D
Case Studies of HHS Chemical, Biological, Radiological, and Nuclear Medical Countermeasure Development Programs, Executive Summary
The following is a white paper prepared for the February 22–24, 2010, workshop on the public health and medical countermeasure enterprise, hosted by the Institute of Medicine Forum on Medical and Public Health Preparedness for Catastrophic Events and Forum on Drug Discovery, Development, and Translation. All opinions expressed in this paper are those of the author and not necessarily of the Institute of Medicine.
By Robert Kadlec
Vice President
Global Public Sector
PRTM
The US government has a long and complex history of medical countermeasure development programs for chemical, biological, radiological and nuclear threats. The goal of this document is to revisit those experiences in order to glean insights into overarching challenges, successful strategies, and areas for improvement across the mission space. Seven medical countermeasure programs were analyzed to identify the most significant contributors to risk for each program, and the factors that led each program to meet or fall short of its goals. Throughout the history of the mission, the US government has actively sought to lower barriers to industry participation and the “rules of the game” have been continually amended and improved. As such, each medical countermeasure program was subject to an evolving framework of rules, challenges, and opportunities.
The following seven medical countermeasure programs were examined for this analysis:
-
BIOTHRAX™ Anthrax Vaccine Adsorbed (AVA)
-
Recombinant Protective Antigen (rPA) Vaccine
-
ACAM2000™ Smallpox Vaccine
-
IMVAMUNE™ Modified Vaccinia Ankara (MVA) Smallpox Vaccine
-
Medical Countermeasures for Hematopoietic Acute Radiation Syn-drome (hARS)
-
Medical Countermeasures for Viral Hemorrhagic Fevers (VHF)
-
Broad Spectrum Antibiotics for Bacterial Threats
This executive summary compiles the key elements of each program across the spectrum of medical countermeasure activities. The key risk factors that impacted each program are summarized. Overarching conclusions from the case studies are drawn, and potential areas for future solutions are outlined.
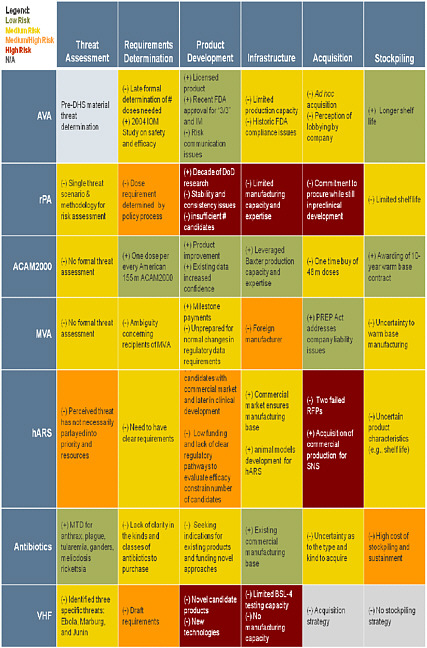
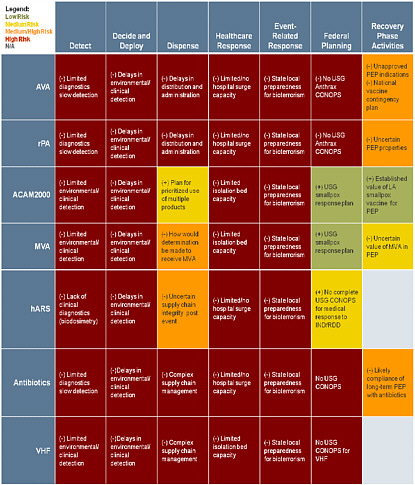
CASE STUDY SUMMARY TABLES
The following tables are intended to depict the entire value chain for medical countermeasure development, from initial threat assessment and requirements determination to response and recovery activities. These tables demonstrate the dependencies throughout the operational spectrum for each medical countermeasure analyzed. The color coding assesses the relative risk associated with each medical countermeasure for each component of the framework. While the focus of this study is the advanced development and procurement of medical countermeasure, the risks of failure do not end when a product enters into the SNS but ultimately reside on whether that medical countermeasure can be distributed and administered to those who need it when they need it.
Case Study Summary: Left Side/Preparedness Activities Preparedness Assessment Framework Mission Components
The Elements of Risk
Scientific/Technical Risks
Assessing the risks of commercial drug development is complicated by the source of the candidate product, therapeutic class, and product type. There are generally higher clinical approval success rates for in-licensed candidates as compared to candidates that are self-originated. In-licensed drugs may benefit from screening or testing prior to licensing, and many have been acquired after
clinical testing has begun.1 Moreover, some argue that in-licensed candidates are subject to a more rigorous analysis than products that have been self- originated.
