control |
2.15 |
0.70 |
0.24 |
18.98 |
0.23 |
|
Diesel bus Conventional control |
1.29 |
4.12 |
0.59 |
55.56 |
0.61 |
|
SOURCE: Wu et al. (2008). |
|||||
Such algorithms consist of an instantaneous optimization (Sciarretta et al., 2004; Rodatz et al., 2005; Pisu and Rizzoni, 2007); the objective function in this optimization is fuel consumption and emissions. Decisions on the energy flow path (engine path and electrical path) can be evaluated based on an Equivalence Consumption Minimization Strategy. The equivalence between electrical energy and fuel energy can be evaluated by comparing the cost of energy produced at any instant. An instantaneous objective function combines the weighted sum of electrical energy and fuel energy and is evaluated with regard to selection of a proper equivalence factor value at any instant. If the engine can produce the required power more efficiently than the electrical system (sweet-spot operating points), the engine energy path is favored, and when the motor can produce power more efficiently than the engine (low speed/low load), the electrical path is favored.
The key in a real-time power management system is simultaneous optimization of the operating points of the entire system as a whole and not just the engine alone. It aims for the best overall efficiency from the engine to the wheels or from the battery pack to the wheels as the case may be. However, owing to greater interactions among the involved subsystems (engine, motor, battery), the control complexity can rise rapidly with the number of agents or their behavioral sophistication. This increasing complexity has motivated continuing research on computational learning methods toward making autonomous intelligent systems that can learn how to improve their performance over time while interacting with the driver. These propulsion systems need to be able to sense their environment and also integrate information from the environment into all decision making.
This challenge can be effectively addressed by applying principles of cognitive optimization techniques. The problem can be formulated as sequential decision making under uncertainty in which an intelligent system (e.g., hybrid-electric vehicle, power train system) learns how to select control actions so as to reduce fuel consumption over time for any different driving cycle (Malikopoulos, 2009).
Dynamic Programming
Power management control algorithms employing dynamic programming (Scordia et al., 2005; Lin et al., 2003; Perez et al., 2006; Ogawa et al., 2008; Karbowski et al., 2009) rely on computing off-line the optimal control policy with respect to the available power train variables—that is, the power split between the thermal (engine) and electrical
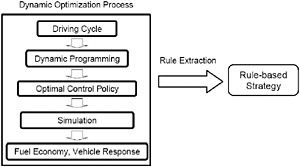
FIGURE 4-25 Dynamic programming process and rule extraction from the result. SOURCE: Lin et al. (2003).
paths (motor, generator, and battery), gear selection, etc., over a given driving cycle or a family of given driving cycles. Even though dynamic programming is not directly implementable, its results can be used to form an efficient rule-based control algorithm (as shown in Figure 4-25) or to develop vehicle-level control using neural networks (Delprat et al., 2001). The derived optimum policy is then approximated with simple rules and shift logic functions in order to be implemented in real time.
Figure 4-26 shows an example of a rule-based algorithm used in real time on an engine map to determine which of the power devices are being used depending on visitation points during the duty cycle. The main shortcoming of this approach is that it is efficient only for the driving cycles used in deriving the optimal policy. In addition, due to the high computational cost of dynamic programming, only simplified models of HEVs can be used. As a result, the extracted optimum policy omits a significant number of HEV dynamics that affects the efficiency of the derived policy even for the given driving cycle derived. Despite the aforementioned shortcomings, power management results based on deterministic dynamic programming methods are useful to serve as the benchmark of possible performance. Table 4-14 compares the predicted fuel consumption of a conventional vehicle against a hybrid power management optimized using dynamic programming and rule-based algorithms.
Crosscutting Issues and Future Outlook for Hybrids
During the committee’s discussions with manufacturers and suppliers, a number of overarching themes emerged with respect to hybrid technology discussed.
Brake O&M Benefits
In addition to saving fuel, hybridization significantly reduces brake costs. Suppliers and OEMs expect that hybrids will more than double brake life. For some applications these savings can outweigh the fuel savings.
