Summary
Tetrachloroethylene is a volatile, chlorinated organic hydrocarbon that is widely used as a solvent in the dry-cleaning and textile-processing industries and as an agent for degreasing metal parts. It is an environmental contaminant that has been detected in the air, groundwater, surface waters, and soil. In June 2008, the U.S. Environmental Protection Agency (EPA) released its draft Toxicological Review of Tetrachloroethylene (Perchloroethylene) (CAS No. 127-18-4) in Support of Summary Information on the Integrated Risk Information System (IRIS). The draft IRIS assessment provides quantitative estimates of cancer and noncancer effects of exposure to tetrachloreothylene, which will be used to establish air-quality and water-quality standards to protect public health and to set cleanup standards for hazardous-waste sites.
At the request of EPA, the National Research Council convened a committee to conduct an independent scientific review of the draft IRIS assessment of tetrachloroethylene from toxicologic, epidemiologic, and human clinical perspectives. The committee was asked to evaluate the adequacy of the EPA assessment, the data and methods used for deriving the noncancer values for inhalation and oral exposures and the oral and inhalation cancer unit risks posed by tetrachloroethylene; to evaluate whether the key studies underlying the draft IRIS assessment are of requisite quality, reliability, and relevance to support the derivation of the reference values and cancer risks; to evaluate whether the uncertainties in EPA’s risk assessment were adequately described and, where possible, quantified; and to identify research that could reduce the uncertainty in the current understanding of human health effects associated with tetrachloroethylene exposure.
COMMITTEE’S ASSESSMENT
The committee appreciates the extensive work that EPA has invested in the development of its draft assessment of tetrachloroethylene. However, the committee has identified concerns about some of the approaches that EPA used to evaluate the data on tetrachloroethylene and subjects about which inadequate information or rationales are used to support its risk assessment—factors that
call into question the soundness and reliability of EPA’s proposed reference values and cancer risk estimates for tetrachloroethylene. One of the overarching weaknesses of the draft assessment was a lack of critical analysis of the data on which EPA relied in evaluating methodologic strengths and weaknesses. That lack was particularly evident in the assessment of the epidemiologic data: study selection and conclusions appeared to be based heavily on results that showed positive associations, and other data and the strengths and weaknesses of the selected studies were not adequately taken into consideration. The committee observed similar problems in its review of EPA’s evaluation of the genotoxicity evidence, in which preference appeared to be given to studies that reported positive results. Specifically, EPA did not analyze studies critically with respect to their methodologic strengths and weaknesses, nor did it organize its discussion clearly to provide an integrated consideration of the weight of evidence on the genotoxicity of tetrachloroethylene. Other mode of action evaluations were also hampered in this way.
In the sections below, the committee evaluates EPA’s noncancer and cancer assessments of tetrachloroethylene. The committee’s recommendations focus on improvements that should be made by EPA in producing its final assessment and on improvements that EPA should pursue in the future when tetrachloroethylene is due for another update.
Noncancer Assessment
For noncancer effects of tetrachloroethylene, EPA proposes to set an inhalation reference concentration (RfC) and oral reference dose (RfD). Those are estimates (with uncertainty spanning perhaps an order of magnitude) of a continuous inhalation exposure and a daily oral exposure of the human population (including sensitive subgroups), respectively, that are likely to be without appreciable risk of deleterious effects during a lifetime. EPA’s proposed RfC is 0.016 mg/m3 (2 ppb), and its proposed RfD is 0.004 mg/kg per day. Those values are based on the neurobehavioral outcomes of visual dysfunction and cognitive deficits observed in epidemiologic studies. A 1995 study by Altmann et al., in which adverse neurotoxic effects (as measured by deficits in vigilance, reaction time, and visual memory) were observed in people who lived near dry-cleaning facilities, was selected as the basis of the derivation of the RfC and RfD. The committee was asked to evaluate the selection of neurobehavioral outcomes in support of the RfC and RfD, the key study used, approaches to route-to-route extrapolation, and the characterization of the uncertainties associated with the data.
Critical Noncancer End Point and Studies
The committee found that EPA adequately supported its selection of neurotoxicity as the critical effect on which to base the RfC and RfD. The draft IRIS
document illustrates that neurotoxic effects are the most sensitive effects of tetrachloroethylene and that reference values based on neurotoxic effects would be protective against other noncancer effects that occur at higher concentrations.