Success rates for new drugs also vary by therapeutic class.2,3 The table below illustrates the variability between three classes of compounds.
|
Therapeutic Class |
||
|
From Discovery |
From Phase III |
|
|
Systemic Anti-Infective |
15% |
79% |
|
Anti-Neoplastic/Immunologic |
7.1% |
55% |
|
Central Nervous System |
3.8% |
46% |
These probabilities emphasize the dramatic increases in risk for early-stage candidates as compared to products later in advanced development. However, even when clinical safety studies have been completed success is far from guaranteed. The findings also suggest that product development programs and associated pipeline strategies must be tailored for the desired product category. While the anti-infective statistics above provide some optimism for medical countermeasures for biodefense indications, the many challenges of working with these threat agents limit any real ability to compare across categories of threat agents. Additional differences in the data were noted when comparing small and large molecules, such as monoclonal antibodies.6
Probability of Success to Licensure
Numerous technical factors impact the chance of success of a product. Failure of large pharmaceutical company commercial drug trials are principally attributed to lack of efficacy (approximately 30%), adverse safety and toxicity profiles (an additional 30%), as well as commercial considerations (cost of development and estimated return on investments).7 While failure is undesirable, the pharmaceutical industry views it part of the cost of doing business. Companies prefer when a candidate fails early in its development, as it limits actual and opportunity costs. For the development of CBRN medical countermeasures, failure must be viewed similarly: inevitable, acceptable, and ideally occurring at an early stage in development.
The lack of validated animal models emerged as a significant and recurring contributor to risk across all case studies. US government partners can do much more to coordinate across organizations, ensuring appropriate data sharing agreements, efficient validation processes, and more rapid means of finalizing animal model reports.
The main tool to mitigate scientific and technical risks is in building from existing products and product candidates—pursuing dual-use of commercially available products, or improving the characteristics of an already available medical countermeasure. Any efforts to move away from developing novel single-purpose products will help to reduce the research and development risks. The use of flexible platforms for development and production also represent a promising mechanism for reducing risk throughout the lifecycle of a product.
Legislative/Legal Risks
Project BioShield procurements have strict requirements on approval and contracting mechanisms that severely restrict the ability for the US government
to offer the most appropriate contracting vehicle to each performer. These acquisitions were given greater flexibility through the use of milestone payments authorized in the milestone funding that was enabled by the legislation contained in the Pandemic and All-Hazards Preparedness Act of 2006. BARDA has not yet utilized the Other Transaction Authority that has been granted to it, but NIAID has shown some success in using MTAs, CDAs, and modified clinical trial agreements to effectively collaborate with private sector partners.
The liability protections enacted in the PREP Act8 has assuaged the concerns of many firms that develop medical countermeasures. This legislation mandates that manufacturers have total immunity from liability surrounding use of their products unless there is evidence of willful misconduct on the part of the company.
Financial Risks
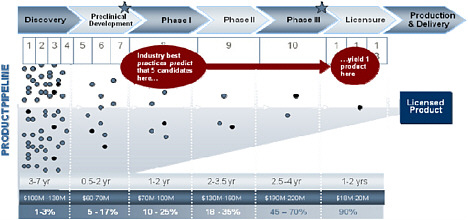
The commercial pharmaceutical research and development of FDA approved vaccines, drugs and diagnostics are acknowledged as expensive and long processes with a substantial risk for failure. The current costs of bringing a new medicine from discovery through licensure are estimated at between $570 million to $1.7 billion.9,10 In addition to the inherent costs, data derived from surveys of the major pharmaceutical companies indicate significant attrition. The current commercial trend indicates that 13 preclinical candidates are necessary to yield a single successful approved product—an approximately 8% chance of success.11 For those candidates entering phase I clinical trials, only one in six (17%) make it to the marketplace.12 Once a candidate enters Phase III clinical trials, it may only have a 64% chance of becoming an approved product.13 Failures in Phase III are particularly expensive in both real and opportunity costs.14 Finally, the time to identify and develop a new candidate vaccine or drug can be between seven to twelve years. The pre-clinical drug
discovery process can last from one to five years and the average time between the authorization of an Investigational New Drug (IND) entering into phase I clinical trials to the finalizing of the New Drug Application (NDA) or Biologics License Application (BLA) is approximately 6.4 years.15
The small number of successful CBRN medical countermeasure compounds the above industry percentages and adds significantly greater difficulty in attracting industry partners. In addition to the direct costs, commercial product developers potentially miss out on successful commercial products by investing time and capital into this niche market. Therefore, the opportunity costs alone limit the number of industry partners interested in initiating CBRN medical countermeasure programs.
Companies involved in the CBRN medical countermeasure market may also encounter unexpected financial challenges that require additional assistance from the USG. In addition, companies in this sector typically have limited financial resilience; without advance payments or milestone funding, typical technical or regulatory setbacks can threaten the viability of these companies. On the other hand, full US government support of single product manufacturers is an expensive venture. One developer of one product can easily require billions of dollars of investments for product development and licensure, warm-base manufacturing, stockpiling and replenishment activities.
