2
Overview of the Toxicokinetics of Tetrachloroethylene
It is important to be familiar with the toxicokinetics of tetrachloroethylene when evaluating the Environmental Protection Agency’s draft Integrated Risk Information System (IRIS) assessment because many of the chemical’s effects are thought to be associated with metabolites rather than with tetrachloroethylene itself. The draft IRIS assessment includes a thorough cataloging of the published literature on tetrachloroethylene metabolism, including consideration of the specific metabolite isoforms that may be involved and polymorphic variants. This chapter presents a brief overview of the absorption, distribution, metabolism, and excretion of tetrachloroethylene to provide context for discussions in this report. More specific toxicokinetic issues associated with specific outcomes and the committee’s review of how they are handled in the draft IRIS assessment are discussed in later chapters.
Tetrachloroethylene is a volatile, lipophilic small molecule that is rapidly and extensively absorbed after inhalation and oral exposure. It can also be rapidly absorbed through the skin (Stewart and Dodd 1964), but dermal absorption appears to be a less important route of exposure. In humans, inhalation exposure to tetrachloroethylene typically results, within a few hours of exposure, in a pseudoequilibrium between inspired air and blood although there can be substantial interindividual differences in absorption behavior (Chiu et al. 2007). After oral dosing in animals, peak blood tetrachloroethylene concentrations are typically reached within 15-30 min, and systemic bioavailability is typically greater than 80% (Dallas et al. 1995); once absorbed, tetrachloroethylene is rapidly distributed throughout the body, and well-perfused tissues reach a pseudo-equilibrium with blood within a few minutes. For example, after oral administration of a 10-mg/kg dose of tetrachloroethylene in rats, peak tissue concentrations occurred within 10-15 min in blood, brain, heart, lungs, kidneys, and liver (Dallas et al 1994). The elimination half-life of tetrachloroethylene was comparable
among those tissues, between 6 and 7 hours (Dallas et al 1994). In poorly perfused tissues, such as fat and muscle, peak tetrachloroethylene concentrations are reached after a longer delay, which may be an hour or more than a day for adipose tissue. The elimination of tetrachloroethylene from fat is also much slower than that from other tissues and can take twice as long (Dallas et al. 1994). Because of its lipophilicity, the highest concentrations of tetrachloroethylene are found in adipose tissue (Savolainen et al. 1977; Dallas et al. 1994). In humans, the fat-to-blood concentration ratio has been estimated to be as high as 90:1 (Monster et al. 1979). Relatively high concentrations are also observed in the liver and brain (Savolainen et al. 1977). On the basis of animal studies and sparse human data, the brain concentration of tetrachloroethylene is 4-8 times the blood concentration (Dallas et al. 1994; Lukaszewski 1979).
The disposition of an absorbed dose of tetrachloroethylene occurs primarily through pulmonary excretion; metabolism is less important than for other chlorinated solvents, such as trichloroethylene. Mass-balance studies in rats with 14C-labeled tetrachloroethylene indicated that 70% or more of an oral or inhaled dose can be recovered in expired air as the parent compound (Pegg et al. 1979; Frantz and Watanabe 1983). The next most important excreted fraction occurs in urine and feces, which may collectively account for up to 23% of an administered dose. A small portion of the dose (less than 3%) may be converted to CO2 and exhaled. Most of the radioactivity recovered in urine can be attributed to formation of trichloroacetic acid, a nonvolatile metabolite of tetrachloroethylene that is excreted primarily in urine. That general pattern of disposition of tetrachloroethylene appears to be consistent after both oral and inhalation dosing (Pegg et al. 1979). However, it is important to note that the highest urinary and fecal elimination coincide with lower administered doses of tetrachloroethylene.
Despite the low overall metabolism of tetrachloroethylene compared with other chlorinated solvents, its metabolism has been studied extensively in both human volunteers and laboratory animals, using both in vivo and in vitro techniques. The studies showed that many metabolites are produced, including some known to be cytotoxic, mutagenic or both. Tetrachloroethylene metabolism can be viewed as having three pathways. The first is cytochrome P-450-mediated (CYP-mediated) oxidation. The second and third share a starting point: direct conjugation with glutathione to S-(1,2,2-trichlorovinyl)glutathione (TCVG) and then further processing to S-(1,2,2-trichlorovinyl)-L-cysteine (TCVC). For the second pathway, β-lyase catalyzes the formation of reactive products from TCVC. The third pathway is independent of β-lyase: TCVC is processed further by acetylation and sulfoxidation reactions. Genotoxic and cytotoxic metabolites are formed by each of these pathways. The predominant metabolic pathway is the CYP path, followed by the β-lyase pathway and then the β-lyase independent pathway. The TCVC derivatives are toxicologically important but quantitatively minor metabolites. A simplified scheme is shown in Figure 2-1.