EPA provides descriptions of the relevant neurotoxicity studies, but its evaluation of the epidemiologic literature could be improved by providing a critical evaluation of the validity of study designs and evaluation of the methods used for data collection and analysis, which the committee judges to be most important in selecting key studies. EPA chose the 1995 study by Altmann et al. as the critical one for determining the RfC and RfD because it involved an environmental exposure and used a standardized computer-assisted testing battery. Those are reasonable bases for the choice, but they do not outweigh methodologic deficiencies that seriously compromised the results of the study. Most important, the referent group was not appropriate. The group had more education than the exposed group and appeared to have pre-existing differences in cognitive abilities, which could account for its better test results. Evidence of residual confounding by education can be seen in the variability in reported results. For example, there was no association between tetrachloroethylene and visual evoked potentials; this is important because changes in the visual system and abnormalities in visual evoked potentials have been associated with tetrachloroethylene and other related solvents, and they are essentially unrelated to education. Other limitations of the study included the lack of a rationale for initial selection of study subjects, inadequacy of exposure characterization, and lack of a dose-response relationship. Finally, even though the test battery was performed properly, some of the tests have not been well validated with regard to what they reveal about brain damage.
Thus, the committee disagrees with EPA’s selection of the 1995 Altmann et al. study as the basis of its risk calculations. In reviewing the database, the committee gave greater weight to studies that had the strongest methods; it neither chose nor excluded studies on the basis of their results. The set of studies that the committee judged to be more appropriate for supporting the RfC and RfD include those of Altmann et al. (1990), Cavalleri et al. (1994), Gobba et al. (1998), Echeverria et al. (1995), and Boyes et al. (2009).
Derivation of Reference Values
EPA derived sample inhalation reference values by using results from several supporting neurotoxicity studies for comparison with its principal study by Altmann et al. The committee found that some uncertainty factors were applied inconsistently; specifically, the application of the uncertainty factor to account for subchronic exposures in epidemiologic studies should be justified better. In some cases, EPA did not use such a factor; in other cases, it applied a value of 10 with weak justification.
The committee derived candidate values by using the same studies as EPA and additional studies. The committee found that the reference values from the strongest studies were in the range of 6-50 ppb (or 0.04-0.34 mg/m3). That range is higher than the RfC of 0.016 mg/m3 derived by EPA and is further supported when considered in the context of the full database (see further discussion below).
EPA extrapolated the results of inhalation studies to derive the oral RfD for tetrachloroethylene. Physiologically based pharmacokinetic (PBPK) modeling was used to support the route-to-route extrapolation. The rationale behind that approach is sound and adequately explained by EPA, and the choice of dose metric (blood area-under-the-curve) was appropriate and adequately supported by the available evidence. However, the three models used by EPA were formulated and validated with data from inhalation exposures; none was validated against blood concentrations that result from oral exposure. EPA empirically assumed a value for the rate of oral absorption of tetrachloroethylene; this assumption is inferior to direct estimation. Other PBPK models that use direct estimation are available, and their use may help to reduce the uncertainty in the assumed values; or additional PBPK models could be developed (see recommendation below for a harmonized PBPK model).
Graphical Presentation
EPA provides graphical comparisons of reference values, values that could be derived from supporting studies. Reference values derived from neurotoxicity data are presented, as are values based on other noncancer effects to illustrate dose dependence of multiple forms of observed toxicity. Overall, the committee supports the approach of presenting the evidence in this visual format. However, the committee recommends some revisions to improve illustration of the uncertainties being represented and to expand the presentation to include the larger body of literature on a particular end point to show how the RfC compares with sample reference values derived from studies that are methodologically sound but not judged to be critical for the RfC. Consistency between the RfC and such studies would provide additional support.
Figure S-1 provides an example illustration developed by the committee. It shows that the majority of sample values is centrally clustered, but there is a wide spread at the lower and higher ends. The overall range of the 19 sample reference values is 0.03-333 ppb (0.0002-2.6 mg/m3), but the range is reduced to about 6-50 ppb (0.04-0.34 mg/m3) when consideration is restricted to the five strongest studies. The RfC of 0.016 mg/m3 calculated by EPA on the basis of the 1995 Altmann et al. study falls below the range. The figure shows that sample reference values that could be derived from the full database of neurotoxicity studies provide some support for the range.