Dramatic differences in portfolio spending have been noted, even among the top priority medical countermeasure programs highlighted in the 2007 HHS PHEMCE Implementation Plan. Medical countermeasures for radiological and nuclear threats have received a small fraction of the funding allocated for anthrax medical countermeasures. While the radiological/threat medical countermeasure community has responded with increased collaboration and exploration of alternative development and stockpiling strategies, this lack of investment at all stages of the development pipeline continues to discourage most potential industry partners.
Regulatory Risks
Uncertainty about regulatory endpoints (definition of “usable product”) and unanswered regulatory related science issues create further challenges. Technical limitations and significant scientific gaps increase the challenges of navigating the regulatory pathway. As animal models mature along with candidate products, additional knowledge that is uncovered during the process can significantly impact the pathway to licensure or approval of a product. Performers need to be prepared for such uncertainty.
The USG should examine ways to assist companies during the regulatory process. FDA has expended extraordinary energy in ushering inexperienced companies through the development process. Such companies are often characterized as requiring extensive “hand-holding” through assay development and product stability submission, and still encounter significant problems. Regulatory submissions are often of poor quality, with highly unrealistic timelines. These problems are compounded by the inability to attract large and experienced performers to this space.
Expanding indications for already existing products, such as broad-spectrum antibiotics, should be the most straightforward path for building the medical countermeasure stockpile. However, these efforts have been fraught with a lack of clear guidance and fractured government development processes. Interagency coordination and a clearly articulated goal are essential to success.
Organizational Risks
Besides the scientific and regulatory factors cited above, there are risk factors that are inherent to participating companies that affect the likelihood of successful CBRN medical countermeasure development. The quality of its management, the staff’s technical experience, familiarity with the regulatory process, any past experience with successful licensure of a product, and the company’s financial soundness affect the perceived likelihood of success. There are extrinsic factors that relate to the company’s interface with the United States Government (USG), particularly through publication of medical countermeasure requirements and product characteristics, the Federal Acquisitions Regulations (FAR) contracting or research grant processes, and adherence to the FDA regulatory process. There are also policy and legislative considerations that play a role that are extrinsic to the company. Executive Branch and Congressional efforts to promote research and development of CBRN medical countermeasure reflect the desire by the USG to reduce the barriers to entry and offer incentives to increase the participation of companies.
There are other considerations as well. Perceptions of the CBRN medical countermeasures market, and hence profit potentials, have their own set of influences as to the kinds of companies and investors attracted to the CBRN medical countermeasure enterprise. To date, with few exceptions, large, experienced pharmaceutical companies have not entered the CBRN medical countermeasure market because of lack of sufficient profit or other incentives. This leaves the market to smaller and less experienced companies. The perception is that relying on companies, some without ever having successfully marketed a drug or vaccine, increases the risk of failure. Small to mid-sized companies are viewed as having limited financial reserves, inexperienced management, limited technical expertise, and limited experience with the FDA regulatory process. These numerous factors may have had synergistically negative effects on the outcome of a range of CBRN medical countermeasure programs.
Other Risks
Many medical countermeasures have been pursued without a clear vision of their effective use or acceptability to the public. Engagement with all stakeholders, from researchers and products developers to public health officials, medical providers, and the public is essential to an effective medical countermeasure enterprise. As an example, diagnostics are under-valued yet a crucial enabling capability that allow for more effective use of limited medical countermeasures. Without a clear understanding of utilization policies for diagnostics, informed by all necessary partners, diagnostics capabilities will never receive the high prioritization that they deserve.
In light of historic CBRN threats, the concern of terrorist acquisition of such weapons has lead to a sense of urgency. There is a perceived need to accelerate the development and production of CBRN medical countermeasure. According to the 2008 report by the Commission on Weapons of Mass Destruction (WMD), a WMD attack somewhere in the world, more likely biological than nuclear, is anticipated by 2013.16 Their report mirrors internal USG assessments.17 While this sense of urgency increases pressure, and expectations on these programs, there has not been a corresponding groundswell of public support or interest among US citizens. This results in programs that have substantial visibility but little support, and that require significant resources yet to date have low rates of return from the public’s perspective.
The national security risks reflect the perceived catastrophic political, military and economic consequences that a large scale CBRN incident could have on the nation. For national security policy-makers, the greatest risk may be not having sufficient types and quantities of CBRN medical countermeasure when the situation demands. Given such high stakes, it is imperative that policy-makers fully understand the risks inherent to commercial drug or vaccine development, and support the appropriate resourcing and managing of such programs.
There is an association between the risks of medical countermeasure development and perceived national security risk. High risks associated with CBRN medical countermeasure development intuitively translate to greater risk from a national security perspective. However, the converse may not necessarily be true. Perceived high national security risks should not necessarily result in accepting greater technical risks of medical countermeasure development. On the contrary, it would seem essential, in light of national security risks, to mitigate the technical risks of medical countermeasure development and
procurement. The objective should be to control and limit technical risks so as to modify the national security risks perceived. The risk calculations for CBRN medical countermeasure development are therefore complex, multi-factorial and cross private and public domains subject to factors with and without historical precedent. It is incumbent upon the USG to provide the necessary assistance to mitigate as many risks as possible.