TABLE 4-14 Predicted Fuel Consumption Comparison: Conventional (nonhybrid), Dynamic Programming (DP), and Rule-Based (RB)
|
|
DP |
RB |
Conventional |
|
Mpg |
13.85 |
12.65 |
10.39 |
|
Fuel (gallon) |
0.5259 |
0.5757 |
0.7005 |
|
SOURCE: Lin et al. (2003). |
|||
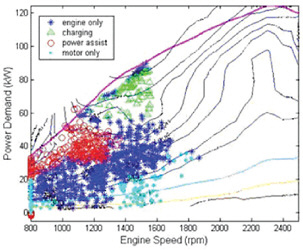
FIGURE 4-26 Implementing dynamic programming as a rule-based algorithm in SIMULINK. SOURCE: Lin et al. (2003).
System Integration
As hybrid systems become more fully integrated into overall vehicle architecture, there are a number of opportunities to further optimize the system:
-
Depending on the application, hybridizing a vehicle can enable engine downsizing. Currently, this is not widely done. Part of the reason is that industry is still figuring out which applications can use a downsized engine without sacrificing mission performance. Another important factor is that smaller engines that meet medium-/heavy-duty warranty requirements may be unavailable. One supplier suggested that they would be able to offer optimized, right-sized power trains at volumes of 5,000 to 10,000 vehicles per year.
-
There is opportunity for substantial integration between hybrid systems and exhaust aftertreatment systems. For example, a system designer could vary engine load to manage exhaust gas temperature, thereby offering emissions benefits. However, current standards measure engine-out emissions. To get credit for any benefit, EPA would need to switch to measuring emissions on a vehicle basis or develop a method to credit the lower vehicle emissions.
-
Improved system power density, component efficiency, and design integration are expected to offer additional fuel-saving opportunities.
-
Electrification is viewed as an enabler for more efficient waste-heat recovery systems, such as electric turbo-compounding or electric bottoming cycles. In a hybrid vehicle, electric waste-heat systems can offer an additional 1 to 2 percent efficiency benefit at neutral cost compared to an equivalent mechanical waste-heat system.
-
By narrowing the design window, hybrid systems enable the engine to be optimized to deliver peak fuel economy within a narrow operating band. This opportunity applies primarily to dual-mode or series systems.
Taken as a whole, improved systems integration can offer an additional 5 to 10 percent improvement in fuel efficiency in future systems in the years 2015-2020. In tandem, higher sales volume can reduce costs by a factor of 2.
Hybrid Power Train Summary
In its report for the committee, TIAX (2009) summarized the hybrid fuel consumption potential reductions by range of years and by application, as shown in Table 4-15.
Based on work discussed in this chapter as well as on the TIAX summary for hybrid power trains, the committee estimated potential fuel consumption reduction as shown in Table 4-16.
TABLE 4-15 Hybrid Fuel Consumption Reduction Potential (percentage) Compared to a Baseline Vehicle Without a Hybrid Power Train, by Range of Years and Application
TABLE 4-16 Estimated Fuel Consumption Reduction Potential (percentage) for Hybrid Power Trains
|
Tractor trailer |
5-10a |
|
Class 6 box truck |
20-35 |
|
Class 6 bucket truck |
30-45 |
|
Refuse truck |
20-35 |
|
Urban bus |
12-50 |
|
Motor coach |
5-40 |
|
Class 2b pickup and van |
18-30 |
|
aIncludes some reduction in hotel load, some idle reduction, and some electrification of accessories. |
|
FINDINGS AND RECOMMENDATIONS
Diesel Engine Technologies
Finding 4-1. Many individual technologies for reducing load-specific fuel consumption of diesel engines were identified. Some technologies are being used in 2010 by nearly all manufacturers (common rail fuel injection and selective catalytic reduction, SCR), and some are being used by a limited number of manufacturers (turbocompounding and multiple turbochargers). One manufacturer, Cummins, has shown a roadmap for 49.1 percent thermal efficiency by 2016 and 52.9 percent by 2019, which are 14.5 and 20.6 percent reductions in fuel consumption, respectively, from a 2008 baseline, compared to current diesel fuel consumption. Significant technical challenges remain to be overcome before many of the fuel-saving technologies described in this section can be successfully implemented in production.