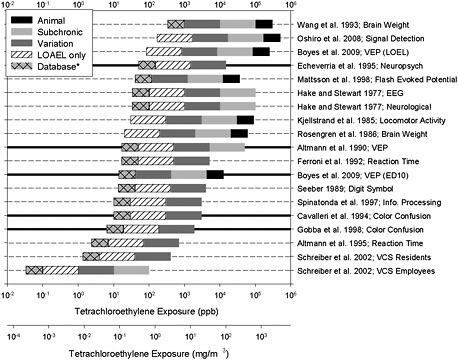
FIGURE S-1 Distribution of sample reference values. Each horizontal bar represents a single study. Thick, horizontal lines represent studies identified by the committee as most applicable to the development of an RfC. The right end of a bar is at the "point of departure" and is based on concentrations used in the referenced study after conversion to “human equivalencies” or, in the case of animal studies, after adjustment for continuous exposure. Uncertainty factors are illustrated in different shadings: a factor of 3 if it is necessary to extrapolate from animals to humans (black); a factor of 10 if it is necessary to extrapolate from acute or subchronic exposure to chronic exposure (light gray); a factor of 10 for individual variation to account for sensitive individuals (dark gray); a factor of 10 if the study did not contain a NOAEL (diagonal lines) and a factor of 3 for uncertainty in the data base as applied by EPA (light gray, cross-hatched). *A maximum total uncertainty factor of 3,000 was applied for the purpose of this exercise. Where this might be exceeded, the maximum was achieved by omitting the “database” uncertainty so that other uncertainties could be visualized. The committee has recommended that EPA review the uncertainty factors to ensure that they are appropriately explained and used consistently, so some of the individual values used here could be subject to change. In some cases, EPA might judge that the total uncertainty exceeds 3,000 and would, therefore, not use that study to derive a sample reference value. Source: Graphic developed by M. Christopher Newland.
Cancer Assessment
EPA faced a formidable challenge in its effort to characterize the carcinogenic properties of tetrachloroethylene both qualitatively and quantitatively.
There appears to be general agreement in the scientific community that tetrachloroethylene is carcinogenic in laboratory animals, but there is a longstanding debate about how to interpret and use the laboratory findings to predict human cancer risks. The debate is reflected in the committee’s inability to reach consensus on some aspects of the tetrachloroethylene assessment, which are discussed below.
Classification
EPA classified tetrachloroethylene as “likely to be carcinogenic to humans.” The committee reviewed the classification guidance in EPA’s 2005 Guidelines for Carcinogen Risk Assessment and the bioassay data available on tetrachloroethylene and concluded that EPA adequately documented that its classification has been based on the results of bioassays that found increased incidences of hepatocelluar tumors, mononuclear-cell leukemia (MCL), renal tumors, and hemangiosarcomas in laboratory animals and to a lesser extent on epidemiologic evidence. EPA’s decision to characterize tetrachloroethylene as likely to be a human carcinogen as opposed to “carcinogenic to humans” appropriately reflects the possibility that there are deficiencies or potential inaccuracies in interpretation of the data. Some of the possible deficiencies and inaccuracies are discussed below for each of the datasets.
Mononuclear-Cell Leukemia
An increased incidence of MCL in F344 rats has been reported in two bioassays. The biologic significance of the increases was debated by the committee because increases were observed in only one strain of rat, which is known to have a high background incidence of MCL, and because MCL’s relevance to humans and the mode of action of tetrachloroethylene causing it are not understood. In considering the high background of MCL, the committee found a published assessment by Thomas et al. (2007) that applied statistical approaches (life-table analyses) to bioassays of the National Toxicology Program (NTP) to interpret dose response relationships. Tetrachloroethylene was one of five chemicals of 500 tested by NTP that showed statistically significant increases in MCL in both male and female rats despite the high background rates. The publication advocated that such statistical evidence be supported with a weight-of-evidence analysis of biologic data before conclusions were drawn.
The committee found some support from epidemiologic studies that suggested an association between tetrachloroethylene and lymphoma, but the data were relatively weak and inconsistent. A difficulty in interpreting the findings is a difference of opinion about the human relevance of MCL. Some committee members judged that similarities between a form of human leukemia (natural killer-cell large granular lymphocyte leukemia) and rat MCL and results of mechanistic studies that the committee recommended be added to EPA’s as-
sessment were adequate to establish human relevance; others believed that more research was needed to establish the relevance. The committee agreed that there was little information on a mode of action of tetrachloroethylene in increasing MCL and that it therefore was not possible to determine whether exposure to tetrachloroethylene results in initiation of new tumors or enhances the expansion or promotion of existing tumors.