US GOVERNMENT RESPONSES TO RISK
The USG has conducted research and development of CBRN medical countermeasures since World War II. The Department of Defense (DOD) has historically had the principal role in CBRN medical countermeasure development. DOD’s requirements are to ensure the protection of its military force conducting assigned military operations. The preponderance of this research and development effort has been focused on vaccines and drugs for pre-exposure protection. Despite its long-standing efforts in this endeavor, external assessments determined that DOD was not organized to develop and license vaccines, therapeutic drugs, and antitoxins. Their efforts were characterized as disjointed, with the following factors cited: fragmentation of responsibility and authority, changing strategies, a lack of strong management, limited technical expertise, and a financial commitment that was not commensurate with the requirements of its program goals.18,19
In 1998, in response to the growing concern about the risk of WMD terrorism, President Clinton designated the Department of Health and Human Services (HHS) as the Lead Federal Agency in planning and preparing for response to domestic WMD-related medical emergencies.20 HHS, in conjunction with the Department of Veterans Affairs, was directed to stockpile antidotes and pharmaceuticals in the event of a WMD incident. The National Pharmaceutical Stockpile (later renamed the SNS) was the first ever civilian medical stockpile, containing necessary medication to treat those exposed to biological or chemical weapons.21 Contrary to DOD’s pre-exposure approach, HHS research and development efforts focus primarily on post-exposure prophylaxis and therapeutics. In addition to its different strategic approach, HHS has to consider the greater diversity of potential recipients of these medical countermeasures, including special populations such as children, the elderly, and the immunocompromised.
Following the terrorist airline and anthrax letter attacks of 2001, the USG significantly increased its investments in basic research and development of CBRN medical countermeasures, as well as the acquisition of countermeasures, for the expansion the SNS. The USG has appropriated and spent approximately $55 billion for biodefense preparedness, including researching, developing, and acquiring CBRN medical countermeasures.22,23 Approximately $1.6 B per year has been spent by the NIAID in basic research since FY 2004. Congress also appropriated $5.6 B for acquisition of CBRN medical countermeasures through Project BioShield.
President Bush issued several Homeland Security Presidential Directives (HSPD) pertaining to medical countermeasure development: HSPD-10, National Biodefense Policy; HSPD-18, WMD Countermeasures; and HSPD-21, Medical and Public Health Preparedness. These policy documents provided guidance pertaining to the importance, approach to research and development, and utilization of CBRN medical countermeasures. HPSD-18 states “the development and acquisition of effective medical countermeasures to mitigate illness, suffering, and death resulting from CBRN is central to our consequence management efforts.” This document also states “it is the policy of the U.S. to draw upon the considerable potential of the scientific community in the public and private sectors to address our medical countermeasure requirements relating to CBRN threats.” However, none of the policy documents specifically address the inherent challenges in either developing or acquiring such products. HSPD-18 simply states that creating such medical countermeasures is a “time-consuming and costly process.”24
In the Public Health Security and Bioterrorism Preparedness and Response Act of 2002 (PL 107-188) Congress affirmed the priority of CBRN medical countermeasures by directing the Secretary of HHS to
-
ensure development and acquisition of smallpox vaccine;
-
accelerate the approval of priority medical countermeasures as a fast-track product through the FDA;
-
direct the FDA to develop the Animal Rule; and
-
accelerate research and development on medical countermeasures against top-priority threat agents.
Congress also played a significant role in incrementally lowering the perceived barriers to medical countermeasure development. The initial
|
22 |
See http://www.armscontrolcenter.org/policy/biochem/articles/fy09_biodefense_ funding. |
|
23 |
Franco, C. 2009. Billions for biodefense: Federal agency biodefense funding, FY2009-FY2010. Biosecurity and Bioterrorism: 7. 1-19. |
|
24 |
HSPD-18 Weapons of Mass Destruction Countermeasures, The White House, January 31, 2007. |
legislative actions were directed to entice large pharmaceutical companies to participate in CBRN medical countermeasure development. The requisite measures to overcome perceived barriers to their participation were several: guaranteeing a medical countermeasure market,25,26 limiting liability, improving USG contracting practices, clarifying regulatory guidance, and expediting the regulatory process.
President Bush proposed the Project BioShield Act in 2003, which attempted to address the need for a guaranteed USG market for CBRN medical countermeasures. The original proposal would have permitted payment for acquisition directly from the US Treasury, which would have circumvented the annual Congressional authorization and appropriation process. This approach would have provided unlimited funds for medical countermeasure acquisition that were deemed necessary and sufficiently mature for inclusion into the SNS. Avoiding the annual appropriations process and permitting unlimited expenditures was believed to be sufficient incentive to induce large, more experienced companies to enter the CBRN medical countermeasure market. As part of this initiative, an extensive oversight mechanism was created, requiring the Secretaries of HHS and Homeland Security (DHS) and the President to certify that the acquisition of the medical countermeasure was necessary.