Gasoline Engine Technologies
Finding 4-2. Technologies exist today, or are under development, that offer the potential to reduce the fuel consumption of gasoline-powered vehicles operating in the medium-duty vehicle sector. The most beneficial technologies and the
magnitude of fuel savings will be dependent on the configuration of the engine and the duty cycle of its application. Under optimal matching of technology and duty cycle, fuel consumption reductions of up to 20 percent appear to be possible compared to 2008 gasoline engines in the 2015 to 2020 time frame. The economic merit of integrating different fuel-saving technologies will be an important consideration for operators and owners in choosing whether to implement these technologies.
Recommendation 4-1. Development of fuel-saving engine technologies and their effective integration into the engine/power train is critical for reducing fuel use by medium- and heavy-duty vehicles and helping the nation to meet national goals related to energy security and the environment. The federal government should continue to support such programs in industries, national labs, private consulting companies, and universities.
Diesel Engines Versus Gasoline Engines
Finding 4-3. Diesel engines can provide fuel consumption advantages, compared to gasoline engines, of 6 to 24 percent depending on application, duty cycle, and baseline gasoline engines.
Finding 4-4. Diesel engines are increasing in cost primarily due to emissions aftertreatment equipment (DPF and SCR), which can cost over $17,000. Because of this cost increase (and diesel fuel prices), dieselization of Class 6 trucks in the new sales fleet went from 75.8 percent in 2004 to 58.0 percent in 2008. The effect of 2010 emission regulations has yet to be felt, but it is expected to accelerate the trend toward gasoline engines in medium-duty trucks.
Recommendation 4-2. Because the potential for fuel consumption reduction through dieselization of Class 2b to 7 vehicles is high, the U.S. Department of Transportation/National Highway Traffic Safety Administration (NHTSA) should conduct a study of Class 2b to 7 vehicles regarding gasoline versus diesel engines considering the incremental fuel consumption reduction of diesels, the price of diesel versus gasoline engines in 2010-2011, especially considering the high cost of diesel emission control systems, and the diesel advantage in durability, with a focus on the costs and benefits of the dieselization of this fleet of vehicles.
Transmission and Driveline Technologies
Finding 4-5. The transmission ratio and axle ratio affect fuel consumption by determining the engine speed versus road speed of the vehicle. A properly specified transmission and axle will allow the engine to run at its best fuel consumption operating range for a given road speed.
Finding 4-6. Manual transmissions have the least mechanical losses. An automated manual transmission can reduce fuel consumption by reducing driver variability (4 to 8 percent benefit). The fully automatic transmission can improve productivity by reducing the shift time (power shift) and by avoiding engine transient response delays and can reduce fuel consumption (up to 5 percent) by reducing driver variability, but the AT has higher parasitic losses.
Recommendation 4-3. The industry should continue its practice of training dealers and provide training materials for truck specifications affecting fuel consumption, such as transmission ratios, axle ratios, and tire size.
Hybrid Power Trains
Finding 4-7. Fuel consumption reductions on hybrid vehicles 5 to 50 percent have been reported by enabling optimum engine operation, downsizing in certain cases, braking energy recovery, accessory electrification, and engine shutdown at idle.
Finding 4-8. A wide range of hybrid electric and hydraulic architectures have been demonstrated. The selection of a particular system architecture depends mainly on application, duty cycle, and cost-benefit trade-offs.
Finding 4-9. The realized fuel consumption benefits of a particular hybrid technology and architecture implementation are strongly dependent on application and duty cycle. Optimization of component sizing and power management are keys to maximizing the potential for fuel consumption reductions while satisfying performance and emission constraints.
Finding 4-10. Computer simulation of medium- and heavy-duty vehicles is an effective way to predict fuel consumption reductions considering the additional variables in a hybrid vehicle system, but such systems are not standardized, leading to a wide variety of results and unpredictability.
Recommendation 4-4. NHSTA should support the formation of an expert working group charged with evaluating available computer simulation tools for predicting fuel consumption reduction in medium- and heavy-duty vehicles and developing standards for further use and integration of these simulation tools.
BIBLIOGRAPHY
Alamgir, M., and A.M. Sastry. 2008. Efficient batteries for transportation applications. Paper No. 08CNVG-0036. Presented at SAE International, Convergence Transportation Electronics Conference 2008, Detroit, Mich. Available at http://amsl.engin.umich.edu/publications/08SAE_Alamgir_Sastry.pdf.