Hepatic Cancer
Statistically significant increases in hepatic tumors were observed in male and female mice after oral or inhalation exposure. As in the case of MCL, the biologic significance of the increases was debated by the committee because B6C3F1 mice have a high background incidence of hepatic cancer. However, the findings were reproduced in several studies conducted in different laboratories and showed a dose-response relationship. There is also fairly substantial information for characterizing potential modes of action of hepatic-tumor formation relative to the data available on MCL and renal cancer. Although the committee recommended that EPA revise its presentation of the mode-of-action evidence on tetrachloroethylene-related hepatic cancer to clarify its position, most of the members agreed with EPA that the mode of action is complex and remains to be established. The latter members also agreed that there was insufficient evidence to rule out human relevance. One member objected to those conclusions and to the committee’s support of using hepatic cancer to quantify risk. He argued that in the absence of evidence of other contributing modes of action, the evidence is sufficient to conclude that the mode of action in mice is predominantly through activation of the peroxisome proliferator-activated receptor-alpha, a mode of action that he considered to be of little relevance to humans. His arguments are presented in a dissenting statement in Appendix B of the report.
Renal Cancer
Tetrachloroethylene caused a low rate of induction of renal tumors in rats. Although the increases were not statistically significant when compared with concurrent controls, EPA has used historical controls to calculate the chances of two of these rare carcinomas to occur by chance to be less than 0.001. Furthermore, a dose-response trend was shown against the low background and the tumors in the treated rats were malignant whereas the tumors in the controls were not. EPA provided a strong evaluation of the potential modes of action for tetrachloroethylene-induced kidney cancer. The committee agrees with EPA that the mode of action of tetrachloroethylene tumorigenesis is not understood but that a mutagenic mode of action cannot be ruled out. Thus, renal tumors observed in tetrachloroethylene-treated rats were considered relevant to humans although additional characterization of quantitative relevance is desirable.
Selection of Tumor Type for Quantitative Assessment
The committee was unable to reach consensus on the selection of the critical cancer end point. The majority of the members judged that the uncertainties associated with MCL (particularly the high background incidence, uncertainty about the dose-response relationship, and poor understanding of mode of action) were too great to support using MCL data rather than data on hepatic or renal cancer for determining quantitative estimates of risk. Those members judged that the use of the MCL data could be justified only if it is EPA’s policy to choose the most conservative unit risk when considering options but that such justification should be distinguished as a policy decision, not a scientific one. They believed that a more scientifically defensible approach would be to use the dataset that has the least uncertainty rather than the dataset that yields the highest estimate of risk. In their judgment, the hepatic-cancer data would have the least uncertainty, followed by the data on renal cancer and MCL.
Other members judged that the MCL data should be used for cancer-risk estimation. Their opinions were based on the observation that reproducible, statistically significant increases in MCL in male and female rats above the background incidence of MCL were found and that MCL was the cancer end point with the highest magnitude of response. They believed that use of the most sensitive response to quantify cancer risk decreases the uncertainty associated with potential differences in metabolism and susceptibility to tetrachloroethylene among exposed populations. They concluded that additional statistical analyses of the dose-response data and the addition of supporting mechanistic information identified by the committee would strengthen the existing support of the use of MCL in the draft assessment.
Mode-of-Action Considerations
The modes of action1 by which tetrachloroethylene produces increases in
MCL, hepatic cancer, and renal cancer were an important consideration in EPA’s and the committee’s evaluations of the evidence. The analytic framework described in EPA’s cancer guidelines for considering hypothesized modes of action was best applied in the draft IRIS assessment’s consideration of renal cancer. The evaluation focused on synthesizing the evidence to support the idea that multiple modes of action may play a role. However, for hepatic cancer, the committee found that the assessment lacked the organization to present and provide appropriate context for the evidence clearly. It therefore recommended that EPA revise its mode-of-action assessment for hepatic cancer to support better the conclusions that were drawn. Specifically, the committee suggested that the mode-of-action analyses would be improved by outlining the proposed sequence of hypothesized tetrachloroethylene-associated key events (possibly with a diagram). Transparency would be improved by presenting the details of experimental results in tabular form to allow the reader to understand more easily the relative potency of tetrachloroethylene, or its metabolites, in inducing both key events and tumors. In this context, species and strain differences could also be considered more easily. The goals of the presentation should be to lay out the timeline of key events explicitly in the context of dose, to evaluate concordance between early and late events, and to consider the relative contribution of chemical-specific data compared with information on categories of chemicals. This approach should be applied to each hypothesized mode of action. Even if the data are ultimately judged to be insufficient to support a hypothesis, the exercise can be used to identify critical data gaps and to inform the direction of future research.