Congress balked at such a proposition based on its Constitutional responsibilities for conducting oversight and providing regular appropriations. Congress eventually passed a bill in 2004 that created a $5.6 B discretionary reserve fund for procuring CBRN medical countermeasures for the SNS. Though the indefinite mandatory authorization was not passed, the extensive oversight mechanisms requiring DHS, HHS and Presidential concurrence were retained. In addition to these funds, the Act included provisions to increase NIH (NIAID) authorities and flexibility to expedite basic research and development of medical countermeasures; it also created the Emergency Use Authorization process for FDA.27
While BioShield created a guaranteed Federal market for CBRN, it was a limited one-time appropriation. Large established pharmaceutical companies were not attracted to the CBRN medical countermeasure market. Other significant barriers remained, such as product liability, that increased the perceived and potential risks to established companies. Since these medical countermeasures would not likely have extensive human testing or use prior to an emergency, companies feared that widespread use of their products in an emergency could result in previously unrecognized adverse events that the companies could be held liable for.
|
25 |
Miller, H., and S. Kazman. 2002. Federalize vaccine production? We’d be taking a shot in the dark. Hoover Digest, No. 2. www.hoover.org/publications/digest/3437401.html. |
|
26 |
Johnson-Winegar Testimony, 2000. |
|
27 |
Project BioShield Act of 2004. PL 108-276. |
Additionally, BioShield initially mandated that payment was conditional upon product delivery; the only exceptions were advance payments—capped at 10% of contract value—that could be made before delivery. BioShield contracts were fixed price contracts that were viewed by HHS and their industry partners as overly restrictive. In later legislation, Congress authorized HHS to award milestone payments of 5% at various points in medical countermeasure development, with cumulative awards limited to 50% of the total contract value.28
In 2005, the threat of a looming H5N1 influenza pandemic prompted Congress to pass the Public Readiness Emergency Preparedness Act (PL 109-148). The Act limits claims that result from injuries or death from public health medical countermeasures (for pandemic and CBRN threats) when used during a declared public health emergency, or if there is credible risk of such an emergency. It specifically protects manufacturers, distributors, program planners, persons who prescribe, administer, or dispense countermeasures, and employees of any of the above. It provides manufacturers absolute immunity, except if injury or death resulted from willful misconduct.29 Limiting liability significantly affected the willingness of major seasonal influenza vaccine manufacturers to develop and manufacture pandemic influenza vaccines. In contrast, it did little to incentivize companies to participate in CBRN development.
The Pandemic All-Hazard Preparedness Act of 2006 (PL 109-417) specifically addressed the reality that large experienced companies generally have not entered the CRBN medical countermeasure market. Rather than seeking to create further incentives for large pharmaceutical manufacturers, the bill attempted to mitigate the perceived financial, contracting, legal, and regulatory risks of the medical countermeasure development process for the smaller, less experienced companies that were participating. The bill also granted HHS limited antitrust exemptions to facilitate collaboration between companies involved in medical countermeasure development.
The bills’ sponsors, Senators Burr and Kennedy, proposed creating the Biomedical Advanced Research and Development Authority (BARDA) under the Assistant Secretary of Preparedness and Response (ASPR) to support, coordinate, and provide oversight of advanced development of pandemic vaccines and CBRN medical countermeasures. The act required that HHS facilitate and increase communication between the HHS, FDA and the participating companies, and establish strategic initiatives to accelerate development and promote innovation in CBRN medical countermeasure. It provided HHS with the authority to award contracts, grants, and cooperative agreements or other transactions to promote innovation, development of research tools and research into rapid diagnostics, broad spectrum antimicrobials, and vaccine manufacturing technologies. BARDA was granted
the same authorities as DOD’s Defense Advanced Research Project Agency (DARPA).
Over time, all these executive and legislative actions shaped and modified the medical countermeasure landscape. Each separate action attempted to clarify the strategic priorities of and perceived obstacles to the USG medical countermeasure effort. The cumulative result of these efforts is a loose coalition of Executive Branch agencies that attempt to align and leverage their activities in medical countermeasure development, acquisition, distribution, and utilization.
President Obama announced before Congress during his 2010 State of the Union Address the “launching a new initiative that will give us the capacity to respond faster and more effectively to bioterrorism or an infectious disease—a plan that will counter threats at home and strengthen public health abroad.”30 His commitment reinforces the earlier announcement by Secretary of HHS to evaluate the capacity and capabilities of the United States to develop and manufacture vaccines and other products against influenza pandemics and bioterrorism.31
The US Government now retains CBRN medical countermeasure assets valued at nearly $4 billion and has committed significant additional funding for infrastructure including biocontainment, manufacturing, animal models, public health and hospital preparedness. There have been successes in the HHS medical countermeasure enterprise, but also ample opportunity to make improvements in its efficiency and effectiveness. The definition of success for the development of CBRN medical countermeasures can be broadly characterized as having a licensed, safe and efficacious medical countermeasure that can be rapidly distributed and administered to mitigate sickness, injury, or death resulting from exposure to CBRN agents.