Allison Transmission. 2008. Superior Fuel Efficiency. Allison Transmission Brochure SA5704EN. Indianapolis, Ind.
Allison Transmission. 2009. Allison fuel efficiency improvements. PowerPoint presentation provided to the committee by Allison Transmission. July.
Allison Transmission. 2009. Field Test Data on Class 8 Distribution Tractors. Excel spreadsheet summary provided to the committee by Allison Transmission. July.
Altoona Bus Research and Testing Center, Online Bus Database. Available at http://www.altoonabustest.com/.
Anderson, S.R., D.M. Lamberson, T.J. Blohm, and W. Turner. 2005. Hybrid route vehicle fuel economy. Advanced Hybrid Vehicle Powertrains 2005. SAE Paper 2005-01-1164. Warrendale, Pa.: SAE International.
Andrew, M. 2006. Lithium-ion: Enabling a spectrum of alternate fuel vehicles. Presentation by Johnson Controls-Saft to the Zero-Emission Vehicles (ZEV) Technology Symposium, Sacramento, Calif., Sept. 25-27.
ANL (Argonne National Laboratory). 2008. EnerDel/Argonne Advanced High-Power Battery for Hybrid Electric Vehicles. August. Available at http://www.transportation.anl.gov/batteries/enerdel.html.
ANL. 2009. Evaluation of Fuel Consumption Potential of Medium and Heavy Duty Vehicles Through Modeling and Simulation.
Baseley, S.J., C. Ehret, E. Greif, and M. Kliffken. 2007. Hydraulic hybrid systems for commercial vehicles. SAE Paper 2007-01-4150. Warrendale, Pa.: SAE International.
Chu, L., W. Wang, and Q. Wang. 2003 Energy management strategy and parametric design for hybrid electric military vehicle. SAE Paper 2003-01-0086. Warrendale, Pa.: SAE International.
Coryell, R. 2008. PACCAR—Eaton Hybrid Collaboration: Taking HD Hybrid from Concept to Commercialization. Presented at the NESCCAF/ICCT Workshop on Improving the Fuel Economy of Heavy-Duty Fleets II, San Diego, Calif. Feb. 20.
Delprat, S., T.M. Guerra, and J.Rimaux. 2001. Optimal Control of a Parallel Powertrain. Presented at the International Electric Vehicle Symposium (EVS18), Berlin. October.
DOE, EERE. 2009. 2008 Vehicle Technologies Market Report. Golden, Colo.: National Renewable Energy Laboratory. July. Available at http://www.nrel.gov/docs/fy09osti/46018.pdf.
Eaton EPRI press release. Available at http://www.reuters.com/article/pressRelease/idUS195396+19-Mar-2009+BW20090319.
Eaton-HTUF (Hybrid Truck User’s Forum) Conference. 2009. Eaton hybrid hydraulic technology & development. Slide 3. Atlanta. October 27-29.
Eaton. 2009. Series Hybrid Hydraulic. Available at http://www.eaton.com/EatonCom/ProductsServices/Hybrid/SystemsOverview/SeriesHydraulic/index.htm.
EPA (U.S. Environmental Protection Agency). 2004. Hydraulic Hybrid Technology—A Proven Approach. EPA brochure 420-F-04-024. March. Available at http://www.epa.gov/oms/technology/420f04024.pdf.
EPA. 2009. Region 9: Air Programs. Hydraulic Hybrid Vehicles. Available at http://www.epa.gov/region09/air/hydraulic-hybrid/.
EPRI (Electric Power Research Institute). 2008. Plug-In Hybrid Trouble Truck: An EPRI/Utility Alliance with Eaton Corporation and Ford Motor Company. Palo Alto, Calif.: EPRI. Available at http://mydocs.epri.com/docs/public/000000000001016496.pdf.
FTA (Federal Transit Administration). 2005. Analysis of Electric Drive Technologies for Transit Applications: Battery-Electric, Hybrid-Electric, and Fuel Cells. U.S. Department of Transportation, Report FTA-MA-26-7100-05.1. August. Available at http://www.navc.org/Electric_Drive_Bus_Analysis.pdf.