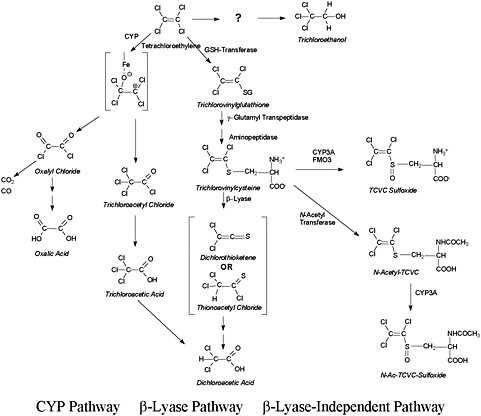
FIGURE 2-1 Simplified illustration of the metabolic pathways of tetrachloroethylene.
THE CYTOCHROME P-450 PATHWAY
The two major products of tetrachloroethylene metabolism by the CYP pathway are trichloroacetyl chloride and oxalyl chloride (Yoshioka et al. 2002). Trichloroacetyl chloride is mutagenic in the Ames test (DeMarini et al. 1994). Trichloroacetyl chloride reacts with lysine on protein to form stable trichloro adducts that can be detected with a specific antibody (Pahler et al. 1998). Trichloroacetyl chloride hydrolyzes to trichloroacetic acid (TCA), which produces liver cancer in mice (Nagano et al. 1998). Oxalyl chloride forms oxalic acid (possibly via oxalyl phosphate) or decomposes to CO2 and CO. Oxalic acid has long been known to be nephrotoxic; calcium oxalate complexes result in tubular toxicity (Guo and McMartin 2005) and nephrolithiasis (Bushinsky et al. 2008).
Mechanistic studies on the products of CYP oxidation of tetrachloroethylene indicate that trichloroacetyl chloride is the predominant product of the CYP-tetrachloroethylene complex; formation of tetrachloroethylene epoxide is much less favored (Yoshioka et al. 2002). Formation of chloral by rearrangement of tetrachloroethylene epoxide has been postulated, as a pathway to trichloroetha
nol in analogy with trichloroethylene. Neither chloral nor chloral hydrate has been identified after tetrachloroethylene exposure. Chloral is a product of trichloroethylene oxidation by CYP although not through an epoxide intermediate (Miller and Guengerich 1982). Chlorine migration of the CYP-oxygenated trichloroethylene results in formation of chloral, whereas the product of tetrachloroethylene is trichloroacetyl chloride.
Rats and mice given tetrachloroethylene by gavage were reported to excrete trichloroethanol in urine (Dekant et al. 1986a). The formation of trichloroethanol from tetrachloroethylene has been reported after occupational exposure (Birner et al. 1996), but it was not confirmed in human volunteers exposed to tetrachloroethylene (Volkel et al. 1998; Chiu et al. 2007). Birner et al. (1996) noted that—on the basis of studies by Larson and Bull (1992)—TCA does not undergo reduction to trichloroethanol and could not explain trichloroethanol formation; a later publication from the same group concluded that trichloroethanol was an artifact of trichloroethylene exposure (Volkel et al. 1998).
Small amounts of dichloroacetic acid (DCA) may be produced by dechlorination of TCA (Larson and Bull 1992), but most DCA arises from the β-lyase pathway (Volkel et al. 1998; Dekant et al. 1988).
THE β-LYASE PATHWAY
Tetrachloroethylene is conjugated with glutathione to S-(1,2,2-trichlorovinyl) glutathione and is later processed by β-glutamyl transpeptidase and aminopeptidase to TCVC (see Anders et al. 1988; Lash and Parker 2001). β-Glutamyl transpeptidase is a brush-border enzyme that is found primarily in the renal proximal tubule and to a lesser extent in the bile canalicular membrane. B-Lyase forms 1-mercapto-1,2,2-trichloroethene, which can tautomerize to dichlorothionacetyl chloride or lose HCl to form dichlorothioketene. Dichloro-thionacetyl chloride and dichlorothioketene both yield dichloroacetic acid (Dekant et al. 1988). Dichlorothioketene reacts with lysine on protein to form stable dichloro adducts that can be detected with a specific antibody (Pahler et al. 1998).
Genotoxicity by the β-lyase pathway is supported by several studies. TCVG induces unscheduled DNA synthesis in mammalian kidney cells, and this response is blocked by inhibiting γ-glutamyltranspeptidase or β-lyase; such inhibition indicates that the genotoxic metabolite arises by the β-lyase pathway (Vamvakas et al. 1989a). The dichlorothioketene adenine and cytosine adducts, formed in vitro in organic solvents, do have stability under physiologic conditions and are potential mutagens (Muller et al. 1998a). The chlorofluoro analogue forms adducts with calf-thymus DNA and produces strand breaks. That analogue has chemical properties similar to those of dichlorothioketene; 19Fl was substituted for a Cl to increase the sensitivity of detection (Muller et al. 1998b).