OVERARCHING CONCLUSIONS
-
The commercial research and development pathway for drugs and biologics is lengthy, expensive, and risky; the CBRN medical countermeasure pathway compounds these challenges with additional financial and regulatory risks.
Failures in the commercial drug development process are principally due to shortfalls in product efficacy, adverse safety and toxicity profiles, and undesirable returns on investment. The industry has developed sustainable
|
30 |
President Barack Obama, Remarks by the President in State of the Union Address, U.S. Capitol, January 27, 2010, http://www.whitehouse.gov/the-press-office/remarks-president-state-union-address. |
|
31 |
The White House. National Strategy for Countering Biological Threats. By President Barack H. Obama. November 23, 2009. http://www.whitehouse.gov/sites/default/files/National_Strategy_for_Countering_BioThreats.pdf. |
-
business models that account for substantial research and development costs and significant candidate attrition. The scientific, regulatory, and financial foundations of CBRN medical countermeasures are significantly more unstable than for mainstream drug and biologic products. In addition, the failure of a CBRN medical countermeasure candidate has been viewed as a catastrophic event by both government and industry participants; these failures need to be incorporated into the system as inevitable and acceptable risks of the business, as they are in mainstream pharmaceutical development. The goal should be to ensure that potential failures occur at the earliest possible stages of the product development process.
-
If considered solely on the basis of financial risk, most CBRN medical countermeasures would never be developed.
The national security consequences of a large scale CBRN attack compel the US government to lead the pursuit of safe and effective medical countermeasures to protect the United States against these threats. However, the current risks of medical countermeasure development are complex and cross private and public domains. It is essential that all partners work aggressively to mitigate potential risks throughout the development process. These risk categories include scientific/technical, organizational, regulatory, financial, and policy.
-
Despite some high-profile failures, the CBRN medical countermeasure development process has improved over time, and there are clear success stories to learn from. In responding to the threat of smallpox, HHS provided strong Secretarial leadership, created specific requirements for the desired product, and focused necessary resources to develop, manufacture, and sustain a smallpox vaccine capability to protect every United States citizen. Since the Project BioShield Act of 2004, successive legislative actions have also expanded authorities for contractual flexibility, and provided appropriations, as well as limit liability, and created long-term (warm-base manufacturing) contracts, and milestone payments. Many challenges remain in developing a sustainable medical countermeasure industry, but there are positive lessons to be drawn from past experience, and a wealth of tools that the US government can use to improve the process.
-
Senior US government officials must reaffirm the high priority of the CBRN medical countermeasure mission. Leadership from the highest levels is essential for motivating and focusing the wide range of stakeholders to successfully prepare for these threats, and for attracting a large and diverse population of product developers. A clear signal of a long-term commitment to this market is essential for investors and industry partners. The ongoing shortfalls in available resources for product development leads to the perception that these activities are unimportant. Pharmaceutical and biotechnology companies and the investment community know the costs of commercial drug and vaccine development. US government funding to date has not been
-
commensurate with commercial industry standards or with the perceived high risk of these programs. Appropriate investments have not been appropriated or allocated for any one, much less all thirteen, of the identified material threat agents.
-
Increased clarity and transparency in product requirements and desired characteristics are essential for every CBRN medical countermeasure. The 2007 HHS PHEMCE Strategy and Implementation Plan for CBRN Threats represented the first effort by the US government to project future CBRN medical countermeasure needs. However, this guidance was only at the level of product category, and provided no additional detail as to the desired characteristics or quantities of each class of product. Past ambiguities concerning USG priorities, commitment, and requirements have undermined the confidence of participating companies and their investors, and have limited the interest of potential industry participants.
-
The HHS oversight and management of CBRN medical countermeasure development has improved over time, but still does not reflect comprehensive end-to-end portfolio and product management. Participating companies large and small will always encounter challenges in developing these novel products as a result of the myriad scientific and technical hurdles. However, it is in the vital interest of the mission that the US government do everything possible to mitigate all foreseeable and avoidable risks. The US government management of programs has substantial room for improvement. Prioritization by senior US government officials, combined with extensive in-house product development expertise, is central to improving oversight and management. The oversight and management of medical countermeasure basic research, through advanced development to stockpiling and sustainment, remains fragmented across several different agencies and budgets. Relationships across departments and agencies must be strengthened and formalized. The basic research portfolio does not appear to be optimally aligned to support the top-priority medical countermeasures outlined in the 2007 HHS PHEMCE Implementation Plan. A consequence of that suboptimal alignment is a far smaller number of candidate products than is necessary to counteract the expected rate of candidate attrition. At the other end of the pipeline, costs of sustaining and replenishing the Strategic National Stockpile (SNS) have not been factored into current and future budget estimates, highlighting the need for a planning and budgeting process longer than the annual appropriations cycle.