Low-Dose Extrapolation
EPA’s dose-response analyses of the various cancer datasets involved using several models to extrapolate to doses below the experimental range. EPA considered six datasets: hepatocellular adenoma or carcinoma in male and female mice, hemangiosarcoma in male mice, MCL in male and female rats, and renal tumors in male rats. It used the multistage model for each dataset because mode-of-action information was lacking or uncertain and the model was able to fit a broad array of dose-response patterns. However, because the studies used small numbers of dose groups and because the benchmark-dose software automatically fixed some parameters to zero to obtain convergence in model-fitting, the fitted models were nearly linear in the low-dose range. The imposed linearity explains the similarity among the slopes of the models and among the unit risks derived from the models. In the case of hepatocellular adenoma and carcinoma in male mice and MCL in female rats, EPA considered the fitted models acceptable solely on the grounds that statistical tests for goodness of fit had nonsignificant results (p > 0.10). The committee considers this to be a weak rationale in that the statistical significance of goodness-of-fit tests may not detect a poor fit when the number of animals per dose group is small. The questionable fitting of
the multistage model to some candidate datasets and insufficient consideration of alternative models contribute to underestimation of the overall uncertainties.
EPA adopted linear low-dose extrapolation, the default option, with several justifications. First, nonlinear, mechanistic models are unavailable for dose-response modeling because mode-of-action information on tetrachloroethylene is insufficient and support for dynamic models is unavailable. Second, because mathematical models are subject to uncertainties for low-dose extrapolation beyond the experimental dose range, linear extrapolation is more conservative than all sublinear (curvilinear) models. When individual thresholds in the human population are plausible, wide variation in threshold values typically implies a curvilinear shape of the dose-response relationship. Thus, linear extrapolation protects susceptible subpopulations. Third, a few of the candidate data, especially EPA’s preferred male-rat MCL data, exhibit a linear dose-response relationship. Whereas those arguments are consistent with EPA’s Guidelines for Carcinogen Risk Assessment, there is evidence in the candidate datasets that the underlying dose-response relationship can be supralinear (for example, in MCL in female rats). When that is the case, low-dose linear extrapolation is not conservative. EPA does not present the full ranges of variation and uncertainty in relation to model choice, in large part because it applied only linear or nearly linear dose-response models to all candidate datasets.
Age-Adjustment Factor
EPA did not apply an age-adjustment factor to its cancer risk assessment, because there is little evidence that tetrachloroethylene or its oxidative metabolites directly damage DNA, because information about genotoxicity of glutathione (GSH) metabolites in cell assays other than Salmonella or in vitro experiments is lacking, and because the mode of action of tetrachloroethylene has not been established. In addition, there are no data on differential sensitivity to tetrachloroethylene carcinogenicity among life stages. The committee agrees that those are adequate reasons for not using an age-adjustment factor but suggests that the rationale can be strengthened if EPA follows the committee’s suggestions for improving its analysis of the genotoxicity data and mode-of-action evidence.
Physiologically Based Pharmacokinetic Models
Tetrachloroethylene can be viewed as being metabolized by three pathways. The predominant pathway is the cytochrome P-450 (CYP) pathway that produces metabolites that have been associated with hepatic cancer. Two other pathways involve the GSH conjugation pathway that produces metabolites that are further metabolized by the β-lyase pathway or the β-lyase-independent pathway, each of which produce metabolites that have been associated with renal cancer. To take those metabolic factors into account, EPA used three PBPK
models to estimate human equivalent doses from animal studies and to perform route-to-route extrapolations. Each of the models used total metabolism of tetrachloroethylene as the dose metric. In some instances, EPA used a single model; in others, it used all three. The justification for using single or multiple models is not always clear. The committee observed that the models could yield different results because they were calibrated with different datasets, so comparisons among them were not straightforward. For consistency and to allow for better comparisons among end points, the committee recommends that EPA use a single PBPK model for its assessment. Ideally, the model would be a “harmonized” version of the three models used by EPA or of other relevant models (that is, a single model that integrates multiple exposure routes and tissue compartments).