TCVC is cytotoxic to proximal tubule cells (Vamvakas et al. 1989b; McGoldrick et al. 2003). The toxicity is decreased by inhibition of β-lyase with aminooxyacetic acid. Elfarra and Krause (2007) reported potentiation of TCVC
toxicity in rats by aminooxyacetic acid, which provides evidence for a β-lyase-independent mechanism in TCVC toxicity in rats in vivo.
Dichloroacetate is produced primarily through the β-lyase pathway and produces liver cancer in rats.
THE β-LYASE-INDEPENDENT PATHWAY
TCVC undergoes acetylation to its mercapturate N-acetyl-TCVC and then sulfoxidation to N-acetyl-S-(1,2,2-trichlorovinyl)-L-cysteine (N-Ac-TCVCS), which is mediated by CYP3A or flavin-containing monooxygenase (FMO). In addition, TCVC undergoes sulfoxidation to TCVC-sulfoxide (TCVCS); this is also mediated by CYP3A or FMO (Ripp et al. 1997).
TCVCS is a more potent nephrotoxicant than TCVC in vivo (Elfarra and Krause 2007). TCVC toxicity is increased by inhibition of β-lyase with aminooxyacetic acid (Elfarra and Krause 2007), underscoring the importance of the β-lyase-independent pathway for kidney toxicity. TCVCS mutagenicity appears to be untested. N-Acetyl-TCVC is not mutagenic in the Ames test but is more cytotoxic than N-acetyl-TCVC, which is mutagenic in the Ames test (Werner et al. 1996).
SPECIES DIFFERENCES
There are important differences between species in the metabolism and toxicity of tetrachloroethylene. Much work has focused on differences between humans and rats, particularly on differences that would influence the human risk of renal cancer that has been observed in rat bioassays. Comparison studies between rats and humans indicate that humans metabolize tetrachloroethylene less than rats; this is based on measurement of metabolites (Birner et al. 1996; Volkel et al. 1998) and on the formation of adducts that are detected by antibodies that are specific for either the CYP-derived trichloro adduct or the dichlorothioketene-derived dichloro adduct (Pahler et al. 1998).
The CYP Pathway
The CYP pathway is the predominant route of tetrachloroethylene metabolism in rats and humans. Plasma albumin adducted with the trichloro derivative, indicating metabolism by the CYP pathway, was found in rats and humans exposed to tetrachloroethylene at 40 ppm for 6 hours. Immunochemical staining was used; the staining of protein from rats was 15-20 times more intense than that of protein from humans (Pahler et al. 1999). Cumulative excretion of TCA in urine was measured in rats and humans after similar controlled exposure to tetrachloroethylene at occupationally relevant concentrations (Volkel et al. 1998). The committee used that data to calculate the ratio of urinary TCA excre-
tion corrected for body mass in rats and humans. TCA excretion by rats was about 23 fold that of humans; or humans excreted about 4.4% of the amount excreted by rats.
The β-Lyase Pathway
Metabolism by the β-lyase pathway results in formation of dichloro protein adducts and DCA. Dichloro albumin adducts were detected in rat, but not human, blood samples after tetrachloroethylene exposure (Pahler et al. 1999). Even after immunoaffinity-column enrichment, the dichloro adduct was not detected in human samples. DCA is a stable product of the β-lyase pathway and is excreted in urine. Rats excreted DCA in urine at about one-tenth the amount of TCA, but DCA was not detected in urine collected from human volunteers after exposure to tetrachloroethylene (Volkel et al. 1998). That outcome is consistent with the lower activity of β-lyase in humans (McGoldrick et al. 2003).
The β-Lyase-Independent Pathway
Protein adducts resulting from the β-lyase-independent pathway have not been reported. N-Acetyl-TCVC, the mercapturate, is excreted in urine. Volkel et al. (1998) also measured urinary excretion of N-acetyl-TCVC after similar exposure to occupationally relevant concentrations of tetrachloroethylene. The Committee calculated the ratio of cumulative urinary excretion of N-acetyl-TCVC by rats to be about 5.5 fold that of humans; or humans excreted about 20% of the amount of N-acetyl-TCVC excreted by rats. Both rats and humans excrete much more TCA, the CYP-pathway product, than N-Ac-TCVC, but the ratio of N-acetyl-TCVC to TCA in humans is about 5 fold that of rats. That is, humans excrete relatively more tetrachloroethylene metabolites as N-Ac-TCVC than rats. That, too, is consistent with the lower activity of β-lyase in humans (McGoldrick et al. 2003); relatively more TCVC is metablized by the β-lyase-independent pathway in humans.