-
The regulatory process supporting CBRN medical countermeasure approval and licensure demands improvement. Incomplete and sometimes conflicting guidance to participating companies from HHS agencies has created confusion that has led to unnecessary or duplicative studies, and potentially wasted time and resources. As the contracting agencies, BARDA and NIAID offer companies regulatory advice that can be inconsistent or conflict with FDA
-
guidance. A lack of regulatory expertise from industry developers only compounds these difficulties, as many companies are completely reliant on government partners for navigating the regulatory process. Improvements in the science that underpins the regulatory process is also essential. The understanding of the pathobiology of these diseases and the host responses to CBRN agents is lacking in many cases. Further clarity and guidance with respect to effective use of the animal rule is also necessary; most threat agents do not yet have validated animals models, or correlates of protection from animals to humans. Additionally, the FDA has been tasked with this substantial new mission since 2002, and has received little to no additional funding to support development of the necessary human capital to review submissions and provide guidance. At present FDA does not receive funding either through its annual budget or user fees to support its regulatory review of CBRN medical countermeasure products.
-
With few exceptions, experienced pharmaceutical companies have not entered the CBRN medical countermeasure market. Smaller and less experienced companies predominate, and present a variety of partnering challenges. Without expert assistance, vaccine and drug development by inexperienced companies is associated with a significant risk for failure. These companies generally have not successfully achieved FDA approval or licensure of any products, have limited technical expertise or experience with the FDA regulatory process, and require coordinated assistance from experts at BARDA, NIH and FDA.32 Building a cadre of HHS experts in product development and manufacturing is critical to supporting this endeavor, and requires the government to place a high priority on developing an elite workforce.
In addition, the performers in the CBRN market generally have limited financial resilience and are almost completely dependent on external funding.33 Their financial standing can be severely compromised when faced with additional studies and trials; more established performers would have sufficient resources to weather these anticipatable delays. The financial weakness of each separate performer is compounded into a significant vulnerability for the sustainment of the long-term medical countermeasure mission.
LESSONS LEARNED AND POTENTIAL SOLUTIONS
These case study analyses evaluate the perceived relative risks associated with the development and production of selected CBRN medical countermeasures. The risks are qualitatively weighed to reflect the preponderance of data collected through interviews, literature and official document reviews. It is worth noting that a risk resides in the eye of the beholder, and reflects the perspective of the observer. Participants in different components of the medical countermeasure enterprise will weigh relative risks very differently. Furthermore, the CBRN medical countermeasure development landscape is not static. Since 2000, the USG has actively sought to lower barriers to industry participation and the “rules of the game” have been continually amended and improved. As such, each medical countermeasure analyzed was subject to a changing set of rules, challenges, and opportunities.
Despite the process volatility, there are several practices that the USG has done right. For example, HHS created specific requirements, providing clear priority and strong leadership from the Secretary of HHS, and the necessary resources to develop, manufacture, and sustain a medical countermeasure capability against smallpox. The successive legislative actions—the 2002 Bioterrorism Preparedness, Project BioShield, PREP, and PAHPA Acts—all expanded authorities to increase contractual flexibility, increase appropriations, limit liability, create long-term (warm-base manufacturing) contracts, and introduce milestone payments. The summation of these actions incrementally improved the process by which the HHS pursues CBRN medical countermeasure development and procurement.
These successes can be replicated and built upon. However, the remaining challenges must be recognized and addressed. HHS can improve the likelihood of success for current and future programs by exploring some of the following approaches.
First, the perceived USG priority and need for CBRN medical countermeasures is inconsistent and unclear. The USG and the Secretary of HHS must reaffirm the priority and need for these products. Companies partially base their decisions on entering the medical countermeasure market on their determination of the USG’s commitment and level of dedicated investment. Ambiguity on the part of the USG creates uncertainty for the companies and their investors. Beyond the strategic ambiguity, there is also ambiguity as it relates to the overall requirements and the prospective product characteristics that can result in unsatisfactory or incomplete Request for Proposals (RFP). The lack of specificity in RFPs can introduce potential candidates that do not meet the real needs or are at the wrong stage of product development for consideration. All these factors can dramatically extend the administrative time and cost of medical countermeasure development.