Freightliner vehicle specification information provided by the Columbia Truck Center, a Freightliner dealership in Columbia, S.C., July 2009. Freightliner Business Class Data Book PRL-72E.013
GM BMW DaimlerChrysler. 2006. Hybrid Kooperation. Internationales Wiener Motorensymposium.
Gotting, G. 2007. Hydraulic Launch Assist HLA System. Eaton. Presented to the Houston Advanced Research Center.
Hanson, R., R. Reitz, D. Splitter, and S. Kokjohn. 2010. An experimental investigation of fuel reactivity controlled PCCI combustion in a heavy-duty engine. SAE Paper 2009-01-0864. Warrendale, Pa.: SAE International.
ICCT (International Council on Clean Transportation). 2008. Japan’s “Top Runner” Fuel Economy Standards for Heavy-Duty Vehicles. Washington, D.C.: ICCT. February 11.
Kalhammer, F., B. Kopf, D. Swan, V. Roan, and M. Walsh. 2007. Status and Prospects for Zero Emissions Vehicle Technology. Report of the ARB Independent Expert Panel 2007. Prepared for the State of California Air Resources Board (ARB), Sacramento, Calif. April 13.
Karbowski, D. 2007. Plug-in Vehicle Control Strategy: From Global Optimization to Real Time Application. Presented at the International Electric Vehicle Symposium, EVS23, Anaheim, Calif. December 2-5.
Karbowski, K., F. von Pechmann, S. Pagerit, J. Kwon, and A. Rousseau. 2009. Fair comparison of powertrain configurations for plug-in hybrid operation using global optimization. SAE Paper 2009-01-1383. Presented at the SAE World Congress, Detroit, April.
Kepner, R.P. 2002. Hydraulic power assist—A demonstration of hydraulic hybrid vehicle regenerative braking in a road vehicle application. SAE Paper 2002-01-3128. Warrendale, Pa.: SAE International.
Kim, Y.J., and Z.S. Filipi. 2007. Simulation study of a series hydraulic hybrid propulsion system for a light truck. SAE Paper 2007-01-4151. Warrendale, Pa.: SAE International.
Kruiswyk, R. 2008. Annual Progress Report 2008 Vehicle Program: Advanced Combustion Engine Technologies. Prepared for the U.S. Department of Energy by Caterpillar. Washington, D.C.: U.S. Department of Energy.
Lin, C.-C., H. Peng, J.W. Grizzle, and J.-M. Kang. 2003. Power management strategy for a parallel hybrid electric truck. IEEE Transactions on Control Systems Technology, 11(6): 839-849.
Lin, C., et al. 2003. Control system development for an advanced-technology medium-duty hybrid electric truck. SAE Paper 2003-01-3369. Warrendale, Pa.: SAE International.
Liu, J., and H. Peng. 2008. Modeling and control of power-split vehicle, IEEE Transactions on Control Systems Technology, 16(6): 1242-1251.
Malikopoulos, A.A. 2009. Convergence properties of a computational learning model for unknown Markov chains. Journal of Dynamic Systems, Measurement and Control, 131(4).
NESCCAF/ICCT, Southwest Research Institute, and TIAX. 2009. Reducing Heavy-Duty Long Haul Combination Truck Fuel Consumption and CO2 Emissions. ICCT. October.
NHTSA (National Highway Traffic Safety Administration) 2009. Average Fuel Economy Standards Passenger Cars and Light Trucks Model Year 2011. Docket No. NHTSA-2009-0062. Washington, D.C.: U.S. Department of Transportation. March.
NRC (National Research Council). 1992. Automotive Fuel Economy: How Far Should We Go? Washington D.C.: National Academy Press.
NRC. 2008. Review of the 21st Century Truck Partnership. Washington, D.C.: The National Academies Press.
NRC. 2010. Transitions to Alternative Transportation Technologies: Plug-in Hybrid Electric Vehicles. Washington, D.C.: The National Academies Press.
NREL (National Renewable Energy Laboratory). 2004. Milestone Report 20040901. Golden, Colo.: NREL.
Ogawa, T., H. Yoshihara, S. Wakao, K. Kondo, and M. Kondo. 2008. Design estimation of the hybrid power source railway vehicle based on the multiobjective optimization by the dynamic programming. IEEE Transactions on Electrical and Electronic Engineering, 3(1): 48-55.