The committee notes that the use of total metabolism as the dose metric for carcinogenicity reflects primarily the CYP metabolic pathway because of large differences in the flux of the metabolism between it and the GSH pathway. Using that dose metric does not reflect the contribution of the GSH conjugation pathway, which has been implicated in the development of renal cancer. EPA did not pursue the addition of the GSH pathway to any of the PBPK models, arguing that data on GSH-dependent metabolism are from in vitro studies or constitute measurements of urinary excretion products and do not represent toxic species in vivo. The committee agrees that the available data on the GSH pathway are more limited than the available data on the CYP pathway but notes that in vitro and urinary metabolite data were used in the development of the CYP-based PBPK models chosen by EPA. Thus, better justification is necessary to rule out modeling the GSH pathway.
The committee recommends that EPA explore the possibility of adding the GSH pathway to a harmonized PBPK model. If such modeling is determined to be infeasible, total metabolism can be used as a reasonably conservative dose metric. The modeling exercise would be useful in identifying data gaps that prevent successful modeling, which can be used to guide research that will allow more comprehensive PBPK models to be developed in support of the next IRIS reassessment of tetrachloroethylene.
Uncertainty Analysis
EPA has clearly identified key sources of uncertainty as part of its process of assessing the cancer risk posed by exposure to tetrachloroethylene, including human population variation, low-dose extrapolation, dose metrics, extrapolation from animals to humans, and the use of PBPK models for route-to-route extrapolation. The effect of uncertainties on risk estimates is assessed qualitatively in most parts of the IRIS draft except in dealing with such issues as the choice of dose-response models, the use of PBPK models, and, to a small degree, variation between studies. That approach reflects the current state of practice of uncertainty analysis.
In a few respects, the committee disagrees with EPA’s presentation on uncertainties. For example, EPA notes narrow variation between cancer risks derived from four dose-response models. However, in its comparison, EPA used only data on male rats, and all four models were linear or nearly linear at lower doses. Failure to consider a wider array of feasible dose-response models, including multistage models of various orders, could lead to inadequate quantification of uncertainty associated with the choice of dose-response model.
The committee supports EPA’s quantitative assessments of uncertainty with regard to choice of dose-response models, the use of PBPK models, and variation between studies. In particular, the committee found EPA’s consideration of uncertainty due to different forms of dose-response models to be valuable, and it recommends that such quantitative evaluations be extended to all candidate datasets so that a fuller array of uncertainties can be assessed.
CONSIDERATIONS FOR FUTURE RE-EVALUATIONS OF TETRACHLOROETHYLENE
The committee found several parts of the draft IRIS assessment that could be improved on in the future. Such changes are not necessary for completing the current assessment but should be considered when tetrachloroethylene is reevaluated in the future. They include improving transparency in selection and analysis of data, particularly with regard to uncertainty analysis. The committee encourages EPA to consider the most recent guidance from the National Research Council report Science and Decisions.
Organization and Approach
There is a vast amount of literature on tetrachloroethylene, and the draft IRIS assessment was hampered by having to manage the sheer volume of information on the chemical. Any new reassessment should begin with problem formulation and issue identification, consideration of whether to rely on previous reviews, determination of the focus of the new effort, and identification of the specific issues on which the reassessment is likely to focus. That would help to identify where multidisciplinary input at early stages of reanalysis should be sought, such as in data selection and mode-of-action evaluations in the context of risk-assessment practices. The process would include a delineation of criteria for selecting studies, approaches for conducting a weight-of-evidence evaluation, and options for dose-response assessment and the characterization of uncertainties. EPA should also consider ways to reorganize the document to streamline presentation of the data and analyses.
Uncertainty Analysis
EPA’s assessment of tetrachloroethylene follows a traditional approach to developing cancer slope factors and hazard indexes that takes uncertainties into account qualitatively and via uncertainty factors. EPA states that it has introduced a new method for uncertainty analysis in the context of the dose-response assessments for tetrachloroethylene, but the only notable differences between its tetrachloroethylene assessment and those of other chemicals are the consideration of multiple end points and the limited use of bootstrap simulation for only a portion of uncertainties. EPA’s uncertainty analysis remained typically focused on individual sources of uncertainty, and the analysis was often qualitative without presenting a full range of the uncertainty. Without an in-depth illustration of the propagation and cumulative effect of the uncertainties on the final risk estimate, quantification of the overarching uncertainty surrounding the final risk assessment is not possible. The committee notes that the current state of practice in quantitative uncertainty analysis does not fully meet the spirit of principles, guidelines, and recommendations that have accrued in recent years.