Changes in anticipated acquisitions, such as the cancellation of RFPs, undermine the confidence of companies and the investment community. The ambiguity in priorities is enhanced by a perception that the USG has devoted an
unrealistically low level of funding to advanced development and stockpile sustainment of medical countermeasure. The investment community is well acquainted with the cost of commercial drug and vaccine development. Investors assess that the USG funding is not consistent with commercial industry standards and is not commensurate with the rhetoric concerning the perceived risk. Furthermore, the 13 published Material Threat Determinations (MTD) have not been prioritized. By neglecting to do so, the USG fails to convey a relative importance among the 13. HHS has not budgeted or appropriated sufficient advanced development funding for any one medical countermeasure program, much less for all medical countermeasures for all 13 identified material threats.
There is a significant level of concern regarding the long-term outlook for the existing USG funding for CBRN medical countermeasures. In the FY2010 budget request, the Obama administration approved transferring roughly $609 million from the Project BioShield Special Reserve Fund to NIAID ($304 million) and BARDA ($305 million).34 While the support of additional advanced development activities is essential for success, removing the necessary resources from the only dedicated procurement fund sends mixed signals to potential developers.
After this transfer, the Project BioShield Special Reserve Fund has approximately $2.4 billion remaining through fiscal year 2013. This raises an even more significant question: what will the USG biodefense procurement budget be after 2013? Uncertainty surrounding the USG commitment to biodefense affects both company interest and investor confidence in this sector.
There are significant opportunities for improving the HHS organizational element of the medical countermeasure effort. The USG oversight and management of CBRN medical countermeasure development has evolved and improved but still does not reflect an “end to end” or “cradle to grave” comprehensive approach. When employed, this comprehensive approach has had tangible success; interviewees characterized development of ACAM2000™ as an “end to end” effort that resulted in successful stockpiling of a new smallpox vaccine.
Strong leadership and priority from senior USG officials, combined with extensive technical expertise in product development, is central to improving that oversight and management. Responsibilities for oversight and management of medical countermeasure basic research, through advanced development to stockpiling and sustainment, remain fragmented across several different agencies and budgets. The integration of efforts across participating agencies must be strengthened. The NIAID CBRN research portfolio does not appear to be optimally aligned to support the priorities of medical countermeasure development outlined in the 2007 HHS PHEMCE Implementation Plan for CBRN Threats. A likely consequence of that suboptimal alignment is that there
are too few prospective CBRN medical countermeasure candidates to overcome the probabilities of failure for the development process. Furthermore, the costs of sustaining and replenishing the SNS have not been factored into current and future budget estimates; this highlights the need for a longer term planning and budgeting process than the annual appropriations cycle.
The regulatory process supporting the CBRN medical countermeasure development enterprise demands improvement. Incomplete and sometimes conflicting guidance to participating companies from HHS agencies has created confusion and may have increased costs. BARDA and NIAID offer companies regulatory related suggestions that can conflict with those that they receive from FDA. This guidance does not appear to be aligned across federal agencies or synchronized with the FDA.
Companies desire clear regulatory guidance to facilitate their product development. However, the regulatory science supporting CBRN medical countermeasure approval and rules for the development of CBRN medical countermeasure is not mature. The basic science underpinning CBRN medical countermeasure is an evolving field. As more knowledge and data is accrued, the ability of the FDA to provide regulatory guidance will likely improve. The animal rule requires further clarity and guidance, as there are not validated animal models for all the CBRN threat agents. Improving regulatory science is essential to improving the overall CBRN medical countermeasure enterprise. However, the FDA does not receive funding either through its annual budget or user fees to expand its scientific knowledge or support its regulatory review of CBRN medical countermeasure candidates.
Finally, the majority of medical countermeasure contracts (from BARDA and NIAID) are awarded to less experienced biotech companies, which represents a significant additional risk to successful product development. These companies generally have limited financial resilience and are dependent on external funding. They have limited assets, limited or non-existent revenue streams, and are heavily dependent on USG funding.35 Their financial standing can be severely compromised when technical or regulatory issues are encountered that require additional studies or trials, thus increasing the costs of development. These companies may lack the financial resilience to enable survival through the product development process. They may require USG grants or subsidies to not only successfully develop a product, but also to become financially viable companies to ensure long-term sustainment of that product.
These companies also lack broad or in-depth in-house technical expertise and also lack the appropriate supporting infrastructure for manufacturing or testing and evaluation.36 These companies require technical assistance that may or may not be available from BARDA, NIH, or FDA.37 Similar to the limited
technical expertise, there appears to be limited experience with the regulatory and licensure process. The simple summation of these individual risks may not entirely reflect the cumulative risk that these companies face. The risk of less experienced companies are perceived to exceed the “ordinary and expected” risks associated with commercial drug and vaccine development by large, experienced pharmaceutical companies.
Commercial drug and vaccine development is challenging. It requires managing the scientific, technical, and regulatory risks to produce a profitable outcome. The US government’s effort to successfully develop, procure, stockpile, and effectively use CBRN medical countermeasures is even more challenging. The pressing national security risks should compel all stakeholders to maximize resources, coordinate efficiently, and pursue all possible avenues to ensure that medical countermeasures are available to protect the public from the catastrophic outcomes of a potential CBRN event.