Patton, K.J., A.M. Sullivan, R.B. Rask, and M.A. Theobald. 2002. General Motors Corporation aggregating technologies for reduced fuel consumption: A review of the technical content in the 2002 National Research Council report on CAFE. SAE Paper 2002-01-0628. Warrendale, Pa.: SAE International.
Perez, L.V., G.R. Bossio, D. Moitre, and G.O. Garcia. 2006. Optimization of power management in an hybrid electric vehicle using dynamic programming. Mathematics and Computers in Simulation, Vol. 73, Special Issue, pp. 244-254.
Pesaran, A., T. Markel, M. Zolot, and S. Sprik. 2005. Ultracapacitors and Batteries in Hybrid Vehicles. Presented at Advanced Capacitors, San Diego, Calif. July 11-13. National Renewable Energy Laboratory document NREL/PR-540-40630. Available at http://www.nrel.gov/vehiclesandfuels/energystorage/pdfs/38484.pdf.
Pisu, P., and G. Rizzoni. 2007. A comparative study of supervisory control strategies for hybrid electric vehicles, IEEE Transactions on Control Systems Technology,15(3): 506-518.
Rodatz, P., G. Paganelli, A. Sciarretta, and L.Guzzella. 2005. Optimal power management of an experimental fuel cell/supercapacitor-powered hybrid vehicle. Control Engineering Practice, 13(1): 41-53.
SAE (Society of Automotive Engineers). 2004. Analysis of the performance and emissions of different bus technologies on the city of San Francisco Routes. SAE Paper 2004-01-2605. Warrendale, Pa.: SAE International.
Sciarretta, A., M. Back, and L. Guzzella. 2004. Optimal control of parallel hybrid electric vehicles. IEEE Transactions on Control Systems Technology, 12(3): 352-363.
Scordia, J., M. Desbois-Renaudin, R. Trigui, B. Jeanneret, F. Badin, and C. Plasse. 2005. Global optimisation of energy management laws in hybrid vehicles using dynamic programming. International Journal of Vehicle Design, 39(4): 349-367.
Snyder, K.A., X.G. Yang, and T.J. Miller. 2009 Hybrid vehicle battery technology—The transition from NiMH to Li-Ion. SAE Paper 2009-01-1385. Warrendale, Pa.: SAE International.
Stein, R., C. House, and T. Leone. 2009. Optimal use of E85 in a turbocharged direct injection engine. SAE Paper 2009-01-01490. Warrendale, Pa.: SAE International.
Tavares, Fernando, Rajit Johri, and Zoran Filipi. 2009. Simulation study of advanced variable displacement engine coupled to power-split hydraulic hybrid powertrain. ICES2009-76113. Proceedings of the ASME Internal Combustion Engine Division 2009 Spring Technical Conference ICES2009 May 3-6, Milwaukee, Wisc.
TIAX. 2009. Assessment of Fuel Economy Technologies for Medium- and Heavy-Duty Vehicles. Report prepared for the National Academy of Sciences by TIAX LLC, Cupertino, Calif. July 31.
Vuk, C. 2006. Electric Turbo Compounding. A Technology Whose Time Has Come. Presented at the Diesel Engine-Efficiency and Emissions Research (DEER) Conference, August 20-24, Detroit, Mich.
Vyas, A, C. Saricks, and F. Stodolsky. 2002. The Potential Effect of Future Energy-Efficiency and Emissions-Improving Technologies on Fuel Consumption of Heavy Trucks. Argonne National Laboratory (ANL) Report ANL/ESD/02-4. Argonne, Ill.: ANL.
Wu, B., C.-C. Lin, Z. Filipi, H. Peng, and D. Assanis. 2004. Optimal Power Management for a Hydraulic Hybrid Delivery Truck. Available at http://www-personal.umich.edu/~hpeng/VSD_from_AVEC_HHV.pdf.
Wu, Y.-Y., B.-C. Chen, and K.D. Huang. 2008. The effects of control strategy and driving pattern on the fuel economy and exhaust emissions of a hybrid electric bus. SAE Paper 2008-01-0306. Warrendale, Pa.: SAE International.
Zhao et al. (eds.) 2003. Homogeneous Charge Compression Ignition (HCCI) Engines: Key Research and Development Issues. Warrendale, Pa.: SAE International.